Abstract
Background
Mouth opening/breathing during sleep is common in patients with obstructive sleep apnea (OSA), which is probably associated with more water loss and higher risk for nocturnal ischemic heart attack. This study aimed to evaluate nocturnal changes in hematocrit/hemoglobin levels and estimated plasma volume loss in OSA patients and its relation to their OSA severity and mouth open/breathing.
Methods
Sixty OSA patients and fifteen healthy controls were enrolled and underwent overnight polysomnography. Mouth status was evaluated via an infrared camera and nasal/mouth airflow. Hematocrit and hemoglobin levels in peripheral venous blood were measured before and after sleep to estimate the change of plasma volume.
Results
Compared to controls, OSA patients had a greater nocturnal increase in hematocrit (1.35% vs. 1.0%, p = 0.013), hemoglobin (0.50% vs. 0.30%, p = 0.002) and more estimated water loss (5.5% vs 3.7% of plasma volume, p < 0.013). The extent of increase was correlated to apnea-hypopnea index (AHI)_the marker of OSA severity (Spearman's ρ = 0.332, p = 0.004; ρ = 0.367, p = 0.001 for hematocrit, hemoglobin, respectively), which remained significant after serial multivariate adjustment. OSA patients had more sleep time with mouth open (96.7% vs 26.7% of total sleep time, p < 0.001) and time with complete mouth breathing (14.1% vs 2.7%, p < 0.001). The extent of mouth breathing was correlated to AHI (ρ=0.487, p < 0.001), nocturnal increase in hematocrit/hemoglobin levels (ρ = 0.236, p = 0.042; ρ = 0.304, p = 0.008, respectively) and estimated plasma volume loss (ρ = 0.262, p = 0.023).
Conclusion
OSA patients had a greater increase in hematocrit/hemoglobin levels after sleep, which is probably linked to more water loss and more sleep time with mouth open/breathing.
Keywords: Obstructive sleep apnea, Sleep-disordered breathing, Hematocrit, Hemoconcentration, Mouth breathing, Water loss
Introduction
At a glance commentary
Scientific background on the subject
Obstructive sleep apnea (OSA) is characterized by repeated episodes of complete or partial obstructions of the upper airway during sleep, which leads to pause of breathing. Study suggests mouth opening/breathing during sleep is common in patients with OSA, which likely contributes to more water loss than nose breathing.
What this study adds to the field
Compared to controls, OSA patients had more mouth breathing during sleep and greater nocturnal increase of hematocrit and hemoglobin levels, which were related to the severity of OSA. Mouth open/breathing was common in OSA and linked to more water loss during sleep.
Obstructive sleep apnea (OSA) is a disorder characterized by repetitive cessation of breathing during sleep owing to collapse of the upper airway. Its prevalence was estimated to be 26% among adult population, and as high as 45–57% in obese subjects [1,2]. There is accumulated evidence showing correlation of OSA to a variety of cardiovascular diseases, including hypertension, coronary artery disease, venous thromboembolism heart failure, stroke and sudden death [3,4]. Particularly, OSA patients tend to have ischemic heart attacks [5], even fatal events [6], across the sleep period.
Dry mouth upon awakening is a common manifestation of OSA with prevalence ranging from 22.4% to 40.7%, which increased linearly with the severity of OSA [7]. Dry mouth had been attributed to more sleep time spent with mouth open in such patients [7], yet, evidence in support of this postulation is scant although studies had shown that breathing through mouth contributed to more water loss than through nostrils [8]. Moreover, dehydration-related hemoconcentration is a known risk factor for cardiovascular events [9] and may be implicated in the predisposition of nocturnal cardiac events among OSA patients.
In this study, we hypothesized that patients with OSA are subject to increased nocturnal water loss due to more sleep time with mouth/breathing. We evaluated nocturnal changes in hematocrit/hemoglobin levels and estimated plasma volume loss in OSA patients and its relation to their OSA severity and mouth status during sleep.
Materials and methods
Study design and patients
This case–control study was conducted at Taipei Veterans General Hospital, a tertiary medical center in Taiwan. The study was approved by the Institutional Review Board of Taipei Veterans General Hospital. Untreated adult OSA patients and healthy controls were enrolled after obtaining signed informed consents. In this study, OSA is defined as an AHI of 5 or greater with associated symptoms (eg, excessive daytime sleepiness, fatigue, or impaired cognition). Healthy controls came from subjects visiting our center for self-pay health check-up, who were free of sleep disorders. Subjects with rhinitis symptoms (a congested, drippy nose or chronic sneezing), acute illness (infection, diarrhea, poor intake, etc), chronic diseases (including hypertension, diabetes, cardiovascular disease, kidney disease, uremia etc), sleep disorders other than OSA (insomnia, narcolepsy etc.) or on diuretics were excluded.
Enrollees were instructed to avoid conditions possibly leading to body fluid imbalance (such as starvation, binge eating or drinking, strenuous exercise, alcohol/coffee/tea consumption) on the same day for the polysomnography. Enrollees’ demographics, body mass index (BMI), and objective sleepiness graded by Epworth Sleepiness Scale (ESS) were acquired. Obesity is defined as a BMI≥27 kg/m2 [10,11], which is consistent with the official definition by Health Promotion Administration, Ministry of Health and Welfare, Taiwan [12].
Blood tests
At the night for polysomnography, 5 ml blood samples were drawn from forearm veins before sleep (9–10 PM) and immediately transferred into ethylenediaminetetraacetic acid collection (EDTA) tubes. After blood draw, oral intake/drinking and taking a bath/shower were prohibited during the night. The complete blood count (CBC) was measured with the Beckman Coulter (LH 750 hematology analyzer) within 2 h of collection. Another blood draw was repeated in the next morning immediately after subjects awoke from sleep (5–6 AM).
Estimation of water loss
If the body water is lost without replenishment, the percentage of plasma in the blood will decrease with concomitant elevation of the hematocrit and hemoglobin levels in blood, a process called hemoconcentration [13]. In this study, water loss during sleep was assessed by the nocturnal change of hematocrit and hemoglobin levels in the peripheral blood. Percent changes in plasma volume were estimated using the Dill and Costill equation [14].
Reliability and reproducibility of measurement of hemoglobin levels in blood
The reproducibility of hematocrit and hemoglobin values was accessed by duplicate measurements from the blood samples in twelve individuals showed strong correlation between the two measurements (hematocrit: r = 0.987, p < 0.001; hemoglobin: r = 0.997, p < 0.001). If the validity of Pearson correlation was concerned due to small case number (n = 12), the non-parametric tests were also performed and similar results were obtained (Spearman's ρ = 1.0, p < 0.001 for hematocrit, Spearman's ρ = 0.996, p < 0.001 for hemoglobin). The paired differences (absolute values) between duplicate measurements were trivial for both hematocrit (median 0.6%, IQR:0.2–0.75%) and hemoglobin (median 0.05 g/dl, IQR:0–0.1 g/dl).
Polysomnography (PSG)
Attended overnight PSG was performed at the Center of Sleep Medicine in Taipei Veterans General Hospital by qualified sleep technicians, which included electro-encephalogram, electro-oculogram, chin/tibialis electromyogram, electrocardiography, oronasal airflow assessment, recording of thoracic/abdominal movements, oxyhemoglobin saturation by pulse oximetry (SpO2), body position monitoring and observation of activity/behavior via a video camera.
Overnight recordings were obtained using a digital data acquisition system (Alice software versions 5, Respironics, Murrysville, PA). Sleep stages were scored according to the criteria of Rechtschaffen and Kales [15]. Cessation of airflow lasting at least 10 s was defined as apnea. Hypopneas were identified based on airflow reduced by more than 30% for at least 10 s, followed by at least 4% desaturation [16,17].
The apnea–hypopnea index (AHI) was defined as the average number of obstructive apneas and hypopneas per hour of sleep. PSG with sleep efficiency [(total sleep time/total time in bed) x 100%] less than 70% were excluded to avoid interference from insomnia [18]. The sleep technician who performed polysomnography was blinded to the results of blood tests.
Evaluation of mouth status
During the sleep study, the status of mouth closing/opening was monitored by the sleep technician via a day & night infrared camera with pan/tilt/zoom and audio (AXIS 214 PTZ Camera) and was classified into three categories: mouth closed, mouth open<50% or >50% of total sleep time (TST).
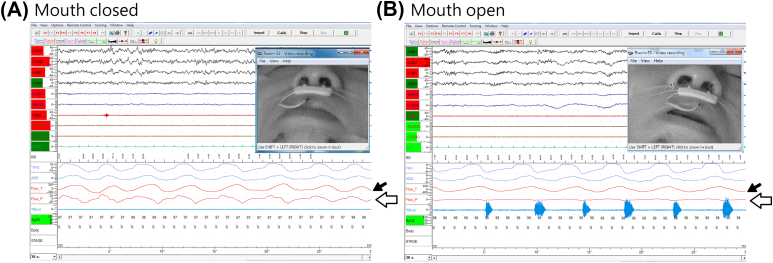
Pure mouth breathing pattern can be identified based on a polysomnogram with the recording of preserved oral airflow signal detected by the (above-the-mouth) thermistor (Pro-Tech® #P1274, Respironics) without concurrent nasal airflow signals detected by nasal airflow sensors in nasal cannulas (Pro-Tech® #P1257, Respironics) [Fig. 1]. Time spent with pure mouth breathing can be measured and expressed as percent of TST. Consistency of the two approaches were tested for trend with the Jonckheere–Terpstra test.
Fig. 1.
Evaluation of mouth status. The subject's face was monitored via a video camera. The image in the left (A) showed his mouth was closed. On the polysomnogram, airflow signals were detected both from the (one branch above-the-mouth) thermistor (black arrow) and from nasal airflow sensors in nasal cannulas (white arrow). For the same subjects with mouth open during sleep (B), oral airflow signals detected by the thermistor (black arrow) were preserved, but no nasal airflow signals were seen (white arrow).
Statistics
Statistical analysis was conducted utilizing the commercial software IBM SPSS Statistics for Windows, Version 22.0 (Released 2013. Armonk, NY: IBM Corp.). Data were presented as median (quartile). All the variables were of non-Gaussian distribution (Shapiro–Wilk test for all the p < 0.05), except age (p = 0.694) and data in reliability assessment tests (n = 12). The correlation analyses were performed with Pearson correlation for variables with Gaussian distribution (only in the reliability assessment tests) and with Spearman's correlation for variables with non-Gaussian distribution. Categorical variables between two groups were compared with Pearson's χ2 test whereas numerical variables were compared with Mann–Whitney U test or Fisher's exact test when appropriate. Paired differences were evaluated with Wilcoxon signed-rank test. Ordered differences across groups were tested with the Jonckheere–Terpstra test for trend.
In evaluating correlation of nocturnal change in hematocrit/hemoglobin to AHI (marker of OSA severity), partial Spearman's rank correlation analysis was further employed for multi-variate adjustment. The variables selected for adjustment included common demographic characteristics (age, gender) and potential confounders, such as BMI (it differed between OSA and control groups), or factors correlated to nocturnal change in hematocrit/hemoglobin (hematocrit/hemoglobin level in the morning, Table S1). ESS and hypoxic markers (SpO2 nadir, ODI, Time spent with SpO2 85–95%) were not included due to its significant correlation to AHI (data not shown), which may arise a concern of collinearity. The adjustment on sequence was shown on Table S2. Statistical significance was inferred at a two-sided p value of <0.05.
Results
Study population and patient characteristics
After exclusion of eight subjects with sleep efficiency less than 70%, 60 OSA patients and 15 healthy controls were enrolled. Clinical characteristics were not significantly different between OSA patients and controls, except controls having a lower BMI (Table 1). Regarding polysomnography, patients with OSA had a lower SpO2 nadir, a higher oxygen desaturation index (ODI) and more time experiencing hypoxemia (time spent for SpO2<95%, <90% and <85%). As to blood tests, hematocrit, hemoglobin level, and platelet count did not differ significantly between groups, but white blood cell count before and after sleep was higher in OSA patients (Morning, 6950/μL vs 5400/μL, p = 0.014; Night, 7700/μL vs 5700/μL, p = 0.033). OSA patients had greater nocturnal differences of hematocrit (1.35% vs 1.0%, p = 0.013), hemoglobin (0.5 vs 0.3 mg/dL, p = 0.002), and more estimated plasma volume loss (5.5% vs 3.7% of plasma volume, p < 0.005) than controls (Table 1).
Table 1.
Characteristics of study subjects.
| n | OSA |
Control |
p value |
|---|---|---|---|
| 60 | 15 | ||
| Age, yr | 47 (39.3–56.8) | 44 (35.0–49.0) | 0.228 |
| Male, % | 88.3 | 80.0 | 0.408† |
| BMI, kg/m2 | 28.0 (25.5–31.1) | 24.5 (22.77–25.83) | <0.001 |
| ESS score | 8 (5–12) | 6 (4–8) | 0.052 |
| Dry mouth, % | 38.3% | 13.3% | 0.066‡ |
| Polysomnography | |||
| Time in bed (min) | 363 (362–367) | 362 (359–364) | 0.065 |
| Total sleep time (min) | 321.8 (305–338.3) | 312 (271–338) | 0.223 |
| Sleep efficiency (%) | 89.2 (83.7–92.7) | 85.9 (75.8–92.9) | 0.305 |
| AHI (/hr) | 41.7 (18.0–65.1) | 1.8 (1.4–3.4) | – |
| SpO2, nadir (%) | 76.5 (69–81.8) | 90 (88–94) | <0.001 |
| ODI (/hr) | 40.2 (15.1–57.0) | 1.4 (0.5–2.7) | <0.001 |
| Time spent for | |||
| SpO2<95% (min) | 138.3 (90.1–226.2) | 4.5 (0.3–61.9) | <0.001 |
| SpO2<90% (min) | 24 (4.9–66.3) | 0 (0–0.2) | <0.001 |
| SpO2<85% (min) | 4.4 (0.5–16.6) | 0 (0–0) | <0.001 |
| Blood tests | |||
| White blood cell (103/μL) | |||
| Night | 7.70 (6.40–9.53) | 5.70 (5.40–8.70) | 0.033 |
| Morning | 6.95 (5.80–9.25) | 5.40 (4.70–6.80) | 0.014 |
| Difference | −0.5 (−1.08–−0.1) | −0.7 (−1.3–−0.3) | 0.292 |
| Hemoglobin (g/dL) | |||
| Night | 14.7 (13.70–15.28) | 14.5 (13.40–15.40) | 0.827 |
| Morning | 15.0 (14.10–15.88) | 14.8 (13.70–15.40) | 0.260 |
| Difference | 0.50 (0.20–0.78) | 0.30 (−0.20–0.40) | 0.002 |
| Hematocrit (%) | |||
| Night | 43.05 (40.75–45.23) | 43.20 (39.20–45.80) | 0.889 |
| Morning | 44.55 (42.15–47.03) | 44.20 (39.9–45.50) | 0.286 |
| Difference | 1.35 (0.50–2.30) | 1.0 (−0.2–1.20) | 0.013 |
| Platelet (103/μL) | |||
| Night | 235 (191.3–276.5) | 245 (212–305) | 0.525 |
| Morning | 235.5 (193.5–271.0) | 225 (197–262) | 0.848 |
| Difference | −4.5 (−14.0–1.75) | −14 (−25–−9) | 0.002 |
| Estimated change in plasma volume (%) | −5.5 (−2.3∼ −8.7) | −3.7 (−4.7–1.7) | <0.005 |
Data are given as median (interquartile range). p values for comparisons between two groups are determined by Mann–Whitney U test unless noted otherwise. †Fisher's exact test. ‡Pearson's χ2 test. Abbreviations: OSA: obstructive sleep apnea; AHI: apnea-hypopnea index; BMI: body mass index; ESS: Epworth Sleepiness Scale; SpO2: arterial oxyhemoglobin saturation by pulse oximetry; ODI: oxygen desaturation index.
Correlations
Nocturnal differences of hematocrit were significantly correlated to AHI (Spearman's ρ = 0.332, p = 0.004)、ESS score (ρ = 0.260, p = 0.024)、SpO2 nadir (ρ = −0.390, p = 0.001)、ODI (ρ = 0.343, p = 0.003) and time experiencing hypoxemia (time spent for SpO2<95%, <90% and <85%, ρ = 0.300, 0.406 and 0.394, p = 0.009, <0.001 and < 0.001, respectively). The correlation of nocturnal differences in hematocrit with AHI remained significant even after serial multi-variate adjustment (ρ = 0.274, p = 0.021 if adjusted with age, gender, BMI, hematocrit in the morning, please refer to Table S2). Similar results were obtained for nocturnal differences in hemoglobin (correlation to AHI, ρ = 0.367, p = 0.001, please refer to Table S1 & S2).
Nocturnal differences in hematocrit and hemoglobin levels
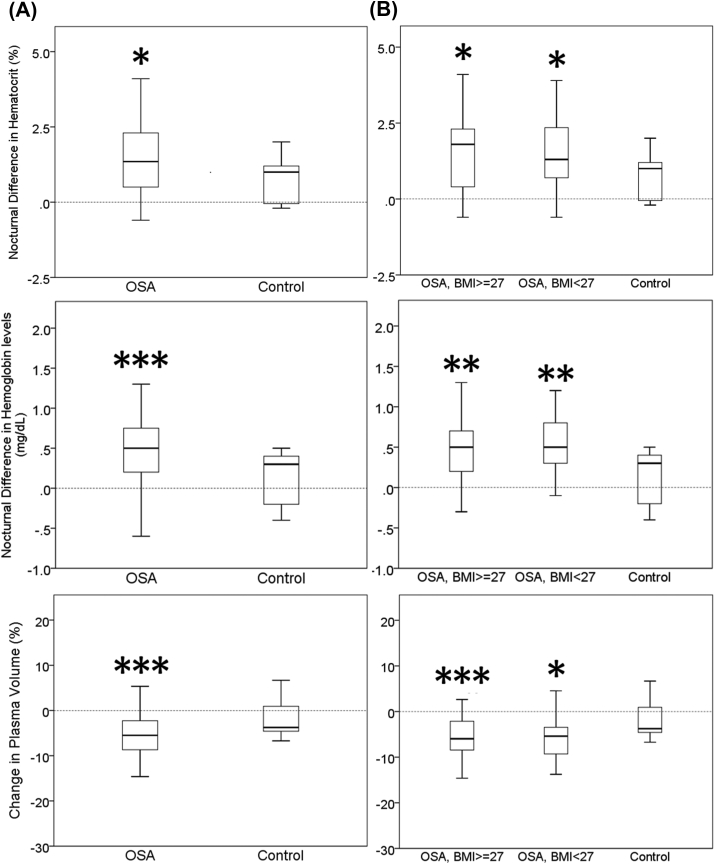
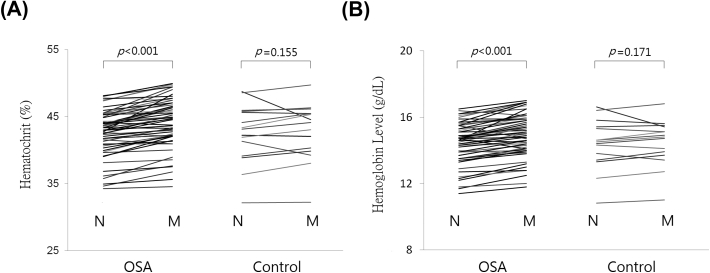
Compared with controls, OSA patients, regardless of obese or non-obese, have significantly greater nocturnal differences in hematocrit and hemoglobin levels [Fig. 2]. Similarly, paired nocturnal differences in hematocrit and hemoglobin levels were significant in OSA patients whereas changes in the control group were not significant [Fig. 3].
Fig. 2.
Nocturnal difference in hematocrit, hemoglobin level and estimated plasma volume. (A) OSA patients versus controls. (B) OSA patients were further classified into obese vs non-obese for comparison. (vs control, ∗p < 0.05; ∗∗<0.01; ∗∗∗ <0.005, Mann–Whitney U test). Obese and non-obese OSA patients did not differ significantly in nocturnal difference in hematocrit, hemoglobin level and estimated plasma volume. Abbreviations: OSA: obstructive sleep apnea.
Fig. 3.
Paired nocturnal change of hematocrit and hemoglobin levels among OSA patients versus controls. N = night, M = morning. Abbreviations: OSA: obstructive sleep apnea.
Mouth opening/breathing
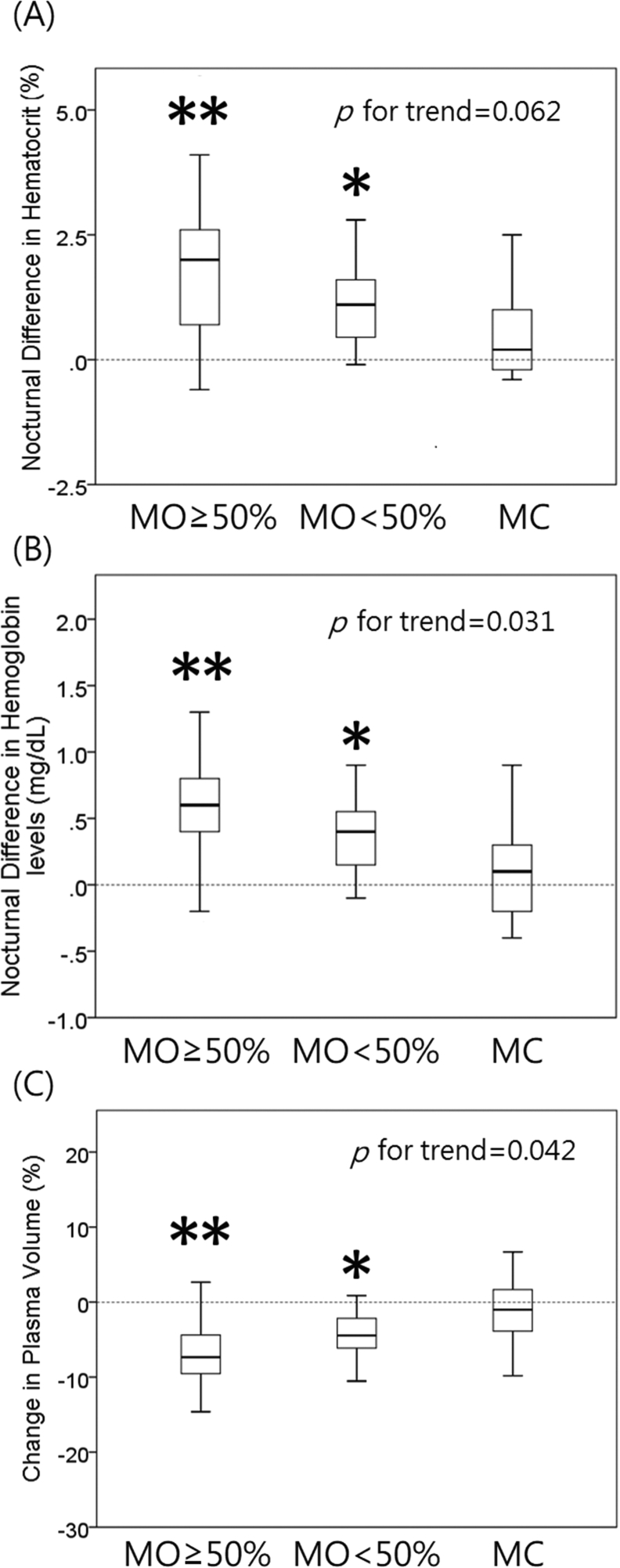
With respect to mouth status, OSA patients tended to have more sleep time with mouth open or mouth breathing in comparison to controls (Mouth open <50% TST, 41.7% vs 20%; Mouth open >50% TST, 55% vs 6.7%; p < 0.001; Table 2). Nocturnal changes in hematocrit, hemoglobin levels and estimated plasma volume accorded with the extent of mouth opening (Fig. 4, p for trend = 0.062, 0.031, 0.042, respectively). Besides, time spent with pure mouth breathing pattern was significantly correlated to nocturnal differences in hematocrit (ρ = 0.236, p = 0.042) and hemoglobin levels (ρ = 0.304, p = 0.008).
Table 2.
Mouth status during sleep among OSA patients and controls.
| OSA (n = 60) | Control (n = 15) | p valuea | |
|---|---|---|---|
| Mouth opening classified by observation via video monitoring | <0.001 | ||
| None (mouth closed) | 2 (3.3%) | 11 (73.3%) | |
| Mouth open <50% TST | 25 (41.7%) | 3 (20%) | |
| Mouth open >50% TST | 33 (55%) | 1 (6.7%) | |
| Pure mouth breathing pattern on polysomnogram, %TST [median, IQT] | 14.1 [8.7–24.2] | 2.7 [1.2–4.6] | <0.001 |
Pearson's χ2 test, Abbreviations: TST: total sleep time; IQT: interquartile range.
Fig. 4.
Relationship of mouth status and parameters of nocturnal water loss. Nocturnal difference of hematocrit (A), hemoglobin levels (B) and estimated plasma volume (C) across subjects with different extent of mouth opening, which were observed via a day & night infrared camera during polysomnography. p values for trend = 0.062, 0.031, 0.042, respectively. MO ≥ 50%: time spent with mouth open ≥50% of total sleep time, MO<50%: time spent with mouth open <50% of total sleep time, MC: mouth closed (vs MC: ∗p < 0.05, ∗∗<0.005; MO ≥ 50% vs MO<50%: p < 0.05, Mann–Whitney U test).
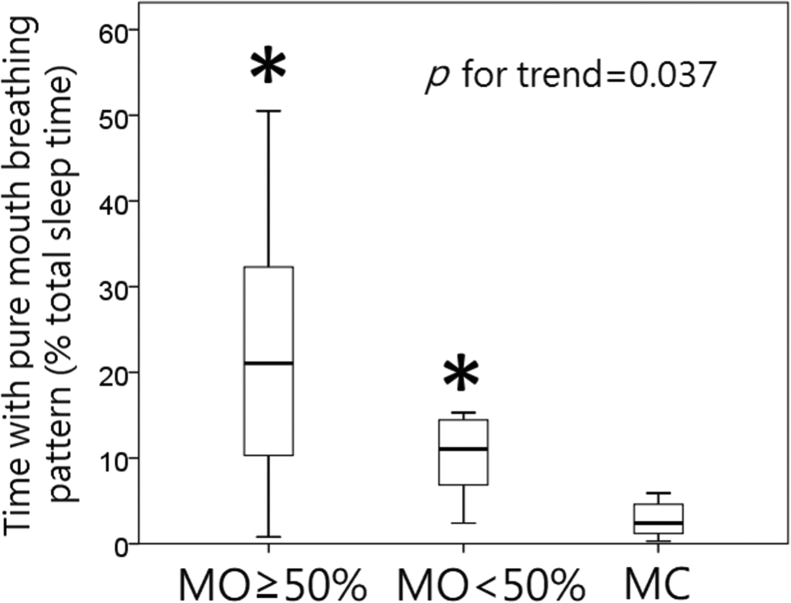
The data of mouth opening (identified via camera) and pure mouth breathing pattern (obtained from PSG) were consistent (p value for trend = 0.037, Fig. 5).
Fig. 5.
Consistency of data regarding mouth open and breathing. The data of mouth opening (identified via camera) and pure mouth breathing pattern (obtained via PSG) were consistent (p values for trend = 0.037). MO ≥ 50%: time spent with mouth open ≥50% of total sleep time, MO<50%: time spent with mouth open <50% of total sleep time, MC: mouth closed (vs MC: ∗ <0.001; MO ≥ 50% vs MO<50%: p < 0.05, Mann–Whitney U test).
Discussion
In this study, we found that OSA patients have a significant increase in hematocrit/hemoglobin levels after sleep, the extent of which was correlated to the severity of OSA, the extent of mouth open/mouth breathing and estimated plasma volume loss. It is noteworthy that the estimated plasma volume loss (median: 5.5%, IQR: 2.3–8.7%, Table 1) was greater than 8.7% in about one fourth of OSA during sleep, which may approximate the extent of body water loss after a marathon run [19]. The nocturnal change may be mild in most OSA patient and readily fixed after awakening. For subjects with defected function for replenishing water or with poor organ reserve (such as renal insufficiency), deficit of plasma volume can further compromise organ function and contribute to more severe dehydration and adverse outcomes.
Dehydration is expected to occur during sleep since no further oral intake of food or fluid in that period. For normal individuals, the extent of dehydration is mild and easily overlooked; however, the condition would be exaggerated in some circumstances, such as mouth breathing. Evidence has showed breathing through mouth contributed to more water loss than through nostrils [8]. It has been postulated that dehydration resulting from mouth open/breathing played a role in the pathogenesis of OSA, but the supporting evidence is scant. This is the reason why this study was conducted. In the current study, we demonstrated that OSA patients are prone to develop hemoconcentration during sleep, which is probably linked to a greater degree of mouth open/breathing. The finding is compatible with the previous research reporting that a higher percentage of OSA patients complained of dry mouth upon awakening and the percentage increased with severity of OSA [7].
Hemoconcentration has been identified as a risk factor for developing several cardiovascular diseases and stroke9. Besides dehydration, hemoconcentration can be induced in situations with physical and mental stress9, which could work with increased catecholamine levels, as seen in OSA [20], to promote blood aggregation [21], and therefore potentially triggers deleterious cardiovascular/cerebrovascular events [22]. Thus, hemoconcentration may provide another link to increased risks of nocturnal ischemic heart events among OSA patients and its prevention may be needed.
In the literature involving mouth breathing, its definition largely relied on history taking or on a clinical assessment in the daytime [23,24]. However, some persons who breathe through mouth during sleep may not be recognized, especially when they do not present signs of mouth breathing on daytime. In our study, mouth breathing during sleep was identified via direct observation with a camera in the sleep laboratory and confirmed with compatible flow signals, assuring the accuracy. Given that not all the mouth breathers keep breathing via nose throughout the whole night, it seems improper to simply dichotomize the subjects into nose or mouth breathers as did in the literature [23,24].
In our attempt on further classification of mouth breathing according to duration the subjects spent with mouth open during sleep, differences in hemoconcentration across groups were also demonstrated.
This study still has some limitations. Firstly, this study looked at the overnight difference in hemogram, which is a short-term change and may be modified by other factors in the long term. For example, such patients who experience dehydration will drink more water in response to thirsty during the night or on the following day. Provided the response maintaining body fluid homeostasis is intact [25], the effect of nocturnal hemoconcentration will be resolved on daytime and may not accumulate day by day theoretically. Despite this, a study by Choi et al. reported patients with severe OSA (AHI>30, 43.5%) had significantly higher hematocrit (after-sleep, p < 0.01) than patients with mild/moderate OSA or non-apneic controls [26]. It's unclear whether other mechanisms than water loss are implicated in maintenance of a higher hematocrit in severe cases, such as mental stress [9] or persistent spleen emptying [27].
Another study by Sökücü et al. also supported our conclusion. It showed that 6-month therapy of CPAP (continuous positive airway pressure) decreased hemoglobin levels and hematocrit in patients with severe OSA [28], which suggests that relief of OSA may mitigate their water loss or hemoconcentration. Yet, the authors did not disclose the baseline differences between OSA patients and controls. They also did not mention whether a humidifier had been used along with CPAP. Further research is needed to clarify the long-term consequences of nocturnal water loss among OSA patients and the impact of CPAP or humidifiers.
Moreover, nocturia is likely another cause of water loss, and we did not measure the urine amount for those who urinated during the sleep test, which is a limitation. However, intake of fluid and food intake of the enrollees were limited during preparation of polysomnography, which often lasted around 3 h (for introduction, allocation to an examination room, changing clothing, filling in [Berlin/STOP-Bang/Epworth Sleepiness Scale/Comorbidity] questionnaires, hooking-up of leads and bio-calibration) prior to the test and we also routinely ask the examinees to take a piss before sleep. Hence, the urine amount of our enrollees was minimized and may not be as much as usual. We've also performed further analyses restricted for those who did not urinate during the sleep test (36 subjects with OSA and 13 controls: 65% of total enrollees). Even though the sample size is shrunk, the conclusion is not changed. (Correlation of nocturnal change of hemoglobin/hematocrit to AHI: ρ = 0.448, p = 0.001; ρ = 0.396, p = 0.005, respectively. nocturnal change of hemoglobin/hematocrit between OSA and controls, p = 0.009, 0.065, respectively.)
Additionally, this study is also limited by its small sample size, particularly for the control group, hence lowering its power. It is partly due to exclusion of 8 subjects with poor sleep efficiency (<70%) in both groups (6 OSA subjects and 2 controls). A substantial portion (39%–58%) of OSA patients suffered from insomnia [29], which may lower the sleep efficiency, especially when tested in an unfamiliar environment. Shorter sleep time may compromise the reliability of measured time with mouth breathing and AHI values. However, even though we performed the analyses again with the 8 subjects included, similar results and conclusion were obtained (data not shown).
Lastly, even though we have identified mouth open/breathing in a simple and economic way, the exact amount of airflow from mouth and the time spent with mouth open were not measured quantitatively, which may await more elaborate research for further analysis.
Conclusion
OSA patients had a greater increase in hematocrit/hemoglobin levels after sleep, which is probably linked to more water loss and more sleep time with mouth open/breathing.
Funding
This work was supported by Grant 103DHA0100600 from Taipei Veterans General Hospital, Taipei, Taiwan.
Conflict of interest
The authors declared no potential conflict of interests.
Acknowledgement
The Institutional Review Board of Taipei Veterans General Hospital approved the study (VGHIRB No. 2013-12-008AC). All the authors declare that there is no potential conflict of interest. This work was supported by the Grant 103DHA0100600 from Taipei Veterans General Hospital, Taipei, Taiwan.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2022.05.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Romero-Corral A., Caples S.M., Lopez-Jimenez F., Somers V.K. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiestand D.M., Britz P., Goldman M., Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130:780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S., Barbe F., Campos-Rodriguez F., Dempsey J.A., Khayat R., Javaheri S., et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley T.D., Floras J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 5.Patel D.J., Knight C.J., Holdright D.R., Mulcahy D., Clarke D., Wright C., et al. Pathophysiology of transient myocardial ischemia in acute coronary syndromes. Characterization by continuous ST-segment monitoring. Circulation. 1997;95:1185–1192. doi: 10.1161/01.cir.95.5.1185. [DOI] [PubMed] [Google Scholar]
- 6.Gami A.S., Howard D.E., Olson E.J., Somers V.K. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 7.Oksenberg A., Froom P., Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res. 2006;15:317–320. doi: 10.1111/j.1365-2869.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 8.Svensson S., Olin A.C., Hellgren J. Increased net water loss by oral compared to nasal expiration in healthy subjects. Rhinology. 2006;44:74–77. [PubMed] [Google Scholar]
- 9.Allen M.T., Patterson S.M. Hemoconcentration and stress: a review of physiological mechanisms and relevance for cardiovascular disease risk. Biol Psychol. 1995;41:1–27. doi: 10.1016/0301-0511(95)05123-r. [DOI] [PubMed] [Google Scholar]
- 10.Chang H.C., Yang H.C., Chang H.Y., Yeh C.J., Chen H.H., Huang K.C., et al. Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh C.J., Chang H.Y., Pan W.H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pac J Clin Nutr. 2011;20:292–300. [PubMed] [Google Scholar]
- 12.Definition of adult obesity for Taiwaners. Health Promotion Administration Ministry of Health and Welfare; Taiwan: 2019. accessed 26 February 2019. [Google Scholar]
- 13.Lipowsky H.H., Firrell J.C. Microvascular hemodynamics during systemic hemodilution and hemoconcentration. Am J Physiol. 1986;250:H908–H922. doi: 10.1152/ajpheart.1986.250.6.H908. [DOI] [PubMed] [Google Scholar]
- 14.Dill D.B., Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 15.Hori T., Sugita Y., Koga E., Shirakawa S., Inoue K., Uchida S., et al. Proposed supplements and amendments to 'A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects', the rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci. 2001;55:305–310. doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- 16.Iber C.A.-I.S., Chesson A.L., Jr., Quan S.F. 1st ed. American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 17.Chou K.T., Liu C.C., Hsu H.S., Chang S.C., Chen Y.M., Perng D.W., et al. Nocturnal stem cell mobilization in patients with obstructive sleep apnoea: a pilot study. Eur J Clin Invest. 2014;44:1189–1196. doi: 10.1111/eci.12353. [DOI] [PubMed] [Google Scholar]
- 18.Chou K.T., Chang Y.T., Chen Y.M., Su K.C., Perng D.W., Chang S.C., et al. The minimum period of polysomnography required to confirm a diagnosis of severe obstructive sleep apnoea. Respirology. 2011;16:1096–1102. doi: 10.1111/j.1440-1843.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- 19.Maughan R.J., Whiting P.H., Davidson R.J. Estimation of plasma volume changes during marathon running. Br J Sports Med. 1985;19:138–141. doi: 10.1136/bjsm.19.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnardottir E.S., Mackiewicz M., Gislason T., Teff K.L., Pack A.I. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokinsky G., Miller M., Ault K., Husband P., Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. A preliminary investigation. Chest. 1995;108:625–630. doi: 10.1378/chest.108.3.625. [DOI] [PubMed] [Google Scholar]
- 22.Andrews N.P., Gralnick H.R., Merryman P., Vail M., Quyyumi A.A. Mechanisms underlying the morning increase in platelet aggregation: a flow cytometry study. J Am Coll Cardiol. 1996;28:1789–1795. doi: 10.1016/S0735-1097(96)00398-1. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H., Tada S., Nakanishi Y., Kawaminami S., Shin T., Tabata R., et al. Association between mouth breathing and atopic dermatitis in Japanese children 2-6 years old: a population-based cross-sectional study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izuhara Y., Matsumoto H., Nagasaki T., Kanemitsu Y., Murase K., Ito I., et al. Mouth breathing, another risk factor for asthma: the Nagahama Study. Allergy. 2016;71:1031–1036. doi: 10.1111/all.12885. [DOI] [PubMed] [Google Scholar]
- 25.McKinley M.J., Johnson A.K. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- 26.Choi J.B., Loredo J.S., Norman D., Mills P.J., Ancoli-Israel S., Ziegler M.G., et al. Does obstructive sleep apnea increase hematocrit? Sleep Breath. 2006;10:155–160. doi: 10.1007/s11325-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 27.Schagatay E., Andersson J.P., Hallen M., Palsson B. Selected contribution: role of spleen emptying in prolonging apneas in humans. J Appl Physiol. 2001;90:1623–1629. doi: 10.1152/jappl.2001.90.4.1623. [DOI] [PubMed] [Google Scholar]
- 28.Sokucu S.N., Ozdemir C., Dalar L., Karasulu L., Aydin S., Altin S. Complete blood count alterations after six months of continuous positive airway pressure treatment in patients with severe obstructive sleep apnea. J Clin Sleep Med. 2014;10:873–878. doi: 10.5664/jcsm.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luyster F.S., Buysse D.J., Strollo P.J. Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.