Abstract
Background:
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous pollutants originating from petrogenic and pyrogenic sources. PAH compounds can cross the placenta, and prenatal PAH exposure is linked to adverse infant and childhood health outcomes.
Objective:
In this first human transcriptomic assessment of PAHs in the placenta, we examined associations between prenatal PAH exposure and placental gene expression to gain insight into mechanisms by which PAHs may disrupt placental function.
Methods:
The ECHO PATHWAYS Consortium quantified prenatal PAH exposure and the placental transcriptome from 629 pregnant participants enrolled in the CANDLE study. Concentrations of 12 monohydroxy-PAH (OH-PAH) metabolites were measured in mid-pregnancy urine using high performance liquid chromatography tandem mass spectrometry. Placental transcriptomic data were obtained using paired-end RNA sequencing. Linear models were fitted to estimate covariate-adjusted associations between maternal urinary OH-PAHs and placental gene expression. We performed sex-stratified analyses to evaluate whether associations varied by fetal sex. Selected PAH/gene expression analyses were validated by treating HTR-8/SVneo cells with phenanthrene, and quantifying expression via qPCR.
Results:
Urinary concentrations of 6 OH-PAHs were associated with placental expression of 8 genes. Three biological pathways were associated with 4 OH-PAHs. Placental expression of SGF29 and TRIP13 as well as the vitamin digestion and absorption pathway were positively associated with multiple metabolites. HTR-8/SVneo cells treated with phenanthrene also exhibited 23% increased TRIP13 expression compared to vehicle controls (p = 0.04). Fetal sex may modify the relationship between prenatal OH-PAHs and placental gene expression, as more associations were identified in females than males (45 vs 28 associations).
Discussion:
Our study highlights novel genes whose placental expression may be disrupted by OH-PAHs. Increased expression of DNA damage repair gene TRIP13 may represent a response to double-stranded DNA breaks. Increased expression of genes involved in vitamin digestion and metabolism may reflect dietary exposures or represent a compensatory mechanism to combat damage related to OH-PAH toxicity. Further work is needed to study the role of these genes in placental function and their links to perinatal outcomes and lifelong health.
Keywords: Polycyclic aromatic hydrocarbons, Placenta, Transcriptomics, Developmental origins of health and disease, TRIP13
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants that originate from petrogenic sources (non-combusted natural gas, crude oil and petroleum products) and pyrogenic sources (incomplete combustion of organic matter (Li et al., 2008a). The developing fetus is particularly vulnerable to environmental toxicants, and prenatal exposure to PAHs quantified in placental tissue or maternal urine has been linked to a variety of adverse perinatal and early childhood outcomes, including decreased birthweight (Agarwal et al., 2020; Doroodzani et al., 2021) and increased risk of preterm birth (Agarwal et al. 2018; Freije et al. 2022; Padula et al. 2014). Prenatal exposure to airborne PAHs has also been positively associated with increased odds of cognitive developmental delays at age 3 in a population of inner city children (Perera et al. 2006). Prenatal exposure to PAH metabolites 1-hydroxypyrene (1-OH-PYR) and 2-naphthol was associated with decreased mental development scores (Ha et al. 2011). In the CANDLE cohort, 1-OH-PYR was associated with increased risk of neuro-developmental delay, and mixtures of OH-PAH metabolites were associated with lower language scores at age 2 (Wallace et al. 2022). Taken together, these observational studies suggest that maternal PAH exposure during pregnancy may cause alterations to the placental in-utero environment that result in perinatal complications and lasting effects on offspring into childhood.
Common dietary sources of PAH exposure include fruits and vegetables, grain, seafood, roasted beans, and smoked meat (Zelinkova and Wenzl 2015). PAHs also represent an important component of air pollution that is often bound to particulate matter (Yang et al., 2021). Ingestion and inhalation are the major routes of PAH exposure, resulting in widespread detection of PAHs in the US population (Ramesh et al. 2004; Woodruff Tracey, Zota Ami, and Schwartz Jackie 2011). After ingestion, PAHs are first oxidized via cytochrome p450 enzymes (phase 1) to form epoxide intermediate metabolites, which are then reduced or hydrolyzed to their hydroxylated metabolites which are excreted in urine and feces (Ramesh et al. 2004). The half-life of urinary OH-PAH metabolites are relatively short (<12 h) so the urinary concentrations of these metabolites reflect recent exposures, including both inhalation and dietary exposures(Li et al. 2012). Accordingly, spot urine measures of maternal urinary OH-PAH metabolites at different timepoints in pregnancy exhibit low to moderate intraclass correlation coefficients (A. L. Cathey et al. 2020; A. Cathey et al. 2018; Gaylord et al. 2022).Despite their short half-lives, urinary concentrations OH-PAH metabolites are higher in individuals with known industrial exposure to PAHs (Gao et al. 2016; Wu et al. 2021) in smokers (Li et al. 2008b), and in individuals with known dietary PAH exposure (Li et al. 2010; Dobraca et al. 2018), highlighting the reliability of these metabolites quantified in urine as indicators of PAH exposure. OH-PAH metabolites measured in urine are commonly used surrogates for PAH exposures in epidemiological assessments (A.L. Cathey et al. 2020; Alghamdi et al. 2015; Gao et al. 2016; Dobraca et al. 2018; Wu et al. 2021; Li et al. 2010; Z. Yang et al. 2021).
The fetal placenta is an ideal organ to study the molecular mechanisms underlying perturbations in fetal development caused by in-utero environmental exposures, including PAHs. The placenta is a transient but essential fetal organ that is the master-regulator of the fetal environment (Myatt 2006). The placenta controls gas exchange, transports nutrients and waste, provides immunological defense, and is involved in maternal-fetal communication (Burton and Jauniaux 2015), and produces neuropeptides, growth factors, and steroid hormones, which are released into the maternal and fetal circulation (Mesiano 2009). Both low and high molecular weight PAH metabolites have been detected in the placenta (Drwal, Rak, and Gregoraszczuk 2019). One study identified detectable levels of 16 PAH metabolites in umbilical cord blood, with low molecular weight PAH metabolites having higher concentrations in cord blood than in the placenta and maternal blood (Dong et al. 2018). The authors posited that this indicates PAHs cross the placenta and accumulate in the undeveloped fetus, potentially due to the high levels of lipoproteins in the fetus (Dong et al. 2018).
PAHs disrupt cellular processes that are important for placental function and fetal development. The reactive metabolites of PAHs form covalent adducts with DNA, which causes DNA damage and can lead to pro-carcinogenic pathways (Henkler, Stolpmann, and Luch 2012) This may have indirect effects of slowing cell cycle progression and triggering DNA repair mechanisms such as TP-53 (Henkler, Stolpmann, and Luch 2012). PAHs can activate the aryl hydrocarbon receptor (AHR), and thus upregulate the expression and activity of enzymes that metabolize xenobiotics (Machala et al. 2001), which may alter the metabolism of other xenobiotics or endocrine disrupting chemicals. PAHs have also been shown to impact immune functions, including the inhibition of B cell, T-cell and myeloid cell development, and alteration of cytokine production (Patel et al. 2020). PAH metabolites have multifaceted effects on placental physiology, including decreasing angiogenesis, inducing trophoblast differentiation, and modulating proliferation (Drwal, Rak, and Gregoraszczuk 2019). In-vitro studies have revealed that PAHs disrupt placental growth and function. The PAH compound Benzo (a) pyrene (BAP) induced secretion of human chorionic gonadotropin and differentiation of the trophoblastic BeWo cells in a AHR and p53 dependent manner (Le Vee et al. 2014), and was associated with increased CYP1A1 expression and inhibition of cellular proliferation in JEG-3 choriocarcinoma cells (L. Zhang and Shiverick 1997). Extravillous trophoblast HTR-8/SVneo cells treated with benzo[k]fluoranthene, BAP, and 4,4-(9-fluorenylidene) diphenol exhibited decreased cell viability and increased necrosis (Y. S. Jo et al. 2022). Concentrations of naphthalene, pyrene, and phenanthrene that were equivalent to levels measured in maternal blood and/or placental tissue caused increased cytotoxicity and exhibited endocrine disrupting effects, based on progesterone and estradiol secretion in JEG-3 cells (Drwal et al. 2017).
Taken together, these disruptions to placental formation and structure may alter placental function, ultimately resulting in disruptions to fetal development. For example, placental levels of three PAHs (BAP, benzo[b]fluorene, and dibenz[a,h]anthracene) were inversely associated with both gestational age at delivery and birthweight, as well as increased PAH-DNA adducts and differences in placental metabolite concentrations in 104 subjects residing near superfund sites in Harris county, Texas (Suter et al. 2019). Moreover, PAH exposure is associated with increased concentrations of important hormones that are involved in pregnancy and parturition, including corticotrophin releasing hormone, estriol, and T3, and decreased concentrations of progesterol (A. L. Cathey et al. 2020). This body of evidence highlights several key mechanisms by which PAHs compromise fetal development through the placenta.
The generation and analysis of -omics data from the placenta can reveal the effects of maternal environmental exposures on placental function and fetal development (Breton Carrie et al. 2017a; Everson and Marsit 2018; Lapehn and Paquette 2022). The transcriptome captures the quantity of all genes that transcribed from the genome at a given time, thus providing insight into underlying molecular signals within the cell (Pertea 2012). Prior studies have identified associations between the placental epigenome and/or transcriptome and other markers of air pollution, including PM2.5, (Deyssenroth et al. 2021; Enquobahrie et al. 2022; S.-I. Yang et al. 2020; Y. Zhao et al. 2021) PM10 (Abraham et al. 2018), and NO2 (Abraham et al. 2018; Ladd-Acosta et al. 2019). This body of literature suggests that air pollution may disrupt placental functions, but chemical exposures within airborne particles are not as well studied in the context of placental -omics (Lapehn and Paquette 2022). However, a limited number of studies have identified associations between exposure to PAHs and differences in gene and long non-coding (lnc)RNA expression in other tissues. In an experimental model, treatment with a mixture of PAHs induced changes involving cell cycle regulation, inflammation, DNA damage, and cellular adhesion in human bronchial epithelial cells (Chang, Rager, and Tilton 2021). In a candidate gene study conducted in industrial workers highly exposed to PAHs, urinary concentrations of 1-OH-PYR were significantly correlated with expression of lncRNAs MALAT1 and HOTAIR in peripheral blood leukocytes (Gao et al. 2016).
Fetal sex is a substantial contributor to differences in placental physiology and function, where male placentas are smaller in relation to body size than females, making them more efficient but with less reserve capacity and potentially more susceptible to environmental perturbations (Eriksson et al. 2010). Widespread sex differences in gene expression have been noted at the transcriptomic level (Braun et al. 2021; Buckberry et al. 2014), including genes involved in immune signaling, cell growth and proliferation, and placental development. Increasing evidence suggests that fetal sex may also have an impact on placental response to environmental exposures (Marsit 2015; Tarrade et al. 2015), and we have identified sex-specific differences in the placental transcriptome in relation to maternal phthalate exposure (Paquette. et al., 2021) as well as maternal PM2.5 exposure (Enquobahrie et al. 2022). We have also observed sex-specific relationships between specific OH-PAH metabolites and gestational duration (Freije et al. 2022), with 1-OH-PYR associated with increased risk of preterm birth and 2-OH-NAP associated with earlier gestational length in females, and 2/3/9 OH-FLOU associated with decreased risk of preterm birth in males. Sex specific transcriptomic responses to phenanthrene have been observed in fathead minnows (Loughery et al. 2021), but to our knowledge this has not been investigated in humans.
Based on this body of evidence, we hypothesized that OH-PAH metabolite concentrations would be associated with differences in the placental transcriptome, including genes and lncRNAs. Herein, we comprehensively characterize the relationship between concentrations of individual OH-PAH metabolites quantified in urine collected in mid pregnancy and the placental transcriptome at birth. Based on sexual dimorphisms in the placental transcriptomic landscape (Buckberry et al. 2014) and other studies demonstrating sex-specific differences in responses to other environmental chemicals, we further considered fetal sex as a potential modifier of the relationship between PAH metabolites and the placental transcriptome. These findings provide insight into how prenatal exposure to PAHs may disrupt placental function at a molecular level.
2. Methods
2.1. Study participants
The CANDLE study (Conditions Affecting Neurocognitive Development and Learning in Early Childhood) is a prospective pregnancy cohort study set in Shelby County TN from 2006 to 2011, and has been described in detail previously (Sontag-Padilla et al., 2015). Within the CANDLE Cohort, the ECHO-PATHWAYS consortium generated placental RNA sequencing data from 794 participants and measured maternal urinary PAH concentrations from 904 participants. (LeWinn et al. 2022) Exclusion criteria for the CANDLE study included confirmed clinical chorioamnionitis, oligohydramnios, infarction or previa, fetal chromosomal abnormalities. In this analysis we also excluded individuals with placental abruptio as well as individuals with tobacco exposure during pregnancy, based on maternal self-report or a prenatal urinary cotinine>200 ng/dl, which is commonly used to define smokers (Schick et al. 2017). Covariate data including maternal race, ethnicity, age, and education were self-reported at the enrollment visit; fetal sex, labor type and delivery status were ascertained from medical record abstraction by a registered nurse (Sontag-Padilla et al., 2015). Analyses included participants with complete RNA sequencing data and PAH measurements and no missing covariate data (N = 629). All research activities for the CANDLE cohort were approved by the Institutional Review Board (IRB) of the University of Tennessee Health Sciences Center. Analyses were conducted as part of the ECHO PATHWAYS study and were approved by the University of Washington IRB.
2.2. Collection of maternal urine and quantification of OH-PAH metabolite concentrations
Maternal urine was collected using polypropylene containers from participants during clinical visits, typically during the second trimester of pregnancy (range: 15.3 weeks-28.7 weeks, mean 22.9 weeks) (Sontag-Padilla et al., 2015). Samples were processed and stored at −80 °C in the study repository of the University of Tennessee Health Science Center Department of Pathology. Detailed methods of OH-PAH measurements within this cohort have been described previously (Freije et al. 2022; Wallace et al. 2022; Loftus et al. 2022). Twelve OH-PAH metabolites were determined using a Waters Acquity I-class ultra-performance liquid chromatography system (Waters; Milford, MA, USA). Identification and quantification of OH-PAH metabolites was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). These 12 OH-PAH metabolites included two metabolites of naphthalene (1-hydroxynaphthalene [1-OH-NAP], 2-hydroxynaphthalene [2-OH-NAP]), four metabolites of phenanthrene (combined 2-hydroxyfluorene, 3-hydroxyfluorene, and 9-hydroxyfluorene (2/3/9-OH-FLUO), combined 1-hydroxyphenanthrene and 9-hydroxyphenanthrene [1/9-OH-PHEN]), 2-hydroxyphenanthrene [2-OH-PHEN], 3-hydroxyphenanthrene [3-OH-PHEN], 4-hydroxyphenanthrene [4-OH-PHEN], 1-hydroxypyrene (1-OH-PYR), 3-hydroxybenzo[c] phenanthrene (3-OH-BCP), two metabolites of hydroxychrysene (1-hydroxychrysene [1-OH-CHRY], 6-hydroxychrysene [6-OH-CHRY]), and 1-hydroxybenz[a]anthracene (1-OH-BAA). Procedural and instrument blanks were included for quality control. Specific gravity was determined using a handheld refractometer. For samples with concentrations below the limit of detection (LOD), the concentration was reported as the LOD/√2. Only those OH-PAH metabolites that were detected in > 70% of samples were included in the analysis, in alignment with prior studies by our group and others which have analyzed urinary metabolites of PAHS (Freije et al. 2022; A. L. Cathey et al. 2020; Wallace et al. 2022; Dobraca et al. 2018; Loftus et al. 2022). Cotinine was measured in maternal urine, as previously described (Ni Yu et al., 2021; Paquette et al., 2021).
2.3. Placental sample processing and RNA sequencing
Placental tissue sampling, RNA isolation, RNA sequencing have previously been described for CANDLE participants (Enquobahrie et al. 2022; Paquette et al., 2021.). Briefly, immediately following delivery, placental villous tissue was dissected from the placental parenchyma and cut into four approximately 0.5 cm cubes. The tissue cubes were stored in RNAlater and refrigerated at 4 °C overnight (at least 8 h but no >24 h), then transferred to an individual 1.8 ml cryovial containing fresh RNAlater. The cryovials were stored at − 80 °C, and the fetal villous tissue was manually dissected, and cleared of maternal decidua. Following dissection, the fetal samples were placed into RNAlater and stored at −80 °C. Approximately 30 mg of fetal villous placental tissue was used for RNA isolation. Tissue was homogenized in tubes containing 600 μL buffer RLT Plus with β-mercaptoethanol using a TissueLyser LT instrument (Qiagen; Germantown, MD). RNA was isolated using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Germantown, MD) according to the manufacturer’s recommended protocol. RNA integrity was determined with a Bioanalyzer 2100 using RNA 6000 Nanochips (Agilent; Santa Clara, CA). Only RNA samples with an RNA Integrity Number (RIN) > 7 were sequenced.
RNA sequencing was performed at the University of Washington Northwest Genomics Center (NWGC). Total RNA was poly-A enriched and cDNA libraries were prepared using the TruSeq Stranded mRNA kit (Illumina, San Diego, CA). Each library was sequenced to an approximate depth of 30 million reads on an Illumina HiSeq 4000 instrument. De-multiplexed BAM files were converted to FASTQ format using Samtools bam2fq. RNA sequencing quality control was performed using both the FASTX-toolkit (v0.0.13) and FastQC (v0.11.2) (Brown, Pirrung, and McCue 2017). Transcript abundances were estimated by aligning to the GRCh38 transcriptome (Gencode v33) using Kallisto (Bray et al. 2016), then collapsed to the gene level using the Bioconductor tximport package, scaling to the average transcript length (Soneson, Love, and Robinson 2015). Only protein-coding genes (N = 12,839) and lncRNAs (N = 1,079) were included in this analysis. Data is available upon reasonable request, in alignment with ECHO PATHWAYS collaboration guidelines (LeWinn et al. 2022).
2.4. Identification of differentially expressed genes (DEGs)
Differentially expressed mRNAs associated with each OH-PAH metabolite were identified using the limma-voom pipeline (Law et al. 2014). Gene counts were scaled to library size (normalized using a trimmed mean of M–values) (Robinson and Oshlack 2010) and converted to log counts/million (log CPM). After filtering to remove unreliably expressed genes (defined as average logCPM < 0), observation-level weights were computed based on the relationship between the mean and variance of the log CPM values. Separate models were run for each OH-PAH metabolite, which was analyzed as a continuous variable, and our effect estimates represent log CPM/OH-PAH concentration in ng/ml. For each separate model, we identified unmeasured confounding (such as from unmeasured covariates or cellular heterogeneity) using surrogate variable analysis (SVA), and adjusted for these surrogate variables (N = 35) in our model (Leek and Storey 2007). We performed adjustment for multiple comparisons using the Benjamini-Hochberg approach (Benjamini and Hochberg 1995), and genes were considered statistically significant at a false discovery rate (FDR) adjusted p < 0.05.
We selected potential confounders a priori by reviewing research literature to identify factors associated with PAHs and the placental transcriptome. We also adjusted for experimental variables. Our models included urine specific gravity, RNA sequencing batch, fetal sex, labor status, delivery method, maternal smoke exposure reflected in maternal urinary cotinine concentration (continuous), maternal age (continuous), maternal pre-pregnancy BMI, and maternal education, maternal race (Black vs other) and maternal ethnicity (Non-Latino/Hispanic vs Latino/Hispanic). Covariate adjustment and groupings for race was performed in a manner consistent with prior transcriptome analyses in the CANDLE cohort (Enquobahrie et al., 2022; Paquette et al, 2021.). To assess effect modification by child sex, we performed a sex-stratified analysis, and adjusted for all variables described above. A complete overview of the sample collection and a directed acyclic diagram are provided in Supplemental Figure S1.
2.5. Pathway enrichment analysis
To identify pathways with significant associations between gene expression and each individual OH-PAH metabolite, we applied a self-contained gene set test using the FRY method (Giner and Smyth 2016). The FRY method tests that the average t-statistic for each gene set is larger than expected under the null hypothesis. We included all Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways (Kanehisa et al. 2016) except disease pathways (KEGG release 98.1). As this was an exploratory analysis, pathways were considered statistically significant at FDR < 0.1.
2.6. Cell culture and PAH treatment conditions
HTR-8/SVneo cells were obtained from ATCC (#CRL-3271, Batch: 70016636) and were cultured at 37 °C with 5% CO2 in 6-well tissue culture dishes using RPMI-1640 with L-glutamate supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (P—S), 1 mM sodium pyruvate, and 10 mM HEPES. Phenanthrene was acquired from Sigma Aldrich (#P11409) and prepared fresh in 100% DMSO before dilution in cell culture media. HTR-8/SVneo cells were treated with DMSO (0.05%), 5 ng/ml or 50 ng/ml phenanthrene (containing a final concentration of 0.05% DMSO) with three replicates per condition for 24 hours.
2.7. RNA isolation, reverse transcription, and qPCR validation
RNA isolation was performed with the Pure Link RNA Mini Kit (Invitrogen) following manufacturer’s instructions using 300 μL lysis buffer per sample well. RNA quality and concentration was assessed using a nanodrop with average RNA concentration across samples of 183.6 ng/μL and average 260/280 ratios of 2.07. cDNA was reverse transcribed from 1 μg RNA using the iScript cDNA Synthesis Kit (Bio-Rad). No reverse transcriptase (NRT) controls were included in the reverse transcription reaction with nuclease free H2O used in place of the reverse transcriptase enzyme. Real time quantitative PCR (qPCR) was performed using TaqMan Fast Advanced Mastermix (Thermo Fisher) with TaqMan Gene Expression Assays (FAM-MGB) following the manufacturer’s protocol. SDHA was used as a housekeeping gene (TaqMan Assay ID: Hs00188166_m1) and TRIP13 was the gene of interest (TaqMan Assay ID: Hs01020073_m1). Each sample was run in technical triplicates with duplicates of NRT controls and two no template controls (NTC) per TaqMan gene expression assay. The qPCR was run on a CFX96A machine (Bio-Rad) following the protocol outlined in the TaqMan instruction manual for single-tube assays. Fold Changes were calculated relative to DMSO with normalization performed based on the SDHA housekeeping gene expression following the ΔΔCt calculation method. No amplification was detected in NRT or NTC controls.
3. Results
Urine was collected from CANDLE participants (Ages 16–41, median age 28) at a clinic visit conducted between 15.29 and 28.71 weeks into the pregnancy (median urine collection timepoint 22 weeks 6 days, standard deviation 3 weeks), which was used for quantification of OH-PAH metabolites. Placental samples were collected at the end of gestation (median gestational age 39.14 weeks, standard deviation 1.5 weeks) with 578 (92.6%) participants delivering after 37 weeks of gestation. Covariate data is described in Table 1. The majority of individuals in this cohort identified as Black (56.8% of participants) or White (37.7% of participants). The remaining participants (5.56%) identified as either multiple race, Asian, or other. Most participants underwent labor (81.4%) and delivered vaginally (60.73%. The distribution of sex across placental samples was similar. While confounding and precision variables were selected a priori (See Figure S1), we also performed differential gene expression analyses in relation to these variables and identified DEGs associated with every confounding variable included, based on a cutoff of FDR-adjusted p < 0.05 (Table S1). The covariates associated most strongly with the placental transcriptome included RNA sequencing batch, maternal race, maternal education, and labor status.
Table 1.
Continuous and categorical information about CANDLE cohort participants used in the present analyses (N = 629 Total), recruited from Shelby County TN from 2006 to 2011.
| N (%) | Range (Min-Max) |
Median | Std. Dev |
|
|---|---|---|---|---|
| Maternal Age (Years) | 16–41 | 28 | 5.53 | |
| Maternal BMI (kg/m2) | 14–62 | 26 | 7.59 | |
| Maternal Cotinine (ng/mL) | 0–197.24 | 0.17 | 21.88 | |
| Gestational Age at Birth (Weeks) | 26.86–41.86 | 39.14 | 1.50 | |
| Gestational Age at PAH Collection | 133 (21.14%) | 15.29–28.71 | 22.86 | 3.04 |
| RNA Sequencing Batch | ||||
| 1 | 133 (21.14%) | |||
| 2 | 21 (3.34%) | |||
| 3 | 187 (29.73%) | |||
| 4 | 288 (45.79%) | |||
| Fetal Sex | ||||
| Female | 331 (52.62%) | |||
| Male | 298 (47.38%) | |||
| Labor Status | ||||
| Labor | 512 (81.40%) | |||
| No Labor | 117 (18.60%) | |||
| Delivery Method | ||||
| C Section | 247 (39.27%) | |||
| Vaginal | 382 (60.73%) | |||
| Preterm Birth (<37 Weeks)* | ||||
| Yes | 51 (8.1%) | |||
| No | 578 (91.9%) | |||
| Preterm Premature Membrane Rupture* | ||||
| Yes | 16 (2.5%) | |||
| No | 613 (97.5%) | |||
| Preeclampsia or Gestational Hypertension* | ||||
| Yes | 50 (8.6%) | |||
| No | 578 (92%) | |||
| Maternal Education | ||||
| Less Than High School | 43 (6.84%) | |||
| High School or GED | 272 (43.24%) | |||
| Graduated Technical School or College | 219 (34.82%) | |||
| Some Graduate work or Graduate Professional Degree | 95 (15.10%) | |||
| Maternal Race | ||||
| Black | 357 (56.76%) | |||
| White | 237 (37.68%) | |||
| Multiple Race | 26 (4.13%) | |||
| Asian | 6 (0.95%) | |||
| Other | 3 (0.48%) | |||
| Maternal Ethnicity | ||||
| Non-Hispanic/Latino | 619 (98.41%) | |||
| Hispanic/Latino | 10 (1.59%) | |||
Data was generated from medical record abstraction and self-reported data (Paquette et al. 2022).
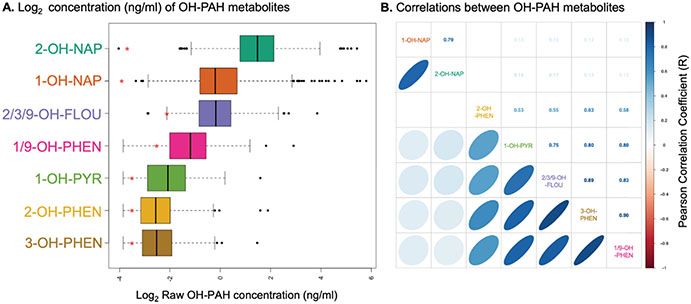
Seven OH-PAH metabolites were detectable (above the LOD) in > 70% of samples (Table S2). Complete OH-PAH metabolite data is shown in Fig. 1 and described in Table 2. 2-hydroxynaphthalene (2-OH-NAP) and 1-hydroxynaphthalene (1-OH-NAP) had the highest concentrations in maternal urine (mean concentration 7.66 ng/ml and 4.06 ng/ml respectively). 1-OH-NAP and 2-OH-NAP were strongly correlated with each other (R = 0.79), but not as strongly positively correlated with other OH-PAH metabolites (R 0.12–0.17, see Fig. 1B). 1-OH-PYR, 2/3/9-OH-FLUO, 3-OH-PHEN, and 1/9-OH-PHEN were all strongly positively correlated with each other (R 0.75–0.9). 2-OH-PHEN was not significantly correlated with 1-OH-NAP or 2-OH-NAP, but was correlated with other OH-PAH metabolites (R 0.53–0.63).
Fig. 1.
(A) Boxplot depicting concentrations of PAH metabolites in CANDLE participants detectable in urine. Red asterisk indicates the log LOD for each metabolite, and samples that were below the LOD were reported as . (B) Correlogram depicting correlations between OH-PAH metabolites within CANDLE participants, based on Pearson correlation coefficients. right upper quadrant depicts correlation coefficient (R), and left bottom quadrant visually depicts these correlations. Only correlations that are statistically significant (P < 0.05) are shown.
Table 2.
Distribution of raw OH-PAH metabolite concentrations (ng/mL) in CANDLE participants.
| Name /Acronym | Parent Compound | Molecular Weight | Range (Min-Max) |
1st Qu. | Median | Mean | 3rd Qu. | SD |
|---|---|---|---|---|---|---|---|---|
| 1-hydroxypyrene (1-OH-PYR) | Pyrene | 218.26 | 0.02–4.91 | 0.06 | 0.12 | 0.19 | 0.25 | 0.27 |
| 1/9-hydroxyphenanthrene (1/9-OH-PHEN) | Phenanthrene | 194.23 | 0.02–18.39 | 0.13 | 0.30 | 0.47 | 0.57 | 0.88 |
| 2-hydroxyphenanthrene (2-OH-PHEN) | Phenanthrene | 194.23 | 0.02–6.61 | 0.04 | 0.08 | 0.13 | 0.14 | 0.34 |
| 3-hydroxyphenanthrene (3-OH-PHEN) | Phenanthrene | 194.23 | 0.02–4.34 | 0.05 | 0.08 | 0.12 | 0.15 | 0.21 |
| 2/3/9-hydroxyfluorene (2/3/9-OH-FLUO) | Fluorene | 182.22 | 0.06–47.10 | 0.43 | 0.84 | 1.34 | 1.53 | 2.39 |
| 1-hydroxynaphthalene (1-OH-NAP) | Naphthalene | 144.17 | 0.04–329.02 | 0.45 | 0.82 | 4.06 | 1.96 | 20.73 |
| 2-hydroxynaphthalene (2-OH-NAP) | Naphthalene | 144.17 | 0.02–227.99 | 2.19 | 4.39 | 7.66 | 8.40 | 16.42 |
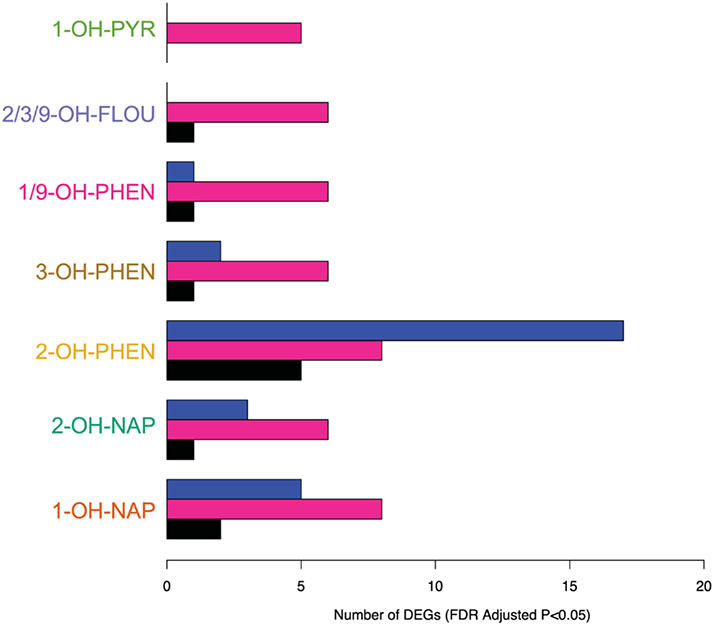
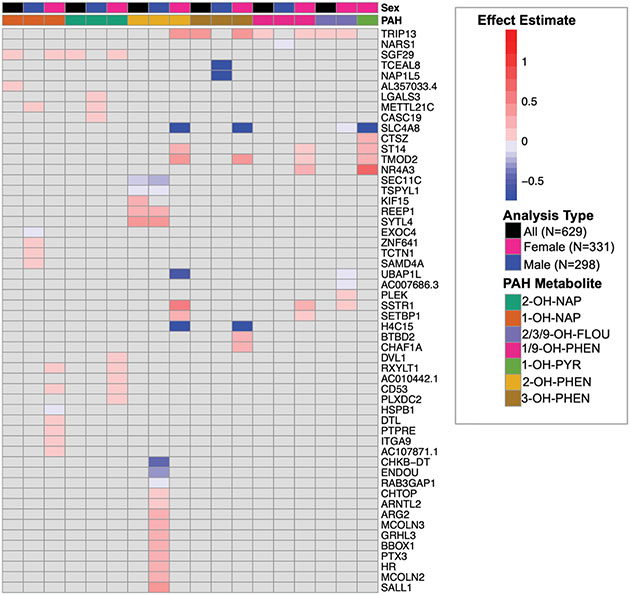
In our fully adjusted models, placental expression of eight genes was significantly associated with urinary concentrations of six OH-PAH metabolites (FDR adjusted p < 0.05; Table 3, Supplemental Fig. 2). These 11 significant relationships between OH-PAH metabolites and genes (from Table 3) are visually depicted in Supplemental Fig. 3. Seven of the genes were protein coding, and one (AL357033.4) was a IncRNA. Of these 7 genes, one gene (NARS1, negatively associated with 1/9-OH-PHEN) was categorized as a trophoblast group enriched gene (At least fourfold higher average mRNA levels in a group of 2–10 cell types compared to any other cell type) by the Human Protein Atlas (HPA)(Uhlen et al. 2010). TRIP13 was positively associated with the urinary concentrations of 3 OH-PAH metabolites (2/3/9-OH-FLOU, 1/9-OH-PHEN, and 3-OH-PHEN), and SGF29 was positively associated with 1-OH-NAP and 2-OH-NAP. 2-OH-PHEN was associated with the highest number of DEGs, including 2 genes (TSPYL1, SECL11C) with decreased expression, and 3 genes (KIF15, REEP1, SYTL4) with increased expression (Figs. 2 and 3). 1-OH-PYR was the only OH-PAH metabolite that was not significantly associated with any differences in gene expression in our primary model.
Table 3.
Genes whose placental expression was significantly associated with OH-PAH metabolites (FDR adjusted P < 0.05).
| Gene | Description | Gene Type | Chromosomal Location | PAH Metabolite |
Effect Estimate |
FDR Adjusted P |
|---|---|---|---|---|---|---|
| TRIP13 | Thyroid hormone receptor interactor 13 | protein coding | Chr: 5: 892884–919357 | 2/3/9-OH-FLOU | 0.032 | 2.08E-02 |
| 1/9-OH-PHEN | 0.079 | 4.39E-02 | ||||
| 3-OH-PHEN | 0.376 | 1.98E-03 | ||||
| SGF29 | SAGA complex associated factor 29 | protein coding | Chr 16: 28553915–28591790 | 1-OH-NAP | 0.004 | 3.83E-22 |
| 2-OH-NAP | 0.004 | 4.65E-08 | ||||
| SEC11C | SEC11 homolog C, signal peptidase complex subunit | protein coding | Chr 18: 59139866-59158832 | 2-OH-PHEN | −0.145 | 3.70E-03 |
| TSPYL1 | TSPY like 1 | protein coding | Chr6:116267760–116279930 | 2-OH-PHEN | −0.090 | 5.01E-04 |
| AL357033.4 | Novel transcript, antisense to SLCO4A1 | lncRNA | Chr 20: 62648961–62650767 | 1-OH-NAP | 0.004 | 2.50E-02 |
| KIF15 | Kinesin family member 15 | protein coding | Chr 3: 44761721–44873376 | 2-OH-PHEN | 0.189 | 4.19E-02 |
| REEP1 | Receptor accessory protein 1 | protein coding | Chr 2: 86213993–86338083 | 2-OH-PHEN | 0.216 | 2.56E-02 |
| SYTL4 | synaptotagminlike 4 | protein coding | Chr X: 100671783–100732123 | 2-OH-PHEN | 0.326 | 5.01E-04 |
Fig. 2.
Summary of the number of DEGs associated with OH-PAH Metabolites in primary model (N = 629 participants, black) and male stratified model (N = 298 participants, blue) and female stratified (N = 331, pink). Genes were considered statistically significant at FDR adjusted P < 0.05.
Fig. 3.
Effect estimates (log CPM/[ng/ml]) of fully adjusted model for all analyses (overall and sex-specific) that were statistically significant, which are presented in Table 2 and Supplemental Table 6. As noted in the figure legend, the top 2 bars of the heatmap depict OH-PAH metabolite and the model type (primary = black, female stratified = pink, male stratified = blue). Within the heatmap, the color of the box represents the magnitude of the effect estimate, with positive associations between OH-PAH metabolite concentration and placental gene expression depicted in red, and negative associations depicted in blue. Only genes/OH-PAH relationships that are statistically significant (FDR adjusted P < 0.05) are depicted. Relationships that were not statistically significant are depicted in grey.
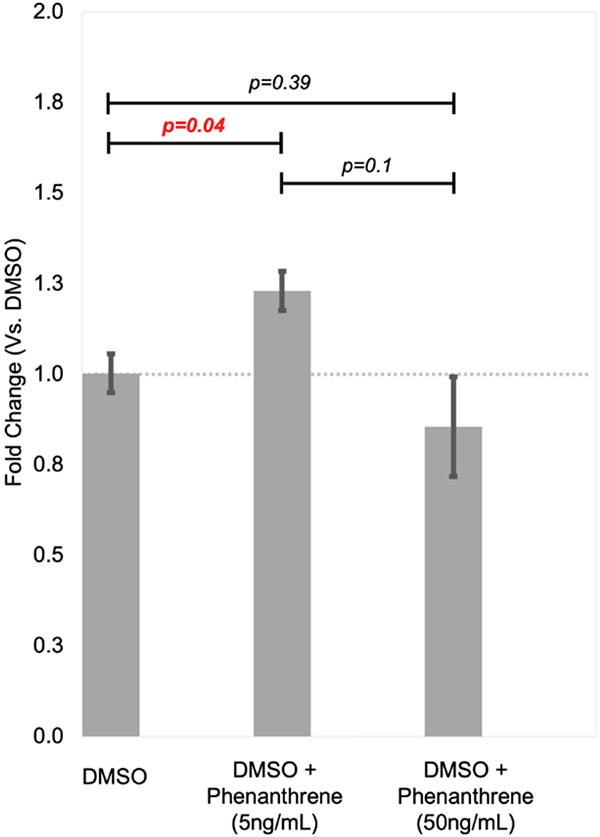
We assessed the relationship between TRIP13 and phenanthrene in the HTR-8/SVneo cell line, which has previously been used for in vitro studies of other PAHs (J.-Y. Jo et al. 2011) and expresses receptors and enzymes responsible for metabolism of phenanthrene (data not shown). We selected 5 ng/ml and 50 ng/ml concentrations based on prior studies investigating relative concentrations of PAH compounds in urine and placental samples (Drwal, Rak, and Gregoraszczuk 2019), and we observed borderline statistically significant differences between groups (P = 0.068, ANOVA). We did not note any changes in cell viability. The expression of TRIP13 was 23% higher in cells treated with 5 ng/ml of phenanthrene compared to DMSO controls (p = 0.041), but we did not observe statistically increased expression at 50 ng/ml compared to controls (Fig. 4).
Fig. 4.
Relative TRIP13 expression after treatment with 5 ng/ml and 50 ng/ml of phenanthrene vs DMSO vehicle control. Expression was calculated using the ΔΔCt method, and all experiments were run in triplicate and qCPR reactions were also run with technical triplicates.
In addition to assessing the association of PAH exposures with individual genes, we also used the FRY gene set testing method as a complementary approach to explore possible associations with biological pathways. Gene set testing does not rely on statistical significance of individual genes but rather takes a comprehensive look at all the genes in a given pathway. Using FRY gene set testing, we identified 3 pathways with genes associated with four OH-PAH metabolites (Table 4). On the pathway level, the KEGG vitamin digestion and absorption pathway was significantly positively associated with increased concentrations of 2/3/9-OH-FLUO, 3-OH-PHEN, 1/9-OH-PHEN, and 1-OH-PYR. Pathway mapping of logfold changes of genes in the pathway (irrespective of P value) highlighted similarities as well as differences between the placental expression of genes within this pathway and different PAH metabolites (Supplemental Fig. 4, with all results presented in Supplemental Table 3). All PAH metabolites exhibited positive relationships between placental expression of APOB, which is involved in the final stage of nutrient transport, as well as THTR, which is involved in the transport of thamin, although these were not statistically significant in our genome scale model described above. 1-OH-PYR, 1/9-OH-PHEN, and 1-OH-PHEN were also had positive relationships with placental expression of PCFT and RFC, which are involved in the transport of folic acid. Genes within the KEGG insulin secretion pathway were positively associated with maternal urinary concentrations of PAH metabolites 3-OH-PHEN and 1/9-OH-PHEN (Table 4, Supplemental Table 4). Genes within the lysosome pathway were positively associated with maternal urinary concentrations of 1-OH-PYR (Table 4, Supplemental Table 5).
Table 4.
KEGG Biological Pathways significantly associated with OH-PAH metabolites (FDR adjusted P < 0.1) based on FRY rotational gene set testing.
| KEGG Pathway |
OH-PAH Metabolite |
Genes in Pathway |
Direction | P Value |
FDR adjusted P |
|---|---|---|---|---|---|
| Vitamin digestion absorption | 2/3/9-OH-FLOU | 15 | Up | 2.37E-05 | 0.01 |
| 3-OH-PHEN | 15 | Up | 2.00E-04 | 0.04 | |
| 1/9-OH-PHEN | 15 | Up | 3.23E-04 | 0.07 | |
| 1-OH-PYR | 15 | Up | 6.83E-04 | 0.09 | |
| Insulin secretion | 3-OH-PHEN | 48 | Up | 3.06E-04 | 0.04 |
| 1/9-OH-PHEN | 48 | Up | 5.28E-04 | 0.07 | |
| Lysosome | 1-OH-PYR | 114 | Up | 5.99E-05 | 0.02 |
We examined sex-specific associations between prenatal OH-PAH exposure and the placental transcriptome through stratified analyses. Figs. 2 and 3 compares the differentially expressed genes identified in our overall and sex-specific analyses. In our male specific analysis (N = 295), we identified 28 associations between 4 OH-PAH metabolites and placental expression of 27 different genes. (Supplemental Table S6). Three of these genes (METTL221C, ARG2, ENDOU) were characterized as synctiotrophoblast enhanced genes by the HPA (At least fourfold higher mRNA level in a certain cell type compared to the average level in all other cell types), and METTL21C was also characterized as a cytotrophoblast enhanced gene. Nine of these associations were negative, with the effect estimates ranging from −0.73 (TCEAL8 with 3-OH-PHEN) to −0.002. 18 of these associations were positive, with effect estimates ranging from 0.002 to 0.43 (SYTL4 with 2-OH-PHEN). SEC11C and REEP1 were negatively associated with OH-PAH metabolites and TSPYL1 and SYTL4 were positively associated with OH-PAH metabolites in our overall and male stratified model, but with stronger effect estimates the stratified models (Figs. 2 and 3, Supplemental Fig. 3), indicating that relationships between the expression of these 4 genes and prenatal PAH exposure may be driven by stronger associations in male participants. Placental expression of METTL21C was positively associated with maternal urinary concentrations of 2-OH-NAP and 2-OH-PHEN. 2-OH-PHEN overall had the highest number of statistically significant associations, including 5 genes with decreased expression and 14 genes with increased expression.
Using FRY gene set testing, we identified significant positive associations between genes within 20 KEGG pathways and negative associations between genes in 1 pathway (cysteine and methionine metabolism) and prenatal exposure to 2-OH-PHEN in males. (Supplemental Table S7) The most significant of these positive associations were within the neurotrophin and T-cell signaling pathways. We observed a positive association between genes in the lysosome pathway and 1-OH-PYR, which was also significant in our primary model.
In our subanalysis of only female placentas, we identified 45 associations across 7 PAH metabolites and 25 unique genes (Supplemental Table S6). Ten of these associations were negative, with the effect estimates ranging from −1.39 (H4C15 with 3-OH-PHEN) to −0.006. 35 of these associations were positive, with effect estimates ranging from 0.002 to 0.72 (SLC4A8 with 1-OH-PYR). Of these 25 genes, only 2 (TRIP13 and SGF29) were also significantly positively associated in our overall analysis, and they had stronger effect estimates in our female only model (Fig. 3). 11 of the DEGs significant in females were associated with more than one PAH metabolite, with TRIP13, TMOD2, SLC4A8 associated with 4 different OH-PAH metabolites in females. Of these 25 genes, DTL and RXYLT1 (both positively associated with 1-OH-NAP) were characterized as enhanced in extravillous trophoblast cells, and RXYLT1 was also enhanced in syncytiotrophoblast cells, based on definitions from the HPA. In the female stratified analysis, we identified 4 genes with increased expression and 1 gene (SLC4A8) with decreased expression in relation to 1-OH-PYR. All genes were autosomal.
Using FRY gene set testing, we observed positive associations between OH-PAH metabolites and genes in the insulin secretion and vitamin digestion and absorption pathways in females (Supplemental Table S7), which were also significant in our primary model. The only pathway that was uniquely significant in our female stratified analysis was hematopoetic cell lineage, which was positively associated with 2-OH-PHEN.
4. Discussion
The main findings of this study included (1) identification of placental expression differences associated with 6 OH-PAH metabolites, including associations between placental expression of TRIP13 and SGF29 and multiple OH-PAH metabolites, (2) increased expression of genes within pathways involving vitamin digestion and absorption, insulin secretion, and the lysosome related to 4 OH-PAH metabolites and (3) evidence that sex may modify the relationship between OH-PAH metabolites and gene expression, with females exhibiting a higher number of unique DEGs in relation to multiple OH-PAH metabolites than males. This study represents the first human analysis of relationships between prenatal exposure to OH-PAH metabolites and the placental transcriptome. It was conducted in a large socioeconomically and racially diverse cohort with well-phenotyped covariate data allowing for rigorous adjustment for confounding variables, and incorporated techniques to mitigate unmeasured sources of confounding such as cellular heterogeneity. Several of the differentially expressed genes were uniquely expressed in different trophoblast cell types based on classifications from the HPA (Uhlen et al. 2010), suggesting that these genes may have molecular functions specific to the placenta. These findings provide unique insight into how prenatal OH-PAH exposure might influence placental function, potentially impacting fetal development.
Different PAHs have different recorded levels of carcinogenicity, with naphthalene classified as a known carcinogen, whereas fluorene, phenanthrene, and pyrene noted as not carcinogenic in humans based on available evidence (Sun et al. 2021). However, limited information is known about shared and distinct mechanisms of toxicity, and comparative omics studies such as this can help provide this insight based on the number and type of differentially expressed genes. We found statistically significant associations between placental gene expression and six of the seven detected PAH metabolites in our combined model (FDR adjusted P < 0.05). In our combined and male only models, we did not observe any significant associations between placental gene expression and maternal urinary concentrations of 1-OH-PYR, a metabolite of pyrene, but in our analysis of female samples we identified 5 DEGs. Many of the significant genes and pathways overlapped between individual OH-PAH metabolites, which suggests that different PAH metabolites have similar effects on the placenta. Alternatively, this could be related to correlations between PAH metabolites.
In our combined analysis (N = 629) we observed the highest number of associations between the placental transcriptome with 2-OH PHEN (5 genes), a metabolite of phenanthrene, and 1-OH-NAP (2 genes), a metabolite of naphthalene. Sources of phenanthrene exposure include tobacco smoke and other combustion aerosols, as well as contamination of combustion effluents in food or water (Gad 2014). Other studies in this cohort have observed positive associations between other metabolites of phenanthrene (1/9-OH-PHEN) and cognitive scores at age 3 (Wallace et al. 2022). Sources of naphthalene exposures include vehicle exhaust, ambient air (from vehicular traffic), and residential use of naphthalene products (mothballs), as well as occupational exposures, such as from petroleum production and refining, asphalt industries, and creosote production and use (Griego et al. 2008).While 1-OH-NAP is a metabolite of both naphthalene and the insecticide carbaryl, 2-OH-NAP is only derived from naphthalene (Meeker et al. 2007). We observed 1 DEG associated with 2-OH-NAP, which was also significantly associated with 1-OH-NAP (SGF29). While the sources of these metabolites may differ, this may suggest shared mechanisms of toxicity. Other studies in this cohort have observed positive associations between other metabolites of phenanthrene (1/9-OH-PHEN) and cognitive scores at age 3 (Wallace et al. 2022). We have also observed associations between other metabolites of naphthalene (2-OH-NAP) and earlier gestational age at birth (Freije et al. 2022). This body of work suggests that metabolites of naphthalene and phenanthrene within this cohort may be related to changes in placental function and fetal development.
We observed associations between SGF29 and TRIP13 and multiple OH-PAH metabolites in our models. Placental expression of TRIP13 was positively associated with maternal urinary concentrations of 2/3/9 OH-FLUO, 1/9-OH-PHEN, and 3-OH-PHEN in both the combined and female stratified model, as well as 2-OH-PHEN in only the female stratified model. We were able to validate these findings in placental cell lines, as HTR-8/SVneo cells treated with 5 ng/ml (but not 50 ng/ml) exhibited increased expression of TRIP13. TRIP13 encodes the Thyroid Hormone receptor interactor 13, which is involved in DNA double strand break repair as well as the mitotic checkpoint complex and homologous recombination during meiosis. TRIP13 negatively regulates the REV7 complex, which dissociates the complex to promote homology directed repair instead of non-homologous end joining (Clairmont et al. 2020). Homology directed repair is a mechanism that cells use to repair double stranded DNA breaks (Dudas and Chovanec 2004), which PAH metabolites are known to cause (Henkler, Stolpmann, and Luch 2012). Other studies have identified increased of TRIP13 expression (Y. Zhang et al. 2017), as well as decreased methylation in the TRIP13 gene body (Bjaanres et al. 2016) in lung adenocarcinomas, which authors suggest may be related to PAH exposure in participants. In an in vivo study, 1-OH-PYR was associated with repression of TRIP13 expression in the liver of European flounders (T. D. Williams et al. 2014). The increased expression of this gene that we observed may suggest upregulation of repair mechanisms in the placenta to mitigate risks due to double stranded DNA breaks. Our in-vitro follow up analysis may suggest that this occurs during low but not high levels of PAH exposure. However, these results should be interpreted with regards to inherent limitations in extrapolating between these approaches. We used immortalized HTR-8/SVneo cells, which represent extra trophoblast and stromal/mesenchymal cells (Abou-Kheir et al. 2017), and not syncytiotrophoblast cells which are the major cell type present in term placental tissue (Jaremek et al. 2021). We treated with only 2 concentrations of phenanthrene which was selected to match placental concentrations in a different population (Drwal et al. 2017). While these levels are within range of phenanthrene metabolites in this population (see table 2), it is not possible to directly compare the acute dosage of the parent (phenanthrene) used in this experiment with the chronic exposures reflected in measurements of its metabolites in urine, which also may relate to the timing of exposure. We were unable to obtain all phenanthrene metabolites measured in our study so we elected to treat with phenanthrene after confirming that the HTR-8/SVneo cell line produced enzymes that could metabolize phenanthrene into these products, but it is also challenging to compare perturbations induced by parent compounds compared to their metabolites, as in some cases metabolites can be more biologically active. A more sophisticated toxicological follow up study is needed to that incorporates multiple doses and the effects of acute vs chronic treatment, and differing effects on different placental cell types, which is beyond the scope of this study.
Placental expression of SGF29 was positively associated with maternal urinary concentrations of 1-OH-NAP and 2-OH-NAP in the combined and female stratified models. SGF29 encodes SAGA Complex associated factor 29, which is a chromatin reader that binds to H3k4me chromatin and mediates the recruitment of the SAGA complex (Schram et al. 2013) The SAGA complex is induced in response to endoplasmic reticulum stress (Nagy Zita et al. 2009) In vitro studies conducted in endothelial cells have shown that other PAH compounds including benzo[ghi]perylene and 1-nitropyrene can induced endoplasmic reticulum stress (Andersson et al. 2009; Zaragoza-Ojeda et al. 2022). The endoplasmic reticulum is involved in the intra and extracellular transport of proteins and lipids. Another cellular trafficking gene, SYTL4, was positively associated with maternal urinary concentrations of 2-OH-PHEN. These changes in cellular trafficking and endocytosis may alter the transfer of nutrients and disrupt maternal-fetal communication (Cooke et al. 2021), which may have lasting impacts on fetal development. To our knowledge, this represents the first work to link OH-PAH exposure to expression of genes involved in repair mechanisms to stress including homology directed repair and endoplasmic reticulum stress response. More work is needed to understand how the placenta may regulate these genes in the context of PAH exposure, as well as their potential impacts on placental function.
Several of the genes associated with OH-PAH metabolites in our study have also been associated with PAHs in experimental models or other related environmental exposures in other cohort studies. In a zebrafish model of PAH toxicity, ARG2 expression (associated with 2-OH-PHEN in our study in males) was significantly decreased after exposure to a combination of 7 nitrated PAHs (Chlebowski et al. 2017). Other genes significant in our study were associated with other chemical or metal exposures, such as KIF15 and bisphenol A (Bredhult, Sahlin, and Olovsson 2009). In male samples we also observed a positive association between 2-OH-PHEN and expression of ARNTL2, which is a homologue of ARNT (Okano et al. 2001), which encodes the aryl hydrocarbon receptor (AHR) nuclear translocator. PAH metabolism is induced by their binding to the AHR, which then forms a complex with ARNT, which acts as a nuclear transcriptional regulator of xenobiotic metabolism enzymes (Goedtke et al. 2020). Taken together, this suggests our transcriptomic signatures of OH-PAH exposure may be functionally related to other environmental exposures, with evidence from in to vitro and mechanistic studies.
We observed positive associations between four OH-PAH metabolites and placental expression of genes involved in the vitamin digestion and absorption pathway. Specifically, PAH metabolites were associated with increased expression of genes involved in the transport of folic acid, thiamin, riboflavin, and biotin, as well as APOB, which is involved in the transport of nutrients via exocytosis. The transport of these nutrients across the placental barrier is crucial for many aspects of fetal development, including central nervous system development (thiamine) (Bâ 2011), and plays a role in the prevention of preterm birth (folate) (Greenberg et al. 2011). The placenta plays a critical role in transporting these nutrients to the developing fetus, so alterations in this pathway may influence fetal growth and development. Dietary intake of vitamins A, C, E and folate modified the relationship between exposure to BAP and spontaneous preterm birth in a nested case-control study from Taiyuan China, with lower dietary intake of vitamins associated with stronger associations between prenatal BAP exposure and preterm birth (N. Zhao et al. 2022). Another study found that maternal fruit and vegetable intake modified the relationship between exposure to 2-napthol and 1-hydroxypyrene and markers of oxidative stress quantified in urine, with significant positive correlations between oxidative stress and OH-PAH metabolites that were only significant for participants in the lowest tertile of fruit and vegetable intake, but not in participants with higher fruit and vegetable intake (Kim et al. 2011). The increased transport of these essential vitamins to the placenta in relation to OH-PAH exposure may represent a compensatory mechanism to combat damage due to OH-PAH toxicity. More work is needed in an experimental system to validate this hypothesis. Alternatively, PAHs enter and are absorbed via oral, dermal or inhalation routes (Ramesh et al. 2004), and so changes in these biological pathways could be a reflection of dietary exposures that are related to both PAH metabolites and these vitamins. For example, processed grains are a high dietary source of PAH exposure, (Phillips 1999) and they also are frequently enriched with folate and thiamine (Anderson, Vickery, and Nicol 1986). Further studies are needed to assess the role of diet as a potential confounding variable, as well as to understand its effects on the placental transcriptome.
We examined sex-specific differences in relation to OH-PAH metabolites because fetal sex is a potential modifier of the relationship between exposure to environmental toxins and developmental defects (DiPietro and Voegtline 2017). In our sex-stratified analyses, we observed 24 DEGs and 20 KEGG pathways that were significantly associated with PAH metabolites only in males, and 23 DEGs and one KEGG pathway (hematopoietic cell lineage) that were only significantly associated with PAH metabolites in females, but not in our combined analysis. Several of the pathways that were significant only in males were related to known mechanisms of PAH toxicity, such as immune disruption (T-cell receptor signaling, Th1 and Th2 cell differentiation, Th17 differentiation) and oxidative stress (FoxO signaling pathway). Notably, several of these pathways (neuroptropin, and adherens junction) were also associated with prenatal phthalate exposure in the same cohort (Paquette. et al., 2021). This work suggests that individual OH-PAH metabolites may disrupt placental gene expression in male or female placentas differently, potentially leading to sex-specific alterations of the biological functions of the placenta, but more work is needed to understand the reason behind these sex-specific differential responses. In this study, we observed 5 genes whose placental expression was uniquely associated with 1-OH-PYR only in females, but not in our overall cohort. Within this same population of CANDLE participants, 1-OH-PYR was associated with increased risk of PTB in female infants (Freije et al. 2022). This work suggests that differential placental transcription in male and females may in part contribute to sex-specific differences in infant health outcomes related to prenatal PAH exposure.
Results of this investigation should be interpreted in the context of inherent limitations in sample collection and RNA sequencing-based analyses. Gene expression was quantified using bulk RNA sequencing data, and one established caveat of this approach is unmeasured confounding due to the potential for differences in the distribution of cell types collected within each sample, (including syncytiotrophoblast, trophoblasts, stromal cells, etcetera) which is a well-established challenge of all bulk RNA sequencing analyses of the placenta (Breton Carrie V. et al. 2017b; Konwar et al. 2019). While our sampling procedure ensures uniform placental material collected within this cohort, there is still some potential for minor discrepancies in cellular heterogeneity that we cannot quantify visually or through computational means, which we addressed by applying surrogate variable analysis, which allows researchers to incorporate unmeasured sources of heterogeneity that are independent of their outcome of interest, including from cell type proportions (Leek and Storey 2007). OH-PAHs are not strongly correlated across pregnancy (A. Cathey et al. 2018; Gaylord et al. 2022; A. L. Cathey et al. 2020), and multiple urinary measurements of PAHs have been shown to be more accurate markers of PAH exposure during pregnancy than a single measure (A. L. Cathey et al. 2020), so future studies could consider capturing and summarizing individual OH-PAH metabolites across multiple time points during pregnancy as a more accurate measure of exposure. Additionally, we only measured monohydroxylated PAH metabolites, which may not capture the complex mixture of PAH metabolites or other conjugated PAH parent compound exposures, including those conjugated with hydroxy, oxo, nitro and organic functional groups, which may represent different mechanisms of toxicity (Luo, Stepanov, and Hecht 2019). Additionally, OH-PAH metabolite concentrations are related to individual variations in enzymes involved in the metabolism of PAHs (Yang et al., 2021). Furthermore, the use of urine for our exposure assessment limited our analysis to smaller PAHs, and we could not evaluate transcriptomic associations with larger PAHs primarily excreted fecally, so future studies may consider expanding on this analysis by investigation of these other PAH metabolites. Another limitation of our approach is uncertainty due to OH-PAH concentrations below the LOD for some metabolites. We applied a standard approach to estimating these values (Freije et al. 2022; Loftus et al. 2022; Wallace et al. 2022; 2023), though this may have contributed bias to the findings. More work is needed to develop appropriate multiple imputation strategies that are compatible with high dimensional transcriptomic data (Song et al. 2020). As we identified associations between multiple OH-PAH metabolites and expression of some genes, future studies could consider utilizing a summary OH-PAH metric or modeling of complex mixtures of PAH metabolites, which was beyond the scope of our study. Finally, our sample is restricted to a population in the US Urban south, thus associations may reflect regionally specific exposures. Further work is needed to replicate this in an independent population.
Our study stands apart as the first transcriptome-wide assessment of OH-PAH metabolites during pregnancy and has a number of unique strengths, including a large sample size and rigorous adjustment for confounding variables. To avoid confounding by maternal smoking, we excluded all smokers and quantified smoking based on cotinine measurements rather than self-report, which can lead to under-estimation of smoking prevalence within a population (J. Williams et al. 2020). We also applied novel RNA sequencing approaches to adjust for unmeasured confounding (Leek and Storey 2007) which overall decreases our chances of type 1 and type 2 error. Unadjusted levels of urinary OH-PAH metabolites in the CANDLE population were generally comparable to those from the US National Health and Nutrition Examination Survey, which is a nationwide survey that study evaluates exposure to a wide range of chemicals including PAHs in a statistically representative sample of the US population (Li et al. 2008b).
In Summary, PAHs are hazardous compounds known to be mutagenic, carcinogenic, and teratogenic (Patel et al. 2020), and maternal PAH metabolite concentrations are associated with infant and childhood health outcomes. Our study demonstrates relationships between different OH-PAH metabolites and the placental transcriptome, which may suggest that PAHs disrupt placental function and ultimately fetal development. We also provide evidence that fetal sex may be a significant modifier of OH-PAH exposure and the placental transcriptome. More work is needed to confirm our findings in other cohorts with similar data, and to explore how OH-PAHs induce transcriptional regulation, with a focus on ER stress and DNA damage repair. Future directions also include integration with other studies investigating the placental transcriptome and birth outcomes to explore the role of the placenta as a functional mediator to understand how the relationships we observed may result in altered placental function and subsequent perinatal and child health outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank the participants of the CANDLE study, as well as CANDLE research staff and investigators. We would particularly like to thank Maureen Sorrells and Lisa Younglove for co-ordination of the biospecimens as well as the ECHO PATHWAYS Data Center for compiling the analytic dataset. We acknowledge the Kobor lab (University of British Columbia, BC CA) for support with RNA preprocessing. We would like to thank Dr. David Beier for generous use of lab space to complete in-vitro validation. We would also like to thank Dr. Melissa Melough for her thoughtful comments. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ECHO PATHWAYS is funded by NIH (1UG3OD023271-01, 4UH3OD023271-03). The University of Washington EDGE center is supported by the NIH (P30ES007033-PI Joel Kaufman). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH (R01 HL109977). This study was funded by the NICHD (K99/R00HD096112).
Footnotes
CRediT authorship contribution statement
Alison G. Paquette: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing – original draft, Visualization. Samantha Lapehn: Methodology, Validation, Formal analysis, Investigation. Sophie Freije: Methodology. James MacDonald: Methodology, Software, Project administration. Theo Bammler: Methodology, Resources, Project administration. Drew B. Day: . Christine T. Loftus: Data curation, Project administration. Kurunthachalam Kannan: Methodology. W. Alex Mason: Resources, Project administration, Funding acquisition. Nicole R. Bush: Resources, Project administration, Funding acquisition. Kaja Z Le Winn: Resources, Project administration, Funding acquisition. Daniel A. Enquobahrie: . Carmen Marsit: . Sheela Sathyanarayana: Conceptualization, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.107763.
Data availability
Data will be made available on request.
References
- Abou-Kheir W, Barrak J, Hadadeh O, Daoud G, 2017. HTR-8/SVneo Cell Line Contains a Mixed Population of Cells. Placenta 50 (February), 1–7. 10.1016/j.placenta.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Abraham E, Rousseaux S, Agier L, Giorgis-Allemand L, Tost J, Galineau J, Hulin A, et al. , 2018. Pregnancy Exposure to Atmospheric Pollution and Meteorological Conditions and Placental DNA Methylation. Environment International 118 (September), 334–347. 10.1016/j.envint.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Singh L, Anand MD, Taneja A, 2018. Association Between Placental Polycyclic Aromatic Hydrocarbons (PAHS), Oxidative Stress, and Preterm Delivery: A Case-Control Study. Archives of Environmental Contamination and Toxicology 74 (2), 218–227. 10.1007/s00244-017-0455-0. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Anand MD, Chakraborty P, Singh L, Masih J, Taneja A, 2020. Placental Levels of Polycyclic Aromatic Hydrocarbons (PAHs) and Their Association with Birth Weight of Infants. Drug and Chemical Toxicology June, 1–10. 10.1080/01480545.2020.1783285. [DOI] [PubMed] [Google Scholar]
- Alghamdi MA, Alam MS, Stark C, Mohammed N, Harrison RM, Shamy M, Khoder MI, Shabbaj II, Göen T, 2015. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons in Saudi Arabian Schoolchildren in Relation to Sources of Exposure. Environmental Research 140 (July), 495–501. 10.1016/j.envres.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Anderson StuartH., Vickery CherryA., and Nicol AndrewD.. 1986. “ADULT THIAMINE REQUIREMENTS AND THE CONTINUING NEED TO FORTIFY PROCESSED CEREALS.” Originally Published as Volume 2, Issue 8498 328 (8498): 85–89. 10.1016/S0140-6736(86)91618-1. [DOI] [PubMed] [Google Scholar]
- Andersson H, Piras E, Demma J, Hellman B, Brittebo E, 2009. Low Levels of the Air Pollutant 1-Nitropyrene Induce DNA Damage, Increased Levels of Reactive Oxygen Species and Endoplasmic Reticulum Stress in Human Endothelial Cells. Toxicology 262 (1), 57–64. 10.1016/j.tox.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Ba A, 2011. Comparative Effects of Alcohol and Thiamine Deficiency on the Developing Central Nervous System. Behavioural Brain Research 225 (1), 235–242. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Statist Soc B 57. [Google Scholar]
- Bjaanæs MM, Fleischer T, Halvorsen AR, Daunay A, Busato F, Solberg S, Jørgensen L, et al. , 2016. Genome-Wide DNA Methylation Analyses in Lung Adenocarcinomas: Association with EGFR, KRAS and TP53 Mutation Status, Gene Expression and Prognosis. Molecular Oncology 10 (2), 330–343. 10.1016/j.molonc.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AE, Muench KL, Robinson BG, Wang A, Palmer TD, Winn VD, 2021. “Examining Sex Differences in the Human Placental Transcriptome During the First Fetal Androgen Peak”. 28 (3), 801–818. 10.1007/s43032-020-00355-8. Reproductive Sciences (Thousand Oaks, Calif. [DOI] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L, 2016. Near-Optimal Probabilistic RNA-Seq Quantification. Nat Biotech 34 (5), 525–557. [DOI] [PubMed] [Google Scholar]
- Bredhult C, Sahlin L, Olovsson M, 2009. Gene Expression Analysis of Human Endometrial Endothelial Cells Exposed to Bisphenol A. Reproductive Toxicology 28 (1), 18–25. 10.1016/j.reprotox.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Breton CV, Marsit CJ, Elaine F, Kari N, Goodrich JM, Dolinoy DC, Julie H, et al. , 2017. Small-Magnitude Effect Sizes in Epigenetic End Points Are Important in Children’s Environmental Health Studies: The Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environmental Health Perspectives 125 (4), 511–526. 10.1289/EHP595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Pirrung M, McCue LA, 2017. FQC Dashboard: Integrates FastQC Results into a Web-Based, Interactive, and Extensible FASTQ Quality Control Tool. Bioinformatics 33 (19), 3137–3319. 10.1093/bioinformatics/btx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT, 2014. Integrative Transcriptome Meta-Analysis Reveals Widespread Sex-Biased Gene Expression at the Human Fetal-Maternal Interface. Molecular Human Reproduction 20 (8), 810–889. 10.1093/molehr/gau035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, 2015. What Is the Placenta? American Journal of Obstetrics and Gynecology 213 (4 Suppl): S6.e1, S6–S8. 10.1016/j.ajog.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD, 2018. Distribution and Predictors of Urinary Polycyclic Aromatic Hydrocarbon Metabolites in Two Pregnancy Cohort Studies. Environmental Pollution 232 (January), 556–562. 10.1016/j.envpol.2017.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, Watkins DJ, Rosario ZY, Vélez CM, Vega R-C, Alshawabkeh AN, Cordero JF, Meeker JD, 2020. Polycyclic Aromatic Hydrocarbon Exposure Results in Altered CRH, Reproductive, and Thyroid Hormone Concentrations during Human Pregnancy. Science of The Total Environment 749 (December), 141581. 10.1016/j.scitotenv.2020.141581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Rager JE, Tilton SC, 2021. Linking Coregulated Gene Modules with Polycyclic Aromatic Hydrocarbon-Related Cancer Risk in the 3D Human Bronchial Epithelium. Chemical Research in Toxicology 34 (6), 1445–1455. 10.1021/acs.chemrestox.0c00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski Anna C, Garcia Gloria R, Du Jane K La, Bisson William H, Truong Lisa, Simonich Staci L Massey, and Tanguay Robert L. 2017. “Mechanistic Investigations Into the Developmental Toxicity of Nitrated and Heterocyclic PAHs.” Toxicological Sciences: An Official Journal of the Society of Toxicology 157 (1): 246–59. 10.1093/toxsci/kfx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairmont CS, Sarangi P, Ponnienselvan K, Galli LD, Csete I, Moreau L, Adelmant G, Chowdhury D, Marto JA, D’Andrea AD, 2020. TRIP13 Regulates DNA Repair Pathway Choice through REV7 Conformational Change. Nature Cell Biology 22 (1), 87–96. 10.1038/s41556-019-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke LDF, Tumbarello DA, Harvey NC, Sethi JK, Lewis RM, Cleal JK, 2021. Endocytosis in the Placenta: An Undervalued Mediator of Placental Transfer. Trophoblast Research - Volume 38 113 (September), 67–73. 10.1016/j.placenta.2021.04.014. [DOI] [PubMed] [Google Scholar]
- Deyssenroth MA, Rosa MJ, Eliot MN, Kelsey KT, Kloog I, Schwartz JD, Wellenius GA, et al. , 2021. Placental Gene Networks at the Interface between Maternal PM2.5 Exposure Early in Gestation and Reduced Infant Birthweight. Environmental Research 199 (August), 111342. 10.1016/j.envres.2021.111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Voegtline KM, 2017. The Gestational Foundation of Sex Differences in Development and Vulnerability. Neuroscience 342 (February), 4–20. 10.1016/j.neuroscience.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobraca D, Lum R, Sjödin A, Calafat AM, Laurent CA, Kushi LH, Windham GC, 2018. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons in Pre- and Peri-Pubertal Girls in Northern California: Predictors of Exposure and Temporal Variability. Environmental Research 165 (August), 46–54. 10.1016/j.envres.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Wang Q, Peng J, Min W.u., Pan B.o., Xing B, 2018. Transfer of Polycyclic Aromatic Hydrocarbons from Mother to Fetus in Relation to Pregnancy Complications. Science of The Total Environment 636 (September), 61–68. 10.1016/j.scitotenv.2018.04.274. [DOI] [PubMed] [Google Scholar]
- Doroodzani K, Atefeh SD, Akhbarizadeh R, Raeisi A, Rahmani E, Mahmoodi M, Nabipour I, et al. , 2021. Diet, Exposure to Polycyclic Aromatic Hydrocarbons during Pregnancy, and Fetal Growth: A Comparative Study of Mothers and Their Fetuses in Industrial and Urban Areas in Southwest Iran. Environmental Pollution 276 (May), 116668. 10.1016/j.envpol.2021.116668. [DOI] [PubMed] [Google Scholar]
- Drwal E, Rak A, Grochowalski A, Milewicz T, Gregoraszczuk EL, 2017. Cell-Specific and Dose-Dependent Effects of PAHs on Proliferation, Cell Cycle, and Apoptosis Protein Expression and Hormone Secretion by Placental Cell Lines. Toxicology Letters 280 (October), 10–19. 10.1016/j.toxlet.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Drwal E, Rak A, Gregoraszczuk EL, 2019. Review: Polycyclic Aromatic Hydrocarbons (PAHs)—Action on Placental Function and Health Risks in Future Life of Newborns. Toxicology 411 (January), 133–142. 10.1016/j.tox.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Dudás A, Chovanec M, 2004. DNA Double-Strand Break Repair by Homologous Recombination. Mutation Research 566 (2), 131–167. 10.1016/j.mrrev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Enquobahrie DA, MacDonald J, Hussey M, Bammler TK, Loftus CT, Paquette AG, Byington N, et al. , 2022. Prenatal Exposure to Particulate Matter and Placental Gene Expression. Environment International 165 (July). 107310. 10.1016/j.envint.2022.107310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP, 2010. Boys Live Dangerously in the Womb. American Journal of Human Biology : The Official Journal of the Human Biology Council 22 (3), 330–335. 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson TM, Marsit CJ, 2018. Integrating -Omics Approaches into Human Population-Based Studies of Prenatal and Early-Life Exposures. Current Environmental Health Reports, July, 10.1007/s40572-018-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freije SL, Enquobahrie DA, Day DB, Loftus C, Szpiro AA, Karr CJ, Trasande L, et al. , 2022. Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Gestational Age at Birth. Environment International 164 (June), 107246. 10.1016/j.envint.2022.107246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad SE, 2014. Phenanthrene. In: Wexler P (Ed.), Encyclopedia of Toxicology, (Third Edition),. Academic Press, Oxford, pp. 865–887. 10.1016/B978-0-12-386454-3.00901-5. [DOI] [Google Scholar]
- Gao C, He Z, Li J, Li X, Bai Q, Zhang Z, Zhang X, et al. , 2016. Specific Long Non-Coding RNAs Response to Occupational PAHs Exposure in Coke Oven Workers. Toxicology Reports 3 (January), 160–216. 10.1016/j.toxrep.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord A, Kannan K, Lakuleswaran M, Zhu H, Ghassabian A, Jacobson MH, Long S, et al. , 2022. Variability and Correlations of Synthetic Chemicals in Urine from a New York City-Based Cohort of Pregnant Women. Environmental Pollution 309 (September), 119774. 10.1016/j.envpol.2022.119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner Goknur, and Smyth Gordon K. 2016. “FRY: A Fast Approximation to ROAST Gene Set Test with Mean Aggregated Set Statistics.” F1000Research 5. [Google Scholar]
- Goedtke L, Sprenger H, Hofmann U, Schmidt FF, Hammer HS, Zanger UM, Poetz O, Seidel A, Braeuning A, Hessel-Pras S, 2020. Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells. International Journal of Molecular Sciences 22 (1), 372. 10.3390/ijms22010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, Bell SJ, Guan Y, Yan-Hong Y.u., 2011. Folic Acid Supplementation and Pregnancy: More than Just Neural Tube Defect Prevention. Reviews in Obstetrics & Gynecology 4 (2), 52–59. [PMC free article] [PubMed] [Google Scholar]
- Griego FY, Bogen KT, Price PS, Weed DL, 2008. Exposure, Epidemiology and Human Cancer Incidence of Naphthalene. Supplement: Naphthalene State of the Science Symposium 51 (2, Supplement), 22–26. 10.1016/j.yrtph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Ha M, Ha E-H, Lee B-E, Park E-Y, Kim E-J, Park H, Kim Y, Hong Y-C, Kim B-N, 2011. Prenatal Exposure to Polycyclic Aromatic Hydrocarbons, Bisphenol A, and Phthalates and Growth and Neurodevelopment in Infants and Children. Epidemiology 22 (1). [Google Scholar]
- Henkler Frank, Stolpmann Kristin, and Luch Andreas. 2012. “Exposure to Polycyclic Aromatic Hydrocarbons: Bulky DNA Adducts and Cellular Responses.” In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology, edited by Luch Andreas, 107–31. Basel: Springer Basel. 10.1007/978-3-7643-8340-4_5. [DOI] [PubMed] [Google Scholar]
- Jaremek A, Jeyarajah MJ, Bhattad GJ, Renaud SJ, 2021. Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast. Frontiers in Cell and Developmental Biology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J-Y, Kim T-H, Jeong H-Y, Lim S-M, Kim H-S, Im D-S, 2011. Effect of Di-(2-Ethylhexyl)-Phthalate on Sphingolipid Metabolic Enzymes in Rat Liver. Toxicological Research 27 (3), 185–190. 10.5487/TR.2011.27.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Ko HS, Kim AY, Jo HG, Kim WJ, Choi SK, 2022. Effects of Polycyclic Aromatic Hydrocarbons on the Proliferation and Differentiation of Placental Cells. Reproductive Biology and Endocrinology 20 (1), 47. 10.1186/s12958-022-00920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M, 2016. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Research 44 (Dl), D457–D462. 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Hwang J-Y, Ha E-H, Park H, Ha M, Lee S-H, Hong Y-C, Chang N, 2011. Fruit and Vegetable Intake Influences the Association between Exposure to Polycyclic Aromatic Hydrocarbons and a Marker of Oxidative Stress in Pregnant Women. European Journal of Clinical Nutrition 65 (10), 1118–1125. 10.1038/ejcn.2011.77. [DOI] [PubMed] [Google Scholar]
- Konwar C, Del Gobbo G, Yuan V, Robinson WP, 2019. Considerations When Processing and Interpreting Genomics Data of the Placenta. Placenta 84 (September), 57–62. 10.1016/j.placenta.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C, Feinberg JI, Brown SC, Lurmann FW, Croen LA, Hertz-Picciotto I, Newschaffer CJ, Feinberg AP, Daniele Fallin M, Volk HE, 2019. Epigenetic Marks of Prenatal Air Pollution Exposure Found in Multiple Tissues Relevant for Child Health. Environment International 126 (May), 363–376. 10.1016/j.envint.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapehn S, Paquette AG, 2022. The Placental Epigenome as a Molecular Link Between Prenatal Exposures and Fetal Health Outcomes Through the DOHaD Hypothesis. Current Environmental Health Reports, April. 10.1007/s40572-022-00354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK, 2014. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biology 15 (2), 1–17. 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Storey JD, 2007. Capturing Heterogeneity in Gene Expression Studies by Surrogate Variable Analysis. PLOS Genetics 3 (9), e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Karr CJ, Hazlehurst M, Carroll K, Loftus C, Nguyen R, Barrett E, et al. , 2022. Cohort Profile: The ECHO Prenatal and Early Childhood Pathways to Health Consortium (ECHO-PATHWAYS). BMJ Open 12 (10), e064288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG, 2008. Concentration and Profile of 22 Urinary Polycyclic Aromatic Hydrocarbon Metabolites in the US Population. Environmental Research 107 (3), 320–331. 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD, Sjödin A, 2010. Assessment of Non-Occupational Exposure to Polycyclic Aromatic Hydrocarbons through Personal Air Sampling and Urinary Biomonitoring. Journal of Environmental Monitoring : JEM 12 (5), 1110–1118. 10.1039/c000689k. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A, 2012. Excretion Profiles and Half-Lives of Ten Urinary Polycyclic Aromatic Hydrocarbon Metabolites after Dietary Exposure. Chemical Research in Toxicology 25 (7), 1452–1461. 10.1021/tx300108e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus CT, Szpiro AA, Workman T, Wallace ER, Hazlehurst MF, Day DB, Ni Y. u., et al. , 2022. Maternal Exposure to Urinary Polycyclic Aromatic Hydrocarbons (PAH) in Pregnancy and Childhood Asthma in a Pooled Multi-Cohort Study. Environment International 170 (September), 107494. 10.1016/j.envint.2022.107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughery JR, Crowley E, Kidd KA, Martyniuk CJ, 2021. Behavioral and Hypothalamic Transcriptome Analyses Reveal Sex-Specific Responses to Phenanthrene Exposure in the Fathead Minnow (Pimephales Promelas). Comparative Biochemistry and Physiology. Part D, Genomics & Proteomics 40 (December), 100905. 10.1016/j.cbd.2021.100905. [DOI] [PubMed] [Google Scholar]
- Luo Kai, Stepanov Irina, and Hecht Stephen S.. 2019. “Chemical Biomarkers of Exposure and Early Damage from Potentially Carcinogenic Airborne Pollutants.” 2019 3. https://ace.amegroups.com/article/view/5135. [Google Scholar]
- Machala M, Vondrácek J, Bláha L, Ciganek M, Neca JV, 2001. Aryl Hydrocarbon Receptor-Mediated Activity of Mutagenic Polycyclic Aromatic Hydrocarbons Determined Using in Vitro Reporter Gene Assay. Mutation Research 497 (1–2), 49–62. 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, 2015. Influence of Environmental Exposure on Human Epigenetic Regulation. The Journal of Experimental Biology 218 (Pt 1), 71–79. 10.1242/jeb.106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R, 2007. Utility of Urinary 1-Naphthol and 2-Naphthol Levels to Assess Environmental Carbaryl and Naphthalene Exposure in an Epidemiology Study. Journal of Exposure Science & Environmental Epidemiology 17 (4), 314–320. 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- Mesiano Sam. 2009. “CHAPTER 11 - The Endocrinology of Human Pregnancy and Fetoplacental Neuroendocrine Development A2 - Strauss, Jerome F.” In Yen & Jaffe’s Reproductive Endocrinology (Sixth Edition), edited by Barbieri Robert L., 249–81. Philadelphia: W.B. Saunders. 10.1016/B978-1-4160-4907-4.00011-5. [DOI] [Google Scholar]
- Myatt L, 2006. Placental Adaptive Responses and Fetal Programming. The Journal of Physiology 572 (1), 25–30. 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Yu, Szpiro Adam A, Young Michael T, Loftus Christine T, Bush Nicole R, LeWinn Kaja Z, Sathyanarayana Sheela, et al. n.d. “Associations of Pre- and Postnatal Air Pollution Exposures with Child Blood Pressure and Modification by Maternal Nutrition: A Prospective Study in the CANDLE Cohort.” Environmental Health Perspectives 129 (4): 047004. 10.1289/EHP7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Yamamoto K, Okano K, Hirota T, Kasahara T, Sasaki M, Takanaka Y, Fukada Y, 2001. Chicken Pineal Clock Genes: Implication of BMAL2 as a Bidirectional Regulator in Circadian Clock Oscillation. Genes to Cells 6 (9), 825–836. 10.1046/j.1365-2443.2001.00462.x. [DOI] [PubMed] [Google Scholar]
- Padula AM, Noth EM, Katharine Hammond S, Lurmann FW, Yang W, Tager IB, Shaw GM, 2014. Exposure to Airborne Polycyclic Aromatic Hydrocarbons during Pregnancy and Risk of Preterm Birth. Environmental Research 135 (November), 221–226. 10.1016/j.envres.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Alison G, MacDonald James, Lapehn Samantha, Bammler Theo, Kruger Laken, Day Drew B, Price Nathan D, et al. n.d. “A Comprehensive Assessment of Associations between Prenatal Phthalate Exposure and the Placental Transcriptomic Landscape.” Environmental Health Perspectives 129 (9): 097003. 10.1289/EHP8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Macdonald J, Bammler T, Day DB, Loftus CT, Erin Buth W, Mason A, et al. , 2022. Placental Transcriptomic Signatures of Spontaneous Preterm Birth. American Journal of Obstetrics and Gynecology, July. 10.1016/j.ajog.2022.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D, 2020. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Frontiers in Microbiology 11, 2675. 10.3389/fmicb.2020.562813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, et al. , 2006. Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environmental Health Perspectives 114 (8), 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, 2012. The Human Transcriptome: An Unfinished Story. Genes 3 (3), 344–360. 10.3390/genes3030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DH, 1999. Polycyclic Aromatic Hydrocarbons in the Diet. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 443 (1), 139–147. 10.1016/S1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH, 2004. Bioavailability and Risk Assessment of Orally Ingested Polycyclic Aromatic Hydrocarbons. International Journal of Toxicology 23 (5), 301–333. 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A, 2010. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biology 11 (3), R25. 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob PR, Saliba NA, Bernert JT, El Hellani A, Jatlow P, et al. , 2017. “Biomarkers of Exposure to New and Emerging Tobacco Delivery Products”. American Journal of Physiology. Lung Cellular and Molecular. Physiology 313 (3), L425–L452. 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram AW, Baas R, Jansen PWTC, Riss A, Tora L, Vermeulen M, Marc Timmers HT, 2013. A Dual Role for SAGA-Associated Factor 29 (SGF29) in ER Stress Survival by Coordination of Both Histone H3 Acetylation and Histone H3 Lysine-4 Trimethylation. PLOS ONE 8 (7), e70035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C, Love MI, and Robinson MD. 2015. “Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences [Version 1; Referees: 2 Approved].” F1000Research 4 (1521). 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Meng, Greenbaum Jonathan, Luttrell Joseph, Zhou Weihua, Wu Chong, Shen Hui, Gong Ping, Zhang Chaoyang, and Deng Hong-Wen. 2020. “A Review of Integrative Imputation for Multi-Omics Datasets.” Frontiers in Genetics 11. https://www.frontiersin.org/articles/10.3389/fgene.2020.570255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla LM, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, Tylavsky F, 2015. The Urban Child Institute CANDLE Study Methodological Overview and Baseline SampleDescription. RAND Corporation. [Google Scholar]
- Sun K, Song Y, He F, Jing M, Tang J, Liu R, 2021. A Review of Human and Animals Exposure to Polycyclic Aromatic Hydrocarbons: Health Risk and Adverse Effects, Photo-Induced Toxicity and Regulating Effect of Microplastics. Science of The Total Environment 773 (June), 145403. 10.1016/j.scitotenv.2021.145403. [DOI] [PubMed] [Google Scholar]
- Suter MA, Coarfa C, Robertson M, Zhou G, Jackson BP, Thompson D, Putluri V, et al. , 2019. Association between Elevated Placental Polycyclic Aromatic Hydrocarbons (PAHs) and PAH-DNA Adducts from Superfund Sites in Harris County, and Increased Risk of Preterm Birth (PTB). Biochemical and Biophysical Research Communications 516 (2), 344–439. 10.1016/j.bbrc.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A, Panchenko P, Junien C, Gabory A, 2015. “Placental Contribution to Nutritional Programming of Health and Diseases: Epigenetics and Sexual Dimorphism”. Edited by Hoppeler Hans H.. Journal of Experimental Biology 218 (1), 50–58. 10.1242/jeb.110320. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, et al. , 2010. Towards a Knowledge-Based Human Protein Atlas. Nature Biotechnology 28 (12), 1248–1250. 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Vee L.e., Marc EK, Jouan E, Collet N, Fardel O, 2014. Differentiation of Human Placental BeWo Cells by the Environmental Contaminant Benzo(a)Pyrene. Chemico-Biological Interactions 210 (March), 1–11. 10.1016/j.cbi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Wallace ER, Ni Y.u., Loftus CT, Sullivan A, Masterson E, Szpiro AA, Day DB, et al. , 2022. Prenatal Urinary Metabolites of Polycyclic Aromatic Hydrocarbons and Toddler Cognition, Language, and Behavior. Environment International 159 (January), 107039. 10.1016/j.envint.2021.107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ER, Buth E, Szpiro AA, Ni Y.u., Loftus CT, Masterson E, Day DB, et al. , 2023. Prenatal Exposure to Polycyclic Aromatic Hydrocarbons Is Not Associated with Behavior Problems in Preschool and Early School-Aged Children: A Prospective Multi-Cohort Study. Environmental Research 216 (January), 114759. 10.1016/j.envres.2022.114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD, Davies IM, Huifeng W.u., Diab AM, Webster L, Viant MR, Kevin Chipman J, et al. , 2014. Molecular Responses of European Flounder (Platichthys Flesus) Chronically Exposed to Contaminated Estuarine Sediments. Chemosphere 108 (August), 152–218. 10.1016/j.chemosphere.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Williams J, Rakovac I, Loyola E, Sturua L, Maglakelidze N, Gamkrelidze A, Mauer-Stender K, Mikkelsen B, Breda J, 2020. A Comparison of Self-Reported to Cotinine-Detected Smoking Status among Adults in Georgia. European Journal of Public Health 30 (5), 1007–1012. 10.1093/eurpub/ckaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environmental Health Perspectives 119 (6), 878–885. 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Liu C, Wang H, Nie J, Yang J, 2021. Dose-Response Relationship between Urinary PAH Metabolites and Blood Viscosity among Coke Oven Workers: A Cross-Sectional Study. BMJ Open 11 (11), e046682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Guo C, Li Q, Zhong Y.i., Ma S, Zhou J, Li X, Huang R, Yingxin Y.u., 2021b. Human Health Risks Estimations from Polycyclic Aromatic Hydrocarbons in Serum and Their Hydroxylated Metabolites in Paired Urine Samples. Environmental Pollution 290 (December), 117975. 10.1016/j.envpol.2021.117975. [DOI] [PubMed] [Google Scholar]
- Yang S-I, Lee S-H, Lee S-Y, Kim H-C, Kim H-B, Kim J-H, Lim H, et al. , 2020. Prenatal PM2.5 Exposure and Vitamin D-Associated Early Persistent Atopic Dermatitis via Placental Methylation. Annals of Allergy, Asthma & Immunology 125 (6), 665–673.e1. 10.1016/j.anai.2020.09.008. [DOI] [PubMed] [Google Scholar]
- Yang L.u., Zhang H, Zhang X, Xing W, Wang Y, Bai P, Zhang L, Hayakawa K, Toriba A, Tang N, 2021a. Exposure to Atmospheric Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Health Effects: A Review. International Journal of Environmental Research and Public Health 18 (4). 10.3390/ijerph18042177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza-Ojeda M, Torres-Flores U, Rodríguez-Leviz A, Arenas-Huertero F, 2022. Benzo[Ghi]Perylene Induces Cellular Dormancy Signaling and Endoplasmic Reticulum Stress in NL-20 Human Bronchial Epithelial Cells. Toxicology and Applied Pharmacology 439 (March), 115925. 10.1016/j.taap.2022.115925. [DOI] [PubMed] [Google Scholar]
- Zelinkova Z, Wenzl T, 2015. The Occurrence of 16 EPA PAHs in Food–a Review. Polycyclic Aromatic Compounds 35 (2–4), 248–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shiverick KT, 1997. Benzo(a)Pyrene, but Not 2,3,7,8-Tetrachlorodibenzo-p-Dioxin, Alters Cell Proliferation and c-Myc and Growth Factor Expression in Human Placental Choriocarcinoma JEG-3 Cells. Biochemical and Biophysical Research Communications 231 (1), 117–120. 10.1006/bbrc.1997.6053. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xue Q, Pan G, Meng QH, Tuo X, Cai X, Chen Z, et al. , 2017. Integrated Analysis of Genome-Wide Copy Number Alterations and Gene Expression Profiling of Lung Cancer in Xuanwei, China. PloS One 12 (1), e0169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang P, Zhou Y, Xia B, Zhu Q, Ge W, Li J, Shi H, Xiao X, Zhang Y, 2021. Prenatal Fine Particulate Matter Exposure, Placental DNA Methylation Changes, and Fetal Growth. Environment International 147 (February), 106313. 10.1016/j.envint.2020.106313. [DOI] [PubMed] [Google Scholar]
- Zhao N, Weiwei W.u., Cui S, Li H, Feng Y, Guo L, Zhang Y, Wang S, 2022. Effects of Benzo[a]Pyrene-DNA Adducts, Dietary Vitamins, Folate, and Carotene Intakes on Preterm Birth: A Nested Case-Control Study from the Birth Cohort in China. Environmental Health 21 (1), 48. 10.1186/s12940-022-00859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zita Nagy, Anne Riss, Christophe Romier, Xavier le Guezennec, Dongre Ashok R., Meritxell Orpinell, Jiahuai Han, Henk Stunnenberg, and Lászlò Tora. 2009. “The Human SPT20-Containing SAGA Complex Plays a Direct Role in the Regulation of Endoplasmic Reticulum Stress-Induced Genes.” Molecular and Cellular Biology 29 (6): 1649–60. 10.1128/MCB.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.