Abstract
Objective
Intrinsic skin aging is an inevitable process with reduced extracellular matrix deposition and impaired mechanical integrity in the dermal‐epidermal junction (DEJ). Hyaluronan is one of the most promising natural ingredients. In this research, multiple mechanisms of a novel hyaluronan complex against intrinsic skin aging were revealed.
Method
Immunohistochemical analysis and enzyme‐linked immunosorbent assay were employed to evaluate the effect of low‐molecular weight sodium hyaluronan, its acetylated derivative and HA complex on expression of matrix metalloproteinase‐1 (MMP‐1) and type I collagen in normal human fibroblasts. Then, immunohistochemical analysis and hematoxylin and eosin staining was carried out to evaluate identical effects of HA complex in reconstructed skin equivalents, as well as its benefits on histological structure and DEJ.
Result
In normal human dermal fibroblasts, the hyaluronan complex, which contains low‐molecular weight sodium hyaluronate and its acetylated derivative, has synergistic effects by increasing type I collagen expression. At the same time, MMP‐1 production was inhibited. This was confirmed in subsequent experiments with skin equivalent, and intriguingly, the hyaluronan complex was also found to increase the expression of two DEJ proteins.
Conclusion
The multimechanism hyaluronan complex in this proof‐of‐concept study exhibited skin antiaging effects in vitro through inhibiting the expression of MMP‐1 and enhancing type I collagen accumulation and the expression of DEJ proteins, which reveals new avenues for investigating more biological activities of various types of hyaluronan.
Keywords: dermal‐epidermal junction, extracellular matrix, hyaluronan complex, intrinsic skin aging, multiple mechanisms
1. INTRODUCTION
As the largest organ of the human body, the skin serves as the foremost natural barrier to the outside world. 1 It is critical in protecting the body against pathogenic microorganisms, regulating water loss, and preventing solar ultraviolet radiation or chemical penetration. 2 Additionally, healthy skin is a manifestation of beauty. 3
The skin is divided into three major layers anatomically,epidermis, dermis and hypodermis. 4 The epidermis is made up of two layers: the outermost stratum corneum (10–30 μm, which contains dying and flattened dead cells) and a living keratinocytes layer (100–150 μm, the active functional part of the epidermis). The dermis (1.5–4 mm in different body areas) can be divided into two sublayers, from the outside to the inside: the papillary layer, the reticular layer respectively. 5 The thinner papillary dermis has a higher fibroblast density, whereas the much thicker reticular dermis is mostly composed of extracellular matrix (ECM) proteins released by fibroblasts, and the deepest white adipocytes‐rich hypodermis connects the skin to the muscle and fascia. 6 Notably, the epidermis and dermis are connected by a highly specialized ECM basement membrane termed the dermal‐epidermal junction (DEJ), which acts as both structural support and a specific signaling niche in cell‐matrix interactions. 7
The skin thins, 8 dries, 9 wrinkles, and looses resilience when the inevitable and progressive physiological process of intrinsic or chronological skin aging happens, and the mechanisms of it are currently believed to be predominantly due to changes in the ECM and DEJ. 10 , 11 , 12
Despite its heterogeneity, the skin ECM is primarily composed of fibrillar collagen and elastin, these proteins offer dermal strength and flexibility, as well as provide the skin's scaffolding fibers to maintain the structural integrity of the tissue. 13 Different types of collagens (mainly types I and III) and elastin are tightly connected and interwoven by a specific glycosaminoglycan (GAG) termed hyaluronan (HA, also known as hyaluronic acid). 14 Given the crucial role of the ECM in maintaining tissue integrity, altering its deposition can lead to tissue dysfunction, 15 aging, 16 and even disease. 17 The molecular mechanisms of these alterations in the ECM are collagen fragmentation and density reduction, 18 as well as a decrease in the renewal of the collagen cross‐linking proteins fibrillin, elastin, 19 and HA. 20 , 21 , 22 Collagen fragmentation, one of the most conspicuous hallmarks of skin aging, 23 is initially caused by matrix metalloproteinase‐1 (MMP‐1). 24 , 25 MMPs are ECM‐degrading zinc‐containing endopeptidases, their activity increases with biological age, affecting ECM deposition, leading to ECM remodeling and consequent skin aging. 26 , 27 , 28 , 29 MMP‐1 is up‐regulated in dermal fibroblasts by the c‐Jun/AP‐1 signaling pathway during intrinsic skin aging 30 and is responsible for increased collagen I and III fragmentation. 31 , 32
As for DEJ, it is rich in collagens, laminins, fibrillins, nidogen, and heparan sulfate proteoglycan perlecan, of which laminin‐332 (previously known as laminin 5) is its fundamental component. 33 , 34 Laminin‐332 is a heterotrimeric glycoprotein comprised of α3, β3 and γ2 chains encoded by the genes LAMA3, LAMB3, and LAMC2, respectively. 35 , 36 It interconnects with collagen XVII and integrin α6β4 on the membranes of basal epidermal cells, as well as collagen VII and anchoring filaments originating from hemidesmosomes in the ECM to construct a complex fibrillar meshwork that provides the most fundamental biomechanical support for DEJ. 33 Fibrillin‐1, another component in DEJ, 37 is involved in the formation of fibrillin microfibrils, which insert vertically from the deep dermis into the basement membrane and is one of the proteins that impart skin elasticity. 38 The decline in the distribution of proteins including laminin‐332 39 and fibrillin‐1 40 was reported in intrinsically aged skin. This may account for the thinning of the DEJ in aged skin and partially explains the increased skin fragility.
Albeit intrinsic skin aging is inevitable, inhibiting ECM remodeling by preserving its deposition while enhancing structural proteins expression in the DEJ has the potential to thwart this process, and HA, a traditional ingredient in cosmetic and aesthetic medicine, may offer a brand‐new option. HA is a linear GAG composed of simple repeats of N‐acetyl‐glucosamine β‐(1‐4) and glucuronic acid β‐(1‐3) disaccharide units. 41 As the most abundant component of the ECM (concentration of 0.5 mg/kg) and with the capacity to retain 1000 times its own weight in water, 42 HA is not just a filler, but it can also affect physiological and pathological processes such as fibroblast migration, inflammation, aging, wound healing, and tumor invasion by binding to proteins including cell surface receptors (CD44, LYVE1, RHAMM, etc.) as well as matrix proteins. 43 , 44 In recent years, HA has grown in popularity and is now the most widely used cosmetic ingredient due to its versatility and nonimmunogenicity. However, the antiaging mechanisms of HA still need to be explored, which is not only valuable to the research on its biological activities, but also of tremendous benefit to the development of new products.
In this study, a novel HA complex combining low molecular weight sodium hyaluronate (30 kDa) and its acetylated derivatives (Figure 1) of the same molecular weight was created. It has been shown to significantly increase type I collagen accumulation by suppressing MMP‐1 production and enhancing type I collagen secretion in fibroblasts. In addition to the HA complex having comparable effects as described above, the structural proteins laminin‐332 and fibrillin‐1 in the DEJ were markedly elevated in the reconstructed full‐thickness skin equivalent. In conclusion, preventing or reversing the age‐related alterations in the ECM and DEJ composition and architecture remains a significant challenge, by integrating HA with different structures, the multiple‐mechanism HA complex works synergistically, shedding light on skin rejuvenation.
FIGURE 1.

The structures of sodium hyaluronate and its acetylated form.
2. MATERIALS AND METHODS
2.1. Materials
Sodium hyaluronate (HA) and acetylated sodium hyaluronate (AcHA) are commercial products provided by Bloomage Biotechnology Co., Ltd., their average molecular weight is 30 kDa, and the acetyl substitution degree of acetylated sodium hyaluronate is 75%. The normal human dermal fibroblast (NHDF) and human epidermal keratinocyte (HEKn) were provided by American Type Culture Collection.
2.2. Cell culture
The NHDF cells were cultured in Dulbecco's modified Eagle's medium (Gibco), supplied with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C with a humid atmosphere containing 5% CO2. When reaching the density of 70%−80%, cells were digested with 0.05% trypsin (Gibco) and seeded into 24‐well or 96‐well plates for further investigation.
2.3. Enzyme‐linked immunosorbent assay
The enzyme‐linked immunosorbent assay (ELISA) was used to determine the quantity of type I collagen secretion. NHDF cells were digested and seeded into a 96‐well plate after achieving a density of 70%−80%, then HA and AcHA at the concentration of 0.0001% (wt%) were added separately. After incubation for 48 h, the supernatant was collected and centrifuged at 1000 rpm for 5 min. The content of type I collagen in the supernatant was then quantified using Type I Collagen ELISA Kit (CUSABIO) according to the protocol. The total protein of cells was extracted by using RIPA buffer (Solarbio). The quantity of total protein was measured by using a BCA kit (ThermoFisher Scientifc). The final amount of type I collagen secretion of each group was normalized by the quantity of total protein. All the data were analyzed with an unpaired t‐test, p < 0.05 was identified as a statistical difference.
2.4. Cellular immunohistochemical analysis
The quantity of MMP‐1 was measured by the cell immunofluorescence method. NHDF cells were digested and seeded into a 24‐well plate after achieving a density of 70%−80%, then HA and AcHA at the concentration of 0.0001% (wt%) and 0.05% (wt%) were added separately. After incubation for 48 h, cells were fixed with cold methyl alcohol for 15 min. Then the supernatant was discarded. Cells were washed with phosphate buffered solution (PBS). 5% Bovine serum albumin (BSA) was added as a blocking solution and incubated for 1 h at room temperature. After that, the anti‐MMP‐1 antibody (Abcam, ab138492) diluted by blocking solution was added and incubated overnight at 4°C. On the following day, cells were washed with PBS and incubated with the secondary antibody for 1.5 h at room temperature. Cells were covered with mounting medium containing DAPI. Photos were taken under a fluorescence microscope. The mean fluorescence intensity of every photograph was analyzed using ImageJ software. All the data were analyzed with an unpaired t‐test. p < 0.05 was identified as a statistical difference.
2.5. Establishment of reconstructed full‐thickness skin equivalent
The reconstructed full‐thickness human skin equivalent is composed of normal human keratinocytes and human dermal fibroblasts that have been fused together via tissue engineering to generate a highly differentiated dermal‐epidermal complex that is regularly utilized as an active screening model. The establishment of reconstructed full‐thickness skin equivalents was carried out according to the Gangatirkar's protocol. 45 NHDF cells mixed with collagen were cultured to establish dermis. After collagen lattice was formed, HEKn were seeded onto the surface of collagen lattice and media was changed every 2–3 days for the next 7 days. Then the skin equivalents were cultivated at an air‐liquid interface for 8 days and media was changed every 2–3 days for the next 8 days. On the last time of renewing culture medium in the stage of liquid cultivation, 0.1% (wt %) HA complex was added into the culture medium until the air‐liquid culture was ended.
2.6. Histology and immunohistochemical analysis
At the end of the air‐liquid cultivation, skin equivalents were fixed with 4% paraformaldehyde (PFA). Dehydration was carried out by gradient ethanol, followed by paraffin embedding. Sections were cut into slices of 5 μm and mounted onto the glass slides. After deparaffinized with methyl cyclohexane and rehydrated with decreasingly gradient ethanol, the tissue sections were stained with hematoxylin and eosin stain (H&E). Observation and photography were performed under a microscope.
For immunohistochemical analysis, skin equivalents were coated with O.C.T and cut into slices of 5 μm and mounted onto the glass slides. The sections were fixed with cold methyl alcohol for 15 min. BSA (5%) was added as a blocking solution and incubated for 1 h at room temperature. After that, the anti‐type I collagen antibody (Abcam, ab138492), anti‐MMP‐1 antibody (Abcam, ab52631), anti‐laminin‐332 antibody (Abcam, ab78286), and anti‐fibrillin‐1 antibody (Sigma, MAB2499) diluted by blocking solution were added separately and incubated overnight at 4°C. On the following day, sections were washed with PBS and incubated with the corresponding secondary antibody for 1.5 h at room temperature, then covered with a mounting medium containing DAPI. Photos were taken under a fluorescence microscope. The mean fluorescence intensity of every photograph was analyzed using ImageJ software. All the data were analyzed with an unpaired t‐test, p < 0.05 was identified as a statistical difference.
2.7. Statistical analysis
P values were calculated with Student's t‐test. p < 0.05 was considered to be statistically significant, and p values are designated as follows: *p < 0.05; **p < 0.01. Values are expressed as mean ± SD of calculated from at least three replicates.
3. RESULTS
3.1. Inhibitory effect of sodium hyaluronan on MMP‐1 expression in fibroblasts
The inhibitory effect of sodium hyaluronate (HA) and acetylated sodium hyaluronate (AcHA) on normal human dermal fibroblasts (NHDF) MMP‐1 was determined by the cell immunofluorescence method. The expression of MMP‐1 decreased after fibroblasts were treated with HA for 48 h, while the corresponding indexes did not decrease significantly in the AcHA group after treatment (Figure 2A). Quantitatively, 0.0001% and 0.05% HA reduced the expression of MMP‐1 in fibroblasts by 12.6% and 19.9%, respectively, while there was no significant change in the AcHA treated group at the same
FIGURE 2.
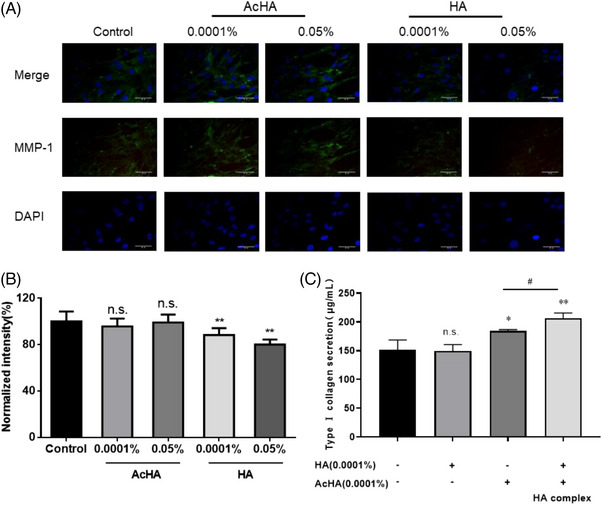
Reduction of matrix metalloproteinase‐1 (MMP‐1) expression and enhancement of type I collagen secretion in normal human dermal fibroblast (NHDF). (A and B) Inhibitory effects of sodium hyaluronate (HA) and acetylated sodium hyaluronate (AcHA) on MMP‐1 expression in NHDF. (A) Decreased MMP‐1 expression was observed in 0.0001% and 0.05% HA treated NHDF by cellular immunohistochemical analysis compared to AcHA groups and the control group (scale bar = 50 μm). (B) Normalized intensity analysis by ImageJ software showed a significant decrease in 0.0001% and 0.05% HA treated groups. (C) Enzyme‐linked immunosorbent assay (ELISA) showed increased type I collagen secretion in 0.0001% AcHA and 0.0001% HA and AcHA co‐treated groups, and the latter indicated a synergistic effect. Values are expressed as mean ± SD. P values were determined by unpaired t‐test. *Significance between control and treated group; #significance between AcHA and HA complex treated group (n = 3, n.s., no significance; *p < 0.05; **p < 0.01; #p < 0.05).
3.2. Quantitative determination of type I collagen secretion in fibroblasts
Fibroblasts type I collagen secretion was quantified by ELISA. The 0.0001% AcHA treatment group had a 21.5% up‐regulation on the secretion of type I collagen; while 0.0001% HA had no significant effect on the synthesis of type I collagen; 0.0001% AcHA was incorporated with the same concentration of HA to yield 0.0002% HA complexes, which increased type I collagen secretion by 36.5% (Figure 2C). A between‐group t‐test (p < 0.05) was performed between the AcHA‐treated group and the HA complex‐treated group, confirming that the combination of HA and AcHA had a synergistic effect.
3.3. Effects of HA complex in skin equivalent
The reconstructed full‐thickness skin equivalents have the same epidermal, DEJ and dermal structures as normal human skin, equivalent to in vivo environments, and are routinely employed for in vitro efficacy evaluation of skincare products or pharmaceuticals. After 11 days of treatment of the skin equivalents with the 0.1% HA complex, H&E staining demonstrated a rise in their epidermal thickness, as well as enhanced tightness between basal keratinocytes (Figure 3A). Consistent with the above results, immunofluorescence assays of frozen tissue sections revealed that HA complexes increased type I collagen expression by 45.3% (p < 0.001) (Figure 3B, C), while dermal MMP‐1 expression was significantly reduced by approximately 10.7% (p < 0.01) (Figure 3D, E).
FIGURE 3.
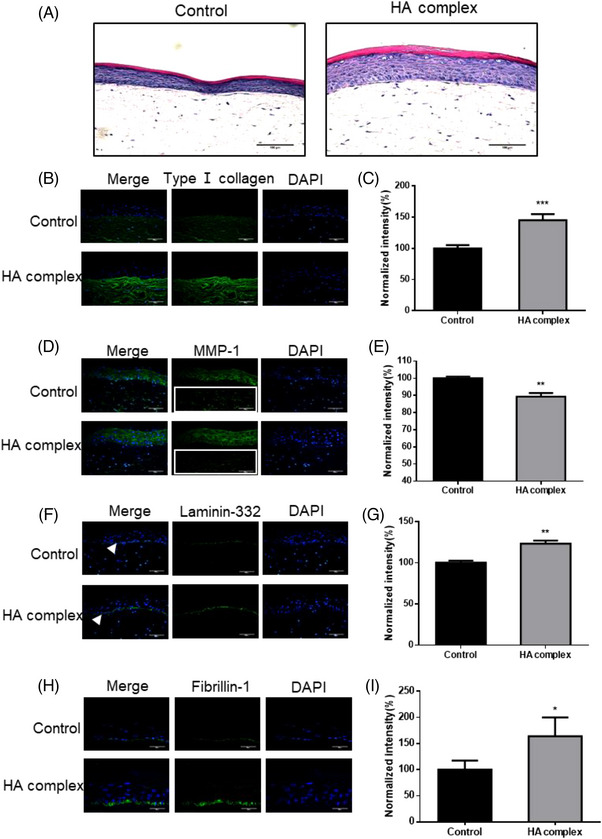
The beneficial effects of HA complex on reconstructed full‐thickness skin equivalents. (A) H&E staining in reconstructed full‐thickness skin equivalents. Increased epidermal thickness and tightness of basal keratinocytes were observed in 0.1% HA complex treated skin equivalent (scale bar = 100 μm). (B–I) Qualitative and quantitative analysis of the expression changes of type I collagen and other proteins in skin equivalents by immunohistochemical method. (B and C) Type I collagen expression was enhanced in the 0.1% HA complex treated group (scale bar = 100 μm). (D and E) Dermal matrix metalloproteinase‐1 (MMP‐1) expression was inhibited in 0.1% HA complex treated group (scale bar = 100 μm). (F and G) Laminin‐332 expression was increased in the DEJ in 0.1% HA complex treated group (scale bar = 100 μm). (H and I) Fibrillin‐1 expression was also observed to be significantly increased (scale bar = 50 μm). Normalized intensity analysis by ImageJ software showed a significant increase in type I collagen expression (C), decrease in dermal MMP‐1 expression (E), and enhancement in laminin‐332 (G) and fibrillin‐1 (I) on DEJ, respectively. Values are expressed as mean ± SD. P values were determined by unpaired t‐test. (n = 3, *p < 0.05; **p < 0.01; ***p < 0.001).
3.4. Effects of HA complex on DEJ in skin equivalent
After treatment of skin equivalent with 0.1% HA complex for 11 days, immunofluorescence assays revealed a more compact and continuous DEJ structure (Figure 3F, H), in which laminin‐332 expression was significantly increased by 23.3% (p < 0.01) (Figure 3G), the expression of fibrillin‐1 was significantly increased by 64.0% (p < 0.05) (Figure 3I).
4. DISCUSSION
Intrinsic skin aging is a natural physiological process and slowing or even reversing this process will be a significant challenge for a long time to come, both for academia and the beauty industry. In this study, a novel complex combining low molecular weight sodium hyaluronate (30 kDa) and its acetylated derivatives was developed with the potential to address this issue. The effects of the HA complex on type I collagen accumulation through inhibiting MMP‐1 production and increasing type I collagen secretion in fibroblasts were revealed. For further verification, the reconstructed full‐thickness skin equivalents were used, and not only consistent results were obtained, but also intriguingly, the role of promoting DEJ protein expression was discovered for the first time, particularly as a significant increase of laminin‐332 (p < 0.01) and fibrillin‐1 (p < 0.05).
The tissue‐strengthening properties of the versatile HA have been extensively demonstrated. Quan et al. reported the effects of a cross‐linked HA to stimulate type I collagen production in fibroblasts to strengthen the structural support of the ECM by injecting it into the skin of individuals over 70 years of age. 46 Wu et al. discovered that the expression of MMP‐1 and −3 were significantly downregulated (p < 0.001) after treatment of IL‐1β‐stimulated rat tenocytes with high molecular weight HA (3000 kDa, 2.5 mg/mL). 47 Based on the abovementioned works, we postulated that low molecular weight HA may have similar effects on collagen secretion and MMPs expression, and thus be used to combat the intrinsic skin aging.
To test this hypothesis, fibroblasts were treated separately with sodium hyaluronate (30 kDa) and its acetylated derivatives of the same molecular weight. In immunofluorescence assays, sodium hyaluronate reduced MMP‐1 expression by 12.6% (p < 0.01) at the concentration of 0.0001%, and by 19.9% at the concentration of 0.05%, suggesting a dosage correlation. At the same concentrations, acetylated sodium hyaluronate had no obvious effect. The two kinds of HA showed reversal effects in the ELISA experiments quantifying type I collagen secretion. Acetylated sodium hyaluronate at the concentration of 0.0001% significantly increased fibroblast type I collagen secretion by 21.5%, whereas the same concentration of sodium hyaluronate had no obvious effect. It is easy to deduce that combining them may be beneficial. As expected, 0.0002% HA complex increased type I collagen secretion by 36.5%, indicating a synergistic effect. This may be owing to that while acetylated sodium hyaluronate stimulates type I collagen secretion, sodium hyaluronate suppresses the expression of MMP‐1 and prevents type I collagen fragmentation.
Next, the reconstructed full‐thickness skin equivalents were treated with 0.1% HA complex and increased epidermal thickness was observed in H&E staining experiments, as was basement membrane tightness. After immunofluorescence analysis, it was revealed that dermal MMP‐1 expression in the dermis was substantially down‐regulated by 10.7%, while collagen I deposition was significantly boosted by up to 45.3%. More notably, laminin‐332 and fibrillin‐1 were significantly increased in DEJ by 23.3% and 64.0%, respectively. This is the first time that HA has been found to have a DEJ‐enhancing effect.
Taken together, this work demonstrated via cellular and skin equivalents that the novel HA complex combines the advantages of two low‐molecular‐weight HA that increase ECM deposition and enhance DEJ structural strength simultaneously. The ECM remodeling driven by the loss of deposition and the weakening of mechanical strength induced by the reduction of DEJ proteins expression are the key factors for intrinsic skin aging. Therefore, the multimechanism HA complex has a potential antiaging effect. In addition, given the detrimental feature of ECM remodeling in wound repair 48 and cancer metastasis, 49 the HA complex may also play a role.
Although the HA complex exhibited robust antiaging effects in fibroblast and skin equivalent, several limitations in this research must be addressed. First, this study does not incorporate molecular biology research on the multiple mechanisms of HA complex and the synergistic phenomenon. Besides, to be more persuasive, the effects of HA complex against intrinsic skin aging remain to be validated by animal models and even clinical trials. Finally, in addition to the traditional external application and injection, oral administration of HA has also been demonstrated to be feasible, 50 and further study on the administration method of the HA complex can be carried out. We believe this work will act as an excellent starting point to launch such experiments.
5. CONCLUSION
In conclusion, this research revealed that the novel low molecular weight HA complex can simultaneously suppress MMP‐1 expression and promote type I collagen, laminin‐332 and fibrillin‐1 production. This robust improvement of ECM deposition and enhanced DEJ structural strength through multiple mechanisms has potential ameliorating effects on intrinsic skin aging. Even if more in‐depth mechanism investigations are required, this work not only opens a new avenue for the exploration of the biological activities of HA and moreover establishes a theoretical foundation for the development of new antiaging products.
ETHICS STATEMENT
An ethics statement was not required for this study type; no human or animal subjects were use.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial supports by Bloomage Biotechnology Corporation Limited.
Chen F, Guo X, Wu Y. Skin antiaging effects of a multiple mechanisms hyaluronan complex. Skin Res Technol. 2023;29:e13350. 10.1111/srt.13350
Contributor Information
Xueping Guo, Email: guoxp@Bloomagebiotech.com.
Yue Wu, Email: wuy@Bloomagebiotech.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to their containing information that could compromise the data privacy of Bloomage Biotechnology Co., Ltd but are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rittié, L , Fisher, GJ . Natural and sun‐induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. doi: 10.1101/cshperspect.a015370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers, ES , Vukmanovic‐Stejic, M . Skin barrier immunity and ageing. Immunology. 2020;160(2):116–125. doi: 10.1111/imm.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajgai, J , Lee, K‐J , Rahman, MH , Fadriquela, A , Kim, C‐S . Role of molecular hydrogen in skin diseases and its impact in beauty. Curr Pharm Des. 2021;27(5):737–746. doi: 10.2174/1381612826666200925124235 [DOI] [PubMed] [Google Scholar]
- 4. Watt, FM . Mammalian skin cell biology: at the interface between laboratory and clinic. Science (New York, N.Y.). 2014;346(6212):937–940. doi: 10.1126/science.1253734 [DOI] [PubMed] [Google Scholar]
- 5. Woodley, DT . Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol Clin. 2017;35(1):95–100. doi: 10.1016/j.det.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 6. Lephart, ED . Skin aging and oxidative stress: equol's anti‐aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31:36–54. doi: 10.1016/j.arr.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Roig‐Rosello, E , Rousselle, P . The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules. 2020;10(12):1607. doi: 10.3390/biom10121607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Purohit, T , He, T , Qin, Z , et al. Smad3‐dependent regulation of type I collagen in human dermal fibroblasts: impact on human skin connective tissue aging. J Dermatol Sci. 2016;83(1):80–83. doi: 10.1016/j.jdermsci.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 9. Farage, MA , Miller, KW , Elsner, P , Maibach, HI Structural characteristics of the aging skin: a review. Cutaneous and Ocular Toxicology. 2007;26(4):343–357. doi: 10.1080/15569520701622951 [DOI] [PubMed] [Google Scholar]
- 10. Shin, J‐W , Kwon, SH , Choi, JY , et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20(9):2126. doi: 10.3390/ijms20092126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong, S , Yoon, S , Kim, S , et al. Anti‐wrinkle benefits of peptides complex stimulating skin basement membrane proteins expression. Int J Mol Sci. 2019;21(1):73. doi: 10.3390/ijms21010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newton, VL , Bradley, RS , Seroul, P , et al. Novel approaches to characterize age‐related remodelling of the dermal‐epidermal junction in 2D, 3D and in vivo. Skin Res Technol. 2017;23(2):131–148. doi: 10.1111/srt.12312 [DOI] [PubMed] [Google Scholar]
- 13. Lynch, MD , Watt, FM . Fibroblast heterogeneity: implications for human disease. J Clin Invest. 2018;128(1):131‐148. doi: 10.1172/JCI93555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watt, FM , Fujiwara, H . Cell‐extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011;3(4):a005124. doi: 10.1101/cshperspect.a005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wynn, TA , Vannella, KM . Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kohl, E , Steinbauer, J , Landthaler, M , Szeimies, R‐MS . Skin ageing. JEADV. 2011;25(8):873–884. doi: 10.1111/j.1468-3083.2010.03963.x [DOI] [PubMed] [Google Scholar]
- 17. Kaur, A , Ecker, BL , Douglass, SM , et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9(1):64–81. doi: 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher, GJ , Sachs, DL , Voorhees, JJ . Ageing: collagenase‐mediated collagen fragmentation as a rejuvenation target. Br J Dermatol. 2014;171(3):446–449. doi: 10.1111/bjd.13267 [DOI] [PubMed] [Google Scholar]
- 19. Amano, S . Characterization and mechanisms of photoageing‐related changes in skin. Damages of basement membrane and dermal structures. Exp Dermatol. 2016;25 Suppl 3):14–19. doi: 10.1111/exd.13085 [DOI] [PubMed] [Google Scholar]
- 20. Tzellos, TG , Klagas, I , Vahtsevanos, K , et al. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009;18(12):1028–1035. doi: 10.1111/j.1600-0625.2009.00889.x [DOI] [PubMed] [Google Scholar]
- 21. Rinnerthaler, M , Bischof, J , Streubel, MK , Trost, A , Richter, K . Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi: 10.3390/biom5020545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee, DH , Oh, J‐H , Chung, JH . Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83(3):174–181. doi: 10.1016/j.jdermsci.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 23. Quan, T , Fisher, GJ . Role of age‐associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini‐review. Gerontology. 2015;61(5):427–434. doi: 10.1159/000371708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia, W , Hammerberg, C , Li, Y , et al. Expression of catalytically active matrix metalloproteinase‐1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell. 2013;12(4):661–671. doi: 10.1111/acel.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher, GJ , Quan, T , Purohit, T , et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase‐1 in fibroblasts in aged human skin. Am J Pathol. 2009;174(1):101–114. doi: 10.2353/ajpath.2009.080599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freitas‐Rodríguez, S , Folgueras, AR , López‐Otín, C . The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochimica et Biophysica Acta. Molecular cell Research. 2017;1864(11 Pt A):101‐114. doi: 10.1016/j.bbamcr.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 27. Sbardella, D , Fasciglione, GF , Gioia, M , et al. Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med. 2012;33(2):119–208. doi: 10.1016/j.mam.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 28. Fligiel, SEG , Varani, J , Datta, SC , Kang, S , Fisher, GJ , Voorhees, JJ . Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase‐1 in vitro. J Invest Dermatol. 2003;120(5):842–848. doi: 10.1046/j.1523-1747.2003.12148.x [DOI] [PubMed] [Google Scholar]
- 29. Panwar, P , Butler, GS , Jamroz, A , Azizi, P , Overall, CM , Brömme, D . Aging‐associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018;65:842‐848. doi: 10.1016/j.matbio.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 30. Qin, Z , Voorhees, JJ , Fisher, GJ , Quan, T . Age‐associated reduction of cellular spreading/mechanical force up‐regulates matrix metalloproteinase‐1 expression and collagen fibril fragmentation via c‐Jun/AP‐1 in human dermal fibroblasts. Aging Cell. 2014;13(6):30‐44. doi: 10.1111/acel.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naylor, EC , Watson, REB , Sherratt, MJ. Molecular aspects of skin ageing. Maturitas. 2011;69(3):249–256. doi: 10.1016/j.maturitas.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 32. Dumas, M , Chaudagne, C , Bonté, F , Meybeck, A . In vitro biosynthesis of type I and III collagens by human dermal fibroblasts from donors of increasing age. Mech Ageing Dev. 1994;73(3):179–187. doi: 10.1016/0047-6374(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 33. Aumailley, M . Laminins and interaction partners in the architecture of the basement membrane at the dermal‐epidermal junction. Exp Dermatol. 2021;30(1):17–24. doi: 10.1111/exd.14239 [DOI] [PubMed] [Google Scholar]
- 34. Watson, RE , Parry, EJ , Humphries, JD , et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138(6):931–937. doi: 10.1046/j.1365-2133.1998.02257.x [DOI] [PubMed] [Google Scholar]
- 35. Hirsch, T , Rothoeft, T , Teig, N , et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–332. doi: 10.1038/nature24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugawara, K , Tsuruta, D , Ishii, M , Jones, JCR , Kobayashi, H . Laminin‐332 and ‐511 in skin. Exp Dermatol. 2008;17(6):327‐332. doi: 10.1111/j.1600-0625.2008.00721.x [DOI] [PubMed] [Google Scholar]
- 37. Marionnet, C , Pierrard, C , Vioux‐Chagnoleau, C , Sok, J , Asselineau, D , Bernerd, F . Interactions between fibroblasts and keratinocytes in morphogenesis of dermal epidermal junction in a model of reconstructed skin. J Invest Dermatol. 2006;126(5):971–979. doi: 10.1038/sj.jid.5700230 [DOI] [PubMed] [Google Scholar]
- 38. Adamo, CS , Zuk, AV , Sengle, G . The fibrillin microfibril/elastic fibre network: a critical extracellular supramolecular scaffold to balance skin homoeostasis. Exp Dermatol. 2021;30(1):25–37. doi: 10.1111/exd.14191 [DOI] [PubMed] [Google Scholar]
- 39. Langton, AK , Halai, P , Griffiths, CEM , Sherratt, MJ , Watson, REB . The impact of intrinsic ageing on the protein composition of the dermal‐epidermal junction. Mech Ageing Dev. 2016;156:14‐16. doi: 10.1016/j.mad.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 40. Watson, RE , Griffiths, CE , Craven, NM , Shuttleworth, CA , Kielty, CM . Fibrillin‐rich microfibrils are reduced in photoaged skin. Distribution at the dermal‐epidermal junction. J Invest Dermatol. 1999;112(5):782–787. doi: 10.1046/j.1523-1747.1999.00562.x [DOI] [PubMed] [Google Scholar]
- 41. Day, AJ , Prestwich, GD . Hyaluronan‐binding proteins: tying up the giant. J Biol Chem. 2002;277(7):4585–4588. doi: 10.1074/jbc.R100036200 [DOI] [PubMed] [Google Scholar]
- 42. Anderegg, U , Simon, JC , Averbeck, M . More than just a filler ‐ the role of hyaluronan for skin homeostasis. Exp Dermatol. 2014;23(5):295–303. doi: 10.1111/exd.12370 [DOI] [PubMed] [Google Scholar]
- 43. Šínová, R , Pavlík, V , Ondrej, M , Velebný, V , Nešporová, KH . A key player or just a bystander in skin photoaging? Exp Dermatol. 2022;31(4):442–458. doi: 10.1111/exd.14491 [DOI] [PubMed] [Google Scholar]
- 44. Vasvani, S , Kulkarni, P , Rawtani, D . Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. 2020;151:1012‐1029. doi: 10.1016/j.ijbiomac.2019.11.066 [DOI] [PubMed] [Google Scholar]
- 45. Gangatirkar, P , Paquet‐Fifield, S , Li, A , Rossi, R , Kaur, P . Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc. 2007;2(1):178–186. doi: 10.1038/nprot.2006.448 [DOI] [PubMed] [Google Scholar]
- 46. Quan, T , Wang, F , Shao, Y , et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133(3):658–667. doi: 10.1038/jid.2012.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu, Po‐T , Kuo, Li‐C , Su, F‐C , et al. High‐molecular‐weight hyaluronic acid attenuated matrix metalloproteinase‐1 and ‐3 expression via CD44 in tendinopathy. Sci Rep. 2017;7:40840. doi: 10.1038/srep40840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lagares, D , Ghassemi‐Kakroodi, P , Tremblay, C , et al. ADAM10‐mediated ephrin‐B2 shedding promotes myofibroblast activation and organ fibrosis. Nat Med. 2017;23(12):1405–1415. doi: 10.1038/nm.4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuzhalin, AE , Lim, SY , Kutikhin, AG , Gordon‐Weeks, AN . Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochimica et Biophysica Acta. Reviews on Cancer. 2018;1870(2):1405‐1415. doi: 10.1016/j.bbcan.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 50. Souza, AB , Chaud, MV , Santana, MHA . Hyaluronic acid behavior in oral administration and perspectives for nanotechnology‐based formulations: a review. Carbohydr Polym. 2019;222:115001. doi: 10.1016/j.carbpol.2019.115001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the data privacy of Bloomage Biotechnology Co., Ltd but are available from the corresponding author upon reasonable request.


