Abstract
The systemic accumulation of both hydrogen peroxide (H2O2) and proteinase inhibitor proteins in tomato leaves in response to wounding was inhibited by the NADPH oxidase inhibitors diphenylene iodonium (DPI), imidazole, and pyridine. The expression of several defense genes in response to wounding, systemin, oligosaccharides, and methyl jasmonate also was inhibited by DPI. These genes, including those of four proteinase inhibitors and polyphenol oxidase, are expressed within 4 to 12 hr after wounding. However, DPI did not inhibit the wound-inducible expression of genes encoding prosystemin, lipoxygenase, and allene oxide synthase, which are associated with the octadecanoid signaling pathway and are expressed 0.5 to 2 hr after wounding. Accordingly, treatment of plants with the H2O2-generating enzyme glucose oxidase plus glucose resulted in the induction of only the later-expressed defensive genes and not the early-expressed signaling-related genes. H2O2 was cytochemically detected in the cell walls of vascular parenchyma cells and spongy mesophyll cells within 4 hr after wounding of wild-type tomato leaves, but not earlier. The cumulative results suggest that active oxygen species are generated near cell walls of vascular bundle cells by oligogalacturonide fragments produced by wound-inducible polygalacturonase and that the resulting H2O2 acts as a second messenger for the activation of defense genes in mesophyll cells. These data provide a rationale for the sequential, coordinated, and functional roles of systemin, jasmonic acid, oligogalacturonides, and H2O2 signals for systemic signaling in tomato plants in response to wounding.
INTRODUCTION
Reactive oxygen species (ROS) are common components of the defense responses of plants against pathogen and herbivore attacks. Inoculation of plant tissues with pathogens or treatment of cell cultures with microbial elicitors causes an oxidative burst characterized by the rapid generation of hydrogen peroxide (H2O2; reviewed by Low and Merida, 1996; Lamb and Dixon, 1997; Bolwell, 1999). Similarly, ROS are generated in plant tissues in response to wounding (Angelini et al., 1990; Bradley et al., 1992; Olson and Varner, 1993; Felton et al., 1994; Bi and Felton, 1995; Orozco-Cárdenas and Ryan, 1999). Mechanical stimulation of isolated cells (Yahraus et al., 1995; Gus-Mayer et al., 1998) and the treatment of cell suspension cultures with plant cell wall–derived oligogalacturonic acid (OGA; Legendre et al., 1993; Stennis et al., 1998) also generate H2O2 accumulation. Wound-induced H2O2 accumulation is observed both locally and systemically in leaves of several plant species, apparently caused by OGA that was released by a systemically wound-induced polygalacturonase (PG; Bergey et al., 1999; Orozco- Cárdenas and Ryan, 1999).
H2O2 can act as a local signal for hypersensitive cell death and also as a diffusible signal for the induction of defensive genes in adjacent cells (Alvarez et al., 1998). For example, transgenic potato plants that overexpress a fungal glucose oxidase gene and protein contain a constitutive increase in sublethal levels of H2O2 and exhibit enhanced disease resistance (Wu et al., 1995, 1997). Similarly, transgenic tobacco plants engineered to have low levels of antioxidant defenses (enzymes) show higher constitutive and inducible levels of H2O2 and pathogenesis-related (PR) proteins, together with increased sensitivity and general resistance to pathogen challenge (Chamnongpol et al., 1998; Mittler et al., 1999).
In several model systems investigated in plants, the oxidative burst and the accumulation of H2O2 appear to be mediated by the activation of a membrane-bound NADPH oxidase complex (Doke et al., 1996; Lamb and Dixon, 1997; Ogawa et al., 1997; Del Río et al., 1998; Potikha et al., 1999; Pei et al., 2000). In animal cells, this enzymatic complex consists of two membrane-associated polypeptides (gp91-phox and gp22-phox) that become active when at least three proteins from the cytosol (p47-phox, p67-fox, and rac) bind to the membrane components (Jones, 1994; Henderson and Chappel, 1996). Plant homologs of the animal NADPH oxidase protein subunits have been identified (Desikan et al., 1996; Xing et al., 1997; Potikha et al., 1999), and some of their genes have been sequenced (Groom et al., 1996; Keller et al., 1998; Torres et al., 1998). How the plant NADPH oxidase is regulated is still unknown. Some chemical inhibitors of the NADPH oxidase enzyme complex found in mammalian neutrophils inhibit the pathogen-, elicitor-, and wound-induced accumulation of H2O2 derived from the oxidative burst in plants (Levine et al., 1994; Auh and Murphy, 1995; Alvarez et al., 1998; Piedras et al., 1998; Orozco-Cárdenas and Ryan, 1999). In mammalian cells, the induction of defense genes by H2O2 involves the activation of the NF-κB transcription factor by mediating its release from the inhibitory IκB proteins (Li and Karin, 1999). In plants, the mechanisms by which H2O2 activates genes are not understood.
A model has been presented (Farmer and Ryan, 1992) for the expression of defense-related genes in tomato leaves in response to wounding and systemin. In this model, systemin initiates a cascade of intracellular events resulting in the activation of a cytoplasmic phospholipase that releases linolenic acid from membranes. Linolenic acid is converted to jasmonic acid, which is a powerful activator of genes coding for both signal pathway enzymes and defensive proteinase inhibitors and polyphenol oxidase. The model was modified recently to note that signal pathway genes are expressed within 0.5 hr after wounding, whereas defensive genes are expressed within 4 hr (Ryan, 2000). Recently, PG was shown to be among the early-expressed genes (Bergey et al., 1999), which raised questions concerning its role in the signal transduction pathway, because it was known to produce oligogalacturonide fragments from plant cell walls that are activators of both the defensive genes (Ryan and Farmer, 1991) and of the production of H2O2 (Stennis et al., 1998) in tomato leaves.
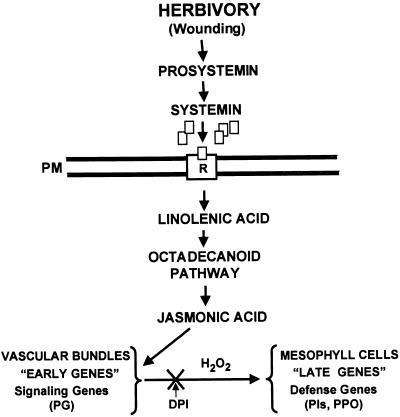
In this study, H2O2 was generated in vascular bundles of tomato leaves in response to wounding and behaved as if it was a diffusible signal for the expression of defense genes in mesophyll cells but not for signaling pathway genes in vascular bundle cells. Based on these results, a modified signal transduction pathway model is proposed (Figure 1) for the wound- and elicitor-induced defense response in which H2O2 is generated by wound-inducible PG and acts as a second messenger for the induction of defensive genes in mesophyll cells.
Figure 1.
A Model for the Differential Regulation of Signal Pathway Genes and Defensive Genes in Leaves of Tomato Plants in Response to Wounding and Systemin.
In this model, JA activates the signal pathway genes (early genes) in the vascular bundles, whereas H2O2, produced by cell wall–derived oligogalacturonides released by PG is a second messenger that activates defense genes (late genes) in mesophyll cells. DPI, diphenyl iodinium chloride; PG, polygalacturonase; PIs, proteinase inhibitors; PM, plasma membrane; PPO, polyphenol oxidase; R, receptor.
RESULTS
The localized and systemic activation of a leaf PG gene and its enzyme activity occur in response to wounding of the leaves of several plant families (Bergey et al., 1999). The time course of induction of PG activity in leaves correlates well with the accumulation of ROS, namely H2O2 (Orozco-Cárdenas and Ryan, 1999). Because oligogalacturonide fragments produced by PG are known to induce a substantial oxidative burst and the activation of defense gene expression (Legendre et al., 1993; Stennis et al., 1998), a relationship between the wound-inducible H2O2 and the wound-inducible defense gene activation after PG induction was investigated (Orozco-Cárdenas and Ryan, 1999).
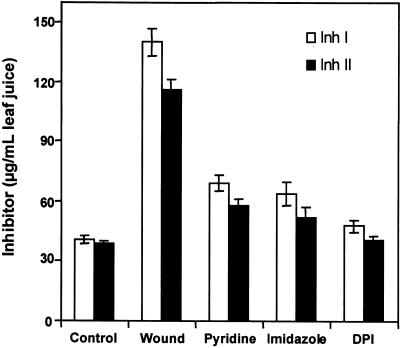
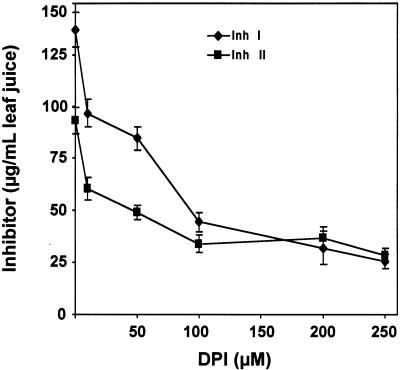
A membrane-bound NADPH oxidase has been implicated in the production of ROS during the defense response of plants against pathogen and herbivore attacks (Doke et al., 1996; Low and Merida, 1996; Lamb and Dixon, 1997; Orozco-Cárdenas and Ryan, 1999). Accordingly, some known chemical inhibitors of the mammalian neutrophil NADPH oxidase inhibit the generation of ROS after pathogen infection in plants (Levine et al., 1994; Auh and Murphy, 1995; Piedras et al., 1998) and the accumulation of H2O2 in wounded or systemin-treated tomato leaves (Orozco-Cárdenas and Ryan, 1999). The production of H2O2 from ROS in tomato leaves likely results from the action of the enzyme SOD (Auh and Murphy, 1995). Therefore, the effect of three different NADPH oxidase inhibitors on the accumulation of proteinase inhibitor I and II proteins in leaves of young tomato plants in response to wounding were investigated. As shown in Figure 2, when young, excised tomato plants were supplied through their cut stems for 30 min with solutions of the inhibitors diphenylene iodonium (DPI), pyridine, or imidazole, the accumulation of proteinase inhibitors I and II in response to wounding was severely diminished compared with untreated controls. Among the three inhibitors, DPI had the greatest inhibitory effect. Figure 3 shows that increasing concentrations of DPI (10 to 250 μM) progressively inhibited the accumulation of wound-inducible proteinase inhibitors in a concentration-dependent manner. The concentration of DPI required for half-maximal inhibition was ∼50 μM (Figure 3).
Figure 2.
Inhibition of Wound-Inducible Accumulation of Proteinase Inhibitors I and II Proteins by Different Chemical Inhibitors of NADPH Oxidase.
Fourteen-day-old tomato plants were excised at the base of the stems and supplied with solutions of phosphate buffer alone (Control and Wound), 40 mM pyridine, 20 mM imidazole, or 100 μM DPI in phosphate buffer for 30 min. Plants, except controls, then were wounded and incubated in water under light as described in Methods. Proteinase inhibitors I (Inh I) and II (Inh II) were assayed immunologically in leaf juice 24 hr later. Data are means ±sd; n 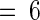 .
.
Figure 3.
Inhibition of Wound-Inducible Accumulation of Proteinase Inhibitors by the NADPH Oxidase Inhibitor DPI at Different Concentrations.
Plants were treated and assayed as described for Figure 1. Inh I, proteinase inhibitor I; Inh II, proteinase inhibitor II. Data are means ±sd; n 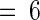 .
.
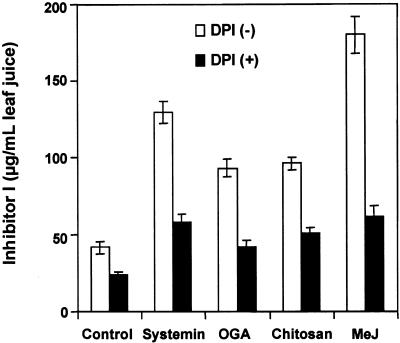
DPI also inhibited the accumulation of proteinase inhibitors induced in tomato plants after treatment with different chemical elicitors of the wound response (Figure 4). DPI-pretreated plants accumulated significantly lower levels of proteinase inhibitor I in their leaves than control plants in response to systemin, OGA, chitosan, and the plant hormone methyl jasmonate (Figure 4). Together, these results suggest that an active NADPH oxidase enzyme is necessary for defense gene activation in response to wounding and chemical elicitors and the generation of H2O2 and may be involved as a signaling pathway component.
Figure 4.
Inhibition of Elicitor-Induced Accumulation of Inhibitor I by DPI.
Plants were supplied with either phosphate buffer alone or 100 μM DPI in phosphate buffer for 30 min and transferred for 30 min to the same buffer solution containing 25 nM systemin, 250 μg/mL OGA, or 125 μg/mL chitosan or exposed to methyl jasmonate (MeJ) vapor as described in Methods. After each treatment, plants were incubated in water for 24 hr and then immunologically assayed for proteinase inhibitor I content in leaf juice. Data are means ±sd; n 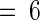 .
.
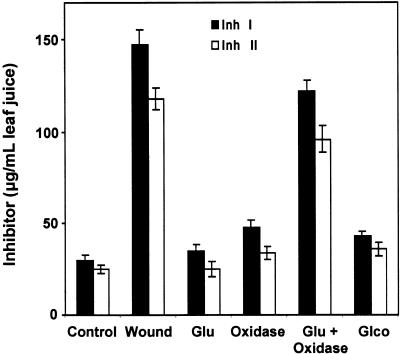
Glucose oxidase has been used to generate H2O2 in plants (Wu et al., 1995, 1997; Alvarez et al., 1998). Supplying plants with glucose oxidase together with glucose causes a continuous production of H2O2 within plant tissues (Levine et al., 1994; Alvarez et al., 1998). Excised tomato plants were incubated in a buffer solution containing glucose, gluconate, glucose oxidase, or a mixture of both glucose and glucose oxidase, and the accumulation of proteinase inhibitors I and II was assayed. Treatment with either compound or with the enzyme alone caused a slight to moderate increase of both proteinase inhibitor proteins I and II over levels observed in untreated controls (Figure 5). However, when the plants were supplied with glucose plus glucose oxidase, the levels of inhibitors I and II were induced to accumulate to ∼80% of the levels found in wounded plants (Figure 5). This result suggests that the H2O2 generated by glucose oxidase within the apoplast was able to trigger signaling events leading to the induction of proteinase inhibitor protein accumulation.
Figure 5.
H2O2-Mediated Accumulation of Proteinase Inhibitors I and II Proteins in Tomato Leaves.
Fourteen-day-old tomato plants were excised and incubated in phosphate buffer alone (Control and Wound) or in buffer containing 50 μM glucose (Glu), 2.5 units/mL glucose oxidase (Oxidase), glucose plus glucose oxidase (Glu + Oxidase), or 50 μM gluconate (Glco) for 2 hr. Thereafter, plants were incubated in water for 24 hr and assayed immunologically for proteinase inhibitors I (Inh I) and II (Inh II) content in leaf juice. Data are means ±sd; n 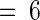 .
.
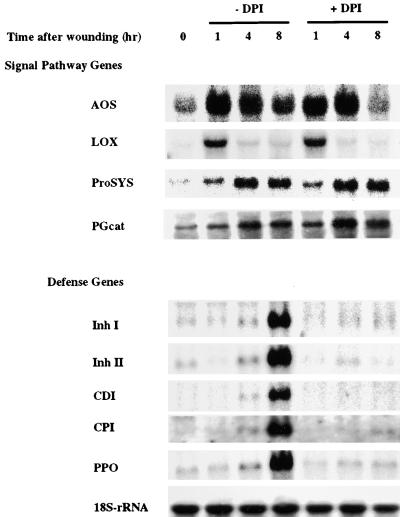
To further investigate the role of H2O2 in defense gene activation, the effect of DPI on the wound induction of defense genes at the mRNA level was investigated. Gel blot analyses of total RNA isolated from wounded leaves of plants that had been preincubated in buffer alone (untreated controls) or pretreated with DPI were performed using cDNA clones of different wound-inducible genes as probes. These cDNAs encoded for two groups of functionally related proteins (Ryan, 2000). The first group included the signaling pathwayassociated proteins prosystemin, lipoxygenase, and allene oxide synthase (AOS). The second group included the defense-related proteinase inhibitors I and II, cathepsin D inhibitor, carboxypeptidase inhibitor, and polyphenol oxidase. The mRNA corresponding to the signaling pathway–related genes, which were first expressed 0.5 to 2 hr after wounding and maximized at 4 hr, were not affected by DPI (Figure 6). On the other hand, the RNA encoding defense genes, which were first expressed ∼4 hr after wounding and maximized at 8 to 12 hr, were inhibited by DPI. Therefore, DPI appeared to specifically block the wound-induced expression of defensive genes but not the activation of signaling genes. Interestingly, the wound-induced expression of the gene encoding the tomato leaf PG occurs early along with that of signaling genes, and the synthesis of its mRNA was not affected by DPI. This finding suggests that this cell wall pectin-degrading enzyme has a signaling role by generating oligouronides, which are known chemical elicitors of the oxidative burst (Legendre et al., 1993; Stennis et al., 1998; Lee et al., 1999). The resulting H2O2 appears to act as a second messenger for the expression of defensive genes, but not for the signal pathway genes. In an earlier study of the effects of systemin on the oxidative burst in tomato suspension cultured cells, Stennis et al. (1998) found that when systemin alone was added to the cells, it did not cause an oxidative burst. Within a few hours after addition, however, systemin did potentiate a severalfold increase of the oxidative burst caused by OGA. This indicates that the components required to produce the oxidative burst by OGA were induced by systemin. The components were not identified, and NADPH oxidase activity was not measured in these cells.
Figure 6.
Differential Inhibition of Systemic Wound Response Genes by DPI.
Young excised tomato plants with two expanding leaves and a young growing leaf were supplied with phosphate buffer alone or with 100 μM DPI for 0.5 hr, wounded on the lower leaf at time 0, transferred to water, and assayed by RNA gel blotting at the times indicated (see Methods). 18S-rRNA, rRNA (loading control); AOS, allene oxide oxidase; CDI, aspartic proteinase inhibitor; CPI, metallocarboxypeptidase inhibitor; Inh I, proteinase inhibitor I; Inh II, proteinase inhibitor II; LOX, lipoxygenase; PGcat, leaf polygalacturonase catalytic subunit; PPO, polyphenol oxidase; ProSYS, prosystemin.
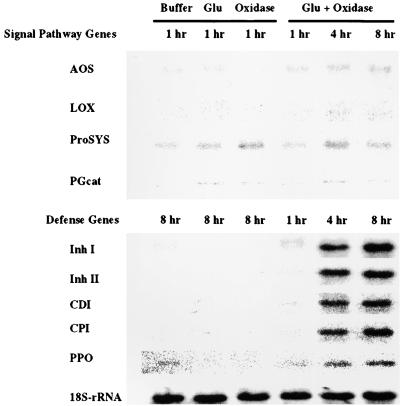
The expression of defensive and signaling genes was investigated after the treatment of excised tomato plants with the glucose oxidase–glucose H2O2-generating system. The plants were assayed for mRNAs for both the genes associated with signaling and those associated with defense. In leaves of plants treated with both glucose and glucose oxidase, the five genes involved in plant defense were induced, with an activation kinetics similar to that observed after wounding (Figure 7). However, none of the signaling genes was induced after the glucose oxidase treatment (Figure 7). Glucose, glucose oxidase, and the gluconate product alone (data not shown) did not cause the accumulation of detectable levels of defense or signaling gene mRNAs. Together, the analyses of wound-inducible defense gene expression indicate that H2O2 plays a key role as a second messenger in the induction of the defense genes but not in the regulation of the signaling genes.
Figure 7.
Differential Induction of mRNAs of Systemic Wound Response Proteins by H2O2 Generated with Glucose Oxidase in Leaves of Tomato Plants.
At time 0, plants were excised and supplied with phosphate buffer alone or with buffer containing 50 μM glucose (Glu), 2.5 units/mL glucose oxidase (Oxidase), or both (Glu + Oxidase). Total leaf RNA was extracted and examined by RNA gel blotting at the times indicated. For the independent (control) treatments with glucose or glucose oxidase alone, only the results obtained after 1 hr (for the signaling genes) and 8 hr (for the defense genes) are shown. Abbreviations are as in Figure 5.
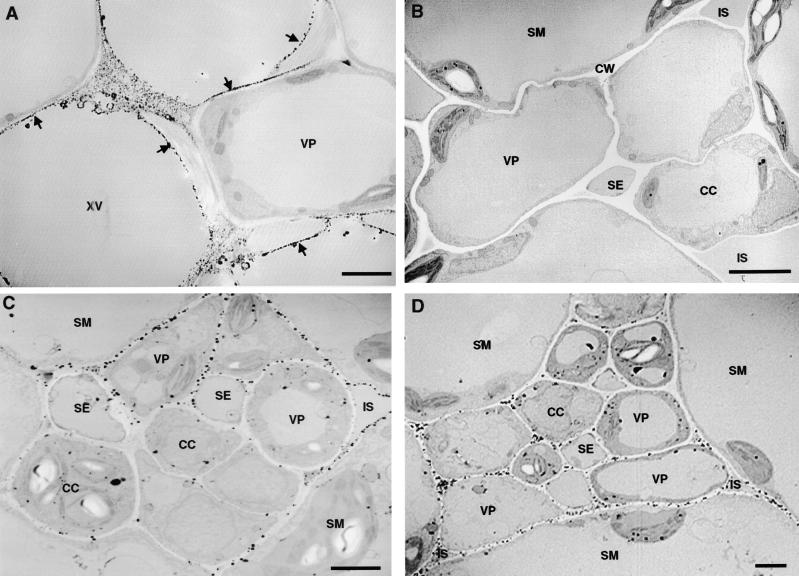
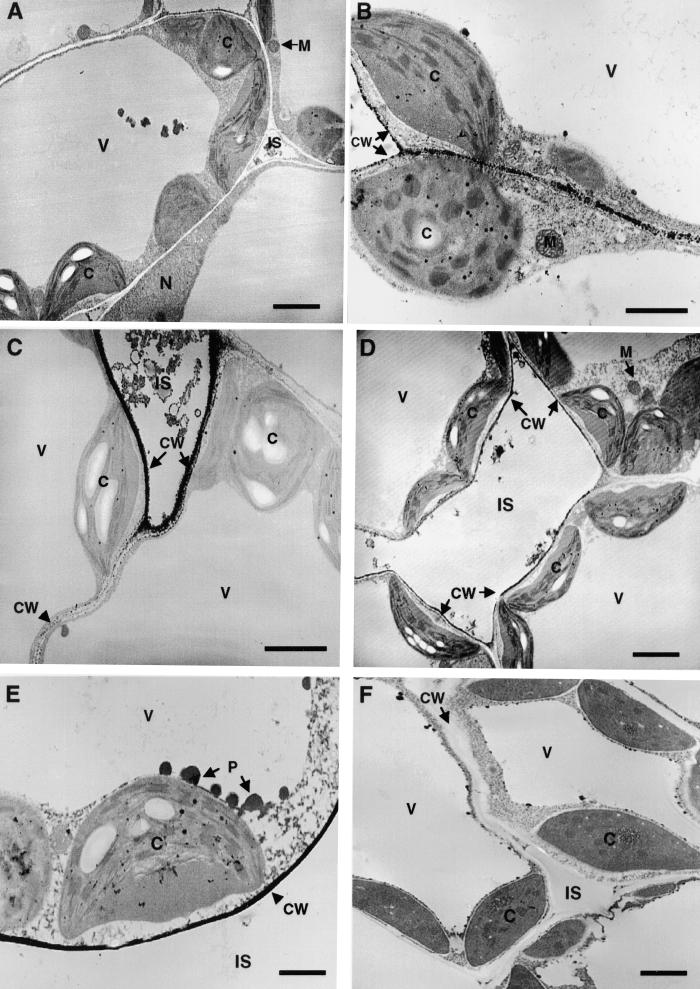
The cytochemical dye 3,3-diaminobenzidine (DAB) visually localizes wound-inducible H2O2 throughout the leaves of wounded tomato plants (Orozco-Cárdenas and Ryan, 1999). H2O2 accumulates mainly around the wound sites and within the major and minor veins of the leaves, reaching levels of ∼1 to 10 μM. DAB staining is blocked by pretreatment of the plants with different NADPH oxidase inhibitors and by catalase treatment after wounding of the leaves (M.L. Orozco-Cárdenas and C.A. Ryan, unpublished results). To further investigate the subcellular location of H2O2 generation and/or accumulation in the wounded leaves, we used a cerium perhydroxide (CeCl3)–based cytochemical technique (Bestwick et al., 1997). In leaves of unwounded young tomato plants, CeCl3 deposits, indicative of the presence of H2O2, were found in developing lignified secondary cell walls of xylem vessels (Figure 8A) but were not observed in the cell walls of vascular parenchyma (Figure 8B) or mesophyll cells (Figure 9A). On the other hand, in leaves of wounded plants, electron-dense CeCl3 deposits were detected in the cell walls of vascular parenchyma (Figures 8C and 8D) and in nearby spongy mesophyll cells in both wounded and unwounded (systemic) leaves (Figures 9B to 9D). Similar deposits were found in cell walls of leaves of transgenic tomato plants overexpressing prosystemin (Figure 9E). Heavy CeCl3 staining often was observed in the cell walls of spongy mesophyll cells facing intercellular spaces, a few cells away from the vascular traces (Figures 9C and 9D). The cytosol and internal cell organelles, including the chloroplasts, mitochondria, peroxisomes, nuclei, and tonoplasts of these cells, were almost completely free of electron- dense material. However, CeCl3 deposits were observed readily within the chloroplasts of palisade and spongy mesophyll cells of plant leaves pretreated with paraquat, a ROS-inducing herbicide (data not shown), indicating that CeCl3 could penetrate throughout the leaves and permeate cell membranes, as has been reported (Pellinen et al., 1999). In addition, supplying excised tomato plants with the DPI inhibitor before wounding did not result in an accumulation of CeCl3 deposits in the cell walls of the vascular parenchyma and the mesophyll cells of the leaves (Figure 9F).
Figure 8.
Cytochemical Localization of Wound-Inducible H2O2 in Vascular Bundles of Tomato Leaves.
(A) Electron-dense deposits of CeCl3 indicative of the presence of H2O2 in developing secondary cell walls of xylem vessels (XV) of control unwounded leaves (arrows show typical deposits). Note that the cell walls of an adjacent vascular parenchyma cell (VP) show little CeCl3 staining.
(B) Absence of CeCl3 staining in the cell walls of vascular parenchyma (VP) and neighboring spongy mesophyll (SM) cells associated with the phloem in control unwounded leaves.
(C) H2O2 generation in the vascular bundle of a wounded tomato leaf 4 hr after wounding. H2O2 accumulates strongly in the cell walls of vascular parenchyma cells bordering spongy mesophyll cells and at the intercellular spaces (IS).
(D) Systemic accumulation of H2O2 in vascular bundles of upper unwounded leaves of young tomato plants 4 hr after wounding of the lower leaf.
CC, companion cell; CW, cell wall; SE, sieve element. 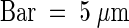 .
.
Figure 9.
Cytochemical Localization of Wound-Inducible H2O2 in Mesophyll Cells of Tomato Leaves.
(A) Spongy mesophyll cells of a control leaf from an unwounded plant does not exhibit H2O2 in the cell walls or intercellular spaces (IS).
(B) Accumulation of H2O2 in the bordering cell walls of a vascular parenchyma and a spongy mesophyll cell of a wounded leaf 4 hr after wounding.
(C) Accumulation of H2O2 in the cell walls of two spongy mesophyll cells facing an intercellular space 4 hr after wounding of the leaf.
(D) Systemic H2O2 accumulation in the cell walls of two spongy mesophyll cells facing an intercellular space 4 hr after wounding of the lower leaf.
(E) Constitutive accumulation of H2O2 in the cell wall of a spongy mesophyll cell of a leaf from a transgenic tomato plant overexpressing prosystemin. The proteinaceous material (P) within the central vacuole corresponds to aggregates containing proteinase inhibitor proteins that constitutively accumulate in transgenic tissue.
(F) Inhibition of wound-induced H2O2 accumulation by DPI. Leaf samples were obtained 4 hr after wounding.
C, chloroplast; CW, cell wall; M, mitochondrion; N, nucleus; P, protein aggregates of defensive inhibitor proteins; V, central vacuole. Bar in (A), (C), (D), and (F) = 2 μm; bar in (B) and (E) = 1 μm.
DISCUSSION
ROS are generated in plant tissues and organs during plant growth and development and also in response to environmental and biological stress (reviewed by Dangl et al., 1996; Greenberg, 1996; Pennell and Lamb, 1997). A previous study using several known chemical inhibitors of NADPH oxidase implicated this enzyme complex in the generation of ROS and the accumulation of H2O2 in wounded and unwounded leaves of several plant species in response to mechanical wounding (Orozco-Cárdenas and Ryan, 1999). The accumulation of H2O2 occurs near wound sites and also in distal unwounded leaves, indicating that the process is regulated by a systemic signaling system. In the present study, NADPH oxidase inhibitors were used to investigate their effects on the wound induction of defense proteinase inhibitor proteins in leaves of tomato plants. All of the NADPH oxidase inhibitors tested had an inhibitory effect not only on the generation of H2O2 but on the accumulation of proteinase inhibitors I and II as well (Figures 2 and 3). This finding supported the hypothesis that the H2O2 produced by the NADPH oxidase in response to wounding may have a regulatory role in the wound inducibility of defensive proteins.
DPI, a suicide inhibitor that binds irreversibly to the flavonoid group of the membrane-associated gp91-phox subunit of the NADPH oxidase complex (O'Donnell et al., 1993), was the most effective of all of the inhibitors tested. As with wounding, DPI inhibited the accumulation of proteinase inhibitor I that was induced after treatment of the plants with chemical elicitors of the wound response, namely systemin, OGA, and chitosan, as well as methyl jasmonate (Figure 4). Although high concentrations of DPI can affect other enzymes potentially involved in the generation of ROS, including extracellular peroxidases and nitric oxide (NO) synthase (Bolwell, 1999), the similar results obtained with different specific inhibitors strongly suggested that the wound-inducible accumulation of H2O2 was the result of the activation of the NADPH oxidase in the leaves. In addition, pretreatment of tomato plants with the NO synthase inhibitors 1,3-phenylene-bis(1,2-ethanediyl)-bisisothiourea and Nω-nitro-l-arginine as well as the NO scavenger 2-(4-carboxylphenyl)-4,4,5,5-tetraimidazoline-1-oxyl-3-oxide did not affect the accumulation of the inhibitor proteins after wounding (data not shown).
Additional evidence that the ROS produced by the NADPH oxidase has a regulatory role in the induction of inhibitor proteins was obtained from experiments with the H2O2-generating system of glucose and glucose oxidase (Alvarez et al., 1998). When glucose plus glucose oxidase were supplied to the excised tomato plants through their cut stems to produce H2O2 within the vascular bundles, proteinase inhibitors I and II accumulated in the leaves 24 hr later, as after wounding (Figure 5). This response was not observed in the presence of glucose, glucose oxidase, or gluconate alone, and the accumulation of proteinase inhibitors was diminished by the addition of catalase (data not shown), demonstrating that the observed effects were due only to the presence of H2O2 and not to glucose or the gluconate product of the enzyme. This H2O2-generating enzymatic system previously was shown to cause the constitutive accumulation of sublethal levels of H2O2 in the apoplast of plant tissues and in the medium of suspension cell cultures, with the concomitant activation of a defense response equivalent to systemic acquired resistance (SAR). This treatment caused the plants to be more resistant to pathogen attacks (Wu et al., 1995, 1997; Alvarez et al., 1998).
Analysis of defense gene expression by using gel blots showed that DPI selectively inhibited the wound induction of defense genes involved directly in deterring pest attacks (i.e., proteinase inhibitors and polyphenol oxidase), but it did not inhibit the expression of genes that are part of the wound-signaling pathway (Figure 6). Glucose/glucose oxidase treatment of the young excised tomato plants induced the expression of only the defense genes that were inhibited by DPI and not the transcription of the signaling genes (Figure 7). By comparing the timing of the wound-inducible expression of the genes that have a direct role in plant defense and those of the signal transduction pathway, it is clear that the two classes of wound-inducible genes are differentially regulated in a temporal manner, as reported previously (Ryan, 2000).
H2O2 is known to directly regulate the expression of numerous genes, some of which are involved in plant defense and the hypersensitive response (Levine et al., 1994; Korsmeyer et al., 1995; Alvarez et al., 1998; Desikan et al., 2000; Kovtun et al., 2000). However, it also has been reported that H2O2 alone is not the only agent involved in the hypersensitive response or programmed cell death and that salicylic acid (SA) and NO both play important roles in the onset of pathogen-induced programmed cell death in plants and the establishment of SAR (Draper, 1997; Shirasu et al., 1997; Delledonne et al., 1998; Durner et al., 1998; Dorey et al., 1999). As discussed above, NO does not appear to be involved in the wound-inducible defense response. Furthermore, because SA-inducible PR genes such as PR-1 (Malamy and Klessig, 1992) were not found in wounded tomato leaves (data not shown), the accumulation of H2O2 in leaves in response to wounding probably does not directly involve SA or NO.
Histochemical techniques have been used for the detection of H2O2 generated in plant tissues during plant–pathogen interactions (Bestwick et al., 1997; Thordal-Christensen et al., 1997) and in response to abiotic stress (Pellinen et al., 1999). In lettuce leaves inoculated with the bacterial pathogen Pseudomonas syringae pv phaseolicola, Bestwick et al. (1997) reported the appearance of CeCl3 deposits of variable intensity in the cell walls of spongy mesophyll cells facing intercellular spaces adjacent to infecting bacteria. In the wounded and unwounded leaves of tomato plants, scattered and localized dense deposits of CeCl3 were observed in the walls of vascular parenchyma cells (Figures 8C and 8D) and nearby spongy mesophyll cells (Figures 9B to 9E). The intensity of the CeCl3 staining would correspond to sublethal levels of H2O2, not high enough to cause cell collapse and death, as observed by Bestwick et al. (1997). As noted above, using the DAB histochemical dye to detect H2O2 in wounded tomato leaves, we calculated the levels of wound-inducible H2O2 to be ∼1 to 10 μM (Orozco-Cárdenas and Ryan, 1999), which again might not be high enough to cause hypersensitive cell death. In fact, no signs of necrotic tissue were observed in the transgenic tomato plants overexpressing prosystemin (McGurl et al., 1994), which constitutively accumulate H2O2 in their leaves (Figure 9E; Orozco-Cárdenas and Ryan, 1999).
The greatest accumulation of H2O2 was observed in the walls of spongy mesophyll cells facing large intercellular spaces (Figures 9C and 9D). These gas-filled cavities, which in some leaves accounted for as much as 70% of the leaf volume, constituted a labyrinth that surrounds the cells and is continuous with the spaces under stomata. The cell walls bordering these spaces were quite thin and were evolved to allow gas exchange (i.e., O2 and CO2) and substantial water loss through transpiration. The water and salts that make up the transpiration stream come from the xylem elements of the vascular traces, usually only a few cells away from any mesophyll element. If H2O2 is generated or overproduced in response to wounding in the cell walls of the vascular bundle cells, it can be readily transported in water through the apoplast and diffuse initially into the cells adjacent to each vein. The accumulation of H2O2 at high levels in cell walls adjacent to intercellular spaces in the spongy mesophyll can be explained by the rapid evaporation of water in these air-filled spaces, together with the lower ROS-scavenging activity at these sites. This also might represent a defense strategy for the plant, because stomata and the intercellular spaces are paths for invasion by microbial pathogens (Bestwick et al., 1997). In a recent report, H2O2 generated after treatment of tomato leaf epidermis with the oligosaccharide elicitors from plant (OGA) and fungal (chitosan) cell walls are shown to cause the closure of stomata (Lee et al., 1999), which can limit the entrance of pathogens into the plant. Stomata closure also is mediated by H2O2 through the activation of calcium channels (Pei et al., 2000).
Low-frequency systemic secondary oxidative bursts and hypersensitive cell death associated with the establishment of SAR have been observed in distant tissues and uninoculated leaves of Arabidopsis plants challenged with an avirulent pathogen (Alvarez et al., 1998). Interestingly, the primary and secondary oxidative bursts, which in this incompatible plant–pathogen interaction leads to hypersensitive cell death, were observed primarily adjacent to the veins, in so-called periveinal cells. These periveinal cells die more rapidly than other cells in the inoculated tissues and before the appearance of the hypersensitive response lesion (Alvarez et al., 1998). The rapid death of periveinal cells also has been observed in tomato leaves inoculated with race-specific elicitors from the fungus Cladosporium (Hammond-Kosack et al., 1994). The occurrence of specialized ROS-generating cells associated with differentiated vascular tissues also is suggested by the observations that the induction of defense-related responses and the hypersensitive cell death are developmentally regulated in tomato plants and cell cultures (Hammond-Kosack et al., 1994; Honne et al., 1998). Together, these observations suggest that the vascular bundles must play an important role in the generation of signals, such as H2O2, that regulate the defense response of plants against pathogens and herbivores.
The model shown in Figure 1 is consistent with what is known at present of both the signals and the signal transduction pathway enzymes that regulate systemic wound induction of signaling and defensive genes in tomato plants (Farmer and Ryan, 1992; Ryan, 2000). Upon wounding of the leaves by herbivores, systemin is released into the vascular system, where it activates the biosynthesis of jasmonic acid in vascular parenchyma cells, upregulating the synthesis of signal pathway genes, including PG. The gene products include prosystemin and some jasmonic acid biosynthetic enzymes, including isoforms of lipoxygenase and AOS, which are known to be synthesized in the vascular bundle cells (Jacinto et al., 1997; Kubigsteltig et al., 1999). PG is likely to be found in the cell walls of the vascular bundles, where H2O2 is localized (Figure 7). The production of OGA by PG results in the synthesis of H2O2, which then diffuses out of the vascular bundles to mesophyll cells, where it activates the expression of genes coding for proteinase inhibitors and polyphenol oxidase. Previous ultrastructural studies of tomato leaves have shown that proteinase inhibitors synthesized in response to wounding are sequestered in the central vacuoles of mesophyll cells (Shumway et al., 1976; Narváez-Vásquez et al., 1993).
Interestingly, OGA initially was found to signal the induction of proteinase inhibitors several years ago (Ryan, 1988). Because the OGAs were not mobile, they were not considered further as systemic signals. The recent discovery of wound-inducible H2O2 in plant leaves and its relationships with OGA and defense gene activation has brought a new perspective and a more rational explanation of the temporal, spatial, and functional relationships among systemic wound signals. These include the polypeptide signal systemin, the lipid signal jasmonic acid, the oligosaccharide signal OGA, and the inorganic chemical signal H2O2.
METHODS
Plant Growth
Tomato plants (Lycopersicon esculentum cv Castlemart) were grown from seed for 2 weeks in a growth chamber with 18-hr days (light at 300 μE·m−2·sec−1) at 28°C and 6-hr nights at 18°C. Transgenic tomato plants expressing a prosystemin cDNA gene under the control of the cauliflower mosaic virus 35S promoter (McGurl et al., 1994) were grown under the same conditions.
Assays
To assay chemical elicitors of the wound response, plants with two expanding leaves and a small terminal leaf were used. The plants were excised at the base of the stem with a razor blade, and the cut stem was placed in 10−3 M potassium phosphate buffer, pH 6.0, or in a solution of systemin (25 nM), oligogalacturonic acid (OGA; 0.5 mg/mL), or chitosan (125 μg/mL) in phosphate buffer for 30 min. After treatment, the plants were incubated in water under constant light at 300 μE·m−2·sec−1 at 28°C within closed Plexiglas boxes as described previously (Orozco-Cárdenas and Ryan, 1999). For experiments in which plants were induced by glucose oxidase plus glucose, excised plants were incubated in buffer alone or in buffer containing glucose (50 μM), gluconate (50 μM), glucose oxidase (2.5 units/mL; [all from Sigma, St. Louis, MO], or glucose (50 μM) plus glucose oxidase (2.5 units/mL) for 2 hr.
Excised plants also were exposed to methyl jasmonate vapors by applying 1 μL of absolute methyl jasmonate (Bedoukian Research, Danbury, CT) to a cotton wick inside a Plexiglas box, as described previously (Farmer and Ryan, 1992). The box was sealed, placed in a growth chamber, and incubated under the light and temperature conditions described above. Wounding was performed by crushing the leaflets of the lower leaf with a hemostat once near their tips and once across the main veins. Inhibitors of NADPH oxidase, including diphenylene iodonium (DPI), pyridine, and imidazole (all from Sigma, St. Louis, MO), also were supplied through the excised stem in the same phosphate buffer for 0.5 to 4 hr before wounding or elicitor treatments.
Proteinase inhibitor concentrations in leaf juice were assayed after 24 hr by immunoradial diffusion as reported previously (Ryan, 1967; Trautman et al., 1971).
RNA Extraction and RNA Gel Blot Analyses
At different time intervals after treatment of detached tomato plants, leaves were excised, immersed in liquid nitrogen, ground to a fine powder, and stored at −80°C to isolate total RNA. Total leaf RNA was extracted using the Trizol reagent (Gibco BRL) according to the manufacturer's recommendations. Total RNA pellets were dissolved in 25 μL of RNase-free water and quantified spectrophotometrically. RNA quality was determined by gel fractionation in agarose/formaldehyde followed by ethidium bromide staining and UV light visualization before analyzing for specific mRNAs (Maniatis et al., 1982).
Total leaf RNA (10 to 20 μg) was fractionated by electrophoresis on 1.4% agarose gels with formaldehyde, blotted onto nitrocellulose membranes, and incubated at 65°C for 1 hr in hybridization buffer (5 × SSPE [1 × SSPE is 0.115 M NaCl, 10 mM sodium phosphate, and 1 mM EDTA, pH 7.4], 5 × Denhardt's solution [1 × Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA], 1% SDS, and 10% dextran sulfate). Radioactive 32P-dCTP–labeled probes were generated by random priming according to the manufacturer's recommendations (DECA prime II kit; Ambion, Austin, TX) by using tomato cDNAs encoding allene oxide synthase (AOS) (Howe et al., 2000), lipoxygenase (Heitz et al., 1997), prosystemin (McGurl et al., 1992), leaf polygalacturonase catalytic subunit (Bergey et al., 1999), serine proteinase inhibitor I (Graham et al., 1985a), serine proteinase inhibitor II (Graham et al., 1985b), cathepsin D inhibitor (Hildemann et al., 1992), carboxypeptidase inhibitor (Moura and Ryan, 2000), and polyphenol oxidase (Constabel et al., 1995). An 18S rRNA gene probe was used as a loading control. Synthetic oligonucleotide probes were purified using Bio-spin P6 chromatography columns (Bio-Rad). The probes were heat denatured, added to the hybridization buffer, and incubated with the blocked membranes overnight at 65°C. Membranes were washed once with 2 × SSPE for 20 min at room temperature, twice with 2 × SSPE and 1% SDS for 15 to 30 min at 65°C, and then exposed for 15 to 32 hr to x-ray film or to a PhosphorImager (Bio-Rad).
Cytochemical Detection of H2O2
H2O2 was visualized at the subcellular level using CeCl3 for localization (Bestwick et al., 1997; Pellinen et al., 1999). Electron-dense CeCl3 deposits are formed in the presence of H2O2 and are visible by transmission electron microscopy. Briefly, tissue pieces (2 × 5 mm2) were excised from leaves of wounded and unwounded wild-type and transgenic tomato plants and then vacuum infiltrated with freshly prepared 5 mM CeCl3 in 50 mM 3-(N-morpholino)-propanesulfonic acid at pH 7.2 for 30 min. Tissues then were fixed in 1.25% (v/v) glutaraldehyde and 1.25% (v/v) paraformaldehyde in 50 mM sodium cacodylate buffer (CAB), pH 7.2, for 1 hr at room temperature and kept overnight at 4°C. After fixation, tissues were washed twice for 10 min in CAB and postfixed for 45 min in 1% (v/v) osmium tetroxide in CAB. Tissues were then washed twice for 10 min in CAB and dehydrated in a graded acetone series (30, 50, 70, 80, 90, and 100% [v/v]), progressively embedded in rising concentrations of acetone-resin mixtures, and finally incubated in two 24-hr changes of pure epoxy resin (Eponate 12 resin; Ted Pella Inc., Redding, CA) before polymerization at 60°C for 48 hr. Ultrathin sections (50 to 100 nm) were obtained on a Reichert Ultracut E Microtome (Leica AG, Wein, Austria) using a diamond knife (Delaware Diamond Knives, Wilmington, DE), mounted on nickel grids (200 mesh), and examined without staining with a transmission electron microscope (model JEM-1200Ex; JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 80 kV.
Acknowledgments
Transmission electron microscopy was performed at the Electron Microscopy Center (EMC) of Washington State University. We thank Sue Vogtman for growing the plants for this study, and the EMC staff for their technical advice and collaboration. This research was supported by Washington State University College of Agriculture and Home Economics (Project No. 1791) and by grants from the United States Department of Agriculture Competitive Grants Program (Grant No. 9801502) and the National Science Foundation (Grant No. 9601099).
References
- Alvarez, M.E., Penell, R.I., Meijer, P.-J., Ishikawa, A., Dixon, R.A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92 773–784. [DOI] [PubMed] [Google Scholar]
- Angelini, R., Manes, F., and Federico, R. (1990). Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 182 89–96. [DOI] [PubMed] [Google Scholar]
- Auh, C.-K., and Murphy, T.M. (1995). Plasma membrane redox enzyme is involved in the synthesis of O2 and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 107 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey, D.R., Orozco-Cárdenas, M.L., de Moura, D.S., and Ryan, C.A. (1999). A wound- and systemic-inducible polygalacturonase in tomato leaves. Proc. Natl. Acad. Sci. USA 96 1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick, C.S., Brown, I.R., Bennett, M.H.R., and Mansfield, J.W. (1997). Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, J.L., and Felton, G.W. (1995). Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 21 1511–1530. [DOI] [PubMed] [Google Scholar]
- Bolwell, G.P. (1999). Role of active oxygen species and NO in plant defense responses. Curr. Opin. Plant Biol. 2 287–294. [DOI] [PubMed] [Google Scholar]
- Bradley, D.J., Kjellbom, P., and Lamb, C.J. (1992). Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell 70 21–30. [DOI] [PubMed] [Google Scholar]
- Chamnongpol, S., Willekens, H., Moeder, W., Langebartels, C., Sandermann, H., Van Montagu, M., Inze, D., and Van Camp, W. (1998). Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. USA 95 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constabel, C.P., Bergey, D.R., and Ryan, C.A. (1995). Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc. Natl. Acad. Sci. USA 92 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L., Dietrich, R.A., and Richberg, M.H. (1996). Death don't have no mercy: Cell death programs in plant–microbe interactions. Plant Cell 8 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne, M., Xia, Y., Dixon, R.A., and Lamb, C. (1998). Nitric oxide functions as a signal in plant disease resistance. Nature 394 585–588. [DOI] [PubMed] [Google Scholar]
- Del Río, L.A., Pastori, G.M., Palma, J.M., Sandalio, L.M., Sevilla, F., Corpas, F.J., Jiménez, A., López-Huertas, E., and Hernández, J.A. (1998). The activated oxygen role of peroxisomes in senescence. Plant Physiol. 116 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R., Hancock, J.T., Coffey, M.J., and Neill, S.J. (1996). Generation of active oxygen species in elicited cells of Arabidopsis thaliana is mediated by NADPH oxidase-like enzyme. FEBS Lett. 382 213–217. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Neill, S.J., and Hancock, J.T. (2000). Hydrogen peroxide–induced gene expression in Arabidopsis thaliana. Free Radic. Biol. Med. 28 773–778. [DOI] [PubMed] [Google Scholar]
- Doke, N., Miura, Y., Sanchez, L.M., Park, H.J., Noritake, T., Yoshioka, H., and Kawakita, K. (1996). The oxidative burst protects plants against pathogen attack: Mechanism and role as an emergency signal for plant bio-defence. A review. Gene 179 45–51. [DOI] [PubMed] [Google Scholar]
- Dorey, S., Kopp, M., Geoffroy, P., Fritig, B., and Kauffmann, S. (1999). Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol. 121 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper, D. (1997). Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 2 162–165. [Google Scholar]
- Durner, J., Wendehenne, D., and Klessig, D. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, E.E., and Ryan, C.A. (1992). Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton, G.W., Summers, C.B., and Mueller, A.J. (1994). Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J. Chem. Ecol. 20 639–649. [DOI] [PubMed] [Google Scholar]
- Graham, J.S., Pearce, G., Merryweather, J., Titani, K., Ericson, L., and Ryan, C.A. (1985. a). Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA deduced primary structure of pre-inhibitor I and its post-translational processing. J. Biol. Chem. 260 6555–6560. [PubMed] [Google Scholar]
- Graham, J.S., Pearce, G., Merryweather, J., Titani, K., Ericson, L., and Ryan, C.A. (1985. b). Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA deduced primary structure of pre-inhibitor II and its post-translational processing. J. Biol. Chem. 260 6561–6564. [PubMed] [Google Scholar]
- Greenberg, J.T. (1996). Programmed cell death: A way of life for plants. Proc. Natl. Acad. Sci. USA 93 12094–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom, O.J., Torres, M.A., Fordham-Skeleton, P., Hammond-Kosak, K.E., Robinson, N.J., and Jones, J.D.G. (1996). RbohA, a rice homologue of the mammalian pg91phox respiratory burst oxidase gene. Plant J. 10 515–522. [DOI] [PubMed] [Google Scholar]
- Gus-Mayer, S., Naton, B., Hahlbrock, K., and Schmelzer, E. (1998). Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc. Natl. Acad. Sci. USA 95 8398–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., Harrison, K., and Jones, J.D.G. (1994). Developmentally regulated cell death on expression of the fungal avirulence gene Avr9 in tomato seedlings carrying the disease-resistance gene Cf-9. Proc. Natl. Acad. Sci. USA 91 10445–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz, T., Berger, D.R., and Ryan, C.A. (1997). A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol. 114 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., and Chappel, J.B. (1996). NADPH oxidase of neutrophils. Biochim. Biophys. Acta 1273 87–107. [DOI] [PubMed] [Google Scholar]
- Hildemann, T., Ebneth, M., Pena-Cortes, H., Sanchez-Serrano, J.J., Wilmitzer, L., and Prat, S. (1992). General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell 4 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honne, G., Buitink, J., Jabs, T., De Kloe, J., Sijbolts, F., Apotheker, M., Weide, R., Sije, T., Stuiver, M., and De Wit, P.J.G.M. (1998). Induction of defense-related responses in Cf9 tomato cells by the AVR9 elicitor peptide of Cladosporium fulvum is developmentally regulated. Plant Physiol. 117 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, G., Lee, G.I., Itoh, A., Li, L., and DeRocher, A.E. (2000). Cytochrome P450-dependent metabolism of oxylipins in tomato: Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol. 123 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto, T., McGurl, B., Franceschi, V., Delano-Freier, J., and Ryan, C.A. (1997). Tomato prosystemin confers wound-inducible, vascular bundle–specific expression of the β-glucoronidase gene in transgenic tomato plants. Planta 203 406–412. [Google Scholar]
- Jones, O.T.G. (1994). The regulation of superoxide production by the NADPH oxidase of neutrophils and other mammalian cells. Bioessays 16 919–923. [DOI] [PubMed] [Google Scholar]
- Keller, T., Damude, H.G., Werner, D., Doaner, P., Dixon, R.A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer, S.J., Yin, X.M., Oltuai, Z.N., Veis-Novack, D.J., and Linette, G.P. (1995). Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim. Biophys. Acta 1271 63–66. [DOI] [PubMed] [Google Scholar]
- Kovtun, Y., Chiu, W.L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress–activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 97 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubigsteltig, I., Laudert, D., and Weiler, E.W. (1999). Structure and regulation of the Arabidosis thaliana allene oxide synthase gene. Planta 208 463–471. [DOI] [PubMed] [Google Scholar]
- Lamb, C., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 251–275. [DOI] [PubMed] [Google Scholar]
- Lee, S., Choi, H., Suh, S., Doo, I.-S., Oh, K.-Y., Choi, E.J., Taylor, A.T.S., Low, P.S., and Lee, Y. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre, L., Rueter, S., Heinstein, P.F., and Low, P.S. (1993). Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 102 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593. [DOI] [PubMed] [Google Scholar]
- Li, N., and Karin, M. (1999). Is NF-κB the sensor of oxidative stress? FASEB J. 13 1137–1143. [PubMed] [Google Scholar]
- Low, P.S., and Merida, J.R. (1996). The oxidative burst in plant defense: Function and signal transduction. Physiol. Plant. 96 533–542. [Google Scholar]
- Malamy, J., and Klessig, D.F. (1992). Salicylic acid and plant disease resistance. Plant J. 2 643–654. [Google Scholar]
- Maniatis, T., Fritsch, E.F., and Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- McGurl, B., Pearce, G., Orozco-Cárdenas, M.L., and Ryan, C.A. (1992). Structure, expression, and antisense inhibition of the systemin precursor gene. Science 255 1570–1573. [DOI] [PubMed] [Google Scholar]
- McGurl, B., Orozco-Cárdenas, M.L., Pearce, G., and Ryan, C.A. (1994). Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc. Natl. Acad. Sci. USA 91 9799–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R., Herr, E.H., Orvar, B.L., van Camp, W., Willekens, H., and Ellis, B.E. (1999). Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA 96 14165–14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura, D., Bergey, D.R., and Ryan, C.A. (2000). Characterization and localization of a wound inducible type I serine-carboxypeptidase from tomato (Lycopersicon esculentum Mill.) leaves. Planta, in press. [DOI] [PubMed]
- Narváez-Vásquez, J., Franceschi, V.R., and Ryan, C.A. (1993). Proteinase-inhibitor synthesis in tomato plants: Evidence for extracellular deposition in roots through the secretory pathway. Planta 189 257–266. [Google Scholar]
- O'Donnell, V.B., Tew, D.G., Jones, O.T.G., and England, P.J. (1993). Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, K., Kanematsu, S., and Asada, K. (1997). Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: Their association with lignification. Plant Cell Physiol. 38 1118–1126. [DOI] [PubMed] [Google Scholar]
- Olson, P.D., and Varner, J.E. (1993). Hydrogen peroxide and lignification. Plant J. 4 887–892. [Google Scholar]
- Orozco-Cárdenas, M.L., and Ryan, C. (1999). Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 96 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. [DOI] [PubMed] [Google Scholar]
- Pellinen, R., Palva, T., and Kangasjarvi, J. (1999). Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 20 349–356. [DOI] [PubMed] [Google Scholar]
- Pennell, R.I., and Lamb, C. (1997). Programmed cell death in plants. Plant Cell 9 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras, P., Hammond-Kosack, K.E., Harrison, K., and Jones, J.D.G. (1998). A rapid Cf-9 and Avr9-dependent production of active oxygen species in tobacco suspension cultures. Mol. Plant-Microbe Interact. 11 1155–1166. [Google Scholar]
- Potikha, T.S., Collins, C.C., Johnson, D.I., Delmer, D.P., and Levine, A. (1999). The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 119 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C.A. (1967). Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal. Biochem. 19 434–440. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A. (1988). Oligosaccharides as recognition signals for the expression of defensive genes in plants. Biochemistry 27 8879–8883. [Google Scholar]
- Ryan, C.A. (2000). The systemin signaling pathway: Differential activation of plant defensive genes. Biochem. Biophys. Acta 1477 112–121. [DOI] [PubMed] [Google Scholar]
- Ryan, C.A., and Farmer, E.E. (1991). Oligosaccharide signals in plants: A current assessment. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 651–674. [Google Scholar]
- Shirasu, K., Nakajima, H., Rajasekhar, V.K., Dixon, R.A., and Lamb, C. (1997). Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway, L.K., Yang, V.V., and Ryan, C.A. (1976). Evidence for the presence of proteinase inhibitor I in vacuolar protein bodies of plant cells. Planta 129 161–165. [DOI] [PubMed] [Google Scholar]
- Stennis, M.J., Chandra, S., Ryan, C.A., and Low, P.S. (1998). Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 117 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen, H., Zhang, Z., Wei, Y., and Collinge, D.B. (1997). Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11 1187–1194. [Google Scholar]
- Torres, M.A., Onouchi, H., Hamada, S., Machida, C., Hammond-Kosack, K.E., and Jones, J.D.G. (1998). Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 14 365–370. [DOI] [PubMed] [Google Scholar]
- Trautman, R., Cowan, K.M., and Wagner, G.G. (1971). Data processing for radial immunodiffusion. Immunochemistry 8 901–916. [DOI] [PubMed] [Google Scholar]
- Wu, G., Shortt, B.J., Lawrence, E.B., Fitzsimmons, K.C., Levine, E.B., and Shah, D.P. (1995). Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell 7 1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G., Shortt, B.J., Lawrence, E.B., Leon, J., Fitzsimmons, K.C., Levine, E.B., Raskin, I., and Shah, D.P. (1997). Activation of host defense mechanisms by elevated production of H2O2 in transgenic plants. Plant Physiol. 115 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, T., Higgins, V.J., and Blumwald, E. (1997). Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell 9 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahraus, T., Chandra, S., Legendre, L., and Low, P.S. (1995). Evidence for mechanically induced oxidative burst. Plant Physiol. 109 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]