Abstract
Purpose
Derangements of liver transcriptional factors and enzymes have important implications in diabetes-induced related complications. Hence, this study which consists of two experimental phases was aimed at evaluating the possible underlying molecular mechanisms of intermittent fasting (IF), exercise starvation and honey in streptozotocin (STZ)-mediated liver damage in diabetic rats.
Methods
The diabetic rats were treated orally with distilled water (0.5 ml/kg), IF, starvation and honey at 1 g/kg body weight in the non-diabetic phase for four (4) weeks. After STZ injections, four (4) weeks of IF, exercise, starvation, and honey therapy were used as interventions prior to a biochemical evaluation of the liver.
Results
IF and exercise greatly decreased liver transcription factor (resistin, SREBP-1c), inflammatory cytokines/enzyme (TNF-α, IL-6, IL-1ß, MPO) as well as oxidative and nitrergic stress with correspondence increased liver PPAR-γ, IL-10, SOD, CAT and GSH in diabetic rats unlike starvation and honey regimen relative to diabetic controls. Furthermore, IF and exercise significantly improved hepatic glycogen synthase and decreased glycogen phosphorylase in diabetic rats compared to the diabetic control group, but starvation and honey therapy had no such influence. IF and exercise strategically reduces STZ-induced liver metabolic disorder via through modulation of liver transcriptional factors and inhibition of pro-inflammatory cytokines, oxido-nitrergic and adipokine signaling pathway.
Keywords: Diabetes mellitus, Exercise, Fasting, Honey, Liver cirrhosis, Oxidative stress
Introduction
Diabetes is a type of metabolic syndrome marked by a sustained rise in blood glucose levels caused by insulin insufficiency or an inappropriate insulin response [1]. There are two types of diabetes: type I diabetes, which result from depleted levels of insulin, while the type II diabetes is associated with low sensitive to insulin [1]. In 2021, it was estimated that the cases of diabetes would increase up to 537 million cases particularly in adults (20–79 years) according to the IDF Diabetes Atlas 10th Edition 2021. Moreover, diabetes has been adjudged among the top 10 causes of organ complication and death globally. As a result, investigations are therefore imperative to develop an optimum diabetes treatment with particular focus on natural strategies [2].
Preclinical and clinical evidence have shown that prolonged sedentary lifestyle plays important role in aging-related metabolic disorders, and it is one of the important key players involved in the diabetes pathogenesis and associated complications. Of note, oxidative and inflammatory-related mechanisms have been increasingly postulated to be implicated in the pathogenesis of sedentary lifestyle-induced diabetes [3]. Free radical production from elevated blood sugar levels is thought to enhance the release of pro-inflammatory cytokines and contribute to the deterioration of metabolic systems that control glucose homeostasis and liver function. Notably, insulin desensitization or hypofunction, obesity, and hunger have all been linked in clinical settings with pro-inflammatory cytokines such tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-12, and IL-18 as well as diminished antioxidant armories [3, 4].
Investigations have shown that increased sedentary life with reduce exercise have been postulated as possible factors promoting obesity and T2DM which are characterized of low-grade inflammatory activity [5, 6]. Intermittent fasting and exercise have been hypothesized as viable strategies to improve the quality of metabolic activity in persons who are vulnerable to T2DM [7, 8]. Indeed, intermittent fasting, which is a low-grade metabolic dietary strategy consisting of consumption of low-grade calories over a long period of time has been suggested as a possible health approach to mitigate the negative impacts of diabetes in vulnerable individuals [8].
Honey which is organically synthesized by Apis mellifera bees, is a natural sweetener with diverse nutritional and health promoting functions. Studies showed that the multipronged activities of honey include but not limited to antioxidant, anti-inflammatory [9], central nervous system adaptogenic effect [10], immune booster, promotes weight loss and therefore, it is used as an apitherapeutic agent [9, 10]. Honey has also been previously reported to demonstrate neuropharmacological properties such as anxiolytic, antidepressant and memory enhancing activity [10]. Although previous studies have shown that honey possesses hepatoprotective property specifically examining some liver enzymes and lipid profiles [2, 10, 11], however its effects on liver transcriptional factors, inhibition of proinflammatory cytokines, oxido-nitrergic and adipokine signaling pathways on streptozotocin-induced diabetes-mediated liver damage remain obscured. Additionally, it is uncertain how exercise, famine, and intermittent fasting affect these pathways to cause liver damage after streptozotocin-induced diabetes. This study is aimed to investigate the effects of intermittent fasting, starvation, exercise, and honey on streptozotocin-induced liver damage in rats through modulation of liver transcriptional factors, inhibition of proinflammatory cytokines, oxido-nitrergic, and adipokine signaling pathway given the variety of beneficial effects of these practices in neurological and metabolic disease conditions [9, 10].
Materials and methods
Animals
The Delta State University Ethical Use of Animals Research Committee (RC) prepared protocols that were followed for using adult male Wistar rats in this work. The reference number for these protocols was given as REC/FBMS/DELSU/21/121. Sixty-six (66) adult Sprague–Dawley rats were used in the investigation, all of which were similar in age (10–12 weeks) and weight (180–250 g). After two-week acclimatization period, the rats were housed in a normal environment with uniform housing, photoperiod conditions (12 h of light and 12 h of darkness), and a room temperature of 280–300 °C. All the rats used in the study were housed in hygienic wooden cages with free access to water and a regular diet of rat chow.
Chemicals
In Abraka, Delta State, honey was acquired from a neighborhood market (Golden Glory, Australia). The honey was mixed with distilled water and administered to the rats as a gavage (1 g/kg/day). Sigma-Aldrich provided the streptozotocin (STZ, 99 percent purity) (Germany). All additional compounds were also bought from Sigma-Aldrich and were of analytical quality (Germany).
Induction of diabetes
To induce diabetes, streptozotocin (STZ, 50 mg/kg) was mixed with a newly prepared 0.1 M citrate buffer (pH 4.5). The night following STZ injections in a sucrose solution (10 g/100 mL), rats were allowed unlimited access to conventional rat chow to prevent hypoglycemia, as determined by a glucometer. An intraperitoneal injection of freshly made 0.1 M citrate buffer (pH 4.5) without STZ was given to the non-diabetic normal control group. Treatments began four weeks after STZ or citrate buffer injections.
Drugs and their preparations
The doses of honey [2, 11], STZ [11] and distilled water [11] were selected on the ground of historical dose and response effects and preliminary studies.
Animal grouping after diabetogenic induction
Sixty-six (66) rats in total were split into the experimental diabetic and non-diabetic groups. Unlike the diabetic tests, which are separated into six cohorts (n = 6), the non-diabetic phase was shared to five cohorts (n = 5). The groups in this phase include the non-diabetic control group, the starving group, the intermittent fasting group, and the honey (1 g/kg body weight) group. Diabetics and honey (1 g/kg body weight) are both considered to be in the diabetic phase.
Physiological intervention approach
Intermittent fasting (IF) intervention
The IF intervention group underwent a 16-h period of complete food restriction, followed by an additional 8 h of unlimited access to rat chow, but only between 12 p.m. and 8 p.m. The experiment was conducted according to previous methodology for 28 days. The IF group had unlimited access to water throughout the experiment. The study monitored changes in body weight and food intake.
Starvation intervention
To assess the effects of starvation, rat chow was withheld for two weeks. Rats were only given food every 48 h for 12 h after fasting for 36 h. The rats were thus denied food for 36 consecutive hours. Rats were then only provided for 12 h. It is important to note that none of the rats died during the prolonged starvation.
Exercise intervention
According to the Szalai et al. [12] technique, the trained animals were housed in individual wheeled cages (Accelerator Ltd., Budapest, Hungery) to which they had free access 24 h a day. The exercise strategy was designed to differentiate between the health benefits of exercise and the added burden of forced exercise programs, a paradigm of voluntary balance cycling. For consistency, each animal was allowed to jog a maximum of 4 km per day.
Collection and preparation of Blood/tissue sample
The rats were put to sleep under diethyl ether anesthesia following an overnight fast at the end of the fourth (4th) week. Heparinized tubes were used to take blood from the retroorbital venous plexus. The acquired blood samples were centrifuged to obtain plasma samples. The adipokine (resistin) was assayed by ELISA reader. The dissected liver tissues were then rinsed in cold physiological saline after being freed from any attached tissue.
The rat tissues were homogenized in a Heidolph Silent Crusher M Teflon homogenizer before being centrifuged at 10,000 g for 15 min. at 4C. A spectrophotometer was used to detect the levels of neutrophils, pro-inflammatory cytokines (TNF-, IL-6, IL-1, and IL-10), liver glycogen, glycogen synthase, glycogen phosphorylase, liver transcription factor (SREBP1c and PPAR-), and oxido-nitrogen stress (LPO, nitrite, SOD, CAT, and GSH) (Shimadzu UV 1700, Kyoto, Japan).
Biochemical assay
The homogenate of rat liver tissues was used to evaluate glycogen, glycogen synthase, glycogen phosphorylase, and liver transcription factors (SREBP1c and PPAR-). The amounts were determined and quantified using ELISA (R&D Systems, USA and Thermo Fisher Scientific, respectively).
Determination of proinflammatory cytokines levels in rat liver tissue homogenate
Using the ELISA technique (pg/mg protein), the pro-inflammatory enzymes and cytokines C-reactive protein (CRP), TNF-α, and IL-1 were assessed in testicular cells (R&D Systems, USA or Thermo Fisher Scientific).
Estimation of neutrophil content in rat liver tissue homogenate
The myeloperoxidase (MPO) assay was used to count the neutrophils in the testes. The amount of polymorphonuclear neutrophils assessed correlates with the concentration of MPO, a biomarker mainly used as a tissue neutrophil accumulation [13]. Testicular tissue was centrifuged at 400 g for 10 min while being treated with 50 mM potassium PB at pH 6.0 to detect MPO activity in testes treated with a solution of 20 mM H2O2 and o-dianisidine [14]. After reading at 460 nm, the tissue MPO activity was calculated in units per gram.
Assessment of oxido-nitrergic marker and antioxidant enzymes concentration
The van Doorn et al. [15] method was used to calculate the amount of reduced glutathione (GSH) based on the development of a long-lasting yellow color when DTNB was added to sulfhydryl compounds. Nandi and Chatterjee method, a suppression of auto-oxidation of pyrogallol at pH 8.5 was used to measure the superoxide dismutase (SOD) activity [13]. The activity of catalase (CAT) was determined by assessing the tissue capacity to break down H2O2, the amount of which can be measured at 240 nm. To infer the nitrogen oxide (NO) content as a nitrite concentration, Miranda et al. [12] assay method was used to assay for nitrite level based on vanadium chloride (VC13).
Statistical analysis
Biostatistical analysis software was used to analyse the data (Graph pad prism 8 Software, Inc., Lajolla, USA, version 8.0). The data were presented using the mean and standard error of the mean (SEM). One-way ANOVA is used for multiple comparisons, followed by a post hoc test with Benferroni. For each test, the significance level was set at P less than 0.05.
Results
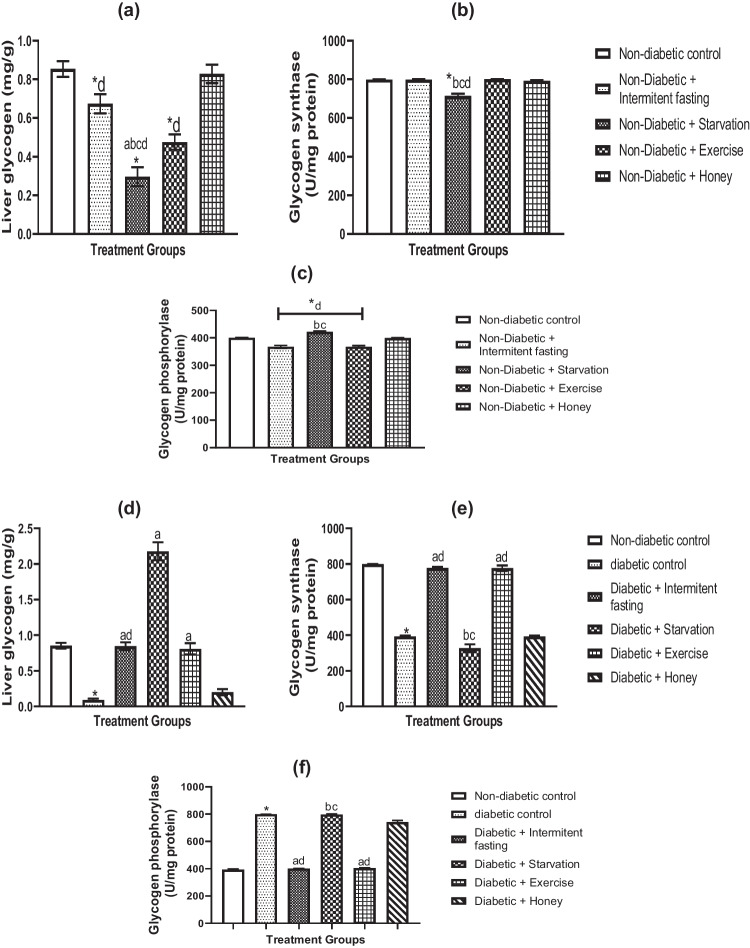
Impacts of IF, starvation, exercise and honey on liver glycogen, glycogen synthase and glycogen phosphorylase in naïve and streptozotocin-mediated diabetes in male rats
In untreated and STZ-induced diabetic male rats, the effects of fasting, exercise, IF, and honey on hepatic glycogen, glycogen synthase, and glycogen phosphorylase are depicted in Fig. 1a–c. 1af. Both IF and exercise significantly (p > 0.05) decreased liver glycogen (Fig. 1a), glycogen phosphorylase (Fig. 1c), but did not affect glycogen synthase (Fig. 1b), however decreased hepatic glycogen (Fig. 1a), increased glycogen phosphorylase (Fig. 1c) and decreased glycogen synthase (Fig. 1b).
Fig. 1.
Effects of IF, starvation, exercise and honey on liver glycogen (a, d), glycogen synthase (b, e) and glycogen phosphorylase (c, f) activities in naïve and diabetic male Wistar rats. Bars show the Mean and S.E.M. (n = 6) data. *P less than 0.05 in comparison to the control group; ap less than 0.05 in comparison to the diabetes group; bp less than 0.05 in relation to the intermittent fasting group; cp less than 0.05 in relation to the exercise group; dp less than 0.05 in relation to the honey group
As shown in Fig. 1d-f, glycogen amount, glycogen synthase activity and glycogen phosphorylase activity were determined in the livers of diabetic rats. The amount of liver glycogen (Fig. 1d) and glycogen synthase activity (Fig. 1e) were reduced dramatically (p < 0.05) in the livers of diabetic rats, but glycogen phosphorylase (Fig. 1f) was significantly (p < 0.001) elevated. These alterations were nearly restored to normal levels by IF and exercise. However, fasting and honey therapies in diabetic rats did not significantly alter glycogen phosphorylase and glycogen synthase activities other from a large increase in glycogen quantity (Fig. 1d-f).
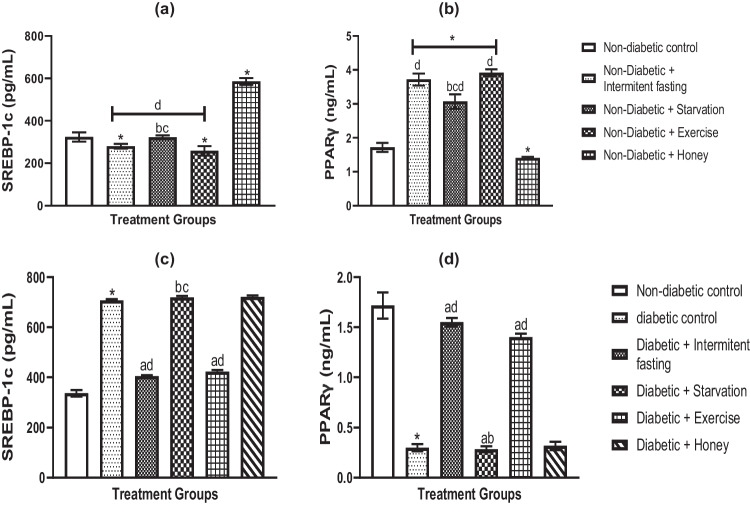
Effects of IF, starvation, exercise and honey on liver transcriptional factors (SREBP1c and PPAR-γ) in naïve and streptozotocin-induced diabetes in male rats
The pictures show how STZ-induced diabetes in male rats and naive rats affected the hepatic sterol regulatory element-binding protein 1c (SREBP1c) and peroxisome proliferator-activated receptor-y (PPAR-y). These variables were used to calculate the regulatory component of insulin signaling, glucose homeostasis, and the adiponectin promoter-mediated TNF suppressor. Exercise and IF together produced a significant (p < 0.05) reduction in SREBP1c and an increase in PPAR- γ as seen in (Fig. 2b). Liver PPAR-y levels were also increased by starvation, although not to the same level as IF and exercise, while SREBP1c levels remained unchanged compared to non-diabetic controls. Liver PPAR decreased but SREBP1c increased significantly. Compared to starvation and honey, intermittent fasting and exercise showed more pronounced significant differences in liver PPAR- γ and SREBP1c.
Fig. 2.
Effects of IF, starvation, exercise and honey on liver sterol regulatory element binding protein 1c (SREBP1c) (a, c) and peroxisome proliferator-activated receptor-γ (PPAR-γ) (b, d) in naïve and diabetic male Wistar rats. Bars show the Mean and S.E.M. (n = 6) data. *P less than 0.05 in comparison to controls. ap less than 0.05 in relation to the diabetes group. bp less than 0.05 in relation to the intermittent fasting group, and cp less than 0.05, cp less than 0.05 in relation to the exercise group. dp less than 0.05 in relation to the honey group
The livers of diabetic rats with SREBP1c and PPAR were examined, as seen in Fig. 2c-d. When compared to non-diabetic rats, diabetic rats' liver PPAR-γ concentration (Fig. 2c) was significantly lower, although SREBP1c activity (Fig. 2d) was dramatically (p 0.05) increased. Exercise and IF treatments effectively reversed STZ-induced alterations in PPAR-γ and SREBP1c levels as compared to diabetic controls. However, PPAR- and SREBP1c activity of diabetic rats did not show significant change in response to fasting or honey administration (Fig. 2c-d).
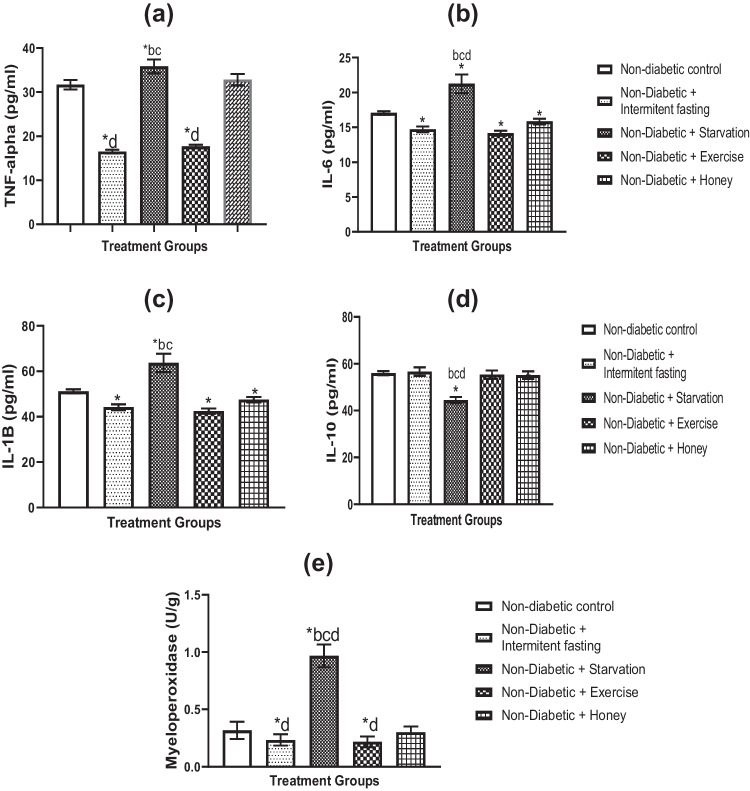
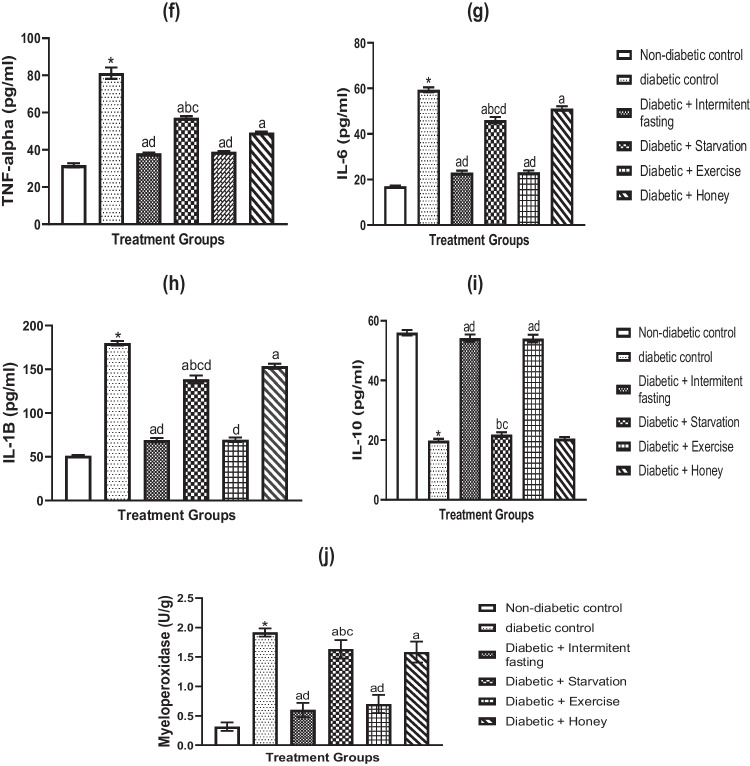
Effects of IF, starvation, exercise and honey on inflammatory enzyme and pro-inflammatory cytokines in naïve and STZ-induced diabetes in male rats
Figures 3a–e and f–j illustrate the impacts of starvation, IF, exercise, and honey on pro-inflammatory cytokines and liver enzymes in untreated and STZ–induced diabetic male rats, respectively. TNF-α (Fig. 3a), IL-6 (Fig. 3b), IL-1β (Fig. 3c) and MPO (Fig. 3e) levels were all considerably (p < 0.05) decreased by IF and exercise, however IL-10 levels were not significantly affected (Fig. 3d) in contrasts to starvation with IF, exercise, and honey. Figure 3d demonstrates that starvation markedly elevated TNF-α, IL-6, IL-1, and decreased IL-10. Rats given honey did not differ statistically significantly from non-diabetic controls in terms of TNF-α, IL-10, or MPO levels (Fig. 3a, d, e). We also found that IL-1β and IL-6 levels did not change significantly. However, the IF and exercise intervention showed a clearly significant decrease in TNF-α, IL-6, IL-1β, and MPO levels and an increase in IL-10 levels compared to starvation and honey.
Fig. 3.
a-e Effects of IF, starvation, exercise and honey on liver tumor necrotic factor-alpha (TNF-α) (a), interleukin-6 (IL-6) (b), interleukin-1 beta (IL-1β) (c), interleukin10 (IL-10) (d) and myeloperoxidase (e) concentrations in naïve male Wistar rats. Bars show Mean S.E.M. (n = 6). *P less than 0.05 in comparison to controls; bp less than 0.05 in relation to the intermittent fasting group; cp less than 0.05 in relation to the exercise group; dp less than 0.05 in relation to the honey group. f-j Effects of IF, starvation, exercise and honey on TNF-α (f), IL-6 (g), IL-1β (h), IL-10 (i) and myeloperoxidase (j) concentrations in diabetic Wistar rats. Bars shows Mean and S.E.M. (n = 6). *p less than 0.05 relative to controls; ap less than 0.05 in relation to diabetic group; bp less than 0.05 in relation to intermittent fasting group; cp < 0.05 in relation to exercise group; dp less than 0.05 relative to Honey group
Additionally, STZ exposure led to significantly increased levels of TNF-α, IL-6, IL-1, and MPO (Fig. 3f, g, h, and j) and significantly lower levels of the anti-inflammatory cytokine IL-10 (Fig. 3i). In diabetic rats, daily IF and exercise intervention significantly reduced TNF-α, IL-6, IL-1, and MPO levels (Fig. 3f, g, h, and j) while boosting the anti-inflammatory cytokine IL-10 (Fig. 3i). In comparison to IF and exercise therapy, the fasting and honey intervention groups had a less significant impact on the levels of TNF-α, IL-6, IL-1, and MPO in the liver of diabetic rats. But IL-10 levels remained unchanged.
Effects of IF, starvation, exercise and honey on lipid peroxidation and nitrergic stress in naïve and STZ-induced diabetes in male rats
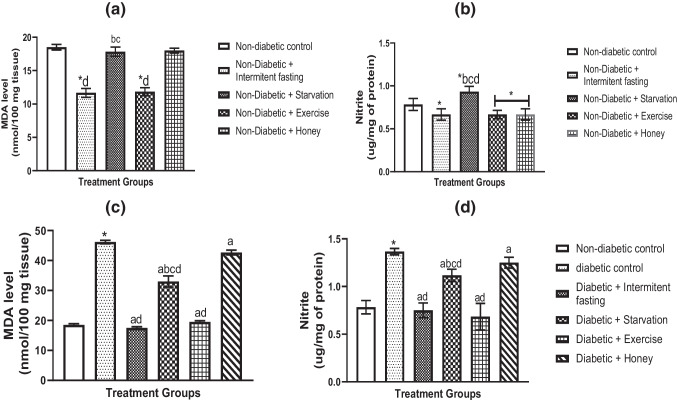
Compared to the non-diabetic control, IF and exercise significantly decreased MDA (Fig. 4a) and nitrite (Fig. 4b). While there were no variations in MDA levels compared to non-diabetic rats, fasting dramatically increased nitrite levels (Fig. 4b), which is an important distinction between IF, exercise, and honey. Exercise intervention and statistically IF were absent. Furthermore, rats treated with honey and non-diabetic controls showed no statistically significant variations in MDA levels (Fig. 4a-b).
Fig. 4.
Effects of IF, starvation, exercise and honey on liver MDA and nitrite concentration in naïve (a, b) and diabetic (c, d) male Wistar rats. Bars reflect Mean and S.E.M. (n = 6); *p less than 0.05 in relation to controls; ap less than 0.05 in relation to diabetic group; bp less than 0.05 in relation to intermittent fasting group; cp less than 0.05 in relation to exercise group. d p less than 0.05 in relation to honey group
However, diabetic rats revealed a substantial raised of oxido-nitrogen stress, as seen by elevated levels of MDA (Fig. 4c) and nitrite (Fig. 4d) in the livers of diabetic rats compared to those of the non-diabetic control group. On the other hand, daily intervention with IF and exercise in diabetic rats significantly reduced MDA and nitrite concentrations in comparison to the starvation and honey groups and diabetic rats (Fig. 4c-d).
Effects of IF, starvation, exercise and honey on antioxidant status in naïve and STZ-induced diabetes in male rats
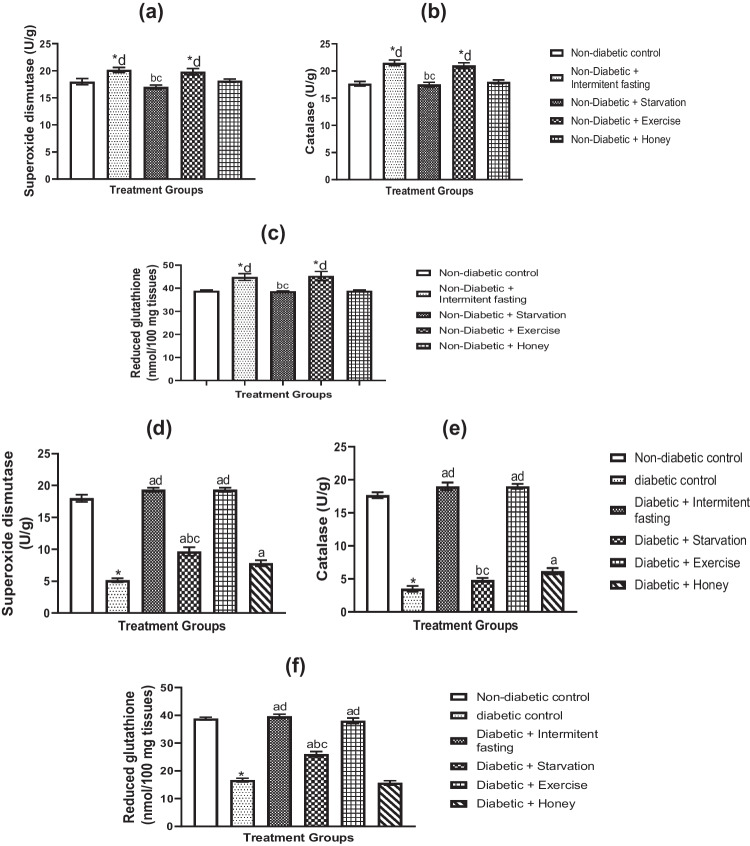
In the naive and STZ-induced diabetic rats, the effects of fasting, exercise, IF, and honey on hepatic antioxidant status (SOD, CAT, and GSH) are depicted in Fig. 5a-f. The rise in SOD, CAT, and glutathione following the intervention with IF and exercise shown in Fig. 5a–c. In comparison to non-diabetic controls, the SOD, CAT, and GSH levels in honey-fed and fasted rats were not statistically significantly different.
Fig. 5.
Effects of IF, starvation, exercise and honey on STZ-induced liver oxidative stress: SOD, CAT and GSH activities in naïve (a, b, c) and diabetic (d, e, f) male Wistar rats. Bars rillustrate Mean and S.E.M. (n = 6). *p less than 0.05 in relation to controls; ap less than 0.05 in relation to diabetic group; bp less than 0.05 in relation to intermittent fasting group; cp less than 0.05 in relation to exercise group; dp less than 0.05 in relation to honey group
When compared to non-diabetic control groups, rats exposed to STZ showed decreased levels of SOD (Fig. 5d), CAT (Fig. 5e), and GSH (Fig. 5f) in the liver. But as compared to diabetic rats, daily exposure to IF and exercise dramatically enhanced the concentrations of SOD (Fig. 5d), CAT (Fig. 5e), and GSH (Fig. 5f). Diabetes rats who received intervention with fasting and honey similarly experienced less pronounced increases in liver SOD, CAT, and GSH.
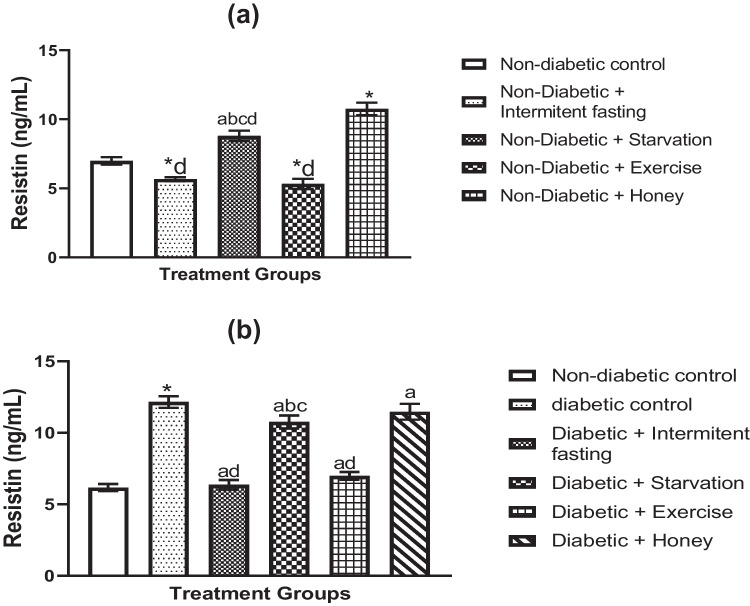
Effect of IF, starvation, exercise and honey on serum resistin in naïve and streptozotocin-induced diabetes in male rats
In male rats with naive and STZ-induced diabetes, Fig. 6a–b depicts the effects of IF, starvation, exercise, and honey on serum resistance activity. Resistance was significantly reduced as a result of the IF and exercise intervention, as seen in Fig. 6a. Starvation also produced a notable rise in resistin level when compared to non-diabetic control groups, though not to the same degree as intermittent fasting and exercise. Honey, IF and exercise intervention shown a significant shift in resistin levels. Figure 6a shows that rats given STZ had higher levels of resistin than non-diabetic controls. However, the STZ-induced rise in resistin levels was dramatically reversed by the use of IF, starvation, exercise, and honey.
Fig. 6.
Effects of IF, starvation, exercise and honey on resistin activities in naïve (a) and diabetic (b) male Wistar rats. Bars depicts Mean and S.E.M. (n = 6). *p less than 0.05 in relation to controls; ap less than 0.05 in relation to diabetic group; bp less than 0.05 in relation to exercise group; dp less than 0.05 in relation to honey group
Discussion
Diabetes, particularly T2DM, is one of the most common disabling diseases in the world and one of the most frequently associated with organ complications. Despite extensive research, the exact mechanism of diabetes pathophysiology remains complete. As a result, we investigated the role of different natural strategies (IF, starvation, and exercise intervention) as well as the effect of honey in controlling STZ-induced liver damage in rats by examining oxidative stress and inflammatory-mediated changes via hepatic transcription factors, glycogen content, and immunological factors Reaction. In this study, STZ, a popular prodiabetic agent, caused a significant decrease in liver glycogen, glycogen synthase, PPAR-γ, with a corresponding increase in SREBP1c and glycogen phosphorylase levels in the diabetic rats. However, interventions involving IF and exercise significantly reversed these changes. Also, there was an increase in LPO, nitrite, TNF-α, IL-6, IL-1ß levels but with decreased IL-10 levels after induction of diabetes by STZ. Notably, the daily intervention with IF and exercise also significantly attenuated the disturbed oxido-nitrogen status, the release of pro-inflammatory cytokine concentrations, and the increase in anti-inflammatory cytokine levels, particularly the IL-10 cytokine. Furthermore, in STZ rats, daily exposures to IF and exercise, but not honey, significantly increased the concentrations of the antioxidants, as evidenced by increased SOD, CAT, and GSH compared to STZ rats. Interestingly, starvation significantly normalized living homeostasis by decreasing liver glycogen, glycogen synthase, and increased glycogen phosphorylase, pro-inflammatory cytokines, compared to non-STZ controls.
The pathogenesis of insulin resistance, the progression of the development of T2DM, and the associated organ toxicity are all known to be significantly influenced by oxidative stress, inflammation, and other conditions collectively known as oxido-inflammation. Chronic oxido-inflammation is a cellular mechanism that is known to disrupt metabolic homeostasis and is associated with the pathophysiology of numerous metabolic diseases [16, 17]. It is characterized by increased oxidative stress and increased production of pro-inflammatory cytokines. In response to free radical overload and inflammatory reactions, there is a particular promotion of cellular and tissue dysfunction by increased migration and infiltration of macrophages into peripheral tissues such pancreatic islets, liver, and adipose tissue. As a result, insulin resistance and insulin sensitivity both decrease [18, 19]. According to the results of this study, STZ decreased the amount of hepatic glycogen and glycogen synthase while increasing glycogen phosphorylase in comparison to the non-diabetic group, indicating a deregulated hepatic metabolism. This outcome is further reinforced by other studies that demonstrate how STZ causes a decrease in the amount of glycogen synthase and hepatic glycogen in mice [20, 21]. However, IF and exercise have been found to increase hepatic glycogen content, glycogen synthase, and decreased glycogen phosphorylase in rats with STZ diabetes. The amount of glycogen in the liver is a good indicator to elucidate whether treatment with hypoglycemic agent or strategy are effective. Insulin secretion and activity increases glycogen synthase while inhibiting glycogen phosphorylase. Thus, resulting in tissue glycogen accumulation [22].
In male rats with naive and STZ-induced diabetes, the effects of IF, starvation, exercise, and honey on serum resistance activity are differentially expressed. Resistance was significantly lowered by the IF and exercise intervention. Starvation also caused a sharp rise in resistin level when compared to non-diabetic control groups, however not to the same extent as IF and exercise intervention. Rats given STZ exhibit higher levels of resistin than non-diabetic controls. This is consistent with research conducted in a clinical setting [22]. Changes in glycogen levels, glycogen phosphorylase, and glycogen synthase are associated with a loss of insulin sensitivity and responses, as IF and exercise restored glycogen content, glycogen phosphorylase, and glycogen synthase activity in diabetic rats. Previous research has shown that the length and intensity of exercise controls muscle glycogenolysis and glucose metabolism [23]. A global increase in enzymes and transport proteins involved in glucose uptake and metabolism, including hormonal glucose utilization and allosteric control, has been observed post-exercise. The possibility of glucose being converted to adenosine triphosphate (ATP) formation, which improves muscle blood flow and survival, is a likely reason for the positive benefits of IF and exercise in this study. It is unlikely that the GLUT4 overexpression plays a role in the beneficial use of glucose during exercise. However, phosphofructokinase-derived processes has been identified as responsible mechanism for exercise-mediated glycolysis may mediate glycolysis during exercise [23].
It is believed that reactive oxygen species contribute to the development of diabetic complications by promoting oxidative processes. They are produced in the mitochondria of cells that have been overactive, and exposed to excess glucose [24]. The citric acid cycle in the mitochondria and the enzyme nicotinamide adenine dinucleotide phosphate oxidase (NADPH) are said to become more active when people consume large amounts of calories more than what their bodies require to function. This has been linked to the production of reactive oxygen species (ROS) [25]. However, one of the known effective methods of reducing insulin-mediated nutrient absorption through a food restriction strategy involves inhibition of mitochondrial NADPH, prevention of single electron transport to oxygen, and formation of superoxide anions. This prevents the build-up of chemicals known to increase oxidation and stress, such as pyruvates and fatty acids [26]. The sustained production of ROS has been linked to increased oxidative stress during diabetes or diabetes-related organ damage [27]. This allows aberrant glucose autoxidation and hyperglycemia-induced liver damage. Also known to be significantly affected by hyperglycemia-induced oxidative stress are glucose and fatty acid metabolism, insulin signaling, and insulin resistance CAT antioxidants than nondiabetic control rats, indicating oxidative stress. This result lends further credence to other studies that showed that elevated levels of oxidative stress are also associated with STZ-induced hyperglycemia [28]. [26]. This suggests that fluctuations in antioxidant levels affect how severely pro-oxidant-mediated organ damage is manifested. Mahmoud et al. [28] found increased lipid peroxidation and decreased antioxidant levels in the liver of type 2 diabetic rats, are consistent with our results. Additionally, the results of Ju et al. [29], who showed that IF and exercise modifies the endogenous antioxidant machinery in diabetic mice, support these results. CAT and GSH) while reducing MDA levels. As previously studied in rat brains, diabetic rats fed honey showed a small significant decrease in oxidative stress and improved antioxidant activity. Previous studies had earlier shown that honey contain numerous adaptogens such as apigenin, hesperitin, naringenin, quercetin and kaempferol known to possess antioxidant properties [24]. Honey has been shown to demonstrate potent antioxidant property in rodents and human subjects [24].
Inflammation is a key pathogenic characteristic, which is connected to hyperglycemia and malnutrition [30]. As demonstrated herein, the persistent inflammatory flux as well as the involvements of chemokine and acute phase proteins such as C-reactive protein, have been well established to cause development of insulin resistance in tissues [31]. Reduced liver TNF-α, IL-6, and IL-1, as well as elevated IL-10, have been connected to the anti-inflammatory effects of intermittent fasting and exercise on oxidative stress, indicating an hepta-protective anti-inflammatory mechanism. These findings are supported by previous studies investigations showing that diabetes-induced liver damage include promotion of release of pro-inflammatory cytokines [32]. As previously stated, STZ diabetic rats had higher levels of serum proinflammatory cytokines [3]. Consequently, insulin signaling and sensitivity are proposed to be disrupted by these cytokines. Insulin resistance, low glucose tolerance, and type 2 diabetes have all been linked to upregulation of TNF-α, IL-6, and IL-1β [33]. Notably, over-consumption of nutrients beyond the levels needed by the body have been postulated to regularly activate immune system thereby causing abnormal release of these cytokines in the systemic circulation. Proinflammatory cytokines that influence protein kinase B (PKB/AKT) and insulin receptor substrate 1 (IRS-1) phosphorylation have been shown to decrease insulin signaling [33] and overt levels of these cytokines and chemokines have been reported in the serum of diabetic patients in clinical settings. Of note, excessive production of IL-1β from pancreatic β-cells and induction of IL-1β expression in the pancreatic β-cells due to high concentration of glucose have been strongly linked to pancreatic β-cell dysfunction [34]. Furthermore, accumulation of cytokines and macrophages in adipose tissues due to tissue hypoxia have also been associated with the development of systemic inflammation, via mechanisms related to induction of endoplasmic reticulum redox stress, decreased adenosine triphosphate (ATP) concentration and induction of apoptotic induction factors, thereby resulting in β-cell apoptosis. However, it has been proposed that excessive influx of glucose and hypoxia can be attenuated by reducing feeding tendency and by conditions that stimulates oxygenation [35]. TNF-α, IL-1β and IL-6 levels were decreased in the STZ-exposed rats treated with intermittent fasting and exercise, while levels of the anti-inflammatory cytokine IL-10 were increased. The anti-inflammatory mechanisms of intermittent fasting and exercise influence their antihyperglycemic and insulin sensitizing effects. It's interesting to note that recent research has shown that exercise and intermittent fasting can both reduce low-grade inflammation and improve insulin sensitivity.
It is known that the metabolic regulation of glucose homeostasis is strongly influenced by a number of tissue transcription factors [36]. PPAR-γ, a key transcription factor involved in the growth and operation of adipose tissue, is also known to have an impact on inflammation, insulin sensitivity and adipocyte differentiation [35]. During differentiation, PPAR-γ promotes the expression of genes exclusive to adipose tissue and involved in glucose metabolism and phenotype. Furthermore, active PPAR-γ suppresses pro-inflammatory transcription factors, lowering pro-inflammatory cytokine expression and reducing adipose tissue inflammation and hypoxia [35]. Likewise, SREBP1c is a major transcription factor involved in adipogenesis, insulin sensitivity, and lipid metabolism, as well as an insulin mediator [36]. Findings indicate that SREBP-1c is highly expressed in the pancreatic β-cells and hepatocytes of animal’s exposure to different models of diabetes. While SREBP-1 gene is involved in the regulation fatty acid synthesis, SREBP-2 is specifically implicated in the synthesis of cholesterol [35]. Importantly, SREBP-1c is involved in modulating the effects of insulin on adipose and hepatocyte tissues by regulating lipogenic genes. However, altered SREBP-1c activity has been suggested in the pathogenesis of T2DM via β-cell dysfunction.
Although obesity-dependent induction of SREBP-1c is reliant on hepatic insulin signaling, the increased SREBP-1c levels in the STZ rats could be attributed to the high insulin level and resistance in T2DM. Mechanistically, TNF-β is one of the potential molecules for insulin resistance, as previously established [37–39]. By deactivating PPAR-γ activity and activating SREBP-1c, TNF-α lowered insulin sensitivity and glycogen synthase levels in diabetic rats [36]. IF has been implicated as a PPAR-γ activator in earlier investigations, which confirms our current findings. Our findings support prior study that showed that STZ causes increased SREBP-1c expression, which promotes T2DM [37–39]. However, when compared to the non-diabetic group, SREBP-1c was observed to be reduced with a corresponding rise in PPAR-γ in IF and exercise interventions in the STZ-model of induced diabetic rats. STZ-induced increase of SREBP-1c expression was decreased by IF and exercise, with enhanced PPAR-γ-mediated signaling.
The adipokine resistin, which is connected to adipose tissue dysfunction, is regulated by PPAR-γ. Resistin has been linked to inflammation, insulin resistance, eating behavior [40], and energy metabolism. Herein, STZ-induced diabetic rats had greater levels of resistin. This increase could be attributed to STZ-induced hyperglycemia, which results in PPAR-γ deactivation and an increase in SREBP, as well as a decline in glycogen content and synthase [44]. In this study, however, IF and exercise therapy were found to reduce the effects of STZ on the adipokine signaling system, as seen by lower hepatic resistin levels. These results are comparable to those of a recent study, which indicated that following fasting and exercise, hepatic resistin mRNA expression was lowered. However, some limitations of this present study include lack of evidence of liver morphological changes following intervention of diabetic rats to IF, starvation, exercise and honey, lack of liver enzyme determination as well as immunohistochemical expressions of some of the transcription factor assayed.
Conclusions
Conclusively, our findings clearly show that intermittent fasting and exercise strategies reduce streptozotocin-induced liver metabolic impairment via modulation of liver transcriptional factors, sterol biosynthesis and inhibition of pro-inflammatory cytokines, oxido-nitrergic and adipokine signaling pathway.
Acknowledgements
The authors thank the technical staff at the Department of Physiology at Delta State University in Abraka, Nigeria.
Abbreviations
- STZ
Streptozotocin,
- IF
Intermittent fasting
- TNF-α
Tumor necrotic factor-alpha,
- IL-6:
Interleukin-6
- IL-1β
Interleukin-1 beta
- IL-10
Interleukin-10
- GSH
Glutathione reductase
- SOD
Superoxide dismutase
- CAT
Catalase
- MDA
Malonaldehyde
- LPO
Lipid peroxidation
- NO
Nitric oxide,
- MPO
Myeloperoxidase
- SREBP1c
Sterol regulatory element binding protein 1c
- PPAR- γ
Peroxisome proliferator-activated receptor-y
Author contributions
Conceptualization E.A.C., N.E.K; data curation, writing original draft preparation. E.A.E., O.M.O., BBA B.O.O., EGM; review and editing BBA, O.M.O., E.G.M., N.E.K and E.V.; supervision.N.E.K.; validation N.E.K.; funding acquisition E.A.C., B.O.O and O.M.O. All authors have read and agreed to the publishing of the manuscript.
Data availability
The corresponding author can provide all of the data used in this article upon request.
Code availability
Not applicable.
Declarations
Ethical approval
The Ethical Review Committee of Delta State University gave their approval to perform this study on 09/11/2021, with the reference number REC/FBMS/DELSU/21/121.
Consent to participate
Not applicable.
Consent for publication
All authors gave their consents for the article to be published.
Conflicts of interest
There were no conflicts of interest decleared by the authors.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hudish LI, Reusch JEB, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Investig. 2019;129(10):4001–4008. doi: 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erejuwa O, Nwobodo N, Akpan J, Okorie U, Ezeonu C, Ezeokpo B, Nwadike K, Erhiano E, Abdul Wahab M, Sulaiman S. Nigerian honey ameliorates hyperglycemia and dyslipidemia in Alloxan-induced diabetic rats. Nutrients. 2016;8(3):95. doi: 10.3390/nu8030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang Y, Tang J, Guo H, Zhang JH. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPKpathway in neonatal rats. Neuropharmacology. 2018;133:415–428. doi: 10.1016/j.neuropharm.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Henson J, Yates T, Edwardson CL, Khunti K, Talbot D, Gray LJ, Davies MJ. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE. 2013;8(10):78350–007835. doi: 10.1371/journal.pone.0078350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects-a narrative review of human and animal evidence. Behav Sci (Basel, Switzerland). 2017;7(1). [DOI] [PMC free article] [PubMed]
- 8.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26(2):254–268. doi: 10.1002/oby.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almasaudi SB, El-Shitany NA, Abbas, AT, Abdel-dayem UA, Ali SS, al Jaouni SK, Harakeh S. Antioxidant, anti-inflammatory, and antiulcer potential of Manuka Honey against gastric ulcer in rats. Oxidative Med Cell Longev. 2016;3643824. [DOI] [PMC free article] [PubMed]
- 10.Akanmu M, Olowookere T, Atunwa S, Ibrahim B, Lamidi O, Adams P, Ajimuda B, Adeyemo L. Neuropharmacological effects of Nigerian honey in mice. Afr J Trad Compl Alt Med. 2011;8(3). [DOI] [PMC free article] [PubMed]
- 11.Al Aamri ZM, Ali BH. Does honey have any salutary effect against streptozotocin - induced diabetes in rats. J Diabetes Metab Disord. 2017;16(1). [DOI] [PMC free article] [PubMed]
- 12.Szalai Z, Szász A, Nagy I, Puskás LG, Kupai K, Király A, ... , Varga C. Anti-inflammatory effect of recreational exercise in TNBS-induced colitis in rats: role of NOS/HO/MPO system. Oxidative Med Cell Longev. 2014. [DOI] [PMC free article] [PubMed]
- 13.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Investig Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 14.Hillegass L, Griswold D, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–295. doi: 10.1016/0160-5402(90)90013-B. [DOI] [PubMed] [Google Scholar]
- 15.Van Doorn R, Leijdekkers CM, Henderson PT. Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology. 1978;11:225–233. doi: 10.1016/S0300-483X(78)91389-6. [DOI] [PubMed] [Google Scholar]
- 16.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 17.Evans JL, Maddux B, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 18.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 20.Whitton PD, Hems DA. Glycogen synthesis in the perfused liver of streptozotocin-diabetic rats. Biochem J. 1975;150(2):153–165. doi: 10.1042/bj1500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao PV, Pugazhenthi S, Khandelwal RL. The effects of streptozotocin-induced diabetes and insulin supplementation on expression of the glycogen phosphorylase gene in rat liver. J Biol Chem. 1995;270(42):24955–24960. doi: 10.1074/jbc.270.42.24955. [DOI] [PubMed] [Google Scholar]
- 22.Solini A, Di Virgilio F, Chiozzi P, Fioretto P, Passaro A, Fellin R. A defect in glycogen synthesis characterizes insulin resistance in hypertensive patients with type2 diabetes. Hypertension. 2001;37(6):1492–1496. doi: 10.1161/01.HYP.37.6.1492. [DOI] [PubMed] [Google Scholar]
- 23.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- 24.Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–10027. doi: 10.1074/jbc.M207787200. [DOI] [PubMed] [Google Scholar]
- 25.Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J. 1988;336(1):19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdulwahab DA, El-Missiry MA, Shabana S, Othman AI, Amer ME. Melatonin protects the heart and pancreas by improving glucose homeostasis, oxidative stress, inflammation and apoptosis in T2DM-induced rats. Heliyon. 2021;7(3):06474. doi: 10.1016/j.heliyon.2021.e06474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griendling KK, FitzGerald GA. Oxidative Stress and Cardiovascular Injury. Circulation. 2003;108(16):1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 28.Yubero-Serrano, EM, Delgado-Lista J, Peña-Orihuela P, Perez-Martinez P, Fuentes F, Marin C, Tunez I, Jose Tinahones F, Perez-Jimenez F, Roche HM, Lopez-Miranda J. Oxidative stress is associated with the number of components of. 2013 [DOI] [PMC free article] [PubMed]
- 29.El-Missiry MA, Amer MA, Hemieda FA, Othman AI, Sakr DA, Abdulhadi HL. Cardioameliorative effect of punicalagin against streptozotocin-induced apoptosis, redox imbalance, metabolic changes and inflammation. Egypt J Basic Appl Sci. 2015;2(4):247–260. [Google Scholar]
- 30.Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complicat. 2012;26(6):483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Kogel V, Trinh S, Gasterich N, Beyer C, Seitz J. Long-term glucose starvation induces inflammatory responses and phenotype switch in primary cortical rat astrocytes. J Mol Neurosci. 2021;71(11):2368–2382. doi: 10.1007/s12031-021-01800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–398. doi: 10.1093/cvr/cvx012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Shen Q, Chen Y, Pan R, Kuang S, Liu G, Sun G, Sun X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. 2017;7(1). [DOI] [PMC free article] [PubMed]
- 34.ALmohaimeed HM, Mohammedsaleh ZM, Batawi AH, Balgoon MJ, Ramadan OI, Baz HA, al Jaouni S, Ayuob NN. Synergistic anti-inflammatory and neuroprotective effects of Cinnamomum cassia and Zingiber officinale Alleviate diabetes-induced hippocampal changes in male Albino rats: structural and molecular evidence. Front Cell Dev Biol. 2021;9. [DOI] [PMC free article] [PubMed]
- 35.Bastard JP, Maachi M, van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87(5):2084–2089. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J Intern Med. 2010;269(1):16–28. doi: 10.1111/j.1365-2796.2010.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114(3):525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 38.Jayaraman S, Devarajan N, Rajagopal P, Babu S, Ganesan SK, Veeraraghavan VP, Palanisamy CP, Cui B, Periyasamy V, Chandrasekar K. Sitosterol circumvents obesity induced inflammation and insulin resistance by down-regulating IKK_/NF-_B and JNK signaling pathway in adipocytes of Type 2 diabetic rats. Molecules. 2021;26:2101. doi: 10.3390/molecules26072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kheiripour N, Karimi J, Khodadadi I, Tavilani H, Goodarzi MT, Hashemnia M. Silymarin prevents lipid accumulation in the liver of rats with type 2 diabetes via sirtuin1 and SREBP-1c. J Basic Clin Physiol Pharmacol. 2018;29(3):301–308. doi: 10.1515/jbcpp-2017-0122. [DOI] [PubMed] [Google Scholar]
- 40.Jumli MN, Nadeem MI. The Mechanistic and Pathophysiological Role of Adiponectin and Resistin towards Regulation of Food Intake and Appetite in Cardiovascular Associated Risk Factor of Metabolic Syndrome, Type 2 Diabetes - From Pathophysiology to Cyber Systems. Anca Pantea Stoian, IntechOpen, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author can provide all of the data used in this article upon request.
Not applicable.