Abstract
Heart failure (HF) is associated with a reduction of skeletal muscle mass. Whey protein isolate (WPI) has been beneficial in increasing muscle mass and strength, in addition to improving body composition. The goal of this research was to evaluate the effect of WPI on the body composition, muscle mass, and strength of chronic HF patients. For this purpose, twenty-five patients of both genders with predominantly NYHA I functional class and a median age of 65.5 (60.5–71.0) years were used to conduct a randomized, single-blind, placebo-controlled clinical trial and received 30 g per day of WPI for 12 weeks. Anthropometric measurements, body composition analysis, and biochemical exams were performed at the beginning and end of the study. An increase in skeletal muscle mass was observed in the intervention group after 12 weeks. A reduction in waist circumference, body fat percentage, and an increase in skeletal muscle index was observed when compared to the placebo group. No significant effect on muscle strength was observed after 12 weeks of intervention. These data demonstrate that WPI consumption contributed to the increase of skeletal muscle mass, strength, and reduction of body fat in HF patients.
Keywords: heart failure, diet, whey proteins, muscle strength, randomized clinical trial
1. Introduction
Heart failure (HF) is one of the main causes of morbidity and mortality worldwide [1]. The impairment of cardiac contractile function leads to several neurohormonal and metabolic disorders, including an imbalance between anabolic and catabolic processes [2], and, in many cases, leads to a reduction of skeletal muscle mass. HF has a significant impact on patient muscle function and body composition, which has been clearly associated with considerable morbidity and institutionalization [3].
Nutritional support in patients with HF should be considered, mainly as a way to prevent progressive weight loss, since restoration of muscle mass may not be achievable [4]. Muscle loss is a strong predictor of frailty and reduced survival in patients with HF, and this process can be mitigated through early nutritional support [5].
Loss of muscle mass affects a large proportion of patients with HF [6], contributing to exercise intolerance and impairment of daily life activities, reduced quality of life, and increased mortality [7,8]. Protein-rich meals stimulate muscle protein synthesis [9], and dietary protein supplementation constitutes a possible intervention for this condition for which no medications are available.
Nutritional recommendations for an older adult (>65 years) population propose an increase in daily protein intake (1–1.2 g/kg/day; 1.2–1.5 g/kg/day in case of inflammatory disease), preferably of high-quality protein (i.e., whey protein), containing large amounts of essential amino acids (EAAs) such as leucine. With advancing age, there is resistance to muscle anabolism, so a daily intake of approximately 25–30 g of high-quality protein is recommended in order to maximize muscle protein synthesis (MPS) [10].
Whey protein isolate (WPI) is a high-quality protein that has been shown to stimulate muscle protein synthesis compared to other protein sources, is highly digestible, and has a high concentration of leucine, which plays an important role in the stimulation of postprandial MPS [11]. Moreover, some studies show that WPI is more effective in stimulating MPS compared to other sources of protein [12,13].
The use of WPI, associated with exercise (especially resistance training), has already been shown to lead to increased muscle protein synthesis and skeletal muscle mass [14], as well as enhanced exercise recovery [15]. Recently, Haß et al. [16], in a pilot study, demonstrated that vibration and resistance exercise plus a high-protein diet based on WPI supplementation (with or without omega-3 fatty acids) increased muscle power in older adults, leading to improved leg strength and chair raise time.
However, many HF patients are unable to exercise for several reasons, ranging from physical restrictions to limitations in the access to cardiac rehabilitation programs, and the effect of the isolated supplementation of WPI in HF patients without concomitant exercise training, although desirable, is still unclear. Therefore, this study aimed to evaluate whether supplementation with WPI, compared to placebo, promotes changes in body composition, especially muscle mass, as well as skeletal muscle strength, in patients with chronic HF.
2. Materials and Methods
2.1. Patients
This study was approved by the Research Ethics Committee of the National Institute of Cardiology under protocol number 03218512.0.2005.5272. Written informed consent was obtained from all subjects/patients. This was a single-blind, randomized, placebo-controlled clinical trial. Patients with a clinical diagnosis of HF, who had been referred for cardiac rehabilitation by their attending physicians and were awaiting scheduling, were assessed. Inclusion criteria were age ≥50 years, HF class I or II NYHA, clinical stability (symptoms and medications) for at least 4 weeks before inclusion, and left ventricular ejection fraction (LVEF) ≤ 50% (echocardiographically defined). Exclusion criteria were creatinine clearance <50 mL/min/1.73 m2, impaired hepatic function (alanine aminotransferase > 150 U/L), hepatic cirrhosis, or allergy to milk proteins.
2.2. Supplementation
Participants were randomly allocated to receive supplementation with WPI (30 g/day [27 g protein; 120 kcal/serving]) or placebo, which consisted of maltodextrin (30 g/day [30 g carbohydrates; 120 kcal/ serving]). Patients were instructed to consume the supplements once a day for 12 weeks. The packaging and distribution of the supplements were performed by personnel not involved in the research, and researchers directly involved in patient care and follow-up were unaware of the supplements taken.
2.3. Biochemical and Anthropometric Measurements
All patients had fasting blood biochemical evaluation, handgrip-strength assessment, and anthropometric and body composition evaluations. All initial evaluations were repeated after 12 weeks of supplementation.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were evaluated using a sphygmomanometer. The SBP and DBP were measured twice by a trained professional, with a 1 min interval between the two measurements, and the average value was used as the patient’s blood pressure.
Blood sampling was performed after a 12 h fasting period. Serum urea, creatinine, glycemia, triglycerides, total cholesterol, and high-density lipoprotein cholesterol were measured by using a biochemistry analyzer (ARCHITECT ci8200, Abbott ARCHITECT®, Abbott Park, IL, EUA. The LDL-cholesterol (LDL-c) was calculated using the formula by Friedewald et al. [17]. The estimated glomerular filtration rate (eGFR) was calculated by using the equation from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [18].
The anthropometric evaluation included body weight and height, waist, and hip measurements, as well as body mass index (BMI) calculation. All measurements were made in duplicate and the average values were used. Body composition was evaluated using a bioimpedance system (Inbody 720®, Bioespace Co. Ltd., Seoul, Republic of Korea), which registered total weight (kg), total skeletal muscle mass (SMM) (kg), total fat mass (kg), and body fat percentage.
Muscle strength was assessed by measuring handgrip strength with a Jamar® hand dynamometer. Participants were instructed to use the non-dominant hand first, followed by the dominant hand. Three measurements were taken for each hand, with a 10–20 s rest interval between measures to avoid fatigue. The average value of the three measures was used.
Sarcopenia was evaluated by calculating the skeletal muscle index (SMI), considered as the ratio of total SMM in kg to the square of the height in meters [19]. The definition of sarcopenia using the SMI was based on the criteria proposed by Cruz-Jentoft et al., which categorizes sarcopenia into three stages according to gender. Moderate to severe sarcopenia was considered present when SMI was <10.75 kg/m2 for men and <6.75 kg/m2 for women [20].
2.4. Food Consumption
The assessment of the patients’ dietary intake was performed by completing a 24-h dietary recall (R24h). The questionnaire was applied at visits T0 and T12 to longitudinally assess food intake and control possible confounding factors. The domestic measures of the foods consumed described in the 24-h dietary recall were converted to weight or milliliters according to the table of equivalents and home measures. Then these data were entered into the Food Processor version 7.2 program (EshaResearch, Salem, MA, USA, 1998) to calculate the energy intake, macronutrients (carbohydrates, protein, total lipids, and fatty acids), total fiber, and micronutrients.
2.5. Statistical Analysis
Randomization occurred in blocks of 2, based on a table of random numbers generated in Openepi®. The main outcome of the study was the gain of muscle mass. Considering a gain of 500 g in the WP group compared to the placebo, with a standard deviation (SD) of 409.7 g, 11 patients were required in each group (5% alpha, 80% power). The results from a similar study with WP supplementation were used as a reference [21].
Data are presented as the number (percentage) or mean (standard deviation, SD) as appropriate. Categorical variables were presented as values and percentages and compared with a chi-square test. Continuous variables were tested for normality using the Shapiro–Wilk test, were presented as mean and SD or median and interquartile interval, and tested with the Student’s t-test or Paired t-test (for parametric variables) and Mann–Whitney and Wilcoxon tests (nonparametric variables). Statistical analyses were conducted with IBM® SPSS® Statistics software version 23. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics and Recruitment
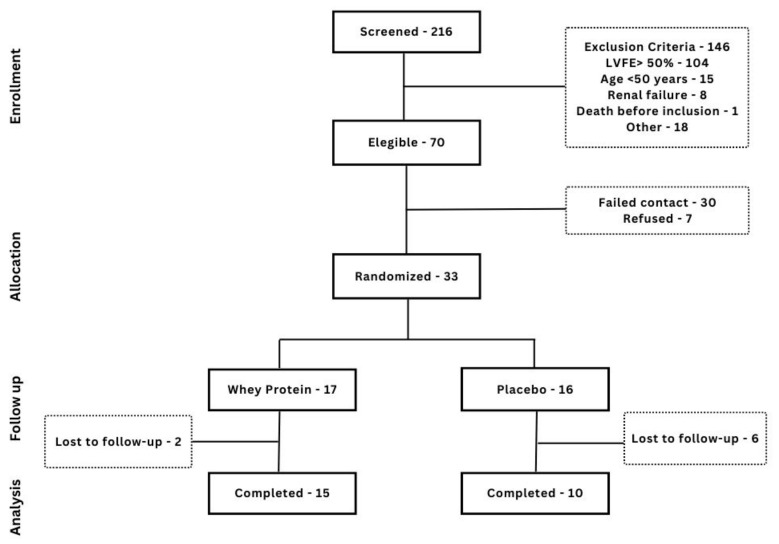
Thirty-three patients were included, 17 randomized for WPI and 16 for placebo. Eight patients were lost to follow-up, and only 15 and 10 patients, respectively, completed the 12 weeks of the study. The flowchart of patient inclusion and follow-up is depicted in Figure 1. A comparison between patients who completed the 12 weeks of supplementation and those who did not was performed (Supplementary Material), and the only differences found were the prevalence of diabetes (44.0% vs. 87.5%, p = 0.032), glycemia (104 (93.5–123.5) vs. 145 (123.0–173.7), p = 0.033), and prior myocardial infarction (100% vs. 75%, p = 0.01). No significant difference regarding LVEF, NYHA functional class, or BMI was found between patients who completed supplementation and those who did not.
Figure 1.
Flowchart of patient inclusion and follow-up.
Patients from both groups (WPI or placebo) were most frequently male, with hypertension, dyslipidemia, overweight/obesity, a history of myocardial infarction, mildly reduced mean LVEF, mostly in class I NYHA, and slightly elevated fasting glycemia and triglycerides (Table 1). A high prevalence of sarcopenia (which was moderate to severe in all cases) was found, especially in the placebo group, although no significant difference was found in the prevalence of sarcopenia between groups.
Table 1.
Baseline characteristics.
| WPI (n = 17) | Placebo (n = 16) | p-Value | |
|---|---|---|---|
| Age (years) | 64 (61–67) | 61 (58–78) | 0.466 |
| Male | 13 (76.5) | 13 (81.3) | 0.737 |
| Hypertension | 13 (76.5) | 10 (62.5) | 0.383 |
| Diabetes | 8 (47.1) | 10 (62.5) | 0.373 |
| Dyslipidemia | 12 (70.6) | 9 (56.3) | 0.392 |
| Overweight/obesity | 15 (88.2) | 11 (68.8) | 0.171 |
| Prior myocardial infarction | 17 (100.0) | 14 (87.5) | 0.133 |
| Percutaneous coronary intervention | 7 (41.2) | 5 (31.3) | 0.554 |
| Coronary artery bypass grafting | 10 (58.8) | 5 (31.3) | 0.112 |
| NYHA I | 15 (88.2) | 12 (75.0) | 0.325 |
| LVEF (%) | 42.0 ± 9.0 | 44.0 ± 7.5 | 0.479 |
| Smoking/former smoking | 13 (76.5) | 14 (87.5) | 0.616 |
| BMI (kg/m2) | 28.6 ± 4.6 | 26.8 ± 3.5 | 0.221 |
| WC (cm) | 101.7 ± 13.2 | 100.4 ± 9.2 | 0.742 |
| Glycemia (mg/dL) | 107 (100.0–138.0) | 123 (98.0–154.8) | 0.605 |
| Triglycerides (mg/dL) | 156.4 ± 58.5 | 176.7 ± 94.2 | 0.458 |
| Total cholesterol (mg/dL) | 159.6 ± 28.3 | 170.4 ± 41.1 | 0.382 |
| HDL-cholesterol (mg/dL) | 43.9 ± 11.3 | 40.6 ± 11.8 | 0.414 |
| LDL-cholesterol (mg/dL) | 102 (71.0–125.5) | 100 (86.5–130.2) | 0.874 |
| Total fat mass (kg) | 28.9 (23.9–34.7) | 27.6 (18.6–30.9) | 0.367 |
| % Body fat | 34.9 ± 8.3 | 32.3 ± 7.8 | 0.361 |
| SMM (kg) | 28.2 ± 5.3 | 28.6 ± 5.1 | 0.789 |
| MMI (kg/m2) | 10.1 ± 1.2 | 9.8 ± 1.1 | 0.487 |
| Handgrip strength (kgf) | |||
| Men | 31.8 ± 8.0 | 31.8 ± 6.9 | 0.979 |
| Women | 13.2 ± 3.1 | 22.3 ± 11.0 | 0.169 |
| Sarcopenia | 7 (41.2%) | 10 (62.5%) | 0.690 |
| Moderate-severe sarcopenia | 7 (41.2%) | 10 (62.5%) | 0.690 |
Values are n (%), mean ± SD, or median (25th–75th percentiles). Chi-square test, Student’s t-test (BMI, % Body fat, Handgrip strength, HDL cholesterol, LVEF, SMM, Total cholesterol, Triglycerides, WC), Mann–Whitney U test (Age, Glycemia, LDL cholesterol, Total fat mass). BMI: body mass index; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; MMI: muscle mass index; NYHA: New York Heart Association; SMM: skeletal muscle mass; WC: waist circumference.
3.2. Dietary Assessment
Table 2 depicts the consumption of macro- and micronutrients between the study groups, according to the 24-h recall without considering supplement intake. The analysis of the dietary data showed an adequate percentage of protein intake, but when we evaluated the protein intake per kilogram of body weight per day, it was found to be under appropriate levels for patients with HF [22]. When the supplementation is accounted for in the value of protein intake in T12, it changes to 1.3 ± 0.51 g/kg/day, which is statistically significant compared to the baseline (p = 0.007). Carbohydrate intake was close to adequate and both groups had inadequate consumption of lipids [23].
Table 2.
Evaluation of the consumption of macro and micronutrients between the study groups.
| Whey Protein (n = 15) | Placebo (n = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | p-Value | Baseline | 12 Weeks | p-Value | |
| Energy intake, kcal/d | 1408.4 (840.2–1605.2) | 1056.6 (989.6–1565.0) | 0.826 | 1295.0 (965.2–1902.7) | 1259.0 (812.2–1673.3) | 0.445 |
| Protein, % of energy | 18.1 (16.0–22.1) | 19.0 (14.8–30.5) | 0.363 | 19.9 (16.8–24.7) | 17.6 (14.3–21.5) | 0.445 |
| Protein, g.kg BW–1. d–1 | 0.8 (0.5–1.0) | 0.8 (0.6–1.3) | 0.363 | 0.9 (0.4–1.1) | 0.7 (0.5–1.0) | 0.203 |
| Carbohydrate, % of energy | 60.6 (49.5–65.7) | 59.9 (51.3–70.2) | 0.397 | 58.2 (53.2–60.6) | 54.3 (49.4–59.7) | 0.508 |
| Fat, % of energy | 20.4 (14.4–28.5) | 18.2 (11.0–22.2) | 0.056 | 23 (16.1–28.4) | 26 (21.4–31.5) | 0.333 |
| SFA, % of energy | 8.3 (4.1–10.8) | 5.0 (4.2–8.3) | 0.056 | 8.1 (4.7–12.6) | 8.5 (6.7–11.1) | 0.799 |
| MUFA, % of energy | 5.0 (3.2–7.0) | 4.3 (3.2–7.2) | 0.683 | 7.7 (3.2–8.6) | 7.5 (5.1–9.3) | 0.445 |
| PUFA, % of energy | 1.7 (1.4–2.8) | 2.0 (1.3–3.3) | 0.638 | 2.3 (1.4–3.7) | 2.5 (2.1–3.8) | 0.721 |
| n-3 PUFA, g | 0.4 (0.2–0.8) | 0.3 (0.2–0.8) | 0.875 | 0.6 (0.2–0.7) | 0.4 (0.3–0.5) | 0.203 |
| n-6 PUFA, g | 2.3 (1.1–4.8) | 2.4 (1.2–3.3) | 0.198 | 2.4 (1.1–7.0) | 3.1 (1.9–4.8) | 0.508 |
| Trans fatty acids, g | 1.1 (0.2–2.0) | 0.4 (0.1–1.6) | 0.133 | 0.4 (0.1–0.5) | 0.8 (0.2–1.8) | 0.074 |
| Cholesterol, mg | 137.6 (71.3–176.0) | 157.5 (66.7–242.3) | 0.331 | 123 (64.4–217.2) | 164.8 (80.7–207.8) | 0.333 |
| Total fiber, g | 12.5 (8.8–22.2) | 14.6 (11.0–24.2) | 0.925 | 16 (7.1–20.9) | 14.6 (8.0–23.6) | 0.878 |
| Vitamin A, μg | 339.7 (204.0–1191.9) | 735.6 (227.0–1867.8) | 0.551 | 696 (301.0–1300.1) | 1577.8 (459.6–2443.3) | 0.131 |
| Vitamin C, mg | 55.4 (9.2–182.4) | 54.5 (21.9–134.4) | 0.826 | 32.2 (15.7–67.6) | 85.1 (8.1–190.7) | 0.333 |
| Vitamin E, mg | 1.1 (0.3–2.3) | 1.3 (0.8–1.9) | 0.778 | 1.3 (0.7–4.1) | 2.4 (1.2–3.5) | 0.646 |
| Sodium, mg | 1495 (810.5–1979.6) | 933 (567.4–1547.4) | 0.041 * | 1249.9 (686.3–2099) | 1159.5 (873.7–1369.1) | 0.594 |
| Zinc, mg | 4.0 (2.2–7.6) | 5.3 (3.4–7.3) | 0.124 | 8.0 (3.8–8.9) | 4.1 (2.5–9.9) | 0.214 |
Values are expressed median (25th–75th) percentiles * p < 0.05. Wilcoxon test. Considered statistically significant values: p < 0.05. MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acid.
No changes were observed in food consumption for macro- and micronutrients when comparing the baseline and final results of the study, except for sodium consumption, which was reduced in the intervention group throughout the study.
3.3. Body Composition
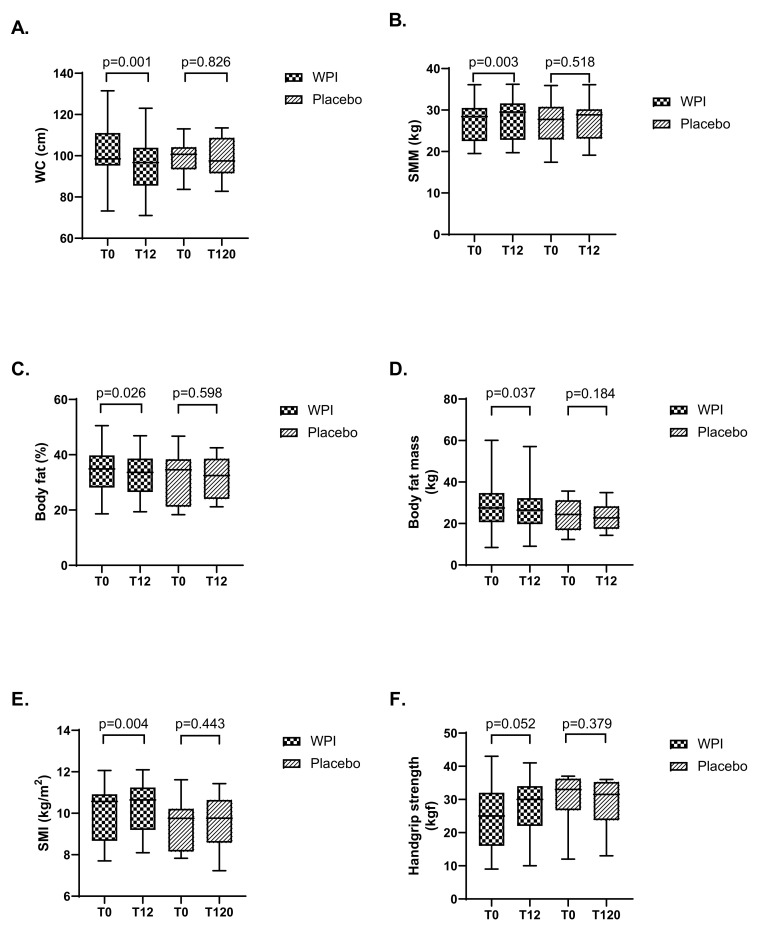
After 12 weeks of WPI supplementation, waist circumference, fat mass, and % fat mass decreased, while skeletal muscle mass and skeletal muscle index increased when compared to placebo (Figure 2). Handgrip strength showed a tendency towards increase (mean 25.7 kgf before and 28.1 kgf after WPI supplementation, p = 0.05). Of note, among patients who received the placebo, no significant change was observed. No changes were observed in body mass index for WPI supplementation and placebo when comparing the baseline and final results of the study.
Figure 2.
Boxplot of anthropometric and muscle strength data before and after supplementation. (A): Waist circumference; (B): Skeletal muscle mass; (C): Percentual body fat; (D): Body fat mass; (E): Skeletal muscle index; (F): Handgrip strength. Boxplot and 95% CI. Paired t-test. SMI, skeletal muscle index; SMM, skeletal muscle mass; WC, waist circumference.
Serum creatinine increased in two groups although it did not show statistical significance. After WPI supplementation, serum urea showed a significant increase when compared to baseline (p = 0.045), although this was not clinically relevant (Table 3). The glomerular filtration rate was calculated at baseline and after 12 weeks of supplementation in order to assess the possible negative effect of WPI on renal function of the study participants, but no statistically significant difference was found [79.7 ± 16.6 mL/min/1.73 m2 vs. 74.6 ± 15.7 mL/min/1.73 m2, showing no statistical difference (p = 0.052)].
Table 3.
Biochemical measurements before and after supplementation.
| Whey Protein (n = 15) | Placebo (n = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | p-Value | Baseline | 12 Weeks | p-Value | |
| Glucose (mg/dL) | 107 (100.0–138.0) | 119 (101.0–137.0) | 0.570 | 123 (98.0–154.7) | 103.5 (96.5–149.3) | 0.674 |
| TC (mg/dL) | 157.3 ± 29.3 | 156.1 ± 41.2 | 0.826 | 165.8 ± 36.8 | 154.7 ± 22.1 | 0.318 |
| Creatinine (mg/dL) | 0.98 ± 0.21 | 1.06 ± 0.17 | 0.126 | 1.08 ± 0.25 | 1.18 ± 0.53 | 0.632 |
| Urea (mg/dL) | 40 ± 12.8 | 47.2 ± 22.0 | 0.045 * | 38.6 ± 7.9 | 43 ± 29.3 | 0.435 |
| TG (mg/dL) | 147.5 ± 44.8 | 143.3 ± 57.5 | 0.785 | 162.2 ± 36.8 | 218.3 ± 94.0 | 0.081 |
| HDL-c (mg/dL) | 43.2 ± 11.7 | 44.7 ± 11.5 | 0.327 | 41.2 ± 13.8 | 39.2 ± 12.5 | 0.244 |
| LDL-c (mg/dL) | 96.9 ± 28.4 | 99.7 ± 37.0 | 0.597 | 108.6 ± 27.5 | 91.5 ± 10.5 | 0.086 |
Values are mean ± SD, or median (25th–75th percentiles) * p < 0.05. Paired t-test; Wilcoxon tests (Glucose). HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
3.4. Other Follow-Up Data
Neither clinical destabilizations (with or without the need for hospital admission) nor changes in patients’ medications were reported during the 12 weeks of the study. No side effects related to gastrointestinal discomfort, such as nausea, vomiting, diarrhea, or constipation, were experienced after drinking the supplement or placebo throughout the study.
4. Discussion
In the scenario of HF, loss of muscle mass and strength are of special concern, as they may impair overall quality of life and even survival, and interventions that improve muscle mass and strength are therefore desirable. This study showed that, in patients with chronic HF, the use of WPI, even without concomitant exercise training, was associated with positive outcomes in terms of body composition and skeletal muscle mass. The benefits obtained even in the absence of exercise display an especially important opportunity, as HF patients are often unable to exercise, either due to inherent physical limitations or due to reduced access to cardiac rehabilitation services.
In this study, a high proportion of the study participants had sarcopenia. This is in line with data showing an elevated prevalence of sarcopenic obesity in HF [24,25]. In a recent study, Chen et al. examined the prevalence of sarcopenia in patients with HF and its association with adverse clinical outcomes and found that the overall prevalence of sarcopenia was 31%, and 18% in HF with preserved ejection fraction [26].
Sarcopenia was associated with an increased risk of poor prognosis (mortality and hospitalizations), with a combined hazard ratio of 1.64. Besides these harder outcomes, loss of muscle mass may contribute to frailty and reduction of exercise capacity of patients with HF [27]. Indeed, exercise intolerance is a core component of HF syndrome [28]. Zhang et al., in another large meta-analysis, described a pooled prevalence of sarcopenia in patients with HF of 34%, ranging from 10% to 69% [3]. These numbers underscore the importance of this problem and the need for strategies to increase muscle mass in this patient population.
Regarding dietary composition, the daily protein intake per kilogram of body weight was found to be below appropriate levels for patients with HF at baseline, even though the percentage of protein intake was within normal limits. This reinforces the importance of routine dietary assessment of patients with HF, as nutritional deficits may go unnoticed, although it has long been demonstrated that patients with chronic stable HF often have an inadequate intake of calories and protein [22]. This also underscores the value of protein supplementation for these patients.
Results from a 57-patient nitrogen balance study in non-obese patients with HF, compared with 49 control subjects, suggested that a higher protein goal of 1.1 g/kg or greater may be necessary [22]. Accordingly, the Academy of Nutrition and Dietetics recommends that protein intake should be individualized, but patients with HF should aim for the general population minimum of 0.8 g/kg per day protein intake to prevent cachexia [29].
Interestingly, inadequate consumption of lipids was observed [23]. This may reflect a substitution of fat for carbohydrates, which may be associated with previous concepts concerning the association between fat intake and cardiovascular disease [30], which have been incorporated into medical practice for years and have led to restrictive orientations regarding lipid consumption up to the present time [31].
Some small studies have shown that supplementation of unsaturated fatty acids from dietary sources can have a beneficial effect in patients with HF [32]. A cross-sectional analysis of 23 patients with HF with Preserved Ejection Fraction showed that dietary intake of saturated, monounsaturated, polyunsaturated, and unsaturated fatty acids as a total amount and percentage of daily calories correlated with increased cardiorespiratory fitness as measured by peak VO2 in maximal cardiopulmonary testing [33].
Of note, HF patients were frequently overweight or obese at baseline. It is known that sarcopenia can occur earlier than cachexia in the body-wasting process in HF and may not be associated with weight loss [6]. Muscle damage may be caused by local or systemic factors, such as decreased physical activity, reduced protein and caloric intake, malabsorption, systemic inflammation, oxidative stress, apoptosis, overactivation of the ubiquitin–proteasome system, low muscle blood flow, and endothelial dysfunction [34].
A nutritional solution to minimize the loss of muscle mass in heart failure, even in obese individuals, would be of great relevance in clinical practice. Ideally, it would be interesting for the nutritional supplement not only to stimulate net body protein gain, but also to do so with minimal caloric intake. HF is increasingly being associated with obesity, and a nutritional formulation that significantly increases caloric intake would be undesirable [35].
With WPI, there was a significant increase in skeletal muscle mass (0.6 ± 0.7 kg) after 12 weeks. Prior studies had described the effects of WPI on muscle mass gain, but with associated exercise training [15,36]. The current results are promising as they show results in terms of muscle gain irrespective of the addition of exercise, which may be viewed as a reality in many settings in which cardiac rehabilitation or other methods of supervised training are not available. It is worth noting that WPI did not result in a significant increase in muscle strength, measured by the handgrip. This result is different from a study of elderly patients who participated in high-intensity resistance training, which showed an increase in knee extension strength after supplementation of 24 g WPI [37].
In another report, 23 obese elderly patients with HF with preserved ejection fraction were randomized into three groups: control, whey protein supplementation alone, and whey protein plus light exercise. As in the current study, protein supplementation alone failed to improve physical performance; however, when combined with light exercise, it improved walking speed and quadriceps strength [38]. Differences in functional responses between studies might be attributed either to the lack of exercise training in the current study or to the different muscle groups that were evaluated. Nonetheless, increased muscle mass may be still considered an achieved goal in this scenario, as patients with HF may have benefited from other muscle groups, such as those from the legs, with improved walking capacity, for example, which was not evaluated in this study.
Overall, WPI supplementation has demonstrated beneficial effects on body composition, with a decrease in body fat mass, BMI, and WC, and an increase in lean body mass [39]. In overweight and obese individuals, the benefits of supplementation with WPI on both body composition and cardiometabolic risk by improving the lipid and glycemic profile and reducing body adiposity have been widely demonstrated. A meta-analysis, comprising nine randomized controlled trials and 455 patients, showed the benefit of WPI in reducing body weight, increasing lean body mass, and improving lipid profile and glycemic control [40].
In patients with HF, high-protein diets have also been demonstrated to be advantageous for weight loss and adipose tissue reduction [41]. However, to date, no studies have been found to assess the effect of isolated supplementation with WPI on body composition in this specific patient population. In this study, patients who received WPI had significant reductions in the percentage of body fat, total body fat, and WC. The improvement of these cardiometabolic risk markers may be considered a huge additional gain obtained from WPI, besides the most expected—increased muscle mass. It remains to be explored if these additional favorable effects of WPI supplementation may consistently determine an improvement of outcomes in this particular patient population, who are of high cardiovascular risk.
5. Limitations
This study is limited by the small sample size and relatively high dropout rate, especially in the placebo group. However, it offers important new data on the use of WPI for patients with HF, such that that the supplementation of WPI may be useful to increase muscle mass and decrease body fat in a patient population with a high prevalence of overweight/obesity and sarcopenia, who are often unable to exercise.
6. Conclusions
In patients with chronic HF with reduced LVEF, 12-week WPI supplementation promoted an improvement of body composition, evidenced by an increase in skeletal muscle mass and a decrease in body fat. Isolated (without accompanying physical exercise) WPI supplementation may be useful for these patients, who are frequently unable to exercise or are limited in their access to supervised exercise programs. The current results may help change the paradigm of the need for exercise during WPI supplementation, which is particularly useful in the specific population of HF patients.
Acknowledgments
We thank staff in National Institute of Cardiology for their assistance with the study. The authors would like to thank Marcio Marinho Gonzalez and Juliana Maradei for their excellent technical assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15102320/s1, Table S1: Comparison between patients who complete dor not the 12 weeks of supplementation.
Author Contributions
Conceptualization, E.M.d.S. and A.S.B.M.; methodology, E.M.d.S., A.S.B.M., G.V.B.H. and A.D.L.; formal analysis, E.M.d.S. and G.V.B.H.; investigation E.M.d.S.; writing—original draft preparation, E.M.d.S.; writing—review and editing, A.S.B.M., G.V.B.H., E.T. and A.D.L.; supervision, A.D.L.; funding acquisition, E.M.d.S. and A.D.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Research Ethics Committee of the National Institute of Cardiology under protocol number 03218512.0.2005.5272. Written informed consent was obtained from all subjects/patients. The protocol for this study was registered at ClinicalTrials.gov under number NCT03142399, the study was registered under the title “Effect of Whey Protein’ Supplementation and Exercise in Patients with Heart Failure” and the URL of the study is https://www.clinicaltrials.gov/ct2/show/NCT03142399?term=NCT03142399&draw=2&rank=1. Accessed on April 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All datasets generated and analyzed are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The isolated whey protein was donated by Supley Laboratory which had no role in the design, analysis, or writing of this article. The consumables (placebo, packaging) used in this study were funded by Fundação Pró Coração (FUNDACOR).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Josiak K., Jankowska E.A., Piepoli M.F., Banasiak W., Ponikowski P. Skeletal myopathy in patients with chronic heart failure: Significance of anabolic-androgenic hormones. J. Cachexia Sarcopenia Muscle. 2014;5:287–296. doi: 10.1007/s13539-014-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Zhang J., Ni W., Yuan X., Zhang H., Li P., Xu J., Zhao Z. Sarcopenia in heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2021;8:1007–1017. doi: 10.1002/ehf2.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotta K., Taniguchi R., Nakayama H., Yamaguchi F., Sato Y. The Effects of an Oral Nutritional Supplement with Whey Peptides and Branched-Chain Amino Acids for Cardiac Rehabilitation of Patients with Chronic Heart Failure. Int. Heart J. 2021;62:1342–1347. doi: 10.1536/ihj.21-102. [DOI] [PubMed] [Google Scholar]
- 5.Lena A., Anker M.S., Springer J. Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science. Int. J. Mol. Sci. 2020;21:6549. doi: 10.3390/ijms21186549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Haehling S., Ebner N., Dos Santos M.R., Springer J., Anker S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017;14:323–341. doi: 10.1038/nrcardio.2017.51. [DOI] [PubMed] [Google Scholar]
- 7.Saitoh M., Dos Santos M.R., Ebner N., Emami A., Konishi M., Ishida J., Valentova M., Sandek A., Doehner W., Anker S.D., et al. Nutritional status and its effects on muscle wasting in patients with chronic heart failure: Insights from Studies Investigating Co-morbidities Aggravating Heart Failure. Wien. Klin. Wochenschr. 2016;128:497–504. doi: 10.1007/s00508-016-1112-8. [DOI] [PubMed] [Google Scholar]
- 8.Anker S.D., Ponikowski P., Varney S., Chua T.P., Clark A.L., Webb-Peploe K.M., Harrington D., Kox W.J., Poole-Wilson P.A., Coats A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 9.Koopman R., Walrand S., Beelen M., Gijsen A.P., Kies A.K., Boirie Y., Saris W.H., van Loon L.J. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009;139:1707–1713. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- 10.Cereda E., Pisati R., Rondanelli M., Caccialanza R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients. 2022;14:1524. doi: 10.3390/nu14071524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopman R., Wagenmakers A.J., Manders R.J., Zorenc A.H., Senden J.M., Gorselink M., Keizer H.A., van Loon L.J. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 2005;288:E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 12.Phillips S.M., Tipton K.D., Ferrando A.A., Wolfe R.R. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am. J. Physiol. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- 13.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 14.Chalé A., Cloutier G.J., Hau C., Phillips E.M., Dallal G.E., Fielding R.A. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulmi J.J., Lockwood C.M., Stout J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010;7:51. doi: 10.1186/1743-7075-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haß U., Kochlik B., Herpich C., Rudloff S., Norman K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients. 2022;14:4274. doi: 10.3390/nu14204274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondanelli M., Klersy C., Terracol G., Talluri J., Maugeri R., Guido D., Faliva M.A., Solerte B.S., Fioravanti M., Lukaski H., et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 22.Aquilani R., Opasich C., Verri M., Boschi F., Febo O., Pasini E., Pastoris O. Is nutritional intake adequate in chronic heart failure patients? J. Am. Coll. Cardiol. 2003;42:1218–1223. doi: 10.1016/S0735-1097(03)00946-X. [DOI] [PubMed] [Google Scholar]
- 23.Rohde L.E.P., Montera M.W., Bocchi E.A., Clausell N.O., Albuquerque D.C., Rassi S., Colafranceschi A.S., Freitas A.F.J., Ferraz A.S., Biolo A., et al. Diretriz Brasileira de Insuficiência Cardíaca Crônica e Aguda. Arq. Bras. Cardiol. 2018;111:436–539. doi: 10.5935/abc.20180190. [DOI] [PubMed] [Google Scholar]
- 24.Delmonico M.J., Harris T.B., Lee J.S., Visser M., Nevitt M., Kritchevsky S.B., Tylavsky F.A., Newman A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 25.Rao V.N., Zhao D., Allison M.A., Guallar E., Sharma K., Criqui M.H., Cushman M., Blumenthal R.S., Michos E.D. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis) JACC Heart Fail. 2018;6:999–1007. doi: 10.1016/j.jchf.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R., Xu J., Wang Y., Jiang B., Xu X., Lan Y., Wang J., Lin X. Prevalence of sarcopenia and its association with clinical outcomes in heart failure: An updated meta-analysis and systematic review. Clin. Cardiol. 2023;46:260–268. doi: 10.1002/clc.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narumi T., Watanabe T., Kadowaki S., Takahashi T., Yokoyama M., Kinoshita D., Honda Y., Funayama A., Nishiyama S., Takahashi H., et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur. J. Intern. Med. 2015;26:118–122. doi: 10.1016/j.ejim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Haykowsky M.J., Tomczak C.R., Scott J.M., Paterson D.I., Kitzman D.W. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J. Appl. Physiol. 2015;119:739–744. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vest A.R., Chan M., Deswal A., Givertz M.M., Lekavich C., Lennie T., Litwin S.E., Parsly L., Rodgers J.E., Rich M.W., et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019;25:380–400. doi: 10.1016/j.cardfail.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Page I.H., Stare F.J., Corcoran A.C., Pollack H., Wilkinson C.F., Jr. Atherosclerosis and the fat content of the diet. J. Am. Med. Assoc. 1957;164:2048–2051. doi: 10.1001/jama.1957.62980180004013. [DOI] [PubMed] [Google Scholar]
- 31.Lima T., Silva D.G.D., Barreto I.D.C., Oliveira J.C., Oliveira L.C.S., Arcelino L.A.M., Sousa A.C.S., Barreto Filho J.A.S. Quality of Intra-Hospital Nutritional Counseling in Patients with STEMI in the Public and Private Health Networks of Sergipe: The VICTIM Register. Arq. Bras. Cardiol. 2019;113:260–269. doi: 10.5935/abc.20190124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billingsley H.E., Hummel S.L., Carbone S. The role of diet and nutrition in heart failure: A state-of-the-art narrative review. Prog. Cardiovasc. Dis. 2020;63:538–551. doi: 10.1016/j.pcad.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbone S., Canada J.M., Buckley L.F., Trankle C.R., Billingsley H.E., Dixon D.L., Mauro A.G., Dessie S., Kadariya D., Mezzaroma E., et al. Dietary Fat, Sugar Consumption, and Cardiorespiratory Fitness in Patients With Heart Failure With Preserved Ejection Fraction. JACC Basic Transl. Sci. 2017;2:513–525. doi: 10.1016/j.jacbts.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curcio F., Testa G., Liguori I., Papillo M., Flocco V., Panicara V., Galizia G., Della-Morte D., Gargiulo G., Cacciatore F., et al. Sarcopenia and Heart Failure. Nutrients. 2020;12:211. doi: 10.3390/nu12010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim I.Y., Park S., Smeets E., Schutzler S., Azhar G., Wei J.Y., Ferrando A.A., Wolfe R.R. Consumption of a Specially-Formulated Mixture of Essential Amino Acids Promotes Gain in Whole-Body Protein to a Greater Extent than a Complete Meal Replacement in Older Women with Heart Failure. Nutrients. 2019;11:1360. doi: 10.3390/nu11061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takata M., Amiya E., Watanabe M., Hosoya Y., Nakayama A., Fujiwara T., Taya M., Oguri G., Hyodo K., Takayama N., et al. An exploratory study on the efficacy and safety of a BCAA preparation used in combination with cardiac rehabilitation for patients with chronic heart failure. BMC Cardiovasc. Disord. 2017;17:205. doi: 10.1186/s12872-017-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niccoli S., Kolobov A., Bon T., Rafilovich S., Munro H., Tanner K., Pearson T., Lees S.J. Whey Protein Supplementation Improves Rehabilitation Outcomes in Hospitalized Geriatric Patients: A Double Blinded, Randomized Controlled Trial. J. Nutr. Gerontol. Geriatr. 2017;36:149–165. doi: 10.1080/21551197.2017.1391732. [DOI] [PubMed] [Google Scholar]
- 38.Azhar G., Raza S., Pangle A., Coleman K., Dawson A., Schrader A., Wolfe R.R., Wei J.Y. Potential Beneficial Effects of Dietary Protein Supplementation and Exercise on Functional Capacity in a Pilot Study of Individuals with Heart Failure with Preserved Ejection Fraction. Gerontol. Geriatr. Med. 2020;6:2333721420982808. doi: 10.1177/2333721420982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepandi M., Samadi M., Shirvani H., Alimohamadi Y., Taghdir M., Goudarzi F., Akbarzadeh I. Effect of whey protein supplementation on weight and body composition indicators: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN. 2022;50:74–83. doi: 10.1016/j.clnesp.2022.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Wirunsawanya K., Upala S., Jaruvongvanich V., Sanguankeo A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018;37:60–70. doi: 10.1080/07315724.2017.1344591. [DOI] [PubMed] [Google Scholar]
- 41.Evangelista L.S., Heber D., Li Z., Bowerman S., Hamilton M.A., Fonarow G.C. Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: A feasibility study. J. Cardiovasc. Nurs. 2009;24:207–215. doi: 10.1097/JCN.0b013e31819846b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated and analyzed are available from the corresponding author upon reasonable request.