Abstract
Cocaine self-administration can disrupt the capacity of humans and rodents to flexibly modify familiar behavioral routines, even when they become maladaptive or unbeneficial. However, mechanistic factors, particularly those driving long-term behavioral changes, are still being determined. Here, we capitalized on individual differences in oral cocaine self-administration patterns in adolescent mice and revealed that the post-synaptic protein PSD-95 was reduced in the orbitofrontal cortex (OFC) of escalating, but not stable, responders, which corresponded with later deficits in flexible decision-making behavior. Meanwhile, NMDA receptor GluN2B subunit content was lower in the OFC of mice that were resilient to escalatory oral cocaine seeking. This discovery led us to next co-administer the GluN2B-selective antagonist ifenprodil with cocaine, blocking the later emergence of cocaine-induced decision-making abnormalities. GluN2B inhibition also prevented cocaine-induced dysregulation of neuronal structure and function in the OFC, preserving mature, mushroom-shaped dendritic spine densities on deep-layer pyramidal neurons, which were otherwise lower with cocaine, and safeguarding functional BLA→OFC connections necessary for action flexibility. We posit that cocaine potentiates GluN2B-dependent signaling, which triggers a series of durable adaptations that result in the dysregulation of post-synaptic neuronal structure in the OFC and disruption of BLA→OFC connections, ultimately weakening the capacity for flexible choice. And thus, inhibiting GluN2B-NMDARs promotes resilience to long-term cocaine-related sequelae.
Subject terms: Operant learning, Reward
Introduction
Behavioral flexibility, meaning, the capacity to deviate from routine behavioral sequences, is necessary for navigating dynamic environments. It is important for planning actions and making choices both large and small, and deficits in flexible action are thought to be core features of multiple neuropsychiatric diseases, including cocaine use disorder (CUD) [1].
The orbitofrontal cortex (OFC) performs myriad functions in support of flexible behavior [2–5]. A prevailing model posits that the OFC encodes a cognitive map of task space [6, 7], a schematic representation of task-relevant variables and associations within a particular environment [8]. Cognitive maps allow for the efficient integration of new learning into existing knowledge, enabling adaptive modification of action strategies when familiar expectancies are violated [9]. These OFC-dependent task space representations are destabilized by cocaine, which may be a mechanism by which cocaine results in rigid behavioral patterns that are resistant to change [10]. Neurobiological mechanisms remain unclear.
While there is debate regarding the precise nature of a potential etiological role for aberrant decision-making processes in drug misuse [1, 11, 12], it is clear that impairments in behavioral flexibility represent a durable consequence of cocaine in both humans and model organisms [13, 14], persisting despite abstinence [14, 15], and often in parallel with cocaine craving [16, 17]. Progressive alterations in decision-making processes may be particularly insidious when coinciding with sensitive developmental periods [14, 18, 19]. For instance, adolescent-onset, versus adult-onset, cocaine use decreases the likelihood that individuals will seek treatment across the lifespan [20, 21].
Here we aimed to understand how cocaine, particularly during adolescence, affects OFC-dependent action flexibility. Cognizant that the transition from recreational cocaine use to CUD is highly heterogeneous between individuals [21], and drug self-administration patterns can differ greatly between mice [22], we hypothesized that individual differences in cocaine-seeking behavior may help to reveal neurobiological factors by which cocaine disrupts action flexibility, or that confer resilience to the drug. We found that adolescent mice apparently resilient to cocaine had low GluN2B content in the OFC. This observation led to the discovery that GluN2B blockade obstructs the capacity of cocaine to degrade OFC neural structure and decision-making capacity, including its capacity for functional interaction with the basolateral amygdala (BLA), later in life. Thus, GluN2B levels or activity in adolescence have long-term ramifications for the structure and function of mature excitatory OFC neurons.
Materials and Methods
Animals
Experiments used C57BL/6J mice (Jackson Laboratory, #000664), except for dendritic spine imaging experiments, which used B6.Cg-Tg(Thy1-YFP)HJrs/J mice (#003782). Male mice were used due to previously reported sex differences in the long-term effects of oral cocaine self-administration on the behaviors examined here [22]. Mice were provided food and water ad libitum, except during food-reinforced testing, when they were food-restricted until body weights were ~93% of baseline. Procedures were performed in accordance with NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Emory University IACUC.
Oral cocaine self-administration
Operant conditioning chambers were equipped with two nose-poke apertures, a retractable lever, and a separate magazine for liquid dipper access or food pellet delivery (Med Associates). Mice were trained using an instrumental response sequence whereby two nose-poke apertures (active vs. inactive) were initially available. A nose-poke response in the active aperture extended the lever, pressing on which provided brief (10 s) access to 100 µL of a 10% w/v sucrose solution with or without 75 µg/mL cocaine (Sigma-Aldrich) (Fig. 1a) [22]. Mice underwent 16 daily self-administration sessions starting at postnatal day (P) 31. Sessions lasted 60 min or until 30 dipper access bouts were earned.
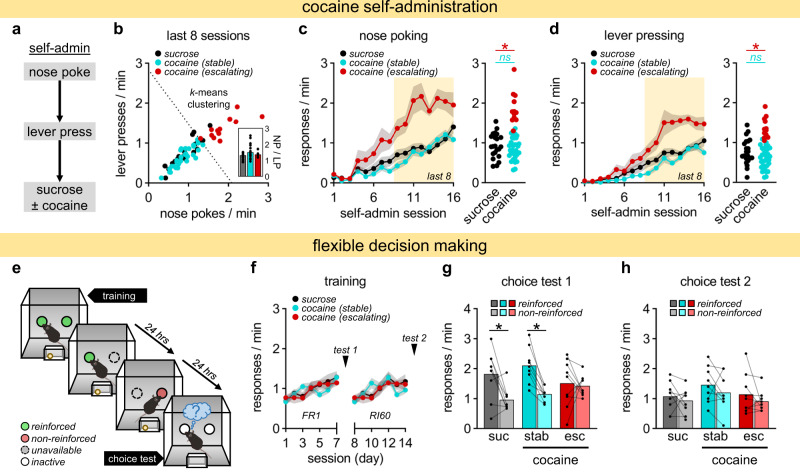
Fig. 1. Cocaine persistently disrupts flexible decision-making behavior.
a Response sequencing during oral cocaine self-administration sessions, conducted in adolescence. b Average nose poke (NP) and lever press (LP) responses across last 8 sessions. Stable and escalatory response groups defined by k-means clustering. Inset. Ratio of NP to LP responses. c, d Left panels. NP and LP responses per session. Right panels. Average NP and LP responses across last 8 sessions. e Behavioral procedure assessing flexible decision making later in life. f Responses across training. g, h Choice test responses after one action was unexpectedly not reinforced, first following FR1 training (test 1; impaired in escalating cocaine responders) and then RI training (test 2). Data presented as individual points or mean ± S.E.M. *p < 0.05 (post-hoc). See Table S2for complete statistics and group sizes.
In order to group mice according to response patterns, we performed an unbiased two-dimensional k-means clustering analysis, using average response rates across the final eight self-administration sessions, ultimately categorizing cocaine-exposed mice into stable and escalating responder groups (Fig. 1b–d; Fig. S1). Escalating responders displayed both heightened seeking and taking responses compared to “stable” or control mice (Fig. 1c, d), resulting in a ~50% increase in the estimated effective dose of cocaine for escalating vs. stable responders across all sessions (Fig. S2). Mice were then left undisturbed and tested in a food-reinforced task 3 weeks later.
Food-reinforced response training
Starting between P60-70, mice were trained to nose poke for 20 mg grain pellets (Bio-Serv) in operant conditioning chambers (Med Associates). Initial training used a fixed ratio (FR1) reinforcement schedule whereby each nose poke delivered one food pellet reinforcer. For experiments when mice underwent multiple training conditions, we next utilized 30- or 60-second random interval (RI30 or RI60, respectively) reinforcement schedules whereby each reinforcer delivery was followed by a random interval of time in which nose-poke apertures were inactive. Mice underwent daily training sessions lasting either 70 min or until mice earned 30 reinforcers from responding on each aperture (60 total).
Test for behavioral flexibility (Fig. 1e)
Following food-reinforced training, we assessed the capacity of mice to modify response strategies based on reinforcer availability. Mice underwent two, 25-minute sessions occurring on consecutive days. On the first day, one nose-poke aperture was occluded while responding on the available aperture remained reinforced. On the second day, the opposite aperture was occluded, but responding on the available aperture was no longer reinforced. Instead, pellets were delivered into the magazine at a rate equal to what each mouse earned during the previous day’s reinforced session. On a third day, mice performed a 10-minute choice test, conducted in extinction, when both apertures were again available. For experiments when mice underwent multiple-choice tests, the aperture that was to be non-reinforced was reversed so that mice could not utilize information from prior tests to inform subsequent responding.
Instrumental reversal learning
Following the test immediately above, some mice were returned to the operant conditioning chambers with access to both nose-poke apertures and a newly available lever. Both apertures were inactive, while lever-pressing was reinforced on an FR1 schedule with the same food pellet as above. Sessions lasted 25 min. For each session, a reversal rate was computed as reinforced responses (lever presses) divided by total responses (nose pokes+lever presses).
Protein quantification
Mice were anesthetized with isoflurane and decapitated. Brains were extracted, flash frozen, and sectioned into 1 mm coronal slices and dissected using a 1 mm tissue core. Tissue was homogenized. Protein concentrations were determined using a Pierce BCA Protein Assay kit (Thermo Fisher). 15 µg of total protein was separated by SDS-PAGE on 4–20% gradient Tris-glycine gels (Bio-Rad), transferred to a PVDF membrane (Bio-Rad), blocked with 5% non-fat dry milk, and incubated overnight at 4 °C in primary antibody solution (Table S1). Membranes were incubated in horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Immunoreactivity was assessed using Pierce ECL chemiluminescence substrate (Thermo Fisher) and measured using a ChemiDoc XRS + Imaging System (Bio-Rad). Densitometry values were normalized to each sample’s respective loading control (HSP-70). Protein levels were expressed as a fold change compared to control sample means from each membrane replicate.
Experimenter-administered cocaine and ifenprodil
Cocaine (10 mg/kg; Sigma-Aldrich) and ifenprodil (10 mg/kg; Tocris) in saline were administered i.p. daily from P31-35, with ifenprodil administered 30 min prior to cocaine.
Dendritic spine imaging and reconstruction
Immediately following the choice test of the behavioral flexibility assay, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and trans-cardially perfused and fixed in 4% paraformaldehyde. Brains were sectioned into 50 µm coronal sections using a freezing microtome. Basal dendritic segments located 50–150 µm from the soma on YFP-expressing neurons were imaged with a spinning disk confocal microscope (VisiTech International). Z-stack series (0.1 µm steps) were collected using a 100×1.4 NA oil-emersion objective. Three-dimensional dendritic morphology reconstructions were performed using the FilamentTracer module in Imaris (Oxford Instruments). Dendritic spines were classified using established parameters for pyramidal prefrontal cortex neurons [23]. Eight cells were analyzed/mouse and one dendrite was analyzed/cell.
Projection-selective chemogenetic manipulations
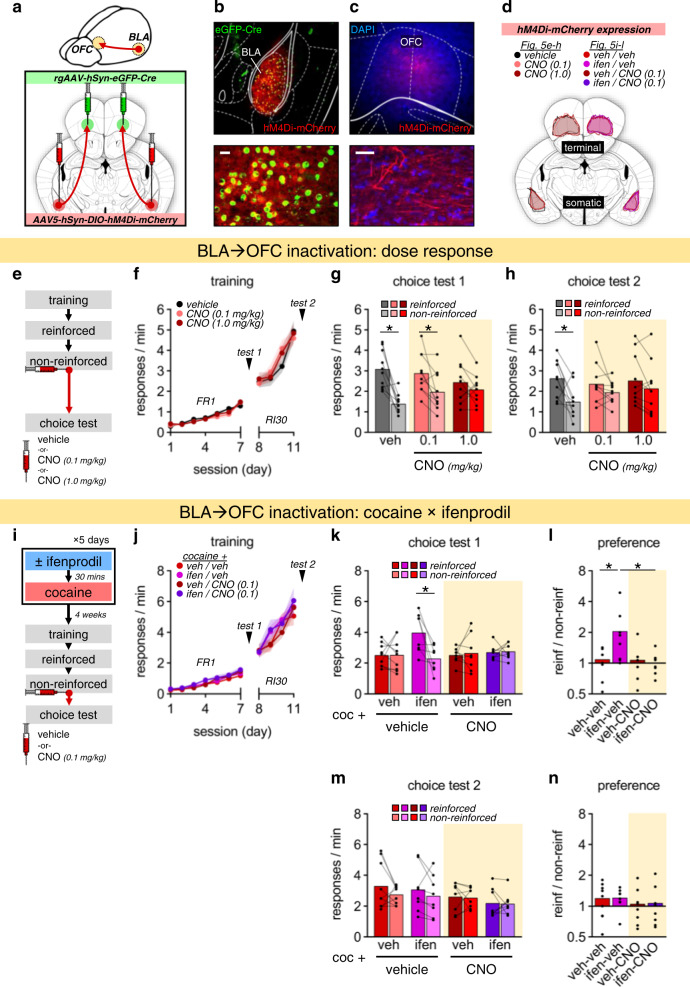
Intracranial surgeries were performed two weeks prior to behavioral testing. Mice were anesthetized with ketamine (80 mg/kg) and dexmedetomidine (0.5 mg/kg). Viral vectors were infused over 5 min using a microliter syringe (Hamilton). Infusion coordinates, relative to bregma (mm): OFC (ML:±1.40, AP:+2.50, DV: −2.80), BLA (ML:±3.00, AP: −1.50, DV: −4.90). A retrogradely transported Cre-recombinase construct (AAVrg-hSyn-HI-eGFP-Cre-WPRE-SV40; Addgene, #105540) was infused into the OFC alongside an anterogradely transported Cre-dependent, inhibitory chemogenetic receptor construct in the BLA (AAV5-hSyn-DIO-hM4D(Gi)-mCherry; Addgene, #44362). Clozapine-N-oxide (CNO; 0.1 or 1.0 mg/kg; 2% DMSO and saline; RTI International) was administered i.p. immediately following non-reinforced sessions.
Experiment overview (outlined Fig. S3)
Experiment 1 identified individual differences in oral cocaine self-administration and measured response flexibility and protein content in the OFC following drug washout (Figs. 1 and 2).
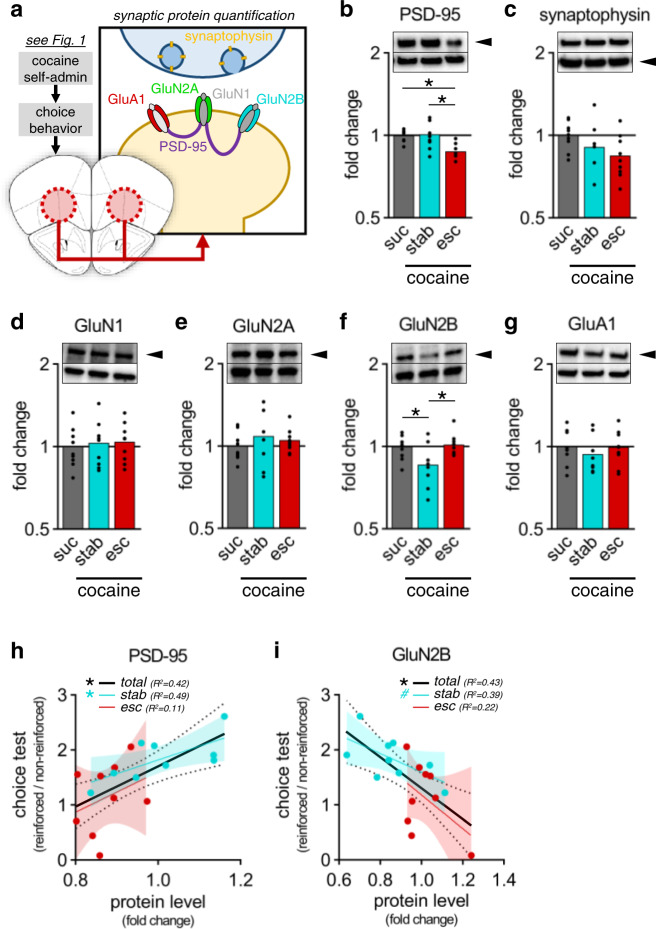
Fig. 2. Quantification of synaptic and plasticity-related protein levels in the OFC following oral cocaine self-administration.
a Synaptic localization of quantified proteins in the OFC. b, c Quantification of synaptic marker protein levels in the OFC: PSD-95 (lower among escalating responders) and synaptophysin. d–g Quantification of glutamate receptor subunit levels in the OFC: GluN1, GluN2A, GluN2B (lower among stable responders), and GluA1. Values normalized to loading controls (HSP-70) and expressed as fold change from sucrose controls. Representative blots show target protein (black arrow) and HSP-70 loading controls (no arrow). h, i Correlation between choice test preference (from Fig. 1g) and PSD-95 or GluN2B levels. 95% confidence intervals (shading). Data presented as individual points. *p < 0.05 (post-hoc), #p = 0.07. See Table S3for complete statistics and group sizes.
Experiment 2 utilized experimenter-administered cocaine and ifenprodil and measured response flexibility and dendritic spine morphology following drug washout (Figs. 3 and 4).
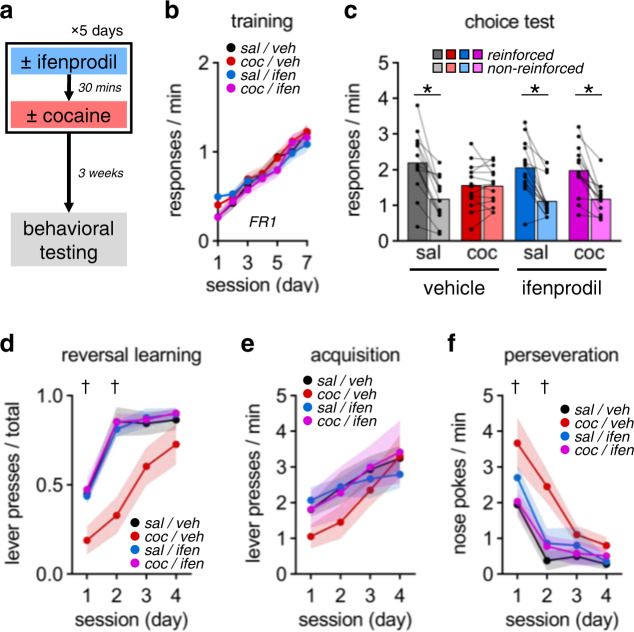
Fig. 3. GluN2B inhibition by ifenprodil blocks cocaine-induced decision-making deficits.
a Ifenprodil and cocaine administration. b Responses across training. (c) Choice test responses. Cocaine impaired action flexibility, which was protected by ifenprodil. d–f Similar patterns were detected in instrumental reversal learning responses: reversal rate, response acquisition, and perseverative responding. Data presented as individual points or mean ± S.E.M. *p < 0.05 (post-hoc). †p < 0.05 (2-factor ANOVA, cocaine × ifenprodil). See Table S4for complete statistics and group sizes.
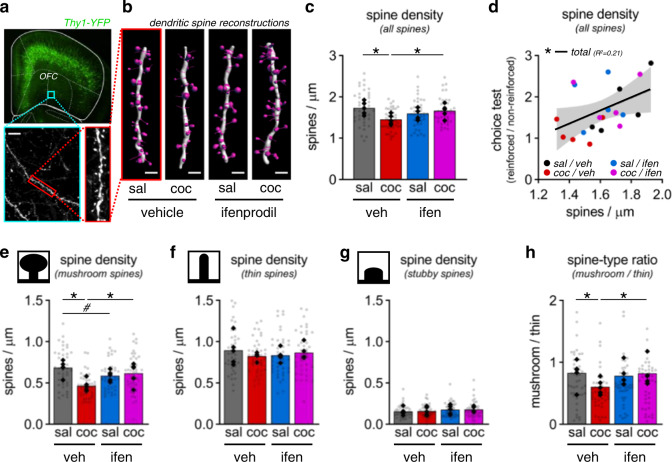
Fig. 4. GluN2B inhibition blocks cocaine-induced dendritic spine loss in the OFC.
a Dendritic spine imaging from the OFC. Scale bar = 100 µm. b Representative three-dimensional dendritic spine reconstructions. Scale bar = 2 µm. c Dendritic spine density for all spines, lower following cocaine, and protected by ifenprodil. d Correlation between individual choice behavior and dendritic spine density across all groups. 95% confidence interval (grey shading). e–g Dendritic spine density stratified by mushroom-, thin-, or stubby-type spines. h Mushroom-to-thin spine-type ratio. Data presented as individual points (solid = per animal; transparent = per dendrite). *p < 0.05, #p = 0.0.038 (post-hoc). See Table S5for complete statistics and group sizes.
Experiment 3 utilized experimenter-administered cocaine and ifenprodil and chemogenetically manipulated BLA-OFC interactions (Fig. 5).
Fig. 5. Preservation of flexible decision making following cocaine by GluN2B inhibition requires functionally intact BLA→OFC projections.
a Combinatorial viral targeting of BLA→OFC projections. b–d eGFP-Cre driving hM4Di-mCherry expression in cell bodies in the BLA and axon terminals in the OFC. Scale bar = 25 µm. e Timing of CNO administration (0.1 or 1.0 mg/kg) occurs following a session when responding is unexpectedly not reinforced, this experimental design allowing us to selectively manipulate projection activity during a period of new memory encoding. f Responses across training. g, h Choice test responses following FR and moderate RI training, which has additive impact with 0.1 mg/kg CNO in obstructing action flexibility. i Timing of ifenprodil, cocaine, and CNO administration. j Responses across training. k Choice test responses following FR training, with effects of ifenprodil obstructed by subthreshold CNO. l Choice test response preference ratios revealing the same pattern. m Choice test responses following moderate RI training. n Choice test response preference ratios. Data presented as individual points or mean ± S.E.M. *p < 0.05 (post-hoc or planned comparison). See Table S6for complete statistics and group sizes.
Data analysis
Statistical analysis was performed using SPSS v.27 (IBM). Tests were two-tailed with α < 0.05. Response rates were compared using ANOVA, with repeated measures when appropriate. For dendritic spine analysis, hierarchical data structure was controlled using linear mixed-effect modeling (LMM) [24]. Intra-class correlation coefficients (ICC) were computed using random-intercept models to assess correlated error from subject-wise clustering. We then performed a 2-factor (cocaine × ifenprodil) LMM with an interaction term to test differences in dendritic spine parameters. Post-hoc analysis following interaction effects was performed for pair-wise comparisons as a 1-factor LMM.
Results
Individual differences in cocaine-seeking behavior
We first examined cocaine self-administration in adolescent mice using a self-paced response sequence task [nose-poke (NP)→lever-press (LP)], which provided mice brief access to a sucrose solution with or without cocaine (Fig. 1a). NP and LP patterns revealed a broad spectrum of both seeking (NP) and taking (LP) behaviors among cocaine-exposed mice, including individual response rates that were similar to, or even lower than, those of control mice that received only sucrose. Mice were thus divided into “low” and “escalating” responders, which significantly differed (Fig. 1b: main effects comparing the last 8 sessions, used to classify mice: NP: F1,43 = 97.2, p < 0.001; LP: F1,43 = 75.7, p < 0.001; NP/LP: F2,64 = 1.63, p = 0.204). Figure 1c depicts NPs across training (session × group: F30,960 = 3.64, p < 0.001). Figure 1d depicts LPs across training (session × group: F30,960 = 3.63, p < 0.001). Average NP and LP rates also differed (Fig. 1c: F2,64 = 51.4, p < 0.001; Fig. 1d: F2,64 = 32.4, p < 0.001). Throughout this report, key ANOVA terms are reported in the main text, and complete statistics can be found in Tables S2–S6.
Mice were left undisturbed for 3 weeks, into adulthood, then we assessed flexible choice behavior, first following training using an FR reinforcement schedule, which generates response patterns that are sensitive to changes in outcome expectancies [25] (Fig. 1e). Prior cocaine did not impact initial training (Fig. 1f; Fig. S4: session × group: F13,312 = 1.10, p = 0.338). Mice were then given isolated access to one aperture, and responding remained reinforced. The next day, they had access to the opposite aperture, and responding was not reinforced; instead, pellets were delivered non-contingently. Prior cocaine did not alter responding when nose-poking was unexpectedly non-reinforced (Fig. S5), but cocaine-exposed mice categorized as escalating responders failed to later prefer the reinforced vs. non-reinforced behavior in a choice test–indicating an inability to update action strategies (Fig. 1g: test 1: reinforcement × group: F1,24 = 3.47, p = 0.048).
We next trained mice using an RI schedule, which over time weakens behavioral sensitivity to changes in outcome expectancies, causing organisms to persist in established behavioral sequence, even if not explicitly reinforced [25, 26]. Here, all mice displayed inflexible choice behavior, as expected (Fig. 1h: reinforcement × group: F1,24 < 1), thus confirming that our procedure is capable of detecting failures in flexible action due to drugs or reinforcement conditions alike.
Cocaine alters post-synaptic protein levels in the OFC
We next dissected OFC tissue from these same mice, focusing on the ventrolateral OFC, which is sensitive to changes in outcome expectancy [4, 27], and quantified several synaptic and plasticity-related proteins (Fig. 2a). PSD-95, a post-synaptic marker of excitatory synapses, was reduced in escalating, but not stable, responders (Fig. 2b: F2,24 = 8.31, p = 0.002), a presumed dose-dependent effect of cocaine mirroring the pattern of behavioral inflexibility. Meanwhile, the pre-synaptic marker synaptophysin was unchanged (Fig. 2c: F2,24 = 2.32, p = 0.120). Given that PSD-95 can regulate synaptic plasticity via interaction with glutamate receptors [28], we next quantified glutamate receptor subunit levels. Levels of the obligate GluN1 or the GluN2A subunit of NMDARs did not differ (Fig. 2d, e: GluN1: F2,24 < 1; GluN2A: F2,24 < 1). However, GluN2B was reduced in stable responders, which cannot be obviously explained by cocaine dose, given that GluN2B levels in escalating responders were similar to controls (Fig. 2f: F2,24 = 4.81, p = 0.017). This also did not reflect general alterations in glutamate receptor density, as AMPAR subunit GluA1 also did not differ (Fig. 2g: F2,24 < 1). Further, several other structural and functional synaptic regulatory proteins did not differ (Fig. S6). Meanwhile, PSD-95 and GluN2B correlated with individual choice behavior, with increased PSD-95 and decreased GluN2B associating with flexible action updating (Fig. 2h, i: PSD-95: F1,16 = 11.7, p = 0.004; GluN2B: F1,16 = 12.0, p = 0.003).
GluN2B inhibition preserves behavioral flexibility following cocaine
These discoveries led us to test whether attenuating GluN2B-mediated signaling would confer resilience to cocaine-induced impairments in flexible choice (Fig. 3a). We administered the GluN2B-selective antagonist ifenprodil shortly before i.p. cocaine injections. We opted for experimenter-administered cocaine to ensure that mice received a dose that would reliably induce action inflexibility, in order to then attempt to combat it. Neither cocaine nor ifenprodil affected response training (Fig. 3b: session × cocaine × ifenprodil: F6,198 = 1.68, p = 0.127). As with cocaine self-administration, experimenter-administered cocaine disrupted flexible choice behavior, and this phenomenon was blocked by ifenprodil (Fig. 3c: reinforcement × cocaine × ifenprodil: F1,33 = 5.85, p = 0.021). To further solidify this evidence that GluN2B blockade buoys OFC function, we also tested these mice in an instrumental reversal task, another OFC-dependent task [29], in which pressing a previously unavailable lever was reinforced, while nose-poking was not. Here, ifenprodil also mitigated cocaine-induced deficits in reversal learning (Fig. 3d: session × cocaine × ifenprodil: F3,99 = 3.71, p = 0.014). Specifically, while cocaine had no effects on the acquisition of a new response (Fig. 3e: session × cocaine × ifenprodil: F3,99 = 1.13, p = 0.295), it perpetuated nose-poking, which was blocked by ifenprodil (Fig. 3f: session × cocaine × ifenprodil: F3,99 = 2.78, p = 0.045).
GluN2B inhibition blocks cocaine-induced dendritic spine dysregulation in the OFC
We next examined in the same mice the density and structure of dendritic spines, the principal sites of excitatory neurotransmission in the brain [30], on ventrolateral OFC pyramidal neurons (Fig. 4a, b; Fig. S7). We visualized deep-layer neurons [31], which send and receive the bulk of long-range cortical projections [32] and appear to be especially vulnerable to cocaine compared to other prefrontal cortical cell types [33]. Cocaine reduced overall spine density (as with self-administered cocaine [34]), while ifenprodil prevented spine loss (Fig. 4c: cocaine × ifenprodil: F1,16 = 7.35, p = 0.015).
Notably, spine densities correlated with choice behavior, with a greater abundance of dendritic spines associated with response flexibility (Fig. 4d: F1,18 = 4.68, p = 0.044). Stratification of dendritic spines by morphologic subtypes revealed that cocaine-induced spine loss was driven by reduction of mushroom-shaped spines (Fig. 4e: cocaine × ifenprodil: F1,16 = 6.96, p = 0.009), while thin- and stubby-shaped spines were unaffected (Fig. 4f: thin: cocaine: F1,16 < 1; ifenprodil: F1,16 < 1; cocaine × ifenprodil: F1,16 = 1.12, p = 0.306; Fig. 4g: stubby: cocaine: F1,16 < 1; ifenprodil: F1,16 < 1; cocaine × ifenprodil: F1,16 < 1). This pattern of spine loss resulted in a reduction in the relative abundance of mushroom-shaped spines (Fig. 4h: cocaine × ifenprodil: F1,16 = 5.20, p = 0.033), containing more mature, stable synaptic connections [35]. This pattern is significant because the presence of these mature spines, and the cellular factors that stabilize them, in the ventrolateral OFC are necessary for action flexibility [14, 27, 36–38].
GluN2B-mediated resilience to cocaine requires BLA→OFC projections
Our findings lead to the question: Might GluN2B blockade confer behavior resilience to cocaine by preserving the ability of OFC neurons to receive inputs necessary for action flexibility? A prominent source of input arises from the BLA [39, 40]. BLA lesions disrupt reward-related activity of OFC neurons [41, 42], and BLA→OFC projections are required for goal-seeking action [43]. Thus, we expressed inhibitory (Gi-coupled) chemogenetic receptors on BLA→OFC projection neurons (Fig. 5a–d). We administered the chemogenetic ligand CNO immediately following the non-reinforced session, when familiar reinforcement conditions were violated (Fig. 5e), and excitatory OFC neuron plasticity is necessary for the consolidation of adaptive action strategies [44]. We tested multiple CNO doses and training conditions to identify a behaviorally sub-threshold CNO dose that weakens, but does not completely abolish, the capacity for flexible learning and memory. Groups did not differ during response acquisition (Fig. 5f: session × CNO condition: F20,270 < 1). A 0.1 mg/kg CNO dose did not disrupt flexible choice following FR training but did exhibit an additive effect with brief RI training, while 1.0 mg/kg induced inflexible choice throughout (Fig. 5g: test 1: reinforcement × CNO: F2,27 = 4.92, p = 0.015; Fig. 5h: test 2: main effect of reinforcement: F1,27 = 15.8, p < 0.001; reinforcement × CNO: F2,27 = 2.39, p = 0.111, followed by planned comparisons).
Next, we used this 0.1 mg/kg dose to attenuate BLA→OFC projection activity in cocaine-exposed mice that had been treated with ifenprodil (Fig. 5i). Groups again did not differ during training (Fig. 5j: session × ifenprodil × CNO: F10,290 < 1). Ifenprodil again preserved flexible choice behavior in cocaine-exposed mice, while dampening BLA→OFC projections abolished this protective effect (Fig. 5k: reinforcement × ifenprodil × CNO: F1,29 = 4.38, p = 0.045). The same data can be reported as preference ratios (simply, responses in the reinforced/non-reinforced condition, such that scores >1 reflect action flexibility). Again, an interaction effect was detected, with post-hoc comparisons indicating that ifenprodil preserved action flexibility (Fig. 5l: ifenprodil × CNO: F1,29 = 4.79, p = 0.037). And again, we confirmed that all groups developed response inflexibility with extended training, as expected (Fig. 5m, n: reinforcement × ifenprodil × CNO: F1,29 < 1; preference ratio: ifenprodil × CNO: F1,29 < 1). Thus, the mechanism by which ifenprodil confers long-term resilience to cocaine-induced behavioral inflexibility appears to involve the preservation of functional BLA→OFC connections.
Discussion
We demonstrate that both self- and experimenter-administered cocaine during adolescence disrupt the capacity of mice to later flexibly modify familiar behaviors when reinforcement conditions unexpectedly change. We capitalized on individual differences in cocaine self-administration patterns and revealed that the post-synaptic protein PSD-95 was lower in the OFC of mice that displayed escalating cocaine-seeking behavior, which corresponded with long-term deficits in flexible decision-making behavior. Meanwhile, GluN2B content was lower in the OFC of mice that were apparently resilient to escalatory cocaine-seeking behaviors. This discovery led us to pharmacologically inhibit GluN2B, attempting to confer resilience to cocaine. GluN2B blockade indeed obstructed the long-term behavioral and neuronal structural effects of cocaine, maintaining mature, mushroom-shaped dendritic spine densities on deep-layer pyramidal neurons in the OFC and preserving functional BLA→OFC connections. Cocaine can rapidly induce the insertion of GluN2B-containing NMDARs into post-synaptic membranes, resulting in long-lasting synaptic changes that persist even following prolonged abstinence [45, 46]. Because GluN2B inhibition coincident with cocaine administration here prevented the emergence of behavioral inflexibilities, we posit that inhibiting GluN2B-mediated signaling promotes resilience to long-term cocaine-related sequelae by interrupting the acute, early-stage synaptic response to cocaine. GluN2B inhibition in this context thus blocks cascading cocaine-induced morphological changes among deep-layer OFC neurons, which weaken the capacity for flexible choice before these deficits emerge and become entrenched.
Individual differences in cocaine seeking reveal biological-behavioral correlates
We utilized an oral cocaine self-administration procedure to resolve individual differences in drug-seeking behavior in mice. Orally-ingested cocaine readily penetrates the brain [47], and has reinforcing properties in both mice [22] and rats [48]. One advantage is that it allows for a non-drug control group that robustly responds for reinforcement (as opposed to animals receiving i.v. saline, which largely do not respond). We replicate existing evidence that some mice respond for a cocaine + sucrose solution comparably to mice receiving sucrose alone; meanwhile, other cocaine-reinforced mice escalate in responding [22]. Only these escalating responders later displayed deficits in an OFC-dependent decision-making task, concurrent with PSD-95 loss in the OFC. Meanwhile, stable responders had lower GluN2B content in the OFC, which could not be explained by intrinsic differences in motivation or reward processing per se, given that these mice responded equivalently to sucrose-reinforced mice by the end of the training period, and the chained response was comparably efficient between groups, meaning, the number of nose pokes generated for access to the reinforced lever was indistinguishable. This discovery would have evaded us were it not for attention to individual differences, since these distinctions would not have been detected.
This unexpected outcome—a potential role for reduced GluN2B-mediated signaling in conferring resilience to cocaine – led us to administer the GluN2B-selective antagonist ifenprodil coincident with cocaine to attempt to block long-term deleterious cocaine-induced sequelae. We opted here for experimenter-administered cocaine to ensure that mice uniformly received a “high” dose that would reliably induce action inflexibility. Ifenprodil meanwhile preserved action flexibility, potentially attributable to the preservation of dendritic spine densities on deep-layer OFC neurons. NMDA receptors are ionotropic glutamate receptors composed of two GluN1 subunits and two GluN2 or GluN3 subunits. Ifenprodil can preferentially inactivate extra-synaptic dimers and block LTD [49]. Reducing extra-synaptic NMDA receptor activity is also thought to improve signal-to-noise ratio of synaptic NMDA receptor signaling [50]. Both of these mechanisms would be associated with the anchoring and maintenance of mature dendritic spine subtypes [30], though it is worth noting that while low GluN2B apparently aids, it is not necessary, for action flexibility, given that sucrose self-administering mice were able to update action strategies without the ‘benefit’ of low GluN2B. It is also likely one of multiple outcomes associated with drug resilience, even potentially compensating for other neurobiological sequelae.
Resilience to cocaine requires BLA→OFC projections
Dendritic spines house the post-synaptic component of a vast majority of synaptic connections in the mammalian brain [30], leading to the question: Which functional inputs to the OFC are preserved by GluN2B blockade? BLA lesions alter reward-related activity of OFC neurons [41, 42], and BLA-OFC interactions are involved in many facets of reinforcement learning [51, 52], leading us to investigate functional BLA→OFC connections, the goal being to test whether the behavioral resilience conferred by ifenprodil required functionally intact BLA→OFC connections. A challenge in designing this experiment is that inactivating BLA→OFC projections in typical, healthy mice obstructs response flexibility in the same task [43], which would make it difficult to draw conclusions regarding the protective actions of GluN2B inhibition with previously established procedures. To circumvent this issue, we established a behaviorally sub-threshold dose of the chemogenetic ligand CNO – meaning, a dose that was not sufficient to disrupt flexible action in cocaine-naïve mice expressing the same chemogenetic receptors. Here, sub-threshold silencing obscured the protective effects of ifenprodil, suggesting that GluN2B blockade preserves functional BLA→OFC connections rendered vulnerable by cocaine. Notably, other inputs to the OFC, such as the ventral hippocampus, support aspects of response flexibility in this task [53] and play major roles OFC-dependent representations of task variables [54–56]. The BLA is thought to broadcast a salience signal regarding task features most relevant to reward acquisition; thus, BLA→OFC inputs might organize multiplexed inputs by biasing the allocation of certain OFC neurons into the memory traces that subserve later adaptive responding.
Conclusions
Here, we administered cocaine during adolescence, given that adolescent-onset drug use is more likely to be associated with relapse across the lifespan [57]. During this late developmental epoch, cortical GluN2B levels decline, in favor of GluN2A receptor subunits, a process thought to optimize synapse and spine stabilization and refinement [58–60]. Meanwhile, cocaine self-administration can increase cortical GluN2B content [61], and developmental cocaine alters dendritic spine developmental trajectories on layer V OFC neurons [14]. Our findings suggest that GluN2B blockade is sufficient to protect connections necessary for flexible goal-seeking behavior, conceivably safeguarding neurodevelopmental processes otherwise disrupted by cocaine. Our investigation is complementary to others in which pharmacologic GluN2B antagonism occluded cocaine- and heroin-seeking behavior [14, 62], cocaine-induced locomotor sensitization [63, 64], and drug-induced habit [14, 65]. In these cases, though, GluN2B inhibitors were largely administered following some degree of drug exposure, and/or in mature organisms. Here, we reveal a novel, prophylactic effect of developmental treatment. A key next question is whether GluN2B blockade preserves learning-related structural plasticity in the OFC [27], and following drug self-administration; resolving this issue would more fully reveal ontogenetic factors that impact OFC function later in life.
Supplementary information
Author contributions
Conceptualization: DCL, EGP, SLG. Investigation: DCL, EGP, NMD. Formal analysis: DCL. Writing: DCL, SLG. Funding acquisition: DCL, SLG. Supervision: SLG.
Funding
This work was supported by NIH grants F30 MH117873 (DCL), R01 MH117103 (SLG), R01 DA044297 (SLG), and R03 DA036737 (SLG), and by the Children’s Healthcare of Atlanta and Emory University’s Pediatric Integrated Cellular Imaging Core and Pediatric Research Alliance Biostatistics Core. The Emory National Primate Research Center is supported by the Office of Research Infrastructure Programs grant P51 OD011132.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01437-8.
References
- 1.Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 2.Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 2017;37:10529–40. doi: 10.1523/JNEUROSCI.1678-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner MPH, Conroy JC, Sanchez DC, Zhou J, Schoenbaum G. Real-time value integration during economic choice is regulated by orbitofrontal cortex. Curr Biol. 2019;29:4315–4322.e4. doi: 10.1016/j.cub.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkes SL, Ravassard PM, Cerpa JC, Wolff M, Ferreira G, Coutureau E. Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cereb Cortex. 2018;28:2313–25. doi: 10.1093/cercor/bhx132. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee A, Parente G, Teutsch J, Lewis C, Voigt FF, Helmchen F. Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature. 2020;585:245–50. doi: 10.1038/s41586-020-2704-z. [DOI] [PubMed] [Google Scholar]
- 6.Schuck NW, Cai MB, Wilson RC, Niv Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron. 2016;91:1402–12. doi: 10.1016/j.neuron.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–79. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niv Y. Learning task-state representations. Nat Neurosci. 2019;22:1544–53. doi: 10.1038/s41593-019-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens TEJ, Muller TH, Whittington J, Mark S, Baram AB, Stachenfeld KL, et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron. 2018;100:490–509. doi: 10.1016/j.neuron.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–66. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogarth L. Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology. 2020;45:720–35. doi: 10.1038/s41386-020-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandaele Y, Ahmed SH. Habit, choice, and addiction. Neuropsychopharmacology. 2021;46:689–98. doi: 10.1038/s41386-020-00899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LH, Luijten M, et al. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–71. doi: 10.1126/science.aaf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePoy LM, Zimmermann KS, Marvar PJ, Gourley SL. Induction and blockade of adolescent cocaine-induced habits. Biol Psychiatry. 2017;81:595–605. doi: 10.1016/j.biopsych.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–8. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation cocaine craving withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–23. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC, Aguilar-Gaxiola S, Berglund PA, Caraveo-Anduaga JJ, DeWit DJ, Greenfield SF, et al. Patterns and predictors of treatment seeking after onset of a substance use disorder. Arch Gen Psychiatry. 2001;58:1065–71. doi: 10.1001/archpsyc.58.11.1065. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30:1006–18. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 22.DePoy LM, Allen AG, Gourley SL. Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl Psychiatry. 2016;6:e875. doi: 10.1038/tp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci. 2013;33:14379–91. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Guindani M, Grieco SF, Chen L, Holmes TC, Xu X. Beyond t test and ANOVA: applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron. 2022;110:21–35. doi: 10.1016/j.neuron.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson A, Nicholas DJ, Adams AD. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q J Exp Psychol B. 1983;35:35–51. doi: 10.1080/14640748308400912. [DOI] [Google Scholar]
- 26.de Wit S, Ostlund SB, Balleine BW, Dickinson A. Resolution of conflict between goal-directed actions: outcome encoding and neural control processes. J Exp Psychol Anim Behav Process. 2009;35:382–93. doi: 10.1037/a0014793. [DOI] [PubMed] [Google Scholar]
- 27.Whyte AJ, Kietzman HW, Swanson AM, Butkovich LM, Barbee BR, Bassell GJ, et al. Reward-related expectations trigger dendritic spine plasticity in the mouse ventrolateral orbitofrontal cortex. J Neurosci. 2019;39:4595–605. doi: 10.1523/JNEUROSCI.2031-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 29.Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–34. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasai H, Ziv NE, Okazaki H, Yagishita S, Toyoizumi T. Spine dynamics in the brain, mental disorders and artificial neural networks. Nat Rev Neurosci. 2021;22:407–22. doi: 10.1038/s41583-021-00467-3. [DOI] [PubMed] [Google Scholar]
- 31.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/S0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 32.DeNardo LA, Berns DS, DeLoach K, Luo L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat Neurosci. 2015;18:1687–97. doi: 10.1038/nn.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacherjee A, Djekidel MN, Chen R, Chen W, Tuesta LM, Zhang Y. Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat Commun. 2019;10:4169. doi: 10.1038/s41467-019-12054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, et al. The contingency of cocaine administration accounts for structural and functional medial prefrontal deficits and increased adrenocortical activation. J Neurosci. 2015;35:11897–910. doi: 10.1523/JNEUROSCI.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry KP, Nedivi E. Spine dynamics: are they all the same? Neuron. 2017;96:43–55. doi: 10.1016/j.neuron.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whyte AJ, Trinoskey-Rice G, Davies RA, Woon EP, Foster SL, Shapiro LP, et al. Cell adhesion factors in the orbitofrontal cortex control cue-induced reinstatement of cocaine seeking and amygdala-dependent goal seeking. J Neurosci. 2021;41:5923–36. [DOI] [PMC free article] [PubMed]
- 37.DePoy LM, Shapiro LP, Kietzman HW, Roman KM, Gourley SL. beta1-integrins in the developing orbitofrontal cortex are necessary for expectancy updating in mice. J Neurosci. 2019;39:6644–55. doi: 10.1523/JNEUROSCI.3072-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann KS, Yamin JA, Rainnie DG, Ressler KJ, Gourley SL. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by brain-derived neurotrophic factor. Biol Psychiatry. 2017;81:366–77. doi: 10.1016/j.biopsych.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barreiros IV, Panayi MC, Walton ME. Organization of afferents along the anterior-posterior and medial-lateral axes of the rat orbitofrontal Cortex. Neuroscience. 2021;460:53–68. doi: 10.1016/j.neuroscience.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy MJM, Deutch AY. Organization of afferents to the orbitofrontal cortex in the rat. J Comp Neurol. 2018;526:1498–526. doi: 10.1002/cne.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–67. doi: 10.1016/S0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 42.Rudebeck PH, Mitz AR, Chacko RV, Murray EA. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron. 2013;80:1519–31. doi: 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li DC, Dighe NM, Barbee BR, Pitts EG, Kochoian B, Blumenthal SA, et al. A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory. Nature Neurosci. 2022; in press. [DOI] [PMC free article] [PubMed]
- 44.Zimmermann KS, Li CC, Rainnie DG, Ressler KJ, Gourley SL. Memory retention involves the ventrolateral orbitofrontal cortex: comparison with the basolateral amygdala. Neuropsychopharmacology. 2018;43:373–83. doi: 10.1038/npp.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L, et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci. 2020;23:32–46. doi: 10.1038/s41593-019-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–7. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan WJ, Hedaya MA. An animal model for simultaneous pharmacokinetic/pharmacodynamic investigations: application to cocaine. J Pharm Toxicol Methods. 1998;39:1–8. doi: 10.1016/S1056-8719(97)00097-X. [DOI] [PubMed] [Google Scholar]
- 48.Jentsch JD, Henry PJ, Mason PA, Ziriax JM. Establishing orally self-administered cocaine as a reinforcer in rats using home-cage pre-exposure. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:229–39. doi: 10.1016/S0278-5846(97)00105-X. [DOI] [PubMed] [Google Scholar]
- 49.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 50.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharm. 2009;157:1301–17. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, et al. Orbitofrontal circuits control multiple reinforcement-learning processes. Neuron. 2019;103:734–746.e3. doi: 10.1016/j.neuron.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013;33:6434–43.. doi: 10.1523/JNEUROSCI.3971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barfield ET, Gourley SL. Glucocorticoid-sensitive ventral hippocampal-orbitofrontal cortical connections support goal-directed action - Curt Richter Award Paper 2019. Psychoneuroendocrinology. 2019;110:104436. doi: 10.1016/j.psyneuen.2019.104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuck NW, Niv, Y. Sequential replay of nonspatial task states in the human hippocampus. Science. 2019;364:eaaw5181. [DOI] [PMC free article] [PubMed]
- 55.Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron. 2017;95:1197–1207.e3. doi: 10.1016/j.neuron.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J, Montesinos-Cartagena M, Wikenheiser AM, Gardner M, Niv Y, Schoenbaum G. Complementary task structure representations in hippocampus and orbitofrontal cortex during an odor sequence task. Curr Biol. 2019;29:3402–3409. e3. doi: 10.1016/j.cub.2019.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 58.Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, et al. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29:10869–82. doi: 10.1523/JNEUROSCI.5531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 60.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–7. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA. 2011;108:19407–12. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schumann J, Matzner H, Michaeli A, Yaka R. NR2A/B-containing NMDA receptors mediate cocaine-induced synaptic plasticity in the VTA and cocaine psychomotor sensitization. Neurosci Lett. 2009;461:159–62. doi: 10.1016/j.neulet.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2314–23. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morisot N, Phamluong K, Ehinger Y, Berger AL, Moffat JJ, Ron D, et al. mTORC1 in the orbitofrontal cortex promotes habitual alcohol seeking. Elife, 2019;8:e51333. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.