Abstract
Evidence obtained from studies with yeast and Xenopus indicate that the initiation of DNA replication is a multistep process. The origin recognition complex (ORC), Cdc6p, and minichromosome maintenance (MCM) proteins are required for establishing prereplication complexes, upon which initiation is triggered by the activation of cyclin-dependent kinases and the Dbf4p-dependent kinase Cdc7p. The identification of human homologues of these replication proteins allows investigation of S-phase regulation in mammalian cells. Using centrifugal elutriation of several human cell lines, we demonstrate that whereas human Orc2 (hOrc2p) and hMcm proteins are present throughout the cell cycle, hCdc6p levels vary, being very low in early G1 and accumulating until cells enter mitosis. hCdc6p can be polyubiquitinated in vivo, and it is stabilized by proteasome inhibitors. Similar to the case for hOrc2p, a significant fraction of hCdc6p is present on chromatin throughout the cell cycle, whereas hMcm proteins alternate between soluble and chromatin-bound forms. Loading of hMcm proteins onto chromatin occurs in late mitosis concomitant with the destruction of cyclin B, indicating that the mitotic kinase activity inhibits prereplication complex formation in human cells.
The molecular mechanism that restricts firing of origins of replication to once per cell cycle invokes the ordered binding to and/or release of different replication proteins from specific DNA sequences (replicators) located in the vicinity of the actual origins of DNA replication. Following separation of sister chromatids at mitosis and during the subsequent G1 phase, prereplication complexes (pre-RCs) are formed at origins of DNA replication. Initiation of DNA replication is triggered by the action of at least two sets of protein kinase activities, cyclin-dependent kinases (CDKs) and Dbf4p-Cdc7p. After initiation, the protein complex at each origin changes to a postreplication state (post-RC), thereby preventing further initiation events for the rest of the cell cycle (reviewed in references 15 and 57).
The origin recognition complex (ORC), a six-subunit initiator protein (2), is present in both pre- and post-RCs (10), and one of its functions is to mark the position of replication origins in the genome. The pre-RC is established by the regulated binding of additional factors, which include Cdc6p and the minichromosome maintenance (MCM) proteins. Yeast CDC6 displays a genetic interaction with the ORC and is a critical factor for establishing the competence of replication origins once per cell cycle (12, 33, 47, 48). Besides its function in DNA replication, it may also be involved in a mitotic checkpoint control, because Cdc6-deprived yeast cells that do not replicate DNA still undergo a reductional mitosis (4, 47, 63).
Cdc6p is a member of the large AAA+ superfamily of ATPases, which includes Orc1p, Orc4p, Orc5p, MCM, proteins and replication factor C (42). Based on sequence similarities between Cdc6p, replication factor C, and other AAA+ family members and on the characterization of a dominant-negative CDC6 mutant, it has been proposed that yeast Cdc6p might function as an ATP-dependent MCM protein loader (45, 63). Indeed, the association of MCM proteins with chromatin is dependent on Cdc6p (1, 12, 34, 59). Biochemical studies with Xenopus provided additional support for the idea of Xenopus Cdc6p (XCdc6p) being an essential factor for establishing pre-RCs. In Xenopus egg cell extracts, XCdc6 could bind to chromatin only in the presence of XOrc2, and it was absolutely required for the subsequent loading of XMcm3 (6).
Yeast Cdc6p is a highly unstable protein, and many factors seem to be involved in its degradation, including the CDC4-CDC34-CDC53-Skp1 pathway (13, 14, 54). However, ectopic expression of Cdc6p in G2 cells is not deleterious for the cell, and it has been shown that Cdc6p cannot induce MCM protein binding to chromatin at this point unless CDKs are inactivated (9, 59). Interestingly, a dominant gain-of-function allele of CDC6 causes persistent MCM protein binding to chromatin and overreplication of the genome in a single cell cycle (34).
Cdc18+ is the Schizosaccharomyces pombe homologue of CDC6 and performs similar functions in regulating initiation of DNA replication and possibly entry into mitosis (28, 40, 43). Gross overexpression of Cdc18+ results in repeated rounds of DNA replication in the absence of mitosis (23, 43). p65Cdc18 is also a very labile protein targeted for destruction by CDK phosphorylation after cells enter S phase (24). At least in S. pombe and Xenopus, another protein, called Cdt1, participates in the assembly of pre-RCs (37, 44).
MCM proteins are essential for DNA replication (reviewed in reference 27). A subcomplex of human MCM proteins may function as a replicative helicase (22). This idea has been recently reinforced by the finding that yeast MCM proteins are required for replication fork progression (32) and by the characterization of a processive helicase activity in an archaeal MCM protein (5, 29).
The regulation of initiation of replication in mammalian cells is much less understood, mainly for two reasons: discrete replicator sequences have not been defined, and the information regarding mammalian proteins involved in this process has been limited. The recent identification of human homologues of ORC subunits (18, 49, 50, 62), Cdc6 (65), and MCM proteins (reference 27 and references therein) suggests that the overall mechanism for replication initiation is conserved between mammals and yeast.
We have therefore investigated the dynamics of chromatin association of endogenous human Orc2, Cdc6, and Mcm proteins (referred to as hOrc2p, hCdc6p, and hMcm proteins) throughout the cell cycle. We used centrifugal elutriation as a method to synchronize cells without interfering with their normal metabolism, and we raised new monoclonal anti-hCdc6p antibodies and polyclonal anti-hMcm antibodies. Our data show that a fraction of hCdc6p is targeted to chromatin during the entire cell cycle and help to define the point at which the hMcm proteins are loaded onto chromatin during late mitosis. We also report a previously unrevealed regulated destruction of hCdc6p and discuss the possible execution points for hCdc6p during the cell cycle.
MATERIALS AND METHODS
Antibodies.
To generate anti-hCdc6p monoclonal antibodies, full-length hCdc6p was overexpressed in Escherichia coli and purified as a glutathione S-transferase (GST) fusion protein. After cleavage of the GST moiety, hCdc6p was further purified in a Prep Cell gel (Bio-Rad) and used as an immunogen. Standard techniques for immunization of mice, analysis of test bleeds, generation of hybridoma cell lines, and production of ascitic fluid were used (19). A double screening of hybridomas was done by dot blotting, using either native or denatured hCdc6p as the antigen immobilized to nitrocellulose. Hybridoma cell lines hCdc6-26 and hCdc6-37 were positive in both tests. Several other positive cell lines (hCdc6-34, hCdc6-39, and hCdc6-41) turned out to produce immunoglobulin Ms (IgMs) and were not used in this work (unpublished data). Anti-hOrc2p and anti-hCdc6p polyclonal antibodies have been described before (18, 65). Different anti-hMcm3p, anti-hMcm4p, and anti-hMcm5p polyclonal sera were raised in rabbits against synthetic peptides corresponding to amino acids 674 to 693 of hMcm3p, amino acids 21 to 40 of hMcm4p, and amino acids 19 to 38 of hMcm5p, conjugated to keyhole limpet hemocyanin. Anti-pan-Mcm polyclonal serum was raised against a synthetic peptide that is highly conserved in all MCM proteins (amino acids 405 to 421 of hMcm3p). Antibodies against TFIIB were a gift from N. Hernandez (Cold Spring Harbor Laboratory). Monoclonal antibody 12CA5 (antihemagglutinin [anti-HA]) was obtained from C. Bautista (Cold Spring Harbor Laboratory). Anti-MEK2 (M24520) was obtained from Transduction Laboratories, anti-cyclin A and anti-p53 were obtained from Santa Cruz Biotechnology, and Texas Red-conjugated anti-rabbit IgG was obtained from Jackson Immunoresearch Laboratories, Inc.
Cell manipulations and centrifugal elutriation.
Cell lines HeLa, U2OS, 293, Manca (B-cell lymphoma), and Raji (Burkitt lymphoma) were obtained from the Cold Spring Harbor Laboratory cell culture facility or the American Type Culture Collection. Separation of logarithmically growing cells into distinct cell cycle phases was accomplished by centrifugal elutriation in a Beckman J2-21 M centrifuge and a JE-6B rotor with a large (40-ml) separation chamber. The rotor was kept at a speed of 1,500 rpm, the temperature was 20°C, and the medium flow was controlled with a Cole-Parmer Masterflex pump. Consecutive fractions of 250 ml were collected at increasing flow rates, ranging from 40 to 120 ml/min. Cells in all fractions were counted, and extracts were prepared after normalization by cell number. For cytofluorometric analyses, an aliquot of 106 cells was fixed by rapid submersion in 1 ml of ice-cold 90% ethanol. After at least 1 h of fixation at 4°C, cells were collected by centrifugation and incubated for 30 min at 37°C in 0.5 ml of staining solution (25 μg of propidium iodide per ml and 10 μg of RNase per ml in phosphate-buffered saline [PBS]). Stained cells were analyzed on a Becton-Dickinson FACScan.
To synchronize a cell culture in prometaphase, 50 ng of nocodazole per ml was added to 1 liter of HeLa cells growing in suspension medium (Joklik's modification to minimum essential medium supplemented with 10% calf serum). After 25 h, the culture was centrifuged at low speed (5 min at 3,000 rpm in a Beckman CS-6R centrifuge), washed with PBS, and resuspended in prewarmed fresh medium without nocodazole. Every 20 min after the release, an aliquot was taken from the main culture, and the cells were collected, washed once in PBS, and used to prepare total cell extracts or subjected to biochemical fractionation as described below. A total of 106 cells from each fraction were used for cytofluorometric analysis of DNA content.
Total cell extracts, nuclear extracts, and chromatin isolation.
To prepare total cell extracts, tissue-cultured cells were harvested by centrifugation, washed in PBS, and directly resuspended in Laemmli buffer, followed by sonication for 15 s in a Tekmar CV26 sonicator set at 25% amplitude. To prepare nuclear extracts, the cells were washed once with PBS and lysed by Dounce homogenization in hypotonic buffer (20 mM Hepes-KOH [pH 8.0], 5 mM KCl, 1.5 mM MgCl2, 5 mM Na butyrate, 0.1 mM dithiothreitol [DTT]). Nuclei were collected by centrifugation (10 min, 16,000 × g, 4°C) and resuspended in nuclear extraction buffer (15 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.4 M NaCl, 10% sucrose, 1 mM DTT). After 30 min on ice, insoluble proteins were removed from the nuclear extract by high-speed centrifugation (40 min, 100,000 × g, 4°C).
To isolate chromatin, cells were resuspended (4 × 107 cells/ml) in buffer A (10 mM HEPES, [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, 5 μg of aprotinin per ml, 5 μg of leupeptin per ml, 0.5 μg of pepstatin A per ml 0.1 mM phenylmethylsulfonyl fluoride). Triton X-100 (0.1%) was added, and the cells were incubated for 5 min on ice. Nuclei were collected in pellet 1 (P1) by low-speed centrifugation (4 min, 1,300 × g, 4°C). The supernatant (S1) was further clarified by high-speed centrifugation (15 min, 20,000 × g, 4°C) to remove cell debris and insoluble aggregates. Nuclei were washed once in buffer A, and then lysed in buffer B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease inhibitors as described above). Insoluble chromatin was collected by centrifugation (4 min, 1,700 × g, 4°C), washed once in buffer B, and centrifuged again under the same conditions. The final chromatin pellet (P3) was resuspended in Laemmli buffer and sonicated for 15 s in a Tekmar CV26 sonicator using a microtip at 25% amplitude (see Fig. 3A). To release chromatin-bound proteins by nuclease treatment, cell nuclei (P1) were resuspended in buffer A plus 1 mM CaCl2 and 0.2 U of micrococcal nuclease (Sigma). After incubation at 37°C for 1 min, the nuclease reaction was stopped by the addition of 1 mM EGTA. Nuclei were collected by low-speed centrifugation and lysed according to the chromatin isolation protocol described above.
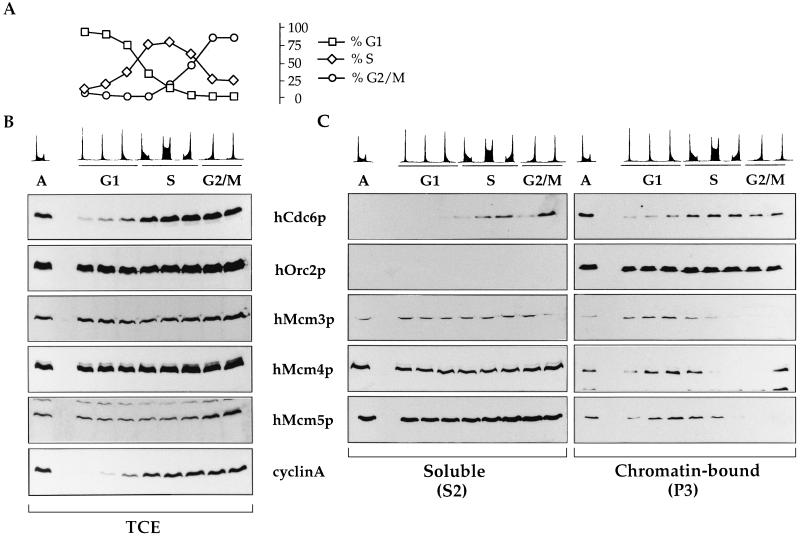
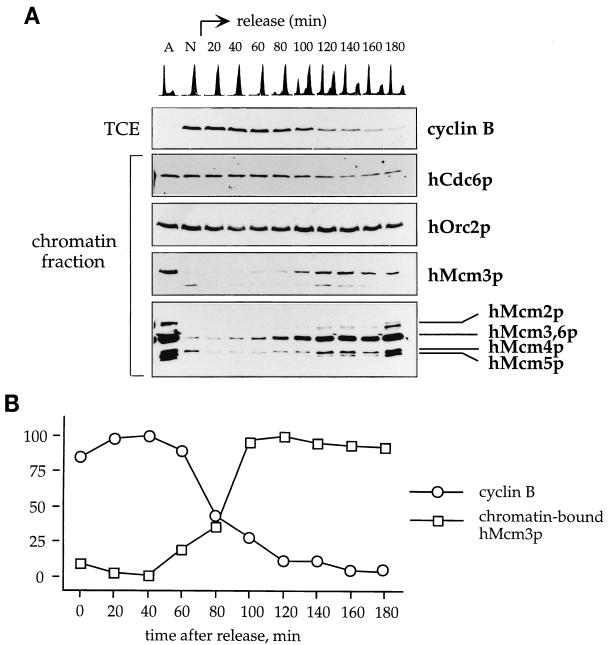
FIG. 3.
Protein levels and chromatin association of hCdc6p, hOrc2p, and hMcm proteins during the cell cycle. An asynchronous culture of human Raji cells was subjected to centrifugal elutriation to isolate cells at different points of the cell cycle. The DNA content for the cells in each fraction is shown. (A) Percentage of cells in each phase of the cell cycle, estimated with the CellFIT computer program. (B) Levels of initiator factors in total cell extracts (TCE). Equivalent amounts of each total cell extract (normalized by cell number) were subjected to SDS-PAGE and transferred to nitrocellulose for immunobloting with the indicated antibodies. The concentration of cyclin A is also shown as a control of cell cycle progression. Lane A, asynchronous cells. (C) Cell cycle-regulated chromatin association of hOrc2p, hCdc6p, and hMcm proteins. Cells at different points in the cell cycle were subjected to the biochemical fractionation described for Fig. 2A. Immunoblots of the soluble protein fraction (S2) or chromatin-enriched fraction (P3) are shown.
Immunoblots, immunoprecipitation, and immunofluorescence.
Standard protocols for immunoblots, immunoprecipitation, and immunofluorescence were used (19), with the following notations. Proteins were blotted to nitrocellulose membranes for 1 h at 0.5 A using the buffer described by Bolt and Mahoney (3) (40 mM Tris, 20 mM sodium acetate, 2 mM EDTA, 20% [vol/vol] methanol, 0.05% sodium dodecyl sulfate [SDS]). For immunofluorescence, HeLa cells growing on coverslips were fixed with 3% paraformaldehyde in PBS (pH 7.4) for 15 min at room temperature and permeabilized with 0.2% Triton X-100 in PBS for 5 min at 4°C. Cells were incubated with PBS containing 1% normal goat serum as a blocking agent for 30 min and then with anti-hMcm4p antibody (1:200 in PBS–1% normal goat serum) for 1 h. After being washed three times for 5 min with PBS, the cells were incubated with Texas Red-conjugated anti-rabbit IgG (from goat) for 1 h. DNA was stained with 1 μg of Hoechst stain per ml for 30 s.
Proteasome inhibition and in vivo ubiquitination assays.
HeLa cells (106) were treated for 12 h with protease inhibitor LLnL, LLM, MG132, or β-lactone or proteasome inhibitor I (PSI) (all inhibitors were from Calbiochem) at a concentration of 25 μM. Cells were then harvested and extracts were prepared exactly as described by Salghetti et al. (53). Ubiquitinated intermediates in human cells were detected using the His6-tagged-ubiquitin (His-Ubi) method of Trier et al. (61) as used by Salghetti et al. (53). HeLa cells (106) were transfected with 2 μg of pCGN.CSH.FL42 (which encodes HA-tagged hCdc6p), pCGN.Myc, or pCGN.TFIIB, either in the absence or in the presence of plasmid pMT107, which encodes His-Ubi (a gift from D. Bohmann, EMBL, Heidelberg, Germany). At 24 h posttransfection, 20 μM MG132 was added to the cells, when indicated. Cells were harvested at 36 h posttransfection, and His-tagged (therefore, ubiquitinated) proteins were purified on Ni-nitrilotriacetic acid–agarose and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). HA-tagged proteins were detected by immunoblotting with anti-HA (12CA5) antibody.
RESULTS
Generation and characterization of monoclonal anti-hCdc6p antibodies.
To study the regulation of CDC6 in mammalian cells, monoclonal antibodies against full-length recombinant hCdc6p were raised (see Materials and Methods). Two monoclonal antibodies (hCdc6-26 and hCdc6-37) were characterized in detail and used throughout this study. In immunoblots, both antibodies recognized a protein of approximately 62 kDa in protein extracts from human cells (Fig. 1A). In some experiments, a second band of slightly lower molecular mass was also detected, most likely corresponding to a modified form or a degradation product of hCdc6p. The specificities of both hCdc6-26 and hCdc6-37 in immunoblots were equal to or better than those of several anti-hCdc6p polyclonal sera tried (Fig. 1A and data not shown). The new antibodies efficiently recognized recombinant hCdc6p expressed in E. coli or baculovirus-infected insect cells (not shown).
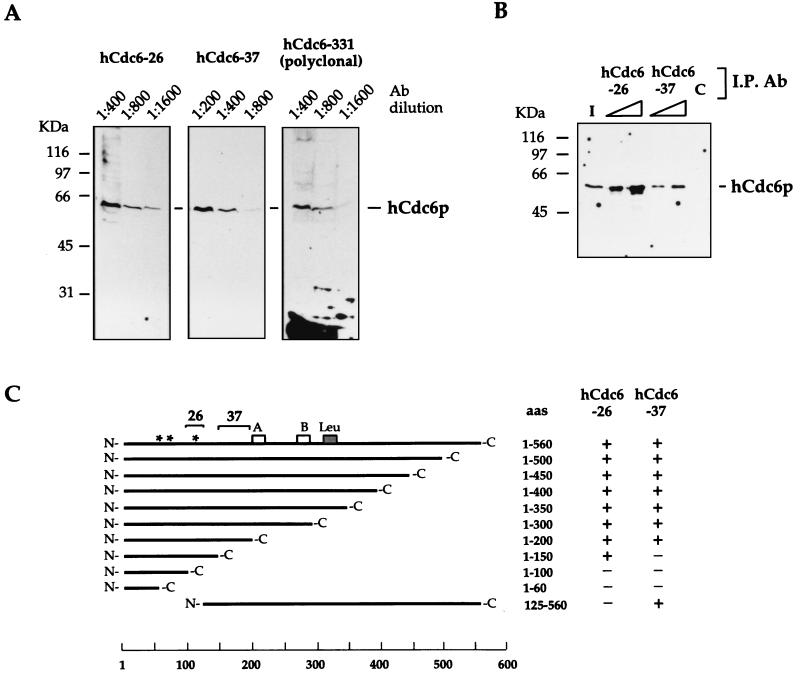
FIG. 1.
Specificities of new monoclonal anti-hCdc6p antibodies (Ab). (A) Nuclear extracts from asynchronous 293 cells were subjected to SDS-PAGE, and proteins were transferred to nitrocellulose. After protein staining with Ponceau-S red, individual lanes were cut and immunoblotted with the indicated dilution of monoclonal antibody hCdc6-26 or hCdc6-37 or a polyclonal antibody, anti-hCdc6p. A major signal corresponding to a 62-kDa protein was detected in all cases. (B) Immunoprecipitation (I.P.). Fifty microliters of nuclear extract from 293 cells (0.5 mg of total protein) was incubated for 1 h with 1 or 3 μl of ascitic fluid of the corresponding monoclonal antibody or with 3 μl of an unrelated control antibody (lane C). Immunocomplexes were purified with protein G-Sepharose 4B, subjected to SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-hCdc6p polyclonal antibodies. Lane I, 10% of the input sample. (C) Epitope mapping. Constructs expressing different C-terminal deletions and one N-terminal deletion of hCdc6p were made and expressed in E. coli as GST fusion proteins. Monoclonal antibodies hCdc6-26 and hCdc6-37 were used to detect the different truncated proteins by immunoblotting. The asterisks represent consensus sites for CDK phosphorylation. Boxes A and B indicate the position of Walker A and Walker B motifs, required for ATP binding and/or hydrolysis. The gray box marked Leu represents the hCdc6p leucine zipper. The regions that contain the epitopes recognized by hCdc6-26 and hCdc6-37 are indicated with brackets. aa, amino acid.
The antibodies also recognized hCdc6p in its native form, as shown by immunoprecipitation from human nuclear protein extracts: both hCdc6-26 and hCdc6-37, but not a control antibody, immunoprecipitated a 62-kDa protein, which was subsequently identified as hCdc6p by immunoblotting with a polyclonal antibody (Fig. 1B).
Monoclonal antibodies hCdc6-26 and hCdc6-37 recognize different epitopes in hCdc6p, as indicated by their ability to recognize in immunoblots a series of deletion derivatives of hCdc6p (Fig. 1C). The hCdc6-26 epitope is located between amino acids 100 and 125 of hCdc6p (possibly overlapping with Ser106, a known phosphorylation site [26]), and the hCdc6-37 epitope is located between amino acids 150 and 200. Within this region, amino acids 170 to 178 display the highest score for hydrophilicity and surface probability. This region does not contain any potential phosphorylation sites. The experiments included in this work have been performed with both monoclonal antibodies, and the results with hCdc6-37 are shown.
Association of hOrc2, hCdc6p, and hMcm proteins with chromatin.
After biochemical fractionation of human cells, hCdc6p was recovered preferentially in nuclear extracts and not in cytosolic extracts (65). We have investigated whether hCdc6p associates with chromatin, because in yeast and Xenopus, the presence of the ORC and Cdc6p on chromatin is a prerequisite for the subsequent loading of MCM proteins. A simple chromatin-binding assay was developed for this purpose (Fig. 2A) (see Materials and Methods for details). In brief, cells were lysed with Triton X-100 in a sucrose-rich buffer. Nuclei were collected by low-speed centrifugation, washed, and then lysed for 30 min in a no-salt buffer. A second centrifugation step separated remaining soluble nuclear proteins from an insoluble fraction. Proteins found in the final pellet were likely to be bound to chromatin or the nuclear matrix.
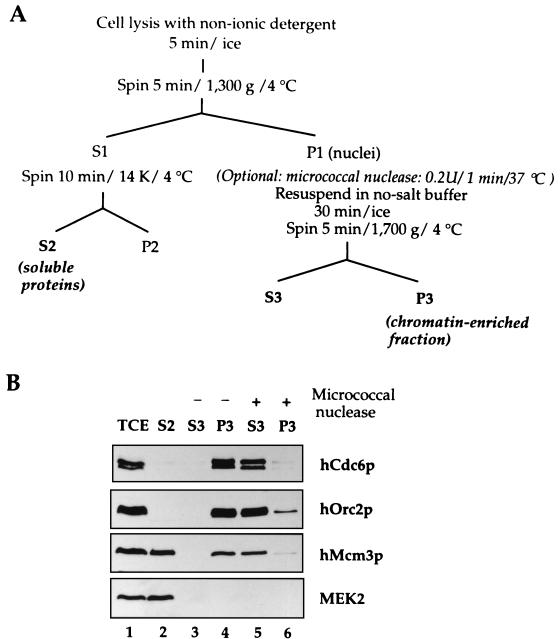
FIG. 2.
Chromatin binding of initiation proteins. (A) Scheme of the biochemical fractionation method. See Materials and Methods for details. (B) An asynchronous culture of Raji cells was subjected to the biochemical fractionation described in panel A. After cell lysis, the nuclei were divided in two aliquots. One of them was incubated for 1 min at 37°C with 0.2 U of micrococcal nuclease, and the other one was incubated in the same conditions without nuclease. After this treatment, nuclei were lysed and the solubilized nuclear proteins (S3) were separated from the chromatin-bound proteins (P3) by centrifugation. The distributions of different proteins in the total cell extract (TCE) soluble fraction (S2), solubilized nuclear proteins fraction (S3), and chromatin-nuclear matrix-bound fraction (P3) are shown.
The distribution of several proteins in the different fractions was tested after biochemical fractionation of an asynchronous cell culture of Raji cells (Fig. 2B). hOrc2p and hCdc6p were recovered preferentially in the chromatin-nuclear matrix fraction (P3). A small fraction of hCdc6p was detected in the soluble cytosolic (S2) and nucleoplasmic (S3) fractions. In contrast to hOrc2p and hCdc6p, hMcm3p was split between the soluble (S2) and chromatin-bound fractions. On the other hand, MEK2, a cytosolic kinase involved in signal transduction, was recovered exclusively in the S2 fraction, indicating that the extraction of cytosolic proteins after cell lysis was complete. The hOrc2, hCdc6, and hMcm3 proteins present in the final insoluble fraction were solubilized almost completely by treatment of the nuclei with micrococcal nuclease (see Fig. 4B, lanes 5 and 6), suggesting that they are associated with chromatin and not a nuclear matrix structure. This simple protocol resulted in good biochemical fractionation and could be used with samples containing as few as 2 × 106 to 5 × 106 cells, making it suitable for cell cycle analysis.
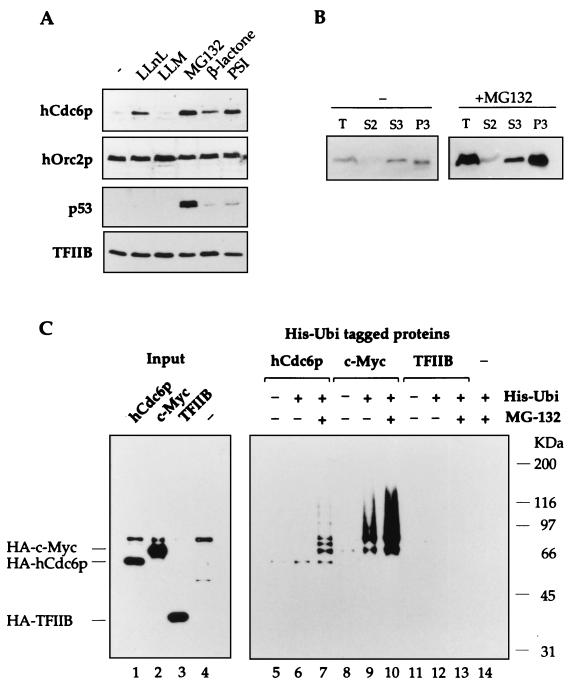
FIG. 4.
hCdc6p is targeted by ubiquitination for destruction by the proteasome. (A) Stabilization of hCdc6p by inhibitors of the proteasome. After treatment of HeLa cells with the indicated inhibitor, the cells were harvested and used to prepare total cell extracts. The steady-state levels of hCdc6p, as well as p53, increased in the presence of proteasome inhibitors but not in the presence of calpain inhibitor LLM. The levels of hOrc2p or TFIIB did not change significantly. (B) HeLa cells treated with dimethyl sulfoxide (control) or MG132 were subjected to the biochemical fractionation described for Fig. 2A. The levels of hCdc6p in the different fractions are shown. (C) In vivo polyubiquitination of hCdc6p. HeLa cells were transfected with plasmids expressing HA-tagged versions of hCdc6p, c-Myc, or TFIIB in the absence or in the presence of a plasmid that expresses His-Ubi. When indicated, 20 μM MG132 was added at 24 h posttransfection. Cells were harvested and lysed at 36 h after transfection. His-tagged proteins were purified and subjected to SDS-PAGE. After transfer to nitrocellulose, the samples were immunoblotted with anti-HA antibodies. Lanes 1 to 4 show 6% of the input protein.
Levels of hCdc6p fluctuate throughout the cell cycle.
To analyze the relative levels of hCdc6p throughout the cell cycle, logarithmically growing Raji cells were separated according to cell size by centrifugal elutriation. The advantage of this synchronization procedure is that cells which have never been forced to leave the cell cycle are separated without the use of drugs that interfere with normal cell metabolism (reviewed in reference 30). The DNA content of each elutriated fraction was determined by staining with propidium iodide, and the percentage of cells in each phase of the cell cycle was calculated (Fig. 3A). Immunoblot analyses of whole-cell extracts (Fig. 3B) showed that the levels of hCdc6p are much lower in the first elutriated fractions (corresponding to small, early-G1 cells) than during the rest of the cell cycle. In contrast, levels of other proteins involved in initiation of replication, such as hOrc2p or hMcm proteins, did not fluctuate during the cell cycle. Very similar results have been obtained after centrifugal elutriation of 293 or Manca cells (not shown). These data suggest that the levels of hCdc6p fluctuate throughout the mammalian cell cycle, but in contrast to yeast Cdc6p, most hCdc6p is degraded as cells progress through mitosis.
Association of hOrc2p, hCdc6p, and hMcm proteins with chromatin across the cell cycle.
An aliquot of each elutriated fraction was subjected to biochemical fractionation (Fig. 2A). The soluble protein fraction (S2) and chromatin-enriched fractions (P3) were tested for the presence of hOrc2p, hCdc6p, and hMcm proteins (Fig. 3C, left and right panels, respectively). The nucleoplasmic fractions (S3) contained very low amounts of protein and are not shown.
Virtually all hOrc2p was associated with chromatin throughout the entire cell cycle, the same as for its yeast counterpart. Interestingly, hCdc6p was also targeted to chromatin across the cell cycle. Soluble hCdc6p was also detected, but only during S phase and G2/M. This observation possibly reflects the fact that a fraction of hCdc6p is rapidly translocated to the cytosol after the G1/S transition, as has been proposed on the basis of immunostaining studies (26, 46, 52). The levels of soluble hCdc6p in this experiment seem higher than those detected in asynchronous cells. This can be explained because the later-elutriated fractions are enriched in late S and G2 cells, which contain soluble hCdc6p, whereas in the asynchronous population, most cells are in G1 or early S phase.
In contrast to the case for hOrc2p and hCdc6p, the association of hMcm3p, hMcm4p, and hMcm5p with chromatin was tightly regulated during the cell cycle. These proteins were detected on chromatin since early G1 and kept accumulating during G1 until the G1/S transition. All of them were then released as cells progressed through S phase. Some hMcm4p was detected in chromatin in the last elutriated fraction, suggesting that hMcm4p could reassociate with chromatin slightly before hMcm3p and hMcm5p, as has been reported recently for Xenopus (36). Very similar results were obtained with elutriated HeLa and Manca cells (not shown). These observations match other reports on the dynamics of chromatin association of hMcm proteins (11, 16, 21, 31, 51, 60) and further support the validity of the biochemical fractionation protocol that we used.
hCdc6p can be polyubiquitinated in vivo and is stabilized by the presence of proteasome inhibitors.
Considering the cell cycle fluctuation of hCdc6p levels described above, we have analyzed the possibility that hCdc6p could be a substrate for ubiquitin-mediated proteolysis. The mitotic degradation of hCdc6p via the 25S proteasome would explain why the protein is more abundant in G2 cells than in early G1.
Two types of experiments were done to address this issue. First, it was determined whether the inhibition of the proteasome with specific inhibitors would increase the steady-state levels of hCdc6p in proliferating cells. Incubation of HeLa cells with a variety of proteasome inhibitors (LLnL, MG132, β-lactone, and PSI) increased the level of hCdc6p between two- and ninefold, whereas incubation with LLM, a calpain inhibitor, did not stabilize hCdc6p (Fig. 4A). In contrast, inhibition of the proteasome did not affect hOrc2p levels. Stabilization of p53, a known substrate of ubiquitin-mediated proteolysis, was also observed as a positive control. As an additional negative control, the proteasome inhibitors did not affect the levels of TFIIB, a very stable transcription factor. Similar results were obtained with U2OS cells (not shown).
To test whether a specific subpopulation of hCdc6p was stabilized, control or MG132-treated HeLa cells were subjected to biochemical fractionation. In HeLa cells, nucleoplasmic (S3) hCdc6p was slightly more abundant than in Raji cells (compare Fig. 2B and 4B). Upon treatment with MG132, both the chromatin-associated and the soluble hCdc6p were strongly stabilized (Fig. 4B), suggesting that both populations of hCdc6p can be degraded by the proteasome.
In a second approach, hCdc6p was found to be polyubiquitinated in vivo. HeLa cells were cotransfected with a plasmid expressing HA-tagged hCdc6p and a plasmid expressing His-Ubi. At 36 h after transfection, the cells were harvested and lysed, and the ubiquitinated proteins were purified from the total protein extract by affinity chromatography (see Materials and Methods). Ubiquitinated molecules were detected after SDS-PAGE by immunoblotting with an antibody directed to the HA epitope. A plasmid expressing HA-cMyc, a known substrate of the proteasome (53), was used as a positive control, and a plasmid expressing HA-TFIIB was used as a negative control.
Comparable levels of HA-hCdc6p, HA-cMyc, or HA-TFIIB were detected 36 h after transfection (Fig. 4C, lanes 1 to 3). When cells were cotransfected with plasmids expressing HA-hCdc6p and His-Ubi, several products with higher apparent masses than HA-hCdc6p were detected, corresponding to polyubiquitinated forms of hCdc6p (lane 7). These products were detected only in the presence of His-Ubi and the proteasome inhibitor MG132 (compare lanes 5 to 7). The requirement for MG132 may reflect the extremely short half-life of hCdc6p once it has been polyubiquitinated. Lanes 8 to 10 show the polyubiquitination of c-Myc, which can be detected even in the absence of MG132. On the other hand, TFIIB was not ubiquitinated under these conditions (lanes 11 to 13).
hMcm proteins are loaded onto chromatin during late mitosis.
In the experiment shown in Fig. 3C, chromatin-bound hMcm proteins were detected even in early G1 cells, which is the stage at which hCdc6p is less abundant. The elutriation technique did not permit detailed investigation of the events that take place during mitosis and the M/G1 transition. To better define the hMcm loading step during this window of the cell cycle, a culture of HeLa cells was synchronized at prometaphase with nocodazole, and cells were collected at different time points after release from the block (Fig. 5A). For each sample, the cellular DNA content was analyzed by flow cytometry after propidium iodide staining. Comparison of the DNA profiles corresponding to the asynchronous (lane A) and the nocodazole-arrested (lane N) cells shows that the mitotic block was complete. After the culture was reseeded in fresh medium, the cells progressed through mitosis and started entering G1 at around 100 min postrelease. The top immunoblot in Fig. 5A shows the mitotic destruction of cyclin B, the activator subunit of the Cdc2 kinase. Quantitation of this blot (Fig. 5B) revealed that the total levels of cyclin B dropped to below 50% after 80 min and to around 10% after 120 min without nocodazole.
FIG. 5.
Chromatin association of hMcm proteins at the M/G1 transition. (A) HeLa cells were synchronized at early mitosis with nocodazole (see Materials and Methods), and a fraction of cells was collected at different time points after release from the block. An aliquot of the cells isolated at each time point was used to determine the DNA content by flow cytometry (top panel), and the rest were subjected to the biochemical fractionation described for Fig. 4A. The presence of hCdc6p, hOrc2p, and hMcm proteins in the chromatin-enriched fraction (P3) was analyzed. The top panel shows the progressive degradation of cyclin B in total cell extracts (TCE) as the cells progress through mitosis. Lane A, asynchronous culture; lane N, nocodazole-arrested cells. (B) Quantitation of total cyclin B and chromatin-bound hMcm3p in the experiment shown in panel A. The results are expressed as percentages of the maximum signal in each curve.
The chromatin-nuclear matrix fraction was isolated, and the presence of hOrc2p, hCdc6p, and hMcm proteins was analyzed by immunoblotting. hOrc2p and hCdc6p were detected on chromatin in all samples. Virtually no hMcm3 protein was associated with chromatin in the nocodazole-arrested cells (Fig. 5A, lane N). However, at 100 min after release from the block, almost 100% of the Mcm3p subpopulation that associates with chromatin had been loaded (see also Fig. 5B). A sharp increase in other chromatin-bound MCM proteins, such as hMcm2p and hMcm5p, was detected around the same time with anti-pan-Mcm, an antibody that cross-reacts with different members of the MCM protein family. This result has two implications: (i) despite the mitotic destruction of the bulk of cellular hCdc6p, a fraction of it remained stable on chromatin during mitosis, and (ii) even though hOrc2p and hCdc6p are present on chromatin during early mitosis, the bulk of hMcm proteins were not loaded until a later stage, concomitant with the destruction of cyclin B.
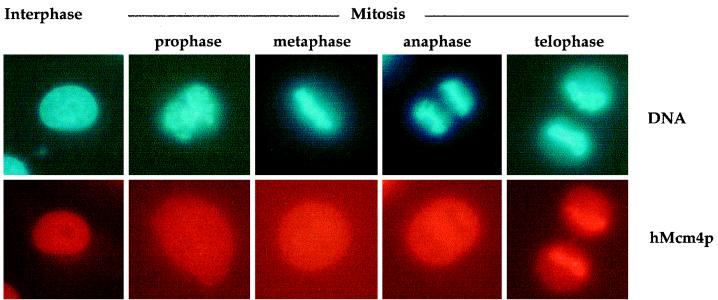
We complemented this observation by examining the subcellular distribution of endogenous hMcm4p protein in HeLa cells by indirect immunofluorescence (Fig. 6). In most interphase cells, a uniform nuclear staining was observed, as has been reported for other hMcm proteins (55, 56). In prophase cells, however, when the nuclear envelope broke down, hMcm4p was uniformly distributed in the cell. Interestingly, during metaphase or anaphase, no hMcm4p staining could be detected on the condensed chromosomes, whereas in telophase, hMcm4p was transported back into the new nuclei. A similar result was observed with the pan-Mcm antibody (not shown). This result (combined with those of Fig. 3 and 5) indicates that hMcm proteins start to be loaded on chromatin during late telophase and early G1, when the mitotic CDK activity was reduced by destruction of cyclin B but before cytokinesis was complete.
FIG. 6.
hMcm4p is loaded onto chromatin during late mitosis and early G1. The subcellular localization of hMcm4p was addressed by indirect immunofluorescence in HeLa cells. Cells were identified as interphasic or mitotic by direct observation of the chromatin condensation state, and the different stages of mitosis were determined by observation of the chromosome distribution. Representative photographs of each stage are shown. hMcm4p stains the nuclei of interphasic cells and the whole cell in prophase, metaphase, or anaphase. In the latter two phases, exclusion of staining in the chromatin is observed. In contrast, strong hMcm4p staining is detected in the chromatin of late telophase cells, which have not yet completed cytokinesis.
DISCUSSION
We have addressed the question of whether the model developed from studies with yeast to explain how replication origins are activated once and only once in each cell cycle is also valid for mammalian cells. According to this model, Cdc6p, Mcm proteins, and possibly other factors such as Cdt1 bind during early G1 to specific locations within the genome via interactions with the ORC. Several protein kinases, including CDKs and Dbf4p-Cdc7p, activate the pre-RCs, leading to entry into S phase. A good deal of evidence indicates that CDKs are also involved in the inhibition of rereplication during the G2 and M phases of the cell cycle, by exerting a negative control on the formation of functional pre-RCs (reviewed in reference 25).
We have analyzed the dynamics of chromatin association of several initiator proteins across the mammalian cell cycle in rapidly proliferating cells. By synchronizing the cultures with centrifugal elutriation instead of drugs, interference with the cellular metabolism was minimized. Moreover, we used new antibodies that detect the endogenous levels of these proteins, thus avoiding possible artifacts derived from protein overexpression. Our data support the notion that, albeit with some significant differences, the mechanisms that control initiation of DNA replication in yeast are conserved in human cells. The finding that hOrc2p remains bound to chromatin throughout the entire cell cycle suggests that the potential for the ORC to serve as the DNA-bound landing pad for other initiation proteins is conserved. Formation of pre-RCs, if they can be defined by the loading of hMcm proteins onto chromatin, starts at late mitosis and continues during early G1, a window of opportunity during which the CDKs are inactive (48). Our data show that hMcm proteins do not bind to chromatin even in the presence of chromatin-bound hOrc2p and hCdc6p, until cyclin B is degraded and therefore the mitotic kinase activity is reduced. From this moment on, hMcm proteins are present on chromatin until the onset of S phase and are progressively released afterwards. This alternation of hMcm proteins between soluble and chromatin-bound states is consistent with previous immunostaining and biochemical data obtained with several hMcm protein subunits (16, 21, 31, 51, 60). Our results also parallel the recent finding that the Chinese hamster Mcm2p starts to bind to both early- and late-replicating chromatin in late telophase, before the so-called origin decision point (66), and keeps accumulating during G1 in CHO-400 cells (11). Therefore, the timing of pre-RC formation is almost identical in different mammalian systems.
In contrast to the case for the ORC and the MCM proteins, the regulation of Cdc6p across the cell cycle is very different in proliferating yeast and mammalian cells. In both budding and fission yeasts, Cdc6 and Cdc18 proteins are degraded shortly after the G1/S transition. Expression of hCdc6 gene is E2F regulated (67), and the levels of hCdc6 mRNA peak at the G1/S transition (65). HCdc6p levels, however, are fairly constant in the cell cycle, with the exception of early G1 cells, in which the hCdc6p concentration is much lower. Therefore, most hCdc6p might be degraded during mitosis. We found that hCdc6p can indeed be polyubiquitinated in vivo and that inhibition of the proteasome results in increased steady-state levels of hCdc6p. These data combined indicate that hCdc6p is a bona fide substrate for ubiquitin-mediated proteolysis. The region of hCdc6p that targets it for degradation by the proteasome has been recently mapped to the N terminus of the protein (B. O. Petersen, C. Wagener, M. Melixetian, F. Marinori, E. L. Denchi, C. Matteucci, and K. Helin, personal communication).
Our study also addresses the issue of hCdc6p subcellular localization. Some groups have concluded that the protein is mostly nuclear in G1 but is translocated to the cytosol after the G1/S transition (26, 46, 52). This conclusion is mostly supported by experiments in which epitope-tagged versions of hCdc6p were introduced in human cells and the overexpressed proteins were detected by indirect immunofluorescence. The nuclear-cytoplasmic translocation seems to correlate with the phosphorylation of hCdc6p by cyclin A-Cdk2 (26, 46) and depends on exportin-1 (26). On the other hand, other reports indicate that endogenous hCdc6p is mostly nuclear (64, 65) and is actually associated with a nuclear structure (17).
Our present analysis with elutriated Raji cells revealed that a significant fraction of endogenous hCdc6p remained associated with chromatin from G1 until mitosis. It should be noted that this is compatible with the previously published observations, because the immunostaining technique cannot rule out the possibility that some hCdc6p remains in the nuclei during S and G2. Some soluble hCdc6p was detected after cells enter S phase, in agreement with the translocation model. Coverley et al. (8) recently reported a series of experiments performed with a cell-free system in which DNA replication was induced in isolated 3T3 cell nuclei by incubation with cytosolic extracts from the same cells in the presence of defined amounts of exogenous XCdc6p. The main conclusion from this study was that chromatin-bound Cdc6p persists in S and G2, while soluble Cdc6p is destroyed in a cyclin A-Cdk2 dependent process. This report is consistent with our own findings with endogenous hCdc6p and indicates that proteolysis of hCdc6p is likely another regulatory step in the mammalian cell cycle.
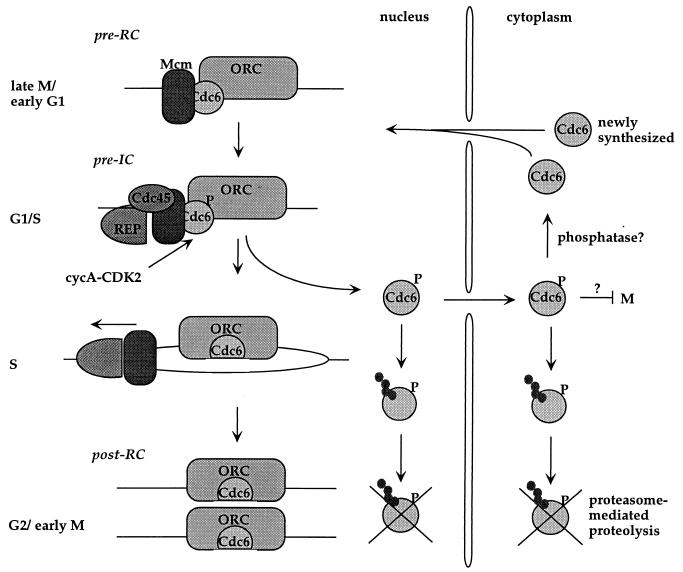
A model for hCdc6p regulation during the cell cycle that integrates the new data with all the previous observations is presented in Fig. 7. In late mitosis and early G1, chromatin-bound hORC and hCdc6p are required to load the hMcm proteins onto chromatin, forming the pre-RCs at origins of replication. Later, just before the origins fire, other factors are loaded to form the preinitiation complex. One of these factors is hCdc45p, a protein that in turn could recruit replisome proteins to the origins, as happens in yeast (69). When cyclin E-Cdk2 triggers S phase and cyclin A-Cdk2 becomes active, most hCdc6p is phosphorylated and detached from chromatin, and some is translocated to the cytosol. However, a fraction of hCdc6p remains associated with chromatin, perhaps because it is not accessible to the CDKs. A specific cellular mechanism might contribute to keep a fraction of hypophosphorylated hCdc6p, because hCdc6p has been found to associate with a novel regulatory subunit of protein phosphatase 2A (68). Nucleoplasmic and cytosolic hCdc6p are degraded by ubiquitin-mediated proteolysis.
FIG. 7.
A model for the regulation of hCdc6p in the cell cycle. See the text for details. IC, initiation complex.
Whether there is a role for cytosolic hCdc6p remains unknown, but it could send a signal that prevents mitosis until it is degraded by the 26S proteasome. The possibility of Cdc6p coordinating DNA replication and mitosis has been previously suggested for yeast (4, 28, 47), although the molecular mechanism of this mitotic inhibition has not been elucidated.
In our model, the post-RC in human cells would contain the hORC as well as hCdc6p. But interestingly, this chromatin-bound pool of hCdc6p did not recruit hMcm proteins onto chromatin during G2 phase, or during mitosis until cyclin B was degraded. Therefore, the negative control exerted by active CDKs on the formation of pre-RCs seems to be conserved. Consistent with this idea, inhibition of the CDKs permitted the MCM proteins to reload onto chromatin when mammalian G2 nuclei were incubated in S-phase Xenopus extracts (7). It is now clear that multiple mechanisms coexist to ensure the inhibition of pre-RC formation after S phase. These additional mechanisms likely include the regulated instability of the largest subunit of the hORC (41; H. Zou-Yang, H., J. Méndez, M. Hidaka, and B. Stillman, unpublished data) and geminin, a protein first identified in Xenopus that is destroyed as cells progress through mitosis (38).
What is the execution point of human Cdc6p?
Based on the evidence accumulated with yeast and Xenopus, it seems reasonable to assume that hCdc6p will be responsible for the recruitment of hMcm proteins onto ORC-bound chromatin at the M/G1 transition. However, if the sole function of hCdc6p was to load Mcm proteins onto pre-RCs during the M/G1 transition, it would be difficult to explain why phosphorylation of hCdc6p by cyclin A-Cdk2 at the G1/S transition seems to be essential for entry into S phase (26). This conclusion is based on the observation that overexpression of a nonphosphorylatable form of hCdc6p (bearing the triple mutation S54A/S74A/S114A) inhibits cellular DNA replication. This result is different from that of Petersen et al. (46), where no significant defect in DNA replication was observed after transfection or microinjection of the same hCdc6p mutant derivative. Interestingly, microinjection of mutant versions of hCdc6p which impair ATP binding and/or hydrolysis also results in G1/S arrest (20), reinforcing the hypothesis that hCdc6p has a function at the G1/S-phase transition. One simple explanation that we favor is that hCdc6p is required to load MCM proteins onto chromatin in late G1 phase, particularly in cells that are entering into the proliferation cycle from G0 phase or in cells that have an extended G1 phase. This situation would resemble that in S. cerevisiae cells undergoing a prolonged G1 phase (e.g., after treatment with α-factor), where there is a new peak of Cdc6p expression just before the G1/S transition. Therefore, we propose that hCdc6p is essential and rate limiting for initiation of DNA replication, as has been observed (35, 58)
Is there another function for the chromatin-bound hCdc6p during S phase and G2? Very recent data obtained with yeast indicate that Cdc6p cooperates with the ORC to determine origin specificity, in an ATP-dependent manner (39). It is tempting to speculate that the chromatin-bound form of hCdc6p could cooperate with the hORC to bind to specific replicator sequences. As the nature of such sequences in higher eukaryotes remains unknown, this may be an exciting topic for future research.
ACKNOWLEDGMENTS
We thank W. Tansey for his help with the proteasome inhibition and in vivo ubiquitination experiments, R. S. Williams for providing purified recombinant hCdc6p, C. Bautista for her work at the Cold Spring Harbor Laboratory monoclonal antibody facility, K. Brown for her help with epitope mapping of the anti-hCdc6p antibodies, A. Koff for his help with the elutriation protocol, N. Hernandez for anti-TFIIB antibodies, D. Bohmann for plasmid pMT107, A. Verreault for his suggestions on the fractionation protocol, K. Cronin for excellent technical assistance, and A. Losada and J. Chong for useful comments on the manuscript.
This work was supported by the National Cancer Institute (grant CA13106). J.M. was the recipient of postdoctoral fellowships from Fundación Ramón Areces (Spain) and the Human Frontier Science Program Organization.
REFERENCES
- 1.Aparicio O M, Weinstein D M, Bell S P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 2.Bell S P, Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- 3.Bolt M W, Mahoney P A. High-efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1997;247:185–192. doi: 10.1006/abio.1997.2061. [DOI] [PubMed] [Google Scholar]
- 4.Bueno A, Russell P. Dual functions of CDC6: a yeast protein required for DNA replication also inhibits nuclear division. EMBO J. 1992;11:2167–2176. doi: 10.1002/j.1460-2075.1992.tb05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong J P, Hayashi M K, Simon M N, Xu R M, Stillman B. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 2000;97:1530–1535. doi: 10.1073/pnas.030539597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman T R, Carpenter P B, Dunphy W G. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 7.Coverley D, Wilkinson H R, Madine M A, Mills A D, Laskey R A. Protein kinase inhibition in G2 causes mammalian Mcm proteins to reassociate with chromatin and restores ability to replicate. Exp Cell Res. 1998;238:63–69. doi: 10.1006/excr.1997.3829. [DOI] [PubMed] [Google Scholar]
- 8.Coverley D, Pelizon C, Trewick S, Laskey R A. Chromatin-bound Cdc6 persists in S and G2 phases in human cells, while soluble Cdc6 is destroyed in a cyclin A-Cdk2 dependent process. J Cell Sci. 2000;113:1929–1938. doi: 10.1242/jcs.113.11.1929. [DOI] [PubMed] [Google Scholar]
- 9.Dahmann C, Diffley J F, Nasmyth K A. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 10.Diffley J F, Cocker J H, Dowell S J, Rowley A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrova D S, Todorov I T, Melendy T, Gilbert D M. Mcm2, but not RPA, is a component of the mammalian early G1-phase prereplication complex. J Cell Biol. 1999;146:709–722. doi: 10.1083/jcb.146.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan S, Harwood J, Drury L S, Diffley J F. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drury L S, Perkins G, Diffley J F. The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 1997;16:5966–5976. doi: 10.1093/emboj/16.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drury L S, Perkins G, Diffley J F. The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Curr Biol. 2000;10:231–240. doi: 10.1016/s0960-9822(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 15.Dutta A, Bell S P. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Kiyono T, Hayashi Y, Ishibashi M. hCDC47, a human member of the MCM family. Dissociation of the nucleus-bound form during S phase. J Biol Chem. 1996;271:4349–4354. doi: 10.1074/jbc.271.8.4349. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Yamada C, Goto H, Yokoyama N, Kuzushima K, Inagaki M, Tsurumi T. Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human MCM complex, and CDC2 kinase-mediated hyperphosphorylation. J Biol Chem. 1999;274:25927–25932. doi: 10.1074/jbc.274.36.25927. [DOI] [PubMed] [Google Scholar]
- 18.Gavin K A, Hidaka M, Stillman B. Conserved initiator proteins in eukaryotes. Science. 1995;270:1667–1671. doi: 10.1126/science.270.5242.1667. [DOI] [PubMed] [Google Scholar]
- 19.Harlow E, Lane D. Using antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 20.Herbig U, Marlar C A, Fanning E. The Cdc6 nucleotide-binding site regulates its activity in DNA replication in human cells. Mol Biol Cell. 1999;10:2631–2645. doi: 10.1091/mbc.10.8.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holthoff H P, Baack M, Richter A, Ritzi M, Knippers R. Human protein MCM6 on HeLa cell chromatin. J Biol Chem. 1998;273:7320–7325. doi: 10.1074/jbc.273.13.7320. [DOI] [PubMed] [Google Scholar]
- 22.Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J Biol Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 23.Jallepalli P V, Kelly T J. Rum1 and Cdc18 link inhibition of cyclin-dependent kinase to the initiation of DNA replication in Schizosaccharomyces pombe. Genes Dev. 1996;10:541–552. doi: 10.1101/gad.10.5.541. [DOI] [PubMed] [Google Scholar]
- 24.Jallepalli P V, Brown G W, Muzi-Falconi M, Tien D, Kelly T J. Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev. 1997;11:2767–2779. doi: 10.1101/gad.11.21.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jallepalli P V, Kelly T J. Cyclin-dependent kinase and initiation at eukaryotic origins: a replication switch? Curr Opin Cell Biol. 1997;9:358–363. doi: 10.1016/s0955-0674(97)80008-7. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Wells N J, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Natl Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearsey S E, Labib K. MCM proteins: evolution, properties, and role in DNA replication. Biochim Biophys Acta. 1998;1398:113–136. doi: 10.1016/s0167-4781(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 28.Kelly T J, Martin G S, Forsburg S L, Stephen R J, Russo A, Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- 29.Kelman Z, Lee J K, Hurwitz J. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum DeltaH contains DNA helicase activity. Proc Natl Acad Sci USA. 1999;96:14783–14788. doi: 10.1073/pnas.96.26.14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krek W, DeCaprio J A. Cell synchronization. Methods Enzymol. 1995;254:114–124. doi: 10.1016/0076-6879(95)54009-1. [DOI] [PubMed] [Google Scholar]
- 31.Krude T, Musahl C, Laskey R A, Knippers R. Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J Cell Sci. 1996;109:309–318. doi: 10.1242/jcs.109.2.309. [DOI] [PubMed] [Google Scholar]
- 32.Labib K, Tercero J A, Diffley J F. Uninterrupted MCM2–7 function required for DNA replication fork progression. Science. 2000;288:1643–1647. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- 33.Liang C, Weinreich M, Stillman B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- 34.Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev. 1997;11:3375–3386. doi: 10.1101/gad.11.24.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madine M A, Swietlik M, Pelizon C, Romanowski P, Mills A D, Laskey R A. The roles of the MCM, ORC, and cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J Struct Biol. 2000;129:198–210. doi: 10.1006/jsbi.2000.4218. [DOI] [PubMed] [Google Scholar]
- 36.Maiorano D, Lemaître J M, Mechali M. Stepwise regulated chromatin assembly of MCM2–7 proteins. J Biol Chem. 2000;275:8426–8431. doi: 10.1074/jbc.275.12.8426. [DOI] [PubMed] [Google Scholar]
- 37.Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 38.McGarry T J, Kirschner M W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 39.Mizushima T, Takahashi N, Stillman B. Cdc6p modulates the structure and DNA binding activity of the origin recognition complex in vitro. Genes Dev. 2000;14:1631–1641. [PMC free article] [PubMed] [Google Scholar]
- 40.Muzi-Falconi M, Brown G W, Kelly T J. cdc18+ regulates initiation of DNA replication in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1996;93:1566–1570. doi: 10.1073/pnas.93.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natale D A, Li C-J, Sun W-H, DePamphilis M L. Selective instability of Orc1 protein accounts for the absence of functional origin recognition complexes during the M-G1 transition in mammals. EMBO J. 2000;19:2728–2738. doi: 10.1093/emboj/19.11.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuwald A F, Aravind L, Spouge J L, Koonin E V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 43.Nishitani H, Nurse P. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell. 1995;83:397–405. doi: 10.1016/0092-8674(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 44.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 45.Perkins G, Diffley J F. Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol Cell. 1998;2:23–32. doi: 10.1016/s1097-2765(00)80110-0. [DOI] [PubMed] [Google Scholar]
- 46.Petersen B O, Lukas J, Sorensen C S, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piatti S, Lengauer C, Nasmyth K. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a ‘reductional’ anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 1995;14:3788–3799. doi: 10.1002/j.1460-2075.1995.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piatti S, Bohm T, Cocker J H, Diffley J F, Nasmyth K. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- 49.Quintana D G, Hou Z, Thome K C, Hendricks M, Saha P, Dutta A. Identification of HsORC4, a member of the human origin of replication recognition complex. J Biol Chem. 1997;272:28247–28251. doi: 10.1074/jbc.272.45.28247. [DOI] [PubMed] [Google Scholar]
- 50.Quintana D G, Thome K C, Hou Z H, Ligon A H, Morton C C, Dutta A. ORC5L, a new member of the human origin recognition complex, is deleted in uterine leiomyomas and malignant myeloid diseases. J Biol Chem. 1998;273:27137–27145. doi: 10.1074/jbc.273.42.27137. [DOI] [PubMed] [Google Scholar]
- 51.Ritzi M, Baack M, Musahl C, Romanowski P, Laskey R A, Knippers R. Human minichromosome maintenance proteins and human origin recognition complex 2 protein on chromatin. J Biol Chem. 1998;273:24543–24549. doi: 10.1074/jbc.273.38.24543. [DOI] [PubMed] [Google Scholar]
- 52.Saha P, Chen J, Thome K C, Lawlis S J, Hou Z H, Hendricks M, Parvin J D, Dutta A. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol Cell Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salghetti S E, Kim S Y, Tansey W P. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 1999;18:717–726. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sánchez M, Calzada A, Bueno A. The Cdc6 protein is ubiquitinated in vivo for proteolysis in Saccharomyces cerevisiae. J Biol Chem. 1999;274:9092–9097. doi: 10.1074/jbc.274.13.9092. [DOI] [PubMed] [Google Scholar]
- 55.Schulte D, Burkhart R, Musahl C, Hu B, Schlatterer C, Hameister H, Knippers R. Expression, phosphorylation and nuclear localization of the human P1 protein, a homologue of the yeast Mcm 3 replication protein. J Cell Sci. 1995;108:1381–1389. doi: 10.1242/jcs.108.4.1381. [DOI] [PubMed] [Google Scholar]
- 56.Schulte D, Richter A, Burkhart R, Musahl C, Knippers R. Properties of the human nuclear protein p85Mcm. Expression, nuclear localization and interaction with other Mcm proteins. Eur J Biochem. 1996;235:144–151. doi: 10.1111/j.1432-1033.1996.00144.x. [DOI] [PubMed] [Google Scholar]
- 57.Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 58.Stoeber K, Mills A D, Kubota Y, Krude T, Romanowski P, Marheineke K, Laskey R A, Williams G H. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka T, Knapp D, Nasmyth K. Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi: 10.1016/s0092-8674(00)80526-7. [DOI] [PubMed] [Google Scholar]
- 60.Todorov I T, Attaran A, Kearsey S E. BM28, a human member of the MCM2–3-5 family, is displaced from chromatin during DNA replication. J Cell Biol. 1995;129:1433–1445. doi: 10.1083/jcb.129.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trier M, Staszewski L M, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 62.Tugal T, Zou-Yang X H, Gavin K, Pappin D, Canas B, Kobayashi R, Hunt T, Stillman B. The Orc4p and Orc5p subunits of the Xenopus and human origin recognition complex are related to Orc1p and Cdc6p. J Biol Chem. 1998;273:32421–32429. doi: 10.1074/jbc.273.49.32421. [DOI] [PubMed] [Google Scholar]
- 63.Weinreich M, Liang C, Stillman B. The Cdc6p nucleotide-binding motif is required for loading Mcm proteins onto chromatin. Proc Natl Acad Sci USA. 1999;96:441–446. doi: 10.1073/pnas.96.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams G H, Romanowski P, Morris L, Madine M, Mills A D, Stoeber K, Marr J, Laskey R A, Coleman N. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA. 1998;95:14932–14937. doi: 10.1073/pnas.95.25.14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams R S, Shohet R V, Stillman B. A human protein related to yeast Cdc6p. Proc Natl Acad Sci USA. 1997;94:142–147. doi: 10.1073/pnas.94.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J R, Gilbert D M. A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science. 1996;271:1270–1272. doi: 10.1126/science.271.5253.1270. [DOI] [PubMed] [Google Scholar]
- 67.Yan Z, DeGregori J, Shohet R, Leone G, Stillman B, Nevins J R, Williams R S. Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells. Proc Natl Acad Sci USA. 1998;95:3603–3608. doi: 10.1073/pnas.95.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan Z, Fedorov S A, Mumby M C, Williams R S. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol Cell Biol. 2000;20:1021–1029. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou L, Stillman B. Assembly of a complex containing Cdc45p, RPA, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol Cell Biol. 2000;20:3086–3096. doi: 10.1128/mcb.20.9.3086-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]