Abstract
Objective
The aim of this study was to assess the effect of flap design for impacted mandibular third molar extraction on the distal periodontal tissue of their neighbors clinically, immunologically, and microbiologically.
Study design
This randomized controlled study comprised 100 patients who were allocated randomly to receive either a triangular flap or a modified triangular flap. The distal periodontal pocket depth, plaque index, bleeding on probing, the presence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia, and the level of interleukin-1β, interleukin-8 and matrix metalloproteinase-8 of adjacent second molars were measured at baseline, and 1, 4 and 8 weeks after surgery.
Results
After 1 and 4 weeks, distal periodontal conditions of adjacent second molars deteriorated, along with an increase in subgingival microbiota and inflammatory factors in both groups. And compared to the modified triangular flap group, the triangular flap group significantly increased (p < 0.05). Prevotella intermedia, interleukin-1β and probing depth were positively correlated in both groups. After 8 weeks, they returned to the preoperative level.
Conclusions
In this study, both flap designs for impacted mandibular third molar extractions was associated with worse clinical periodontal indices, increased inflammatory biomarkers of gingival crevicular fluid, and more subgingival pathogenic microbiota within 4 weeks. But compared with the triangular flap, the modified triangular flap was better for distal periodontal health of adjacent second molars, which provides certain directions for clinical treatment.
Keywords: Impacted mandibular third molar, Tooth extraction, Flap design, Periodontal conditions, Subgingival microbiota
1. Introduction
Impacted mandibular third molars (IMTM) are the most commonly impacted teeth in the adult oral cavity, with an incidence rate of 66%–77% [1]. In order to prevent a number of problems caused by impacted teeth, early prophylactic extraction of IMTM is mostly recommended. In recent years, the effect of prophylactic IMTM extraction on the periodontal tissue surrounding the adjacent second molars (ASMs) has become a controversial clinical issue [2]. However, based on the existing clinical literature and on practical observations, there are conflicting results as to whether IMTM extraction impact the ASMs periodontal health. A study by Petsos et al. showed that prophylactic early MITMs extraction significantly reduced distal attachment loss and probing depth (PD) in ASMs [3]. Pham et al. also drew a similar conclusion that the probing depth, attachment loss, and alveolar bone height of ASMs were dramatically ameliorated after IMTM extraction [4]. In contrast, others have reported periodontal destruction after prophylactic IMTM extraction. A significant increase in the distal PD of adjacent second molars was found by Aniko-Włodarczyk et al. [5] Consistent with this, Kugelberg et al. observed the incidence of similar damage two years after IMTM extraction, amounting to 43.3% in PD ≥ 7 mm, and 32.1% in bone loss≥4 mm [6].
It is currently believed that periodontal disease results from the interaction between oral microbiota and the host [7]. The progression from health to periodontal disease is not caused by specific exogenous pathogens, but is more likely due to a homeostatic disruption of the preexisting oral microbiota [8]. The use of a flap design for third molar extraction may destroy the gingival epithelium and epithelial attachment, and thereby affect the homeostasis of the subgingival microbiota, leading to the development of chronic periodontal inflammation [9]. Microorganisms closely related to periodontal disease include Actinobacillus actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg) and Prevotella intermedia (Pi) [10]. These periodontal pathogens colonize and multiply in the sulcus and produce toxic substances such as endotoxins and proteases that directly damage the periodontal tissue [11]. This triggers a series of host immune responses in which inflammatory mediators such as interleukin-1β (IL-1β), interleukin-8 (IL-8) and matrix metalloproteinase-8 (MMP-8) are produced, and this indirectly promotes periodontal connective tissue destruction and alveolar bone resorption [12]. However, it has not been reported in previous studies that the effect of IMTM extraction flap design on subgingival microbial and host immune responses in ASMs.
During IMTM extraction, the traditional standard triangular flap is cut from the distal second molar along the gingival sulcus to the median buccal site [13]. In this method, the incision of the gingival fibers and junctional epithelium may contribute to the formation of an adjacent periodontal pocket, resulting in increased expression of subgingival microbiota and inflammatory factors. In this study, the standard triangular flap was modified by moving the incision on the buccal and distal site of the second molar 1–2 mm apically from the gingival margin, thus, leaving the gingival sulcus intact. After the extraction, clinical periodontal indices, the presence of the subgingival microbiota Aa, Pg, Pi and expression of the inflammatory factors IL-1β, IL-8, and MMP-8 in gingival crevicular fluid were used to evaluate the effect of using triangular flaps and modified triangular flaps on the distal periodontal tissue of ASMs. The results of this study enhance our the understanding on how the mechanism of periodontal tissue destruction is related to incision design.
2. Materials and methods
2.1. Ethics and trial registration
This study was conducted in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of the Stomatological Hospital of Shanxi Medical University (No: 2021SLL022). This study protocol was registered in the China Clinical Trials Registry (NO. ChiCTR2200057020). All patients provided written informed consent before making a decision to participate.
2.2. Study design
To detect the effect of flap design on the distal periodontal tissue of adjacent second molars after IMTM extraction, with PD as the primary outcome, which was in agreement with the study of Menziletoglu et al. [14] with a two-sided 5% significance level and a power of 80%, a sample size of at least 35 patients per group was required. In pre-experiments and references to similar articles [15], it was found that about 20% of the patients were lost to follow-up. Considering the 20% dropout rate, at least 42 patients per group was required for this study. So from March 2022 to May 2022, 100 patients were continuously recruited and randomly assigned to receiving either triangular flap (n = 50) or modified triangular flap (n = 50) by IMTM extraction for a randomized controlled study. Subjects were divided into groups for treatment using Internet based (IWR) randomization, who have to meet the following inclusion criteria: ① Good general condition; The third molar did not erupt completely. ② The mandibular dentition is complete; Second molar without decay, loosing, attachment loss and PD < 3 mm [16]; ③ CBCT showed that IMTM were classified as meso-impacted by Winter, with median position and class II [17]. The distance between the IMTM's crown and the second molar distal was 1∼2 mm, which alveolar bone was no obvious absorption. ④ All the patients were 20–25 years old, without abnormal enlargement of the root of the tooth neck, and difficulty in extraction was Class I [18].
Exclusion criteria: ① with contraindications to tooth extraction; ② people with high fear of teeth extraction or mental diseases; ③postoperative wound dehiscence and infection; ④ pregnant and lactating women; ⑤ Tobacco and alcohol addicts; ⑥ inability to follow doctor's orders, imperfect follow-up procedures. ⑦ surgery time for ≤10 min or ≥30 min ⑧ mesial and buccal bone from the third molar was removed. Inform all patients of the treatment plan, cost, postoperative complications and research process, and sign informed consent before participation. Each subject did not know which flap design to use, the surgeon and examiner knew.
2.3. Methods
2.3.1. Methods of anesthesia
Only one IMTM was extracted for each patients. All patients were performed by the same experienced physician (Dr. X. W). Routine mucosal disinfection with 0.5% iodophor was followed by 2 mm 2% lidocaine hydrochloride for block anesthesia of inferior alveolar, buccal and lingual nerve, then local infiltration anesthesia with 1 mL of articaine hydrochloride containing 1:100,000 epinephrine.
2.3.2. Flap type and incision design
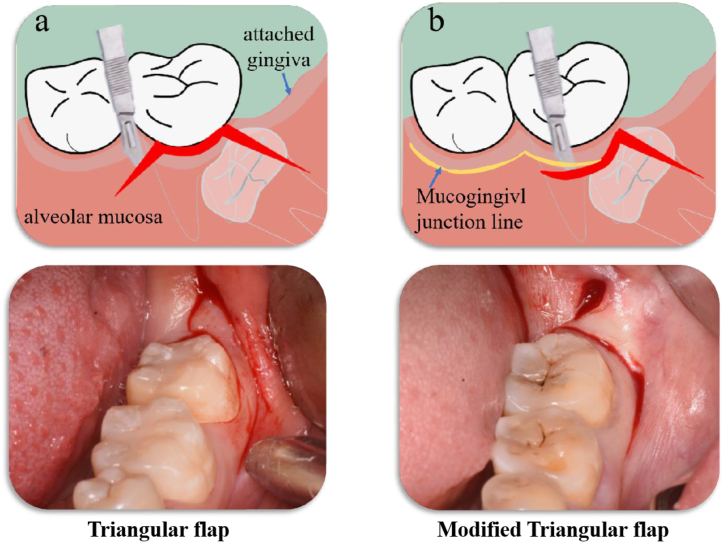
(1) Triangular flap: use a 15# sharp knife to make a distal incision started from the external oblique ridge and extended proximally to the middle of the distal surface of the second molar, Further, a sulcular incision made from the distal surface to the near middle-buccal line angle of the second molar. And then a relieving incision made in the mesial region, not exceeding the vestibular sulcus (Fig. 2a); (2) Modified triangular flap: use a 15# sharp knife to make a distal incision started from the external oblique ridge and extended proximally to the middle of the distal surface of the second molar, and then a vertical incision continued from the distal surface of the second molar downwards, then turn buccally and cut forward along the mucogingival junction line. (Fig. 2b).
Fig. 2.
Schematic diagram of flap design a. Triangular flap b. Modified triangular flap.
2.3.3. Surgical methods
After collecting the subgingival pathogenic microbiotas and gingival crevicular fluid in the two groups before surgery. Then, a full-thickness mucoperiosteal flap was created, distal mild osteotomy (1/3 of dental crown height) [19] and/or tooth sectioning were performed by using a high-speed elevation turbine handpiece combined with a minimally invasive extraction to remove the resistance. Last, tension-free interrupted 4–0 non-absorbable silk suturing (Ethicon, Johnson & Johnson, USA) [20]. Instruct the patient about routine precautions after surgery. All patients did not take any antibiotics after surgery, with only oral hygiene instruction. If necessary, postoperative analgesics (Loxoprofen Sodium Capsules, 60 mg; QIDU Pharmaceutical Co., Ltd., Shandong, China) was prescribed 3 times/day for 3 days [21].
2.4. Variables and data collection methods
Subgingival plaque, gingival crevicular fluid and clinical indices were collected before surgery, 1 week, 4 weeks and 8 weeks after surgery.
2.4.1. Subgingival plaque collection and Pi, Aa, g detection
Remove the supragingival plaque with a sterile curette, sterile lap wet insulation, dry the tooth surface with an air gun, and the subgingival plaque in the distal surface of the adjacent second molar was scraped with a Gracey scaler, placed in a sterile Eppendorf (EP) tube, Stored at - 80 °C [22]. After all samples were collected, they were sent to Novogene company for 16 S rRNA high-throughput sequencing. The V3–V4 regions of the 16 S rRNA gene were sequenced:515 F: 5′-GAGTGCCAGCMG CCGCGGTAA-3′ and 806 R: 5′-ACGGACTACHVGGGTWTCTAT-3′. After sequencing, the relative abundance of the Pi, Aa and Pg was analyzed at the species level.
2.4.2. Gingival crevicular fluid collection and IL-8, MMP-8, IL-1β detection
Remove the supragingival plaque with a sterile curette, sterile lap wet insulation. Two 3 mmx8mm moisture absorbent paper tips were slowly inserted into the distal buccal and lingual gingival sulcus of the second molar, and kept for 30s when encountering resistance. After removal, the paper tips were placed into EP tubes containing 200 μL PBS buffer. 1 h shock; Centrifuged at 3000r/min for 10min, the supernatant was taken and stored at - 80 °C. IL-8, MMP-8 and IL-1β were detected by Enzyme-linked immunosorbent assay (ELISA) [23]. The kits were purchased from Shanghai Kanglang Technology Co., LTD. The sensitivities for IL-8, MMP-8 and IL-1β ELISAs were 2.87 pg/mL, 0.03 ng/mL and 1.02 pg/mL, respectively.
2.4.3. Collection of clinical periodontal indices
Clinical periodontal indices include Plaque index (PLI), Bleeding on probing (BOP), and Probing depth (PD). Preoperatively, there was no significant difference in full-mouth PLI and BOP. However, in this study, PLI and BOP were divided into the average scores of distal surfaces of adjacent second molars. PD was assessed at three sites (distal buccal, distal, distal lingual) of adjacent teeth. All measurements were repeated three times by 2 experienced Periodontist (Dr. J. Z and Y. Z), who performed an inter-examiner calibration based on the Intraclass Correlation Index (ICC) [24] to obtain a variable value of 0.97, and averaged.
-
a.
Plaque index (PLI) [25]: The plaque index proposed by Silness and Loe was adopted, and the specific scoring criteria were as follows: 0 = no plaque in the gingival margin; 1 = Thin plaque can be scraped on the gingival margin surface with probe; 2 = Medium amount of plaque on gingival margin or adjacent surface; 3 = There is a large amount of soft scale in the gingival sulcus or perigingival area and adjacent surface.
-
b.
Bleeding on bleeding (BOP) [25]: Probe gently into the gingival sulcus, 30 s after probing was removed, observed: 0 = no bleeding; 1 = bleeding.
-
c.
Probing depth (PD) [26]: William's periodontal probe (Hu-Friedy, Chicago, IL, USA) in millimeters was inserted parallel to the long axis of the tooth from the free gingival margin to the distance until it encounters slight resistance.
2.5. Data analysis
Data results that were repeated three times were expressed as mean ± standard deviation (mean ± STD). Data analysis used GraphPad Prism software (version 8.0). The Shapiro-Wilk normality test was used to clarify that all measurements were normal distribution. Two independent sample T tests were used to compare Intra/inter-group differences of measurement data samples. The Pearson correlation test was used to evaluate the correlations between subgingival microbiota and inflammatory factors and periodontal clinical indices. p < 0.05 was considered to be statistically significant.
3. Results
3.1. General condition
A total of 100 patients were collected and randomly assigned into two groups, 50 patients in the triangular flap group and 50 patients in the modified triangular flap group, excluded 20 patients due to loss of follow-up and postoperative wound dehiscence and infection. Ultimately, 80 patients completed the study. There were 39 patients (39 teeth) in the triangular flap group and 41 patients (41 teeth) in the modified triangular flap group (Fig. 1), including 38 males and 42 females, with an average age of 21.3 ± 2.6 years. There were no significant differences in age, sex, and operation time between the two groups (p > 0.05). Furthermore, no infection, grade 3 or higher pain or swelling occurred in all patients.
Fig. 1.
Flow chart of study design.
3.2. Clinical results
3.2.1. Changes in distal periodontal clinical indices of adjacent second molars before and after surgery
Preoperatively, there was no significant difference in PD, PLI and BOP indices between the triangular flap group (2.88 ± 0.31 mm; 0.94 ± 0.78; 0.00 ± 0.00) and the modified triangular flap group (2.83 ± 0.37 mm, 0.77 ± 0.50, 0.00 ± 0.00) (p = 0.5024; p = 0.3412). At 1 week and 4 weeks postoperatively, the clinical indices in both groups increased compared with those before surgery, and PD in the triangular flap group (5.83 ± 1.24 mm; 5.31 ± 1.10 mm) and the modified triangular flap group (4.36 ± 0.85 mm; 4.14 ± 0.89 mm) was statistically different from that before surgery (p < 0.0001; p < 0.0001). At 8 weeks, the clinical indices basically recovered the preoperative levels. As shown in Table 1.
Table 1.
Comparison of periodontal clinical indices between both groups (mean ± STD).
| Periodontal index | Time | Triangular flap group | Modified triangular flap group | p value |
|---|---|---|---|---|
| PD (mm) | Before | 2.88 ± 0.31 | 2.83 ± 0.37 | 0.5024 |
| 1 week | *5.83 ± 1.24 | *4.36 ± 0.85 | 0.0001 | |
| 4 weeks | *5.31 ± 1.10 | *4.14 ± 0.89 | 0.0026 | |
| 8 weeks | 2.90 ± 0.36 | 2.75 ± 0.32 | 0.6383 | |
| PLI | Before | 0.94 ± 0.78 | 0.77 ± 0.50 | 0.3412 |
| 1 weeks | *1.42 ± 0.79 | *1.14 ± 0.67 | 0.1191 | |
| 4 weeks | 1.28 ± 0.78 | 1.02 ± 0.70 | 0.1551 | |
| 8 weeks | 0.88 ± 0.53 | 0.83 ± 0.66 | 0.7347 | |
| BOP | Before | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 1 weeks | *0.67 ± 0.47 | *0.53 ± 0.49 | 0.2355 | |
| 4 weeks | *0.58 ± 0.49 | *0.39 ± 0.38 | 0.1016 | |
| 8 weeks | 0.00 ± 0.00 | 0.00 ± 0.00 |
Note: Compared with preoperative, *p < 0.05; PD: Probing Depth; PLI: Plaque Index; BOP: Bleeding on Probing.
3.2.2. Comparison of postoperative periodontal clinical indices between the groups
After 1 week, PD, PLI, and BOP in the triangular flap group respectively were (5.83 ± 1.24 mm; 1.42 ± 0.79; 0.67 ± 0.47), which were significantly higher than those in the modified triangular flap group (4.36 ± 0,85 mm; 1.14 ± 0.67; 0.53 ± 0.49), with statistically significant the difference in PD (p < 0.0001). At 4 weeks, PD, PLI, and BOP in both groups decreased compared with that at 1 week, but the PD in the triangular flap group (5.31 ± 1.10) was still significantly different from that in the modified triangular flap group (4.14 ± 0.89) (p < 0.0026). There was no significant difference in the clinical indices at 8 weeks between groups. As shown in Table 1.
3.3. Microbiological results
Before surgery, no difference was observed comparing triangular flap group and modified triangular flap group in term of the relative abundances of Pi, Aa, and Pg. After 1 week, the relative abundances of Pi, Aa, and Pg significantly increased in both groups compared with those before surgery, and Pi increased particularly significantly in the triangular flap group (p = 0.0066). Compared with 1 week after surgery, the contents of Pi, Aa, and Pg decreased at 4 weeks, but Pi was more abundant in the triangular flap group than in the modified triangular flap group (p = 0.0229). 8 weeks after surgery, the relative abundances of Pi, Aa, and Pg basically returned to the preoperative levels, as shown in Fig. 3.
Fig. 3.
The relative abundance of (a) Pi, (b) Aa, and (c) Pg in the two groups (Compared with preoperative, *p < 0.05).
3.4. Immunological results
Preoperatively, there was no difference in the levels of IL-8, MMP-8, and IL-1β in the gingival crevicular fluid between the groups. At 1 week and 4 weeks, the contents of three inflammatory factors significantly increased, but significantly reduced in the modified triangular flap compared with the triangular flap (p = 0.0266). IL-8, MMP-8, and IL-1β in both groups basically restored to the preoperative levels at 8 weeks, as shown in Table 2.
Table 2.
The levels of IL-8, MMP-8, and IL-1β in the gingival crevicular fluid of both groups (mean ± STD).
| Index | Time | Triangular flap group | Modified triangular flap group | p value |
|---|---|---|---|---|
| IL-8 | Before | 11.04 ± 0.93 | 10.87 ± 0.89 | 0.6864 |
| 1 week | *23.78 ± 0.74 | *22.92 ± 0.77 | 0.0266 | |
| 4 weeks | *18.12 ± 0.67 | *17.32 ± 0.76 | 0.0283 | |
| 8 weeks | 10.96 ± 0.92 | 10.83 ± 0.83 | 0.7623 | |
| MMP-8 | Before | 3.63 ± 1.48 | 3.12 ± 1.34 | 0.5221 |
| 1 week | *15.37 ± 1.39 | *13.67 ± 1.70 | 0.0321 | |
| 4 weeks | *10.46 ± 1.50 | *8.88 ± 1.34 | 0.0305 | |
| 8 weeks | 3.69 ± 1.36 | 3.34 ± 1.63 | 0.6270 | |
| IL-1β | Before | 13.33 ± 0.88 | 12.96 ± 0.90 | 0.2786 |
| 1 week | *33.71 ± 0.78 | *32,48 ± 1.07 | 0.0120 | |
| 4 weeks | *20.47 ± 0.84 | *19.28 ± 1.18 | 0.0250 | |
| 8 weeks | 13.04 ± 1.46 | 13.02 ± 1.37 | 0.9741 |
Note: Compared with preoperative, *p < 0.05; IL-8: interleukin-8; MMP-8: matrix metalloproteinase-8; IL-1β: interleukin-1β.
3.5. Correlation analysis of Pi with inflammatory factors and periodontal clinical indices between groups
The Pearson correlation analysis showed that the expression of Pi was positively correlated with the levels of PD, PLI, BOP, IL-8, MMP-8 and IL-1β in both groups, among which the correlation coefficients between the expression of Pi and PD, PLI and IL-1β was statistically significant (p < 0.05), as can be seen in Table 3.
Table 3.
Correlation analysis of Pi expression with inflammatory factors and periodontal clinical indexes between the groups.
| Relative indexes |
Pi |
|||
|---|---|---|---|---|
| Triangular flap group |
Modified triangular flap group |
|||
| r | p | r | p | |
| PD | 0.7955 | *0.0012 | 0.7415 | *0.0029 |
| PLI | 0.6653 | *0.0074 | 0.4363 | 0.0528 |
| BOP | 0.1015 | 0.4034 | 0.1226 | 0.3556 |
| IL-8 | 0.0080 | 0.8192 | 0.01057 | 0.7924 |
| MMP-8 | 0.4293 | 0.0554 | 0.3166 | 0.1147 |
| IL-1β | 0.9056 | *<0.0001 | 0.8669 | *0.0003 |
Note: Compared with preoperative, *p < 0.05; PD: Probing Depth; PLI: Plaque Index; BOP: Bleeding on Probing; IL-8: interleukin-8; MMP-8: matrix metalloproteinase-8; IL-1β: interleukin-1β; Pi: Prevotella intermedia.
4. Discussion
Extraction of impacted mandibular third molars is one of the most common procedures in oral and maxillofacial surgery. Although minimally invasive techniques have been widely used, complications often still occur after surgery. Among these, the effect of no erupted IMTM extraction on the periodontal tissue of the adjacent second molars has become a controversial clinical issue [2]. The relevant literature indicates that multiple factors, including preoperative periodontal condition, patient age, type of IMTM impaction, intraoperative flap design, tooth extraction instruments used, suture type and other variables affect the distal periodontal tissue of adjacent second molars after IMTM extraction [[27], [28], [29], [30], [31], [32], [33]], and suggests that intraoperative flap design plays a major role. Flap designs, as a basic operation during surgery, not only determine the surgical area and access but are also closely related to periodontal tissue healing [34]. Various flap designs, including standard flaps and modified flaps, have been proposed and studied, but some controversy about their use remains.
In this study, no erupted IMTM were used to investigate the effect of using triangular flaps and modified triangular flaps on periodontal tissues. To avoid the influence of mesial and buccal bone removal on the operation, only distal bones were removed intraoperatively in any of the patients; The crowns were then extracted separately to minimize as much as possible the influence of factors other than incision design. The traditional standard triangular flap, which is commonly used in IMTM extraction and is favored by clinicians, including distal incisions, sulcular incisions and vertical releasing incisions, and together these provide a wide range of surgical area and operation spaces [14]. Studies have revealed that an important factor that affects the periodontal healing of second molars after IMTM extraction is the amount of residual periodontal ligament and gingival fibers [35]. To minimize the effect of sulcular incision on periodontal tissue, in this study the standard triangular flap was modified to preserve the gingival fibers, junctional epithelium and the buccal attached gingiva around the second molar. But the modified triangular flap also has some disadvantages, such as limited area of the elevated gingival flap, small intraoperative field of view. The results of the study showed that although both flap designs caused an increase in the periodontal pocket depth of the adjacent second molars within 4 weeks, the pockets in the group in which a modified triangular flap group was used were significantly lower in depth than those in the triangular flap group.
The formation of a distal periodontal pocket around the second molar is a common complication after IMTM surgery [36]. In our research, compared with the modified triangular flap, the triangular flap resulted in a larger periodontal pocket and obviously increased probing depth. However, this difference was only observed 1 and 4 weeks after surgery, which may be due to the immaturity of the collagen fibers of the triangular flap and the still fragile connection between the gingival flap and the tooth surface, resulting in a still larger probing depth.; There was no significant difference at 8 weeks. It has been reported that periodontal protective membrane reset may take up to 45 days [37], Castagna et al. [38] also evaluated the effect of different flap designs on periodontal parameters and found no significant differences 2 months postoperatively, further suggesting that after flap surgery, the periodontal healing process interfered with the results. In addition, the plaque index and bleeding on probing increased without statistical significance in the both groups, indicating that the difference in probing depth observed 1 and 4 weeks after surgery did not contribute to plaque accumulation, but was caused by the use of different flap designs.
Bacteria are the initiators of periodontal disease, and periodontal pathogens such as Aa, pg, Pi are commonly found near the third molars of healthy patients [39]. The formation of complex communities of subgingival microbiota depends on environmental factors, and changes in these factors may encourage the growth of pathogenic bacteria and the expression of virulence factors that then become a primary cause of periodontal tissue destruction [40]. The effect of flap design, which may disturb local environmental homeostasis by undermining epithelial attachment, on periodontal tissue is related to subgingival microbiota dysbiosis and the host immune response. Flap design may also directly stimulate the body's immune response, triggering increased secretion of inflammatory factors in gingival crevicular fluid.
This research revealed that colonization by Aa, Pg, and Pi, bacteria that are closely related to periodontal disease, was significantly increased at 1 and 4 weeks after surgery, with Pi showing a significant difference between the two flap designs. Current studies [41] suggest that Pi, which correlates moderately with the occurrence and development of periodontal disease, can inhibit bone formation and accelerate bone absorption, leading to the formation of periodontal pockets, increasing probing depth, and causing a metabolic disorder of alveolar bone. The events triggers a series of host immune responses in which virulence factors and various metabolites are released, giving rise to the production of inflammatory factors such as IL-1β and IL-8 as well as matrix metalloproteinases [42]. Some of the biological effects of Pi involve in stimulation of fibroblast proliferation and activation of different cell populations, causing them to synthesize and release specific collagenases that promote the degradation of collagen fibers and the destruction of periodontal supporting tissues. This may be part of the reason for the increased probing depth observed after surgery [43]. Pearson correlation analysis showed that the degree of Pi infection caused by the use of flap designs was positively correlated with PD and IL-1β levels in gingival crevicular fluid, indicating that the increase in probing depth after surgery may be associated with subgingival microbiota and the host's immune inflammatory response.
The innovation of this study is that it explores the mechanism of the effect of flap designs on periodontal tissue by performing extraction in which a modification of the standard triangular flap is used and observing the resulting changes in subgingival microbiota and inflammatory factors in gingival crevicular fluid. This study still has many shortcomings. (1) Although strict inclusion and exclusion criteria we established and used, these criteria are still not comprehensive enough to fully define the specific tooth location, such as partial buccal, partial lingual or dentition, root number, and root form. (2) Because the follow-up time was short, only 8 weeks, long-term assessment of soft tissue and effect on bone tissue was not possible. (3) Studies have shown that IMTM extraction before age 25 (regardless of sex) has significantly less impact on periodontal tissue than extraction after age 25 [44]. We selected patients between the ages of 20–25 years to minimize the effects of other factors on the periodontal tissue. Therefore, our results may only apply to a small number of people. Future, experiments will address the deficiencies of the this research.
5. Conclusion
Within the limitations of the present study, it is concluded that the use of either type of flap during IMTM extraction resulted in an increase in probing depth and in changes in the relative abundance of subgingival microbiota and the levels of inflammatory factors within 4 weeks; the changes in the modified triangular flap group were significantly less marked than those in the triangular flap group. Pearson correlation analysis revealed that Pi was positively correlated with IL-1β and PD. This indicates that short-term application of the modified triangular flap significantly mitigates distal periodontal damage to the adjacent second molar. Our study provides inferential evidence that the subgingival microbiota and the host's inflammatory response may be mediated by the incision design.
Funding statement
This work was supported by National Natural Science Foundation of China [82071155 and 82271023], the Shanxi Applied Basic Research Program Science-Youth Technology Research Fund [grant numbers 20210302124398 and 202103021223218], the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2022L176), Shanxi Province Key Research and Development Program [201903D321148], Project of Clinical Medical Research Center of Oral Diseases of Shanxi Province [RC2023-01], Project of Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials [RC2021-02].
Statement of clinical relevance
The use of flap design for third molar extraction may destroy the gingival epithelium and epithelial attachment, and thereby affect the homeostasis of the subgingival microbiota, leading to the development of chronic periodontal inflammation.
Author contribution statement
Jing Zhao: Yuan Zhang: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Yongfeng Cheng: Performed the experiments; Analyzed and interpreted the data.
Si Xie: Peng-Fei Zhang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Dian-Dian Li: Contributed reagents, materials, analysis tools or data.
Xiu-Yun Ren: Xing Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Upon reasonable request, the data generated in this study are available from our corresponding author Dr. Xing Wang.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16161.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Lee C.T., Hum L., Chen Y.W. The effect of regenerative periodontal therapy in preventing periodontal defects after the extraction of third molars: a systematic review and meta-analysis. J. Am. Dent. Assoc. 2016;147:709–719. doi: 10.1016/j.adaj.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Kumari C.B.N., Ramakrishnan T., Devadoss P., Vijayalakshmi R., Alzahrani K.J., Almasri M.A., Al-Ahmari M.M., Al Dira H.S., Suhluli M., Bhati A.K., Ahmad Z.H., Raj A.T., Bhandi S., Patil S. Use of collagen membrane in the treatment of periodontal defects distal to mandibular second molars following surgical removal of impacted mandibular third molars: a comparative clinical study. Biology. 2021;10:1348. doi: 10.3390/biology10121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petsos H., Fleige J., Korte J., Eickholz P., Hoffmann T., Borchard R. Five-years periodontal outcomes of early removal of unerupted third molars referred for orthodontic purposes. J. Oral Maxillofac. Surg. 2021;79:520–531. doi: 10.1016/j.joms.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Pham T.A.V., Nguyen N.H. Periodontal status of the adjacent second molar after impacted mandibular third molar surgical extraction. Contemp. Clin. Dent. 2019;10:311–318. doi: 10.4103/ccd.ccd_634_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aniko-Włodarczyk M., Jaroń A., Preuss O., Grzywacz A., Trybek G. Evaluation of the effect of surgical extraction of an impacted mandibular third molar on the periodontal status of the second molar-prospective study. J. Clin. Med. 2021;10:2655. doi: 10.3390/jcm10122655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kugelberg C.F., Ahlström U., Ericson S., Hugoson A., Thilander H. The influence of anatomical, pathophysiological and other factors on periodontal healing after impacted lower third molar surgery. A multiple regression analysis. J. Clin. Periodontol. 1991;18:37–43. doi: 10.1111/j.1600-051x.1991.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 7.Valm A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019;431:2957–2969. doi: 10.1016/j.jmb.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemiec B.A. Periodontal disease. Top. Companion Anim. Med. 2008;23:72–80. doi: 10.1053/j.tcam.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Bodet C., Chandad F., Grenier D. Potentiel pathogénique de Porphyromonas gingivalis, Treponema denticola et Tannerella forsythia, le complexe bactérien rouge associé à la parodontite [Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis] Pathol. Biol. 2007;55:154–162. doi: 10.1016/j.patbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Ng H.M., Kin L.X., Dashper S.G., Slakeski N., Butler C.A., Reynolds E.C. Bacterial interactions in pathogenic subgingival plaque. Microb. Pathog. 2016;94:60–69. doi: 10.1016/j.micpath.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Belibasakis G.N., Johansson A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine. 2012;59:124–130. doi: 10.1016/j.cyto.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Pani P., Tsilioni I., McGlennen R., Brown C.A., Hawley C.E., Theoharides T.C., Papathanasiou E. IL-1B(3954) polymorphism and red complex bacteria increase IL-1β (GCF) levels in periodontitis. J. Periodontal. Res. 2021;56:501–511. doi: 10.1111/jre.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menziletoglu D., Guler A.Y., Basturk F., Isik B.K., Erdur E.A. Comparison of two different flap designs for bilateral impacted mandibular third molar surgery. J. Stomatol. Oral Maxillofac. Surg. 2020;121:368–372. doi: 10.1016/j.jormas.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Haukka A., Heikkinen A.M., Haukka J., Kaila M. Oral health indices predict individualised recall interval. Clin Exp Dent Res. 2020;6:585–595. doi: 10.1002/cre2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamimi Z., Al Habashneh R., Hamad I., Al-Ghazawi M., Roqa'a A.A., Kharashgeh H. Efficacy of serratiopeptidase after impacted third molar surgery: a randomized controlled clinical trial. BMC Oral Health. 2021;21:91. doi: 10.1186/s12903-021-01451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almendros-Marqués N., Berini-Aytés L., Gay-Escoda C. Evaluation of intraexaminer and interexaminer agreement on classifying lower third molars according to the systems of Pell and Gregory and of Winter. J. Oral Maxillofac. Surg. 2008;66:893–899. doi: 10.1016/j.joms.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Yanine N., Sabelle N., Vergara-Gárate V., Salazar J., Araya-Cabello I., Carrasco-Labra A., Martin C., Villanueva J. Effect of antibiotic prophylaxis for preventing infectious complications following impacted mandibular third molar surgery. A randomized controlled trial. Med. Oral Patol. Oral Cir. Bucal. 2021;26:e703–e710. doi: 10.4317/medoral.24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.Y., Yong H.S., H Park K., Huh J.K. Modified difficult index adding extremely difficult for fully impacted mandibular third molar extraction. J. Korean Assoc. Oral Maxillofac. Sur.g. 2019;45:309–315. doi: 10.5125/jkaoms.2019.45.6.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Que G. Advance in study on 16S rRNA gene sequencing technology in oral microbial diversity. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45:849–855. doi: 10.11817/j.issn.1672-7347.2020.190236. [DOI] [PubMed] [Google Scholar]
- 20.Balakrishna R., Poojary D.A., Sali S., Moharana A.K., Ts D. Single blind, randomized study comparing clinical equivalence of Trusilk ® and Mersilk ® silk sutures for mucosal closure following surgical removal of mesioangular impacted mandibular third molar. F1000Res. 2022;11:689. doi: 10.12688/f1000research.122678.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L.J., Qu H.L., Tian Y., Bi C.S., Zhang S.Y., Chen F.M. Impacts of non-impacted third molar removal on the periodontal condition of adjacent second molars. Oral Dis. 2020;26:1010–1019. doi: 10.1111/odi.13314. [DOI] [PubMed] [Google Scholar]
- 22.Akman A.C., Buyukozdemir Askin S., Guncu G.N., Nohutcu R.M. Evaluation of gingival crevicular fluid and peri-implant sulcus fluid levels of periostin: a preliminary report. J. Periodontol. 2018;89:195–202. doi: 10.1902/jop.2017.170315. [DOI] [PubMed] [Google Scholar]
- 23.Silness J., Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal CONDTION. Acta Odontol. Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Xiao M., Li Y., Ma F., Zhang G., Qiang J. Multiparametric MRI radiomics nomogram for predicting lymph-vascular space invasion in early-stage cervical cancer. Br. J. Radiol. 2022;95 doi: 10.1259/bjr.20211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bui F.Q., Almeida-da-Silva C.L.C., Huynh B., Trinh A., Liu J., Woodward J., Asadi H., Ojcius D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai S., Zhou Y., Pathak J.L., Piao Z., Zhou L. The association of mandibular third molar impaction with the dental and periodontal lesions in the adjacent second molars. J. Periodontol. 2021;92:1392–1401. doi: 10.1002/JPER.20-0424. [DOI] [PubMed] [Google Scholar]
- 27.Caymaz M.G., Buhara O. Association of oral hygiene and periodontal health with third molar pericoronitis: a cross-sectional study. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6664434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candotto V., Oberti L., Gabrione F., Scarano A., Rossi D., Romano M. Complication in third molar extractions. J. Biol. Regul. Homeost. Agents. 2019;33 [PubMed] [Google Scholar]
- 29.Mistry F.K., Hegde N.D., Hegde M.N. Postsurgical consequences in lower third molar surgical extraction using micromotor and piezosurgery. Ann Maxillofac Surg. 2016;6:251–259. doi: 10.4103/2231-0746.200334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dragovic M., Pejovic M., Stepic J., Colic S., Dozic B., Dragovic S., Lazarevic M., Nikolic N., Milasin J., Milicic B. Comparison of four different suture materials in respect to oral wound healing, microbial colonization, tissue reaction and clinical features-randomized clinical study. Clin. Oral Invest. 2020;24:1527–1541. doi: 10.1007/s00784-019-03034-4. [DOI] [PubMed] [Google Scholar]
- 31.Bailey E., Kashbour E.W., Shah N., Worthington H.V., Renton T.F., Coulthard P. Surgical techniques for the removal of mandibular wisdom teeth. Cochrane Database Syst. Rev. 2020;7:CD004345. doi: 10.1002/14651858.CD004345.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledano-Serrabona J., Ruiz-Romero V., Camps-Font O., Gay-Escoda C., Sánchez-Garcés M.Á. A systematic review and meta-analysis on the effectiveness of xenograft to prevent periodontal defects after mandibular third molar extraction. Med. Oral Patol. Oral Cir. Bucal. 2021;26:e414–e421. doi: 10.4317/medoral.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muntean M.V., Oradan A., Achimas-Cadariu P.A. Using noncontact vein visualization to optimize venous flap design. J. Plast. Reconstr. Aesthetic Surg. 2020;73:608–620. doi: 10.1016/j.bjps.2019.11.059. [DOI] [PubMed] [Google Scholar]
- 34.Alqahtani N.A., Khaleelahmed S., Desai F. Evaluation of two flap designs on the mandibular second molar after third molar extractions. J. Oral Maxillofac. Pathol. 2017;21:317–318. doi: 10.4103/jomfp.JOMFP_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasparro R., Sammartino G., Mariniello M., diLauro A.E., Spagnuolo G., Marenzi G. Treatment of periodontal pockets at the distal aspect of mandibular second molar after surgical removal of impacted third molar and application of L-PRF: a split-mouth randomized clinical trial. Quintessence Int. 2020;51:204–211. doi: 10.3290/j.qi.a43947. [DOI] [PubMed] [Google Scholar]
- 36.Pinto G., Silva M.D., Peddey M., Sillankorva S., Azeredo J. The role of bacteriophages in periodontal health and disease. Future Microbiol. 2016;11:1359–1369. doi: 10.2217/fmb-2016-0081. [DOI] [PubMed] [Google Scholar]
- 37.Jamal S., Gul M., Khan F.R., Ghafoor R. Effect of full sulcular versus papilla-sparing flap on periodontal parameters in periradicular surgeries: a systematic review and meta-analysis. J. Indian Soc. Periodontol. 2021;25:186–192. doi: 10.4103/jisp.jisp_290_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castagna V., Pardo A., Lanaro L., Signoriello A., Albanese M. Periodontal healing after lower third molars extraction: a clinical evaluation of different flap designs. Healthcare. 2022;10:1587. doi: 10.3390/healthcare10081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2018;76:85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 40.Curtis M.A., Diaz P.I., Van Dyke T.E. The role of the microbiota in periodontal disease. Periodontol. 2020;83:14–25. doi: 10.1111/prd.12296. [DOI] [PubMed] [Google Scholar]
- 41.Cong S., Tong Q., Peng Q., Shen T., Zhu X., Xu Y., Qi S. In vitro anti-bacterial activity of diosgenin on Porphyromonas gingivalis and Prevotella intermedia. Mol. Med. Rep. 2020;22:5392–5398. doi: 10.3892/mmr.2020.11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajishengallis G., Lamont R.J. Polymicrobial communities in periodontal disease: their quasi-organismal nature and dialogue with the host. Periodontol. 2000;86(2021):210–230. doi: 10.1111/prd.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogrel M.A. What is the effect of timing of removal on the incidence and severity of complications? J. Oral Maxillofac. Surg. 2012;70:S37–S40. doi: 10.1016/j.joms.2012.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request, the data generated in this study are available from our corresponding author Dr. Xing Wang.