Abstract
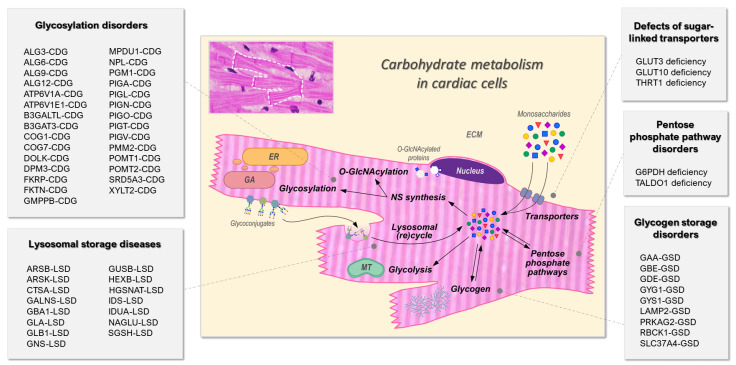
Heart failure (HF) is a progressive chronic disease that remains a primary cause of death worldwide, affecting over 64 million patients. HF can be caused by cardiomyopathies and congenital cardiac defects with monogenic etiology. The number of genes and monogenic disorders linked to development of cardiac defects is constantly growing and includes inherited metabolic disorders (IMDs). Several IMDs affecting various metabolic pathways have been reported presenting cardiomyopathies and cardiac defects. Considering the pivotal role of sugar metabolism in cardiac tissue, including energy production, nucleic acid synthesis and glycosylation, it is not surprising that an increasing number of IMDs linked to carbohydrate metabolism are described with cardiac manifestations. In this systematic review, we offer a comprehensive overview of IMDs linked to carbohydrate metabolism presenting that present with cardiomyopathies, arrhythmogenic disorders and/or structural cardiac defects. We identified 58 IMDs presenting with cardiac complications: 3 defects of sugar/sugar-linked transporters (GLUT3, GLUT10, THTR1); 2 disorders of the pentose phosphate pathway (G6PDH, TALDO); 9 diseases of glycogen metabolism (GAA, GBE1, GDE, GYG1, GYS1, LAMP2, RBCK1, PRKAG2, G6PT1); 29 congenital disorders of glycosylation (ALG3, ALG6, ALG9, ALG12, ATP6V1A, ATP6V1E1, B3GALTL, B3GAT3, COG1, COG7, DOLK, DPM3, FKRP, FKTN, GMPPB, MPDU1, NPL, PGM1, PIGA, PIGL, PIGN, PIGO, PIGT, PIGV, PMM2, POMT1, POMT2, SRD5A3, XYLT2); 15 carbohydrate-linked lysosomal storage diseases (CTSA, GBA1, GLA, GLB1, HEXB, IDUA, IDS, SGSH, NAGLU, HGSNAT, GNS, GALNS, ARSB, GUSB, ARSK). With this systematic review we aim to raise awareness about the cardiac presentations in carbohydrate-linked IMDs and draw attention to carbohydrate-linked pathogenic mechanisms that may underlie cardiac complications.
Keywords: heart failure, cardiomyopathies, arrhythmogenic disorders, congenital heart disease, inborn errors of metabolism, disorders of sugar transporters, glycogen storage disorders, disorders of pentose phosphate pathway, lysosomal storage disorders, congenital disorders of glycosylation
1. Introduction
Heart failure (HF) is a chronic, progressive condition in which heart functionality becomes heavily compromised and cannot sustain the oxygen demands of the body, ultimately resulting in death. Several studies have established that 40–50% of patients with HF die within 5 years from diagnosis [1]. It has been estimated to affect over 64 million people worldwide and the societal burden has been estimated at around $108 billion per year globally, representing an unacceptably high medical and societal burden [2].
The pathogenesis of HF is extremely complex, heterogeneous and multifactorial. HF can result from environmental factors (e.g., lifestyle), genetic predisposition, hereditary disorders or a combination of these three. Nonetheless, the most common causes of HF are cardiomyopathies (CMs) and congenital cardiac defects [1].
CMs represent a clinically heterogeneous group of disorders resulting in abnormal heart structure and functionality [3]. They are conventionally divided into familial (genetic, inherited) and non-familial (acquired) forms [4]. During the past three decades, a number of disease-causing genes of different CMs have been identified and further subdivided CMs based on their poly-, oligo- or mono-genic origin [3].
A relevant portion of genetic cardiomyopathies are monogenic. Considering the two most frequent forms of CMs, it has been estimated that over 60% of hypertrophic cardiomyopathies (HCMs) and 30–35% of dilated cardiomyopathies (DCMs) have monogenic etiology [5].
Among the CMs and structural heart defects arising from monogenic diseases, the number of those linked to genetic defects affecting proteins and enzymes linked to cellular metabolism has been steadily growing. Genetic disorders of the metabolism represent their own class of rare diseases, often referred to as inborn metabolic disorders (IMDs), or inborn errors of metabolism. To date, over 1000 IMDs have been described. They can be caused by inherited or de novo genetic mutations that disrupt one or more pathways of the cellular metabolism [6,7]. Although IMDs are very rare, taken collectively they affect 1 in 2500 births and account for 0.4% of all child deaths worldwide [8,9]. Cardiac manifestations in IMDs are largely variable in pattern and severity but encompass CMs, cardiac structural defects, arrhythmogenic disorders and can be the cause of HF [10]. Cardiac manifestations have been linked to genetic alteration of several pathways of cellular metabolism, including fatty acid oxidation, mitochondrial respiration, ion transporters and, relevant to the scope of this review, carbohydrate metabolism [10].
Carbohydrates play a central role in the physiology, development and metabolism of cardiac muscle tissue, or myocardium. Glucose, galactose, fructose and other sugars can serve as substrates for different metabolic pathways linked to cardiac tissue development, metabolism and functionality. First, carbohydrates contribute to the energy production in cardiac cells. Specifically, sugars represent the second most important energy source for the heart, covering 30–40% of the cardiac energy demand in the adult myocardium. Via glycolysis, glucose is transformed anaerobically into pyruvate while producing a net gain of two molecules of adenosine-5′-triphosphate (ATP). ATP provides readily releasable energy, stored in the bond between the second and third phosphate groups, to myosin heads in the contractile machinery of cardiac cells. The hydrolysis of ATP allows myosin to attach to actin filaments, starting a new contractile cycle, whose reiteration enables the pumping function of the myocardium. Second, carbohydrates can be used to store energy in the form of glycogen. Glycogen is a branched polymer of glucose molecules that constitutes an endogenous metabolic reserve able to grant up to 16 kilojoules (KJ) of energy per gram (g) oxidized, via the release of glucose into glycolysis [11]. Third, carbohydrates contribute to the biosynthesis of nucleotides and thus of DNA and RNA, by providing ribose 5-phosphate. The synthesis of this building block is enabled by the so called pentose phosphate pathway (PPP), which also produces nicotinamide adenine dinucleotide phosphate (NADPH) to use in reductive biosynthesis, such as that of fatty acids [12]. Lastly, carbohydrates can modulate protein and lipid maturation by fueling the synthesis of nucleotide sugars, such as UDP–glucose, UDP–galactose and GDP–mannose, which represent the precursors of essential post-translational modifications such as glycosylation and O-GlcNAcylation [13]. Glycosylation consists in the attachment of oligosaccharide chains, called glycans, to acceptor molecules, such as proteins and lipids, to enable their final configuration and functionality. In the context of the heart, glycoconjugates are mostly important for signal transduction, depolarization, and cell adhesion. O-GlcNAcylation, by contrast, indicates the process of attachment of N-acetylglucosamine onto serine or threonine residues of proteins and is responsible for the modulation of intracellular proteins, such as transcriptional factors [13].
This brief overview only roughly outlines the main key roles played by carbohydrates in the development and functionality of cardiac tissue. However, the knowledge of the exact molecular mechanisms by which these metabolic alterations generate cardiac defects remain mostly elusive and often understudied. The availability of more advanced cardiological clinical assays and the growing awareness of metabolic diseases have further widened the list of IMDs with cardiac involvement, but clinical information from clinical literature and case reports remains fragmented.
In this review, we offer a systematic and comprehensive overview of the different classes of monogenic carbohydrate-linked IMDs that present with cardiomyopathies, arrhythmogenic disorders and/or cardiac defects.
2. Method and Search Strategies
2.1. Definition of the Search Terms
To set up our systematic literature search, we needed to define the categorization systems for IMDs and for cardiac manifestations. Concerning IMDs, despite the lack of universal consensus when it comes to systematic classification, we chose the recently proposed International Classification of Inherited Metabolic Disorders (ICIMD) [7]. Of the 1450 disorders described by the ICIMD, we identified six categories of IMDs connected to carbohydrate metabolism and homeostasis: (1) disorders of sugar transporters; (2) disorders of fructose metabolism; (3) disorders of the pentose phosphate pathway; (4) disorders affecting glycogen metabolism; (5) disorders of glycosylation and galactose metabolism; (6) lysosomal storage diseases linked to carbohydrate homeostasis (Figure 1a).
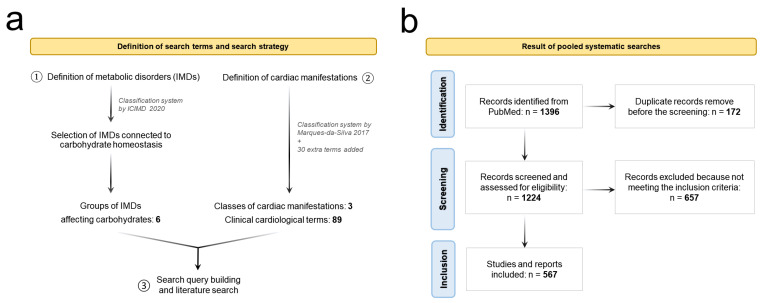
Figure 1.
PRISMA flow diagram of the systematic search strategy. (a) Definition of the terms used for the systematic search performed for each IMD [7,14]. (b) Pooled results of the systematic searches, including the total number of studies found, screened and included. The terms used to build the search queries are reported in Supplementary Table S1. The details of the separate systematic searches performed for each disorder (e.g., excluded and included articles) are reported in Supplementary Tables S2 and S3. For four disorders, G6PDH-, GAA-. GLA- and GALNS-deficiency, respectively, we selected the articles manually and not systematically due to the high volume of records identified (>300 articles/each).
Likewise, for the classification of the cardiac manifestations, we used as a base the system proposed in Marques-da-Silva et al. [14], which we further expanded by the addition of 33 extra terms. In total, our system includes 89 different cardiological clinical manifestations, divided into three categories: (1) arrhythmogenic disorders, (2) cardiomyopathies, and (3) structural cardiac and valvular defects (Figure 1a, Table 1).
Table 1.
Classification of cardiomyopathies, arrhythmogenic disorders and structural defects used in the systematic search.
| Arrhythmogenic Disorders | Cardiomyopathies | Cardiac and Valvular Defects | |||
|---|---|---|---|---|---|
| AT | Arrhythmia | ASH | Atrial septal hypertrophy | AD | Aortic dilation |
| AF | Atrial fibrillation | BVD | Biventricular dilation | AC | Aortic coarctation |
| BR | Bradycardia | BVH | Biventricular hypertrophy | AF | Atrial fibrosis |
| HF | Heart failure | CD | Cardiac dilation | AI | Aortic insufficiency |
| LQTS | Long-QT syndromes | CH | Cardiac hypertrophy | ARe | Aortic regurgitation |
| TC | Tachycardia | DI | Diastolic impairment (or dysfunction) | ASD | Atrial septal defects |
| VF | Ventricular fibrillation | DCM | Dilated cardiomyopathy | AVB | Atrioventricular block |
| HCM | Hypertrophic cardiomyopathy | AVD | Aortic valve dysplasia | ||
| HOCM | Hypertrophic obstructive cardiomyopathy | AVP | Atrial valve prolapse | ||
| HoRV | Hypoplastic right ventricle | AVS | Aortic valve stenosis | ||
| HoLV | Hypoplastic left ventricle | BAV | Bicuspid aortic valve | ||
| IC | Ischemic cardiomyopathy | BPV | Bicuscpid pulmonary valve | ||
| IOC | Iron overload cardiomyopathy | CMG | Cardiomegaly | ||
| LCDM | Dilated ventricular cardiomyopathy | CVD | Cardiac valve defects | ||
| LHCM | Left HCM | DAR | Dilated aortic root | ||
| LVHCM | Left ventricular HCM | DC | Dextrocardia | ||
| LVCO | Left ventricular cavity obliteration | DOV | Double outlet ventricle | ||
| LVD | Left ventricular dilation | EA | Ebstein anomaly | ||
| LVH | Left ventricular hypertrophy | ECD | Endocardial cushion defect | ||
| LVRWMA | Left ventricular regional wall motion abnormality * | HSS | Hypertrophic subaortic stenosis | ||
| NCM | Non-compaction cardiomyopathy | LAE | Left atrial enlargement | ||
| RAD | Right atrial dilation | LBBB | Left bundle branch block | ||
| RCM | Restrictive cardiomyopathy | LVCR | Left ventricular concentric remodeling | ||
| RHCM | Right HCM | MF | Myocardial fibrosis | ||
| RVD | Right ventricular dilation | MI | Mitral insuffiency | ||
| RVDe | Right ventricular defect | MVP | Mitral valve prolapse | ||
| RVH | Right ventricular hypertrophy | MVR | Mitral valve regurgitation | ||
| RVHCM | Right ventricular HCM | MVS | Mitral valve stenosis | ||
| SH | Septal hypertrophy | OA | Overriding aorta | ||
| SI | Systolic impairment (or dysfunction) | PDA | Patent (persistent) ductus arteriosus | ||
| VD | Ventricular dysfunction | PFO | Patent (persistent) foramen ovale | ||
| VEFR | Ventricular ejection fraction reduced | PMV | Parachute mitral valve | ||
| VH | Ventricular hypertrophy | PPS | Peripheral pulmonary stenosis | ||
| PVSD | Perimembranous ventricular septal defect | ||||
| RAE | Right atrial enlargement | ||||
| RBBB | Right bundle branch block (associated with structure) | ||||
| RDA | Right-descending aorta | ||||
| RHHS | Right hypoplastic heart syndrome | ||||
| SAI | Small aortic isthmus | ||||
| SD | Septal defect | ||||
| SCA | Single coronary artery | ||||
| TGA | Transposition of the great arteries | ||||
| TI | Tricuspid insufficiency | ||||
| ToF | Tetralogy of Fallot (VSD, aortic dextroposition, PPS, RVH) | ||||
| TVR | Tricuspid valve regurgitation | ||||
| TVS | Tricuspid valve stenosis | ||||
| VC | Valvular calcification | ||||
| VF | Ventricular fibrosis | ||||
| VSD | Ventricular septal defect | ||||
* Left ventricular regional wall motion abnormality is defined as a LV segment in which the systolic motion score is below normal.
2.2. Systematic Literature Search
A comprehensive literature search based on PRISMA guidelines [15] was conducted in February 2023 using PubMed (MeSH term-based search and free text search), in concert with the online databases Inborn Errors of Metabolism Knowledgebase (IEMbase) and Online Mendelian Inheritance in Man (OMIM) (Figure 1).
Each disorder included in the selected IMD groups was investigated in the databases mentioned hereinabove to identify articles and case reports reporting IMD patients displaying cardiac symptoms. The details on the search query building are reported in Supplementary Table S1. Articles were included only when presenting genetic diagnosis of the IMDs and clinical details on the cardiac manifestations in patients. Articles reporting experimental findings from animal models or in vitro experiments which did not mention any cardiac clinical data in patients were excluded. Articles not written in English were also excluded.
In cases where the same patients were mentioned across multiple studies, we counted them only once each to avoid bias in the estimation of the affected patients, but all linked articles were nevertheless reported.
3. Results
Our systematic search produced 567 included articles, which led to 58 IMDs reported with cardiac manifestations in patients (Figure 1), divided into 5 classes: 3 disorders affecting cytosolic sugar-linked transporters, 2 disorders of the pentose phosphate pathway, 9 diseases of glycogen metabolism, 29 congenital disorders of glycosylation and galactose metabolism and 15 carbohydrate-linked lysosomal storage diseases. For one of the selected carbohydrate-linked IMD groups, namely the disorders of fructose metabolism, no reports of patients displaying cardiac manifestations have been found.
3.1. Disorders of Plasma Membrane Transporters of Sugars and Linked Metabolites
Monosaccharides such as glucose, galactose and mannose are neutral hydrophilic molecules and cannot easily navigate through the lipophilic bilayer of cell membranes. Transport of monosaccharides, especially glucose, is the rate-limiting step in carbohydrate metabolism and energy homeostasis. Besides anchored proteins that function as hydrophilic pores that enable passive exchange between cytoplasm and ECM (unspecific transcellular transport), monosaccharides, can be absorbed by the cell via specialized transporters. Three classes of eukaryotic sugar transporters have been characterized, namely glucose transporters (GLUTs), sodium–glucose symporters (SGLTs) and the most recently discovered family, SWEETs [16]. SGLTs are sodium–sugar symporters, meaning that they transport glucose and other monosaccharides against their concentration gradient. Conversely, GLUTs and SWEETs are uniporters, which facilitate sugar transport along the sugar gradient. Interestingly, SGLT1 and SGLT2 have been associated with the development of diabetes-linked cardiac symptoms and SGLT2 inhibitors have been trialed in recent years as a preventive treatment for HF and other cardiovascular complications [17,18,19,20]. In the context of congenital metabolic disorders, out of the six disorders affecting carbohydrate transporters reported to date, three present with cardiac manifestations [21] (Table 2).
3.1.1. GLUT3 Deficiency
Mutations affecting the solute carrier family 2 member 3 (SLC2A3) gene, located on chromosome 12p13.31, have been linked to congenital syndromic heart defects, although the molecular mechanisms still remain elusive [22]. SLC2A3 encodes glucose transporter 3 (GLUT3), a membrane uniporter with a high affinity for glucose, which is predominantly expressed in the brain, pharyngeal arches and heart (highest expression detected in the left ventricular outflow tract during development) [22,23]. So far, a limited number of clinical reports have been published, but Ma et al. [22] reported 14 patients with SLC2A3 mutation, of which 6 presented with secundum ASD, right heart enlargement with pulmonary hypertension, TC and multi-hole PVSD, and one patient died due to heart disease (Table 2, Supplementary Tables S2 and S3).
3.1.2. GLUT10 Deficiency
Arterial tortuosity syndrome (ATORS) [OMIM:208050] is caused by an AR mutation in the SLC2A10 gene at location 20q13.12, resulting in GLUT10 deficiency. GLUT10 is critical for cardiovascular development by facilitating both TGFβ signaling and mitochondrial respiration [24]. We found 24 patients affected by ATORS and with confirmed genetic diagnoses of SLC2A10 mutations [25,26,27,28,29]. ATORS is characterized by tortuosity of the aorta and middle-sized arteries, elongation, stenosis and aneurysm formation in major arteries, leading to disrupted elastic fibers in the medial layers of arterial walls [24,25]. Other symptoms include joint laxity and defects of the connective tissue and one patient also reported stomach displacement and bilateral hip dislocation [26]. In our review, we identified four patients with ATORS presenting alongside cardiac symptoms, including cardiac murmur, severe kinking of the aortic arch and arteries, aggressive DAR and progressive RDA, MVP, CM, ICM and PPS [28,29,30] (Table 2, Supplementary Tables S2 and S3).
3.1.3. THTR1 Deficiency
Thiamine-responsive megaloblastic anemia (TRMA) syndrome [OMIM:249270] is an AR disorder of the thiamine (also known as vitamin B1) transporter. It is caused by homozygous mutations of the SLC19A2 gene on location 1q24.2, which encodes thiamine transporter type 1 (THTR1). Thiamine is important for the oxidation of carbohydrates, especially glucose. Upon its conversion to thiamine–pyrophosphate, thiamine acts as a coenzyme for three key enzymes involved in carbohydrate metabolism: transketolase (TK) in the pentose phosphate pathway (PPP), pyruvate dehydrogenase (PDH) in the terminal part of glycolysis and α-ketoglutarate dehydrogenase complex (α-KDH) in the tricarboxylic acid cycle. Moreover, thiamine is involved in reduced glutathione generation and thus contributes to the counteraction of oxidative stress [31]. We identified about 189 cases reported worldwide [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], mostly characterized by a triad of non-type 1 diabetes mellitus, sensorineural deafness, megaloblastic anemia, optic nerve atrophy, retinal dystrophy, short stature and complete reversal of positions of major thoracic and abdominal organs (situs inversus totalis) [33]. We identified 24 patients in whom cardiac manifestation have been observed and whose cardiac symptom spectrum encompasses the following: DC, AF, barely visible P waves, RBBB, wandering pacemaker, endocardial cushion defect, BVH, VSD, (secundum) ASD, atrial dysrhythmias, DCM, congestive HF, supraventricular TC, TI (including that which is secondary to Ebstein’s anomaly), EA, subendocardial ischemia and ST-T changes consistent with diaphragmatic wall myocardial infarction [32,33,34,35,36,37,38,39,40,41,42,43,45,46,47] (Table 2, Supplementary Tables S2 and S3).
Table 2.
Disorders of cytosolic transporters of sugars and sugar-linked metabolites presenting with cardiac manifestations (abbreviations described in Table 1).
| Affected Gene | Affected Protein | Inheritance | Heart Defects & Manifestations | No. Patients Identified by Our Search | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cardiomyopathies | Structural Defects | Arrhythmogenic Disorders | |||||
| SLC2A3 | Glucose transporter type 3 (GLUT3) | AR | - | ASD, PVSD | TC, HF | 6 | [22] |
| SLC2A10 | Glucose transporter type 10 (GLUT10) | AR | CM, ICM, RDA | DAR, MVP, PPS | - | 4 | [28,29,30] |
| SLC19A2 | THTR1 transporter | AR | BVH, DCM | DC, ECD, RBBB, VSD, ASD, EA, TI (Ebstein) | TC, AF, HF | 24 | [32,33,34,35,36,37,38,39,40,41,42,43,45,46,47] |
3.2. Disorders of the Pentose Phosphate Pathway
The pentose phosphate pathway (PPP), also known as the pentose phosphate shunt, is a ubiquitous pathway highly conserved in living organisms, which branches from glucose 6-phosphate. It is essential for the synthesis of ribose-5-phosphate, a precursor to amino acid and nucleic acid production and NADPH, a metabolite critical to reduction–oxidation (redox) balance and detoxification of intracellular radical oxygen species [48,49]. The PPP is divided into two biochemical branches: an oxidative branch and a non-oxidative branch [12]. The oxidative branch converts glucose 6-phosphate into ribulose-5-phosphate with generation of CO2 and NADPH. The non-oxidative branch yields glycolytic intermediates, such as fructose 6-phosphate and glyceraldehyde 3-phosphate and sedoheptulose sugars, which contribute to the production of sugar phosphate precursors to amino acid synthesis and ribose-5-phosphate [12]. Under healthy growth conditions, the PPP regulates cell cycle progression, myelin formation and the maintenance of the structure of many organs. In pathologic conditions, the PPP is defective and cells may adopt alternative metabolic pathways to generate NADPH that do not depend on the immediate supply of glucose and are largely mediated by the activation of AMPK [50]. Of the three reported inborn errors of the PPP in our research, two were reported in association with cardiac manifestations (Table 3).
3.2.1. TALDO Deficiency
Transaldolase deficiency (TALDOD) [OMIM:606003] is caused by AR-inherited homozygous or compound heterozygous mutations affecting the TALDO1 gene on chromosome 11p15.5. Transaldolase is involved in the irreversible, non-oxidative part of the PPP, where pentose phosphates are recycled into hexose phosphates in concerted action with transketolase [51,52,53]. Therefore, TALDOD leads to the accumulation of polyols derived from PPP intermediates: arabitol, ribitol and erythriol. To date, only ten patients from three unrelated families have been reported [52]. Clinical manifestations include hydrops foetalis with oligoaminos, dysmorphia, severe recurrent anemia, hepatosplenomegaly and variable liver, renal and cardiac involvement [54]. Specifically, cardiac symptoms arise in early infancy and may either gradually improve or lead to infantile death or adult cirrhosis [54]. Fewer than 50 cases of TALDOD have been reported to date, of which we identified 35 patients described with congenital heart disease, VSD and/or ASD, BAV, DC, AC, CM, LVH and RVH, or TVR [53,55,56,57] (Table 3, Supplementary Tables S2 and S3).
Table 3.
Disorders of the pentose phosphate pathway for which patients displaying cardiac manifestations have been reported (abbreviations described in Table 1).
| Affected Gene | Affected Protein | Inheritance | Heart Defects & Manifestations | No. Patients Identified by Our Search | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cardiomyopathies | Structural Defects | Arrhythmogenic Disorders | |||||
| TALDO | Transaldolase (TALDO) | AR | CM, LVH | VSD, ASD, BAV, DC, MVP, TR | HF | 35 | [53,55,56,57] |
| G6PDH | Glucose-6-phosphate dehydrogenase (G6PDH) |
XL | CD | PDA, PVSD, MVS, TR | HF | >300 | [58,59,60,61,62,63,64,65,66,67] (selected) * |
* For G6PDH deficiency our search produced over 300 entries (articles), thus only some meaningful articles were selected.
3.2.2. G6PDH Deficiency
Glucose-6-phosphate dehydrogenase (G6PDH) deficiency [OMIM:300908] is one of the most common forms of enzyme deficiency and although prevalence estimations vary in different studies, it is considered to affect over 400 million people worldwide [58]. It is a dominant X-linked (XL) disorder caused by mutations in the G6PDH gene at location Xq28. G6PDH is the rate-limiting step of the oxidative PPP and catalyzes the conversion of glucose 6-phosphate to 6-phosphoglucolacetone, accompanied by NADPH production. In G6PDH deficiency, the first, irreversible step of the PPP is compromised, with consequential decreased production of NADPH in the hexose monophosphate pathway. Molecular investigations in mice revealed that G6PDH and NADPH levels are involved in modulating myocardial contractility under physiological and pathophysiological conditions by interacting with L-type Ca2+ channel activity [59]. The lack of the G6PDH enzyme leads to hemolysis and, when compensation is not possible, anemia develops. Patients are mostly asymptomatic however when exposed to triggers (drugs, infectious diseases and fava bean consumption), they may develop life threatening acute hemolytic anemia. Our literature search produced several reports of single or few G6PH-deficient patients describing with cardiac symptoms, which included: CD, HF, TVR, severe MVS, PVSD, PDA, coronary artery disease and ST-segment elevation [58,60,61,62,63,64,65,66] (Table 3). More recently, studies on large patient cohorts have also been performed to investigate whether patients with G6PDH-deficiency present a higher predisposition to cardiovascular diseases and the results are still controversial. For instance, in the study from Dore et al. [67], 324 elderly G6PDH-deficient patients (out of 1123) have been confirmed to have cardiovascular defects, while Meloni et al. [68] found that cardiovascular defects are less frequently present among G6PDH-deficient patients than in controls (11.8% vs. 18.6%, respectively). Moreover, several studies suggest that patients’ age plays a role in the development of cardiovascular symptoms in G6PDH deficiency [67,69]. This evidence has also been observed in G6PDX mice, which showed higher susceptibility to age-associated cardiac hypertrophy and ventricular dilation in response to myocardial infarction or pressure overload-induced heart failure [69,70]. Definitive clinical studies in large populations are needed to determine the effects of G6PDH deficiency on the development of cardiovascular disease and subsequent outcomes.
3.3. Disorders of Glycogen Metabolism
Glycogen storage diseases (GSDs) are a group of IMDs which arise from congenital defects that affect glycogen synthesis (glycogenesis) or breakdown (glycogenolysis) primarily in hepatic and muscle tissues [71]. Glycogen is a multibranched polysaccharide that acts as a readily mobilized storage form of glucose mostly in liver, muscle and heart cells to different extents [11]. When systemic glucose levels are low, for example during fasting, starvation or intense physical exercise, glycogen is mobilized and broken down to yield glucose molecules that are used by the muscle and cardiac tissues or released from the liver into the bloodstream to support the energy demand of several tissues, while it is directly used in the muscle to support contraction [71]. In a healthy situation, this process is highly controlled and serves as a buffer to maintain blood glucose levels. Conversely, when genetic defects lead to the inability of the body to store or break down glycogen, resulting in very low blood glucose levels during fasting periods, those defects are indicated as GSDs. GSDs are mostly AR-inherited and have been divided into subtypes based on genotypic and phenotypic heterogeneity. The lack of activity in glycogen processing enzymes result in a wide range of clinical manifestations including hypoglycemia, hypotonia, muscle weakness, myoglobinuria, hyperlipidemia, elevated liver aminotransferases, elevated creatine kinase, CMG and other cardiac manifestations [71]. Of the 14 GSDs described to date, eight have been found to present with cardiac manifestations (Table 4).
3.3.1. GAA-GSD
Pompe disease is an AR GSD type IIa [OMIM:232300] with infantile onset, caused by acid maltase deficiency (AMD). AMD is due to a homozygous or compound heterozygous mutations in the GAA gene, localized on chromosome 17q25.3. Its prevalence is estimated at between 1/30,000–140,000 births worldwide, depending on the form [72]. GAA mutations result in the loss of function of the acid maltase enzyme and ultimately in intra-lysosomal accumulation [73]. Classically, GAA-GSD is classified in three forms. The infantile form (also known as “classic” Pompe disease) has neonatal onset and average life expectancy does not exceed one year of age. Clinically, it manifests with skeletal myopathy, muscle weakness, hepatomegaly and prominent CM and hypertrophic DCM that result in lethal cardiorespiratory failure [72,73,74]. The childhood/juvenile form (also known as “non-classical” infantile Pompe disease) has an onset at around six months of age and life expectancy longer than two years, as the clinical spectrum is similar to the infantile form but with milder cardiac involvement [72]. Lastly, the adult form has the mildest clinical presentation, mostly consisting of progressive proximal myopathy and usually without cardiac involvement [72].
Focusing on the non-classical form of Pompe disease, a comprehensive systematic review from van Kooten et al. [75] collected information on 750 Pompe patients and described several cardiac phenotypes reported significantly more frequently in these patients than in the normal population and these included DCM, HCM, sinus AT, AF, TC, VLD, prolonged QRS interval and QT interval, short PR interval, sick sinus syndrome, incomplete bundle branch block, LBBB, RBBB, LAE, AVB, LVH (in some cases with left ventricular outflow tract obstruction), VEFR, ventricular repolarization disorder, BVH, MVP and hypertension (Table 4).
Enzyme replacement therapy (ERT) is now a consolidated treatment for Pompe disease and can efficiently prevent deterioration of cardiac symptoms while partially helping to recover some functional dysfunction [73].
3.3.2. GBE-GSD
Andersen’s disease [OMIM:232500], or AR GSD type IV, is caused by mutations in the GBE1 gene at location 3p12, which encodes the glycogen branching enzyme (GBE, or 1,4-α-glucan branching enzyme type 1). This disorder alone accounts for 3% of all GSDs [76,77]. Loss of function of GBE translates to an accumulation of abnormal glycogen chains with fewer branch points, called polyglucosans. These polyglucosans aggregate in metabotoxic polyglucosan bodies that impair the function of organs and tissues, particularly the central neuromuscular system and liver. Clinical presentation of Andersen’s disease is vastly heterogenous. The most severe form presents as fetal perinatal neuromuscular disorder, which may include polyhydramnios causative of fetal akinesia deformation sequence that lead to arthrogryposis after birth [78]. After birth, patients develop severe muscular hypotonia and atrophy, along with cardiac symptoms often linked to deposition of amylopectin-like polysaccharides in the myocardium. Our systematic search produced 35 patients with GBE deficiency with cardiac involvement, including LVH, CMG, DCM or HCM, the latter two often reported as the cause of HF [76,77,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] (Table 4, Supplementary Tables S2 and S3). In most cases, patients do not survive past the neonatal period due to cardiorespiratory complications [102,103].
3.3.3. GDE-GSD
Cori–Forbes disease [OMIM:232400] is an AR glycogen storage disorder (type III) caused by homozygous or compound heterozygous mutations affecting the amylo-α-1,6-glucosidase/4-α-glucanotransferase (AGL) gene located on chromosome 1p21. This gene encodes the glycogen debrancher enzyme (GDE), which is involved in glycogen degradation. This enzyme has two independent catalytic activities that occur at different sites on the protein: a 4-α-glucotransferase activity and an amylo-1,6-glucosidase activity. When genetic mutations cause a loss of function of GDE, accumulation of abnormal glycogen with short outer chains occurs, which often cause hypertrophy in the affected tissues. GSDIII has been traditionally divided into two subtypes: in GSDIIIa, the most common, the enzymatic deficiency affects the liver, heart and skeletal muscle, while in GSDIIIb, only the liver is affected [104]. Focusing on GSDIIIa, our systematic search produced 204 patients with cardiac involvement, predominantly represented by DCM and HCM, but also RVH, LVH, LVD, SH and heart murmur [80,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146] (Table 4, Supplementary Tables S2 and S3).
3.3.4. GYG1-GSD
GSD type XV [OMIM:613507] is a rare AR disorder caused by compound heterozygous mutations in the glycogenin type 1 (GYG1) gene on chromosome 3q24. The prevalence of GSD type XV is estimated to be at lower than 1/1,000,000. Glycogenin 1 is a glycosyltransferase that catalyzes the synthesis of short glucose polymers, indicated as (1,4-α-D-glucosyl)n or polyglucosans, from UDP-glucose. These short polymers act as substrates for glycogen synthase and branching enzyme for the synthesis of new glycogen macro-polymers [102]. Clinical manifestations include profound glycogen depletion in skeletal muscle and abnormal accumulation in the heart. Seven patients have been reported with cardiac symptoms, including HCM, AT, VEFR, late-onset coronary artery disease and HF [147,148,149,150] (Table 4, Supplementary Tables S2 and S3). Endomyocardial biopsy often shows hypertrophic cardiomyocytes (suggestive of HCM) with enlarged nuclei, large centrally located vacuoles with periodic acid Schiff (PAS)-positive material and glycogen depletion in the cytoplasm [147,151].
3.3.5. GYS1-GSD
GSD type 0b [OMIM:611556] is an AR disorder caused by homozygous mutations in the muscle glycogen synthase type 1 (GYS1) gene, located at 19q13.33 and has an estimated prevalence of <1/1,000,000 [152,153]. These mutations cause loss of function of the GYS1 protein, which leads to a lack of glycogen biosynthesis in skeletal muscle and the heart. This results in symptoms such as severe syncope (following even moderate exercise), hypoglycemia, muscle pain, lethargy, loss of consciousness, arrythmias and CM [153]. Furthermore, GYS1 deficiency compromises the cardiac ability to pump blood and increases the risk of cardiac arrest and sudden death during exercise [152,153,154]. Our search identified four patients affected by GYS1 deficiency [152,153]. In 2007, Kolberg et al. reported 3 siblings from consanguineous parents who showed severe cardiac symptoms with exercise intolerance [152] (Table 4, Supplementary Tables S2 and S3). In detail, the eldest sibling developed tonic–clonic seizures during infancy and died of cardiac arrest at age 10.5 years as result of thickened LV and HCM. The second sibling also exhibited signs of HCM, abnormal heart rate and pressure. Cardiac examination in the youngest sibling revealed LAE and DI at rest [152].
3.3.6. LAMP2-GSD
GSD type II(b), more commonly known as Danon disease, [OMIM:300257] is a variant of Pompe disease with XLD inheritance, characterized by severe cardiac manifestations, along with skeletal and neurological symptoms, with estimated prevalence of <1/1,000,000. Genetically, it is caused by mutations of the LAMP2 gene, localized on chromosome Xq24, which encodes a lysosome-associated membrane protein-2. LAMP2 is a critical component of lysosomal membranes and plays a role in autophagosome–lysosome fusion, lysosome biogenesis and lysosomal membrane permeabilization (LMP) [155,156]. Its loss of function results in a wide spectrum of symptoms, including vacuolar cardioskeletal myopathy. Classically, the onset in males is around 10 years old, but occurs later in females and to date it has been identified in over 20 families [157]. Two specific LAMP2 mutations have been associated with prominent hypertrophy and electrophysiological abnormalities [157,158]. Our systematic review resulted in 200 Danon patients predominantly showing severe HCM (less often DCM), CMG, Wolf–Parkinson–White syndrome with complete AVB and LBBB, syncope, AF and LVH (with ventricular preexcitation), which often led to death or heart transplant [110,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192] (Table 4, Supplementary Tables S2 and S3).
3.3.7. PRKAG2-GSD
Glycogen glycogen-associated cardiomyopathy [OMIM:602743] is an autosomal dominant GSD caused by mutations in the protein kinase, AMP-activated, noncatalytic, gamma-2 (PRKAG2) gene on chromosome 7q36.1, which is associated with Wolff–Parkinson–White syndrome (WPW) [OMIM:194200]. The affected protein is a serine/threonine AMP-activated protein kinase (AMPK) that in response to activation by several cellular stressors induces increased AMP production and ATP catabolism [193]. In 2001, two families with severe HCM were identified as PRKAG2 mutation carriers. Molecular studies in these patients suggested that, since AMPK provides a central sensing mechanism that protects cells from ATP exhaustion, disruption of energy homeostasis could be a unifying pathogenic mechanism in all forms of HCM [194]. Overall, we found 103 clinically affected patients with cardiac involvement (and skeletal muscle glycogenosis and myopathy in some cases), who were investigated often along with family members who were carriers of PRKAG2 mutations [195,196,197,198,199,200,201,202,203,204,205]. Besides HCM due to defective glycogen storage, cardiac symptoms reported WPW (progressive dysfunction of the conduction system), conduction system degeneration (RBBB, LBBB, AVB), ventricular preexcitation, LVH, supraventricular TC, atrial premature beat, maximal left ventricular wall thickness stoke episodes and HF (Table 4, Supplementary Tables S2 and S3).
3.3.8. RBCK1-GSD
This congenital disorder, also referred to as polyglucosan body myopathy-1 (PGBM1) [OMIM:610924], is an AR GSD caused by mutations on the RANBP-type and C3HC4-type zinc finger-containing 1 (RBCK1) gene located on chromosome 20p13 [206,207]. The encoded protein is an E3 ubiquitin ligase complex responsible for adding head-to-tail polyubiquitin chains to substrate proteins and it plays a role in NFkB and JNK signaling pathways [208]. Although the molecular mechanisms remain mostly elusive, deficiency of this enzyme induces accumulations of polyglucosan in cardiac and muscle tissues (also known as amylopectinosis). This peculiar characteristic has suggested a link between this disease and GSDs. Of 22 patients reported, 18 have been reported with severe cardiac manifestations. The first report from 2012 described three patients that, among several other symptoms, all developed CM with congestive HF in early childhood [207]. Following studies collectively reported 16 patients who developed progressive DCM, which led to heart transplants in nine cases and to death due to heart failure in two patients [206,209,210,211] (Table 4, Supplementary Tables S2 and S3).
3.3.9. SLC37A4-GSD
Homozygous and heterozygous compound mutations of the SLC37A4 gene on chromosome 11q23.3 cause an AR disorder that compromises both glycogen metabolism and glycosylation and thus can be defined both as glycogen storage disease type Ib (GSDIb) and as congenital disorder of glycosylation type IIw (CDG2W) [OMIM:619525]. This gene encodes glucose 6-phosphate translocase type I (G6PT1), which is responsible for regulating the rate-limiting step of glucose 6-phosphate transport through the membrane of the ER. Hence, G6PT1 functions as a glucose 6-phosphate receptor/sensor in ATP-mediated calcium sequestration in the ER lumen [212]. Biochemically, deficiency of G6PT1 leads to excessive fat and glycogen in the liver, kidneys and intestinal mucosa and lactic acidosis and profound abnormalities in the N-glycosylation of serum specific proteins [213]. Clinically, this disorder is characterized by liver dysfunction and hepatomegaly, renomegaly, neutropenia, hypoglycemia and coagulation defects [213,214]. Unlike GSD type II and III, the heart is not primarily affected in this GSDIb. The most common cardiovascular abnormality in patients is systemic hypertension, which usually occurs in the context of renal disease. We identified four patients reported with diagnosed SLC37A4 deficiency and cardiac abnormalities, manifesting as ToF, VSD, PPS and RVH [213,214] (Table 4, Supplementary Tables S2 and S3).
Table 4.
Glycogen storage diseases for which patients displaying clinical cardiac manifestations have been reported (abbreviations described in Table 1).
| Affected Gene | Affected Protein | Inheritance | Heart Defects & Manifestations | No. Patients Identified by Our Search | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cardiomyopathies | Structural Defects | Arrhythmogenic Disorders | |||||
| GAA | α-1,4-glucosidase (GAA) | AR | DCM, HCM, LVH, VEFR, BVH | VLD, LAE, LBBB, RBBB, AVB, MVP | AT, AF, TC, HF, LQTS | >300 | [72,73,74,75] (selected) * |
| GBE1 | Glycogen branching enzyme (GBE1) |
AR | DCM, HCM, LVH | CMG | AT, HF | 35 | [76,77,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| AGL | Glycogen debranching enzyme (GDE) |
AR | DCM/HCM, LVH, RDH, LVD | SD | - | 204 | [80,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146] |
| GYG1 | Glycogenitype 1 (GYG1) | AR | HCM | - | VF, TC, HF | 7 | [147,148,149,150] |
| GYS1 | Muscle glycogen synthase | AR | HCM, LVH | LAE | HF | 4 | [152,153] |
| LAMP2 | Lysosome-associated membrane protein-2 (LAMP2) | XLD | DCM, HCM, CH | - | - | 200 | [110,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192] |
| PRKAG2 | AMP-activated protein kinase (AMPK) |
AD | HCM, LVH, DI | AVB, LBBB, RBBB |
HF, TC | 103 | [195,196,197,198,199,200,201,202,203,204,205] |
| RBCK1 | E3 Ubiquitin ligase | AR | DCM | - | HF | 16 | [206,209,210,211] |
| SCL37A4 | Glucose 6-phosphate translocase type I (G6PT1) | AD | RVH | ASD, VSD, ToF, PPS | HF | 4 | [213,214] |
* For GAA deficiency our search produced over 300 entries (articles), thus only some meaningful articles were selected.
3.4. Congenital Disorders of Glycosylation
Glycosylation is a complex post-translational modification that consists in the attachment of one or more chains made of monosaccharides onto acceptor molecules, such as proteins and lipids. The attachment of these carbohydrate chains allows the acceptor molecules to acquire their functional three-dimensional folding and final physicochemical properties, such as solubility. Glycoconjugates, like glycoproteins, are critical for cell recognition and adhesion, cell migration, protease resistance and many other biological functions [14]. Glycomics-based studies of cardiac cells, also known as cardiomyocytes, have suggested that glycosylation is a critical process for the regulation and modulation of cardiac structural development and functionality [14].
Congenital disorders of glycosylation (CDGs) represent a clinically and genetically heterogenous group of rare monogenic disorders affecting the synthesis, processing, attachment and degradation of glycans. CDGs consist of more than 160 disorders [215], of which we identified 29 that clinically present with cardiac manifestations in patients (Table 5).
3.4.1. Disorders Affecting N-Glycosylation
ALG3-CDG
Alpha-1,3-mannosyltransferase (ALG3) deficiency [OMIM:601110] is a CDG (type 1d) with AR inheritance and an estimated prevalence of <1/1,000,000 live births [216]. It is caused by a homozygous or compound heterozygous mutation in the ALG3 gene on chromosome 3q27, which encodes the enzyme that is responsible for the addition of the 6th mannose to the dolichol-linked oligosaccharide in the endoplasmic reticulum [217]. About 44 patients have been reported to date [218], with a broad phenotypical spectrum that mainly includes neurological, skeletal, gastrointestinal and urogenital symptoms [217]. We identified 15 patients described with cardiac symptoms, which collectively involved HOCM, RDA, TVR, VSD/AVD, MVS, PDA, PFO, PDA, truncus arteriosus type II, poor biventricular function and congenital heart disease [216,218,219,220] (Table 5, Supplementary Tables S2 and S3).
ALG6-CDG
Alpha-1,3-glucosyltransferase deficiency [OMIM:603147] is a hyper-rare CDG (type Ic) caused by the loss of function of the enzyme encoded by the ALG6 gene, located on chromosome 1p31. ALG6 enzyme, also called glucosyltransferase 1, catalyzes the addition of the first glucose residue to the growing lipid-linked oligosaccharide precursor of N-linked glycosylation. The CDG resulting from this deficiency manifests in muscular hypotonia, ataxia, motor developmental retardation and severe neurological involvement and more rarely retinal degeneration, deep vein thrombosis and pseudotumor cerebri [221]. However, one patient was reported with a novel mutation (c.482A>G; p.Y161C) and unusual presentation, including very mild neurological symptoms but the presence of DCM and LV dysfunction [221] (Table 5, Supplementary Tables S2 and S3).
ALG9-CDG
Alpha-1,2-mannosyltransferase (ALG9) deficiency [OMIM:607143] is a CDG (type Il) has a prevalence of <1/1,000,000 live births. It is caused by an AR loss-of-function mutation in the ALG9 gene on chromosome 11q23. The encoded protein, alpha-1,2-mannosyltransferase (ALG9), catalyzes the transfer of the 7th and 9th mannose residues to the growing lipid-linked glycan chains in the endoplasmic reticulum [7,14]. The overall clinical spectrum of this CDG includes progressive microcephaly, hypotonia, developmental delay, hepatomegaly and drug-resistant infantile epilepsy. Additional features may include skeletal dysplasia and pericardial effusion. A total of 19 patients have been reported to date [222], of which 12 have been described as displaying cardiac symptoms, including TVR, BAV, SAI, ASD, pericardial effusion, RVD and, in one case, severely reduced biventricular function and PDA [14,219,222,223,224,225,226] (Table 5, Supplementary Tables S2 and S3).
ALG12-CDG
Alpha-1,6-mannosyltransferase (ALG12) deficiency [OMIM:607143] is another very rare AR (CDG type Ig) caused by a loss-of-function mutation in the ALG12 gene on chromosome 22q13.33. The encoded protein is Dolichol-P-mannose: Man7GlcNAc2-PP-Dol-alpha-6-mannosyltransferase, responsible for the addition of the 8th mannose residue on the immature glycan chain Man7GlcNAc2-PP-Dol. This CDG is characterized by generalized hypotonia, feeding difficulties and facial dysmorphism, which can present along with skeletal anomalies, seizures, and cardiac anomalies in some cases [14,227,228,229]. To date, fewer than 15 cases have been reported in the literature, of which nine displayed HCM, PVSD, misalignment of the interventricular septum, deviation of the left ventricular outflow tract, PDA, VSD and AT [14,227,228,230] (Table 5, Supplementary Tables S2 and S3). One of these patients died before the age of two as a result of cardiorespiratory failure associated with CM [227].
GMPPB-CDG
GDP-mannose pyrophosphorylase B (GMPPB) deficiency [OMIM:615320] is an AR-inherited CDG caused by homozygous or compound heterozygous mutations in the GMPPB gene on chromosome 3p21.31. This gene encodes the beta subunit of the enzyme that catalyzes the conversion of mannose-1-phosphate and GTP to inorganic diphosphate and GDP-mannose (GDP-Man), which is an essential mannosyl-donor required for N-, O- and C-linked glycosylation [231]. Pathological reduction of GDP-Man manifests similarly to other dystroglycanopathies, with muscle phenotypes ranging from severe congenital muscular dystrophy to Limb–Girdle Muscular Dystrophy (LGMD) [232]. To date, fewer than 15 patients have been described with GMPPB deficiency and mostly displayed severe muscle phenotypes, hypotonia, microcephaly, epilepsy, strabismus, nystagmus and cataracts. Our search identified four patients reported with cardiac clinical features, specifically LVD, RBBB, sino-atrial block with atrial ectopics, aberrant ventricular conduction and cardiorespiratory compromise [232,233] (Table 5, Supplementary Tables S2 and S3). In addition, one patient was reported by Oestergaard et al. with VEFR; however, no more details on underlying cardiac causes were provided [234].
NPL-CDG
N-acetylneuraminate pyruvate lyase (NPL) deficiency [OMIM:611412] is a very rare CDG with AR inheritance due to compound heterozygous mutations resulting in the loss of function of the NPL gene. NPL enzyme, also known as sialic acid aldolase, regulates the cellular concentration of sialic acid by catalyzing SA conversion into N-acetylmannosamines (ManNAc) and pyruvate. Sialic acids are essential components of glycoproteins and glycolipids, which enable critical cellular processes. Sialic acid catabolism has been proven recently to be important for cardiac and skeletal muscle function and development [235]. In fact, NPL-CDG leads to NPL myopathy, exercise intolerance and elevated urinary sialic acid [235]. ManNAc and N-acetylmannosamine 6-phosphate (ManNAc-6P) were found to be significantly reduced in the cells of two siblings with compound heterozygous mutations in the NPL gene, pointing to affected NPL enzyme activity and defected SA catabolism [236]. Cardiac symptoms were observed in one of the patients, who developed fetal AT with hydrops and after birth was diagnosed with progressive DCM, LVH, VEFR and cardiac arrest [236] (Table 5, Supplementary Tables S2 and S3). Interestingly, in an NPL-knockdown zebrafish model, the cardiac phenotype was rescued with ManNAc, suggesting the possibility of monosaccharide replacement therapy in human patients [236].
PGM1-CDG
Genetic mutations affecting the phosphoglucomutase 1 (PGM1) gene located on chromosome 1p31.3 are causative of CDG type It [OMIM:614921], a rare disorder with AR inheritance and unknown prevalence in the population. PGM1 protein is an enzyme belonging to the phosphohexose mutase family. Although several PGM isoenzymes have been described in humans, PGM1 alone accounts for 90% of phosphoglucomutase activity in the body [237]. Specifically, PGM1 catalyzes the transfer of phosphate between position 1 and 6 of glucose. Glucose 6-phosphate flows mostly into glycolysis for energy production, while glucose 1-phosphate is the substrate for UDP-glucose synthesis, which is the building block of glycogen (PGM1-CDG is also considered a glycogen storage disorder) and of glycosylation [238]. PGM1-CDG is characterized by high clinical heterogeneity and multi-organ involvement, but the most frequently diagnosed symptoms are cleft palate/uvula, hepatopathy, growth delay, endocrine deficiency, exercise intolerance, myopathy (with or without rhabdomyolysis) and cardiac defects (which proved fatal in some cases) [239]. To date, about 60 patients with confirmed PGM1 deficiency have been reported [239,240,241,242,243]. Of these, 30 have been reported with cardiac involvement and at least six required heart transplantation [239,241,243]. Cardiac defects vary in type and severity and include: DCM (in some cases RCM), LVD, LVH, VSD, AC, MP, AT, AF, CMG, heart rhythm alterations and, in the most severe case, cardiac arrest [14,239,241,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257] (Table 5, Supplementary Tables S2 and S3). A potential molecular link between PGM1 and cardiac symptoms (particularly DCM) was proposed by Arimura et al. [258], who found that in stressed rat myocardium, PGM1 binds to an anchoring protein named Z-band alternatively spliced PDS-motif protein (ZASP, homolog of LDB3 in human) and suggested this to be a compensatory cardioprotective mechanism which would be compromised in PGM1-deficient patients.
PMM2-CDG
Phosphomannomutase 2 (PMM2) deficiency [OMIM:212065] is the most frequently diagnosed CDG. It has AR inheritance and its estimated incidence reaches up to 1/20,000 newborns and it affects over 800 patients worldwide [215,240]. It is caused by homozygous or heterozygous compound mutations in the PMM2 gene on chromosome 16p13. The affected PMM2 enzyme converts mannose-6-phosphate to mannose-1-phosphate, which is the immediate precursor of GDP-Mannose. PMM2-CDG can be classified into three forms: the infantile multisystemic form, the late infantile/childhood form presenting with ataxia and intellectual disability and the adult form that presents with stable disability. Clinically, PMM2 mutations cause psychomotor delay, seizures, cerebellar hypoplasia, coagulopathy and, in the most severe (yet rare) cases CM and other cardiac symptoms. Through our research, we found 70 PMM2-CDG patients described with HCM (more rarely DCM), ToF, ASD, PDA, PFO, pericarditis and pericardial effusion and truncus arteriosus, but also PPS, vascular ring anomaly based on a right aortic arch and aberrant left subclavian artery have also been reported [14,230,243,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284] (Table 5, Supplementary Tables S2 and S3).
3.4.2. Disorders Affecting O-Glycosylation
B3GALTL-CDG
Enzymatic deficiency of O-Fucose-specific β-1,3-N-glucosyltransferase (B3GALTL, sometimes referred to as B3GLCT or B3GTL) [OMIM:261540], also known as Peters plus syndrome, is a rare AR disorder that presents with syndromic developmental defects mostly affecting the eye. It is caused by mutations in the B3GALTL gene that encodes an O-Fucose-specific β-1,3-N-glucosyltransferase responsible for transferring glucose to fucose with a β-1,3 linkage, thus contributing to the elongation of O-linked fucosylglycans on thrombospondin type-1 repeats (TSRs) of several proteins [285]. It is characterized by a variable phenotype including Peters anomaly (corneal defects), short limbs, characteristic facial features, mild to severe mental delay and genitourinary system disorders [14]. Our search resulted in the identification of 19 patients displaying cardiac anomalies, predominantly ASD, VSD, HoLV and, in a few cases, cardiac murmur, absence of right pulmonary vein and BPV [14,286,287,288,289,290,291,292] (Table 5, Supplementary Tables S2 and S3).
B3GAT3-CDG
Beta-1,3-glucuronyltransferase 3 (B3GAT3) deficiency [OMIM:245600], also known as Larsen-like syndrome, is a rare AR and idiopathic CDG with a mutation in the B3GAT3 gene on chromosome 11q12.3 [14]. The affected beta-1,3-glucoronyltransferase 3 protein is a member of the glucoronyltransferase family, which exhibits strict acceptor specificity, recognizing nonreducing terminal sugars and their anomeric linkages—a critical step for proteoglycan synthesis. B3GAT3-CDG is characterized by skeletal dysplasia, multiple joint dislocations, joint laxity and other alterations of connective tissue, short stature, craniofacial dysmorphism, limb malformations, ocular defects, and cardiac symptoms [293]. Overall, we found 32 patients reported with this CDG, of which 15 exhibited variable cardiac presentations, including BAV, DAR, VSD, ASD, MVP, PDA and PPS [14,293,294,295,296,297,298,299,300,301] (Table 5, Supplementary Tables S2 and S3).
FKRP-CDG
Muscular dystrophy–dystroglycanopathy type C5 [OMIM:607155] (also called limb–girdle muscular dystrophy type 2I, or more recently type R9), muscular dystrophy–dystroglycanopathy type A5 (also known as Walker–Warburg syndrome) [OMIM:613153] and muscular dystrophy–dystroglycanopathy type B5 [OMIM:606612] are AR dystroglycanopathies caused by mutations in the fukutin-related protein gene (FKRP) on chromosome 19q13.32. The encoded protein, FKRP, is a ribitol-5-phosphate transferase involved in the functional glycosylation of α-dystroglycan (α-DG), which is a significant component in the link between the cytoskeleton and the extracellular matrix [302]. Mutations causing loss of function in FKRP lead to a broad spectrum of dystroglycanopathy-associated symptoms, primarily affecting the development of the muscles and heart, but also the brain and eyes. FKRP-linked disorders have been defined as the most severe forms of dystroglycanopathy and it has been suggested that a full loss of function of this protein results in embryonic lethality [303]. Our systematic search identified 220 FKRP-deficient patients with cardiac involvement [14,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329] (Supplementary Tables S2 and S3). Cardiac manifestations are common clinical symptoms of FKRP-CDG, are especially severe in patients with homozygous mutations and can cause dyspnea, peripheral edema and cardiac arrest. These symptoms include DCM, LVD, VEFR (also linked to reduced cardiac torsion), ventricular extrasystoles, RBBB leading to HF; less frequently, LBBB, TGA and valvular defects have also been reported (Table 5).
FKTN-CDG
Fukuyama congenital muscular dystrophy [OMIM:253800] is a rare AR CDG originating from mutations in the fukutin (FKTN) gene located on chromosome 9q31.2. The estimated prevalence of this disorder has been estimated in Japan at between 6–11/100,000 live births, while estimations for other countries are still not available. Fukutin is responsible for the addition of ribitol-5-phosphate to a special type of glycan named called α-dystroglycan, which is usually conjugated to membrane proteins and lipids. α-dystroglycans are essential for the maintenance of muscle integrity, cortical histogenesis and normal ocular development. Therefore, defects in the FKTN gene lead to a congenital progressive muscular dystrophy characterized by brain malformation, dystrophic changes in skeletal muscle, severe intellectual deficit, epilepsy, and motor impairment [14,330]. Other features with later onset include myopathic facial appearance, pseudohypertrophy of the calves and forearms and progressive cardiac involvement [330]. Cardiac symptoms have been more often reported during the second decade of life, especially in patients with subtypes caused by p.Q358P and p.R179T mutations, who experience DCM with minimal muscle weakness [14]. Our systematic search results in 77 patients with FKTN deficiency and cardiac manifestations, encompassing DCM, VD, DI, SI, AF, PFO, double subaortic ventricular defect, HoLV, PPS, MF and infundibular TGA (with no innominate vein) [14,185,330,331,332,333,334,335,336,337,338,339,340,341,342,343] (Table 5, Supplementary Tables S2 and S3).
POMT1-CDG
Muscular dystrophy–dystroglycanopathy type A1 [OMIM:236670] is another severe AR congenital muscular dystrophy with a prevalence of <1/1,000,000. The mutation is found in the POMT1 gene on chromosome 9q34.13. The protein encoded by this gene is O-mannosyltransferase 1 which catalyzes the first step in O-mannosyl glycan synthesis with attaching mannose to the serine or threonine residue of α-dystroglycans (α-DG) via O-glycosyl linkage [344]. O-mannosyltransferase 1 is localized in the ER and the structural role of α-DGs is in muscle fiber integrity, connecting the dystrophin–glycoprotein complex to the ECM. Defects in this enzyme result in reduced α-DG glycosylation in skeletal muscles, which leads to Walker–Warburg syndrome (WWS), congenital muscular dystrophy (CMD) and LGMD type 2 [345]. Symptoms usually include brain and eye malformations, severe mental disability, early death and varying degrees of severity [14]. In total, five patients have been described in the clinical literature as also displaying cardiac features. In the cohort of Pane et al. [321], only one POMT1-deficient patient was described with DCM, similar to the patient from Devisme et al. [346]. Instead, Bello et al. [345] reported four POMT1-deficient patients with wider cardiac manifestation: LVD, VEFR and LVH with moderate-to-severe SI (Table 5, Supplementary Tables S2 and S3).
POMT2-CDG
Muscular dystrophy–dystroglycanopathy type A2 [OMIM:613150] is a rare AR dystrophy that occurs as result of mutations in the O-mannosyltransferase 2 (POMT2) gene on chromosome 14q24.3. This gene encodes a protein called O-mannosyltransferase 2, which requires the activity of its homolog, POMT1, to initiate O-mannosylglycan synthesis in the endoplasmic reticulum, making it essential for the glycosylation of α-DGs [347]. Clinically, POMT2 deficiency is often associated with a wide range of clinical involvement, ranging from severe muscle–eye–brain disease and Walker–Warburg syndrome to limb–girdle muscular dystrophy without structural brain or ocular involvement. Although cardiovascular anomalies are thought to be uncommon in congenital muscular dystrophy, we identified seven patients reported with aortopathy (ascending aorta, AD and DAR, dilation of the annulus and sinotubular junction), reduced LV systolic function, LVH (non-progressive) and DCM [14,321,346,348,349] (Table 5, Supplementary Tables S2 and S3).
XYLT2-CDG
Spondyloocular syndrome (SOS) [OMIM:605822] is a rare AR CDG caused by mutations in the XYLT2 gene located on chromosome 17q21.33. The affected protein, xylosyltransferase 2 (XYLT2), catalyzes the first step in the biosynthesis of chondroitin sulfate, heparan sulfate and dermatan sulfate proteoglycans. Proteoglycans are present in almost all ECMs of connective tissues and derive their major biochemical function from the physiochemical characteristics of the glycosaminoglycan component of the molecule, which provide hydration and swelling pressure to the tissue to withstand compressional forces [350,351]. Characteristic symptoms of SOS are bone fractures, cataracts, hearing loss, retinal detachment, and neurological defects. To date, only 22 patients have been reported [352]. Of these, three patients from two unrelated families were diagnosed with bone fragility, learning disabilities and cardiac symptoms, including ASD, MVP, AVD and mild MI [14,353,354] (Table 5, Supplementary Tables S2 and S3).
3.4.3. Dolichol-Phosphate Synthesis Defects
DOLK-CDG
Dolichol kinase 1 (DOLK, or DK1) deficiency [OMIM:610768] is an AR CDG caused by homozygous or heterozygous compound mutations in the DOLK gene on chromosome 9q34.11. Its prevalence is estimated as <1/1,000,000 live births. DOLK is one of the enzymes involved in the de novo biosynthesis of dolichol phosphate mannose (Dol-P-Man). Dol-P-Man is an essential glycosyl carrier lipid for C- and O-mannosylation and N- and O-linked glycosylation of proteins and for the biosynthesis of glycosyl phosphatidylinositol anchors in the ER. Loss of function of this kinase clinically manifests with muscular hypotonia, ichthyosis, nervous system symptoms, and cardiac defects, predominantly CMs [355]. In most cases, patients required heart transplant or died due to heart complications. In total, 26 patients with DOLK-CDG with different cardiac manifestations were described in the literature: DCM and more rarely HCM, severe BVD, mild/severe CD, LVD, HF (acute congestive), non-sustained ventricular TC, BR, AT, CMG with right deviation of the heart, PDA, VSD, myocyte hypertrophy and interstitial fibrosis [14,227,355,356,357,358,359,360] (Table 5, Supplementary Tables S2 and S3).
DPM3-CDG
Limb–girdle muscular dystrophy–dystroglycanopathy type C15 (MDDGC15) [OMIM:612937] is an AR CDG caused by mutation on the dolichol-phosphate mannose synthase subunits 3 (DPM3) gene on chromosome 1q22. DPM3, together with DPM1 and DPM2, forms the DPM complex, which is responsible for the production of mannosyl donors for glycosylphosphatidylinositols, N-glycan synthesis, and protein O-/C-mannosylation [361]. Loss of function due to DPM3 gene mutations clinically results in a rare type of limb–girdle muscular dystrophy–dystroglycanopathy, presenting with progressive proximal muscle weakness and DCM. Out of the 11 patients reported to date, four were described with DCM as the most predominant cardiac symptom and less frequently with mild LVD and LVRWMA [361,362,363] (Table 5, Supplementary Tables S2 and S3).
MPDU1-CDG
Mutations in the mannose-phosphate-dolichol utilization defect 1 (MPDU1) gene lead to a very rare AR CDG (type If). This gene, located on chromosome 17p13, encodes an ER membrane protein essential for the flipping of DPM and dolichol-phosphate-glucose (DPG) across the ER membrane and for the regulation of DPM and DPG within the ER lumen [364]. When defective, the synthesis of glycosylphosphatidylinositols and of lipid-linked oligosaccharides (LLO) is compromised, the latter resulting in the lack of complete N-glycans. MPDU1-CDG patients are mostly characterized by epilepsy, psychomotor retardation and skin abnormalities. Furthermore, four MDPU1-CDG patients out of six found in the literature shown either DCM or NCM [364,365,366] (Table 5, Supplementary Tables S2 and S3). In addition, the infantile patient described by Thiel et al. [365] had an older brother, who suffered from an undefined neonatal-onset disease with facial dysmorphism, skin ichthyosis and cardiac malformations (including truncus arteriosus communis) that died during neonatal cardiac surgery.
SRD5A3-CDG
Dolichol Steroid 5 α-reductase 3 (SRD5A3) deficiency [OMIM:612379] is a rare X-linked CDG resulting from mutations in the SRD5A3 gene on chromosome 4q12. Loss of function of SRD5A3 enzyme compromises the conversion of polyprenol into dolichol, a necessary substrate for the beginning of multiple glycosylation processes [367]. Phenotypes in SRD5A3-CDG are highly variable and include ocular anomalies, mental retardation, cerebellar malformations and coagulation defects. Although cardiac involvement is sporadic, our search identified seven patients exhibiting heart symptoms, specifically CM, palpitations, AT, (secundum) ASD, LQTS, TGA and PFO [14,368,369,370,371] (Table 5, Supplementary Tables S2 and S3).
3.4.4. Glycosylphosphatidylinositol (GPI)-Anchor Biosynthesis Defects
PIGA-CDG
Multiple congenital anomalies–hypotonia–seizures syndrome-2 (MCAHS2) [OMIM: 300868], caused by mutations on the phosphatidylinositol-glycan-anchor biosynthesis class A (PIGA) gene, is an idiopathic X-linked recessive (XL) neurodevelopmental disorder. Mutations in the PIGA gene on chromosome Xp22.2 2 lead to the loss of function of the PIGA protein, which participates in the synthesis of N-acetylglucosaminyl phosphatidylinositol on the ER membrane. This reaction represents the first step in the GPI anchor synthesis, and when deficient, it results in defective glycan synthesis [372]. PIGA-CDG has been identified in over 100 patients and is mainly characterized by dysmorphic features, neonatal hypotonia, early-onset myoclonic seizures and variable congenital anomalies affecting the urinary system and central nervous system [14,372,373,374]. Via our search, we identified 19 patients reported with a wide range of cardiac clinical features, namely ASD, CM, AR, PFO, atrial septal aneurysm, BAV, mildly AD (ascending), and first-degree AVB; in some cases these symptoms became the primary cause of spontaneous death due to cardiac arrest, especially in childhood [372,373,374,375,376,377] (Table 5, Supplementary Tables S2 and S3).
PIGL-CDG
Coloboma–congenital heart disease–ichthyosiform dermatitis–mental retardation–ear anomalies (CHIME) syndrome [OMIM:280000] is an AR CDG resulting from mutations in the phosphatidylinositol glycan anchor biosynthesis class L (PIGL) gene on chromosome 17p11.2. The loss of function of PIGL enzyme disrupts the second step of GPI synthesis in the ER, namely the de-N-acetylation of the N-acetylglucosaminylphosphatidylinositol (GlcNAc-PI), which results in the disruption of glycan synthesis and unoccupied glycosylation sites on proteins and other molecules. PIGL-CDG presents as a multisystemic disorder clinically characterized by colobomas, migratory ichthyosiform dermatosis, mental retardation, ear anomalies and congenital heart defects. Concerning the latter, to date eight patients have been reported with cardiac manifestations, ranging from TGA, HSS and ToF to VSD with pulmonary hypertension, DOV, PPS and systolic murmur [14,378,379,380,381] (Table 5, Supplementary Tables S2 and S3).
PIGN-CDG
Multiple congenital anomalies–hypotonia–seizures syndrome 1 (MCAHS1) [OMIM:614080] is an AR CDG due to mutations in the phosphatidylinositol glycan anchor biosynthesis class N (PIGN) gene. This gene encodes the GPI ethanolamine phosphate tranferase-1, which is also involved in GPI-anchor synthesis on the ER membrane. The backbone of GPI synthesis is assembled by the coordinated addition of sugar and phosphoethanolamine (EtNP) components to phosphatidylinositol. The disruption of this reaction leads to neonatal hypotonia, seizures, multiple congenital anomalies, and often premature death [14]. A total of 18 patients have been reported with heart defects in the form of VSD/ASD, PVSD, DC, LV noncompaction (NCM), PDA, ToF, PPS, PFO, RVD, DAR, OA and reduced left ventricular inotropy [14,382,383,384,385,386,387,388] (Table 5, Supplementary Tables S2 and S3).
PIGT-CDG
Multiple congenital anomalies–hypotonia–seizures syndrome 3 (MCAHS3) [OMIM:615398] is an AR CDG that originates from homozygous mutations on chromosome 20q13.12 in the phosphatidylinositol-glycan biosynthesis class T (PIGT) gene. The encoded enzyme is a subunit of a heteropentameric transamidase complex involved in the attachment of proteins to the GPI anchor, which functions as a plasma membrane anchor for extracellular proteins [389,390]. Fewer than 30 patients have been reported as having this disease, which is mostly characterized as manifesting with hypotonia, delayed psychomotor development, seizures, dysmorphic facial features, decreased serum alkaline phosphatase, kidney defects and skeletal abnormalities. Kvarnung et al. reported three patients belonging to a consanguineous family with PIGT-CDG manifesting the cardiac symptoms RCM, minor PDA and cardiac disease [389]. Later reports described five other patients with heart involvement, which further widened the cardiac symptom spectrum to other heart defects such as PFO and an atrial septal aneurysm [391,392] (Table 5, Supplementary Tables S2 and S3).
PIGV-CDG & PIGO-CDG
Homozygous and compound heterozygous mutations affecting the genes encoding phosphatidylinositol glycan (GPI) anchor biosynthetic enzymes class V (PIGV, located on chromosome 1p36.11) and class O (PIGO, located on 9p13.3) cause two very rare CDGs that manifest with facial dysmorphism, skin abnormalities, mental retardation, epilepsy and gastrointestinal defects. PIGV is localized in the ER, where it transfers the second mannose to the GPI backbone [393]. PIGO, together with another enzyme called PIGF, instead catalyzes the attachment of an ethanolamine phosphate to the third mannose of the three-mannosyl glycan core [394].
From our search, we identified one PIGV-deficient patient and three PIGO-deficient patients reported with cardiac symptoms. The PIGV-deficient patient and one of the PIGO-deficient patients were described with ASD, whilst the second and third PIGO-deficient patients exhibited ToF and TC, respectively [393,395,396,397] (Table 5, Supplementary Tables S2 and S3).
3.4.5. COG Complex Defects
COG1-CDG & COG7-CDG
Genetic defects of the component of oligomeric Golgi complex 1 (COG1) [OMIM:611209] and complex 7 (COG-7) [OMIM:608779] genes lead to AR CDG type IIg and CDG type IIe, respectively. The GOG1 gene is located on chromosome 17.q25.1, while the COG7 gene is on chromosome 16p12.2. Glycoprotein modification and intracellular transport are key functions of the Golgi apparatus (GA), which depend on multiprotein complexes such as the Golgi transport complex, LDLC complex and SEC34 complex. These complexes participate in glycosylation reactions and vesicular transport. Together they are termed the conserved oligomeric Golgi complex (COG). COG1 and COG7 are thus necessary for GA structure and activity. To date, fewer than ten COG1-CDG patients have been described, presenting with dwarfism, facial dysmorphism, microcephaly and psychomotor delay. Of these, we identified four cases with HCM, PFO, SD, PMV and pulmonary hypertension [14,398,399,400,401] (Table 5, Supplementary Tables S2 and S3). For COG7-CDG eight patients were found in total, characterized by dysmorphic features and liver, gastrointestinal, skeletal and neurologic involvement. Cardiac involvement was found in six cases and included PVSD, (secundum) ASD and TI [14,402,403,404] (Table 5, Supplementary Tables S2 and S3).
3.4.6. V-ATPase Complex Defects
ATP6V1A-CDG & ATP6V1E1-CDG
Defects of ATPase H+-transporting V1 subunit A (ATP6V1A) gene on chromosome 3q13.31 are causative of an AR CDG also known as cutis laxa type IId [OMIM:617403]. The acidification of endosomes, lysosomes, GA and other intracellular organelles are dependent on the vacuolar-type H+-ATPase (V-ATPase), a multimeric complex acting as an ATP-dependent protein pump, of which the protein encoded by ATP6V1A is a subunit. This complex is critical for the transport of hydrogen ions across the plasma membrane into the extracellular space and protein glycosylation. An imbalance in this process leads to acidification. So far, 4 patients have been reported, of which two exhibited SD (with tortuous aortic arch), LQTS with incomplete RBBB, HCM and progressive cardiac failure [14,405,406] (Table 5, Supplementary Tables S2 and S3). Likewise, mutations affecting the ATP6V1E1 gene on chromosome 22q11.21 encoding another subunit of this complex are the cause of an AR inherited CDG, known as cutis laxa type IIC [OMIM:617402]. This CDG presents more severe cardiac manifestations. Out of six reported patients, five were described with a variety of cardiac symptoms: severe DAR, PFO, SD, MVR, TVR, HCM, AI and TI, RHHS with HoRV, TVS, small PDA, MVP, RVD with reduced diastolic compliance (DI) and RBBB [14,405,407] (Table 5, Supplementary Tables S2 and S3).
Table 5.
Congenital disorders of glycosylation for which patients displaying cardiac manifestations have been reported (abbreviations described in Table 1).
| Affected Gene | Affected Protein | Inheritance | Heart Defects & Manifestations | No. Patients Identified by Our Search | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cardiomyopathies | Structural Defects | Arrhythmogenic Disorders | |||||
| Defects of N-Glycosilation | |||||||
| ALG3 | Dolichol-P-mannose: Man5GlcNAc2-PP-dolichol mannosyltransferase | AR | HOCM, RDA, TR | VSD, AVD, PFO, PDA, MVS | - | 15 | [200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,216,217,218] |
| ALG6 | α-1,3-glucosyltransferase | AR | DCM, LVD | - | - | 1 | 221 |
| ALG9 | α-1,2-mannosyltransferase | AR | RVD | ASD, BAV, SAI, PDA, TVR | - | 12 | [14,219,222,223,224,225,226] |
| ALG12 | Dolichol-P-mannose: Man7GlcNAc2-PP-dolichol mannosyltransferase | AR | HCM, PVSD | PDA, SD, VSD, PFO | AT, HF | 9 | [14,227,228,230] |
| GMPPB | GDP-mannose pyrophosphorylase B (GMPPB) | AR | VD, LVD, VEFR | RBBB | - | 5 | [232,233,234] |
| NPL | N-acetylneuraminate pyruvate lyase (NPL) | AR | DCM, LVH, VEFR | - | AT, HF | 1 | 236 |
| PGM1 | Phosphoglucomutase 1 (PGM1) | AR | DCM, RCM, LVD, LVH, AC, CMG | VSD, MP | AT, AF, HF | 30 | [14,239,241,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257] |
| PMM2 | Phosphomannomutase 2 (PMM2) | AR | HCM, ToF | ASD, PDA, PFO, PPS | - | 70 | [14,230,243,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284] |
| Defects of O-Glycosilation | |||||||
| B3GALTL | O-fucose-specific β-1,3-N-glucosyltransferasen (B3GALTL) | AR | HoLV | ASD, VSD, BVP | - | 19 | [14,286,287,288,289,290,291,292] |
| B3GAT3 | β-1,3-glucuronyltransferase 3 (B3GAT3) | AR | BAV, VSD, ASD, MVP, PDA, PPS, DAR | - | 15 | [14,293,294,295,296,297,298,299,300,301] | |
| FKRP | Fukutin-related protein (FKRP) | AR | LVD, LVRWMA, VEFR, DCM | VSD, TI, RBBB, TGA | HF | 220 | [14,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329] |
| FKTN | Fukutin (FKTN) | AR | DCM, VEFR, CD | PPS, TGA, CMG | - | 77 | [14,183,330,331,332,333,334,335,336,337,338,339,340,341,342,343] |
| POMT1 | O-mannosyltransferase 1 (POMT1) | AR | DCM, VD, VEFR, LVH, SI | - | - | 5 | [14,321,345,346] |
| POMT2 | O-mannosyltransferase 2 (POMT2) | AR | LVH, DCM, VEFR | AD, DAR | - | 7 | [14,321,346,348,349] |
| XYLT2 | Xylosyltransferase 2 (XYLT2) | AR | - | ASD, AVD, MVP | - | 3 | [14,353,354] |
| Dolichol-phosphate synthesis defects | |||||||
| DOLK | Dolichol kinase (DOLK) | AR | DCM, HCM, BVD, CD, LVD | CMG, PDA, VSD | HF, TC, BR, AT | 26 | [14,227,355,356,357,358,359,360] |
| DPM3 | Dolichol-phosphate mannose synthase subunit 3 (DPM3) | AR | DCM, LVD, LVRWMA | - | - | 3 | [361,362,363] |
| MPDU1 | Mannose-phosphate-dolichol utilization defect 1 (MPDU1) | AR | DCM, NCM | - | - | 4 | [364,365,366] |
| SRD5A3 | Steroid 5 α-reductase 3 (SRD5A3) |
AR | CM | ASD, TGA, PFO | AT, LQTS | 7 | [14,368,369,370,371] |
| Glycosylphosphatidylinositol anchor olichol-phosphate synthesis defects | |||||||
| PIGA | Phosphatidylinositol glycan anchor biosynthesis class A protein (PIGA) | XL | HCM | AD, BAV, PFO, ASD | AR. HF | 19 | [14,372,373,374,375,376,377] |
| PIGL | Phosphatidylinositol glycan anchor biosynthesis class L protein (PIGL) | AR | - | HSS, TGA, ToF, VSD, DOV, PPS | - | 8 | [14,378,379,380,381] |
| PIGN | Phosphatidylinositol glycan anchor biosynthesis class N protein (PIGN) | AR | NCM, RVD | DAR, PVSD, DC, PDA, PFO, ToF, PPS, DC, VSD, ASD, OA | - | 18 | [14,383,384,385,386,387,388] |
| PIGT | Phosphatidylinositol glycan anchor biosynthesis class T protein (PIGT) | AR | RCM | PDA, PFO, VSD, ASD | - | 8 | [14,389,391,392] |
| PIGV | Phosphatidylinositol glycan anchor biosynthetic enzymes class V (PIGV) | AR | - | ASD | - | 1 | [393] |
| PIGO | Phosphatidylinositol glycan anchor biosynthetic enzymes class O (PIGO) | AR | - | ASD, ToF | TC | 2 | [395,396,397] |
| COG complex defects | |||||||
| COG1 | Subunit 1 of the COG complex in Golgi trafficking (COG1) | AR | HCM | PFO, ASH, AI, PMV | - | 4 | [14,398,399,400,401] |
| COG7 | Subunit 7 of the COG complex in Golgi trafficking (COG7) | AR | - | PVSD, TI, ASD | - | 6 | [14,398,399,400,401] |
| V-ATPase complex defects | |||||||
| ATP6V1A | V-ATPase A subunit 1 (ATP6V1A) |
AR | HCM | SD, RBBB | HF, LQTS | 4 | [14,405,406] |
| ATP6V1E1 | ATPase subunit E (ATP6V1E1) |
AR | HoRV, RVD, HCM | AD, MVR, TVR, PFO, SD, TI, AI, RHHS, TVS, PDA, MVP, RBBB | - | 5 | [14,405,407] |
3.5. Disorders of Lysosomal Carbohydrate-Processing
Lysosomes are specialized organelles where complex components, such as glycans, proteins and other molecules, are chemically degraded in their fundamental components. Several lysosomal enzymes are involved in the finely organized degradation cascades of glycoconjugates and other complex molecules. The lysosomal catabolism of glycoproteins is an essential part of the cellular homeostasis of glycosylation. Once inside the lysosomes, glycoproteins are catabolized by acidic hydrolases. Here, the glycans are broken down and other posttranslational modifications such as sulfation, phosphorylation and esterification are removed as well [408]. All cellular glycoproteins continuously under turn-over and the degradationproducts (such as monosaccharides and amino acids) pass through the lysosomal membrane to be recycled by the cells. When this process is dysregulated, glycoproteins and other undegraded molecules accumulate in the lysosomes. Lysosomal storage diseases (LSDs) are inherited metabolic diseases which are caused by a deficiency of degradation enzymes, leading to abnormal build-up of a variety of toxic compounds [409]. Thus far, over 40 LSDs have been described and reported to mostly involve the skeleton, skin, brain and central nervous system [410]. Within this large family, we identified 15 LSDs that present with cardiac manifestations (Table 6).
3.5.1. CTSA-LSD
Galactosialidosis (GSL), or Goldberg syndrome [OMIM#256540], is an AR LSD caused by homozygous or compound heterozygous mutations affecting the gene encoding cathepsin A (CTSA, also known as PPCA) on chromosome 20q13. CTSA is a carboxypeptidase exerting a protective and stabilizing function on β-galactosidase and neuraminidase. In fact, GSL is associated with a combined loss of function of these two enzymes and manifests in three different phenotypic subtypes: early infantile form, late infantile form and juvenile/adult form. Although the three forms share some clinical features (e.g., coarse facies, cherry red spots and foam cells in the bone marrow), they display some phenotypic differences and very different levels of severity and life expectancy. Cardiac involvement is usually reported in the infantile forms, which are also the most severe. Through our search, we identified 10 GLS patients, 8 infantile and 2 older in age (11 and 18 years old, respectively), described with cardiac involvement, specifically DC, heart murmur, SD (mainly thickened cardiac septa), altered LV wall thickness (with decreased ventricular function), CM, HF, VDL, arterial hypertension and presence of vacuolated myocardial fibers (Table 6, Supplementary Tables S2 and S3) [411,412,413,414,415,416,417,418,419,420]. Complex cyanotic congenital heart disease and situs inversus have also been reported in one of these cases [413].
3.5.2. GBA1-LSD
Gaucher disease (GD) [OMIM:231000] is a rare AR subtype of the classical subacute/chronic neuronopathic GD caused by homozygous or compound heterozygous mutations affecting the glucosylceramidase-beta type 1 (GBA1, also known as acid beta-glucosidase) gene on chromosome 1q22. Within the lysosomal lumen, this enzyme hydrolyzes glucosylceramides, playing a central role in the glucosylation of cholesterol, degradation of complex lipids and turnover of the cellular membrane. This results in the accumulation of glucocerebrosides within cells such as immune cells, which can infiltrate several tissues such as heart, liver and muscles [421]. In addition to a severe perinatal–lethal form associated with ichthyosiform or collodion skin abnormalities or with nonimmune hydrops fetalis, three main types have been described [421]. GD type 1 is characterized by bone defects, hepatosplenomegaly, anemia, thrombocytopenia and lung disease without primary central nervous system disease. GD types 2 and 3 are instead predominantly characterized by primary neurologic disease and were once divided based on the onset age. In addition, GD type 3 can present with a cardiovascular form (GD3c) primarily associated with a specific GBA1 mutation, namely p.D409H. Our search resulted in 141 patients with GD with cardiac involvement (mostly GD3c) [415,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464]. The described cardiac manifestations include VC, valve thickening, ARe, MVR, AD, AVS, MVS, CMG, MF, LVH, LAE, RAE, TC, AF, BR, which escalates to HF (often congestive). Less frequently HCM, DCM, high–left ventricular wall thickness and intraventricular septum, AVB, recurrent syncope, palpitation and hemorrhagic pericarditis have also been reported (Table 6). Cardiovascular symptoms such as coronary artery disease and pulmonary hypertension have also often been described in GD patients.
3.5.3. GLA-LSD
Fabry disease [OMIM:301500] is an X-linked inherited LSD caused by mutations on the α-galactosidase A (GLA) gene on chromosome Xq22. It has an estimated prevalence of approximately 1–5/10 000 live births. Genetic mutations on this gene induce deficient or absent activity of lysosomal enzyme GLA, which mediates glycosphingolipid catabolism. As a result, accumulation of globotriaoslyceramide (Gb3) and related glycosphingolipids have been detected in the plasma and cellular lysosomes of vessels, nerves, and other tissues [465,466]. Thus, GLA-LSD is a systemic disease that manifests with renal failure, cerebrovascular disease, small-fiber peripheral neuropathy, skin lesions and cardiac defects. Several thousand patients affected by GLA-LSD have been reported since its discovery in 1898, and many cardiac symptoms have been diagnosed as associated with this disease [467,468]. Cardiovascular manifestations predominantly include LVH (regional, apical, global), increased endomyocardial trabeculation, LV apical aneurysm, LVD, reduced strain (global and regional/segmental), RVH, RCM, reduced RV free wall strain, immune-mediate myocarditis, MF, congestive HF, increased filling pressure, DI, ARe, MVR, AVP, MVP, aorta and mitral valve thickening, LV outflow tract obstruction, reduced LA function (reservoir strain and strain rate), mild AD, coronary microvascular dysfunction, AT, BR, beat-to-beat variation in heart rate, chronotropic incompetence, AVB, short PR interval, increased arterial stiffness and elevated cardiac biomarkers (NT-proBNP, high-sensitivity troponins) [468]. Although cardiac involvement has been reported in patients affected by different mutations, one mutation affecting exon 5 of GLA gene, p.N215S (c.644A>G), has been confirmed to be causative of predominantly cardiac symptoms, mostly late-onset LVH, AT and conduction disturbances (Table 1) [469].
Many case reports and large cohort studies have been published thus far. For instance, one of the first large studies was published by Mehta et al., who investigated a cohort of 1453 GLA-LSD patients of which 798 (422 males and 376 females) presented cardiac symptoms such as LVH, LVM, valvular heart disease, conduction disturbances and hypertension (Table 6) [470]. In this cohort, cardiac complications represented the primary cause of death in both male (34%) and female (57%) patients.
Enzyme replacement therapy (ERT) is an established treatment for GLA LSD, with or without chaperone therapy (migalastat), which helps in preventing or delaying the progression of some complications such as LV mass increase; however, its effects on other cardiac symptoms such as LVH and on fibrotic damage are less clear [468].
3.5.4. GLB1-LSD
Homozygous or compound heterozygous mutations of the gene encoding for beta-galactosidase-1 (GLB1) on chromosome 3p22.3 can originate two clinically distinct, albeit allelic, disorders: GM1-gangliosidosis type 1 and mucopolysacharidosis type IVB, also known by the eponym Morquio syndrome type B.
This enzyme hydrolyzes the terminal β-linked galactoside moieties from the glycans of gangliosides, glycosaminoglycans and other glycoconjugates within the lysosome, enabling their catabolism and recycling of monosaccharides. Loss of function of GLB1 leads to accumulation of gangliosides and sphingolipids in several organs, causing rapid psychomotor degeneration. Moreover, an alternative spliced transcript of the GLB1 gene generates an elastin-binding protein (EBP), and some EBP mutations that compromise elastin fiber assembly have been associated with the development of cardiovascular symptoms [471,472].
GM1-gangliosidosis type 1 (GM1G1) [OMIM:230500] is an AR LSD with an estimated prevalence of about 1/100,000–200,000 live births. Clinically, GM1G1 is characterized by variable degrees of neurodegeneration and skeletal abnormalities, while cardiac symptoms are a less common occurrence, mostly associated with the infantile form and with specific GLB1 mutations. The first clinical report of a GM1G1 case with cardiac involvement dates back to 1971 and describes an infantile patient with CM, incomplete RBBB, vacuolated and hypertrophied myofibers, thickened mitral valve leaflets with fibrous tissue and right coronary occlusion due to atherosclerotic plaque [473]. Later reports described 10 infantile patients with similar cardiac symptoms, predominantly DCM or HCM, LVH, CMG, systolic murmur and cases of death due to heart failure [474,475,476,477,478]. More recently, the spectrum of cardiac symptoms has been expanded by the description of 25 other patients presenting DCM or HCM, LVH, LVD, HSS and right interventricular conduction delay [472,479,480] (Table 6, Supplementary Tables S2 and S3).
Morquio syndrome type B [OMIM: 253010] is an AR LSD with a prevalence of 1/250,000–1,000,000 live births, with multiorgan involvement due to compromised keratan sulfate catabolism which results in intralysosomal accumulation in connective tissues and several other organs [481]. Clinically, this disease manifests with skeletal dysplasia, coarse facial features, corneal clouding, hearing disorder and cardiac (valvular) defects, usually without central nervous system involvement. Through our search, we identified eight patients with cardiac involvement, presenting MVS, AVS, VC and VF, MF, MVR, mitral dysplasia, ARe, DAR, SH, LVH, LV outflow tract obstruction and dyspnea with severe pulmonic autograft regurgitation [481,482,483] (Table 6, Supplementary Tables S2 and S3).
3.5.5. HEXB-LSD
Sandhoff disease [OMIM:268800] is an AR LSD caused by mutations in the beta-hexosaminidase (HEXB) gene on chromosome 5q13. It has an estimated prevalence of 1–9/1,000,000 live births. This lysosomal hexosaminidase after activation catalyzes the degradation of the ganglioside GM2 and other molecules containing terminal N-acetyl hexosamines. In the case of HEXB loss of function, GM2 ganglioside is abnormally stored in several tissues, primarily neurons and peripheral nervous tissues (in fact, it is also classified as GM2-gangliosidosis type II). This translates to early blindness, progressive central nervous system degeneration, hepatosplenomegaly, macrocephaly and cherry red spots on the macula. Although less common in Sandhoff disease, nine infantile patients have been reported with cardiac manifestations [484,485,486]. All patients displayed MVP with MVR, ASD, VSD, CMG with LVD and HF [484,485,486,487,488,489,490] (Table 6, Supplementary Tables S2 and S3). A few patients also showed heart murmur and mild ARe from AVP, along with asymmetric hypertrophy of the interventricular septum without left ventricular outflow tract obstruction. These studies suggest that cardiac involvement in Sandhoff disease (though rare) can appear as an early sign during infancy.
3.5.6. IDUA-LSD
Mucopolysaccharidosis type I is an AR LSD due to mutation of the gene encoding alpha-L-iduronidase (IDUA) on chromosome 4p16. IDUA hydrolyzes the terminal alpha-L-iduronic acid residues of two glycosaminoglycans (GAGs, also known as mucopolysaccharides), dermatan sulfate and heparan sulfate. From a clinical standpoint, MPS-I has been traditionally classified in subtypes based on the severity of the symptoms: the mild subtype called Scheie syndrome (MPS-IS) [OMIM: 607016], the moderate form called Hurler–Scheie syndrome (MPS-IH/S) [OMIM:607015] and the severe form called Hurler syndrome MPS-IH [OMIM:607014]. At present, however, MPS-I is considered a spectrum of disorders from attenuated to severe with many phenotypes in between [491]. Overall, it presents with neurological delay, coarse facial features, corneal clouding, hernias, hepatosplenomegaly, hearing impairment, skeletal malformations (e.g., scoliosis) and risk of infection, with pneumonia being one of the main causes of mortality along with cardiac failure [491]. Our systematic review results in 440 IDUA-deficient patients with cardiac manifestations, predominantly valvular disease (encompassing MVS, AVS, MVR, ARe), but also LVD, LVH leading to SI and DI, along with characteristic cardiovascular symptoms such coronary artery narrowing and/or occlusion [492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551]. In addition to standard pharmacological and surgical management, systemic therapies are also available for MPS-I (and other MPS types), namely hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT). Long-term metabolic correction by HSCT results in preservation of cardiac function and regression of cardiac, whereas long-term ERT may improve systolic ventricular function and resolution of LVD. Both therapies, though, did not show clear amelioration of valvular thickening, stenosis or regurgitation [491].
3.5.7. IDS-LSD
Mucopolysaccharidosis type II [OMIM:309900], also known by the eponym Hunter syndrome, is an XLR LSD resulting from mutations of the gene encoding the enzyme iduronate 2-sulfatase (IDS), located on chromosome Xq28. IDS hydrolyzes the 2-sulfate groups of the L-iduronate 2-sulfate units of dermatan sulfate, heparan sulfate and heparin. When IDS is deficient, similarly to other MPS types, GAGs accumulate in the lysosomes in many tissues, leading to a multiorgan syndrome. Main clinical manifestations are represented by neurological decline, severe airway obstruction, skeletal deformities (e.g., scoliosis), hepatosplenomegaly, hearing impairment, cardiomyopathy and other valvulopathy. Unlike other MPS types, in MPS-II the lifespan can increase from the second decade up to the sixth decade, depending on the severity of the clinical presentation and the treatments received (e.g., ERT) [552]. Pneumonia and respiratory and cardiac failures are the most common causes of death in these patients [552,553]. Through our systematic review, we identified 742 MPS-II patients with cardiac symptoms, encompassing MVS, MVR, MI, AVS, ARe, AI, TI, DAR, ASD, AVB, CM/HCM, SH, AF, AT and cardiac murmur [497,509,512,540,544,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598] (Table 6, Supplementary Tables S2 and S3). ERT and substrate reduction therapy in MPS-II patients prevent the accumulation of GAGs in peripheral tissues, thus reducing inflammation, risk of respiratory infections and coarseness of facial features and improving joint mobility, although no clear improvement in neurological deterioration and cardiac functionality have been documented [599]. HSCT instead appears to normalize hepatosplenomegaly and aortic valve deterioration [599].
3.5.8. SGSH-LSD
Mucopolysaccharidosis type III subtype A [OMIM:252900], or Sanfilippo syndrome subtype A, is an AR LSD caused by homozygous or compound heterozygous mutations in the gene encoding N-sulfoglucosamine sulfohydrolase (SGSH) on chromosome 17q25. It is the most common subtype of MPS-III, with a prevalence of 1–1.62/100,000 newborns, depending on ethnicity and country [600,601]. The loss of function of this enzyme causes impaired degradation of GAGs, such as heparan sulfate and dermatan sulfate, which accumulate in the cell lysosomes of multiple tissues and compromise their functionality, leading to progressive deterioration of patients’ conditions. MPS-IIIA can be defined as an aggressive infantile-onset neurodegenerative disease traditionally presenting with intellectual disability, sleep disturbances, loss of ambulation, hepatomegaly, dysmorphism and early death [602]. Death usually occurs as a consequence of aggressive infections (mostly pneumonia) and of cardiac complications [603]. Through our search, we gathered (at least) 47 patients reported with CVD (MVS, MVR, AVS, ARe), BR, AT, VSD, asymmetric SH and CH and arrhythmogenic RVHCM, which can result in cardiac arrest and recommendation for heart transplant [532,589,604,605,606,607,608,609] (Table 6, Supplementary Tables S2 and S3). Additionally, we found eight studies with patients displaying cardiac involvement, but the subtype of Sanfilippo syndrome (A, B, C or D) was not specified, and thus they have not been included [512,540,544,556,564,591,600,610].
3.5.9. NAGLU-LSD
Mucopolysaccharidosis type III subtype B [OMIM:252920], or Sanfilippo syndrome subtype B, is another AR LSD caused by compromised GAG degradation due to mutations affecting the N-alpha-acetylglucosaminidase (NAGLU) gene, located on chromosome 17q21. Its prevalence is estimated to be 0.5–1/100,000 live births [601]. Similarly to subtype A, NAGLU deficiency also results in lysosomal accumulation of partially degraded heparan sulfate in several tissues and manifests clinically with severe neurodegenerative symptoms and low life expectancy (second decade) [611]. However, subtype B is also characterized by severe skeletal defects and facial dysmorphism [611]. Our systematic search identified (at least) 39 MPS-IIIB patients described with cardiac symptoms, including CVD (MVS, MVR, AVS, ARe), to BR, AT, VSD, SH (asymmetric), CH and arrhythmogenic RVHCM, which in some cases resulted in cardiac arrest and/or recommendation for heart transplant [589,604,605,606,609,612,613,614] (Table 6, Supplementary Tables S2 and S3). As mentioned in the previous paragraph, we found eight additional studies with MPS_III patients described with cardiac involvement, but the subtype was not specified, thus these studies have been excluded (Supplementary Table S3) [512,540,544,556,564,591,600,610].
3.5.10. HGSNAT-LSD and GNS-LSD
The rarest subtypes of mucopolysaccharidosis type III, namely subtype C [OMIM:252930] and D [OMIM:252940], are both hyper-rare AR LSDs with an unknown prevalence, caused by genetic mutations of two different genes: the HGSNAT gene (8p11.2-p11.1) and the GNS gene (12q14.3), respectively. The HGSNAT gene encodes heparan acetyl-CoA:alpha-glucosaminide N-acetyltransferase, while the GNS gene encodes N-acetylglucosamine-6-sulfatase.
HGSNAT is a lysosomal acetyltransferase that acetylates the non-reducing terminal alpha-glucosamine residue of heparin and heparan sulfate in the presence of acetyl-CoA. When defective, intralysosomal GAG accumulation occurs and compromises the functionality of multiple organs, resulting in MPS-IIIC. This subtype has an estimated prevalence of 0.07–0.42/100,000 live births, depending on ethnicity and country [601,615]. MPS-IIIC associates with skeletal malformations and dysmorphic features, progressively delaying psychomotor development, hearing loss and sleep disturbances [616]. Through our systematic research, we gathered 10 patients who were also described with cardiac symptoms, such as CVD (MVS, MVR, AVS, ARe), LVH and SH; in at least one case, acute cardiac failure has also been reported [589,606,607,617] (Table 6, Supplementary Tables S2 and S3).
GNS is a glucosamine (N-acetyl)-6-sulfatase that hydrolyzes N-sulfate esters from heparin, heparan sulphate and keratan sulphate. This subtype has a prevalence estimated at around 0.10/100,000 [601] and is characterized by psychomotor delay, behavioral issues and dysmorphic features. Our systematic search produced two patients with MPS-IIID and cardiac involvement, specifically MR and SH (asymmetric) [609] (Table 6, Supplementary Tables S2 and S3). As mentioned in Section 3.5.8 and Section 3.5.9, eight additional studies with MPS-III patients with cardiac involvement were found, but in these studies the subtype was not specified, thus they have been excluded (Supplementary Table S3).
3.5.11. GALNS-LSD
Mucopolysaccharidosis type IV is divided into two types: type A, also known by the eponym of Morquio syndrome A [OMIM:253000], and type B, or Morquio disease B [OMIM:253010, see Section 3.5.4], which originates from genetic defects on two different genes. Focusing on the former, MPS-IVA has a prevalence of 0.15–0.47/100,000 [618] and is caused by the absence of functional galactosamine-6-sulfatase, which results in the intralysosomal deposition and accumulation of several glycosylated substrates, primarily keratan sulfate and chondroitin sulfate. The spectrum of clinical features encompasses dwarfism, growth delay, skeletal malformations, dysplasia, cardiac symptoms such as cardiac valve thickening, CVD, MF, VF, MVR, ARe, TVR, MVS, AVS, TVS and CD [482,618,619,620,621,622] (Table 6). ERT and HSCT have both been applied and investigated in MAP-IVA patients and seems to at best normalize hypertrophy by reducing GAG accumulation and heart/valve fibrosis by controlling fibroblast infiltration, but in some cases, the valvulopathy worsened [623].
3.5.12. ARSB-LSD
Mucopolysaccharidosis type VI [OMIM:253200], also known as Maroteaux–Lamy syndrome, is a progressive AR LSD with an estimated prevalence of 1/43,000–1,500,000 [624,625], caused by mutations affecting the ARSB gene on chromosome 5q14.1. This gene encodes arylsulfatase B (ARSB, or N-acetylgalactosamine 4-sulfatase), a sulfatase involved in the lysosomal catabolism of GAGs and which is mainly present in the liver and pancreas. When mutated, it results in the accumulation of partially degraded dermatan-sulphate and chondroitin-sulphate, in tissues and organs, which in turn causes an array of clinical manifestations that progressively worsen with age [625]. From a clinical standpoint, MPS-IV is divided into severe and attenuated forms, the latter further separated into cardiac type and osteoarticular type (although mixed cases can be found in the literature). Few specific mutations, predoinantly p.R152W, have been proved to specifically associated with the cardiac form [626]. Our search resulted in (at least) 520 MPS-IV patients presenting cardiac symptoms (in some comparative studies in which multiple MPS types were studied together, it was not always possible to determine the exact number of MPS-VI, and thus our count could be slightly underestimated; see Supplementary Table S3). The cardiac symptoms consisted primerily of cardiac valvular disease including VLD, valve thickening, DAR, MVP, AVP, TVP, MVR, ARe, TVR, MI, AI, TI, MVS, TVS and less frequently VSD (intraventricular septal hypertrophy), LHV, HCM and LVCR [497,512,532,540,544,556,564,570,574,579,589,590,591,596,625,626,627,628,629,630,631,632,633,634,635,636,637,638,639,640,641,642,643,644,645,646,647,648,649,650,651,652,653,654,655,656,657,658,659,660,661,662,663] (Table 6, Supplementary Tables S2 and S3). Cardiac involvement often escalated to congestive HF. ERT has been proven to stabilize and in some cases ameliorate hypertrophy-linked symptoms by reducing GAG accumulation; however, the beneficial effects on valvular defects are less clear and cases of worsened valvulopathies have also been reported [637,664].
3.5.13. GUSB-LSD
Mucopolysaccharidosis type VII [OMIM:253220], also called Sly syndrome, is an ultra-rare AR LSD caused by mutations affecting the gene encoding beta-glucuronidase (GUSB) located on chromosome 7q11. The loss of function of GUSB results in the lysosomal accumulation of three main GAGs, dermatan sulfate, heparan sulfate and chondroitin sulfate, leading to tissue hypertrophy and function disruption of many organs [665]. MPS-VII is clinically characterized by degenerative neurological symptoms, severe skeletal malformations (mostly thoracic deformity, scoliosis, and hip dysplasia), facial dysmorphism, hepatosplenomegaly, susceptibility to infections of the ear and respiratory tract, pulmonary complications and cardiac defects [665]. Our search resulted in 46 MPS-VII patients with cardiac involvement consisting of progressive CVD, ARe, AVS, AI, DAR, MVR, MVS, MI, TVR, MF, LVD, LVH, CH, early repolarization and T wave inversion and, in several cases, congestive HF [540,564,590,654,666,667,668,669,670,671,672,673,674,675] (Table 6, Supplementary Tables S2 and S3). ERT in MPS-VII seems to stabilize some of the cardiac symptoms (although worsening cases have also been reported), while the effects on these symptoms due to HSCT are still under scrutiny in few clinical trials [676].
3.5.14. ARSK-LSD
Mucopolysaccharidosis type X [OMIM:619698] is a very recently described type of AR LSD caused by homozygous or compound heterozygous mutations of the ARSK gene on chromosome 5q15. This gene encodes arylsulfatase K, which is responsible for the hydrolysis of N-sulfate esters from sulfated multiple substrates such as GAGs, steroids, carbohydrates, proteoglycans and glycolipids. When loss of function occurs, the catabolism of GAGs (e.g. heparan sulfate, chondroitin sulfate, dermatan sulfate) and other substrates is compromised, and they accumulate in peripheral tissues disrupting their functionality [677]. Thus far, only 4 cases from two different families have been reported with this disease. Of these, 2 patients (siblings) were described with cardiac complications, in the form of AVS, MVS, ARe, MVR, thickened ends of aortic cusps, LVH and systolic murmur [678] (Table 6, Supplementary Tables S2 and S3).
Table 6.
Lysosomal storage diseases for which patients displaying cardiac manifestations have been reported (abbreviations described in Table 1).
| Affected Gene | Affected Protein | Inheritance | Heart Defects & Manifestations | No. Patients Identified by Our Search | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cardiomyopathies | Structural Defects | Arrhythmogenic Disorders | |||||
| CTSA | Cathepsin A (CTSA) |
AR | CM, LVRWMA | DC, SD, VLD | HF | 10 | [411,412,413,414,415,416,417,418,419,420] |
| GBA1 | Glucocerebrosidase (GBA) |
AR | LVH, HCM, DCM | VC, ARe, MVR, CMG, MF, AVS, MVS, LAE, RAE, AVB | AF, TC, BR, HF | 141 | [415,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464] |
| GLA | α-galactosidase (GLA) |
XL | LVH, LVD, RVH, RCM | ARe, MVR, AD, MF, DI, MVP, AVP, AVB | AT, BR | >300 | [467,468,469,470] * selected |
| GLB1 | β-galactosidase (GLB1) |
AR (GM1 gangliosidosis) |
DCM, LVH, LVD, HCM | RBBB, HSS | HF | 25 | [472,473,474,475,476,477,478,479,480] |
| GLB1 | β-galactosidase (GLB1) |
AR (mucopolysaccharidosis) |
LVH, SH | ARe, DAR, MVR, MVS, AVS, MF, VF | - | 8 | [481,482,483] |
| HEXB | β-hexosaminidase B (HEXB) |
AR | HCM, LVD | CMG, ARe, MVR, MVP, MVS, AVP, VLD |
HF | 9 | [484,485,486,487,488,489,490] |
| IDUA | α-L-iduronidase (IDUA) |
AR | LVD, LVH | ARe, MVR, MVS, AVS, SI, DI | HF | 440 | [492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551] |
| IDS | 2-sulfatase (IDS) | XLR | HCM, SH | Are, MVR, DAR, MVS, MI, AVS, AI, TI | AF, AT | 742 | [497,509,512,540,544,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598] |
| SGSH | N-sulfoglucosamine sulfohydrolase | AD | SH, CH, RVHCM | CVD, ARe, MVR, MVS, AVS, VSD | BR, AT, HF | 47 | [532,589,604,605,606,607,608,609] |
| NAGLU | N-α-acetylglucosaminidase (NAGLU) | AD | SH, CH, RVHCM | ARe, MVR, CVD, MVS, AVS, VSD | BR, AT | 39 | [589,604,605,606,609,612,613,614] |
| HGSNAT | heparan acetyl-CoA:alpha-glucosaminide N-acetyltransferase (HGSNAT) |
AD | SH, CH RVHCM |
ARe, MVR, CVD, MVS, AVS, VSD | BR, AT, HF | 10 | [589,606,607,617] |
| GNS | glucosamine (N-acetyl)-6-sulfatase (GNS) |
AD | SH | MVR | - | 2 | [609] |
| GALNS | galactosamine (N-acetyl)-6-sulfatase (GALNS) |
AD | CD | ARe, MVR, TVR, CVD, MF, VF, AVS, MVS, TVS | - | >300 | [482,618,619,620,621,622] *selected |
| ARSB |
N-acetylgalactosamine 4-sulfatase (ARSB) |
AD | VSD, SH, HCM | DAR, MVR, ARe, TVR, VLD, MVP, AVP, TVP, AI, MI, MVS, TVS, LVCR | HF | 520 | [497,512,532,540,544,556,564,570,574,579,589,590,591,596,625,626,627,628,629,630,631,632,633,634,635,636,637,638,639,640,641,642,643,644,645,646,647,648,649,650,651,652,653,654,655,656,657,658,659,660,661,662,663] |
| GUSB | β-glucuronidase (GUSB) |
AD | LVD, LVH, CH | ARe, DAR, MVR, TVR, CVD, AVI, AVS, MVS, MI, MF | HF | 46 | [540,564,590,654,666,667,668,669,670,671,672,673,674,675] |
| ARSK | arylsulfatase K (ARSK) |
AD | LVH | ARe, MVR, AVS, MVS | = | 1 | [678] |
* For GAA deficiency our search produced over 300 entries, thus only some meaningful articles were selected.
4. Conclusions
The heart is the organ responsible for providing and maintaining the blood supply to all tissues of the body. Its muscular component, called myocardium, is formed by highly specialized contractile cells, extremely resistant to fatigue and characterized by unparalleled metabolic plasticity. To meet the high energy demand required by the contractile machinery of these cells, the biochemistry of the human myocardium has evolved to ensure constant energy production from a variety of substrates, mainly lipids and carbohydrates [679,680]. Carbohydrates do not only serve as substrates for energy production, but also as precursors for energy storage (via glycogen), macromolecule synthesis (via ribose 5-phosphate) and maturation (via glycosylation). In our review of the literature, we identified 58 congenital metabolic disorders affecting carbohydrate metabolism which are associated with cardiomyopathies, arrhythmogenic disorders and/or structural defects, variable in severity and presentation (Figure 2). Although for some of the diseases discussed hereinabove the cardiac complications may be secondary to other mechanism (such as dysfunctional autophagy in LAMP2 deficiency), our results stress the pivotal role of carbohydrate-linked mechanisms in the formation, development and contractile function of the heart.
Figure 2.
Summary of the inherited disorders of carbohydrate metabolism associated to cardiac manifestations, contextualized with the main carbohydrate metabolic pathways in cardiac cells. Abbreviations: CDG, congenital disorder of glycosylation; ECM, extracellular matrix (yellow); ER, endoplasmic reticulum (orange); GA, Golgi apparatus (red); GSD, glycogen storage disorder; MT, mitochondrion (green). Other symbols: fucose molecules, red triangles; galactose molecules, yellow circles; glucose molecules, blue circles; GlcNAc molecules, blue squares; mannose molecules, green circles; sialic acid molecules, purple diamonds; transporters, grey ovals; cell nucleus colored in purple; cytoplasm colored in pink.
With this systematic review, on one hand, we intend to raise awareness among clinicians about the potential association between rare IMDs and cardiac symptoms, as aid for diagnostics and therapeutics. On the other hand, by systematically gathering information on the enzymatic deficiencies that lead to cardiac manifestations, we aim to provide relevant knowledge on the molecular mechanisms underlying specific processes involved in cardiac development and functionality, which could be targeted for pharmacologic therapy and metabolic interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24108632/s1.
Author Contributions
Conceptualization: F.C.; methodology, F.C. and J.-E.S.; investigation, J.-E.S. and F.C.; writing—original draft preparation, F.C. and J.-E.S.; writing—review and editing, D.J.L., R.P. and F.C.; visualization, F.C.; supervision, D.J.L. and R.P.; funding acquisition, D.J.L., R.P. and F.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
R.P. is a cofounder of Pluriomics (Ncardia) and River BioMedics BV. The other authors have no conflicts of interest to disclose.
Funding Statement
This study was financially supported by the Twente University & RadBoudumc Opportunities (TURBO) Initiative (grant 2020-21 to R.P. and D.J.L.), the Prinses Beatrix Spierfonds (Grant W.OR22-14 to F.C. and D.J.L.) and the Stichting Stofwisselkracht (Grant no. 2019-2766-001 to F.C.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tayal U., Prasad S., Cook S.A. Genetics and genomics of dilated cardiomyopathy and systolic heart failure. Genome Med. 2017;9:20. doi: 10.1186/s13073-017-0410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbich M., Globe G., Pantiri K., Heisen M., Bennison C., Wirtz H.S., Di Tanna G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020) PharmacoEconomics. 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacoby D., McKenna W.J. Genetics of inherited cardiomyopathy. Eur. Heart J. 2012;33:296–304. doi: 10.1093/eurheartj/ehr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czepluch F.S., Wollnik B., Hasenfuß G. Genetic determinants of heart failure: Facts and numbers. ESC Heart Fail. 2018;5:211–217. doi: 10.1002/ehf2.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., Eesa M., Fischer U., Hausegger K., Hirsch J.A., et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018;13:612–632. doi: 10.1016/j.jvir.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira C.R., van Karnebeek C.D.M. Inborn errors of metabolism. Handb. Clin. Neurol. 2019;162:449–481. doi: 10.1016/b978-0-444-64029-1.00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira C.R., Rahman S., Keller M., Zschocke J. An international classification of inherited metabolic disorders (ICIMD) J. Inherit. Metab. Dis. 2021;44:164–177. doi: 10.1002/jimd.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeanmonod R., Asuka E., Jeanmonod D. Inborn Errors Of Metabolism. StatPearls Publishing LLC; Treasure Island, FL, USA: 2022. StatPearls Publishing: Copyright © 2022. [PubMed] [Google Scholar]
- 9.Waters D., Adeloye D., Woolham D., Wastnedge E., Patel S., Rudan I. Global birth prevalence and mortality from inborn errors of metabolism: A systematic analysis of the evidence. J. Glob. Health. 2018;8:021102. doi: 10.7189/jogh.08.021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott P., Limongelli G. Cardiac Aspects of Inherited Metabolic Diseases. In: Hollak C.E.M., Lachmann R., editors. Inherited Metabolic Disease in Adults: A Clinical Guide. Oxford University Press; Oxford, UK: 2016. [Google Scholar]
- 11.Kassiotis C., Rajabi M., Taegtmeyer H. Metabolic reserve of the heart: The forgotten link between contraction and coronary flow. Prog. Cardiovasc. Dis. 2008;51:74–88. doi: 10.1016/j.pcad.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y., Kim E.Y., Seo Y.M., Yoon T.K., Lee W.S., Lee K.A. Function of the pentose phosphate pathway and its key enzyme, transketolase, in the regulation of the meiotic cell cycle in oocytes. Clin. Exp. Reprod. Med. 2012;39:58–67. doi: 10.5653/cerm.2012.39.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loaeza-Reyes K.J., Zenteno E., Moreno-Rodríguez A., Torres-Rosas R., Argueta-Figueroa L., Salinas-Marín R., Castillo-Real L.M., Pina-Canseco S., Cervera Y.P. An Overview of Glycosylation and its Impact on Cardiovascular Health and Disease. Front. Mol. Biosci. 2021;8:751637. doi: 10.3389/fmolb.2021.751637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques-da-Silva D., Francisco R., Webster D., Dos Reis Ferreira V., Jaeken J., Pulinilkunnil T. Cardiac complications of congenital disorders of glycosylation (CDG): A systematic review of the literature. J. Inherit. Metab. Dis. 2017;40:657–672. doi: 10.1007/s10545-017-0066-y. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L.Q., Cheung L.S., Feng L., Tanner W., Frommer W.B. Transport of sugars. Annu. Rev. Biochem. 2015;84:865–894. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 17.Kalra J., Mangali S.B., Dasari D., Bhat A., Goyal S., Dhar I., Sriram D., Dhar A. SGLT1 inhibition boon or bane for diabetes-associated cardiomyopathy. Fundam. Clin. Pharmacol. 2020;34:173–188. doi: 10.1111/fcp.12516. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S.K., McGaffin K.R., Pastor-Soler N.M., Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc. Res. 2009;84:111–118. doi: 10.1093/cvr/cvp190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packer M. Potential Interactions When Prescribing SGLT2 Inhibitors and Intravenous Iron in Combination in Heart Failure. JACC Heart Fail. 2022;11:106–114. doi: 10.1016/j.jchf.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Jhund P.S. SGLT2 Inhibitors and Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2022;18:579–586. doi: 10.1016/j.hfc.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Santer R., Klepper J. Disorders of Glucose Transport. In: Saudubray J.-M., Baumgartner M.R., Walter J., editors. Inborn Metabolic Diseases: Diagnosis and Treatment. Springer; Berlin/Heidelberg, Germany: 2016. pp. 175–183. [Google Scholar]
- 22.Ma L., Xu J., Tang Q., Cao Y., Kong R., Li K., Liu J., Jiang L. SLC2A3 variants in familial and sporadic congenital heart diseases in a Chinese Yunnan population. J. Clin. Lab. Anal. 2022;36:e24456. doi: 10.1002/jcla.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grover-McKay M., Walsh S.A., Thompson S.A. Glucose transporter 3 (GLUT3) protein is present in human myocardium. Biochim. Biophys. Acta. 1999;1416:145–154. doi: 10.1016/S0005-2736(98)00216-8. [DOI] [PubMed] [Google Scholar]
- 24.Willaert A., Khatri S., Callewaert B.L., Coucke P.J., Crosby S.D., Lee J.G., Davis E.C., Shiva S., Tsang M., De Paepe A., et al. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFβ signaling. Hum. Mol. Genet. 2012;21:1248–1259. doi: 10.1093/hmg/ddr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coucke P.J., Willaert A., Wessels M.W., Callewaert B., Zoppi N., De Backer J., Fox J.E., Mancini G.M., Kambouris M., Gardella R., et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat. Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi S.H., Meyer S., Peltekova V.D., Lindinger A., Teebi A.S., Faiyaz-Ul-Haque M. A novel non-sense mutation in the SLC2A10 gene of an arterial tortuosity syndrome patient of Kurdish origin. Eur. J. Pediatr. 2009;168:867–870. doi: 10.1007/s00431-008-0839-2. [DOI] [PubMed] [Google Scholar]
- 27.Zoppi N., Chiarelli N., Cinquina V., Ritelli M., Colombi M. GLUT10 deficiency leads to oxidative stress and non-canonical αvβ3 integrin-mediated TGFβ signalling associated with extracellular matrix disarray in arterial tortuosity syndrome skin fibroblasts. Hum. Mol. Genet. 2015;24:6769–6787. doi: 10.1093/hmg/ddv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castori M., Ritelli M., Zoppi N., Molisso L., Chiarelli N., Zaccagna F., Grammatico P., Colombi M. Adult presentation of arterial tortuosity syndrome in a 51-year-old woman with a novel homozygous c.1411+1G>A mutation in the SLC2A10 gene. Am. J. Med. Genet. Part A. 2012;158:1164–1169. doi: 10.1002/ajmg.a.35266. [DOI] [PubMed] [Google Scholar]
- 29.Kocova M., Kacarska R., Kuzevska-Maneva K., Prijic S., Lazareska M., Dordoni C., Ritelli M., Colombi M. Clinical Variability in Two Macedonian Families with Arterial Tortuosity Syndrome. Balk. J. Med. Genet. BJMG. 2018;21:47–52. doi: 10.2478/bjmg-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boel A., Veszelyi K., Németh C.E., Beyens A., Willaert A., Coucke P., Callewaert B., Margittai É. Arterial Tortuosity Syndrome: An Ascorbate Compartmentalization Disorder? Antioxid. Redox Signal. 2021;34:875–889. doi: 10.1089/ars.2019.7843. [DOI] [PubMed] [Google Scholar]
- 31.Beltramo E., Berrone E., Tarallo S., Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol. 2008;45:131–141. doi: 10.1007/s00592-008-0042-y. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S., Qiao Y., Wang Z., Zhuang J., Sun Y., Shang X., Li G. Identification of novel compound heterozygous variants in SLC19A2 and the genotype-phenotype associations in thiamine-responsive megaloblastic anemia. Clin. Chim. Acta Int. J. Clin. Chem. 2021;516:157–168. doi: 10.1016/j.cca.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Bay A., Keskin M., Hizli S., Uygun H., Dai A., Gumruk F. Thiamine-responsive megaloblastic anemia syndrome. Int. J. Hematol. 2010;92:524–526. doi: 10.1007/s12185-010-0681-y. [DOI] [PubMed] [Google Scholar]
- 34.Viana M.B., Carvalho R.I. Thiamine-responsive megaloblastic anemia, sensorineural deafness, and diabetes mellitus: A new syndrome? J. Pediatr. 1978;93:235–238. doi: 10.1016/S0022-3476(78)80503-4. [DOI] [PubMed] [Google Scholar]
- 35.Mandel H., Berant M., Hazani A., Naveh Y. Thiamine-dependent beriberi in the “thiamine-responsive anemia syndrome”. N. Engl. J. Med. 1984;311:836–838. doi: 10.1056/NEJM198409273111307. [DOI] [PubMed] [Google Scholar]
- 36.Abboud M.R., Alexander D., Najjar S.S. Diabetes mellitus, thiamine-dependent megaloblastic anemia, and sensorineural deafness associated with deficient alpha-ketoglutarate dehydrogenase activity. J. Pediatr. 1985;107:537–541. doi: 10.1016/S0022-3476(85)80011-1. [DOI] [PubMed] [Google Scholar]
- 37.Poggi V., Rindi G., Patrini C., De Vizia B., Longo G., Andria G. Studies on thiamine metabolism in thiamine-responsive megaloblastic anaemia. Eur. J. Pediatr. 1989;148:307–311. doi: 10.1007/BF00444120. [DOI] [PubMed] [Google Scholar]
- 38.Scharfe C., Hauschild M., Klopstock T., Janssen A.J., Heidemann P.H., Meitinger T., Jaksch M. A novel mutation in the thiamine responsive megaloblastic anaemia gene SLC19A2 in a patient with deficiency of respiratory chain complex I. J. Med. Genet. 2000;37:669–673. doi: 10.1136/jmg.37.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gritli S., Omar S., Tartaglini E., Guannouni S., Fleming J.C., Steinkamp M.P., Berul C.I., Hafsia R., Jilani S.B., Belhani A., et al. A novel mutation in the SLC19A2 gene in a Tunisian family with thiamine-responsive megaloblastic anaemia, diabetes and deafness syndrome. Br. J. Haematol. 2001;113:508–513. doi: 10.1046/j.1365-2141.2001.02774.x. [DOI] [PubMed] [Google Scholar]
- 40.Lorber A., Gazit A.Z., Khoury A., Schwartz Y., Mandel H. Cardiac manifestations in thiamine-responsive megaloblastic anemia syndrome. Pediatr. Cardiol. 2003;24:476–481. doi: 10.1007/s00246-002-0215-3. [DOI] [PubMed] [Google Scholar]
- 41.Lagarde W.H., Underwood L.E., Moats-Staats B.M., Calikoglu A.S. Novel mutation in the SLC19A2 gene in an African-American female with thiamine-responsive megaloblastic anemia syndrome. Am. J. Med. Genet. Part A. 2004;125:299–305. doi: 10.1002/ajmg.a.20506. [DOI] [PubMed] [Google Scholar]
- 42.Saedi S., Maleki M., Pezeshki S. Right ventricular dysfunction in thiamine-responsive megaloblastic anaemia syndrome: A case report. Heart Asia. 2011;3:140–142. doi: 10.1136/heartasia-2011-010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aycan Z., Baş V.N., Cetinkaya S., Ağladioğlu S.Y., Kendirci H.N., Senocak F. Thiamine-responsive megaloblastic anemia syndrome with atrial standstill: A case report. J. Pediatr. Hematol./Oncol. 2011;33:144–147. doi: 10.1097/MPH.0b013e31820030ae. [DOI] [PubMed] [Google Scholar]
- 44.Xian X., Liao L., Shu W., Li H., Qin Y., Yan J., Luo J., Lin F.Q. A Novel Mutation of SLC19A2 in a Chinese Zhuang Ethnic Family with Thiamine-Responsive Megaloblastic Anemia. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;47:1989–1997. doi: 10.1159/000491467. [DOI] [PubMed] [Google Scholar]
- 45.Argun M., Baykan A., Hatipoğlu N., Akın L., Şahin Y., Narin N., Kurtoğlu S. Arrhythmia in thiamine responsive megaloblastic anemia syndrome. Turk. J. Pediatr. 2018;60:348–351. doi: 10.24953/turkjped.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Akbari M.T., Zare Karizi S., Mirfakhraie R., Keikhaei B. Thiamine-responsive megaloblastic anemia syndrome with Ebstein anomaly: A case report. Eur. J. Pediatr. 2014;173:1663–1665. doi: 10.1007/s00431-013-2237-7. [DOI] [PubMed] [Google Scholar]
- 47.Li X., Cheng Q., Ding Y., Li Q., Yao R., Wang J., Wang X. TRMA syndrome with a severe phenotype, cerebral infarction, and novel compound heterozygous SLC19A2 mutation: A case report. BMC Pediatr. 2019;19:233. doi: 10.1186/s12887-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman M., Hasan M. Pentose Phosphate Pathway in Disease and Therapy. Adv. Mater. Res. 2014;995:1–27. doi: 10.4028/www.scientific.net/AMR.995.1. [DOI] [Google Scholar]
- 49.Debasis B., Sreejayan N. Nutritional and therapeutic interventions for diabetes and metabolic syndrome. Acta Endocrinol. 2018;14:438. doi: 10.4183/aeb.2018.438. [DOI] [Google Scholar]
- 50.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhoeven N.M., Wallot M., Huck J.H., Dirsch O., Ballauf A., Neudorf U., Salomons G.S., van der Knaap M.S., Voit T., Jakobs C. A newborn with severe liver failure, cardiomyopathy and transaldolase deficiency. J. Inherit. Metab. Dis. 2005;28:169–179. doi: 10.1007/s10545-005-5261-6. [DOI] [PubMed] [Google Scholar]
- 52.Verhoeven N.M., Jakobs C. Disorders of the Pentose Phosphate Pathway. In: Fernandes J., Saudubray J.-M., van den Berghe G., Walter J.H., editors. Inborn Metabolic Diseases: Diagnosis and Treatment. Springer; Berlin/Heidelberg, Germany: 2006. pp. 131–134. [Google Scholar]
- 53.Williams M., Valayannopoulos V., Altassan R., Chung W.K., Heijboer A.C., Keng W.T., Lapatto R., McClean P., Mulder M.F., Tylki-Szymańska A., et al. Clinical, biochemical, and molecular overview of transaldolase deficiency and evaluation of the endocrine function: Update of 34 patients. J. Inherit. Metab. Dis. 2019;42:147–158. doi: 10.1002/jimd.12036. [DOI] [PubMed] [Google Scholar]
- 54.Kishnani P.S., Chen Y.-T. Chapter 156. Disorders of Pentose Phosphate Pathway. In: Rudolph C.D., Rudolph A.M., Lister G.E., First L.R., Gershon A.A., editors. Rudolph′s Pediatrics, 22e. The McGraw-Hill Companies; New York, NY, USA: 2011. [Google Scholar]
- 55.Verhoeven N.M., Huck J.H., Roos B., Struys E.A., Salomons G.S., Douwes A.C., Van der Knaap M.S., Jakobs C. Transaldolase deficiency: Liver cirrhosis associated with a new inborn error in the pentose phosphate pathway. Am. J. Hum. Genet. 2001;68:1086–1092. doi: 10.1086/320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyaid W., Al Harbi T., Anazi S., Wamelink M.M., Jakobs C., Al Salammah M., Al Balwi M., Alfadhel M., Alkuraya F.S. Transaldolase deficiency: Report of 12 new cases and further delineation of the phenotype. J. Inherit. Metab. Dis. 2013;36:997–1004. doi: 10.1007/s10545-012-9577-8. [DOI] [PubMed] [Google Scholar]
- 57.Jassim N., Alghaihab M., Saleh S.A., Alfadhel M., Wamelink M.M., Eyaid W. Pulmonary manifestations in a patient with transaldolase deficiency. JIMD Rep. 2014;12:47–50. doi: 10.1007/8904_2013_243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feghaly J., Al Hout A.R., Mercieca Balbi M. Aspirin safety in glucose-6-phosphate dehydrogenase deficiency patients with acute coronary syndrome undergoing percutaneous coronary intervention. BMJ Case Rep. 2017;2017:bcr2017220483. doi: 10.1136/bcr-2017-220483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawat D.K., Hecker P., Watanabe M., Chettimada S., Levy R.J., Okada T., Edwards J.G., Gupte S.A. Glucose-6-phosphate dehydrogenase and NADPH redox regulates cardiac myocyte L-type calcium channel activity and myocardial contractile function. PLoS ONE. 2012;7:e45365. doi: 10.1371/journal.pone.0045365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rigattieri S., Silvestri P., Minucci A., Di Russo C., Ferraiuolo G., Giardina B., Capoluongo E., Loschiavo P. Drug-eluting stents in a patient with favism: Is the aspirin administration safe? J. Cardiovasc. Med. 2008;9:1159–1162. doi: 10.2459/JCM.0b013e32831103c3. [DOI] [PubMed] [Google Scholar]
- 61.Dogra N., Puri G.D., Rana S.S. Glucose-6-phosphate dehydrogenase deficiency and cardiac surgery. Perfusion. 2010;25:417–421. doi: 10.1177/0267659110380770. [DOI] [PubMed] [Google Scholar]
- 62.Porto I., Leo A., Crea F. Glucose-6-phosphate dehydrogenase (G6PDH) deficiency in a patient with ST-segment elevation acute myocardial infarction successfully treated by simple thrombectomy. J. Atheroscler. Thromb. 2011;18:425–430. doi: 10.5551/jat.6858. [DOI] [PubMed] [Google Scholar]
- 63.Chowdhry V., Bisoyi S., Mishra B. Perioperative challenges in a patient of severe G6PD deficiency undergoing open heart surgery. Ann. Card. Anaesth. 2012;15:50–53. doi: 10.4103/0971-9784.91483. [DOI] [PubMed] [Google Scholar]
- 64.Albertsen J., Ommen H.B., Wandler A., Munk K. Fatal haemolytic crisis with microvascular pulmonary obstruction mimicking a pulmonary embolism in a young African man with glucose-6-phosphate dehydrogenase deficiency. BMJ Case Rep. 2014;2014:bcr2013201432. doi: 10.1136/bcr-2013-201432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balderia P.G., Wongrakpanich S., Patel M., Stanek M. Healing the orphaned heart: Heart failure in a patient with glucose-6-phosphate dehydrogenase deficiency. BMJ Case Rep. 2015;2015:bcr2015209365. doi: 10.1136/bcr-2015-209365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padakanti A., Shenoy A., Kamath A., Chakrapani M. Drug-induced Hemolysis in G6PD Deficiency: An Unusual Presentation of a Common Clinical Condition. Acta Med. 2019;62:166–169. doi: 10.14712/18059694.2020.7. [DOI] [PubMed] [Google Scholar]
- 67.Dore M.P., Portoghese M., Pes G.M. The Elderly with Glucose-6-Phosphate Dehydrogenase Deficiency are More Susceptible to Cardiovascular Disease. J. Atheroscler. Thromb. 2021;28:604–610. doi: 10.5551/jat.56531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meloni L., Manca M.R., Loddo I., Cioglia G., Cocco P., Schwartz A., Muntoni S., Muntoni S. Glucose-6-phosphate dehydrogenase deficiency protects against coronary heart disease. J. Inherit. Metab. Dis. 2008;31:412–417. doi: 10.1007/s10545-008-0704-5. [DOI] [PubMed] [Google Scholar]
- 69.Hecker P.A., Leopold J.A., Gupte S.A., Recchia F.A., Stanley W.C. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am. J. Physiology. Heart Circ. Physiol. 2013;304:H491–H500. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain M., Brenner D.A., Cui L., Lim C.C., Wang B., Pimentel D.R., Koh S., Sawyer D.B., Leopold J.A., Handy D.E., et al. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ. Res. 2003;93:e9–e16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 71.Brown A. 457Glycogen and Energy Metabolism. In: Kettenmann H., Ransom B.R., editors. Neuroglia. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 72.Di Rocco M., Buzzi D., Tarò M. Glycogen storage disease type II: Clinical overview. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2007;26:42–44. [PMC free article] [PubMed] [Google Scholar]
- 73.Lim J.A., Li L., Raben N. Pompe disease: From pathophysiology to therapy and back again. Front. Aging Neurosci. 2014;6:177. doi: 10.3389/fnagi.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuishi T., Yoshino M., Terasawa K., Nonaka I. Childhood acid maltase deficiency. A clinical, biochemical, and morphologic study of three patients. Arch. Neurol. 1984;41:47–52. doi: 10.1001/archneur.1984.04050130053022. [DOI] [PubMed] [Google Scholar]
- 75.Van Kooten H.A., Roelen C.H.A., Brusse E., Van der Beek N., Michels M., Van der Ploeg A.T., Wagenmakers M., Van Doorn P.A. Cardiovascular disease in non-classic Pompe disease: A systematic review. Neuromuscul. Disord. NMD. 2021;31:79–90. doi: 10.1016/j.nmd.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Szymańska E., Szymańska S., Truszkowska G., Ciara E., Pronicki M., Shin Y.S., Podskarbi T., Kępka A., Śpiewak M., Płoski R., et al. Variable clinical presentation of glycogen storage disease type IV: From severe hepatosplenomegaly to cardiac insufficiency. Some discrepancies in genetic and biochemical abnormalities. Arch. Med. Sci. AMS. 2018;14:237–247. doi: 10.5114/aoms.2018.72246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ndugga-Kabuye M.K., Maleszewski J., Chanprasert S., Smith K.D. Glycogen storage disease type IV: Dilated cardiomyopathy as the isolated initial presentation in an adult patient. BMJ Case Rep. 2019;12:e230068. doi: 10.1136/bcr-2019-230068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shin Y.S. Glycogen storage disease: Clinical, biochemical, and molecular heterogeneity. Semin. Pediatr. Neurol. 2006;13:115–120. doi: 10.1016/j.spen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 79.Ishihara T., Uchino F., Adachi H., Takahashi M., Watanabe S., Tsunetoshi S., Fuji T., Ikee Y. Type IV glycogenosis—A study of two cases. Acta Pathol. Jpn. 1975;25:613–633. doi: 10.1111/j.1440-1827.1975.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 80.Kawaguchi Y., Shirasawa K., Yotsumoto S., Nagahara S. Type III glycogenosis with deposition of urate and amyloid. Acta Pathol. Jpn. 1980;30:599–612. doi: 10.1111/j.1440-1827.1980.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 81.Servidei S., Riepe R.E., Langston C., Tani L.Y., Bricker J.T., Crisp-Lindgren N., Travers H., Armstrong D., DiMauro S. Severe cardiopathy in branching enzyme deficiency. J. Pediatr. 1987;111:51–56. doi: 10.1016/S0022-3476(87)80341-4. [DOI] [PubMed] [Google Scholar]
- 82.Hemsrichart V., Karalak S., Thakerngpol K., Stitnimankarn T. Type IV glycogen storage disease: First reported case in Thailand. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 1989;72:697–700. [PubMed] [Google Scholar]
- 83.Sokal E.M., Van Hoof F., Alberti D., De Ville de Goyet J., De Barsy T., Otte J.B. Progressive cardiac failure following orthotopic liver transplantation for type IV glycogenosis. Eur. J. Pediatr. 1992;151:200–203. doi: 10.1007/BF01954384. [DOI] [PubMed] [Google Scholar]
- 84.Schröder J.M., May R., Shin Y.S., Sigmund M., Nase-Hüppmeier S. Juvenile hereditary polyglucosan body disease with complete branching enzyme deficiency (type IV glycogenosis) Acta Neuropathol. 1993;85:419–430. doi: 10.1007/BF00334454. [DOI] [PubMed] [Google Scholar]
- 85.Van Noort G., Straks W., Van Diggelen O.P., Hennekam R.C. A congenital variant of glycogenosis type IV. Pediatr. Pathol. 1993;13:685–698. doi: 10.3109/15513819309048254. [DOI] [PubMed] [Google Scholar]
- 86.Starzl T.E., Demetris A.J., Trucco M., Ricordi C., Ildstad S., Terasaki P.I., Murase N., Kendall R.S., Kocova M., Rudert W.A., et al. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher′s disease. N. Engl. J. Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herrick M.K., Twiss J.L., Vladutiu G.D., Glasscock G.F., Horoupian D.S. Concomitant branching enzyme and phosphorylase deficiencies. An unusual glycogenosis with extensive neuronal polyglucosan storage. J. Neuropathol. Exp. Neurol. 1994;53:239–246. doi: 10.1097/00005072-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 88.Alshak N.S., Cocjin J., Podesta L., Van de Velde R., Makowka L., Rosenthal P., Geller S.A. Hepatocellular adenoma in glycogen storage disease type IV. Arch. Pathol. Lab. Med. 1994;118:88–91. [PubMed] [Google Scholar]
- 89.Nase S., Kunze K.P., Sigmund M., Schroeder J.M., Shin Y., Hanrath P. A new variant of type IV glycogenosis with primary cardiac manifestation and complete branching enzyme deficiency. In vivo detection by heart muscle biopsy. Eur. Heart J. 1995;16:1698–1704. doi: 10.1093/oxfordjournals.eurheartj.a060797. [DOI] [PubMed] [Google Scholar]
- 90.Rosenthal P., Podesta L., Grier R., Said J.W., Sher L., Cocjin J., Watanabe F., Vasiliauskas E., Van de Velde R., Makowka L. Failure of liver transplantation to diminish cardiac deposits of amylopectin and leukocyte inclusions in type IV glycogen storage disease. Liver Transplant. Surg. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 1995;1:373–376. doi: 10.1002/lt.500010607. [DOI] [PubMed] [Google Scholar]
- 91.Giuffrè B., Parini R., Rizzuti T., Morandi L., Van Diggelen O.P., Bruno C., Giuffrè M., Corsello G., Mosca F. Severe neonatal onset of glycogenosis type IV: Clinical and laboratory findings leading to diagnosis in two siblings. J. Inherit. Metab. Dis. 2004;27:609–619. doi: 10.1023/B:BOLI.0000042980.45692.bb. [DOI] [PubMed] [Google Scholar]
- 92.Das B.B., Narkewicz M.R., Sokol R.J., Chen Y.T., Bali D., Li S.C., Matthews M.R., Mierau G.W., Ivy D.D. Amylopectinosis disease isolated to the heart with normal glycogen branching enzyme activity and gene sequence. Pediatr. Transplant. 2005;9:261–265. doi: 10.1111/j.1399-3046.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 93.Raju G.P., Li H.C., Bali D.S., Chen Y.T., Urion D.K., Lidov H.G., Kang P.B. A case of congenital glycogen storage disease type IV with a novel GBE1 mutation. J. Child Neurol. 2008;23:349–352. doi: 10.1177/0883073807309248. [DOI] [PubMed] [Google Scholar]
- 94.Shandling A.H., Safani M. Coexistent manifestations of the Andersen-Tawil and Brugada syndromes. J. Electrocardiol. 2008;41:102–106. doi: 10.1016/j.jelectrocard.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Eminoglu T.F., Tumer L., Okur I., Olgunturk R., Hasanoglu A., Gonul I.I., Dalgic B. Multisystem involvement in a patient due to accumulation of amylopectin-like material with diminished branching enzyme activity. J. Inherit. Metab. Dis. 2008;31((Suppl. S2)):S255–S259. doi: 10.1007/s10545-008-0819-8. [DOI] [PubMed] [Google Scholar]
- 96.Lamperti C., Salani S., Lucchiari S., Bordoni A., Ripolone M., Fagiolari G., Fruguglietti M.E., Crugnola V., Colombo C., Cappellini A., et al. Neuropathological study of skeletal muscle, heart, liver, and brain in a neonatal form of glycogen storage disease type IV associated with a new mutation in GBE1 gene. J. Inherit. Metab. Dis. 2009;32((Suppl. 1)):S161–S168. doi: 10.1007/s10545-009-1134-8. [DOI] [PubMed] [Google Scholar]
- 97.Willot S., Marchand V., Rasquin A., Alvarez F., Martin S.R. Systemic progression of type IV glycogen storage disease after liver transplantation. J. Pediatr. Gastroenterol. Nutr. 2010;51:661–664. doi: 10.1097/MPG.0b013e3181d29780. [DOI] [PubMed] [Google Scholar]
- 98.Magoulas P.L., El-Hattab A.W., Roy A., Bali D.S., Finegold M.J., Craigen W.J. Diffuse reticuloendothelial system involvement in type IV glycogen storage disease with a novel GBE1 mutation: A case report and review. Hum. Pathol. 2012;43:943–951. doi: 10.1016/j.humpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Aksu T., Colak A., Tufekcioglu O. Cardiac Involvement in Glycogen Storage Disease Type IV: Two Cases and the Two Ends of a Spectrum. Case Rep. Med. 2012;2012:764286. doi: 10.1155/2012/764286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu W., Brundler M.A., Wright J.R., Jr. Polyglucosan Bodies in Placental Extravillious Trophoblast for the Diagnosis of Fatal Perinatal Neuromuscular-type Glycogen Storage Disease Type IV. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2018;21:423–427. doi: 10.1177/1093526617707852. [DOI] [PubMed] [Google Scholar]
- 101.Lyo S., Miles J., Meisner J., Guelfguat M. Case report: Adult-onset manifesting heterozygous glycogen storage disease type IV with dilated cardiomyopathy and absent late gadolinium enhancement on cardiac magnetic resonance imaging. Eur. Heart J. Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cenacchi G., Papa V., Costa R., Pegoraro V., Marozzo R., Fanin M., Angelini C. Update on polyglucosan storage diseases. Virchows Arch. Int. J. Pathol. 2019;475:671–686. doi: 10.1007/s00428-019-02633-6. [DOI] [PubMed] [Google Scholar]
- 103.Ellingwood S.S., Cheng A. Biochemical and clinical aspects of glycogen storage diseases. J. Endocrinol. 2018;238:R131–R141. doi: 10.1530/JOE-18-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mili A., Ben Charfeddine I., Mamaï O., Abdelhak S., Adala L., Amara A., Pagliarani S., Lucchiarri S., Ayadi A., Tebib N., et al. Molecular and biochemical characterization of Tunisian patients with glycogen storage disease type III. J. Hum. Genet. 2012;57:170–175. doi: 10.1038/jhg.2011.122. [DOI] [PubMed] [Google Scholar]
- 105.DiMauro S., Hartwig G.B., Hays A., Eastwood A.B., Franco R., Olarte M., Chang M., Roses A.D., Fetell M., Schoenfeldt R.S., et al. Debrancher deficiency: Neuromuscular disorder in 5 adults. Ann. Neurol. 1979;5:422–436. doi: 10.1002/ana.410050504. [DOI] [PubMed] [Google Scholar]
- 106.Moses S.W., Gadoth N., Bashan N., Ben-David E., Slonim A., Wanderman K.L. Neuromuscular involvement in glycogen storage disease type III. Acta Paediatr. Scand. 1986;75:289–296. doi: 10.1111/j.1651-2227.1986.tb10201.x. [DOI] [PubMed] [Google Scholar]
- 107.Moses S.W., Wanderman K.L., Myroz A., Frydman M. Cardiac involvement in glycogen storage disease type III. Eur. J. Pediatr. 1989;148:764–766. doi: 10.1007/BF00443106. [DOI] [PubMed] [Google Scholar]
- 108.Labrune P., Huguet P., Odievre M. Cardiomyopathy in glycogen-storage disease type III: Clinical and echographic study of 18 patients. Pediatr. Cardiol. 1991;12:161–163. doi: 10.1007/BF02238523. [DOI] [PubMed] [Google Scholar]
- 109.Carvalho J.S., Matthews E.E., Leonard J.V., Deanfield J. Cardiomyopathy of glycogen storage disease type III. Heart Vessel. 1993;8:155–159. doi: 10.1007/BF01744800. [DOI] [PubMed] [Google Scholar]
- 110.Tada H., Kurita T., Ohe T., Shimomura K., Ishihara T., Yamada Y., Osawa N. Glycogen storage disease type III associated with ventricular tachycardia. Am. Heart J. 1995;130:911–912. doi: 10.1016/0002-8703(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 111.Akazawa H., Kuroda T., Kim S., Mito H., Kojo T., Shimada K. Specific heart muscle disease associated with glycogen storage disease type III: Clinical similarity to the dilated phase of hypertrophic cardiomyopathy. Eur. Heart J. 1997;18:532–533. doi: 10.1093/oxfordjournals.eurheartj.a015283. [DOI] [PubMed] [Google Scholar]
- 112.Lee P.J., Deanfield J.E., Burch M., Baig K., McKenna W.J., Leonard J.V. Comparison of the functional significance of left ventricular hypertrophy in hypertrophic cardiomyopathy and glycogenosis type III. Am. J. Cardiol. 1997;79:834–838. doi: 10.1016/S0002-9149(96)00885-5. [DOI] [PubMed] [Google Scholar]
- 113.Cuspidi C., Sampieri L., Pelizzoli S., Pontiggia G., Zanchetti A., Nappo A., Caputo V., Matturri L. Obstructive hypertrophic cardiomyopathy in type III glycogen-storage disease. Acta Cardiol. 1997;52:117–123. [PubMed] [Google Scholar]
- 114.Hashimoto M., Watanabe G., Yokoyama T., Tsutsumi K., Dohi T., Matsuda M., Okubo M., Nakamura N., Tsurumaru M. Case report: Rupture of a gastric varix in liver cirrhosis associated with glycogen storage disease type III. J. Gastroenterol. Hepatol. 1998;13:232–235. doi: 10.1111/j.1440-1746.1998.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 115.Okuda S., Kanda F., Takahashi K., Kawanami C., Kinoshita Y., Fujita M., Maeda S., Jinnai K., Matsushita T., Sugio T., et al. Fatal liver cirrhosis and esophageal variceal hemorrhage in a patient with type IIIa glycogen storage disease. Intern. Med. 1998;37:1055–1057. doi: 10.2169/internalmedicine.37.1055. [DOI] [PubMed] [Google Scholar]
- 116.Sugie H., Fukuda T., Ito M., Sugie Y., Kojoh T., Nonaka I. Novel exon 11 skipping mutation in a patient with glycogen storage disease type IIId. J. Inherit. Metab. Dis. 2001;24:535–545. doi: 10.1023/A:1012459625902. [DOI] [PubMed] [Google Scholar]
- 117.Toda G., Yoshimuta T., Kawano H., Yano K. Glycogen storage disease associated with left ventricular aneurysm in an elderly patient. Jpn. Circ. J. 2001;65:462–464. doi: 10.1253/jcj.65.462. [DOI] [PubMed] [Google Scholar]
- 118.Mohart D., Russo P., Tobias J.D. Perioperative management of a child with glycogen storage disease type III undergoing cardiopulmonary bypass and repair of an atrial septal defect. Paediatr. Anaesth. 2002;12:649–654. doi: 10.1046/j.1460-9592.2002.00942.x. [DOI] [PubMed] [Google Scholar]
- 119.Moon J.C., Mundy H.R., Lee P.J., Mohiaddin R.H., Pennell D.J. Images in cardiovascular medicine. Myocardial fibrosis in glycogen storage disease type III. Circulation. 2003;107:e47. doi: 10.1161/01.CIR.0000050691.73932.CB. [DOI] [PubMed] [Google Scholar]
- 120.Ogimoto A., Okubo M., Okayama H., Shin Y.S., Endo Y., Ebara T., Inoue K., Ohtsuka T., Tahara H., Murase T., et al. A Japanese patient with cardiomyopathy caused by a novel mutation R285X in the AGL gene. Circ. J. Off. J. Jpn. Circ. Soc. 2007;71:1653–1656. doi: 10.1253/circj.71.1653. [DOI] [PubMed] [Google Scholar]
- 121.Dagli A.I., Zori R.T., McCune H., Ivsic T., Maisenbacher M.K., Weinstein D.A. Reversal of glycogen storage disease type IIIa-related cardiomyopathy with modification of diet. J. Inherit. Metab. Dis. 2009;32((Suppl. S1)):S103–S106. doi: 10.1007/s10545-009-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vertilus S.M., Austin S.L., Foster K.S., Boyette K.E., Bali D.S., Li J.S., Kishnani P.S., Wechsler S.B. Echocardiographic manifestations of Glycogen Storage Disease III: Increase in wall thickness and left ventricular mass over time. Genet. Med. Off. J. Am. Coll. Med. Genet. 2010;12:413–423. doi: 10.1097/GIM.0b013e3181e0e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LaBarbera M., Milechman G., Dulbecco F. Premature coronary artery disease in a patient with glycogen storage disease III. J. Invasive Cardiol. 2010;22:E156–E158. [PubMed] [Google Scholar]
- 124.Clemente M., Gussinyer M., Arranz J.A., Riudor E., Yeste D., Albisu M., Carrascosa A. Glycogen storage disease type III with hypoketosis. J. Pediatr. Endocrinol. Metab. JPEM. 2010;23:833–836. doi: 10.1515/jpem.2010.134. [DOI] [PubMed] [Google Scholar]
- 125.Valayannopoulos V., Bajolle F., Arnoux J.B., Dubois S., Sannier N., Baussan C., Petit F., Labrune P., Rabier D., Ottolenghi C., et al. Successful treatment of severe cardiomyopathy in glycogen storage disease type III with D,L-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatr. Res. 2011;70:638–641. doi: 10.1203/PDR.0b013e318232154f. [DOI] [PubMed] [Google Scholar]
- 126.Lee T.M., Berman-Rosenzweig E.S., Slonim A.E., Chung W.K. Two Cases of Pulmonary Hypertension Associated with Type III Glycogen Storage Disease. JIMD Rep. 2011;1:79–82. doi: 10.1007/8904_2011_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramachandran R., Wedatilake Y., Coats C., Walker F., Elliott P., Lee P.J., Lachmann R.H., Murphy E. Pregnancy and its management in women with GSD type III—A single centre experience. J. Inherit. Metab. Dis. 2012;35:245–251. doi: 10.1007/s10545-011-9384-7. [DOI] [PubMed] [Google Scholar]
- 128.Austin S.L., Proia A.D., Spencer-Manzon M.J., Butany J., Wechsler S.B., Kishnani P.S. Cardiac Pathology in Glycogen Storage Disease Type III. JIMD Rep. 2012;6:65–72. doi: 10.1007/8904_2011_118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sentner C.P., Vos Y.J., Niezen-Koning K.N., Mol B., Smit G.P. Mutation Analysis in Glycogen Storage Disease Type III Patients in the Netherlands: Novel Genotype-Phenotype Relationships and Five Novel Mutations in the AGL Gene. JIMD Rep. 2013;7:19–26. doi: 10.1007/8904_2012_134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Preisler N., Pradel A., Husu E., Madsen K.L., Becquemin M.H., Mollet A., Labrune P., Petit F., Hogrel J.Y., Jardel C., et al. Exercise intolerance in Glycogen Storage Disease Type III: Weakness or energy deficiency? Mol. Genet. Metab. 2013;109:14–20. doi: 10.1016/j.ymgme.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 131.Brambilla A., Mannarino S., Pretese R., Gasperini S., Galimberti C., Parini R. Improvement of Cardiomyopathy After High-Fat Diet in Two Siblings with Glycogen Storage Disease Type III. JIMD Rep. 2014;17:91–95. doi: 10.1007/8904_2014_343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayorandan S., Meyer U., Hartmann H., Das A.M. Glycogen storage disease type III: Modified Atkins diet improves myopathy. Orphanet J. Rare Dis. 2014;9:196. doi: 10.1186/s13023-014-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rousseau-Nepton I., Okubo M., Grabs R., Mitchell J., Polychronakos C., Rodd C. A founder AGL mutation causing glycogen storage disease type IIIa in Inuit identified through whole-exome sequencing: A case series. Can. Med. Assoc. J. J. L′Assoc. Med. Can. 2015;187:E68–E73. doi: 10.1503/cmaj.140840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mogahed E.A., Girgis M.Y., Sobhy R., Elhabashy H., Abdelaziz O.M., El-Karaksy H. Skeletal and cardiac muscle involvement in children with glycogen storage disease type III. Eur. J. Pediatr. 2015;174:1545–1548. doi: 10.1007/s00431-015-2546-0. [DOI] [PubMed] [Google Scholar]
- 135.Sentner C.P., Hoogeveen I.J., Weinstein D.A., Santer R., Murphy E., McKiernan P.J., Steuerwald U., Beauchamp N.J., Taybert J., Laforêt P., et al. Glycogen storage disease type III: Diagnosis, genotype, management, clinical course and outcome. J. Inherit. Metab. Dis. 2016;39:697–704. doi: 10.1007/s10545-016-9932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ben Chehida A., Ben Messaoud S., Ben Abdelaziz R., Mansouri H., Boudabous H., Hakim K., Ben Ali N., Ben Ameur Z., Sassi Y., Kaabachi N., et al. A lower energetic, protein and uncooked cornstarch intake is associated with a more severe outcome in glycogen storage disease type III: An observational study of 50 patients. J. Pediatr. Endocrinol. Metab. JPEM. 2018;31:979–986. doi: 10.1515/jpem-2018-0151. [DOI] [PubMed] [Google Scholar]
- 137.Nazari F., Sinaei F., Nilipour Y., Petit F., Oveisgharan S., Nassiri-Toosi M., Razzaghy-Azar M., Mahmoudi M., Nafissi S. Distinct Clinical and Genetic Findings in Iranian Patients with Glycogen Storage Disease Type 3. J. Clin. Neuromuscul. Dis. 2018;19:203–210. doi: 10.1097/CND.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 138.Francini-Pesenti F., Tresso S., Vitturi N. Modified Atkins ketogenic diet improves heart and skeletal muscle function in glycogen storage disease type III. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2019;38:17–20. [PMC free article] [PubMed] [Google Scholar]
- 139.Laforêt P., Inoue M., Goillot E., Lefeuvre C., Cagin U., Streichenberger N., Leonard-Louis S., Brochier G., Madelaine A., Labasse C., et al. Deep morphological analysis of muscle biopsies from type III glycogenesis (GSDIII), debranching enzyme deficiency, revealed stereotyped vacuolar myopathy and autophagy impairment. Acta Neuropathol. Commun. 2019;7:167. doi: 10.1186/s40478-019-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Du C., Wei H., Zhang M., Hu M., Li Z., Zhang C., Luo X., Liang Y. Genetic analysis and long-term treatment monitoring of 11 children with glycogen storage disease type IIIa. J. Pediatr. Endocrinol. Metab. JPEM. 2020;33:923–930. doi: 10.1515/jpem-2019-0453. [DOI] [PubMed] [Google Scholar]
- 141.Olgac A., İnci A., Okur İ., Biberoğlu G., Oğuz D., Ezgü F.S., Kasapkara Ç.S., Aktaş E., Tümer L. Beneficial Effects of Modified Atkins Diet in Glycogen Storage Disease Type IIIa. Ann. Nutr. Metab. 2020;76:233–241. doi: 10.1159/000509335. [DOI] [PubMed] [Google Scholar]
- 142.Rossi A., Hoogeveen I.J., Bastek V.B., De Boer F., Montanari C., Meyer U., Maiorana A., Bordugo A., Dianin A., Campana C., et al. Dietary lipids in glycogen storage disease type III: A systematic literature study, case studies, and future recommendations. J. Inherit. Metab. Dis. 2020;43:770–777. doi: 10.1002/jimd.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Focardi M., Bosco A., Bugelli V., Defraia B., Donati M.A., Pinchi V. “On air” diagnosis of sudden cardiac death with dynamic Holter ECG in glycogen storage disease type III young female. Minerva Pediatr. 2020;72:142–144. doi: 10.23736/S0026-4946.19.05496-3. [DOI] [PubMed] [Google Scholar]
- 144.Marusic T., Zerjav Tansek M., Sirca Campa A., Mezek A., Berden P., Battelino T., Groselj U. Data highlighting effects of Ketogenic diet on cardiomyopathy and hepatopathy in Glycogen storage disease Type IIIA. Data Brief. 2020;32:106205. doi: 10.1016/j.dib.2020.106205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hijazi G., Paschall A., Young S.P., Smith B., Case L.E., Boggs T., Amarasekara S., Austin S.L., Pendyal S., El-Gharbawy A., et al. A retrospective longitudinal study and comprehensive review of adult patients with glycogen storage disease type III. Mol. Genet. Metab. Rep. 2021;29:100821. doi: 10.1016/j.ymgmr.2021.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumru Akin B., Ozturk Hismi B., Daly A. Improvement in hypertrophic cardiomyopathy after using a high-fat, high-protein and low-carbohydrate diet in a non-adherent child with glycogen storage disease type IIIa. Mol. Genet. Metab. Rep. 2022;32:100904. doi: 10.1016/j.ymgmr.2022.100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moslemi A.R., Lindberg C., Nilsson J., Tajsharghi H., Andersson B., Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N. Engl. J. Med. 2010;362:1203–1210. doi: 10.1056/NEJMoa0900661. [DOI] [PubMed] [Google Scholar]
- 148.Hedberg-Oldfors C., Glamuzina E., Ruygrok P., Anderson L.J., Elliott P., Watkinson O., Occleshaw C., Abernathy M., Turner C., Kingston N., et al. Cardiomyopathy as presenting sign of glycogenin-1 deficiency-report of three cases and review of the literature. J. Inherit. Metab. Dis. 2017;40:139–149. doi: 10.1007/s10545-016-9978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hedberg-Oldfors C., De Ridder W., Kalev O., Böck K., Visuttijai K., Caravias G., Töpf A., Straub V., Baets J., Oldfors A. Functional characterization of GYG1 variants in two patients with myopathy and glycogenin-1 deficiency. Neuromuscul. Disord. NMD. 2019;29:951–960. doi: 10.1016/j.nmd.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 150.Visuttijai K., Hedberg-Oldfors C., Thomsen C., Glamuzina E., Kornblum C., Tasca G., Hernandez-Lain A., Sandstedt J., Dellgren G., Roach P., et al. Glycogenin is Dispensable for Glycogen Synthesis in Human Muscle, and Glycogenin Deficiency Causes Polyglucosan Storage. J. Clin. Endocrinol. Metab. 2020;105:557–566. doi: 10.1210/clinem/dgz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malfatti E., Nilsson J., Hedberg-Oldfors C., Hernandez-Lain A., Michel F., Dominguez-Gonzalez C., Viennet G., Akman H.O., Kornblum C., Van den Bergh P., et al. A new muscle glycogen storage disease associated with glycogenin-1 deficiency. Ann. Neurol. 2014;76:891–898. doi: 10.1002/ana.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kollberg G., Tulinius M., Gilljam T., Ostman-Smith I., Forsander G., Jotorp P., Oldfors A., Holme E. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. N. Engl. J. Med. 2007;357:1507–1514. doi: 10.1056/NEJMoa066691. [DOI] [PubMed] [Google Scholar]
- 153.Sukigara S., Liang W.C., Komaki H., Fukuda T., Miyamoto T., Saito T., Saito Y., Nakagawa E., Sugai K., Hayashi Y.K., et al. Muscle glycogen storage disease 0 presenting recurrent syncope with weakness and myalgia. Neuromuscul. Disord. NMD. 2012;22:162–165. doi: 10.1016/j.nmd.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Cameron J.M., Levandovskiy V., MacKay N., Utgikar R., Ackerley C., Chiasson D., Halliday W., Raiman J., Robinson B.H. Identification of a novel mutation in GYS1 (muscle-specific glycogen synthase) resulting in sudden cardiac death, that is diagnosable from skin fibroblasts. Mol. Genet. Metab. 2009;98:378–382. doi: 10.1016/j.ymgme.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 155.Cui L., Zhao L.P., Ye J.Y., Yang L., Huang Y., Jiang X.P., Zhang Q., Jia J.Z., Zhang D.X., Huang Y. The Lysosomal Membrane Protein Lamp2 Alleviates Lysosomal Cell Death by Promoting Autophagic Flux in Ischemic Cardiomyocytes. Front. Cell Dev. Biol. 2020;8:31. doi: 10.3389/fcell.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eskelinen E.L., Illert A.L., Tanaka Y., Schwarzmann G., Blanz J., Von Figura K., Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol. Biol. Cell. 2002;13:3355–3368. doi: 10.1091/mbc.e02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arad M., Maron B.J., Gorham J.M., Johnson W.H., Jr., Saul J.P., Perez-Atayde A.R., Spirito P., Wright G.B., Kanter R.J., Seidman C.E., et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N. Engl. J. Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 158.Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J.E., Oh S.J., Koga Y., et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 159.Sugie K., Yamamoto A., Murayama K., Oh S.J., Takahashi M., Mora M., Riggs J.E., Colomer J., Iturriaga C., Meloni A., et al. Clinicopathological features of genetically confirmed Danon disease. Neurology. 2002;58:1773–1778. doi: 10.1212/WNL.58.12.1773. [DOI] [PubMed] [Google Scholar]
- 160.Takahashi M., Yamamoto A., Takano K., Sudo A., Wada T., Goto Y., Nishino I., Saitoh S. Germline mosaicism of a novel mutation in lysosome-associated membrane protein-2 deficiency (Danon disease) Ann. Neurol. 2002;52:122–125. doi: 10.1002/ana.10235. [DOI] [PubMed] [Google Scholar]
- 161.Lacoste-Collin L., Garcia V., Uro-Coste E., Arné-Bes M.C., Durand D., Levade T., Delisle M.B. Danon′s disease (X-linked vacuolar cardiomyopathy and myopathy): A case with a novel Lamp-2 gene mutation. Neuromuscul. Disord. NMD. 2002;12:882–885. doi: 10.1016/S0960-8966(02)00179-7. [DOI] [PubMed] [Google Scholar]
- 162.Sugie K., Koori T., Yamamoto A., Ogawa M., Hirano M., Inoue K., Nonaka I., Nishino I. Characterization of Danon disease in a male patient and his affected mother. Neuromuscul. Disord. NMD. 2003;13:708–711. doi: 10.1016/S0960-8966(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 163.Charron P., Villard E., Sébillon P., Laforêt P., Maisonobe T., Duboscq-Bidot L., Romero N., Drouin-Garraud V., Frébourg T., Richard P., et al. Danon′s disease as a cause of hypertrophic cardiomyopathy: A systematic survey. Heart (Br. Card. Soc.) 2004;90:842–846. doi: 10.1136/hrt.2003.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Balmer C., Ballhausen D., Bosshard N.U., Steinmann B., Boltshauser E., Bauersfeld U., Superti-Furga A. Familial X-linked cardiomyopathy (Danon disease): Diagnostic confirmation by mutation analysis of the LAMP2gene. Eur. J. Pediatr. 2005;164:509–514. doi: 10.1007/s00431-005-1678-z. [DOI] [PubMed] [Google Scholar]
- 165.Echaniz-Laguna A., Mohr M., Epailly E., Nishino I., Charron P., Richard P., Guiraud-Chaumeil C., Tranchant C. Novel Lamp-2 gene mutation and successful treatment with heart transplantation in a large family with Danon disease. Muscle Nerve. 2006;33:393–397. doi: 10.1002/mus.20471. [DOI] [PubMed] [Google Scholar]
- 166.Fanin M., Nascimbeni A.C., Fulizio L., Spinazzi M., Melacini P., Angelini C. Generalized lysosome-associated membrane protein-2 defect explains multisystem clinical involvement and allows leukocyte diagnostic screening in Danon disease. Am. J. Pathol. 2006;168:1309–1320. doi: 10.2353/ajpath.2006.050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sugimoto S., Shiomi K., Yamamoto A., Nishino I., Nonaka I., Ohi T. LAMP-2 positive vacuolar myopathy with dilated cardiomyopathy. Intern. Med. 2007;46:757–760. doi: 10.2169/internalmedicine.46.6265. [DOI] [PubMed] [Google Scholar]
- 168.Taylor M.R.G., Ku L., Slavov D., Cavanaugh J., Boucek M., Zhu X., Graw S., Carniel E., Barnes C., Quan D., et al. Danon disease presenting with dilated cardiomyopathy and a complex phenotype. J. Hum. Genet. 2007;52:830–835. doi: 10.1007/s10038-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 169.Nadeau A., Therrien C., Karpati G., Sinnreich M. Danon disease due to a novel splice mutation in the LAMP2 gene. Muscle Nerve. 2008;37:338–342. doi: 10.1002/mus.20930. [DOI] [PubMed] [Google Scholar]
- 170.Dougu N., Joho S., Shan L., Shida T., Matsuki A., Uese K., Hirono K., Ichida F., Tanaka K., Nishino I., et al. Novel LAMP-2 mutation in a family with Danon disease presenting with hypertrophic cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2009;73:376–380. doi: 10.1253/circj.CJ-08-0241. [DOI] [PubMed] [Google Scholar]
- 171.Maron B.J., Roberts W.C., Arad M., Haas T.S., Spirito P., Wright G.B., Almquist A.K., Baffa J.M., Saul J.P., Ho C.Y., et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. Jama. 2009;301:1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Regelsberger G., Höftberger R., Pickl W.F., Zlabinger G.J., Körmöczi U., Salzer-Muhar U., Luckner D., Bodamer O.A., Mayr J.A., Muss W.H., et al. Danon disease: Case report and detection of new mutation. J. Inherit. Metab. Dis. 2009;32((Suppl. S1)):S115–S122. doi: 10.1007/s10545-009-1097-9. [DOI] [PubMed] [Google Scholar]
- 173.Toib A., Grange D.K., Kozel B.A., Ewald G.A., White F.V., Canter C.E. Distinct clinical and histopathological presentations of Danon cardiomyopathy in young women. J. Am. Coll. Cardiol. 2010;55:408–410. doi: 10.1016/j.jacc.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 174.Miani D., Taylor M., Mestroni L., D′Aurizio F., Finato N., Fanin M., Brigido S., Proclemer A. Sudden death associated with danon disease in women. Am. J. Cardiol. 2012;109:406–411. doi: 10.1016/j.amjcard.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 175.Cheng Z., Cui Q., Tian Z., Xie H., Chen L., Fang L., Zhu K., Fang Q. Danon disease as a cause of concentric left ventricular hypertrophy in patients who underwent endomyocardial biopsy. Eur. Heart J. 2012;33:649–656. doi: 10.1093/eurheartj/ehr420. [DOI] [PubMed] [Google Scholar]
- 176.Majer F., Vlaskova H., Krol L., Kalina T., Kubanek M., Stolnaya L., Dvorakova L., Elleder M., Sikora J. Danon disease: A focus on processing of the novel LAMP2 mutation and comments on the beneficial use of peripheral white blood cells in the diagnosis of LAMP2 deficiency. Gene. 2012;498:183–195. doi: 10.1016/j.gene.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 177.Fidzianska A., Madej-Pilarczyk A., Walczak E., Kuch M. Morphologic and clinical aspects of Danon disease in a patient with a mutation c.137G > A in the LAMP-2 gene. Neuropediatrics. 2013;44:276–280. doi: 10.1055/s-0033-1336017. [DOI] [PubMed] [Google Scholar]
- 178.Majer F., Pelak O., Kalina T., Vlaskova H., Dvorakova L., Honzik T., Palecek T., Kuchynka P., Masek M., Zeman J., et al. Mosaic tissue distribution of the tandem duplication of LAMP2 exons 4 and 5 demonstrates the limits of Danon disease cellular and molecular diagnostics. J. Inherit. Metab. Dis. 2014;37:117–124. doi: 10.1007/s10545-013-9617-z. [DOI] [PubMed] [Google Scholar]
- 179.Kim J., Parikh P., Mahboob M., Arrighi J.A., Atalay M.K., Rowin E.J., Maron M.S. Asymptomatic young man with Danon disease. Tex. Heart Inst. J. 2014;41:332–334. doi: 10.14503/THIJ-13-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sugie K., Yoshizawa H., Onoue K., Nakanishi Y., Eura N., Ogawa M., Nakano T., Sakaguchi Y., Hayashi Y.K., Kishimoto T., et al. Early onset of cardiomyopathy and intellectual disability in a girl with Danon disease associated with a de novo novel mutation of the LAMP2 gene. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2016;36:561–565. doi: 10.1111/neup.12307. [DOI] [PubMed] [Google Scholar]
- 181.Ng K.M., Mok P.Y., Butler A.W., Ho J.C., Choi S.W., Lee Y.K., Lai W.H., Au K.W., Lau Y.M., Wong L.Y., et al. Amelioration of X-Linked Related Autophagy Failure in Danon Disease with DNA Methylation Inhibitor. Circulation. 2016;134:1373–1389. doi: 10.1161/CIRCULATIONAHA.115.019847. [DOI] [PubMed] [Google Scholar]
- 182.Marino M., Musumeci O., Paleologo G., Cucinotta M., Migliorato A., Rodolico C., Toscano A. Ischemic stroke due to hypoperfusion in a patient with a previously unrecognized Danon disease. Neuromuscul. Disord. NMD. 2016;26:890–894. doi: 10.1016/j.nmd.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 183.Kitahara H., Nawata K., Kinoshita O., Itoda Y., Shintani Y., Fukayama M., Ono M. Implantation of a Left Ventricular Assist Device for Danon Cardiomyopathy. Ann. Thorac. Surg. 2017;103:e39–e41. doi: 10.1016/j.athoracsur.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 184.Samad F., Jain R., Jan M.F., Sulemanjee N.Z., Menaria P., Kalvin L., Bush M., Jahangir A., Khandheria B.K., Tajik A.J. Malignant cardiac phenotypic expression of Danon disease (LAMP2 cardiomyopathy) Int. J. Cardiol. 2017;245:201–206. doi: 10.1016/j.ijcard.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 185.Nguyen T.V., Tran Vu M.T., Do T.N.P., Tran T.H.N., Do T.H., Nguyen T.M.H., Tran Huynh B.N., Le L.A., Nguyen Pham N.T., Nguyen T.D.A., et al. Genetic Determinants and Genotype-Phenotype Correlations in Vietnamese Patients with Dilated Cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2021;85:1469–1478. doi: 10.1253/circj.CJ-21-0077. [DOI] [PubMed] [Google Scholar]
- 186.Sugie K., Komaki H., Eura N., Shiota T., Onoue K., Tsukaguchi H., Minami N., Ogawa M., Kiriyama T., Kataoka H., et al. A Nationwide Survey on Danon Disease in Japan. Int. J. Mol. Sci. 2018;19:3507. doi: 10.3390/ijms19113507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Y., Chen X., Wang F., Liang Y., Deng H., Liao H., Zhang Q., Zhang B., Zhan X., Fang X., et al. Prevalence and clinical characteristics of Danon disease among patients with left ventricular hypertrophy and concomitant electrocardiographic preexcitation. Mol. Genet. Genom. Med. 2019;7:e638. doi: 10.1002/mgg3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Di Nora C., Miani D., D′Elia A.V., Poli S., Iascone M., Nucifora G., Finato N., Sponga S., Proclemer A., Livi U. Heart transplantation in Danon disease: Long term single centre experience and review of the literature. Eur. J. Med. Genet. 2020;63:103645. doi: 10.1016/j.ejmg.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 189.Meinert M., Englund E., Hedberg-Oldfors C., Oldfors A., Kornhall B., Lundin C., Wittström E. Danon disease presenting with early onset of hypertrophic cardiomyopathy and peripheral pigmentary retinal dystrophy in a female with a de novo novel mosaic mutation in the LAMP2 gene. Ophthalmic Genet. 2019;40:227–236. doi: 10.1080/13816810.2019.1627464. [DOI] [PubMed] [Google Scholar]
- 190.Nguyen H.T., Noguchi S., Sugie K., Matsuo Y., Nguyen C.T.H., Koito H., Shiojima I., Nishino I., Tsukaguchi H. Small-Vessel Vasculopathy Due to Aberrant Autophagy in LAMP-2 Deficiency. Sci. Rep. 2018;8:3326. doi: 10.1038/s41598-018-21602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miliou A., Antonopoulos A.S., Kouris N., Lazaros G., Tsioufis K., Vlachopoulos C. Danon Cardiomyopathy: Specific Imaging Signs. JACC Case Rep. 2022;4:1496–1500. doi: 10.1016/j.jaccas.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hashida Y., Wada T., Saito T., Ohta K., Kasahara Y., Yachie A. Early diagnosis of Danon disease: Flow cytometric detection of lysosome-associated membrane protein-2-negative leukocytes. J. Cardiol. 2015;66:168–174. doi: 10.1016/j.jjcc.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 193.Gollob M.H. Glycogen storage disease as a unifying mechanism of disease in the PRKAG2 cardiac syndrome. Biochem. Soc. Trans. 2003;31:228–231. doi: 10.1042/bst0310228. [DOI] [PubMed] [Google Scholar]
- 194.Blair E., Redwood C., Ashrafian H., Oliveira M., Broxholme J., Kerr B., Salmon A., Ostman-Smith I., Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: Evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 195.Arad M., Benson D.W., Perez-Atayde A.R., McKenna W.J., Sparks E.A., Kanter R.J., McGarry K., Seidman J.G., Seidman C.E. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Investig. 2002;109:357–362. doi: 10.1172/JCI0214571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vaughan C.J., Hom Y., Okin D.A., McDermott D.A., Lerman B.B., Basson C.T. Molecular genetic analysis of PRKAG2 in sporadic Wolff-Parkinson-White syndrome. J. Cardiovasc. Electrophysiol. 2003;14:263–268. doi: 10.1046/j.1540-8167.2003.02394.x. [DOI] [PubMed] [Google Scholar]
- 197.Murphy R.T., Mogensen J., McGarry K., Bahl A., Evans A., Osman E., Syrris P., Gorman G., Farrell M., Holton J.L., et al. Adenosine monophosphate-activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome: Natural history. J. Am. Coll. Cardiol. 2005;45:922–930. doi: 10.1016/j.jacc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 198.Burwinkel B., Scott J.W., Bührer C., Van Landeghem F.K., Cox G.F., Wilson C.J., Grahame Hardie D., Kilimann M.W. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am. J. Hum. Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laforêt P., Richard P., Said M.A., Romero N.B., Lacene E., Leroy J.P., Baussan C., Hogrel J.Y., Lavergne T., Wahbi K., et al. A new mutation in PRKAG2 gene causing hypertrophic cardiomyopathy with conduction system disease and muscular glycogenosis. Neuromuscul. Disord. NMD. 2006;16:178–182. doi: 10.1016/j.nmd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 200.Akman H.O., Sampayo J.N., Ross F.A., Scott J.W., Wilson G., Benson L., Bruno C., Shanske S., Hardie D.G., Dimauro S. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Pediatr. Res. 2007;62:499–504. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 201.Tan H.L., Van der Wal A.C., Campian M.E., Kruyswijk H.H., Ten Hove Jansen B., Van Doorn D.J., Oskam H.J., Becker A.E., Wilde A.A. Nodoventricular accessory pathways in PRKAG2-dependent familial preexcitation syndrome reveal a disorder in cardiac development. Circ. Arrhythmia Electrophysiol. 2008;1:276–281. doi: 10.1161/CIRCEP.108.782862. [DOI] [PubMed] [Google Scholar]
- 202.Yang K.Q., Lu C.X., Zhang Y., Yang Y.K., Li J.C., Lan T., Meng X., Fan P., Tian T., Wang L.P., et al. A novel PRKAG2 mutation in a Chinese family with cardiac hypertrophy and ventricular pre-excitation. Sci. Rep. 2017;7:2407. doi: 10.1038/s41598-017-02455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thevenon J., Laurent G., Ader F., Laforêt P., Klug D., Duva Pentiah A., Gouya L., Maurage C.A., Kacet S., Eicher J.C., et al. High prevalence of arrhythmic and myocardial complications in patients with cardiac glycogenosis due to PRKAG2 mutations. EP Eur. 2017;19:651–659. doi: 10.1093/europace/euw067. [DOI] [PubMed] [Google Scholar]
- 204.Hu J., Tang B., Wang J., Huang K., Wang Y., Lu S., Gowreesunkur H.B., Wang Y., Wu D., Mayala H.A., et al. Familial Atrial Enlargement, Conduction Disorder and Symmetric Cardiac Hypertrophy Are Early Signs of PRKAG2 R302Q. Curr. Med. Sci. 2020;40:486–492. doi: 10.1007/s11596-020-2207-z. [DOI] [PubMed] [Google Scholar]
- 205.Beyzaei Z., Ezgu F., Geramizadeh B., Imanieh M.H., Haghighat M., Dehghani S.M., Honar N., Zahmatkeshan M., Jassbi A., Mahboubifar M., et al. Clinical and genetic spectrum of glycogen storage disease in Iranian population using targeted gene sequencing. Sci. Rep. 2021;11:7040. doi: 10.1038/s41598-021-86338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nilsson J., Schoser B., Laforet P., Kalev O., Lindberg C., Romero N.B., Dávila López M., Akman H.O., Wahbi K., Iglseder S., et al. Polyglucosan body myopathy caused by defective ubiquitin ligase RBCK1. Ann. Neurol. 2013;74:914–919. doi: 10.1002/ana.23963. [DOI] [PubMed] [Google Scholar]
- 207.Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z., Abhyankar A., Israël L., Trevejo-Nunez G., Bogunovic D., et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yamanaka K., Ishikawa H., Megumi Y., Tokunaga F., Kanie M., Rouault T.A., Morishima I., Minato N., Ishimori K., Iwai K. Identification of the ubiquitin-protein ligase that recognizes oxidized IRP2. Nat. Cell Biol. 2003;5:336–340. doi: 10.1038/ncb952. [DOI] [PubMed] [Google Scholar]
- 209.Wang K., Kim C., Bradfield J., Guo Y., Toskala E., Otieno F.G., Hou C., Thomas K., Cardinale C., Lyon G.J., et al. Whole-genome DNA/RNA sequencing identifies truncating mutations in RBCK1 in a novel Mendelian disease with neuromuscular and cardiac involvement. Genome Med. 2013;5:67. doi: 10.1186/gm471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krenn M., Salzer E., Simonitsch-Klupp I., Rath J., Wagner M., Haack T.B., Strom T.M., Schänzer A., Kilimann M.W., Schmidt R.L.J., et al. Mutations outside the N-terminal part of RBCK1 may cause polyglucosan body myopathy with immunological dysfunction: Expanding the genotype-phenotype spectrum. J. Neurol. 2018;265:394–401. doi: 10.1007/s00415-017-8710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Phadke R., Hedberg-Oldfors C., Scalco R.S., Lowe D.M., Ashworth M., Novelli M., Vara R., Merwick A., Amer H., Sofat R., et al. RBCK1-related disease: A rare multisystem disorder with polyglucosan storage, auto-inflammation, recurrent infections, skeletal, and cardiac myopathy-Four additional patients and a review of the current literature. J. Inherit. Metab. Dis. 2020;43:1002–1013. doi: 10.1002/jimd.12234. [DOI] [PubMed] [Google Scholar]
- 212.Belkaid A., Copland I.B., Massillon D., Annabi B. Silencing of the human microsomal glucose-6-phosphate translocase induces glioma cell death: Potential new anticancer target for curcumin. FEBS Lett. 2006;580:3746–3752. doi: 10.1016/j.febslet.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 213.Ng B.G., Sosicka P., Fenaille F., Harroche A., Vuillaumier-Barrot S., Porterfield M., Xia Z.J., Wagner S., Bamshad M.J., Vergnes-Boiteux M.C., et al. A mutation in SLC37A4 causes a dominantly inherited congenital disorder of glycosylation characterized by liver dysfunction. Am. J. Hum. Genet. 2021;108:1040–1052. doi: 10.1016/j.ajhg.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meimand S.E., Azizi G., Yazdani R., Sanadgol N., Rezaei N. Novel mutation of SLC37A4 in a glycogen storage disease type Ib patient with neutropenia, horseshoe kidney, and arteriovenous malformation: A case report. Immunol. Res. 2022;71:107–111. doi: 10.1007/s12026-022-09320-w. [DOI] [PubMed] [Google Scholar]
- 215.Lefeber D.J., Freeze H.H., Steet R., Kinoshita T. Congenital Disorders of Glycosylation. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Mohnen D., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. 4th ed. Cold Spring Harbor; Long Island, NY, USA: 2022. pp. 599–614. [Google Scholar]
- 216.Alsharhan H., Ng B.G., Daniel E.J.P., Friedman J., Pivnick E.K., Al-Hashem A., Faqeih E.A., Liu P., Engelhardt N.M., Keller K.N., et al. Expanding the phenotype, genotype and biochemical knowledge of ALG3-CDG. J. Inherit. Metab. Dis. 2021;44:987–1000. doi: 10.1002/jimd.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bian Y., Qiao C., Zheng S., Qiu H., Li H., Zhang Z., Yin S., Jiang H., Li-Ling J., Liu C., et al. ALG3-CDG: Lethal phenotype and novel variants in Chinese siblings. J. Hum. Genet. 2020;65:1129–1134. doi: 10.1038/s10038-020-0798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Farolfi M., Cechova A., Ondruskova N., Zidkova J., Kousal B., Hansikova H., Honzik T., Liskova P. ALG3-CDG: A patient with novel variants and review of the genetic and ophthalmic findings. BMC Ophthalmol. 2021;21:249. doi: 10.1186/s12886-021-02013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alsubhi S., Alhashem A., Faqeih E., Alfadhel M., Alfaifi A., Altuwaijri W., Alsahli S., Aldhalaan H., Alkuraya F.S., Hundallah K., et al. Congenital disorders of glycosylation: The Saudi experience. Am. J. Med. Genet. Part A. 2017;173:2614–2621. doi: 10.1002/ajmg.a.38358. [DOI] [PubMed] [Google Scholar]
- 220.Himmelreich N., Dimitrov B., Geiger V., Zielonka M., Hutter A.M., Beedgen L., Hüllen A., Breuer M., Peters V., Thiemann K.C., et al. Novel variants and clinical symptoms in four new ALG3-CDG patients, review of the literature, and identification of AAGRP-ALG3 as a novel ALG3 variant with alanine and glycine-rich N-terminus. Hum. Mutat. 2019;40:938–951. doi: 10.1002/humu.23764. [DOI] [PubMed] [Google Scholar]
- 221.Al-Owain M., Mohamed S., Kaya N., Zagal A., Matthijs G., Jaeken J. A novel mutation and first report of dilated cardiomyopathy in ALG6-CDG (CDG-Ic): A case report. Orphanet J. Rare Dis. 2010;5:7. doi: 10.1186/1750-1172-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Himmelreich N., Dimitrov B., Zielonka M., Hüllen A., Hoffmann G.F., Juenger H., Müller H., Lorenz I., Busse B., Marschall C., et al. Missense variant c.1460 T > C (p.L487P) enhances protein degradation of ER mannosyltransferase ALG9 in two new ALG9-CDG patients presenting with West syndrome and review of the literature. Mol. Genet. Metab. 2022;136:274–281. doi: 10.1016/j.ymgme.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 223.Weinstein M., Schollen E., Matthijs G., Neupert C., Hennet T., Grubenmann C.E., Frank C.G., Aebi M., Clarke J.T., Griffiths A., et al. CDG-IL: An infant with a novel mutation in the ALG9 gene and additional phenotypic features. Am. J. Med. Genet. Part A. 2005;136:194–197. doi: 10.1002/ajmg.a.30851. [DOI] [PubMed] [Google Scholar]
- 224.Vleugels W., Keldermans L., Jaeken J., Butters T.D., Michalski J.C., Matthijs G., Foulquier F. Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology. 2009;19:910–917. doi: 10.1093/glycob/cwp067. [DOI] [PubMed] [Google Scholar]
- 225.Tham E., Eklund E.A., Hammarsjö A., Bengtson P., Geiberger S., Lagerstedt-Robinson K., Malmgren H., Nilsson D., Grigelionis G., Conner P., et al. A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach–Nishimura skeletal dysplasia due to pathogenic variants in ALG9. Eur. J. Hum. Genet. 2016;24:198–207. doi: 10.1038/ejhg.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Davis K., Webster D., Smith C., Jackson S., Sinasac D., Seargeant L., Wei X.C., Ferreira P., Midgley J., Foster Y., et al. ALG9-CDG: New clinical case and review of the literature. Mol. Genet. Metab. Rep. 2017;13:55–63. doi: 10.1016/j.ymgmr.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kranz C., Basinger A.A., Güçsavaş-Calikoğlu M., Sun L., Powell C.M., Henderson F.W., Aylsworth A.S., Freeze H.H. Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): Sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am. J. Med. Genet. Part A. 2007;143:1371–1378. doi: 10.1002/ajmg.a.31791. [DOI] [PubMed] [Google Scholar]
- 228.De la Morena-Barrio M.E., Sabater M., De la Morena-Barrio B., Ruhaak R.L., Miñano A., Padilla J., Toderici M., Roldán V., Gimeno J.R., Vicente V., et al. ALG12-CDG: An unusual patient without intellectual disability and facial dysmorphism, and with a novel variant. Mol. Genet. Genom. Med. 2020;8:e1304. doi: 10.1002/mgg3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tahata S., Gunderson L., Lanpher B., Morava E. Complex phenotypes in ALG12-congenital disorder of glycosylation (ALG12-CDG): Case series and review of the literature. Mol. Genet. Metab. 2019;128:409–414. doi: 10.1016/j.ymgme.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 230.Kristiansson B., Stibler H., Conradi N., Eriksson B.O., Ryd W. The heart and pericardial effusions in CDGS-I (carbohydrate-deficient glycoprotein syndrome type I) J. Inherit. Metab. Dis. 1998;21:112–124. doi: 10.1023/A:1005387408009. [DOI] [PubMed] [Google Scholar]
- 231.Ning B., Elbein A.D. Cloning, expression and characterization of the pig liver GDP-mannose pyrophosphorylase. Evidence that GDP-mannose and GDP-Glc pyrophosphorylases are different proteins. Eur. J. Biochem. 2000;267:6866–6874. doi: 10.1046/j.1432-1033.2000.01781.x. [DOI] [PubMed] [Google Scholar]
- 232.Cabrera-Serrano M., Ghaoui R., Ravenscroft G., Johnsen R.D., Davis M.R., Corbett A., Reddel S., Sue C.M., Liang C., Waddell L.B., et al. Expanding the phenotype of GMPPB mutations. Brain J. Neurol. 2015;138:836–844. doi: 10.1093/brain/awv013. [DOI] [PubMed] [Google Scholar]
- 233.Carss K.J., Stevens E., Foley A.R., Cirak S., Riemersma M., Torelli S., Hoischen A., Willer T., Van Scherpenzeel M., Moore S.A., et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 2013;93:29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Oestergaard S.T., Stojkovic T., Dahlqvist J.R., Bouchet-Seraphin C., Nectoux J., Leturcq F., Cossée M., Solé G., Thomsen C., Krag T.O., et al. Muscle involvement in limb-girdle muscular dystrophy with GMPPB deficiency (LGMD2T) Neurol. Genet. 2016;2:e112. doi: 10.1212/NXG.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Willems A.P., Van Engelen B.G., Lefeber D.J. Genetic defects in the hexosamine and sialic acid biosynthesis pathway. Biochim. Biophys. Acta. 2016;1860:1640–1654. doi: 10.1016/j.bbagen.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 236.Wen X.Y., Tarailo-Graovac M., Brand-Arzamendi K., Willems A., Rakic B., Huijben K., Da Silva A., Pan X., El-Rass S., Ng R., et al. Sialic acid catabolism by N-acetylneuraminate pyruvate lyase is essential for muscle function. JCI Insight. 2018;3:e122373. doi: 10.1172/jci.insight.122373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jin G.Z., Zhang Y., Cong W.M., Wu X., Wang X., Wu S., Wang S., Zhou W., Yuan S., Gao H., et al. Phosphoglucomutase 1 inhibits hepatocellular carcinoma progression by regulating glucose trafficking. PLoS Biol. 2018;16:e2006483. doi: 10.1371/journal.pbio.2006483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Conte F., van Buuringen N., Voermans N.C., Lefeber D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta. Gen. Subj. 2021;1865:129898. doi: 10.1016/j.bbagen.2021.129898. [DOI] [PubMed] [Google Scholar]
- 239.Conte F., Morava E., Bakar N.A., Wortmann S.B., Poerink A.J., Grunewald S., Crushell E., Al-Gazali L., de Vries M.C., Mørkrid L., et al. Phosphoglucomutase-1 deficiency: Early presentation, metabolic management and detection in neonatal blood spots. Mol. Genet. Metab. 2020;131:135–146. doi: 10.1016/j.ymgme.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 240.Altassan R., Péanne R., Jaeken J., Barone R., Bidet M., Borgel D., Brasil S., Cassiman D., Cechova A., Coman D., et al. International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: Diagnosis, treatment and follow up. J. Inherit. Metab. Dis. 2019;42:5–28. doi: 10.1002/jimd.12024. [DOI] [PubMed] [Google Scholar]
- 241.Donoghue S.E., White S.M., Tan T.Y., Kowalski R., Morava E., Yaplito-Lee J. Galactose treatment of a PGM1 patient presenting with restrictive cardiomyopathy. JIMD Rep. 2021;57:29–37. doi: 10.1002/jmd2.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lipiński P., Bogdańska A., Tylki-Szymańska A. Congenital disorders of glycosylation: Prevalence, incidence and mutational spectrum in the Polish population. Mol. Genet. Metab. Rep. 2021;27:100726. doi: 10.1016/j.ymgmr.2021.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lipiński P., Cielecka-Kuszyk J., Czarnowska E., Bogdańska A., Socha P., Tylki-Szymańska A. Congenital disorders of glycosylation in children–Histopathological and ultrastructural changes in the liver. Pediatr. Neonatol. 2021;62:278–283. doi: 10.1016/j.pedneo.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 244.Timal S., Hoischen A., Lehle L., Adamowicz M., Huijben K., Sykut-Cegielska J., Paprocka J., Jamroz E., van Spronsen F.J., Körner C., et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum. Mol. Genet. 2012;21:4151–4161. doi: 10.1093/hmg/dds123. [DOI] [PubMed] [Google Scholar]
- 245.Tegtmeyer L.C., Rust S., van Scherpenzeel M., Ng B.G., Losfeld M.E., Timal S., Raymond K., He P., Ichikawa M., Veltman J., et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N. Engl. J. Med. 2014;370:533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Küçükçongar A., Tümer L., Ezgü F.S., Kasapkara Ç.S., Jaeken J., Matthijs G., Rymen D., Dalgiç B., Bıdecı A., Hasanoğlu A. A case with rare type of congenital disorder of glycosylation: PGM1-CDG. Genet. Couns. 2015;26:87–90. [PubMed] [Google Scholar]
- 247.Loewenthal N., Haim A., Parvari R., Hershkovitz E. Phosphoglucomutase-1 deficiency: Intrafamilial clinical variability and common secondary adrenal insufficiency. Am. J. Med. Genet. Part A. 2015;167:3139–3143. doi: 10.1002/ajmg.a.37294. [DOI] [PubMed] [Google Scholar]
- 248.Wong S.Y., Beamer L.J., Gadomski T., Honzik T., Mohamed M., Wortmann S.B., Brocke Holmefjord K.S., Mork M., Bowling F., Sykut-Cegielska J., et al. Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. J. Pediatr. 2016;175:130–136.e138. doi: 10.1016/j.jpeds.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 249.Schrapers E., Tegtmeyer L.C., Simic-Schleicher G., Debus V., Reunert J., Balbach S., Klingel K., Du Chesne I., Seelhöfer A., Fobker M., et al. News on Clinical Details and Treatment in PGM1-CDG. JIMD Rep. 2016;26:77–84. doi: 10.1007/8904_2015_471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zeevaert R., Scalais E., Muino Mosquera L., De Meirleir L., De Beaufort C., Witsch M., Jaeken J., De Schepper J. PGM1 deficiency diagnosed during an endocrine work-up of low IGF-1 mediated growth failure. Acta Clin. Belg. 2016;71:435–437. doi: 10.1080/17843286.2016.1142043. [DOI] [PubMed] [Google Scholar]
- 251.Wong S.Y., Gadomski T., van Scherpenzeel M., Honzik T., Hansikova H., Holmefjord K.S.B., Mork M., Bowling F., Sykut-Cegielska J., Koch D., et al. Oral D-galactose supplementation in PGM1-CDG. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017;19:1226–1235. doi: 10.1038/gim.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Voermans N.C., Preisler N., Madsen K.L., Janssen M.C., Kusters B., Abu Bakar N., Conte F., Lamberti V.M., Nusman F., van Engelen B.G., et al. PGM1 deficiency: Substrate use during exercise and effect of treatment with galactose. Neuromuscul. Disord. NMD. 2017;27:370–376. doi: 10.1016/j.nmd.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 253.Nolting K., Park J.H., Tegtmeyer L.C., Zühlsdorf A., Grüneberg M., Rust S., Reunert J., Du Chesne I., Debus V., Schulze-Bahr E., et al. Limitations of galactose therapy in phosphoglucomutase 1 deficiency. Mol. Genet. Metab. Rep. 2017;13:33–40. doi: 10.1016/j.ymgmr.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ding Y., Li N., Chang G., Li J., Yao R., Shen Y., Wang J., Huang X., Wang X. Clinical and molecular genetic characterization of two patients with mutations in the phosphoglucomutase 1 (PGM1) gene. J. Pediatr. Endocrinol. Metab. JPEM. 2018;31:781–788. doi: 10.1515/jpem-2017-0551. [DOI] [PubMed] [Google Scholar]
- 255.Tian W.T., Luan X.H., Zhou H.Y., Zhang C., Huang X.J., Liu X.L., Chen S.D., Tang H.D., Cao L. Congenital disorder of glycosylation type 1T with a novel truncated homozygous mutation in PGM1 gene and literature review. Neuromuscul. Disord. NMD. 2019;29:282–289. doi: 10.1016/j.nmd.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 256.Fernlund E., Andersson O., Ellegård R., Årstrand H.K., Green H., Olsson H., Gunnarsson C. The congenital disorder of glycosylation in PGM1 (PGM1-CDG) can cause severe cardiomyopathy and unexpected sudden cardiac death in childhood. Forensic Sci. Int. Genet. 2019;43:102111. doi: 10.1016/j.fsigen.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 257.Perales-Clemente E., Liedtke K., Studinski A., Radenkovic S., Gavrilov D., Oglesbee D., Matern D., Rinaldo P., Tortorelli S., Morava E., et al. A new D-galactose treatment monitoring index for PGM1-CDG. J. Inherit. Metab. Dis. 2021;44:1263–1271. doi: 10.1002/jimd.12406. [DOI] [PubMed] [Google Scholar]
- 258.Arimura T., Inagaki N., Hayashi T., Shichi D., Sato A., Hinohara K., Vatta M., Towbin J.A., Chikamori T., Yamashina A., et al. Impaired binding of ZASP/Cypher with phosphoglucomutase 1 is associated with dilated cardiomyopathy. Cardiovasc. Res. 2009;83:80–88. doi: 10.1093/cvr/cvp119. [DOI] [PubMed] [Google Scholar]
- 259.Feldman B.J., Rosenthal D. Carbohydrate-deficient glycoprotein syndrome-associated pericardial effusion treated with corticosteroids and salicylic acid. Pediatr. Cardiol. 2002;23:469–471. doi: 10.1007/s00246-002-1497-1. [DOI] [PubMed] [Google Scholar]
- 260.Marquardt T., Hülskamp G., Gehrmann J., Debus V., Harms E., Kehl H.G. Severe transient myocardial ischaemia caused by hypertrophic cardiomyopathy in a patient with congenital disorder of glycosylation type Ia. Eur. J. Pediatr. 2002;161:524–527. doi: 10.1007/s00431-002-1029-2. [DOI] [PubMed] [Google Scholar]
- 261.Gehrmann J., Sohlbach K., Linnebank M., Böhles H.J., Buderus S., Kehl H.G., Vogt J., Harms E., Marquardt T. Cardiomyopathy in congenital disorders of glycosylation. Cardiol. Young. 2003;13:345–351. doi: 10.1017/S1047951103000702. [DOI] [PubMed] [Google Scholar]
- 262.Kusa J., Pyrkosz A., Skiba A., Szkutnik M. Cardiac manifestations of carbohydrate-deficient glycoprotein syndrome. Pediatr. Cardiol. 2003;24:493–494. doi: 10.1007/s00246-002-0399-6. [DOI] [PubMed] [Google Scholar]
- 263.Damen G., de Klerk H., Huijmans J., den Hollander J., Sinaasappel M. Gastrointestinal and other clinical manifestations in 17 children with congenital disorders of glycosylation type Ia, Ib, and Ic. J. Pediatr. Gastroenterol. Nutr. 2004;38:282–287. doi: 10.1097/00005176-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 264.Aronica E., van Kempen A.A., van der Heide M., Poll-The B.T., van Slooten H.J., Troost D., Rozemuller-Kwakkel J.M. Congenital disorder of glycosylation type Ia: A clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol. 2005;109:433–442. doi: 10.1007/s00401-004-0975-3. [DOI] [PubMed] [Google Scholar]
- 265.Noelle V., Knuepfer M., Pulzer F., Schuster V., Siekmeyer W., Matthijs G., Vogtmann C. Unusual presentation of congenital disorder of glycosylation type 1a: Congenital persistent thrombocytopenia, hypertrophic cardiomyopathy and hydrops-like aspect due to marked peripheral oedema. Eur. J. Pediatr. 2005;164:223–226. doi: 10.1007/s00431-004-1611-x. [DOI] [PubMed] [Google Scholar]
- 266.Van de Kamp J.M., Lefeber D.J., Ruijter G.J., Steggerda S.J., den Hollander N.S., Willems S.M., Matthijs G., Poorthuis B.J., Wevers R.A. Congenital disorder of glycosylation type Ia presenting with hydrops fetalis. J. Med. Genet. 2007;44:277–280. doi: 10.1136/jmg.2006.044735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vermeer S., Kremer H.P., Leijten Q.H., Scheffer H., Matthijs G., Wevers R.A., Knoers N.A., Morava E., Lefeber D.J. Cerebellar ataxia and congenital disorder of glycosylation Ia (CDG-Ia) with normal routine CDG screening. J. Neurol. 2007;254:1356–1358. doi: 10.1007/s00415-007-0546-3. [DOI] [PubMed] [Google Scholar]
- 268.Schollen E., Keldermans L., Foulquier F., Briones P., Chabas A., Sánchez-Valverde F., Adamowicz M., Pronicka E., Wevers R., Matthijs G. Characterization of two unusual truncating PMM2 mutations in two CDG-Ia patients. Mol. Genet. Metab. 2007;90:408–413. doi: 10.1016/j.ymgme.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 269.Truin G., Guillard M., Lefeber D.J., Sykut-Cegielska J., Adamowicz M., Hoppenreijs E., Sengers R.C.A., Wevers R.A., Morava E. Pericardial and abdominal fluid accumulation in congenital disorder of glycosylation type Ia. Mol. Genet. Metab. 2008;94:481–484. doi: 10.1016/j.ymgme.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 270.Coman D., Bostock D., Hunter M., Kannu P., Irving M., Mayne V., Fietz M., Jaeken J., Savarirayan R. Primary skeletal dysplasia as a major manifesting feature in an infant with congenital disorder of glycosylation type Ia. Am. J. Med. Genet. Part A. 2008;146:389–392. doi: 10.1002/ajmg.a.32119. [DOI] [PubMed] [Google Scholar]
- 271.Romano S., Bajolle F., Valayannopoulos V., Lyonnet S., Colomb V., de Baracé C., Vouhe P., Pouard P., Vuillaumier-Barrot S., Dupré T., et al. Conotruncal heart defects in three patients with congenital disorder of glycosylation type Ia (CDG Ia) J. Med. Genet. 2009;46:287–288. doi: 10.1136/jmg.2008.057620. [DOI] [PubMed] [Google Scholar]
- 272.Thong M.K., Fietz M., Nicholls C., Lee M.H., Asma O. Congenital disorder of glycosylation type Ia in a Malaysian family: Clinical outcome and description of a novel PMM2 mutation. J. Inherit. Metab. Dis. 2009;32((Suppl. S1)):S41–S44. doi: 10.1007/s10545-009-1031-1. [DOI] [PubMed] [Google Scholar]
- 273.Verstegen R.H., Theodore M., van de Klerk H., Morava E. Lymphatic edema in congenital disorders of glycosylation. JIMD Rep. 2012;4:113–116. doi: 10.1007/8904_2011_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rudaks L.I., Andersen C., Khong T.Y., Kelly A., Fietz M., Barnett C.P. Hypertrophic cardiomyopathy with cardiac rupture and tamponade caused by congenital disorder of glycosylation type Ia. Pediatr. Cardiol. 2012;33:827–830. doi: 10.1007/s00246-012-0214-y. [DOI] [PubMed] [Google Scholar]
- 275.Resende C., Carvalho C., Alegria A., Oliveira D., Quelhas D., Bandeira A., Proença E. Congenital disorders of glycosylation with neonatal presentation. BMJ Case Rep. 2014;2014:bcr2013010037. doi: 10.1136/bcr-2013-010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Serrano M., de Diego V., Muchart J., Cuadras D., Felipe A., Macaya A., Velázquez R., Poo M.P., Fons C., O′Callaghan M.M., et al. Phosphomannomutase deficiency (PMM2-CDG): Ataxia and cerebellar assessment. Orphanet J. Rare Dis. 2015;10:138. doi: 10.1186/s13023-015-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Al Teneiji A., Bruun T.U., Sidky S., Cordeiro D., Cohn R.D., Mendoza-Londono R., Moharir M., Raiman J., Siriwardena K., Kyriakopoulou L., et al. Phenotypic and genotypic spectrum of congenital disorders of glycosylation type I and type II. Mol. Genet. Metab. 2017;120:235–242. doi: 10.1016/j.ymgme.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 278.Schiff M., Roda C., Monin M.L., Arion A., Barth M., Bednarek N., Bidet M., Bloch C., Boddaert N., Borgel D., et al. Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J. Med. Genet. 2017;54:843–851. doi: 10.1136/jmedgenet-2017-104903. [DOI] [PubMed] [Google Scholar]
- 279.Wu R.H., Li D.F., Tang W.T., Qiu K.Y., Li Y., Liao X.Y., Tang D.X., Qin L.J., Deng B.Q., Luo X.Y. Atrial septal defect in a patient with congenital disorder of glycosylation type 1a: A case report. J. Med. Case Rep. 2018;12:17. doi: 10.1186/s13256-017-1528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Qian Z., Van den Eynde J., Heymans S., Mertens L., Morava E. Vascular ring anomaly in a patient with phosphomannomutase 2 deficiency: A case report and review of the literature. JIMD Rep. 2020;56:27–33. doi: 10.1002/jmd2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Görlacher M., Panagiotou E., Himmelreich N., Hüllen A., Beedgen L., Dimitrov B., Geiger V., Zielonka M., Peters V., Strahl S., et al. Fatal outcome after heart surgery in PMM2-CDG due to a rare homozygous gene variant with double effects. Mol. Genet. Metab. Rep. 2020;25:100673. doi: 10.1016/j.ymgmr.2020.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Malhotra A., Pateman A., Chalmers R., Coman D., Menahem S. Prenatal cardiac ultrasound finding in congenital disorder of glycosylation type 1a. Fetal Diagn. Ther. 2009;25:54–57. doi: 10.1159/000196816. [DOI] [PubMed] [Google Scholar]
- 283.Footitt E.J., Karimova A., Burch M., Yayeh T., Dupré T., Vuillaumier-Barrot S., Chantret I., Moore S.E., Seta N., Grunewald S. Cardiomyopathy in the congenital disorders of glycosylation (CDG): A case of late presentation and literature review. J. Inherit. Metab. Dis. 2009;32((Suppl. S1)):S313–S319. doi: 10.1007/s10545-009-1262-1. [DOI] [PubMed] [Google Scholar]
- 284.Bogdańska A., Lipiński P., Szymańska-Rożek P., Jezela-Stanek A., Rokicki D., Socha P., Tylki-Szymańska A. Clinical, biochemical and molecular phenotype of congenital disorders of glycosylation: Long-term follow-up. Orphanet J. Rare Dis. 2021;16:17. doi: 10.1186/s13023-020-01657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vasudevan D., Takeuchi H., Johar S.S., Majerus E., Haltiwanger R.S. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr. Biol. CB. 2015;25:286–295. doi: 10.1016/j.cub.2014.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lesnik Oberstein S.A., Kriek M., White S.J., Kalf M.E., Szuhai K., den Dunnen J.T., Breuning M.H., Hennekam R.C. Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am. J. Hum. Genet. 2006;79:562–566. doi: 10.1086/507567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Reis L.M., Tyler R.C., Abdul-Rahman O., Trapane P., Wallerstein R., Broome D., Hoffman J., Khan A., Paradiso C., Ron N., et al. Mutation analysis of B3GALTL in Peters Plus syndrome. Am. J. Med. Genet. Part A. 2008;146:2603–2610. doi: 10.1002/ajmg.a.32498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Haldeman-Englert C.R., Naeem T., Geiger E.A., Warnock A., Feret H., Ciano M., Davidson S.L., Deardorff M.A., Zackai E.H., Shaikh T.H. A 781-kb deletion of 13q12.3 in a patient with Peters plus syndrome. Am. J. Med. Genet. Part A. 2009;149:1842–1845. doi: 10.1002/ajmg.a.32980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Dassie-Ajdid J., Causse A., Poidvin A., Granier M., Kaplan J., Burglen L., Doummar D., Teisseire P., Vigouroux A., Malecaze F., et al. Novel B3GALTL mutation in Peters-plus Syndrome. Clin. Genet. 2009;76:490–492. doi: 10.1111/j.1399-0004.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 290.Shimizu R., Saito R., Hoshino K., Ogawa K., Negishi T., Nishimura J., Mitsui N., Osawa M., Ohashi H. Severe Peters Plus syndrome-like phenotype with anterior eye staphyloma and hypoplastic left heart syndrome: Proposal of a new syndrome. Congenit. Anom. 2010;50:197–199. doi: 10.1111/j.1741-4520.2010.00282.x. [DOI] [PubMed] [Google Scholar]
- 291.Faletra F., Athanasakis E., Minen F., Fornasier F., Marchetti F., Gasparini P. Vertebral defects in patients with Peters plus syndrome and mutations in B3GALTL. Ophthalmic Genet. 2011;32:256–258. doi: 10.3109/13816810.2011.587082. [DOI] [PubMed] [Google Scholar]
- 292.Weh E., Reis L.M., Tyler R.C., Bick D., Rhead W.J., Wallace S., McGregor T.L., Dills S.K., Chao M.C., Murray J.C., et al. Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin. Genet. 2014;86:142–148. doi: 10.1111/cge.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li Y., Zhang C., Zhang H., Feng W., Wang Q., Fan R. Severe phenotypes of B3GAT3-related disorder caused by two heterozygous variants: A case report and literature review. BMC Med. Genom. 2022;15:27. doi: 10.1186/s12920-022-01160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Baasanjav S., Al-Gazali L., Hashiguchi T., Mizumoto S., Fischer B., Horn D., Seelow D., Ali B.R., Aziz S.A., Langer R., et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am. J. Hum. Genet. 2011;89:15–27. doi: 10.1016/j.ajhg.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Von Oettingen J.E., Tan W.H., Dauber A. Skeletal dysplasia, global developmental delay, and multiple congenital anomalies in a 5-year-old boy-report of the second family with B3GAT3 mutation and expansion of the phenotype. Am. J. Med. Genet. Part A. 2014;164:1580–1586. doi: 10.1002/ajmg.a.36487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jones K.L., Schwarze U., Adam M.P., Byers P.H., Mefford H.C. A homozygous B3GAT3 mutation causes a severe syndrome with multiple fractures, expanding the phenotype of linkeropathy syndromes. Am. J. Med. Genet. Part A. 2015;167:2691–2696. doi: 10.1002/ajmg.a.37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Job F., Mizumoto S., Smith L., Couser N., Brazil A., Saal H., Patterson M., Gibson M.I., Soden S., Miller N., et al. Functional validation of novel compound heterozygous variants in B3GAT3 resulting in severe osteopenia and fractures: Expanding the disease phenotype. BMC Med. Genet. 2016;17:86. doi: 10.1186/s12881-016-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bloor S., Giri D., Didi M., Senniappan S. Novel Splicing Mutation in B3GAT3 Associated with Short Stature, GH Deficiency, Hypoglycaemia, Developmental Delay, and Multiple Congenital Anomalies. Case Rep. Genet. 2017;2017:3941483. doi: 10.1155/2017/3941483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yauy K., Tran Mau-Them F., Willems M., Coubes C., Blanchet P., Herlin C., Taleb Arrada I., Sanchez E., Faure J.M., Le Gac M.P., et al. B3GAT3-related disorder with craniosynostosis and bone fragility due to a unique mutation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2018;20:269–274. doi: 10.1038/gim.2017.109. [DOI] [PubMed] [Google Scholar]
- 300.Ritelli M., Cinquina V., Giacopuzzi E., Venturini M., Chiarelli N., Colombi M. Further Defining the Phenotypic Spectrum of B3GAT3 Mutations and Literature Review on Linkeropathy Syndromes. Genes. 2019;10:631. doi: 10.3390/genes10090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ranza E., Huber C., Levin N., Baujat G., Bole-Feysot C., Nitschke P., Masson C., Alanay Y., Al-Gazali L., Bitoun P., et al. Chondrodysplasia with multiple dislocations: Comprehensive study of a series of 30 cases. Clin. Genet. 2017;91:868–880. doi: 10.1111/cge.12885. [DOI] [PubMed] [Google Scholar]
- 302.Ortiz-Cordero C., Azzag K., Perlingeiro R.C.R. Fukutin-Related Protein: From Pathology to Treatments. Trends Cell Biol. 2021;31:197–210. doi: 10.1016/j.tcb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Van Reeuwijk J., Olderode-Berends M.J., Van den Elzen C., Brouwer O.F., Roscioli T., Van Pampus M.G., Scheffer H., Brunner H.G., Van Bokhoven H., Hol F.A. A homozygous FKRP start codon mutation is associated with Walker-Warburg syndrome, the severe end of the clinical spectrum. Clin. Genet. 2010;78:275–281. doi: 10.1111/j.1399-0004.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 304.Poppe M., Cree L., Bourke J., Eagle M., Anderson L.V., Birchall D., Brockington M., Buddles M., Busby M., Muntoni F., et al. The phenotype of limb-girdle muscular dystrophy type 2I. Neurology. 2003;60:1246–1251. doi: 10.1212/01.WNL.0000058902.88181.3D. [DOI] [PubMed] [Google Scholar]
- 305.Mercuri E., Brockington M., Straub V., Quijano-Roy S., Yuva Y., Herrmann R., Brown S.C., Torelli S., Dubowitz V., Blake D.J., et al. Phenotypic spectrum associated with mutations in the fukutin-related protein gene. Ann. Neurol. 2003;53:537–542. doi: 10.1002/ana.10559. [DOI] [PubMed] [Google Scholar]
- 306.Poppe M., Bourke J., Eagle M., Frosk P., Wrogemann K., Greenberg C., Muntoni F., Voit T., Straub V., Hilton-Jones D., et al. Cardiac and respiratory failure in limb-girdle muscular dystrophy 2I. Ann. Neurol. 2004;56:738–741. doi: 10.1002/ana.20283. [DOI] [PubMed] [Google Scholar]
- 307.Louhichi N., Triki C., Quijano-Roy S., Richard P., Makri S., Méziou M., Estournet B., Mrad S., Romero N.B., Ayadi H., et al. New FKRP mutations causing congenital muscular dystrophy associated with mental retardation and central nervous system abnormalities. Identification of a founder mutation in Tunisian families. Neurogenetics. 2004;5:27–34. doi: 10.1007/s10048-003-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Müller T., Krasnianski M., Witthaut R., Deschauer M., Zierz S. Dilated cardiomyopathy may be an early sign of the C826A Fukutin-related protein mutation. Neuromuscul. Disord. NMD. 2005;15:372–376. doi: 10.1016/j.nmd.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 309.Boito C.A., Melacini P., Vianello A., Prandini P., Gavassini B.F., Bagattin A., Siciliano G., Angelini C., Pegoraro E. Clinical and molecular characterization of patients with limb-girdle muscular dystrophy type 2I. Arch. Neurol. 2005;62:1894–1899. doi: 10.1001/archneur.62.12.1894. [DOI] [PubMed] [Google Scholar]
- 310.Schwartz M., Hertz J.M., Sveen M.L., Vissing J. LGMD2I presenting with a characteristic Duchenne or Becker muscular dystrophy phenotype. Neurology. 2005;64:1635–1637. doi: 10.1212/01.WNL.0000157654.59374.E5. [DOI] [PubMed] [Google Scholar]
- 311.Frosk P., Greenberg C.R., Tennese A.A., Lamont R., Nylen E., Hirst C., Frappier D., Roslin N.M., Zaik M., Bushby K., et al. The most common mutation in FKRP causing limb girdle muscular dystrophy type 2I (LGMD2I) may have occurred only once and is present in Hutterites and other populations. Hum. Mutat. 2005;25:38–44. doi: 10.1002/humu.20110. [DOI] [PubMed] [Google Scholar]
- 312.Gaul C., Deschauer M., Tempelmann C., Vielhaber S., Klein H.U., Heinze H.J., Zierz S., Grothues F. Cardiac involvement in limb-girdle muscular dystrophy 2I: Conventional cardiac diagnostic and cardiovascular magnetic resonance. J. Neurol. 2006;253:1317–1322. doi: 10.1007/s00415-006-0213-0. [DOI] [PubMed] [Google Scholar]
- 313.Sveen M.L., Schwartz M., Vissing J. High prevalence and phenotype-genotype correlations of limb girdle muscular dystrophy type 2I in Denmark. Ann. Neurol. 2006;59:808–815. doi: 10.1002/ana.20824. [DOI] [PubMed] [Google Scholar]
- 314.D′Amico A., Petrini S., Parisi F., Tessa A., Francalanci P., Grutter G., Santorelli F.M., Bertini E. Heart transplantation in a child with LGMD2I presenting as isolated dilated cardiomyopathy. Neuromuscul. Disord. NMD. 2008;18:153–155. doi: 10.1016/j.nmd.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 315.Wahbi K., Meune C., Hamouda E.H., Stojkovic T., Laforêt P., Bécane H.M., Eymard B., Duboc D. Cardiac assessment of limb-girdle muscular dystrophy 2I patients: An echography, Holter ECG and magnetic resonance imaging study. Neuromuscul. Disord. NMD. 2008;18:650–655. doi: 10.1016/j.nmd.2008.06.365. [DOI] [PubMed] [Google Scholar]
- 316.Kefi M., Amouri R., Chabrak S., Mechmeche R., Hentati F. Variable cardiac involvement in Tunisian siblings harboring FKRP gene mutations. Neuropediatrics. 2008;39:113–115. doi: 10.1055/s-2008-1081465. [DOI] [PubMed] [Google Scholar]
- 317.Sveen M.L., Thune J.J., Køber L., Vissing J. Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch. Neurol. 2008;65:1196–1201. doi: 10.1001/archneur.65.9.1196. [DOI] [PubMed] [Google Scholar]
- 318.Margeta M., Connolly A.M., Winder T.L., Pestronk A., Moore S.A. Cardiac pathology exceeds skeletal muscle pathology in two cases of limb-girdle muscular dystrophy type 2I. Muscle Nerve. 2009;40:883–889. doi: 10.1002/mus.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bourteel H., Vermersch P., Cuisset J.M., Maurage C.A., Laforet P., Richard P., Stojkovic T. Clinical and mutational spectrum of limb-girdle muscular dystrophy type 2I in 11 French patients. J. Neurol. Neurosurg. Psychiatry. 2009;80:1405–1408. doi: 10.1136/jnnp.2007.141804. [DOI] [PubMed] [Google Scholar]
- 320.Yilmaz A., Gdynia H.J., Ponfick M., Ludolph A.C., Rösch S., Sechtem U. The proteoglycan-dystrophin complex in genetic cardiomyopathies—Lessons from three siblings with limb-girdle muscular dystrophy-2I (LGMD-2I) Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2011;100:611–615. doi: 10.1007/s00392-011-0291-6. [DOI] [PubMed] [Google Scholar]
- 321.Pane M., Messina S., Vasco G., Foley A.R., Morandi L., Pegoraro E., Mongini T., D′Amico A., Bianco F., Lombardo M.E., et al. Respiratory and cardiac function in congenital muscular dystrophies with alpha dystroglycan deficiency. Neuromuscul. Disord. NMD. 2012;22:685–689. doi: 10.1016/j.nmd.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hollingsworth K.G., Willis T.A., Bates M.G., Dixon B.J., Lochmüller H., Bushby K., Bourke J., MacGowan G.A., Straub V. Subepicardial dysfunction leads to global left ventricular systolic impairment in patients with limb girdle muscular dystrophy 2I. Eur. J. Heart Fail. 2013;15:986–994. doi: 10.1093/eurjhf/hft057. [DOI] [PubMed] [Google Scholar]
- 323.Liang W.C., Hayashi Y.K., Ogawa M., Wang C.H., Huang W.T., Nishino I., Jong Y.J. Limb-girdle muscular dystrophy type 2I is not rare in Taiwan. Neuromuscul. Disord. NMD. 2013;23:675–681. doi: 10.1016/j.nmd.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 324.Schottlaender L.V., Petzold A., Wood N., Houlden H. Diagnostic clues and manifesting carriers in fukutin-related protein (FKRP) limb-girdle muscular dystrophy. J. Neurol. Sci. 2015;348:266–268. doi: 10.1016/j.jns.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 325.Wang D.N., Wang Z.Q., Chen Y.Q., Xu G.R., Lin M.T., Wang N. Limb-girdle muscular dystrophy type 2I: Two Chinese families and a review in Asian patients. Int. J. Neurosci. 2018;128:199–207. doi: 10.1080/00207454.2017.1380640. [DOI] [PubMed] [Google Scholar]
- 326.El-Battrawy I., Zhao Z., Lan H., Li X., Yücel G., Lang S., Sattler K., Schünemann J.D., Zimmermann W.H., Cyganek L., et al. Ion Channel Dysfunctions in Dilated Cardiomyopathy in Limb-Girdle Muscular Dystrophy. Circ. Genom. Precis. Med. 2018;11:e001893. doi: 10.1161/CIRCGEN.117.001893. [DOI] [PubMed] [Google Scholar]
- 327.Ten Dam L., Frankhuizen W.S., Linssen W., Straathof C.S., Niks E.H., Faber K., Fock A., Kuks J.B., Brusse E., de Coo R., et al. Autosomal recessive limb-girdle and Miyoshi muscular dystrophies in the Netherlands: The clinical and molecular spectrum of 244 patients. Clin. Genet. 2019;96:126–133. doi: 10.1111/cge.13544. [DOI] [PubMed] [Google Scholar]
- 328.Murphy L.B., Schreiber-Katz O., Rafferty K., Robertson A., Topf A., Willis T.A., Heidemann M., Thiele S., Bindoff L., Laurent J.P., et al. Global FKRP Registry: Observations in more than 300 patients with Limb Girdle Muscular Dystrophy R9. Ann. Clin. Transl. Neurol. 2020;7:757–766. doi: 10.1002/acn3.51042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Libell E.M., Richardson J.A., Lutz K.L., Ng B.Y., Mockler S.R.H., Laubscher K.M., Stephan C.M., Zimmerman B.M., Edens E.R., Reinking B.E., et al. Cardiomyopathy in limb girdle muscular dystrophy R9, FKRP related. Muscle Nerve. 2020;62:626–632. doi: 10.1002/mus.27052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nakanishi T., Sakauchi M., Kaneda Y., Tomimatsu H., Saito K., Nakazawa M., Osawa M. Cardiac involvement in Fukuyama-type congenital muscular dystrophy. Pediatrics. 2006;117:e1187–e1192. doi: 10.1542/peds.2005-2469. [DOI] [PubMed] [Google Scholar]
- 331.Miura K., Shirasawa H. Congenital muscular dystrophy of the Fukuyama type (FCMD) with severe myocardial fibrosis. A case report with postmortem angiography. Acta Pathol. Jpn. 1987;37:1823–1835. doi: 10.1111/j.1440-1827.1987.tb02875.x. [DOI] [PubMed] [Google Scholar]
- 332.Murakami T., Hayashi Y.K., Noguchi S., Ogawa M., Nonaka I., Tanabe Y., Ogino M., Takada F., Eriguchi M., Kotooka N., et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann. Neurol. 2006;60:597–602. doi: 10.1002/ana.20973. [DOI] [PubMed] [Google Scholar]
- 333.Cotarelo R.P., Valero M.C., Prados B., Peña A., Rodríguez L., Fano O., Marco J.J., Martínez-Frías M.L., Cruces J. Two new patients bearing mutations in the fukutin gene confirm the relevance of this gene in Walker-Warburg syndrome. Clin. Genet. 2008;73:139–145. doi: 10.1111/j.1399-0004.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 334.Rosales X.Q., Moser S.J., Tran T., McCarthy B., Dunn N., Habib P., Simonetti O.P., Mendell J.R., Raman S.V. Cardiovascular magnetic resonance of cardiomyopathy in limb girdle muscular dystrophy 2B and 2I. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2011;13:39. doi: 10.1186/1532-429X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Fiorillo C., Moro F., Astrea G., Morales M.A., Baldacci J., Marchese M., Scapolan S., Bruno C., Battini R., Santorelli F.M. Novel mutations in the fukutin gene in a boy with asymptomatic hyperCKemia. Neuromuscul. Disord. NMD. 2013;23:1010–1015. doi: 10.1016/j.nmd.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 336.Amiya E., Morita H., Hatano M., Nitta D., Hosoya Y., Maki H., Motozawa Y., Sato N., Ishiura H., Numakura S., et al. Fukutin gene mutations that cause left ventricular noncompaction. Int. J. Cardiol. 2016;222:727–729. doi: 10.1016/j.ijcard.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 337.Ishigaki K., Ihara C., Nakamura H., Mori-Yoshimura M., Maruo K., Taniguchi-Ikeda M., Kimura E., Murakami T., Sato T., Toda T., et al. National registry of patients with Fukuyama congenital muscular dystrophy in Japan. Neuromuscul. Disord. NMD. 2018;28:885–893. doi: 10.1016/j.nmd.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 338.Carnevale A., Rosas-Madrigal S., Rosendo-Gutiérrez R., López-Mora E., Romero-Hidalgo S., Avila-Vazzini N., Jacobo-Albavera L., Domínguez-Pérez M., Vargas-Alarcón G., Pérez-Villatoro F., et al. Genomic study of dilated cardiomyopathy in a group of Mexican patients using site-directed next generation sequencing. Mol. Genet. Genom. Med. 2020;8:e1504. doi: 10.1002/mgg3.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vad O.B., Paludan-Müller C., Ahlberg G., Kalstø S.M., Ghouse J., Andreasen L., Haunsø S., Tveit A., Sajadieh A., Christophersen I.E., et al. Loss-of-Function Variants in Cytoskeletal Genes Are Associated with Early-Onset Atrial Fibrillation. J. Clin. Med. 2020;9:372. doi: 10.3390/jcm9020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Villarreal-Molina M.T., Rosas-Madrigal S., López-Mora E., Calderón-Avila A.L., Rodríguez-Zanella H., Romero-Hidalgo S., Rosendo-Gutierrez R., Carnevale A. Homozygous Fukutin Missense Mutation in Two Mexican Siblings with Dilated Cardiomyopathy. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2020;73:132–137. doi: 10.24875/RIC.20000277. [DOI] [PubMed] [Google Scholar]
- 341.Al-Shafai K.N., Al-Hashemi M., Manickam C., Musa R., Selvaraj S., Syed N., Vempalli F., Ali M., Yacoub M., Estivill X. Genetic evaluation of cardiomyopathies in Qatar identifies enrichment of pathogenic sarcomere gene variants and possible founder disease mutations in the Arabs. Mol. Genet. Genom. Med. 2021;9:e1709. doi: 10.1002/mgg3.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Gaertner A., Burr L., Klauke B., Brodehl A., Laser K.T., Klingel K., Tiesmeier J., Schulz U., Knyphausen E.Z., Gummert J., et al. Compound Heterozygous FKTN Variants in a Patient with Dilated Cardiomyopathy Led to an Aberrant α-Dystroglycan Pattern. Int. J. Mol. Sci. 2022;23:6685. doi: 10.3390/ijms23126685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lesurf R., Said A., Akinrinade O., Breckpot J., Delfosse K., Liu T., Yao R., Persad G., McKenna F., Noche R.R., et al. Whole genome sequencing delineates regulatory, copy number, and cryptic splice variants in early onset cardiomyopathy. NPJ Genom. Med. 2022;7:18. doi: 10.1038/s41525-022-00288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Willer T., Valero M.C., Tanner W., Cruces J., Strahl S. O-mannosyl glycans: From yeast to novel associations with human disease. Curr. Opin. Struct. Biol. 2003;13:621–630. doi: 10.1016/j.sbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 345.Bello L., Melacini P., Pezzani R., D′Amico A., Piva L., Leonardi E., Torella A., Soraru G., Palmieri A., Smaniotto G., et al. Cardiomyopathy in patients with POMT1-related congenital and limb-girdle muscular dystrophy. Eur. J. Hum. Genet. EJHG. 2012;20:1234–1239. doi: 10.1038/ejhg.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Devisme L., Bouchet C., Gonzalès M., Alanio E., Bazin A., Bessières B., Bigi N., Blanchet P., Bonneau D., Bonnières M., et al. Cobblestone lissencephaly: Neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain J. Neurol. 2012;135:469–482. doi: 10.1093/brain/awr357. [DOI] [PubMed] [Google Scholar]
- 347.Sparks S.E., Escolar D.M. Congenital muscular dystrophies. Handb. Clin. Neurol. 2011;101:47–79. doi: 10.1016/b978-0-08-045031-5.00004-9. [DOI] [PubMed] [Google Scholar]
- 348.Martinez H.R., Craigen W.J., Ummat M., Adesina A.M., Lotze T.E., Jefferies J.L. Novel cardiovascular findings in association with a POMT2 mutation: Three siblings with α-dystroglycanopathy. Eur. J. Hum. Genet. EJHG. 2014;22:486–491. doi: 10.1038/ejhg.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Yanagisawa A., Bouchet C., Van den Bergh P.Y., Cuisset J.M., Viollet L., Leturcq F., Romero N.B., Quijano-Roy S., Fardeau M., Seta N., et al. New POMT2 mutations causing congenital muscular dystrophy: Identification of a founder mutation. Neurology. 2007;69:1254–1260. doi: 10.1212/01.wnl.0000268489.60809.c4. [DOI] [PubMed] [Google Scholar]
- 350.Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol. Jpn. 1993;43:283–293. doi: 10.1111/j.1440-1827.1993.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 351.Wang Q., Chi L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers. 2022;14:5014. doi: 10.3390/polym14225014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Doddato G., Fabbiani A., Fallerini C., Bruttini M., Hadjistilianou T., Landi M., Coradeschi C., Grosso S., Tomasini B., Mencarelli M.A., et al. Spondyloocular Syndrome: A Novel XYLT2 Variant with Description of the Neonatal Phenotype. Front. Genet. 2021;12:761264. doi: 10.3389/fgene.2021.761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Munns C.F., Fahiminiya S., Poudel N., Munteanu M.C., Majewski J., Sillence D.O., Metcalf J.P., Biggin A., Glorieux F., Fassier F., et al. Homozygosity for frameshift mutations in XYLT2 result in a spondylo-ocular syndrome with bone fragility, cataracts, and hearing defects. Am. J. Hum. Genet. 2015;96:971–978. doi: 10.1016/j.ajhg.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Taylan F., Costantini A., Coles N., Pekkinen M., Héon E., Şıklar Z., Berberoğlu M., Kämpe A., Kıykım E., Grigelioniene G., et al. Spondyloocular Syndrome: Novel Mutations in XYLT2 Gene and Expansion of the Phenotypic Spectrum. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016;31:1577–1585. doi: 10.1002/jbmr.2834. [DOI] [PubMed] [Google Scholar]
- 355.Kranz C., Jungeblut C., Denecke J., Erlekotte A., Sohlbach C., Debus V., Kehl H.G., Harms E., Reith A., Reichel S., et al. A defect in dolichol phosphate biosynthesis causes a new inherited disorder with death in early infancy. Am. J. Hum. Genet. 2007;80:433–440. doi: 10.1086/512130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lefeber D.J., de Brouwer A.P., Morava E., Riemersma M., Schuurs-Hoeijmakers J.H., Absmanner B., Verrijp K., van den Akker W.M., Huijben K., Steenbergen G., et al. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 2011;7:e1002427. doi: 10.1371/journal.pgen.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kapusta L., Zucker N., Frenckel G., Medalion B., Ben Gal T., Birk E., Mandel H., Nasser N., Morgenstern S., Zuckermann A., et al. From discrete dilated cardiomyopathy to successful cardiac transplantation in congenital disorders of glycosylation due to dolichol kinase deficiency (DK1-CDG) Heart Fail. Rev. 2013;18:187–196. doi: 10.1007/s10741-012-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lieu M.T., Ng B.G., Rush J.S., Wood T., Basehore M.J., Hegde M., Chang R.C., Abdenur J.E., Freeze H.H., Wang R.Y. Severe, fatal multisystem manifestations in a patient with dolichol kinase-congenital disorder of glycosylation. Mol. Genet. Metab. 2013;110:484–489. doi: 10.1016/j.ymgme.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yu S., Zhang Y., Chen Z., Song J., Wang C. Case Report: A Novel Compound Heterozygous Gene Mutation of Dolichol Kinase Deficiency (DOLK-CDG) Endocr. Metab. Immune Disord. Drug Targets. 2022;23:235–241. doi: 10.2174/1871530322666220607123739. [DOI] [PubMed] [Google Scholar]
- 360.Komlosi K., Claris O., Collardeau-Frachon S., Kopp J., Hausser I., Mazereeuw-Hautier J., Jonca N., Zimmer A.D., Sanlaville D., Fischer J. Fatal Neonatal DOLK-CDG as a Rare Form of Syndromic Ichthyosis. Front. Genet. 2021;12:719624. doi: 10.3389/fgene.2021.719624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nagy S., Lau T., Alavi S., Karimiani E.G., Vallian J., Ng B.G., Noroozi Asl S., Akhondian J., Bahreini A., Yaghini O., et al. A recurrent homozygous missense DPM3 variant leads to muscle and brain disease. Clin. Genet. 2022;102:530–536. doi: 10.1111/cge.14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lefeber D.J., Schönberger J., Morava E., Guillard M., Huyben K.M., Verrijp K., Grafakou O., Evangeliou A., Preijers F.W., Manta P., et al. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 2009;85:76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Van Tol W., Michelakakis H., Georgiadou E., van den Bergh P., Moraitou M., Papadimas G.K., Papadopoulos C., Huijben K., Alsady M., Willemsen M.A., et al. Toward understanding tissue-specific symptoms in dolichol-phosphate-mannose synthesis disorders; insight from DPM3-CDG. J. Inherit. Metab. Dis. 2019;42:984–992. doi: 10.1002/jimd.12095. [DOI] [PubMed] [Google Scholar]
- 364.Van Tol W., Ashikov A., Korsch E., Abu Bakar N., Willemsen M.A., Thiel C., Lefeber D.J. A mutation in mannose-phosphate-dolichol utilization defect 1 reveals clinical symptoms of congenital disorders of glycosylation type I and dystroglycanopathy. JIMD Rep. 2019;50:31–39. doi: 10.1002/jmd2.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Thiel C., Wortmann S., Riedhammer K., Alhaddad B., Mayatepek E., Prokisch H., Distelmaier F. Severe ichthyosis in MPDU1-CDG. J. Inherit. Metab. Dis. 2018;41:1293–1294. doi: 10.1007/s10545-018-0189-9. [DOI] [PubMed] [Google Scholar]
- 366.Bastaki F., Bizzari S., Hamici S., Nair P., Mohamed M., Saif F., Malik E.M., Al-Ali M.T., Hamzeh A.R. Single-center experience of N-linked Congenital Disorders of Glycosylation with a Summary of Molecularly Characterized Cases in Arabs. Ann. Hum. Genet. 2018;82:35–47. doi: 10.1111/ahg.12220. [DOI] [PubMed] [Google Scholar]
- 367.Cantagrel V., Lefeber D.J., Ng B.G., Guan Z., Silhavy J.L., Bielas S.L., Lehle L., Hombauer H., Adamowicz M., Swiezewska E., et al. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kasapkara C.S., Tümer L., Ezgü F.S., Hasanoğlu A., Race V., Matthijs G., Jaeken J. SRD5A3-CDG: A patient with a novel mutation. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2012;16:554–556. doi: 10.1016/j.ejpn.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 369.Tuysuz B., Pehlivan D., Özkök A., Jhangiani S., Yalcinkaya C., Zeybek Ç.A., Muzny D.M., Lupski J.R., Gibbs R., Jaeken J. Phenotypic Expansion of Congenital Disorder of Glycosylation Due to SRD5A3 Null Mutation. JIMD Rep. 2016;26:7–12. doi: 10.1007/8904_2015_478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wheeler P.G., Ng B.G., Sanford L., Sutton V.R., Bartholomew D.W., Pastore M.T., Bamshad M.J., Kircher M., Buckingham K.J., Nickerson D.A., et al. SRD5A3-CDG: Expanding the phenotype of a congenital disorder of glycosylation with emphasis on adult onset features. Am. J. Med. Genet. Part A. 2016;170:3165–3171. doi: 10.1002/ajmg.a.37875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kamarus Jaman N., Rehsi P., Henderson R.H., Löbel U., Mankad K., Grunewald S. SRD5A3-CDG: Emerging Phenotypic Features of an Ultrarare CDG Subtype. Front. Genet. 2021;12:737094. doi: 10.3389/fgene.2021.737094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bayat A., Kløvgaard M., Johannesen K.M., Barakat T.S., Kievit A., Montomoli M., Parrini E., Pietrafusa N., Schelhaas J., van Slegtenhorst M., et al. Deciphering the premature mortality in PIGA-CDG–An untold story. Epilepsy Res. 2021;170:106530. doi: 10.1016/j.eplepsyres.2020.106530. [DOI] [PubMed] [Google Scholar]
- 373.Fauth C., Steindl K., Toutain A., Farrell S., Witsch-Baumgartner M., Karall D., Joset P., Böhm S., Baumer A., Maier O., et al. A recurrent germline mutation in the PIGA gene causes Simpson-Golabi-Behmel syndrome type 2. Am. J. Med. Genet. Part A. 2016;170:392–402. doi: 10.1002/ajmg.a.37452. [DOI] [PubMed] [Google Scholar]
- 374.Bayat A., Knaus A., Pendziwiat M., Afenjar A., Barakat T.S., Bosch F., Callewaert B., Calvas P., Ceulemans B., Chassaing N., et al. Lessons learned from 40 novel PIGA patients and a review of the literature. Epilepsia. 2020;61:1142–1155. doi: 10.1111/epi.16545. [DOI] [PubMed] [Google Scholar]
- 375.Van der Crabben S.N., Harakalova M., Brilstra E.H., van Berkestijn F.M., Hofstede F.C., van Vught A.J., Cuppen E., Kloosterman W., Ploos van Amstel H.K., van Haaften G., et al. Expanding the spectrum of phenotypes associated with germline PIGA mutations: A child with developmental delay, accelerated linear growth, facial dysmorphisms, elevated alkaline phosphatase, and progressive CNS abnormalities. Am. J. Med. Genet. Part A. 2014;164:29–35. doi: 10.1002/ajmg.a.36184. [DOI] [PubMed] [Google Scholar]
- 376.Swoboda K.J., Margraf R.L., Carey J.C., Zhou H., Newcomb T.M., Coonrod E., Durtschi J., Mallempati K., Kumanovics A., Katz B.E., et al. A novel germline PIGA mutation in Ferro-Cerebro-Cutaneous syndrome: A neurodegenerative X-linked epileptic encephalopathy with systemic iron-overload. Am. J. Med. Genet. Part A. 2014;164:17–28. doi: 10.1002/ajmg.a.36189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tarailo-Graovac M., Sinclair G., Stockler-Ipsiroglu S., Van Allen M., Rozmus J., Shyr C., Biancheri R., Oh T., Sayson B., Lafek M., et al. The genotypic and phenotypic spectrum of PIGA deficiency. Orphanet J. Rare Dis. 2015;10:23. doi: 10.1186/s13023-015-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Shashi V., Zunich J., Kelly T.E., Fryburg J.S. Neuroectodermal (CHIME) syndrome: An additional case with long term follow up of all reported cases. J. Med. Genet. 1995;32:465–469. doi: 10.1136/jmg.32.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tinschert S., Anton-Lamprecht I., Albrecht-Nebe H., Audring H. Zunich neuroectodermal syndrome: Migratory ichthyosiform dermatosis, colobomas, and other abnormalities. Pediatr. Dermatol. 1996;13:363–371. doi: 10.1111/j.1525-1470.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 380.Ng B.G., Hackmann K., Jones M.A., Eroshkin A.M., He P., Wiliams R., Bhide S., Cantagrel V., Gleeson J.G., Paller A.S., et al. Mutations in the glycosylphosphatidylinositol gene PIGL cause CHIME syndrome. Am. J. Hum. Genet. 2012;90:685–688. doi: 10.1016/j.ajhg.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Knight Johnson A., Schaefer G.B., Lee J., Hu Y., Del Gaudio D. Alu-mediated deletion of PIGL in a Patient with CHIME syndrome. Am. J. Med. Genet. Part A. 2017;173:1378–1382. doi: 10.1002/ajmg.a.38181. [DOI] [PubMed] [Google Scholar]
- 382.Maydan G., Noyman I., Har-Zahav A., Neriah Z.B., Pasmanik-Chor M., Yeheskel A., Albin-Kaplanski A., Maya I., Magal N., Birk E., et al. Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J. Med. Genet. 2011;48:383–389. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- 383.Chen C.P., Lin H.M., Leung C., Lin S.P., Su Y.N., Su J.W., Chen Y.T., Wang W. Partial monosomy 9p (9p22.2→pter) and partial trisomy 18q (18q21.32→qter) in a female infant with anorectal malformations. Genet. Couns. 2012;23:201–206. [PubMed] [Google Scholar]
- 384.Brady P.D., Moerman P., De Catte L., Deprest J., Devriendt K., Vermeesch J.R. Exome sequencing identifies a recessive PIGN splice site mutation as a cause of syndromic congenital diaphragmatic hernia. Eur. J. Med. Genet. 2014;57:487–493. doi: 10.1016/j.ejmg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 385.Couser N.L., Masood M.M., Strande N.T., Foreman A.K., Crooks K., Weck K.E., Lu M., Wilhelmsen K.C., Roche M., Evans J.P., et al. The phenotype of multiple congenital anomalies-hypotonia-seizures syndrome 1: Report and review. Am. J. Med. Genet. Part A. 2015;167:2176–2181. doi: 10.1002/ajmg.a.37129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Fleming L., Lemmon M., Beck N., Johnson M., Mu W., Murdock D., Bodurtha J., Hoover-Fong J., Cohn R., Bosemani T., et al. Genotype-phenotype correlation of congenital anomalies in multiple congenital anomalies hypotonia seizures syndrome (MCAHS1)/PIGN-related epilepsy. Am. J. Med. Genet. Part A. 2016;170:77–86. doi: 10.1002/ajmg.a.37369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Xiao S.Q., Li M.H., Meng Y.L., Li C., Huang H.L., Liu C.X., Lyu Y., Na Q. Case Report: Compound Heterozygous Phosphatidylinositol-Glycan Biosynthesis Class N (PIGN) Mutations in a Chinese Fetus with Hypotonia-Seizures Syndrome 1. Front. Genet. 2020;11:594078. doi: 10.3389/fgene.2020.594078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Siavrienė E., Maldžienė Ž., Mikštienė V., Petraitytė G., Rančelis T., Dapkūnas J., Burnytė B., Benušienė E., Sasnauskienė A., Grikinienė J., et al. PIGN-Related Disease in Two Lithuanian Families: A Report of Two Novel Pathogenic Variants, Molecular and Clinical Characterisation. Medicina. 2022;58:1526. doi: 10.3390/medicina58111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kvarnung M., Nilsson D., Lindstrand A., Korenke G.C., Chiang S.C., Blennow E., Bergmann M., Stödberg T., Mäkitie O., Anderlid B.M., et al. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J. Med. Genet. 2013;50:521–528. doi: 10.1136/jmedgenet-2013-101654. [DOI] [PubMed] [Google Scholar]
- 390.Ohishi K., Inoue N., Kinoshita T. PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J. 2001;20:4088–4098. doi: 10.1093/emboj/20.15.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Lam C., Golas G.A., Davids M., Huizing M., Kane M.S., Krasnewich D.M., Malicdan M.C.V., Adams D.R., Markello T.C., Zein W.M., et al. Expanding the clinical and molecular characteristics of PIGT-CDG, a disorder of glycosylphosphatidylinositol anchors. Mol. Genet. Metab. 2015;115:128–140. doi: 10.1016/j.ymgme.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bayat A., Knaus A., Juul A.W., Dukic D., Gardella E., Charzewska A., Clement E., Hjalgrim H., Hoffman-Zacharska D., Horn D., et al. PIGT-CDG, a disorder of the glycosylphosphatidylinositol anchor: Description of 13 novel patients and expansion of the clinical characteristics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019;21:2216–2223. doi: 10.1038/s41436-019-0512-3. [DOI] [PubMed] [Google Scholar]
- 393.Reynolds K.K., Juusola J., Rice G.M., Giampietro P.F. Prenatal presentation of Mabry syndrome with congenital diaphragmatic hernia and phenotypic overlap with Fryns syndrome. Am. J. Med. Genet. Part A. 2017;173:2776–2781. doi: 10.1002/ajmg.a.38379. [DOI] [PubMed] [Google Scholar]
- 394.Morren M.A., Jaeken J., Visser G., Salles I., Van Geet C., Simeoni I., Turro E., Freson K. PIGO deficiency: Palmoplantar keratoderma and novel mutations. Orphanet J. Rare Dis. 2017;12:101. doi: 10.1186/s13023-017-0654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Krawitz P.M., Murakami Y., Hecht J., Krüger U., Holder S.E., Mortier G.R., Delle Chiaie B., De Baere E., Thompson M.D., Roscioli T., et al. Mutations in PIGO, a member of the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation. Am. J. Hum. Genet. 2012;91:146–151. doi: 10.1016/j.ajhg.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Kuki I., Takahashi Y., Okazaki S., Kawawaki H., Ehara E., Inoue N., Kinoshita T., Murakami Y. Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology. 2013;81:1467–1469. doi: 10.1212/WNL.0b013e3182a8411a. [DOI] [PubMed] [Google Scholar]
- 397.Tanigawa J., Mimatsu H., Mizuno S., Okamoto N., Fukushi D., Tominaga K., Kidokoro H., Muramatsu Y., Nishi E., Nakamura S., et al. Phenotype-genotype correlations of PIGO deficiency with variable phenotypes from infantile lethality to mild learning difficulties. Hum. Mutat. 2017;38:805–815. doi: 10.1002/humu.23219. [DOI] [PubMed] [Google Scholar]
- 398.Foulquier F., Vasile E., Schollen E., Callewaert N., Raemaekers T., Quelhas D., Jaeken J., Mills P., Winchester B., Krieger M., et al. Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc. Natl. Acad. Sci. USA. 2006;103:3764–3769. doi: 10.1073/pnas.0507685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zeevaert R., Foulquier F., Dimitrov B., Reynders E., Van Damme-Lombaerts R., Simeonov E., Annaert W., Matthijs G., Jaeken J. Cerebrocostomandibular-like syndrome and a mutation in the conserved oligomeric Golgi complex, subunit 1. Hum. Mol. Genet. 2009;18:517–524. doi: 10.1093/hmg/ddn379. [DOI] [PubMed] [Google Scholar]
- 400.Salazar M., Miyake N., Silva S., Solar B., Papazoglu G.M., Asteggiano C.G., Matsumoto N. COG1-congenital disorders of glycosylation: Milder presentation and review. Clin. Genet. 2021;100:318–323. doi: 10.1111/cge.13980. [DOI] [PubMed] [Google Scholar]
- 401.Quelhas D., Martins E., Azevedo L., Bandeira A., Diogo L., Garcia P., Sequeira S., Ferreira A.C., Teles E.L., Rodrigues E., et al. Congenital Disorders of Glycosylation in Portugal-Two Decades of Experience. J. Pediatr. 2021;231:148–156. doi: 10.1016/j.jpeds.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 402.Wu X., Steet R.A., Bohorov O., Bakker J., Newell J., Krieger M., Spaapen L., Kornfeld S., Freeze H.H. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat. Med. 2004;10:518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 403.Spaapen L.J., Bakker J.A., van der Meer S.B., Sijstermans H.J., Steet R.A., Wevers R.A., Jaeken J. Clinical and biochemical presentation of siblings with COG-7 deficiency, a lethal multiple O- and N-glycosylation disorder. J. Inherit. Metab. Dis. 2005;28:707–714. doi: 10.1007/s10545-005-0015-z. [DOI] [PubMed] [Google Scholar]
- 404.Morava E., Zeevaert R., Korsch E., Huijben K., Wopereis S., Matthijs G., Keymolen K., Lefeber D.J., De Meirleir L., Wevers R.A. A common mutation in the COG7 gene with a consistent phenotype including microcephaly, adducted thumbs, growth retardation, VSD and episodes of hyperthermia. Eur. J. Hum. Genet. EJHG. 2007;15:638–645. doi: 10.1038/sj.ejhg.5201813. [DOI] [PubMed] [Google Scholar]
- 405.Van Damme T., Gardeitchik T., Mohamed M., Guerrero-Castillo S., Freisinger P., Guillemyn B., Kariminejad A., Dalloyaux D., van Kraaij S., Lefeber D.J., et al. Mutations in ATP6V1E1 or ATP6V1A Cause Autosomal-Recessive Cutis Laxa. Am. J. Hum. Genet. 2017;100:216–227. doi: 10.1016/j.ajhg.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Vogt G., El Choubassi N., Herczegfalvi Á., Kölbel H., Lekaj A., Schara U., Holtgrewe M., Krause S., Horvath R., Schuelke M., et al. Expanding the clinical and molecular spectrum of ATP6V1A related metabolic cutis laxa. J. Inherit. Metab. Dis. 2021;44:972–986. doi: 10.1002/jimd.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Alazami A.M., Al-Qattan S.M., Faqeih E., Alhashem A., Alshammari M., Alzahrani F., Al-Dosari M.S., Patel N., Alsagheir A., Binabbas B., et al. Expanding the clinical and genetic heterogeneity of hereditary disorders of connective tissue. Hum. Genet. 2016;135:525–540. doi: 10.1007/s00439-016-1660-z. [DOI] [PubMed] [Google Scholar]
- 408.Winchester B. Lysosomal metabolism of glycoproteins. Glycobiology. 2005;15:1r–15r. doi: 10.1093/glycob/cwi041. [DOI] [PubMed] [Google Scholar]
- 409.Stütz A.E., Wrodnigg T.M. Carbohydrate-Processing Enzymes of the Lysosome: Diseases Caused by Misfolded Mutants and Sugar Mimetics as Correcting Pharmacological Chaperones. Adv. Carbohydr. Chem. Biochem. 2016;73:225–302. doi: 10.1016/bs.accb.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 410.Mistry P.K., Kishnani P., Wanner C., Dong D., Bender J., Batista J.L., Foster J. Rare lysosomal disease registries: Lessons learned over three decades of real-world evidence. Orphanet J. Rare Dis. 2022;17:362. doi: 10.1186/s13023-022-02517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sewell A.C., Pontz B.F., Weitzel D., Humburg C. Clinical heterogeneity in infantile galactosialidosis. Eur. J. Pediatr. 1987;146:528–531. doi: 10.1007/BF00441610. [DOI] [PubMed] [Google Scholar]
- 412.Strisciuglio P., Sly W.S., Dodson W.E., McAlister W.H., Martin T.C. Combined deficiency of beta-galactosidase and neuraminidase: Natural history of the disease in the first 18 years of an American patient with late infantile onset form. Am. J. Med. Genet. 1990;37:573–577. doi: 10.1002/ajmg.1320370431. [DOI] [PubMed] [Google Scholar]
- 413.Say B., Hommes F.A., Malik S.A., Carpenter N.J. An infant with multiple congenital abnormalities and biochemical findings suggesting a variant of galactosialidosis. J. Med. Genet. 1992;29:423–424. doi: 10.1136/jmg.29.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Kyllerman M., Månsson J.E., Westphal O., Conradi N., Nellström H. Infantile galactosialidosis presenting with congenital adrenal hyperplasia and renal hypertension. Pediatr. Neurol. 1993;9:318–322. doi: 10.1016/0887-8994(93)90073-L. [DOI] [PubMed] [Google Scholar]
- 415.Senocak F., Sarçlar M., Ozkutlu S. Echocardiographic findings in some metabolic storage diseases. Jpn. Heart J. 1994;35:635–643. doi: 10.1536/ihj.35.635. [DOI] [PubMed] [Google Scholar]
- 416.Haverkamp F., Jacobs D., Cantz M., Hansmann M., Fahnenstich H., Zerres K. Nonimmune hydrops fetalis with galactosialidosis: Consequences for family planning. Fetal Diagn. Ther. 1996;11:114–119. doi: 10.1159/000264289. [DOI] [PubMed] [Google Scholar]
- 417.Claeys M., Van der Hoeven M., de Die-Smulders C., Bakker J.A., Offermans J.P., Forget P.P., Groener J.E., Spaapen L.J. Early-infantile type of galactosialidosis as a cause of heart failure and neonatal ascites. J. Inherit. Metab. Dis. 1999;22:666–667. doi: 10.1023/A:1005546501043. [DOI] [PubMed] [Google Scholar]
- 418.Bursi F., Osranek M., Seward J.B., O′Leary P.W. Mitral and aortic valve thickening associated with galactosialidosis: Echocardiographic features of a lysosomal storage disease. Echocardiography. 2003;20:605–606. doi: 10.1046/j.1540-8175.2003.02139.x. [DOI] [PubMed] [Google Scholar]
- 419.Matsumoto N., Gondo K., Kukita J., Higaki K., Paragison R.C., Nanba E. A case of galactosialidosis with a homozygous Q49R point mutation. Brain Dev. 2008;30:595–598. doi: 10.1016/j.braindev.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 420.Kartal A., Aydın K. A Turkish case of galactosialidosis with a new homozygous mutation in CTSA gene. Metab. Brain Dis. 2017;32:973–975. doi: 10.1007/s11011-017-0042-0. [DOI] [PubMed] [Google Scholar]
- 421.Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Uyama E., Takahashi K., Owada M., Okamura R., Naito M., Tsuji S., Kawasaki S., Araki S. Hydrocephalus, corneal opacities, deafness, valvular heart disease, deformed toes and leptomeningeal fibrous thickening in adult siblings: A new syndrome associated with beta-glucocerebrosidase deficiency and a mosaic population of storage cells. Acta Neurol. Scand. 1992;86:407–420. doi: 10.1111/j.1600-0404.1992.tb05109.x. [DOI] [PubMed] [Google Scholar]
- 423.Mester S.W., Weston M.W. Cardiac tamponade in a patient with Gaucher′s disease. Clin. Cardiol. 1992;15:766–767. doi: 10.1002/clc.4960151015. [DOI] [PubMed] [Google Scholar]
- 424.Saraçlar M., Atalay S., Koçak N., Ozkutlu S. Gaucher′s disease with mitral and aortic involvement: Echocardiographic findings. Pediatr. Cardiol. 1992;13:56–58. doi: 10.1007/BF00788233. [DOI] [PubMed] [Google Scholar]
- 425.Sharratt G.P., Price D., Curtis J.A., Cornel G. Gaucher′s disease with mitral valve calcification. Pediatr. Cardiol. 1992;13:127–128. doi: 10.1007/BF00798223. [DOI] [PubMed] [Google Scholar]
- 426.Abrahamov A., Elstein D., Gross-Tsur V., Farber B., Glaser Y., Hadas-Halpern I., Ronen S., Tafakjdi M., Horowitz M., Zimran A. Gaucher′s disease variant characterised by progressive calcification of heart valves and unique genotype. Lancet. 1995;346:1000–1003. doi: 10.1016/S0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- 427.Beutler E., Kattamis C., Sipe J., Lipson M. 1342C mutation in Gaucher′s disease. Lancet. 1995;346:1637. doi: 10.1016/S0140-6736(95)91975-9. [DOI] [PubMed] [Google Scholar]
- 428.Chabás A., Cormand B., Grinberg D., Burguera J.M., Balcells S., Merino J.L., Mate I., Sobrino J.A., Gonzàlez-Duarte R., Vilageliu L. Unusual expression of Gaucher′s disease: Cardiovascular calcifications in three sibs homozygous for the D409H mutation. J. Med. Genet. 1995;32:740–742. doi: 10.1136/jmg.32.9.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Erduran E., Mocan H., Gedik Y., Kamaci R., Okten A., Değer O. Hydrocephalus, corneal opacities, deafness, left ventricle hypertrophy, clinodactyly in an adolescent patient. A new syndrome associated with glucocerebrosidase deficiency. Genet. Couns. 1995;6:211–215. [PubMed] [Google Scholar]
- 430.Hill S.C., Damaska B.M., Tsokos M., Kreps C., Brady R.O., Barton N.W. Radiographic findings in type 3b Gaucher disease. Pediatr. Radiol. 1996;26:852–860. doi: 10.1007/BF03178036. [DOI] [PubMed] [Google Scholar]
- 431.Hrebícek M., Zeman J., Musilová J., Hodanová K., Renkema G.H., Vepreková L., Ledvinová J., Hrebícek D., Sokolová J., Aerts J.M., et al. A case of type I Gaucher disease with cardiopulmonary amyloidosis and chitotriosidase deficiency. Virchows Arch. Int. J. Pathol. 1996;429:305–309. doi: 10.1007/BF00198347. [DOI] [PubMed] [Google Scholar]
- 432.Pasmanik-Chor M., Laadan S., Elroy-Stein O., Zimran A., Abrahamov A., Gatt S., Horowitz M. The glucocerebrosidase D409H mutation in Gaucher disease. Biochem. Mol. Med. 1996;59:125–133. doi: 10.1006/bmme.1996.0077. [DOI] [PubMed] [Google Scholar]
- 433.Uyama E., Uchino M., Ida H., Eto Y., Owada M. D409H/D409H genotype in Gaucher-like disease. J. Med. Genet. 1997;34:175. doi: 10.1136/jmg.34.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Damiano A.M., Pastores G.M., Ware J.E., Jr. The health-related quality of life of adults with Gaucher′s disease receiving enzyme replacement therapy: Results from a retrospective study. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1998;7:373–386. doi: 10.1023/A:1008814105603. [DOI] [PubMed] [Google Scholar]
- 435.Chabás A., Gort L., Montfort M., Castelló F., Domínguez M.C., Grinberg D., Vilageliu L. Recurrence of the D409H mutation in Spanish Gaucher disease patients: Description of a new homozygous patient and haplotype analysis. J. Med. Genet. 1998;35:775–777. doi: 10.1136/jmg.35.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Guemes A., Kosmorsky G.S., Moodie D.S., Clark B., Meisler D., Traboulsi E.I. Corneal opacities in Gaucher disease. Am. J. Ophthalmol. 1998;126:833–835. doi: 10.1016/S0002-9394(98)00249-9. [DOI] [PubMed] [Google Scholar]
- 437.Bohlega S., Kambouris M., Shahid M., Al Homsi M., Al Sous W. Gaucher disease with oculomotor apraxia and cardiovascular calcification (Gaucher type IIIC) Neurology. 2000;54:261–263. doi: 10.1212/WNL.54.1.261. [DOI] [PubMed] [Google Scholar]
- 438.Cho L., Lytle B.W., Moodie D.S. Type IIIC Gaucher′s disease. Circulation. 2000;102:E69–E70. doi: 10.1161/01.CIR.102.10.e69. [DOI] [PubMed] [Google Scholar]
- 439.Stone D.L., Tayebi N., Coble C., Ginns E.I., Sidransky E. Cardiovascular fibrosis, hydrocephalus, ophthalmoplegia, and visceral involvement in an American child with Gaucher disease. J. Med. Genet. 2000;37:E40. doi: 10.1136/jmg.37.11.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Inui K., Yanagihara K., Otani K., Suzuki Y., Akagi M., Nakayama M., Ida H., Okada S. A new variant neuropathic type of Gaucher′s disease characterized by hydrocephalus, corneal opacities, deformed toes, and fibrous thickening of spleen and liver capsules. J. Pediatr. 2001;138:137–139. doi: 10.1067/mpd.2001.109789. [DOI] [PubMed] [Google Scholar]
- 441.Ono H., Fujiwara M., Ito K., Ueda H., Mizoguchi N., Sakura N. Neurological features in Gaucher′s disease during enzyme replacement therapy. Acta Paediatr. 2001;90:229–231. doi: 10.1111/j.1651-2227.2001.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 442.George R., McMahon J., Lytle B., Clark B., Lichtin A. Severe valvular and aortic arch calcification in a patient with Gaucher′s disease homozygous for the D409H mutation. Clin. Genet. 2001;59:360–363. doi: 10.1034/j.1399-0004.2001.590511.x. [DOI] [PubMed] [Google Scholar]
- 443.Michelakakis H., Skardoutsou A., Mathioudakis J., Moraitou M., Dimitriou E., Voudris C., Karpathios T. Early-onset severe neurological involvement and D409H homozygosity in Gaucher disease: Outcome of enzyme replacement therapy. Blood Cells Mol. Dis. 2002;28:1–4. doi: 10.1006/bcmd.2001.0477. [DOI] [PubMed] [Google Scholar]
- 444.Fujiwaki T., Yamaguchi S., Tasaka M., Sakura N., Taketomi T. Application of delayed extraction-matrix-assisted laser desorption ionization time-of-flight mass spectrometry for analysis of sphingolipids in pericardial fluid, peritoneal fluid and serum from Gaucher disease patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002;776:115–123. doi: 10.1016/S1570-0232(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 445.Torloni M.R., Franco K., Sass N. Gaucher′s disease with myocardial involvement in pregnancy. Sao Paulo Med. J. Rev. Paul. De Med. 2002;120:90–92. doi: 10.1590/S1516-31802002000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Brautbar A., Abrahamov A., Hadas-Halpern I., Elstein D., Zimran A. Gaucher disease in Arab patients at an Israeli referral clinic. Isr. Med. Assoc. J. IMAJ. 2008;10:600–602. [PubMed] [Google Scholar]
- 447.Mireles S.A., Seybold J., Williams G. Undiagnosed type IIIc Gaucher disease in a child with aortic and mitral valve calcification: Perioperative complications after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2010;24:471–474. doi: 10.1053/j.jvca.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 448.Cindik N., Ozcay F., Süren D., Akkoyun I., Gökdemir M., Varan B., Alehan F., Ozbek N., Tokel K. Gaucher disease with communicating hydrocephalus and cardiac involvement. Clin. Cardiol. 2010;33:E26–E30. doi: 10.1002/clc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Perić Z., Kardum-Skelin I., Puskarić B.J., Letilović T., Vrhovac R., Jaksić B. An unusual presentation of Gaucher′s disease: Aortic valve fibrosis in a patient homozygous for a rare G377S mutation. Coll. Antropol. 2010;34:275–278. [PubMed] [Google Scholar]
- 450.Kozelj M., Zver S., Zadnik V. Echocardiographic Assessment of Left Ventricular Function in Type 1 Gaucher′s Disease. Adv. Hematol. 2010;2010:820843. doi: 10.1155/2010/820843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Talluto C.J., Silverman N.H. Aortic and mitral valve stenosis with regurgitation: Not due to rheumatic heart disease. Echocardiography. 2011;28:E24–E27. doi: 10.1111/j.1540-8175.2010.01253.x. [DOI] [PubMed] [Google Scholar]
- 452.Aksu T., Baysal E., Bıyıkoğlu F., Tüfekçioğlu O. Gaucher′s disease with valvular, myocardial and aortic involvement in a patient with oculomotor apraxia. Anadolu Kardiyol. Derg. AKD Anatol. J. Cardiol. 2011;11:E4–E5. doi: 10.5152/akd.2011.023. [DOI] [PubMed] [Google Scholar]
- 453.Altunbas G., Ercan S., Inanç I.H., Ozer O., Kervancıoğlu S., Davutoğlu V. Extensive vascular and valvular involvement in Gaucher disease. Asian Cardiovasc. Thorac. Ann. 2015;23:446–448. doi: 10.1177/0218492313513598. [DOI] [PubMed] [Google Scholar]
- 454.Lo Iudice F., Barbato A., Muscariello R., Di Nardo C., de Stefano F., Sibilio M., Strazzullo P., de Simone G., Galderisi M. Left ventricular diastolic dysfunction in type I Gaucher disease: An echo Doppler study. Echocardiography. 2015;32:890–895. doi: 10.1111/echo.12759. [DOI] [PubMed] [Google Scholar]
- 455.Rastogi P., Rao S., Kaur J., Malhotra P., Varma S., Das R. Gaucher′s Disease with Cardiac Valve Calcification and Stenosis: A Rare Presentation due to Homozygous p.D409H Mutation in a North Indian Family. Indian J. Pediatr. 2016;83:877–878. doi: 10.1007/s12098-015-2025-7. [DOI] [PubMed] [Google Scholar]
- 456.Roghi A., Poggiali E., Cassinerio E., Pedrotti P., Giuditta M., Milazzo A., Quattrocchi G., Cappellini M.D. The role of cardiac magnetic resonance in assessing the cardiac involvement in Gaucher type 1 patients: Morphological and functional evaluations. J. Cardiovasc. Med. 2017;18:244–248. doi: 10.2459/JCM.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 457.Kör Y., Keskin M., Başpınar O. Severe cardiac involvement in Gaucher type IIIC: A case report and review of the literature. Cardiol. Young. 2017;27:1426–1429. doi: 10.1017/S1047951117000579. [DOI] [PubMed] [Google Scholar]
- 458.Weinreb N.J., Barbouth D.S., Lee R.E. Causes of death in 184 patients with type 1 Gaucher disease from the United States who were never treated with enzyme replacement therapy. Blood Cells Mol. Dis. 2018;68:211–217. doi: 10.1016/j.bcmd.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 459.Alzahrani A., Alghamdi A.A., Waggass R. A Saudi Infant with Vici Syndrome: Case Report and Literature Review. Open Access Maced. J. Med. Sci. 2018;6:1081–1084. doi: 10.3889/oamjms.2018.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Alsahli S., Bubshait D.K., Rahbeeni Z.A., Alfadhel M. Aortic calcification in Gaucher disease: A case report. Appl. Clin. Genet. 2018;11:107–110. doi: 10.2147/TACG.S180995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Karakoyun M., Canda E., Kiran Tasci E., Dogan E., Coker M., Aydogdu S. Two siblings with Gaucher type 3c: Different clinical presentations. J. Pediatr. Endocrinol. Metab. JPEM. 2019;32:533–536. doi: 10.1515/jpem-2018-0549. [DOI] [PubMed] [Google Scholar]
- 462.Kurolap A., Del Toro M., Spiegel R., Gutstein A., Shafir G., Cohen I.J., Barrabés J.A., Feldman H.B. Gaucher disease type 3c: New patients with unique presentations and review of the literature. Mol. Genet. Metab. 2019;127:138–146. doi: 10.1016/j.ymgme.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 463.Lazea C., Bucerzan S., Al-Khzouz C., Zimmermann A., Vesa Ș.C., Nașcu I., Creț V., Crișan M., Asăvoaie C., Miclea D., et al. Cardiac Manifestations in a Group of Romanian Patients with Gaucher Disease Type 1 (a Monocentric Study) Diagnostics. 2021;11:989. doi: 10.3390/diagnostics11060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Demirci I., Demir T., Dagdelen S., Haymana C., Tasci I., Atmaca A., Ertugrul D., Ata N., Sahin M., Salman S., et al. No association of Gaucher disease with COVID-19-related outcomes: A nationwide cohort study. Intern. Med. J. 2022;52:379–385. doi: 10.1111/imj.15673. [DOI] [PubMed] [Google Scholar]
- 465.Schiffmann R. Fabry disease. Pharmacol. Ther. 2009;122:65–77. doi: 10.1016/j.pharmthera.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 466.Li X., Ren X., Zhang Y., Ding L., Huo M., Li Q. Fabry disease: Mechanism and therapeutics strategies. Front. Pharmacol. 2022;13:1025740. doi: 10.3389/fphar.2022.1025740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Mehta A., Beck M., Linhart A., Sunder-Plassmann G., Widmer U. History of lysosomal storage diseases: An overview. In: Mehta A., Beck M., Sunder-Plassmann G., editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford PharmaGenesis; Oxford, UK: 2006. Copyright © 2006, Oxford PharmaGenesis™. [PubMed] [Google Scholar]
- 468.Saeed S., Imazio M. Fabry disease: Definition, Incidence, Clinical presentations and Treatment–Focus on cardiac involvement. Pak. J. Med. Sci. 2022;38:2337–2344. doi: 10.12669/pjms.38.8.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Germain D.P., Brand E., Burlina A., Cecchi F., Garman S.C., Kempf J., Laney D.A., Linhart A., Maródi L., Nicholls K., et al. Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: A multicenter Fabry Registry study. Mol. Genet. Genom. Med. 2018;6:492–503. doi: 10.1002/mgg3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Mehta A., Clarke J.T., Giugliani R., Elliott P., Linhart A., Beck M., Sunder-Plassmann G. Natural course of Fabry disease: Changing pattern of causes of death in FOS–Fabry Outcome Survey. J. Med. Genet. 2009;46:548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- 471.Caciotti A., Donati M.A., Boneh A., d′Azzo A., Federico A., Parini R., Antuzzi D., Bardelli T., Nosi D., Kimonis V., et al. Role of beta-galactosidase and elastin binding protein in lysosomal and nonlysosomal complexes of patients with GM1-gangliosidosis. Hum. Mutat. 2005;25:285–292. doi: 10.1002/humu.20147. [DOI] [PubMed] [Google Scholar]
- 472.Morrone A., Bardelli T., Donati M.A., Giorgi M., Di Rocco M., Gatti R., Parini R., Ricci R., Taddeucci G., D′Azzo A., et al. beta-galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum. Mutat. 2000;15:354–366. doi: 10.1002/(SICI)1098-1004(200004)15:4<354::AID-HUMU8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 473.Hadley R.N., Hagstrom J.W. Cardiac lesions in a patient with familial neurovisceral lipidosis (generalized gangliosidosis) Am. J. Clin. Pathol. 1971;55:237–240. doi: 10.1093/ajcp/55.2.237. [DOI] [PubMed] [Google Scholar]
- 474.Gilbert E.F., Varakis J., Opitz J.M., ZuRhein G.M., Ware R., Viseskul C., Kaveggia E.G., Hartmann H.A. Generalized gangliosidosis type II (juvenile GM1 gangliosidosis). A pathological, histochemical and ultrastructural study. Z. Kinderheilkd. 1975;120:151–180. doi: 10.1007/BF00439006. [DOI] [PubMed] [Google Scholar]
- 475.Benson P.F., Barbarik A., Brown S.P., Mann T.P. GM1-generalized gangliosidosis variant with cardiomegaly. Postgrad. Med. J. 1976;52:159–165. doi: 10.1136/pgmj.52.605.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Kohlschütter A., Sieg K., Schulte F.J., Hayek H.W., Goebel H.H. Infantile cardiomyopathy and neuromyopathy with beta-galactosidase deficiency. Eur. J. Pediatr. 1982;139:75–81. doi: 10.1007/BF00442086. [DOI] [PubMed] [Google Scholar]
- 477.Rosenberg H., Frewen T.C., Li M.D., Gordon B.L., Jung J.H., Finlay J.P., Roy P.L., Grover D., Spence M. Cardiac involvement in diseases characterized by beta-galactosidase deficiency. J. Pediatr. 1985;106:78–80. doi: 10.1016/S0022-3476(85)80472-8. [DOI] [PubMed] [Google Scholar]
- 478.Miall-Allen V.M., Morgan B., Cooper P., Shinebourne E.A. Peripheral arteriovenous fistula as a cause of neonatal cardiac failure. Int. J. Cardiol. 1986;10:177–179. doi: 10.1016/0167-5273(86)90226-3. [DOI] [PubMed] [Google Scholar]
- 479.Yang C.F., Wu J.Y., Tsai F.J. Three novel beta-galactosidase gene mutations in Han Chinese patients with GM1 gangliosidosis are correlated with disease severity. J. Biomed. Sci. 2010;17:79. doi: 10.1186/1423-0127-17-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Brunetti-Pierri N., Scaglia F. GM1 gangliosidosis: Review of clinical, molecular, and therapeutic aspects. Mol. Genet. Metab. 2008;94:391–396. doi: 10.1016/j.ymgme.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 481.Caciotti A., Cellai L., Tonin R., Mei D., Procopio E., Di Rocco M., Andaloro A., Antuzzi D., Rampazzo A., Rigoldi M., et al. Morquio B disease: From pathophysiology towards diagnosis. Mol. Genet. Metab. 2021;132:180–188. doi: 10.1016/j.ymgme.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 482.Barry M.O., Beardslee M.A., Braverman A.C. Morquio′s syndrome: Severe aortic regurgitation and late pulmonary autograft failure. J. Heart Valve Dis. 2006;15:839–842. [PubMed] [Google Scholar]
- 483.Dostalova G., Hlubocka Z., Lindner J., Hulkova H., Poupetova H., Vlaskova H., Sikora J., Linhart A., Zeman J., Magner M. Late diagnosis of mucopolysaccharidosis type IVB and successful aortic valve replacement in a 60-year-old female patient. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2018;35:52–56. doi: 10.1016/j.carpath.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 484.Venugopalan P., Joshi S.N. Cardiac involvement in infantile Sandhoff disease. J. Paediatr. Child Health. 2002;38:98–100. doi: 10.1046/j.1440-1754.2002.00765.x. [DOI] [PubMed] [Google Scholar]
- 485.Villamizar-Schiller I.T., Pabón L.A., Hufnagel S.B., Serrano N.C., Karl G., Jefferies J.L., Hopkin R.J., Prada C.E. Neurological and cardiac responses after treatment with miglustat and a ketogenic diet in a patient with Sandhoff disease. Eur. J. Med. Genet. 2015;58:180–183. doi: 10.1016/j.ejmg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 486.Lee H.F., Chi C.S., Tsai C.R. Early cardiac involvement in an infantile Sandhoff disease case with novel mutations. Brain Dev. 2017;39:171–176. doi: 10.1016/j.braindev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 487.Sakpichaisakul K., Taeranawich P., Nitiapinyasakul A., Sirisopikun T. Identification of Sandhoff disease in a Thai family: Clinical and biochemical characterization. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2010;93:1088–1092. [PubMed] [Google Scholar]
- 488.Tim-Aroon T., Wichajarn K., Katanyuwong K., Tanpaiboon P., Vatanavicharn N., Sakpichaisakul K., Kongkrapan A., Eu-Ahsunthornwattana J., Thongpradit S., Moolsuwan K., et al. Infantile onset Sandhoff disease: Clinical manifestation and a novel common mutation in Thai patients. BMC Pediatr. 2021;21:22. doi: 10.1186/s12887-020-02481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ozaal S., Jayasena S., Jayakody S., Schröder S., Jayawardana A., Jasinge E. Clinical Presentation and Genetic Heterogeneity Including Two Novel Variants in Sri Lankan Patients with Infantile Sandhoff Disease. Child Neurol. Open. 2022;9:2329048x221139495. doi: 10.1177/2329048X221139495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Sahyouni J.K., Odeh L.B.M., Mulla F., Junaid S., Kar S.S., Al Boot Almarri N.M.J. Infantile Sandhoff disease with ventricular septal defect: A case report. J. Med. Case Rep. 2022;16:317. doi: 10.1186/s13256-022-03550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Hampe C.S., Eisengart J.B., Lund T.C., Orchard P.J., Swietlicka M., Wesley J., McIvor R.S. Mucopolysaccharidosis Type I: A Review of the Natural History and Molecular Pathology. Cells. 2020;9:1838. doi: 10.3390/cells9081838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Butman S.M., Karl L., Copeland J.G. Combined aortic and mitral valve replacement in an adult with Scheie′s disease. Chest. 1989;96:209–210. doi: 10.1378/chest.96.1.209. [DOI] [PubMed] [Google Scholar]
- 493.Keith O., Scully C., Weidmann G.M. Orofacial features of Scheie (Hurler-Scheie) syndrome (alpha-L-iduronidase deficiency) Oral Surg. Oral Med. Oral Pathol. 1990;70:70–74. doi: 10.1016/0030-4220(90)90181-Q. [DOI] [PubMed] [Google Scholar]
- 494.Demirsoy S., Gücüyener K., Olguntürk R., Tunaoğlu S., Oğuz D. A case of mucopolysaccharidoses type I with heart involvement during infancy. Turk. J. Pediatr. 1990;32:49–52. [PubMed] [Google Scholar]
- 495.Nicolson S.C., Black A.E., Kraras C.M. Management of a difficult airway in a patient with Hurler-Scheie syndrome during cardiac surgery. Anesth. Analg. 1992;75:830–832. doi: 10.1213/00000539-199211000-00032. [DOI] [PubMed] [Google Scholar]
- 496.Du Cret R.P., Weinberg E.J., Jackson C.A., Braunlin E.A., Boudreau R.J., Kuni C.C., Carpenter B.M., Hunter D.W., Krivit W., Bodeau G. Resting Tl-201 scintigraphy in the evaluation of coronary artery disease in children with Hurler syndrome. Clin. Nucl. Med. 1994;19:975–978. doi: 10.1097/00003072-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 497.Imaizumi M., Gushi K., Kurobane I., Inoue S., Suzuki J., Koizumi Y., Suzuki H., Sato A., Gotoh Y., Haginoya K., et al. Long-term effects of bone marrow transplantation for inborn errors of metabolism: A study of four patients with lysosomal storage diseases. Acta Paediatr. Jpn. Overseas Ed. 1994;36:30–36. doi: 10.1111/j.1442-200X.1994.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 498.Minakata K., Konishi Y., Matsumoto M., Miwa S. Surgical treatment for Scheie′s syndrome (mucopolysaccharidosis type I-S): Report of two cases. Jpn. Circ. J. 1998;62:700–703. doi: 10.1253/jcj.62.700. [DOI] [PubMed] [Google Scholar]
- 499.Kakkis E.D., Muenzer J., Tiller G.E., Waber L., Belmont J., Passage M., Izykowski B., Phillips J., Doroshow R., Walot I., et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 500.Braunlin E.A., Stauffer N.R., Peters C.H., Bass J.L., Berry J.M., Hopwood J.J., Krivit W. Usefulness of bone marrow transplantation in the Hurler syndrome. Am. J. Cardiol. 2003;92:882–886. doi: 10.1016/S0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 501.Vijay S., Wraith J.E. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94:872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 502.Lin H.Y., Lin S.P., Chuang C.K., Chen M.R., Chen B.F., Wraith J.E. Mucopolysaccharidosis I under enzyme replacement therapy with laronidase—A mortality case with autopsy report. J. Inherit. Metab. Dis. 2005;28:1146–1148. doi: 10.1007/s10545-005-0211-x. [DOI] [PubMed] [Google Scholar]
- 503.Hingston E.J., Hunter M.L., Hunter B., Drage N. Hurler′s syndrome: Dental findings in a case treated with bone marrow transplantation in infancy. Int. J. Paediatr. Dent. 2006;16:207–212. doi: 10.1111/j.1365-263X.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 504.Braunlin E.A., Berry J.M., Whitley C.B. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am. J. Cardiol. 2006;98:416–418. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 505.Sifuentes M., Doroshow R., Hoft R., Mason G., Walot I., Diament M., Okazaki S., Huff K., Cox G.F., Swiedler S.J., et al. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol. Genet. Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 506.Tokic V., Barisic I., Huzjak N., Petkovic G., Fumic K., Paschke E. Enzyme replacement therapy in two patients with an advanced severe (Hurler) phenotype of mucopolysaccharidosis I. Eur. J. Pediatr. 2007;166:727–732. doi: 10.1007/s00431-006-0316-8. [DOI] [PubMed] [Google Scholar]
- 507.Soliman O.I., Timmermans R.G., Nemes A., Vletter W.B., Wilson J.H., ten Cate F.J., Geleijnse M.L. Cardiac abnormalities in adults with the attenuated form of mucopolysaccharidosis type I. J. Inherit. Metab. Dis. 2007;30:750–757. doi: 10.1007/s10545-007-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Hirth A., Berg A., Greve G. Successful treatment of severe heart failure in an infant with Hurler syndrome. J. Inherit. Metab. Dis. 2007;30:820. doi: 10.1007/s10545-007-0613-z. [DOI] [PubMed] [Google Scholar]
- 509.Hansen M.D., Filipovich A.H., Davies S.M., Mehta P., Bleesing J., Jodele S., Hayashi R., Barnes Y., Shenoy S. Allogeneic hematopoietic cell transplantation (HCT) in Hurler′s syndrome using a reduced intensity preparative regimen. Bone Marrow Transplant. 2008;41:349–353. doi: 10.1038/sj.bmt.1705926. [DOI] [PubMed] [Google Scholar]
- 510.Nemes A., Timmermans R.G., Wilson J.H., Soliman O.I., Krenning B.J., ten Cate F.J., Geleijnse M.L. The mild form of mucopolysaccharidosis type I (Scheie syndrome) is associated with increased ascending aortic stiffness. Heart Vessel. 2008;23:108–111. doi: 10.1007/s00380-007-1013-x. [DOI] [PubMed] [Google Scholar]
- 511.Khedhiri S., Chkioua L., Bouzidi H., Dandana A., Ben Turkia H., Miled A., Laradi S. Mucopolysaccharidoses type I and IVA: Clinical features and consanguinity in Tunisia. Pathol.-Biol. 2009;57:392–397. doi: 10.1016/j.patbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 512.Fesslová V., Corti P., Sersale G., Rovelli A., Russo P., Mannarino S., Butera G., Parini R. The natural course and the impact of therapies of cardiac involvement in the mucopolysaccharidoses. Cardiol. Young. 2009;19:170–178. doi: 10.1017/S1047951109003576. [DOI] [PubMed] [Google Scholar]
- 513.Yano S., Moseley K., Pavlova Z. Postmortem studies on a patient with mucopolysaccharidosis type I: Histopathological findings after one year of enzyme replacement therapy. J. Inherit. Metab. Dis. 2009;32((Suppl. S1)):53–57. doi: 10.1007/s10545-009-1057-4. [DOI] [PubMed] [Google Scholar]
- 514.Goksel O.S., El H., Tireli E., Dayioglu E. Combined aortic and mitral valve replacement in a child with mucopolysaccharidosis type I: A case report. J. Heart Valve Dis. 2009;18:214–216. [PubMed] [Google Scholar]
- 515.Mercimek-Mahmutoglu S., Reilly C., Human D., Waters P.J., Stoeckler-Ipsiroglu S. Progression of organ manifestations upon enzyme replacement therapy in a patient with mucopolysaccharidosis type I/Hurler. World J. Pediatr. WJP. 2009;5:319–321. doi: 10.1007/s12519-009-0062-x. [DOI] [PubMed] [Google Scholar]
- 516.Bahadir C., Kurtulus D., Cihandide E. Mucopolysaccharidosis type-IS presenting with onset of carpal tunnel syndrome at adolescence. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2009;15:402–404. doi: 10.1097/RHU.0b013e3181bedf12. [DOI] [PubMed] [Google Scholar]
- 517.Gabrielli O., Clarke L.A., Bruni S., Coppa G.V. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5-year follow-up. Pediatrics. 2010;125:e183–e187. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- 518.Thomas J.A., Beck M., Clarke J.T., Cox G.F. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J. Inherit. Metab. Dis. 2010;33:421–427. doi: 10.1007/s10545-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Furukawa Y., Hamaguchi A., Nozaki I., Iizuka T., Sasagawa T., Shima Y., Demura S., Murakami H., Kawahara N., Okuyama T., et al. Cervical pachymeningeal hypertrophy as the initial and cardinal manifestation of mucopolysaccharidosis type I in monozygotic twins with a novel mutation in the alpha-L-iduronidase gene. J. Neurol. Sci. 2011;302:121–125. doi: 10.1016/j.jns.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 520.Watanabe N., Anagnostopoulos P.V., Azakie A. Aortic stenosis in a patient with Hurler′s syndrome after bone marrow transplantation. Cardiol. Young. 2011;21:349–350. doi: 10.1017/S1047951110002015. [DOI] [PubMed] [Google Scholar]
- 521.Van den Broek L., Backx A.P., Coolen H., Wijburg F.A., Wevers R., Morava E., Neeleman C. Fatal coronary artery disease in an infant with severe mucopolysaccharidosis type I. Pediatrics. 2011;127:e1343–e1346. doi: 10.1542/peds.2009-2047. [DOI] [PubMed] [Google Scholar]
- 522.Harada H., Uchiwa H., Nakamura M., Ohno S., Morita H., Katoh A., Yoshino M., Ikeda H. Laronidase replacement therapy improves myocardial function in mucopolysaccharidosis I. Mol. Genet. Metab. 2011;103:215–219. doi: 10.1016/j.ymgme.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 523.Muñoz-Rojas M.V., Bay L., Sanchez L., van Kuijck M., Ospina S., Cabello J.F., Martins A.M. Clinical manifestations and treatment of mucopolysaccharidosis type I patients in Latin America as compared with the rest of the world. J. Inherit. Metab. Dis. 2011;34:1029–1037. doi: 10.1007/s10545-011-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Grigull L., Sykora K.W., Tenger A., Bertram H., Meyer-Marcotty M., Hartmann H., Bültmann E., Beilken A., Zivicnjak M., Mynarek M., et al. Variable disease progression after successful stem cell transplantation: Prospective follow-up investigations in eight patients with Hurler syndrome. Pediatr. Transplant. 2011;15:861–869. doi: 10.1111/j.1399-3046.2011.01595.x. [DOI] [PubMed] [Google Scholar]
- 525.Yosunkaya E., Karaca E., Yilmaz S.B., Gezdirici A., Guven G., Seven M., Yuksel A. Sudden vision loss in a mucopolysaccharidosis I patient receiving enzyme replacement therapy. Genet. Couns. 2011;22:371–376. [PubMed] [Google Scholar]
- 526.Rocha R.V., Alvarez R.J., Bermudez C.A. Valve surgery in a mucopolysaccharidosis type I patient: Early prosthetic valve endocarditis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012;41:448–449. doi: 10.1016/j.ejcts.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 527.Arn P., Whitley C., Wraith J.E., Webb H.W., Underhill L., Rangachari L., Cox G.F. High rate of postoperative mortality in patients with mucopolysaccharidosis I: Findings from the MPS I Registry. J. Pediatr. Surg. 2012;47:477–484. doi: 10.1016/j.jpedsurg.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 528.Cabrera G.H., Fernández I., Dominguez M., Clarke L.A. Left ventricular aneurysm in an adult patient with mucopolysaccharidosis type I: Comment on pathogenesis of a novel complication. Mol. Genet. Metab. 2012;106:470–473. doi: 10.1016/j.ymgme.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 529.Jurecka A., Marucha J., Jurkiewicz E., Różdżyńska-Świątkowska A., Tylki-Szymańska A. Enzyme replacement therapy in an attenuated case of mucopolysaccharidosis type I (Scheie syndrome): A 6.5-year detailed follow-up. Pediatr. Neurol. 2012;47:461–465. doi: 10.1016/j.pediatrneurol.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 530.Wiseman D.H., Mercer J., Tylee K., Malaiya N., Bonney D.K., Jones S.A., Wraith J.E., Wynn R.F. Management of mucopolysaccharidosis type IH (Hurler′s syndrome) presenting in infancy with severe dilated cardiomyopathy: A single institution′s experience. J. Inherit. Metab. Dis. 2013;36:263–270. doi: 10.1007/s10545-012-9500-3. [DOI] [PubMed] [Google Scholar]
- 531.Abelin Genevois K., Garin C., Solla F., Guffon N., Kohler R. Surgical management of thoracolumbar kyphosis in mucopolysaccharidosis type 1 in a reference center. J. Inherit. Metab. Dis. 2014;37:69–78. doi: 10.1007/s10545-013-9630-2. [DOI] [PubMed] [Google Scholar]
- 532.Braunlin E., Orchard P.J., Whitley C.B., Schroeder L., Reed R.C., Manivel J.C. Unexpected coronary artery findings in mucopolysaccharidosis. Report of four cases and literature review. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2014;23:145–151. doi: 10.1016/j.carpath.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 533.Aldenhoven M., Wynn R.F., Orchard P.J., O′Meara A., Veys P., Fischer A., Valayannopoulos V., Neven B., Rovelli A., Prasad V.K., et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: An international multicenter study. Blood. 2015;125:2164–2172. doi: 10.1182/blood-2014-11-608075. [DOI] [PubMed] [Google Scholar]
- 534.Al-Sannaa N.A., Bay L., Barbouth D.S., Benhayoun Y., Goizet C., Guelbert N., Jones S.A., Kyosen S.O., Martins A.M., Phornphutkul C., et al. Early treatment with laronidase improves clinical outcomes in patients with attenuated MPS I: A retrospective case series analysis of nine sibships. Orphanet J. Rare Dis. 2015;10:131. doi: 10.1186/s13023-015-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Brazier A., Hasan R., Jenkins P., Hoschtitzky A. Urgent resection of a giant left atrial appendage aneurysm and mitral valve replacement in a complex case of Hurler-Scheie syndrome. BMJ Case Rep. 2015;2015:bcr2015211551. doi: 10.1136/bcr-2015-211551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Ghosh A., Miller W., Orchard P.J., Jones S.A., Mercer J., Church H.J., Tylee K., Lund T., Bigger B.W., Tolar J., et al. Enzyme replacement therapy prior to haematopoietic stem cell transplantation in Mucopolysaccharidosis Type I: 10 year combined experience of 2 centres. Mol. Genet. Metab. 2016;117:373–377. doi: 10.1016/j.ymgme.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Gabrielli O., Clarke L.A., Ficcadenti A., Santoro L., Zampini L., Volpi N., Coppa G.V. 12 year follow up of enzyme-replacement therapy in two siblings with attenuated mucopolysaccharidosis I: The important role of early treatment. BMC Med. Genet. 2016;17:19. doi: 10.1186/s12881-016-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Horovitz D.D., Acosta A.X., Giugliani R., Hlavatá A., Hlavatá K., Tchan M.C., Lopes Barth A., Cardoso L., Jr., Embiruçu de Araújo Leão E.K., Esposito A.C., et al. Alternative laronidase dose regimen for patients with mucopolysaccharidosis I: A multinational, retrospective, chart review case series. Orphanet J. Rare Dis. 2016;11:51. doi: 10.1186/s13023-016-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Laraway S., Mercer J., Jameson E., Ashworth J., Hensman P., Jones S.A. Outcomes of Long-Term Treatment with Laronidase in Patients with Mucopolysaccharidosis Type I. J. Pediatr. 2016;178:219–226.e1. doi: 10.1016/j.jpeds.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 540.Bolourchi M., Renella P., Wang R.Y. Aortic Root Dilatation in Mucopolysaccharidosis I-VII. Int. J. Mol. Sci. 2016;17:2004. doi: 10.3390/ijms17122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Robinson C.R., Roberts W.C. Outcome of Combined Mitral and Aortic Valve Replacement in Adults with Mucopolysaccharidosis (the Hurler Syndrome) Am. J. Cardiol. 2017;120:2113–2118. doi: 10.1016/j.amjcard.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 542.Kiely B.T., Kohler J.L., Coletti H.Y., Poe M.D., Escolar M.L. Early disease progression of Hurler syndrome. Orphanet J. Rare Dis. 2017;12:32. doi: 10.1186/s13023-017-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Lum S.H., Stepien K.M., Ghosh A., Broomfield A., Church H., Mercer J., Jones S., Wynn R. Long term survival and cardiopulmonary outcome in children with Hurler syndrome after haematopoietic stem cell transplantation. J. Inherit. Metab. Dis. 2017;40:455–460. doi: 10.1007/s10545-017-0034-6. [DOI] [PubMed] [Google Scholar]
- 544.Andrade M.F.A., Guimarães I.C.B., Acosta A.X., Leão E., Moreira M.I.G., Mendes C.M.C. Left ventricular assessment in patients with mucopolysaccharidosis using conventional echocardiography and myocardial deformation by two-dimensional speckle-tracking method. J. Pediatr. 2019;95:475–481. doi: 10.1016/j.jped.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 545.Braunlin E., Miettunen K., Lund T., Luquette M., Orchard P. Hematopoietic cell transplantation for severe MPS I in the first six months of life: The heart of the matter. Mol. Genet. Metab. 2019;126:117–120. doi: 10.1016/j.ymgme.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 546.Yamazaki N., Kosuga M., Kida K., Takei G., Fukuhara Y., Matsumoto H., Senda M., Honda A., Ishiguro A., Koike T., et al. Early enzyme replacement therapy enables a successful hematopoietic stem cell transplantation in mucopolysaccharidosis type IH: Divergent clinical outcomes in two Japanese siblings. Brain Dev. 2019;41:546–550. doi: 10.1016/j.braindev.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 547.Moghadam S.H., Ghahvechi M., Mozafari F., Sayarifard F., Mousavi M.S., Rostami R., Ziaee V. Mucopolysaccharidosis Type I in Children, a Forgotten Diagnosis Responsible for Undiagnosed Musculoskeletal Complaints: Report of Two Cases. Acta Med. 2019;62:161–165. doi: 10.14712/18059694.2020.6. [DOI] [PubMed] [Google Scholar]
- 548.Encarnacion C.O., Hang D., Earing M., Mitchell M.E. Mucopolysaccharidoses Causing Valvular Heart Disease: Report and Review of Surgical Management. World J. Pediatr. Congenit. Heart Surg. 2020;11:Np22–Np24. doi: 10.1177/2150135117690105. [DOI] [PubMed] [Google Scholar]
- 549.Zhou Y.A., Li P., Zhang Y., Xiong Q., Li C., Zhao Z., Wang Y., Xiao H. Identification of a novel compound heterozygous IDUA mutation underlies Mucopolysaccharidoses type I in a Chinese pedigree. Mol. Genet. Genom. Med. 2020;8:e1058. doi: 10.1002/mgg3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Sugiura K., Kubo T., Ochi Y., Baba Y., Hirota T., Yamasaki N., Kitaoka H. Cardiac manifestations and effects of enzyme replacement therapy for over 10 years in adults with the attenuated form of mucopolysaccharidosis type I. Mol. Genet. Metab. Rep. 2020;25:100662. doi: 10.1016/j.ymgmr.2020.100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sadeghian H., Sadeghian A., Eslami B., Abbasi S.H., Lotfi-Tokaldany M. Combined Aortic and Mitral Valve Stenosis in Mucopolysaccharidosis Syndrome Type I-S: A Report of a Rare Case. J. Tehran Heart Cent. 2021;16:31–33. doi: 10.18502/jthc.v16i1.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wraith J.E., Scarpa M., Beck M., Bodamer O.A., De Meirleir L., Guffon N., Meldgaard Lund A., Malm G., Van der Ploeg A.T., Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): A clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lin H.Y., Chuang C.K., Huang Y.H., Tu R.Y., Lin F.J., Lin S.J., Chiu P.C., Niu D.M., Tsai F.J., Hwu W.L., et al. Causes of death and clinical characteristics of 34 patients with Mucopolysaccharidosis II in Taiwan from 1995–2012. Orphanet J. Rare Dis. 2016;11:85. doi: 10.1186/s13023-016-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Kurihara M., Kumagai K., Goto K., Imai M., Yagishita S. Severe type Hunter′s syndrome. Polysomnographic and neuropathological study. Neuropediatrics. 1992;23:248–256. doi: 10.1055/s-2008-1071352. [DOI] [PubMed] [Google Scholar]
- 555.Kettles D.I., Sheppard M., Liebmann R.D., Davidson C. Left ventricular aneurysm, aortic valve disease and coronary narrowing in a patient with Hunter′s syndrome. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2002;11:94–96. doi: 10.1016/S1054-8807(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 556.Mohan U.R., Hay A.A., Cleary M.A., Wraith J.E., Patel R.G. Cardiovascular changes in children with mucopolysaccharide disorders. Acta Paediatr. 2002;91:799–804. doi: 10.1111/j.1651-2227.2002.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 557.Bhattacharya K., Gibson S.C., Pathi V.L. Mitral valve replacement for mitral stenosis secondary to Hunter′s syndrome. Ann. Thorac. Surg. 2005;80:1911–1912. doi: 10.1016/j.athoracsur.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 558.Pinto L.L., Schwartz I.V., Puga A.C., Vieira T.A., Munoz M.V., Giugliani R. Prospective study of 11 Brazilian patients with mucopolysaccharidosis II. J. Pediatr. 2006;82:273–278. doi: 10.2223/JPED.1512. [DOI] [PubMed] [Google Scholar]
- 559.Neely J., Carpenter J., Hsu W., Jordan L., Restrepo L. Cerebral infarction in Hunter syndrome. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2006;13:1054–1057. doi: 10.1016/j.jocn.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 560.Guffon N., Bertrand Y., Forest I., Fouilhoux A., Froissart R. Bone marrow transplantation in children with Hunter syndrome: Outcome after 7 to 17 years. J. Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 561.Antoniou T., Kirvassilis G., Tsourelis L., Ieromonachos C., Zarkalis D., Alivizatos P. Mitral valve replacement and Hunter syndrome: Case report. Heart Surg. Forum. 2009;12:E54–E56. doi: 10.1532/HSF98.20081108. [DOI] [PubMed] [Google Scholar]
- 562.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F., Eto Y., Orii T. Japan Elaprase Treatment (JET) study: Idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 563.Kampmann C., Beck M., Morin I., Loehr J.P. Prevalence and characterization of cardiac involvement in Hunter syndrome. J. Pediatr. 2011;159:327–331.e322. doi: 10.1016/j.jpeds.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 564.Wang R.Y., Covault K.K., Halcrow E.M., Gardner A.J., Cao X., Newcomb R.L., Dauben R.D., Chang A.C. Carotid intima-media thickness is increased in patients with mucopolysaccharidoses. Mol. Genet. Metab. 2011;104:592–596. doi: 10.1016/j.ymgme.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Sohn Y.B., Choi E.W., Kim S.J., Park S.W., Kim S.H., Cho S.Y., Jeong S.I., Huh J., Kang I.S., Lee H.J., et al. Retrospective analysis of the clinical manifestations and survival of Korean patients with mucopolysaccharidosis type II: Emphasis on the cardiovascular complication and mortality cases. Am. J. Med. Genet. Part A. 2012;158:90–96. doi: 10.1002/ajmg.a.34371. [DOI] [PubMed] [Google Scholar]
- 566.Grinberg H., Quaio C.R., Avila M.S., Ferreira S.M., Vieira M.L., Benvenuti L.A., Kim C.A., Bocchi E.A. The first cardiac transplant experience in a patient with mucopolysaccharidosis. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2012;21:358–360. doi: 10.1016/j.carpath.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 567.Uluganyan M., Velibey Y., Karaca G., Güngör B. Echocardiographic demonstration of isolated mitral valve involvement in a patient with mucopolysaccharidosis. Turk Kardiyol. Dern. Ars. Turk Kardiyol. Dern. Yayin. Organidir. 2012;40:199. doi: 10.5543/tkda.2012.01795. [DOI] [PubMed] [Google Scholar]
- 568.Tanaka A., Okuyama T., Suzuki Y., Sakai N., Takakura H., Sawada T., Tanaka T., Otomo T., Ohashi T., Ishige-Wada M., et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: A nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 569.Quaio C.R., Grinberg H., Vieira M.L., Paula A.C., Leal G.N., Gomy I., Leistner-Segal S., Giugliani R., Bertola D.R., Kim C.A. Report of a Large Brazilian Family with a Very Attenuated Form of Hunter Syndrome (MPS II) JIMD Rep. 2012;4:125–128. doi: 10.1007/8904_2011_90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Brands M.M., Frohn-Mulder I.M., Hagemans M.L., Hop W.C., Oussoren E., Helbing W.A., van der Ploeg A.T. Mucopolysaccharidosis: Cardiologic features and effects of enzyme-replacement therapy in 24 children with MPS I, II and VI. J. Inherit. Metab. Dis. 2013;36:227–234. doi: 10.1007/s10545-011-9444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Tajima G., Sakura N., Kosuga M., Okuyama T., Kobayashi M. Effects of idursulfase enzyme replacement therapy for Mucopolysaccharidosis type II when started in early infancy: Comparison in two siblings. Mol. Genet. Metab. 2013;108:172–177. doi: 10.1016/j.ymgme.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 572.Sato Y., Fujiwara M., Kobayashi H., Ida H. Massive accumulation of glycosaminoglycans in the aortic valve of a patient with Hunter syndrome during enzyme replacement therapy. Pediatr. Cardiol. 2013;34:2077–2079. doi: 10.1007/s00246-013-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Lampe C., Bosserhoff A.K., Burton B.K., Giugliani R., de Souza C.F., Bittar C., Muschol N., Olson R., Mendelsohn N.J. Long-term experience with enzyme replacement therapy (ERT) in MPS II patients with a severe phenotype: An international case series. J. Inherit. Metab. Dis. 2014;37:823–829. doi: 10.1007/s10545-014-9686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Lin S.M., Lin H.Y., Chuang C.K., Lin S.P., Chen M.R. Cardiovascular abnormalities in Taiwanese patients with mucopolysaccharidosis. Mol. Genet. Metab. 2014;111:493–498. doi: 10.1016/j.ymgme.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 575.Tomanin R., Zanetti A., D′Avanzo F., Rampazzo A., Gasparotto N., Parini R., Pascarella A., Concolino D., Procopio E., Fiumara A., et al. Clinical efficacy of enzyme replacement therapy in paediatric Hunter patients, an independent study of 3.5 years. Orphanet J. Rare Dis. 2014;9:129. doi: 10.1186/s13023-014-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Madireddi J., Sarada P., Shetty R.K., Prabhu M., Girish K.M. Hunter syndrome with its typical heart: A close mimic to rheumatic heart. BMJ Case Rep. 2015;2015:bcr2015209359. doi: 10.1136/bcr-2015-209359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Dalmau Serra J., Vitoria Miñana I., Calderón Fernández R., Cortell Aznar I. Clinical response to long term enzyme replacement treatment in children, adolescent and adult patients with Hunter syndrome. Med. Clin. 2015;145:392–398. doi: 10.1016/j.medcli.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 578.Parini R., Rigoldi M., Tedesco L., Boffi L., Brambilla A., Bertoletti S., Boncimino A., Del Longo A., De Lorenzo P., Gaini R., et al. Enzymatic replacement therapy for Hunter disease: Up to 9 years experience with 17 patients. Mol. Genet. Metab. Rep. 2015;3:65–74. doi: 10.1016/j.ymgmr.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Lin H.Y., Chuang C.K., Chen M.R., Lin S.M., Hung C.L., Chang C.Y., Chiu P.C., Tsai W.H., Niu D.M., Tsai F.J., et al. Cardiac structure and function and effects of enzyme replacement therapy in patients with mucopolysaccharidoses I, II, IVA and VI. Mol. Genet. Metab. 2016;117:431–437. doi: 10.1016/j.ymgme.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 580.Alkhzouz C., Lazea C., Bucerzan S., Nascu I., Kiss E., Denes C.L., Grigorescu-Sido P. Clinical and Genetic Characteristics of Romanian Patients with Mucopolysaccharidosis Type II. JIMD Rep. 2017;33:19–25. doi: 10.1007/8904_2016_535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Bounds R.L., Kuebler J., Cholette J.M., Alfieris G.M., Emani S.M., Wittlieb-Weber C.A. Left Main Coronary Artery Atresia in an Infant with Inclusion-Cell Disease. World J. Pediatr. Congenit. Heart Surg. 2018;9:246–250. doi: 10.1177/2150135116664701. [DOI] [PubMed] [Google Scholar]
- 582.Chlebowski M.M., Heese B.A., Malloy-Walton L.E. Early childhood onset of high-grade atrioventricular block in Hunter syndrome. Cardiol. Young. 2018;28:786–787. doi: 10.1017/S1047951118000215. [DOI] [PubMed] [Google Scholar]
- 583.Suzuki K., Sakai H., Takahashi K. Perioperative airway management for aortic valve replacement in an adult with mucopolysaccharidosis type II (Hunter syndrome) JA Clin. Rep. 2018;4:24. doi: 10.1186/s40981-018-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Selvanathan A., Ellaway C., Wilson C., Owens P., Shaw P.J., Bhattacharya K. Effectiveness of Early Hematopoietic Stem Cell Transplantation in Preventing Neurocognitive Decline in Mucopolysaccharidosis Type II: A Case Series. JIMD Rep. 2018;41:81–89. doi: 10.1007/8904_2018_104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Curran L., Davison J., Shaughnessy L., Shore D., Franklin R.C. Visual Loss Post Ross Procedure in an Adolescent with Newly Diagnosed Mucopolysaccharidosis Type II. Ann. Thorac. Surg. 2019;108:e297–e299. doi: 10.1016/j.athoracsur.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 586.Broomfield A., Davison J., Roberts J., Stewart C., Hensman P., Beesley C., Tylee K., Rust S., Schwahn B., Jameson E., et al. Ten years of enzyme replacement therapy in paediatric onset mucopolysaccharidosis II in England. Mol. Genet. Metab. 2020;129:98–105. doi: 10.1016/j.ymgme.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 587.Do Valle D.A., Cirino R.H.D., Santos M., Pellissari E.C., Scola R.H. Enzyme Replacement Therapy Decreases Left Ventricular Mass Index in Patients with Hunter Syndrome? Pediatr. Cardiol. 2020;41:361–365. doi: 10.1007/s00246-019-02267-0. [DOI] [PubMed] [Google Scholar]
- 588.Suzuki Y., Taylor M., Orii K., Fukao T., Orii T., Tomatsu S. Assessment of Activity of Daily Life in Mucopolysaccharidosis Type II Patients with Hematopoietic Stem Cell Transplantation. Diagnostics. 2020;10:46. doi: 10.3390/diagnostics10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Lin H.Y., Chuang C.K., Lee C.L., Chen M.R., Sung K.T., Lin S.M., Hou C.J., Niu D.M., Chang T.M., Hung C.L., et al. Cardiac Evaluation using Two-Dimensional Speckle-Tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics. 2020;10:62. doi: 10.3390/diagnostics10020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Ayuna A., Stepien K.M., Hendriksz C.J., Balerdi M., Garg A., Woolfson P. Cardiac rhythm abnormalities–An underestimated cardiovascular risk in adult patients with Mucopolysaccharidoses. Mol. Genet. Metab. 2020;130:133–139. doi: 10.1016/j.ymgme.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 591.Lin H.Y., Chen M.R., Lee C.L., Lin S.M., Hung C.L., Niu D.M., Chang T.M., Chuang C.K., Lin S.P. Aortic Root Dilatation in Taiwanese Patients with Mucopolysaccharidoses and the Long-Term Effects of Enzyme Replacement Therapy. Diagnostics. 2020;11:16. doi: 10.3390/diagnostics11010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Lin H.Y., Chen M.R., Lee C.L., Lin S.M., Hung C.L., Niu D.M., Chang T.M., Chuang C.K., Lin S.P. Natural progression of cardiac features and long-term effects of enzyme replacement therapy in Taiwanese patients with mucopolysaccharidosis II. Orphanet J. Rare Dis. 2021;16:99. doi: 10.1186/s13023-021-01743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Poitier B., Amrane M., Bruneval P., Achouh P. Surgical management of an aortic root dilatation in a patient suffering from Hunter syndrome. Interact. Cardiovasc. Thorac. Surg. 2021;33:819–821. doi: 10.1093/icvts/ivab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Racoma M.J.C., Calibag M., Cordero C.P., Abacan M.A.R., Chiong M.A.D. A review of the clinical outcomes in idursulfase-treated and untreated Filipino patients with mucopolysaccharidosis type II: Data from the local lysosomal storage disease registry. Orphanet J. Rare Dis. 2021;16:323. doi: 10.1186/s13023-021-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Mori N., Kitahara H., Muramatsu T., Matsuura K., Nakayama T., Matsumiya G., Kobayashi Y. Transcatheter aortic valve implantation for severe aortic stenosis in a patient with mucopolysaccharidosis type II (Hunter syndrome) accompanied by severe airway obstruction. J. Cardiol. Cases. 2022;25:49–51. doi: 10.1016/j.jccase.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Sestito S., Rinninella G., Rampazzo A., D′Avanzo F., Zampini L., Santoro L., Gabrielli O., Fiumara A., Barone R., Volpi N., et al. Cardiac involvement in MPS patients: Incidence and response to therapy in an Italian multicentre study. Orphanet J. Rare Dis. 2022;17:251. doi: 10.1186/s13023-022-02396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Stephan B.O., Quaio C.R., Spolador G.M., de Paula A.C., Curiati M.A., Martins A.M., Leal G.N., Tenorio A., Finzi S., Chimelo F.T., et al. Impact of ERT and follow-up of 17 patients from the same family with a mild form of MPS II. Clinics. 2022;77:100082. doi: 10.1016/j.clinsp.2022.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Zhang Z., Ma M., Zhang W., Zhou Y., Yao F., Zhu L., Wei M., Qiu Z. Phenotypic and genetic characteristics of 130 patients with mucopolysaccharidosis type II: A single-center retrospective study in China. Front. Genet. 2023;14:1103620. doi: 10.3389/fgene.2023.1103620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Stapleton M., Kubaski F., Mason R.W., Yabe H., Suzuki Y., Orii K.E., Orii T., Tomatsu S. Presentation and Treatments for Mucopolysaccharidosis Type II (MPS II.; Hunter Syndrome) Expert Opin. Orphan Drugs. 2017;5:295–307. doi: 10.1080/21678707.2017.1296761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Cohen M.A., Stuart G.M. Delivery of anesthesia for children with Mucopolysaccharidosis Type III (Sanfilippo syndrome): A review of 86 anesthetics. Paediatr. Anaesth. 2017;27:363–369. doi: 10.1111/pan.13075. [DOI] [PubMed] [Google Scholar]
- 601.Zelei T., Csetneki K., Vokó Z., Siffel C. Epidemiology of Sanfilippo syndrome: Results of a systematic literature review. Orphanet J. Rare Dis. 2018;13:53. doi: 10.1186/s13023-018-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Seker Yilmaz B., Davison J., Jones S.A., Baruteau J. Novel therapies for mucopolysaccharidosis type III. J. Inherit. Metab. Dis. 2021;44:129–147. doi: 10.1002/jimd.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Lavery C., Hendriksz C.J., Jones S.A. Mortality in patients with Sanfilippo syndrome. Orphanet J. Rare Dis. 2017;12:168. doi: 10.1186/s13023-017-0717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Mueller P., Attenhofer Jost C.H., Rohrbach M., Valsangiacomo Buechel E.R., Seifert B., Balmer C., Kretschmar O., Baumgartner M.R., Weber R. Cardiac disease in children and young adults with various lysosomal storage diseases: Comparison of echocardiographic and ECG changes among clinical groups. Int. J. Cardiol. Heart Vessel. 2013;2:1–7. doi: 10.1016/j.ijchv.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Wilhelm C.M., Truxal K.V., McBride K.L., Kovalchin J.P., Flanigan K.M. Natural history of echocardiographic abnormalities in mucopolysaccharidosis III. Mol. Genet. Metab. 2018;124:131–134. doi: 10.1016/j.ymgme.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Nijmeijer S.C.M., de Bruin-Bon R., Wijburg F.A., Kuipers I.M. Cardiac disease in mucopolysaccharidosis type III. J. Inherit. Metab. Dis. 2019;42:276–285. doi: 10.1002/jimd.12015. [DOI] [PubMed] [Google Scholar]
- 607.Lin H.Y., Chen M.R., Lin S.M., Hung C.L., Niu D.M., Chang T.M., Chuang C.K., Lin S.P. Cardiac characteristics and natural progression in Taiwanese patients with mucopolysaccharidosis III. Orphanet J. Rare Dis. 2019;14:140. doi: 10.1186/s13023-019-1112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Cirino Ballantyne M., Chiu B., Sergi C.M. Sanfilippo syndrome type A: Early cardiac involvement of two patients with cardiac manifestations. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2022;60:107430. doi: 10.1016/j.carpath.2022.107430. [DOI] [PubMed] [Google Scholar]
- 609.Altin H., Dorum S., Ture E. Tissue doppler echocardiographic evaluation of cardiac functions in children with mucopolysaccharidosis type III disease. Niger. J. Clin. Pract. 2022;25:1717–1724. doi: 10.4103/njcp.njcp_195_22. [DOI] [PubMed] [Google Scholar]
- 610.Shawky R.M., Abd el-Monim M.T., el-Sebai A.A., el-Sayed S.M. Cardiac and ocular manifestations in Egyptian patients with mucopolysaccharidoses. East. Mediterr. Health J. Rev. Sante Mediterr. Orient. Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2001;7:981–991. doi: 10.26719/2001.7.6.981. [DOI] [PubMed] [Google Scholar]
- 611.Zhao H.G., Li H.H., Bach G., Schmidtchen A., Neufeld E.F. The molecular basis of Sanfilippo syndrome type B. Proc. Natl. Acad. Sci. USA. 1996;93:6101–6105. doi: 10.1073/pnas.93.12.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Muenzer J., Beekman R.H., Profera L.M., Bove E.L. Severe mitral insufficiency in mucopolysaccharidosis type III-B (Sanfilippo syndrome) Pediatr. Cardiol. 1993;14:130–132. doi: 10.1007/BF00796996. [DOI] [PubMed] [Google Scholar]
- 613.Kourouklis S., Chatzis D., Skafida M., Liagkas K., Paradellis G., Kyriakides Z. Outlet type of interventricular septal defect in SanFilippo type-B syndrome. Int. J. Cardiol. 2007;122:e4–e5. doi: 10.1016/j.ijcard.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 614.Moog U., van Mierlo I., van Schrojenstein Lantman-de Valk H.M., Spaapen L., Maaskant M.A., Curfs L.M. Is Sanfilippo type B in your mind when you see adults with mental retardation and behavioral problems? Am. J. Med. Genet. Part C Semin. Med. Genet. 2007;145:293–301. doi: 10.1002/ajmg.c.30142. [DOI] [PubMed] [Google Scholar]
- 615.Ruijter G.J., Valstar M.J., van de Kamp J.M., van der Helm R.M., Durand S., van Diggelen O.P., Wevers R.A., Poorthuis B.J., Pshezhetsky A.V., Wijburg F.A. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in The Netherlands. Mol. Genet. Metab. 2008;93:104–111. doi: 10.1016/j.ymgme.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 616.Fedele A.O. Sanfilippo syndrome: Causes, consequences, and treatments. Appl. Clin. Genet. 2015;8:269–281. doi: 10.2147/TACG.S57672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Kurihara M., Kumagai K., Yagishita S. Sanfilippo syndrome type C: A clinicopathological autopsy study of a long-term survivor. Pediatr. Neurol. 1996;14:317–321. doi: 10.1016/0887-8994(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 618.Kampmann C., Abu-Tair T., Gökce S., Lampe C., Reinke J., Mengel E., Hennermann J.B., Wiethoff C.M. Heart and Cardiovascular Involvement in Patients with Mucopolysaccharidosis Type IVA (Morquio-A Syndrome) PLoS ONE. 2016;11:e0162612. doi: 10.1371/journal.pone.0162612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Ireland M.A., Rowlands D.B. Mucopolysaccharidosis type IV as a cause of mitral stenosis in an adult. Br. Heart J. 1981;46:113–115. doi: 10.1136/hrt.46.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.John R.M., Hunter D., Swanton R.H. Echocardiographic abnormalities in type IV mucopolysaccharidosis. Arch. Dis. Child. 1990;65:746–749. doi: 10.1136/adc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Nicolini F., Corradi D., Bosio S., Gherli T. Aortic valve replacement in a patient with morquio syndrome. Heart Surg. Forum. 2008;11:E96–E98. doi: 10.1532/HSF98.20071197. [DOI] [PubMed] [Google Scholar]
- 622.Pagel P.S., Almassi G.H. Perioperative implications of Morquio syndrome in a 31-year-old woman undergoing aortic valve replacement. J. Cardiothorac. Vasc. Anesth. 2009;23:855–857. doi: 10.1053/j.jvca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 623.Politei J., Porras-Hurtado G.L., Guelbert N., Fainboim A., Horovitz D.D.G., Satizábal J.M. Enzyme replacement therapy interruption in mucopolysaccharidosis type IVA patients and its impact in different clinical outcomes. JIMD Rep. 2021;58:104–113. doi: 10.1002/jmd2.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Golda A., Jurecka A., Tylki-Szymanska A. Cardiovascular manifestations of mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome) Int. J. Cardiol. 2012;158:6–11. doi: 10.1016/j.ijcard.2011.06.097. [DOI] [PubMed] [Google Scholar]
- 625.Aminzadeh M., Malekpour N., Ghandil P. Identification of arylsulfatase B gene mutations and clinical presentations of Iranian patients with Mucopolysaccharidosis VI. Gene. 2019;706:1–5. doi: 10.1016/j.gene.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 626.Jurecka A., Zakharova E., Cimbalistiene L., Gusina N., Kulpanovich A., Golda A., Opoka-Winiarska V., Piotrowska E., Voskoboeva E., Tylki-Szymańska A. Mucopolysaccharidosis type VI: A predominantly cardiac phenotype associated with homozygosity for p.R152W mutation in the ARSB gene. Am. J. Med. Genet. Part A. 2013;161:1291–1299. doi: 10.1002/ajmg.a.35905. [DOI] [PubMed] [Google Scholar]
- 627.Marwick T.H., Bastian B., Hughes C.F., Bailey B.P. Mitral stenosis in the Maroteaux-Lamy syndrome: A treatable cause of dyspnoea. Postgrad. Med. J. 1992;68:287–288. doi: 10.1136/pgmj.68.798.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Tan C.T., Schaff H.V., Miller F.A., Jr., Edwards W.D., Karnes P.S. Valvular heart disease in four patients with Maroteaux-Lamy syndrome. Circulation. 1992;85:188–195. doi: 10.1161/01.CIR.85.1.188. [DOI] [PubMed] [Google Scholar]
- 629.Herskhovitz E., Young E., Rainer J., Hall C.M., Lidchi V., Chong K., Vellodi A. Bone marrow transplantation for Maroteaux-Lamy syndrome (MPS VI): Long-term follow-up. J. Inherit. Metab. Dis. 1999;22:50–62. doi: 10.1023/A:1005447232027. [DOI] [PubMed] [Google Scholar]
- 630.Dilber E., Celiker A., Karagöz T., Kalkanoğlu H.S. Permanent transfemoral pacemaker implantation in a child with Maroteaux Lamy syndrome. Pacing Clin. Electrophysiol. PACE. 2002;25:1784–1785. doi: 10.1046/j.1460-9592.2002.01784.x. [DOI] [PubMed] [Google Scholar]
- 631.Oudit G.Y., Butany J., Williams W.G., Siu S.C., Clarke J.T., Iwanochko R.M. Left ventricular aneurysm in a patient with mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome): Clinical and pathological correlation. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2007;16:237–240. doi: 10.1016/j.carpath.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 632.Scarpa M., Barone R., Fiumara A., Astarita L., Parenti G., Rampazzo A., Sala S., Sorge G., Parini R. Mucopolysaccharidosis VI: The Italian experience. Eur. J. Pediatr. 2009;168:1203–1206. doi: 10.1007/s00431-008-0910-z. [DOI] [PubMed] [Google Scholar]
- 633.Scarpa M., Buffone E., Marca P.L., Campello M., Rampazzo A. Difficulties in diagnosing slowly progressive mucopolysaccharidosis VI: A case series. J. Pediatr. Rehabil. Med. 2010;3:71–75. doi: 10.3233/PRM-2010-0104. [DOI] [PubMed] [Google Scholar]
- 634.Jurecka A., Golda A., Opoka-Winiarska V., Piotrowska E., Tylki-Szymańska A. Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome) with a predominantly cardiac phenotype. Mol. Genet. Metab. 2011;104:695–699. doi: 10.1016/j.ymgme.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 635.Furujo M., Kubo T., Kosuga M., Okuyama T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI. Mol. Genet. Metab. 2011;104:597–602. doi: 10.1016/j.ymgme.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 636.Hendriksz C.J., Giugliani R., Harmatz P., Lampe C., Martins A.M., Pastores G.M., Steiner R.D., Leão Teles E., Valayannopoulos V. Design, baseline characteristics, and early findings of the MPS VI (mucopolysaccharidosis VI) Clinical Surveillance Program (CSP) J. Inherit. Metab. Dis. 2013;36:373–384. doi: 10.1007/s10545-011-9410-9. [DOI] [PubMed] [Google Scholar]
- 637.Braunlin E., Rosenfeld H., Kampmann C., Johnson J., Beck M., Giugliani R., Guffon N., Ketteridge D., CM S.M., Scarpa M., et al. Enzyme replacement therapy for mucopolysaccharidosis VI: Long-term cardiac effects of galsulfase (Naglazyme®) therapy. J. Inherit. Metab. Dis. 2013;36:385–394. doi: 10.1007/s10545-012-9481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Yano S., Li C., Pavlova Z. The transforming growth factor-Beta signaling pathway involvement in cardiovascular lesions in mucopolysaccharidosis-I. JIMD Rep. 2013;7:55–58. doi: 10.1007/8904_2012_141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Brands M.M., Oussoren E., Ruijter G.J., Vollebregt A.A., van den Hout H.M., Joosten K.F., Hop W.C., Plug I., van der Ploeg A.T. Up to five years experience with 11 mucopolysaccharidosis type VI patients. Mol. Genet. Metab. 2013;109:70–76. doi: 10.1016/j.ymgme.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 640.Horovitz D.D., Magalhães T.S., Acosta A., Ribeiro E.M., Giuliani L.R., Palhares D.B., Kim C.A., de Paula A.C., Kerstenestzy M., Pianovski M.A., et al. Enzyme replacement therapy with galsulfase in 34 children younger than five years of age with MPS VI. Mol. Genet. Metab. 2013;109:62–69. doi: 10.1016/j.ymgme.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 641.Leal G.N., de Paula A.C., Morhy S.S., Andrade J.L., Kim C.A. Advantages of early replacement therapy for mucopolysaccharidosis type VI: Echocardiographic follow-up of siblings. Cardiol. Young. 2014;24:229–235. doi: 10.1017/S1047951113000152. [DOI] [PubMed] [Google Scholar]
- 642.Kampmann C., Lampe C., Whybra-Trümpler C., Wiethoff C.M., Mengel E., Arash L., Beck M., Miebach E. Mucopolysaccharidosis VI: Cardiac involvement and the impact of enzyme replacement therapy. J. Inherit. Metab. Dis. 2014;37:269–276. doi: 10.1007/s10545-013-9649-4. [DOI] [PubMed] [Google Scholar]
- 643.Giugliani R., Lampe C., Guffon N., Ketteridge D., Leão-Teles E., Wraith J.E., Jones S.A., Piscia-Nichols C., Lin P., Quartel A., et al. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)—10-year follow-up of patients who previously participated in an MPS VI Survey Study. Am. J. Med. Genet. Part A. 2014;164:1953–1964. doi: 10.1002/ajmg.a.36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Choy Y.S., Bhattacharya K., Balasubramaniam S., Fietz M., Fu A., Inwood A., Jin D.K., Kim O.H., Kosuga M., Kwun Y.H., et al. Identifying the need for a multidisciplinary approach for early recognition of mucopolysaccharidosis VI (MPS VI) Mol. Genet. Metab. 2015;115:41–47. doi: 10.1016/j.ymgme.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 645.Franco J.F., Soares D.C., Torres L.C., Leal G.N., Cunha M.T., Honjo R.S., Bertola D.R., Kim C.A. Short Communication Impact of early enzyme-replacement therapy for mucopolysaccharidosis VI: Results of a long-term follow-up of Brazilian siblings. Genet. Mol. Res. GMR. 2016;15 doi: 10.4238/gmr.15017850. [DOI] [PubMed] [Google Scholar]
- 646.Lin H.Y., Chuang C.K., Wang C.H., Chien Y.H., Wang Y.M., Tsai F.J., Chou Y.Y., Lin S.J., Pan H.P., Niu D.M., et al. Long-term galsulfase enzyme replacement therapy in Taiwanese mucopolysaccharidosis VI patients: A case series. Mol. Genet. Metab. Rep. 2016;7:63–69. doi: 10.1016/j.ymgmr.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Torre S., Scarpelli M., Salviati A., Buffone E., Faggian G., Luciani G.B. Aortic and Mitral Valve Involvement in Maroteaux-Lamy Syndrome VI: Surgical Implications in the Enzyme Replacement Therapy Era. Ann. Thorac. Surg. 2016;102:e23–e25. doi: 10.1016/j.athoracsur.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 648.Kılıç M., Dursun A., Coşkun T., Tokatlı A., Özgül R.K., Yücel-Yılmaz D., Karaca M., Doğru D., Alehan D., Kadayıfçılar S., et al. Genotypic-phenotypic features and enzyme replacement therapy outcome in patients with mucopolysaccharidosis VI from Turkey. Am. J. Med. Genet. Part A. 2017;173:2954–2967. doi: 10.1002/ajmg.a.38459. [DOI] [PubMed] [Google Scholar]
- 649.Furujo M., Kosuga M., Okuyama T. Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI: 10-Year follow up. Mol. Genet. Metab. Rep. 2017;13:69–75. doi: 10.1016/j.ymgmr.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Bell D.J., He C., Pauli J.L., Naidoo R. Maroteaux-Lamy syndrome: A rare and challenging case of mitral valve replacement. Asian Cardiovasc. Thorac. Ann. 2018;26:560–562. doi: 10.1177/0218492316675533. [DOI] [PubMed] [Google Scholar]
- 651.Lin H.Y., Lee C.L., Lo Y.T., Wang T.J., Huang S.F., Chen T.L., Wang Y.S., Niu D.M., Chuang C.K., Lin S.P. The relationships between urinary glycosaminoglycan levels and phenotypes of mucopolysaccharidoses. Mol. Genet. Genom. Med. 2018;6:982–992. doi: 10.1002/mgg3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Harmatz P.R., Lampe C., Parini R., Sharma R., Teles E.L., Johnson J., Sivam D., Sisic Z. Enzyme replacement therapy outcomes across the disease spectrum: Findings from the mucopolysaccharidosis VI Clinical Surveillance Program. J. Inherit. Metab. Dis. 2019;42:519–526. doi: 10.1002/jimd.12079. [DOI] [PubMed] [Google Scholar]
- 653.Lampe C., Harmatz P.R., Parini R., Sharma R., Teles E.L., Johnson J., Sivam D., Sisic Z. Enzyme replacement therapy initiated in adulthood: Findings from the mucopolysaccharidosis VI Clinical Surveillance Program. Mol. Genet. Metab. 2019;127:355–360. doi: 10.1016/j.ymgme.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 654.Honjo R.S., Vaca E.C.N., Leal G.N., Abellan D.M., Ikari N.M., Jatene M.B., Martins A.M., Kim C.A. Mucopolysaccharidosis type VI: Case report with first neonatal presentation with ascites fetalis and rapidly progressive cardiac manifestation. BMC Med. Genet. 2020;21:37. doi: 10.1186/s12881-020-0972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Horovitz D.D.G., Leão E., Ribeiro E.M., Martins A.M., Barth A.L., Neri J., Kerstenetzky M., Siqueira A.C.M., Ribeiro B.F.R., Kim C.A., et al. Long-term impact of early initiation of enzyme replacement therapy in 34 MPS VI patients: A resurvey study. Mol. Genet. Metab. 2021;133:94–99. doi: 10.1016/j.ymgme.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 656.Garcia P., Phillips D., Johnson J., Martin K., Randolph L.M., Rosenfeld H., Harmatz P. Long-term outcomes of patients with mucopolysaccharidosis VI treated with galsulfase enzyme replacement therapy since infancy. Mol. Genet. Metab. 2021;133:100–108. doi: 10.1016/j.ymgme.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 657.Zauk J., Wyatt K. Transversus thoracis muscle plane blocks for a patient with Maroteaux-Lamy syndrome undergoing mitral valve replacement. J. Clin. Anesth. 2021;72:110269. doi: 10.1016/j.jclinane.2021.110269. [DOI] [PubMed] [Google Scholar]
- 658.Salik I., Rodhouse H.B., Mehta B., Villion A. Preoperative cardiac POCUS for urgent surgery in a patient with Maroteaux-Lamy syndrome. J. Clin. Anesth. 2021;72:110296. doi: 10.1016/j.jclinane.2021.110296. [DOI] [PubMed] [Google Scholar]
- 659.Demis A.A., Oikonomidou S., Daglis F., Polymenakos S., Panagiotou M. Double valve replacement in a patient with Maroteaux–Lamy syndrome as an ultimate team challenge. J. Cardiothorac. Surg. 2021;16:141. doi: 10.1186/s13019-021-01530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.İnci A., Okur İ., Tümer L., Biberoğlu G., Öktem M., Ezgü F. Clinical and event-based outcomes of patients with mucopolysaccharidosis VI receiving enzyme replacement therapy in Turkey: A case series. Orphanet J. Rare Dis. 2021;16:438. doi: 10.1186/s13023-021-02060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Guffon N., Chowdary P., Teles E.L., Hughes D., Hennermann J.B., Huot-Marchand P., Faudot-Vernier E., Lacombe O., Fiquet A., Richard M.P., et al. Oral treatment for mucopolysaccharidosis VI: Outcomes of the first phase IIa study with odiparcil. J. Inherit. Metab. Dis. 2022;45:340–352. doi: 10.1002/jimd.12467. [DOI] [PubMed] [Google Scholar]
- 662.Andrade I., Ribeiro R., Carneiro Z.A., Giugliani R., Pereira C., Cozma C., Grinberg D., Vilageliu L., Lourenco C.M. Fifteen years of enzyme replacement therapy for mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome): A case report. J. Med. Case Rep. 2022;16:46. doi: 10.1186/s13256-021-03240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Haleem A.A. Clinical, Endocrine and Genetic spectrums of Mucopolysaccharidoses type VI in Duhok city, Kurdistan Region, Iraq. Cell. Mol. Biol. 2022;68:63–69. doi: 10.14715/cmb/2022.68.7.11. [DOI] [PubMed] [Google Scholar]
- 664.Parini R., Deodato F. Intravenous Enzyme Replacement Therapy in Mucopolysaccharidoses: Clinical Effectiveness and Limitations. Int. J. Mol. Sci. 2020;21:2975. doi: 10.3390/ijms21082975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Sands M.S. Mucopolysaccharidosis type VII: A powerful experimental system and therapeutic challenge. Pediatr. Endocrinol. Rev. PER. 2014;12((Suppl. S1)):159–165. [PubMed] [Google Scholar]
- 666.Vogler C., Levy B., Kyle J.W., Sly W.S., Williamson J., Whyte M.P. Mucopolysaccharidosis VII: Postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 1994;7:132–137. [PubMed] [Google Scholar]
- 667.Toda Y., Takeuchi M., Morita K., Iwasaki T., Oe K., Yokoyama M., Hirakawa M. Complete heart block during anesthetic management in a patient with mucopolysaccharidosis type VII. Anesthesiology. 2001;95:1035–1037. doi: 10.1097/00000542-200110000-00041. [DOI] [PubMed] [Google Scholar]
- 668.Fox J.E., Volpe L., Bullaro J., Kakkis E.D., Sly W.S. First human treatment with investigational rhGUS enzyme replacement therapy in an advanced stage MPS VII patient. Mol. Genet. Metab. 2015;114:203–208. doi: 10.1016/j.ymgme.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Gniadek T.J., Singer N., Barker N.J., Spevak P.J., Crain B.J., Valle D., Halushka M.K. Cardiovascular pathologies in mucopolysaccharidosis type VII (Sly Syndrome) Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2015;24:322–326. doi: 10.1016/j.carpath.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 670.Montaño A.M., Lock-Hock N., Steiner R.D., Graham B.H., Szlago M., Greenstein R., Pineda M., Gonzalez-Meneses A., Çoker M., Bartholomew D., et al. Clinical course of sly syndrome (mucopolysaccharidosis type VII) J. Med. Genet. 2016;53:403–418. doi: 10.1136/jmedgenet-2015-103322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Lew V., Pena L., Edwards R., Wang R.Y. Cardiovascular Histopathology of a 11-Year Old with Mucopolysaccharidosis VII Demonstrates Fibrosis, Macrophage Infiltration, and Arterial Luminal Stenosis. JIMD Rep. 2018;39:31–37. doi: 10.1007/8904_2017_43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Kantaputra P.N., Smith L.J., Casal M.L., Kuptanon C., Chang Y.C., Nampoothiri S., Paiyarom A., Veerasakulwong T., Trachoo O., Ketudat Cairns J.R., et al. Oral manifestations in patients and dogs with mucopolysaccharidosis Type VII. Am. J. Med. Genet. Part A. 2019;179:486–493. doi: 10.1002/ajmg.a.61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Bilginer Gurbuz B., Aypar E., Coskun T., Alehan D., Dursun A., Tokatli A., Sivri H.S. The effectiveness of enzyme replacement therapy on cardiac findings in patients with mucopolysaccharidosis. J. Pediatr. Endocrinol. Metab. JPEM. 2019;32:1049–1053. doi: 10.1515/jpem-2019-0293. [DOI] [PubMed] [Google Scholar]
- 674.Marek J., Kuchynka P., Mikulenka V., Palecek T., Sikora J., Hulkova H., Lambert L., Linkova H., Zemanek D., Tesarova M., et al. Combined valve replacement and aortocoronary bypass in an adult mucopolysaccharidosis type VII patient. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2021;50:107297. doi: 10.1016/j.carpath.2020.107297. [DOI] [PubMed] [Google Scholar]
- 675.Oldham A., Oxborrow N.J., Woolfson P., Jenkins P., Gadepalli C., Ashworth J., Saxena A., Rothera M., Hendriksz C.J., Tol G., et al. MPS VII–Extending the classical phenotype. Mol. Genet. Metab. Rep. 2022;33:100922. doi: 10.1016/j.ymgmr.2022.100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Poswar F.O., Henriques Nehm J., Kubaski F., Poletto E., Giugliani R. Diagnosis and Emerging Treatment Strategies for Mucopolysaccharidosis VII (Sly Syndrome) Ther. Clin. Risk Manag. 2022;18:1143–1155. doi: 10.2147/TCRM.S351300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Trabszo C., Ramms B., Chopra P., Lüllmann-Rauch R., Stroobants S., Sproß J., Jeschke A., Schinke T., Boons G.J., Esko J.D., et al. Arylsulfatase K inactivation causes mucopolysaccharidosis due to deficient glucuronate desulfation of heparan and chondroitin sulfate. Biochem. J. 2020;477:3433–3451. doi: 10.1042/BCJ20200546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Verheyen S., Blatterer J., Speicher M.R., Bhavani G.S., Boons G.J., Ilse M.B., Andrae D., Sproß J., Vaz F.M., Kircher S.G., et al. Novel subtype of mucopolysaccharidosis caused by arylsulfatase K (ARSK) deficiency. J. Med. Genet. 2022;59:957–964. doi: 10.1136/jmedgenet-2021-108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Depre C., Vanoverschelde J.L., Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.CIR.99.4.578. [DOI] [PubMed] [Google Scholar]
- 680.Kolwicz S.C., Jr., Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc. Res. 2011;90:194–201. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.