Abstract
MicroRNAs (miRNAs or miRs) are non-coding, single-stranded, endogenous RNAs that regulate various biological processes, most notably the pathophysiology of many human malignancies. It process is accomplished by binding to 3′-UTR mRNAs and controlling gene expression at the post-transcriptional level. As an oncogene, miRNAs can either accelerate cancer progression or slow it down as a tumor suppressor. MicroRNA-372 (miR-372) has been found to have an abnormal expression in numerous human malignancies, implying that the miRNA plays a role in carcinogenesis. It is both increased and downregulated in various cancers, and it serves as both a tumor suppressor and an oncogene. This study examines the functions of miR-372 as well as the LncRNA/CircRNA-miRNA-mRNA signaling pathways in various malignancies and analyses its potential prognostic, diagnostic, and therapeutic implications.
Keywords: MicroRNA-372, Function, Importance, Cancer, Tumor suppressor, Oncogene
1. Introduction
Cancer is a complicated genetic disease that is the most rampant death in many nations after heart disease [[1], [2], [3]]. It is estimated that approximately 28.4 million new cancer cases will be diagnosed in 2040, which will be drastically growing in comparison with the rate of cancer cases which was roughly 19.3 million in 2020 [4]. Most rifle cancer among women is comprised of breast, lung, and colorectal cancer, while cancer, the most common among men, is such as prostate, lung, and bronchus cancer [2]. Despite significant research on the diagnosis and treatment of cancer and its pain relief drugs, it remains the leading health concern [5]. As a result, novel tumor-targeted therapies with greater efficiency and specificity are required. During the past few years, working on genes generating non-coding RNA (ncRNA) has been revolutionized in diagnosing and treating cancers [1,6]. MicroRNAs are small (18–25 nucleotides), endogenous, single-stranded, ncRNAs expressed in cells to control gene expression at the post-transcription level through binding with the 3’ untranslated region (3’-UTR) of target mRNA molecules [7,8]. In addition, some studies demonstrated that miRs could bind to the 5’ UTR of mRNAs to activate the translation of the target mRNA [9,10]. MiRs, which were first discovered in 1993, has been proposed to play a critical role in various biological functions, comprising cell growth, apoptosis, migration, differentiation, and angiogenesis [7,11,12]. Dysregulation of miRs is involved in many diseases, including cancer, cardiovascular disease, multiple sclerosis and diabetic nephropathy [[13], [14], [15], [16], [17], [18], [19]]. The role of miRs in cancer was investigated for the first time in 2002 [20]. They play the opposite role as either oncogenic miRs (oncomiRs) or tumor suppressors [21]. OncomiRs are highly expressed in the cancer cells and enhance cancer cell division through decreased tumor suppressor genes, while tumor suppressor miRs regulate the processes of survival and cell cycle division [22]. This discrimination depends on miR molecules' ability to intervene in carcinogenesis processes, comprising mechanisms associated with cell proliferation, invasion, metastasis, and apoptosis of cancer cells [21]. The growing body of research on miRs and cancer is at an extraordinary rate. During the past few years, research on the association between miRs and cancer has drastically increased, and they can be used as prognostic or therapeutic markers in human cancers [23].
Over the past decade, research interest in the relationship between miRs and various malignancies has grown exponentially. The discovery of the potential of miRs in the prognosis, diagnosis, and treatment of human cancers has fueled this interest. Currently, miRs are being tightly investigated in various preclinical and clinical studies for their therapeutic potential in cancer treatment. These findings have the potential to lead to the development of new, more effective cancer treatments, and improve outcomes for cancer patients. Although there have been several studies that reviewed the role of various miRs in cancers, such as miR-1297 [24], the dualistic role of miR-372 in cancer has not been evaluated. Therefore, for the first time, we aimed to review the role of miR-372 in various cancers comprehensively.
1.1. miR-372
MiRNA-372 (Fig. 1) is a small ncRNA with one exosome count, located on the 19q13.42 chromosome [25]. The function and expression pattern of miR-372 was first discovered in testicular germ cell tumors in 2006 [26]. Since this miR was identified, several researchers have worked on its diverse function. Yasukawa et al. revealed that miR-372 can reduce the production of both IFN-β and pro-inflammatory cytokines in response to viral infection and also controls MAVS-mediated antiviral innate immunity by regulating mitochondrial metabolism via the SLC25A12 transporter. They found that it could be a therapeutic antiviral target for the RNA viruses such as influenza A [27]. Besides, Shan et al. acknowledged that miR-372 was upregulated in hyperlipidemic acute pancreatitis (HTGAP) and had a positive relationship with the severity of the disease. These findings could lead to an innovative strategy for diagnosing and assessing the severity of HTGAP [28].
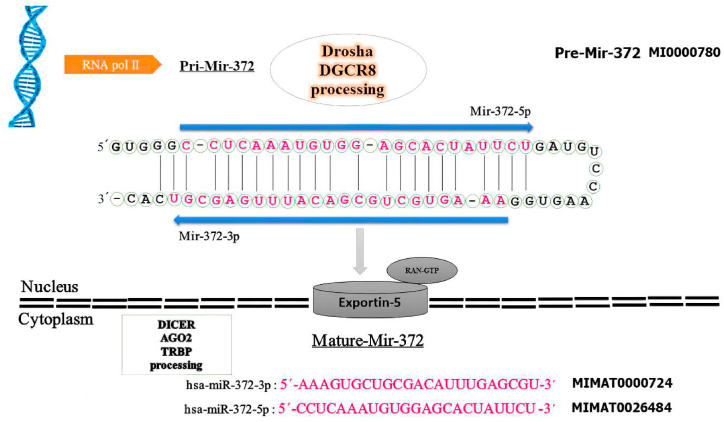
Fig. 1.
The hairpin structure of pre-miR-372 and the sequence of mature miR-372-3p & -5p. The miR-372 gene is transcribed into a precursor (pre-miR-372) with 66 nucleotides in the nucleus, transferred to the cytoplasm by Exportin-5 for further processing to yield mature miRNAs with 23 nucleotides for mature miR-372-3p & -5p. The sequence of mature miR-372 is colored in red. The arrow indicates the orientations from 5′ to 3′. The codes of miR-372 were extracted from miRBase.
Furthermore, miR-372 increases primordial germ cell numbers by repressing multiple pathways, including cell cycle (CDKN1A, RBL2, CDC2L6), EMT (RHOC, TGFBR2), and epigenetic regulators (MECP2, SMARCC2) [29]. Inhibiting these targets can also boost the formation of human-induced pluripotent stem cells [30]. Dysregulation of miR-372 has been found in a distinct range of human tumors (such as breast cancer, colorectal cancer, hepatocellular carcinoma) [[31], [32], [33]]. The expression of microRNA in various cancers has two contrasting roles as either oncogenic or anti-oncogenic. In this review, we summarize the recent research results on the role of miR-372 in various human cancers and describe its potential diagnostic and therapeutic implications.
1.2. MiRNA-372 in cancerous diseases
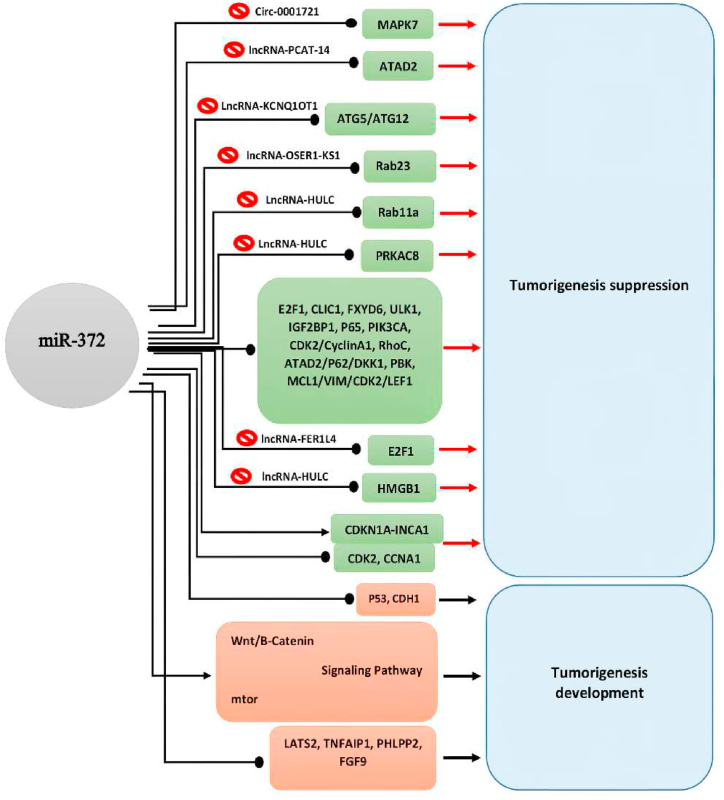
MiR-372 has been shown to play a crucial role in the carcinogenesis of a variety of human cancers, including Colorectal Cancer (CRC), Renal Cell Carcinoma (RCC), Lung Adenocarcinoma (LA), Hepatocellular Carcinoma (HCC), Gallbladder Carcinoma (GBC), Oral Cancer (OC), Cervical Cancer (CC), Testicular Germ Cell Tumor (TGCT), Pancreatic Adenocarcinoma (PAC), Gastric Cancer (GC), Cervical Cancer (CC), Ovarian Carcinoma (OVC), Breast Cancer (BC), Prostate Cancer (PC), Nasopharyngeal Carcinoma (NFC), Melanoma, Osteosarcoma (OS), and Glioma Cell (GLC). MiR-372 plays distinct roles in different malignancies, such as inhibiting or promoting proliferation, migration, invasion, epithelial–mesenchymal transition (EMT), induction or suppression of apoptosis, and cell-cycle arrest. Furthermore, its expression level in malignant tissues is commonly measured to be lower than in normal margin tissues, suggesting that miR-372 is a tumor suppressor. Since miR-372 behaves as an oncogene in some malignancies, its role as a tumor suppressor or oncogene in human neoplasms can be classified as cancer-type specific. MiR-372 induced both functionalities by targeting various genes, as detailed in Table 1. The action of Long non-coding RNAs (lncRNAs) or Circular RNAs (CircRNAs), which target miR-372s and operate as a competitive endogenous RNA (ceRNA), causes carcinogenesis in certain malignancies, the so-called sponge effect (Table 2). The interaction of miR-372 with its target genes, LncRNA and circRNA, and the results reported in Fig. 2.
Table 1.
Target Genes of miRNA-372 Responsible for Its Tumor Suppressive/Oncogenic Action in Different Cancers.
| Gene | Location | Malignancy | Cell Lines | No. of Tissue sample | Outcomes | Protein | Mir- 372 expresstion | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| RUVBL2 | 19q13.3 | Non-small cell lung cancer | N/A | CL 1-0 | N/A | Increases regulation of cell growth and transcription | RuvB-like 2 | ↑ | [165] |
| – | – | Lung adenocarcinoma | BEAS-2B | A549 | N/A | Increases growth, migration, invasion and decreases apoptosis | Wnt/β-catenin | ↑ | [166] |
| MTOR | 1p36.2 | Lung adenocarcinoma | BEAS-2B | A549 | N/A | Increases growth, migration, invasion and decreases apoptosis | mTOR | ↑ | [166] |
| CA12 | 15q22.2 | Lung adenocarcinoma | N/A | A549 | N/A | Patients with reduced CA12 in tumors have an increased risk of recurrence | CA12 proteins | ↑ | [167] |
| FGF9 | 13q12.11 | Lung squamous cell carcinoma | BEAS-2B | NCI–H520, SK-MES-1 and NCI–H1703 |
20 | Induces cell cycle arrest in G0/G1 stage Increases apoptosis |
FGF9 | ↑ | [168] |
| TP53 | 17p13.1 | nasopharyngeal carcinoma | N/A | 5–8F,C666-1, TW01 | N/A | Enhancing radiosensitivity while suppressing invasion and metastasis | p53 | ↓ | [169,170] |
| BAX | 19q13.33 | Nasopharyngeal Carcinoma | N/A | 5–8F,C666-1,TW01 | N/A | Induces mitochondrial dysfunction and death | BAX | ↓ | [169,170] |
| BCL2 | 18q21.33 | Nasopharyngeal Carcinoma | N/A | 5–8F,C666-1,TW01 | N/A | inhibition of cell progression due to a prolong duration of G1 phase, anti-apoptotic gene | Bcl-2 | ↓ | [169,170] |
| CDK2 | 12q13.2 | Nasopharyngeal Carcinoma | N/A | TW01 | N/A | Cell proliferation | Cdk-2 | ↓ | [170] |
| CCNA1 | 13q13.3 | Nasopharyngeal Carcinoma | N/A | TW01 | N/A | Cell proliferation | Cyclin-A1 | ↓ | [170] |
| PRKACB | 1p31.1 | Hepatocellular carcinoma | QSG-7701, Chang liver and HL-7702 | Huh-1 Huh-4, Hep3B, HepG2, Huh-7, SNU-449, SNU-475 |
14 | Decreases phosphorylation of CREB and CERB down regulates HULC | – | ↓ | [171] |
| ATAD2 | 8q24.13 | Hepatocellular carcinoma | LO2 | SMMC7721, QGY-7701, Bel-7402, PLC5, Huh7, HCCLM3, HepG2 |
129 | reduced their capacity for invasion and proliferation and led to a G1 phase arrest | tRNA-specific adenosine deaminase 2 | ↓ | [172] |
| MCL-1 | 1q21.2 | Hepatocellular carcinoma | N/A | HepG2 | 88 | Anti-apoptosis | Mcl-1 | ↓ | [173] |
| E2F1 | 20q11.22 | Hepatocellular carcinoma | N/A | HUH-7/DDP | 95 | Anti-apoptosis | E2f-1 | ↓ | [174] |
| CUL4B | Chromosome X | Bladder cancer | N/A | T24 253J 5637 RT112 RT4 SV-HUC-1 |
77 | Increased motility, invasiveness, stemness and chemoresistence | Cullin 4 B | ↓ | [125] |
| FXYD | 11q23.3 | Osteosarcoma | hfOB1.19 | O2OS SOSP-9607 MG-63 |
30 | Promotes tumor growth | FXYD6 | ↓ | [117] |
| LATS2 | 13q12.11 | Ovarian cancer | N/A | OVCAR3 A2780 |
N/A | Cell proliferation | Large tumor suppressor kinase2 | ↓ | [137] |
| LATS2 | 13q12.11 | Prostate cancer | N/A | DU145 PC3 |
25 | Tumor suppressor | Large tumor suppressor kinase2 | ↑ | [150] |
| LATS2 | 13q12.11 | Testicular germ cell tumor | N/A | N/A | N/A | Tumor suppressor | Large tumor suppressor kinase2 | ↑ | [26] |
| RhoC | 1p13.2 | Endometrial carcinoma | N/A | N/A | N/A | Cell growth Migration and invasion |
RAS homolog family member C | ↓ | [134] |
| TP65 | 11q3 | Prostate cancer | N/A | DU145 | 20 | Cell proliferation Invasion and migration |
P65 | ↓ | [149] |
| Sequestosome 1 | 5q35.3 | Ovarian cancer | N/A | OVCAR3 A2780 |
N/A | Cell proliferation | P62 | ↓ | [137] |
| CDK2 | 12q13.2 | Cervical cancer | N/A | HeLa C33A |
18 | Cell proliferation | Cyciln-dependent kinase 2 | ↓ | [129] |
| CDK2 | 12q13.2 | Endometrial cancer | N/A | N/A | N/A | Cell proliferation | Cyciln-dependent kinase 2 | ↓ | [134] |
| CCNA1 | 13q13.3 | Cervical cancer | N/A | HeLa C33A |
18 | Cell proliferation | Cyclin A1 | ↓ | [129] |
| CCNA1 | 13q13.3 | Endometrial caner | N/A | N/A | N/A | Cell proliferation | Cyclin A1 | ↓ | [134] |
| CCNA1 | 13q13.3 | Ovarian cancer | N/A | OVCAR3 A2780 |
N/A | Cell proliferation | Cyclin A1 | ↓ | [137] |
| ATAD2 | 8q24 | Ovarian cancer | N/A | OVCAR3 A2780 |
N/A | Cell proliferation and metastasis | ATPase Family AAA Domain Containing 2 | ↓ | [137] |
| ATAD2 | 8.q24 | Renal cell carcinoma | HK2 | Caki-1 786-O A498 ACHN Caki-2 |
52 | Cell proliferation | ATPase Family AAA Domain Containing 2 | ↓ | [143] |
| DKK1 | 10q21.1 | Ovarian cancer | N/A | OVCAR3 A2780 |
N/A | Cell proliferation | Dickkopf-related protein 1 | ↓ | [137] |
| IGF2BP1 | 17q21.32 | Renal cell carcinoma | HK2 | 769-P 786-O A498 SN12-PM6 |
30 | Cell proliferation and invasion | Insulin-like growth factor2 mRNA-binding protein1 | ↓ | [142] |
N/A: Not Applicable.
↑: Increased expression of miR-372.
↓: Decreased expression of miR-372.
Table 2.
Oncogenic Circular RNA and Long Non-Coding RNAs (lncRNA), Which Act as Competitive Endogenous RNA (ceRNA) for miR-372 (So-Called Sponging Effect) in Different Cancers.
| RNA | Malignancy | Cell Lines |
No. of Tissue Samples (Pair) | Outcomes | References | |
|---|---|---|---|---|---|---|
| Normal | Cancerous | |||||
| KCNQ1OT1 | Lung adenocarcinoma | HEK293 | A549, H1975 |
50 | larger tumor, advanced clinical stage, and a lower response rate to concurrent therapy | [175] |
| OSER1-AS1 | Hepatocellular Carcinoma | N/A | HepG2 and Hep3b | 34 | the proliferation, invasion and migration of HCC cells, and induced the apoptosis |
[176] |
| LncRNA HULC | Hepatocellular Carcinoma | N/A | N/A | 100 | tumor node and metastases (TNM) higer stage, HCC recurrence, intra-hepatic metastases, and Lower postoperative survival. |
[[62], [171]] |
| PCAT-14 | Hepatocellular Carcinoma | LO2 | Huh7, HCCLM3, HepG2, SMMC7721, PLC5, and QGY7701 |
120 | proliferation and invasion of hepatocellular carcinoma cells | [66] |
| LncRNA HULC | Osteosarcoma | HfoB | SAOS-2 U2OS HoS MG63 |
32 | Cell proliferation, migration and invasion of OS cells | [119] |
| Circ_0001721 | Osteosarcoma | HfoB1.19 | U2OS HoS |
56 | Tumor growth, proliferation, invasion, migration, EMT and promote cell apoptosis | [118] |
N/A: Not Applicable.
Fig. 2.
Effects of miR-372 on its target genes for regulating tumorigenesis biological behaviors. In the figure, all the target genes of miR-372 with tumorigenesis of human cancers have been shown. For example, miR-372 represses tumorigenesis by suppressing the PRKAC8 gene. In this regard, lncRNA-HULC inhibits the anti-tumor effect of miR-372. On the other hand, it has been reported that miR-372 via suppressing the FGF9 as well as activation of Wnt/B-Catenin and mtor signaling pathway leads to increased tumorigenesis biological behaviors.
1.3. MiRNA-372 and nervous system cancers
Central nervous system (CNS) tumors involve mainly the brain (>90%) and the lesser other parts of the CNS. Malignancies that spread rapidly develop robust angiogenesis, invade normal brain tissue, reveal a short median survival time, and subsequently have a poor prognosis [24]. The globocan 2020 database provides the latest global data on cancer burden cancer deaths. Its result revealed brain and other nervous system accounts for 1.6% of new cases of all cancer site (19292789 all sites) and 2.5% of new deaths in all cancer site of new deaths 9958133 all deaths [4].
Glioma is one of the lethal types of brain tumor originating from the glial cell. The glial cell consists of astrocyte oligodendrocyte and ependymal cells. Gliomas account for approximately 50% of all malignant primary brain tumors in adults. According to tumor growth potential and aggressiveness, WHO classified 4 grades for glioma that 1,2 grade is low, and 3,4 are high. Surgery, radiation therapy, and chemotherapy are treatments that take the situation of the patient into account, although, despite these treatments, the short median survival time is just approximately 14.6 months [24].
Understanding the disease's pathogenesis to develop new therapeutic and diagnostic strategies for glioma is crucial. miRNAs are a class of non-coding RNA that influence various physiological and pathological processes like differentiation, proliferation, apoptosis, and invasion by regulating the expression of genes at the post-transcriptional level. The profiling of miRNA in gliomas has been studied in several studies, and a series of miRNA, including mir-9, miR-17, miR-21, miR-184, and miR-342-3p, be predictive biomarkers associated with glioma progression and clinical outcome. There is also evidence that miR-26a, miR100, miR155, and miR −218 are both tumor suppressors and oncogenes in glioma [34].
Even so, far better therapeutic opportunities for glioma patients and more insight into the molecular mechanisms of glioma miRNAs are needed. miR-372 is one of the members of miRNAs that the latest evidence has shown. It has a tumor-suppressing role in glioma. This review focuses on different mechanisms of miR-372 activity in glioma cells.
A study has proved that the expression of miR-372 was higher in glioma tissue compared to normal brain tissue. The high miR-372 expression was associated with the advanced pathological grade of the patient. Chen et al. assessment unveiled that downregulation of miR-372 considerably inhibited cell proliferation by modulating PHLPP2. They transfected cells with miR-372 inhibitor, and analysis showed that inhibition of miR-372 increased the level of PHLPP2 mRNA and protein expression, thus inhibiting the PI3K/AKT signaling pathway. In other words, inhibition of miR-372 led to a decrease in the phosphorylation levels of critical components of the PI3K/Akt pathway, such as Akt, mechanistic target of rapamycin (mTOR), and P70S6K, suggesting that miR-372 may be an important regulator of PI3K/Akt pathway in glioma tissues. Also, luciferase reporter assay results showed that luciferase activity of cells transfected with miR-372 inhibitor was significantly increased. Furthermore, PHLPP2 has been shown to induce apoptosis and limit tumor growth in glioblastoma cell lines [34].
Recent research indicates that long noncoding RNAs are key regulatory factors in cancers. FER1L4, one of the important members of LnRNA investigated in the literature, regulates the cell progression in G2-M through regulating E2F transcription factor 1 (E2F1). Xia et al. found that FER1L4 and E2F1 were highly expressed in grade 4 glioma tissues. Also, their expression significantly differs between grade Ⅲ glioma tissues and grade Ⅱ. Thus, FER1L4 and E2F1 overexpression predict poor prognosis of gliomas, and FER1L4 knocking down can inhibit the proliferation and cell progression of glioma cells. This study proved that overexpression of hsa-miR-372 caused the downregulation of both FER1L4 and E2F1 expression, and it has a remarkably negative effect on glioma cell proliferation [35].
One of the dangerous side effects of vascular surgeries and thoracic surgeries is spinal cord ischemia/reperfusion injury (SCII). The pathological effect of SCII is nerve cell apoptosis which causes limb movement and sensory dysfunction. In-vivo and in-vitro examinations conducted by Moradi et al. unveiled that in spinal cord tissue of SCII rats, expression of miR-34a considerably increased. Also, they observed that miR-372 knocking down caused upregulation of becline-1 as a regulator of autophagy and subsequently reduced spinal cord neurons apoptosis [36].
One of the most prevalent neoplasms worldwide is head and neck squamous cell carcinoma (HNSCC); hypoxia occurs during most tumors’ late stage. Under hypoxic conditions, specific transcription factors, including hypoxia-inducible factors (HIFs), control transcription programs that promote cell survival in an adverse microenvironment. As a result of Yeh et al. observation of head and neck tumors in late stages. Hypoxia upregulates HIF-1α in head and neck keratinocytes. Then HIF-1α upregulate miR372. They identified that miR-372 could stimulate migration and increase ROS by targeting p62 through binding to its 3'-UTR site and causing downregulation of p62 (also called sequestosome1 or SQSTM1 is a ubiquitin-binding protein). Eventually, this study elucidates those high levels of plasma and salivary miR-372 corresponded to the progression of HNSCC and higher mortality of the patient [37].
The contradictory expression pattern of miR-372 in different tissue is what a thing makes this field interesting, and it needs more research for therapeutic and prognostic purposes.
1.4. MiRNA-372 and respiratory system cancers
Lung carcinoma has progressed from an uncommon disease to one of the most common cancers globally and a leading cause of death from cancer in the last century [38]. Furthermore, a global statistical analysis demonstrated that 1.8 million new cases were diagnosed worldwide in 2012 [39]. Lung cancer risk factors include genetic and behavioral factors, chronic inflammation from infections, ionizing radiation, air pollution, and other risk factors [40]. It is becoming increasingly difficult to ignore the low overall 5-year survival rate for lung cancer and its minimally changes in decades [[41], [42], [43]]. Thus, several attempts have been made to recommend possible therapeutic targets. In 2012, Lai et al. reported that miR-372 increased the invasive ability of CL 1–0, and comparative proteomic profiling was used to identify the possible mechanism. Findings of the study showed that the possible mechanism is a complement of the RNA sequence of miR-372 with ribonucleic acid sequences of eukaryotic initiation factor 4A-I, heat shock protein 90-alpha, and RuvB-like 2 [44]. Three years later, Mallick et al. suggested that cells with overexpression of MiR-372 proliferated faster with a higher percentage of the cells in the S phase of the cell cycle and had more capability for migrating and invading the extracellular matrix. Also, these cells formed bigger xenograft tumors in mice, and microarray analysis demonstrated that overexpression of MiR-372 altered the expression of 1,186 genes. One of the critical genes investigated in this study was the anhydrase-encoding CA12 gene which downregulation of this gene by MiR-372 plays a crucial role in the risk of cancer recurrence. Finally, they concluded that a higher miR-372 level correlates with a poorer prognosis in NSCLC [45]. In 2017, Wang et al. trans-infected lung squamous cell carcinoma (LSCC) with Mir-372-3p, which led to suppression of Fibroblast Growth Factor 9 (FGF9) expression. Thus, Mir-372-p has an oncogenic effect by promoting cell growth and metastasis via targeting FGF9 [46].
Furthermore, in 2018 Sun et al. demonstrated that in comparison to the propofol + negative control group, the expression levels of Wnt3a, p/total β-catenin, p/total p70S6K, and p/total mTOR proteins in human lung cancer cells were increased in the propofol + miR-372 mimic group and decreased in the propofol + miR-372 inhibitor group. These results indicate that propofol suppressed growth, migration, and invasion of lung adenocarcinoma cells and inactivation of Wnt/β-catenin and mTOR pathways by down-regulation of miR-372 [47].
A recently published paper by Moradi et al. investigated possible causes of resistance to stereotactic body radiotherapy in the lung. This study detected downstream miRNA expression and upregulated miR-372-3p after sh-KCNQ1OT1 transfection by microarray. The possible mechanism of KCNQ1OT1-induced SBRT resistance mentioned in the promotion of ATG5-and ATG12-dependent autophagy via sponging miR-372-3p. In addition, overexpression of miR-372-3p inhibited autophagy and enhanced the radiosensitivity of IR-resistant cells [48].
Nasopharyngeal carcinoma (NPC) is a rare malignancy worldwide, but it is endemic in some geographical regions, including Southeast Asia, North Africa, and the Arctic [49]. Also, epidemiological trends have shown that its incidence has declined gradually in the past decade [50] The main risk factors of NPC include Epstein-Barr virus infection, high consumption of salt-preserved fish, smoking, and lack of fresh fruit and vegetable intake [51] However, the major problem with NPC is that >70% of patients were diagnosed at advanced stages, and the median survival of these patients is three years. Thus, improving diagnostic and therapeutic methods is necessary [52,53]. Meanwhile, microRNAs have been reported to regulate tumor radio-sensitivity by modulating DNA damage repair, cell cycle checkpoint, tumor microenvironment, and apoptosis [54]. Therefore, several studies have been conducted to identify the role of mir-372 on radiosensitivity, invasion, and metastasis of NPC. In 2014, Tan et al. transfected NPC TW01 cells with the miR-372 precursor molecules and conducted gene expression studies using RT-PCR assays for nine cancer-related genes. Furthermore, they investigated the effects of miR-372 on cell proliferation, cell cycle arrest, and apoptosis. Findings demonstrated that a "high level of MiR-372 resulted in a significant increase of cells at the S phase and reduced cells in both the G2/M phase". Also, a comparison between transfected TW01 cells and untransfected cells revealed that four genes, including INCA1, CDKN1A, BIRC5, and LATS2, were up-regulated, and five genes including CCNA1, CDK2, BAX, TP53, and BCL2 were down-regulated in the transfected TW01 cells. The possible mechanism for decreasing TW01 cell proliferation and S phase cell arrest is down-regulating both CDK2 and CCNA1 by directly binding to their 3'UTR regions [55]. In 2018, Wang, Z. et al. studied the role of miR‐372 in radio-sensitivity, invasion, and metastasis of NPC by using microarray analysis to explore NPC‐related differentially expressed genes. They also investigated the PBK‐dependent p53 signaling pathway. Findings demonstrated that miR‐372 expression enhanced radiosensitivity while suppressing invasion and metastasis via the expression of PBK and activating the p53 signaling pathway. Besides, over‐expressed miR‐372 down‐regulated PKB and Bcl‐2 expression and the extent of Akt phosphorylation while up‐regulated the expression of Bax and p53 [56]. Surprisingly, we found that miR-372 plays an anti-tumor role in NPC, unlike its oncogenic role in lung carcinoma. These findings may suggest novel ideas for future NPC treatments. Overall, our study shows that mir-372 plays an oncogenic role in different types of lung cancer, which would be a potential therapeutic target to improve lung cancer treatments.
1.5. MiRNA-372 and hepatocellular carcinoma
Primary liver cancer is the fifth most common cancer and the third cause of death among different cancers worldwide [57]. Furthermore, the incidence of liver cancer is becoming a warning state, with 841,080 cases globally [58]. Several studies were accomplished to illuminate molecular pathways involving primary liver cancer. In this regard, Wu et al. found the anti-tumor role of MiR-372 in hepatocellular carcinoma (HCC) via four experimental shreds of evidence, including (a) downregulation of MiR-372 in HCC cells; (b) aberrantly high DNA methylation of gene promoter in the MiR-372 that induces epigenetic silencing of MiR-372; (c) MiR-372 can inhibit proliferation and invasion of HCC cell lines; (d) low Mir-372 was related with tumor metastasis and poor prognosis [59]. These findings contradict a previous study accomplished by Gu et al., demonstrating that the expression of miR-372 in HCC is higher than in normal cells and correlates with advanced tumor progression [33]. However, more recent evidence supported the anti-tumor role of miR-372 in HCC [[60], [61], [62]]. In 2020 Soliman et al. published a paper that described that over-expression of MiR-372-p in HepG2 indirectly reduced the expression of Mcl-1(myeloid cell leukemia 1) at both gene and protein levels. Mcl-1 is a key anti-apoptotic protein member of the Bcl-2 family and is identified to have an important role in tumor pathogenesis [60]. Furthermore, in 2019 Fan et al. expressed that MiR-372-3p was under-expressed in HCC tissues and negatively correlated with the level of OSER1-AS1. OSER1-AS1 is a long non-coding RNA (lncRNA) that might play an oncogenic role in HCC, and the high expression of OSER1-AS1 was closely associated with advanced tumor stages, larger tumor size, and lower disease survival in HCC patients. The possible mechanism is a molecular sponge role of OSER1-AS1 for miR-372-3p to promote Rab23 expression [61]. Previous studies support this claim that higher expression of Rab23 was detected in HCC and was related to the poor overall survival of patients [63,64]. LncRNA, highly up-regulated in liver cancer (HULC), is another Competitive Endogenous RNA (ceRNA) for miR-372 investigated by Wang et al. nine years before. This study demonstrated that inhibition of miR-372 reduces translational repression of its target gene, PRKACB, which in turn induces phosphorylation of CREB (cAMP response element binding protein). CERB up-regulates the HULC expression level, which in turn starts a cascade of molecular events to increase the chromatin accessibility for the general transcription in liver HCC [65]. In 2020 Shaker et al. also reported the inhibitory role of HULC for MiR-372 and lower expression levels of MiR-372 in both HCC and HCV groups compared to the control group [62]. PCAT-14 is another lncRNA that inhibits miR-372 expression by inducing methylation of CpG islands in the miR-372 promoter. Inhibition of MiR-372 promotes the ATAD2 gene and Hedgehog (Hh) pathway key proteins, including PTCH-1, SMO, and Gli2. Finally, the Hh pathway induces proliferation, invasion, and cell cycle in HCC cells [66]. Another study also confirmed that the ATAD2 mRNA 3′ non-coding region (3′ UTR) has a binding site for miR-372 and ATAD2 knockdown, decreasing liver cancer cells' invasive and migratory capacity. Also, this study mentioned the up-regulated APC gene and downregulated CTNNA1 gene after knocking down ATAD2, so concluded it might be the mechanism of ATAD2 gene action [67]. In 2021, Wang et al. investigated the potential role of Fer-1 like family member 4 (FER1L4) in chemotherapy resistance and liver cancer development. They applied dual-luciferase reporter gene assay, bioinformatics analysis, and RNA pull-down were to compare possible binding relationships between clinically collected liver cancer tissues and the HUH-7/DDP cell line. Results demonstrated that FER1L4 promotes drug-resistant liver cancer progression by inhibiting miR-106a and miR- 372-5p expression. MiR-106a and miR- 372-5p knock-down results to upregulation of E2F1 and activation of nuclear factor-κB (NF-κB). NF-kB up-regulates Bcl-2, PCNA, MMP9, and P50, which inhibits apoptosis pathway. Therefore, suppression of NF-κB may be new therapeutic target for the drug-resistant HCC treatment [68]. Altogether, our study provides evidence that miR-372 plays an anti-tumor role in HCC. These findings may shed light on the improvement of HCC treatments.
1.6. MiRNA-372 and gastrointestinal cancers
The prevalent head and neck carcinoma are oral cavity cancers whose death rate is exponentially high despite improving diagnosis. The five-year survival of this disease is about 50%. As a result, detecting biomarker plays a staple role in the early diagnosis of such cancer. One of the biomarkers worked over the past decade is the micro-RNA form of tumor genesis and tumor suppressor [69]. In what follows, the impact of miR-372 on oral cancer worked by some investigators is reviewed. The commonality between the outcome of these studies was upregulating miR-372 in oral squamous cell carcinoma (OSCC). Yeh et al. (2018) asserted that the target gene of miR-372 is Zinc finger and BTB domain-containing 7A (ZBTB7A), which is inversely related to miR-372. ZBTB7A expression augments drug (CDDP) sensitivity through activating three death receptors named Trail-R1, Trail-R2, and Fans expression [70]. In addition to mentioned issues, Yeh et al. (2020) underlined that ZBTB7A can trans-activate merely TRAIL-R2. Besides, they declined in ZBTB7A expression, leading to aggravating prognosis and boosting the migration of OSCC cells, proliferation cells in the G2/M stage, and invasion of tumor cells [71]. Yeh et al. (2014) reported that hypoxia is conducive to the overexpression of miR-372, which is associated with the development of OSCC. This study identified a new target of miR-372 named P62, upsetting ROS equilibrium and leading to decreasing migration of tumor cells [72]. Some scholars emphasized that nodal metastasis, lympho-vascular invasion, and poor survival correlated with the high level of miR-372 [73,74]. Plus, Tu et al. emphasize that LATS2 expression, the target gene of miR-372, is negatively related to miR-372 [73]. Furthermore, another study maintained that mono-2-ethyhexyl phthalate (MEHP) contributed to aggrandizing oral cancer cells and proliferating cell nuclear antigens (PCNA). However, it lowers miR-372-5p expression. In fact, the target of miR-372-5p is PCNA (3’UTR domain) which is inversely associated with miR-372-5p [75].
Gastric Cancer (GC) is the fifth most diagnosed cancer and the fourth most common cause of cancer death. In 2020, approximately 769,000 out of over one million died from stomach cancer worldwide. Research shows that the incidence rate among men is two times higher than women. GCs can be fundamentally divided into two main categories consisting of cardia (CGC) and non-cardia (NCGC) [4,76]. The risk factors for gastric cancer are helicobacter pylori infection, low socioeconomic status, tobacco smoking, alcohol consumption, diet, and genetic factors [77,78]. In 2006, the connection between microRNA and GC was firstly identified, which could play an essential role in the early detection of gastric cancer [79,80]. Evidence from disparate research shows a high correlation between Gastric cancers and overexpression of miR-372, whose role is oncogenicity.
According to Cho et al. and Ghasemi et al., LATS2, the target of miR-372, is negatively associated with miR-372. Either overexpression of LATS2 or the inhibition of miR-372 by AS-miR-372 use leads to lowering the proliferation of tumor cells, ceasing the cell cycle at the stage of G2M, and boosting AGS cells apoptosis [81,82]. Besides, Cho et al. suggested that the increased level of LAST2 is conducive to reducing Cyclin B, Cds2, Bcl, and ICAD expression and increasing AGS cell apoptosis [81]. Furthermore, as reported by Ghasemi et al., the decrease of LATS2 brings about the unsteadiness of the genome throughout cell division and making micronuclei [82]. In the other study reported by Zhou et al., miR-372 is attached to the 3'UTR site of tumor necrosis factor α-induced protein 1 (TNFAIP1), which is inversely associated with miR-372 expression; MiR-372 leads to the development of tumor cells and the inhibition of apoptosis. Moreover, they professed that TNFAIP1 acts as a mediator between miR-372 and NFκB activation, resulting in enhanced cell survival through the activation of anti-apoptotic genes downstream of NFκB. Collectively, our data indicate that an oncogenic role of miR-372 may be through control of cell growth and cell apoptosis by downregulating the TNFAIP1 and positively regulating NFκB expression.
Plus, they asserted that miR-372 expression is unmeasurable, as well as the high level of TNFAIP1 in the HGS cell line, but the level of miR-372 is high in the AGS cell line [83]. Staedel et al. evaluated the effect of 8-mer-locked nucleic acid (LNA) on miR-372 seeds leading to the inhibition of miR-372 and the increase of LAST2, which is a target of miR-372 expression. All mentioned factors contribute to reducing the growth of cells [84]. Following one research investigation conducted by Belair et al., after approximately 24 h of the helicobacter pylori, infection causes the miR-372 expression to lower and LAST2 to augment. In a sense, the mentioned process is conducive to terminating the cell cycle at the phase of the G1/S. This downregulation of miR-372 expression relies on the place of bacteria effector cog-A getting attached to the host cells. The augmentation of miR-372 expression may contribute to the proliferation of the cell cycle throughout the increased level of p21cip1/waf1, which fundamentally builds up in AGS cells [85]. Vo et al. came up with the innovative idea, which is new small RNA ligands including two RNA binding motives conjugation. Small RNA precludes the synthesis of tumorigenesis miR-372 by inhibiting the Dicer enzymes process playing a role in constructing miRNA, which is made of pre-miRNAs. Moreover, they asserted that the target of this miRNA is the LATS-2 protein [86].
The third most prevalent cancer, diagnosed in women and men, is called CRC, divided into the colon, rectal and anal cancers. The evidence of many kinds of research acknowledges that CRCs will have grown drastically among the vast majority of older people by 2035 [2,4,76]. With regard to the high prevalence and importance of CRC, different dimensions of pathogenesis have been taken into account at a molecular level in recent years [87,88]. Many researchers have analyzed the relationship between microRNA-372 and colorectal cancer during the past few years. A growing body of evidence indicates that those patients diagnosed with CRC fundamentally have high expression of microRNA-372 in their body, act as a carcinogen and play an essential role in tumorigenesis in CRC [32,[89], [90], [91], [92], [93]]. According to Yamashito’s research outcome, a target of miR-372 is a large tumor suppressor 2 (LATS2), which is inversely correlated with miR-372.
Furthermore, they asserted that upregulation of miR-372 has a fundamental relationship with distant metastasis, particularly in, Liver. In a sense, those patients diagnosed with liver metastasis have drastically higher expression of miR-372 and lower expression of LAST2 than other patients without liver metastasis. Besides, Yamashito’s research found that patients with high miR-372 expression are exposed to shorter five-year survival rates and are considered a poor prognosis factor [32]. Peng et al. obtained the same outcome showing LATS2 is a target of miR-372-3p, which had an inverse correlation with LATS2. The high expression of miR-372-3p is closely tied up with tumor size and differentiation of CRC cells, and it pertains to the lowest five-year relative survival. The role of LATS2 is to suppress tumor genes, mitigating the proliferation, migration, and apoptosis of CRC cells through a Hippo signaling pathway [89]. Based on Yu’s and his colleagues’ research, the level of miR-372 remarkably upgraded both CRC and colon precancerous lesions (CPL). The increased level of miR-372 expression is related to tumor size, Tumor-Nodes-Metastasis (TNM) stage, leading to worse overall survival. The serum levels of miR-372 drastically decreased in those ECRC patients undergoing surgery relative to preoperative serum miR-372 levels. Taken together, the serum levels of miR-372 are of significant importance in the early diagnosis and prognosis of CRC [90]. According to Zhang et al., miR-372 is the predictor factor for the response to chemotherapy in patients with CRC [91]. Ragusa et al. contended that the miR-372 is higher in CRC patients with mutated KRAS than those with wild-type genotype. They introduced another signaling pathway called mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), which regulates miR-372 through TRBP phosphorylation. The outcome of their research cast light on the fact that the target of the miR-372 gene is TXNIP which is inversely correlated with miR-372. While TXNIP is upgraded through MAPK/ERK inhibitors (name as U0126, FR180204, and WAY265506), miR-372 expression remarkably reduces in CRC cell lines [92]. The other study conducted by Wang et al. (2018) is concerned with other dimensions of miR-372 features. Their research indicated that decreasing some pathways and signals enhances the stemness of CRCs. The overexpression of miR-372 causes phenotype cancer stem cells (CSC) to enhance in the body. Besides, the researchers realized that the high level of miR-372 leads to resistance to the chemotherapeutic drug in patients with CRC. In a sense, even miR-372 inhibitor (TuD) could not remove the remarkable effect of drug resistance. CRC cell invasion, migration, tumor formation, and the protein levels of CSC markers (CD44 and CD26) were boosted by overexpression of miR-372. A set of signaling pathways called Hedgehog, c-Myc, and Nanog enhance by enforced expression of miR-372. However, some signaling pathways (NFĸB, SP1, MAPK/Erk, and vitamin D) and a series of genes such as RELA, VDR, SETD7, SPOP, TRERF1, ZNF367, and MTUS1 lower with the existence of overexpression of miR-372. The investigator elicited three genes (VDR, SPOP, and SETD7) amid mentioned genes to meticulously be considered. Furthermore, they asserted that decreasing these genes contributes to upgrading the CSD population. In fact, such genes play a fundamental role in suppressing tumor formation in the body. Also, the researchers worked on another signaling pathway named Wnt/β-catenin, which is significant in maintaining CRC stem cells and a sharp decline in the NFĸB pathway. In effect, cross-talking between Wnt/β-catenin and NFĸB in CRC cells is created by miR-372 expression, as key effectors of Wnt/β-catenin signaling [93].
Gallbladder cancer (GBC) is the most common and lowest survival cancer among all biliary tract cancers [94,95]. An estimated 11980 new cases and 4310 deaths of biliary tract cancers will be diagnosed in 2021 [2]. There is merely one study focusing on the association between miR-372 and GBC. Zhou et al. researched the relationship of hsa-miR-372 with GBC, and they indicated that in GBC, the level of hsa-miR-372 expression, whose target is likely CLIC1 (Chloride intracellular channel 1), is lower. Even though the decline in miR-372 expression is associated with an advanced and aggressive tumor, shorter overall survival, and metastasis, the decrease of hsa-miR-372 expression is not correlated with age, sex, tumor size, and gallbladder stones [96].
Toll death of pancreatic cancer is the highest among other gastrointestinal cancers since most patients are diagnosed at the advanced stage and metastasis [97,98]. The 5-year survival rate is approximately 10% worldwide [2,76,97]. As reported in 2020, the number of new cases and deaths are 49,5773 and 46,6003, respectively [4]. In doing so, early detection and treatment of this insidious cancer are important in saving patients and increasing their overall survival. s, many experimental studies are working on the biomarker of pancreas cancer, a microRNA, to find a way to diagnose and treat in the earlier stage of the disease. Just one research indicates the association between miR-372 and pancreas cancer described in the process of autophagy [99]. Chen et al. demonstrated miR-372 expression decline in human pancreatic adenocarcinoma cells (HPAC). The function of miR-372 is to inhibit proliferation, invasion, migration, and autophagy of HPAC and increase cell apoptosis. A UNC5Q-like kinase (ULK1), a target of miR-372, is inversely related to miR-372. The augmentation of miR-372 expression reduces both mRNA and protein levels of ULK1 and phosphorylation. ULK1 causes the autophagy process to begin. The inhibitor of ULK1 is mTOR. However, this process is regulated by the adenosine monophosphate-activated protein kinase (AMPK). Autophagy is in relationship with LC3, whose subgroup is named LC3-II, which boosts HPAC. The autophagy substrate is P62, increasing in HPAC. Both LC3-II and P62 levels are inverse associated with miR-372. Si-ULK1, which is an inhibitor of the autophagy process, inhibits ULK1 and LC3II but causes an increase of P62 and LC3-I. All the impacts of miR-372 on HPAC are reversed through the inhibition of ULK1 with chloroquine treatment. The decline in LC3-II is through the knockdown of ULK1, but LC3-II increases by exogenous ULK1 and chloroquine. The level of p62 increases by the knockdown of ULK1. Nevertheless, the extent of P62 is lowered by exogenous ULK1. These processes highlight that miR-372 affects HPAC survival via targeting p62 and adjusting autophagy by targeting ULK1 [99]. A plethora of research has yet to be conducted in this field. However, another study investigated by Yu et al. shed light on the fact that the level of miR-372 expression decreases in pancreatic intraepithelial neoplasia (PanIN) [100].
1.7. MiR-372 and breast cancer
Breast cancer (BC) is considered the most ubiquitous cancer diagnosed among women worldwide, except for skin cancer. Approximately 41,760 out of 268,600 women diagnosed with invasive breast cancer will be dead. In the same vein, worldwide, a large population of women, constituting roughly 13%, will be diagnosed with invasive breast cancer [[101], [102], [103]]. Many studies shed light on the fact that the mortality rate of BC, which is common among women, is exceedingly high after lung cancer in the US [101,104]. However, the rate of the BC toll remarkably mitigates in developed countries like the US because of innovative therapeutic and diagnostic methods [105]. Fundamentally, breast cancer is categorized in terms of molecular and histological subtypes. The former is contingent on two hormone receptors (estrogen and progesterone) and the expression of the human epidermal growth factor receptor 2 (HER2-positive/HER2-negative) protein. The latter is divided into invasive ductal carcinoma, invasive lobular carcinoma, and mixed ductal/lobular carcinomas. The most common breast cancer subtype is HR-positive/HER2-negative and invasive ductal carcinoma [101,106]. Over the past few years, one of the biomarkers that have stood out in breast cancer progression is essentially microRNA playing an essential role in carcinogenesis [107]. In this study, we review assorted research regarding the role of miR-372 in BC cases.
The outcome of one research conducted by Zhao et al. asserted that the expression of miR-372, whose target is E2F1, is downregulated in both BC specimens and cell lines since the responsibility of miR-372 is tumor suppressive in BC development. In a sense, this research demonstrated that E2F1 is upregulated in human breast cancer tissues compared to their close normal tissues, and also, there is a significantly negative correlation between the mRNA level of E2F1 and miR-372 levels in BC tissues. Moreover, their research asserted that miR-372 prohibits proliferation and results in apoptosis in breast cancer through targeting E2F1 [31]. In contrast with such research, another research investigated by Cheng et al. and Fan et al. provided a different perspective on miR-372. They contended that miR-372 is considerably upgraded in the BC tissues and cell lines. In doing so, the downregulation of miR-372 impedes the increase of BC cells in the body. They shed light on that miR-372 plays an oncogenic role in the BC cells [108,109].
What’s more, Cheng et al. maintained that large tumor suppressor kinase 2 (LATS2) is the target gene of miR-372 in the BC tissues. LATS2 downregulation reverses the anti-proliferation effect of AS-miR-327 in breast cancer cells [108]. Along similar lines, Fan et al. presented that the increase of miR-372-3p expression culminated in leading to shorter survival time in breast carcinoma patients, and also, although it is tied up with two leading factors called tumor size and tumor stage, it is by no means related to age, gender, and lymph node metastasis. In doing so, miR-372-3p is considered a prognostic factor for BC cases. Besides, they stated that miR-372-3p stimulates breast carcinoma cells’ epithelial-mesenchymal transition (EMT) via activating the pathway of Wnt. MiR-372-3p got attached to DKK1, which is the inhibitor of the Wnt pathway. In reality, there is a reverse relationship between mir-372 and DKK1 [109]. Nevertheless, taken together, it is still required that other prospective researchers work on the impact of miR-372 on breast cancer cases.
1.8. miR-372 and osteosarcoma
Osteosarcoma (OS) is the most prevalent malignant bone tumor that frequently occurs in children and young adults dedicated %3.4 of all childhood cancers to itself [110]. OS mainly occurs in the long bones’ metaphysis, especially the distal femur or proximal tibia around the knee [111]. Some risk factors related to OS occurrence: Age, gender, socio-economic status, height, environmental features, and genetics [112]. Genetic research is fundamental in OS prevention and treatment strategies, in light of the most cardinal cause of OS is genetic mutations [113]. Countless mutations could potentially predispose to OS [114]. It is found that miRNA dysregulation may promote or inhibit OS metastasis [115]. It has been realized that RNA dysregulation, including miRNA dysregulation, has a decisive role in OS initiation and progression [116]. Xu et al. revealed that miR-372 is presented as a tumor suppressor gene in OS. They proved that miR-372 over-expression inhibits the proliferation, migration, and invasion of OS cells in vivo and in vitro. MiR-372 did its tumor suppressor part by suppressing FXYD6 mRNA [117]. Two years later, Gao et al. unveiled that Circ_0001721, a circular RNA, was upregulated in OS more than in normal tissues. Circ_0001721 knockdown inhibited glycolysis, cell proliferation, cell migration, invasion, and epithelial-to-mesenchymal transition (EMT) and promoted apoptosis by regulating miR-372 through the MAPK7 pathway. MiR-372 appeared as a tumor suppressor through the MAPK7 axis [118]. In another study, Li et al. proved that HULC has a common suppressive effect on miR-372 expression. Also, the knockdown of miR-372 leads to the proliferation, migration, and invasion of OS cells in vitro. HULC could cause OS tumor growth and metastasis through the miR-372/HMGB1 axis. Thus, miR-372 has a suppressive role in OS by inhibiting proliferation, migration, and invasion by targeting HMGB1 [119].
1.9. miR-372 and genitourinary cancers
Genitourinary (GU) system cancers are one of the most prevalent malignancies in clinical practice, accounting for an estimated 540,970 new cancer patients and 101,050 deaths in 2021 in the United States [2,120]. Furthermore, GU malignancies are higher in men than in women, especially in bladder cancer subjects; it is as much as three times higher. GU neoplasms are still responsible for millions of fatalities annually, despite current diagnosis breakthrough procedures, notably imaging techniques and therapy opportunities [120]. In GU cancers, many investigations demonstrate that miR-372 possesses tumor-suppressive characteristics.
Bladder cancer is the second most prevalent urinary tract malignancy, assigned the highest lifetime treatment costs per patient in all cancers [121]. Bladder cancer is responsible for %3 of cancer diagnoses worldwide and is the sixth most incident neoplasm in the US. It mainly occurs in old ages, whereas %90 of new diagnoses are 55 years of age and older [122]. Some risk factors, including smoking, environmental and occupational exposures, chronic bladder irritation/infections, and congenital abnormalities, are responsible for bladder cancer [123]. Gene abnormalities ranging from single DNA mutations to complete or partial chromosomal deletions can come to cell cycle disorders. Several studies proposed that PI3K signaling pathway may relate to bladder cancer development and progression [124]. In 2020 Liu et al. concluded that CLUB4 levels over-expression is concerning bladder cancer and could cause increased motility, invasiveness, stemness, and chemoresistance in bladder cancer cells. CLUB4 plays its role by upregulating PIK3CA by repressing miR-372. It is better to mention that miR-372 as a tumor suppressor could decrease PIK3CA expression and inhibit the migration and invasion of bladder cancer cells. So, they established that CLUB4B-miR-372/373-PIK3CA/AKT axis has a crucial role in bladder cancer pathogenesis with important prognostic and therapeutic implications [125].
Cervical cancer has taken third place among the most common cancers in women worldwide, mainly occurring in low and middle-income countries with the peak age of 35–45 [126,127]. Most cervical cancer cases are infected by the human papillomavirus (HPV) [126]. Most infections are cleared by the immune system, but a few subtypes, including HPV16 and HPV18, continue to exist and express E6 and E7. These viral oncogenes (E6 and E7) could cause increased genomic instability, somatic mutation accumulation, and HPV integration into the host genome, leading to cervical cancer [128]. Typically, it takes 5–10 years between precancer diagnoses and the initial oncogenic infection. %30 of high-grade precancers will turn into invasive cancers finally [127]. In 2011, Tian et al. indicated that miR-372 has a tumor suppressor function in cervical cancer. They found that miR-372 was down-regulated in cancer cells compared to adjacent normal tissues, whereas cyclinA1 and CDK2 were up-regulated as the targets of miR-372. When miR-372 was over-expressed, expression of the EGFP receptor, which contains 3′-UTR of cyclinA1 and CDK2, was inhibited. In addition, they demonstrated that CDK2 and cyclinA1 over-expression quickened DNA replication in S-phase, entry into G2, and vice versa. So miR-372 does its anti-oncogenic role by down-regulating the cell cycle genes, CDK2, and cyclinA1, controlling cell cycle growth and its progression [129].
Endometrial cancer – the most gynecologic cancer in developed countries – is women's 4th most prevalent cancer. Unbalanced increase of estrogen via early menarche, late menopause, exposure to tamoxifen, nulliparity, and obesity as significant risk factors have a crucial role in developing endometrial cancer [130,131]. Typically post-menopausal obese women are the most common cases of endometrial cancer, but its incidence is dramatically rising among young women due to earlier-onset obesity [132]. Carcinoma is the most common form of endometrial cancer [133]. In 2015, Liu et al. found that miR-372 has a tumor suppressor role in endometrial carcinoma. In that study, miR-372 expression was lower in cancer cells than in normal cells, and miR-372 over-expression suppressed the proliferation of endometrial carcinoma cells. RhoC, Cyclin A1, and CDK2 as miR-372 targets with oncogene characteristics were down-regulated when miR-372 over-expressed. So miR-372/RhoC. miR-372/Cyclin A1 and miR-372/CDK2 pathways may collectively be a part of miR-372 anti-oncogenic properties in endometrial carcinoma [134].
Ovarian cancer (OC) is the third-ranked of the most common gynecologic cancers, has the worst prognosis and the highest mortality rates, three times more lethal than breast cancer [135]. OC is comprehended by heterogeneous malignancies with various etiology and molecular biology; its prevalence may differ between races and countries because of several factors, including genetics and economy (126). The most common form of ovarian cancer is serous carcinoma [136]. In 2017, Guan et al. stated that miR-372 over-expression has a prominent role in tumor growth inhibiting as a tumor suppressor in ovarian carcinoma. In that study, they concluded that miR-372 over-expression could result in three major outcomes: 1) suppress ovarian cancer cells proliferation and induce cell cycle arrest, 2) ATAD2, LATS2, P62, DKK1, and Cyclin A1 down-regulation as responsible for cell proliferation and 3) cell apoptosis promotion. Thus miR-372 could be a critical target in future ovarian carcinoma treatments [137].
Renal cell carcinoma (RCC) is the most common malignancy originating from the kidney. Typically, RCC is asymptomatic, leading to late diagnosis, making RCC the most lethal genitourinary cancer [138]. The 5-year survival is below %10, and more than %20 of patients who went through nephrectomy will experience metastasis during follow-up, making RCC a poor prognosis [139]. The most common type of RCC is the clear cell type accounting for %80 of all RCC cases [140]. MiRNAs, as the most well-known non-coding RNAs, are extensively investigated for their critical role in cancer to make it a potential target for advancing the diagnosis, prognosis, and therapeutic implications [141]. In 2015, Huang et al. concluded that miR-372 has an anti-oncogenic role in RCC. miR-372 over-expression could inhibit cell proliferation and invasion in vitro. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), a target of miR-372, was responsible for cell proliferation and invasion. miR-372 over-expression could lead to IGF2BP1 down-regulation to do its anti-oncogenic part in RCC [142]. In another study in 2018, Ji et al. revealed another pathway of miR-372. They presented that miR-372 expression was down-regulated in RCC tissues, and it is significantly associated with the poor survival rate and adverse clinical characteristics in RCC patients. miR-372 suppressed EMT and metastasis by ATAD2 targeting, suggesting that miR-372/ATAD2 axis have an anti-oncogenic role in RCC and may be a potential therapeutic biomarker for RCC treatment [143].
Prostate cancer (PC) ranks first among the most cancers diagnosed in men worldwide [144]. Age and family history are the most critical risk factors for PC [145]. Prostate malignant neoplasms mostly originated and differentiated from epithelial tissue and are carcinomas [146]. Multifocal nature of the disease has made the diagnosis challenging [147]. Multiple studies have defined a genetic route in prostate cancer, including several genetic and epigenetic pathways [148]. In 2016, Kong et al. indicated that miR-372 has tumor suppressor function by regulating P65. They resulted that miR-372 could decrease migration and invasion in prostate cancer cells by negative regulation of P65. Thus miR-372 as an anti-oncogenic could be set for the prevention, diagnosis, and treatment of prostate cancer patients [149].
Conversely in another study, Cao et al. conclude that over-expression of miR-372 boosts tumor cell proliferation and migration via targeting LATS2. They indicated that LATS2 works as a tumor suppressor gene. They indicated that repressing the over-expression of miR-372 by arsenic sulfide (As4S4) could prevent cancer cell proliferation and migration [150].
Testicular germ cell tumors (TGCTs) are the most common malignancy in young men, extensively classified into seminomatous and non-seminomatous groups [151]. Generally, to speak freestanding, Testicular tumors follow %90 rules; more than %90 are malignant, more than %90 are of germ cells origin (TGCTs), and more than %90 are sporadic and diagnosed in 20–45 years of age [152]. TGCTs have an outstanding overall cure rate and prognosis [153]. Cryptorchidism, prior GCT, subfertility, sexual differentiation disorder, and positive family history are related risk factors for TGCT. TGCT is likely driven by polygenic variation. Serum miRNA is starting to appear as a diagnostic and therapeutic biomarker in TGCT patients [151]. Despite most of the studies about genitourinary tumors mentioned above, miR-372 has an oncogenic role in testicular germ cell tumors. In 2006, Voorhoeve et al. identified miR-372 in an oncogenic way, letting the primary human cells' proliferation and tumorigenesis. miR-372 could conceal RAS and active wild-type P53 by neutralizing P53-mediated CDK inhibition via targeting the expression of the tumor suppressor LATS2. So, numbing the P53 pathway allows tumorigenicity which could introduce miR-372 as an oncogene in TGCT [154]. Syring et al. stated that miRNA detection was precise for testicular cancer. The mean serum miR-372 was significantly increased in TGCT patients. miR-372 levels had a %74 diagnostic sensitivity with a %100 specificity, while tumor markers including Alpha Fito Protein, Human Chorionic Gonadotropin and Lactate Dehydrogenase had a combined sensitivity of %60 for testicular cancers [155,156].
2. Conclusions and perspectives
The frequency of many cancers in humans has increased over the past few decades, despite the fact that there has been a general improvement in lifestyle, consciousness, and the living environment of humans. However, despite the fact that many researchers are always striving to understand the detailed processes of carcinogenesis and that each year new generations of anticancer medications are launched, the survival rate of cancer patients has remained steady, and only a little amount of improvement has been accomplished.
Studies conducted in recent years have revealed that restoring normal levels of improperly produced miRNA can influence the growth of malignant tumors, and as a result, can function as an effective molecular targeted medicine for the treatment of cancers [157,158].
Naked miRNAs have a short half-life in the blood due to the breakdown of nucleases that occur naturally in the body. This is despite the fact that it has been demonstrated that miRNAs are promising and effective therapeutic medicines in vitro. Therefore, the limited bio-availability of nucleic acid therapeutics in vivo presents a significant obstacle for the development of miRNAs as potential anti-cancer medications. It is impossible to ignore the fact that numerous systems or carriers for miRNA have been widely advocated. These systems or carriers include nanoparticles, modifications to ncRNA, and oncolytic adenovirus methods [159]. Carriers for microRNA that are based on nanoparticles are the most common approach [160]. For instance, miR-21 has been found to play a role in the progression of multiple cancers as a tumor promoter [161]. Several researchers attempted to transport miR-21 antisense oligonucleotides in order to exhibit an anti-cancer function. Satisfying effects were reached in cases of pancreatic cancer, breast cancer, glioblastoma, and other cancers [158,162,163]. In light of the intriguing function that miR-372 plays in suppressing the growth of tumors, we think that in the future, therapeutic nucleic acid medications based on miR-372 could be very effective.
It has been hypothesized that the miR-372 has significant tumor-suppressing effects in a variety of human malignancies. Notably, a number of studies suggested that the ceRNA mechanism would play an essential role in miR-372-mediated tumor suppression. Based on our analysis of the lncRNA/CircRNA-miR-372 networks, we come to the conclusion presented in Table 2. The ceRNA mechanism primarily incorporates three different types of RNAs, including mRNA, pseudogene transcripts, and lncRNA; however, circRNA has recently followed in the footsteps of lncRNA to become a novel hotspot in the field of ceRNA family. The next step in elucidating the role of miR-372 in human cancers will involve determining whether or not circRNAs are collaborators who take part in the miR-372-mediated regulation of tumor growth.
In conclusion, the research to far show that miR-372 is both up- and down-regulated in various malignancies. Additionally, it has a dual function in various types of cancer, acting both as an oncogene and a tumor suppressor in different organs under different circumstances. Future studies should delve deeper into this enormous controversy. Particularly, the focus of current research is mostly on clinical tissue samples and cell culture. However, more studies with a clinical focus and animal models are needed to clarify miR-372's precise function in various malignancies. Also, the involvement of miR-372 has not yet been explored in some of the tumors, particularly those coming from the hematopoietic and lymphoid tissues, thus it is strongly advised to investigate the functional role of miR-372 in tumor of hematopoietic and lymphoid tissues.
It is a widely held belief that the treatment of human tumors typically involves a synergistic approach that includes elements of surgery, radiotherapy, and chemotherapy [164]. Because of this, doing in-depth research into the roles that miR-372 plays in chemo-sensitization and radio-sensitization are of the utmost relevance.
2.1. Limitations of the study
The most important limitation of research related to miR-372 is the lack of clinical trial studies, so it seems that designing and conducting clinical trial studies is necessary to confirm the in vitro and in vivo research done. Also, the authors should consider genomic-directed stratifications during the design and enrollment of clinical trials.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Forogh Mohammadi, Email: F_mohammadi@iauksh.ac.ir, forogh_mo58@yahoo.com.
Maryam Karimi-Dehkordi, Email: ma_karimivet58@yahoo.com.
Shahin Alizadeh-Fanalou, Email: alizadeh.sh@umsu.ac.ir, shahin.alizadeh60@yahoo.com.
References
- 1.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., et al. Cancer statistics, 2021. CA A Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 4.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Sahebi-Fakhrabad A., Sadeghi A.H., Handfield R. Evaluating state-level prescription drug monitoring program (PDMP) and pill mill effects on opioid consumption in pharmaceutical supply chain. Healthcare. 2023;11 doi: 10.3390/healthcare11030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahabady M.K., et al. Noncoding RNAs and their therapeutics in paclitaxel chemotherapy: mechanisms of initiation, progression, and drug sensitivity. J. Cell. Physiol. 2022;237(5):2309–2344. doi: 10.1002/jcp.30751. [DOI] [PubMed] [Google Scholar]
- 7.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 9.Vasudevan S., Tong Y., Steitz J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 10.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 11.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 12.Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Sall A., Yang D. MicroRNA: an emerging therapeutic target and intervention tool. Int. J. Mol. Sci. 2008;9(6):978–999. doi: 10.3390/ijms9060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y.S., Dutta A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alizadeh-Fanalou S., et al. MiR-613 promotes cell death in breast cancer cells by downregulation of nicotinamide phosphoribosyltransferase and reduction of NAD. DNA Cell Biol. 2021;40(7):1026–1036. doi: 10.1089/dna.2021.0330. [DOI] [PubMed] [Google Scholar]
- 16.Behl T., et al. Intercalating the role of MicroRNAs in cancer: as enemy or protector. Asian Pac. J. Cancer Prev. APJCP: Asian Pac. J. Cancer Prev. APJCP. 2020;21(3):593–598. doi: 10.31557/APJCP.2020.21.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizadeh-Fanalou S., et al. Dysregulation of microRNAs regulating survivin in CD4+ T cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102303. [DOI] [PubMed] [Google Scholar]
- 18.Ghaffari T., et al. Captopril and spironolactone can attenuate diabetic nephropathy in wistar rats by targeting ABCA1 and microRNA-33. Curr. Pharmaceut. Des. 2022;28(16):1367–1372. doi: 10.2174/1381612828666220401143249. [DOI] [PubMed] [Google Scholar]
- 19.Tavakoli S., et al. Transcriptional regulation of T-bet, GATA3, ROR<gamma>T, HERV-K env, Syncytin-1, microRNA-9, 192 and 205 induced by nisin in colorectal cancer cell lines (SW480, HCT116) and human peripheral blood mononuclear cell. Gene Reports. 2021;23 [Google Scholar]
- 20.Jorge A.L., et al. MicroRNAs: understanding their role in gene expression and cancer. Einstein (Sao Paulo) 2021;19:eRB5996. doi: 10.31744/einstein_journal/2021RB5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B., et al. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Dalmay T., Edwards D.R. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25(46):6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 23.Gheytanchi E., et al. Circulating exosomal microRNAs as potential prognostic biomarkers in gastrointestinal cancers: a systematic review and meta-analysis. Cancer Cell Int. 2023;23(1):10. doi: 10.1186/s12935-023-02851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alizadeh-Fanalou S., et al. Dual role of microRNA-1297 in the suppression and progression of human malignancies. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111863. [DOI] [PubMed] [Google Scholar]
- 25.Pfister S., et al. Novel genomic amplification targeting the microRNA cluster at 19q13.42 in a pediatric embryonal tumor with abundant neuropil and true rosettes. Acta Neuropathol. 2009;117(4):457–464. doi: 10.1007/s00401-008-0467-y. [DOI] [PubMed] [Google Scholar]
- 26.Voorhoeve P.M., et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124(6):1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Yasukawa K., et al. The microRNAs miR-302b and miR-372 regulate mitochondrial metabolism via the SLC25A12 transporter, which controls MAVS-mediated antiviral innate immunity. J. Biol. Chem. 2020;295(2):444–457. doi: 10.1074/jbc.RA119.010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan Y., et al. Increased levels of miR-372 correlate with disease progression in patients with hyperlipidemic acute pancreatitis. Exp. Ther. Med. 2020;19(6):3845–3850. doi: 10.3892/etm.2020.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran N.D., et al. A miR-372/let-7 Axis regulates human germ versus somatic cell fates. Stem Cell. 2016;34(7):1985–1991. doi: 10.1002/stem.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanyam D., et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011;29(5):443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y.X., et al. microRNA-372 inhibits proliferation and induces apoptosis in human breast cancer cells by directly targeting E2F1. Mol. Med. Rep. 2017;16(6):8069–8075. doi: 10.3892/mmr.2017.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita S., et al. MicroRNA-372 is associated with poor prognosis in colorectal cancer. Oncology. 2012;82(4):205–212. doi: 10.1159/000336809. [DOI] [PubMed] [Google Scholar]
- 33.Gu H., et al. Upregulation of microRNA-372 associates with tumor progression and prognosis in hepatocellular carcinoma. Mol. Cell. Biochem. 2013;375(1–2):23–30. doi: 10.1007/s11010-012-1521-6. [DOI] [PubMed] [Google Scholar]
- 34.Chen X., et al. miR-372 regulates glioma cell proliferation and invasion by directly targeting PHLPP2. J. Cell. Biochem. 2015;116(2):225–232. doi: 10.1002/jcb.24949. [DOI] [PubMed] [Google Scholar]
- 35.Xia L., et al. FER1L4/miR-372/E2F1 works as a ceRNA system to regulate the proliferation and cell cycle of glioma cells. J. Cell Mol. Med. 2019;23(5):3224–3233. doi: 10.1111/jcmm.14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moradi N., et al. Lower expression of miR-10a in coronary artery disease and its association with pro/anti-inflammatory cytokines. Clin. Lab. 2018;64(5):847–854. doi: 10.7754/Clin.Lab.2018.171222. [DOI] [PubMed] [Google Scholar]
- 37.Yeh L.Y., et al. miR-372 inhibits p62 in head and neck squamous cell carcinoma in vitro and in vivo. Oncotarget. 2015;6(8):6062–6075. doi: 10.18632/oncotarget.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debakey M. Carcinoma of the lung and tobacco smoking: a historical perspective. Ochsner J. 1999;1(3):106–108. [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold M., et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra J., et al. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016;48(3):889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 41.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 42.Dela Cruz C.S., Tanoue L.T., Matthay R.A. Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med. 2011;32(4):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Groot P.M., et al. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018;7(3):220–233. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai J.H., et al. Comparative proteomic profiling of human lung adenocarcinoma cells (CL 1-0) expressing miR-372. Electrophoresis. 2012;33(4):675–688. doi: 10.1002/elps.201100329. [DOI] [PubMed] [Google Scholar]
- 45.Mallick R., et al. MicroRNA miR-372 enhances the malignant potential of lung cancer cells. J. Am. Coll. Surg. 2015;221(4):S151. [Google Scholar]
- 46.Wang Q., et al. MiR-372-3p promotes cell growth and metastasis by targeting FGF9 in lung squamous cell carcinoma. Cancer Med. 2017;6(6):1323–1330. doi: 10.1002/cam4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun H., Gao D. Propofol suppresses growth, migration and invasion of A549 cells by down-regulation of miR-372. BMC Cancer. 2018;18(1):1252. doi: 10.1186/s12885-018-5175-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Moradi N., et al. Emerging role of miR-372 and miR-101a in head and neck squamous cell carcinoma. Clin. Lab. 2020;66(4) doi: 10.7754/Clin.Lab.2019.190734. [DOI] [PubMed] [Google Scholar]
- 49.Wu L., Li C., Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp. Ther. Med. 2018;15(4):3687–3692. doi: 10.3892/etm.2018.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y.P., et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 51.Ma B.B.Y., Hui E.P., Chan A.T.C. Investigational drugs for nasopharyngeal carcinoma. Expet Opin. Invest. Drugs. 2017;26(6):677–685. doi: 10.1080/13543784.2017.1324568. [DOI] [PubMed] [Google Scholar]
- 52.Tang L.Q., et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2016;108(1) doi: 10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 53.Chua D.T., et al. Treatment outcome after radiotherapy alone for patients with Stage I-II nasopharyngeal carcinoma. Cancer. 2003;98(1):74–80. doi: 10.1002/cncr.11485. [DOI] [PubMed] [Google Scholar]
- 54.Zhao L., et al. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis. 2012;33(11):2220–2227. doi: 10.1093/carcin/bgs235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan J.K., Tan E.L., Gan S.Y. Elucidating the roles of miR-372 in cell proliferation and apoptosis of nasopharyngeal carcinoma TW01 cells. Exp. Oncol. 2014;36(3):170–173. [PubMed] [Google Scholar]
- 56.Wang Z., et al. MicroRNA-372 enhances radiosensitivity while inhibiting cell invasion and metastasis in nasopharyngeal carcinoma through activating the PBK-dependent p53 signaling pathway. Cancer Med. 2019;8(2):712–728. doi: 10.1002/cam4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGlynn K.A., London W.T. Epidemiology and natural history of hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2005;19(1):3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Goodarzi E., et al. Global incidence and mortality of liver cancers and its relationship with the human development index (HDI): an ecology study in 2018. World Cancer Res J. 2019;6:12. [Google Scholar]
- 59.Wu G., et al. Low mir-372 expression correlates with poor prognosis and tumor metastasis in hepatocellular carcinoma. BMC Cancer. 2015;15:182. doi: 10.1186/s12885-015-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soliman M.H., et al. MicroRNA-372-3p predicts response of TACE patients treated with doxorubicin and enhances chemosensitivity in hepatocellular carcinoma. Anti Cancer Agents Med. Chem. 2021;21(2):246–253. doi: 10.2174/1871520620666200516145830. [DOI] [PubMed] [Google Scholar]
- 61.Fan J., et al. lncRNA OSER1-AS1 acts as a ceRNA to promote tumorigenesis in hepatocellular carcinoma by regulating miR-372-3p/Rab23 axis. Biochem. Biophys. Res. Commun. 2020;521(1):196–203. doi: 10.1016/j.bbrc.2019.10.105. [DOI] [PubMed] [Google Scholar]
- 62.Shaker O., et al. Long non-coding HULC and miRNA-372 as diagnostic biomarkers in hepatocellular carcinoma. Rep. Biochem. Mol. Biol. 2020;9(2):230–240. doi: 10.29252/rbmb.9.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z., et al. Circular RNA MYLK promotes hepatocellular carcinoma progression by increasing Rab23 expression by sponging miR-362-3p. Cancer Cell Int. 2019;19(1):1–11. doi: 10.1186/s12935-019-0926-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Jiao Z.H., Wang J.D., Wang X.J. MicroRNA-16 suppressed the invasion and migration of osteosarcoma by directly inhibiting RAB23. Eur. Rev. Med. Pharmacol. Sci. 2018;22(9):2598–2605. doi: 10.26355/eurrev_201805_14953. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38(16):5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., et al. Long noncoding RNA PCAT-14 induces proliferation and invasion by hepatocellular carcinoma cells by inducing methylation of miR-372. Oncotarget. 2017;8(21):34429–34441. doi: 10.18632/oncotarget.16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu G., et al. miR-372 down-regulates the oncogene ATAD2 to influence hepatocellular carcinoma proliferation and metastasis. BMC Cancer. 2014;14:107. doi: 10.1186/1471-2407-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., et al. Effects of FER1L4-miR-106a-5p/miR-372-5p-E2F1 regulatory axis on drug resistance in liver cancer chemotherapy. Mol. Ther. Nucleic Acids. 2021;24:449–461. doi: 10.1016/j.omtn.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sethi N., et al. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur. J. Cancer. 2014;50(15):2619–2635. doi: 10.1016/j.ejca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Yeh L.Y., et al. miR-372 enhances tumorigenesis and drug resistance in oral carcinoma by targeting ZBTB7A transcription factor. Cancer Res. 2018;78(13) [Google Scholar]
- 71.Yeh L.Y., et al. The miR-372-ZBTB7A oncogenic Axis suppresses TRAIL-R2 associated drug sensitivity in oral carcinoma. Front. Oncol. 2020;10:47. doi: 10.3389/fonc.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeh L.Y., et al. Hypoxia-induced miR-372 targets p62 to affect the progression of oral carcinoma. Cancer Res. 2014;74(19) [Google Scholar]
- 73.Tu H.F., et al. Upregulation of miR-372 and -373 associates with lymph node metastasis and poor prognosis of oral carcinomas. Laryngoscope. 2015;125(11):E365–E370. doi: 10.1002/lary.25464. [DOI] [PubMed] [Google Scholar]
- 74.Liu C.J., et al. Upregulation of microRNA-372 & 373 associates with cervical lymph node metastasis and poor prognosis of oral carcinomas. Cancer Res. 2014;74(19) [Google Scholar]
- 75.Wang M., et al. MEHP promotes the proliferation of oral cancer cells via down regulation of miR-27b-5p and miR-372-5p. Toxicol. Vitro. 2019;58:35–41. doi: 10.1016/j.tiv.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 76.Arnold M., et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wroblewski L.E., Peek R.M., Jr. Helicobacter pylori, cancer, and the gastric microbiota. Adv. Exp. Med. Biol. 2016;908:393–408. doi: 10.1007/978-3-319-41388-4_19. [DOI] [PubMed] [Google Scholar]
- 78.Lyons K., et al. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur. J. Cancer Prev. 2019;28(5):397–412. doi: 10.1097/CEJ.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 79.Volinia S., et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U. S. A. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Necula L., et al. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019;25(17):2029–2044. doi: 10.3748/wjg.v25.i17.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho W.J., et al. miR-372 regulates cell cycle and apoptosis of ags human gastric cancer cell line through direct regulation of LATS2. Mol. Cell. 2009;28(6):521–527. doi: 10.1007/s10059-009-0158-0. [DOI] [PubMed] [Google Scholar]
- 82.Ghasemi S., Mozdarani H. The Effect of miR-372 on genome instability in MKN-45 cell line. J. Isfahan Med. School. 2015;32(311) M.J.J.o.I.M.S. Soleimani. [Google Scholar]
- 83.Zhou C., et al. microRNA-372 maintains oncogene characteristics by targeting TNFAIP1 and affects NFκB signaling in human gastric carcinoma cells. Int. J. Oncol. 2013;42(2):635–642. doi: 10.3892/ijo.2012.1737. [DOI] [PubMed] [Google Scholar]
- 84.Staedel C., et al. Inhibition of gastric tumor cell growth using seed-targeting LNA as specific, long-lasting MicroRNA inhibitors. Mol. Ther. Nucleic Acids. 2015;4(7):e246. doi: 10.1038/mtna.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belair C., et al. Helicobacter pylori interferes with an embryonic stem cell micro RNA cluster to block cell cycle progression. Silence. 2011;2(1):7. doi: 10.1186/1758-907X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vo D.D., et al. Targeting the production of oncogenic microRNAs with multimodal synthetic small molecules. ACS Chem. Biol. 2014;9(3):711–721. doi: 10.1021/cb400668h. [DOI] [PubMed] [Google Scholar]
- 87.Corté H., et al. MicroRNA and colorectal cancer. Dig. Liver Dis. 2012;44(3):195–200. doi: 10.1016/j.dld.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Schetter A.J., Okayama H., Harris C.C. The role of microRNAs in colorectal cancer. Cancer J. 2012;18(3):244–252. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng H., et al. MiR-372-3p promotes tumor progression by targeting LATS2 in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2019;23(19):8332–8344. doi: 10.26355/eurrev_201910_19144. [DOI] [PubMed] [Google Scholar]
- 90.Yu J., et al. Serum miR-372 is a diagnostic and prognostic biomarker in patients with early colorectal cancer. Anti Cancer Agents Med. Chem. 2016;16(4):424–431. doi: 10.2174/1871520615666150716110406. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., et al. Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anti Cancer Drugs. 2014;25(3):346–352. doi: 10.1097/CAD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 92.Ragusa M., et al. Specific alterations of the microRNA transcriptome and global network structure in colorectal cancer after treatment with MAPK/ERK inhibitors. J. Mol. Med. (Berl.) 2012;90(12):1421–1438. doi: 10.1007/s00109-012-0918-8. [DOI] [PubMed] [Google Scholar]
- 93.Wang L.Q., et al. miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol. Oncol. 2018;12(11):1949–1964. doi: 10.1002/1878-0261.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alkhayyat M., et al. Epidemiology of gallbladder cancer in the Unites States: a population-based study. Chin. Clin. Oncol. 2021;10(3):25. doi: 10.21037/cco-20-230. [DOI] [PubMed] [Google Scholar]
- 95.Valle J.W., et al. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 96.Zhou N., et al. Decreased expression of hsa-miR-372 predicts poor prognosis in patients with gallbladder cancer by affecting chloride intracellular channel 1. Mol. Med. Rep. 2017;16(5):7848–7854. doi: 10.3892/mmr.2017.7520. [DOI] [PubMed] [Google Scholar]
- 97.Mizrahi J.D., et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 98.Huang J., et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160(3):744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Chen H., et al. Downregulation of ULK1 by microRNA-372 inhibits the survival of human pancreatic adenocarcinoma cells. Cancer Sci. 2017;108(9):1811–1819. doi: 10.1111/cas.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu J., et al. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 2012;18(4):981–992. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeSantis C.E., et al. Breast cancer statistics. CA A Cancer J. Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 102.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 103.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 104.Momenimovahed Z., Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer. 2019;11:151–164. doi: 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wöckel A., et al. Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-level, AWMF registry number 032/045OL, december 2017) - Part 1 with recommendations for the screening, diagnosis and therapy of breast cancer. Geburtshilfe Frauenheilkd. 2018;78(10):927–948. doi: 10.1055/a-0646-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 107.Yang Z., Liu Z. The emerging role of MicroRNAs in breast cancer. J. Oncol. 2020;2020:9160905. doi: 10.1155/2020/9160905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheng X., Chen J., Huang Z. miR-372 promotes breast cancer cell proliferation by directly targeting LATS2. Exp. Ther. Med. 2018;15(3):2812–2817. doi: 10.3892/etm.2018.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan X., et al. MicroRNA-372-3p promotes the epithelial-mesenchymal transition in breast carcinoma by activating the Wnt pathway. J buon. 2018;23(5):1309–1315. [PubMed] [Google Scholar]
- 110.Harrison D.J., et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018;18(1):39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 111.Eaton B.R., et al. Osteosarcoma. Pediatr. Blood Cancer. 2021;68(2) doi: 10.1002/pbc.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sadykova L.R., et al. Epidemiology and risk factors of osteosarcoma. Cancer Invest. 2020;38(5):259–269. doi: 10.1080/07357907.2020.1768401. [DOI] [PubMed] [Google Scholar]
- 113.Zhao X., et al. Osteosarcoma: a review of current and future therapeutic approaches. Biomed. Eng. Online. 2021;20(1):24. doi: 10.1186/s12938-021-00860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Czarnecka A.M., et al. Molecular biology of osteosarcoma. Cancers. 2020;12(8) doi: 10.3390/cancers12082130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheng G., et al. Osteosarcoma and metastasis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.780264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Z., et al. An update on the roles of circular RNAs in osteosarcoma. Cell Prolif. 2021;54(1) doi: 10.1111/cpr.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu S.Y., Xu P.F., Gao T.T. MiR-372-3p inhibits the growth and metastasis of osteosarcoma cells by targeting FXYD6. Eur. Rev. Med. Pharmacol. Sci. 2018;22(1):62–69. doi: 10.26355/eurrev_201801_14101. [DOI] [PubMed] [Google Scholar]
- 118.Gao Y., et al. CircRNA Circ_0001721 promotes the progression of osteosarcoma through miR-372-3p/MAPK7 Axis. Cancer Manag. Res. 2020;12:8287–8302. doi: 10.2147/CMAR.S244527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y., et al. LncRNA HULC induces the progression of osteosarcoma by regulating the miR-372-3p/HMGB1 signalling axis. Mol. Med. 2020;26(1):26. doi: 10.1186/s10020-020-00155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nazarizadeh A., et al. MicroRNA-154: a novel candidate for diagnosis and therapy of human cancers. OncoTargets Ther. 2020;13:6603–6615. doi: 10.2147/OTT.S249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fankhauser C.D., Mostafid H. Prevention of bladder cancer incidence and recurrence: nutrition and lifestyle. Curr. Opin. Urol. 2018;28(1):88–92. doi: 10.1097/MOU.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 122.Saginala K., et al. Epidemiology of bladder cancer. Med. Sci. 2020;8(1) doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong V.K., et al. Imaging and management of bladder cancer. Cancers. 2021;13(6) doi: 10.3390/cancers13061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y., et al. Frontiers in bladder cancer genomic research. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.670729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X., et al. The CUL4B-miR-372/373-PIK3CA-AKT axis regulates metastasis in bladder cancer. Oncogene. 2020;39(17):3588–3603. doi: 10.1038/s41388-020-1236-1. [DOI] [PubMed] [Google Scholar]
- 126.Bedell S.L., et al. Cervical cancer screening: past, present, and future. Sex Med. Rev. 2020;8(1):28–37. doi: 10.1016/j.sxmr.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Eun T.J., Perkins R.B. Screening for cervical cancer. Med. Clin. 2020;104(6):1063–1078. doi: 10.1016/j.mcna.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Revathidevi S., et al. APOBEC: a molecular driver in cervical cancer pathogenesis. Cancer Lett. 2021;496:104–116. doi: 10.1016/j.canlet.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian R.Q., et al. MicroRNA-372 is down-regulated and targets cyclin-dependent kinase 2 (CDK2) and Cyclin A1 in human cervical cancer, which may contribute to tumorigenesis. J. Biol. Chem. 2011;286(29):25556–25563. doi: 10.1074/jbc.M111.221564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Tucci C., et al. Immunotherapy in endometrial cancer: new scenarios on the horizon. J Gynecol Oncol. 2019;30(3):e46. doi: 10.3802/jgo.2019.30.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raglan O., et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int. J. Cancer. 2019;145(7):1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 132.Moore K., Brewer M.A. Endometrial cancer: is this a new disease? Am. Soc. Clin. Oncol. Educ. Book. 2017;37:435–442. doi: 10.1200/EDBK_175666. [DOI] [PubMed] [Google Scholar]
- 133.Urick M.E., Bell D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer. 2019;19(9):510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu B.L., et al. MicroRNA-372 inhibits endometrial carcinoma development by targeting the expression of the Ras homolog gene family member C (RhoC) Oncotarget. 2016;7(6):6649–6664. doi: 10.18632/oncotarget.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Momenimovahed Z., et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Torre L.A., et al. Ovarian cancer statistics. CA A Cancer J. Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guan X., et al. The role of miR-372 in ovarian carcinoma cell proliferation. Gene. 2017;624:14–20. doi: 10.1016/j.gene.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 138.Shingarev R., Jaimes E.A. Renal cell carcinoma: new insights and challenges for a clinician scientist. Am. J. Physiol. Ren. Physiol. 2017;313(2):F145–f154. doi: 10.1152/ajprenal.00480.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Petejova N., Martinek A. Renal cell carcinoma: review of etiology, pathophysiology and risk factors. Biomed Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 2016;160(2):183–194. doi: 10.5507/bp.2015.050. [DOI] [PubMed] [Google Scholar]
- 140.Perazella M.A., Dreicer R., Rosner M.H. Renal cell carcinoma for the nephrologist. Kidney Int. 2018;94(3):471–483. doi: 10.1016/j.kint.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 141.Ding L., et al. The emerging role of small non-coding RNA in renal cell carcinoma. Transl. Oncol. 2021;14(1) doi: 10.1016/j.tranon.2020.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang X., et al. miR-372 suppresses tumour proliferation and invasion by targeting IGF2BP1 in renal cell carcinoma. Cell Prolif. 2015;48(5):593–599. doi: 10.1111/cpr.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ji S., et al. MicroRNA-372 functions as a tumor suppressor in cell invasion, migration and epithelial-mesenchymal transition by targeting ATAD2 in renal cell carcinoma. Oncol. Lett. 2019;17(2):2400–2408. doi: 10.3892/ol.2018.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pernar C.H., et al. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med. 2018;8(12) doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Merriel S.W.D., Funston G., Hamilton W. Prostate cancer in primary care. Adv. Ther. 2018;35(9):1285–1294. doi: 10.1007/s12325-018-0766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Humphrey P.A. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7(10) doi: 10.1101/cshperspect.a030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haffner M.C., et al. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021;18(2):79–92. doi: 10.1038/s41585-020-00400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang G., et al. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17–18):1105–1140. doi: 10.1101/gad.315739.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong X., et al. microRNA-372 suppresses migration and invasion by targeting p65 in human prostate cancer cells. DNA Cell Biol. 2016;35(12):828–835. doi: 10.1089/dna.2015.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao H., Feng Y., Chen L. Repression of MicroRNA-372 by arsenic sulphide inhibits prostate cancer cell proliferation and migration through regulation of large tumour suppressor kinase 2. Basic Clin. Pharmacol. Toxicol. 2017;120(3):256–263. doi: 10.1111/bcpt.12687. [DOI] [PubMed] [Google Scholar]
- 151.Singla N., et al. Genetics of testicular germ cell tumors. Curr. Opin. Urol. 2019;29(4):344–349. doi: 10.1097/MOU.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Damjanov I. Testicular germ cell tumors: serological and immunohistochemical diagnosis. Acta Med. Acad. 2021;50(1):58–70. doi: 10.5644/ama2006-124.326. [DOI] [PubMed] [Google Scholar]
- 153.Mele T., Reid A., Huddart R. Recent advances in testicular germ cell tumours. Fac. Rev. 2021;10:67. doi: 10.12703/r/10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Voorhoeve P.M., et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv. Exp. Med. Biol. 2007;604:17–46. doi: 10.1007/978-0-387-69116-9_2. [DOI] [PubMed] [Google Scholar]
- 155.Abstracts of the 65th congress of the German society for urology. September 25-28, 2013. Dresden, Germany] Urologe. 2013;52(Suppl 1):10–151. doi: 10.1007/s00120-013-3273-7. [DOI] [PubMed] [Google Scholar]
- 156.Syring I., et al. Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer. J. Urol. 2015;193(1):331–337. doi: 10.1016/j.juro.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Iman M., et al. HOXB7 and hsa-miR-222 as the potential therapeutic candidates for metastatic colorectal cancer. Recent Pat. Anti-Cancer Drug Discov. 2016;11(4):434–443. doi: 10.2174/1574892811999160628114857. [DOI] [PubMed] [Google Scholar]
- 158.Shu D., et al. Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano. 2015;9(10):9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W.T., et al. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019;12(1):55. doi: 10.1186/s13045-019-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ganju A., et al. miRNA nanotherapeutics for cancer. Drug Discov. Today. 2017;22(2):424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Javanmardi S., et al. miR-21, an oncogenic target miRNA for cancer therapy: molecular mechanisms and recent advancements in chemo and radio-resistance. Curr. Gene Ther. 2017;16(6):375–389. doi: 10.2174/1566523217666170102105119. [DOI] [PubMed] [Google Scholar]
- 162.Lee T.J., et al. RNA nanoparticle-based targeted therapy for glioblastoma through inhibition of oncogenic miR-21. Mol. Ther. 2017;25(7):1544–1555. doi: 10.1016/j.ymthe.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y., et al. Co‐delivery of micro RNA‐21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017;108(7):1493–1503. doi: 10.1111/cas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jabbari N., et al. Assessment of synergistic effect of combining hyperthermia with irradiation and calcium carbonate nanoparticles on proliferation of human breast adenocarcinoma cell line (MCF-7 cells) Artif. Cells, Nanomed. Biotechnol. 2018;46(sup2):364–372. doi: 10.1080/21691401.2018.1457537. [DOI] [PubMed] [Google Scholar]
- 165.Lai J.H., et al. Comparative proteomic profiling of human lung adenocarcinoma cells (CL 1–0) expressing miR‐372. Electrophoresis. 2012;33(4):675–688. doi: 10.1002/elps.201100329. [DOI] [PubMed] [Google Scholar]
- 166.Sun H., Gao D. Propofol suppresses growth, migration and invasion of A549 cells by down-regulation of miR-372. BMC Cancer. 2018;18(1):1–11. doi: 10.1186/s12885-018-5175-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Mallick R., et al. MicroRNA miR-372 enhances the malignant potential of lung cancer cells. J. Am. Coll. Surg. 2015;221:S151. [Google Scholar]
- 168.Wang Q., et al. MiR‐372‐3p promotes cell growth and metastasis by targeting FGF 9 in lung squamous cell carcinoma. Cancer Med. 2017;6(6):1323–1330. doi: 10.1002/cam4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Z., et al. MicroRNA‐372 enhances radiosensitivity while inhibiting cell invasion and metastasis in nasopharyngeal carcinoma through activating the PBK‐dependent p53 signaling pathway. Cancer Med. 2019;8(2):712–728. doi: 10.1002/cam4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tan J., Tan E., Gan S. Elucidating the roles of miR-372 in cell proliferation and apoptosis of nasopharyngeal carcinoma TW01 cells. Exp. Oncol. 2014;36(3):170–173. [PubMed] [Google Scholar]
- 171.Wang J., et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38(16):5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu G., et al. miR-372 down-regulates the oncogene ATAD2 to influence hepatocellular carcinoma proliferation and metastasis. BMC Cancer. 2014;14(1):1–11. doi: 10.1186/1471-2407-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soliman M.H., et al. MiR-372-3p predicts response of TACE patients treated with doxorubicin and enhances chemosensitivity in hepatocellular carcinoma. Anti Cancer Agents Med. Chem. 2021;21(2):246–253. doi: 10.2174/1871520620666200516145830. [DOI] [PubMed] [Google Scholar]
- 174.Wang X., et al. Effects of FER1L4-miR-106a-5p/miR-372-5p-E2F1 regulatory axis on drug resistance in liver cancer chemotherapy. Mol. Ther. Nucleic Acids. 2021;24:449–461. doi: 10.1016/j.omtn.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.He H., et al. Upregulation of KCNQ1OT1 promotes resistance to stereotactic body radiotherapy in lung adenocarcinoma by inducing ATG5/ATG12-mediated autophagy via miR-372-3p. Cell Death Dis. 2020;11(10):1–14. doi: 10.1038/s41419-020-03083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan J., et al. lncRNA OSER1-AS1 acts as a ceRNA to promote tumorigenesis in hepatocellular carcinoma by regulating miR-372-3p/Rab23 axis. Biochem. Biophys. Res. Commun. 2020;521(1):196–203. doi: 10.1016/j.bbrc.2019.10.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.