Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had worldwide repercussions for health care and research. In spring 2020, most non-COVID-19 research was halted, hindering research across the spectrum from laboratory-based experimental science to clinical research. Through the second half of 2020 and the first half of 2021, biomedical research, including cardiovascular science, only gradually restarted, with many restrictions on onsite activities, limited clinical research participation, and the challenges associated with working from home and caregiver responsibilities. Compounding these impediments, much of the global biomedical research infrastructure was redirected toward vaccine testing and deployment. This redirection of supply chains, personnel, and equipment has additionally hampered restoration of normal research activity. Transition to virtual interactions offset some of these limitations but did not adequately replace the need for scientific exchange and collaboration. Here, we outline key steps to reinvigorate biomedical research, including a call for increased support from the National Institutes of Health. We also call on academic institutions, publishers, reviewers, and supervisors to consider the impact of COVID-19 when assessing productivity, recognizing that the pandemic did not affect all equally. We identify trainees and junior investigators, especially those with caregiving roles, as most at risk of being lost from the biomedical workforce and identify steps to reduce the loss of these key investigators. Although the global pandemic highlighted the power of biomedical science to define, treat, and protect against threats to human health, significant investment in the biomedical workforce is required to maintain and promote well-being.
Keywords: AHA Scientific Statements, cardiovascular research, COVID-19, early career, health literacy, research training, workforce
For much of the world, the global pandemic resulted in shelter-in-place orders for several months in spring 2020. In the United States, these orders restricted onsite research activities to essential personnel with cessation of most active experimentation. Although shelter-in-place orders were largely lifted by summer 2020, as of summer 2021, many research activities still have not returned to full operation because of the need to observe US Centers for Disease Control and Prevention guidance on social distancing and other safety precautions. During 2020 and 2021, considerable effort was redirected to coronavirus disease 2019 (COVID-19)–related investigation, from treatments to vaccine development and response. For dry laboratory research, the initial transition to remote activities required ensuring access to proper equipment and technology, and it was often limited by the many needs and distractions of home life during the pandemic. For clinical and translational research, patient interactions were severely limited through much of 2020, causing some clinical trials and data collection to be delayed or halted. These restrictions, along with increased home demands for parents and caregivers, as well as many pandemic-induced adaptations, led to underpopulated and inefficient research operations across the United States.
In addition to pandemic-related research disruption, the United States experienced social and political unrest after the murder of George Floyd on May 25, 2020. This unrest continued through much of the second half of 2020 and in some regions of the country led to curfews and other forms of restricted access. The emotional stress induced by the primary event, as well as the resulting social and political instability, further challenged the United States and the world, including the cardiovascular research enterprise. Additional challenges engendered by disagreements related to the optimal way to balance the effects and management of the pandemic with other priorities, including economic recovery and schooling, also may have hampered research activity.
Each of these components–restricted access and working virtually, redirection of the research enterprise, and social and political unrest–diminished the efficiency of research. Furthermore, disruptions in research, training, and education are having a protracted adverse impact across the entire scientific workforce, disproportionately so for early career researchers. For research trainees and early career researchers, who are more likely to be from diverse and underrepresented backgrounds, the disruption of academic curricula and limitations on activities essential for project objectives have the potential to threaten future job prospects, promotion/tenure, and grant competitiveness. In this advisory, we highlight key areas of impact. In response, we call for key actions to help address this crisis so that the cardiovascular research workforce can regain its footing to build science back better.
IMPACT ON SCIENCE: GENERAL CONSIDERATIONS
A number of broad effects of the pandemic on biomedical science are worthy of consideration, including institutional financial and related strains, the shift from in-person to virtual scientific meetings, and the impact on international research collaborations.
In October 2020, 7 months into the pandemic in the United States, the National Institutes of Health surveyed research administration leaders from institutions across the country. A total of 224 institutions (32% of invited institutions) responded, representing doctorate-granting universities with or without professional schools, independent research institutions, and institutions serving underrepresented groups, among others. Overall, 83% of research leaders expected moderate to major impacts on research productivity, and 66%, including leaders at three-fourths of doctorate-granting universities and institutions serving underrepresented groups, expressed that they were very or extremely concerned about their institution’s financial status. Among the reasons cited was the substantial impact from loss in endowment. Indeed, 41% of respondents said it is likely that the financial repercussions of COVID-19 will jeopardize their institution’s ability to maintain research functions.1 Other potential threats to the financial health of such institutions include loss of research-funding revenue streams from charitable organizations, diversion of existing and new funding streams exclusively to COVID-19–related research,2 and greater debt and more difficulty borrowing funds at affiliated academic medical centers.3 In addition, research institutions face financial and productivity threats from loss of skilled personnel in research and regulatory and research administration, in addition to loss of faculty researchers attributable to clinical duties, home caregiving responsibilities, and personal health and mental health issues. These issues may particularly threaten women and groups underrepresented in medicine and biomedical research.1
Beginning in March 2020, major scientific meetings transitioned from in-person to fully virtual meetings. These meetings represent the most critical venues for advancing cardiovascular science through the exchange of ideas and for advancing cardiovascular clinical care by sharing and debating the latest trials and treatment advances. Whereas there have been some benefits of virtual meetings, including no need for travel, lower attendance costs, and availability of enduring content for extended learning opportunities, other significant costs threaten the future exchange of scientific ideas. Bidirectional scientific exchange benefits from in-person connections and in-person networking with thought leaders, collaborators, and potential mentors. This is especially true for junior investigators and trainees, for whom virtual networking opportunities are limited and insufficient. The loss of the habit of meeting attendance and networking could have a substantial and reverberating impact on careers and research more broadly.
The unpredictable nature of persistent waves of the pandemic across the globe has also posed a particular challenge for international scientific collaborations. At any given time, laboratories and trials have struggled to maintain research operations across countries with high rates of infections and overwhelmed health systems. Well into 2021, research supply chains are unpredictably disrupted, with many typical laboratory materials, reagents, and equipment being diverted to vaccine production and deployment. The following sections highlight specific impacts on basic, clinical, and population research in cardiovascular science (Figure 1).
Figure 1.
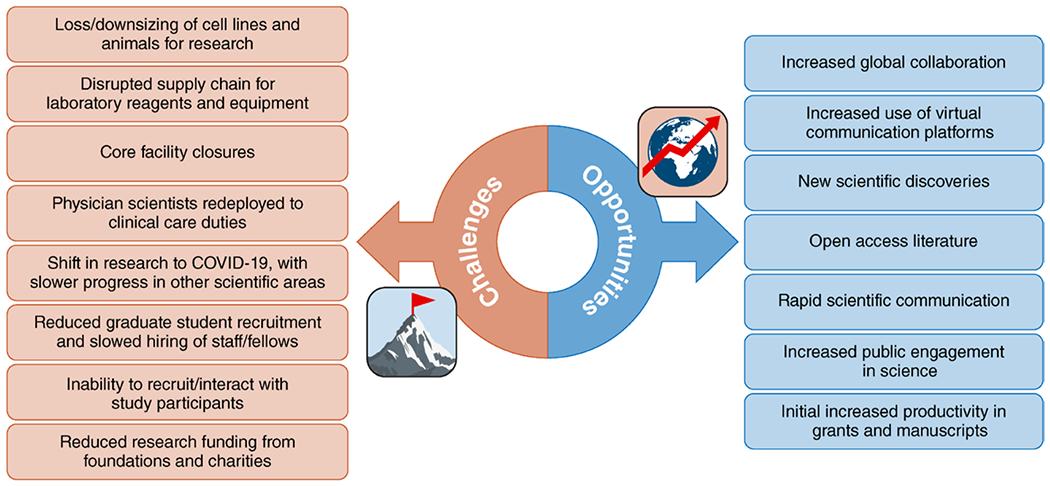
Broad impact of the coronavirus disease 2019 (COVID-19) pandemic on basic, clinical, and population research in cardiovascular science.
IMPACT ON BASIC SCIENCE
The impact of the COVID-19 pandemic on basic science has been significant because most basic science research is conducted in the laboratory setting. When major universities and research institutions shuttered their doors, laboratory staff were required to work from home, which caused major disruption in experimental timelines and disproportionally affected bench researchers.4 Many investigators reduced animal colonies because of limited onsite availability of veterinary and animal handling personnel. Furthermore, supplies such as gloves and personal protective equipment, as well as disposable plasticware, were diverted for clinical care and testing needs. This caused significant loss to the scientific enterprise in terms of loss of both data and time. For example, it takes months to years to establish a line of genetically engineered mice and to expand their colonies. Because of a lack of staff for weaning and genotyping mice, many colonies were lost and needed to be re-established, a process that can take 1 to 2 years. For those performing experiments using cell lines, the work-from-home directive required investigators to urgently end long-term cell culture experiments. In addition to loss of animals and cell lines, complex laboratory skills, which are honed only through continuous use, have deteriorated for many researchers. Research core facilities were closed; histological and microscopic studies were halted; polymerase chain reaction machines were idle or repurposed to intensive clinical use for COVID-19 testing; and surgical procedures, a source of tissue for research, simply stopped. Once laboratories were allowed to reopen, many had to use a “shift structure” with strict social distancing, which reduced opportunities for scientific collaboration and interaction essential to training and education. Many funders, including the National Institutes of Health, allowed technicians and trainees to continue to draw salary from sponsored projects, despite output being limited. Although this provided initial protection from job loss, the potential remains that this use of funds will limit the possibility of completing projects later. The long-term effects of COVID-19 continue, with unpredictable availability of key reagents and drastic increases in the prices of equipment and supplies.2 In addition, many scientists had to juggle unexpected family responsibilities, including caring for family members who were ill or helping children who now had limited time in school or studied entirely at home. Despite the resilience of the scientific workforce, it will take years for many scientists to recover.
Despite the challenges, there are some unexpected and noteworthy positives of the pandemic.5 The intensive research on coronaviruses, immune response, and response to vaccines has improved our understanding of infectious disease; in particular, the technology of mRNA vaccines has demonstrated its remarkable value and potential. The immediate- and long-term cardiovascular effects of COVID-19 have led to advances in our foundational knowledge of vascular disease and immunology. Initially, work-from-home mandates allowed many researchers to focus on completing unfinished manuscripts that were begun well before the pandemic. Grant submissions also initially increased as researchers began to prepare for the return to work in their laboratories on campus. These positive aspects may mitigate some of the adverse effects of the pandemic on the scientific workforce, both improving morale and facilitating a faster restart once institutions are fully operational.
IMPACT ON CLINICAL TRIALS, CLINICAL RESEARCH, AND POPULATION SCIENCE
By necessity, clinical trials have been affected by the pandemic for 2 main reasons. First, to protect the safety and well-being of participants and research staff, measures were taken to suspend clinical trials or to significantly limit unsafe exposures.6 Second, a dramatic swing in personnel resources was redirected to address the pandemic directly and to support rapid research on COVID-19.
Throughout the world and in the United States, trials were altered, suspended, or terminated. Most research operations shut down almost completely, if not completely, for several months in many centers. Among 1052 suspended clinical trials from March 1, 2020, to April 26, 2020, COVID-19 was reported as the reason in 86%.7 If trials continued, research visits were altered to tele-visits when possible or delayed. In the worst case, trials were terminated as a result of lack of recruitment, uncertainty about the ability to deliver the investigational product safely, and concerns about when trial operations would resume normally. To address these issues, a US Food and Drug Administration guidance document recognized investigational product supply chain disruptions, travel restrictions, site closures, and quarantines contributing to challenges in clinical trials and provided guidance to support the eventual completion of studies.6 The US Food and Drug Administration guidance focused on supporting the integrity of clinical trials, including modifications in procedures, locations, use of telemedicine, and statistical analysis plans to account for missing data and changes in study visits or discontinuation and to keep study participants informed of changes and potential needs to stop recruitment.
As the ecosystem of clinical trials shifted to studies on COVID-19 therapeutics, vaccines, and population science, there has been a massive shift away from non–COVID-19 research. In contrast to the huge pivot toward COVID-19 research (5716 COVID-19 studies listed on ClinicalTrials.gov as of May 21, 2021), ≈80% of non–COVID-19 trials have been stopped or interrupted.8 From ClinicalTrials.gov data, during the period of February 2020 through May 2020, US monthly trial activations were 57% of the expected number estimated from January 2015 to September 2020; with reopening from June 2020 to September 2020, a rebound occurred, but it was weaker for US-based than for non–US-based trials.9 Population studies have been affected by delays in pivotal in-person surveillance visits. Follow-up data may be less rigorous, with participants dropping out of studies or having safety concerns about returning to a site. Delays are expected in non–COVID-19 medical product development, obtaining regulatory approval, and bringing new products to market. Continuing or restarting study operations has been limited by hiring freezes related to pandemic-related hospital financial losses, shift of clinicians to clinical care for patients with COVID-19, and delays in approvals for non–COVID-19 research attributable to shifts to COVID-19 research taking priority.
Although there have been many challenges for clinical trials, the pandemic likely will have an enduring impact on clinical trial design and conduct going forward, some of which may be beneficial. These changes include acceleration of implementation of telemedicine, with remote and digital consent processes and follow-up visits (eg, by telemedicine or mobile application [app]-based follow-up, as has been done by the Centers for Disease Control and Prevention for COVID-19 vaccine follow-up); potential enhancement of geographic diversity; increased enrollment of women, underrepresented racial and ethnic groups, and rural participants; and streamlining of testing with marginal benefits. We have witnessed the speed of the development and implementation of COVID-19 vaccine clinical trials and successful implementation of research procedures previously thought to be logistically untenable (eg, home delivery of randomized therapies within hours of randomization). Such pandemic-inspired advances may pave the way for trial design and pragmatic approaches that could accelerate therapeutic approval for drugs tested even in large clinical trials in the future. Last, advances in integration and analysis of vast data sources related to the study of COVID-19 will have a long-lasting impact on research, from clinical trials to observational studies and population health studies.
IMPACT ON RESEARCH CAREER DEVELOPMENT AND TRAINING
Although pandemic-related research inefficiency affects the entire research workforce, research trainees–predoctoral, postdoctoral, MD, and PhD–are particularly adversely affected. By design, training intervals and funding are time limited, and trainees are expected to graduate and transition to full employment within a standard interval. Time to terminal degree and length of postdoctoral training are metrics used to evaluate institutional training grants and programs and individual eligibility for early career funding and benefits.10 Failing to appropriately transition within a given time window can result in termination from a program or appear unfavorable to prospective employers and thereby impede progression to the next career step. Fundamentally, research training requires scientific discussions, onsite training, and participation in scientific meetings with bidirectional exchange of ideas. Virtual formats, although useful for information dissemination, are often insufficient for the dialog and multilog that propel scientific advancement.
Graduate training programs for predoctoral fellows are often well formulated, with mentoring committees and other career development mechanisms; thus, predoctoral trainees may have advisors providing guidance on how to navigate lengthened time to degree. However, formal mentoring committees are not universal for postdoctoral trainees, and these trainees may be less likely to experience extendable financial support to continue in their position. Many academic institutions suspended new faculty hiring through 2020, with a relatively slow uptick in 2021, because of institutional financial uncertainties and the logistics of interview visits. Therefore, postdoctoral trainees may be at especially high risk for leaving the research workforce because of limited academic job availability.
Junior faculty also have time-limited duration of their positions, especially for tenure track faculty. Faculty at all levels will experience hindered career development in the face of slowed research productivity because research output is necessary to publish, secure research funding, and achieve promotion. Many academic institutions have attempted to support faculty during the pandemic by extending tenure clocks by 1 year, expanding a frequently used extension policy for new parents or caregivers. However, promotion and tenure clock extensions are costless gestures without accompanying financial support. Often, such policies widen disparities between women and men in that women are differentially penalized for productivity losses compared with men.11 These policies can decrease earning potential and can put faculty out of sync with funding mechanisms with rank or time restrictions.12 Thus, physician-scientists are likely to be at higher risk for being lost from the research workforce because, in addition to the aforementioned impediments to research, they may have experienced increased clinical demands through transitions to telemedicine and time spent staffing COVID-19 wards.
Challenges of work-life balance affect trainees and researchers at all levels. Working from home, when possible, may have reduced commute times and travel, in principle allowing more time for research. Any increased time for research was offset by increased stress and distractions at home such as poor internet bandwidth, virtual school, and a lack of dependent care. With resolution of at-home distractions and impediments, as was seen by spring 2021, some have continued working from home more than in prepandemic times. Whether this perceived efficiency of at-home work will translate into greater productivity remains to be seen. In other disciplines, reduced at-work interaction is already believed to impede career advancement.13,14 Multiple studies highlight the disproportionate manner in which the pandemic affected women, early career investigators, and those underrepresented in biomedical science.15–21
PUBLIC ATTITUDES TOWARD SCIENCE
One particularly notable feature of the pandemic has been the public’s increased engagement in health-related science, for better and for worse, over the past year. This trend began before the pandemic as movements toward citizen science, community-based participatory research, and open science have grown. The pandemic accelerated this tendency, with much of the public growing familiar with epidemiological principles, statistical terms, and vaccinology. As a result, perhaps, of this more direct engagement with science, confidence in science has increased. Since the early days of the crisis in March 2020, there has been an increase in the proportion of people in the United States and worldwide who expressed trust in scientists, and this increase was greater than that seen for other institutions.22 In an international survey, fielded among citizens of countries including the United States, Canada, Brazil, Russia, and across Europe and the Asia-Pacific region, a median of 82% said that they considered government investment in scientific research worthwhile, and majorities across countries stated that it is important to be a leader in scientific achievements.22 One year into the pandemic, most people believed that science would provide solutions to the crisis, and almost 80% believed that science broadly has the ability to improve lives and provide for a better future for society.23 Indeed, the pandemic provided remarkable success stories for science: mRNA vaccines, the potential of assaying wastewater to identify viral burden and variants, and cooperation among scientists using large international databases to better understand COVID-19, among others. Most people (68%) believe that news media did a very or somewhat good job of communicating science, although a majority also indicated that there is a limited public understanding of scientific issues.23 Traditional media and public health organizations such as the American Heart Association (AHA) played an important role in transmitting information about hygienic and other health promotion practices such as hand washing, social distancing, mask wearing, and vaccination,24 as well as the value of telehealth. Social media also played an important role in spreading both information and misinformation about COVID-19, as it did in prior disease outbreaks such as Ebola and Zika, although other factors—including demographic factors and lack of scientific literacy—also contributed.25
Quickly after the onset of the pandemic, however, attitudes in the United States became heavily politicized, and public health needs were often pitted against the health of the economy or the need to reopen in-person education.26 Although politicization was seen in many countries throughout the pandemic, the effect in the United States was more extreme than in other countries.27 Several countries, including the United States, the United Kingdom, and Spain, also provided evidence of mixed opinions among citizens, breaking by ideology and political group, about the ability of their governments to handle the pandemic.28 The pandemic brought into sharp relief long-brewing tensions among members of the public, political leaders, and scientific experts. Notable failures in the public health community such as the early recommendations against wearing masks further accentuated skepticism about the trustworthiness of public health experts and specifically government organizations that oversee public health.29 Even within the scientific community, the rapid pace of discovery led at times to missteps. The increased reliance on preprints to provide rapid access to data about this new disease, for example, carried with it the risk of incompletely or poorly reviewed evidence, in some cases leading to retractions or distractions, further threatening the confidence of the public.30 At the same time, preprints helped disseminate critical information such as viral sequences, which directly enabled the earliest phases of vaccine development and without which vaccines could not have been developed so quickly.
The enhancement of broad public trust in biomedical science and the medical community is crucial and will require concerted actions on several fronts to lead to enduring change. Foremost, negative attitudes toward vaccinations have emerged as a barrier to stemming the global pandemic and its economic impact, especially in communities at higher risk for infection. In a survey of 32 361 adults in the United Kingdom, 16% of respondents expressed highly negative attitudes toward vaccines, including worries about side effects, a preference for natural immunity, and concern for profiteering by large corporations.31 Mistrust was higher in underrepresented racial and ethnic groups. Furthermore, the disparities in global availability and distribution of vaccines between high-income and low- and middle-income countries are likely to further exacerbate these negative public attitudes.
THE AHA’S RESPONSE TO COVID-19
Flexible Rebudgeting and No-Cost Extensions
As the largest nonprofit, nongovernmental funder of cardiovascular and cerebrovascular research in the country, the AHA is typically supporting 1500 to 2000 active research awards totaling roughly $500 million. Not surprisingly, the effect of COVID-19 on researchers funded by the AHA has been significant. The vast majority of investigators reported that their AHA-funded research was either moderately (30.2%) or significantly (43.9%) affected by the pandemic, and 3.6% reported that it had been completely shut down.32 To mitigate at least some of the challenges that AHA-funded researchers were facing, early in the crisis, the AHA instituted a number of measures to provide enhanced flexibility in managing AHA funding. For instance, the AHA expanded the availability of No-Cost Extensions, allowed continued payment of salaries during slowdown or stoppage of research, and supported rebudgeting of award funds by investigators so that they could optimally manage their projects.
New Support for COVID-19 Research
As the health impact of coronavirus infection became clear, the AHA recognized the urgent need to better understand the pathobiology of severe acute respiratory syndrome coronavirus 2 infection, especially its clinical cardiovascular implications. By June 2020, the AHA had developed and posted a request for applications, recruited >150 volunteer scientists to review 694 applications, and funded $2.5 million in novel research on COVID-19.33 Although these grants were funded less than a year ago, a number of key discoveries, including identification of potential therapeutic targets for treatment of COVID-19, have been made.32,34,35 The AHA also leveraged the network of hospitals participating in its Get With The Guidelines quality improvement programs to create a large registry of hospitalized patients with COVID-19.36 Research on data from the registry has led to several important insights into the pandemic.37,38
Cost Extension for Early Career Awardees
As the effect of the crisis on the research enterprise and AHA-funded investigators has persisted, the AHA’s Research Committee and its Funding Subcommittee explored the potential for allocating supplemental funding to those awardees most likely to be adversely affected by disruptions to research. In April 2021, up to $3 million in supplemental funding was provided to enhance support for eligible awardees. Eligible applicants must be either in the last active year of their award or in No-Cost Extension; those prioritized for supplemental funding include those shown to be most adversely affected by the pandemic, including early career investigators, women, and investigators from underrepresented racial and ethnic groups.12–18
COVID-19–Related Publishing
In addition to its role as a funder of research, a key aspect of the AHA’s mission is disseminating new research discoveries to the scientific community. The AHA does this in part by publishing new research findings in its 13 scientific journals. Since the start of the pandemic, nearly 4000 manuscripts focused on COVID-19 have been submitted to the AHA journals (>1500 to the AHA flagship journal, Circulation). More than 400 submissions are already published or in press.39 The AHA also convenes scientific meetings through which leading researchers share their most recent and impactful results, including new COVID-19–related findings. For example, >50 oral presentations focused on COVID-19 were given at the AHA’s preeminent research conferences, Scientific Sessions 2020 and International Stroke Conference 2021. Thus, through both its publication and scientific conference arms, the AHA has played an important role in ensuring that key discoveries related to COVID-19 are rapidly and clearly communicated to the scientific community. In addition, through establishment of an online COVID-19 compendium,40 the AHA has established a collection of resources for health care systems, clinicians, patients, and the general public.
CALL TO ACTION: INVESTING TO REBUILD SCIENCE
What should be the call to action for the scientific and biomedical community? First, building confidence in the scientific enterprise should begin locally: People generally trust their physicians and health care professionals most strongly, then regional public health officials, then the scientific community more generally, and last political leaders. The communication of science must also transcend evidence and data; social scientists recognize the importance of stories in communicating the essential message of scientific discoveries. Science journalists and public health scientists should incorporate whenever possible narrative and human dimensions when communicating scientific or statistical results. At the same time, we need a long-term commitment to increasing funding for enhancing education in science, technology, engineering, and mathematics, which, by improving scientific and statistical literacy, will make our future society more resilient when the next pandemic strikes. These efforts must be spread equitably throughout society, however, to ensure that all segments of society benefit and remain protected from disinformation (Figure 2).
Figure 2.
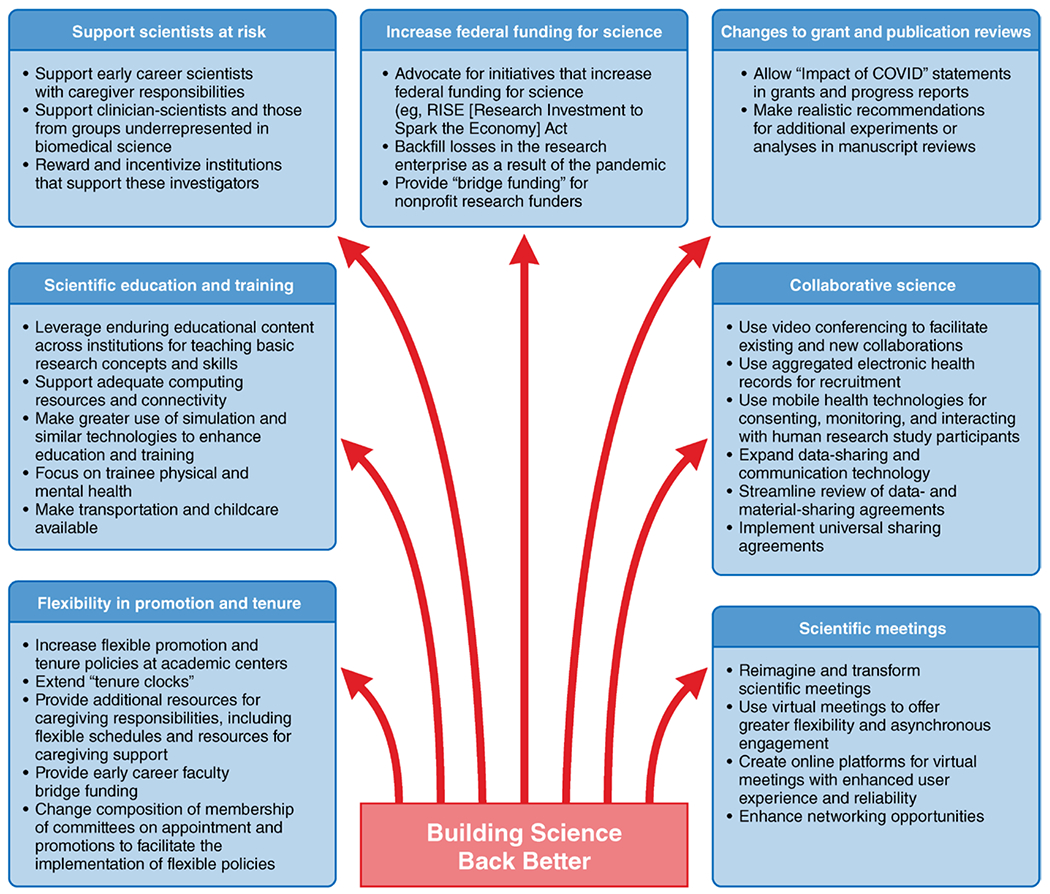
A number of actions are recommended to facilitate rebuilding of the research enterprise from the effects of COVID-19. COVID-19 indicates coronavirus disease 2019.
Supporting Scientists at Risk
As noted, scientists who also have caregiving responsibilities are at particular risk of being adversely affected by the pandemic. With caregiving increased for many during the pandemic, there is considerable concern that the careers of many of these researchers may be sidetracked. Recognizing the difficulties faced by women and those of racial and ethnic groups underrepresented in medicine even before the pandemic, we should make building science back better a priority. As one approach to address this, the AHA is participating with other nonprofit funders in a novel mechanism that will provide funding for these early career researchers with caregiving responsibilities.37 Building on a program originally offered by the Doris Duke Charitable Foundation, this multifunder collaborative is awarding grants to select institutions that demonstrate a commitment to supporting early career scientists who have caregiver responsibilities that have been exacerbated by the pandemic. This mechanism aims to support clinician-scientists and those from underrepresented groups. The goal of this program is to facilitate retention of these early career investigators within the research ecosystem; it also serves to reward and incentivize institutions that support these investigators so that their contributions to research can be fully realized.
Increasing Federal Funding for Science
We support the AHA using its trusted voice in support of similar creative national efforts to enhance opportunities for those from groups most affected by the pandemic. For instance, the AHA helped to secure–together with our partners in the National Heart, Lung, and Blood Institute Constituency Group (co-chaired by the AHA and the American Thoracic Society)–$1.15 billion in emergency supplemental funding for the National Institutes of Health to study long COVID as part of the Consolidated Appropriations Act of 2021 (PL 116–260) enacted in December 2020.41 We also join with Research!America and many other entities in advocating for the Research Investment to Spark the Economy Act (H.R.869/S.289), a proposed $26 billion bill that will backfill losses in the research enterprise as a result of the pandemic.42 In addition, we continue to urge federal lawmakers to provide “bridge funding” for nonprofit research funders, with the goal of offsetting their losses in dollars available to fund research.
Changes to Grant and Publication Reviews
Peer review of academic productivity for both grants and publications also should be updated. Investigators should be given the opportunity to provide “Impact of COVID-19” statements in their grant applications and progress reports because the pandemic was uneven in its effect on individual researchers. Journal editors and manuscript peer reviewers should also adjust expectations when reviewing articles and consider making more realistic recommendations for further experiments or analyses before accepting articles because some revisions may have been rendered prohibitively difficult by pandemic-related delays or changes in project feasibility, including loss of cell lines and animal models, incomplete clinical datasets, or other issues.
Flexibility in Promotion and Tenure
The volunteer scientific leadership of the AHA, most of whom are in positions of leadership at their own academic medical centers and research institutes, also supports efforts to increase flexible promotion and tenure policies at academic centers. Some institutions have already extended their tenure clocks in response to the pandemic by giving faculty an extra year. Others have provided additional resources for caregiving responsibilities, including flexible schedules and resources for caregiving support. When possible, it would be ideal to provide early career faculty with bridge funding to allow them to continue in academia until funding levels rebound and investigators can fully renew their research commitments. In some institutions, policy changes may require changing the composition of membership of committees on appointments and promotions to facilitate the implementation of more flexible policies. Specific solutions will depend on local practices and policies, as well as resource availability, but the AHA remains committed to ensuring that the academic research community can continue its effort to conduct cardiovascular and cerebrovascular science to the fullest extent possible.
Scientific Education and Training
In the short term, resources will need to be made available to keep individuals in the scientific workforce, including support through periods of lapsed funding and decreased productivity and extension of training and tenure clocks. Over the longer term, there may be opportunities to leverage enduring educational content across institutions for teaching basic research concepts and skills, realizing economies of scale. Productivity for some scientists and trainees can be enhanced when working partly from home, given adequate computing resources and connectivity. The research enterprise should make greater use of simulation and similar technologies to enhance education and training opportunities. There should be greater focus on trainee physical and mental health and on issues such as availability of transportation and childcare to support a more diverse and innovative future research workforce.
Scientific Meetings
Scientific meetings should continue to be reimagined and transformed, becoming more available to more attendees. Virtual meetings offer greater flexibility to interact with the educational and scientific content asynchronously, sometimes days or weeks after a meeting ends. A recent poll of 900 readers of the journal Nature revealed that 74% thought that scientific meetings should continue to be virtual or at least have a virtual component.43 Respondents to the Nature survey did, however, note the inability to network effectively through virtual platforms. We should thus plan for a future that includes both virtual and in-person meetings, and online platforms for virtual meetings should continue to evolve to ensure better user experience and reliability, especially for junior investigators who use conferences for important networking opportunities.
Collaborative Science
Within and across institutions, collaborations have been catalyzed by innovations forced on us or rapidly disseminated as a result of the pandemic. Geographically dispersed research teams were less common before the pandemic, but innovations in video conferencing have greatly facilitated existing and new collaborations that were not previously possible. Likewise, quantum leaps forward in the conduct of clinical and population research are within our reach, and some have already been demonstrated, through the use of aggregated electronic health records for recruitment and of mobile health technologies for consenting, monitoring, and interacting with human research study participants. There is thus the opportunity to further enhance multisite collaborative science through expanded data sharing and communication technologies. Administrators can also streamline the formation of collaborative research through more rapid review of data and material sharing agreements, including the implementation of universal sharing agreements. Collaboration with these groups in the design, conduct, and dissemination of science across the translational spectrum will enhance the relevance of cardiovascular research and ensure greater public support for its continued funding.
CONCLUSIONS
Much as it has affected the rest of society, the COVID-19 pandemic has changed the cardiovascular research enterprise for the foreseeable future. Basic, clinical, and population science has been altered as research stopped, slowed, or shifted to COVID-19. Researchers, particularly early career investigators, women, and those from underrepresented groups in medicine, experienced challenges in completing education and training, using or obtaining funding, or advancing along their scheduled career timelines. Scientific meetings and the nature of scientific collaboration changed substantially, and it may be some time until optimal means of collaboration return or are forged anew.
The relationship between the scientific community and the broader society was challenged. Although biomedical science is increasingly appreciated as a means to improve public health, disunity and pockets of misinformation and resistance to science have contributed to skepticism about vaccine efficacy and science more broadly in some quarters. Nonetheless, the pandemic has also demonstrated that science can bring great advances and insights and that new ways of conducting science and collaborating may be in the offing. A renewed public commitment to funding science and scientists is warranted if society is to reap the benefits of all that science has to offer. The AHA, with its partners, intends to lead the way.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on August 27, 2021, and the American Heart Association Executive Committee on October 14, 2021. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/ honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Elizabeth M. McNally | Northwestern University | None | None | None | None | Ikaika Therapeutics* | None | None |
| Mitchell S.V. Elkind | Columbia University | None | None | None | None | None | None | None |
| Ivor J. Benjamin | Medical College of Wisconsin | None | None | None | None | None | None | None |
| Mina K. Chung | Cleveland Clinic | NIH†; AHA† | None | None | None | None | None | None |
| Glenn H. Dillon | American Heart Association | None | None | None | None | None | None | None |
| Adrian F. Hernandez | Duke University | AstraZeneca*; Boehringer Ingelheim*; Merck*; Novartis*; Verily* | None | None | None | None | AstraZeneca*; Bayer*; Boston Scientific*; Merck*; Myokardia* | None |
| Chinwe Ibeh | Columbia University Irving Medical Center and New York–Presbyterian Hospital | None | None | None | None | None | None | None |
| Donald M. Lloyd-Jones | Northwestern University | None | None | None | None | None | None | None |
| Louise D. McCullough | The University of Texas Health Science Center at Houston | None | None | None | None | None | None | None |
| Loren E. Wold | The Ohio State University | NIH (R01 and U01)†; AHA† | None | None | None | None | None | None |
| Davene R. Wright | HMS and Harvard Pilgrim Health Care Institute | None | None | None | None | None | None | None |
| Joseph C. Wu | Stanford University | NIH† | None | None | None | Khloris Bio-sciences† | None | None |
REFERENCES
- 1.Bernard MA, Lauer M. The impact of the COVID-19 pandemic on the extramural scientific workforce: outcomes from an NIH-led suvey. 2021. National Institutes of Health: Office of Extramural Research, Extramural Nexus. Accessed May 27, 2021. https://nexus.od.nih.gov/all/2021/03/25/the-impact-of-the-covid-19-pandemic-on-the-extramural-scientific-workforce-outcomes-from-an-nih-led-survey/ [Google Scholar]
- 2.Sohrabi C, Mathew G, Franchi T, Kerwan A, Griffin M, Soleil C Del Mundo J, Ali SA, Agha M, Agha R. Impact of the coronavirus (COVID-19) pandemic on scientific research and implications for clinical academic training: a review. Int J Surg. 2021;86:57–63. doi: 10.1016/j.ijsu.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colenda CC, Applegate WB, Reifler BV, Blazer DG 2nd. COVID-19: financial stress test for academic medical centers. Acad Med. 2020;95:1143–1145. doi: 10.1097/ACM.0000000000003418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korbel JO, Stegle O. Effects of the COVID-19 pandemic on life scientists. Genome Biol. 2020;21:113. doi: 10.1186/s13059-020-02031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaul P, Garg PK. Crowdsourcing and global collaboration during COVID-19 pandemic: a silver lining. J Surg Oncol. 2021;123:1204–1205. doi: 10.1002/jso.26417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during the COVID-19 public health emergency: guidance for industry, investigators, and institutional review boards. 2020. Accessed May 31, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency
- 7.Asaad M, Habibullah NK, Butler CE. The impact of COVID-19 on clinical trials. Ann Surg. 2020;272:e222–e223. doi: 10.1097/SLA.0000000000004113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dorn A COVID-19 and readjusting clinical trials. Lancet. 2020;396: 523–524. doi: 10.1016/S0140-6736(20)31787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger JM, Xiao H. The COVID-19 pandemic and new clinical trial activations. Trials. 2021;22:260. doi: 10.1186/s13063-021-05219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber C New investigator grants expand research opportunities. IEEE Pulse. 2011;2:22–23. doi: 10.1109/MPUL.2011.942060 [DOI] [PubMed] [Google Scholar]
- 11.King M, Frederickson M. The pandemic penalty: the gendered effects of COVID-19 on scientific productivity. Socius. 2021;7:1–24. [Google Scholar]
- 12.Malisch JL, Harris BN, Sherrer SM, Lewis KA, Shepherd SL, McCarthy PC, Spott JL, Karam EP Moustaid-Moussa N, Calarco JM, et al. Opinion: in the wake of COVID-19, academia needs new solutions to ensure gender equity. Proc Natl Acad Sci USA. 2020;117:15378–15381. doi: 10.1073/pnas.2010636117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feintzeig R The uneven odds for promotions with hybrid work. 2021. Dow Jones & Company, Inc. Accessed August 9, 2021. https://www.wsj.com/articles/the-uneven-odds-for-promotions-with-hybrid-work-11626062462 [Google Scholar]
- 14.Kessler S Will remote workers get left behind in the hybrid office? 2021. New York Times Co. Accessed August 9, 2021. https://www.nytimes.com/2021/08/05/business/dealbook/remote-work-bias.html [Google Scholar]
- 15.Levine RL, Rathmell WK. COVID-19 impact on early career investigators: a call for action. Nat Rev Cancer. 2020;20:357–358. doi: 10.1038/s41568-020-0279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabster BP, van Daalen K, Dhatt R, Barry M. Challenges for the female academic during the COVID-19 pandemic. Lancet. 2020;395:1968–1970. doi: 10.1016/S0140-6736(20)31412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Termini CM, Traver D. Impact of COVID-19 on early career scientists: an optimistic guide for the future. BMC Biol. 2020;18:95. doi: 10.1186/s12915-020-00821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen LH, Tan-McGrory A, Oh AY, Barreto EA, Bartels SJ, Armstrong KA, Chan AT, Warner ET. Diversifying the biomedical workforce during the COVID-19 pandemic. Nat Med. 2020;26:1811. doi: 10.1038/s41591-020-1134-7 [DOI] [PubMed] [Google Scholar]
- 19.Arora VM, Wray CM, O’Glasser AY, Shapiro M, Jain S. Leveling the playing field: accounting for academic productivity during the COVID-19 pandemic. J Hosp Med. 2021;16:120–123. doi: 10.12788/jhm.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr RM, Lane-Fall MB, South E, Brady D, Momplaisir F, Guerra CE, Montoya-Williams D, Dalembert G, Lavizzo-Mourey R, Hamilton R. Academic careers and the COVID-19 pandemic: reversing the tide. Sci Transl Med. 2021;13:eabe7189. doi: 10.1126/scitranslmed.abe7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muric G, Lerman K, Ferrara E. Gender disparity in the authorship of biomedical research publications during the COVID-19 pandemic: retrospective observational study. J Med Internet Res. 2021;23:e25379. doi: 10.2196/25379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk C, Tyson A, Kennedy B, Johnson C. Science and Scientists Held in High Esteem Across Global Publics. 2020. Pew Research Center. Accessed May 31, 2021. https://www.pewresearch.org/science/2020/09/29/science-and-scientists-held-in-high-esteem-across-global-publics/ [Google Scholar]
- 23.3M State of Science Index survey. 2021. Accessed May 31,2021. https://www.3m.com/3M/en_US/state-of-science-index-survey/interactive-3m-state-of-science-survey/
- 24.Anwar A, Malik M, Raees V, Anwar A. Role of mass media and public health communications in the COVID-19 pandemic. Cureus. 2020;12:e10453. doi: 10.7759/cureus.10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury N, Khalid A, Turin TC. Understanding misinformation infodemic during public health emergencies due to large-scale disease outbreaks: a rapid review. Z Gesundh Wiss. 2021:1–21. doi: 10.1007/s10389-021-01565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abd-Alrazaq A, Schneider J, Mifsud B, Alam T, Househ M, Hamdi M, Shah Z. A comprehensive overview of the COVID-19 literature: machine learning-based bibliometric analysis. J Med Internet Res. 2021;23:e23703. doi: 10.2196/23703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimock M, Wike R. America is exceptional in the nature of its political divide. 2020. Pew Research Center. Accessed May 23, 2021. https://pewrsr.ch/2JZY9fb [Google Scholar]
- 28.Devlin K, Connaughton A. Most Approve of National Response to COVID-19 in 14 Advanced Economies. 2020; Pew Research Center, https://www.pewresearch.org/global/2020/08/27/most-approve-of-national-response-to-covid-19-in-14-advanced-economies/. Accessed May 23, 2021. [Google Scholar]
- 29.Boschele M COVID-19 is a crisis in planetary health and politics of expertise: time to think critically and innovate both. OMICS. 2021;25:279–284. doi: 10.1089/omi.2021.0038 [DOI] [PubMed] [Google Scholar]
- 30.Smith EM. Reimagining the peer-review system for translational health science journals. Clin Transl Sci. 2021;14:1210–1221. doi: 10.1111/cts.13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul E, Steptoe A, Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: implications for public health communications. Lancet Reg Health Eur. 2021;1:100012. doi: 10.1016/j.lanepe.2020.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An J, Wei R, Zhou H, Luong TQ, Gould MK, Mefford MT, Harrison TN, Creekmur B, Lee MS, Sim JJ, et al. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers use and cOVID-19 infection among 824 650 patients with hypertension from a US integrated healthcare system. J Am Heart Assoc. 2021;10:e019669. doi: 10.1161/JAHA.120.019669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Heart Association. AHA Rapid Response Grant COVID-19 and its cardiovascular impact. Accessed May 31, 2021. https://professional.heart.org/en/research-programs/strategically-focused-research/aha-rapid-response-grant-covid19
- 34.Garcia G Jr, Sharma A, Ramaiah A, Sen C, Purkayastha A, Kohn DB, Parcells MS, Beck S, Kim H, Bakowski MA, et al. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021;35:108940. doi: 10.1016/j.celrep.2021.108940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morselli Gysi D, do Valle Í, Zitnik M, Ameli A, Gan X, Varol O, Ghiassian SD, Patten JJ, Davey RA, Loscalzo J, et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc Natl Acad Sci USA. 2021;118:e2025581118. doi: 10.1073/pnas.2025581118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alger HM, Rutan C, Williams JH 4th, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID-19 CVD registry powered by Get With The Guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. doi: 10.1161/CIRCOUTCOMES.120.006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez F, Solomon N, de Lemos JA, Das SR, Morrow DA, Bradley SM, Elkind MSV, Williams JH, Holmes D, Matsouaka RA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth GA, Emmons-Bell S, Alger HM, Bradley SM, Das SR, de Lemos JA, Gakidou E, Elkind MSV, Hay S, Hall JL, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e218828. doi: 10.1001/jamanetworkopen.2021.8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Heart Association. American Heart Association and the global COVID-19 pandemic. Accessed June 14, 2021. https://www.ahajournals.org/coronavirus.
- 40.American Heart Association. COVID 19 content: an AHA compendium. Accessed June 14, 2021. https://professional.heart.org/en/covid-19-content-an-aha-compendium
- 41.PL. 116-260(H.R. 133) - Consolidated Appropriations Act, 2021. Accessed September 14, 2021. https://officeofbudget.od.nih.gov/pdfs/FY21/cy/FY%202021%20Appropriations%20(PL-116-260).pdf
- 42.Research!America. Statement of Research!America President & CEO Mary Woolley on introduction of the RISE Act. 2021. Accessed May 31, 2021. https://www.researchamerica.org/news-events/news/statement-resear-chamerica-president-ceo-mary-woolley-introduction-rise-act
- 43.Remmel A Scientists want virtual meetings to stay after the COVID pandemic. Nature. 2021;591:185–186. doi: 10.1038/d41586-021-00513-1 [DOI] [PubMed] [Google Scholar]


