Abstract
Ozone therapy (OT), a medical procedure, has been showing good results during the coronavirus disease (COVID-19). We aimed to build an evidence and gaps map (EGM) of OT in the COVID-19 ranking the articles found according to levels of evidence and outcomes. The EGM brings bubbles of different sizes and different colors according to the articles. The OT intervention used was major or minor autohemotherapy, rectal insufflation and ozonized saline solution. EGM was based on 13 clinical studies using OT for COVID-19 involving a total of 271 patients. We found 30 outcomes related to OT in COVID-19. Our EGM divided the outcomes into six groups: 1-clinical improvement; 2-hospitalization; 3-inflammatory, thromboembolic, infectious, or metabolic markers; 4-radiological aspects, 5-viral infection and 6-adverse events. Major autohemotherapy was present in 19 outcomes, followed by rectal insufflation. Improvement in clinical symptoms of COVID-19, improvement of respiratory function, improvement of oxygen saturation, reduction in hospital internment, decrease in C-reactive protein, decrease in ferritin, decrease in lactate dehydrogenase, decrease in interleukin 6, decrease in D-dimer, radiological improvement of lung lesions and absence of reported adverse events were related in the papers. The most commonly used concentrations of OT in major autohemotherapy and in rectal insufflation were 40 μg/mL and 35 μg/mL, respectively. Here, we bring the first EGM showing the efficacy and safety of OT in the treatment of COVID-19. OT can be used as integrative medical therapy in COVID-19 at a low cost and improve the health conditions of the patients.
Keywords: complementary therapies, coronavirus, COVID-19, evidence and gaps map, evidence map, medical ozone therapy, medicinal ozone, ozone therapy, ozone, SARS-COV-2
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the global pandemic. The scientific community has been looking for effective and safe treatments for COVID-19.
Ozone is triatomic oxygen with high oxidizing power. Ozone has been used for a variety of purposes, including ozone therapy (OT). OT is the therapeutic administration of medicinal ozone to treat some diseases. OT is available and recognized as a medical procedure in Germany, Italy, Russia, Portugal, Spain, Turkey, Switzerland, Austria, Greece, Egypt, China, Japan, Cuba, Mexico, Honduras and several Eastern European countries.[1]
OT can be administered by different routes according to the desired therapeutic purpose, considering safety and efficacy concentrations that ranged from 1 μg/mL to 100 μg/mL.[2] OT has been used as an alternative for the treatment of dentistry,[3] osteoarthritis,[4] wound healing,[5,6,7] inactivation of viruses and bacteria8 and backache.[9,10] The ozone has also effectiveness in general viral infections,[11,12,13,14,15,16] including cornovarius.[17] Evidence and gap maps (EGMs) are an innovative rapid review method that involves a systematic search and characterization of a topic of interest, identifying scientific evidence, trends, knowledge gaps and future research needs.[18] The EGMs apply a reproducible and clear method, in addition to an explicit bibliographic search process and inclusion and exclusion criteria.[18] EGMs allow descriptive and/or visual analysis of the database, such as bubble charts, to identify gaps in clinical research and support the formulation of evidence-based policies to provide accessible resources in health decision-making. Here, we present, for the first time, an overview of existing evidence and gaps on the effects of OT in COVID-19, based on case reports, clinical trials, case-control studies, prospective case-control studies, quasi-experimental prospective cohort studies, randomized clinical trials and non-randomized clinical trials to promote evidence-based practice. We also sought to identify the possible mechanisms of action of OT in the treatment of SARS-CoV-2.
DATA AND METHODS
The map was built with the articles found between December 2020 and March 2022. The systematic evidence search process was adapted from the EGM of the International Initiative for Impact Evaluation (3iE) and included: 1-definition of limits and map contexts; 2-research and selection of relevant scientific studies; 3-characterization of the studies.
The results are presented in the form of an evidence map, highlighting positive points related to the potential gains from the clinical application of OT in COVID-19.
Research methods to identify studies
The electronic databases MEDLINE (PubMed), Virtual Health Library (VHL) and WHO-COVID19 (Table 1) were used. The main terms included in the search were: ozonotherapy, ozonetherapy, ozone therapy and medical ozone, COVID-19 and Coronavirus.
Table 1:
Search strategies applied to identify OT studies at COVID-19
| Database | URL | Search strategy |
|---|---|---|
| VHL | http://www.bvsalud.org | ("ozone therapy" OR "ozonioterapia" OR (("oxygen-ozone" OR ozono* OR ozone* OR ozonio* OR "ionized medical oxygen" OR ozone*) (treat* OR tratam* OR therap* OR terap* OR medic* OR clinic*))) AND (((("2019-2020" OR 2019 OR da:202*) ("New Coronavirus" OR "Novel Coronavirus" OR "Nuevo Coronavirus" OR "Novo Coronavirus" OR "Coronavirus disease" OR "Enfermedad por Coronavirus" OR "severe acute respiratory syndrome coronavirus 2")) OR ((2019-ncov) OR (ncov 2019) OR 2019ncov OR covid19 OR (covid-19) OR covid2019 OR (covid-2019) OR (covid 2019)) OR ((srag-cov-2 OR sars-cov-2 OR sars2 OR (sars 2) OR (sars cov 2) OR cov19 OR cov2019 OR Coronavirus* OR "Severe Acute Respiratory Infections" OR "Severe Acute Respiratory Infection" OR "Coronavirus 2" OR "acute respiratory disease" OR mh:Betacoronavirus OR mh:"Coronavirus infections" OR mh:"sars virus") AND (tw:2019 OR da:202*) AND NOT da:201*) OR (Wuhan market virus) OR (virus mercado Wuhan) OR "Wuhan Coronavirus" OR "Coronavirus de Wuhan")) |
| MEDLINE (PubMed) | http://pubmed.gov | (("ozone therapy" OR "Ozonated Autohemotherapy" OR (("oxygen-ozone" OR ozone* OR "ionized medical oxygen") (treat* OR therap* OR medic* OR clinic*))) AND (Severe Acute Respiratory Syndrome Coronavirus 2[Supplementary Concept] OR COVID-19[Supplementary Concept] OR coronavirus[MeSH Terms] OR coronavirus infections[MeSH Terms] OR betacoronavirus[MeSH Terms] OR Betacoronavirus*[TIAB] OR Coronavirus Infection[TIAB] OR Severe Acute Respiratory Syndrome Coronavirus 2[TIAB] OR Coronavirus Disease 2019[TIAB] OR 2019 Novel Coronavirus[TIAB] OR Wuhan Coronavirus[TIAB] OR COVID- 19[TIAB] OR SARS-CoV-2[TIAB] OR 2019-nCoV[TIAB] OR 2019 Novel Coronavirus[TIAB] OR Coronavirus Infection Disease 2019[TIAB] OR 2019 nCoV Infection[TLAB] OR Novel Coronavirus Pneumonia[TIAB] OR nCoV[TIAB])) |
| WHO-COVID19 | https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/ | ("ozone therapy" OR "Ozonated Autohemotherapy" OR (("oxygen-ozone" OR ozone* OR "ionized medical oxygen") (treat* OR therap* OR medic* OR clinic*))) |
Note: COVID-19: Coronavirus disease 2019; OT: ozone therapy.
Unpublished articles from other sources were also identified and included for final evaluation.
Study inclusion criteria
Case reports, clinical trials, case-control studies, prospective case-control studies, quasi-experimental prospective cohort studies, randomized clinical trials and non-randomized clinical trials, in any language or publication date, reporting cases of clinical application of OT in patients affected by the SARS-CoV2 virus were also included, according to Additional Table 1.
Additional Table 1.
List of included studies
| Title | Author | Source |
|---|---|---|
| A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. | Zheng, Zhishui; Dong, Minglin; Hu, Ke | J Med Virol; 92(11): 2348-2350, 2020 11. |
| Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. | Tascini, Carlo; Sermann, Giovanni; Pagotto, Alberto; Sozio, Emanuela; De Carlo, Chiara; Giacinta, Alessandro; Sbrana, Francesco; Ripoli, Andrea; Castaldo, Nadia; Merelli, Maria; Cadeo, Barbara; Macor, Cristiana; De Monte, Amato | Intern Emerg Med; 2021 Feb 03. |
| Case report: recovery of one ICU-acquired COVID-19 patient via ozonated autohemotherapy. | Junping Wu; Cherie S. Tan; Hongzhi Yu, M.M; Youwei Wang; Yutao Tian; Wenwei Shao; Yifei Zhang; Kuo Zhang; Hongxia Shao; Guangjian Ni; Jun Shen; Qi Wu, M.M; Dong Ming. | The Innovation; 2020 |
| Complementary application of the ozonized saline solution in mild and severe patients with pneumonia COVID-19: A non-randomized pilot study | Schwartz, A.; Martínez-Sánchez, G.; de Lucía, A. M.; Viana, S. M.; Constanta, A. M. | J Pharm Pharmacognosy Res; 2021 2(9):126-46 |
| Effect of Rectal Ozone (O3) in Severe COVID-19 Pneumonia: Preliminary Results. | Fernández-Cuadros, Marcos Edgar; Albaladejo-Florín, María Jesús; Álava-Rabasa, Sandra; Usandizaga-Elio, Isabel; Martinez-Quintanilla Jimenez, Dolores; Peña-Lora, Daiana; Neira-Borrajo, Inmaculada; López-Muñoz, María Jesús; Rodríguez-de-Cía, Javier; Pérez-Moro, Olga Susana | SN Compr Clin Med: 1-9, 2020 Aug 03. |
| Oxygen-ozone (O(2)-O(3)) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. | Franzini, Marianno; Valdenassi, Luigi; Ricevuti, Giovanni; Chirumbolo, Salvatore; Depfenhart, Markus; Bertossi, Dario; Tirelli, Umberto | Int Immunopharmacol; 88: 106879, 2020 Nov. |
| Ozone therapy for patients with COVID-19 pneumonia: Preliminary report of a prospective case-control study. | Hernández, Alberto; Viñals, Montserrat; Pablos, Asunción; Vilás, Francisco; Papadakos, Peter J.; Wijeysundera, Duminda N.; Bergese, Sergio D.; Vives, Marc | Int Immunopharmacol; 88: 106879, 2020 Nov. |
| Potential Role of Oxygen-Ozone Therapy in Treatment of COVID-19 Pneumonia. | Hernández, Alberto; Viñals, Montserrat; Isidoro, Tomas; Vilás, Francisco | Am J Case Rep; 21: e925849, 2020 Aug 17. |
| Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: A phase 1/11 randomized control trial (SEOT study). | Shah, Mili; Captain, Jignasha; Vaidya, Vidyadhar; Kulkarni, Arvind; Valsangkar, Kedar; Nair, Pradeep M. K.; Ganu, Gayatri | Int Immunopharmacol; 91: 107301, 2021 Feb. |
| Uso de ozonoterapia rectal en paciente anciana con neumonía grave por COVID-19 | Peña-Lora, Daiana Yulissa; Albaladejo-Florín, María Jesús; Fernández-Cuadros, Marcos Edgar | Rev Esp Geriatr Gerontol; 55(6): 362-364, 2020. |
| Utilization of Ozone as a Complementary Therapy for COVID-19 Patients. | Husham A. Razzaq, Mohammad S. Hasan, Muthanna F. Al-Dhalemy, Wurood M. Al-Silaykhee, Hekmat B. Alhmadi, Zaid A. Majeed | Int J Psychosoc Rehab; 24(7):10577-88, 2020. |
| Old Treatment for a New Disease: Can Rectal Ozone Insufflation Be Used for COVID-19 Management? A Case Report. | Hendawy, H.A.; Mosallam, W.; Abuelnaga, M.E.; Sabry, A.M. | SN Compr Clin Med. 3(6):1424-1427, 2021. |
| A pilot study for treatment of COVID-19 patients in moderate stage using intravenous administration of ozonized saline as an adjuvant treatment-registered clinical trial. | Sharma A, Shah M, Lakshmi S, Sane H, Captain J, Gokulchandran N, Khubchandani P, Pradeep MK, Gote P, Tuppekar B, Kulkarni P, Paranjape A, Pradhan R, Varghese R, Kasekar S, Nair V, Khanbande U. | Int Immunopharmacol. 96:107743, 2021. |
Exclusion criteria from the study
Bibliographic and editorial reviews, comments and opinions papers were excluded, as well as articles that used OT associated with other therapies and in pre- or post-COVID-19 patients, according to Additional Table 2.
Additional Table 2.
List of exclusion studies
| Reason for exclusion | Title | Author | Source |
|---|---|---|---|
| Ozone associated with other therapies | A novel approach to treating COVID-19 using nutritional and oxidative therapies | David Brownstein; Richard Ng; Robert Rowen; Jennie-Dare Drummond; Taylor Eason; Hailey Brownstein; Jessica Brownstein | Science, Public Health Policy, and The Law, 2020, 2:4-22 |
| Ozone associated with other therapies | Ozone as adjuvant support in the treatment of COVID-19: A preliminary report of probiozovid trial | Araimo, Fabio; Imperiale, Carmela; Tordiglione, Paolo; Ceccarelli, Giancarlo; Borrazzo, Cristian; Alessandri, Francesco; Santinelli, Letizia; Innocenti, Giuseppe Pietro; Pinacchio, Claudia; Mauro, Vera; Recchia, Gregorio Egidio; Zancla, Serena; Calò, Andrea; Poscia, Roberto; Ruberto, Franco; d'Ettorre, Gabriella; Bilotta, Federico; Mastroianni, Claudio; Pugliese, Francesco | Journal of medical virology, 2020, 4(93):2210-20 |
| Ozone associated with other therapies | Ozone gas applied through nebulization as adjuvant treatment for lung respiratory diseases due to COVID-19 infections: a prospective randomized trial | Dengiz, E.; Özcan, Ç.; Güven, Y.İ.; Uçar, S.; Ener, B.K.; Sözen, S.; Yağcı, B.; Güzel, İ.A.; Yiğit, B.; Andaç, A.; Güneş, B.; Bor, E.; Karabudak, U.; Kaya, A. | Med Gas Res. 2022 Apr-Jun;12(2):55-59 |
| Ozone associated with other therapies | Effectiveness of ozone therapy in addition to conventional treatment on mortality in patients with COVID-19 | Çolak, S.; Genç, Y B.; Yavuz, M.; Özçelik, B.; Öner, M.; Özgültekin, A.; Senbayrak, S. | Int J Clin Pract; 75(8): e14321, 2021 Aug. |
| Ozone associated with other therapies | CORonavirus-19 mild to moderate pneumonia Management with blood Ozonization in patients with Respiratory failure (CORMOR) multicentric prospective randomized clinical trial | Sozio, E.; De Monte, A.; Sermann, G.; Bassi, F.; Sacchet, D.; Sbrana, F.; Ripoli, A.; Curcio, F.; Fabris, M.; Marengo, S.; Italiani, D.; Luciana, Boccalatte-Rosa, D.; Tascini, C. | Int Immunopharmacol. 2021 Sep;98:107874. |
| Ozone associated with other therapies | Compassionate Use of Rectal Ozone (O 3) in Severe COVID-19 Pneumonia: a Case-Control Study | Fernández-Cuadros, M.E.; Albaladejo-Florín, M.J.; Álava-Rabasa, S.; Gallego-Galiana, J.; Pérez-Cruz, G.F.; Usandizaga-Elio, I.; Pacios, E.; Torres-García, D.E.; Peña-Lora, D.; Casique-Bocanegra, L.; López-Muñoz, M.J.; Rodríguez-de-Cía, J.; Pérez-Moro, O. S. | SN Compr Clin Med. 2021 Mar 22;1-15. |
| Pre-covid study | Intravenous ozonized saline therapy as prophylaxis for healthcare workers (HCWs) in a dedicated COVID-19 hospital in India – A retrospective study | Sharma, A.; Shah, M.; Sane, H.; Gokulchandran, N.; Paranjape, A.; Khubchandani, P.; Captain, J.; Shirke, S.; Kulkarni, P. | Eur Rev Med Pharmacol Sci, 2021; 25 (9): 3632-3639 |
| Pre-covid study | Could the minor autohemotherapy be a complementary therapy for healthcare professionals to prevent COVID-19 infection? | Orscelik, A.; Karaaslan, B.; Agiragac, B.;Solmaz, I.; Parpucu, M. | Annals of Medical of Research, 28(10):1863-1869, 2021 |
| Post-covid study | Amelioration of symptoms and oxidative stress in hospitalized convalescent post SARS-COV-2 patients treated with rectal ozonetherapy and nutritional supplementation | Lizette Gil-del-Valle; Olga Elena López-Fernández; Joniel Arnoldo Sánchez- Márquez; Zullyt ZamoraRodríguez; Ana Librada Carballo- Reyes; Lidia Asela Fernández-García; Mario Manuel Delgado-Guerra; Faustina Fonseca-Betancourt; Ernesto Miyares-Díaz; Yasmina Piloto, Orraca.; Yusimit, B.A.; Maria Carla Hdez Glez-Abreu; Sarahi Mendoza-Castaño; Yamila Ramona de Armas-Aguila. | International Journal of Modern Pharmaceutical Research, 2020, 4(6):94-107 |
| Post-covid study | Fatigue in post-acute sequelae of SARS-CoV2 (PASC) treated with oxygen-ozone autohemotherapy - preliminary results on 100 patients | Tirelli, U.; Franzini, M.; Valdenassi, L.; Pisconti, S.; Taibi, R.; Torrisi, C.; Pandolfi, S.; Chirumbolo, S. | Eur Rev Med Pharmacol Sci, 2021 Sep;25(18):5871-5875 |
| Editorials | Homeopathic use of Ozonum for some post-Covid dyspnea and/or asthenia. Study based on a cases serie | Renoux, H. | La Revue d'Homéopathie, 2021, 12 (4), 222-227 |
| Editorials | Oxygen–ozone treatment and COVID-19: antioxidants targeting endothelia lead the scenery | Varesi, A.; Chirumbolo, S.; Ricevuti, G. | Intern Emerg Med, 2022, 17(2):593-596 |
| Editorials | COVID-19 profilaxis with ozonetherapy | Falzoni, W.; Senvaitis, M. I.; Iwasa, S. | Acupuncture and Electro-Therapeutics Research, 2021, 46(1):35-36 |
| Editorials | Persistent anosmia after COVID-19 Infection: Treatment options according to BDORT | Dotta Barros, F. C.; Iwasa, S. | Acupuncture and Electro-Therapeutics Research, 2021, 46(1):33-34 |
| Editorials | Ozone therapy may be an option for COVID-19 patients | Şahin, M.; Eryilmaz, F.; Keser Şahin, H.H. | Eur Rev Med Pharmacol Sci, 2021; 25 (6): 2470-2472 |
| Editorials | Oxygen-ozone autohemotherapy against COVID-19 needs to fit highly experienced, customized, and standardized protocols to succeed | Chirumbolo, S.; Franzini, M.; Simonetti, V.; Valdenassi, L.; Ricevuti, G.; Bertossi, D.; Pandolfi, S. | J Med Virol, 2021, 93(5):2580-2582 |
| Editorials | Estado vegetativo persistente por ozonoterapia contra Covid-19 | Zavala Jonguitud, L.F.; Solís, J.G.; Noyola García, M. | Salud Publica Mex, 2021, 63:155 |
| Editorials | A Plausible "Penny" Costing Effective Treatment for Corona Virus - Ozone Therapy | Robert Jay Rowen; Howard Robins | Infectious Diseases and Epidemiology, 2020, 6(2):1-5 |
| Editorials | Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. | Yano, H.; Nakano, R.; Suzuki, Y; Nakano, A.; Kasahara, K.; Hosoi, H. | The Journal of hospital infection, 2020, 4(106)837-8 |
| Editorials | Oxygen-ozone immunoceutical therapy in COVID-19 outbreak: facts and figures | Giovanni Ricevuti; Marianno Franzini; Luigi Valdenassi | Ozone Therapy, 2020, 1(5) |
| Editorials | Oxygen-ozone therapy as adjuvant in the current emergency in SARS-COV-2 infection: a clinical study. | Marini, S.; Maggiorotti, M.; Dardes, N.; Bonetti, M.; Martinelli, M.; Re, L.; Carinci, F.; Tavera, C. | Journal of biological regulators and homeostatic agents, 2020, 3(34):757-66 |
| Editorials | Ozone Therapy as a Possible Option in COVID-19 Management. | Gavazza, Alessandra; Marchegiani, Andrea; Rossi, Giacomo; Franzini, Marianno; Spaterna, Andrea; Mangiaterra, Sara; Cerquetella, Matteo | Frontiers in Public Health, 2020, 8:417 |
| Editorials | Ozonioterapia como adjuvante no tratamento da COVID-19 / Ozone as a complement therapy in the treatment of COVID-19 | Jorge Bomfim Fróes de Farias; Antonio Pedro Fróes de Farias; Anelize Gimenez de Souza | Revista Brasileira de Fisiologia do Exercicio, 2020, 19(2):5-8 |
| Editorials | Potential mechanisms by which the oxygen-ozone (O2-O3) therapy could contribute to the treatment against the coronavirus COVID-19. | Valdenassi, L.; Franzini, M.; Ricevuti, G.; Rinaldi, L.; Galoforo, A. C.; Tirelli, U. | European review for medical and pharmacological sciences, 2020, 8(24):4059-61 |
| Editorials | Recovery of Four COVID-19 Patients via Ozonated Autohemotherapy. | Wu, Junping; Tan, Cherie S.; Yu, Hongzhi; Wang, Youwei; Tian, Yutao; Shao, Wenwei; Zhang, Yifei; Zhang, Kuo; Shao, Hongxia; Ni, Guangjian; Shen, Jun; Galoforo, Antonio Carlo; Wu, Qi; Ming, Dong | Innovation (New York, N.Y.), 2020, 3(1)100060 |
| Editorials | The need for a correct oxygen-ozone autohemotherapy (O(3)-AHT) in patients with mild to moderate COVID -19 pneumo nia | Chirumbolo, Salvatore; Pandolfi, Sergio; Valdenassi, Luigi; Bertossi, Dario; Franzini, Marianno | Internal and Emergency Medicine, 2021, P.1-2 |
| Editorials | Utilização do gás ozônio e da ozonioterapia no combate à disseminação da COVID-19 submetidas pela Sociedade Brasileira de Ozonioterapia Médica (SOBOM) | Brasil. Ministério da Saúde. Secretaria de Ciencia, Tecnologia, Inovação e Insumos Estratégicos em Saúde. | Brasília; Brasil. Ministério da Saúde; abr. 2020. |
| Literature review | Dos terapias conocidas podrían ser efectivas como adyuvantes en el paciente crítico infectado por COVID-19 | Hernández, A.; Papadakos, P. J.; Torres, A.; González, D. A.; Vives, M.; Ferrando, C.; Baeza, J. | Rev. esp. anestesiol. reanim, 2020, 5(67):245-242 |
| Literature review | Implicações sobre o uso do ozônio (O3) no tratamento coadjuvante do COVID-19 | Jorge Bomfim Fróes de Farias; Antonio Pedro Fróes de Farias; Anelize Gimenez de Souza | Revista Brasileira de Fisiologia do Exercício, 2020, 4(106):5-8 |
| Literature review | Ozone (O3) and SARS-CoV-2: Physiological Bases and Their Therapeutic Possibilities According to COVID-19 Evolutionary Stage. | Fernández-Cuadros, Marcos Edgar; Albaladejo-Florín, María Jesús; Peña-Lora, Daiana; Álava-Rabasa, Sandra; Pérez-Moro, Olga Susana | SN comprehensive clinical medicine, 2020, p.1-9 |
| Literature review | Ozone potential to fight against SAR-COV-2 pandemic: facts and research needs. | Blanco, Angeles; Ojembarrena, Francisco de Borja; Clavo, Bernardino; Negro, Carlos | Environmental science and pollution research international, 2021, p.1-15 |
| Literature review | Ozone therapy for the treatment of COVID-19 pneumonia: A scoping review. | Izadi, Morteza; Cegolon, Luca; Javanbakht, Mohammad; Sarafzadeh, Ali; Abolghasemi, Hassan; Alishiri, Gholamhossein; Zhao, Shi; Einollahi, Behzad; Kashaki, Mandana; Jonaidi-Jafari, Nematollah; Asadi, Mosa; Jafari, Ramezan; Fathi, Saeid; Nikoueinejad, Hassan; Ebrahimi, Mehrdad; Imanizadeh, Sina; Ghazale, Amir Hosein | Int Immunopharmacol, 2021, 92:107307 |
| Literature review | Ozone therapy in COVID-19: A narrative review. | Francesco Cattel; Susanna Giordano; Cecilia Bertiond; Tommaso Lupia; Silvia Corcione; Matilde Scaldaferri; Lorenzo Angelone; Francesco Giuseppe De Rosa | Virus Research, 2021, 291:198207 |
| Literature review | Ozone: A Potential Oxidant for COVID-19 Virus (SARS-CoV-2) | Chedly Tizaoui | Ozone: Science & Engineering, 2020, 42(5):378-85 |
| Literature review | Ozonetherapy: a multirole weapon, topical pathway against SARS-COV-2 | Giovanni Ranaldi; Emanuele Rocco; VillaniLaura Franza; Giulia Motola | Frenxiv, 2020, p.1-11 |
| Literature review | Ozonoterapia enteral: una posible opción segura y económica para pacientes con COVID-19 | Vivian Borroto Rodríguez; Antonio Jiménez Tapia; María Esther Dragustinovis Ruiz | Revista Cubana de Medicina Física y Rehabilitación, 2020, 12(3):1-16 |
| Literature review | Ozonoterapia para el tratamiento de pacientes adultos con COVID-19 | Peru. EsSalud Instituto de Evaluación de Tecnologías en Salud e Investigación | Peru. EsSalud. Instituto de Evaluación de Tecnologías en Salud e Investigación. s.l; IETSI; 1 jun. 2020. |
| Literature review | Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. | Martínez-Sánchez, Gregorio; Schwartz, Adriana; Donna, Vincenzo Di | Antioxidants (Basel, Switzerland), 2020, 9(5)1-12 |
| Literature review | Potential use of ozone in SARS-CoV-2 / COVID-19 | Therapy, International Scientific Committee of Ozone | International Scientific Committee of Ozone Therapy. Madrid; s.n; mar. 2020. p.2-15 |
| Literature review | Rationale for ozone-therapy as an adjuvant therapy in COVID-19: a narrative review. | Ranaldi, Giovanni Tommaso; Villani, Emanuele Rocco; Franza, Laura | Medical Gas Research, 2020, 3(10):134-8 |
| Literature review | Recent case studies on the use of ozone to combat coronavirus: Problems and perspectives. | Manjunath, Soumya Nagashri; Sakar, M.; Katapadi, Manmohan; Geetha Balakrishna, R. | Environmental Technology & Innovation, 2021, 21:101313 |
| Literature review | Safety, pitfalls, and misunderstandings about the use of ozone therapy as a regenerative medicine tool. A narrative review. | Re, L.; Noci, J. B.; Gadelha Serra, M. E.; Mollica, P.; Bonetti, M.; Travagli, V. | Journal of biological regulators and homeostatic agents, 2020, 4(34):1-3 |
| Literature review | SARS-COV-2 and Ozonetherapyin microcirculation: Devils and Angels~lll | Giovanni T. Ranaldi; Emanuele Rocco Villani; Laura Franza; Giulia Motola. | Frenxiv, 2020 |
| Literature review | Therapeutic Effects of Ozone Therapy that Justifies Its Use for the Treatment of COVID-19 | Silvia Menendez-Cepero; José Antonio Marques-Magallanes-Regojo; Alberto Hernandez-Martinez; Francisco Javier Hidalgo Tallón; JoséBaeza-Noci | Journal of Neurology and Neurocritical Care, 2020, 3(1):1-6 |
| Literature review | Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. | Hernández, A.; Papadakos, P. J.; Torres, A.; González, D. A.; Vives, M.; Ferrando, C.; Baeza, J. | Revista espanola de anestesiologia y reanimacion, 2020, 5(67):245-52 |
| Literature review | Observancia de reacciones adversas y análisis de cumplimiento de bioseguridad en la aplicación rectal de ozonoterapia en pacientes cubanos con infección aguda o convalecientes de COVID-19. | Lizette Gil del Valle, Mario M. Delgado Guerra, Ana L. Carballo- Reyes, Joniel A. Sánchez Márquez, Olga E. López Fernández, Faustina Fonseca Betancourt, Zullyt Zamora-Rodríguez, Lidia A. Fernández García, Rodolfo Suárez Iznaga, Raiza Martinez Casanueva, Lisandra Castro de la Fe, Niorkidis Gonzalez Carvajal, Silvia V. Castellanos Veitia, Yamila R. de Armas-Aguila | J Pharm Pharmacogn Res, 2021, 9(4): 465-473 |
| Literature review | Promising gas therapies for severe COVID-19 | Wang, T.; Xiang, Q.; Bian, Jinjun. | Journal of Intensive Medicine; 2021. |
| Literature review | The possibilities of using the effects of ozone therapy in neurology | Masan, J.; Sramka, M.; Rabarova, D. | Neuro Endocrinol Lett. 2021 Mar;42(1):13-21. |
| Literature review | COVID-19 and ozone | Yilmaz, N.; Eren, E.; Oz, C. | Cyprus Journal of Medical Sciences, 2020, 5(4):365-372 |
| Literature review | Ozonum—The Global Impact | Gajdos, P. S. | Homoeopathic Links, 2020, 33(4):283-296 |
| Literature review | Potential therapeutic effect of oxygen-ozone in controlling of COVID-19 disease. | Yousefi, B.; Banihashemian, S.Z.; Feyzabadi, Z.K.; Hasanpour, S.; Kokhaei, P.; Abdolshahi, A.; Emadi, A.; Eslami, M. | Med Gas Res, 2022, 12(2):33-40 |
| Literature review | Insights on the mechanisms of action of ozone in the medical therapy against COVID-19 | Chirumbolo, S.; Valdenassi, L.; Simonetti, V; Bertossi, D.; Ricevuti, G.; Franzini, M.; Pandolfi, S. | Int Immunopharmacol, 2021, 96:107777 |
| Literature review | Using Ozone Therapy as an Option for Treatment of COVID-19 Patients: A Scoping Review | Radvar, S.; Karkon-Shayan, S.; Motamed-Sanaye, A.; Majidi, M.; Hajebrahimi, S.; Taleschian-Tabrizi, N.; Pashazadeh, F.; Sahebkar, A. | Adv Exp Med Biol, 2021, 1327:151-160 |
| Literature review | Drug Repurposing in the COVID-19 Era: Insights from Case Studies Showing Pharmaceutical Peculiarities | Gatti, M.; De Ponti, F. | Pharmaceutics, 2021, 25;13(3):302 |
| Literature review | Alternative therapies for Covid-19 | Sundararajan, G.; Isaac, PJ.; Andal, V.; Lakshmipathy, R. | Mater Today Proc, 2021, 5;55:327-9 |
| Literature review | Ozonoterapia: una propuesta terapéutica para la prevención y el tratamiento de la infección por coronavirus SARS-COV-2 "COVID 19 | Longas Vélez, B.; Muñoz Mejía, C. A.; Ramírez Amaya, C. A.; Gaviria Morales, C.; Sarasti Vanegas, D. A.; Ramírez Zuluaga, E.; Trujillo Escobar, G.; Ríos Arenas, J. A.; Gaitán Sánchez, J. F.; Londoño Murillo, M. C.; Martínez Cadavid, M. M.; Soto Velásquez, M. L.; Álvarez Upegui, N.; Pérez Garcíí, C. L.; Mejía Alfaro, J. H. | Medellin – Colombia 2020 |
| Clinical trials registers | Ozone Plasma on Lung Function and Inflammatory Parameters in Pulmonary Sequelae Associated With Coronavirus 19 Infection | Fernando Grover Paez, Centro Universitario de Ciencias de la Salud, Mexico | https://clinicaltrials.gov/show/NCT05089305 |
| Clinical trials registers | Rectal ozone therapy in high-risk symptomatic SARS-CoV-2 positive patients | National Center for Scientific Research (CNIC) | https://rpcec.sld.cu/en/trials/RPCEC00000383-En |
| Clinical trials registers | Evaluation of Post-covid 19 Patients Who Receive Ozonetheraphy With Thorax CT | Gaziosmanpasa Research and Education Hospital | https://clinicaltrials.gov/show/NCT04789395 |
| Clinical trials registers | Study on ozonated blood in COVID-19 severe pneumonia | Políclinica Nuestra Señora del Rosario | https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-005020-11 |
| Full paper in Russian | Ozone Therapy In Patients With The New Coronavirus Infection Covid-19 | Hammad, E.V.; Nikitin, I.G.; Fedorova, K.V | Bulletin of Rehabilitation Medicine, 2020, 5 (99): 94-100 |
| Full paper in Russian | Ozone therapy as a component of a comprehensive rehabilitation program for patients after polysegmental pneumonia associated with SARS-CoV2 infection | Baranova, I. V; Gumeniuk, A. F.; Semenenko, A. I.; Iliuk, I. A.; Osypenko, I. P. | Zaporozhye Medical Journal, 2021, 23(6):752-758 |
Study analysis
The titles and abstracts of the 606 studies identified were analyzed. 341 duplicates were excluded, and 190 studies did not use OT for medical purposes. Subsequently, the full text of 75 pre-selected studies was read, of which only 13 met the inclusion criteria, as shown in Figure 1. Additional Tables 1 and 2 detail the included and excluded studies, respectively.
Figure 1:
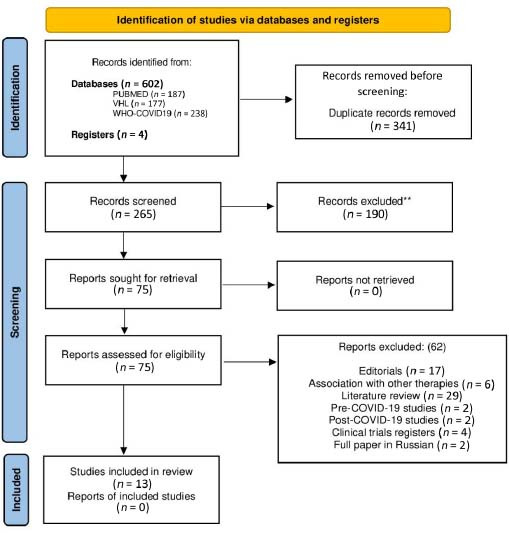
Flow of OT literatures according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow diagram.
Note: OT: Ozone therapy.
Characterization of the studies
The characterization of the studies depends on the identification of the data: type of study (methodology), countries where the study was conducted, type of intervention (routes of application of OT) and the results obtained.
Size of points on the bubble chart
According to the number of studies found, the “Result” versus “Routes of administration” items received different point sizes. The larger points were related to three or more studies, the medium points were related to two studies and the small points were only related to one study.
Bubble chart color
Circles located at the intersections between “interventions” and “outcomes” items represent the identified studies and quantity. The color of the circle represents the study design: green for randomized clinical trials, yellow for non-randomized clinical trials and quasi-experimental clinical studies, and red for case reports and case studies.
Administration routes
The routes of administration were identified in the 13 studies (Table 2).
Table 2:
Routes of administration of the ozone therapy
| Routes | Method |
|---|---|
| Intravenous systemic (venipuncture for collection of the blood, followed by reinfusion of ozonated blood with oxygen-ozone gas mixture using the same venous access) | Major autohemotherapy |
| Systemic by intramuscular administration (venipuncture for collection of the blood, followed by injection of ozonated blood with oxygen-ozone gas mixture intramuscularly) | Minor autohemotherapy |
| Intestinal systemic (rectal oxygen-ozone gas mixture insufflation) | Rectal insufflation |
| Parenteral ozonated saline solution | Intravenous |
Outcomes mentioned in scientific studies
The main topics observed in the original articles were analyzed following:
A) Clinical improvement: (1) General improvement of clinical symptoms of COVID-19; (2) Improvement of cough; (3) Reduction of body temperature; (4) Improvement of shortness of breath; (5) Improvement of respiratory function; (6) Improvement of oxygen saturation; (7) Early weaning from oxygen support; (8) Reduction in the need for invasive mechanical ventilation; (9) Faster recovery from acute respiratory distress syndrome; (10) Prevention of clinical worsening; (11) Reduction of mortality.
B) Hospitalization: (1) Reduction of hospital stay; (2) Reduction in the need for admission to the intensive care unit.
C) Inflammatory/thromboembolic/infectious/metabolic markers: (1) Decrease in C-reactive protein (CRP);(2) Reduction of ferritin; (3) Decreased lactate dehydrogenase (LDH); (4) Decreased fibrinogen; (5) Decrease in interleukin (IL) 6; (6) D-dimer decrease; (7) Leukocyte normalization; (8) Normalization of lymphocytes; (9) Decrease in procalcitonin; (10) Decreased neutrophil-lymphocyte ratio; (11) Normalization of alanine aminotransferase or glutamate-pyruvate transaminase.
D) Radiological aspects: Radiological improvement of lung lesions.
E) Viral infection: (1) Reduction of viral load; (2) Faster reverse transcriptase polymerase chain reaction negative.
F) Adverse events: (1) Discrete meteorism (gas sensation); (2) Epistaxis associated with the concomitant use of heparin; (3) Improvement of refractory hypoxemia; (4) Mild pain at the injection site and headache.
RESULTS
The results of existing evidence and gaps in the effects of OT for the treatment of COVID-19 are shown in the colored bubble graph (Figure 2). The colored bubble chart summarizes the results of the 13 selected studies (date of survey: March 15, 2022).
Figure 2:

Medical evidence of OT and EGM in the COVID-19.
Note: COVID-19: Coronavirus disease 2019; EGM: evidence and gaps map; OT: ozone therapy.
All included studies were primary studies, being case reports (n = 6), randomized clinical trials (n = 2), case and control studies (n = 2), quasi-experimental clinical study (n = 1), observational study (n = 1) and non-randomized clinical trial (n = 1). Publications occurred in 2022. The countries that carried out the studies were: Spain (n = 5), China (n = 2), Italy (n = 2), India (n = 2), Iraq (n = 1) and not identified (n = 1).
The 13 selected clinical studies, involving a total of 271 patients with COVID-19 that received OT showed positive results, both in mild to severe cases, being mild to moderate in 3 studies, moderate to severe in 1 study, mild to severe in 1 study, moderate in 1 study, severe in 7 studies. The outcomes found were: general improvement of clinical symptoms of COVID-19 (13 studies); improvement of cough (2 studies); reduction of body temperature (3 studies); improvement of shortness of breath (2 studies); improvement of respiratory function (6 studies); improvement of oxygen saturation (6 studies); early weaning from oxygen support (2 studies); reduction in the need for invasive mechanical ventilation (3 studies); faster recovery from acute respiratory distress syndrome (2 studies); prevention of clinical worsening (4 studies); reduction of mortality (2 studies); reduction of hospital stay (6 studies); reduction in the need for admission to the intensive care unit (2 studies); decrease in CRP (10 studies); reduction of ferritin (7 studies); decreased LDH (8 studies); decreased fibrinogen (2 studies); decrease in IL-6 (6 studies); D-dimer decrease (8 studies); leukocyte normalization (1 study); normalization of lymphocytes (2 studies); decrease in procalcitonin (1 study); decreased neutrophil-lymphocyte ratio (1 study); normalization of alanine aminotransferase or glutamate-pyruvate transaminase (1 study); radiological improvement of lung lesions (7 studies); reduction of viral load (1 study) and faster reverse transcriptase polymerase chain reaction negative (1 study). The information compilation of the 13 articles is shown in Additional Table 3.
| Clinical improvement | Hospitalization | Inflammatory/thromboembolic/infectious/metabolic markers | Radiological aspects | Viral infection | Adverse events | |||||||||||||||||||||||||||||||
| General improvement in clinicalsymptoms of COVID-19 | Improvement of cough | Reduction of body temperature | Improvement of shortness of breath | Improvement of respiratory function | Improvement of oxygen saturation | Early weaning from oxygen support | Reduction in the need for invasivemechanical ventilation | Faster recovery from acute respiratorydistress syndrome (ARDS) | Prevention of clinical worsening | Reduction of mortality | Reduction of hospital stay | Reduction in the need for admission tothe intensive care unit (ICU) | Decrease in C-reactive protein | Reduction of ferritin | Decreased lactate dehydrogenase | Decreased fibrinogen | Decrease in interleukin 6 | D-dimer decrease | Leukocyte normalization | Normalization of lymphocytes | Decrease in procalcitonin | Decreased Neutrophil-LymphocyteRatio | Normalization of alanineaminotransferase | Radiological improvement of lunglesions | Reduction of viral load | Faster RT-PCR negative | Presence | Absence | Unmentioned | |||||||
| Study | Name | Country | Therapy | Type of study | Number of hospitalized patients who received Ozone Therapy | Pneumonia severity level | ||||||||||||||||||||||||||||||
| A | Wu et al. (2020)* | China | GAHT, 40 mcg / ml, 100 ml, 1: 1, once a day on the first day and twice a day on the second to the fifth day, for 5 days, totaling 9 sessions | Report of a case | 1 | Severe | X | X | X¶ | X | X | X | X | X | X | X | X | |||||||||||||||||||
| B | Zheng et al. (2020)* | China | GAHT, 20 mcg / ml, 100 ml, 1: 1, once a day for 7 days, totaling 7 sessions | Report of a case | 2 | Severe | X | X | X | X | X | X | X | X | ||||||||||||||||||||||
| C | Franzini et al. (2020) | Italy | Clinical trial | 50 | Moderate | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| D | Hernández et al. (2020)* | Spain | GAHT, 40 mcg / ml, 200 ml, 1: 1, 2 timer a day, for 3 days, totaling 6 sessions | Report of a case | 3 | Severe | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| E | Tascini et al. (2020) | Italy | GAHT, 40 mcg / ml, 200 ml, 1: 1, once a day, for 3 days, totaling 3 sessions | Cases and cortrols study | 30 | Mild to moderate | X | X | X | X | X | |||||||||||||||||||||||||
| F | Hernández et al. (2021) | Spain | GAHT, 40 mcg / ml, 200 ml, 1: 1, 2 times a day for 5 days, totaling 10 sessions | Prospective case-control study | Severe | X | X | X | X | X | X | X | ||||||||||||||||||||||||
| G | Fernández-Cuadros et al.(2020)* | Spain | IR, 35 mcg / ml, 100 ml, once a day for 5 to 10days, for a total of 5 to 10 sessions | Quasi-experimental prospective cohort study | Severe | X | X | X | X | X | X | X | X | XΔ | ||||||||||||||||||||||
| H | Pena-Lora et al. (2020)* | Spain | IR, 35 mcg / ml, 100 ml, once a day for 5 days, totaling 5 sessions | Report of a case | 1 | Severe | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| I | Shah et al. (2020) | India | 1st) IR, 40 mcg / mL, 150mL, 2 times a day for9 days, totaling 18 sessions 2nd) PAHT 25mcg /mL, 5mL of O3 and 2 to 3 ml of blood, once aday for 9 days, totaling 9 sessions | Randomized clinical trial | 30 | Mild to moderate | X | X | X | X | X | X | X¤ | X¤ | X¤ | X | X | |||||||||||||||||||
| J | Razzaq et al. (2020)* | Iraq | SFO3, 35-45mcg / mL, 500mL, 3 to 5 minutes of preparation in continuous bubbling, infusion in15 minutes, 1 to 2 times a day, for 5-10 days. | Clinical trial | 104 | Moderate to severe | X | X | X | X | ||||||||||||||||||||||||||
| K | Schwartz et al. (2020) | Spain | SFO3, 3-5mcg / mL, 200mL, 10 minutes of preparation in continuous bubbling, infusion of15 to 30 minutes, once a day, for 10 days. | Non-randomized clinical trial | 35 | Severe | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||
| J | Sharma et al. (2021) | India | SFO3. 5mcg / mL, 200mL, 20 minutes of preparation in continuous bubbling, infusion of 1hour, once a day, for 8 days. | Clinical trial | 10 | Moderate | X | X | X | X | X | X | X | X | X | X | X | X | X | XΦ | ||||||||||||||||
| L | Hendawy et al. (2021) | Egypt | 1) IR, 12.6 mcg / mL, 2 liters, two sessions2) IR, 12.6mcg/mL, 2 liters, one session | Report of a case | 3 | Mild and severe | X | X | X | X | X | X | X | |||||||||||||||||||||||
| Total Results | 13 | 2 | 3 | 2 | 6 | 6 | 2 | 3 | 2 | 4 | 2 | 6 | 2 | 10 | 7 | 8 | 2 | 6 | 8 | 1 | 2 | 1 | 1 | 1 | 7 | 1 | 1 | 3 | 6 | 4 | ||||||
Note: * Studies without statistics; Δ Discreet weather, gas sensation; . Epistaxis associated with the use of heparin; ¤ Falling of inflammatory markers, without significant statistics; ¶ Improvement of refractory hypoxemia; Φ Mild pain at the injection site and headache.
The most reported type of intervention was major autohemotherapy (MAH), followed by rectal insufflation (RI). The reported outcomes were: general improvement in clinical symptoms of COVID-19 (n = 13), improvement of respiratory function (n = 6), improvement of oxygen saturation (n = 6), reduction in hospital stay (n = 6), decrease in CRP (n = 10), decrease in ferritin (n = 7), decrease in LDH (n = 8), decrease in IL-6 (n = 6), decreased D-dimer (n = 8), radiological improvement of lung lesions (n = 7) and absence of reported adverse events (n = 10). The concentration of medicinal ozone in the MAH ranged from 20 to 45 μg/mL, with 40 mcg/mL being the most used concentration, and the number of sessions was 3–10. For the RI route, a concentration of 12.6 to 40 μg/mL was used, with the concentration of 35 μg/mL being the most used, and the average of sessions was only one session.
DISCUSSION
The best findings of this work are high evidence (randomized clinical trials) for MAH in decreasing ferritin, D-dimer, IL-6, LDH, procalcitonic, normalization of alanine aminotransferase, improved respiratory function, faster acute respiratory distress syndrome recovery, improved oxygen saturation and improves symptoms of COVID-19. OT can be beneficial in reducing the length of hospital stay and decreasing inflammatory markers. RI results in negative reverse transcriptase polymerase chain reaction, breathlessness relief, cough relief, prevention of clinical worsening and decrease in clinical symptoms of COVID-19.
The advantages of OT in COVID-19 are:
General improvement in oxygen metabolism;
Increasing oxygenation and gas exchange in the lungs and peripheral tissues;
Restoring the balance of the cellular redox state;
Increasing the synthesis of intracellular antioxidant enzymes;
Promoting interferon-gamma induction;
Reducing pro-inflammatory cytokines;
Increasing the effectiveness of antigen-antibody dynamics;
Having antiviral action, vasodilation, increase of tissue perfusion, modification and attenuation of the viruses’ structural conformation, favoring the immune response;
Increasing hemorheological, reducing the aggregation of red blood cells, decreasing blood viscosity and optimizing blood flow;
Favoring the regeneration of injured tissues, due to the release of growth factors and obtaining a feeling of well-being, as reported by most patients, through the activation of the neuroendocrine system.
OT can improve hemorheological properties,[19] favoring blood flow,[20] reducing erythrocyte aggregation and systemic and erythrocyte oxidative stress[21] in patients with COVID-19.
COVID-19 patients are also likely to have mild nonspecific hepatitis[22,23] and pulmonary fibrosis.[23] OT stabilizes hepatic metabolism and plasma fibrinogen and prothrombin levels tend to normalize in infected patients, suggesting an improvement in hepatic protein synthesis.[24]
There is also a demonstration that OT protects against oxidative damage to the lung,[25] kidney,[26] heart,[27] intestine,[28] muscles[29] and brain.[30]
OT causes an increase in the glycolysis rate of red blood cells, which produce more ATP. This leads to the stimulation of 2,3-diphosphoglycerate, which can shift the oxyhemoglobin dissociation curve to the right, increase the partial pressure of arterial oxygen and decrease the partial pressure of venous oxygen called Bohr effect, which generates an increase in the amount of oxygen released to the tissues. This effect improves the oxygen supply to ischemic tissues. OT also improves oxygenation and gas exchange in the lungs and peripheral tissues due to vasodilation provided by nitrosothiols, activation of nitric oxide synthase (with increased production of nitric oxide, a potent vasodilator) and produces more red blood cells because of better functioning of the Na+/K+ ATPase pump.[31] Ozone can improve the circulation and perfusion of the lung and all organs in a state of hypoxia, as it improves oxygen metabolism.[32]
In summary, the mechanisms of action of OT applicable to the main complications of COVID-19 are based on: (1) General improvement in oxygen metabolism, increasing oxygenation and gas exchange in the lungs and peripheral tissues. (2) Restoring the balance of the cellular redox state. (3) Increased synthesis of intracellular antioxidant enzymes. (4) Promotion of interferon-gamma induction (main endogenous humoral mechanism of antiviral control). (5) Reduction of pro-inflammatory cytokines. (6) Increasing the effectiveness of antigen-antibody dynamics. (7) Antiviral action. (8) Vasodilation and increased tissue perfusion. (9) Modification and attenuation of the structural conformation of virions, favoring the antiviral immune response (“autovaccine”). (10) Hemorheological improvement, reducing the aggregation of red blood cells, decreasing blood viscosity, and optimizing blood flow. (11) Regeneration of injured tissues, due to the release of growth factors. (12) Obtaining a feeling of well-being, as reported by most patients, through the activation of the neuroendocrine system.
The most widely used administration routes for OT are MAH, minor autohemotherapy (MiAH) and RI, described in the clinical studies for COVID-19.[33,34,35,36,37,38,39,40,41] In MAH, approximately 250 mL of blood is collected from the participant and gently homogenized with the same amount of oxygen-ozone mixture, and then injected again into the participant intravenously. In MiAH, a smaller volume of blood (5 mL) is taken from the participant, homogenized with the same amount of the oxygen-ozone mixture, and the application is made intramuscularly.[31] In the pathophysiological context of thrombogenesis of COVID-19, in which there may be diffuse occlusive arterial disease, we find another potential applicability of OT. Clinical practice has already consolidated in the treatment of peripheral arterial occlusive disease. MAH is useful in improving both the rheological properties of blood and oxygen release in ischemic tissues,[19] in addition to the dose-dependent vasodilator mechanism related to the production of prostacyclin.
The OT used by RI has local and systemic action, since the gas is quickly dissolved in the contents of the intestinal lumen, where there are mucoproteins and other secretion products with a strong antioxidant activity, so that it reacts quickly and produces reactive oxygen species and lipid peroxidation products, which, once generated, are absorbed and pass into the systemic circulation, being able to promote therapeutic effects. To avoid damage to the rectal mucosa, it is not convenient to reach a concentration above 40 μg/mL in the RI. The volume of the gas mixture to be applied is variable, depending on the disease and the different institutions of OT, which varies between 100 to 500 mL per session for adults. RI is able to achieve similar results to MAH, but it needs a high number of sessions and a higher dose.[42] RI was already used in the treatment of patients infected with Ebola, obtaining a significant improvement after 5 sessions, without deaths.[43] It was believed that this benefit could be extrapolated to patients with COVID-19.[44] In a case-control study evaluating 14 patients with COVID-19 severe bilateral accompanied by pneumonia, the patients were treated with standard of care plus OT via RI, for 10 days. The OT protocol consisted of 8 sessions (1 session per day), at a volume of 150 mL and a concentration of 35 μg/mL. The standard of care protocol included oxygen delivery, antivirals (remdesivir), corticosteroids (dexamethasone/methylprednisolone), monoclonal antibodies (anakinra/tocilizumab), antibiotics (azithromycin) and anticoagulants (enoxaparin). The lymphocyte count improved statistically in the OT group (P < 0.05). Inflammation biomarkers (fibrinogen, D-dimer, urea, LDH, CRP and IL-6) were decreased in both groups, but only significantly in the OT group (P < 0.05). Ferritin showed a significant decrease in the OT group and an increase in the standard of care group. Pneumonia decreased in both groups, but it was significant only in the OT group (improved Taylor’s radiological score) (P < 0.0001). Mortality and length of stay, although not significant, were lower in the OT group. Therefore, OT via RI improved oxygen saturation, decreased inflammation biomarkers and improved Taylor’s radiological scale in the treatment of COVID-19.[45]
The precise mechanism of COVID-19 disease is not completely defined; however the link between mitochondria and viruses is known. The virus SARS-CoV-2 silences the body’s innate inflammatory response and does so by diverting mitochondrial genes from their normal function. SARS-CoV-2 has been shown to reduce levels of a group of mitochondrial proteins, known as Complex One, which are encoded by nuclear DNA. This effect can "calm" the cell's metabolic production and the generation of reactive oxygen species, which, when functioning properly, produce an inflammatory response that can kill the virus.[46] It has also been observed that the SARS-CoV-2 virus can be even more complex, suggesting that in the peripheral blood mononuclear cells of patients with COVID-19, the virus deliberately manipulates the metabolic functions of mitochondria to its own benefit. Ajaz et al.[47] found increases in the fibroblast growth factor 21 mitokine and in glycolysis. Since mitochondria play a key role in the initiation and development of the cytokine storm, specific mitochondrial pathways in immune cells can be targeted clinically.
Mitochondria have a high sensitivity to oxidative damage, caused by free radicals that can compromise their structure and function, reducing their activity.[48] Systemic OT can activate the action of mitochondria.[49]
The pathogenesis of COVID-19 is also associated with a hyperinflammatory response. The protein spike (S) potently induces inflammatory cytokines and chemokines, including IL-6, IL-1β and tumor necrosis factor α. Protein S triggers inflammation by activating the nuclear factor κB pathway in a MyD88-dependent manner.[50] OT is able to inhibit the NF-κβ gene,[51] a pathway that would play an important role in stimulating hyperinflammation or cytokine storm.[52] These findings would explain why inflammatory variables such as ferritin, IL-6 and CRP decrease in patients with COVID-19 treated with OT via RI.[37]
The detection and control of the pro-inflammatory response are crucial in the early stages of COVID-19 and IL-6 is one of the main pro-inflammatory cytokines. The control of systemic levels of IL-6 in patients infected with SARS-CoV-2 may signal an improvement in the disease.[53] Systemic OT can decrease IL-6 and other pro-inflammatory cytokines.[54] It has also been measured in vivo that the release of numerous cytokines from ozonated blood, at a concentration between 30 and 55 μg/mL, causes an increase in interferon production and less production of tumor necrosis factor α[55] and IL-2.[56]
OT is also associated with the production of interferon alpha, beta and gamma, as well as ILs, granulopoietin, and in the transformation of growth factor transforming growth factor β and possibly other proteins are also stimulated.[31] These changes can be observed for days after ozonation, suggesting that when leukocytes are activated by OT, they migrate to lymphoid environments where the release of cytokines triggers the activation of other immune cells, reactivating the depressed immune system.[57]
The activation of nuclear factor erythroid 2-related factor 2 can also be a strategy against COVID-19, since its action promotes the resolution of inflammation and, in parallel, restores cellular redox and protein homeostasis, and favors tissue repair. The possible benefits of pharmacological activation of nuclear factor erythroid 2-related factor 2 in the context of SARS-CoV-2 infection are: to increase fitness and provide protection to the host cell; promote the anti-inflammatory phenotype during the activation of macrophages, avoiding the uncontrolled production of pro-inflammatory cytokines and pyroptosis; and inhibit viral spread.[58] They may, as a result, be important in mitigating the severity of COVID-19, acting through endoplasmic reticulum stress or angiotensinconverting enzyme-angiotensin-II-ATVia of axis 1 receptor.[59]
OT also modulates the nuclear factor erythroid 2-related factor 2 system[60] producing three effects. First, it normalizes the redox balance by increasing antioxidants in the cytoplasm, in the mitochondria and finally in the plasma, mainly glutathione peroxidase, but also glutathione reductase, NADPH and superoxide dismutase. Second, it induces the production of heme oxygenase-1, a protective enzyme, along with heat shock proteins (HSPs) like HSP-60, HSP-70 and HSP-90.[61] Third, it activates the nuclear factor κB system, which modulates the production of pro-inflammatory ILs in damaged tissues.[62] All effects contribute to restoring the normal functioning of inflamed tissues and decreasing the plasma ILs.
HSPs also play a role at different levels in viral infections. They can regulate viral infections through interaction with the virus, including cell entry and nuclear import, viral replication and gene expression, viral protein folding/assembly, regulation of apoptosis, and host immunity. They are also effective carrier molecules for cross-presentation to antigen-presenting cells. Thus, HSPs and especially HSP70 can increase the development of innate and adaptive immune responses against infectious agents.[63] Mortality in a rat model with sepsis-induced acute respiratory distress syndrome) has been reduced by overexpression of HSP70 chaperone adenovirus. A natural increase in body temperature during low fever can naturally accumulate high cellular levels of HSP70 that can stop apoptosis and protect alveolar lung cells from inflammatory damage. However, in addition to 1–2 hours of fever, no HSP70 is being produced and a decrease in body temperature is needed to restore the cell’s ability to produce more HSP70 in a subsequent fever cycle. Therefore, it is suggested that antipyretics may be beneficial in patients with COVID-19, allowing lung cells to accumulate protective HSP70 against damage from the inflammatory response to the SARS-CoV-2 virus.[64]
OT can also induce HSP70.[32] The confluence of human vascular endothelial cells was challenged with different concentrations of these inducers and the production of nitric oxide (NO); and heme oxygenase-1 was measured by nitrite or bilirubin formation, or/and protein immune reactivity by Western blot using a rabbit anti-human heme oxygenase-1 and HSP70. The production of NO and heme oxygenase-1 was quite dose-dependent and it was particularly high using human plasma after exposure to ozone. The HSP70 induction was also observed. Therefore, the results clarify that OT can also induce HSP70. OT also increased the length of the mitochondrial ridges and the content of the mitochondrial HSP70.[65]
An important source of cytokines and chemokines are mast cells, which are ubiquitous in the body, especially in the lungs, and are essential for allergic and lung diseases.[66] Activated mast cells were recently detected in the lungs of deceased patients with COVID-19 and were associated with pulmonary edema, inflammation and thrombosis.[67] Hyperinflammation was also observed in patients with COVID-19 and post-COVID-19, suggesting that this hyperinflammation is linked to mast cell activation syndrome.[68]
Mast cells are typically activated by allergic triggers, but they can also be triggered by pathogen-associated molecular patterns via the activation of Toll-like receptors. In addition, mast cells express the renin-angiotensin system, the ectoprotease angiotensin-converting enzyme 2 required for SARS-CoV-2 binding, and serine proteases, including transmembrane protease serine 2, required for priming of the corona spike protein.[65] Such triggers could lead to the secretion of multiple proinflammatory mediators selectively, without the release of histamine or tryptase.[69]
In addition to the proinflammatory cytokines and chemokines, activated mast cells could release matrix metalloproteinases and transforming growth factor beta, which could contribute to lung fibrosis, including thromboxanes and platelet-activating factor, leading to the recently reported microthromboses in the lungs of deceased patients with COVID-19.[67] Moreover, mast cells communicate with endothelial cells, fibroblasts, and macrophages, further stimulating the release of proinflammatory, fibrotic, thrombogenic, and vasoactive mediators.
Peden and Dailey[70] showed the immunoregulatory action of OT on mast cells in vitro and verified that during OT, the plasma histaminopexic and serotoninopexic activity increased. These findings can be explained by the elimination of histamine and serotonin from the body.
For the prescription of a medication, by a qualified professional, but for an unapproved indication, and therefore, that is not included in the package leaflet, this practice is called off-label use. The definition for off-label by the Brazilian National Health Surveillance Agency (ANVISA) is a use in situations that differ from the package insert for a drug registered. It may include differences in indication, age/weight, dose, frequency, presentation, or route of administration. In Brazil, off-label use is not illegal, and since it is supported by robust scientific evidence and the absence of a therapeutic alternative, it is considered legitimate or even necessary when considering the clinical situation that demanded it.
In view of the seriousness of the medical-political-social and economic situation and in the absence of known treatments for COVID-19, some treatments are being researched and used as off-label (hydroxychloroquine, tocilizumab, invermectin, colchicine, remdesivir, etanercept, corticosteroids, antibiotics, convalescent heterologous plasma, immunoglobulins, antivirals and immunomodulators), however, bring with them, to a greater or lesser degree, a high cost, be it financial or a potential adverse effect. OT is a feasible health resource, if judiciously practiced, it can achieve excellent therapeutic results associated with a very low rate of complications and risks.[71]
In our study, we verified the presence of very few adverse events, which were: mild meteorism (sensation of gas) and epistaxis that was associated with the use of heparin. These results agree with the Jacobs’s study[72] which tabulated data from nearly 5.6 million OT treatments and found only 0.0007% risk of complications and 0.0001% risk of death.
OT can also be used in patients after COVID-19,[73] evaluated the effects of nutritional supplementation and RI with ozone. The 40 patients received nutritional supplementation capsule (400 mg) daily or 2 capsule nutritional supplementation plus RI with ozone daily during 1 month. Twenty healthy patients were enrolled as controls. Improvements in symptoms of COVID-19 were observed in both groups, resulting in 85% of patients for nutritional supplementation plus RI with ozone and 37% for nutritional supplementation (P < 0.05). The symptoms that persist in COVID-19 were reduced and no side effects were observed. These results corroborate that substantial oxidative stress and symptoms persist in COVID-19 powders can be improved by the treatments of RI with ozone plus nutritional supplementation.
Our EGM has some limitations such as the fact that we only used published studies to provide an overview of the research and that no further evidence was included. We did not calculate effect sizes in a Meta-analysis, nor provide risk of bias assessments, but tried to overcome these limitations by relying on the authors’ skills in conducting and evaluating the study quality, choice of outcomes, analysis of effects and susceptibility to publication and outcome reporting bias. We suggest that future studies should adopt health and economic impact assessments for health services, as well as robust methodologies for evaluating clinical trials.
The EGM showed that all routes of administration (MAH, MiAH, RI) have high evidence levels in some outcomes. The MAH presented high evidence levels for the improvement of clinical symptoms of COVID-19, of oxygen saturation, in the recovery from acute respiratory distress syndrome, in the respiratory function, and decrease D-dimer, CRP, IL-6, procalcitonin, LDH. MAH also normalized ALT levels. The high evidence levels for MiAH were found for the decrease of LDH, CRP and ferritin. And finally, RI improves clinical symptoms of COVID-19, including cough, prevents clinical worsening and shortness of breath, and faster reverse transcriptase polymerase chain reaction negative.
In this way, the main high evidence levels were found with the use of MAH route. MAH was able to improve the clinical signs of the patients and inflammatory markets. The EGM demonstrates in a preliminary way (13 articles) that OT can be beneficial in reducing the length of hospital stay and decreasing inflammatory markers with few adverse events. These benefits are seen in patients at a mild and/or moderate stage of the disease and may accelerate recovery to avoid the entry into severe stages of COVID-19.
Author contributions
Conceptualization: MEGS, MML, APA; methodology, software, formal analysis: CVMA; validation: MEGS, APA; investigation: MEGS, JBN, CDB, APA; resources: MEGS, CDB, APA; data curation: MEGS, CVMA, CDB, APA; writing – original draft: MEGS, MML, CDB, APA; writing – review & editing: MEGS, JBN, CDB, APA; visualization: MEGS, JBN, MML, CVMA, DFS, CDB, APA; supervision: CDB, APA; project administration, and funding acquisition: MEGS. All authors approved the final manuscript for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files
Additional Table 1: List of included studies.
Additional Table 2: List of exclusion studies.
Additional Table 3: Methodology and outcomes observed in the 13 final article.
REFERENCES
- 1.Bocci V, Di Paolo N. Oxygen-ozone therapy in medicine: an update. Blood Purif. 2009;28:373–376. doi: 10.1159/000236365. [DOI] [PubMed] [Google Scholar]
- 2.Viebahn-Hänsler R, León Fernández OS, Fahmy Z. Ozone in medicine: the low-dose ozone concept—guidelines and treatment strategies. Ozone Sci Eng. 2012;34:408–424. [Google Scholar]
- 3.Sen S, Sen S. Ozone therapy a new vista in dentistry: integrated review. Med Gas Res. 2020;10:189–192. doi: 10.4103/2045-9912.304226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra SK, Pramanik R, Das P, et al. Role of intra-articular ozone in osteo-arthritis of knee for functional and symptomatic improvement. Ind J Phys Med Rehabil. 2011;22:65–69. [Google Scholar]
- 5.Kim HS, Noh SU, Han YW, et al. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368–374. doi: 10.3346/jkms.2009.24.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indurkar MS, Verma R. Effect of ozonated oil and chlorhexidine gel on plaque induced gingivitis: A randomized control clinical trial. J Indian Soc Periodontol. 2016;20:32–35. doi: 10.4103/0972-124X.170806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel PV, Kumar S, Vidya GD, Patel A, Holmes JC, Kumar V. Cytological assessment of healing palatal donor site wounds and grafted gingival wounds after application of ozonated oil: an eighteen-month randomized controlled clinical trial. Acta Cytol. 2012;56:277–284. doi: 10.1159/000336889. [DOI] [PubMed] [Google Scholar]
- 8.Burleson GR, Murray TM, Pollard M. Inactivation of viruses and bacteria by ozone, with and without sonication. Appl Microbiol. 1975;29:340–344. doi: 10.1128/am.29.3.340-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaillant JD, Fraga A, Díaz MT, et al. Ozone oxidative postconditioning ameliorates joint damage and decreases pro-inflammatory cytokine levels and oxidative stress in PG/PS-induced arthritis in rats. Eur J Pharmacol. 2013;714:318–324. doi: 10.1016/j.ejphar.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Steppan J, Meaders T, Muto M, Murphy KJ. A metaanalysis of the effectiveness and safety of ozone treatments for herniated lumbar discs. J Vasc Interv Radiol. 2010;21:534–548. doi: 10.1016/j.jvir.2009.12.393. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Huang J, Xiang Y, Gao L, Pan Y, Lu J. Topical ozone therapy: an innovative solution to patients with herpes zoster. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:168–172. doi: 10.11817/j.issn.1672-7347.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Neronov VA. Experience with the use of ozone for the treatment of chronic viral hepatitis. Vopr Kurortol Fizioter Lech Fiz Kult. 2009:14–17. [PubMed] [Google Scholar]
- 13.Cespedes-Suarez J, Martin-Serrano Y, Carballosa-Peña MR, Dager-Carballosa DR. The immune response behavior in HIV-AIDS patients treated with ozone therapy for two years. J Ozone Ther. 2018:2. [Google Scholar]
- 14.Herbold K, Flehmig B, Botzenhart K. Comparison of ozone inactivation, in flowing water, of hepatitis A virus, poliovirus 1, and indicator organisms. Appl Environ Microbiol. 1989;55:2949–2953. doi: 10.1128/aem.55.11.2949-2953.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brié A, Boudaud N, Mssihid A, Loutreul J, Bertrand I, Gantzer C. Inactivation of murine norovirus and hepatitis A virus on fresh raspberries by gaseous ozone treatment. Food Microbiol. 2018;70:1–6. doi: 10.1016/j.fm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Khadre MA, Yousef AE. Susceptibility of human rotavirus to ozone, high pressure, and pulsed electric field. J Food Prot. 2002;65:1441–1446. doi: 10.4315/0362-028x-65.9.1441. [DOI] [PubMed] [Google Scholar]
- 17.Hudson JB, Sharma M, Vimalanathan S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci Eng. 2009;31:216–223. [Google Scholar]
- 18.Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28. doi: 10.1186/s13643-016-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giunta R, Coppola A, Luongo C, et al. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann Hematol. 2001;80:745–748. doi: 10.1007/s002770100377. [DOI] [PubMed] [Google Scholar]
- 20.Turczyński B, Sroczyński J, Antoszewski Z, et al. Ozone therapy and viscosity of blood and plasma, distance of intermittent claudication and certain biochemical components in patients with diabetes type II and ischemia of the lower extremities. Pol Tyg Lek. 1991;46:708–710. [PubMed] [Google Scholar]
- 21.Shinriki N, Suzuki T, Takama K, et al. Susceptibilities of plasma antioxidants and erythrocyte constituents to low levels of ozone. Haematologia (Budap) 1998;29:229–239. [PubMed] [Google Scholar]
- 22.Chau TN, Lee KC, Yao H, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bocci V. Ozone as a bioregulator. Pharmacology and toxicology of ozonetherapy today. J Biol Regul Homeost Agents. 1996;10:31–53. [PubMed] [Google Scholar]
- 25.Kaldirim U, Uysal B, Yuksel R, et al. Ozone therapy ameliorates paraquat-induced lung injury in rats. Exp Biol Med (Maywood) 2014;239:1699–1704. doi: 10.1177/1535370214543060. [DOI] [PubMed] [Google Scholar]
- 26.Kurtoglu T, Durmaz S, Akgullu C, et al. Ozone preconditioning attenuates contrast-induced nephropathy in rats. J Surg Res. 2015;195:604–611. doi: 10.1016/j.jss.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Hernández F, Menéndez S, Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic Biol Med. 1995;19:115–119. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- 28.Kesik V, Uysal B, Kurt B, Kismet E, Koseoglu V. Ozone ameliorates methotrexate-induced intestinal injury in rats. Cancer Biol Ther. 2009;8:1623–1628. doi: 10.4161/cbt.8.17.9203. [DOI] [PubMed] [Google Scholar]
- 29.Ozkan H, Ekinci S, Uysal B, et al. Evaluation and comparison of the effect of hypothermia and ozone on ischemia-reperfusion injury of skeletal muscle in rats. J Surg Res. 2015;196:313–319. doi: 10.1016/j.jss.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Lintas G, Liboni W, Simonetti V, et al. Long-term cerebrovascular reactivity mediated by ozone autohemotherapy: A NIRS study. Paper presented at: Terzo Congresso del Gruppo Nazionale di Bioingegneria. 2012 [Google Scholar]
- 31.Bocci V. 2nd. Netherlands: Springer; 2011. Ozone: A New Medical Drug. [Google Scholar]
- 32.Bocci V, Aldinucci C, Mosci F, Carraro F, Valacchi G. Ozonation of human blood induces a remarkable upregulation of heme oxygenase-1 and heat stress protein-70. Mediators Inflamm. 2007;2007:26785. doi: 10.1155/2007/26785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Z, Dong M, Hu K. A preliminary evaluation on the efficacy of ozone therapy in the treatment of COVID-19. J Med Virol. 2020;92:2348–2350. doi: 10.1002/jmv.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tascini C, Sermann G, Pagotto A, et al. Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. Intern Emerg Med. 2021;16:669–675. doi: 10.1007/s11739-020-02542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Tan CS, Yu H, et al. Recovery of four COVID-19 patients via ozonated autohemotherapy. Innovation (Camb) 2020;1:100060. doi: 10.1016/j.xinn.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz A, Martínez-Sánchez G, Menassa de Lucía A, Mejía Viana S, Alina Mita C. Complementary application of the ozonized saline solution in moderate and severe patients with pneumonia Covid-19: efficacy and tolerability. Preprint. 2020:2020060233. [Google Scholar]
- 37.Fernández-Cuadros ME, Albaladejo-Florín MJ, Álava-Rabasa S, et al. Effect of rectal ozone (O(3)) in severe COVID-19 pneumonia: preliminary results. SN Compr Clin Med. 2020;2:1328–1336. doi: 10.1007/s42399-020-00374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franzini M, Valdenassi L, Ricevuti G, et al. Oxygen-ozone (O(2)-O(3)) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int Immunopharmacol. 2020;88:106879. doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández A, Viñals M, Pablos A, et al. Ozone therapy for patients with COVID-19 pneumonia: Preliminary report of a prospective case-control study. Int Immunopharmacol. 2021;90:107261. doi: 10.1016/j.intimp.2020.107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández A, Viñals M, Isidoro T, Vilás F. Potential role of oxygen-ozone therapy in treatment of COVID-19 pneumonia. Am J Case Rep. 2020;21:e925849. doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah M, Captain J, Vaidya V, et al. Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: A phase 1/11 randomized control trial (SEOT study) Int Immunopharmacol. 2021;91:107301. doi: 10.1016/j.intimp.2020.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernández Rosales FA, Calunga Fernández JL, Turrent Figueras J, Menéndez Cepero S, Montenegro Perdomo A. Ozone therapy effects on biomarkers and lung function in asthma. Arch Med Res. 2005;36:549–554. doi: 10.1016/j.arcmed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Rowen RJ, Robins H. A plausible "penny" costing effective treatment for corona virus - ozone therapy. J Infect Dis Epidemiol. 2020;6:113. [Google Scholar]
- 44.Fernández-Cuadros ME, Albaladejo-Florín MJ, Peña-Lora D, Álava-Rabasa S, Pérez-Moro OS. Ozone (O3) and SARS-CoV-2: physiological bases and their therapeutic possibilities according to COVID-19 evolutionary stage. SN Compr Clin Med. 2020;2:1094–1102. doi: 10.1007/s42399-020-00328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Cuadros ME, Albaladejo-Florín MJ, Álava-Rabasa S, et al. Compassionate use of rectal ozone (O(3)) in severe COVID-19 pneumonia: a case-control study. SN Compr Clin Med. 2021;3:1185–1199. doi: 10.1007/s42399-021-00849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller B, Silverstein A, Flores M, et al. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci Rep. 2021;11:3. doi: 10.1038/s41598-020-79552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajaz S, McPhail MJ, Singh KK, et al. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol. 2021;320:C57–C65. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scassellati C, Galoforo AC, Bonvicini C, Esposito C, Ricevuti G. Ozone: a natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res Rev. 2020;63:101138. doi: 10.1016/j.arr.2020.101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costanzo M, Cisterna B, Vella A, et al. Low ozone concentrations stimulate cytoskeletal organization, mitochondrial activity and nuclear transcription. Eur J Histochem. 2015;59:2515. doi: 10.4081/ejh.2015.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan S, Shafiei MS, Longoria C, Schoggins JW, Savani RC, Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife. 2021;10:e68563. doi: 10.7554/eLife.68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-alpha and tissue injury are dependent on NF-kappaB p50. Am J Physiol Lung Cell Mol Physiol. 2004;287:L279–285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- 52.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vatansever HS, Becer E. Relationship between IL-6 and COVID-19: to be considered during treatment. Future Virol. 2020;15:817–822. [Google Scholar]
- 54.Vinnik IS, Salmina AB, Tepliakova OV, et al. The results of combined ozone therapy using in complex treatment of soft tissues infections in patients with diabetes mellitus type II. Khirurgiia (Mosk) 2015:63–69. doi: 10.17116/hirurgia2015263-69. [DOI] [PubMed] [Google Scholar]
- 55.Bocci V, Luzzi E, Corradeschi F, Paulesu L. Studies on the biological effects of ozone: 5. Evaluation of immunological parameters and tolerability in normal volunteers receiving ambulatory autohaemotherapy. Biotherapy. 1993;7:83–90. doi: 10.1007/BF01877731. [DOI] [PubMed] [Google Scholar]
- 56.Nowicka D. Positive effect of ozonotherapy on serum concentration of soluble interleukin-2 receptor and neopterin in patients with systemic sclerosis. Postepy Dermatol Alergol. 2019;36:158–163. doi: 10.5114/ada.2019.83651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bocci V. Dordrecht: Kluwer Academic Publishers; 2002. Oxygen-Ozone Therapy: A Critical Evaluation. [Google Scholar]
- 58.Cuadrado A, Pajares M, Benito C, et al. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci. 2020;41:598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M, Wang Y, Ndiwane N, et al. The association of COVID-19 occurrence and severity with the use of angiotensin converting enzyme inhibitors or angiotensin-II receptor blockers in patients with hypertension. PLoS One. 2021;16:e0248652. doi: 10.1371/journal.pone.0248652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galiè M, Covi V, Tabaracci G, Malatesta M. The role of Nrf2 in the antioxidant cellular response to medical ozone exposure. Int J Mol Sci. 2019;20:4009. doi: 10.3390/ijms20164009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bocci V. The case for oxygen-ozonetherapy. Br J Biomed Sci. 2007;64:44–49. doi: 10.1080/09674845.2007.11732755. [DOI] [PubMed] [Google Scholar]
- 62.Xing B, Chen H, Wang L, Weng X, Chen Z, Li X. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury via modulation of the TLR4-NF-κB pathway. Acta Cir Bras. 2015;30:60–66. doi: 10.1590/S0102-86502015001000008. [DOI] [PubMed] [Google Scholar]
- 63.Rébé C, Ghiringhelli F, Garrido C. Can the hyperthermia-mediated heat shock factor/heat shock protein 70 pathway dampen the cytokine storm during SARS-CoV-2 infection? Br J Pharmacol. 2022;179:4910–4916. doi: 10.1111/bph.15343. [DOI] [PubMed] [Google Scholar]
- 64.Guihur A, Rebeaud ME, Fauvet B, Tiwari S, Weiss YG, Goloubinoff P. Moderate fever cycles as a potential mechanism to protect the respiratory system in COVID-19 patients. Front Med (Lausanne) 2020;7:564170. doi: 10.3389/fmed.2020.564170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galiè M, Costanzo M, Nodari A, et al. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic Biol Med. 2018;124:114–121. doi: 10.1016/j.freeradbiomed.2018.05.093. [DOI] [PubMed] [Google Scholar]
- 66.Theoharides TC. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors. 2020;46:306–308. doi: 10.1002/biof.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motta Junior JDS, Miggiolaro A, Nagashima S, et al. Mast cells in alveolar septa of COVID-19 patients: a pathogenic pathway that may link interstitial edema to immunothrombosis. Front Immunol. 2020;11:574862. doi: 10.3389/fimmu.2020.574862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Afrin LB, Weinstock LB, Molderings GJ. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theoharides TC. Potential association of mast cells with coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:217–218. doi: 10.1016/j.anai.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peden DB, Dailey L. Modulation of mast cell functions by in vitro ozone exposure. Am J Physiol. 1995;268:L902–910. doi: 10.1152/ajplung.1995.268.6.L902. [DOI] [PubMed] [Google Scholar]
- 71.Bocci V, Zanardi I, Michaeli D, Travagli V. Mechanisms of action and chemical-biological interactions between ozone and body compartments: a critical appraisal of the different administration routes. Curr Drug Ther. 2009;4:159–173. [Google Scholar]
- 72.Jacobs MT. Investigation into pitfalls and typical complications in ozoneoxygen therapy. OzoNachrichten. 1982;5:1–5. [Google Scholar]
- 73.Gil-del-Valle L, López-Fernández OE, Márquez JAS-, et al. Amelioration of symptoms and oxidative stress in hospitalized convalescent post SARS-CoV-2 patients treated with rectal ozonetherapy and nutritional supplementation. Int J Mod Pharm Res. 2020;4:94–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


