Abstract
Proliferation of T cells via activation of the T-cell receptor (TCR) requires concurrent engagement of accessory costimulatory molecules to achieve full activation. The best-studied costimulatory molecule, CD28, achieves these effects, in part, by augmenting signals from the TCR to the mitogen-activated protein (MAP) kinase cascade. We show here that TCR-mediated stimulation of MAP kinase extracellular-signal-regulated kinases (ERKs) is limited by activation of the Ras antagonist Rap1. CD28 increases ERK signaling by blocking Rap1 action. CD28 inhibits Rap1 activation because it selectively stimulates an extrinsic Rap1 GTPase activity. The ability of CD28 to stimulate Rap1 GTPase activity was dependent on the tyrosine kinase Lck. Our results suggest that CD28-mediated Rap1 GTPase-activating protein activation can help explain the augmentation of ERKs during CD28 costimulation.
Maximal activation of T lymphocytes following antigen presentation is thought to require at least two signals. One signal is generated by engagement of the T-cell receptor (TCR). A costimulatory molecule mediates the second signal. The best-studied costimulatory molecule is CD28, which is engaged by antigen-presenting cells during antigen presentation. Costimulation enhances production of interleukin-2 (IL-2) and T-cell proliferation. The importance of costimulation is demonstrated by the fact that TCR engagement in the absence of costimulation leads to a state of T-cell unresponsiveness termed anergy.
One signaling pathway that is required for IL-2 production is the Ras–Raf-1–extracellular-signal-regulated kinase (ERK) pathway or the mitogen-activated protein (MAP) kinase cascade (23, 34, 75) through the actions of the transcription factor AP-1 (69). Interfering mutant Ras (2, 57), Raf-1 (34), and MAP kinase kinase MEK (17) proteins can block IL-2 transcription following CD28 costimulation. The activation of ERK is thought to result in the activation of AP-1 (21, 74), presumably via the transcriptional activation of c-fos through the transcription factor Elk-1 (49). Indeed, a role for CD28 in c-fos expression has been demonstrated (31). The MAP kinase cascade has also been implicated in other aspects of T-cell function (22), including the regulation of T-cell development (10, 73).
In primary T cells, CD3 stimulation by itself can activate ERK (54). However, ERK activation is enhanced by CD28 coengagement (52). Activation of the Ras-ERK pathway is strongly inhibited under experimental conditions of T-cell unresponsiveness, or anergy, induced following stimulation via the TCR in the absence of CD28 costimulation (4, 19, 45). One candidate effector of this blockade of Ras signaling is Rap1, a small G protein that was initially cloned as an antagonist of Ras-dependent transformation in fibroblasts (37). Rap1 is constitutively activated in anergic T cells, and activation of Rap1 inhibits both ERK activation and IL-2 expression (4). Interestingly, TCR cross-linking activates Rap1 (4, 58) while coengagement of CD28 blocks this activation (58). In this study, we examine the mechanism by which CD28 activates the ERK signaling cascade in T cells via CD28's inhibition of Rap1.
MATERIALS AND METHODS
Cell culture, transfections, and stimulations.
The human T-cell leukemia Jurkat cell line and JCaM1.6 T-cell isolates (stably expressing wild-type Lck and Lck with the mutation W97A [LckW97A]) were maintained in RPMI medium with 10% fetal calf serum (FCS) at 37°C with 5% CO2. Jurkat cells expressing the mouse CD28 (mCD28) receptor (31) were maintained in RPMI medium–10% FCS–50 μg of G418 per ml. For transient transfections, 5 × 107 cells were resuspended in 400 μl of cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4–KH2PO4 [pH 7.6], 25 mM HEPES [pH 7.6], 2 mM EGTA [pH 7.6], 5 mM MgCl2, 5 mM glutathione) with the appropriate cDNAs and electroporated (250 V, 950 μF). All cDNAs were transfected at a concentration of 5 μg per 5 × 107 cells, except dominant negative Lck (dn.Lck) (10 μg), Rap1GAP1 (10 μg), and fos-luciferase (20 μg), and the total DNA transfected held constant with the addition of pCDNA3.1 (vector). The transfection efficiencies of transfected plasmids were monitored using 5 μg of cDNA encoding green fluorescent protein (GFP; Clonetech). In all experiments, transfection efficiency was greater than 60%. After a 24-h recovery in RPMI medium with 10% FCS, cells were incubated for 30 min on ice with or without anti-TCR-CD3 monoclonal antibody (MAb) (C305 MAb 1/40 hybridoma supernatant; gift from A. Weiss, University of California, San Francisco), anti-human CD28 MAb (CD28.2, 5 μg/ml; Pharmingen, San Diego, Calif.), and/or anti-mCD28 MAb (PV-1, 5 μg/ml; gift from C. June, Naval Medical Research Institute, Bethesda, Md.). Cells were stimulated by addition of 10 μg of goat anti-mouse secondary antibody (Southern Biotech) at 37°C for the indicated times. Where indicated in Fig. 1, PD98059 (50 μM) or UO126 (20 μM) was added to cells for 30 min of pretreatment and remained in the incubation medium for the duration of the experiment.
FIG. 1.
Costimulation with antibodies to CD28 augments signals to ERKs. (A) Jurkat T cells (left gel) were stimulated with anti-TCR-CD3 antibody (α-CD3) with or without anti-CD28 antibody (α-CD28) or left untreated, as indicated. Primary splenocytes (right gel) were stimulated with α-CD3 and/or α-CD28 as indicated, and phorbol myristate acetate (PMA) was used as a positive control. Incubation of cells with secondary antibody alone (2° Ab) served as a negative control. In both gels, phospho-ERK (pERK1/2) was measured using phosphorus-specific pERK antibodies. The positions of pERK1 and pERK2 are indicated. (B) Jurkat cells were incubated with anti-TCR-CD3 antibody, anti-CD28 antibody, or PMA (50 ng/ml) for 30 min on ice or left untreated as indicated and then stimulated at 37°C for 5 min following addition of 10 μg of a cross-linking antibody per ml. Secondary antibody alone (2°) was used as a negative control. Cells were lysed, and ERK2 was immunoprecipitated. Activation of immunoprecipitated ERK2 was measured by an in vitro kinase assay. Samples were subjected to SDS-PAGE and analyzed with a PhosphorImager. A representative gel with the position of the substrate MBP is presented. (C) Wild-type Jurkat cells were transfected with a fragment of the c-fos promoter coupled to luciferase (c-fos–luciferase) (70) as indicated and incubated with anti-human CD3 and anti-human CD28 antibodies for 6 h in the absence and presence of the MEK inhibitor PD98059 or UO126, as indicated. For all luciferase assays, lysates were prepared and assayed for luciferase activity. The data reflect the fold activation above basal luciferase activity (lane 1), with standard errors (n = 3).
T-cell isolation and antibody stimulation.
T cells were purified from C57BL6 splenocytes using a murine T-cell enrichment column (R&D Systems) according to the manufacturer's instructions. Ten million T cells were then incubated with 5 μg of anti-CD3 antibody (145-2C11; Pharmingen) with or without costimulation with 10 μg of anti-CD28 antibody (37.51; Pharmingen) on ice for 30 min, washed, and then incubated with 20 μg of goat anti-hamster immunoglobulin (Fisher) for 5 min at 37°C and lysed for the RalGDS assay, as described below.
DNA constructs and mutant proteins.
Wild-type bovine Rap1b or wild-type human Ha-Ras (71) was tagged at the amino terminus with 2× FLAG epitope (Kodak) by PCR and introduced into the BamHI and XbaI sites of pcDNA3.1 vector (Invitrogen). Rap1GAP1 (RG9T; gift from P. Polakis) was N-terminally tagged with the 2× FLAG epitope by PCR and introduced into the BamHI and XbaI sites of pcDNA3.1. pcDNA3.FLAG-RapE63/V12 (RapE63/V12) and dn.Rap1GAP1 were generated from pcDNA3. FLAG-RapWT and wild-type Rap1GAP1, respectively, using a Quick-change PCR mutagenesis kit (Stratagene). For dn.Rap1GAP1, this resulted in the replacement of residues 284 to 286 (RKR) with LIG (59). Constitutively active and dominant negative versions of Lck and Fyn were constructed using PCR-directed mutagenesis.
Raf-1 pull-down assays.
Experiments using FLAG-tagged Rap1 (FLAG-Rap1) and FLAG-tagged Ras (FLAG-Ras) were performed by transfecting Jurkat cells with cDNAs encoding either FLAG-Rap1 or FLAG-Ras and by immunoprecipitating the resulting protein complexes with anti-FLAG antibodies. The proteins within the pellets were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Raf-1 was detected by Western blotting (71). Equal amounts of FLAG-Rap1 and FLAG-Ras were confirmed by Western blotting.
In vitro kinase assays.
For ERK and FLAG-ERK assays, treated and untreated cells were lysed in a buffer containing 1% NP-40, 10% sucrose, 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 mM sodium orthovanadate, and 10 mM sodium fluoride. The lysates were spun at low speed to remove nuclei, and the supernatant was assayed for kinase activity. ERK activity was assayed as described previously (71) using myelin basic protein (MBP) and [γ-32P]ATP as substrates and equal protein amounts per treatment condition. For FLAG-ERK2 assays, FLAG antibodies were used to immunoprecipitate the kinase prior to assay.
In vivo Rap and Ras activation assays.
Activated Rap1 was isolated from cell lysates using a protocol adapted from the work of Franke et al. (20). Jurkat cells (5 × 107/ml) were stimulated with anti-TCR-CD3 and/or anti-CD28 MAb as previously described for the times indicated in Fig. 6 at 37°C. Cells were lysed in 400 μl of ice-cold Rap lysis buffer (10% glycerol, 1% NP-40, 50 mM Tris-HCl [pH 8.0], 200 mM NaCl, 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 10 μg of soybean trypsin inhibitor per ml, 10 mM NaF, 0.5 mM aprotinin, 1 mM Na3VO4). Lysates were clarified by centrifugation, and supernatants containing 0.5 mg of total protein were incubated with 60 μg of the glutathione S-transferase (GST)–RalGDS–Ras-binding domain (RBD) fusion protein (gift of J. L. Bos, Utrecht University, Utrecht, The Netherlands) coupled to glutathione agarose beads for 1 h at 4°C. Beads were pelleted and rinsed three times with lysis buffer, and protein was eluted from the beads with Laemmli buffer. Activated Ras was isolated from stimulated cell lysates using agarose-coupled GST-Raf1-RBD provided in a Ras activation assay kit (Upstate Biotechnology, Inc., Lake Placid, N.Y.) according to the manufacturer's recommended protocol. Proteins were separated by electrophoresis in a 12% gel, followed by transfer to a polyvinylidine difluoride membrane. Membranes were blocked in 5% milk and probed with either anti-Rap1 polyclonal antibody (anti-Krev-1; Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) or anti-Ras MAb (Upstate Biotechnology, Inc.) and then with a horseradish peroxidase-conjugated antimouse monoclonal secondary antibody (Amersham). Proteins were detected by enhanced chemiluminescence. Activation of ERK1 and ERK2 was detected from 30 μg of total-cell-stimulated lysates by immunoblotting with a phospho-specific MAP kinase MAb (New England Biolabs, Beverly, Mass.). The densitometric analysis of the bands was performed using NIH Image software.
FIG. 6.
Lck is required for CD28's inhibition of Rap1 and augmentation of ERK. (A) dn.Lck inhibits mobilization of intracellular calcium. Jurkat T cells were transfected with the vector (black line), dn.Lck (dark-gray line), or dn.Fyn (light-gray line) and preloaded with Indo-1. Cells were then stimulated with anti-CD3 MAb as indicated. Changes in the mobilization of intracellular free calcium, presented as ratios of 405 nm to 480 nm (representing Ca2+-associated Indo-1 [405 nm] and free Indo-1 [480 nm]), are shown as a function of time. Expression of dn.Fyn had no effect on Ca2+ flux. However, expression of dn.Lck blocked Ca2+ in these cells. (Inset) Successful loading of Jurkat cells, expressing dn.Lck, with Indo-1 was confirmed by treating cells with anti-CD3 MAb and ionomycin (+ iono), which resulted in a Ca2+ flux, or treating them with anti-CD3 antibody alone (− iono), which resulted in a block in Ca2+ flux. Results of a representative experiment are shown (n = 3). (B) Jurkat cells were transfected with LckR273 (dn.Lck) or the vector alone and incubated with anti-human CD3 antibody (α-CD3) and/or anti-human CD28 antibody (α-CD28) for 2 min or left untreated as indicated. T-cell lysates were prepared and assayed for Rap1 activation using GST-RalGDS, and Western blotting was performed using Rap1 antiserum. The position of Rap1 in control lysates and following isolation of glutathione-bound proteins is shown. Representative Western blots are shown (n = 3). (C) Jurkat cells were transfected with dn.lck or the vector along with FLAG-ERK2 and treated with antibodies to CD3 and/or CD28 for 5 min as indicated. The phosphorylation of FLAG-ERK2 was monitored by pERK Western blotting. The position of pFlag-ERK2 is shown. A representative Western blot is shown (n = 3). (D) JCaM1.6 cells stably expressing the vector (JCaM/vector), wild-type Lck (JCaM/LckWT) or LckW97A (JCaM/LckW97A) were incubated with anti-human CD3 antibody and/or anti-human CD28 antibody for 5 min or left untreated as indicated. Cell lysates were prepared and assayed for Rap1 activation using GST-RalGDS, and Western blotting was performed using Rap1 antiserum (upper blot). A representative western blot with the position of Rap1 is shown following a GST-RalGDS pull-down assay (n = 3). The lower Western blot shows the relative levels of expression of Rap1 in these cell lines. (E) The data in panel D are presented as the averages of results of three independent experiments with standard errors. Untr., untreated cells.
GAP assay.
Cos7 cells were transfected with FLAG-Rap1b, FLAG-Ras, FLAG-RapE63, or the pcDNA3.1 vector alone using Lipofectamine (GIBCO, BRL). Cells were allowed to recover for 48 h and then lysed in Rap lysis buffer, and FLAG epitope-tagged proteins were immunoprecipitated with 10 μg of M2-FLAG antibody (Sigma) coupled to protein A-Sepharose for 2 h at 4°C. Expression of FLAG-Rap and FLAG-Ras was confirmed by Western blotting. Immune complexes retained on protein A-agarose beads were washed twice in lysis buffer and once with Rap loading buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 1 mM MgCl2, 1 mM dithiothreitol, 5 μg of bovine serum albumin [BSA] per ml, 5% glycerol, 0.1% NP-40, 1 μg of leupeptin per ml, 0.5 μg of aprotinin per ml) or with Ras loading buffer (20 mM Tris [pH 7.5], 5 mM EDTA, 10 mM NaCl, 5 μg of BSA per ml, 1 μg of leupeptin per ml, 0.5 μg of aprotinin per ml). FLAG-Rap complexes were loaded with 0.1 μM [γ-32P]GTP (3,000 Ci/mmol) at 30°C for 20 min, and FLAG-Ras complexes were loaded with 0.1 μM [γ-32P]GTP at 30°C for 10 min. The MgCl2 concentration was adjusted to 10 mM to stabilize the FLAG-Rap-[γ-32P]GTP or FLAG-Ras-[γ-32P]GTP complex. Unincorporated GTP was removed by rinsing the complexes four times in ice-cold loading buffer containing 10 mM MgCl2. Jurkat cells were stimulated as described above and lysed in Rap lysis buffer. For each assay condition, 10 μg of total cellular protein was added to 100 μl of exchange buffer (25 mM Tris [pH 7.5], 5 mM MgCl2, 100 mM NaCl, 1 mM GTP, 1 μg of BSA per ml, 1 μg of leupeptin per ml, 0.5 mg of aprotinin per ml) along with the [γ-32P]GTP-loaded FLAG-Rap or FLAG-Ras complex and incubated at 30°C for the times indicated in the legends to Fig. 8 and 9. The reaction was stopped with 1 ml of stop buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 10 mM MgCl2). The complexes were washed four times with 1 ml of ice-cold stop buffer to remove released 32P. Total radioactivity remaining associated with the immune complexes was measured by scintillation counting. [α-32P]GTP was used as a negative control for all GTPase-activating protein (GAP) assays.
FIG. 8.
Enhancement of Rap1 GTPase activity by CD28. (A) Specificity of Rap1GAP1 for Ras and selected Rap1 mutants in vitro. Human Rap1GAP1 was expressed in Cos7 cells and incubated with recombinant Rap1 mutant proteins loaded with [γ-32P]GTP. The percentages of hydrolysis of [γ-32P]GTP from wild-type Rap1(Rap1 WT), RapV12/E63 (V12/E63), Ras, and buffer alone are indicated by the percentage of γ-32P released from Rap or Ras loaded in vitro. (B) CD28 stimulation of Rap1 GAP activity and introduction of E63 into RapV12, which blocks GTPase activity. Jurkat cells were incubated with anti-human CD3 antibody (α-CD3) and/or anti-human CD28 antibody (α-CD28) for 5 min or left untreated (Untr.) as indicated. Lysates were prepared and incubated for 20 min with recombinant FLAG-tagged Rap1 (left graph) or RapV12/E63 (right graph) loaded in vitro with [γ-32P]GTP, as indicated. The percentages of release of [γ-32P]GTP are shown with standard errors. (n = 3). (C) Cells were treated as described for panel B and were assayed with [γ-32P]GTP. Release of α-32P was monitored as described in Materials and Methods, and the percentages of release of [γ-32P]GTPase is shown with standard errors (n = 6).
FIG. 9.
Involvement of Lck in CD28's enhancement of Rap1 GTPase activity. (A) The 16 carboxy-terminal amino acid residues of CD28 are required for stimulating Rap1GAP activity. Jurkat cells stably expressing full-length wild-type mCD28 (mCD28-WT) or Jurkat cells stably expressing mCD28-CΔ16 were treated with anti-human CD3 antibody (α-hCD3) and/or anti-mCD28 antibody (α-mCD28) or left untreated (Untr.) as indicated, and GTPase assays were performed as described in Materials and Methods. Percentages of release of [γ-32P]GTP are shown with standard errors (n = 3). (B) Lck, but not Fyn, stimulates Rap1GAP activity. Jurkat cells were transfected with the vector alone, ca.Lck, or ca.Fyn and incubated with recombinant [γ-32P]GTP-loaded Rap1 (left) and either the vector alone or ca.Lck and incubated with recombinant [γ-32P]GTP-loaded Ras (right) as indicated. Percentages of release of [γ-32P]GTP are shown with standard errors (n = 5). (C) JCaM/vector, JCaM/LckWT, or JCaM/LckW97A cells were incubated with anti-human CD3 antibody (α-CD3) and/or anti-human CD28 antibody (α-CD28) for 5 min or left untreated as indicated. Cell lysates were prepared and assayed for Rap1 GAP activity as described for panel B. Percentages of release of [γ-32P]GTP are shown with standard errors (n = 3).
Guanine nucleotide exchange factor (GEF) assay.
FLAG-Rap and FLAG-Ras immune complexes were prepared as described above and resuspended in Rap exchange buffer (20 mM Tris [pH 7.5], 1 mM MgCl2, 20 mM EDTA, 100 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol, and 1 mg of BSA per ml) or Ras exchange buffer (20 mM Tris [pH 7.5], 1 mM MgCl2, 10 mM NaCl, 5 μg of BSA per ml, 1 μg of leupeptin per ml, 0.5 mg of aprotinin per ml). FLAG-Rap immune complexes were loaded with 5 μCi of [3H]GDP (34 Ci/mmol) at 30°C for 20 min, and FLAG-Ras immune complexes were loaded with 5 μCi of [3H]GDP (34 Ci/mmol) at 37°C for 10 min. FLAG-Rap-[3H]GDP or FLAG-Ras-[3H]GDP immune complexes were stabilized by adjusting the MgCl2 concentration to 25 mM. Unincorporated [3H]GDP was removed by washing the beads four times in loading buffer containing 25 mM MgCl2. Jurkat cells were stimulated as described above and lysed in Rap lysis buffer. For each assay condition, 50 μg of total cellular protein was added to 250 μl of exchange buffer containing 100 μM GDP, 1.5 mM GTP, and 50,000 to 100,000 cpm of the FLAG-Rap-[3H]GDP or FLAG-Ras-[3H]GDP complex and incubated at 30°C for the times indicated in the legend to Fig. 7. The reaction was stopped by adding 1 ml of ice-cold stop buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 25 mM MgCl2), and the reaction mixture was immediately applied to nitrocellulose filters under vacuum. The filters were rinsed four times with 3 ml of stop buffer, and retained radioactivity was measured by scintillation counting.
FIG. 7.
Lack of regulation of Rap1 GEF activity by CD28. Jurkat cells were incubated with anti-human CD3 antibody (α-CD3) and/or anti-human CD28 antibody (α-CD28) for 5 min or left untreated (Untr.) as indicated. Lysates were prepared and incubated for 10 min at 30°C with recombinant [3H]GDP-loaded Rap1 protein (left panel) or Ras protein (right panel) bound to agarose beads. The percentages of [3H]GDP released from the beads are indicated. Standard errors are shown (n = 3).
Calcium flux analyses.
Calcium measurements were performed as previously described (33). Briefly, Jurkat cells were cotransfected with (i) dn.Lck and cDNA encoding GFP, (ii) dn.Fyn and GFP, or (iii) pcDNA3.1 (vector) and GFP as described above. After recovery, cells were resuspended to 2 × 106/ml in complete medium supplemented with a 2 mM concentration of a solution containing the Ca2+ indicator Indo-1 and 0.02% pluronic F127 (Molecular Probes, Eugene, Oreg.) and incubated at 37°C for 30 min. Cells were then washed in ice-cold buffer (150 mM NaCl, 1 mM CaCl2, 1 mM MgSO4, 5 mM KCl, 10 mM glycine, 15 mM HEPES [pH 7.4]), resuspended to 2 × 106 cells/ml, and then kept on ice until use. Ca2+ fluctuations, before and after the addition of anti-CD3 MAb (Pharmingen) at 10 μg/ml, were monitored using a fluorescence-activated cell sorter (FACS) Vantage flow cytometer by gating on the GFP-positive cells. Cells were excited at 355 nm, and emission was measured at 480 nm, representing free Indo-1, and at 405 nm, representing Ca2+-associated Indo-1, to give a ratio of 405 nm to 480 nm.
Luciferase assays.
Luciferase assays were performed as previously described (71).
RESULTS
CD28 costimulation augments ERK activation through the TCR and CD3.
In Jurkat cells (Fig. 1A, left gel) and in primary T cells harvested from mouse splenocytes (Fig. 1A, right gel), activation of the TCR and CD3 by antibody cross-linking produced a modest activation of ERKs as measured by phosphorus-specific antibodies recognizing pT202pY204 of human ERK1 and -2 (pERK). However, activation of ERKs was strongly augmented upon activation of CD28 by cross-linking antibodies (Fig. 1A). Similar results were seen for ERK activity by in vitro kinase assay (Fig. 1B). The data demonstrate the profound synergy between CD3 and CD28 on ERK activation in primary T cells, as well as in Jurkat T cells. One potential downstream target of ERKs is the expression of c-fos. Activation of the c-fos promoter coupled to luciferase (70) can also be enhanced by CD28 costimulation. This action of CD28 on the fos promoter was blocked by the MEK inhibitors PD98059 (14) and UO126 (15) (Fig. 1C), suggesting that the enhancement of fos expression by CD3-CD28 reflected CD3-CD28's ability to augment ERK activity.
Rap1 limits ERK activation.
It has been previously demonstrated that CD3 engagement stimulates activation of Rap1 (58). Rap1 is a small G protein that antagonizes Ras signaling to ERKs in a cell type-specific manner (71) through its antagonism of the MAP kinase kinase kinase Raf-1. In cells that express the Raf isoform B-Raf, as in PC12 cells, Rap1 has the opposite effect: it activates ERKs (71). In Jurkat T cells (Fig. 2A) and primary T cells (data not shown), Rap1 is expressed but B-Raf is not. Therefore, we predict that Rap1 activation may antagonize ERK signaling in Jurkat cells, as has been shown for primary T cells (4, 58). Indeed, expression of the constitutively active mutant protein of Rap1 Rap1E63 blunts CD3-CD28 activation of ERKs (Fig. 2B). That CD3-CD28's activation of ERKs requires Ras was demonstrated by the ability of the interfering mutant protein RasN17 to block ERK activation through CD3-CD28 (Fig. 2B). To examine the role of endogenous Rap1 to limit ERK activation following TCR-CD3 stimulation, Rap1 activity was inhibited by transfection of Rap1GAP1, a Rap1-specific GAP (35, 56). The inhibition of Rap1 by Rap1GAP1 augmented CD3's activation of ERKs (Fig. 2C), suggesting that endogenous Rap1 serves to limit CD3 activation of ERKs. It has been proposed that the antagonism of signals to ERKs by Rap1 may be due to the sequestration of Raf-1 by activated Rap1 (53, 61). Consistent with this model, CD3 stimulation of Jurkat cells promoted the association of Raf-1 and Rap1 (Fig. 2D). Interestingly, CD3 also promoted the association of Raf-1 with Ras (Fig. 2D), consistent with the ability of CD3 to activate both Rap1 and Ras (58). This association of Ras and Raf-1 was augmented following the inhibition of endogenous Rap1 by the Rap1 inhibitor Rap1GAP1 (Fig. 2D), presumably because Rap1 sequesters Raf-1 only in the GTP-loaded state (53). This finding suggests that activation of Rap1 by CD3 limits ERK signaling by limiting Ras-dependent recruitment of Raf-1.
FIG. 2.
Rap1 limits signals from the TCR to ERKs. (A) Jurkat T cells express Rap1 but not B-Raf. Western blots for B-Raf and Rap1 expression in both PC12 cells and Jurkat cells are shown. In the left gel, low levels of Rap1 are detected in Jurkat cells compared to levels in PC12 cells. In the right gel, B-Raf expression is very high in PC12 cells, in which Rap1 activates ERK (71), but absent in lymphocytes, in which Rap1 inhibits ERK (4). (B) Constitutively active Rap1 blocks ERK activation by CD28 costimulation in Jurkat cells. Jurkat cells were transfected with the vector, Rap1E63, or RasN17 as indicated. Cells were treated with anti-CD3 antibody (α-CD3) and/or anti-CD28 antibody (α-CD28) for 5 min or not treated (lanes 0) as indicated. Phosphorylation of ERK1 and -2 as monitored by Western blotting with pERK is shown. The positions of pERK1 and -2 are shown. In the lower gel, control shows equivalent levels of protein loading. (C) Jurkat cells were transfected with 10 μg each of cDNAs encoding the vector or Rap1GAP1 as indicated. All cells received 10 μg of FLAG-ERK2 cDNA. Subsequently, cells were incubated with anti-TCR-CD3 antibody for 30 min on ice or left untreated. Cells were then activated by incubation at 37°C for 10 min. Cells were lysed, and FLAG-ERK2 was immunoprecipitated. Activation of immunoprecipitated ERK2 was measured by in vitro kinase assay. Samples were subjected to SDS-PAGE and analyzed with a PhosphorImager. A representative gel with the position of the substrate MBP indicated is presented. (D) Ras activation of Raf-1 is limited by endogenous Rap1. Jurkat cells were transfected with either FLAG-Rap1 or FLAG-Ras and treated with α-CD3 or left untreated as indicated. The associated endogenous Raf-1 was measured following FLAG immunoprecipitation and anti-Raf-1 antibody Western blotting. For FLAG-Ras, cells were also transfected with the vector or Rap1GAP1 as indicated. A representative gel with the position of Raf-1 indicated is shown (n = 3).
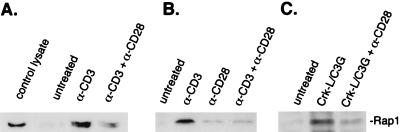
CD28 inhibits Rap1 activation.
It was previously demonstrated that CD3's activation of Rap1 can be inhibited by coengagement of CD28 in phytohemagglutinin blasts and in some T-cell lines (58). This finding was confirmed using primary splenic T cells (Fig. 3A) and the Jurkat T-cell line (Fig. 3B). This inhibition was likely a direct effect on Rap1 rather than on an upstream activator of Rap1, as CD28 also blocked the activation of Rap1 triggered by overexpression of C3G, a Rap1-specific GEF (78) (Fig. 3C).
FIG. 3.
Inhibition of Rap1 by CD28 costimulation in primary splenic T cells and in human Jurkat cells. (A) Activation of Rap1 in primary splenic T cells. Primary splenic T cells were harvested and incubated with anti-mCD3 antibody (α-CD3) and/or anti-mCD28 antibody (α-CD28) for 5 min or left untreated as indicated. (B) Activation of Rap1 in human Jurkat T cells. Jurkat cells were incubated with anti-human CD3 antibody (α-CD3) and/or anti-human CD28 antibody (α-CD28) for 5 min or left untreated as indicated. (C) Inhibition of Rap1 by CD28 following transfection of human Jurkat T cells. Wild-type Jurkat cells were transfected with CrkL/C3G or the vector alone and incubated with α-CD28 as indicated. In all experiments, T-cell lysates were prepared and assayed for Rap1 activation using GST-RalGDS and Western blotting was performed using Rap1 antiserum. The position of Rap1 in control lysates and following isolation of glutathione-bound proteins is shown. Representative Western blots are shown (n = 3).
Mapping of sequences of CD28 required to inhibit Rap1 activation.
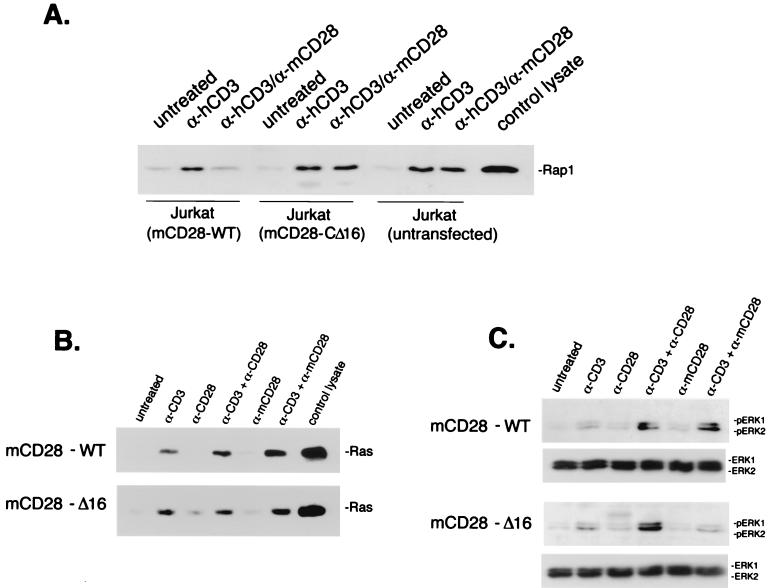
To determine which residues of CD28 were required to inhibit Rap1 activation, we used Jurkat cells stably expressing either full-length wild-type mCD28 (mCD28-WT) or a truncated form of mCD28 lacking 16 C-terminal residues (mCD28-CΔ16) (31). These cells express equivalent numbers of mCD28 molecules, as measured by FACS analysis (data not shown). Similar to the results with endogenous human CD28 in Jurkat cells, engagement of mCD28 in Jurkat cells was able to inhibit Rap1 activation by anti-CD3 antibody (Fig. 4A). Importantly, engagement of mCD28-CΔ16 using anti-mCD28 antibody was unable to inhibit Rap1 activation by anti-CD3 antibody (Fig. 4A). This result demonstrates that the 16 carboxy-terminal residues of CD28 were required to inhibit Rap1 activation by CD3.
FIG. 4.
CD28's inhibition of Rap 1 and enhancement of ERKs map to the 16 carboxy-terminal residues of CD28. (A) Requirement of the carboxy terminus of CD28 in the inhibition of Rap1 by CD28. Jurkat cells and Jurkat cells stably expressing wild-type mCD28 [Jurkat (mCD28-WT)] or mCD28 with its 16 carboxy-terminal amino acids deleted [Jurkat (mCD28-CΔ16)] were incubated with anti-human CD3 antibody (α-hCD3) and/or anti-mCD28 antibody (α-mCD28) for 5 min or treated with secondary antibody alone (untreated). In all experiments, T-cell lysates were prepared and assayed for Rap1 activation using GST-RalGDS and Western blotting was performed using polyclonal Rap1 antiserum. The position of Rap1 in control lysates (A and D) and following isolation of glutathione-bound proteins is shown. Representative Western blots are shown (n = 3). (B) Ras activation does not require the carboxy terminus of CD28. Jurkat mCD28-WT cells and mCD28-CΔ16 cells were incubated with anti-human CD3 antibody (α-CD3), anti-human CD28 antibody (α-CD28), and/or anti-mCD28 antibody (α-mCD28) for 5 min or left untreated as indicated. Lysates were prepared and assayed for Ras activation using GST–Raf-1–RBD, and Western blotting was performed using Ras antiserum. The position of Ras in control lysates and following isolation of glutathione-bound proteins is shown. (C) The 16 carboxy-terminal amino acids residues of CD28 that are required for inhibiting Rap1 are required for stimulating ERK activity. Jurkat mCD28-WT cells and mCD28-CΔ16 cells were incubated with anti-human CD3 antibody (α-CD3), anti-human CD28 antibody (α-CD28), and/or anti-mCD28 antibody (α-mCD28) for 5 min or left untreated as indicated. Lysates were prepared and assayed for ERK activation using phospho-specific ERK (pERK) antibodies. Representative Western blots with the positions of pERK1 and pERK2 indicated are shown (n = 3).
The 16 carboxy-terminal residues of CD28 are required for CD28's augmentation of ERKs but not Ras.
We examined the actions of CD3-CD28 on Ras activation using a Raf-1 fragment containing the RBD linked to GST (GST–Raf-1–RBD). Anti-CD3 antibody was able to stimulate Ras activity; this stimulation was only slightly enhanced by the addition of antibodies to CD28 (Fig. 4B, upper gel), similar to what occurred with mCD28-CΔ16 cells (Fig. 4B, lower gel). In contrast, ERK activation under the same conditions was strongly augmented by CD28 (Fig. 4C, upper gel). However, CD28 costimulation of mCD28-CΔ16 cells was not sufficient to activate ERKs (Fig. 4C, lower gel). This finding suggests that full ERK activation is not solely dependent on Ras but also requires negation of the actions of Rap1.
The tyrosine kinase Lck mediates CD28's inhibition of Rap1.
Recently, the 16 carboxy-terminal amino acids of CD28 were shown to be important for binding and activation of Lck (31). To directly test whether activation of Lck is involved in inhibiting Rap1 activation, we expressed a constitutively active form of Lck (LckF505 or ca.Lck) and analyzed its effects on Rap1 (Fig. 5A). Expression of LckF505 was able to block the activation of Rap1 by CD3. This was a specific effect, as expression of a constitutively active form of Fyn, a related src family kinase (FynF531 or ca.Fyn), was unable to inhibit CD3's activation of Rap1 (data not shown). Indeed, overexpression of FynF531 stimulated Rap1 activation by itself, suggesting that the activation of Rap1 by anti-CD3 antibody might be mediated via the activation of Fyn, as has been suggested (4). Importantly, LckF505 was also able to block the activation of Rap1 by FynF531 (Fig. 5B). These results suggest that activation of Fyn is sufficient to activate Rap1 but that activation of Lck blocks this activation.
FIG. 5.
Lck is sufficient to inhibit TCR stimulation of Rap1. (A) Jurkat cells were transfected with ca.Lck or the vector alone and incubated with anti-human CD3 antibody (α-CD3) for 2 min or left untreated as indicated. (B) Jurkat cells were transfected with LckF505 (ca.Lck) and/or FynF531 (ca.Fyn) or the vector alone. In both experiments, T-cell lysates were prepared and assayed for Rap1 activation using GST-RalGDS and Western blotting was performed using Rap1 antiserum. The position of Rap1 in control lysates and following isolation of glutathione-bound proteins is shown. Representative Western blots are shown (n = 3).
We next tested whether an interfering mutant Lck (LckR273 or dn.Lck) could block CD28's actions on Rap1. In this mutant protein, the essential lysine at residue 273 has been replaced with an arginine (7) to create a kinase-dead protein that has been shown to block Lck function selectively in vivo (1, 28, 29, 41). After transfection of the Lck and Fyn genes in Jurkat cells, we found that expression of kinase-inactive Lck, but not expression of kinase-inactive Fyn (dn.Fyn) (9), was able to completely block TCR-induced calcium flux in Jurkat T cells (Fig. 6A), suggesting that this mutant protein is selective for Lck-dependent actions. Expression of dn.Lck in Jurkat cells blocked the ability of CD28 to inhibit Rap1 activation (Fig. 6B), demonstrating that Lck mediates CD28's regulation of Rap1. Expression of dn.Lck in Jurkat cells also blocked the ability of CD28 to augment ERK activation (Fig. 6C). A similar result was found using JCaM1.6 cells that lack functional Lck (67). In JCaM1.6 cells, stimulation through neither the TCR nor CD28 was able to regulate Rap1 activity (Fig. 6D and E). Interestingly, stable expression of Lck (using JCaM/LckWT cells [11, 12]) restored Rap1 regulation by both TCR and CD28. This result suggests that Lck is required for both Rap1 activation by CD3 and Rap downregulation by CD28. The ability of Lck to restore Rap1 activation by CD3 may reflect the role of Src family kinases in this process (4, 58) and, in part, in the increased expression of Rap1 seen in Lck-expressing cells (Fig. 6D, lower gel).
The ability of CD28 to inhibit Rap1 requires the expression of an intact Lck SH3 domain.
In addition to containing the kinase domain (SH1 domain), Lck contains an SH2 and SH3 domain. Binding of Lck to the proline-rich domain (PRD) of CD28 is thought to require an intact SH3 domain (31), as is Lck-dependent activation of ERKs (11). The importance of the SH3 domain in ERK signaling is suggested by experiments examining the expression of a mutant Lck SH3 (LckW978A). For example, wild-type Lck, but not LckW978A, can restore ERK activation in Lck-defective JCaM1.6 cells (11). Using JCaM1.6 cells expressing either wild-type Lck (JCaM/LckWT) or LckW97A (JCaM/LckW97A), we examined whether the SH3 domain was required in CD28's inhibition of Rap1. Activation of CD28 inhibited Rap1 activation in the JCaM/LckWT cells but not in the related JCaM/LckW97A cells (Fig. 6D, upper gel, and E). These data show that CD28 inhibition of Rap1 requires an intact SH3 domain of Lck.
CD28 inhibits Rap1 by stimulating extrinsic Rap1-specific GTPase activity.
The activity of small G proteins like Rap1 is regulated by both positive and negative factors. GEFs activate Rap1 by catalyzing the exchange of GDP for GTP. GAPs negatively regulate Rap1 by enhancing the intrinsic GTPase activity of the G protein. CD28's inhibitory effects on Rap1 may therefore be mediated either by inhibition of Rap1 GEF activity or by enhancement of Rap1 GAP activity.
We first examined the ability of CD28 to modulate Rap1 GEF activity. GEF activity was measured in an in vitro assay, using recombinant Rap1 loaded with [3H]GDP, as previously described (42, 43). While anti-CD3 antibody stimulated GEF activity, anti-CD28 antibody had no effect on Rap1 GEF activity. More importantly, CD28 did not inhibit the GEF activity stimulated by anti-CD3 antibody (Fig. 7, left panel). Therefore, inhibition of Rap1 GEF was not likely to contribute to CD28's inhibition of Rap1. CD28, however, did have a modest effect on Ras exchange (right panel).
To examine the possibility that a Rap1 GAP was stimulated by CD28, we used an in vitro GAP assay. For these experiments, we used recombinant wild-type Rap1 loaded with [γ-32P]GTP and measured the release of 32P catalyzed by exogenous GAP activities. To validate this assay, we expressed the human Rap1GAP1 protein, which displays Rap1-specific activity (60). The specificity of Rap1GAP1 for Rap1 was shown by its inability to hydrolyze GTP bound to Ras (Fig. 8A). In addition, we assayed the release of [α-32P]GTP to confirm that CD28 was not stimulating exchange under the GAP assay conditions. Using this assay, we measured Rap1 GAP activity from Jurkat cell lysates before and after treatment with antibodies to CD3, CD28, or both (56). CD28 stimulation by itself was able to enhance a Rap1-specific GAP activity (Fig. 8B, left graph). Anti-CD28 antibody treatment stimulated the release of 32P from [γ-32P]GTP-loaded recombinant wild-type Rap1, but it had no effect on [γ-32P]GTP-loaded RapV12/E63, a mutated form of Rap1 that cannot be regulated by Rap1GAP (4) (Fig. 8A and B, right graph). CD28 did not have any effect on Rap1 loaded with [α-32P]GTP under the conditions of this GAP assay (Fig. 8C), consistent with the inability of CD28 to regulate Rap1 exchange activity (Fig. 7). Consistent with this, the Rap1 GAP activity from anti-CD28 antibody-treated cell extracts was heat sensitive (data not shown), suggesting that the activity in anti-CD28 antibody-treated cell extracts represented a bona fide Rap1 GAP activity. Because the activity expressed from the transfected cDNA encoding Rap1GAP1 may be distinct from the Rap1GAP activity identified in stimulated Jurkat T-cell lysates, we refer to the cDNA as Rap1GAP1 and the GAP activity from the lysates as Rap1 GAP activity.
We confirmed that the last 16 residues of CD28 were required to stimulate Rap1 GTPase activity (Fig. 9A). Truncation of the last 16 residues of CD28 blocked its ability to stimulate Rap1 GAP activity. Furthermore, expression of LckF505 (ca.Lck) in Jurkat cells was also able to stimulate Rap1 GTPase activity but not Ras GTPase activity (Fig. 9B). This activation was specific because overexpression of a constitutively active form of the highly related Src family kinase (ca.Fyn) was unable to stimulate Rap1 GAP activity (Fig. 9B). Therefore, although some Lck-dependent functions are partially rescued by Fyn (12), Fyn cannot rescue Rap1 GAP activation by Lck. We next investigated the requirement of the SH3 domain of Lck for CD28's activation of Rap1GAP activity using JCaM/LckWT or JCaM/LckW97A cells. As shown in Fig. 9C, JCaM/LckWT cells demonstrated elevated Rap1GAP activity following CD28 stimulation to a significantly higher degree than did JCaM/LckW97A cells. These data support a model where CD28's ability to stimulate Rap1 GAP activity is mediated by the last 16 residues of CD28 and the SH3 domain of Lck.
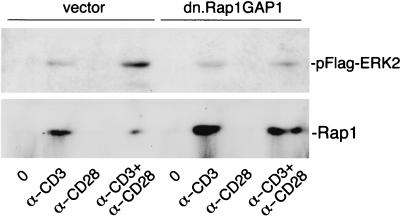
Mutation of the essential arginines within the catalytic active site of all known GAPs produces an interfering mutant protein that can block GAP function (30). We next tested the ability of Rap1GAP1-R284L/K285I/R286G (dn.Rap1GAP1) (59) to regulate CD28's activation of ERK (Fig. 10). As expected, dn.Rap1GAP1 blocked CD28's augmentation of ERK, suggesting that Rap1GAP1 (or a related Rap1-specific GAP) played an important role in CD28's augmentation of ERKs. Rap1 assays performed in parallel confirmed that expression of dn.Rap1GAP1 stimulated Rap1 activation (Fig. 10). Taken together, these data show that CD3 activation of Rap1 limits ERK signals but that CD28 activation of a Rap1 GAP augments ERK signals (Fig. 11).
FIG. 10.
Interfering with Rap1 GAP blocks CD28 enhancement of ERKs. Jurkat cells were transfected with FLAG-ERK2 along with the vector and dn.Rap1GAP1 or the vector alone as indicated. Cells were treated with anti-CD3 antibody (α-CD3) and/or anti-CD28 antibody (α-CD28) or left untreated (lanes 0), as indicated. In the upper blot, the activation of FLAG-ERK2 was monitored using FLAG immunoprecipitation followed by pERK Western blotting. The position of pFlag-ERK2 is shown. A representative gel is shown (n = 4). In the lower blot, lysates prepared as described above were subjected to a GST-RalGDS pull-down assay and Rap1 Western blotting. The position of Rap1 is shown.
FIG. 11.
Diagram of Rap1 regulation by CD28/Lck. Rap1 is regulated by the balance between the actions of Rap1 GEFs and Rap1 GAPs. During TCR-CD3 engagement, the activation of Rap1 limits signals generated by activated Ras. Costimulation of CD28 recruits Lck to its C terminus, where it can activate a Rap1 GAP. This activity functions to reverse the Rap1-dependent antagonism of Ras signaling to strongly potentiate Ras-dependent signals to ERKs.
DISCUSSION
The small G protein Rap1 has been proposed to be an antagonist of Ras-dependent signaling pathways (4, 36, 62, 71) in multiple cell types. However, two papers have also suggested that Rap1 may not limit Ras signaling (6, 79). The data presented here show that Rap1 limits CD3 signals to ERKs in Jurkat T cells. Moreover, we show that CD3-dependent activation of Rap1 is associated with the sequestration of Raf-1 away from Ras and propose that this provides a mechanism for Rap1 antagonism of Ras function. Furthermore, activated Rap1 is a target for regulation by additional intracellular signals. In particular, inhibition of Rap1 via CD28 costimulation enhances ERK activation. Therefore, Rap1 is a negative regulator of the ERK signaling pathway in T cells and its inhibition by CD28 augments signals to ERK.
We show here that Lck is both necessary and sufficient for CD28's inhibition of Rap1 and its enhancement of ERKs. A structure-function analysis of CD28 revealed that the last 16 residues of the CD28 cytoplasmic domain were required for these effects. Contained within these residues is a PRD capable of associating with the SH3 domain of Lck (31). Therefore, we tested whether constitutively active forms of Lck could also affect Rap1 activation. Constitutively, activation of Lck strongly inhibited Rap1 activation by TCR engagement. Furthermore, interfering with endogenous Lck function blocked both CD28's inhibition of Rap1 and augmentation of ERKs.
Lck activation by CD28 is mediated by interactions between Lck's SH3 domain and the PRD of CD28 (31). Interestingly, the SH3 domain of Lck has previously been shown to be important for Lck's activation of ERK (11) but not for other actions of Lck, including the activation and phosphorylation of ZAP-70 and LAT (66). Here, we show that Lck is also required for the ability of CD28 to inhibit Rap1 activation, suggesting that CD28's ability to enhance ERK activation is mediated by its ability to inhibit Rap1. Our data demonstrate that both the SH3 domain of Lck and the PRD of CD28 are required for this action. Since the activation of Rap1 is negatively regulated by Rap1-specific GAPs, Rap1 GAPs are potential targets of CD28 action. Indeed, measurements of Rap1 GAP activity in cell extracts after CD28 engagement revealed that CD28 engagement does result in significant stimulation of Rap1 GAP activity and that this stimulation requires both an intact PRD of CD28 and the SH3 domain of Lck.
Despite extensive research into its mechanism of action, the exact function of CD28 remains unclear. Most current models propose that CD28 provides an essential second signal required for T-cell activation. In these models, T-cell activation requires two distinct signals for full activation (27, 40). The first signal is transduced by the TCR, while CD28 provides the second signal, a process termed costimulation (48). However, the exact signal mediated by CD28 engagement has remained elusive. While it has been proposed that CD28 regulates signals like that activating jun N-terminal protein kinase (JNK) or NF-κB, these signals require coengagement of the TCR; CD28 engagement by itself does not activate JNK or NF-κB (68). Furthermore, JNK activation utilizes the membrane-proximal region of CD28, a region that is dispensable for IL-2 production (3).
Recently, it was shown that CD28 engagement by itself can induce tyrosine phosphorylation as well as stimulate a c-fos receptor construct in an Lck-dependent fashion (31). This finding suggested that CD28 may not transduce a distinct signal but that rather it functions to potentiate signals initiated by the TCR. In this model, although both TCR and CD28 can couple to Lck, CD28 also utilizes Lck in a distinct way to further augment TCR signaling (16, 64). It has been proposed that Lck function is dictated not only through its activation but also by association with specific receptors. Here we extend those findings and demonstrate that Lck activation by CD28 results in the generation of a specific signal, activation of a Rap1 GAP, which is distinct from Lck's other functions, which are thought to be triggered by binding proteins associated with the TCR (63, 66). The findings presented here can potentially reconcile these two models and provide new insight into the function of CD28 costimulation.
In the absence of signaling by the TCR, CD28 is still capable of activating Lck, resulting in Rap1 GAP activation. However, in the absence of Rap1 activation, the stimulation of a Rap1 GAP is without effect on ERK signaling. This can explain why little to no signaling had previously been detected by CD28 engagement itself. In contrast, in the presence of Rap1 activation, CD28 engagement may have a significant effect. Consistent with previous reports, we found that TCR engagement is a potent activator of Rap1 (4, 58). Because Rap1 opposes the action of Ras, this suggests that the magnitude of signals transduced by the TCR is self-limiting in the absence of CD28. Thus, in the presence of activated Rap1, the stimulation of a Rap1 GAP may have a powerfully synergistic effect (Fig. 11), allowing ERK signaling to reach its maximal potential. This provides a model by which coengagement of CD28 with the TCR can have such a profound effect on T-cell activation.
Rap1 activation is a mechanism utilized by multiple cells, including both T and B cells, to modulate signals downstream of Ras (4, 25, 26, 50, 62, 71). The ability of Rap1 to antagonize Ras-dependent actions requires Rap1 activation. This antagonism of signaling pathways to ERK contrasts with the actions of Rap1 in other cell types that express the Raf-1 isoform B-Raf, a positive effector of Rap1 (62, 71). Neither peripheral lymphocytes nor Jurkat cells express B-Raf, and Rap1 antagonism of Raf-1 appears to be its major action in these cells. Rap1 activation is triggered by a growing family of Rap1-specific GEFs that can be activated by a diverse set of intracellular-signaling pathways (13, 24, 50, 78). This activation of Rap1 may account for the ability of multiple intracellular signals like that of cyclic AMP (cAMP) to inhibit Ras-dependent pathways in lymphocytes (32, 46, 47), as well as other cell types (5, 8, 77). cAMP can potently activate Rap1 in multiple cell types (71, 72), including lymphocytes (data not shown) (72), and the hydrolysis of cAMP via CD28-regulated phosphodiesterases has recently been proposed as a mechanism for CD28 costimulation (44).
Here, we identify Rap1 GAPs as novel targets of CD28 signaling. Dysregulation of Rap1 GAPs may underscore some of the signaling defects seen in states of T-cell hyporesponsiveness, such as anergy (4). Although largely unexplored, the recent identification of a growing family of Rap1-specific GAPs demonstrates that they are widely expressed in multiple cells types, including T cells (38, 51). Some have a ubiquitous pattern of expression, while other Rap1 GAPs are restricted in their pattern of expression (60, 76). For example, the Rap1 GAP SPA-1 appears to be expressed predominantly in lymphocytes (38). The structural diversity of these Rap1 GAPs suggest that they will have multiple mechanisms of regulation. At present, the mechanism of Rap1 GAP regulation is not well known (55); however, roles for heterotrimeric G proteins have been suggested (35, 51). Our results using dn.Rap1GAP1 suggest that this protein (or a related GAP) may be the target of CD28's actions in Jurkat cells. Because CD28's ability to regulate both Rap1 and ERKs is common to both Jurkat and primary T cells, it is possible that regulation of Rap1GAP activity represents an important mechanism for modulating ERK activation in vivo as well.
The activation of Rap1 may play an important role in regulating signals transduced by the TCR. Rap1, which is activated by engagement of the TCR alone, is likely to set a threshold level for ERK activation that prevents activation of T cells by nonspecific ligands. This is important because it can potentially explain how the TCR achieves its exquisite sensitivity and specificity (39, 65). Although it is generally assumed that inhibitory signals function to terminate signaling processes, inhibitory molecules can also play important roles in shaping the character of the signaling response. Activation of inhibitory molecules during the signaling process can suppress weak signals, ensuring that nonspecific ligands are unable to activate the T cell. The CD28-Lck signaling pathway allows the T cell a unique mechanism for amplifying signals that overcome a specific threshold. CD28 and Lck not only reverse the Rap1 signal but may also stimulate the Ras pathway on their own. This process allows signaling by the TCR to achieve a switch-like character (18); i.e., all signals below a specific threshold are suppressed, and all signals above a specific threshold are amplified to their maximal potential. The requirement of a second molecule, CD28, to relieve the inhibition allows the T cell a second layer of temporal control and may help to explain the function of CD28 in T-cell costimulation. It can also help to explain the persistent activation of Rap1 in anergic cells (4).
ACKNOWLEDGMENTS
We are grateful to Johannes Bos, Paul Polakis, Frank McCormick, and Michael Gold for providing reagents. We thank David Parker, Scott Wetzel, Paul Allen, and Hong Yao for their helpful scientific discussions and the Oregon Cancer Center and Anthony Baake for help with the FACS analysis.
Support for P.J.S.S. was from the N.C.I. and for A.S.S. was from the Washington University Monsanto Agreement and NIAID.
REFERENCES
- 1.Anderson S J, Levin S D, Perlmutter R M. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor beta-chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 2.Bldari C T, Heguy A, Telford J L. ras protein activity is essential for T-cell antigen receptor signal transduction. J Biol Chem. 1993;268:2693–2698. [PubMed] [Google Scholar]
- 3.Barz C, Nagel T, Truitt K E, Imboden J B. Mutational analysis of CD28-mediated costimulation of Jun–N-terminal kinase and IL-2 production. J Immunol. 1998;161:5366–5372. [PubMed] [Google Scholar]
- 4.Boussiotis V A, Freeman G J, Berezovskaya A, Barber D L, Nadler L M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 5.Burgering B M, Pronk G J, van Weeren P C, Chardin P, Bos J L. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 1993;12:4211–4220. doi: 10.1002/j.1460-2075.1993.tb06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne J P, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrera A C, Alexandrov K, Roberts T M. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc Natl Acad Sci USA. 1993;90:442–446. doi: 10.1073/pnas.90.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook S J, McCormick F. Inhibition by cAMP of ras-dependent activation of raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 9.Cooke M P, Abraham K M, Forbush K A, Perlmutter R M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 10.Delgado P, Fernandez E, Dave V, Kappes D, Alarcon B. CD3delta couples T-cell receptor signalling to ERK activation and thymocyte positive selection. Nature. 2000;406:426–430. doi: 10.1038/35019102. [DOI] [PubMed] [Google Scholar]
- 11.Denny M F, Kaufman H C, Chan A C, Straus D B. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J Biol Chem. 1999;274:5146–5152. doi: 10.1074/jbc.274.8.5146. [DOI] [PubMed] [Google Scholar]
- 12.Denny M F, Patai B, Straus D B. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol Cell Biol. 2000;20:1426–1435. doi: 10.1128/mcb.20.4.1426-1435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 14.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncia J V, Santella III J B, Higley C A, Pitts W J, Wityak J, Frietze W E, Rankin F W, Sun J H, Earl R A, Tabaka A C, Teleha C A, Blom K F, Favata M F, Manos E J, Daulerio A J, Stradley D A, Horiuchi K, Copeland R A, Scherle P A, Trzaskos J M, Magolda R L, Trainor G L, Wexler R R, Hobbs F W, Olson R E. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 16.Dustin M L, Shaw A S. Costimulation: building an immunological synapse. Science. 1999;283:649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- 17.Faris M, Kokot N, Lee L, Nel A E. Regulation of interleukin-2 transcription by inducible stable expression of dominant negative and dominant active mitogen-activated protein kinase kinase kinase in jurkat T cells. Evidence for the importance of Ras in a pathway that is controlled by dual receptor stimulation. J Biol Chem. 1996;271:27366–27373. doi: 10.1074/jbc.271.44.27366. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell J E J. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 19.Fields P E, Gajewski T F, Fitch F W. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 20.Franke B, Akkerman J-W, Bos J L. Rapid Ca2+-mediated activation of rap1 in human platelets. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost J A, Geppert T D, Cobb M H, Feramisco J R. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genot E, Cantrell D A. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–294. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 23.Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 24.Gotoh T, Hattori S, Nakamura S, Kitayama H, Noda M, Takai Y, Kaibuchi K, Matsui H, Hatase O, Takahashi H, Kurata T, Matsuda M. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15:6746–6753. doi: 10.1128/mcb.15.12.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grewal S, Horgan A M, York R D, Withers G S, Banker G A, Stork P J S. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase (PKA) J Biol Chem. 2000;275:3722–3728. doi: 10.1074/jbc.275.5.3722. [DOI] [PubMed] [Google Scholar]
- 26.Grewal S S, York R D, Stork P J S. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 27.Guerder S, Flavell R A. Costimulation in tolerance and autoimmunity. Int Rev Immunol. 1995;13:135–146. doi: 10.3109/08830189509061743. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K, Sohn S J, Levin S D, Tada T, Perlmutter R M, Nakayama T. Requirement for p56lck tyrosine kinase activation in T cell receptor-mediated thymic selection. J Exp Med. 1996;184:931–943. doi: 10.1084/jem.184.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Hoyos G, Sohn S J, Rothenberg E V, Alberola-Ila J. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity. 2000;12:313–322. doi: 10.1016/s1074-7613(00)80184-3. [DOI] [PubMed] [Google Scholar]
- 30.Hillig R C, Renault L, Vetter I R, Drell IV T, Wittinghofer A, Becker J. The crystal structure of rna1p: a new fold for a GTPase-activating protein. Mol Cell. 1999;3:781–791. doi: 10.1016/s1097-2765(01)80010-1. [DOI] [PubMed] [Google Scholar]
- 31.Holdorf A D, Green J M, Levin S D, Denny M F, Straus D B, Link V, Changelian P S, Allen P M, Shaw A S. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hordijk P L, Verlaan I, Jalink K, van Corven E J, Moolenaar W H. cAMP abrogates the p21ras-mitogen-activated protein kinase pathway in fibroblasts. J Biol Chem. 1994;269:3534–3538. [PubMed] [Google Scholar]
- 33.Huby R D, Weiss A, Ley S C. Nocodazole inhibits signal transduction by the T cell antigen receptor. J Biol Chem. 1998;273:12024–12031. doi: 10.1074/jbc.273.20.12024. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo M, Bowden S, Cantrell D. The role of Raf-1 in the regulation of extracellular signal-regulated kinase 2 by the T cell antigen receptor. J Exp Med. 1994;180:401–406. doi: 10.1084/jem.180.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan J D, Carey K D, Stork P J S, Iyengar R. Modulation of Rap activity by direct interaction of Galpha(o) with Rap1 GTPase-activating protein. J Biol Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 36.Kitayama H, Matsuzaki T, Ikawa Y, Noda M. Genetic analysis of the Kirsten-ras-revertant 1 gene: potentiator of its tumor suppressor activity by specific point mutations. Proc Natl Acad Sci USA. 1990;87:4284–4288. doi: 10.1073/pnas.87.11.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 38.Kurachi H, Wada Y, Tsukamoto N, Maeda M, Kubota H, Hattori M, Iwai K, Minato N. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J Biol Chem. 1997;272:28081–28088. doi: 10.1074/jbc.272.44.28081. [DOI] [PubMed] [Google Scholar]
- 39.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 40.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 41.Levin S D, Anderson S J, Forbush K A, Perlmutter R M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Subleski M, Fusaki N, Yamamoto T, Copeland T, Princler G L, Kung H, Kamata T. Catalytic activity of the mouse guanine nucleotide exchanger mSOS is activated by Fyn tyrosine protein kinase and the T-cell antigen receptor in T cells. Proc Natl Acad Sci USA. 1996;93:1001–1005. doi: 10.1073/pnas.93.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B Q, Kaplan D, Kung H F, Kamata T. Nerve growth factor stimulation of the Ras-guanine nucleotide exchange factor and GAP activities. Science. 1992;256:1456–1459. doi: 10.1126/science.1604323. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Yee C, Beavo J A. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Whaley C D, Mondino A, Mueller D L. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 46.Lin K, Abraham K M. Targets of p56(lck) activity in immature thymoblasts: stimulation of the Ras/Raf/MAPK pathway. Int Immunol. 1997;9:291–306. doi: 10.1093/intimm/9.2.291. [DOI] [PubMed] [Google Scholar]
- 47.Lingk D S, Chan M A, Gelfand E W. Increased cyclic adenosine monophosphate levels block progression but not initiation of human T cell proliferation. J Immunol. 1990;145:449–455. [PubMed] [Google Scholar]
- 48.Liu Y, Jones B, Brady W, Janeway C A, Jr, Linley P S. Costimulation of murine CD4 T cell growth: cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992;22:2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- 49.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 50.McLeod S J, Ingham R J, Bos J L, Kurosaki T, Gold M R. Activation of the Rap1 GTPase by the B cell antigen receptor. J Biol Chem. 1998;273:29218–29223. doi: 10.1074/jbc.273.44.29218. [DOI] [PubMed] [Google Scholar]
- 51.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Activation of the ERK/MAPK pathway by an isoform of rap1GAP associated with G alpha(i) Nature. 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 52.Nunes J A, Collette Y, Truneh A, Olive D, Cantrell D A. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7–1, activates p21ras. J Exp Med. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okada T, Hu C D, Jin T G, Kariya K, Yamawaki-Kataoka Y, Kataoka T. The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases. Mol Cell Biol. 1999;19:6057–6064. doi: 10.1128/mcb.19.9.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez V L, Van Parijs L, Biuckians A, Zheng X X, Strom T B, Abbas A K. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 55.Polakis P, Rubinfeld B, McCormick F. Phosphorylation of rap1GAP in vivo and by cAMP-dependent kinase and the cell cycle p34cdc2 kinase in vitro. J Biol Chem. 1992;267:10780–10785. [PubMed] [Google Scholar]
- 56.Polakis P G, Rubinfeld B, Evans T, McCormick F. Purification of a plasma membrane-associated GTPase-activating protein specific for rap1/Krev-1 from HL60 cells. Proc Natl Acad Sci USA. 1991;88:239–243. doi: 10.1073/pnas.88.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rayter S I, Woodrow M, Lucas S C, Cantrell D A, Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO J. 1992;11:4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reedquist K A, Bos J L. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J Biol Chem. 1998;273:4944–4949. doi: 10.1074/jbc.273.9.4944. [DOI] [PubMed] [Google Scholar]
- 59.Reedquist K A, Ross E, Koop E A, Wolthuis R M, Zwartkruis F J, van Kooyk Y, Salmon M, Buckley C D, Bos J L. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinfeld B, Munemitsu S, Clark R, Conroy L, Watt K, Crosier W J, McCormick F, Polakis P. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991;65:1033–1042. doi: 10.1016/0092-8674(91)90555-d. [DOI] [PubMed] [Google Scholar]
- 61.Sakoda T, Kaibuchi K, Kishi K, Kishida S, Doi K, Hoshino M, Hattori S, Takai Y. smg/rap1/Krev-1 p21 inhibit the signal pathway to the c-fos promoter/enhancer from c-Ki-ras p21 but not from c-raf-1 kinase in NIH3T3 cells. Oncogene. 1992;7:1705–1711. [PubMed] [Google Scholar]
- 62.Schmitt J M, Stork P J S. β2-adrenergic receptor activates extracellular regulated kinases (ERKs) via the small G protein Rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 63.Shaw A S, Amrein K E, Hammond C, Stern D F, Sefton B M, Rose J K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- 64.Shaw A S, Dustin M L. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 65.Sloan-Lancaster J, Evavold B D, Allen P M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 66.Straus D B, Chan A C, Patai B, Weiss A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J Biol Chem. 1996;271:9976–9981. doi: 10.1074/jbc.271.17.9976. [DOI] [PubMed] [Google Scholar]
- 67.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 68.Su J H, Anderson A J, Cummings B J, Cotman C W. Immunohistochemical evidence for DNA fragmentation in neurons in the AD brain. Neuroreport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 69.Tuosto L, Acuto O. CD28 affects the earliest signaling events generated by TCR engagement. Eur J Immunol. 1998;28:2131–2142. doi: 10.1002/(SICI)1521-4141(199807)28:07<2131::AID-IMMU2131>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 70.Visvader J, Sassone-Corsi P, Verma I. Two adjacent promoter elements mediate nerve growth factor activation of the c-fos gene and bind distinct nuclear complexes. Proc Natl Acad Sci USA. 1988;85:1031–1040. doi: 10.1073/pnas.85.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vossler M, Yao H, York R, Rim C, Pan M-G, Stork P J S. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 72.Wan Y, Huang X Y. Analysis of the Gs/mitogen-activated protein kinase pathway in mutant S49 cells. J Biol Chem. 1998;273:14533–14537. doi: 10.1074/jbc.273.23.14533. [DOI] [PubMed] [Google Scholar]
- 73.Werlen G, Hausmann B, Palmer E. A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 74.Westwick J K, Cox A D, Der C J, Cobb M H, Hibi M, Karin M, Brenner D A. Oncogenic ras activates c-jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci USA. 1994;91:6030–6034. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitehurst C E, Geppert T D. MEK1 and the extracellular signal-regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020–1029. [PubMed] [Google Scholar]
- 76.Wienecke R, Konig A, DeClue J E. Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J Biol Chem. 1995;270:16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- 77.Wu J, Dent P, Jelinek T, Wolfman A, Weber M J, Sturgill T W. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–1068. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 78.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J S. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–625. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 79.Zwartkruis F J, Wolthuis R M, Nabben N M, Franke B, Bos J L. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 1998;17:5905–5912. doi: 10.1093/emboj/17.20.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]