Abstract
In flowering plants, two cells are fertilized in the haploid female gametophyte. Egg and sperm nuclei fuse to form the embryo. A second sperm nucleus fuses with the central cell nucleus, which replicates to generate the endosperm, a tissue that supports embryo development. The FERTILIZATION-INDEPENDENT ENDOSPERM (FIE) and MEDEA (MEA) genes encode WD and SET domain polycomb proteins, respectively. In the absence of fertilization, a female gametophyte with a loss-of-function fie or mea allele initiates endosperm development without fertilization. fie and mea mutations also cause parent-of-origin effects, in which the wild-type maternal allele is essential and the paternal allele is dispensable for seed viability. Here, we show that FIE and MEA polycomb proteins interact physically, suggesting that the molecular partnership of WD and SET domain polycomb proteins has been conserved during the evolution of flowering plants. The overlapping expression patterns of FIE and MEA are consistent with their suppression of gene transcription and endosperm development in the central cell as well as their control of seed development after fertilization. Although FIE and MEA interact, differences in maternal versus paternal patterns of expression, as well as the effect of a recessive mutation in the DECREASE IN DNA METHYLATION1 (DDM1) gene on mutant allele transmission, indicate that fie and mea mutations cause parent-of-origin effects on seed development by distinct mechanisms.
INTRODUCTION
Flowering plant reproduction involves fertilization of two cells (reviewed in van Went and Willemse, 1984). Within the Arabidopsis ovule, the female gametophyte consists of an egg cell and two synergid cells at the micropylar end, a central cell in the middle, and three antipodal cells at the chalazal end. All are haploid except for the central cell, which contains two polar nuclei that fuse to form a diploid nucleus. Reproduction is initiated when an entering pollen tube discharges two genetically identical haploid sperm cells. Fertilization of the egg generates the diploid embryo, which passes through morphologically defined stages (globular, heart, torpedo, walking stick, early maturation, and maturation) (Goldberg et al., 1994; Jurgens and Mayer, 1994). During embryo development, two organ systems (axis and cotyledon) and three tissue layers (protoderm, procambium, and ground meristem) are specified (Lindsey and Topping, 1993; Jurgens, 1994; Meinke, 1994).
Fertilization of the central cell generates the triploid endosperm, for which the pattern of development differs dramatically from that of the embryo. Arabidopsis endosperm development is characteristic of nuclear endosperm development in angiosperms (Mansfield and Briarty, 1990a; Webb and Gunning, 1991; Berger, 1999; Brown et al., 1999). The Arabidopsis primary endosperm nucleus replicates without cytokinesis to form a syncytium of nuclear–cytoplasmic domains that migrate to the periphery of the expanding central cell (Brown et al., 1999). When the embryo is at the globular/heart transition stage, the endosperm surrounding the embryo at the micropylar end of the seed begins to cellularize. Cellularization progresses from the micropylar to the chalazal pole of the seed. Finally, periclinal divisions produce successive layers of endosperm that fill most of the central cell (Brown et al., 1999). As the seed matures, the endosperm, except for a thin aleurone layer, is degraded and absorbed by the embryo. The endosperm is thought to support embryo development by producing large amounts of storage proteins, starch, and lipids and by sequestering nutrients from maternal tissue (Lopes and Larkins, 1993).
Loss-of-function mutations in the FERTILIZATION-INDEPENDENT ENDOSPERM (FIE) gene allow diploid endosperm development in the absence of fertilization (Ohad et al., 1996, 1999; Chaudhury et al., 1997). This phenotype suggests that the wild-type FIE gene functions to suppress endosperm development until fertilization occurs. The FIE polypeptide (Ohad et al., 1999) has seven tryptophan-aspartate (WD) motifs (Ng et al., 1997). FIE is related to polycomb proteins from Drosophila (EXTRA SEX COMBS [ESC; Gutjahr et al., 1995; Sathe and Harte, 1995]), mammals (EMBRYONIC ECTODERM DEVELOPMENT [EED; Schumacher et al., 1996; Sewalt et al., 1998]), and Caenorhabditis (MATERNAL EFFECT STERILE6 [MES6; Korf et al., 1998]). Drosophila ESC and mammalian EED promote interactions with other polycomb proteins that repress gene transcription at specific sites within the genome (Wall et al., 1995; Sondek et al., 1996; Ng et al., 1997).
After fertilization, mutations in the FIE gene cause parent-of-origin effects on seed development (Ohad et al., 1996; Chaudhury et al., 1997). For example, when a heterozygous fie/FIE plant is pollinated with wild-type pollen, half of the seed inherit a maternal mutant fie allele and subsequently abort their development, even in the presence of the wild-type paternal allele. These aborted seed contain endosperm and a heart-shaped embryo. However, no seed abortion is observed when the reciprocal cross is performed. Thus, the wild-type maternal FIE allele is essential, whereas the paternal FIE allele plays little or no role during seed development. The mechanism responsible for these parent-of-origin effects is unknown. Perhaps maternal FIE gene expression, which is essential for embryo or endosperm development, occurs before fertilization in the female gametophyte. Alternatively, a gene dosage–dependent mechanism might underlie the parent-of-origin effect of fie on seed viability; that is, a single copy of the paternal wild-type FIE gene may not be able to compensate for the lack of gene activity associated with two maternal mutant fie alleles within the triploid endosperm. Finally, FIE could be an imprinted gene in which the maternal allele is expressed and the paternal allele is silenced.
Plants with mutations in either the MEDEA (MEA) or FERTILIZATION-INDEPENDENT SEED2 (FIS2) gene display phenotypes similar to those of fie mutant plants (Chaudhury et al., 1997; Grossniklaus et al., 1998; Kiyosue et al., 1999). The MEA gene encodes a SET (Jenuwein et al., 1998) domain polycomb protein (Grossniklaus et al., 1998; Kiyosue et al., 1999; Luo et al., 1999), whereas FIS2 encodes a zinc finger protein (Luo et al., 1999). The relationships between the functions performed by the FIE, FIS2, and MEA genes have not been elucidated.
To better understand how female gametophyte, endosperm, and embryo development is controlled, we compared the functions of the FIE and MEA polycomb genes. Here, we show that the FIE WD polycomb protein interacts physically with the MEA SET domain polycomb protein. Their overlapping expression patterns are consistent with their suppression of endosperm development in the central cell as well as their control of seed development after fertilization. Although FIE and MEA probably function together in a polycomb protein complex, differences in maternal versus paternal patterns of expression, as well as the effect of a recessive mutation in the DECREASE IN DNA METHYLATION1 (DDM1) gene on mutant allele transmission, indicate that fie and mea mutations cause parent-of-origin effects on seed development by distinctly different mechanisms.
RESULTS
FIE and MEA Proteins Interact
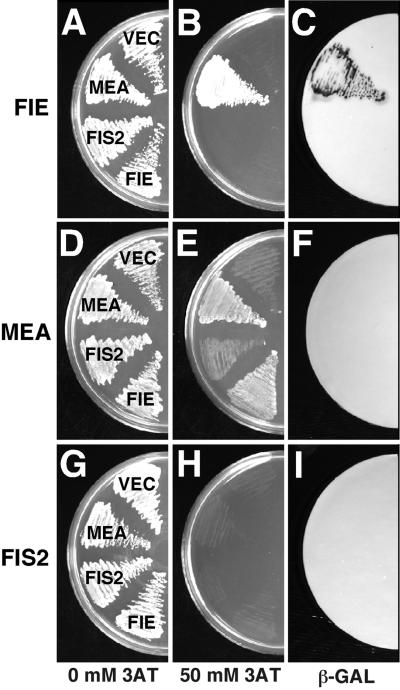
Because the phenotypes associated with mutations in the FIE, MEA, and FIS2 genes are similar, we considered that the FIE WD polycomb might interact directly with the MEA SET domain polycomb or the FIS2 zinc finger protein. To investigate this possibility, we used the yeast two-hybrid system, in which interacting proteins bring a GAL4 activation domain (GAL4AD) and a DNA binding domain (GAL4BD) together to activate transcription of β-GALACTOSIDASE (β-GAL) and HISTIDINE3 (HIS3) reporter genes (Chevray and Nathans, 1992). To this end, we expressed all pairwise combinations of FIE, MEA, and FIS2 fused to GAL4AD and GAL4BD. As shown in Figure 1, yeast expressing both GAL4BD-FIE and GAL4AD-MEA activated strong expression of the HIS3 (Figure 1B) and β-GAL (Figure 1C) genes. In contrast, control yeast containing individual constructs did not activate reporter expression. Although MEA was shown to bind weakly to itself (Figure 1E), analogous to the self-association of SET domain polypeptides in Drosophila and human (Rozovskaia et al., 2000), no other strong interactions, including pairwise combinations with FIS2, were detected in the yeast two-hybrid experiments (Figure 1). Therefore, these results suggest that the FIE and MEA proteins interact specifically in a yeast two-hybrid system.
Figure 1.
FIE and MEA Interaction in Yeast.
Yeast were transformed with genes encoding GAL4BD fused with full-length FIE ([A] to [C]), MEA ([D] to [F]), and FIS2 ([G] to [I]). Yeast were also transformed with genes encoding GAL4AD fused to MEA, FIS2, and FIE, as shown in (A), (D), and (G), respectively. In (B), (E), and (H), yeast were grown on plates containing synthetic complete medium minus leucine, tryptophan, and histidine. 3-Amino- 1,2,4-triazole (3AT) (50 mM) was added to repress the basal activity of the HIS3 reporter gene, which had resulted in the nonspecific background growth visible in (A), (D), and (G) in the absence of exogenous histidine (Kishore and Shah, 1988). The colony colors of the transformants on the plates shown in (B), (E), and (H) were determined by the filter lift assay shown in (C), (F), and (I), respectively.
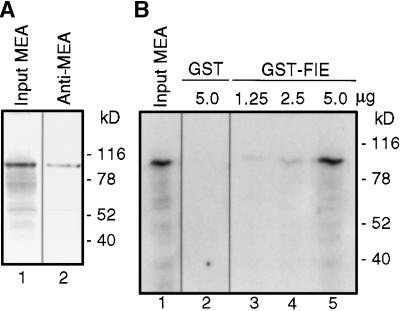
In the reciprocal two-hybrid experiment, expression of GAL4BD-MEA and GAL4AD-FIE activated HIS3 reporter expression (Figure 1E), but no β-GAL reporter expression was detected (Figure 1F). Because this may have been a result of decreased expression or stability of the fusion proteins in this experiment (Fields and Sternglanz, 1994), we used an independent method to verify whether FIE binds to MEA. Glutathione S-transferase–tagged FIE fusion protein (GST-FIE) was purified from Escherichia coli and tested for binding to in vitro–synthesized radiolabeled MEA protein. As shown in Figure 2A (lane 1), radiolabeled full-length MEA migrated as an ∼100-kD polypeptide in our gel electrophoresis system and reacted with an anti-MEA antibody (Figure 2A, lane 2). Radiolabeled MEA was retained by the immobilized GST-FIE (Figure 2B, lanes 3 to 5) but not by control immobilized GST (Figure 2B, lane 2). Moreover, the quantity of radiolabeled MEA retained correlated with the amount of immobilized GST-FIE used (Figure 2B, lanes 3 to 5). These results indicate that FIE is capable of binding to MEA in vitro.
Figure 2.
In Vitro Interaction of FIE and MEA Polycomb Proteins.
(A) In vitro–transcribed and –translated 35S-methionine–labeled MEA (lane 1) was subjected to SDS-PAGE, blotted, and incubated with anti-MEA antibody (lane 2).
(B) In vitro–transcribed and –translated 35S-methionine–labeled MEA was incubated with immobilized GST (lane 2) or GST-FIE (lanes 3 to 5), washed, eluted, and subjected to SDS-PAGE and autoradiography. The quantities of GST and GST-FIE proteins immobilized (μg) are indicated. The input MEA (lane 1) represents 10% of the amount of 35S-methionine–labeled MEA incubated with immobilized proteins.
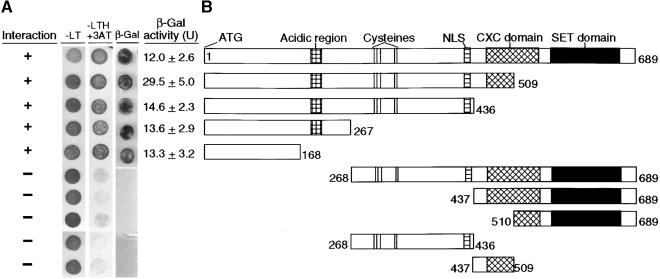
To investigate the specificity of the interaction between WD and SET domain polycomb proteins, we localized the portion of the MEA polypeptide that binds to FIE. As shown in Figure 3B, we constructed different deletions of GAL4DB-MEA and analyzed their capacity to bind GAL4AD-FIE in the yeast two-hybrid system (Figure 3A). Our results show that the N-terminal 168 amino acids of MEA are sufficient to interact with FIE in the yeast two-hybrid system. Together, these results indicate that the FIE and MEA proteins interact specifically and that this interaction is mediated by the N-ter-minal region of the MEA protein.
Figure 3.
Interaction Domain Analysis of MEA in the Yeast Two-Hybrid System.
(A) Yeast were transformed with GAL4BD-FIE and the GAL4AD plasmid containing the deletion constructs indicated in (B). −LT, synthetic complete medium minus leucine and tryptophan; −LTH +3AT, synthetic complete medium minus leucine, tryptophan, and histidine and supplemented with 50 mM 3-amino-1,2,4-triazole; (+), positive interaction; (−), no detectable interaction; β-Gal activity (U), β-galactosidase activity in liquid cultures measured in units (Kim et al., 1997).
(B) Scheme showing the deletion constructs of MEA. Numbers indicate the positions of the deletions relative to the full-length MEA protein. NLS, nuclear localization sequence.
FIE::GFP and FIE::GUS Expression during Ovule, Endosperm, and Embryo Development
If the interaction between FIE and MEA is biologically relevant, then their expression patterns should overlap. Previously, we showed that both FIE and MEA were expressed in reproductive organ systems, immature floral buds, mature flowers, and young siliques containing developing seed (Kiyosue et al., 1999). Moreover, MEA mRNA is detected in the female gametophyte (i.e., central, egg, and synergid cells) (Vielle-Calzada et al., 1999) as well as in the embryo and endosperm (Kiyosue et al., 1999; Vielle-Calzada et al., 1999). To obtain information about the spatial regulation of FIE gene expression within the female gametophyte, and after fertilization in the endosperm and embryo, we transformed Arabidopsis plants with three chimeric genes: either 1.3 or 4.9 kb of FIE 5′ flanking sequences ligated to a GREEN FLUORESCENT PROTEIN (GFP) reporter gene (FIE::GFP) (Heim et al., 1995; Chiu et al., 1996), and 1.3 kb of FIE 5′ flanking sequences ligated to a β-GLUCURONIDASE (GUS) reporter gene (FIE::GUS) (Jefferson et al., 1987). As shown in Figure 4A, in a line bearing the 1.3-kb FIE::GFP gene, GFP reporter fluorescence was detected in mature female gametophytes within the central cell. No GFP fluorescence was detected in ovules at earlier stages (data not shown). Similar results were observed in multiple transgenic lines and in lines bearing a FIE::GFP gene with 4.9 kb of 5′ flanking sequences. In contrast, histochemical localization of GUS activity in FIE::GUS plants indicated an earlier stage of female gametophyte development, before the polar nuclei fusion, for the onset of FIE::GUS activity (Figure 5A). Most likely, GFP and GUS accumulated in the cytoplasm because the FIE-GFP and FIE-GUS fusion proteins did not include the putative FIE nuclear localization sequence (Ohad et al., 1999). Full-length FIE protein fused translationally with GFP localized in the central cell nucleus (T. Kinoshita and R.L. Fischer, unpublished results). This pattern of FIE::GFP and FIE::GUS expression is consistent with the hypothesis that FIE protein is present in the central cell, along with MEA (Vielle-Calzada et al., 1999), and functions to prevent the central cell from replicating and forming an endosperm.
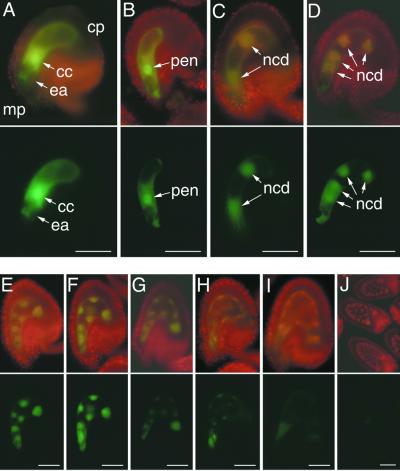
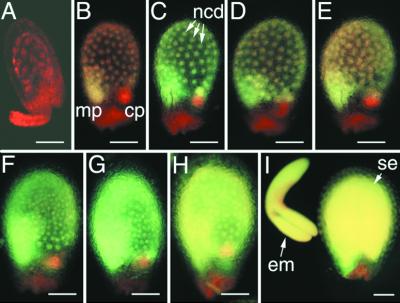
Figure 4.
Pattern of FIE::GFP Expression in an Unfertilized Ovule and Early Endosperm Development.
Unfertilized ovules and developing seeds from transformed plants bearing a transgene with 1.3 kb of FIE 5′ flanking sequences fused to GFP were analyzed by using fluorescence microscopy, as described in Methods.
(A) Fluorescence micrograph of an intact unfertilized ovule with a mature female gametophyte, indicating FIE::GFP expression in the cytoplasm of the central cell.
(B) to (J) Fluorescence micrographs of intact developing seed corresponding to early stages of endosperm development, including the formation of the primary endosperm nucleus after fertilization (B) and its subsequent division ([C] to [J]) to form a syncytium of nuclear cytoplasmic domains (Mansfield and Briarty, 1990a; Brown et al., 1999).
Images were obtained by using fluorescein isothiocyanate (FITC) (chloroplast plus GFP fluorescence; top rows) and GFP (GFP fluorescence; bottom rows) filter sets on a compound microscope (see Methods). The images of the ovules and the developing seed in this figure and in Figures 5, 6, and 8 are oriented in a uniform manner, with the micropylar pole oriented toward the left side and the chalazal pole oriented toward the right side of each figure. cc, central cell; cp, chalazal pole; ea, egg apparatus; mp, micropylar pole; ncd, nuclear cytoplasmic domain; pen, primary endosperm nucleus.  ;
; 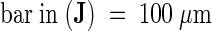 .
.
Figure 5.
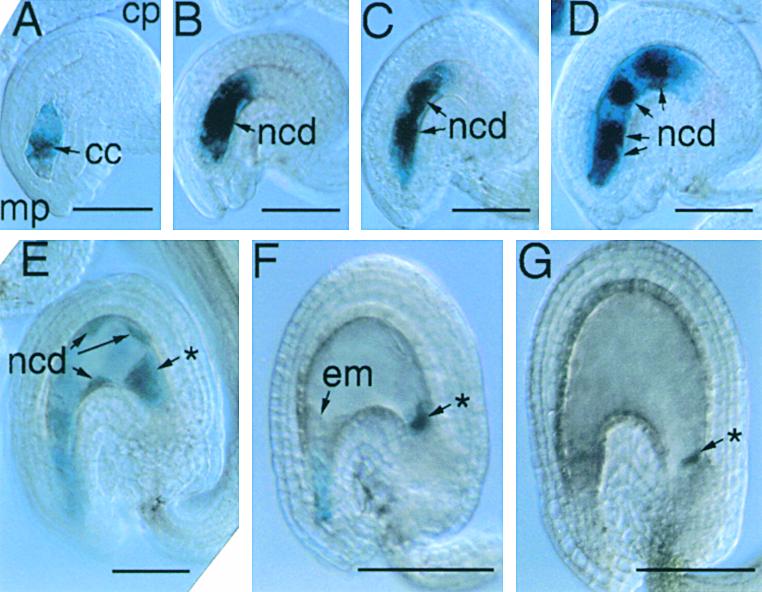
Pattern of FIE::GUS Expression in an Unfertilized Ovule and Early Endosperm Development.
Unfertilized ovules and developing seeds from transformed plants bearing a transgene with 1.3 kb of FIE 5′ flanking sequences fused to GUS were analyzed by using light microscopy, as described in Methods.
(A) Light micrograph of an intact unfertilized ovule with an eight-nucleate stage of female gametophyte development (Christensen et al., 1997), indicating FIE::GUS expression in the cytoplasm of the central cell.
(B) to (G) Light micrographs of intact developing seed corresponding to early stages of endosperm development, including the formation of the primary endosperm nucleus after fertilization (B) and its subsequent division ([C] to [G]) to form a syncytium of nuclear cytoplasmic domains (Mansfield and Briarty, 1990a; Brown et al., 1999).
Images were obtained by using differential interference contrast microscopy of unfixed, intact ovules and early seeds after a histochemical reaction for the detection of GUS activity (see Methods). cc, central cell; cp, chalazal pole; em, embryo; mp, micropylar pole; ncd, nuclear cytoplasmic domain; (*), chalazal cyst. 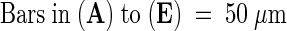 ;
; 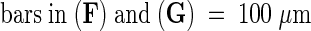 .
.
After fertilization, FIE::GFP and FIE::GUS expression was detected during very early endosperm development, from the primary endosperm nuclear-cytoplasmic domain stage to the eight– to 16–nuclear-cytoplasmic domain stage (Figures 4B to 4G and 5B to 5E). After this stage, GFP fluorescence and GUS activity decreased (Figures 4H to 4J, 5F, 5G, and 6A) until a second phase of FIE::GFP and FIE::GUS expression was observed in the endosperm, when the ∼100 nuclear-cytoplasmic domains formed a single layer surrounding the endosperm cavity (Figures 6B to 6E and data not shown). GFP fluorescence persisted in the endosperm during the cellularization stage (Figures 6F to 6H) and was also observed in the developing embryo at the torpedo, walking stick, and early maturation stages (Figure 6I and data not shown). Before endosperm cellularization, the only evidence for any transgene activity within the developing seed was a persistent localization of GUS activity in the chalazal cyst (Figures 5E to 5G). The biphasic expression pattern in the endosperm described above was specific to FIE::GFP and FIE::GUS and was not detected in endosperm in an enhancer trap line (data not shown), in which GFP transcription during endosperm development was controlled by an uncharacterized transcriptional enhancer element (C. Dever and G.N. Drews, unpublished results). The mRNA transcribed from the FIE::GFP and FIE::GUS constructs used in this study included essentially all of the FIE 5′ untranslated sequences (see Methods). Therefore, these patterns of GFP fluorescence and GUS activity may reflect both transcriptional and translational regulatory processes conferred by FIE 5′ flanking sequences. Together, these results indicate a complex pattern of expression for FIE::GFP and FIE::GUS before and after fertilization, reflecting the essential role of the FIE protein in seed development.
Figure 6.
Pattern of FIE::GFP Expression during Mid to Late Endosperm and Embryo Development.
Developing seed from transformed plants bearing a transgene with 1.3 kb of FIE 5′ flanking sequences fused to GFP were analyzed by fluorescence microscopy, as described in Methods. Fluorescence micrographs are shown for intact developing seed at various stages of development (Mansfield and Briarty, 1990c; Brown et al., 1999).
(A) Preglobular stage embryo/free nuclear endosperm.
(B) Late globular or transition stage embryo.
(C) to (F) Heart stage embryos/cellularizing endosperm.
(G) and (H) Late heart or early torpedo stage embryos.
(I) Maturation stage embryo/degenerating cellular endosperm.
Images were obtained using an FITC filter set (chloroplast and GFP fluorescence) on a fluorescence stereomicroscope (see Methods). cp, chalazal pole; em, embryo; mp, micropylar pole; ncd, nuclear cytoplasmic domain; se, intact seed containing an embryo and endosperm. 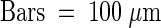 .
.
Parent-of-Origin Effects of fie and mea Mutations on Seed Development
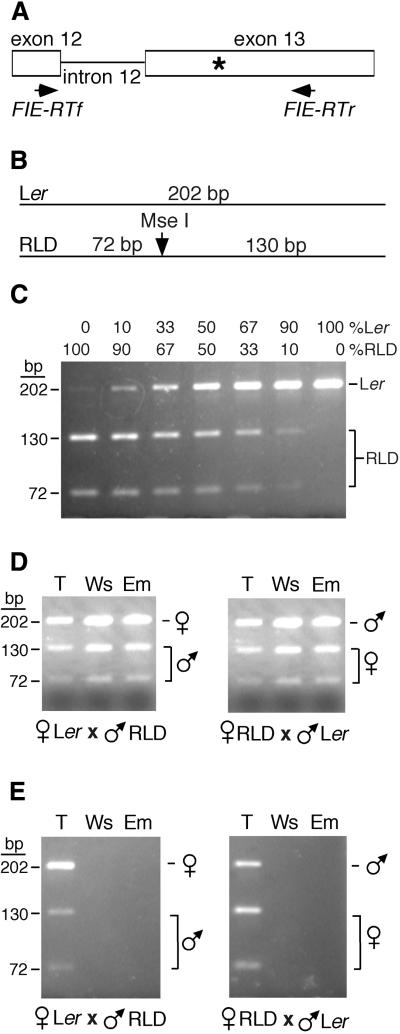
Mutations in either the FIE or MEA genes cause parent-of-origin effects on seed development. For mea mutations, this probably results, at least in part, from the fact that MEA is an imprinted gene in which the paternal MEA allele is silenced (Kinoshita et al., 1999; Vielle-Calzada et al., 1999). In particular, previous studies revealed that the paternal MEA allele is silenced specifically in the endosperm of dissected seed from the torpedo to maturation stages (Kinoshita et al., 1999). To determine whether the paternal FIE allele is silenced in the same way, we directly measured the amounts of maternal and paternal FIE mRNAs in dissected seed. As shown in Figure 7A, to distinguish maternal and paternal FIE RNAs, we identified a restriction (MseI) fragment length DNA sequence polymorphism in the FIE gene isolated from two Arabidopsis ecotypes, Landsberg erecta (Ler) and RLD (Hardtke et al., 1996). Reverse transcription–polymerase chain reaction (RT-PCR) amplification of Ler FIE sequences followed by MseI digestion was predicted to produce a 202-bp DNA sequence (Figure 7B). For RLD, the same treatment was predicted to produce 130- and 72-bp DNA sequences (Figure 7B). To test this strategy, RNA from Ler and RLD floral buds was mixed, and the FIE sequences were amplified and digested as described above. As shown in Figure 7C, restriction fragments of the expected size and abundance were detected, indicating that we can distinguish between FIE RNAs transcribed from the Ler and the RLD genomes and that the assay is semiquantitative.
Figure 7.
Pattern of Paternal and Maternal FIE mRNA Accumulation in Dissected Seed.
(A) Strategy for distinguishing maternal and paternal MEA mRNA. FIE-RTf and FIE-RTr represent primers used for RT-PCR amplification of FIE sequences. The asterisk indicates the DNA sequence polymorphism between the Ler and RLD MEA genes. Positions of exons and introns are indicated.
(B) Predicted sizes of restriction fragments after RT-PCR amplification and digestion with MseI restriction endonuclease.
(C) Amplification of FIE sequences from Ler and RLD floral RNA. RNA was isolated from Ler and RLD floral buds at stages 0 to 13 (Smyth et al., 1990), mixed in the indicated proportions, and subjected to RT-PCR amplification, MseI restriction endonuclease digestion, and agarose gel electrophoresis. Ler- and RLD-specific bands are indicated at right, and the lengths of the restriction fragments (in base pairs) are indicated at left.
(D) Pattern of paternal and maternal FIE mRNA accumulation in embryos. Reciprocal crosses between Ler and RLD plants were made, and F1 seed were harvested at 6, 7, and 8 days after pollination, corresponding to the torpedo (T), walking stick (Ws), and early maturation (Em) embryo stages, respectively. Embryos were dissected from seed. RNA was isolated and subjected to RT-PCR amplification, restriction endonuclease digestion, and agarose gel electrophoresis. Restriction fragments associated with maternal and paternal alleles are indicated at left.
(E) Pattern of paternal and maternal FIE mRNA accumulation in endosperm plus seed coat. Reciprocal crosses were performed as described in (D). Embryos at the indicated stages were removed from seed. RNA was isolated from the remaining endosperm-plus-seed-coat portion and analyzed as described in (D). Restriction fragments associated with maternal and paternal alleles are indicated at left.
To examine the expression of paternal and maternal FIE alleles in seed, reciprocal crosses were performed between Ler and RLD Arabidopsis plants. RNA was isolated from dissected F1 seed harvested at 6, 7, and 8 days after pollination—times corresponding to the torpedo, walking stick, and early maturation stages of embryo development (Jurgens and Mayer, 1994). Seed were dissected, and RNA was isolated from the embryo and endosperm-plus-seed-coat components. Using the procedures described above, we measured the amounts of maternal and paternal FIE mRNA. Both maternal and paternal FIE allele expression was detected in embryos at all stages tested (Figure 7D) and in endosperm plus seed coat at the torpedo stage of embryo development (Figure 7E). In contrast, using the same RNAs, we had previously detected biallelic MEA expression in the embryo but only the maternal MEA mRNA in the endosperm of seeds containing torpedo, walking stick, and early-maturation embryos (Kinoshita et al., 1999). Thus, the paternal MEA allele, and not the paternal FIE allele, is silenced at this stage of endosperm development. These results support the hypothesis that different mechanisms are responsible for fie and mea parent-of-origin effects on seed development.
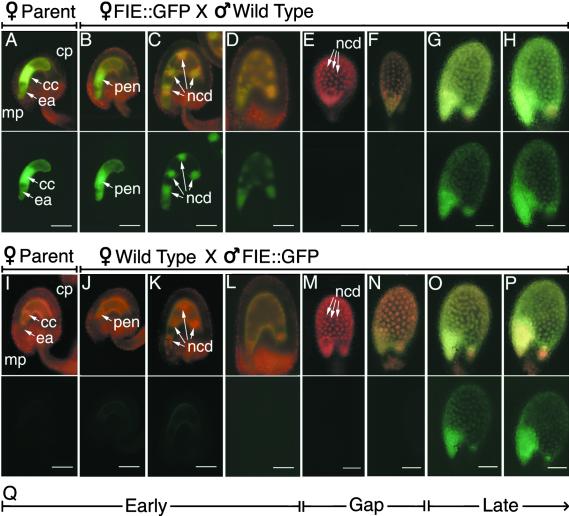
To obtain clues about earlier patterns of allele-specific FIE gene expression, we performed reciprocal crosses between wild-type and transgenic plants bearing a FIE::GFP transgene and then monitored the GFP fluorescence in F1 progeny seed. As described below, similar results were observed with transgenes having either 1.3 or 4.9 kb of FIE 5′ flanking sequences. As shown in Figure 8A (before fertilization), we detected GFP fluorescence in the central cell of a FIE::GFP ovule before pollination. After pollination of the FIE::GFP line with wild-type pollen, maternal FIE::GFP expression was detected up to the 16–nuclear cytoplasmic domain stage (Figures 8B to 8D) in seed with a four- to 16-cell embryo (data not shown). After an interval during which no GFP was detected (Figures 8E and 8F), maternal FIE::GFP expression resumed in the 100–nuclear cytoplasmic domain stage endosperm (Figures 8G and 8H). Thus, the maternal FIE::GFP allele was expressed in a biphasic pattern (Figure 8Q), as had been observed in self-pollinated FIE::GFP plants (Figures 4 and 6).
Figure 8.
Pattern of Paternal and Maternal FIE::GFP Expression during Endosperm and Embryo Development.
Developing F1 progeny seed resulting from reciprocal crosses between wild-type and transgenic plants bearing a transgene with 4.9 kb of the FIE 5′ flanking sequences fused to GFP were analyzed by fluorescence microscopy, as described in Methods.
(A) and (I) Intact unfertilized ovules with mature female gametophytes are shown from the maternal parents in each cross, one bearing a FIE::GFP transgene (A) and the other a wild-type/nontransgenic plant (I).
(B) to (H) Intact developing seed resulting from fertilizing a transgenic plant (A) with wild-type pollen.
(J) to (P) Intact developing seed resulting from fertilizing a wild-type plant (I) with pollen from a transgenic plant (A).
(Q) Scheme of early and late periods of maternal FIE::GFP allele expression separated by a gap.
Images were obtained by using FITC (chloroplast plus GFP fluorescence; top rows) and GFP (GFP fluorescence; bottom rows) filter sets on a compound microscope ([A] to [D]) and ([I] to [L]) and a stereomicroscope ([E] to [H]) and ([M] to [P]) as described in Methods. cc, central cell; cp, chalazal pole; ea, egg apparatus; mp, micropylar pole; ncd, nuclear cytoplasmic domain; pen, primary endosperm nucleus. 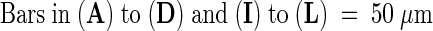 ;
; 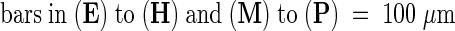 .
.
For the reciprocal cross, as expected, no GFP fluorescence was observed in a wild-type nontransgenic ovule before pollination (Figure 8I). After pollination with FIE::GFP pollen, no GFP fluorescence was detected until the 100–nuclear cytoplasmic domain stage (Figure 8O), corresponding to the latter phase of FIE::GFP expression observed in self-pollinated FIE::GFP plants (Figure 6). In particular, the early phase of maternal FIE::GFP expression, from fertilization in the central cell until the 16–nuclear cytoplasmic stage of endosperm development, was not detected for the paternal FIE::GFP allele (cf. Figures 8B to 8D with 8J to 8L). These results show that the expression of the maternal and paternal FIE::GFP alleles is not equivalent.
Differential Effects of ddm1 on fie- and mea-Mediated Seed Abortion
Gene silencing is known to be associated with hypermethylation, and a recessive mutation in the DECREASE IN DNA METHYLATION1 (DDM1) gene, ddm1-2, encoding a chromatin-remodeling SWI2/SNF2-like protein (Jeddeloh et al., 1999), has been shown to rescue mutant mea seed in self-pollinated plants (Vielle-Calzada et al., 1999). To determine whether a similar relationship exists between genome hypomethylation and fie phenotype, we analyzed the ability of the ddm1-2 mutation to rescue fie mutant seed. As shown in Table 1, when control mea/MEA heterozygous plants were pollinated with wild-type pollen, most of the F1 seed inheriting a maternal mutant mea allele aborted, and only 6% of the F1 progeny were mea/MEA heterozygotes. In contrast, pollination of mea/MEA heterozygous plants with homozygous ddm1/ddm1 pollen decreased the proportion of seed abortion, and 33% of the F1 progeny inherited the maternal mutant mea allele (Table 1). This fivefold increase is consistent with DDM1 playing a role in silencing the paternal wild-type MEA allele during pollen development. In contrast to MEA, when fie/FIE heterozygous plants were pollinated with either wild-type or homozygous ddm1 mutant pollen, all of the F1 seed inheriting a maternal fie allele aborted, and none of the F1 progeny were fie/FIE heterozygotes (Table 1). Thus, ddm1 pollen suppressed mea-mediated, but not fie-mediated, seed abortion. Therefore, the ddm1-2 mutation distinguishes the distinct mechanisms responsible for fie and mea parent-of-origin effects on seed development.
Table 1.
Effect of a Paternal ddm1-2 Mutant Allele on Transmission of a Maternal fie-1 or mea-3 Mutant Allele
| Maternal Parent | Paternal Parent | Progeny with Maternal Mutant Allele | Progeny with Maternal Wild-Type Allele | Percenta |
|---|---|---|---|---|
| mea/MEA | MEA/MEA (wild type) | 9 mea/MEA | 150 MEA/MEA | 6 |
| mea/MEA | MEA/MEA, ddm1/ddm1 | 44 mea/MEA, ddm1/DDM1 | 89 MEA/MEA, ddm1/DDM1 | 33 |
| fie/FIE | FIE/FIE (wild type) | 0 fie/FIE | 52 FIE/FIE | 0 |
| fie/FIE | FIE/FIE, ddm1/ddm1 | 0 fie/FIE, ddm1/DDM1 | 46 FIE/FIE, ddm1/DDM1 | 0 |
Percentage of transmission is 100 × (number of fie or mea heterozygotes/total progeny of cross).
DISCUSSION
Molecular Function of the FIE Protein
The Arabidopsis FIE (Ohad et al., 1999) polycomb protein belongs to a family of WD repeat polypeptides conserved during the evolution of diverse organisms (Ng et al., 1997). The WD repeats in this family of proteins form a circular structure known as a β-propeller, which forms surface loops used as a scaffold for the generation of protein complexes (Komachi et al., 1994; Wall et al., 1995; Sondek et al., 1996). The closest related members to FIE in model genetic organisms are the polycomb proteins ESC in Drosophila (Sathe and Harte, 1995), EED in mouse (Schumacher et al., 1996), and MES6 in Caenorhabditis (Korf et al., 1998). Drosophila ESC and mouse EED have been shown to interact with the SET domain (Jenuwein et al., 1998) polycomb proteins ENHANCER OF ZESTE (E[Z]) (Jones et al., 1998) and ENX1/ENX2 (van Lohuizen et al., 1998), respectively. In the case of Drosophila, yeast two-hybrid experiments, in vitro binding studies, demonstration of coimmunoprecipitation of proteins from embryos, colocalization of proteins on polytene chromosomes, and identification of mutations that disrupt both binding and function all indicate that ESC and E(Z) interact directly and that this interaction is an essential aspect of their function (Jones et al., 1998; Tie et al., 1998). An analogous set of studies demonstrates a direct physical interaction between the mouse WD and SET domain homologs (Denisenko et al., 1998; Jones et al., 1998; Sewalt et al., 1998; van Lohuizen et al., 1998).
Here, we show that the Arabidopsis homologs FIE WD polycomb and MEA SET domain polycomb interact in the yeast two-hybrid system (Figure 1) and in vitro (Figure 2). We also show that an interacting domain is present at the N terminus of MEA (Figure 3), similar to that found in Drosophila and mouse SET domain homologs (Tie et al., 1998; van Lohuizen et al., 1998). In addition, these results imply that the MEA interaction domain is distinct from other conserved regions of the protein, such as the cysteine-rich domain, the nuclear localization domain, the CXC domain, and the SET domain (Grossniklaus et al., 1998). Moreover, the pattern of FIE::GFP and FIE::GUS expression (Figures 4 to 6) and FIE mRNA accumulation (Figure 7) overlaps with the pattern of MEA RNA accumulation in the central cell of the female gametophyte, endosperm, and embryo (Kinoshita et al., 1999; Vielle-Calzada et al., 1999). Thus, FIE and MEA polypeptides probably are present in the same cells, which allows for interaction. Together, these results suggest that FIE and MEA interact and that a fundamental biochemical mechanism, the molecular partnership of WD and SET domain polycomb proteins, has been conserved during the evolution of flowering plants.
In Drosophila and mouse, long-term repression of homeotic genes requires the activity of polycomb group proteins (Pirrotta, 1998), and a distinct temporal sequence of events involved in the assembly of the complex has been defined. Initially recruited, although not in a direct interaction (Kehle et al., 1998; Zhang et al., 1998), by a zinc finger transcription factor (HUNCHBACK), ESC functions transiently (Struhl and Brower, 1992; Gutjahr et al., 1995; Sathe and Harte, 1995) to recruit other polycomb group proteins, including the SET domain protein E(Z), which form large, stable complexes at specific sites within the genome (Sewalt et al., 1998; van der Vlag and Otte, 1999; Ng et al., 2000). The complexes, perhaps by the action of a histone deacetylase component (van der Vlag and Otte, 1999), are thought to remodel chromatin into a condensed form that prevents gene transcription (Pirrotta, 1998). By analogy, a similar process may occur in plants, with FIS2 taking the role of the zinc finger protein to indirectly recruit FIE. FIE, in turn, may function transiently to establish a stable complex that includes the SET domain polycomb MEA and ultimately results in the stable repression of gene transcription.
Function of FIE in the Female Gametophyte
Although the biochemical mode of action of interacting pairs of WD and SET domain polycomb proteins is highly conserved, the processes that they regulate are not conserved during evolution. The Drosophila and mouse WD and SET domain polycomb proteins have a somatic role and control pattern formation during embryogenesis by suppressing the transcription of homeotic genes (Gutjahr et al., 1995; Schumacher et al., 1996). In contrast, the Caenorhabditis WD and SET domain homologs MES6 and MES2, respectively, act as transcriptional repressors (Kelly and Fire, 1998) and are essential for germ line development (Holdemann et al., 1998; Korf et al., 1998). Thus, it is not surprising that the plant FIE and MEA interacting pair has been used to repress distinct developmental pathways, as described below.
Loss-of-function mutations in the FIE (Ohad et al., 1999) and MEA (Kiyosue et al., 1999; Luo et al., 1999) genes allow for the central cell in the female gametophyte to replicate and begin endosperm development. Moreover, FIE and MEA interact (Figures 1 to 3) and are expressed in the central cell (Figures 4 and 5) (Vielle-Calzada et al., 1999). These results suggest that before fertilization, FIE and MEA participate in the formation of a polycomb complex that represses the transcription of genes required for replication of the central cell nucleus and subsequent endosperm development. The fact that FIE::GFP expression was detected in the central cell and not in the egg cell (Figure 4) suggests that FIE may not be expressed in the egg cell, which is consistent with the observation that concomitant autonomous embryo development was not observed in fie mutant ovules (Ohad et al., 1996). Together, these results suggest that different proteins and mechanisms may be responsible for the activation of endosperm and embryo development.
Function of FIE after Fertilization
The FIE::GFP and FIE::GUS genes display a complex pattern of expression during seed development. They display a biphasic pattern during endosperm development (Figures 4 to 6 and 8), with the latter phase of activity also present in the embryo (Figure 6 and data not shown). This complex pattern suggests that FIE might function, and have multiple roles, during endosperm and embryo development. Because MEA is also expressed in the developing embryo and endosperm (Kinoshita et al., 1999; Vielle-Calzada et al., 1999), FIE and MEA may interact after fertilization. We had shown previously that the MEA polycomb protein functions to suppress endosperm growth after fertilization (Kiyosue et al., 1999). Possibly FIE recruits MEA into a polycomb complex in the endosperm for that purpose.
Many studies have focused on the regulation of early embryogenesis by the Drosophila ESC and mouse EED WD proteins. However, Drosophila ESC is also detected in mid and late embryogenesis as well as in larval and pupal stages, during which it is thought to play a role in imaginal disc formation (Struhl and Brower, 1992; Ng et al., 2000). The mouse EED gene also is expressed in many tissues during mouse development (Schumacher et al., 1996), and EED protein has been associated with the chromatin-remodeling enzyme histone deacetylase (van der Vlag and Otte, 1999). These studies suggest that ESC and EED may be globally involved in chromatin regulation at many points during the development of Drosophila and mouse, respectively (Ng et al., 2000).
The broad pattern of FIE RNA accumulation in both reproductive and vegetative tissues (Kiyosue et al., 1999; Ohad et al., 1999; R. Yadegari and R.L. Fischer, unpublished results) suggests that the FIE polycomb protein may also participate in a general repression mechanism, perhaps by interacting with various SET domain polypeptides or histone deacetylase genes at different times during plant development (Goodrich et al., 1997; Wu et al., 2000). However, because loss-of-function mutations in the FIE gene are not transmitted to the next generation (Table 1), it has not been possible to address this point by generating homozygous mutant fie plants to study the function of FIE during vegetative plant development.
Mechanism for Parent-of-Origin Effects of fie Mutations on Seed Development
Mutations in both the FIE and MEA polycomb genes result in parent-of-origin effects on seed development. Specifically, a seed inheriting a mutant mea or fie allele will abort, even if the paternal allele is wild type. For MEA, this is probably a result, at least in part, of the silencing of the paternal MEA allele (Kinoshita et al., 1999; Vielle-Calzada et al., 1999). However, several lines of evidence suggest that fie- and mea-mediated parent-of-origin effects are caused by distinct mechanisms. First, the parent-of-origin effects associated with fie mutations are more stringent than those associated with mea mutations. That is, the maternal mutant mea allele is occasionally transmitted (Chaudhury et al., 1997; Kiyosue et al., 1999; Vielle-Calzada et al., 1999), whereas the maternal mutant fie allele is not (Table 1) (Ohad et al., 1996; Chaudhury et al., 1997). Second, despite a report that homozygosity of ddm1 in the developing seed is required to suppress the mea mutant phenotype (Vielle-Calzada et al., 1999), we find that pollen from a homozygous ddm1 paternal parent is sufficient for suppression (Table 1). In contrast, pollen from a ddm1 parent does not suppress the fie mutant phenotype (Table 1), indicating that FIE, unlike MEA, is not influenced by DDM1 during pollen donor development. Third, the wild-type paternal MEA is silenced specifically in the endosperm during the mid to late stages of endosperm development (Kinoshita et al., 1999). In contrast, the paternal FIE allele is expressed during these stages (Figure 7). The different patterns of paternal FIE and MEA allele expression within the endosperm (Figures 7 and 8) indicate differences in the regulation of their expression. For example, paternal FIE and MEA alleles might be initially silenced at the same time before fertilization, but the underlying molecular mechanisms responsible for silencing might be sufficiently different to include a longer period of silencing for the paternal MEA allele. Together, these results suggest that the mechanisms for fie and mea parent-of-origin effects on seed development differ.
Why is the maternal FIE allele essential and the paternal FIE allele dispensable? Only the maternal FIE::GFP allele is expressed in the unfertilized central cell of the female gametophyte and during very early endosperm development until approximately the 16–nuclear cytoplasmic domain stage (Figure 8). During this interval, the paternal FIE::GFP allele is either not present (i.e., in the unfertilized ovule) or not active (Figure 8). Perhaps the parent-of-origin phenotype of fie mutants results from a similar difference in the expression of maternal and paternal FIE alleles during this interval. In support of this hypothesis, a transgene consisting of 1.6 kb of 5′ flanking sequences ligated to a full-length FIE cDNA fused to GFP showed a pattern of early GFP expression similar to that of the FIE::GFP transgenes and rescued a mutant fie seed only when inherited maternally (T. Kinoshita and R.L. Fischer, unpublished results). Therefore, we propose that maternal FIE allele gene expression—transcription, translation, protein modification (Ng et al., 2000)—during this interval results in the generation of maternal FIE protein, which is necessary for seed development. As a result, mutations in fie cause parent-of-origin effects on seed development. As described below, three models can be considered.
One model suggests that essential expression of the FIE maternal allele occurs in the central cell of the female gametophyte before the introduction of the paternal FIE allele by fertilization. Indeed, the early pattern of GFP fluorescence (Figures 8A to 8D and 8Q) might reflect the translation of maternal FIE::GFP mRNA transcribed before fertilization. If so, FIE would be similar to PROLIFERA (PRL), an Mcm7 protein essential for DNA replication; accumulation of PRL in the female gametophyte is thought to be responsible for parent-of-origin effects of prl mutations (Springer et al., 2000).
An alternative model suggests that a gene dosage-dependent mechanism regulates the expression of FIE (and possibly other regulatory genes). That is, a single copy of the paternal wild-type FIE gene might be incapable of compensating for the lack of gene activity associated with two mutant fie maternal alleles within the triploid endosperm of a fie/FIE heterozygous plant. However, fie/FIE maternal plants, when fertilized with pollen having a wild-type FIE allele plus extra FIE transgenes capable of complementing the fie mutation when maternally inherited (Ohad, et al., 1999), did not display any decrease in the fie-mediated seed abortion phenotype (M. Hannon and R.L. Fischer, unpublished data). Moreover, in reciprocal crosses between wild-type and FIE::GFP transgenic plants (Figure 8), pollen donors with single or multiple copies of the transgene exhibited the same pattern of early paternal silencing during early endosperm development, followed by reactivation of FIE::GFP activity before endosperm cellularization (R. Yadegari and R.L. Fischer, unpublished data). We think it unlikely, therefore, that a FIE gene dosage model would account for the parent-of-origin effect observed for fie during seed development.
A final model to account for the fie mutation's parent-of-origin effects on seed development suggests that essential expression of the FIE maternal allele takes place in the endosperm soon after fertilization, although not later than the 16–nuclear cytoplasmic domain stage (Figure 7). Vielle-Calzada et al. (2000) proposed that during this interval, paternal allele expression of many Arabidopsis genes may be delayed. However, the scarcity of “embryo-lethal” mutations that cause parent-of-origin effects on seed viability (Jurgens et al., 1991) implies that late expression of the paternal wild-type allele is sufficient for almost all genes required for seed viability. Therefore, if this model of fie-mediated parent-of-origin effects is correct, then the essential early requirement for FIE expression must be very stringent to explain why later expression of the paternal FIE allele does not result in viable seed. Experiments designed to elucidate more precisely when FIE is transcribed and translated and when active FIE protein accumulates will make it possible to distinguish between these models of fie-mediated parent-of-origin effects on seed development.
In summary, although the mechanisms responsible for parent-of-origin effects caused by fie and mea mutations are probably different, we propose that FIE and MEA interact directly in wild-type plants to control female gametophyte, endosperm, and embryo development.
METHODS
Growth Conditions
Arabidopsis thaliana plants were grown in greenhouses under a 16-hr light/8-hr dark photoperiod generated by supplemental lighting.
Yeast Two-Hybrid Clones and Assays
Full-length FERTILIZATION-INDEPENDENT ENDOSPERM (FIE), MEDEA (MEA), and FERTILIZATION-INDEPENDENT SEED2 (FIS2) cDNA clones were subcloned into the pBI880 vector (pGAL4BD) containing the GAL4 DNA binding domain and the pBI771 vector (pGAL4AD) containing the GAL4 activation domain (Kohalmi et al., 1997). Interactions between FIE, MEA, and FIS2 proteins were tested by introducing the appropriate pairs of constructs into Y190 yeast cells. Transformants were plated on synthetic complete medium that lacked leucine, tryptophan, and histidine amino acids and was supplemented with 3-amino-1,2,4-triazole as noted. The surviving yeast colonies were then transferred to filters containing 1 mg/mL 5-bromo- 4-chloro-3-indolyl-β-d-galactopyranoside, and the amount of β-galactosidase activity was determined by scoring the intensity of blue pigment produced. Deletions of MEA were constructed by using restriction endonuclease sites and were ligated in frame in pBI771. β-Galactosidase assays were performed as described previously (Kohalmi et al., 1997).
In Vitro Binding of FIE and MEA
A cDNA encoding a full-length FIE protein (Ohad et al., 1999) was ligated into the pGEX-4T-1 vector (Amersham Pharmacia Biotechnology, Piscataway, NJ), giving rise to glutathione S-transferase (GST)–FIE, which was introduced into Escherichia coli BL21 cells. GST-FIE fusion protein expression was induced by adding 1 mM isopropyl-β-d-thiogalactopyranoside (Bio Lab Ltd., Falmouth, MA) at 30°C for 12 to 18 hr. Similarly, control GST protein was produced from Escherichia BL21 cells containing the pGEX-4T-1 vector. Cells were harvested and frozen at −20°C. Cells were resuspended in one-tenth volume of PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3) containing protease inhibitor mix (Complete; Roche Molecular Biochemicals, Indianapolis, IN) and were lysed by using a French press. After centrifugation at 11,000g, the soluble fraction was incubated with glutathione agarose beads (Sigma, St. Louis, MO) for 30 min at 4°C while being rotated. Agarose beads were washed two times with PBS plus 1% (w/v) Triton X-100 and three times in PBS. GST-FIE protein was shown to migrate as a single band after PAGE and staining with Gel-Code (Pierce, Rockford, IL).
Full-length MEA cDNA sequences (Kiyosue et al., 1999) were amplified by polymerase chain reaction (PCR) and ligated into pGEM-T Easy vector (Promega, Madison, WI) at the BamHI-XhoI site in the polylinker. The pGEM-MEA plasmid was digested with SalI and used as a template for in vitro transcription and translation in a wheat germ extract system (Promega) by procedures provided by the manufacturer. Redivue 35S-methionine (Amersham Pharmacia Biotechnology) was used to label the MEA protein.
Purified GST-FIE (1.25, 2.5, or 5.0 μg) or control GST (5.0 μg) proteins were immobilized on glutathione agarose beads, washed with binding buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40), resuspended in 250 μL of binding buffer, and incubated for 2 hr at 4°C with equal amounts of 35S-methionine–labeled MEA protein. Beads were washed four times with the binding buffer and resuspended in SDS–polyacrylamide gel sample buffer (60 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 0.002% bromphenol blue, and 100 mM DTE). Samples were boiled for 2 min at 98°C, separated by electrophoresis on a 10% SDS–polyacrylamide gel, and transferred to a polyvinylidene difluoride (PVDF) Immobilion-P membrane (Millipore, Bedford, MA). Labeled MEA protein was detected with a PhosphorImager (model BAS-1000; Fuji, Tokyo, Japan) using trans-screen-LE intensifying screens (Kodak, Rochester, NY).
For protein blot analysis, PVDF membranes were blocked for 1 hr in TBS (25 mM Tris-HCl, pH 7.5, 136 mM NaCl, and 2.68 mM KCl) containing 5% powdered milk and incubated with anti-MEA antibody for 1 hr at room temperature. Membranes were washed three times for 10 min in TBS. A second anti–goat antibody conjugate (Sigma) was added to the membrane after the third wash and incubated for 30 min at room temperature. Blots were then washed three times with TBS. Antibodies were detected by alkaline phosphatase reaction.
To generate MEA antibodies, a 1.3-kb MEA cDNA clone encoding amino acids 1 through 436 was inserted into the pET28a vector (Novagen, Madison, WI). The resulting histidine tag (HIS)–MEA fusion protein was purified by SDS-PAGE. A single protein band was eluted from the gel and used as an immunogen in rabbits. MEA antibodies were affinity-purified by reacting sera with GST-MEA fusion protein bound to PVDF membranes. Affinity-purified MEA antibodies were tested for their ability to bind specifically to HIS-MEA and GST-MEA proteins produced in Escherichia.
Generation of FIE::GFP and FIE::GUS Transgenic Plants
The GREEN FLUORESCENT PROTEIN (GFP) gene used in the construction of reporter transgenes is the synthetic variant S65T (sGFP[S65T]) and lacks any subcellular localization sequences (Heim et al., 1995; Chiu et al., 1996; Niwa et al., 1999). The complete coding sequence of sGFP(S65T) and a polyadenylation signal from the nopaline synthase gene (nos3′) were excised together from a donor plasmid (CaMV35S-sGFP[S65T]-nos3′) by use of BamHI and EcoRI and were cloned into the corresponding sites of the Agrobacterium tumefaciens binary vector pBI101.1 (Jefferson et al., 1987), replacing the β-glucuronidase–nos3′ reporter cassette (pBI-GFP[S65T]; M. Pastuglia and R.L. Fischer, unpublished data). The FIE::GFP transgenes used in this study were created by PCR amplification of two sets of FIE upstream sequences, restriction modification of the ends, and direct cloning into pBI-GFP(S65T). A genomic clone (p12Cla) was used as a template to amplify ∼1.3 kb of FIE 5′ flanking sequences in a PCR reaction with a genome-based primer, RY013 (5′-CCCGGGATCCCTTCGACATTCGATATTCG-3′), and a plasmid-based primer, T3 (5′-AATTAACCCTCACTAAAGGG-3′). The insert of p12Cla is identical to the genomic cosmid pCLD36, which was previously shown to complement the fie mutation (Ohad et al., 1999). Another genomic clone (Cer2) was used as a template to amplify ∼4.9 kb of FIE 5′ flanking sequences in a PCR reaction with two genome-based primers, RY013 and RY014 (5′-ACTAGTCGACGGTGGCTT-GTGAAGATGGTTAG-3′). The 1.3- and 4.9-kb amplification products were restriction-digested with BamHI and with BamHI plus SalI, respectively, and then cloned into the corresponding sites upstream of the sGFP(S65T) cassette in pBI-GFP(S65T). Therefore, the resulting binary constructs contained variable 5′ sites positioned at −1273 bp (1.3kbFIE::GFP) and −4942 bp (4.9kbFIE::GFP) relative to the putative start codon, and both contained a fixed 3′ site at position +9 bp fused in frame to sGFP(S65T). The FIE::β-GLUCURONIDASE (GUS) transgenes were created similarly by PCR amplification of 1.3 kb of FIE 5′ flanking sequences in a PCR reaction using p12Cla as a template and primers RY013-G (5′-CCCGGATCCTTCGACATTCGATATTCG-3′) and T3, followed by restriction modification of the ends and direct cloning into pBI101.2 (Jefferson et al., 1987). Agrobacterium GV3101 pMP90 strains (Koncz and Schell, 1986) containing the binary vectors were then used to transform (Century et al., 1997) three Arabidopsis ecotypes, Landsberg erecta (Ler), Columbia-0 (Col-0), and Columbia-PRL, gl1 (Col-PRL, gl1), to generate 10 to 50 independent kanamycin-resistant T1 plants for each FIE::GFP and FIE::GUS construct.
Analysis of FIE::GFP Expression during Development and in Reciprocal Crosses
Approximately 22 and 10 independently transformed T1 lines each for the 1.3kbFIE::GFP and 4.9kbFIE::GFP constructs, respectively, and ∼30 independently transformed T1 lines for the 1.3kbFIE::GUS construct were used to determine the overall pattern of FIE::GFP and FIE::GUS expression during development. Four T1 lines each for the 1.3kbFIE::GFP and 4.9kbFIE::GFP constructs were used to determine the overall pattern of FIE::GFP expression after reciprocal crosses. Subsequently, more detailed analysis of FIE::GFP gene expression was performed with T2 sibling plants that were heterozygous or homozygous for a single FIE::GFP locus. For analysis of FIE::GFP and FIE::GUS expression in mature female gametophytes and to prepare pistils for reciprocal crosses, flowers were emasculated during stages 12A to 12C to prevent self-fertilization (Smyth et al., 1990; Christensen et al., 1997). Seed development was correlated to morphological changes occurring during endosperm and embryo development (Mansfield and Briarty, 1990a, 1990b, 1990c; Mansfield et al., 1990; Jurgens and Mayer, 1994). Intact unfertilized ovules and developing seeds from FIE::GFP transformants were excised, mounted in water, and observed immediately with a Zeiss Axioskop 2 fluorescence microscope (Carl Zeiss, Thornwood, NY) or an Olympus SZX12 fluorescence stereomicroscope (Olympus America, Melville, NY). The former was equipped with a fluorescein isothiocyanate (FITC) filter set (exciter 470/40, dichroic 505, emitter OG515LP; Chroma Technology, Inc., Brattleboro, VT) and a GFP filter set (wtGFP Longpass Emission: exciter HQ450/50x, dichroic Q480LP, emitter HQ48LP; Chroma Technology), and the latter was equipped with two analogous filter sets (GFP Longpass: exciter 460 to 490, dichroic 505, emitter 510; GFP Bandpass: exciter 460 to 490, dichroic 505, emitter 510 to 550; Olympus America). Histochemical localization of GUS activity in FIE::GUS plants was performed on intact pistils and developing siliques, which were excised longitudinally, vacuum-infiltrated for 10 to 15 min on ice with staining solution (50 mM sodium phosphate buffer, pH 7.0, 0.2% Triton X-100, 10 mM potassium ferrocyanide, 10 mM potassium ferricyanide, and 1 mM X-gluc), and incubated for 12 to 16 hr at 37°C (Sessions et al., 1999). Individual ovules and developing seeds were then dissected out, mounted in 50 mM sodium phosphate buffer, pH 7.0, and observed with a Zeiss Axioskop microscope equipped with differential contrast interference microscopy optics. Images were captured on 35-mm slide film (Kodak Elite Chrome, ISO 100) or with an Optronics Color 3 charge-coupled device camera (Optronics, Goleta, CA) and Scion 1.62c image acquisition software (Scion, Frederick, MD). Image processing and reproduction were performed with Photoshop 5.0.2 (Adobe Systems, San Jose, CA).
Distinguishing Ler and RLD FIE mRNAs in Dissected Seed
The two Arabidopsis ecotypes used in these experiments were Ler and RLD (Hardtke et al., 1996). The DNA sequences for the Ler and RLD FIE genes were 99.8% identical (data not shown) over a 442-bp region, and parent-of-origin effects on seed development of the fie-1 mutation were observed in both the Ler (Ohad et al., 1996) and RLD (data not shown) genetic backgrounds. Plants were selected for reciprocal crosses 1 week after bolting. Flowers were pollinated 2 days after removal of anthers. F1 seed were harvested from siliques at 4, 6, 7, or 8 days after pollination. Seed were dissected into embryo and endosperm-plus-seed-coat components under a stereomicroscope. To confirm seed stage, seed were cleared and visualized with a Zeiss Axioskop microscope with Nomarski optics (Ohad et al., 1999). RNA was isolated as described previously (Kinoshita et al., 1999). cDNA synthesized with the primer FIE-RTr (5′-CTGTAATCAGGCAAACAG-CC-3′) and amplified by PCR with primers FIE-RTr and FIE-RTf (5′-CTGTAATCAGGCAAACAGCC-3′) were as described by Kinoshita et al. (1999). To determine the ratio of RLD and Ler FIE sequences, purified PCR products were digested with the MseI restriction endonuclease and subjected to electrophoresis on a 3% Metaphor agarose gel (FMC BioProducts, Rockland, ME).
Measuring the Effect of ddm1-2 on Transmission of a Maternal fie-1 or mea-3 Mutant Allele
Heterozygous mea-3 (Kiyosue et al., 1999) (backcrossed six times to the Col-0 ecotype) and fie-1 (Ohad et al., 1999) (Ler ecotype) were pollinated with pollen from homozygous decrease in DNA methylation (ddm)1-2 (Col-0 ecotype) plants (Vongs et al., 1993). Progeny from these crosses were grown, DNA was isolated (Murray and Thompson, 1980), and genotypes were determined by using derived cleaved amplified polymorphic sequence primers (Neff et al., 1998). For ddm1-2, DDM1f (5′-CAGATCTCTACCCTCCTGT-3′) and ddm1-2dRsa (5′-TGAGCTACGAGCCATGGGTTTGTGAAACGTA-3′) primers were used to amplify DNA by PCR. Amplified fragments were digested with RsaI restriction endonuclease and subjected to agarose gel electrophoresis (3% Metaphor agarose). For mea-3, the f644dXba (5′-CATGCAACGACGGCAATGACGTCTATCAGCAAAATTCT-3′) and UCB3SR85 (5′-CGAAGTGGATGTTTCGGAC-3′) primers were used; for fie-1, the 579dXba (5′-CATTACTGCCATTGGTGTATCTCTTATTATCTA-3′) and 48S4 (5′-CACTGTTGACGTCAATGACTCGG-3′) primers were used. The amplified DNAs were digested with XbaI restriction enzyme and analyzed by agarose gel electrophoresis. PCR conditions were as described by Kinoshita et al. (1999).
Acknowledgments
We thank Eric J. Richards for providing us with a ddm1-2 mutant line and Yasuo Niwa for a 35S-sGFP(S65T) construct. We thank Gary Drews for a GFP-enhancer trap line and for critically reading the manuscript. We express our appreciation to Barbara Rotz for supplying excellent greenhouse services. This work was funded by a grant to R.L.F. from the National Research Institute Competitive Grants Program of the United States Department of Agriculture (Grant No. 97-35304-4941), by Grant No. 677/97 to N.O. from the Israel Science Foundation, and by grants to R.L.F. and N.O. from the U.S.–Israel Binational Agricultural Research and Development Fund (Grant No. IS-3158-99C) and the U.S.–Israel Binational Science Foundation (Grant No. 96-00242). T.K. is a recipient of a fellowship from the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad program. R.Y. is a Department of Energy-Biosciences Research Fellow of the Life Sciences Research Foundation.
References
- Berger, F. (1999). Endosperm development. Curr. Opin. Plant Biol. 2 28–32. [DOI] [PubMed] [Google Scholar]
- Brown, R.C., Lemmon, B.E., Nguyen, H., and Olsen, O.-A. (1999). Development of endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12 32–42. [Google Scholar]
- Century, K.S., Shapiro, A.D., Repetti, P.P., Dahlbeck, D., Holub, E., and Staskawicz, J.B. (1997). NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278 1963–1965. [DOI] [PubMed] [Google Scholar]
- Chaudhury, A.M., Ming, L., Miller, C., Craig, S., Dennis, E.S., and Peacock, W.J. (1997). Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94 4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevray, P.M., and Nathans, D. (1992). Protein interaction cloning in yeast: Identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. USA 89 5789–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, W.-L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6 325–330. [DOI] [PubMed] [Google Scholar]
- Christensen, C.A., King, E.J., Jordan, J.R., and Drews, G.N. (1997). Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex. Plant Reprod. 10 49–64. [Google Scholar]
- Denisenko, O., Shnyreva, M., Suzuki, H., and Bomsztyk, K. (1998). Point mutations in the WD40 domain of Eed block its interactions with Ezh2. Mol. Cell. Biol. 18 5634–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, S., and Sternglanz, R. (1994). The two-hybrid system: An assay for protein–protein interactions. Trends Genet. 10 286–292. [DOI] [PubMed] [Google Scholar]
- Goldberg, R.B., de Paiva, G., and Yadegari, R. (1994). Plant embryogenesis: Zygote to seed. Science 266 605–614. [DOI] [PubMed] [Google Scholar]
- Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M., and Coupland, G. (1997). A polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386 44–51. [DOI] [PubMed] [Google Scholar]
- Grossniklaus, U., Vielle-Calzada, J.-P., Hoeppner, M.A., and Gagliano, W.B. (1998). Maternal control of embryogenesis by MEDEA, a polycomb-group gene in Arabidopsis. Science 280 446–450. [DOI] [PubMed] [Google Scholar]
- Gutjahr, T., Frei, E., Spicer, C., Baumgartner, S., White, R.A.H., and Noll, M. (1995). The Polycomb-group gene, extra sex combs, encodes a nuclear member of the WD-40 repeat family. EMBO J. 14 4296–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke, C., Mullter, J., and Berleth, T. (1996). Genetic similarity among Arabidopsis thaliana ecotypes estimated by DNA sequence comparison. Plant Mol. Biol. 32 915–922. [DOI] [PubMed] [Google Scholar]
- Heim, R., Cubitt, A.B., and Tsien, R.Y. (1995). Improved green fluorescence. Nature 373 663–664. [DOI] [PubMed] [Google Scholar]
- Holdemann, R., Nehrt, R., and Strome, S.S. (1998). MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila polycomb group protein. Development 125 2457–2467. [DOI] [PubMed] [Google Scholar]
- Jeddeloh, J.A., Stokes, T.L., and Richards, E.J. (1999). Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 22 94–97. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.V. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein, T., Laible, G., Dorn, R., and Reuter, G. (1998). SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol. Life Sci. 54 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C.A., Ng, J., Peterson, A.J., Morgan, K., Simon, J., and Jones, R.S. (1998). The Drosophila Esc and E(z) proteins are direct partners in polycomb group–mediated repression. Mol. Cell. Biol. 18 2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens, G. (1994). Pattern formation in the embryo. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 297–312.
- Jurgens, G., and Mayer, U. (1994). Arabidopsis. In Embryos: Color Atlas of Development, J.B.L. Bard, ed (London: Wolfe), pp. 7–21.
- Jurgens, G., Mayer, U., Torres Ruiz, R., Berleth, T., and Misera, S. (1991). Genetic analysis of pattern formation in the Arabidopsis embryo. In Molecular and Cellular Basis of Pattern Formation, K. Roberts, E.S. Coen, C. Dean, J. Jones, K. Chater, R. Flavell, A. Wilkins, and N. Holder, eds. (Cambridge, UK: The Company of Biologists), pp. 27–38.
- Kehle, J., Beuchle, D., Treuheit, S., Christen, B., Kennison, J.A., Bienz, M., and Muller, J. (1998). dMi-2, a Hunchback-interacting protein that functions in polycomb repression. Science 282 1897–1900. [DOI] [PubMed] [Google Scholar]
- Kelly, W.G., and Fire, A. (1998). Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development 125 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., Harter, K., and Theologis, A. (1997). Protein–protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. USA 94 11786–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T., Yadegari, R., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (1999). Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell 11 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore, G.M., and Shah, D.M. (1988). Amino acid biosynthesis inhibitors as herbicides. Annu. Rev. Biochem. 57 627–663. [DOI] [PubMed] [Google Scholar]
- Kiyosue, T., Ohad, N., Yadegari, R., Hannon, M., Dinneny, J., Wells, D., Katz, A., Margossian, L., Harada, J., Goldberg, R.B., and Fischer, R.L. (1999). Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc. Natl. Acad. Sci. USA 96 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohalmi, S.E., Nowak, J., and Crosby, W.L. (1997). A practical guide to using the yeast 2-hybrid system. In Differentially Expressed Genes in Plants, E. Hansen and G. Harper, eds (London: Taylor and Francis), pp. 63–82.
- Komachi, K., Redd, M.J., and Johnson, A.D. (1994). The WD repeats of Tup1 interact with the homeodomain protein a2. Genes Dev. 8 2857–2867. [DOI] [PubMed] [Google Scholar]
- Koncz, C., and Schell, J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204 383–396. [Google Scholar]
- Korf, I., Fan, Y., and Strome, S. (1998). The polycomb group in Caenorhabditis elegans and maternal control of germline development. Development 125 2469–2478. [DOI] [PubMed] [Google Scholar]
- Lindsey, K., and Topping, J.F. (1993). Embryogenesis: A question of pattern. J. Exp. Bot. 44 359–374. [Google Scholar]
- Lopes, M.A., and Larkins, B.A. (1993). Endosperm origin, development, and function. Plant Cell 5 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, M., Bilodeau, P., Koltunow, A., Dennis, E.S., Peacock, W.J., and Chaudhury, A.M. (1999). Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1990. a). Development of the free-nuclear endosperm in Arabidopsis thaliana. Arabidopsis Inf. Serv. 27 53–64. [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1990. b). Early embryogenesis in Arabidopsis thaliana: The developing embryo. Can. J. Bot. 69 461–475. [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1990. c). Endosperm cellularization in Arabidopsis thaliana L. Arabidopsis Inf. Serv. 27 65–72. [Google Scholar]
- Mansfield, S.G., Briarty, L.G., and Erni, S. (1990). Early embryogenesis in Arabidopsis thaliana: The mature embryo sac. Can. J. Bot. 69 447–460. [Google Scholar]
- Meinke, D.W. (1994). Seed development in Arabidopsis thaliana. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 253–295.
- Murray, M.G., and Thompson, W.F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M.M., Neff, J.D., Chory, J., and Pepper, A.E. (1998). dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 14 387–392. [DOI] [PubMed] [Google Scholar]
- Ng, J., Li, R., Morgan, K., and Simon, J. (1997). Evolutionary conservation and predicted structure of the Drosophila extra sex combs repressor protein. Mol. Cell. Biol. 17 6663–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, J., Hart, C.M., Morgan, K., and Simon, J.A. (2000). A Drosophila ESC–E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC. Mol. Cell. Biol. 20 3069–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M., and Kobayashi, H. (1999). Non-invasive quantitative detection and applications of non-toxic S65T-type green fluorescent protein in living plants. Plant J. 18 455–463. [DOI] [PubMed] [Google Scholar]
- Ohad, N., Margossian, L., Hsu, Y.-C., Williams, C., Repetti, P., and Fischer, R.L. (1996). A mutation that allows endosperm development without fertilization. Proc. Natl. Acad. Sci. USA 93 5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad, N., Yadegari, R., Margossian, L., Hannon, M., Michaeli, D., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (1999). Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 11 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta, V. (1998). Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93 333–336. [DOI] [PubMed] [Google Scholar]
- Rozovskaia, T., Rozenblatt-Rosen, O., Sedkov, Y., Burakov, D., Yano, T., Nakamura, T., Petruck, S., Ben-Simchon, L., Croce, C.M., and Mazo, A. (2000). Self-association of the SET domains of human ALL-1 and of Drosophila TRITHORAX and ASH1 proteins. Oncogene 19 351–357. [DOI] [PubMed] [Google Scholar]
- Sathe, S.S., and Harte, P.J. (1995). The Drosophila extra sex combs protein contains WD motifs essential for its function as a repressor of homeotic genes. Mech. Dev. 52 77–87. [DOI] [PubMed] [Google Scholar]
- Schumacher, A., Faust, C., and Magnuson, T. (1996). Positional cloning of a global regulator of anterior–posterior patterning in mice. Nature 383 250–253. [DOI] [PubMed] [Google Scholar]
- Sessions, A., Weigel, D., and Yanofsky, M.F. (1999). The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J. 20 259–263. [DOI] [PubMed] [Google Scholar]
- Sewalt, R.G., van der Vlag, J., Gunster, M.J., Hamer, K.M., der Blaauwen, J.L., Satijn, D.P., Hendrix, T., van Driel, R., and Otte, A.P. (1998). Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes. Mol. Cell. Biol. 18 3586–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondek, J., Boh, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. (1996). Crystal structure of a GA protein dimer at 2.1 Å resolution. Nature 379 369–374. [DOI] [PubMed] [Google Scholar]
- Springer, P.S., Holding, D.R., Groover, A., Yordan, C., and Martienssen, R.A. (2000). The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during G1 phase and is required maternally for early Arabidopsis development. Development 127 1815–1822. [DOI] [PubMed] [Google Scholar]
- Struhl, G., and Brower, D. (1992). Early role of the esc+ gene product in the determination of segments in Drosophila. Cell 31 285–292. [DOI] [PubMed] [Google Scholar]
- Tie, G., Furuyama, T., and Harte, P.J. (1998). The Drosophila polycomb group proteins ESC and E(Z) bind directly to each other and co-localize multiple chromosomal sites. Development 125 3483–3496. [DOI] [PubMed] [Google Scholar]
- van der Vlag, J., and Otte, A.P. (1999). Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat. Genet. 23 474–478. [DOI] [PubMed] [Google Scholar]
- van Lohuizen, M., Tijms, M., Voncken, J.J., Schumacher, A., Magnuson, T., and Wientjens, E. (1998). Interaction of mouse polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: Indication for separate Pc-G complexes. Mol. Cell. Biol. 18 3572–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Went, J.L., and Willemse, M.T.M. (1984). Fertilization. In Embryology of Angiosperms, B. Johri, ed (Berlin: Springer-Verlag), pp. 273–318.
- Vielle-Calzada, J.-P., Thomas, J., Spillane, C., Coluccio, A., Hoeppner, M.A., and Grossniklaus, U. (1999). Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev. 13 2971–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielle-Calzada, J.-P., Baskar, R., and Grossniklaus, U. (2000). Delayed activation of the paternal genome during seed development. Nature 404 91–94. [DOI] [PubMed] [Google Scholar]
- Vongs, A., Kakutani, T., Martienssen, R.A., and Richards, E.J. (1993). Arabidopsis thaliana DNA methylation mutants. Science 260 1926–1928. [DOI] [PubMed] [Google Scholar]
- Wall, M.A., Coleman, D.E., Lee, E., Iniguez-Lluhi, J.J., Posner, B.A., Gilman, A.G., and Sprang, S.R. (1995). The structure of the G protein heterotrimer Gia1β1γ2. Cell 83 1047–1058. [DOI] [PubMed] [Google Scholar]
- Webb, M.C., and Gunning, B.E.S. (1991). The microtubular cytoskeleton during development of the zygote, proembryo and free-nuclear endosperm in Arabidopsis thaliana. Planta 184 187–195. [DOI] [PubMed] [Google Scholar]
- Wu, K., Tian, L., Malik, K., Brown, D., and Miki, B. (2000). Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. Plant J. 22 19–27. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., LeRoy, G., Seelig, H.-P., Lane, W.S., and Reinberg, D. (1998). The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95 279–289. [DOI] [PubMed] [Google Scholar]