Abstract
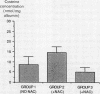
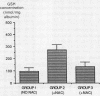
N-acetylcysteine (600 mg/day) was given to patients by mouth for five days before bronchoscopy and bronchoalveolar lavage to determine whether N-acetylcysteine could increase the concentrations of the antioxidant reduced glutathione in plasma and bronchoalveolar lavage fluid. Bronchoalveolar lavage was performed 1-3 hours (group 2, n = 9) and 16-20 hours (group 3, n = 10) after the last dose of N-acetylcysteine and the values were compared with those in a control group receiving no N-acetylcysteine (group 1, n = 8). N-acetylcysteine was not detected in plasma or lavage fluid. Plasma concentrations of cysteine, the main metabolite of N-acetylcysteine and a precursor of reduced glutathione, were greater in the groups receiving treatment (groups 2 and 3) than in group 1. Cysteine concentrations in lavage fluid were similar in the three groups. Concentrations of reduced glutathione were greater in both plasma and lavage fluid in group 2 than in group 1. These data suggest that N-acetylcysteine given by mouth is rapidly deacetylated to cysteine, with resulting increases in the concentrations of cysteine in plasma and of reduced glutathione in plasma and the airways, which thus temporarily increase the antioxidant capacity of the lung.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew J. E., Pavia D., Clarke S. W. Mucus clearance from peripheral and central airways of asymptomatic cigarette smokers. Bull Eur Physiopathol Respir. 1986 May-Jun;22(3):263–267. [PubMed] [Google Scholar]
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren M., Dawson J., Moldéus P. Glutathione biosynthesis in the isolated perfused rat lung: utilization of extracellular glutathione. FEBS Lett. 1984 Oct 15;176(1):189–192. doi: 10.1016/0014-5793(84)80938-2. [DOI] [PubMed] [Google Scholar]
- Boman G., Bäcker U., Larsson S., Melander B., Wåhlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis. 1983 Aug;64(6):405–415. [PubMed] [Google Scholar]
- Borgström L., Kågedal B., Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- Burgunder J. M., Varriale A., Lauterburg B. H. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis. 1989 Aug;140(2):513–524. doi: 10.1164/ajrccm/140.2.513. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., Hubbard R. C., Crystal R. G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989 Feb;139(2):370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cotgreave I. A., Eklund A., Larsson K., Moldéus P. W. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur J Respir Dis. 1987 Feb;70(2):73–77. [PubMed] [Google Scholar]
- Cotgreave I. A., Moldéus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J Biochem Biophys Methods. 1986 Nov;13(4-5):231–249. doi: 10.1016/0165-022x(86)90102-8. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Vähäkangas K., Jernström B., Moldéus P. Glutathione conjugation by isolated lung cells and the isolated, perfused lung. Effect of extracellular glutathione. Eur J Biochem. 1984 Feb 1;138(3):439–443. doi: 10.1111/j.1432-1033.1984.tb07935.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson S. G., Black R. D., Lawrence R. A. Glutathione concentrations in rat lung bronchoalveolar lavage fluid: effects of hyperoxia. J Lab Clin Med. 1988 Sep;112(3):345–351. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Kelly C. A., Fenwick J. D., Corris P. A., Fleetwood A., Hendrick D. J., Walters E. H. Fluid dynamics during bronchoalveolar lavage. Am Rev Respir Dis. 1988 Jul;138(1):81–84. doi: 10.1164/ajrccm/138.1.81. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van Twisk C., Klasen E. C., Dijkman J. H. Interactions among stimulated human polymorphonuclear leucocytes, released elastase and bronchial antileucoprotease. Clin Sci (Lond) 1988 Jul;75(1):53–62. doi: 10.1042/cs0750053. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock J. Biological properties of acetylcysteine: assay development and pharmacokinetic studies. Eur J Respir Dis Suppl. 1980;111:52–58. [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. B., Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J. 1988 Apr;1(4):351–355. [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Sheffner A. L., Medler E. M., Bailey K. R., Gallo D. G., Mueller A. J., Sarett H. P. Metabolic studies with acetylcysteine. Biochem Pharmacol. 1966 Oct;15(10):1523–1535. doi: 10.1016/0006-2952(66)90197-3. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Suttorp N. Lung cell oxidant injury: decrease in oxidant mediated cytotoxicity by N-acetylcysteine. Eur J Respir Dis Suppl. 1985;139:132–135. [PubMed] [Google Scholar]
- Tsan M. F., Danis E. H., Del Vecchio P. J., Rosano C. L. Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun. 1985 Feb 28;127(1):270–276. doi: 10.1016/s0006-291x(85)80154-6. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Mathieu-Costello O., Bebout D. E., Gray A. T., Natterson P. D., Glennow C. Protection against pulmonary O2 toxicity by N-acetylcysteine. Eur Respir J. 1989 Feb;2(2):116–126. [PubMed] [Google Scholar]
- Ziment I. Acetylcysteine: a drug with an interesting past and a fascinating future. Respiration. 1986;50 (Suppl 1):26–30. doi: 10.1159/000195085. [DOI] [PubMed] [Google Scholar]