Abstract
The Saccharomyces cerevisiae Cdc42p GTPase interacts with multiple regulators and downstream effectors through an ∼25-amino-acid effector domain. Four effector domain mutations, Y32K, F37A, D38E, and Y40C, were introduced into Cdc42p and characterized for their effects on these interactions. Each mutant protein showed differential interactions with a number of downstream effectors and regulators and various levels of functionality. Specifically, Cdc42D38Ep showed reduced interactions with the Cla4p p21-activated protein kinase and the Bem3p GTPase-activating protein and cdc42D38E was the only mutant allele able to complement the Δcdc42 null mutant. However, the mutant protein was only partially functional, as indicated by a temperature-dependent multibudded phenotype seen in conjunction with defects in both septin ring localization and activation of the Swe1p-dependent morphogenetic checkpoint. Further analysis of this mutant suggested that the multiple buds emerged consecutively with a premature termination of bud enlargement preceding the appearance of the next bud. Cortical actin, the septin ring, Cla4p-green fluorescent protein (GFP), and GFP-Cdc24p all predominantly localized to one bud at a time per multibudded cell. These data suggest that Cdc42D38Ep triggers a morphogenetic defect post-bud emergence, leading to cessation of bud growth and reorganization of the budding machinery to another random budding site, indicating that Cdc42p is involved in prevention of the initiation of supernumerary buds during the cell cycle.
Saccharomyces cerevisiae Cdc42p, a highly conserved member of the Rho family of GTPases, is required for bud site selection, bud emergence, cell cycle progression, and rearrangement of the actin cytoskeleton to regions of polarized growth (16). Mutational and biochemical characterization of Cdc42p revealed that regulation of the guanine nucleotide state of Cdc42p is essential for its proper function in these processes (9, 40). Moreover, biochemical and two-hybrid studies showed that GTP-bound Cdc42G12Vp displayed enhanced interactions with effectors and regulators (4, 5, 8–10, 15, 20, 29, 30, 33, 37). Mutational analysis of the Cdc42p effector domain, which consists of amino acids 26 to 50 (Fig. 1), indicated that this region is required for function and lends specificity to interactions with a number of Cdc42p regulators and effectors (9, 23, 33). However, how these various specific interactions lead to downstream events such as actin reorganization and bud emergence still needs to be explored.
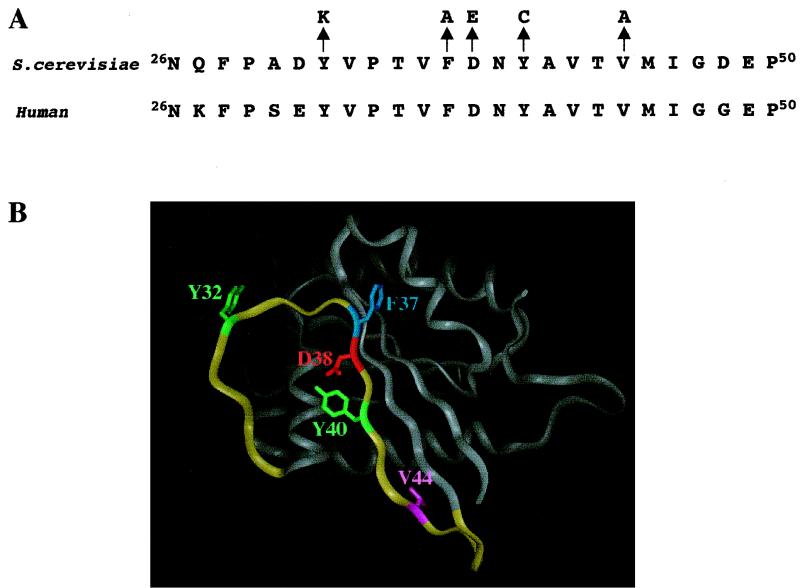
FIG. 1.
(A) Alignment of the S. cerevisiae and human Cdc42p effector domains. Arrows point to the specific amino acid changes (one-letter code) made at positions 32, 37, 38, 40, and 44. (B) Cdc42p crystal structure (adapted from reference 27). Highlighted in yellow are amino acids 26 to 50 of the effector domain. Highlighted in green are Tyr32 and Tyr40, in blue is Phe37, in red is Asp38, and in purple is Val44.
Characterization of Cdc42p regulators and effectors has provided valuable insight into how Cdc42p functions (16). Both the S. cerevisiae guanine nucleotide exchange factor Cdc24p and GTPase-activating proteins (GAPs) Bem3p and Rga1p were shown to interact with Cdc42p through its effector domain, as well as other domains (9); T. J. Richman and D. I. Johnson, unpublished results, suggesting that competition for binding is important for maintenance of a balance of active and inactive Cdc42p at the proper time(s) during the cell cycle. There are also a number of downstream effectors, including the p21-activated protein kinases (PAKs) Ste20p, Cla4p, and Skm1p, novel effectors Gic1p and Gic2p, formin homolog Bni1p, and IQGAP homolog Iqg1p/Cyk1p, that interact with Cdc42p in S. cerevisiae and are involved in various cellular functions, including actin polarization, budding, mating, and cytokinesis (16). Characterization of some of these effectors suggested that Cdc42p is involved in their localization (20) or in the regulation of their activation (2, 25). However, how Cdc42p balances interactions with all of these known effectors to specifically regulate cell cycle events is largely unknown.
Characterization of effector domain mutant cdc42V44A highlighted the effects of altered effector and regulator interactions on the budding cycle. Cdc42V44Ap showed altered interactions with Cla4p, Gic1p, Gic2p, and Cdc24p, which led to defects in the apical-isotropic bud growth switch, localization and structural integrity of the septin ring, and cytokinesis and a delay in nuclear division (9, 33). To build on the cdc42V44A study, four additional effector domain mutations, Y32K, F37A, D38E, and Y40C, were introduced into CDC42 and characterized for interactions with regulators and effectors and for functionality. Only the cdc42D38E allele was functional as the sole copy of CDC42. The cdc42D38E mutant showed a multibudded phenotype with a new bud emerging from a random site after a preceding bud prematurely terminated growth within a single cell cycle. This phenotype implicated Cdc42p in the regulation of bud growth and limitation of bud emergence to once per cell cycle. Analysis of cdc42D38E also reinforced Cdc42p's role in the Swe1p-dependent morphogenetic checkpoint in G2/M. Together, these results stressed the relationship between interactions with regulators-effectors and Cdc42p function and showed that Cdc42p is required for maintenance of bud growth following bud emergence.
MATERIALS AND METHODS
Reagents, media, and strains.
Enzymes, PCR kits, and other reagents were obtained from standard commercial sources and used as specified by the suppliers. Oligonucleotide primers for sequencing and PCR were obtained from Genosys (The Woodlands, Tex.). Growth media and maintenance of bacterial strains have been described previously (34, 35). The S. cerevisiae strains used are listed in Table 1. Yeast transformations were performed as described previously (35). Selection of transformants was on synthetic complete (SC) dropout media lacking a specified amino acid(s) and containing 2% glucose as a carbon source.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference or sourcea |
|---|---|---|
| C276-4A | MATa gal2 | 39 |
| DJTD2-16A | MATa cdc42-1 his4 leu2 trp1 ura3 | 17 |
| PMYD9-1B | MATa ura3-52:cdc42W97R:URA3 Δcdc42::TRP1 trp1-Δ101 leu2 ade2-101lys2-801 his | 26 |
| EGY48-p1840 | MATα ura3 his3 trp1 integrated lexAop-LEU2 integrated lexAop-lacZ | 11 |
| DJD6-11 | MATa/MATα Δcdc42::TRP1/+ his3Δ200/+ his4/+ leu2/+ can1/+ lys2-801/lys2-801 trp1-Δ1/trp1-Δ101 ade2-101/+ ura3-52/ura3-52 | 26 |
| TRY11-7D | MATα leu2 ura3 trp1 his | 33 |
| DLY1028 | MATa Δswe1::LEU2 ade2 ura3 trp1 his2 | 33 |
| TRY13-5A | MATa his3 leu2 ura3 trp1 | This study |
| TRY32 | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42Y32K:URA3/ura3-52 leu2/+ lys2-801/lys2-801 his3Δ200/+ his4/+ trp1-Δ1/trp1-Δ101 ade2-101/+ can1/+ | This study |
| TRY37 | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42F37A:URA3/ura3-52 leu2/+ lys2-801/lys2-801 his3Δ200/+ his4/+ trp1-Δ1/trp1-Δ101 ade2-101/+ can1/+ | This study |
| TRY38 | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42D38E:URA3/ura3-52 leu2/+ lys2-801/lys2-801 his3Δ200/+ his4/+ trp1-Δ1/trp1-Δ101 ade2-101/+ can1/+ | This study |
| TRY38-2B | MATa Δcdc42::TRP1 ura3-52:cdc42D38E:URA3 leu2 lys2-801 his3Δ200 trp1Δ1 | This study |
| TRY38-8A | MATα Δcdc42::TRP1 ura3-52:cdc42D38E:URA3 leu2 lys2-801 his3Δ200 his4 trp1Δ1 ade2-101 | This study |
| TRY39 | MATa/MATα Δcdc42::TRP1/+ ura3:cdc42D38E:URA3/ura3 ade2-101/ade2 Δswe1::LEU2/+ leu2/leu2 trp1-Δ101/trp1 his2/+ his3/+ his4/+ lys2-801/+ | This study |
| TRY40 | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42Y40C:URA3/ura3-52 leu2/+ lys2-801/lys2-801 his3Δ200/+ his4/+ trp1-Δ1/trp1-Δ101 ade2-101/+ can1/+ | This study |
| TRY41A | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42D38E:URA3/ura3 leu2/leu2 his3/his3 trp1Δ1/trp1 ade2/ade2 +/Δcla4::trp1::HIS3 | This study |
| TRY46 | MATa/MATα Δcdc42::TRP1/+ ura3-52:cdc42D38E:URA3/ura3 +/ade1 leu2/leu2 trp1/trp1 Δbem3::LEU2/+ his3/his3 lys2-801/+ | This study |
| TRY48 | MATa/MATα ade1/++/ade2 ura3/ura3 leu2/leu2-3 his3/his3 trp1/trp1 +/Δcla4::trp1::HIS3 bem3::LEU2/+ | This study |
TRY32 was generated by integrating linearized pRS306(cdc42Y32K) into DJD6-11. TRY37, TRY38, and TRY40 were created in the same manner as TRY32 with pRS306(cdc42F37A), pRS306(cdc42D38E), and pRS306(cdc42Y40C), respectively. TRY39 was generated by crossing TRY38-8A with DLY1028. TRY41A was generated by crossing TRY38-2B with TRY41-3A (a Δcla4::trp1::HIS3 strain generated by tetrad dissection of TRY41, which was generated by crossing TRY38-8B with TRY3-H [33]). TRY46 was generated by crossing TRY38-2B with TRY4-1B (a Δbem3::LEU2 strain generated by tetrad dissection of SY3032 [37]). TRY48 was generated by crossing TRY3-H with TRY4-1B.
To determine if cdc42Y32K, cdc42F37A, cdc42D38E, or cdc42Y40C could function as the sole copy of CDC42, these mutant alleles including the CDC42 promoter were cloned into integrating vector pRS306 that was then cut within the URA3 locus. The linearized plasmids were transformed into the CDC42/Δcdc42::TRP1 ura3-52/ura3-52 diploid DJD6-11; stable Ura+ transformants had mutant cdc42 integrated at the ura3 locus. Spores from dissected tetrads were grown at 23°C and then streaked on selective media to determine marker distributions; segregants that contained the cdc42 mutant allele in a Δcdc42 background would be Ura+ Trp+. No Ura+ Trp+ segregants were recovered from tetrads containing cdc42Y32K, cdc42F37A, and cdc42Y40C, suggesting that these alleles could not act as the sole copy of CDC42. In contrast, eight Ura+ Trp+ segregants were recovered from strain TRY38 which contained integrated cdc42D38E, indicating that this allele could act as the sole copy of CDC42. In heterozygous diploids, the cdc42D38E mutant was recessive to the wild type (data not shown). Cell viability was determined by growing cells in yeast extract-peptone-dextrose (YEPD) liquid, micromanipulating 50 single cells on YEPD plates, and quantitating their ability to form colonies.
Plasmids, DNA manipulations, PCR, and site-directed mutagenesis.
Recombinant DNA manipulations (34) and plasmid isolation from Escherichia coli (3) were performed as previously described. Site-directed mutagenesis was performed with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). The sequences of the mutagenic primers are available upon request. Automated sequencing at the Vermont Cancer Center DNA Sequencing Facility was used to sequence all gene constructs. Plasmids pRS315(cdc42V44A), pRS315(GFP-CDC3), pRS315(GFP-CDC12), YEp351(CLA4), YEp13(BEM3), YEp351(CDC42), and pRS425 were described previously (7, 33, 37, 40). pBRS115(CLA4-GFP) was kindly provided by Malcolm Whiteway. Plasmid pRS306(cdc42Y32K) was created by inserting the NotI-plus-SalI fragment containing cdc42Y32K from plasmid pRS315(cdc42Y32K) into pRS306 (36) cut with NotI plus SalI. pRS306(cdc42F37A), pRS306(cdc42D38E), and pRS306(cdc42Y40C) were created in the same manner. Plasmid pAD11(CLA4) was created by inserting the EagI-plus-EcoRI fragment containing CLA4 and its own promoter from plasmid pBB130 (33) into pAD11 cut with EagI plus EcoRI.
To create pEG202(cdc42Y32K, C188S), cdc42Y32K was amplified from pRS315(cdc42Y32K) using Taq-based PCR and primers that incorporated the C188S mutation (to override the normal plasma membrane localization of Cdc42p) into the resulting 576-bp PCR fragment. PCR fragments were digested with EcoRI plus BamHI and inserted into EcoRI/BamHI-digested pEG202 (11). pEG202(cdc42F37A, C188S), pEG202(cdc42D38E, C188S), and pEG202(cdc42Y40C, C188S) were created in the same manner. pEG202(cdc42G12V, Y32K, C188S) was created using the QuikChange kit using pEG202(cdc42Y32K, C188S) as the DNA template. pEG202(cdc42G12V, F37A, C188S), pEG202(cdc42G12V, D38E, C188S), and pEG202(cdc42G12V, Y40C, C188S) were created in the same manner. The QuikChange mutagenesis kit was also used to create p416MET(GFPS65T-A8-cdc42D38E) using p416MET(GFPS65T-A8-CDC42) (33) as the DNA template. All of resulting constructs described above were sequenced to confirm the fusion and mutant cdc42 sequence.
Two-hybrid protein interactions and protein analysis.
The methods used for two-hybrid analysis have been described previously (6, 11). Strain EGY48-p1840 containing pJG4-5(CLA4) (8), pJG4-5(SKM1) (33), pJG4-5(GIC1) and pJG4-5(GIC2) (5), or pJG4-5(BNI1 1-1214 aa) (10) and the various pEG202(CDC42) plasmids were selected on SC-His-Trp media containing 2% galactose and 2% raffinose at 23°C. Strain EGY48-p1840 containing pRL222(STE20) (33) or pGADC2(IQG1) (29) and the various constructs were selected on SC-His-Leu medium containing 2% glucose. LexA-DBD fusions in vector pEG202 are under the control of the ADH constitutive promoter. GAL4-AD fusions in vector pJG4-5 are under the control of the pGAL1 inducible promoter. β-Galactosidase lift assays were performed with at least three transformants for each interaction tested and performed as previously described (9). β-Galactosidase liquid assays were performed in triplicate, and β-galactosidase units were calculated as previously described (32).
Protein preparation and Cdc42p cell fractionation were performed as previously described (41), with the following changes. Cells were grown at 30°C, and ∼109 cells (optical density at 600 nm, 50) were harvested, washed with water, and resuspended in membrane buffer (10 mM Tris acetate [pH 7.6], 1 mM magnesium acetate, 0.1 mM EDTA, 8% glycerol [13]) with the protease inhibitor concentrations described previously (41). After cell fractionation, pellets were resuspended in a volume of membrane buffer equivalent to that of the supernatant. Equal volumes of all fractions were loaded onto a sodium dodecyl sulfate–12% polyacrylamide gel for immunoblot analysis. Cdc42p was detected by immunoblot analysis using a 1:500 dilution of Cdc42p antibodies and a 1:1,000 dilution of goat anti-rabbit antibodies as described previously (41).
Photomicroscopy and flow cytometry.
Cells were grown in the appropriate liquid media at 23, 30, or 37°C to mid-log phase and then collected, sonicated, and examined morphologically. The methods used to prepare and stain cells with 4′,6-diamidino-2-phenylindole (DAPI), calcofluor, and rhodamine-phalloidin have been described previously (31). Green fluorescent protein (GFP)-Cdc3p- and GFP-Cdc12p-containing cells were grown to mid-log phase, sonicated, and observed. Photomicroscopy using Hoffman modulation optics to visualize calcofluor, rhodamine-phalloidin staining, and GFP fluorescence was performed on an Olympus BH-2 epifluorescence microscope. Photomicroscopy using phase-contrast optics and Omega optical filter cube XF06 to visualize DAPI fluorescence was performed on an E400 Nikon microscope (Omega Optical, Brattleboro, Vt.). Digital cell images were obtained and analyzed as previously described (33). Time-lapse microscopy was performed on the E400 Nikon microscope: cdc42D38E (TRY38-2B) cells were grown to stationary phase at 23°C and sonicated, and an aliquot of cells was layered onto a microscope slide thin layered with a 1% YEPD agarose slab. The slide was then placed on the microscope stage heated to 30°C, and cell division was monitored and recorded for 7 h. Still frames from the video recording were captured and analyzed. Where indicated, cells from the same culture but different fields were assembled into collages using Adobe Photoshop 5.0. Cells used in flow cytometry were prepared as previously described (12), and flow cytometry was performed at the Harry Hoodbassett Flow Cytometry Facility at the University of Vermont.
RESULTS
Cdc42 effector domain mutant proteins differentially interact with regulators and downstream effectors.
In order to further study the correlation between Cdc42p interactions and function, four effector domain mutations, Y32K, F37A, D38E, and Y40C, were generated (Fig. 1). Analogous mutations in mammalian Cdc42p have been characterized (see Discussion). These mutant proteins were then screened for interactions with Cla4p, Ste20p, Skm1p, Gic1p, Gic2p, Bni1p, Iqg1p, and the Bem3p and Rga1p GAPs using the two-hybrid system (Table 2). Cdc42Y32K, C188Sp showed reduced or no interactions with most effectors and regulators, except Ste20p, Bni1p, and Rga1p, even in the context of the G12V activated mutation, although Cdc42G12V, Y32K, C188Sp interactions with Ste20p were slightly reduced. Cdc42Y40C, C188Sp showed a similar interaction pattern, except that it retained interactions with Iqg1p. The only change seen with the incorporation of the G12V mutation was a partial recovered interaction with Gic1p and Gic2p that was comparable to the interaction seen with the Cdc42D118Ap negative control. In contrast, Cdc42F37A, C188Sp showed no interactions with any effectors except Bni1p but Cdc42G12V, F37A, C188Sp showed reduced or no interactions with only Iqg1p, Bem3p, and Ste20p. Finally, Cdc42D38E, C188Sp had reduced interactions with Cla4p and retained all of the other interactions compared to controls. Incorporation of the G12V mutation restored interactions between Cdc42G12V, D38E, C188S and Cla4p but reduced interactions with Bem3p. These diminished interactions seen with Cla4p and Bem3p were not complete losses of interaction, as confirmed by β-galactosidase liquid assays (Table 3). The interactions with Bem3p were also affected by the V44A mutation (Table 2). Taken together, these data suggested that conserved amino acids Tyr32, Phe37, Asp38, Tyr40, and Val44 are required for Cdc42p specificity for PAK kinases, Gic1p, Gic2p, Iqg1p, and the Bem3p GAP but not the Bni1p formin homolog or the Rga1p GAP. In addition, locking these mutant Cdc42p proteins in a GTP-bound state via the G12V mutation enhanced interactions with only a subset of effectors.
TABLE 2.
Screen of Cdc42p effector domain mutantsa
| Mutation(s) | CLA4 | STE20 | SKM1 | GIC1 | GIC2 | BNI1 | IQG1 | BEM3 | RGA1 |
|---|---|---|---|---|---|---|---|---|---|
| None (wild type) | +++ | ++ | − | +++ | +++ | +++ | + | ||
| Y32K | − | ++ | − | − | − | +++ | − | ||
| F37A | − | − | − | − | − | +++ | − | ||
| D38E | + | ++ | − | +++ | +++ | +++ | + | ||
| Y40C | − | + | − | − | − | +++ | + | ||
| V44A | + | ++ | − | + | + | +++ | + | ||
| G12V | +++ | +++ | ++ | +++ | +++ | +++ | ++ | +++ | +++ |
| G12V, Y32K | − | ++ | − | − | − | +++ | − | − | +++ |
| G12V, F37A | +++ | ++ | ++ | +++ | +++ | +++ | − | − | +++ |
| G12V, D38E | +++ | +++ | ++ | +++ | +++ | +++ | ++ | + | +++ |
| G12V, Y40C | − | ++ | − | + | + | +++ | ++ | + | +++ |
| G12V, V44A | ++ | +++ | ++ | + | + | +++ | ++ | + | +++ |
| D118A | − | − | − | + | + | − | − | − | − |
The C188S mutation was present in all Cdc42 constructs to override the normal plasma membrane localization of Cdc42p. β-Galactosidase lift assays were performed with at least three transformants for each interaction. −, no interaction detected; +, weak interaction detected; ++, intermediate interaction detected; +++, strong interaction detected. Direct comparison of Ste20p and Iqg1p interactions with other interactions is not possible because different promoter systems were used (see Materials and Methods).
TABLE 3.
Cdc42D38Ep quantitative two-hybrid interactions with Cla4p and Bem3pa
| LexA binding domain (pEG202) | GAL activation domain (pJG4-5)
|
|
|---|---|---|
| CLA4 | BEM3 | |
| cdc42C188S | 1,088.9 ± 139.8 | 3.6 ± 0.4 |
| cdc42D38E, C188S | 139.6 ± 5.7 | 4.1 ± 0.8 |
| cdc42G12V, C188S | 1,854.3 ± 209.4 | 1,006.6 ± 13.2 |
| cdc42G12V, D38E, C188S | 1,538.2 ± 73.9 | 124.8 ± 4.1 |
| cdc42D118A, C188S | 2.8 ± 4.3 | 1.0 ± 1.8 |
β-Galactosidase liquid assays were performed in triplicate. These data are averages and standard deviations of one triplicate assay for each interaction and are representative of three independent β-galactosidase liquid assays for each interaction. The units of measurement are Miller units.
cdc42Y32K, cdc42F37A, cdc42D38E, and cdc42Y40C are partially functional alleles.
To test the functionality of these mutant proteins, the cdc42Y32K, cdc42F37A, cdc42D38E, and cdc42Y40C alleles (driven by the CDC42 endogenous promoter) were tested for complementation of various cdc42 loss-of-function mutants (Table 4). Low-copy plasmids containing these mutant alleles were transformed into cdc42-1 (DJTD2-16A) and cdc42W97R (PMYD9-1B) temperature-sensitive (ts) strains and tested for complementation at 37°C. None of the transformants showed any abnormal morphological phenotypes except the large, round, unbudded cells of these ts strains typically seen at restrictive temperatures. All four alleles could at least partially complement the cdc42-1 strains, suggesting that these alleles were partially functional. However, cdc42Y32K, cdc42F37A, and cdc42Y40C were not able to complement cdc42W97R at 37°C or the Δcdc42 null mutant (see Materials and Methods), indicating that these alleles could not function as the sole cellular copy of CDC42. The inability of these mutants to complement the Δcdc42 mutant provided a strong correlation between loss of Cdc42p function and the inability of these mutants to interact with multiple effectors and regulators, as seen in the two-hybrid screen. In contrast, cdc42W97R strains containing cdc42D38E showed growth at 37°C and strains containing cdc42D38E in a Δcdc42 background were viable, indicating that this allele could function as the sole cellular copy of CDC42. The functionality of this allele was not completely surprising in that the D38E mutation diminished interactions with only Cla4p and Bem3p, as opposed to multiple effectors and regulators, as seen with the three other mutant proteins. These results also showed that complementation of cdc42W97R at 37°C more closely mimics complementation of the Δcdc42 null mutant, reinforcing the observation that this allele was a better ts allele than cdc42-1 for determination of functionality (26).
TABLE 4.
Functionality of effector domain mutant allelesa
| Mutation | Growth
|
||
|---|---|---|---|
| cdc42-1ts, 37°C | cdc42W97R, 37°C | Δcdc42, 23°C | |
| None (vector) | − | − | − |
| CDC42 | + | + | + |
| Y32K | ± | − | − |
| F37A | ± | − | − |
| D38E | + | ± | + |
| Y40C | + | − | − |
| V44A | + | + | + |
See Materials and Methods for a description of the complementation of the Δcdc42 mutant. pRS315(CDC42), pRS315(cdc42Y32K), pRS315(cdc42F37A), pRS315(cdc42D38E), pRS315(cdc42Y40C), and pRS315(cdc42V44A) were transformed into strains DJTD2-16A (cdc42-1 ts) and PMYD9-1B (cdc42W97R ts) and selected on SC-Leu medium. Transformants were then streaked on SC-Leu medium at 23 and 37°C to test for viability at restrictive temperatures. −, no growth; ±, partial growth; +, normal growth.
cdc42D38E mutant cells displayed a temperature-dependent morphological defect.
Since cdc42D38E could function as the sole copy of CDC42, this mutant was further characterized for morphological defects. cdc42D38E mutant strain TRY38-2B was viable at 16, 23, 30, and 37°C; however, cell morphology changed as the temperature increased. During mid-log phase at 16 or 23°C, ∼41% of the budded cell population showed an abnormal phenotype, with ∼14% having elongated buds and ∼27% having more than one bud (one of which may be elongated) and an overall cell viability of ∼90%. At 30°C, the severity of these abnormalities increased such that ∼85% of the budded cell population showed an abnormal phenotype, with ∼38% having only an elongated bud and ∼47% showing the multibudded phenotype (Fig. 2A) and an overall cell viability of ∼80%. In addition to the presence of one or more buds that were either normal or elongated, a number of buds emerging from the same cell were small and seemingly underdeveloped, suggesting that these buds were not enlarging. At 37°C, the morphology changed again such that in addition to the elongated-bud and multibudded cells seen at 30°C, mother cells, in general, became larger and rounder and ∼24% of the cells were large, round, and unbudded (data not shown), indicating a defect in bud emergence in these cells. These results suggested that the cdc42D38E morphological defects increased in severity with temperature.
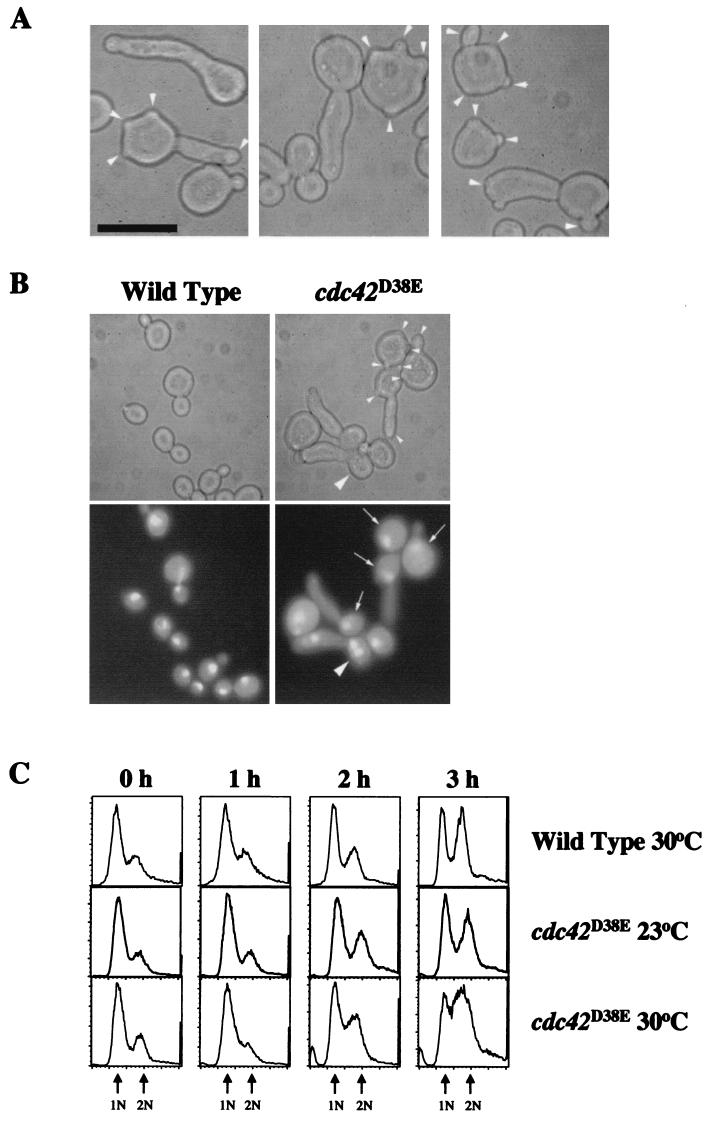
FIG. 2.
Morphological characterization of the cdc42D38E mutant. (A) Morphology of cdc42D38E strain TRY38-2B at 30°C. Arrowheads indicate buds on multibudded cells. (B) Morphology and DNA contents of cdc42D38E mutant strain TRY38-2B and wild-type C276-4A cells at 30°C shown by phase-contrast optics (top) and DAPI staining (bottom). Small arrowheads indicate buds on multibudded cells. Arrows indicate elongated-budded or multibudded cells containing single nuclei. Large arrowheads indicate multinucleate cells. Cells were grown in YEPD liquid medium to mid-log phase and sonicated briefly before observation. Bars, 10 μm. (C) Flow cytometric analysis of synchronized wild-type and cdc42D38E mutant cell populations released at 23 or 30°C over 3 h. Arrows point to peaks representing cells with a DNA content of 1 or 2 N based on propidium iodide fluorescence. The broadness of the cdc42D38E peaks at 30°C resulted from a population of multibudded cells with dimmer fluorescence than the standard fluorescence seen with cells with replicating DNA or 2-N DNA content.
At 30°C, the majority (∼82%) of the cdc42D38E budded cells had a single DAPI-stained nucleus and ∼16% had two nuclei while only ∼2% had multiple nuclei indicative of a minor cytokinesis defect in the cell population (Fig. 2B). Similar nuclear staining was seen with cells grown at 37°C. Furthermore, both single elongated-bud and multibudded cdc42D38E cells contained only one distinguishable nucleus, suggesting that there may be a nuclear division delay in these mutant cells. The presence of only a single nucleus in the multibudded cell population also suggested the possibility that cdc42D38E cells have a defect in DNA replication. To determine the DNA content of these mutant cells, flow cytometry was performed on propidium iodide-stained cdc42D38E cells synchronized in stationary phase at 23°C and shifted into fresh media at 30°C. Cells at the time of the shift were ∼85% unbudded, and flow cytometry confirmed that the majority of cells contained 1 N of DNA (Fig. 2C). Cells with small buds were apparent after ∼1 h, and within 2 h at 30°C, the multibudded phenotype was apparent with ∼57% of the budded population showing multiple buds (∼66% of the cell population was budding by T = 2 h). After 3 h at 30°C, the quantitative morphology was comparable to that of cells observed in mid-log phase, as the elongated-budded morphology became more prominent. Flow cytometry and cell sorting (data not shown) revealed that both multibudded and elongated-budded cells were entering S phase after 1 to 2 h at 30°C and eventually completed DNA replication, resulting in 2-N DNA content by 3 h at 30°C (Fig. 2C). These data indicated that cdc42D38E cells did not have a defect in DNA replication despite defects in budding. Furthermore, the multibudded phenotype appeared prior to the completion of DNA replication, suggesting that multiple buds were being formed within a single cell cycle and prior to the G2/M transition. Similar DNA content profiles were obtained with asynchronous cdc42D38E cells during mid-log phase (data not shown), suggesting that the majority of the cells also did not have >2-N DNA content despite the presence of multiple buds.
To determine if the multiple small buds observed were attached cytoplasmically, cdc42D38E cells were grown to mid-log phase at 30°C, fixed, and treated with the cell wall-digesting enzyme glusulase. The starting cell population contained ∼21% elongated-budded, ∼55% multibudded, and ∼13% unbudded cells pre-glusulase treatment. Glusulase treatment resulted in a significant decrease in elongated-budded cells (to ∼10%) and a small decrease in multibudded cells (to ∼47%) with a corresponding increase in unbudded cells (to ∼32%). These results suggested that ∼50% of the elongated-budded cells were defective for cell separation and ∼50% were defective for cytokinesis, while the majority of the multiple small buds were cytoplasmically connected to the mother cell. Taken together, these morphological and nuclear mutant phenotypes suggested that the cdc42D38E mutant had pleiotropic defects in cell cycle progression.
cdc42D38E mutant cells localized actin and septin rings to one bud per cell and triggered the Swe1p-dependent morphogenetic checkpoint.
Levels of Cdc42D38Ep were comparable to those of endogenous Cdc42p in wild-type strains, as was its fractionation pattern (Fig. 3A). In addition, GFP-Cdc42D38Ep localized to sites of polarized growth in wild-type cells (data not shown), suggesting that the mutant defects were not due to abnormal expression or mislocalization of Cdc42D38Ep. The elongated-budded phenotype of cdc42D38E cells and the reduced interactions with Cla4p suggested that this mutant could have a defect in the apical-isotropic switch and G2/M morphogenetic checkpoint similar to that seen with the cdc42V44A mutant (33). This was indeed shown to be the case, in that cortical actin was persistently polarized to the tips of elongated buds (Fig. 3B); septin protein localization, visualized by both GFP-Cdc3p (Fig. 3B) and GFP-Cdc12p (data not shown), was diffuse or delocalized to tips of elongated buds; and the Δswe1 mutation could suppress the elongated-budded phenotype (data not shown). However, the multibudded population showed variable phenotypes in which ∼44% had actin polarized or persistently polarized (if elongated) to one of the multiple buds (Fig. 3B) while ∼20% had actin polarized to more than one bud. The presence of cortical actin at more than one bud appeared to be only in cells with multiple elongated buds, suggesting that cytokinesis or cell separation had not occurred even though a new budding cycle had begun. In ∼6% of cdc42D38E mutant cells, actin was seen at the bud tip and the mother bud neck region simultaneously, suggesting that there could also be a loss of coordination of the retargeting of actin in these cells during the budding cycle. There was also an increase in cortical actin distribution in mother cells that may reflect an overall defect in proper localization of actin during budding. In addition, the septin ring appeared to localize to one bud per multibudded cell either normally (Fig. 3B) or diffusely if an elongated bud was present. Surprisingly, ∼23% of the cells had septin rings localized to more than one bud on the same cell (Fig. 3B). However, it was unclear whether these buds were nonenlarging buds (see below) with residual rings or actively growing buds. Chitin ring localization mirrored the septin ring localization pattern (data not shown).
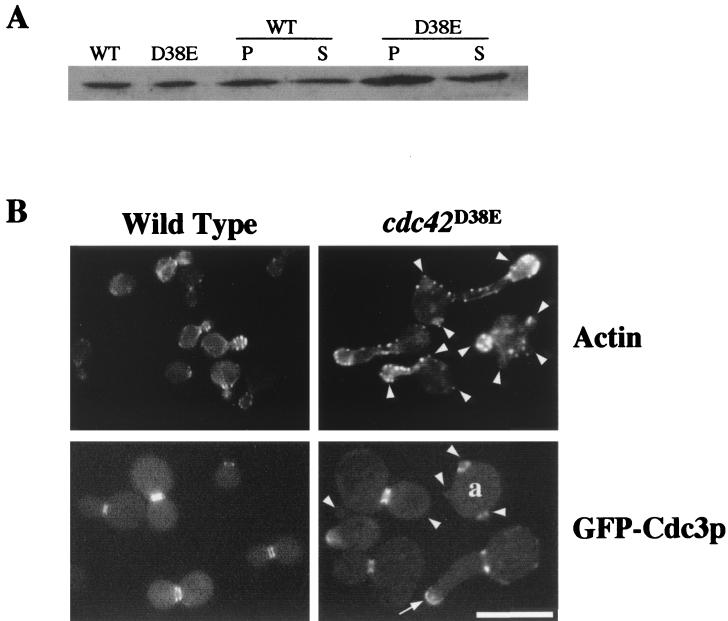
FIG. 3.
(A) Cdc42D38Ep protein levels. The first two lanes represent 150 μg of total protein from wild-type strain TRY11-7D (WT) and cdc42D38E mutant strain TRY38-2B (D38E), respectively. The next four lanes represent 10,000 × g pellets (P) and supernatants (S) from wild type and cdc42D38E mutant cells. (B) Actin and GFP-Cdc3p localization in cdc42D38E cells. TRY11-7D (wild-type) and TRY38-2B (cdc42D38E mutant) cells at 30°C were fixed and stained with the actin-fluorescent stain rhodamine-phalloidin using standard fixation and staining procedures (top). To observe GFP-Cdc3p localization in cdc42D38E cells, pRS315(GFP-CDC3) was transformed into TRY11-7D (wild-type) and TRY38-2B (cdc42D38E mutant) cells and transformants were selected on SC-Leu plates and grown in liquid medium at 30°C for observation (bottom). Arrowheads indicate where buds are located on multibudded cells. The arrow indicates diffuse and mislocalized GFP-Cdc3p. Cell a is a multibudded cell with septins localized to two mother bud neck regions. All cells were sonicated briefly before observation. The images shown are collages from the same cell culture assembled in Adobe Photoshop 5.0. Bar, 10 μM.
Interestingly, the multibudded phenotype did persist in the cdc42D38E Δswe1 double mutant, making up ∼52% of the population. DAPI staining of the double mutant showed that ∼43% of the cell population now had two or more nuclei, compared to ∼18% of cdc42D38E mutant cells. These results suggested that the D38E mutation, through reduced interactions with Cla4p, affected the G2/M morphogenetic checkpoint but that the presence of multibudded cells was independent of Swe1p.
cdc42D38E multibudded cells budded consecutively.
To determine whether buds were emerging from multibudded cells either consecutively or concurrently, haploid cdc42D38E cells were synchronized in stationary phase at 23°C and released at 30°C and cell division was observed and recorded for 7 h. Two representative cells from these time-lapse experiments are shown in Fig. 4 and are representative of typical cdc42D38E multibudded cells. One cell was observed to have one bud enlarge to a medium size before growth stopped prematurely and indefinitely (Fig. 4A; bud 1 grew for ∼14 min, from time 12:04 to 12:18). After ∼48 min (time 13:06), a second bud (no. 2) began to emerge from a site that was not axial to the first bud (Fig. 4A), suggesting that selection of this second bud site was random and that bud emergence occurred after bud 1 stopped growing. Bud 2 enlarged normally within the first 20 min of growth (Fig. 4A, time 13:19) but then began to elongate for ∼2 h (Fig. 4A, time 13:41 to 15:15), suggesting an ∼2-h delay in the apical-isotropic switch. After ∼2 h of apical growth, the tip of the elongated bud began to enlarge (Fig. 4A, time 15:25 to 16:10). Finally, this bud, as well as the original mother cell, began another cycle of normal budding (in Fig. 4A, the number 3 indicates a third bud emerging from the original mother during time 17:00 to 17:15). The third bud emerging from the mother cell was also not axially positioned. In addition, the first emerging bud never changed in size or shape over the entire time course from when the second bud started to emerge, suggesting that bud growth had ceased and was never reactivated to continue growth at this site.
FIG. 4.
Buds of multibudded cdc42D38E mutant cells emerge in a consecutive manner. cdc42D38E (TRY38-2B) cells were grown to stationary phase at 23°C and sonicated, and an aliquot of cells was layered onto a microscope slide thinly layered with a 1% YEPD agarose slab. The slide was then placed on a microscope stage heated to 30°C, and cell division was monitored and recorded for 7 h. (A) Representative cell in which bud growth ceased after a medium bud size was reached. (B) Representative cell that ceased bud growth after the formation of small buds. Cells are representative of a field of cdc42D38E cells within which at least nine multibudded cells were observed to bud consecutively. The white numbers show the order in which buds emerged from the mother cell. The hour and minute at which each image was captured is shown.
Figure 4B shows a cell with multiple small buds. The first bud (no. 1) was observed at time 12:08, a second bud (no. 2) was observed at a random site by time 12:23, and a third bud (no. 3) had appeared by time 13:29, again at a nonaxial site (Fig. 4B). The size of these three small buds was never observed to increase over the entire course of the time-lapse experiments after they first were seen to have appeared, suggesting that bud growth had ceased. A fourth bud (no. 4) was seen emerging from a random site ∼1 h after the third bud was observed and continued to elongate for >1 h before the tip began enlarging. Taken together, these time-course experiments indicated that multiple buds emerged sequentially at random sites from cdc42D38E mother cells and grew to various sizes before bud enlargement ceased.
To determine whether the random bud site selection defect was specific to multibudded cdc42D38E cells, bud scars were visualized with the chitin stain calcofluor and bud scar patterns were quantitated in both wild-type and cdc42D38E budded cells at 30°C. In wild-type cells, ∼96% of the normal-budded cells had an axial pattern of budding. In contrast, ∼17% of the cdc42D38E multibudded cells showed an axial budding pattern although the normal and single-elongated-budded cells more often showed an axially budded pattern (∼68 and ∼78%, respectively). Therefore, cdc42D38E cells have a bud site selection defect that is predominantly in the multibudded cell population.
Cla4p-GFP and GFP-Cdc24p localized to one bud per cell in cdc42D38E cells.
Characterization of the multibudded cdc42D38E phenotype suggested that growth was redirected to a new bud site after a previous bud ceased enlarging. To determine where proteins involved in regulation of the budding pathway were directed in multibudded cells, localization of Cla4p-GFP and GFP-Cdc24p was observed in cdc42D38E cells grown at 30°C. Cla4p-GFP localized to prebud sites and bud tips in wild-type cells (Fig. 5A), as previously described (14). In cdc42D38E cells, Cla4p-GFP partially suppressed the elongated phenotype but not the multibudded phenotype, which was also found to be the case with Cla4p alone expressed from a plasmid (see below). In the cells that were multibudded, Cla4p-GFP appeared to localize properly and to only one bud at a time (Fig. 5A), suggesting that Cla4p localization was normal and directed to one bud per cell. GFP-Cdc24p, expressed from the methionine-repressible promoter, localized similarly to Cla4p-GFP to prebud sites, to the tips and sides of growing buds, and to the mother bud neck region in wild-type cells (Fig. 5B), as previously described (38). Expression of GFP-Cdc24p did not affect the morphology of cdc42D38E cells, and it localized to only one bud on multibudded cells (Fig. 5B), suggesting that, like that of Cla4p, Cdc24p localization was normal and directed to one bud per cell. Altogether, the localization patterns of Cla4p-GFP and GFP-Cdc24p suggested that Cdc42D38Ep did not affect Cla4p or Cdc24p localization and supported the consecutive formation of multiple buds in that the bud emergence regulatory proteins were directed to a single bud per cell. Finally, in elongated-budded cells, both Cla4p-GFP and GFP-Cdc24p were persistently localized to bud tips, similar to the predominant actin localization pattern, suggesting that Cla4p and Cdc24p regulated, or their localization was dependent on, the apical-isotropic switch.
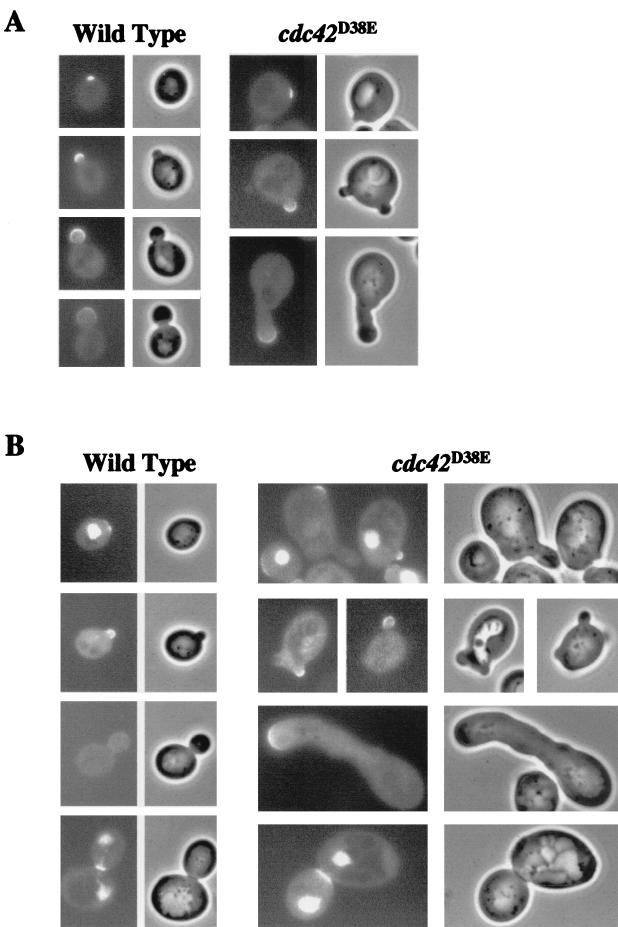
FIG. 5.
Cla4p-GFP and GFP-Cdc24p localization in cdc42D38E mutant cells. (A) pBRS115(CLA4-GFP) was transformed into TRY13-5A (wild-type) and TRY38-2B (cdc42D38E) cells, and transformants were selected on SC-His plates, grown at 30°C to mid-log phase in SC-His liquid medium, sonicated, and observed. (B) p415MET(GFP-CDC24) was transformed into TRY13-5A and TRY38-2B, and transformants were selected on SC-Leu plates, grown at 30°C to mid-log phase in SC-Leu liquid medium, sonicated, and observed.
The cdc42D38E morphology was partially suppressed by overexpression of Cla4p and altered by Δbem3 and Δcla4.
Cla4p and Bem3p had altered interactions with Cdc42D38Ep, suggesting that the cdc42D38E mutant morphologies could be a result of these altered interactions. To determine if overexpression of either Cla4p or Bem3p could suppress cdc42D38E-encoded phenotypes at 30°C, high-copy plasmids pRS425, YEp351(CDC42), YEp351(CLA4), and YEp13(BEM3) were transformed into cdc42D38E mutant strain TRY38-2B. Wild-type CDC42 complemented cdc42D38E, with ∼96% of the budded population showing a normal budded phenotype. Overexpression of Cla4p partially suppressed the cdc42D38E morphology with ∼66% of the budded population showing a normal budded phenotype, as was seen with Cla4p-GFP. In this cell population, there was a significant decrease in the number of cells solely exhibiting elongated buds (∼31% seen in the pRS425 vector alone versus ∼8% when Cla4p was overexpressed). However, the multibudded phenotype was still prevalent with ∼30% of the budded population being multibudded (a decrease from the ∼48% seen in the vector-only cells). Taken together, these results suggested that overexpression of Cla4p suppressed the elongated-budded phenotype of cdc42D38E but only slightly affected the multibudded phenotype. Overexpression of Bem3p in cdc42D38E cells resulted in a small increase in the number of large, round, unbudded cells (∼6% versus 0% in cdc42D38E alone), suggesting that overexpression of Bem3p slightly exacerbates the cdc42D38E phenotype. Co-overexpression of Cla4p and Bem3p, using plasmids pAD11(CLA4) and YEp13(BEM3), resulted in a decrease in both elongated-budded and multibudded cells that was similar to that observed with overexpression of Cla4p alone. However, there was a significant increase in the number of cells exhibiting the large, round, unbudded phenotype (∼17% of the population) associated with overexpression of Bem3p.
Δcla4 cdc42D38E and Δbem3 cdc42D38E double mutants were both viable, suggesting that the cdc42D38E cells did not require Cla4p or Bem3p for viability. However, the Δcla4 cdc42D38E mutant showed an exacerbated elongated-budded phenotype at 23°C, compared to the cdc42D38E mutant, with ∼46% of the budded population showing an elongated or highly branched morphology. At 30 and 37°C, highly branched cells were also apparent in the double-mutant population, although the prevalence of elongated-budded cells was comparable to that in the Δcla4 single-mutant cells. These results indicated that loss of Cla4p exacerbated cdc42D38E defects in the apical-isotropic switch and suggested that Cla4p, in conjunction with Cdc42p, is required for proper regulation of the apical-isotropic switch in this mutant strain.
The morphology of the Δbem3 cdc42D38E mutant at 30°C was also different from that of the cdc42D38E mutant. The total number of multibudded cells in the population was similar to that seen in the cdc42D38E population; but the total number of cells having elongated buds, with and without multiple buds, was decreased in the double mutant (∼70% in cdc42D38E cells versus ∼27% in cdc42D38E Δbem3 cells). However, there was no significant difference in the total number of buds per cell, in which ∼49% of cdc42D38E multibudded cells had more than four buds and ∼55% of Δbem3 cdc42D38E cells had more than four buds. These results suggested that loss of Bem3p in the cdc42D38E background either caused some cells not to progress through the cell cycle to the checkpoint or caused a partial bypass of the Swe1p-dependent checkpoint. These results reinforced the likelihood that the altered interaction between Cdc42D38Ep and Bem3p contributes to the mutant morphology of cdc42D38E cells. If the defects of the cdc42D38E mutant were simply related to the diminishment of interaction with Cla4p and Bem3p, then one would predict that a Δcla4 Δbem3 double mutant would phenocopy a cdc42D38E mutant. A Δcla4 Δbem3 double mutant displayed a more highly branched, elongated-budded phenotype at 30°C and was synthetically lethal at 37°C. However, there was no increase in small-budded cells as in the cdc42D38E mutant, suggesting that this phenotype is not due solely to diminished interactions with Bem3p and Cla4p.
DISCUSSION
Bud emergence normally occurs only once during the mitotic cell cycle, poststart, at a nonrandom location on the cell periphery. Within a cdc42D38E cell grown at a restrictive temperature, bud emergence was initiated but then bud enlargement ceased during early-to-mid stages of bud growth and prior to the apical-isotropic switch. After cessation of bud growth, another bud site was chosen randomly, as opposed to in an axial or bipolar pattern of selection, and the bud emergence phase began again, which resulted in either another nonenlarging bud or a normal or elongated-budded daughter cell. The time-lapse experiments, as well as the glusulase experiment, also indicated that the nonenlarging buds remained attached to the mother cell but never enlarged after growth ceased. The presence of a single nucleus in a large percentage of cdc42D38E multibudded cells also suggested that multiple buds were emerging within a single mitotic cell cycle. The analysis of DNA content in synchronous and asynchronous cdc42D38E cells indicated that cells began DNA replication, that the multibudded phenotype arose before the completion of a round of DNA replication, and that the majority of multibudded cells did not have >2-N DNA content. These results suggested that multiple buds were emerging between G1 and G2/M and that bud emergence was taking place more than once during a single DNA replication cycle. Taken together, the analysis of this mutant phenotype uncovered roles of Cdc42p specifically in maintenance of bud growth and in prevention of bud emergence more than once per cell cycle.
The time-lapse observations, taken together with the actin, Cla4p-GFP, GFP-Cdc42p, and septin ring localization mainly to one bud per multibudded cell, suggested that the multiple buds emerged consecutively and involved retargeting of cytoskeletal structures and regulatory proteins within a single cell cycle. Septin rings at multiple mother bud neck regions in a small percentage of cells may represent residual rings that had not disassembled at the previous neck regions. If this were the case, then disassembly of the septin ring was most likely not responsible for the cessation of bud growth.
One mechanism that may underlie this multibudded phenotype is mutant Cdc42D38Ep affecting a number of protein-protein interactions, directly or indirectly, such that as a bud emerges, essential protein complex dynamics or stability is altered and the bud ceases enlarging due to dysfunctional protein interactions. Evidence that supports this hypothesis is that Cdc42D38Ep showed altered interactions with at least two known Cdc42p-interacting proteins, Cla4p and Bem3p. Secondly, overexpression of Cla4p reduced the penetrance of the multibudded phenotype and overexpression of Bem3p caused slight exacerbation of the cdc42D38E phenotype. Furthermore, Δcla4 exacerbated the cdc42D38E elongated-bud morphology and Δbem3 led to a significant decrease in elongated buds, suggesting that loss of these proteins partially affected cdc42D38E cell morphology. Altered Cla4p interactions, which are important for the localization and structure of the septin ring, as well as regulation of the apical-isotropic switch, contributed to the elongation of the buds. The reduced interactions with the Bem3p GAP suggested that perhaps cdc42D38E defects were related to the GTP-bound state of Cdc42p. It is possible that the reduced interactions with Bem3p resulted in increased levels of GTP-bound Cdc42p at particular steps of the cell cycle. This is also consistent with the observation that the G12V mutation can reverse the diminished interaction seen with Cla4p. If this were the case, the protein-protein dynamics between Cdc42p and its effectors and regulators would be altered. The multibudded phenotype associated with constitutively GTP-bound Cdc42G12Vp (40) also supports a model in which an increase in GTP-bound Cdc42p would lead to a multibudded phenotype.
The analysis of the cdc42Y32K, cdc42F37A, cdc42D38E, and cdc42Y40C mutant alleles further emphasized the differential effects effector domain mutations have on Cdc42p interactions and function. Both Cdc42V44Ap and Cdc42D38Ep retained interactions with at least two PAK kinases, Bni1p, Iqg1p, and Rga1p, and both proteins were sufficiently functional to act as the sole copy of CDC42. Cdc42Y32Kp, Cdc42F37Ap, and Cdc42Y40Cp, however, showed altered interactions with at least two of the PAK kinases, Gic1p, Gic2p, and Bem3p, and none of these mutant alleles could function as the sole cellular copy of CDC42, suggesting that collectively maintaining interactions with the PAKs, Gic1p, Gic2p, and Bem3p was required for Cdc42p function. Interestingly, no effector mutations studied to date affect Cdc42p interactions with Bni1p or Rga1p, suggesting that different amino acids or another domain of Cdc42p is required for Bni1p and Rga1p specificity.
Incorporation of the Y32K, F37A, D38E, and Y40C mutations into mammalian Cdc42p has also uncovered differential interactions with various effectors and regulators. The Y32K mutation in mammalian Cdc42p interfered with binding to human Cdc42-GAP (22), S. cerevisiae Cdc24p (24), human Cdc42p actin-binding effector Wiscott-Aldrich syndrome protein, and IQGAP but not with the mPAK Cdc42p/Rac interactive binding domain (22, 23). In S. cerevisiae, the Y32K mutation interfered with interactions with most effectors and regulators, except Ste20p (which shows the most homology to mPAK), Bni1p, and Rga1p, suggesting that the Y32K mutation affects Cdc42p specificity similarly in yeast and mammalian cells. The Y40C mutation also interfered similarly with interactions between Cdc42p and the PAK kinases (19). The F37A mutation interfered with Cdc42p interactions with all effectors except Bni1p, and the Iqg1, Cla4p, and Skm1p interaction profiles seen with the incorporation of the G12V mutation were consistent with how the F37A mutation affected mammalian Cdc42p binding to IQGAP but not mPAK (23). Finally, the D38E mutation effects on interactions with Cla4p and Iqg1p were similar to results obtained with mammalian Cdc42D38Ep (23), although mammalian Cdc42D38Ep did not affect interactions with Cdc42-GAP (21). Overall, these results suggested that the amino acid specificity within the effector domain at these specific residues was highly conserved between the S. cerevisiae and mammalian Cdc42p proteins.
The effector domain mutations studied herein were relatively conserved amino acid changes but led to dramatic effects in effector and regulator specificity, as well as Cdc42p cellular function. This observation suggested either that the amino acid change altered the structure of Cdc42p such that protein interactions and/or Cdc42p function was lost or that the change in amino acid side chains affected specific protein-protein contact points required for proper interactions. Interestingly, the cdc42Y32K mutant was found to be conditionally viable in a different S. cerevisiae strain background (18), suggesting that the genetic background could affect the function of these mutant proteins. Furthermore, mutation of Asp38 to alanine instead of glutamate in S. cerevisiae Cdc42p led to a nonfunctional protein (18), suggesting that amino acid specificity and the charged nature of the amino acid at position 38 are important for Cdc42p function. Asp38 in the human Ras protein was shown to be critical for hydrogen bond contacts with the binding domain of the Ras effector Raf kinase (28), suggesting that substitutions at position 38 may sterically affect effector and regulator interactions. The altered amino acids were mapped onto the Cdc42p crystal structure to determine the positioning of the amino acid side chains (Fig. 1B). The Tyr32, Phe37, Asp38, and Tyr40 side chains extended outward from the protein, suggesting that all of these residues would be accessible for protein-protein interactions and that even slight changes in the side chain could affect the dynamics of Cdc42p interactions. Interestingly, the Val44 side chain was buried within the protein and only partially exposed on the surface, suggesting that an amino acid change at this position may lead to conformation changes within the entire protein and/or effector domain that affect interactions, as opposed to the residue itself specifically altering interactions.
Previously, it was shown that Cdc42p and its functional interactions are required for bud emergence (1, 9) and for timely cell cycle progression through the G2/M transition (33). Herein, Cdc42p was shown to be required for maintenance of bud growth and prevention of the initiation of multiple buds during the cell cycle. Further study of the cdc42D38E mutant should be useful in uncovering how proteins are targeted to the bud site, how bud growth is monitored during the cell cycle, and how Cdc42p itself is regulated during the cell cycle.
ACKNOWLEDGMENTS
We thank Malcolm Whiteway for sharing valuable reagents and Collette Charland for performing flow cytometry. We also thank members of the Johnson lab for valuable discussions and critical comments on the manuscript.
This work was supported by NSF grant MCB-9728218 and by National Institutes of Health Cancer Biology Training Grant T32-CAO9286-19 (T.J.R.).
REFERENCES
- 1.Adams A E M, Johnson D I, Longnecker R M, Sloat B F, Pringle J R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton B K, Tinkelenberg A, Gonzalez I, Cross F R. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol Cell Biol. 1997;17:5067–5076. doi: 10.1128/mcb.17.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown J L, Jaquenoud M, Gulli M-P, Chant J, Peter M. Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 1997;11:2972–2982. doi: 10.1101/gad.11.22.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G-C, Kim Y-J, Chan C S M. The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 1997;11:2958–2971. doi: 10.1101/gad.11.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien C-T, Bartel P L, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 8.Cvrcková F, De Virgilio C, Manser E, Pringle J R, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- 9.Davis C R, Richman T J, Deliduka S B, Blaisdell J O, Collins C C, Johnson D I. Analysis of the mechanisms of action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J Biol Chem. 1998;273:849–858. doi: 10.1074/jbc.273.2.849. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista M, Blundell K, Longtine M S, Chow C J, Adames N, Pringle J R, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 11.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 12.Haase S B, Lew D J. Cytometric analysis of DNA content in budding yeast. Methods Enzymol. 1997;283:322–332. doi: 10.1016/s0076-6879(97)83026-1. [DOI] [PubMed] [Google Scholar]
- 13.Hirschman J E, DeZutter G S, Simonds W F, Jenness D D. The G βγ complex of the yeast pheromone response pathway: subcellular fractionation and protein-protein interactions. J Biol Chem. 1997;272:240–248. doi: 10.1074/jbc.272.1.240. [DOI] [PubMed] [Google Scholar]
- 14.Holly S P, Blumer K J. PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J Cell Biol. 1999;147:845–856. doi: 10.1083/jcb.147.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaquenoud M, Gulli M P, Peter K, Peter M. The Cdc42p effector Gic2p is targeted for ubiquitin-dependent degradation by the SCFGrr1 complex. EMBO J. 1998;17:5360–5373. doi: 10.1093/emboj/17.18.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D I. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D I, Pringle J R. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozminski K G, Chen A J, Rodal A A, Drubin D G. Functions and functional domains of the GTPase Cdc42p. Mol Biol Cell. 2000;11:339–354. doi: 10.1091/mbc.11.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65(PAK) and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 20.Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall J E, Thomas D Y. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 1997;16:83–97. doi: 10.1093/emboj/16.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard D A, Lin R, Cerione R A, Manor D. Biochemical studies of the mechanism of action of the Cdc42-GTPase-activating protein. J Biol Chem. 1998;273:16210–16215. doi: 10.1074/jbc.273.26.16210. [DOI] [PubMed] [Google Scholar]
- 22.Leonard D A, Satoskar R S, Wu W J, Bagrodia S, Cerione R A, Manor D. Use of a fluorescence spectroscopic readout to characterize the interactions of Cdc42Hs with its target/effector, mPAK-3. Biochemistry. 1997;36:1173–1180. doi: 10.1021/bi9622837. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Debreceni B, Jia B, Gao Y, Tigyi G, Zheng Y. Localization of the PAK1-, WASP-, and IQGAP1-specifying regions of Cdc42. J Biol Chem. 1999;274:29648–29654. doi: 10.1074/jbc.274.42.29648. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Zheng Y. Residues of the Rho family GTPases Rho and Cdc42 that specify sensitivity to Dbl-like guanine nucleotide exchange factors. J Biol Chem. 1997;272:4671–4679. doi: 10.1074/jbc.272.8.4671. [DOI] [PubMed] [Google Scholar]
- 25.Martín H, Mendoza A, Rodríguez-Pachón J M, Molina M, Nombela C. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol Microbiol. 1997;23:431–444. doi: 10.1046/j.1365-2958.1997.d01-1870.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller P J, Johnson D I. Characterization of the S. cerevisiae cdc42–1ts allele and new temperature-conditional-lethal cdc42 alleles. Yeast. 1997;13:561–572. doi: 10.1002/(SICI)1097-0061(199705)13:6<561::AID-YEA114>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Nassar N, Hoffman G R, Manor D, Clardy J C, Cerione R A. Structures of Cdc42 bound to the active and catalytically compromised forms of Cdc42GAP. Nat Struct Biol. 1998;5:1047–1052. doi: 10.1038/4156. [DOI] [PubMed] [Google Scholar]
- 28.Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A. The 22 Å crystal structure of the Ras-binding domain of the serine-threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature. 1995;375:554–560. doi: 10.1038/375554a0. [DOI] [PubMed] [Google Scholar]
- 29.Osman M A, Cerione R A. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates Cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peter M, Neiman A M, Park H-O, van Lohuizen M, Herskowitz I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 1996;15:7046–7059. [PMC free article] [PubMed] [Google Scholar]
- 31.Pringle J R, Preston R A, Adams A E M, Stearns T, Drubin D G, Haarer B K, Jones E W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds A, Lundblad V. Assay for β-galactosidase in liquid cultures. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1989. pp. 13.6.2–13.6.4. [Google Scholar]
- 33.Richman T J, Sawyer M M, Johnson D I. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J Biol Chem. 1999;274:16861–16870. doi: 10.1074/jbc.274.24.16861. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 36.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson B J, Ferguson B, De Virgilio C, Bi E, Pringle J R, Ammerer G, Sprague G F. Mutation of RGA1, which encodes a putative GTPase-activating protein for the polarity-establishment protein Cdc42p, activates the pheromone-response pathway in the yeast Saccharomyces cerevisiae. Genes Dev. 1995;9:2949–2963. doi: 10.1101/gad.9.23.2949. [DOI] [PubMed] [Google Scholar]
- 38.Toenjes K A, Sawyer M M, Johnson D I. The guanine-nucleotide-exchange factor Cdc24p is targeted to the nucleus and polarized growth sites. Curr Biol. 1999;9:1183–1186. doi: 10.1016/S0960-9822(00)80022-6. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson L E, Pringle J R. Transient G1 arrest of S. cerevisiae cells of mating type α by a factor produced by cells of mating type α. Exp Cell Res. 1974;89:175–187. doi: 10.1016/0014-4827(74)90200-6. [DOI] [PubMed] [Google Scholar]
- 40.Ziman M, O'Brien J M, Ouellette L A, Church W R, Johnson D I. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol Cell Biol. 1991;11:3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziman M, Preuss D, Mulholland J, O'Brien J M, Botstein D, Johnson D I. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]