Abstract
Low back pain accounts for the most years lost to disability of any malady worldwide but most cases of disc herniation (DH) and degenerative disc disease (DDD) resolve with conservative methods. Numerous tissue sources of pain affecting the degenerative/herniated disc have been identified, with changes secondary to the influence of inflammation figuring prominently among them. Due to the proven linkage of inflammation to the pain and progression of disc degeneration, anti-inflammatory/anti-catabolic and pro-anabolic repair strategies are gaining prominence for novel therapeutic approaches. Current treatments include conservative therapies such as modified rest, exercise, anti-inflammatory treatments, and analgesics. There is no accepted proposed mechanism of action to support the direct role of spinal manipulation for the treatment of the degenerative and/or herniated disc. However, there are published accounts of very serious adverse events accompanying such treatments leading to the question; ‘should a patient with suspected painful IVD be treated with manipulation?
Keywords: chiropractic, disc, herniation, safety
Abstract
Les douleurs lombaires sont responsables du plus grand nombre d’années perdues pour cause d’invalidité, toutes pathologies confondues, mais la plupart des cas de hernie discale (HD) et de discopathie dégénérative (DD) sont résolus par des méthodes conventionnelles. De nombreuses sources tissulaires de douleur affectant le disque dégénératif/herniaire ont été identifiées, les changements secondaires à l’influence de l’inflammation figurant en bonne place parmi elles. En raison du lien avéré entre l’inflammation et la douleur et la progression de la dégénérescence discale, les stratégies de réparation anti-inflammatoires/anticataboliques et pro-anaboliques sont de plus en plus utilisées comme nouvelles approches thérapeutiques. Les traitements actuels comprennent des thérapies conventionnelles telles que le « repos modifié », l’exercice, les traitements anti-inflammatoires et les analgésiques. Il n’existe pas de mécanisme d’action proposé et accepté pour soutenir le rôle direct de la manipulation de la colonne vertébrale dans le traitement de la dégénérescence et/ou de la hernie discale. Toutefois, des comptes rendus publiés font état d’effets indésirables très graves liés à ces traitements, ce qui amène à se poser la question suivante : « Un patient soupçonné de souffrir d’une discopathie dégénérative douloureuse doit-il être traité par manipulation? »
MOTS CLÉS: chiropratique, hernie discale, sécurité

Back and/or neck pain associated with disorders of the intervertebral disc (IVD) are a well-recognized source of spinal pain with and without radicular symptoms and disabling spinal pain is the world’s leading cause of years lost to disability.1 The causes of pain and disability associated with disc disease (herniated and/or degenerative disc disease or ‘DDD’) are difficult to accurately diagnose even with sophisticated imaging including CT, MRI scanning and provocative testing such as discography. A thorough history, careful physical examination and appropriate imaging can lead to a presumptive diagnosis of ‘discogenic pain’ in each patient, however certainty of diagnosis unless reproduced such as through provocative discography, remains elusive. Most episodes of back and/or neck pain are self-limiting and respond to rest, modified activity, analgesics and/or anti-inflammatory interventions. Patients suffering from symptoms of axial and/or radicular pain commonly seek such treatment advice from their family physician, physical therapists, massage therapist, chiropractors, and acupuncturists. Treatments provided by these professionals predictably varies according to the unique perspective /skill set /bias of the respective provider. The chiropractor ought to consider a treatment plan to address pain of discogenic origin based upon a contemporary understanding of the pathobiology of the intervertebral disc and an appropriate risk /reward analysis. This review provides an updated overview of the pathobiology of degeneration of the intervertebral disc and mechanisms of pain with a view to treatment decision making with an emphasis upon the treatment of disc herniation.
Pathology of disc degeneration
The anatomical aspects of the IVD are well known and many review papers that describe the molecular and cellular events integral to disc degeneration have been published previously.2–6 There are numerous inter-related cellular and biological processes that summate to result in degeneration of the IVD and contribute to a loss in IVD integrity, impaired biomechanical properties, disability, pain, and the possible advent of a herniated disc. These processes include a loss of homeostatic regulation of the IVD whereby pro-inflammatory signaling within the IVD lead to progressive cell death, impaired extracellular matrix and cellular communication, all of which potentiates further degeneration.6–10 It is impossible for the clinician to know the precise cellular/molecular signaling status of a given patient’s degenerative disc since the temporal aspects of these biological processes vary from person to person as well as with differing degrees of degeneration. However, what is a certainty, is that disc degeneration impairs loadbearing of the disc and is associated with osteoarthritis of the facet joints, varying degrees of hypertrophy of segmental ligamentous structures and disorders affecting the endplates; all of which may contribute to some degree to the patient’s status and pain.11–14 Impaired loadbearing may contribute to spinal pain of discogenic origin for several reasons including activated nociceptors within the outer annulus, facet joints and joint capsules, and paraspinal muscles and is one of the reasons why discogenic pain is often relieved by rest (lying supine or side lying) or by de-loading such as by the patient supporting themselves to ‘offload’ their back. Pain relief by offloading is also one of the principles underlying such treatments as traction where some patients report significant pain relief at least while the traction/offloading therapy is underway.15 Other aspects of disc-related pain include contributions of neoinnervation and neovascularization that can occur presumably secondary to pro-inflammatory changes within the disc.8,16–18 It is important to recognize that a herniated disc (unless through overt trauma) is almost always downstream of internally dysregulated cellular/extracellular matrix integrity, a series of processes that can present through recurrent episodes of spinal and/or radicular pain or from an acute episode without a history of past events.
Pain of disc origin
It has been extensively demonstrated that under a variety of circumstances, which predominantly include degeneration and damage, the IVD can be a cause of pain.19–22 However, determining that the disc is the source of pain has been a clinical and experimental challenge for many years. Surgical removal of herniated disc tissue that compresses a spinal nerve can provide significant pain relief of up to 90% of patients in the immediate post-op period or within four weeks, however the pain reduction seems to lose significance at approximately one year post surgery.23 With respect to the herniated disc, various non-operative treatments such as rest, extension-based exercise, epidural steroid injections, NSAIDS/analgesics also show good results with up to 90% experiencing relief within four to six weeks.23 Non-operative treatments may result in resolution in most patients suggesting that the presence of the herniated fragment(s) alone may not account for the genesis of the patient’s pain. Nonetheless, pain and disability do not resolve in a smaller number of patients for reasons that remain to be determined. In what may come as a surprise to many clinicians, imaging studies of patients suffering from disc herniation have shown that the size of the herniated disc material does not correlate with symptoms, suggesting that other causes of the patient’s pain are at play, including macrophage mediated tissue digestion and immune modulatory mechanisms.24
Disc degeneration is common in persons over 30 years of age and in many cases is largely asymptomatic (aside from possible contribution to self-limiting back pain afforded perhaps by muscular adaptation to some degree of loadbearing anomalies).25 In the event of a symptomatic herniated disc, there are myriad causes of pain both local and radicular that are more closely associated with the pathology affecting the disc. For example, distortion of and/or pressure/contact with the annulus fibrosus by herniated fragments may activate mechanoreceptors and nociceptors within the annulus, posterior longitudinal ligament and activate reflexive muscular activity leading to muscle spasm and the generation of pain.22,26,27 Pressure placed upon spinal nerve roots plus inflammation associated with such contact often results in the advent of radicular pain within the dermatome of the specific nerve root(s) that may develop within hours, days, weeks, or months. A recent report by Gupta et al examined the size of lumbar disc herniation and predictive value for the success of non-operative therapy.24 This was a retrospective study of 368 patients who had a diagnosis of primary lumbar radicular pain with MRI documentation of a lumbar disc herniation and who also had completed at least a six-month course of non-operative, conservative management. The conservative management inclusion criteria included nonsteroidal anti-inflammatory medication, gabapentin or pregabalin, or pain medication, steroid injection, or physical therapy (not defined) in patients followed for a minimum of two-years. Interestingly the authors report no association between the size of the disc herniation and non-operative recovery. The odds ratio between herniation size and the likelihood of surgical treatment in this study was 1.003 indicating no statistical association between disc herniation size and failure (or success) of conservative treatment.24 A similar report by Benson et al.28 found that large disc herniations could be managed conservatively, including massive disc herniations with up to 85% dural sac stenosis. In addition, a prospective cohort study by Gugliotta et al.29 showed that surgical treatment had better pain relief in the short term (three months post op) but such benefits were no longer seen at one year without regard to the size of the herniated disc. It is noteworthy that in none of the above conservative vs surgical care published papers was spinal manipulation included within the conservative care treatment groups. Taken together these studies indicate that an optimal treatment to address disc herniation continues to remain elusive and that the resolution of a herniated disc follows a largely favorable natural history (of course associated with appropriate symptom management).
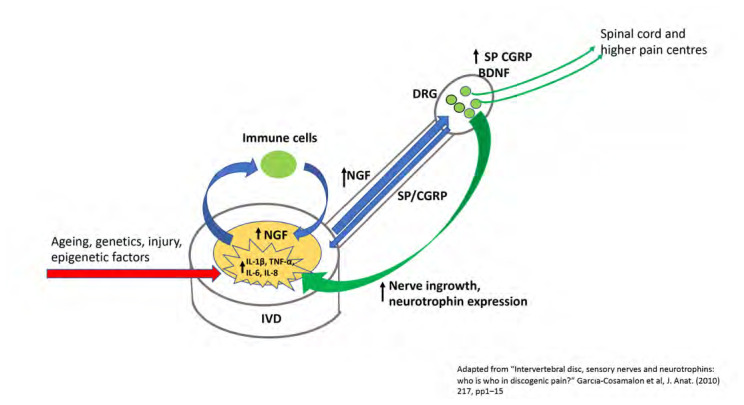
With respect to the genesis of pain from the disc, several mechanisms have been proposed and some have been validated using in vivo animal experiments, but much remains to be determined. However, pain emanating from the IVD must be facilitated via nociceptive capable neurons primarily within the annulus fibrosus but also the vertebral endplates and in some cases, from the nucleus pulposus. With respect to nociception, a class of proteins known as neurotrophins, and neuropeptides have been implicated in the development/modulation of pain including pain of IVD origin.30–32 It has been shown that almost all painful human degenerative discs increase their expression of various neurotrophins such as Nerve Growth Factor (NGF), the Nerve Growth Factor Receptor (TrkA), Brain Derived Factor (BDNF), the BDNF receptor (TrkB), and the neuropeptides Substance P and Calcitonin Gene Related Peptide (CGRP) and its receptor, Calcitonin Receptor Like receptor (CALCRL).30,32,33 Neurotrophins are factors important in the regulation of neuronal survival, development and nociception and are central to cellular responses to inflammation and pain.21,30,34,35 Importantly, neurotrophin expression is also associated with neovascularization and neoinnervation that occurs with the degenerative disc that is a central aspect to the activity of IVD nociceptors and discogenic pain.36 Low back pain of IVD origin has been associated with neural ingrowth and the expression of neurotrophic factors including NGF, BDNF, TrkA, and TrkB that are thought to contribute to primary disc pain.36 Freemont et al.16 were the first to report that nonmyelinated nerve fibres grew into intervertebral discs thought to be painful and that these fibres expressed Substance P. Freemont et al.37 subsequently determined that these IVD-penetrating unmyelinated nerve fibres expressed TrkA as well as NGF. In another report Yamauchi et al.38 showed that conditioned media developed by culturing human IVD tissues obtained from spinal surgery for painful DDD when cultured with neonatal rat dorsal root ganglia (DRG) cells led to axonal growth in the cultured DRG cells. In contrast to untreated media, the authors showed that neuropeptides such as substance P were induced within cultured DRGs only in the presence of media conditoned by degenerative human IVD cells.38 It has been widely reported that inflammation associated with DDD such as increased expression of Il-1α and TNF-α are also associated with increases in pro-inflammatory cytokines within the IVD such as IL-6 and IL-8. These pro-inflammatory events together, potentiate the pro-catabolic, degenerative cascade that summate in a positive feedback cycle of inflammation, cellular/extracellular matrix degradation and elevated levels of neurotrophins/neuropeptides within the IVD, DRG and spinal cord and association with the development of a painful IVD.9,30–32,38,39 Figure 1 depicts a pictorial representation of neurotrophin/neuropeptide expression and putative roles in nociception.
Figure 1.
Pictorial representation of the interplay between pro-inflammatory cytokine expression within the degenerative disc, interaction within the peripheral nerovous system (DRG innervation and retrograde neurotrophin expression), neoinnervation and pain in a theorized mechanism of neurotrophin/neuropeptide/inflammatory cytokine induced IVD pain.
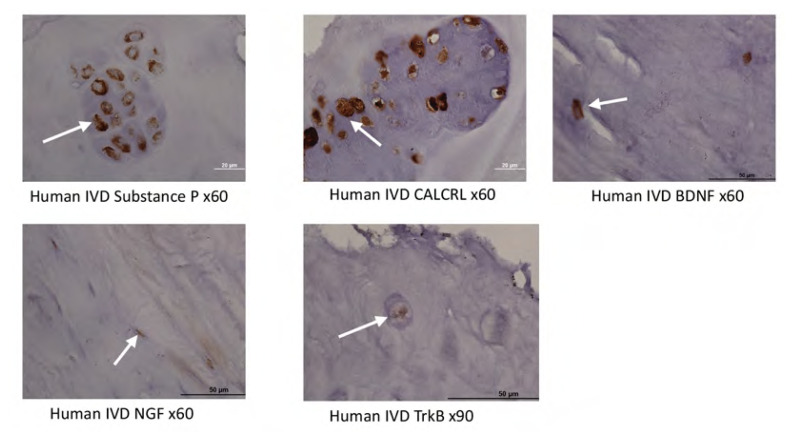
In a needle puncture-induced rat model of DDD, Sugiura et al.40, reported that post IVD puncture and saline injection, there was a significant upregulation of the neuropeptide CGRP in the respective DRGs subserving the affected discs. This study provided support for the hypothesis that disc damage/inflammation may potentiate the expression of pain-related neurotrophins/neuropeptides and provide a basis for disc-mediated pain40. Further, Yamauichi et al.41, reported that proliferation of sensory nerves that innervate the degenerative disc is induced by NGF, itself expressed within the NP38. Additionally, in a study examining healthy and degenerative human IVDs, Purmessur et al.32, reported that the expression of TrkA and TrkB within the IVD are increased in cases of more severe disease. The author’s group has recently published a manuscript in which, amongst other experiments, we assessed the expression of NGFr, BDNF, TrkB, and CALCRL (Calcitonin Receptor Like receptor) in human IVD tissue obtained in cases of discogenic pain and found that virtually all samples strongly expressed these neurotrophins/neuropeptides (Figure 2).33 Tissues used in Figure 2 kindly provided by Dr. Ivan Cheng, Stanford University, and prepared as described in Matta et al 2022.33
Figure 2.
Immunohistochemical staining (DAB chromogen) of human IVD tissues obtained at the time of spinal surgery. (a) Substance P, (b) CALCRL, (c) BDNF, (d) NGF, (e) TrkB. Immunohistochemical staining utilized the DAB chromogen with positive staining depicted by brown deposits (white arrows).
In an in vivo study involving rodents subjected to painful whole body vibration, Kartha et al.42 showed that cervical spine IVDs significantly increased their expression of pain-related neurotrophins as compared to controls. In the Kartha et al.42 study, it was shown that after painful, whole body vibration, BDNF levels within the IVD were increased that also significantly correlated with increased levels of pain, thus supporting the hypothesis that BDNF may play a central role in the generation of discogenic pain.42 BDNF is thought to play a central role in the modulation of nociception via anterograde transport from the DRG that in turn increases neuronal hyperexcitability in the spinal dorsal horn.43 With respect to the important role played by BDNF in disc pain, the expression of BDNF in the disc increases with degenerative grade.31,32,44
Most recently in a paper by the author’s group using a large animal study of needle puncture induced DDD, in injured IVDs that subsequently received a single intradiscal injection of saline, the annulus fibrosus showed significantly increased expression of neurotrophins/neuropeptides associated with pain.33 However, a single injection of a molecular therapy that has been shown to inhibit DDD, significantly suppressed the expression of neurotrophins/neuropeptides at levels indistinguishable from untreated control discs.33,45 Taken together, the preceding cited papers strongly support the hypothesis that disc injury is associated with inflammation and increased neurotrophin/neuropeptide levels that increase nociception and disc-related pain. Therefore, if pain arises as a consequence of cellular/molecular events unique to the damaged disc, how can an externally applied physical load (such as SMT) positively influence the expression of pain by these tissues? Furthermore, a seminal paper by Burke et al.46 showed that the discs obtained from patients who had surgical treatment for disc disease (herniated/degenerative disc) and back/leg pain expressed significantly increased levels of interleukin-6 and -8 (IL-6 and IL-8) as compared to normals or persons experiencing only radiculopathy. The author’s group published a large animal trial of needle puncture-induced DDD and showed that in animals that received needle puncture followed one month later by saline injection the treated discs also expressed significantly increased IL-6 and IL-8 as compared to no treatment control discs.33
Furthermore, recent work has determined that changes within the IVD NP milieu including the percentages of lactic acid, alanine, and propionic acid as well as alterations in the proteoglycan/collagen ratio may account for primary disc pain.47 The determination of these changes uses a new (and investigative) technology termed ‘MRI spectroscopy’ (MRS). MRS is a non-invasive method of determining molecular alterations with the NP that has a high correlation with provocative discography without the downside of inducing disc damage.48 In the future, it may be that such imaging will greatly assist the diagnosis of disc-centric pain. For the present however, in addition to the use of provocative discography (although due to significant inter-examiner reliability and possibility of downstream procedurally induced DDD), a combination of imaging such as MRI, physical examination and patient history remain the only methods available to determine the specific role played by the IVD in the generation of pain.
Vertebral endplate-mediated pain
In addition to MR spectroscopy that can identify IVD NP biochemical sources of pain, recent research has focused upon the vertebral endplates as possible sources of pain, over and above pain of annulus/nucleus origin. Using modified MRI analytics, it has been shown in investigative reports, that Modic changes can be a source of pain and that Modic type 1 changes (inflammation related as compared to fatty infiltration such as is the case with Modic type 2) are more associated with LBP.49,50 It has also been reported that Modic type 1 changes are more amenable to conservative management whereas Modic type 2 (fatty bone marrow infiltration) are refractory to therapy.51 More emerging imaging platforms are currently under development that utilize short-echo time (TE) or ultrashort TE(UTE) MRI sequences that can detect pathological changes in vertebral endplate morphology.52 The possible utility of these imaging platforms may be to replace the semi-quantitative Pfirrmann grading system with (in addition to MRI spectroscopy) and quantitate changes within the vertebral endplates that may significantly contribute to spinal pain associated with DDD. The report by Berg-Johansen52 suggests that vertebral endplate thickness is correlated with Pfirrmann grade, and that new MRI imaging technology may provide objective assessment of tissue sources of back pain.
Clinical decision making
Clinical decisions with respect to the best treatment for a patient suffering from presumptive disc-related pain (a herniated disc in particular) will depend upon patient-specific conditions including age, overall health, neurological status, pain, function, and orthopedic testing plus relevant imaging. Once a presumptive diagnosis of disc-related pain has been made (axial and/or radicular), a treatment plan would be devised based upon available evidence, where the benefit of a given treatment should outweigh its risk These decisions are particularly important when contemplating spinal manipulation (SMT) since these types of treatments involve external loads applied to the involved spinal segment(s) plus areas remote to the injured/degenerative segment. The contribution of a degenerative disc to mechanical spinal pain is difficult to assess in that impaired loadbearing could certainly result in forces acting upon local and more remote spinal joints/tissues that result in adaptive muscular reflex action and presumably associated mechanical pain. Furthermore, it has been reported that the application of spinal manipulation to a given segment also loads segments remote from the ‘targeted’ vertebral segment.53 It is likely that these conditions are a consequence of muscle spindle/golgi tendon organ mediated reflexive muscle splinting or ‘spasm’ when the patient’s pain is of an axial character.27 Such conditions have been reported to be amenable to physical therapeutic maneuvers including stretching, mobilization, manipulation, modified rest, and exercise.54
Resolution of the herniated disc
As cited earlier, numerous published manuscripts detail that approximately 90% of herniated discs recover with ‘conservative therapy’ and have a favorable natural history. Various mechanisms have been proposed to explain the ability of herniated disc to resolve that include pressure upon the herniated mass from the posterior longitudinal ligament, macrophage invasion and resorption, resorption associated with inflammation and even dehydration. 55,56 There have also been hypotheses that the herniated material may retract back inside the IVD because of dehydration, although this has yet to be conclusively demonstrated.24,28,56 The herniated disc can be considered to be a downstream effect of degeneration of the IVD (apart from overt trauma) that leads to loss of water binding within the IVD extracellular matrix, impaired loading tolerance, the development of fissures and tears within the annulus fibrosus that may extend into the nucleus pulposus.2,12,57 It is therefore difficult to understand how a herniated disc could retract into the IVD NP and if so, how the material would remain in place given the degradation of the cellular/extracellular matrix, impaired water binding, fragmented proteoglycan core proteins and increased degradative enzymatic activity.12,58 Therefore, the herniated disc may resolve/resorb over time, or it may not appear to change in size at all. However, many patients (up to 90%) experience relief within three months or more without surgery; raising the question of the relevance of actual herniated material to the pain experienced by the patient and is there any utility in attempting to affect the herniated material? Alternatively, if the herniated material persists, what might be the risk of dislodging such material through externally applied loads? At the present the precise mechanisms involved with resolution of the herniated disc remain to be clarified.
Spinal manipulation and the intervertebral disc
In a case whereby the patient’s pain is thought to be associated with an ‘active’ disc disorder, clinicians may consider the use of spinal manipulation as a treatment option. In the case of spinal manipulation, contemporary theories with respect to its mechanism of action include a gate-controlled theory involving stretch receptors and spinal/supraspinal pain regulation that may be associated with gapping of synovial joints and rapid pressure reduction within the joint and gaseous events associated with this maneuver.27,59 However, the disc is not a synovial joint therefore the gapping of joints theory would not apply. Despite many years of use and several prospective studies (lacking controls), a cogent theory that may seek to explain a reason to contemplate the use of spinal manipulation with respect to the herniated disc remains elusive.27,59 This form of treatment has in some publications, reported to be safe with a very low risk of adverse events.60 With respect to the treatment of disc herniations by spinal manipulation Leeman et al.61 published a manuscript that detailed the treatment of patients with MRI confirmed lumbar disc herniation and low back and/or leg pain associated with the disc herniation. In this manuscript, the authors presented data showing that patients treated with one of two different forms of manipulation yielded a favorable result with no patients experiencing untoward outcomes.61 This study contains several limitations, chief among them is a lack of control group, therefore it is not possible to quantify any reported improvements that may be a product of natural history. This aspect is an important one in that the natural history of lumbar disc herniation is quite favorable. Numerous studies have reported a very favorable natural history with over 90% recovery between four to six weeks.23 The Leeman et al.61 study involving spinal manipulation, reported a very good recovery within 12 weeks, arguably well within the period where natural history may account for symptom resolution. Also, there were no post-treatment/symptom resolution MRI scans making it difficult to discern whether the manipulations performed had any discernible effect upon the herniated disc. A randomized, controlled study by Brontfort et al.62 involving leg pain of ‘back origin’ reported that using spinal manipulative therapy (SMT) and home exercise/advice, and reported that there was an advantage of SMT plus home exercise and advice over home exercise and advice only after 12 weeks. In this study the authors reported that at 12 weeks, 37% of patients receiving SMT plus home exercise and advice had a minimum of 75% reduction in leg pain, as compared to a 19% reduction in the home exercise and advice group. There were no adverse events reported in this study. However, there was no discussion of any imaging results and there was not mention of diagnosis (Spinal stenosis? Herniated disc? Disc protrusion? Other?) thus creating difficulties with data interpretation. 62 Another report involving a series of patients with cervical spine disc herniations that were treated with spinal manipulation claimed to have offered relief with no instances of adverse events.63 On the other hand, spinal manipulation has been found to be linked to worsening of the condition including cauda equina or spinal cord compression. 53,64,65
It has been reported that various physical maneuvers such as lumbar joint mobilizations, prone press ups as well as spinal manipulation has an effect upon water movement within the disc (assessed by pre and post MRI) that has an association with changes in back pain.66,67 These evaluations use a form of MRI assessment and a determination of “associated diffusion coefficient’ (ADC) that ostensibly determines the migration of water within the disc. As much as there is an association with changes in water movement within the disc, currently there is no hypothesis concerning whether such changes in fluid flow within the disc affects pain or how this change in water content might have any effect(s) upon pain.
From the perspective of clinical decision making, due to a series of litigation cases, recent publications within the jurisprudence area has recommended that spinal manipulation treatments not be performed in acute cases of disc injury and that such treatment ought to be reserved for after a period of ‘watchful waiting’/conservative care.53 In particular, the courts found that lumbar disc herniation may be aggravated/caused by spinal manipulation even upon vertebral segments remote from the involved area and this was cited to be particularly the case when a disc herniation was already present.53 A prior manuscript published by the author presented a review of the cellular/molecular biology of the intervertebral disc and included a clinical vignette of a patient who presented with spontaneous neck and arm pain some 15 years following a motor vehicle accident.2 In this manuscript the patient in question was found to have a large disc herniation compressing the cervical spinal cord and associated nerve root that upon clinical examination showed elements of cervical spondylotic myelopathy. The patient was treated initially with a neck brace but was then referred for an anterior cervical discectomy and fusion following which she made a full recovery with complete resolution of the symptoms of myelopathy (abnormal gait, Hoffman sign).2 The salient question in this case was what might have occurred should the patient’s cervical spine been manipulated. In this case of course the pathology was spinal cord compression that is clearly more of a risk than dural sac compression that may occur in the lumbar spine. Of course, this publication is only a single case observational one and global conclusions would be inappropriate, nonetheless, the risk/reward ratio ought to be considered when the clinical encounters a symptomatic disc herniation-particularly in the cervical spine.
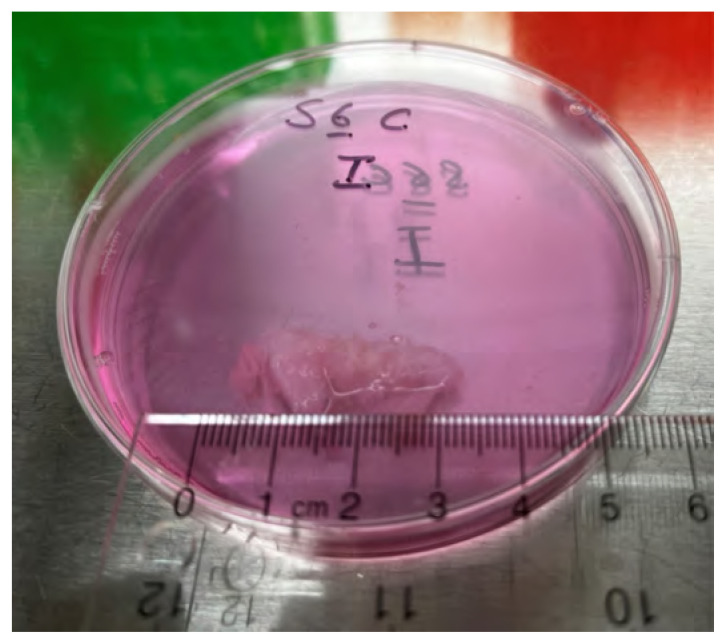
Taken together, there is a robust body of published data showing that with damage/degeneration, IVDs develop changes in their morphology and cellularity that increase the expression of pain-related neurotrophins/neuropeptides that are associated with IVD pain.30–32 Further, disc damage and degeneration increase IVD NP expression of pro-inflammatory and catabolic molecules (IL-6, IL-8, lactic acid, alanine, and propionic acid that are associated with IVD pain) and develop pathological changes within the IVD endplates.9,45–51,68,69 These and associated biomechanical abnormalities likely account for a large amount of the pain and disability associated with IVD pathologies and are clearly tissue level pathological changes that would not be amenable to spinal manipulative techniques. The cases of IVD pain that are refractory to conservative care account for most of the expense and disability associated with disc pathology that urgently require novel, effective therapies that can address the pro-catabolic, pro-inflammatory and anti-anabolic effects that occur in the presence of disc disease. However, the salient question remains, what is the most effective treatment method to use in the case of the herniated disc? Ought spinal manipulation be considered and if so, why? A ‘chunk’ of herniated nucleus pulposus tissue may be precariously attached to the disc itself, therefore the question of what externally applied loads may do to such tissue is an important consideration (Figure 3) (material graciously provided by Drs Paul Salo and Ganesh Swamy, University of Calgary). The tissue in Figure 3 is gross surgical material within tissue culture media (Dulbeccos’ Modified Eagle Medium containing 10% fetal bovine serum, penicillin/streptomycin).
Figure 3.
Gross operative specimen from a human herniated lumbar (L4/5) IVD. The specimen is approximately 3 x 1 cm in size, contained within a 70mm Petri dish in Dulbecco’s Modified Eagle Medium.
Conclusions
The herniated disc with or without radiculopathy, presents a clinical challenge to the treating practitioner and the patient alike as the pain can be excruciating and debilitating. Fortunately, natural history is favorable with a large percentage of patients reporting recovery within three months largely regardless of the applied therapy. It remains to be seen whether non-surgical treatment hastens recovery although in some cases interventional care such as selective nerve root block injections can help to suppress pain until the disc injury heals on its own.70 The same can be said about oral steroid medications that are used acutely with a steep decline in dose over a short period of time. The goal with these treatments is to suppress inflammation either directly by injection in the case of an epidural or transforaminal nerve root block or via systemic delivery. The commonality of these treatments is an attempt to modulate the inflammatory pathway(s) thought to be responsible for a good deal of the pain/disability in the patient suffering from disc herniation. Tissue level changes of the herniated disc downstream of the acute phase, whether it be resorption, dehydration, or macrophage-mediated digestion is likely variable and certainly incompletely understood. It may be that a combination of inflammation and increased nociception of the damaged disc (inflammation/neurotrophin expression?) are key players in the acute phase, with inflammation thought to contribute to IVD endplate changes in the case of Modic type 1 changes.
As discussed within the body of this paper, there is a paucity of evidence to support the use of spinal manipulation to treat disc degeneration/herniation AND a relative lack of convincing supportive data for its use. Within the context of the pathologies known to affect the degenerative/herniated disc as presented herein, the clinician is therefore, left to ponder why the use of SMT would be contemplated at all? Published cases whereby SMT was used to treat the patient suffering from spinal pain and/or radicular pain in the presence of disc herniation for example, often lack suitable controls providing objective evidence that the herniated disc in question was causative of the patient’s pain/disability (no provocative discography, MRI spectroscopy, electrodiagnostic evidence). Further, to the author’s knowledge, there are no published studies that examine the appearance of the herniated disc (such as MRI) post treatment, leading unresolved the question of what SMT may accomplish and how. These continue to be unresolved and important questions; however, the clinician would be well advised to consider the risk/reward ratio when considering manipulating a patient’s spine in the presence of a herniated disc and justifying such an approach in an absence of any proven mechanism of action.
From the author’s perspective, when deciding upon a treatment for a patient suffering from a suspected herniated disc, particularly if there is a radiculopathy, it is wise to remember the salient ‘first do no harm’ principal contained within the Hippocratic Oath. There is no harm in watchful waiting when the patient can be provided with measures that they can follow (exercise, positional relief) to help manage their pain coupled, when necessary, with a multi-disciplinary plan of action such as medication and/or interventional measures. The use of spinal manipulation may have a role to play, however at present, there is insufficient data available to know when and how to apply such therapy and what to expect.
Footnotes
The author has no disclaimers, competing interests, or sources of support or funding to report in the preparation of this manuscript.
References
- 1.Hoy D, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheumat Dis. 2014 doi: 10.1136/annrheumdis-2013-204428. https://doi.org:10.1136/annrheumdis-2013-204428 . [DOI] [PubMed] [Google Scholar]
- 2.Erwin WM, Hood KE. The cellular and molecular biology of the intervertebral disc: a clinician’s primer. J Can Chiropr Assoc. 2014;58:246–257. [PMC free article] [PubMed] [Google Scholar]
- 3.Erwin WM, et al. The biological basis of degenerative disc disease: proteomic and biomechanical analysis of the canine intervertebral disc. Arthritis Res Ther. 2015;17 doi: 10.1186/s13075-015-0733-z. https://doi.org:0.1186/s13075-015-0733-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freemont AJ, Watkins A, Le Maitre C, Jeziorska JAH. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh Adam H, Twomey Julianne D. Cellular mechanobiology of the intervertebral disc: New directions and approaches. J Biomech. 2010;43 doi: 10.1016/j.jbiomech.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang JD, Stefanovic-Racic M, Mcintyre LA, Georgescu H, Evans H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kluba T, Niemeyer T, Christoph GC, Grunder T. Human annulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005;30:2743–2748. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 8.Lotz JC, Ulrich JA. Innervation inflammation and hypermobility may characterize pathological disc degeneration: review of animal model data. J Bone Joint Surg. 2006;88-A:76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 9.Risbud Makarand V, Shapiro Irving M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. https://doi.org:10.1038/nrrheum.2013.160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao CQ, Wang LM, Jiang LS, LY D. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Pain Headache Rep. 2004;8:512–517. doi: 10.1007/s11916-004-0075-z. [DOI] [PubMed] [Google Scholar]
- 12.Sharon Brown, et al. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Eur Spine J. 2012;21:S154–S159. doi: 10.1007/s00586-012-2179-1. https://doi.org:10.1007/s00586-012-2179-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Cho S, et al. Intervertebral disc degeneration and repair. Neurosurg. 2017;80:S46–54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aybala Kocak Fatmanur, et al. Comparison of the short-term effects of the conventional motorized traction with non-surgical spinal decompression performed wtih a DRX9000 device on pain, functionality, depression, and quality of life in patients with low back pain associated with lumbar disc herniation: a single-blind randomized-controlled trial. Turk J Phys Med Rehabil. 2018;64:17–27. doi: 10.5606/tftrd.2017.154. https://doi.org:10.5606/tftrd.2017.154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freemont AJ, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;9072 doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 17.Freemont AJ, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. https://doi.org:10.1002/path.1108 . [DOI] [PubMed] [Google Scholar]
- 18.García-Cosamalón J, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi: 10.1111/j.1469-7580.2010.01227.x. https://doi.org:10.1111/j.1469-7580.2010.01227.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki Y, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine. 2004;29:1077–1081. doi: 10.1097/00007632-200405150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Khan Aysha N, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci. 2017;1410:68–84. doi: 10.1111/nyas.13551. https://doi.org:10.1111/nyas.13551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emerson Krock, et al. Painful degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Molec Med. 2014;18:1213– 1225. doi: 10.1111/jcmm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg. 2007;89-B:1135–1139. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Lopez PD, Rodriguez Salazar A, Martin-Alonso J, Martin-Velasco V. Lumbar disc herniation: natural history, role of physical examination, timing of surgery, treatment options and conflicts of interests. Neurocirug. 2017;8:124–134. doi: 10.1016/j.neucir.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, et al. Does size matter? An analysis of the effect of lumbar disc herniation size on the success of nonoperative Tteatment. Global Spine J. 2020;10:881–887. doi: 10.1177/2192568219880822. https://doi.org:10.1177/2192568219880822 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benoist M. The natural history of lumbar disc herniation and radiculopathy. Joint Bone Spine. 2002:155–160. doi: 10.1016/s1297-319x(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 26.Gen Inoue, et al. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar interverebral discs in rats. Spine. 2006;31:1443–1438. doi: 10.1097/01.brs.0000219946.25103.db. [DOI] [PubMed] [Google Scholar]
- 27.Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine. 2003;70:336–341. doi: 10.1016/s1297-319x(03)00074-5. https://doi.org:10.1016/s1297-319x(03)00074-5 . [DOI] [PubMed] [Google Scholar]
- 28.Benson RT, Tavares SP, Robertson SC, Sharp R, Marshall RW. Conservatively treated massive prolapsed discs: a 7-year follow-up. Ann R Coll Surg Engl. 2010;92:147–153. doi: 10.1308/003588410X12518836438840. https://doi.org:10.1308/003588410x12518836438840 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gugliotta M, et al. Surgical versus conservative treatment for lumbar disc herniation: a prospective cohort study. BMJ Open. 2016;6:e012938. doi: 10.1136/bmjopen-2016-012938. https://doi.org:10.1136/bmjopen-2016-012938 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Cosamalon J, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010 doi: 10.1111/j.1469-7580.2010.01227.x. https://doi.org:doi:10.1111/j.1469-7580.2010.01227.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Ann Rev Neurosc. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. https://doi.org:10.1146/annurev.neuro.29.051605.112929 . [DOI] [PubMed] [Google Scholar]
- 32.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthr Res Ther. 2008;10:R99. doi: 10.1186/ar2487. https://doi.org:10.1186/ar2487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matta A, et al. A single injection of NTG-101 reduces the expression of pain-related neurotrophins in a canine model of degenerative disc disease. Int J Mol Sci. 2022:23. doi: 10.3390/ijms23105717. https://doi.org:10.3390/ijms23105717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. https://doi.org:10.1146/annurev.neuro.24.1.677 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pezet S, MacMahon SB. Neurotrophins: mediators and modulators of pain. Ann Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 36.Binch Abbie LA, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertbral disc degeneration. Arthr Res Ther. 2014;16 doi: 10.1186/s13075-014-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic pain. Rheumatol. 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi IG, et al. Nerve growth factor of cultured medium extracted from human degenerative nucleus pulposus promotes sensory nerve growth and induces substance P in vitro. Spine. 2009;34:2263–2269. doi: 10.1097/BRS.0b013e3181a5521d. [DOI] [PubMed] [Google Scholar]
- 39.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. https://doi.org:10.22203/ecm.v030a08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ai Sugiura, et al. Effect of applying p75NTR saporin to a punctured intervertebral disc on calcitonin generelated peptide expression in rat dorsal root ganglions. J Orthopaed Sc. 2010;15:407–413. doi: 10.1007/s00776-010-1469-x. [DOI] [PubMed] [Google Scholar]
- 41.Navone SE, et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J Orthop Res. 2012;30:1470–1477. doi: 10.1002/jor.22098. https://doi.org:10.1002/jor.22098 . [DOI] [PubMed] [Google Scholar]
- 42.Kartha S, et al. Upregulation of BDNF and NGF in cervical intervertebral discs exposed to painful whole body vibration. Spine. 2014:1542–1548. doi: 10.1097/BRS.0000000000000457. https://doi.org:10.1097/BRS.0000000000000457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hee-Jung Cho, Jeong-Ki Kim, Xin-Fu Zhou, Rush RA. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997;764:269–272. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- 44.Gruber HE, et al. Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther. 2008;10 doi: 10.1186/ar2456. https://doi.org:10.1186/ar2456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matta A, et al. NTG-101: a novel molecular therapy that halts the progression of degenerative disc disease. Sci Reports. 2018;8 doi: 10.1038/s41598-018-35011-4. https://doi.org:10.1038/s41598-018-35011-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke JG, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg. 2002;84-B:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 47.Keshari KR, et al. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine. 2008;33:312–317. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 48.Gornet MG, et al. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur Spine J. 2019;28:674–687. doi: 10.1007/s00586-018-05873-3. https://doi.org:10.1007/s00586-018-05873-3 . [DOI] [PubMed] [Google Scholar]
- 49.Jarvinen J, et al. Association between changes in lumbar modic changes and low back symptoms over a two-year period. BMC Musculoskel Dis. 2015;16 doi: 10.1186/s12891-015-0540-3. https://doi.org:10.1186/s12891-015-0540-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen C, Poiraudeau S, Francois R. From Modic 1 vertebral-endplate subchondral bone signal changes detected by MRI to the concept of ‘active discopathy’. Ann Rheumat Dis. 2015;74:1488–1494. doi: 10.1136/annrheumdis-2015-207317. https://doi.org:10.1136/annrheumdis-2015-207317 . [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Yang H, Zhang L, Wang Y, Zou J. Analyzing the influence of modic changes on patients with lower back pain undergoing conservative treatment. Pain Res Manag. 2019;8185316 doi: 10.1155/2019/8185316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg-Johansen B, et al. Cartilage endplate thickness variation measured by ultrashort echo-time MRI Is associated with adjacent disc degeneration. Spine. 2018;43:e592–e600. doi: 10.1097/BRS.0000000000002432. https://doi.org:10.1097/brs.0000000000002432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boucher P, Robidoux S. Lumbar disc herniation and cauda equina syndrome following spinal manipulative therapy: a review of six court decsions in Canada. J Forens Legal Med. 2014;22:159–169. doi: 10.1016/j.jflm.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Cao DY, Pickar JG. Effect of spinal manipulation on the development of history-dependent responsiveness of lumbar paraspinal muscle spindles in the cat. J Can Chiropr Assoc. 2014;58:149–159. [PMC free article] [PubMed] [Google Scholar]
- 55.Grunhagen T, Shirazi AA, Fairbank JC, Urban JP. Intervertebral disc nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthopaed Clin North Am. 2011;42:465–477. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Turk O, Antar V, Yaldiz C. Spontaneous regression of herniated nucleus pulposus. Med. 2019;98 doi: 10.1097/MD.0000000000014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthr Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erwin WM, et al. The biological basis of degenerative disc disease: proteomic and biomechanical analysis of the canine intervertebral disc. Arth Res Ther. 2015;17 doi: 10.1186/s13075-015-0733-z. https://doi.org:10.1186/s13075-015-0733-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bialosky JE, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthopaed Sports Phys Ther. 2018;48:8–18. doi: 10.2519/jospt.2018.7476. [DOI] [PubMed] [Google Scholar]
- 60.Oliphant D. Safety of spinal manipulation in the treatment of lumbar disc herniations-a systematic review and risk assessment. J Manip Physiol Ther. 2004;27:197–210. doi: 10.1016/j.jmpt.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Leeman S, Peterson CK, Schmid C, Anklin B, Humphreys BK. Outcomes of acute and chronic patients with magnetic resonance imaging-confirmed symptomatic lumbar disc herniations receiving high-velocity, low-amplitude, spinal manipulative therapy: a prospective observational cohort study with one-year follow-up. J Manip Physiol Ther. 2014;37:155–163. doi: 10.1016/j.jmpt.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Bronfort G, et al. Spinal manipulation and home exercise with advice for subacute and chronic back-related leg pain. Ann Inter Med. 2014;161:381–391. doi: 10.7326/M14-0006. https://doi.org:10.7326/M14-0006 . [DOI] [PubMed] [Google Scholar]
- 63.Peterson CK, Schmid C, Leeman S, Anklin B, Humphreys BK. Outcomes from magnetic resonance imaging-confirmed symptomatic cervical disc herniation patients treated with high-velocity, low amplitude spinal manipulative therapy: a prospective cohort study with 3-month follow-up. J Manip Physiol Ther. 2013;36:461–467. doi: 10.1016/j.jmpt.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Tamburreli FC, Maurizo G. Cauda equina syndrome and spine manipulation: case report and review of the literature. Eur Spine J. 2011;20:S128–S131. doi: 10.1007/s00586-011-1745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheng-Li Huang, et al. Characteristics of lumbar disc herniation with exacerbation of presentation due to spinal manipulative therapy. Med. 2015;94 doi: 10.1097/MD.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beattie PF, Arnot CF, Donley JW, Noda H, Lane B. The immediate reduction in low back pain intensity following lumbar joint mobilization and prone press-ups is associated with increased diffusion of water in the L5–S1 intervertebral disc. J Orthopaed Sports Phys Ther. 2010;40:256–264. doi: 10.2519/jospt.2010.3284. [DOI] [PubMed] [Google Scholar]
- 67.Beattie PF, Butts R, Donley JW, Lizuaao DM. The withinsession change in ow back pain intensity following spinal manipulative therapy is related to differences in diffusion of water in the intervertebral discs of the upper lumbar spine and L5–S1. J Orthopaed Sports Phys Ther. 2014;44:19–29. doi: 10.2519/jospt.2014.4967. [DOI] [PubMed] [Google Scholar]
- 68.Keshari KR, Lotz JC, Kurhanewicz J, Majumdar S. Correlation of HR-MAS spectroscopy derived metabolite concentrations with collagen and proteoglycan levels and Thompson grade in the degenerative disc. Spine. 2005;30:2683–2688. doi: 10.1097/01.brs.0000188256.88859.9c. [DOI] [PubMed] [Google Scholar]
- 69.Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of modic changes. Eur Spine J. 2016;25:3723–3734. doi: 10.1007/s00586-016-4459-7. https://doi.org:10.1007/s00586-016-4459-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckel TS, Bartynski WS. Epidural steroid injections and selective nerve root blocks. Tech Vasc Interv Radiol. 2009;12:11–21. doi: 10.1053/j.tvir.2009.06.004. https://doi.org:10.1053/j.tvir.2009.06.004 . [DOI] [PubMed] [Google Scholar]