Abstract
One of the major causes of death on the globe is cancer. It has remained a significant obstacle for current therapies and has not yet been effectively treated. Conventional treatment strategies available for cancer such as surgery, chemotherapy, radiation therapy etc. have severe adverse effects. The use of herbal active constituents in cancer treatment has tremendous potential to increase the effectiveness of conventional cancer therapy. Natural plant active components have been reported to have strong in vitro pharmacological activity but narrow in vivo absorption. In order to increase their bioavailability and absorption and get around the drawbacks and negative effects of traditional herbal extracts, Phytosomes are one of the growing nanotechnologies that can be used to improve the miscibility of bioactive phytoconstituents in lipid-rich barriers and overcome their poor bioavailability. Many novel drug delivery carriers are employed for targeted delivery of phytoconstituent at the site of action. Phytosomes are well-known biocompatible nanocarriers that can be employed to increase the solubility and permeability of phytopharmaceuticals among various novel drug delivery systems (NDDS). This review mainly focused on various conventional as well as novel approaches and various Nano carrier used in cancer therapies. Also comprising summary of the most recent research on the development and use of phytosomes as a better carrier for herbal constituents in the treatment of cancer. Additionally provides information about the formulation, characterization technique and mechanism of drug release from phytosome. Some of the major herbal active constituents made of phytosome which have shown proven anticancer activity are also studied. Finally, challenges and future perspective related to phytosome in cancer treatment are also discussed.
Keywords: Phytosomes, Cancer therapies, Targeted drug delivery, Herbal extract, Nanocarrier
Graphical abstract
1. Introduction
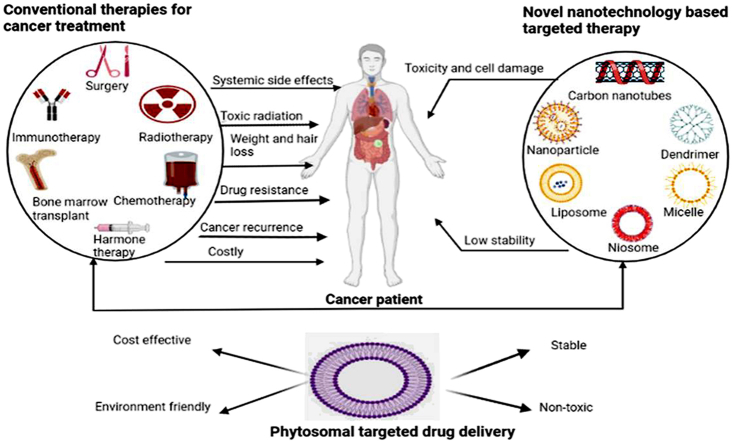
The rate of new cancer cases or cancer death rates are increasing in the digital world, and in the coming decades, it is anticipated that the number of people diagnosed with cancer will double, Therefore, to increase their therapeutic potential, there is also an increasing need for the creation of more potent anticancer medications from conventional sources [1]. There has been a significant advancement in the creation of new anti-cancer medications in recent years, particularly novel drug delivery systems that improve the pharmacodynamics and bioavailability of medications and deliver precise drug concentrations to cancer cells with little cytotoxicity to healthy cells [2]. Conventional cancer therapeutic approaches such as surgery, chemotherapy, radiation therapy, hormone therapy, immunotherapy, targeted therapy, or combination of them, have made considerable advancements in the past few years and to a certain degree, improved the outlook for cancer patients [3]. Due to the lack of medicine bioavailability at the intended location, which can result in several different side effects, including death, these procedures are heavily criticised. Many scientists and researchers have sought to create targeted or site-specific medication delivery methods to address the mentioned difficulties. Nanotechnology is the novel and targeted drug delivery system for treatment of cancer and the future of medical science. Nanostructures including nanoparticles, liposomes, dendrimers, and micelles have been employed as controlled delivery systems for the treatment of cancer [4]. For many years, herbal remedies have been used and are still used as the primary form of healthcare in developing countries. Plants have been utilised in medicine because of their inherent antibacterial characteristics. Therefore, research has concentrated on the possible applications and characteristics of tropical herbal extracts for the development of prospective nano-based medicines for diseases like cancer [5]. Natural materials in the form of extracts are frequently examined for intended bioactivities. Extracts exhibiting the desired bioactivity are next fractionated to isolate and identify the bioactive ingredients. Plants, in particular, are a natural source that has been thoroughly examined for anti-cancer properties [6]. Natural plant active components have been reported to have strong in vitro pharmacological effects but only moderate in vivo absorption [7]. There is a strong need to develop a new drug delivery method to address the issue of low water solubility i.e., less in vivo absorption. Nanotechnology-based medication delivery methods are developing to solve the problem of low-solubility compounds’ bioavailability [8]. Among all effective methods, Phytophospholipid complexes, also known as phytosomes, have become a successful method to enhance the active ingredients' bioavailability [7].
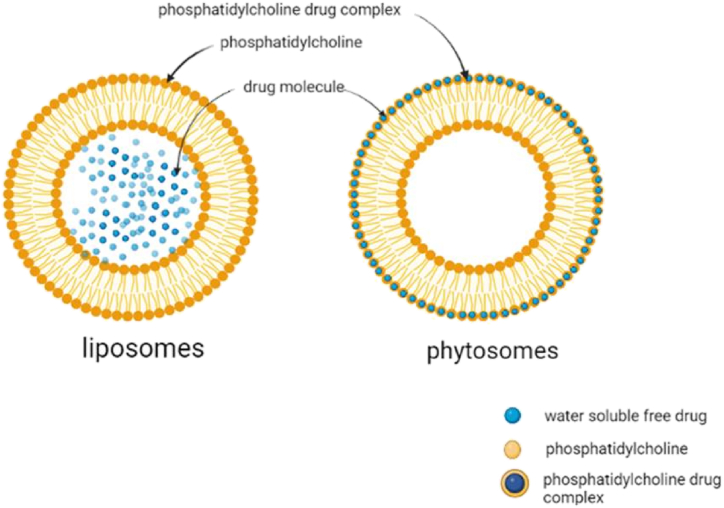
The words "phyto" and "some," which relate to both plants and cell-like structures, are combined to form the term "phytosome," which is also known as "herbosome" in some literature. Phytosomes and liposomes are quite similar. In order to improve their bioavailability and absorption and get around the drawbacks and negative effects of traditional herbal extracts, phytosomes, an unique developing vesicular drug delivery technology, include plant extracts or hydrophilic phytochemicals in phospholipids [9]. They are micelles formed when phospholipids and water interaction. The addition of polyphenolic plant extracts enhances the interaction. The polar functional groups of lipophilic compounds conjugate with the charged phosphate head of phospholipids via hydrogen bonds and polar interaction, forming a micelle arrangement. The polyphenolic compounds are encapsulated into these micelles. Phytosomes differ from liposomes in that the bioactive compound is dissolved in a medium and is contained in the core cavity or between the shell membrane layers. In phytosomes, the bio active compound forms an integral part of the micelle where the molecules are anchored to the polar heads of the phospholipids through chemical bonds. Liposomes are used to deliver only water-soluble compounds, whereas phytosomes are deliver both water and lipid soluble compounds over the liposomes. Phytosomes are used as a vesicular drug delivery system for cancer. Vesicular drug delivery systems in common are passive targeting drug carriers by evading the immune system. However, in the case of tumour therapy, phytosomes with a molecular weight greater than 40 kDa and a nano-metric size range of 100–1200 nm actively target tumour cells due to enhanced permeation and retention. Passive targeting increases drug bioavailability, while active targeting specifically delivers drugs to the site of action. These strategies are combined in phytosomes to deliver bioactive compounds [10]. They are produced via a patented process whereby the individual components of an herbal extract are bound to lipid (e.g. phosphatidylcholine) [70]. A phytosome is a complex of polar polyphenolics and dietary phospholipids with distinct physicochemical and spectroscopic properties. Phytosome were initially studied for cosmetic purposes, but mounting evidence of drug delivery potential has accumulated over the last two decades, with beneficial activity in the areas of cardiovascular, anti-inflammatory, hepatoprotective, and anticancer applications [11]. The idea of phytosomes was initially applied to the administration of medication in 1991, with complexation between the phospholipid's polar region and its phytoconstituent [8]. To increase their bioavailability and absorption and get around the drawbacks and negative effects of traditional herbal extracts, phytosomes, an innovative developing vesicular drug delivery technology, incorporate plant extracts and hydrophilic phytocomponents in phospholipids [9]. A novel cancer therapeutic approach was developed by combining phytochemicals from herbal plant extract with a carrier made of phospholipids to decrease the harmful effects of traditional chemotherapy and enhance its pharmacological effects [1]. The manufacturing process of phytosomes is straightforward, does not require difficult or expensive equipment, and does not interact with the encapsulated herbal ingredients due to strong chemical interaction among phytosome phospholipid and phytochemical [12]. Thus this review highlights the application and advancement of phytosome in cancer diagnosis, their formulation, characterization, mechanism of action, challenges and future perspective in cancer therapy.
2. Role of ayurveda in cancer therapy
Cancer is one of the major cause of mortality around the globe. It is distinguished by extremely rapid growth, evasion of growth inhibitors, replicative immortality, and immune system evasion. Most cancer patients had surgical intervention, radiation, or standard chemotherapy. Patients who receive this systemic therapy experience severe adverse effects such as extreme exhaustion, hair loss, bruises, bleeding, anaemia, nausea, and vomiting. Consequently, alternative cancer medicines and treatments must be developed [13]. Herbal remedies have been and continue to be the mainstay of medical care in underdeveloped nations for many years. Due to their inherent antibacterial characteristics, plants have been used in medicines. Consequently, research has advanced to examine the possible application of extracts from terrestrial plants for the development of prospective nanomaterial-based medicines for diseases like cancer. In developed countries, many plants are ingested for their health advantages, whereas for thousands of years, people in Asia and Africa have used natural herbs in traditional medicine. According to the World Health Organization (WHO), some nations still primarily use plant-based medicines, and developing nations are utilising the therapeutic advantages of substances derived from sources that occur naturally. Polyphenols, brassinosteroids, and taxols are among the substances that have been discovered and isolated from terrestrial plants for their anticancer characteristics. According to sushrut, the vata, pitta, and Kapha doshas especially the vata and kapha doshas when inflamed, have the potential to create a cancerous tumour. This has an impact on the tissue, which could lead to the growth of a cancerous tumour [5]. The ayurvedic classification of neoplasms is based on the relationship between numerous clinical signs and tridoshas. Group I: Conditions that are categorically classified as malignancies. Group II: Conditions like growths and ulcers that have the potential to develop into cancer. Group III includes conditions including intractable jaundice, intractable leukorrhea, and intractable sinusitis that have the potential to develop into cancer [14]. Because they have several health benefits for people, extracts that are high in nutraceuticals and have medicinal value are employed in ayurvedic treatments. These ayurvedic preparations can be utilised in a variety of ways, including chewing, applying to the skin, swallowing, ingesting milk, ghee, tea, honey, etc. More than 25,000 plant-based or herbal remedies have been employed in Indian traditional medicine up to this point. On the indian market, there are over 75 formulations for promoting human health and vitality. More than 219 formulations contain triphala which has powerful anticancer properties. Various plant extracts are renowned for their capacity to improve therapeutic outcomes and serve as adjuvants in cancer therapy regimens [15]. An Ayurvedic strategy emphasising increasing tissue metabolism, tonifying digestion, removing toxins, and limiting tumour growth is viewed as helpful in situations where the biomedical treatment has failed or is unavailable [16]. Phytomedicines have been observed to have reduced in vivo activity for a variety of reasons, including low water solubility, insufficient molecular size, and reduced systemic availability. Furthermore, accurate identification and isolation of phytoconstituents in active form demand considerable time and expense. Extracts of many phytoconstituents display structural biological fragility, early drug loss via quick clearance, frequent biotransformation, and gastric or enzymatic destruction [17]. Thus, a novel strategy that encourages cancer cell-specific targeting through the use of nanotechnology is a current research trend to overcome these negative effects [18]. Nanotechnology is a new field that first gained ground a few decades ago and has the potential to solve these biopharmaceutical problems. In order to meet unmet requirements, nanotechnology has transformed the pharmaceutical and medical industries. Enhancing solubility, bioavailability, toxicity, pharmacological activity, stability, maintaining the release profile, and physical and chemical stability are further benefits of nano-based herbal development that help achieve desired safety and efficacy [17].
3. Various approaches for cancer treatment
3.1. Conventional approaches for cancer treatment
One of the most popular forms of cancer treatment includes chemotherapy, radiation therapy, hormone therapy, immunotherapy, targeted therapy, or a combination of them. There are a variety of treatments available depending on the type of cancer, the severity of the condition, how quickly it is progressing, the patient's health, and how the treatment is working [71].
3.1.1. Surgery
Surgery is a common method of cancer treatment for non-haematological malignancies. The surgeon takes the cancerous tissues out of the body surgically. Cancer may be completely or partially cured with surgery. There is no surgical procedure that can entirely remove cancer if it has spread to other places of the body. Surgery is a viable option for the treatment of cancer in cases where the tumour is small and confined. Surgical cancer therapies include prostatectomy for prostate cancer, brain tumour through neurosurgery, mastectomy for breast cancer, and kidney, lung, and liver cancers, among others [19]. Surgery might involve cutting, abrading, suturing, managing acute illnesses and injuries, or removing obstructions from chronic and slowly advancing disorders [20].
3.1.2. Chemotherapy
Chemotherapy mostly affects cancer cells by preventing them from proliferating and growing. Cancer cells often reproduce and expand at a rate that is significantly faster than that of normal cells, and they also physiologically have very high levels of endogenous stress. Therefore, compared to other cells in the area, the medications can quickly and more efficiently kill them. The kind and stage of cancer heavily influence the decision to use chemo-preventive medications alone or in combination. These medications main goals are to combat malignant cells and lessen the stress brought on by the development of the tumour. Two crucial factors to think about are the treatment's dosage and duration. It has been noted that the drugs are administered at extremely high levels, which results in a variety of adverse effects and injury to other healthy cells. The recurrence of the illness is a significant obstacle [21]. Chemotherapy drugs cause cell death by apoptosis either by interfering directly with DNA or by targeting the essential proteins needed for cell division. Sadly, they can also be "cytotoxic" to healthy cells that are dividing, especially those with a high turnover rate like the bone marrow and mucous membranes [20].
3.1.3. Radiation therapy
Radiation is used as a physical agent to kill cancer cells. Because it creates ions (electrically charged particles) and deposits energy in the cells of the tissues it passes through, the radiation employed is known as ionising radiation. This accumulated energy may either kill cancer cells or change their genetic makeup [22]. By directly killing cancer cells or by inflicting DNA damage that results in tumour cell death, radiation has proven to be beneficial. Thus, therapeutic radiation has emerged as one of the main cancer treatment techniques, alongside chemotherapy, immunotherapy, hormone therapy, and surgery. Cancer sufferers will have the chance to live longer and have a higher quality of life because of ongoing research and technological advancements, which will also help cancer become less common as a cause of death globally [23]. For the majority of tumour types, including malignancies of the brain, breast, cervix, larynx, liver, lung, pancreas, prostate, skin, stomach, and uterine, radiation therapy is frequently used to treat these conditions. Leukemia and lymphoma are two more malignancies that can be treated with radiation treatment. The radiation dose to be administered to the cancer or tumour site according to several variables, comprising the patient's age, the type of tumour, its location, and any potential radiation source side effects on neighbouring tissues and organs [19]. Conversely, radiation kills cells that divide quickly and have trouble fixing their DNA [20].
3.2. Novel approaches for cancer treatment
3.2.1. Immunotherapy
The cancer-fighting ability of the immune system is enhanced by immunotherapy. This is often referred to as biological therapy since it activates the body's natural defences against sickness to combat malignancy. Numerous studies have been done on the use of monoclonal antibodies, which teach the defence mechanism to recognise and cause the death of cancer cells and to treat cancer. These antibodies disrupt a specific protein's activity by attaching to cancer cells. This therapy approach is secure [19]. Immunotherapies are frequently restricted by immunological-related adverse events, an inflammatory reaction against the host's healthy tissues, and immune activation. Although immune activity against the host tumour is the intended result, it might be difficult to predict, identify, and treat [24].
3.2.2. Hormone therapy
Oestrogen and progesterone receptor-positive breast cancer and prostate cancer can both be successfully treated with hormone therapy since it is both safe and effective. Almost all patients with metastatic breast and prostate cancer who respond to hormone therapy develop resistance., which causes the disease to advance. Resistance has numerous, diverse, and poorly understood molecular origins. Increased hormone receptor sensitivity and pathway activation without hormone exposure are two possible mechanisms [25]. To treat specific cancers that rely on these substances to thrive and spread, hormone treatment reduces the number of hormones in the body. Breast, reproductive system, and prostate cancers are all treated using this procedure. The adverse effects vary according to the cancer kind, age, sex, and type of medication used in the treatment [19].
3.2.3. Gene therapy
Gene-based cancer therapies in clinical trials include ex vivo and in vivo cytokine gene transfer, drug sensitization using prodrug delivery genes, and protection from high-dose chemotherapy using drug-resistant genes in the bone marrow. Two methods for attacking the underlying genetic damage in cancer cells are gene substitution and oncogene as well as tumour suppressor gene inactivation [26]. The term "gene therapy" describes a broad spectrum of medical procedures that all alter cells using genetic material (either in vitro or in vivo) to treat the disease. Numerous preclinical and in vitro animal models used to test a wide range of gene therapy drugs have demonstrated amazing success. The development of cancer vaccines, the targeting of viruses to cancer cells for lysis and destruction, the reduction of the blood supply to tumours, and the introduction of genes into cancer cells that bring out death or re-establish the normal cellular phenotype have all shown to increase survival [27].
3.2.4. Targeted therapy
Due to their superior effectiveness and safety compared to conventional chemotherapy drugs, targeted therapeutic drugs have gained popularity as cancer treatments. Since the US Food and Drug Administration (FDA) approved the first tyrosine kinase inhibitor, imatinib, for sale in 2001, an increasing number of small-molecule targeted drugs for cancer treatment have been developed. Targeted medications can roughly be divided into two groups: macromolecules and small molecules (e.g, monoclonal antibodies, polypeptides, antibody–drug conjugates, and nucleic acids) [28]. Lack of accurate preclinical models to forecast the effectiveness of anticancer drugs, inefficient clinical development, only a few single-agent activities are some difficulties associated with targeted cancer therapy [29].
3.2.5. Nanocarrier for cancer therapy
In cancer therapy, nanotechnology-based treatments are widely utilised to improve drug solubility, stability, and multidrug resistance as well as to increase the safety and effectiveness of cancer treatment. Exosomes, polymersomes, dendrimers, nanoparticles, polymeric drug conjugates, polymeric micelles, and dendrimers are a few examples of effective carriers used in nanotechnology-based drug delivery systems [30]. These man-made non-bioactive nonviral vectors offer a reliable method of transferring therapeutic material to cells. Unique benefits of this strategy include low immunogenicity, reduced toxicity, and flexibility for chemical alterations [31].
3.2.5.1. Nanoparticles
Depending on their intended use, nanoparticles are structured that typically range in size from 10 to 1000 nm. The medication is solubilised, enclosed, trapped, or bonded to a nanoparticle matrix in the drug delivery area [32]. Deep tissue penetration of NPS has been discovered to enhance the permeability and retention (EPR) effect. Furthermore, surface properties affect the bioavailability and half-life by successfully crossing epithelial fenestration. By adjusting the particle polymer properties, it is possible to maximise the release rate of medications or active moieties. Together, the unique characteristics of NPS control their therapeutic impact in the prevention and treatment of cancer [33]. Although there are various restrictions on nanoparticles during the formulation, drug loading, and scale-up processes, they have a great chance of receiving FDA/EMA approval. FDA-approved nanoparticles were at their maximum level between 2001 and 2005; during this time, a decline began that may have been caused by the 2008 global financial crisis. But even so, it has the largest proportion of all the nano-carriers [32]. Due to their potential for use in the treatment and diagnosis of cancer, inorganic nanoparticles have received a lot of attention. Inorganic nanoparticles have unique, size-dependent physical characteristics like magnetism, electrical and optical effects, and an effective contrasting effect. Nanometer-sized quantum dots, manganese phosphate nanoparticles, noble metals, carbon nanotubes, silica nanoparticles, and magnetic nanoparticles are a few examples of inorganic nanoparticles. They also have outstanding preservation qualities and good microbiological resistance [34].
3.2.5.2. Dendrimer
Spherical polymeric macromolecules known as dendrimers have a well-defined hyperbranched topology. Dendrimers are characterised by their highly branching architectures [33]. These dendrimers' special properties, which include tunable surface functionality, multi-valency, water solubility, mono-disperse size, and internal drug space, promote drug administration [26].
3.2.5.3. Micelle
When compared to other methods, polymeric micelles are effective at transporting anticancer medications that are not very water-soluble, they improve drug stability, and they are also more site-specific, which increases therapeutic efficacy. A weakly water-soluble medication can be delivered via micelles, which have a size range of about 5–100 nm [30]. Polymeric micelles are spherical, colloidal, and supramolecular nano-constructs (10–100 nm) typically formed by self-assembly of amphiphilic block copolymers that contain both hydrophilic and hydrophobic groups in an aqueous environment. Numerous characteristics of polymeric micelles favor their use in cancer drug delivery applications. The ability of polymeric micelles to solubilize poorly water soluble or hydrophobic drugs within their core, thereby increasing their bioavailability, is their most significant advantage [35].
3.2.5.4. Cyclodextrins
Cyclodextrin are interesting macromolecules that are found naturally capable of improving the structure and attributes of the multifunctional Cyclodextrin-based delivery systems described. They are a remarkable excipient to improve drug apparent solubility, stability, and bioavailability as well as a useful tool for the construction of new drug delivery systems due to their exceptional capacity to act as molecular containers by entrapping a variety of guest molecules in their internal cavity. These characteristics are particularly helpful when it comes to chemotherapy because the majority of anticancer medications have both low permeability and decreased water solubility. It has proven to improve drug apparent solubility, lessen toxic side effects, and improve the bioavailability of the reported anticancer agents, making it a useful anticancer therapy strategy [36].
3.2.5.5. Carbon nanotubes
Because of their unique properties, CNTs were extensively researched in targeted drug delivery for cancer therapy. Because of their inherent optical properties, CNTs can be used as outstanding radiation treatment mediators in addition to being drug carriers for a variety of anticancer agents. The versatility of CNTs allows for a wide range of therapeutic uses in the treatment of different cancers. Even after their benefits, CNT-based therapies still face several challenges in the clinical setting. The safety of CNTs in the human body has not been thoroughly investigated. Consequently, additional research work is still needed to confirm the long-term safety of CNT-based nanotechnology in human bodies before their potential for use in clinics. Given that CNTs are fairly difficult to produce on a large scale, another issue preventing the clinical application of functionalized CNTs is the lack of easily controlled manufacturing production with high replicability [37].
3.2.5.6. Liposomes
A centre aqueous area is encircled by an outer lipid bilayer in liposomes, which are self-assembled, closed colloidal entities made of lipid layers. By applying various techniques, these lipid-based systems are now useful for the operation of a variety of cancer treatments. The use of Immunoliposomes, which have enhanced drug delivery selectivity, is a prospect for liposomes [26]. Doxorubicin, paclitaxel, and nucleic acid can all be delivered via liposomes, which also exhibit improved absorption and greater anti-tumour activity [33].
3.2.5.7. Niosomes
Niosomes have a bilayer strong resemblance to liposomes, but they also have nonionic surfactants in an aqueous phase. They exhibit better stability, have a long shelf life, are biodegradable, nonimmunogenic, biocompatible, and can transport a treatment to its target site over time. Tamoxifen and doxorubicin-loaded self-assembled niosomes were created by Kulkarni et al. for breast cancer management. Tamoxifen and doxorubicin were reported to have entrapment efficiencies of 74.3% and 72.7%, respectively. Breast cancer treatment with niosomes seems to be possible [20]. Niosomes have the potential to be a more effective treatment than traditional drug-delivery platforms and are a highly effective technique for drug delivery in the therapeutic regime of many diseases [38].
3.2.5.8. Ethosomes
Ethosomes are appealing, preferred, and novel drug delivery systems. Using ethosomes, drugs with poor skin penetration capacity can be given. In essence, ethosomes are ethanolic liposomes made of lipid vesicles with phospholipids; large quantities of ethanol or isopropyl alcohol may be employed [20]. Ethosomes are non-invasive transporters that let pharmaceuticals reach deep skin layers and the circulatory system. Soft vesicles called ethosomes are designed to enhance the transport of active substances like medicines and natural channels [39]. A well-known permeation enhancer called ethanol gives ethosomes special properties like high elasticity and deformability that allow them to penetrate deeply across the skin and improve drug permeation and deposition, with respect to treating various pathologies, such as alopecia, psoriasis, acne, and skin cancers, the improved composition of ethosomes offers important advantages over conventional liposomes in particular [40].
3.2.5.9. Transferosomes
Early in the 1990s, transfersomes, a class of deformable or elastic nanocarriers first appeared. Regular liposomes are restricted to the stratum corneum layer of the skin and do not penetrate deeper levels. As an upgraded form of liposomes, other varieties of lipid carriers, like transfersomes, have been created [39]. The recently introduced innovative drug carriers are transfersomes, which are highly deformable vesicles capable of transporting big molecules over intact mammalian skin. In its broadest meaning, a transfersome is a device that cans instinctively pass-through skin and deliver medications from the application to the target place. The use of transfersomes in the diagnosis of cancer includes melanoma, basal cell carcinoma etc. The transfersomes may be an effective medication delivery method that can penetrate the skin's layers, making them helpful for the treatment of skin cancer [41].
3.2.5.10. Exosomes
A nanosphere with a bilayered membrane known as an exosome is capable of efficiently delivering drugs and is nonimmunogenic due to its composition, which is comparable to that of the body's cells [30]. These are naturally occurring nanoparticles that are known to play crucial roles in intercellular communication. They carry a wide range of lipids, proteins, metabolites, RNAs (mRNA, miRNA, tRNA, long non-coding RNA), and DNAs as part of their cargo. (mtDNA, ssDNA, dsDNA). Exosomes are involved in complex biological processes and have been suggested for use in drug delivery because they can carry both small molecules and large molecules, supporting their use as therapeutic agents for a variety of diseases, including cancer [42].
3.2.5.11. Aquasomes
The pharmaceutical and biotechnology vesicles known as aquasomes are submicron and based on the self-assembling principle. It has a polyhydroxy oligomeric film on top and a particulate core made of nano-crystalline calcium phosphate or ceramic diamond. Even in the case of conformationally sensitive ones, the medication candidates supplied by aquasomes show greater biological activity. Aquasome is an effective delivery system transporting biological compounds such as a peptide, proteins, hormones, antigens, and genes to specific places due to its features that protect and preserve delicate biomolecules, maintain structural integrity and expose surface area [43].
3.2.5.12. Phytosomes
The plant extracts complexed with phosphatidylcholine that forms a new drug delivery system known as phytosome, which showed better phytopharmacological profiles of many plant medicines. The phytosome showed promising bioavailability of various phytomedicine present in milk thistle, grape seed, green tea, olive, and turmeric [44]. Phytosomes are well-known biocompatible nanocarriers that can be employed to increase the solubility and permeability of phytopharmaceuticals among various NDDS [45]. Phytosomes because of its improved ability to cross the lipid-rich bio-membranes and get through the blood circulation, is found to exhibit more bioavailability as compared to simple herbal extracts [44]. Formulations containing phytosomes have significant pharmacological benefits, like anti-inflammatory, antioxidant, and neuroprotective properties, and they can enhance the penetration and bioavailability of phytoconstituents. Although it has been studied as an anticancer agent in the treatment of many types of tumours [9]. The synthesis of Phyto-phospholipid complexes is simple, and they have a superior drug complexation rate than other types of complexes. Additionally, they exhibit greater stability due to the synthesis of chemical linkages between phosphatidylcholine and plant extracts. They enhance liver targeting by enhancing the solubility of bile to active components [7]. Phytosomes are one of the growing nanotechnologies that can be used to improve the miscibility of bioactive phytoconstituents in lipid-rich barriers and overcome their poor bioavailability [12].
4. Phytosomes as a novel approach for cancer management
4.1. Definition
Phytosomes are a pioneering lipid-based delivery system with a structure resembling liposome (Table 1), that can be used to entrap various phytoconstituents with polyphenolic bases to enhance their absorption when administered. Indena company (Milan, Italy) created the first phytosomes in the late 1980s with the intention of increasing the bioavailability of medications by complexing them with phospholipids [12]. It is a patented technique to combine phytoconstituents with phospholipids like phosphatidylcholine (PC) to create lipid-compatible molecular complexes that can increase the phytochemical’s absorption and bioavailability. In pharmacokinetic investigations, phytosomes have been effectively used to improve the formulation of many kinds of herbal extracts (ginkgo, green tea and milk thistle) and phytochemicals (e.g. silybin, curcumin and ginkgolides) [46]. It is a nano-delivery system which consist phospholipid-phytocompound conjugates. The main difference between phytosomes and liposomes is that the active ingredient in liposomes is distributed in the medium contained in the cavity or in the layers of the membrane, whereas in phytosomes, it is an integral part of the membrane, being the molecules stabled through hydrogen bonds to the polar head of the phospholipids (Fig. 1) [7]. In this, trapped phytochemicals interact on a molecular level with the phospholipids that make up the liposome. The polar head group of the phospholipid and the phytochemical that has been trapped are said to establish a hydrogen bond [47]. The phytosomes is a mixture of phospholipids and plant compounds, including phosphatidylcholine (PC), phosphatidylethanolamine, phosphatidylserine, soy phospholipid, and egg lecithin. The most popular phospholipid among these is PC since it can be applied topically or ingested and is miscible and absorbable [9]. The development of phytosome nanotechnology may have an impact on medicine delivery by removing obstacles caused by inadequate lipid solubility and increasing the bioavailability of bioactive phytochemicals [12]. The efficacy of phytosomal carriers allows them to be applied topically to increase the bioavailability of phytoconstituents [43].
Table 1.
Difference between phytosome and liposome.
| Property | Phytosome | Liposome | Reference |
|---|---|---|---|
| Structure | The phytochemicals are conjugated to the hydrophilic choline head and it becomes a part of the phospholipid. | The active compounds are dissolved in intermate aqueous core or bilayer lipid membrane. | [2,9] |
| Nature of bond | Chemical bond (Hydrogen bonding) | No chemical bond | [2,48] |
| Drug: Phospholipid ratio | 1:1 or 2:1 molecular complex | thousands of phospholipid molecules gathered around the drug | [2,48] |
| Encapsulation | H-bonds hold the bioactive molecules to the polar tip of the phospholipids. | The lipid bilayer membrane or the aqueous interior of the vesicles both contain active components. | [12] |
| Route of administation | Oral and topical | Topical and parenteral (No evidence exists for oral delivery.) | [48] |
| Bioavailability | High | Lower than phytosome | [48] |
| Stability | High | Lower than phytosome | [9,12] |
Fig. 1.
Structural difference between liposome and phytosome.
4.2. Method of preparation
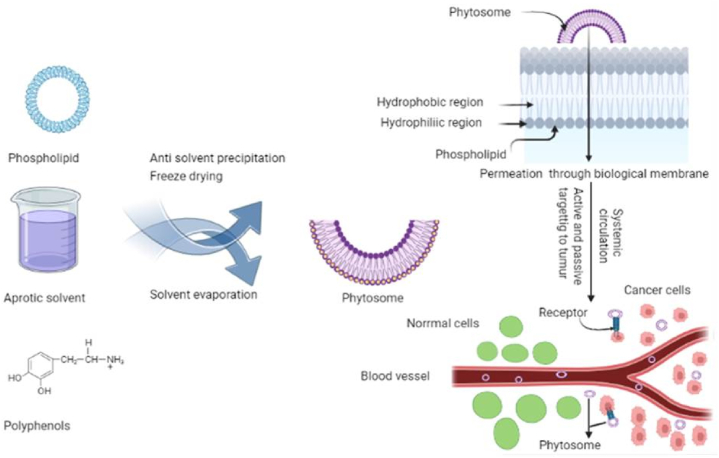
To make phyto-phospholipid complexes, there are three main techniques: anti-solvent precipitation, freeze drying, solvent evaporation (Fig. 2) [7]. To create phytosomes complexes, standardised herbal extracts require stoichiometric interactions with natural or synthetic phospholipids. Molar ratios can be between 0.5 and 3. (phospholipid: phytoconstituents). The preferred molar ratio, however, was typically given as 1:1 [2]. The process of making a phytosome involves five key steps: mixing biomaterials, making a clear solution of the biomaterial using phospholipids or an inorganic solvent, evaporating the solvent and forming a thin film, hydrating the mixture, and sonicating the mixture. As aprotic solvents, dioxane, acetone, methylene chloride, tetrahydrofuran, or ethyl acetate are employed to solute bioactive components. Additionally, several aprotic solvents for solubilizing bioactive components, such as n-hexane, are utilised to precipitate the initial solvent [9]. Solvent, the stoichiometric ratio of active ingredients, reaction temperature, and reaction duration are the primary variables that influence the formation of Phyto-phospholipid complexes [7].
Fig. 2.
Synthesis and mechanism of drug release from phytosomes.
4.2.1. Solvent evaporation/thin film hydration method
A traditional and widely used technique for making phospholipid complexes is solvent evaporation. Phosphatidylcholines and active ingredients were combined in the shared round bottom and dissolved in a good solvent by heating at the ideal constant temperature for a predetermined amount of time. Complexes that have been prepared can be obtained by evaporating the solvent under vacuum [7]. Zhenqing Hou et al. developed phytosomes loaded with mitomycin C (MMC) soybean phosphatidylcholine complex by solvent evaporation method. In this they took 12.5 mL of tetrahydrofuran (THF), 10 mg of MMC powder and 30 mg of soyabean phosphatidylcholine (SPC) were codissolved, and the resulting solution was then transferred to a glass pressure vessel with 4 h of vigorous stirring in a water bath at 40 °C resulted in the creation of a clear magenta mixture. Then, using a rotary evaporator and vacuum rotary evaporation, THF was eliminated [49].
4.2.2. Anti-solvent precipitation
It is second mostly used method for preparation of phytosomes. During this procedure, lawsone and soy lecithin were refluxed with dichloromethane at a temperature that did not go above 60 °C. N-hexane was then added to the precipitate in order to get it stored overnight in vacuum desiccators [39]. Nabil A. Alhakamy et al. formulate icariin (ICA) phytosome by anti-solvent precipitation in this refluxing were used to create ICA-phytosomes. According to the experimental plan, accurately weighed amounts of ICA (27 mg) and Phospholipon® 90H (32, 64, or 96 mg) were dissolved in dichloromethane (20 mL). A concentrate of about 5 mL was produced by refluxing the solution at the temperature and for the duration specified by the experimental design. The phytosomal complex was obtained by lyophilizing the concentrate for 72 h. The dried complex was then stored in an airtight amber coloured glass container at 4 °C until further use [50].
4.2.3. Freeze drying or lyophilization
In this the phytosome are prepared by using freeze dryer or lyophilizer. May S Freag et al. synthesized diosmin (DSN) phytosome by freeze drying technique in this Dimethylsulphoxide (DMSO) completely dissolved DSN. The resulting DSN solution was added to a t-butylalchol-dissolved SPC solution, which was then stirred for 3 h on a magnetic stirrer until complex formation occurred. Lyophilization was used to isolate the complex. The vials were frozen for 4 h at 80 °C before being placed in a Cryodos-50 lyophilizer with a condenser temperature of 70 °C. Lyophilization was carried out for one day at a pressure of 40 mbar and a shelf temperature of 40 °C, followed by another day of secondary drying at 25 °C.Then the sample was removed from freeze dryer and kept in desiccator. Factors affecting these techniques are phospholipid type, drug:lipid ratio and co-solvent type [51].
4.3. Characterization of phytosomes
4.3.1. Visualization
The morphology of phytosomes can be investigated using atomic force microscopy (AFM), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Finding the trapping mechanisms and potential contaminants on the surface of phytosomes can frequently be determined by analysing their surface shape [2]. Identification of entrapment behavior, surface characteristics, and the presence or absence of impurities on the surface are frequently determined through the study of surface morphology. Following a very thin gold coating, the SEM provides photomicrographs of the phytosomes at the appropriate magnification. Phytosomes surfaces typically don't have any crystalline particles or other surface impurities. The majority of times, the spherical bulging on the surface are obtained, confirming the phytosomes' spherical shape. The TEM study can be used to clearly investigate the internal environment where the drug is entrapped and its distribution within the phospholipid mesh [52].
4.3.2. Particle size and zeta potential
Particle size and zeta potential are crucial complicated qualities that are connected to stability and repeatability. The typical particle size of phospholipid complexes varied from 50 nm to 100 nm [7]. Dynamic Light Scattering (NANO ZS Malvern instrument) was used to measure the phytosomes' particle size, and electrophoretic mobility in an electric field was used to estimate the phytosomes' zeta potential [53].
4.3.3. Entrapment efficiency
This is assessed using the ultra-centrifugation method. The formula below is used to compute the drug entrapment percentage (%) [54]. By extracting the phytosomes using suitable solvent systems and centrifuging them for a shorter period of time or longer at a higher rpm value, the percentage of drug entrapment can be calculated. The supernatant is estimated for detection of drug either by UV–Visible spectroscopy or high performance liquid chromatography (HPLC) method [52]. Drug entrapment efficiency can be calculated by using Eq. (1).
| (1) |
4.3.4. Vesicle stability
The basic methods for evaluating the stability of phytosomes vesicles in relation to changes in size and form across relevant time intervals are DLS, SEM, and TEM. DLS and SEM are useful for determining the typical vesicular size. The TEM and SEM used to keep track of how the structure and form of phytosomes change over time [2].
4.3.5. Transition temperature
By utilising differential scanning calorimetry (DSC), it is determined [48]. In DSC, interactions can be seen by contrasting the transition temperature, new peaks appearing and disappearing, melting points, and variations in the relative peak area. When compared to a physical mixture, phyto-phospholipid complexes typically show radically different characteristic peaks. Strong interactions between the active ingredients are thought to occur in addition to the two fatty chains of phospholipids, and the polar portion of phospholipids also prevents free rotation [7].
4.3.6. Crystallinity
The complexation of phytoactive chemicals results in the loss of crystallinity, which is what balances the hydrophilicity and hydrophobicity. The two primary methods that are typically used to evaluate the interaction of phospholipid with phytoconstituents, as well as the crystallinity, are DSC and X-ray diffraction (XRD) studies [2].
4.3.7. Spectroscopic conformation
Various spectroscopic methods, including 1H NMR, 13C NMR, 31P NMR, and IR spectroscopy, are used to study phyto-phospholipid complexation and molecular interactions in solution. Typically, the emergence of new bands in IR spectra and changes in chemical shift and line broadening in NMR spectra are connected with complex formation and interactions [54].
4.3.8. Retention time
The HP-TLC was described as a straightforward method for the characterization of phytosomes from the perspective of chromatography. When examined separately, phytosomes had a different retention period than phospholipids and phytoconstituents, which again supports the creation of the new complex [2].
4.3.9. Drug release
Since the release profile achieved in vitro may serve as an index of the efficacy of the carrier in vivo, drug release behavior of vesicle carriers has been the focus of intensive investigation over the recent past (Fig. 2). The most common traditional methods used to ascertain the release rate of active substances are continuous flow, sample and separate strategies, in situ processes, and membrane diffusion strategies (dialysis, micro-dialysis, fractionallises, and reverse dialysis) [39].
4.3.10. Stability study
Any formulation must maintain stability during its shelf life, and a stability study was conducted to determine this [55]. Another crucial element in the successful design of a carrier is phytosomal stability. To investigate the phytochemical alterations of phytosomes during storage and general circulation, stability studies are carried out. By measuring the mean vesicle size, zeta potential, size distribution, and trap efficiency over a period of several months, stability can be evaluated [39].
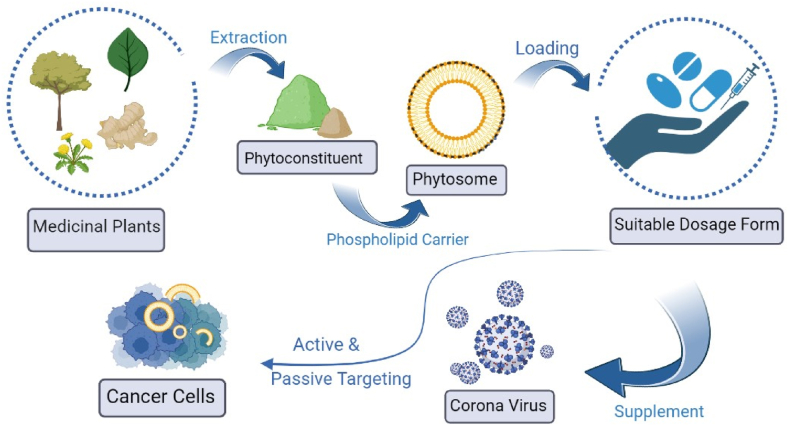
5. Advancement and application of phytosomes in cancer
The phytosomes technology is growing in the pharmaceutical industry for its ability to deliver drugs targeted both passive and active (Fig. 4). Phytosomes has several benefits (Fig. 3). The formulation method of phytosomes is simpler and easier than most drug carrier preparations. This strategy is more innovative and viable because the plant materials needed to create the phytosomes themselves serve as active pharmaceutical ingredients. Future development of phytosome technology has an immense potential [10]. The antioxidant qualities of medicinal plants chemical constituents, such as flavones, isoflavones, flavonoids, anthocyanins, coumarins, lignins, catechins, and isocatechins, primarily contribute to their anticancer potential. Numerous side effects are associated with the currently available, pricey traditional cancer therapies including radiotherapy and chemotherapy may have a significant negative impact on life quality. Bipolar moiety helps plant-derived pharmaceuticals become more soluble, dispersible, and permeable, making them an effective anti-cancer agent [56]. Various active herbal constituents showing anticancer activity on different cancer cell lines are mentioned in Table 2. Nabil A. Alhakamy et al. developed Quercetin Phytosomes with Scorpion Venom Function for Breast Cancer Management; he developed optimised phytosome and checked their effectiveness in combating MCF-7 cells. He concluded that the optimised phytosomes had vesicles with a size of 116.9 nm and a zeta potential of 31.5 mV, respectively. According to cell cycle research, the treatment with the improved QRT formula significantly arrested the cell cycle at the S phase. The study's conclusions demonstrated that a QRT phytosome formulation would represent an effective therapeutic strategy for the management of breast cancer. This study's conclusions have many important applications in real life [57]. Additionally, doxorubicin's effectiveness at inhibiting the proliferation of MCF-7 human breast cancer cells was enhanced by quercetin phytosomes [33]. Yasmiwar Susilawati et al. in their article reported that the phytosomal drug delivery system performed exceptionally well in enhancing quercetin performance; erythema was significantly (P 0.003) reduced; redness, itching, and inflammation were reduced; skin layers were improved; hydration was increased; and quercetin solubility and absorption were also increased [58]. Dina A. Hafez et al. reported that in another controlled study, lecithin curcumin phytosomal complex (Meriva®) was administered to 160 patients. Lecithinized curcumin minimise the negative effects of chemotherapy and radiotherapy, demonstrating the usefulness of curcumin phytosomes as a supporter of cancer treatment [59]. Reyhaneh Moradi Marjaneh et al. evaluated the anticancer potential of phytosomal curcumin, CT26 cells were subjected to enhancing the concentrations of curcumin (0–1000 M) and 5-FU (1–50 mg/ml) for 24, 48, and 72 h, both separately and in combination. They demonstrated how dose-dependent cell growth inhibition was caused by phytosomal curcumin and 5-FU. Additionally, co-treatment with 5-FU and phytosomal curcumin decreased the 5-FU IC50 value. They concluded that the anti-antiproliferative of 5-FU was increased by phytosomal curcumin in both in vitro and in vivo systems [60]. Curcumin conjugated with phosphatidylcholine's effectiveness in treating mammary gland tumours was examined by Ibrahim et al. In patients with solid tumours, Panahi et al. looked at the effectiveness of using phytosomal curcumin in addition to chemotherapy [46]. Zhenqing Hou et al. developed Mitomycin (MMC) c soybean phosphatidylcholine complex loaded phytosomes. To create an MMC drug delivery system, phytosomes are prepared using a solvent evaporation method paired with a nanoprecipitation procedure. The author reported that the anticancer activity of MMC-loaded phytosomes was incredible. When compared to free MMC, MMC-loaded phytosomes inhibited tumour growth more effectively and in a dose-dependent manner, without causing weight loss. According to these results, MMC-loaded phytosomes may be an encouraging and efficient formulation for drug administration and cancer treatment. A simple yet effective approach was developed to create a novel formulation of MMC-loaded phytosomes with improved formulation properties such as smaller size, reduced size distribution, higher zeta potential, and better stability. In contrast to MMC, the MMC-loaded phytosomes showed noticeably more cytotoxicity and a stronger inhibitory effect [49]. Nabil A. Alhakamy et al. developed optimised icariin (ICA) phytosomes and displayed enhanced cytotoxicity and apoptosis-inducing potential in ovarian cancer cells (OVCAR). He concluded that phytosome is a very good indicator of apoptosis. The cytotoxic properties of ICA against OVCAR-3 cells are markedly improved by its phytosome composition [50]. Nabil A. Alhakamy et al. developed thymoquinone (TQ) loaded soy-phospholipid-phytosomes and show their anticancer activity against human lung cancer cells, To transport TQ, he concludes that this phytosomal delivery would be an auspicious nanocarrier cargo [8]. Mehdi Sabzichi et al. reported that MDA-MB 231 cells were sensitive to doxorubicin when luteolin was placed in phytosomes as an advanced nanoparticle carrier. To increase the bioavailability of luteolin and boost passive targeting in breast cancer cells, researchers in this study created nanophytosomes of luteolin. The author concluded that phytosome not only made luteolin more water soluble but also improved its therapeutic effectiveness. Improvements in phytosome technology's pharmacokinetic and pharmacological parameters make them a promising option for therapeutic goals like cardiovascular, anti-inflammatory, immunomodulator, and anticancer drugs [61]. Manikkampatti et al. developed a novel phytosome-loaded gel in which aloe vera extract is encapsulated with phospholipids. The phospholipid that is bound to aloe vera has a larger molecular size, and the zeta potential values imply that the stable nature is still present. Aloe vera that has been loaded with phospholipids has an inhibiting effect on the MCF-7 cell line which is very concentration-dependent. Studies conducted in vitro have demonstrated that phytosome gel has effective anticancer effects. The higher anticancer activity is indicated by a lower IC-50 value [1]. Sharma Shalini et al. developed phytosomes made from Terminalia arjuna bark that were studied for their antiproliferative effects on the human MCF-7 cell line using the MTT test. The findings imply that quercetin phytosomes and Terminalia arjuna bark extract phytosomes are pharmacologically active and exert greater antiproliferative effects on MCF-7 cells than do pure methanolic plant extract and pure quercetin, respectively. The author concluded that compared to pure extract, the phytosome formulation has greater antiproliferative efficacy [62]. The first phytosome study on skin cancer found that the aforementioned sinigrin-phytosome complex was cytotoxic to A-375 melanoma cells. At 0.14 mg/mL, the complex reduced cell viability by more than 74%, compared to more than 46% for free sinigrin. The impact of silymarin on in vitro nanostructured lipid carriers (NLC) was investigated in the second study. In compared to commercial formulation of an unidentified phytosome (IC50: 26 g/mL), silymarin-NLC demonstrated a greater suppression of cell viability (IC50: 21 g/mL) [39]. Also Silybin and its phospholipid complex IdB1016 have been shown to have antitumor activity and to potentiate the effect of cisplatin. When IdB1016 was repeatedly administered to mice with human ovarian cancer xenografts, tumour growth was markedly reduced. When it comes to its potential benefits in the treatment of cancer, turmeric is likely the dietary supplement that has undergone the most extensive research. In rats, the bioavailability of MerivaTM (curcumin-PHYTOSOME®), the active ingredient in turmeric, and pure curcumin was compared. MerivaTM was found to be five times more bioavailable than the parent compound. Therefore, MerivaTM seems to be the best option to increase the bioavailability of curcumin and thereby enhance its biological action [11].
Fig. 4.
Advancement of phytosomal drug delivery system.
Fig. 3.
Significance of phytosomal drug delivery.
Table 2.
Phytosome showing anticancer activity.
| Active Herbal constituent | Biological source | Preparation technique | Major findings | Type of cancer | Cell line study | Reference |
|---|---|---|---|---|---|---|
| Quercetin | Terminalia Arjuna bark | Anti-solvent precipitation | The optimised QRT formulation have potential to treat breast cancer. | Breast cancer | MCF-7 cells | [2,57,62] |
| Icariin | Epimedium grandiflorum | Anti-solvent precipitation | ICA-phytosomes can cause cell death by increasing apoptosis, caspase 3, ROS production, and MMP disruption. | ovarian cancer | OVCAR-3 cells | [50] |
| Curcumin | Curcuma Longa | Rotary evaporation technique | The curcumin loaded phytosome have capability of efficiently controlling the release of medication. | Pancreatic cancer | Myeloma cell lines | [2,63] |
| Thymoquinone | Nigella sativa L | Anti-solvent precipitation method | The apoptotic potential of TQ-phytosomes against A549 cells for lung cancer was increased threefold. | Lung cancer | Human lung cancer cell line (A549) | [8] |
| Mitomycin | Streptomyces caespitosus | Solvent evaporation method | Both oral bioavailability and accumulation in cancer tissue have improved. | Anticancer | H22 cells (solid tumour-bearing mice) | [2,49] |
| Luteolin | Vegetables and fruits | Thin layer hydration method | Increased luteolin’s water solubility and therapeutic effectiveness. | breast cancer | MDA-MB231 cells | [61] |
| Aloe | Aloe vera extract | Thin film hydration | The aloe vera loaded phytosome are biocompatible and had a growth inhibiting impact on MCF-7 cancer cell line. | Anticancer | MCF 7 cell line | [1] |
| Genistein | Dyer's Genista tinctoria L. | Solvent evaporation method | Breast cancer specific Gen phytosome have stronger chemotherapeutic effects on breast cancer. | Breast cancer | Ehrlich Ascites Carcinoma (EAC) | [64] |
| Chrysophanol | Colubrina greggii | Anti-solvent precipitation | Increased superior solubility, dissolution and amorphous properties. Also increses therapeutic usefulness and GI tract absorption. | Anticancer | – | [65] |
| Emodin | Rhubarb | Solvent evaporation | Solubility and dissolution rate of emodin was increased. | Anticancer | – | [65,66] |
5.1. Curent phytosomal delivery against various cancers
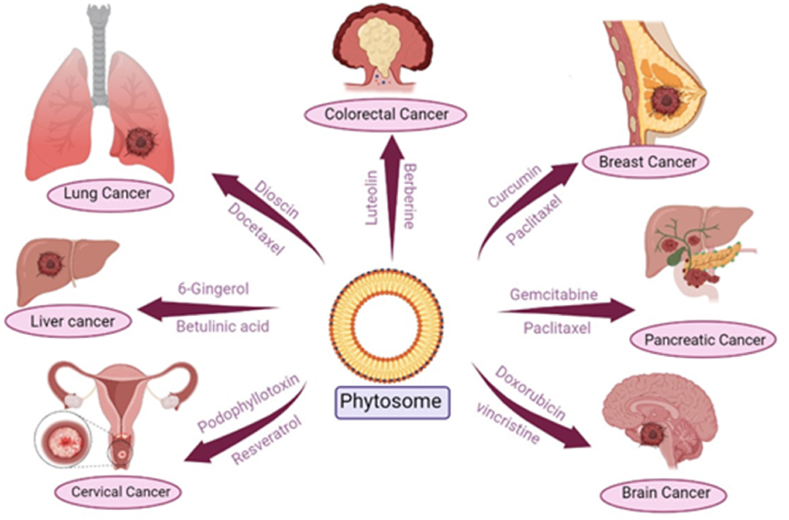
Lung cancer is the second most prevalent cancer. The phytochemicals -elemene, dioscin, docetaxel, hydroxycamptothecin, paclitaxel, and vinorelbine are studied most for their potential in the treatment of lung cancer. The most common cancer in women is breast cancer. The phytochemicals with potential to treat breast cancer include curcumin, docetaxel, paclitaxel, piperine, and resveratrol. The third most common cancer in the world is colorectal cancer. The phytochemicals luteolin, berberine, curcumin, and galbanic acid have the potential to fight colon cancer. Curcumin, phytol, quercetin, and vincristine are the major phytochemicals used to treat leukemia. One of the leading causes of death for women is cervical cancer. Podophyllotoxin, resveratrol, curcumin showed potential to treat cervical cancer. Liver cancer is the fourth most common cancer that kills people worldwide, according to the WHO. The phytochemicals 6-gingerol, betulinic acid, resveratrol, and triptolide may be effective in treating hepatocellular carcinoma. The development of malignancy in pancreatic tissue is known as pancreatic cancer. Curcumin, gemcitabine and paclitaxel showed potential to treat pancreatic cancer. Phytochemicals such as vincristine and doxorubicin have potential to treat brain cancer [18]. Fig. 5 illustrates the current phytosomal approach against various cancers, the phytoconstituents in the form of phytosomes can be given for the treatment of cancer.
Fig. 5.
Phytosomes used in various cancer therapy.
5.2. Phytosomes on COVID 19
The World Health Organization (WHO) designated the viral virus, which started in december 2019 and spread globally on March 11, 2020, to be a pandemic. It is brought on by the SARS-CoV-2 coronavirus, which has produced an alarmingly high number of infections and deaths (4.9 million) from acute severe respiratory syndromes worldwide [67]. The human body is unable to generate quercetin, which is a member of the flavanols class. It has a yellow tint and is not very soluble in boiling water, although it is quite soluble in lipids and alcohol. Widely used as a nutritional supplement, quercetin may protect against several ailments. In COVID-19 quercetin phytosome is used as a supplement (Fig. 4). The relatively recent confirmation of quercetin's reasonable potency as a 3CLpro inhibitor using biophysical techniques. Any effort to get over this restriction should be welcomed as quercetin's relatively low oral bioavailability profile already puts a lot of strain on its potential antiviral activities. The oral bioavailability of quercetin has recently been evaluated in humans using a phospholipid delivery form (Quercetin Phytosome®). After quercetin's conjugated form, namely glucuronide, was hydrolyzed, whole quercetin's bioavailability was determined to be 20 times higher, according to pharmacokinetics research. Additionally, it was noted that a particular type of glucuronidase can cause the body to produce free systemic quercetin. A bioavailable form of quercetin, such as Quercetin Phytosome®, should be thought of as a potential candidate to combat COVID-19 in a clinical setting while also taking into account its anti-inflammatory and thrombin-inhibitory effects [68]. Quercetin Phytosome® (250 mg twice a day) multivitamin supplements for 3 months as a defence against symptomatic COVID-19 was studied by Mariangela Rondanelli et al. to determine its potential effects. There were 120 participants in total (63 men and 57 women, ages 49 and 12), with 60 in the supplementation group and 60 in the placebo group. In terms of gender, smoking, and chronic illness, no variations between groups were found to be statistically significant. Every three weeks, participants underwent quick COVID-19 diagnostic exams. Out of 60 participants in the quercetin group and 4 out of 60 in the control group, 5 had COVID-19 during our study. In the quercetin and placebo groups, complete clinical complete recovery was noted at 7 and 15 days, respectively. Analysis revealed that at 5 months, quercetin-supplemented participants had a COVID-free survival function (risk of infection) of 99.8%, compared to 96.5% in the control condition. According to the value of EXP(B), the patients who taken the supplement had a protection factor 14% higher compared to those who had taken a placebo to avoid contracting the COVID-19 infestation [67].
6. Challenges
More attention should be placed on thorough characterization with optimization, quantitative and qualitative study of the, phytosomal drug delivery system and its influence in various disease conditions [69]. Though the domain has been extensively researched, the scope of the study must be expanded to focus on the challenge of the formulation process, stability, and real clinical superiority of such drug delivery systems. As a conventional approach, the solvent evaporation method has been commonly used for the formulation of Phyto-phospholipid complexes. However, the technique includes numerous unit operations, which is a time-consuming task, and the finished product's quality in terms of particle size, appearance, and hygroscopicity frequently depends on the drying technique used, which has not been optimised during any of the research. Because particle size and dispersion can be more precisely controlled at extremely low temperatures, the supercritical fluid approach can be used to address the shortcomings of conventional technologies. There have been no studies that show a link between improved in vivo and in vitro pharmacokinetic characteristics and the pharmacological activity of medicinal compounds in their phospholipid complex forms. Large attention has been placed on the characterization and evaluation of pharmacokinetic characteristics of phyto-phospholipid complexes, whereas clinical aspects of developed formulations have been avoided. More research is needed to bridge this gap and link the increase in bioavailability to clinical effectiveness [66]. Large-scale phytosome synthesis is the second difficulty. However, while scaling up, the product's qualities should be preserved. This relates to how useful laboratory procedures are in an industrial setup. Even though the formulation technique for numerous kinds of phytosomes are frequently easy, the poor physicochemical stability of pH-sensitive phytosomes made their commercial manufacture difficult. From product development to successful commercialization, a lot of time elapses. Despite all the advantages, very few phytosomal products have been introduced to the market. A major obstacle to the commercialization of phytosomes is the confirmation of safety after the development of an efficient formulation. However, before they are marketed, certain criteria such as bioaccumulation, biocompatibility, metabolism, and excretion should be evaluated. Furthermore, after developing a phyto-some, pharmacokinetic and pharmacodynamic characteristics in animals and people should be evaluated to demonstrate their superiority over pure phyto-constituents. Another step in the marketing process is determining the best dosage form to improve the finished product's absorption and efficacy [39]. Despite the apparent potential of phytosome technology, there are just a few anti-cancer studies involving phytosome as a carrier in cancer therapy. As a result, only a few products, such as Meriva® (curcumin phytosomes) and Siliphos®, have come on the market (Silybin phytosomes). Another important factor in this aspect is the larger-scale manufacture of nanophytosomes. Although not yet utilised on an industrial scale, the simplicity and easy production techniques of phytosomes made them more powerful to be scaled up. The pH sensitivity of phytosome structures may be the primary cause for the non-industrial scale-up. This is a significant difficulty that affects the physicochemical stability of phytosomes and should be resolved in the future for industrial manufacturing of these types of nanocarriers. Aside from recent advancements in industrial-level manufacturing of vesicular systems, such as extruding technologies, which provide hopeful prospects for commercial fabrication of these systems, the high cost of raw materials remains a challenge, Potential threats to this advancement include pegylated soy phosphatidylcholine [2].
7. Future outlook
The development of the phytophospholipid complex technology as a progressive component in determining systemic absorption of herbal extracts. This method has successfully addressed the unreasonable concerns about plant-based medications. These innovative compounds are excellent candidates for enhanced medication dose therapy. Phytosome initially employed in cosmetics, are now widely used in treatments for cancer, heart disease, inflammation, tumours, and other diseases that affect the liver. Phytosomes has re-explained the importance of herbals in contemporary medication targeting approaches with this newly developed formulation tool [69]. In addition to passive targeting, Phyto-phospholipid complexes can be effective candidates for active targeting by attaching certain ligands and antigens to the cellular structures. This will increase the range of diseases for which phyto-phospholipid complexes can be used to treat, including cancer, osteoarthritis, and rheumatism. By utilising more recent methods, such as supercritical fluid systems, and optimising the temperature, pressure, and several other factors, the dimension of the product can be changed to a variety of limited ranges. Because of their improved penetrability and increased retention, such size controlled product will be useful in targeting different microbial areas such as inflammation and tumours more specifically. To achieve the highest level of entrapment efficiency and the best drug release profile, the molar ratios of drug candidates with phospholipids, as well as the temperature and other variables, can be optimised using additional statistical tools such as factorial design, spherical symmetric designing, and others [66]. The development of nanotechnology-based phytosomes may have an impact on medicine delivery by removing obstacles caused by inadequate lipid solubility and enhancing the bioavailability of bioactive phytochemicals including silybin, ginkgo, and polyphenolic substances present in olive oil. Numerous phytochemicals have been successfully manufactured as phytosomes, and it is expected that additional phytochemicals can gain from similar formulations. The use of phytosomes in combination with other phytochemicals or the combination of a medicine and a phytochemical in a nano-vesicle in future the study could have synergistic effects [12]. When compared to chemically similar non-complexed forms, phospholipids considerably increase bioavailability. With the help of physicians and other researchers, the potential of phyto-phospholipid complex has a promising future for use in the pharmaceutical industry [7]. This may open up a big window of opportunity to use the medication for other medicinal purposes. Indeed, utilising secure phytoconstituents like curcumin with clever drug delivery techniques may open the way for the creation of eco-friendly and secure treatments for the most common human diseases. The ingestion of whole curcumin NPs for targeted distribution to different organs, including malignancies, is one important field that might be investigated [34]. In summary, Phytosomes® is a gift for naturally occurring extracts that are poorly bioavailable and have proven analytical methodologies and well-established processing methods. In comparison to other conventional kinds of medicine, it offers a wide range of benefits. There are several pharmaceutical items with registered patents on the market. This research demonstrates that Phytosomes® have introduced a new dimension to pharmaceutical research and development, with a wealth of untapped potential [48]. Another element in the effective product commercialization is its popularity. Along with the biocompatibility, affordability, and safety of natural products have increased people's desire for this kind of treatment in recent years. Additionally, because of the straightforward manufacturing method and straightforward encouraging the use of phytosomal technology to an industrial scale, the commercialization of phytosomes is a quick process. Numerous drug manufacturers investigated the benefits and biological activities of phytosome formulations as well as the increased bioavailability of polar phytoconstituents. The researchers are encouraged to continue their field research by the overall evidence for these formulations. Clinical research on standardised products that demonstrate greater efficacy in comparison to unformulated components or extracts will be important in the future to promote awareness of these technologies [39]. Because of its ability to mediate controlled release systems, targeted delivery systems, and the ability to increase the stability of active compounds, it is the first choice for increasing the effectiveness and becoming a promising approach for cosmeceutical products [58]. A few nano-particulate drug delivery systems for cancer therapy have received FDA approval. Lipid-based nanoparticles, one of the many new nanocarriers, have many significance than traditional drug carriers, particularly in regards to biocompatibility and biodegradability, affordability and raw material accessibility, and a long history of research. Nanophytosomal delivery systems for cancer therapy are expected to advance and expand in the shortly clear that incorporating natural and synthetic anti-cancer agents into nanophytosomes significantly boosts oral bioavailability and slows tumour growth. The use of phytosome technology in the nano-formulation of nutraceuticals has the potential to revolutionise the way hydrophilic plant chemicals are used in cancer treatment [2]. The use of phytosomes in combination with other phytochemicals or the combination of a medicine and a phytochemical in a nano-vesicle in future study could have stimulatory effects [12].
8. Conclusion
Phytosomes are well-known biocompatible nanocarriers that can be employed to increase the solubility and permeability of phytopharmaceuticals among various NDDS. In order to improve their bioavailability and absorption and get around the drawbacks and negative effects of traditional herbal extracts, phytosomes, an unique developing vesicular drug delivery technology, include plant extracts or hydrophilic phytochemicals in phospholipids. The formulation technique and characterization of phytosomes has been well established. Phytosome has several benefits such as increased stability, bioavailability, hepatoprotective etc, however there are some drawbacks to this technology, such as the quick removal of phytoconstituents from the Phytosome, PH-sensitivity. A major obstacle to the commercialization of phytosomes is the confirmation of safety after the development of an efficient formulation. It is clear that incorporating natural and synthetic anti-cancer agents into nano-phytosomes significantly boosts oral bioavailability and slows tumour growth. The use of phytosome technology in the nano-formulation of nutraceuticals has the potential to revolutionise the way hydrophilic plant chemicals are used in cancer treatment. The use of phytosomes in combination with other phytochemicals or the combination of a medicine and a phytochemical in a nano-vesicle in future study could have stimulatory effects.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
This is review article so no supplimentary data is there.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are also thankful to management of SRES, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, India, for providing all the necessary requirements to complete this work.
Abbreviations
- NDDS
Novel drug delivery system
- WHO
World health organization
- DNA
Deoxyribonucleic acid
- FDA
Food drug administration
- NPs
Nanoparticles
- CNTs
Carbon nanotubes
- PC
Phosphatidylcholine
- THF
Tetrahydrofuran
- SPC
Soyabean phosphatidylcholine
- DSN
Diosmin
- DMSO
Dimethylsulphoxide
- AFM
Atomic force microscopy
- SEM
Scanning electron microscopy
- TEM
Transmission electron microscopy
- DLS
Dynamic light scattering
- DSC
Differential scanning calorimetry
- NMR
Nuclear magnetic resonance
- X-RD
X-ray diffraction
- IR
Infrared
- HPTLC
High-performance thin layer chromatography
- QRT
Quercetin
- 5-FU
5-fluorouracil
- MMC
Mitomycin
- ICA
Icariin
- OVCAR
Ovarian cancer
- NLC
Nanostructured lipid carriers
- ROS
Reactive oxygen species
- MMP
Matrix metalloproteinase
- TQ
Thymoquinone
References
- 1.Murugesan M.P., Ratnam M.V., Mengitsu Y., Kandasamy K. Evaluation of anti-cancer activity of phytosomes formulated from aloe vera extract. Mater. Today: Proc. 2020;42(2):631–636. doi: 10.1016/j.matpr.2020.11.047. [DOI] [Google Scholar]
- 2.Baba Zadeh A., Zenial M., Hamishehkar H. Nano-phytosome: a developing platform for herbal anti-cancer agents in cancer therapy. Curr. Drug Targets. 2018;19(2):170–180. doi: 10.2174/1389450118666170508095250. [DOI] [PubMed] [Google Scholar]
- 3.Tao F., Zhang Y., Zhang Z. The role of herbal bioactive components in mitochondria function and cancer therapy. Evid. Based Complementary Altern. Med. 2019;2019:1–12. doi: 10.1155/2019/3868354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateti T., Aswath S., Vatti A.K., Kamath A., Laha A. A review on allopathic and herbal nanofibrous drug delivery vehicles for cancer treatments. Biotechnol. Rep. 2021;31:1–9. doi: 10.1016/j.btre.2021.e00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmier A. Cancer & ayurveda as a complementary treatment. J. Compl. Integr. Med. 2017;6 doi: 10.15406/ijcam.2017.06.00202. [DOI] [Google Scholar]
- 6.Yap K.M., Sekar M., Fuloria S., Wu Y.S., Gan S.H., Rani N.N.I.M., Subramaniyan V., Kokare C., Lump P.T., Begum, S M.Y., et al. Drug delivery of natural products through nanocarriers for effective breast cancer therapy: a comprehensive review of literature. Int. J. Nanomed. 2021;16:7891–7941. doi: 10.2147/ijn.s328135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu M., Qiu Q., Luo X., Liu X., Sun J., Wang C., Lin X., Deng Y., Song Y. Phyto-phospholipid complexes (phytosomes): a novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. 2019;14(3):265–274. doi: 10.1016/j.ajps.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhakamy N.A., Badr-Eldin S.M., Fahmy U.A., Alruwaili N.K., Awan Z.A., Caruso G., Alfaleh M.A., L Alaofi A., Arif F.O., Ahmed O.A.A., et al. Thymoquinone-loaded soy-phospholipid-based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics. 2020;12(8):1–17. doi: 10.3390/pharmaceutics12080761. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Karpuz M., Gunay M.S., Ozer A.Y. Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents. 2020. Liposomes and phytosomes for phytoconstituents; pp. 525–553. [DOI] [Google Scholar]
- 10.Azeez N.A., Deepa V.S., Sivapriya V. Phytosomes: emergent promising nano vesicular drug delivery system for targeted tumor therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9(3):1–6. doi: 10.1088/2043-6254/aadc50. [DOI] [Google Scholar]
- 11.Semalty A., Semalty M., M Rawat M.S., Franceschi F. Supramolecular phospholipids-polyphenolics interactions: the PHYTOSOME strategy to improve the bioavailability of phytochemicals. Fitoterapia. 2010;81(5):306–314. doi: 10.1016/j.fitote.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Alharbi W.S., Almughem F.A., Almehmady A.M., Jarallah S.J., Alsharif W.K., Alzahrani N.M., Alshehri A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics. 2021;13(9):1–20. doi: 10.3390/pharmaceutics13091475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anwar D.M., El-Sayed M., Reda A., Fang J.Y., Khattab S.N., Elzoghby A.O. Recent advances in herbal combination nanomedicine for cancer: delivery technology and therapeutic outcomes. Expet Opin. Drug Deliv. 2021;18(11):1609–1625. doi: 10.1080/17425247.2021.1955853. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal B.B., Ichikawa H., Garodia P., Weerasinghe P., Sethi G., D Bhatt I., Pandey M.K., Shishodia S., Nair M.G. From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin. Ther. Targets. 2006;10(1):87–118. doi: 10.1517/14728222.10.1.87. [DOI] [PubMed] [Google Scholar]
- 15.Koul B. 2020. Herbs for Cancer Treatment; pp. 1–1174. [DOI] [Google Scholar]
- 16.Dhruva A., Hecht F.M., Miaskowski C., Kaptchuk T.J., Bodeker G., Abrams D., Lad V., Adler S.R. Correlating traditional ayurvedic and modern medical perspectives on cancer: results of a qualitative study. J. Alternative Compl. Med. 2014;20(5):364–370. doi: 10.1089/2Facm.2013.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handa M., Beg S., Shukla R., Barkat M.A., Choudhry H., Singh K.K. Recent advances in lipid-engineered multifunctional nanophytomedicines for cancer targeting. J. Contr. Release. 2021;340:48–59. doi: 10.1016/j.jconrel.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Chavda V.P., Vihol D., Mehta B., Shah D., Patel M., Vora L.K., Pereira-Silva M., Paiva-Santos A.C. Phytochemical-loaded liposomes for anticancer therapy: an updated review. Nanomedicine. 2022;17(8):547–568. doi: 10.2217/nnm-2021-0463. [DOI] [PubMed] [Google Scholar]
- 19.Wang J.J., Lei K.F., Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018;22(12):3855–3864. doi: 10.26355/eurrev_201806_15270. [DOI] [PubMed] [Google Scholar]
- 20.Gautam L., Jain A., Shrivastava P., Vyas S., Vyas S.P. Nano Drug Delivery Strategies for the Treatment of Cancers. 2021. Emergence of novel targeting systems and conventional therapies for effective cancer treatment; pp. 1–35. [DOI] [Google Scholar]
- 21.Anand U., Dey A., Chandel A.K.S., Sanyal R., Mishra A., Pandey D.K., Falco V.D., Upadhyay A., Kandimalla R., Choudhary A., et al. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. GENES DIS. 2022 doi: 10.1016/j.gendis.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskar R., Lee K.A., Yeo R., Yeoh K.W. Cancer and radiation therapy: current advances and future directions. Int. J. Medical Sci. 2012;9(3):193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abshire D., Lang M.K. The evolution of radiation therapy in treating cancer. Semin. Oncol. Nurs. 2018;34(2):151–157. doi: 10.1016/j.soncn.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Esfahani K., Roudaia L., Buhlaiga N., Rincon S.V.D., Papneja N., H Miller W. A review of cancer immunotherapy: from the past, to the present, to the future. Curr. Oncol. 2020;27(2):S87–S97. doi: 10.3747/co.27.5223. 10.3747/2Fco.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham J., Staffurth J. Hormonal therapy for cancer. Medicine. 2020;48(2):103–107. doi: 10.1016/j.mpmed.2019.11.007. [DOI] [Google Scholar]
- 26.Zaimy M.A., Saffarzadeh N., Mohammadi A., Pourghadamyari H., Izadi P., Sarli A., Moghaddam L.K., Paschepari S.R., Azizi H., Torkamandi S., et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017;24(6):233–243. doi: 10.1038/cgt.2017.16. [DOI] [PubMed] [Google Scholar]
- 27.Cross D., Burmester J.K. Gene therapy for cancer treatment: past, present and future. Clin. Med. Res. 2006;4(3):218–227. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong L., Li Y., Xiong L., Wang W., Wu M., Yuan T., Yang W., Tian C., Miao Z., Wang T., et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Targeted Ther. 2021;6(1):201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan L., Rosen N., Arteaga C. Targeted cancer therapies. Chin. J. Cancer. 2011;30(1):1–4. doi: 10.5732/cjc.010.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hani U., Rahamathulla M., Osmani R.A., Kumar H.Y., Urolagin D., Ansari M.Y., Pandey K., Devi K., Yasmin S. Recent advances in novel drug delivery systems and approaches for management of breast cancer: a comprehensive review. J. Drug Deliv. Sci. Technol. 2020;56 doi: 10.1016/j.jddst.2020.101505. [DOI] [Google Scholar]
- 31.Mitra A.K., Agrahari V., Mandal A., Cholkar K., Natarajan C., Shah S., Joseph M., Trinh H.M., Vaishya R., Yang X., et al. Novel delivery approaches for cancer therapeutics. J. Contr. Release. 2015;219:248–268. doi: 10.1016/j.jconrel.2015.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozturk-Atar K., Eroglu H., Calıs S. Novel advances in targeted drug delivery. J. Drug Target. 2018;26(8):633–642. doi: 10.1080/1061186x.2017.1401076. [DOI] [PubMed] [Google Scholar]
- 33.Gavas S., Quazi S., Karpiński T.M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 2021;16(1):1–21. doi: 10.1186/s11671-021-03628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ipar V.S., Dsouza A., Devarajan P.V. Enhancing curcumin oral bioavailability through nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019;44(4):459–480. doi: 10.1007/s13318-019-00545-z. [DOI] [PubMed] [Google Scholar]
- 35.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. APR. Front. Pharmacol. 2014;25:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos A.C., Costa D., Ferreira L., Guerra C., Pereira-Silva M., Pereira I., Peixoto D., Ferreira N.R., Veiga F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2021;11(1):49–71. doi: 10.1007/s13346-020-00778-5. [DOI] [PubMed] [Google Scholar]
- 37.Tang L., Xiao Q., Mei Y., He S., Zhang Z., Wang R., Wang W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021;19(1):423. doi: 10.1186/s12951-021-01174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo P.L., Lim C.L., Chye S.M., Ling A.P.K., Koh R.Y. Niosomes: a review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017;11(4):301–313. doi: 10.1515/abm-2018-0002. [DOI] [Google Scholar]
- 39.Barani M., Sangiovanni E., Angarano M., Rajizadeh M.A., Mehrabani M., Piazza S., Gangadharappa H.V., Pardakhty A., Mehrbani M., Dell’Agli M., et al. Phytosomes as innovative delivery systems for phytochemicals: a comprehensive review of literature. Int. J. Nanomed. 2021;16:6983–7022. doi: 10.2147/ijn.s318416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paiva-Santos A.C., Silva A.L., Guerra C., Peixoto D., Pereira-Silva M., Zeinali M., Mascarenhas-Melo F., Castro R., Veiga F. Ethosomes as nanocarriers for the development of skin delivery formulations. Pharm. Res. (N. Y.) 2021;38(6):947–970. doi: 10.1007/s11095-021-03053-5. [DOI] [PubMed] [Google Scholar]
- 41.Rai S., Pandey V., Rai G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev. Exp. 2017;8(1) doi: 10.1080/20022727.2017.1325708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aqil F., Gupta R.C. Exosomes in cancer therapy. Cancers. 2022;14(3):1–4. doi: 10.3390/cancers14030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rana M., Kumar A., Rana A.J. Role of Novel Drug Delivery Vehicles in Nanobiomedicine, IntechOpen. 2020. Drug delivery through targeted approach with special references to phytosomes. [DOI] [Google Scholar]
- 44.Beg S., Barkat MdA., Ahmad F.J. 2020. Nanophytomedicine; pp. 1–386. [Google Scholar]
- 45.Sundaresan N., Kaliappan I. Development and characterization of a nano-drug delivery system containing vasaka phospholipid complex to improve bioavailability using quality by design approach. Res. Pharm. Sci. 2021;16(1):103–117. doi: 10.4103/1735-5362.305193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirzaei H., Shakeri A., Rashidi B., Jalili A., Banikazemi Z., Sahebkar A. Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017;85:102–112. doi: 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 47.Vu H.T.H., Hook S.M., Siqueira S.D., Müllertz A., Rades T., McDowell A. Are phytosomes a superior nanodelivery system for the antioxidant rutin. Int. J. Pharm. 2018;548(1):82–91. doi: 10.1016/j.ijpharm.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal A., Chakraborty P., Chakraborty D.D., Saharan V.A. Phytosomes: complexation, utilisation and commerical status. J. Biol. Act. Prod. Nat. 2012;2(2):65–77. doi: 10.1080/22311866.2012.10719111. [DOI] [Google Scholar]
- 49.Hou Z., Li Y., Huang Y., Zhou C., Lin J., Wang Y., Cui F., Zhou S., Jia M., Ye S., et al. Phytosomes loaded with mitomycin C-soybean phosphatidylcholine complex developed for drug delivery. Mol. Pharm. 2013;10(1):90–101. doi: 10.1021/mp300489p. [DOI] [PubMed] [Google Scholar]
- 50.Alhakamy N.A., Fahmy U.A., Badr-Eldin S.M., Ahmed O.A.A., Asfour H.Z., Aldawsari H.M., Algandaby M.M., Eid B.G., Abdel-Naim A.B., Awan Z.A., et al. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. Pharmaceutics. 2020;13(1):346. doi: 10.3390/pharmaceutics12040346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Freag M.S., Elnaggar Y.S.R., Abdallah O.Y. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex vivo permeation. Int. J. Nanomed. 2013;8:2385–2397. doi: 10.2147/ijn.s45231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathy S., Patel D.K., Baro L., Nair S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Therapeut. 2013;3(3):147–152. doi: 10.22270/jddt.v3i3.508. [DOI] [Google Scholar]
- 53.Sharma S., Sahu A. Development, characterization, and evaluation of hepatoprotective effect of abutilon indicum and piper longum phytosomes. Pharmacogn. Res. 2016;8(1):29–36. doi: 10.4103/0974-8490.171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gnananath K., Nataraj K.S., Rao B.G. Phospholipid complex technique for superior bioavailability of phytoconstituents. Adv. Pharmaceut. Bull. 2017;7(1):35–42. doi: 10.15171/apb.2017.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jain P., Taleuzzaman M., Kala C., Kumar D.G., Ali A., Aslam M. Quality by design (Qbd) assisted development of phytosomal gel of aloe vera extract for topical delivery. J. Liposome Res. 2021;31(4):381–388. doi: 10.1080/08982104.2020.1849279. [DOI] [PubMed] [Google Scholar]
- 56.Chivte P.S., Pardhi V.S., Joshi V.A., Rani A. A review on therapeutic applications of phytosomes. J. Drug Deliv. Therapeut. 2017;7(5) doi: 10.22270/jddt.v7i5.1513. [DOI] [Google Scholar]
- 57.Alhakamy N.A., Fahmy U.A., Badr Eldin S.M., Ahmed O.A.A., Aldawsari H.M., Okbazghi S.Z., Alfaleh M.A., Abdulaal W.H., Alamoudi A.J., Mady F.M. Scorpion venom-functionalized quercetin phytosomes for breast cancer management: in vitro response surface optimization and anticancer activity against MCF-7 cells. Polymer. 2022;14(1):1–21. doi: 10.3390/polym14010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Susilawati Y., Chaerunisa A.Y., Purwaningsih H. Phytosome drug delivery system for natural cosmeceutical compounds: whitening agent and skin antioxidant agent. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2021;12(4):327–334. doi: 10.4103/japtr.JAPTR_100_20. 10.4103/2Fjaptr.JAPTR_100_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hafez D.A., Elkhodairy K.A., Teleb M., Elzoghby A.O. Nanomedicine-based approaches for improved delivery of phyto-therapeutics for cancer therapy. Expet Opin. Drug Deliv. 2020;17(3):279–285. doi: 10.1080/17425247.2020.1723542. [DOI] [PubMed] [Google Scholar]
- 60.Marjaneh R.M., Rahmani F., Hassanian S.M., Rezaei N., Hashemzehi M., Bahrami A., Ariakia F., Fiuji H., Sahebkar A., Avan A. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J. Cell. Physiol. 2018;233(10):6785–6798. doi: 10.1002/jcp.26538. [DOI] [PubMed] [Google Scholar]
- 61.Sabzichi M., Hamishehkar H., Ramezani F., Sharifi S., Tabasinezhad M., Pirouzpanah M., Ghanbari P., Samadi N. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac. J. Cancer Prev. APJCP. 2014;15(13):5311–5316. doi: 10.7314/apjcp.2014.15.13.5311. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S., Roy R., Shrivastava B. Antiproliferative effect of phytosome complex of methanolic extact of Terminalia arjuna bark on human breast cancer cell lines (MCF-7) Int. J. Drug Dev. Res. 2015;7(1):173–182. [Google Scholar]
- 63.Sachin K.S., Nesalin J.A.J., Mani T.T. Preparation and evaluation of curcumin phytosomes by rotary evaporation method. Int. j. pharm. biomed. res. 2019;6(1):29–34. doi: 10.14445/23942576/IJPBE-V6I1P104. [DOI] [Google Scholar]
- 64.Komeil I.A., Abdallah O.Y., El-Refaie W.M. Surface modified genistein phytosome for breast cancer treatment: in-vitro appraisal, pharmacokinetics, and in-vivo antitumor efficacy. Eur. J. Pharmaceut. Sci. 2022;179:1–9. doi: 10.1016/j.ejps.2022.106297. [DOI] [PubMed] [Google Scholar]
- 65.Singh D., Rawat M.S.M., Semalty A., Semalty M. Chrysophanol-phospholipid complex: a drug delivery strategy in herbal novel drug delivery system (HNDDS) J. Therm. Anal. Calorim. 2013;111:2069–2077. doi: 10.1007/s10973-012-2448-6. [DOI] [Google Scholar]
- 66.Khan J., Alexander A., Saraf Ajazuddin S., Saraf S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Contr. Release. 2013;168(1):50–60. doi: 10.1016/j.jconrel.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Rondanelli M., Perna S., Gasparri C., Petrangolini G., Allegrini P., Cavioni A., Faliva M.A., Mansueto F., Patelli Z., Peroni G., et al. Promising effects of 3-month period of quercetin Phytosome® supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: a pilot study. Life. 2022;12(1):7–11. doi: 10.3390/life12010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dipierro F.D., Khan A., Bertuccioli A., Maffioli P., Derosa G., Khan S., Khan B.A., Nigar R., Ujjan I., Devrajani B.R. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. 2021;67(2):190–195. doi: 10.23736/s2724-5985.20.02771-3. [DOI] [PubMed] [Google Scholar]
- 69.Kumar S., Baldi A., Sharma D.K. Phytosomes: a modernistic approach for novel herbal drug delivery - enhancing bioavailability and revealing endless frontier of phytopharmaceuticals. J. Dev. Drugs. 2020;9(2):1–8. 10.3390/2Fpharmaceutics13091475. [Google Scholar]
- 70.https://in.iherb.com/blog/phytosomes/123 [20 Dec.2022.].
- 71.https://www.britannica.com/science/cancer-disease/Conventional-therapies. [25 Dec.2022.].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is review article so no supplimentary data is there.