Abstract
Purpose
Most index cases with novel coronavirus infections transmit disease to just one or two other individuals, but some individuals “super-spread”—they infect many secondary cases. Understanding common factors that super-spreaders may share could inform outbreak models, and be used to guide contact tracing during outbreaks.
Methods
We searched in MEDLINE, Scopus, and preprints to identify studies about people documented as transmitting pathogens that cause SARS, MERS, or COVID-19 to at least nine other people. We extracted data to describe them by age, sex, location, occupation, activities, symptom severity, any underlying conditions, disease outcome and undertook quality assessment for outbreaks published by June 2021.
Results
The most typical super-spreader was a male age 40+. Most SARS or MERS super-spreaders were very symptomatic, the super-spreading occurred in hospital settings and frequently the individual died. In contrast, COVID-19 super-spreaders often had very mild disease and most COVID-19 super-spreading happened in community settings.
Conclusions
SARS and MERS super-spreaders were often symptomatic, middle- or older-age adults who had a high mortality rate. In contrast, COVID-19 super-spreaders tended to have mild disease and were any adult age. More outbreak reports should be published with anonymized but useful demographic information to improve understanding of super-spreading, super-spreaders, and the settings in which super-spreading happens.
Keywords: Coronavirus, Super-spreading, Heterogeneity of transmission, Index cases
Introduction
During the COVID-19 pandemic, the role of super-spreaders in the transmission of the disease became a widely discussed topic within the scientific community and in the media. Throughout 2020, the media reported COVID-19 super-spreading in fitness classes, religious worship, skiing trips, and public transport. It has long been noted that for a variety of diseases, 20% of the host population has the potential to cause 80% of transmission (20/80 rule) [1]. Fully understanding the role of super-spreaders could enable more effective containment of disease outbreaks and more accurate modeling of epidemics. Studies have described super-spreading events for SARS [2], [3], MERS [4], [5], and COVID-19 [6], [7]. However, to date, there is little focus on the characteristics of the individual super-spreader and what factors might make an individual into a super-spreader [9], [10], [8]. By compiling details from super-spreading events across three novel coronavirus (nCoV) outbreaks, we aimed to assess whether there are any common factors between super-spreaders. This information may be used to guide contact tracing during outbreaks, aid early identification of super-spreaders, and prevent super-spreading, therefore helping to reduce total transmission and reduce harm from future pandemics [9], [10], [11].
Reasons offered to explain why only some cases become super-spreaders can be grouped into four categories: transmission pathways suited to that pathogen (e.g., droplet, fecal-oral, contact with bodily fluids, etc.), biological/individual factors (e.g., age, sex, ethnicity, viral load, duration of shedding), behavioral factors (e.g., type of work, number of contacts), and environmental factors (e.g., air circulation in workplace) [12]. We undertook this review to research attributes of individuals believed to be index patients in super-spreading events of nCoVs that cause any of MERS, SARS, or COVID-19, using evidence published by June 2021. We were interested in specific individual, behavioral and environmental factors that were likely to be most available in outbreak reports: transmission setting, sex, age, ethnicity, occupation, how many cases were in the cluster, severity of symptoms, disease course outcomes, and comorbidities.
Methods
We defined a “super-spreader” as an index case that was described in the scientific literature as linked to at least nine secondary infections with eligible viruses. We chose nine as the threshold to be indisputably much higher than the commonly cited effective reproductive numbers (between 2 and 5) for the nCoV diseases: SARS, MERS, and COVID-19 [14], [15], [13]. There is no formal consensus on how to define super-spreaders relative to the typical reproductive number for a stated pathogen [16]. Super-spreading was defined as “above the average number of secondary cases” for SARS [15]. Others suggested that super-spreaders should be defined as the 1% of index cases that generated the most secondary infections [9], [17], [18]. We acknowledge that other definitions of “super-spreader” have merits.
We were only interested in transmission between humans outside of deliberate experiments. We refer to “super-spreading events” as events where super-spreading is believed to have happened. Events ranged from a small birthday party to the annual Hajj pilgrimage in size and scope.
There was ambiguity in primary studies about how many secondary infections were directly linked to each index case. Different studies identified different numbers of secondary cases for the same index case, sometimes fewer than nine. We tried to be inclusive about this eligibility criteria: if at least one peer-reviewed assessment identified a minimum of nine secondary infections, we included the individual as a super-spreader. However, some sources that describe the same specific index case, may have suggested fewer secondary cases. Therefore, the range of secondary cases reported sometimes includes values under nine. We report the full range of possible counts of secondary cases from each specific index case as identified in relevant literature even if some of these were less than nine.
We undertook a structured search of scientific bibliographic databases using the search terms in Box 1. Note that how phrases in this search were interpreted by the search engine is that partial matches are returned; that is, searching for SARS would match to any of SARS (the disease), SARS-CoV (original name of the virus that causes SARS), SARS-CoV-1 (the new name of the virus that causes SARS) or SARS-CoV-2 (the virus that causes COVID-19). Searches were completed in June 2021. The search results were combined into a single database and de-duplicated. We chose not to use data from public-access inventories of apparent nCoV super-spreader events because we wanted to confine our efforts to the most validated evidence available to date, using evidence that had been collected by applying criteria that we understood, was designed to have minimal bias, and we could describe transparently. This review has PROSPERO registration CRD42020190596.
Box 1. Phrases used for scientific bibliographic database searches.
SCOPUS SEARCH.
( TITLE-ABS-KEY ( coronavirus OR covid* OR wuhan OR mers* OR "Middle East Respiratory" OR sars OR "sudden acute respiratory") AND ALL ( super*)) AND PUBYEAR>1999 AND ( LIMIT-TO ( SUBJAREA, "MEDI")).
medrxiv/bioRxiv/Preprint.org servers.
(coronavirus or COVID* or MERS* or “Middle East Respiratory” or SARS* or “sudden acute respiratory syndrome”).
And.
Super*.
OVID MEDLINE & EMBASE.
The search phrases was.
((coronavirus or COVID* or MERS* or “Middle East Respiratory” or SARS* or “sudden acute respiratory syndrome”).ti. or.
((coronavirus or COVID* or MERS* or “Middle East Respiratory” or SARS* or “sudden acute respiratory syndrome”).kw. or.
((coronavirus or COVID* or MERS* or “Middle East Respiratory” or SARS* or “sudden acute respiratory syndrome”).ab.).
and.
((super*).tx.).
Screening
Eligible publications were published in 2002 or later. Our latest search date was June 18, 2021. We considered publications in all languages if we could translate them into English or Spanish. Two independent screeners applied eligibility criteria, with a third person deciding if no consensus could be reached. Studies had to be chosen by at least two reviewers to go to full-text review. Full-text review confirmed eligibility criteria. The study had to describe infection(s) confirmed by a reverse transcription-polymerase chain reaction test (rt-PCR), cell culture or clinical presentation, and known exposure history. Eligibility criteria were:
-
•
The study must describe one of these three nCoV diseases: MERS, SARS, COVID-19.
-
•
Study design: almost any, but case or case-cluster studies were preferred. Preprints or gray literature from credible sources could be used and could be supported by sources such as public statements by individuals themselves, interviews, and press releases.
-
•
Any unplanned non-laboratory setting (e.g., clinical, schools, homes, places of worship, etc.).
Extraction and synthesis
One investigator undertook initial extractions which were checked by another researcher, with differences resolved by discussion or a third opinion. We tabulated and descriptively summarized this information separately for each disease. The extracted information was grouped by virus and/or linked disease (MERS, SARS, COVID-19). Pooled summaries were only undertaken if there were at least 20 super-spreaders found for any disease. No pooling of data beyond descriptive statistics (such as median age or percentage female) was attempted for smaller groups. We expected that much information would be heterogeneous and need to be reported as stated by the authors. Throughout the results, we often report raw numbers (such as numbers of persons found in a specific age group) but note that because the COVID-19 pandemic was much bigger than SARS or MERS outbreaks, the results should be considered relative to all cases (proportionately) for each specific disease.
Activities when super-spreading
We reported the activities that super-spreaders seem to have been doing when spreading infection (such as being an inpatient, working in a call center, attending a fitness class, etc.).
Setting
We determined where super-spreader had their impact, in settings such as households, hospitals, places of worship, etc. Additionally, the country was reported, as was a city or other regional information when available. For reporting purposes, the setting was grouped with activities when super-spreading.
Age and sex of the super-spreader
Age (in years) and gender were reported.
Ethnicity and occupation
We knew from preliminary searches that this information is largely unavailable, but report the information we found narratively.
Secondary cases
We recorded the direct count of first-generation cases and took this to equal the number of people that each super-spreader was believed to transmit to. This was expressed as individual case counts. This estimate was sometimes described as a range, as counts for index cases occasionally varied across different sources. We report all available information.
Symptoms and disease outcomes
Outcomes (especially mortality rates) for the super-spreaders were summarized in a simple narrative format.
Underlying conditions
We recorded narratively which underlying conditions the super-spreader individuals were reported to have (such as diabetes or heart disease). There is a hypothesis that super-spreaders are more likely (than the general population) to be people with compromised immune systems [19]. Conversely, there was also widespread public perception early in the COVID-19 pandemic [20] that super-spreaders might often be nearly or completely asymptomatic. We collected data that might address either hypothesis.
Deviations from protocol
We intended to report on tertiary and onward transmission counts, broken into percentiles or median estimates. We extracted this information but found that it was reported with such variability and inconsistency that it was not possible to summarize, and is not elaborated upon further. A post-hoc sensitivity analysis was undertaken.
Quality assessment
We designed a customized quality assessment of the primary studies ( Table 1). This checklist was designed to assess the credibility of sources and the rigor of their contact tracing methods and transmission chain reconstructions. We defined studies with six “Yes” answers as “Most credible,” studies with four or five “Yes” answers as “Fairly credible” and those with three or fewer “Yes” answers as “Less credible.”
Table 1.
Quality assessment checklist for included studies
| Category | Answers (from cluster of sources about each event) | |
|---|---|---|
| 1. Primary | Is the document a primary source of information, i.e., have they conducted the study themselves or are they reporting on the work of others? | YES: collected the majority of info themselves, but may mention some secondary data to inform their summary. Primary needs to be original interviews or review of original medical records. |
| NO: Obviously mostly or entirely citing others | ||
| UNCLEAR: other answers, including unclear sources or not clear that the majority of the info being reported was primary data collection | ||
| 2. Document | Has the main source of information been subject to peer review? | YES: Publication title = in Scimago or Pubmed |
| NO: other source | ||
| 3. Case definition | Is there a formal case definition (ideally chain based upon a PCR/antigen/lab test among at least one of the index/secondaries) as opposed to only symptoms etc? If not explicitly stated this could be surmised if the study was from a health agency. | YES: There is a
statement what case definition is, and this involves either
|
| NO: No case definition provided | ||
| UNCLEAR: Other case definition | ||
| 4. Rigor | Do sources state that a formal contact tracing procedure was in place? If not explicitly stated this could be surmised if the study is from a health agency. | YES: Describes interview(s) with most/all original index case(s) |
| NO: clearly No Interviews with index cases or valid proxies | ||
| UNCLEAR: Few/minority interviews with index/contacts, or can't tell, or not easy to answer YES or NO | ||
| 5. Exposure | Is there evidence that there has been close (enough) contact between the super-spreader and infected individuals (e.g., same hospital ward, apartment) **This may be difficult to define** | YES: Is plausible from contact history that had the opportunity to spend at least 10 min within 2 m of each other; or another mechanism for super-spreading has been suitably described and defended (e.g., Amoy Gardens aerosolized) |
| NO: Contact history not describing plausible high-risk contact opportunities | ||
| UNCLEAR: If contact history not sufficiently described | ||
| 6. Timeliness | Do the dates of contacts and illness match likely incubation periods (i.e., MERS median 5 range 2–14 days, SARS variable 2–7 days, COVID median 5 range 2–14 days) | YES: see windows <- |
| NO: outside those windows | ||
| UNCLEAR: not stated, can’t be sure |
Scoring: number of YES answers: Most credible: 6 YES answers, Fairly credible: 4–5 YES answers, Less credible: 0–3 YES answers.
Additional analyses and interpretations
We examined the information collected to attempt to establish whether people identified as super-spreaders of nCoVs “could be anyone” or tend to fit a profile, such as being late middle age males or especially socially connected individuals. We were interested in whether super-spreading depended on certain types of people or the situations they were in. This information was only interpreted narratively but with respect to relevant literature or statistics such as the age distribution of people in the country where the super-spreader lived.
We did not collect information on the viral load carried by identified super-spreaders because this information is not systematically reported. However, we collected information to address the hypotheses that super-spreaders tend to be especially asymptomatic or especially severely ill. The severity of the disease was indicated by mortality outcomes. Survival rates for super-spreaders were calculated for each disease. The survival rates of patients who are super-spreaders were compared to publicly available survival rates of known MERS, SARS, or COVID-19 cases.
A sensitivity analysis was undertaken. We considered if broad findings about age profile, sex balance, settings, or symptomatic status were especially different (segregated by disease) for individuals linked to a higher number of secondary cases (specified as at least 12 in all reports) and where the evidence base was at least fairly credible, that is, quality scores ≥4. We compare these subgroup findings narratively to results from the main analysis.
Results
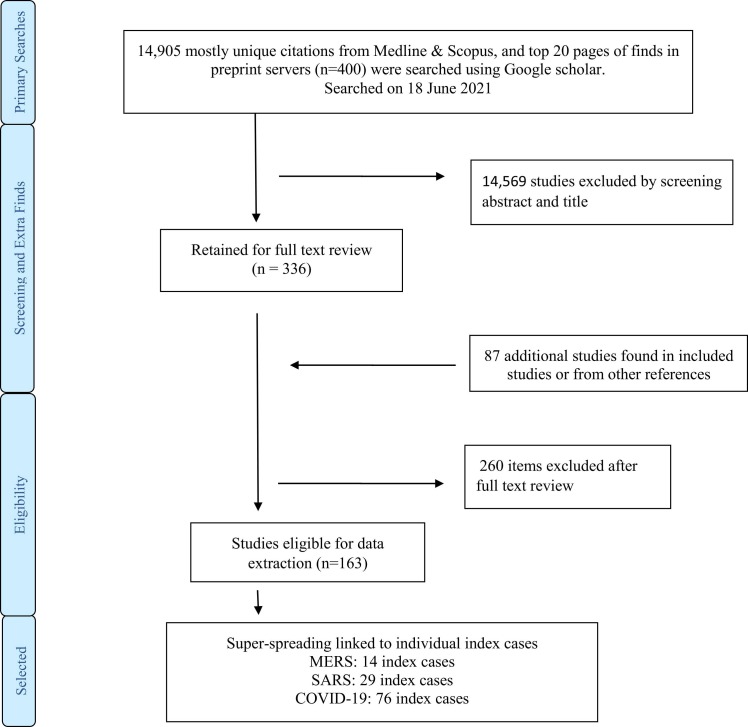
The search procedure is illustrated in Figure 1. Counts of super-spreaders identified for each disease were: COVID-19, 76; SARS, 29; MERS, 14. There were many more COVID-19 super-spreading individuals documented, partly due to the much wider spread of COVID-19, and long pandemic duration, starting in 2020, as well as better ascertainment methods available in 2020 compared to earlier years and greater numbers of papers published around this disease compared to MERS and SARS. The data summaries are discussed narratively and also presented in a condensed format in Table 2 (with sensitivity analysis).
Figure 1.
Study selection procedure.
Table 2.
Age, sex, symptomatic status, and transmission settings for subgroups
| n | Age median: IQR | Sex%M | % Symptomatic at time of transmission | % In community or mixed settings | |
|---|---|---|---|---|---|
| All studies, when at least some reports say ≥9 secondary infections | |||||
| SARS | 29 | 54: 42–70 | 71.4 | 100 | 12.0 |
| MERS | 14 | 48: 38: 60 | 83.3 | 100 | 0 |
| COVID-19 | 76 | 56: 43: 67 | 63.9 | 73.7 | 84.2 |
| When all reports say ≥12 secondary infections | |||||
| SARS | 18 | 57: 42–64 | 76.5 | 100 | 11.8 |
| MERS | 5 | 47: 41–53 | 100 | 100 | 0 |
| COVID-19 | 32 | 55: 43–59 | 44.0 | 72.0 | 87.5 |
| Only studies with quality score ≥4 | |||||
| SARS | 18 | 54: 43–71 | 72.2 | 100 | 12.5 |
| MERS | 10 | 50: 42–63 | 90.0 | 100 | 10 |
| COVID-19 | 32 | 55: 44–58 | 94.4 | 70.4 | 75.0 |
n = number of studies that contributed any useable data to any of age, sex etc., but often did not provide useable data for all of these fields. All non-clinical settings (including care homes or prisons, for instance) were treated as community settings; persons who were linked to transmission in both community and clinical settings were treated as “mixed” settings.
Quality assessment
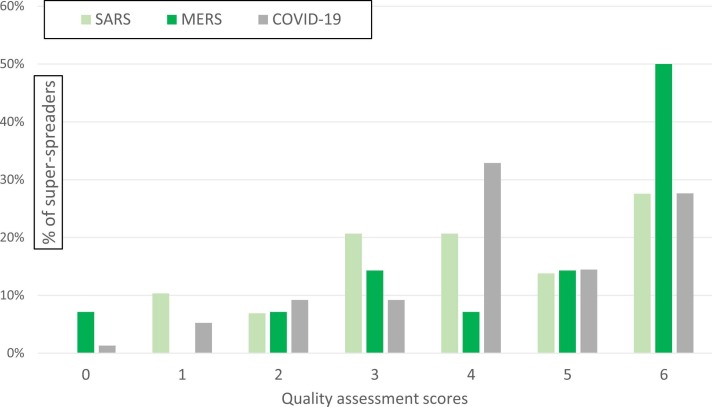
Figure 2 shows information about the distribution of quality scores for the individuals identified. The quality score data are shown as percentages with each score, to make it possible to compare the quality of evidence associated with each disease, in spite of the different case counts (many more for COVID-19). Altogether, 30% (36/119) of super-spreader individuals had epidemiologic descriptions that scored 6 in the quality assessment exercise (most credible rating). Proportionately, MERS super-spreaders had the highest number of “most credible” ratings (7/14, 50%). Around 28% (8/29) of SARS super-spreaders had epidemiologic evidence that was most credible (scores ≥6); this proportion was similar for COVID-19 super-spreaders (26% [21/76]). Proportionately, more COVID-19 outbreak reports were good or better quality than those for SARS or MERS, which is reassuring in light of much commentary about poor quality research published during the COVID-19 pandemic [21].
Figure 2.
Quality assessment scores for epidemiologic information on nCoV super-spreaders. Note: Questions in Table 1 were used to determine the quality assessment scores shown in Figure 2. nCoV, novel coronavirus.
MERS
We provide a narrative summary because only 14 eligible MERS super-spreader individuals were identified. See Table S1 in Supplemental Material for MERS super-spreader details. Eight index cases were in Saudi Arabia, five in South Korea, and one in the UAE. These transmissions happened from 2014 to 2017. The five South Korean super-spreaders were linked to the May–July 2015 MERS outbreak.
Activities when super-spreading and settings
All MERS super-spreading was linked to clinical settings, usually from hospital inpatients admitted for their recognized respiratory illness, although day patients who attended the clinic for other reasons (e.g., receiving dialysis) were also identified. Most secondary cases were other hospital patients and healthcare workers.
Age and sex
Index case age and sex were available for 12 individuals, who had a median age of 48 years (range 23–75 years, inter quartile range (IQR) 38–60.5). There were 2 females and 10 males.
Ethnicity and occupation
Traits such as ethnicity and occupation were mostly not reported. Two super-spreaders were stated to be Korean, one was Yemeni, and the patient in UAE was described as “ex-patriate.” The occupation was described for three MERS super-spreaders in the Middle East: police storage room staff, camel butcher, and factory worker. Most secondary cases were health care workers.
Secondary case counts
Estimated counts of secondary cases caused by these 14 index patients ranged in published studies (sometimes quite preliminary) from 2 to 89. The largest counts of identified secondary cases and total people in epidemiological clusters were in South Korea.
Symptom severity and outcomes
No MERS super-spreaders were described as asymptomatic; specific symptoms of severe illness were documented for nine super-spreaders among whom pneumonia was the single most common diagnosis (n = 5). No information was given on the survival or otherwise of two MERS super-spreaders. Of the remaining 12 super-spreaders, 4 survived to discharge and 8 were recorded as deceased from MERS (mortality rate 67%). The crude case fatality rate for MERS has been reported as 34.8% [22].
Underlying conditions
Two of the MERS super-spreaders were investigated for comorbidities but reported to have no underlying health conditions; information was not provided for the two others. Among the 10 super-spreaders with documented underlying conditions, a range of conditions was mentioned, including hypertension and kidney disease. Diabetes mellitus was the most commonly mentioned comorbidity (4 of the 14 super-spreaders).
SARS
Altogether, 29 eligible SARS super-spreader individuals were identified. Of these, 16 were in China, 5 in Singapore, 3 each in Canada and Hong Kong, and 1 each in Vietnam and Taiwan. All transmissions occurred in the period December 2002 to April 2003 (most were in March 2003). See Table S2 in Supplemental Material for a summary of extracted SARS super-spreader details.
Activities when super-spreading and settings
Almost all transmissions occurred while patients were in clinical settings (usually when they were admitted to the hospital for treatment related to their SARS, but also when being treated as outpatients, visiting other inpatients, or transiting between hospitals). Some transmission in the community was deemed likely for five super-spreaders. Air travel was identified as the transmission location for one patient.
Age and sex
Index case sex was available for 27 individuals. There were 7 females and 20 males. Index case age was available for 23 individuals who had median age of 54 years (range 22–91 years, IQR 42–70).
Ethnicity and occupation
Ethnicity or national origin was only reported for nine SARS super-spreaders: five were described as Chinese, one Malay, one Filipino, one Asian American, and one of “non-Asian” descent. There was no evidence that ethnic representation was different from ethnic distribution in the origin areas. The occupation was mostly not reported. Five individuals had occupational links to clinical settings: an ambulance driver, clinical professor, nurse, hospital laundry worker, and family physician. There were two business people, one seafood merchant and one vegetable seller. A patient aged 73–74 was described as retired. Similar to large MERS outbreaks, where occupational data were available for secondary cases, the secondary cases tended to be health professionals. Other occupations listed among the secondary cases were taxi drivers and market stall traders.
Secondary case counts
Estimated counts of secondary cases generated by these 29 index patients ranged from 4 to 51. Most cases were in the Far East (China, Hong Kong, and Taiwan) plus Canada.
Symptom severity and outcomes
No SARS super-spreaders were described as asymptomatic. Information was available on symptom severity for 22 SARS super-spreaders. Altogether, 18 were described as having a fever; symptoms of respiratory illness were reported for 17. No information was given on mortality for 13 SARS super-spreaders. Of the remaining 16 patients, 3 survived to discharge and 13 were recorded as deceased from SARS (mortality rate 81.3%). SARS mortality rates are sex- and age-dependent and have been estimated at 26% for Chinese patients age ≥80 years [15].
Underlying conditions
There was no information about possible underlying health conditions in 16 of the 29 SARS super-spreaders. Two were described as having unremarkable or “previously healthy” histories. Underlying health conditions were reported for 11 patients. A total of 8 of the 11 had cardiovascular conditions, 6 had forms of diabetes.
COVID-19
In total, 76 eligible super-spreaders of COVID-19 were found. Of these, 38 were in East or Southeast Asia, 15 were in Europe, 14 were in North America, and 9 were elsewhere in the world. The USA contributed 12 super-spreaders to the database, the largest count from a single territory. The majority of super-spreader events (n = 61) reported in this study occurred before the end of May 2020. See Table S3 in Supplemental Material for a summary of extracted COVID-19 super-spreader details.
Activities when super-spreading and settings
Information on activities that were happening when super-spreading events happened was available for 56 individuals. In contrast to the MERS and SARS outbreaks, most super-spreading COVID-19 index patients were active in the community at the time that they were index patients, not receiving medical care. Just 12 of the super-spreaders were linked to clinical settings, either as patients or healthcare workers interacting with patients and colleagues. Other activities or settings linked to super-spreading were social/business in nature (n = 22), religious worship (n = 7), fitness or sport (n = 5), education (n = 4), public transport (n = 3) and residential settings (one each of prison, summer camp, and long-term care facility).
Age and sex
Index case age was mostly unavailable; where age data were available this was predominately in Asian countries. Approximate or specific age information was provided for 28 individuals who had a median age of 56 years (range 18–85 years, IQR 43–67). From the 36 cases where sex was reported, there were 23 males and 13 females. All 13 super-spreaders whose sex was reported as female were from countries in East and Southeast Asia (where males were equally represented, n = 12). This sex imbalance for COVID-19 super-spreaders is likely an artifact of a widespread lack of reporting index patient sex rather than evidence of underlying sex disparity in representation.
Ethnicity and occupation
Ethnicity was rarely reported (only for six individuals). Where ethnicity was reported it was typically described by nationality: “American” (n = 3), “British” (n = 2), and Korean (n = 1). The occupation was mostly not reported but was described for 26 individuals (34%). There were six health care workers, four fitness instructors, four school staff, three religious leaders, two retired individuals, two businesspeople, and one person each described as an office worker, summer camp staff, sales representative, pilot, and meat processing plant worker. One person may be plausibly inferred as working as a church organist or piano player [23].
Secondary case counts
Estimated counts of secondary cases ranged from 8 to 78. The index cases with the largest counts of secondary infections were in Jordan, South Korea, Myanmar, Hong Kong, and the USA.
Symptom severity and outcomes
Symptom status was recorded for 50 individuals. Seven were described as asymptomatic at the time of super-spreading. Three were described as entirely asymptomatic, while four subsequently developed symptoms [24], [25], [26], [27], [28], [29]. Additionally, 13 super-spreaders were described as pre-symptomatic and three as mildly symptomatic. Other individuals had symptoms at the time of their super-spreading. No information on survival was available for 41 index cases. Three super-spreaders were reported to have died, while 32 were reported to have survived. This suggests a case fatality rate for super-spreaders of about 8.8%, which is 3.27 times higher than the all-age COVID-19 case fatality rate of 2.7% suggested by a pooled analysis of data available in the first 9 month of the COVID-19 pandemic [30].
Underlying conditions
Individual underlying health status was rarely reported for COVID-19 super-spreader individuals. It is likely that in some cases this lack of information means the absence of underlying conditions, but we cannot be sure without explicit statements. Just four super-spreaders were reported to have underlying conditions, while one was reported to have had a previous “record of good health.”
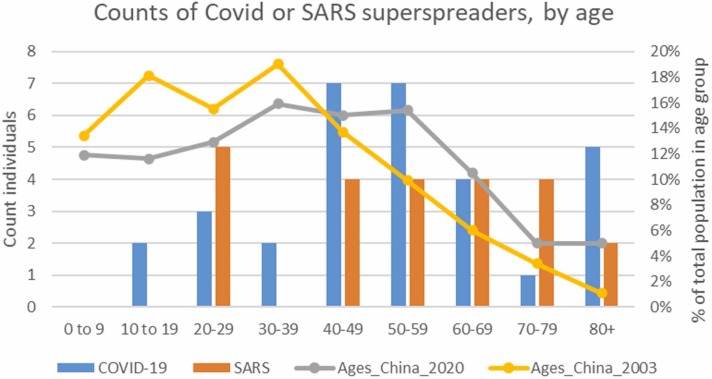
The lack of specific age data on most super-spreader individuals resulted in small country-level datasets, making a robust comparison with age distribution in each country untenable. The country that contributed the most specific age data was China. We, therefore, undertook a China-only comparison with the age-distribution estimates within the Chinese population of 2003 (SARS) or 2020 (COVID-19) (see Supplemental Fig. S4). This limited comparison suggests that older individuals (age 40+) were over-represented for both SARS and COVID-19 compared with age-distribution estimates within the Chinese population of 2003 (SARS) or 2020 (COVID-19). These data suggest that in China individuals more prone to super-spreading are relatively older, at least age 40+.
Figure S4.
Age distribution of super-spreader cases compared to concurrent proportion of population in each age group in China. Note: All super-spreader individuals are counted here but the population proportions in each age group are only for China in 2003 and 2020 because China contributed most of the age-specific data for either SARS or COVID super-spreaders detailed breakdown or comparison for each contributing country is not informative when ages were generally unavailable outside of China. Chinese Population distribution source: https://www.populationpyramid.net/china.
Sensitivity analysis
In our sensitivity analysis, we considered the age distribution, sex balance, transmission settings, and symptomatic status of super-spreader individuals for each disease, where the subgroups were defined by those individuals linked to at least 12 secondary cases in all reports, or where the evidence base had quality score ≥4. The results are in Table 2. There was a divergence from the main analysis findings, in that females slightly dominated the COVID-19 super-spreaders linked to ≥12 secondary cases; but this result was not confirmed for the most credibly documented COVID-19 super-spreaders (where males strongly dominated, as they did for all SARS and MERS subgroups). The median age was about 55 in these subgroups (compared to 48, 54, or 56 in all-data findings). Almost all settings for subsets of SARS or MERS super-spreaders were clinical, but community settings were the large majority for COVID-19 super-spreading. All subset SARS or MERS index cases were symptomatic, but about 30% of COVID-19 index cases in these subgroups were pre-symptomatic or never developed symptoms. Overall, this sensitivity analysis has results that agree with and reinforce conclusions that may be drawn from the results gained by analyzing the larger dataset.
Discussion
We produced a dataset that would provide empirical parameters for anyone attempting to model the role of super-spreading individuals related to nCoVs. The data we provide help to indicate which persons (by age or sex category, at least) in a population seem to be most likely to become super-spreaders. This information could help indicate which persons in a specific population (with known age/sex distribution and contact patterns) might contribute to epidemic growth. However, this is an appropriate opportunity to state that no one chooses to be highly infectious and hence in no way do we mean to imply stigma on any persons with such demographic traits; no one chooses to be highly infectious.
Observable mortality rates for super-spreaders of all the nCoV we examined were much higher than mortality statistics for the same diseases. However, we note that mortality outcomes for COVID-19 index cases were particularly incompletely reported. The count of COVID-19 super-spreaders who died was only three—so we may have an inaccurate picture of how often COVID-19 super-spreaders tend to die from the illness.
For MERS and SARS, super-spreaders tended to be quite ill. No asymptomatic super-spreaders were described for MERS and SARS, in spite of the prevalence of asymptomatic or mild infection among MERS cases being 26.9% [22]. This may be because for MERS and SARS only symptomatic people were tested. This contrasts with COVID-19, where super-spreaders who were completely asymptomatic throughout their disease course, or who were pre-symptomatic at the time that they transmitted the disease to others, were commonly reported. A recent meta-analysis found at least one-third of all COVID-19 cases were estimated to be truly asymptomatic [31]. This high prevalence of undetected infection severely challenged COVID-19 control. However, we acknowledge that biases in ascertainment may have affected epidemiologic reporting of symptom severity, especially for COVID-19. Individuals may have been motivated to incorrectly describe their symptoms as asymptomatic if they had social contact, due to legal proscriptions against social contact for people with COVID-19 symptoms in their jurisdictions at the time of their super-spreading.
Only two individuals aged under 20 years old were identified as super-spreaders for any of these nCoVs (both had COVID-19). One of these was age 18, another was described as a teenage staff member (so can be inferred to be close to or already reached adult age). In addition, not many super-spreaders were aged 19–39. Because younger people tend to have much higher social contact rates than older individuals [32], more youthful super-spreaders might have been expected. As asymptomatic infection of MERS [34], [33] is more common in younger people, and droplet spread of infectious respiratory disease tends to closely correlate with symptom severity [35], this might explain why we found relatively few super-spreaders under the age of 40 for MERS. However given that COVID-19 asymptomatic infection is also most common in younger people [36] and that many COVID-19 super-spreaders were asymptomatic, it is surprising there were not more younger COVID-19 super-spreaders. For SARS, it is unclear why there are fewer younger super-spreaders. There is little information on how asymptomatic SARS infection varies with age, although a study in Singapore indicates almost no difference in age between symptomatic and asymptomatic cases [37]. There is no clear explanation as to why most nCoV super-spreaders were aged over 40.
We tried to identify super-spreaders who generated at least nine secondary infections. However, the counts of secondary infections from individual index cases varied in the epidemiological studies. We did not aspire to evaluate which studies were most accurate (in absence of all primary epidemiological data). Identifying “super-spreaders” is challenging when there is much uncertainty about the true count of secondary cases.
There was insufficient information about the occupation to link specific lines of work to super-spreading. There was also very limited information about patient ethnicity. There were some large differences in settings or traits for MERS or SARS super-spreaders compared to COVID-19 index patients. MERS and SARS super-spreaders were much more likely to have been spreading in clinical settings, whilst COVID-19 spreading appeared to occur more commonly in the community. Alongside this, MERS and SARS super-spreaders were more likely to experience severe symptoms, while non-symptomatic super-spreading from COVID-19 index patients was common. These findings reiterate the value of high awareness of asymptomatic COVID-19 infection.
Limitations
Ascertainment bias affected our efforts. Universal screening of all MERS and SARS contacts rarely happened, in contrast to much broader testing of all COVID-19 contacts. Therefore, it is unsurprising that we collected data about more COVID-19 super-spreaders. Even when a super-spreading individual is detected within a surveillance system, a decision is made whether or not to publish as a case study (or academic publication) and it is unknown what biases this introduces. Case studies may be more likely for diseases perceived to be rare, which might explain why relatively more studies on MERS and SARS were produced. It is also possible that super-spreaders who are considered unusual or have already generated publicity are preferentially published. We have reported attributes, such as symptom severity, as described in the original studies. It may be that some attributes have not been reported faithfully. When extracting data from individual publications the details presented varied greatly between studies. More complete reporting would be desirable.
To systematically collect data about super-spreading individuals we had to create consistent criteria to define them. Slightly different criteria would have identified a different group of people. We did not collect data that would allow a sensitivity analysis using lower eligibility thresholds (such as only 3 or 5 secondary cases). After our searches and analysis were completed, a useful database of infection trees was published that could be used to support confirmatory and replicated analyses of nCoV super-spreading events using different definitions of what is a “super spreading individual,” at outbreaktrees.ecology.uga.edu [38].
COVID-19 is fast moving and our findings are only relevant to the time period investigated. Our latest searches were in June 2021 and all our super-spreaders were active in 2020; data about super-spreading linked to the Delta and Omicron variants of COVID-19 would not have been included.
Conclusion
A key question is what makes individuals super-spreaders [9], [10], [8]. We demonstrate that this question is challenging to answer due to the limited number of published studies on super-spreader individuals and inconsistencies in the available details about index cases in available studies. One solution would be to encourage the publication of more outbreak reports with suitably anonymized, consistent descriptions of the index and secondary patients. Since early 2020, detailed contact tracing databases have been assembled in many jurisdictions to facilitate COVID-19 control: these may form a rich resource for identifying super-spreaders. Should stronger data emerge, they could be incorporated into disease modeling or be used to guide intensive contact tracing in outbreak situations.
For all three diseases, we found that males and people aged 40+ were traits most typical of super-spreaders, while people under age 18 years were unlikely to be identified as super-spreaders. However, characteristics varied between the coronaviruses. Most super-spreading from MERS or SARS was observed in clinical environments, while COVID-19 super-spreading happened predominantly in community settings. Generally, MERS and SARS super-spreaders were highly symptomatic, and had poor disease outcomes and underlying health conditions. In contrast, many individuals who super-spread COVID-19 were observed to have mild or no symptoms at the time of their high transmission rate, and where survival status was documented, the majority survived the infectious period.
Approval to use the data to undertake the research
This was a secondary analysis of data in the public domain and therefore ethics approval was not required for the study.
Author contributions
JB and IRL conceived of the research. JB designed and ran the searches of bibliographic databases. All authors screened scientific studies and resolved differences using discussion or by consulting another author. IRL and NRJ designed the quality assessment which was undertaken by JB, NRJ and FCDH. JB designed the database, JB and IRL coordinated the project. All authors extracted data. JB summarized the dataset with descriptive statistics. JB and FCDH wrote the first draft and all authors revised for content.
Acknowledgments
This study was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emergency Preparedness and Response at King’s College London in partnership with the UK Health Security Agency (UK HSA) and collaboration with the University of East Anglia. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, UEA, the Department of Health or UK HSA.
Footnotes
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Iain Lake reports that he was grant recipient for financial support for all authors, as provided by the National Institute for Health Research.
Appendix
Supplemental Material, Table S1, Table S2, Table S3 and Fig. S4 .
Table S1.
Traits of MERS super-spreader individuals
| Name, age, sex | Dates/country | Activities/setting | Estimated #secondaries | Symptoms & outcome | Underlying condition | Quality score | Key source (s) |
|---|---|---|---|---|---|---|---|
| Pt III-A, 45, M | 2014, UAE | Patient in emergency department (ED) then critical care | 11 | Breathing difficulties. Died | No information | 5 | [39] |
| Pt1, 53, M | 2014, Saudi Arabia | While receiving kidney dialysis, clinic | 14 | Symptoms not reported. Died, died | In receipt of regular dialysis | 5 | [40] |
| Index, no info | 2015, Saudi Arabia | Patient in ED | 10 | No information | No information | 3 | [41] |
| Case 02, 56, M | 2015, Saudi Arabia | Hospital ED and inpatient | 8–9 | Symptoms not reported. Died | Diabetes, hypertension, hypothyroidism, coronary artery disease | 4 | [42], [43], [44] |
| Pt 1664, ∼48, F | 2016, Saudi Arabia | Hospital inpatient | 17–23 | Symptoms not reported. Died | Unspecified comorbidities | 2 | [45] |
| Pt1, 68, M | 2015, S.Korea | Seeking treatment in ED, later as hospital inpatient | 19–38 | Pneumonia, severe cough, hospitalized for months. Survived | Hypertension | 6 | [46], [47], [48], [49] |
| Pt14, 35, M | 2015, S.Korea | Seeking treatment in ED, later as hospital inpatient | 73–93 | Pneumonia, severe cough, respiratory failure, diarrhea. Survived | Ascertained but none known | 6 | [46], [47], [48], [49] |
| Pt15, 35, M | 2015, S.Korea | As hospital inpatient | 4–9 | Pneumonia without severe cough. Survived | Liver failure | 3 | [50], [51] |
| Pt16, 41, M | 2015, S.Korea | As hospital inpatient | 21–25 | Pneumonia, severe cough. Survived | Pancreatitis | 6 | [52], [48] |
| Pt76, 75, F | 2015, S.Korea | As hospital inpatient | 10–12 | Pneumonia, slight cough. Died | Multiple myeloma; diabetes mellitus | 6 | [52], [48] |
| Case 5, 65, M | 2017, Saudi Arabia | Seeking treatment in ED, later as hospital inpatient | 9–11 | Symptoms not reported. Died | Diabetes mellitus, hypertension | 6 | [4] |
| Index A, ∼47, M | 2017, Saudi Arabia | Seeking treatment in ED, later as hospital inpatient | 16–19 | Cough, shortness of breath, chest pain. No fever. History of diarrhea prior to admission. Died | Diabetes mellitus, hypertension, chronic kidney disease | 6 | [4] |
| Index B, 23, M | 2017, Saudi Arabia | Seeking treatment in ED, later as hospital inpatient | 9 | Fever, cough, rhinorrhea. Died | Ascertained but none known | 6 | [4] |
| Index, no info | 2017, Saudi Arabia | While receiving dialysis and within household | Up to 10 | No information | Was receiving dialysis | 0 | [22] |
Table S2.
Traits of SARS super-spreader individuals
| Name, age, sex | Dates/country | Activities/setting | Estimated #secondaries | Symptoms & outcome | Underlying condition (s) | Quality score | Key source (s) |
|---|---|---|---|---|---|---|---|
| Heyuan index, adult, M | Dec 17, 2002, China | As an inpatient | 10+ | High fever, mild respiratory symptoms. Outcome not stated | None known | 4 | [53] |
| Case A, 46, M | Late Jan 2003, China | As an inpatient, multiple hospitals | 74 | Fever, cough, sputum, chest abnormalities, shortness of breath, respiratory difficulties. Outcome not stated | No information | 5 | [54], [53] |
| Pt ZH, no info | Unstated, China | As an inpatient | ≥9 | No information | No information | 1 | [55] |
| Pt Y, no info | Unstated, China | As an inpatient | 11 | No information | No information | 1 | [55] |
| Case C, 42, F | Early Feb, China | As an inpatient | 11 | Cough and shortness of breath, hypoxemia. Died | Acute myocardial infarction and third-degree atrioventricular block, acute renal failure | 4 | [54] |
| HK index, 64, M | Early Feb, Hong Kong | Hotel guest, shopping, as an inpatient | 10–12 | Prolonged period of mild symptoms, followed by respiratory failure, cough, dyspnea, pleurisy, malaise, myalgia, rigor, fever, headache, ventilated. Died | "Unremarkable medical history" | 2 | [56] |
| Case B, 55, M | Early Feb, China | As an inpatient | 9 | Fever, chills, myalgia, malaise, rhinorrhea, cough, congested pharynx, swollen tonsils, ventilated. Died | No information | 4 | [54] |
| Case 1, 27, F | Late Feb, China | While seeking treatment, social contacts, as an inpatient | 12–18 | No information | No information | 5 | [57], [58] |
| Pt B, 47, M | Late Feb, Viet Nam | In the community and as an inpatient | 9–12 | Lower respiratory symptoms, medical ventilation. Died | No information | 1 | [59] |
| Index A, ∼22, M | Late Feb, Singapore | In the community and as an inpatient | 21 | Fever, dry cough, cough, dyspnea, vomiting, required oxygen, Critical care, headache. Survived | No information | 3 | [60], [61] |
| Case A, ∼73, M | Early March, China | As inpatient | 4–9 | No information | No information | 5 | [3], [57] |
| Case A wife, adult, F | March, China | No information, likely in the community | 19 | No information | No information | 6 | [3] |
| Pt, 26, M | Early March, Hong Kong | As inpatient | 43 | Fever, chills, rigor, cough, vomiting, diarrhea. Unstated outcome | “previously healthy” | 6 | [62], [63] |
| Case C, adult, M | Early March, Canada | In the community, as an inpatient, while in transit between hospitals | 18 | Myocardial infarction symptoms, also treated with renal dialysis. Died | Shortness of breath secondary to a pleural effusion | 4 | [64] |
| Index B, 27, F | Mid-March 2003, Singapore | As inpatient | 11–23 | Fever, sore throat, vomiting, diarrhea, not ventilated. Survived | None known, young health care professional | 3 | [60], [61] |
| Case B, 76, M | Mid-March, Canada | In the community and as an inpatient | 23 | Fever, diaphoresis, fatigue, intubated. Died | Atrial fibrillation, type 2 diabetes, coronary heart disease, hypertension | 3 | [65], [64] |
| Index C, 53, F | Mid-March, Singapore | As inpatient | 18–25 | Dyspnea, fever, pneumonia gram-negative bacteremia. heart failure, cough, dyspnea diarrhea, Critical care, intubated. Died | T2 diabetes and ischemic heart disease | 3 | [60], [61] |
| Flight 2 index, 72, M | Mid-March, China | Air travel (as a passenger) and as an inpatient | 10–24 | Fever, cough. Died | No information | 6 | [58], [66] |
| Tai Po index, age?, M | Late March, Hong Kong | As an inpatient | 24 | Diarrhea and abdominal pain, fever. Outcome not stated | No information | 5 | [67] |
| Index 1, 91, M | Late March, China | As an inpatient | 12–13 | Stroke, fever. Died | No information | 6 | [3] |
| Case Z, 88, M | Late March, China | As an inpatient | 13 | Cerebral infarction. Outcome not stated | No information | 6 | [3] |
| Index D, 60, M | Late March and Early April, Singapore | As an inpatient | 37–51 | Fever, pneumonia. Survived | Ischemic heart disease, diabetes mellitus, renal impairment, steroid-induced gastrointestinal bleeding | 3 | [60], [61] |
| Hospital C Index Pt, 54, M | Early April, Canada | As an inpatient | 9 | Fever, myalgia, headache, mild diarrhea, cough. Outcome not stated | Hyperlipidemia, hypertension, noninsulin-dependent diabetes | 4 | [68], [69] |
| Pt A, 62, F | Early April, China | As an inpatient | 33 | Fever, headache. Died | Diabetes mellitus | 6 | [2] |
| Index E, ∼64, M | Early April, Singapore | As an inpatient | 7–15 | Myalgia, cough, fever, dyspnea. Died | Ischemic heart disease hypertension, atrial fibrillation | 3 | [60], [61] |
| Pt D, 70, F | Mid-April, China | Visitor to hospital inpatient | 10 | No information | No information | 6 | [2] |
| Pt M, 54, M | Mid-April, China | As an inpatient | 33 | Myalgia, sore throat, mild productive cough, fever. Died | Coronary disease, type II diabetes, chronic renal failure | 4 | [57] |
| Hospital A Index Pt, 42, M | Mid-April, Taiwan | Lived and worked at the hospital | Up to 49 | Fever, diarrhea, shortness of breath. Died | Type 2 diabetes, peripheral vascular disease, treated for presumed salmonella infection | 2 | [70], [71] |
| 23, M | Late April, China | Mostly in community | 12 | No information | None reported | 6 | [2] |
The year is 2003 in all SARS cases except if noted otherwise.
? : information not found.
Table S3.
Traits of COVID-19 super-spreader individuals
| Name, age, sex | Dates/country | Activities/setting | Estimated direct # secondaries | Symptoms & outcome | Underlying condition | Quality score | Key source (s) |
|---|---|---|---|---|---|---|---|
| Pt 1, adult, M | Early Jan 2020, China | As an inpatient | 11–13 | Unclear if had symptoms. Died within 28 days of diagnosis | Yes but not specified | 3 | [72] |
| Pt A, 24, M | Jan 2020, China | Socializing | 13 | Occasional chest discomfort and dyspnea (only for 2 d). Survived | Hepatitis B, dyslipidemia, hypoglycemia, and slight liver dysfunction | 6 | [29] |
| Dr. B, adult, ?sex | Likely Jan 2020, China | Being at work at the hospital, mostly transmitted to colleagues | 16 | Onset after SSing. Mild symptoms. Survived | No information | 4 | [73] |
| C-1, 50s, M | Early Jan 2020, China | Visiting hospital to support inpatient | 11–21 | Fever, headache, chest pain, and myalgias. Died | No information | 4 | [74] |
| C-29, 50s, M | Mid-Jan 2020, China | Working as health care professional | 12 | Had symptoms. Survived | No information | 4 | [74] |
| Mrs. S, 64, F | Mid-Jan 2020, China | Pilgrimage, public transport, communal meal | 28–32 | Onset after SSing. Fever, cough, chills, myalgia. Survived | No information | 6 | [75], [76], [77] |
| Index, no info | ∼Jan 20, 2020, China | Meals together | 9–10 | No information | No information | 1 | [78] |
| Pt A, age?, M | Late Jan 2020, China | Riding public bus | 9–11 | Onset after SSing: yes symptoms but not specified. Outcome not stated | No information | 6 | [79] |
| Pt A1, no info | Late Jan 2020, China | Restaurant meal | 9 | Onset after SSing started: fever, cough. Outcome not stated | No information | 3 | [80] |
| Case 4, likely adult, M | Late Jan 2020, China | Working as supermarket staff; socializing | 12 | Onset after SSing started: fever. Outcome not stated | No information | 2 | [81], [82] |
| Pt 1, 24, M | Late Jan 2020, Hong Kong | Meals with family, socializing | 11 | Onset after SSing started: fever & cough. Survived | “record of good health” | 5 | [26] |
| UK-A, 53, M | Jan–Feb 2020, France and UK | Skiing, socializing | 9 | Unspecified mild-moderate severity. Survived | No information | 6 | [83] |
| Z-21, 61, F | Jan–Feb 2020, China | Mostly within household socializing | 10 | No information | No information | 1 | [17], [18] |
| Pt 12–31, ∼60, F | Jan–Feb 2020, S. Korea | Church services, church community socializing, church singing, group meal | 8–40 | Headache, fever, pneumonia (later). Survived | No information | 1 | [6], [84], [85] |
| Case 102, 43, M | Early Feb 2020, Hong Kong | Leading religious worship, socializing | 9–18 | No symptoms ever. Survived | No information | 4 | [27], [86] |
| Carnival index, likely adult, sex? | Mid-Feb 2020, Germany | Carnival, social & work contacts | 26–37 | Symptomatic. Outcome not stated | No information | 4 | [87] |
| Index, no info | Feb 2020, S. Korea | Link to the psychiatric inpatient ward | 11+ | No information | No information | 1 | [6], [88] |
| Instructor A, mid 40s, F | Feb 2020, S. Korea | Teaching dance fitness classes | 24 | Mild, just a cough. Survived | No information | 6 | [6], [89] |
| Instructor B, mid 40s, F | Feb 2020, S. Korea | Teaching dance fitness classes | 25–26 | Mild, just a cough. Survived | No information | 6 | [6], [89] |
| Pt 1–125, 56–57, F | Feb 2020, S. Korea | Working within call-center | 18 | No information on symptoms. Survived | No information | 0 | [6], [90] |
| Pt1, 57, M | Late Feb 2020, Germany | Scientific conferences and while attending hospital | 15 | Onset after SSing started: fever, cough, sneezing, loss of smell and taste. Survived | No information | 6 | [91] |
| S3, adult, sex? | Late Feb 2020, Italy | Possibly agricultural work | 17 | Unclear symptoms and outcome | No information | 5 | [92] |
| Flight-index, 27, F | Feb–Mar 2020, UK->Viet Nam | As aeroplane passenger and in the community | 19 | Unclear when symptomatic. Cough, fever, sore throat, fatigue, and shortness of breath. Outcome not stated | No information | 6 | [93] |
| A1.1, age? M | Feb–Mar 2020, USA | Restaurant meal, funeral, birthday party | 10 | Mild respiratory symptoms. Survived | No information | 5 | [94] |
| Pt A, adult, sex? | Early 2020, USA | Nosocomial, as patient and/or staff | Up to 14 | Fever, bilateral lower lobe interstitial infiltrates. Unclear outcome | Diabetes mellitus type 2, an active cancer | 4 | [95] |
| Pt 2–125, 82, M | ∼Mar 7, 2020, S. Korea | In community including religious worship | 11 | No information on symptoms. Survived | No information | 2 | [6] |
| E1, adult, M | Early March 2020, Switzerland | Social event(s) at workplace (office) | 8–10 | Fatigue, slight cough at the time he was SSring. Survived | No information | 6 | [96] |
| Choir index, no info | Mar 10, 2020, USA | Choir practice events | 10+ | Sore throat. Survived | No information | 3 | [97] |
| Bride’s father, 58, M | Mid-March 2020, Jordan | Large gathering, wedding, dancing, greetings, prewedding activities | Up to 76 | Fever, cough. Survived | No information | 4 | [98] |
| Pt 2–167, 44, F | Mid-March 2020, S. Korea | Unclear, possibly providing health care | 24 | No information on symptoms. Survived | No information | 2 | [6] |
| Pt 2–205, 74, F | Mid-March 2020, S. Korea | In community including religious worship | 51 | No information on symptoms. Survived | No information | 2 | [6] |
| Pt 2–309, 85, F | Mid-March 2020, S. Korea | Unclear | 21 | Was symptomatic. Survived | No information | 2 | [6] |
| Pt 1, ∼42, M | Mid-March 2020, Viet Nam | Socializing, traveling | 13 | Onset after SSing started: fever, cough, muscle aches, fatigue, and headache. Survived after a long severe illness | No information | 6 | [99] |
| Pastor, 57, M | Mid-March 2020, USA | Religious leader, church and bible study | 35 | Headache, body aches, lethargy, chills, nausea, mild fever, and cough. Survived | No information | 6 | [100] |
| Pt 2–476, 82, F | Late March 2020, S. Korea | Unclear, possibly providing health care | 9 | Was symptomatic. Survived | No information | 2 | [6] |
| Missionary-index, 68, M | Late March/early April 2020, Thailand | Acting as religious and community leader | 11–26 | Onset after SSing started: unspecified symptoms. Survived | No information | 5 | [101] |
| Case 590, no info | Jan–May 2020 Tunisia | No information | 9 | No information | No information | 4 | [7] |
| Case 399, no info | Jan–May 2020 Tunisia | No information | 12 | No information | No information | 4 | [7] |
| Case 75, no info | Jan–May 2020 Tunisia | Family gathering, wedding, meal | 10 | No information | No information | 4 | [7] |
| Case 106, no info | Jan–May 2020 Tunisia | Family gathering, wedding, meal | 14 | No information | No information | 4 | [7] |
| Case 704, no info | Jan–May 2020 Tunisia | No information | 9 | No information | No information | 4 | [7] |
| Case 342, no info | Jan–May 2020 Tunisia | No information | 9 | No information | No information | 4 | [7] |
| Case 453, no info | Jan–May 2020 Tunisia | Family gathering | 21 | No information on symptoms. Died | No information | 4 | [7] |
| Pt 2–508, 47, F | ∼Apr 1, 20, S. Korea | Unclear | 15 | No information on symptoms or outcome | No information | 2 | [6] |
| Imported primary, no info | Mar–Apr 2020, Faroes | Unclear | 14–15 | No information | No information | 4 | [102] |
| Domestic primary, no info | Mar–Apr 2020, Faroes | Unclear | 15 | No information | No information | 4 | [102] |
| School-linked, no info | Mar–Apr 2020, Faroes | Unclear | 10 | No information | No information | 4 | [102] |
| Resident, adult?, sex? | Mid-April 2020, USA | Recipient of dialysis in nursing home | 14–16 | Elevated temperature and malaise. Outcome unclear | In receipt of dialysis | 6 | [103] |
| B1, adult | May 2020, Germany | meat processing plant worker and community contacts | 18–29 | Always asymptomatic. Survived | No information | 6 | [104] |
| CS010, no info | May–Jul 2020, Mexico | Unclear | 17 | No information | No information | 5 | [105] |
| CS120, no info | May–Jul 2020, Mexico | Unclear | 9 | No information | No information | 5 | [105] |
| Index, no info | Summer 2020, Ireland | Social events | 25 | No information | No information | 3 | [106] |
| Team A player, adult, M | Jun 16, 2020, USA | Indoor team sport (ice hockey player) | 14 | Onset after main SSring: fever, cough, sore throat, and headache. Survived | No information | 6 | [107] |
| Staff index, teens, sex? | Late June 2020, USA | Residential camp staff | 26+ | Chills. Survived | No information | 6 | [108] |
| Inst A, adult, M | Jun 29, 2020, USA | Fitness class instruction | 10 | Onset after main SSring: fatigue, chills, body aches, cough, congestion, sore throat, and headache. Outcome unclear | No information | 6 | [25] |
| Inst B, adult, M | Jul 1–2, 2020, USA | Fitness class instruction | 11 | Onset after main SSring: body aches sore throat, fever, chills, cough, shortness of breath, fatigue, admitted to critical care. Outcome unclear | No information | 6 | [25] |
| School staff 1, adult | Jun–Jul 2020, UK | Working in a school | 9 | No information | No information | 4 | [109] |
| School staff 2, adult | Jun–Jul 2020, UK | Working in a school | 13 | No information | No information | 4 | [109] |
| School staff 4, adult | Jun–Jul 2020, UK | Working in a school | 11–12 | No information | No information | 4 | [109] |
| School staff 4, adult | Jun–Jul 2020, UK | Working in a school | 10 | No information | No information | 4 | [109] |
| Pianist, 18, M | Jul16–17, 2020, Australia | Playing instruments and singing in churches | 12 | Cough, fever. Survived | No information | 5 | [23] |
| Index, 85, M | Before August 2020, Japan | As inpatient | 12 | Fever, cough, and dyspnea. Chest computed tomography revealed unilateral pleural effusion and no findings of pneumonia. Outcome not stated | No information | 4 | [110] |
| Case-1, no info | Between March 23 and July 13, 2020, Myanmar | No information | Up to 11 | No information | No information | 3 | [111] |
| Case-24, no info | Between March 23 and July 13, 2020, Myanmar | No information | Up to 78 | No information | No information | 3 | [111] |
| Case-24, no info | Between March 23 and July 13, 2020, Myanmar | No information | 17 | No information | No information | 3 | [111] |
| HCW1, adult, sex? | Before Apr 27, 2020, Germany | At work in the pediatric dialysis unit | 11 | Fever, body aches, cough, headache, fatigue, blocked nose, loss of taste/smell. Outcome not stated | No information | 5 | [112] |
| Imported index, no info | Before August 2020, Hong Kong | Socializing; attended a wedding | 10 | No information | No information | 4 | [86] |
| Bar and band index, no info | Before August 2020, Hong Kong | No information | Up to 60 | No information | No information | 4 | [86] |
| Group D index, no info | Before August 2020, Hong Kong | No information | 11 | No information | No information | 4 | [86] |
| Wedding guest, no info | Aug 7, 2020, USA | Wedding preparations and wedding party | 30 | Onset after main SSring: On 8 Aug had onset of fever, runny nose, cough, and fatigue. Survived | No information | 6 | [28] |
| Pt B1, adult, sex? | Aug 11–12, 2020, USA | Healthcare worker at long-term care facility | 38–39 | Fever, chills, cough, myalgia, runny nose, and headache. Survived | No information | 6 | [28] |
| Pt A3, adult, sex? | Aug15–19, 2020, USA | Working as prison staff | At least 64 | Cough, myalgia, runny nose, sore throat, and a new onset loss of taste sensation. Survived | No information | 6 | [28] |
| Case-123, no info | Before Aug 24, 2020, Thailand | No information | 17 | No information | No information | 5 | [113] |
| Case-169, no info | Before Aug 24, 2020, Thailand | No information | 17 | No information | No information | 5 | [113] |
| Index, 33, M | Before Nov 9, 2020, India | At home, community socializing, at work as health care professional in a hospital | 16 | Fever, cough, and sore throat at presentation, but unclear at point of exposures. Outcome not stated | No health information; he lived in a poor area | 6 | [114] |
| P2, 84, F | Dec 2020, Hong Kong | As inpatient | 21 | Never had symptoms. Survived | Diabetic ketoacidosis | 5 | [24] |
? : information not found.
NOTES for all tables: Quality scores are based on count of “Yes” answers to quality checklist shown as Table 1. #secondaries means the number of direct secondary cases caused by the specified index case, according to the group of related articles that describe this index case; hence, the answer can be a range if multiple sources have different estimates for the number of secondary cases linked to the index patient.
References
- 1.Woolhouse Mark E.J., Dye C., Etard J.-F., Smith T., Charlwood J.D., Garnett G.P., et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Zhuang, Ning Fang, Zhou Weigong, He Xiong, Lin Changying, Chin Daniel P., et al. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng Guang, Xie Shu-Yun, Li Qin, Ou Jian-Ming. Infectivity of severe acute respiratory syndrome during its incubation period'. Biomed Environ Sci. 2009;22:502–510. doi: 10.1016/S0895-3988(10)60008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alanazi Khalid H., Marie E.Killerby, Biggs Holly M., Abedi Glen R., Jokhdar Hani, Alsharef Ali A., et al. Scope and extent of healthcare-associated Middle East respiratory syndrome coronavirus transmission during two contemporaneous outbreaks in Riyadh, Saudi Arabia, 2017. Infect Control Hosp Epidemiol. 2019;40:79–88. doi: 10.1017/ice.2018.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang Chang Kyung, Song Kyoung-Ho, Choe Pyoeng Gyun, Park Wan Beom, Bang Ji Hwan, Kim Eu Suk, et al. Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Korean Med Sci. 2017;32:744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Yejin, Jiang Xiaoqian. Evolving transmission network dynamics of COVID-19 cluster infections in South Korea: a descriptive study. medRxiv. 2020 [Google Scholar]
- 7.Safer Mouna, Letaief Hejer, Hechaichi Aicha, Harizi Chahida, Dhaouadi Sonia, Bouabid Leila, et al. Identification of transmission chains and clusters associated with COVID-19 in Tunisia. BMC Infect Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Paul Z., Koopmans Marion, Fisman David N., Gu Frank X. Understanding why superspreading drives the COVID-19 pandemic but not the H1N1 pandemic. Lancet Infect Dis. 2021;21:1203–1204. doi: 10.1016/S1473-3099(21)00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Smith J.O., Schreiber S.J., Kopp P.E., Getz W.M. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein Richard Albert. The 2019 coronavirus: Learning curves, lessons, and the weakest link. Int J Clin Pract. 2020;74 doi: 10.1111/ijcp.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kain Morgan P., Childs Marissa L., Becker Alexander D., Mordecai Erin A. Chopping the tail: how preventing superspreading can help to maintain COVID-19 control. Epidemics. 2021;34 doi: 10.1016/j.epidem.2020.100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frieden Thomas R., Lee Christopher T. Identifying and interrupting superspreading events—implications for control of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1059–1066. doi: 10.3201/eid2606.200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S., Jung E., Choi B.Y., Hur Y.J., Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2018;99:162–168. doi: 10.1016/j.jhin.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viceconte Giulio, Petrosillo Nicola. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516. doi: 10.4081/idr.2020.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). 2003, p. 47.
- 16.Al-Tawfiq Jaffar A., Rodriguez-Morales Alfonso J. Super-spreading events and contribution to transmission of MERS, SARS, and SARS-CoV-2 (COVID-19) J Hosp Infect. 2020;105:111–112. doi: 10.1016/j.jhin.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Xiao-Ke, Liu Xiao Fan, Wu Ye, Ali Sheikh Taslim, Du Zhanwei, Bosetti Paolo, et al. Reconstruction of transmission pairs for novel coronavirus disease 2019 (COVID-19) in mainland China: estimation of superspreading events, serial interval, and hazard of infection. Clin Infect Dis. 2020;71:3163–3167. doi: 10.1093/cid/ciaa790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X.-K., Liu X.-F., Wang L., Ali S.T., Du Z., Bosetti P., et al. Household transmissions of SARS-CoV-2 in the time of unprecedented travel lockdown in China. medRxiv. 2020 [Google Scholar]
- 19.Lakdawala Seema S., Menachery Vineet D. Catch me if you can: superspreading of COVID-19. Trends Microbiol. 2021;29:919–929. doi: 10.1016/j.tim.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grey, Heather. Just one person can transmit COVID-19 to dozens in a fewhours. Healthline. https://www.healthline.com/health-news/how-one-person-can-transmit-covid19-to-dozens-in-just-a-few-hours; 2020 [accessed 29.09.2020].
- 21.Bramstedt Katrina A. The carnage of substandard research during the COVID-19 pandemic: a call for quality. J Med Ethics. 2020;46:803–807. doi: 10.1136/medethics-2020-106494. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. MERS-CoV global summary and assessment of risk 2017. 2017, 8pp.
- 23.Katelaris Anthea L., Wells Jessica, Clark Penelope, Norton Sophie, Rockett Rebecca, Arnott Alicia, et al. Epidemiologic evidence for airborne transmission of SARS-CoV-2 during church singing, Australia, 2020. Emerg Infect Dis. 2021;27:1677. doi: 10.3201/eid2706.210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Vincent Chi-Chung, Fung Kitty Sau-Chun, Siu Gilman Kit-Hang, Wong Shuk-Ching, Cheng Lily Shui-Kuen, Wong Man-Sing, et al. Nosocomial outbreak of coronavirus disease 2019 by possible airborne transmission leading to a superspreading event. Clin Infect Dis. 2021;73:e1356–e1364. doi: 10.1093/cid/ciab313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groves Laura M., Usagawa Lauren, Elm Joe, Low Eleanor, Manuzak Augustina, Quint Joshua, et al. Community transmission of SARS-CoV-2 at three fitness facilities—Hawaii, June–July 2020. Morb Mortal Wkly Rep. 2021;70:316. doi: 10.15585/mmwr.mm7009e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam Ho Yeung, Lam Tsz Sum, Wong Chi Hong, Lam Wing Hang, Mei Emily Leung Chi, Kuen Yonnie Lam Chau, et al. A superspreading event involving a cluster of 14 coronavirus disease 2019 (COVID-19) infections from a family gathering in Hong Kong Special Administrative Region SAR (China) West Pac Surveill Response J. 2020;11:36. doi: 10.5365/wpsar.2020.11.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung Kenneth Siu-Sing, Ng Timothy Ting-Leung, Wu Alan Ka-Lun, Yau Miranda Chong-Yee, Lao Hiu-Yin, Choi Ming-Pan, et al. A territory-wide study of COVID-19 cases and clusters with unknown source in Hong Kong community: a clinical, epidemiological and phylogenomic investigation. SSRN: preprint. 2020 [Google Scholar]
- 28.Mahale Parag, Rothfuss Craig, Bly Sarah, Kelley Megan, Bennett Siiri, Huston Sara L., et al. Multiple COVID-19 outbreaks linked to a wedding reception in rural Maine—August 7–September 14, 2020. Morb Mortal Wkly Rep. 2020;69:1686. doi: 10.15585/mmwr.mm6945a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Xuejing, Ran Dongchuan, Wang Jinhui, Qin Yuan, Liu Ruishan, Shi Xueli, et al. Unclear but present danger: an asymptomatic SARS-CoV-2 carrier. Genes Dis. 2020;7:558–566. doi: 10.1016/j.gendis.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahammed Tanvir, Anjum Aniqua, Rahman Mohammad Meshbahur, Haider Najmul, Kock Richard, Uddin Md. Jamal. Estimation of novel coronavirus (COVID‐19) reproduction number and case fatality rate: a systematic review and meta‐analysis. Health Sci Rep. 2021;4 doi: 10.1002/hsr2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sah Pratha, Fitzpatrick Meagan C., Zimmer Charlotte F., Abdollahi Elaheh, Juden-Kelly Lyndon, Moghadas Seyed M., et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118(34) doi: 10.1073/pnas.2109229118. e2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossong Joël, Hens Niel, Jit Mark, Beutels Philippe, Auranen Kari, Mikolajczyk Rafael, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Tawfiq Jaffar A., Gautret Philippe. Asymptomatic Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: extent and implications for infection control: a systematic review. Travel Med Infect Dis. 2019;27:27–32. doi: 10.1016/j.tmaid.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan S., El Morabet R., Khan R.A., Bindajam A., Alqadhi S., Alsubih M., et al. Where we missed? Middle East Respiratory Syndrome (MERS-CoV) epidemiology in Saudi Arabia; 2012–2019. Sci Total Environ. 2020;747:141369. doi: 10.1016/j.scitotenv.2020.141369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff Werner E., Swett Katrina, Leng Iris, Peters Timothy R. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 36.McDonald Scott A., Miura Fuminari, Vos Eric R.A., van Boven Michiel, de Melker Hester E., van der Klis Fiona R.M., et al. Estimating the asymptomatic proportion of SARS-CoV-2 infection in the general population: analysis of nationwide serosurvey data in the Netherlands. Eur J Epidemiol. 2021;36:735–739. doi: 10.1007/s10654-021-00768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilder-Smith Annelies, Teleman Monica D., Heng Bee H., Earnest Arul, Ling Ai E., Leo Yee S. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taube Juliana C., Miller Paige B., Drake John M. An open-access database of infectious disease transmission trees to explore superspreader epidemiology. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter J.C., Nguyen D., Aden B., Al Bandar Z., Al Dhaheri W., Elkheir K.A., et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis. 2016;22(4):647. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assiri A., Abedi G.R., Saeed A.A.B., Abdalla M.A., Al-Masry M., Choudhry A.J., et al. Multifacility outbreak of middle east respiratory syndrome in Taif, Saudi Arabia. Emerg Infect Dis. 2016;22(1):32. doi: 10.3201/eid2201.151370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alenazi T.H., Al Arbash H., El-Saed A., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y.M., et al. Identified transmission dynamics of Middle East respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65(4):675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry M., Phan M.V., Akkielah L., Al-Majed F., Alhetheel A., Somily A., et al. Nosocomial outbreak of the Middle East Respiratory Syndrome coronavirus: a phylogenetic, epidemiological, clinical and infection control analysis. Travel Med Infect Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Bushra H.E., Al Arbash H.A., Mohammed M., Abdalla O., Abdallah M.N., Al-Mayahi Z.K., et al. Outcome of strict implementation of infection prevention control measures during an outbreak of Middle East respiratory syndrome. Am J Infect Control. 2017;45(5):502–507. doi: 10.1016/j.ajic.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adegboye O.A., Elfaki F. Network analysis of MERS coronavirus within households, communities, and hospitals to identify most centralized and super-spreading in the Arabian peninsula, 2012 to 2016. Can J Infect Dis Med Microbiol. 2018;2018:6725284. doi: 10.1155/2018/6725284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowling B.J., Park M., Fang V.J., Wu P., Leung G.M., Wu J.T. Preliminary epidemiologic assessment of MERS-CoV outbreak in South Korea, May–June 2015. Euro Surveill: Eur Commun Dis Bull. 2015;20:25. doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim K.M., Ki M., Cho S.-i, Sung M., Hong J.K., Cheong H.-K., et al. Epidemiologic features of the first MERS outbreak in Korea: focus on Pyeongtaek St. Mary’s Hospital. Epidemiol Health. 2015;37:e2015041. doi: 10.4178/epih/e2015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korea Centers for Disease Control and Prevention Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6(4):269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park G.E., Ko J.-H., Peck K.R., Lee J.Y., Lee J.Y., Cho S.Y., et al. Control of an outbreak of Middle East respiratory syndrome in a tertiary hospital in Korea. Ann Intern Med. 2016;165(2):87–93. doi: 10.7326/M15-2495. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.W., Park J.W., Jung H.-D., Yang J.-S., Park Y.-S., Lee C., et al. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin Infect Dis. 2017;64(5):551–557. doi: 10.1093/cid/ciw768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiura H., Endo A., Saitoh M., Kinoshita R., Ueno R., Nakaoka S., et al. Identifying determinants of heterogeneous transmission dynamics of the Middle East respiratory syndrome (MERS) outbreak in the Republic of Korea, 2015: a retrospective epidemiological analysis. BMJ Open. 2016;6(2) doi: 10.1136/bmjopen-2015-009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choe S., Kim H.-S., Lee S. Exploration of superspreading events in 2015 MERS-CoV outbreak in Korea by branching process models. Int J Environ Res Pub Health. 2020;17(17):6137. doi: 10.3390/ijerph17176137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang S., Huang L., Chen X., Wang J., Wu W., Yin S., et al. Ventilation of wards and nosocomial outbreak of severe acute respiratory syndrome among healthcare workers. Chin Med J. 2003;116(9):1293–1297. [PubMed] [Google Scholar]
- 55.WHO Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) Epidemic Alert Response. 2003:44. [Google Scholar]
- 56.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 57.Cooper B.S., Fang L.Q., Zhou J.P., Feng D., Lv H., Wei M.T., et al. Transmission of SARS in three Chinese hospitals. Trop Med Int Health. 2009;14:71–78. doi: 10.1111/j.1365-3156.2009.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang W., Zhu Z., Guo J., Liu Z., He X., Zhou W., et al. Severe acute respiratory syndrome, Beijing, 2003. Emerg Infect Dis. 2004;10(1):25. doi: 10.3201/eid1001.030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsang T., Lai-Yin T., Pak-Yin L., Lee M. Update: outbreak of severe acute respiratory syndrome-worldwide, 2003. Morbid Mortal Wkly Rep. 2003;52(12):241. [PubMed] [Google Scholar]
- 60.Chen M.I., Leo Y.-S., Ang B.S., Heng B.-H., Choo P. The outbreak of SARS at Tan Tock Seng Hospital-relating epidemiology to control. Ann Acad Med Singap. 2006;35(5):317. [PubMed] [Google Scholar]
- 61.Goh K.-T., Cutter J., Heng B.-H., Ma S., Koh B.K., Kwok C., et al. Epidemiology and control of SARS in Singapore. Ann-Acad Med Singap. 2006;35(5):301. [PubMed] [Google Scholar]
- 62.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 63.Wong R.S., Hui D.S. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339. doi: 10.3201/eid1002.030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varia M., Wilson S., Sarwal S., McGeer A., Gournis E., Galanis E. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. Can Med Assoc J. 2003;169(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 65.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 66.Olsen S.J., Chang H.-L., Cheung T.Y.-Y., Tang A.F.-Y., Fisk T.L., Ooi S.P.-L., et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 67.Ho A.S., Sung J.J., Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med. 2003;139(7):564–567. doi: 10.7326/0003-4819-139-7-200310070-00008. [DOI] [PubMed] [Google Scholar]
- 68.Ofner-Agostini M., Gravel D., McDonald L.C., Lem M., Sarwal S., McGeer A., et al. Cluster of cases of severe acute respiratory syndrome among Toronto healthcare workers after implementation of infection control precautions: a case series. Infect Control Hosp Epidemiol. 2006;27(5):473–478. doi: 10.1086/504363. [DOI] [PubMed] [Google Scholar]
- 69.Ofner M., Lem M., Sarwal S., Vearncombe M., Simor A. Cluster of severe acute respiratory syndrome cases among protected health-care workers-Toronto, Canada, April 2003. Morbid Mortal Wkly Rep. 2003;52(19):433. [Google Scholar]
- 70.Centers for Disease Control and Prevention Severe acute respiratory syndrome--Taiwan, 2003. Morbid Mortal Wkly Rep. 2003;52(20):461–466. [PubMed] [Google Scholar]
- 71.Simmerman J.M., Chu D., Chang H. Implications of unrecognized severe acute respiratory syndrome. Nurse Pract. 2003;28(11):21–22. doi: 10.1097/00006205-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 72.Li Y.-K., Peng S., Li L.-Q., Wang Q., Ping W., Zhang N., et al. Clinical and transmission characteristics of Covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40(2):295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei C., Yuan Y., Cheng Z. A super-spreader of SARS-CoV-2 in incubation period among health-care workers. Respir Res. 2020;21(1):1–3. doi: 10.1186/s12931-020-01592-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu K., Zhao Y., Wang M., Zeng Q., Wang X., Wang M., et al. Identification of a super-spreading chain of transmission associated with COVID-19 at the early stage of the disease outbreak in Wuhan. Arch Clin Biomed Res. 2021;5(4):598–612. [Google Scholar]
- 75.Lin J., Yan K., Zhang J., Cai T., Zheng J. A super-spreader of COVID-19 in Ningbo city in China. J Infect Public Health. 2020;13(7):935–937. doi: 10.1016/j.jiph.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Y., Li C., Dong H., Wang Z., Martinez L., Sun Z., et al. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA Intern Med. 2020;180(12):1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin G., Jin H. Comparison of transmissibility of coronavirus between symptomatic and asymptomatic patients: reanalysis of the Ningbo COVID-19 data. JMIR Pub Health Surveill. 2020;6(2) doi: 10.2196/19464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y., Eggo R.M., Kucharski A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395(10227) doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo K., Lei Z., Hai Z., Xiao S., Rui J., Yang H., et al. Transmission of SARS-CoV-2 in public transportation vehicles: a case study in Hunan province, China. Open Forum Infect Dis. 2020;7(10):ofaa430. doi: 10.1093/ofid/ofaa430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu J., Gu J., Li K., Xu C., Su W., Lai Z., et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian S., Wu M., Chang Z., Wang Y., Zhou G., Zhang W., et al. Epidemiological investigation and intergenerational clinical characteristics of 24 coronavirus disease patients associated with a supermarket cluster: a retrospective study. BMC Public Health. 2021;21(1):1–9. doi: 10.1186/s12889-021-10713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J., Zhou P., Han D., Wang W., Cui C., Zhou R., et al. Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41 doi: 10.3760/cma.j.cn112338-20200228-00206. E055. [DOI] [PubMed] [Google Scholar]
- 83.Danis K., Epaulard O., Bénet T., Gaymard A., Campoy S., Botelho-Nevers E., et al. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin Infect Dis. 2020;71(15):825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang Y.-J. Characteristics of the COVID-19 outbreak in Korea from the mass infection perspective. J Prev Med Public Health. 2020;53(3):168. doi: 10.3961/jpmph.20.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J.Y. Spatial visualization of cluster-specific Covid-19 transmission network in South Korea during the early epidemic phase. medRxiv. 2020 [Google Scholar]
- 86.Adam D.C., Wu P., Wong J.Y., Lau E.H., Tsang T.K., Cauchemez S., et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26(11):1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 87.Müller L., Ostermann P.N., Walker A., Wienemann T., Mertens A., Adams O., et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur J Clin Microbiol Infect Dis. 2021;40(5):1063–1071. doi: 10.1007/s10096-021-04169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jang S., Han S.H., Rhee J.-Y. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg Infect Dis. 2020;26(8):1917. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park S.Y., Kim Y.-M., Yi S., Lee S., Na B.-J., Kim C.B., et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hijnen D., Marzano A.V., Eyerich K., GeurtsvanKessel C., Giménez-Arnau A.M., Joly P., et al. SARS-CoV-2 transmission from presymptomatic meeting attendee, Germany. Emerg Infect Dis. 2020;26(8):1935. doi: 10.3201/eid2608.201235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valent F., Gallo T., Mazzolini E., Pipan C., Sartor A., Merelli M., et al. A cluster of COVID-19 cases in a small Italian town: a successful example of contact tracing and swab collection. Clin Microbiol Infect. 2020;26(8):1112–1114. doi: 10.1016/j.cmi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khanh N.C., Thai P.Q., Quach H.-L., Thi N.-A.H., Dinh P.C., Duong T.N., et al. Transmission of SARS-CoV 2 during long-haul flight. Emerg Infect Dis. 2020;26(11):2617. doi: 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghinai I., Woods S., Ritger K.A., McPherson T.D., Black S.R., Sparrow L., et al. Community transmission of SARS-CoV-2 at two family gatherings—Chicago, Illinois, February–March 2020. Morbid Mortal Wkly Rep. 2020;69(15):446. doi: 10.15585/mmwr.mm6915e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thompson E.R., Williams F.S., Giacin P.A., Drummond S., Brown E., Nalick M., et al. Universal masking to control healthcare-associated transmission of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) Infect Control Hosp Epidemiol. 2022;43(3):344–350. doi: 10.1017/ice.2021.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weissberg D., Böni J., Rampini S.K., Kufner V., Zaheri M., Schreiber P.W., et al. Does respiratory co-infection facilitate dispersal of SARS-CoV-2? Investigation of a super-spreading event in an open-space office. Antimicrob Resist Infect Control. 2020;9(1):1–8. doi: 10.1186/s13756-020-00861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamner L. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR: Morbid Mortal Wkly Rep. 2020;69(19):606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 98.Yusef D., Hayajneh W., Awad S., Momany S., Khassawneh B., Samrah S., et al. Large outbreak of coronavirus disease among wedding attendees, Jordan. Emerg Infect Dis. 2020;26(9):2165. doi: 10.3201/eid2609.201469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chau N.V.V., Hong N.T.T., Ngoc N.M., Thanh T.T., Khanh P.N.Q., Nguyet L.A., et al. Superspreading event of SARS-CoV-2 infection at a Bar, Ho Chi Minh City, Vietnam. Emerg Infect Dis. 2021;27(1):310. doi: 10.3201/eid2701.203480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G., Ward J.L., Hudson L., et al. Susceptibility to and transmission of COVID-19 amongst children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phiriyasart F., Chantutanon S., Salaeh F., Roka A., Thepparat T., Kaesaman S., et al. Outbreak investigation of coronavirus disease (COVID-19) among islamic missionaries in Southern Thailand, April 2020. OSIR J. 2020;13(2):48–54. [Google Scholar]
- 102.Kristiansen M.F., Heimustovu B.H., Borg S.Á., Mohr T.H., Gislason H., Møller L.F., et al. Epidemiology and clinical course of first wave coronavirus disease cases, Faroe Islands. Emerg Infect Dis. 2021;27(3):749. doi: 10.3201/eid2703.202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bigelow B.F., Tang O., Toci G.R., Stracker N., Sheikh F., Slifka K.M.J., et al. Transmission of SARS-CoV-2 involving residents receiving dialysis in a nursing home—Maryland, April 2020. Morb Mortal Wkly Rep. 2020;69(32):1089. doi: 10.15585/mmwr.mm6932e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Günther T., Czech‐Sioli M., Indenbirken D., Robitaille A., Tenhaken P., Exner M., et al. SARS‐CoV‐2 outbreak investigation in a German meat processing plant. EMBO Mol Med. 2020;12(12) doi: 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez-Fierro M.L., Ríos-Jasso J., Garza-Veloz I., Reyes-Veyna L., Cerda-Luna R.M., Duque-Jara I., et al. The role of close contacts of COVID-19 patients in the SARS-CoV-2 transmission: an emphasis on the percentage of nonevaluated positivity in Mexico. Am J Infect Control. 2021;49(1):15–20. doi: 10.1016/j.ajic.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy N., Boland M., Bambury N., Fitzgerald M., Comerford L., Dever N., et al. A large national outbreak of COVID-19 linked to air travel, Ireland, summer 2020. Euro Surveill. 2020;25(42) doi: 10.2807/1560-7917.ES.2020.25.42.2001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Atrubin D., Wiese M., Bohinc B. An outbreak of COVID-19 associated with a recreational hockey game—Florida, June 2020. Morb Mortal Wkly Rep. 2020;69(41):1492. doi: 10.15585/mmwr.mm6941a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szablewski C.M., Chang K.T., Brown M.M., Chu V.T., Yousaf A.R., Anyalechi N., et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp—Georgia, June 2020. Morbid Mortal Wkly Rep. 2020;69(31):1023. doi: 10.15585/mmwr.mm6931e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ismail S.A., Saliba V., Bernal J.L., Ramsay M.E., Ladhani S.N. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21(3):344–353. doi: 10.1016/S1473-3099(20)30882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sugimoto H., Kohama T. Chest tube with air leaks is a potential “super spreader” of COVID-19. Am J Infect Control. 2020;48(8):969. doi: 10.1016/j.ajic.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thway A.M., Tayza H., Win T.T., Tun Y.M., Aung M.M., Win Y.N., et al. Epidemiological characteristics of SARS-COV-2 in Myanmar. medRxiv. 2020 [Google Scholar]
- 112.Schwierzeck V., König J.C., Kühn J., Mellmann A., Correa-Martínez C.L., Omran H., et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 in a pediatric dialysis unit. Clin Infect Dis. 2021;72(2):265–270. doi: 10.1093/cid/ciaa491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Phucharoen C., Sangkaew N., Stosic K. The characteristics of COVID-19 transmission from case to high-risk contact, a statistical analysis from contact tracing data. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohindra R., Ghai A., Brar R., Khandelwal N., Biswal M., Suri V., et al. Superspreaders: a Lurking Danger in the Community. J Prim Care Community Health. 2021;12 doi: 10.1177/2150132720987432. 2150132720987432. [DOI] [PMC free article] [PubMed] [Google Scholar]