Abstract
Purpose:
The diagnosis of pulmonary blastomycosis is usually delayed because of its non-specific presentation. We aimed to assess the extent of diagnostic delay in hospitalized patients and detect the step in the diagnostic process that requires the most improvement.
Methods:
Adult patients diagnosed with pulmonary blastomycosis during a hospital admission between January 2010 through November 2021 were eligible for inclusion. Patients who did not have pulmonary involvement and who were diagnosed before admission were excluded. Demographics and comorbid conditions, specifics of disease presentation, and interventions were evaluated. The timing of the diagnosis, antifungal treatment, and patient outcomes were noted. Descriptive analytical tests were performed.
Results:
A total of 43 patients were diagnosed with pulmonary blastomycosis during their admissions. The median age was 47 years, with 13 (30%) females. Of all patients, 29 (67%) had isolated pulmonary infection, while 14 (33%) had disseminated disease, affecting mostly skin and musculoskeletal system. The median duration between the initial symptoms and health care encounters was four days, and the time to hospital admission was nine days. The median duration from the initial symptoms to the diagnosis was 20 days. Forty patients (93%) were treated with empirical antibacterials before a definitive diagnosis was made. In addition, corticosteroid treatment was empirically administered to 15 patients (35%) before the diagnosis, with indications such as suspicion of inflammatory processes or symptom relief. In 38 patients (88%), the first performed fungal diagnostic test was positive. Nineteen patients (44%) required admission to the intensive care unit, and 11 patients (26%) died during their hospital stay.
Conclusions:
There was a delay in diagnosis of patients with pulmonary blastomycosis, largely attributable to the lack of consideration of the etiological agent. Novel approaches to assist providers in recognizing the illness earlier and trigger evaluation are needed.
Keywords: Advanced informatics technology, atypical microorganisms, blastomycosis, diagnostic delay, intelligent alert systems
INTRODUCTION
Worldwide, pneumonia is one of the leading causes of mortality[1]. Most bacterial pneumonia cases can be treated with first-line empirical antibiotic agents[2]. However, the clinical overlap between bacterial and non-bacterial pneumonia presents a significant challenge. Mis-identification of non-bacterial pneumonia can lead to antibiotic overuse[3] and mistreatment[4, 5]. Moreover, fungal causes are usually not considered promptly in the differential diagnosis since they have a similar clinical appearance to bacterial pneumonia but are less common[5, 6]. The diagnostic delay thus presents a significant clinical challenge in fungal pneumonia.
Blastomycosis is a systemic disease caused by inhalation of conidia of Blastomyces., a dimorphic fungus[7]. Although not all diagnostic tests have high sensitivity and specificity, tests with relatively high performance are available. Still, the diagnosis is delayed even in endemic regions because of the non-specific presentation and, thus, lack of consideration[8–11].Accordingly, varying degrees of diagnostic delay has been reported in literature. Depending on the characteristics of the studies and the patients included, the median duration between the initial presentation and eventual diagnosis ranges between 23 to 128 days [3, 6, 9, 12]. This delay was shown to cause a considerable number of patients to become critically ill and increase mortality[3, 6, 9, 12, 14, 15]. Although the problem is relatively well described, there is a dearth of literature evaluating methods to shorten the diagnostic delay. The yield of diagnostic tests and the median time to first healthcare institution visit by the patient are reported to be acceptable[6, 10]. However, it is not very clear, yet, which phase of the diagnosis process accounts for most of the delay in patients with moderate to severe illness.
We embarked on this study to investigate the extent of diagnostic delay in patients diagnosed with pulmonary blastomycosis during admission. Our purpose was to determine the longest phase of delay for patients with moderate to severe pulmonary blastomycosis before a definite diagnosis was rendered. Thus, we aimed to identify the best target for improving the inpatient diagnostic process for pulmonary blastomycosis. We also sought to explore the possible implications of the delay in terms of individual patient outcomes as well as healthcare resources.
MATERIALS AND METHODS
Study Design and Cases:
This study was designed as a retrospective case series. We studied adult patients diagnosed with pulmonary blastomycosis during their hospitalization at one of the Mayo Clinic Health System hospitals. The study was approved by Mayo Clinic Institutional Review Board (IRB:18–007115). To ensure the quality and accessibility of the available data and the use of standard diagnostic and therapeutic approaches, we exclusively screened more recent diagnoses, including those between January 1, 2010, through November 7, 2021. To determine the cases, we performed a query of International Classification of Diseases (ICD) codes 9 and 10 through the Mayo Data Explorer tool on all Mayo Clinic Enterprise hospitals (B40.0, B40.2, B40.7, B40.9, 116.0)[16]. Subsequently, a free-text filter was applied using the keyword “Blastomycosis” using the Advanced Text Explorer instrument[17]. Then, a further screening was performed via retrospective chart review by two physician-researchers (AT, YP).
The inclusion criteria were:
Detection of Blastomyces spp. by culture, polymerase chain reaction (PCR) test, or histopathology in a respiratory specimen,
Detection of the organism in a specimen from an extrapulmonary site in the presence of a clinical course compatible with pneumonia and an abnormal finding on a chest radiograph, or
Diagnosis strongly suggested by the clinical course in conjunction with positive results of immunodiffusion and/or antigen testing[6, 10].
The exclusion criteria were:
Patients who did not provide research authorization,
Patients who did not have pulmonary involvement,
Patients who were diagnosed in outpatient settings or already had the diagnosis before admission.
Blastomyces PCR assay, though not widely available in laboratories outside of Mayo Enterprise, is an in-house developed test with demonstrated efficacy in blastomycosis diagnosis[11].
To assess the reliability of case selection, an Infectious Diseases physician (JO) evaluated the charts of included patients and a random sample of the excluded patients (n=10) in terms of compatibility. The agreement was 98.1%, with a kappa value of 93.6%.
Data extraction and variable definitions:
Demographic variables (age, sex, race, and ethnicity) and comorbidities and the specifics regarding the admission (i.e., admission and discharge dates, specific Mayo Clinic location) were automatically retrieved from the Mayo Data Explorer tool and confirmed via chart reviews. The data about symptomatology were extracted from free text physician notes. Symptoms and encounters were limited to those that were within six months of diagnosis. Patients for whom symptomatology or the first encounter related to blastomycosis was unclear (patients who were repeatedly seen for other chronic conditions and diagnosed incidentally or already had chronic symptoms due to unrelated conditions) were excluded from the relevant analyses. The empirical antimicrobial treatment prescribed as an outpatient or administered as an inpatient were recorded separately, and corticosteroids administered were recorded along with their route, dose, and durations. The number of imaging studies and microbiological tests were recorded as categories (e.g., X-ray, computerized tomography, culture, or PCR). Interventions were searched through the period starting from the first encounter to the time of the first fungal diagnostic test order, which suggested that a diagnosis of blastomycosis was considered[6]. Time of hospital admission was gathered from timestamps as date and time. The first fungal diagnostic test order and the timing of the first positive result indicating blastomycosis were noted separately. The extent of the disease (i.e., pulmonary or disseminated), as well as the diagnostic method that led to the correct diagnosis, were recorded. Timing of the initially started antifungal agent and the second antifungal agent (if the treatment was changed) were noted[7]. We considered the time of the first positive laboratory test as the time of diagnosis. Timestamps were gathered from internal or external physician notes and recorded as date and time. If the encounter was at an external institution with no reliable timestamp, the timing was noted as midnight on that specific date. De-identified data were collected through Research Electronic Data Capture software (REDCap, version 8.11.11, Vanderbilt University, Nashville, Tennessee) internal for Mayo Clinic[18]. The REDCap electronic data capture system is a secure, web-based application for research data capture that includes an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.
Our primary outcome was the time between the beginning of symptoms and definite diagnosis, which was defined as time to diagnosis [19]. As secondary outcomes, we assessed the diagnostic and therapeutic modalities that patients underwent before the definite diagnosis of pulmonary blastomycosis, requirement of invasive mechanical ventilation and intensive care unit admission, length of hospital stay, and in-hospital mortality.
Statistical analysis:
To measure the impact of different phases, we also assessed the individual components of the diagnostic delay, i.e., symptom start to the first encounter, first encounter to first fungal diagnostic test order, and first positive result to first treatment. The durations were also schematized separately for patient groups according to the extent of the disease, presentation with symptoms involving different systems, and hospital discharge status. Descriptive analyses were performed using summary statistics. Continuous variables were summarized by median and interquartile range, while categorical variables were expressed as numbers and frequencies. We did not perform any statistical comparisons due to the small sample size. Analyses were performed using JMP Pro 14.1.0 software (SAS Institute Inc., Cary, NC, 1989–2021).
RESULTS
After applying free-text filters and evaluating exclusion criteria by chart reviews, the final sample comprised 43 cases. All included patients were enrolled from Mayo Clinic Enterprise Hospitals located in the Midwestern United States (Mayo Clinic Rochester, Mayo Clinic Health System Mankato, Eau Claire, and La Crosse) (Figure 1). Baseline characteristics are outlined in Table 1.
Figure 1.
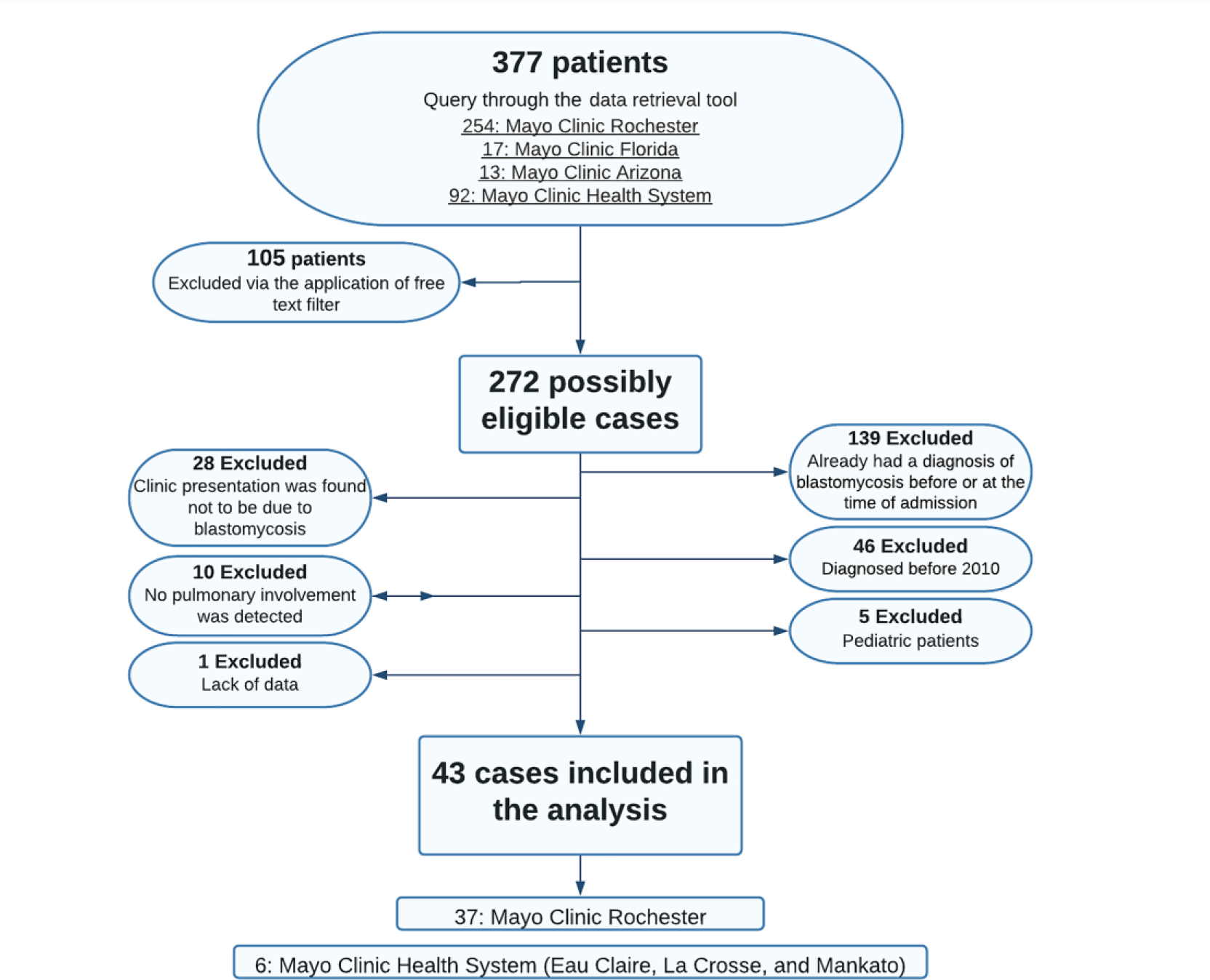
Flowchart for the identification of cases
Table 1.
Baseline characteristics:
| Variables | Total (n=43) |
|---|---|
| Age, median (IQR) | 47 (33–64) |
| Sex, no. (%) | |
| Female | 13 (30.2%) |
| Male | 30 (69.8%) |
| Race/ethnicity, no. (%) | |
| Hispanic (all races) | 2 (4.7%) |
| White, non-Hispanic | 34 (79.1%) |
| African American, non-Hispanic | 3 (7%) |
| All others | 4 (9.3%) |
| Comorbidities (any), no. (%) | 15 (34.9%) |
| Renal disease | 8 (18.6%) |
| Peripheral vascular disease | 4 (9.3%) |
| Mild liver disease* | 4 (9.3%) |
| Diabetes without chronic complication | 3 (7%) |
| Any malignancy | 3 (7%) |
| Charlson comorbidity index score, no. (%) | |
| 0–1 | 31 (72.1%) |
| ≥2 | 12 (27.9%) |
IQR: interquartile range
Mild liver disease was defined as chronic hepatitis or cirrhosis without portal hypertension. In our cases, one patient had a history of alcoholic cirrhosis while others had the findings of hepatitis.
Ten of the 29 patients with the pulmonary-only disease (34%) had localized pneumonia involving a single lobe, one patient (3%) had accompanying pleural involvement, and the remaining 18 (62%) had multifocal or diffuse lung spread. Among 14 patients with disseminated blastomycosis, the most common location was skin and soft tissue (7 patients, 50%), followed by bone and joints (4 patients, 29%). Two patients (14%) had both cutaneous and musculoskeletal involvement. Three patients had peritoneal, esophageal, and central nervous system infections alongside pneumonia; one in each group. The remaining two patients were diagnosed with disseminated infection due to their clinical presentations (organ involvement not further specified). In 8 of the 12 patients with specified accompanying infection foci (67%), Blastomyces spp. were demonstrated in relevant samples; in the remaining 4, diagnoses were based on clinical presentation and biochemistry results. In 35 patients (81%) a diagnosis was concluded by detecting the pathogen in respiratory specimens. For 18 of these patients (51%) the primary sample that Blastomyces spp. was shown was bronchoalveolar lavage, for 11 (31%) it was sputum, for 4 (11%) it was tissue sample, and for 2 (6%) it was body fluid (Table 2).
Table 2.
Clinical information:
| Variables | Total (n=43) |
|---|---|
| Extent of the disease, no. (%) | |
| Isolated pulmonary | 29 (67.4%) |
| Disseminated | 14 (32.6%) |
| Symptoms at the time of presentation, no. (%) | |
| Fever | 21 (48.8%) |
| Cough | 21 (48.8%) |
| Shortness of breath | 15 (34.9%) |
| Arthralgia | 4 (9.3%) |
| Rash | 4 (9.3%) |
| Headache | 4 (9.3%) |
| Asymptomatic | 1 (2.3%) |
| Transferred from another hospital, no. (%) | 16 (37.2%) |
| Outside of Mayo Clinic | 10 (23.3%) |
| Within Mayo Clinic Enterprise | 6 (14%) |
| Diagnostic method | |
| Blastomyces spp. was detected in a respiratory specimen by culture or PCR | 35 (81.4%) |
| The organism was isolated from an extrapulmonary site in the presence of a compatible clinical course and abnormal chest radiograph finding | 3 (7%) |
| No organism was isolated, but the diagnosis was strongly suggested by the clinical course, abnormal chest radiograph finding, and positive results of immunodiffusion and/or antigen testing | 5 (11.6%) |
| The number of pulmonary imaging studies per patient, median (IQR) ** | |
| The number of X-Rays | 2 (1–3) |
| The number of computed tomography imagings | 1 (1–2) |
| The most common findings in pulmonary imaging studies, no. (%) | |
| Nodular opacities | 23 (53.5%) |
| Multilobar involvement | 14 (32.6%) |
| Ground glass opacities | 8 (18.6%) |
| Cavitation | 7 (16.3%) |
| Effusion | 2 (4.7%) |
| Treated with corticosteroids before diagnosis, no. (%) | 15 (34.9%) |
| The first fungal diagnostic test, no. (%) | |
| Culture | 24 (55.8%) |
| Antibody or antigen | 9 (20.9%) |
| PCR | 4 (9.3%) |
| Histology | 4 (9.3%) |
| Fungal smear | 2 (4.7%) |
| Initial antifungal treatment, no. (%) | |
| Amphotericin B | 26 (60.5%) |
| Itraconazole | 13 (30.2%) |
| Voriconazole | 3 (7%) |
| Required IMV, no. (%) | 14 (32.6%) |
| Indications for IMV, no. (%) | |
| Respiratory failure | 12 (85.7%) |
| Post-surgery, due to intraoperative complications | 2 (14.3%) |
| ICU admission, no. (%) | 19 (44.2%) |
| Indications for ICU admission**, no. (%) | |
| Respiratory failure | 14 (73.7%) |
| Post-surgery follow up | 3 (15.8%) |
| Hypotension, shock | 2 (10.5%) |
| Acute kidney injury, volume overload | 2 (10.5%) |
| In-hospital mortality, no. (%) | 11 (25.6%) |
| Cause of death ** , no. (%) | |
| Respiratory failure | 4 (36.4%) |
| Cardiovascular, circulatory failure | 4 (36.4%) |
| Multiorgan failure | 4 (36.4%) |
ICU: intensive care unit, IMV: invasive mechanical ventilation, PCR: polymerase chain reaction
Imaging studies that were done between the time of symptom start and the definitive diagnosis was made.
Categories are not mutually exclusive.
During the interval from initial symptoms to diagnosis, 15 (35%) patients received systemic corticosteroid treatments for a median of 8 days (IQR: 6–14 days). The most commonly stated indication for corticosteroid treatment was the concern for possible inflammatory syndromes, e.g., inflammatory central nervous system disease or temporal arteritis, in 5 of the 15 patients (33%), followed by symptom control such as pleuritic pain in 4 patients (27%). In three patients (20%) they were administered for possible exacerbation of underlying conditions like asthma or anti-glomerular basement membrane disease. In the remaining three patients the indications for corticosteroid treatment were acute respiratory failure, suspicion of allergic diathesis, and pneumonia (7%, each). As for the details of the corticosteroid regimens, they were unavailable for two patients since the treatments were initiated at outside institutions. Of the remaining 13, 5 patients (38%) received intravenous steroids, and the remainder was treated orally. Nine patients received prednisone (69%), and two patients each received dexamethasone and methylprednisolone (15%). The median dose for daily corticosteroids was 7.5 milligrams (IQR: 4.25–15 milligrams) (for standardization purposes, the dose was converted to the equivalent dexamethasone dose).
Out of the included patients, 93% (40/43) received antimicrobial treatment in concordance with community-acquired pneumonia treatment guidelines by the American Thoracic Society and Infectious Diseases Society of America [2], of which 81% (35/43) were in the outpatient setting. The most commonly administered antibiotics were vancomycin (23 patients, 53%), azithromycin (22 patients, 51%), levofloxacin (19 patients, 44%), cefepime (16 patients, 37%), ceftriaxone (14 patients, 33%), and piperacillin (12 patients, 28%). The median antimicrobial treatment duration was 9 days (IQR: 5–13 days). The median number of imaging studies obtained before the fungal-specific microbiological test was ordered was 3 (IQR:2–5). Eleven patients (25,6%) had two or more computerized tomography scanning studies. As for the culture and serology tests with regards to non-fungal microorganisms, the median number performed per patient was 7 (IQR: 2–11). Twenty-one patients received two or more PCR tests for non-fungal etiologies before a diagnosis of fungal infection was considered. A bronchoscopy was performed in 31 patients (72%), most commonly to obtain bronchoalveolar lavage sampling. Four patients also had a bronchoscopic biopsy (9%), one patient had video-assisted thoracoscopic surgery and an open decortication due to pleural involvement (2%), and one patient underwent a pulmonary lobectomy (with suspicion of malignancy) (2%). As per non-pulmonary diagnostic measures, 8 patients had skin biopsy (19%), two patients had a cerebrospinal fluid analysis done (5%), two patients had synovial fluid sampling (5%), and one patient had a thyroidectomy (for work-up of a thyroid nodule) (2%). Additionally, one patient each underwent peritoneal fluid sampling and esophageal brush (2%).
All patients were started on antifungals, the most common one being liposomal amphotericin B (26 patients, 60.5%). In 29 patients, the antifungal treatment was changed, most commonly from amphotericin B to itraconazole (58.6%). It is known that amphotericin B is usually reserved for more severe patients. When we looked further into the patients who started the treatment with Amphotericin B, we noted that all but four patients (86%) either required critical care unit admission or were patients with invasive disease. Twenty-one patients who started the treatment with amphotericin B were switched to another antifungal agent in a median of 14 days (IQR: 5–24). Among those who were discharged alive (n=31), the median hospital stay at a Mayo hospital was 10 days (IQR: 5–22), and the total hospital stay was 11 days (IQR: 7–22). Other details are provided in Table 2.
The median time to diagnosis in our case series was found to be 20 days (IQR: 10–42). Of note, 14 patients had a delay of more than 4 weeks. When we explored the distribution of this duration, the longest time was between the first encounter to the first fungal diagnostic test order, being a median of 15 days (IQR: 8–28). The duration between symptom start and the first encounter was 4 days (IQR: 0–6). Six patients (14%) were admitted on the first day of their healthcare institution encounter, and the median duration between the first healthcare encounter and the hospital admission was 9 days (IQR: 1–22). For 38 patients (88.4%), the first fungal diagnostic test that was ordered was positive, and the median duration between the test order to antifungal treatment was 1 day (IQR: 0–2) (Figure 2). Though no statistical comparison tests were conducted, we noted that patients presenting with dermatological or musculoskeletal symptoms tended to be diagnosed earlier compared to those presenting without these symptoms. The distribution of median diagnostic delay is represented in the Supplementary Figure.
Figure 2.
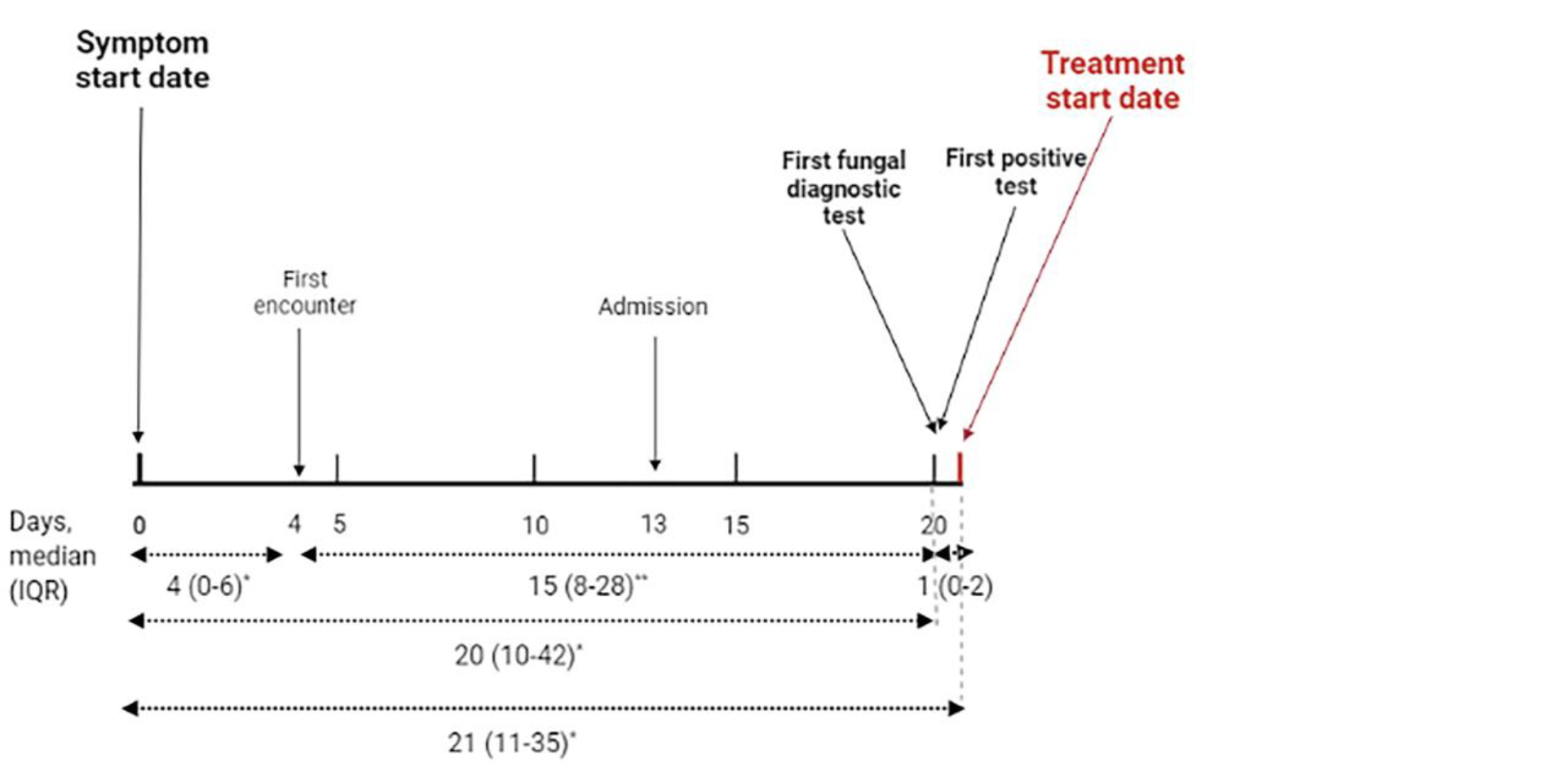
The median durations between significant events
* Patients who were asymptomatic or did not have an unequivocal symptom start date (n=5) were excluded from the analyses regarding the date of symptomatology.
**One patient who had a prolonged admission for a hematological indication and was diagnosed with pulmonary blastomycosis during the hospital stay was excluded from the relevant analysis.
DISCUSSION
We observed considerable diagnostic delay in our study despite most patients seeking healthcare relatively quickly after symptom onset. During the delay period, patients underwent numerous diagnostic tests as well as antimicrobial courses. Furthermore, more than a third of patients received corticosteroid treatments.
In accordance with the literature, most of our cases were middle-aged male patients who were previously healthy[3, 6, 9, 12, 20, 21]. Similar to other studies, the most common symptoms at the time of disease presentation were cough, fever, and shortness of breath[6, 9, 20]. As was reported by Ireland et al. and Benedict et al., cultures of respiratory samples were the most common diagnostic method utilized[6, 9]. In line with other reports, amphotericin B was the most common initial treatment, with a step-down to triazoles[13].
In our case series, the median time to diagnosis was 20 days. This duration was shorter than those reported by previous studies, ranging from 23 to 128 days[3, 6, 9, 12]. Ireland et al. found that being hospitalized was associated with receiving a fungal diagnostic test[6], which could account for the relatively short time to diagnosis in our study, which was conducted exclusively on patients diagnosed during hospitalization. The same analysis also showed that patients who had a fatal course were diagnosed earlier than those who survived. They concluded that a more severe disease might have resulted in a more thorough diagnostic follow-up[6]. In our study, the mortality rate was higher than the most of the other studies[6, 9, 12, 22], which could explain the shorter time to diagnosis than those reported earlier.
In the majority of patients, the first ordered diagnostic test was positive. This finding was in concordance with the reports evaluating the diagnostic capacity of blastomycosis tests as well as the PCR test, which was developed institutionally [10, 11]. Our results indicated a tendency for the patients presenting with accompanying dermatological and musculoskeletal symptoms have a shorter time to diagnosis. This was concordant with studies by Ireland et al. and Lemos et al., who attributed the relatively prompt diagnosis to visible symptoms[6, 15]. The skin involvement mainly occurs due to dissemination from a pulmonary source, if not resulting from direct inoculation following trauma. Patients with isolated skin or skeletal disease (without concurrent pulmonary involvement) were not targeted in this study. Nevertheless, patients with isolated respiratory symptoms tended to have a more extended delay. This may be a consequence of patients with conventional pneumonia symptoms getting more delayed with empirical treatments rather than being evaluated more aggressively. Our results showed that the target for improving the diagnostic delay of pulmonary blastomycosis should be the interval between the first encounter and fungal test order.
In our case group, being exposed to antibacterial treatments before the diagnosis was almost uniform. Alpern et al. also showed that antibiotic exposure was common for patients with blastomycosis[3]. While empirically treating community-acquired pneumonia based on compatible symptoms is an appropriate course, failure to re-evaluate the diagnosis in the face of new evidence or inappropriate clinical response can subject patients to prolonged and unnecessary antibiotic courses as well as delayed diagnosis. Additionally, prolonged and unwarranted antibiotic therapy might expose the patients to potential side effects while potentially contributing to increased antibiotic resistance[23]. Furthermore, more than a third of patients received empirical corticosteroid treatments during their disease course with or without antimicrobials. Although case reports of acute respiratory distress syndrome patients with Blastomyces pneumonia showed successful results with steroid treatments[24], and some studies suggested that steroid treatment might be useful in patients with severe pulmonary blastomycosis[25, 26], the latest guideline by Infectious Diseases Society of America does not recommend corticosteroids in the treatment of blastomycosis. Furthermore, empirical employment of steroids without accompanying effective antimicrobial treatment might potentially have a counterproductive effect[2]. Also, patients received repeated laboratory and imaging studies during their evaluation, other than fungal diagnostic tests. Community-acquired pneumonia and fungal infections already pose a heavy burden on healthcare resources[4, 27]. Implementing unnecessary tests increases the burden further while not providing any helpful information. Additionally, continued aggressive diagnostic procedures alongside the obscure disease might cause significant distress for the patient[5].
The major strength of our study is that it provides information from referral centers located in the endemic regions. The standardized care and laboratory practices facilitate the evaluation of possible underlying causes of the delay. Furthermore, limiting the analysis to the relatively homogenous group of cases who were diagnosed as inpatients facilitates the evaluation of the processes that need to be targeted in terms of decreasing the diagnostic delay. Also, having access to extensive and high-quality data serves as another advantage for our analyses.
The most important limitation of our study is that, being a case series, we included the patients who had a diagnosis of blastomycosis. However, it is known that the disease is often underdiagnosed[9]. Due to the characteristics of our sampling, we are missing the patient group who never received the correct diagnosis, which would have had the most significant impact on diagnostic delay as well as other unfavorable events. Furthermore, we performed the screening based on ICD codes, which might have caused the omission of some cases. Nevertheless, in terms of those who were detected, we tried to control the sampling bias by checking the reliability of our evaluation, and the interrater agreement was almost perfect. Furthermore, being tertiary care centers, the Mayo Clinic case group is subject to referral bias. However, this also provided us with the opportunity of focusing on a more severely affected group, which might benefit more from the improvement in care practices.
CONCLUSION
In a case series of patients who received a diagnosis of pulmonary blastomycosis during their hospital stay, we demonstrated a delay in diagnosis. Extensive diagnostic tests were performed between the first symptom and eventual diagnosis, and antimicrobial therapies and corticosteroids were used. The yield of the fungal diagnostic tests was high, and the lack of consideration accounted for most of the delay, which seems to be the appropriate target for improving the diagnostic practices. Thus, there is an urgent need to develop novel approaches to help trigger appropriate evaluation and recognition of blastomycosis earlier.
Supplementary Material
Funding:
There are no financial disclosures relevant to this article.
Footnotes
Competing interests:
JCO has received grants from Nference, Inc. and the MITRE corporation outside the present work. He has also received personal consulting fees from Bates college and Elsevier, inc, also outside the present work. These funds had no influence on acquisition, analysis, interpretation and reporting of pooled data for this manuscript.
BWP has received fees for board membership, has patents (planned, pending, or issued, funds paid to his institution), has received royalties, and has stock/stock options (all not related to the current work) from Ambient Clinical Analytics. JCO Has received grants from nference, inc and the MITRE corporation outside the present work. The other authors report no competing interests.
Data availability statement:
The data that support the findings of this study are available from the corresponding author, JO, upon reasonable request.
REFERENCES
- 1.Heron M Deaths: Leading Causes for 2019. National Vital Statistics Reports Centers for Disease Control and Prevention; 2021. [PubMed] [Google Scholar]
- 2.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American Journal of Respiratory and Critical Care Medicine. 2019;200(7):e45–e67. 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpern JD, Bahr NC, Vazquez-Benitez G, Boulware DR, Sellman JS, Sarosi GA. Diagnostic Delay and Antibiotic Overuse in Acute Pulmonary Blastomycosis. Open Forum Infectious Diseases. 2016;3(2). 10.1093/ofid/ofw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin Infect Dis. 2019;68(11):1791–7. 10.1093/cid/ciy776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace J Pulmonary blastomycosis: a great masquerader. Chest. 2002;121(3):677–9. 10.1378/chest.121.3.677. [DOI] [PubMed] [Google Scholar]
- 6.Ireland M, Klumb C, Smith K, Scheftel J. Blastomycosis in Minnesota, USA, 1999–2018. Emerg Infect Dis. 2020;26(5):866–75. 10.3201/eid2605.191074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(12):1801–12. 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 8.Patel RG, Patel B, Petrini MF, Carter RR 3rd, Griffith J. Clinical presentation, radiographic findings, and diagnostic methods of pulmonary blastomycosis: a review of 100 consecutive cases. South Med J. 1999;92(3):289–95. 10.1097/00007611-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Benedict K, Gibbons-Burgener S, Kocharian A, Ireland M, Rothfeldt L, Christophe N, et al. Blastomycosis Surveillance in 5 States, United States, 1987–2018. Emerg Infect Dis. 2021;27(4):999–1006. 10.3201/eid2704.204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martynowicz MA, Prakash UB. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121(3):768–73. 10.1378/chest.121.3.768. [DOI] [PubMed] [Google Scholar]
- 11.O’Dowd TR, Mc Hugh JW, Theel ES, Wengenack NL, O’Horo JC, Enzler MJ, et al. Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study. J Fungi (Basel). 2021;7(11). 10.3390/jof7110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin MS, Duckro AN, Proia L, Semel JD, Huhn G. The Epidemiology of Blastomycosis in Illinois and Factors Associated with Death. Clinical Infectious Diseases. 2005;41(12):e107–e11. 10.1086/498152. [DOI] [PubMed] [Google Scholar]
- 13.McBride JA, Sterkel AK, Matkovic E, Broman AT, Gibbons-Burgener SN, Gauthier GM. Clinical Manifestations and Outcomes in Immunocompetent and Immunocompromised Patients With Blastomycosis. Clinical Infectious Diseases. 2020;72(9):1594–602. 10.1093/cid/ciaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman SW, Lin AC, Hendricks KA, Nolan RL, Currier MM, Morris KR, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12(3):219–28. [PubMed] [Google Scholar]
- 15.Lemos LB, Baliga M, Guo M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann Diagn Pathol. 2002;6(3):194–203. 10.1053/adpa.2002.34575. [DOI] [PubMed] [Google Scholar]
- 16.Mayo Data Explorer. Mayo Clinic, 2021. https://mde.mayo.edu/explorer. Accessed 5/7/2021.
- 17.Advanced Text Explorer. Mayo Clinic, 2021. https://ate.mayo.edu/. Accessed 5/7/2021.
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infectious Diseases. 2009;9(1):91. 10.1186/1471-2334-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir Med. 2012;106(6):769–76. 10.1016/j.rmed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Seitz AE, Younes N, Steiner CA, Prevots DR. Incidence and Trends of Blastomycosis-Associated Hospitalizations in the United States. PLOS ONE. 2014;9(8):e105466. 10.1371/journal.pone.0105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush B, Lother S, Paunovic B, Mooney O, Kumar A. Outcomes With Severe Blastomycosis and Respiratory Failure in the United States. Clinical Infectious Diseases. 2020;72(9):1603–7. 10.1093/cid/ciaa294. [DOI] [PubMed] [Google Scholar]
- 23.Organization WH. Antibiotic resistance. (2020). https://www.who.int/news-room/factsheets/detail/antibiotic-resistance. Accessed November 22 2021.
- 24.Lahm T, Neese S, Thornburg AT, Ober MD, Sarosi GA, Hage CA. Corticosteroids for blastomycosis-induced ARDS: a report of two patients and review of the literature. Chest. 2008;133(6):1478–80. 10.1378/chest.07-2778. [DOI] [PubMed] [Google Scholar]
- 25.Plamondon M, Lamontagne F, Allard C, Pépin J. Corticosteroids as adjunctive therapy in severe blastomycosis-induced acute respiratory distress syndrome in an immunosuppressed patient. Clin Infect Dis. 2010;51(1):e1–3. 10.1086/653429. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz IS, Embil JM, Sharma A, Goulet S, Light RB. Management and Outcomes of Acute Respiratory Distress Syndrome Caused by Blastomycosis: A Retrospective Case Series. Medicine (Baltimore). 2016;95(18):e3538. 10.1097/MD.0000000000003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–9. 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, JO, upon reasonable request.


