Abstract
During the investigation of lignicolous freshwater fungi in the Tibetan Plateau habitat, fifteen collections were isolated from submerged decaying wood. Fungal characteristics are commonly found as punctiform or powdery colonies with dark pigmented and muriform conidia. Multigene phylogenetic analyses of combined ITS, LSU, SSU and TEF DNA sequences showed that they belong to three families in Pleosporales. Among them, Paramonodictys dispersa, Pleopunctum megalosporum, Pl. multicellularum and Pl. rotundatum are established as new species. Paradictyoarthrinium hydei, Pleopunctum ellipsoideum and Pl. pseudoellipsoideum are reported as new records on the freshwater habitats in Tibetan Plateau, China. The morphological descriptions and illustrations of the new collections are provided.
Keywords: freshwater fungi, Paramonodictys, Pleopunctum, hyphomycetes, Pleosporales
1. Introduction
The Tibetan Plateau locates in Central Asia with a mean elevation of more than 4000 m above sea level and an area of about 2,300,000 km2 [1]. The Tibetan Plateau encompasses remarkable endemic biodiversity as one of the largest and most unique geographical units on earth [2,3,4,5]. The Tibetan Plateau is rich in water resources, from which almost all major rivers in Asia originate and these rivers serve nearly 1.4 billion people [6]. Furthermore, the Tibetan Plateau, acting as an environmental and ecological barrier [7], has an important effect on ecological security in Asia [8]. It is called the “third pole” of the world [9]. Therefore, it is important to investigate and study the Tibetan Plateau.
Freshwater fungi are a critical component of aquatic ecosystems, playing key roles in the cycling of carbon, nutrients and, energy and are involved in the decomposition of dead organic matter [10,11,12,13]. Despite their ecological importance, freshwater fungi have been often overlooked in studies of freshwater ecosystems [10,12]. The unique environmental conditions of the Tibetan Plateau provide an ideal and unmatched setting for the diversity and distribution of freshwater fungi. The plateau’s high altitude, cold temperatures, and low precipitation create a distinctive set of environmental conditions that shape the uniqueness of the fungal diversity communities and their interactions with other organisms [7,14].
Pleosporales is one of largest orders in Dothideomycetes comprising 94 families, with more than 400 genera [15,16,17,18,19,20]. Parabambusicolaceae was introduced by Tanaka et al. [21], with the generic type Parabambusicola. Currently, this family covers 12 genera, including Aquastroma, Lonicericola, Multilocularia, Multiseptospora, Neoaquastroma, Neomultiseptospora, Parabambusicola, Paramonodictys, Paramultiseptospora, Paratrimmatostroma, Pseudomonodictys and Scolecohyalosporium [19,22]. Paramonodictys was first introduced by Hyde et al. [23] with P. solitarius as the type species, which is saprobic on decaying wood in terrestrial habitats. Paramonodictys is characterized by superficial subcylindrical or truncated-cone-form stromata; absent conidiophores; monoblastic conidiogenous cells; muriform, globose or subglobose; dark-pigmented conidia [23]. Paramonodictys species occur on decaying wood in both freshwater and terrestrial habitats [20,23].
Paradictyoarthriniaceae was introduced by Liu et al. [24], accommodating an asexual morph genus Paradictyoarthrinium based on its unique morphology and distinct lineage in the phylogenetic analysis. Subsequently, Xenomassariosphaeria, a sexual morph genus in this family, was established by Wanasinghe et al. [25]. To date, two genera have been accepted in the family viz. Paradictyoarthrinium and Xenomassariosphaeria [19,24,25]. Paradictyoarthrinium is characterized by superficial, gregarious, black, powdery and macronematous conidiophores with unevenly dictyoseptate, subglobose to ellipsoidal, dark brown conidia [26]. Paradictyoarthrinium was initially established as a monotypic genus, with Pd. diffractum as the type species [27]. Subsequently, species of this genus have been continuously described and illustrated [24,26,28,29,30]. So far, four species have been accepted in Paradictyoarthrinium, viz. Pd. aquatica, Pd. diffractum, Pd. hydei and Pd. Tectonicola [24,26,27], http://www.indexfungorum.org/Names/Names.asp accessed on 6 March 2023.
Phaeoseptaceae was established by Hyde et al. [31] to accommodate three genera: Lignosphaeria, Neolophiostoma and Phaeoseptum. Wanasinghe et al. [32] suggested the classification of Phaeoseptaceae includes five genera: Decaisnella, Lignosphaeria, Phaeoseptum, Pleopunctum and Thyridaria. The type of genus Phaeoseptum was initially introduced by Zhang et al. [33] and is known for its sexual morph [31,33,34,35], http://www.indexfungorum.org/Names/Names.asp, accessed on 6 Match 2023. Pleopunctum, the first hyphomycetous genus in Phaeoseptaceae, was introduced by Liu et al. [36], with Pl. ellipticum as the type species. The genus is characterized by colonies that are scattered, gregarious and, punctiform; macronematous, cylindrical, branched conidiophores; monoblastic, cylindrical conidiogenous cells; and acrogenous, solitary, muriform conidia, often with a hyaline, elliptical to globose basal cell. Currently, seven species have been accepted, all of which are asexual morphs found in China and Thailand, including Pl. bauhiniae, Pl. clematidis, Pl. ellipsoideum, Pleopunctum heveae, Pl. menglaense, Pl. pseudoellipsoideum and Pl. thailandicum [32,35,36,37].
In this study, we studied the lignicolous freshwater fungi of the Tibetan Plateau, China. Seven species were collected with the common features of punctiform colonies and muriform conidia. Although these species are morphologically indistinguishable, they belong to three different families within Pleosporales: Parabambusicolaceae, Paradictyoarthriniaceae, and Phaeoseptaceae, as revealed by multi-gene phylogenetic analyses.
2. Materials and Methods
2.1. Collection, Morphological Examination and Isolation
Submerged decaying wood samples were collected from freshwater habitats in Hengduan Mountains of the Tibetan Plateau, China. Fresh specimens were studied following the methods of Senanayake et al. [38]. Microscopic structures were examined by using a stereomicroscope (SteREO Discovery.V12, Carl Zeiss Microscopy GmBH, Göttingen, Germany), photographed by using a Nikon ECLIPSE 80i compound microscope fitted with a Nikon DS-Ri2 digital camera, and measured by using the Tarosoft (R) Image Framework program. Illustrated figures were processed by using Adobe Photoshop CS6 version 10.0 software (Adobe Systems, San Jose, CA, USA).
Single spore isolation was performed on potato dextrose agar (PDA) plates following the methods described in Senanayake et al. [38]. Fungal herbarium specimens and axenic living cultures were deposited into the Herbarium of Cryptogams of the Kunming Institute of Botany, Chinese Academy of Sciences (KUN-HKAS) and Kunming Institute of Botany Culture Collection (KUNCC), Kunming, China. The novel species were registered in the Faceoffungi [39] and Index Fungorum databases (Index Fungorum 2023).
2.2. DNA Extraction, PCR Amplification and Sequencing
Fresh mycelia scraped from colonies growing on PDA plates were used for DNA extraction by using a total DNA extraction kit according to the manufacturer’s instructions (TOLOBIO Plant Genomic DNA Extraction Kit, Shanghai Co., Ltd., Shanghai, China). Polymerase Chain Reaction (PCR) amplifications were performed by using primer pairs ITS5/ITS4 for internal transcribed spacer rDNA region and covered 5.8S ribosomal (ITS), LR0R/LR5 for the nuclear ribosomal large subunit 28S rDNA gene (LSU), NS1/NS4 for the nuclear ribosomal small subunit 18S rDNA gene (SSU) and TEF1-983F/TEF1-2218R for TEF1, respectively [40,41]. The DNA template was carried out in 25 μL reaction volume containing 21 μL of 1 × Power Taq PCR Master Mix, 1 μL of each primer (10 μL stock) and 2 μL of genomic DNA template. Amplifications were carried out by using the BioTeke GT9612 thermocycler (Beijing City, China). The PCR amplification conditions for ITS, LSU and SSU consisted of initial denaturation at 98 °C for 3 min, followed by 35 cycles of denaturation at 98 °C for 20 s, annealing at 53 °C for 10 s, extension at 72 °C for 20 s and the final extension at 72 °C for 5 min. The PCR amplification conditions for TEF1 consisted of initial denaturation at 98 °C for 3 min, followed by 35 cycles of denaturation at 98 °C for 20 s, annealing at 64 °C for 10 s, extension at 72 °C for 20 s, final extension at 72 °C for 5 min. PCR products were visualized by using 1% agarose gel electrophoresis and distinct bands were checked in Gel documentation system (Compact Desktop UV Transilluminator analyzer GL-3120). The PCR products were sequenced by Tsingke Company, Beijing, China.
2.3. Phylogenetic Analyses
The sequences were blasted to search for related taxa in the GenBank database (http://www.ncbi.nlm.nih.gov/blast/, accessed on 6 Match 2023). Sequences generated from the LSU, TEF1, SSU and ITS gene regions were carefully verified before further analysis. The new sequences were submitted to GenBank, and the strain information used in this paper was provided in Table 1 [19,20,36]. Multiple sequence alignments were aligned with MAFFT v.7 [42] http://mafft.cbrc.jp/alignment/server/index.html, accessed on 6 Match 2023, and the dataset was trimmed by TrimAlv.1.3 using the gappyout option [43] http://phylemon.bioinfo.cipf.es/utilities.html, accessed on 6 Match 2023. A combined sequence dataset was performed with the SquenceMatrix v.1.7.8 [44].
Maximum likelihood (ML) analysis was performed by RAxML-HPC2 v.8.2.12 [45] in the CIPRES Science Gateway web server [46] http://www.phylo.org/portal2, accessed on 6 Match 2023, by using 1000 rapid bootstrap replicates and the GTRGAMMA+I model. Bootstrap support values for ML equal to or greater than 75% were given above the nodes in the phylogenetic tree (Figure 1).
Figure 1.
RAxML tree based on analysis of a combined LSU, TEF1, SSU and ITS sequence dataset from three families in Pleosporales, viz. Parabambusicolaceae, Paradictyoarthriniaceae and Phaeoseptaceae. Bootstrap support values for ML equal to or greater than 75% were given above the nodes (black). PP values equal to or greater than 0.95 were given above the nodes (yellow). The tree was rooted to Ceratostomella cuspidata (ICMP 17629) and C. pyrenaica (CBS 129343). The type strains were shown in bold, and the newly generated isolates were shown in red.
The model of evolution for the Bayesian inference (BI) analysis was performed by using MrModeltest v2.3 [47]. GTR+I+G was selected as the best-fitting model for LSU, TEF1, SSU and ITS dataset. The Markov chain Monte Carlo sampling (BMCMC) was carried out to assess posterior probabilities (PP) by using MrBayes v.3.2.7 [48]. Six simultaneous Markov chains were run for random trees for 1,000,000 generations, and trees were sampled every 200th generation. Bayesian posterior probabilities (PP) equal to or greater than 0.95 were given above the nodes in the phylogenetic tree (Figure 1). Phylograms were visualized by using FigTree v1.4.0 [49] and rearranged in Adobe Photoshop CS6 software (Adobe Systems, USA). The new sequences were deposited in GenBank (Table 1), and the final alignments and phylogenetic tree were registered in TreeBASE under the submission ID: 30133 (http://www.treebase.org/, accessed on 6 Match 2023).
Table 1.
Taxa used in the phylogenetic analyses and their corresponding GenBank accession numbers.
| Species | Isolate No. | GenBank Accession No. | ||||
|---|---|---|---|---|---|---|
| ITS | LSU | SSU | TEF1-α | References | ||
| Aquastroma magniostiolatum | MFLUCC 20-0112 | MT772005 | MT772011 | – | MT777678 | [50] |
| Aquastroma magniostiolatum | CBS 139680 | NR_153583 | NG_056936 | NG_061000 | – | [21] |
| Ceratostomella cuspidata | ICMP 17629 | KT991671 | FJ617558 | KT991642 | – | [51] |
| Ceratostomella pyrenaica | CBS 129343 | KT991672 | KY931835 | KY931893 | – | [51] |
| Decaisnella formosa | BCC 25616 | – | GQ925846 | GQ925833 | GU479851 | [52] |
| Decaisnella formosa | BCC 25617 | – | GQ925847 | GQ925834 | GU479850 | [52] |
| Lignosphaeria fusispora | MFLUCC 11-0377 | NR_164233 | KP888646 | – | – | [53] |
| Lignosphaeria thailandica | MFLUCC 11-0376 | KP899139 | NG_069268 | – | – | [53] |
| Lonicericola fuyuanensis | MFLU 19-2850 | NR_172419 | NG_073809 | NG_070329 | MN938324 | [54] |
| Lonicericola hyaloseptispora | KUMCC 18-0149 | NR_164294 | NG_066434 | NG_067680 | – | [55] |
| Lonicericola hyaloseptispora | KUMCC 18-0150 | MK098194 | MK098200 | MK098206 | MK098210 | [55] |
| Multilocularia bambusae | MFLUCC 11-0180 | NR_148099 | NG_059654 | NG_061229 | KU705656 | [56] |
| Multiseptospora thailandica | MFLUCC 11-0183 | KP744447 | KP744490 | KP753955 | KU705657 | [24] |
| Multiseptospora thailandica | MFLUCC 12-0006 | KU693448 | KU693441 | KU693445 | KU705660 | [24] |
| Multiseptospora thysanolaenae | MFLUCC 11-0202 | – | NG_059655 | NG_063600 | KU705658 | [56] |
| Neoaquastroma bauhiniae | MFLUCC 16-0398 | MH025952 | MH023319 | MH023315 | MH028247 | [57] |
| Neoaquastroma cylindricum | MFLUCC 19-0489 | MN473060 | MN473054 | MN473048 | MN481600 | [58] |
| Neoaquastroma guttulatum | MFLUCC 14-0917 | KX949739 | KX949740 | KX949741 | KX949742 | [59] |
| Neoaquastroma krabiense | MFLUCC 16-0419 | NR_165218 | NG_067815 | NG_067670 | MH028249 | [57] |
| Parabambusicola aquatica | MFLUCC 18-1140 | NR_171877 | NG_073791 | – | – | [16] |
| Parabambusicola bambusina | KH 139 | LC014579 | AB807537 | AB797247 | AB808512 | [21] |
| Parabambusicola bambusina | KT 2637 | LC014580 | AB807538 | AB797248 | AB808513 | [21] |
| Parabambusicola thysanolaenae | KUMCC 18-0147 | NR_164044 | NG_066435 | NG_067681 | MK098209 | [55] |
| Parabambusicola thysanolaenae | KUMCC 18-0148 | MK098193 | MK098198 | MK098202 | MK098211 | [55] |
| Paramonodictys dispersa | KUNCC 10788 | ON261165 | OQ146988 | OQ135189 | OQ943185 | This study |
| Paramonodictys dispersa | KUNCC 10782 | ON261159 | OQ146982 | OQ135187 | OQ943183 | This study |
| Paramonodictys dispersa | KUNCC 10783 | ON261160 | OQ146983 | OQ135188 | OQ943184 | This study |
| Paramonodictys hongheensis | KUMCC 21-0343 | OL436229 | OL436227 | OL436232 | OL505582 | [20] |
| Paramonodictys hongheensis | KUMCC 21-0346 | OL436235 | OL436224 | OL436225 | OL505583 | [20] |
| Paramonodictys solitarius | GZCC 20-0007 | MN901152 | MN897835 | MN901118 | MT023012 | [18] |
| Paramonodictys yunnanensis | KUMCC 21-0337 | OL436231 | OL436226 | OL436230 | OL505585 | [20] |
| Paramonodictys yunnanensis | KUMCC 21-0347 | OL436233 | OL436228 | OL436234 | OL505586 | [20] |
| Paratrimmatostroma kunmingensis | HKAS 102224A | MK098192 | MK098196 | MK098204 | MK098208 | [55] |
| Paratrimmatostroma kunmingensis | HKAS 102224B | MK098195 | MK098201 | MK098207 | – | [55] |
| Paradictyoarthrinium aquatica | MFLUCC 16-1116 | MG747496 | MG747495 | – | – | [26] |
| Paradictyoarthrinium diffractum | MFLUCC 13-0466 | KP744455 | KP744498 | KP753960 | – | [24] |
| Paradictyoarthrinium diffractum | MFLUCC 12-0557 | KP744454 | KP744497 | – | – | [24] |
| Paradictyoarthrinium hydei | MFLUCC 17-2512 | MG747498 | MG747497 | – | – | [26] |
| Paradictyoarthrinium hydei | KUNCC 10440 | OQ135178 | OQ146990 | OQ135190 | OQ943182 | This study |
| Paradictyoarthrinium hydei | KUNCC 10441 | OQ135179 | OQ146991 | OQ135191 | OQ943181 | This study |
| Paradictyoarthrinium tectonicola | MFLUCC 13-0465 | KP744456 | KP744500 | – | – | [24] |
| Phaeoseptum aquaticum | CBS 123113 | KY940803 | JN644072 | – | – | [33] |
| Phaeoseptum carolshearerianum | NFCCI 4221 | MK307810 | MK307813 | MK307816 | MK309874 | [34] |
| Phaeoseptum carolshearerianum | NFCCI 4384 | MK307812 | MK307815 | MK307818 | MK309876 | [34] |
| Phaeoseptum hydei | MFLUCC 17-0801 | MT240622 | MT240623 | MT240624 | MT241506 | [60] |
| Phaeoseptum mali | MFLUCC 17-2108 | MK659580 | MK625197 | – | MK647990 | [61] |
| Phaeoseptum mali | KUMCC 21-0335 | OL413027 | OL413028 | – | OL690512 | [61] |
| Phaeoseptum manglicola | NFCCI-4666 | MK307811 | MK307814 | MK307817 | MK309875 | [34] |
| Phaeoseptum terricola | MFLUCC 10-0102 | MH105778 | MH105779 | NG_065749 | MH105781 | [31] |
| Pleopunctum clematidis | MFLUCC 17-2091 | MT310618 | MT214573 | – | MT394632 | [35] |
| Pleopunctum ellipsoideum | MFLUCC 19-0390 | MK804512 | MK804517 | MK804514 | MK828510 | [36] |
| Pleopunctum ellipsoideum | KUNCC 10784 | ON261161 | OQ146984 | – | OQ943188 | This study |
| Pleopunctum megalosporum | KUNCC 10785 | ON261162 | OQ146985 | – | OQ943186 | This study |
| Pleopunctum megalosporum | KUNCC 10442 | OQ135180 | OQ146986 | – | OQ943187 | This study |
| Pleopunctum menglaense | KUMCC 21-0025 | ON009118 | ON009102 | ON009086 | – | [32] |
| Pleopunctum menglaense | KUMCC 21-0026 | ON009119 | ON009103 | ON009087 | – | [32] |
| Pleopunctum multicellularum | KUNCC 10789 | ON261166 | OQ146989 | – | OQ943190 | This study |
| Pleopunctum multicellularum | KUNCC 10781 | ON261158 | OQ146981 | – | OQ943189 | This study |
| Pleopunctum multicellularum | KUNCC 10778 | ON261155 | OQ146978 | – | – | This study |
| Pleopunctum pseudoellipsoideum | MFLUCC 19-0391 | MK804513 | MK804518 | – | MK828511 | [36] |
| Pleopunctum pseudoellipsoideum | KUNCC 10779 | ON261156 | OQ146979 | – | OQ943191 | This study |
| Pleopunctum pseudoellipsoideum | KUNCC 10786 | ON261163 | – | – | OQ943192 | This study |
| Pleopunctum rotundatum | KUNCC 10787 | ON261164 | OQ146987 | – | OQ943194 | This study |
| Pleopunctum rotundatum | KUNCC 10780 | ON261157 | OQ146980 | – | OQ943193 | This study |
| Pleopunctum thailandicum | MFLUCC 21-0039 | MZ198894 | MZ198896 | – | MZ172461 | [37] |
| Pseudomonodictys tectonae | MFLUCC 12-0552 | – | NG_059590 | NG_061213 | KT285571 | [62] |
| Thyridaria macrostomoides | GKM 1033 | – | GU385190 | – | GU327776 | [53] |
| Thyridaria macrostomoides | GKM 224N | – | GU385191 | – | GU327777 | [53] |
| Xenomassariosphaeria clematidis | MFLUCC 14-0923 | MT310616 | MT214571 | – | MT394630 | [35] |
| Xenomassariosphaeria rosae | MFLUCC 15-0179 | – | NG_059883 | MG829192 | – | [25] |
| Xenomassariosphaeria rosae | CBS 612.86 | MH862004 | MH873692 | EF165035 | [25] | |
The newly generated sequences are indicated in red and the ex-type strains are bold and “–” indicated unavailable sequences.
3. Results
3.1. Phylogenetic Analyses
The concatenated sequence datasets of LSU, TEF1, SSU and ITS comprised 71 strains and two outgroup taxa, Ceratostomella cuspidata (ICMP 17629) and C. pyrenaica (CBS 129343) [51]. The datasets contained 3625 characters including gaps after alignments (LSU: 1–850 bp, TEF1 = 851–1764 bp, SSU: 1765–2769 bp, ITS: 2770–3625 bp). The RAxML analysis of the combined datasets yielded the best scoring tree with a final ML optimization likelihood value of −24,434.495681. The aligned sequences matrix comprised 1588 distinct alignment patterns with 30.90% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.233967, C = 0.259535, G = 0.275768, T = 0.230731, with substitution rates AC = 1.078519, AG = 2.612360, AT = 1.312049, CG = 1.289214, CT = 6.139967, GT = 1.000000; gamma distribution shape parameter α = 0.0010000000. The tree topologies of combined sequence data obtained from ML and BI analyses were not significantly different Figure 1.
The phylogenetic analysis showed that our taxa are distributed in three families i.e., Parabambusicolaceae, Paradictyoarthriniaceae and Phaeoseptaceae into Pleosporales. Three isolates of Paramonodictys dispersa (KUNCC 10782, KUNCC 10783 and KUNCC 10788) formed a sister clade with isolates of Pa. yunnanensis (KUMCC 21-0337 and KUMCC 21-0347) and Pa. solitarius (MFLUCC 17-2353) with 95% ML/1.00 PP support. Two isolates of Paradictyoarthrinium hydei (KUNCC 10440 and KUNCC 10441) clustered with the ex-type strain of Pd. hydei MFLU 17-2512 (Paradictyoarthriniaceae) with 100% ML/1.00 PP bootstrap support. Ten isolates (KUNCC 10442, KUNCC 10778, KUNCC 10779, KUNCC 10780, KUNCC 10781, KUNCC 10784, KUNCC 10785, KUNCC 10786, KUNCC 10787 and KUNCC 10789) belong to Pleopunctum (Phaeoseptaceae) and were identified as two known species, i.e., Pl. ellipsoideum and Pl. pseudoellipsoideum. The three new species, i.e., Pl. megalosporum, Pl. multicellularum and Pl. rotundatum with high statistical support, are shown in Figure 1.
3.2. Taxonomy
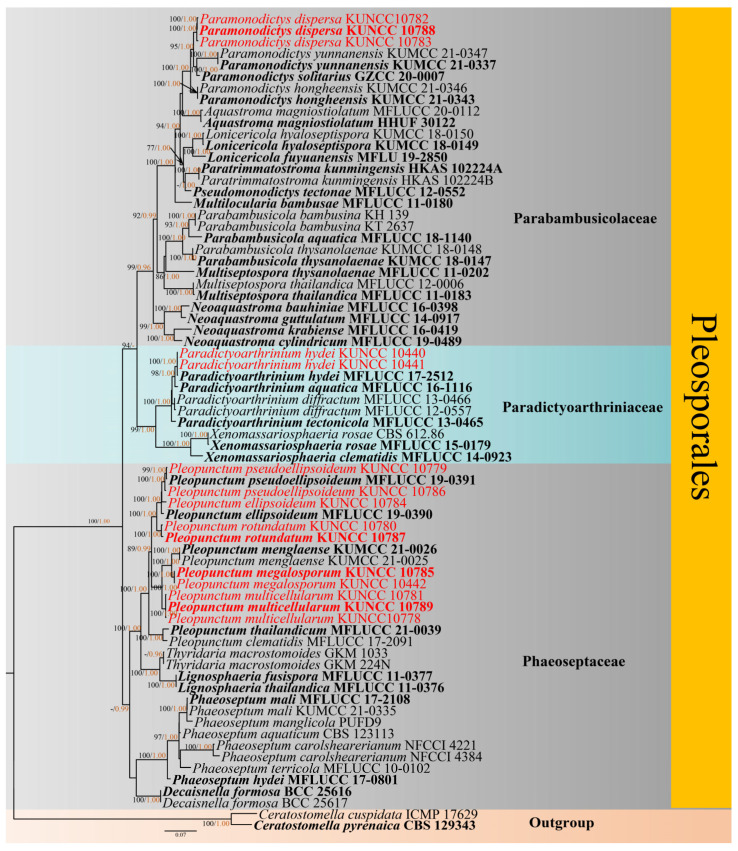
Paramonodictys dispersa R.J. Xu, Q. Zhao and Boonmee, sp. nov., Figure 2.
Figure 2.
Paramonodictys dispersa (HKAS 124267, holotype). (a) Scattered, punctiform colonies on natural substrates. (b) Conidia on substrate. (c–g) Conidia with or without multiple hyaline basal cells. (h) Germinated conidium. (i) Colonies on PDA. Scale bars: (b–h) = 20 μm.
MycoBank number: MB 847825; Facesoffungi number: FoF 14062
Etymology: Referring to the dispersed colonies.
Holotype: HKAS 124267.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on natural substrate superficial, sporodochial, dark brown to black, scattered, punctiform, glistening. Mycelium immersed in the substratum, composed of septate, branched, subhyaline to light brown hyphae. Conidiophores micronematous. Conidiogenous cells monoblastic, terminal, integrated, light brown. Conidia 52–61 × 35–43 μm ( = 57 × 38 μm, n = 30), acrogenous, solitary, muriform, irregular septation, subglobose to elliptical, thick-walled, dark brown, basal cells 4–10 × 4–12 μm ( = 6 × 7 μm, n = 20), 0–multiple, sometimes extension at the surrounding (Figure 2c), hyaline, elliptical to globose.
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from conidia around. Mycelia superficial, velvet, irregular circular, gray in the central cycle from above, dark brown in the central cycle from below.
Material examined: CHINA, Yunnan Province, Lushui City, Sanhe village (25°55′36″ N, 98°46′9″ E), on submerged wood in a freshwater stream, 30 April 2021, R.J Xu, WS-830 (HKAS 124267, holotype), ex-type living culture, (KUNCC 10788). Furthermore, ibid.; (25°55′36″ N, 98°46′9″ E), on submerged wood in a freshwater stream, 30 April 2021, R.J Xu, WS-803 (HKAS 124261), living culture, (KUNCC 10783). Furthermore, ibid.; (25°58′9″ N, 98°41′1″ E), on submerged wood in a freshwater stream, 29 April 2021, R.J. Xu, GLG-37 (HKAS 124270), living culture, (KUNCC 10782).
GenBank accession numbers: KUNCC 10788: ITS = ON261165, LSU = OQ146988, SSU = OQ135189, TEF1-α = OQ943185. KUNCC 10783: ITS = ON261160, LSU = OQ146983, SSU = OQ135188, TEF1-α = OQ943184. KUNCC 10782: ITS = ON261159, LSU = OQ146982, SSU = OQ135187, TEF1-α = OQ943183.
Notes: Morphologically, the scattered, punctiform colonies and muriform, globose or subglobose conidia are the typical characteristics in Paramonodictys. Simultaneously, Pa. dispersa is highly similar to Pa. hongheensis (HKAS 122190) [20]. However, Pa. dispersa is different from Pa. hongheensis by larger conidia (52–61 × 35–43 μm vs. 19–26 × 19–22 µm) [20]. Moreover, Pa. dispersa has 0–multiple basal cell extension at the surrounding, while Pa. hongheensis has multicellular with regular or more often irregular septation. Phylogenetically, Pa. dispersa formed a distinct lineage and was sister to Pa. solitarius and Pa. yunnanensis with good bootstrap support (95% ML/1.00 PP, Figure 1). However, P. solitarius has a globose or subglobose, olivaceous conidia, while Pa. yunnanensis has a brownish to reddish-brown, cylindrical conidiogenous cell. Therefore, we dealt with Pa. dispersa as a new species based on morphological and phylogenetic analyses.
Paradictyoarthrinium hydei N.G. Liu and J.K. Liu, Phytotaxa 338: 290 (2018), Figure 3.
Figure 3.
Paradictyoarthrinium hydei (HKAS 124272). (a) Colonies on natural substrates. (b–d) Conidiophores and conidia (e) Germinated conidia. (f) Colonies on PDA. Scale bars: (b–d) = 20 μm, e = 10 μm.
Index Fungorum number: IF554082; Facesoffungi number: FoF 03933
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate superficial, black, gregarious, powdery. Mycelium is mostly immersed, composed of pale brown to dark brown, septate, branched hyphae. Conidiophores 2.5–6 μm wide, macronematous, mononematous, short, green, straight to slightly curved, branched, septate, unevenly cylindrical, thick-walled. Conidiogenous cells monoblastic, integrated, terminal, determinate, dark green. Conidia 14–23 × 10–19 μm ( = 17 × 15 μm, n = 25), solitary or catenate, muriform, deeply constricted at the septa, subglobose to ellipsoidal to irregular, green to dark brown in the maturity periods, verrucose.
Culture characters: Conidium germinated on PDA within 24 h. Germ tubes are produced from around. Mycelia circular, gray and dense, with the entire edge. brown to dark brown, black on the reverse.
Material examined: CHINA, Yunnan Province, Lushui City, Gaoligong Mountains, (25°55′36″ N, 98°46′9″ E), on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, XS-31 (HKAS 124272); living culture, (KUNCC 10441). Furthermore, ibid.; (25°55′36″ N, 98°46′9″ E), on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, XS-09 (HKAS 124271); living culture, (KUNCC 10440).
GenBank accession numbers: KUNCC 10441: ITS = OQ135179, LSU = OQ146991, SSU = OQ135191, TEF1-α = OQ943181. KUNCC 10440: ITS = OQ135178, LSU = OQ146990, SSU = OQ135190, TEF1-α = OQ943182.
Notes: Paradictyoarthrinium hydei was introduced by Liu et al. [26] from decaying wood in Chiang Mai Province, Thailand. Later Hyde et al. [18] reported Pd. hydei on Quercus variabilis from Yunnan Province, China. Morphological and phylogenetic analyses showed that Pd. hydei (KUNCC 10441) and Pd. hydei (KUNCC 10440) clustered with ex-type strain of Pd. hydei (MFLUCC 17-2512) with 100% ML/1.00 PP bootstrap support (Figure 1). Therefore, the isolates were identified as Pd. hydei and as a new freshwater habitat record to the Tibetan Plateau, China.
Pleopunctum ellipsoideum N.G. Liu, K.D. Hyde and J.K. Liu, Mycosphere 10: 767 (2019), Figure 4.
Figure 4.
Pleopunctum ellipsoideum (HKAS 124262). (a,b) Colonies on natural substrates. (c–g) Conidia with or without basal hyaline cells. (h,i) Colonies on PDA. Scale bars: (c) = 50 μm, (d–g) = 20 μm.
Index Fungorum number: IF 556523; Facesoffungi number: FoF 06114
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on wood substrate superficial, sporodochial, brown, scattered, gregarious, punctiform. Mycelium immersed in the substratum, composed of septate, branched, subhyaline to grayish brown hyphae. Conidiophores macronematous, mononematous, cylindrical, branched, septate, medium brown, smooth and thick-walled. Conidiogenous cells monoblastic, terminal, integrated, medium brown. Conidia 32–40 × 17–23 μm ( = 36 × 19 μm, n = 40), acrogenous, solitary, muriform, constricted at the septa, oval to ellipsoidal, smooth-walled, pale brown when immature, broadly obtuse and dark brown at apex, truncate and paler brown at the base when mature, often with a hyaline, elliptical to globose basal cell, 4–12 × 8–13 μm ( = 8 × 9 μm, n = 35).
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from basal cells. Mycelia superficial, velvet, pale in the central cycle and pale gray or white in the outer circle from above. Dark brown to black in the central cycle and dark brown in the outer circle from below.
Material examined: CHINA, Yunnan Province, Lushui City, Dishui River (25°56′3″ N, 98°45′40″ E), on submerged decaying wood in a freshwater river, 28 April 2021, R.J Xu, WS-820 (HKAS 124262), living culture, (KUNCC 10784).
GenBank accession numbers: KUNCC 10784: ITS = ON261161, LSU = OQ146984, TEF1-α = OQ943188.
Notes: Pleopunctum ellipsoideum was introduced by Liu et al. [36], which is isolated from decaying woods in Guizhou Province, China. Based on phylogenetic analyses, our new isolate KUNCC 21-10784 was placed among two species, i.e., Pl. ellipsoideum and Pl. pseudoellipsoideum [36] with 100% ML/1.00 PP support (Figure 1). However, our new isolate shares identical morphological characters to the holotype of Pl. ellipsoideum. Thus, we identified the two strains as Pl. ellipsoideum, and as a new record to the Tibetan Plateau, China.
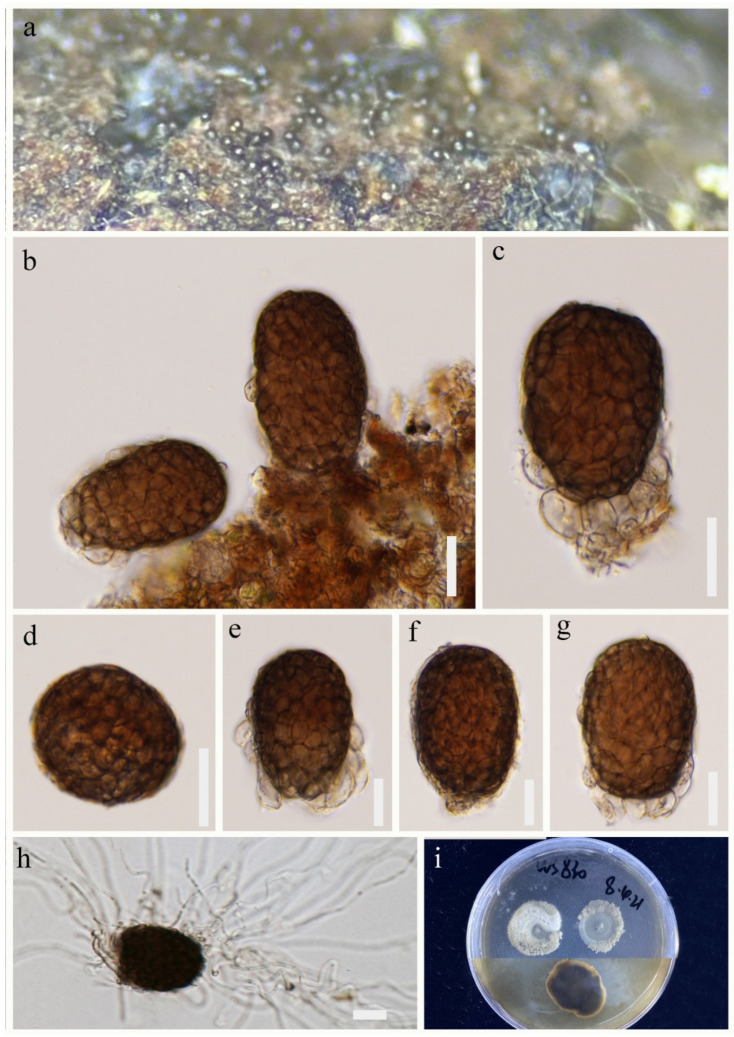
Pleopunctum megalosporum R.J. Xu, Q. Zhao and Boonmee, sp. nov., Figure 5.
Figure 5.
Pleopunctum megalosporum (HKAS 124263, holotype). (a,b) Colonies on natural substrates. (c) Conidia on substrate. (d–g) Conidiophores and conidia. (h) Basal hyaline cells. (i,j) Colonies on PDA. Scale bars: (c–g) = 20 μm, (h) = 10 μm.
MycoBank number: MB 847826; Facesoffungi number: FoF 14063
Etymology: Referring to the macro-conidia.
Holotype: HKAS 124263.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on natural substrate sporodochial, superficial, light brown, scattered, gregarious, punctiform, glistening. Mycelium immersed in the substratum, composed of septate, branched, subhyaline to light brown hyphae. Conidiophores macronematous, mononematous, cylindrical, unbranched, septate, short, light brown, smooth-walled. Conidiogenous cells monoblastic, terminal, integrated, light brown. Conidia 51–72 × 28–36 μm ( = 63 × 33 μm, n = 40), acrogenous, solitary, muriform, constricted at the septa, oval to long elliptical, smooth-walled, subhyaline to light brown when immature, dark brown when mature, basal cell 10–18 × 7–19 μm ( = 14 × 12 μm, n = 20), 0–multiple-basal cells, hyaline, elliptical to globose.
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from basal cell. Mycelia is superficial, velvet, irregular circular, grey in the central cycle from above. Dark brown in the central cycle and yellowish brown in the outer circle from below.
Material examined: CHINA, Yunnan Province, Lushui City, Sanhe village, (25°55′36″ N, 98°46′9″ E), on submerged decaying wood in a freshwater stream, 30 April 2021, R.J Xu, WS-823, (HKAS 124263, holotype), ex-type living culture, (KUNCC 10785). Furthermore, ibid.; (25°30′28″ N, 97°55′3″ E) on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, WS-823-1, (HKAS 124264), living culture, (KUNCC 10442).
GenBank accession numbers: KUNCC 10785: ITS = ON261162, LSU = OQ146985, TEF1-α = OQ943186. KUNCC 10442: ITS = OQ135180, LSU = OQ146986, TEF1-α = OQ943187.
Notes: Phylogenetic analysis shows that Pleopunctum megalosporum clustered into a distinctly separated clade and was sister to Pl. menglaense with good bootstrap support (100% ML/1.00 PP, Figure 1). However, Pl. megalosporum differs from Pl. menglaense in having larger conidia (51–72 × 28–36 μm vs. 18–25 × 10–14 µm or 38–55 × 20–26 µm) [32]. Therefore, Pl. megalosporum is identified as a new species based on the morphological and phylogenetic analyses.
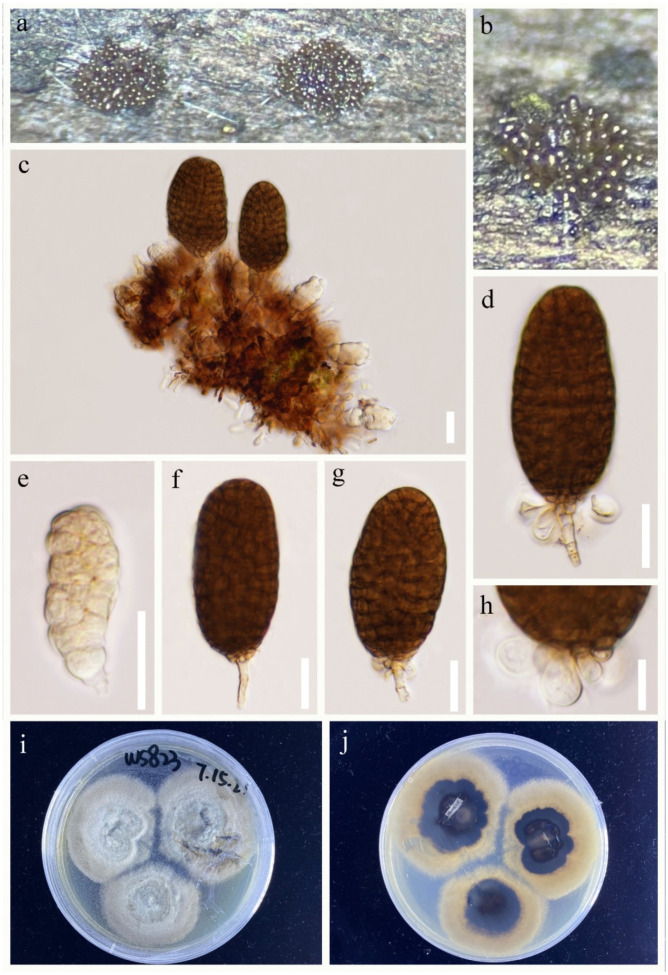
Pleopunctum multicellularum R.J. Xu, Q. Zhao and Boonmee, sp. nov., Figure 6.
Figure 6.
Pleopunctum multicellularum (HKAS 124268, holotype). (a,b) Colonies on natural substrates. (c,d) Conidia mass. (e,f) Conidia with hyaline basal cells. (g) Germinated conidium. (h) Colonies on PDA. Scale bars: (c–g) = 20 μm.
MycoBank number: MB 847827; Facesoffungi number: FoF 14064
Etymology: “multicellularum” meaning pluricellular; referring to multiple basal cells.
Holotype: HKAS 124268.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on natural substrate, superficial, dark brown to black, scattered, gregarious, punctiform, glistening. Mycelium immersed in the substratum, composed of septate, branched, subhyaline to light brown hyphae. Conidiophores 3–5 μm ( = 4 μm, n = 20), macronematous, mononematous, cylindrical, unbranched, septate, light brown, smooth-walled. Conidiogenous cells monoblastic, terminal, integrated, light brown. Conidia 55–71 × 27–39 μm ( = 62 × 32 μm, n = 25), acrogenous, solitary, muriform, constricted at the septa, oval to ellipsoidal, smooth-walled, dark brown when mature, broadly obtuse at apex, basal cell 14–20 × 10–17 μm ( = 16 × 13 μm, n = 25), 0–3-basal cells, hyaline, elliptical to globose.
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from basal cells. Mycelia superficial, circular, gray from above and pale brown from below.
Material examined: CHINA, Yunnan Province, Lushui City, Gaoligong Mountains, (25°59′4″ N, 98°48′50″ E), on submerged decaying wood in a freshwater stream, 30 April 2021, R.J Xu, WS-831, (HKAS 124268, holotype), ex-type living culture, (KUNCC 10789). Furthermore, ibid.; (24°16′8″ N, 101°15′38″ E), on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, ALS-04, (HKAS 124273), living culture, (KUNCC 10778). Furthermore, ibid.; (25°58′9″ N, 98°41′1″ E), on submerged decaying wood in a freshwater stream, 30 April 2021, R.J Xu, GLG-29, (HKAS 124269), living culture, (KUNCC 10781).
GenBank accession numbers: KUNCC 10789: ITS = ON261166, LSU = OQ146989, TEF1-α = OQ943190. KUNCC 10778: ITS = ON261155, LSU = OQ146978. KUNCC 10781: ITS = ON261158, LSU = OQ146981, TEF1-α = OQ943189.
Notes: Morphologically, Pleopunctum multicellularum fits well with the generic concept of Pleopunctum. However, Pl. multicellularum is distinctly different from other species of Pleopunctum in having hyaline, 0–3-basal cells and larger conidia. Additionally, phylogenetic analyses show that three Pl. multicellularum strains (KUNCC 10781, KUNCC 10789 and KUNCC 10778) clustered into a distinctly separated clade and sharing a sister relationship to Pl. menglaense (KUMCC 210026 and KUMCC 210025) and Pl. megalosporum (KUNCC 10785 and KUNCC 10442) with good bootstrap support (100% ML/1.00 PP, Figure 1). Therefore, Pl. multicellularum is introduced as a new species.
Pleopunctum pseudoellipsoideum N.G. Liu, K.D. Hyde and J.K. Liu, Mycosphere 10: 768 (2019), Figure 7.
Figure 7.
Pleopunctum pseudoellipsoideum (HKAS 124274). (a) Colonies on natural substrates. (b,c,e) Conidia. (d,f) Conidiophores and conidia with basal hyaline cells. (g) Germinated conidium. (h) Colonies on PDA. Scale bars: (b) = 50 μm, (c–g) = 20 μm.
Index Fungorum number: IF 556524, Facesoffungi number: FoF 06115
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on wood substrate superficial, sporodochial, black, gregarious, punctiform. Mycelium immersed in the substratum, composed of septate, branched hyphae. Conidiophores macronematous, mononematous, cylindrical, septate, medium brown, smooth and thick-walled. Conidiogenous cells monoblastic, terminal, integrated, cylindrical, light brown. Conidia 36–51 × 22–34 μm ( = 44 × 26 μm, n = 25), acrogenous, solitary, muriform, constricted at the septa, oval to ellipsoidal, smooth-walled, broadly obtuse at apex, dark brown, truncate at the base and paler brown, often with hyaline, elliptical to subglobose basal cell, 12–21 × 13–21 μm ( = 16 × 16 μm, n = 30).
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from basal cells. Mycelia superficial, irregular circular, flat, entire, dark brown in the center and grayish white near the edge from above. Dark brown in the center with paler towards the edge.
Material examined: CHINA, Yunnan Province, Lushui City, Gaoligong Mountains, (24°16′8″ N, 101°15′38″ E), on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, ALS-14, (HKAS 124274), living cultures, (KUNCC 10779). Furthermore, ibid.; (25°56′3″ N, 98°45′40″ E), on submerged decaying wood in a freshwater stream, 5 May 2021, R.J Xu, WS-824, (HKAS 124265), living culture, KUNCC 10786.
GenBank accession numbers: KUNCC 10779: ITS = ON261156, LSU = OQ146979, TEF1-α = OQ943191. KUNCC 10786: ITS = ON261163, TEF1-α = OQ943192.
Notes: Pleopunctum pseudoellipsoideum was isolated from decaying woods in Guizhou Province, China [36]. In a recent study, Wanasinghe et al. [32] described Pl. pseudoellipsoideum on decaying wood from Yunnan Province, China. Morphologically, our collections have largely overlapped conidial size with the holotype (36–51 × 22–34 μm vs. 39–59 × 19–28 μm), except for slightly larger basal cell (12–21 × 13–21 μm vs. 6.5–13.5 × 11–15.5 μm) [36]. Furthermore, Phylogenetic analyses provide strong support for this classification, placing the two new strains and the ex-type strain MFLUCC 19-0391 of Pl. pseudoellipsoideum in a single clade with 100% ML/1.00 PP support (Figure 1). Therefore, the isolates are identified as Pl. pseudoellipsoideum, and they are new record of Pl. pseudoellipsoideum in Tibetan Plateau, China.
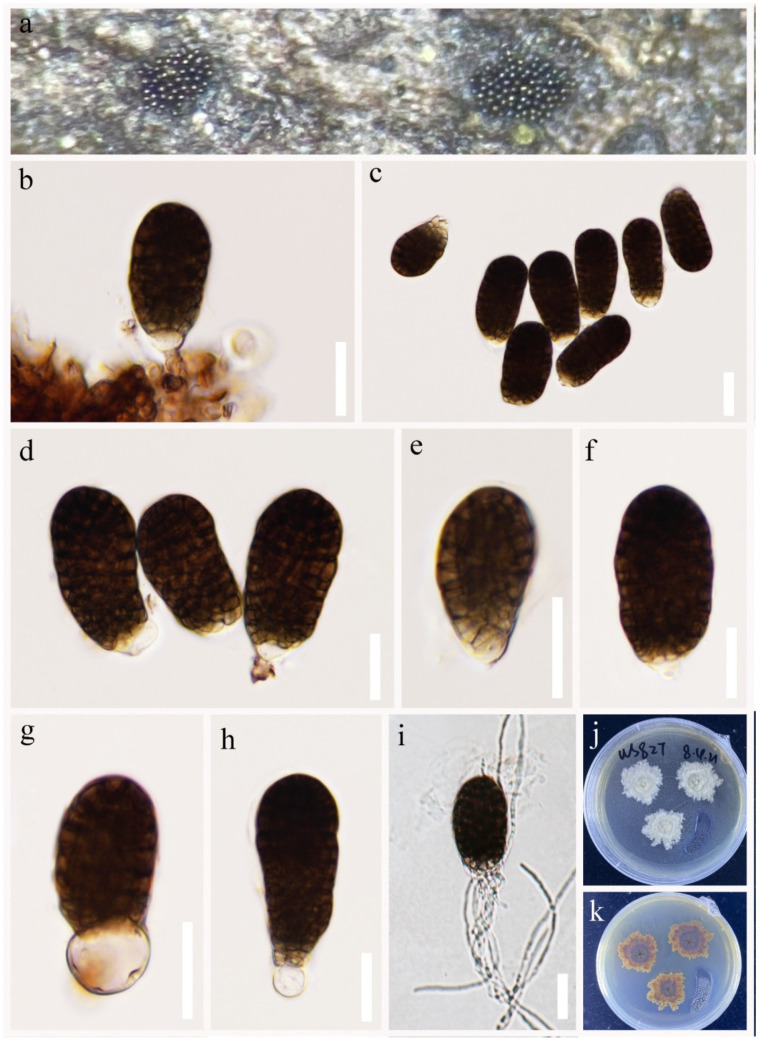
Pleopunctum rotundatum R.J. Xu, Q. Zhao and Boonmee, sp. nov., Figure 8.
Figure 8.
Pleopunctum rotundatum (HKAS 124266, holotype). (a) Colonies on natural substrates. (b) Conidiophores. (c–f) Conidiogenous cells and conidia. (g,h) Conidia with hyaline basal cells. (i) Germinated conidium. (j,k) Colonies on PDA. Scale bars: (b–i) = 20 μm.
MycoBank number: MB 847828; Facesoffungi number: FoF 14065
Etymology: in reference to the rounded base cell.
Holotype: HKAS 124266.
Saprobic on decaying wood submerged in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on natural substrate sporodochial, superficial, brown to black, scattered and gregarious, punctiform, glistening. Mycelium immersed in the substratum, composed of septate, branched, subhyaline to light brown hyphae. Conidiophores are often reduced to conidiogenous cells, short, simple, cylindrical, septate, light brown, smooth-walled. Conidiogenous cells monoblastic, terminal, integrated, light brown. Conidia 37–52 × 19–27 μm ( = 49 × 23 μm, n = 30), acrogenous, solitary, muriform, constricted at septa, oval to ellipsoidal, smooth-walled, broadly obtuse and dark brown at apex, truncate and pale brown at base, basal cell 5–10 × 8–13 μm ( = 8 × 11 μm, n = 20), hyaline or light brown, elliptical to globose.
Culture characteristics: Conidium germinated on PDA within 48 h. Germ tubes are produced from basal cells. Mycelia superficial, irregular circular, gray to pale brown in the central cycle from above and pale brown to yellowish to brown from below.
Material examined: CHINA, Yunnan Province, Lushui City, Gaoligong Mountains (25°59′4″ N, 98°48′50″ E), on submerged decaying wood in a freshwater stream, 20 April 2021, R.J Xu, WS-827, (HKAS 124266, holotype), ex-type living culture, (KUNCC 10787). Furthermore, ibid.; (25°66′3″ N, 98°52′4″ E), on submerged decaying wood in a freshwater stream, 6 May 2021, R.J Xu, ALS-30, (HKAS 124275), living culture, (KUNCC 10780).
GenBank accession numbers: KUNCC 10787: ITS = ON261164, LSU = OQ146987, TEF1-α = OQ943194. KUNCC 10780: ITS = ON261157, LSU = OQ146980, TEF1-α = OQ943193.
Notes: Pleopunctum rotundatum shares common features in Pleopunctum and is similar to Pl. ellipsoideum and Pl. pseudoellipsoideum in having scattered, gregarious, punctiform, colonies, monoblastic, terminal, integrated conidiogenous cells and acrogenous, solitary, muriform, oval to ellipsoidal conidia. However, Pl. rotundatum differs from Pl. ellipsoideum in having smaller basal cells (5–10 × 8–13 μm vs. 8–20 × 8.5–18.5 μm), and differs from Pl. pseudoellipsoideum in Pl. pseudoellipsoideum has a short, septate conidiophores [36]. Furthermore, multi-gene phylogenetic analyses have shown that Pl. rotundatum can be classified into a distinct clade, which is the sister group of Pl. ellipsoideum and Pl. pseudoellipsoideum with high bootstrap support (100% ML/1.00 PP, Figure 1).
4. Discussion
The Tibetan Plateau is a region known for its unique biological diversity. Our investigation focuses on the freshwater fungal diversity in the Hengduan Mountains Range, because it is an almost virgin field for the discovery of freshwater fungal diversity. We found seven species from the collections from Hengduan Mountains into Pleosporales based on morphological and multi-gene phylogenetic analyses. The colonies of Pleopunctum are gregarious and widely distributed on the substrate [32,35,36,37], while Paramonodictys are dispersed and scattered [20,23]. In Paradictyoarthrinium, colonies are generally a powdery mass [26]. Thus far, only asexual morphs have been documented in these three genera. Therefore, to gain a more comprehensive understanding of their systematic relationships, it is imperative to obtain further collections and conduct research on their sexual morphs.
The Tibetan Plateau harbors a wide range of aquatic habitats, including lakes, rivers, and wetlands, which support different fungal communities [7]. Although freshwater fungi play an essential role in the ecosystem, they have been understudied in this region due to few researchers have studied freshwater fungi in this region. It is necessary to continue more studies on their diversity, distribution and adaptation to the harsh environmental conditions of the Tibetan Plateau, which has significant implications for our understanding of the functioning in freshwater ecosystems and for the conservation and sustainable use of freshwater resources in this critical region.
Acknowledgments
We would like to thank Shaun Pennycook for checking the nomenclature.
Author Contributions
Conceptualization, Q.Z. and S.B.; methodology, R.-J.X.; formal analysis, R.-J.X.; investigation, R.-J.X. and Y.-A.Z.; resources, Q.Z.; data curation, R.-J.X.; writing—original draft preparation, R.-J.X.; writing—review and editing, Y.-A.Z., N.-G.L., S.B. and D.-Q.Z.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study is supported by the Second Tibetan Plateau Scientific Expedition and Research (STEP) Program (Grant No. 2019QZKK0503), Major science and technology projects and key R&D plans/programs, Yunnan Province (202202AE090001); Natural Science Foundation of Guizhou Province (Grant No. Qian Ke Zhong Yin Di [2021]4031, Qian Ke He Zhi Cheng [2021] Generally 200), the open research project of “Cross-Cooperative Team” of the Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences (Grant No. 292019312511043), Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-STS-QYZD-171), the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, PR China (2019HJ2096001006).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Liu X., Chen B. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. J. R. Meteorol. Soc. 2000;20:1729–1742. doi: 10.1002/1097-0088(20001130)20:14<1729::AID-JOC556>3.0.CO;2-Y. [DOI] [Google Scholar]
- 2.Guo B., Han B., Yang F., Chen S., Liu Y., Yang W. Determining the Contributions of Climate Change and Human Activities to the Vegetation NPP Dynamics in the Qinghai-Tibet Plateau, China, from 2000 to 2015. Environ. Monit. Assess. 2020;192:1–18. doi: 10.1007/s10661-020-08606-6. [DOI] [PubMed] [Google Scholar]
- 3.Manish K., Pandit M.K. Geophysical Upheavals and Evolutionary Diversification of Plant Species in the Himalaya. PeerJ. 2018;6:e5919. doi: 10.7717/peerj.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z., Zhang Y., Yang Y., Zhou W., Gang C., Zhang Y., Li J., An R., Wang K., Odeh I., et al. Quantitative Assess the Driving Forces on the Grassland Degradation in the Qinghai-Tibet Plateau, in China. Ecol. Inf. 2016;33:32–44. doi: 10.1016/j.ecoinf.2016.03.006. [DOI] [Google Scholar]
- 5.Xu W., Dong W.J., Fu T.T., Gao W., Lu C.Q., Yan F., Wu Y.H., Jiang K., Jin J.Q., Chen H.M., et al. Herpetological Phylogeographic Analyses Support a Miocene Focal Point of Himalayan Uplift and Biological Diversification. Natl. Sci. Rev. 2021;8:nwaa263. doi: 10.1093/nsr/nwaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibi S., Wang L., Li X., Zhou J., Chen D., Yao T. Climatic and Associated Cryospheric, Biospheric, and Hydrological Changes on the Tibetan Plateau: A Review. Int. J. Climatol. 2018;38:e1–e17. doi: 10.1002/joc.5411. [DOI] [Google Scholar]
- 7.Yao T.D., Wu G.J., Xu B.Q., Wang W.C., Gao J., An B.S. Asian Water Tower Change and Its Impacts. Bull. Chin. Acad. Sci. 2019;34:1203–1209. doi: 10.16418/j.issn.1000-3045.2019.11.003. [DOI] [Google Scholar]
- 8.Sun H.L., Zheng D., Yao T.D., Zhang Y. Protection and construction of the national ecological security shelter zone on Tibetan Plateau. Acta Geogr. Sin. 2012;67:3–12. [Google Scholar]
- 9.Qiu J. China: The Third Pole. Nature. 2008;454:393–396. doi: 10.1038/454393a. [DOI] [PubMed] [Google Scholar]
- 10.Krauss G.-J., Solé M., Krauss G., Schlosser D., Wesenberg D., Bärlocher F. Fungi in Freshwaters: Ecology, Physiology and Biochemical Potential. FEMS Microbiol. Rev. 2011;35:620–651. doi: 10.1111/j.1574-6976.2011.00266.x. [DOI] [PubMed] [Google Scholar]
- 11.Sridhar K.R., Karamchand K.S., Seena S. Fungal Assemblage and Leaf Litter Decomposition in Riparian Tree Holes and in a Coastal Stream of the South-West India. Mycol. Int. J. Fungal Biol. 2013;4:118–124. doi: 10.1080/21501203.2013.825657. [DOI] [Google Scholar]
- 12.Tsui C.K.M., Baschien C., Goh T.-K. Biology of Microfungi. Fungal Biology. Springer; Cham, Switzerland: 2016. Biology and Ecology of Freshwater Fungi; pp. 285–313. [DOI] [Google Scholar]
- 13.Wurzbacher C., Rösel S., Rychła A., Grossart H.-P. Importance of Saprotrophic Freshwater Fungi for Pollen Degradation. PLoS ONE. 2014;9:e94643. doi: 10.1371/journal.pone.0094643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Souza L.M.D., Ogaki M.B., Câmara P.E.A.S., Pinto O.H.B., Convey P., Carvalho-Silva M., Rosa C.A., Rosa L.H. Assessment of Fungal Diversity Present in Lakes of Maritime Antarctica Using DNA Metabarcoding: A Temporal Microcosm Experiment. Extremophiles. 2021;25:77–84. doi: 10.1007/s00792-020-01212-x. [DOI] [PubMed] [Google Scholar]
- 15.Calabon M.S., Hyde K.D., Jones E.B.G., Luo Z.L., Dong W., Hurdeal V.G., Gentekaki E., Rossi W., Leonardi M., Thiyagaraja V., et al. Freshwater Fungal Numbers. Fungal Divers. 2022;114:3–235. doi: 10.1007/s13225-022-00503-2. [DOI] [Google Scholar]
- 16.Dong W., Wang B., Hyde K.D., McKenzie E.H.C., Raja H.A., Tanaka K., Abdel-Wahab M.A., Abdel-Aziz F.A., Doilom M., Phookamsak R., et al. Freshwater Dothideomycetes. Fungal Divers. 2020;105:319–575. doi: 10.1007/s13225-020-00463-5. [DOI] [Google Scholar]
- 17.Hongsanan S., Hyde K.D., Phookamsak R., Wanasinghe D.N., McKenzie E.H.C., Sarma V.V., Boonmee S., Lücking R., Bhat D.J., Liu N.G., et al. Refined Families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere. 2020;11:1553–2107. doi: 10.5943/mycosphere/11/1/13. [DOI] [Google Scholar]
- 18.Hyde K.D., Norphanphoun C., Maharachchikumbura S.S.N., Bhat D.J., Jones E.B.G., Bundhun D., Chen Y.J., Bao D.F., Boonmee S., Calabon M.S., et al. Refined Families of Sordariomycetes. Mycosphere. 2020;11:305–1059. doi: 10.5943/mycosphere/11/1/7. [DOI] [Google Scholar]
- 19.Wijayawardene N., Hyde K., Dai D., Sánchez-García M., Goto B., Saxena R., Erdoğdu M., Selçuk F., Rajeshkumar K., Aptroot A., et al. Outline of Fungi and Fungus-like Taxa—2021. Mycosphere. 2022;13:53–453. doi: 10.5943/mycosphere/13/1/2. [DOI] [Google Scholar]
- 20.Yang E.F., Tibpromma S., Karunarathna S.C., Phookamsak R., Xu J.C., Zhao Z.X., Karunanayake C., Promputtha I. Taxonomy and Phylogeny of Novel and Extant Taxa in Pleosporales Associated with Mangifera indica from Yunnan, China (Series I) J. Fungi. 2022;8:152. doi: 10.3390/jof8020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K., Hirayama K., Yonezawa H., Sato G., Toriyabe A., Kudo H., Hashimoto A., Matsumura M., Harada Y., Kurihara Y., et al. Revision of the Massarineae (Pleosporales, Dothideomycetes) Stud. Mycol. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie N., Phookamsak R., Jiang H., Zeng Y.-J., Zhang H., Xu F., Lumyong S., Xu J., Hongsanan S. Morpho-Molecular Characterization of Five Novel Taxa in Parabambusicolaceae (Massarineae, Pleosporales) from Yunnan, China. J. Fungi. 2022;8:108. doi: 10.3390/jof8020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde K.D., Dong Y., Phookamsak R., Jeewon R., Bhat D.J., Jones E.B.G., Liu N.-G., Abeywickrama P.D., Mapook A., Wei D., et al. Fungal Diversity Notes 1151–1276: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungal Taxa. Fungal Divers. 2020;100:5–277. doi: 10.1007/s13225-020-00439-5. [DOI] [Google Scholar]
- 24.Liu J.K., Hyde K.D., Jones E.B.G., Ariyawansa H.A., Bhat D.J., Boonmee S., Maharachchikumbura S.S.N., McKenzie E.H.C., Phookamsak R., Phukhamsakda C., et al. Fungal Diversity Notes 1–110: Taxonomic and Phylogenetic Contributions to Fungal Species. Fungal Divers. 2015;72:1–197. doi: 10.1007/s13225-015-0324-y. [DOI] [Google Scholar]
- 25.Wanasinghe D.N., Phukhamsakda C., Hyde K.D., Jeewon R., Lee H.B., Gareth Jones E.B., Tibpromma S., Tennakoon D.S., Dissanayake A.J., Jayasiri S.C., et al. Fungal Diversity Notes 709–839: Taxonomic and Phylogenetic Contributions to Fungal Taxa with an Emphasis on Fungi on Rosaceae. Fungal Divers. 2018;89:1–236. doi: 10.1007/s13225-018-0395-7. [DOI] [Google Scholar]
- 26.Liu J.K., Luo Z.L., Liu N.G., Cheewangkoon R., To-Anun C. Two Novel Species of Paradictyoarthrinium from Decaying Wood. Phytotaxa. 2018;338:285–293. doi: 10.11646/phytotaxa.338.3.6. [DOI] [Google Scholar]
- 27.Matsushima T. Matsushima Mycological Memoirs. Volume 9. Matsushima Fungus Collection; Kobe, Japan: 1996. pp. 1316–1321. [Google Scholar]
- 28.Doilom M., Dissanayake A.J., Wanasinghe D.N., Boonmee S., Liu J.-K., Bhat D.J., Taylor J.E., Bahkali A., McKenzie E.H.C., Hyde K.D. Microfungi on Tectona Grandis (Teak) in Northern Thailand. Fungal Divers. 2017;82:107–182. doi: 10.1007/s13225-016-0368-7. [DOI] [Google Scholar]
- 29.Prabhugaonkar A., Bhat D.J. New record of Megacapitula villosa and Paradictyoarthrinium diffractum from India. Mycosphere. 2011;2:463–467. [Google Scholar]
- 30.Wijayawardene N.N., Hyde K.D., Lumbsch H.T., Liu J.K., Maharachchikumbura S.S.N., Ekanayaka A.H., Tian Q., Phookamsak R. Outline of Ascomycota: 2017. Fungal Divers. 2018;88:167–263. doi: 10.1007/s13225-018-0394-8. [DOI] [Google Scholar]
- 31.Hyde K.D., Chaiwan N., Norphanphoun C., Boonmee S., Camporesi E., Chethana K.W.T., Dayarathne M.C., de Silva N.I., Dissanayake A.J., Ekanayaka A.H., et al. Mycosphere Notes 169-224. Mycosphere. 2018;9:271–430. doi: 10.5943/mycosphere/9/2/8. [DOI] [Google Scholar]
- 32.Wanasinghe D.N., Ren G.-C., Xu J.-C., Cheewangkoon R., Mortimer P.E. Insight into the Taxonomic Resolution of the Pleosporalean Species Associated with Dead Woody Litter in Natural Forests from Yunnan, China. J. Fungi. 2022;8:375. doi: 10.3390/jof8040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Fournier J., Phookamsak R., Bahkali A.H., Hyde K.D. Halotthiaceae Fam. Nov. (Pleosporales) Accommodates the New Genus Phaeoseptum and Several Other Aquatic Genera. Mycologia. 2013;105:603–609. doi: 10.3852/11-286. [DOI] [PubMed] [Google Scholar]
- 34.Dayarathne M.C., Jones E.B.G., Maharachchikumbura S.S.N., Devadatha B., Sarma V.V., Khongphinitbunjong K., Chomnunti P., Hyde K.D. Morpho-Molecular Characterization of Microfungi Associated with Marine Based Habitats. Mycosphere. 2020;11:1–188. doi: 10.5943/mycosphere/11/1/1. [DOI] [Google Scholar]
- 35.Phukhamsakda C., McKenzie E.H.C., Phillips A.J.L., Gareth Jones E.B., Jayarama Bhat D., Stadler M., Bhunjun C.S., Wanasinghe D.N., Thongbai B., Camporesi E., et al. Microfungi Associated with Clematis (Ranunculaceae) with an Integrated Approach to Delimiting Species Boundaries. Fungal Divers. 2020;102:1–203. doi: 10.1007/s13225-020-00448-4. [DOI] [Google Scholar]
- 36.Liu N.G., Hyde K.D., Bhat D.J., Jumpathong J., Liu J.K. Morphological and Phylogenetic Studies of Pleopunctum gen. Nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere. 2019;10:757–775. doi: 10.5943/mycosphere/10/1/17. [DOI] [Google Scholar]
- 37.Boonmee S., Wanasinghe D.N., Calabon M.S., Huanraluek N., Chandrasiri S.K.U., Jones G.E.B., Rossi W., Leonardi M., Singh S.K., Rana S., et al. Fungal Diversity Notes 1387–1511: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungal Taxa. Fungal Divers. 2021;111:1–335. doi: 10.1007/s13225-021-00489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senanayake I., Rathnayaka A., Marasinghe D., Calabon M., Gentekaki E., Lee H., Hurdeal V., Pem D., Dissanayake L., Wijesinghe S., et al. Morphological Approaches in Studying Fungi: Collection, Examination, Isolation, Sporulation and Preservation. Mycosphere. 2020;11:2678–2754. doi: 10.5943/mycosphere/11/1/20. [DOI] [Google Scholar]
- 39.Jayasiri S.C., Hyde K.D., Ariyawansa H.A., Bhat J., Buyck B., Cai L., Dai Y.-C., Abd-Elsalam K.A., Ertz D., Hidayat I., et al. The Faces of Fungi Database: Fungal Names Linked with Morphology, Phylogeny and Human Impacts. Fungal Divers. 2015;74:3–18. doi: 10.1007/s13225-015-0351-8. [DOI] [Google Scholar]
- 40.Vilgalys R., Hester M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White T.J., Bruns T., Lee S., Taylor J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990;18:315–322. [Google Scholar]
- 42.Katoh K., Standley D.M. A Simple Method to Control Over-Alignment in the MAFFT Multiple Sequence Alignment Program. Bioinformatics. 2016;32:1933–1942. doi: 10.1093/bioinformatics/btw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capella-Gutierrez S., Silla-Martinez J.M., Gabaldon T. TrimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidya G., Lohman D.J., Meier R. SequenceMatrix: Concatenation Software for the Fast Assembly of Multi-Gene Datasets with Character Set and Codon Information. Cladistics. 2011;27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees; Proceedings of the 2010 Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; pp. 1–8. [Google Scholar]
- 47.Nylander J.A.A. MrModeltest v2, Program Distributed by the Author Evolutionary Biology Centre. Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 48.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rambaut A. FigTree v1. 4. University of Edinburgh; Edinburgh, UK: 2012. [Google Scholar]
- 50.Chethana K.W.T., Niranjan M., Dong W., Samarakoon M.C., Bao D.F., Calabon M.S., Chaiwan N., Chuankid B., Dayarathne M.C., de Silva N.I., et al. AJOM New Records and Collections of Fungi: 101–150. Asian J. Mycol. 2021;4:113–260. doi: 10.5943/ajom/4/1/8. [DOI] [Google Scholar]
- 51.Réblová M. Molecular Systematics of Ceratostomella Sensu Lato and Morphologically Similar Fungi. Mycologia. 2006;98:68–93. doi: 10.1080/15572536.2006.11832714. [DOI] [PubMed] [Google Scholar]
- 52.Suetrong S., Schoch C.L., Spatafora J.W., Kohlmeyer J., Volkmann-Kohlmeyer B., Sakayaroj J., Phongpaichit S., Tanaka K., Hirayama K., Jones E.B.G. Molecular Systematics of the Marine Dothideomycetes. Stud. Mycol. 2009;64:155–173. doi: 10.3114/sim.2009.64.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thambugala K.M., Hyde K.D., Tanaka K., Tian Q., Wanasinghe D.N., Ariyawansa H.A., Jayasiri S.C., Boonmee S., Camporesi E., Hashimoto A., et al. Towards a Natural Classification and Backbone Tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae Fam. Nov. Fungal Divers. 2015;74:199–266. doi: 10.1007/s13225-015-0348-3. [DOI] [Google Scholar]
- 54.Yasanthika E., Dissanayake L.S., Wanasinghe D.N., Karunarathna S.C., Mortimer P.E., Samarakoon B.C., Monkai J., Hyde K.D. Lonicericola fuyuanensis (Parabambusicolaceae) a new terrestrial pleosporalean ascomycete from Yunnan Province, China. Phytotaxa. 2020;446:103–113. doi: 10.11646/phytotaxa.446.2.3. [DOI] [Google Scholar]
- 55.Phookamsak R., Hyde K.D., Jeewon R., Bhat D.J., Jones E.B.G., Maharachchikumbura S.S.N., Raspé O., Karunarathna S.C., Wanasinghe D.N., Hongsanan S., et al. Fungal Diversity Notes 929–1035: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungi. Fungal Divers. 2019;95:1–273. doi: 10.1007/s13225-019-00421-w. [DOI] [Google Scholar]
- 56.Li G.J., Hyde K.D., Zhao R.L., Hongsanan S., Abdel-Aziz F.A., Abdel-Wahab M.A., Alvarado P., Alves-Silva G., Ammirati J.F., Ariyawansa H.A., et al. Fungal Diversity Notes 253–366: Taxonomic and Phylogenetic Contributions to Fungal Taxa. Fungal Divers. 2016;78:1–237. doi: 10.1007/s13225-016-0366-9. [DOI] [Google Scholar]
- 57.Phukhamsakda C., Bhat D.J., Hongsanan S., Xu J.-C., Stadler M., Hyde K.D. Two Novel Species of Neoaquastroma (Parabambusicolaceae, Pleosporales) with Their Phoma-like Asexual Morphs. MycoKeys. 2018;34:47–62. doi: 10.3897/mycokeys.34.25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samarakoon M.C., Wanasinghe D.N., Liu J.K., Hyde K.D., Promputtha I. The Genus Neoaquastroma Is Widely Distributed; a Taxonomic Novelty, N. Cylindricum sp. nov. (Parabambusicolaceae, Pleosporales) from Guizhou, China. Asian J. Mycol. 2019;2:235–244. doi: 10.5943/ajom/2/1/14. [DOI] [Google Scholar]
- 59.Wanasinghe D.N., Hyde K.D., Konta S., To-Anun C., Jones E.G. Saprobic Dothideomycetes in Thailand: Neoaquastroma gen. nov. (Parabambusicolaceae) introduced based on morphological and molecular data. Phytotaxa. 2017;302:133–144. doi: 10.11646/phytotaxa.302.2.3. [DOI] [Google Scholar]
- 60.Wanasinghe D.N., Mortimer P.E., Senwanna C., Cheewangkoon R. Saprobic Dothideomycetes in Thailand: Phaeoseptum hydei sp. nov., a new terrestrial ascomycete in Phaeoseptaceae. Phytotaxa. 2020;449:149–163. doi: 10.11646/phytotaxa.449.2.3. [DOI] [Google Scholar]
- 61.Phukhamsakda C., Jeewon R., McKenzie E.H.C., Xu J.C. Morphology and phylogeny of Phaeoseptum mali sp. nov. (Phaeoseptaceae, Pleosporales) on bark of Malus halliana. Asian J. Mycol. 2019;2:118–128. doi: 10.5943/ajom/2/1/6. [DOI] [Google Scholar]
- 62.Ariyawansa H.A., Hyde K.D., Jayasiri S.C., Buyck B., Chethana K.W.T., Dai D.Q., Dai Y.C., Daranagama D.A., Jayawardena R.S., Lücking R., et al. Fungal Diversity Notes 111–252—Taxonomic and Phylogenetic Contributions to Fungal Taxa. Fungal Divers. 2015;75:27–274. doi: 10.1007/s13225-015-0346-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.