Abstract
Periodontal diseases and dental caries are the most common infectious oral diseases impacting oral health globally. Oral cavity health is crucial for enhancing life quality since it serves as the entranceway to general health. The oral microbiome and oral infectious diseases are strongly correlated. Gram-negative anaerobic bacteria have been associated with periodontal diseases. Due to the shortcomings of several antimicrobial medications frequently applied in dentistry, the lack of resources in developing countries, the prevalence of oral inflammatory conditions, and the rise in bacterial antibiotic resistance, there is a need for reliable, efficient, and affordable alternative solutions for the prevention and treatment of periodontal diseases. Several accessible chemical agents can alter the oral microbiota, although these substances also have unfavorable symptoms such as vomiting, diarrhea, and tooth discoloration. Natural phytochemicals generated from plants that have historically been used as medicines are categorized as prospective alternatives due to the ongoing quest for substitute products. This review concentrated on phytochemicals or herbal extracts that impact periodontal diseases by decreasing the formation of dental biofilms and plaques, preventing the proliferation of oral pathogens, and inhibiting bacterial adhesion to surfaces. Investigations examining the effectiveness and safety of plant-based medicines have also been presented, including those conducted over the past decade.
Keywords: herbal medicine, plant extracts, periodontal diseases, anti-infective agents, plants
1. Introduction
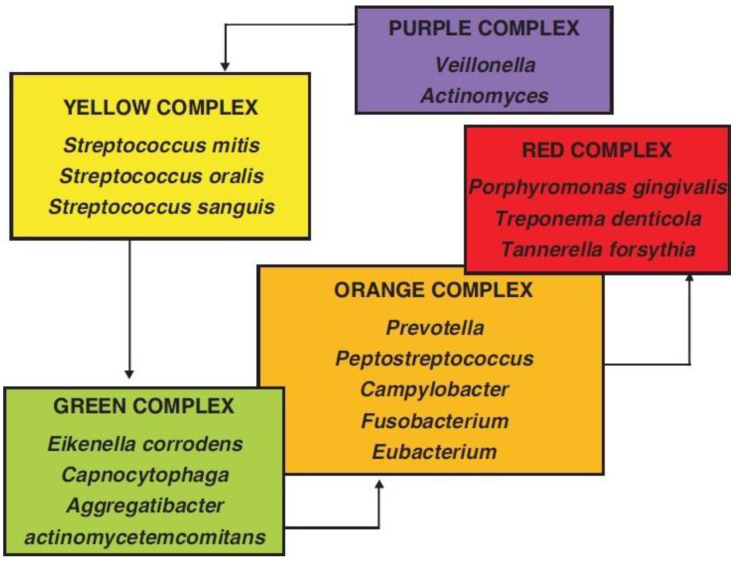
One of the most prevalent diseases influencing the teeth and the supporting tissues, such as the bone, periodontal ligaments (PDL), and cementum, is periodontitis [1]. The word “periodontitis” originates from the Ancient Greek words περί (perí means “around”) + ὀδoύς (odoús means “a tooth”) + -itis. When left untreated, periodontitis spreads from the gingival inflammatory response to the deeper tissues, changing the bone’s homeostasis and destroying the connective tissue attachment, resulting in the loss of alveolar bone, ultimately leading to tooth loss [2]. In adult populations, the prevalence of periodontal disease, particularly in its mild to moderate forms, is significant [3]. In contrast, the incidence of its severe form increases with age, particularly between the third and fourth decades of life [4]. Several factors contribute to periodontal disease [5]. The bacterial biofilm that develops on dental surfaces and its byproducts have been recognized as the primary cause of periodontitis [6,7,8,9]. By demolishing the attachments surrounding the tooth, the toxins released by periodontal pathogens have a crucial impact on the onset of periodontal disease [10]. Periodontal pathogens classified as the “red complex” are most frequently associated with the commencement and development of periodontal diseases among all the bacterial complexes found in biofilm. T. denticola, P. gingivalis, and T. forsythia are the three bacterial species that make up the red complex. The coexistence and elevated levels of all the pathogens in the red complex have been identified in stage III and IV periodontitis [11,12]. On the other hand, stage IV periodontitis of the molar incisor and some stage III and IV periodontitis are commonly associated with Aggregatibacter actinomycetemcomitans (A. a) [12,13] (Figure 1).
Figure 1.
Microbial complexes involved in the progression and development of periodontal diseases [14].
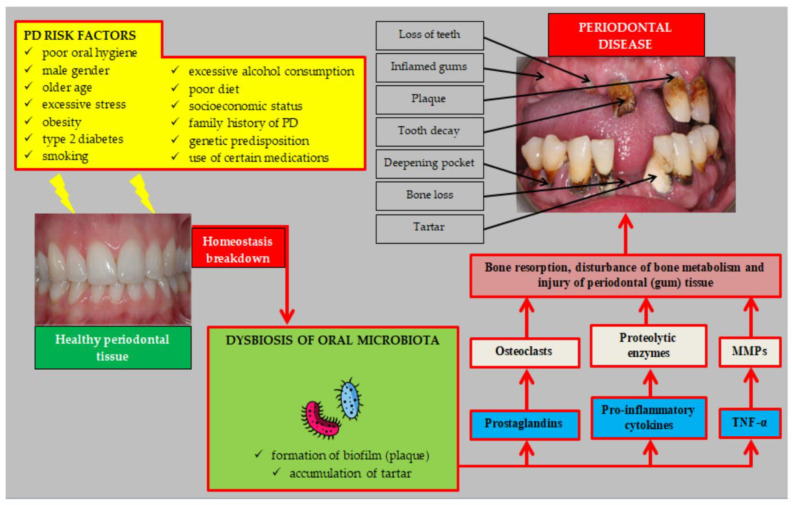
Along with local factors, including plaque and calculus, the patient’s systemic health, socioeconomic status, lifestyle choices, age, gender, ethnicity, genetics, environmental factors, and host response also affect how the disease progresses [5]. Metabolic syndrome, smoking, diabetes, and obesity are additional significant contributors (Figure 2) [15,16]. Periodontopathogens have detrimental effects on patients’ overall health, in addition to their damaging effects on the periodontium [17]. Leaving periodontal disease untreated may predispose the patient to various systemic diseases. Cardiovascular disease, diabetes, insulin resistance, oral and colon cancer, digestive disorders, adverse pregnancy outcomes, respiratory tract infections, pneumonia, and Alzheimer’s disease are among these conditions [18].
Figure 2.
Personal, social, systemic, and local risk factors associated with oral dysbiosis lead to periodontal disease development and progression through activating pathogenic pathways [19].
Periodontal treatment seeks to create a root surface that is “biologically acceptable” by eliminating the etiological bacteria and their metabolites [20]. Mechanical debridement is the primary method for treating and preventing periodontal disease, which entails scaling and root planning (SRP). Additionally, chemical plaque control procedures may be used as adjuvants to sustain long-term findings [21]. While surgical periodontal therapy is required in cases of progressive disease, non-surgical approaches can be practical in mild–moderate periodontitis [22]. Irrigating solutions, long-term drug delivery mechanisms, various drug delivery techniques, and mouthwashes are frequently employed as alternative solutions to non-surgical debridement in managing periodontitis [22,23]. In clinical interventions, the most commonly used adjunctive therapies for periodontitis cases are tetracycline, azithromycin, amoxicillin, and metronidazole [24,25,26]. The exponential increase in multidrug-resistant (MDR) bacteria to current antibiotics is a significant concern because it is the leading cause of treatment failure [27]. Therefore, it is crucial to develop antimicrobial medications that stop the emergence of drug resistance and improve the outcomes of treating infectious diseases.
Since time immemorial, plants and their extracts have been employed to achieve therapeutic objectives [21]. When it comes to preventing the emergence of antibiotic resistance in bacterial pathogens, these compounds have shown encouraging results [28]. Herbal medications are suitable substitutes for synthetic medicines in preventing and treating periodontal diseases due to their considerable natural action, broader biological activity, substantial safeness, and lower price [29,30,31]. A large class of chemical compounds discovered naturally in plants are referred to as plant-derived chemicals. These substances are widely present, and their anti-inflammatory, antibacterial, and antioxidant effects have proven advantageous [32]. Antibacterial compounds are widely distributed among plant species [33], and ethnobotanical knowledge can aid in the identification of plant extracts for developing new antibacterials [34,35]. In addition, older antibiotics can be increased in potency to restore their clinical application through the adjuvant effects of herbal extracts added to them, thus preventing the emergence of resistance [36].
Herbal alternatives are an untapped source of potential compounds beneficial in treating many human ailments, such as periodontal diseases, and benefiting overall health. As dietary supplements, herbal products are increasingly used to treat or prevent common diseases [37]. However, regarding the clinical use of these compounds in periodontics, investigation in the field of herbal science is still in its infant stages. The goal of this study was to summarize the research on medicinal plants that has been conducted to support their application as traditional medicine for the management of periodontitis.
2. Methods
The Scopus and PubMed/MEDLINE databases and Google Scholar were thoroughly searched. Two reviewers (S.A.M. and H.T.) searched the databases independently. A preliminary investigation was performed of medical botany to list all the plant-based compounds studied in periodontal diseases. The search keywords then comprised a list of medicinal herbs included in the present study combined with the terms “periodontitis” and “periodontal diseases”. The search protocol was conducted separately for each herbal compound using its related keywords. The keywords were searched in English-published papers in journals up to December 2022. To improve the search’s accuracy, the reference lists of the listed articles were manually searched as well.
After screening the retrieved papers, the eligible articles regarding the study’s subject were included. Clinical, in vitro, and in vivo studies published within the last 30 years that investigated any relationship between periodontal diseases and medicinal herbs were included. Non-English papers, posters, abstracts, and studies with inadequate data were all excluded. Two reviewers (H.T. and S.A.M.) then performed the data extraction, extracting the necessary data and recording it on a standardized Excel datasheet. For each plant species, data were compiled on its family, genus, species, parts utilized for medicine, and applications. The type of study, studied samples, methodology, and findings were all included in the extraction form.
3. Periodontitis
In the past, periodontal diseases were classified as aggressive or chronic [38]. In a new classification scheme, periodontitis is grouped under one category (“periodontitis”). A multidimensional grading and staging system further characterizes periodontitis. The stages determine both the complexity of the disease management and the severity of the disease at presentation. At the same time, the grades provide additional information on biological characteristics, such as an evaluation of the periodontitis progression rates, an evaluation of the risk of further advancement, and an assessment of the likelihood of poor treatment outcomes and adverse health effects associated with periodontitis [39].
Adaptive and innate immunity are involved in periodontitis. The inflammatory response has four consecutive phases: (1) silence, in which the first proinflammatory mediators are synthesized and released; (2) vascular, where the vascular wall increases in permeability and dilation; (3) cellular, during which inflammatory cells infiltrate the injury site; and (4) inflammatory responses subside [40].
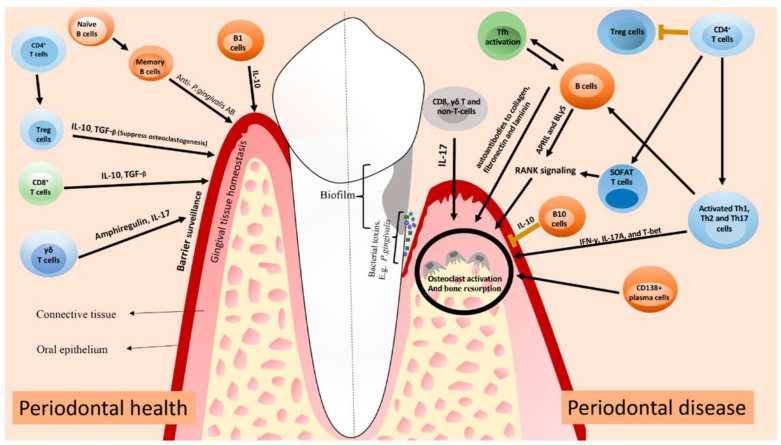
In the periodontium, neutrophils, antigen-presenting cells, and T and B lymphocytes form complex networks that interact with the humoral systems, including the complement system, which initiate immune and inflammatory responses [41]. The complement system has many other functions besides tagging and eliminating microorganisms. By synergizing with the Toll-like receptors on innate leukocytes, the complement system can increase immune and inflammatory responses and regulate B and T cell differentiation [42]. Several protein interactions occur in periodontitis, which is responsible for inflammation-induced bone loss. These proteins include the decoy receptor (osteoprotegerin), the functional receptor (RANK), and the receptor activator of the nuclear factor-κB ligand (RANKL). When the periodontium becomes inflamed, the activated lymphocytes (T and B) and osteoblasts produce RANKL [43]. Pre-osteoclasts mature and activate by contacting the RANK receptors on their cell membrane with RANKL. However, osteoprotegerin, a decoy receptor, antagonizes this binding process [44]. Figure 3 discloses the role of immune cells in a healthy periodontium versus periodontitis.
Figure 3.
An overview of how the mentioned T and B cells can affect periodontal health. Treg and CD8+ T cells produce IL-10 and TGF-β to maintain periodontal health. To maintain periodontal health, T cells produce amphiregulin and IL-17. Antibodies produced by B cells limit periodontal inflammation. Pro-inflammatory cytokines are released by activated Th1, Th2, and Th17 cells during periodontal disease. A combination of T and B cells produce RANKL, which activates osteoclasts. By clonally activating B cells, Tfh cells can cause local tissue destruction by producing autoantibodies against collagen, fibronectin, and laminin. A lack of Tregs or impaired function probably causes periodontitis. Other cells can also activate osteoclasts by producing IL-17 [45].
The inflammatory response should be terminated before it becomes chronic and adversely affects the individual. It is crucial to coordinate a series of steps to treat inflammation effectively, as chronic inflammation contributes to many chronic conditions, including periodontitis [46]. In addition to inhibiting neutrophil recruitment, tissue phagocytes clear apoptotic neutrophils via efferocytosis and initiate tissue repair. These processes involve downregulating proinflammatory mediators and upregulating regulatory or pre-resolution mediators [47].
To restore gingival health while protecting the residual periodontal tissues, periodontal therapy minimizes local factors and the bacterial load and corrects behavioral variables such as tobacco use and smoking cessation [48]. The non-surgical treatments for periodontitis include SRP, mouthwashes, dentifrices, and locally and systematically administered antimicrobial medications [49]. Combining mechanical root debridement with the patient’s oral hygiene practices prevents bacterial recolonization and the production of supragingival biofilms [50].
In addition to SRP, chemoplaque management techniques can be used as adjuvants in treating periodontal disease [21]. Patients with periodontitis have been shown to have improved outcomes with SRP and adjuvant antimicrobial agents [51]. The important issue is antimicrobial resistance [52]. Research on dental biofilms has found that antibiotic resistance increases in biofilms when exposed to clindamycin, doxycycline, metronidazole, and moxifloxacin. Herbal medications are recommended as alternatives to synthetic agents because of their natural action, high safety, and lower cost [29,30,31].
4. Plant-Based Antimicrobials against Periodontitis
Traditional therapies for various human diseases have used medicinal plants for centuries and in many regions worldwide [53]. Traditional medicines are used for health care by approximately 80% of people in developing nations [54]. Numerous biologically active substances have been developed into new lead chemicals for pharmaceuticals employing natural substances formulated from therapeutic herbs, which have been shown to be rich sources of these substances. There is a lot of potential for identifying new bioactive compounds because roughly 1% of the approximately 500,000 plant species worldwide have undergone phytochemical research [55]. Many cases of oral infections and diseases have been reported to have been treated with traditional plants and natural products [55]. In particular, phytochemicals and extracts from traditional medicinal plants have been shown to reduce dental plaque deposition, inhibit the proliferation of oral pathogenic organisms, and impact their adhesion to dental surfaces, alleviating the consequences of oral diseases [55].
4.1. Acacia arabica (Babul)
A commonly used chewing stick in India is Acacia arabica, known as “Babul” or “Kikar” datun. Many societies use Acacia arabica gum to maintain oral hygiene [56]. The main ingredient is arabica, a complex blend of Arabic acid’s calcium, magnesium, and potassium salts. In addition, tannins, cyanogenic glycosides, oxidases, peroxidases, and pectinases with antibacterial properties are present [57]. Acacia arabica’s antibacterial and antiprotease abilities have been established in vitro [58]. Clinical studies comparing Acacia arabica gum to CHX have demonstrated its comparable effectiveness in preventing plaque, lowering bacteria counts, and treating gingivitis without any of CHX’s side effects [59,60]. As a result, long-term Acacia arabica use is advised.
4.2. Acacia nilotica
The tree Acacia nilotica, also known in Sudanese folk medicine as “Garad or Sunt”, is found in this country’s central and northern regions. Tannin [61], gallic acid, catechin, epigallocatechin-7-gallate, catechin derivatives [62,63], ellagic acid, kaempferol, and quercetin [64] have all been found in the leaves and bark of A. nilotica. Additionally, numerous investigations have demonstrated that it possesses a variety of pharmacological effects, such as anti-HIV-1 protease [65], antibacterial [66], antioxidant, anticarcinogenic [67], and anti-inflammatory characteristics [68]. According to evidence, A. nilotica bark has antibacterial potential and inhibitory activity. Moreover, it can be utilized as an adjuvant antioxidant in mouthwashes and to develop future treatment options for periodontal diseases [69].
4.3. Allium sativum (Garlic)
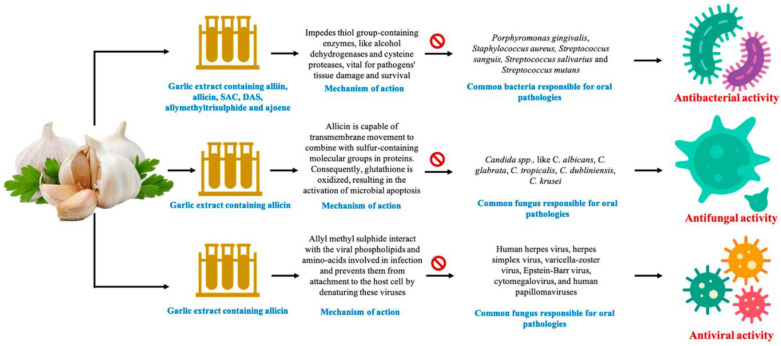
As a medicine, garlic (Allium sativum) has been recognized for centuries as having antibacterial, antifungal, and antiviral properties [70,71]. Allium sativum is traditionally used in treating infection, diabetes, and cardiac disease. Fresh raw garlic are composed mainly of water (66%), carbohydrates (27%), proteins (2.5%), amino acids (1.3%), fiber (1.6%), phenols, and trace minerals (2.4%) [72]. Garlic extract (GE) may benefit health because of its phytochemicals, including alliin, methiin, and sodium acetate [73]. Alliinase converts garlic alliin into allicin, an antibacterial compound that shows promise for treating periodontal disease, dental caries, and oral cancer [74]. Innovative concepts have emerged with fresh discoveries, such as aged garlic extract (AGE), which has been applied as medicine since 3000 BC. Researchers found that AGE lowered patients’ periodontitis levels more effectively than a placebo [75]. It is widely known that garlic can prevent inflammation, attack bacteria, viruses, and fungi, and prevent mutagenesis [76,77]. Numerous oral microbial diseases may be treated with garlic. Several novel garlic-based products, such as gels, gums, toothpaste, and pharmaceutical strips, have been reported as cost-effective and consumer-friendly solutions for improving oral health [78]. Figure 4 demonstrates the antimicrobial effects of GE against oral microorganisms. Allicin takes 1000 times longer than antibiotics to acquire resistance. Alcohol dehydrogenases and cysteine proteases (vital for tissue destruction) are among the thiol-containing enzymes inhibited by allicin’s antibacterial chemical [73]. A study discovered that taking GE orally reduced both the gingival (GI) and the bleeding index (BI), demonstrating that GE can also reduce periodontal conditions [79]. Tannins, flavonoids, and alkaloids are responsible for GE’s antibacterial activities [80]. As a result, periodontal diseases and dental caries can be successfully treated using garlic bulbs. When administered directly, garlic irritates the mucosa and so must be used carefully [81]. DAS, a sulfur-containing amino acid found in AGE, was shown to suppress the development of periodontal bacteria and reduce the P. gingivalis-induced inflammatory responses in human gingival fibroblast cells [82]. Taking AGE tablets helped prevent and enhance periodontal diseases in the long term [83]. According to studies, gingival inflammation and bleeding are reduced when AGE is consumed regularly for at least four months [79]. In recent investigations, garlic has been discovered to have anti-proteolytic properties against P. gingivalis protease, as evidenced by AGE’s intense bacteriostatic activity against P. gingivalis and gelatin liquefaction after 250 μL/mL dose administration [84]. In 200 individuals with good health, the effectiveness and effects of AGE on periodontitis were examined. Compared to the baseline value (1.50 ± 0.46), the mean PD for AGE after ten months was 1.06 ± 0.49, showing that AGE might help prevent or decrease periodontitis. Garlic’s bioactive components may suppress oral infections and some proteases, which may benefit patients with periodontitis [83].
Figure 4.
Different mechanisms of action through which garlic extract’s compounds assert antibacterial, antifungal, and antiviral effects [78].
4.4. Aloe barbadensis Miller (Aloe Vera)
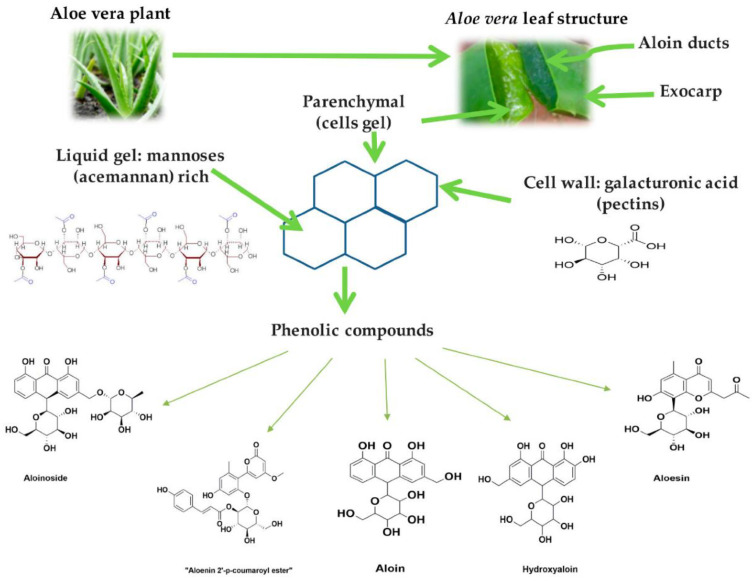
Therapeutic uses of Aloe vera date back thousands of years. In addition to treating bruising, X-ray burns, skin infections, hemorrhoids, sinusitis, gastrointestinal pain [85], and insect bites, this medicinal plant is also an anti-helminthic, somatic, and anti-arthritic [86,87]. Among the 75 constituents of Aloe vera are minerals, enzymes, sugars, anthraquinone, and salicylic acid [88]. Approximately 99.5% of Aloe vera leaves contain water and 0.0013% protein [87]. Figure 5 shows the primary constituents of an Aloe vera plant. Aloe vera gel has been shown to have pharmacokinetic activities that include anti-inflammatory, antibacterial, antioxidant, immune-stimulating, and hypoglycemic effects [89,90]. Aloe vera has antimicrobial effects on Streptococcus pyogenes and Enterococcus faecalis [91,92]. Isorabaichromone, feruoylaloesin, and p-coumaryl aloesin, three aloesin derivatives, have demonstrated the potential to scavenge radicals and superoxide anions [93,94]. It is perfect for treating gingivitis and periodontitis due to having an anti-inflammatory compound (C-glucosyl chromone), inhibiting the COX pathway, reducing PGE2, and breaking down the bradykinin inflammatory agent responsible for pain generation [93,95,96]. Edema, bleeding, and irritation of the gingival tissues are reduced by using it. It is beneficial in deep pockets where routine cleansing is challenging, and its antifungal properties also help treat denture stomatitis, aphthous ulcers, and angular cheilitis [97]. Using it after extractions is a powerful healer [98]. In root canal therapy, it has been used as a sedative dressing and file lubricant [99]. Many studies have been performed to determine if Aloe vera effectively cures gingivitis. In a double-blinded trial, 120 volunteers were requested to skip two weeks of tooth brushing. After being separated into three groups, 100% Aloe vera, distilled water as a placebo, and 0.2% CHX were given to the patients. The Aloe vera mouthwash was beneficial in lowering plaque and gingivitis, although when compared to CHX, its effects were not as noticeable [100]. Another study investigated how toothpaste with a high Aloe vera content affected the remission of plaque and gingivitis. The subjects were observed over three six-month periods using either Aloe vera toothpaste or a regular one. After the clinical experiment, the plaque and gingivitis indices decreased by roughly 20%, with no significant difference between the two study groups. Individuals motivated to improve their dental hygiene practices did not experience extra anti-plaque or -gingivitis when using an Aloe vera toothpaste [101]. Using Aloe vera as a medication in periodontal pockets was highlighted in a study performed by Geetha et al. [85].
Figure 5.
The main phenolic compounds of the Aloe vera plant and their chemical structures [102].
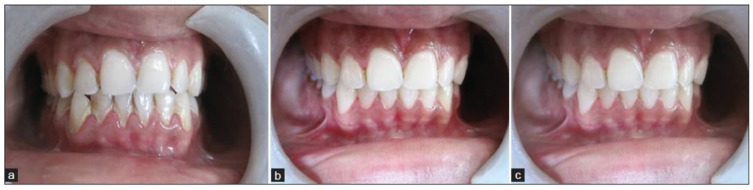
In Ajmera et al.’s study, Aloe vera mouthwash reduced plaque-induced gingivitis inflammation. Three months of Aloe vera mouthwash (BID) were administered to Group 1. Group 2 was scaled only. Group 3 received Aloe vera mouthwash and scaling. In contrast to the other two groups, Aloe vera mouthwash and scaling were more effective in reducing gingival inflammation. Consequently, Aloe vera was found to be anti-inflammatory, and combined with mechanical therapy, it helped treat plaque-induced gingivitis (Figure 6) [103].
Figure 6.
Results of a study on beneficial anti-inflammatory effects of Aloe vera + scaling treatment [(a) baseline; (b) one-month post-op; and (c) three-month post-op] to reduce plaque-induced gingivitis [103].
4.5. Amphipterygium adstringens
A Mexican endemic species of the Julianaceae family called “Cuachalalate” is Amphipterygium adstringens [104]. Anacardic acid [105], which has antioxidant, anti-inflammatory [106], anticancer [107], antiulcer, and antibacterial effects [105,108], is the primary ingredient responsible for the plant’s capabilities, according to recent studies.
4.6. Azadirachta indica [109]
A member of the Meliaceae family of mahogany trees, the neem tree (Azadirachta indica) is an evergreen that grows naturally in India and Myanmar’s subcontinent [110,111]. It has been found that extracts from various portions of this tree contain a variety of polyphenols, such as tannins, lignins, and flavonoids, that are potent antioxidants, antibacterials, anti-inflammatory agents, and immunomodulators [110,111,112,113,114,115,116,117,118,119,120]. The chewing sticks produced from twigs of the tree may play a role in oral care due to their mechanical cleansing properties, stimulation of saliva secretion, and antibacterial and antioxidant properties [121]. Aqueous preparations of neem have shown antimicrobial properties by reducing the surface adhesion of specific bacteria, destructing bacterial cell membranes, and inhibiting bacterial growth [115,116,122,123]. The plaque buildup and bacterial counts were significantly reduced after oral neem extract therapy [124]. With antioxidant properties, a neem extract may reduce the oxidative stress associated with periodontal disease and have anti-inflammatory potential [110,111,125]. Neem may have anti-inflammatory properties by suppressing prostaglandin E and 5 HT, reducing inflammation [123].
4.7. Berberis vulgaris
Extracts of Berberis vulgaris (Berberidaceae family) root exhibit antibacterial activity against periodontal bacteria due to berberine, the principal active ingredient. The growth of P. gingivalis and A. a has been shown by researchers to be inhibited by berberine [126,127,128]. P. intermedia, Actinomyces naeslundii, and Prevotella nigrescens do not grow due to the bacteriostatic properties of berberine [126,129]. The microbiological activity of a dental gel containing barberry root extract was investigated [130]. It was demonstrated that the protoberberine alkaloids had a synergistic antibacterial action, which can be used to explain why P. gingivalis growth was suppressed at 0.015 mg/g [130]. The plaque index (PI) was found to have decreased in a trial of the efficacy of a dental gel containing 1% berberine. Comparatively, applying a 5% gel reduced the growth of invading bacteria [129].
4.8. Camellia sinensis (Green Tea)
Camellia sinensis belongs to the Theaceae family and has small perennial shrubs widely used to produce green and black teas [131]. Its beneficial properties are attributed to green tea’s polyphenol components (catechins). Epicatechin-3-gallate and epigallocatechin-3 gallate are the two significant catechins. Compared to black tea, green tea contains higher polyphenols (30–40% vs. 3–10%), with enhanced antioxidant capacity and strong anti-inflammation, antibacterial, antiviral, antimutagenic, and anti-aging activities [132,133,134]. Inflammation and periodontitis are positively affected by green tea. Thus, research supports green tea as a curative and preventive agent for periodontal disease [135].
4.9. Cinnamomum zeylanicum (Ceylon Cinnamon)
Cinnamon has been utilized as a culinary herb in traditional medicine. Cinnamon has been researched in pregnancy, diabetes management [136], and gynecological disorders [137]. It has anti-inflammatory, cardioprotective, antioxidative, and antibacterial activities and anti-inflammatory capabilities [138]. Cinnamon refers to a collection of around 250 evergreen trees belonging to the Lauraceae family [139]. Several species have been studied, including those linked to oral medicine. Cinnamomum verum and Cinnamomum zeylanicum are two of the most studied cinnamon types. Cassia cinnamon, often known as Chinese cinnamon or Cinnamomum aromaticum, is a well-studied spice. Cinnamomum burmannii and Cinnamomum loureiroi are two more major cinnamon species [138,140]. The EO of Cinnamomum bark (CBEO) contains many aromatic compounds and high concentrations of cinnamaldehyde and eugenol. CBEO and cinnamaldehyde have antibacterial, antifungal, anti-inflammatory, and anticancer properties [141,142,143]. According to Wang et al., the cinnamaldehyde in C. zeylanicum bark EO works against P. gingivalis [144]. According to reports, cinnamaldehyde is responsible for CBEO’s antibacterial effect [144]. The relative mechanism of cinnamaldehyde was uncovered by examining the cell microstructure, membrane integrity, and membrane properties [145]. CBEO and cinnamaldehyde may irreversibly damage bacterial membranes, thus compromising membrane integrity. The metabolism will err when the cell membrane depolarizes, and the bacteria will die [144]. As determined by propidium iodide uptake tests, the CBEO and cinnamaldehyde treatments interrupted the integrity of the bacterial membranes. The confocal microscopy analysis of P. gingivalis detected PI incorporation, indicating a cell membrane disruption [144]. Microorganisms can be killed by this principal mechanism, which is known as membrane damage [146]. P. gingivalis may therefore be susceptible to membrane permeabilization caused by CBEO and cinnamaldehyde.
Eugenol, a compound more commonly associated with clove, is also a potent component of cinnamon EO [147]. Due to its powerful antibacterial properties and abundance in cinnamon EO and extracts, it has been demonstrated to be beneficial to periodontal health. In addition to having antibacterial properties, eugenol has multiple mechanisms of action [148] through the destruction of the cell membrane in a dose-dependent fashion and reducing the presence and formation of the biofilm [148]. Cinnamaldehyde has also been declared safe and non-toxic by the FDA. Cinnamaldehyde can be absorbed quickly by the gastrointestinal system [149]. Nearly no residues are left when the body removes the metabolites [150].
4.10. Citrus sinensis
In the Rutaceae family, oranges are classified as Citrus sinensis, a sweet and juicy fruit. Orange trees are often grown in tropical and subtropical climates because of their medicinal properties and sweet juice. Aside from preventing and treating vitamin deficiency, colds, flu, and scurvy, it also fights bacterial and viral infections [151]. Antibacterial properties have also been reported for orange peel [152]. Dubey et al. demonstrated the robust antibacterial properties of orange peel extracts against different bacteria using the disk diffusion method [153]. The effectiveness of orange peel extract against Klebsiella pneumoniae has been demonstrated by Jabuk et al. [154]. Numerous studies [109,151,152,155] have revealed that Citrus sinensis can also treat periodontal disease (Table 1 and Table 2).
Table 1.
Clinical trials studying plant-based antimicrobials in periodontal diseases.
| Natural Compound | Study Type | Samples Studied | Methods | Result(s)/Conclusion(s) | Limitations | Ref./Year |
|---|---|---|---|---|---|---|
| Acacia arabica | RCT | Nc: 40 Ns: 40 [Patients with mild–moderate periodontitis] |
Gc: SRP + placebo Gs: SRP + Acacia arabica [PD and CAL were compared at baseline and after 15 and 90 days] |
Acacia arabica’s antiplaque and antigingivitis properties were significantly valuable (p < 0.05). A reduction in sites with moderate PD was observed more among Gs than Gc (p = 0.001). The treatment may be prescribed with SRP for mild to moderate chronic periodontitis. | 1. No assessment of bone defect fill. 2. Short follow-up period. |
[56]/2018 |
| RCT | Nc: 30 Ns: 30 [Patients with gingivitis] |
Gc: Regular toothpaste Gs: Toothpaste containing Acacia arabica [PI, GI, and BOP were compared at the baseline and after 28 days] |
Gs showed statistically significantly better results regarding PI, GI, and BOP (p < 0.001). Gingivitis may be prevented with toothpaste that contains Acacia arabica. Using it daily can help improve oral health. | Not assessing the combination therapy using herbal toothpastes and mouthwashes. | [156]/2012 | |
| Allium sativum | RCT | Nc: 100 Ns: 100 [Patients with mild–moderate periodontitis] |
Gc: Placebo tablets Gs: Tablets containing 300 mg of AGE powder [Subjects were examined at the start and 12 and 18 months post-op. GR and PD were measured] |
The mean value of pocket depth was 1.06 ± 0.49 in comparison with the baseline value of 1.89 ± 0.74 (p < 0.001). The corresponding value was 1.50 ± 0.46 for the placebo group (p < 0.001). Periodontal disease can be prevented or improved with aged garlic extract. | Not determining the exact dosage, required duration of usage, or the principal mode of action. | [83]/2020 |
| Aloe barbadensis Miller | RCT | N1: 10 N2: 10 [Patients with gingivitis] |
G1: First, Aloe vera toothpaste for 14 days, then fluoride toothpaste for another 14 days G2: First, fluoride toothpaste for 14 days, then Aloe vera toothpaste for another 14 days [PI and GI were assessed] |
PI was 2.14 ± 1.3 at baseline and 1.84 ± 1.02 at 30 days (p < 0.098). GI was 0.62 ± 0.74 at baseline and 0.25 ± 0.46 at 30 days (p < 0.068). In comparison with fluoride toothpaste, Aloe vera toothpaste had similar effects on PI and GI, and it seems that it can be used as an alternative. |
1. Small sample size 2. Short follow-up period |
[157]/2021 |
| RCT | Nc: 20 Ns: 20 [Patients with chronic periodontitis] |
Gc: SRP Gs: SRP + Aloe vera gel [GI, PI, and PD were evaluated at baseline and after 15 and 30 days] |
The mean reduction in GI: baseline day, 1.98 ± 0.10; 15 days, 1.6 ± 0.10; 30 days, 1.05 ± 0.10. After and before treatment measurements. Aloe vera treatment significantly reduced PI. The plaque index was meaningfully decreased from 2.15 ± 0.271 to 1.60 ± 0.34 after 30 days. Periodontitis significantly decreased in areas treated with Aloe vera gel. |
1. Small sample size 2. Short follow-up period |
[158]/2019 | |
| RCT | Nc: 18 Ns: 18 [Healthy individuals] |
Gc: Close-up tooth gel Gs: Aloe vera tooth gel [After 3 weeks, PI and GI were measured at baseline] |
It was demonstrated that toothpaste containing Aloe vera significantly improved GI and PI; the results were similar to those achieved with tooth gel applied close up. | 1. Small sample size 2. Short follow-up period |
[159]/2018 | |
| RCT | N1: 130 N2: 130 N3: 130 [Patients with PI > 1.9, and GI > 1.1] |
G1: Aloe vera mouthwash G2: CHX mouthwash G3: Placebo [GI and PI were measured at baseline and after 15 and 30 days] |
Compared to the CHX and Aloe vera groups, all parameters presented considerable reductions (p < 0.05). Neither Aloe vera nor CHX presented a significant difference (p < 0.05). Aloe vera and placebo and CHX and placebo had significantly different mean PI and GI scores (p < 0.000). | Short follow-up period | [160]/2016 | |
| RCT | N1: 30 N2: 30 N3: 30 [Healthy individuals] |
G1: Aloe vera mouthwash G2: CHX mouthwash G3: Placebo (normal saline) [After 15 and 30 days, GI and PI were evaluated at baseline] |
Both Aloe vera and CHX significantly reduced plaque and gingivitis. There was no statistically significant difference between groups (p > 0.05). CHX mouthwash can be replaced with Aloe vera as an affordable and suitable alternative. | 1. Small sample size 2. Short follow-up period |
[161]/2016 | |
| RCT | N1: 30 N2: 30 N3: 30 [Patients with visible plaque and gingivitis in at least 30% of their teeth] |
G1: Aloe vera mouthwash G2: Chlorine dioxide mouthwash G3: CHX mouthwash [GI and PI were evaluated at baseline and after 15 days] |
At follow-up, all three groups had significantly lower plaque and gingival scores than at baseline (p < 0.001). In comparison with Aloe vera, CHX significantly reduced PI and GI (p < 0.05). | 1. Small sample size 2. Short follow-up period |
[162]/2016 | |
| RCT | N1: 30 N2: 30 N3: 30 [Patients with periodontitis] |
G1: 0.2% CHX mouthwash G2: Green tea–Aloe vera mouthwash G3: Distilled water [After 14 days, GI, BI, and PI were evaluated at baseline] |
G1, G2, and G3 reduced the PI by 0.17 ± 0.14, 0.10 ± 0.08, and 0.02 ± 0.18, respectively (p = 0.008). Between G1 and G2 with G3 were a significant difference. BIs between these three groups were significantly different, with p = 0.001 between G2 and G1 with G3. Periodontal health was improved by green tea–Aloe vera mouthwash. This can lead to improved dental and oral health. | 1. Small sample size 2. Short follow-up period |
[163]/2016 | |
| RCT | N1: 115 N2: 115 N3: 115 [Healthy individuals with baseline DMFT index of 2.5 to 5 and baseline PI > 1.5] |
G1: Mouthwash containing Aloe vera G2: CHX mouthwash G3: Placebo mouthwash [After 15 and 30 days, GI and PI were evaluated at baseline and] |
CHX and Aloe vera groups reduced plaque and gingivitis significantly, but there was no statistically significant difference (p > 0.05). The ability of Aloe vera to reduce periodontal indexes makes it an effective mouthwash. |
Short follow-up period | [164]/2014 | |
| RCT | N1: 40 N2: 40 N3: 40 [Healthy individuals] |
G1: 100% Aloe vera mouthwash G2: CHX mouthwash G3: Placebo mouthwash [At 7, 14, and 22 days, GI, BI, and PI were evaluated at baseline] |
The PI, GI, and BI scores of G1 and G2 decreased statistically significantly after rinse regimens were initiated compared with G3. Aloe vera mouthwash significantly decreased gingivitis and plaque, but not as much as CHX. | 1. Canceling regular oral hygiene was an inconvenient and embarrassing prerequisite in this mouth rinse study. 2. Short follow-up period |
[100]/2012 | |
| RCT | Nc: 20 Ns: 20 [Patients with chronic periodontitis] |
Gc: SRP Gs: SRP + local administration of Aloe vera gel [GI, PI, and PD were measured at baseline and after 30 and 60 days] |
There was no statistically significant difference between the control and experimental groups in PI in any of the three stages. Both groups treated with SRP combined with Aloe vera or SRP indicated substantial improvement in all 3 stages regarding GI and PD. As a result, Gs had significantly lower GI than the control group (p = 0.0001) and PD (p = 0.009). | 1. Small sample size 2. Short follow-up period |
[165]/2017 | |
| Berberis vulgaris | RCT | N1: 25 N2: 10 N3: 10 [Patients were healthy dormitory students] |
G1: Barberry gel G2: Placebo gel without an active ingredient G3: Colgate® antiplaque toothpaste [GI and PI were measured at baseline and after 21 days] |
Between placebo and barberry gel groups, Colgate® and placebo groups, there were significant differences in PI and GI (p < 0.01). However, there was no statistically significant difference between barberry and Colgate® groups. By applying barberry dental gel, school-aged children can effectively control microbial plaque and gingivitis. | 1. Lack of randomization 2. Small sample size 3. Lack of blinding 4. Short follow-up period |
[166]/2007 |
| Camellia sinensis | RCT | Nc: 15 Ns: 15 [Patients with generalized gingivitis] |
Gc: Full-mouth prophylaxis Gs: Green tea extract and oral lycopene for 45 days + oral prophylaxis. [BI, salivary UA, and PI levels were measured at baseline and after 45 days] |
After treatment, a comparison of the test and control groups revealed statistically significant results in BI (p ≤ 0.001), salivary UA levels (p ≤ 0.01), and PI (p ≤ 0.001). Gingivitis can be treated with lycopene and green tea extract as adjunctive prophylactic and therapeutic methods. | 1. Small sample size 2. Short follow-up period |
[167]/2019 |
| RCT | Nc: 15 Ns: 15 [Patients with chronic periodontitis] |
Gc: No intervention Gs: Green tea herbal [After six weeks, BI, PI, and PD were evaluated at baseline] |
Before and after SRP, both groups presented significant reductions in PD and BI; the intervention group presented a greater reduction (p = 0.003 and 0.031, respectively). The effect of reducing PI between the two groups was not significant, despite being significant within each group (p = 0.135). According to this study, green tea herbal may effectively treat periodontal diseases and improve the benefits of phase I periodontal therapy. |
1. Lack of blinding 2. Small sample size 3. Lack of randomization 4. Short follow-up period |
[168]/2018 | |
| RCT | Nc: 20 Ns: 20 [Patients with a gingival index ≥ 1] |
Gc: 0.12% CHX mouthwash Gs: Green tea mouthwash with 1% tannin [BI, PI, GI, and staining were measured at baseline and after one and four weeks] |
After 1 and 4 weeks, significant differences were detected between groups, but not between groups, in all indices (p < 0.0001). Significantly less tooth staining was observed with the test mouthwash than with the control mouthwash. An adjunct to mechanical plaque reduction could be 1% tannin green tea mouthwash. | 1. Small sample size 2. Observation bias (Hawthorne effect) 3. Short follow-up period |
[169]/2017 | |
| RCT | Nc: 22 Ns: 23 [Patients with marginal gingivitis] |
Gc: Placebo gum Gs: Chewing gum containing green tea [BI, PI, and salivary IL-1β were measured at baseline and after 7 and 21 days] |
There was a significant impact of chewing gum on BI and PI (p < 0.001). BI and PI mean changes at different observation periods were significantly different between the two groups (p < 0.001). Chewing gum had a significant effect on IL-1 β (p < 0.001). A significant difference in mean IL-1 β changes within 1–21 days was not observed (p = 0.086). | 1. Small sample size 2. Short follow-up period |
[170]/2016 | |
| RCT | Nc: 55 Ns: 55 [Patients with PI and GI of at least 1.5 and 1, respectively] |
Gc: Placebo mouthwash Gs: Mouthwash containing 2% green tea [GI and PI were measured at baseline and after 28 days] |
Between baseline and 28 days, mean GI and PI scores decreased significantly among the test group, but not in the control group (p < 0.05). GI scores in the test group (0.67 ± 0.22) were statistically significantly reduced as compared with the control group (0.05 ± 0.11), and PI scores (1.65 ± 0.68) were statistically significantly reduced as compared to the control group (0.45 ± 0.99). |
1. Short follow-up period 2. Small sample size |
[171]/2015 | |
| RCT | N1: 20 N2: 20 N3: 20 [Patients were healthy dormitory students] |
G1: 0.2% CHX G2: 0.05% sodium fluoride G3: 0.5% Camellia sinensis extract [Salivary pH, PI, GI, and OHI scores were measured at baseline and after one and two weeks] |
The experimental groups showed a reduction in mean PI and GI over the 2-week trial period. In all groups, antiplaque effectiveness was highest in G3 (p < 0.05). G1 and G3 were more effective than sodium fluoride at cleaning gingiva (p < 0.05). As compared to G1, the salivary pH increased in G2 and G3. In G1 and G3, the improvement in oral hygiene was more apparent. Due to its minimal side effects and prophylactic benefits, Camellia sinensis can be utilized as an adjunct to oral self-care. |
1. Small sample size 2. Short follow-up period |
[172]/2015 | |
| RCT | Nc: 25 Ns: 25 [Patients with chronic gingivitis] |
Gc: Normal saline Gs: Mouthwash containing 5% green tea [After five weeks, GI, PI, and BI were measured at baseline] |
The periodontal indices showed significant improvement throughout this study (p < 0.001). The changing alteration patterns of indices were contrasted between two groups (p < 0.05). Even though the mouthwash group showed a greater overall improvement, the differences did not reach statistical significance (p > 0.05, observed power for GI: 0.09, PI: 0.11, and BI: 0.07). Green tea mouthwash is effective and safe for treating inflammatory periodontal diseases. | 1. Short follow-up period 2. Small sample size |
[173]/2012 | |
| RCT | Nc: 10 Ns: 10 [Patients with chronic periodontitis] |
Gc: SRP Gs: SRP + green tea catechin application [GI, PI, and PD were measured at baseline and after one and five weeks. After subgingival plaque sampling, red-complex bacteria were studied via PCR] |
Between baseline and 1 week and baseline and 5 weeks, both study and control groups showed significant differences in PD, GI, and PI and substantial reductions in red-complex organisms. (p < 0.001). PD, PI, and GI did not show statistically significant intergroup differences. In Gs, T. forsythus was significantly reduced at one week and five weeks and P. gingivalis was significantly reduced at one week compared to Gc. Chronic periodontitis can be effectively treated with green tea catechins in combination with SRP. | 1. Short follow-up period 2. Split-mouth design 3. Small sample size |
[174]/2013 | |
| Citrus sinensis | RCT | Nc: 10 Ns: 10 [Patients with moderate-severe gingivitis] |
Gc: 2% CHX mouthwash Gs: Citrus sinensis (4% ethanolic extract) mouthwash [GI, PI, and BI were measured at baseline and after 7 and 14 days] |
A mouthwash containing citrus sinensis 4% reduced PI (p = 0.095) as well as a mouthwash containing CHX 0.2% and BI (p = 0.42). However, the extract was more efficient in lowering GI (p = 0.04). | 1. Lack of blinding 2. Small sample size 3. Lack of randomization 4. Short follow-up period |
[109]/2018 |
| Curcuma longa | RCT | Nc: 15 Ns: 15 [Patients with chronic periodontitis] |
Gc: SRP + CHX gel Gs: SRP + curcumin gel [After 30 and 45 days, GI, PI, BI, and PD were evaluated at baseline] |
A significant difference in PI, PD, BI, and GI was found between Gs and Gc (p < 0.001). SRP can be administered with the control and experimental gel, but curcumin gel performed better than CHX gel in reducing periodontal pockets in mild to moderate cases. | 1. Short follow-up period 2. Lack of microbiological evaluations 3. Small sample size |
[175]/2016 |
| Pilot Study | Ten patients with severe gingivitis | Curcuma gel was consumed orally by the subjects for 21 days; BI was measured after three weeks | Statistical significance was found in the results (p < 0.001). By reducing gingival inflammation, Curcuma longa extract gel was effective. | 1. Short follow-up period 2. Pilot study 3. Small sample size |
[176]/2014 | |
| Garcinia mangostana | RCT | Nc: 25 Ns: 25 [Patients with generalized chronic periodontitis] |
Gc: SRP + placebo gel Gs: mangostana gel + SRP [At baseline and after three months, PI, BI, PD, CAL, and red-complex bacteria were evaluated] |
Between baseline and three months after the study began, Gs had significantly lower PD, CAL, BI, PI, and Treponema denticola values than the placebo group (p ≤ 0.05). | 1. Small sample size 2. Short follow-up period |
[177]/2017 |
| Glycyrrhiza glabra | RCT | Nc: 52 Ns: 52 [Patients with mild–moderate periodontitis] |
Gc: No intervention Gs: 10% G. glabra gum paint [GI, PD, and CAL were evaluated at baseline and after four weeks] |
Patients in the study group showed significant improvements in their periodontal health. G. glabra prevented periodontal diseases. | 1. Small sample size 2. Short follow-up period |
[178]/2019 |
| Juglans regia | RCT | N: 20 [Patients with mild gingivitis] |
2% and 3% ether extracts of Juglans regia, 2% and 3% petroleum extracts of Juglans regia, 2% water-soluble extract of Juglans regia, and propylene glycol vehicles were evaluated. [PI was measured at baseline and after three days] |
There was 32.12% and 31.56% antiplaque activity in 2% and 3% ether fractions of Juglans regia, respectively. Inhibition of plaque was observed in 30.32% of cases with the 2% aqueous solution of Juglans regia, and in 17.62% and 19.45% of cases with the 2% and 3% petroleum ether fractions. A high level of statistical significance was found in all the findings. The researchers concluded that Juglans regia could be used as an adjunct to oral hygiene regimens since it displayed potent anti-plaque properties. | 1. Small sample size 2. Short follow-up period |
[179]/2009 |
| Lippia sidoides | RCT | N1: 10 N2: 10 N3: 10 [Healthy individuals] |
G1: Lippia sidoides EO G2: CHX G3: Placebo [PI and BI were measured at baseline and after three months] |
In the test groups, plaque and gingivitis were significantly reduced (p < 0.05), Statistically, there was no significant differences (p > 0.05). Gel preparations containing Lippia sidoides essential oil were effective against plaque and gingivitis. | 1. Small sample size 2. Short follow-up period |
[180]/2013 |
| RCT | Nc: 28 Ns: 27 [Patients with PI and GI of at least 1.5 and 1, respectively] |
G1: CHX mouthwash G2: Lippia sidoides EO mouthwash [PI, BI, GI, and salivary S. mutans colony counts were measured at baseline and after 7 and 30 days] |
Clinical and microbiological parameters were significantly reduced by both mouth rinses. Both groups showed no significant differences (p > 0.05). Both groups had considerable reductions in the number of colonies of S. mutans (p < 0.05). Although CHX treatment reduced more efficiently than L. sidoides, there was no statistical difference between the two groups, and both treatments reduced the bacteria equally (p = 0.3). The results of this study demonstrate that Lippia sidoides EO mouth rinses reduce gingival inflammation and microbial plaque. | 1. Small sample size 2. Short follow-up period |
[181]/2009 | |
| Mangifera indica | RCT | Nc: 10 Ns: 10 [Healthy individuals] |
Gc: CHX mouthwash Gs: Mango leaf mouthwash [After five days, PI, GI, and salivary S. mutans, S. mitis, and S. salivarius counts were evaluated at baseline] |
Mango leaf and CHX mouthwashes significantly reduced the microbial count and improved gingival health and plaque control, with CHX showing a greater reduction in the microbial count and better plaque control. | 1. Small sample size 2. Short follow-up period |
[182]/2017 |
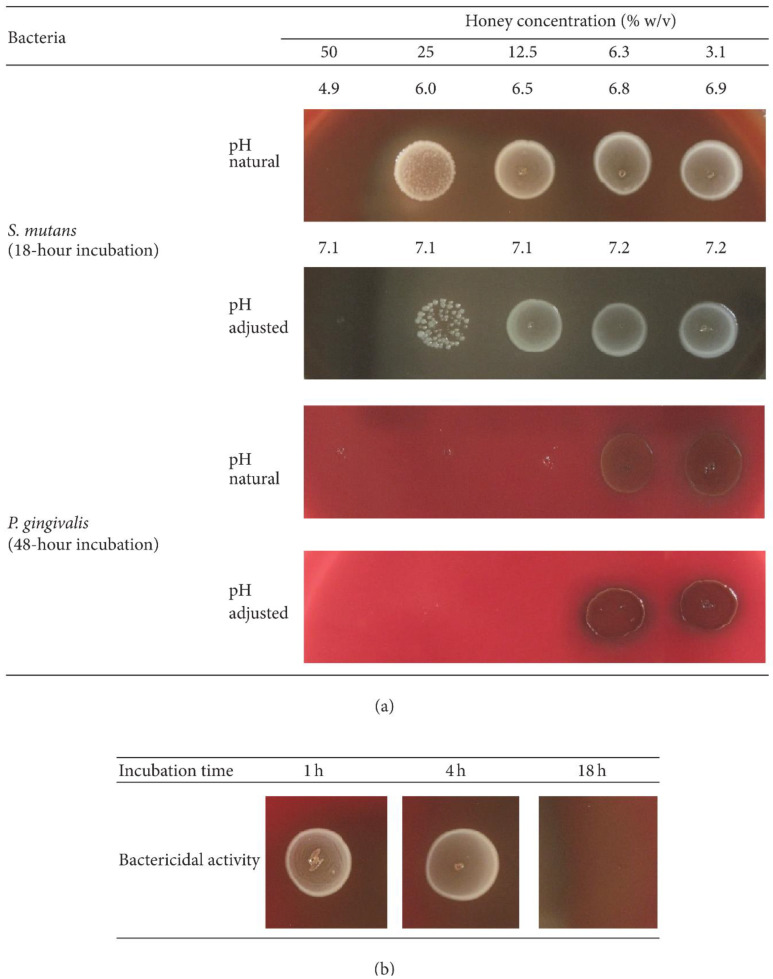
| Manuka honey | RCT | N1: 7 N2: 7 N3: 6 [Orthodontic patients] |
G1: Honey G2: 10% sucrose G3: 10% sorbitol [Plaque pH, bacterial count, and antibacterial properties of honey against S. mutans, L. acidophilus, and P. gingivalis were measured at baseline and after 2, 5, 10, 20, and 30 min] |
As compared to sorbitol, honey and sucrose had significantly different plaque pH values (p ≤ 0.001). Honey was the only substance that significantly reduced pH after 30 min of exposure, despite sucrose, sorbitol, and honey significantly reducing bacteria recovery (p < 0.001). There was a significant decrease in the growth of all bacterial strains when honey was added (p ≤ 0.001). By applying honey topically, pH can be modified, bacteria counts can be reduced, and bacterial growth can be inhibited. | 1. No follow-up 2. Small sample size |
[183]/2014 |
| Pilot study | Nc: 15 Ns: 15 [Healthy individuals] |
Gc: Sugar-free chewing gum Gs: Manuka honey products [PI and GI were measured at baseline and after 21 days] |
Manuka honey reduced plaque scores and bleeding sites (48% reduced to 17%; p = 0.001) statistically significantly compared to the control group. The results suggest that manuka honey may have therapeutic potential in treating gingivitis and periodontitis. | 1. Short follow-up period 2. Pilot study 3. Small sample size |
[184]/2004 | |
| Matricaria chamomilla | RCT | N1: 25 N2: 25 N3: 25 [Patients with chronic periodontitis] |
G1: SRP + placebo G2: 0.12% CHX + SRP G3: 1% Matricaria chamomilla mouthwash + SRP [PI, BI, GI, PD, CAL, GR, stain index, and microbial colony counts were measured at baseline and after six weeks and three months] |
All parameters (except GR in the placebo group) changed significantly between baseline and three months. Compared to the placebo group, meditative mouth rinses containing chamomilla exhibited significant benefits. In comparison to baseline, the CHX rinse resulted in slightly higher improvements in both PD (3.68 mm vs. 3.36 mm) and CAL (3.00 mm vs. 2.72 mm) than CHX rinse. Non-surgical periodontal therapy for chronic periodontitis can use Matricaria chamomilla as an adjunct to CHX mouthwash. | 1. Small sample size 2. Short follow-up period |
[185]/2020 |
| Pilot study | N1: 10 N2: 10 N3: 10 [Orthodontic patients with fixed appliances] |
G1: Placebo G2: 0.12% CHX G3: 1% Matricaria chamomilla mouthwash [PI and BI were measured at baseline and after 15 days] |
G1 exhibited increases in PI and BI (10.2% and 23.1%, respectively). The PI and BI levels in G3(−25.6% and −29.9%, respectively) and G2 (−39.9% and −32.0%, respectively) were considerably lower than those in the placebo group. Biofilm formation and BI were reduced in gingivitis patients. This was probably a result of Matricaria chamomilla’s anti-inflammatory and antimicrobial properties | 1. Short follow-up period 2. Pilot study 3. Small sample size |
[186]/2016 | |
| Psidium guajava | RCT | N total: 15 patients (30 sites) Nc: 15 sites Ns: 15 sites [Patients with chronic periodontitis] |
Gc: SRP Gs: 3% P. guajava gel [After one and three months, PI, GI, BI, PD, CAL, and colony counts of A. a and P. gingivalis were evaluated at baseline] |
Clinical parameters improved significantly throughout the study. Three months after testing, site-specific indices, PD (2.74 ± 0.283), and CAL (2.8 ± 0.152) showed statistically significant reductions. A. a. (17.4 ± 0.026) and P. gingivalis (22.7 ± 1.225) colony counts were significantly reduced at one and three months in the test sites (p < 0.001). Local delivery of 3% P. guajava gel treated chronic periodontitis with clinical and microbiological parameter improvements. | 1. Short follow-up period 2. Small sample size 3. Split-mouth design |
[187]/2021 |
| Punica granatum | RCT | N total: 10 patients (20 sites) Nc: 10 sites Ns: 10 sites [Patients with moderate–severe chronic periodontitis] |
Gc: Placebo gel Gs: Punica granatum gel [after 15 days, PI, GI, and BI were measured at baseline] |
After 15 days following gel application, mean BI, GI, and PI were significantly reduced. According to microbiological results, Punica granatum oral gel suppresses microbial growth. Test specimens revealed mild perivascular inflammation and increased collagen fibers, while controls showed dense inflammatory infiltration and collagen fiber destruction. In combination with SRP, Punica granatum gel reduced chronic periodontitis clinical symptoms. | 1. Small sample size 2. Short follow-up period |
[188]/2019 |
| RCT | N1: 20 N2: 20 N3: 20 N4: 20 [Healthy individuals] |
G1: Pomegranate extract gel G2: CHX gel G3: Ornidazole–CHX gel G4: Placebo gel [After 14 and 60 days, GI, PI, BOP, PD, and GCF levels of IL-8, IL-1β and chemokine ligand 28 were measured at baseline] |
Inhibition of inflammatory cytokines and chemokines was observed in G1. G1 levels of IL-1β and IL-8 increased less than CCL28 levels (p = 0.003, 0.002), which remained unchanged from baseline (p = 0.15). G1 subjects showed a lower increase in BOP and GI (p = 0.01, 0.05) compared to other groups (p < 0.001) after 14 days. It was similar in terms of PI reduction between G1 and G3 gels (p = 0.96). For the treatment of gingivitis, PEG effectively reduced inflammatory markers. | 1. In an experimental gingivitis model, all products were tested, which may differ from the natural gingivitis model. 2. In order to avoid bias caused by variable host responses, a cross-over design would have been more appropriate. |
[189]/2017 | |
| RCT | Nc: 40 Ns: 40 [Diabetic patients with gingivitis] |
Gc: CHX 0.2% Gs: Punica granatum mouthwash [After 14 days, PI, GI, BI, and PD were measured at baseline] |
Both interventions significantly improved gingival and plaque indices (p < 0.001 for all indices). Primary outcome measures showed no significant differences between Gc and Gs, except for GI, where Gs mouthwash had superiority over Gc after two weeks (p = 0.039). It is safe and effective to use Punica granatum mouthwash as an alternative to CHX for diabetic patients with gingivitis. | 1. Short follow-up period 2. Lack of a placebo group 3. Small sample size |
[190]/2016 | |
| Rosmarinus officinalis | RCT | Nc: 23 Ns: 23 [Patients with moderate chronic periodontitis] |
Gc: SRP + placebo Gs: SRP + EO mouth rinse [After three and six months, PD, CAL, BOP, and BI were evaluated at baseline; the subgingival plaque was sampled to evaluate principal periodontitis-associated bacteria] |
A significant improvement in CAL was observed after 3 and 6 months compared to the control group (p < 0.001). Following SRP, adding essential oils to mouthwashes decreases subgingival bacterial levels and improves clinical outcomes. | Small sample size | [191]/2016 |
| Salvadora persica | RCT | Nc: 47 Ns: 47 [School students] |
Gc: Fluoridated toothpaste + brushing Gs: SP sticks [Baseline, three-week, and 12-week PI measurements were conducted as well as saliva sampling] |
Plaque scores decreased statistically significantly in both groups (p = 0.007 and p = 0.001, respectively). After three months, the number of subjects with abundant S. sanguinis increased from zero to six. | 1. Small sample size 2. Short follow-up period |
[192]/2020 |
| RCT | N total: 44 [Pediatric patients receiving chemotherapy] |
Gc: Normal saline Gs: Persica oral drops [Oral conditions were recorded at baseline and after 8 and 15 days] |
A comparison of the severity of mucositis and oral health status of patients in both examination sessions did not reveal significant differences between treatment groups (p > 0.05). Mucositis, plaque accumulation, and gingival health improved statistically significantly in both treatment groups after 14 days following mouth rinse administration (p < 0.05). SP oral drops significantly improve plaque and gingival health | 1. Small sample size 2. Short follow-up period |
[193]/2020 | |
| RCT | N1: 12 N2: 13 [Patients with grade two or three plaque score] |
G1: Toothpaste with tea tree oil G2: Miswak-based toothpaste [PI was recorded at baseline and after 24 h of follow-up] |
Both herbal-based toothpastes reduced plaque scores, but when compared with G1, G2 resulted in significantly lower plaque scores. | 1. Short follow-up period 2. Lack of gingival inflammation assessment 3. Small sample size |
[194]/2018 | |
| Cross-sectional | N1: 115 N2: 93 N3: 79 |
G1: SP sticks (Miswak) G2: Conventional toothpaste/toothbrush G3: SP sticks + toothbrush [GI, OHI, and PI were recorded] |
G1 had a higher mean GI than G2, and G3 had a lower mean PI than G2. Between G1 and G2, the mean GI score was statistically significant (p = 0.001). Oral hygiene did not differ statistically significantly between groups. | Small sample size | [195]/2012 | |
| Terminalia chebula | RCT | N1: 30 N2: 30 N3: 30 [Healthy students] |
G1: T. chebula mouthwash G2: CHX G3: Distilled water At baseline and after 15 and 30 days, PI and GI were evaluated] |
At 15 and 30 days, PI and GI decreased significantly in G1 and G2 (p < 0.05). There was a significant reduction in G2, but not statistically significant in comparison to G1. The GI between G1 and G2 was not statistically significant (p = 0.837 for 15 days and p = 0.909 for 30 days) and PI (p = 0.592 at 15 days and p = 1.096 at 30 days). Using the T. chebula mouth rinse reduced dental plaque and gingivitis as effectively as CHX without the adverse effects of CHX. |
1. Small sample size 2. Short follow-up period |
[196]/2015 |
| RCT | N1: 26 N2: 26 N3: 26 [Patients with PI > 1.5] |
G1: 0.12% CHX G2: Terminalia chebula 10% mouthwash G3: Saline rinse [At baseline and after 7 and 14 days, PI and GI were evaluated] |
Clinical parameters were significantly reduced in both G1 and G2 even though there were no significant differences between them (p > 0.05). Studies have shown that Terminalia chebula mouth rinses reduce microbial plaques and gingival inflammations, as well as neutralize salivary pH levels. | 1. Small sample size 2. Short follow-up period |
[197]/2014 | |
| RCT | Nc: 40 Ns: 40 [Patients with chronic gingivitis] |
Gc: Oral prophylaxis alone Gs: Oral prophylaxis + gingival massage with T. chebula powder [At baseline and after one month, PI, GI, and BI were measured] |
Significant reductions in the PI, GI, and BI scores were observed after gum massage with T. chebula powder. Chronic gingivitis patients can benefit from T. chebula powder. | 1. Short follow-up period 2. No comparison with other studies 3. Small sample size |
[198]/2017 |
RCT: Randomized Clinical Trial; CAL: Clinical Attachment Level; Nc: Number of Subjects in the Control Group; Ns: Number of Subjects in the Study Group; Gc: Control Group; Gs: Study Group; GI: Gingival Index, GR: Gingival Recession; AGE: Aged Garlic Extract; SRP: Scaling and Root Planning; PD: Pocket Depth; CHX: Chlorhexidine; BI: Bleeding Index; UA: Uric Acid; OHI: Oral Hygiene Index; BOP: Bleeding on Probing; PCR: Polymerase Chain Reaction; EO: Essential Oil; GCF: Gingival Crevicular Fluid; PI: Plaque Index, SP: Salvadora persica.
4.11. Coffea canephora (Coffee)
The primary phenolic acid in coffee, chlorogenic acid, acts on human health due to its various effects, such as its antioxidant, anti-inflammatory, and antibacterial properties [199,200,201,202,203]. The safety of chlorogenic acid in rats and dogs is well documented, although there are no reports about humans, except for a potential allergic reaction [204]. Green coffee extract’s chlorogenic acid reduced the quantity of the oral bacteria S. mutans in a clinical experiment [205]. There is evidence that coffee extract is antibacterial and inhibits the activity of proteases produced by periodontitis-causing organisms, such as P. gingivalis [206].
4.12. Copaifera pubiflora
Copaifera pubiflora (Fabaceae-Caesalpinioideae) plants are indigenous to tropical areas of Western Africa and Latin America. Copaiba is the common name given to these plants in Brazil. The plants produce oléoresin as a byproduct of their secondary metabolism to protect themselves against animals, fungi, and bacteria [207,208,209,210,211,212,213,214,215]. Numerous studies have suggested that Copaifera can act against the bacteria responsible for endodontic infections and dental caries [208,209,210,213]. The antibacterial and antivirulence activity was tested against P. gingivalis and A. a by Abrão et al. These compounds were helpful as antimicrobials against periodontal pathogens [216].
4.13. Coptidis rhizoma
The medicinal plant Coptidis rhizoma (CR) is a member of the Ranunculaceae family [126]. Current investigations indicate that a chemical called berberine (BBR) is the principal active ingredient in CR extract [126]. CR and BBR have various antimicrobial, anti-inflammatory, antifungal, antidiarrheic, and other functions [126,217,218]. BBR therapy exerts anti-inflammatory action by inhibiting MMP-2 and MMP-9 activation, thus reducing periodontal tissue damage in periodontitis [219]. By reducing the synthesis of monocyte chemoattractant protein-1 from affected PDL cells, BBR might reduce leucocyte infiltration into the periodontium [220]. A rat periodontitis model was treated with oral BBR therapy for seven weeks, significantly reducing alveolar bone resorption [220,221]. BBR effectively reduced local and systemic inflammation in a periodontitis rat model by lowering TNF-α and IL-17 production and the number of IL-17A+ cells in the alveolar bone [222]. An in vivo experiment by Gu and colleagues on rats with ligation-induced periodontitis showed that BBR prevents alveolar bone loss caused by inflammation [223]. It was discovered that the enzyme PCSK9, which stimulates inflammatory reactions in the body, was a novel target of BBR’s anti-inflammatory effect. P. gingivalis-induced periodontitis was significantly reduced by BBR therapy by lowering PCSK9 production, which was also associated with the suppression of inflammatory responses [224]. Activated T cells in periodontitis produce the cytokine RANKL, which leads to osteoclastic activity and the destruction of alveolar bone [43]. The formation of RANKL is reduced by BBR, preventing bone loss in periodontitis [224].
4.14. Curcuma longa (Turmeric)
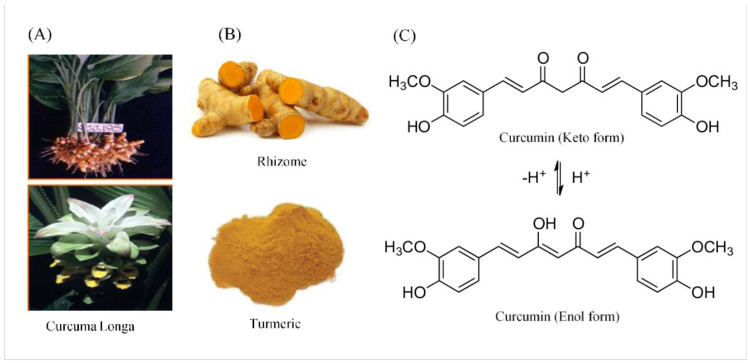
Southeast Asia is home to the ginger family member Curcuma longa. Curcumin’s capacity to inhibit LOX and COX activity in people is the basis for its well-documented anti-inflammatory action [225,226]. By controlling inflammatory pathways and activating transcription factors such as activator protein-1, mitogen-activated protein kinase (MAP Kinas), and NF-κB of activated B cells, curcumin has anti-inflammatory actions [176,227]. Additionally, evidence suggests that curcumin exerts healing effects on periodontal conditions and gingival inflammation by efficiently inhibiting the activation of inflammatory mediators [176,228]. Turmeric has been discovered to be a potent anti-inflammatory when used as mouthwash [176]. Curcumin combined with SRP has been shown to boost periodontal parameters. The periodontal indices are also better when curcumin is compared to ornidazole gel [175,176,228,229,230,231]. Kandwal et al. found no appreciable differences in the plaque or GI between CHX and curcumin gels [232]. A possible treatment for periodontitis is being explored by researchers thanks to curcumin’s ability to block the effects of Toll-like receptors [233]. Figure 7 demonstrates the chemical structures of various forms of curcumin. To determine its effects on alveolar bone loss, curcumin was studied in a meta-analysis study. The best results regarding the bone volume fraction and millimeters were obtained with chemically modified curcumin [234].
Figure 7.
The botanical source of turmeric (A). Powdered curcumin (B). Curcumin in enol and keto forms (C) [235].
4.15. Cymbopogon citratus (Lemongrass)
The medicinal plant Cymbopogon citratus is used to cure various illnesses [236]. According to reports, its chemical constituents, including phenol and flavonoids, exhibit antioxidant, anti-inflammatory, and antimutagenic properties [237]. Lemongrass EO can prevent bacterial growth at a concentration of ≤2% [238]. Hongkhunthian et al. found that it had antibacterial properties against periodontal pathogens, which formerly were resistant to tetracycline [239]. Gingivitis can be effectively treated with lemongrass EO mouthwash as a non-surgical adjunct to standard remedies [31,240]. Mucoadhesive polymer-based semi-solid formulations have been proposed to enhance contact quality and lengthen the dosage form’s persistence in the deep periodontal pocket, where conventional mouthwashes have difficulty penetrating [241]. The antioxidant properties of these EOs may account for their anti-clastogenic effects [242].
4.16. Eucalyptus globulus
The fever tree, or Eucalyptus globulus, belongs to the Myrtaceae family. Eucalyptus EOs have widespread use throughout the globe, are considered safe and non-toxic, and are approved for use as a food flavoring ingredient [243]. Eucalyptus leaves’ EOs, flavonoids, and tannins are considered responsible for their antioxidant, larvicidal, anthelmintic, antibacterial, and fumigant properties [244]. Oral pathogens such as streptococci are frequent among the oral bacteria that antibacterial ethanol extracts from the leaves of E. globulus target [245]. Additionally, the extracellular glucosyltransferase from these bacteria is inhibited by the extracts from producing insoluble glucan [246]. Ethanol extracts of E. globulus leaves also showed antibacterial action against two periodontal bacteria: P. gingivalis and P. intermedia. P. gingivalis, a periodontopathic bacterium, was significantly suppressed at modest concentrations [247]. A study demonstrated statistically significant positive effects on gingivitis outcome indicators with chewing gum containing 0.6% extract of E. globulus leaves [247].
4.17. Garcinia mangostana
The Guttiferae family includes Garcinia mangostana, more commonly referred to as mangosteen or the “queen of fruits”. It is an evergreen tree that originated in Southeast Asia [177]. The pericarp of this plant contains chrysanthemum, garcinone, sesquiterpenoids, gartanin, fructose, sucrose, tannins, and other beneficial chemicals [177]. Among its many properties, mangostana has antibacterial, antioxidative, anticancer, antiproliferative, and pro-apoptotic effects, and it exhibits aromatase inhibition [248,249]. Mangosteen is high in xanthones, a polyphenol molecule with significant biological properties. It is also high in flavonoids and anthocyanins [250,251]. Additionally, regular mangostana may be beneficial in preventing numerous pathological illnesses caused by oxidative stress and inflammation [252]. One study reduced the growth of P. gingivalis using an 80% ethanolic extract of mangostana pericarp gel at an MIC of 3.91 g/mL [253]. Researchers observed significant improvements in the periodontal parameters of patients with chronic periodontitis after locally applying 4% mangostana gel in their periodontal pockets [177]. According to a recent study, combining mangosteen and propolis extract significantly reduced the production of IL-6, IL-8, and PGE2 in immortalized human cells treated with P. gingivalis lipopolysaccharides. Furthermore, it stimulated human osteoblast-like cells to produce the highest bone-forming activity [254].
4.18. Glycyrrhiza glabra and Glycyrrhiza uralensis (Chinese Licorice)
Chinese and Ayurvedic medicine have used licorice root for centuries. The Glycyrrhiza species native to Europe and Asia contain licorice, a sweet, moist, alleviating plant [255]. Licoricidin and licorisoflavan A, the primary isoflavans from Chinese licorice (Glycyrrhiza uralensis), inhibited the proliferation of P. gingivalis, the generation of volatile sulfur compounds (VSCs), and the protease activity resulting in halitosis [256]. Research suggests that using licorice can prevent gingivitis and promote oral health. After pre-treatment of P. gingivalis with licorice root polysaccharides, Witttschier et al. discovered that these polysaccharides might inhibit bacterial binding from host cells. According to the study, polysaccharides from G. glabra inhibit bacterial adhesion [257]. When macrophages are activated with A. a and P. gingivalis, licorice extract demonstrates powerful anti-inflammatory activities by suppressing the periodontopathogen LPS-induced IL-1β, IL-6, IL-8, and TNF-α responses [258]. MMPs and inflammatory cytokines are well inhibited by licoricidin and licorisoflavan A, according to La et al.; thus, they can treat periodontitis [259]. The host immunological response and biofilm development by P. gingivalis are inhibited by licochalcone A [260]. Recently, the efficiency of licorice extract in inhibiting MMP production by host cells in patients with chronic periodontitis was established [261].
4.19. Hibiscus sabdariffa
In English, Hibiscus sabdariffa, often called roselle or red sorrel, belongs to the Malvaceae family and is an extensively cultivated plant in Southeast Asia and Central and West Africa. Tropical or subtropical climates favor its growth [262,263]. Many secondary metabolites are present in the calyx of roselle, such as flavonoids, alkaloids, saponins, and hibiscetin [264,265]. Roselle also contains delphinidin-3-sambubioside, which inhibits osteoclastogenesis by decreasing inflammatory mediator synthesis. Due to its anti-inflammatory and antibacterial properties, it may be used to address alveolar bone loss [266,267,268,269]. Roselle’s antibacterial potential may help prevent plaque development, leading to the prevention of further bone destruction [270,271,272,273]. Its anti-inflammatory properties have also been shown in previous research on its extract [274,275,276].
4.20. Inula viscosa (False Yellowhead)
The Asteraceae family includes I. viscosa (Dittrichia viscosa), which grows mainly in the Mediterranean region [277]. I. viscosa was demonstrated to exhibit minimal bactericidal concentrations (MBC) of 0.15 mg/mL against obligate anaerobes such as P. gingivalis, with minimum inhibitory concentrations (MIC) ranging from 0.07 mg/mL (P. gingivalis) to 2.50 mg/mL (S. sobrinus) against selected oral bacterial species [278]. In situ, early oral biofilms are not yet known to be susceptible to I. viscosa’s antibacterial effect. Scabies and skin irritations such as eczema are treated with I. viscosa as a folk medicine plant [279,280]. I. viscosa extract was reported to have anticancer, antioxidant, antifungal, antibacterial, and hypoglycemic properties [281]. In addition, I. viscosa tea decreased the adhering bacteria in the primary in vivo oral biofilm without harming the salivary pellicle’s ability to protect against acid [282]. I. viscosa, based on the research, shows significant potential to preserve oral health, especially when its diverse components come into contact with the oral mucosa.
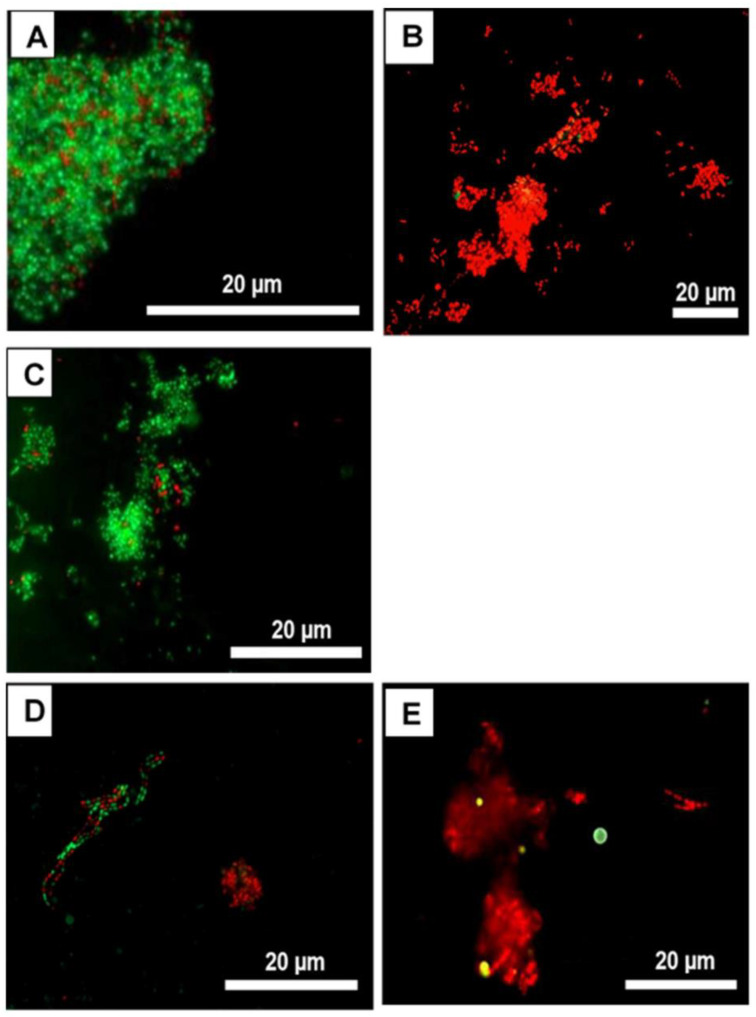
Using Inula viscosa extract to inhibit microbial adhesion in the oral cavity, Kurz et al. studied its antimicrobial effect. In this study, bovine enamel samples were attached to individual test splints for each participant. Fluorescence microscopy, colony-forming units (CFU), and vitality testing were used to assess microbiological parameters. Figure 8 displays live/dead samples of oral biofilms after applying I. viscosa extract at different concentrations. The untreated control and the DMSO-treated control (Figure 8) exhibited condensed accumulation of viable [39] bacteria. Almost no avital (red) bacteria were detected, and the bacterial arrangements were diverse. CHX and I. viscosa extract (Figure 8) significantly affected the oral biofilms. Initially, most adhering bacteria were red (avital) [283].
Figure 8.
Live/dead bacLight® fluorescent microscopy images. Avital fluoresces are in red, while vital bacteria are in green. (A) NaCl treatment as negative control, (B) CHX treatment as positive control, and (C) DMSO treatment as toxicity control, I. viscosa groups in concentrations: 10 mg/mL (D) and 30 mg/mL (E) [283].
4.21. Juglans regia
One of the most valuable medicinal plants is the walnut tree, or Juglans regia, which is beneficial in the therapeutic and cosmetic domains [284]. Various regional names are also used in other civilizations, including Derum, Dandasa, and Sewak. Multiple studies have examined the shells, kernels, seeds, and bark of Juglans regia, among other aspects [285]. The bark of Juglans regia may be used as a teeth-cleaning agent or a lip colorant in the cosmetic industry [286]. As a fibrous, resinous, and fragrant part, Juglans regia bark comes in several forms and sizes [287]. A variety of disorders can be treated with the bark of Juglans regia, which has anti-inflammatory, blood purification, anticancer, depurative, diuretic, and antioxidant properties [288]. Its antifungal and antibacterial properties have been proven to exert inhibitory action [289]. Juglans regia bark extracts showed broad-spectrum antibacterial efficacy against various pathogens, including Gram-positive and Gram-negative bacteria, in a dose-dependent manner [290]. Several studies showed Juglans regia’s antimicrobial activity (Table 1 and Table 2). Juglans regia contains terpenoids, alkaloids, steroids, phenols, and flavonoids used in oral hygiene products [290]. A recent study showed that juglone, a bioactive component of Juglans regia, inhibits P. gingivalis growth and antibiofilm action (S. sobrinus, A. viscosus, and S. mutans). In mice and rats, septa and leaf extracts demonstrated minimal toxicity [155,291]. Juglans regia is a good product for enhancing dental and oral health based on its antiplaque activity [291].
Table 2.
In vitro and in vivo investigations of plant-based antimicrobials in periodontal diseases.
| Natural Compound | Study Type | Samples Studied | Methods | Result(s)/Conclusion(s) | Ref./Year |
|---|---|---|---|---|---|
| Acacia nilotica | In vivo | Albino rabbits with ligature-induced periodontitis | G1: Distilled water G2: Positive control group G3: A. nilotica aqueous extract (dosage 300 mg/kg) G4: A. nilotica aqueous extract (dosage 500 mg/kg) G5: Amoxicillin (15 mg/kg) [CBC, ESR, serum creatinine, ALT, and AST were measured after 14 days] |
A. nilotica extract significantly cured periodontitis to a great extent after 14 consecutive days of oral administration. | [292]/2019 |
| Allium sativum | In vitro | P. gingivalis, F. nucleatum, A. a. | Gs: An aqueous extract of Allium sativum [Disc diffusion technique, microspindle dilution method, and assessment of MIC and MBC were performed] |
Allium sativum extract at 55.2% w/v produced inhibition zones of 17.3 ± 1.0, 30.3 ± 1.7, and 21.2 ± 2.3 mm with A. a, F. nucleatum, and P. gingivalis, respectively. MIC of 17.2, 1.1, and 4.3 mg/mL was obtained for A. a, F. nucleatum, and P. gingivalis, respectively. The MBC was 34.4, 1.1, and 8.6 mg/mL, respectively. Allium sativum aqueous extract may be a therapeutic alternative for treating periodontal disease based on the results obtained in this study. |
[293]/2021 |
| In vitro | L. acidophilus, S. aureus, S. sanguis, S. mutans, S. salivarius | Gs: Allium sativum bulb [MIC and MBC were measured] |
A. sativum bulbs are effective in treating periodontitis and dental caries. MBC value ranged from 60 ± 5 to 215 ± 7 mg/mL and MIC value ranged between 20 ± 2 and 120 ± 6 mg/mL. | [80]/2020 | |
| In vitro | P. gingivalis | G1: Aqueous garlic extract G2: 0.2% CHX [Groups were compared regarding MIC and MBC] |
A significant difference was observed between G1 and G2 (0.29 ± 0.1 μL; p < 0.001) regarding the MIC (1.21 ± 0.37 μL) and MBC (1.44 ± 0.67 μL) against P. gingivalis. As compared to G1 (20.1 ± 1.4 mm), G2 (27.3 ± 1.8 mm) showed a significantly larger inhibitory zone against P. gingivalis (p < 0.000). Garlic extracts performed well as antimicrobial agents against P. gingivalis; however, they were not superior to CHX as antimicrobial agents. | [294]/2019 | |
| Aloe barbadensis Miller | In vitro | C. albicans, S. mutans, L. acidophilus, E. faecalis, P. intermedia, P. anaerobius | G1: Aloe vera tooth gel G2: Pepsodent toothpaste G3: Colgate toothpaste [Zone of inhibition was measure] |
In preliminary tests, Aloe vera tooth gel and other toothpastes had similar antibacterial effects. S. mitis benefited from an enhanced antibacterial impact by Aloe vera tooth gel (p = 0.034). | [295]/2009 |
| Amphipterygium adstringens | In vitro | S. mutans, P. gingivalis, A. a, E. coli, C. albicans, C. dubliniensis | Gc: 0.12% CHX Gs: A methanolic extract of A. Adstringens [MIC, MBC, and total growth inhibition were measured] |
All microbial strains tested with methane extracts of A. adstringens exhibited antimicrobial activity between 0.125 and 63 mg/mL. MIC of S. mutans was 0.125 mg/mL, and MBC was 0.31 mg/mL, making it the most sensitive strain. The MIC and MFC of Candida strains were 0.4 and 1.6 mg/mL, respectively. An MIC/MBC of 37 mg/mL was observed for both P. gingivalis and E. coli. With an inhibitory concentration of 63 mg/mL, A. a and E. coli also exhibited similar results. An MBC of 2.4 mg/L was found for chlorhexidine. | [296]/2015 |
| Azadirachta indica, Syzygium aroticum, and Cinnamomum zeylanicum | Ex vivo | Actinobacillus sp. | Gc: Tetracycline and azithromycin (30 mcg/mL) Gs: Neem, clove, and cinnamon in aqueous and acetone solvents (2%, 4%, 6%, 8%, and 10%) [Zone of inhibition was measured] |
Actinobacillus sp. were inhibited at 10% concentration by aqueous extracts of clove and neem (24 and 22 mm, respectively). At the same concentration, aqueous cinnamon extracts displayed only a moderate inhibition zone (16 mm). Acetone extracts of neem and clove showed effective inhibition of Actinobacillus sp. (20 and 18 mm, respectively) compared with cinnamon, which showed a moderate inhibition zone (14 mm). Neem, clove, and cinnamon extracts could be used as an alternative treatment for chronic periodontitis. | [297]/2020 |
| Berberis vulgaris | In vivo | Rats with ligature-induced periodontitis | Gc: Cholisal gel Gs: A dental gel containing barberry extract. [Histopathology and ultrasound dopplerography were performed] |
Periodontitis can be effectively treated with a dental gel containing 0.015 mg/g of barberry root extract. | [130]/2020 |
| Cinnamomum burmanii | In vitro | An A. a. or E. coli LPS-stimulated macrophage model | Gs: Cinnamon bark aqueous extract [Cytokine production, binding of LPS cells, and PPAR-γ binding were studied] |
IL-6, TNF-α, and IL-8 secretion was reduced by the cinnamon fraction in a dose-dependent manner. A cinnamon fraction may have anti-inflammatory properties by reducing LPS binding to monocytes. A natural PPAR-γ ligand may exist in the cinnamon fraction as well. A cinnamon fraction has been shown to contain anti-inflammatory properties that can be used to treat periodontal disease due to its anti-inflammatory properties. | [298]/2021 |
| Cinnamomum zeylanicum | In vitro | P. gingivalis | Gs: Different concentrations of cinnamon with oil solvent (10, 50, 100, 250, 500, 750, and 1500 mg/mL) Gc: Amoxicillin, metronidazole, ciprofloxacin, amikacin, and gentamycin [MBC and MIC were evaluated] |
Cinnamon at an MIC value of 750 mg/mL inhibited bacteria, while cinnamon at an MIC value of 1500 mg/mL killed them. The antibacterial activity was, however, much weaker than that of common antibiotics (p < 0.001). The antimicrobial activity of cinnamon against the pathogen P. gingivalis was demonstrated in patients with chronic periodontitis with deep pockets. | [299]/2018 |
| In vitro | A. a, F. nucleatum, P. gingivalis, S. salivarius, S. mitis, and S. mutans | Gs: EO from cinnamon tree bark + cinnamaldehyde [MIC was measured] |
An MIC of 0.21–0.63 mg/mL was observed for cinnamon oil and 0.8–0.15 mg/mL for cinnamaldehyde against the tested bacteria. Changes in cell membranes were observed after two hours of exposure to the oil. Bacterial infections of the oral cavity can be prevented by cinnamon bark oil. | [300]/2013 | |
| Citrus sinensis | In vitro | P. gingivalis | Gs: Citrus sinensis [MIC, SI, and IC50 were measured] |
Citrus sinensis exhibited low cytotoxicity and good antibacterial activity. It demonstrated an IC50 value of 512 μg/mL. | [155]/2020 |
| Coffea canephora | In vitro | P. gingivalis | Gs: Coffee extract and chlorogenic acid [The inhibitory effect, protease activity, and viability of P. gingivalis were evaluated] |
Chlorogenic acid had an MIC of 4 mg/mL and an MBC of 16 mg/mL. When chlorogenic acid is applied above the MIC, the viability of P. gingivalis is inhibited for a longer period of time and the activity of the associated protease is significantly reduced. Different roast levels of coffee had no effect on the antibacterial activity of the extract. | [206]/2019 |
| Coptidis rhizoma | In vitro | A. naeslundii A. a, P. gingivalis, P. nigrescens, P. intermedia | Gs: C. rhizoma extract. [MIC and IC50 were measured] |
MICs of 0.031–0.25 mg/mL inhibited the growth of the mentioned bacteria, while MICs of 0.5–2 mg/mL inhibited the growth of Lactobacillus and Streptococcus. C. rhizoma extract inhibited periodontopathogenic bacteria. Clinical application of these results may be possible for treating periodontal diseases. | [126]/2000 |
| Curcuma longa | In vivo | Rats with induced periodontitis | Gs: Curcumin-loaded nanoparticles | The μCT analysis demonstrated significant attenuation of NF-kB activation and p38 MAPK activity resulting from curcumin local administration. Inflammatory bone resorption, osteoclast counts, and inflammation infiltrates were significantly reduced. Experimental periodontal disease was effectively treated with curcumin-loaded nanoparticles. | [301]/2018 |
| In vivo | Wistar rats with ligature-induced periodontitis | G1: Placebo G2: Resveratrol G3: Curcumin G4: Resveratrol + curcumin [Morphometric analysis of bone loss was performed histologically; TNF-α, IL-4, IFN-γ, and IL-1β were studied] |
As compared with the other groups, G1 showed greater bone loss than the other groups based on intergroup comparisons (p < 0.05). G2, G3, and G4 did not have different bone-loss values (p > 0.05). In G4, IL-1 β levels were lower than in G1 based on the immunoenzymatic assay of gingival tissue (p < 0.05). In comparison with G1, G2, and G3, G4 showed higher IL-4 values (p < 0.05). The levels concerning IFN-γ were only reduced by G2 (p < 0.05). Among the four groups, the TNF-α concentrations did not differ (p > 0.05). There was a reduction in alveolar bone loss due to resveratrol and curcumin. It was not found that these agents combined or synergized in any way. |
[302]/2017 | |
| Cymbopogon citratus | In vitro | S. mutans, S. epidermidis, Lactobacillus | Gc: Tetracycline Gs: Lemongrass EO [Inhibition zone measurement] |
The minimal inhibitory concentration of lemongrass EO was estimated to be 10 μL. A statistically significant zone of inhibition, and the antibacterial zone was more marked in Gs than Gc for S. mutans and S. epidermis (p < 0.001). Tetracycline had less antibacterial activity than lemongrass. Therefore, the herbal EO may be an adjunctive treatment for periodontitis or an alternative to tetracycline. |
[303]/2019 |
| In vitro | A. naeslundii, P. gingivalis | Gs: Cymbopogon citratus EO [MIC was measured] |
Based on the results, EO had MIC values of 0.44 and 0.22 mg/mL against A. naeslundii and P. gingivalis. Both reference strains and most clinical isolates, especially the tetracycline-resistant strains, are sensitive to Cymbopogon citratus EO. | [239]/2009 | |
| Eucalyptus globulus | In vitro | P. gingivalis, F. nucleatum, A. a | Gs: Eucalyptus globulus EOs [Their antioxidant capacity and MIC were measured] |
In the analyzed oils, the antioxidant activity was weak, although the antibacterial activity was significant, especially against F. nucleatum (MIC = 1.14 mg/mL) and P. gingivalis (MIC = 0.28 mg/mL). A potential therapeutic application for E. globulus EOs may be periodontal disease treatment. | [304]/2015 |
| Garcinia mangostana | In vivo | Wistar rats with administered A. a | G1: Tetracycline gel (0.7%) G2: Mucoadhesive patch G2: An extract of mangosteen peel applied to a mucoadhesive patch [Histopathological examinations were performed to quantify osteoblasts and osteoclasts] |
Osteoclasts and osteoblasts were significantly reduced in all groups by G3 (p < 0.05). Mangosteen peel extract inhibited osteoclasts and stimulated osteoblasts, thus preventing alveolar bone damage in periodontitis. | [305]/2021 |
| Glycyrrhiza uralensis | In vitro | P. gingivalis | Gs: Glycyrrhiza uralensis root extract [MIC and MBC were evaluated] |
It was found that the licorice root extract had antimicrobial activity against P. gingivalis at an MIC value of 62.5 μg/mL and an MBC value of 25 μg/mL. Biofilms of P. gingivalis were also affected by licorice root extract. A potential therapeutic application of licorice root extract could be for periodontal disease. | [306]/2017 |
| In vivo | GCF samples from patients with mild–moderate periodontitis | G1: Doxycycline G2: Licorice G3: Placebo [MMP-8 concentration was measured] |
There was a statistically significant difference between G1 and G2 and G3 in the mean MMP-8 concentrations (p < 0.001). A statistically significant difference was not detected between G2 and G1 in the mean MMP-8 concentration. Licorice extract is a powerful natural remedy for periodontitis and inflammation, as well as preventing MMPs from being released by the host cells. There were no side effects associated with the use of licorice extract. | [261]/2013 | |
| Glycyrrhiza glabra | In vitro | Pathogens responsible for plaque colonization and periodontitis | Gs: Glycyrrhiza uralensis bark extract [Zone of inhibition was measured] |
A potential antibacterial effect was observed for G. glabra against primary plaque colonizers and periodontal pathogens (ZOI = 9.2 ± 1.09 and 10.6 ± 0.54 mm, respectively). Statistically, there was no significant difference between G. glabra and standard antibiotics for periodontal pathogens. | [307]/2016 |
| Juglans regia | In vitro | G. adiacens, S. sciuri, Kocuria spp. | Gc: Ciprofloxacin (5 μg/mL) + cefotaxime (30 μg/mL) Gs: Crude aqueous extracts from Juglans regia (100 mg/mL, 250 mg/mL, 500 mg/mL). [Measurement of zone of inhibition] |
Compared to the other extracts, the 250 mg/mL extract was more effective. The extract showed the greatest impact on Kocuria spp. The extract’s active components increased biological activities, thus aiding in fighting bacterial infections. | [308]/2021 |
| In vitro | P. gingivalis | G1: Immature fruit ethanol extraction G2: Immature fruit methanol extraction G3: Woody parts ethanol extraction G4: Woody stems ethanol extraction G5: Woody stems methanol extraction [MIC, SI, and IC50 were measured] |
The MIC and SI of the five extracts of J. regia studied were as follows: G5 (MIC 64 μg/mL, SI > 16), SI > 16), G4 (MIC 64 μg/mL, SI > 16), G3 (MIC 32 μg/mL, SI > 32), G2 (MIC 32 μg/mL, SI > 32), and G1 (MIC 64 μg/mL. | [155]/2020 | |
| In vitro | S. mutans, S. salivarius, S. sanguis, S. aureus | Gc(+): Erythromycin 15 μg + tetracycline 30 μg Gc(-): Water Gs: Aqueous and ethanolic extracts of Juglans regia bark [MIC was measured] |
Aqueous and ethanolic extracts were found to be the most potent against S. sanguis and S. mutans, respectively. All strains of bacteria tested were significantly inhibited by the ethanolic extract. An antibacterial effect was not observed on S. mutans in the aqueous extract in comparison with the ethanolic extract. In comparison with the control, the aqueous extract significantly inhibited S. sanguis, S. salivarius, and S. aureus (p < 0.0001), In comparison with erythromycin, it did not affect S. mutans. The growth of oral bacteria was significantly inhibited by ethanolic and aqueous bark extracts of Juglans regia. | [290]/2013 | |
| Lippia sidoides | In vivo | Wistar rats with ligature-induced periodontitis | Gc(+): Diethylammonium diclofenac gel at 10 mg/g Gc(-): Saline gel Gs: Thymol gel [Histopathological analyses were performed] |
Compared with a control of saline gel, Gs reduced histopathological lesions in gingival tissue and reduced myeloperoxidase activity (p < 0.05). | [309]/2016 |
| Manuka honey | In vitro In vivo |
E. nodatum, S. mutans, C. rectus, S. sangiunis, A. a, P. gingivalis | In vitro section (G1: 0.2% CHX, G2: honey mouthwash, G3: saline) [MIC was measured] In vivo section: Plaque regrowth was simulated for four days. Four days after baseline, PI was measured |
Among the six microorganisms tested, honey mouth rinses inhibited their growth effectively. All test species showed the lowest MICs with CHX rinses over honey and saline rinses. As a result of in vivo testing, CHX and honey rinses inhibited or reduced plaque formation. Testing showed honey to be antibacterial and antiplaque. | [310]/2012 |
| Myristica fragrans | In vitro | Ten tissue samples from patients with chronic periodontitis undergoing a flap surgery | Gc: Doxycycline Gs: Myristica fragrans [Zone of inhibition and antiprotease activity were measured] |
Myristica fragrans, when added to the tissue sample, showed no zone of clearance compared to a significant zone of clearance of the tissue sample alone. Doxycycline demonstrated a small zone of clearance. Myristica fragrans possesses a better antiprotease activity as compared to doxycycline in vitro. | [311]/2016 |
| Myristica fragrans | In vitro | P. gingivalis | Gs: Myristica fragrans extract [Zone of inhibition was measured] |
A 13.5 mm inhibitory zone was found in nutmeg extract. Myristica fragrans inhibited Porphyromonas gingivalis | [312]/2016 |
| In vitro | P. gingivalis | Gs: Isolated malabaricone C from nutmeg (Myristica fragrans) [MIC was measured] |
Arg-gingipain was irreversibly inhibited by malabaricone C at 0.7 μg/mL, and P. gingivalis was selectively inhibited. | [313]/2014 | |
| Ocimum sanctum | In vitro | A. a, P. intermedia, P. gingivalis | Gc(+): Doxycycline Gc(-): Dimethyl formamide Gs: Ethanolic extract of Tulsi leaves (0.5%, 1%, 2%, 5%, and 10%) [Zone of inhibition was measured] |
It was found that Tulsi extracts showed similar inhibition zones to doxycycline at concentrations of 5% and 10%, with similar antimicrobial activity against A. a (p > 0.05). However, P. gingivalis and P. intermedia resisted Tulsi extract, showing significantly smaller inhibition zones (p < 0.05). Due to its antimicrobial properties, Tulsi may be used as a complementary therapy to standard periodontal care. |
[314]/2016 |
| In vivo | Wistar albino rats with ligature-induces periodontitis | G1: Control G2: Plain gel G3: 2% O. sanctum gel. [GI, PD, and morphometric analysis were performed] |
Inhibition of edema by 2% Tulsi (O. sanctum) gel was 33.66% at 24 h. The GI and PD demonstrated statistical significance. No significant differences were found between the groups based on the morphometric analysis. Tulsi extract 2000 mg/kg was not found to have any toxic effects when administered orally. The O. sanctum gel was effective in treating experimental periodontitis. | [315]/2015 | |
| Pistacia atlantica Kurdica | In vitro In vivo |
P. gingivalis Wistar rats |
Gs: EO extracted from the gum of Pistacia atlantica Kurdica [MIC and MBC were measured; histological analyses were performed] |
The experimental gel produced adequate wound healing and exhibited inhibitory and bactericidal activity against P. gingivalis. | [316]/2019 |
| Salvadora persica | In vitro | P. gingivalis and HSV-1 | Gs: Miswak raw extract [MIC, IC50, and MTT antiviral assays were measured] |
An MIC of 62.5 μg/mL was determined against P. gingivalis. A therapeutic index of 11.3 μg/mL was observed against HSV-1. A concentration of 18.6 μg/mL was calculated as the IC50. A concentration of 210 μg/mL caused cytotoxicity in 50% of Vero cells. The SP films significantly inhibit P gingivalis and the HSV-1. | [317]/2020 |
| In vitro | S. mutans, S. mitis, Candida albicans, L. acidophilus, P. intermedia, and Peptostreptococcus | Gc(+): CHX Gc(-): Distilled water Gs: Aqueous and alcoholic extracts of SP (200 μg/mL and 400 μg/mL) [MIC was measured] |
No significant results were obtained when Salvadora persica’s water extracts were tested, except for the minimum inhibitory effect against bacteria. Salvadora persica alcoholic extract exhibited relatively significant inhibitory effects. On all tested pathogens, alcoholic extract from SP showed antimicrobial activity. | [318]/2016 | |
| Satureja hortensis | In vitro |
A. a, P. gingivalis, P. micra, T. forsythia, F. nucleatum, P. Intermedia, P. nigrescens |
Gc: CHX Gs: Satureja hortensis EO [MIC and antibiofilm effects were measured] |
All tested bacteria were inhibited by S. hortensis EO, despite its low MIC value. All strains of bacteria tested showed inhibition of proliferation at 0.125 µL/mL. In tests against periodontal bacteria, S. hortensis EO had limited antibiofilm activity (0.01 µL/mL), inhibiting only P. nigrescens biofilm formation. | [319]/2009 |
| Syzygium aromaticum | In vitro | A. a, F. nucleatum, and P. intermedia | G1: Hydro-ethanolic extracts G2: Delipidated hydro-ethanolic extracts G3: Fresh extract [MIC, MBC, and zone of inhibition were measured] |
According to the MIC values, the tested organisms were antibacterial when tested at 6.25–25 mg/mL. On all bacteria subjected to the extract, the non-delipidated dry extract had a bactericidal effect. F. nucleatum was also shown to be bactericidal by delipidated extracts, as well as A. actinomycetemcomitans by fresh extracts. | [320]/2021 |
| In vitro | P. gingivalis | Gc(+): Tinidazole Gs: Syzygium aromaticum leaf essential oil (CLEO)-derived eugenol [MIC, MBC, CFU count, SEM, PI uptake, nucleic acid and protein leakage, biofilm quantification, and PCR were performed] |
The amount of eugenol in CLEO, 90.84%, was found to have antibacterial activity against P. gingivalis at a concentration of 31.25 μM. The presence of eugenol at different concentrations inhibited the formation of biofilms and reduced the preformed ones of P. gingivalis. | [148]/2017 | |
| Terminalia chebula | In vitro | S. mutans, A. a | Gc: Dimethyl sulfoxide (0.01%) Gs: Ethanol extract of Terminalia chebula (EETC) [MIC, susceptibility test, cytotoxicity assay, PGE2 assay, PCR, inflammation antibody array, protease array, ECM degradation, osteoclast formation, and pit formation were studied] |
By inhibiting the growth of bacteria, EETC also inhibited the stimulation of PGE2, COX-2, and inflammatory cytokines. In the osteoblasts, EETC stimulated lipopolysaccharide derived from dental plaque to inhibit bone resorption and inhibit osteoclast formation. | [321]/2017 |
| Vaccinium macrocarpon | In vitro | P. gingivalis | Gc: Phosphate-buffered saline Gs: Cranberry juice concentrate prepared as a non-dialysable material [Growth, adherence properties, and biofilm formation of P. gingivalis were studied] |
With cranberry concentrations exceeding 62.5 mg/mL, significant inhibition was observed (p < 0.05). With cranberry, P. gingivalis could not adhere effectively to collagen-, fibrinogen- or human serum-coated surfaces. Cranberry constituents may help prevent and treat periodontitis by preventing P. gingivalis from colonizing periodontal sites. | [322]/2006 |
| Vicia faba | In vitro | P. gingivalis | Gs: Vicia faba ethanolic and methanolic extracts [MIC, SI, and IC50 were measured] |
Vicia faba exhibited low cytotoxicity and antibacterial activity. | [155]/2020 |
| Vitis vinifera | In vivo | Rats with ligature-induced periodontitis | G1: Laboratory diet G2: GSE for eight weeks G3: GSE for six weeks G4: GSE for two weeks [Histopathological studies were performed to determine ICN, CAL, OD, IL-10, and TGF-β] |
GSE groups had lower ICN, higher CAL, and lower OD (p < 0.05). In the GSEs and GEs, IL-10 levels were higher (p < 0.05). In group B, periodontal ligament IL-10 levels were highest (p < 0.05). All groups had higher levels of TGF-ß in the gingival epithelium (p < 0.017). | [323]/2017 |
| Zingiber officinale | In vitro | P. gingivalis, P. endodontalis, P. intermedia | Gs: Ethanol and n-hexane extracts of ginger [MIC and MBC were measured] |
The two alkylated gingerols, [10]-gingerol and [12]-gingerol, inhibited oral pathogen growth at MICs of 6–30 μg/mL. At an MBC range of 4–20 μg/mL, these ginger compounds also killed oral pathogens, but not 5-acetoxy-[6]-gingerol, galanolactone, or 3,5-diacetoxy-[6]-gingerdiol. | [324]/2008 |
CBC: Complete Blood Count; ESR: Erythrocyte Sedimentation Rate; ALT: Alanine Transaminase; AST: Aspartate Transaminase; Gs: Study Group; Gc: Control Group; MBC: Minimum Bactericidal Concentration; EPS: Extracellular Polysaccharides; MFC: Minimum Fungicidal Concentration; MIC: Minimum Inhibitory Concentrations; CBEO: Cinnamomum zeylanicum Bark Essential Oil; PI: Propidium Iodide; SI: Selectivity Index; IC50: Half MIC; MAPK: Mitogen-Activated Protein Kinase; EO: Essential Oil; MEC: Ethanol Extracts of Garcinia mangostana Peel and Propolis; ZOI: Zone of Inhibition; SEM: Scanning Electron Microscope; GCF: Gingival Crevicular Fluid; CFU: Colony-Forming Units; PI: Plaque Index; MSE: M. alba Stem Extract; LPS: Lipopolysaccharide; hPDL: Human Periodontal Ligament; GI: Gingival Index; PD: Pocket Depth; PYC: Pycnogenol®; SP: Salvadora persica; HSV: Herpes Simplex Virus; MTT: (3-[4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide); CLEO: Syzygium aromaticum Leaf Essential Oil; EETC: Ethanol Extract of Terminalia chebula; PGE2: Prostaglandin E2; AC-PACs: A-Type Cranberry Proanthocyanidins; GSE: Grape Seed Extract; ICN: Inflammatory Cell Number; CAL: Connective Tissue Attachment Level; OD: Osteoclast Density.
4.22. Lippia sidoides
An aromatic Verbenaceae shrub known as Lippia sidoides is commonly found in the northeastern part of Brazil (where it thrives in semiarid conditions) and is referred to as “Pepper-Rosmarin” [325,326]. The EOs and other types of extracts derived from various parts of this plant contain monoterpenes, such as thymol and carvacrol, which have antimicrobial properties [181,327]. Traditional Brazilian medicine uses L. sidoides extracts as a topical antiseptic to treat skin and mucous membrane lesions [328,329]. The use of this plant in dentistry has reportedly produced satisfactory results, particularly in managing supragingival biofilm, as well as antiplaque and antigingivitis effects in humans [180,181,330,331] and animal investigations [332,333,334]. Researchers found that periodontal inflammation could be controlled by reducing pro-inflammatory cytokines such as IL-1β and TNF-α and suppressing gingival neutrophil infiltration by reducing myeloperoxidase activity [332].
4.23. Mangifera indica (Mango)
Mangifera indica (mango), composed of 10% mangiferin, belongs to the Anacardiaceae family. Various medicinal purposes have been attributed to this tropical and subtropical herb [335]. One of the mechanisms involved with mangiferin is that it is a glycosylated xanthone in nature with immunomodulatory and anti-inflammatory properties [336,337]. An experiment showed bone anti-resorption effects in lumbar vertebrae [338]. This plant is also effective against certain periodontal bacteria [339].
4.24. Manuka Honey
Since ancient times, honey has treated infections and other medical conditions [340]. Researchers revived interest in honey because of its antibacterial properties, particularly against antibiotic-resistant microorganisms and wound infections [341,342]. This led to manuka honey being approved to treat bacterial infections, ulcers, and burns [343,344,345]. Various proteinaceous substances, the hyperosmolarity effect, an acidic pH, bee defensin-1, hydrogen peroxide, flavonoids, and methylglyoxal, phenolic compounds [343,346,347] are all antibacterial components of honey. However, hydrogen peroxide has the most antimicrobial impact among most kinds of honey [348]. Manuka honey exhibits antibacterial activity, including biofilm and planktonic bacteria [349,350,351]. When cultured as planktonic bacteria [352,353], P. gingivalis [354] and A. a [355] are sensitive to manuka honey, whereas when developed as a biofilm, P. gingivalis is substantially more resilient [356]. English et al. discovered that chewing manuka honey strips reduced plaque development and gingival bleeding [184].
Safii et al. assessed manuka honey’s antibacterial activity against plaque-associated bacteria to evaluate its potential use in periodontal therapy. The differences between white clover honey and manuka honey were studied at neutral and natural pH levels using the MIC and MBC. Their MBCs were 12.5–50% (w/v) in manuka honey and between 6.3% to 50% (w/v) in clover honey. It took 18 h for both types of honey to be bactericidal. The pH-adjusted manuka honey retained its bactericidal activity (Figure 9), while the pH-adjusted clover honey exhibited variable bactericidal effects [357].
Figure 9.
An evaluation of manuka honey’s bactericidal effect (a) and effective manuka honey incubation period (b) [357].
4.25. Matricaria aurea and Matricaria chamomilla
The ancient medicinal plant Matricaria chamomilla (MTC) (chamomile) is an aromatic daisy from the Asteraceae family whose flower extracts and oil can be used to treat a variety of ailments [358]. It is rich in active constituents such as spiroether, flavonoids, coumarins, and terpenoids [358,359,360]. Spigenin, chamazulene, and bisabolol are the anti-inflammatory components of MTC extract, inhibiting NO generation, hyaluronidase, collagenase, cyclooxygenase prostaglandin E2, interleukins (1β, 6, 12), and TNF-α [361,362]. It has been shown that using MTC oral rinse improved plaque buildup, gingival irritation, and recurrent stomatitis [186,363,364,365,366]. Matricaria aurea (M. aurea), a plant native to Saudi Arabia that belongs to the genus Matricaria, has recently been studied for its therapeutic effects and potential to be a rich source of antimicrobials and antioxidants [367,368]. Matricaria chamomilla shares many similar chemical characteristics with this species [369]. Ahmad et al. revealed recently that this extract could be a source of numerous substances that may benefit the development of the next phase of the drugs used in treating chronic periodontitis [370].
4.26. Morus alba (M. alba)
Morus alba has traditionally been used to cure fevers, enhance eyesight, strengthen joints, and decrease blood pressure [371]. Mulberry fruit is also used to cure weakness, exhaustion, anemia, and premature hair greying, to replenish the blood, and to aid the kidneys. Mulberry root bark possesses anti-inflammatory, hypoglycemia, and antibacterial, antibacterial qualities, and it exhibits anti-inflammatory capabilities [372]. The ethanolic extract of the stems of the mulberry tree contains the most oxyresveratrol (2,3′,4,5′-tetrahydroxystilbene), whereas the ethanolic extract from the leaves contains the least [373]. M. alba’s oxyresveratrol has antioxidant and radical-scavenging properties. Several components, including prenylated flavonoids, may have anti-inflammatory effects by preventing the generation of nitric oxide (NO) [374]. In several studies, M. alba has been reported to be beneficial for treating periodontitis (Table 1 and Table 2).
4.27. Myristica fragrans (Nutmeg)
Myristica fragrans belongs to the family Myristicaceae and is frequently planted for spices on Malaysia’s Penang Island. Its four components are skin, flesh, seeds, and mace [375]. Myristica fragrans contains a variety of alkyl benzene derivatives [376,377]. Scientists from many fields have researched the chemical constituents of Myristica fragrans for their hypolipidemic and hypocholesterolemic, antimicrobial, antidepressive, and antioxidant characteristics, etc. [375]. The seed kernel possesses antithrombotic, antiplatelet, and antifungal properties, among others [378], while the mace has antipapillomagenic, anticarcinogenic [379], and anti-inflammatory activities [380].
Trimyristin, a chemical derived from the seeds of Myristica fragrans, has been shown to have antibacterial effects against Gram-negative and -positive bacteria [381]. In extracts of the flesh, seeds, and mace of Myristica fragrans, Zaleha Shafiei et al. found decreased bacterial concentrations [382]. Nutmeg’s essential oil contains the 5-lipoxygenase inhibitors limonene, β-pinene, α-pinene, and sabinene [383]. Limonene is a specific COX-2 inhibitor with notable inhibitory effects on PGE2 synthesis [384]. Terpene-4-ol, found in seed oils at 7.2% and mace oils at 23.6%, inhibits the production of IL-10, IL-1β, TNF-α, IL-8, and PGE2 [385]. Although it does not decrease IL-1β, α-pinene lowered the pro-inflammatory IL-6 produced in the mouse colon [386]. Sabinene, eugenol, and α-pinene prevent the synthesis of TNF-α [387]. Additionally, sabinene blocks IL-1β and IL-6 [383]. The presence of macelignan also inhibits the expression of MMP-9 [388]. Myristica fragrans may be a supplemental treatment for periodontitis due to its anti-inflammatory characteristics [389]. It is still necessary for further research to determine whether Myristica fragrans can act as an anticollagenolytic agent in periodontitis.
4.28. Ocimum sanctum (Tulsi)
Holy basil, or Tulsi or Ocimum sanctum, is an aromatic plant from the Labiatae family. This tiny plant is used in terms of its various parts for its medicinal properties. It has antioxidant abilities due to its COX2 inhibitory functions and the ability to protect against radiation poisoning and cataract formation [390,391,392]. In periodontal diseases, it has been demonstrated to have antimicrobial [393], antioxidant (by scavenging free radicals through phenolic compounds such as cirsilineol, apigenin, rosmarinic acid, and eugenol), and antigingivitis (due to the presence of compounds such as civsilineol, civsimavatine, isothymonin, apigenin, rosavinic acid, and eugenol) properties by inhibiting COX activity [394]. Tulsi’s methanolic and aqueous extracts are also pronounced analgesics, antipyretics, and anti-inflammatory agents [395,396]. The findings of a study on the antibacterial effects of Tulsi extract and doxycycline on periodontal microorganisms are shown in Figure 10. Tulsi extracts at 5% and 10% had doxycycline-like antibacterial action against A. a; however, P. gingivalis and P. intermedia had markedly greater resistance to Tulsi extract [314].
Figure 10.
Different concentrations of Tulsi on growth inhibition of P. gingivalis (a); 5% Tulsi against A.a (b); 10% Tulsi against A. a (c); and against P. intermedia (d) [314].
4.29. Pinus pinaster
Pycnogenol® (PYC), a standardized bark extract, is produced from the French maritime pine Pinus pinaster (previously Pinus maritime), which is found along the southwest French coast. Procyanidins constitute 65–75% of PYC extract. Procyanidins themselves are composed of catechins and epicatechins. Other compounds include phenolics, cinnamic acids, glycosides, and polyphenolic monomers [397]. Studies have shown that the extract and specific fractions have potential antioxidant properties in cultured cells, perfused organs, and when used in vivo [398,399]. The anti-inflammatory activities of the PYC compound have been demonstrated in human studies [397,400,401]. One study showed chewing gum with PYC may prevent gingival bleeding and plaque formation [402].
4.30. Piper marginatum and Ilex guayusa
Piper marginatum may be found in the Caribbean from Guatemala to Brazil. It belongs to the Piperaceae family. There are also names for this plant in Colombia, including “small cord” and “tooth healer” [403,404]. Against diseases in humans, animals, and plants, P. marginatum leaf extracts have shown antibacterial, antimycotic, and antiviral properties [403,404]. Guayusa is the common name for “Ilex guayusa”, an Aquifoliaceae plant that grows in tropical and subtropical climates [405]. When its leaves are consumed in infusions, they stimulate nerves and muscles and treat colds, respiratory problems, and gastrointestinal disorders [405]. Gamboa et al. reported the antibacterial properties of the extract of these plant leaves against microorganisms involved in periodontal diseases [406].
4.31. Pistacia lentiscus (Mastic Gum)
Pistacia lentiscus (PL), a member of the Anacardiaceae family and a species native to the Mediterranean region that is primarily found in Sardinia (Italy), is a wild-growing plant [407]. Pistacia lentiscus plant and processed products were traditionally used for their antiseptic, anti-inflammatory, and analgesic properties [407,408]. Pistacia lentiscus has antibacterial and antifungal properties throughout many applications [409]. Several volatile chemicals in mastic gum include α-pinene, β-caryophyllene, β-myrcene, and limonene [410]. Triterpenic acids are the principal active components of mastic gum, which has strong bactericidal properties, especially against H. pylori [411]. A recent study demonstrated that mastic gum is effective against anaerobic oral infections such as F. nucleatum, P. intermedia, and P. gingivalis [278]. Likewise, the resin of Pistacia lentiscus is used to make dental powder, which is used to eliminate bad breath and to clean teeth [14]. However, due to its limited solubility, it may be more effective for local application as opposed to mouthwash [412]. Additionally, Pistacia vera extracts have inhibited adhesion and glycolysis in oral streptococci [413]. An EO of PL leaves showed antimicrobial activity against periodontal bacteria. COX-1/2 and lipoxygenase were tested to investigate the anti-inflammatory activity, while the antioxidant capacity was assessed electrochemically and by an MTT assay. As a result of COX-1/2 inhibition, PL EO was shown to have anti-inflammatory activity in a dose-dependent manner (Figure 11) [414].
Figure 11.
Anti-inflammatory, oxidative stress, cytotoxicity, and antimicrobial characteristics of using Pistacia lentiscus essential oils against periodontal bacteria and Candida albicans (GK: gingival keratinocytes, PDLF: periodontal ligament fibroblasts; GF: gingival fibroblasts; and DOK: dysplastic oral keratinocytes) [414].
Experimentally induced periodontitis in rats was treated with EO of Pistacia atlantica kurdica gel and its effect on osteoclastogenic bone markers examined in a study by Azeez et al. H&E slides were processed from the mandibular central incisors 30 days after sacrifice, and histologically, the inflammation, osteoclasts, and PDL were examined. An ELISA was also used to measure the RANKL and IL-1β concentrations. Despite the presence of mild inflammation, the junctional epithelium was intact. In addition, the bone form had a regular shape and good density. As shown in Figure 12A, the PDL space was wide and filled with proliferating ligament tissue attached to a normal cementum layer. The control group filled the PDL space with less organized proliferating PDL tissues, as demonstrated in Figure 12B [415].
Figure 12.
A histological section of a rat incisor tooth and its surrounding periodontal tissues. Treatment control group (A): mild inflammatory cells (black arrows) with intact junctional epithelium and a stable bony surface with dense, well-formed bone and multiple blood vessels (red arrows) (H&E, scale bar 10 μm in section (a), and 20 μm in section (b,c)). Testing group (B): mild inflammatory cells (black arrowheads) with intact junctional epithelium and well-formed, dense bone (H&E, scale bar 10 μm in section (a), and 20 μm in section (b–d)) (AB; alveolar bone, PL; periodontal ligament and C; cementum) [415].
4.32. Psidium guajava (Guava)
This tiny tree may reach 20 feet in the Amazon rainforest [416]. The opposite, oblong leaves range in size from three to seven inches, and the undersides have noticeable veins. It has white flowers that are about an inch wide. Pear-shaped, oval, or spherical fruit are produced by P. guajava (guava). Two varieties exist, one with a thin shell and numerous seeds encapsulated in a solid pulp and one with a thick shell and few seeds [417]. Guava is an antiplaque, anti-inflammatory, antioxidant, and wound-healer agent. A paste made from fragile guava leaves is traditionally used to improve oral hygiene [418].
In particular, the flavonoids guaijaverin and quercetin have been linked to the antibacterial action of guava against bacteria [419,420,421]. The bark also has antimicrobial qualities because of tannins [422]. There has been evidence of the inhibitory effects of quercetin on P. gingivalis, P. intermedia, F. nucleatum, and Actinomyces species. Quercetin acts against bacteria by forming irreversible complexes that disrupt membranes and inactivate extracellular proteins [423,424]. As a result of its antiplaque properties, guaijaverin derived from guava leaves inhibits S. mutans and S. aureus [418,425,426,427]. Guaijaverin reduces the ability of pathogenic oral bacteria to attach to the tooth surface due to their reduced hydrophobicity [418] by binding to cell surface proteins and decreasing the total hydrophobicity of a cell, suggesting that guava might be developed as a natural antiplaque agent [418]. Researchers have shown that mouthwash containing an aqueous extract of the leaves is particularly efficient in preventing the proliferation of S. aureus and E. coli bacteria [428]. Gingivitis was reduced significantly after using guava leaf extract in a mouthwash [429]. Guava’s cytotoxic ability would make it more advantageous to use it as an additive in manufacturing products for oral health care [430]. Therefore, guava might complement conventional periodontal treatment with its antibacterial and antiplaque properties.
Guava’s anti-inflammatory properties come from its capacity to suppress prostaglandin, kinin, and histamine [431]. It was found that guava extract completely neutralized the cytotoxic, pro-inflammatory reaction caused by A. a leukotoxin [432]. In addition to their anti-inflammatory properties, guava leaf and stem extract lower CRP levels, modulating the inflammatory response [433]. Additionally, guava’s immunomodulatory activity on NF-kβ has been demonstrated [434]. Periodontal disease can be treated with guava by blocking NF-kβ, inducible NO synthase, and COX-2, suggesting an effective way to reduce inflammation-induced bone resorption [435,436].
Periodontal inflammation may also trigger excessive production of free radicals from neutrophils, resulting in tissue destruction [437]. Diets rich in antioxidants have been suggested to prevent and manage periodontal diseases [438,439]. Antioxidant micronutrients are vital to reduce excessive cytokine production and control oxidative and tissue damage [440]. In addition to being rich in vitamin C, guavas are also excellent antioxidants [441,442]. The quercetin, carotenoids, vitamin C, and polyphenols in guava are responsible for the antioxidant effect [438,443,444]. Consequently, guava might be used to treat periodontal diseases using antioxidant-based mechanisms.
Fibroblasts characterize periodontal connective tissue as the most common cell type. Collagen fibers compose the periodontal and gingival ligaments [445]. The effect of ascorbic acid on the extracellular matrix modulates procollagen gene expression, which leads to collagen synthesis [446]. Guava extracts may facilitate tissue healing [447], as they are a potential source of vitamin C and bioflavonoids [448]. In addition to maintaining the functional and structural integrity of the epithelium and the physiologic or metabolic parameters important for periodontal health, vitamin C also supports immunological function [449]. Swollen gums are recommended to be treated with a mouthwash produced from its root bark, while swollen and bleeding gums could be treated with a gargle made with a decoction of leaves [450].
4.33. Punica granatum (Pomegranate)
Punica granatum is the scientific name for pomegranate, which belongs to the Punicaceae family [451]. It is characterized by its spiny branches, lance-shaped glossy leaves, gray aged barks, large red or white flowers, ripe fruit that has a leathery and deep red skin five inches wide and with a grenade shape with a pointy calyx, and seeds that are enclosed in a white, membranous pericarp. A tart, red liquid surrounds each seed [452]. Due to their benefits, pomegranates are considered “a pharmacy unto themselves” [452]. Pomegranate offers a variety of possible health benefits, including those for bacterial, fungal, viral, immunological, vermifuge, stimulant, refrigerant, stomachic, styptic, diuretic, and helminthic infections [452]. Punicic acid, the primary component of pomegranate fatty acids, is an excellent anti-inflammatory agent, inhibiting prostaglandin synthesis [453]. Both cyclooxygenase (COX-1, COX-2) and lipoxygenase enzymes (critical enzymes in producing several inflammatory mediators) have been inhibited in vitro by cold-pressed pomegranate seed oil [452], and they are also inhibited by orally ingesting the extract of the pomegranate fruit rich in polyphenols [454]. Numerous polyphenolic substances, including ferulic acid, ellagic acid, chlorogenic acid, punicalagin, gallic acid, punicalin, epicatechin, caffeic acid, catechin, delphinidin, and rutin, are present in pomegranate fruit [455]. The fruit extract inhibits IL-1β-induced tissue destruction and the expression of matrix metalloproteinase (MMP) [452]. In addition, it reduces NO and PGE2 production [456]. In addition to the methods above, pomegranate’s anti-inflammatory effects may also result from its immunoregulatory effects on macrophages, T lymphocytes, and B lymphocytes [457]. It can prevent inflammation-induced bone resorption as another mechanism in treating periodontitis by blocking the NF-κB signaling pathway, generating many damaging factors [435,458]. Gingival bleeding was significantly reduced when pomegranate-based dentifrices were used [454]. Macrophages may be protected from oxidative stress and lipid peroxidation by the free radical-scavenging properties of pomegranate extract [452]. Pomegranate flavonoids, which have antioxidant potential, are thought to be responsible for the fruit’s antigingivitis effects [459].
Delivering Punica granatum extracts locally after SRP demonstrated that the pocket depths [433] and clinical attachments were improved, and the levels of IL-1β and IL-6 were reduced compared to the baseline [460]. In addition to enhancing oral health, pomegranates may lower the risk of gingivitis. When mouthwashes with dissolved pomegranate extract were used three times a day, antioxidant activity was increased, while aspartate aminotransferase activity (an indicator of cell injury, elevated in periodontitis) decreased [461,462]. A correlation exists between the saliva protein levels and plaque-forming bacterial content in periodontitis patients. The protein levels decreased significantly in saliva samples obtained after using pomegranate mouthwash, indicating antibacterial activity [463]. It has been demonstrated in vitro that pomegranate flavonoids have antibacterial properties [464]. In the formation of dental plaque, S. sanguis is thought to be the first colonizer [465] and was shown to be sensitive to pomegranate extract, which has a prevention effect comparable to CHX [466]. The antibacterial action of tannins is thought to be the main reason for their ability to enhance bacteriolysis and interfere with bacterial adhesion processes [466]. The extract of the pomegranate’s fruit bark has also shown promising results compared to CHX in inhibiting the plaque-former bacteria [467]. Compared to CHX, mouth rinses containing pomegranate hydroalcoholic extract reduced the plaque-forming bacteria’s colony-forming units by 84% in 60 healthy individuals. The plaque-forming bacteria’s ability to adhere to tooth surfaces decreased, suggesting that the extract can be beneficial in preventing and treating tooth plaque [468].
Gels containing 10% Punica granatum extract were shown to be ineffective in reducing dental plaque and gingivitis [451]. However, combined with mechanical debridement, pomegranate gel improved the clinical and microbiological markers in gingivitis [469]. In 92 subjects, the plaque, gingival, and bleeding indices improved significantly after using pomegranate toothpaste [470]. Its antibacterial properties may make pomegranate a great addition to traditional periodontal care as a plaque-fighting agent [471]. Patients with periodontitis have higher levels of H. pylori in deep pockets [472,473,474]. The antibacterial properties of pomegranate have also been demonstrated against Helicobacter [475]. The herpes virus-fighting properties of pomegranate extract have also been discovered [453]. Recent research suggested that herpes viruses may cause periodontal damage. Herpes viruses can induce and accelerate periodontitis by triggering the release of cytokines from the cells, disrupting host defense systems, and enhancing the virulence of local periodontal bacteria [458]. Thus, the antiviral properties of pomegranate make it a potential treatment for periodontitis. According to a recent study, pomegranate peel extract reduced the growth of Trichomonas tenax, and it can be used to treat acute ulcerative gingivitis [476]. Enteric probiotic microorganisms also benefit from pomegranate administration [477]. Several Bifidobacterium and Lactobacillus species have developed increased growth in their presence [478]. By affecting the development, adhesion, and colonization of the pathogenic bacteria responsible for periodontitis, decreasing the level of interleukins and MMPs, and improving the epithelium barrier’s role in resisting infections and bacterial invasion, these probiotic species have shown therapeutic properties in treating periodontitis [479,480,481,482]. Combined with methicillin-resistant staphylococcus aureus (MRSA)-fighting antibiotics, pomegranate extract has synergistic activity [483]. The added effect of pomegranate peel’s methanolic extract combined with ciprofloxacin was improved regarding the antibacterial activity [484]. Due to its ability to improve antibiotic sensitivity, pomegranate may be an effective treatment for periodontitis.
4.34. Rosmarinus officinalis
A little aromatic bush from the Lamiaceae family, Rosmarinus officinalis is also referred to as “Alecrim” (rosemary) in Brazil. It is a native of the Mediterranean region. It has several therapeutic potentials, including antioxidant, antimicrobial, and antifungal properties. Several components of its leaves, including terpenoids, flavonoids, phenols, and essential oils, contribute to the plant’s characteristics [33,55,485,486]. An investigation by Santoyo et al. showed the EOs responsible for the antimicrobial effect. They concluded that borneol produced better results in terms of antibacterial activity, followed by camphor and verbenone [487]. Lee et al. studied rosmarinic acid in bone cells. They found that it significantly induced alkaline phosphatase activity and enhanced mineralization in osteoblasts, suggesting that it could be used to prevent bone destruction [488].
4.35. Salvadora persica (Miswak)
This chewing stick is also called the Miracle Twig or Brushtree in the Muslim world, and it is also known as Miswak, Arak, Meswak, or the Toothbrush Tree [192]. The WHO’s recommendation allows the use of fibrous branches of Salvadora persica (SP) in oral hygiene because of its antimicrobial properties [489]. Salvadoraceae plants are found in this plant family [490]. The plant is mainly found in Africa’s arid and subtropical regions, the Middle East, and the Indian subcontinent [491]. As a predecessor of toothbrushes, SP was used by Arab and Islamic communities throughout the pre-Islamic and Islamic periods to clean the teeth and improve oral hygiene [492]. When used for brushing, SP’s beneficial properties for dental and oral health result from its mechanical action and pharmacologic active ingredients. An example of its chemically active compounds is tannins, which reduce plaque and periodontal disease by blocking the glucosyltransferase enzyme [493].
Additionally, several compounds found in its natural extracts, including vitamin C, potassium and sodium chloride, silica, salvadorine, salvadourea, saponins, and various minerals, have been linked to SP’s antibacterial, anti-inflammatory and antioxidant properties [493,494]. Studies have examined the efficacy and benefits that SP may have for periodontal care and the mechanism of action through its anti-inflammatory [493,495,496], antioxidant [494,495,496,497,498], and antibacterial effects [493,499,500], as well as the regenerative modulatory activity it has. SP’s clinical therapeutic impact on periodontal health and inflammation (Table 1 and Table 2) has been reviewed [192,193,194,195,501,502,503,504,505,506,507,508,509]. All trials that employed SP (Table 1) showed a substantial decrease in gingival inflammation and plaque buildup, suggesting the effectiveness of SP herbal supplementary treatment in treating or preventing inflammation and plaque, which are significant contributors to periodontal diseases [510]. Comparing the clinical outcomes of SP to those of chlorhexidine (CHX) as a principal mouthwash used in periodontal therapy [511] showed that SP provided equal or better results [507,508]. Moreover, Rezaei et al. discovered that herbal mouthwash considerably reduced GI more effectively than chlorhexidine (p < 0.05) [508]. However, Prasad et al. [507] reported that neither 0.2% chlorohexidine nor herbal mouthwash significantly affected GI or PI (p = 0.969 and 0.427, respectively).
SP extract showed potent antimicrobial and bactericidal effects on the periodontal pathogens examined, especially on Gram-negative bacteria, including P. gingivalis [512,513,514]. It was more effective in organic solvent extracts than in water extracts [21,318,515], and it had a coactive mechanism when administered with antibiotics [21] (Figure 13, Table 1 and Table 2). It has also been suggested that SP produces phytochemicals such as β-sitosterol, which may help prevent the formation of genotoxic bacteria compounds on teeth [516]. Moreover, dissolved anionic compounds may damage bacterial cell walls, inhibit oxygen absorption, and cause severe oxidative stress, leading to toxicity [516]. The essential and volatile oils and nonpolar chemicals in SP have also been demonstrated to buffer saliva pH, decrease bacterial activity, and break down plaque and biofilm [516]. According to reports, the antibacterial action was dose- and time-dependent, with the most potent effects occurring right after SP application [512]. Salvadora persica’s modes of action in periodontal disease are summarized in Figure 13.
Figure 13.
The chemotherapeutic effects of Salvadora persica on periodontal diseases [517].
In addition, SP’s diverse effects may affect periodontal therapy and encourage regeneration and repair [496,518,519,520]. According to researchers, SP may increase regenerative and stem cell activities by stimulating the expression of transforming growth factor-1 [496,521]. SP increased mesenchymal stem cells, fibroblast proliferation, and survival, and it reduced collagen degradation, a fundamental cause of periodontal diseases [519,520,522]. Other research found that applying SP extract to defects improved the healing capacity [497,518]. These findings imply that when SP is combined with periodontal therapy, regeneration may significantly speed up. In vivo and in vitro investigations are still required, especially regarding PDL-derived stem cells (Table 2).
4.36. Satureja hortensis (Summer Savory)
The annual herbaceous crop species known as summer savory (Satureja hortensis) is heavily branching, has linear leaves, and is a member of the Lamiaceae family. The principal biomolecules in S. hortensis extracts and EOs, including phenolic compounds, pyrocatechols, mucilage, tannins, flavonoids, steroids, volatile oils, acids, and gums, have a variety of antioxidant, antimicrobial, and anti-inflammatory potentials used in treating some systemic diseases [523]. According to Gursoy et al.’s research, S. hortensis EO, when applied in a dose safe for keratinocytes, had a marginal antibiofilm effect at subinhibitory levels. Still, it inhibited periodontal bacterial growth [319].
4.37. Syzygium aromaticum (Clove)
The dried blossom buds of the clove tree, Syzygium aromaticum (Myrtaceae family), are used to make the spice clove [524]. They are composed of approximately 14–20% EOs [524]. Many pathogens and bacteria that cause tooth decay and periodontal diseases are sensitive to clove [525]. Additionally, investigations have shown Syzygium aromaticum has antifungal, anticarcinogenic, antiallergic, and antimutagenic properties [147,526]. Among the main components of clove oil is eugenol, which is both an antioxidant and anti-inflammatory agent [524,527]. Clove is antibacterial against P. gingivalis and P. intermedia, two Gram-negative anaerobic infections of the periodontal pockets [525]. The NF-κB signaling pathway may be modulated, and IL-6, COX-2, and TNF-α are suppressed by clove, which may reduce periodontal inflammation [528,529,530,531]. The anti-inflammatory properties of cloves are also accompanied by their antioxidant properties [532,533]. Oxidative stress is joint in periodontal diseases, which could be reduced by its antioxidant potential [532,533,534]. Furthermore, Karmarkar et al. found that dried clove buds are high in eugenol and that its derivatives prevent bone loss, which are favorable properties for treating periodontal diseases [535].
4.38. Terminalia chebula
In addition to its anti-inflammatory and antioxidant actions, it has been claimed that the plant Terminalia chebula (family Combretaceae) has antibacterial and anticariogenic capabilities. The fruit of this plant have been utilized to prevent and treat dental caries, gingivitis, and stomatitis, and mouth rinses containing this agent have been shown to have antibacterial properties against oral pathogens [536]. Numerous phytochemical compounds, such as polyphenols, terpenes, anthocyanins, flavonoids, alkaloids, and glycosides, appear responsible for these positive effects [537]. An essential component of Ayurvedic medicine is the “Triphala” formulation [538]. Triphala’s bulk powder, pill, or liquid extract is used for oral and dental diseases [539].
Additionally, it can heal wounds when applied externally [540]. A key component of Triphala’s treatment for periodontal disease is its anticollagenase activity. Degradation of periodontal tissues occurs in part due to MMPs. Doxycycline inhibits collagenases and gelatinases more effectively than any other tetracycline. On the other hand, tetracycline is not an ideal medicine for long-term use. It is impossible to experience the same adverse effects of tetracycline-like side effects from using herbal extracts to treat periodontal disease. Triphala significantly inhibits PMN-type collagenase, especially when MMP-9 presents in high concentrations (1500 μg/mL) [541]. Triphala is also used as a periodontal mouthwash due to its broad range of activities. It is also suggested to gargle with Triphala for oral diseases. Compared to CHX after oral prophylaxis, Triphala mouthwash after SRP significantly reduced the PI, GI, and oral hygiene index with no sign of tooth discoloration [542]. When taken with 400 mg of metronidazole (TID), Triphala mouthwash ought to be applied BID. Reduced tooth mobility, PD, bleeding, sensitivity to heat and cold, and calculus formation were among the clinical indices that indicated improvements in the results, with minimum recurrence in all of the clinical parameters when taken in this manner, as opposed to those exhibited by 0.2% CHX (BID) + metronidazole 400 mg (TID) [543]. The findings of a study utilizing an ethanolic extract of Terminalia chebula and ampicillin to reduce bone resorption and inflammation brought on by dental plaque bacteria are shown in Figure 14. The extract suppressed the growth of A. a, dental plaque bacteria, and S. mutans [321].
Figure 14.
A comparison of the effect of Terminalia chebula ethanolic extract and ampicillin (Amp) on the growth of A. a, S. mutans, and other dental plaque bacteria (DPB#1, DPB#2, and DPB#3) [321].
4.39. Vaccinium macrocarpon
The native North American fruit, the cranberry (Vaccinium macrocarpon), has become increasingly popular due to its health benefits. The cranberry is a rich source of bioactive flavonoids, such as anthocyanins, flavanols, and proanthocyanidins, making it a potentially useful medicinal herb. It has been demonstrated that cranberries inhibit P. gingivalis and F. nucleatum colonization in the gingival crevice and prevent P. gingivalis from adhering to various proteins, including type I collagen, hence lowering bacterial coaggregation in periodontal disorders [322,544]. Several studies have also demonstrated that cranberries inhibit the red complex’s proteolytic activity [258]. The host macrophages release pro-inflammatory cytokines after being stimulated with lipopolysaccharides derived from bacteria (Figure 15). Bodet et al. argued in 2006 that these cytokines were inhibited [545] since the activator protein 1 (AP-1) regulation was decreased by a fraction when it interfered with the cellular signaling proteins [258,545]. MMPs and elastase, which are involved in the breakdown of tissues, are created by inflammation-producing cells [546]. With the arrest of MMP1 and MMP9 catalytic activity following treatment with the cranberry fraction, the phosphorylation of intracellular kinases was reduced and NF-kβ.P65 activity was suppressed [547]. In regulating fibroblast inflammatory responses in aggressive periodontitis, cranberry components prevent NF-κB and MMP-3 [548]. Tanabe studied the effects of A-type cranberry proanthocyanidins (AC-PACs) on bone resorption and osteoclast development. It was shown that AC-PACs at high concentrations were not toxic to osteoclastic cells. The physiology and maturation of osteoclastic cells can be affected by AC-PACs, which raises the possibility that they might prevent bone resorption [549].
Figure 15.
An illustration of flavan-3-ols and proanthocyanidin immunomodulatory activities in periodontitis [550].
4.40. Vicia faba
In the Mediterranean and the Far East, these plants are primarily used as cattle feed rather than human food or as soil nitrogen enhancers [551]. Vicia faba is divided into three types based on seed size: var. minor, var. quina, and var. major [552]. Fiber, vitamins, and antioxidants in broad fava beans lower triglycerides and cholesterol [553,554]. Proteins in Vicia faba reach their isoelectric point at pH 4.0, where their solubility is the lowest. However, as the pH increases, the solubility rises steadily until it reaches a maximum of 8.0 [555]. The primary components of Vicia faba are flavan-3-ols, including catechin and epicatechin, flavonols, and flavones, and all of which contribute to the plant’s anti-inflammatory and antioxidant capabilities [556]. Despite preliminary study findings, several publications provide data about periodontal disorders [557].
4.41. Vitis vinifera
Natural antioxidants are abundant in the byproducts of Vitis vinifera [202]. Grapes are one of the most significant fruit crops in the world, producing more than 79 million tons of fruit annually [202]. In winery residues, bioactive chemicals have been discovered to possess anti-inflammatory, antioxidant, and cytoprotective properties [558,559]. Most of the health advantages of vine extracts are attributed to phenolic compounds, which are regarded as the most significant active chemicals. Numerous studies have noted that phenolic compounds have antibacterial, antifungal, and antiviral activity, either directly against oral infections or by inhibiting virulence factors [560,561].
Additionally, it has been demonstrated that V. vinifera extracts can control the bacterial-induced inflammatory response and oxidative stress imbalance in periodontal diseases [562]. Grape seed extract (GSE) is the most researched byproduct of V. vinifera. Furiga et al. discovered that GSE had antibacterial action against two anaerobic bacteria linked to periodontal diseases [563]. It has been demonstrated that human PDL cells exposed to P. gingivalis may produce fewer pro-inflammatory cytokines when treated with the phenolic acid resveratrol [564], which was found to decrease IL-1β, IL-6, IL-8, and TNF-α levels as well as alveolar bone loss in an animal model of periodontitis [302]. Resveratrol significantly improves periodontal health, according to research conducted on human volunteers to evaluate the benefits of resveratrol supplementation in diabetic patients [565].
Additionally, Özden et al. revealed GSE as an essential player in periodontal inflammatory processes using histomorphometric and immunohistochemical analyses [323]. Inflammation levels were lower, connective tissue levels were improved, and bone healing was higher [323]. Cranberry proanthocyanidins have also helped treat periodontitis by inhibiting MMPs and pro-inflammatory cytokines, biofilm formation, P. gingivalis adhesion to various proteins, and growth of pathogenic bacteria in periodontal pockets [566].
4.42. Zanthoxylum armatum
Zanthoxylum armatum is a member of the Rutaceae family. Its distribution is from Kashmir to Bhutan, at up to 2500 m in altitude [567]. Several species of Zanthoxylum are used to improve oral health [568,569]. Several plant parts of Zanthoxylum species contain abundant alkaloids, sterols, phenolic acids, lignins, and terpenoids. Zanthoxylum armatum bark powder relieves gingival bleeding when mixed with honey. Inflammatory tooth pain is also treated using an extract from Zanthoxylum, which is known as the toothache tree [570]. EOs isolated from Zanthoxylum armatum leaves were active against all the tested bacterial strains [571]. Due to their terpenoids, Zanthoxylum armatum EOs may have antibacterial properties [572].
4.43. Zingiber officinale
One medicinal plant used in many nations is ginger (Zingiber officinale), which belongs to the Zingiberaceae family [573]. Ginger’s active compounds, including β-bisabolene, shogaol, gingerol, and paradol, have glycemic-controlling, anti-inflammatory, antioxidant, anticancer, and antiobesity properties [574,575]. The effectiveness of herbal mouthwash containing hydroalcoholic extracts of Z. officinale, Rosmarinus officinalis, and Calendula officinalis was recently investigated and compared to CHX by Mahyari et al. The polyherbal mouthwash had similar effectiveness to CHX [576].
5. Constraints
Various factors, including anecdotes, cultural practices, societal repercussions, inaccurate information about health, cost, availability, lucrative businesses, innovative advertising, and consumer choice, influence the use of herbal supplements. Natural medicine is used widely, irrespective of geographical and demographic differences. However, the encouraging outcomes of most herbal supplements in contemporary treatment are few, conflicting, or inconsistent [577]. Instead, there is more evidence that herbal supplements may have potentially harmful effects, leading to public health concerns [578].
Several promising studies have shown the efficacy of herbal products in treatments; however, many of them have not been tested and are relatively unmonitored [578]. Due to the lack of knowledge regarding their modes of action, potential adverse effects, contraindications, and interactions with conventional pharmaceuticals and foods, it would not be easy to establish a rationale for the safe usage of these agents. It is, therefore, challenging to determine which therapies are the safest and most effective. Herbal products could also be unsafely promoted due to inadequate quality controls, labeling, and patient education. A regulatory flaw exists regarding herbal products, as premarketing approval is not required by the Food and Drug Administration [326], unlike the situation for conventional pharmaceuticals such as prescription and OTC treatments [577]. Regulatory authorities must establish appropriate measures to protect public health, as safety remains a significant concern with traditional remedies. A safe and adequate quality of all-natural medicines is essential to accomplish this goal.
On the other hand, despite frequent public use of herbal medications, communication between physicians and patients is still limited. Physicians generally have limited knowledge of herbal supplements, and patients frequently conceal or underreport their usage of herbal supplements out of concern that their doctors would not be interested [579]. In addition, practitioners usually emphasize potential toxicities one-sidedly, even though determining the toxic properties of natural preparations is often challenging because patients usually self-medicate and are reluctant to disclose their information.
Most adverse reactions associated with plant-based products are associated with hepatotoxicity [580], often from the simultaneous use of other hepatotoxic agents, such as acetaminophen and nonsteroidal anti-inflammatory agents or hepatotoxic botanical components [581]. However, no toxic effects were reported by the reviewed investigations in this study. The list of medicinal plants presented here were declared safe according to the corresponding investigations, possibly due to the nature of the natural compounds and their low dosage in different oral hygiene aids, such as mouthwashes, toothpaste, chewing gums, gels, and patches. However, evidence concerning the safety of herbal-based oral hygiene aids in dentistry is still unsatisfactory.
Physicians and healthcare providers must increase their knowledge of these products, pharmacokinetics, and potential interactions with conventional medications. There should not be generalizations regarding herbal supplements’ effectiveness and safety in society. By cooperating with educational institutions, scientific organizations, mainstream media, and legislators, healthcare professionals can significantly contribute to increasing herbal supplements’ overall safety. Additional clinical research, formal education, legislative modifications, and international partnerships are essential for reducing the overall burden of herbal supplements in clinical settings.
6. Conclusions
This paper aimed to review the currently available information on the effects of herbal drugs used as supplements or treatments for periodontitis. According to the review’s findings, herbal medicine is an effective alternative to contemporary medicine. There is an abundance of evidence that pure phytochemicals, essential oils, and plant extracts have the potential to be converted into medications that can be used to treat or prevent periodontitis. More studies on the safety and efficacy of these products are required to ascertain whether they have medicinal value, either alone or in combination with conventional treatment options, which can help reduce the overall burden of oral diseases globally, although the numerous clinical trials for these products are encouraging.
Acknowledgments
The authors would like to acknowledge the colleagues who gave valuable comments.
Abbreviations
AC-PACs: A-Type Cranberry Proanthocyanidins; AGE: Aged Garlic Extract; BBR: Berberine; BI: Bleeding Index; BID: Twice Daily; BOP: Bleeding on Probing; CAL: Clinical Attachment Level; CBEO: Cinnamomum Bark Essential Oil; CFU: Colony-Forming Unit; CHX: Chlorhexidine; CR: Coptidis rhizoma; CS: Camellia sinensis; EO: Essential Oil; GE: Garlic Extract; GI: Gingival Index; GSE: Grape Seed Extract; LPS: Lipopolysaccharide; MBC: Minimal Bactericidal Concentration; MDR: Multidrug-Resistant; MIC: Minimum Inhibitory Concentration; MMP: Matrix Metalloproteinase; MRSA: Methicillin-Resistant Staphylococcus Aureus; MTC: Matricaria chamomilla; NO: Nitric Oxide; PD: Pocket Depth; PDL: Periodontal Ligament PI: Plaque Index; PL: Pistacia lentiscus; PYC: Pycnogenol®; SP: Salvadora persica; SRP: Scaling and Root Planning; TID: Three Times Daily; VSC: Volatile Sulfur Compounds.
Author Contributions
Conceptualization: A.H. and H.T.; methodology: S.A.M. and H.T.; software: S.A.M. and H.T.; validation: A.H.; formal analysis: S.A.M. and H.T.; investigation: H.T. and S.A.M.; resources: A.H. and S.A.M.; data curation: H.T. and S.A.M.; writing—original draft preparation: S.A.M., H.T. and A.H.; writing—review and editing: H.T. and S.A.M.; visualization: S.A.M.; supervision: A.H. and H.T.; project administration: A.H. All the authors have reviewed and accepted the final draft and are in charge of the content and similarity index of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
This article is a review and does not contain any studies with humans or animals performed by the authors.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Petersen P.E., Baehni P.C. Periodontal health and global public health. Periodontology 2000. 2012;60:7–14. doi: 10.1111/j.1600-0757.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- 2.Flemmig T.F. Periodontitis. Ann. Periodontol. 1999;4:32–38. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- 3.Eke P.I., Dye B.A., Wei L., Thornton-Evans G.O., Genco R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 4.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J., Marcenes W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartold P.M., Van Dyke T.E. Van Dyke, Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000. 2013;62:203–217. doi: 10.1111/j.1600-0757.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 7.Darveau R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 8.Boyd R.L., Leggott P., Quinn R., Buchanan S., Eakle W., Chambers D. Effect of self-administered daily irrigation with 0.02% SnF2 on periodontal disease activity. J. Clin. Periodontol. 1985;12:420–431. doi: 10.1111/j.1600-051X.1985.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 9.Listgarten M.A., Lindhe J., Hellden L. Effect of tetracycline and/or scaling on human periodontal disease. Clinical, microbiological, and histological observations. J. Clin. Periodontol. 1978;5:246–271. doi: 10.1111/j.1600-051X.1978.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 10.Slots J. Subgingival microflora and periodontal disease. J. Clin. Periodontol. 1979;6:351–382. doi: 10.1111/j.1600-051X.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 11.Dzink J.L., Socransky S.S., Haffajee A.D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J. Clin. Periodontol. 1988;15:316–323. doi: 10.1111/j.1600-051X.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 12.Wara-aswapati N., Pitiphat W., Chanchaimongkon L., Taweechaisupapong S., Boch J.A., Ishikawa I. Red bacterial complex is associated with the severity of chronic periodontitis in a Thai population. Oral Dis. 2009;15:354–359. doi: 10.1111/j.1601-0825.2009.01562.x. [DOI] [PubMed] [Google Scholar]
- 13.Newman M.G., Takei H., Klokkevold P.R., Carranza F.A. Carranza’s Clinical Periodontology. Elsevier Health Sciences; London, UK: 2011. [Google Scholar]
- 14.Chandki R., Banthia P., Banthia R. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011;15:111–114. doi: 10.4103/0972-124X.84377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genco R.J., Borgnakke W.S. Risk factors for periodontal disease. Periodontology 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 16.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 17.Kwon T., Lamster I.B., Levin L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021;71:462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bui F.Q., Almeida-da-Silva C.L.C., Huynh B., Trinh A., Liu J., Woodward J., Asadi H., Ojcius D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czerniuk M.R., Surma S., Romańczyk M., Nowak J.M., Wojtowicz A., Filipiak K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis–Plaque Destabilization? From the Teeth to a Coronary Event. Biology. 2022;11:272. doi: 10.3390/biology11020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spellberg B., Bartlett J.G., Gilbert D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saquib S.A., AlQahtani N.A., Ahmad I., Kader M.A., Al Shahrani S.S., Asiri E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics. 2019;8:89. doi: 10.3390/antibiotics8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelmagyd H.A.E., Shetty S.R., Al-Ahmari M.M.M. Herbal medicine as adjunct in periodontal therapies—A review of clinical trials in past decade. J. Oral Biol. Craniofac. Res. 2019;9:212–217. doi: 10.1016/j.jobcr.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer R.G., Lira R., Jr., Retamal-Valdes B., Figueiredo L.C., Malheiros Z., Stewart B., Feres M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral Res. 2020;34((Suppl. S1)):e026. doi: 10.1590/1807-3107bor-2020.vol34.0026. [DOI] [PubMed] [Google Scholar]
- 24.Olsvik B., Tenover F.C. Tetracycline resistance in periodontal pathogens. Clin. Infect. Dis. 1993;16((Suppl. S4)):S310–S313. doi: 10.1093/clinids/16.Supplement_4.S310. [DOI] [PubMed] [Google Scholar]
- 25.Pajukanta R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol. Immunol. 1993;8:325–326. doi: 10.1111/j.1399-302X.1993.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 26.Slots J. Selection of antimicrobial agents in periodontal therapy. J. Periodontal. Res. 2002;37:389–398. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 27.Nascimento G.G., Locatelli J., Freitas P.C., Silva G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000;31:247–256. doi: 10.1590/S1517-83822000000400003. [DOI] [Google Scholar]
- 28.Rossiter S.E., Fletcher M.H., Wuest W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandita V., Patthi B., Singla A., Singh S., Malhi R., Vashishtha V. Dentistry meets nature-role of herbs in periodontal care: A systematic review. J. Indian Assoc. Public Health Dent. 2014;12:148–156. doi: 10.4103/2319-5932.144784. [DOI] [Google Scholar]
- 30.Kaur A., Kapoor D., Soni N., Gill S. Phytodentistry—A boon. Arch. Dent. Med. Res. 2016;2:35–41. [Google Scholar]
- 31.Anand B. Herbal therapy in periodontics: A review. J. Res. Pharm. Sci. 2017;3:1–7. [Google Scholar]
- 32.Khameneh B., Iranshahy M., Soheili V., Fazly Bazzaz B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019;8:118. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva L.N., Zimmer K.R., Macedo A.J., Trentin D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016;116:9162–9236. doi: 10.1021/acs.chemrev.6b00184. [DOI] [PubMed] [Google Scholar]
- 34.Gyllenhaal C., Kadushin M.R., Southavong B., Sydara K., Bouamanivong S., Xaiveu M., Xuan L.T., Hiep N.T., Hung N.V., Loc P.K., et al. Ethnobotanical approach versus random approach in the search for new bioactive compounds: Support of a hypothesis. Pharm. Biol. 2012;50:30–41. doi: 10.3109/13880209.2011.634424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox P.A., Balick M.J. The ethnobotanical approach to drug discovery. Sci. Am. 1994;270:82–87. doi: 10.1038/scientificamerican0694-82. [DOI] [PubMed] [Google Scholar]
- 36.Barbieri R., Coppo E., Marchese A., Daglia M., Sobarzo-Sánchez E., Nabavi S.F., Nabavi S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Ramesh A., Varghese S.S., Doraiswamy J.N., Malaiappan S. Herbs as an antioxidant arsenal for periodontal diseases. J. Intercult. Ethnopharmacol. 2016;5:92–96. doi: 10.5455/jice.20160122065556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albandar J.M., Susin C., Hughes F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018;45((Suppl. S20)):S171–S189. doi: 10.1111/jcpe.12947. [DOI] [PubMed] [Google Scholar]
- 39.Papapanou P.N., Sanz M., Buduneli N., Dietrich T., Feres M., Fine D.H., Flemmig T.F., Garcia R., Giannobile W.V., Graziani F., et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018;45((Suppl. S20)):S162–S170. doi: 10.1111/jcpe.12946. [DOI] [PubMed] [Google Scholar]
- 40.José Luis M.-C., Viridiana Elizabeth H.-R., Oscar Eduardo G.-H., Francisca C.-R., María Isabel C.-R., Karla Mariana C.-R., Lizbeth D.-A. In: Pathogenesis of Periodontal Disease, in Periodontal Disease. Nermin Mohammed Ahmed Y., editor. IntechOpen; Rijeka, Croatia: 2019. Chapter 1. [Google Scholar]
- 41.Hajishengallis G., Chavakis T., Lambris J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000. 2020;84:14–34. doi: 10.1111/prd.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajishengallis G., Reis E.S., Mastellos D.C., Ricklin D., Lambris J.D. Novel mechanisms and functions of complement. Nat. Immunol. 2017;18:1288–1298. doi: 10.1038/ni.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukasaki M. RANKL and osteoimmunology in periodontitis. J. Bone Miner. Metab. 2021;39:82–90. doi: 10.1007/s00774-020-01165-3. [DOI] [PubMed] [Google Scholar]
- 44.Belibasakis G.N., Bostanci N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 45.Figueredo C., Lira R., Jr., Love R. T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. Int. J. Mol. Sci. 2019;20:3949. doi: 10.3390/ijms20163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kourtzelis I., Mitroulis I., von Renesse J., Hajishengallis G., Chavakis T. From leukocyte recruitment to resolution of inflammation: The cardinal role of integrins. J. Leukoc. Biol. 2017;102:677–683. doi: 10.1189/jlb.3MR0117-024R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gingivitis P.I. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. J. Periodontol. 2001;72:1790–1800. doi: 10.1902/jop.2001.72.12.1790. [DOI] [PubMed] [Google Scholar]
- 49.Walker C.B. The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000. 1996;10:79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 50.Sanz I., Alonso B., Carasol M., Herrera D., Sanz M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012;12((Suppl. S3)):76–86. doi: 10.1016/S1532-3382(12)70019-2. [DOI] [PubMed] [Google Scholar]
- 51.Bonito A.J., Lux L., Lohr K.N. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: A systematic review. J. Periodontol. 2005;76:1227–1236. doi: 10.1902/jop.2005.76.8.1227. [DOI] [PubMed] [Google Scholar]
- 52.Haffajee A.D., Socransky S.S. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontology 2000. 2006;42:7–12. doi: 10.1111/j.1600-0757.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 53.Chitme H.R., Chandra M., Kaushik S. Studies on anti-diarrhoeal activity of Calotropis gigantea R. Br. in experimental animals. J. Pharm. Pharm. Sci. 2004;7:70–75. [PubMed] [Google Scholar]
- 54.Kim H.-S. Do not put too much value on conventional medicines. J. Ethnopharmacol. 2005;100:37–39. doi: 10.1016/j.jep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Palombo E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid.-Based Complement. Altern. Med. 2011;2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singhal R., Agarwal V., Rastogi P., Khanna R., Tripathi S. Efficacy of Acacia arabica gum as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A randomized controlled clinical trial. Saudi Dent. J. 2018;30:53–62. doi: 10.1016/j.sdentj.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirtikar K., Basu B. Indian medicinal plan Leader road. Allahabad India. 1984;2:1347–1348. [Google Scholar]
- 58.Clark D., Gazi M., Cox S., Eley B., Tinsley G. The effects of Acacia arabica gum on the in vitro growth and protease activities of periodontopathic bacteria. J. Clin. Periodontol. 1993;20:238–243. doi: 10.1111/j.1600-051X.1993.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 59.Pradeep A., Agarwal E., Bajaj P., Naik S., Shanbhag N., Uma S. Clinical and microbiologic effects of commercially available gel and powder containing Acacia arabica on gingivitis. Aust. Dent. J. 2012;57:312–318. doi: 10.1111/j.1834-7819.2012.01714.x. [DOI] [PubMed] [Google Scholar]
- 60.Pradeep A., Happy D., Garg G. Short-term clinical effects of commercially available gel containing Acacia arabica: A randomized controlled clinical trial. Aust. Dent. J. 2010;55:65–69. doi: 10.1111/j.1834-7819.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- 61.Mnisi C.M., Mlambo V. Influence of harvesting site on chemical composition and potential protein value of Acacia erioloba, A. nilotica and Ziziphus mucronata leaves for ruminants. J. Anim. Physiol. Anim. Nutr. 2017;101:994–1003. doi: 10.1111/jpn.12535. [DOI] [PubMed] [Google Scholar]
- 62.Kaur K., Michael H., Arora S., Härkönen P., Kumar S. In vitro bioactivity-guided fractionation and characterization of polyphenolic inhibitory fractions from Acacia nilotica (L.) Willd. ex Del. J. Ethnopharmacol. 2005;99:353–360. doi: 10.1016/j.jep.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Singh R., Singh B., Singh S., Kumar N., Kumar S., Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. In Vitro. 2008;22:1965–1970. doi: 10.1016/j.tiv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Al-Nour M.Y., Ibrahim M.M., Elsaman T. Ellagic acid, Kaempferol, and Quercetin from Acacia nilotica: Promising combined drug with multiple mechanisms of action. Curr. Pharmacol. Rep. 2019;5:255–280. doi: 10.1007/s40495-019-00181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussein G., Miyashiro H., Nakamura N., Hattori M., Kawahata T., Otake T., Kakiuchi N., Shimotohno K. Inhibitory effects of Sudanese plant extracts on HIV-1 replication and HIV-1 protease. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999;13:31–36. doi: 10.1002/(SICI)1099-1573(199902)13:1<31::AID-PTR381>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 66.Abd El Nabi O.M., Reisinger E.C., Reinthaler F.F., Still F., Eibel U., Krejs G.J. Antimicrobial activity of Acacia nilotica (L.) Willd. ex Del. var. nilotica (Mimosaceae) J. Ethnopharmacol. 1992;37:77–79. doi: 10.1016/0378-8741(92)90006-D. [DOI] [PubMed] [Google Scholar]
- 67.Maldini M., Montoro P., Hamed A.I., Mahalel U.A., Oleszek W., Stochmal A., Piacente S. Strong antioxidant phenolics from Acacia nilotica: Profiling by ESI-MS and qualitative–quantitative determination by LC–ESI-MS. J. Pharm. Biomed. Anal. 2011;56:228–239. doi: 10.1016/j.jpba.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Dafallah A.A., Al-Mustafa Z. Investigation of the anti-inflammatory activity of Acacia nilotica and Hibiscus sabdariffa. Am. J. Chin. Med. 1996;24:263–269. doi: 10.1142/S0192415X96000323. [DOI] [PubMed] [Google Scholar]
- 69.Muddathir A.M., Mohieldin E.A.M., Mitsunaga T. In vitro activities of Acacia nilotica (L.) Delile bark fractions against Oral Bacteria, Glucosyltransferase and as antioxidant. BMC Complement. Med. Ther. 2020;20:360. doi: 10.1186/s12906-020-03147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block E. The chemistry of garlic and onions. Sci. Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 71.Ankri S., Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 72.Ceccanti C., Rocchetti G., Lucini L., Giuberti G., Landi M., Biagiotti S., Guidi L. Comparative phytochemical profile of the elephant garlic (Allium ampeloprasum var. holmense) and the common garlic (Allium sativum) from the Val di Chiana area (Tuscany, Italy) before and after in vitro gastrointestinal digestion. Food Chem. 2021;338:128011. doi: 10.1016/j.foodchem.2020.128011. [DOI] [PubMed] [Google Scholar]
- 73.Harini K., Babu S., Ajila V., Hegde S. Garlic: It’s role in oral and systemic health. Nitte Univ. J. Health Sci. 2013;3:17–22. [Google Scholar]
- 74.Fenwick G.R., Hanley A.B. The genus Allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985;22:199–271. doi: 10.1080/10408398509527415. [DOI] [PubMed] [Google Scholar]
- 75.Mann J., Bernstein Y., Findler M. Periodontal disease and its prevention, by traditional and new avenues (Review) Exp. Ther. Med. 2020;19:1504–1506. doi: 10.3892/etm.2019.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai C.-W., Chen H.-W., Sheen L.-Y., Lii C.-K. Garlic: Health benefits and actions. BioMedicine. 2012;2:17–29. doi: 10.1016/j.biomed.2011.12.002. [DOI] [Google Scholar]
- 77.Ahmad T.A., El-Sayed B.A., El-Sayed L.H. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016;5:38–47. doi: 10.1016/j.trivac.2016.02.001. [DOI] [Google Scholar]
- 78.Sasi M., Kumar S., Kumar M., Thapa S., Prajapati U., Tak Y., Changan S., Saurabh V., Kumari S., Kumar A., et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants. 2021;10:1847. doi: 10.3390/antiox10111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zini A., Mann J., Mazor S., Vered Y. The Efficacy of Aged Garlic Extract on Gingivitis—A Randomized Clinical Trial. J. Clin. Dent. 2018;29:52–56. [PubMed] [Google Scholar]
- 80.Bin C., Al-Dhabi N.A., Esmail G.A., Arokiyaraj S., Arasu M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020;27:1428–1434. doi: 10.1016/j.sjbs.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muniz I.A.F., Campos D.E.S., Shinkai R.S.A., Trindade T.G.D., Cosme-Trindade D.C. Case report of oral mucosa garlic burn during COVID-19 pandemic outbreak and role of teledentistry to manage oral health in an older adult woman. Spec. Care Dent. 2021;41:639–643. doi: 10.1111/scd.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohtani M., Nishimura T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp. Ther. Med. 2020;19:1507–1510. doi: 10.3892/etm.2019.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zini A., Mann J., Mazor S., Vered Y. Beneficial effect of aged garlic extract on periodontitis: A randomized controlled double-blind clinical study. J. Clin. Biochem. Nutr. 2020;67:297–301. doi: 10.3164/jcbn.20-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shetty S., Thomas B., Shetty V., Bhandary R., Shetty R.M. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. Ayu. 2013;34:445–451. doi: 10.4103/0974-8520.127732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhat G., Kudva P., Dodwad V. Aloe vera: Nature’s soothing healer to periodontal disease. J. Indian Soc. Periodontol. 2011;15:205–209. doi: 10.4103/0972-124X.85661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi S.W., Son B.W., Son Y.S., Park Y.I., Lee S.K., Chung M.H. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br. J. Dermatol. 2001;145:535–545. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 87.Reynolds T., Dweck A. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999;68:3–37. doi: 10.1016/S0378-8741(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 88.Gottlieb K. Aloe Vera Heals: The Scientific Facts. Cancer Book House; Sydney, Australia: 1980. [Google Scholar]
- 89.Budavari S., O’Neil M.J., Smith A., Heckelman P.E. The Merck Index. Volume 11 Merck & Co., Inc.; Merck Rahway, NJ, USA: 1989. [Google Scholar]
- 90.Vogler B.K., Ernst E. Aloe vera: A systematic review of its clinical effectiveness. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 1999;49:823–828. [PMC free article] [PubMed] [Google Scholar]
- 91.Heggers J., Pineless G., Robson M. Dermaide aloe aloe vera gel-comparison of the anti-microbial effects. J. Am. Med. Technol. 1979;41:293–294. [Google Scholar]
- 92.Schleifer K.H., Kilpper-Bälz R. Transfer of Streptococcus faecalis and Streptococcus faecium to the Genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Evol. Microbiol. 1984;34:31–34. doi: 10.1099/00207713-34-1-31. [DOI] [Google Scholar]
- 93.Grindlay D., Reynolds T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 1986;16:117–151. doi: 10.1016/0378-8741(86)90085-1. [DOI] [PubMed] [Google Scholar]
- 94.Saoo K., Miki H., Ohmori M., Winters W. Antiviral activity of aloe extracts against cytomegalovirus. Phytother. Res. 1996;10:348–350. doi: 10.1002/(SICI)1099-1573(199606)10:4<348::AID-PTR836>3.0.CO;2-2. [DOI] [Google Scholar]
- 95.Hutter J.A., Salman M., Stavinoha W.B., Satsangi N., Williams R.F., Streeper R.T., Weintraub S.T. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J. Nat. Prod. 1996;59:541–543. doi: 10.1021/np9601519. [DOI] [PubMed] [Google Scholar]
- 96.Ito S., Teradaira R., Beppu H., Obata M., Nagatsu T., Fujita K. Properties and pharmacological activity of carboxypeptidase in Aloe arborescens Mill var. natalensis Berger. Phytother. Res. 1993;7:S26–S29. doi: 10.1002/ptr.2650070710. [DOI] [Google Scholar]
- 97.Tello C.G., Ford P., Iacopino A. In vitro evaluation of complex carbohydrate denture adhesive formulations. Quintessence Int. 1998;29:585–593. [PubMed] [Google Scholar]
- 98.Poor M.R., Hall J.E., Poor A.S. Reduction in the incidence of alveolar osteitis in patients treated with the SaliCept patch, containing Acemannan hydrogel. J. Oral Maxillofac. Surg. 2002;60:374–379. doi: 10.1053/joms.2002.31222. [DOI] [PubMed] [Google Scholar]
- 99.Sudworth R. The Use of Aloe Vera in Dentistry. Positive Health Publications Ltd.; Philadelphia, PA, USA: 2002. [Google Scholar]
- 100.Chandrahas B., Jayakumar A., Naveen A., Butchibabu K., Reddy P.K., Muralikrishna T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J. Indian Soc. Periodontol. 2012;16:543. doi: 10.4103/0972-124X.106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Namiranian H., Serino G. The effect of a toothpaste containing aloe vera on established gingivitis. Swed. Dent. J. 2012;36:179–185. [PubMed] [Google Scholar]
- 102.Tornero-Martínez A., Cruz-Ortiz R., Jaramillo-Flores M.E., Osorio-Díaz P., Ávila-Reyes S.V., Alvarado-Jasso G.M., Mora-Escobedo R. In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules. 2019;24:3605. doi: 10.3390/molecules24193605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ajmera N., Chatterjee A., Goyal V. Aloe vera: It’s effect on gingivitis. J. Indian Soc. Periodontol. 2013;17:435–438. doi: 10.4103/0972-124X.118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cronquist A., Takhtadzhian A.L. An Integrated System of Classification of Flowering Plants. Columbia University Press; New York City, NY, USA: 1981. [Google Scholar]
- 105.Rivero-Cruz B.E., Esturau N., Sánchez-Nieto S., Romero I., Castillo-Juárez I., Rivero-Cruz J.F. Isolation of the new anacardic acid 6-[16’Z-nonadecenyl]-salicylic acid and evaluation of its antimicrobial activity against Streptococcus mutans and Porphyromonas gingivalis. Nat. Prod. Res. 2011;25:1282–1287. doi: 10.1080/14786419.2010.534996. [DOI] [PubMed] [Google Scholar]
- 106.Sung B., Pandey M.K., Ahn K.S., Yi T., Chaturvedi M.M., Liu M., Aggarwal B.B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111:4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y., He L., Zhang L., Chen J., Yi Z., Zhang J., Liu M., Pang X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011;339:403–411. doi: 10.1124/jpet.111.181891. [DOI] [PubMed] [Google Scholar]
- 108.Robles-Zepeda R.E., Velázquez-Contreras C.A., Garibay-Escobar A., Gálvez-Ruiz J.C., Ruiz-Bustos E. Antimicrobial activity of Northwestern Mexican plants against Helicobacter pylori. J. Med. Food. 2011;14:1280–1283. doi: 10.1089/jmf.2010.0263. [DOI] [PubMed] [Google Scholar]
- 109.Mandal A., Manohar B., Shetty N., Mathur A., Makhijani B., Sen N. A Comparative Evaluation of Anti-Inflammatory and Antiplaque Efficacy of Citrus Sinesis Mouthwash and Chlorhexidine Mouthwash. J. Nepal. Soc. Periodontol. Oral Implantol. 2018;2:9–13. doi: 10.3126/jnspoi.v2i1.23602. [DOI] [Google Scholar]
- 110.Brahmachari G. Neem—An omnipotent plant: A retrospection. Chembiochem. 2004;5:408–421. doi: 10.1002/cbic.200300749. [DOI] [PubMed] [Google Scholar]
- 111.Prakash D., Suri S., Upadhyay G., Singh B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007;58:18–28. doi: 10.1080/09637480601093269. [DOI] [PubMed] [Google Scholar]
- 112.Sakagami H., Oi T., Satoh K. Prevention of oral diseases by polyphenols. In Vivo. 1999;13:155–171. [PubMed] [Google Scholar]
- 113.Alzoreky N., Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003;80:223–230. doi: 10.1016/S0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 114.SaiRam M., Ilavazhagan G., Sharma S., Dhanraj S., Suresh B., Parida M., Jana A., Devendra K., Selvamurthy W. Anti-microbial activity of a new vaginal contraceptive NIM-76 from neem oil (Azadirachta indica) J. Ethnopharmacol. 2000;71:377–382. doi: 10.1016/S0378-8741(99)00211-1. [DOI] [PubMed] [Google Scholar]
- 115.Wolinsky L., Mania S., Nachnani S., Ling S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J. Dent. Res. 1996;75:816–822. doi: 10.1177/00220345960750021301. [DOI] [PubMed] [Google Scholar]
- 116.Vanka A., Tandon S., Rao S., Udupa N., Ramkumar P. The effect of indigenous Neem Azadirachta indica [correction of (Adirachta indica)] mouth wash on Streptococcus mutans and lactobacilli growth. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2001;12:133–144. [PubMed] [Google Scholar]
- 117.Dasgupta T., Banerjee S., Yadava P., Rao A. Chemopreventive potential of Azadirachta indica (Neem) leaf extract in murine carcinogenesis model systems. J. Ethnopharmacol. 2004;92:23–36. doi: 10.1016/j.jep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 118.Baral R., Chattopadhyay U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004;4:355–366. doi: 10.1016/j.intimp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Raji Y., Ogunwande I.A., Osadebe C.A., John G. Effects of Azadirachta indica extract on gastric ulceration and acid secretion in rats. J. Ethnopharmacol. 2004;90:167–170. doi: 10.1016/j.jep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 120.Bandyopadhyay U., Biswas K., Sengupta A., Moitra P., Dutta P., Sarkar D., Debnath P., Ganguly C.K., Banerjee R.K. Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 2004;75:2867–2878. doi: 10.1016/j.lfs.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 121.Wu C.D., Darout I.A., Skaug N. Chewing sticks: Timeless natural toothbrushes for oral cleansing. J. Periodontal Res. 2001;36:275–284. doi: 10.1034/j.1600-0765.2001.360502.x. [DOI] [PubMed] [Google Scholar]
- 122.Prashant G., Chandu G., Murulikrishna K., Shafiulla M. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J. Dent. Res. 2007;18:148. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 123.Robinson T. In: The Organic Constituents of Higher Plants, Diterjemahkan Oleh Padmawinata. Kosasih P., editor. ITB; Bandung, Indonesia: 1995. [Google Scholar]
- 124.Pai M.R., Acharya L.D., Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel—A 6-week clinical study. J. Ethnopharmacol. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 125.Schumacher M., Cerella C., Reuter S., Dicato M., Diederich M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149–160. doi: 10.1007/s12263-010-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu J.P., Takahashi N., Yamada T. Coptidis rhizoma inhibits growth and proteases of oral bacteria. Oral Dis. 2000;6:297–302. doi: 10.1111/j.1601-0825.2000.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 127.Lamont R.J., Jenkinson H.F. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/MMBR.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pandit N., Changela R., Bali D., Tikoo P., Gugnani S. Porphyromonas gingivalis: Its virulence and vaccine. J. Int. Clin. Dent. Res. Organ. 2015;7:51. [Google Scholar]
- 129.Moeintaghavi A., Shabzendedar M., Parissay I., Makarem A., Orafaei H., Hosseinnezhad M. Berberine gel in periodontal inflammation: Clinical and histological effects. J. Adv. Periodontol. Implant. Dent. 2012;4:7–11. [Google Scholar]
- 130.Strusovskaya A., Poroysky S., Smirnov A., Firsova I., Sirotenko V., Kirichenko L., Strusovskaya O. A study of the influence of barbaris root (Berberis vulgaris L., Berberidaceae) extract dental gel on the dynamics of the inflammatory process in periodontal tissues of rats on the model of induced gingivitis; Proceedings of the AIP Conference Proceedings; Yekaterinburg, Russia. 13–16 November 2019. [Google Scholar]
- 131.Passos V.F., Melo M.A.S.d., Lima J.P.M., Marçal F.F., Costa C.A.G.d.A., Rodrigues L.K.A., Santiago S.L. Active compounds and derivatives of camellia sinensis responding to erosive attacks on dentin. Braz. Oral Res. 2018;32:e40. doi: 10.1590/1807-3107bor-2018.vol32.0040. [DOI] [PubMed] [Google Scholar]
- 132.Kushiyama M., Shimazaki Y., Murakami M., Yamashita Y. Relationship between intake of green tea and periodontal disease. J. Periodontol. 2009;80:372–377. doi: 10.1902/jop.2009.080510. [DOI] [PubMed] [Google Scholar]
- 133.Koyama Y., Kuriyama S., Aida J., Sone T., Nakaya N., Ohmori-Matsuda K., Hozawa A., Tsuji I. Association between green tea consumption and tooth loss: Cross-sectional results from the Ohsaki Cohort 2006 Study. Prev. Med. 2010;50:173–179. doi: 10.1016/j.ypmed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 134.Ide R., Fujino Y., Hoshiyama Y., Mizoue T., Kubo T., Pham T.-M., Shirane K., Tokui N., Sakata K., Tamakoshi A. A prospective study of green tea consumption and oral cancer incidence in Japan. Ann. Epidemiol. 2007;17:821–826. doi: 10.1016/j.annepidem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 135.Mazur M., Ndokaj A., Jedlinski M., Ardan R., Bietolini S., Ottolenghi L. Impact of Green Tea (Camellia Sinensis) on periodontitis and caries. Systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2021;57:1–11. doi: 10.1016/j.jdsr.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wazaify M., Afifi F.U., El-Khateeb M., Ajlouni K. Complementary and alternative medicine use among Jordanian patients with diabetes. Complement. Ther. Clin. Pract. 2011;17:71–75. doi: 10.1016/j.ctcp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Yanakiev S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules. 2020;25:4184. doi: 10.3390/molecules25184184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kawatra P., Rajagopalan R. Cinnamon: Mystic powers of a minute ingredient. Pharmacogn. Res. 2015;7((Suppl. S1)):S1–S6. doi: 10.4103/0974-8490.157990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jayaprakasha G.K., Rao L.J. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011;51:547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 140.Chen P., Sun J., Ford P. Differentiation of the four major species of cinnamons (C. burmannii, C. verum, C. cassia, and C. loureiroi) using a flow injection mass spectrometric (FIMS) fingerprinting method. J. Agric. Food Chem. 2014;62:2516–2521. doi: 10.1021/jf405580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fischer C.L., Walters K.S., Drake D.R., Dawson D.V., Blanchette D.R., Brogden K.A., Wertz P.W. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int. J. Oral Sci. 2013;5:130–140. doi: 10.1038/ijos.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mendes S.J.F., Sousa F.I.A.B., Pereira D.M.S., Ferro T.A.F., Pereira I.C.P., Silva B.L.R., Pinheiro A.J.M.C.R., Mouchrek A.Q.S., Monteiro-Neto V., Costa S.K.P., et al. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016;34:60–70. doi: 10.1016/j.intimp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 143.Yang X.Q., Zheng H., Ye Q., Li R.Y., Chen Y. Essential oil of Cinnamon exerts anti-cancer activity against head and neck squamous cell carcinoma via attenuating epidermal growth factor receptor—Tyrosine kinase. J. Buon. 2015;20:1518–1525. [PubMed] [Google Scholar]
- 144.Wang Y., Zhang Y., Shi Y.Q., Pan X.H., Lu Y.H., Cao P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018;116:26–32. doi: 10.1016/j.micpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y., Liu X., Wang Y., Jiang P., Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 146.Meng X., Li D., Zhou D., Wang D., Liu Q., Fan S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016;194:698–705. doi: 10.1016/j.jep.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 147.Chaieb K., Hajlaoui H., Zmantar T., Kahla-Nakbi A.B., Rouabhia M., Mahdouani K., Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y., Wang Y., Zhu X., Cao P., Wei S., Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017;113:396–402. doi: 10.1016/j.micpath.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 149.Bickers D., Calow P., Greim H., Hanifin J.M., Rogers A.E., Saurat J.H., Sipes I.G., Smith R.L., Tagami H. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005;43:799–836. doi: 10.1016/j.fct.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 150.Cocchiara J., Letizia C.S., Lalko J., Lapczynski A., Api A.M. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005;43:867–923. doi: 10.1016/j.fct.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 151.Hussain K.A., Tarakji B., Kandy B.P., John J., Mathews J., Ramphul V., Divakar D.D. Antimicrobial effects of citrus sinensis peel extracts against periodontopathic bacteria: An in vitro study. Rocz. Panstw. Zakl. Hig. 2015;66:173–178. [PubMed] [Google Scholar]
- 152.Lawal D., Bala J.A., Aliyu S.Y., Huguma M.A. Phytochemical Screening and In Vitro Anti-Bacterial Studies of the Ethanolic Extract of Citrus Senensis (Linn.) Peel against some Clinical Bacterial Isolates. Int. J. Innov. Appl. Stud. 2013;2:138–145. [Google Scholar]
- 153.Dubey D., Balamurugan K., Agrawal R., Verma R.K., Jain R. Evalution of Antibacterial and Antioxidant Activity of Methanolic and Hydromethanolic Extract of Sweet Orange Peels. Recent Res. Sci. Technol. 2011;3:22–25. [Google Scholar]
- 154.Jabuk S., Chabuck A., Adil N., Chabuck G. In vitro and in vivo effect of three aqueous plant extract on pathogenicity of Klebsiella pneumonia isolated from patient with urinary tract infection. World J. Pharm. Res. 2014;5:160–179. [Google Scholar]
- 155.Carrol D.H., Chassagne F., Dettweiler M., Quave C.L. Antibacterial activity of plant species used for oral health against Porphyromonas gingivalis. PLoS ONE. 2020;15:e0239316. doi: 10.1371/journal.pone.0239316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tangade P.S., Mathur A., Tirth A., Kabasi S. Anti-gingivitis effects of Acacia arabica-containing toothpaste. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 2012;15:49–53. [PubMed] [Google Scholar]
- 157.Sayar F., Farahmand A.H., Rezazadeh M. Clinical Efficacy of Aloe Vera Toothpaste on Periodontal Parameters of Patients with Gingivitis—A Randomized, Controlled, Single-masked Clinical Trial. J. Contemp. Dent. Pract. 2021;22:242–247. [PubMed] [Google Scholar]
- 158.Nazir S., Kumar C. The Effect of Aloe Vera in Patient with Chronic Periodontitis. Pak. J. Med. Dent. 2019;7:5. [Google Scholar]
- 159.Kanika M. Efficacious Evaluation of Aloe Vera Tooth Gel and Commercially Available Tooth Gel on Patients with Gingivitis. J. Oral Health Dent. Sci. 2018;2:203. [Google Scholar]
- 160.Vangipuram S., Jha A., Bhashyam M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: A randomized controlled trial. J. Clin. Exp. Dent. 2016;8:e442–e447. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nair A.A., Malaiappan S. The comparison of the antiplaque effect of aloe vera, chlorhexidine and placebo mouth washes on gingivitis patients. J. Pharm. Sci. Res. 2016;8:1295–1300. [Google Scholar]
- 162.Yeturu S.K., Acharya S., Urala A.S., Pentapati K.C. Effect of Aloe vera, chlorine dioxide, and chlorhexidine mouth rinses on plaque and gingivitis: A randomized controlled trial. J. Oral Biol. Craniofac. Res. 2016;6:54–58. doi: 10.1016/j.jobcr.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sargolzaie N., Rajabi O., Arab H., Esmaele H., Ehteshamfar A. Comparative evaluation of Green Tea-Aloe Vera mouthwash and chlorhexidine 0.2% on gingival indices (A randomized clinical trial) J. Dent. Mater. Tech. 2016;5:31–35. [Google Scholar]
- 164.Karim B., Bhaskar D.J., Agali C., Gupta D., Gupta R.K., Jain A., Kanwar A. Effect of Aloe vera mouthwash on periodontal health: Triple blind randomized control trial. Oral Health Dent. Manag. 2014;13:14–19. [PubMed] [Google Scholar]
- 165.Ashouri Moghaddam A., Radafshar G., Jahandideh Y., Kakaei N. Clinical Evaluation of Effects of Local Application of Aloe vera Gel as an Adjunct to Scaling and Root Planning in Patients with Chronic Periodontitis. J. Dent. 2017;18:165–172. [PMC free article] [PubMed] [Google Scholar]
- 166.Makarem A., Asodeh N.K.R. Efficacy of barberry aqueous extracts dental gel on control of plaque and gingivitis. Acta Med. Iran. 2007;45:91–94. [Google Scholar]
- 167.Tripathi P., Blaggana V., Upadhyay P., Jindal M., Gupta S., Nishat S. Antioxidant therapy (lycopene and green tea extract) in periodontal disease: A promising paradigm. J. Indian Soc. Periodontol. 2019;23:25–30. doi: 10.4103/jisp.jisp_277_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taleghani F., Rezvani G., Birjandi M., Valizadeh M. Impact of green tea intake on clinical improvement in chronic periodontitis: A randomized clinical trial. J. Stomatol. Oral Maxillofac. Surg. 2018;119:365–368. doi: 10.1016/j.jormas.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 169.Radafshar G., Ghotbizadeh M., Saadat F., Mirfarhadi N. Effects of green tea (Camellia sinensis) mouthwash containing 1% tannin on dental plaque and chronic gingivitis: A double-blinded, randomized, controlled trial. J. Investig. Clin. Dent. 2017;8:e12184. doi: 10.1111/jicd.12184. [DOI] [PubMed] [Google Scholar]
- 170.Behfarnia P., Aslani A., Jamshidian F., Noohi S. The Efficacy of Green Tea Chewing Gum on Gingival Inflammation. J. Dent. 2016;17:149–154. [PMC free article] [PubMed] [Google Scholar]
- 171.Sarin S., Marya C., Nagpal R., Oberoi S.S., Rekhi A. Preliminary clinical evidence of the antiplaque, antigingivitis efficacy of a mouthwash containing 2% green tea—A randomised clinical trial. Oral Health Prev. Dent. 2015;13:197–203. doi: 10.3290/j.ohpd.a33447. [DOI] [PubMed] [Google Scholar]
- 172.Hambire C.U., Jawade R., Patil A., Wani V.R., Kulkarni A.A., Nehete P.B. Comparing the antiplaque efficacy of 0.5% Camellia sinensis extract, 0.05% sodium fluoride, and 0.2% chlorhexidine gluconate mouthwash in children. J. Int. Soc. Prev. Community Dent. 2015;5:218–226. doi: 10.4103/2231-0762.158016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jenabian N., Moghadamnia A.A., Karami E., Mir A P.B. The effect of Camellia Sinensis (green tea) mouthwash on plaque-induced gingivitis: A single-blinded randomized controlled clinical trial. DARU J. Pharm. Sci. 2012;20:39. doi: 10.1186/2008-2231-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hattarki S., Pushpa S., Bhat K. Evaluation of the efficacy of green tea catechins as an adjunct to scaling and root planing in the management of chronic periodontitis using PCR analysis: A clinical and microbiological study. J. Indian Soc. Periodontol. 2013;17:204–209. doi: 10.4103/0972-124X.113071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hugar S.S., Patil S., Metgud R., Nanjwade B., Hugar S.M. Influence of application of chlorhexidine gel and curcumin gel as an adjunct to scaling and root planing: A interventional study. J. Nat. Sci. Biol. Med. 2016;7:149. doi: 10.4103/0976-9668.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farjana H.N., Chandrasekaran S., Gita B. Effect of oral curcuma gel in gingivitis management—A pilot study. J. Clin. Diagn. Res. JCDR. 2014;8:ZC08. doi: 10.7860/JCDR/2014/8784.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahendra J., Mahendra L., Svedha P., Cherukuri S., Romanos G.E. Clinical and microbiological efficacy of 4% Garcinia mangostana L. pericarp gel as local drug delivery in the treatment of chronic periodontitis: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2017;8:e12262. doi: 10.1111/jicd.12262. [DOI] [PubMed] [Google Scholar]
- 178.Madan S., Kashyap S., Mathur G. Glycyrrhiza glabra: An efficient medicinal plant for control of periodontitis—A randomized clinical trial. J. Int. Clin. Dent. Res. Organ. 2019;11:32–35. doi: 10.4103/jicdro.jicdro_7_19. [DOI] [Google Scholar]
- 179.Saimbi C., Shubh N., Kapoor K., Kaushal S. Clinical effect of Juglans regia on developing dental plaque. J. Int. Clin. Dent. Res. Organ. 2009;1:1. [Google Scholar]
- 180.Pereira S.L., Praxedes Y.C., Bastos T.C., Alencar P.N., da Costa F.N. Clinical effect of a gel containing Lippia sidoides on plaque and gingivitis control. Eur. J. Dent. 2013;7:28–34. [PMC free article] [PubMed] [Google Scholar]
- 181.Botelho M.A., dos Santos R.A., Martins J.G., Carvalho C.O., Paz M.C., Azenha C., Ruela R.S., Queiroz D.B., Ruela W.S., Marinho G., et al. Comparative effect of an essential oil mouthrinse on plaque, gingivitis and salivary Streptococcus mutans levels: A double blind randomized study. Phytother. Res. 2009;23:1214–1219. doi: 10.1002/ptr.2489. [DOI] [PubMed] [Google Scholar]
- 182.Bhat S.S., Hegde K.S., Mathew C., Bhat S.V., Shyamjith M. Comparative evaluation of Mangifera indica leaf mouthwash with chlorhexidine on plaque accumulation, gingival inflammation, and salivary streptococcal growth. Indian. J. Dent. Res. 2017;28:151–155. doi: 10.4103/ijdr.IJDR_583_15. [DOI] [PubMed] [Google Scholar]
- 183.Atwa A.L.D.A., AbuShahba R.Y., Mostafa M., Hashem M.I. Effect of honey in preventing gingivitis and dental caries in patients undergoing orthodontic treatment. Saudi Dent. J. 2014;26:108–114. doi: 10.1016/j.sdentj.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.English H.K.P., Pack A.R.C., Molan P.C. The effects of manuka honey on plaque and gingivitis: A pilot study. J. Int. Acad. Periodontol. 2004;6:63–67. [PubMed] [Google Scholar]
- 185.Agarwal A., Chaudhary B. Clinical and microbiological effects of 1% Matricaria chamomilla mouth rinse on chronic periodontitis: A double-blind randomized placebo controlled trial. J. Indian Soc. Periodontol. 2020;24:354–361. doi: 10.4103/jisp.jisp_441_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Goes P., Dutra C.S., Lisboa M.R., Gondim D.V., Leitão R., Brito G.A., Rego R.O. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J. Oral Sci. 2016;58:569–574. doi: 10.2334/josnusd.16-0280. [DOI] [PubMed] [Google Scholar]
- 187.Manohar Sharma H., Deepika P., Venkatesh M., Chandan S., Shashikumar P. Efficacy of 3% Psidium guajava local drug delivery in the treatment of chronic periodontitis: A randomized controlled trial. J. Int. Oral Health. 2021;13:17–23. doi: 10.4103/jioh.jioh_249_20. [DOI] [Google Scholar]
- 188.Dobayan B.A., Ayeid F.a.A., Guraid I.A., Bassiouny G., Nasser S.A. The Effect of Punica Granatum Gel as An Adjunctive Therapy in Patients with chronic Periodontitis: A Clinical, Microbiological and histological Study. J. Am. Sci. 2019;19:12–15. [Google Scholar]
- 189.Prakash J., Bhatnagar V., Nath S., Jacob Pulikkotil S., Prajapati V. Effect of Punica granatum Extract Gel on Gingival Crevicular Fluid Levels of Interleukin-1β, Interleukin-8 and CCL28 Levels: Randomised Controlled Clinical Trial. J. Clin. Diagn. Res. 2017;11:ZC12–ZC17. doi: 10.7860/JCDR/2017/31035.10845. [DOI] [Google Scholar]
- 190.Sedigh-Rahimabadi M., Fani M., Rostami-chijan M., Zarshenas M.M., Shams M. A Traditional Mouthwash (Punica granatum var pleniflora) for Controlling Gingivitis of Diabetic Patients: A Double-Blind Randomized Controlled Clinical Trial. J. Evid.-Based Complement. Altern. Med. 2016;22:59–67. doi: 10.1177/2156587216633370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Azad M.F., Schwiertz A., Jentsch H.F.R. Adjunctive use of essential oils following scaling and root planing—A randomized clinical trial. BMC Complement. Altern. Med. 2016;16:171. doi: 10.1186/s12906-016-1117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sabbagh H.J., AlGhamdi K.S., Mujalled H.T., Bagher S.M. The effect of brushing with Salvadora persica (miswak) sticks on salivary Streptococcus mutans and plaque levels in children: A clinical trial. BMC Complement. Med. Ther. 2020;20:53. doi: 10.1186/s12906-020-2847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bahrololoomi Z., Sadat-Hashemi A., Hassan-Akhavan-Karbassi M., Khaksar Y. Evaluating the additive effect of Persica and chlorhexidine mouthwashes on oral health status of children receiving chemotherapy for their hematomalignancy: A randomized clinical trial. J. Clin. Exp Dent. 2020;12:e574–e580. doi: 10.4317/jced.56104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Varma S.R., Sherif H., Serafi A., Fanas S.A., Desai V., Abuhijleh E., Al Radaidah A. The Antiplaque Efficacy of Two Herbal-Based Toothpastes: A Clinical Intervention. J. Int. Soc. Prev. Community Dent. 2018;8:21–27. doi: 10.4103/jispcd.JISPCD_411_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saha S., Mohammad S., Saha S., Samadi F. Efficiency of traditional chewing stick (miswak) as an oral hygiene aid among Muslim school children in Lucknow: A cross-sectional study. J. Oral Biol. Craniofac. Res. 2012;2:176–180. doi: 10.1016/j.jobcr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta D., Gupta R., Bhaskar D., Gupta V. Comparative Evaluation of Terminalia chebula Extract Mouthwash and Chlorhexidine Mouthwash on Plaque and Gingival Infl ammation—4-week Randomised Control Trial. Oral Health Prev. Dent. 2015;13:5–12. doi: 10.3290/j.ohpd.a32994. [DOI] [PubMed] [Google Scholar]
- 197.Gupta D., Bhaskar D.J., Gupta R.K., Karim B., Gupta V., Punia H., Batra M., Jain A., Agarwal A., Singh P. Effect of Terminalia chebula extract and chlorhexidine on salivary pH and periodontal health: 2 weeks randomized control trial. Phytother. Res. 2014;28:992–998. doi: 10.1002/ptr.5075. [DOI] [PubMed] [Google Scholar]
- 198.Esther P., Elanchezhiyan S., Daniel R., Meenalochani T., Pavithra T., Surya D. Evaluation of clinical effificacy of Terminalia chebula inplaque-induced gingivitis: A randomized control trial. Indian J. Multidiscip. Dent. 2017;7:21–24. doi: 10.4103/ijmd.ijmd_9_17. [DOI] [Google Scholar]
- 199.Joët T., Salmona J., Laffargue A., Descroix F., Dussert S. Use of the growing environment as a source of variation to identify the quantitative trait transcripts and modules of co-expressed genes that determine chlorogenic acid accumulation. Plant Cell Environ. 2010;33:1220–1233. doi: 10.1111/j.1365-3040.2010.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Higdon J.V., Frei B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 201.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., FangFang X., Modarresi-Ghazani F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 202.Bogdan C., Pop A., Iurian S.M., Benedec D., Moldovan M.L. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants. 2020;9:502. doi: 10.3390/antiox9060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bouayed J., Rammal H., Dicko A., Younos C., Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007;262:77–84. doi: 10.1016/j.jns.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 204.Chaube S., Swinyard C.A. Teratological and toxicological studies of alkaloidal and phenolic compounds from Solanum tuberosum L. Toxicol. Appl. Pharmacol. 1976;36:227–237. doi: 10.1016/0041-008X(76)90002-8. [DOI] [PubMed] [Google Scholar]
- 205.Yadav M., Kaushik M., Roshni R., Reddy P., Mehra N., Jain V., Rana R. Effect of Green Coffee Bean Extract on Streptococcus mutans Count: A Randomised Control Trial. J. Clin. Diagn. Res. 2017;11:Zc68–Zc71. doi: 10.7860/JCDR/2017/25743.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsou S.-H., Hu S.-W., Yang J.-J., Yan M., Lin Y.-Y. Potential Oral Health Care Agent from Coffee Against Virulence Factor of Periodontitis. Nutrients. 2019;11:2235. doi: 10.3390/nu11092235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arruda C., Mejía J.A.A., Ribeiro V.P., Borges C.H.G., Martins C.H.G., Veneziani R.C.S., Ambrosio S.R., Bastos J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019;109:1–20. doi: 10.1016/j.biopha.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 208.Bardají D.K.R., da Silva J.J.M., Bianchi T.C., de Souza Eugênio D., de Oliveira P.F., Leandro L.F., Rogez H.L.G., Venezianni R.C.S., Ambrosio S.R., Tavares D.C. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe. 2016;40:18–27. doi: 10.1016/j.anaerobe.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 209.Abrão F., de Araújo Costa L.D., Alves J.M., Senedese J.M., de Castro P.T., Ambrósio S.R., Veneziani R.C.S., Bastos J.K., Tavares D.C., Martins C.H.G. Copaifera langsdorffii oleoresin and its isolated compounds: Antibacterial effect and antiproliferative activity in cancer cell lines. BMC Complement. Altern. Med. 2015;15:443. doi: 10.1186/s12906-015-0961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.da S. Moraes T., Leandro L.F., de O. Silva L., Santiago M.B., Souza A.B., Furtado R.A., Tavares D.C., Veneziani R.C.S., Ambrósio S.R., Bastos J.K. In vitro evaluation of Copaifera oblongifolia oleoresin against bacteria causing oral infections and assessment of its cytotoxic potential. Curr. Pharm. Biotechnol. 2016;17:894–904. doi: 10.2174/1389201017666160415155359. [DOI] [PubMed] [Google Scholar]
- 211.Borges C.H., Cruz M.G., Carneiro L.J., da Silva J.J., Bastos J.K., Tavares D.C., de Oliveira P.F., Rodrigues V., Veneziani R.C., Parreira R.L. Copaifera duckei oleoresin and its main nonvolatile terpenes: In vitro schistosomicidal properties. Chem. Biodivers. 2016;13:1348–1356. doi: 10.1002/cbdv.201600065. [DOI] [PubMed] [Google Scholar]
- 212.Alves J.M., Senedese J.M., Leandro L.F., Castro P.T., Pereira D.E., Carneiro L.J., Ambrósio S.R., Bastos J.K., Tavares D.C. Copaifera multijuga oleoresin and its constituent diterpene (−)-copalic acid: Genotoxicity and chemoprevention study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2017;819:26–30. doi: 10.1016/j.mrgentox.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 213.Abrão F., Alves J.A., Andrade G., De Oliveira P.F., Ambrósio S.R., Veneziani R., Tavares D.C., Bastos J.K., Martins C.H. Antibacterial effect of Copaifera duckei Dwyer oleoresin and its main diterpenes against oral pathogens and their cytotoxic effect. Front. Microbiol. 2018;9:201. doi: 10.3389/fmicb.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Furtado R.A., de Oliveira P.F., Senedese J.M., Ozelin S.D., de Souza L.D.R., Leandro L.F., de Oliveira W.L., da Silva J.J.M., Oliveira L.C., Rogez H. Assessment of genotoxic activity of oleoresins and leaves extracts of six Copaifera species for prediction of potential human risks. J. Ethnopharmacol. 2018;221:119–125. doi: 10.1016/j.jep.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 215.De Souza M.G.M., Leandro L.F., da Silva Moraes T., Abrão F., Veneziani R.C.S., Ambrosio S.R., Martins C.H.G. ent-Copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius. Anaerobe. 2018;52:43–49. doi: 10.1016/j.anaerobe.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 216.Abrão F., Silva T.S., Moura C.L., Ambrósio S.R., Veneziani R.C.S., de Paiva R.E.F., Bastos J.K., Martins C.H.G. Oleoresins and naturally occurring compounds of Copaifera genus as antibacterial and antivirulence agents against periodontal pathogens. Sci. Rep. 2021;11:4953. doi: 10.1038/s41598-021-84480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ivanovska N., Philipov S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996;18:553–561. doi: 10.1016/S0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 218.Nakamoto K., Sadamori S., Hamada T. Effects of crude drugs and berberine hydrochloride on the activities of fungi. J. Prosthet. Dent. 1990;64:691–694. doi: 10.1016/0022-3913(90)90298-Q. [DOI] [PubMed] [Google Scholar]
- 219.Tu H.-P., Fu M.M., Kuo P.-J., Chin Y.-T., Chiang C.-Y., Chung C.-L., Fu E. Berberine’s effect on periodontal tissue degradation by matrix metalloproteinases: An in vitro and in vivo experiment. Phytomedicine. 2013;20:1203–1210. doi: 10.1016/j.phymed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 220.Zhang F., Yu Z.-H. Effect of berberine hydrochloride on the secretion of monocyte chemoattractant protein-1 from human periodontal ligament cells in vitro. Zhonghua Kou Qiang Yi Xue Za Zhi= Zhonghua Kouqiang Yixue Zazhi= Chin. J. Stomatol. 2012;47:610–613. doi: 10.3760/cma.j.issn.1002-0098.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 221.Yucel-Lindberg T., Båge T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
- 222.Jia X., Jia L., Mo L., Yuan S., Zheng X., He J., Chen V., Guo Q., Zheng L., Yuan Q. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J. Dent. Res. 2019;98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 223.Gu L., Ke Y., Gan J., Li X. Berberine suppresses bone loss and inflammation in ligature-induced periodontitis through promotion of the G protein-coupled estrogen receptor-mediated inactivation of the p38MAPK/NF-κB pathway. Arch. Oral Biol. 2021;122:104992. doi: 10.1016/j.archoralbio.2020.104992. [DOI] [PubMed] [Google Scholar]
- 224.Sun H.L., Wu Y.R., Song F.F., Gan J., Huang L.Y., Zhang L., Huang C. Role of PCSK9 in the development of mouse periodontitis before and after treatment: A double-edged sword. J. Infect. Dis. 2018;217:667–680. doi: 10.1093/infdis/jix574. [DOI] [PubMed] [Google Scholar]
- 225.Kohli K., Ali J., Ansari M., Raheman Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005;37:141. doi: 10.4103/0253-7613.16209. [DOI] [Google Scholar]
- 226.Suhag A., Dixit J., Dhan P. Role of curcumin as a subgingival irrigant: A pilot study. Periodontal Pract. Today. 2007;4:115–121. [Google Scholar]
- 227.Guimarães M.R., Coimbra L.S., de Aquino S.G., Spolidorio L.C., Kirkwood K.L., Rossa C., Jr. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J. Periodontal Res. 2011;46:269–279. doi: 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Guimaraes-Stabili M.R., de Aquino S.G., de Almeida Curylofo F., Tasso C.O., Rocha F.R.G., de Medeiros M.C., de Pizzol J.P., Cerri P.S., Romito G.A., Rossa C. Systemic administration of curcumin or piperine enhances the periodontal repair: A preliminary study in rats. Clin. Oral Investig. 2019;23:3297–3306. doi: 10.1007/s00784-018-2755-9. [DOI] [PubMed] [Google Scholar]
- 229.Anuradha B., Bai Y.D., Sailaja S., Sudhakar J., Priyanka M., Deepika V. Evaluation of anti-inflammatory effects of curcumin gel as an adjunct to scaling and root planing: A clinical study. J. Int. Oral Health JIOH. 2015;7:90. [PMC free article] [PubMed] [Google Scholar]
- 230.Nagasri M., Madhulatha M., Musalaiah S., Kumar P.A., Krishna C.M., Kumar P.M. Efficacy of curcumin as an adjunct to scaling and root planning in chronic periodontitis patients: A clinical and microbiological study. J. Pharm. Bioallied Sci. 2015;7((Suppl. S2)):S554. doi: 10.4103/0975-7406.163537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ravishankar P., Kumar Y.P., Anila E., Chakraborty P., Malakar M., Mahalakshmi R. Effect of local application of curcumin and ornidazole gel in chronic periodontitis patients. Int. J. Pharm. Investig. 2017;7:188. doi: 10.4103/jphi.JPHI_82_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kandwal A., Mamgain R.K., Mamgain P. Comparative evaluation of turmeric gel with 2% chlorhexidine gluconate gel for treatment of plaque induced gingivitis: A randomized controlled clinical trial. Ayu. 2015;36:145. doi: 10.4103/0974-8520.175537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jacob P., Nath S., Sultan O. Use of curcumin in periodontal inflammation. Interdiscip. J. Microinflamm. 2014;1:2. [Google Scholar]
- 234.Borges J.S., Paranhos L.R., de Souza G.L., de Souza Matos F., de Macedo Bernardino Í., Moura C.C.G., Soares P.B.F. Does systemic oral administration of curcumin effectively reduce alveolar bone loss associated with periodontal disease? A systematic review and meta-analysis of preclinical in vivo studies. J. Funct. Foods. 2020;75:104226. doi: 10.1016/j.jff.2020.104226. [DOI] [Google Scholar]
- 235.Nebrisi E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021;22:11248. doi: 10.3390/ijms222011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shah G., Shri R., Panchal V., Sharma N., Singh B., Mann A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass) J. Adv. Pharm. Technol. Res. 2011;2:3–8. doi: 10.4103/2231-4040.79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Crawford M., Hanson S.W., Koker M.E. The structure of cymbopogone, a novel triterpenoid from lemongrass. Tetrahedron Lett. 1975;16:3099–3102. doi: 10.1016/S0040-4039(00)75085-4. [DOI] [Google Scholar]
- 238.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 239.Khongkhunthian S., Sookkhee S., Okonogi S. Antimicrobial activities against periodontopathogens of essential oil from lemon grass (Cymbopogon citratus (DC.) Stapf.) CMU J. Nat. Sci. 2009;8:11–22. [Google Scholar]
- 240.Susanto S., Oktavianti T., Wijaya Y., Wira V., Paramitta V. Increased glutathione level in saliva of moderate gingivitis patients after lemongrass (cymbopogoncitratus) essential oil gargling. Asia Pac. Dent. Stud. J. 2010;1:45–52. [Google Scholar]
- 241.Bruschi M.L., Jones D.S., Panzeri H., Gremião M.P., de Freitas O., Lara E.H. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007;96:2074–2089. doi: 10.1002/jps.20843. [DOI] [PubMed] [Google Scholar]
- 242.Rabbani S.I., Devi K., Khanam S., Zahra N. Citral, a component of lemongrass oil inhibits the clastogenic effect of nickel chloride in mouse micronucleus test system. Pak. J. Pharm. Sci. 2006;19:108–113. [PubMed] [Google Scholar]
- 243.Batish D.R., Singh H.P., Kohli R.K., Kaur S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008;256:2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 244.Topiar M., Sajfrtova M., Pavela R., Machalova Z. Comparison of fractionation techniques of CO2 extracts from Eucalyptus globulus—Composition and insecticidal activity. J. Supercrit. Fluids. 2015;97:202–210. doi: 10.1016/j.supflu.2014.12.002. [DOI] [Google Scholar]
- 245.Nagata H., Inagaki Y., Yamamoto Y., Maeda K., Kataoka K., Osawa K., Shizukuishi S. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol. Immunol. 2006;21:159–163. doi: 10.1111/j.1399-302X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 246.Osawa K., Yasuda H., Morita H., Takeya K., Itokawa H. Macrocarpals H, I, and J from the Leaves of Eucalyptus globulus. J. Nat. Prod. 1996;59:823–827. doi: 10.1021/np9604994. [DOI] [PubMed] [Google Scholar]
- 247.Nagata H., Inagaki Y., Tanaka M., Ojima M., Kataoka K., Kuboniwa M., Nishida N., Shimizu K., Osawa K., Shizukuishi S. Effect of Eucalyptus Extract Chewing Gum on Periodontal Health: A Double-Masked, Randomized Trial. J. Periodontol. 2008;79:1378–1385. doi: 10.1902/jop.2008.070622. [DOI] [PubMed] [Google Scholar]
- 248.Steinmetz K.A., Potter J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 249.Yang J., Liu R.H., Halim L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT Food Sci. Technol. 2009;42:1–8. doi: 10.1016/j.lwt.2008.07.007. [DOI] [Google Scholar]
- 250.Obolskiy D., Pischel I., Siriwatanametanon N., Heinrich M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009;23:1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- 251.Suttirak W., Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014;51:3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shan T., Ma Q., Guo K., Liu J., Li W., Wang F., Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011;11:666–677. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shankaranarayan D., Gopalakrishnan C., Kameswaran L. Pharmacological profile of mangostin and its derivatives. Arch. Int. Pharmacodyn. Ther. 1979;239:257–269. [PubMed] [Google Scholar]
- 254.Lim Y.K., Yoo S.Y., Jang Y.Y., Lee B.C., Lee D.S., Kook J.-K. Anti-inflammatory and in vitro bone formation effects of Garcinia mangostana L. and propolis extracts. Food Sci. Biotechnol. 2020;29:539–548. doi: 10.1007/s10068-019-00697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Messier C., Epifano F., Genovese S., Grenier D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012;18:32–39. doi: 10.1111/j.1601-0825.2011.01842.x. [DOI] [PubMed] [Google Scholar]
- 256.Tanabe S.-i., Desjardins J., Bergeron C., Gafner S., Villinski J.R., Grenier D. Reduction of bacterial volatile sulfur compound production by licoricidin and licorisoflavan A from licorice. J. Breath Res. 2012;6:016006. doi: 10.1088/1752-7155/6/1/016006. [DOI] [PubMed] [Google Scholar]
- 257.Wittschier N., Faller G., Beikler T., Stratmann U., Hensel A. Polysaccharides from Glycyrrhiza glabra L. exert significant anti-adhesive effects against Helicobacter pylori and Porphyromonas gingivalis. Planta Med. 2006;72:P_238. doi: 10.1055/s-2006-950038. [DOI] [Google Scholar]
- 258.Bodet C., La V.D., Gafner S., Bergeron C., Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J. Periodontol. 2008;79:1752–1761. doi: 10.1902/jop.2008.080052. [DOI] [PubMed] [Google Scholar]
- 259.La V.D., Tanabe S.i., Bergeron C., Gafner S., Grenier D. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: Potential therapeutic approach for periodontitis. J. Periodontol. 2011;82:122–128. doi: 10.1902/jop.2010.100342. [DOI] [PubMed] [Google Scholar]
- 260.Feldman M., Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J. Appl. Microbiol. 2012;113:438–447. doi: 10.1111/j.1365-2672.2012.05329.x. [DOI] [PubMed] [Google Scholar]
- 261.Farhad S.Z., Aminzadeh A., Mafi M., Barekatain M., Naghney M., Ghafari M.R. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent. Res. J. 2013;10:624. [PMC free article] [PubMed] [Google Scholar]
- 262.Segura-Carretero A., Puertas-Mejía M.A., Cortacero-Ramírez S., Beltrán R., Alonso-Villaverde C., Joven J., Dinelli G., Fernández-Gutiérrez A. Selective extraction, separation, and identification of anthocyanins from Hibiscus sabdariffa L. using solid phase extraction-capillary electrophoresis-mass spectrometry (time-of-flight/ion trap) Electrophoresis. 2008;29:2852–2861. doi: 10.1002/elps.200700819. [DOI] [PubMed] [Google Scholar]
- 263.Rodríguez-Medina I.C., Beltrán-Debón R., Molina V.M., Alonso-Villaverde C., Joven J., Menéndez J.A., Segura-Carretero A., Fernández-Gutiérrez A. Direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J. Sep. Sci. 2009;32:3441–3448. doi: 10.1002/jssc.200900298. [DOI] [PubMed] [Google Scholar]
- 264.Hirunpanich V., Utaipat A., Morales N.P., Bunyapraphatsara N., Sato H., Herunsalee A., Suthisisang C. Antioxidant Effects of Aqueous Extracts from Dried Calyx of Hibiscus sabdariffa L INN.(Roselle) in Vitro Using Rat Low-Density Lipoprotein (LDL) Biol. Pharm. Bull. 2005;28:481–484. doi: 10.1248/bpb.28.481. [DOI] [PubMed] [Google Scholar]
- 265.Olaleye M. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. Antioxidant effect of Cysticus scoparius against carbon tetrachloride treated liver injury in rats. J. Ethonopharmacol. 2007;109:41–47. doi: 10.1016/j.jep.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 266.Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 267.Jung E., Kim Y., Joo N. Physicochemical properties and antimicrobial activity of Roselle (Hibiscus sabdariffa L.) J. Sci. Food Agric. 2013;93:3769–3776. doi: 10.1002/jsfa.6256. [DOI] [PubMed] [Google Scholar]
- 268.Chandra Shekar B.R., Nagarajappa R., Suma S., Thakur R. Herbal extracts in oral health care—A review of the current scenario and its future needs. Pharmacogn. Rev. 2015;9:87–92. doi: 10.4103/0973-7847.162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Portillo-Torres L.A., Bernardino-Nicanor A., Gómez-Aldapa C.A., González-Montiel S., Rangel-Vargas E., Villagómez-Ibarra J.R., González-Cruz L., Cortés-López H., Castro-Rosas J. Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria. Antibiotics. 2019;8:218. doi: 10.3390/antibiotics8040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Baena-Santillán E.S., Piloni-Martini J., Santos-López E.M., Gómez-Aldapa C.A., Rangel-Vargas E., Castro-Rosas J. Comparison of the Antimicrobial Activity of Hibiscus sabdariffa Calyx Extracts, Six Commercial Types of Mouthwashes, and Chlorhexidine on Oral Pathogenic Bacteria, and the Effect of Hibiscus sabdariffa Extracts and Chlorhexidine on Permeability of the Bacterial Membrane. J. Med. Food. 2021;24:67–76. doi: 10.1089/jmf.2019.0273. [DOI] [PubMed] [Google Scholar]
- 271.Subhaswaraj P., Sowmya M., Bhavana V., Dyavaiah M., Siddhardha B. Determination of antioxidant activity of Hibiscus sabdariffa and Croton caudatus in Saccharomyces cerevisiae model system. J. Food Sci. Technol. 2017;54:2728–2736. doi: 10.1007/s13197-017-2709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Riaz G., Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018;102:575–586. doi: 10.1016/j.biopha.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 273.Djeussi D.E., Noumedem J.A.K., Seukep J.A., Fankam A.G., Voukeng I.K., Tankeo S.B., Nkuete A.H.L., Kuete V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 2013;13:164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Shen C.-Y., Zhang T.-T., Zhang W.-L., Jiang J.-G. Anti-inflammatory activities of essential oil isolated from the calyx of Hibiscus sabdariffa L. Food Funct. 2016;7:4451–4459. doi: 10.1039/C6FO00795C. [DOI] [PubMed] [Google Scholar]
- 275.Hassan S.T., Berchová K., Majerová M., Pokorná M., Švajdlenka E. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016;54:1736–1740. doi: 10.3109/13880209.2015.1126618. [DOI] [PubMed] [Google Scholar]
- 276.Builders P.F., Kabele-Toge B., Builders M., Chindo B.A., Anwunobi P.A., Isimi Y.C. Wound healing potential of formulated extract from Hibiscus sabdariffa calyx. Indian J. Pharm. Sci. 2013;75:45–52. doi: 10.4103/0250-474X.113549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Omezzine F., Daami-Remadi M., Rinez A., Ladhari A., Haouala R. In vitro assessment of Inula sporganic extracts for their antifungal activity against some pathogenic and antagonistic fungi. Afr. J. Microbiol. Res. 2011;5:3527–3531. [Google Scholar]
- 278.Karygianni L., Cecere M., Skaltsounis A.L., Argyropoulou A., Hellwig E., Aligiannis N., Wittmer A., Al-Ahmad A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. BioMed Res. Int. 2014;2014:839019. doi: 10.1155/2014/839019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Danino O., Gottlieb H.E., Grossman S., Bergman M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009;42:1273–1280. doi: 10.1016/j.foodres.2009.03.023. [DOI] [Google Scholar]
- 280.Hernández V., Recio M.C., Máñez S., Giner R.M., Ríos J.-L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007;81:480–488. doi: 10.1016/j.lfs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 281.Andolfi A., Zermane N., Cimmino A., Avolio F., Boari A., Vurro M., Evidente A. Inuloxins A–D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry. 2013;86:112–120. doi: 10.1016/j.phytochem.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 282.Hertel S., Graffy L., Pötschke S., Basche S., Al-Ahmad A., Hoth-Hannig W., Hannig M., Hannig C. Effect of Inula viscosa on the pellicle’s protective properties and initial bioadhesion in-situ. Arch. Oral Biol. 2016;71:87–96. doi: 10.1016/j.archoralbio.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 283.Kurz H., Karygianni L., Argyropoulou A., Hellwig E., Skaltsounis A.L., Wittmer A., Vach K., Al-Ahmad A. Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients. 2021;13:4029. doi: 10.3390/nu13114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Oğuz H., Gökdoğan O., Baran M., Oğuz I. XXX International Horticultural Congress IHC2018: XIX Symposium on Horticultural Economics and Management, VII Symposium on 1258. ISHS; Fort Collins, CO, USA: 2018. Potential of walnut (Juglans regia L.) nursery production and its economic importance in Turkey. [Google Scholar]
- 285.Ribeiro A.S., Estanqueiro M., Oliveira M.B., Sousa Lobo J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics. 2015;2:322–341. doi: 10.3390/cosmetics2020048. [DOI] [Google Scholar]
- 286.Strelyaeva A.V., Lezhava D.I., Luferov A.N., Kuznetsov R.M., Bobkova N.V., Lazareva Yu B., Kostikova E.N. Study of Quality Medicinal Plants Bark Walnuts and Extract from it. Pharmacogn. J. 2020;12:282–286. doi: 10.5530/pj.2020.12.44. [DOI] [Google Scholar]
- 287.Croitoru A., Ficai D., Craciun L., Ficai A., Andronescu E. Evaluation and Exploitation of Bioactive Compounds of Walnut, Juglans regia. Curr. Pharm. Des. 2019;25:119–131. doi: 10.2174/1381612825666190329150825. [DOI] [PubMed] [Google Scholar]
- 288.Jahanban-Esfahlan A., Ostadrahimi A., Tabibiazar M., Amarowicz R. A Comprehensive Review on the Chemical Constituents and Functional Uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019;20:3920. doi: 10.3390/ijms20163920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Alkhawajah A.M. Studies on the antimicrobial activity of juglans regia. Am. J. Chin. Med. 1997;25:175–180. doi: 10.1142/S0192415X97000202. [DOI] [PubMed] [Google Scholar]
- 290.Zakavi F., Golpasand Hagh L., Daraeighadikolaei A., Farajzadeh Sheikh A., Daraeighadikolaei A., Leilavi Shooshtari Z. Antibacterial Effect of Juglans Regia Bark against Oral Pathologic Bacteria. Int. J. Dent. 2013;2013:854765. doi: 10.1155/2013/854765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pandita N., Mantri M., Alice V. Antiplaque Activity of Juglans Regia L. and Characterization of Juglone from Juglans Regia L. Am. J. Biochem. Biotechnol. 2011;7:29–31. [Google Scholar]
- 292.Farooq Z. Evaluation of the Acacia nilotica extract in periodontitis induced Albino rabbit. Int. J. e-Healthc. Inf. Syst. 2019;6:164–172. [Google Scholar]
- 293.Ntep Ntep D., Bong A., Ngokwe B., Nokam Kamdem G.S., Hortense G., Charles B. In Vitro Evaluation of the Efficacy of an Aqueous Extract of Allium Sativum as an Antibacterial Agent on Three Major Periodontal Pathogen. J. Oral Dent. Health Res. 2021;3:121. [Google Scholar]
- 294.Alirezaei S., Godarzi H., Moezi Ghadim N., Maheri A. Antimicrobial Activity of Aqueous Garlic Extract (Allium sativum) Against Porphyromonas gingivalis: An In-Vitro Study. J. Res. Dent. Maxillofac. Sci. 2019;4:17–22. doi: 10.29252/jrdms.4.4.17. [DOI] [Google Scholar]
- 295.George D., Bhat S.S., Antony B. Comparative evaluation of the antimicrobial efficacy of aloe vera tooth gel and two popular commercial toothpastes: An in vitro study. Gen. Dent. 2009;57:238–241. [PubMed] [Google Scholar]
- 296.Rodriguez-Garcia A., Peixoto I.T.A., Verde-Star M.J., De la Torre-Zavala S., Aviles-Arnaut H., Ruiz A.L.T.G. In Vitro Antimicrobial and Antiproliferative Activity of Amphipterygium adstringens. Evid.-Based Complement. Altern. Med. Ecam. 2015;2015:175497. doi: 10.1155/2015/175497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ambarish S.S., Anmol G.K., Bellad A.S., Krishna K. In-vitroantibacterial activity of neem, clove, and cinnamon against Actinobacillus sp., isolatedfrom chronic periodontitis patients. Biomedicine. 2020;40:214–219. [Google Scholar]
- 298.Ben Lagha A., Azelmat J., Vaillancourt K., Grenier D. A polyphenolic cinnamon fraction exhibits anti-inflammatory properties in a monocyte/macrophage model. PLoS ONE. 2021;16:e0244805. doi: 10.1371/journal.pone.0244805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Amoian B. Assessment of Antibacterial Effect of Cinnamon on Growth of Porphyromons Gingivalis in Chronic Periodontitis Patients with Deep Pockets. Babol University of Medical Sciences; Babol, Iran: 2018. [Google Scholar]
- 300.Zainal-Abidin Z., Mohd-Said S., Adibah F., Abdul Majid F., Aida W., Wan Mustapha W., Jantan I. Anti-Bacterial Activity of Cinnamon Oil on Oral Pathogens. Open Conf. Proc. J. 2013;4:12–16. doi: 10.2174/2210289201304020012. [DOI] [Google Scholar]
- 301.Zambrano L.M.G., Brandao D.A., Rocha F.R.G., Marsiglio R.P., Longo I.B., Primo F.L., Tedesco A.C., Guimaraes-Stabili M.R., Rossa C., Jr. Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease. Sci. Rep. 2018;8:6652. doi: 10.1038/s41598-018-24866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Corrêa M., Pires P., Ribeiro F., Pimentel S., Casarin R., Cirano F., Tenenbaum H., Casati M. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 2017;52:201–209. doi: 10.1111/jre.12382. [DOI] [PubMed] [Google Scholar]
- 303.Paavai I., Vasugi S., Ayswarya V.V., Vanessa R., Veejai C., Arulpari M., Vineela Katam R. Evaluation of Antibacterial Activity of Lemongrass Oil against Oral Clinical Isolates—An In vitro Study. Pharmacogn. J. 2019;11:1023–1028. [Google Scholar]
- 304.Harkat-Madouri L., Asma B., Madani K., Said Z.B.-O.S., Rigou P., Grenier D., Allalou H., Remini H., Adjaoud A., Boulekbache-Makhlouf L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015;78:148–153. doi: 10.1016/j.indcrop.2015.10.015. [DOI] [Google Scholar]
- 305.Ridwan R.D., Yuliati Y., Sidarningsih S., Sholihah F.M., Aljunaid M., Lashari D.M. A study of the mucoadhesive patches loaded with mangosteen peel extract in periodontitis. J. Taibah Univ. Med. Sci. 2021;16:864–869. doi: 10.1016/j.jtumed.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Suwannakul S., Chaibenjawong P. Antibacterial Activities of Glycyrrhiza gabra Linn. (Licorice) Root Extract against Porphyromonas gingivalis rand Its Inhibitory Effects on Cysteine Proteases and Biofilms. J. Dent. Indones. 2017;24:85–92. doi: 10.14693/jdi.v24i3.1075. [DOI] [Google Scholar]
- 307.Sharma H., Yunus G., Mohapatra A., Kulshrestha R., Agrawal R., Kalra M. Antimicrobial efficacy of three medicinal plants Glycyrrhiza glabra, Ficus religiosa, and Plantago major on inhibiting primary plaque colonizers and periodontal pathogens: An in vitro study. Indian J. Dent. Res. 2016;27:200–204. doi: 10.4103/0970-9290.183135. [DOI] [PubMed] [Google Scholar]
- 308.Khawlah A.S., Sahar M.J., Najla Naji C., Alaa S.J.A.-B. Efficacy of Bark (Juglans regia L.) Extracts Against Periodontitis Bacteria: An In Vitro Study. Indian J. Forensic Med. Toxicol. 2021;15:5492–5498. [Google Scholar]
- 309.Botelho M.A., Barros G., Queiroz D.B., Carvalho C.F., Gouvea J., Patrus L., Bannet M., Patrus D., Rego A., Silva I., et al. Nanotechnology in Phytotherapy: Antiinflammatory Effect of a Nanostructured Thymol Gel from Lippia sidoides in Acute Periodontitis in Rats. Phytother. Res. 2016;30:152–159. doi: 10.1002/ptr.5516. [DOI] [PubMed] [Google Scholar]
- 310.Aparna S., Srirangarajan S., Malgi V., Setlur K.P., Shashidhar R., Setty S., Thakur S. A comparative evaluation of the antibacterial efficacy of honey in vitro and antiplaque efficacy in a 4-day plaque regrowth model in vivo: Preliminary results. J. Periodontol. 2012;83:1116–1121. doi: 10.1902/jop.2012.110461. [DOI] [PubMed] [Google Scholar]
- 311.Jangid K., Bhargava V., Jayakumar D. Anti-protease activity of Myristica fragrans compared to doxycycline on periodontal tissues: An ex-vivo study. Res. J. Pharm. Biol. Chem. Sci. 2016;7:984–988. [Google Scholar]
- 312.Kaawoan P.T., Abidjulu J., Siagian K.V. Uji daya hambat ekstrak buah pala (Myristica fragrans Houtt) terhadap bakteri penyebab periodontitis porphyromonas gingivalis secara in vitro. e-GiGi. 2016;4:13504. doi: 10.35790/eg.4.2.2016.13504. [DOI] [Google Scholar]
- 313.Shinohara C., Mori S., Ando T., Tsuji T. Arg-Gingipain Inhibition and Anti-bacterial Activity Selective for Porphyromonas gingivalis by Malabaricone C. Biosci. Biotechnol. Biochem. 1999;63:1475–1477. doi: 10.1271/bbb.63.1475. [DOI] [PubMed] [Google Scholar]
- 314.Mallikarjun S., Rao A., Rajesh G., Shenoy R., Pai M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: An in vitro study. J. Indian Soc. Periodontol. 2016;20:145–150. doi: 10.4103/0972-124X.175177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hosadurga R.R., Rao S.N., Edavanputhalath R., Jose J., Rompicharla N.C., Shakil M., Raju S. Evaluation of the efficacy of 2% Ocimum sanctum gel in the treatment of experimental periodontitis. Int. J. Pharm. Investig. 2015;5:35–42. doi: 10.4103/2230-973X.147231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Azeez S.H., Gaphor S.M. Evaluation of antibacterial effect against Porphyromonas gingivalis and biocompatibility of essential oil extracted from the gum of Pistacia atlantica kurdica. BioMed Res. Int. 2019;2019:9195361. doi: 10.1155/2019/9195361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sekar S., Jacob S., Suthanthiran T.K., Dhasthaheer S., Vikraman S., Kaliappan K. Characterization and Formulation of Miswak Film for the Treatment of Chronic Periodontitis: An In Vitro Study. J. Pharm. Bioallied. Sci. 2020;12((Suppl. S1)):S199–S203. doi: 10.4103/jpbs.JPBS_59_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Siddeeqh S., Parida A., Jose M., Pai V. Estimation of Antimicrobial Properties of Aqueous and Alcoholic Extracts of Salvadora Persica (Miswak) on Oral Microbial Pathogens—An Invitro Study. J. Clin. Diagn. Res. 2016;10:Fc13–Fc16. doi: 10.7860/JCDR/2016/22213.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gursoy U.K., Gursoy M., Gursoy O.V., Cakmakci L., Könönen E., Uitto V.J. Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe. 2009;15:164–167. doi: 10.1016/j.anaerobe.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 320.Fotsing Kwetché P.R., Essama Eno Belinga L., Yawat Djogang A.M., Chamdjeu V.T., Agbor M.A., Tchoukoua S.H., Tekam J.M. Antibacterial Potential of Hydro-Ethanolic Extracts from Syzygium Aromaticum (Myrtaceae) on Periodontal Bacteria. Health Sci. Dis. 2021;22:2530. [Google Scholar]
- 321.Lee J., Nho Y.H., Yun S.K., Hwang Y.S. Use of ethanol extracts of Terminalia chebula to prevent periodontal disease induced by dental plaque bacteria. BMC Complement. Altern. Med. 2017;17:113. doi: 10.1186/s12906-017-1619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Labrecque J., Bodet C., Chandad F., Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J. Antimicrob. Chemother. 2006;58:439–443. doi: 10.1093/jac/dkl220. [DOI] [PubMed] [Google Scholar]
- 323.Özden F.O., Sakallioğlu E.E., Sakallioğlu U., Ayas B., Erişgin Z. Effects of grape seed extract on periodontal disease: An experimental study in rats. J. Appl. Oral Sci. 2017;25:121–129. doi: 10.1590/1678-77572016-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Park M., Bae J., Lee D.S. Antibacterial activity of [10} gingerol and [12} gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- 325.Funari C.S., Gullo F.P., Napolitano A., Carneiro R.L., Mendes-Giannini M.J., Fusco-Almeida A.M., Piacente S., Pizza C., Silva D.H. Chemical and antifungal investigations of six Lippia species (Verbenaceae) from Brazil. Food Chem. 2012;135:2086–2094. doi: 10.1016/j.foodchem.2012.06.077. [DOI] [PubMed] [Google Scholar]
- 326.Pinto Cda P., Rodrigues V.D., Pinto Fda P., Pinto Rda P., Uetanabaro A.P., Pinheiro C.S., Gadea S.F., Silva T.R., Lucchese A.M. Antimicrobial activity of lippia species from the brazilian semiarid region traditionally used as antiseptic and anti-infective agents. Evid.-Based Complement. Altern. Med. 2013;2013:614501. doi: 10.1155/2013/614501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Fontenelle R.O., Morais S.M., Brito E.H., Kerntopf M.R., Brilhante R.S., Cordeiro R.A., Tomé A.R., Queiroz M.G., Nascimento N.R., Sidrim J.J., et al. Chemical composition, toxicological aspects and antifungal activity of essential oil from Lippia sidoides Cham. J. Antimicrob. Chemother. 2007;59:934–940. doi: 10.1093/jac/dkm066. [DOI] [PubMed] [Google Scholar]
- 328.Gomes G.A., Monteiro C.M., Senra Tde O., Zeringota V., Calmon F., Matos Rda S., Daemon E., Gois R.W., Santiago G.M., de Carvalho M.G. Chemical composition and acaricidal activity of essential oil from Lippia sidoides on larvae of Dermacentor nitens (Acari: Ixodidae) and larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae) Parasitol. Res. 2012;111:2423–2430. doi: 10.1007/s00436-012-3101-9. [DOI] [PubMed] [Google Scholar]
- 329.Pascual M.E., Slowing K., Carretero E., Sánchez Mata D., Villar A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001;76:201–214. doi: 10.1016/S0378-8741(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 330.Botelho M.A., Bezerra Filho J.G., Correa L.L., Fonseca S.G., Montenegro D., Gapski R., Brito G.A., Heukelbach J. Effect of a novel essential oil mouthrinse without alcohol on gingivitis: A double-blinded randomized controlled trial. J. Appl. Oral Sci. 2007;15:175–180. doi: 10.1590/S1678-77572007000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rodrigues I.S., Tavares V.N., Pereira S.L., Costa F.N. Antiplaque and antigingivitis effect of Lippia Sidoides: A double-blind clinical study in humans. J. Appl. Oral Sci. 2009;17:404–407. doi: 10.1590/S1678-77572009000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Botelho M.A., Rao V.S., Carvalho C.B., Bezerra-Filho J.G., Fonseca S.G., Vale M.L., Montenegro D., Cunha F., Ribeiro R.A., Brito G.A. Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. J. Ethnopharmacol. 2007;113:471–478. doi: 10.1016/j.jep.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 333.Girão V.C., Nunes-Pinheiro D.C., Morais S.M., Sequeira J.L., Gioso M.A. A clinical trial of the effect of a mouth-rinse prepared with Lippia sidoides Cham essential oil in dogs with mild gingival disease. Prev. Vet. Med. 2003;59:95–102. doi: 10.1016/S0167-5877(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 334.Gomes de Alencar-Araripe M., Sousa Nunes-Pinheiro D.C., Odebrecht Costa B., Sampaio Batista L., Silva Feitosa M., Gama de Almeida G.K., da Rocha Tomé A., Carneiro Girão V. A Clinical Trial and Oral Wound Treated by Essential Oil of Lippia sidoides Mouthrinse in Horses. Acta Sci. Vet. 2014;42:1249. [Google Scholar]
- 335.Coe F.G., Anderson G.J. Screening of medicinal plants used by the Garífuna of eastern Nicaragua for bioactive compounds. J. Ethnopharmacol. 1996;53:29–50. doi: 10.1016/0378-8741(96)01424-9. [DOI] [PubMed] [Google Scholar]
- 336.Andreu G.P., Delgado R., Velho J.A., Curti C., Vercesi A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005;513:47–55. doi: 10.1016/j.ejphar.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 337.Leiro J.M., Alvarez E., Arranz J.A., Siso I.G., Orallo F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-alpha and transforming growth factor-beta genes. Biochem. Pharmacol. 2003;65:1361–1371. doi: 10.1016/S0006-2952(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 338.Li H., Miyahara T., Tezuka Y., Namba T., Nemoto N., Tonami S., Seto H., Tada T., Kadota S. The effect of Kampo formulae on bone resorption in vitro and in vivo. I. Active constituents of Tsu-kan-gan. Biol. Pharm. Bull. 1998;21:1322–1326. doi: 10.1248/bpb.21.1322. [DOI] [PubMed] [Google Scholar]
- 339.Bairy I., Reeja S., Siddharth, Rao P.S., Bhat M., Shivananda P.G. Evaluation of antibacterial activity of Mangifera indica on anaerobic dental microglora based on in vivo studies. Indian J. Pathol. Microbiol. 2002;45:307–310. [PubMed] [Google Scholar]
- 340.Israili Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014;21:304–323. doi: 10.1097/MJT.0b013e318293b09b. [DOI] [PubMed] [Google Scholar]
- 341.Schneider M., Coyle S., Warnock M., Gow I., Fyfe L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013;27:1162–1168. doi: 10.1002/ptr.4844. [DOI] [PubMed] [Google Scholar]
- 342.Al-Waili N., Salom K., Al-Ghamdi A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. TheScientificWorldJournal. 2011;11:766–787. doi: 10.1100/tsw.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Al-Waili N., Al-Ghamdi A., Ansari M., Al-Attar Y., Osman A., Salom K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013;44:307–316. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 344.Jull A.B., Cullum N., Dumville J.C., Westby M.J., Deshpande S., Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015;2015:CD005083. doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Charalambous M., Raftopoulos V., Lambrinou E., Charalambous A. The effectiveness of honey for the management of radiotherapy-induced oral mucositis in head and neck cancer patients: A systematic review of clinical trials. Eur. J. Integr. Med. 2013;5:217–225. doi: 10.1016/j.eujim.2013.01.003. [DOI] [Google Scholar]
- 346.Alvarez-Suarez J.M., Gasparrini M., Forbes-Hernández T.Y., Mazzoni L., Giampieri F. The composition and biological activity of honey: A focus on Manuka honey. Foods. 2014;3:420–432. doi: 10.3390/foods3030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Molan P.C. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World. 1992;73:5–28. doi: 10.1080/0005772X.1992.11099109. [DOI] [Google Scholar]
- 348.Allen K.L., Molan P.C., Reid G.M. A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 1991;43:817–822. doi: 10.1111/j.2042-7158.1991.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 349.Cooper R., Molan P., Harding K. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002;93:857–863. doi: 10.1046/j.1365-2672.2002.01761.x. [DOI] [PubMed] [Google Scholar]
- 350.Maddocks S.E., Lopez M.S., Rowlands R.S., Cooper R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology. 2012;158:781–790. doi: 10.1099/mic.0.053959-0. [DOI] [PubMed] [Google Scholar]
- 351.Hammond E.N., Donkor E.S., Brown C.A. Biofilm formation of Clostridium difficile and susceptibility to Manuka honey. BMC Complement. Altern. Med. 2014;14:329. doi: 10.1186/1472-6882-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Badet C., Quero F. The in vitro effect of manuka honeys on growth and adherence of oral bacteria. Anaerobe. 2011;17:19–22. doi: 10.1016/j.anaerobe.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 353.Schmidlin P.R., English H., Duncan W., Belibasakis G.N., Thurnheer T. Antibacterial potential of Manuka honey against three oral bacteria in vitro. Swiss Dent. J. 2014;124:922–924. doi: 10.61872/sdj-2014-09-01. [DOI] [PubMed] [Google Scholar]
- 354.Haffajee A.D. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 355.Slots J., Reynolds H.S., Genco R.J. Actinobacillus actinomycetemcomitans in human periodontal disease: A cross-sectional microbiological investigation. Infect. Immun. 1980;29:1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Eick S., Schäfer G., Kwieciński J., Atrott J., Henle T., Pfister W. Honey—A potential agent against Porphyromonas gingivalis: An in vitro study. BMC Oral Health. 2014;14:24. doi: 10.1186/1472-6831-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Safii S.H., Tompkins G.R., Duncan W.J. Periodontal Application of Manuka Honey: Antimicrobial and Demineralising Effects In Vitro. Int. J. Dent. 2017;2017:9874535. doi: 10.1155/2017/9874535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Srivastava J.K., Shankar E., Gupta S. Chamomile: A herbal medicine of the past with a bright future. Mol. Med. Rep. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 360.Avallone R., Zanoli P., Puia G., Kleinschnitz M., Schreier P., Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59:1387–1394. doi: 10.1016/S0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 361.Srivastava J.K., Pandey M., Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Martins M.D., Marques M.M., Bussadori S.K., Martins M.A.T., Pavesi V.C.S., Mesquita-Ferrari R.A., Fernandes K.P.S. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009;23:274–278. doi: 10.1002/ptr.2612. [DOI] [PubMed] [Google Scholar]
- 363.Pourabbas R., Delazar A., Chitsaz M.T. The effect of German chamomile mouthwash on dental plaque and gingival inflammation. Iran. J. Pharm. Res. 2010:105–109. [Google Scholar]
- 364.Willershausen B., Kasaj A., Sculean A., Wehrbein H. Influence of an Herbal Mouthwash on Inflammatory Changes of the Gingiva in Patients with Fixed Orthodontic Appliance. Periodontal Pract. Today. 2004;1:255–262. [Google Scholar]
- 365.Batista A.L.A., Lins R.D.A.U., de Souza Coelho R., do Nascimento Barbosa D., Belém N.M., Celestino F.J.A. Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complement. Ther. Clin. Pract. 2014;20:93–98. doi: 10.1016/j.ctcp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 366.Tiemann P., Toelg M., Ramos F M.H. Administration of Ratanhia-based herbal oral care products for the prophylaxis of oral mucositis in cancer chemotherapy patients: A clinical trial. Evid.-Based Complement. Altern. Med. 2007;4:361–366. doi: 10.1093/ecam/nel070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Al-Mustafa A.H., Al-Thunibat O.Y. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci. 2008;11:351–358. doi: 10.3923/pjbs.2008.351.358. [DOI] [PubMed] [Google Scholar]
- 368.Siddiqui N.A. Chemical constituents of essential oil from flowers of Matricaria aurea grown in Saudi Arabia. Indian J. Drugs. 2014;2:164–168. [Google Scholar]
- 369.Singh O., Khanam Z., Misra N., Srivastava M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011;5:82. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ahmad I., Wahab S., Nisar N., Dera A.A., Alshahrani M.Y., Abullias S.S., Irfan S., Alam M.M., Srivastava S. Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020;28:1203–1209. doi: 10.1016/j.jsps.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Chan E.W.C., Lye P.-Y., Wong S.-K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016;14:17–30. doi: 10.3724/SP.J.1009.2016.00017. [DOI] [PubMed] [Google Scholar]
- 372.Gunjal S., Ankola A., Bhat K. In vitro antibacterial activity of ethanolic extract of Morus alba leaf against periodontal pathogens. Indian J. Dent. Res. 2015;26:533–536. doi: 10.4103/0970-9290.172082. [DOI] [PubMed] [Google Scholar]
- 373.Soonthornsit N., Pitaksutheepong C., Hemstapat W., Utaisincharoen P., Pitaksuteepong T. In Vitro Anti-Inflammatory Activity of Morus alba L. Stem Extract in LPS-Stimulated RAW 264.7 Cells. Evid.-Based Complement. Altern. Med. 2017;2017:3928956. doi: 10.1155/2017/3928956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yang Z.-G., Matsuzaki K., Takamatsu S., Kitanaka S. Inhibitory Effects of Constituents from Morus alba var. multicaulis on Differentiation of 3T3-L1 Cells and Nitric Oxide Production in RAW264.7 Cells. Molecules. 2011;16:6010–6022. doi: 10.3390/molecules16076010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Jaiswal P., Kumar P., Singh V.K., Singh D.K. Biological effects of Myristica fragrans. Annu. Rev. Biomed. Sci. 2009;11:21–29. [Google Scholar]
- 376.Yang X.-W., Huang X., Ahmat M. New neolignan from seed of Myristica fragrans. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Medica. 2008;33:397–402. [PubMed] [Google Scholar]
- 377.Chung J.Y., Choo J.H., Lee M.H., Hwang J.K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006;13:261–266. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 378.Sonavane G.S., Sarveiya V.P., Kasture V.S., Kasture S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002;71:239–244. doi: 10.1016/S0091-3057(01)00660-8. [DOI] [PubMed] [Google Scholar]
- 379.Hussain S., Rao A. Chemopreventive action of mace (Myristica fragrans, Houtt) on methylcholanthrene-induced carcinogenesis in the uterine cervix in mice. Cancer Lett. 1991;56:231–234. doi: 10.1016/0304-3835(91)90007-5. [DOI] [PubMed] [Google Scholar]
- 380.Ozaki Y., Soedigdo S., Wattimena Y.R., Suganda A.G. Antiinflammatory effect of mace, aril of Myristica fragrans Houtt., and its active principles. Jpn. J. Pharmacol. 1989;49:155–163. doi: 10.1016/S0021-5198(19)43064-3. [DOI] [PubMed] [Google Scholar]
- 381.Narasimhan B., Dhake A.S. Antibacterial principles from Myristica fragrans seeds. J. Med. Food. 2006;9:395–399. doi: 10.1089/jmf.2006.9.395. [DOI] [PubMed] [Google Scholar]
- 382.Shafiei Z., Shuhairi N.N., Md Fazly Shah Yap N., Harry Sibungkil C.-A., Latip J. Antibacterial Activity of Myristica fragrans against Oral Pathogens. Evid.-Based Complement. Altern. Med. Ecam. 2012;2012:825362. doi: 10.1155/2012/825362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Yoon W.J., Kim S.S., Oh T.H., Lee N.H., Hyun C.G. Torreya nucifera Essential Oil Inhibits Skin Pathogen Growth and Lipopolysaccharide-Induced Inflammatory Effects. Int. J. Pharmacol. 2009;5:37–43. doi: 10.3923/ijp.2009.37.43. [DOI] [Google Scholar]
- 385.Hart P.H., Brand C., Carson C.F., Riley T.V., Prager R.H., Finlay-Jones J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000;49:619–626. doi: 10.1007/s000110050639. [DOI] [PubMed] [Google Scholar]
- 386.Juhás Š., Bukovska A., Čikoš Š., Czikková S., Fabian D., Koppel J. Anti-Inflammatory Effects of Rosmarinus officinalis Essential Oil in Mice. Acta Vet. Brno. 2009;78:121–127. doi: 10.2754/avb200978010121. [DOI] [Google Scholar]
- 387.Gomes A., Fernandes E., Lima J.L., Mira L., Corvo M.L. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr. Med. Chem. 2008;15:1586–1605. doi: 10.2174/092986708784911579. [DOI] [PubMed] [Google Scholar]
- 388.Anggakusuma, Yanti, Hwang J.-K. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J. Dermatol. Sci. 2010;57:114–122. doi: 10.1016/j.jdermsci.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 389.Lee K.E., Mun S., Pyun H.B., Kim M.S., Hwang J.K. Effects of macelignan isolated from Myristica fragrans (Nutmeg) on expression of matrix metalloproteinase-1 and type I procollagen in UVB-irradiated human skin fibroblasts. Biol. Pharm. Bull. 2012;35:1669–1675. doi: 10.1248/bpb.b12-00037. [DOI] [PubMed] [Google Scholar]
- 390.Sethi J., Sood S., Seth S., Talwar A. Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian J. Clin. Biochem. 2004;19:152–155. doi: 10.1007/BF02894276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Devi P.U., Ganasoundari A. Modulation of glutathione and antioxidant enzymes by Ocimum sanctum and its role in protection against radiation injury. Indian J. Exp. Biol. 1999;37:262–268. [PubMed] [Google Scholar]
- 392.Sharma P., Kulshreshtha S., Sharma A. Anti-cataract activity of Ocimum sanctum on experimental cataract. Indian J. Pharmacol. 1998;30:16. [Google Scholar]
- 393.Agarwal P., Nagesh L. Evaluation of the antimicrobial activity of various concentrations of Tulsi (Ocimum sanctum) extract against Streptococcus mutans: An in vitro study. Indian J. Dent. Res. 2010;21:357–359. doi: 10.4103/0970-9290.70800. [DOI] [PubMed] [Google Scholar]
- 394.Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 395.Mondal S., Varma S., Bamola V.D., Naik S.N., Mirdha B.R., Padhi M.M., Mehta N., Mahapatra S.C. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J. Ethnopharmacol. 2011;136:452–456. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 396.Prakash P., Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J. Physiol. Pharmacol. 2005;49:125–131. [PubMed] [Google Scholar]
- 397.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002;40:158–168. doi: 10.5414/CPP40158. [DOI] [PubMed] [Google Scholar]
- 398.Packer L., Rimbach G., Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999;27:704–724. doi: 10.1016/S0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 399.Cho K.J., Yun C.H., Packer L., Chung A.S. Inhibition mechanisms of bioflavonoids extracted from the bark of Pinus maritima on the expression of proinflammatory cytokines. Ann. N. Y. Acad. Sci. 2001;928:141–156. doi: 10.1111/j.1749-6632.2001.tb05644.x. [DOI] [PubMed] [Google Scholar]
- 400.Hosseini S., Pishnamazi S., Sadrzadeh S.M.H., Farid F., Farid R., Watson R.R. Pycnogenol® in the Management of Asthma. J. Med. Food. 2001;4:201–209. doi: 10.1089/10966200152744472. [DOI] [PubMed] [Google Scholar]
- 401.Lau B.H., Riesen S.K., Truong K.P., Lau E.W., Rohdewald P., Barreta R.A. Pycnogenol as an adjunct in the management of childhood asthma. J. Asthma. 2004;41:825–832. doi: 10.1081/JAS-200038433. [DOI] [PubMed] [Google Scholar]
- 402.Kimbrough C., Chun M., dela Roca G., Lau B.H.S. Pycnogenol® chewing gum minimizes gingival bleeding and plaque formation. Phytomedicine. 2002;9:410–413. doi: 10.1078/09447110260571643. [DOI] [PubMed] [Google Scholar]
- 403.Sequeda-Castañeda L., Célis C., Gutiérrez S., Gamboa F. Piper marginatum jacq.(piperaceae): Phytochemical, therapeutic, botanical insecticidal and phytosanitary uses. Pharmacol. Online. 2015;3:136–145. [Google Scholar]
- 404.Delgado-Paredes G.E., Kato M.J., Vásquez-Dueñas N., Minchala-Patiño J., Rojas-Idrogo C. Cultivo de tejidos de Piper sp. (Piperaceae): Propagación, organogénesis y conservación de germoplasma in vitro. Rev. Colomb. Biotecnol. 2012;14:49–60. [Google Scholar]
- 405.Dueñas J.F., Jarrett C., Cummins I., Logan–Hines E. Amazonian Guayusa (Ilex guayusa Loes.): A historical and ethnobotanical overview. Econ. Bot. 2016;70:85–91. doi: 10.1007/s12231-016-9334-2. [DOI] [Google Scholar]
- 406.Gamboa F., Muñoz C.-C., Numpaque G., Sequeda-Castañeda L.G., Gutierrez S.J., Tellez N. Antimicrobial activity of Piper marginatum Jacq and Ilex guayusa Loes on microorganisms associated with periodontal disease. Int. J. Microbiol. 2018;2018:4147383. doi: 10.1155/2018/4147383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Barra A., Coroneo V., Dessi S., Cabras P., Angioni A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. J. Agric. Food Chem. 2007;55:7093–7098. doi: 10.1021/jf071129w. [DOI] [PubMed] [Google Scholar]
- 408.Di Rosa A. Erbe e Piante Medicinali in Sardegna. La Nuova; Sassari, Italy: 2018. [Google Scholar]
- 409.Sakagami H., Kishino K., Kobayashi M., Hashimoto K., Iida S., Shimetani A., Nakamura Y., Takahashi K., Ikarashi T., Fukamachi H. Selective antibacterial and apoptosis-modulating activities of mastic. In Vivo. 2009;23:215–223. [PubMed] [Google Scholar]
- 410.Paraschos S., Mitakou S., Skaltsounis A.-L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012;19:2292–2302. doi: 10.2174/092986712800229014. [DOI] [PubMed] [Google Scholar]
- 411.Paraschos S., Magiatis P., Mitakou S., Petraki K., Kalliaropoulos A., Maragkoudakis P., Mentis A., Sgouras D., Skaltsounis A.-L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007;51:551–559. doi: 10.1128/AAC.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sterer N. Antimicrobial effect of mastic gum methanolic extract against Porphyromonas gingivalis. J. Med. Food. 2006;9:290–292. doi: 10.1089/jmf.2006.9.290. [DOI] [PubMed] [Google Scholar]
- 413.Kamrani Y., Amanlou M., Esmaeelian B., Bidhendi S.M., SahebJamei M. Inhibitory effects of a flavonoid-rich extract of Pistacia vera hull on growth and acid production of bacteria involved in dental plaque. Int. J. Pharmacol. 2007;3:219–226. [Google Scholar]
- 414.Milia E., Usai M., Szotáková B., Elstnerová M., Králová V., D’Hallewin G., Spissu Y., Barberis A., Marchetti M., Bortone A., et al. The Pharmaceutical Ability of Pistacia lentiscus L. Leaves Essential Oil Against Periodontal Bacteria and Candida sand Its Anti-Inflammatory Potential. Antibiotics. 2020;9:281. doi: 10.3390/antibiotics9060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Azeez S.H., Gaphor S.M., Sha A.M., Garib B.T. Effect of Pistacia atlantica subskurdica Gum in Experimental Periodontitis Induced in Wistar Rats by Utilization of Osteoclastogenic Bone Markers. Molecules. 2020;25:5819. doi: 10.3390/molecules25245819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Joseph B., Priya R. Phytochemical and biopharmaceutical aspects of Psidium guajava (L.) essential oil: A review. Res. J. Med. Plants. 2011;5:432–442. doi: 10.3923/rjmp.2011.432.442. [DOI] [Google Scholar]
- 417.Rishika D., Sharma R. An update of pharmacological activity of Psidium guajava in the management of various disorders. Int. J. Pharm. Sci. Res. 2012;3:3577. [Google Scholar]
- 418.Prabu G., Gnanamani A., Sadulla S. Guaijaverin—A plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006;101:487–495. doi: 10.1111/j.1365-2672.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- 419.Ushimaru P.I., Silva M.T.N.d., Di Stasi L.C., Barbosa L., Fernandes A., Jr. Antibacterial activity of medicinal plant extracts. Braz. J. Microbiol. 2007;38:717–719. doi: 10.1590/S1517-83822007000400024. [DOI] [Google Scholar]
- 420.Joseph B., Priya R.M., Helen P.M., Sujatha S. Bio-active compounds in essential oil and its effects of antimicrobial, cytotoxic activity from the Psidium guajava (L.) leaf. J. Adv. Biotechnol. 2010;9:10–14. [Google Scholar]
- 421.Gashe F., Belete A., Gebre-Mariam T. Evaluation of antimicrobial and anti-inflammatory activities and formulation studies on the leaf extracts of Psidium guajava L. Ethiop. Pharm. J. 2010;28:131–142. [Google Scholar]
- 422.Tona L., Kambu K., Ntondele D., Cimanga K. Antimicrobial activity of tannins. Filoterapia. 1994;65:276–278. [Google Scholar]
- 423.Shu Y., Liu Y., Li L., Feng J., Lou B., Zhou X., Wu H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011;5:5358–5361. [Google Scholar]
- 424.Geoghegan F., Wong R., Rabie A. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytother. Res. 2010;24:817–820. doi: 10.1002/ptr.3014. [DOI] [PubMed] [Google Scholar]
- 425.Abdelrahim S., Almagboul A., Omer M., Elegami A. Antimicrobial activity of Psidium guajava L. Fitoterapia. 2002;73:713–715. doi: 10.1016/S0367-326X(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 426.Limsong J., Benjavongkulchai E., Kuvatanasuchati J. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J. Ethnopharmacol. 2004;92:281–289. doi: 10.1016/j.jep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 427.Brötz-Oesterhelt H., Beyer D., Kroll H.-P., Endermann R., Ladel C., Schroeder W., Hinzen B., Raddatz S., Paulsen H., Henninger K. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 2005;11:1082–1087. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 428.Esimone C., Nworu C., Ekong U., Iroha I., Okolin C. A case for the use of herbal extracts in oral hygiene: The efficacy of Psidium guajava-based mouthwash formulations. Res. J. Appl. Sci. 2007;2:1143–1149. [Google Scholar]
- 429.Kraivaphan V., Boonyamanound L., Amornchat C., Trirantana T., Kraivaphan P. The effect of a mouthrinse containing Psidium guajava leaf extract on gingivitis. J. Dent. Assoc. Thai. 1991;41:323–328. [Google Scholar]
- 430.Fathilah A., Sujata R., Norhanom A., Adenan M. Antiproliferative activity of aqueous extract of Piper betle L. and Psidium guajava L. on KB and HeLa cell lines. J. Med. Plants Res. 2010;4:987–990. [Google Scholar]
- 431.Kavimani S., Ilango R., Vertichelvan T. Anti–Inflammatory activity of volatile oil of Psidium guajava. Anc. Sci. Life. 1998;17:300. [PMC free article] [PubMed] [Google Scholar]
- 432.Kwamin F., Gref R., Haubek D., Johansson A. Interactions of extracts from selected chewing stick sources with Aggregatibacter actinomycetemcomitans. BMC Res. Notes. 2012;5:203. doi: 10.1186/1756-0500-5-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Joyce O., Chinwe N., Kwaku J., Tabot P. Psidium guajava’s effect on acute phase protein levels during acute inflammation. Am. J. Pharm.Tech. Res. 2012;2:424–433. [Google Scholar]
- 434.Kaileh M., Berghe W.V., Boone E., Essawi T., Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J. Ethnopharmacol. 2007;113:510–516. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 435.Koide M., Kinugawa S., Takahashi N., Udagawa N. Osteoclastic bone resorption induced by innate immune responses. Periodontology 2000. 2010;54:235–246. doi: 10.1111/j.1600-0757.2010.00355.x. [DOI] [PubMed] [Google Scholar]
- 436.Choi S.Y., Hwang J.H., Park S.Y., Jin Y.J., Ko H.C., Moon S.W., Kim S.J. Fermented guava leaf extract inhibits LPS-induced COX-2 and iNOS expression in Mouse macrophage cells by inhibition of transcription factor NF-κB. Phytother. Res. 2008;22:1030–1034. doi: 10.1002/ptr.2419. [DOI] [PubMed] [Google Scholar]
- 437.Åsman B., Bergström K. Expression of Fc-γ-RIII and fibronectin in peripheral neutrophils with increased response to Fc stimulation in patients with Juvenile periodontitis. Arch. Oral Biol. 1992;37:991–995. doi: 10.1016/0003-9969(92)90030-C. [DOI] [PubMed] [Google Scholar]
- 438.Battino M., Bullon P., Wilson M., Newman H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit. Rev. Oral Biol. Med. 1999;10:458–476. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- 439.Figuero E., Soory M., Cerero R., Bascones A. Oxidant/antioxidant interactions of nicotine, Coenzyme Q10, Pycnogenol and phytoestrogens in oral periosteal fibroblasts and MG63 osteoblasts. Steroids. 2006;71:1062–1072. doi: 10.1016/j.steroids.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 440.Carnelio S., Khan S.A., Rodrigues G. Definite, probable or dubious: Antioxidants trilogy in clinical dentistry. Br. Dent. J. 2008;204:29–32. doi: 10.1038/bdj.2007.1186. [DOI] [PubMed] [Google Scholar]
- 441.Fasola T.R., Oloyede G.K., Aponjolosun B.S. Chemical composition, toxicity and antioxidant activities of essential oils of stem bark of Nigerian species of guava (Psidium guajava Linn.) Excli J. 2011;10:34. [PMC free article] [PubMed] [Google Scholar]
- 442.Vyas N., Tailang M., Gavatia N.P., Gupta B.K. Antioxidant potential of Psidium guajava Linn. Int. J. PharmTech Res. 2010;2:417–419. [Google Scholar]
- 443.He Q., Venant N. Antioxidant power of phytochemicals fromPsidium guajava leaf. J. Zhejiang Univ. Sci. A. 2004;5:676–683. doi: 10.1007/BF02840979. [DOI] [PubMed] [Google Scholar]
- 444.Dakappa S.S., Adhikari R., Timilsina S.S., Sajjekhan S. A review on the medicinal plant Psidium guajava Linn. (Myrtaceae) J. Drug Deliv. Ther. 2013;3 doi: 10.22270/jddt.v3i2.404. [DOI] [Google Scholar]
- 445.Oguntibeju O.O. The biochemical, physiological and therapeutic roles of ascorbic acid. Afr. J. Biotechnol. 2008;7 [Google Scholar]
- 446.Brown L., Jones D. Handbook of Antioxidants. Marcel Dekker; New York, NY, USA: 1996. [Google Scholar]
- 447.Fernandes K.P.S., Bussadori S.K., Marques M.M., Wadt N.S.Y., Bach E., Martins M.D. Healing and cytotoxic effects of Psidium guajava (Myrtaceae) leaf extracts. Braz. J. Oral Sci. 2010;9:449–454. [Google Scholar]
- 448.Kumar P., Ansari S.H., Ali J. Herbal remedies for the treatment of periodontal disease—A patent review. Recent Pat. Drug Deliv. Formul. 2009;3:221–228. doi: 10.2174/187221109789105603. [DOI] [PubMed] [Google Scholar]
- 449.Chapple I.L. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin. Mol. Pathol. 1996;49:M247. doi: 10.1136/mp.49.5.M247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Mittal P., Gupta V., Kaur G., Garg A.K., Singh A. Phytochemistry and pharmacological activities of Psidium guajava. IJPSR. 2010;1:9–19. [Google Scholar]
- 451.Salgado A.D., Maia J.L., Pereira S.L., de Lemos T.L., Mota O.M. Antiplaque and antigingivitis effects of a gel containing Punica granatum Linn extract: A double-blind clinical study in humans. J. Appl. Oral Sci. 2006;14:162–166. doi: 10.1590/S1678-77572006000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Jurenka J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 453.Arun N., Singh D. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012;3:1240. [Google Scholar]
- 454.Pereira J., Pereira M., Higino J., Sampio F., Alves P., Araujo C. Studies with the extract of the Punica granatum Linn. (Pomegranate): Antimicrobial effect “in vitro” and clinical evaluation of a toothpaste upon microorganisms of the oral biofilm. J. Dent. Sci. 2005;20:262–269. [Google Scholar]
- 455.Teniente S.L., Flores-Gallegos A.C., Esparza-González S.C., Campos-Múzquiz L.G., Nery-Flores S.D., Rodríguez-Herrera R. Anticancer Effect of Pomegranate Peel Polyphenols against Cervical Cancer. Antioxidants. 2023;12:127. doi: 10.3390/antiox12010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Shukla M., Gupta K., Rasheed Z., Khan K.A., Haqqi T.M. Bioavailable constituents/metabolites of pomegranate (Punica granatum L.) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J. Inflamm. 2008;5:9. doi: 10.1186/1476-9255-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Ross R.G., Selvasubramanian S., Jayasundar S. Immunomodulatory activity of Punica granatum in rabbits—A preliminary study. J. Ethnopharmacol. 2001;78:85–87. doi: 10.1016/S0378-8741(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 458.Ishikawa I. Host responses in periodontal diseases: A preview. Periodontology 2000. 2007;43:9–13. doi: 10.1111/j.1600-0757.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 459.Bhadbhade S.J., Acharya A.B., Rodrigues S.V., Thakur S.L. The antiplaque efficacy of pomegranate mouthrinse. Quintessence Int. 2011;42:29–36. [PubMed] [Google Scholar]
- 460.Sastravaha G., Gassmann G., Sangtherapitikul P., Grimm W.D. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts in supportive periodontal therapy. J. Int. Acad. Periodontol. 2005;7:70–79. [PubMed] [Google Scholar]
- 461.DiSilvestro R.A., DiSilvestro D.J., DiSilvestro D.J. Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivitis risk. Phytother. Res. 2009;23:1123–1127. doi: 10.1002/ptr.2759. [DOI] [PubMed] [Google Scholar]
- 462.Nomura Y., Tamaki Y., Tanaka T., Arakawa H., Tsurumoto A., Kirimura K., Sato T., Hanada N., Kamoi K. Screening of periodontitis with salivary enzyme tests. J. Oral Sci. 2006;48:177–183. doi: 10.2334/josnusd.48.177. [DOI] [PubMed] [Google Scholar]
- 463.Närhi T.O., Tenovuo J., Ainamo A., Vilja P. Antimicrobial factors, sialic acid, and protein concentration in whole saliva of the elderly. Eur. J. Oral Sci. 1994;102:120–125. doi: 10.1111/j.1600-0722.1994.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 464.Badria F.A., Zidan O.A. Natural products for dental caries prevention. J. Med. Food. 2004;7:381–384. doi: 10.1089/jmf.2004.7.381. [DOI] [PubMed] [Google Scholar]
- 465.Newman M.G., Carranza F.A., Takei H.H., Klokkevold P.R. Carranza’s Clinical Periodontology. Elsevier Brasil; Amsterdam, The Netherlands: 2006. [Google Scholar]
- 466.Pereira J.V., Silva Filho S.C., LS H.J. Antimicrobial activity of hyroalcoholic extract of Punica granatum Linn. on plaque forming microorganisms. Periodontics Rev. 2001;12:57–64. [Google Scholar]
- 467.Pereira J.V., Pereira M.S.V., Sampaio F.C., Sampaio M.C.C., Alves P.M., Araújo C.R., Higino J.S. In vitro antibacterial and antiadherence effect of the extract of the Punica granatum Linn. upon dental biofilm microrganisms. Rev. Bras. Farmacogn. 2006;16:88–93. doi: 10.1590/S0102-695X2006000100016. [DOI] [Google Scholar]
- 468.Menezes S.M., Cordeiro L.N., Viana G.S. Punica granatum (pomegranate) extract is active against dental plaque. J. Herb. Pharmacother. 2006;6:79–92. doi: 10.1080/J157v06n02_07. [DOI] [PubMed] [Google Scholar]
- 469.Somu C.A., Ravindra S., Ajith S., Ahamed M.G. Efficacy of a herbal extract gel in the treatment of gingivitis: A clinical study. J. Ayurveda Integr. Med. 2012;3:85–90. doi: 10.4103/0975-9476.96525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Mazumdar M., Chatterjee A., Majumdar S., Chandrika M., Patki P. Evaluation of the safety and efficacy of complete care herbal toothpaste in controlling dental plaque, gingival bleeding and periodontal diseases. J. Homeop. Ayurv. Med. 2013;2:1000124. [Google Scholar]
- 471.Dye B.A., Kruszon-Moran D., McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am. J. Public Health. 2002;92:1809–1815. doi: 10.2105/AJPH.92.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Umeda M., Kobayashi H., Takeuchi Y., Hayashi J., Morotome-Hayashi Y., Yano K., Aoki A., Ohkusa T., Ishikawa I. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J. Periodontol. 2003;74:129–134. doi: 10.1902/jop.2003.74.1.129. [DOI] [PubMed] [Google Scholar]
- 473.Gebara E.C., Pannuti C., Faria C.M., Chehter L., Mayer M.P., Lima L.A. Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol. Immunol. 2004;19:277–280. doi: 10.1111/j.1399-302X.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 474.Riggio M.P., Lennon A. Identification by PCR of Helicobacter pylori in subgingival plaque of adult periodontitis patients. J. Med. Microbiol. 1999;48:317–322. doi: 10.1099/00222615-48-3-317. [DOI] [PubMed] [Google Scholar]
- 475.Hajimahmoodi M., Shams-Ardakani M., Saniee P., Siavoshi F., Mehrabani M., Hosseinzadeh H., Foroumadi P., Safavi M., Khanavi M., Akbarzadeh T., et al. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat. Prod. Res. 2011;25:1059–1066. doi: 10.1080/14786419.2010.501763. [DOI] [PubMed] [Google Scholar]
- 476.El-Sherbini G.T., Shoukry N.M. In vitro effect of pomegranate peel extract on Trichomonas tenax. Life Sci. J. 2012;9:791–797. [Google Scholar]
- 477.Howell A.B., Dsouza D.H. The pomegranate: Effects on bacteria and viruses that influence human health. Evid.-Based Complement. Altern. Med. 2013;2013:606212. doi: 10.1155/2013/606212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Bialonska D., Ramnani P., Kasimsetty S.G., Muntha K.R., Gibson G.R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 479.Stamatova I., Meurman J.H. Probiotics and periodontal disease. Periodontology 2000. 2009;51:141–151. doi: 10.1111/j.1600-0757.2009.00305.x. [DOI] [PubMed] [Google Scholar]
- 480.Stamatova I., Meurman J.H. Probiotics: Health benefits in the mouth. Am. J. Dent. 2009;22:329–338. [PubMed] [Google Scholar]
- 481.Twetman S., Derawi B., Keller M., Ekstrand K., Yucel-Lindberg T., Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol. Scand. 2009;67:19–24. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 482.Staab B., Eick S., Knöfler G., Jentsch H. The influence of a probiotic milk drink on the development of gingivitis: A pilot study. J. Clin. Periodontol. 2009;36:850–856. doi: 10.1111/j.1600-051X.2009.01459.x. [DOI] [PubMed] [Google Scholar]
- 483.Braga L.C., Leite A.A., Xavier K.G., Takahashi J.A., Bemquerer M.P., Chartone-Souza E., Nascimento A.M. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 2005;51:541–547. doi: 10.1139/w05-022. [DOI] [PubMed] [Google Scholar]
- 484.Dey D., Debnath S., Hazra S., Ghosh S., Ray R., Hazra B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram-negative bacilli. Food Chem. Toxicol. 2012;50:4302–4309. doi: 10.1016/j.fct.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 485.Begum A., Sandhya S., Shaffath Ali S., Vinod K.R., Reddy S., Banji D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae) Acta Sci. Pol. Technol. Aliment. 2013;12:61–73. [PubMed] [Google Scholar]
- 486.Satyal P., Jones T.H., Lopez E.M., McFeeters R.L., Ali N.A., Mansi I., Al-Kaf A.G., Setzer W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods. 2017;6:20. doi: 10.3390/foods6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Santoyo S., Cavero S., Jaime L., Ibañez E., Señoráns F.J., Reglero G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 2005;68:790–795. doi: 10.4315/0362-028X-68.4.790. [DOI] [PubMed] [Google Scholar]
- 488.Lee J.W., Asai M., Jeon S.K., Iimura T., Yonezawa T., Cha B.Y., Woo J.T., Yamaguchi A. Rosmarinic acid exerts an antiosteoporotic effect in the RANKL-induced mouse model of bone loss by promotion of osteoblastic differentiation and inhibition of osteoclastic differentiation. Mol. Nutr. Food Res. 2015;59:386–400. doi: 10.1002/mnfr.201400164. [DOI] [PubMed] [Google Scholar]
- 489.Amir Alireza R.G., Afsaneh R., Seied Hosein M.S., Siamak Y., Afshin K., Zeinab K., Mahvash M.J., Amir Reza R. Inhibitory activity of Salvadora persica extracts against oral bacterial strains associated with periodontitis: An in-vitro study. J. Oral Biol. Craniofac. Res. 2014;4:19–23. doi: 10.1016/j.jobcr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Khatak M., Khatak S., Siddqui A.A., Vasudeva N., Aggarwal A., Aggarwal P. Salvadora persica. Pharm. Rev. 2010;4:209–214. doi: 10.4103/0973-7847.70920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Mansour H., Alsamadany H., Al-Hasawi Z.M. Genetic diversity and genetic structure of Salvadora persica L., rare plant species in Rabigh province, Saudi Arabia: Implications for conservation. J. Taibah Univ. Sci. 2020;14:881–888. doi: 10.1080/16583655.2020.1787640. [DOI] [Google Scholar]
- 492.Haque M.M., Alsareii S.A. A review of the therapeutic effects of using miswak (Salvadora Persica) on oral health. Saudi Med. J. 2015;36:530–543. doi: 10.15537/smj.2015.5.10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Al Bratty M., Makeen H.A., Alhazmi H.A., Syame S.M., Abdalla A.N., Homeida H.E., Sultana S., Ahsan W., Khalid A. Phytochemical, Cytotoxic, and Antimicrobial Evaluation of the Fruits of Miswak Plant, Salvadora persica L. J. Chem. 2020;2020:4521951. doi: 10.1155/2020/4521951. [DOI] [Google Scholar]
- 494.Mohamed S.A., Khan J.A. Antioxidant capacity of chewing stick miswak Salvadora persica. BMC Complement. Altern. Med. 2013;13:40. doi: 10.1186/1472-6882-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Ibrahim M.M., Al Sahli A.A., Alaraidh I.A., Al-Homaidan A.A., Mostafa E.M., El-Gaaly G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi. J. Biol. Sci. 2015;22:168–175. doi: 10.1016/j.sjbs.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Lebda M.A., El-Far A.H., Noreldin A.E., Elewa Y.H.A., Al Jaouni S.K., Mousa S.A. Protective Effects of Miswak (Salvadora persica) against Experimentally Induced Gastric Ulcers in Rats. Oxid. Med. Cell. Longev. 2018;2018:6703296. doi: 10.1155/2018/6703296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Nordin A., Bin Saim A., Ramli R., Abdul Hamid A., Mohd Nasri N.W., Bt Hj Idrus R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020;27:1801–1810. doi: 10.1016/j.sjbs.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Hosseini M., Vazirian M., Nomani A., Monsef-Esfahani H. Evaluation of anti-inflammatory effect of Salvadora persica in IBD-induced rat. Res. J. Pharmacogn. 2017;4:27. [Google Scholar]
- 499.Khalil M.A., El-Sabbagh M.S., El Naggar E.B., El-Erian R.H. Antibacterial activity of Salvadora persica against oral pathogenic bacterial isolates. Niger. J. Clin. Pract. 2019;22:1378–1387. doi: 10.4103/njcp.njcp_413_14. [DOI] [PubMed] [Google Scholar]
- 500.Arshad H., Sami M.A., Sadaf S., Hassan U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep. 2021;11:5996. doi: 10.1038/s41598-021-85584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Abdulbaqi H.R., Himratul-Aznita W.H., Baharuddin N.A. Evaluation of Salvadora persica L. and green tea anti-plaque effect: A randomized controlled crossover clinical trial. BMC Complement. Altern. Med. 2016;16:493. doi: 10.1186/s12906-016-1487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Patel P.V., Shruthi S., Kumar S. Clinical effect of miswak as an adjunct to tooth brushing on gingivitis. J. Indian Soc. Periodontol. 2012;16:84–88. doi: 10.4103/0972-124X.94611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Aspalli S., Shetty V.S., Devarathnamma M.V., Nagappa G., Archana D., Parab P. Evaluation of antiplaque and antigingivitis effect of herbal mouthwash in treatment of plaque induced gingivitis: A randomized, clinical trial. J. Indian Soc. Periodontol. 2014;18:48–52. doi: 10.4103/0972-124X.128208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Bhate D., Jain S., Kale R., Muglikar S. The comparative effects of 0.12% chlorhexidine and herbal oral rinse on dental plaque-induced gingivitis: A randomized clinical trial. J. Indian Soc. Periodontol. 2015;19:393–395. doi: 10.4103/0972-124X.153478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Gupta P., Agarwal N., Anup N., Manujunath B.C., Bhalla A. Evaluating the anti-plaque efficacy of meswak (Salvadora persica) containing dentifrice: A triple blind controlled trial. J. Pharm. Bioallied Sci. 2012;4:282–285. doi: 10.4103/0975-7406.103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Deshmukh M.A., Dodamani A.S., Karibasappa G., Khairnar M.R., Naik R.G., Jadhav H.C. Comparative Evaluation of the Efficacy of Probiotic, Herbal and Chlorhexidine Mouthwash on Gingival Health: A Randomized Clinical Trial. J. Clin. Diagn. Res. 2017;11:Zc13–Zc16. doi: 10.7860/JCDR/2017/23891.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Prasad K.A., John S., Deepika V., Dwijendra K.S., Reddy B.R., Chincholi S. Anti-Plaque Efficacy of Herbal and 0.2% Chlorhexidine Gluconate Mouthwash: A Comparative Study. J. Int. Oral Health. 2015;7:98–102. [PMC free article] [PubMed] [Google Scholar]
- 508.Rezaei S., Rezaei K., Mahboubi M., Jarahzadeh M.H., Momeni E., Bagherinasab M., Targhi M.G., Memarzadeh M.R. Comparison the efficacy of herbal mouthwash with chlorhexidine on gingival index of intubated patients in Intensive Care Unit. J. Indian Soc. Periodontol. 2016;20:404–408. doi: 10.4103/0972-124X.194269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Tadikonda A., Pentapati K.C., Urala A.S., Acharya S. Anti-plaque and anti-gingivitis effect of Papain, Bromelain, Miswak and Neem containing dentifrice: A randomized controlled trial. J. Clin. Exp. Dent. 2017;9:e649–e653. doi: 10.4317/jced.53593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Mekhemar M., Hassan Y., Dörfer C. Nigella sativa and Thymoquinone: A Natural Blessing for Periodontal Therapy. Antioxidants. 2020;9:1260. doi: 10.3390/antiox9121260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Butera A., Gallo S., Maiorani C., Molino D., Chiesa A., Preda C., Esposito F., Scribante A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms. 2020;9:69. doi: 10.3390/microorganisms9010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Albabtain R., Azeem M., Wondimu Z., Lindberg T., Borg-Karlson A.K., Gustafsson A. Investigations of a Possible Chemical Effect of Salvadora persica Chewing Sticks. Evid.-Based Complement. Altern. Med. 2017;2017:2576548. doi: 10.1155/2017/2576548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Rafiei M., Kiani F., Sayehmiri F., Sayehmiri K., Sheikhi A., Zamanian Azodi M. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran. 2017;31:62. doi: 10.14196/mjiri.31.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Al-Ayed M.S., Asaad A.M., Qureshi M.A., Attia H.G., AlMarrani A.H. Antibacterial Activity of Salvadora persica L. (Miswak) Extracts against Multidrug Resistant Bacterial Clinical Isolates. Evid.-Based Complement. Altern. Med. 2016;2016:7083964. doi: 10.1155/2016/7083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Al-sieni A.I. The antibacterial activity of traditionally used Salvadora persica L. (miswak) and Commiphora gileadensis (palsam) in Saudi Arabia. Afr. J. Tradit. Complement. Altern. Med. 2014;11:23–27. doi: 10.4314/ajtcam.v11i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Abhary M., Al-Hazmi A.-A. Antibacterial activity of Miswak (Salvadora persica L.) extracts on oral hygiene. J. Taibah Univ. Sci. 2016;10:513–520. doi: 10.1016/j.jtusci.2015.09.007. [DOI] [Google Scholar]
- 517.Mekhemar M., Geib M., Kumar M., Radha, Hassan Y., Dörfer C. Salvadora persica: Nature’s Gift for Periodontal Health. Antioxidants. 2021;10:712. doi: 10.3390/antiox10050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Faruk E.M., Nafea O.E., Fouad H., Ebrahim U.F.A., Hasan R.A.A. Possible healing effects of Salvadora persica extract (MISWAK) and laser therapy in a rabbit model of a caustic-induced tongue ulcers: Histological, immunohistochemical and biochemical study. J. Mol. Histol. 2020;51:341–352. doi: 10.1007/s10735-020-09884-7. [DOI] [PubMed] [Google Scholar]
- 519.Moezizadeh M., Javand F., Tabatabaei F.S. Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J. Conserv. Dent. 2015;18:315–320. doi: 10.4103/0972-0707.159740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Khunkar S., Hariri I., Alsayed E., Linjawi A., Khunkar S., Islam S., Bakhsh T.A., Nakashima S. Inhibitory effect of Salvadora persica extract (Miswak) on collagen degradation in demineralized dentin: In vitro study. J. Dent. Sci. 2021;16:208–213. doi: 10.1016/j.jds.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Xu X., Zheng L., Yuan Q., Zhen G., Crane J.L., Zhou X., Cao X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018;6:2. doi: 10.1038/s41413-017-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Checchi V., Maravic T., Bellini P., Generali L., Consolo U., Breschi L., Mazzoni A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health. 2020;17:4923. doi: 10.3390/ijerph17144923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M., Sohraby M. Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Tradit. Complement. Med. 2014;4:140–144. doi: 10.4103/2225-4110.136540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Zheng G.-Q., Kenney P.M., Lam L.K.T. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992;55:999–1003. doi: 10.1021/np50085a029. [DOI] [PubMed] [Google Scholar]
- 525.Cai L., Wu C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996;59:987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- 526.Kamatou G.P., Vermaak I., Viljoen A.M. Eugenol—From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules. 2012;17:6953–6981. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Pramod K., Ansari S.H., Ali J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010;5:1934578X1000501236. doi: 10.1177/1934578X1000501236. [DOI] [PubMed] [Google Scholar]
- 528.Lee Y.Y., Hung S.L., Pai S.F., Lee Y.H., Yang S.F. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J. Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 529.Bachiega T.F., de Sousa J.P., Bastos J.K., Sforcin J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012;64:610–616. doi: 10.1111/j.2042-7158.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 530.Thompson D., Eling T. Mechanism of inhibition of prostaglandin H synthase by eugenol and other phenolic peroxidase substrates. Mol. Pharmacol. 1989;36:809–817. [PubMed] [Google Scholar]
- 531.Raghavenra H., Diwakr B.T., Lokesh B.R., Naidu K.A. Eugenol—The active principle from cloves inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostaglandins Leukot. Essent. Fat. Acids. 2006;74:23–27. doi: 10.1016/j.plefa.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 532.Yadav A.S., Bhatnagar D. Free radical scavenging activity, metal chelation and antioxidant power of some of the Indian spices. Biofactors. 2007;31:219–227. doi: 10.1002/biof.5520310309. [DOI] [PubMed] [Google Scholar]
- 533.Jirovetz L., Buchbauer G., Stoilova I., Stoyanova A., Krastanov A., Schmidt E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 534.Yoshimura M., Ito H., Miyashita K., Hatano T., Taniguchi S., Amakura Y., Yoshida T. Flavonol glucuronides and C-glucosidic ellagitannins from Melaleuca squarrosa. Phytochemistry. 2008;69:3062–3069. doi: 10.1016/j.phytochem.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 535.Karmakar S., Choudhury M., Das A.S., Maiti A., Majumdar S., Mitra C. Clove (Syzygium aromaticum Linn) extract rich in eugenol and eugenol derivatives shows bone-preserving efficacy. Nat. Prod. Res. 2012;26:500–509. doi: 10.1080/14786419.2010.511216. [DOI] [PubMed] [Google Scholar]
- 536.Jagtap A., Karkera S. Potential of the aqueous extract of Terminalia chebula as an anticaries agent. J. Ethnopharmacol. 1999;68:299–306. doi: 10.1016/S0378-8741(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 537.Rathinamoorthy R., Thilagavathi G. Terminalia chebula—Review on pharmacological and biochemical studies. Int. J. Pharm. Tech. Res. 2014;6:97–116. [Google Scholar]
- 538.Bajaj N., Tandon S. The effect of Triphala and Chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth. Int. J. Ayurveda Res. 2011;2:29–36. doi: 10.4103/0974-7788.83188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Baliga M.S., Meera S., Mathai B., Rai M.P., Pawar V., Palatty P.L. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin. J. Integr. Med. 2012;18:946–954. doi: 10.1007/s11655-012-1299-x. [DOI] [PubMed] [Google Scholar]
- 540.Kumar M.S., Kirubanandan S., Sripriya R., Sehgal P.K. Triphala promotes healing of infected full-thickness dermal wound. J. Surg. Res. 2008;144:94–101. doi: 10.1016/j.jss.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 541.Prabhakar J., Senthilkumar M., Priya M.S., Mahalakshmi K., Sehgal P.K., Sukumaran V.G. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J. Endod. 2010;36:83–86. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 542.Naiktari R.S., Gaonkar P., Gurav A.N., Khiste S.V. A randomized clinical trial to evaluate and compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. J. Periodontal Implant. Sci. 2014;44:134–140. doi: 10.5051/jpis.2014.44.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Maurya D., Mittal N., Sharma K., Nath G. Role of triphala in the management of Periodontal disease. Anc. Sci. Life. 1997;17:120–127. [PMC free article] [PubMed] [Google Scholar]
- 544.Yamanaka-Okada A., Sato E., Kouchi T., Kimizuka R., Kato T., Okuda K. Inhibitory effect of cranberry polyphenol on cariogenic bacteria. Bull. Tokyo Dent. Coll. 2008;49:107–112. doi: 10.2209/tdcpublication.49.107. [DOI] [PubMed] [Google Scholar]
- 545.Bodet C., Chandad F., Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J. Dent. Res. 2006;85:235–239. doi: 10.1177/154405910608500306. [DOI] [PubMed] [Google Scholar]
- 546.Bodet C., Chandad F., Grenier D. Cranberry components inhibit interleukin-6, interleukin-8, and prostaglandin E2 production by lipopolysaccharide-activated gingival fibroblasts. Eur. J. Oral Sci. 2007;115:64–70. doi: 10.1111/j.1600-0722.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 547.La V., Howell A., Grenier D. Cranberry proanthocyanidins inhibit MMP production and activity. J. Dent. Res. 2009;88:627–632. doi: 10.1177/0022034509339487. [DOI] [PubMed] [Google Scholar]
- 548.Tipton D.A., Babu J.P., Dabbous M.K. Effects of cranberry components on human aggressive periodontitis gingival fibroblasts. J. Periodontal Res. 2013;48:433–442. doi: 10.1111/jre.12023. [DOI] [PubMed] [Google Scholar]
- 549.Tanabe S., Santos J., La V.D., Howell A.B., Grenier D. A-type cranberry proanthocyanidins inhibit the RANKL-dependent differentiation and function of human osteoclasts. Molecules. 2011;16:2365–2374. doi: 10.3390/molecules16032365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Nawrot-Hadzik I., Matkowski A., Kubasiewicz-Ross P., Hadzik J. Proanthocyanidins and Flavan-3-ols in the Prevention and Treatment of Periodontitis—Immunomodulatory Effects, Animal and Clinical Studies. Nutrients. 2021;13:239. doi: 10.3390/nu13010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Otegui I., Fernández-Quintela A., Diego A.D., Cid C., Macarulla M.T., Partearroyo M.A. Properties of spray-dried and freeze-dried faba bean protein concentrates. Int. J. Food Sci. Technol. 1997;32:439–443. doi: 10.1111/j.1365-2621.1997.tb02118.x. [DOI] [Google Scholar]
- 552.Baginsky C., Peña-Neira Á., Cáceres A., Hernández T., Estrella I., Morales H., Pertuzé R. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J. Food Compos. Anal. 2013;31:1–6. doi: 10.1016/j.jfca.2013.02.003. [DOI] [Google Scholar]
- 553.Liu Y., Wu X., Hou W., Li P., Sha W., Tian Y. Structure and function of seed storage proteins in faba bean (Vicia faba L.) 3 Biotech. 2017;7:74. doi: 10.1007/s13205-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Warsame A.O., O’Sullivan D.M., Tosi P. Seed Storage Proteins of Faba Bean (Vicia faba L): Current Status and Prospects for Genetic Improvement. J. Agric. Food Chem. 2018;66:12617–12626. doi: 10.1021/acs.jafc.8b04992. [DOI] [PubMed] [Google Scholar]
- 555.Arogundade L.A., Tshay M., Shumey D., Manazie S. Effect of ionic strength and/or pH on Extractability and physico-functional characterization of broad bean (Vicia faba L.) Protein concentrate. Food Hydrocoll. 2006;20:1124–1134. doi: 10.1016/j.foodhyd.2005.12.010. [DOI] [Google Scholar]
- 556.Mejri F., Selmi S., Martins A., Benkhoud H., Baati T., Chaabane H., Njim L., Serralheiro M.L.M., Rauter A.P., Hosni K. Broad bean (Vicia faba L.) pods: A rich source of bioactive ingredients with antimicrobial, antioxidant, enzyme inhibitory, anti-diabetic and health-promoting properties. Food Funct. 2018;9:2051–2069. doi: 10.1039/C8FO00055G. [DOI] [PubMed] [Google Scholar]
- 557.Lewis W., Elvin-Lewis M. Oral Hygiene, Medical Botany. John Willy and Sons; New York, NY, USA: 1977. [Google Scholar]
- 558.Xia E.-Q., Deng G.-F., Guo Y.-J., Li H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010;11:622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 560.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 561.Esteban-Fernandez A., Zorraquin-Pena I., de Llano D.G., Bartolome B., Moreno-Arribas M.V. The role of wine and food polyphenols in oral health. Trends Food Sci. Technol. 2017;69:118–130. doi: 10.1016/j.tifs.2017.09.008. [DOI] [Google Scholar]
- 562.Pizzorno J.E., Murray M.T., Joiner-Bey H. The Clinician’s Handbook of Natural Medicine E-Book. Elsevier Health Sciences; London, UK: 2016. [Google Scholar]
- 563.Furiga A., Lonvaud-Funel A., Badet C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009;113:1037–1040. doi: 10.1016/j.foodchem.2008.08.059. [DOI] [Google Scholar]
- 564.Rizzo A., Bevilacqua N., Guida L., Annunziata M., Carratelli C.R., Paolillo R. Effect of resveratrol and modulation of cytokine production on human periodontal ligament cells. Cytokine. 2012;60:197–204. doi: 10.1016/j.cyto.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 565.Zare Javid A., Hormoznejad R., Yousefimanesh H.A., Zakerkish M., Haghighi-zadeh M.H., Dehghan P., Ravanbakhsh M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2017;31:108–114. doi: 10.1002/ptr.5737. [DOI] [PubMed] [Google Scholar]
- 566.Bonifait L., Grenier D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. J. (Can. Dent. Assoc.) 2010;76:a130. [PubMed] [Google Scholar]
- 567.Gewali M.B., Awale S. Aspects of Traditional Medicine in Nepal. Institute of Natural Medicine University of Toyama; Toyama, Japan: 2008. pp. 140–142. [Google Scholar]
- 568.Patiño L.O.J., Prieto R.J.A., Cuca S.L.E. Zanthoxylum genus as potential source of bioactive compounds. Bioact. Compd. Phytomed. 2012;10:184–218. [Google Scholar]
- 569.Ocheng F., Bwanga F., Joloba M., Borg-Karlson A.-K., Gustafsson A., Obua C. Antibacterial activities of extracts from Ugandan medicinal plants used for oral care. J. Ethnopharmacol. 2014;155:852–855. doi: 10.1016/j.jep.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 570.Kumar M., Prakash S., Radha, Kumari N., Pundir A., Punia S., Saurabh V., Choudhary P., Changan S., Dhumal S., et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants. 2021;10:1061. doi: 10.3390/antiox10071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Mukhtar H.M., Kalsi V. A review on medicinal properties of zanthoxylum armatum DC. Res. J. Pharm. Technol. 2018;11:2131–2138. doi: 10.5958/0974-360X.2018.00395.5. [DOI] [Google Scholar]
- 572.Negi J.S., Bisht V., Bhandari A., Bisht R., Kandari Negi S. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. essential oil. Iran. J. Pharmacol. Ther. 2012;11:68. [Google Scholar]
- 573.Abuajah C.I., Ogbonna A.C., Osuji C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Shidfar F., Rajab A., Rahideh T., Khandouzi N., Hosseini S., Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015;12:165–170. doi: 10.1515/jcim-2014-0021. [DOI] [PubMed] [Google Scholar]
- 575.Srinivasan K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition. 2017;5:18–28. doi: 10.1016/j.phanu.2017.01.001. [DOI] [Google Scholar]
- 576.Mahyari S., Mahyari B., Emami S.A., Malaekeh-Nikouei B., Jahanbakhsh S.P., Sahebkar A., Mohammadpour A.H. Evaluation of the efficacy of a polyherbal mouthwash containing Zingiber officinale, Rosmarinus officinalis and Calendula officinalis extracts in patients with gingivitis: A randomized double-blind placebo-controlled trial. Complement. Ther. Clin. Pract. 2016;22:93–98. doi: 10.1016/j.ctcp.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 577.Hassen G., Belete G., Carrera K.G., Iriowen R.O., Araya H., Alemu T., Solomon N., Bam D.S., Nicola S.M., Araya M.E., et al. Clinical Implications of Herbal Supplements in Conventional Medical Practice: A US Perspective. Cureus. 2022;14:e26893. doi: 10.7759/cureus.26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Del Prete A., Scalera A., Iadevaia M.D., Miranda A., Zulli C., Gaeta L., Tuccillo C., Federico A., Loguercio C. Herbal Products: Benefits, Limits, and Applications in Chronic Liver Disease. Evid.-Based Complement. Altern. Med. 2012;2012:837939. doi: 10.1155/2012/837939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Stickel F., Patsenker E., Schuppan D. Herbal hepatotoxicity. J. Hepatol. 2005;43:901–910. doi: 10.1016/j.jhep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 581.Abebe W. Herbal medication: Potential for adverse interactions with analgesic drugs. J. Clin. Pharm. Ther. 2002;27:391–401. doi: 10.1046/j.1365-2710.2002.00444.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article is a review and does not contain any studies with humans or animals performed by the authors.