Abstract
Background:
Broadly neutralizing monoclonal antibodies (bNAbs) suppress HIV-1 RNA and may deplete residual viral reservoirs. We evaluated the safety and pharmacokinetics (PK) of dual intravenous VRC01LS and 10–1074 in very early-treated children with HIV-1 on suppressive antiretroviral treatment (ART).
Setting:
Botswana
Methods:
Children with HIV-1 (median age 3.1 years) on antiretroviral treatment (ART) from <7 days old were enrolled. In Phase A, six children received 10–1074 (30 mg/kg at Day 0, 28, and 56) and 6 children received VRC01LS (30mg/kg at Day 0, 10mg/kg at Days 28 and 56) by intravenous infusion. In Phase B, six children received the two bNAbs combined (with higher VRC01LS maintenance dose, 15mg/kg) every 4 weeks for 32 weeks with PK evaluations over 8 weeks. Population PK models were developed to predict steady-state concentrations.
Results:
BNAb infusions were well tolerated. There were no infusion reactions nor any bNAb related grade ¾ events. The median (range) first dose Cmax and trough (Day 28) combined from both phases were 1,405 (876–1,999) mcg/mL and 133 (84–319) mcg/mL for 10–1074 and 776 (559–846) mcg/mL and 230 (158–294) mcg/mL for VRC01LS. No large differences in bNAb clearances were observed when given in combination. The estimated VRC01LS half-life was shorter than in adults. Predicted steady-state troughs [median (90% prediction interval)] were 261 (95–565) and 266 (191–366) mcg/mL for 10–1074 and VRC01LS, respectively, when given in combination.
Conclusions:
10–1074 and VRC01LS were safe and well-tolerated among children receiving ART. Troughs exceeded minimal targets with every 4 week administration of 10–1074 at 30mg/kg and VRC01LS at 15mg/kg.
Keywords: VRC01LS, 10–1074, pharmacokinetics, HIV-1 infection, pediatrics, broadly neutralizing antibodies
INTRODUCTION
Long-term viral suppression with conventional antiretroviral medications is difficult to maintain over a lifetime. This is particularly true in the pediatric setting when dosing and adherence challenges add complexity to standard antiretroviral treatment (ART). Novel treatment strategies such as the use of broadly neutralizing monoclonal antibodies (bNAbs) against HIV-1, which have the potential to maintain HIV-1 viral suppression with infrequent administration, could provide a break from standard ART, reduce long-term toxicities, improve long-term treatment adherence, and potentially help to deplete residual viral reservoirs.[1–5]
Because of limited HIV-1 diversity and low viral reservoirs, [6] early-treated children with HIV-1 may be ideal candidates for use of bNAbs as an alternative to ART. VRC01, a recombinant human immunoglobulin G1 (IgG1) monoclonal antibody that targets the CD4 binding site of gp120, has demonstrated activity in reducing plasma viremia among ART-untreated adults[1] and delaying viral rebound after periods of ART interruption, compared with historical controls (chronically infected adults).[7] The long-acting version, VRC01LS, improves tissue levels and may improve efficacy,[8] and both VRC01 and VRC01LS have been administered to infants subcutaneously.[9, 10] The bNAb 10–1074 targets a different Env binding site from VRC01, the V3 glycan supersite on the HIV-1 envelope (Env) protein, and has achieved 1.52 log HIV-1 RNA reductions when used in chronically infected adults.[11] This agent has not previously been studied in children.
In preparation for a trial of dual bNAb use as a treatment alternative to ART in pediatric patients, we evaluated the pharmacokinetics (PK) of intravenous 10–1074 and VRC01LS, dosed individually and in combination every 4 weeks for 3 doses in each Phase (up to 6 doses total), among children on suppressive ART; safety was evaluated for up to 32 weeks.
METHODS
Trial design
The Tatelo study, “A Clinical Trial to Evaluate the Impact of Broadly Neutralizing Antibodies VRC01LS and 10–1074 on Maintenance of HIV-1 Suppression in a Cohort of Early-Treated Children in Botswana,” is an ongoing phase I/II, multi-site clinical trial among virally suppressed children with HIV-1 (NCT03707977). The primary overall aim is to evaluate dual anti-HIV-1 bNAb treatment as an alternative to ART for up to 24 weeks. This manuscript covers the first two phases of the study for dose-finding and safety of 10–1074 and VRC01LS while maintaining ART. In the initial PK and safety phase (Phase A), six participants on suppressive ART received three intravenous (IV) doses (every 4 weeks) of 10–1074, and six participants received three (IV) doses (every 4 weeks) of VRC01LS and underwent safety and PK testing for 12 weeks. Safety, PK, and viral suppression data were then reviewed by a Safety Monitoring Committee (SMC) before opening the study to its second phase (Phase B). In Phase B, ART was continued and 6 participants received both bNAbs every 4 weeks, with PK evaluation of dual bNAb dosing for 8 weeks, and safety evaluations for 32 weeks. Based on SMC review of the Phase A and B results, additional phases of the trial are ongoing; we report here the complete Phase A and B PK and safety results.
Study population
All Tatelo Study participants had previously participated in the Early Infant Treatment (EIT) Study, a clinical trial of early infant diagnosis and treatment in Gaborone and Francistown, Botswana (NCT02369406).[12] Eligible participants for Phase A and B of the Tatelo Study had started on ART <7 days of life, were on continuous ART for at least 96 weeks, and had HIV-1 RNA <40 copies/mL for at least 24 weeks prior to enrollment. Phase B participants were selected from Phase A participants based on prior follow-up history and proximity to clinics (3 in Gaborone and 3 in Francistown) for frequent follow-up, without consideration of Phase A results.
Study procedures
In Phase A, participants were assigned to receive either three doses of 10–1074 (30 mg/kg IV on Days 0, 28 and 56) or three doses of VRC01LS (30 mg/kg IV on Day 0, then 10mg/kg IV on Days 28 and 56). A 28-day dosing interval was chosen to allow both bNAb formulations to be dosed at every visit, recognizing that while a longer interval might be possible for VRC01LS, monthly administration would be beneficial from a pharmacodynamic perspective. Participants continued their ART during Phase A, and blood samples for bNAb serum PK were collected before and 5 times after each infusion starting at the end of each infusion, 1 hour post-infusion, 1 day post-infusion, 1 week post-infusion, 2 weeks post-infusion and at Day 84 (four weeks after the third dose).
In Phase B, participants received dual bNAbs and ART for up to 32 weeks, with PK testing for 8 weeks. 10–1074 remained at 30 mg/kg IV throughout. Based on review of data from Phase A, VRC01LS maintenance dosing was increased to 15 mg/kg IV after the 30mg/kg IV load on Day 0. As in Phase A, participants remained on ART in Phase B. PK samples were collected pre-infusion, at the end of the second product infusion, 1 hour post-infusions, 1 day post-infusions, and 1 week post-infusions in relation to the Day 0 and Day 28 doses; a pre-dose trough sample was collected at Day 56. Additional safety data were collected up to 32 weeks. The general study design is summarized in Supplemental Figure 1.
Administration of bNAbs was overseen by a study clinician. Study products were administered intravenously over approximately 60 minutes each. 10–1074 was administered using a 0.2 micron in-line filter infusion set. VRC01LS was administered using a 1.2 micron in-line filter infusion set with a DEHP-free, latex-free polyethersulfone filter membrane. When both study products were administered on the same day, 10–1074 was given first followed by VRC01LS with a gap of approximately 10–15 minutes between infusions. Vital signs were measured before, between, and after infusions. Post-infusion monitoring for 2–4 hours occurred following the first infusion (4 hours for the first cohort receiving 10–1074 in Phase A), and one hour with subsequent infusions.
Laboratory tests included HIV-1 RNA (every 4 weeks), hematology, CD4+/CD8+ cell count, and serum chemistry. All adverse events (Grade 1 or higher), including any infusion reactions, were recorded. Adverse events were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (DAIDS AE Grading Table) version 2.1 dated July 2017.[13] Adverse events were assessed by site investigators and the protocol team as “related” or “not related” to study products.
Study products
10–1074 is a recombinant fully human IgG1 bNAb expressed in Chinese hamster ovary (CHO) cells and purified using standard methods. The 10–1074 used in this study was manufactured by Mass Bio under contract to the National Institute of Allergy and Infectious Diseases (NIAID). 10–1074 was stored at study site pharmaceutical refrigerators at 2°−8°C.
VRC01LS is manufactured under cGMP using a stably transfected CHO cell line, purified, and the drug product vials filled and labeled at the Vaccine Research Center (VRC), Vaccine Clinical Material Program operated by Leidos Biomedical Research, Inc., Frederick, MD. VRC01LS was stored in study site pharmacy freezers with a temperature range of −45°C to −10°C.
Pharmacokinetic Evaluation
PK analysis for 10–1074 concentrations was performed at Duke University (Durham, NC). 10–1074 serum concentrations were determined by a validated sandwich ELISA as previously described.[14] Briefly, high bind polystyrene plates were coated overnight with an anti-idiotypic antibody specifically recognizing 10–1074 (anti-ID 3A1–4E11 mAb; produced by the Duke Protein Production Facility). A horseradish peroxidase (HRP)-conjugated goat antihuman IgG Fc-specific antibody (Jackson ImmunoResearch) was used to detect 10–1074. A 5-PL curve fitting-algorithm (Softmax Pro, v. 5.4.5) was used to calculate serum 10–1074 concentrations from respective standard curves run on the same plate. Standards and positive controls were created from a clinical grade, GMP manufactured drug product lot of 10–1074.
PK analysis for VRC01LS concentrations was performed at the Vaccine Research Center (Gaithersburg, MD). Quantification of VRC01LS concentrations in serum was performed by ELISA with a Beckman Biomek based integrated automation platform. The anti-idiotype 5C9 monoclonal antibody was added to Immulon-4HXB microtiter overnight prior to blocking. Plates were washed and blocked (10%FBS in PBS) for 2 hours at room temperature. Duplicate serial 3-fold dilutions covering the range of 1:100 to 1:218,700 of the test sample were incubated for 2 hours at 37 o C. Biotin-labeled anti-human IgG1, and Streptavidin conjugated with horseradish peroxidase and TMB (3, 5’, 5, 5’-tetra-methylbenzidine) substrate was used to develop the reaction. Sulfuric acid was then added to halt color development. Plates were then read within 30 minutes at 450nm with a Molecular Devices Paradigm plate reader. After dilution sample concentrations were quantified using a linear regression of a standard curve of VRC01LS covering a range between 5–125 ng/mL.
10–1074 and VRC01LS pharmacokinetics were evaluated by a descriptive non-compartmental analysis. First dose Cmax, AUC0–28D, Cave and trough (C28D) were taken from observed concentrations from Phases A and B. Phase A troughs after the third dose (Day 84) were also summarized. Cave was calculated as the AUC divided by the dose interval. 10–1074 and VRC01LS target trough concentrations of >7.5 mcg/mL and >160 mcg/mL, respectively, were pre-specified for Phase B of the study based on expected prior PK findings in adults and viral sensitivity.[11, 15]
A limited population PK (popPK) analysis was performed on the combined PK results from Phases A and B. For the popPK analysis, a separate two-compartment model was developed for each bNAb using NONMEM ver. 7.2 and the FOCEI subroutine. Pharmacokinetic parameters were allometrically scaled and normalized to 70kg. Specifically, elimination clearance (CL) and inter-compartmental clearance (Q) were scaled to participants weight (WT) as (WT/70)0.85 and volume of distribution (Vss) as (WT/70)1.0.[16] Due to the limited number of participants and PK evaluations, a limited covariate assessment was performed to detect the potential impact of repeat dosing (first vs second or third dose) and single bNAb versus dual bNAb on volume of distribution (Vss) or clearance (CL). A bootstrap analysis was performed to assess model appropriateness and generate parameter confidence intervals. The popPK model was used to simulate 5000 virtual participants and the distribution of steady-state concentrations of the bNAbs used in combination.
Trial ethics and oversight
The study was approved by Institutional Review Boards in Botswana and at the Harvard T.H. Chan School of Public Health. A parent or guardian provided written informed consent for all participants. The study is monitored by an independent Safety Monitoring Committee.
RESULTS
Participant Characteristics
Baseline characteristics of the 12 participants enrolled in Phase A from June to July, 2019 are shown in Table 1. The median age at baseline was 3.1 years (range 2.1–4.1) and 9 (75%) were female. All were receiving lopinavir/ritonavir (LPV-r)/zidovudine (ZDV)/lamivudine (3TC); one was also receiving abacavir (ABC). All children entered Phase A on the LPV-r solution formulation; 7 switched to granules and/or tablets over the course of the study. The baseline median CD4+ cell count and CD4+ percent were 1,211 (range 635–2,547) cells/mm3 and 34% (range 21%−45%) and all were virologically suppressed (HIV-1 RNA<40 copies/mL). One child had transient viral rebound while non-adherent to ART during follow-up in Phase A. Six Phase A participants, all of whom remained virologically suppressed (<40 copies/mL), subsequently enrolled into Phase B in March 2020 (three from each bNAb group in Phase A). At the beginning of Phase B, participant median age was 4.5 years (range 3.4–4.9), and throughout Phase B all remained virologically suppressed, while continuing to receive LPV-r/ZDV/3TC.
Table 1.
Baseline Characteristics of Participants Evaluated for Initial Safety and PK of 10–1074 and/or VRC01LS.
| Characteristic | N=12 |
|---|---|
| Median age (range) | 3.1 years (2.1–4.1) |
| Female Sex (%) | 9 (75%) |
| Median CD4 cell count (range) Median CD4 % (range) |
1,211 cells/ mm3 (635–2,547 cells/mm3) 34% (21%−45%) |
| HIV-1 RNA | <40 copies/mL (required) |
| Concomitant Antiretrovirals | |
| LPV-r* | 12/12 (100%) |
| ZDV | 12/12 (100%) |
| 3TC | 12/12 (100%) |
| ABC | 1/12 (8%) |
LPV-r, lopinavir-ritonavir; ZDV, zidovudine; 3TC, lamivudine; ABC, abacavir
All children entered Phase A on the liquid formulation of LPV-r.
Study visit attendance in both Phases was excellent with no missed visits. No concerns with IV access or administration were reported by staff of participants. One participant received an incomplete dose (~70% of intended) of VRC01LS due to leaking infusion set at Day 28 in Phase A and PK results affected by this dose were excluded from summaries. All other infusions were administered on schedule and to completion.
Safety of 10–1074 and VRC01LS
The bNAb infusions were well tolerated in both Phase A (given individually) and in Phase B (administered together). There were no infusion reactions. There were 94 unique adverse events, Eighty-three (88%) were grade 1, nine (10%) were grade 2, and two (2%) were grade 3 and none were grade 4 events. Forty-five (48%) were abnormal laboratory results and 49 (52%) were clinical diagnoses (with or without associated signs and symptoms).. The two grade 3 events were considered unrelated to bNAbs: one event of high blood pressure following an incorrect measurement procedure, and one event of hyperkalemia following delayed specimen processing. Grade 1–2 adverse events were common in this pediatric population, and most were considered unrelated to bNAbs. All adverse events are summarized in Table 2.
Table 2.
Graded adverse events following receipt of 10–1074 and/or VRC01LS among 12 children during 12 weeks of follow-up in Phase A and in the subset of 6 children during 32 weeks of follow-up in Phase B.
| Toxicity Grade (N) | ||||
|---|---|---|---|---|
|
| ||||
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Total |
|
| ||||
| Overall, N (%) | 83 (88%) | 9 (10%) | 2 (2%) | 94 |
|
| ||||
| Abnormal laboratory results | 38 | 6 | 1 | 45 |
| Neutrophil count decreased | 8 | 2 | 0 | 10 |
| Hemoglobin decreased | 8 | 1 | 0 | 9 |
| Glucose decreased | 5 | 2 | 0 | 7 |
| Creatinine increased | 5 | 0 | 0 | 5 |
| Sodium decreased | 5 | 0 | 0 | 5 |
| Glucose increased | 3 | 0 | 0 | 3 |
| Potasium increased | 1 | 0 | 1 | 2 |
| Bicarbonate decreased | 2 | 0 | 0 | 2 |
| Calcium decreased | 1 | 0 | 0 | 1 |
| Carbon dioxide decreased | 0 | 1 | 0 | 1 |
|
| ||||
| Clinical diagnoses | 45 | 3 | 1 | 49 |
|
| ||||
| Upper respiratory tract infection | 14 | 1 | 0 | 15 |
| Isolated cough | 8 | 0 | 0 | 8 |
| Fungal skin infection | 5 | 0 | 0 | 5 |
| Rash, dermatitis or eczema | 4 | 1 | 0 | 5 |
| Abdominal pain | 4 | 0 | 0 | 4 |
| Gastroenteritis or diarrhea | 2 | 1 | 0 | 3 |
| Conjunctivitis | 2 | 0 | 0 | 2 |
| Abscess | 1 | 0 | 0 | 1 |
| Acquired phimosis | 1 | 0 | 0 | 1 |
| Blood pressure increased | 0 | 0 | 1 | 1 |
| Extremity pain | 1 | 0 | 0 | 1 |
| Oral fungal infection | 1 | 0 | 0 | 1 |
| Penile pain | 1 | 0 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 0 | 1 |
There were seven grade 1 or 2 events considered related to bNAbs in Phase A. For 10–1074, these included two events (in the same participant) of grade 1 elevated creatinine. For VRC01LS, these included one grade 1 elevated creatinine, one grade 2 dermatitis, one grade 1 eczema, and two events of grade 1 cough within a day of infusion. All events deemed to be related to bNAbs resolved before subsequent doses or the end of Phase A, and no dosing modifications were required. In Phase B, following receipt of both 10–1074 and VRC01LS, there were seven adverse events considered related to bNAbs: three events of low hemoglobin among two participants (two grade 1 and one grade 2), one grade 1 low glucose, and three events of grade 1 decreased neutrophil count among two participants. These events resolved on subsequent lab testing.
Pharmacokinetics of 10–1074 and VRC01LS
The first dose median (range) Cmax and trough for 10–1074 were 1,405 (876–1,999) mcg/mL and 133 (84–319) mcg/mL and first dose median (range) Cmax and trough for VRC01LS were 776 (559–846) mcg/mL and 230 (158–294) mcg/mL. These concentrations were similar whether given as single bNAbs or in combination (Figure 1). There was little accumulation of 10–1074 Cmax with repeated doses of 30mg/kg in phase A and B. Cmax after the second dose (10–15mg/kg) of VRC01LS was lower than the Cmax after the first dose of 30mg/kg. Participants’ Cave concentrations following the first dose were 432 (324–706) mcg/mL for 10–1074 and 360 (range 287–410) mcg/mL for VRC01LS. The 10–1074 trough concentrations increased with each 30mg/kg dose and the median 10–1074 Day 84 concentration in Phase B was 258 (122–467) mcg/mL. Based on adult VRC01LS clearance the steady-state VRC01LS concentrations were expected to be at least 200 mcg/mL with 10mg/kg IV every 4 weeks.[15] However, the median VRC01LS Day 84 concentration in Phase A was 156.9 (range 125.8–201.4) mcg/mL. Therefore, the VRC01LS maintenance dose was increased to 15 mg/kg for Phase B.
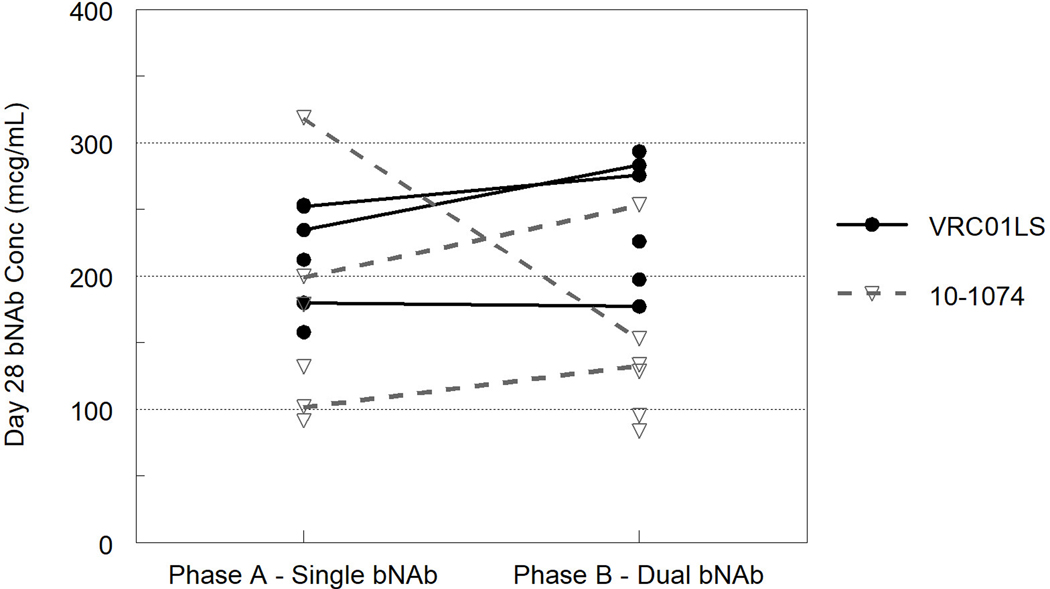
Fig. 1.
Observed pre-dose concentrations of 10–1074 and VRC01LS at 28 days following single and dual bNAb administration. Lines connect the values from participants evaluated in both Phase A and B.
The combined PK results from Phases A and B for 10–1074 and VRC01LS were well described by the population pharmacokinetics models (Figure 2). The non-compartmental PK, population PK parameters, and simulated steady-state troughs are summarized in Table 3. As expected, there was much smaller Cmax/trough fluctuation and higher steady-state troughs with VRC01LS than for 10–1074, despite the lower maintenance dosage of VRC01LS, due to the slower elimination associated with the LS modification.[15]
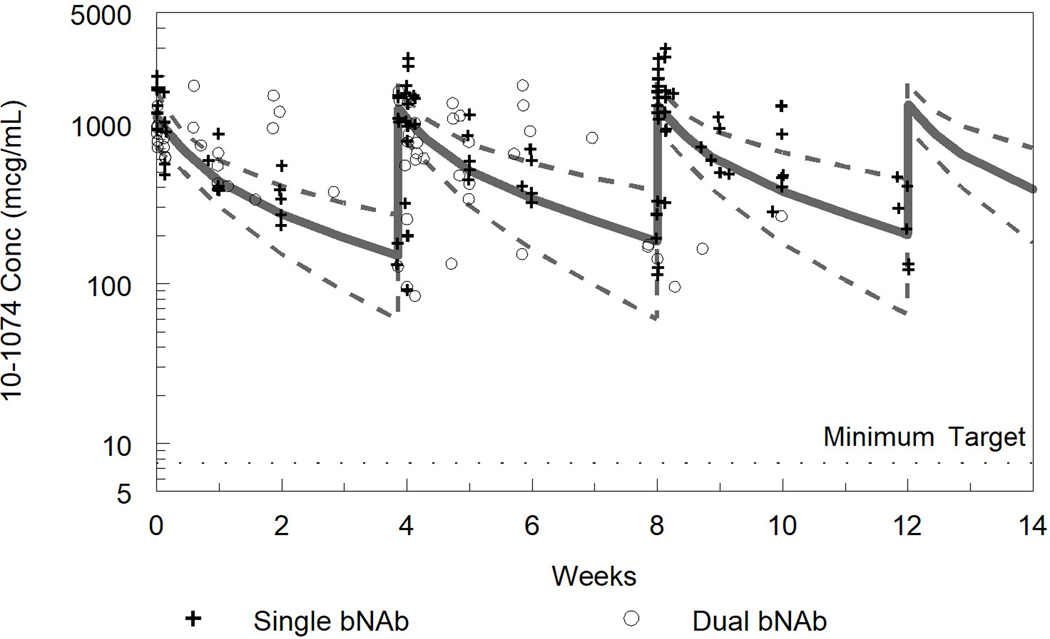
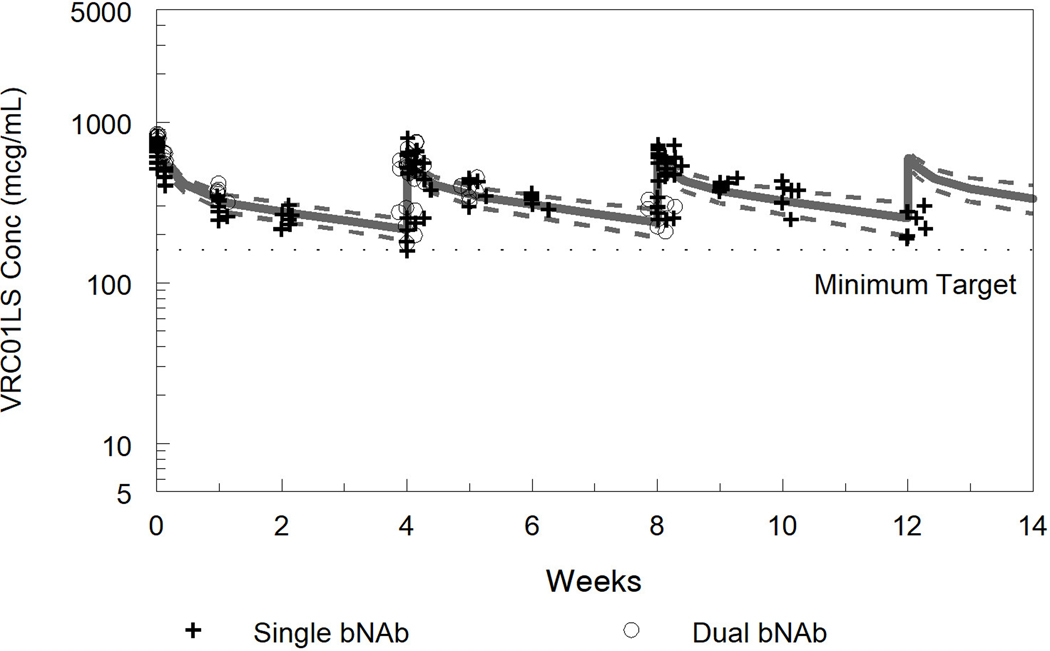
Fig. 2. Measured and model predicted 10–1074 concentrations (a) and VRC01LS concentrations (b).
For the model predicted concentrations the solid line represents the median and dashed lines the 5th and 95th percentiles from the popPK model simulations. Observed concentrations from Phase A, bNAb alone, are represented as “+” and Phase B, bNAb in combination, as “o”. For VRC01LS Phase A, concentrations after the second and third doses were normalized to the 15 mg/kg dose in Phase B.
Table 3.
Observed Concentrations and Modeled Pharmacokinetic (PK) Parameters
| 10–1074 | VRC01LS | |
|---|---|---|
| Observed Concentration | ||
| Cmax (mcg/mL) | 1,405 (876–1,999) | 776 (559–846) |
| C28D (mcg/mL) | 133 (84–319) | 230 (158–294) |
| Cave0–28D (mcg/mL) | 432 (324–706) | 360 (287–410) |
| Population PK Parameters | ||
| CL (mL/d/70kg) | 119 (90–150) | 81 (73–88) |
| Vss (L/70kg) | 2.7 (2.3–4.0) | 6.0 (5.7–6.3) |
| T1/2β (d) | 19 (14–35) | 50 (48–58) |
| Dual bNAb Factor on Vss | 1.46 (1.23–1.70) | 0.85 (0.77–0.92) |
| Ctrss (mcg/mL)* | 261 (95–565) | 266 (191–366) |
| Between Subject Variability | ||
| BSV-CL | 33.9% | 15.2% |
| BSV-Vss | 19.2% | - |
| Residual Variability | ||
| 48.6% | 35.9% |
Observed Cmax, C28D and Cave concentrations – median (range). Population pharmacokinetic parameter estimates with 95% confidence interval from bootstrap analysis and estimated between subject variabilities (BSV) for CL and Vss and residual error. Vss and T1/2βeta values represent single bNAb parameters without “Dual bNAb Factor” covariate, which is a scalar multiplier with 1.0 representing no effect.
Predicted steady-state troughs and 90% prediction intervals represent VRC01LS dosed at 15mg/kg every 4 weeks and 10–1074 dosed at 30mg/kg every 4 weeks in combination.
Cmax, maximum concentration; C28D, concentration at day 28; Cave0–28D, average concentration over the first 28 days; CL, clearance; Vss, volume of distribution; T1/2β, half-life (beta); Ctrss,, predicted steady-state trough; BSV, between subject variability
When used in combination during Phase B, 10–1074 and VRC01LS concentrations were similar to the individual concentrations observed in Phase A (Figure 2). In the population PK model, combined therapy was associated with a modest impact on volume of distribution (Vss) for 10–1074 (increased) and VRC01LS (decreased) but these were not clinically meaningful had there no significant effects of combination therapy on clearance. No changes in 10–1074 or VRC01LS pharmacokinetics were observed following first versus subsequent doses. The predicted median steady-state troughs with Phase B dosing were 261 mcg/mL (10–1074) and 266 mcg/mL (VRC01LS). Both 10–1074 and VRC01LS met the pre-specified targets for Phase B and this dosing was approved by the Safety Monitoring Committee without modification for the final phase of the Tatelo Study (dual bNAbs without ART).
DISCUSSION
These initial safety and PK data from the Tatelo Study provide the first information regarding intravenous use of combined bNAbs in children, and the first data for 10–1074 use in children. Our study demonstrated reassuring safety profiles for both 10–1074 and VRC01LS, and we have established dosing that meets targets set by adult studies. However, there were some differences from values based on adult studies, most notably a considerably shorter estimated half-life of VRC01LS than expected.
10–1074 and VRC01LS were both well tolerated when given intravenously, with no related grade 3 or 4 events. In addition, there were no concerns or difficulties with IV administration reported by staff of participants. These findings are similar to the excellent safety profile of these bNAbs in adults[14,15] and in the initial studies of subcutaneous VRC01 and VRC01LS in infants.[9, 10] Only a small number of grade 1 or 2 events were considered potentially related to either or both bNAbs. Six children on dual bNAbs were assessed through 32 weeks in Phase B for safety and tolerability of dual bNAbs, which provides reassuring long-term data, and a larger sample size will be assessed in the next phase of the study. Testing for anti-drug antibodies (ADA) also will be performed in the next phase of the study.
The pharmacokinetics of 10–1074 and VRC01LS have been described in healthy HIV-1 negative adults and adults living with HIV-1. Both 10–1074 and VRC01 show increased elimination in adults living with HIV-1 compared to healthy participants but it is unclear if this is due to bNAb binding to HIV-1, chronic inflammation altering bNAb metabolism, or other factors.[11, 17] The observed half-lives seen in our children were shorter than adults for VRC01LS (50 vs. 71 days) and 10–1074 (19 vs. 24 days)[11,15]. A shorter half-life in children is commonly seen and expected for many drugs and biologics based on allometry alone. There are limited pediatric PK data on subcutaneous VRC01 and VRC01LS in infants from the IMPAACT Study P1112.[9, 10] However, comparison of the P1112 PK results with the current study is difficult due to significant differences in age, dose, and incomplete absorption following subcutaneous administration. Also in the current study VRC01LS was administered every 4 weeks despite its long half-life to allow administration of VRC01LS and 10–1074 at the same visit. In P1112, lower, less frequent doses and exposure were targeted based on its design to prevent transmission from breastfeeding (rather than to treat HIV-1).
The current study assessed PK parameters for 10–1074 and VRC01LS following intravenous administration given alone or together. There was no impact of dual bNAb or repeat dosing on clearance for either agent and there was limited antibody accumulation with the repeat dosing. Both bNAbs achieved the target trough range with the dosing used in Phase B. The variability in 10–1074 trough concentrations was greater than for VRC01LS, in part due to its shorter half-life. The VRC01LS concentrations in Phase A were lower than expected based on allometric scaling of PK from healthy adults, but the maintenance dose increase from 10 mg/kg in Phase A to 15 mg/kg in Phase B subsequently achieved the desired PK target. Simulations for both agents indicate 90% prediction intervals greater than 95 and 191 mcg/mL for 10–1074 and VRC01LS, respectively, which is more than an order of magnitude greater than the IC80 for sensitive viruses.
The PK data were well described by the popPK model. Despite the limited size of the study, we found find a slight reduction in VRC01LS volume (Vss) when co-administered with 10–1074 and conversely the Vss for 10–1074 was larger when given with VRC01LS. However, these differences had minimal effects on observed concentrations. First dose troughs (C28D) were less than 20% different between Phase A (single bNAb) and B (dual bNAb) for both 10–1074 and VRC01LS. As expected with the LS modification of the Fc region, the elimination rate of VRC01LS was significantly slower than for 10–1074. However, VRC01LS elimination in children was faster (50 days) than reported in heathy adults (71 days).[15] This was not unexpected since many drugs and biologics have shorter half-lives in young children compared to adults based on allometry theory and size differences.
Our study had several limitations. The small number of subjects in Phase A and B limited our precision in analyzing PK and safety in this population. The study population size was restricted by requiring prior participation in EIT, to ensure a well characterized population with very early standardized ART and excellent virologic suppression. This well defined population is necessary for objectives in subsequent phases in this trial. The relatively intensive sampling design generated robust estimation of popPK parameters with the bootstrapped 95% confidence intervals for CL of both bNAbs spanning_+/−25% or less from the population mean, The between subject variance (BSV) precision was more limited and VRC01LS BSV could not be characterized for Vss. While we achieved the a priori study target concentrations, the optimal concentrations of VRC01LS and 10–1074 in this setting are not known. Viral rebound was frequently seen in previously suppressed adults switched to VRC01 at concentrations over 50 mcg/mL[7], and VRC01 target concentration in the AMP studies were 5 and 15 mcg/mL and only prevented transmission for highly sensitive viruses.;[18] Although the VRC01LS target trough concentration in the current study was more than an order of magnitude greater than those from the “high” dose arm of the AMP studies, optimal dosing for combination bNAbs to achieve virologic suppression remains uncertain.
Although ART is highly effective in children, strategies are needed to maintain life-long virologic suppression and optimal immunologic response. Small studies of combined bNAbs directed toward different viral epitopes have demonstrated promising virologic results in adult trials,[19] and similar combination bNAb treatment strategies have promise for the pediatric population. Our study demonstrates initial safety and favorable PK parameters for 10–1074 and VRC01LS, and supports further evaluation of these bNAbs as a combination treatment strategy for children with HIV-1.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Tatelo study participants and their families, and the Tatelo Study team and collaborators, including: Dorcus Babuile, Oganne Batlang, Rachel Bowman, Caroline Brackett, Loveness Bunhu, Alex Carnacchi, Lars Colson, Jack Disaro, Lorato Esele, Tshepho Frank, Olebile Kgakge, Tsholofelo Kebopetswe, Nametso Kelentse, Judith Lucas, Kaia Lyons, Abraham Maigwa, Ria Madison, Princess Mapenshi, Mogomotsi Matshaba, Simon Masopa, Sam McMillan, Charlotte Mdluli, Lendsey Melton, Lindsey Miller, Mompati Mmalane, Maureen Mosetlhi, Akanyang Motlhanka, Sarah Mudrak, Thabani Ncube, Sandra Ndongwe, Martha Ngwaca, Maduo Oabona, Salome Othusitse, Gaoele Pelontle, Obonwe Pule, Christina Reding, Marcella Sarzotti-Kelsoe, Tumalano Sekoto, Ngozana Seonyatseng, Dineo Tumagole, Xu Yu. We thank Frontier Science, and Christina Reding in particular, for providing excellent data management services. We also thank the entire Vaccine Immunology Program (VIP) team at VRC and especially Bob Lin for his painstaking efforts with the automated pseudo virus neutralizing antibody assay. We also thank the Botswana Ministry of Health and Wellness, the Botswana Health Research Development Committee, the Harvard Office of Human Research Administration, the Tatelo SMC Members (Terry Fenton, Grace John-Stewart, Jane Lindsey, Katherine Luzuriaga, Loeto Mazhani, Pablo Tebas), and the NIH (Patrick Jean-Philippe, Judi Miller, Dwight Yin, Lynette Purdue).
E.V.C., R.L.S., and M.P.H. prepared the first draft of the manuscript. G.A. and K.M. collected the data and followed participants. E.V.C., K.B., R.L.S., and M.D.H. analyzed and interpreted the data. K.M.P., S.L., P.J-P., J.M., M.C., M.P.H., E.V.C., M.D.H., R.L.S., M.L., and D.R.K. provided overall guidance on study conduct, design, and interpretation of results. S.M., T.M., C.M., K.E.S., G.T., S.M., A.T., S.O., S.N., A.M., and L.G. supervised and conducted laboratory experiments. R.L.S., M.L., and D.R.K. conceived of and designed the study.
Sources of support:
This study was funded by NIH/NIAID (U01 AI135940). PK testing at Duke Center for AIDS Research was also supported by 5P30 AI064518.
REFERENCES
- 1.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 2015; 7(319):319ra206. [DOI] [PubMed] [Google Scholar]
- 2.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; 522(7557):487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 2016; 352(6288):997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 2016; 352(6288):1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niessl J, Baxter AE, Mendoza P, Jankovic M, Cohen YZ, Butler AL, et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med 2020; 26(2):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma P, Zangari P, Alteri C, Tchidjou HK, Manno EC, Liuzzi G, et al. Early antiretroviral treatment (eART) limits viral diversity over time in a long-term HIV viral suppressed perinatally infected child. BMC Infect Dis 2016; 16(1):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 2016; 375(21):2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 2014; 514(7524):642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland EJ, Cunningham CK, Muresan P, Capparelli EV, Perlowski C, Morgan P, et al. Safety, Tolerability, and Pharmacokinetics of a Long-Acting Broadly Neutralizing HIV-1 Monoclonal Antibody VRC01LS in HIV-1-Exposed Newborn Infants. J Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham CK, McFarland EJ, Morrison RL, Capparelli EV, Safrit JT, Mofenson LM, et al. Safety, Tolerability, and Pharmacokinetics of the Broadly Neutralizing Human Immunodeficiency Virus (HIV)-1 Monoclonal Antibody VRC01 in HIV-Exposed Newborn Infants. J Infect Dis 2020; 222(4):628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 2017; 23(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maswabi K, Ajibola G, Bennett K, Capparelli EV, Jean-Philippe P, Moyo S, et al. Safety and efficacy of starting antiretroviral therapy in the first week of life. Clin Infect Dis Epub ahead of print 12 Jan 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events version 2.1, July 2017. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed July 8, 2021.
- 14.Cohen YZ, Butler AL, Millard K, Witmer-Pack M, Levin R, Unson-O’Brien C, et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10–1074 in healthy adults: A randomized, phase 1 study. PLoS One 2019; 14(8):e0219142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15(1):e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs 2011; 3(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Nikanjam M, Cunningham CK, McFarland EJ, Coates EE, Houser KV, et al. Model Informed Development of VRC01 in Newborn Infants Using a Population Pharmacokinetics Approach. Clin Pharmacol Ther 2021; 109(1):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corey L, Gilbert PB, Juraska M, Montefiori DC, Morris L, Karuna ST, et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N Engl J Med 2021; 384(11):1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561(7724):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.