Abstract
Tintelnot et al.1 identified enrichment of indole-3-acetic acid (3-IAA), a tryptophan metabolite produced by gut microbiota, as a predictor of chemotherapy response in pancreatic adenocarcinoma. Recapitulated in mouse models, 3-IAA represents a novel potential therapeutic approach for chemotherapy sensitization.
Tintelnot et al.1 identified enrichment of indole-3-acetic acid (3-IAA), a tryptophan metabolite produced by gut microbiota, as a predictor of chemotherapy response in pancreatic adenocarcinoma. Recapitulated in mouse models, 3-IAA represents a novel potential therapeutic approach for chemotherapy sensitization.
Main text
Despite recent advances in systemic therapy for solid tumors, pancreatic adenocarcinoma (PDAC) remains difficult to treat, with less than half of patients responding to first-line chemotherapy.2 While tumor markers like CA19-9 have proved useful in estimating dynamics of response, there has been little cohesive genomic signal that predicts efficacy of chemotherapy.3 The gut microbiome and diet have been implicated in tumor immunity and response to systemic therapy across multiple malignancies,4,5 and strategies to modulate the gut microbiota (using fecal microbiota transplantation [FMT] from responders) have shown benefit in treating patients with metastatic melanoma that have progressed on immunotherapy.6,7 Additionally, microbiota in the tumor microenvironment (TME) of PDAC have been shown to influence response to chemotherapy, the tumor immunity, and clinical outcomes8,9 with clinical trials using FMT modulation underway (NCT04975217). However, the relationship of gut microbiota in response to chemotherapy for PDAC has been less well studied.
In a recent issue of Nature, Tintelnot et al.1 deeply explored the interplay between the gut microbiota, the TME, and response to chemotherapy in PDAC, using translational studies in human cohorts and mechanistic studies in pre-clinical models. They first evaluated the gut microbiota in patients with metastatic PDAC prior to their treatment with first-line chemotherapy (either FOLFIRINOX or gemcitabine/nab-paclitaxel). Clinical response was defined by radiographic changes or progression-free survival with decreasing tumor markers. The gut microbiome of responders (R) and non-responders (NR) were significantly different in composition.
To test whether the difference in gut microbiome was functionally mediating response to chemotherapy, gnotobiotic mice underwent FMT with R and NR stool, followed by implantation of KPC pancreatic cancer cells and subsequent treatment with FIRINOX (FOLFIRINOX without folinic acid). Only mice treated with R FMT demonstrated a reduction in tumor after FIRINOX. Of note, there was no widespread translocation of bacteria to the tumor after R FMT and chemotherapy, suggesting that the gut microbiota impact was mediated by systemic factors.
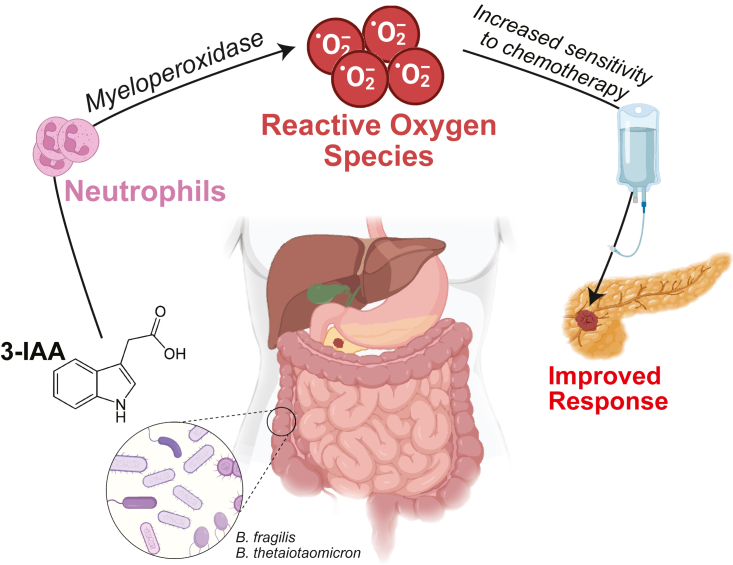
Next, metabolomic profiling was performed on both human pre-treatment and the R/NR FMT mice sera; there was significant increase in levels of indole-3-acetic acid (3-IAA) in those that responded to chemotherapy in both patients and mice. 3-IAA is a tryptophan metabolite derived from aromatic amino acid fermentation in the gut, driven by species in the Bacteroidetes and Firmicutes phyla; indeed, two Bacteroides species (B. fragilis and B. thetaiotaomicron) were increased in R microbiota compared to that of NR.
Because 3-IAA is a known metabolite of tryptophan, the authors then modulated the amount of tryptophan in the diet of R FMT-treated mice and found that serum 3-IAA levels correlated with tryptophan intake. Furthermore, this was sufficient to increase the response to FIRINOX, with subsequent 3-IAA serum levels correlating negatively with tumor volume. They then tested oral 3-IAA against R FMT in mice during FIRINOX treatment and showed that 3-IAA alone was able to enhance chemotherapy response.
Drawing on previous findings that neutrophils’ production of myeloperoxidase (MPO) is able to oxidize 3-IAA,10 the authors showed that depletion of MPO-producing neutrophils abrogated the 3-IAA effect in a transgenic model. Notably, T cell depletion in mouse models did not negate the effectiveness of 3-IAA to enhance FIRINOX response. Furthermore, the group demonstrated that oxidation of 3-IAA via MPO induces radical oxygen species (ROS), which induce tumor cell death. Mice treated with N-acetylcysteine, a ROS scavenger, abrogated the anti-tumor effect of 3-IAA.
Finally, bringing the study back into clinical relevance, the authors utilized two separate cohorts of patients with metastatic PDAC treated with first-line chemotherapy and noted low neutrophil counts in patients who responded to treatment. Their 3-IAA levels were inversely correlated with neutrophils after chemotherapy but showed no correlations with other immune populations (e.g., lymphocytes or monocytes). In addition, there was a direct correlation with progression-free survival days and serum 3-IAA levels but not with other metabolites.
Taken together, this work highlights multiple therapeutic opportunities, either by modulating gut microbial production of 3-IAA or its downstream targets, to enhance chemotherapy response (Figure 1). Such strategies warrant thoughtful validation in the pre-clinical and clinical setting.
Figure 1.
Proposed mechanism of action of gut microbiota-mediated 3-IAA chemosensitization
Indole-3-acetic acid (3-IAA) is a metabolite of tryptophan broken down by gut microbiota, and higher serum 3-IAA levels were found in pancreatic cancer patients who responded to chemotherapy. The gut microbiome of responders was enhanced in 3-IAA producers (including Bacteroides). Through multiple loss-of- and gain-of-function pre-clinical experiments, the authors demonstrate neutrophil-mediated accumulation of reactive oxygen species, which led to improved response to chemotherapy.
Nonetheless, despite this exciting work, there are some mechanistic and clinical questions that remain. One of the main challenges of treating PDAC is the late stage of detection; we need to understand the impact of the gut and tumor microbiota in earlier stages of development to facilitate more effective treatment and interception of disease. Studying the role of gut (and tumor) microbiota and their relationship to metabolites and the immune microenvironment in earlier-stage disease (in transgenic models and in relevant patient cohorts) would be highly fruitful, with opportunities for better cancer treatment and interception (and ultimately even avenues for prevention).
Another consideration not addressed was the potential toxicity when targeting microbial 3-IAA to enhance chemotherapy response. FOLFIRINOX and gemcitabine/nab-paclitaxel are both aggressive cytotoxic regimens with high incidence of adverse events. While the mechanism of 3-IAA via increased ROS accumulation was elegantly demonstrated, dose-related toxicity was not directly assessed. Testing off-target toxicity with varying degrees of 3-IAA supplementation would clearly be warranted prior to launching this treatment approach into the clinical realm.
Finally, the specific connection between specific gut microbes and 3-IAA based on the current study remains less clear. While R FMT was able to produce higher levels of 3-IAA in mice, and while two Bacteroides species were higher in R versus NR stool, direct causality was not established, and engraftment was not measured after FMT. As our knowledge in the microbiome field has expanded, we are moving beyond these taxonomic associations and focusing more on the functional consequences of these microbes. This is highly relevant as we enter into the emerging field of microbiome therapeutics (“bugs as drugs”), with opportunities to engineer microbes to improve and enhance their functional attributes to treat and intercept disease, and ultimately to improve overall health and immunity.
Acknowledgments
Declaration of interests
J.A.W. is an inventor on a US patent application (PCT/US17/53.717); reports compensation for speaker’s bureau and honoraria from Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, MedImmune, and Bristol-Myers Squibb; serves as a consultant/advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, Merck, Biothera Pharmaceuticals, and Micronoma. J.A.W. holds stock options from Micronoma.
References
- 1.Tintelnot J., Xu Y., Lesker T.R., Schonlein M., Konczalla L., Giannou A.D., Pelczar P., Kylies D., Puelles V.G., Bielecka A.A., et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615:168–174. doi: 10.1038/s41586-023-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Chone L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or gemcitabine as Adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 3.Sturm N., Ettrich T.J., Perkhofer L. The impact of Biomarkers in pancreatic Ductal adenocarcinoma on Diagnosis, Surveillance and therapy. Cancers. 2022;14:217. doi: 10.3390/cancers14010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer C.N., McQuade J.L., Gopalakrishnan V., McCulloch J.A., Vetizou M., Cogdill A.P., Khan M.A.W., Zhang X., White M.G., Peterson C.B., et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 7.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K., et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riquelme E., Zhang Y., Zhang L., Montiel M., Zoltan M., Dong W., Quesada P., Sahin I., Chandra V., San Lucas A., et al. Tumor microbiome Diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkes L.K., Dennis M.F., Stratford M.R., Candeias L.P., Wardman P. Peroxidase-catalyzed effects of indole-3-acetic acid and analogues on lipid membranes, DNA, and mammalian cells in vitro. Biochem. Pharmacol. 1999;57:375–382. doi: 10.1016/s0006-2952(98)00323-2. [DOI] [PubMed] [Google Scholar]