Abstract
Many diseases are caused by insufficient expression of mutated genes and would benefit from increased expression of the corresponding protein. However, in drug development, it has been historically easier to develop drugs with inhibitory or antagonistic effects. Protein replacement and gene therapy can achieve the goal of increased protein expression but have limitations. Recent discoveries of the extensive regulatory networks formed by non-coding RNAs offer alternative targets and strategies to amplify the production of a specific protein. In addition to RNA-targeting small molecules, new nucleic acid-based therapeutic modalities that allow highly specific modulation of RNA-based regulatory networks are being developed. Such approaches can directly target the stability of mRNAs or modulate non-coding RNA-mediated regulation of transcription and translation. This Review highlights emerging RNA-targeted therapeutics for gene activation, focusing on opportunities and challenges for translation to the clinic.
Subject terms: Target identification, Oligo delivery
Many diseases involve reduced or absent levels of a particular protein and would benefit from therapies that increase gene expression. In their Review, Khorkova et al. discuss the growing range of RNA-targeted therapies in development that aim to boost gene expression, including nucleic acid-based therapeutics targeting the complex regulatory network of non-coding RNA species.
Introduction
Traditionally, therapeutic development involved the discovery of protein-targeting small molecules. In general, it has been easier to find such molecules with inhibitory or antagonistic effects1. While efficacious in many contexts, this paradigm had not been easily applied to the multitude of diseases that are caused by insufficient expression of biologically vital proteins. The breakthrough moment in this area came in the early 2000s, after the completion of the human genome project. The research revealed that, surprisingly, while approximately three-quarters of the genome sequence is transcribed, only around 1% of the human genome codes for proteins1. It has since become clear that these newly discovered long non-coding RNA (lncRNA) transcripts have important biological functions and are core players in the vast RNA-based regulatory networks that affect all aspects of intracellular protein synthesis2,3. Simultaneously, innovations in chemical structure and manufacturing processes of nucleic acid-based therapeutics (NBTs) have added a powerful modality to small-molecule approaches to access these networks4.
Historically, one of the first clinically available NBT types for protein upregulation, namely protein replacement, employed cloning of the insufficient protein followed by its expression in cultured bacterial, human, or insect cells, purification and injection into patients (Fig. 1). Subsequent innovations in large-scale recombinant protein production and purification techniques opened the way for the advancement of this approach into the clinic. The therapeutic use of recombinant proteins and peptides, such as insulin administration for diabetes or monoclonal antibody treatments, has been successful in the clinic for many years5. However, this approach is mostly suitable for secreted proteins or enzymes and is hindered by the complex pharmacokinetics of these molecules and cost-related issues. Notably, recombinant proteins require cold storage and frequent injections, increasing the burden on patients. Furthermore, synthetic polypeptides are unlikely to fully recapitulate the diversity of endogenous functions of a protein that arise from alternative splicing, post-translational modifications, subcellular targeting and other regulatory mechanisms.
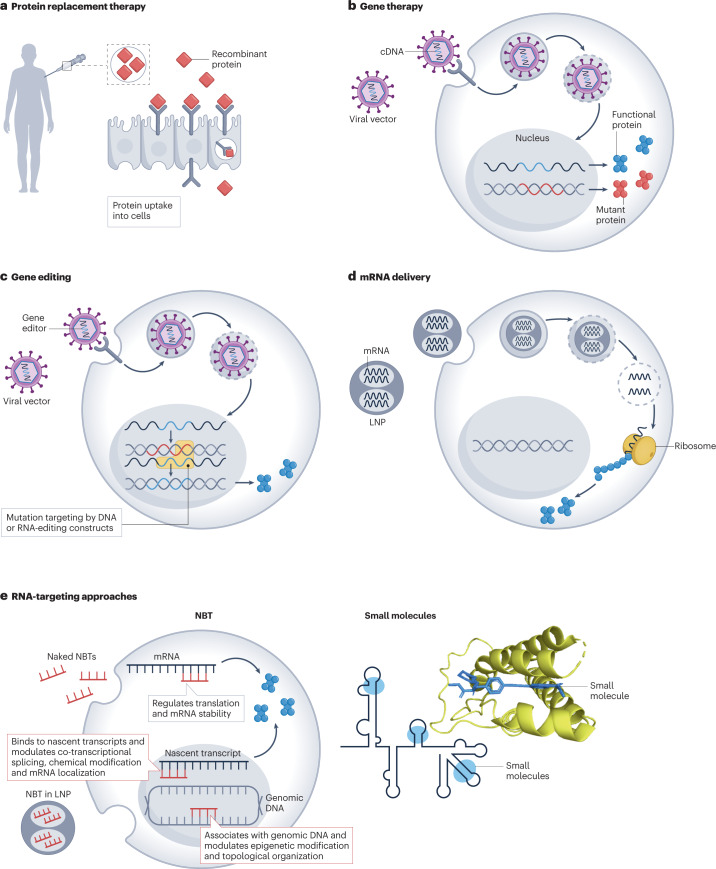
Fig. 1. Overview of strategies to increase protein production.
Currently, several strategies are approved or in development to increase protein production. a, In protein-replacement therapy, recombinant proteins are administered to replace a mutant variant or to supplement for a deficient variant in a patient. b, For gene therapy, viral vectors are used to deliver cDNA encoding functional proteins. c, For gene editing, specific mutations are corrected in situ by targeted DNA-editing or RNA-editing constructs (for example, transcription activator-like effector nucleases, zinc finger nucleases, CRISPR–Cas, base editors). d, For mRNA delivery, functional mRNA is delivered to cells to increase protein levels using various delivery modalities such as lipid nanoparticles (LNPs). e, RNA-targeted approaches include nucleic acid-based therapeutic (NBT) and small-molecule strategies. NBTs, such as antisense oligonucleotides, natural antisense transcript-specific oligonucleotides (AntagoNATs), small activating RNAs and microRNA blockers (antagomirs), modulate non-coding RNA-mediated regulation of transcription and translation through various mechanisms involving, for example, RNAse H, RNA-induced silencing complex-mediated RNA interference and steric blocking. Small molecules directly target RNA–protein interactions or recruit endogenous enzymes to target RNA, leading to protein upregulation.
These shortcomings of protein-replacement therapy were partially addressed by the development of gene therapy approaches. In this paradigm, cDNA or RNA encoding the desired protein is delivered to cells in patients through the use of a plasmid or an engineered viral vector. It is then expressed in the cell, using endogenous protein synthesis and post-translational modification machinery, thus avoiding many problems associated with protein replacement (Fig. 1). The gene therapy field has an extensive history6 and significant recent successes in reaching the clinic (Supplementary Table 1) that are beyond the scope of this Review. However, multiple gene therapy-specific impediments also exist, including overexpression of the therapeutic protein, expression in unintended cell and tissue types due to the use of constitutive promoters, and vector-associated immunogenicity7,8. Another interesting and up-and-coming approach to protein upregulation, namely gene editing, is briefly described in Box 1. This approach is, however, only applicable to a subset of diseases.
Thus, there is still an enormous unmet medical need for alternative therapeutic approaches that can specifically and controllably increase the expression of coding and non-coding genes and reduce development and manufacturing costs, thus increasing the range of treatable diseases (Fig. 1).
In recent years, multiple RNA-targeted therapeutic modalities have emerged as potent and specific activators of endogenous gene expression. RNA-targeted therapeutics have distinguished themselves from parallel approaches to supplement gene expression by their ability to selectively modulate a wide network of endogenous RNA-mediated cellular mechanisms (such as transcription, splicing, translation, and mRNA stability and subcellular localization) and enhance protein production. Furthermore, several therapies that target splicing mechanisms to modulate inclusion of mutated exons are already approved by the FDA and EMA (Supplementary Table 2).
The majority of treatments under investigation in this space are NBTs. Catalysed by significant advancements in understanding of the regulatory roles of non-coding RNAs (ncRNAs)2, developments in synthetic nucleotide chemistry4,9 (Box 2) and a few monumental clinical successes (Supplementary Table 2), this burgeoning drug class is rapidly expanding and redefining the meaning of a druggable target10. In recent years, several small-molecule drugs targeting RNA-mediated processes have also been approved (Supplementary Table 2).
Nevertheless, significant obstacles remain that have hampered progress in the field despite considerable research and investment over the past decade. Some issues are related to oral and central nervous system (CNS) delivery, which impose clinical limitations in terms of administration routes and frequency of dosing, particularly in chronic diseases. These limitations join other problems shared by small-molecule drugs such as insufficient knowledge of disease biology (especially in rare diseases), poor selection of molecular targets and clinical trial challenges.
In this Review, we discuss key aspects of gene expression regulation that provide opportunities for therapeutic upregulation of disease-associated proteins. We highlight the most promising NBTs and small molecules for gene activation, which have already demonstrated clinical potential or are in the discovery phase, and discuss remaining obstacles and possible solutions for their successful development.
Box 1 Gene editing.
In contrast to gene therapy, which uses vectors to deliver a healthy gene copy, gene editing is intended to target disease-causing mutations in situ, directly in the host genome or host RNA. An important benefit of gene editing is the availability of the engineered gene or RNA to all endogenous regulatory mechanisms and thus the absence of potentially detrimental spurious expression. Several methods of gene editing have been proposed and some of them have reached clinical trial stage134. However, the biology of gene-editing processes and the consequences of the resulting interventions as well as multiple problems with the delivery and tissue distribution of vectors are not completely understood. These factors restrict current use of gene-editing treatments to ultra-rare diseases and limited numbers of patients.
Most therapeutics that are currently under development apply gene editing using CRISPR–Cas9 ex vivo. Several clinical trials employing this technique that are currently under way in cancer, HIV infection, β-thalassaemia and sickle cell disease have been recently reviewed135,136. Base editing is a related approach based on CRISPR-guided genome editing, in which a modified Cas9 fused to a deaminase enzyme converts C to G and A to T without making double-stranded DNA breaks. Base editors are currently in clinical trials for indications including familial hypercholesterolaemia and cancer137.
A gene-editing method based on vectorized zinc finger nucleases (ZFNs) is now being tested in clinical trials. ZFNs are engineered proteins combining the DNA recognition specificity of zinc finger proteins with the nuclease domain of Fok1, an endonuclease from Flavobacterium okeanokoites, to create double-strand breaks and edit mutated genes at precise sites in the genome. This approach has been applied to autologous CD34+ haematopoietic stem and progenitor cells from patients with severe sickle cell disease. Ex vivo editing of the erythroid-specific enhancer of BCL11A increased endogenous fetal haemoglobin (HbF) production and total Hb in five patients, according to preliminary clinical trial results (NCT03653247)138. However, several other ZFN programmes, including those in mucopolysaccharidosis type IH (also known as Hurler syndrome), HIV1 and several neurological diseases, have been discontinued138.
Another gene-editing method is mediated by transcription activator-like effector nucleases (TALENs). TALENs are fusion proteins that combine the catalytic module of Fok1 nucleases with the DNA-binding domain of TALEs, naturally occurring virulence proteins secreted by a plant bacterial pathogen, Xanthomonas. Several clinical trials of chimeric antigen receptor (CAR)-T cell therapy using TALEN technology139 are ongoing in various cancers (NCT04142619, NCT04150497 and NCT03190278). CAR-T cell therapy usually involves ex vivo engineering of autologous T cells, which are then returned to the patient. An allogeneic, off-the-shelf, genome-edited anti-CD19 CAR-T cell product (UCART19) is also now in clinical trials140.
Techniques utilizing the ability of endogenous human double-stranded RNA-specific adenosine deaminases (ADARs) to convert adenosine residues to inosine, which is interpreted by translation machinery as guanosine, are now being actively explored. This change can reverse G->A mutation post-transcriptionally in multiple known diseases. Advantages of this approach include achieving mutation correction without permanently altering genomic DNA141.
An ADAR-based approach termed Axiomer RNA-editing technology uses editing oligonucleotides to guide A to I editing by ADAR. Editing oligonucleotides are 25–30 nucleotides long and have a chemically modified backbone to increase stability in vivo and optimize ADAR function. Another innovation in ADAR approaches is the use of Benner base Z instead of a mismatched C usually found opposite the target A in endogenous ADAR editing sites. This modification imitates a naturally occurring E488Q mutation in ADAR2 that boosts enzymatic activity (see ‘Related links’ for further information on Axiomer technology). Over 20,000 disease-causing mutations could be remediated by A-to-I editing142,143.
Wave Life Sciences is investigating stereopure phosphoramidate oligonucleotides, called AIMers, for ADAR-mediated editing for various indications. WVE-006 is a phosphoryl guanidine-modified N-acetyl galactosamine-conjugated oligomer that aims to correct the single base mutation in mRNA coded by the SERPINA1 Z allele to treat alpha 1 antitrypsin deficiency144. An Investigational New Drug application for WVE-006 is expected in 2023.
Another gene-editing approach, now in the discovery stage, is termed ‘leveraging endogenous ADAR for programmable editing of RNA’ (LEAPER). Investigators are developing short engineered ‘ADAR-recruiting RNAs’ (arRNAs) to recruit native ADAR1 or ADAR2 to restore IDUA activity in fibroblasts from patients with Hurler syndrome. arRNAs can be delivered as naked antisense oligonucleotides or by a plasmid or viral vector145. Use of covalently closed circular arRNAs delivered by an adeno-associated virus improved LEAPER editing efficiency and reduced bystander editing146.
Early discovery studies of circular ADAR-recruiting guide RNAs incorporating interspersed loops in the antisense domains showed 53% RNA editing of the mPCSK9 transcript in mouse liver and 12% UAG-to-UGG RNA correction of the amber nonsense mutation in the IDUA-W392X mouse model of Hurler syndrome147.
Box 2 Chemical modification of NBTs.
As with any other therapeutic strategy, the clinical productivity of nucleic acid-based therapeutics (NBTs) relies on biodistribution, target specificity, activity, and evasion of immunosurveillance and clearance mechanisms. These characteristics, to a large extent, depend on the pharmacokinetic properties of NBTs that are determined by their common nucleic acid-based chemistry. Unmodified oligonucleotides do not have optimal pharmacokinetic and pharmacodynamic profiles. The polyanionic hydrophilic structure of the nucleic acids stalls cellular membrane penetrance and endosomal escape while being highly vulnerable to endonuclease degradation and off-target interactions. Altering the chemical architecture of NBTs via the introduction of backbone modifications, ribose sugar substitutions, nucleobase derivatives, stabilizing or cleavable internucleotide linkages, and functionalization with various conjugates (see the figure) has been beneficial in the development of clinically mature NBT candidates9.
Internucleotide bond modifications, such as phosphorothioate (PS) bonds, and 2′ribose substitutions, such as 2′-O-methyl (2′-OMe), 2′-O-methoxyethyl (2′-MOE) or 2′-fluoro (see the figure), enhance endonuclease resistance and serum stability of NBTs, which results in prolonged drug retention in tissues. Furthermore, replacement of the non-bridging oxygen of the natural phosphodiester bond with a sulfur atom in PS bonds reduces the negative charge and increases the hydrophobicity of the NBT, facilitating protein binding and enhanced endosomal uptake148. Substitutions at 2′-OH in ribose, such as 2′-MOE, 2′-OMe and 2′-fluoro, or introduction of nucleotides modified with conformationally constrained ribose sugars, broadly referred to as bridged nucleic acids, increase the affinity of NBTs to the target sequence. Modifications such as locked nucleic acids (LNAs), 2′-O,4′-C ethylene bridged nucleic acid, tri-cyclo DNA or 7′,5′-α-bi-cyclo DNA (bcDNA; see the figure) form oligomer-target RNA duplexes with greater stability, enhancing their target affinity in vivo149. As a result, changes in chemical structure introduced by sugar modifications can significantly influence the potency of the drugs by modulating their cellular uptake and activity150.
Many of the ribose modifications are incompatible with RNase H or RNA interference (RNAi) activity but are suitable for designing oligonucleotides for non-cutting applications such as splice modulation or double-stranded RNA-specific adenosine deaminase recruitment151.
Another extensively studied field of NBT chemistry explores the utility of alternative, more hydrophobic backbones such as phosphorodiamidate morpholino oligomers (PMO), thiomorpholino oligonucleotides (TMO) and peptide nucleic acid, replacing the charged RNA and DNA building blocks in the oligonucleotides152 (see the figure). These structures improve the pharmacokinetic properties of NBTs but fail to recruit RNase H and RNAi machinery and are hence used in the design of steric blockers and mixmers.
Novel customized backbone chemistries, such as alkylphosphonates, phosphoryl guanidine (PN) and mesyl phosphoramidate (see the figure), substituting PS bonds in gapmers, were found to enhance nuclease resistance and ameliorate cytotoxicity and immunostimulation153–155. However, these new modifications can modulate target affinity.
Terminal modifications stabilizing the 5′-ribose with phosphatase-resistant 5′-phosphate analogues, such as 5′-(E)-vinylphosphonate, are used in small interfering RNAs to enhance Argonaut 2 interactions and thereby RNAi activity156.
In addition to the off-target binding of NBTs to loci sharing sequence similarities, hybridization-independent effects, such as adhesion to cellular proteins, are known to build cytotoxicity. Altering the nucleobase chemistry is a promising strategy to reduce off-target binding and improve tissue half-life of the drug. Methylated pyrimidines, such as 5-methylcytosine and N1-methylpseudouracil, are known to evade interaction with biomolecules involved in immunosurveillance157,158. Liver-targeting LNA gapmers optimized with synthetic nucleobase derivatives C1 (5-hydroxycytosine), T1 (2-thiothymine) and G1 (8-bromoguanine) that replace C, T and G nucleotides, respectively, demonstrated milder hepatotoxicity in vivo159. Recently, Ionis Pharmaceuticals demonstrated the utility of abasic nucleotides in modulating the affinity and interaction of antisense oligonucleotides with nuclear protein complexes160.
Functionalization with various conjugates, such as lipids (cholesterol, 2′-O-hexadecyl (2′-O-C16)), cell targeting or penetrating peptides (RVG, transferrin), sugars (N-acetyl galactosamine (GalNAc)), antibodies and small molecules, has demonstrated unprecedented success in ensuring efficient delivery and enhanced pharmacokinetics of NBTs9,161. Development of brain-targeting conjugates, such as α-tocopherol, anti-transferrin receptor Fab and 2′-O-C16, compatible with non-invasive administration routes, including inhalation, oral or minimally invasive nasal depot, are helping to expand the use of NBTs for neurological disorders109,124,162 (see the figure and Table 1).
The multiplicity of possible enantiomers for oligomers assembled with bridged nucleic acids, novel backbone chemistries and conjugated ligands challenges chemists to devise commercial methods for the stereocontrolled synthesis of such NBTs. In vivo preclinical and several clinical studies have assessed stereopure antisense oligonucleotides and small interfering RNAs with superior efficacy, potency and durability compared to their stereorandom counterparts155,163 (Table 1).
Therapeutic mRNAs that are currently in clinical development also carry multiple chemical modifications in the coding sequence as well as 5′ and 3′ untranslated regions (UTRs). Coding sequences are optimized through elimination of rare codons, increases in GC content and use of modified nucleotides such as N6-methyladenosine, 5-methylcytosine, pseudouridine and 2′-OMe to improve transcription and translation efficiency27. Introduction of the modified nucleotides and elimination of U-rich RNA patches have the additional benefit of reducing nucleic acid-induced stimulation of Toll-like receptors (TLRs) and diminishing the innate immunogenicity of mRNAs164. Guided adenosine-to-inosine RNA editing (Box 1) by endogenous mechanisms can further reduce immune reaction to exogenous mRNAs30. In some of the current therapeutic mRNAs, cap structure, consisting of an inverted 7-methylguanosine linked to the 5′ terminal nucleotide, is also optimized. The cap is essential for mRNA protection from 5′-exonucleases and for recruiting multiple factors necessary for all steps of mRNA processing32. Caps containing chemically modified dinucleotides, such as m7GpppG, 2′,4′-locked nucleic acids or 3′-O-benzyl-modified nucleotides, were shown to increase resistance to decapping, leading to higher mRNA stability164. Furthermore, nucleotides in the poly-A tail of therapeutic mRNA could be chemically modified or replaced with nucleotide mimics (ribose-modified adenosines, cordycepin, 8-azaadenosine) to reduce 3′-to-5′ exonuclease-mediated degradation164. Although such modifications potentially increase translation efficiency and mRNA stability, their effects could be dependent on position and cell type95. Moreover, in vivo delivery of mRNA requires a carrier, for example, a lipid nanoparticle, to facilitate cellular uptake and protect it from endogenous nucleases. In recent years, significant progress has been achieved in designing lipid nanoparticles for mRNA delivery (Box 3).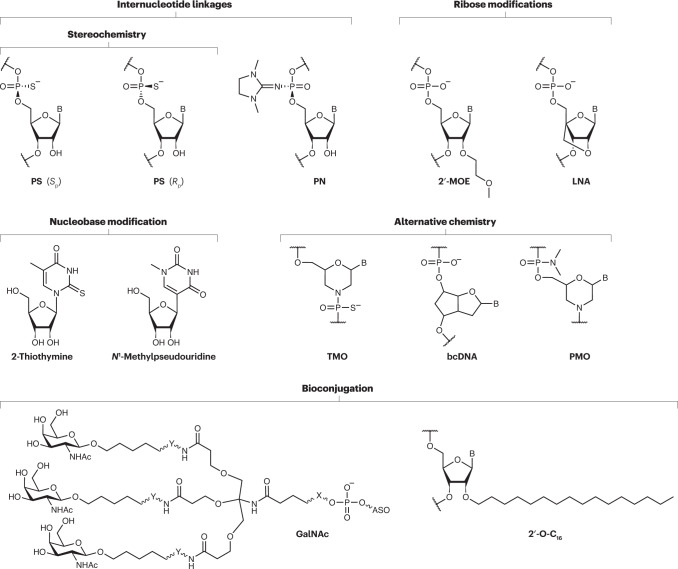
Biology of protein upregulation
The goal of therapeutic protein upregulation could be achieved by modulating biological processes at any stage of protein production in the cell, including transcription, splicing, translation or post-translational modification (Fig. 2). As many of these processes involve DNA or mRNA and are regulated by ncRNA networks, they are particularly amenable to modulation by NBTs.
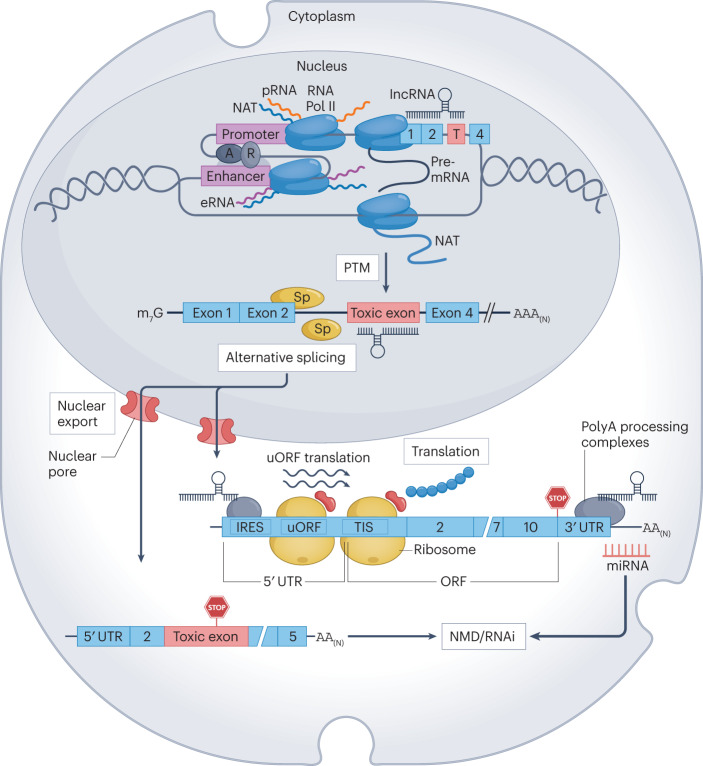
Fig. 2. Biology of protein upregulation.
Physical interaction of distal enhancer elements with active gene promoters via chromosomal looping recruits transcription factors, chromatin modifiers, Mediator complex and RNA polymerase II (Pol II), leading to transcription activation. Bidirectional transcription from the promoter region and at enhancers gives rise to non-coding promoter RNAs (pRNAs) and enhancer RNAs (eRNAs), respectively. Natural antisense transcripts (NATs) transcribed from the antisense strand of protein-coding loci, trans-acting long non-coding RNA (lncRNA), pRNAs and eRNAs can scaffold epigenetic modifiers and transcription regulatory proteins at the target gene locus. Alternatively, they can pair directly with complementary regions in mRNA, inducing distinct stimulatory and inhibitory effects on transcription. Nascent pre-mRNA undergoes co-transcriptional and post-transcriptional modification (PTM) such as 5′-capping, 3′-end processing and polyadenylation, alternative splicing, in situ introduction of N6-methyladenosine, 5-methylcytosine, N1-methyladenosine, pseudouridine and 2′-O-methyl modifications and deamination of adenosine to inosine by adenosine deaminases acting on RNA. The resulting mature mRNA isoforms are exported to the cytoplasm through nuclear pores. Translation of mRNA by the ribosome begins at a translation initiation site (TIS) and is regulated by proteins recruited by mRNA structural elements such as an internal ribosome entry site (IRES), and NATs and lncRNAs targeting untranslated regions (UTRs). miRNAs targeting unique sequences in 3′ UTRs can induce mRNA degradation through the RNA interference (RNAi) mechanism. Short upstream open reading frames (uORFs) within the 5′ UTR compete for the ribosome with the productive open reading frame (ORF) that gives rise to the functional full-length protein, thus slowing down its translation. Non-productive transcripts with inclusion of toxic exons characterized by the presence of dysfunctional termination codons are degraded by the nonsense-mediated decay (NMD) pathway. A, activator; R, repressor; Sp, splice-promoting or inhibiting factors.
While proteins regulating transcription and translation have been studied for a long time, the roles of ncRNA in this process have been discovered only recently10. Transcriptional activation is the most explored way to increase protein abundance. Natural antisense transcripts (NATs), promoter RNAs (pRNAs), enhancer RNAs (eRNAs) and super-enhancer RNAs (seRNAs) have been shown to play both inhibitory and stimulatory roles through direct transcriptional interference or via coordinating and scaffolding epigenetic modifiers or transcription-promoting protein complexes at the target gene loci. ncRNA, including NATs and microRNAs (miRNAs), are also involved in the regulation of mRNA and protein stability and translation2,10. It should be noted that the ncRNA field is still relatively young and many of the mechanisms of action proposed for ncRNAs are at the hypothesis stage and require further elucidation. Importantly, the activity of these regulatory ncRNAs can be modulated using NBTs or small-molecule compounds.
Unexpectedly, under normal physiological conditions, both transcription and translation of multiple cellular proteins are partially suppressed or enhanced by endogenous RNA-based regulatory elements, including regulatory sequences in mRNAs, toxic exons, lncRNAs, pRNAs, eRNAs and miRNAs. The multiple transcriptional and translational layers of control can be beneficial for the cell, for example, when an instant increase in protein synthesis is required utilizing a pre-existing pool of mRNA11. Notably, under disease conditions caused by the shortage of a specific protein, disengaging the inhibitory transcription or translation control mechanisms could lead to therapeutic benefits. Therefore, significant effort is being directed at understanding the biology of these inhibitory mechanisms in order to identify new RNA-based upregulation targets. The findings also form the foundation for optimizing therapeutic mRNAs for increased protein production.
As the lncRNA field is relatively young, the nomenclature of lncRNAs is not yet finalized. Currently, lncRNAs are tentatively divided into two large groups based on their position relative to protein-coding genes: long intergenic ncRNAs (lincRNAs) and NATs. If NATs or lincRNAs are transcribed from enhancers, super-enhancers or promoters they are referred to as eRNA, seRNA or pRNA, respectively. In addition to full-length RNAs, these regulatory elements also generate abundant short transient transcripts referred to by the same names.
lincRNAs represent very long transcripts that are expressed from stretches of the chromosome that do not express any coding genes. lincRNAs, such as MALAT1 and HOTAIR, have been shown to modulate sets of developmentally regulated genes in trans through scaffolding protein binding and altering chromosome topology12. Although lincRNAs can be precisely targeted by NBTs, their wide-ranging effects and incompletely understood biology complicate the development of lincRNA-targeted therapies.
NATs are a class of lncRNAs that are transcribed from the antisense strand of coding gene loci (Fig. 2). A key defining feature of NATs is that they can specifically regulate transcription, RNA processing and translation of their sense gene partners in cis or in trans. While NATs have diverse regulatory functions, including sponging miRNA and pairing with mRNA to increase its stability, many of them act to inhibit expression of their target coding gene through coordination of repressive factors. Accordingly, targeting NATs with antisense oligonucleotides (ASOs) can result in de-repression of the sense gene and increased protein expression13,14.
eRNAs, also called non-coding pervasive transcripts, are ncRNAs generated by enhancers that are transcribed bidirectionally by RNA polymerase II (RNA Pol II) from enhancer regions of many genes. These transcripts include both lncRNA and short transcripts that are degraded rapidly by the nuclear exosome complex15. Analysis of expression profiles of 27,919 human lncRNA genes across 1,829 samples of human primary cell types and tissues has demonstrated that enhancers initiate the transcription of the majority of intergenic lncRNAs16. eRNAs can determine chromatin accessibility, histone modification and gene expression by scaffolding chromatin loops that bring enhancers within the proximity of their target genes. Furthermore, eRNAs were shown to displace NELF and other factors from paused RNA Pol II complex, resulting in the activation of pause-controlled genes. Additionally, eRNAs may increase their target coding gene transcription rates by binding Argonaut 1 (AGO1) and stimulating the histone acetyltransferase activity of CREB-binding protein (CBP), for example, during myogenic differentiation15. eRNAs have also been shown to coordinate multiple transcription factors, such as YY1, BRD4 and others, at cognate regulatory elements17. Notably, many enhancers also initiate and regulate the transcription of lncRNAs18.
Development of methods such as chromatin immunoprecipitation sequencing (ChIP-seq) and DNase I hypersensitivity sequencing (DNase-seq) revealed regions with extremely high levels of transcription factor binding, chromatin modification and DNase I hypersensitivity that were termed super-enhancers19. Super-enhancers are clusters of enhancers that regulate sets of genes mostly linked to cell fate and are therefore crucial to cancer biology20. Super-enhancers are known to generate seRNAs. Due to the current state of knowledge, we do not always make the distinction between seRNA and eRNA in this Review. It is likely that eRNA and seRNA share some mechanisms of action such as RNA Pol II pause release or heterogeneous nuclear ribonucleoprotein L (hnRNPL) binding21,22.
Similar to eRNAs, short pRNAs (also known as promoter antisense RNA or promoter upstream transcripts) as well as lncRNAs are generated bidirectionally from coding gene promoters23. pRNA-specific transcription is promoted by the presence of R-loops and a high density of poly(A) sites in the vicinity of their transcription start site. pRNA transcription may be terminated early by the Integrator complex, which also controls transcription termination of eRNAs. Early termination targets these ncRNAs for fast degradation by the nuclear RNA exosome complex24. eRNA and pRNA may have similar functions, including RNA Pol II promoter–proximal pause release24,25.
The final protein abundance in the cell is regulated by the exact sequence of the expressed mRNA isoforms, which is determined by alternative splicing and/or selection of alternative promoters and polyadenylation sites. These processes in turn are regulated by the availability, repertoire and stoichiometry of the splicing, transcription and polyadenylation site cleavage factors26.
In addition to target mRNA and miRNA, and RNA components of these factors, diverse lncRNA may also be involved in regulation of alternative splicing and polyadenylation processes in a gene-specific manner (Fig. 2).
Furthermore, naturally occurring modified nucleotides, such as N6-methyladenosine, 5-methylcytosine, N1-methyladenosine, pseudouridine and 2′-O-methylated ribose, are incorporated in mRNAs and lncRNAs co-transcriptionally. These modifications have regulatory functions that can be harnessed using NBTs27. For example, the effects of pseudouridylation on splicing and stop codon readthrough can be modulated using synthetic pseudouridylation guide RNAs28,29.
Adenosine-to-inosine RNA editing mediated by endogenous double-stranded RNA (dsRNA)-specific adenosine deaminases (ADARs) occurs naturally in cells and is tightly regulated. The resulting inosine residue is interpreted by translation machinery as guanosine, thus changing the protein sequence. This modification can also alter post-transcriptional processing of the target mRNA and block activation of the dsRNA sensor MDA5 and subsequent interferon responses and inflammation30,31. It has also been employed for therapeutic purposes to reverse disease-causing mutations and alter protein interactions (Box 1).
However, as has been recently shown, increases in mRNA levels do not always result in proportional increases in protein production, revealing additional levels of regulation. As described in the following sections, besides the relatively well-studied regulatory proteins, translation efficiency can be affected by mRNA structural features that can be roughly divided into two types: (1) linear (such as 5′ caps, short upstream open reading frames (uORFs) located in 5′ UTRs, ‘toxic exons’ or polyadenylation signals) and (2) three-dimensional (3D; such as internal ribosome entry sites (IRESs) or other protein-binding sites formed by RNA secondary or tertiary conformation). The regulatory 3D structures can be folded or unfolded by RNA remodelers such as RNA helicases32–34. Optimization of both structure types in therapeutic mRNAs has been shown to increase translation efficiency and thus their efficacy34–41. Furthermore, blocking or enhancing the activity of these structures using NBTs could lead to therapeutic protein upregulation.
The 5′ UTR region of mRNA is crucial for controlling translation as it encodes uORFs and multiple types of 3D structures that bind regulatory proteins. uORFs are present in approximately half of mammalian transcripts and might inhibit translation of the main ORF by ‘sponging’ initiating ribosomes (Fig. 2). Some of the uORFs also encode bioactive microproteins33. Translation and stability of uORF products can be modulated by NBTs.
Furthermore, 5′ UTRs are known to interact with NATs through base-pairing. 5′ UTRs also contain target sites for multiple regulatory proteins (for example, ADARs) and three-dimensional DNA structures, including cap-proximal hairpins, pseudoknots (formed by several intercalated stem-loop structures) or G-quadruplex (RG4) structures (formed by (CGG)4 repeats) that can physically block the assembly of translation complexes (Fig. 2). Unwinding of the 5′ UTR secondary structures by DNA helicases, for example, eIF4A, or their stabilization by proteins, such as fragile X mental retardation protein (FMRP), represents another mechanism of translational control. Additionally, some 5′ UTRs contain IRES. When cap-mediated translation is inhibited, for example, during stress, IRES-initiated translation can be activated by diverse IRES-binding factors34. All these mechanisms are amenable to modulation using NBTs.
Multiple features of the mRNA coding sequences themselves affect translation efficiency, including mutated or naturally occurring ‘toxic’ exons that trigger immediate transcript degradation, splicing regulatory elements, secondary structures and other protein-binding sites35 (Fig. 2). These sequences can be modified in therapeutic mRNAs for optimum translation or modulated in endogenous transcripts using NBTs.
3′ UTR sequences determine mRNA stability, translation, and subcellular and tissue localization via the presence or absence of the binding sites for diverse RNA binding proteins, miRNAs or lncRNAs26. AU-rich elements, short sequences (50–150 nucleotides long) that include several copies of the AUUUA repeat, and other imperfect repeat sequences present in 3′ UTRs recruit the degradation machinery to their host mRNAs. At the same time, U-rich motifs in 3′ UTRs can bind the mRNA-stabilizing factor HuR (ELAVL1), while AC-rich sequences recruit stabilizing hnRNPL. miRNAs associate with their specific binding sequences frequently located in 3′ UTRs to initiate mRNA degradation through activation of the RNA-induced silencing complex26. NBTs can be designed to modulate the effects of these sequences.
NBTs for protein upregulation
RNA-targeted NBTs that are currently being used to upregulate gene expression address both transcriptional and translational mechanisms and can be roughly divided into two groups: (1) NBTs that increase mRNA abundance by enhancing transcription or increasing mRNA stability (Fig. 3) and (2) NBTs that optimize translation (Fig. 4). Strategies in the first group include therapeutic mRNA delivery, NBTs to regulate transcription via modulation of NAT, pRNA, seRNA and eRNA activity, NBTs to regulate RNA stability via modulation of miRNAs and NATs, as well as splice-switching applications that prevent nonsense-mediated decay (NMD) of mRNAs. Strategies in the second group include NBTs modulating uORF translation and activity of other mRNA structural elements and of ncRNAs that regulate translation initiation and elongation.
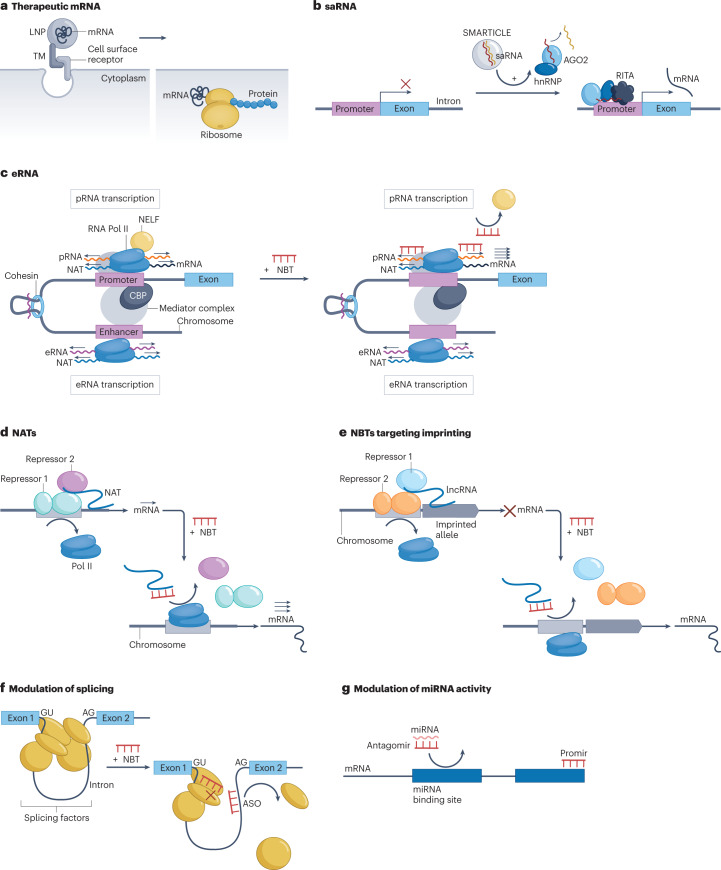
Fig. 3. NBTs that increase mRNA abundance.
a, Therapeutic mRNA transcribed in vitro and encapsulated in lipid nanoparticles (LNPs) is introduced into cells and initiates translation. Targeting molecules (TMs) that bind specific cell surface receptors can be added for cell type-specific delivery. b, Small activating RNAs (saRNAs) are double-stranded synthetic RNAs, formed by guide (red) and passenger (yellow) strands encapsulated in LNPs (known as SMARTICLEs), which are delivered to cells. saRNAs are initially recognized by double-stranded RNA loading factors, followed by argonaute 2 (AGO2) protein binding. The passenger strand of the saRNA is discarded, and a complex consisting of the guide saRNA strand, AGO2 and heterogeneous nuclear ribonucleoproteins (hnRNPs) is imported into the nucleus, where it binds directly to DNA and participates in the RNA-induced transcriptional activation (RITA) complex. RITA interacts with RNA polymerase II (RNA Pol II) to initiate transcription. c, Enhancer RNA (eRNAs), bidirectionally transcribed from an enhancer region, interact with proteins such as BRD4, CREB-binding protein (CBP) or NELF to maintain chromatin in an active state and initiate RNA Pol II pause release. Transcriptional activation of some proteins involves interaction of eRNAs and enhancer-associated natural antisense transcripts (NATs) with the Mediator complex and cohesin. Nucleic acid-based therapeutics (NBTs) mimicking eRNAs or inducing their expression can facilitate transcriptional regulation by blocking eRNA interaction with inhibitory factors. d, NAT scaffold transcriptional repressors at target gene loci. NBT treatment blocks NAT interaction with repressors and/or chromosomes and accelerates transcription. e, NBTs targeting NATs involved in silencing imprinted alleles can derepress transcription of target mRNA. f, NBTs that interact with splice factors that promote or inhibit splicing, or with their binding sites on pre-mRNA, can modulate splice factor binding and/or activity. g, NBTs designed to bind to natural miRNAs (antagomirs) serve as decoys to prevent microRNA (miRNA) binding to mRNAs. Alternatively, NBTs can be designed to function as miRNA mimics (promirs). ASO, antisense oligonucleotide; lncRNA, long non-coding RNA; pRNA, promoter RNA.
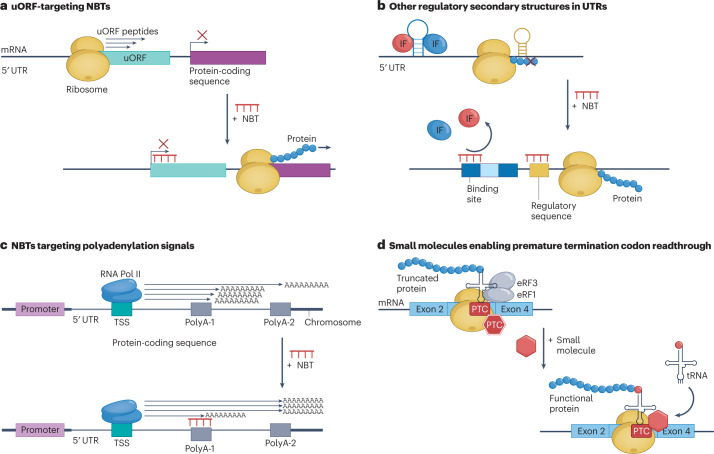
Fig. 4. NBTs and small molecules targeting mRNA translation.
a, Upstream open reading frame (uORF)-targeting nucleic acid-based therapeutics (NBTs) increase protein expression through blocking uORF translation and thus redirecting the ribosome to the main start site. b, Other regulatory secondary structures in untranslated regions (UTRs) can be targeted. NBTs targeting stem-loop and other secondary structures within 5′ UTRs that inhibit translation can increase protein expression by hybridizing them and releasing inhibitory factors (IFs) that recognize them or by removing obstacles for binding or movement of ribosomes and translation-enhancing factors. c, NBTs targeting polyadenylation (polyA) signals can preferentially prevent expression of mutated or toxic isoforms and redirect expression towards beneficial isoform ratios. d, Small molecules can enable premature termination codon (PTC) readthrough. Upon encountering a PTC, ribosomes recruit proteins such as eukaryotic translation termination factor 1 (eRF1) and eRF3, facilitating premature termination of translation. Small molecules interact with ribosomes to enable recruitment of near-cognate tRNAs and facilitate readthrough, allowing translation of full-length protein. RNA Pol II, RNA polymerase II; TSS, translation start site.
Although the field of NBT-mediated protein upregulation is young, in the last 5–7 years, it has undergone explosive growth and some of these strategies have been approved (Supplementary Table 2), while multiple other NBTs are in clinical trials (Table 1). Examples of these strategies are described below, starting with more advanced clinical cases and moving towards prospective future NBT targets.
Table 1.
Selected RNA-targeted drugs in development for gene expression amplification
| Drug | Company | Type | Target | Indication | Delivery route | Status |
|---|---|---|---|---|---|---|
| Splice-modulating NBTs | ||||||
| SRP-5051 (vesleteplirsen) | Sarepta | PPMO ASO | DMD | Exon 51-related DMD | IV | Phase I/II |
| WVE-N531 | Wave | Stereopure ASO | DMD | Exon 53-related DMD | IV | Phase I/II |
| scAAV9.U7.ACCA | Audentes/Astellas | U7 in AAV9 vector | Modified U7 snRNA (4 copies) | DMD with exon 2 duplications | IV | Phase I/II |
| Sepofarsen (QR-110) | ProQR | PS 2′-OMe ASO | CEP290 | Leber congenital amaurosis 10 | Intravitreal injection | Phase II/III |
| QR-421a (ultevursen) | ProQR | ASO | USH2A | Retinitis pigmentosa due to exon 13 mutations | Intravitreal injection | Phase I/II |
| STK-001 | Stoke | Splice-optimizing 2′-MOE PS ASO | SCN1A | Dravet syndrome | IT | Phase I/II |
| mRNA delivery | ||||||
| MRT5005 | Translate Bio | mRNA in LNP | CFTR | Cystic fibrosis | Inhalation | Phase I/II |
| mRNA-3927 | Moderna | Dual mRNAs | PCCA and PCCB | Propionic acidaemia | IV | Phase I/II |
| mRNA-3705 | Moderna | mRNA in LNP | MMUT | Methylmalonic acidaemia | IV | Phase I/II |
| mRNA-3745 | Moderna | mRNA | G6PC | Glycogen storage disease type 1a | IV | Phase I/II |
| mRNA-2752 | Moderna | 3 mRNAs in LNP | OX40 ligand, IL-23, IL-36γ | Cancer | Intratumoural injection | Phase I |
| BNT131 (SAR44100) | BioNTech/Sanofi | 4 modified mRNAs | IL-12sc, IL-15sushi, IFNA and GM-CSF | Solid tumours | Intratumoural injection | Phase I/II |
| CV8102 | CureVac | Single-stranded RNA | Non-coding, immunogenic | Cancer | Intratumoural injection | Phase I/II |
| mRNA-6231 | Moderna | mRNA in LNPs | Human IL-2 fused to human serum albumin | Autoimmune diseases | SC | Phase I/II |
| RNA-targeting small molecules | ||||||
| Dovitinib-RIBOTAC | NA | RIBOTAC | Pre-miR-21 | Triple-negative breast cancer and Alport syndrome | NA | Preclinical |
| miR-200c rSM | NA | rSM | Pre-miR-200c | Type 2 diabetes | NA | Preclinical |
| TEC-1 | Reborna biosciences | Splice-modulating rSM | SMN2 pre-mRNA | Spinal muscular atrophy | Oral | Preclinical |
| DT-216 | Design Therapeutics | GeneTAC™, gene-targeted chimera small molecules | FXN repeat expansion | Friedreich ataxia | IV injection | Phase I/II |
| miRNA-targeted NBTs | ||||||
| AMT-130 | UniQure | AAV5-miRNA | HTT | Huntington disease | Convection-enhanced stereotactic delivery | Phase I |
| Remlarsen (MRG-201, MRG-229) | miRagen/Viridian | LNA promir | miR-29b | Pathological fibrosis | Intradermal injection at biopsy site | Discontinued |
| RGLS8429 | Regulus | Antagomir | miR-17 | Autosomal dominant polycystic kidney disease | SC | Phase I/II |
| Lademirsen (SAR33937) | Genzyme/Sanofi | Antagomir | miR-21 | Alport syndrome | SC | Discontinued |
| Cobomarsen | miRagen/Viridian | Antagomir | miR-155 | Cancer | Intratumoural injection | Discontinued |
| saRNA | ||||||
| MTL-CEBPA | MiNA Therapeutics | saRNA modulator, dsRNA in SMARTICLEs | CEBPA | Hepatocellular carcinoma | IV | Phase II |
2′-OMe, 2′-O-methyl; 2′-MOE, 2′-O-methoxyethyl; AAV, adeno-associated virus; ASO, antisense oligonucleotide; CFTR, cystic fibrosis transmembrane regulator; DMD, Duchenne muscular dystrophy; dsRNA, double-stranded RNA; FXN, frataxin; HTT, huntingtin; IFN-α, interferon-α; IL, interleukin; IV, intravenous; IT, intrathecal; LNA, locked nucleic acid; LNP, lipid nanoparticle; miR, microRNA; NA, not available; NBT, nucleic acid-based therapeutic; PPMO, peptide-conjugated phosphorodiamidate morpholino oligonucleotide; PS, phosphorothioate; rSM, RNA-targeting small molecule; saRNA, small activating RNA; SC, subcutaneous; snRNA, small nuclear RNA.
Clinical-stage NBTs
Several protein-upregulating NBTs have already reached the clinical stage, including both approved drugs and drug candidates in clinical trials. mRNA delivery is perhaps the most clinically advanced modality if COVID-19 vaccines, which induce viral protein synthesis in human cells, are considered. However, development of therapeutic mRNA for the treatment of diseases associated with protein insufficiency is progressing slowly. Several splice-modulating NBTs have been approved for use in genetic diseases and constitute a more clinically mature (in terms of the number of patients treated) and more reversible alternative to gene therapy. Splice-switching NBTs that omit a ‘toxic’ exon, as well as NBTs and small molecules that enhance ribosomal readthrough and other aspects of translation, may be widely used to augment protein production in the absence of insufficiency-causing mutations. Although such NBTs have not yet been approved, some small-molecule readthrough drugs are already on the market.
Splice-modulating NBTs
Aberrant RNA splicing caused by mutations frequently leads to non-functional transcripts that are quickly destroyed by NMD, leading to the shortage of the affected protein, and is known to underlie many diseases. Furthermore, normal alternative splicing of pre-mRNA can lead to inclusion of so-called ‘toxic exons’, resulting in transcripts that are quickly degraded via NMD, thus reducing protein levels. ASOs binding to specific sequences on pre-mRNA that regulate splicing events can prevent the generation of mutated or naturally non-productive transcripts and subsequently increase target protein levels42 (Fig. 3). Given that a significant number of protein-coding genes contain toxic exons, and that many of the known disease-causing mutations can be rescued through skipping mutated exons, this strategy can have wide applicability. Indeed, therapeutic splice-modifying oligonucleotides have received a lot of attention in recent years, with some significant clinical successes (Supplementary Table 2).
Nusinersen, perhaps the most well-known splice-switching ASO, was approved by the FDA in 2016 to treat spinal muscular atrophy (SMA) and has resulted in monumental improvements in duration and quality of life for patients43. SMA is caused by insufficient levels of SMN protein due to loss-of-function mutations in the SMN1 gene. A duplicate gene (SMN2) produces no protein due to aberrant splicing out of exon 7. Nusinersen inhibits skipping of exon 7 in SMN2 by binding to the intronic splicing silencer N1 (ISS-N1) and thus increases production of corrected SMN2 mRNA and SMN protein.
As the pharmacokinetic and pharmacodynamic characteristics of ASO drugs are similar, results obtained with nusinersen can help evaluate future prospects of the protein-upregulating NBT field in general. Overall, the long-term efficacy and safety data for nusinersen are positive44–46, although the intrathecal route of administration may be associated with adverse events47,48 and has potentially reduced treatment adherence, highlighting the need to develop non-invasive methods for NBT delivery49.
Notably, both gene therapy and oligonucleotide therapy options are available in SMA. Clinical reports on combined and consecutive treatments with the two modalities may help in the evaluation of the potential of NBTs to compete with gene therapy treatments that have become more widely accepted in recent years. A related question is whether combining NBTs that address independent biological mechanisms leading to upregulation of a target protein would improve patient outcomes. This question might be addressed through clinical experience with nusinersen and gene therapy as these approaches have different gene targets (SMN2 vs SMN1) and could potentially exhibit additive effects. Furthermore, combined treatment with a splice-switching ASO and a small-molecule splicing modifier, RG7800, had an additive effect on SMN protein production, resulting in improved motor unit function in ∆7SMA mice even when given late in development50.
The FDA-approved splice-switching ASOs eteplirsen, golodirsen, viltolarsen and casimersen are intended to treat Duchenne muscular dystrophy (DMD) associated with exon 51, 53 or 45 mutations (Supplementary Table 2). Skipping these exons does not alter dystrophin function, although it possibly leads to partial sequestration and extended half-life of the truncated protein51. Following a rocky start, NBTs targeted at the upregulation of dystrophin in DMD are now showing great promise.
Although, initially, the efficacy of eteplirsen was questioned, evaluation at 6 years post-treatment demonstrated longer median time to loss of ambulation (+2.09 years; P < 0.01) and attenuated rates of pulmonary decline (P < 0.0001) compared to standard-of-care controls52. The biology of the disease, mechanism of action of the particular NBT modality used and the pharmacokinetic properties of NBTs might have all contributed to the extended time needed to observe the clinical effects. These developments also highlight the poor applicability of clinical trial paradigms developed for small-molecule drugs to NBT modalities due to their unusual pharmacokinetic properties and novel mechanisms of action. Importantly, treatment with a next-generation peptide-conjugated phosphorodiamidate morpholino oligomer vesleteplirsen (SRP-5051) led to 18-fold more exon skipping and eightfold higher dystrophin levels compared to eteplirsen in a clinical trial (NCT04004065). The trial was temporarily placed on hold due to observed hypomagnesaemia but later resumed with expanded monitoring of urine biomarkers and magnesium supplementation.
Golodirsen is an NBT designed to induce exon 53 skipping in DMD. Notably, positive clinical results have also been reported for golodirsen after 3-year follow-up., wherein loss of ambulation occurred in 9% of patients treated with golodirsen versus 26% in controls53.
Another treatment option in exon 53-related DMD is a splice-switching phosphorodiamidate morpholino oligomer, viltolarsen. Preliminary trial results after 2-year treatment with viltolarsen demonstrated preservation of motor function, while the DMD natural history controls showed significant functional decline54,55.
The exon 45-skipping ASO casimersen was approved in 2021 with a warning of kidney side effects. However, data from an extension study showed good tolerability in 12 patients followed for a mean period of 139.6 weeks56.
Among the promising novel chemistry investigational treatments for DMD is WVE-N531, a stereopure phosphoramidate splice-modulating oligonucleotide administered systemically, which is in a clinical trial in 15 patients with DMD amenable to exon 53 skipping (NCT04906460). Potential benefits of the stereopure oligonucleotides may involve higher potency. As chemical modifications of oligonucleotides used to increase their nuclease resistance introduce a chiral centre, the standard process leads to the synthesis of a large number of stereoisomers that have variable affinity to the target RNA sequence9,10. An approach developed by Wave Therapeutics leads to selective synthesis of one stereoisomer, thus potentially increasing the concentration of the active ingredient in the oligonucleotide product. Interim results from the initial cohort of three boys evaluated after dose escalation followed for 6 weeks of biweekly injections of WVE-N531 demonstrated high muscle concentration of the drug and 53% mean exon skipping. Pharmacokinetic data demonstrated a half-life of 25 days, which may support monthly dosing. Although mean dystrophin expression was below the limit of quantification, the company expects protein production to lag behind the increase in RNA synthesis. The initial clinical results for WVE-N531 indicate possible pharmacological improvement due to phosphoryl guanidine compared to first-generation DMD splice-switching NBTs and knockdown NBTs in Huntington disease programmes.
Several companies are conducting clinical trials for gene therapy treatments in DMD, but no data comparing their efficacy with exon-skipping oligonucleotides is yet available.
A combination strategy that might mitigate the disadvantages of exon-skipping ASOs and gene therapy approaches in the treatment of DMD with exon 2 duplications is under investigation. ASOs require repeated administration and are not readily taken up by muscle — the target tissue in DMD — while the limited viral vector packaging capacity requires the use of a significantly shortened dystrophin sequence. scAAV9.U7.ACCA, now in a clinical trial (NCT04240314), employs two innovations. It contains four copies of U7 small nuclear RNA, normally involved in 3′-end processing of histone pre-mRNAs. These U7 small nuclear RNAs were modified to replace histone-binding segments with sequences targeting the splice donor (two copies) and splice acceptor (two copies) sites of dystrophin exon 2, which leads to enhanced splicing out of exon 2 and upregulation of dystrophin. Furthermore, these sequences are delivered by a muscle-targeting adeno-associated virus, amplified in situ and incorporated into endogenous small nuclear ribonucleoprotein particles, which protect it from nucleases and facilitate its accumulation in the nucleus, where splicing occurs57. Preliminary data showed that, in the youngest of the three treated patients (7 months old), 99% of muscle fibres produced full-length dystrophin at about 70% of normal amounts. The two other patients (9 and 14 years old) demonstrated stabilization of disease and production of full-length protein (scAAV9.U7.ACCA (See Related links)).
Among splice-modulating NBTs for the treatment of other diseases, sepofarsen (also known as QR-110) is showing positive results in early clinical trials for Leber congenital amaurosis 10 (NCT03913143). Sepofarsen targets the c.2991+1655A>G variant in intron 26 of CEP290 that introduces a cryptic splice donor site and causes insertion of a pseudoexon, resulting in a premature stop codon (p.Cys998*). Sepofarsen is a 17-mer 2′-O-methyl/phosphorothioate-modified single-stranded RNA molecule targeting splicing of CEP290 mRNA, which is delivered by intravitreal injection58. Intravitreal injection offers some advantages, including protection from nucleases and reduced possibility of off-target effects and kidney and liver toxicity, due to sequestration of the NBT in the eye. However, this route of administration is invasive and burdensome for patients, as was illustrated by the clinical trial results. Of the 11 patients, 90.9% developed mild ocular adverse events in the treated eye versus 9.1% in the untreated eye. Eight patients developed cataracts, 75.0% of whom required lens replacement. The effects were dose dependent; therefore; higher doses were discontinued or not initiated. However, given statistically significant improvements in visual acuity and retinal sensitivity, it was concluded that the risk–benefit profile of sepofarsen supports the continuation of clinical development59.
The SCN1A-targeting splice-switching oligonucleotide STK-001 for Dravet syndrome developed by Stoke Therapeutics is currently in phase I/IIa clinical trials (NCT04442295 and NCT04740476)60. This treatment uses a different approach to SMA and DMD therapies, termed targeted augmentation of nuclear gene output (TANGO) platform. TANGO is based on the discovery of ‘toxic’ or ‘poison’ exons naturally occurring in many mRNAs. The majority of mRNA molecules containing such exons are destroyed through the NMD pathway and are not available for translation35,61. ASOs from Stoke Therapeutics are designed to prevent the inclusion of toxic exons, thus increasing the corresponding protein levels60. Preliminary results from the clinical trial show good safety and tolerability of the treatment as well as a 55% median reduction from baseline in convulsive seizure frequency after three doses of 45 mg in six patients refractory to standard medications. Reductions in seizure frequency began after the first dose and were maintained during treatment in the open-label extension study. Trends towards improvement in non-seizure readouts as measured by the BRIEF-P, an assessment of executive function, were also observed (see Related links).
Importantly, Stoke Therapeutics developed a population pharmacokinetic model for intrathecal STK-001 administration using non-human primate data. STK-001 levels in plasma and cerebrospinal fluid of patients treated with STK-001 correlated well with model predictions62. This represents encouraging results for the toxic exon approach, which can be applicable to thousands of disease-relevant genes35.
Therapeutic mRNA delivery
Introducing exogenous mRNA into diseased cells has several advantages compared to injections of purified protein, including correct post-translational modifications and subcellular localization of the generated protein product, lower immunogenicity, a simpler manufacturing process, and lower cost27.
The use of mRNA reduces the chances of insertion mutagenesis, which is a potential risk associated with viral gene therapy. A short half-life makes therapeutic mRNAs well suited for applications that require transient effects such as RNA vaccines. At the same time, for the treatment of genetic diseases, duration of the effect could be extended if the mRNAs are chemically modified or expressed by a plasmid or viral vector (Box 2).
Despite significant advances in chemistry, the use of mRNA as a therapeutic approach has some technical challenges. It is more difficult to synthesize and deliver full-length mRNA than shorter NBTs due to poor cell permeability and instability of exogenous mRNAs63. In vivo delivery of mRNA requires a carrier (Box 3). Furthermore, immunostimulatory properties of mRNAs could be a major safety consideration in gene upregulation applications. Multiple strategies to overcome the immunostimulatory activity are now being developed, including modifications to the RNA backbone, optimization of the manufacturing and purification processes, and supplementation with immune inhibitors64.
The therapeutic mRNA manufacturing and purification processes are also more complex than those of short oligonucleotides that can be manufactured using fully automated chemical synthesis. The currently used manufacturing processes start with in vitro transcription of a linearized plasmid or PCR template containing the gene of interest and a strong promoter region. Transcription is usually conducted by bacteriophage T7, SP6 or T3 RNA polymerases. Recently, significant improvements have been made to this step, including optimization of the cDNA template for transcription, improved mRNA product stability and advanced purification techniques to eliminate dsRNA fragments and other immunogenic by-products. These improvements are crucial for the development of the field as specifics of the manufacturing process significantly contribute to mRNA therapy efficiency (Moderna SEC filing (see Related links)).
The recent innovations in therapeutic mRNA technology (Box 2) resulted in highly positive results for mRNA vaccines in the clinic (for example, COVID-19 vaccines) and in advancement of several protein-replacement therapeutic mRNAs to clinical trials63,65 (Table 1). Progress in the understanding of mRNA biology obtained in the therapeutic mRNA field can be applied to the development of other NBTs.
One of the representative examples of clinical application of therapeutic mRNA technology for protein replacement is the CFTR mRNA therapy MRT5005 for cystic fibrosis developed by Translate Bio/Sanofi, which was tested in a phase II clinical trial (NCT03375047). MRT5005 consists of in vitro-transcribed, unmodified CFTR mRNA encased in lipid nanoparticles and aerosolized to permit inhalation delivery to the lung. The nanoparticles are composed of hyperbranched poly(β-amino esters) (hPBAEs) and enable wide expression of cargo mRNA in lung tissue after inhalation66,67. Importantly, the exogenous mRNA expression is largely localized to the lung, limiting possible off-target effects. The inhalation delivery is non-invasive, and is compatible with frequent dosing and addition of other cargo molecules. The interim results from a phase I/II clinical trial of MRT5005 in patients with cystic fibrosis have shown good tolerability (see Related links). While exploratory efficacy in ppFEV1 (percent predicted forced expiratory volume in 1 s) from the interim analysis of the single-ascending dose portion of the study looked promising, the next set of results, from the multiple-dose portion, did not demonstrate a pattern of increases in ppFEV1. These results underline the necessity of further studies on NBT pharmacokinetics and improvements in drug product composition and clinical study design.
Other examples of therapeutic protein-replacement mRNA in clinical trials further demonstrate rapid progress that is being made in this particular modality. Among them are several early-stage clinical trials of mRNA therapies for rare metabolic diseases conducted by Moderna.
mRNA-3927 developed by Moderna delivers dual mRNAs for propionyl-CoA carboxylase subunits α and β in patients with propionic acidaemia (NCT04159103)68. Repeated intravenous doses were well tolerated in 10 patients, and preliminary data showed a decrease in the number of clinical crises that occur in the natural course of the disease due to build-up of toxic metabolites.
mRNA-3705, delivering mRNA for a mitochondrial enzyme methylmalonyl-coenzyme A mutase, is being tested in patients with methylmalonic acidaemia. The enzyme sequence has been engineered to improve protein translation. In vitro, the therapeutic mRNA showed physiologically correct mitochondrial localization69. mRNA-3705 has replaced mRNA-3704 in clinical trials (NCT04899310) due to its greater potency and extended lowering of methylmalonic acid in mutase-deficient mice compared to mRNA-3704 (Moderna SEC filing (see Related links)). Initial results from the mRNA-3705 study are expected in 2023.
mRNA-3745, also in early clinical trials conducted by Moderna, delivers mRNA for glucose 6-phosphatase in patients with glycogen storage disease type 1a (NCT05095727).
In an alternative approach, mRNA therapeutics are designed to locally or systemically enhance the expression of regulatory proteins that are not mutated in a given disease but needed to induce a desired therapeutic outcome. One example is the investigational cancer therapy mRNA-2752 developed by Moderna, which consists of lipid nanoparticles containing three mRNAs (OX40 ligand, IL-23, IL-36γ). Together, these mRNAs can boost expansion of CD4 and CD8 T cells as well as enhance priming and maturation of dendritic cells. mRNA-2752 is injected directly into tumours to induce a tumour-targeted immune response. A phase I clinical study (NCT03739931) in patients with accessible solid tumours and lymphomas has reported positive preliminary results (see related links). As of July 2022, 88 patients were treated with mRNA-2752, 69 in combination with durvalumab, an immune-checkpoint inhibitor. Analyses of plasma and tumour tissues show that mRNA-2752 treatment was associated with elevated pro-inflammatory cytokines, including IL-23, IL-36γ, IFNγ and TNF, and an increase in proliferating CD8+ T cells, dendritic cell recruitment and T cell activation compared to baseline. Increases in immune response positively correlated with clinical benefit70.
A similar multiplex approach is being advanced by BioNTech/Sanofi in their BNT131 (SAR441000) therapy, which contains a combination of IL-12sc, IL-15sushi, IFNα and GM–CSF nucleoside-modified mRNAs engineered for minimal immunogenicity. BNT131, administered by weekly intratumoural injections in combination with the immune-checkpoint inhibitor cemiplimab, is currently in phase I clinical trials for advanced melanoma (NCT03871348)65,71.
At the same time, as a testament to challenges in NBT clinical development, work on AZD8601, a naked VEGF-A mRNA developed by Moderna/AstraZeneca that was in a phase II trial for enhancing post-surgical angiogenesis after bypass surgery, was discontinued despite showing good safety and positive trends in efficacy end points72.
Overall, direct delivery of protein-encoding mRNA has gained a great deal of attention owing to its utility in generating the COVID-19 vaccines65. Additionally, as demonstrated by the current examples, it also has therapeutic potential for supplementing the insufficient expression of endogenous genes, which will likely significantly expand its applications in the future.
Box 3 Optimization of LNPs for NBT delivery.
In recent years, significant progress has been achieved in designing lipid nanoparticles (LNPs) for mRNA or small interfering RNA (siRNA) delivery63. As opposed to hollow liposomes, LNPs are particles densely packed with lipids and nucleic acids. LNPs are usually assembled by dissolving their lipid components (ionizable amino lipids, phospholipids, cholesterol and PEG-lipids) in ethanol, then mixing this solution with mRNA in an aqueous buffer. The amino lipid and phospholipid components are thought to facilitate both cellular uptake and endosomal escape of the mRNA. The development of ionizable lipids was a major breakthrough in LNP design. Ionizable lipids maintain neutral charge at neutral pH present in the blood, and thus have low toxicity, but become ionized upon uptake into the acidic endosomes. The appearance of a positive charge triggers a change in the shape of the nanoparticle, which ultimately leads to mRNA release into the cytoplasm. Cholesterol and the PEG-lipid components contribute to the stability of LNPs in vitro and in vivo63.
When delivered intravenously, LNPs are opsonized by apolipoprotein E, which leads to their uptake predominantly into hepatocytes via the low-density lipoprotein receptor. Specific ligands can be added to the particles if liver targeting is not desired165. An additional consideration in designing LNPs, especially for treatments that require repeat administration, is the speed of degradation of the lipid components in vivo, which can depend on lipid chemistry, pharmacogenomic factors and disease pathophysiology.
Fully assembled LNPs have to be purified from contaminants such as unincorporated molecular components (especially mRNA fragments that could activate the immune system), degradation products and particles outside of the desired size range. Furthermore, multiple chemical reactions, such as oxidation, hydrolysis or transesterification, may occur among LNP components during storage and handling, leading to mRNA degradation, formation of lipid–mRNA adducts by covalent binding of reactive lipid species and nucleobases, or generation of toxic by-products. These reactions are particularly important in repeat dosing paradigms, needed in most mRNA-treatable diseases166. Further progress in developing analytical techniques in the context of this process is essential for the advancement of therapeutic mRNAs and siRNAs. Building on extensive work in nanoparticle design for siRNA delivery carried out by Alnylam, Protiva, Inex and others in the early 2000s, Moderna conducted extensive screening to optimize LNP components and their ratios for mRNA delivery and evaluate their efficacy as well as repeat dosing toxicity in rats and non-human primates167. Other efforts in this area have been recently reviewed63.
Small molecules facilitating ribosomal readthrough
While NBTs interact with RNA based on their sequence complementarity, RNA-targeting small molecules (rSMs) target the 3D structure of RNAs. RNA molecules form distinct 3D folds similar to pockets of 3D protein structures that can be specifically bound by small molecules to modify biological function. Advantages of using rSMs instead of NBTs to modulate RNA targets include oral availability and, in some cases, blood–brain barrier permeability. However, potential disadvantages include low target specificity and high development costs73.
The first clinical success in the rSM field, ataluren (developed by PTC Therapeutics), was designed to facilitate ribosome readthrough of missense mutations by increasing recruitment of near-cognate tRNAs. The drug received marketing authorization in Europe for the treatment of DMD in 2014. However, it later failed to gain FDA approval and did not show sufficient efficacy in clinical trials in Dravet and CDKL5-deficiency syndromes74.
In 2020, the FDA approved a splicing modifier rSM, risdiplam, for the treatment of SMA. Developed by Roche, risdiplam exerts its unique mechanism of action by interacting with exon 7 of SMN2 pre-mRNA and increasing the binding affinity of spliceosome components. This results in proper inclusion of exon 7 in the final mRNA transcript and subsequent increases in the yield of functional SMN protein. However, risdiplam has significant off-target effects on splicing of non-relevant RNAs, which may increase its toxicity75.
Success of risdiplam has further fuelled interest in the rational design of rSMs. PTC518, a brain-penetrant splice-modifying rSM, is in a phase II clinical trial for Huntington disease (NCT05358717)76. PTC518 degrades huntingtin mRNA by promoting the inclusion of a poison exon (see Related links). Notably, another rSM called TEC-1 has also displayed promising preclinical results for SMA by increasing functional SMN protein. TEC-1 has the same splice-modifying mechanism of action as risdiplam. Yet, unlike risdiplam, TEC-1 did not affect splicing of another well-known target of risdiplam, FOXM1, and did not induce micronucleus formation77.
DT-216 is an rSM in phase I/II clinical trials for Friedreich ataxia (NCT05285540 and NCT05573698). It was developed using a platform known as GeneTAC™ (gene-targeted chimera small molecules). GeneTAC™ molecules comprise a DNA-binding moiety connected via a linker to ligand moieties that engage transcription elongation complexes. A rationally designed DNA-binding moiety binds specifically at the site of the disease-causing nucleotide repeat expansion that normally stalls transcription machinery. The ligand moiety recruits elongation complexes that facilitate transcription through repeat expansion, thus increasing production of the deficient protein. Preliminary results in 39 patients with Friedreich ataxia indicated that treatment with DT-216 was well tolerated. Single doses of DT-216, ranging from 100 to 600 mg, resulted in a 1.2-fold to 2.6-fold increase in frataxin (FXN) mRNA at 24 h post-dose (see Related links).
Other developments in the rSM space are building on the work on riboswitches conducted in the early 2000s78,79. Riboswitches are naturally occurring structures in RNA molecules that change conformation upon binding of small-molecule metabolites such as vitamin derivatives, metal ions and others. They function as endogenous environmental sensors regulating protein expression. Synthetic riboswitches have been applied in the development of biodetectors, molecular diagnostics and computational hardware with a biological component (wetware)80. Endogenous structures similar to riboswitches that can potentially bind rSMs — changing the conformation of the RNA transcript that harbours them and thus affecting its intracellular functions — are present in many RNA transcripts79. Given the unique nature of such interactions, rSMs are typically found via extensive screening. Recent advances in computational modelling of RNA have facilitated in silico identification of rSMs that can directly bind RNA molecules to modify the function of a given RNA structural moiety, inactivate the RNA transcript by generating covalent bonds or even trigger degradation of the RNA transcript81.
Disney’s group used a mechanism similar to proteolysis-targeting chimaeras (PROTACs) or small molecules that tag proteins for proteasomal degradation to design rSMs linked to an RNase-recruiting moiety that can target the RNA for degradation. The RNA-degrading rSMs, termed RIBOTACs, that target inhibitory or repressive RNA molecules, such as miRNAs, are capable of upregulating disease-associated proteins. Expansion Therapeutics, which was founded in 2016 following the work by Costales et al., has been focusing on RIBOTACs that degrade a causative RNA transcript for myotonic dystrophy type 1 (ref. 82). By binding to the hairpin structure of the toxic CUG repeat expansion, which causes myotonic dystrophy type 1, ERX-963 allows translocation of mutant CUG repeat-containing mRNA from the nucleus to the cytoplasm, ultimately stimulating translation of the mutant mRNA83. However, their lead compound, ERX-963, failed to provide any significant therapeutic effect in a clinical trial84.
Disney et al. have further advanced RNA computational modelling by designing an online computational platform, INFORNA, for in silico identification of lead small molecules for RNA targets85. They have utilized INFORNA and more traditional binding assays to identify an approved drug, the receptor tyrosine kinase inhibitor dovitinib, as a selective binder of oncogenic pre-miR-21. By modifying dovitinib into a RIBOTAC, Zhang et al. were able to inhibit metastasis of breast cancer cells to the lungs in a mouse model after 30 days of treatment with the RIBOTAC, with no reports of toxicity86.
As miR-21 represses translation of apoptosis-related genes, including PTEN, TPM1 and PDCD4 (encoding programmed cell death 4), degrading pre-miR-21 with dovitinib-PROTAC derepresses translation of these apoptosis-related genes.
Another rSM designed by Haniff et al. selectively targets miR-200c and represses pancreatic β-cell apoptosis by derepressing translation of miR-200c targets Rps6kb1 and Dnajc3. Surprisingly, the rSM did not inhibit other miR-200 family members while an oligonucleotide targeting the RNA sequence did87.
Arrakis Therapeutics is at the early discovery stage investigating rSMs that modulate intrinsic function of RNA targets, rSMs that form deactivating covalent bonds with RNA targets, and RIBOTACs88 (see Related links). Gene upregulation can be achieved with this approach by deactivating or degrading repressive RNA transcripts.
These developments demonstrate that, although even the most promising rSMs have limitations, there is potential for further development of rSM therapeutics. Notably, identifying molecules that do not have off-target effects resulting in toxicity remains the most pressing challenge for rSMs. Aside from risdiplam, most rSMs are utilized as chemical probes, more useful for experimental, rather than therapeutic, purposes. However, proponents of the approach think that there are specific RNA structures that can be targeted without off-target interactions89.
NBTs in preclinical and early-stage clinical development
miRNA-targeted NBTs
In recent years, miRNAs have emerged as attractive therapeutic targets90. As their activity results in a reduction in abundance of specific mRNAs, blocking this activity leads to an increase in protein production.
miRNAs are initially transcribed as long primary miRNA transcripts containing double-stranded hairpin structures and are then processed to generate RNA duplexes. The duplexes associate with AGO proteins of the RNA-induced silencing complex that remove the ‘passenger’ RNA strand and retain the ‘guide’ strand. The ‘seed’ region of the guide strand (positions 2–8 at the 5′ end) can then anneal to specific response elements in target mRNAs, leading to their degradation directly by AGOs or by additional proteins recruited by AGOs90.
Synthetic oligonucleotides have been used to interfere with this pathway as both miRNA mimics (promirs) and miRNA blockers (antagomirs or antimirs or miRNA sponges; Fig. 3). Depending on the mechanism of action of a given miRNA, both of these intervention types may lead to target protein upregulation.
Promirs are synthetic oligonucleotides that have the same sequence as a naturally occurring miRNA and are used to amplify their effects. In cases when the target mRNA codes for an inhibitory protein, its destruction should lead to de-repression of a disease-relevant protein. Although a similar effect can be achieved by oligonucleotides complementary to any part of the target mRNA sequence because miRNAs usually have multiple targets and can be modulated by endogenous regulatory mechanisms, miRNA mimics could achieve context-dependent pleiotropic effects without the need to apply multiple oligonucleotides91. However, clinical development of such molecules has encountered multiple challenges involving low efficacy and adverse events in clinical trials92.
Antagomirs are single-stranded oligonucleotides that can abolish miRNA activity by competing with target mRNA for binding to the guide strand–AGO complex. miRNA blockers are usually designed with mispairing or modified bases at the AGO2 cleavage site, which inhibits mRNA degradation.
Although development of miRNA blockers has been under way for a long time, the field has been plagued by multiple failures. Regulus discontinued clinical trials of RGLS4326, designed to inhibit miR-17, which suppresses expression of polycystins 1 and 2, for autosomal dominant polycystic kidney disease and replaced it with the next-generation candidate RGLS8429, shown to have a more favourable risk–benefit profile. Off-target CNS events were observed at the top doses of RGLS4326 in chronic preclinical toxicology studies, while RGLS8429 did not show such effects. Notably, miR-17 is known to repress multiple targets besides polycystins and thus has a high possibility of off-target toxicity (see Related links). Topline results from the RGLS8429 study showed good tolerability and an expected pharmacokinetic profile, providing support for initiation of a phase Ib trial (NCT02855268).
At the same time, lademirsen (RG-012/SAR339375), an anti-miR-21 antagomir, was tested in a phase II clinical trial in Alport syndrome, a rare kidney disease (NCT05521191) (Table 1). miR-21 is upregulated in Alport syndrome and blocks the expression of multiple apoptosis-related genes, including PTEN, TPM1, PDCD4 and genes involved in renal tubulointerstitial injury pathways. Although the drug was well tolerated, the results of the interim futility analysis led to study termination.
miRagen/Viridian also halted clinical development of MRG-110, a locked nucleic acid (LNA) ASO against miR-92a-3p for the treatment of heart failure (NCT03601052). Inhibition of miR-92a was shown to improve endothelial cell function and recovery after acute myocardial infarction via endothelial cell-specific regulation of autophagy-related genes93.
miR-155 is a master regulator of inflammation that is overexpressed in multiple cancers and in the spinal cord of patients with amyotrophic lateral sclerosis. In vitro blockade of miR-155 using LNA antagomirs (MRG-107 and cobomarsen/MRG-106) de-repressed numerous direct and downstream miR-155 targets in JAK–STAT, MAPK–ERK and PI3K–AKT pathways, leading to reduced survival signalling and apoptosis94. However, development of cobomarsen in cancer was discontinued due to inconclusive efficacy.
Despite the recent setbacks in the miRNA-targeting NBT space, their preclinical development continues, mostly aimed at gene silencing95–97.
Small activating RNAs
Small dsRNAs are widely recognized for their ability to induce degradation of homologous mRNA transcripts. Surprisingly, it was discovered that synthetic dsRNAs containing a guide strand complementary to promoter or enhancer regions of a target gene — coined small activating RNAs (saRNAs) — can induce gene expression98 (Fig. 3).
The mechanisms of action of saRNAs are not completely understood but could be related to the mechanism of promoter activation by endogenous miRNA, referred to as RNA activation99,100. RNA activation could be mediated by miRNA binding to pRNAs (discussed below) and association with the AGO2–TNRC6 complex but does not require pRNA degradation. Using the induction of ZMYND10 expression by miR-34a as a test case, it was shown that this complex interacts with positive transcription elongation factors CDK9 and DDX21, thus bringing them in the vicinity of the target promoter, which leads to the release of paused promoter–proximal RNA Pol II and upregulation of ZMYND10 expression101.
Furthermore, saRNA binding to pRNA or lncRNA in sense and/or antisense orientation was shown to be associated with epigenetic changes within the promoter region, including histone demethylation and acetylation102–104. These changes can explain the prolonged upregulation of gene expression induced by saRNAs, which can last several weeks101,105.
Alternatively, saRNAs were proposed to bind AGO2 and hnRNPs in the cytoplasm. This complex can then be imported into the nucleus, where it binds directly to promoter DNA and participates in the RNA-induced transcriptional activation (RITA) complex. RITA interacts with RNA Pol II to initiate transcription. Other components of RITA are RNA helicase A (RHA), RNA polymerase-associated protein CTR9 homologue (CTR9) and DEAD-box helicase 5 (DDX5)99,100.
The saRNA MTL-CEBPA developed by MiNA Therapeutics is being tested as a supplemental therapy to enhance the efficacy of standard-of-care cancer drugs106. The mechanism of action of MTL-CEBPA likely involves inactivation of immune-suppressive myeloid cells by upregulation of its target transcription factor CEBPA107. MTL-CEBPA is composed of amphoteric iminolipid nanoparticles, called SMARTICLES, and a 21-mer 2′-O-methylated RNA oligonucleotide duplex. Interim results showed regression of tumours in 26.7% of patients with hepatocellular carcinoma103. A phase I trial of MTL-CEBPA combined with pembrolizumab, an anti-PD1 immune-checkpoint inhibitor, is ongoing in 50 patients resistant to or ineligible for standard therapies (NCT04105335). The treatment ameliorated immune suppression and improved infiltration of cytotoxic T cells in tumours compared to baseline. Objective tumour responses were observed in four patients who were treated108.
If successful, saRNA treatments could be applicable to a wide range of disorders.
NAT-targeted NBTs
NATs are known to inhibit expression in 50–80% of all gene loci, which gives this NAT-targeted approach high potential in therapeutic protein upregulation2. Several strategies targeting NAT-mediated regulation have been proposed.
Single-stranded NAT-specific oligonucleotides, termed AntagoNATs, targeted to a NAT in the BDNF locus (BDNF-AS) induced gene-specific upregulation of the neurotrophic factor BDNF both in vitro and in vivo14. Furthermore, intranasal delivery via the minimally invasive nasal depot (MIND) technique induced upregulation of BDNF protein in multiple regions, including deep structures, in rat brain109. The MIND procedure involves the use of routine outpatient techniques, representing a significantly safer and more patient-friendly method for oligonucleotide delivery to the brain compared with intracerebroventricular and intrathecal delivery strategies.
The AntagoNAT CMP-SCN targets a NAT in the SCN1A gene locus110. SCN1A codes for the α-subunit of the voltage-gated sodium channel NaV1.1. Heterozygous mutations in SCN1A lead to Dravet syndrome, a rare form of childhood epilepsy. Treatment with CMP-SCN and its mouse analogue induced specific upregulation of SCN1A both in vitro and in vivo and significantly reduced the occurrence of seizures in a mouse model of Dravet syndrome110. Preclinical toxicity studies in non-human primates demonstrated a good safety profile and significant increases in SCN1A mRNA and protein in various brain regions after treatment with CMP-SCN111.
Putative NBTs of the future
pRNAs, eRNAs and seRNAs
Although currently mostly untapped, pRNA, eRNAs and seRNAs present multiple opportunities for therapeutic intervention with saRNA-type molecules17. Interestingly, promoters and enhancers produce a similar repertoire of bidirectionally transcribed short and long non-coding transcripts that can regulate transcription, which could indicate their common evolutionary origins, with enhancers initiating the transcription of the majority of intergenic lncRNAs16. Recent studies have demonstrated the existence of pervasive bidirectional transcription of short ncRNAs from DNase-hypersensitive regions that do not have canonical enhancer or promoter histone modification profiles that could represent other, as yet unidentified, regulatory elements18,112,113. However, the current knowledge of the mechanisms of action and biological effects of transcripts generated by promoters, enhancers, super-enhancers and other regulatory elements is still evolving.
Notably, expression patterns of these RNAs are known to be tissue specific, which makes them attractive as potential therapeutic targets114. Expression of eRNAs is also stimulus specific; for instance, particular subsets of eRNAs are preferentially expressed at inflammation-related genes in response to inflammatory stimuli, in neurons during the stimulus-induced expression of immediate early genes and after exposure to early-life undernutrition115–117. An experience-induced enhancer lncRNA, ADRAM (activity-dependent lncRNA associated with memory), is generated by an enhancer of Nr4a2 (nuclear receptor subfamily 4, group A, member 2). ADRAM localized to the Nr4a2 promoter, increased deposition of H3K4me3 marks and coordinated binding of 14-3-3 protein. This facilitated the dissociation of HDAC3 and HDAC4, followed by CBP-mediated activation of Nr4a2 expression. ADRAM was required for the formation of fear extinction memory118. NBTs that act as ADRAM mimics to boost Nr4a2 expression may have therapeutic significance in anxiety disorders.
A possible way to modulate eRNA activity is based on the fact that eRNA expression can be regulated by lncRNAs. For example, the lncRNA KHPS1 (sphingosine kinase 1a antisense transcript) forms a triple-helical RNA–DNA–DNA structure at the sphingosine kinase 1a (SPHK1) enhancer119. The triplex facilitates recruitment of activation factors E2F1 and p300 and expression of eRNA-Sphk1. eRNA-Sphk1 displaces CTCF (CCCTC-binding factor), a zinc finger protein that insulates the SPHK1 enhancer from the SPHK1 promoter and thus upregulates SPHK1 transcription. Interestingly, triplex-forming regions of NATs are sequence specific and can be repurposed to target genes of interest. For example, a KHPS1 transcript engineered to contain the triplex-forming region of lncRNA MEG3 binds to a region in the MEG3 target gene TGFBR1 (ref. 119).
Super-enhancers are known to generate seRNA. seRNA functions include promoting enhancer–promoter looping, recruiting transcription factors and facilitating epigenetic histone modifications. For example, muscle injury triggers expression of Pax7-associated muscle lncRNA (PAM) from a myoblast-specific super-enhancer in muscle satellite cells. PAM interacts with DEAD‐box helicase 5 (Ddx5) to align the super-enhancer to its target genes Timp2 (encoding tissue inhibitor of metalloproteinase 2) and VIM (encoding vimentin). This activates the expression of the extracellular matrix proteins required for satellite cell differentiation into myotubes120. Upregulation of PAM expression may be beneficial in muscle diseases and in promoting muscle regeneration after injury.
Furthermore, hnRNPL has been shown to bind diverse eRNAs and seRNAs in several cell types. In myogenic super-enhancer-generated seRNA1, this binding occurs via a CAAA tract. Disruption of seRNA1–hnRNPL interaction decreases RNA Pol II binding and H3K36me3 deposition and transcription at the myoglobin locus, which impairs muscle formation21. seRNA1 mimics could therefore promote muscle regeneration.
Importantly, activation of seRNAs is cell and tissue specific and can serve as a target for fine modulation of cell fate, essential, for example, in the treatment of cancers, ischaemia and neurodegenerative diseases22,121,122.
Several methods for modulating eRNA and seRNA expression, including ASOs, dsRNAs and repurposed RNA-guided RNA-targeting CRISPR–Cas13 machinery, have been proposed.
Targeting imprinting
In some cases, targeting lncRNAs that are known to participate in silencing of imprinted transcripts can be utilized in disease treatment. In Angelman syndrome, mutations affecting the maternally inherited ubiquitin-protein ligase E3A (UBE3A) can be rescued by de-repression of the paternal copy silenced by Ube3a NAT (Ube3a-ATS). NBTs targeting Ube3a-ATS activated the imprinted copy, increased UBE3A levels in the brain to up to 74% of wild type and normalized phenotypes in a mouse disease model123. Although still in discovery stage, this approach can be applicable in many cases of mutations in imprinted genes and epimutations.
Targeting uORFs
NBTs targeting several translation regulatory elements in 5′ and 3′ UTRs have been proposed as a protein upregulation strategy (Fig. 4). Although none of these approaches is in clinical testing, fine modulation of translation may be beneficial in the treatment of many diseases. Early preclinical studies have shown that ASOs targeting uORFs increase protein expression, likely by base-pairing with the uORF upstream AUG regions and redirecting translation to the main start site36 (Fig. 4).
Targeting regulatory elements in UTRs
Stem-loop and other secondary structures in 5′ UTRs can inhibit translation of the gene. Treatment with ASOs designed to hybridize to such structures was shown to result in up to 2.7-fold increases in the levels of RNASEH1, LDLR and ACP1 proteins in different cell types and species, in vitro and in vivo, with minimal off-target effects as determined by microarray analyses37.
Targeting polyadenylation
Selection of alternative polyadenylation sites during splicing can determine the biological activity and subcellular distribution of proteins produced by mRNA transcripts. Shifting the balance between isoforms with different physiological functions without increasing the overall mRNA levels may have the additional benefit of reducing the possibility of target isoform overexpression.
Such a situation can occur in IgE-mediated allergies that are caused by an increased proportion of secreted versus membrane-bound isoforms of IgE, which differ in their polyadenylation sites40. Morpholino ASOs targeting the polyadenylation signal of IgE that are coupled to Pip6a, an arginine-rich cell-penetrating peptide, alter IgE transcript polyadenylation to produce more membrane-bound isoform and less secreted isoform. This leads to decreased IgE secretion and increased apoptosis of IgE+ antibody-secreting cells in transgenic mice expressing humanized IgE (Fig. 4). The change in IgE isoform composition may be beneficial for the reduction of IgE-related allergic symptoms and could promote allergen tolerance through apoptosis of IgE+ antibody-secreting cells40.
Another example is NEAT1_2, one of the isoforms of lncRNA NEAT1. NEAT1_2 is known to scaffold paraspeckles, essential membrane-less nuclear structures thought to sequester RNAs and proteins. Another isoform, NEAT1_1, is not involved in this process but promotes the proliferation of cancer cells. Treatment with ASOs that block a NEAT1 polyadenylation site results in a shift in the ratio of NEAT1_2 to NEAT1_1 towards the NEAT1_2 isoform, increased paraspeckle formation, induction of differentiation and suppression of tumorigenesis in vitro41.
Combinatorial treatments
Using combinations of NBTs targeting different biological pathways with synergistic effects is an approach that is receiving considerable attention. In a clinically relevant example, it has been shown that treatment with a combination of ASOs that bind to FXN 5′ or 3′ UTR can enhance the upregulation of FXN protein in vitro compared to individual ASO treatment. The mechanism is possibly related to the increased stability of FXN mRNA without changes in transcription of the FXN gene38.
An interesting case of NBT–small molecule synergy occurs after combined treatment with a 5′ UTR-targeting ASO and a small-molecule drug Symdeko (ivacaftor plus tezacaftor). Tezacaftor facilitates the folding and membrane localization of CFTR, and ivacaftor shifts the chloride channel encoded by CFTR towards the open state, while treatment with the ASO increases CFTR protein production. The combination treatment increases CFTR function in primary human bronchial epithelial cells with multiple cystic fibrosis genotypes compared to treatment with Symdeko alone39.
Advantages and challenges for NBTs
Some of the advantages of NBTs make them well suited for the upregulation of insufficient protein expression that underlies numerous known diseases. NBTs are characterized by high target specificity and greatly extended half-lives (such as weeks or months) that allow for infrequent dosing. This stability is due to the recently developed chemical modifications of NBTs (Box 2) and/or NBT sequestration in synthetic delivery particles (Box 3) and intracellular vesicular compartments. Multiple clinical trials and clinical experience with approved NBTs have demonstrated their high tolerability. Reduced development timelines and costs of NBTs permit individualized treatments for fast-developing cancers and rare genetic diseases4.
However, as for any emergent drug class in the early stages of development, NBTs present several challenges compared to traditional therapeutic modalities. A common drawback of current NBTs is their inability to cross the intestinal lining or blood–brain barrier. Currently, this problem is addressed by the use of intravenous, subcutaneous, intracerebral, intracerebroventricular or intrathecal administration. Unfortunately, these methods are invasive, especially for delivery to the CNS, and have some probability of causing adverse effects on their own. Furthermore, such administration routes require hospital visits for drug administration and additional testing to monitor for potential adverse events. Therefore, extensive studies are being conducted to develop chemical modifications, carriers, vectors and administration techniques that will permit less invasive delivery routes. Novel clinical approaches and formulations, such as inhalation, oral or MIND administration, are helping to bring these technological achievements to the clinic109,124.
Another common feature of NBTs, which has both positive and negative implications, is their post-uptake sequestration in late endosomes. A small proportion of the sequestered NBT pool is gradually released (so-called ‘productive uptake pathway’) and can participate in regulation of target RNA functions. The resulting sustained release of the NBTs has some clinical benefit in permitting infrequent administration. Importantly, channelling more of the administered dose towards productive uptake pathways could reduce the risk of detrimental overloading of the kidney and liver, the organs that preferentially accumulate NBTs. Some early-stage work in this area is targeted at optimizing oligonucleotide sequences and chemistry and at developing delivery agents and compounds that would facilitate the release of NBTs from endosomes125,126. Another area in the early stages of investigation is modifying organ and tissue distribution of NBTs to avoid a potentially toxic high accumulation in non-target cells. These studies involve chemical modifications, synthetic carriers (Box 3), exosomes, apoptotic bodies, vectorized delivery of NBTs and other methods127.
Furthermore, compared to most small molecules, NBTs have complex and currently poorly understood pharmacokinetics and pharmacodynamics resulting from their frequently epigenetic mechanisms of action, extended half-lives and several rate-limiting steps in their uptake mechanism4,125. The situation is further complicated by the paucity of existing clinical pharmacokinetic data and incomplete understanding of its relationship to pharmacokinetic or pharmacodynamic data obtained in animal studies. Although several attempts to build pharmacokinetic models for NBTs have been made utilizing reporters or HPLC and hybridization assays to directly determine tissue concentrations of NBTs, this area requires significant further investigation, especially in viral vector-mediated applications128–131.
As demonstrated by the clinical examples described above, the current stage of NBT development also necessitates extensive improvements and optimization in clinical trial design and approval processes, especially in orphan indications where statistical power is limited by the prevalence of the target disease. Furthermore, methods and infrastructure for manufacturing and quality control of NBTs need to be further developed and optimized to help realize the potential of this novel therapeutic modality.
Despite these limitations, the overall success rate of NBT development programmes was reported to be on par with or higher than the pharmaceutical industry average. Analysis of 7,455 drug development programmes conducted between 2006 and 2015 has shown that only approximately 6% of new molecular entity drugs (excluding reformulations and generics) that enter clinical trials were approved. In this analysis, novel biologics had a success rate of 11.5%. Similar trends have been reported in other studies132,133. The observed higher approval rates of NBT drugs may reflect their ability to satisfy a significant unmet clinical need and their largely rational design process.
At the same time, the high approval rate of NBTs is offset by the economic realities of small companies and rare diseases that have led to discontinuation of drug production in several cases. A positive trend in this area is an increased interest in NBTs among pharmaceutical companies after the success of COVID-19 vaccines.
Another encouraging trend is that some of the next-generation NBT chemistries and delivery methods show improved safety and efficacy in clinical studies compared to earlier versions of the same drug as described above. Overall, the limitations of NBTs, when counterbalanced by safety, unique mechanisms of action and cost-effective development process, may still not preclude their clinical use in diseases with a high unmet need.
Conclusions and outlook
Recent discoveries of RNA-based mechanisms have opened vast new opportunities for protein upregulation, which historically was largely inaccessible via protein-targeted small-molecule drugs. Advances in NBT chemistry and biology have provided a tool for specific modulation of RNA-based mechanisms. The accelerated development cycles of NBTs expand their applicability towards the treatment of fast-developing cancers with unique mutations, rare and ultra-rare diseases caused by protein paucity, and personalized medicine approaches.
However, protein-upregulating NBTs face the same challenges as other pharmaceutical modalities, including the need to better understand genetic determinants and pathophysiology of disease, improvements in the rational design of drug molecules, advanced studies of their pharmacokinetic and pharmacodynamic characteristics, more convenient routes of administration, and the requirement for specific regulatory procedures.
Despite the growing pains of the protein upregulation field described above, the experience of the companies that are working to improve NBT technology has demonstrated that such advances lead directly to better treatment outcomes. The success of COVID-19 mRNA vaccines has pushed RNA-targeted protein upregulation technology into the spotlight and will likely accelerate vital breakthroughs in the field. Improvements in gene sequencing techniques, and possibly the new hope inspired by the feasibility of NBTs in genetic disorders, will lead to the expansion of genetic studies. Significant progress in human genomics is continuously increasing the number of diseases with known genetic aetiology. Subsequently, an increasing number of rare disorders and subpopulations of ‘common’ diseases will be attributed to specific genetic defects. As many of the disease-associated mutations lead to insufficiency of target proteins, the applicability of protein-upregulating NBTs will increase.
Overall, developments in understanding of disease genomics and pathophysiology and improvements in NBT technology, manufacturing and regulatory infrastructure could expand the clinical application of protein-upregulating NBTs, opening paths to better treatment of ‘common’ diseases and individualized precision medicine.
Supplementary information
Acknowledgements
This work was supported in part by NIH grants AA29924 and AG079373 and the State of Florida grant 23A17. The authors thank Michael Brown for helpful advice, comments and ideas and for critically reading the manuscript.
Glossary
- Benner base Z
A synthetic nucleotide analogue that can form a non-hydrogen-bonded base pair with another non-natural base F; the shapes of the Z and F bases are similar to A and T, respectively.
- Enhancer–promoter looping
Repositioning of enhancers in physical proximity of promoters necessary for activation of gene expression, made possible by the formation of a chromosomal DNA loop.
- Enhancer RNAs
(eRNAs). Long and short non-coding RNAs transcribed from enhancer regions.
- Integrator complex
Multi-protein complex containing IntS11 RNA endonuclease, which cleaves nascent RNAs transcribed by RNA polymerase II; cleavage generates small nuclear RNAs and enhancer RNAs.
- Internal ribosome entry sites
(IRESs). Three-dimensional RNA structures that facilitate cap-independent transcription.
- Long non-coding RNA
(lncRNA). RNA transcripts that are >200 nucleotides and do not have long open reading frames.
- MicroRNAs
(miRNAs). Short (20–30 nucleotides) double-stranded regulatory non-coding RNAs that can be generated endogenously; can also refer to synthetic miRNA mimics.
- Natural antisense transcripts
(NATs). Long non-coding RNAs expressed from the chromosome strand opposite to the protein-coding gene.
- Nucleic acid-based therapeutics
(NBTs). Therapeutic agents derived from nucleic acids, including antisense oligonucleotides, small interfering RNAs, therapeutic mRNAs and vectorized constructs expressing any combination of the above.
- Pause-controlled genes
Genes of which the expression is regulated by RNA polymerase II pausing.
- Polyadenylation
Addition of a polyA tail to the 5′ end of an RNA.
- Promoter RNAs
(pRNAs). Long and short non-coding RNA (ncRNA) transcribed from promoter regions.
- Proteolysis-targeting chimaeras
(PROTACs). Small molecules that tag proteins for proteasomal degradation.
- Toxic exons
Naturally occurring exons that contain a premature stop codon or otherwise interfere with protein translation from the transcripts that incorporate them.
- Upstream open reading frames
(uORFs). Short open reading frames present in 5′ untranslated regions of some mRNAs.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Arthur Krieg, Ruchira Glaser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
C.W. is a co-founder of Epigenetix and Jupiter Neurosciences, Inc., and serves on scientific advisory boards of Camp4 Therapeutics, Cascade Biotechnology, Galatea Bio and Ribocure. O.K. is employed by OPKO Health.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Arrakis Therapeutics enters strategic collaboration and license agreement with Roche for multi-target program utilizing RNA-targeted small-molecule drug discovery platform: https://go.nature.com/45sV2lq
Axiomer technology: https://www.proqr.com/axiomer-technology
Design Therapeutics reports positive data from single-ascending dose trial of DT-216 for the treatment of Friedreich ataxia and portfolio progress: https://go.nature.com/3oojm7l
Moderna announces presentation of interim data from phase 1 study of mRNA triplet program at 2021 SITC annual meeting: https://go.nature.com/45sygu0
Moderna SEC filing: https://go.nature.com/3MtuoA4
Nationwide researchers announce restoration of full-length dystrophin in humans: https://go.nature.com/3MRJYa8
PTC provides update on ongoing global PIVOT-HD trial For PTC518: https://go.nature.com/43EEchR
Regulus Therapeutics announces positive topline safety and pharmacokinetic (PK) data from the phase 1 single-ascending dose (SAD) clinical trial of RGLS8429 for the treatment of autosomal dominant polycystic kidney disease (ADPKD): https://go.nature.com/3MCo0qu
Stoke Therapeutics presents data from a combined interim analysis of the phase I/IIa MONARCH and ADMIRAL studies of STK-001 in children and adolescents with Dravet syndrome at the American Epilepsy Society (AES) 2022 Annual Meeting: https://go.nature.com/45Aso1R
Translate Bio announces results from second interim data analysis from ongoing phase 1/2 clinical trial of MRT5005 in patients with cystic fibrosis (CF): https://go.nature.com/3MPmsKY
Supplementary information
The online version contains supplementary material available at 10.1038/s41573-023-00704-7.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat. Rev. Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat. Rev. Drug Discov. 2013;12:433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- 3.Katayama S, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 4.Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 2021;6:427–453. doi: 10.1038/s41573-021-00162-z. [DOI] [PubMed] [Google Scholar]
- 5.Riggs AD. Making, cloning, and the expression of human insulin genes in bacteria: the path to humulin. Endocr. Rev. 2021;42:374–380. doi: 10.1210/endrev/bnaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone P, et al. Long-term follow-up after gene therapy for Canavan disease. Sci. Transl. Med. 2012;4:165–163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Alstyne M, et al. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat. Neurosci. 2021;7:930–940. doi: 10.1038/s41593-021-00827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belbellaa B, Reutenauer L, Messaddeq N, Monassier L, Puccio H. High levels of frataxin overexpression lead to mitochondrial and cardiac toxicity in mouse models. Mol. Ther. Meth. Clin. Dev. 2020;19:120. doi: 10.1016/j.omtm.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;10:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khorkova O, Wahlestedt C. Oligonucleotide therapies for disorders of the nervous system. Nat. Biotechnol. 2017;35:249–263. doi: 10.1038/nbt.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byeon GW, et al. Functional and structural basis of extreme conservation in vertebrate 5′ untranslated regions. Nat. Genet. 2021;53:729–741. doi: 10.1038/s41588-021-00830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009;10:637e643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modarresi F, et al. Natural antisense inhibition results in transcriptional de-repression and gene upregulation. Nat. Biotechnol. 2012;30:453. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallatah B, et al. Ago1 controls myogenic differentiation by regulating eRNA-mediated CBP-guided epigenome reprogramming. Cell Rep. 2021;37:110066. doi: 10.1016/j.celrep.2021.110066. [DOI] [PubMed] [Google Scholar]
- 16.Hon CC, et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, et al. The PAF1 complex promotes 3′ processing of pervasive transcripts. Cell Rep. 2022;38:110519. doi: 10.1016/j.celrep.2022.110519. [DOI] [PubMed] [Google Scholar]
- 18.Nair SJ, et al. Transcriptional enhancers at 40: evolution of a viral DNA element to nuclear architectural structures. Trends Genet. 2022;38:1019–1047. doi: 10.1016/j.tig.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hnisz D, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, et al. Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers. Nat. Genet. 2016;48:176–182. doi: 10.1038/ng.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, et al. MyoD induced enhancer RNA interacts with hnRNPL to activate target gene transcription during myogenic differentiation. Nat. Commun. 2019;10:5787. doi: 10.1038/s41467-019-13598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao S, Huang Q, Ren H, Yang M. The mechanism and function of super enhancer RNA. Genesis. 2021;59:e23422. doi: 10.1002/dvg.23422. [DOI] [PubMed] [Google Scholar]
- 23.Seila AC, et al. Divergent transcription from active promoters. Science. 2008;322:1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F. Promoter antisense RNAs: beyond transcription by-products of active promoters. RNA Biol. 2022;19:533–540. doi: 10.1080/15476286.2022.2062177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, et al. Shape of promoter antisense RNAs regulates ligand-induced transcription activation. Nature. 2021;595:444–449. doi: 10.1038/s41586-021-03589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro E, Mallén A, Hueso M. Dynamic variations of 3′UTR length reprogram the mRNA regulatory landscape. Biomedicines. 2021;9:1560. doi: 10.3390/biomedicines9111560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SC, et al. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell. Toxicol. 2021 doi: 10.1007/s13273-021-00171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerneckis J, Cui Q, He C, Yi C, Shi Y. Decoding pseudouridine: an emerging target for therapeutic development. Trends Pharmacol. Sci. 2022;43:522–535. doi: 10.1016/j.tips.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Borchardt EK, Martinez NM, Gilbert WV. Regulation and function of RNA pseudouridylation in human cells. Annu. Rev. Genet. 2020;54:309–336. doi: 10.1146/annurev-genet-112618-043830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg E, Levanon EY. A-to-I RNA editing — immune protector and transcriptome diversifier. Nat. Rev. Genet. 2018;1:473–490. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature. 2022;608:569–577. doi: 10.1038/s41586-022-05052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kachaev ZM, Lebedeva LA, Kozlov EN, Shidlovskii YV. Interplay of mRNA capping and transcription machineries. Biosci. Rep. 2020;40:BSR20192825. doi: 10.1042/BSR20192825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renz PF, Valdivia-Francia F, Sendoel A. Some like it translated: small ORFs in the 5′UTR. Exp. Cell Res. 2020;396:112229. doi: 10.1016/j.yexcr.2020.112229. [DOI] [PubMed] [Google Scholar]
- 34.Leppek K, Das R, Barna M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018;19:158–174. doi: 10.1038/nrm.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim KH, et al. Antisense oligonucleotide modulation of non-productive alternative splicing upregulates gene expression. Nat. Commun. 2020;11:3501. doi: 10.1038/s41467-020-17093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang XH, Shen W, Crooke ST. Specific increase of protein levels by enhancing translation using antisense oligonucleotides targeting upstream open frames. Adv. Exp. Med. Biol. 2017;983:129–146. doi: 10.1007/978-981-10-4310-9_9. [DOI] [PubMed] [Google Scholar]
- 37.Liang XH, et al. Antisense oligonucleotides targeting translation inhibitory elements in 5′ UTRs can selectively increase protein levels. Nucleic Acids Res. 2017;45:9528–9546. doi: 10.1093/nar/gkx632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, et al. Targeting 3′ and 5′ untranslated regions with antisense oligonucleotides to stabilize frataxin mRNA and increase protein expression. Nucleic Acids Res. 2021;49:11560–11574. doi: 10.1093/nar/gkab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki S, et al. Steric inhibition of 5′ UTR regulatory elements results in upregulation of human CFTR. Mol. Ther. 2019;27:1749–1757. doi: 10.1016/j.ymthe.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchalot A, et al. Targeting IgE polyadenylation signal with antisense oligonucleotides decreases IgE secretion and plasma cell viability. J. Allergy Clin. Immunol. 2022;149:1795–1801. doi: 10.1016/j.jaci.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Naveed A, et al. NEAT1 polyA-modulating antisense oligonucleotides reveal opposing functions for both long non-coding RNA isoforms in neuroblastoma. Cell Mol. Life Sci. 2021;78:2213–2230. doi: 10.1007/s00018-020-03632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iftikhar M, et al. Current and emerging therapies for Duchenne muscular dystrophy and spinal muscular atrophy. Pharmacol. Ther. 2021;220:107719. doi: 10.1016/j.pharmthera.2020.107719. [DOI] [PubMed] [Google Scholar]
- 43.Finkel RS, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 2017;377:1723. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 44.De Wel B, et al. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J. Neurol. 2021;268:923–935. doi: 10.1007/s00415-020-10223-9. [DOI] [PubMed] [Google Scholar]
- 45.Finkel RS, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc. Health. 2021;5:491–500. doi: 10.1016/S2352-4642(21)00100-0. [DOI] [PubMed] [Google Scholar]
- 46.Coratti G, et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta-analysis. Orphanet J. Rare Dis. 2021;16:430. doi: 10.1186/s13023-021-02065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wataya T, et al. Real-world safety of nusinersen in Japan: results from an interim analysis of a post-marketing surveillance and safety database. Int. J. Neurosci. 2021 doi: 10.1080/00207454.2021.1995382. [DOI] [PubMed] [Google Scholar]
- 48.Stolte B, et al. Nusinersen treatment in adult patients with spinal muscular atrophy: a safety analysis of laboratory parameters. J. Neurol. 2021;268:4667–4679. doi: 10.1007/s00415-021-10569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauthier-Loiselle M, et al. Nusinersen for spinal muscular atrophy in the United States: findings from a retrospective claims database analysis. Adv. Ther. 2021;38:5809–5828. doi: 10.1007/s12325-021-01938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kray KM, McGovern VL, Chugh D, Arnold WD, Burghes AHM. Dual SMN inducing therapies can rescue survival and motor unit function in symptomatic ∆7SMA mice. Neurobiol. Dis. 2021;159:105488. doi: 10.1016/j.nbd.2021.105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novak JS, et al. Interrogation of dystrophin and dystroglycan complex protein turnover after exon skipping therapy. J. Neuromuscul. Dis. 2021 doi: 10.3233/JND-210696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitelman O, et al. A Combined prospective and retrospective comparison of long-term functional outcomes suggests delayed loss of ambulation and pulmonary decline with long-term eteplirsen treatment. J. Neuromuscul. Dis. 2021 doi: 10.3233/JND-210665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Servais L, et al. Long-Term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 2021 doi: 10.1089/nat.2021.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komaki H, et al. Viltolarsen in Japanese Duchenne muscular dystrophy patients: a phase 1/2 study. Ann. Clin. Transl. Neurol. 2020;7:2393–2408. doi: 10.1002/acn3.51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clemens PR, et al. Long-term functional efficacy and safety of viltolarsen in patients with Duchenne muscular dystrophy. J. Neuromuscul. Dis. 2022 doi: 10.3233/JND-220811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner KR, et al. Safety, tolerability, and pharmacokinetics of casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: a randomized, double-blind, placebo-controlled, dose-titration trial. Muscle Nerve. 2021;64:285–292. doi: 10.1002/mus.27347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muscular Dystrophy Association. Expression of Apparent Full-length Dystrophin in Skeletal Muscle in a First-in-human Gene Therapy Trial Using the SCAAV9.U7-ACCA Vectorhttps://www.mdaconference.org/abstract-library/expression-of-apparent-full-length-dystrophin-in-skeletal-muscle-in-a-first-in-human-gene-therapy-trial-using-the-scaav9-u7-acca-vector/ (2021).
- 58.Dulla K, et al. Antisense oligonucleotide-based treatment of retinitis pigmentosa caused by USH2A exon 13 mutations. Mol. Ther. 2021;29:2441–2455. doi: 10.1016/j.ymthe.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell SR, et al. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: a phase 1b/2 trial. Nat. Med. 2022;28:1014–1021. doi: 10.1038/s41591-022-01755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Z, et al. Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci. Transl. Med. 2020;12:558. doi: 10.1126/scitranslmed.aaz6100. [DOI] [PubMed] [Google Scholar]
- 61.Wengert ER, et al. Targeted augmentation of nuclear gene output (TANGO) of Scn1a rescues parvalbumin interneuron excitability and reduces seizures in a mouse model of Dravet syndrome. Brain Res. 2022;1775:147743. doi: 10.1016/j.brainres.2021.147743. [DOI] [PubMed] [Google Scholar]
- 62.Laux, L. et al. Positive Interim Safety, PK, and CSF Exposure Data from the Phase 1/2a MONARCH study of STK-001, An Antisense Oligonucleotide (ASO), in Children and adolescents with Dravet Syndrome (DS)https://www.stoketherapeutics.com/wp-content/uploads/AES-2021-Interim-Safety-PK-and-CSF-Exposure-Data-from-the-Phase-1-2a-MONARCH-Study-of-STK-001.pdf (2021).
- 63.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021 doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minnaert AK, et al. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: getting the message across. Adv. Drug Deliv. Rev. 2021;176:113900. doi: 10.1016/j.addr.2021.113900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbier AJ, et al. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022;40:840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 66.Patel AK, et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31:e1805116. doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bangel-Ruland N, et al. Cystic fibrosis transmembrane conductance regulator-mRNA delivery: a novel alternative for cystic fibrosis gene therapy. J. Gene Med. 2013;15:414–426. doi: 10.1002/jgm.2748. [DOI] [PubMed] [Google Scholar]
- 68.Jiang L, et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat. Commun. 2020;11:5339. doi: 10.1038/s41467-020-19156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An D, et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. eBioMedicine. 2019;45:519–528. doi: 10.1016/j.ebiom.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olson D, et al. 767 Safety and preliminary efficacy of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L/IL-23/IL-36γ for intratumoral (ITu) injection, and durvalumab (IV) in TNBC, HNSCC, and melanoma. J. Immunother. Cancer. 2022;10:A797–A797. [Google Scholar]
- 71.Hotz C, et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 2021;13:eabc7804. doi: 10.1126/scitranslmed.abc7804. [DOI] [PubMed] [Google Scholar]
- 72.Zangi L, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Childs-Disney JL, et al. Targeting RNA structures with small molecules. Nat. Rev. Drug Discov. 2022;21:736–762. doi: 10.1038/s41573-022-00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Devinsky O, King L, Bluvstein J, Friedman D. Ataluren for drug-resistant epilepsy in nonsense variant-mediated Dravet syndrome and CDKL5 deficiency disorder. Ann. Clin. Transl. Neurol. 2021;8:639–644. doi: 10.1002/acn3.51306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh RN, Ottesen EW, Singh NN. The first orally deliverable small molecule for the treatment of spinal muscular atrophy. Neurosci. Insights. 2020;15:2633105520973985. doi: 10.1177/2633105520973985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhattacharyya A, et al. Small molecule splicing modifiers with systemic HTT-lowering activity. Nat. Commun. 2021;12:7299. doi: 10.1038/s41467-021-27157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ando S, et al. Discovery of a CNS penetrant small molecule SMN2 splicing modulator with improved tolerability for spinal muscular atrophy. Sci. Rep. 2020;10:17472. doi: 10.1038/s41598-020-74346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serganov A, Nudler E. A decade of riboswitches. Cell. 2013;152:17–24. doi: 10.1016/j.cell.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lennon SR, Batey RT. Regulation of gene expression through effector-dependent conformational switching by cobalamin riboswitches. J. Mol. Biol. 2022 doi: 10.1016/j.jmb.2022.167585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chau THT, Mai DHA, Pham DN, Le HTQ, Lee EY. Developments of riboswitches and toehold switches for molecular detection-biosensing and molecular diagnostics. Int. J. Mol. Sci. 2020;21:3192. doi: 10.3390/ijms21093192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimberg H, et al. Machine learning approaches to optimize small-molecule inhibitors for RNA targeting. J. Cheminform. 2022;14:4. doi: 10.1186/s13321-022-00583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costales MG, Childs-Disney JL, Haniff HS, Disney MD. How we think about targeting RNA with small molecules. J. Med. Chem. 2020;17:8880–8900. doi: 10.1021/acs.jmedchem.9b01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rzuczek SG, et al. Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nat. Chem. Biol. 2017;13:188–193. doi: 10.1038/nchembio.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sampson, J. et al. Results of double-blind, placebo-controlled, dose range finding, crossover study of single day administration of ERX-963 (IV flumazenil) in adults with myotonic dystrophy type 1. Neurology96 (Suppl. 15) (2021).
- 85.Disney MD, et al. Inforna 2.0: a platform for the sequence-based design of small molecules targeting structured RNAs. ACS Chem. Biol. 2016;11:1720. doi: 10.1021/acschembio.6b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang P, et al. Reprogramming of protein-targeted small-molecule medicines to RNA by ribonuclease recruitment. J. Am. Chem. Soc. 2021;33:13044–13055. doi: 10.1021/jacs.1c02248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haniff HS, et al. A structure-specific small molecule inhibits a miRNA-200 family member precursor and reverses a type 2 diabetes phenotype. Cell Chem. Biol. 2022;29:300–311. doi: 10.1016/j.chembiol.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mustoe A, et al. Pervasive regulatory functions of mRNA structure revealed by high-resolution SHAPE probing. Cell. 2018;173:1–15. doi: 10.1016/j.cell.2018.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheridan C. First small-molecule drug targeting RNA gains momentum. Nat. Biotechnol. 2021;39:6–8. doi: 10.1038/s41587-020-00788-1. [DOI] [PubMed] [Google Scholar]
- 90.Fu Z, et al. MicroRNA as an important target for anticancer drug development. Front. Pharmacol. 2021;12:736323. doi: 10.3389/fphar.2021.736323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang P, Zhou Y, Richards AM. Effective tools for RNA-derived therapeutics: siRNA interference or miRNA mimicry. Theranostics. 2021;11:8771–8796. doi: 10.7150/thno.62642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallant-Behm CL, et al. A microRNA-29 mimic (remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J. Invest. Dermatol. 2019;139:1073–1081. doi: 10.1016/j.jid.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Rogg EM, et al. Analysis of cell type-specific effects of microRNA-92a provides novel insights into target regulation and mechanism of action. Circulation. 2018;138:2545–2558. doi: 10.1161/CIRCULATIONAHA.118.034598. [DOI] [PubMed] [Google Scholar]
- 94.Seto AG, et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018;183:428–444. doi: 10.1111/bjh.15547. [DOI] [PubMed] [Google Scholar]
- 95.Raue R, Frank AC, Syed SN, Brüne B. Therapeutic targeting of microRNAs in the tumor microenvironment. Int. J. Mol. Sci. 2021;22:2210. doi: 10.3390/ijms22042210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Innao V, Allegra A, Pulvirenti N, Allegra AG, Musolino C. Therapeutic potential of antagomiRs in haematological and oncological neoplasms. Eur. J. Cancer Care. 2020;29:e13208. doi: 10.1111/ecc.13208. [DOI] [PubMed] [Google Scholar]
- 97.Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics — challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20:629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li LC, et al. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl Acad. Sci. USA. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L, et al. miR-2337 induces TGF-β1 production in granulosa cells by acting as an endogenous small activating RNA. Cell Death Discov. 2021;7:253. doi: 10.1038/s41420-021-00644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li H, et al. Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ. Res. 2019;125:1106–1120. doi: 10.1161/CIRCRESAHA.119.314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohno SI, et al. Nuclear microRNAs release paused Pol II via the DDX21-CDK9 complex. Cell Rep. 2022;39:110673. doi: 10.1016/j.celrep.2022.110673. [DOI] [PubMed] [Google Scholar]
- 102.Wang XY, et al. RNA activation technique and its applications in cancer research. Am. J. Cancer Res. 2018;8:584–593. [PMC free article] [PubMed] [Google Scholar]
- 103.Tan CP, Sinigaglia L, Gomez V, Nicholls J, Habib NA. RNA activation — a novel approach to therapeutically upregulate gene transcription. Molecules. 2021;26:6530. doi: 10.3390/molecules26216530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsui M, et al. Activation of LDL receptor expression by small RNAs complementary to a noncoding transcript that overlaps the LDLR promoter. Chem. Biol. 2010;17:1344–1355. doi: 10.1016/j.chembiol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park KH, et al. Targeted induction of endogenous VDUP1 by small activating RNA inhibits the growth of lung cancer cells. Int. J. Mol. Sci. 2022;23:7743. doi: 10.3390/ijms23147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarker D, et al. MTL-CEBPA, a small activating rna therapeutic upregulating C/EBP-α, in patients with advanced liver cancer: a first-in-human, multicenter, open-label, phase I trial. Clin. Cancer Res. 2020;26:3936–3946. doi: 10.1158/1078-0432.CCR-20-0414. [DOI] [PubMed] [Google Scholar]
- 107.Hashimoto A, et al. Upregulation of C/EBPα inhibits suppressive activity of myeloid cells and potentiates antitumor response in mice and patients with cancer. Clin. Cancer Res. 2021;27:5961–5978. doi: 10.1158/1078-0432.CCR-21-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plummer, R. et al. 850 Interim results for phase 1b dose expansion of MTL-CEBPA in combination with pembrolizumab in patients with advanced solid tumour malignancies. J. Immunother. Cancer10, 10.1136/jitc-2022-SITC2022.0850 (2022).
- 109.Padmakumar S, et al. Minimally invasive nasal depot (MIND) technique for direct BDNF AntagoNAT delivery to the brain. J. Control. Rel. 2021;331:176. doi: 10.1016/j.jconrel.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsiao J, et al. Upregulation of haploinsufficient gene expression in the brain by targeting a long non-coding RNA improves seizure phenotype in a model of Dravet syndrome. eBioMedicine. 2016;9:257. doi: 10.1016/j.ebiom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brynczka, C. et al. Upregulation of SCN1A by regRNA-targeting Antisense Oligonucleotide CMP-SCNChttps://www.camp4tx.com/wp-content/uploads/2022/04/DIA-FDA-poster-final.pdf (2022).
- 112.Young RS, Kumar Y, Bickmore WA, Taylor MS. Bidirectional transcription initiation marks accessible chromatin and is not specific to enhancers. Genome Biol. 2017;18:242. doi: 10.1186/s13059-017-1379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanciano S, Cristofari G. Measuring and interpreting transposable element expression. Nat. Rev. Genet. 2020;21:721–736. doi: 10.1038/s41576-020-0251-y. [DOI] [PubMed] [Google Scholar]
- 114.Greulich F, et al. Enhancer RNA expression in response to glucocorticoid treatment in murine macrophages. Cells. 2021;11:28. doi: 10.3390/cells11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wan L, et al. Inflammatory immune-associated eRNA: mechanisms, functions and therapeutic prospects. Front. Immunol. 2022;13:849451. doi: 10.3389/fimmu.2022.849451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gorbovytska V, et al. Enhancer RNAs stimulate Pol II pause release by harnessing multivalent interactions to NELF. Nat. Commun. 2022;13:2429. doi: 10.1038/s41467-022-29934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, et al. Early-life undernutrition induces enhancer RNA remodeling in mice liver. Epigenetics Chromatin. 2021;14:18. doi: 10.1186/s13072-021-00392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei W, et al. ADRAM is an experience-dependent long noncoding RNA that drives fear extinction through a direct interaction with the chaperone protein 14-3-3. Cell Rep. 2022;38:110546. doi: 10.1016/j.celrep.2022.110546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blank-Giwojna A, Postepska-Igielska A, Grummt I. lncRNA KHPS1 activates a poised enhancer by triplex-dependent recruitment of epigenomic regulators. Cell Rep. 2019;26:2904–2915. doi: 10.1016/j.celrep.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 120.So KKH, et al. seRNA PAM controls skeletal muscle satellite cell proliferation and aging through trans regulation of Timp2 expression synergistically with Ddx5. Aging Cell. 2022;21:e13673. doi: 10.1111/acel.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu M, Shen J. From super-enhancer non-coding rna to immune checkpoint: frameworks to functions. Front. Oncol. 2019;9:1307. doi: 10.3389/fonc.2019.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan Y, Li Y, Tang F. Oncogenic seRNA functional activation: a novel mechanism of tumorigenesis. Mol. Cancer. 2020;19:74. doi: 10.1186/s12943-020-01195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Milazzo C, et al. Antisense oligonucleotide treatment rescues UBE3A expression and multiple phenotypes of an Angelman syndrome mouse model. JCI Insight. 2021;6:e145991. doi: 10.1172/jci.insight.145991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gennemark P, et al. An oral antisense oligonucleotide for PCSK9 inhibition. Sci. Transl. Med. 2021;13:593. doi: 10.1126/scitranslmed.abe9117. [DOI] [PubMed] [Google Scholar]
- 125.Juliano RL. Chemical manipulation of the endosome trafficking machinery: implications for oligonucleotide delivery. Biomedicines. 2021;9:512. doi: 10.3390/biomedicines9050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gökirmak T, et al. Overcoming the challenges of tissue delivery for oligonucleotide therapeutics. Trends Pharmacol. Sci. 2021;42:588–604. doi: 10.1016/j.tips.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 127.Van de Vyver T, De Smedt SC, Raemdonck K. Modulating intracellular pathways to improve non-viral delivery of RNA therapeutics. Adv. Drug Deliv. Rev. 2021 doi: 10.1016/j.addr.2021.114041. [DOI] [PubMed] [Google Scholar]
- 128.Habtemariam BA, et al. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin. Pharmacol. Ther. 2021;109:372–382. doi: 10.1002/cpt.1974. [DOI] [PubMed] [Google Scholar]
- 129.Ayyar VS, Song D, Zheng S, Carpenter T, Heald DL. Minimal physiologically based pharmacokinetic-pharmacodynamic (mPBPK-PD) model of N-acetylgalactosamine-conjugated small interfering RNA disposition and gene silencing in preclinical species and humans. J. Pharmacol. Exp. Ther. 2021;379:134–146. doi: 10.1124/jpet.121.000805. [DOI] [PubMed] [Google Scholar]
- 130.Willmann S, et al. PK/PD modeling of FXI antisense oligonucleotides to bridge the dose-FXI activity relation from healthy volunteers to end-stage renal disease patients. CPT Pharmacomet. Syst. Pharmacol. 2021;10:890–901. doi: 10.1002/psp4.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monine M, Norris D, Wang Y, Nestorov I. A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J. Pharmacokinet. Pharmacodyn. 2021;48:639–654. doi: 10.1007/s10928-021-09761-0. [DOI] [PubMed] [Google Scholar]
- 132.Mullard A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016;15:447. doi: 10.1038/nrd.2016.136. [DOI] [PubMed] [Google Scholar]
- 133.Yamaguchi S, Kaneko M, Narukawa M. Approval success rates of drug candidates based on target, action, modality, application, and their combinations. Clin. Transl. Sci. 2021;14:1113–1122. doi: 10.1111/cts.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.González-Castro N, Bjelic J, Malhotra G, Huang C, Alsaffar SH. Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int. J. Mol. Sci. 2021;22:10355. doi: 10.3390/ijms221910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheng H, Zhang F, Ding Y. CRISPR/Cas9 delivery system engineering for genome editing in therapeutic applications. Pharmaceutics. 2021;13:1649. doi: 10.3390/pharmaceutics13101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ernst MPT, et al. Ready for Repair? Gene editing enters the clinic for the treatment of human disease. Mol. Ther. Methods Clin. Dev. 2020;18:532–557. doi: 10.1016/j.omtm.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Porto EM, Komor AC, Slaymaker IM, Yeo GW. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug Discov. 2020;19:839–859. doi: 10.1038/s41573-020-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alavi, A. et al. Interim Safety and Efficacy Results from a Phase 1/2 Study of Zinc Finger Nuclease-modified Autologous Hematopoietic Stem Cells for Sickle Cell Disease (PRECIZN-1)https://www.sangamo.com/wp-content/uploads/2022/12/22-1566-ASH-BIVV003-Phase-I-II-Interim-results_v6.pdf (2022).
- 139.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 140.Benjamin R, et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet Haematol. 2022;9:e833–e843. doi: 10.1016/S2352-3026(22)00245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhakta S, Tsukahara T. Artificial RNA editing with ADAR for gene therapy. Curr. Gene Ther. 2020;20:44–54. doi: 10.2174/1566523220666200516170137. [DOI] [PubMed] [Google Scholar]
- 142.Doherty EE, et al. Rational design of RNA editing guide strands: cytidine analogs at the orphan position. J. Am. Chem. Soc. 2021;143:6865–6876. doi: 10.1021/jacs.0c13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Platenburg, G. Progress on Development of RNA Base Editing Technologies for Precision Medicineshttps://www.proqr.com/files/2022-11/ProQR_Axiomer_TIDES-EU_2022_Presentation.pdf (2022).
- 144.Monian P, et al. Endogenous ADAR-mediated RNA editing in non-human primates using stereopure chemically modified oligonucleotides. Nat. Biotechnol. 2022;40:1093–1102. doi: 10.1038/s41587-022-01225-1. [DOI] [PubMed] [Google Scholar]
- 145.Qu L, et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat. Biotechnol. 2019;37:1059–1069. doi: 10.1038/s41587-019-0178-z. [DOI] [PubMed] [Google Scholar]
- 146.Yi Z, et al. Engineered circular ADAR-recruiting RNAs increase the efficiency and fidelity of RNA editing in vitro and in vivo. Nat. Biotechnol. 2022;40:946–955. doi: 10.1038/s41587-021-01180-3. [DOI] [PubMed] [Google Scholar]
- 147.Katrekar D, et al. Efficient in vitro and in vivo RNA editing via recruitment of endogenous ADARs using circular guide RNAs. Nat. Biotechnol. 2022;40:938–945. doi: 10.1038/s41587-021-01171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crooke ST, Vickers TA, Liang XH. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020;48:5235–5253. doi: 10.1093/nar/gkaa299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhattacharyya J, et al. Effect of locked nucleic acid modifications on the thermal stability of noncanonical DNA structure. Biochemistry. 2011;50:7414–7425. doi: 10.1021/bi200477g. [DOI] [PubMed] [Google Scholar]
- 150.Baker YR, et al. An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides. Nat. Commun. 2022;13:4036. doi: 10.1038/s41467-022-31636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Christou M, et al. Systemic evaluation of chimeric LNA/2′-O-methyl steric blockers for myotonic dystrophy type 1 therapy. Nucleic Acid Ther. 2020;30:80–93. doi: 10.1089/nat.2019.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Evéquoz D, et al. 7′,5′-α-bicyclo-DNA: new chemistry for oligonucleotide exon splicing modulation therapy. Nucleic Acids Res. 2021;49:12089–12105. doi: 10.1093/nar/gkab1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Migawa MT, et al. Site-specific replacement of phosphorothioate with alkyl phosphonate linkages enhances the therapeutic profile of gapmer ASOs by modulating interactions with cellular proteins. Nucleic Acids Res. 2019;47:5465–5479. doi: 10.1093/nar/gkz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang L, et al. The combination of mesyl-phosphoramidate inter-nucleotide linkages and 2′-O-methyl in selected positions in the antisense oligonucleotide enhances the performance of RNaseH1 active PS-ASOs. Nucleic Acid Ther. 2022;32:401–411. doi: 10.1089/nat.2022.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kandasamy P, et al. Impact of guanidine-containing backbone linkages on stereopure antisense oligonucleotides in the CNS. Nucleic Acids Res. 2022;50:5401–5423. doi: 10.1093/nar/gkac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elkayam E, et al. siRNA carrying an (E)-vinylphosphonate moiety at the 5΄ end of the guide strand augments gene silencing by enhanced binding to human Argonaute-2. Nucleic Acids Res. 2017;45:3528–3536. doi: 10.1093/nar/gkw1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jurk M, et al. Structure-activity relationship studies on the immune stimulatory effects of base-modified CpG toll-like receptor 9 agonists. Chem. Med. Chem. 2006;1:1007–1014. doi: 10.1002/cmdc.200600064. [DOI] [PubMed] [Google Scholar]
- 158.Svitkin YV, et al. N1-methyl-pseudouridine in mRNA enhances translation through eIF2α-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017;45:6023–6036. doi: 10.1093/nar/gkx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yoshida T, et al. Identification of nucleobase chemical modifications that reduce the hepatotoxicity of gapmer antisense oligonucleotides. Nucleic Acids Res. 2022;50:7224–7234. doi: 10.1093/nar/gkac562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vickers TA, Migawa MT, Seth PP, Crooke ST. Interaction of ASOs with PC4 Is highly influenced by the cellular environment and ASO chemistry. J. Am. Chem. Soc. 2020;142:9661–9674. doi: 10.1021/jacs.0c01808. [DOI] [PubMed] [Google Scholar]
- 161.Brown KM, et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotech. 2022;40:1500–1508. doi: 10.1038/s41587-022-01334-x. [DOI] [PubMed] [Google Scholar]
- 162.Hammond SM, et al. Antibody-oligonucleotide conjugate achieves CNS delivery in animal models for spinal muscular atrophy. JCI Insight. 2022;7:e154142. doi: 10.1172/jci.insight.154142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Da Silva Sanchez AJ, et al. Substituting racemic ionizable lipids with stereopure ionizable lipids can increase mRNA delivery. J. Control. Rel. 2022;353:270–277. doi: 10.1016/j.jconrel.2022.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao M, Zhang Q, Feng XH, Liu J. Synthetic modified messenger RNA for therapeutic applications. Acta Biomater. 2021;131:1–15. doi: 10.1016/j.actbio.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim M, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021;7:eabf4398. doi: 10.1126/sciadv.abf4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Packer M, Gyawali D, Yerabolu R, Schariter J, White P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021;12:6777. doi: 10.1038/s41467-021-26926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sabnis S, et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.