Abstract
In 1892, J.L. Wolff proposed that bone could respond to mechanical and biophysical stimuli as a dynamic organ. This theory presents a unique opportunity for investigations on bone and its potential to aid in tissue repair. Routine activities such as exercise or machinery application can exert mechanical loads on bone. Previous research has demonstrated that mechanical loading can affect the differentiation and development of mesenchymal tissue. However, the extent to which mechanical stimulation can help repair or generate bone tissue and the related mechanisms remain unclear. Four key cell types in bone tissue, including osteoblasts, osteoclasts, bone lining cells, and osteocytes, play critical roles in responding to mechanical stimuli, while other cell lineages such as myocytes, platelets, fibroblasts, endothelial cells, and chondrocytes also exhibit mechanosensitivity. Mechanical loading can regulate the biological functions of bone tissue through the mechanosensor of bone cells intraosseously, making it a potential target for fracture healing and bone regeneration. This review aims to clarify these issues and explain bone remodeling, structure dynamics, and mechano-transduction processes in response to mechanical loading. Loading of different magnitudes, frequencies, and types, such as dynamic versus static loads, are analyzed to determine the effects of mechanical stimulation on bone tissue structure and cellular function. Finally, the importance of vascularization in nutrient supply for bone healing and regeneration was further discussed.
Keywords: Bone, mechanical loading, remodeling, fracture healing, vascularization, bone regeneration, bone structure
Introduction
Bone is a mineralized connective tissue that provides a structural framework for vertebrates, serving functions such as locomotion, support and protection of soft tissues, minerals storage, and hemopoiesis.1,2 As a metabolically active organ, bone undergoes continuous remodeling, repair, and regeneration throughout life. After a fracture, the formation of new bone involves the interaction between molecules and different cell lineages. 3 The ultimate goal of healing is to improve the load-bearing ability and restore bone strength, 4 which may be affected by factors such as unhealthy habits like smoking, lack of nutrients, biological factors like growth hormones and cytokines, and genetic factors, and physical stimuli like ultrasound, mechanics, and electrical fields. 4 Among the abovementioned factors, mechanical loading has gathered extensive attention as a potential therapeutic strategy for promoting bone regeneration, owing to its ubiquitous nature, non-invasiveness, and maneuverability. The impact of mechanical loads on bone regeneration has been widely studied since Wolff discovered that mechanical loads could promote bone regeneration. 5 Another well-known “Mechanostat” hypothesis invented by Harold Frost in 1960 described how mechanical loading influences bone structure by changing the bone mass and architecture to provide a design that resists habitual loads with optimal use of material accordingly.6–8 Mechanical forces can stimulate bone marrow mesenchymal cell congregation in the initial fracture healing phase, promote callus tissue formation during the repairing phase, and improve tissue reconstruction in the remodeling phase. 9 The osteoblastic cells are also sensitive to mechanical loading and respond to it by altered proliferation, extracellular matrix synthesis, and secretion/expression of cytokines. 10
However, the outcome of bone remodeling relies on the balance of osteogenic and osteoclastic activity. The effects of mechanical loading on osteoblastic cells depend on the type and magnitude of the stimulation. Inappropriate stimulation can hinder osteogenic functions while promoting the overactivation of osteoclasts. 11 Furthermore, osteoblasts from patients with osteoporosis failed to increase their proliferation and TGF-β release in response to a mechanical loading regimen that stimulated normal donor osteoblasts, suggesting that the response mode of bone formation-related cells to mechanical loading is not fixed but highly correlated with the overall health condition of the host such as age, sex, disease, etc. 12 It also indicates that mechanical loading, as an initiating factor of bone remodeling, cannot function independently regardless of bone tissue’s biophysical and biochemical microenvironment. Instead, such regulation is more likely achieved through the modulation of mechanotransduction signaling pathways, the interaction between physiological, biochemical, and mechanobiological signals, and the local cytokine profile.13,14 In addition to its effects on bone remodeling, mechanical loading can also affect bone vascularization, which is critical for nutrient supply, waste exchange, and the long-term stability of bone. 15
This work aims to comprehensively review current studies on regulating bone healing and regeneration through mechanical loading. Regarding previous studies based on different mechanical models, a consensus on the biological functions of mechanical stimuli and relevant mechanisms has not been reached. To address this issue, bone structure, bone cells, the processes involved in bone remodeling, mechanoconduction, and responses to mechanical loading were defined and explained first. Then, the effects and related mechanisms of mechanical loading on key events such as fracture healing and vascularization in vitro/in vivo were then systematically analyzed. Finally, potential therapeutic strategies and future research directions for improving bone healing/regeneration by optimizing the parameters of mechanical stimulation were further discussed.
Biology and mechanosensation of bone matrix and bone cells
There are two types of bone: dense cortical bone and spongy cancellous bone (Figure 1). Cortical bone forms a dense protective shell around the medullary canal and stores yellow marrow. The osteons within the dense cortical bone are arranged in concentric rings called lamellae, which contain cells crucial for bone formation and remodeling. The Haversian canal, located in the center of each osteon, houses blood vessels, lymphatic vessels, and nerve fibers. 16 Cancellous bone comprises a cellular network of trabeculae grouped in arrangements that follow the lines of stress points, allowing maximum strength with minimal mass. Red bone marrow is located between each trabecular pore and contains hematopoietic stem cells, which play a critical role in hematopoiesis. 16 Bone is composed of 60% inorganic minerals, 30% organic components, and 10% water.17,18 As a vital and uniquely biodynamic organ, bone contains a matrix supporting its spatial structure and bone cells. The bone matrix consists of organic components and inorganic minerals. 17 The organic matrix contains ~90% collagens (mainly type I collagen) and non-collagenous proteins including osteocalcin (OCN), bone sialoprotein (BSP), osteopontin (OPN), and bone morphogenetic proteins (BMPs), etc. 19 While phosphate and calcium ions comprise the primary inorganic substance of bone, other minerals such as bicarbonate, sodium, potassium, citrate, magnesium, carbonate, fluorite, zinc, barium, and strontium are also present in significant amounts and contribute to the structure and strength of bone.20,21 Hydroxyapatite (HA) is the main inorganic crystal in bone tissue, forming through the nucleation of calcium and phosphate. Collagen and non-collagenous matrix proteins work together to create a cross-linked framework for HA deposition and matrix mineralization, forming the structural basis for bone tissue’s characteristic stiffness and resistance. 2 Alongside the supporting functions of bone strength and homeostasis, the bone matrix provides several soluble or adhesion molecules that regulate the bioactivity of bone cells, thereby participating in bone remodeling and metabolism. 22 Moreover, depending on the arrangement of the hierarchical structural units, the mineralized bone matrix can decompose and transform the mechanical loadings into cells in the form of compressive stress, tensile strain, or fluid shear stress (FSS). 23
Figure 1.
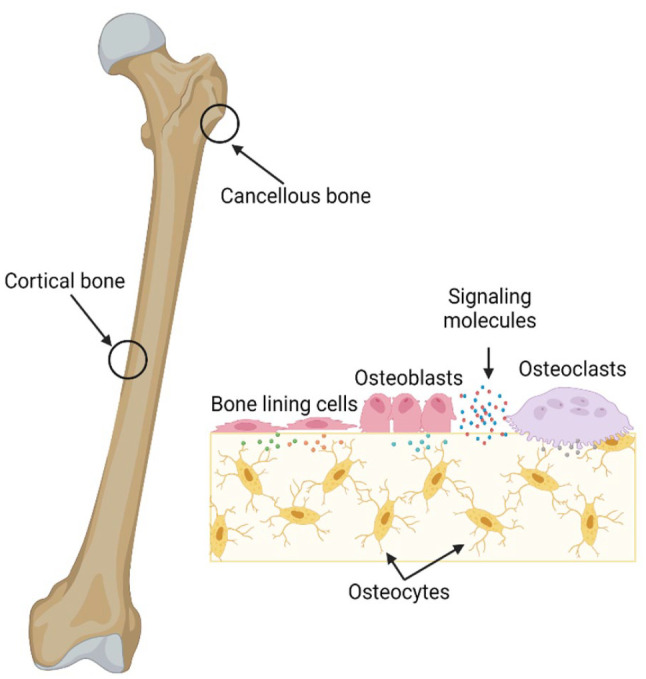
Bone includes both cortical and trabecular bone. In the cellular area, bone comprises osteoblasts, osteocytes, bone lining cells, and osteoclasts created by BioRender 2023.
Four key cell types are responsible for bone’s dynamic development, remodeling, and healing: osteoblasts, osteoclasts, bone lining cells, and osteocytes.
(1) Osteoblasts, known for their bone formation functions, locate along the bone surface, secrete osteoid toward the bone matrix and make up around 4%–6% of the total resident bone cells.24,25 These cuboidal cells (diameter of 9.33–29.91 μm) have abundant rough endoplasmic reticulum, a prominent Golgi apparatus, and various secretory vesicles, indicating their role in protein synthesis in the bone matrix.26,27 Osteoblasts are derived from mesenchymal stem cells (MSCs) under the conservative timely programmed steps (MSCs-osteoblast progenitors-osteoblasts), such as BMPs synthesis, Wnt pathways activation, and upregulated expression of Runx2.28–30 The maturation of osteoblasts is characterized by increased secretion of collagen I and non-collagen bone matrix proteins, such as OPN, OCN, and BSP.30–32 Osteoblasts synthesize bone matrix in two stages: osteoblasts secrete collagens, non-collagenous proteins, and proteoglycans, including decorin and biglycan in the first stage. Then, the bone matrix undergoes mineralization in two phases: the vesicular and fibrillar phases.33,34 In the vesicular phase, matrix vesicles (30–200 nm in diameter) are released from the apical membrane domain of the osteoblasts into the newly formed bone matrix, where they bind to proteoglycans and other organic components. 35 The negatively charged sulfated proteoglycans immobilize calcium ions within the vesicles. 34 When proteoglycans are degraded by enzymes produced by osteoblasts, the calcium ions are released and enter into vesicles through the annexin-associated calcium channels. 33 Concurrently, ALP secreted by osteoblasts degrades phosphate-containing compounds, releasing phosphate ions into the matrix vesicles. The phosphate and calcium ions inside the vesicles nucleate and form HA crystals. 36 During the fibrillar phase, matrix vesicles rupture because of the supersaturation of calcium and phosphate ions inside, allowing the HA crystals to spread to the surrounding matrix. 37 At the end of the bone-forming phase and with the maturation of the bone matrix, osteoblasts will enter into three different fates: (i) embedded in the bone matrix and differentiate into osteocytes, (ii) transform into quiescent flat-shaped bone lining cells that cover the bone surfaces, (iii) undergo apoptosis. 38 As the main contributor to bone formation, osteoblasts have been proven to respond to mechanical stimuli. Hyper gravity at 3×g could upregulate the osteogenic mRNA expression, including ALP, Runx2, OPN, OCN, and Osterix of osteoblasts.39,40 Meanwhile, microgravity inhibits the osteoprotegerin (OPG, a potent decoy receptor/inhibitor of receptor activator of nuclear factor kappa-B ligand, RANKL) production from osteoblasts and leads to high RANKL/OPG ratio and increased osteoclasts formation. 41 Their osteogenic functions could also be promoted under the stimulation of tensile strain. 42
(2) Bone lining cells are quiescent osteoblasts with a flat shape and a diameter of around 15 μm. They cover the bone surface and inhibit bone resorption by preventing the direct interaction between bone matrix and osteoclasts. 43 There is a layer of unmineralized osteoid between bone lining cells and mineralized bone. With various surface receptors, bone lining cells could respond to signaling molecules (e.g. Parathyroid Hormone-PTH, prostaglandin E2 - PGE2) by removing the unmineralized covering osteoid, thereby exposing the mineralized underlying bone matrix to osteoclasts and initiating the bone resorption. 44 By anchoring hematopoietic stem cells, bone lining cells also provide appropriate signals to keep these stem cells in an undifferentiated state. 45 On the other hand, bone lining cells play a crucial role in the transitions involved with bone remodeling by communicating through gap junctions with osteocytes deep inside the bone matrix. They also participate in the formation of osteoclasts by producing RANKL and OPG.43,45 Although bone lining cells do not synthesize new bone, they regulate osteoblastic and osteoclastic activity and mechanosensation. 46 They may change back to an osteoblastic phenotype in the presence of parathyroid hormone or specific physiological status of bone.47,48
(3) Osteocytes are the most abundant and long-lived bone cells (up to 25 years lifespan), making up 90%–95% of the total bone cells. They are derived from the MSCs lineage through osteoblastic differentiation and undergo four identifiable stages: osteoid-osteocytes, pre-osteocytes, young osteocytes, and mature osteocytes. 38 During the osteoblasts-osteocytes transition, cytoplasmic processes begin to appear, followed by progressive encapsulation of osteocytes into the bone matrix. 26 Morphological and ultrastructural changes occur during this process, such as a reduced size of rounded osteocytes, a decreased number of organelles (e.g. Golgi apparatus), and an increase in the ratio of nucleus-to-cytoplasm, reflecting a decline in protein synthesis and secretion. When mature osteocytes are fully embedded in the mineralized bone matrix, the expression of osteoblast-specific markers is downregulated. In contrast, osteocytic markers such as dentine matrix protein 1 (DMP1) and sclerostin (Sost) are highly expressed.49,50 Osteocytes display a dendritic morphology in the lacunae (a typical dimension of 9–29 μm in length and 2–8 μm in width), which are wrapped by the mineralized bone matrix (Figure 1). Their cytoplasmic processes cross tiny tubes named canaliculi (with a diameter of 100–700 nm), forming the osteocyte lacunar-canalicular system.51,52 These processes are connected by gap junctions to adjacent osteocytic processes, the cytoplasmic protrusions of osteoblasts, and bone lining cells on the bone surface, allowing the intercellular exchange of small molecules such as NO. 53 Intercellular communication is also achieved by interstitial fluid flowing between the osteocytes processes and canaliculi (50–100 nm channel size). 54 The osteocytes function as mechanosensor through the lacunar-canalicular system, as their interconnected network can detect mechanical loading, aiding in the adaptation of bone to daily mechanical forces. 55 The morphology of embedded osteocytes varies by bone type. Osteocytes from trabecular bone are more rounded than elongated cortical bone osteocytes. 56 Such difference is not only affected by the arrangement of the basic unit of bone substance but also more likely to be the differential feedback of osteocytes to the stress distribution in different bone types. By altering the synthesis of various signaling molecules such as BMPs, Wnts, PGE2, and NO, osteocytes orchestrate bone remodeling by manipulating the differentiation, activation, and recruitment of osteoblasts and osteoclasts in response to mechanical stimuli.57–61 Experimental evidence from Xiong et al. and Nakashima et al. indicated that RANKL deletion in osteocytes leads to resistance to bone loss induced by mechanical unloading and osteopetrosis phenotype in mouse model.62,63 Tatsumi et al. reported that mice exhibited fragile bone with intracortical porosity, microfractures, and other hallmarks in aging bone tissue after selective ablation of osteocytes. these osteocytes-defect mice were highly resistant to the mechanical unloading-induced bone loss, which directly support the role of osteocytes in mechanotransduction. 64 As a source of OPG, osteocytes regulate osteoclast-mediated bone resorption through differential secretion profiles of OPG and RANKL. 65 Additionally, osteocytes also respond to fluid pressure. Kulkarni et al. established an in vitro model to study the remodeling capacity of osteocytes under pulsating fluid flow (PFF), which ubiquitously exists in bone matrix. PPF (0.7 ± 0.3 Pa, 5 Hz) application upregulated OPG expression via a matrix extracellular phosphoglycoprotein (MEPE)-related manner, thereby inhibiting mouse osteoclasts formation dramatically. 66
4) Osteoclasts are terminally differentiated cells. They originate from monocytic cells of the hematopoietic stem cell lineage in the bone marrow and appear as large (varying diameter: 10–300 μm) multinucleated cells. The development of osteoclasts is influenced by several factors, such as macrophage colony-stimulating factor (M-CSF) and RANKL secreted from osteoblastic lineage cells and stromal cells.67,68 As the main cellular component mediating bone resorption (Figure 1), osteoclasts release protons and proteases (cathepsin, tartrate-resistant acid phosphatase (TRAP), and matrix metalloproteinases (MMPs) et al.) to create an acidic environment conducive to mineral dissolution and bone matrix proteins’ degradation.69–71 In addition to their well-studied osteolytic functions, osteoclasts were also reported as mechanosensitive cells. The fluid surrounding the osteoclasts in the lacunar-canalicular matrix enables the exchange of metabolic and biochemical signaling molecules and generates flow-based mechanical stimuli throughout skeletal loading. 24 Therefore, current studies on the response of osteoclasts to mechanical stimuli were mainly conducted through hydrodynamic models. FSS has been reported to alter the cell shape and ATP6V1A and TCIRG1 expression in rat osteoclasts without affecting cell viability. 72 FSS could further inhibit the osteoclasts’ differentiation and bone resorption functions of mature osteoclasts via the ERK5 pathway. 73 Another possible explanation is that FSS mediates the influx of calcium ions through STIM and transient receptor potential vanilloid 4 (TRPV4) Ca2+ channels on osteoclast progenitors at the early and late stages of osteoclast differentiation separately, thereby affecting osteoclast formation. 74 The application of FSS does not always inhibit the differentiation and function of osteoclasts but depends on the magnitude and duration of FSS per cycle. 75 Physiological FSS loading (loading amplitude of 0.7 ± 0.3 Pa) could inhibit osteoclast differentiation from hematopoietic progenitor cells, whereas higher FSS loading (loading amplitude of 3.0 ± 0.2 Pa) dramatically increased osteoclast formation. Similarly, under higher FSS load, longer loading duration per cycle resulted in more osteoclasts formation, ATP release, and bone resorption areas. 75 In addition to hydrodynamic models, tension stimulation (stretching model) was also reported to affect osteoclast differentiation. Different studies have produced inconsistent findings, indicating that the intensity of mechanical strain, loading frequency and duration of loading application per cycle, and the total duration of force application utilized in various models may play a crucial role in the differentiation and functionalization of osteoclasts.76,77
Bone remodeling, mechanosensation, and mechanotransduction under mechanical loadings
Bone remodeling in response to mechanical loadings
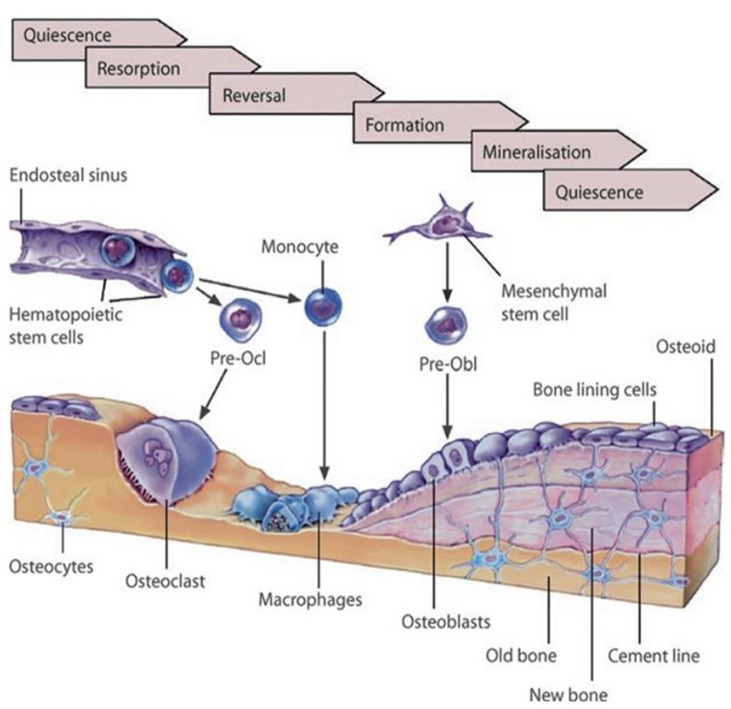
Bone remodeling is a continuous and dynamic process to resorb and replace tiny tissue packets involving the coordination of osteogenic and osteolytic activities, which is achieved by the concerted functions of osteoblasts, osteocytes, bone lining cells, and osteoclasts cells in anatomical structures named “basic multicellular units” (BMU, also known as bone remodeling units, BRU). Bone remodeling occurs on all kinds of bone surfaces, including the bone on the periosteal and endosteal sides, Haversian canals, and the surface of the trabecular bone. Etc. Trabecular bone has a dramatically higher remodeling rate (5–10 times) than cortical bone in adults. The rate of cortical bone remodeling may be up to 50% per year in the first 2 years of life and then reduce to 2%–5% per year in older individuals. 78 Various factors, including system health conditions, age, hormone level, cytokine profiles, and mechanical loading, tightly regulate the activities of bone cells and ultimately decide the fate of bone remodeling. The total bone quantity will decrease if bone resorption exceeds bone formation over the years.
Three possible explanations have been suggested for this negative skewing of bone metabolism: (i) enhanced osteoclastic activity without enhanced osteoblastic activity (high turnover), (ii) regular osteoclastic activity but with decreased osteoblastic activity (low turnover), and (iii) decreased osteoclastic activity with decreased osteoblastic activity (atrophic or adynamic bone). The decrease in bone quantity primarily attributes to the lack of coordination between BMUs, which comprise the cutting cone formed first by osteoclasts and the closing cone formed subsequently by osteoblasts (Figure 2) accompanied by the participation of blood vessels and the peripheral innervation. 79 Both loss and bone gain result from skewed bone remodeling. Anabolic remodeling can increase net bone mass in response to more significant physical activity. For example, the playing arms (humeri) of professional tennis players have 20% more bone mineral mass than the non-playing arms, mainly due to increased diaphyseal thickness.80,81 In contrast, bone loss is associated with prolonged bed rest, spinal cord injury, or space travel.82–84 A typical remodeling process takes about 120 days and is divided into 6 steps/phases. 85
Figure 2.
The schematic of the bone remodeling process 85 was adapted from Reiner and Christoph and reprinted with permission from © 2020 Springer Nature Switzerland AG by license number 501718237.
(i) Quiescence phase: a layer of bone lining cells over a thin collagenous membrane covers the bone surface.
(ii) Activation phase: the quiescent surface of the bone is prepared for resorption, which involves the retraction of the bone lining cells and the elimination of the collagenous membrane covering the bone’s surface. MMPs produced by osteoblasts are involved in this process. The site-specific activation might be obtained by the mechanical stresses, which are transmitted to the endosteal lining cells by the osteocytes via the lacunar-canalicular network.
(iii) Resorption phase: osteoclastic precursors (e.g. monocytes, macrophages, multinuclear giant cells, etc.). Osteoclasts develop ruffled membranes, form cutting cones, and resorb the bone, forming lacunae or pits. Meanwhile, osteoclasts immigrate slowly or undergo apoptosis.
(iv) Reversal phase: Osteoblast progenitors are driven to the resorption pit. At the same time, macrophages prepare the surface of the resorption pit for new bone formation by eliminating the debris of osteoclasts.
(v) Early and late formation phase: active osteoblasts produce osteoid, followed by osteoid mineralization.
(vi) Quiescence phase: finally, the osteoblasts undergo apoptosis or differentiate into flat bone lining cells or osteocytes if trapped inside the newly formed bone matrix. 85
Mechanical loading-mediated bone remodeling is not an independent process involving a single factor. More and more investigations have been focused on the cytokines, genes encoding the enzymes, bone matrix proteins, and transcription factors regulating local bone remodeling. Besides, exercise-generated loads can regulate the level of PTH, estrogen, and glucocorticoids, which mediates cytokines production and skews the anabolic/catabolic balance of bone remodeling at the system level. 86 For example, estrogen can prevent bone resorption by inhibiting RANKL secretion and TRPV5 (a non-selective Ca2+ ion channel) expression while promoting osteoblastic OPG production.87,88 Physical exercises inhibit the secretion of proinflammatory cytokines (such as IL-1, IL-6, and TNF-α. etc.), facilitating bone resorption while stimulating the protective cytokines production against bone resorption (such as IL-10, IL-2, IL-12, and IL-4) by OPG/RANKL/RANK-independent pathways.89–91 It was reported that the osteocytes and osteoblasts in the bone could respond to both fluid flow and mechanical deformation, which result from mechanical loading in vivo. 92 Famous mediators of mechanical loading-induced bone formation include NO, PGE2, prostaglandin I2 (PGI2), and glucose-6-phosphate dehydrogenase (G6PD). 93 In vitro investigations on osteoblasts and osteocytes have demonstrated that the level of prostanoids and NO increased after exposure to physiological fluid flow and mechanical strain. 94 Mechanical stimuli acting on bone marrow stromal cells could suppress RANKL expression and osteoclast formation. Osteoblastic lineage cells are likely to inhibit bone resorption via NO production. 61 Two active prostaglandins, PGI2 and PGE2, are released from osteocytes or osteoblasts shortly after mechanical loading and mediate the recruitment of osteoblasts from bone marrow.95,96 Subcutaneous administration of PGE2 in canines considerably enhances bone formation on periosteal and endocortical surfaces, with apparent trabecular bone formation inside bone marrow. 97
Only a few interventional strategies have been proposed to address the problems associated with adverse bone remodeling. One strategy is avoiding bone resorption or improving osteoblast activity, which can be achieved by manipulating bone remodeling through biochemical mediators or hormones (estrogens and anticatabolic drugs, such as calcitonin, bisphosphonates, and selective estrogen receptor modulators (SERMs)). 98 Nevertheless, these strategies fail to utilize the intrinsic ability of bone tissue to adapt and respond to external loading, which is based on the natural and appropriate coordination between osteolysis and osteogenesis at specific bone sites under mechanical loadings. However, at the cellular level, osteoblasts, osteoclasts, and osteocytes have different mechano-sensitivity. Different research models of mechanical loadings (loading types, frequency, magnitude, etc.) also lead to controversial impacts on bone remodeling. Therefore, the structural basis and mechanisms of mechanosensation and mechanotransduction in bone tissue need to be discussed detailedly.
Mechanosensation and mechanotransduction: Structures and mechanisms
With typical macro-micron-nano hierarchical architectural structures, bone matrix can transmit and transform mechanical loadings to bone cells in forms of compressive stress, tensile strain, FSS, etc. 23 Bone tissue deformation during everyday locomotion ranges from 0.04% to 0.3%, with a rare occurrence exceeding 0.1%. 99 In vitro studies reveal that the deformation required for bone cells to react to mechanical stimulation is significantly higher, ranging from 1% to 10%, which is 10 to 100 times greater than that needed for bone tissue. It is important to note that using the same relative deformation to stimulate bone cells in natural bone tissue would result in a fracture.100,101 You et al.’s experimental mathematical model explains the contradiction between macroscopic and microscopic stimulation levels. The model suggests that the canalicular system, where osteocytes are embedded, acts as an amplifier for the mechanical deformation generated by physical activity. According to Weinbaum’s model, mechanical loading-mediated fluid flow goes through the canalicular space. It deforms the shape of tethering elements (dendritic processes of osteocytes are tethered to the canalicular wall and anchored to hexagonal actin bundles within the cell processes), generating a drag force that then applies a hoop strain on the central actin bundles inside the cell processes of osteocytes. 102 This system allows for more significant deformation at the cellular level than using the same level of deformation at the macroscopic level. 101 In addition, FSS is also significant in affecting the bone matrix components and tailors the functions of bone cells in vivo by acting on the endosteal bone surfaces, walls of lacunar-canalicular system, cell membranes as well as collagens in as-formed osteoid.23,103–105 A recent study revealed that osteocytes do not always connect permanently with the bone surface cells but with highly dynamic structures. 106 Mechanical loading-mediated fluid flow exerts FSS on osteocytes, resulting in the deformation of cells and dendric processes within the lacunar-canalicular system. 107 The theoretical model predicts that peak physiological loadings will effectively make wall shear stress on osteocytes in vivo from 0.8 to 3.0 Pa (8–30 dyn/cm2). 107
Three levels of porosity in the bone matrix are hierarchically nested within microcirculatory pathways and contribute to the generation of fluid flow under mechanical loadings. 108 The largest pore size is related to vascular porosity (VP), which consists of the volume of all tunnels in the bone that contain blood vessels, including all bony canals (primary and secondary) as well as transverse (Volkmann) canals. Lacunar-canalicular porosity (LCP) includes the second-largest porous structure associated with osteocytic lacunae and canalicular channels. The glycocalyx and interstitial fluid of the osteocyte fill the space between the osteocytes and the lacunar-canalicular walls. Finally, the smallest pore size in bone exists in the collagen-apatite porosity (CAP). Most of the water is bound to ionic crystals in the bones at this level. 109 Oxygenated and nutrient-rich blood passes through the bone capillaries. Blood components then leave with less oxygen, nutrients, and cellular wastes. Various substances, including glucose, amino acids, fatty acids, hormones, neurotransmitters, and inorganic compounds, are exchanged from capillaries into the interstitial fluid in the VP. LCPs are occupied by osteocytes, connecting neighbor cells with elongated cell processes, thereby permitting communication between bone cells. Due to the small pore size and low permeability of LCP (10−20 to 10−25 m2), the lacunar-canalicular system has dramatically higher fluid pressure than VP (similar to blood pressure), leading to a longer relaxation time (~10−6 s) compared with VP (~10−3 s) after pressure pulse. 51 In situ measurement of solute transport in the bone lacunar-canalicular system has provided direct evidence for load-induced fluid flow in real-time within the lacunar-canalicular system.110,111 The interstitial fluid flow in the lacunar-canalicular system could be enhanced by everyday mechanical loading.112,113
Mechanoreceptors sense various external and internal mechanical forces. Detecting external mechanical signals requires mechanoreceptors to be in direct contact with the external environment or to sense changes in intermediate cellular structures (e.g. cell membrane, intracellular plasm movement, etc.) caused by tension, pressure, and FSS. Various cell surface proteins or membrane structures, including focal adhesion, ion channels, connexons, G protein-coupled receptors (GPCRs), and primary cilia, have been identified as potentially mechanosensitive structures (Figure 3). These structures can directly sense single or multiple mechanical signals and change their conformation or activity in response to mechanical stimuli to activate downstream signaling pathways and guide cell behaviors. Below, we discuss these mechanosensitive structures, downstream signaling pathways, and corresponding cellular behaviors in bone. Considering the critical role of osteocytes and FSS in transforming macroscopic mechanical loading to the cellular level, we mainly focus the osteocytes and FSS-related mechanosensation and mechanotransduction.
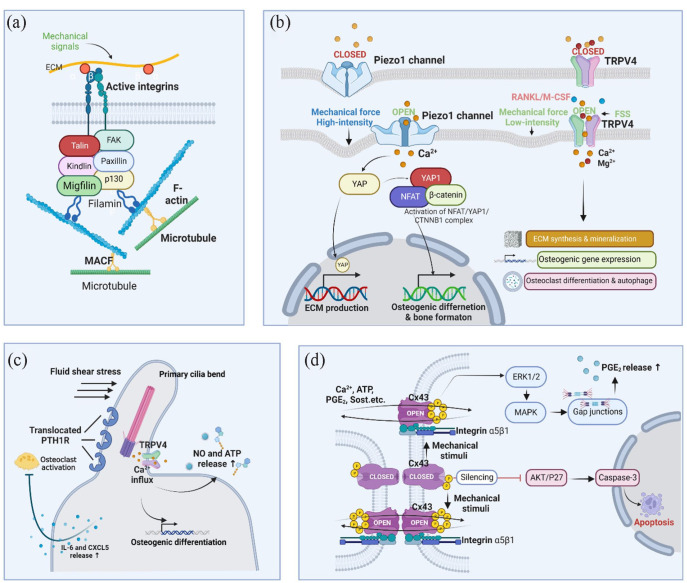
Figure 3.
Mechanosensitive structures. (a) Focal adhesions. Focal adhesions connect ECM mechanical signals to the cytoskeleton, affecting cytoskeleton arrangement and crosslinking; (b) Piezo1 and TRPV4. Activation of ion channels by mechanical stimuli elicits specific ion flow, especially calcium influx, to modulate downstream signaling pathways and cell differentiation; (c) Primary cilium. When primary cilia bend under FSS, TRPV4 ion channels open, allowing Ca2+ influx and MSCs osteogenic differentiation. PTH1R translocation on primary cilia prevents osteoclast activation by releasing IL-6 and CXCL5; (d) Cx43. When osteocytes experience mechanical stimulation, the Cx43 protein is phosphorylated, and the connexon is opened, allowing the exchange of several effectors, such as calcium, ATP, PGE2, and Sost, between connecting cells through gap junctions. Osteocytes with Cx43-silencing undergo apoptosis via AKT/P27/Caspase-3 pathway. The graph was created with BioRender.com.
Mechanosensitive structures
Extracellular matrix
The fate and function of bone cells are influenced by their niche, which consists of extracellular matrix (ECM) components and surrounding cells. The ECM contains various molecules, such as collagen, fibronectin, elastin, laminin, glycosaminoglycans, and glycoproteins. It provides the cell with a 3D microenvironment, variable stiffness, and signaling molecules. The mechanical properties of the ECM play a significant role in osteocyte behavior. A compact preosteoblast-derived matrix (PDM) can promote the maturation of osteoblasts, whereas loose PDM contributes to the overactivation of osteoclasts. 114 Changing the stiffness of the matrix can induce osteogenic differentiation of adipogenic human mesenchymal stem cells (hMSCs). 115 Precise regulation of calcification and elongation is crucial for osteocytes, which are embedded in a bone matrix and extend through the LCP network with cell processes. Osteocytes on a stiffer bone matrix (mineralized) tend to pull more than those on a softer matrix (as-build osteoid), leading to increased tension on stress-bearing elements such as F-actin. 59 F-actin acts as a mechanosensor, mechanotransduction effector, and primary regulator of YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif). 116
Focal adhesion
Focal contacts are direct mechanical linkers between the extracellular matrix (ECM) and the cell, formed by focal adhesion kinase (FAK), integrins, cadherins, and other ECM and cytoskeletal proteins (Figure 3(a)). These contacts facilitate the transfer of signals from the external matrix to the cytoskeleton, promoting cell adhesion, stretching, and migration. Integrins are transmembrane receptors consisting of alpha and beta subunits that form heterodimers. They serve to connect the extracellular matrix (ECM) to the cytoskeleton, enabling the transmission of mechanical stimuli from the extracellular to the intracellular components.
Human primary bone cells express several integrin subunits, including α1, α2, α3, α4, α5, α6, αv, β1, β3, and β5.117,118 The α2, αv, β1, and β3 subunits have been proven to participate in sensing mechanical stimuli.119,120 Integrin heterodimers possess specific affinities for extracellular matrix (ECM) ligands like collagens, fibronectin, laminin, and other non-collagenous proteins. The aggregation of Integrins promotes the activation and phosphorylation of FAK, which facilitate intermediate proteins like MAPK/ERK/JNK and GTPases to mediate mechanotransduction. 121 In vitro studies revealed that the blockade of integrin αvβ3 in osteocytic MLO-Y4 cells reduced their sensitivity to the stimulation of laminar oscillatory fluid flow, resulting in impaired COX-2 and PGE2 production. 122 FSS regulates the activity of the RUNX2 transcription factor by ERK activation, leading to the upregulated integrin β1 expression in hMSCs via the NF-kB pathway. 123 Integrins are also highly expressed in osteoclasts (αvβ3 and α2β1), but it is unclear whether they are mechanosensitive therein.124,125 In vivo studies show that integrin β1 conditional knockout (CKO) mice did not experience bone loss compared to wild-type mice in response to mechanical unloading. 126 Similarly, mice with CKO of OPN, a ligand for integrins in the ECM, also showed resistance to mechanical unloading-induced bone loss, indicating the significance of the interaction between integrins and their ligands in the bone matrix for mechanosensation and signal transduction. 127
FAK is a protein that integrates extracellular stimuli with intracellular events and senses mechanical forces generated inside or outside the cell. 128 Loss of FAK impairs focal contact turnover and disrupts intracellular microtubule polarization via FAK-mediated regulation of Rho-family GTPases. 129 Rho family GTPases control the assembly and disassembly of the actin cytoskeleton. The RhoA/ROCK pathway involves multiple mechanosensitive signaling pathways, downstream-related ERK activity regulation, and osteogenic differentiation. 130 Activated RhoA signaling can activate the p38/MAPK and Akt signaling pathways, creating a link between integrins and phosphoinositide 3-kinase (PI3K)/MAPK signaling. 131 In mandibular stem cells, FAK-mediated mechanotransduction activates new bone formation. 132 FSS dephosphorylates FAK and inhibits the phosphorylation of histone deacetylase (HDAC) 5 tyrosine 642, which inhibits the expression of sclerostin (Sost) in bone cells via an epigenetic mechanism. 133 FAK catalytic inhibitors can similarly reduce Sost expression in vivo and in vitro. 133 Sost, as a BMP antagonist, can bind to BMP receptors and reduce BMP signaling activity, thereby inhibiting the mineralization functions of osteoblasts. 134 These findings indicate that FAK is crucial in bone remodeling in response to mechanical loading.
Ion channels: PIEZO and TRPV4
Bone is highly responsive to mechanical stimuli, and recent research has highlighted the potential role of PIEZO proteins in skeletal mechanosensation. PIEZO1 and PIEZO2 are mechanosensitive cation channels with similar structures but only 42% sequence identity 135 (Figure 3(d)). In vitro studies have shown that PIEZO1/2 stimulates calcineurin by activating Ca2+ influx in osteoblasts, resulting in the coordinated activation of NFATc1, YAP1, and β-catenin in response to mechanical loading. 136 In vivo studies have demonstrated the crucial role of PIEZO1 in the osteoblast lineage. Reduced protein levels of PIEZO1 and several single nucleotide polymorphisms (SNPs) may be associated with osteoporosis and fractures. 137 A PIEZO1 CKO (osteoblastic lineage) mouse model confirmed that loss of PIEZO1 impairs bone formation by inhibiting the expression of RUNX2, type I collagen, and OCN. 138 Moreover, osteoclasts are overactive in Prx1-Cre and Dmp1-Cre guided PIEZO1 CKO (osteoblastic linage) mice, leading to dysregulated interactions between osteoblasts and osteoclasts and subsequent bone loss.139,140 Although PIEZO1 and PIEZO2 share similar structures, their mechanosensory functions in bone are not identical. Loss of both PIEZO1 and PIEZO2 results in severe bone defects, whereas loss of PIEZO2 alone has minimal effects on bone, indicating that PIEZO1 is critical for mechanosensation in bone. 136 However, PIEZO2 has been reported essential for the Merkel-cell mediated mechanotransduction (gentle touch) and proprioception.141,142
TRPs are a family of nonselective cation channels that play a crucial role in bone mechanosensation. Among the seven subgroups of this superfamily, TRPV4 is a significant regulator of bone metabolism, determining bone strength and potentially predicting the risk of fractures. 143 TRPV4 can mediate mechanosensation in osteocytes, chondrocytes, and epithelial cells.144–146 Lee et al. reported that FSS in the lacunae activates TRPV4 (not PIEZO1) to increase calcium concentrations in the cellular plasma, accelerating collagen deposition and mineralization.144,147 TRPV4 is also involved in mediating oscillatory FSS and laminar shear stress-induced calcium signaling and osteogenic gene expression in bone marrow stromal cells (BMSCs).144,147,148 The mechanosensitivity of PIEZO1 and TRPV4 varies with the intensity of mechanical stimuli, with high-intensity mechanical stimuli recognized and input by PIEZO1 and low-intensity mechanical stimuli by TRPV4 (Figure 3(b)). 149 Notably, TRPV4 is predominantly localized in regions with primary ciliary structures and loses its mechanosensitive in BMSCs with defective primary cilia. 147 TRPV4 is also expressed in osteoclasts, where it manipulates autophagy and activates NFATc1 signaling to regulate terminal differentiation through Ca2+ influx.150,151 In the mouse model, TRPV4-knockout leads to marked resistance to hindlimb unloading by inhibiting the increase in bone resorption, 150 suggesting that such resistance may attribute to TRPV4-deficiency-mediated dysfunction of osteoclasts.
Primary cilium
The primary cilium is a microtubule-based, antenna-like sensory organelle found in various bone cells, including osteocytes, osteoblasts, and hMSCs. 152 Primary cilia protrude into the outer space of the cell and perceive mechanical stimuli. 152 In osteocytes, primary cilia respond to extracellular fluid pulses generated by physical activities. When primary cilia bend, mechanosensitive ion channels, such as TRPV4, are activated, leading to intracellular Ca2+ influx, membrane depolarization, and nerve fiber activation, and the cell then undergoes mechanical stimulation 153 (Figure 3(c)). The formation of primary cilia was positively correlated with the mechanosensitivity of osteocytes, manifested by the more release of ATP and NO by osteocytes as the length of primary cilia increased. 154 However, Shi et al. reported that a simulated microgravity (SMG) environment abolished primary cilia formation and shortened the residual cilia, inhibiting the formation and mineralization of rat calvaria. 155 Periosteal osteochondroprogenitors can perceive FSS via primary cilia and differentiate into osteoblasts. This response can be invalidated almost entirely in the absence of primary cilia. 156 Similarly, the normal osteogenic response to FSS is also reduced in MC3T3-E1 and MLO-Y4 cells after abrogating primary cilia.157,158 Osteocytes with PTH1R translocation to primary cilia can prevent osteoclast formation under FSS by manipulating CXCL5 and IL-6 secretion. 159 Therefore, restoring or enhancing the function of primary cilia may be a potential strategy to combat bone loss associated with mechanical disuse, such as microgravity. Nevertheless, current studies do not support the existence of primary cilia in osteoclasts. 160
Primary cilium
The primary cilium is a microtubule-based, antenna-like sensory organelle found in various bone cells, including osteocytes, osteoblasts, and hMSCs. 152 Primary cilia protrude into the outer space of the cell and perceive mechanical stimuli. 152 In osteocytes, primary cilia respond to extracellular fluid pulses generated by physical activities. When primary cilia bend, mechanosensitive ion channels, such as TRPV4, are activated, leading to intracellular Ca2+ influx, membrane depolarization, and nerve fiber activation, and the cell then undergoes mechanical stimulation 153 (Figure 3(c)). The formation of primary cilia was positively correlated with the mechanosensitivity of osteocytes, manifested by the more release of ATP and NO by osteocytes as the length of primary cilia increased. 154 However, Shi et al. reported that a simulated microgravity (SMG) environment abolished primary cilia formation and shortened the residual cilia, inhibiting the formation and mineralization of rat calvaria. 155 Periosteal osteochondroprogenitors can perceive FSS via primary cilia and differentiate into osteoblasts. This response can be invalidated almost entirely in the absence of primary cilia. 156 Similarly, the normal osteogenic response to FSS is also reduced in MC3T3-E1 and MLO-Y4 cells after abrogating primary cilia.157,158 Osteocytes with PTH1R translocation to primary cilia can prevent osteoclast formation under FSS by manipulating CXCL5 and IL-6 secretion. 159 Therefore, restoring or enhancing the function of primary cilia may be a potential strategy to combat bone loss associated with mechanical disuse, such as microgravity. Nevertheless, current studies do not support the existence of primary cilia in osteoclasts. 160
Connexon 43 (Cx43)
Gap junctions act as intercellular channels, facilitating the passive diffusion of small molecules (<1 kDa) and electrical currents between neighboring cells in response to extracellular stimuli (Figure 3(d)). They consist of two docked, hexagonal connexons, each comprising six connexin molecules. 161 Connexin 43 (Cx43) is a prevalent isoform expressed in humans and rodents’ osteoblasts, osteocytes, and osteoclasts. 162 Cheng et al. found that both pulsating and steady fluid shear stress (FSS) can induce the redistribution of intracellular Cx43 from the perinuclear region to the cytoplasm and processes of osteocytes. 163 Osteocytes exposed to FSS at 1.6 Pa (16 dyn/cm2) increased Cx43 expression on the cell membrane, leading to the formation of hemichannels, thereby facilitating the release of PGE2 and the construction of intercellular gap junctions.163–166 Interestingly, mechanical stretching of osteoblasts can promote the phosphorylation level of Cx43 without affecting its mRNA expression. 167 Furthermore, the oscillating fluid flow facilitates the development of new gap junctions (GJs) between mouse osteocytes by an ERK1/2-MAPK-dependent mechanism, while the dye transfer between existing GJs remains unchanged. 168 However, Cx43 cannot sense mechanical stress independently but requires interaction with conformationally activated integrin α5β1 (C-terminal) to open the Cx43 hemichannel. 169 These stress-sensing structures work together to enhance cellular mechanosensitivity. The findings suggest that Cx43 and integrin α5β1 are tightly coordinated in sensing mechanical stimuli and improving cellular mechanosensitivity.
Several studies have emphasized the importance of Cx43 in normal bone formation. Cx43-silenced MLO-Y4 cells underwent apoptosis through the AKT/P27/Caspase-3 pathway. 170 However, different Cx43 CKO mouse models have yielded different conclusions. Specifically, Col1-Cre or Dmp1-Cre-guided Cx43 CKO resulted in bone loss, impaired osteoblast function, and reduced mechanical loading-mediated bone anabolism.171–173 In contrast, an Ocn-Cre-guided osteocyte/osteoblast Cx43 CKO mouse model showed increased osteolytic function by manipulating the RANKL/OPG ratio and enhancing anabolic responses mediated by mechanical loading. 174 Another study found that CKO of Cx43 in osteocytes and osteoblasts prevented mechanical unloading-mediated loss of trabecular bone but failed to maintain the mechanical properties of cortical bone without suppressing cortical bone formation. 175 Interestingly, Cx43 CKO in osteocytes (guided by Dmp1-Cre) enhanced the mechanoresponsiveness in mice. 172 Compared with WT mice, Cx43 CKO mice exhibited a higher rate of periosteal bone formation, manifested by elevated mineralized surface and enhanced mineral deposition rate, which may be due to the loss of Cx43 in osteocytes promoting stretch-induced expression of β-catenin and its target genes. 172 The complicated results from different systems may attribute to the unspecific CKO cell coverage guided by different Cre molecules and the dual functions of Cx43 in gap junctions and hemichannels.
GPCRs
G protein-coupled receptors (GPCRs) have been proposed as mechanosensitive structures. However, only specific GPCRs can sense mechanical forces, such as Angiotensin II receptor 1 (AGTR1), bradykinin receptor B2 (BDKRB2), and GPR68.176,177 Mechanosensitivity is determined by the presence of the C-terminal helix 8 (H8) domain, which is absent in mechano-insensitive GPCRs but can be linked to confer mechanosensitivity. 178 The activation pathway of GPCRs involves agonist binding and subsequent conformational change, activating guanine nucleotide exchange (GEF) activity toward one of the potentially interacting heterotrimeric Gαβγ protein elements. Then, GDP on the α subunit is replaced by GTP, leading to the activation and dissociation of Gα from the βγ subunit. The activated α/β/γ subunit activates different downstream effectors, such as phospholipase C, adenylyl cyclase, GIRK channels, and PI3K. 179 Mechanosensitive GPCRs can induce downstream signaling events upon activation by mechanical stress, including increases in intracellular calcium concentrations via PLC-IP3 and DG pathways. 180 GPCRs often function as a multi-sensitive surface structure. GPR68, for example, responds to extracellular acidification during membrane stretching. Its activity level reflects the degree of membrane stretching and acidification. 181 Further research is needed to comprehend their involvement in physiological and pathological states within bone remodeling.
The mechanotransduction pathways
Mechanotransduction converts physical load to biochemical signals, 182 which change the morphology and function of cells, gene expression, and ECM synthesis. 183 This process involves four steps: (i) mechanocoupling, (ii) biomechanical coupling, (iii) transmission of signals from the sensor cells to the effector cells, and (iv) responses of the effector cells. 184 As illustrated in Figure 4, this process involves receptors (e.g. cadherins and integrins), mechanosensors (e.g. stretchable proteins such as p130CAS and talin), and nuclear cues factors, which alter protein and gene expression profiles. Other factors, such as gender and age, can also regulate the mechanotransduction process. 185 For example, the impact of age has been investigated previously; research on rats of diverse ages showed that inducing bone formation in older rats was over 16-fold less than in younger ones by applying a load of 64 N. Thus, age can be considered an inhibitory factor of bone formation. 184 Gender also acts as a contributor since men have less mechano-responsiveness than women. 186
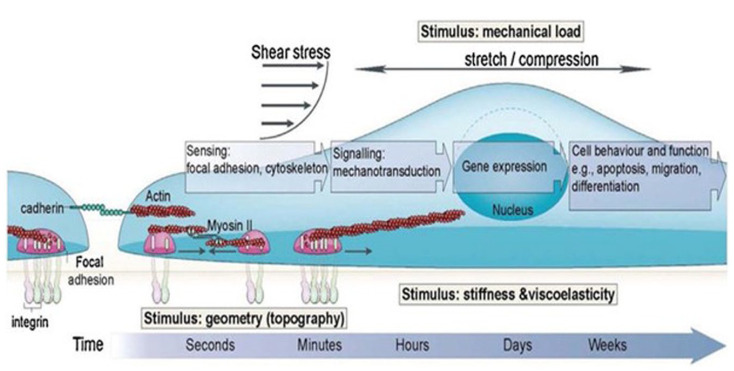
Figure 4.
Showing biological response to different mechanical stimuli to regulate cell function and behavior. Figure reprinted from Iskratsch et al. 185 with permission under license number 501718235. Copyright © 2014, Nature Publishing Group. All Rights Reserved.
When a load is applied to the bone, osteocytes detect the fluid flow and then generate and transmit signaling molecules that modulate the osteogenic/osteoclastic functions of osteoclasts and osteoblasts, respectively, thereby affecting bone remodeling consequently. 60 Mechanotransduction has been investigated from two perspectives: the micro level and the macro level. The macro perspective deals with the system that mechanical stimuli can form by compression or stretching on cells between neighboring cells or cell membrane interfaces. Mechanotransduction from a macroscopic perspective involves mechanical loads imparting varying degrees of deformation to the bone matrix through compression and stretching or imparting fluid flow to the lacunae-tubular network, transmitting mechanical stimuli to mechanosensitive cells represented by osteocytes. On the other hand, the micro perspective has sought to focus on characterizing specialized molecular signaling pathways in specialized tissues. Both these perspectives are involved in particular theories. 187 The one theory investigating the impact of mechanical stresses on the living cells’ function and molecular structure is tensegrity. Tissue and living cells use a form of architecture called tensegrity. 188 The factors involved in this theory are structure (3D structure) and the prestress level. 189 This type of architecture obtains its mechanical stability via the transmission of continuous tension by the geodesic path and through an internal prestress’ presence. Regarding living cells, internal compression elements create this prestress that resists the inward pull of surrounding tensile actomyosin filament networks. 188 Therefore, it can protect the cells against damage by disturbing the forces; furthermore, a mechanical stimulus, even on a small scale, can affect many cells and various cellular functions.190,191 In this theory, the above-mentioned focal adhesions, integrins, ion channels, connexons, primary cilia, and GPCRs are considered to mediate the mechanosensation of bone cells. At the same time, multiple pathways or mechanisms are involved in intracellular mechanotransduction and corresponding functional responses, including cytoskeleton, RhoA/ROCK, YAP/TAZ, etc.
Cytoskeleton
Cytoskeleton is a fibrous network formed by the nuclear skeleton, cytoplasmic skeleton, cell membrane skeleton, cross-linking factors, and extracellular matrix. It provides the framework of basic cell morphology and connects all mechanosensitive components. Among the main cytoskeletal elements, F-actin can sense and transmit mechanical stimuli in osteocytes. 59 Myosin II acts like a cross-linker, strengthening or softening the actin network by directing filament sliding, disassembly, and rearrangement.192,193 This ability to stiffen or soften provides cells with an intrinsic mechanism for maintaining global morphology in response to mechanical stimuli in different magnitudes. Myosin II activity is determined by the phosphorylation of its light and heavy chains mediated by multiple kinases, which are activated by Ca2+ (MLCK), RhoA(citron kinase), Cdc-42 (myotonic dystrophy kinase-related Cdc-42-binding kinase, MRCK). 194 As a cytoskeletal linker between F-actin and microtubules (Figure 3(a)), microtubule-actin cross-linking factor 1 (MACF1) is known as a mechanosensitive structure due to its reduced expression in response to mechanical unloading both in vitro and in vivo. 195 MACF1 mediates the phosphorylation of EB1 at Y247. p-EB1 moves along microtubular bundles, contributing to the polarization, motility, and focal adhesion turnover of pre-osteoblasts. 196 Hu et al. reported that MACF1 significantly enhances the mineralization of MC3T3-E1 cells by promoting the β-catenin/TCF1-RUNX2 signaling pathway.147,197 However, loss of MACF1 results in dysfunction of microtubule organization.143,144
The cytoskeleton determines bone cell morphology and mechanosensitivity. Cultured osteocytes in round shape appear more responsive to mechanical stimuli than adherent flat osteocytes (MLO-Y4). 198 The less stiff cytoskeleton of round cells may facilitate the response of cells to tiny strains mediated by mechanical loading. 198 However, because dendritic osteocytes in the lacunar-canalicular system can amplify and perceive the micro-deformation of bone tissue, the significance of this low-stiffness cytoskeleton in the round cells to bone health needs further study. Microgravity (as well as SMG) leads to cytoskeleton depolymerization and misarrangement of microfilaments and microtubules. 199 In osteoblasts, cytochalasin B-induced SMG impedes BMP2-induced Smad1/5/8 activation and RUNX2 expression by hindering the F-actin polymerization. 200 Our recent study also showed that nanotopography-mediated M1 polarization of human primary macrophages on Titanium implants was impaired under an SMG environment (induced by cytochalasin D), suggesting that F-actin plays an essential role in the mechanosensation/mechanotransduction of macrophages. 201 The crosstalk between BMSCs and M1/M2 polarized macrophages can further manipulate the balance of osteogenesis and osteoclastogenesis in the local milieu, ultimately determining the outcome of bone remodeling. 202
RhoA/ROCK
Small GTPases undergo conformational changes between their active GTP-bound and inactive GDP-bound states to transduce information through signaling pathways. Such process is accelerated by GEFs and GTPase activating proteins (GAPs), which assist GDP dissociation and GTP hydrolysis, respectively. 203 In addition, guanine dissociation inhibitors (GDIs) can bind to small GTPases and redistribute them to the membrane or cytoplasm. 203 The most well-studied GTPases include RhoA, Rac1, and Cdc42. 204 As a member of the Rho family of 20 small GTPases encoded in mammalian genomes, 205 the RhoA signaling pathway is essential for mechanotransduction as it regulates the response of the actin cytoskeleton to mechanical forces. 206 The activation and inactivation of RhoA are controlled by upstream signals from various receptors, including GPCRs, integrins, and growth factor receptors (TGF-βR). Mechanical stimuli such as FSS can activate small RhoA via a GEF-dependent mechanism. GEF binds to the inactive RhoA-GDP to form a RhoA-GEF dimer, which promotes the dissociation of GDP from Rho and facilitates the binding of GTP, leading to RhoA activation. Activated RhoA then interacts with its essential effectors (Rho-associated protein kinase family, ROCK; particularly ROCK1 and ROCK2) and phosphorylates myosin phosphatase, resulting in the contraction of the actin cytoskeleton by activating myosin light chain.207,208
RhoA/ROCK2 regulates the osteogenic differentiation of C3H10T1/2 cells and MSCs and has additive effects on RUNX2 expression under oscillatory fluid flow.207,209 Myocardin-related transcription factor (MRTF) and YAP/TAZ have been identified as transcription factors activated by mechanical stimulation. 210 When external forces or endogenous cell stress act on the cell, the mechanosensor is stimulated by the cytoskeleton and cell membrane tension, leading to the activation of related pathways and changes in gene expression through Rho/ROCK mediated activation of actin-MRTF-serum response factor (SRF) signaling pathway.211,212 Stretching can activate the RhoA/ROCK signaling pathway and YAP/TAZ, resulting in the polymerization of F-actin, promoting osteogenic differentiation of MSCs while inhibiting adipogenic differentiation. 213 A similar RhoA-YAP/TAZ pathway also participates in sensing and transducing the ECM stiffness signals, thereby manipulating the mechanosensitivity of osteoblasts through cytoskeleton reorganization. 214 Moreover, the activation of P2Y2 receptors mediated by FSS regulates the mechanosensitivity of MC3T3-E1 cells via RhoA/ROCK signaling pathway. 215
YAP/TAZ
The Hippo pathway regulates crucial cellular processes through YAP and TAZ activity by integrating various signals. 216 YAP and TAZ are transcriptional coregulators lacking a DNA-binding domain, necessitating their interaction with DNA-binding proteins to regulate transcriptional activity. The Hippo pathway can limit tissue growth and cell proliferation by phosphorylating YAP/TAZ. In mammals, SAV1 and MST1/2 form heterodimers that phosphorylate SAV1, MOB1, and LATS1/2 kinases, leading to direct phosphorylation of YAP and TAZ at multiple sites via LATS1/2. 217 Then, the phosphorylated YAP/TAZ is trapped in the cytoplasm and undergoes degradation through the ubiquitin-proteasome system.218,219 Conversely, when the Hippo pathway is off, YAP/TAZ are kept dephosphorylated and translocated into the nucleus, interacting with co-transcriptional factors to initiate transcriptional programs associated with cell proliferation, survival, and migration.220,221
Various upstream inputs regulate the nuclear localization of YAP/TAZ in response to mechanical stresses. Low stiffness increases intracellular phosphatidylinositol 4,5-bisphosphate and phosphatidic acid levels through phospholipase Cγ1 (PLCγ1), which activates RAP2, a Ras-related GTPase to relay ECM rigidity signals and control the mechanosensitive cellular activities. 222 RAP2 triggers the LATS1/2 activation, leading to the phosphorylation and degradation of YAP/TAZ. 222 In cells experiencing low mechanical signaling, the ARID1A/SWI/SNF-YAP/TAZ complex inhibitory interaction also predominates. Conversely, nuclear F-actin binds to ARID1A/SWI/SNF at high mechanical stress, preventing the formation of the ARID1A/SWI/SNF-YAP/TAZ complex and promoting YAP/TAZ association with TEAD (their DNA binding platform).223,224 It is reported that ECM with high stiffness increases the abundance of vinculin, which promotes the nuclear accumulation of YAP/TAZ independent of LATS1 and following osteogenic differentiation of MSCs. 225 Vinculin deletion with shRNA abrogates rigid ECM-mediated osteogenic differentiation of MSCs while promoting adipogenic differentiation. 225 Therefore, promoting YAP/TAZ nuclear accumulation by inactivating Hippo signaling and enhancing YAP/TAZ binding to TEAD by genetically deactivating ARID1A/SWI/SNF or raising cellular mechanics may be effective strategies to strengthen the responsiveness of YAP/TAZ to mechanical stimuli.
YAP/TAZ in osteocytes is crucial for maintaining bone mass and regulating matrix collagen content and organization, affecting bone mechanical properties. 226 In a recent study, Zarka et al. investigated the significance of YAP/TAZ in osteocyte mechanotransduction. They found that YAP/TAZ translocated to the nucleus and activated their target genes in 3D cultured osteocytes under mechanical compression. 227 Silencing of YAP/TAZ with shRNA partially blocked the mechanical-loading-induced M-CSF and Cxcl3 genes expression, indicating that YAP/TAZ function as a mediator of mechanically-induced chemokine expression in osteocytes. 227 Furthermore, transcriptomic analysis of YAP/TAZ-depleted osteocytes under compressive strain revealed several key factors in initiating dendrites formation associated with YAP/TAZ. 227 These findings suggest that YAP/TAZ plays a central role in forming the perilacunar/canalicular network and osteocyte-mediated mechanotransduction/bone remodeling.
YAP and TAZ play intricate roles in osteogenesis. TAZ is generally considered a transcriptional coactivator that interacts with Runx2 and serves as a key regulator of osteoblastogenesis. 228 siRNA silencing of TAZ abolishes osteogenic differentiation induced by FGF-2 and IGF-1 in cultured rat bone marrow. In contrast to TAZ, YAP inhibits Runx2 activity in ROS 17/2.8 osteoblast-like cells and regulates osteoblastogenesis through Wnt/β-catenin signaling in vitro and in vivo.229,230 SMG significantly weakens the osteogenic differentiation of rat MSCs via the downregulation of TAZ activity. However, by activating ROCK signaling, TAZ activated by lipophosphatidic acid can counteract the inhibitory effects of SMG on osteogenic differentiation in MSCs. 231 Recent studies have utilized mouse models to investigate the roles of YAP/TAZ in bone formation and have revealed their diverse functions depending on the stage of osteoblastogenesis. Induction of YAP/TAZ double deletion in Prx1Cre MSCs was found to promote osteoblastogenesis and bone formation in 12-week-old mouse vertebral cortical bone. 232 Conversely, conditional deletion of YAP in fully differentiated osteoblasts in YAPfl/fl-OcnCre mice resulted in bone loss due to decreased osteoblast proliferation and differentiation. 232 Furthermore, YAPfl/fl/TAZfl/fl-OsxCre mice showed increased osteogenic differentiation with upregulated Osx, osteocalcin, and collagen I levels. Such double deletion-induced enhancement of osteogenesis was associated with activation of the Wnt/β-catenin signaling and increased Runx2 expression. 232 However, YAP or TAZ single knockout in Osx+ cells or YAP/TAZ double knockout at the mature osteoblast/osteocyte stage (YAPfl/fl/TAZfl/fl-Dmp1Cre) led to decreased bone formation and increased osteoclast activity.232,233 In summary, YAP/TAZ can promote osteogenic activity in fully differentiated osteoblasts/osteocytes while inhibiting the commitment of stem cells into the osteoblastic lineage.
Wnt/β-catenin
The Wnt signaling pathway has diverse functions in bone remodeling and homeostasis. 234 Canonical Wnt signaling is triggered by the binding of Wnt ligands to Frizzled and Lrp5/6 receptors on the cell membrane. This signaling promotes β-catenin accumulation by inhibiting GSK-3β-induced β-catenin phosphorylation, and translocated β-catenin then induces transcription of LEF/TCF-responsive genes. 235 β-catenin is a critical mediator of mechanotransduction, and its activity is modulated by mechanical loading and unloading via activation of the nitric oxide, FAK, and Akt signaling pathways. 236 Strength and power training can increase Wnt-related gene expression in human subjects, while mechanical strain induces MSCs to switch from adipogenic to osteogenic differentiation by preserving β-catenin in the nucleus.237,238
In osteocytes, Wnt/β-catenin signaling plays a vital role in mechanotransduction. Mice with β-catenin deletion in osteocytes exhibit severe osteopenia and fragile bones. 239 Wnt signaling-activated transgenic mice (LRP5 G171V) show upregulated Wnt/β-catenin target gene expression and increased bone formation under physiological and mechanical loading conditions. 240 Conversely, the absence of Wnt inhibitors (FRZB and Sost) enhances the anabolic activity of bone in response to mechanical loading.241,242 Furthermore, mechanical loading promotes Postn expression and inhibits Sost expression through the Postn-integrin αVβ3 interaction, while unloading produces the opposite effect. 243 However, high-intensity mechanical loading can inhibit the PI3K/Akt pathway, leading to β-catenin phosphorylation and impaired osteoblast differentiation. 244 Mechanical loadings can also activate non-canonical Wnt signaling. Oscillating fluid flow induces the expression of Wnt5a and its non-canonical tyrosine kinase receptor Ror2, which are required for mechanically mediated RhoA signaling activation and osteogenesis. 245 Overexpression of Ror2 enhances osteogenesis, indicating that non-canonical Wnt signaling plays a crucial role in mechanotransduction. 246 These findings support the involvement of canonical and non-canonical Wnt signaling in bone mechanotransduction and provide insights into the mechanisms underlying the effects of mechanical loading on bone remodeling.
Potential pathways and mediators
Various signaling pathways and factors have been discovered to mediate the transduction of mechanical signals in bone cells, in addition to the molecules and pathways previously mentioned. One of these is the Ras/ERK-mediated mitogen-activated protein kinase (MAPK) signaling, which can be activated by mechanical forces, promoting hypoxia-inducible factor 1-alpha (HIF-1α) expression in osteoblasts. 247 Osteoblast-targeted delivery of miR-33-5p, a noncoding RNA, has been found to enhance osteogenesis and partially counteract the reduction of osteogenic genes and mineral apposition rate in the hindlimb unloading mouse model. 248 Furthermore, during the commitment of hMSCs to the osteogenic lineage, cell shape has been observed to modulate the ability of BMP2 to activate RhoA, ROCK, and cytoskeletal tension. RhoA/ROCK activity and associated cytoskeletal tension can regulate hMSC commitment to the BMP-induced osteogenic phenotype. 249 As further studies are conducted, more transcription factors involved in metabolic and hypoxic modulation in response to mechanical loading are expected to be identified. HIF-1α CKO in osteoblasts has been reported to result in the formation of thinner cortical bone, highlighting the importance of such factors in the process of bone formation. 247 The epigenetic mechanism also involves the mechanical loading mediated bone formation. In MSCs with osteogenic differentiation induced by cyclic stretching and compression loading, histone deacetylase (HDAC) activity decreases, accompanied by increased histone acetylation and remodeled chromatin. Deleting nuclear matrix protein lamin A and C abrogates mechanical loading-induced alteration in histone acetylation. 250
Effect of mechanical loading on bone healing and regeneration
Bone healing and regeneration involve a variety of bone defects, including fractures, traumatic bone defects, and medical-related bone injuries (implantation of endosseous medical devices), which have different mechanical properties. In this section, the in vivo evidence and the in vitro mechanism research data on the influence of mechanical loading on the healing of fracture and bone trauma were summarized and analyzed to obtain potential clinical intervention strategies.
Mechanoresponses of bone healing/regeneration in vivo
A vast diversity of mechanical factors has been recognized to affect fracture healing. The predominant factors in this process include rigid fixation, fracture geometry, fracture type, direction, and magnitude. All these factors determine local stress distribution at the fracture site and provide mechano-biological signals to regulate fracture healing and elicit cellular reactions. 10 Not only the amount of interfragmentary movement but also its direction influences the healing process. Moderate axial interfragmentary movement enhances fracture repair by promoting periosteal callus formation and accelerating healing. 251 Conversely, tensile or shear movements of similar magnitude do not appear to promote fracture healing. While induced cyclic tensile strains can stimulate periosteal callus formation but fail to promote bone healing. 252 Shear movements at the fracture site have been shown to impede healing, manifested by decreased periosteal callus formation, delayed bone formation in the fracture gap, and inferior mechanical stability compared to the axial movement in a sheep model after loading (immediate post-surgery to 8 weeks). 253 However, in a clinical case, the shear movement induced by 15 kg loading 2 weeks (full body weight applied after 8 weeks) after closed, low-energy diaphyseal tibial fractures is shown to be compatible with successful healing. 254 In vivo investigation in rabbit model also demonstrated that shear movement resulted in superior healing outcomes 4 weeks after fracture but inferior outcomes 2 weeks after fracture compared to axial interfragmentary movement. Such shear movement-induced improvement in fracture healing occurs through enhanced endochondral ossification. 255 Therefore, the shear movement appears more sensitive to timing, magnitude, and gap geometry than axial movement.
Liu et al. investigated the impact of the timing phase of force application on bone defect healing in a mouse model. This study showed that applying daily loading of 5 N peak load, 2 Hz, 4 consecutive days, 60 cycles within inflammation and hematoma consolidation disrupted the traumatic site and activated cartilage formation surrounding, which impedes stabilization of the trauma site. On the contrary, loading throughout the matrix deposition phase improved cartilage and bone formation; Loading within the matrix deposition phase enhanced both bone and cartilage formation; Loading within the remodeling phase increased woven bone formation. 256 Another rat in vivo study reported the effect of delayed and immediate cyclic axial load (0.05 Hz, 30 g loading with 2.2% graft elongation) on the tendon graft-bone interface healing. The results demonstrated that delayed loading improved biological and mechanical parameters of tendon-to-bone healing compared to immediate loading. 257 Gardner et al. reported similar results from a mouse model that both timing and loading magnitude affected fracture healing. Compared to the immediate loading model, the low magnitude (0.5 N, 1 Hz for 100 cycles/day, 5 days/week for 2 weeks) axial cyclic compression with a short delay (4 days delay) led to significantly improved fracture healing, evidenced by increased callus strength which vanishes with the increase in loading amplitudes (2 N). Therefore, mechanical loading in inappropriate timing and overloading can potentially impair fracture healing. 258 Wehrle et al. reported that the bone remodeling (from week 4 to 7) behaviors are more responsive to cyclic mechanical loading. Cyclic strain (8–16 N, 10 Hz, 3000 cycles; 3 times/week for 4 weeks) applied on the mouse fracture model led to significantly higher callus formation and mineralization, which may associate with Wnt signaling activation and reduced distribution of sclerostin and RANKL in fracture callus. 259 Such time- and magnitude-dependent acceleration of fracture healing may attribute to the enhanced exchange of cells and bioactive factors mediated by loading-mediated callus deformation and altered interstitial fluid flow. Ghimire et al. established a finite elemental model to analyze the impact of dynamic loadings (150 N, 1 Hz for 5 h) on fracture healing (human tibia bone) under various locking compression plate configurations. Dynamic loading increased the transport of bone cells (280% for chondrocytes and 180% for osteoblasts) and growth factors (220% for chondrogenic growth factors and 120% for osteogenic growth factors) in the callus compared to the free diffusion. Similarly, a moderate transport improvement was observed for the MSCs and fibroblasts, around 22% and 17%, respectively. 260 Another study on the sheep metatarsus fracture model showed that mechanical loading with low amplitude and high frequency (0.02 mm of compression displacement with frequencies between 50 and 100 Hz) significantly improved the osteogenic activity of the callus. Regarding the four mechanical variables (deviatoric strain, octahedral strain, pore pressure, and fluid flow velocity) tested within the callus, only interstitial fluid flow velocity underwent significant increases in amplitude and peak value when the frequency of the external stimulus was altered. 261 The regulation of bone formation by mechanical loading is also influenced by overall health status. An in-depth study conducted by Maycas et al. applied the combination of parathyroid hormone-related protein (PTHrP)-derived peptides and mechanical loading to treat skeletal deterioration in a diabetic mouse model. In diabetic mice, mechanical loading induced less bone formation than in healthy mice. The combination of mechanical stimuli and PTHrP peptide can overcome bone loss, fragility, and reduced mechanoresponsiveness caused by diabetes. 262 Li et al. also reported the impact of spinal compression loading (4 N, 10 Hz, 5 min/day for 2 weeks) on bone formation in ovariectomized (OVX) mice. The results supported the hypothesis that Wnt3a-mediated signaling was involved in the effects of spinal loading on enhancing bone formation/angiogenesis and repressing bone resorption in OVX mice. Wnt3a may work as a potential mechanosensitive therapeutic target for postmenopausal osteoporosis. 263 Applying bend loadings (31, 43, 53, and 65 N, single boat for 36 or 360 cycles) also facilitated bone formation at the endosteal surface. The lamellar bone formation rate (BFR) was enhanced in all categories (Maximum bone formation obtained after loading of 65 N), suggesting that bone lining cells could be stimulated by bend loading and contribute to the anabolic responses on the bone surface. 264
The type of mechanical loadings is supposed to be crucial for the response of bone. For instance, bone cannot adapt to loading unless applied cyclically (as physiological movement or physical exercises). Hert et al. found that static bending on the tibiae of rabbits for 30 days impaired bone formation. In contrast, rabbits subjected to dynamic loading of the equivalent magnitude were shown to have enhanced bone formation on both endosteal and periosteal surfaces. 265 Similarly, rats with static loading at 8.5 and 17 N (10 min/day, day 1–5 and 8–12) showed the same bone formation on the periosteal bone surface. Static loading could not generate an anabolic bone response but even suppress the appositional growth of the skeleton with the increase of loading magnitude. However, applying a dynamic force at 17 N (haversine waveform, 2 Hz, 1200 cycles/day) for a similar period significantly enhanced bone formation. 266 Bone cells can rapidly desensitize under static loading and lose mechanosensitivity before mechanosensation and mechanotransduction are complete. 267 Therefore, cyclic and intermittent loading may be more beneficial for maintaining bone mechanosensitivity than continuous loading because more rest phases are presented. 268 Furthermore, if loading cycles are divided into discrete bouts with hour intervals, the mechanical loading protocol may be more osteogenic than the cycles applied within one uninterrupted bout. Robling et al. evaluated the effect of discrete mechanical loading bouts on the biomechanical and structural properties of the rat ulna. The right ulnas of 26 adult female rats were exposed to a haversine waveform at 17 N peak value, 360 cycles/day, 3 days/week for 16 weeks. In half of the experimental subjects, all 360 daily cycles were applied in a single bout (uninterrupted, 360 ×1). The other subjects were applied 90 cycles four times per day (90 × 4), with an interval of 3 h between bouts. The loaded ulnas showed 5.4% (360 ×1) and 8.6% (90 ×4) greater areal bone mineral density than the control. Bone mineral content was enhanced by 6.9% and 11.7% in the 360 × 1 and 90 ×4 loaded ulnas, respectively. 269
In addition to fracture and bone defect healing, osteogenesis in normal bone and the osseointegration of implants have also been shown to be highly dependent on mechanical loading. A comparative rat study evaluated the effect of ultrasound and mechanical compression on normal bone in three different groups: (i) transcutaneous low-intensity pulsed ultrasound (1 MHz sine waves with an intensity of 30 mW/cm2) applied on the left ulnae; (ii) ultrasound and compression loading (0.003 at 0.12/s, with a 0.46 s rest period at peak strain and a 10 s rest period between each cycle, 3 times/week for 2 weeks) applied on the left ulna simultaneously; (iii) compression loading applied on the left ulna. The bone formation was evaluated by measuring the periosteal bone surface by the double label (dLS/BS, %). All groups showed a considerably increased mineral apposition rate (MAR) and enhanced dLS/BS % from less than 10% in the control samples to more than 80% in the treated samples. 270 The study conducted by Chavarri-Prado et al. provides evidence of the impact of mechanical loads and exercise on osseointegration. Four dental implants were placed in both tibiae of 10 New Zealand rabbits, which were divided into two groups. The test group underwent 20 min of daily treadmill running during the osseointegration period (with a 2-week progressing adaptation phase), while the other group served as control. The test group had more significant vertical bone growth (1.26 ± 0.48vs 0.32 ± 0.47 mm, p < 0.001), higher ISQ values (11.25 ± 6.10vs 5.80 ± 5.97 p = 0.006), higher BIC (25.14 ± 5.24%vs 18.87 ± 4.45%), and higher bone neoformation (280.50 ± 125.40vs 228.00 ± 141.40 mm2, p = 0.121). 271 Zhang et al. evaluated different factors for bone-implant contact (BIC) and a peri-implant bone fraction (BF). In the rat tibia compression model with titanium implant placement, the high and low frequencies (HF, LF) with high and low magnitudes (HM, LM) were applied as follows: HF/LM (40 Hz, 0.5 N); HF/HM (40 Hz, 1 N), LF/LM (2 Hz, 10 N), and LF/HM (2 Hz, 20 N). Both HF/LM and LF/HM effectively improved the BF and BIC at the cortical level. However, BIC at the medullar level was positively influenced only in the case of HF-LM loading. 272 Such HF/LM-mediated improvement could attribute to changes in the interstitial fluid flow velocity, which promotes endochondral ossification, cell proliferation, migration, and ECM synthesis. 273 The relationship between the peri-implant bone, osseointegration, and mechanical loading can be found in the animal model and clinical research. 274 Nevertheless, due to the limit of the device access and ethical issues, experimental mechanical loadings with specific types, magnitudes, and frequencies are hard to be conducted on the clinical implant. On the contrary, there is no difference in the success rate between immediate loading (immediate occlusal vs non-occlusal loading) and conventional loading (3–6 months delay).274,275 Occlusal loading does not lead to improved implant osteointegration. On the other hand, immediate loading seems harmful (2.7 times more risk compared with delayed loading) to the survival of implants 1 year after surgery. 276 According to the studies of mechanical loading with a delay (matrix deposition or bone remodeling phases), more detailed comparative clinical studies are needed to clarify if delayed loading can benefit bone healing around the implant. In strategies to improve fracture healing or implant osseointegration using mechanical stimulation, the priority is maintaining primary stability (bone-implant interface or between fracture fragments). On this basis, high-frequency and low-level loading with resting intervals can be employed to stimulate the osteogenic response of the callus and avoid adverse movement of the trauma site. Since the existing studies are based on different animals and mechanical models, it is difficult to unify the parameters and models of mechanical loading, which is detrimental to forming a theoretical consensus on the biological response of bone to mechanical loading. Finite element analysis and mathematical modeling based on big data help to obtain more uniform mechanical parameters. In addition, additional systemic factors should be considered, especially in elderly patients with reduced mental performance and coordination. These patients require early mobilization but are often unable to avoid uncontrolled full weight bearing and thus may experience adverse interfragmentary motion. The porotic bone of the elderly will also increase the shear movement (Table 1).
Table 1.
The summary of in vivo studies analyzed in this review on the impacts of mechanical loading on fracture healing/regeneration.
| Mechanical loading model and parameters | Main findings | Immediate /delay loading post-trauma |
|---|---|---|
| Fracture healing | ||
| Clinical trial with axial sliding model (82 human subjects). 1st stage: axial displacement (1 mm, 0.5 hz, 20 min/day); 2nd stage: axial movement allowed over the level of 12 kg. | Both clinical healing and mechanical healing were enhanced in the group subjected to axial micromovement, compared to the control group in a fixed mode | Delay (0–7 days) 251 |
| Clinical trial with diaphyseal tibial shaft fractures (3 volunteers, 2 oblique fractures and 1 transverse fracture). 1st stage: 15 kg loading between week 2 and 8; 2nd stage: full body weight loading. | No difference between transverse fractures and oblique fractures. The shear movement induced by 15 kg loading is shown to be compatible with successful healing | Delay (2 weeks) 254 |
| Sheep fix-sliding model with diaphyseal osteotomy (24 subjects). Nonuniform cyclic tensile strains (0.2 and 0.8 mm displacement, 1,5 and 10 Hz for 500 cycles/day) | The external stimulation applied in this study did not significantly enhance the fracture healing process. However, 0.5 mm/10 Hz stimulation induced the highest periosteal callus area. | Delay (7 days) 252 |
| Sheep model with diaphyseal osteotomy (24 subjects). Axial movement: 1.5 mm displacement with preloaded spring with a force of 40 N; Shear movement: allowing 1.5 mm sliding between distal and proximal bone segment, with 2% rotational slackness. | Shear movement considerably delayed the fracture healing, with only 60% bridging of osteotomy fragments in the Shear group, whereas 100% in the Axial group. Peripheral callus formation in the Shear group also reduced to 64% compared to the Axial group. | Immediate 253 |
| Rabbit fix-sliding model with diaphyseal osteotomy (64 subjects). Rabbits in the Axial and Shear group were subjected to self-body-weight induced compression, with different directions restrained by the fix-sliding devices. | The shear movement led to superior healing 4 weeks after fracture but inferior outcomes 2 weeks after fracture compared to axial interfragmentary movement. | Immediate 255 |
| Mouse model with tibia osteotomy followed by intramedullary nailing (80 subjects). Cyclic compression loading (0.5, 1 or 2 N; 1 Hz for 100 cycles/day, 5 days/week for 2 weeks) | Compared to the immediate loading model, the low magnitude (0.5 N, 1 Hz for 100 cycles/day, 5 days/week for 2 weeks) axial cyclic compression with a short delay (4 days delay) significantly improved fracture healing with increased callus strength. Such improvement diminished with increased loading amplitudes (0.5–2 N). | Immediate and delayed (4 days) 258 |
| Mouse model with femur osteotomy (20 subjects). Cyclic strain (8–16 N, 10 Hz, 3000 cycles, 3 times/week for 4 weeks) | Cyclic strain applied on the mouse fracture model led to significantly higher callus formation and mineralization within the remodeling phase, which is associated with Wnt signaling activation and reduced distribution of sclerostin and RANKL in fracture callus. | Delayed (3 weeks) 259 |
| Human tibia bone surrogates model with transverse osteotomy*. Dynamic compression loading (150/200 N−1 Hz for 5 h) | Dynamic loading increased the transport of bone cells (280% for chondrocytes and 180% for osteoblasts) and growth factors (220% for chondrogenic growth factors and 120% for osteogenic growth factors) in the callus compared to the free diffusion. A moderate transport improvement was observed for the MSCs (22%) and fibroblasts (17%). | Simulated loading 260 |
| Sheep metatarsus fracture model*. Cyclic compression loading (0.02 mm displacement of amplitude; 1, 50, and 100 Hz for 15 min) | Mechanical loading with low amplitude and high frequency (0.02 mm displacement, 50 and 100 Hz) significantly improved the osteogenic activity of the callus. Interstitial fluid flow velocity was the only mechanical variable undergoing a significant increase in amplitude and peak value when the frequency of the external stimulus increased. | Simulated loading 261 |
| Sheep fracture model*. HF/LM Vibration (1% displacement, 90 Hz for 5 min, 2 times/day) | HF/LM-mediated improvement of bone formation could attribute to an increase in the interstitial fluid flow velocity, which promotes endochondral ossification, cell proliferation, migration, and ECM synthesis | Simulated loading 273 |
| Defect healing, bone loss regeneration, and implant healing | ||
| Mouse model with critical-sized tibia defect. The defective limb was subjected to daily loading of 5 N peak load, 2 Hz, 60 cycles for 4 consecutive days. | Loading during the inflammatory phase (post-surgery day, PSD 2–5) delayed hematoma clearance and bone matrix deposition and stimulated cellular proliferation and osteoclast activity. Loading during the matrix deposition phase (PSD 5–8) stimulated cellular proliferation and promoted cartilage and bone formation. Finally, loading during the remodeling phase (PSD 10–13) stimulated cellular proliferation and prolonged the remodeling phase. | Delayed (1 day) 256 |
| Rat model (156 subjects) with anterior cruciate ligament reconstruction. Cyclic axial loading (0.05 Hz, 30 g with 2.2% graft elongation tensile). | Delayed application of cyclic axial loading after anterior cruciate ligament reconstruction resulted in improved mechanical and biological parameters of tendon-to-bone healing, manifested by improved bone formation, less ED1+ inflammatory macrophages, more ED2+ resident macrophages, fewer osteoclasts, and reduced tissue vascularity. | Immediate, early delayed (4 days), and late delayed (10 days) 257 |
| Healthy rat ulna model (29 subjects). Static axial compression (8.5 and 17 N, 10 min/day for day 1–5 and 8–12) and axial dynamic compression (17 N, 2 Hz, 1200 cycles/day for day 1–5 and 8–12) | Static loading could not generate an anabolic bone response, whereas it suppresses skeleton growth. Dynamic loading significantly promotes periosteal and endocortical bone formation compared with static loading. | Immediate 266 |
| Healthy rat ulna model (29). Axial dynamic compression (haversine waveform at 17 N peak value, 360 ×1 cycles/day or 90 ×4 cycles/day, 3 days/week for 16 weeks) | The loaded ulnas showed 5.4% (360 ×1) and 8.6% (90 ×4) greater areal bone mineral density than the control. In addition, bone mineral content was enhanced by 6.9% and 11.7% in the 360 × 1 and 90 ×4 loaded ulnas. | Immediate. 269 |
| Mouse model with type I diabetes-induced bone loss (133 subjects). Cyclic axial compression (1.2–2.4 N, 2 Hz, 120 cycles/day for 3 days) | The combination of mechanical loading and PTHrP-derived peptide overcame the bone loss, fragility, and reduced mechanoresponsiveness caused by diabetes. | Immediate 262 |
| OVX Mouse model with undamaged bone (150 subjects). Compression loading (4 N, 10 Hz, 5 min/d for 2 weeks) was applied on the lumbar spine in the dorsal-ventral direction. | The loaded OVX mice showed a significant increase in the number of osteoblasts and a decrease in the number of osteoclasts via Wnt3a signaling. Spinal loading also elevated the volume of microvascular and VEGF levels. | Immediate (2 weeks after OVX surgery) 263 |
| Rat tibia model with titanium implant placement (77 subjects). Compression loading: HF/LM (40 Hz, 0.5 N); HF/HM (40 Hz, 1 N); LF/LM (2 Hz, 10 N); and LF/HM (2 Hz, 20 N). | Bone fraction and bone-implant-contact rate were increased at the cortical level in response to HF/LM and LF/HM loading. However, BIC at the medullar level was positively influenced only in response to HF-LM loading | Immediate 272 |
| Rabbit tibia model with titanium implants (4 implants/rabbit) placement (10 subjects). Movement loading (20 min daily treadmill running from 0 to 6 weeks) | The test group showed more significant vertical bone growth (1.26 ± 0.48vs 0.32 ± 0.47 mm, p < 0.001), higher ISQ values (11.25 ± 6.10vs 5.80 ± 5.97 p = 0.006), higher BIC (25.14 ± 5.24%vs 18.87 ± 4.45%), and higher bone neoformation (280.50 ± 125.40 mm2 vs. 228.00 ± 141.40 mm2, p = 0.121). | Immediate (with 2 weeks progressive adaption phase) 271 |
Finite elemental analysis and mathematical modeling.
Mechanoresponses of bone regeneration in vitro
Mechanical load is a type of physical signaling that can impact the host cell functions, including proliferation, migration, matrix orientation, and enzyme secretion. 277 Applying external mechanical stimulation to bone tissue engineering (BTE) could improve bone tissue development.278,279 Bioreactors have been reported to provide mechanical stimuli, such as fluid flow, to allow nutrient migration to cells, thereby increasing cell viability and promoting bone regeneration (Figure 5).267,268,280 Different bioreactors (such as rotating wall vessel reactor, pinner flask, and flow perfusion) have been introduced for applying mechanical stimuli.267,268 A previous study compared the impact of static and bioreactor cultures on scaffolds-based polycaprolactone-tricalcium phosphate (PCL-TCP) seeded with human fetal mesenchymal stem cells (hfMSC). Compared to the static culture environment, the biaxial rotating bioreactor considerably enhanced the osteogenic differentiation and proliferation of hfMSC. 267 Liu et al. provided a 3D-culture system for human bone mesenchymal stromal cells (hBMSC) encapsulated in a scaffold-based polyurethane. The results indicated that both differentiation and proliferation of hBMSC were improved under the exertion of on-off cyclic mechanical compressions (10% strain) and perfusion (10 ml/min) for about 2 weeks of culture. 281 Another study reported that cyclic mechanical stimuli improved the osteogenic differentiation of MSCs in demineralized bone scaffolds. 282 Ignatius et al. investigated the effects of cyclic strain on human fetal osteoblasts (hFOB 1. 19). The results showed that uniaxial mechanical strain (1%, 1 Hz, 1800 cycles/day for 3 weeks) promoted the proliferation, differentiation, and osteogenic gene expression on osteoblasts. 283 In addition, continuous compression (0–10.0 g/cm2 for 48 h) stimulated the OPG production of mouse osteoblasts (MC3T3-E1) via a non-canonical Wnt/Ca2+ pathway. The enriched OPG inhibited osteoclastogenesis by blocking the RANK/RANKL interaction. 278 van Eijk et al. also evaluated the effect of the timing of mechanical stimuli on the proliferation and differentiation of BMSCs cultured on braided poly (lactic-co-glycolic acid) (PLGA) scaffolds. The application of loading during cell seeding appears to be influential in the differentiation and proliferation as opposed to applying loading immediately after cell seeding or with a delay. 284 A previous study reviewed the impact of mechanical loading on hMSCs and focused on BTE challenges. 285 This study reviewed four types of mechanical loads, including compression, perfusion, vibration, and stretching. Various mechanical loadings can induce osteogenesis in hMSCs via different or similar metabolic routes. For instance, dynamic compression might activate calcium signaling that upregulates the phosphorylation of ERK1/2, therefore, enhancing the FOSB expression in hMSCs. 286 C-Jun protein and FOSB protein then can form a complex known activator protein-1 (AP-1) that readily links to DNA, causing an enhancement in the transcription rates of RUNX2 and other osteogenic genes. 285 In addition to the conventional osteogenic functions, mechanical loading also leads to epigenetic changes in bone cells, which is central to cellular differentiation and stem cell lineage commitment. FSS (rocking platform at 0.5 Hz, 1.5 cm amplitude for 24 h) was reported to suppress DNA methylation for late-stage osteogenic markers (OPN) in mouse osteocytes (MLO-Y4) and MSCs, increasing gene availability for expression.287,288
Figure 5.
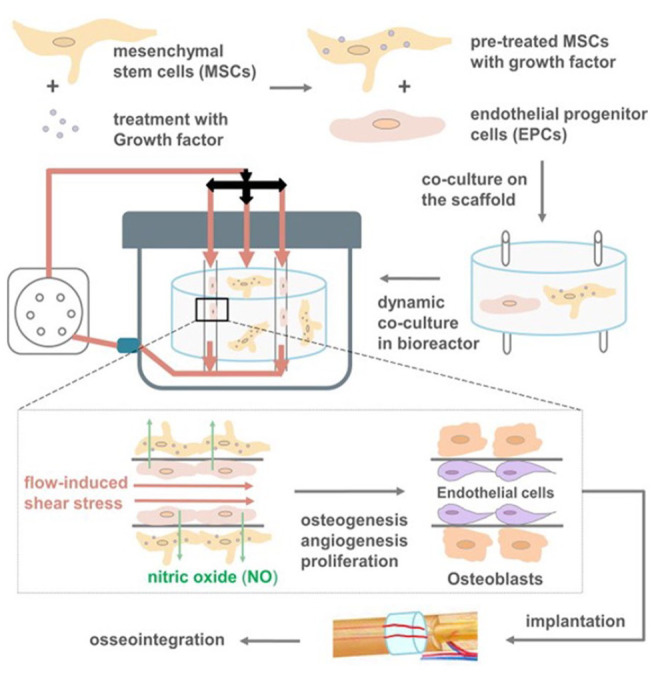
The role of the bioreactor in vascular network progression. Figure reused with permission from Mokhtari-Jafari et al. 289 according to license agreement 5271260413964. © 2020 Elsevier Ltd. All rights reserved.
Several recent studies have reported the improved osteogenic functions of osteoblasts and MSCs in response to mechanical loading (Table 2). These studies based on stretching or compression models have non-negligible limits. As mentioned above, bone tissue deformation during everyday locomotion and physical exercises ranges from 0.04% to 0.3%, with a rare occurrence exceeding 0.1%. 99 In vitro studies reveal that the deformation required for bone cells to react to mechanical stimulation is significantly higher, ranging from 1% to 10%, which is 10 to 100 times greater than that needed for bone tissue, which would result in a fracture when stimulating bone cells in natural bone.100,101 Therefore, the deformation model with high magnitude may not be ideal for studying the mechanoresponsiveness of bone cells. Instead, the effects of FFS or low-magnitude deformation on osteocytes could be a potential and promising point to help us understand the mechanism of bone mechanoresponsiveness. As a bony mechanosensor, dendritic osteocytes and lacunar-canalicular systems work together to perceive and amplify subtle deformation or FSS generated by mechanical loading.102,107 This mechanism allows osteocytes to sense micro deformation generated by macroscopic and physiological mechanical loading. 101 Furthermore, given the central regulatory role of osteocytes on osteogenic and osteoclastic functions,57–61 functional changes of osteocytes induced by FSS or deformation may be an ideal model to study bone remodeling in response to mechanical loading. However, how to translate macroscopic mechanical loading into deformation and fluid shear stress received by osteocytes remains unclear. Biosensors with high sensitivity are needed to quantify the pressure and deformation inside the bone matrix. At the same time, it is imperative to establish a unified mathematical model platform based on different mechanical loading parameters and animal models (species, location, and physical properties of bone). Using such platforms, researchers can calculate the loading parameters generated by physiological movement and fierce exercises at the cellular level. Such parameters facilitate establishing an in vitro model of the effect of “real world” mechanical loading on bone cells, which is of great significance for elucidating the mechanism of bone mechanoresponsiveness and establishing a therapeutic mechanical loading strategy that promotes anabolic bone remodeling.
Table 2.
The summary of recent in vitro studies on the impacts of three types of mechanical loading on bone cells. 290 .
| Amount of load/Type of system used | Result | Publish year | Reference |
|---|---|---|---|
| Compressive Pressure* | |||
| 490.33 Pa (5.0 g/cm2) | No considerable impact was found on the viability of MC3T3-E1 cells | 2017 | Shen et al. 291 |
| 196.13–392.26 Pa (2.0 g/cm2–4.0 g/cm2) | The differentiation of osteoblast was increased with increasing compressive stress | 2013 | Tripuwabhrut et al. 292 |
| 98.07 Pa (1.0 g/cm2) | It is considered an optimal parameter for the osteoblastic differentiation | 2008 | Yanagisawa et al. 293 |
| Tensile Strain & | |||
| Cyclic stretching (5% strain at a frequency of 1.0 Hz for up to 12 h by the FX-4000 Flexcell) was exerted on macrophages. The stretched macrophages have been co-cultured with BMSCs | The expression of RUNX2 and OPN was considerably increased with induced YAP activation and nuclear translocation, which later manipulated the expression of downstream BMP2 to promote BMSCs osteogenesis | 2021 | Dong et al. 294 |
| 10% uniaxial static strain was exerted on osteoblasts by a homemade multiunit cell stretching and compressing device | The osteoblasts’ proliferation and the expression levels of mRNA and protein of alkaline phosphatase (ALP), osteocalcin (OCN), Runt-related transcription factor 2 (Runx2), collagen type I, hypoxia-inducible factor 1-alpha (HIF-1α), and vascular endothelial growth factor (VEGF) were considerably more than in the non-stretch control categories | 2020 | Li et al. 42 |
| Flexcell tension system in vitro for applying mechanical stretching of human jaw bone marrow MSCs | Considerably increased calcium deposition and ALP activity. The expression levels of Runx2 and osterix were markedly upregulated, while NF- kB was downregulated | 2018 | Chen et al. 295 |
| Fluid Shear Stress # | |||
| 1.2 Pa (12 dyn/cm2) Fluid shear stress for 30, 60, and 90 min was applied to MC3T3-E1 cells | The expression level of long-coding (RNA) lncRNA taurine
upregulated 1 (TUG1) enhanced in a time-dependent
behavior. LncRNA TUG1 upregulated expression of fibroblast growth factor receptor 1 (FGFR1) by sponging miR34a, which promoted the proliferation of osteoblast and prevented osteoblast apoptosis |
2021 | Wang et al. 296 |
| Laminar flow 1.2 Pa (12 dyn/cm2) was applied on the MC3T3-E1 cells for 1 h after incubation in serum-free medium for 6 h | Downregulated miR-140–5p and promoted the proliferation of osteoblast by activating the VEGFA/ERK5 signaling pathway | 2021 | Wang et al. 297 |
| 1.2 Pa (12 dyn/cm2) | Inducing proliferation of MG-63 cells and up-regulated the expression level of focal adhesion kinase (FAK) % | 2021 | Lei et al. 298 |
| The mouse osteoblast cell line, MC3T3-E1, was cultured in α-minimum essential medium (α-MEM). Fluid shear stress was applied to cells by placing T25 flasks or 6-well culture dishes on a horizontal shaking apparatus fixed in a culture incubator and shaken at 100–120 rpm | Fluid shear stress increased the Piezo1 in MC3T3-E1 cells’ expression, activated the AKT- serine-threonine protein kinase glycogen synthase kinase 3 (GSK3)/β-catenin pathway and regulated the expression level of RUNX-2 | 2020 | Song et al. 299 |
| Mouse osteoblasts (MC3T3-E1) were exposed to the pulsating fluid flow (peak shear stress rate: 6.5 Pa/s, amplitude: 1.0 Pa, frequency: 1 Hz) for 1 h | FSS induced changes in the arrangement of F-actin in one direction, causing them to be uniform and more compact, and enhanced the expression level of phospho-paxillin and integrin α5 | 2020 | Jin et al. 300 |
| FSS at 2 Pa (20 dyn/cm2) | Inducing a 126% enhancement in hMSC proliferation over static controls | 2006 | Riddle et al. 301 |
| Mouse osteocytes (MLO-Y4) on exposed to FSS (rocking platform) at 0.5 Hz with an amplitude of 1.5 cm for 24 h | With FSS stimulation, the DNA methylation decreased for osteogenic markers, facilitating the availability of genes for expression. | 2015 | Chen et al. 287 |
The results showed that continuous compression positively impacts the differentiation of MC3T3-E1 cells and osteoblasts. However, the exact dose of continuous compression for MC3T3-E1 cells and specific impacts are currently unclear. 290
Different kinds of stress can create relative strain when exerted on an object. The ratio of the shape, length, and volume of the object’s variation before and after the action of tensile stress (single/bidirectional tensile stress) is known as tensile strain. 302
Fluid shear stress is mechanical stress from the extracellular fluid, like tissue fluid, flowing in the cell membrane surface. 303 Applying loads (including muscle contractions, blood pressure, mechanical loads, and lymphatic drainage) to the bones leads the interstitial fluid to flow, compressing the lacunar-canalicular system and inducing different mechanical stimuli like fluid shear stress.100,111,304
FAK may play an essential role in the mechanical leading of the implant-bone interface.
The effect of mechanical loads on vascularization
Why vascularization and angiogenesis are important?
One of the major challenges in bone tissue engineering (BTE) is achieving successful and sufficient vascularization after implantation, which is crucial for providing the necessary nutrients to support cell growth within the scaffolds. 305 Host tissue can provide blood vessels and nutrients to aid healing after implanting scaffolds. In addition, biomaterials and composites can create an environment that facilitates the release of specific biochemical cues after implantation, which targets wound healing. These signals initiate blood vessel ingrowth. Consequently, more blood vessels are directed into the injured tissue. 8
Blood vessels are produced through two biological processes, which are crucial to osteoporosis. Hemangioblasts are mesodermal cells that migrate to a specific location during early development and assemble to create the primary vessels in angiogenesis. 306 Most of the newly formed blood vessels sprout through angiogenesis, accompanied by the growth of the current vascular networks through several processes such as endothelial cell migration, sprouting vessel pruning, and anastomosis.307,308 Endochondral and intramembranous ossification are separate processes through which bones are formed. Osteoblasts that can form bone must be present, and bone growth must be accompanied by neovascularization. Angiogenesis-osteogenesis coupling describes how bone production happens in a spatial and temporal link with the vascularization of the ossifying tissue.309–311 Throughout the angiogenesis process, endothelial cells (ECs) develop, migrate, form tubes, and eventually create conduits where blood flows and supplies the essential nutrients, growth factors, hormones, and oxygen for the bone cells. Also, blood vessels deliver the hematopoietic precursors of osteoclasts to the site of bone resorption and cartilage to eliminate the consequences of ECM degradation. Additionally, the subendothelial walls of vessels include pericytes, which seem crucial in the linkage between osteogenesis and angiogenesis.312,313 Blood vessel formation in osteoporosis occurs through two critical biological processes. Hemangioblasts, mesodermal cells, assemble at specific locations during early development and create primary vessels in angiogenesis. Most blood vessels are formed through angiogenesis, which involves the growth of current vascular networks through endothelial cell migration, sprouting, vessel pruning, and anastomosis. Bones are formed through endochondral and intramembranous ossification, which require the presence of osteoblasts and neovascularization. The coupling of angiogenesis and osteogenesis describes the spatial and temporal link between bone formation and vascularization of the ossifying tissue. Throughout angiogenesis, endothelial cells develop, migrate, form tubes, and create conduits for blood flow, delivering essential nutrients, growth factors, hormones, and oxygen to bone cells. Additionally, blood vessels transport hematopoietic precursors of osteoclasts to sites of bone resorption and cartilage to remove the consequences of extracellular matrix degradation. The subendothelial walls of vessels contain pericytes, which play a crucial role in the linkage between osteogenesis and angiogenesis.
Angiogenesis is necessary for bone growth and development, bone health maintenance, and post-fracture repair.314,315 For instance, a previous study discovered that the blood supply in individuals with osteopenia or osteoporosis is significantly lower than that in individuals with healthy bone mass, demonstrating a strong correlation between bone density and blood supply.316,317 Moreover, it has been reported that endothelial Notch signaling encourages osteogenesis and angiogenesis in the bone microenvironment. It was demonstrated by the presence of 5-ethynyl-2′-deoxyuridine (EdU) labeled vascular ECs in the region where long bones in mice grew rapidly. 318 Hence, promoting angiogenesis and vascularization can benefit bone formation/remodeling. The vasculogenesis stage is completed with primary vascular plexus formation. All transformations of the vascular net proceed within angiogenesis when new vessels are created from existing ones. At the angiogenesis stage, the initial vascular plexus considerably expands through capillary branching and changes into a highly organized vascular network. 319 Angiogenesis starts from the local elimination of the wall of the pre-existing blood vessel as well as the activation of ECs proliferation and migration. ECs are recruited in tubular structures around which the blood vessel walls are created. During further maturation of the vascular network, capillaries fuse into larger vessels, veins, and arteries. 319 The capillaries walls and fine vessels include a single layer of cells (pericytes), while walls of arteries and veins are formed by various smooth muscle cell layers. 320 There are two key cell types in vessels: mural cells and endothelial cells. Therefore, it is prominent to understand the mechanism of angiogenesis to characterize which processes regulate the bioactivity of these cells and their interaction. 319 In flat bones, bone thickness affects microvasculature’s patterning significantly. Thinner regions (less than 0.4 mm) possess only dural networks and periosteal, with larger vessels connecting the two sides of bone, lacking an actual vascular network. 321 However, thicker and flat bones contain a microvascular network similar to long bones.321,322 Blood vessels of various regions in bone include distinct structures. 322 Due to the close relationship between osteogenesis and angiogenesis, angiogenic growth factors are involved in endochondral ossification and neovascularization, making them prominent therapeutic targets for bone regeneration. For example, VEGF, a key angiogenic growth factor associated with bone healing, has a pivotal role in bone repair by promoting angiogenesis and stimulating significant skeletal cell populations, osteoblasts, chondrocytes, and osteoclasts.323,324
How mechanical loading regulates vascularization
Previous research has demonstrated that mechanical loading increased osteogenic and angiogenic responses in bone. Matsuzaki et al. reported that skeletal fatigue cyclic compression (18.7 N, 2 Hz, 1650–5287 cycles) increased periosteal vascularity and regional bone area, with the coordination of angiogenesis and osteogenesis. 325 Additionally, mechanical loading applies compressive forces to specific bone areas, allowing interstitial bone fluid to migrate from a high fluid-pressure area to a low fluid-pressure area, which promotes osteogenesis and inhibits the development of osteoclasts. 326 The increased intramedullary fluid pressure enhances transcortical fluid flow, generating fluid shear stresses on bone cells and activating mechanoresponses. Since lower extremities exercises (done while standing upright) were more influential in bone mass augmenting than the same exercise in done-supine, 327 it is supposed that gradients of fluid pressure affect bone remodeling. 327 Frangos et al. reported that enhanced vascularization was associated with the increased interstitial fluid flow because of the leaky nature of capillaries. 329 , 331 Vascularization can change osteoblastic functions by releasing endothelial-derived factors like endothelin and NO. Endothelin enhances DNA synthesis and inhibits alkaline phosphatase activity in osteoblasts, which may reflect an enhancement in the proliferation of osteoblasts.328,329 Endothelin also inhibits bone resorption and osteoclast margin ruffling (Q effect).329,330 On the other hand, NO released from endothelial cells also suppresses bone resorption by disturbing the osteoclast spreading. On exposure to NO, osteoclasts undergo significant retraction without the action of margin ruffling.329,330 Notably, the release of NO and endothelin in cultured ECs can be regulated by FSS via protein-kinase-C (PKC) and cGMP pathways.329,331 Another case study also found that delayed mechanical loading (body weight loading by daily activity, lasting 3 weeks, 4 weeks delay post-trauma) promoted critical-sized (8 mm displacement) fracture healing (20% more bone formation) and stimulated vascular remodeling in a rat model by increasing the number of large vessels while decreasing the number of small vessels. Whereas early mechanical loading inhibited vascular invasion into the defect (66% less) and reduced bone formation (75% less) compared to the non-loading control. 332 Therefore, Vascular network remodeling and its coupling effect on bone regeneration are highly time-dependent in response to mechanical loading. 332 Mechanical loads can affect some factors, including nephronectin (NPNT), VEGF, HIF-1, epidermal growth factor-like domain (EGFL), and Notch ligands. Such factors can regulate the differentiation and proliferation of ECs, encourage bone vascularization, and improve angiogenic and osteogenic coupling in the local bone microenvironment.333,334 Some investigations focused on how mechanical loading affects ECs and their angiogenic ability in vitro. It has been demonstrated that specific forces, such as hemodynamics forces (shear stress and cyclic strain generated by the blood flow), manipulate the commencement and development of angiogenesis, along with the function of ECs.277,280,335 For instance, Li and Sumpio reported that the proliferation of bovine aortic endothelial cells (BAECs) was enhanced by cyclic strain (10% strain, 1 Hz for ⩽24 h). 280 Another investigation showed an enhancement in the migration (1.83 ± 0.1 folds) and tube formation of BAECs in response to cyclic strain (5% strain, 1 Hz for 24 h). Such enhancement can be weakened or abolished with the treatment of Pertussis toxin (a Gi-protein inhibitor), cRGD peptide (an integrin blocker), and siRNA silencing of MMP9 and urokinase-type plasminogen activator (uPA). 335 Furthermore, Iba and Sumpio observed that cyclic strain regulated the ECs elongation by reorganizing the actin filaments network. 336 A comparative study reported that the type of mechanical loading determined the response of mechanoreceptors in BAECs. In this study, ERK1 and ERK2 were activated 2- and 1.6-fold at 30 min by cyclic strain, whereas they were activated 11.7- and 14.4-fold at 5 min by FSS. FSS leads to more robust and rapid activation of ERK and p38 compared with cyclic strain. 337
Although previous studies have clarified the positive effects of mechanical loading on osteogenesis and angiogenesis, few studies have focused on the mutual regulation and crosstalk of osteogenesis and angiogenesis under mechanical loading. Cheung et al. reported that mouse osteocytes (MLO-Y4) exposed to the physiologic fluid flow (1.0 Pa) were preserved from TNF-α mediated apoptosis. 338 However, the absence of fluid flow led to the prevalence of osteocyte apoptosis, resulting in the release of VEGF.338,339 Apoptotic bone cells fail to inhibit osteoclast activation,58,59,61 resulting in the colocalization of bone resorption and angiogenesis promoted by VEGF. 338 This study clarifies the relationship between inflammatory environment-mediated bone resorption and vascularization and provides substantial experimental evidence for mechanical loading against bone resorption but with certain shortcomings. TNFα may directly affect VEGF release, based on cell line and phenotype,340,341 and other inducers of apoptosis should be tested (especially mediators associated with unloading-associated bone resorption). Mechanical compression loading can activate human umbilical vein endothelial cells (HUVECs) on the demineralized bone scaffold embedded with alginate microspheres through increased VEGF release. VEGF and mechanical loading synergically activate HUVECs with elevated expression of MMP-2/9 and Flk-1 (a VEGF receptor and cytoskeletal component participating in mechanotransduction), thereby improving angiogenesis in vivo. 15 Claes and Meyers hypothesized the relationship between interfragmentary movement direction and vascularization in fracture healing. Cycle compressive strain resulted in more vessel formation than the shearing or tensile strain. 342
Although mechanical loading has been proven to improve angiogenesis, how to make the vascular network structure more conducive to bone formation through mechanical loading is still inconclusive. Certainly, the overabundance of the capillary network impairs bone formation. In contrast, the hierarchical structure of large blood vessels-small blood vessels-capillaries closer to healthy tissues is more beneficial for nutrient transport and waste exchange. In addition, due to the elasticity of blood vessels, whether the vascular deformation generated by blood flow can be employed to activate the mechanoresponsiveness of bone cells inside the bone defects deserves further study.
Conclusions
Mechanical stimulation is essential for bone regeneration as it affects the biological functions of bone cells and endothelial cells. This review provided an overview of the basic structure of bone and the biological structures and functions related to mechanical loading, focusing mainly on the regulatory effects of mechanical loading on bone fracture/regeneration. The mechanosensation and mechanotransduction-related molecular structures and signaling pathways were analyzed in detail. Current in vitro and in vivo models used for research on the effects of mechanical loading on bone healing/regeneration were collected and compared. In addition, we briefly reviewed the role of mechanical loading in angiogenesis during tissue healing. Overall, the hierarchical structure of bone matrix can convert mechanical loading into deformation or fluid shear with different magnitudes and types, stimulating bone cells (osteocytes, as the primary bone remodeling regulator) and regulating following bone remodeling via skewing the balance of osteogenic/osteolytic functions. The signaling pathways related to mechanotransduction do not act independently but synergistically with the overall health status, hormone levels, and cytokine patterns in local tissue. Therefore, enhancing the activity of target molecules related to mechanosensation and mechanotransduction (such as integrins, FAK, Cx43, TAP/TAZ., etc.) may improve or restore the mechanosensitivity of bone cells under specific conditions, leading to ideal bone gain/maintenance.
In the study of mechanical loading-mediated bone fracture healing and regeneration, the molecular pathways and targets identified in vitro cannot be fully validated in animal models due to the unspecificity of cell lineage with conditional gene knockout in vivo. Moreover, the current in vivo and in vitro models are remarkably distinct, requiring an excellent mathematical model to convert the mechanical parameters between them. Only in this way can the parameters be standardized for future research, which also applies to the study of mechanical loading-mediated angiogenesis. Furthermore, the current research on osteogenesis and angiogenesis induced by mechanical loading is primarily based on coupled experiments. Therefore, from a logistical perspective, it is crucial to establish experimental models in which mechanical loading modulates osteogenesis through angiogenesis and vice versa, which helps to clarify the primary and secondary relationships between angiogenesis and osteogenesis.
In conclusion, although mechanical loading is considered a promising strategy for bone repair and regeneration, more studies are needed to elucidate their roles, limitations, shortcomings, and challenges for future research and application. The focus of research in this field is to match the models and parameters of in vivo and in vitro studies, screen out highly sensitive molecular targets that can improve the mechanoresponsiveness of bone cells, and form therapeutic strategies that are truly clinically applicable.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge financial support from the Baltic Research Programme Project No. EEA-RESEARCH-85 “Waste-to-resource: eggshells as a source for next generation biomaterials for bone regeneration (EGGSHELL)” under the EEA Grant of Iceland, Liechtenstein and Norway No. EEZ/BPP/VIAA/2021/1 and National Natural Science Foundation of China (No. 81971752). The authors acknowledge the access to the infrastructure and expertise of the BBCE – Baltic Biomaterials Centre of Excellence (European Union’s Horizon 2020 research and innovation programme under grant agreement No. 857287).
ORCID iDs: Qianli Ma  https://orcid.org/0000-0002-7044-8172
https://orcid.org/0000-0002-7044-8172
Håvard Jostein Haugen  https://orcid.org/0000-0002-6690-7233
https://orcid.org/0000-0002-6690-7233
References
- 1.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 2006; 8: 455–498. [DOI] [PubMed] [Google Scholar]
- 2.Datta HK, Ng WF, Walker JA, et al. The cell biology of bone metabolism. J Clin Pathol 2008; 61(5): 577–587. [DOI] [PubMed] [Google Scholar]
- 3.Glatt V, Evans CH, Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front Physiol 2017; 7: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinkamer R, Eberl C, Fratzl P. Mechanoregulation of bone remodeling and healing as inspiration for self-repair in materials. Biomimetics 2019; 4(3): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff J. Concept of the law of bone remodelling. The law of bone remodelling. Berlin, Heidelberg: Springer, 1986. pp.1. [Google Scholar]
- 6.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab 2000; 18(6): 305–316. [DOI] [PubMed] [Google Scholar]
- 7.Rahmati M, Silva EA, Reseland JE, et al. Biological responses to physicochemical properties of biomaterial surface. Chem Soc Rev 2020; 49(15): 5178–5224. [DOI] [PubMed] [Google Scholar]
- 8.Ishaug-Riley SL, Crane GM, Gurlek A, et al. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res 1997; 36(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Haffner-Luntzer M, Liedert A, Ignatius A. Mechanobiology of bone remodeling and fracture healing in the aged organism. Innov Surg Sci 2016; 1(2): 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augat P, Simon U, Liedert A, et al. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int 2005; 16(Suppl 2): S36–S43. [DOI] [PubMed] [Google Scholar]
- 11.Kadow-Romacker A, Hoffmann JE, Duda G, et al. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs 2009; 190(2): 61–68. [DOI] [PubMed] [Google Scholar]
- 12.NeidlingerWilke C, Stalla I, Claes L, et al. Human osteoblasts from younger normal and osteoporotic donors show differences in proliferation and TGFβ-release in response to cyclic strain. J Biomech 1995; 28(12): 1411–1418. [DOI] [PubMed] [Google Scholar]
- 13.Watabe H, Furuhama T, Tani-Ishii N, et al. Mechanotransduction activates α₅β₁ integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell Res 2011; 317(18): 2642–2649. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB J 2010; 24(10): 3625–3632. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Hou T, Zhao J, et al. Vascular endothelial growth factor release from alginate microspheres under simulated physiological compressive loading and the effect on human vascular endothelial cells. Tissue Eng Part A 2011; 17(13-14): 1777–1785. [DOI] [PubMed] [Google Scholar]
- 16.Bilgiç E, Boyacıoğlu Gizer M, et al. Chapter 6 - architecture of bone tissue and its adaptation to pathological conditions. In: Angin S, Şimşek IE. (eds) Comparative kinesiology of the human body. Cambridge: Academic Press, 2020, pp.71–90. [Google Scholar]
- 17.Boskey AL, Spevak L, Paschalis E, et al. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 2002; 71(2): 145–154. [DOI] [PubMed] [Google Scholar]
- 18.Morgan E, Barnes G, Einhorn T. The bone organ system: form and function. In: Marcus R, Feldman D, Nelson D, et al. (eds) Osteoporosis. Amsterdam: Elsevier, 2008. [Google Scholar]
- 19.Aszódi A, Bateman JF, Gustafsson E, et al. Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice? Cell Struct Funct 2000; 25(2): 73–84. [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter JA, Glimcher MJ, Cooper RR, et al. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect 1996; 45: 371–386. [PubMed] [Google Scholar]
- 21.Downey PA, Siegel MI. Bone biology and the clinical implications for osteoporosis. Phys Ther 2006; 86(1): 77–91. [DOI] [PubMed] [Google Scholar]
- 22.Green J, Schotland S, Stauber DJ, et al. Cell-matrix interaction in bone: type I collagen modulates signal transduction in osteoblast-like cells. Am J Physiol 1995; 268(5 Pt 1): C1090–C1103. [DOI] [PubMed] [Google Scholar]
- 23.Ma C, Du T, Niu X, et al. Biomechanics and mechanobiology of the bone matrix. Bone Res 2022; 10(1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce MR, Burr DB, Sharkey NA, et al. Skeletal tissue mechanics. Skeletal tissue mechanics. 2nd ed.New York; Heidelberg; Dordrecht; London: Springer, 2015. pp.142. [Google Scholar]
- 25.Damoulis PD, Hauschka PV. Nitric oxide acts in conjunction with proinflammatory cytokines to promote cell death in osteoblasts. J Bone Miner Res 1997; 12(3): 412–422. [DOI] [PubMed] [Google Scholar]
- 26.Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: Games without frontiers. Arch Biochem Biophys 2014; 561: 3–12. [DOI] [PubMed] [Google Scholar]
- 27.Astakhova NM, Korel AV, Shchelkunova EI, et al. Analysis of the basic characteristics of osteogenic and chondrogenic cell lines important for tissue engineering implants. Bull Exp Biol Med 2018; 164(4): 561–568. [DOI] [PubMed] [Google Scholar]
- 28.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol 1988; 106(6): 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997; 89(5): 755–764. [DOI] [PubMed] [Google Scholar]
- 30.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997; 89(5): 747–754. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 2002; 108(1): 17–29. [DOI] [PubMed] [Google Scholar]
- 32.Hu H, Hilton MJ, Tu X, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005; 132(1): 49–60. [DOI] [PubMed] [Google Scholar]
- 33.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep 2003; 5(3): 222–226. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiko Y, Candeliere GA, Maeda N, et al. Osteoblast autonomous Pi regulation via pit1 plays a role in bone mineralization. Mol Cell Biol 2007; 27(12): 4465–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florencio-Silva R, Sasso GR, Sasso-Cerri E, et al. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015; 2015: 421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glimcher MJ. The nature of the mineral phase in bone: biological and clinical implications. In: Avioli LV, Krane SM. (eds) Metabolic bone disease and clinically related disorders. Amsterdam: Elsevier, 1998, pp.23–52e. [Google Scholar]
- 37.Boivin G, Meunier PJ. The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif Tissue Int 2002; 70(6): 503–511. [DOI] [PubMed] [Google Scholar]
- 38.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn 2006; 235(1): 176–190. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S, Zu Y, Sun Z, et al. Effects of hypergravity on osteopontin expression in osteoblasts. PLoS One 2015; 10(6): e0128846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawao N, Morita H, Iemura S, et al. Roles of dkk2 in the linkage from muscle to bone during mechanical unloading in mice. Int J Mol Sci 2020; 21(7): 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxena R, Pan G, Dohm ED, et al. Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J Bone Miner Metab 2011; 29(1): 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Zheng J, Wan D, et al. Uniaxial static strain promotes osteoblast proliferation and bone matrix formation in distraction osteogenesis in vitro. Biomed Res Int 2020; 2020: 3906426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen TL, Sondergaard TE, Skorzynska KE, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 2009; 174(1): 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JL, Kumbar SG, Laurencin CT. Chapter II.6.7 - Bone tissue engineering. In: Ratner BD, Hoffman AS, Schoen FJ, et al. (eds) Biomaterials science. Cambridge: Academic Press, 2013, pp.1194–1214, 3rd ed. [Google Scholar]
- 45.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006; 12(6): 657–664. [DOI] [PubMed] [Google Scholar]
- 46.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 2010; 11(4): 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SW, Pajevic PD, Selig M, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res 2012; 27(10): 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donahue HJ, McLeod KJ, Rubin CT, et al. Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res 1995; 10(6): 881–889. [DOI] [PubMed] [Google Scholar]
- 49.Bonewald LF. The Amazing Osteocyte. J Bone Miner Res 2011; 26(2): 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ubaidus S, Li M, Sultana S, et al. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc 2009; 58(6): 381–392. [DOI] [PubMed] [Google Scholar]
- 51.Cardoso L, Fritton SP, Gailani G, et al. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J Biomech 2013; 46(2): 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manolagas SC. Choreography from the tomb: an emerging role of dying osteocytes in the purposeful, and perhaps not so purposeful, targeting of bone remodeling. IBMS Bonekey 2006; 3: 5–14. [Google Scholar]
- 53.Civitelli R, Lecanda F, Jørgensen N, et al. Principles of Bone Biology. 2002. [Google Scholar]
- 54.Mullender MG, vanderMeer DD, Huiskes R, et al. Osteocyte density changes in aging and osteoporosis. Bone 1996; 18(2): 109–113. [DOI] [PubMed] [Google Scholar]
- 55.Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 2010; 21(9): 1457–1469. [DOI] [PubMed] [Google Scholar]
- 56.Currey JD. The many adaptations of bone. J Biomech 2003; 36(10): 1487–1495. [DOI] [PubMed] [Google Scholar]
- 57.You L, Temiyasathit S, Lee P, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 2008; 42(1): 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan SD, de Vries TJ, Kuijpers-Jagtman AM, et al. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 2007; 41(5): 745–751. [DOI] [PubMed] [Google Scholar]
- 59.Klein-Nulend J, Bacabac RG, Bakker AD. Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater 2012; 24: 278–291. [DOI] [PubMed] [Google Scholar]
- 60.Santos A, Bakker AD, Zandieh-Doulabi B, et al. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res 2009; 27(10): 1280–1287. [DOI] [PubMed] [Google Scholar]
- 61.Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact 2006; 6(4): 354. [PubMed] [Google Scholar]
- 62.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med 2011; 17(10): 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011; 17(10): 1231–1234. [DOI] [PubMed] [Google Scholar]
- 64.Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 2007; 5(6): 464–475. [DOI] [PubMed] [Google Scholar]
- 65.Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 2010; 30(12): 3071–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kulkarni RN, Bakker AD, Everts V, et al. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int 2010; 87(5): 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyce BF, Hughes DE, Wright KR, et al. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest 1999; 79(2): 83–94. [PubMed] [Google Scholar]
- 68.Crockett JC, Mellis DJ, Scott DI, et al. New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporos Int 2011; 22(1): 1–20. [DOI] [PubMed] [Google Scholar]
- 69.Mancuso ME, Wilzman AR, Murdock KE, et al. Effect of external mechanical stimuli on human bone: a narrative review. Prog Biomed Eng 2022; 4(1): 012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone Res 2013; 1(1): 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiedemann K, Le Nihouannen D, Fong JE, et al. Regulation of osteoclast growth and fusion by mTOR/raptor and mTOR/rictor/Akt. Front Cell Dev Biol 2017; 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo D, Zhang Q, Li J, et al. Fluid shear stress changes cell morphology and regulates the expression of ATP6V1A and TCIRG1 mRNA in rat osteoclasts. Mol Med Rep 2010; 3(1): 173–178. [DOI] [PubMed] [Google Scholar]
- 73.Ma C, Geng B, Zhang X, et al. Fluid shear stress suppresses osteoclast differentiation in RAW264.7 cells through extracellular signal-regulated kinase 5 (ERK5) signaling pathway. Med Sci Monit 2020; 26: e918370–e918371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li P, Bian X, Liu C, et al. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium 2018; 71: 45–52. [DOI] [PubMed] [Google Scholar]
- 75.Bratengeier C, Liszka A, Hoffman J, et al. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. FASEB J 2020; 34(3): 3755–3772. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki N, Yoshimura Y, Deyama Y, et al. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med 2008; 21(3): 291–296. [PubMed] [Google Scholar]
- 77.Kurata K, Uemura T, Nemoto A, et al. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res 2001; 16(4): 722–730. [DOI] [PubMed] [Google Scholar]
- 78.Parfitt AM. Bone remodeling. Henry Ford Hosp Med J 1988; 36(3): 143–144. [PubMed] [Google Scholar]
- 79.Cao X. Targeting osteoclast-osteoblast communication. Nat Med 2011; 17(11): 1344–1346. [DOI] [PubMed] [Google Scholar]
- 80.Mellon SJ, Tanner KE. Bone and its adaptation to mechanical loading: a review. Int Mater Rev 2012; 57(5): 235–255. [Google Scholar]
- 81.Calbet JAL, Moysi JS, Dorado C, et al. Bone mineral content and density in professional tennis players. Calcif Tissue Int 1998; 62(6): 491–496. [DOI] [PubMed] [Google Scholar]
- 82.Amin S. Mechanical factors and bone health: effects of weightlessness and neurologic injury. Curr Rheumatol Rep 2010; 12(3): 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Troy K, Mancuso M, Butler T, et al. Exercise early and often: Effects of physical activity and exercise on women’s bone health. Int J Env Res Pub Health 2018; 15(5): 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science 1978; 201(4361): 1138–1141. [DOI] [PubMed] [Google Scholar]
- 85.Reiner B, Christoph B. Modelling and remodelling of bone. In: Bone disorders. Cham: Springer, 2017, pp.21–30, 1st ed. [Google Scholar]
- 86.Tong X, Chen X, Zhang S, et al. The effect of exercise on the prevention of osteoporosis and bone angiogenesis. Biomed Res Int 2019; 2019: 8171897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen F, OuYang Y, Ye T, et al. Estrogen inhibits RANKL-induced osteoclastic differentiation by increasing the expression of TRPV5 channel. J Cell Biochem 2014; 115(4): 651–658. [DOI] [PubMed] [Google Scholar]
- 88.Hofbauer LC, Kühne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 2004; 4(3): 268–275. [PubMed] [Google Scholar]
- 89.Santos RV, Viana VA, Boscolo RA, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine 2012; 60(3): 731–735. [DOI] [PubMed] [Google Scholar]
- 90.Yuan Y, Chen X, Zhang L, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol 2016; 122(2): 122–130. [DOI] [PubMed] [Google Scholar]
- 91.Leelarungrayub D, Saidee K, Pothongsunun P, et al. Six weeks of aerobic dance exercise improves blood oxidative stress status and increases interleukin-2 in previously sedentary women. J Bodyw Mov Ther 2011; 15(3): 355–362. [DOI] [PubMed] [Google Scholar]
- 92.Mavčič B, Antolič V. Optimal mechanical environment of the healing bone fracture/osteotomy. Int Orthop 2012; 36(4): 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int 2002; 13(9): 688–700. [DOI] [PubMed] [Google Scholar]
- 94.Liedert A, Kaspar D, Augat P, et al. Mechanobiology of bone tissue and bone cells. In: Kamkin A, Kiseleva I. (eds) Mechanosensitivity in cells and tissues. Moscow: Academia Publishing House Ltd, 2005, pp.83–95. [PubMed] [Google Scholar]
- 95.Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts’ and osteocytes’ early responses to mechanical strain. Bone 1996; 19(6): 609–614. [DOI] [PubMed] [Google Scholar]
- 96.Keila S, Kelner A, Weinreb M. Systemic prostaglandin E2 increases cancellous bone formation and mass in aging rats and stimulates their bone marrow osteogenic capacity in vivo and in vitro. J Endocrinol 2001; 168(1): 131–139. [DOI] [PubMed] [Google Scholar]
- 97.Li XJ, Jee WS, Li YL, et al. Transient effects of subcutaneously administered prostaglandin E2 on cancellous and cortical bone in young adult dogs. Bone 1990; 11(5): 353–364. [DOI] [PubMed] [Google Scholar]
- 98.Lyritis GP, Georgoulas T, Zafeiris CP. Bone anabolic versus bone anticatabolic treatment of postmenopausal osteoporosis. Ann N Y Acad Sci 2010; 1205: 277–283. [DOI] [PubMed] [Google Scholar]
- 99.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech 2000; 33(3): 317–325. [DOI] [PubMed] [Google Scholar]
- 100.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 1995; 57(5): 344–358. [DOI] [PubMed] [Google Scholar]
- 101.You L, Cowin SC, Schaffler MB, et al. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech 2001; 34(11): 1375–1386. [DOI] [PubMed] [Google Scholar]
- 102.Han Y, Cowin SC, Schaffler MB, et al. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA 2004; 101(47): 16689–16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Niu X, Fan R, Guo X, et al. Shear-mediated orientational mineralization of bone apatite on collagen fibrils. J Mater Chem B 2017; 5(46): 9141–9147. [DOI] [PubMed] [Google Scholar]
- 104.Du T, Niu X, Hou S, et al. Highly aligned hierarchical intrafibrillar mineralization of collagen induced by periodic fluid shear stress. J Mater Chem B 2020; 8(13): 2562–2572. [DOI] [PubMed] [Google Scholar]
- 105.Shuai C, Yang W, Peng S, et al. Physical stimulations and their osteogenesis-inducing mechanisms. Int J Bioprinting 2018; 4(2): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Veno PA, Nicolella DP, Sivakumar P, et al. Live imaging of osteocytes within their lacunae reveals cell body and dendrite motions. J Bone Miner Res 2006; 21: S38–S9. [Google Scholar]
- 107.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 1994; 27(3): 339–360. [DOI] [PubMed] [Google Scholar]
- 108.Cowin SC, Gailani G, Benalla M. Hierarchical poroelasticity: movement of interstitial fluid between porosity levels in bones. Philos Trans A Math Phys Eng Sci 2009; 367(1902): 3401–3444. [DOI] [PubMed] [Google Scholar]
- 109.Wehrli FW, Fernández-Seara MA. Nuclear magnetic resonance studies of bone water. Ann Biomed Eng 2005; 33(1): 79–86. [DOI] [PubMed] [Google Scholar]
- 110.Gardinier JD, Townend CW, Jen KP, et al. In situ permeability measurement of the mammalian lacunar-canalicular system. Bone 2010; 46(4): 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Price C, Zhou X, Li W, et al. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res 2011; 26(2): 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol 2000; 19(2): 91–96. [DOI] [PubMed] [Google Scholar]
- 113.Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature 1977; 269(5623): 80–82. [DOI] [PubMed] [Google Scholar]
- 114.Hwang MP, Subbiah R, Kim IG, et al. Approximating bone ECM: crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials 2016; 103: 22–32. [DOI] [PubMed] [Google Scholar]
- 115.Das RK, Gocheva V, Hammink R, et al. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater 2016; 15(3): 318–325. [DOI] [PubMed] [Google Scholar]
- 116.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013; 154(5): 1047–1059. [DOI] [PubMed] [Google Scholar]
- 117.Gronthos S, Stewart K, Graves SE, et al. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res 1997; 12(8): 1189–1197. [DOI] [PubMed] [Google Scholar]
- 118.Bennett JH, Carter DH, Alavi AL, et al. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch Oral Biol 2001; 46(3): 229–238. [DOI] [PubMed] [Google Scholar]
- 119.Orr AW, Ginsberg MH, Shattil SJ, et al. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell 2006; 17(11): 4686–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bachmann M, Schäfer M, Mykuliak VV, et al. Induction of ligand promiscuity of αVβ3 integrin by mechanical force. J Cell Sci 2020; 133(9): jcs242404. [DOI] [PubMed] [Google Scholar]
- 121.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005; 6(1): 56–68. [DOI] [PubMed] [Google Scholar]
- 122.Haugh MG, Vaughan TJ, McNamara LM. The role of integrin alpha(V)beta(3) in osteocyte mechanotransduction. J Mech Behav Biomed Mater 2015; 42: 67–75. [DOI] [PubMed] [Google Scholar]
- 123.Liu L, Zong C, Li B, et al. The interaction between β1 integrins and ERK1/2 in osteogenic differentiation of human mesenchymal stem cells under fluid shear stress modelled by a perfusion system. J Tissue Eng Regen Med 2014; 8(2): 85–96. [DOI] [PubMed] [Google Scholar]
- 124.Tucci M, De Palma R, Lombardi L, et al. Beta(3) integrin subunit mediates the bone-resorbing function exerted by cultured myeloma plasma cells. Cancer Res 2009; 69(16): 6738–6746. [DOI] [PubMed] [Google Scholar]
- 125.Zou W, Teitelbaum SL. Absence of Dap12 and the alphavbeta3 integrin causes severe osteopetrosis. J Cell Biol 2015; 208(1): 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phillips JA, Almeida EA, Hill EL, et al. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol 2008; 27(7): 609–618. [DOI] [PubMed] [Google Scholar]
- 127.Ishijima M, Rittling SR, Yamashita T, et al. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med 2001; 193(3): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019; 35(3): 347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Palazzo AF, Eng CH, Schlaepfer DD, et al. Localized stabilization of microtubules by integrin- and FAK-facilitated rho signaling. Science 2004; 303(5659): 836–839. [DOI] [PubMed] [Google Scholar]
- 130.Khatiwala CB, Kim PD, Peyton SR, et al. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res 2009; 24(5): 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamamura K, Swarnkar G, Tanjung N, et al. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res 2012; 53(5): 398–406. [DOI] [PubMed] [Google Scholar]
- 132.Ransom RC, Carter AC, Salhotra A, et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature 2018; 563(7732): 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sato T, Verma S, Andrade CDC, et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat Commun 2020; 11(1): 3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003; 22(23): 6267–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang L, Zhou H, Zhang M, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019; 573(7773): 225–229. [DOI] [PubMed] [Google Scholar]
- 136.Zhou T, Gao B, Fan Y, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-satenin. eLife 2020; 9: e52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morris JA, Kemp JP, Youlten SE, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 2019; 51(2): 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun W, Chi S, Li Y, et al. The mechanosensitive piezo1 channel is required for bone formation. eLife 2019; 8: e47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L, You X, Lotinun S, et al. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun 2020; 11(1): 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X, Han L, Nookaew I, et al. Stimulation of piezo1 by mechanical signals promotes bone anabolism. eLife 2019; 8: e49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woo SH, Ranade S, Weyer AD, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509(7502): 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woo SH, Lukacs V, de Nooij JC, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 2015; 18(12): 1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van der Eerden BC, Oei L, Roschger P, et al. TRPV4 deficiency causes sexual dimorphism in bone metabolism and osteoporotic fracture risk. Bone 2013; 57(2): 443–454. [DOI] [PubMed] [Google Scholar]
- 144.Lee KL, Guevarra MD, Nguyen AM, et al. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 2015; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci 2014; 111(4): 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pochynyuk O, Zaika O, O’Neil RG, et al. Novel insights into TRPV4 function in the kidney. Pflug Arch Eur J Phy 2013; 465(2): 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Corrigan MA, Johnson GP, Stavenschi E, et al. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci Rep 2018; 8(1): 3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu K, Sun H, Gui B, et al. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother 2017; 91: 841–848. [DOI] [PubMed] [Google Scholar]
- 149.Du G, Li L, Zhang X, et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp Biol Med 2020; 245(3): 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Masuyama R, Vriens J, Voets T, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 2008; 8(3): 257–265. [DOI] [PubMed] [Google Scholar]
- 151.Cao B, Dai X, Wang W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca 2+ –calcineurin–nfatc1 pathway. J Cell Physiol 2019; 234(5): 6831–6841. [DOI] [PubMed] [Google Scholar]
- 152.Mirvis M, Stearns T, James Nelson W. Cilium structure, assembly, and disassembly regulated by the cytoskeleton. Biochem J 2018; 475: 2329–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whitfield JF. Primary cilium–is it an osteocyte’s strain-sensing flowmeter? J Cell Biochem 2003; 89(2): 233–237. [DOI] [PubMed] [Google Scholar]
- 154.Ding D, Yang X, Luan HQ, et al. Pharmacological regulation of primary cilium formation affects the mechanosensitivity of osteocytes. Calcif Tissue Int 2020; 107(6): 625–635. [DOI] [PubMed] [Google Scholar]
- 155.Shi W, Zhang Y, Chen K, et al. Primary cilia act as microgravity sensors by depolymerizing microtubules to inhibit osteoblastic differentiation and mineralization. Bone 2020; 136: 115346. [DOI] [PubMed] [Google Scholar]
- 156.Moore ER, Zhu YX, Ryu HS, et al. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res Ther 2018; 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Malone AMD, Anderson CT, Tummala P, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci 2007; 104(33): 13325–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun 2011; 412(1): 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tirado-Cabrera I, Martin-Guerrero E, Heredero-Jimenez S, et al. PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion. J Cell Physiol 2022; 237(10): 3927–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yuan X, Serra RA, Yang S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci 2015; 1335: 78–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 2003; 4(4): 285–294. [DOI] [PubMed] [Google Scholar]
- 162.Riquelme MA, Cardenas ER, Xu H, et al. The role of connexin channels in the response of mechanical loading and unloading of bone. Int J Mol Sci 2020; 21(3): 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheng B, Zhao S, Luo J, et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res 2001; 16(2): 249–259. [DOI] [PubMed] [Google Scholar]
- 164.Burra S, Nicolella DP, Francis WL, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci 2010; 107(31): 13648–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang JX, Cherian PP. Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E-2 by osteocytes in response to mechanical strain. Cell Commun Adhes 2003; 10(4-6): 259–264. [DOI] [PubMed] [Google Scholar]
- 166.Song L. Effects of exercise or mechanical stimulation on bone development and bone repair. Stem Cells Int 2022; 2022: 5372229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ziambaras K, Lecanda F, Steinberg TH, et al. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res 1998; 13(2): 218–228. [DOI] [PubMed] [Google Scholar]
- 168.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone 2003; 33(1): 64–70. [DOI] [PubMed] [Google Scholar]
- 169.Batra N, Burra S, Siller-Jackson AJ, et al. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci USA 2012; 109(9): 3359–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davis HM, Pacheco-Costa R, Atkinson EG, et al. Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell 2017; 16(3): 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chung DJ, Castro CH, Watkins M, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 2006; 119(Pt 20): 4187–4198. [DOI] [PubMed] [Google Scholar]
- 172.Bivi N, Pacheco-Costa R, Brun LR, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res 2013; 31(7): 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grimston SK, Brodt MD, Silva MJ, et al. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res 2008; 23(6): 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One 2011; 6(8): e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lloyd SA, Lewis GS, Zhang Y, et al. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res 2012; 27(11): 2359–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schnitzler MMY, Storch U, Meibers S, et al. G(q)-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 2008; 27(23): 3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci 2006; 103(42): 15463–15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Erdogmus S, Storch U, Danner L, et al. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat Commun 2019; 10: 5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol 2011; 7(6): 362–372. [DOI] [PubMed] [Google Scholar]
- 180.Ishida T, Takahashi M, Corson MA, et al. Fluid shear stress-mediated signal transduction: How do endothelial cells transduce mechanical force into biological responses? Ann N Y Acad Sci 1997; 811: 12–24. [DOI] [PubMed] [Google Scholar]
- 181.Wei WC, Bianchi F, Wang YK, et al. Coincidence detection of membrane stretch and extracellular pH by the Proton-sensing receptor OGR1 (GPR68). Curr Biol 2018; 28(23): 3815–3823.e4. [DOI] [PubMed] [Google Scholar]
- 182.Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol 2004; 287(1): C1–C11. [DOI] [PubMed] [Google Scholar]
- 183.Luu YK, Pessin JE, Judex S, et al. Mechanical signals as a Non-Invasive means to influence mesenchymal stem cell fate, promoting bone and suppressing the fat phenotype. Bonekey Osteovision 2009; 6(4): 132–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res 1995; 10(10): 1544–1549. [DOI] [PubMed] [Google Scholar]
- 185.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 2014; 15(12): 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Robling AG, Warden SJ, L Shultz K, et al. Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res 2007; 22(7): 984–991. [DOI] [PubMed] [Google Scholar]
- 187.Bilgen B, Odabas S. Effects of mechanotransduction on stem cell behavior. In: Tiwari A, Garipcan B, Uzun L. (eds) Advanced surfaces for stem cell research. Hoboken, NJ: John Wiley, 2016, pp.45–65, 1st ed. [Google Scholar]
- 188.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol 2008; 97(2-3): 163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 2003; 116(8): 1397–1408. [DOI] [PubMed] [Google Scholar]
- 190.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 1997; 59: 575–599. [DOI] [PubMed] [Google Scholar]
- 191.Myers KA, Rattner JB, Shrive NG, et al. Hydrostatic pressure sensation in cells: integration into the tensegrity model. Biochem Cell Biol 2007; 85(5): 543–551. [DOI] [PubMed] [Google Scholar]
- 192.Humphrey D, Duggan C, Saha D, et al. Active fluidization of polymer networks through molecular motors. Nature 2002; 416(6879): 413–416. [DOI] [PubMed] [Google Scholar]
- 193.Haviv L, Gillo D, Backouche F, et al. A cytoskeletal demolition worker: Myosin II acts as an actin depolymerization agent. J Mol Biol 2008; 375(2): 325–330. [DOI] [PubMed] [Google Scholar]
- 194.Vicente-Manzanares M, Ma X, Adelstein RS, et al. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10(11): 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yin C, Zhang Y, Hu L, et al. Mechanical unloading reduces microtubule actin crosslinking factor 1 expression to inhibit β-catenin signaling and osteoblast proliferation. J Cell Physiol 2018; 233(7): 5405–5419. [DOI] [PubMed] [Google Scholar]
- 196.Su P, Yin C, Li D, et al. MACF1 promotes preosteoblast migration by mediating focal adhesion turnover through EB1. Biol Open 2020; 9(3): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hu L, Su P, Yin C, et al. Microtubule actin crosslinking factor 1 promotes osteoblast differentiation by promoting beta-catenin/TCF1/Runx2 signaling axis. J Cell Physiol 2018; 233(2): 1574–1584. [DOI] [PubMed] [Google Scholar]
- 198.Bacabac RG, Mizuno D, Schmidt CF, et al. Round versus flat: bone cell morphology, elasticity, and mechanosensing. J Biomech 2008; 41(7): 1590–1598. [DOI] [PubMed] [Google Scholar]
- 199.Vorselen D, Roos WH, MacKintosh FC, et al. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J 2014; 28(2): 536–547. [DOI] [PubMed] [Google Scholar]
- 200.Xu H, Wu F, Zhang H, et al. Actin cytoskeleton mediates BMP2-Smad signaling via calponin 1 in preosteoblast under simulated microgravity. Biochimie 2017; 138: 184–193. [DOI] [PubMed] [Google Scholar]
- 201.Fu Z, Hou Y, Haugen HJ, et al. TiO(2) nanostructured implant surface-mediated M2c polarization of inflammatory monocyte requiring intact cytoskeleton rearrangement. J Nanobiotechnol 2023; 21(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ma QL, Fang L, Jiang N, et al. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterials 2018; 154: 234–247. [DOI] [PubMed] [Google Scholar]
- 203.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene 2012; 503(2): 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70(3): 389–399. [DOI] [PubMed] [Google Scholar]
- 205.Burridge K, Wennerberg K. Rho and rac take center stage. Cell 2004; 116(2): 167–179. [DOI] [PubMed] [Google Scholar]
- 206.Lessey EC, Guilluy C, Burridge K. From mechanical force to RhoA activation. Biochemistry 2012; 51(38): 7420–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arnsdorf EJ, Tummala P, Kwon RY, et al. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 2009; 122(Pt 4): 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Alfieri R, Vassalli M, Viti F. Flow-induced mechanotransduction in skeletal cells. Biophys Rev 2019; 11(5): 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol 2010; 9(6): 659–670. [DOI] [PubMed] [Google Scholar]
- 210.Cai X, Wang KC, Meng Z. Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front Cell Dev Biol 2021; 9: 673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 2006; 7(4): 265–275. [DOI] [PubMed] [Google Scholar]
- 212.Zhao XH, Laschinger C, Arora P, et al. Force activates smooth muscle alpha-actin promoter activity through the rho signaling pathway. J Cell Sci 2007; 120(Pt 10): 1801–1809. [DOI] [PubMed] [Google Scholar]
- 213.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474(7350): 179–183. [DOI] [PubMed] [Google Scholar]
- 214.Wagh K, Ishikawa M, Garcia DA, et al. Mechanical regulation of transcription: Recent Advances. Trends Cell Biol 2021; 31(6): 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gardinier J, Yang W, Madden GR, et al. P2Y2 receptors regulate osteoblast mechanosensitivity during fluid flow. Am J Physiol Cell Physiol 2014; 306(11): C1058–C1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov 2020; 19(7): 480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mo JS, Yu FX, Gong R, et al. Regulation of the Hippo-yap pathway by protease-activated receptors (PARs). Genes Dev 2012; 26(19): 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Udan RS, Kango-Singh M, Nolo R, et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 2003; 5(10): 914–920. [DOI] [PubMed] [Google Scholar]
- 219.Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011; 144(5): 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tang Y, Feinberg T, Keller ET, et al. Snail/slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat Cell Biol 2016; 18(9): 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Murakami M, Nakagawa M, Olson EN, et al. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt–Oram syndrome. Proc Natl Acad Sci 2005; 102(50): 18034–18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meng Z, Qiu Y, Lin KC, et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature 2018; 560(7720): 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rando OJ, Zhao K, Janmey P, et al. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc Natl Acad Sci 2002; 99(5): 2824–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chang L, Azzolin L, Di Biagio D, et al. The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature 2018; 563(7730): 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kuroda M, Wada H, Kimura Y, et al. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J Cell Sci 2017; 130(5): 989–1002. [DOI] [PubMed] [Google Scholar]
- 226.Kegelman CD, Coulombe JC, Jordan KM, et al. YAP and TAZ Mediate Osteocyte perilacunar/canalicular remodeling. J Bone Miner Res 2020; 35(1): 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zarka M, Etienne F, Bourmaud M, et al. Mechanical loading activates the YAP/TAZ pathway and chemokine expression in the MLO-Y4 osteocyte-like cell line. Lab Invest 2021; 101(12): 1597–1604. [DOI] [PubMed] [Google Scholar]
- 228.Cui CB, Cooper LF, Yang X, et al. Transcriptional coactivation of bone-specific transcription factor cbfa1 by TAZ. Mol Cell Biol 2003; 23(3): 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zaidi SK, Sullivan AJ, Medina R, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J 2004; 23(4): 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pan JX, Xiong L, Zhao K, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res 2018; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen Z, Luo Q, Lin C, et al. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem Biophys Res Commun 2015; 468(1-2): 21–26. [DOI] [PubMed] [Google Scholar]
- 232.Xiong J, Almeida M, O’Brien CA. The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation. Bone 2018; 112: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kegelman CD, Mason DE, Dawahare JH, et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB J 2018; 32(5): 2706–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013; 19(2): 179–192. [DOI] [PubMed] [Google Scholar]
- 235.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149(6): 1192–1205. [DOI] [PubMed] [Google Scholar]
- 236.Santos A, Bakker AD, Zandieh-Doulabi B, et al. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun 2010; 391(1): 364–369. [DOI] [PubMed] [Google Scholar]
- 237.Leal ML, Lamas L, Aoki MS, et al. Effect of different resistance-training regimens on the WNT-signaling pathway. Eur J Appl Physiol 2011; 111(10): 2535–2545. [DOI] [PubMed] [Google Scholar]
- 238.Sen B, Xie Z, Case N, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 2008; 149(12): 6065–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone 2008; 42(4): 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/β-Catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 2006; 281(42): 31720–31728. [DOI] [PubMed] [Google Scholar]
- 241.Pflanz D, Birkhold AI, Albiol L, et al. Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci Rep 2017; 7(1): 9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lories RJU, Peeters J, Bakker A, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum 2007; 56(12): 4095–4103. [DOI] [PubMed] [Google Scholar]
- 243.Gerbaix M, Vico L, Ferrari SL, et al. Periostin expression contributes to cortical bone loss during unloading. Bone 2015; 71: 94–100. [DOI] [PubMed] [Google Scholar]
- 244.Song F, Jiang D, Wang T, et al. Mechanical stress regulates osteogenesis and adipogenesis of rat mesenchymal stem cells through PI3K/Akt/GSK-3β/β-Catenin signaling pathway. Biomed Res Int 2017; 2017: 6027402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 2009; 4(4): e5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu Y, Bhat RA, Seestaller-Wehr LM, et al. The orphan receptor tyrosine kinase ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol 2007; 21(2): 376–387. [DOI] [PubMed] [Google Scholar]
- 247.Kanno T, Takahashi T, Tsujisawa T, et al. Mechanical stress-mediated runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 2007; 101(5): 1266–1277. [DOI] [PubMed] [Google Scholar]
- 248.Wang H, Hu Z, Shi F, et al. Osteoblast-targeted delivery of miR-33-5p attenuates osteopenia development induced by mechanical unloading in mice. Cell Death Dis 2018; 9(2): 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang YK, Yu X, Cohen DM, et al. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev 2012; 21(7): 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li Y, Chu JS, Kurpinski K, et al. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J 2011; 100(8): 1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kenwright J, Richardson JB, Cunningham JL, et al. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br 1991; 73-B(4): 654–659. [DOI] [PubMed] [Google Scholar]
- 252.Augat P, Merk J, Wolf S, et al. Mechanical stimulation by external application of cyclic tensile strains does not effectively enhance bone healing. J Orthop Trauma 2001; 15(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 253.Augat P, Burger J, Schorlemmer S, et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res 2003; 21(6): 1011–1017. [DOI] [PubMed] [Google Scholar]
- 254.Sarmiento A, McKellop HA, Llinas A, et al. Effect of loading and fracture motions on diaphyseal tibial fractures. J Orthop Res 1996; 14(1): 80–84. [DOI] [PubMed] [Google Scholar]
- 255.Park SH, O’Connor K, McKellop H, et al. The influence of active shear or compressive motion on fracture-healing. J Bone Joint Surg 1998; 80(6): 868–878. [DOI] [PubMed] [Google Scholar]
- 256.Liu C, Carrera R, Flamini V, et al. Effects of mechanical loading on cortical defect repair using a novel mechanobiological model of bone healing. Bone 2018; 108: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bedi A, Kovacevic D, Fox AJ, et al. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg 2010; 92(14): 2387–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gardner MJ, van der Meulen MC, Demetrakopoulos D, et al. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res 2006; 24(8): 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wehrle E, Paul GR, Tourolle Né, Betts DC, et al. Individualized cyclic mechanical loading improves callus properties during the remodelling phase of fracture healing in mice as assessed from time-lapsed in vivo imaging. Sci Rep 2021; 11(1): 23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ghimire S, Miramini S, Richardson M, et al. Effects of dynamic loading on fracture healing under different locking compression plate configurations: A finite element study. J Mech Behav Biomed Mater 2019; 94: 74–85. [DOI] [PubMed] [Google Scholar]
- 261.González-Torres LA, Gómez-Benito MJ, Doblaré M, et al. Influence of the frequency of the external mechanical stimulus on bone healing: A computational study. Med Eng Phys 2010; 32(4): 363–371. [DOI] [PubMed] [Google Scholar]
- 262.Maycas M, McAndrews KA, Sato AY, et al. PTHrP-Derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J Bone Miner Res 2017; 32(3): 486–497. [DOI] [PubMed] [Google Scholar]
- 263.Li X, Liu D, Li J, et al. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J 2019; 33(8): 8913–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Forwood MR, Owan I, Takano Y, et al. Increased bone formation in rat tibiae after a single short period of dynamic loading in vivo. Am J Physiol Endocrinol Metab 1996; 270(3): E419–E23. [DOI] [PubMed] [Google Scholar]
- 265.Hert J, Lisková M, Landa J. Reaction of bone to mechanical stimuli. 1. Continuous and intermittent loading of tibia in rabbit. Folia Morphol 1971; 19(3): 290–300. [PubMed] [Google Scholar]
- 266.Robling AG, Robin D, Fuchs RK, et al. Mechanical adaptation. In: Burr DB, Allen MR. (eds) Basic and applied bone biology. Cambridge, MA: Academic Press, 2019, pp.203–233, 2nd ed. [Google Scholar]
- 267.Zhang ZY, Teoh SH, Chong WS, et al. A biaxial rotating bioreactor for the culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 2009; 30(14): 2694–2704. [DOI] [PubMed] [Google Scholar]
- 268.Bancroft GN, Sikavitsas VI, Mikos AG. Technical note: Design of a flow perfusion bioreactor system for bone tissue-engineering applications. J Tissue Eng 2003; 9(3): 549–554. [DOI] [PubMed] [Google Scholar]
- 269.Robling AG, Hinant FM, Burr DB, et al. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res 2002; 17(8): 1545–1554. [DOI] [PubMed] [Google Scholar]
- 270.Perry MJ, Parry LK, Burton VJ, et al. Ultrasound mimics the effect of mechanical loading on bone formation in vivo on rat ulnae. Med Eng Phys 2009; 31(1): 42–47. [DOI] [PubMed] [Google Scholar]
- 271.Chavarri-Prado D, Brizuela-Velasco A, Álvarez-Arenal Á, et al. The Bone Buttress Theory: the effect of the mechanical loading of bone on the osseointegration of dental implants. Biology 2021; 10(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang X, Vandamme K, Torcasio A, et al. In vivo assessment of the effect of controlled high- and low-frequency mechanical loading on peri-implant bone healing. J R Soc Interface 2012; 9(72): 1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gómez-Benito MJ, González-Torres LA, Reina-Romo E, et al. Influence of high-frequency cyclical stimulation on the bone fracture-healing process: mathematical and experimental models. Phil Trans R Soc A 2011; 369(1954): 4278–4294. [DOI] [PubMed] [Google Scholar]
- 274.Duyck J, Vandamme K. The effect of loading on peri-implant bone: a critical review of the literature. J Oral Rehabil 2014; 41(10): 783–794. [DOI] [PubMed] [Google Scholar]
- 275.Velasco-Ortega E, Wojtovicz E, España-Lopez A, et al. Survival rates and bone loss after immediate loading of implants in fresh extraction sockets (single gaps). A clinical prospective study with 4 year follow-up. Med Oral Patol Oral Cir Bucal 2018; 23(2): e230–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Susarla SM, Chuang SK, Dodson TB. Delayed versus immediate loading of implants: survival analysis and risk factors for dental implant failure. J Oral Maxillofac Surg 2008; 66(2): 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nguyen LH, Annabi N, Nikkhah M, et al. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev 2012; 18(5): 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kaneuji T, Ariyoshi W, Okinaga T, et al. Mechanisms involved in regulation of osteoclastic differentiation by mechanical stress-loaded osteoblasts. Biochem Biophys Res Commun 2011; 408(1): 103–109. [DOI] [PubMed] [Google Scholar]
- 279.Huo B, Lu XL, Guo XE. Intercellular calcium wave propagation in linear and circuit-like bone cell networks. Philos Trans A Math Phys Eng Sci 2010; 368(1912): 617–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li W, Sumpio BE. Strain-induced vascular endothelial cell proliferation requires PI3K-dependent mTOR-4E-BP1 signal pathway. Am J Physiol Heart Circ Physiol 2005; 288(4): H1591–H7. [DOI] [PubMed] [Google Scholar]
- 281.Liu C, Abedian R, Meister R, et al. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials 2012; 33(4): 1052–1064. [DOI] [PubMed] [Google Scholar]
- 282.Mauney JR, Sjostorm S, Blumberg J, et al. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int 2004; 74(5): 458–468. [DOI] [PubMed] [Google Scholar]
- 283.Ignatius A, Blessing H, Liedert A, et al. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials 2005; 26(3): 311–318. [DOI] [PubMed] [Google Scholar]
- 284.van Eijk F, Saris DBF, Creemers LB, et al. The effect of timing of mechanical stimulation on proliferation and differentiation of goat bone marrow stem cells cultured on braided PLGA scaffolds. Tissue Eng Part A 2008; 14(8): 1425–1433. [DOI] [PubMed] [Google Scholar]
- 285.Yong KW, Choi JR, Choi JY, et al. Recent advances in mechanically loaded human mesenchymal stem cells for bone tissue engineering. Int J Mol Sci 2020; 21(16): 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gharibi B, Cama G, Capurro M, et al. Gene expression responses to mechanical stimulation of mesenchymal stem cells seeded on calcium phosphate cement. Tissue Eng Part A 2013; 19(21-22): 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chen JC, Chua M, Bellon RB, et al. Epigenetic changes during mechanically induced osteogenic lineage commitment. J Biomech Eng 2015; 137(2): 020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Arnsdorf EJ, Tummala P, Castillo AB, et al. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech 2010; 43(15): 2881–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mokhtari-Jafari F, Amoabediny G, Dehghan MM. Role of biomechanics in vascularization of tissue-engineered bones. J Biomech 2020; 110: 109920. [DOI] [PubMed] [Google Scholar]
- 290.Liu P, Tu J, Wang W, et al. Effects of mechanical stress stimulation on function and expression mechanism of osteoblasts. Front Bioeng Biotechnol 2022; 10: 830722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shen XQ, Geng YM, Liu P, et al. Magnitude-dependent response of osteoblasts regulated by compressive stress. Sci Rep 2017; 7(1): 44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tripuwabhrut P, Mustafa M, Gjerde CG, et al. Effect of compressive force on human osteoblast-like cells and bone remodelling: an in vitro study. Arch Oral Biol 2013; 58: 826–836. [DOI] [PubMed] [Google Scholar]
- 293.Yanagisawa M, Suzuki N, Mitsui N, et al. Compressive force stimulates the expression of osteogenesis-related transcription factors in ROS 17/2.8 cells. Arch Oral Biol 2008; 53: 214–219. [DOI] [PubMed] [Google Scholar]
- 294.Dong L, Song Y, Zhang Y, et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol 2021; 236: 6376–6390. [DOI] [PubMed] [Google Scholar]
- 295.Chen X, Liu Y, Ding W, et al. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-κB pathway. Cell Death Dis 2018; 9: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang X, He J, Wang H, et al. Fluid shear stress regulates osteoblast proliferation and apoptosis via the lncRNA TUG1/miR-34a/FGFR1 Axis. J Cell Mol Med 2021; 25: 8734–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang X, Geng B, Wang H, et al. Fluid shear stress-induced down-regulation of microRNA-140-5p promotes osteoblast proliferation by targeting VEGFA via the ERK5 pathway. Connect Tissue Res 2022; 63(2): 156–168. [DOI] [PubMed] [Google Scholar]
- 298.Lei X, Liu Q, Li S, et al. Effects of fluid shear stress on expression of focal adhesion kinase in MG-63 human osteoblast-like cells on different surface modification of titanium. Bioengineered 2021; 12: 4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Song J, Liu L, Lv L, et al. Fluid shear stress induces runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int 2020; 44: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 300.Jin J, Jaspers RT, Wu G, et al. Shear stress modulates osteoblast cell and nucleus morphology and volume. Int J Mol Sci 2020; 21: 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Riddle RC, Taylor AF, Genetos DC, et al. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol 2006; 290(3): C776–C84. [DOI] [PubMed] [Google Scholar]
- 302.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Clin Orthop Relat Res 1989; 238: 249–281. [PubMed] [Google Scholar]
- 303.Kumar R, Tiwari AK, Tripathi D, et al. Anatomical variations in cortical bone surface permeability: tibia versus femur. J Mech Behav Biomed Mater 2021; 113: 1–7. [DOI] [PubMed] [Google Scholar]
- 304.Tanaka SM, Sun HB, Roeder RK, et al. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int 2005; 76: 261–271. [DOI] [PubMed] [Google Scholar]
- 305.Checa S, Prendergast PJ. Effect of cell seeding and mechanical loading on vascularization and tissue formation inside a scaffold: a mechano-biological model using a lattice approach to simulate cell activity. J Biomech 2010; 43(5): 961–968. [DOI] [PubMed] [Google Scholar]
- 306.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995; 11: 73–91. [DOI] [PubMed] [Google Scholar]
- 307.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011; 12(9): 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development 2011; 138(21): 4569–4583. [DOI] [PubMed] [Google Scholar]
- 309.Riddle RC, Khatri R, Schipani E, et al. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med 2009; 87(6): 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Schipani E, Maes C, Carmeliet G, et al. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 2009; 24(8): 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Maes C. Role and regulation of vascularization processes in Endochondral Bones. Calcif Tissue Int 2013; 92(4): 307–323. [DOI] [PubMed] [Google Scholar]
- 312.Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 2010; 19(2): 329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007; 131(2): 324–336. [DOI] [PubMed] [Google Scholar]
- 314.Ramasamy SK, Kusumbe AP, Schiller M, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun 2016; 7(1): 13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys 2014; 561: 109–117. [DOI] [PubMed] [Google Scholar]
- 316.Alagiakrishnan K, Juby A, Hanley D, et al. Role of vascular factors in osteoporosis. J Gerontol A Biol Sci 2003; 58(4): M362–M366. [DOI] [PubMed] [Google Scholar]
- 317.Vogt MT, Cauley JA, Kuller LH, et al. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res 1997; 12(2): 283–289. [DOI] [PubMed] [Google Scholar]
- 318.Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014; 507(7492): 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry 2008; 73(7): 751–762. [DOI] [PubMed] [Google Scholar]
- 320.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 2003; 314(1): 15–23. [DOI] [PubMed] [Google Scholar]
- 321.Pannarale L, Morini S, DUbaldo E, et al. SEM corrosion-casts study of the microcirculation of the flat bones in the rat. Anat Rec 1997; 247(4): 462–471. [DOI] [PubMed] [Google Scholar]
- 322.Watson EC, Adams RH. Biology of bone: the vasculature of the skeletal system. Cold Spring Harb Perspect Med 2018; 8(7): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tarkka T, Sipola A, Jämsä T, et al. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med 2003; 5(7): 560–566. [DOI] [PubMed] [Google Scholar]
- 324.Santos MI, Reis RL. Vascularization in Bone Tissue Engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 2010; 10(1): 12–27. [DOI] [PubMed] [Google Scholar]
- 325.Matsuzaki H, Wohl GR, Novack DV, et al. Damaging fatigue loading stimulates increases in periosteal vascularity at sites of bone formation in the rat ulna. Calcif Tissue Int 2007; 80(6): 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Knothe Tate ML. Whither flows the fluid in bone?" an osteocyte’s perspective. J Biomech 2003; 36(10): 1409–1424. [DOI] [PubMed] [Google Scholar]
- 327.Turner CH. Site-specific skeletal effects of exercise: importance of interstitial fluid pressure. Bone 1999; 24(3): 161–162. [DOI] [PubMed] [Google Scholar]
- 328.Takuwa Y, Masaki T, Yamashita K. The effects of the endothelin family peptides on cultured osteoblastic cells from rat calvariae. Biochem Biophys Res Commun 1990; 170(3): 998–1005. [DOI] [PubMed] [Google Scholar]
- 329.Kuchan MJ, Frangos JA. Shear-stress regulates endothelin-1 release via protein-kinase-C and cGMP in cultured endothelial-cells. Am J Physiol 1993; 264(1 Pt 2): H150–H156. [DOI] [PubMed] [Google Scholar]
- 330.Zaidi M, Alam AS, Bax BE, et al. Role of the endothelial cell in osteoclast control: new perspectives. Bone 1993; 14(2): 97–102. [DOI] [PubMed] [Google Scholar]
- 331.Hillsley MV, Frangos JA. Bone tissue engineering - the role of interstitial fluid-flow - review. Biotechnol Bioeng 1994; 43(7): 573–581. [DOI] [PubMed] [Google Scholar]
- 332.Boerckel JD, Uhrig BA, Willett NJ, et al. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci USA 2011; 108(37): E674–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhu S, Yao F, Qiu H, et al. Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biol Rev Camb Philos Soc 2018; 93(1): 469–480. [DOI] [PubMed] [Google Scholar]
- 334.Kular J, Tickner J, Chim SM, et al. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem 2012; 45(12): 863–873. [DOI] [PubMed] [Google Scholar]
- 335.Sweeney NV, Cummins PM, Cotter EJ, et al. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 2005; 329(2): 573–582. [DOI] [PubMed] [Google Scholar]
- 336.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res 1991; 42(3): 245–254. [DOI] [PubMed] [Google Scholar]
- 337.Azuma N, Duzgun SA, Ikeda M, et al. Endothelial cell response to different mechanical forces. J Vasc Surg 2000; 32(4): 789–794. [DOI] [PubMed] [Google Scholar]
- 338.Cheung WY, Liu C, Tonelli-Zasarsky RM, et al. Osteocyte apoptosis is mechanically regulated and induces angiogenesis in vitro. J Orthop Res 2011; 29(4): 523–530. [DOI] [PubMed] [Google Scholar]
- 339.Aguirre JI, Plotkin LI, Stewart SA, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 2006; 21(4): 605–615. [DOI] [PubMed] [Google Scholar]
- 340.Terasaki H, Kase S, Shirasawa M, et al. TNF-α decreases VEGF secretion in highly polarized RPE cells but increases it in non-polarized RPE cells related to crosstalk between JNK and NF-κB pathways. PLoS One 2013; 8(7): e69994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cha HS, Bae EK, Koh JH, et al. Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes. J Rheumatol 2007; 34(1): 16–19. [PubMed] [Google Scholar]
- 342.Claes LE, Meyers N. The direction of tissue strain affects the neovascularization in the fracture-healing zone. Med Hypotheses 2020; 137: 109537. [DOI] [PubMed] [Google Scholar]