Abstract
Introduction: Currently, faecal calprotectin (FC) is the predominate faecal biomarker utilised in clinical practice to monitor Crohn’s disease (CD) activity. However, there are several potential faecal biomarkers described in the literature. We performed a meta-analysis to determine the accuracy of faecal biomarkers in discriminating endoscopic activity and mucosal healing in CD. Methods: We searched the medical literature using MEDLINE, EMBASE, and PubMed from 1978 to 8 August 2022. Descriptive statistics, including sensitivity, specificity of the primary studies, their positive and negative likelihood ratios, and their diagnostic odds ratio (DOR), were calculated. The methodological quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS) criteria. Results: The search found 2382 studies, of which 33 were included for analysis after screening. FC was found to have a pooled sensitivity and specificity, DOR, and negative predictive value (NPV) in discriminating active endoscopic disease (versus inactive) of 81%, 74%, 13.93, and 0.27, respectively. Faecal lactoferrin (FL) had a pooled sensitivity and specificity, DOR, and NPV in discriminating active endoscopic disease of 75%, 80%, 13.41, and 0.34, respectively. FC demonstrated a pooled sensitivity and specificity, DOR, and NPV of 88%, 72%, 18.17, and 0.19 in predicting mucosal healing. Conclusion: FC remains an accurate faecal biomarker. Further evaluation of the utility of novel faecal biomarkers is needed.
Keywords: Crohns’ Disease, biomarkers, endoscopy, accuracy
1. Introduction
Crohn’s disease (CD) is a chronic immune-inflammatory condition with a growing incidence in developed countries [1,2,3]. Whilst inflammatory changes including ulceration can manifest throughout the GIT, the most commonly affected luminal regions include the small bowel in 70% of patients and CD limited to the colon in 20% [4]. Left untreated, the inflammatory burden of CD can result in complications including stenosis formation and subsequent intestinal obstruction, fistula formation, as well as increased risk of infective complications such as intra-abdominal abscess and development of colorectal cancer [5,6]. Thus, current therapy for CD is geared toward an overall reduction in inflammatory burden and prevention of complications associated with longstanding disease [7,8,9,10]. Current treatment targets in CD include endoscopic remission assessed via the simplified endoscopic score for CD (SES-CD) and/or the CD endoscopic index of severity (CDEIS) and/or radiological targets such as transmural healing which is best evaluated via magnetic resonance enterography (MRE) and/or intestinal ultrasound (IUS) [11,12]).
Current STRIDE-2 guidelines recommend serial, objective assessment of disease activity at 6–12 monthly intervals [13,14]. Whilst ileocolonoscopic assessment may be desirable, multiple limitations preclude regular and repeated endoscopic assessment, including cost and resource constraints, the need for bowel preparation and sedation anaesthesia, plus procedural risks including bowel perforation and bleeding. Thus, there is a need for cheap, rapid, objective, and patient-friendly alternatives for disease assessment. Faecal biomarkers are attractive in this setting, and their capacity for discriminating endoscopic activity in CD has been studied extensively, thus promoting their utility in serial monitoring of CD activity. Currently, faecal calprotectin (FC) is the most commonly used faecal biomarker in CD. Calprotectin is a calcium- and zinc-binding protein of the s-100 protein family released predominantly by neutrophils that migrate to the small and large intestines during periods of active bowel inflammation [15,16]. The concentration of FC has been shown to correlate with active neutrophilic inflammation within the bowel, although it is not specific to CD or inflammatory bowel disease (IBD) [15,16]. Furthermore, a number of other faecal biomarkers of promising utility for discriminating endoscopic CD activity have been described in the literature with the potential to be incorporated more widely into routine clinical practice, including faecal lactoferrin (FL), faecal immunochemical test (FIT), neopterin, metalloprotease-9 (MMP-9), myeloperoxidase, faecal lipocalin-2 (FCLN-2), chitinase 3-like 1 (CHI3L1), polymorphonuclear neutrophil elastase (PMN-e), microRNA, S100A12, and alpha-1 antitrypsin. Therefore, this meta-analysis was conducted to assess and reaffirm the accuracy of FC and examine other studied faecal biomarkers in discriminating endoscopic activity in CD.
2. Materials and Methods
2.1. Literature Search
We searched the medical literature using MEDLINE (via Ovid), EMBASE, and PubMed from 1 January 1978 to 8 August 2022 inclusive. To identify published abstracts, we hand searched conference proceedings from the United European Gastroenterology Week, European Crohn’s and Colitis Organisation, British Society of Gastroenterology, and Digestive Diseases Week from September 2016 to August 2022 inclusive.
We searched the medical literature using the terms in the appendix using both medical subject headings [MeSH] and free-text terms. Only English language manuscripts were reviewed. We hand searched references from eligible studies and reviews for any further studies to be included. This process adhered to a standard, prespecified study protocol which was registered on the International Prospective Register of Systematic Reviews(PROSPERO) and given the study identification number: CRD42022354526 [17].
2.2. Study Selection
The search was uploaded to Covidence, a webtool for systematic reviews, where eligibility was assessed by two independent reviewers (A.B and G.M.). Articles were first screened independently (A.B. and G.M.) on the basis of title and abstract. A subsequent full-text review was performed by the same reviewers to determine inclusion. All disagreements occurring at the title, abstract, and/or full-text review went to a third reviewer (J.P.S.) for a consensus.
A study was included if it met one or more of the following inclusion criteria: (1) the study evaluated an FC, FL, FIT, neopterin, MMP-9, myeloperoxidase, FCLN-2, CHI3L1, PMN-e, microRNA, S100A12, or alpha-1 antitrypsin for discriminating endoscopically active versus inactive Crohn’s disease in adults (age > 18 years); (2) an endoscopic scoring system or endoscopic description of activity was documented as a reference standard to assess inflammatory activity; (3) the study provided sufficient details to calculate true-positive (TN), false-positive (FP), false-negative (FN), and true-negative (TN) results to (re)construct a two-by-two table.
2.3. Data Extraction and Quality Assessment
Two reviewers (A.B. and G.M.) independently retrieved specific data from each full-text article using a standard data extraction form, which included the author, year of publication, nation, method of endoscopic examination (ileocolonoscopy versus balloon assisted enteroscopy), type of faecal biomarker, and endoscopic cut-off values used for each biomarker to discriminate active versus inactive endoscopic CD. The published values for TP, FP, TN, and FN were identified and used to construct a 2 × 2 contingency table. Disagreements between the two reviewers were resolved by consensus. If consensus between the two reviewers could not be reached, a third investigator (J.P.S.) was referred for consensus.
The methodological quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS) criteria [18].
2.4. Statistical Analysis
Descriptive statistics were calculated based on the sensitivity, specificity, and false-positive rate of the primary studies, their positive, and negative likelihood ratios (LR+, LR−) and their diagnostic odds ratios (DOR). χ2 tests were performed to assess the heterogeneity of sensitivities and specificities. Crosshair plots were performed, and summary receiver operating characteristic (sROC) curves were produced to graphically demonstrate sensitivity and specificity.
Univariate diagnostic odds ratios and negative likelihood ratios were calculated using a fixed effects model and the Mantel–Haensz method with the i2 statistic applied to assess heterogeneity. A bivariate model was used to generate pooled sensitivity and specificities. However, as illustrated by Glas et al., these paired indicators are not valid for comparing multiple diagnostic tests, especially if one test was not superior to the other across both indicators [19]. In addition, these parameters cannot be analysed in the traditional framework of network meta-analysis or indirect comparison models. We therefore performed a bivariate analysis of the diagnostic odds ratio (DOR) which is a single global indicator for diagnostic test performance and is defined as the ratio of the odds of positive test results in subjects with the disease, compared to the odds in those without the disease. This bivariate model applied logit transformation of pairs of sensitivity and specificity to provide pooled sensitivity and specificity estimates and to derive the area under the curve analyses.
If 2 × 2 data were reported at multiple thresholds, data related to the optimal cut-off value were extracted. Global statistical heterogeneity was assessed across all comparisons using the i2 measure with the meta-statistical package [20]. Heterogeneity was defined as the following: 0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; and 75% to 100%, considerable heterogeneity [21].
The meta-analysis was performed using the statistical package meta in R (version 4.1.3, R Core Team, The R Foundation, Vienna, Austria). The results were reported according to the PRISMA extension statement for meta-analyses [22]. Finally, comparison-adjusted funnel plots were generated to evaluate publication bias and small-study bias, where sufficient studies (≥10) existed [23].
2.5. Deviations from Protocol
In the prespecified protocol for this review [17], the target population was described as patients with IBD. Given the differences in disease patterns and distribution, we focused solely on patients with CD, with a future scope for another review with ulcerative colitis as the target population. In addition, it was originally proposed that the reference standard for diagnosis should include radiological and histological endpoints. However, given that cross-sectional imaging treatment targets and histological treatment targets remained poorly defined at the time of writing, this review was focused on ileocolonoscopy or balloon-assisted endoscopy assessment, which is the current gold standard of CD assessment, as the reference standard. A subanalysis of faecal biomarkers in determining mucosal healing in CD utilising endoscopic descriptions and/or definitions was also performed (where possible) or was otherwise reported with original sensitivities and specificities. In addition, to avoid the risk of duplicate patient inclusion, where studies from the same author in the same year were found, this analysis included the study with the larger number of patients.
Finally, the review also planned to determine the accuracy of faecal biomarkers based on IBD location. However, given the limited number of location-specific studies that were performed across multiple different modalities (e.g., ileocolonoscopy, radiology, and capsule endoscopy), this was not undertaken because the accuracy of faecal biomarker assessment was degraded using the variable application of multiple modalities as the reference standard.
3. Results
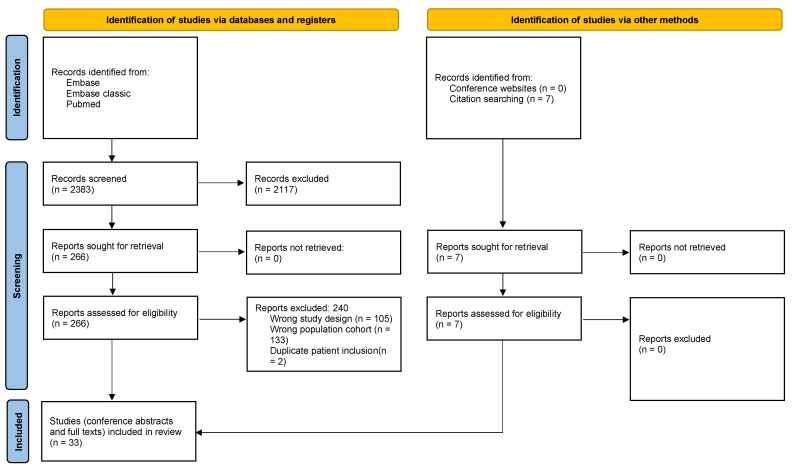
The initial literature search identified a total of 2382 reports; of these, 2117 were excluded based on their titles and abstracts. A review of the full text of the remaining 266 articles led to an additional 240 being excluded due to failure to meet the inclusion criteria, lacking the data required to create a contingency table, or due to the same author publishing twice within the same year. A further seven studies were identified through reference checking of the included studies. Hence, 33 eligible studies were included (see Figure 1: PRISMA flow chart).
Figure 1.
PRISMA flow chart.
3.1. Study Characteristics
The 33 eligible studies included a population of CD patients who undertook faecal biomarker assessment with FC, FL, CHI3L1, MMP-9, FCLN-2, FIT, PMN-e, neopterrin, and/or myeloperoxidase against endoscopic assessment as the reference standard. Thirty-two studies assessed FC, four studies assessed FL, two studies assessed FCLN-2 and FIT, and one study assessed each of PMN-e, MMP-9, neopterrin, CHI3L1, and myeloperoxidase, respectively. A total of 2511 and 230 patients with CD were included in the FC and FL analyses, discriminating between active and inactive endoscopic disease. A total of 1086 patients were included in a separate subanalysis evaluating the capacity of FC to discriminate endoscopic mucosal healing. Endoscopic definitions of CD activity were via the simplified endoscopy score for CD (SES-CD), Crohn’s disease endoscopic index of severity (CDEIS), presence of endoscopic ulcers, and one study used an internally developed author’s score. SES-CD scores to discriminate between endoscopic CD activity and mucosal healing ranged between 0 and 3, and CDEIS between 0 and 6. Han et al. utilised a partial SES-CD whereby the presence of stenosis was excluded from the original SES-CD in determining endoscopic activity [24]. Iwamoto et al. utilised an extended SES-CD whereby CD activity from the proximal ileum and jejunum were included in determining endoscopic activity [25].
3.2. Study Quality
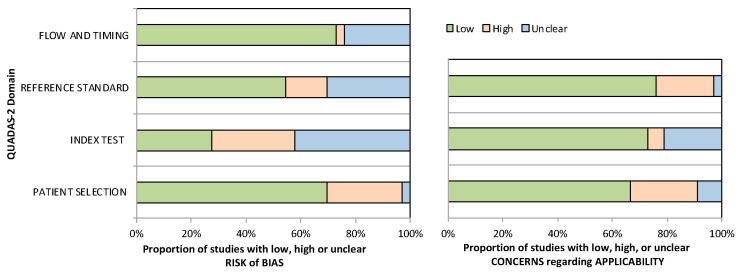
The 33 included studies underwent quality assessment with the QUADAS-2 criteria for diagnostic studies, with a summary presented in Figure 2. Twenty-three studies randomly and/or consecutively enrolled patients with CD, whereas the remaining studies were either unclear about or engendered bias via their method selection of patients. Moreover, in 15 studies it was unclear whether blinding of the reference standard was performed, and in 24 studies it was unclear whether blinding of the index test was performed. Overall, concerns regarding the applicability of the included studies across all domains were minimal.
Figure 2.
Graphical display of QUADAS-2 results.
3.3. Faecal Calprotectin
There were 32 studies that were eligible for assessment of faecal calprotectin [15,16,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Faecal calprotectin assays were performed via a lateral flow method in six studies, enzyme-linked immunosorbent assay (ELISA) in 25 studies, and the method was not described in one study. Multiple different proprietary faecal calprotectin kits were used, including Calprest (Eurospital Spa, Trieste, Italy), Bühlmann Calprotectin ELISA kit (Bühlmann, Schönenbuch, Switzerland), CALPRO ELISA (Calpro AS, Lysaker, Norway), EliA Calprotectin 2 (Thermo Fisher Scientific, Tokyo, Japan), Quantum Blue test (Buhlmann Laboratories, Schönenbuch, Switzerland), PhiCal (Immundiagnostik AG, Bensheim, Germany), RIDASCREEN® CALPROTECTIN (R-Biopharm AG, Darmstadt, Germany), and PhiCal (Genova Diagnostics Laboratories, Asheville, NC, USA). Definitions of endoscopic activity varied within the included studies, with nine studies applying an SES-CD > 2, five using SES-CD > 3, three using the presence of endoscopic ulcers, two using a CDEIS ≥ 3, and one using a CDEIS > 3. The remaining definitions (extended SES-CD ≥ 1, partial SES-CD ≥ 1, and an internally developed authors’ score) were each used in one study, respectively. Additionally, definitions of mucosal healing varied within the included studies, with three studies applying an SES-CD ≤ 2, three using an SES-CD = 0, and one each using a CDEIS ≤ 3, pSES-CD = 0, and the absence of endoscopic ulcers and/or inflammation.
3.3.1. Detection of Endoscopic Activity
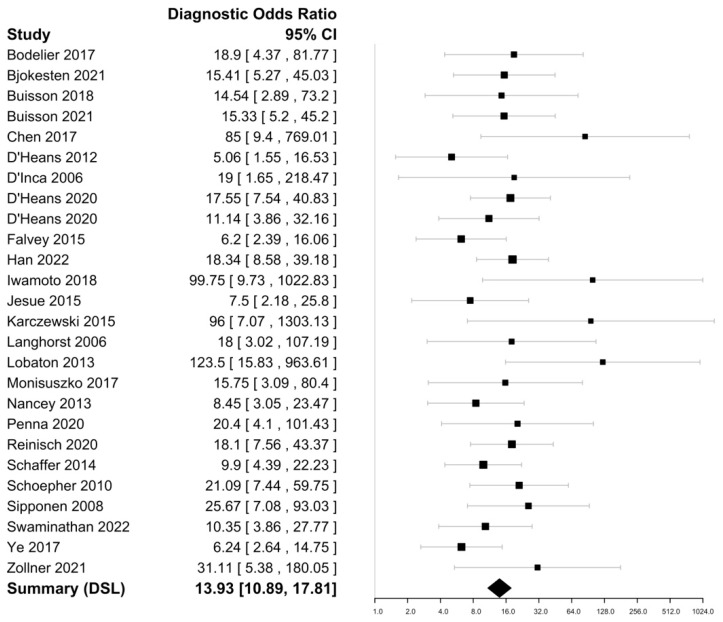
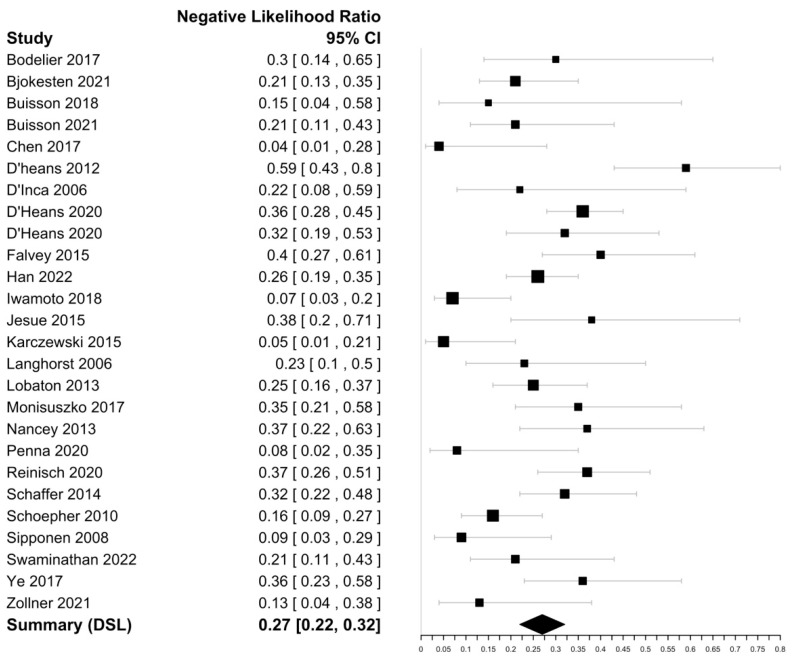
Within the included 25 studies (Table 1), FC sensitivities in discriminating endoscopically active CD ranged from 52 to 97% with a specificity ranging from 45 to 98%. The highest sensitivity was reported by Chen et al. (97%), with the highest specificity reported by Lobaton et al. (98%) [30,39]. The DOR was 13.93 (95% CI 10.89–17.81) with an i2 value of 1.34%, suggesting an insignificant heterogeneity and a negative likelihood ratio of 0.27 (95% CI 0.22–0.33) (Figure 3 and Figure 4). The Spearman’s correlation between sensitivity and false positive rate was 0.52. Using bivariate analysis, the pooled sensitivity was 81% (95% CI, 77–84%) with a specificity of 74% (95% CI, 70–80%) and an AUC of 0.85. On assessment using Deek’s funnel plot, there was no evidence of publication bias (p = 0.41).
Table 1.
Summary of FC studies assessing endoscopic activity in Crohn’s Disease(CD).
| Study (Name, Year) |
Nation of Origin | CD Patients (n=) | Definition of Endoscopic Activity (Inactive/Active) |
Optimal FC Cut-off | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| Björkesten 2021 [15] | Finland | 126 | SES-CD ≤ 2/SES-CD > 2 | 94 µg/g | 84% (76–90) |
74% (54–87) |
| Bodelier 2017 [26] |
Netherlands | 50 | SES-CD ≤ 3/SES-CD > 3 | 250 µg/g | 74% (51–88) |
87% (71–95) |
| Buisson 2021 [16] | France | 83 | Absence of ulcers/ presence of ulcers |
250 µg/g | 84% (70–91) |
74% (58–85) |
| Buisson 2018 [27] |
France | 54 | Absence of ulcers/presence of ulcers | 250 µg/g | 91% (73–98) |
58% (41–73) |
| Chen 2017 [28] |
China | 56 | SES-CD ≤ 2/SES-CD > 2 | 250 µg/g | 97% (85–99) |
71% (50–86) |
| D’Haens 2012 [29] |
Netherlands | 87 | Absence of ulcers/presence of ulcers | 250 µg/g | 52% (40–63) |
83% (63–93) |
| D’Haens 2020 * [30] | Netherlands | 247 81 |
SES-CD ≤ 2/SES-CD > 2 SES-CD ≤ 2/SES-CD > 2 |
250 µg/g 50 µg/g |
68% (61–75) 75% (61–85) |
88% (78–94) 78% (62–89) |
| D’Inca 2006 [31] |
Italy | 31 | SES-CD ≤ 3/SES-CD > 3 | 80 µg/g | 83% (63–93) |
80% (38–96) |
| Falvey 2015 [32] |
New Zealand | 108 | SES-CD ≤ 2/SES-CD > 2 | 125 µg/g | 71% (60–80) |
71% (53–84) |
| Han 2022 [24] |
China | 254 | pSES-CD = 0/pSES-CD ≥ 1 | 156 µg/g | 78% (72–83) |
83% (72–90) |
| Iwamoto 2018 [25] |
Japan | 69 | eSES-CD = 0/eSES-CD ≥ 1 | 92 mg/kg | 93% (84–97) |
88% (53–91) |
| Jesue 2018 [33] |
Spain | 52 | SES-CD = 0/SES-CD ≥ 1 | 54 µg/g | 71% (53–84) |
75% (55–88) |
| Karczewski 2015 [34] | Poland | 55 | CDEIS < 3/CDEIS ≥ 3 | 76 µg/g | 96% (87–99) |
80% (38–96) |
| Langhorst 2006 [35] |
Germany | 43 | Authors’ score | 48 µg/mL | 82% (66–91) |
80% (49–94) |
| Lobaton 2013 [36] |
Spain | 115 | CDEIS < 3/CDEIS ≥ 3 | 274 µg/g | 76% (65–84) |
98% (87–99) |
| Monisuszko 2017 [37] | Poland | 57 | SES-CD ≤ 3/SES-CD > 3 | 238.5 µg/g | 69% (53–81) |
88% (64–97) |
| Nancey 2013 [38] | France | 78 | SES-CD ≤ 2/SES-CD > 2 | 250 µg/g | 71% (55–82) |
77% (62–87) |
| Penna 2020 [39] |
Italy | 80 | SES-CD ≤ 2/SES-CD > 2 | 155 µg/g | 96% (87–99%) |
45% (28–63) |
| Reinisch 2020 [40] |
Austria | 156 | CDEIS ≤ 3/CDEIS > 3 | 250 µg/g | 67% (56–76) |
90% (81–95) |
| Schaffer 2014 [41] |
Switzerland | 136 | SES-CD ≤ 3/SES-CD > 3 | 250 µg/g | 75% (65–83%) |
76% (63–86) |
| Schoepher 2010 [42] | Switzerland | 122 | SES-CD ≤ 3/SES-CD > 3 | 70 µg/g | 89% (81–93) |
72% (53–86) |
| Sipponen 2008 [43] |
Finland | 116 | CDEIS ≤ 3/CDEIS > 3 | 200 µg/g | 94% (84–98) |
61% (48–73) |
| Swaminathan 2022 [44] | New Zealand | 100 | SES-CD ≤ 2/SES-CD > 2 | 58 µg/g | 87% (76–93) |
61% (45–74) |
| Ye 2017 [45] |
China | 109 | SES-CD ≤ 2/SES-CD > 2 | 213 µg/g | 76% (65–84) |
66% (51–79) |
| Zollner 2021 [46] |
Austria | 72 | SES-CD ≤ 2/SES-CD > 2 | 78 µg/g | 90% (75–97) |
77% (50–92) |
* D’Haens 2020 had 2 groups measured separately. Abbreviations: SES-CD, simplified endoscopy score for Crohn’s disease; CDEIS, Crohn’s disease endoscopic index of severity; pSES-CD, partial simplified endoscopy score for Crohn’s disease; eSES-CD, extended simplified endoscopy score for Crohn’s disease.
Figure 3.
Forest plots of diagnostic odds ratio of faecal calprotectin for CD endoscopic activity assessment [15,16,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Figure 4.
Forest plots of negative likelihood ratio estimate of faecal calprotectin for CD endoscopic activity assessment [15,16,24,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
3.3.2. Prediction of Mucosal Healing
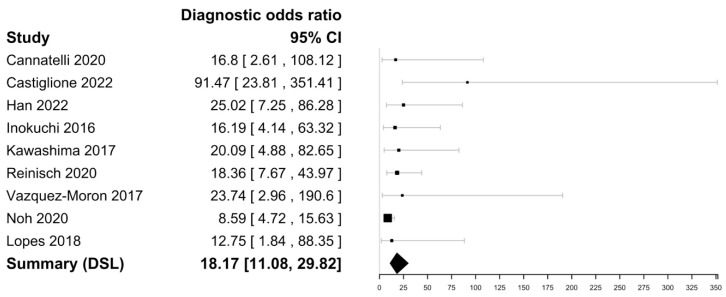
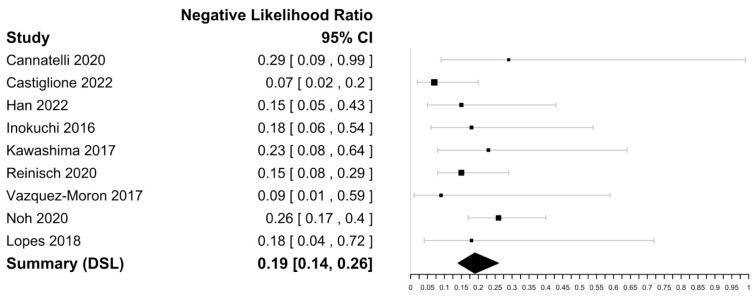
Within the nine included studies (Table 2), FC sensitivities in mucosal healing in CD ranged from 75 to 95% with a specificity ranging from 53 to 85%. The highest sensitivity was reported by Vazquez-Moron et al. (95%), and the highest specificity was reported by Castiglione and Cannatelli et al. (85%) [47,53,54]. The DOR was 18.17 (95% CI [11.08–29.82] with an i2 value of 0% implying no significant heterogeneity and a negative likelihood ratio of 0.19 [0.14–0.26]) (Figure 5 and Figure 6). The Spearman’s correlation between sensitivity and false positive rate was 0.48. Using bivariate analysis, the pooled sensitivity was 88% (84–90), specificity was 72% (64–79), and AUC was 0.88. There were not enough studies to assess for publication bias.
Table 2.
Summary of FC studies assessing mucosal healing in CD.
| Study (Name, Year) | Nation of Origin | Definition of Endoscopic Mucosal Healing | Optimal FC Cut-off | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| Cannatelli 2021 [53] | Italy | SES-CD ≤ 2 | 96 mcg/g | 75% (41–93%) | 85% (69–93) |
| Castiglione 2022 [54] | Italy | SES-CD ≤ 2 | 94 µg/g | 94% (84–98) | 85% (74–92) |
| Han 2022 [24] | China | pSES-CD = 0 | 117.48 µg/g | 89% (72–86) | 76% (70–81) |
| Inokuchi 2016 [48] | Japan | SES-CD = 0 | 180 µg/g | 87% (68–95) | 71% (57–82) |
| Kawashima 2017 [49] | Japan | SES-CD ≤ 2 | 162.2 µg/g | 81% (57–93) | 82% (71–90) |
| Lopes 2018 [50] | Spain | SES = CD = 0 | 100 µg/g | 89% (69–97) | 60% (31–83) |
| Noh 2018 [51] | Korea | No ulcers and/or inflammation | 234 µg/g | 84% (76–90) | 62% (54–69) |
| Reinisch 2020 [40] | Austria | CDEIS ≤ 3 | 250 µg/g | 90% (81–95) | 67% (56–77) |
| Vazquez-Moron 2017 [47] | Spain | SES-CD ≤ 2 | 71 µg/g | 95% (78–99) | 53% (39–66) |
Abbreviations: SES-CD, simplified endoscopy score for Crohn’s disease: CDEIS, Crohn’s disease endoscopic index of severity; pSES-CD, partial simplified endoscopy score for Crohn’s disease.
Figure 5.
Forest plots of diagnostic odds ratio of faecal calprotectin for CD mucosal healing assessment [24,40,47,48,49,50,51,53,54].
Figure 6.
Forest plots of negative likelihood ratio estimate of faecal calprotectin for CD mucosal healing assessment [24,40,47,48,49,50,51,53,54].
3.4. Faecal Lactoferrin
Lactoferrin is a component of polymorphonuclear neutrophils, which are implicated in the acute inflammatory response as seen in CD [35]. Four studies were eligible for the assessment of sensitivity and specificity for faecal lactoferrin in discriminating endoscopic activity (see Table 3) [31,34,35,43]. All lactoferrin assays were performed via ELISA, with four studies using the IBD-SCAN® (Techlab, Blacksburg, VA, USA) kit and one study using the IBD-CHEK® (Techlab, Blacksburg, VA, USA) kit [31,34,35,43]. Definitions of endoscopic activity varied within the included studies, with one study each using a CDEIS > 3, CDEIS > 2, SES-CD > 3, and an internally developed authors’ score to discriminate between active and inactive endoscopic CD [31,34,35,43].
Table 3.
Summary of studies assessing accuracy of FL to CD endoscopic activity.
| Study (Name, Year) | Nation of Origin | CD Patients (n=) | Definition of Endoscopic Activity | Optimal FL Cut-off | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| D’Inca 2006 [31] | Italy | 31 | SES-CD ≤ 3/SES-CD > 3 | 0.007 optical density | 77% (57–89) |
80% (38–96) |
| Karczewski 2015 [34] | Poland | 55 | CDEIS ≤ 2/CDEIS > 2 | 25 µg/g | 75% (62–85) |
80% (38–96) |
| Langhorst 2006 [35] |
Germany | 43 | Authors score | 7.1 µg/mL | 81% (65–91) |
59% (32–82) |
| Sipponen 2008 [43] |
Finland | 116 | CDEIS ≤ 3/CDEIS > 3 | 10 µg/g | 66% (54–76) |
91% (77–96) |
Abbreviations: SES-CD: simplified endoscopy score for Crohn’s disease, CDEIS: Crohn’s disease endoscopic index of severity.
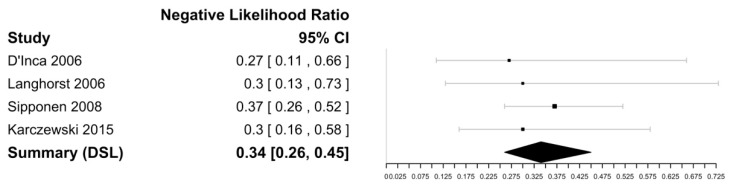
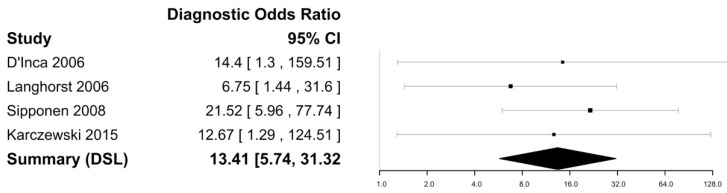
Figure 7 and Figure 8 show the forest plots for the negative likelihood ratio and DOR of FL in discriminating endoscopically active and inactive CD. Sensitivities ranged from 66 to 81%, with the highest sensitivity reported as 81% by Langhorst et al. [35]. The specificity ranged from 59 to 91%, with the highest specificity of 91% reported by Sipponen et al. [43]. The negative likelihood ratio was 0.34 (95% CI [0.26–0.45] and the DOR was 13.42 (95% CI: 5.74–31.32) with an I2 value of 0%, suggesting no heterogeneity. The Spearman’s correlation between sensitivity and false positivity was 0.89. Using bivariate analysis, the pooled sensitivity was 75% (65–83) and the pooled specificity was 80% (57–92), with an AUC of 0.81. There were not enough studies to assess for publication bias.
Figure 7.
Forest plot for the negative likelihood ratio of FL in discriminating endoscopically active and inactive CD [33,36,38,45].
Figure 8.
Forest plot for the diagnostic odds ratio of FL in discriminating endoscopically active and inactive CD [31,34,35,43].
3.5. Other Biomarkers
Faecal Lipocalin-2
FCLN-2 is a glycoprotein produced by intestinal epithelial cells and released into the gut lumen in response to proinflammatory stimuli, as occurs in CD [27,46]. FCLN-2 assays are performed via ELISA and have been described in two studies as potential discriminant faecal biomarkers of endoscopically active CD [27,46]. In a cohort of 54 patients with CD, Buisson et al. demonstrated an optimal cut-off level of 6700 ng/g for discriminating between the absence and presence of endoscopic ulcers, with a sensitivity and specificity of 86% and 46%, respectively [27]. More recently, in a cohort of 72 patients with CD, Zollner et al. demonstrated an optimal cut-off of 0.56 µg/g for discriminating endoscopically active and inactive CD (defined as an SES-CD > 3 for active CD), with a sensitivity and specificity of 91% and 77%, respectively [46]. The discrepancy in optimal cut-offs between the two studies may be explained by the different endoscopic standards applied (i.e., ulceration versus defined SES-CD score).
Faecal metalloprotease 9
Matrix metalloproteases are known to activate or degrade a variety of substrates within an immune response [27]. MMP-9 is expressed in inflamed intestinal mucosa by macrophages and neutrophils and released into faeces during active CD [27]. MMP-9 assays are performed via ELISA and have been described in one study as a potential discriminant faecal biomarker of endoscopically active CD. In a cohort of 54 patients with CD, Buisson et al. demonstrated an optimal cut-off level of 350 ng/g for discriminating between the absence and presence of endoscopic ulcers, with a sensitivity and specificity of 90% and 64%, respectively [27].
Neopterin
Neopterin is produced and released primarily from macrophages in response to stimulation from activated T-cells, as occurs in CD [38]. Neopterin assays are performed via ELISA and have been described in one study as a potential faecal biomarker for discriminating endoscopically active CD [38]. In a cohort of 78 patients with CD, Nancey et al. demonstrated sensitivity and specificity of 74% and 73%, utilising a neopterin cut-off value of 200 pmol/g for discriminating endoscopically active CD (defined as an SES-CD > 3) [38].
Faecal myeloperoxidase
Myeloperoxidase is an enzyme present within neutrophils with a putative role in inflammatory tissue damage [44]. Its presence within faeces is detectable via ELISA, with one study demonstrating its potential as a biomarker for discriminating endoscopically active CD [44]. In a cohort of 100 patients with CD, Swaminathan et al. demonstrated an optimal faecal myeloperoxidase cut-off level of 10.25 µg/g for discriminating endoscopically active CD (defined as an SES-CD > 3) [44]. At this cut-off, faecal myeloperoxidase demonstrated a sensitivity and specificity of 63 and 69%, respectively [44].
Faecal chitinase 3-like 1
Chitinase 3-like 1(CH3L1) is expressed in a variety of cells such as macrophages and neutrophils and is upregulated in the colonic epithelial cells and lamina propria macrophages of inflamed mucosa in CD [52]. Its presence within faeces is detectable via ELISA, with one study demonstrating its potential as a biomarker for discriminating endoscopically active CD [52]. In a study of 54 patients with CD, Buisson et al. determined an optimal faecal CH3L1 cut-off level of 15 ng/g for discriminating endoscopic ulceration in CD [52]. At this cut-off level, faecal CH3L1 demonstrated a sensitivity and specificity of 100% and 64%, respectively [52].
PMN-e
Polymorphonuclear neutrophil elastase (PMN-e) is an enzyme stored in polymorphonuclear neutrophils and released during the activation of these cells as a mediator of inflammation [35]. Its presence within faeces is detectable via ELISA, with one study demonstrating its potential as a biomarker capable of discriminating endoscopically active CD [35]. In a study of 43 patients with CD, Langhorst et al. determined an optimal faecal PMN-e cut-off level of 0.062 µg/mL for discriminating endoscopically active CD (using an internally developed endoscopic score by the authors as the reference standard) [35]. At this cut-off level, faecal PMN-E demonstrated a sensitivity and specificity of 82% and 70%, respectively [35].
FIT
FIT quantitatively measures faecal haemoglobin concentrations and forms the backbone of colon cancer surveillance programs worldwide [48]. Quantitative measurements are widely performed using the proprietary test from the Eiken Chemical Co. (Tokyo, Japan). In a cohort of 69 patients, Iwamoto et al. determined an FIT cut-off of 13 ng/mL to have a sensitivity and specificity of 73% and 71% in discriminating endoscopically active CD (defined as an eSES-CD ≥ 1) [25]. Regarding mucosal healing, in a cohort of 71 patients, Inokuchi et al. determined that a FIT cut-off level of 52 ng/mL was predictive of mucosal healing in CD (defined as an SES-CD = 0), with a sensitivity and specificity of 96% and 48%, respectively [48]. Other biomarkers used to detect endoscopic activity in CD are summarized in Table 4.
Table 4.
Summary of other faecal biomarkers used to assess endoscopic activity in CD.
| Study (Name, Year) | Nation of Origin | Number of CD Patients | Definition of Endoscopic Activity | Faecal Biomarker | Optimal Faecal Biomarker Cut-off | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| Buisson 2016 [52] | France | 54 | Absence of ulcers/Presence of ulcers | Faecal chitinase 3-like 1 | 15 ng/g | 100% (84–100) | 64% (41–80) |
| Buisson 2018 [27] | France | 54 | Absence of ulcers/Presence of ulcers Absence of ulcers/Presence of ulcers |
FCLN-2 Faecal metalloprotease 9 |
6700 ng/g 350 ng/g |
86% (64–97) 90% (64–97) |
45% (24–68) 64% (41–80) |
| Iwamoto 2018 [25] | Japan | 69 | eSES-CD = 0/eSES-CD ≥ 1 | FIT | 13 ng/mL | 73% (95% CI na) | 71% (95% CI na) |
| Langhorst 2006 [35] | Germany | 43 | Authors score | PMN-e | 0.062 µg/mL | 82% (65–93) | 70% (35–93) |
| Nancey 2013 [38] | France | 78 | SES-CD ≤ 2/SES-CD > 2 | Neopterin | 200 pmol/g | 74% (57–87) | 73% (56–85) |
| Swaminathan 2022 [44] | New Zealand | 100 | SES-CD ≤ 2/SES-CD > 2 | Faecal Myeloperoxidase | 10.25 µg/g | 63% (50–75) | 68% (51–83) |
| Zollner 2021 [46] | Austria | 72 | SES-CD ≤ 2/SES-CD > 2 | FLCN-2 | 0.56 µg/g | 91% (74–98) | 77% (46–95) |
Abbreviations: SES-CD, simplified endoscopy score for Crohn’s disease; CDEIS, Crohn’s disease endoscopic index of severity; pSES-CD, partial simplified endoscopy score for Crohn’s disease; eSES-CD, extended simplified endoscopy score for Crohn’s disease; FIT, faecal immunochemical test; FCLN-2, faecal lipocalin-2; PMN-e, polymorphonuclear neutrophil elastase, na = not available.
4. Discussion
With regular objective assessment now recommended by international guidelines, there is a growing imperative for cheaper, rapidly accessible yet reliable tests for assessing disease activity in CD. Clinical activity scores, such as the Harvey-Bradshaw index or CD activity index (CDAI), are predominantly symptom-based, and therefore subjective and prone to providing falsely reassuring results [32]. Moreover, biochemical tests, such as C-reactive protein, remain nonspecific to IBD and are subject to confounding in multiple clinical settings. Whilst ileocolonoscopy and cross-sectional imaging remain important options due to their informational capacity, neither are cheap nor necessarily rapidly available. Furthermore, both require significant patient participation, preparation, and risks that might be deemed unacceptable for repetitive testing over short six-monthly assessment intervals, as recommended by STRIDE-2 guidelines. Thus, faecal biomarkers play a significant role in the routine, serial monitoring of IBD patients.
This meta-analysis strengthens the existing knowledge regarding FC as a capable biomarker in discriminating between active/inactive endoscopic disease and mucosal healing and provides context for FC amongst other more novel faecal biomarkers and their respective performance in discriminating endoscopically active versus inactive CD. This meta-analysis reaffirmed that both FC and FL are highly sensitive and specific for discriminating active versus inactive endoscopic disease in CD, with the inclusion of a number of more recent studies since the previous meta-analysis was performed many years ago [55]. In this meta-analysis, FC demonstrated a pooled sensitivity of 81% and specificity of 74% (AuROC and DOR of 0.85 and 13.93, respectively), implying that FC retains good diagnostic capacity across all disease subtypes and locations of the heterogeneous entity that is CD. These pooled data are similar to those previously reported for FC in the literature [55]. Moreover, FC exhibits an excellent capacity for prediction of mucosal healing with a summary DOR of 18.17 (95% CI 11.08–29.82) in this study.
Many FC manufacturers recommend 50 µg/g as a cut-off value for representing active intestinal inflammation. However, in this meta-analysis, 15 of 25 (60%) studies reported an FC > 200 µg/g as their optimal cut-off when discriminating between endoscopically active and inactive disease, albeit with heterogeneous definitions of endoscopically active CD. Comparatively, with respect to mucosal healing, only two studies derived a FC > 200 µg/g as optimal and most (5/9) applied a normal endoscopy (SES-CD = 0 and/or no ulcers/inflammation) as their definition of mucosal healing. Hence, the capacity of a faecal biomarker to surrogately represent mucosal healing may be a more appropriate ‘harder’ endpoint by which to determine its accuracy and utility when correlating to endoscopy as a reference standard. While determining the optimal cut-off for FC in detecting endoscopically active or mucosal healing in CD was beyond the scope of this analysis, it appears that a lower calprotectin cut-off at a higher sensitivity is demonstrated in studies addressing mucosal healing compared to endoscopic disease activity in CD. Ultimately, further studies examining newer calprotectin assays and similar definitions of endoscopic activity (and/or histological activity as some recent studies have explored) are needed.
Within our meta-analysis, FL demonstrated a pooled sensitivity, specificity, AuROC, and DOR of 75%, 80%, 0.81, and 13.41, respectively. Compared to FC, the performance of FL was similar, yet the role of FL, for unknown reasons beyond the scope of this study, has not been incorporated into widespread clinical practice. The diagnostic performance of alternative faecal biomarkers was included in the study, yet most were limited to single studies. Thus, it remains difficult to draw meaningful conclusions from these biomarkers regarding their capacity to discriminate between active and inactive endoscopic CD or mucosal healing. Further studies of biomarkers beyond FC and FL are clearly needed to determine and establish their potential role in CD assessment, especially concerning whether they offer advantages over FC in terms of cost, accuracy, and/or convenience. In addition, combinations of low-cost faecal biomarkers should be explored in an effort to enhance diagnostic performance.
This meta-analysis was subject to several weaknesses and limitations. The data were drawn from predominately retrospective studies with their inherent biases. Moreover, our endoscopic reference standard was variably applied via multiple, different endoscopic scores and cut-off levels therein used to discriminate CD activity which may have impacted the observed results. In addition, FC and FL assays were performed via different methods (i.e., lateral flow and ELISA) which may not be of equivalent accuracy.
5. Conclusions
Based on this rigorous meta-analysis, FC and FL were both shown to perform robustly in discriminating between active and inactive CD. Both are relatively cheap, rapid, and reliable tests that are therefore amenable to serial testing at frequent intervals to confidently monitor CD activity. Newer and novel faecal biomarkers have been proposed but require further evaluation before any could be considered as a replacement, let alone superior, to FC in routine clinical practice. There is clearly an unmet need to incisively examine the capacity of faecal biomarkers to assess CD activity according to disease location, extent, and specific subtypes. Moreover, in this context, the application of multiple biomarkers (faecal, serum, and/or other) in a combined diagnostics matrix, perhaps harnessing artificial intelligence-based algorithms, may provide even greater diagnostic and predictive power. Faecal biomarkers are here to stay and, with further research, are likely to continue to increasingly dominate the diagnostics landscape in routine management of CD.
Acknowledgments
Wendy Wong, Eastern Health Library Services for assistance with the literature search.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11051408/s1, Table S1: FC to CD endoscopic activity dataset; Table S2: FC to CD mucosal healing activity dataset; Table S3: FL to CD endoscopic dataset; Table S4: Literature search strategy; Table S5: Tabular presentation of QUADAS-2 results; PRISMA Checklist. Ref. [56] is cited in the supplementary materials.
Author Contributions
A.B.: literature search, data collation and extraction, writing, editing, statistical analysis; D.R.V.L.: critical revision and supervision; A.V.: critical revision and supervision; G.M.: literature search, data collation and extraction; D.L.: statistical analysis, J.P.S.: literature search, data collation and extraction, writing, editing, statistical analysis and critical revision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available in body of text and Supplementary Materials.
Conflicts of Interest
D.R.V.L. has received educational grants or research support from Janssen, Medtronic, and Pfizer, as well as speaker fees from Janssen, Takeda, and Pfizer, and is on the advisory boards for Janssen, Takeda, Pfizer, and AbbVie. J.P.S. has received speaker fees for Janssen, Abbvie, and Takeda.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal M., Christensen H.S., Bøgsted M., Colombel J.F., Jess T., Allin K.H. The Rising Burden of Inflammatory Bowel Disease in Denmark Over Two Decades: A Nationwide Cohort Study. Gastroenterology. 2022;163:1547–1554.e5. doi: 10.1053/j.gastro.2022.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.W., Eun C.S. Inflammatory bowel disease in Korea: Epidemiology and pathophysiology. Korean J. Intern. Med. 2022;37:885–894. doi: 10.3904/kjim.2022.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argüelles-Arias F., Rodríguez-Oballe J., Duarte-Chang C., Castro-Laria L., García-Montes J.M., Caunedo-Álvarez A., Herrerías-Gutiérrez J.M. Capsule endoscopy in the small bowel Crohn’s disease. Gastroenterol. Res. Pract. 2014;2014:529136. doi: 10.1155/2014/529136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyrin-Biroulet L., Loftus E.V., Colombel J.F., Sandborn W.J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 2011;17:471–478. doi: 10.1002/ibd.21417. [DOI] [PubMed] [Google Scholar]
- 6.Cho C.W., You M.W., Oh C.H., Lee C.K., Moon S.K. Long-term Disease Course of Crohn’s Disease: Changes in Disease Location, Phenotype, Activities, and Predictive Factors. Gut Liver. 2022;16:157–170. doi: 10.5009/gnl210118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamina M., Bonovas S., Raine T., Spinelli A., Warusavitarne J., Armuzzi A., Bachmann O., Bager P., Biancone L., Bokemeyer B., et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn's Colitis. 2020;14:155–168. doi: 10.1093/ecco-jcc/jjz187. [DOI] [PubMed] [Google Scholar]
- 8.Rieder F., Latella G., Magro F., Yuksel E.S., Higgins P.D., Di Sabatino A., de Bruyn J.R., Rimola J., Brito J., Bettenworth D., et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohn's Colitis. 2016;10:873–885. doi: 10.1093/ecco-jcc/jjw055. [DOI] [PubMed] [Google Scholar]
- 9.Torres J., Bonovas S., Doherty G., Kucharzik T., Gisbert J.P., Raine T., Adamina M., Armuzzi A., Bachmann O., Bager P., et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn's Colitis. 2020;14:4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Cole A., Segal J., Smith P., Limdi J.K. A review of the therapeutic management of Crohn’s disease. Therap. Adv. Gastroenterol. 2022;15:17562848221078456. doi: 10.1177/17562848221078456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maaser C., Sturm A., Vavricka S.R., Kucharzik T., Fiorino G., Annese V., Calabrese E., Baumgart D.C., Bettenworth D., Borralho Nunes P., et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn's Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 12.Geyl S., Guillo L., Laurent V., D’Amico F., Danese S., Peyrin-Biroulet L. Transmural healing as a therapeutic goal in Crohn’s disease: A systematic review. Lancet Gastroenterol. Hepatol. 2021;6:659–667. doi: 10.1016/S2468-1253(21)00096-0. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L., Sandborn W., Sands B.E., Reinisch W., Bemelman W., Bryant R.V., D’Haens G., Dotan I., Dubinsky M., Feagan B., et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 14.Turner D., Ricciuto A., Lewis A., D’Amico F., Dhaliwal J., Griffiths A.M., Bettenworth D., Sandborn W.J., Sands B.E., Reinisch W., et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 15.af Björkesten C.G., Nieminen U., Turunen U., Arkkila P., Sipponen T., Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand. J. Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 16.Buisson A., Mak W.Y., Andersen M.J., Lei D., Pekow J., Cohen R.D., Kahn S.A., Pereira B., Rubin D.T. Fecal Calprotectin Is Highly Effective to Detect Endoscopic Ulcerations in Crohn’s Disease Regardless of Disease Location. Inflamm. Bowel Dis. 2021;27:1008–1016. doi: 10.1093/ibd/izaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed G., Bohra A., Segal J. Sensitivity and Specificity of Stool Markers in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. PROSPERO 2022 CRD42022354526. [(accessed on 13 April 2023)]. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022354526.
- 18.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M., QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 19.Glas A.S., Lijmer J.G., Prins M.H., Bonsel G.J., Bossuyt P.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003;56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 20.Doebler P. Comprehensive R Archive Network Mada: Meta-Analysis of Diagnostic Accuracy. [(accessed on 13 April 2023)]. Available online: https://cran.r-project.org/web/packages/mada/index.html.
- 21.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks J.J., Macaskill P., Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Han W., Wu J., Zhang P., Hu N., Mei Q., Hu J. Fecal calprotectin predicts endoscopic activity and mucosal healing of small bowel Crohn’s disease evaluated by double-balloon endoscopy. Int. J. Color. Dis. 2022;37:1953–1961. doi: 10.1007/s00384-022-04232-5. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto F., Matsuoka K., Motobayashi M., Takenaka K., Kuno T., Tanaka K., Tsukui Y., Kobayashi S., Yoshida T., Fujii T., et al. Prediction of disease activity of Crohn’s disease through fecal calprotectin evaluated by balloon-assisted endoscopy. J. Gastroenterol. Hepatol. 2018;33:1984–1989. doi: 10.1111/jgh.14310. [DOI] [PubMed] [Google Scholar]
- 26.Bodelier A.G., Jonkers D., van den Heuvel T., de Boer E., Hameeteman W., Masclee A.A., Pierik M.J. High Percentage of IBD Patients with Indefinite Fecal Calprotectin Levels: Additional Value of a Combination Score. Dig. Dis. Sci. 2017;62:465–472. doi: 10.1007/s10620-016-4397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buisson A., Vazeille E., Minet-Quinard R., Goutte M., Bouvier D., Goutorbe F., Pereira B., Barnich N., Bommelaer G. Fecal Matrix Metalloprotease-9 and Lipocalin-2 as Biomarkers in Detecting Endoscopic Activity in Patients With Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2018;52:e53–e62. doi: 10.1097/MCG.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 28.Chen J.M., Liu T., Gao S., Tong X.D., Deng F.H., Nie B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: A prospective study in China. World J. Gastroenterol. 2017;23:8235–8247. doi: 10.3748/wjg.v23.i46.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Haens G., Ferrante M., Vermeire S., Baert F., Noman M., Moortgat L., Geens P., Iwens D., Aerden I., Van Assche G., et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 30.D’Haens G., Kelly O., Battat R., Silverberg M.S., Laharie D., Louis E., Savarino E., Bodini G., Yarur A., Boland B.S., et al. Development and Validation of a Test to Monitor Endoscopic Activity in Patients With Crohn’s Disease Based on Serum Levels of Proteins. Gastroenterology. 2020;158:515–526.e10. doi: 10.1053/j.gastro.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 31.D’Incà R., Dal Pont E., Di Leo V., Ferronato A., Fries W., Vettorato M.G., Martines D., Sturniolo G.C. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int. J. Color. Dis. 2007;22:429–437. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 32.Falvey J.D., Hoskin T., Meijer B., Ashcroft A., Walmsley R., Day A.S., Gearry R.B. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflamm. Bowel Dis. 2015;21:824–831. doi: 10.1097/MIB.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 33.Jusué V., Chaparro M., Gisbert J.P. Accuracy of fecal calprotectin for the prediction of endoscopic activity in patients with inflammatory bowel disease. Dig. Liver Dis. 2018;50:353–359. doi: 10.1016/j.dld.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Karczewski J., Swora-Cwynar E., Rzymski P., Poniedziałek B., Adamski Z. Selected biologic markers of inflammation and activity of Crohn’s disease. Autoimmunity. 2015;48:318–327. doi: 10.3109/08916934.2015.1016221. [DOI] [PubMed] [Google Scholar]
- 35.Langhorst J., Elsenbruch S., Koelzer J., Rueffer A., Michalsen A., Dobos G.J. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am. J. Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 36.Lobatón T., López-García A., Rodríguez-Moranta F., Ruiz A., Rodríguez L., Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J. Crohn's Colitis. 2013;7:e641–e651. doi: 10.1016/j.crohns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Moniuszko A., Głuszek S., Rydzewska G. Rapid fecal calprotectin test for prediction of mucosal inflammation in ulcerative colitis and Crohn disease: A prospective cohort study. Pol. Arch. Intern. Med. 2017;127:312–318. doi: 10.20452/pamw.4009. [DOI] [PubMed] [Google Scholar]
- 38.Nancey S., Boschetti G., Moussata D., Cotte E., Peyras J., Cuerq C., Haybrard J., Charlois A.L., Mialon A., Chauvenet M., et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2013;19:1043–1052. doi: 10.1097/MIB.0b013e3182807577. [DOI] [PubMed] [Google Scholar]
- 39.Penna F.G.C., Rosa R.M., Pereira F.H., Cunha P.F.S., Sousa S.C.S., Ferrari T.C.A., Cara C., Ferrari M.L.A. Combined evaluation of fecal calprotectin and C-reactive protein as a therapeutic target in the management of patients with Crohn’s disease. Gastroenterol. Hepatol. 2021;44:87–95. doi: 10.1016/j.gastrohep.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Reinisch W., Panaccione R., Bossuyt P., Baert F., Armuzzi A., Hébuterne X., Travis S., Danese S., Sandborn W.J., Schreiber S., et al. Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn’s Disease: A Post Hoc Analysis From the CALM Study. Inflamm. Bowel Dis. 2020;26:1562–1571. doi: 10.1093/ibd/izaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaffer T., Schoepfer A.M., Seibold F., Swiss IBD Cohort Study Group Serum ficolin-2 correlates worse than fecal calprotectin and CRP with endoscopic Crohn’s disease activity. J. Crohns Colitis. 2014;8:1125–1132. doi: 10.1016/j.crohns.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Schoepfer A.M., Beglinger C., Straumann A., Trummler M., Vavricka S.R., Bruegger L.E., Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 43.Sipponen T., Savilahti E., Kolho K.L., Nuutinen H., Turunen U., Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan A., Borichevsky G.M., Edwards T.S., Hirschfeld E., Mules T.C., Frampton C.M.A., Day A.S., Hampton M.B., Kettle A.J., Gearry R.B. Faecal Myeloperoxidase as a Biomarker of Endoscopic Activity in Inflammatory Bowel Disease. J. Crohn's Colitis. 2022;16:1862–1873. doi: 10.1093/ecco-jcc/jjac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye L., Cheng W., Chen B.Q., Lan X., Wang S.D., Wu X.C., Huang W., Wang F.Y. Levels of Faecal Calprotectin and Magnetic Resonance Enterocolonography Correlate with Severity of Small Bowel Crohn’s Disease: A Retrospective Cohort Study. Sci. Rep. 2017;7:1970. doi: 10.1038/s41598-017-02111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zollner A., Schmiderer A., Reider S.J., Oberhuber G., Pfister A., Texler B., Watschinger C., Koch R., Effenberger M., Raine T., et al. Faecal Biomarkers in Inflammatory Bowel Diseases: Calprotectin Versus Lipocalin-2-a Comparative Study. J. Crohn's Colitis. 2021;15:43–54. doi: 10.1093/ecco-jcc/jjaa124. [DOI] [PubMed] [Google Scholar]
- 47.Vázquez Morón J.M., Pallarés Manrique H., Machancoses F.H., Ramos Lora M., Ruiz Frutos C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn’s disease with fecal calprotectin. Rev. Esp. Enferm. Dig. 2017;109:130–136. doi: 10.17235/reed.2017.4542/2016. [DOI] [PubMed] [Google Scholar]
- 48.Inokuchi T., Kato J., Hiraoka S., Takashima S., Nakarai A., Takei D., Sugihara Y., Takahara M., Kawano S., Harada K., et al. Fecal Immunochemical Test Versus Fecal Calprotectin for Prediction of Mucosal Healing in Crohn’s Disease. Inflamm. Bowel Dis. 2016;22:1078–1085. doi: 10.1097/MIB.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima K., Ishihara S., Yuki T., Fukuba N., Sonoyama H., Kazumori H., Yamashita N., Tada Y., Kusunoki R., Oka A., et al. Fecal Calprotectin More Accurately Predicts Endoscopic Remission of Crohn’s Disease than Serological Biomarkers Evaluated Using Balloon-assisted Enteroscopy. Inflamm. Bowel Dis. 2017;23:2027–2034. doi: 10.1097/MIB.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 50.Lopes S., Andrade P., Afonso J., Cunha R., Rodrigues-Pinto E., Ramos I., Macedo G., Magro F. Monitoring Crohn’s disease activity: Endoscopy, fecal markers and computed tomography enterography. Therap. Adv. Gastroenterol. 2018;11:1756284818769075. doi: 10.1177/1756284818769075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noh S.M., Oh E.H., Park S.H., Lee J.B., Kim J.Y., Park J.C., Kim J., Ham N.S., Hwang S.W., Yang D.H., et al. Association of Faecal Calprotectin Level and Combined Endoscopic and Radiological Healing in Patients With Crohn’s Disease Receiving Anti-tumour Necrosis Factor Therapy. J. Crohn's Colitis. 2020;14:1231–1240. doi: 10.1093/ecco-jcc/jjaa042. [DOI] [PubMed] [Google Scholar]
- 52.Buisson A., Vazeille E., Minet-Quinard R., Goutte M., Bouvier D., Goutorbe F., Pereira B., Barnich N., Bommelaer G. Faecal chitinase 3-like 1 is a reliable marker as accurate as faecal calprotectin in detecting endoscopic activity in adult patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2016;43:1069–1079. doi: 10.1111/apt.13585. [DOI] [PubMed] [Google Scholar]
- 53.Cannatelli R., Bazarova A., Zardo D., Nardone O.M., Shivaji U., Smith S.C.L., Gkoutos G., Ricci C., Gui X.S., Ghosh S., et al. Fecal Calprotectin Thresholds to Predict Endoscopic Remission Using Advanced Optical Enhancement Techniques and Histological Remission in IBD Patients. Inflamm. Bowel Dis. 2021;27:647–654. doi: 10.1093/ibd/izaa163. [DOI] [PubMed] [Google Scholar]
- 54.Castiglione F., Imperatore N., Testa A., de Sire R., Nardone O.M., Ricciolino S., Di Luna I., Patturelli M., Villani G.D., Olmo O., et al. Exploring the concept of deep remission in Crohn’s disease: Correlation between transmural healing and biomarkers. Therap. Adv. Gastroenterol. 2022;15:17562848221110643. doi: 10.1177/17562848221110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rokkas T., Portincasa P., Koutroubakis I.E. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: A diagnostic accuracy meta-analysis. J. Gastrointestin. Liver Dis. 2018;27:299–306. doi: 10.15403/jgld.2014.1121.273.pti. [DOI] [PubMed] [Google Scholar]
- 56.56 Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available in body of text and Supplementary Materials.