Abstract
A finding commonly observed in human immunodeficiency virus type 1 (HIV-1)-infected patients is invasion of the brain by activated T cells and infected macrophages, eventually leading to the development of neurological disorders and HIV-1-associated dementia. The recruitment of T cells and macrophages into the brain is likely the result of chemokine expression. Indeed, earlier studies revealed that levels of different chemokines were increased in the cerebrospinal fluid of HIV-1-infected patients whereas possible triggers and cellular sources for chemokine expression in the brain remain widely undefined. As previous studies indicated that HIV-1 Tat, the retroviral transactivator, is capable of inducing a variety of cellular genes, we investigated its capacity to induce production of chemokines in astrocytes. Herein, we demonstrate that HIV-1 Tat72aa is a potent inducer of MCP-1, interleukin-8 (IL-8), and IP-10 expression in astrocytes. Levels of induced IP-10 protein were sufficiently high to induce chemotaxis of peripheral blood lymphocytes. In addition, Tat72aa induced IL-8 expression in astrocytes. IL-8 mRNA induction was seen less then 1 h after Tat72aa stimulation, and levels remained elevated for up to 24 h, leading to IL-8 protein production. Tat72aa-mediated MCP-1 and IL-8 mRNA induction was susceptible to inhibition by the MEK1/2 inhibitor UO126 but was only modestly decreased by the inclusion of the p38 mitogen-activated protein kinase (MAPK) inhibitor SB202190. In contrast, Tat-mediated IP-10 mRNA induction was suppressed by SB202190 but not by the MEK1/2 inhibitor UO126. These findings indicate that MAPKs play a major role in Tat72aa-mediated chemokine induction in astrocytes.
Human immunodeficiency virus type 1 (HIV-1) infection causes neurological disorders in up to 90% of infected patients, either by opportunistic infections of the brain (i.e., toxoplasmosis) or by development of HIV-1-associated dementia (HAD). HAD, a subcortical dementia characterized by cognitive deficits, as well as motor and behavioral impairment, occurs in up to 30% of all patients infected with HIV-1 (for reviews, see references 21 and 50). HAD develops independently of opportunistic infections and is caused by direct infection of cells in the central nervous system (CNS) by HIV-1. The main sources of infected cells in the brain are infiltrating macrophages and resident microglia cells (15, 32). Besides the presence of infected cells in the brain, some characteristic findings in HAD patients include astrogliosis, microgliosis, the presence of activated T cells and macrophages in the brain, and the loss of specific neuronal populations (for reviews, see references 17, 21, and 50).
A prerequisite for the recruitment of T cells and macrophages to the brain is the secretion of chemokines, small proteins of 5 to 12 kDa. Thus far, four major subfamilies of chemokines have been characterized (1, 5, 29, 30). Of those, the CXC and CC chemokines are distinguished according to the position of the first two cysteines from four conserved cysteines linked by disulfide bonds. Those two cysteine residues are adjacent in CC chemokines, whereas they are separated by one amino acid in CXC chemokines. Gamma interferon (IFN-γ)-inducible protein 10 (IP-10) belongs to the family of CXC chemokines, along with monokine induced by IFN-γ (Mig) and stromal cell-derived factor (SDF-1). IP-10 was first discovered as an IFN-γ-induced gene product found to be expressed in delayed-type hypersensitivity reactions of the skin (26). In the human system, IP-10 is reported to attract NK cells, monocytes, and T lymphocytes (63) although some of the reported results are controversial (41, 52). IL-8, the prototypic CXC chemokine, was initially described to be a monocyte-derived factor known to attract neutrophils (2). Subsequently, T cells, neutrophils, fibroblasts, endothelial cells, and epithelial cells were also identified as sources for IL-8 production. IL-8 attracts T cells, neutrophils, basophils, and endothelial cells (for review, see reference 3). In addition, IL-8 mediates shear flow-resistant adhesion of monocytes (20).
In recent publications, the role of chemokines in the development of HAD and the correlation between their presence in the cerebrospinal fluid (CSF) and the degree of neurological disorder observed in HIV-1-infected patients have been described. Fontana and coworkers reported that IP-10 is the only chemokine to be present in the CSF of all HIV-1-infected patients tested and is absent in uninfected control individuals (33). Other groups demonstrated that expression of MCP-1 or RANTES in the CSF correlated with HIV-1 infection (7, 11, 13, 28), with some of those studies having conflicting results. In addition, the chemokines MIP-1α and MIP-1β were detected in the brains of HIV-1 patients using in situ PCR (58). For all of the above chemokines except IP-10, the cellular sources were identified as microglia and/or astrocytes. The cellular source of IP-10 in the brains of HAD patients has not been determined.
HIV-1 Tat, the retroviral transactivator, is essential for viral replication and directs viral gene expression by binding to the TAR element within the long terminal repeat of the integrated viral genome. HIV-1 Tat exists as different splice variants, of which Tat72aa (one-exon Tat) and Tat86aa or Tat101aa (two-exon Tat) are the most prominent (31). Tat can be released from HIV-1-infected cells (16) and then interact with nearby cells. Expression of Tat in cells by transient or stable transfection, as well as stimulation of cells with extracellular Tat, has been demonstrated to have different effects, including inhibition of antigen-induced T-cell responsiveness (62), apoptosis (39), and induction of several cytokines or chemokines, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-8, and monocyte chemoattractant protein 1 (MCP-1) (10, 25, 36, 40, 49, 55, 67). Putative receptors for Tat that would mediate the effects of extracellularly applied Tat include integrins and the CD26 molecule (22, 65). In addition, Tat has been reported to be capable of penetrating the cell membrane without interacting with any receptor (59).
In this study, we investigated the potential of HIV-1 Tat72aa to stimulate chemokine expression in human astrocytes and demonstrated the involvement of the mitogen-activated protein kinase (MAPK) signaling pathway in Tat-mediated induction of MCP-1, IP-10, and IL-8 in human astrocytes.
MATERIALS AND METHODS
Reagents.
The p38 MAPK inhibitor SB202190 and the MEK1/2 inhibitor UO126, as well as the respective controls SB202474 and UO124 were obtained from Calbiochem (San Diego, Calif.) and dissolved in dimethyl sulfoxide to achieve a stock concentration of 20 mM. HIV-1 Tat72aa was produced as described earlier (14, 46) and was >98% pure. For some experiments, Tat protein was heat inactivated by incubation at 85°C for 30 min. Ficoll-Paque for the preparation of peripheral blood lymphocytes (PBL) was obtained from Pharmacia (Uppsala, Sweden). Neutralizing anti-TNF-α antibody and recombinant human IL-2 and IP-10 were obtained from R&D Systems (Minneapolis, Minn.).
Cell culture.
Human CRT-MG astroglioma cells were maintained in RPMI medium with 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum (FBS) as previously described (47). Human primary adult astrocytes were obtained from biopsy material of patients undergoing surgery to treat intractable epilepsy as described earlier (4). These cells are 95% positive for glial fibrillary acidic protein expression. For passage of CRT-MG cells and human primary astrocytes, medium was removed and cells were disloged by trypsinization (0.05% trypsin).
PBL were isolated from healthy donors by Ficoll-Paque density gradient centrifugation, followed by plastic panning to remove macrophages, and were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. PBL were stimulated with phytohemagglutinin (5 μg/ml; Boehringer GmbH, Mannheim, Germany) and 100 U of human IL-2 per ml for 10 days.
RNA isolation and RPA.
Incubation of human primary adult astrocytes and CRT-MG cells with Tat72aa for RNase protection assays (RPA) was performed in 75-ml flasks containing 2 ml of RPMI medium, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml, either in the absence or in the presence of 10% FBS. Stimulation in the absence or presence of serum revealed the same results (data not shown). After addition of Tat72aa protein, cells were incubated for the indicated times on a rocker at 37°C. Adherent cells were then rinsed once with ice-cold phosphate-buffered saline and dislodged by brief exposure to trypsin-EDTA (0.05% trypsin, 0.02% EDTA; Gibco BRL). Cells were washed twice with ice-cold phosphate-buffered saline, pelleted, and frozen for subsequent RNA extraction. Cell pellets were lysed, and RNA was extracted with guanidinium isothiocyanate and phenol and precipitated with ethanol as described previously (60). A linearized human chemokine multiprobe set (hCK-5; catalog no. 45035P; Pharmingen, San Diego, Calif.) was transcribed with T7 RNA polymerase, resulting in 10 antisense RNA probes of different lengths for lymphotactin, RANTES, IP-10, MIP-1β, MIP-1α, MCP-1, IL-8, I-309, and L32. As an internal control standard, a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe is provided. RPA were performed as previously described (47). Ten to 15 μg of total RNA was hybridized with dUTP-labeled hCK-5 riboprobes and separated on a denaturing (8 M urea) 5% polyacrylamide gel. For quantification of protected RNA fragments, the gels were analyzed using a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). Values for each chemokine mRNA were normalized to GAPDH mRNA expression for each experimental condition as previously described (47).
ELISA.
To determine secretion of chemokines by Tat-activated primary astrocytes and astroglioma cells, enzyme-linked immunosorbent assays (ELISAs) were performed. Primary astrocytes or CRT-MG cells (3 × 105) were plated in six-well plates, incubated for 24 h, and then stimulated with Tat72aa for 24 h. All experiments were done in the absence of serum. Expression of MCP-1 and IL-8 in the supernatants was quantitated using a dual-antibody solid-phase ELISA (Biosource International, Camarillo, Calif.) in accordance with the manufacturer's instructions, as previously described (47). The dual-antibody solid-phase ELISA used for IP-10 was based on anti-human IP-10 antibodies (catalog no. 266-IP and BAF266; R&D Systems), and the instructions of the manufacturer were followed. Each supernatant sample was analyzed in duplicate.
Migration assays.
Migration assays were performed using 24-well Transwell plates (Costar). One milliliter of conditioned medium from unstimulated astrocytes or astrocytes stimulated for 24 h with HIV-1 Tat72aa was added to the lower chamber. IL-2-activated PBL (106) in 200 μl of RPMI medium were placed in the upper chamber. Chambers were separated by a 3-μm-pore-size polycarbonate membrane. The chambers were incubated for 4 h at 37°C in 5% CO2, and then the Transwell inserts were removed and 0.5 × 105 fluorescein isothiocyanate-conjugated Calibrite-Beads (Becton Dickinson) were added as a standard. Cells were then transferred to tubes, and ratios of Calibrite-Beads to unstained, migrated cells were used to calculate the total number of migrated cells per well. To investigate the role of IP-10 in cell migration, 10 μg of neutralizing anti-IP-10 antibody (catalog no. BAF266; R&D Systems) per ml or isotype-matched control antibody (10 μg/ml) was added 30 min prior to the onset of the migration assay.
Statistical analysis.
Levels of significance for comparisons between samples were determined using Student's t-test distribution.
RESULTS
Dose-dependent regulation of chemokine mRNA expression by HIV-1 Tat72aa in primary human astrocytes and human astroglioma cells.
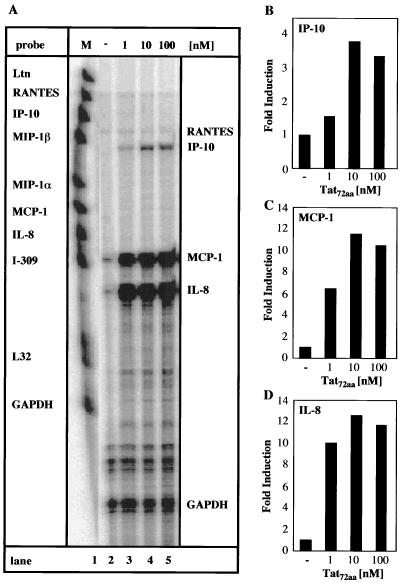
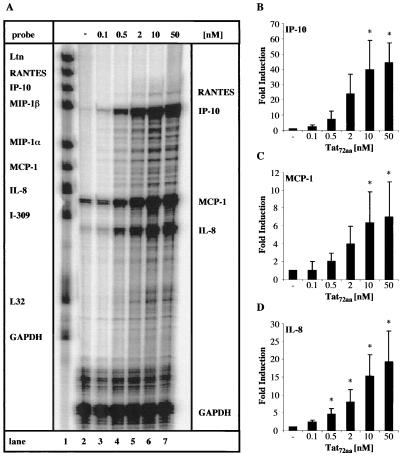
Primary human adult astrocytes and the human astroglioma cell line CRT-MG were tested for the ability to respond to extracellular HIV-1 Tat72aa stimulation by the induction of chemokine mRNA expression. Cells were stimulated for 6 h with various amounts of HIV-1 Tat72aa, and then multiprobe RPA was used to analyze chemokine expression. MCP-1 and IL-8 mRNAs were constitutively expressed at low levels in primary astrocytes, whereas there was no basal expression of any of the other chemokines (Fig. 1A, lane 2). In response to Tat72aa (1 nM), primary human astrocytes showed induction of mRNA for MCP-1, IL-8, and IP-10 (Fig. 1A, lane 3). Increased expression of MCP-1, IL-8, and IP-10 mRNAs was observed when 10 nM Tat72aa was used, while a higher concentration of Tat72aa (100 nM) did not further enhance expression (Fig. 1A, lanes 4 and 5, and 1B, C, and D). Depending on the donor or the passage number of the primary astrocytes, the degree of chemokine mRNA induction after Tat72aa treatment was variable (Table 1). Nevertheless, a consistent pattern of chemokine mRNA induction in response to Tat72aa stimulation was observed. CRT-MG astroglioma cells reacted in a fashion comparable to that of primary astrocytes. CRT-MG cells constitutively expressed small amounts of MCP-1 and IL-8 mRNAs (Fig. 2A, lane 2). A Tat72aa concentration of 0.5 nM stimulated MCP-1, IL-8, and IP-10 mRNA expression (Fig. 2A, lane 4). In a dose-dependent manner, Tat72aa increased the expression of MCP-1, IL-8, and IP-10 mRNAs (Fig. 2A, lanes 5 to 7), reaching saturation between 10 and 50 nM (Fig. 2B, C, and D). RANTES mRNA was found to be induced only at high concentrations of Tat72aa in CRT-MG cells (Fig. 2A, lanes 6 and 7). The specificity of the Tat72aa effect was confirmed by immune precipitation of Tat72aa prior to stimulation of the cells, resulting in >70% abrogation of Tat72aa-induced chemokine mRNA expression (data not shown).
FIG. 1.
Induction of IP-10, MCP-1, and IL-8 mRNAs in primary human astrocytes by HIV-1 Tat72aa. (A) Human primary astrocytes were incubated with medium or Tat72aa (1 to 100 nM) for 6 h, and total RNA was isolated and analyzed for induction of chemokine mRNA using RPA. Probe alone is shown in lane 1. Quantitative analysis of chemokine mRNA induction for IP-10 (B), for MCP-1 (C), and for IL-8 (D) is shown. Expression of different chemokine mRNAs was normalized to the respective expression of GAPDH mRNA, and fold induction was calculated in comparison to cells cultured in medium alone. These results are representative of three independent experiments.
TABLE 1.
Variability of chemokine expression after stimulation of primary human astrocytes with HIV-1 Tat72aa
| Donora | Passageb | RANTESc | IP-10c | MCP-1c | IL-8c |
|---|---|---|---|---|---|
| I | 2 | 14 | 183 | 5 | 30 |
| II | 6 | 3 | 10 | 12 | |
| III | 4 | 23 | 5 | 17 | |
| III | 5 | 11 | 4 | 17 |
Cells were derived from three different patients (I to III) who underwent surgery to treat intractable epilepsy.
Depending on availability, cells were assayed at different passages.
Primary human astrocytes were stimulated for 6 h with 100 nM Tat72aa and then analyzed for mRNA induction of various chemokines by RPA. The values shown represent the fold induction of mRNA compared to unstimulated cells.
FIG. 2.
Induction of IP-10, MCP-1, and IL-8 mRNAs in the human astroglioma cell line CRT-MG by HIV-1 Tat72aa. (A) CRT-MG human astroglioma cells were incubated with medium or HIV-1 Tat72aa (0.1 to 50 nM) for 6 h, and total RNA was isolated and analyzed for induction of chemokine mRNA using RPA. Probe alone is shown in lane 1. Quantitative analysis of chemokine mRNA induction for IP-10 (B), for MCP-1 (C), and for IL-8 (D) is shown. Expression of different chemokine mRNAs was normalized to the respective expression of GAPDH mRNA, and fold induction was calculated in comparison to cells cultured in medium alone. Bars represent the mean ± the standard deviation of the experiment shown in panel A and two additional experiments. Statistical analysis was performed comparing chemokine mRNA induction between Tat72aa-stimulated CRT-MG cells and untreated controls (∗, P < 0.05).
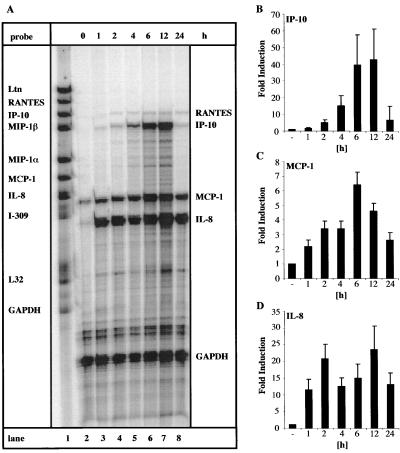
Kinetics of chemokine mRNA induction by HIV-1 Tat72aa in human astroglioma cells.
Kinetic analysis of Tat72aa induction of chemokines revealed differences in the pattern of induction time and stability. CRT-MG cells were incubated with Tat72aa (50 nM) for various amounts of time (1 to 24 h), and then mRNA expression was detected by RPA. MCP-1 and IL-8 mRNAs were induced within the first hour after Tat72aa stimulation (Fig. 3A, lane 3). MCP-1 mRNA peaked at 6 h but was still elevated after 24 h (Fig. 3C). IL-8 mRNA exhibited a biphasic pattern of expression; levels first peaked after 2 h, declined slightly, and then reached a second peak at 12 h (Fig. 3D). Levels of IL-8 mRNA were still increased 24 h after stimulation (Fig. 3D). IP-10 induction was delayed; IP-10 mRNA was first detectable 2 h after Tat72aa stimulation, peaked at 12 h, and returned to basal levels 24 h after stimulation (Fig. 3B).
FIG. 3.
Kinetic analysis of chemokine mRNA expression in the human astroglioma cell line CRT-MG after stimulation with HIV-1 Tat72aa. (A) CRT-MG cells were incubated with medium (lane 2) or with 50 nM Tat for the times indicated (1 to 24 h; lanes 3 to 8). Free probe is shown in lane 1. Total RNA was isolated and analyzed for chemokine expression by RPA. Quantitative analysis of chemokine mRNA expression is shown for IP-10 (B), MCP-1 (C), and IL-8 (D). Expression of different chemokine mRNAs was normalized to the respective expression of GAPDH mRNA, and fold induction was calculated in comparison to cells cultured in medium alone. Bars represent the mean ± the standard deviation of five independent experiments.
As Tat has previously been described to stimulate TNF-α expression (10), and TNF-α, in turn, can induce expression of IL-8, MCP-1, and IP-10 in astrocytes (47), we wanted to exclude a possible influence of TNF-α produced by an autocrine mechanism. Therefore, CRT-MG cells were stimulated with 50 nM Tat72aa in the absence or presence of neutralizing anti-human TNF-α antibody (5 μg/ml). Under these conditions, Tat72aa-mediated induction of IL-8, MCP-1, or IP-10 mRNA was not affected (data not shown), suggesting that endogenous production of TNF-α is not responsible for Tat72aa-induced chemokine expression.
Secretion of chemokine protein after stimulation of primary astrocytes and astroglioma cells with HIV-1 Tat72aa.
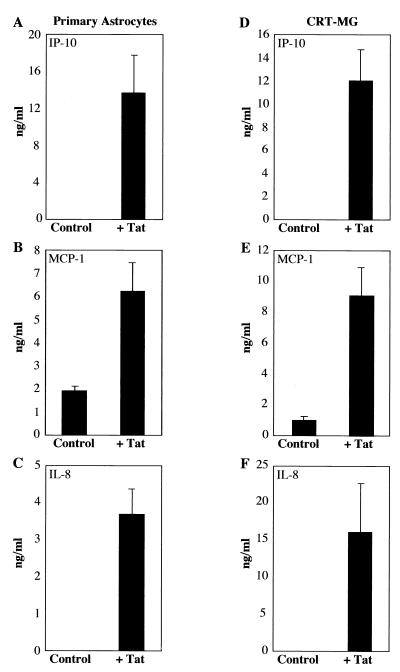
To test whether Tat72aa stimulation of astrocytes would lead not only to the induction of chemokine mRNA but also to the synthesis and secretion of the encoded chemokine proteins, primary human adult astrocytes and CRT-MG cells were stimulated with Tat72aa and secretion of IL-8, IP-10, and MCP-1 was detected after 24 h using ELISA. All three chemokines were efficiently synthesized and secreted into the culture supernatants (Fig. 4).
FIG. 4.
Expression of chemokine protein by HIV-1 Tat72aa-stimulated astrocytes. Primary human astrocytes (A, B, and C) and CRT-MG cells (D, E, and F) were stimulated with 50 nM Tat72aa for 24 h. Supernatants were collected and analyzed for the expression of IP-10 (A and D), MCP-1 (B and E), and IL-8 (C and F) by ELISA. Bars represent the mean ± the standard deviation of three independent experiments.
Capacity of supernatants from Tat72aa-stimulated astrocytes to induce migration of PBL.
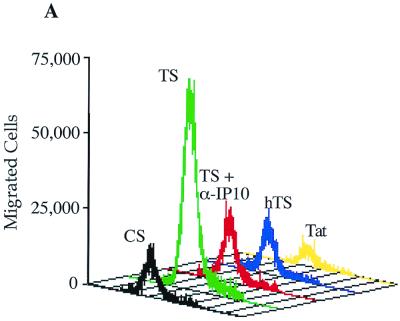
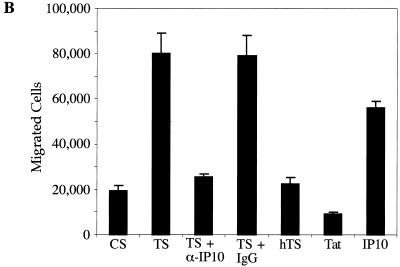
To assess the functionality of the secreted chemokines, we performed migration assays with Transwell plates using PBL which consisted primarily of T cells (90% of the PBL used in the migration assays expressed the T-cell receptor, as assessed by fluorescence-activated cell sorter analysis). Within the 4-h time frame of the experiment, minimal migration of PBL was observed in response to supernatants from unstimulated astrocytes. In response to supernatants from Tat72aa-stimulated CRT-MG cells, PBL exhibited greatly increased migratory activity (Fig. 5). Interestingly, this activity was completely abrogated upon the addition of neutralizing anti-IP-10 antibody to the supernatants of astrocytes stimulated with Tat72aa, whereas a control antibody had no effect on migration. Supernatants from CRT-MG cells stimulated with heat-inactivated Tat did not cause migration. Migration also was not detected when Tat72aa (50 nM) was used directly as a chemoattractant. In contrast, IP-10 (10 ng/ml) induced migration of PBL to a degree similar to that of supernatants from Tat72aa-stimulated astrocytes (Fig. 5). Comparable results were obtained by using supernatants from Tat72aa-stimulated primary astrocytes (data not shown). These findings suggest that IP-10 in the supernatants from Tat72aa-stimulated astrocytes is the major attractant for T cells.
FIG. 5.
Migration of IL-2-activated PBL toward supernatants from HIV-1 Tat72aa-stimulated astrocytes. PBL were stimulated with phytohemagglutinin and cultivated in the presence of IL-2 (100 U/ml) for 5 days. Migration assays to measure the capacity of supernatants from Tat72aa-stimulated CRT-MG cells to induce chemotaxis were performed in 24-well Transwell chambers. Supernatants from unstimulated control cultures (CS) and from Tat-stimulated cultures (TS) were added to the lower chamber of Transwell plates, and 106 PBL were placed in the upper chamber. After 4 h, migration was quantitated by flow cytometry using Calibrite beads as a standard. (A) Histogram analysis of PBL migration induced by supernatants from unstimulated CRT-MG cells (CS; black), supernatants from Tat-stimulated CRT-MG cells (TS; green), supernatants from Tat-stimulated astrocytes in the presence of neutralizing anti-IP-10 antibody (10 μg/ml; TS + α-IP10; red), supernatants derived from CRT-MG cells stimulated with heat-inactivated Tat72aa (hTS; blue), and Tat72aa (50 nM), used as a chemoattractant (Tat; yellow). (B) Quantitative analysis of migrated cells under different conditions. In addition to the culture conditions shown in panel A, the migratory responses of PBL to supernatants from Tat72aa-stimulated CRT-MG cells treated with isotype control antibody (TS + IgG) and to recombinant IP-10 (10 ng/ml), used as a chemoattractant (IP10), are shown. Bars represent the mean ± the standard deviation of three independent experiments.
Inhibition of Tat72aa-induced chemokine expression by MEK and p38 MAPK inhibitors.
We next examined the signal transduction pathways activated upon Tat72aa that are involved in chemokine induction, focusing on the involvement of MAPKs in this response. MAPKs are activated by a variety of extracellular stimuli and can be divided into three major signal transduction cascades: the ERK1/2 MAPK pathway, involving mainly Ras, Raf, and MEK1/2; the JNK/SAPK pathway; and the p38 pathway. The ERK1/2 pathway is thought to be activated mainly by growth factors, whereas the JNK/SAPK pathway is activated by heat shock or inflammatory cytokines, as is the p38 pathway (for a review, see reference 12).
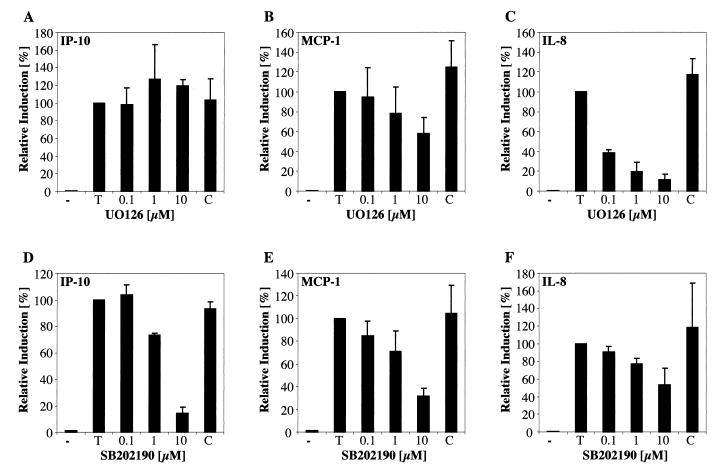
Using the highly specific MEK1/2 inhibitor UO126 and its negative control UO124, IL-8 mRNA induction by Tat was almost completely blocked at a UO126 concentration of 1 μM while UO124 at 10 μM had no inhibitory effect (Fig. 6C). UO126 also partially abrogated MCP-1 mRNA induction by Tat72aa (Fig. 6B), whereas IP-10 induction remained unaffected (Fig. 6A). In accordance with these findings, we have determined that ERK1/2 is phosphorylated 20 min after stimulation with 50 nM Tat72aa (data not shown). Using the p38-specific inhibitor SB202190 (negative control, SB202474), we demonstrate that increasing concentrations of SB202190 strongly inhibit the induction of IP-10 mRNA by Tat72aa (Fig. 6D), with modest inhibition of MCP-1 (Fig. 6E) or IL-8 (Fig. 6F). SB202474 was without effect on IP-10, MCP-1, and IL-8 expression. These findings indicate that the MAPK pathway is of major importance in the signal transduction pathway activated by extracellular Tat72aa.
FIG. 6.
Inhibition of HIV-1 Tat72aa-mediated induction of chemokine mRNA by the MEK1/2 inhibitor UO126 and the p38 inhibitor SB202190. CRT-MG cells were incubated with medium (−) or stimulated with 50 nM Tat (T) for 6 h. Cells were preincubated with the MEK1/2 inhibitor UO126 (A to C) or the p38 inhibitor SB202190 (D to F) for 1 h at the concentrations indicated (0.1 to 10 μM) before stimulation of the cells with 50 nM Tat. As a negative control for UO126, the compound UO124, at a concentration of 10 μM, was used (C; A to C). For inhibition experiments using SB202190, the control compound SB202474, at a concentration of 10 μM, was used (C; D to F). The histograms show the quantitative analysis of chemokine mRNA expression for IP-10 (A and D), MCP-1 (B and E), and IL-8 (C and F). Expression of different chemokine mRNAs was normalized to the respective expression of GAPDH mRNA and corrected for the background, and relative induction was calculated in comparison to the expression of mRNA in cells stimulated with Tat alone (100%). Bars represent the mean ± the standard deviation of four independent experiments.
DISCUSSION
A high percentage of HIV-1 patients develop neurological disorders during the course of the disease, caused either by HAD or by opportunistic infections. HAD is caused by direct effects of HIV-1 infection on the different cell types of the CNS. Pathological hallmarks of HAD are the recruitment of activated T cells and infected macrophages into the CNS, astrogliosis, and apoptosis of defined population of neurons (for a review, see reference 17). Opportunistic infections within the CNS of HIV-1 patients (i.e., toxoplasmosis) occur due to systemic immune suppression of HIV-1 patients. Leukocytes infected with different parasites or virus are not eliminated due to the compromised systemic immune response, thereby potentially serving as vehicles by which infectious agents invade the brain. Chemokines are capable of participating in both syndromes, either by the recruitment of immune cells into the brain or by alteration of vital cellular functions. RANTES, SDF-1, and IP-10 exhibit the ability to recruit T cells (8, 9, 57, 63), while MCP-1 and MIP-1 are major attractants for macrophages (19, 68). SDF-1 also has the capacity to induce apoptosis in neurons (23, 27). Therefore, it is of major importance to elucidate sources and stimulators of chemokine production in the CNS during the course of an HIV-1 infection as control of chemokine expression may be beneficial for HAD patients.
HIV-1-infected cells release the viral Tat protein, either by secretion or due to the cytopathic effect of the virus (16). Externally applied Tat72aa has been shown to be a potent inducer of different cellular genes; in various cell types, it is capable of stimulating expression of IL-6 (45, 56, 69), TNF (10, 55), IL-8 (25, 49), and MCP-1 (13, 43, 67). In this study, we demonstrated that primary human adult astrocytes and CRT-MG human astroglioma cells are stimulated by extracellularly applied Tat72aa to express elevated levels of the CC chemokine MCP-1, as well as of the CXC chemokines IL-8 and IP-10. One nanomolar Tat72aa was sufficient to induce significant levels of all three chemokine mRNAs in both primary astrocytes and CRT-MG astroglioma cells. The kinetics of mRNA induction after Tat72aa stimulation differed for all three chemokines. MCP-1 and IL-8 mRNA induction was evident at 1 h, whereas IP-10 mRNA induction was not strongly detected until 4 h. MCP-1 mRNA induction peaked after 6 h, and MCP-1 mRNA was still present at elevated levels after 24 h. IP-10 mRNA peaked at 12 h but returned to basal levels after 24 h. IL-8 mRNA induction exhibited a first peak after 2 h and a second after 12 h. Due to the fast initial induction of the different chemokine mRNAs, these findings suggest that the initial mRNA induction is due only to the extracellularly applied Tat72aa. As Tat has been reported to induce TNF-α in astrocytes (10) and, in turn, TNF-α is capable of inducing chemokine expression (47), neutralizing anti-TNF-α antibody was added to the Tat-stimulated cultures and found to have no effect on chemokine induction. This supports the idea that Tat can directly stimulate chemokine expression. Nevertheless, we cannot exclude the possibility that secretion of other cytokines due to Tat72aa stimulation activates chemokine expression in astrocytes in an autocrine fashion. The biphasic pattern of IL-8 mRNA expression suggests the involvement of other factors, possibly IL-1β, at a later stage of the response (47).
As induction of chemokines in astrocytes by different stimuli has been demonstrated to be dependent on the MAPK signaling pathway (38) and Tat has been demonstrated to be capable of activating the MAPK signaling pathway in glial cells (44), we investigated the involvement of the MAPK signaling pathway in Tat-mediated chemokine induction. Finding that ERK1/2 became phosphorylated after Tat72aa stimulation (data not shown), UO126, a very potent and highly specific MEK1/2 inhibitor (18), was utilized to demonstrate the involvement of the ERK pathway. We found that UO126 partially inhibited Tat72aa-mediated induction of MCP-1 and abrogated the induction of IL-8 but had no effect on IP-10 mRNA induction. In contrast, SB202190, a specific inhibitor of p38 MAPK activation (37), efficiently suppressed IP-10 mRNA induction by Tat72aa, while expression of MCP-1 and IL-8 mRNAs was affected less. These findings suggest that Tat activation of MCP-1 and IL-8 gene expression is only partially dependent on p38 MAPK activation, as even high concentrations of SB202190 could not completely suppress expression. Tat-mediated IL-8 gene regulation is stringently controlled by the ERK1/2 pathway, while IP-10 regulation by Tat72aa exclusively involves the p38 MAPK pathway. Supernatants from Tat72aa-stimulated astrocyte cultures exhibited a strong capacity to induce migration of IL-2-stimulated PBL, which was completely abrogated using neutralizing anti-IP-10 antibody. This finding is very interesting in the context of a recent publication in which Fontana and coworkers demonstrated that elevated IP-10 levels correlate with the degree of HAD and IP-10 is the main factor in the CSF to cause migration of T cells in HIV-1-infected patients (33). Our findings suggest that astrocytes are a major source of IP-10 in the brains of HIV-1-infected patients. In this regard, the p38 MAPK signal transduction pathway would be an interesting target for pharmaceutical drugs to control IP-10 expression in the brains of HIV-1-infected patients. Other signaling pathways may be involved in Tat-mediated chemokine induction. We found that Tat72aa induction of all three chemokine mRNAs could be abrogated by preincubation of the astrocytes with tolylsulfonyl phenylalanyl chloromethyl ketone (TPCK), a potent inhibitor of NF-κB activation (data not shown). Involvement of NF-κB has been described in the induction of IL-8 (49) and IP-10 (48) in other cell types using other stimuli and in astrocytes for the stimulation of TNF-α (10). Thus, further investigation is needed to definitely determine the involvement of NF-κB in the induction of the monitored chemokines by Tat72aa in astrocytes and possible cross talk with the MAPK signaling pathway.
Recent publications suggest that other T-cell attractants, such as RANTES, are increased in the CSF of HIV-1 patients (28), as well as MIP-1α and MIP-1β (58), two potent macrophage attractants. In our study, Tat72aa did not lead to an increase in MIP-1α or MIP-1β expression in astrocytes and only a minor increase in RANTES production could be seen. An earlier study in our laboratory also demonstrated that MIP-1α and MIP-1β could not be induced in astrocytes by cytokines such as TNF-α, IFN-γ, and IL-1β (47). Therefore, these chemokines may be produced by cells in the CNS other than astrocytes, such as microglia and/or macrophages (42).
The presence of elevated levels of IP-10 can be connected to the migration of T cells into the CNS. IP-10 has been shown to be increased not only in the brains of HIV-1-infected patients (33) and macaques with SIV encephalitis (54) but also in a variety of other diseases, all of which exhibit increased levels of activated T cells in the brain, such as Theiler's virus-mediated demyelination (24, 64), experimental allergic encephalomyelitis (51), or multiple sclerosis (61). A possible role for IL-8 in HAD is less obvious. Thus far, IL-8 has been reported to attract mainly neutrophils, which are absent in the brains of HIV-1-infected patients. Nevertheless, there is evidence that IL-8 is of major importance in the brain. IL-8 release into the CSF after brain injury is associated with blood-brain barrier dysfunction (6) and nerve growth factor production (34). IL-8 is produced by astrocytes under acidosis (66), by blood mononuclear cells after ischemic stroke (35), and in neoplastic and infectious diseases of the human CNS (35). Also, a recent publication suggests that IL-8 contributes to shear flow-resistant adhesion of macrophages to vascular endothelial cells (20). In this context, IL-8 secreted by astrocytes may facilitate the extravasation of macrophages through the blood-brain barrier. Of interest is the finding that depletion of IL-8 by addition of neutralizing antibodies to astrocyte cultures renders astrocytes susceptible to Fas-mediated apoptosis (53). Increased IL-8 expression in the brain could be a response to stress, promoting the survival of astrocytes. A better understanding of chemokine expression in the brain may therefore be of great interest not only for HAD therapy but also as a strategy to prevent opportunistic infections in the brain.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants MH55795, NS36765, and NS29719 (to E.N.B.). O.K. is supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft, and J.-W.O. is supported by a postdoctoral fellowship from the National Multiple Sclerosis Society.
We thank Y. Gillespie (the University of Alabama at Birmingham) for the cultures of primary adult astrocytes and Shaun Sparacio for assistance in running the laboratory.
REFERENCES
- 1.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 4.Barnum S R, Jones J L, Benveniste E N. Interferon-gamma regulation of C3 gene expression in human astroglioma cells. J Neuroimmunol. 1992;38:275–282. doi: 10.1016/0165-5728(92)90020-l. [DOI] [PubMed] [Google Scholar]
- 5.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 6.Bell M D, Taub D D, Perry V H. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74:283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi S, Cinque P, Peri G, Sozzani S, Crociati A, Torri W, Vicenzi E, Vago L, Lazzarin A, Poli G, Mantovani A. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–1101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- 8.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 9.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-α production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272:22385–22388. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 11.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Cobb M H. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 13.Conant K, Garzino-Demo A, Nath A, McArthur J C, Halliday W, Power C, Gallo R C, Major E O. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conant K, Ma M, Nath A, Major E O. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-κB binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eilbott D J, Peress N, Burger H, LaNeve D, Orenstein J, Gendelman H E, Seidman R, Weiser B. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc Natl Acad Sci USA. 1989;86:3337–3341. doi: 10.1073/pnas.86.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R A, Wingfield P, Gallo R C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein L G, Gelbard H A. HIV-1-induced neuronal injury in the developing brain. J Leukoc Biol. 1999;65:453–457. doi: 10.1002/jlb.65.4.453. [DOI] [PubMed] [Google Scholar]
- 18.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 19.Furutani Y, Nomura H, Notake M, Oyamada Y, Fukui T, Yamada M, Larsen C G, Oppenheim J J, Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF) Biochem Biophys Res Commun. 1989;159:249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- 20.Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A, Jr, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 21.Glass J D, Johnson R T. Human immunodeficiency virus and the brain. Annu Rev Neurosci. 1996;19:1–26. doi: 10.1146/annurev.ne.19.030196.000245. [DOI] [PubMed] [Google Scholar]
- 22.Gutheil W G, Subramanyam M, Flentke G R, Sanford D G, Munoz E, Huber B T, Bachovchin W W. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc Natl Acad Sci USA. 1994;91:6594–6598. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 α is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman L M, Fife B T, Begolka W S, Miller S D, Karpus W J. Central nervous system chemokine expression during Theiler's virus-induced demyelinating disease. J Neurovirol. 1999;5:635–642. doi: 10.3109/13550289909021292. [DOI] [PubMed] [Google Scholar]
- 25.Hofman F M, Chen P, Incardona F, Zidovetzki R, Hinton D R. HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. J Neuroimmunol. 1999;94:28–39. doi: 10.1016/s0165-5728(98)00198-2. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan G, Luster A D, Hancock G, Cohn Z A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul M, Lipton S A. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelder W, McArthur J C, Nance-Sproson T, McClernon D, Griffin D E. β-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 29.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy J, Kelner G S, Kleyensteuber S, Schall T J, Weiss M C, Yssel H, Schneider P V, Cocks B G, Bacon K B, Zlotnik A. Molecular cloning and functional characterization of human lymphotactin. J Immunol. 1995;155:203–209. [PubMed] [Google Scholar]
- 31.Klotman M E, Kim S, Buchbinder A, DeRossi A, Baltimore D, Wong-Staal F. Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc Natl Acad Sci USA. 1991;88:5011–5015. doi: 10.1073/pnas.88.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 33.Kolb S A, Sporer B, Lahrtz F, Koedel U, Pfister H W, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-γ inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 34.Kossmann T, Stahel P F, Lenzlinger P M, Redl H, Dubs R W, Trentz O, Schlag G, Morganti-Kossmann M C. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997;17:280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Kostulas N, Kivisakk P, Huang Y, Matusevicius D, Kostulas V, Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mRNA. Stroke. 1998;29:462–466. doi: 10.1161/01.str.29.2.462. [DOI] [PubMed] [Google Scholar]
- 36.Lafrenie R M, Wahl L M, Epstein J S, Yamada K M, Dhawan S. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J Immunol. 1997;159:4077–4083. [PubMed] [Google Scholar]
- 37.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee S J, Zhou T, Choi C, Wang Z, Benveniste E N. Differential regulation and function of Fas expression on glial cells. J Immunol. 2000;164:1277–1285. doi: 10.4049/jimmunol.164.3.1277. [DOI] [PubMed] [Google Scholar]
- 39.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 40.Lim S P, Garzino-Demo A. The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the beta-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-κB consensus sites. J Virol. 2000;74:1632–1640. doi: 10.1128/jvi.74.4.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–327. [PubMed] [Google Scholar]
- 42.McManus C M, Brosnan C F, Berman J W. Cytokine induction of MIP-1α and MIP-1β in human fetal microglia. J Immunol. 1998;160:1449–1455. [PubMed] [Google Scholar]
- 43.McManus C M, Weidenheim K, Woodman S E, Nunez J, Hesselgesser J, Nath A, Berman J W. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menegon A, Leoni C, Benfenati F, Valtorta F. Tat protein from HIV-1 activates MAP kinase in granular neurons and glial cells from rat cerebellum. Biochem Biophys Res Commun. 1997;238:800–805. doi: 10.1006/bbrc.1997.7393. [DOI] [PubMed] [Google Scholar]
- 45.Nath A, Conant K, Chen P, Scott C, Major E O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 46.Nath A, Haughey N J, Jones M, Anderson C, Bell J E, Geiger J D. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- 47.Oh J W, Schwiebert L M, Benveniste E N. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- 48.Ohmori Y, Hamilton T A. The interferon-stimulated response element and a κB site mediate synergistic induction of murine IP-10 gene transcription by IFN-γ and TNF-α. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 49.Ott M, Lovett J L, Mueller L, Verdin E. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-κB factors. J Immunol. 1998;160:2872–2880. [PubMed] [Google Scholar]
- 50.Price R W. Neurological complications of HIV infection. Lancet. 1996;348:445–452. doi: 10.1016/S0140-6736(95)11035-6. [DOI] [PubMed] [Google Scholar]
- 51.Ransohoff R M, Hamilton T A, Tani M, Stoler M H, Shick H E, Major J A, Estes M L, Thomas D M, Tuohy V K. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 52.Roth S J, Carr M W, Springer T A. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon-γ inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995;25:3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- 53.Saas P, Boucraut J, Quiquerez A L, Schnuriger V, Perrin G, Desplat-Jego S, Bernard D, Walker P R, Dietrich P Y. CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: a key role in brain inflammation? J Immunol. 1999;162:2326–2333. [PubMed] [Google Scholar]
- 54.Sasseville V G, Smith M M, Mackay C R, Pauley D R, Mansfield K G, Ringler D J, Lackner A A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 55.Sastry K J, Reddy H R, Pandita R, Totpal K, Aggarwal B B. HIV-1 tat gene induces tumor necrosis factor-β (lymphotoxin) in a human B-lymphoblastoid cell line. J Biol Chem. 1990;265:20091–20093. [PubMed] [Google Scholar]
- 56.Scala G, Ruocco M R, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, Dragonetti E, Quinto I, Venuta S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994;179:961–971. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schall T J, Bacon K, Toy K J, Goeddel D V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 58.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine β peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 60.Shrikant P, Weber E, Jilling T, Benveniste E N. Intercellular adhesion molecule-1 gene expression by glial cells. Differential mechanisms of inhibition by IL-10 and IL-6. J Immunol. 1995;155:1489–1501. [PubMed] [Google Scholar]
- 61.Sorensen T L, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik V A, Qin S, Rottman J, Sellebjerg F, Strieter R M, Frederiksen J L, Ransohoff R M. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Investig. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanyam M, Gutheil W G, Bachovchin W W, Huber B T. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993;150:2544–2553. [PubMed] [Google Scholar]
- 63.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin D J, Oppenheim J J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theil D J, Tsunoda I, Libbey J E, Derfuss T J, Fujinami R S. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler's virus infections. J Neuroimmunol. 2000;104:22–30. doi: 10.1016/s0165-5728(99)00251-9. [DOI] [PubMed] [Google Scholar]
- 65.Vogel B E, Lee S J, Hildebrand A, Craig W, Pierschbacher M D, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the αv β5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe Y, Miura I, Ohgami Y, Fujiwara M. Extracellular presence of IL-8 in the astrocyte-rich cultured cerebellar granule cells under acidosis. Life Sci. 1998;63:1037–1046. doi: 10.1016/s0024-3205(98)00365-8. [DOI] [PubMed] [Google Scholar]
- 67.Weiss J M, Nath A, Major E O, Berman J W. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–2959. [PubMed] [Google Scholar]
- 68.Yoshimura T, Yuhki N, Moore S K, Appella E, Lerman M I, Leonard E J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 69.Zauli G, Furlini G, Re M C, Milani D, Capitani S, La Placa M. Human immunodeficiency virus type 1 (HIV-1) tat-protein stimulates the production of interleukin-6 (IL-6) by peripheral blood monocytes. New Microbiol. 1993;16:115–120. [PubMed] [Google Scholar]