Abstract
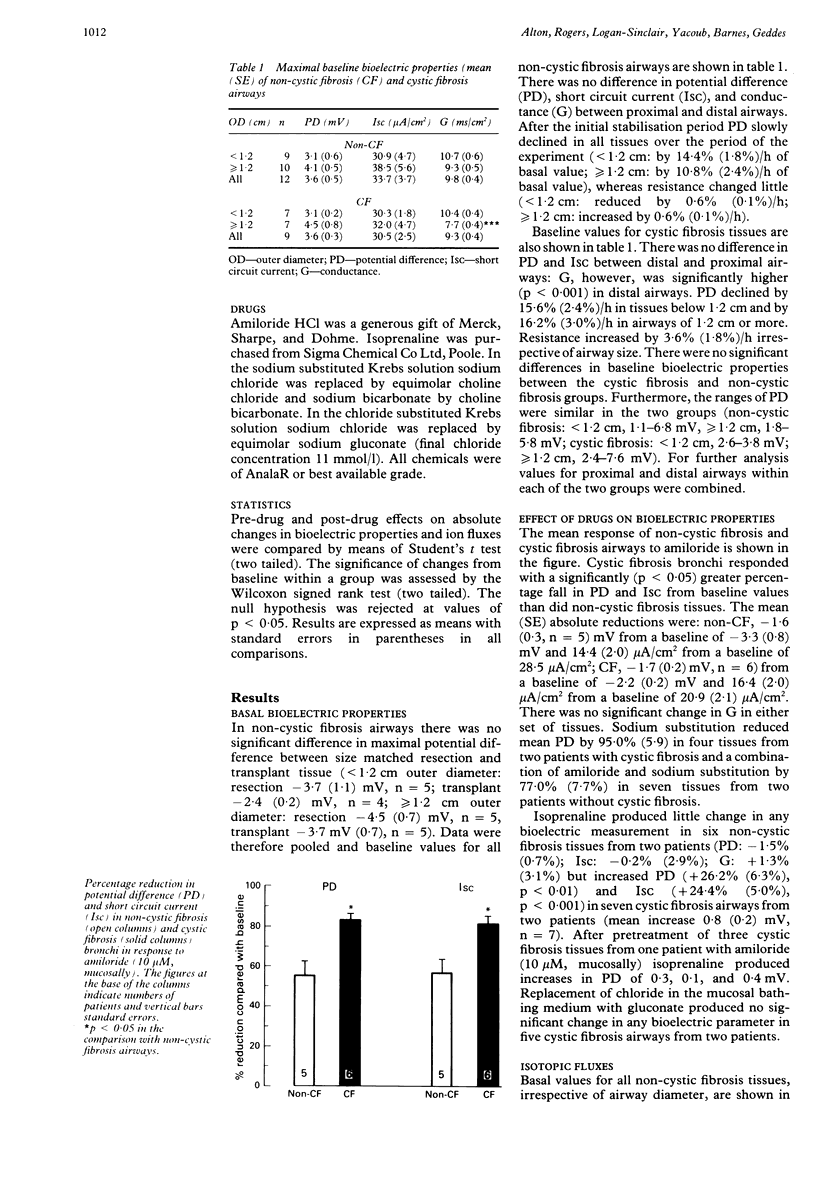
BACKGROUND: The basic defect in cystic fibrosis centres on abnormal ion transport in affected tissues such as the respiratory tract. Heart-lung transplantation provides a limited supply of native lower airways from these patients. The feasibility of in vitro studies of bioelectric properties and ion fluxes in lower airways, obtained at heart-lung transplantation from patients with cystic fibrosis, has been assessed. Comparison was made with airways from patients without cystic fibrosis. METHODS AND RESULTS: Tissue segments were mounted in Ussing chambers under open circuit conditions. The basal potential difference in tissues from nine patients with cystic fibrosis was -3.6 mV (SE 0.3 mV), not different from tissues from 12 patients without cystic fibrosis of -3.6 mV (0.5 mV). Amiloride (10 microM) caused a significantly greater fall in potential difference in bronchi from patients with cystic fibrosis (83.5% (SE 2.9%)) than in those from controls (55.1% (7.1%)). Isoprenaline (100 microM) produced no significant change in bioelectric properties in non-cystic fibrosis tissues, but induced a 26.2% (6.3%) increase in potential difference in cystic fibrosis airways. The latter response was reduced by amiloride pretreatment. Mucosal chloride substituted Krebs-Henseleit solution caused no change in bioelectric properties in cystic fibrosis airways. Sodium substituted Krebs solution produced a substantial fall in potential difference similar in magnitude to that seen after amiloride. Isotropic flux measurements showed no significant differences between non-cystic fibrosis and cystic fibrosis airways. No net movement of Na+ or Cl- was detected under open circuit conditions in either group. CONCLUSIONS: Cystic fibrosis bronchi obtained at heart-lung transplantation provide a viable source of tissue for in vitro studies of bioelectric properties. The increased response to amiloride characteristic of the upper airways in cystic fibrosis is retained in these tissues, as is the reduced chloride conductance. Although no differences in isotopic fluxes were seen between non-cystic fibrosis and cystic fibrosis tissues, heavily infected airways from patients with cystic fibrosis may not be suitable for ion flux measurements.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton E. W., Khagani A., Taylor R. F., Logan-Sinclair R., Yacoub M., Geddes D. M. Effect of heart-lung transplantation on airway potential difference in patients with and without cystic fibrosis. Eur Respir J. 1991 Jan;4(1):5–9. [PubMed] [Google Scholar]
- Berger H. A., Anderson M. P., Gregory R. J., Thompson S., Howard P. W., Maurer R. A., Mulligan R., Smith A. E., Welsh M. J. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest. 1991 Oct;88(4):1422–1431. doi: 10.1172/JCI115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Gatzy J. T. Regional differences in bioelectric properties and ion flow in excised canine airways. J Appl Physiol Respir Environ Exerc Physiol. 1981 Sep;51(3):706–714. doi: 10.1152/jappl.1981.51.3.706. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton C. U., Lawson E. E., Boucher R. C., Gatzy J. T. Bioelectric properties and ion transport of airways excised from adult and fetal sheep. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1542–1549. doi: 10.1152/jappl.1983.55.5.1542. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Brayden D. J., Dunne A., Smyth R. L., Wallwork J. Altered sensitivity to amiloride in cystic fibrosis. Observations using cultured sweat glands. Br J Clin Pharmacol. 1990 Feb;29(2):227–234. doi: 10.1111/j.1365-2125.1990.tb03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Steel D. M., Alton E. W., Geddes D. M. Second-messenger regulation of sodium transport in mammalian airway epithelia. J Physiol. 1992;453:475–491. doi: 10.1113/jphysiol.1992.sp019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Yankaskas J. R., Stutts M. J., Willumsen N. J., Boucher R. C. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989 Jun 23;244(4911):1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981 Dec 17;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Kopelman H., Durie P., Gaskin K., Weizman Z., Forstner G. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med. 1985 Feb 7;312(6):329–334. doi: 10.1056/NEJM198502073120601. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Chloride impermeability in cystic fibrosis. Nature. 1983 Feb 3;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Knowles M. R., Gatzy J. T., Boucher R. C. Oxygen consumption and ouabain binding sites in cystic fibrosis nasal epithelium. Pediatr Res. 1986 Dec;20(12):1316–1320. doi: 10.1203/00006450-198612000-00026. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Davis C. W., Boucher R. C. Cellular Cl- transport in cultured cystic fibrosis airway epithelium. Am J Physiol. 1989 May;256(5 Pt 1):C1045–C1053. doi: 10.1152/ajpcell.1989.256.5.C1045. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Davis C. W., Boucher R. C. Intracellular Cl- activity and cellular Cl- pathways in cultured human airway epithelium. Am J Physiol. 1989 May;256(5 Pt 1):C1033–C1044. doi: 10.1152/ajpcell.1989.256.5.C1033. [DOI] [PubMed] [Google Scholar]


