Summary
Background
Thus far, all approved chimeric antigen receptor (CAR)-T products are manufactured using modified viruses, which increases the risk of tumorigenesis, costs and production time. We aimed to evaluate the safety and efficacy of a kind of virus-free CAR-T cells (PD1-19bbz), in which an anti-CD19 CAR sequence is specifically integrated at the PD1 locus using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, in adults with relapsed/refractory (r/r) B cell non-Hodgkin’s lymphoma (B-NHL).
Methods
This single-arm phase I dose-escalation clinical trial evaluated PD1-19bbz in adult patients with r/r B-NHL from May 3rd 2020 to August 10th 2021. The patients were recruited and treated at the First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China. Patients underwent leukapheresis and lymphodepleting chemotherapy before PD1-19bbz infusion. After the dose-escalation phase including three cohorts: 2 × 106/kg, 4 × 106/kg, 6 × 106/kg with three patients at each dose level, the optimal biological dose was determined to be 2 × 106/kg, which was then applied to an extended cohort of nine patients. The primary endpoint was the incidence of dose-limiting toxicities (DLT). The secondary endpoint was the response and survival. This trial was registered at www.clinicaltrials.gov as #NCT04213469.
Findings
Twenty-one patients received PD1-19bbz infusion. Among all treated patients, 19 (90%) patients were diagnosed with stage III or IV disease. Meanwhile, 19 (90%) were stratified as intermediate risk or worse. Of note, four participants had >50% programmed death ligand-1 (PD-L1) expression in pre-treatment tumour sample, including two with extremely high levels (∼80%). There was no DLT identified. Fourteen patients had low-grade (1–2) cytokine release syndrome and two patients received tocilizumab. Four patients experienced immune effector cell-associated neurotoxicity syndrome of grade 1–2. The most common adverse events were hematologic toxicities, including anaemia (n = 6), lymphocyte count decreased (n = 19), neutrophil count decreased (n = 17), white blood cell count decreased (n = 10), and platelet count decreased (n = 2). All patients had objective response and 18 patients reached complete response. At a median follow-up of 19.2 months, nine patients remained in remission, and the estimated median progression-free survival duration was 19.5 months (95% confidence interval: 9.9–infinity), with the median overall survival not reached.
Interpretation
In this first-in-human study of non-viral specifically integrated CAR-T products, PD1-19bbz exhibited promising efficacy with a manageable toxicity profile. A phase I/II trial of PD1-19bbz in a larger patient cohort is underway.
Funding
National Key R&D Program of China, National Natural Science Foundation of China, Key Project of Science and Technology Department of Zhejiang Province, Shanghai Zhangjiang National Independent Innovation Demonstration Area, Key Projects of Special Development Funds.
Keywords: CAR-T, Non-viral, PD-1, PD-L1, Non-Hodgkin lymphoma, Diffused large B cell lymphoma
Research in context.
Evidence before this study
We searched PubMed for full-text clinical trials written in English published up to March 18, 2023, to identify papers on PD1-ablated CAR-T cells and/or virus-free CAR-T cells. The search terms used were “(non-viral) AND (CAR-T)”, and “(PD-1) AND (CAR-T)”. The search revealed a scarcity of clinical reports of CAR-T cells manufactured via a virus-free procedure except for our previous report, and very few clinical trials involved PD1-ablated CAR-T cells.
Added value of this study
To the best of our knowledge, this is the first clinical trial to evaluate the safety and efficacy of this virus-free CAR-T cells except for our preliminary report. With an additional 13 patients, we further provide support for the feasibility and efficacy of this non-viral CAR-T product, and identified the appropriate dose for a phase II trial in future.
Implications of all the available evidence
Our study findings provide support for the safety and efficacy of this virus-free CAR-T product, which warrant further large-scale and multi-centre clinical trials evaluating it.
Introduction
The past few decades have witnessed the thriving of chimeric antigen receptor (CAR)-T cell therapy, and unprecedented efficacy has been reported, especially in haematological malignancies.1, 2, 3, 4, 5, 6 With an increasing number of receivers, however, limitations have been found for the common CAR-T therapies in current clinical practice. Apart from the general ones, including CAR-T cell-associated toxicities, antigen escape, limited persistence, immunosuppressive tumour microenvironment (TME), among others, the oncogenic potential of genome-edited T cells is another rare but serious issue of concern.7,8 As retrovirus or lentivirus, traditionally used to generate CAR-T cells, randomly integrates a CAR sequence into genomic DNA of T cells, introduction of mutations at oncogenes and/or tumour suppressive genes possibly occurs. In addition, the current virus-dependent manufacturing process is time-consuming, complicated and expensive.9,10
Thus, non-viral methods were developed to produce CAR-T cells. As shown by several reports, by utilizing transposon systems such as piggyBac and Sleeping Beauty, CAR sequences can be efficiently inserted into genome and CAR proteins are stably expressed.11,12 While this strategy simplifies the manufacturing process and reduces the expense of preparing CAR-T cells, it cannot completely avoid the tumorigenesis risk, owing to the random integration caused by transposon-dependent transduction.13 On the other hand, several groups attempted to figure out methods to generate CAR-T cells with precise integration using genome editing technologies, such as zinc-finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN) and clustered regularly interspaced short palindromic repeat (CRISPR).14 In this way, the consistency of CAR-T cell product is significantly increased, and uncertain alterations of endogenous genes are dramatically decreased. Nevertheless, since most of current approaches to generate specifically integrated CAR-T cells rely on the usage of adeno-associated virus (AAV), the cost and safety concerns still remain.
We and others have verified the feasibility and safety of CRISPR-Cas9 application in the manufacture of CAR-T cells, although most of which still involved the random integration of CAR sequences.15, 16, 17, 18 Several recent studies have presented locus-specific integrated anti-tumour T cells with unique functions and features by using new genome-editing technologies, providing new strategies to produce CAR-T cells.19,20 Hence, to solve the problems caused by virus usage and random integration simultaneously, we successfully developed non-viral site-specific integrated CAR-T technology through CRISPR-Cas9 system. A novel type of anti-CD19 CAR-T cells named PD1-19bbz, with the CAR sequence precisely inserted at the PD1 locus, was produced and showed high safety and efficacy in preclinical experiments and in a preliminary clinical trial involving the first eight patients.21 As a virus-free two-in-one strategy, non-viral genome-specific integrated CAR-T technology simplifies manufacturing process, shortens preparation time and reduces production expense. Given the previous encouraging results, in this study, we extended the trial to a larger cohort to further evaluate the safety and efficacy of PD1-19bbz in treating adult patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma (r/r B-NHL).
Methods
Study design and patients
We conducted a single-arm, open-label, dose-escalation phase I study of autologous PD1-integrated anti-CD19 cells (PD1-19bbz) in adults with r/r B-NHL. The clinical protocol was reviewed and approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (2020IIT (85)), and has been registered at ClinicalTrials.gov with the identifier NCT04213469. The patients were recruited and treated at the First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China, in accordance with the Declaration of Helsinki and Good Clinical Practice.
Written informed consents were obtained from all patients after the benefits and risks were properly discussed. All patients received lymphodepleting chemotherapy consisting of cyclophosphamide (day −3 to −2, 500 mg/m2/day) and fludarabine (day −4 to −2, 30 mg/m2/day). Dose escalation was based on 3 + 3 escalation rule, including three cohorts: 2 × 106 CAR-T cells/kg (dose A), 4 × 106 CAR-T cells/kg (dose B), 6 × 106 CAR-T cells/kg (dose C). After dose-escalation segment, an optimal biological dose (OBD) was decided based on both the efficacy and the cost effectiveness, which would be used as the recommended phase II dose in the expansion segment.
Yet we report a protocol deviation in the study. It was supposed to recruit another three patients for DLT evaluation even if there’s no DLT in the former three patients, as mentioned in the protocol. However, given the fact that there’s no DLT occurrence at each dose level, after a discussion among the investigators, we decided to escalate to a higher dose without adding another three patients for DLT evaluation. Only when 1/3 DLT occurs, there should be another three patients added for further DLT evaluation. Therefore, this is a protocol deviation during the study.
CAR-T cell manufacture
The PD1-19bbz preparation procedure was consistent with our previous report,21 and a summary is presented below. Fresh peripheral blood mononuclear cells (PBMCs) from patients were collected by apheresis. PBMCs were isolated by density gradient centrifugation using Ficoll (Sigma–Aldrich). T cells were enriched through magnetic separation using anti-CD4 and anti-CD8 microbeads (Miltenyi Biotec) and activated with T Cell TransAct (Miltenyi Biotec). T cells were cultured in X-VIVO 15 medium (Lonza) supplemented with CTS Immune Cell Serum Replacement (Thermo Fisher) and recombinant human IL-2 (100 U/mL), IL-7 (5 ng/mL) and IL-15 (5 ng/mL). Cells were collected once cell number reached the requirement for administration and then washed, formulated and cryopreserved. Cell products were shipped to the investigational site after meeting release criteria.
Study end points
In this phase I study, the primary endpoint was the incidence of dose-limiting toxicities (DLTs). DLTs were defined as PD1-19bbz-related events, except for haematological AEs, with following features: 1) occurring within 28 days after infusion, 2) grade ≥3 at worst, 3) not resolving to grade 2 or better within seven days.
Secondary endpoints included objective response rate (ORR, i.e., the combined percentage of patients who had a complete or partial response), progression-free survival (PFS), and overall survival (OS).
Exploratory endpoints included the cellular pharmacokinetic characteristics and the persistence of PD1-19bbz, and the consequent long-term effects on haematopoiesis, especially B cells.
Assessment of toxicities
Cytokine release syndrome (CRS) was graded according to the criteria proposed by Lee et al.22 and American Society for Transplantation and Cellular Therapy consensus grading.23
Neurotoxicity was graded according to the criteria of American Society for Transplantation and Cellular Therapy consensus grading.23 Once CRS symptoms such as pyrexia, hypotension and capillary leak or other types of AEs were observed, the patient would be closely monitored for signs of neurological toxicity.
Other toxicities were also graded using National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. All patients were inpatients and were closely monitored throughout the course of treatment.
Assessment of response
Response to the therapy was assessed according to the revised criteria of the Lugano classification24 primarily based on the results of PET/CT scans and bone marrow aspiration/biopsy at sequential time points—27–30 days post infusion, 56–67 days post infusion, 90 days post infusion, and etc. And all patients were further followed-up until relapse, death, or the study cut-off date.
Cellular kinetics
Cellular kinetics was characterised by both flow cytometry (FCM) and quantitative real-time polymerase chain reaction (qPCR). The test methods are consistent with our previous report.21 The in vivo amplification curves were plotted using FCM and qPCR results, respectively. Parameters for in vivo exposure included the maximum of both cell number and DNA copy number. The absolute value of CAR-T cells per microliter of blood was calculated by the cell proportion of CAR-T cells and the count of white blood cells. In this way, the relationship between cell dose and in vivo exposure, and the relationship between in vivo exposure and safety were analysed.
Statistical analysis
Comparisons of two groups were performed by unpaired Wilcoxon two-tailed test. Comparisons of more than two groups were performed by Kruskal–Wallis test. PFS and OS were summarised using the Kaplan–Meier method in all the patients and the log rank test was used to compare groups. All the box-and-whisker plots were used to show the distributions of quantitative variables, and the data points of all the patients unless otherwise indicated. A P-value < 0.05 was considered as statistically significant. R (version 4.2.1) was used for statistical analysis of the experiments, data processing and presentation.
Role of the funding source
The funding sources had no influence on study design; on the collection, analysis, and interpretation of data; on the writing of the report; and on the decision to submit the paper for publication. All authors have access to the entire dataset. The decision to submit for publication was made by Y. H., C. Z., M. Z., W. L., B. D., J. Z., M. L., G. W., and H. H., and all authors agreed with the publication.
Results
Patients
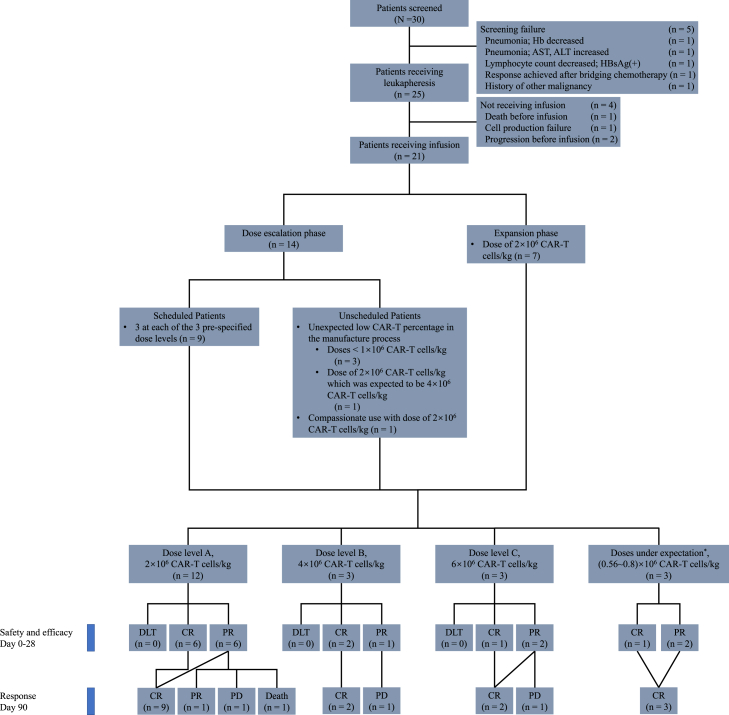
From May 3rd 2020 to August 10th 2021, 30 patients were screened, 25 were eligible for the trial of PD1-19bbz, and 21 eventually received CAR-T cell infusion following lymphodepleting chemotherapy comprised of cyclophosphamide and fludarabine. At the dose-escalation phase, 14 patients were enrolled, including three at each of the three pre-specified dose levels (dose A: 2 × 106 cells/kg; dose B: 4 × 106 cells/kg; dose C: 6 × 106 cells/kg), four with unexpected low CAR-T percentage in the manufacture process (Patients 1, 4, 5, 8), and one for compassionate use (Patient 7). OBD was then determined to be 2 × 106 cells/kg, at which another seven patients for expansion phase were enrolled and infused (Fig. 1, Table 1, and Supplementary Table S1). All infused patients were followed up until the cut-off date, October 29, 2022, or death, with a median follow-up time of 19.2 months (interquartile range [IQR]: 12.6–24.7). For conciseness, patients from both phases were amalgamated in further analysis and presentation according to the actual doses they received.
Fig. 1.
CONSORT diagram. Hb, haemoglobin; AST, aspartate transaminase; ALT, alanine transaminase; HBsAg, hepatitis B surface antigen; CAR-T cells, chimeric antigen receptor T cells; DLT, dose-limiting toxicity; CR, complete response; PR, partial response; PD, progressive disease. ∗These patients received doses other than pre-specified three dose levels because of unexpected low CAR percentages caused by the early and premature manufacturing process.
Table 1.
Baseline characteristics of patients.
| All (n = 21) | Dose A (n = 12) | Dose B (n = 3) | Dose C (n = 3) | Doses under expectationa (n = 3) | |
|---|---|---|---|---|---|
| Age | |||||
| Median (IQR)—yr | 56 (46–62) | 64.5 (53–70) | 36 (36–46) | 57 (45.5–62.5) | 44 (43.5–45) |
| ≥65 yr—No. (%) | 3 (14) | 2 (17) | 0 (0) | 1 (33) | 0 (0) |
| Male sex—No. (%) | 11 (52) | 6 (50) | 2 (67) | 1 (33) | 2 (67) |
| Female sex—No. (%) | 10 (48) | 6 (50) | 1 (33) | 2 (67) | 1 (33) |
| Disease—No. (%) | |||||
| DLBCL | 17 (81) | 10 (83) | 2 (67) | 3 (100) | 2 (67) |
| MCL | 2 (10) | 1 (8) | 1 (33) | 0 (0) | 0 (0) |
| B-LBL | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (33) |
| FL | 1 (5) | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Disease stageb—No. (%) | |||||
| II | 2 (10) | 0 (0) | 1 (33) | 1 (33) | 0 (0) |
| III | 4 (19) | 4 (33) | 0 (0) | 0 (0) | 0 (0) |
| IV | 15 (71) | 8 (67) | 2 (67) | 2 (67) | 3 (100) |
| Risk stratificationc—No. (%) | |||||
| Worse than intermediate | 17 (81) | 11 (92) | 2 (67) | 2 (67) | 2 (67) |
| Intermediate or better | 4 (19) | 1 (8) | 1 (33) | 1 (33) | 1 (33) |
| ≥50% PD-L1 expressiond | 4 (19) | 2 (17) | 0 (0) | 1 (33) | 1 (33) |
| Prior therapies—No. (%) | |||||
| ≥3 prior lines of therapy | 18 (86) | 10 (83) | 3 (100) | 2 (67) | 3 (100) |
| Previous PD-1/PD-L1 mAb | 2 (10) | 1 (8) | 0 (0) | 0 (0) | 1 (33) |
| Previous auto-HSCT | 2 (10) | 1 (8) | 0 (0) | 0 (0) | 1 (33) |
| Bridging therapies applied | 10 (48) | 4 (33) | 3 (100) | 2 (67) | 1 (33) |
Abbreviations: IQR, interquartile range; yr., year(s); DLBCL, diffuse large B cell lymphoma; MCL, mantle cell lymphoma; B-LBL, B-lymphoblastic lymphoma; FL, follicular lymphoma; PCNS DLBCL, primary central nervous system DLBCL; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1; mAb, monoclonal antibody; auto-HSCT, autologous haematopoietic stem cell transplantation; IPI, international prognostic index; aaIPI, age-adjusted IPI; MIPI, mantle cell lymphoma IPI; FLIPI, follicular lymphoma IPI; NCCN, National Comprehensive Cancer Network; ALL, acute lymphoblastic leukemia.
These patients received doses other than pre-specified three dose levels because of unexpected low CAR percentages caused by the early and premature manufacturing process.
Staged as per Ann-Arbor Staging System pre-treatment.
Stratified as per IPI, aaIPI, MIPI, FLIPI score, or NCCN guideline for ALL pre-treatment.
4 patients had no available pre-treatment specimen.
Two (10%) patients were classified as stage II, while 19 (90%) patients as stage III-IV. Risk stratification was performed in 17 patients with diffuse large B cell lymphoma (DLBCL) as per international prognostic index (IPI, >60 year-old) or age-adjusted IPI (aaIPI, ≤60 year-old) score,25 presenting one (6%) with low risk, one (6%) with low-intermediate risk, ten (59%) with high-intermediate risk, and five (29%) with high risk; while a patient with follicular lymphoma (FL) was stratified as high risk as per FLIPI,26 a patient with B lymphoblastic lymphoma (B-LBL) was stratified as poor risk as per the National Comprehensive Cancer Network (NCCN) guideline,27 and two patients with mantle cell lymphoma (MCL) were stratified as high and intermediate risk as per MIPI,28 respectively (Table 1 and Supplementary Table S1). One (5%) patient had primary central nervous system (PCNS)-DLBCL. Of the 17 patients whose pre-treatment tumour samples were available, programmed death-ligand 1 (PD-L1) expression was detected in 11 (65%) patients. Four patients had >50% PD-L1 expression, among whom two had extremely high levels of PD-L1 (∼80%) (Table 1 and Supplementary Table S1). Of note, no patient reported autoimmune diseases before or after PD1-19bbz infusion.
The median age at the time of infusion was 56 years old (IQR: 46–62). All patients had experienced failure or relapses after multiple lines of previous therapy (median 5, IQR: 3–5), among which two (10%) patients had undergone autologous hematopoietic stem cell transplantation (HSCT) while none received previous CAR-T cell infusion. All patients were subjected to anti-CD20 therapy using Rituximab, and two (10%) patients had previously received monoclonal antibody targeting programmed cell death protein 1 (PD-1)/PD-L1 axis. And one (5%) patient had a history of primary refractory disease. Of note, ten (48%) patients required bridging therapy during the manufacture of CAR-T cells because of the rapid progression of primary disease (Table 1 and Supplementary Table S1).
All patients received lymphodepleting chemotherapy comprised of cyclophosphamide and fludarabine. After the preparation of CAR-T cell products, 12 patients received (2 ± 0.6) × 106 CAR-T cells/kg (dose A), three patients received (4 ± 0.4) × 106 CAR-T cells/kg (dose B), and three patients received (6 ± 0.8) × 106 CAR-T cells/kg (dose C). An extended cohort of another nine patients received the dose level of 2 × 106 CAR-T cells/kg (including Patient 7 receiving compassionate use; and Patient 8 whose CAR-T cell product failed to reach the expected dose of 4 × 106 CAR-T cells/kg). And another cohort of three patients received unscheduled doses of 0.56, 0.78, and 0.8 × 106 CAR-T cells/kg because of unexpected low CAR percentages caused by the early and premature manufacturing process (Table 1 and Supplementary Table S1).
Safety
No DLT was observed in all the cohorts.
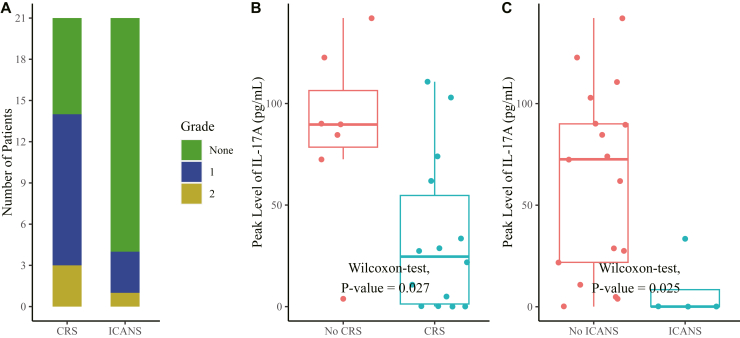
CRS was diagnosed in 14 (67%) patients, with 11 cases of grade 1 and three cases of grade 2. The median onset time of CRS was 2 days post infusion (IQR: 1–4.75) (Fig. 2A). Most CRS patients resolved with supportive antipyretic agents (e.g., ibuprofen, indomethacin), presenting a median duration of 7.5 days (IQR: 4.25–10). In addition to non-steroidal anti-inflammatory drugs (NSAIDs), Patient 6 received tocilizumab and Patient 19 received both methylprednisolone and tocilizumab. The most common symptom of CRS was fever (14/14), and other clinical manifestations included diarrhoea, chest discomfort, cough, expectoration, and chills. CRS featured elevated levels of IL-6, IFN-γ, and CRP, while the elevation of IL-17A seemed to be negatively correlated with CRS (Fig. 2B and Supplementary Fig. S1).
Fig. 2.
The incidence of CRS and ICANS. (A) Occurrence of CRS and ICANS after treatment. (B and C) The peak levels of IL-17A in the peripheral blood according to the occurrence of CRS and ICANS. The horizontal bars inside the boxes indicates the median values, the boxes indicate values range from 25th to 75th percentile, the whiskers extended from the minimum to the maximum values, and the points indicates the individual value of each patient. Unpaired Wilcoxon two-tailed test was used. Abbreviations: CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; IL, interleukin.
ICANS was observed in four (19%) patients, with three of grade 1 and one of grade 2 (Fig. 2A). There was a median interval of eight days (IQR: 7–9.5) between infusion and neurotoxicity onset, with a median duration of 3.5 days (IQR: 2.5–4.5). All the cases of ICANS occurred in the context of CRS, with five, six, eight, and ten days later following CRS onset, respectively. Neurotoxicity mainly manifested as dizziness, hypoesthesia, muscle weakness lower limbs, and sleeping disorders. All the four patients with ICANS spontaneously resolved without any countermeasures. For the patient with PCNS-DLBCL, especially, no neurotoxicity was observed. Similar to CRS, ICANS was also negatively correlated with the elevation of IL-17A (Fig. 2C).
All adverse events occurring within 30 days post infusion were listed in Table 2. The most common adverse events were hematologic toxicities, including anaemia (n = 6, 29%), lymphocyte count decreased (n = 19, 90%), neutrophil count decreased (n = 17, 81%), white blood cell count decreased (n = 10, 48%), and platelet count decreased (n = 2, 10%), among which six, four, eight, seven, and two, respectively, were prolonged cases (>30 days). The majority of cytopenia resolved without specific intervention. Two cases of neutrophil count decreased received granulocyte colony-stimulating factor (G-CSF) and a case of platelet count decreased received thrombopoietin (TPO).
Table 2.
Adverse events.
| AEa | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematologic AEs | ||||
| Anaemia | 4 (19%) | 8 (38%) | 6 (29%) | 0 (0%) |
| Lymphocyte count decreased | 0 (0%) | 2 (10%) | 3 (14%) | 16 (76%) |
| Lymphocyte count increased | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Neutrophil count decreased | 0 (0%) | 4 (19%) | 14 (67%) | 3 (14%) |
| Platelet count decreased | 2 (10%) | 8 (38%) | 2 (10%) | 0 (0%) |
| Immune system disorders | ||||
| CRS | 11 (52%) | 3 (14%) | 0 (0%) | 0 (0%) |
| ICANS | 3 (14%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Nervous system disorders | ||||
| Dizziness | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Headache | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Paraesthesia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Muscle weakness lower limb | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Psychiatric disorders | ||||
| Insomnia | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Metabolism and nutrition disorders | ||||
| Anorexia | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypercalcemia | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypertriglyceridemia | 2 (10%) | 3 (14%) | 2 (10%) | 3 (14%) |
| Hyperuricemia | 0 (0%) | 1 (5%) | 0 (0%) | 1 (5%) |
| Hyponatremia | 1 (5%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Hypoalbuminemia | 3 (14%) | 3 (14%) | 3 (14%) | 3 (14%) |
| Hypocalcaemia | 2 (10%) | 6 (29%) | 2 (10%) | 6 (29%) |
| Hypokalaemia | 4 (19%) | 2 (10%) | 4 (19%) | 2 (10%) |
| Hypomagnesemia | 2 (10%) | 0 (0%) | 2 (10%) | 0 (0%) |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Back pain | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Bone pain | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Flank pain | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pain in extremity | 4 (19%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 7 (33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Productive cough | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | ||||
| Tumour pain | 0 (0%) | 1 (5%) | 1 (5%) | 0 (0%) |
| Skin and subcutaneous tissue disorders | ||||
| Pruritus | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| General disorders and administration site conditions | ||||
| Chills | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Oedema limbs | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 7 (33%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Fever | 13 (62%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Localised oedema | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Non-cardiac chest pain | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gastrointestinal disorders | ||||
| Nausea | 4 (19%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdominal distension | 3 (14%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdominal pain | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Constipation | 3 (14%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diarrhoea | 4 (19%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dysphagia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mucositis oral | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Paraesthesia | 5 (24%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Investigations | ||||
| Activated partial thromboplastin time prolonged | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Blood lactate dehydrogenase increased | 8 (38%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Fibrinogen decreased | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Proteinuria | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Urine output decreased | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| White blood cell decreased | 1 (5%) | 11 (52%) | 4 (19%) | 5 (24%) |
Note: Data are No. (%); All AEs except for CRS were graded according to NCI CTCAE 5.0; CRS was graded according to the criteria of Lee et al.22 and American Society for Transplantation and Cellular Therapy consensus grading.23.
Abbreviations: SOC, System Organ Class; WBC, white blood cell; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome.
Listed are adverse events, which occurred within 30 days post-infusion in the 21 patients, regardless of whether the investigators attributed the events to the CAR-T cell treatment. This table did not report late-onset (with onset more than 30 days post-infusion) adverse events, including a Pneumocystis jirovecii pneumonia nine months after infusion in one patient.
Regarding infections, a patient had grade 2 enterocolitis infectious caused by Candida albicans during hospital stay, manifesting as diarrhoea, which was relieved after oral nystatin use. And the same patient developed Pneumocystis jirovecii pneumonia nine months after infusion, with last evaluation of B cells at Day 201 (6.7 months after infusion) showing continued B cell aplasia, and eventually died of the consequent disseminated infection.
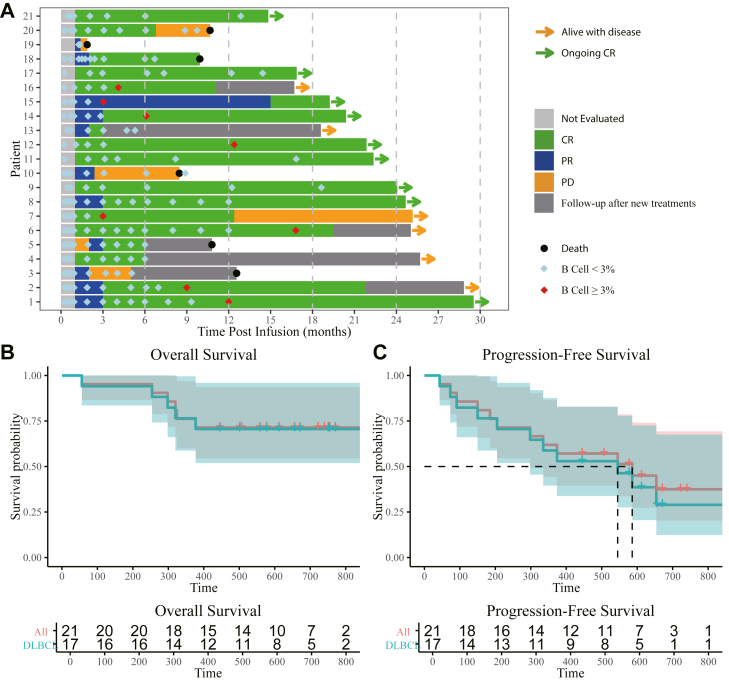
Efficacy
By October 29th 2022, 21 assessable patients were followed up for a median follow-up time of 19.2 months (IQR:12.6–24.7). According to the assessment by investigators, all of 21 (100%) patients had objective response (OR, including complete and partial responses) to PD1-19bbz, among whom 17 (81%) patients achieved a complete response (CR) as the best response while the others had a partial response (PR) (Fig. 3A). The responses started at the first evaluation at one month post infusion for all patients except for patient 5, who reached PR at month 2. Ongoing responses were observed in 17 (81%) patients at three months post infusion, including one with PR and 16 with CR. Notably, in four patients with more than 50% PD-L1 expression (Patient 1, 80%; Patient 3, 80%; Patient 14, 60%; Patient 21, 70%; Table 1 and Supplementary Table S1), three patients achieved and maintained CR at data cut-off (Patient 1, 14, and 21; Fig. 3A). The estimated median PFS was 19.5 months (95% confidence interval [CI]: 9.9–infinity), while the estimated median OS was not reached, with an OS rate of 76% (95% CI: 60%–97%) at 12 months post infusion (Fig. 3B and C). For the 17 patients with DLBCL, an CR rate of 77% (13/17) was documented, and the estimated median PFS was 18.2 months, with estimated median OS not reached (Fig. 3B and C).
Fig. 3.
Clinical response. (A) Swimmer plot showing the clinical responses and follow-up of individual patients treated with PD1-19bbz, as indicated with different colours in the swimmer lanes. Each bar represents one patient. Patients 2, 3, 4, 5, 6, 13, and 16 experienced disease relapse and received new treatments afterwards. The CD19 expression in relapsed diseases of patients 7, 10, and 20 was not available. (B) OS in all patients and the subpopulation with DLBCL. (C) PFS in all patients and the subpopulation with DLBCL. Abbreviations: CR, complete response; PR, partial response; PD, progressive disease; DLBCL, diffused large B cell lymphoma.
The characteristics of Infusion products (percentage of TSCM, CD4:CD8 ratio), infusion dose, CRS, ICANS, or the response at one month didn’t apparently influence the survival (Supplementary Fig. S4).
A total of 11 patients had relapses, among whom seven (64%) received new treatments for the relapses. Notably, CD19 antigen escape was observed in one (9%) patient, and one patient presented down-regulated CD19 expression in relapsed lymphoma. Of the six patients who died, five patients died of progressive primary disease, while one patient died of disseminated infection by P. jirovecii with his primary disease in a status of CR nine months after infusion. For the patient with PCNS DLBCL specifically, relapse in the brain was identified after a 6-month response. And after additional chemotherapies, unfortunately, she didn’t make it through a second infusion of CD19 CAR-T cells (relmacabtagene autoleucel) and died of bone marrow suppression and subsequent multiple infections.
PD1-19bbz expansion and persistence, and B cell aplasia
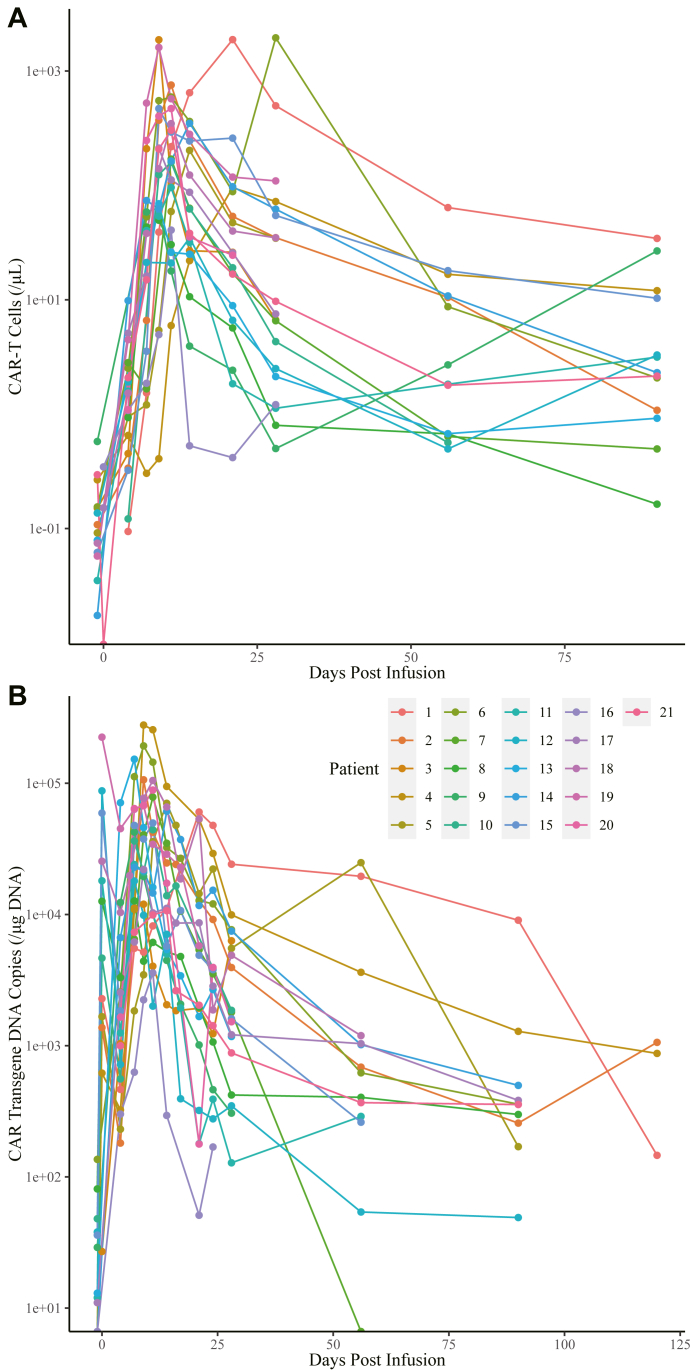
CAR-T cell expansion in peripheral blood was measured by FCM. CAR-T cells peaked between Day 9 and Day 28 with a median number of 211.87 cells/μL (IQR: 95.4–469.49) in blood (Fig. 4A). CAR copy number was monitored via qPCR in 21 patients, which peaked between Day 0 and Day 21 with a median of 61,243 copies/μg DNA (IQR: 24010–104966; Fig. 4B).
Fig. 4.
CAR-T cell expansion. (A) CAR-T cell expansion measured by the absolute number of CAR-T cells. (B) CAR-T cell expansion measured by CAR transgene copy number. Abbreviation: CAR, chimeric antigen receptor.
B cell aplasia reflects the persistence of functional CAR-T cells. During a median follow-up of 9.0 months (IQR: 5.8–12.5) for B cell aplasia, the percentages of ongoing B cell aplasia at six and 12 months were 70% and 40%, respectively (Fig. 3A). B cell reconstitution was observed in eight patients during CR/PR, of whom four patients presented subsequent relapse (three patients had CD19+ expression after relapse, and the other was not evaluated). On the contrary, other six relapsed patients maintained the depletion of B cells at or after the diagnosis of relapse (CD19− in one patient, CD19dim in one patient, CD19+ in two patients, and the other two patients were not evaluated).
The expansion and persistence of PD1-19bbz, measured by the peak of CAR-T cell number or CAR copy number, were not significantly correlated with infusion dose or AEs among patient subgroups (Supplementary Fig. S3).
Discussion
In this first-in-human, dose-escalation, phase I study, we explored the safety, efficacy, and in vivo kinetics of PD1-19bbz in 21 patients with relapsed/refractory B-NHL. In general, the toxicity profile of PD1-19bbz was manageable, with no DLT observed, and the responses were significant and durable. All patients were previously heavily treated with chemotherapies, immunotherapies, and HSCT, thus reflecting the robust efficacy of PD1-19bbz.
CRS was observed in 67% of the patients, ICANS occurred in 19% of the patients, and both were mild (grade 1–2) and self-limiting. In most cases, patients required neither steroid nor cytokine blockade therapy. Although robust CAR-T cell expansion is associated with the development of CRS and ICANS in previous studies, we didn’t find a significant correlation in our study (Supplementary Figs. S3C–S3F). Also, CRS and ICANS have no influence on the response and long-term survival of the patients. Cytopenia was the most common observed high grade (≥3) AE. Both prior treatments, including lymphodepletion, and CAR-T cells might contribute to that, but it was manageable with proper intervention. Although B cell aplasia was observed in all patients, only two cases of infection occurred. Unfortunately, Patient 18 died of Pneumocystis jirovecii pneumonia. Although the occurrence was originally considered to be correlated with defects in cell-mediated immunity, accumulating evidence from the widespread use of Rituximab suggests that B cell depletion interferes with the proper function of T cells against P. jirovecii infection.29 Finney et al. found that longer duration of B cell aplasia was associated with longer PFS after anti-CD19 CAR-T therapy.30 However, no such correlation was identified in our trial (Supplementary Fig. S4).
Excellent clinical efficacy was observed in the 21 patients with r/r B-NHL, with an ORR of 100%, a CR rate of 86%, and an estimated median PFS of 19.5 months (95% CI: 9.9–infinity) in our study. In spite of the failure of multiple prior treatments, especially immunotherapies targeting CD20 and/or PD1/PD-L1 axis and HSCT, PD1-integrated anti-CD19 CAR-T cells induced durable objective responses in all the patients. Up till now, five anti-CD19 CAR-T products have been approved for the treatment of r/r B-lymphoma globally. Among their landmark clinical trials, those conducted in cohorts primarily consisting of patients with DLBCL showed ORRs ranging from 50% to 82%, CR rates from 40% to 54%, and median PFS from <4 months to 7 months.31, 32, 33, 34 Our data suggest that the efficacy of PD1-19bbz compares favourably with other currently available CAR-T cell products.
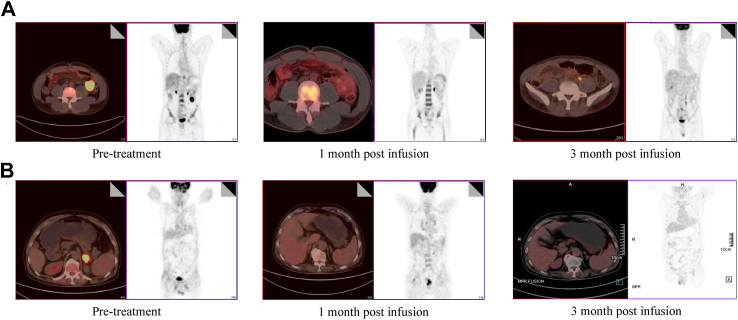
It’s noteworthy that PD1-19bbz remarkably induced enduring CR in three out of four patients with >50% PD-L1 expression in pre-treatment tumour samples (Fig. 5), and all the three patients maintained CR at the data-cut-off. PD-L1 expression has been found to be potentially related to both primary resistance to and relapse after Axicabtagene Ciloleucel treatment.35,36 Given the promising response among PD-L1 over-expressing patients in this study, PD1-19bbz may serve as a prospective option for both CAR-T-naïve or post-CAR-T-relapsed patients. We and others have demonstrated that PD-1/PD-L1 blockade, regardless of the technique used, can enhance CAR-T cell activity and rescue exhausted CAR-T cells in preclinical experiments.21,37, 38, 39 Nevertheless, the clinical results were inconsistent. On one hand, Adusumilli et al. and Heczey et al. demonstrated appreciable clinical efficacy resulting from the co-administration of CAR-T cells and pembrolizumab (a PD-1 inhibitor).40,41 On the other, a phase I study involving CAR-T cells with CRISPR-mediated disruption of PD1 and TCR genes showed poor clinical response.42 One major difference is that while administration of PD-1/PD-L1 inhibitors systemically affects immune functions, PD1 abrogation in a gene editing manner is restricted to CAR-T cells. Boulch et al. revealed massive cross-talks between CAR-T cells and tumour microenvironment (TME), which are proved to be essential for the full activation of CAR-T cells.43 The administration of CAR-T cells is capable of inducing broad activation of the host immune system, including endogenous T cells, which in turn contributes to the remission of primary disease. Consistently, Jiang et al. showed that the quantity and cytotoxic/exhausted status of endogenous T cells are correlated with the response and relapse in patients with MCL.44 Therefore, although CAR-T cell-intrinsic PD-1 blockade is able to strengthen themselves, endogenous T cells may still be suppressed by the TME. This seems to be one important reason for not acquiring high efficacy and survival rate, particularly for the long-term evaluation, since CAR-T cells tend to fade away gradually in vivo after infusion. Thus, it’s valuable to further dissect the collaboration between CAR-T cells and endogenous immune microenvironment, in order to exert the full potential of PD-1/PD-L1 blockade to boost CAR-T cell functions. Regarding gene modification methods, it is noteworthy that multiple pathways are probably altered by PD-1 disruption in CAR-T cells, which differs from the blockade strategy using inhibitors. Also, it should be noted that different preparation methods may affect the property of CAR-T cells, even if the efficiency of PD1 knockout is comparable, which provides a possible explanation for the inconsistency in another aspect. Additional attempts have also been made to switch the inhibitory signal of PD-1 to an activating signal by expressing a receptor consisting of the extracellular domain of PD-1 and the intracellular domain of CD28, which indeed improved the outcomes in patients with r/r PD-L1+ large B-cell lymphoma.45
Fig. 5.
Pre- and Post-treatment PET/CT Images of representative patients with high-level PD-L1 expression. (A) Patient 1 with a PD-L1 expression level of 80%. (B) Patient 21 with a PD-L1 expression level of 70%.
Considering the tumorigenesis risk, although the clinical results to date suggest that CAR-T cell therapy is safe, a potential safety concern still remains, since tumour-driven genes can always possibly be stimulated by the random integration of CAR sequence, which is supported by compelling evidences in rodent studies.46, 47, 48 It is undoubtable that approaches to achieve specific integration of CAR sequence are more favourable. Moreover, precise integration strategy can markedly increase the homogeneity of CAR-T cell products, preventing the over-activation of a small number of CAR-T cells and ensuring consistent potency. Importantly, this study well supports the high safety and efficacy of this kind of non-viral specifically integrated CAR-T cells in clinic. As reported previously, low-frequency off-target events were found in the PHACTR1 locus.21 Our data showed that PD1-19bbz did not cause apparent genotoxicity in treated patients with a median follow-up time near two years, demonstrating that this off-target mutation doesn’t lead to serious consequences, which is also compatible with the understanding that PHACTR1 is not expressed in T cells. Notably, since the electroporation method completes the transduction immediately after manipulation, the manufacturing time can be shortened when compared to the conventional lentivirus method, which mostly needs about 2 days. Moreover, considering the comparatively complicated manufacturing process of virus, and the concern of safety, the production of Cas9 protein, sgRNA and DNA is simpler and safe, thereby significantly reducing the cost. The expense advantage of this two-in-one approach is manifest when generating gene-modified CAR-T cells, which normally needs both virus preparation and genome editing manipulation.49,50
In summary, this phase I study demonstrates the safety and efficacy of PD1-19bbz in treating r/r B-NHL, showing promising clinical applications of non-viral genome-specific integrated CAR-T cells. Large-scale and multi-centre designed clinical trials are needed to further evaluate PD1-19bbz in the future.
Contributors
G. W., B. D., J. Z., M. L., Y. H., and H. H. designed the overall study. G. W., M. Z., S. F., R. H., L. Z., W. W., J. C., Y. H., and H. H. performed the clinical trial and provided medical care to the patients. Y. H., C. Z., M. Z., W. L., and J. Z. collected and analysed the data. G. W., C. Z., M. Z., W. L., D. W., B. D., J. Z., M. L., Y. H., and H. H. discussed the results and wrote the manuscript. B. D., J. Z., M. L., G. W., Y. H., and H. H. supervised the study. All authors contributed to the article and approved the manuscript for submission and publication. All authors confirm that they had full access to all the data in the study and the underlying data was independently verified by Y. H., C. Z., M. Z., W. L., M. L., and H. H. All authors accept responsibility for the decision to submit for publication.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Declaration of interests
This study was partially supported by BRL Medicine, Inc. Patent applications related to this manuscript have been submitted (J.Z., B.D., M.L. ‘sgRNA guiding PD1 gene for cleavage to achieve efficient integration of exogenous sequences’; J.Z., B.D., M.L. ‘Method for performing gene editing on target site in cell’). All of the authors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This study was supported by the National Key R&D Program of China (2019YFA0802802, 2018YFA0507001), the National Natural Science Foundation of China (82130003, 82270234, 81870153, 81772622), the Key Project of Science and Technology Department of Zhejiang Province (2021C03010) and Shanghai Zhangjiang National Independent Innovation Demonstration Area, Key Projects of Special Development Funds (H25).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102010.
Contributor Information
Jiqin Zhang, Email: jqzhang@bio.ecnu.edu.cn.
He Huang, Email: huanghe@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Larson R.C., Maus M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 3.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 4.Schuster S.J., Tam C.S., Borchmann P., et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 5.Munshi N.C., Anderson L.D., Shah N., et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aftab B.T., Sasu B., Krishnamurthy J., Gschweng E., Alcazer V., Depil S. Toward “off-the-shelf” allogeneic CAR T cells. Adv Cell Gene Ther. 2020;3(3):e86. [Google Scholar]
- 8.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann J., Schüßler-Lenz M., Bondanza A., Buchholz C.J. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop D.C., Clancy L.E., Simms R., et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood. 2021;138(16):1504–1509. doi: 10.1182/blood.2021010813. [DOI] [PubMed] [Google Scholar]
- 12.Magnani C.F., Gaipa G., Lussana F., et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. 2020;130(11):6021–6033. doi: 10.1172/JCI138473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnani C.F., Tettamanti S., Alberti G., et al. Transposon-based CAR T cells in acute leukemias: where are we going? Cells. 2020;9(6):E1337. doi: 10.3390/cells9061337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaj T., Gersbach C.A., Barbas C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y., Zhou Y., Zhang M., et al. CRISPR/Cas9-Engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–2772. doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Li S., Gao L., et al. Abstract CT052: clinical safety and efficacy study of TruUCAR™ GC027: the first-in-human, universal CAR-T therapy for adult relapsed/refractory T-cell acute lymphoblastic leukemia (r/r T-ALL) Cancer Res. 2020;80(16_Supplement):CT052. [Google Scholar]
- 17.Hu Y. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;21:995–1007. doi: 10.1038/s41422-022-00721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper M.L., Choi J., Staser K., et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32(9):1970–1983. doi: 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eyquem J., Mansilla-Soto J., Giavridis T., et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai X., Park J.J., Du Y., et al. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat Methods. 2019;16(3):247–254. doi: 10.1038/s41592-019-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Hu Y., Yang J., et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609(7926):369–374. doi: 10.1038/s41586-022-05140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D.W., Gardner R., Porter D.L., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 24.Cheson B.D., Fisher R.I., Barrington S.F., et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 26.Solal-Céligny P., Roy P., Colombat P., et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 27.Acute lymphoblastic leukemia guideline detail. NCCN; 2023. https://www.nccn.org/guidelines/guidelines-detail Available from: [Google Scholar]
- 28.Hoster E., Dreyling M., Klapper W., et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 29.Park J.W., Curtis J.R., Jun K.I., et al. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients receiving Rituximab. Chest. 2022;161(5):1201–1210. doi: 10.1016/j.chest.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Finney O.C., Brakke H.M., Rawlings-Rhea S., et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129(5):2123–2132. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster S.J., Bishop M.R., Tam C.S., et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 32.Abramson J.S., Palomba M.L., Gordon L.I., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet Lond Engl. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 33.Locke F.L., Ghobadi A., Jacobson C.A., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying Z., Yang H., Guo Y., et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. 2021;10(3):999–1011. doi: 10.1002/cam4.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Long-term follow-up ZUMA-1: a pivotal trial of axicabtagene ciloleucel (Axi-Cel; KTE-C19) in patients with refractory aggressive non-hodgkin lymphoma (NHL) Blood. 2017;130:578. [Google Scholar]
- 36.Jacobson C.A., Hunter B., Armand P., et al. Axicabtagene ciloleucel in the real world: outcomes and predictors of response, resistance and toxicity. Blood. 2018;132:92. [Google Scholar]
- 37.Yamaguchi Y., Gibson J., Ou K., et al. PD-L1 blockade restores CAR T cell activity through IFN-γ-regulation of CD163+ M2 macrophages. J Immunother Cancer. 2022;10(6) doi: 10.1136/jitc-2021-004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi B.D., Yu X., Castano A.P., et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7(1):304. doi: 10.1186/s40425-019-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherkassky L., Morello A., Villena-Vargas J., et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adusumilli P.S., Zauderer M.G., Rivière I., et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–2763. doi: 10.1158/2159-8290.CD-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heczey A., Louis C.U., Savoldo B., et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther J Am Soc Gene Ther. 2017;25(9):2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Li N., Feng K., et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. 2021;18(9):2188–2198. doi: 10.1038/s41423-021-00749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulch M., Cazaux M., Loe-Mie Y., et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci Immunol. 2021;6(57) doi: 10.1126/sciimmunol.abd4344. [DOI] [PubMed] [Google Scholar]
- 44.Jiang V.C., Hao D., Jain P., et al. TIGIT is the central player in T-cell suppression associated with CAR T-cell relapse in mantle cell lymphoma. Mol Cancer. 2022;21(1):185. doi: 10.1186/s12943-022-01655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H., Lei W., Zhang C., et al. CD19-specific CAR T cells that express a PD-1/CD28 chimeric switch-receptor are effective in patients with PD-L1-positive B-cell lymphoma. Clin Cancer Res. 2021;27(2):473–484. doi: 10.1158/1078-0432.CCR-20-1457. [DOI] [PubMed] [Google Scholar]
- 46.Dalwadi D.A., Torrens L., Abril-Fornaguera J., et al. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol Ther J Am Soc Gene Ther. 2021;29(2):680–690. doi: 10.1016/j.ymthe.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kustikova O.S., Geiger H., Li Z., et al. Retroviral vector insertion sites associated with dominant hematopoietic clones mark “stemness” pathways. Blood. 2007;109(5):1897–1907. doi: 10.1182/blood-2006-08-044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Themis M., Waddington S.N., Schmidt M., et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol Ther. 2005;12(4):763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- 49.Yang J., He J., Zhang X., et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022;12(7):104. doi: 10.1038/s41408-022-00694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ran T., Eichmüller S.B., Schmidt P., Schlander M. Cost of decentralized CAR T-cell production in an academic nonprofit setting. Int J Cancer. 2020;147(12):3438–3445. doi: 10.1002/ijc.33156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.