Abstract
This study investigated intestinal oxidative damage caused by F18+ Escherichia coli and its amelioration with antibacterial bacitracin fed to nursery pigs. Thirty-six weaned pigs (6.31 ± 0.08 kg BW) were allotted in a randomized complete block design. Treatments were: NC, not challenged/not treated; PC, challenged (F18+ E. coli at 5.2 × 109 CFU)/not treated; AGP challenged (F18+ E. coli at 5.2 × 109 CFU)/treated with bacitracin (30 g/t). Overall, PC reduced (p < 0.05) average daily gain (ADG), gain to feed ratio (G:F), villus height, and villus height to crypt depth ratio (VH:CD), whereas AGP increased (p < 0.05) ADG, and G:F. PC increased (p < 0.05) fecal score, F18+ E. coli in feces, and protein carbonyl in jejunal mucosa. AGP reduced (p < 0.05) fecal score and F18+ E. coli in jejunal mucosa. PC reduced (p < 0.05) Prevotella stercorea populations in jejunal mucosa, whereas AGP increased (p < 0.05) Phascolarctobacterium succinatutens and reduced (p < 0.05) Mitsuokella jalaludinii populations in feces. Collectively, F18+ E. coli challenge increased fecal score and disrupted the microbiota composition, harming intestinal health by increasing oxidative stress, and damaging the intestinal epithelium, ultimately impairing growth performance. Dietary bacitracin reduced reduced F18+ E. coli populations and the oxidative damages they cause, thereby improving intestinal health and the growth performance of nursery pigs.
Keywords: F18+ E. coli, growth performance, intestinal health, oxidative damages, pigs
1. Introduction
In swine production, the post-weaning period is associated with immunological, physiological, psychological, and nutritional challenges that can impair the intestinal immune system and growth performance of pigs [1,2,3]. The impaired intestinal immune system increases pigs susceptibility to pathogen invasion [3,4]. Enterotoxigenic Escherichia coli, which causes post-weaning diarrhea (PWD), is a pathogen of concern for producers around the world. As a consequence of E. coli infection, changes in intestinal microbiota can led to increased inflammation and oxidative damage in the intestine, ultimately resulting in growth retardation [5,6,7]. According to Duarte and Kim [8], the changes in the intestinal microbiota in pigs challenged with F18+ E. coli are positively correlated with oxidative damages in the jejunal mucosa.
Different strategies have been utilized to reduce the susceptibility of pigs to potential pathogens [9,10]. Since the 1950s, antibiotics have been used in swine feed to promote growth by improving intestinal health [11,12]. Bacitracin is an antibiotic commonly used in animal feeds as a growth promoter and to treat and control infections [13]. In the US, bacitracin use as a growth promoter is not subjected to the veterinary feed directive rule and, therefore, does not require veterinary prescription [14]. Although the use of bacitracin has been primarily thought of as effective against Gram-positive pathogens, its use has also been reported to modulate the intestinal microbiota in nursery pigs [7], rabbits [15], and poultry [16,17]. This modulation of microbiota may explain the ability of bacitracin ability to prevent the deleterious effects of E. coli infection [7].
If the damage caused by F18+ E. coli infection is partially due to alterations in intestinal microbiota, which led to increased oxidative damage and increased intestinal inflammatory responses, understanding ways to mediate this is important for improving the efficiency of swine production. Bacitracin may be a useful tool to minimize the disruption of the intestinal microbiota due to F18+ E. coli infection, consequently promoting the growth of challenged pigs. To test this hypothesis, this study evaluated the intestinal oxidative damages caused by F18+ E. coli and its protection with the antibacterial bacitracin fed to nursery pigs.
2. Materials and Methods
The Institutional Animal Care and Use Committee at North Carolina State University approved the experimental protocol used in this study, as stated in the North Carolina State Animal Care and Use Procedures (REG 10.10.01).
2.1. Animals, Experimental Design, Diets, and Inoculation
An amount of 36 newly weaned pigs (18 barrows and 18 gilts) with 6.31 ± 0.08 kg body weight (BW) and 21 d of age were allotted to 3 treatments using a randomized complete block design (RCBD). Sex and initial BW were considered as blocks. The treatments were: NC, not challenged/not treated; PC, challenged (F18+ E. coli at 5.2 × 109 CFU)/not treated; AGP, challenged (F18+ E. coli at 5.2 × 109 CFU)/treated with bacitracin (30 g/t). Pigs were fed diets for 28 d divided into 2 phases (P1 for 14 d, and P2 for 14 d). Basal diets were formulated to meet the nutrient requirements suggested by NRC [18] (Table 1).
Table 1.
Composition of basal diets (Exp. 1; as-fed basis).
| Item | Phase 1 | Phase 2 |
|---|---|---|
| Ingredient, % | ||
| Corn, yellow | 40.45 | 54.47 |
| Soybean meal, 48% CP | 22.00 | 23.50 |
| Whey permeate | 20.00 | 10.00 |
| Blood plasma | 6.00 | 3.00 |
| Poultry meal | 5.00 | 4.00 |
| Poultry fat | 3.50 | 1.80 |
| L-Lys HCl | 0.48 | 0.47 |
| DL-Met | 0.22 | 0.18 |
| L-Thr | 0.15 | 0.13 |
| L-Trp | 0.00 | 0.00 |
| Dicalcium phosphate | 0.60 | 0.85 |
| Limestone | 0.95 | 0.95 |
| Vitamin premix 1 | 0.03 | 0.03 |
| Mineral premix 2 | 0.15 | 0.15 |
| Salt | 0.22 | 0.22 |
| Zinc oxide | 0.25 | 0.25 |
| Calculated composition: | ||
| Dry matter, % | 90.8 | 90.1 |
| ME, kcal/kg | 3481 | 3388 |
| CP, % | 23.00 | 21.60 |
| SID 3 Lys, % | 1.50 | 1.35 |
| SID Met + Cys, % | 0.82 | 0.74 |
| SID Trp, % | 0.25 | 0.22 |
| SID Thr, % | 0.88 | 0.79 |
| Ca, % | 0.86 | 0.81 |
| STTD 4 p, % | 0.45 | 0.40 |
| Total p, % | 0.67 | 0.64 |
1 The vitamin premix provided the following per kilogram of complete diet: 6613.8 IU of vitamin A as vitamin A acetate, 992.0 IU of vitamin D3, 19.8 IU of vitamin E, 2.64 mg of vitamin K as menadione sodium bisulfate, 0.03 mg of vitamin B12, 4.63 mg of riboflavin, 18.52 mg of D-pantothenic acid as calcium pantothenate, 24.96 mg of niacin, and 0.07 mg of biotin. 2 The trace mineral premix provided the following per kilogram of complete diet: 4.0 mg of Mn as manganous oxide, 165 mg of Fe as ferrous sulfate, 165 mg of Zn as zinc sulfate, 16.5 mg of Cu as copper sulfate, 0.30 mg of I as ethylenediamine di-hydroiodide, and 0.30 mg of Se as sodium selenite. 3 SID, standardized ileal digestible. 4 STTD, standardized total tract digestible.
Bacitracin methylene disalicylate (BMD) was added to the diets as a source of bacitracin. After 7 d of feeding (pre-challenge period), all pigs on PC and AGP received an oral dose of F18+ E. coli (5.2 × 109 CFU), and pigs on NC received an oral dose of sterile saline solution. The F18+ E. coli culture was prepared and inoculated to the challenged pigs, as previously reported by Duarte and Kim [8] and Xu et al. [7]. The inoculum was produced by utilizing the F18ac (O147) strain that generates heat-stable toxins A (STa) and B (STb). The strain stock was tested to confirm the expression of F18ac, STa, and STb.
2.2. Growth Performance and Fecal Score
Body weight and feed intake were measured weekly to calculate the average daily gain (ADG), average feed intake (ADFI), and the gain to feed ratio (G:F) in order to evaluate the growth performance of pigs. The fecal scores were recorded every other day using a scoring system where 1 = very hard and dry stool, 2 = firm stool; 3 = normal stool; 4 = loose stool; and 5 = watery stool, as previously reported by [19,20]
2.3. Sample Collection and Processing
Fecal and blood samples were collected from all pigs at d 14 and 28. Fecal samples were freshly collected to evaluate the microbiota composition in the post-challenge period. Blood (10 mL) was collected from the jugular vein into vacutainer tubes without anticoagulant to obtain serum to determine the concentration of tumor necrosis-alpha (TNF-α), as an indicator of inflammatory status [21] and protein carbonyl, as an indicator of oxidative stress status [22]. Sera were stored at −80 °C until analysis.
After 28 d feeding, all pigs were euthanized by penetrating captive bolt followed by exsanguination. Jejunal tissue and mucosa were collected 3 m distal to the pyloric-duodenal junction. Jejunal tissue (5 cm) was collected in 10% buffered formalin, and mucosa was obtained from the next 20 cm of jejunum and snap frozen in liquid nitrogen. The mucosa samples were used to evaluate the microbiota composition, the inflammatory and the oxidative stress status. Protein extracts from the mucosa were obtained by homogenization homogenizer (Tissuemiser; Fisher Scientific Inc., Waltham, MA, USA) in phosphate-buffered saline (PBS). The homogenate was then centrifuged at 10,000× g at 4 °C for 15 min, and the supernatant stored at −80 °C for further analysis.
2.4. Immune and Oxidative Stress Status
Protein concentration of samples were determined using the Protein Assay Kit (23225#, Thermo Fisher Scientific Inc., Wilmington, DE, USA). Prior to analysis, the samples were diluted in PBS at 1:80 and 1:40 for serum and mucosa samples, respectively. Concentrations of TNF-α in mucosa and protein carbonyl in mucosa and sera were normalized to total protein content, as previously reported by Cheng et al. [23]. The concentration of TNF-α was measured in serum and mucosa samples using the Porcine TNF-α Immunoassay Kit (#PTA00; R&D Systems, Minneapolis, MN, USA) as previously described by Holanda and Kim [24]. The concentration of protein carbonyl was measured using the OxiSelect Protein carbonyl ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA) as previously described by Jang et al. [25].
2.5. Intestinal Morphology and Crypt Cell Proliferation
Jejunal tissue samples were sent to the North Carolina State University Histology Laboratory (College of Veterinary Medicine, Raleigh, NC, USA) for Ki-67 staining [21]. Fifteen fields of view at 40× magnification of villi and their respective crypts per pig were used to measure villi height and width and crypt depth. The villi height to crypt depth ratio (VH:CD) was then calculated. Fifteen fields of view at 100× magnification were used to determine the proportion of Ki-67+ to total cells in the crypt as an estimator of cell proliferation rate in crypts, as previously described by Duarte and Kim [22].
2.6. Intestinal Microbiota
DNA was extracted (DNA Stool Mini Kit,#51604, Qiagen; Germantown, MD, USA) from fecal and mucosa samples for 16S rRNA analysis and for quantification of F18+ E. coli by qPCR. The DNA samples were sent to MAKO laboratories (Raleigh, NC, USA) for 16S rRNA and qPCR analysis according to their protocol, as reported by Duarte et al. [26]. The relative abundance of microbiota was calculated, and the OTU (operational taxonomic unit) with <0.5% relative abundance was combined and reported as “Others”.
The F18+ E. coli in the mucosa and fecal samples was quantified by qPCR following the protocol used by MAKO laboratories. Briefly, the plasmid containing the F18 fimbriae genes fedA (NCBI GeneBank, accession no. M61713) was constructed using the GeneArt (Thermo Fisher Scientific). The synthetic F18 gene was assembled from synthetic oligonucleotides. The fragment was inserted into the pMK-RQ-Bs vector GeneArt (Thermo Fisher Scientific). The concentration of plasmid DNA was measured by UV spectroscopy after the purification from the transformed bacteria. The similarity of sequence within the insertion sites was 100%. A TaqMan probe specific to the fedA gene was provided by Thermo Fisher. For quantification of F18+ plasmid in the samples, the assembled vector was used as standard.
The standard vector was linearized using the SmaI digestion (#FD0664, Thermo Fisher Scientific) prior to sequencing using qPCR. The count of the stock standard was calculated based on the vector size (914 bp). Then, the standard was diluted to 2.86 × 107, 2.86 × 106, 2.86 × 105, 2.86 × 104, and 2.86 × 103. The Taqpath qPCR Master Mix CG (#A15297, Thermo Fisher Scientific) and the QuantStudio 12K Flex (Thermo Fisher Scientific) were used for the qPCR of samples and standards following the instructions of the manufacturer. Based on the count of the plasmid on the standard, linear regression was used to calculate the concentration of the F18+ plasmid in the samples. Before statistical analysis, the concentration of F18+ plasmid was Log transformed.
2.7. Statistical Analysis
The Mixed procedure of SAS 9.4 Software (Cary, NC, USA) was used to analyze all data based on a randomized complete block design. The main effect was the treatments, and the random effects were sex and initial BW. Pre-planned contrasts were used to test the effect of the F18+ E. coli challenge (NC vs. PC) and the effect of AGP on challenged pigs (PC vs. AGP). Statistical differences were considered significant with p < 0.05, and the tendency was considered when 0.05 ≤ p < 0.10.
3. Results
3.1. Growth Performance and Fecal Score
Prior to challenge (d 0 to 7), the treatments did not affect BW, ADG, ADFI, or G:F (Table 2). After the E. coli challenge, the PC had lower (p < 0.05) BW at d 14, 21, and 28 when compared with the NC. The AGP-treated pigs had higher (p < 0.05) BW at d 14 and tended to have higher BW (p = 0.066) at d 28 when compared with PC. The PC reduced (p < 0.05) the ADG of pigs post-challenge (d7 to 14, d 14 to 21, and d 7 to 28) and over the entire experiment (d 0 to 28) when compared to the NC. The AGP increased (p < 0.05) the ADG of pigs post-challenge (d 14 to 21, and d 7 to 28) and over the entire experiment (d 0 to 28) when compared with PC. During the last week of the experiment, d 21 to 28, the treatments did not affect the ADG, ADFI, nor the G:F. The PC did not affect the ADFI during the entire experiment, whereas AGP tended to increase ADFI (p = 0.073) from d 7 to 14. The PC reduced (p < 0.05) the G:F of pigs during the post-challenge (d7 to 14, and d 7 to 28) and the overall experiment (d 0 to 28) when compared with NC. The AGP increased (p < 0.05) the G:F of pigs, compared to the PC, from d 14 to 21.
Table 2.
Growth performance of pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| BW, kg | ||||||
| Initial | 6.31 | 6.31 | 6.30 | 0.08 | 0.985 | 0.912 |
| d 7 | 6.91 | 6.90 | 6.93 | 0.15 | 0.958 | 0.852 |
| d 14 | 8.64 | 7.72 | 8.20 | 0.28 | 0.036 | 0.231 |
| d 21 | 11.93 | 10.21 | 11.88 | 0.46 | 0.019 | 0.018 |
| d 28 | 16.25 | 14.19 | 15.94 | 0.64 | 0.040 | 0.066 |
| ADG, kg | ||||||
| Pre-challenge (d 0 to 7) | 0.080 | 0.084 | 0.091 | 0.021 | 0.980 | 0.804 |
| Post-challenge (d 7 to 28) | 0.445 | 0.348 | 0.429 | 0.026 | 0.020 | 0.039 |
| d 7 to 14 | 0.247 | 0.118 | 0.181 | 0.028 | 0.005 | 0.119 |
| d 14 to 21 | 0.470 | 0.356 | 0.526 | 0.035 | 0.038 | 0.003 |
| d 21 to 28 | 0.617 | 0.569 | 0.580 | 0.042 | 0.430 | 0.857 |
| Overall | 0.353 | 0.282 | 0.344 | 0.022 | 0.022 | 0.046 |
| ADFI, kg | ||||||
| Pre-challenge (d 0 to 7) | 0.120 | 0.149 | 0.142 | 0.024 | 0.415 | 0.844 |
| Post-challenge (d 7 to 28) | 0.643 | 0.582 | 0.663 | 0.048 | 0.403 | 0.254 |
| d 7 to 14 | 0.340 | 0.303 | 0.386 | 0.034 | 0.436 | 0.073 |
| d 14 to 21 | 0.627 | 0.572 | 0.681 | 0.064 | 0.467 | 0.149 |
| d 21 to 28 | 0.972 | 0.870 | 0.921 | 0.071 | 0.300 | 0.589 |
| Overall | 0.512 | 0.474 | 0.532 | 0.038 | 0.497 | 0.285 |
| G:F | ||||||
| Pre-challenge (d 0 to 7) | 0.61 | 0.55 | 0.57 | 0.10 | 0.679 | 0.845 |
| Post-challenge (d 7 to 28) | 0.72 | 0.56 | 0.62 | 0.03 | 0.001 | 0.121 |
| d 7 to 14 | 0.76 | 0.36 | 0.46 | 0.07 | 0.001 | 0.276 |
| d 14 to 21 | 0.77 | 0.65 | 0.78 | 0.04 | 0.060 | 0.036 |
| d 21 to 28 | 0.64 | 0.67 | 0.63 | 0.03 | 0.634 | 0.378 |
| Overall | 0.71 | 0.55 | 0.61 | 0.03 | 0.009 | 0.236 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
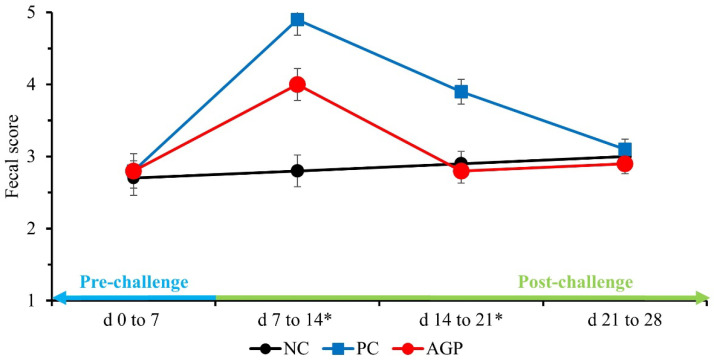
Before the E. coli challenge (d 0 to 7), the treatments did not affect the fecal score of pigs (Figure 1). After the challenge, the PC pigs had higher (p < 0.05) fecal scores during the first- and second-week post-challenge when compared with NC. The AGP pigs had fecal scores that were intermediate to the PC and the NC during the first week post-challenge (p < 0.05), and they were not significantly different than those of the NC during the second week post-challenge. There were no significant differences in fecal score among the treatments during the final week of the experiment.
Figure 1.
The fecal score of pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter. NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t). * d 7 to 14: NC vs. PC: (p = 0.001), PC vs. AGP: (p = 0.004); d 14 to 21: NC vs. PC: (p = 0.001), PC vs. AGP: (p = 0.001).
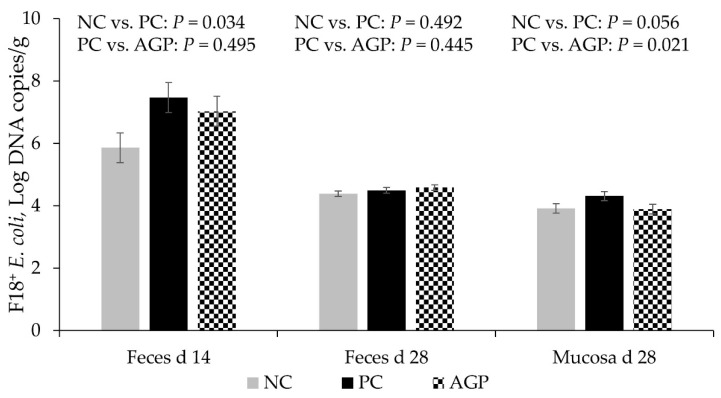
3.2. F18+ E. coli Counting
The PC had increased (p < 0.05) copies of fedA, indicating higher populations of F18+ E. coli in the feces of pigs at d 14 when compared with the NC. There are no significant differences in feces at d 28 (Figure 2). The AGP did not significantly impact the copies of fedA in the feces on d 14. The PC tended to have greater (p = 0.056) concentrations of fedA in samples from jejunal mucosa, compared to the NC at d 28, and AGP appeared to have concentrations that were significantly lower than those of the PC group.
Figure 2.
F18+ E. coli counting in feces and jejunal mucosa of pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter. NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
3.3. Immune and Oxidative Stress Status
The concentration of TNF-α in jejunal mucosa was not affected by the treatments (Table 3). The PC tended to increase the concentration of TNF-α in sera at d 14 when compared with NC. The PC increased (p < 0.05) the concentration of protein carbonyl in serum and jejunal mucosa at d 28 when compared with NC. The AGP tended to reduce (p < 0.05) the concentration of protein carbonyl in the jejunal mucosa of pigs at d 28 when compared with PC.
Table 3.
Immune and oxidative stress status of pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| Tumor necrosis factor-alpha | ||||||
| d 14 serum, pg/mL | 110.4 | 128.7 | 119.4 | 6.3 | 0.054 | 0.365 |
| d 28 serum, pg/mL | 114.7 | 107.9 | 104.8 | 8.6 | 0.586 | 0.796 |
| Jejunal mucosa, pg/mg protein | 1.31 | 1.66 | 1.76 | 0.26 | 0.361 | 0.773 |
| Protein carbonyl, nmol/mg protein | ||||||
| d 14 serum | 2.20 | 2.08 | 2.16 | 0.11 | 0.419 | 0.605 |
| d 28 serum | 1.63 | 2.37 | 2.32 | 0.19 | 0.010 | 0.851 |
| Jejunal mucosa | 2.15 | 3.61 | 2.56 | 0.35 | 0.012 | 0.059 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
3.4. Intestinal Morphology and Cell Proliferation in Crypt
The PC reduced (p < 0.05) the villus height and the VH:CD in the jejunum of pigs when compared with NC (Table 4). The villus width, crypt depth, and cell proliferation in jejunal crypts were not affected by the treatments.
Table 4.
Intestinal morphology and cell proliferation in crypts of pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| Villus height, µm | 527 | 394 | 436 | 32 | 0.003 | 0.296 |
| Villus width, µm | 85 | 91 | 85 | 7 | 0.378 | 0.419 |
| Crypt depth, µm | 245 | 253 | 253 | 12 | 0.591 | 0.975 |
| VH:CD 2 | 2.22 | 1.58 | 1.73 | 0.14 | 0.002 | 0.398 |
| Ki-67+, 2 (%) | 52.0 | 47.3 | 51.6 | 5.3 | 0.473 | 0.511 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t). 2 Cell proliferation rate.
3.5. Relative Abundance and Diversity of the Fecal and Mucosa-Associated Microbiota
The PC reduced (p < 0.05) the relative abundance of Tenericutes and tended to reduce (p = 0.095) the relative abundance of Deferribacteres in the feces of pigs at d 28 when compared with NC (Table 5). The PC tended to increase (p = 0.072) the relative abundance of Firmicutes in the feces of pigs at d 28 when compared with NC. The AGP tended to increase (p = 0.088) the relative abundance of Actinobacteria in the feces of pigs at d 28 when compared with PC. The PC tended to reduce (p = 0.055) the relative abundance of Bacteroidetes in the jejunal mucosa of pigs when compared with NC.
Table 5.
Relative abundance of fecal and mucosa-associated microbiota at the phylum level in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| d 14 (Feces) | ||||||
| Bacteroidetes | 41.3 | 37.2 | 39.3 | 3.3 | 0.380 | 0.651 |
| Firmicutes | 36.2 | 42.9 | 45.6 | 5.1 | 0.375 | 0.705 |
| Proteobacteria | 19.6 | 15.4 | 11.6 | 5.9 | 0.629 | 0.653 |
| Spirochaetes | 2.1 | 3.2 | 2.3 | 1.3 | 0.571 | 0.619 |
| Tenericutes | 0.4 | 1.0 | 0.4 | 0.3 | 0.170 | 0.146 |
| Actinobacteria | 0.1 | 0.1 | 0.6 | 0.2 | 0.878 | 0.121 |
| Deferribacteres | 0.1 | 0.2 | 0.1 | 0.1 | 0.628 | 0.337 |
| d 28 (Feces) | ||||||
| Firmicutes | 46.1 | 53.9 | 55.7 | 2.8 | 0.072 | 0.649 |
| Bacteroidetes | 41.2 | 38.8 | 37.1 | 2.2 | 0.458 | 0.595 |
| Proteobacteria | 10.1 | 5.8 | 5.2 | 2.5 | 0.234 | 0.855 |
| Tenericutes | 1.4 | 0.1 | 0.2 | 0.4 | 0.043 | 0.906 |
| Deferribacteres | 0.5 | 0.0 | 0.0 | 0.2 | 0.095 | 0.912 |
| Spirochaetes | 0.4 | 0.7 | 0.6 | 0.4 | 0.653 | 0.865 |
| Actinobacteria | 0.1 | 0.5 | 1.2 | 0.3 | 0.438 | 0.088 |
| d 28 (Mucosa) | ||||||
| Firmicutes | 50.5 | 46.2 | 51.0 | 14.6 | 0.839 | 0.821 |
| Proteobacteria | 25.9 | 43.1 | 39.3 | 15.6 | 0.452 | 0.867 |
| Bacteroidetes | 22.0 | 9.6 | 8.9 | 4.3 | 0.055 | 0.904 |
| Actinobacteria | 0.8 | 0.8 | 0.4 | 0.5 | 0.946 | 0.571 |
| Spirochaetes | 0.4 | 0.2 | 0.3 | 0.2 | 0.367 | 0.463 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
The PC increased (p < 0.05) the relative abundance of Lachnospiraceae and tended to increase (p = 0.072) the relative abundance of Campylobacteraceae in the feces of pigs at d 14 when compared with NC (Table 6). The AGP tended to reduce (p = 0.094) the relative abundance of Others in the feces of pigs at d 14 when compared with PC. The PC tended to increase (p = 0.099) the relative abundance of Acidaminococcaceae in the jejunal mucosa of pigs when compared with NC (Table 7). The AGP did not affect the relative abundance of mucosa-associated microbiota at the family level in the jejunum of pigs.
Table 6.
Relative abundance of fecal microbiota at the family level in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| d 14 | ||||||
| Prevotellaceae | 29.6 | 29.0 | 26.6 | 3.4 | 0.874 | 0.571 |
| Veillonellaceae | 10.8 | 10.9 | 14.2 | 4.3 | 0.989 | 0.599 |
| Enterobacteriaceae | 8.9 | 6.5 | 5.8 | 5.5 | 0.764 | 0.926 |
| Ruminococcaceae | 7.0 | 6.1 | 6.1 | 2.2 | 0.767 | 0.977 |
| Acidaminococcaceae | 2.5 | 4.5 | 6.2 | 1.6 | 0.402 | 0.480 |
| Porphyromonadaceae | 4.4 | 4.4 | 7.8 | 2.1 | 0.978 | 0.266 |
| Lactobacillaceae | 1.7 | 4.2 | 7.3 | 2.2 | 0.449 | 0.339 |
| Lachnospiraceae | 3.0 | 5.1 | 4.4 | 0.7 | 0.049 | 0.469 |
| Succinivibrionaceae | 6.6 | 3.6 | 2.9 | 2.0 | 0.309 | 0.825 |
| Eubacteriaceae | 2.0 | 2.8 | 3.0 | 0.5 | 0.301 | 0.813 |
| Clostridiaceae | 2.3 | 3.3 | 2.2 | 1.2 | 0.576 | 0.544 |
| Cytophagaceae | 3.3 | 1.3 | 0.9 | 1.3 | 0.302 | 0.813 |
| Campylobacteraceae | 0.6 | 3.7 | 1.9 | 1.2 | 0.072 | 0.284 |
| Erysipelotrichaceae | 1.2 | 1.3 | 1.3 | 0.6 | 0.919 | 0.984 |
| Spirochaetaceae | 1.4 | 1.3 | 1.4 | 1.1 | 0.983 | 0.967 |
| Rikenellaceae | 1.9 | 0.3 | 1.5 | 1.1 | 0.310 | 0.453 |
| Peptococcaceae | 2.6 | 0.9 | 0.1 | 1.4 | 0.414 | 0.709 |
| Bacteroidaceae | 0.6 | 1.4 | 1.8 | 0.6 | 0.368 | 0.627 |
| Others | 9.6 | 9.6 | 5.0 | 1.8 | 0.995 | 0.094 |
| d 28 | ||||||
| Prevotellaceae | 36.6 | 33.5 | 30.8 | 2.5 | 0.407 | 0.456 |
| Veillonellaceae | 21.0 | 27.1 | 21.1 | 2.8 | 0.144 | 0.150 |
| Lactobacillaceae | 7.7 | 4.6 | 3.6 | 2.7 | 0.438 | 0.793 |
| Ruminococcaceae | 4.5 | 5.0 | 7.2 | 0.9 | 0.737 | 0.121 |
| Lachnospiraceae | 3.5 | 4.9 | 6.4 | 1.4 | 0.404 | 0.544 |
| Acidaminococcaceae | 3.4 | 5.1 | 6.3 | 1.4 | 0.390 | 0.533 |
| Porphyromonadaceae | 2.5 | 2.6 | 4.5 | 0.9 | 0.922 | 0.189 |
| Succinivibrionaceae | 5.2 | 2.7 | 2.2 | 2.3 | 0.450 | 0.882 |
| Eubacteriaceae | 1.8 | 2.3 | 3.3 | 0.4 | 0.423 | 0.132 |
| Streptococcaceae | 0.9 | 1.3 | 4.2 | 1.6 | 0.864 | 0.226 |
| Clostridiaceae | 1.4 | 1.5 | 1.5 | 0.4 | 0.772 | 0.949 |
| Campylobacteraceae | 2.3 | 1.1 | 0.4 | 0.8 | 0.312 | 0.567 |
| Others | 9.2 | 8.2 | 8.5 | 1.9 | 0.703 | 0.904 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
Table 7.
Relative abundance of mucosa-associated microbiota at the family level in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| Lactobacillaceae | 25.6 | 23.8 | 21.4 | 12.7 | 0.922 | 0.893 |
| Helicobacteraceae | 14.6 | 35.6 | 34.7 | 15.7 | 0.365 | 0.968 |
| Prevotellaceae | 19.6 | 8.9 | 8.4 | 4.5 | 0.117 | 0.938 |
| Veillonellaceae | 7.6 | 5.38 | 19.2 | 5.7 | 0.763 | 0.115 |
| Streptococcaceae | 9.4 | 12.1 | 6.3 | 6.7 | 0.778 | 0.551 |
| Campylobacteraceae | 6.5 | 6.0 | 2.6 | 4.7 | 0.948 | 0.615 |
| Acidaminococcaceae | 2.6 | 1.0 | 0.8 | 0.7 | 0.099 | 0.862 |
| Enterobacteriaceae | 4.2 | 1.7 | 2.2 | 1.1 | 0.128 | 0.766 |
| Lachnospiraceae | 2.1 | 0.8 | 1.2 | 0.6 | 0.151 | 0.675 |
| Ruminococcaceae | 1.2 | 0.6 | 0.8 | 0.3 | 0.233 | 0.691 |
| Erysipelotrichaceae | 0.4 | 1.3 | 0.2 | 0.6 | 0.379 | 0.271 |
| Clostridiaceae | 0.4 | 0.6 | 0.2 | 0.2 | 0.556 | 0.268 |
| Bifidobacteriaceae | 0.6 | 0.7 | 0.3 | 0.4 | 0.897 | 0.539 |
| Porphyromonadaceae | 1.3 | 0.4 | 0.4 | 0.4 | 0.129 | 0.929 |
| Succinivibrionaceae | 0.7 | 0.5 | 1.0 | 0.5 | 0.749 | 0.479 |
| Others | 4.2 | 1.7 | 2.2 | 1.1 | 0.128 | 0.766 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
The PC tended to reduce (p = 0.079) the relative abundance of Succinivibrio dextrinosolvens in the feces of pigs at d 14 when compared with NC (Table 8). The PC tended to reduce (p = 0.065) the relative abundance of Prevotella stercorea and increased the relative abundance of Mitsuokella jalaludinii in the feces of pigs at d 28 when compared with NC. The AGP increased (p < 0.05) the relative abundance of Phascolarctobacterium succinatutens whereas reduced (p < 0.05) the relative abundance of Mitsuokella jalaludinii in feces of pigs at d 28 when compared with PC.
Table 8.
Relative abundance of fecal microbiota at the specie level in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| d 14 | ||||||
| Prevotella copri | 36.8 | 29.7 | 20.1 | 5.6 | 0.376 | 0.238 |
| Phascolarctobacterium succinatutens | 4.8 | 8.8 | 13.2 | 3.8 | 0.468 | 0.427 |
| Prevotella stercorea | 10.2 | 6.8 | 9.4 | 3.4 | 0.482 | 0.582 |
| Faecalibacterium prausnitzii | 10.1 | 8.0 | 9.1 | 3.2 | 0.648 | 0.811 |
| Succinivibrio dextrinosolvens | 6.8 | 3.0 | 0.7 | 1.5 | 0.079 | 0.288 |
| Dialister succinatiphilus | 2.5 | 1.6 | 6.3 | 3.0 | 0.845 | 0.290 |
| Roseburia faecis | 3.7 | 3.1 | 2.1 | 1.3 | 0.723 | 0.598 |
| Campylobacter coli | 1.1 | 4.8 | 6.0 | 2.4 | 0.275 | 0.720 |
| Prevotella sp. | 3.0 | 1.4 | 2.7 | 1.3 | 0.388 | 0.495 |
| Mitsuokella jalaludinii | 2.8 | 2.3 | 3.9 | 1.2 | 0.783 | 0.364 |
| Brachyspira hampsonii | 1.8 | 3.0 | 2.6 | 1.5 | 0.605 | 0.877 |
| Treponema porcinum | 0.9 | 1.8 | 3.7 | 1.2 | 0.594 | 0.262 |
| Campylobacter lanienae | 0.6 | 3.0 | 3.2 | 1.3 | 0.214 | 0.924 |
| Dorea longicatena | 0.4 | 0.9 | 1.2 | 0.4 | 0.410 | 0.641 |
| Lactobacillus mucosae | 0.5 | 0.4 | 0.4 | 1.2 | 0.939 | 0.985 |
| Mitsuokella multacida | 0.3 | 1.5 | 1.8 | 0.6 | 0.159 | 0.675 |
| Lactobacillus kitasatonis | 0.3 | 1.1 | 0.6 | 0.5 | 0.290 | 0.542 |
| Gemmiger formicilis | 1.7 | 1.2 | 0.6 | 0.6 | 0.585 | 0.472 |
| Others | 12.0 | 17.9 | 12.5 | 3.6 | 0.261 | 0.308 |
| d 28 | ||||||
| Prevotella copri | 39.0 | 35.2 | 30.3 | 6.0 | 0.656 | 0.568 |
| Prevotella stercorea | 9.3 | 5.0 | 8.6 | 1.6 | 0.065 | 0.119 |
| Dialister succinatiphilus | 4.2 | 8.0 | 3.2 | 3.2 | 0.409 | 0.294 |
| Phascolarctobacterium succinatutens | 4.3 | 3.0 | 10.0 | 2.0 | 0.658 | 0.024 |
| Faecalibacterium prausnitzii | 5.2 | 4.0 | 7.3 | 1.5 | 0.551 | 0.121 |
| Mitsuokella jalaludinii | 3.3 | 8.7 | 2.0 | 1.6 | 0.025 | 0.007 |
| Roseburia faecis | 3.1 | 3.6 | 3.6 | 1.1 | 0.754 | 0.992 |
| Prevotella sp. | 2.2 | 4.5 | 2.2 | 1.4 | 0.262 | 0.259 |
| Streptococcus alactolyticus | 1.0 | 1.6 | 4.5 | 2.6 | 0.867 | 0.435 |
| Succinivibrio dextrinosolvens | 4.4 | 2.3 | 1.8 | 2.2 | 0.502 | 0.876 |
| Lactobacillus delbrueckii | 4.4 | 1.1 | 0.5 | 2.6 | 0.373 | 0.873 |
| Lactobacillus kitasatonis | 1.7 | 0.7 | 0.8 | 1.5 | 0.654 | 0.958 |
| Acidaminococcus fermentans | 1.4 | 3.9 | 1.4 | 1.1 | 0.110 | 0.108 |
| Gemmiger formicilis | 1.1 | 1.2 | 1.6 | 0.8 | 0.901 | 0.699 |
| Mitsuokella multacida | 0.7 | 2.3 | 1.5 | 0.8 | 0.194 | 0.523 |
| Campylobacter coli | 2.7 | 0.8 | 0.5 | 0.8 | 0.127 | 0.837 |
| Selenomonas bovis | 1.6 | 1.3 | 2.2 | 0.9 | 0.797 | 0.489 |
| Lactobacillus mucosae | 1.4 | 1.3 | 0.6 | 0.7 | 0.911 | 0.496 |
| Dorea longicatena | 0.2 | 1.3 | 4.4 | 1.5 | 0.595 | 0.168 |
| Megasphaera hominis | 1.5 | 0.9 | 1.3 | 0.4 | 0.367 | 0.568 |
| Selenomonas lipolytica | 0.9 | 1.4 | 2.2 | 0.8 | 0.637 | 0.476 |
| Others | 6.4 | 7.9 | 9.5 | 1.9 | 0.578 | 0.574 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
The PC tended to reduce the relative abundance of Prevotella copri (p = 0.090), Phascolarctobacterium succinatutens (p = 0.053), and Lactobacillus delbrueckii (p = 0.050) in jejunal mucosa of pigs when compared with NC (Table 9). The AGP did not affect the relative abundance of mucosa-associated microbiota in pigs challenged with F18+ E. coli.
Table 9.
Relative abundance of mucosa-associated microbiota at the specie level in pigs challenged with F18+ Escherichia coli and fed diets with bacitacin as growth promoter.
| Treatment 1 | p Value | |||||
|---|---|---|---|---|---|---|
| Item | NC | PC | AGP | SEM | NC vs. PC | PC vs. AGP |
| Helicobacter rappini | 7.3 | 26.3 | 14.2 | 11.1 | 0.240 | 0.449 |
| Helicobacter mastomyrinus | 7.5 | 11.2 | 22.4 | 7.6 | 0.732 | 0.311 |
| Lactobacillus kitasatonis | 9.9 | 10.4 | 5.4 | 7.2 | 0.959 | 0.631 |
| Lactobacillus mucosae | 9.7 | 4.5 | 11.3 | 7.4 | 0.627 | 0.526 |
| Prevotella copri | 17.8 | 9.3 | 12.1 | 3.4 | 0.090 | 0.560 |
| Streptococcus alactolyticus | 10.4 | 10.1 | 4.7 | 4.5 | 0.967 | 0.408 |
| Campylobacter upsaliensis | 2.8 | 3.8 | 2.6 | 5.7 | 0.905 | 0.879 |
| Streptococcus infantarius | 3.2 | 5.4 | 3.9 | 2.6 | 0.549 | 0.679 |
| Dialister succinatiphilus | 2.9 | 1.9 | 2.9 | 1.2 | 0.555 | 0.568 |
| Prevotella stercorea | 5.1 | 1.5 | 1.2 | 1.1 | 0.036 | 0.853 |
| Phascolarctobacterium succinatutens | 4.8 | 1.0 | 0.8 | 1.3 | 0.053 | 0.937 |
| Lactobacillus salivarius | 0.7 | 0.9 | 1.2 | 1.7 | 0.945 | 0.906 |
| Faecalibacterium prausnitzii | 1.7 | 0.8 | 1.4 | 0.7 | 0.346 | 0.524 |
| Lactobacillus delbrueckii | 2.2 | 0.6 | 0.9 | 0.6 | 0.050 | 0.751 |
| Helicobacter sp. | 1.3 | 0.0 | 0.0 | 1.2 | 0.473 | 0.992 |
| Mitsuokella jalaludinii | 0.7 | 1.4 | 1.8 | 0.9 | 0.600 | 0.788 |
| Others | 12.0 | 11.0 | 13.5 | 4.6 | 0.875 | 0.705 |
1 NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
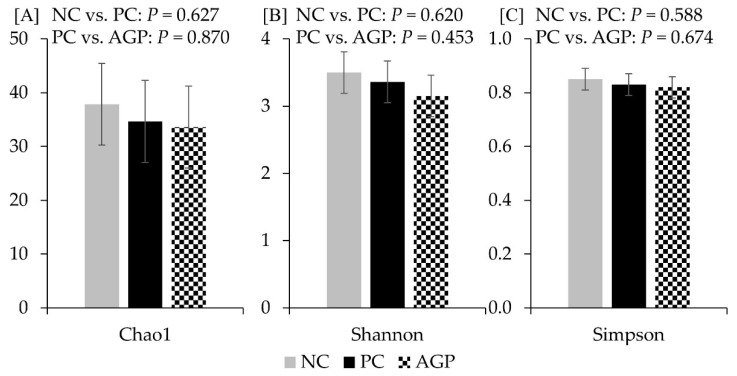
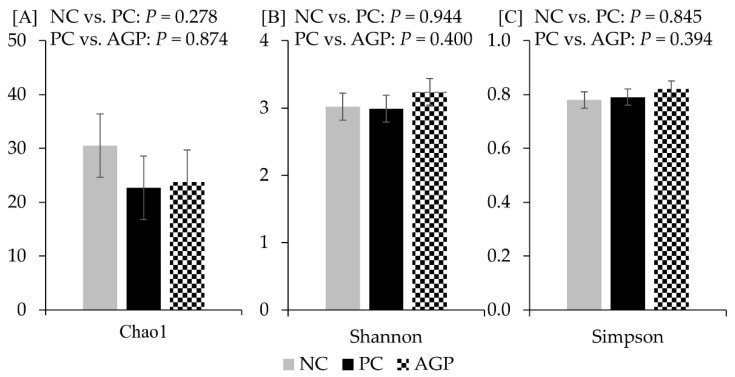
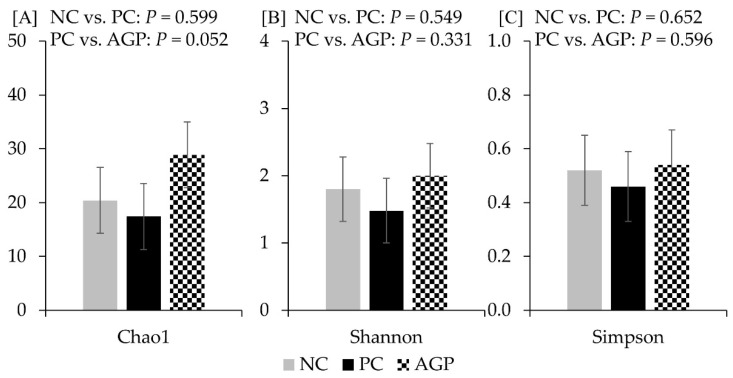
The alpha diversity of fecal microbiota was not affected by the treatments at d 14 and d 28 (Figure 3 and Figure 4). However, AGP tended to increase (p = 0.052) the alpha diversity of mucosa-associated microbiota estimated with Chao1 (Figure 5).
Figure 3.
Alpha diversity of fecal microbiota at d 14 estimated with Chao1 richness (A), Shannon diversity (B), and Simpson diversity (C) in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter. NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
Figure 4.
Alpha diversity of fecal microbiota at d 28 estimated with Chao1 richness (A), Shannon diversity (B), and Simpson diversity (C) in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter. NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
Figure 5.
Alpha diversity of mucosa-associated microbiota at d 28 estimated with Chao1 richness (A), Shannon diversity (B), and Simpson diversity (C) in pigs challenged with F18+ Escherichia coli and fed diets with bacitracin as a growth promoter. NC, not challenged/not treated; PC, challenged (E. coli F18+ at 5.2 × 109 CFU)/not treated; AGP, challenged (E. coli F18+ at 5.2 × 109 CFU)/treated with bacitracin (30 g/t).
4. Discussion
In this study, direct oral challenge with F18+ E. coli caused PWD, increased oxidative damage, and reduced the growth performance of weaned pigs, which is in agreement with previous reports [7,27]. The reduced feed efficiency seen among the E. coli challenged pigs can be attributed to the impaired intestinal health of challenged pigs as observed by increased fecal score, increased inflammation and oxidative stress, and the damaged villi and disrupted microbial community. The health challenged pigs may have had reduced nutrient absorption and/or altered partitioning of nutrients for immune response and growth, resulting in reduced feed efficiency [5,28]. However, bacitracin ameliorated many of the effects of the E. coli challenge, as evidenced by improved fecal scores, reduced oxidative damage and improved the feed efficiency. These benefits were seen without a significant reduction in fecal shedding of E. coli post-challenge.
Changes in diet, environment, social interaction, and the removal of the passive immunity from sow’s milk during a period where the immune system is not fully mature increase the vulnerability of newly weaned pigs to opportunistic pathogens [1,10,29]. The F18+ E. coli attaches to glycoproteins on the brush border in the intestine mediating resistance to flushing and promoting colonization [30,31,32]. In the current study, the increased F18+ E. coli counting in feces at d 14 matches with the increased fecal score in the period of 7 to 14 d of the experiment and may be an indicator of proliferation on the intestinal epithelium. At d 28, 21 d after challenge, the F18+ E. coli counting in feces did not differ among treatments, and fecal scores returned to normal, indicating pigs had controlled the E. coli infection to a less harmful level. The trend toward increased F18+ E. coli in the jejunal mucosa of challenged pigs indicates that F18+ E. coli can persist in the gastrointestinal tract for up to 21 d post-challenge. Duarte and Kim [8] reported that F18+ E. coli has a long-lasting effect in jejunal mucosa when compared with feces.
Interestingly, bacitracin reduced the F18+ E. coli population in jejunal mucosa. Antibiotics have been used to overcome or mitigate the challenges associated with health and nutrition, mainly by impairing the growth of pathogens [11,12]. Bacitracin, produced by Bacillus licheniformis, is an antibiotic with a narrow spectrum against primarily Gram-positive bacteria [33]. Bacitracin inhibits the synthesis of peptidoglycan and teichoic acids in the cell wall of bacteria inhibiting their proliferation [34,35]. Gram-positive bacteria are the main target for bacitracin due to the thicker peptidoglycan layer [35,36]. However, Gram-negative bacteria also contain peptidoglycan on the cell wall [37]. Xu et al. [7] demonstrated that bacitracin can mitigate the effects of PWD caused by F18+ E. coli in nursery pigs.
During proliferation, E. coli can produce enterotoxins, including STa and STb, that induce the secretion of fluid in the lumen of the small intestine, causing diarrhea [7,30]. Pigs challenged with F18+ E. coli in this study had increased fecal scores until d 21 of the experiment. Challenged pigs that received bacitracin showed improved fecal scores at d 14, although they remained higher than those of the unchallenged pigs. By d 21, the fecal scores of the F18+ E. coli challenged pigs treated with bacitracin were similar to those of the unchallenged controls. These results demonstrate the efficacy of bacitracin in mitigating PWD in pigs, as previously reported by Xu et al. [7]. Duarte and Kim [8] reported that, although the diarrhea symptoms ceased 14 d after an F18+ E. coli-challenge, the effects of F18+ E. coli on intestinal health lasted for at least 21 d. In addition to diarrhea, F18+ E. coli infections can also result in an inflammatory response [7,8]. A systemic inflammatory response was seen 14 d after challenge in this study, with a trend toward increased concentration of TNF-α in the serum of challenged pigs. However, there were no differences at d 28 and in TNF-α concentrations in jejunal mucosa. Other studies have reported increased expression of IL-6 and IL-8 in the jejunal mucosa of F18+ E. coli-challenged pigs without a significant change in TNF-α expression [7,38]. Due to the complex timing of cytokine cascades during an immune response, it is not necessarily surprising that TNF-α concentrations in the intestinal mucosa were not elevated at the end of the study. At the completion of the study, sera and mucosal concentrations of protein carbonyl were increased in the challenged pigs. These findings are in agreement with previous works that have reported that a F18+ E. coli challenge increases oxidative stress in nursery pigs [5,7,27]. During infection, ROS, including nitrite, are produced by immune cells to fight the infection [39,40,41]. The antioxidant enzymes scavenge the ROS maintaining homeostasis [40]. When the production of ROS exceeds the antioxidant capacity, products from oxidative stress, including protein carbonyls, are generated [42]. Protein carbonyl has been reported as an important biomarker of oxidative stress because it can be produced by all ROS, and it has higher stability compared with other products of oxidative damage [43]. Protein carbonyls lead to the dysfunction of cellular proteins, which can induce apoptosis [41,44]. In this study, bacitracin treatment tended to reduce protein carbonyl concentrations in challenged pigs, possibly by altering gut microbiota and reducing the intestinal mucosa’s immunoreaction in response to the F18+ E. coli or by altering the production of toxins and other antigens by the E. coli [45].
The altered fluid secretion induced by enterotoxins from E. coli can reduce water absorption and increase the flux of water from the enterocyte into the lumen of the intestine, causing dehydration and cell apoptosis [46,47,48]. Previous studies have shown that the apoptosis induced by cell dehydration and oxidative damage in challenged pigs is associated with the reduction in villus length [46,49]. In this study, pigs challenged with F18+ E. coli had the lower villus height in jejunum, confirming the deleterious effects of the E. coli on the epithelium. Enterocyte damage in the villi can induce cell proliferation in crypts to provide new enterocytes [49]. Increased cell proliferation can increase crypt depth, therefore reducing the villus height to crypt depth ratio [50,51,52], which was seen with E. coli challenge in this study. According to Pluske et al. [50], the atrophy of villi and the hyperplasia of crypts can reduce the digestion and absorption of nutrients, thereby reducing the feed efficiency of pigs. Additionally, undigested nutrients can further contribute to PWD due to the increased amount of substrate available for microbial fermentation [6,53].
Increased fluid secretion, products from an immune response, and undigested nutrients can all modulate the microbiome toward a more inflammatory microbiota, such as increasing the abundance of Proteobacteria [5,6,7,8]. This change in the microbiota composition is associated with the increased production of ROS, including nitrite, released during the immune response. The nitrite is transformed into nitrate in the lumen favoring the growth of bacteria expressing nitrate reductase, such as Proteobacteria [5,54]. However, 7 d after the challenge, there was a trend of increasing Firmicutes on the feces of pigs mainly by increasing Lachnospiraceae while reducing Tenericutes and Deferribacteries. The environmental changes near the mucosa may have exerted pressure on the microbiota, moving Lachnospiraceae toward the luminal content, consequently modulating the luminal and the mucosa-associated microbiota [6,8]. Additionally, it was observed a trend towards increasing Campylobacteraceae in the feces of challenged pigs. Interestingly, at 21 d after the challenge, the abundance of was increased in the feces of challenged pigs. According to Duarte and Kim [17], Mitsuokella spp. and Campylobacter spp. are highly correlated to inflammatory and oxidative stress in pigs challenged with F18+ E. coli.
According to Belkaid et al. [55], the immune system plays a pivotal role in modulating the mucosa-associated microbiota, which in turn modulate the luminal microbiota. The relative abundance of Prevotella spp. and Phascolarctobacterium succinatutens in jejunal mucosa-associated microbiota was reduced in challenged pigs, possibly due to the oxidative environment promoted by the immune response against E. coli. Prevotella is a Bacteroidetes that is associated with health conditions, and its relative abundance increases in pigs after weaning due to the fiber content in the diet [6,56]. The unbalance in the microbiota composition by reducing the abundance of fiber-degrading bacteria, in turn, can increase the immune response in the intestine [5,6]. Interestingly, bacitracin tended to increase the alpha diversity of mucosa-associated microbiota in the jejunum. Previous studies have demonstrated that bacitracin can increase microbial diversity [7,17,57], although, in general, antibiotics are associated with reduced diversity [58]. According to Proctor and Phillips [17], the bacitracin may have inhibited the proliferation of certain bacteria allowing the growth of others. These effects were observed in fecal samples at d 28, where the abundance of P. succinatutens was increased, and M. jalaludinii populations were reduced. M. jalaludinii are Gram-negative bacteria confirming that bacitracin can also affect bacteria other than Gram-positive. Phascolarctobacterium succinatutens, a strict anaerobic bacteria belonging to Firmicutes, are associated with propionate production through the succinate scavenge [59,60]. Succinate is normally produced by different bacteria within the intestine, especially from carbohydrate fermentation [60]. It has been reported that succinate exerts inflammatory [61] and oxidative [62] roles. Therefore, these findings suggest that the reduction in protein carbonyl reported in the current study can also be associated with the increased abundance of bacteria associated with fiber utilization, including P. succinatutens.
5. Conclusions
The F18+ E. coli challenge resulted in increased fecal scores, altered intestinal histology, increased oxidative damage, all demonstrating reduced intestinal health. This resulted in impaired growth performance of pigs challenged with F18+ E. coli. Dietary supplementation with bacitracin ameliorates many of the intestinal health challenges caused by F18+ E. coli, resulting in improved growth performance. Whereas further studies are needed to elucidate the protective mechanisms of bacitracin on a F18+ E. coli infection, alterations in the microbiota towards a less harmful milieu may underlay this effect and ultimately provide greater insight into the role of microbiota on improving growth performance.
Acknowledgments
K. L. Brooks, H. Chen, J. Guo, K. B. Jang, Y. I. Kim; J. K. Lee, I. Park, W. Parnsen, and L. Zheng of the Kim Lab for their assistance in animal management, sampling, and sample analysis.
Author Contributions
Conceptualization, S.W.K.; methodology, S.W.K. and C.H.S.; formal analysis, S.W.K. and M.E.D.; investigation, S.W.K.; resources, S.W.K.; data curation, M.E.D.; and S.W.K.; writing—original draft preparation, M.E.D. and S.W.K.; writing—review and editing, M.E.D., C.H.S., and S.W.K.; supervision, S.W.K.; project administration, S.W.K.; funding acquisition, S.W.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The experimental protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee at North Carolina State University (IACUC #: 19-834). The experiments were performed by trained scientists in full compliance with the North Carolina State Animal Care and Use Procedures (REG 10.10.01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
USDA-NIFA (Hatch #02893) and North Carolina Agricultural Foundation (#660101).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McLamb B.L., Gibson A.J., Overman E.L., Stahl C., Moeser A.J. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS ONE. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013;4:2–5. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moeser A.J., Pohl C.S., Rajput M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017;3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y., Kim S.W. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 2017;3:322–330. doi: 10.1016/j.aninu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duarte M.E., Tyus J., Kim S.W. Synbiotic effects of enzyme and probiotics on intestinal health and growth of newly weaned pigs challenged with enterotoxigenic F18+ Escherichia coli. Front. Vet. Sci. 2020;7:573. doi: 10.3389/fvets.2020.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte M.E., Kim S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2022;8:169–184. doi: 10.1016/j.aninu.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X., Duarte M.E., Kim S.W. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2022;100:skac210. doi: 10.1093/jas/skac210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte M.E., Kim S.W. Significance of mucosa-associated microbiota and its impacts on intestinal health of pigs challenged with F18+ E. coli. Pathogens. 2022;11:589. doi: 10.3390/pathogens11050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang K.B., Purvis J.M., Kim S.W. Dose–response and functional role of whey permeate as a source of lactose and milk oligosaccharides on intestinal health and growth of nursery pigs. J. Anim. Sci. 2021;99:skab008. doi: 10.1093/jas/skab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L., Duarte M.E., Sevarolli Loftus A., Kim S.W. Intestinal health of pigs upon weaning: Challenges and nutritional intervention. Front. Vet. Sci. 2021;8:628258. doi: 10.3389/fvets.2021.628258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter L.E. The effect of antibiotics and vitamin B12 on the growth of swine. Arch. Biochem. Biophys. 1951;32:187–191. doi: 10.1016/0003-9861(51)90252-4. [DOI] [PubMed] [Google Scholar]
- 12.Luecke R.W., Thorp F., Newland H.W., Mcmillen W.N. The growth promoting effects of various antibiotics on pigs. J. Anim. Sci. 1951;10:538–542. doi: 10.2527/jas1951.102538x. [DOI] [PubMed] [Google Scholar]
- 13.Bridges J.H., Hale F., Kunkel H.O., Lyman C.M. The effects of bacitracin, penicillin and arsanilic acid on growth rate and feed efficiency in swine. J. Anim. Sci. 1954;13:912–917. doi: 10.2527/jas1954.134912x. [DOI] [Google Scholar]
- 14.FDA Food and Drug Administration . Guidance for Industry #213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food- Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with. Center for Veterinary Medicine; Rockville, MD, USA: 2013. [Google Scholar]
- 15.Chen Y., Hu S., Li J., Zhao B., Yang N., Zhou T., Liang S., Bai S., Wu X. Bacitracin methylene disalicylate improves intestinal health by modulating its development and microbiota in weaned rabbits. Front. Microbiol. 2021;12:579006. doi: 10.3389/fmicb.2021.579006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson T.A., Sylte M.J., Looft T. In-feed bacitracin methylene disalicylate modulates the turkey microbiota and metabolome in a dose-dependent manner. Sci. Rep. 2019;9:8212. doi: 10.1038/s41598-019-44338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proctor A., Phillips G.J. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 2019;6:114. doi: 10.3389/fvets.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NRC . Nutrient Requirements of Swine. 11th ed. National Academies Press; Washington, DC, USA: 2012. [Google Scholar]
- 19.Deng Z., Duarte M.E., Jang K.B., Kim S.W. Soy protein concentrate replacing animal protein supplements and its impacts on intestinal immune status, intestinal oxidative stress status, nutrient digestibility, mucosa-associated microbiota, and growth performance of nursery pigs. J. Anim. Sci. 2022;100:skac255. doi: 10.1093/jas/skac247.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang K.B., Duarte M.E., Purvis J.M., Kim S.W. Impacts of weaning age on dietary needs of whey permeate for pigs at 7 to 11 kg body weight. J. Anim. Sci. Biotechnol. 2021;12:111. doi: 10.1186/s40104-021-00637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moita V.H.C., Duarte M.E., Kim S.W. Functional roles of xylanase enhancing intestinal health and growth performance of nursery pigs by reducing the digesta viscosity and modulating the mucosa-associated microbiota in the jejunum. J. Anim. Sci. 2022;100:skac116. doi: 10.1093/jas/skac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte M.E., Kim S.W. Phytobiotics from oregano extracts enhance the intestinal health and growth performance of pigs. Antioxidants. 2022;11:2066. doi: 10.3390/antiox11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y.-C., Duarte M.E., Kim S.W. Effects of Yarrowia lipolytica supplementation on growth performance, intestinal health and apparent ileal digestibility of diets fed to nursery pigs. Anim. Biosci. 2022;35:605–613. doi: 10.5713/ab.21.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holanda D.M., Kim S.W. Investigation of the efficacy of mycotoxin-detoxifying additive on health and growth of newly-weaned pigs under deoxynivalenol challenges. Anim. Biosci. 2021;34:405–416. doi: 10.5713/ajas.20.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang K.B., Kim J.H., Purvis J.M., Chen J., Ren P., Vazquez-Anon M., Kim S.W. Effects of mineral methionine hydroxy analog chelate in sow diets on epigenetic modification and growth of progeny. J. Anim. Sci. 2020;98:skaa271. doi: 10.1093/jas/skaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte M.E., Sparks C., Kim S.W. Modulation of jejunal mucosa-associated microbiota in relation to intestinal health and nutrient digestibility in pigs by supplementation of β-glucanase to corn–soybean meal-based diets with xylanase. J. Anim. Sci. 2021;99:skab190. doi: 10.1093/jas/skab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang K.B., Moita V.H.C., Martinez N., Sokale A., Kim S.W. Efficacy of zinc glycinate reducing zinc oxide on intestinal health and growth of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2023;101:skad035. doi: 10.1093/jas/skad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntley N.F., Nyachoti C.M., Patience J.F. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 2018;9:47. doi: 10.1186/s40104-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S.W., Duarte M.E. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 2021;34:338–344. doi: 10.5713/ab.21.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubreuil J.D., Isaacson R.E., Schifferli D.M. Animal enterotoxigenic Escherichia coli. EcoSal Plus. 2016;7:1–47. doi: 10.1128/ecosalplus.ESP-0006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson J.W., Whipp S.C. Comparison of the mechanisms of action of cholera toxin and the heat-stable enterotoxins of Escherichia coli. Infect. Immun. 1995;63:1452–1461. doi: 10.1128/iai.63.4.1452-1461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy B., Whipp S.C., Imberechts H., Bertschinger H.U., Dean-Nystrom E.A., Casey T.A., Salajka E. Biological relationship between F18ab and F18ac fimbriae of enterotoxigenic and verotoxigenic Escherichia coli from weaned pigs with oedema disease or diarrhoea. Microb. Pathog. 1997;22:1–11. doi: 10.1006/mpat.1996.0085. [DOI] [PubMed] [Google Scholar]
- 33.Huyghebaert G., De Groote G. The bioefficacy of zinc bacitracin in practical diets for broilers and laying hens. Poult. Sci. 1997;76:849–856. doi: 10.1093/ps/76.6.849. [DOI] [PubMed] [Google Scholar]
- 34.Tay W.M., Epperson J.D., da Silva G.F.Z., Ming L.-J. H NMR, Mechanism, and Mononuclear oxidative activity of the antibiotic metallopeptide bacitracin: The Role of d -Glu-4, Interaction with Pyrophosphate Moiety, DNA Binding and Cleavage, and Bioactivity. J. Am. Chem. Soc. 2010;132:5652–5661. doi: 10.1021/ja910504t. [DOI] [PubMed] [Google Scholar]
- 35.Mascher T., Margulis N.G., Wang T., Ye R.W., Helmann J.D. Cell wall stress responses in Bacillus subtilis: The regulatory network of the bacitracin stimulon. Mol. Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- 36.Rajagopal M., Walker S. Current Topics in Microbiology and Immunology. Volume 404. Springer; Cham, Switzerland: 2017. Envelope structures of Gram-positive bacteria; pp. 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y.-C., Kim S.W. Use of microorganisms as nutritional and functional feedstuffs for nursery pigs and broilers. Animals. 2022;12:3141. doi: 10.3390/ani12223141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong B.T., Park S., Kovanda L., He Y., Kim K., Xu S., Lingga C., Hejna M., Wall E., Sripathy R., et al. Dietary supplementation of botanical blends enhanced performance and disease resistance of weaned pigs experimentally infected with enterotoxigenic Escherichia coli F18. J. Anim. Sci. 2022;100:skac353. doi: 10.1093/jas/skac353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y., Bazhin A.V., Werner J., Karakhanova S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013;32:249–270. doi: 10.3109/08830185.2012.755176. [DOI] [PubMed] [Google Scholar]
- 40.Morris G., Gevezova M., Sarafian V., Maes M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022;19:1079–1101. doi: 10.1038/s41423-022-00902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celi P., Gabai G. Oxidant/antioxidant balance in animal nutrition and health: The role of protein oxidation. Front. Vet. Sci. 2015;2:48. doi: 10.3389/fvets.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 44.DalleDonne I., Milzani A., Colombo R. The tert -butyl hydroperoxide-induced oxidation of actin cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry. 1999;38:12471–12480. doi: 10.1021/bi990367k. [DOI] [PubMed] [Google Scholar]
- 45.Settle T., Leonard S.S., Falkenstei E., Fix N., Van Dyke K., Klandorf H. Effects of a phytogenic feed additive versus an antibiotic feed additive on oxidative stress in broiler chicks and a possible mechanism determined by electron spin resonance. Int. J. Poult. Sci. 2014;13:62–69. doi: 10.3923/ijps.2014.62.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson A.M., Kaushik R.S., Rotella N.J., Hardwidge P.R. Enterotoxigenic Escherichia coli modulates host intestinal cell membrane asymmetry and metabolic activity. Infect. Immun. 2009;77:341–347. doi: 10.1128/IAI.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubreuil J.D. Enterotoxigenic Escherichia coli and probiotics in swine: What the bleep do we know? Biosci. Microbiota Food Health. 2017;36:75–90. doi: 10.12938/bmfh.16-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy D.J., Greenberg R.N., Dunn J.A., Abernathy R., Ryerse J.S., Guerrant R.L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect. Immun. 1984;46:639–643. doi: 10.1128/iai.46.3.639-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assimakopoulos S.F., Tsamandas A.C., Louvros E., Vagianos C.E., Nikolopoulou V.N., Thomopoulos K.C., Charonis A., Scopa C.D. Intestinal epithelial cell proliferation, apoptosis and expression of tight junction proteins in patients with obstructive jaundice. Eur. J. Clin. Investig. 2011;41:117–125. doi: 10.1111/j.1365-2362.2010.02379.x. [DOI] [PubMed] [Google Scholar]
- 50.Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997;51:215–236. doi: 10.1016/S0301-6226(97)00057-2. [DOI] [Google Scholar]
- 51.Shaw D. Intestinal mucosal atrophy and adaptation. World J. Gastroenterol. 2012;18:6357. doi: 10.3748/wjg.v18.i44.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pluske J.R., Williams I.H., Aherne F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996;62:131–144. doi: 10.1017/S1357729800014417. [DOI] [Google Scholar]
- 53.Kim K., He Y., Xiong X., Ehrlich A., Li X., Raybould H., Atwill E.R., Maga E.A., Jørgensen J., Liu Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019;10:52. doi: 10.1186/s40104-019-0364-3. [DOI] [Google Scholar]
- 54.Bäumler A.J., Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary Fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Díaz Carrasco J.M., Redondo E.A., Pin Viso N.D., Redondo L.M., Farber M.D., Fernández Miyakawa M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed Res. Int. 2018;2018:1879168. doi: 10.1155/2018/1879168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grazul H., Kanda L.L., Gondek D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes. 2016;7:101–114. doi: 10.1080/19490976.2016.1138197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeyama N., Murakami T., Toyoda A., Mori H., Iino T., Ohkuma M., Sakamoto M. Microbial interaction between the succinate-utilizing bacterium Phascolarctobacterium faecium and the gut commensal Bacteroides thetaiotaomicron. Microbiologyopen. 2020;9:e1111. doi: 10.1002/mbo3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernández-Veledo S., Vendrell J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019;20:439–447. doi: 10.1007/s11154-019-09513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.