Abstract
Background
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) confers anti-inflammatory efficacy, which has been suggested to be effective for patients with osteoarthritis (OA). However, previous studies evaluating the influence of n-3 PUFAs supplementation in patients with OA showed inconsistent results. We performed a systematic review and meta-analysis to comprehensively evaluate the influence of n-3 PUFAs on symptom and joint function of patients with OA.
Methods
Relevant randomized controlled trials (RCTs) were obtained by searching PubMed, Embase, and Cochrane Library databases. A random-effects model was employed to combine the results.
Results
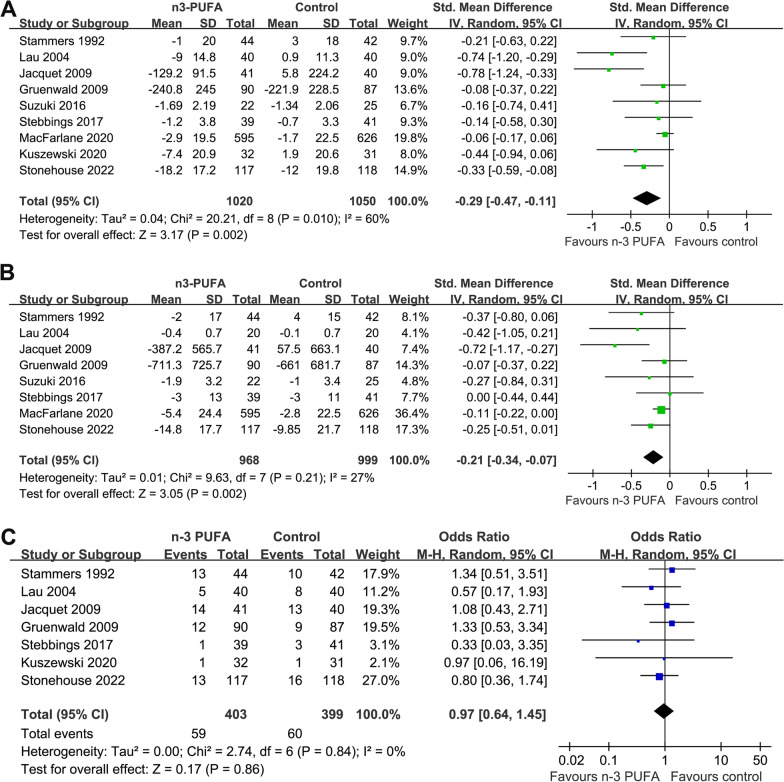
Nine RCTs with 2070 patients with OA contributed to the meta-analysis. Pooled results showed that n-3 PUFAs supplementation could significantly relieve the arthritis pain as compared to placebo (standardized mean difference [SMD]: − 0.29, 95% confidence interval [CI] − 0.47 to − 0.11, p = 0.002, I2 = 60%). Besides, supplementation with n-3 PUFAs was also associated with improved joint function (SMD: − 0.21, 95% CI − 0.34 to − 0.07, p = 0.002, I2 = 27%). Subgroup analysis showed consistent results of studies with arthritis pain and joint function evaluated by the Western Ontario-McMaster University Osteoarthritis Index and other scales (p for subgroup difference = 0.33 and 0.34, respectively). No severe treatment-related adverse events (AEs) were observed in the included patients, and the incidence of overall AEs was similar between groups (odds ratio: 0.97, 95% CI 0.64–1.45, p = 0.86, I2 = 0%).
Conclusions
Supplementation of n-3 PUFAs is effective to relieve pain and improve joint function in patients with OA.
Keywords: Osteoarthritis, Omega-3 polyunsaturated fatty acids, Arthritis pain, Joint function, Meta-analysis
Introduction
Worldwide, osteoarthritis (OA) is the most common degenerative joint disease affecting cartilage and surrounding tissues, which has become a leading cause of disability worldwide, particularly of the older population [1, 2]. With a growing elderly and obese population, the incidence of OA in recent decades has been increasing, and this has also resulted in a substantial economic burden for the global populations [3, 4]. With the improved understanding of the pathogenesis of OA, a variety of medications have been used in these patients, such as analgesics and non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate, hydroxylchloroquine, and tumor necrosis factor (TNF) inhibitors [5, 6]. However, due to the potential adverse events associated with the long-term use of these medications and the poor efficacies of these agents in some patients with OA, substantial patients have been seeking alternative and complementary agents for relieving the symptoms and improving the function of the affected arthritis [7, 8]. Omega-3 polyunsaturated fatty acids (n-3 PUFAs), mainly including eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) [9], have been suggested to be effective for patients with OA because of their efficacy for attenuating the systemic inflammatory response [10] and the catabolic environment that accelerates cartilage degradation [11]. However, previous randomized controlled trials (RCTs) evaluating the efficacy of n-3 PUFAs for patients with OA showed inconsistent results. Some studies suggested that additional supplementation of n-3 PUFAs for patients with OA could improve the arthritis pain [11–13], while the others did not [14–19]. Therefore, we performed a systematic review and meta-analysis to comprehensively evaluate the influence of n-3 PUFAs on symptom and joint function of patients with OA.
Methods
This study was designed and implemented according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [20, 21] and Cochrane Handbook guidelines [22].
Search strategy
The Medline (PubMed), Embase (Ovid), and CENTER (Cochrane Library), databases were searched for relevant studies with a combined strategy of: (1) “omega-3 fatty acids” OR “fish oil” OR fish-oil OR “polyunsaturated fatty acids” OR “marine oil” OR “eicosapentaenoic acid” OR “docosahexanoic acid” OR “DHA” OR “EPA”; (2) “osteoarthritis” OR “osteoarthritic”; and (3) “random” OR “randomly” OR “randomized” OR “randomized” OR “control” OR “placebo”. Relevant studies were limited to the studies that included human subjects. Moreover, references of related reviews and original articles were also searched. The final database searches were conducted on January 6, 2023.
Study selection
Included were studies with the following criteria: (1) full-length English articles; (2) RCTs with parallel groups; (3) patients with OA were randomly assigned to an intervention group of PUFAs supplementation on the basis of conventional treatments, and a control group of conventional treatments only (with placebo or no additional treatment); (4) one or more of the following efficacy and/or safety outcomes were reported, including the changes of arthritis pain, joint function, and the incidence of treatment related adverse events (AEs). The assessment of arthritis pain and joint function was in accordance with the methods and scales of the original studies. Non-randomized studies, studies not with patients of OA, studies not with an intervention group of n-3 PUFA supplementation, studies not with a control group of placebo or no treatment, or studies that failed to report the outcome of interest were excluded. In cases of studies with overlapped patient populations, the study with the largest sample size was included in the meta-analysis.
Data extraction and quality assessment
Data extraction, data mining, and quality evaluation were handled by two independent authors. If disagreements arose, discussions between the two authors were made to reach consensus. The following variables were extracted, such as the publication detail (first author, publication year, and study country), study design (blinded or open-label), patient characteristics (number of patients, mean age, and sex), intervention (daily supplemental dose of n-3 PUFAs, and the contents of EPA and DHA), regimen of controls, follow-up durations, and the outcomes that were reported. We evaluated the quality of the study using Cochrane's Risk of Bias Tool [22] in accordance with the following criteria: (1) randomly generation of sequences; (2) concealing allocations; (3) blinding of participants and staff; (4) blinding outcome assessors; (5) presenting incomplete outcome data; (6) reporting selective results; and (7) other potential bias.
Statistical analysis
Differences for the changes of pain and joint function after treatment between the intervention and control groups were summarized as standardized mean difference (MD) with corresponding 95% confidence interval (CI), because the scales used among the included studies were varying [22]. Influences of treatment on the outcomes of categorized variables were presented as odds ratios (OR) and corresponding CI. For detection of heterogeneity, we used Cochrane Q test [23]. A statistical analysis of heterogeneity was also conducted by estimating the I2 statistic, and an I2 > 50% suggests significant heterogeneity [24]. A random-effects model was used in the pooled analyses to account for potential heterogeneity and provide a more general conclusion [22]. In case of significant heterogeneity, a univariate meta-regression analysis was performed to explore the potential source of heterogeneity [22]. Sensitivity analysis by excluding one study at a time was performed to evaluate the robustness of the finding [22]. Moreover, subgroup analyses were performed to evaluate the potential influences of study characteristics on the outcome, such as the scales used, mean ages and sex of the patients, daily supplemental doses of n-3 PUFAs, EPA and DHA, and follow-up durations. An analysis of funnel plots and Egger’s regression asymmetry test was conducted when at least ten studies were included in order to determine publication bias [25]. Statistically significant differences were defined as p < 0.05. RevMan (version 5.1; Cochrane, Oxford, UK) and Stata (version 12.0; Stata Corporation) software were used for the statistical analyses.
Results
Search results
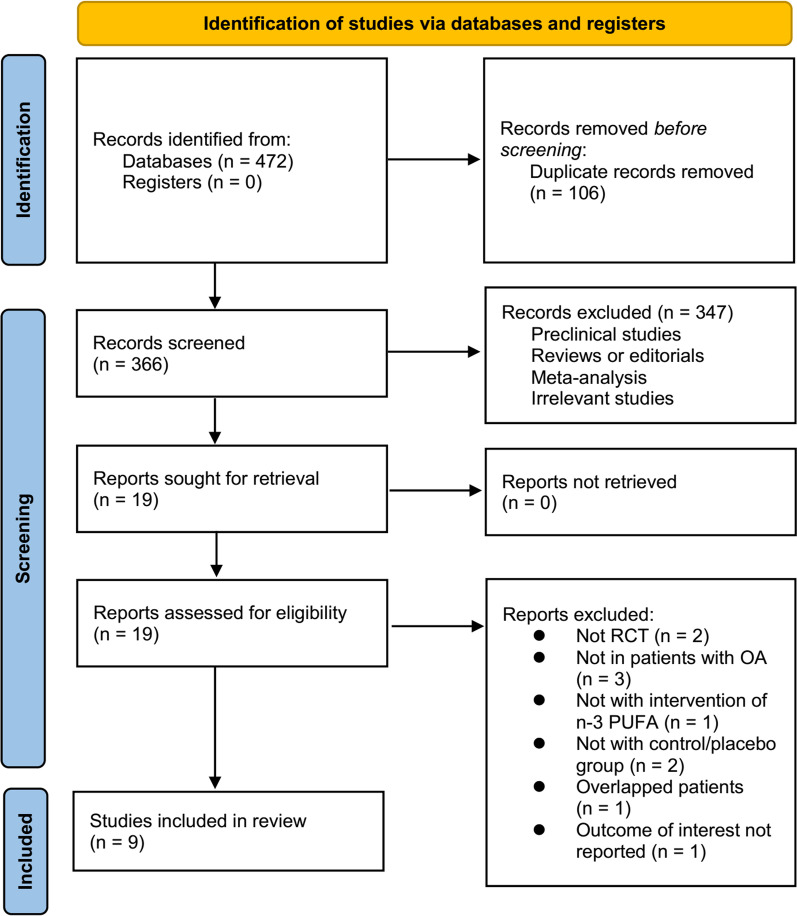
A diagram showing how to search databases and identify studies is shown in Fig. 1. By searching the database, 472 articles were obtained, and 366 were identified after excluding duplicates. Based on title and abstract, 347 of them were subsequently excluded, mainly because their objectives were irrelevant. Ten articles were further excluded from full-text review for the reasons illustrated in Fig. 1. The final analysis included nine RCTs [11–19] in total.
Fig. 1.
Flowchart of literature search
Study characteristics and data quality
An overview of the included studies is presented in Table 1. Overall, nine RCTs [11–19] including 2070 patients with OA contributed to the meta-analysis. These studies were all double-blind RCTs from the UK [14], China [11], France [12], Germany [15], Japan [16], New Zealand [17], the USA [19], and Australia [13, 18], which were published between 1992 and 2022. As for the diagnosis, three studies included patients with knee OA [13, 16, 19], four with hip or knee OA [11, 12, 15, 17], and the other two did not specify the sites of OA [14, 18]. The mean ages of the patients were 55 to 68 years, and the percentiles of men were 12–45%. A total of 1020 patients were allocated to the intervention group, who received a daily supplementation of n-3 PUFA of 350–2400 mg, and a total of 1050 patients were allocated to the control group of placebo or no additional treatment. The follow-up durations were 1 to 63 months. The arthritis pain was evaluated via visual analogue scale (VAS) in four studies [11, 14, 16, 18], and via Western Ontario-McMaster University Osteoarthritis Index (WOMAC) in five studies [12, 13, 15, 17, 19]. The joint function was evaluated via WOMAC in five studies [12, 13, 15, 17, 19], and with VAS [14], the Patients’ Global Assessment [11], and the Japanese Orthopaedic Association score [16] in the other three studies. Details of quality evaluation via the Cochrane Risk of Bias Tool for each included study are summarized in Fig. 2. All of the included studies were double-blind RCTs. Details of random sequence generation were reported in four studies [13, 15, 17, 19], and details of allocation concealment were also reported in four studies [13, 16, 17, 19]. No other publication biases such are those related to incomplete outcome data, selective reporting, or of other sources were detected.
Table 1.
Characteristics of the included studies
| Study | Country | Study design | OA site | Sample size | Mean age | Men | n-3 PUFA dose | EPA dose | DHA dose | Control | Follow-up duration | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| years | % | mg/d | mg/d | mg/d | months | |||||||
| Stammers 1992 | UK | R, DB, PC | Not specified | 86 | 68 | 27.9 | 786 | 786 | 0 | Olive oil | 6 | VAS for pain and VAS for function |
| Lau 2004 | China | R, DB, PC | Hip or knee | 80 | 62.5 | 13.8 | 1360 | 520 | 840 | Olive oil | 6 | VAS for pain and PGA for function |
| Jacquet 2009 | France | R, DB, PC | Hip or knee | 81 | 57.1 | 32.1 | 1360 | 520 | 840 | Placebo | 3 | WOMAC for pain and function |
| Gruenwald 2009 | Germany | R, DB | Hip or knee | 177 | 62.3 | 36.5 | 1332 | 800 | 532 | Palm and sunflower oil | 6 | WOMAC for pain and function |
| Suzuki 2016 | Japan | R, DB, PC | Knee | 47 | 64.7 | 12.8 | 350 | 240 | 110 | Safflower oil | 1 | VAS for pain and JOA for function |
| Stebbings 2017 | New Zealand | R, DB, PC | Hip or knee | 80 | 66.4 | 45 | 460 | 280 | 180 | Corn oil | 4 | WOMAC for pain and function |
| MacFarlane 2020 | USA | R, DB, PC | Knee | 1221 | 67.7 | 34 | 840 | 490 | 350 | Placebo | 63 | WOMAC for pain and function |
| Kuszewski 2020 | Australia | R, DB, PC | Not specified | 63 | 65.4 | NR | 2400 | 400 | 2000 | Corn oil | 4 | VAS for pain |
| Stonehouse 2022 | Australia | R, DB, PC | Knee | 235 | 55.9 | 45.1 | 880 | 600 | 280 | Vegetable oil | 6 | WOMAC for pain and function |
n-3 PUFA omega-3 polyunsaturated fatty acids; OA osteoarthritis; EPA eicosapentaenoic acid; DHA docosahexanoic acid; R randomized; DB double-blind; PC placebo-controlled; VAS visual analogue scale; PGA Patients’ Global Assessment; WOMAC Western Ontario-McMaster University Osteoarthritis Index; JOA Japanese Orthopaedic Association score
Fig. 2.
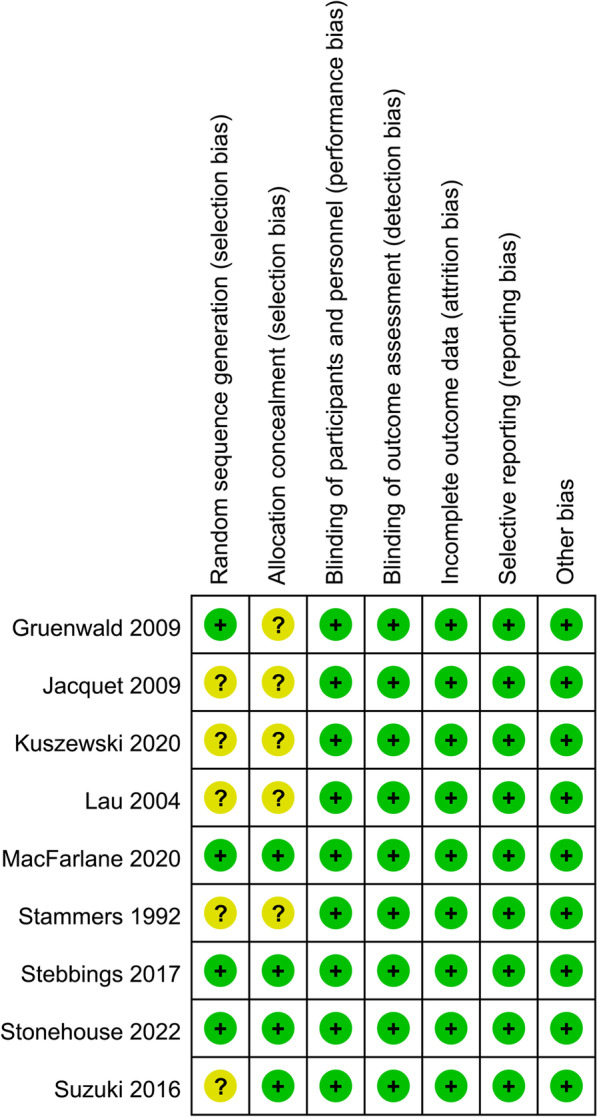
Summarized details of study quality evaluation via the Cochrane Risk of Bias Tool
Meta-analysis of n-3 PUFA supplementation on arthritis pain in patients with OA
Meta-analysis with nine studies evaluated the influences of n-3 PUFA supplementation on arthritis pain in patients with OA [11–19]. Significant heterogeneity was observed among these studies (p for Cochrane Q test = 0.01, I2 = 60%). Pooled results showed that n-3 PUFAs supplementation could significantly relieve the arthritis pain as compared to placebo (SMD: − 0.29, 95% CI − 0.47 to − 0.11, p = 0.002; Fig. 2A). Results of univariate meta-regression analysis failed to show the following variables may significantly contribute to the heterogeneity, including patient number, mean age, percentile of men, dose of n-3 PUFA, EPA, or DHA, and the follow-up duration (Table 2, p all > 0.05). Sensitivity analysis by excluding one study at a time showed consistent results (− 0.22 to − 0.34, p all < 0.05). Subgroup analysis showed that the improvement of pain after n-3 PUFA supplementation was consistent in studies with the symptom evaluated by WOMAC or VAS, in studies with n-3 PUFA supplementation < 1000 mg/d or ≥ 1000 mg/d, with EPA < 500 mg/d or ≥ 500 mg/d, with DHA < 500 mg/d or ≥ 500 mg/d, or with follow-up duration < 6 or ≥ 6 months (p for subgroup difference all > 0.05; Table 3). Interestingly, a more remarkable improvement in arthritis pain was observed in younger patients (< 65 years) than it in older patients (≥ 65 years, p for subgroup difference = 0.03; Table 3).
Table 2.
Univariate meta-regression analysis for the effects of n-3 PUFA on joint pain in patients with OA
| Covariate | Pain | ||
|---|---|---|---|
| Coefficient | 95% CI | p | |
| Patient number | 0.0003 | − 0.0001 to 0.0007 | 0.16 |
| Mean age (years) | 0.031 | − 0.012 to 0.073 | 0.13 |
| Men (%) | 0.0092 | − 0.0117 to 0.0301 | 0.33 |
| n-3 PUFA dose (mg/d) | − 0.0002 | − 0.0006 to 0.0002 | 0.21 |
| EPA dose (mg/d) | 0.0001 | − 0.0012 to 0.0014 | 0.82 |
| DHA dose (mg/d) | − 0.0003 | − 0.0007 to 0.0001 | 0.18 |
| Follow-up duration (months) | 0.005 | − 0.003 to 0.013 | 0.18 |
CI confidence interval; n-3 PUFA omega-3 polyunsaturated fatty acids; OA osteoarthritis; EPA eicosapentaenoic acid; DHA docosahexanoic acid
Table 3.
Subgroup analysis
| Arthritis pain of patients with OA | Joint function of patients with OA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study characteristics | Datasets number | SMD (95% CI) | I2 (%) | P1 | P2 | Datasets number | SMD (95% CI) | I2 (%) | P1 | P2 |
| Scale | ||||||||||
| WOMAC | 5 | − 0.23 [− 0.45, − 0.02] | 67 | 0.03 | 5 | − 0.19 [− 0.36, − 0.02] | 49 | 0.03 | ||
| Others | 4 | − 0.40 [− 0.67, − 0.13] | 19 | 0.003 | 0.33 | 3 | − 0.35 [− 0.65, − 0.05] | 0 | 0.02 | 0.34 |
| Mean age | ||||||||||
| < 65 years | 5 | − 0.40 [− 0.67, − 0.13] | 60 | 0.004 | 5 | − 0.29 [− 0.50, − 0.08] | 31 | 0.006 | ||
| ≥ 65 years | 4 | − 0.09 [− 0.19, 0.02] | 0 | 0.10 | 0.03 | 3 | − 0.12 [− 0.23, − 0.01] | 0 | 0.03 | 0.15 |
| Dose of n− 3 PUFA | ||||||||||
| < 1000 mg/d | 5 | − 0.11 [− 0.21, − 0.01] | 0 | 0.02 | 5 | − 0.14 [− 0.24, − 0.05] | 0 | 0.004 | ||
| ≥ 1000 mg/d | 4 | − 0.49 [− 0.86, − 0.11] | 69 | 0.01 | 0.09 | 3 | − 0.37 [− 0.80, 0.06] | 65 | 0.09 | 0.31 |
| Dose of EPA | ||||||||||
| < 500 mg/d | 4 | − 0.08 [− 0.19, 0.02] | 0 | 0.12 | 3 | − 0.11 [− 0.22, − 0.00] | 0 | 0.04 | ||
| ≥ 500 mg/d | 5 | − 0.39 [− 0.65, − 0.14] | 60 | 0.003 | 0.08 | 5 | − 0.31 [− 0.51, − 0.10] | 33 | 0.003 | 0.09 |
| Dose of DHA (mg/d) | ||||||||||
| < 500 mg/d | 5 | − 0.11 [− 0.21, − 0.01] | 0 | 0.02 | 5 | − 0.14 [− 0.24, − 0.05] | 0 | 0.004 | ||
| ≥ 500 mg/d | 4 | − 0.49 [− 0.86, − 0.11] | 69 | 0.01 | 0.09 | 3 | − 0.37 [− 0.80, 0.06] | 65 | 0.09 | 0.31 |
| Follow− up duration | ||||||||||
| < 6 months | 4 | − 0.39 [− 0.70, − 0.09] | 38 | 0.01 | 3 | − 0.33 [− 0.77, 0.11] | 60 | 0.15 | ||
| ≥ 6 months | 5 | − 0.23 [− 0.44, − 0.03] | 65 | 0.03 | 0.39 | 5 | − 0.14 [− 0.24, − 0.05] | 0 | 0.002 | 0.42 |
P1 p values for subgroup effect; P2 p values for subgroup difference; OA osteoarthritis; SMD standardized mean difference; CI confidence interval; n-3 PUFA omega-3 polyunsaturated fatty acids; EPA eicosapentaenoic acid; DHA docosahexanoic acid; WOMAC Western Ontario-McMaster University Osteoarthritis Index
Meta-analysis of n-3 PUFA supplementation on joint function in patients with OA
Pooled results of eight studies [11–17, 19] showed a significant improved joint function following n-3 PUFA supplementation in patients with OA (SMD: − 0.21, 95% CI: − 0.34 to − 0.07, p = 0.002; Fig. 2B) with mild heterogeneity (p for Cochrane Q test = 0.21, I2 = 27%). Sensitivity analysis by omitting one study at a time did not significantly affect the results (− 0.22 to − 0.34, p all < 0.05). In addition, subgroup analysis showed consistent results of studies with joint function evaluated by WOMAC or other scales (p for subgroup difference = 0.34, Table 3). Moreover, further subgroup analysis according to the mean age of the patients, daily dose of n-3 PUFA, EPA, and DHA, as well as the follow-up durations also showed consistent results (p for subgroup difference all > 0.05; Table 3).
Safety outcome
No severe AEs related to the treatment n-3 PUFA were reported among the included studies. Pooled results with seven studies [11–15, 17, 18] showed that the incidence of treatment-related AEs was not significantly different between groups (OR: 0.97, 95% CI 0.64–1.45, p = 0.86, I2 = 0%; Fig. 2C).
Publication bias
The funnel plots for the meta-analyses of the influences of n-3 PUFA supplementation on arthritis pain, joint function, and the incidence of AEs are shown in Fig. 3A, B, C. These plots were symmetrical on visual inspection, reflecting low risks of publication biases. In addition, results of Egger’s regression tests also confirmed the low risks of publication biases (p = 0.26, 0.31, and 0.19, respectively) (Fig. 4).
Fig. 3.
Forest plots for the meta-analysis of the efficacy and safety of n-3 PUFA supplementation in patients with OA; A, Forest plots for the meta-analysis of arthritis pain; B, Forest plots for the meta-analysis of joint function; and C, Forest plots for the meta-analysis of the incidence of treatment related AEs
Fig. 4.
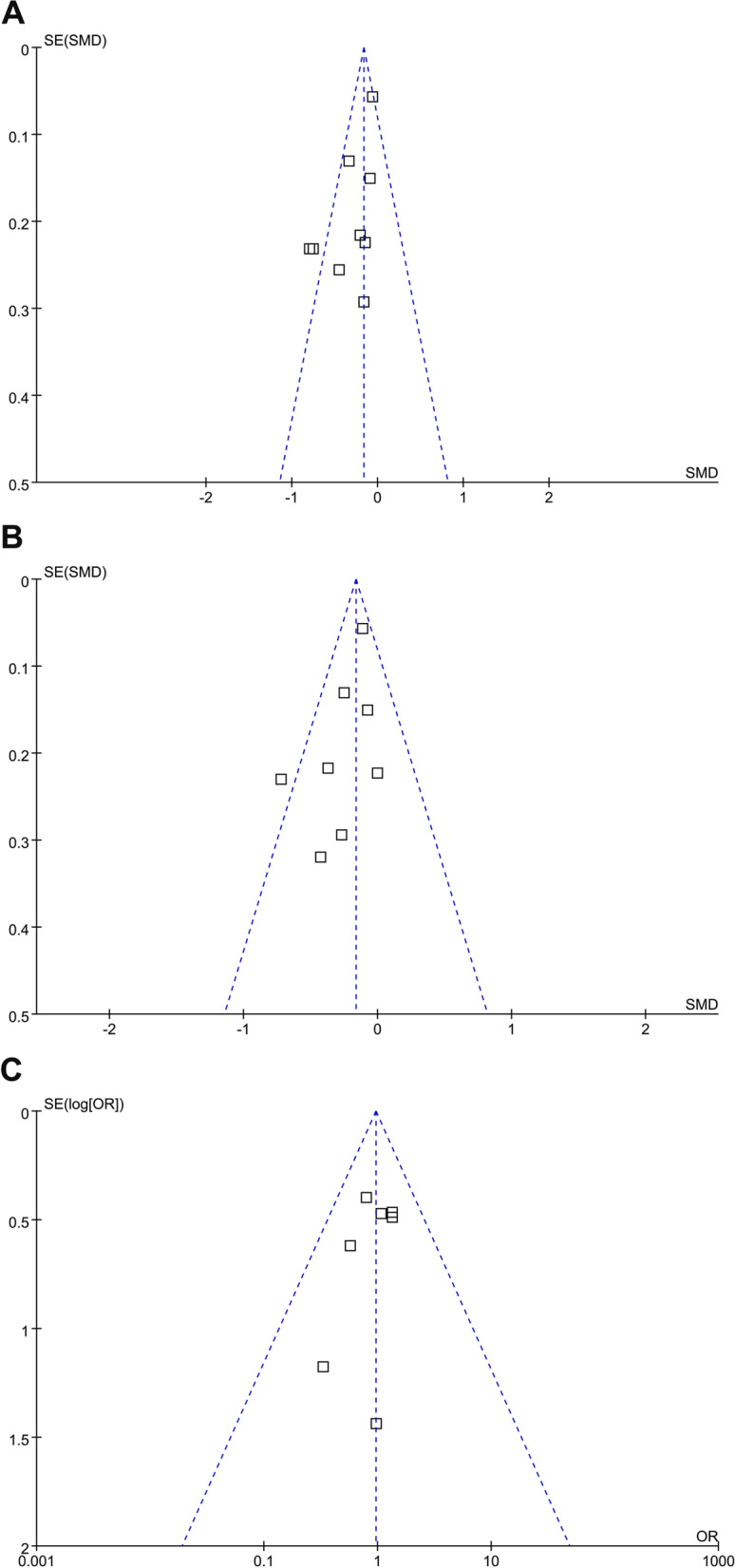
Funnel plots for the detection of the publication biases underlying the meta-analysis; A, Funnel plots for the meta-analysis of arthritis pain; B, Funnel plots for the meta-analysis of joint function; and C, Funnel plots for the meta-analysis of the incidence of treatment related AEs
Discussion
In this systematic review and meta-analysis, we pooled the results of nine relevant RCTs, and the results showed that compared to placebo or no additional treatment, supplementation of n-3 PUFA could significantly relieve arthritis pain and improve joint function in patients with OA. Moreover, no severer treatment related AEs were reported, and the incidence of overall treatment related AEs was comparable between n-3 PUFA and controls. Taking together, these results suggest that supplementation of n-3 PUFAs is effective and safe in patients with OA.
To the best of our knowledge, few meta-analyses have been published which evaluated the role of n-3 PUFAs supplementation as an alternative treatment for patients with OA. An early meta-analysis by Senftleber et al. in 2017 evaluated the influences of marine oil supplements for arthritis pain of various etiologies [26]. Although they found that supplementation of marine oil may be effective to relieve arthritis pain, most of the studies included patients with rheumatoid arthritis, and a subgroup analysis of five studies of patients with OA failed to show the potential efficacy of marine oil on arthritis pain [26]. Compared to the previous meta-analysis, our study has some potential methodological strength. First, extensive literature search was performed in three commonly used electronic databases, which could provide the up-to-date studies regarding the role of n-3 PUFA supplementation in patients with OA. Second, only RCTs were included, and all of the included studies were of double-blind design, which could minimize the influences of patient characteristics on the outcomes of the meta-analysis. Third, besides the symptom of pain, influences of n-3 PUFA supplementation on joint function was also evaluated, as well as the safety outcome. Finally, besides the main meta-analysis, a few sensitivity and subgroup analyses were performed to evaluate the robustness of the findings. The generally consistent results of these analyses further confirmed the stability of the results.
Overall, we found that n-3 PUFA is effective in relieving pain and improving joint function in patients with OA. The mechanisms underlying the benefits of n-3 PUFA may be multifactorial. A previous study in a cellular model for canine OA suggested that EPA and DHA supplementation could reduce the expression of multiple inflammatory markers involved in the pathogenesis of cartilage degeneration, such as interleukin-1 beta (IL-1β) and inducible nitric oxide synthase [27]. A similar mechanism has also been revealed in leptin-induced cartilage degeneration, which showed that supplementation with EPA and DHA could reduce IL-1β-induced activation of nuclear factor-κB and c-Jun N-terminal kinase, thereby attenuating leptin-induced cartilage degeneration [28]. Besides, EPA has also been reported to reduce oxidative stress-induced apoptosis and matrix loss of chondrocytes by inhibiting metalloproteinases13 expression and chondrocyte apoptosis [29]. In addition, a previous study also showed that in a rat model of anterior cruciate ligament transection induced OA, DHA is effective to restrain bone remodeling and vessel formation in the osteochondral unit [30]. Furthermore, a recent study showed that maresin-1, a metabolite of DHA, also confers the therapeutic efficacy for OA in a rat model via its potential anti-inflammatory effects [31]. Studies are warranted to determine the key molecular pathways underlying the potential therapeutic efficacy of n-3 PUFA for OA.
Significant heterogeneity was observed for the meta-analysis of the influence of n-3 PUFA supplementation on arthritis pain in patients with OA. However, subsequent meta-regression and subgroup analysis failed to show that the heterogeneity could be explained by the predefined study characteristics, such as sample size, patient age, dose of n-3 PUFA, DHA, EPA, follow-up duration, or the scales for assessment of pain. From our perspective, the serum level of n-3 PUFA of the patients may be an important determinant for the effects of n-3 PUFA supplementation for OA. For patients with adequate dietary intake of n-3 PUFA (such as patients with habitual intake of fish), additional supplementation is unnecessary and of limited efficacy [32]. Unfortunately, none of the included studies reported the baseline level of n-3 PUFA in these patients. Further studies are warranted our hypothesis. Interestingly, for the outcome of arthritis pain, results of the meta-analysis suggested that benefits of n-3 PUFA supplementation may be more remarkable in younger patients (< 65 years) as compared to older patients (≥ 65 years). This may be explained that in older adults, OA is likely to frequently exist alongside other common chronic conditions which may deteriorate the symptom and function of the joint, such as diabetes [33], which limited the efficacy of n-3 PUFA supplementation. Similarly, studies are warranted in the future for further validation. Additionally, subgroup analyses failed to show more remarkable benefits of high-dose versus low-dose n-3 PUFA in patients with OA, which is also consistent with the finding of a previous clinical study [34].
This meta-analysis also has several limitations. First, the number of included study is limited. The results of the meta-analysis should be validated in large-scale RCTs, and the results of the subgroup analyses should be interpreted with caution. In addition, the optimal dose, components (ratio of EPA to DHA), and treatment duration of n-3 PUFA supplementation for OA remains to be determine. Furthermore, differences of n-3 PUFA doses and follow-up durations in different studies may affect the outcome of the meta-analysis, which may be a limitation of the current meta-analysis. In view of the fact that these variations may lead to clinical heterogeneity among the included studies, a random-effects model was used to pool the results. Besides, we performed meta-regression and subgroup analyses to evaluate if these factors may have significant influence on the outcome. However, these subsequent meta-regression and subgroup analyses failed to show that difference of these factors have a significant influence on the results of the meta-analysis. Moreover, as mentioned previously, influences of dietary habits and concomitant treatment on the efficacy of n-3 PUFA supplementation for OA should also be investigated in the future. Besides, OA of different sites were included. Studies are needed to determine if the benefits of n-3 PUFA supplementation for OA are consistent according to the sites of OA. Finally, long-term clinical studies with adequate sample size may be considered to explore the influence of n-3 PUFA supplementation on clinical outcomes in patients with OA.
Conclusions
To sum up, results of the meta-analysis indicate that supplementation of n-3 PUFAs is effective to relieve pain and improve joint function in patients with OA, without increasing the risk of treatment related AEs. These findings support the use of n-3 PUFAs supplementation as an alternative treatment for OA.
Acknowledgements
None.
Author contributions
WD, HY and XY designed the study. WD and ZY performed database search, literature review, study quality evaluation, and data collection. WD, EY, and RL performed statistical analyses and interpreted the results. WD and XY drafted the manuscript. All authors revised the manuscript and approved the submission of the work.
Funding
This study was supported by the Natural Science Foundation of Hubei Province (NO. ZRMS2021000624).
Availability of data and materials
The present study was a meta-analysis of previous published studies.
Declarations
Ethics approval and consent to participate
Not applicable. This paper does not involve research on humans.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawker GA, King LK. The burden of osteoarthritis in older adults. Clin Geriatr Med. 2022;38(2):181–192. doi: 10.1016/j.cger.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Quicke JG, Conaghan PG, Corp N, Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthr Cartil. 2022;30(2):196–206. doi: 10.1016/j.joca.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthr Cartil. 2022;30(2):184–195. doi: 10.1016/j.joca.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C. Economic and humanistic burden of osteoarthritis: a systematic review of large sample studies. Pharmacoeconomics. 2016;34(11):1087–1100. doi: 10.1007/s40273-016-0424-x. [DOI] [PubMed] [Google Scholar]
- 5.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology (Oxford) 2018;57(suppl_4):iv61–iv74. doi: 10.1093/rheumatology/key011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieu S, Soubrier M, Peirs C, Monfoulet LE, Boirie Y, Tournadre A. A meta-analysis of the impact of nutritional supplementation on osteoarthritis symptoms. Nutrients. 2022 doi: 10.3390/nu14081607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordingley DM, Cornish SM. Omega-3 Fatty acids for the management of osteoarthritis: a narrative review. Nutrients. 2022 doi: 10.3390/nu14163362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppedisano F, Bulotta RM, Maiuolo J, Gliozzi M, Musolino V, Carresi C, et al. The Role of Nutraceuticals in Osteoarthritis Prevention and Treatment: Focus on n-3 PUFAs. Oxid Med Cell Longev. 2021;2021:4878562. doi: 10.1155/2021/4878562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abshirini M, Ilesanmi-Oyelere BL, Kruger MC. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog Lipid Res. 2021;83:101113. doi: 10.1016/j.plipres.2021.101113. [DOI] [PubMed] [Google Scholar]
- 12.Lau CS, Chiu PKY, Chu EMY, Cheng IYW, Tang WM, Man RYK, et al. Treatment of knee osteoarthritis with Lyprinol®, lipid extract of the green-lipped mussel-a double-blind placebo-controlled study. Prog Nutr. 2004;6(1):17–31. [Google Scholar]
- 13.Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192. doi: 10.1186/ar2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stonehouse W, Benassi-Evans B, Bednarz J, Vincent AD, Hall S, Hill CL. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2022;116(3):672–685. doi: 10.1093/ajcn/nqac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128–129. doi: 10.1136/ard.51.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858–871. doi: 10.1007/s12325-009-0060-3. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Fukushima M, Sakuraba K, Sawaki K, Sekigawa K. Krill oil improves mild knee joint pain: a randomized control trial. PLoS One. 2016;11(10):e0162769. doi: 10.1371/journal.pone.0162769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stebbings S, Gray A, Schneiders AG, Sansom A. A randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of a novel green-lipped mussel extract -BioLex(R) -for managing pain in moderate to severe osteoarthritis of the hip and knee. BMC Complement Altern Med. 2017;17(1):416. doi: 10.1186/s12906-017-1907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuszewski JC, Wong RHX, Howe PRC. Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity. Rheumatol Adv Pract. 2020;4(2):rkaa036. doi: 10.1093/rap/rkaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarlane LA, Cook NR, Kim E, Lee IM, Iversen MD, Gordon D, et al. The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol. 2020;72(11):1836–1844. doi: 10.1002/art.41416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. 2021. www.training.cochrane.org/handbook.
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senftleber NK, Nielsen SM, Andersen JR, Bliddal H, Tarp S, Lauritzen L, et al. Marine oil supplements for arthritis pain: a systematic review and meta-analysis of randomized trials. Nutrients. 2017 doi: 10.3390/nu9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler N, Schoeniger A, Fuhrmann H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2018;102(2):e623–e632. doi: 10.1111/jpn.12804. [DOI] [PubMed] [Google Scholar]
- 29.Phitak T, Boonmaleerat K, Pothacharoen P, Pruksakorn D, Kongtawelert P. Leptin alone and in combination with interleukin-1-beta induced cartilage degradation potentially inhibited by EPA and DHA. Connect Tissue Res. 2018;59(4):316–331. doi: 10.1080/03008207.2017.1385605. [DOI] [PubMed] [Google Scholar]
- 30.Sakata S, Hayashi S, Fujishiro T, Kawakita K, Kanzaki N, Hashimoto S, et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J Orthop Res. 2015;33(3):359–365. doi: 10.1002/jor.22767. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Zhou W, Zhong Z, Yu H, Zhang P, Shen H. Docosahexaenoic acid inhibits bone remodeling and vessel formation in the osteochondral unit in a rat model. Biomed Pharmacother. 2019;114:108811. doi: 10.1016/j.biopha.2019.108811. [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Feng X, Zhang H, Wei Y, Yang Y, Tian Y, et al. Maresin-1 suppresses IL-1beta-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-kappaB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect Tissue Res. 2021;62(5):508–518. doi: 10.1080/03008207.2020.1780218. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, Marchand NE, Driban JB, McAlindon T, Eaton CB, Lu B. Dietary patterns and progression of knee osteoarthritis: data from the osteoarthritis initiative. Am J Clin Nutr. 2020;111(3):667–676. doi: 10.1093/ajcn/nqz333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veronese N, Cooper C, Reginster JY, Hochberg M, Branco J, Bruyere O, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49(1):9–19. doi: 10.1016/j.semarthrit.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill CL, March LM, Aitken D, Lester SE, Battersby R, Hynes K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis. 2016;75(1):23–29. doi: 10.1136/annrheumdis-2014-207169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present study was a meta-analysis of previous published studies.