Summary
To find nutrients, mosquitoes use volatile organic compounds (VOCs) emitted by plants and animal hosts. These resources overlap in their chemical composition, and an important layer of information resides in VOCs’ relative abundance in the headspace of each resource. In addition, a large majority of the human species regularly uses personal care products such as soaps and perfumes, which add plant-related VOCs to their olfactory signature. Using headspace sampling and gas chromatography-mass spectrometry, we quantified how human odor is modified by soap application. We showed that soaps alter mosquito host selection, with some soaps increasing the attractiveness of the host and some soaps reducing it. Analytical methods revealed the main chemicals associated with these changes. These results provide proof-of-concept that data on host-soap valences can be reverse-engineered to produce chemical blends for artificial baits or mosquito repellents, and evince the impact of personal care products on host selection processes.
Subject areas: Chemical engineering, Ethology, Bioactive plant product
Graphical abstract
Highlights
-
•
Soaps significantly alter the olfactory signature of human hosts
-
•
Some soaps increase host attractiveness for mosquitoes while others reduce it
-
•
Effects on mosquito attraction are linked to specific chemical compositions
-
•
Artificial mixtures based on analytical results recapitulate the effect of soaps
Chemical engineering; Ethology; Bioactive plant product
Introduction
Mosquitoes are the vectors of several infectious diseases that kill hundreds of thousands of people per year.1,2,3 To detect and locate blood sources, mosquitoes rely to a large extent on olfactory cues released by their hosts.4,5,6,7 The abundance of volatile organic compounds (VOCs) derived from the hosts’ metabolism or produced by the activity of their associated microbiome constitutes an olfactory “odor print” that will enable mosquitoes to discriminate between hosts of different species and between individuals within the same species.7,8,9,10,11,12,13 However, the increasing use of synthetic fragrances, inspired by floral extracts to alter the scent of people (e.g., perfumes and soaps) and items (e.g., laundry detergent), resulted in the introduction of compounds used by insects outside of their natural context.14,15 For example, personal care products (PCPs), such as shampoos and soaps, are widely used on a daily basis and in variable quantities and contain numerous VOCs that naturally mediate plant- and host-mosquito interactions. However, the extent to which soap application alters human odor profiles and results in the modulation of mosquito attraction has yet to be defined.
Here, we selected four commercial soaps (i.e., products of the brands: Dial, Dove, Native, and Simple Truth) based on their high popularity (i.e., 53% of people in the United States favored these brands in 201916). We analyzed their effect on human skin odor profiles using gas chromatography-mass spectrometry and on the behavioral response to human odors by the yellow fever mosquito Aedes aegypti. Relying on a multi-pattern analysis of the chemical profiles of soap-washed skin, we designed attractive and repulsive mixtures as proof of concept that this approach can be leveraged for mosquito control.
Results
Soap application alters the host odor profile
To understand how the application of soap affects human odor profiles, we first analyzed the chemical composition of the headspace above nylon sleeves worn by volunteers on their forearms. A total of 68 odor samples were collected, including from clean control sleeves, with four replicates of each unwashed volunteer, three replicates of each soap-washed volunteer, and one replicate of each soap sample. A total of 123 compounds were identified based on mass spectral library matches (≥80%) and confirmed through the NIST mass spectral library. After chemicals that represent less than 1% of the total abundance across samples were removed from the dataset, 85 compounds were retained for further analyses (See dataset at https://doi.org/10.17632/pdd3f2py8d.2). In addition to the expected inter-individual variability in the odor profile of the volunteers, we found that soap washing significantly modified human body odors in both chemical composition and abundance (ANOSIM, R = 0.23, p = 10−4; Figures 1 and 2). Specifically, the abundance and number of VOCs were increased by soap washes (average total amount: 329 ± 170, 515 ± 246, 125 ± 26.4, and 238 ± 96.6 ng mean ± standard error for Dial, Dove, Simple Truth, and Native, respectively; average number of compounds: 27.8 ± 2.03, 28.5 ± 2.32, 18.3 ± 2.05, and 17.8 ± 2.02 for Dial, Dove, Simple Truth, and Native, respectively) when compared to the unwashed skin (average total amount: 7.19 ± 1.16 ng; average number: 10 ± 1.18 mean ± standard error; Amounts: Student’s t test, t = −3.7218, df = 47.02, p < 0.001; number of compounds: Student’s t test, t = −7.5973, df = 48.633, p < 0.001). Moreover, the two soaps advertised as more “natural” (i.e., Simple Truth and Native) tended to be less chemical-heavy soaps (e.g., lower abundance of saturated and unsaturated hydrocarbons, such as alkanes and alkenes) than Dial and Dove (Figure 1B).
Figure 1.
Soap application alters the odor profile of human hosts
(A) Schematic of the experimental paradigm where nylon sleeves worn by volunteers are used for solid-phase microextraction (SPME) sampling and gas chromatography-mass spectrometry (GC-MS) or behavioral dual-choice assays.
(B) Representative chromatogram for the headspace composition of each volunteer (by row) either unwashed (gray) or washed with standardized amounts of Dial (light blue), Dove (dark blue), Simple Truth (light orange), or Native (dark orange) soap. Numbers indicate: 1) Linalool, 2) Nonanal, 3) D-Limonene, 4) β-Iraldeine, 5) Butylated hydroxytoluene, and 6) Lillal.
Figure 2.
Soap application significantly modifies the scent of human hosts
Nonmetric multidimensional scaling (NMDS) plot (stress = 0.11) of the chemical composition of the scent of all volunteers (washed and unwashed) and pure soaps. Each point represents a sample from an individual nylon sleeve worn by a volunteer. The shape of the points encodes for the identity of the volunteer, and the color of the points encodes for the experimental treatment (i.e., unwashed, or washed with one of the four tested soaps). The ellipses represent the SD around the centroid of their respective cluster. Scent compositions are significantly different between soap treatments (ANOSIM, R = 0.043, p = 0.015).
Multivariate analysis (Nonmetric multidimensional scaling) showed a clear separation between the chemical profiles of the unwashed arm samples and the soap-washed samples (ANOSIM; R = 0.23, p = 10−4; Figure 2). Samples containing only soaps were more similar to samples washed with the corresponding soap, suggesting that soap volatiles dominate the odor signature of soap-washed samples. A significant separation was also found between the clustering of the two “natural” soaps (i.e., Simple Truth and Native) and the more chemical-heavy soaps (i.e., Dial and Dove) (ANOSIM, R = 0.067, p = 0.01; Figure 2).
Specifically, the application of soap on human skin changed which chemical classes dominate the host’s olfactory signature (Figure 3). Regardless of the volunteer’s identity, odor profiles of non-washed samples were primarily dominated by aldehydes (45.96 ± 6.06% of total amounts) and ketones/esters (36.52 ± 7.85%). After soap application, a strong increase in the proportion of terpenes was observed, augmenting from 2.55 ± 0.75% in unwashed samples to 65.86 ± 8.77% across samples, while the relative proportion of aldehydes and ketones/esters decreased to 6.13 ± 0.75% and 13.55 ± 4.90%, respectively (Figure 3). However, when considering the absolute concentrations of these chemical classes, the amount of aldehydes increased by ∼4.1-folds as, in average, 12.89 ± 0.88 ng of aldehydes were found in unwashed volunteers’ samples, and 53.12 ± 10.21 ng were found in soap-washed volunteers’ samples. Similarly, the absolute amount of ketones/esters increased by ∼12.8-fold after the application of soaps, i.e., from 10.99 ± 2.79 ng in unwashed volunteers’ samples to 140.46 ± 50.34 ng in soap-washed samples (see Figure S1). The treatment group, the chemical class, and the interaction between these two variables had significant effects on the abundance of the chemicals found in the headspace (two-way ANOVA, df = 4, F = 553.85 for the effect of the treatment group, df = 7, F = 561.19 for the effect of the chemical class, and df = 28, F = 86.78 for the effect of the interaction. For all, p < 0.001). These changes reflect a significant increase in the emission rates of volunteers after soap applications: from 28.75 ± 2.19 ng per unwashed volunteer’ sample to 905.37 ± 209.60 ng per soap-washed sample (ANOVA, df = 4, F = 18.08, p < 0.001; see Figure S2). Among the soap tested here, Dial and Dove increased the overall abundance of chemicals in the headspace significantly more than the Simple Truth and Native soaps (Tukey multiple comparisons of means, p < 0.01).
Figure 3.
Washed and unwashed samples differ in the relative abundance of key chemical classes
Odor profiles showing the relative abundance of the chemical classes of compounds that made up >1% each sample. Human samples are pooled by treatment prior to the analysis (n = 4 unwashed, n = 3 washed for each soap, n = 1 for pure soaps, indicated with downward pointing triangles).
To further characterize the chemical basis of these differences, we identified the chemicals that dominate the scent of each treatment group, i.e., which made up more than 10% of the chemical composition (See dataset at https://doi.org/10.17632/pdd3f2py8d.2). The scent of unwashed volunteers was largely dominated by (E)-2-nonenal (30.3 ± 1.89%), decanal (23.5 ± 2.57%), sulcatone (20.6 ± 1.22%), geranyl acetone (19.75 ± 0.5%), and nonanal (10.2 ± 0.95%). The scent of Dial-washed volunteers was dominated by ψ-Limonene (38 ± 4.88%), 4-tert-butylcyclohexyl acetate (or Vertenex, 19.9 ± 4.92%), and linalool (12 ± 0.02%). Dove-washed volunteers’ scent was most abundant in ψ-Limonene (39.6 ± 3.15%), 7-octen-2-ol, 2,6-dimethyl- (10.4 ± 2.17%), and linalool (10.2 ± 0.08%). Simple Truth-washed and Native-washed volunteers predominantly smelled ψ-Limonene (61.1 ± 5.40% and 63.1 ± 1.71%, respectively).
The host valence is modulated by soap application
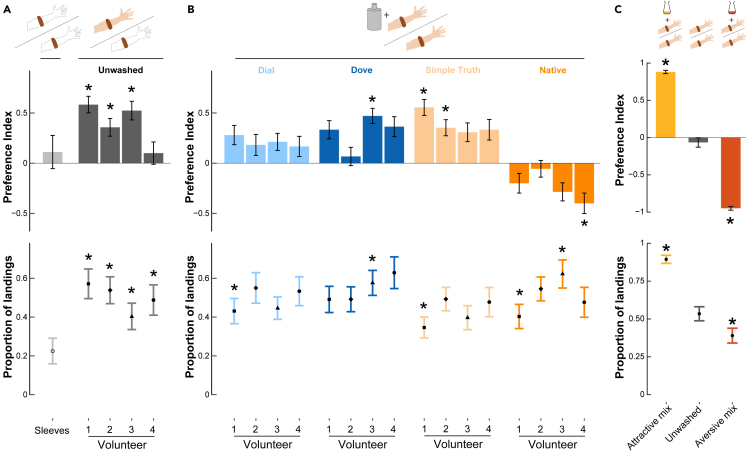
To determine whether these changes in the host odor affect mosquito olfactory preferences and activity level (evaluated as the proportion of landings), we used a free-flight, dual-choice landing assay (Figure 4). When presented with two clean, odorless sleeves (i.e., never worn by a human), a low number of mosquitoes made a choice (22.5%, Binomial Exact test: p = 0.99, n = 40). However, when one of the sleeves was worn by a volunteer, the proportion of landings significantly increased to 50 ± 3.65% (Binomial Exact test: p < 0.001, n = 187). In these conditions, mosquitoes biased their preference toward the sleeve worn by volunteers 1, 2, and 3 (Binomial Exact test: p < 0.05, 41 < n < 52) but did not show any preference toward the sleeve worn by the fourth volunteer (Binomial Exact test: p = 0.82, n = 20, Figure 4A), even though this volunteer’s scent increased the overall proportion of landings. Out of the four soaps tested here, the Dove and Simple Truth body wash significantly increased the attractiveness of some but not all volunteers, suggesting an interactive effect between the host’s olfactory profile and the soap’s chemistry (Figure 4B). Conversely, Native-washed hosts tended to be avoided, and a significant aversion was observed in the case of the fourth volunteer (Binomial Exact test, p < 0.001, n = 20, Figure 4B). Overall, the Native soap biased mosquitoes’ responses in a way that was significantly different from all other treatments (Generalized Linear Model with a binomial distribution of errors, p < 0.0001). Similarly, the effect of soap applications on the proportion of landing observed during the assay was a function of the interaction between the soap and the volunteer. Specifically, fewer mosquitoes landed on either sleeve when volunteer 1 was washed with either Dial, Simple Truth, or Native soap, than when only their unwashed scent was presented (Binomial Exact test, p < 0.05, 55 < n < 78). In the case of volunteer 3, more mosquitoes landed on either sleeve when one of the samples corresponded to their arm’s scent washed with either Dove or Native soaps (Binomial Exact test, p < 0.05, 45 < n < 59; Figure 4B).
Figure 4.
Soap application can modulate mosquito attraction
(A) Behavioral preferences of adult Aedes aegypti females between two clean sleeves (light gray) or between a human-worn nylon sleeve and clean sleeves. A dual-choice assay was used for the behavioral experiments in which mosquitoes are released into a test cage and can choose to land on either of the test or the control sleeve (See Figure 1A for details). Top: A preference index was calculated based on the number of landings on each sleeve (see methods for details). Asterisks indicate distributions of landings that were significantly different from chance (i.e., 50% on each sleeve; Binomial Exact test, p < 0.05). Bottom: Proportion of mosquitoes landing on either sleeve. Asterisks denote a significant difference from the proportion of landings when two clean sleeves are presented (i.e., 0.225; Binomial Exact test, p < 0.05).
(B) Top: Behavioral preferences of mosquitoes when provided with a choice between a sleeve worn on the unwashed arm and a sleeve worn on the washed arm of the same volunteer (colored as a function of the soap brand). Asterisks indicate distributions of landings that were significantly different from chance (i.e., 50% on each sleeve; Binomial Exact test, p < 0.05). Bottom: Proportion of mosquitoes landing on either sleeve. Asterisks denote a significant difference from the proportion of landings observed when only the unwashed sleeve is presented for the corresponding volunteer (i.e., 0.57 for volunteer 1, 0.53 for volunteer 2, 0.40 for volunteer 3, and 0.48 for volunteer 4; Binomial Exact test, p < 0.05). The volunteer identity is represented with the same point shape as in Figure 2. Across treatments: 35 < n < 78. C) Top: Behavioral preferences of mosquitoes when provided a choice between two sleeves worn by the same individual host either unaltered (gray bar) or spiked with 1 mL of an attractive mixture (yellow bar) or an aversive mixture (red bar). Asterisks indicate distributions of landings that were significantly different from chance (i.e., 50% on each sleeve; Binomial Exact test, p < 0.05). Bottom: Proportion of mosquitoes landing on either sleeve. Asterisks denote a significant difference from the proportion of landings when two unwashed sleeves are presented (i.e., 0.53; Binomial Exact test, p < 0.05). Across treatments: 100 < n < 132. Error bars for both the preference index and the proportion of landings represent the standard error to the mean.
Artificial blends based on significant chemical associations recapitulate the effect of soaps
Next, we used a multi-level pattern analysis, commonly used in ecology to quantify the degree of association between species and habitats, for example,17 to leverage the behavioral results and chemical composition of each sample, and identified lilial (a synthetic aromatic aldehyde), α-isomethyl ionone (naturally found in bacterial fermentation18 and flower headspace volatiles19), allyl heptanoate (naturally found in the scent of tropical fruits20 and used in perfumery to imitate pineapple scent21), and 4-tert-butylcyclohexyl acetate (a commonly used fragrance ingredient22) as candidates to design an “attractive” mixture. Not only were these chemicals strongly associated with soaps that increased the attraction of mosquitoes but they were also among the 10 most abundant compounds present in the Dial and Dove soaps (i.e., soaps that induced the highest preference indices, Figure 4B). Similarly, benzyl benzoate (an organic compound found in the scent of flowers and commonly used to treat scabies and lice23), γ-nonalactone (a component of watermelon’s scent24 and identified as a key compound of the aroma of American Bourbon25), and benzaldehyde (an aromatic aldehyde commonly found in plants with a characteristic almond-like odor26,27) were the most abundant compounds and most strongly associated with the Native soap (i.e., soap that induced the lowest preference indices, Figure 4B. Multi-level pattern analysis, p < 0.001; see dataset at https://data.mendeley.com/datasets/pdd3f2py8d/2 for statistical details), and were selected to design a “repellent” mixture. Mosquitoes’ behavioral responses to these mixtures were quantified using the dual-choice assay described above, but using a fifth volunteer who was not part of the first step of the study. When given a choice between two sleeves worn by this volunteer and spiked with mineral oil, mosquitoes landed equally on either sleeve (Binomial Exact test, n = 116, p = 0.73; Figure 4C). However, when one of the sleeves was spiked with the “attractive” mixture, a significant bias was observed toward the mixture-spiked sleeve (Binomial Exact test, n = 132, p < 0.001, Figure 4C). Conversely, mosquitoes significantly avoided the “repulsive” mixture and preferentially landed on the mineral-oil-spiked sleeve (Binomial Exact test, n = 100, p < 0.001, Figure 4C).
Discussion
In this study, we showed that human body odor profiles are significantly altered by PCPs such as soaps, resulting in changes in mosquito attractiveness. We also provided proof of concept that improved knowledge of the chemical ecology of mosquitoes can be leveraged to design artificial blends for control purposes.
Plants emit a wide array of VOCs, including terpenes, esters, aldehydes, alcohols, and acids, that are detectable by mosquitoes.28,29 The main effect of soap application evinced here was a shift in favor of these plant-derived chemicals in the human odor profiles. Soap application also drastically increased the absolute amounts of semiochemicals released in the host’s headspace (Figure S1). This is epidemiologically relevant as, for example, variations in the concentration of aldehydes are known to be linked to modulations of mosquito attraction to humans.6,11,29,30,31,32,33,34,35,36 Interestingly, the difference between the two soaps advertised as more “natural” (i.e., Simple Truth and Native) and the more chemical-heavy soaps (i.e., Dial and Dove) also lies in the proportion of aldehydes, ketones, esters, and alcohols (Figure 3), with the samples washed with the “natural” soaps having proportionally fewer of these chemicals.
The next most remarkable effect of soap applications observed here was the extensive increase in the relative amounts of terpenes in the host profile. Terpene derivatives constitute the largest and most structurally diverse group of compounds37 that can serve as mosquito kairomones. This class of semiochemicals mediates host attraction,38,39 plant location,28,36 and oviposition site detection.40 In addition, certain terpenes (e.g., α-pinene, linalool, and limonene) also have repellent effects.41,42 The soap-specific composition and concentration of terpenes are thus likely to be an important driver of the resulting modulation of mosquito attraction to the host. Finally, the amount of ketones and esters increased by ∼12.8-fold after soap application (Figure S1). Given their well-established role as mosquito semiochemicals, either attractants or repellents,8,43,44,45,46 these changes are also likely to contribute to the modulation of mosquito-host interactions.
It is worth noting that in the present study, CO2, an important cue used for host location,4,47 was not delivered during the behavioral choice assays. Any potential synergistic effect of CO2 and plant-derived compounds present in soaps has therefore not been considered here, and remains to be analyzed.
Chemical parsimony
Following the principle of chemical parsimony formulated by Blum,48 mosquitoes exploit semiochemical odorant molecules that can be produced by different resources (e.g., plants, oviposition sites, and hosts). Mosquitoes resolve this chemical overlap by exploiting differences in the relative abundance and concentration of these volatile compounds among and between resources.28,36,49,50,51 When evaluating the effect of chemical changes in the host scent profile on mosquitoes’ behavior, we found that while the host scent was largely dominated by the soap VOCs, mosquito behavior was significantly modified only for specific soap-volunteer combinations (Figure 4). Altogether, our results suggest that rather than the isolated effect of a change in a single chemical’s concentration (either absolute or relative), the modulation of mosquitoes’ host-seeking behavior is the result of the interplay between changes in the concentration of soap chemicals and the specific composition of the individual host’s scent. Supporting this point, the fact that the well-known mosquito repellent limonene is one of the most abundant chemical compounds in all the soaps we tested but, remarkably, did not elicit aversion in three of the four soaps we tested. The broader implication of these results is that chemical ecology studies in general should give these interactive effects the same consideration given to the chemicals that dominate scents by their absolute abundance.
Human skin emanations, by-products of our metabolic activity and associated skin microbiota,10 constitute an olfactory “odor print” and are key to explaining mosquito preferences for certain individual hosts over others.13 Inter-individual variations in human body odor profiles are also driven by a variety of factors such as genetic differences,13 gender, age, body parts, food consumption, exercise, pregnancy, and health status.11,45,52,53,54,55,56,57,58,59 These factors can also lead to intra-individual variations in body odor profiles, which have, historically, made it very difficult to come up with a simple answer to the question: why are some people bitten more than others?5,13,60,61 In the present study, volunteers have not been subjected to any specific restrictions (e.g., diet, activity) prior to the scent collection. The effect of intra-individual variability on mosquito behavior is controlled by providing mosquitoes with a choice between samples taken from the same volunteer on the same day (i.e., left and right arm), where one arm was washed with soap, while the other one was kept unwashed. In addition, performing multiple replicates over the course of several months allowed us to integrate intra-individual and temporal variabilities. Specifically, we opted here for treating each host-soap combination as the result of a “black box” of modulating factors (e.g., the host microbiome, diet, phase of the menstrual cycle, and activity level). By using pairs of samples to resolve the chemical composition of the host’s scent and the valence of that scent to the mosquito, we identified the chemicals most significantly associated with the valence of each specific host-soap combination.
Recent studies have highlighted the importance of carboxylic acids, detected by ionotropic (IR) receptors on the mosquito antennae, in explaining inter-individual differences in mosquito attractions.5,13,62 Specifically, individual hosts producing higher levels of acidic volatiles, such as pentadecanoic acid, heptadecanoic acid, and nonadecanoic acid, were more attractive to mosquitoes.13 Here, however, we observed relatively low abundances of carboxylic acids, including lactic acid, although it is well established that they are prime components of human odor.8 This discrepancy is likely due to the fact that odor collections from other studies were performed differently, either using glass beads8 or with additional processing of the samples,13 leading to a higher sensitivity for highly polar and less volatile acids associated with perspiration and the skin microbiome than the solid-phase microextraction sampling method used here.
Going further than absolute chemical abundance
A classical approach to the search for the chemical basis of insects’ behavioral responses consists in identifying the physiologically active chemicals, i.e., those that elicit responses by olfactory receptor neurons on the insect olfactory appendages (e.g., [28]). Next, analytical chemistry methods are used to screen for compounds that are enriched in either repulsive or attractive samples, sometimes working under the assumption that chemicals at the highest concentrations are likely to be explaining the observed behavior (e.g., [13]). While electro-antennography coupled with gas chromatography (GC-EAG63) would have certainly allowed us to refine our analysis and focus solely on biologically relevant chemicals, we opted for basing our analytical approach on all chemicals associated with scents driving either attraction or repulsion in our behavioral assays. The rationale behind this decision is that there are converging lines of evidence that blends of chemicals and, particularly, their specific ratios in the blends are critical determinants of the valence of an odor.7,28 Although this does not exclude the possibility of selecting a chemical that would be statistically associated with either mosquito attraction or repulsion but not detected by mosquitoes, this approach is agnostic to the soap, volunteer, or experimental replicate identity, and reveals patterns of chemicals most frequently associated with either attraction or repulsion. It therefore includes potential chemical interactions occurring in the headspace.
The chemical mixtures informed by the multi-level pattern analysis were further prepared so that chemicals are at similar ratios than in the averaged headspace of the corresponding samples. This ensured that chemicals were not only selected for their association with attractive/repulsive samples but also that they were presented to the mosquitoes at adequate relative abundances. However, it is worth noting that in our behavioral assays, the artificial mixtures were presented at a higher concentration than in the original samples, which is relevant in the context of deploying such mixtures for mosquito control.
Ultimately, the blends created in this study led to strong effects on mosquito preference. This serves as a proof of concept that such a statistical approach is efficient at extracting relevant chemicals from natural odor samples. These results constitute a first step toward generating a large dataset based on a wider variety of PCPs, such as perfumes and laundry detergent, to isolate specific chemical patterns which drive mosquito behavior.
Limitations of the study
Even though the same compounds (e.g., monoterpenes emitted by host plants) may serve as cues in plant location by different mosquito species,29,36 it is unclear how chemical patterns that are relevant for Ae. aegypti translate to other mosquito species, especially considering the long history of Ae. aegypti’s potential exposure to soaps while in laboratory colonies. In addition, odorant volatiles often act in a context-dependent manner where the presence of other bioactive volatiles (e.g., CO2) may affect their valence.36,39,50,64,65,66 Increasing the number and diversity of both PCPs and volunteers will thus be critical in fully understanding the effect of soaps in altering mosquito behavior. Finally, the effects we observed here were quantified with samples collected within the first hour after soap application. The duration of these effects will have to be quantified to capture the full measure of the epidemiological impact of PCPs. Overall, our study represents a crucial first step in this direction and represents a new avenue for reducing mosquito bites and, therefore, the transmission of mosquito-borne diseases.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Lilial | Sigma-Aldrich | CAS# 80-54-6 |
| α-isomethyl ionone | Sigma-Aldrich | CAS# 127-51-5 |
| Allyl heptanoate | Sigma-Aldrich | CAS# 142-19-8 |
| 4-tert-Butylcyclohexyl acetate | Sigma-Aldrich | CAS# 32210-23-4 |
| Benzyl benzoate | Sigma-Aldrich | CAS# 120-51-4 |
| γ-nonalactone | Sigma-Aldrich | CAS# 104-61-0 |
| Benzaldehyde | Sigma-Aldrich | CAS# 100-52-7 |
| Deposited data | ||
| Data and code | This paper | https://data.mendeley.com/datasets/pdd3f2py8d/2 |
| Experimental models: Organisms/strains | ||
| Healthy human volunteers: 2 men, 3 women | Recruited amongst students and employees at Virginia Tech | Inform consent: Virginia Tech IRB protocol # 20-037 |
| Mosquitoes: Aedes aegypti Rockefeller strain | Manassas, VA, USA | Rockefeller strain, MR-734, MR4, ATCC® |
| Software and algorithms | ||
| Cobra algorithm (Chromeleon 7) | Thermo Fisher Scientific, Waltham, MA, USA | https://assets.thermofisher.com/TFS-Assets/CMD/Technical-Notes/TN-70698-CDS-SmartPeaks-Cobra-TN70698-EN.pdf |
| NIST library | The NIST Mass Spectrometry Data Center | https://chemdata.nist.gov/ |
| RStudio, vegan package | Oksanen et al.67 | https://cran.r-project.org/web/packages/vegan/index.html |
| RStudio, indicspecies package | De Cáceres et al.68 | https://cran.r-project.org/web/packages/indicspecies/index.html |
| Other | ||
| Soap Dial® | Henkel North American Consumer Goods, The Dial Corporation, Scottsdale, AZ, USA | Dial® Body Wash Marula Oil, 21 fl oz |
| Soap Dove® | Unilever, London, England | Dove® Deep Moisture Nourishing Body Wash, 24 fl oz |
| Soap Simple Truth® | The Kroger Company, Cincinnati, Ohio, USA | Simple Truth® Organic Honey Blossom Baby Shampoo & Body Wash, 16 fl oz |
| Soap Native | Native™ San Francisco, California, USA | Native Coconut and Vanilla Body wash |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Clément Vinauger (vinauger@vt.edu).
Material availability
This study did not generate new unique reagents or materials.
Data and code availability
-
•
Data: All data is available at https://data.mendeley.com/datasets/pdd3f2py8d/2.
-
•
Code: All R code is available at https://data.mendeley.com/datasets/pdd3f2py8d/2.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Human volunteers
Five volunteers (2 men, 3 women, age range 24–33, mean 28) were sampled for this study using the scent collection method described below. Informed consent was received from each of the participants before sampling (Virginia Tech IRB protocol # 20–037).
Mosquitoes
Aedes aegypti mosquitoes of the Rockefeller strain (MR-734, MR4, ATCC®, Manassas, VA, USA) were used for all behavioral assays. Mosquito larvae were raised in plastic trays 34.3 x 25.4 x 3.8 cm (BioQuip, Rancho Dominguez, CA, USA—1425, 1426B) filled with deionized water and fed with Hikari Tropical First Bites (Petco, San Diego, CA, USA). After pupation, the pupae were removed from the larval trays and transferred to mosquito breeding containers (BioQuip, Rancho Dominguez, CA, USA—1425, 1425DG). Adult mosquitoes were synchronized to a 12h/12h Light/Dark cycle for at least three days prior to the experiment and maintained at 25 ± 0.6°C and 60 ± 10% relative humidity (RH). All behavioral experiments were performed during the peak host-seeking and activity time (Zeitgeber Times [ZT] 10–12), i.e., the last two hours of photophase (Eilerts et al., 2018). To increase mating rates, males and females were kept in the container until the experiments. The mating status of females was further confirmed by random selection of 10 females and dissection of their spermatheca (90% were mated). On the day of the experiment, 6–8 days old females were isolated between ZT 8–9 and allowed to adjust to the experimental environment for one hour (ZT 9–10) before being tested during their peak activity period (ZT 10–12; see [69]).
Methods details
Soap selection
Four body washes were selected based on their brand popularity, and fragrance: 1) Dial® Body Wash, Marula Oil, 21 fl oz (Henkel North American Consumer Goods, The Dial Corporation, Scottsdale, AZ, USA); 2) Dove® Deep Moisture Nourishing Body Wash, 24 fl oz (Unilever, London, England); 3) Simple Truth® Organic Honey Blossom Baby Shampoo & Body Wash, 16 fl oz (The Kroger Company, Cincinnati, Ohio, USA); and 4) Native Coconut and Vanilla Body wash (Native™ San Francisco, California, USA). The presence in the ingredient list of chemicals previously described as eliciting behavioral responses by mosquitoes was also used as a selection criterion.
Scent collection
Both of each volunteer’s forearms were rinsed for 30 seconds with deionized (DI) water and dried with a paper towel. On one forearm that received no further treatment (i.e., control arm), two 1-inch-wide strips of nylon sleeves (Hanesbrands Inc., Winston-Salem, NC, USA) were applied and the arm was wrapped in aluminum foil. The experimenter washed the other forearm (i.e., treatment arm) with 1.0 ± 0.5 g of a given test soap for 10 seconds and rinsed with DI water for 10 seconds before applying and wrapping two nylon sleeves in aluminum foil, similarly to the control arm. After 1 hour of scent collection, all nylon sleeves were removed. Two sleeves (one control and one soap treated) were placed in separate 20 mL scintillation vials with screw top lids (#B7800-20 Thermo Fisher Scientific, Waltham, MA, USA) for headspace volatile sampling using solid-phase microextraction (SPME) and chemical identification using GC-MS. The other two sleeves (one control and one soap treated) were placed into separate 59 mL black plastic cups (CAT #B079WRJRNW, Golden Apple, Phoenix, AZ, USA) to be used in behavioral choice assays. For each soap, four volunteers were tested, and each experiment was conducted in triplicate.
In a separate set of experiments, the scents of the soaps alone were analyzed by washing a nylon sleeve with 1.0 ± 0.5 of each test soap for 10 seconds followed by rinsing the soap out of the sleeve for 10 seconds. Sleeves were then placed into 20 mL scintillation vials for SPME sampling. In both experiments, clean nylon sleeves rinsed with DI water were used as negative controls and the volatiles released by the sleeves were identified using the same approach.
Chemical identification and quantification
SPME fibers (57328-U DVB/CAR/PDMS, Millipore Sigma, Saint-Louis, MO, USA and SPME holder 57330-U Millipore Sigma, Saint-Louis, MO, USA) were used to collect volatile chemicals from the headspace above nylon sleeves. Before any odor collection, the SPME fiber was conditioned and cleaned up by being exposed inside the GC inlet at a constant temperature of 250°C for 30 min. To collect volatiles from a given sample, the SPME fiber was inserted through the cap of a scintillation vial containing the nylon sleeve to be sampled. The headspace volatiles above each sleeve were collected for 1 hour and the fiber was desorbed by exposure in the GC inlet (210°C) for 3 minutes. A GC-MS (GC: Trace 1310, MS: ISQ 7000, Thermo Fisher Scientific, Waltham, MA, USA) equipped with a 30 m column (I.D. 0.25 mm, #36096-1420, Thermo Fisher Scientific, Waltham, MA, USA) was used with helium as a carrier gas at a constant flow of 1 cc/min. The GC temperature started at 45°C for 3.75 min, followed by a heating gradient from 45°C to 250°C which was then held isothermally for 10 min. The total run time was 38.75 min. Between each sample, the fiber was exposed inside the GC inlet at 210 °C for 3 minutes to prevent carryover and contamination between samples.
A total of 68 odor samples were analyzed, including one clean sleeve control, four replicates of each volunteer, three replicates of each soap-washed sample per volunteer, and one replicate of each soap sample. Chromatogram peaks were integrated using the Cobra algorithm (Chromeleon 7, Thermo Fisher Scientific, Waltham, MA, USA) and tentatively identified using the online NIST library. NIST library identification parameters were set to a normal search type with a minimum chemical match factor (SI) of 600. To remove additional contaminants or low concentration chemicals from the samples, chemicals that represent less than 1% of the total abundance in each individual sample were removed from the analysis. Additionally, chemicals present in the negative controls (clean, unused sleeves) were also discarded from the analysis (Table 1).
Concentration (ng.μL-1) and mass (ng) were calculated for each compound. Serial dilutions of heptyl acetate (HA) (0.354, 3.54, 35.4, and 354 (ng); CAS #112-06-1, Sigma-Aldrich, Saint-Louis, MO, USA) into hexane (227064-2L, Sigma Aldrich, Saint-Louis, MO, USA) were used to produce a standard curve, and a known amount of the internal standard (0.354 ng.μL-1) was added to each sample by pipetting 10 μL of HA at 0.0354 ng/μL, enabling the conversion of peak areas into chemical amounts.
Dual-choice assay
Behavioral assays were conducted in a 30 x 30 x 30 cm cage (DP1000, BugDorm, Taiwan) (Figure 1A). Groups of 16 < n < 25 female mosquitoes were cold anesthetized on ice for 5–10 minutes and isolated into small containers 1–2 hours before experiments. The effect of soap application on mosquito olfactory preference was tested by comparing the number of landings on soap-free versus soap-washed samples from the same host.
In all assays, nylon sleeves were placed in black cups covered with fabric mesh (to prevent contact between mosquitoes and the nylon sleeves) and symmetrically positioned inside the cage (Figure 1A). Female mosquitoes were released into the cage for 20 minutes during which their behavior was video recorded at 30fps (Logitech C920, Logitech, Lausanne, Switzerland) to avoid potential confounds introduced by the presence of the experimenter in the room. Later, the number of landings on each cup was recorded from the videos. A preference index was determined for each assay, and calculated as the number of mosquito landings on the treatment sleeve minus the number of landings on the control sleeve divided by the total number of landings.70 Four volunteers were used for each soap, and each assay was replicated 3–4 times. To control for potential spatial biases, the position of the control and soap-treated sleeves were randomly swapped between assays, and the cage was cleaned with 95% ethanol after each experiment to remove any potential traces of chemicals emanating from the nylon sleeves. All experiments were performed in a well-ventilated environment at 23 ± 2°C and 50–70% RH.
Attractive and repulsive mixtures
Based on the dual-choice behavioral assays, two mixtures were elaborated with soap chemicals most strongly associated with the soaps that biased mosquitoes’ behavior toward the host. Specifically, we performed a multi-level pattern analysis using the multipatt function of the indicspecies package in R.17,68 In this approach, combinations of chemicals are created in silico and each combination is compared with the chemicals present in the input matrix containing information on the valence of each soap. For each soap, the function chooses the combination with the highest association value and the best matching patterns are tested for statistical significance of the associations. Based on the multi-level pattern analysis results, we selected lilial, α-isomethyl ionone, allyl heptanoate, and 4-tert-Butylcyclohexyl acetate for the “attractive” mixture because these chemicals were most strongly associated with Dial and Dove soaps that tended to increase mosquitoes’ attraction to the host. Similarly, based on their strong association with the Native soap, which tended to reduce the attraction for the host, we selected benzyl benzoate and γ-nonalactone for the “repulsive” mixture. Because benzaldehyde was the third most abundant compound of the pure Native soap’s scent (relative concentration: 9.56%), we added it to the “repulsive” mixture. Mixtures were prepared in mineral oil as a solvent. Raoult’s law was used to determine the concentrations of chemical components in the solution so that their concentration in the vapor phase corresponds to the average of their concentration in the headspace above the nylon sleeves of the corresponding samples (See dataset at https://doi.org/10.17632/pdd3f2py8d.1).
The mixtures were then applied to nylon sleeves (1 mL pipetted onto the sleeve) and used in dual-choice behavioral assays similar to those described above. To reduce the potential effects of using a volunteer whose scent has been used to determine the valence of soap-washed samples, we used a fifth volunteer, whose scent’s chemical composition was not involved in the determination of the mixtures’ composition. We compared the number of landings on control sleeves (i.e., worn by unwashed hosts and spiked with mineral oil) versus sleeves worn by unwashed hosts and spiked with either the attractive or the repulsive mixture.
Quantification and statistical analysis
Differences in the overall scent composition of samples were evaluated using Nonmetric multidimensional scaling (NMDS) and analysis of similarity (ANOSIM), performed in R using the vegan package.67
All comparisons between observed mosquito choices and chance levels, as well as comparisons between proportions of landing between groups, were achieved in R by means of Binomial Exact tests. A multi-level pattern analysis was conducted using the multipatt function of the indicspecies package in R17,68 to identify soap chemicals that are most strongly associated with the soaps that biased mosquitoes’ behavior.
All statistical details can be found in the figure legends and the results section.
Acknowledgments
We are grateful to the volunteers who volunteered their arms for odor sampling. We would like to thank Darren Dougharty as well as the Vinauger and Lahondère Laboratory members (K. Chandrasegaran, D. Eilerts, L. Fryzlewicz, J. Reinhold, I. Upshur, and N. Wynne) for assistance with mosquito care. We would also like to thank Paul Carlier for his helpful suggestions for the chemical analysis. This work was supported by the USDA National Institute of Food and Agriculture, Hatch project 1017860 to C.V. Finally, we would like to thank the three anonymous reviewers and the handling editors for their suggestions on the manuscript.
Author contributions
Conceptualization, M.V.G., A.K.T., C.L., and C.V.; methodology, M.V.G., A.K.T., C.L., and C.V.; formal analysis, M.V.G., A.K.T., and C.V.; investigation, M.V.G., A.K.T., B.D., and C.V.; writing—original draft preparation, M.V.G., A.K.T., B.D., C.L., and C.V.; writing—review and editing, M.V.G., A.K.T., C.L., and C.V.; visualization, M.V.G., A.K.T., C.L., and C.V.; supervision, C.L. and C.V.; project administration, C.V.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. One or more of the authors of this paper self-identifies as a gender minority in their field of research.
Published: May 10, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106667.
Supplemental information
References
- 1.World Health Organization . 2018. World Health Statistics 2018. [Google Scholar]
- 2.Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020;11:2130. doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A.Q.N., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeGennaro M., McBride C.S., Seeholzer L., Nakagawa T., Dennis E.J., Goldman C., Jasinskiene N., James A.A., Vosshall L.B. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raji J.I., Melo N., Castillo J.S., Gonzalez S., Saldana V., Stensmyr M.C., DeGennaro M. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 2019;29:1253–1262.e7. doi: 10.1016/j.cub.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afify A., Potter C.J. Insect repellents mediate species-specific olfactory behaviours in mosquitoes. Malar. J. 2020;19:127. doi: 10.1186/s12936-020-03206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z., Zung J.L., Hinze A., Kriete A.L., Iqbal A., Younger M.A., Matthews B.J., Merhof D., Thiberge S., Ignell R., et al. Mosquito brains encode unique features of human odour to drive host seeking. Nature. 2022;605:706–712. doi: 10.1038/s41586-022-04675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier U.R., Kline D.L., Barnard D.R., Schreck C.E., Yost R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti) Anal. Chem. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- 9.Penn D.J., Oberzaucher E., Grammer K., Fischer G., Soini H.A., Wiesler D., Novotny M.V., Dixon S.J., Xu Y., Brereton R.G. Individual and gender fingerprints in human body odour. J. R. Soc. Interface. 2007;4:331–340. doi: 10.1098/rsif.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhulst N.O., Qiu Y.T., Beijleveld H., Maliepaard C., Knights D., Schulz S., Berg-Lyons D., Lauber C.L., Verduijn W., Haasnoot G.W., et al. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One. 2011;6:e28991. doi: 10.1371/journal.pone.0028991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson A., Busula A.O., Voets M.A., Beshir K.B., Caulfield J.C., Powers S.J., Verhulst N.O., Winskill P., Muwanguzi J., Birkett M.A., et al. Plasmodium-associated changes in human odor attract mosquitoes. Proc. Natl. Acad. Sci. USA. 2018;115:E4209–E4218. doi: 10.1073/pnas.1721610115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wooding M., Naudé Y., Rohwer E., Bouwer M. Controlling mosquitoes with semiochemicals: a review. Parasites Vectors. 2020;13:80. doi: 10.1186/s13071-020-3960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Obaldia M.E., Morita T., Dedmon L.C., Boehmler D.J., Jiang C.S., Zeledon E.V., Cross J.R., Vosshall L.B. Differential mosquito attraction to humans is associated with skin-derived carboxylic acid levels. Cell. 2022;185:4099–4116.e13. doi: 10.1016/j.cell.2022.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaschka U. Naturally toxic: natural substances used in personal care products. Environ. Sci. Eur. 2015;27:1. doi: 10.1186/s12302-014-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díaz-Cruz M.S., Barceló D. The Handbook of Environmental Chemistry The handbook of environmental chemistry. Springer International Publishing; 2015. Concluding remarks and future research needs; pp. 401–407. [Google Scholar]
- 16.U.S. 2022. Most Used Bar Soap Brands 2020 Statista.https://www.statista.com/statistics/275244/us-households-most-used-brands-of-barsoap/ [Google Scholar]
- 17.De Cáceres M., Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- 18.Maturano Y.P., Assof M., Fabani M.P., Nally M.C., Jofré V., Rodríguez Assaf L.A., Toro M.E., Castellanos de Figueroa L.I., Vazquez F. Enzymatic activities produced by mixed Saccharomyces and non-Saccharomyces cultures: relationship with wine volatile composition. Antonie Leeuwenhoek. 2015;108:1239–1256. doi: 10.1007/s10482-015-0578-0. [DOI] [PubMed] [Google Scholar]
- 19.Kiralan M. Use of headspace solid-phase microextraction in rose (Rosa damescena Mill) products for volatile compounds. Journal of Essential Oil Bearing Plants. 2015;18:1266–1270. [Google Scholar]
- 20.Jiang J.-M., Hu W.-S., Hu J.-Z., Chen X.-P., Deng C.-J., Jiang F., Zheng S.-Q. Volatiles in fruits of two loquat cultivars Xiangtian , Jiefangzhong and their two offspring selections. J. Plant Genet. Resour. 2014;15:894–900. [Google Scholar]
- 21.Zhu G., Yu G. A pineapple flavor imitation by the note method. Food Sci. Technol. 2020;40:924–928. [Google Scholar]
- 22.Bhatia S.P., Jones L., Letizia C.S., Api A.M. Fragrance material review on 4-tert-butylcyclohexyl acetate. Food Chem. Toxicol. 2008;46(Suppl 12):S36–S41. doi: 10.1016/j.fct.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Williamson E.M., Priestley C.M., Burgess I.F. An investigation and comparison of the bioactivity of selected essential oils on human lice and house dust mites. Fitoterapia. 2007;78:521–525. doi: 10.1016/j.fitote.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Aboshi T., Musya S., Sato H., Ohta T., Murayama T. Changes of volatile flavor compounds of watermelon juice by heat treatment. Biosci. Biotechnol. Biochem. 2020;84:2157–2159. doi: 10.1080/09168451.2020.1787814. [DOI] [PubMed] [Google Scholar]
- 25.Poisson L., Schieberle P. Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008;56:5813–5819. doi: 10.1021/jf800382m. [DOI] [PubMed] [Google Scholar]
- 26.Opgrande, J.L., Dobratz, C.J., Brown, E., Liang, J., Conn, G.S., Shelton, F.J., and With, J. (2000). Benzaldehyde. Kirk-Othmer Encyclopedia of Chemical Technology (J. Wiley & Sons), pp. 496. 10.1002/0471238961.0205142615160718.a01. [DOI]
- 27.Denis W., Dunbar P.B. The determination of benzaldehyde in almond flavoring extract. J. Ind. Eng. Chem. 1909;1:256–257. [Google Scholar]
- 28.Lahondère C., Vinauger C., Okubo R.P., Wolff G.H., Chan J.K., Akbari O.S., Riffell J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA. 2020;117:708–716. doi: 10.1073/pnas.1910589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyasembe V.O., Torto B. Volatile phytochemicals as mosquito semiochemicals. Phytochem. Lett. 2014;8:196–201. doi: 10.1016/j.phytol.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Healy T.P., Jepson P.C. The location of floral nectar sources by mosquitoes: the long-range responses of Anopheles arabiensis Patton (Diptera: Culicidae) to Achillea millefolium flowers and isolated floral odour. Bull. Entomol. Res. 1988;78:651–657. [Google Scholar]
- 31.Jepson P.C., Healy T.P. The location of floral nectar sources by mosquitoes: an advanced bioassay for volatile plant odours and initial studies with Aedes aegypti (L.) (Diptera: Culicidae) Bull. Entomol. Res. 1988;78:641–650. [Google Scholar]
- 32.Logan J.G., Stanczyk N.M., Hassanali A., Kemei J., Santana A.E.G., Ribeiro K.A.L., Pickett J.A., Mordue Luntz A.J. Arm-in-cage testing of natural human-derived mosquito repellents. Malar. J. 2010;9:239. doi: 10.1186/1475-2875-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker T., Ignell R., Ghebru M., Glinwood R., Hopkins R. Identification of mosquito repellent odours from Ocimum forskolei. Parasites Vectors. 2011;4:183. doi: 10.1186/1756-3305-4-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikbakhtzadeh M.R., Terbot J.W., Otienoburu P.E., Foster W.A. Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J. Vector Ecol. 2014;39:372–383. doi: 10.1111/jvec.12113. [DOI] [PubMed] [Google Scholar]
- 35.Emami S.N., Lindberg B.G., Hua S., Hill S.R., Mozuraitis R., Lehmann P., Birgersson G., Borg-Karlson A.-K., Ignell R., Faye I. A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science. 2017;355:1076–1080. doi: 10.1126/science.aah4563. [DOI] [PubMed] [Google Scholar]
- 36.Nyasembe V.O., Tchouassi D.P., Pirk C.W.W., Sole C.L., Torto B. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Neglected Trop. Dis. 2018;12:e0006185. doi: 10.1371/journal.pntd.0006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degenhardt J., Köllner T.G., Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Bowen M.F. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J. Med. Entomol. 1992;29:843–849. doi: 10.1093/jmedent/29.5.843. [DOI] [PubMed] [Google Scholar]
- 39.Omondi A.B., Ghaninia M., Dawit M., Svensson T., Ignell R. Age-dependent regulation of host seeking in Anopheles coluzzii. Sci. Rep. 2019;9:9699. doi: 10.1038/s41598-019-46220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milugo T.K., Tchouassi D.P., Kavishe R.A., Dinglasan R.R., Torto B. Root exudate chemical cues of an invasive plant modulate oviposition behavior and survivorship of a malaria mosquito vector. Sci. Rep. 2021;11:14785. doi: 10.1038/s41598-021-94043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park B.-S., Choi W.-S., Kim J.-H., Kim K.-H., Lee S.-E. Monoterpenes from thyme (Thymus vulgaris) as potential mosquito repellents. J. Am. Mosq. Control Assoc. 2005;21:80–83. doi: 10.2987/8756-971X(2005)21[80:MFTTVA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Liu F., Chen L., Appel A.G., Liu N. Olfactory responses of the antennal trichoid sensilla to chemical repellents in the mosquito, Culex quinquefasciatus. J. Insect Physiol. 2013;59:1169–1177. doi: 10.1016/j.jinsphys.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Cork A., Park K.C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 44.Bernier U.R., Kline D.L., Schreck C.E., Yost R.A., Barnard D.R. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae) J. Am. Mosq. Control Assoc. 2002;18:186–195. [PubMed] [Google Scholar]
- 45.Curran A.M., Rabin S.I., Prada P.A., Furton K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005;31:1607–1619. doi: 10.1007/s10886-005-5801-4. [DOI] [PubMed] [Google Scholar]
- 46.Nesterkina M., Bernier U.R., Tabanca N., Kravchenko I. Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti. Open Chem. 2018;16:95–98. [Google Scholar]
- 47.McMeniman C.J., Corfas R.A., Matthews B.J., Ritchie S.A., Vosshall L.B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blum M.S. Semiochemical parsimony in the arthropoda. Annu. Rev. Entomol. 1996;41:353–374. doi: 10.1146/annurev.en.41.010196.002033. [DOI] [PubMed] [Google Scholar]
- 49.Hao H., Sun J., Dai J. Dose-dependent behavioral response of the mosquito Aedes albopictus to floral odorous compounds. J. Insect Sci. 2013;13:127. doi: 10.1673/031.013.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majeed S., Hill S.R., Birgersson G., Ignell R. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R. Soc. Open Sci. 2016;3:160467. doi: 10.1098/rsos.160467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghaninia M., Majeed S., Dekker T., Hill S.R., Ignell R. Hold your breath - differential behavioral and sensory acuity of mosquitoes to acetone and carbon dioxide. PLoS One. 2019;14:e0226815. doi: 10.1371/journal.pone.0226815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Himeidan Y.E., Elbashir M.I., Adam I. Attractiveness of pregnant women to the malaria vector, Anopheles arabiensis, in Sudan. Ann. Trop. Med. Parasitol. 2004;98:631–633. doi: 10.1179/000349804225021307. [DOI] [PubMed] [Google Scholar]
- 53.Havlicek J., Lenochova P. Chemical Signals in Vertebrates 11. Springer; 2007. Environmental effects on human body odour; pp. 199–210. [Google Scholar]
- 54.Lenochova P., Havlicek J. Chemical Signals in Vertebrates 11. Springer; 2007. Human body odour individuality; pp. 189–198. [Google Scholar]
- 55.Gallagher M., Wysocki C.J., Leyden J.J., Spielman A.I., Sun X., Preti G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008;159:780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Moraes C.M., Wanjiku C., Stanczyk N.M., Pulido H., Sims J.W., Betz H.S., Read A.F., Torto B., Mescher M.C. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc. Natl. Acad. Sci. USA. 2018;115:5780–5785. doi: 10.1073/pnas.1801512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindsay S., Ansell J., Selman C., Cox V., Hamilton K., Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet. 2000;355:1972. doi: 10.1016/S0140-6736(00)02334-5. [DOI] [PubMed] [Google Scholar]
- 58.Kelly, M., Su, C.-Y., Schaber, C., Crowley, J.R., Hsu, F.-F., Carlson, J.R., and Odom, A.R. (2015). Malaria parasites produce volatile mosquito attractants. mBio 6, pp. 002355–e315. 10.1128/mBio.00235-15. [DOI] [PMC free article] [PubMed]
- 59.Ansell J., Hamilton K.A., Pinder M., Walraven G.E.L., Lindsay S.W. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 2002;96:113–116. doi: 10.1016/s0035-9203(02)90271-3. [DOI] [PubMed] [Google Scholar]
- 60.Kelly D.W. Why are some people bitten more than others? Trends Parasitol. 2001;17:578–581. doi: 10.1016/s1471-4922(01)02116-x. [DOI] [PubMed] [Google Scholar]
- 61.Wang P., Cheng G. Mosquito-borne pathogens hijack human body odors to promote transmission. Sci. China Life Sci. 2023;66:180–182. doi: 10.1007/s11427-022-2231-7. [DOI] [PubMed] [Google Scholar]
- 62.Smallegange R.C., Qiu Y.T., van Loon J.J.A., Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem. Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- 63.Lahondère C. Mosquito electroantennography. Cold Spring Harb. Protoc. 2022;2022:top107679. doi: 10.1101/pdb.top107679. [DOI] [PubMed] [Google Scholar]
- 64.McBride C.S. Genes and odors underlying the recent evolution of mosquito preference for humans. Curr. Biol. 2016;26:R41–R46. doi: 10.1016/j.cub.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majeed S., Hill S.R., Dekker T., Ignell R. Detection and perception of generic host volatiles by mosquitoes : responses to CO2 constrains host-seeking behaviour. R. Soc. Open Sci. 2017;4:170189. doi: 10.1098/rsos.170189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tallon A.K., Hill S.R., Ignell R. Sex and age modulate antennal chemosensory-related genes linked to the onset of host seeking in the yellow-fever mosquito. Sci. Rep. 2019;9:43. doi: 10.1038/s41598-018-36550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., et al. Vegan: community ecology package. R package version 2. 2022;2020:5–7. [Google Scholar]
- 68.De Cáceres M., Legendre P., Moretti M. Improving indicator species analysis by combining groups of sites. Oikos. 2010;119:1674–1684. [Google Scholar]
- 69.Eilerts D.F., VanderGiessen M., Bose E.A., Broxton K., Vinauger C. Odor-specific daily rhythms in the olfactory sensitivity and behavior of Aedes aegypti mosquitoes. Insects. 2018;9:147. doi: 10.3390/insects9040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinauger C., Lahondère C., Wolff G.H., Locke L.T., Liaw J.E., Parrish J.Z., Akbari O.S., Dickinson M.H., Riffell J.A. Modulation of host learning in Aedes aegypti mosquitoes. Curr. Biol. 2018;28:333–344.e8. doi: 10.1016/j.cub.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: All data is available at https://data.mendeley.com/datasets/pdd3f2py8d/2.
-
•
Code: All R code is available at https://data.mendeley.com/datasets/pdd3f2py8d/2.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.