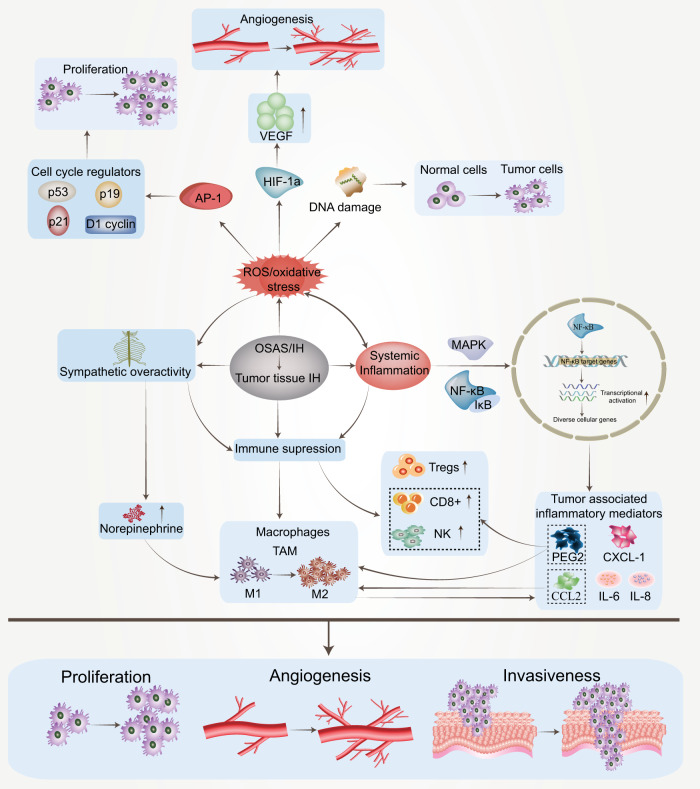
Abstract
Obstructive sleep apnea syndrome (OSAS) is a common breathing disorder in sleep in which the airways narrow or collapse during sleep, causing obstructive sleep apnea. The prevalence of OSAS continues to rise worldwide, particularly in middle-aged and elderly individuals. The mechanism of upper airway collapse is incompletely understood but is associated with several factors, including obesity, craniofacial changes, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck. The main characteristics of OSAS are recurrent pauses in respiration, which lead to intermittent hypoxia (IH) and hypercapnia, accompanied by blood oxygen desaturation and arousal during sleep, which sharply increases the risk of several diseases. This paper first briefly describes the epidemiology, incidence, and pathophysiological mechanisms of OSAS. Next, the alterations in relevant signaling pathways induced by IH are systematically reviewed and discussed. For example, IH can induce gut microbiota (GM) dysbiosis, impair the intestinal barrier, and alter intestinal metabolites. These mechanisms ultimately lead to secondary oxidative stress, systemic inflammation, and sympathetic activation. We then summarize the effects of IH on disease pathogenesis, including cardiocerebrovascular disorders, neurological disorders, metabolic diseases, cancer, reproductive disorders, and COVID-19. Finally, different therapeutic strategies for OSAS caused by different causes are proposed. Multidisciplinary approaches and shared decision-making are necessary for the successful treatment of OSAS in the future, but more randomized controlled trials are needed for further evaluation to define what treatments are best for specific OSAS patients.
Subject terms: Respiratory tract diseases, Metabolic disorders
Introduction
OSAS is a highly prevalent sleep-related breathing disorder characterized by hypopnea and apnea in ventilation. These breathing disturbances cause IH, which leads to blood hypoxemia, hypercapnia, fragmented sleep, recurrent nocturnal arousals, enhanced respiratory effort, and increased sympathetic nerve activity.1,2 Epidemiologic studies have documented the incidence of OSAS in the general population aged 30–60 years to be 24% in men and 9% in women,3,4 and a recent study reported almost 1 billion affected people globally,5 which has aroused extremely important concern (Table 1). Obesity, age, and sex have been identified as risk factors for OSAS, and other risk factors are related to ethnicity, family history, and poor lifestyle habits (alcoholism and smoking).6,7 The risk of OSAS correlates with body mass index (BMI), in which OSAS increases progressively with increases in BMI, most likely related to upper airway narrowing due to excess fat tissue.8 Obesity can induce a decrease in vital capacity, an imbalance in the ventilation-perfusion ratio, and limitations of lung and chest wall movement.8 As a result of this association, the countries with the highest incidence of OSAS are those with high rates of obesity, and thus, the incidence of OSAS increases with increasing levels of obesity.9 OSAS can occur at all ages, the incidence of OSAS has a tendency to increase with age, and the number of apnea events occurring during the night is usually higher in healthy older people than in middle-aged adults, reaching a plateau after approximately 65 years of age.8,10,11 Male sex is an independent risk factor for OSAS, with a male predominance and an estimated male-to-female prevalence of 1.5:1,12 and the reasons for this disparity are incompletely understood. The prevalence of OSAS increases in postmenopausal women, probably because body fat is redistributed to the upper body.13,14 In addition, the protective effects of female hormones, such as progesterone and estrogen, are decreased in the postmenopausal period.15 Symptoms of OSAS appear nonspecific and include snoring, apnea, arousal, and daytime sleepiness. Table 2 shows that day and night can be distinguished with respect to the major signs and symptoms of OSAS.1,16 According to current international recommendations, the diagnosis of OSAS is made after a sleep examination, and polysomnography (PSG) monitoring is applied as a method to diagnose OSAS, with the application of the 2017 scoring rules.17 These rules define apnea as a 90% reduction in airflow that lasts at least 10 s. Hypoventilation is defined as a decrease in flow of at least 50% and a decrease in oxygen saturation of 3% for at least 10 s. The severity of OSAS is distinguished clinically by the number of apnea–hypopneas per hour of sleep and the apnea-hypopnea index (AHI). AHI <5 is defined as no sleep apnea, AHI 5–15 as mild OSAS, AHI 15–30 as moderate OSAS, and AHI >30 as severe OSAS, and sleep apnea events identified in the sleep record of individuals without any symptoms are not considered OSAS unless AHI >15.17,18
Table 1.
Incidences of apnea and hypopnea frequencies in various parts of the world
| Country/Region | Study population | Year(s) of data collection | Age range (years) | Scoring criteria | AHI ≥5 | AHI ≥15 | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||||
| USA | 1520 adult employed individuals in the Wisconsin Sleep Cohort Study | 1988–2011 | 30–70 | AASM 2007 | 33.9% | 17.4% | 13.0% | 5.6% | Peppard et al. (2013)9 |
| USA | 5,804 participants in the Sleep Heart Health Study Cohort | 1995–2006 | å 40 | AASM 2012 | 32.4% | 25.3% | 26% | 12.3% | Donovan et al. (2016)664 |
| Hong Kong | 153 male office-based workers in Hong Kong | 1997–1999 | 30–60 | AASM 2007 | 8.8% | - | 5.3% | - | Mary et al. (2001)665 |
| Hong Kong | 106 female office staff members of public institutions in Hong Kong | 1998–2000 | 30–60 | AASM 2007 | - | 3.7% | - | 1.9% | Mary et al. (2004)666 |
| Australia | 380 residents of the rural town of Busselton in the state of Western Australia who were participants in the Busselton Health Study | 1990 | 40–65 | AASM 2012 | 25.5% | 23.5% | 4.7% | 4.9% | Marshall et al. (2008)667 |
| Japan | 322 male employees of a wholesale company | 2004–2005 | 23–59 | AASM 2012 | 59.7% | - | 22.3% | - | Yukiyo et al. (2008)668 |
| Singapore | 242 individuals in the Singapore Health Study 2012 | 2012 | 21–79 | AASM 2012 | 70.8% | 70.8% | 30.5% | 30.5% | Adeline et al. (2016)669 |
| Switzerland | 2121 participants in the HypnoLaus Sleep Cohort study | 2009–2013 | 40–85 | AASM 2012 | 83.8% | 60.8% | 49.7% | 23.4% | Heinzer et al. (2015)670 |
| Russia | 1050 participants in the ARKH sleep study | 2014–2018 | 30–70 | AASM 2017 | 14.1% | 19.5% | 3.7% | 5.9% | Anna et al. (2020)671 |
| Brazil | 1042 volunteers in the Sao Paulo Epidemiologic Sleep Study | 2008 | 20–80 | AASM 2007 | 46.5% | 30.6% | 24.8% | 9.6% | Sergio et al. (2010)672 |
| Germany | 1208 persons who participated in SHIP‐Trend | 2008–2012 | 20–81 | AASM 2007 | 59% | 33% | 30% | 13% | Ingo et al. (2019)673 |
| Iceland | 415 subjects in the European Community Respiratory Health Survey | 2012–2013 | 40–65 | AASM 2007 | 13.3% | 10.8% | 10.6% | 4.8% | Arnardottir et al. (2016)674 |
| New Zealand | 364 Māori and non-Māori New Zealanders | 1999–2001 | 30–59 | AASM 2007 | 12.5% | 3.4% | 3.9% | 0.2% | Mihaere et al. (2009)675 |
| Norway | 518 subjects in the Akershus Sleep Apnea Project | 2006–2008 | 30–65 | AASM 2007 | 21% | 13% | 11% | 6% | Harald et al. (2011)676 |
| Spain | 2148 subjects from Vitoria-Gasteiz, Basque Country (Spain) | 1993–1997 | 30–70 | AASM 2007 | 26.2% | 28% | 14.2% | 7% | Durán et al. (2001)677 |
| South Korea | 457 participants of a study that included residents of Ansan community (Southwest Seoul) | 2001 | 40–69 | AASM 2007 | 21.7% | 16.8% | 10.1% | 4.7% | Kim et al. (2004)678 |
| Poland | 676 adult inhabitants of Warsaw in the MONICA II study | 1993 | 41–72 | AASM 2007 | 36.2% | 18.4% | 15.8% | 7.6% | Robert et al. (2008)679 |
| India | 365 subjects from the South Delhi district | 2005–2007 | 30–65 | Chicago 1999 | 13.5% | 6.1% | 5.5% | 6.1% | Reddy et al. (2009)680 |
| China | 309 patients with type 2 diabetes mellitus in Beijing | 2016–2017 | 40–70 | AASM 2012 | 68.3% | 62.4% | 38% | 30.7% | Ding et al. (2022)681 |
| Chile | 205 Chilean adults enrolled in the 2016/17 National Health Survey | 2016–2017 | 18–84 | AASM 2007 | 62% | 31% | 21% | 13% | Fernando et al. (2020)682 |
| Canada | 215 individuals in the First Nations Sleep Health Project | 2018–2019 | 18–76 | AASM 2017 | 51.1% | 41.7% | 14.8% | 9.4% | James et al. (2022)683 |
We searched PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov. Finally, high-quality and representative studies from 19 countries or regions were included
AASM American Academy of Sleep Medicine, AHI apnea-hypopnea index
Table 2.
Day and night can be distinguished with respect to the major signs and symptoms of obstructive sleep apnea syndrome (OSAS)
| A. Nocturnal symptoms |
| Snoring and observed apnea are the most frequent and hallmark nocturnal symptoms of OSAS, both of which reflect the critical narrowing of the upper airway. Nocturnal asphyxia also appears to be helpful in identifying patients with OSAS |
| a. Snoring: Snoring is the most characteristic nocturnal symptom of OSAS; patients with OSAS tend to have a long-standing history of snoring, which becomes increasingly intense and irregular over time |
| b. Observed apneas: Apneas are a frequent cause of consultation, since they often cause concern for the partner of the patient, describing them as respiratory pauses that interrupt snoring while the patient continues to struggle to breathe. Apnea alternates with snoring, and apneas occur after cessation of snoring, accounting for ~40% of sleep time |
| c. Arousals: Patients may experience arousal or distress when they experience apnea, feelings of terror, hand swings, or body movements. Arousals are less frequent than observed apneas. This symptom is associated with hypertension, since recurrent arousals are related to sympathetic discharges that elevate blood pressure and heart rate |
| d. Other: Night sweats, nocturia, restless sleep, somniloquy, and symptoms of gastroesophageal reflux are additional nocturnal symptoms related to OSAS |
| B. Daytime symptoms |
| a. Daytime sleepiness: Most patients have significant excessive daytime sleepiness (EDS), poor concentration and tiredness, which is due to sleep fragmentation. In addition, morning distension or headache, apathy, depression, irritability and/or changes in affect, memory loss, social issues, decreased libido, and erectile dysfunction are other characteristic daytime symptoms of patients with OSAS |
Over the past decades, research progress on the pathophysiology of OSAS has been relatively slow due to the limitations of disease models. Reviewing previous studies, we showed that IH can induce alterations in multiple signal transduction pathways that could affect various systems and organs throughout the body. Epidemiological studies have reported a positive association between OSAS and increased risks of cardiovascular diseases,19,20 neurological disorders,21,22 and metabolic diseases.23,24 Additionally, a number of studies have shown that OSAS plays a crucial role in the development of nonalcoholic fatty liver disease.25–27 Recently, increasing evidence from our laboratories and others has shown that OSAS is also involved in a number of other diseases, including insulin resistance,28,29 glucose metabolism,30 kidney disease,31 hypertension,32,33 cancer,1,34 the immune system,35 and gastroesophageal reflux.36 However, the pathogenic mechanisms of OSAS in organs are complex and intertwined and not fully understood. In this review, the pathophysiological mechanism of OSAS and the relationship between the alterations in potential signaling pathways and multiple systemic diseases are described in detail and comprehensively, and the corresponding therapeutic strategies for different pathogeneses are discussed.
Mechanisms/pathophysiology of OSAS
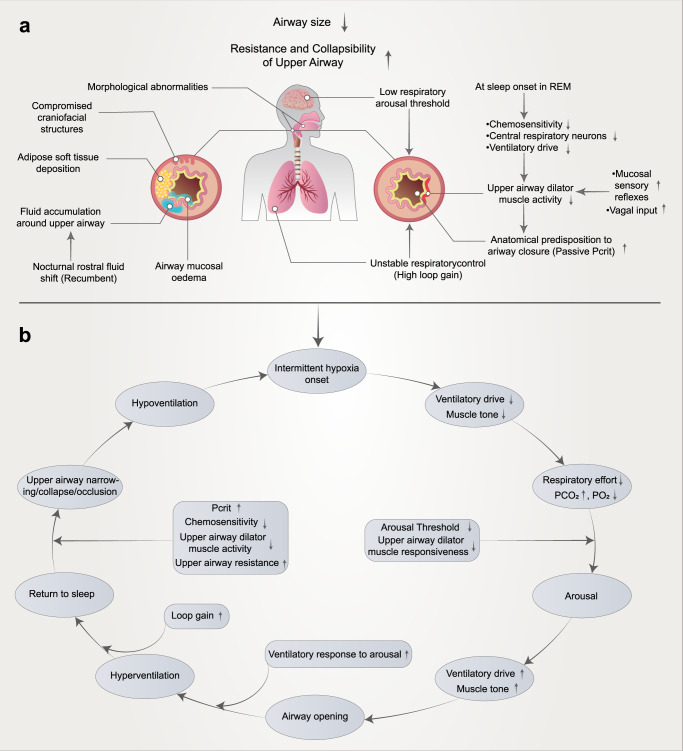
The pathophysiological mechanisms underlying OSAS are complex and multifactorial, and furthermore, the underlying causes of OSAS vary substantially between afflicted individuals, with many unknown and poorly understood aspects. With the increase in OSAS-related research, it is gradually recognized that there are anatomical factors and functional factors involved in the mechanism of upper airway collapse. Based on the involvement of anatomical and nonanatomical factors in the pathogenesis of OSAS, a model of PALM pathogenesis was proposed,37 which can be summarized as pharyngeal critical closing pressure (Pcrit, P), decreased respiratory arousal threshold (arousal threshold, A), increased loop gain (loop gain, L), and upper airway dilator muscle activity (muscle responsiveness, M). (Fig. 1a). Various pathophysiological factors interact to contribute to the pathogenesis of OSAS (Fig. 1b). The following sections will focus on reviewing the key pathophysiological factors of OSAS and their interactions to highlight innovations in our understanding of OSAS pathogenesis.
Fig. 1.
Mechanisms influencing upper airway collapse in the pathogenesis of OSAS (a) and the interplay between various factors (b). The reduction in upper airway volume caused by obesity or craniofacial structural abnormalities and soft tissue changes is an important factor in upper airway collapse. All OSAS patients have different degrees of upper airway anatomical structure injury. A nocturnal rostral fluid shift is defined as fluid accumulated in the legs during the daytime, redistributing to the upper part of the body upon lying down at night, causing an increase in peripheral pressure. In addition, most patients have mucosal edema, and the mechanism is not clear. Furthermore, several mechanisms associated with a low respiratory arousal threshold, poor pharyngeal neuromuscular muscle responsiveness, high loop gain, and high passive Pcrit may involve OSAS. When awake, neuronal activity ensures that the muscles of the dilated throat are activated, thereby preventing collapse. When this muscle loses activation during rapid eye movement (REM) sleep (chemosensitivity, central respiratory neurons, and ventilatory drive), the airway may collapse. Schematic representation of multiple pathological factors interacting to promote cyclical OSAS pathogenesis (b). In addition, these mechanisms might represent therapeutic targets. In the treatment section of this article, we introduce targeted therapies for different mechanisms
Upper airway collapse
Upper airway anatomical abnormalities are a key factor in the pathogenesis of OSAS. Almost all patients have upper airway anatomical abnormalities to varying degrees, that is, upper airway stenosis and collapse caused by abnormal bone structure and soft-tissue hyperplasia. Upper airway anatomical abnormalities include relative stenosis due to fat deposition in the upper airway caused by obesity and absolute stenosis due to abnormalities in the maxillofacial structure, which are important causes of upper airway collapse.38 In addition, patients with leg edema due to cardiac and renal failure or venous insufficiency may experience a shift in leg fluid volume from the leg to the neck during the night, which may lead to upper airway collapse.39 Interestingly, the degree of collapse of a particular airway can be measured by calculating the Pcrit (see below for more details).
Morphological abnormalities
Morphological abnormalities are the most common contributing factor to the development of OSAS, and in adult patients with OSAS, a reduced mandibular body length, inferiorly positioned hyoid bone, posterior displacement of the maxilla, and narrowing of the pharyngeal space all result in oral cavity crowding.40–42 Abnormalities in anatomical features, conditioned by skeletal abnormalities as in Pfeiffer syndromes (craniofacial synostosis) or Pierre Robin syndrome (midface hypoplasia) and Crouzon syndromes and Apert syndromes are also implicated in OSAS.43
Enlargement of soft-tissue structures in and around the airways is an important cause of pharyngeal airway narrowing in most cases of OSAS. Examples include excessive or elongated tissues of the soft palate, retrognathia, macroglossia, enlarged tonsils, increased soft tissue in the neck, and a redundant pharyngeal mucosa.16 The enlarged soft palate and tongue invade the airway diameter in the anteroposterior plane, whereas the thickened pharyngeal wall invades the lateral plane,44 a major site of airway narrowing in most patients with OSAS.45 Obesity rates are high in patients with OSAS. Obesity is a major factor contributing to the compression of the respiratory tract through an increase in the area and volume of fat deposition in the pharynx, and fat deposition in the upper airways and around the thoracic cavity may promote the development of OSAS.45,46 In addition, tongue shape might play an important role in the development of OSAS, and studies have found that the tongue shape in patients with OSAS is different from that in normal subjects in the supine position.47
Nocturnal rostral fluid shift
Fluid retention may contribute to the pathogenesis of OSAS, and nocturnal rostral fluid shift refers to the nighttime redistribution of fluid accumulated in the legs to the upper parts of the body while lying in bed.48 The passive movement of isotonic fluid between capillaries and the interstitial space is determined by capillary hydrostatic versus colloid osmotic pressure.49 When moving from the recumbent to the upright position, the hydrostatic pressure in the leg capillaries (90–120 cmH2O) exceeds the hydrostatic pressure in the interstitial space (15–20 cmH2O) due to gravity, thus causing fluid to seep from the capillaries into the interstitial space.50–52 Thus, while standing, the plasma volume is reduced by 300–400 ml due to venous pooling and fluid infiltration into the interstitial space, but the leg volume is increased by 100–300 ml.39 Fluid that accumulates in the interstitial space enters the circulation through the lymphatic system to maintain a stable interstitial volume. Once the lymphatic excreting capacity is saturated, the fluid accumulated in the interstitium is proportional to the standing time, and the gradient from the foot to the heart decreases.53 Upon lying down, the lower limb blood volume is rapidly reduced, and fluid is redistributed to the chest and neck.54,55 In addition, when lower body positive pressure was applied to the leg, the fluid was removed from the leg, and the neck circumference increased within 1 min, indicating that the fluid was able to move quickly to the neck.56–58 In summary, daytime postures, such as prolonged sitting or standing, causes fluid to accumulate in the intravascular and interstitial spaces distal to the lower extremities. During recumbency, patients may experience a shift in leg fluid capacity from the legs to the neck, increasing tissue pressure and resulting in narrowing of the upper airway, which increases its collapsibility and predisposes them to OSAS.45,46 It has recently been documented that the accumulation of even a relatively small amount (100–200 ml) of edema fluid expands the upper airway soft-tissue structures in patients with OSAS and snorers.59 Changes in leg circumference at night have been shown to correlate strongly with changes in neck circumference and AHI.39
Passive airway collapsibility
Although upper airway obstruction may be due to a variety of factors, such as obesity, there is increasing evidence that individual collapsibility is also a key factor in upper airway obstruction.60–62 The importance of abnormal pharyngeal susceptibility to collapse in the pathogenesis of obstructive apnea was demonstrated by studying the Pcrit in patients with OSAS and in control subjects.63 A highly collapsed upper airway is the leading cause of OSAS pathogenesis, and the passive Pcrit technique is considered the gold standard for measuring the degree of pharyngeal airway collapse.64 The Pcrit is the pressure at which the airway fails to remain open and collapses,61,65 and previous investigators have demonstrated that in normal individuals, Pcrit is negative,66 implying that the upper respiratory airway tends to remain open. In patients with OSAS, the critical pressure is less negative, which means that the upper respiratory airway is more likely to collapse and become occluded during sleep.66,67 Applying a theoretical model of upper airway obstruction, researchers could represent the upper airway as a simple tube with collapsible parts. Any increase in pressure around the tube will exceed the internal pressure in the tube, causing pharyngeal collapse. When the pressure around the tube increases to the level of the pressure inside the tube, it is called the Pcrit of that segment.64 Therefore, the pharyngeal critical closing pressure refers to the pressure acting on the upper airway. In the absence of muscle activity, the pharynx will close, and it could reflect the mechanical properties or collapsibility of the pharynx. The more negative the tube pressure, the less effort is required to open the airway compared to atmospheric pressure. A growing body of literature has shown that Pcrit is higher in patients with greater upper airway collapsibility. The critical closing pressure of the airway was higher in patients with OSAS than in those without the disorder.68,69 Pcrit is a vital part of categorizing subjects with OSAS into various endotype groups, which could provide help for the treatment and response prediction of OSAS patients.70
Decreased respiratory arousal threshold
In recent years, a number of studies have shown that a low respiratory arousal threshold may be an important endotype of OSAS.71–73 Each OSAS event terminates with brief brain activation in a process called arousal or microarousal.1 The tendency of OSAS patients to wake easily during sleep-disordered breathing is called the low arousal threshold. The arousal threshold varies between individuals,74 and studies have found that at least one-third of OSAS patients have a decreased respiratory arousal threshold.75 Arousal plays a dual role in the mechanism of OSAS. On the one hand, arousal from sleep at the end of a respiratory event is an important protective mechanism for restoring pharyngeal patency,76 and patients will resume normal breathing and relieve airway obstruction through neuromuscular and respiratory compensation mechanisms during arousal.77 Thus, respiratory arousal is considered a potentially lifesaving event that could avert asphyxia during sleep. On the other hand, a decreased respiratory arousal threshold is the cause of recurrent microarousal in OSAS patients. Recent studies also suggest that frequent arousals might lead to the interruption of sleep continuity, prevent deeper and more stable sleep, reduce the ability to recruit upper airway dilator muscles, and may contribute to further obstructive respiratory events.72,76–78 Arousal intensity is a unique pathophysiological phenotype, and individuals with a more intense arousal tendency to airway stenosis elicit a greater ventilatory response and are, therefore, more likely to experience instability in ventilatory control.79 Theoretically, hyperventilation during arousal would also reduce pharyngeal muscle activity,76,77 and in many cases, arousal might promote the cyclical breathing pattern of OSAS.78 Experimentally, the respiratory arousal threshold is measured by the lowest pressure in the esophagus produced during a respiratory event or perturbation of a breath taken before awakening. Evidence suggests that the magnitude of the intrapleural pressure generated by breathing is a major stimulus for the initiation of arousal from sleep.80–82 Although arousal thresholds vary widely between individuals, patients with OSAS tend to have diminished arousal responses to airway obstruction compared with controls, which may exacerbate upper airway dilator hypotonia, leading to an inability to recruit dilator muscles to open the airway before arousal occurs.46,79
Increased loop gain
In ventilatory control, loop gain is a measure of respiratory instability, which refers to unstable ventilatory chemoreflex control and is recognized as a key pathophysiological feature that contributes to OSAS.83–85 Eckert’s study has shown that approximately 36% of OSAS patients have high loop gain.37 The loop gain consists of the control gain, plant gain, and cycle time.86 Control gain refers to the response degree of the respiratory system to the change in PaCO2, plant gain is characterized by the efficiency of the respiratory system in responding to the reduction in CO2 by ventilation, and cycle time refers to the feedback time from the change in PaCO2 and PaO2 in blood being received by the sensor to the ventilatory response of the body.87 High control gain represents a strong chemoreceptor response to a small change in PaCO2, and high plant gain indicates that a mild ventilatory response can cause a significant change in PaCO2.88 For example, upper airway muscles are innervated by neuronal fibers from the respiratory center, high ventilation caused by high loop gain can expel more CO2, and low serum CO2 levels reduce the central ventilatory drive in the dilator muscles of the upper airway, thereby reducing pharyngeal muscle activity.89,90 Thus, the higher the loop gain is, the less stable the ventilatory chemoreflex control. Unstable ventilatory chemoreflex control could promote airway collapse in OSAS due to hypocapnic (produced by hyperventilation after obstructive apnea) hypotonia of the upper airways. Obstructive apnea is followed by hyperventilation, producing hypocapnia and respiratory depression, which contribute to the instability of ventilatory chemoreflex control and high loop gain,1,46,83,91 and increased CO2 from hypoventilation leads to the development of rapid and large negative inspiratory pressure, also leading to a collapse of the upper airway. In addition, high loop gain could lead to a mismatch between the driving force of the respiratory center on the respiratory muscles and the driving force of the upper airway dilator muscles; that is, the activity of the upper airway dilator muscles is not sufficient to counter the negative suction generated by the respiratory muscles during inspiration, which leads to upper airway stenosis and collapse.89,90
Decreased upper airway dilator muscle activity during sleep and impaired sympathetic neural activity
Increased pharyngeal dilator muscle activity in OSAS patients compared with matched controls has been interpreted as evidence of a neuromuscular protective compensatory reflex in response to anatomical compromise in OSAS.80 When awake, neuronal activation of the dilator muscles ensures that the pharyngeal dilator muscles are activated, thus preventing pharyngeal narrowing and collapse and protecting pharyngeal patency. When this upper airway dilator muscle activation is lost at the onset of sleep, its ability to maintain a patent airway decreases, and in turn, the airway could narrow and/or collapse.1,45 The genioglossus muscle is the most important pharyngeal dilator and has pharyngeal mechanoreceptors and chemoreceptors that deliver the relevant stimulus signals received (carbon dioxide in the blood) to the brainstem, tuning the upper airway dilator activity. Impairments in this process may lead to a reduction in the expansion forces of the pharyngeal dilator muscles, and the reduced pharyngeal caliber increases the likelihood of an obstructive event, in addition to the incoordination between the inspiratory activity of the muscles and the respiratory effort, increasing the resistance of the upper airway.16,45,80,92,93
Mechanisms of central sleep apnea
Central sleep apnea (CSA) is a sleep-breathing disorder characterized by apnea and hypopnea caused by a lack of drive to breathe during sleep.94 The occurrence of respiratory events can be intermittent or periodic, and patients could also experience obstructive respiratory events. In contrast, OSAS is apnea or hypopnea due to repeated collapse or obstruction of the upper airway during sleep, which is characterized by the weakening or disappearance of oronasal airflow, while chest and abdominal motion or respiratory effort is still present.89 CSA is not as common as OSAS in clinical practice and accounts for less than 10% of all sleep-related breathing disorders,95 so it has received less attention. Similar to OSAS, CSA is associated with important complications, including frequent night awakenings, excessive daytime sleepiness, and an increased risk of adverse cardiovascular outcomes,96 and CSA has been divided into eight categories by the International Classification of Sleep Disorders, Third Edition (ICSD-3).18 Table 3 summarizes the differences between OSAS and CSA. Neurophysiologically, CSA is due to a temporary failure of the pontomedullary pacemaker to generate breathing rhythm. Thus, without brainstem inspiratory nerve output, the nerves innervating all inspiratory muscle groups are silent, which results in a loss of inspiratory ventilatory effort.96,97 Although the exact pathogenesis of different types of CSA might vary considerably, unstable ventilatory drive during sleep is the main characteristic. Sleep phases can be divided into nonrapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and wakefulness. CSA and instability in humans mainly occur in NREM sleep, and the mechanism is related to the high loop gain in NREM sleep.88,98,99 Under the joint action of high control gain and high plant gain, the sensitivity of the ventilation control system would be increased, but only two points cannot cause the occurrence of CSA. There must be a certain time interval between the effect produced by the effector (lung) (increase or decrease in ventilation) and the change in CO2 sensed by the receptor (peripheral or central chemoreceptors), which is the key to the eventual onset of apnea.89 Under the action of some factors, the increased PaCO2 will act on the peripheral chemoreceptors and cause a ventilatory response, which will lead to a decrease in PaCO2. Under normal circumstances, PaCO2 will finally reach the dynamic equilibrium state. Interestingly, elevated PaCO2 is rapidly corrected in patients with CSA, and the initiating factor driving the ventilatory response may have normalized, while due to delayed signal cycling caused by a prolonged cycle time, this signal is not promptly fed back by the receptor to the effector, at which point the effector is still performing ventilatory commands and finally results in hyperventilation.100 If PCO2 falls below the chemoreceptor detection threshold, the respiratory drive is eliminated, and CSA occurs.101–103 In the event of CSA, the oscillatory cycle that leads to the recurrence of CSA is perpetuated by the following factors: pharyngeal stenosis requiring sufficient expansion tension to overcome gravity and tissue adhesion and inconsistencies between normal and actual PCO2 levels at which respiratory rhythm resumes following CSA.104–106 Compared with OSAS, although a large number of studies have been conducted in the past 20 years, the etiology and pathophysiological mechanism of CSA are complex, so the understanding of CSA is still insufficient and needs to be further explored and improved.
Table 3.
Differences between obstructive sleep apnea syndrome (OSAS) and central sleep apnea (CSA)
| OSAS | CSA | |
|---|---|---|
| Definition | OSAS is a sleep-related breathing disorder associated with an obstruction in the upper airway that results in an increased breathing effort and inadequate ventilation. | CSA is defined by the recurrent cessation of respiration during sleep not associated with ventilatory effort |
| Prevalence | The incidence of OSAS was 24% in men and 9% in women aged 30–60 years | It accounts for less than 10% of all sleep-related breathing disorders |
| Common etiology | Obesity; advanced age; male sex; genetic predisposition; menopausal, postmenopausal; upper airway disease. Other associated diseases: hypothyroidism, acromegaly, hypopituitarism, amyloidosis, vocal cord paralysis, sequelae of polio or other neuromuscular disorders (Parkinson’s disease), long-standing gastroesophageal reflux | Neuropathy: nervous system tumors, trauma, angioembolism, intracranial infection; dysautonomia: familial dysautonomia, Shy-Drager syndrome; myopathy: diaphragmatic myopathy, myotonic dystrophy occipital foramen magnum developmental malformation, lateral medullary syndrome. Others: congestive heart failure, nasal obstruction, OSAS after tracheotomy or uvulopalatopharyngoplasty |
| Pathogenesis | After patients with OSAS fall asleep, the central respiration drive is reduced, and the activity of the pharyngeal dilator muscles is diminished, which, combined with defects in airway anatomy, increases upper airway resistance; the balance of forces to maintain airway opening and closing is thus broken, and the airways collapse, with apnea occurring (see text for details) | When transferring from wakefulness to sleep, the responsiveness of the respiratory centers to various stimuli (e.g., high PaCO2 versus low PaO2 and pulmonary and respiratory resistive loads) is reduced, i.e., the threshold for responsiveness is elevated; instability of the central nervous system to respiratory feedback control induced by pathological states such as PaCO2 and hypoxia |
| Clinical manifestations | Common in obese patients; increased daytime sleep; the number of awakenings during sleep is minimal; strong snoring; cognitive decline; morning headache; nocturnal enuresis | Normal weight; insomnia is common, but somnolence is rare; more arousals during sleep; snoring is light and intermittent; depressive symptoms; decreased libido |
Intermittent hypoxic injury induced by OSAS: alterations in signaling pathways
The role of HIF-1α under different oxygen conditions
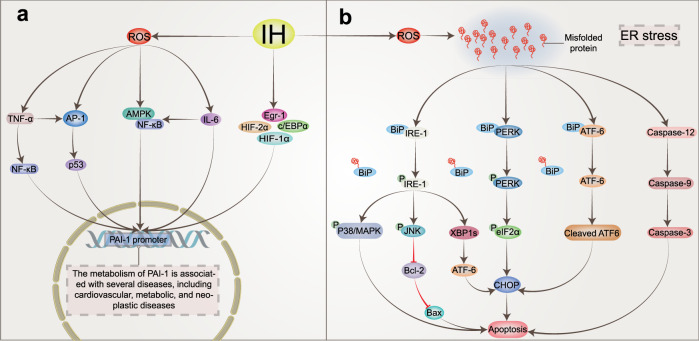
Due to the importance of oxygen for cell survival, metazoans have evolved mechanisms to sense changes in oxygen levels in the cellular microenvironment and trigger adaptive responses during evolution. It is increasingly recognized that the adaptation of organisms to hypoxia depends on the activation of specific oxygen-sensitive genes.107–109 A variety of redox-sensitive transcription factors have been identified, with the key factors being the HIF (hypoxia-inducible factor) family (including HIF-1, HIF-2, and HIF-3).110,111 HIF-1 is ubiquitously expressed in various tissues, whereas HIF-2 shows a tissue-specific expression pattern and is mainly expressed in a variety of immune cell subtypes, such as macrophages, neutrophils, and lymphocytes.112–115 The expression and role of HIF-3 in some immune cells remain unclear. These transcriptional regulators respond to fluctuations in oxygen levels and bind to specific DNA sequences to induce or repress genes, ultimately initiating adaptive transcriptional responses.116 Chief among these is HIF-1, which is a dimer consisting of the HIF-1α and HIF-1β subunits.117 The expression of HIF-1α is regulated at the level of transcription and translation, and multiple factors regulate the stability and activity of HIF-1α in oxygen-dependent or oxygen-independent ways at the posttranslational level.118,119 Under sufficient oxygen conditions, the oxygen sensitivity of the HIF-1α pathway is controlled by prolyl hydroxylase (PHD).120 The hydroxylase induces the hydroxylation of HIF-1α proline residues (Pro402 and Pro564) in the presence of oxygen, 2-oxoglutarate, and iron.121,122 Moreover, acetylation of HIF-1α at Lys532 by arrest-defective-1 (ARD-1) contributes to the reaction of HIF-1α with the von Hippel-Lindau (VHL) protein,123 followed by ubiquitylation of the alpha subunit of HIF-1 and finally ubiquitin-tagged HIF-1α protein degradation by the 26S proteasome124–126 (Fig. 2). During hypoxia, the oxygen required for HIF-1α ubiquitination is lost, and the enzyme activity associated with hydroxylation is weakened. Thus, HIF-1α escapes degradation, moves to the nucleus to bind to HIF-1β,127 and recruits the transcriptional coactivator (CREB)-binding protein (CBP) and p300128,129 to the HIF-1α binding site with hypoxia response elements (HREs)130,131 (Fig. 2). The result is the upregulation of a large number of target genes that promote hypoxia adaptation, and over 100 HIF-1α target genes have been identified thus far.132,133 These genes are involved in various biological processes, including anaerobic glycolysis metabolism,134,135 inflammation and immunity,115,136,137 erythropoiesis,138,139 metabolism,140 angiogenesis,141,142 cell survival and apoptosis,143,144 and cancer metastasis.145 In addition, the downregulation of some genes, such as PDK1, resulted in decreased mitochondrial oxygen consumption.146
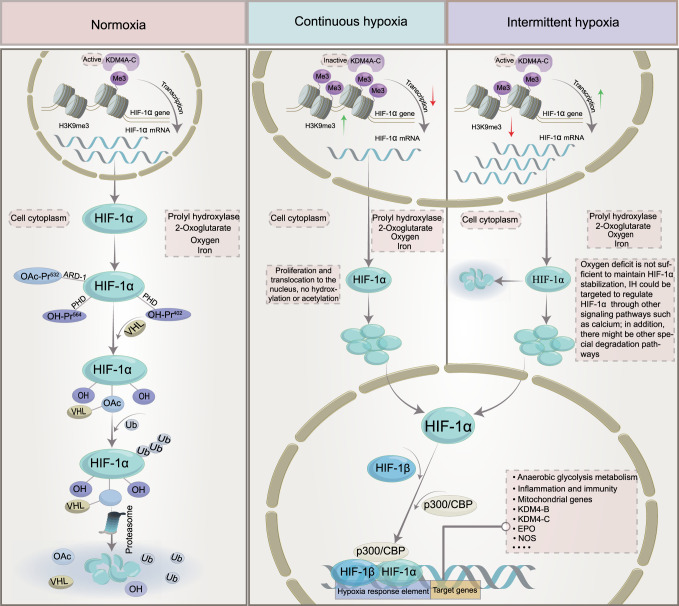
Fig. 2.
The mechanism of HIF‐1α activation and degradation under intermittent and continuous hypoxia conditions. Under normoxic conditions, HIF-1α is transcribed in the nucleus and translated into HIF-1α protein in the cytoplasm, which is normally hydroxylated by PHD. It then interacts with the VHL protein, undergoes ubiquitination, and is destroyed. Under continuous hypoxia, HIF-1α does not degrade but translocates to the nucleus, where it binds with HIF-1β and then recruits p300/CBP on HRE to initiate gene transcription. Among them, the HIF-1α target genes KDM4B and KDM4C were upregulated. Despite the elevated enzyme levels of KDM4A, KDM4B, and KDM4C, KDM activity was not maintained by the limited amount of oxygen, and KDM4A, KDM4B, and KDM4C remained largely inactive. This leads to increased H3K9me3, which ultimately reduces the amount of HIF-1α mRNA transcribed. Under intermittent hypoxia conditions, HIF‐1α was partially degraded during the reoxygenation phase, but the levels of KDM4B and KDM4C were increased but not to the level of continuous hypoxia. However, in contrast to continuous hypoxia, KDM4A, KDM4B, and KDM4C showed increased activity, resulting in higher H3K9me3 demethylation of the HIF‐1α gene than in normoxia or continuous hypoxia. This leads to increased production of HIF‐1α mRNA. KDMs histone lysine demethylases, H3K9me3 histone 3 lysine 9 trimethylation, HIF‐1α hypoxia-inducible factor-1, OAc acetoxy, OH hydroxyl, PHD prolyl hydroxylases, VHL von Hippel‒Lindau, EPO erythropoietin, NOS nitric oxide synthase, CBP coactivator-binding protein, HRE hypoxia response element
Similar to chronic hypoxia (Fig. 2), the essence of intermittent hypoxia is the switching between normoxic and hypoxic states [intermittent hypoxia switching (IHS)], which leads to changes in cellular and molecular functions that are different from chronic hypoxia. Studies have found that prolonged IH (hours to days) increases HIF‐1α activity.147,148 However, the molecular mechanisms driving cell behavior in IH compared to chronic hypoxia are less well understood. For example, proline hydroxylation and subsequent ubiquitination pathways are critical for HIF-1α stabilization in continuous hypoxia, and whether they also play a role in IH requires further study. Furthermore, in IH mode, we speculate that the free oxygen deficit is not sufficient to maintain HIF-1α stabilization, but studies on cell culture models of IH have shown that IH can evoke transcriptional activation more than continuous hypoxia for a given duration and intensity.149,150 Interestingly, HIF-1α protein levels were found to be lower in HCT116 cells treated with IH than in those treated with chronic hypoxia but were still higher than in normoxia.151 When the proteasome inhibitor MG262 was added, the accumulation of HIF-1α was much higher than that observed under chronic hypoxia, indicating that proteasomal degradation occurs at a higher level under IH than under chronic hypoxia,151 suggesting that there is another mechanism for HIF-1α degradation under IH conditions. In an experiment with cells cultured in IH, PC12 (pheochromocytoma-12) cells were exposed to alternating cycles of hypoxia and reoxygenation, with one cycle of 1.5% oxygen for 30 s and 20% oxygen for 4 min, to investigate the activation of HIF-1α by IH.152 HIF-1α protein and transcriptional activity increased in a stimulation-dependent manner as IH increased from 10 to 30 to 60 cycles.149 Interestingly, when cells were subjected to continuous hypoxia for 60 min, equivalent to 120 episodes of IH (30 s each episode), continuous hypoxia for 60 min did not increase HIF-1α protein expression or transcriptional activity.149 However, prolonged hypoxia in experiments increased HIF-1α protein expression and transcriptional activity.149,150 These observations suggest that IH activates HIF-1α more rapidly than continuous hypoxia. Based on current studies, it has been found that there are differences between continuous hypoxia and IH in the kinetics of protein kinase activation, the downstream targets of protein kinases, and the types of activated protein kinases. In addition, molecular responses activated by IH and continuous hypoxia are also different in many pathological conditions. We propose that novel oxygen-sensing mechanisms may exist in organisms that regulate and fine-tune the cellular hypoxic response depending on the duration of hypoxia (Fig. 2) (see below).
Histones regulate the expression of HIF-1α induced by IH
Multiple studies have shown that exposure to hypoxia could alter the epigenetic landscape at the cellular chromatin level.153–160 Similar changes in epigenetic marks (histone modifications,161–163 noncoding RNAs,164,165 and DNA methylation166–168) have been found in developmental and disease states. The number of studies have found increased histone methylation marks in different mammalian cells exposed to severe and continuous hypoxia.169–171 Histone methylation affects gene expression by affecting chromatin structure and altering the accessibility of chromatin to transcription factors.172 The nucleosome core consists of two H2A/H2B dimers and an H3/H4 tetramer whose protruding long tails can be covalently modified by methylation (me). Generally, histones are methylated only at lysine (K) or arginine residues, but methylation most often occurs at the K residues of H3 and H4 in the histone tails.172,173 The state of histone methylation is strongly associated with transcriptional repression or activation, depending on the position of the modified residues and the number of methyl groups.174 For example, lysine 4 methylation of H3 (H3K4me2/3), H3K79me2/3 and H3K36me2/3 is associated with active genes, whereas methylation at H3K9 and H3K27 (H3K9me2/3 and H3K27me2/3) correlates with gene repression.175,176 Histone methylation involves many chromatin remodeling proteins, including histone lysine demethylases (KDMs), histone methyltransferases, and other histone-modifying enzymes, and KDMs play an important role in the methylation process.177,178 Similar to PHD, which regulates HIF-1α degradation, KDMs require 2‐oxoglutarate, Fe, and oxygen as important cofactors for their activity,179,180 and another important feature of KDMs is the presence of a Jumanji-C (JmjC) domain. Given the dependence of this enzyme on oxygen for its activities, KDMs can act as molecular oxygen sensors in cells. Interestingly, Batie et al. found that hypoxia can alter chromatin in a range of human cultured cells by directly affecting JmjC-histone demethylase.170 The genomic locations of H3K4me3 and H3K36me3 after brief exposure of cells in culture to hypoxia allow assessment of the transcriptional response of cells several hours later. In addition, KDM5A inactivation was also found to mimic hypoxia-induced cellular responses. The above findings suggest that chromatin responds to oxygen fluctuations through the repression of JmjC-histone demethylase.170 Another study found that the H3K27 histone demethylase KDM6A is oxygen sensitive, and its deletion results in the same effect as hypoxia, preventing H3K27 demethylation, disrupting cellular differentiation, and reestablishing H3K27 methylation homeostasis in hypoxic cells, which could ameliorate these impairments.171 Upregulation of oxygen-dependent KDMs under persistent hypoxia is thought to increase the demethylation of methylated lysine residues. It has been suggested that the upregulation of KDMs is a compensatory mechanism by increasing the levels of these enzymes to compensate for their reduced activity under oxygen-depleted conditions,153,181,182 but oxygen-dependent KDM activity may not be elevated due to the scarcity of oxygen content. In addition, the effect of IH on histone methylation has been less studied than that of continuous hypoxia, and the specific regulatory mechanism of histone methylation and the changes in downstream molecules under different oxygen concentrations are also unclear.
Beyer et al. found that when KDM3A and KDM4B were overexpressed in HeLa cells cultured in 0.2% oxygen, the cells were differentially sensitive to hypoxia. Demethylation of H3K9me3 by KDM4B was decreased, whereas KDM3A activity remained unchanged under the same conditions.181 This finding implies that the physiological change from normoxia to hypoxia weakens the enzyme activity and additionally reveals a difference in the apparent oxygen sensitivity of the two JmjC-KDMs. Continuous hypoxia induces a decrease in KDM activity, resulting in global hypermethylation of lysine residues in histones, altering the expression of several genes.178 KDMs have been observed to be upregulated (at the mRNA level) in response to continuous hypoxia, but thus far, KDMs have not been identified as HIF-1α target genes.153,169,183 Recent studies have found that IH increases HIF-1α activity through pathways that are distinct from chronic hypoxia. Martinez et al. exposed different cell types to IH. HIF-1α protein and HIF-1α target gene (KDM4B and KDM4C) expression were increased under both chronic hypoxia and IH relative to normoxia, and the degree of gene expression was related to the dose-dependent effect of hypoxia. The increased expression of HIF-1α protein and known HIF-1α target genes under intermittent hypoxia is a generalized cellular response.151,184 Multiple experiments have compared HIF-1α mRNA levels in HCT116 cells, MCF7 cells, and brain (U251), prostate (PC3), and breast (MDA-MB-231) cancer cell lines after normoxic, chronic hypoxia, and IH exposure.151,184 Surprisingly, HIF-1α mRNA expression levels were decreased in chronic hypoxia and increased in IH in all cell lines compared to normoxia.151,184 The data suggest that HIF-1α expression is controlled differently in IH and chronic hypoxia. Further studies found that H3K9me3 increases in different cell types exposed to chronic hypoxia relative to normoxia170,185; however, unlike chronic hypoxia, IH reduced H3K9me3 levels below those observed with normoxia.151 Interestingly, H3K9me3 is associated with heterochromatin and gene silencing,186 so the global reduction in H3K9me3 induced by IH may lead to increased expression of associated genes.178,185 This finding supports the hypothesis that H3K9me3 reduction mediates the IH-induced increase in HIF-1α gene expression (Fig. 2). In parallel, the protein and mRNA expression of KDM4A, KDM4B, and KDM4C was further assessed. The protein levels of KDM4A were found to be unchanged in cells exposed to normoxia, chronic hypoxia or intermittent hypoxia, and the protein levels of KDM4B and KDM4C were significantly increased in chronic hypoxia compared with IH. Given that KDM4A mRNA levels are reduced in chronic hypoxia and do not change in IH compared to normoxia, it is suggested that KDM4A is not an HIF-1α target gene. Interestingly, several studies have found that the degradation of KDM4A in hypoxia is prolonged via an unknown mechanism,185,187,188 resulting in higher levels of KDM4A under hypoxic conditions, although KDM4A may be inactive.180 Although the enzyme levels of KDM4A, KDM4B, and KDM4C are increased under conditions of constant hypoxia, they may lose their activity due to hypoxia.151,178 Compared with continuous hypoxia, there is sufficient oxygenation between hypoxia fluctuations to remain active in IH, resulting in higher H3K9 demethylation levels of the HIF-1α gene than those in normoxia or chronic hypoxia, resulting in increased HIF-1α mRNA production (Fig. 2). Overall, studying the biological response to OSAS-induced IH is difficult because the patterns and types of IH vary widely in vivo, and it remains to be tested whether this response occurs in all forms of IH. Future studies will contribute to further understanding of how novel cellular oxygen sensors react and interact to generate hypoxic responses in IH.
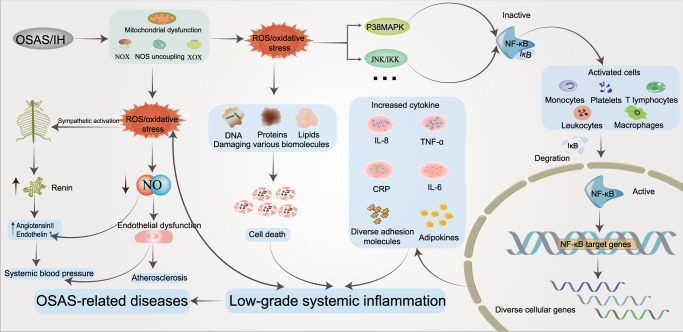
ROS-dependent Ca2+ signaling pathways and IH-induced HIF-1α activation
A number of studies have found that the synthesis and stability of HIF-1α evoked by both IH and continuous hypoxia are closely related to the increase in ROS (reactive oxygen species) produced by NOX activation.189,190 Interestingly, increased levels of ROS can activate PLC-γ (phospholipase C γ)191 to produce IP3 (inositol-3-phosphate) and diacylglycerol (DAG). Hong et al. found that hydrogen peroxide-induced PLC-γ activation and an IP3 receptor-dependent increase in Ca2+ in rat astrocytes.192 In addition, Yuan et al. demonstrated that HIF-1α accumulation involved PLC-γ and protein kinase C (PKC) activation in PC12 cells treated with IH. IH-induced transcriptional activation of HIF-1α was blocked by the Ca2+ chelator BAPTA-AM or a Ca2+/CaMK (calmodulin-dependent kinase) inhibitor, which confirmed the crucial role of the ROS-dependent Ca2+ signaling pathway.149 A previous study reported that continuous hypoxia resulted in transient (15 min) and moderate (1.5-fold) increases in CaMKII activity, which is an important downstream signaling molecule involved in Ca2+-mediated gene regulation, in PC12 cells.193 These observations are in sharp contrast to IH, where IH induced an exponential and nearly sixfold increase in CaMKII activity with increasing IH cycles and correlated with increased phosphorylation of the CAMKII protein.149 Interestingly, both calmodulin and CaMKII inhibitors prevented IH-induced HIF-1α transcriptional activity but not continuous hypoxia-induced HIF-1α transcriptional activity.149 Moreover, CaMKII inhibitors did not effectively inhibit IH-induced HIF-1α protein expression, suggesting that CaMKII-dependent signaling is essential for IH-induced HIF-1α transcriptional activation, while HIF-1α protein expression may be independent of the CaMKII pathway. On the other hand, it was also shown that the signaling pathways associated with HIF-1α activation in response to continuous hypoxia differ significantly from HIF-1α activation in response to IH. Multiple lines of evidence show that p300/CBP proteins194,195 are major coactivators of IH-induced HIF-1α transcriptional activation.196–200 In a hypoxic PC12 cell experiment, it was found that the IP3 receptor-mediated Ca2+ signaling pathway leads to the hyperphosphorylation of p300.201 IH increases the transcriptional activity of p300, confirming that CaMKII specifically phosphorylates p300 in vitro, which was blocked by CaMKII inhibitors.149 These observations indicate that IH-induced HIF-1α transcriptional activation requires a novel signaling pathway involving CaMKII-dependent activation of p300/CBP coactivators (Fig. 3 ①). Increased Ca2+ has been reported to activate classical PKC, which in turn activates mTOR (mammalian target of rapamycin) signaling, a kinase that promotes HIF-1α expression.202 Ca2+-dependent activation of PKC and mTOR could increase HIF-1α protein expression in PC12 cells.203 Interestingly, IH resulted in PKC-dependent mTOR activation compared to continuous hypoxia, and mTOR-dependent increased HIF-1α expression contributed to IH-induced HIF-1α accumulation. At the same time, rapamycin reduced IH-induced HIF-1α stabilization, and IH increased phosphorylated mTOR levels and downstream S6 kinase activation.190 In addition, the effects of IH on mTOR activation and HIF-1α protein activity were inhibited by inhibitors of IP3 receptors and PLC-γ as well as the Ca2+ chelator BAPTA-AM.204 The results further confirmed that IH-induced HIF-1α stabilization was associated with increased protein synthesis and activation of rapamycin-sensitive mTOR signaling (Fig. 3 ②). Similar to the continuous hypoxia report, decreased PHD activity was also found to lead to stable enhancement of HIF-1α after IH, and the negative regulation of PHD activity by PLC-γ/Ca2+/PKC/PHD signaling requires further investigation to elucidate the underlying molecular mechanisms (Fig. 3 ③). Based on the present evidence, the Ca2+ signaling pathway is involved in IH-induced mTOR activation and subsequent HIF-1α protein accumulation, as well as HIF-1α transcriptional activity. Recent studies have found that hypoxia can activate the PI3K (phosphoinositide 3-kinase)/Akt (protein kinase B) signaling pathway in cells.205–207 In addition, the stability of HIF-1α is related to the PI3K/Akt signaling pathway,206 and activation of PI3K is required for continuous hypoxia to activate HIF-1α.208 Several studies have also found that PI3K inhibitors reduce HIF-1α expression.206,209,210 However, neither LY294002 nor wortmannin (two PI3K inhibitors) blocked IH-induced HIF-1α transcriptional activity.149 The correlation between the PI3K/Akt signaling pathway and IH is controversial and may be related to the disease and cell type under hypoxic conditions. There are relatively few related studies, and more studies are needed to clarify the relationship between IH and the PI3K/Akt signaling pathway (Fig. 3 ④).
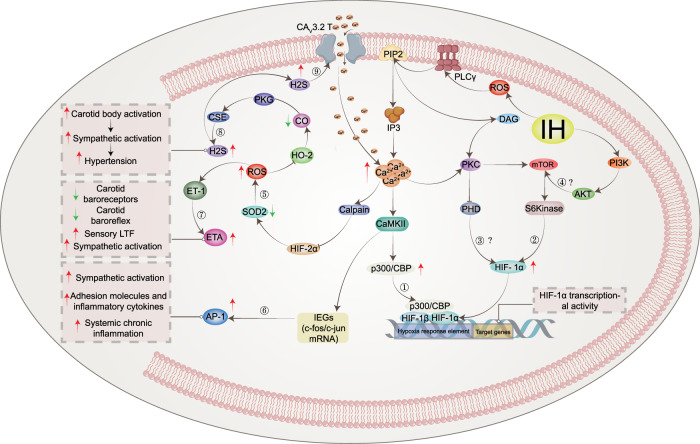
Fig. 3.
Activation of IH-associated signaling pathways. IH causes an increase in intracellular ROS, which can activate PLC-γ to produce IP3 and DAG. These two messengers are involved in intracellular signal transduction pathways and induce HIF-1α protein expression and transcriptional activity, respectively. Pathway ① indicates that IH-induced transactivation of HIF-1α requires ROS-mediated phosphorylation of the CaMKII-dependent coactivator p300. Pathway ② indicates that hypoxia-induced HIF-1α protein expression is caused by increased synthesis of mTOR, which is dependent on the ROS/Ca2+ signaling pathway. However, the mechanism by which PKC inhibits the reduction in PHD and the mechanism of the PI3K/AKT signaling pathway needs to be further confirmed (③④). Pathway ⑤ indicates that calcium-activated calpain promoted the degradation of HIF-2α protein in arterial corpuscles, resulting in a decrease in SOD2 and impaired antioxidant capacity of cells. Pathway ⑥ indicates that CaMKII can activate IEG genes, increase the transcription of c-fox mRNA or c-jun mRNA and increase the expression of AP-1, which is related to the activation of the sympathetic system and systemic inflammation. Pathway ⑦ indicates that increased ROS could stimulate the increased expression of ET and ETA and induce LTF in the carotid body. Pathway ⑧ indicates that IH causes ROS-dependent inhibition of CO production by HO-2, resulting in a decrease in PKG activity and an increase in H2S produced by CSE, which triggers a chemosensory reflex of the carotid body, leading to sympathetic excitation and hypertension. In addition, elevated H2S could activate the CAV3.2 T calcium channel on the cell membrane, causing Ca2+ influx and further aggravating the damage caused by IH (⑨). IH intermittent hypoxia, PLC-γ phospholipase C γ, PIP2 phosphatidylinositol (4,5) bisphosphate, IP3 inositol-3-phosphate, CaMKII calmodulin-dependent kinase II, IEGs immediate early genes, AP-1 activator protein-1, SOD2 superoxide dismutase 2, ET-1 endothelin 1, ETA endothelin receptor, HO-2 heme oxygenase-2, CO carbon monoxide, PKG: protein kinase G, CSE cystathionine γ-lyase, H2S hydrogen sulfide, LTF long-term facilitation
Previous studies have shown that PI3K and mitogen-activated protein kinases (MAPKs) are essential for continuous hypoxia-induced activation of HIF-1α-mediated transcription.200,211 In addition, other studies have shown that MAPK inhibitors attenuate hypoxia-induced transcriptional activation of HIF-1α in PC12 cells.212–214 Inhibitors of PI3K have also been shown to inhibit HIF-1α protein accumulation and attenuate hypoxia-induced transcriptional activation of HIF-1α.215 Although MAPKs (ERK 1/2 kinases; Jun Kinase) could be activated by IH, Yuan et al. examined the effects of MAPKs and PI3K inhibitors on HIF-1α transcriptional activation induced by IH. It was found that neither MAPKs nor PI3K inhibitors prevented HIF-1α transcriptional gene activation induced by IH.149 These studies, although preliminary, suggest that IH is associated with transcription factor activation in signaling pathways that are distinct from those used by continuous hypoxia. Another closely related protein, HIF-2α, is processed similarly to HIF-1α and has been reported to be a potent activator of genes encoding antioxidant enzymes.216 Several studies have shown that antioxidants such as superoxide dismutase 2 (SOD2) are also downregulated in IH-exposed cells.217–219 It has been hypothesized that the downregulation of antioxidants is closely related to HIF-2α downregulation. Interestingly, research has confirmed that IH-induced HIF-2α degradation leads to a significant downregulation of SOD2 transcription, which prevents IH-induced oxidative stress and restores SOD2 activity by ectopic overexpression of transcriptionally active HIF-2α.218 Systemic treatment of IH-exposed rats with ALLM (a potent inhibitor of calpains) not only restored HIF-2α in carotid bodies (CBs) and adrenal medulla but, more importantly, restored SOD2 activity and protected against oxidative stress.218 The reduction in HIF-2α expression by IH is due to increased degradation of the protein by Ca2+-dependent calpain.218,220 The degradation of HIF-2α by calpains involves the C-terminus portion of the HIF-2α protein.117 In addition, inhibitors of ALLM prevented IH-induced HIF-2α degradation, whereas PHD inhibitors or proteasome inhibitors were ineffective. These observations demonstrate that IH leads to HIF-2α downregulation via Ca2+-dependent signaling (Fig. 3 ⑤).
ROS-dependent Ca2+ signaling pathways and IH-induced IEG activation
In the family of proto-oncogenes, there is a class that can be induced by second messengers. These genes are called immediate early genes (IEGs), also known as rapid response genes. The IEG family mainly includes the fos, jun, and myc families.221 At present, the c-fos and c-jun families are the most deeply studied. The c-fos gene is one of the most important members of the IEG family and can be activated by hypoxia.222,223 The AP-1 (activator protein-1) complex is formed from heterodimers of either the Jun or Fos proteins or homodimers of Jun proteins.223,224 The AP-1 binding sequence is a common component of transcriptional regulatory elements that can drive the activation of multiple target genes during hypoxia, including tyrosine hydroxylase (TH), which encodes an important enzyme in catecholamine synthesis.225,226 Because TH is the rate-limiting enzyme for catecholamine synthesis, it is possible that IH-induced TH activation partially induces an increase in catecholamine levels in the body,227,228 leading to a chronic increase in sympathetic activity.229 In addition, the upregulation of AP-1 is involved in the expression of adhesion molecules and inflammatory cytokines, suggesting that AP-1 is also involved in OSAS-induced systemic chronic inflammation.230,231 Yuan et al. reported that IH increased c-fos mRNA expression in PC12 cells in a stimulation-dependent manner, and the IH-induced increase in c-fos mRNA was due in part to an increase in c-fos transcriptional activation.152 Further experiments showed that point mutations in the c-fos promoter indicated that the serum-responsive element and Ca2+ response element are vital for IH-induced c-fos promoter activation.152 Interestingly, several studies have found that IH increases the expression of c-fos mRNA in PC12 cells. However, continuous hypoxia exposure (equal to the accumulated time of IH) had no effect.152,232 In addition, prolonged continuous hypoxia was able to activate c-fos mRNA, and when the c-fos gene was activated by continuous hypoxia, the expression level of c-fos mRNA returned to the control level within 30 min after termination of hypoxic stimulation. Interestingly, c-fos mRNA levels remained high 5 h after the end of IH.233 Another study found that c-fos mRNA continued to increase for at least 3 h after IH intervention but returned to normal levels within 1 h after continuous hypoxia cessation,152 suggesting that different hypoxia modes have significant differences in the regulation of c-fos mRNA. Long-lasting activation of c-fos mRNA by IH is closely related to IH-induced carotid body sensory activity234 and respiration.235,236 A major difference between IH and continuous hypoxia is that IH has a reoxygenation phase, which is absent during continuous hypoxia. Therefore, it has been proposed that the generation of ROS by IH during the reoxygenation phase may mediate the regulation of c-fos mRNA. The amount of c-fos mRNA expression activated by IH was reported to be dependent on the duration of reoxygenation after hypoxia but not on the duration of hypoxia.152 Superoxide ion scavengers [manganese tetrakis methyl porphyrin pentachloride (MnTMPyP)] could inhibit the upregulation of c-fos mRNA and attenuate the transcriptional activation of AP-1 induced by IH.152,237 Studies have shown that the Ca2+ signaling pathway is involved in the hypoxic activation of the c-fos gene and AP-1 in PC12 cells.193,222 RT‒PCR and reporter gene assays showed that hypoxia enhanced c-fos mRNA and promoter activity, which were inhibited by the Ca2+ chelator BAPTA-AM or L-type Ca2+-channel blocker, while the L-type Ca2+-channel agonist BAYK8644 enhanced c-fos gene activation by hypoxia.193 Further immunoblot analysis showed that hypoxia increased the expression of CaMKII protein in PC12 cells, whereas the CaMKII inhibitor inhibited hypoxia-induced stimulation of the c-fos promoter.193 Ectopic expression of CaMKII mutants was also able to stimulate c-fos promoter activity under normoxic conditions. In addition, hypoxia-induced phosphorylation of CREB at the serine residue,133 and CaMKII inhibitors inhibited this effect.193 In summary, Ca2+-dependent signaling pathways play a vital role in hypoxia-regulated c-fos gene expression (Fig. 3 ⑥).
Mechanisms associated with altered carotid body function in response to IH
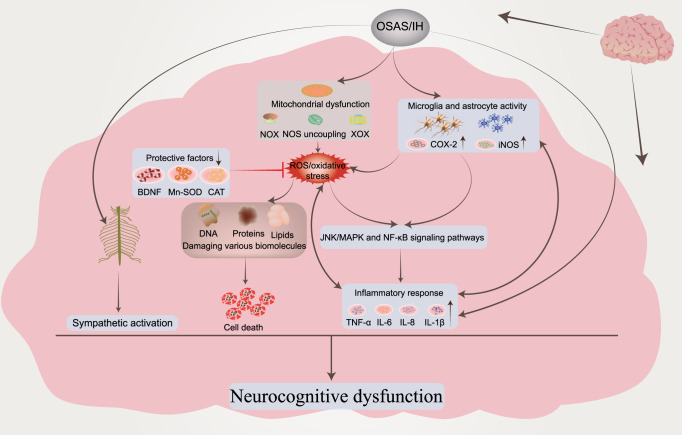
Patients with IH due to recurrent apnea, as well as IH-exposed rodents, develop autonomic abnormalities, including enhanced hypoxic ventilatory responses, elevated plasma catecholamines, persistent activation of the sympathetic nervous system, and systemic hypertension.238,239 The acute response to hypoxia, which occurs within seconds to minutes, is entirely dependent on the oxygen-sensitive capacity of peripheral arterial chemoreceptors, particularly the carotid bodies.240–242 Studies have shown that carotid body chemoreceptor are the “front line” defense system to detect alterations in arterial blood oxygen during apnea, which is more sensitive and rapid than other respiratory chemoreceptors, such as central chemoreceptors.243–245 This is because the time for oxygen to diffuse from the lung to the carotid body (6 s) is shorter than the time to reach the central region, and thus, the carotid body has already responded to hypoxia before the hypoxic stimulus is felt in the central region. Given its location and functional properties, IH-induced carotid body activation is closely related to autonomic dysfunction.
When it is starved of oxygen, the body actively begins to increase ventilation within a few minutes. This physiological response to increase ventilation due to oxygen deficiency is called the hypoxic ventilatory response (HVR).246 OSAS patients and IH-exposed rodents exhibit enhanced HVR,247,248 a hallmark of the carotid body chemoreflex.249,250 In a rodent model, awake rats were exposed to IH (5% O2 for 15 s, 21% O2 for 5 min; 9 sessions per hour, 8 h per day for 10 days). Efferent phrenic nerve activity was used as an indicator of neural respiration to assess HVR. The results showed a 38% increase in baseline minute neural respiration and a 56% increase in ventilatory stimulation induced by acute hypoxia (12% inspired O2 fraction).233 As reported in another experiment, there was no significant increase in HVR in rats exposed to 30 days of IH. It is possible that HVR becomes adaptive after 30 days compared to 2 weeks of IH.251 Exposure of experimental animals (cats,252 dogs,253 rats,254 and goats255) and humans256,257 to repeated hypoxia promotes a compensatory and sustained (>1 h) increase in respiratory motor activity. This prolonged respiratory activation in response to IH is often referred to as respiratory long-term facilitation (LTF),258,259 which is considered to be a marker of IH because a similar duration of continuous hypoxia does not result in prolonged respiratory activation. It was found that rats exposed to IH for 10 days showed a significant enhancement in LTF of respiratory motor output.233 It has been hypothesized that LTF prevents collapse by increasing the tone of the upper airway and that enhanced LTF may contribute to increased basal ventilation in patients with OSAS as well as in animals exposed to IH. Afferent input to the carotid body may be critical for LTF in respiratory motor output resulting from IH. Therefore, a group of researchers further investigated the effect of IH on chemoreceptor sensory discharge in the carotid body of rats, and anesthetized rats were subjected to 10 sessions of hypoxia (12% O2 for 15 s) followed by 5 min of reoxygenation.260 Interestingly, when this hypoxic pattern was repeated in animals subjected to IH for 10 days, it resulted in a prolonged elevation of baseline carotid somatosensory activity for nearly 1 h.260 These observations suggest that IH induces novel functional plasticity of the carotid body, leading to LTF in sensory discharge. However, sensory LTF plays an important role in reflex activation of the sympathetic nervous system and sustained daytime hypertension,261,262 and ablation of the carotid body reduces sympathetic activation and hypertension in intermittently hypoxic rats.263,264
ROS, which are produced during the reoxygenation phase of IH, may play a vital role in eliciting changes in carotid body activity induced by IH.265,266 In contrast to rats exposed to IH, the response of the carotid body was found to be blunted under continuous hypoxia; additionally, there was no induction of LTF in the sensory discharge of the carotid body under continuous hypoxia.260 Physiological studies showed that antioxidants (MnTMPyP and N-acetylcysteine) could ameliorate IH-induced plasma catecholamine elevation227 and decrease hypoxia sensitivity in the carotid body, and the magnitude of the LTF during sensory discharge was also significantly attenuated.204,249 Several studies have also confirmed that intervention with ROS scavengers during exposure of rats to IH could normalize carotid body activity and improve IH-induced hypertension.227,234,267 Increased sensitivity of carotid body chemoreceptors to hypoxic chemotherapy may involve endothelin (ET) and ET receptors,268–270 which are expressed in glomus cells (oxygen-sensitive type I cells) and blood vessels in the carotid body.271 ET acts on two receptors, the ETA receptor and the ETB receptor.272 In rodents exposed to IH, quantitative RT‒PCR confirmed a gradual increase in ET and ETA expression in type I cells and a time-dependent increase in hypoxia-induced carotid receptor activity. The application of a specific ETA antagonist could inhibit or attenuate hypoxia-induced carotid sensory discharge.272 In cats exposed to chronic IH for 4 days, ET-1 expression increased approximately 10-fold in the carotid body, while plasma ET-1 levels were unchanged, and the ETA/ETB receptor antagonist inhibited the chronic IH-induced increase in the carotid body hypoxic chemosensory responses.270 Another study found that the administration of MnTMPyP prevented the IH-induced elevation of ROS, basal release of ET-1 levels, and ETA receptor mRNA and augmented sensory responses. These observations suggest that the IH-induced increase in sensory responses involves a ROS-mediated increase in ET-1 release and upregulation of ETA receptor mRNA.273 A recent study explored chronic IH to increase carotid body chemosensory sensitivity via the ET-1 receptor signaling pathway.274 PKC, PLC, or p38 MAPK antagonists were used to elucidate the signaling pathways involved. The results showed that after chronic IH exposure, the protein levels of p38 MAPK and PKC were increased, and the expression of ETA and ETB receptors was upregulated in the carotid body, but only ETA was involved in ET-1-induced carotid body chemosensory sensitivity.274 It was confirmed that ETA receptor-mediated PLC, PKC and p38 MAPK signaling pathways were responsible for chronic IH-induced carotid body chemosensory sensitivity, and Ca2+ influx was also involved in the increase in carotid sinus nerve activity.274 In addition to ET-1, the renin-angiotensin system is also strongly associated with enhanced carotid body chemosensory sensitivity. Angiotensinogen mRNA and protein have been found to be present in type I cells. Similar to ET-1, IH increased the transcriptional and posttranscriptional expression of angiotensin II type 1 receptor (AT1) in the carotid body.275 Interestingly, the study by Lam and Leung et al.276 found that angiotensin II was able to act directly and enhance carotid body chemosensory sensitivity, rather than being mediated by altered arterial pressure or blood flow, and angiotensin II enhances carotid sinus nerve activity in the carotid artery in vitro. Based on the current study, we hypothesize that IH induces the production of sensory LTF in the carotid body through ROS/Ca2+/AT signaling to increase the sensitivity of the carotid body to hypoxic chemotherapy, which may be an important molecular mechanism of sympathetic activation after IH (Fig. 3 ⑦).
Type I cells in carotid bodies are derived from neurons and are the primary oxygen-sensing cells. Available evidence indicates that type I cells are the initial site of sensory transduction and that they release an excitatory neurotransmitter in response to hypoxia, acting on nearby afferent nerve endings and thus resulting in increased sensory discharge.240,277 One hypothesis suggests that heme and/or redox-sensitive enzymes are oxygen sensors and that biochemical events associated with heme proteins trigger transduction cascades,278 which leads to increased cytosolic Ca2+ concentrations and evokes neurotransmitter release in type I cells. An alternative hypothesis suggests that K+ channel proteins are oxygen sensors and that inhibition and subsequent depolarization of this channel is the initiating event in transduction.278,279 ROS may enhance the hypoxia-induced increase in intracellular Ca2+ concentration in type I cells by affecting voltage-gated Ca2+ channels, thereby enhancing sensitivity to hypoxia. One study showed that ROS enhanced the increase in intracellular Ca2+ concentration in PC12 cells in response to depolarizing stimulation, but the specific triggering mechanism is unclear.280
Recent studies have shown that the sensing of hypoxia in the carotid body requires an O2-dependent interaction between hydrogen sulfide (H2S) and carbon monoxide (CO).281–285 CO produced by heme oxygenase-2 (HO-2) in the carotid body induces a signaling pathway.286 CO inhibits the CSE (cystathionine γ-lyase) activity of the carotid body through protein kinase G (PKG)-dependent phosphorylation of serine residue 377, thereby inhibiting hydrogen sulfide (H2S) synthesis and leading to the inhibition of carotid body activity.283 Interestingly, the IH-increased H2S production was due to ROS-dependent inactivation of HO-2 that reduced CO production in the carotid artery, which in turn reduced the inhibitory effect of PKG on CSE phosphorylation,283 thereby increasing the H2S concentration and stimulating its neural activity.287 Rodents exposed to IH showed a significant increase in the H2S concentration in the carotid body, and this effect was abolished in rats treated with the CSE inhibitor L-propargylglycine (L-PAG).287 Furthermore, CSE-deficient mice showed a significant reduction in basal H2S levels in the carotid body,281 suggesting that IH increased CSE-dependent H2S production. HO-2 knockout mice exhibit more abundant CSE-derived H2S in carotid bodies and enhanced carotid body chemosensitivity, and CSE inhibitors prevent OSAS in HO-2 knockout mice.288 The carotid body of IH-exposed rats showed reduced CO levels, PKG activity, and CSE phosphorylation, whereas all of these effects were abolished after administration of the membrane-permeable ROS scavenger MnTMPyP.287 Therefore, we hypothesized that the activation of H2S signaling in the carotid body under IH is also a key trigger of sympathetic activation and hypertension (Fig. 3 ⑧). In addition, increased H2S may mediate ROS-induced intracellular Ca2+ elevation (Fig. 3 ⑨). Previous studies have shown that voltage-gated Ca2+ channels (VGCCs) are essential for hypoxia-induced Ca2+ elevation in type I cells,289,290 with L-type (high-voltage-activated channel) VGCCs mediating the majority of the hypoxia-induced Ca2+ influx.291,292 A recent study detailed the role of T-type (low-voltage-activated channel) VGCCs in the carotid body and found that the mRNA encoding the α1H subunit and α1H-protein is highly expressed in rat carotid body type I cells, implying that CAV3.2 is the major T-type VGCC isoform in the carotid body.293 Mibefradil and TTA-A2, as selective blockers of T-type VGCCs, significantly reduced the hypoxia-induced increases in intracellular Ca2+ concentration, catecholamine secretion from type I cells, and sensory excitation of the carotid body.293 Studies have also confirmed that H2S, dependent on CSE production, is required for VGCC-mediated Ca2+ influx in type I cells294 and carotid body sensory nerve excitation.281,284 Interestingly, similar to hypoxia, the H2S donor NaHS increased the intracellular Ca2+ concentration and carotid body nerve activity, while these effects were significantly attenuated in CAV3.2 knockout mice.293 In wild-type mice, TTA-A2 significantly reduced the response of type I cells and carotid body sensory nerves to hypoxia, and these effects were abolished in CSE knockout mice.293 Based on the present findings, we hypothesized that the highly expressed CAV3.2 T-type VGCCs in type I cells are involved in H2S-mediated Ca2+ influx and Ca2+ secretion, as well as the response of the carotid body to hypoxia. However, whether other types of calcium channels also play these roles in IH and hypoxia is unknown, and the types of oxygen-sensitive channels need to be further explored in the future.
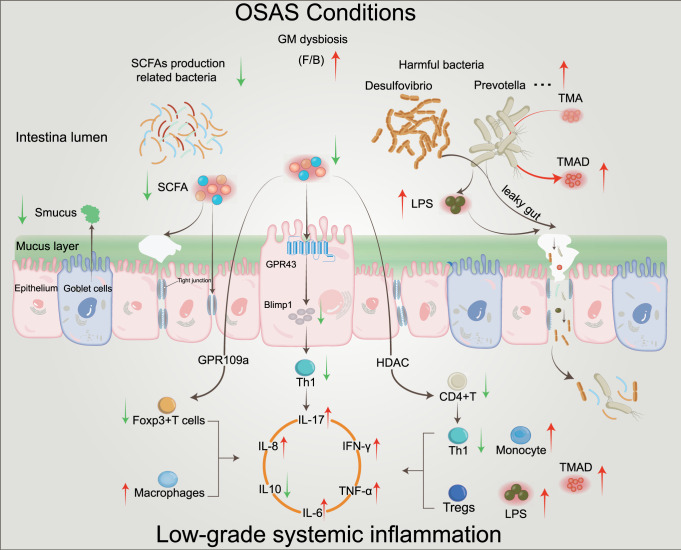
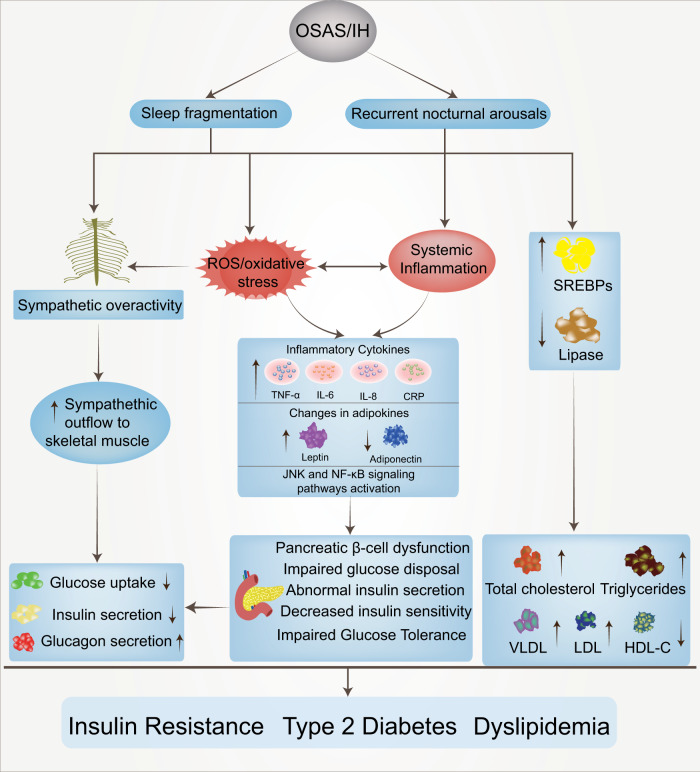
Mechanisms of OSAS-induced gut dysbiosis
In normal physiological states, there is a mutually beneficial relationship between the host and the gut microbiota. The host provides nutrients and a living environment for the microbiota, while bacteria help maintain the host immune response, act as a barrier against invading pathogens, and provide nutrients to the host.295,296 This balanced relationship may be disrupted by changes in the composition of the microbiota, known as dysbiosis. Current studies have found that gut dysbiosis might play a role in OSAS-associated morbidities, such as systemic hypertension,297–300 metabolic disorders,301–303 neurological diseases,304 COVID-19,305 and atherosclerotic heart disease.306 The gut is the largest immune organ and the largest microecosystem in the human body. The gut microbiota contains at least 1500 species of microorganisms with more than 100 trillion bacteria,307,308 and 70% of lymphoid tissue is present in the gut and forms gut-associated lymphoid tissue.309 The five most common bacterial phyla inhabiting the colon are Actinomycetes, Bacteroides, Proteus, Firmicutes, and Cerrucomicrobia.310 Bacteroides and Firmicutes account for 90% of the bacteria in the colon.311 The beneficial and healthy Bacteroidetes (gram-negative) include Lactobacillaceae, Ruminococcaceae, Erysipelotrichaceae, Bifidobacteriaceae, and Clostridium, which play key roles in carbohydrate and fiber fermentation. This process produces short-chain fatty acids [SCFAs (butyrate, acetate, and propionate)], which provide the main source of nutrition and energy for colonic cells and regulate the immune system.312–314 On the other hand, Desulfovibrio, Prevotella, Lachnospiraceae, and Paraprevotella species, which belong to Firmicutes, have local (gut) and systemic harmful characteristics and are capable of disrupting the structural integrity of the gut barrier.315,316 Interestingly, an increased Firmicutes/Bacteroidetes (F/B) ratio has been shown to be a hallmark of gut dysbiosis in almost all animal studies using similar IH exposure models.310,316,317
It is well known that the core of the gut contents is hypoxic, but studies have shown that there is a gradient in the oxygen concentration of the microbiota in the range of ≈150–200 μm near the gut epithelium318 and that the oxygen concentration has an effect on the microbiota.319 In a mouse model of IH intervention, it was found that IH induced a periodic hypoxia/reoxygenation pattern in arterial blood and the lumen of the small intestine. It is possible that there is a physiological process involving oxygen diffusion from the epithelial capillaries into the gut lumen, and a periodic pattern of hypoxia/reoxygenation could be observed within 200 μm of the intestinal epithelial barrier316; that is, IH translates into a hypoxia/reoxygenation pattern in the proximal intestinal epithelial feces (<200 μm). Under these conditions, we hypothesized that an increased duration of hypoxia would favor the survival of obligate anaerobes and that the biological diversity of the gut microorganisms might be altered. In fact, some studies have also confirmed that IH exposure causes changes in the relative abundance of aerobic bacteria in mice that mimic moderate OSAS and causes an increase in the abundance of obligate and facultative anaerobes.319 In addition, dysbiosis was characterized by a changed F/B ratio in many experiments.320,321 Given that arousal is an important component in the pathogenesis of OSAS, a recent study showed that when mice were exposed to sleep fragmentation, it resulted in significant changes in the microbiota, including an increase in Firmicutes and a decrease in Bacteroidetes compared with those of control mice.322 Another consequence of arousal is increased sympathetic activity and catecholamine release,323 and catecholamines could significantly increase the growth of certain bacterial species.324,325 Adrenergic stimulation of enteric neurons regulates intestinal motility and ion transport, thereby altering the microbiota.326,327 In addition, adrenergic release from the intestinal epithelial layer disrupts the integrity of the epithelial barrier.327
In OSAS patients, IH leads to ischemia-reperfusion injury of the intestinal mucosa and insufficient oxygen supply to the intestinal mucosa, resulting in changes in the structure and abundance of the gut bacteria and destruction of the integrity of the intestinal barrier.328–330 Prevotella and Desulfovibrio belong to the specific bacterial phylum Firmicutes, and the abundances of both bacteria increased significantly with IH exposure,316,322 exhibiting mucin-degrading features. The sulfate released during mucin degradation by Prevotella is cleared by Desulfovibrio, a process that further promotes mucin degradation and increases gut permeability.316,331 Disruption of the intestinal wall membrane integrity produces a small-molecule protein (plasma intestinal fatty acid-binding protein) that is considered to be a highly sensitive marker of the ischemic intestinal mucosa.332–334 Interestingly, plasma intestinal fatty acid-binding protein was found to be significantly elevated in OSAS patients.332,335 In addition, it has been found that the plasma D-lactic acid level is closely related to the permeability and degree of damage of the intestinal mucosa in patients with OSAS and is positively correlated with AHI.336 Dysbiosis of the gut microbiota reduces the levels of butyrate and acetate, causing intestinal mucosal nutritional disorders, which could lead to a dysfunctional epithelium.312,313,337 In addition, repeated hypoxia/reoxygenation cycles also damage the epithelium.338,339 Eventually, the tight junctions between colonic epithelial cells are destroyed, resulting in a “leaky gut.” As Prevotella produces endotoxin (lipopolysaccharide)340 and other bacterial components that leak from the gut into the blood circulation, it stimulates the release of inflammatory mediators,341 such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, through monocyte recruitment and Toll-like receptor activation,342 thereby aggravating systemic inflammation.322,343 Interestingly, a positive correlation was found between the abundance of the mucin-degrading bacterium Desulfovibrio and plasma lipopolysaccharide in IH-exposed mice.344 In addition, Prevotella converts nutrients (choline and l-carnitine) containing trimethylamine (TMA) into trimethylamine oxide (TMAO), which promotes inflammation, thrombosis, and the uptake of LDL by macrophages345 and contributes to hypertension346,347 and atherosclerosis.348–351 Multiple gut microfloral analyses demonstrated a reduction in bacteria associated with SCFA production in OSAS animal models320,321 and OSAS patients.328 SCFAs play an important role in maintaining intestinal integrity. Butyrate is a major source of energy and nutrition for enterocytes.352 An in vitro study has shown that butyrate enhances the expression of tight junction proteins, which are located transversally between epithelial cells,353 thereby increasing transepithelial resistance, maintaining gut integrity, and preventing gut permeability.354 Butyrate and propionate could induce the secretion of some mucin glycoproteins necessary for the construction of a mucus layer (which separates the colonocytes from the lumen) to protect intestinal epithelial cells.355 In addition, acetate enhances the differentiation of intestinal epithelial goblet cells and the secretion of mucus,356 which is beneficial for increasing the tight junction of enterocytes and improving the immune defense ability of enterocytes357 to inhibit lipopolysaccharide and bacteria from the gut entering into the systemic circulation. As hormone signaling molecules, SCFAs regulate immunity directly or indirectly through host metabolism through specific receptors.358,359 Butyrate can act on signal transducers of Th1 cells (T-helper 1 cells) and mTOR, an activator of transcription, and can upregulate B lymphocyte-induced maturation protein-1 (Blimp-1). Butyrate may induce the production of highly differentiated Th1 cells by acting on G-protein-coupled receptor 43 (GPR43) on intestinal epithelial cells to cause them to then secrete IL-10 and inhibit the excessive inflammatory response of Th cells.360 Butyrate also activates GPR109A, induces Treg and T-cell differentiation to produce IL-10 and inhibits intestinal inflammation by enhancing the anti-inflammatory properties of colonic macrophages and dendritic cells (DCs).361,362 The normal gut microbiota and its metabolites contribute to the regulation of Th17/Treg cell balance. Studies have found that SCFAs promote the proliferation and differentiation of Treg cells via epigenetic mechanisms.363,364 It has been confirmed that Th17/Treg cell imbalance is associated with the development of several disorders, and it is interesting to note that OSAS patients exhibit an increase in the number of Th17 cells365 and a significantly increased Th17/Treg cell ratio.366 Further studies showed that butyrate treatment of naive T cells could enhance histone H3 acetylation levels in the promoter and noncoding regions of the Foxp3 (forkhead Box p3) gene,363 induce naive CD4+ T cells to differentiate into peripheral Tregs, which secrete IL-10, and suppress the excessive immune response induced by Th1 and Th17 cells.360,367–369 Propionate and butyrate can downregulate the histone deacetylase (HDAC) activity of T cells to regulate immune function.370 This regulation might increase the phosphorylation of ribosomal protein S6, a target of the mTOR pathway, and induce the acetylation of p70 S6 kinase (S6K) and further phosphorylation of S6,371 ultimately promoting the differentiation of CD4+ T cells and the secretion of IL-10, IFN-γ, and IL-17.372 Interestingly, SCFAs can cross the blood‒brain barrier through the circulatory system, affect the growth and development of microglia, control their function and maturation, and enhance immunity and immune defense of the brain.373,374 There is increasing evidence that butyrate may provide neuroprotection by reducing microglial activation, which in turn decreases the levels of proinflammatory mediators and increases the levels of anti-inflammatory mediators.375 SCFA treatment also ameliorated the defective morphology and maturation of microglia in germ-free animals.373 Apparently, SCFAs have an immunomodulatory capacity not only in the gut and periphery but also in the nervous system. The mechanisms of OSAS-induced gut dysbiosis are shown in Fig. 4.
Fig. 4.
OSAS-induced low-grade systemic inflammation by mediating gut dysbiosis. The increased F/B ratio is a hallmark of gut microbiota dysbiosis, which is mainly characterized by a decrease in SCFA production-related bacteria and an increase in harmful bacteria. Decreased mucus secretion and mucin synthesis by dermal goblet cells disrupt the integrity of the intestinal barrier. The intestinal epithelium is dysfunctional due to inadequate nutrition, manifesting as reduced mucus production, decreased mucin secretion, and disrupted intestinal barrier integrity. Increased abundances of Prevotella and Desulfovibrio produce lipopolysaccharide and promote the degradation of mucin, increasing intestinal permeability and leading to a “leaky gut”, which triggers an intrinsic and adaptive immune response that induces low-grade inflammation in the body. Prevotella converts nutrients containing TMA into TMAO, which promotes inflammation. The reduced ability of SCFAs to activate GPR43, GPR109a, and HDAC results in diminished anti-inflammatory and increased proinflammatory capacity. GM gut microbiota, F/B Firmicutes/Bacteroidetes, SCFAs short-chain fatty acids, TMA trimethylamine, TMAO trimethylamine oxide, LPS lipopolysaccharide, HDAC histone deacetylase, GPR G-protein-coupled receptor, Blimp-1 maturation protein-1
IH-induced oxidative stress in OSAS
In recent years, increasing evidence has implicated oxidative stress as a fundamental component of OSAS pathophysiology, which is manifested by increased ROS production and decreased antioxidant capacity.2,376 Oxidative stress is defined as a break in the balance between oxidant-generating systems and antioxidant defense mechanisms, and the oxidative stress associated with OSAS is due to the production of ROS exceeding the antioxidant supply.376 Repeated breathing cessation is characteristic of OSAS, a severe hypoxic episode followed intermittently by rapid blood oxygenations that could be considered to be similar to repeated ischemia-reperfusion events, which affects cellular components and functions, resulting in increased ROS production. In the reperfusion period, the flux of excess ROS can alter their biological functions and induce various pathologies by damaging various biomolecules, such as proteins, lipids, carbohydrates, and DNA.1,2,377,378 In OSAS, the main sources of ROS for these pathologies are derived from damaged mitochondria, activated inflammatory cells, or superoxide production by activated enzyme systems, such as xanthine oxidase, nitric oxide synthase uncoupling and NADPH oxidase2 (Fig. 5). Hypoxia and reoxygenation might also induce complex metabolic and molecular changes, which include changes in gene expression and changes in energy metabolism.230 The disruption of oxidant-producing systems and antioxidant defense mechanisms may also result from decreased antioxidant capacity. A decrease in antioxidant capacity resulting in an increased oxidative stress load has also been described in OSAS. For example, the total antioxidant capacity of serum is decreased in OSAS patients.379
Fig. 5.
Schematic demonstrating the central role played by oxidative stress and inflammation in OSAS. OSAS/IH induces ROS production by inducing mitochondrial dysfunction, activating NOX and XOX, and inducing NOS uncoupling, which results in oxidative stress. The interaction between ROS and NO further promotes oxidative stress and diminishes the bioavailability of NO, thus promoting endothelial dysfunction and inflammation, which is closely related to hypertension, atherosclerosis, and hypercoagulability. Increased ROS-dependent sympathetic activation enhances renin levels, which leads to an increase in angiotensin II, endothelin 1, and hypertension. As a second messenger, ROS can activate multiple signaling pathways (MAPK, JNK), which in turn activate NF-κB and then induce the activation of nuclear transcription factors in a variety of cells. As the main switch of the inflammatory response, NF-κB plays an important role in the pathological process of OSAS, activating and entering the nucleus, regulating the transcription of many kinds of cells (immune cells), causing an increase in cytokines and participating in the inflammatory process of cells. In addition, elevated ROS can damage intracellular macromolecular substances (DNA) and cause cell death. Various pathological processes coordinate with each other and induce low-grade inflammation in the body, which is closely related to the occurrence and progression of a variety of diseases. ROS reactive oxygen species, NOX NADPH oxidase, NOS uncoupling nitric oxide synthase uncoupling, XOX xanthine oxidase, NOS nitric oxide synthase, JNK c‐Jun N‐terminal kinase, MAPK mitogen-activated protein kinase, NF-κB nuclear factor kappa B
Oxidative stress initiates a vicious cycle that facilitates the increased production of inflammatory cytokines, producing a systemic inflammatory state that increases vascular cell adhesion molecules and promotes sympathetic activation and vagal activation.1,379 Sympathetic activation stimulates the renin-angiotensin-aldosterone system (RAAS), which leads to increased levels of angiotensin II and aldosterone in the blood (Fig. 5). In addition, increased sympathetic tone is the key mediator of disrupted glycemic and insulin homeostasis, which may contribute to the development of metabolic risk factors in OSAS.2,380 Studies have found excessive ROS and increased expression of adhesion molecules and inflammatory cytokines, which reduce nitric oxide (NO) activity.2 The main consequences are endothelial dysfunction and hypercoagulability, which are identified as pathogenic mechanisms involved in different clinical and experimental models and affect various conditions and diseases (Fig. 5). However, in each disease, the results may differ according to the most affected organ or cellular function.379 It is estimated that more than 100 pathologies are associated with ROS and oxidative stress. Among them are cerebrovascular disease, cardiovascular disease, metabolic syndrome, type 2 diabetes, carcinogenesis and metastasis, inflammatory diseases (such as glomerulonephritis), atherosclerosis, and hypertension.2
A large body of evidence indicates that under normal physiological conditions, ROS function as signaling molecules, consistently described as regulators of signal transduction and as second messengers in many signaling pathways in all cells.381 Evidence regarding the capacity of ROS as signaling molecules is increasing. ROS regulates biological processes such as proinflammatory, profibrotic, cell proliferation, differentiation, migration, and apoptosis without triggering a requirement for macromolecular damage.382,383 Disruption of the ROS balance may activate a plethora of signaling pathways and inhibit others, affecting gene expression and protein function and leading to changes in signaling output, enzymatic activity, membranes, and intercellular communication.383–385 We present here a few examples of signaling targets.
Increased intracellular ROS were implicated in the PI3K cascade, c‐Jun N‐terminal kinase (JNK), and MAPK pathways that might induce the activation of multiple nuclear transcription factors (Fig. 5), such as nuclear factor kappa B (NF-κB), AP-1, redox factor-1 (Ref-1), HIF-1α, sterol regulatory element binding proteins (SREBPs), p53 and GATA-4.383,386 NF-κB, as a master switch in inflammation, is of special interest in the pathological process of OSAS, which is subject to complex regulation involving many regulatory molecules. At the same time, it orchestrates the production of adhesion molecules, inflammatory cytokines, and adipokines in OSAS.387,388 In addition, AP-1 expression was upregulated in cultured PC12 cells exposed to IH. Given that the upregulation of AP-1 is similar to that of NF-κB, AP-1 might also be involved in the pathogenesis of OSAS.152,230 However, the pathways of activation are not yet fully elucidated. HIF-1α is a transcription factor that plays a major regulatory role in the transcriptional response to decreased oxygen levels, which is essential for oxygen homeostasis and the adaptive response to hypoxia,381,389 and has been found mainly in several experimental models of IH in tissue culture as well as in rodents exposed to chronic IH.390 In addition, it has been stated above that the transduction signals that activate HIF-1α under IH conditions are distinct from those activated by sustained hypoxia.381 IH may cause worse HIF-1α stability, resulting in the activation of NF-κB-induced inflammation, possibly as a result of oxidative stress.391 In addition, it is becoming increasingly clear that there is a large degree of crosstalk between HIF-1α and the NF-κB pathway, and recent studies suggest that the NF-κB pathway plays a key role in inflammation induced by sustained hypoxia.392 OSAS has been shown to activate redox signaling, which may contribute to several systemic and cellular functional changes (including changes in blood pressure, increased release of neurotransmitters, and alterations in sleep and cognitive function) that are associated with the activation of second messenger pathways and HIF-1α, which is potentially important in OSAS pathology.148,393 SREBPs are a group of transcription factors affected by redox imbalance and oxidative stress that regulate the expression of genes required to maintain lipid homeostasis.394 In an experimental model of IH, the SREBPs activating genes regulating lipid metabolism were shown to be upregulated.395,396 Recently, a series of elegant studies has shown that lipid peroxidation and atherosclerosis are closely associated with the severity of chronic IH, and SREBP pathway-mediated hyperlipidemia was observed in this model.397,398 Additional transcription factors that are redox-sensitive and could possibly be implicated in OSAS pathology include NRF2-Keap1, which regulates antioxidant genes with a role in maintaining redox homeostasis.399
IH-induced systemic inflammation in OSAS
IH appears to be an important mechanism triggering inflammatory pathways.400 As outlined above, the main mechanisms of OSAS are hypoxia and oxidative stress, which are potent inducers of a cascade of inflammatory pathways. Furthermore, several studies have confirmed that inflammation also plays a crucial role in the occurrence and development of OSAS401 (Fig. 5). IH is hypothesized to activate the NF-κB-mediated inflammatory pathway that induces the overexpression of adhesion molecules [such as E- and P-selectin, intracellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM)], adipokines and proinflammatory cytokines [TNF-α, IL-1, IL-6, IL-8, and C-reactive protein (CRP)].386,402 Activation of these inflammatory pathways promotes the activation of endothelial cells, immune cells (circulating leukocytes, monocytes, and T lymphocytes), and platelets.403 These activated cells can further promote oxidative stress and injury by releasing ROS and increasing the expression of adhesion molecules on leukocytes, platelets, and endothelial cells, thereby exaggerating the inflammatory response386,404 (Fig. 5). In OSAS pathophysiology, as well as in the conditions and comorbidities that aggregate with it, the presence of inflammation can be considered a potential contributor to OSAS.405
Cytokines are intracellular and extracellular soluble mediators that, by interacting with various transcription factors in a very complex and intermingled network, regulate both the innate and acquired immune systems, orchestrating immune cells and inflammatory responses.406 They stimulate cells to secrete inflammatory cytokines, activate and recruit macrophages, promote the proliferation of smooth muscle cells, interfere with nitric oxide production, and activate endothelial cells to cause vascular dysfunction.403 TNF-α synthesized by macrophages is a cell signaling proinflammatory cytokine that is involved in host defense, immune mechanisms, and the pathogenesis of different infections and participates in a large number of signaling events that, in turn, lead to necrosis and apoptosis.407 In patients with OSAS, circulating TNF-α levels are not only elevated in plasma or serum408 but are also elevated in monocytes and various cytotoxic T lymphocytes.379 In addition, TNF-α stimulates NF-κB activity, promoting increased expression of VCAM in endothelial cells,409 which enables enhanced monocyte adhesion to the endothelium, triggers inflammatory responses in endothelial cells, and promotes the initiation and progression of atherosclerosis. Interestingly, activation of inflammatory pathways via upregulation of NF-κB has recently been found in monocytes from patients with OSAS410,411 (Fig. 5). Several studies have highlighted the persistence of a state of systemic chronic low-grade inflammation in patients with OSAS, mainly characterized by increased levels of TNF-α, IL-6, IL-8, and CRP.407,408 The major proinflammatory cytokines (TNF-α, IL-6, and IL-8) that activate NF-κB and AP-1 are regulated by oxygen tension and free radicals.412 Conversely, these cytokines can further activate inflammatory transcription factors and enhance inflammatory responses by activating various blood cells and endothelial cells. Adhesion molecules are cell surface proteins that play a key role in intercellular associations and are considered to be a major part of the inflammatory response against hypoxia. When facing various stimuli, such as hypoxia/reoxygenation and OSAS, adhesion molecules, and cytokines are upregulated in blood leukocytes and endothelial cells, which promote endothelial cell injury.384 CRP not only upregulates the transcriptional activity of NF-κB but also promotes the expression of ICAM and VCAM, which induces monocyte-endothelial cell adhesion.413 Thus, it is clear that CRP is not only an inflammatory marker but also a functional regulator that might contribute to the development of inflammation in OSAS through oxidative stress.
Other IH-induced signaling pathways in OSAS
Plasminogen activator inhibitor-1 (PAI-1) levels are consistently elevated in OSAS patients,414–416 and there are multiple pathways through which OSAS can trigger PAI-1 upregulation. The metabolism of PAI-1 has been implicated in several diseases and conditions, including cardiovascular disease,417 metabolic diseases,418 and cancer.419 Cells exposed to hypoxia showed increased PAI-1 mRNA expression and stability.420–422 ROS are involved in most of the mechanisms regulating PAI-1 expression. Incubation of endothelial cells with H2O2 induced a significant increase in PAI-1 mRNA and protein expression.423 In contrast, the PAI-1 promoter is repressed by up to 75% in the presence of antioxidants.424 The ROS-induced increased transcription and expression of PAI-1 is mediated by activation of the MAPK and NF-κB pathways, which are tightly linked to proinflammatory pathways.425,426 In addition, in vitro and in vivo experimental studies as well as clinical studies, have identified TNF-α as an important factor in increasing PAI-1 expression.427–429 In endothelial cells, TNF-α upregulates PAI-1 levels and is abolished by N-acetylcysteine, suggesting that ROS are mediators.424 IL-6 is another inflammatory cytokine that regulates PAI-1 upregulation. Animals injected with IL-6 had a significant increase in PAI-1 levels, whereas the use of an IL-6 receptor antagonist decreased PAI-1 expression.430,431 IL-6 can also activate the MAPK/NF-κB signaling pathway, leading to increased transcription of PAI-1.432,433 PAI-1 is one of the major transcriptional targets of HIF-1α. Hypoxic stimulation by IH could promote HIF-1α signaling and the upregulation of PAI-1.434 In addition, IH-induced HIF-2α, CCAAT-enhancer-binding protein-α (C/ΕBPα) and early growth response protein-1 (Egr-1) could also upregulate PAI-1 expression435,436 (Fig. 6a).
Fig. 6.
Other IH-induced signaling pathways in OSAS. IH regulates PAI-1 transcription through multiple pathways (a). IH could induce ROS, which in turn activated TNF-α, AP-1, AMPK/NF-κB pathway, and IL-6. In addition, IH could also promote the expression of Egr-1, HIF-2α, HIF-1α, and C/ΕBPα, ultimately upregulating the transcription of PAI-1. Upregulation of PAI-1 is associated with the development of IH-related disorders. Possible mechanisms of apoptosis induced by IH (b). IH could induce the generation of ROS, which in turn causes ER stress manifested by the production of misfolded proteins that bind BiP released from IRE1, PERK, and ATF6. After BiP release, IRE1, PERK, and ATF6 are activated. The activated IRE1, PERK, and ATF6 further activate their respective downstream pathways to ultimately upregulate the expression of CHOP and promote cell apoptosis. In addition, ER stress activates caspase-12, which in turn activates caspase-9 and caspase-3, leading to cell apoptosis. IH intermittent hypoxia, TNF-α tumor necrosis factor α, AP-1 activator protein-1, MAPK mitogen-activated protein kinase, NF-κB nuclear factor kappa B, C/ΕBPα CCAAT-enhancer-binding protein-α, Egr-1 early growth response protein-1, PAI-1 plasminogen activator inhibitor-1, ER endoplasmic reticulum stress, PERK protein kinase-like kinase, ATF6 transcription factor 6, IRE1 inositol requiring enzyme 1, CHOP C/EBP-homologous protein, XBP1 X-box protein-1, eIF2α e α-subunit of eukaryotic initiation factor 2
Recent studies have demonstrated endoplasmic reticulum (ER) stress in the brain,437,438 heart,439,440 kidney,441 and liver442 of rodents exposed to IH. The ER is an important organelle for protein synthesis, folding, lipid biosynthesis, secretion, and cell homeostasis.443 When cells are stimulated by hypoxia or oxidative stress, homeostasis is disrupted.444 The accumulation of unfolded and misfolded proteins in the ER activates ER stress, which in turn triggers the unfolded protein response (UPR).445 UPR activation is regulated by the chaperone protein glucose-regulated protein BiP/GRP78.446 Prolonged or severe ER stress induces accelerated separation of BiP and GRP78,439 which activates protein kinase-like kinase (PERK), transcription factor 6 (ATF6) and inositol requiring enzyme 1 (IRE1).443,445 Activated ATF6, PERK, and IRE1 accelerate the activation of CHOP protein,447 which mediates apoptosis.448 CHOP deficiency protects cells from apoptosis induced by excessive ER stress.449,450 The UPR in mammals has three branches: the IRE1 pathway, PERK pathway, and ATF6 pathway.451–453 Phosphorylated IRE1 activates the downstream target proteins JNK and p38 MAPK.454,455 A study has shown that phosphorylation of JNK both activates proapoptotic BIM and inhibits antiapoptotic Bcl-2.456 In addition, the activated ATF6 pathway and PERK pathway are also involved in ER stress-related apoptosis. XBP1 is spliced by the endoribonuclease for IRE1 under ER stress,457 acting as a potent transcription factor for CHOP.458 IH in patients with OSAS increases ROS generation, which reduces the production of functional proteins and even leads to apoptosis.446 Several studies have confirmed that the levels of ER stress-related proteins, including JNK, MAPK, GRP78, CHOP, PERK, p-eIF2α, and ATF4, were dramatically increased when exposed to IH.446,459 Cai et al. found that the PERK-eIF2α signaling pathway was involved in apoptosis in rats under IH conditions.460 In addition, the expression of IRE1-XBP1 and ATF6 was significantly increased in rat cardiac tissues after IH exposure for 5 weeks.439 In another study of cardiovascular disease in rats, the protein expression of the ER stress marker proteins BiP, PERK, CHOP, and ATF4 was increased in IH.461 During IH, Bcl-2/Bax is low, and activation of caspase-3, caspase-9, caspase-12, and JNK is induced439,455,462 (Fig. 6b).
Epigenetic alterations in OSAS
Epigenetics is generally defined as heritable phenotypic changes that do not involve DNA sequence changes that are not directly encoded by modifications of the nucleotide genomic sequence but by posttranslational modifications of DNA and histones and the regulation of noncoding RNAs.463 Recent studies have shown that epigenetic changes are associated with the development of OSAS and its pathogenesis, but the specific mechanisms of action are currently unknown. Below, we review relevant studies on the relationship between epigenetics and OSAS, and further understanding of the interplay between genetic and environmental factors through epigenetic regulation will be valuable to gain insight into the mechanisms underlying OSAS-associated oxidative stress, low-grade inflammation, and sympathetic hyperactivity.
Noncoding RNAs include microRNAs (miRNAs) and long noncoding RNAs (lncRNAs).464 MiRNAs, a class of single-stranded RNAs consisting of 19 to 25 nucleotides in length, can regulate gene expression by binding to mRNA. MiRNAs can mediate posttranslational gene silencing and thus negatively regulate target genes.465–467 Recent studies have found that multiple miRNAs can influence the IH process and influence hypoxia-induced apoptosis.468 For example, in a rat model, miR-26b-5p upregulation and miR-207 downregulation were involved in IH-induced cognitive impairment by increasing Bax and cleaved caspase-3 expression and reducing Bcl-2 expression in the hippocampus.469 MiR-155 promoted oxidation and enhanced the IH-induced NLRP3 inflammasome pathway by repressing the target forkhead box protein O3 (FOXO3a) gene in a murine model and HK-2 cells. Interestingly, IH-induced NLRP3 inflammasome activation in renal tubular cells was then suppressed by inhibiting miR-155 expression.470 In addition, miR-155 has been shown to have a proapoptotic function in diseases where other antiapoptotic proteins, such as clusterin, are decreased and correlate with increased clusterin levels in OSAS.471,472 MiR-664a-3p is downregulated in patients with OSAS and is negatively correlated with AHI and carotid intima-media maximum thickness, suggesting that circulating miR-664a-3p has the potential to serve as a noninvasive marker of atherosclerosis in OSAS.473 MiRNAs have been considered ideal biomarkers in the era of precision medicine, and sequencing analysis has shown that the expression levels of miR-199-3p, 107, and 485-5p were downregulated, whereas the expression level of miR-574-5p was upregulated in OSAS patients, suggesting that the differentially expressed miRNAs are closely related to OSAS.474 Based on the current study, miRNAs could be potential indicators for the diagnosis and treatment of OSAS in the future (Table 4).
Table 4.
Main studies on microRNAs in obstructive sleep apnea syndrome (OSAS)/intermittent hypoxia (IH)
| MiRNA name | Expression in IH | Target gene | Original source | Quantification approach | Main findings | Reference |
|---|---|---|---|---|---|---|
| miR-26b-5p | Up | Unknown | Rat hippocampus | miRNA microarray and qRT‒PCR | miR-26b-5p and miR-207 could be involved in cognitive impairments | Gao et al. (2017)469 |
| miR-207 | Down | |||||
| miR-155 | Up | FOXO3a | Renal tissue and HK‐2 cells | RT‒qPCR | miR‐155 might be a positive regulator of the NLRP3 pathway to enhance renal injury | Wu et al. (2018)470 |
| miR-664a-3p | Down | Unknown | Serum of OSAS patients | qRT‒PCR | Negative correlation of miR-664a-3p expression with AHI and maximum carotid intima-media thickness (CIMT) and positive correlation with the lowest oxygen saturation (LOS); miR-664a-3p as a candidate biomarker of atherosclerosis in OSAS | Li et al. (2018)473 |
| miR-199-3p | Down | Unknown | Serum of OSAS patients | LNA oligonucleotide microarrays and qRT‒PCR | Involved in hypoxia, metabolism, and oxidative stress | Li et al. (2017)474 |
| miR-107 | ||||||
| miR-485-5p | Up | |||||
| miR-574-5p | ||||||
| miR-130a | Up | GAX | Blood of OSAS patients; human umbilical vein endothelial cells | qRT‒PCR | miR-130a may contribute to the development of OSAS-associated pulmonary hypertension by downregulating the expression of GAX | An et al. (2017)684 |
| miR-365 | Down | IL-6 | Hepatocyte, stellate cell, and macrophage cell lines; serum of OSAS patients | qRT‒PCR | miR-365 acts as an important trigger for the production of proinflammatory cytokines and activation of macrophages in OSAS patients | Schaefer et al. (2017)685 |
| miR-185 | Down | CoLA1 | Lung tissue of dogs; COPD lung tissue; human primary pulmonary cells | qRT‒PCR | OSAS could inhibit miR-185 and promote CoLA1 expression leading to lung remodeling | Ding et al. (2016)686 |
| miR-34a-5p | Up | Bcl-2 | Human coronary artery endothelial cells | qRT‒PCR | miR‐34a‐5p activated beclin-1 through Bcl‐2 inhibition in IH and participated in IH-induced endothelial cell autophagy | Lv et al. (2019)687 |
| miR-630 | Down | Nrf2, AMP kinase, and tight junction pathways | Plasma of pediatric OSAS patients and human microvascular endothelial cells | miRNA microarrays and qRT‒PCR | miRNA-630 as a putative key mediator of endothelial dysfunction in children with underlying OSAS | Khalyfa et al. (2019)688 |
| miR-145 | Down | Smad3 | Canines; human aortic tissue; vascular smooth muscle cells from rats | qRT‒PCR | OSAS could activate the miR-145/Smad3 signaling pathway to promote aortic fibrosis, apoptosis and sympathetic nerve sprouting, which cause aortic structural and autonomic remodeling | Yu et al. (2017)689 |
| miR-146a-5p | Up | XIAP | H9c2 cells | qRT‒PCR | miR-146a-5p could aggravate IH-induced H9c2 cell injury by attenuating H9c2 viability and promoting its apoptosis by targeting XIAP | Lin et al. (2019)690 |
| miR-30a | Up | Beclin-1 | Mouse endothelial cells | RT‒qPCR | Upregulated miR-30a significantly reduced beclin-1 levels to attenuate endothelial cell autophagy in vitro and in vivo, which aggravated IH-induced endothelial cell injury | Bi et al. (2019)691 |
| miR-31 | Up | PKCε | H9c2 neonatal cardiomyocytes | qRT‒PCR | Upregulation of miR-31 decreased the mRNA and protein expression of PKCε to promote myocardial hypertrophy | Ren et al. (2018)692 |
| miR-224-5p | Down | NLRP3 | Mouse brain tissues and microglial BV2 mouse cells | qRT‒PCR | miR-224-5p reduces microglial inflammatory activation by regulating NLRP3 expression | Du et al. (2020)481 |
| miR-218 | Up | Robo1 | Mice aortic endothelial cells | qRT‒PCR | Upregulated expression of miR-218 promotes IH-induced apoptosis in aortic endothelial cells targeting Robo1 | Liu et al. (2017)693 |
| miR-203 | Down | SELENOP HIP/PAP | Human hepatocytes | qRT‒PCR | IH upregulated the levels of SELENOP in human hepatocytes to potentiate insulin resistance and upregulated the levels of HIP/PAP mRNAs to promote cell proliferation via a miR-203-mediated mechanism. | Uchiyama et al. (2017)694 |
| miR-452 | Down | RETN, TNF-α, and CCL2 | Mouse adipocytes and human liposarcoma adipocytes | qRT‒PCR | IH downregulated miR-452, resulting in increased levels of RETN, TNFα, and CCL2, leading to insulin resistance | Uchiyama et al. (2019)695 |
| miR-126a-3p | Down | HIF-1a | The blood, heart tissues, and abdominal aortas of rats; rat aortic smooth muscle cells | qRT‒PCR | IH decreased miR-126a-3p levels and increased HIF-1α expression, which promoted hypertension in the OSAS rat model | He et al. (2020)696 |
| miR-320b | Down | USP37 | Lung cancer tissues and lung cancer cells | qRT‒PCR | IH-induced miR-320b downregulation promoted the proliferation and invasion capabilities of lung cancer cells through a USP37-mediated mechanism | Li et al. (2021)697 |
| miR-21 | Up | Spry1/ERK/MMP-9, PTEN/PI3K/AKT and NF-κB pathways | Rat atrial tissues | RT‒qPCR | IH-induced upregulation of miR-21 expression promotes atrial remodeling and fibrosis | Zhang et al. (2018)698 |
| miR-214-3p | Up | CTRP9 | Cardiac tissue of IH mice | qRT‒PCR | Myocardial infarction + IH upregulated miR-214-3p, inhibited cardiac CTRP9 expression and exacerbated cardiac remodeling and heart failure | Du et al. (2020)699 |
|
miR-1249 miR-193 miR-218 miR-30B |
Up | Unknown | Mouse aortic endothelial cell | qRT‒PCR | Different miRNA expression patterns could be induced by IH, in which downregulation of miR-193 was associated with the expression of autophagy- and apoptosis-related genes. | Liu et al. (2018)468 |
|
miR-16 miR-718 |
Down |
LncRNAs are composed of RNA strands longer than 200 nucleotides that are not translated into proteins, and experimental evidence has shown that they can regulate gene expression through a variety of mechanisms, including transcriptional activation or repression, chromatin modification, and posttranscriptional regulation.475,476 A microarray study of cardiac samples from rats exposed to IH for 8 weeks identified 157 lncRNAs with upregulated expression and 132 lncRNAs with downregulated expression. Three of the downregulated lncRNAs (XR_600374, XR_590196, and XR_597099) and three of the upregulated lncRNAs (XR_596701, XR_344474, and ENSRNOT00000065561) were validated by quantitative reverse transcription polymerase chain reaction. This study provides novel insights into lncRNAs in the pathogenesis of IH.477 Another study found that overexpressing lncRNA CPS1-IT decreased IL-1β through the transcriptional activity of HIF-1 expression to reduce pulmonary arterial hypertension in OSAS patients.478 Multiple studies have confirmed that the abnormal expression of lncRNAs promotes the occurrence and development of diseases, and some lncRNAs have been identified as biomarkers for diseases.479 LncRNA is not only a repressive regulator but also a source of miRNAs.480 Du et al. found that blocking the lncRNA MALAT1/miR-224-5p/NLRP3 axis suppressed hippocampal inflammation in type 2 diabetes mellitus patients with OSAS.481 Another experiment on aortic endothelial dysfunction in OSAS patients showed that the lncRNA maternally expressed gene 3 (MEG3) altered HIF-1α expression by competitively binding to miR-135a, and silencing MEG3 could inhibit aortic endothelial cell apoptosis and injury.482 More details are given in Table 5. Further studies are needed to clarify the role of lncRNAs as potential biomarkers in OSAS.
Table 5.
Main studies on lncRNA in obstructive sleep apnea syndrome (OSAS)/intermittent hypoxia (IH)
| LncRNA name | Expression in IH | Target | Original source | Quantification approach | Main findings | Reference |
|---|---|---|---|---|---|---|
| Down | Unknown | Heart samples of rats | lncRNA microarray and qRT‒PCR | This study revealed for the first time that OSAS changed the expression profile of lncRNA in the rat heart, which could help us to establish the knowledge base of cardiovascular disease pathogenesis induced by OSAS | Chen et al. (2019)477 | |
|
XR_344474 ENSRNOT00000065561 |
Up | |||||
| CPS1-IT | Down | HIF-1 | Pulmonary artery tissues of rats | RT‒qPCR | Decreased CPS1-IT could enhance the transcriptional activity of HIF-1, enhance the expression of IL-1β through the NF-κB signaling pathway, and promote pulmonary arterial hypertension in OSAS | Zhang et al. (2019)478 |
| MALAT1 | Up | miR-224-5p | Mouse brain tissues and the microglial BV2 mouse cell line | qRT‒PCR | IH increased the expression of MALAT1, further inhibited the expression of miR-224-5p, and finally regulated the NLRP3/IL-1β pathway and promoted hippocampal inflammation | Du et al. (2020)481 |
| MEG3 | Up | miR135a | Aortic endothelial tissues of mice | RT‒qPCR | IH induced increased expression of MEG3, and targeted miR-135a upregulated HIF-1α to promote aortic endothelial injury and apoptosis in IH mice | Ding et al. (2020)482 |
| ROR | Up | miR-145 | HK-2 cells | qRT‒PCR | ROR alleviated CoCl2-induced hypoxia injury through the regulation of miR-145 | Ge et al. (2019)700 |
| ENST00000592016 | Up | Unknown | Plasma exosomes of OSAS patients | qRT‒PCR | The level of ENST00000592016 is correlated with the severity of OSAS and can be used as a diagnostic marker for OSAS | Chen et al. (2022)701 |
| MRPL20-AS1 | Down | Unknown | Male human coronary artery cells | qRT‒PCR | MRPL20-AS1 might serve as a useful tool to identify patients with severe OSAS | Zietzer et al. (2022)702 |
| NONMMUT032513 | Up | ZEB1 and smad5; Cmbl and ADH5 (unverified) | Mouse heart tissues | Microarray and qRT‒PCR | LncRNAs might be responsible for myocardial infarction aggravation under OSAS | Hu et al. (2021)703 |
| NONMMUT074571 | ZEB1 and Smtn; Cmbl and Pfdn6 (unverified) | |||||
| XIST | Up | GRα | The adenoids of patients with OSAS and NP69 cells | qRT‒PCR | XIST reduces the expression of GRα through the NF-κB-dependent signaling pathway, thereby promoting the occurrence and development of OSAS | Zhou et al. (2021)704 |
| XR_595552 | Up | PI3K/AKT pathway | H9c2 cells | qRT‒PCR | XR_595552 may play a protective role in alleviating IH-induced cardiomyocyte injury by regulating the PI3K/AKT pathway | Chen et al. (2023)705 |
DNA methylation, the best-known and best-characterized epigenetic modification, is a heritable, reversible epigenetic change that mediates the transcriptional silencing of genes by altering transcription factors in the promoter regions of genes and activates gene transcription by alternative splicing.483 DNA hypermethylation usually leads to transcriptional repression and decreased gene expression, whereas DNA hypomethylation affects chromosomal stability.484 Currently, there are few studies on the role of DNA methylation in OSAS. A previous study showed that the FOXP3 gene, which regulates T regulatory lymphocyte expression, showed increased DNA methylation in a total cohort of children with OSAS who had increased systemic inflammatory responses, suggesting that epigenetic-mediated downregulation of T regulatory lymphocytes might be an important determinant of OSAS-induced systemic low-grade inflammation.485 IH-exposed neonatal rats exhibit increased DNA methylation in the promoter region of the superoxide dismutase (SOD2) gene, and methylation modification has long-lasting effects on elevated chemoreflex sensitivity and hypertension in adult rats.486 Another study also confirmed that the impairment of respiratory and carotid body chemosensory reflexes by IH is partly the result of inhibition of antioxidant enzyme (AOE) genes via DNA methylation, including peroxiredoxin 4 (Prdx4) and thioredoxin reductase (Txnrd2).487 Previously, in a study of epigenomic DNA methylation, Chen et al. demonstrated multiple differentially methylated genes associated with OSAS and its adverse outcomes. Studies have found that hypomethylated interleukin 1 receptor 2 (IL-1 R2) and hypermethylated androgen receptor (AR) may be important contributors to disease severity, whereas hypomethylated natriuretic peptide receptor 2 (NPR2) and hypermethylated speckled protein 140 (SP140) may be biomarkers that predispose patients with OSAS to excessive daytime sleepiness488 (Table 6).
Table 6.
Main studies on DNA methylation in obstructive sleep apnea syndrome (OSAS)/intermittent hypoxia (IH)
| Gene name | Methylation level | Original source | Main findings | Reference |
|---|---|---|---|---|
| FOXP3 | Hyper | Blood of OSAS children with and without high hsCRP | In OSAS children with increased systemic inflammatory response, methylation of the FOXP3 gene is more likely to increase, which may provide potential biomarkers for terminal organ susceptibility | Kim et al. (2023)485 |
| AOEs | Hyper | Carotid body and adrenal medulla of rats exposed to IH | The persistent cardiopulmonary abnormality caused by IH is due to the long-term inhibition of the AOE gene by DNA methylation, resulting in a continuous increase in ROS levels in the carotid body chemosensory reflex pathway | Nanduri et al. (2017)487 |
| IL1R2 | Hypo | Blood of sleep-disordered breathing (SDB) patients with ODI >30 and SDB patients with ODI ≤30 | IL1R2 hypomethylation and AR hypermethylation might be important determinants of disease severity | Chen et al. (2016)488 |
| AR | Hyper | |||
| NPR2 | Hypo | Blood of SDB patients with excessive daytime sleepiness (EDS) and SDB patients without EDS | NPR2 hypomethylation and SP140 hypermethylation might be biomarkers of EDS in patients with OSAS. | |
| SP140 | Hyper | |||
| eNOS | Hyper | Blood of OSAS children | Endothelial dysfunction caused by eNOS hypermethylation | Kheirandish-Gozal et al. (2016)706 |
|
Ace1 Agt |
Hypo | CD31+ endothelial cells isolated from the mesenteric arteries of IH-exposed mice | IH-exposed mice had higher DNA methylation levels of Ace1 and Agt genes, which led to persistent changes in the renin-angiotensin system regulation and endothelial function, eventually leading to hypertension | Chu et al. (2015)707 |
| FPR1 | Hypo | Blood leukocyte of OSAS patients | Aberrant DNA methylation of the FPR1/2/3 gene in OSAS patients may be involved in the severity of the disease and the occurrence of diabetes mellitus or cardiovascular disease. | Chen et al. (2020)708 |
| FPR2 | Hyper | |||
| FPR3 |
Diseases associated with OSAS
Repeated processes of airway collapse and obstruction caused by various pathological factors in OSAS patients lead to recurrent apnea and periodic arousal during sleep, which eventually cause IH and sleep fragmentation. These core factors stimulate cell and molecular mechanisms, including increased sympathetic nerve activity, metabolic dysregulation, systemic inflammation, oxidative stress, and endothelial dysfunction, which have been identified as pathogenic in different clinical and experimental models and could lead to various OSAS-related complications. Different mechanisms may predominate in specific comorbidities, and the evidence for an independent association between OSAS and comorbidities is stronger for some comorbidities than others. While the detailed molecular mechanisms leading to the development of cardiovascular, cerebrovascular, and other diseases in OSAS are complex and several different mechanisms are involved, it seems that oxidative stress and inflammation are fundamental underlying mechanisms and are closely related to diseases in various systems throughout the body.
OSAS and cardiocerebrovascular disorders
A large body of evidence indicates that OSAS is associated with a number of cardiovascular complications,1,19,489,490 including systemic hypertension, arrhythmias, coronary artery disease, and stroke. The most convincing epidemiologic evidence of a causal relationship between OSAS and hypertension was provided in the 4-year follow-up results from the Wisconsin Sleep Cohort study.491 It is estimated that approximately 50% of patients with OSAS suffer from hypertension, and 30–40% of patients with hypertension suffer from OSAS.492,493 This is particularly true in patients with resistant hypertension, of whom up to 80% may suffer from OSAS. The Sleep Heart Health Study (n = 6132) also showed an increased likelihood of hypertension with increasing severity of OSAS, and the prevalence of hypertension was 59, 62, and 67% in patients with mild, moderate, and severe sleep apnea, respectively.494 In addition, OSAS is also responsible for masked hypertension in many cases.19,491 The ROS-dependent increase in sympathetic nerve activity (SNA) is a prominent feature of OSAS and has been shown to be associated with OSAS-related atrial fibrillation (AF), heart failure, and hypertension.19,386,495 Sympathetic outflow to the kidney is increased and stimulates renin release, which leads to increased circulating levels of angiotensin II and aldosterone, which in turn increases vascular resistance to constrict the vessels and raise blood pressure.496 Circulating and urinary catecholamines, which are biomarkers of elevated SNA, are also elevated in patients with OSAS.148 Emerging evidence implicates transcriptional changes by HIF-1α as an important molecular mechanism by which IH leads to SNA and hypertension.148 Animal studies of OSAS have shown activation of HIF-1α in myocardial tissue and increased expression of its downstream gene endothelin. Endothelin is a potent vasoconstrictor that causes blood pressure elevation.497 Advances in the understanding of cardiovascular disease in OSAS are closely related to the understanding of the development of coronary artery disease, but the underlying mechanisms remain poorly understood. The pathogenesis is likely to be a multifactorial process involving several mechanisms, including SNA, oxidative stress, vascular smooth muscle cell activation, lymphocyte activation, increased lipid levels, and lipid peroxidation within macrophages leading to endothelial dysfunction, which largely contributes to the development of various cardiovascular diseases, particularly atherosclerosis.407 IH triggers a molecular response that generates inflammation and oxidative stress and induces the formation of ROS, which in turn activates the inflammatory cascade by activating the transcription factor NF-κB and downstream genes such as inflammatory cytokines and adhesion molecules.2,386 Various activated blood cells produce more ROS, adhesion molecules, and proinflammatory cytokines. Adhesion molecules promote the accumulation of platelets, leukocytes, and possibly red blood cells on the vascular endothelium.379 Clinical studies have confirmed that blood cells from patients with OSAS present a proinflammatory and prothrombotic phenotype; additionally, the role of monocytes in the initiation and propagation of the progression of atherosclerosis is well established, and resident or circulating leukocytes mediate monocyte adhesion to the endothelium, which might promote thrombosis, endothelial dysfunction, and atherosclerosis.498–502 Growing evidence indicates a concomitant prevalence of AF of 21–74% in patients with OSAS,503 suggesting that OSAS might be a causative factor in AF pathogenesis.504 A potential explanation is the enhanced sympathetic and vagal nerve activities caused by hypoxemia, which triggers AF during acute OSAS.505 Chronic recurrence and sudden negative changes in intrathoracic pressure play a crucial role in atrial autonomic, structural, and electrical remodeling, leading to structural and functional atrial remodeling that triggers AF by contributing to atrial fibrosis.19,506 Multiple prospective studies have demonstrated a strong association between moderate-severe OSAS and stroke. The Wisconsin Sleep Cohort study found that an AHI >20 was significantly associated with an increased risk of stroke,507 while another study found that men with an AHI >15 had a threefold increased risk of stroke.508 Unsurprisingly, concurrent AF substantially increased the risk of stroke in patients with OSAS. Continuous positive airway pressure (CPAP) therapy has been shown to benefit the incidence and recurrence of stroke in patients with OSAS,509 and another study showed that CPAP therapy can reduce the rates of stroke and cardiovascular events in patients with severe OSAS.510 Hypertension or other traditional vascular risk factors do not fully explain the association of OSAS with stroke, and the underlying mechanisms include multiple factors such as hypercoagulability, cardiac arrhythmias, inflammation, oxidative stress, dysautonomia, and dyslipidemia.19
Accumulating evidence suggests that oxidative stress, inflammation, and molecular mechanisms play an important role in the pathophysiology of cardiocerebrovascular disease in patients with OSAS. In addition, a clinical lesson learned from understanding the underlying pathophysiology of OSAS with the accompanying comorbidities is that to prevent cardiovascular morbidity, treatment of breathing disorders during sleep might need to start at the earliest possible age.
OSAS and neurological disorders
Prolonged periods of IH in patients with OSAS could impact multiple CNS systems, all of which ultimately lead to severe neurocognitive and behavioral deficits, including a decline in cognitive functions, such as memory, executive function and comprehension, mood disturbances, insomnia, and/or excessive daytime sleepiness. In addition, OSAS may promote the development of neurodegenerative diseases.511,512 The results of animal studies from our team have shown that IH induces severe neuronal injury (especially in the hippocampal CA1 region), enhances inflammation, and activates astrocytes in the rat brain. The rats in the IH group showed a much longer escape latency when locating the hidden platform and much less time spent in the target quadrant than the normal control group. In addition, we found that IH significantly increased ROS levels, decreased manganese superoxide dismutase (Mn-SOD) and catalase (CAT) expression, increased the levels of lipid peroxidation products [including malondialdehyde (MDA) and DNA damage products, such as 8-hydroxy-2’-deoxyguanosine (8-OHdG)] in the hippocampus and significantly increased caspase-1, IL-1β, and IL-18 expression in the frontal medial cortex in mice.513,514 IH-induced increases in neuroinflammation, oxidative stress, and brain tissue damage in mice might account for the diminished performance in the Morris water maze test. We used the Montreal Cognitive Assessment (MoCA) and Epworth Sleepiness Scale to evaluate the cognitive status of OSAS patients in our previous clinical study. The findings showed significant impairments in attention, delayed memory function, and executive function in patients with OSAS, and the MoCA scores were negatively correlated with the AHI and oxygen desaturation index and positively correlated with the lowest oxygen saturation. In this study, we compared the automatic processing of emotional facial expression patterns between OSAS patients and matched normal controls by evaluating expression-related mismatch negativity (a brain electrophysiological detection tool) and found that OSAS patients suffer from cognitive impairment in the automatic processing of emotional facial expressions under the preattentive condition.21 Structural and functional alterations in brain anatomy and function in OSAS patients provide indirect evidence that OSAS causes damage to brain structures over time. Perhaps these changes underlie cognitive impairment. Studies have suggested a decrease in gray matter in the prefrontal cortex, anterior cingulate cortex, thalamus, parietal cortex, parahippocampal gyrus, inferior temporal gyrus, hippocampus, and cerebellum in patients with OSAS.511,515
It is well known that the brain is more sensitive to hypoxia than other organs and requires more energy and oxygen consumption. Clinical and animal findings suggest that IH resulting from OSAS can lead to structural neuronal damage and dysfunction in the CNS, with oxidative stress and inflammatory damage being the pathophysiological basis.516 Accumulating evidence supports the view that, in the CNS, IH may induce ROS production in the CNS, oxidative stress overactivation, and inflammatory damage leading to neuronal apoptosis and/or necrosis that, in turn, contributes to the development of OSAS-related cognitive impairments.517 Brain tissue NF-κB, TNF-α, CRP, IL-1β, IL-6, and cyclooxygenase-2 (COX-2) levels were measured in IH animal models, which were consistent with the changes seen in human plasma. The standardized regression test showed significant associations between proinflammatory cytokines and neurocognitive performance.516 A recent study confirmed that nocturnal overactivation of the sympathetic nervous system can lead to visuospatial dysfunction in patients with OSAS.518 The most prominent maladaptive effect of IH is neuroinflammation, and although the exact neural cell source of the associated processes is still not fully understood, microglial activation may be important. The findings showed that IH exposure resulted in a significant increase in microglial activity and hippocampal neuronal apoptosis, as well as increased levels of related inflammatory markers (NF-κB, TNF-α, and IL-1β).519 Microglia, the major inflammatory cells of the CNS, mediates oxidative stress and inflammation through mitochondria, NADPH oxidase, and the release of excitotoxic neurotransmitters. Recently, we demonstrated an important role for microglia in the hippocampus in the development of diabetic encephalopathy by single-cell RNA sequencing.520 NADPH oxidase is involved in microglia-mediated neurotoxicity and microglial activation. Activated microglia express high levels of inducible nitric oxide synthase (iNOS) and COX-2 isoforms, ultimately leading to increased ROS generation. Furthermore, activated microglia trigger the NF-κB signaling pathway, which regulates the immune inflammatory response, oxidative stress, and memory. Studies have confirmed that this pathway plays an important role in hypoxia.521 JNK is a member of the MAPK family and has a complex relationship with the NF-κB pathway. IH effectively activated the NF-κB/JNK pathway and its downstream signaling molecules, confirming the role of the NF-κB-mediated JNK pathway in hippocampal injury and cognitive dysfunction in IH model rats.522 p38 MAPK is also a member of the MAPK family, and its activation has adverse effects on learning and memory. In an IH animal model, p38 MAPK levels were significantly increased, which could activate the NF-κB signaling pathway, releasing cytokines such as IL-1 β, IL-6, and TNF-α, oxidative species, and adhesion molecules.523 The release of cytokines, in turn, promotes the production of ROS by microglia, thereby perpetuating inflammation and aggravating ongoing oxidative stress.524,525 CNS neuronal damage and apoptosis from IH might involve other mechanisms. For example, brain-derived neurotrophic factor (BDNF), an important neuromodulator of CNS function, significantly prevents oxidative stress-induced neuronal damage in the CNS.526 In addition, microglia release excitatory toxic neurotransmitters, such as glutamate, and studies have shown that higher glutamate concentrations are found in the cerebral cortex of OSAS patients, leading to excitotoxicity-induced neuronal dysfunction and apoptosis.527
Undoubtedly, most OSAS patients develop cognitive and neurologic dysfunction. Furthermore, these findings suggest a strong link between inflammation and cognitive impairment in OSAS (Fig. 7). At the same time, evidence regarding its links with neurological diseases is similarly accumulating. The evidence for its links with major psychiatric and neurologic disorders is similarly accumulating. However, the exact nature of the mechanisms responsible for these effects remains to be determined and must be investigated further.
Fig. 7.
Proposed interactions between neurological disorders and other pathological processes induced by OSAS/IH-induced elevated ROS levels. OSAS/IH upregulates the expression of ROS in the brain, and the inhibitory effect of protective neurotrophic factors on ROS is weakened, which further leads to an increase in ROS. The macromolecular substances in injured nerve cells cause nerve cell death and activate inflammation-related signaling pathways to release inflammatory factors. Sympathetic nerve activation by OSAS/IH could cause cognitive impairment independently of other mechanisms. In addition, OSAS/IH can directly activate microglia and astrocytes and promote the release of inflammatory cytokines in the central nervous system. Excessive neuroinflammatory responses could, in turn, promote the activation of glial cells, resulting in synaptic damage and loss, neuronal necrosis, and apoptosis and ultimately leading to exaggerated neurocognitive dysfunction. BDNF brain-derived neurotrophic factor, Mn-SOD superoxide dismutase, CAT catalase, COX-2 cyclooxygenase-2, iNOS inducible nitric oxide synthase
OSAS and metabolic diseases
Growing evidence in animal models of OSAS suggests that IH is independently associated with metabolic dysfunction. In particular, OSAS was independently associated with insulin resistance, suggesting that OSAS might be an important factor in the development of type 2 diabetes and so-called metabolic syndrome (MS), namely, obesity, insulin resistance, hypertension, and dyslipidemia. Studies have confirmed that the levels of fasting blood glucose and insulin resistance in OSAS patients are significantly higher than those in non-OSAS patients, and the severity of OSAS is related to an increase in insulin resistance. Moreover, the relationship between OSAS and insulin resistance also applies to nonobese patients.528 In addition, clinical data suggest that the AHI is an independent risk factor for insulin resistance and type 2 diabetes. With each unit increase in the AHI, the level of insulin resistance increased by 0.5%.529,530 In vivo kinetic studies of glucose metabolism have also demonstrated that severe OSAS impairs insulin sensitivity, glucose effectiveness, and pancreatic β-cell function.531 Oxidative stress and inflammation induced by intermittent hypoxemia in patients with OSAS may be key factors in insulin resistance. Inflammatory factors induced by OSAS, including TNF-α, IL-6, and IL-18, which activate NF-κB, JNK, and other downstream signaling pathways, inhibit insulin receptors and the phosphorylation of insulin receptor substrates, leading to insulin resistance.532 IH decreases glucose uptake in muscle, increases β-cell proliferation and β-cell death1 and can also affect ATP synthesis in pancreatic islet β cells, thereby inhibiting insulin secretion.532 Increased sympathetic tone in OSAS patients is a key mediator of deterioration of glycemic and insulin homeostasis, and increased levels of catecholamines after arousal directly stimulate glycogen mobilization and inhibit muscle glucose uptake, stimulate glucagon secretion, and inhibit insulin secretion.533 In addition, IH has been shown to induce lipid abnormalities, such as increased total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), very-low-density lipoprotein (VLDL), and low-density lipoprotein (LDL) levels, and the severity of lipid elevation is proportional to the severity of hypoxic stimulation.532 Several cross-sectional studies have shown that OSAS is independently associated with increased levels of total cholesterol, LDL, and triglycerides and that treatment of OSAS with CPAP may have beneficial effects on the lipid profile.532,534,535 In addition to the promotion of SREBP expression by IH mentioned earlier, IH is also related to lipoprotein lipase inhibition in adipose tissue, which leads to an increase in plasma chylomicron particles and VLDL that may be conducive to the progression of atherosclerosis.536 IH increases leptin gene expression levels, acting centrally and peripherally to inhibit insulin secretion while increasing glucose uptake. A number of reports have demonstrated that serum leptin levels are positively correlated with AHI and hypoxemia in patients with OSAS. The higher the serum leptin level is, the higher the AHI and the longer the duration of hypoxemia.532,537 Conversely, adiponectin’s effects counter those of leptin, an insulin-sensitizing hormone with antiatherogenic, anti-inflammatory, and antidiabetic effects, and IH may inhibit adiponectin secretion; studies have demonstrated significantly lower circulating adiponectin levels in patients with OSAS and a negative correlation with the AHI.538,539
In summary, OSAS leads to metabolic dysfunction (Fig. 8). However, the exact relationship between OSAS and metabolic diseases remains controversial, and most cross-sectional studies lack adequate sample sizes. The specific mechanism remains to be further studied. In addition, there is an urgent need to increase awareness of their strong association, and early detection of comorbidities cannot be overemphasized.
Fig. 8.
Theoretical framework of possible mechanisms by which sleep fragmentation and recurrent nocturnal arousals might contribute to the occurrence of metabolic syndrome. Two key features of OSAS, namely, sleep fragmentation and recurrent nocturnal arousal, could lead to increased sympathetic nerve activity and altered glucose metabolism in skeletal muscle. ROS production in fat and activation of inflammatory pathways lead to the increased release of inflammatory factors and changes in fat-related factors, leading to metabolic dysfunction and impaired islet function. In addition, elevated SREBPs and decreased lipase caused by inflammation and oxidative stress lead to associated lipid/lipoprotein abnormalities
OSAS and cancer
Over the past years, circumstantial, epidemiological, clinical, and animal-based experimental evidence has provided significant support that OSAS affects tumorigenesis and tumor development. A large multicenter cohort of cancer-free patients with OSAS showed that nocturnal hypoxemia was associated with all-cancer incidence in OSAS patients.540 Patients younger than 45 years with severe OSAS have a significantly higher incidence of all types of cancer than the general population.541 Epidemiologic studies have also confirmed that OSAS is associated with increased cancer-related mortality. A dose‒response relationship between OSAS severity and cancer-specific mortality was observed over a 22-year follow-up of 1522 participants in the community-based Wisconsin Sleep Cohort study, with severe OSAS conferring a nearly fivefold risk of death from cancer.542 OSAS appears to elevate the incidence of some tumor types, including lung cancer, breast cancer, prostate cancer, nasopharyngeal tumors, and melanoma. In certain types of tumors, IH exposure that mimics the oxygenation pattern induced by OSAS during sleep promotes the growth, invasion, and metastasis of lung cancer, colon cancer, and melanoma.543
OSAS-associated intermittent hypoxemia may affect tumor biology via several mechanisms, including oxygen-sensing pathways, chronic systemic inflammation, oxidative stress, endothelial dysfunction, and immune dysregulation. The carotid body response to hypoxemia and sleep fragmentation increases sympathetic nervous system activity, which might affect the tumor and its microenvironment and contribute to cancer progression.147 Oxidative stress promotes tumor occurrence and progression, and it has been mentioned previously that increased oxidative stress can cause damage to DNA, proteins, and lipids, leading to gene mutations, altered cell growth patterns, and, ultimately tumorigenesis. It has also been demonstrated that in sleep apnea, oxidative stress-induced DNA damage can increase the probability of genetic mutations and hence increase cell malignant transformation potential.544 In addition, ROS activate the AP-1 and NF-κB signaling pathways,545 with increased levels of AP-1 observed in many human tumor types. AP-1 regulates the expression of cell cycle regulators (p53, p19, p21, and cyclin D1) while also affecting the downregulation of tumor suppressor genes, thereby inducing hyperproliferation and tumorigenesis. NF-κB can induce the expression of cell proliferation molecules, apoptosis inhibitor factors, proangiogenic factors, and enzymes involved in extracellular matrix degradation. The activation of NF-κB increases the expression of genes associated with the inflammatory response and increases the cellular response to proinflammatory factors. In particular, the expression of COX-2, CC motif chemokine ligand 2 (CCL2), CXC motif chemokine ligand (CXCL)1, IL-8, and IL-6 was increased. All are inflammatory mediators involved in various neoplastic processes.546 Thus, NF-κB is regarded as having an important role in tumor development. ROS generated by IH can also activate HIF-1α, which is highly expressed in many solid tumors and plays an important role in many aspects of tumor angiogenesis, cell survival, proliferation, apoptosis, metastasis, invasion, and metabolism.547 Moreover, IH can affect the expression of HIF-1α downstream genes by upregulating the transcription of HIF-1α, for example, upregulating the expression of the vascular endothelial growth factor gene (VEGF), which in turn induces tumor angiogenesis and promotes tumor development, as also demonstrated in animal experiments using IH (or intermittent blood flow).548 Downregulation of immune responses against cancer is an important mechanism by which IH might affect tumor growth and aggressiveness. Data from studies of tumor-specific immune function in patients with OSAS also suggest that IH might contribute to reduced innate antitumor responses. The upregulation of tumor-promoting gene sets in untreated patients with severe OSAS was demonstrated by genome sequencing in circulating leukocytes, and the expression of these genes was downregulated after approximately one month of CPAP treatment.549 A key effector cell in cancer biology is the macrophage, and tumor-associated macrophages (TAMs) have now been identified as a crucial component of the cancer microenvironment, especially those with an anti-inflammatory M2 phenotype, inhibiting the antitumor activity of T cells and NK cells and releasing growth factors, cytokines, inflammatory mediators, and proteolytic enzymes involved in tumor growth and invasion to promote their proliferative development.550 Animal model experiments have found that IH exposure selectively induced a tumor-promoting phenotype, and TAMs explanted from IH-exposed mice enhanced the proliferation and invasiveness of lung epithelial cancer cells in vitro.551 More specifically, IH recruits more TAMs to participate in tumor progression and accelerates their transformation from an antitumor phenotype (M1) to a tumor-promoting phenotype (M2). It is interesting to find that CCL2 is a TAM recruiting factor,552 and PGE2 has an effect against tumor cells, playing an important role in the mechanism of cancer immune evasion. PGE2 inhibits the anticancer function of NK cells and enhances the cancer-promoting function of M2 macrophages and regulatory T (Treg) cells.553 Increased sympathetic activity caused by apnea may also contribute to cancer development. In vitro studies have shown that adrenergic signaling can regulate multiple cellular processes involved in cancer progression and that long-term treatment with β-blockers improves outcomes in several human cancers.554 In addition, evidence suggests that activated sympathetic nerves contribute importantly to changes in macrophage recruitment and differentiation that alter gene expression within the primary tumor.555
In conclusion, the available data suggest that OSAS might be an important risk factor for cancer development and aggressive cancer behavior. Data linking OSAS to the risk of neoplastic disease are scarce, but the above retrospective studies reveal the possibility of a close relationship (Fig. 9), which should stimulate more research on the effects of OSAS on carcinogenesis, tumor progression, and metastasis. In addition, there are currently no relevant studies reporting the complex links between sleep, adrenergic signaling, and cancer biology, suggesting a new direction for future research.
Fig. 9.
Potential mechanisms of the interaction between OSAS and cancer. IH could increase ROS levels in tumor tissues and further regulate cell cycle regulators through AP-1 to promote tumor proliferation. Elevated HIF-1α promotes the expression of VEGF and induces the growth of blood vessels in tumor tissues. The activation of NF-κB leads to the overexpression of tumor-related inflammatory mediators and tumor-related cellular immune dysfunction. In addition, enhanced sympathetic nerve activity releases norepinephrine, which can also change the tumor microenvironment and promote the occurrence of tumor cells. VEGF Vascular endothelial growth factor, CCL2 CC motif chemokine ligand 2, CXCL1 CXC motif chemokine ligand TAMs tumor-associated macrophages, M1 denotes antitumor phenotype macrophages, M2 denotes tumor-promoting phenotype macrophages
OSAS and reproductive disorders
Emerging evidence suggests556 that IH associated with OSAS might contribute to reduced fertility and decreased testicle antioxidant capacity in male patients with this sleep-breathing disorder. In parallel, motility impairment of sperm and increased oxidative stress markers were observed in the testes of middle-aged and young mice subjected to IH, which resulted in reduced sperm motility. In addition, OSAS has been reported to cause alterations in male sexual function, and previous studies using IH in an animal model of OSAS showed that mice subjected to a chronic exposure protocol develop erectile dysfunction accompanied by decreased libido and impaired sexual capability.557 Multiple studies have confirmed that 10 to 60% of patients with OSAS may experience erectile dysfunction, and although erectile dysfunction is a frequently reported sexual dysfunction in males with OSAS,558 notably, OSAS also has a negative impact on sexual function in females.559 Interestingly, erectile dysfunction may be significantly improved after treatment with CPAP.560 As mentioned above, OSAS can cause reduced NO production and elevated levels of endothelin, leading to endothelial dysfunction, which results in increased vasoconstriction and impaired endothelial cell function. It has also been shown that IH increases oxidative stress in erectile tissue through the modulation of NADPH oxidase enzymes, leading to decreased NO production and subsequently to impaired penile tumescence.557 Another potential mechanism is the nocturnal suppression of testosterone release,561 as peak testosterone levels coincide with the onset of REM sleep, but patients with OSAS suffer from disrupted sleep and a reduction in the number and time of REM sleep episodes, which is associated with reduced circulating testosterone concentrations. In addition, hypo- and hypercapnia suppress the increase in blood testosterone levels during the night. The results from a large cohort study suggest that OSAS is associated with an increased risk of preeclampsia, eclampsia, and gestational diabetes, even after controlling for obesity.562 Another retrospective population-based dataset study found an increased risk of preeclampsia among pregnant women with OSAS, and these differences remained significant after controlling for obesity.563 Moreover, experimental studies in animals have found that pregnant rodents subjected to chronic hypoxia developed preeclampsia-like symptoms.564 IH-induced inflammation and oxidative stress are considered major contributors to end-organ damage in preeclamptic patients.565 OSAS-induced inflammation-related factors (TNF-α, IL-6, IL-8, and CRP) might act through synergistic pathways with the pathogenesis of preeclampsia.566 Evidence suggests that hypoxia-related signaling pathways in preeclampsia might be mediated by the immune system.567 At present, the mechanisms linking OSAS to preeclampsia are also not well defined, and we propose some plausible mechanisms, but few studies have investigated these potential pathways. This hypothesis remains to be further studied.
OSAS and COVID-19
Coronavirus disease 2019 (COVID-19) is a severe respiratory-compromising disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus) infection and is currently causing a pandemic. The link between OSAS and COVID-19 is biologically plausible. First, systemic chronic low-grade inflammation in patients with OSAS407,408 might contribute to a more severe immune response to COVID-19. Furthermore, OSAS could exacerbate the core symptoms of severe COVID-19, especially during the night, when oxygen saturation levels in OSAS become lower, resulting in more pronounced hypoxemia-, oxidative stress-, and hypoxia-related manifestations. Studies have shown that the risk of infection with COVID-19 was much higher in OSAS patients than in non-OSAS patients. Among patients with COVID-19 infection, OSAS was associated with an increased risk of hospitalization and could increase the risk of developing respiratory failure.568 OSAS is known to be strongly associated with male sex, obesity, and diabetes, all of which are well-recognized risk factors for severe COVID-19.569 It is inevitable that the limitations of these important confounders influence such conclusions. After addressing possible confounders, the most recent study found that OSAS was associated with a twofold increased risk of severe COVID-19, a finding that could not be explained by obesity or other comorbidities.570 These current findings strongly suggest that OSAS is an independent factor contributing to the risk of more severe COVID-19.568,570,571 The most damaging complication during COVID-19 is the cytokine storm involving IL, TNF-α, CRP, leptin, and ferritin. Similar inflammatory responses observed during OSAS have been described in detail previously. There is a close relationship between hypoxemia and cytokine storms, and hypoxia/reoxygenation in OSAS patients worsens hypoxemia, thereby aggravating cytokine storms.572 Moreover, HIF-1α and NF-κB, which are associated with OSAS, are fully involved in the triggering effect of hypoxemia on cytokine storm development.573 Notably, studies have established that SARS-CoV-2 enters host cells by binding to the angiotensin-converting enzyme-2 (ACE-2) receptor.574,575 ACE-2 is a noncanonical pathway of the renin-angiotensin system (RAS) pathway, and therefore, the RAS itself is involved in the pathogenesis of COVID-19.576 Interestingly, the increased expression of ACE-2 and dysregulation of the RAS in untreated OSAS patients due to IH have been shown,577 which could facilitate the entry of the SARS-CoV-2 virus into host cells, increase its viral load and infectivity, and ultimately lead to severe disease outcomes and mortality. In addition, patients with OSAS might have a higher susceptibility to the SARS-CoV-2 virus and might be more susceptible to the virus.
In conclusion, we propose that dysregulation of the RAS plays an important role in the pathogenesis of COVID-19 in OSAS patients and that IH might exacerbate cytokine storms in COVID-19, leading to acute respiratory distress syndrome and multiorgan failure. Data from the current study are very limited, and further studies are needed to better define the relationship between OSAS and COVID-19.
Treatment
The treatment of OSAS aims to reduce symptoms, improve quality of life, reduce complications, and decrease mortality. Effective treatment of OSAS includes nonsurgical interventions (behavioral therapy, medical devices, and pharmacotherapy) (Table 7) and surgical procedures (Table 8). Behavioral therapy includes psychological education, cigarette smoking cessation, abstinence from alcohol and sedatives, aerobic exercise, weight loss, and avoiding the supine sleeping position. Behavioral therapy can address factors that may exacerbate OSAS. Regarding psychological education, doctors should communicate more with patients, patiently listen to their opinions and requirements, and explain in detail that OSAS is closely related to the occurrence of systemic diseases, which can help OSAS patients achieve a good psychological state to maintain a positive attitude toward the disease, which also contributes to improving patient compliance with subsequent treatment measures. Alcohol selectively decreases airway muscle tone and increases apnea frequency during sleep. In addition, alcohol also prolongs the duration of asphyxia by delaying arousal, and alcohol clearly interferes with the treatment of OSAS.578–580 A previous study showed that cigarette smoking might induce oropharyngeal narrowing and increase the severity of OSAS,581 and a recent meta-analysis found that secondhand smoke exposure is also significantly associated with OSAS.582 Cigarette smoking might increase the severity of OSAS by altering the sleep architecture, inducing upper airway inflammation, and interfering with upper airway neuromuscular function and arousal mechanisms.583 Weight loss may improve AHI in obese OSAS584–586 and should be recommended for all overweight or obese patients who are not suitable for other treatments. It could be used as the sole initial treatment for asymptomatic or minimally symptomatic patients. Recent studies have found that for OSAS patients with obesity, weight loss has been shown to be effective in reducing the tongue fat volume, which is directly related to a reduction in the AHI.587 In another randomized study, a lifestyle intervention that involved weight loss through diet and exercise resulted in a reduction of 10.2 kg and a reduction in the AHI of 9.7 events per hour in obese patients with type 2 diabetes mellitus and OSAS.588
Table 7.
Primary nonsurgical interventions for obstructive sleep apnea syndrome (OSAS)
| Treatment | Description | Indications and advantages | Downsides to treatment |
|---|---|---|---|
| Behavioral intervention | |||
| Psychoeducation | Targeted mental health counseling should be carried out, communication with patients should be strengthened, and knowledge of the disease should be introduced to patients in plain language. Patients should be advised to stop drinking, smoking, and taking sedatives | It can help OSAS patients achieve a good psychological state in order to maintain a positive attitude toward the disease, which also contributes to improving patient compliance for subsequent treatment measures | |
| Weight loss709–711 | Diet control, exercise therapy, and drug treatment | It is recommended for all overweight and obese patients diagnosed with OSAS; weight loss is beneficial for health and can improve cardiovascular and metabolic diseases and improve quality of life | It takes a long time; may not be effective for some patients; weight loss is hard to stick to |
| Exercise589,712 | Choose suitable aerobic exercise, such as jogging, walking, swimming, and ball games | Contributes to weight control; it improves apnea independently of other mechanisms; reduces the risk of chronic diseases | May be difficult for patients with excessive body weight, muscle and joint damage, and severe cardiopulmonary dysfunction |
| Positional treatment607 | Avoid sleeping in the supine position; tennis ball technique; chest position therapy device; neck position therapy device | Alternative treatments for patients with OSAS who are intolerant of PAP therapy; self-positioning has no cost; it is not expensive to wear | It is only applicable to patients with positional OSAS; shoulder problems or other physical disabilities can affect sleep in the side-lying position; adherence to treatment remains an issue |
| Pharmacologic Therapy46 | Medical therapy focuses on improving upper airway muscle tone, ventilatory drive, or the arousal threshold | Complementary therapeutic approaches; to improve the treatment compliance of patients; availability of pharmacologic therapy opens up new directions for the pathophysiological phenotype of OSAS | There are currently no marker pharmacologic treatments available in OSAS; much effort has been made to pharmacologically improve airway patency, but a large number of studies have not been of very high quality; relevant experimental models of OSAS are lacking |
| Noninvasive medical treatment | |||
| Positive airway pressure (PAP)614,713 | PAP treatment delivers pressure to the upper airway by circulating compressed room air via a mask worn over the nose or the nose and mouth. There are three modes of PAP delivery: CPAP, BPAP, and APAP | First-line treatment of OSAS; it can effectively eliminate nocturnal snoring and other respiratory events, correct nocturnal hypoxemia, and improve sleepiness and blood pressure | Approximately one-third of patients have poor tolerability; may cause nasal injury, leading to local compression necrosis; not easily fixed |
| Mandibular advancement device (oral appliances)626,714 | These devices are manufactured to accommodate the upper and lower teeth, are worn in the mouth, and during sleep, the lower jaw is kept in the anterior position | Patients with mild to moderate OSAS; PAP intolerant patients, PAP nonresponder patients, PAP treatment failure patients | There is a high cost and time required to build the equipment; temporomandibular joint discomfort, tooth pain, dryness of the mouth, or excessive saliva production |
Table 8.
Primary surgical treatment for obstructive sleep apnea syndrome (OSAS)
| Treatment | Description | Indications and advantages | Downsides to treatment |
|---|---|---|---|
| Uvulopalatopharyngoplasty (UPPP)636 | UPPP can expand the pharyngeal cavity and relieve the obstruction of the retropalatal plane by removing part of the hypertrophic soft palate tissue, palatal ptosis, the redundant soft tissue of the lateral pharyngeal wall, and hypertrophic palatine tonsil | Surgical methods are widely used; significantly improved symptoms in patients with OSAS | Surgical risks of the procedure; voice change, swallowing disorder, postoperative pain, and nasal regurgitation; recurrence occurs with weight gain; potential retroglossal collapse is not resolved |
| Maxillomandibular advancement645,715 | Maxillary Le Fort I osteotomy, mandibular sagittal split ramus osteotomy, and infrahyoid muscle group transection of the hyoid bone suspension were performed | The forward movement of the upper and lower jaws dilates the upper airway, the tongue falls back, and the collapse of the airway is reduced; mandibular deficiency, severe OSAS with multiple obstructions | Surgical risks of the procedure; the operation is complicated and the recovery time is long; potential complications include poor cosmetic results and facial paresthesia |
| Nasal surgery716 | It mainly includes septoplasty, turbinoplasty, and adenoidectomy | Nasal surgery is mainly used in CPAP-intolerant patients who have no response to medical treatment of nasal obstruction | Surgical risks of the procedure |
| Tracheostomy634,717 | A tracheostomy is a surgical procedure that incises the anterior wall of the trachea at the cervical level to allow a new respiratory passage to be established | Used in emergency situations only; rarely, it is performed in cases where other treatments for severe OSAS are not feasible | An unacceptable cosmetic result; effects on verbal communication; easy intercurrent infection; need for long-term tracheotomy care |
| Bariatric surgery718,719 | The most effective treatment for obesity; the three most common methods of weight loss in the United States are laparoscopic sleeve gastrectomy, Roux-en-Y gastric bypass, and laparoscopic adjustable gastric banding | Patients with OSAS (body mass index ≥35) who failed to achieve sufficient weight loss to achieve target health goals after behavioral therapy with or without medication | Contraindications include poor cardiac function, respiratory insufficiency, poor adherence to medication, and severe psychological disorders |
| Hypoglossal nerve stimulation648,720 | The stimulation device is surgically implanted subcutaneously to stimulate the hypoglossal nerve to increase the tongue protrusion and expand the upper airway and improve airflow in and out | Patients unwilling or unable to tolerate PAP; endoscopy during induction of anesthesia revealed no centripetal collapse of the soft palate location; body mass index <32 | Surgical site pain, infection, stiff tongue, pharyngeal pain, tongue muscle paralysis; expensive compared to alternative therapies |
Exercise is often recommended in conjunction with weight loss. In fact, general exercise, when used as the sole intervention, modestly improved OSAS severity,589 and was independent of weight loss.590–593 In a study of a heart failure population, exercise alone reduced the AHI, and exercise with CPAP was associated with a significantly reduced AHI.594 Interestingly, in another randomized clinical trial of patients with OSAS, exercise was associated with a 24 to 34% reduction in OSAS severity, with no significant change in body weight.591–593 The mechanism of this weight-independent improvement in OSAS is unclear. Redistribution of fat, decreased nocturnal leg fluid absorption, improved sleep quality, and increased pharyngeal muscle strength are thought to be underlying mechanisms of action. In another study of the association between exercise volume and OSAS prevalence, compared with individuals who did not exercise vigorously, those who exercised 1 to 2 h weekly, 3 to 6 h weekly, and at least 7 h weekly had odds ratios for moderate-to-severe OSAS of 0.62, 0.39, and 0.31, respectively.590
Positional OSAS was first defined by the Cartwright criteria,595 that is, the AHI during nonsupine sleep was at least 50% lower than that during supine sleep. Since then, its definition has been reiterated several times.596 A recent study applying Cartwright’s definition of positional OSAS found that 35.3% of a large number of patients with severe OSAS had positional sleep apnea.597 Alternatively, several studies have estimated that approximately half of OSAS cases appear or worsen only during supine sleep.598–600 There are multiple anatomical and physiological changes in the respiratory system capable of increasing the propensity for sleep-disordered breathing when switching from the nonsupine to the supine position. These include an increase in the loop gain,601 a reduction in airway diameter602,603 and a reduction in functional residual capacity.604 Traditional positional therapy is a variation of the “tennis ball technique” (TBT) and involves strapping a bulky object to the patient’s back to discourage supine sleep.605 This technique is effective in reducing supine sleep duration and is simple and affordable, but it is often uncomfortable for patients and therefore has poor long-term adherence. One study found that only 6% of patients adhered to the TBT at 2.5 years, which was stopped mainly due to discomfort.606 Although there are no standardized approaches to positional therapy and prospective data on its efficacy are lacking, for patients with positional OSAS, restricting sleep to the lateral or prone position may be an effective treatment.607,608
In 1981, Collin Sullivan proposed positive airway pressure (PAP) therapy609 as the primary treatment for patients with symptomatic OSAS of any severity.610 PAP treatment delivers pressure to the upper airway by circulating compressed room air via a mask worn over the nose or the nose and mouth. The elevated air pressure acts as a splint to prevent upper airway collapse during inspiration and improve oxygenation, thereby enabling normal breathing.609,611 There are many other different PAP options available, depending on the mode of positive pressure delivery and the setup.612 CPAP devices apply a fixed positive pressure, requiring pressure titration in the laboratory to determine the optimal treatment pressure. In patients with OSAS who cannot tolerate CPAP fixed pressure, autotitrating positive airway pressure (APAP) devices could be used. APAP can monitor airflow and adjust the delivered pressure in response to flow rate changes, airway resistance, and pressure changes,613 which contributes to initiating PAP therapy without laboratory titration, reduces costs, and increases convenience, and there is no significant difference in the efficacy or treatment compliance between laboratory titration and automatic titration.614 However, APAP devices may not be appropriate for patients with CSA or nocturnal hypoxemia due to causes other than sleep apnea. Bilevel positive airway pressure (BPAP) devices deliver higher pressures during inhalation than exhalation and may be considered to improve hypercapnia better in OSAS with other comorbidities (obesity hypoventilation syndrome) but are neither more effective nor more tolerated than CPAP or APAP devices. When an OSAS patient wears the device regularly during sleep, PAP normalizes the AHI to avoid apnea events in more than 90% of patients.614–616 Treatment effectiveness was dependent on adherence to device use, with longer nightly wear associated with greater improvement in symptoms617 and greater blood pressure reduction.618 Although adherence was arbitrary, adequate adherence was generally defined as use for 4 or more hours nightly for at least five nights per week.619 However, many patients with OSAS cannot tolerate PAP devices, resulting in poor compliance.620 Unfortunately, reported nonadherence rates range from 46 to 83%.621 In addition, many studies have also reported low adherence and irregular use status of CPAP.622–624 Measures to improve PAP adherence include informing of OSAS risks and expected benefits of PAP treatment, monitoring PAP use, and enhancing support for technical issues. Each of these measures increased PAP compliance by more than 30 min per night.625
Oral appliances are effective treatment options, especially for patients with mild to moderate OSAS.626,627 In addition, this option is also indicated for patients who are intolerant of CPAP, nonresponders to CPAP, CPAP treatment failure, or patients with more severe OSAS who prefer alternative treatments.586,628 The most common designs are mandibular advancement devices, palate lift devices, and tongue retention devices.629 Mandibular advancement devices have become a popular means of oral appliance treatment due to the poor adherence of palate lift devices and tongue retention devices.630 These devices are constructed of steel plates that fit into the upper and lower teeth. These combined plates can be adjusted to allow the mandible to advance relative to the maxilla, with the aim of enlarging the oropharynx and velopharynx during sleep and activating stretch receptors to reduce airway collapse and improve upper airway patency.631,632 A multicenter study of more than 400 patients treated with mandibular advancement devices found that the AHI of OSAS patients became normal (AHI < 5) in 37% of patients, decreased to <10 in 52%, and was more than halved in 64%.633 A recent meta-analysis of randomized clinical trials found that these devices were strongly associated with improvements in the AHI (mean reduction in the AHI of 13.6 events/hour).626
Tracheotomy was used to treat severe OSAS before the advent of PAP therapy, with the advantage of bypassing airway obstruction and significantly improving OSAS, but it is now rarely used in the management of OSAS.634 The most common surgical treatment for OSAS is uvulopalatopharyngoplasty (UPPP), which expands the oropharyngeal airway and reduces pharyngeal collapse by altering the upper airway soft tissues, including the lateral pharyngeal walls, tongue base, and palate.635,636 According to the available reports, the AHI and lowest oxygen saturation of the blood are significantly improved after surgery, the oropharyngeal cavity diameter is significantly increased, and the surgical treatment rate of UPPP is approximately 33%.637,638 Multiple randomized trials have found significant reductions in the AHI with UPPP compared with observation controls.639,640 In these larger trials (32 surgery patients and 33 control patients), surgery was strongly associated with a mean decrease in the AHI from 53.3 to 21.1 beats per hour, whereas no significant change was observed in the control group.639 However, in patients with severe OSAS, its effect on AHI is limited, and long-term adverse effects have been reported.641–643 The limitations of UPPP include its failure to improve the lateral dimensions of the upper airway, to address retroglossal collapse, or to address the reduction in upper airway dilator muscle tone.643 Therefore, UPPP combined with other surgical treatments is necessary. Liu et al. found that UPPP combined with tongue base radiofrequency ablation increased the total effective rate of OSAS to 71.9%.644 Therefore, patients with retropalatal collapse are more suitable for UPPP, although this is difficult to diagnose definitively.642 Surgical modification of the facial bone structure can also be used to treat OSAS. The most studied procedure is maxillomandibular advancement, which combines a standard Le Fort I osteotomy with a sagittal split mandibular osteotomy to facilitate maxillary and mandibular advancement and to fix the facial skeleton by approximately 10 mm forward. It achieves upper airway dilation by physically expanding the skeletal frame of the face. A recent meta-analysis of individual data from 45 studies, including 455 patients/interventions showed that maxillomandibular advancement surgery was associated with an average 80% reduction in the AHI, consistent with a mean change of -47.8 (25.0) events/hour.645
Hypoglossal nerve stimulation is an advanced surgical treatment that can improve the tone of pharyngeal dilator muscles during sleep.646 At present, the most widely used technique and most used commercial implantation system places the stimulating electrode on the medial branch of the right hypoglossal nerve to enhance the ipsilateral tongue process. The respiration-sensing sensor is placed between the internal and external intercostal muscles to detect inspiratory power, and an implantable pulse generator is implanted in the chest wall to trigger hypoglossal nerve electrodes in response to respiratory effort.647,648 Adult patients with moderate-to-severe OSAS who failed or could not tolerate noninvasive treatment were recruited in a multicenter prospective single-group trial. Patients with OSAS had an AHI of 20 to 50 and a BMI of ≤32. In addition, exclusion criteria included CSA, positional OSAS, severe cardiopulmonary or neuromuscular disease, or concentric collapse of the retropalatal airway on drug-induced sleep endoscopy. When assessed after 12 months, this surgical modality reduced the median AHI from 29.3/h to 9/h.649 Treatment with hypoglossal nerve stimulation was associated with quality of life and improvements in sleepiness after 5 years, with a 63% remission rate.650 There were no serious adverse events. Thus, hypoglossal nerve stimulation is a surgical treatment with sustained benefits. Recently, a novel device known as the GENIO system has been developed to provide bilateral hypoglossal nerve stimulation for moderate-to-severe OSAS, resulting in a 45% decrease in the AHI,651 and transcutaneous stimulation is also under investigation.652 Although treatment with hypoglossal nerve stimulation appears to be effective and well tolerated, it is invasive and more costly than oral appliances and PAP.
Currently, there are no effective drugs available to treat OSAS. However, along with the development of modalities to address the nonanatomical pathogenesis of OSAS (pharyngeal critical closing pressure, muscle responsiveness, loop gain, nocturnal rostral fluid shift, and arousal threshold), it is helpful to guide the pharmacological development of novel OSAS targeted therapies (Table 9). Usually, hypnotic agents are contraindicated in OSAS due to concerns about upper airway muscle relaxation. Nevertheless, recent studies have shown that drugs such as eszopiclone could increase the arousal threshold and reduce the AHI without hypoxemia, which can be used as an adjuvant treatment for OSAS patients with good upper airway muscle activity and a low arousal threshold.653 Furthermore, standard doses of zolpidem affected respiratory arousal thresholds to varying degrees and did not interfere with pharyngeal muscle activity during sleep.654 Acetazolamide, a carbonic anhydrase inhibitor with diuretic properties that stimulates respiratory excitation through metabolic acidosis, has been shown to decrease the loop gain associated with OSAS, thereby improving ventilatory stability.655–657 In a study involving 13 patients with OSAS, acetazolamide (500 mg twice daily) for 1 week resulted in a 40% reduction in loop gain and a 50% reduction in the AHI.655 Another study involving 13 men with moderate-to-severe OSAS randomized participants to acetazolamide alone, CPAP alone, and acetazolamide + CPAP. Two weeks later, the AHI had decreased in all three groups, with the acetazolamide + CPAP group showing the greatest AHI reduction.658 A previous study showed that aminopyridine (a potassium channel blocker) is able to improve genioglossus activity during REM sleep.659 It is well known that potassium conductance mediates the reduction in motor neuron excitability by neuromodulators. Blocking some potassium channels in the hypoglossal motor pool could significantly enhance the activity of the genioglossus in sleep, which provides a novel direction for research on OSAS drug treatment.660 Interestingly, topical administration of potassium channel blockers increased upper airway reflex activity in animals and prevented negative pressure-induced upper airway collapse.661 Further studies are needed to clarify the role of potassium channel blockers in OSAS in humans. For OSAS patients with weaker muscle function, the tricyclic antidepressant desipramine reduces the severity of OSAS by preventing the sleep-induced decrease in genioglossus activity, thereby improving upper airway collapse.662 A recent study evaluated the AHI in patients with significant OSAS with the combination of atomoxetine (a norepinephrine reuptake inhibitor) and oxybutynin (an antimuscarinic agent).663
Table 9.
Targeted pharmacotherapy to treat obstructive sleep apnea syndrome (OSAS)
| Class | Pharmacotherapeutic agents | Reference | Mechanism of action |
|---|---|---|---|
| Anatomical impairment | Liraglutide | Blackman et al. (2016)721 | Reduce body weight, leading to a decrease in upper airway fat (due to obesity) and thus reduce narrowing and/or the propensity for closure during sleep, which may decrease Pcrit in susceptible individuals |
| Spironolactone and furosemide | Blackman et al. (2018)722 | Reduce fluid retention, thereby reducing nighttime fluid transfer from the limbs to the neck | |
| Nasal decongestants (Mometasone alone) | Acar et al. (2013)723 | Reducing nasal resistance can induce pharyngeal dilatation by decreasing the negative suction pressure downstream in the velo- and oropharynx | |
| Fluticasone | Kiely et al. (2004)724 | ||
| Nasal steroid dexamethasone with the decongestant tramazoline | Koutsourelakis et al. (2013)725 | ||
| Low arousal threshold | Triazolam | Berry et al. (1995)726 | Raising the arousal threshold might have the potential to buy time for the upper airway muscle recruitment and the stabilization of airway patency; zolpidem, diphenhydramine, and lorazepam all increased arousal threshold; lorazepam and zolpidem increased genioglossus activity before arousal in response to hypercapnia |
|
Lorazepam Zolpidem Diphenhydramine Eszopiclone |
Carberry et al. (2017)654 Carter et al. (2016)727 Eckert et al. (2011)653 Rosenberg et al. (2006)728 Carberry et al. (2017)654 Park et al. (2008)729 |
||
| Sodium oxybate | George et al. (2011)730 | Sodium oxybate reduces the severity of sleep apnea by increasing deep sleep time and increasing the arousal threshold | |
| Trazodone |
Eckert et al. (2014)731 Smales et al. (2015)732 |
Trazodone can increase the arousal threshold in response to hypercapnia and allow tolerance to higher CO2 levels without arousal, thus stabilizing sleep | |
| High loop gain | Carbonic anhydrase inhibitor: Zonisamide and Acetazolamide |
Eskandari et al. (2014)733 Eskandari et al. (2018)658 Edwards et al. (2013)734 Edwards et al. (2012)655 Schmickl et al. (2020)735 Schmickl et al. (2021)656 Tojima et al. (1988)736 |
Agents targeting loop gain reduce the PCO2 reserve by producing transient metabolic acidosis and relative hyperventilation, thus widening the difference between eupneic paCO2 and the apneic threshold, effectively reducing loop gain by reducing plant gain, stabilizing ventilator drive leading to respiratory tract opening and decreasing obstructive events |
| Oxygen therapy |
Sands et al. (2018)737 Wellman et al. (2008)738 Pokorski et al. (2000)739 Joosten et al. (2021)740 Wang et al. (2018)741 |
Oxygen therapy can reduce the circulation gain by quieting the chemosensory output of an overly sensitive chemoreflex system, which converts the perceived change in gas tension into a smaller change in the ventilatory drive. | |
| Carbon dioxide Rebreathing |
Dempsey et al. (2004)742 Messineo et al. (2018)743 Xie et al. (2013)744 |
CO2 is added during hyperpnea to prevent transient hypocapnia to stabilize periodic respiratory abnormalities. In patients with high loop gain, CO2 rebreathing seems to be a promising treatment | |
| Poor muscle responsiveness | Noradrenergic mechanisms: Desipramine, Protriptyline, Atomoxetine, and Antimuscarinic oxybutynin |
Taranto-Montemurro et al. (2016)662 Taranto-Montemurro et al. (2016)745 Hanzel et al. (1991)746 Smith et al. (1983)747 Bart Sangal et al. (2008)748 |
By identifying the receptor targets that stimulate the upper airway muscles, we can manipulate the airway muscle tone to prevent upper airway muscle relaxation, restore pharyngeal muscle activity, and then restore upper airway patency through reflexive recruitment; desipramine could increase genioglossus activity and reduce upper airway collapse during sleep in humans |
|
Serotonergic mechanisms: Ondansetron, Buspirone, Mirtazapine, Paroxetine, Fluoxetine, and l-Tryptophan |
Veasey et al. (2001)749 Mendelson et al. (1991)750 Carley et al. (2007)751 Berry et al. (1999)752 Hanzel et al. (1991)746 Schmidt et al. (1983)753 |
Serotonergic drive is attenuated centrally from wakefulness to NREM sleep and reaches a minimum during REM sleep, resulting in a relative reduction in ventilatory drive. Central administration of serotonin mediates respiratory excitation through 5-HT2a/c receptors on upper airway motoneurons and 5-HT1a receptors on respiratory neurons. Serotonin has different effects on central and peripheral respiration, but 5-HT3 antagonists and 5-HT1a agonists consistently improve respiration | |
|
K+ channel blockers: 4-aminopyridine, Tetraethylammonium, and Doxapram |
Grace et al. (2013)754 Suratt et al. (1986)755 |
Blocking potassium channels promotes membrane depolarization and cellular excitability, which leads to increased genioglossus activity during REM and NREM sleep; cannabinoids improve respiratory stability by attenuating the feedback of the vagus nerve to the medulla to help stabilize breathing and activate pharyngeal muscles | |
| Cannabinoids |
Guo et al. (2004)756 Prasad et al. (2013)757 |
||
| Nicotine | Gothe et al. (1985)758 | ||
| Other pharmacotherapeutic agents involved in OSAS | |||
| Forskolin | Aoki et al. (1985)759 | During wakefulness and non-REM sleep, forskolin increases cAMP at the hypoglossal motor nucleus, which in turn increases the activity of the pharyngeal muscle | |
| Xanthines | Lagercrantz et al. (1985)760 | Increase ventilation by antagonizing adenosine in the central nervous system and increasing diaphragm contractility | |
Conclusion
The past two decades have seen unprecedented growth in sleep medicine, mostly owing to the growing awareness of OSAS and its profound impact on patient’s quality of life. As described above, epidemiological data and evidence from clinical trials, animal studies, and in vitro experiments indicate that IH caused by OSAS could lead to the activation of different signaling pathways and is closely related to the damage to multiple tissues and organs, in which oxidative stress, inflammation, and sympathetic activation are essential components of OSAS-related diseases, and IH plays an important role in the pathogenesis, development, and prognosis of multiple diseases. More in vitro and animal studies at the cellular level (different cell types) are needed in future studies to uncover new underlying mechanisms of IH and to predict new IH-related diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.82000771), Shandong Provincial Natural Science Foundation (Grant No. ZR2021MH014), Jinan Municipal Science and Technology Project (Grant No. 202134033), and Shandong Provincial Youth Innovation Team Development Plan of Colleges and Universities (2022KJ193).
Author contributions
All authors have read and approved the article. R.L. conceived and drafted the manuscript. X.L., Y.Z., and Y.H. discussed the concepts of the manuscript. R.L., N.D., and X.W. drew the figures. H.Y. and Q.Y. approved the version to be submitted.
Competing interests
The authors declare no competing interests.
Contributor Information
Hongmei Yue, Email: yuehongmei18@sina.com.
Qingqing Yin, Email: yinqingqing@sdfmu.edu.cn.
References
- 1.Lévy P, et al. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Prim. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 2.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med. Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Salzano G, et al. Obstructive sleep apnoea/hypopnoea syndrome: relationship with obesity and management in obese patients. Acta Otorhinolaryngol. Ital. 2021;41:120–130. doi: 10.14639/0392-100X-N1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med. Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Benjafield AV, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin. Chest Med. 2010;31:179–186. doi: 10.1016/j.ccm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, et al. Racial/ethnic differences in sleep disturbances: the multi-ethnic study of atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 9.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Stradling JR, Davies RJ. Sleep. 1: obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax. 2004;59:73–78. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theorell-Haglöw J, et al. Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults - What do we know? A clinical update. Sleep Med. Rev. 2018;38:28–38. doi: 10.1016/j.smrv.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107:362–366. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 14.Resta O, Bonfitto P, Sabato R, De Pergola G, Barbaro MP. Prevalence of obstructive sleep apnoea in a sample of obese women: effect of menopause. Diabetes Nutr. Metab. 2004;17:296–303. [PubMed] [Google Scholar]
- 15.Laouafa S, et al. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep. 2017;40:zsx104. doi: 10.1093/sleep/zsx104. [DOI] [PubMed] [Google Scholar]
- 16.Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, Llamas-Carreras JM, Solano-Reina E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med. Oral. Patol. Oral. Cir. Bucal. 2012;17:e925–e929. doi: 10.4317/medoral.17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur VK, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 19.Yeghiazarians Y, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144:e56–e67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DJ. Sleep apnea and cardiovascular disease. Curr. Diab. Rep. 2021;21:64. doi: 10.1007/s11892-021-01426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv R, et al. Dysfunction in automatic processing of emotional facial expressions in patients with obstructive sleep apnea syndrome: an event-related potential study. Nat. Sci. Sleep. 2020;12:637–647. doi: 10.2147/NSS.S267775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44:zsab076. doi: 10.1093/sleep/zsab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo WB, et al. [Obstructive sleep apnea and metabolic syndrome: an association study based on a large sample clinical database] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;56:1263–1269. doi: 10.3760/cma.j.cn115330-20210531-00314. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Kim B, Han K, Kim SW. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: a nationwide population-based study. Sci. Rep. 2021;11:8751. doi: 10.1038/s41598-021-88233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krolow GK, Garcia E, Schoor F, Araujo FBS, Coral GP. Obstructive sleep apnea and severity of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021;33:1104–1109. doi: 10.1097/MEG.0000000000001920. [DOI] [PubMed] [Google Scholar]
- 26.Chung GE, et al. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci. Rep. 2021;11:13473. doi: 10.1038/s41598-021-92703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Intermittent hypoxia aggravates non-alcoholic fatty liver disease via RIPK3-dependent necroptosis-modulated Nrf2/NFκB signaling pathway. Life Sci. 2021;285:119963. doi: 10.1016/j.lfs.2021.119963. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, et al. Circulating endocannabinoids and insulin resistance in patients with obstructive sleep apnea. Biomed. Res. Int. 2016;2016:9782031. doi: 10.1155/2016/9782031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun S, et al. Insulin resistance is associated with Sfrp5 in obstructive sleep apnea. Braz. J. Otorhinolaryngol. 2019;85:739–745. doi: 10.1016/j.bjorl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Yu Q, Yue H, Zeng S, Cui F. Effect of intermittent hypoxia and rimonabant on glucose metabolism in rats: involvement of expression of GLUT4 in skeletal muscle. Med. Sci. Monit. 2015;21:3252–3260. doi: 10.12659/MSM.896039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Chu Y, Jiang Z, Yu Q. Losartan protects against intermittent hypoxia-induced peritubular capillary loss by modulating the renal renin-angiotensin system and angiogenesis factors. Acta Biochim. Biophys. Sin. 2020;52:38–48. doi: 10.1093/abbs/gmz136. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Yue H, Zhang J, Pu J, Yu Q. Effects of plasma ghrelin, obestatin, and ghrelin/obestatin ratio on blood pressure circadian rhythms in patients with obstructive sleep apnea syndrome. Chin. Med. J. 2014;127:850–855. [PubMed] [Google Scholar]
- 33.Yuan F, Zhang S, Liu X, Liu Y. Correlation between obstructive sleep apnea hypopnea syndrome and hypertension: a systematic review and meta-analysis. Ann. Palliat. Med. 2021;10:12251–12261. doi: 10.21037/apm-21-3302. [DOI] [PubMed] [Google Scholar]
- 34.Kendzerska T, et al. Obstructive sleep apnea and incident cancer: a large retrospective multicenter clinical cohort study. Cancer Epidemiol. Biomark. Prev. 2021;30:295–304. doi: 10.1158/1055-9965.EPI-20-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polasky C, et al. Redistribution of monocyte subsets in obstructive sleep apnea syndrome patients leads to an imbalanced PD-1/PD-L1 cross-talk with CD4/CD8 T Cells. J. Immunol. 2021;206:51–58. doi: 10.4049/jimmunol.2001047. [DOI] [PubMed] [Google Scholar]
- 36.Elfanagely Y, Atsawarungruangkit A, Scharfen J, Pavlech L, Moss SF. Association between obstructive sleep apnea and Barrett’s esophagus: a systematic review and meta-analysis. Dig. Dis. Sci. 2021;66:3689–3697. doi: 10.1007/s10620-020-06709-1. [DOI] [PubMed] [Google Scholar]
- 37.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer P, et al. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur. Respir. J. 1996;9:1801–1809. doi: 10.1183/09031936.96.09091801. [DOI] [PubMed] [Google Scholar]
- 39.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J. Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmann OP, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 41.Riley R, Guilleminault C, Herran J, Powell N. Cephalometric analyses and flow-volume loops in obstructive sleep apnea patients. Sleep. 1983;6:303–311. doi: 10.1093/sleep/6.4.303. [DOI] [PubMed] [Google Scholar]
- 42.Neelapu BC, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017;31:79–90. doi: 10.1016/j.smrv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Tan HL, Kheirandish-Gozal L, Abel F, Gozal D. Craniofacial syndromes and sleep-related breathing disorders. Sleep Med. Rev. 2016;27:74–88. doi: 10.1016/j.smrv.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciscar MA, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur. Respir. J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 45.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol. Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schütz SG, Dunn A, Braley TJ, Pitt B, Shelgikar AV. New frontiers in pharmacologic obstructive sleep apnea treatment: a narrative review. Sleep Med. Rev. 2021;57:101473. doi: 10.1016/j.smrv.2021.101473. [DOI] [PubMed] [Google Scholar]
- 47.Pae EK, Lowe AA. Tongue shape in obstructive sleep apnea patients. Angle Orthod. 1999;69:147–150. doi: 10.1043/0003-3219(1999)069<0147:TSIOSA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Tourneux P, et al. Influence of thermal drive on central sleep apnea in the preterm neonate. Sleep. 2008;31:549–556. doi: 10.1093/sleep/31.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starling EH. On the absorption of fluids from the connective tissue spaces. J. Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krogh A, Landis EM, Turner AH. The movement of fluid through the human capillary wall in relation to venous pressure and to the colloid osmotic pressure of the blood. J. Clin. Invest. 1932;11:63–95. doi: 10.1172/JCI100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youmans JB, Wells HS, Donley D, Miller DG, Frank H. The effect of posture (standing) on the serum protein concentration and colloid osmotic pressure of blood from the foot in relation to the formation of edema. J. Clin. Invest. 1934;13:447–459. doi: 10.1172/JCI100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levick JR, Michel CC. The effects of position and skin temperature on the capillary pressures in the fingers and toes. J. Physiol. 1978;274:97–109. doi: 10.1113/jphysiol.1978.sp012136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winkel J, Jørgensen K. Evaluation of foot swelling and lower-limb temperatures in relation to leg activity during long-term seated office work. Ergonomics. 1986;29:313–328. doi: 10.1080/00140138608968267. [DOI] [PubMed] [Google Scholar]
- 54.Hildebrandt W, et al. Enhanced slow caudad fluid shifts in orthostatic intolerance after 24-h bed-rest. Eur. J. Appl. Physiol. Occup. Physiol. 1994;69:61–70. doi: 10.1007/BF00867929. [DOI] [PubMed] [Google Scholar]
- 55.Baccelli G, et al. Scintigraphic recording of blood volume shifts. J. Nucl. Med. 1995;36:2022–2031. [PubMed] [Google Scholar]
- 56.Chiu KL, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am. J. Respir. Crit. Care Med. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 57.Shiota S, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–872. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su MC, et al. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir. Physiol. Neurobiol. 2008;161:306–312. doi: 10.1016/j.resp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Schwab RJ, Gefter WB, Pack AI, Hoffman EA. Dynamic imaging of the upper airway during respiration in normal subjects. J. Appl Physiol. 1993;74:1504–1514. doi: 10.1152/jappl.1993.74.4.1504. [DOI] [PubMed] [Google Scholar]
- 60.Issa FG, Sullivan CE. Upper airway closing pressures in snorers. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984;57:528–535. doi: 10.1152/jappl.1984.57.2.528. [DOI] [PubMed] [Google Scholar]
- 61.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984;57:520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 62.Morrison DL, et al. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 1993;148:606–611. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 63.Gleadhill IC, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am. Rev. Respir. Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 64.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 65.Kazemeini E, et al. Critical to know Pcrit: a review on pharyngeal critical closing pressure in obstructive sleep apnea. Front. Neurol. 2022;13:775709. doi: 10.3389/fneur.2022.775709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J. Appl. Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 67.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J. Appl. Physiol. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 68.Bosi M, Incerti Parenti S, Fiordelli A, Poletti V, Alessandri-Bonetti G. Upper airway collapsibility in patients with OSA treated with continuous positive airway pressure: a retrospective preliminary study. J. Clin. Sleep Med. 2020;16:1839–1846. doi: 10.5664/jcsm.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep. 2016;39:511–521. doi: 10.5665/sleep.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genta PR, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep. 2014;37:1673–1678. doi: 10.5665/sleep.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geckil AA, Ermis H. The relationship between anxiety, depression, daytime sleepiness in the REM-related mild OSAS and the NREM-related mild OSAS. Sleep Breath. 2020;24:71–75. doi: 10.1007/s11325-019-01838-y. [DOI] [PubMed] [Google Scholar]
- 72.Xiao SC, et al. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep. 2015;38:941–949. doi: 10.5665/sleep.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshino T, et al. Estimated respiratory arousal threshold in patients with rapid eye movement obstructive sleep apnea. Sleep Breath. 2022;26:347–353. doi: 10.1007/s11325-021-02399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee RWW, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22:1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 75.Altree TJ, Chung F, Chan MTV, Eckert DJ. Vulnerability to postoperative complications in obstructive sleep apnea: importance of phenotypes. Anesth. Analg. 2021;132:1328–1337. doi: 10.1213/ANE.0000000000005390. [DOI] [PubMed] [Google Scholar]
- 76.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 77.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 2014;116:302–313. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 78.Younes M, et al. Mechanisms of breathing instability in patients with obstructive sleep apnea. J. Appl. Physiol. 2007;103:1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 79.Amatoury J, et al. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016;39:2091–2100. doi: 10.5665/sleep.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campana L, Eckert DJ, Patel SR, Malhotra A. Pathophysiology & genetics of obstructive sleep apnoea. Indian J. Med. Res. 2010;131:176–187. [PMC free article] [PubMed] [Google Scholar]
- 81.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 82.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am. Rev. Respir. Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 83.Deacon-Diaz N, Malhotra A. Inherent vs. induced loop gain abnormalities in obstructive sleep apnea. Front. Neurol. 2018;9:896. doi: 10.3389/fneur.2018.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deacon-Diaz NL, Sands SA, McEvoy RD, Catcheside PG. Daytime loop gain is elevated in obstructive sleep apnea but not reduced by CPAP treatment. J. Appl. Physiol. 2018;125:1490–1497. doi: 10.1152/japplphysiol.00175.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panza GS, et al. Increased oxidative stress, loop gain and the arousal threshold are clinical predictors of increased apnea severity following exposure to intermittent hypoxia. Nat. Sci. Sleep. 2019;11:265–279. doi: 10.2147/NSS.S228100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naughton MT. Loop gain in apnea: gaining control or controlling the gain? Am. J. Respir. Crit. Care Med. 2010;181:103–105. doi: 10.1164/rccm.200909-1449ED. [DOI] [PubMed] [Google Scholar]
- 87.Waters T, Mehra R. Clinical neurophysiology of apnea. Handb. Clin. Neurol. 2019;161:345–352. doi: 10.1016/B978-0-444-64142-7.00059-X. [DOI] [PubMed] [Google Scholar]
- 88.Rowley JA, Badr MS. Central sleep apnea in patients with congestive heart failure. Sleep Med. Clin. 2017;12:221–227. doi: 10.1016/j.jsmc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 89.White DP. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 90.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog. Cardiovasc. Dis. 2009;51:313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deacon NL, Catcheside PG. The role of high loop gain induced by intermittent hypoxia in the pathophysiology of obstructive sleep apnoea. Sleep Med. Rev. 2015;22:3–14. doi: 10.1016/j.smrv.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Adachi S, Lowe AA, Tsuchiya M, Ryan CF, Fleetham JA. Genioglossus muscle activity and inspiratory timing in obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 1993;104:138–145. doi: 10.1016/S0889-5406(05)81003-0. [DOI] [PubMed] [Google Scholar]
- 93.Marra S, Arnaldi D, Nobili L. The pharmacotherapeutic management of obstructive sleep apnea. Expert Opin. Pharmacother. 2019;20:1981–1991. doi: 10.1080/14656566.2019.1652271. [DOI] [PubMed] [Google Scholar]
- 94.Ishikawa O, Oks M. Central sleep apnea. Clin. Geriatr. Med. 2021;37:469–481. doi: 10.1016/j.cger.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Muza RT. Central sleep apnoea-a clinical review. J. Thorac. Dis. 2015;7:930–937. doi: 10.3978/j.issn.2072-1439.2015.04.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Javaheri S, Dempsey JA. Central sleep apnea. Compr. Physiol. 2013;3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 97.Ginter G, Badr MS. Central sleep apnea. Handb. Clin. Neurol. 2022;189:93–103. doi: 10.1016/B978-0-323-91532-8.00011-2. [DOI] [PubMed] [Google Scholar]
- 98.Landry SA, et al. Ventilatory control sensitivity in patients with obstructive sleep apnea is sleep stage dependent. Sleep. 2018;41:zsy040. doi: 10.1093/sleep/zsy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr. Pulmonol. Rep. 2019;8:14–21. doi: 10.1007/s13665-019-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roberts EG, Raphelson JR, Orr JE, LaBuzetta JN, Malhotra A. The pathogenesis of central and complex sleep apnea. Curr. Neurol. Neurosci. Rep. 2022;22:405–412. doi: 10.1007/s11910-022-01199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J. Appl Physiol. Respir. Environ. Exerc. Physiol. 1983;55:813–822. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- 102.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J. Appl Physiol. 2000;89:192–199. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 103.Ginter G, et al. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J. Appl. Physiol. 2020;128:960–966. doi: 10.1152/japplphysiol.00532.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olson LG, Strohl KP. Airway secretions influence upper airway patency in the rabbit. Am. Rev. Respir. Dis. 1988;137:1379–1381. doi: 10.1164/ajrccm/137.6.1379. [DOI] [PubMed] [Google Scholar]
- 105.Leevers AM, Simon PM, Dempsey JA. Apnea after normocapnic mechanical ventilation during NREM sleep. J. Appl. Physiol. 1994;77:2079–2085. doi: 10.1152/jappl.1994.77.5.2079. [DOI] [PubMed] [Google Scholar]
- 106.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J. Appl. Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 107.Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27:281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Loenarz C, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12:63–70. doi: 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology. 2010;25:272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 110.Luo Z, et al. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduct. Target Ther. 2022;7:218. doi: 10.1038/s41392-022-01080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corrado C, Fontana S. Hypoxia and HIF signaling: one axis with divergent effects. Int. J. Mol. Sci. 2020;21:5611. doi: 10.3390/ijms21165611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Löfstedt T, et al. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 113.Lu X, Prodger A, Sim J, Evans CE. Pulmonary thrombosis promotes tumorigenesis via myeloid hypoxia-inducible factors. Biomolecules. 2022;12:1354. doi: 10.3390/biom12101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cummins EP, Keogh CE, Crean D, Taylor CT. The role of HIF in immunity and inflammation. Mol. Asp. Med. 2016;47-48:24–34. doi: 10.1016/j.mam.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflug. Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 117.Yang C, et al. HIF-1: structure, biology and natural modulators. Chin. J. Nat. Med. 2021;19:521–527. doi: 10.1016/S1875-5364(21)60051-1. [DOI] [PubMed] [Google Scholar]
- 118.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 120.Watts ER, Walmsley SR. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol. Med. 2019;25:33–46. doi: 10.1016/j.molmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 121.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 122.Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol. Chem. 2004;385:223–230. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 123.Jeong JW, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/S0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 124.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jewell UR, et al. Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J. 2001;15:1312–1314. doi: 10.1096/fj.00-0732fje. [DOI] [PubMed] [Google Scholar]
- 126.Heun Y, et al. The phosphatase SHP-2 activates HIF-1α in wounds in vivo by inhibition of 26S proteasome activity. Int. J. Mol. Sci. 2019;20:4404. doi: 10.3390/ijms20184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol. Pharm. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 128.Yu Z, et al. Insights from molecular dynamics simulations and steered molecular dynamics simulations to exploit new trends of the interaction between HIF-1α and p300. J. Biomol. Struct. Dyn. 2020;38:1–12. doi: 10.1080/07391102.2019.1580616. [DOI] [PubMed] [Google Scholar]
- 129.Wu D, et al. A novel function of novobiocin: disrupting the interaction of HIF 1α and p300/CBP through direct binding to the HIF1α C-terminal activation domain. PLoS ONE. 2013;8:e62014. doi: 10.1371/journal.pone.0062014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slemc L, Kunej T. Transcription factor HIF1A: downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumour Biol. 2016;37:14851–14861. doi: 10.1007/s13277-016-5331-4. [DOI] [PubMed] [Google Scholar]
- 131.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem. Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 133.Taylor CT, Doherty G, Fallon PG, Cummins EP. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Invest. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peek CB, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25:86–92. doi: 10.1016/j.cmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 2021;599:23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 136.McGettrick AF, O’Neill LAJ. The role of HIF in immunity and inflammation. Cell Metab. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharm. 2013;13:646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 138.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tomc J, Debeljak N. Molecular insights into the oxygen-sensing pathway and erythropoietin expression regulation in erythropoiesis. Int. J. Mol. Sci. 2021;22:7074. doi: 10.3390/ijms22137074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int. J. Mol. Sci. 2021;22:5703. doi: 10.3390/ijms22115703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 142.Sun J, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020;11:373. doi: 10.1186/s13287-020-01881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J, et al. HIF-1α inhibits mitochondria-mediated apoptosis and improves the survival of human adipose-derived stem cells in ischemic microenvironments. J. Plast. Reconstr. Aesthet. Surg. 2021;74:1908–1918. doi: 10.1016/j.bjps.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 144.Karagiota A, Kourti M, Simos G, Mylonis I. HIF-1α-derived cell-penetrating peptides inhibit ERK-dependent activation of HIF-1 and trigger apoptosis of cancer cells under hypoxia. Cell Mol. Life Sci. 2019;76:809–825. doi: 10.1007/s00018-018-2985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen X, et al. Trim21-mediated HIF-1α degradation attenuates aerobic glycolysis to inhibit renal cancer tumorigenesis and metastasis. Cancer Lett. 2021;508:115–126. doi: 10.1016/j.canlet.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 146.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 147.Hunyor I, Cook KM. Models of intermittent hypoxia and obstructive sleep apnea: molecular pathways and their contribution to cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R669–R687. doi: 10.1152/ajpregu.00036.2018. [DOI] [PubMed] [Google Scholar]
- 148.Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Invest. 2020;130:5042–5051. doi: 10.1172/JCI137560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J. Biol. Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 150.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martinez CA, et al. Intermittent hypoxia enhances the expression of hypoxia inducible factor HIF1A through histone demethylation. J Biol Chem. 2022;298:102536. doi: 10.1016/j.jbc.2022.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yuan G, et al. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J. Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xia X, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc. Natl Acad. Sci. USA. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tausendschön M, Dehne N, Brüne B. Hypoxia causes epigenetic gene regulation in macrophages by attenuating Jumonji histone demethylase activity. Cytokine. 2011;53:256–262. doi: 10.1016/j.cyto.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 155.Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol. Cell Biol. 2006;26:2901–2912. doi: 10.1128/MCB.26.8.2901-2912.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–9016. doi: 10.1158/0008-5472.CAN-06-0101. [DOI] [PubMed] [Google Scholar]
- 157.Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 2008;640:174–179. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou X, et al. Hypoxia induces trimethylated H3 lysine 4 by inhibition of JARID1A demethylase. Cancer Res. 2010;70:4214–4221. doi: 10.1158/0008-5472.CAN-09-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Osumek JE, Revesz A, Morton JS, Davidge ST, Hardy DB. Enhanced trimethylation of histone h3 mediates impaired expression of hepatic glucose 6-phosphatase expression in offspring from rat dams exposed to hypoxia during pregnancy. Reprod. Sci. 2014;21:112–121. doi: 10.1177/1933719113492212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perez-Perri JI, Acevedo JM, Wappner P. Epigenetics: new questions on the response to hypoxia. Int J. Mol. Sci. 2011;12:4705–4721. doi: 10.3390/ijms12074705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cortese R, et al. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: potential role of CD36. Sci. Rep. 2017;7:43648. doi: 10.1038/srep43648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Watson JA, et al. Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum. Mol. Genet. 2009;18:3594–3604. doi: 10.1093/hmg/ddp307. [DOI] [PubMed] [Google Scholar]
- 163.Lee HY, Yang EG, Park H. Hypoxia enhances the expression of prostate-specific antigen by modifying the quantity and catalytic activity of Jumonji C domain-containing histone demethylases. Carcinogenesis. 2013;34:2706–2715. doi: 10.1093/carcin/bgt256. [DOI] [PubMed] [Google Scholar]
- 164.Wang X, Zhao D, Xie H, Hu Y. Interplay of long non-coding RNAs and HIF-1α: a new dimension to understanding hypoxia-regulated tumor growth and metastasis. Cancer Lett. 2021;499:49–59. doi: 10.1016/j.canlet.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 165.Barreca MM, Zichittella C, Alessandro R, Conigliaro A. Hypoxia-induced non-coding RNAs controlling cell viability in cancer. Int. J. Mol.Sci. 2021;22:1857. doi: 10.3390/ijms22041857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nanduri J, Semenza GL, Prabhakar NR. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L1096–L1100. doi: 10.1152/ajplung.00325.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jones ER, Griffitt RJ. Oil and hypoxia alter DNA methylation and transcription of genes related to neurological function in larval Cyprinodon variegatus. Aquat. Toxicol. 2022;251:106267. doi: 10.1016/j.aquatox.2022.106267. [DOI] [PubMed] [Google Scholar]
- 168.McDonnell F, Irnaten M, Clark AF, O’Brien CJ, Wallace DM. Hypoxia-induced changes in DNA methylation alter RASAL1 and TGFβ1 expression in human trabecular meshwork cells. PLoS ONE. 2016;11:e0153354. doi: 10.1371/journal.pone.0153354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shmakova A, Batie M, Druker J, Rocha S. Chromatin and oxygen sensing in the context of JmjC histone demethylases. Biochem. J. 2014;462:385–395. doi: 10.1042/BJ20140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Batie M, et al. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science. 2019;363:1222–1226. doi: 10.1126/science.aau5870. [DOI] [PubMed] [Google Scholar]
- 171.Chakraborty AA, et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363:1217–1222. doi: 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 173.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 175.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 176.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell Biol. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 177.Faundes V, et al. Histone lysine methylases and demethylases in the landscape of human developmental disorders. Am. J. Hum. Genet. 2018;102:175–187. doi: 10.1016/j.ajhg.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hancock RL, Dunne K, Walport LJ, Flashman E, Kawamura A. Epigenetic regulation by histone demethylases in hypoxia. Epigenomics. 2015;7:791–811. doi: 10.2217/epi.15.24. [DOI] [PubMed] [Google Scholar]
- 179.Sánchez-Fernández EM, et al. Investigations on the oxygen dependence of a 2-oxoglutarate histone demethylase. Biochem. J. 2013;449:491–496. doi: 10.1042/BJ20121155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hancock RL, Masson N, Dunne K, Flashman E, Kawamura A. The activity of JmjC histone lysine demethylase KDM4A is highly sensitive to oxygen concentrations. ACS Chem. Biol. 2017;12:1011–1019. doi: 10.1021/acschembio.6b00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J. Biol. Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen YC, Hsu PY, Hsiao CC, Lin MC. Epigenetics: a potential mechanism involved in the pathogenesis of various adverse consequences of obstructive sleep apnea. Int. J. Mol. Sci. 2019;20:2937. doi: 10.3390/ijms20122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Melvin A, Rocha S. Chromatin as an oxygen sensor and active player in the hypoxia response. Cell Signal. 2012;24:35–43. doi: 10.1016/j.cellsig.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martinez CA, Kerr B, Jin C, Cistulli PA, Cook KM. Obstructive sleep apnea activates HIF-1 in a hypoxia dose-dependent manner in HCT116 colorectal carcinoma cells. Int. J. Mol. Sci. 2019;20:445. doi: 10.3390/ijms20020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dobrynin G, et al. KDM4A regulates HIF-1 levels through H3K9me3. Sci. Rep. 2017;7:11094. doi: 10.1038/s41598-017-11658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nicetto D, Zaret KS. Role of H3K9me3 heterochromatin in cell identity establishment and maintenance. Curr. Opin. Genet. Dev. 2019;55:1–10. doi: 10.1016/j.gde.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Black JC, et al. Hypoxia drives transient site-specific copy gain and drug-resistant gene expression. Genes Dev. 2015;29:1018–1031. doi: 10.1101/gad.259796.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van Rechem C, et al. The SKP1-Cul1-F-box and leucine-rich repeat protein 4 (SCF-FbxL4) ubiquitin ligase regulates lysine demethylase 4A (KDM4A)/Jumonji domain-containing 2A (JMJD2A) protein. J. Biol. Chem. 2011;286:30462–30470. doi: 10.1074/jbc.M111.273508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhan G, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am. J. Respir. Crit. Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J. Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.González-Pacheco FR, et al. Mechanism of vascular smooth muscle cells activation by hydrogen peroxide: role of phospholipase C gamma. Nephrol. Dial. Transpl. 2002;17:392–398. doi: 10.1093/ndt/17.3.392. [DOI] [PubMed] [Google Scholar]
- 192.Hong JH, et al. Critical role of phospholipase Cgamma1 in the generation of H2O2-evoked [Ca2+]i oscillations in cultured rat cortical astrocytes. J. Biol. Chem. 2006;281:13057–13067. doi: 10.1074/jbc.M601726200. [DOI] [PubMed] [Google Scholar]
- 193.Premkumar DR, et al. L-type Ca(2+) channel activation regulates induction of c-fos transcription by hypoxia. J. Appl. Physiol. 2000;88:1898–1906. doi: 10.1152/jappl.2000.88.5.1898. [DOI] [PubMed] [Google Scholar]
- 194.Bhattacharya S, et al. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arany Z, et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl Acad. Sci. USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for Hif-1 alpha /CBP recognition in the cellular hypoxic response. Proc. Natl Acad. Sci. USA. 2002;99:5271–5276. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sang N, Fang J, Srinivas V, Leshchinsky I, Caro J. Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 alpha is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol. Cell Biol. 2002;22:2984–2992. doi: 10.1128/MCB.22.9.2984-2992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J. Biol. Chem. 2002;277:38723–38730. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- 199.Freedman SJ, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc. Natl Acad. Sci. USA. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sang N, et al. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J. Biol. Chem. 2003;278:14013–14019. doi: 10.1074/jbc.M209702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zakrzewska A, et al. Hypoxia-activated metabolic pathway stimulates phosphorylation of p300 and CBP in oxygen-sensitive cells. J. Neurochem. 2005;94:1288–1296. doi: 10.1111/j.1471-4159.2005.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF. Calcium signaling stimulates translation of HIF-alpha during hypoxia. FASEB J. 2006;20:466–475. doi: 10.1096/fj.05-5086com. [DOI] [PubMed] [Google Scholar]
- 204.Nanduri J, Nanduri RP. Cellular mechanisms associated with intermittent hypoxia. Essays Biochem. 2007;43:91–104. doi: 10.1042/bse0430091. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia‑ischemia (Review) Mol. Med. Rep. 2018;18:3547–3554. doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xie Y, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol. Med. Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alvarez-Tejado M, et al. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J. Biol. Chem. 2002;277:13508–13517. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 208.Zhou J, Schmid T, Frank R, Brüne B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J. Biol. Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- 209.Wang Z, Jiang L, Wang J, Chai Z, Xiong W. Morphine promotes angiogenesis by activating PI3K/Akt/HIF-1α pathway and upregulating VEGF in hepatocellular carcinoma. J. Gastrointest. Oncol. 2021;12:1761–1772. doi: 10.21037/jgo-20-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu ZP, et al. Troxerutin attenuates oxygen‑glucose deprivation and reoxygenation‑induced oxidative stress and inflammation by enhancing the PI3K/AKT/HIF‑1α signaling pathway in H9C2 cardiomyocytes. Mol. Med. Rep. 2020;22:1351–1361. doi: 10.3892/mmr.2020.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir. Physiol. Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Conrad PW, Freeman TL, Beitner-Johnson D, Millhorn DE. EPAS1 trans-activation during hypoxia requires p42/p44 MAPK. J. Biol. Chem. 1999;274:33709–33713. doi: 10.1074/jbc.274.47.33709. [DOI] [PubMed] [Google Scholar]
- 213.Richard DE, Berra E, Gothié E, Roux D, Pouysségur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 214.Hur E, Chang KY, Lee E, Lee SK, Park H. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not the stabilization or DNA binding ability of hypoxia-inducible factor-1alpha. Mol. Pharm. 2001;59:1216–1224. doi: 10.1124/mol.59.5.1216. [DOI] [PubMed] [Google Scholar]
- 215.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 216.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat. Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 217.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia augments acute hypoxic sensing via HIF-mediated ROS. Respir. Physiol. Neurobiol. 2010;174:230–234. doi: 10.1016/j.resp.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nanduri J, et al. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc. Natl Acad. Sci. USA. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Prabhakar NR, Kumar GK, Nanduri J. Intermittent hypoxia-mediated plasticity of acute O2 sensing requires altered red-ox regulation by HIF-1 and HIF-2. Ann. N. Y Acad. Sci. 2009;1177:162–168. doi: 10.1111/j.1749-6632.2009.05034.x. [DOI] [PubMed] [Google Scholar]
- 220.Nanduri J, et al. Xanthine oxidase mediates hypoxia-inducible factor-2α degradation by intermittent hypoxia. PLoS ONE. 2013;8:e75838. doi: 10.1371/journal.pone.0075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Roussel MF. Regulation of cell cycle entry and G1 progression by CSF-1. Mol. Reprod. Dev. 1997;46:11–18. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 222.Premkumar DR, et al. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv. Exp. Med. Biol. 2000;475:101–109. doi: 10.1007/0-306-46825-5_10. [DOI] [PubMed] [Google Scholar]
- 223.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 224.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 225.Mishra RR, Adhikary G, Simonson MS, Cherniack NS, Prabhakar NR. Role of c-fos in hypoxia-induced AP-1 cis-element activity and tyrosine hydroxylase gene expression. Brain Res. Mol. Brain Res. 1998;59:74–83. doi: 10.1016/S0169-328X(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 226.Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J. Biol. Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 227.Kumar GK, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J. Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ziegler MG, et al. Sleep apnea, norepinephrine-release rate, and daytime hypertension. Sleep. 1997;20:224–231. doi: 10.1093/sleep/20.3.224. [DOI] [PubMed] [Google Scholar]
- 229.Knight WD, et al. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R131–R139. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med. Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 231.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol. Clin. 2005;23:1059–1075. doi: 10.1016/j.ncl.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 232.Adhikary G, et al. Gene regulation during intermittent hypoxia: evidence for the involvement of reactive oxygen species. Adv. Exp. Med. Biol. 2001;499:297–302. doi: 10.1007/978-1-4615-1375-9_47. [DOI] [PubMed] [Google Scholar]
- 233.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J. Appl. Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 234.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl Acad. Sci. USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J. Appl. Physiol. 2003;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- 236.Mitchell GS, et al. Invited review: intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 237.Kuo TB, et al. Reactive oxygen species are the cause of the enhanced cardiorespiratory response induced by intermittent hypoxia in conscious rats. Respir. Physiol. Neurobiol. 2011;175:70–79. doi: 10.1016/j.resp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 238.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp. Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 239.Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L524–L528. doi: 10.1152/ajplung.2001.281.3.L524. [DOI] [PubMed] [Google Scholar]
- 240.Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J. Appl. Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 241.Ott EP, et al. Sympathetic neural recruitment strategies following acute intermittent hypoxia in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R961–R971. doi: 10.1152/ajpregu.00004.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Iturriaga R, Andrade DC, Del Rio R. Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front. Physiol. 2014;5:468. doi: 10.3389/fphys.2014.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lazovic B, et al. T. Med. Pregl. 2016;69:385–390. doi: 10.2298/MPNS1612385L. [DOI] [PubMed] [Google Scholar]
- 244.Dempsey JA, et al. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv. Exp. Med. Biol. 2012;758:343–349. doi: 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- 245.Smith CA, Blain GM, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2 : role of carotid body CO2. J. Physiol. 2015;593:4225–4243. doi: 10.1113/JP270114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pamenter ME, Powell FL. Time domains of the hypoxic ventilatory response and their molecular basis. Compr. Physiol. 2016;6:1345–1385. doi: 10.1002/cphy.c150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol. Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 248.Narkiewicz K, et al. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.CIR.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 249.Peng YJ, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J. Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J. Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J. Appl. Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- 252.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir. Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- 253.Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J. Appl. Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- 254.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am. J. Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- 255.Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J. Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J. Appl. Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- 257.Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir. Physiol. Neurobiol. 2008;160:65–75. doi: 10.1016/j.resp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mendonça-Junior BA, M VF, Zoccal DB. Acute intermittent hypoxia evokes ventilatory long-term facilitation and active expiration in unanesthetized rats. Respir. Physiol. Neurobiol. 2021;294:103768. doi: 10.1016/j.resp.2021.103768. [DOI] [PubMed] [Google Scholar]
- 259.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir. Physiol. 1998;112:123–134. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 260.Peng Y, Kline DD, Dick TE, Prabhakar NR. Chronic intermittent hypoxia enhances carotid body chemoreceptor response to low oxygen. Adv. Exp. Med. Biol. 2001;499:33–38. doi: 10.1007/978-1-4615-1375-9_5. [DOI] [PubMed] [Google Scholar]
- 261.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: relevance to recurrent apneas. Respir. Physiol. Neurobiol. 2007;157:148–153. doi: 10.1016/j.resp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 263.Fletcher EC, et al. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J. Appl. Physiol. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 264.Peng YJ, et al. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J. Physiol. 2014;592:3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir. Physiol. Neurobiol. 2011;178:375–380. doi: 10.1016/j.resp.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Moya EA, et al. Intermittent hypoxia-induced carotid body chemosensory potentiation and hypertension are critically dependent on peroxynitrite formation. Oxid. Med. Cell Longev. 2016;2016:9802136. doi: 10.1155/2016/9802136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur. Respir. J. 2010;36:143–150. doi: 10.1183/09031936.00158109. [DOI] [PubMed] [Google Scholar]
- 268.Peng YJ, et al. Role of oxidative stress-induced endothelin-converting enzyme activity in the alteration of carotid body function by chronic intermittent hypoxia. Exp. Physiol. 2013;98:1620–1630. doi: 10.1113/expphysiol.2013.073700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Iturriaga R. Intermittent hypoxia: endothelin-1 and hypoxic carotid body chemosensory potentiation. Exp. Physiol. 2013;98:1550–1551. doi: 10.1113/expphysiol.2013.075820. [DOI] [PubMed] [Google Scholar]
- 270.Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006;1086:152–159. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 271.Rey S, Corthorn J, Chacón C, Iturriaga R. Expression and immunolocalization of endothelin peptides and its receptors, ETA and ETB, in the carotid body exposed to chronic intermittent hypoxia. J. Histochem. Cytochem. 2007;55:167–174. doi: 10.1369/jhc.6A7079.2006. [DOI] [PubMed] [Google Scholar]
- 272.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L1314–L1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 273.Pawar A, et al. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R735–R742. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li J, Yang S, Yu F, Ji E, Woodrow Weiss J. Endothelin-1 enhanced carotid body chemosensory activity in chronic intermittent hypoxia through PLC, PKC and p38MAPK signaling pathways. Neuropeptides. 2019;74:44–51. doi: 10.1016/j.npep.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 275.Lin L, Finn L, Zhang J, Young T, Mignot E. Angiotensin-converting enzyme, sleep-disordered breathing, and hypertension. Am. J. Respir. Crit. Care Med. 2004;170:1349–1353. doi: 10.1164/rccm.200405-616OC. [DOI] [PubMed] [Google Scholar]
- 276.Lam SY, Leung PS. A locally generated angiotensin system in rat carotid body. Regul. Pept. 2002;107:97–103. doi: 10.1016/S0167-0115(02)00068-X. [DOI] [PubMed] [Google Scholar]
- 277.López-Barneo J, Macías D, Platero-Luengo A, Ortega-Sáenz P, Pardal R. Carotid body oxygen sensing and adaptation to hypoxia. Pflug. Arch. 2016;468:59–70. doi: 10.1007/s00424-015-1734-0. [DOI] [PubMed] [Google Scholar]
- 278.Prabhakar NR, Overholt JL. Cellular mechanisms of oxygen sensing at the carotid body: heme proteins and ion channels. Respir. Physiol. 2000;122:209–221. doi: 10.1016/S0034-5687(00)00160-2. [DOI] [PubMed] [Google Scholar]
- 279.Pardal R, López-Barneo J. Carotid body thin slices: responses of glomus cells to hypoxia and K(+)-channel blockers. Respir. Physiol. Neurobiol. 2002;132:69–79. doi: 10.1016/S1569-9048(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 280.Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc. Natl Acad. Sci. USA. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Peng YJ, et al. H2S mediates O2 sensing in the carotid body. Proc. Natl Acad. Sci. USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Peng YJ, et al. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc. Natl Acad. Sci. USA. 2014;111:1174–1179. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yuan G, et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci. Signal. 2015;8:ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li Q, et al. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox Signal. 2010;12:1179–1189. doi: 10.1089/ars.2009.2926. [DOI] [PubMed] [Google Scholar]
- 285.Telezhkin V, et al. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir. Physiol. Neurobiol. 2010;172:169–178. doi: 10.1016/j.resp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 286.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc. Natl Acad. Sci. USA. 1995;92:1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yuan G, et al. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci. Signal. 2016;9:ra80. doi: 10.1126/scisignal.aaf3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Peng YJ, et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl Acad. Sci. USA. 2017;114:1413–1418. doi: 10.1073/pnas.1620717114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr. Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Makarenko VV, et al. CaV3.2 T-type Ca2+ channels mediate the augmented calcium influx in carotid body glomus cells by chronic intermittent hypoxia. J. Neurophysiol. 2016;115:345–354. doi: 10.1152/jn.00775.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Summers BA, Overholt JL, Prabhakar NR. Augmentation of L-type calcium current by hypoxia in rabbit carotid body glomus cells: evidence for a PKC-sensitive pathway. J. Neurophysiol. 2000;84:1636–1644. doi: 10.1152/jn.2000.84.3.1636. [DOI] [PubMed] [Google Scholar]
- 293.Makarenko VV, et al. CaV3.2 T-type Ca2+ channels in H2S-mediated hypoxic response of the carotid body. Am. J. Physiol. Cell Physiol. 2015;308:C146–C154. doi: 10.1152/ajpcell.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Makarenko VV, et al. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am. J. Physiol. Cell Physiol. 2012;303:C916–C923. doi: 10.1152/ajpcell.00100.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 296.Tiffany CR, Bäumler AJ. Dysbiosis: from fiction to function. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;317:G602–G608. doi: 10.1152/ajpgi.00230.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mashaqi S, Gozal D. Obstructive sleep apnea and systemic hypertension: gut dysbiosis as the mediator? J. Clin. Sleep Med. 2019;15:1517–1527. doi: 10.5664/jcsm.7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ganesh BP, et al. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72:1141–1150. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang C, Chen F, Shen Y, Chen Y, Ma J. Sleep apnea is associated with the increase of certain genera of Ruminococcaceae and Lachnospiraceae in the gut microbiome of hypertensive patients. Expert Rev. Respir. Med. 2022;16:1247–1256. doi: 10.1080/17476348.2022.2147509. [DOI] [PubMed] [Google Scholar]
- 300.Durgan DJ. Obstructive sleep apnea-induced hypertension: role of the gut microbiota. Curr. Hypertens. Rep. 2017;19:35. doi: 10.1007/s11906-017-0732-3. [DOI] [PubMed] [Google Scholar]
- 301.Almendros I, Basoglu ÖK, Conde SV, Liguori C, Saaresranta T. Metabolic dysfunction in OSA: is there something new under the sun? J. Sleep Res. 2022;31:e13418. doi: 10.1111/jsr.13418. [DOI] [PubMed] [Google Scholar]
- 302.Zhang Y, et al. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022;91:84–92. doi: 10.1016/j.sleep.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 303.Tang SS, et al. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022;28:2320–2333. doi: 10.3748/wjg.v28.i21.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.O'Connor KM, Lucking EF, Cryan JF, O'Halloran KD. Bugs, breathing and blood pressure: microbiota-gut-brain axis signalling in cardiorespiratory control in health and disease. J. Physiol. 2020;598:4159–4179. doi: 10.1113/JP280279. [DOI] [PubMed] [Google Scholar]
- 305.Mashaqi S, et al. Obstructive sleep apnea as a risk factor for COVID-19 severity-The gut microbiome as a common player mediating systemic inflammation via gut barrier dysfunction. Cells. 2022;11:1569. doi: 10.3390/cells11091569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hu C, et al. Chronic intermittent hypoxia participates in the pathogenesis of atherosclerosis and perturbs the formation of intestinal microbiota. Front. Cell Infect. Microbiol. 2021;11:560201. doi: 10.3389/fcimb.2021.560201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van. Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 308.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 309.Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008;153(Suppl 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140:1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Canani RB, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin. Epigenetics. 2012;4:4. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Van Hul M, et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am. J. Physiol. Endocrinol. Metab. 2018;314:E334–E352. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 316.Moreno-Indias I, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 2015;45:1055–1065. doi: 10.1183/09031936.00184314. [DOI] [PubMed] [Google Scholar]
- 317.Mariat D, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 319.Albenberg L, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063 e1058. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Durgan DJ, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lucking EF, et al. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine. 2018;38:191–205. doi: 10.1016/j.ebiom.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Poroyko VA, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005;20:332–339. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 325.Lyte M. Microbial endocrinology: an ongoing personal journey. Adv. Exp. Med. Biol. 2016;874:1–24. doi: 10.1007/978-3-319-20215-0_1. [DOI] [PubMed] [Google Scholar]
- 326.Mittal R, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J. Cell Physiol. 2017;232:2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 328.Ko CY, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019;133:905–917. doi: 10.1042/CS20180891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ko CY, et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9:e01287. doi: 10.1002/brb3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Valentini F, et al. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 2020;76:140–147. doi: 10.1016/j.sleep.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 331.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes. Rev. 2012;13:799–809. doi: 10.1111/j.1467-789X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 332.Li Q, et al. Impaired intestinal barrier in patients with obstructive sleep apnea. Sleep Breath. 2021;25:749–756. doi: 10.1007/s11325-020-02178-y. [DOI] [PubMed] [Google Scholar]
- 333.Vollrath JT, et al. I-FABP as a potential marker for intestinal barrier loss in porcine polytrauma. J. Clin. Med. 2022;11:4599. doi: 10.3390/jcm11154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schellekens DH, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J. Clin. Gastroenterol. 2014;48:253–260. doi: 10.1097/MCG.0b013e3182a87e3e. [DOI] [PubMed] [Google Scholar]
- 335.Barceló A, et al. Gut epithelial barrier markers in patients with obstructive sleep apnea. Sleep Med. 2016;26:12–15. doi: 10.1016/j.sleep.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 336.Heizati M, et al. Does increased serum d-lactate mean subclinical hyperpermeability of intestinal barrier in middle-aged nonobese males with OSA? Medicine. 2017;96:e9144. doi: 10.1097/MD.0000000000009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Liu P, et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharm. Res. 2021;165:105420. doi: 10.1016/j.phrs.2021.105420. [DOI] [PubMed] [Google Scholar]
- 338.Singhal R, Shah YM. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020;295:10493–10505. doi: 10.1074/jbc.REV120.011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 340.Scher JU, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kheirandish-Gozal L, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J. Clin. Endocrinol. Metab. 2014;99:656–663. doi: 10.1210/jc.2013-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Moreno-Indias I, et al. Normoxic recovery mimicking treatment of sleep apnea does not reverse intermittent hypoxia-induced bacterial dysbiosis and low-grade endotoxemia in mice. Sleep. 2016;39:1891–1897. doi: 10.5665/sleep.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31:1317–1323. doi: 10.1016/j.nut.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 346.Jiang S, et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021;46:102115. doi: 10.1016/j.redox.2021.102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Duttaroy AK. Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: a review. Nutrients. 2021;13:144. doi: 10.3390/nu13010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.He S, Jiang H, Zhuo C, Jiang W. Trimethylamine/trimethylamine-N-oxide as a key between diet and cardiovascular diseases. Cardiovasc. Toxicol. 2021;21:593–604. doi: 10.1007/s12012-021-09656-z. [DOI] [PubMed] [Google Scholar]
- 350.Zheng Y, He JQ. Pathogenic mechanisms of trimethylamine N-oxide-induced atherosclerosis and cardiomyopathy. Curr. Vasc. Pharm. 2022;20:29–36. doi: 10.2174/1570161119666210812152802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xue J, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine-oxide. Am. J. Respir. Cell Mol. Biol. 2017;57:581–588. doi: 10.1165/rcmb.2017-0086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 353.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 354.Tolhurst G, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Burger-van Paassen N, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 356.Wrzosek L, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Raqib R, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL., Jr Short chain fatty acids and their receptors: new metabolic targets. Transl. Res. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 359.Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 360.Sun M, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Singh N, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Disco. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 363.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 364.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ye J, et al. CD4(+)T-lymphocyte subsets in nonobese children with obstructive sleep apnea syndrome. Pediatr. Res. 2015;78:165–173. doi: 10.1038/pr.2015.76. [DOI] [PubMed] [Google Scholar]
- 366.Ye J, et al. The treg/th17 imbalance in patients with obstructive sleep apnoea syndrome. Mediators Inflamm. 2012;2012:815308. doi: 10.1155/2012/815308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 368.Asarat M, Apostolopoulos V, Vasiljevic T, Donkor O. Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol. Invest. 2016;45:205–222. doi: 10.3109/08820139.2015.1122613. [DOI] [PubMed] [Google Scholar]
- 369.Chen L, et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm. Bowel Dis. 2019;25:1450–1461. doi: 10.1093/ibd/izz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Davie JR. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 371.Park J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Brahmakshatriya V, et al. IL-6 production by TLR-activated APC broadly enhances aged cognate CD4 helper and B cell antibody responses in vivo. J. Immunol. 2017;198:2819–2833. doi: 10.4049/jimmunol.1601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wang Y, et al. The gut-microglia connection: implications for central nervous system diseases. Front. Immunol. 2018;9:2325. doi: 10.3389/fimmu.2018.02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Patnala R, Arumugam TV, Gupta N, Dheen ST. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol. Neurobiol. 2017;54:6391–6411. doi: 10.1007/s12035-016-0149-z. [DOI] [PubMed] [Google Scholar]
- 376.Maniaci A, et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J. Clin. Med. 2021;10:277. doi: 10.3390/jcm10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kang IG, Jung JH, Kim ST. The effect of obstructive sleep apnea on DNA damage and oxidative stress. Clin. Exp. Otorhinolaryngol. 2013;6:68–72. doi: 10.3342/ceo.2013.6.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hopps E, et al. Lipid peroxidation and protein oxidation are related to the severity of OSAS. Eur. Rev. Med. Pharm. Sci. 2014;18:3773–3778. [PubMed] [Google Scholar]
- 379.Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012;4:1391–1403. doi: 10.2741/e469. [DOI] [PubMed] [Google Scholar]
- 380.Olea E, et al. Intermittent hypoxia and diet-induced obesity: effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J. Appl. Physiol. 2014;117:706–719. doi: 10.1152/japplphysiol.00454.2014. [DOI] [PubMed] [Google Scholar]
- 381.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 382.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev. Respir. Med. 2008;2:75–84. doi: 10.1586/17476348.2.1.75. [DOI] [PubMed] [Google Scholar]
- 385.Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol. Cell Biochem. 2020;467:1–12. doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur. Respir. J. 2009;33:1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 387.Israel LP, Benharoch D, Gopas J, Goldbart AD. A pro-inflammatory role for nuclear factor kappa B in childhood obstructive sleep apnea syndrome. Sleep. 2013;36:1947–1955. doi: 10.5665/sleep.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Lee WJ, et al. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-kappaB pathway. Int. J. Obes. 2009;33:465–472. doi: 10.1038/ijo.2009.24. [DOI] [PubMed] [Google Scholar]
- 389.Waypa GB, Smith KA, Schumacker PT. O2 sensing, mitochondria and ROS signaling: the fog is lifting. Mol. Asp. Med. 2016;47-48:76–89. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 391.Yeo EJ. Hypoxia and aging. Exp. Mol. Med. 2019;51:1–15. doi: 10.1038/s12276-019-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Fitzpatrick SF, et al. An intact canonical NF-κB pathway is required for inflammatory gene expression in response to hypoxia. J. Immunol. 2011;186:1091–1096. doi: 10.4049/jimmunol.1002256. [DOI] [PubMed] [Google Scholar]
- 393.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 394.Nguyen TTP, et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice. Mol. Cell. 2021;81:3820–3832 e3827. doi: 10.1016/j.molcel.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 395.Li J, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005;99:1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 396.Li J, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 397.Li J, Nanayakkara A, Jun J, Savransky V, Polotsky VY. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol. Genomics. 2007;31:273–280. doi: 10.1152/physiolgenomics.00082.2007. [DOI] [PubMed] [Google Scholar]
- 398.Li J, et al. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. 2007;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 399.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease (review) Int J. Mol. Med. 2015;35:859–869. doi: 10.3892/ijmm.2015.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev. Endocr. Metab. Disord. 2015;16:25–34. doi: 10.1007/s11154-014-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem. Biophys. Res. Commun. 2007;355:728–733. doi: 10.1016/j.bbrc.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 403.Lavie L, Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome–molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78:361–370. doi: 10.1159/000243552. [DOI] [PubMed] [Google Scholar]
- 404.Lavie L, Dyugovskaya L, Polyakov A. Biology of peripheral blood cells in obstructive sleep apnea–the tip of the iceberg. Arch. Physiol. Biochem. 2008;114:244–254. doi: 10.1080/13813450802306701. [DOI] [PubMed] [Google Scholar]
- 405.Wang J, et al. Association between severity of obstructive sleep apnea and high-sensitivity C-reactive protein in patients with hypertrophic obstructive cardiomyopathy. Clin. Cardiol. 2020;43:803–811. doi: 10.1002/clc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling–regulation of the immune response in normal and critically ill states. Crit. Care Med. 2000;28:N3–N12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 407.McNicholas WT. Obstructive sleep apnea and inflammation. Prog. Cardiovasc. Dis. 2009;51:392–399. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 408.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 409.Feng YM, et al. Glomerular function in relation to circulating adhesion molecules and inflammation markers in a general population. Nephrol. Dial. Transpl. 2018;33:426–435. doi: 10.1093/ndt/gfx256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Htoo AK, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 411.Greenberg H, et al. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem. Biophys. Res. Commun. 2006;343:591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 412.Haddad JJ. Pharmaco-redox regulation of cytokine-related pathways: from receptor signaling to pharmacogenomics. Free Radic. Biol. Med. 2002;33:907–926. doi: 10.1016/S0891-5849(02)00985-1. [DOI] [PubMed] [Google Scholar]
- 413.Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1170–H1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- 414.Zakrzewski M, et al. Evaluation of fibrinolytic inhibitors: alpha-2-antiplasmin and plasminogen activator inhibitor 1 in patients with obstructive sleep apnoea. PLoS ONE. 2016;11:e0166725. doi: 10.1371/journal.pone.0166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Bagai K, et al. Circadian variability of fibrinolytic markers and endothelial function in patients with obstructive sleep apnea. Sleep. 2014;37:359–367. doi: 10.5665/sleep.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Dimsdale JE. Elevated plasminogen activator inhibitor 1 in sleep apnea and its relation to the metabolic syndrome: an investigation in 2 different study samples. Metabolism. 2007;56:969–976. doi: 10.1016/j.metabol.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 417.Song C, Burgess S, Eicher JD, O'Donnell CJ, Johnson AD. Causal effect of plasminogen activator inhibitor type1 on coronary heart disease. J. Am. Heart Assoc. 2017;6:e004918. doi: 10.1161/JAHA.116.004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Altalhi R, Pechlivani N, Ajjan RA. PAI-1 in diabetes: pathophysiology and role as a therapeutic target. Int. J. Mol. Sci. 2021;22:3170. doi: 10.3390/ijms22063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Placencio VR, DeClerck YA. Plasminogen activator inhibitor-1 in cancer: rationale and insight for future therapeutic testing. Cancer Res. 2015;75:2969–2974. doi: 10.1158/0008-5472.CAN-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Lin MT, et al. Involvement of hypoxia-inducing factor-1α-dependent plasminogen activator inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell invasion. J. Biol. Chem. 2016;291:27433. doi: 10.1074/jbc.A116.708933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Sanagawa A, et al. Sphingosine 1‑phosphate induced by hypoxia increases the expression of PAI‑1 in HepG2 cells via HIF‑1α. Mol. Med. Rep. 2016;14:1841–1848. doi: 10.3892/mmr.2016.5451. [DOI] [PubMed] [Google Scholar]
- 422.Uchiyama T, et al. Hypoxia induces transcription of the plasminogen activator inhibitor-1 gene through genistein-sensitive tyrosine kinase pathways in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:1155–1161. doi: 10.1161/01.ATV.20.4.1155. [DOI] [PubMed] [Google Scholar]
- 423.Oszajca K, et al. Effect of oxidative stress on the expression of t-PA, u-PA, u-PAR, and PAI-1 in endothelial cells. Biochem. Cell Biol. 2008;86:477–486. doi: 10.1139/O08-137. [DOI] [PubMed] [Google Scholar]
- 424.Swiatkowska M, Szemraj J, Al-Nedawi KN, Pawłowska Z. Reactive oxygen species upregulate expression of PAI-1 in endothelial cells. Cell Mol. Biol. Lett. 2002;7:1065–1071. [PubMed] [Google Scholar]
- 425.Jaulmes A, et al. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb. Res. 2009;124:439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 426.Kwon IS, Kim J, Rhee DK, Kim BO, Pyo S. Pneumolysin induces cellular senescence by increasing ROS production and activation of MAPK/NF-κB signal pathway in glial cells. Toxicon. 2017;129:100–112. doi: 10.1016/j.toxicon.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 427.Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Takeshita Y, et al. Tumor necrosis factor-alpha-induced production of plasminogen activator inhibitor 1 and its regulation by pioglitazone and cerivastatin in a nonmalignant human hepatocyte cell line. Metabolism. 2006;55:1464–1472. doi: 10.1016/j.metabol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 429.Pandey M, Loskutoff DJ, Samad F. Molecular mechanisms of tumor necrosis factor-alpha-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J. 2005;19:1317–1319. doi: 10.1096/fj.04-3459fje. [DOI] [PubMed] [Google Scholar]
- 430.Kang S, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl Acad. Sci. USA. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Mestries JC, et al. In vivo modulation of coagulation and fibrinolysis by recombinant glycosylated human interleukin-6 in baboons. Eur. Cytokine Netw. 1994;5:275–281. [PubMed] [Google Scholar]
- 432.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb. Haemost. 2008;100:969–975. doi: 10.1160/TH08-04-0269. [DOI] [PubMed] [Google Scholar]
- 433.Rahman FA, Krause MP. PAI-1, the plasminogen system, and skeletal muscle. Int. J. Mol. Sci. 2020;21:7066. doi: 10.3390/ijms21197066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Steffanina A, et al. The plasminogen system and transforming growth factor-β in subjects with obstructive sleep apnea syndrome: effects of CPAP treatment. Respir. Care. 2015;60:1643–1651. doi: 10.4187/respcare.03571. [DOI] [PubMed] [Google Scholar]
- 435.Ahn YT, et al. Rodent-specific hypoxia response elements enhance PAI-1 expression through HIF-1 or HIF-2 in mouse hepatoma cells. Int. J. Oncol. 2010;37:1627–1638. doi: 10.3892/ijo_00000817. [DOI] [PubMed] [Google Scholar]
- 436.Liao H, Hyman MC, Lawrence DA, Pinsky DJ. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007;21:935–949. doi: 10.1096/fj.06-6285com. [DOI] [PubMed] [Google Scholar]
- 437.Chou YT, et al. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep. 2013;36:481–492. doi: 10.5665/sleep.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhou YH, et al. [Effect of endoplasmic reticulum stress in brain injury following chronic intermittent hypoxia in weanling rat] Zhonghua Yi Xue Za Zhi. 2012;92:1706–1710. [PubMed] [Google Scholar]
- 439.Ding W, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS ONE. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhou S, et al. Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol. Lett. 2014;227:113–123. doi: 10.1016/j.toxlet.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 441.Guan P, et al. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi: 10.1016/j.lfs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 442.Hou Y, et al. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp. Ther. Med. 2017;14:2461–2468. doi: 10.3892/etm.2017.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Disco. 2022;21:115–140. doi: 10.1038/s41573-021-00320-3. [DOI] [PubMed] [Google Scholar]
- 444.Zhou L, Chen P, Peng Y, Ouyang R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid. Med. Cell Longev. 2016;2016:9626831. doi: 10.1155/2016/9626831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Xu LH, et al. Critical role of endoplasmic reticulum stress in chronic intermittent hypoxia-induced deficits in synaptic plasticity and long-term memory. Antioxid. Redox Signal. 2015;23:695–710. doi: 10.1089/ars.2014.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Yao Y, et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 2017;8:133. doi: 10.1038/s41467-017-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J. Mol. Cell Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 449.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 2002;109:525–532. doi: 10.1172/JCI0214550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 452.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 456.Kim I, et al. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. J. Biol. Chem. 2009;284:1593–1603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yang X, et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol. Lett. 2015;234:40–49. doi: 10.1016/j.toxlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zhu Y, et al. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J. Neurosci. 2008;28:2168–2178. doi: 10.1523/JNEUROSCI.5232-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Cai XH, et al. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp. Neurol. 2014;257:148–156. doi: 10.1016/j.expneurol.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 461.Jung SY, Kim SS, Yeo SG. Impact of endoplasmic reticulum stress in otorhinolaryngologic diseases. Int. J. Mol. Sci. 2020;21:4121. doi: 10.3390/ijms21114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 463.Zong DD, Ouyang RY, Chen P. Epigenetic mechanisms in chronic obstructive pulmonary disease. Eur. Rev. Med. Pharm. Sci. 2015;19:844–856. [PubMed] [Google Scholar]
- 464.Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5:e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 466.Santamaria-Martos F, et al. Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. Sci. Rep. 2019;9:13456. doi: 10.1038/s41598-019-49940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Guo Y, Sun J, Lai D. Role of microRNAs in premature ovarian insufficiency. Reprod. Biol. Endocrinol. 2017;15:38. doi: 10.1186/s12958-017-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Liu KX, et al. Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 2018;193:194–199. doi: 10.1016/j.lfs.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 469.Gao H, et al. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017;335:80–87. doi: 10.1016/j.bbr.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 470.Wu X, Chang SC, Jin J, Gu W, Li S. NLRP3 inflammasome mediates chronic intermittent hypoxia-induced renal injury implication of the microRNA-155/FOXO3a signaling pathway. J. Cell Physiol. 2018;233:9404–9415. doi: 10.1002/jcp.26784. [DOI] [PubMed] [Google Scholar]
- 471.Meszaros M, et al. Circulating levels of clusterin and complement factor H in patients with obstructive sleep apnea. Biomark. Med. 2021;15:323–330. doi: 10.2217/bmm-2020-0533. [DOI] [PubMed] [Google Scholar]
- 472.Palma CA, et al. MicroRNA-155 as an inducer of apoptosis and cell differentiation in acute myeloid leukaemia. Mol. Cancer. 2014;13:79. doi: 10.1186/1476-4598-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Li K, Chen Z, Qin Y, Wei Y. MiR-664a-3p expression in patients with obstructive sleep apnea: a potential marker of atherosclerosis. Medicine. 2018;97:e9813. doi: 10.1097/MD.0000000000009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Li K, Wei P, Qin Y, Wei Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine. 2017;96:e7917. doi: 10.1097/MD.0000000000007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 476.Joosten SC, et al. Epigenetics in renal cell cancer: mechanisms and clinical applications. Nat. Rev. Urol. 2018;15:430–451. doi: 10.1038/s41585-018-0023-z. [DOI] [PubMed] [Google Scholar]
- 477.Chen Q, et al. Expression profile of long non-coding RNAs in rat models of OSA-induced cardiovascular disease: new insight into pathogenesis. Sleep Breath. 2019;23:795–804. doi: 10.1007/s11325-018-1753-0. [DOI] [PubMed] [Google Scholar]
- 478.Zhang Z, Li Z, Wang Y, Wei L, Chen H. Overexpressed long noncoding RNA CPS1-IT alleviates pulmonary arterial hypertension in obstructive sleep apnea by reducing interleukin-1β expression via HIF1 transcriptional activity. J. Cell Physiol. 2019;234:19715–19727. doi: 10.1002/jcp.28571. [DOI] [PubMed] [Google Scholar]
- 479.Jathar S, Kumar V, Srivastava J, Tripathi V. Technological developments in lncRNA biology. Adv. Exp. Med. Biol. 2017;1008:283–323. doi: 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 480.Dykes IM, Emanueli C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics Proteom. Bioinforma. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Du P, Wang J, Han Y, Feng J. Blocking the LncRNA MALAT1/miR-224-5p/NLRP3 axis inhibits the hippocampal inflammatory response in T2DM with OSA. Front. Cell Neurosci. 2020;14:97. doi: 10.3389/fncel.2020.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Ding H, et al. Silencing of the long non-coding RNA MEG3 suppresses the apoptosis of aortic endothelial cells in mice with chronic intermittent hypoxia via downregulation of HIF-1α by competitively binding to microRNA-135a. J. Thorac. Dis. 2020;12:1903–1916. doi: 10.21037/jtd-19-2472. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 483.Maunakea AK, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Pan Y, Liu G, Zhou F, Su B, Li Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018;18:1–14. doi: 10.1007/s10238-017-0467-0. [DOI] [PubMed] [Google Scholar]
- 485.Kim J, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012;185:330–338. doi: 10.1164/rccm.201106-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Nanduri J, et al. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc. Natl Acad. Sci. USA. 2012;109:2515–2520. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Nanduri J, et al. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J. Physiol. 2017;595:63–77. doi: 10.1113/JP272346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Chen YC, et al. Whole genome DNA methylation analysis of obstructive sleep apnea: IL1R2, NPR2, AR, SP140 methylation and clinical phenotype. Sleep. 2016;39:743–755. doi: 10.5665/sleep.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Martí-Almor J, et al. Obstructive sleep apnea syndrome as a trigger of cardiac arrhythmias. Curr. Cardiol. Rep. 2021;23:20. doi: 10.1007/s11886-021-01445-y. [DOI] [PubMed] [Google Scholar]
- 490.Wang J, Hu L, Wang Z, Yang S, Wu S. Effect of obstructive sleep apnea syndrome on glycolipid metabolism and early atherosclerosis in diabetics. Diabetes Res. Clin. Pr. 2020;159:107999. doi: 10.1016/j.diabres.2020.107999. [DOI] [PubMed] [Google Scholar]
- 491.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 492.Khurana S, et al. Canvassing the aetiology, prognosis and molecular signatures of obstructive sleep apnoea. Biomarkers. 2019;24:1–16. doi: 10.1080/1354750X.2018.1514655. [DOI] [PubMed] [Google Scholar]
- 493.Vrints H, et al. Cardiovascular mechanisms and consequences of obstructive sleep apnoea. Acta Clin. Belg. 2013;68:169–178. doi: 10.2143/ACB.2981. [DOI] [PubMed] [Google Scholar]
- 494.Nieto FJ, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 495.Usui K, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J. Am. Coll. Cardiol. 2005;45:2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 496.Iturriaga R, Castillo-Galán S. Potential contribution of carotid body-induced sympathetic and renin-angiotensin system overflow to pulmonary hypertension in intermittent hypoxia. Curr. Hypertens. Rep. 2019;21:89. doi: 10.1007/s11906-019-0995-y. [DOI] [PubMed] [Google Scholar]
- 497.Belaidi E, et al. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J. Am. Coll. Cardiol. 2009;53:1309–1317. doi: 10.1016/j.jacc.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 498.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 499.Sanner BM, et al. Platelet function in patients with obstructive sleep apnoea syndrome. Eur. Respir. J. 2000;16:648–652. doi: 10.1034/j.1399-3003.2000.16d14.x. [DOI] [PubMed] [Google Scholar]
- 500.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir. Physiol. Neurobiol. 2011;178:475–481. doi: 10.1016/j.resp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 501.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 502.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65:S140–S146. doi: 10.1301/nr.2007.dec.S140-S146. [DOI] [PubMed] [Google Scholar]
- 503.Linz D, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 504.Huang B, et al. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 2021;31:127–132. doi: 10.1016/j.tcm.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 505.Yu L, et al. Atrial fibrillation in acute obstructive sleep apnea: autonomic nervous mechanism and modulation. J. Am. Heart Assoc. 2017;6:e006264. doi: 10.1161/JAHA.117.006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Iwasaki YK, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J. Am. Coll. Cardiol. 2014;64:2013–2023. doi: 10.1016/j.jacc.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 507.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am. J. Respir. Crit. Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Redline S, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am. J. Respir. Crit. Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Martínez-García MA, et al. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest. 2005;128:2123–2129. doi: 10.1378/chest.128.4.2123. [DOI] [PubMed] [Google Scholar]
- 510.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 511.Rosenzweig I, et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir. Med. 2015;3:404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 512.Vanek J, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 513.Jiang Z, et al. Anti-inflammatory effects of paeoniflorin caused by regulation of the hif1a/miR-210/caspase1/GSDMD signaling pathway in astrocytes: a novel strategy for hypoxia-induced brain injury in rats. Immunopharmacol. Immunotoxicol. 2021;43:410–418. doi: 10.1080/08923973.2021.1924194. [DOI] [PubMed] [Google Scholar]
- 514.Ling J, et al. Edaravone improves intermittent hypoxia-induced cognitive impairment and hippocampal damage in rats. Biol. Pharm. Bull. 2020;43:1196–1201. doi: 10.1248/bpb.b20-00085. [DOI] [PubMed] [Google Scholar]
- 515.Desseilles M, et al. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep. 2008;31:777–794. doi: 10.1093/sleep/31.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Liu X, et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflammation. 2020;17:229. doi: 10.1186/s12974-020-01905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Almendros I, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–1133. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Alomri RM, Kennedy GA, Wali SO, Alhejaili F, Robinson SR. Association between nocturnal activity of the sympathetic nervous system and cognitive dysfunction in obstructive sleep apnoea. Sci. Rep. 2021;11:11990. doi: 10.1038/s41598-021-91329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Shi Y, et al. DNA binding protein HMGB1 secreted by activated microglia promotes the apoptosis of hippocampal neurons in diabetes complicated with OSA. Brain Behav. Immun. 2018;73:482–492. doi: 10.1016/j.bbi.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 520.Ma S, et al. Single-cell sequencing analysis of the db/db mouse hippocampus reveals cell-type-specific insights into the pathobiology of diabetes-associated cognitive dysfunction. Front. Endocrinol. 2022;13:891039. doi: 10.3389/fendo.2022.891039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sun X, Feinberg MW. NF-κB and hypoxia: a double-edged sword in atherosclerosis. Am. J. Pathol. 2012;181:1513–1517. doi: 10.1016/j.ajpath.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Liu F, Liu TW, Kang J. The role of NF-κB-mediated JNK pathway in cognitive impairment in a rat model of sleep apnea. J. Thorac. Dis. 2018;10:6921–6931. doi: 10.21037/jtd.2018.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Liu S, Sun JY, Ren LP, Chen K, Xu B. Propofol attenuates intermittent hypoxia induced up-regulation of proinflammatory cytokines in microglia through inhibiting the activation of NF-Bκ/p38 MAPK signalling. Folia Neuropathol. 2017;55:124–131. doi: 10.5114/fn.2017.68579. [DOI] [PubMed] [Google Scholar]
- 524.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 525.Yang Q, Wang Y, Feng J, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr. Dis. Treat. 2013;9:1077–1086. doi: 10.2147/NDT.S49868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Xie H, Yung WH. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: a role for brain-derived neurotrophic factor. Acta Pharm. Sin. 2012;33:5–10. doi: 10.1038/aps.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Macey PM, et al. Obstructive sleep apnea is associated with low GABA and high glutamate in the insular cortex. J. Sleep Res. 2016;25:390–394. doi: 10.1111/jsr.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Ip MS, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 529.Ceccato F, Bernkopf E, Scaroni C. Sleep apnea syndrome in endocrine clinics. J. Endocrinol. Invest. 2015;38:827–834. doi: 10.1007/s40618-015-0338-z. [DOI] [PubMed] [Google Scholar]
- 530.Katz ES, D'Ambrosio CM. Pediatric obstructive sleep apnea syndrome. Clin. Chest Med. 2010;31:221–234. doi: 10.1016/j.ccm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 531.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Li M, Li X, Lu Y. Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology. 2018;159:2670–2675. doi: 10.1210/en.2018-00248. [DOI] [PubMed] [Google Scholar]
- 533.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best. Pr. Res Clin. Endocrinol. Metab. 2010;24:843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 535.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 536.Drager LF, Polotsky VY. Lipid metabolism: a new frontier in sleep apnea research. Am. J. Respir. Crit. Care Med. 2011;184:288–290. doi: 10.1164/rccm.201105-0837ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Söğüt A, et al. Leptin levels in children with obstructive sleep apnea syndrome. Tuberk. Toraks. 2016;64:283–288. doi: 10.5578/tt.21009. [DOI] [PubMed] [Google Scholar]
- 538.Chen DD, Huang JF, Lin QC, Chen GP, Zhao JM. Relationship between serum adiponectin and bone mineral density in male patients with obstructive sleep apnea syndrome. Sleep Breath. 2017;21:557–564. doi: 10.1007/s11325-017-1492-7. [DOI] [PubMed] [Google Scholar]
- 539.Lacedonia D, et al. Evaluation of adiponectin profile in Italian patients affected by obstructive sleep apnea syndrome. Pulm. Pharm. Ther. 2016;40:104–108. doi: 10.1016/j.pupt.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 540.Justeau G, et al. Association between nocturnal hypoxemia and cancer incidence in patients investigated for OSA: data from a large multicenter French cohort. Chest. 2020;158:2610–2620. doi: 10.1016/j.chest.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 541.Brenner R, et al. Increased risk for cancer in young patients with severe obstructive sleep apnea. Respiration. 2019;97:15–23. doi: 10.1159/000486577. [DOI] [PubMed] [Google Scholar]
- 542.Nieto FJ, et al. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Ma L, et al. Intermittent hypoxia induces tumor immune escape in murine S180 solid tumors via the upregulation of TGF-β(1) in mice. Sleep Breath. 2021;25:719–726. doi: 10.1007/s11325-020-02166-2. [DOI] [PubMed] [Google Scholar]
- 544.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Cao J, Feng J, Li L, Chen B. Obstructive sleep apnea promotes cancer development and progression: a concise review. Sleep Breath. 2015;19:453–457. doi: 10.1007/s11325-015-1126-x. [DOI] [PubMed] [Google Scholar]
- 546.Korbecki J, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int. J. Mol. Sci. 2021;22:10701. doi: 10.3390/ijms221910701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 549.Gharib SA, Seiger AN, Hayes AL, Mehra R, Patel SR. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep. 2014;37:709–714. doi: 10.5665/sleep.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Almendros I, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Li F, et al. Retinoblastoma inactivation induces a protumoral microenvironment via enhanced CCL2 secretion. Cancer Res. 2019;79:3903–3915. doi: 10.1158/0008-5472.CAN-18-3604. [DOI] [PubMed] [Google Scholar]
- 553.Park A, et al. Prostaglandin E2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (NK) cell cytotoxicity and NK cell differentiation. Front. Immunol. 2018;9:1859. doi: 10.3389/fimmu.2018.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Lutgendorf SK, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 555.Sloan EK, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Torres M, et al. Male fertility is reduced by chronic intermittent hypoxia mimicking sleep apnea in mice. Sleep. 2014;37:1757–1765. doi: 10.5665/sleep.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Liu K, et al. NADPH oxidase activation: a mechanism of erectile dysfunction in a rat model of sleep apnea. J. Androl. 2012;33:1186–1198. doi: 10.2164/jandrol.112.016642. [DOI] [PubMed] [Google Scholar]
- 558.Budweiser S, et al. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J. Sex. Med. 2009;6:3147–3157. doi: 10.1111/j.1743-6109.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 559.Köseoğlu N, et al. Sexual function status in women with obstructive sleep apnea syndrome. J. Sex. Med. 2007;4:1352–1357. doi: 10.1111/j.1743-6109.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 560.Schulz R, et al. CPAP therapy improves erectile function in patients with severe obstructive sleep apnea. Sleep Med. 2019;53:189–194. doi: 10.1016/j.sleep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 561.Andersen ML, Tufik S. The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function. Sleep Med. Rev. 2008;12:365–379. doi: 10.1016/j.smrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 562.Bourjeily G, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57. doi: 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Lui B, et al. Obstructive sleep apnea is associated with adverse maternal outcomes using a United States multistate database cohort, 2007-2014. Int. J. Obstet. Anesth. 2021;45:74–82. doi: 10.1016/j.ijoa.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 564.Zhou J, et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62:599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J. Obstet. Gynecol. 2006;45:189–200. doi: 10.1016/S1028-4559(09)60224-2. [DOI] [PubMed] [Google Scholar]
- 566.de Lima FF, Mazzotti DR, Tufik S, Bittencourt L. The role inflammatory response genes in obstructive sleep apnea syndrome: a review. Sleep Breath. 2016;20:331–338. doi: 10.1007/s11325-015-1226-7. [DOI] [PubMed] [Google Scholar]
- 567.Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J. Reprod. Immunol. 2010;85:112–116. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2021;25:1155–1157. doi: 10.1007/s11325-020-02203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Rögnvaldsson KG, et al. Obstructive sleep apnea is an independent risk factor for severe COVID-19: a population-based study. Sleep. 2022;45:zsab272. doi: 10.1093/sleep/zsab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.de Kruif MD, Voncken SFJ, Laven S, Feron TMH, Kolfoort-Otte AAB. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2021;25:2103. doi: 10.1007/s11325-020-02271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Machado-Curbelo C. Dangers and management of obstructive sleep apnea syndrome in COVID-19 patients. MEDICC Rev. 2021;23:10. doi: 10.37757/MR2021.V23.N1.17. [DOI] [PubMed] [Google Scholar]
- 573.Vanderhaeghen T, Vandewalle J, Libert C. Hypoxia-inducible factors in metabolic reprogramming during sepsis. FEBS J. 2020;287:1478–1495. doi: 10.1111/febs.15222. [DOI] [PubMed] [Google Scholar]
- 574.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Brevini T, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2022;615:134–142. doi: 10.1038/s41586-022-05594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Ekiz T, İnönü Köseoğlu H, Pazarlı AC. Obstructive sleep apnea, renin-angiotensin system, and COVID-19: possible interactions. J. Clin. Sleep Med. 2020;16:1403–1404. doi: 10.5664/jcsm.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Barceló A, et al. Angiotensin converting enzyme in patients with sleep apnoea syndrome: plasma activity and gene polymorphisms. Eur. Respir. J. 2001;17:728–732. doi: 10.1183/09031936.01.17407280. [DOI] [PubMed] [Google Scholar]
- 578.Yang S, Guo X, Liu W, Li Y, Liu Y. Alcohol as an independent risk factor for obstructive sleep apnea. Ir. J. Med. Sci. 2022;191:1325–1330. doi: 10.1007/s11845-021-02671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J. Neurol. Neurosurg. Psychiatry. 1982;45:353–359. doi: 10.1136/jnnp.45.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Verbraecken J, et al. Non-CPAP therapy for obstructive sleep apnoea. Breathe. 2022;18:220164. doi: 10.1183/20734735.0164-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Kim KS, et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2012;8:367–374. doi: 10.5664/jcsm.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Chang CW, et al. What is the association between secondhand smoke (SHS) and possible obstructive sleep apnea: a meta-analysis. Environ. Health. 2022;21:58. doi: 10.1186/s12940-022-00868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Krishnan V, Dixon-Williams S, Thornton JD. Where there is smoke…there is sleep apnea: exploring the relationship between smoking and sleep apnea. Chest. 2014;146:1673–1680. doi: 10.1378/chest.14-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ashrafian H, et al. Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obes. Surg. 2015;25:1239–1250. doi: 10.1007/s11695-014-1533-2. [DOI] [PubMed] [Google Scholar]
- 585.Hudgel DW, et al. The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 586.Randerath W, et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021;30:210200. doi: 10.1183/16000617.0200-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Wang SH, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am. J. Respir. Crit. Care Med. 2020;201:718–727. doi: 10.1164/rccm.201903-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Foster GD, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch. Intern. Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Aiello KD, et al. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir. Med. 2016;116:85–92. doi: 10.1016/j.rmed.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 590.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 591.Kline CE, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Mendelson M, et al. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur. Respir. J. 2016;48:142–150. doi: 10.1183/13993003.01897-2015. [DOI] [PubMed] [Google Scholar]
- 593.Iftikhar IH, et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7–14. doi: 10.1016/j.sleep.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 594.Servantes DM, et al. Effects of exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest. 2018;154:808–817. doi: 10.1016/j.chest.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 595.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 596.Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pépin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J. Clin. Sleep Med. 2017;13:813–824. doi: 10.5664/jcsm.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Oksenberg A, Gadoth N, Töyräs J, Leppänen T. Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA. Sleep Breath. 2020;24:551–559. doi: 10.1007/s11325-019-01897-1. [DOI] [PubMed] [Google Scholar]
- 598.Douglas NJ, Jan MA, Yildirim N, Warren PM, Drummond GB. Effect of posture and breathing route on genioglossal electromyogram activity in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am. Rev. Respir. Dis. 1993;148:1341–1345. doi: 10.1164/ajrccm/148.5.1341. [DOI] [PubMed] [Google Scholar]
- 599.Omobomi O, Quan SF. Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath. 2018;22:297–304. doi: 10.1007/s11325-017-1561-y. [DOI] [PubMed] [Google Scholar]
- 600.Nokes B, Cooper J, Cao M. Obstructive sleep apnea: personalizing CPAP alternative therapies to individual physiology. Expert Rev. Respir. Med. 2022;16:917–929. doi: 10.1080/17476348.2022.2112669. [DOI] [PubMed] [Google Scholar]
- 601.Joosten SA, et al. Dynamic loop gain increases upon adopting the supine body position during sleep in patients with obstructive sleep apnoea. Respirology. 2017;22:1662–1669. doi: 10.1111/resp.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Kastoer C, et al. Comparison of upper airway collapse patterns and its clinical significance: drug-induced sleep endoscopy in patients without obstructive sleep apnea, positional and non-positional obstructive sleep apnea. Sleep Breath. 2018;22:939–948. doi: 10.1007/s11325-018-1702-y. [DOI] [PubMed] [Google Scholar]
- 603.Pevernagie DA, Stanson AW, Sheedy PF, 2nd, Daniels BK, Shepard JW., Jr. Effects of body position on the upper airway of patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995;152:179–185. doi: 10.1164/ajrccm.152.1.7599821. [DOI] [PubMed] [Google Scholar]
- 604.Joosten SA, et al. Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology. 2015;20:819–827. doi: 10.1111/resp.12549. [DOI] [PubMed] [Google Scholar]
- 605.de Vries GE, et al. Usage of positional therapy in adults with obstructive sleep apnea. J. Clin. Sleep Med. 2015;11:131–137. doi: 10.5664/jcsm.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Bignold JJ, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J. Clin. Sleep Med. 2009;5:428–430. doi: 10.5664/jcsm.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Srijithesh PR, Aghoram R, Goel A, Dhanya J. Positional therapy for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2019;5:CD010990. doi: 10.1002/14651858.CD010990.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Bouloukaki I, et al. Intensive versus standard follow-up to improve continuous positive airway pressure compliance. Eur. Respir. J. 2014;44:1262–1274. doi: 10.1183/09031936.00021314. [DOI] [PubMed] [Google Scholar]
- 609.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/S0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 610.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 611.Lance CG. Positive airway pressure: making an impact on sleep apnea. Cleve Clin. J. Med. 2019;86:26–33. doi: 10.3949/ccjm.86.s1.05. [DOI] [PubMed] [Google Scholar]
- 612.Selim B, Ramar K. Sleep-related breathing disorders: when CPAP is not enough. Neurotherapeutics. 2021;18:81–90. doi: 10.1007/s13311-020-00955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Morgenthaler TI, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31:141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Patil SP, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med. 2019;15:301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Qaseem A, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2013;159:471–483. doi: 10.7326/0003-4819-159-11-201312030-00009. [DOI] [PubMed] [Google Scholar]
- 616.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 617.Weaver TE, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Martínez-García MA, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 619.Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest. 2019;155:1272–1287. doi: 10.1016/j.chest.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 620.Sawyer AM, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med. Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc. Am. Thorac. Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Reid ML, et al. The role of sham continuous positive airway pressure as a placebo in controlled trials: best apnea interventions for research trial. Sleep. 2019;42:zsz099. doi: 10.1093/sleep/zsz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Tamisier R, et al. Impact of a multimodal telemonitoring intervention on CPAP adherence in symptomatic OSA and low cardiovascular risk: a randomized controlled trial. Chest. 2020;158:2136–2145. doi: 10.1016/j.chest.2020.05.613. [DOI] [PubMed] [Google Scholar]
- 624.Grewe FA, et al. Patterns of nightly CPAP usage in OSA patients with suboptimal treatment adherence. Sleep Med. 2020;74:109–115. doi: 10.1016/j.sleep.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 625.Smith I, Nadig V, Lasserson TJ. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines for adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2009;4:CD007736. doi: 10.1002/14651858.CD007736. [DOI] [PubMed] [Google Scholar]
- 626.Ramar K, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J. Clin. Sleep Med. 2015;11:773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Ng JH, Yow M. Oral appliances in the management of obstructive sleep apnea. Sleep Med. Clin. 2020;15:241–250. doi: 10.1016/j.jsmc.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 628.Ilea A, et al. Oral appliance therapy in obstructive sleep apnea and snoring - systematic review and new directions of development. Cranio. 2021;39:472–483. doi: 10.1080/08869634.2019.1673285. [DOI] [PubMed] [Google Scholar]
- 629.Mickelson SA. Oral appliances for snoring and obstructive sleep apnea. Otolaryngol. Clin. North Am. 2020;53:397–407. doi: 10.1016/j.otc.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 630.Marklund M, Braem MJA, Verbraecken J. Update on oral appliance therapy. Eur. Respir. Rev. 2019;28:190083. doi: 10.1183/16000617.0083-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Sutherland K, Cistulli PA. Oral appliance therapy for obstructive sleep apnoea: state of the art. J. Clin. Med. 2019;8:2121. doi: 10.3390/jcm8122121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Edwards BA, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Sutherland K, et al. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J. Clin. Sleep Med. 2015;11:861–868. doi: 10.5664/jcsm.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Guilleminault C, et al. Obstructive sleep apnea syndrome and tracheostomy. Long-term follow-up experience. Arch. Intern. Med. 1981;141:985–988. doi: 10.1001/archinte.1981.00340080025009. [DOI] [PubMed] [Google Scholar]
- 635.Randerath WJ, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur. Respir. J. 2011;37:1000–1028. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 636.Sheen D, Abdulateef S. Uvulopalatopharyngoplasty. Oral. Maxillofac. Surg. Clin. North Am. 2021;33:295–303. doi: 10.1016/j.coms.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 637.Khan A, et al. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the mayo clinic experience. Mayo Clin. Proc. 2009;84:795–800. doi: 10.4065/84.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Tschopp S, Tschopp K. Tonsil size and outcome of uvulopalatopharyngoplasty with tonsillectomy in obstructive sleep apnea. Laryngoscope. 2019;129:E449–E454. doi: 10.1002/lary.27899. [DOI] [PubMed] [Google Scholar]
- 639.Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax. 2013;68:846–853. doi: 10.1136/thoraxjnl-2012-202610. [DOI] [PubMed] [Google Scholar]
- 640.Sommer UJ, et al. Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea. Dtsch Arztebl Int. 2016;113:1–8. doi: 10.3238/arztebl.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Sundaram, S., Bridgman, S. A., Lim, J. & Lasserson, T. J. Surgery for obstructive sleep apnoea. Cochrane Database Syst. Rev. CD001004 (2005). [DOI] [PubMed]
- 642.Caples SM, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Puccia R, Woodson BT. Palatopharyngoplasty and palatal anatomy and phenotypes for treatment of sleep apnea in the twenty-first century. Otolaryngol. Clin. North Am. 2020;53:421–429. doi: 10.1016/j.otc.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 644.Zhang QF, et al. [Coblation-assisting uvulopalatopharyngoplasty combining coblation-channeling of the tongue for patients with severe OSAHS] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:114–117. doi: 10.13201/j.issn.1001-1781.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 645.Zaghi S, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 2016;142:58–66. doi: 10.1001/jamaoto.2015.2678. [DOI] [PubMed] [Google Scholar]
- 646.Yan Q, Guan B. [Hypoglossal nerve stimulation therapy for obstructive sleep apnea hypopnea syndrome] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:796–799. doi: 10.3760/cma.j.issn.1673-0860.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 647.Manchanda S, Neupane P, Sigua NL. Upper airway stimulation/hypoglossal nerve stimulator. Am. J. Respir. Crit. Care Med. 2020;202:P23–P24. doi: 10.1164/rccm.2028P23. [DOI] [PubMed] [Google Scholar]
- 648.Mashaqi S, et al. The hypoglossal nerve stimulation as a novel therapy for treating obstructive sleep apnea—A literature review. Int. J. Environ. Res. Public Health. 2021;18:1642. doi: 10.3390/ijerph18041642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Strollo PJ, Jr., et al. Upper-airway stimulation for obstructive sleep apnea. N. Engl. J. Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 650.Woodson BT, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol. Head. Neck Surg. 2018;159:194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 651.Eastwood PR, et al. Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. Eur. Respir. J. 2020;55:1901320. doi: 10.1183/13993003.01320-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.He B, et al. Domiciliary use of transcutaneous electrical stimulation for patients with obstructive sleep apnoea: a conceptual framework for the TESLA home programme. J. Thorac. Dis. 2019;11:2153–2164. doi: 10.21037/jtd.2019.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Eckert DJ, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin. Sci. 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Carberry JC, et al. Role of common hypnotics on the phenotypic causes of obstructive sleep apnoea: paradoxical effects of zolpidem. Eur. Respir. J. 2017;50:1701344. doi: 10.1183/13993003.01344-2017. [DOI] [PubMed] [Google Scholar]
- 655.Edwards BA, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J. Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Schmickl CN, et al. Effects of acetazolamide on control of breathing in sleep apnea patients: mechanistic insights using meta-analyses and physiological model simulations. Physiol. Rep. 2021;9:e15071. doi: 10.14814/phy2.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Ni YN, Yang H, Thomas RJ. The role of acetazolamide in sleep apnea at sea level: a systematic review and meta-analysis. J. Clin. Sleep Med. 2021;17:1295–1304. doi: 10.5664/jcsm.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Eskandari D, Zou D, Grote L, Hoff E, Hedner J. Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: a randomized controlled trial. J. Clin. Sleep Med. 2018;14:309–317. doi: 10.5664/jcsm.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Taranto-Montemurro L, et al. Effect of 4-aminopyridine on genioglossus muscle activity during sleep in healthy adults. Ann. Am. Thorac. Soc. 2017;14:1177–1183. doi: 10.1513/AnnalsATS.201701-006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep. 2014;37:41–50. doi: 10.5665/sleep.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Wirth KJ, Steinmeyer K, Ruetten H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: investigations with AVE0118 in anesthetized pigs. Sleep. 2013;36:699–708. doi: 10.5665/sleep.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Taranto-Montemurro L, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am. J. Respir. Crit. Care Med. 2016;194:878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Taranto-Montemurro L, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am. J. Respir. Crit. Care Med. 2019;199:1267–1276. doi: 10.1164/rccm.201808-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39:1353–1359. doi: 10.5665/sleep.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Ip MS, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 666.Ip MS, et al. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125:127–134. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 667.Marshall NS, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 668.Nakayama-Ashida Y, et al. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep. 2008;31:419–425. doi: 10.1093/sleep/31.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Tan A, et al. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: a community-based study. Respirology. 2016;21:943–950. doi: 10.1111/resp.12747. [DOI] [PubMed] [Google Scholar]
- 670.Heinzer R, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir. Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Khokhrina A, Andreeva E, Degryse JM. The prevalence of sleep-disordered breathing in Northwest Russia: the ARKHsleep study. Chron. Respir. Dis. 2020;17:1479973120928103. doi: 10.1177/1479973120928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 673.Fietze I, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - Results of SHIP-Trend. J. Sleep Res. 2019;28:e12770. doi: 10.1111/jsr.12770. [DOI] [PubMed] [Google Scholar]
- 674.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur. Respir. J. 2016;47:194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 675.Mihaere KM, et al. Obstructive sleep apnea in New Zealand adults: prevalence and risk factors among Māori and non-Māori. Sleep. 2009;32:949–956. doi: 10.1093/sleep/32.7.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Hrubos-Strøm H, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP) J. Sleep Res. 2011;20:162–170. doi: 10.1111/j.1365-2869.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 677.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am. J. Respir. Crit. Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 678.Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am. J. Respir. Crit. Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 679.Pływaczewski R, Bednarek M, Jonczak L, Zieliński J. Sleep-disordered breathing in a middle-aged and older Polish urban population. J. Sleep Res. 2008;17:73–81. doi: 10.1111/j.1365-2869.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 680.Reddy EV, et al. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: a community-based study. Sleep Med. 2009;10:913–918. doi: 10.1016/j.sleep.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 681.Ding S, et al. Prevalence of obstructive sleep apnea syndrome in hospitalized patients with type 2 diabetes in Beijing, China. J. Diabetes Investig. 2022;13:1889–1896. doi: 10.1111/jdi.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Saldías Peñafiel F, et al. [Prevalence of obstructive sleep apnea syndrome in Chilean adults. a sub-study of the national health survey, 2016/17] Rev. Med. Chil. 2020;148:895–905. doi: 10.4067/S0034-98872020000700895. [DOI] [PubMed] [Google Scholar]
- 683.Dosman JA, et al. STOP-Bang score and prediction of severity of obstructive sleep apnea in a first nation community in Saskatchewan, Canada. Clocks Sleep. 2022;4:535–548. doi: 10.3390/clockssleep4040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.An Z, et al. Role of microRNA-130a in the pathogeneses of obstructive sleep apnea hypopnea syndrome-associated pulmonary hypertension by targeting the GAX gene. Medicine. 2017;96:e6746. doi: 10.1097/MD.0000000000006746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 685.Schaefer E, et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol. Commun. 2017;1:326–337. doi: 10.1002/hep4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Ding X, et al. Chronic obstructive sleep apnea accelerates pulmonary remodeling via TGF-β/miR-185/CoLA1 signaling in a canine model. Oncotarget. 2016;7:57545–57555. doi: 10.18632/oncotarget.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Lv X, et al. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J. Cell Biochem. 2019;120:18871–18882. doi: 10.1002/jcb.29207. [DOI] [PubMed] [Google Scholar]
- 688.Khalyfa A, et al. Circulating plasma extracellular microvesicle microRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2016;194:1116–1126. doi: 10.1164/rccm.201602-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Yu C, et al. Chronic obstructive sleep apnea promotes aortic remodeling in canines through miR-145/Smad3 signaling pathway. Oncotarget. 2017;8:37705–37716. doi: 10.18632/oncotarget.17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Lin G, et al. miR-146a-5p mediates intermittent hypoxia-induced injury in H9c2 cells by targeting XIAP. Oxid. Med. Cell Longev. 2019;2019:6581217. doi: 10.1155/2019/6581217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Bi R, et al. Endothelial cell autophagy in chronic intermittent hypoxia is impaired by miRNA-30a-mediated translational control of Beclin-1. J. Cell Biochem. 2019;120:4214–4224. doi: 10.1002/jcb.27708. [DOI] [PubMed] [Google Scholar]
- 692.Ren J, et al. Atorvastatin attenuates myocardial hypertrophy induced by chronic intermittent hypoxia in vitro partly through miR-31/PKCε pathway. Curr. Med. Sci. 2018;38:405–412. doi: 10.1007/s11596-018-1893-2. [DOI] [PubMed] [Google Scholar]
- 693.Liu KX, et al. Inhibition of microRNA-218 reduces HIF-1α by targeting on Robo1 in mice aortic endothelial cells under intermittent hypoxia. Oncotarget. 2017;8:104359–104366. doi: 10.18632/oncotarget.22239. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 694.Uchiyama T, et al. Up-regulation of selenoprotein P and HIP/PAP mRNAs in hepatocytes by intermittent hypoxia via down-regulation of miR-203. Biochem. Biophys. Rep. 2017;11:130–137. doi: 10.1016/j.bbrep.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Uchiyama T, et al. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019;20:1960. doi: 10.3390/ijms20081960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.He L, Liao X, Zhu G, Kuang J. miR-126a-3p targets HIF-1α and alleviates obstructive sleep apnea syndrome with hypertension. Hum. Cell. 2020;33:1036–1045. doi: 10.1007/s13577-020-00404-z. [DOI] [PubMed] [Google Scholar]
- 697.Li W, et al. Intermittent hypoxia-induced downregulation of microRNA-320b promotes lung cancer tumorigenesis by increasing CDT1 via USP37. Mol. Ther. Nucleic Acids. 2021;24:528–541. doi: 10.1016/j.omtn.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Zhang K, et al. Beneficial effects of tolvaptan on atrial remodeling induced by chronic intermittent hypoxia in rats. Cardiovasc. Ther. 2018;36:e12466. doi: 10.1111/1755-5922.12466. [DOI] [PubMed] [Google Scholar]
- 699.Du Y, et al. miRNA-mediated suppression of a cardioprotective cardiokine as a novel mechanism exacerbating post-MI remodeling by sleep breathing disorders. Circ. Res. 2020;126:212–228. doi: 10.1161/CIRCRESAHA.119.315067. [DOI] [PubMed] [Google Scholar]
- 700.Ge H, Liu J, Liu F, Sun Y, Yang R. Long non-coding RNA ROR mitigates cobalt chloride-induced hypoxia injury through regulation of miR-145. Artif. Cells Nanomed. Biotechnol. 2019;47:2221–2229. doi: 10.1080/21691401.2019.1620759. [DOI] [PubMed] [Google Scholar]
- 701.Chen X, et al. Screening of plasma exosomal lncRNAs to identify potential biomarkers for obstructive sleep apnea. Ann. Transl. Med. 2022;10:936. doi: 10.21037/atm-22-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Zietzer A, et al. The lncRNA MRPL20-AS1 is associated with severe OSAS and downregulated upon hypoxic injury of endothelial cells. Int. J. Cardiol. 2022;369:65–68. doi: 10.1016/j.ijcard.2022.08.035. [DOI] [PubMed] [Google Scholar]
- 703.Hu C, et al. Impact of chronic intermittent hypoxia on the long non-coding RNA and mRNA expression profiles in myocardial infarction. J. Cell Mol. Med. 2021;25:421–433. doi: 10.1111/jcmm.16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Zhou Z, Ni H, Li Y, Jiang B. LncRNA XIST promotes inflammation by downregulating GRα expression in the adenoids of children with OSAHS. Exp. Ther. Med. 2021;21:500. doi: 10.3892/etm.2021.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Chen Q, et al. LncRNA XR_595552 inhibition alleviates intermittent hypoxia-induced cardiomyocyte damage via activating the PI3K/AKT pathway. Sleep Breath. 2023;27:129–136. doi: 10.1007/s11325-022-02584-4. [DOI] [PubMed] [Google Scholar]
- 706.Kheirandish-Gozal L, Khalyfa A, Gozal D, Bhattacharjee R, Wang Y. Endothelial dysfunction in children with obstructive sleep apnea is associated with epigenetic changes in the eNOS gene. Chest. 2013;143:971–977. doi: 10.1378/chest.12-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Chu A, Gozal D, Cortese R, Wang Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatr. Res. 2015;77:425–433. doi: 10.1038/pr.2014.197. [DOI] [PubMed] [Google Scholar]
- 708.Chen YC, et al. Aberrant DNA methylation levels of the formyl peptide receptor 1/2/3 genes are associated with obstructive sleep apnea and its clinical phenotypes. Am. J. Transl. Res. 2020;12:2521–2537. [PMC free article] [PubMed] [Google Scholar]
- 709.Mokhlesi B, et al. Evaluation and management of obesity hypoventilation syndrome. an official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019;200:e6–e24. doi: 10.1164/rccm.201905-1071ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in sleep AHEAD. Am. J. Respir. Crit. Care Med. 2021;203:161–162. doi: 10.1164/rccm.202007-2906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Carneiro-Barrera A, Díaz-Román A, Guillén-Riquelme A, Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: systematic review and meta-analysis. Obes. Rev. 2019;20:750–762. doi: 10.1111/obr.12824. [DOI] [PubMed] [Google Scholar]
- 712.Andrade FM, Pedrosa RP. The role of physical exercise in obstructive sleep apnea. J. Bras. Pneumol. 2016;42:457–464. doi: 10.1590/s1806-37562016000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Lee JJ, Sundar KM. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung. 2021;199:87–101. doi: 10.1007/s00408-021-00426-w. [DOI] [PubMed] [Google Scholar]
- 714.Uniken Venema JAM, et al. Mandibular advancement device design: a systematic review on outcomes in obstructive sleep apnea treatment. Sleep Med. Rev. 2021;60:101557. doi: 10.1016/j.smrv.2021.101557. [DOI] [PubMed] [Google Scholar]
- 715.Rocha NS, de França AJB, Niño-Sandoval TC, do Egito Vasconcelos BC, Filho JRL. Efficiency of maxillomandibular advancement for the treatment of obstructive apnea syndrome: a comprehensive overview of systematic reviews. Clin. Oral. Investig. 2022;26:4291–4305. doi: 10.1007/s00784-022-04489-8. [DOI] [PubMed] [Google Scholar]
- 716.Mickelson SA. Nasal surgery for obstructive sleep apnea syndrome. Otolaryngol. Clin. North Am. 2016;49:1373–1381. doi: 10.1016/j.otc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 717.Camacho M, et al. Mini tracheostomy for obstructive sleep apnea: an evidence based proposal. Int. J. Otolaryngol. 2016;2016:7195349. doi: 10.1155/2016/7195349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.de Raaff CAL, de Vries N, van Wagensveld BA. Obstructive sleep apnea and bariatric surgical guidelines: summary and update. Curr. Opin. Anaesthesiol. 2018;31:104–109. doi: 10.1097/ACO.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 719.Ming X, Yang M, Chen X. Metabolic bariatric surgery as a treatment for obstructive sleep apnea hypopnea syndrome: review of the literature and potential mechanisms. Surg. Obes. Relat. Dis. 2021;17:215–220. doi: 10.1016/j.soard.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 720.Olson MD, Junna MR. Hypoglossal nerve stimulation therapy for the treatment of obstructive sleep apnea. Neurotherapeutics. 2021;18:91–99. doi: 10.1007/s13311-021-01012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Blackman A, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J. Obes. 2016;40:1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Fiori CZ, et al. Diuretic or sodium-restricted diet for obstructive sleep apnea-a randomized trial. Sleep. 2018;41:zsy016. doi: 10.1093/sleep/zsy016. [DOI] [PubMed] [Google Scholar]
- 723.Acar M, et al. The effects of mometasone furoate and desloratadine in obstructive sleep apnea syndrome patients with allergic rhinitis. Am. J. Rhinol. Allergy. 2013;27:e113–e116. doi: 10.2500/ajra.2013.27.3921. [DOI] [PubMed] [Google Scholar]
- 724.Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004;59:50–55. [PMC free article] [PubMed] [Google Scholar]
- 725.Koutsourelakis I, Minaritzoglou A, Zakynthinos G, Vagiakis E, Zakynthinos S. The effect of nasal tramazoline with dexamethasone in obstructive sleep apnoea patients. Eur. Respir. J. 2013;42:1055–1063. doi: 10.1183/09031936.00142312. [DOI] [PubMed] [Google Scholar]
- 726.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 727.Carter SG, et al. Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. Sleep. 2016;39:757–766. doi: 10.5665/sleep.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Med. 2007;8:464–470. doi: 10.1016/j.sleep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 729.Park E, Younes M, Liu H, Liu X, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep. 2008;31:355–365. doi: 10.1093/sleep/31.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.George CF, et al. A 2-week, polysomnographic, safety study of sodium oxybate in obstructive sleep apnea syndrome. Sleep Breath. 2011;15:13–20. doi: 10.1007/s11325-009-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37:811–819. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Smales ET, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann. Am. Thorac. Soc. 2015;12:758–764. doi: 10.1513/AnnalsATS.201408-399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Eskandari D, et al. Zonisamide reduces obstructive sleep apnoea: a randomised placebo-controlled study. Eur. Respir. J. 2014;44:140–149. doi: 10.1183/09031936.00158413. [DOI] [PubMed] [Google Scholar]
- 734.Edwards BA, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–285. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Schmickl CN, et al. Acetazolamide for OSA and central sleep apnea: a comprehensive systematic review and meta-analysis. Chest. 2020;158:2632–2645. doi: 10.1016/j.chest.2020.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Tojima H, et al. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43:113–119. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Sands SA, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018;52:1800674. doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Wellman A, et al. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir. Physiol. Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Pokorski M, Jernajczyk U. Nocturnal oxygen enrichment in sleep apnoea. J. Int Med Res. 2000;28:1–8. doi: 10.1177/147323000002800101. [DOI] [PubMed] [Google Scholar]
- 740.Joosten SA, et al. A randomized controlled trial of oxygen therapy for patients who do not respond to upper airway surgery for obstructive sleep apnea. J. Clin. Sleep Med. 2021;17:445–452. doi: 10.5664/jcsm.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Wang D, et al. Predicting response to oxygen therapy in obstructive sleep apnoea patients using a 10-minute daytime test. Eur. Respir. J. 2018;51:1701587. doi: 10.1183/13993003.01587-2017. [DOI] [PubMed] [Google Scholar]
- 742.Dempsey JA, et al. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J. Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Messineo L, et al. Breath-holding as a means to estimate the loop gain contribution to obstructive sleep apnoea. J. Physiol. 2018;596:4043–4056. doi: 10.1113/JP276206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Xie A, et al. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J. Appl. Physiol. 2013;115:22–33. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Taranto-Montemurro L, et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur. Respir. J. 2016;48:1340–1350. doi: 10.1183/13993003.00823-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100:416–421. doi: 10.1378/chest.100.2.416. [DOI] [PubMed] [Google Scholar]
- 747.Smith PL, Haponik EF, Allen RP, Bleecker ER. The effects of protriptyline in sleep-disordered breathing. Am. Rev. Respir. Dis. 1983;127:8–13. doi: 10.1164/arrd.1983.127.1.8. [DOI] [PubMed] [Google Scholar]
- 748.Bart Sangal R, Sangal JM, Thorp K. Atomoxetine improves sleepiness and global severity of illness but not the respiratory disturbance index in mild to moderate obstructive sleep apnea with sleepiness. Sleep Med. 2008;9:506–510. doi: 10.1016/j.sleep.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 749.Veasey SC, Chachkes J, Fenik P, Hendricks JC. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep. 2001;24:155–160. doi: 10.1093/sleep/24.2.155. [DOI] [PubMed] [Google Scholar]
- 750.Mendelson WB, Maczaj M, Holt J. Buspirone administration to sleep apnea patients. J. Clin. Psychopharmacol. 1991;11:71–72. doi: 10.1097/00004714-199102000-00019. [DOI] [PubMed] [Google Scholar]
- 751.Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 752.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–1092. doi: 10.1093/sleep/22.8.1087. [DOI] [PubMed] [Google Scholar]
- 753.Schmidt HS. L-tryptophan in the treatment of impaired respiration in sleep. Bull. Eur. Physiopathol. Respir. 1983;19:625–629. [PubMed] [Google Scholar]
- 754.Grace KP, Hughes SW, Shahabi S, Horner RL. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir. Physiol. Neurobiol. 2013;188:277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 755.Suratt PM, Wilhoit SC, Brown ED, Findley LJ. Effect of doxapram on obstructive sleep apnea. Bull. Eur. Physiopathol. Respir. 1986;22:127–131. [PubMed] [Google Scholar]
- 756.Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharm. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- 757.Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. Front. Psychiatry. 2013;4:1. doi: 10.3389/fpsyt.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Gothe B, Strohl KP, Levin S, Cherniack NS. Nicotine: a different approach to treatment of obstructive sleep apnea. Chest. 1985;87:11–17. doi: 10.1378/chest.87.1.11. [DOI] [PubMed] [Google Scholar]
- 759.Aoki CR, Liu H, Downey GP, Mitchell J, Horner RL. Cyclic nucleotides modulate genioglossus and hypoglossal responses to excitatory inputs in rats. Am. J. Respir. Crit. Care Med. 2006;173:555–565. doi: 10.1164/rccm.200509-1469OC. [DOI] [PubMed] [Google Scholar]
- 760.Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, von Euler C. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr. Res. 1984;18:387–390. doi: 10.1203/00006450-198404000-00018. [DOI] [PubMed] [Google Scholar]