Abstract
Although the amygdala plays an important part in the pathogenesis of anxiety and generation of exteroceptive fear, recent discoveries have challenged the directionality of this brain-behavior relationship with respect to interoceptive fear. Here we highlight several paradoxical findings including: (1) amygdala lesion patients who experience excessive fear and panic following inhalation of carbon dioxide (CO2), (2) clinically anxious patients who have significantly smaller (rather than larger) amygdalae and a pronounced hypersensitivity toward CO2, and (3) epilepsy patients who exhibit apnea immediately following stimulation of their amygdala yet have no awareness that their breathing has stopped. The above findings elucidate an entirely novel role for the amygdala in the induction of apnea and inhibition of CO2-induced fear. Such a role is plausible given the strong inhibitory connections linking the central nucleus of the amygdala with respiratory and chemoreceptive centers in the brainstem. Based on this anatomical arrangement, we propose a model of Apnea-induced Anxiety (AiA) which predicts that recurring episodes of apnea are being unconsciously elicited by amygdala activation, resulting in transient spikes in CO2 that provoke fear and anxiety, and lead to characteristic patterns of escape and avoidance behavior in patients spanning the spectrum of anxiety. If this new conception of AiA proves to be true, and activation of the amygdala can repeatedly trigger states of apnea outside of one’s awareness, then it remains possible that the chronicity of anxiety disorders is being interoceptively driven by a chemoreceptive system struggling to maintain homeostasis in the midst of these breathless states.
Keywords: respiration, brainstem, interoception, CO2, fear, panic, suffocation alarm
Introduction
Anxiety disorders are the most common form of mental illness, affecting over a quarter of the population and representing the sixth leading cause of disability, worldwide (Bandelow & Michaelis, 2015; Baxter et al., 2014; Kessler et al., 2005). Age of onset is typically in adolescence and young adulthood, with symptoms often persisting throughout life without treatment, making anxiety one of the most chronic health conditions (Kessler et al., 2012). More than three-quarters of patients never receive treatment (Collins et al., 2004), and meta-analyses and large-scale clinical trials suggest that only about half of patients improve with existing treatments (Loernic et al., 2015; Springer, Levy, & Tolin, 2018; Warden et al., 2007).
Given the ubiquitous and debilitating nature of anxiety, and the insufficient treatment response to currently available therapies, it is imperative that we gain a better understanding of the pathophysiological mechanisms underpinning anxiety so that more targeted treatments can be developed (LeDoux & Pine, 2016). Yet, the etiology driving the chronic and unrelenting nature of anxiety continues to elude neuroscience, psychology, and psychiatry. This point was recently underscored in an article that interviewed a panel of the top researchers in the field and found very little consensus on the basic definition of what constitutes fear and anxiety, let alone the best way to probe its neural underpinnings (Mobbs et al., 2019). In order to avoid confusion, some neuroscientists have recommended that the terms fear and anxiety be used in an undifferentiated manner, and the focus should instead be on differentiating the specific parameters of the threat (Shackman & Fox, 2016), a recommendation which we intend to follow throughout this paper.
No threat is more proximal to survival than a threat from within the body. Yet, most neuroscience research investigating fear has focused almost exclusively on studying exteroceptive threats conveyed through canonical visual, auditory, olfactory, or somatosensory channels. In contrast, interoception is the process by which the nervous system senses, interprets, and integrates signals originating from within the body, especially the viscera, but very little is known about the neural basis of interoceptive fear (Berntson & Khalsa, 2021; Khalsa et al., 2018; Paulus, Feinstein, & Khalsa, 2019). This paper focuses on the chemoreceptive system, an often-neglected branch of interoceptive neurobiology that is foundational to our survival, and a potential source of the fear and anxiety that so often pervades the consciousness of clinically anxious patients.
Respiratory Chemoreception
Every moment of the day, breath by breath, the chemoreceptive system is regulating the pH of our blood and extracellular fluids within a tightly constrained range between 7.35–7.45 (Duffin, 2005; Shirakabe et al., 2012). Even small shifts in pH will rapidly trigger compensatory changes in respiration, as disruptions in acid-base homeostasis can quickly lead to organ failure and eventually death. Carbon dioxide (CO2) is the primary driver of these respiratory changes. Increases in CO2 reduce blood pH (via carbonic acid), triggering acidosis. CO2 also provokes pronounced changes throughout the cardiovascular system including vasodilation and an increase in blood pressure and cerebral blood flow (Battisti-Charbonney, Fisher, & Duffin, 2011; Chang & Glover, 2009; Vickers et al., 2012). As aptly summarized by one of the original CO2 physiologists, “Carbon dioxide is the chief [para]hormone of the entire body; it is the only one that is produced by every tissue and that probably acts on every organ… carbon dioxide is, in fact, a more fundamental component of living matter than is oxygen” (Henderson, 1940).
Respiration is regulated by the chemoreceptive system via feedback from peripheral and central chemoreceptors (Del Negro, Funk, & Feldman, 2018; Guyenet & Bayliss, 2015), in addition to acid-sensing ion channels found in neurons throughout the nervous system (Wemmie, Price, & Welsh, 2006). Peripheral chemoreceptors monitor the arterial blood for changes in the partial pressure of oxygen (PO2), the partial pressure of carbon dioxide (PCO2), and pH, and can be found in two primary locations: (1) the carotid body located near the bifurcation of the carotid arteries, and (2) the aortic bodies on the aortic arch. Signals from the peripheral chemoreceptors are directly transmitted to the nucleus tractus solitarii (NTS) in the brainstem, with signals from the carotid body conveyed via the glossopharyngeal nerve and signals from the aortic bodies conveyed via the vagus nerve. The carotid body is the primary peripheral chemoreceptor responsible for detecting states of hypoxemia (Iturriaga et al., 2021), with recent work also highlighting its role in both inflammation and metabolism (Conde, Sacramento, & Martin, 2020). Central chemoreceptors are largely located on the ventrolateral surface of the medulla, in regions such as the medullary raphe and the retrotrapezoid nucleus (Nattie & Li, 2012; Richerson, 2004). Since central chemoreceptors sense changes in PCO2 and pH within the cerebrospinal fluid, the central nervous system is remarkably insensitive to detecting acute short-term changes in oxygen (Nattie & Li, 2012; but see Gestreau et al., 2010 for an example of how central chemoreceptors can respond to chronic hypoxia). In contrast, changes in CO2 are readily detected by both central and peripheral chemoreceptors and rapidly alter respiration such that an increase in PCO2 of 2–5 mmHg can increase ventilation more than twofold (Del Negro, Funk, & Feldman, 2018; Duffin, 2005; Rassovsky, Abrams, & Kushner, 2006). As PCO2 rises and pH becomes more acidic, respiratory chemoreceptors will emit a cascade of “suffocation alarms” (Klein, 1993), a proverbial primal scream aimed at alerting the nervous system of impending demise. If corrective action is not immediately taken, unconsciousness can occur within as little as 30 to 40 seconds, and death within a matter of minutes (Sharp, Azar, & Lawson, 2006).
Fear and panic in patients with bilateral amygdala lesions
There is a large body of evidence in both humans and other animals showing that the amygdala plays a central role in the generation of fear and pathogenesis of anxiety (Davis, 1992; Etkin & Wager, 2007; Gorman et al., 2000; Kalin, Shelton, & Davidson, 2004; Oler et al., 2010; Shackman & Fox, 2016; Tovote, Fadok, & Lüthi, 2015). Much of this research utilized external threats, such as exposure to predators, in order to probe the behavioral manifestations of fear. For example, animals with amygdala lesions typically display reduced freezing and a striking lack of avoidance as exemplified by rats with amygdala lesions that readily approach cats (e.g., Blanchard & Blanchard, 1972), and monkeys with amygdala lesions that approach snakes (e.g., Meunier et al., 1999). Much less is known about the amygdala’s “necessary” role in the conscious experience of fear (LeDoux & Pine, 2016). This is in large part because nonhuman animals with amygdala lesions are unable to verbally report on their internal subjective experience, and humans with amygdala lesions are extremely rare.
An exception is patient SM, a middle-aged woman with Urbach–Wiethe disease who is one of the best-characterized human cases with focal bilateral amygdala lesions. Over the past three decades, there have been numerous attempts to probe SM’s reaction to a host of different exteroceptive threats, in both the laboratory and the real-world (for a review of these publications, see Feinstein, Adolphs, & Tranel, 2016). Similar to amygdala-lesioned animals, SM exhibits a deficit in fear conditioning (Bechara et al., 1995), and an overall lack of fear and avoidance behavior to external threats including when exposed to snakes and tarantulas, horror films, haunted houses, and a range of traumatic life events (Feinstein et al., 2011). Beyond her inability to recognize and respond to dangerous situations, SM also reported that such situations failed to evoke feelings of fear. Her highly impoverished experience of fear dates back to adolescence, around the time when her amygdala lesions likely began to emerge. However, during early childhood, SM remembers several incidents that did evoke fear, and as a consequence, she maintains a basic conceptual understanding of what fear feels like (Feinstein et al., 2011).
After many unsuccessful attempts to scare SM using exteroceptive threats, we shifted course and decided to probe her reaction to interoceptive threat. In comparison to the wide range of paradigms probing exteroceptive fear, there are far fewer options for safely inducing interoceptive fear in humans. One method that has been well-studied entails the inhalation of an air mixture containing 35% CO2 (Vickers et al., 2012), a quantity of CO2 that is 875 times greater than the amount of CO2 in the air we typically breathe (~.04%). Given such a high concentration, participants only take a single vital capacity inhalation, triggering a brief hypercapnic state that rapidly activates both central and peripheral chemoreceptors causing a marked increase in ventilation (Griez, van den Hout, & Verstappen, 1987; Rassovsky & Kushner, 2003; Vickers et al., 2012). The most commonly reported side effect of this chemoreceptive perturbation is a profound sense of ‘air hunger’ that is felt almost immediately after the inhalation and lasts for about a minute (Vickers et al., 2012). Since the gas mixture contains a normal amount of oxygen, O2 levels within the body are unaffected, which means that the CO2 manipulation triggers an illusion of air hunger. Despite its illusory nature, the feeling induced by 35% CO2 is very potent and capable of inducing fear, anxiety, and even panic, in up to one-quarter of healthy individuals who undergo this challenge (Colasanti et al., 2012). In individuals with a history of panic disorder, the manipulation readily produces full-blown panic attacks that closely parallel those occurring in everyday life (Colasanti et al., 2012; Schruers, Van de Mortel, Overbeek, & Griez, 2004).
The amygdala has been shown to have the ability to directly detect changes in CO2 and pH through acid-sensing ion channels, leading to CO2-evoked fear behaviors (Ziemann et al., 2009). Moreover, several theories have highlighted a central role for the amygdala in the generation of panic attacks (Coplan & Lydiard, 1998; Gorman, Kent, Sullivan, & Coplan, 2000). Based on these theories and findings, we hypothesized that patient SM would experience abnormally low levels of CO2-evoked fear and panic as a result of the damage incurred to her amygdala. To test this hypothesis, we arranged for SM to undergo a 35% CO2 challenge, marking the first time we had ever directly exposed her to an interoceptive threat (Feinstein et al., 2013).
Immediately following the inhalation of 35% CO2, SM began breathing at a rapid pace and gasping for air, exclaiming, “I can’t breathe”. She attempted to escape from the air mask (even though it was no longer delivering CO2). During debriefing, she reported that she felt an immense fear, labeling it as the “worst” fear she had ever felt in life. In one breath, our hypothesis was proven wrong, and we learned that the amygdala could not be the brain’s quintessential and sole fear center. To test whether this result was reproducible, we collaborated with Dr. René Hurlemann, who had identified monozygotic twin sisters (AM and BG) who both had focal bilateral amygdala lesions due to the same genetic disorder as SM (Becker et al., 2012). Replicating SM’s reaction, inhalation of 35% CO2 triggered panic attacks in both twins characterized by an intense fear of suffocation, concomitant thoughts of death, heightened physiological arousal (including hyperventilation), and prominent signs of escape behavior (Feinstein et al., 2013). The amygdala-lesioned patients exhibited a significantly higher rate of CO2-evoked fear and panic than a sample of demographically-matched healthy participants who did not panic when exposed to CO2 (Figure 1). The procedure entailed single vital capacity inhalations of 35% CO2 or compressed air, each administered twice in a blinded order. Subjective changes were assessed several minutes after each inhalation where participants reported how they felt during and immediately following the inhalation when symptoms were at their peak. Physiological changes were concurrently measured throughout each inhalation. The threshold for defining a panic attack used conservative criteria (Rassovsky & Kushner, 2003) that required a participant to endorse at least four DSM-IV symptoms of panic, exhibit signs of escape behavior, and report at least a 25% increase in self-reported panic.
Figure 1. Heightened fear and panic to 35% CO2 in three rare lesion patients with focal bilateral amygdala damage.
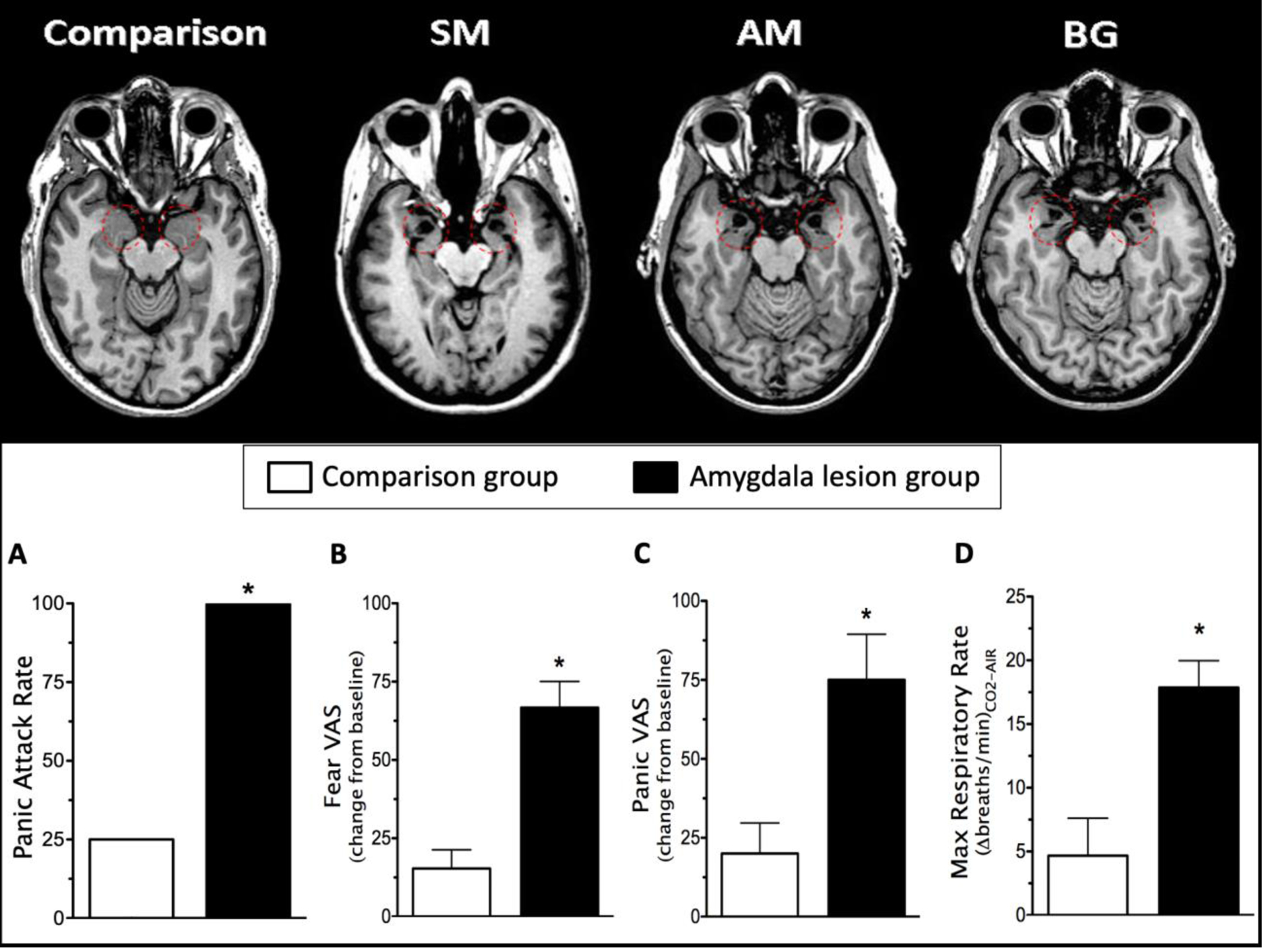
(Feinstein et al., 2013). The amygdala is highlighted by red-dashed circles on the MRI scans in the top panel. A single vital capacity inhalation of 35% CO2 triggered a panic attack in all of the patients with amygdala damage (A), as well as significantly higher levels of self-reported fear (B) and panic (C), and a significantly higher rate of respiration (D). *p < .05; error bars represent the standard error of the mean. VAS, visual analogue scale.
The significantly higher level of panic experienced by the amygdala-lesioned patients raises the possibility that instead of inducing panic, the amygdala may be integrally involved in inhibiting panic, especially panic evoked by elevated levels of CO2. Such an inhibitory role might also help explain how another patient with amygdala lesions spontaneously developed panic attacks in everyday life (Wiest, Lehner-Baumgartner, & Baumgartner, 2006). Likewise, amygdala-lesioned mice were recently found to exhibit an excessive amount of escape behavior (frenetically jumping off the walls and ceiling) in tandem with a lack of freezing behavior when placed in a chamber with 10% CO2 (Taugher et al., 2020). Notably, this pattern of excessive escape behavior was eliminated by also lesioning the dorsal periaqueductal gray (PAG), suggesting that an intact amygdala normally inhibits CO2-induced escape and promotes CO2-induced freezing via its connections with the PAG (Kim et al., 2013). Interestingly, bilateral lesions to the bed nucleus of the stria terminalis (BNST) in mice also reduced freezing to 10% CO2, as well as conditioned-place avoidance, however the BNST-lesioned mice did not show any sign of the excessive escape-like behavior that was seen in the amygdala-lesioned mice, suggesting that the amygdala may exert a preferential regulatory role over CO2-induced escape (Taugher et al., 2014; Taugher et al., 2020).
In a follow-up study with the amygdala-lesioned twins (Khalsa et al., 2016), we examined their response to a different form of interoceptive threat entailing intravenous infusions of isoproterenol, a beta-adrenergic agonist akin to adrenaline. Shortly after receiving a 4 microgram bolus infusion of isoproterenol, both patients exhibited a large physiological response as evidenced by a rapid acceleration in their heart rate, on the order of 30 beats per minute faster than baseline. During this time period, both patients reported experiencing heightened levels of anxiety. One of the patients (BG) had a panic attack that was accompanied by intense feelings of breathlessness, a partial replication of what was found during the CO2 challenge. However, BG’s level of self-reported panic was only about half the intensity of what she experienced during 35% CO2, and neither patient reported having a fear of dying during isoproterenol, signifying that CO2 was a more potent panicogen. One potential explanation for this difference in panic response could be due to the fact that isoproterenol bronchodilates the lungs (Tattersfield & McNicol, 1969) and thus would not be expected to elicit hypercapnia.
While definitive conclusions from the above lesion experiments with CO2 and isoproterenol are limited by the small number of rare patients that were studied, the findings demonstrate that the amygdala is not always required for the conscious experience of fear, anxiety, and panic. Brain regions outside the amygdala may be the source of interoceptive fear and identifying their locus could provide key neural modulation targets for future anxiolytic treatments. These findings also stand in sharp contrast to prior research from our group demonstrating that these same lesion patients showed a marked absence of fear in response to threatening stimuli from the external environment (Becker et al., 2012; Feinstein et al., 2011; Feinstein et al., 2016; Scheele et al., 2012). Thus, there appears to be an important neural distinction between threats conveyed through exteroceptive sensory channels (e.g., visual and auditory pathways) versus threats conveyed through interoceptive sensory channels (e.g., chemoreceptive and cardiorespiratory pathways). Sensory and association cortices required for representing external stimuli are intact in the brain of SM and the other lesion patients, as are the brainstem and hypothalamic circuitry necessary for orchestrating the action program of a fear response. Their amygdala lesion in effect disconnects these two components, making it improbable, if not impossible, for external sensory representations of threat to trigger full-blown fear responses, leading to their deficits in the realm of exteroceptive fear. On the other hand, interoceptive threats are able to bypass the amygdala and directly stimulate the brainstem. Since the lesion patients have lost the amygdala’s inhibitory control over brainstem circuitry, certain interoceptive threats (such as CO2 and isoproterenol) can lead to a disinhibited fear response that likely engages an interoceptive pathway projecting from the brainstem to the diencephalon, insular cortices, anterior cingulate, and a network of other limbic and cortical structures, culminating in their conscious experience of fear, anxiety, and panic (Berntson & Khalsa, 2021; Davenport & Vovk, 2009). In summary, the amygdala may be critical for the generation of exteroceptive fear, but it does not appear to be necessary for the generation of interoceptive fear. Instead, the amygdala may play an important role in the inhibition of interoceptive fear, especially chemoreceptive fear evoked by elevations in CO2.
Amygdala-driven apnea and the loss of awareness for elevations in CO2
Recent findings from the field of neurosurgery (Dlouhy et al. 2015; Lacuey et al., 2017; Nobis et al. 2018; Rhone et al., 2020) have identified a remarkable brain-behavior relationship that has largely been overlooked for the better part of the past century; stimulation of the human amygdala inhibits breathing and triggers apnea, yet surprisingly, the patients had no awareness that their breathing had stopped, and did not report any fear or dyspnea as their CO2 levels rose during the period of apnea. In other words, amygdala stimulation can quite literally ‘take one’s breath away’ and they will not even know that it has happened. The neurosurgical studies defined an apnea as the involuntary cessation of breathing following the onset of electrode stimulation where at least 2 breaths were missed with a drop in peak signal excursion ≥ 90% of baseline breathing (Lacuey et al., 2017) or at least 1 breath was missed with a flattened airflow trace (oral/nasal thermistor or nasal pressure transducer) and verified by the absence of chest wall movement as measured with plethysmography belts or video (Rhone et al., 2020). In this next section we review these findings in detail and discuss how they further highlight the strong inhibitory role of the amygdala with regard to both respiration and chemoreception.
The first study to clearly demonstrate amygdala-driven apnea was published in 2015 by Dlouhy and his colleagues at the University of Iowa (Dlouhy et al., 2015). They found that stimulation of electrodes in the amygdala would cause breathing to come to an immediate halt and often remain in a state of apnea until the stimulation was turned off (Figure 2). All three patients who were tested were awake and conscious throughout the stimulation, but were completely unaware that their breathing had stopped, and showed no signs of discomfort or distress. In one patient, the apnea lasted for 48 seconds, causing their oxygen saturation to drop from 97% to 87%. When this same patient was asked to voluntarily hold their breath for the same amount of time, but without the amygdala stimulation, they had great difficulty doing so and reported experiencing severe dyspnea during the breathhold, suggesting that the amygdala stimulation was somehow inhibiting their normal response to elevations in CO2.
Figure 2. Intracranial stimulation of the human amygdala induces apnea.
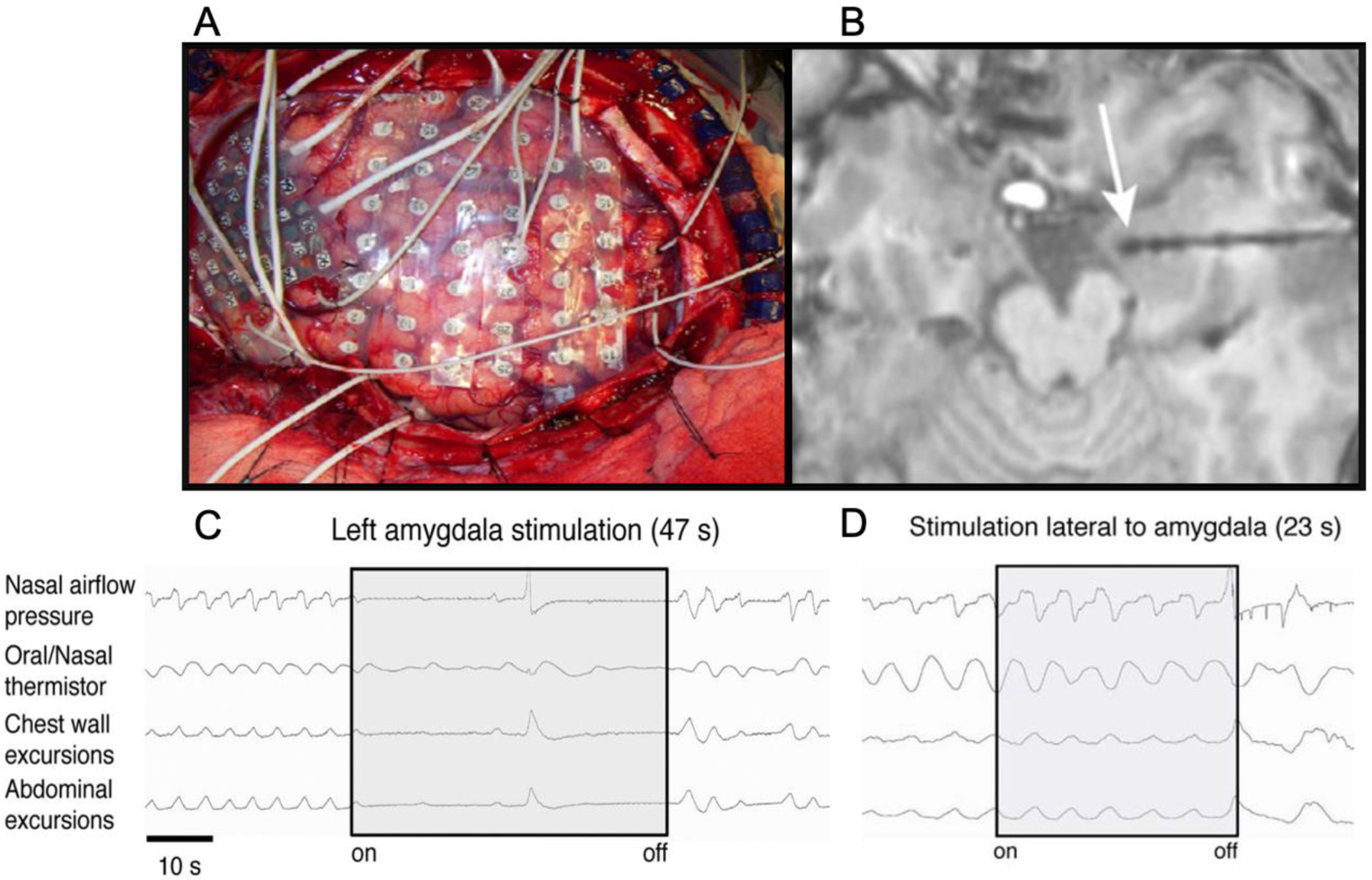
(images from Dlouhy et al., 2015). (A) For many years, neurosurgeons have applied large arrays of electrodes inside the brain of epilepsy patients to identify the source of their seizures prior to resecting the epileptogenic tissue. These intracranial electrodes were mainly used for passively recording neuronal activity. More recently, neurosurgeons have started using the electrodes to stimulate the underlying tissue by applying extended trains of electrical pulses at 50 Hz while simultaneously recording changes in respiration. (B) Depth electrodes stimulated in the vicinity of the amygdala (white arrow) reliably trigger apnea (C), whereas stimulation of electrodes outside of the amygdala does not disrupt respiration (D).
This finding of amygdala-driven apnea has now been replicated by two other neurosurgical departments (Lacuey et al., 2017; Nobis et al. 2018), and in all cases the patients were completely unaware that their breathing had stopped. In one case the apnea lasted for 56 seconds and caused the patient’s end-tidal CO2 to increase by 10 mmHG (Lacuey et al., 2017). Most recently, these results were replicated in patients with pediatric epilepsy (Rhone et al., 2020), which found that all patients “were completely unaware that they had stopped breathing and did not report any shortness of breath, air hunger, desire to breathe, or display any visible signs of respiratory distress during or after the periods of stimulation-induced apnea” (Rhone et al., 2020, p. 3). The researchers stimulated a number of different electrodes, both within and outside of the amygdala, and found a remarkable degree of specificity such that apnea only occurred when the amygdala was stimulated. Using machine learning with a multiclass support vector, the researchers identified a specific site in the basomedial amygdala that consistently induced apnea. Notably, the basomedial amygdala has been shown to mediate the top-down control of fear and freezing via projections from the ventromedial prefrontal cortex (Adhikari et al., 2015). In the study by Nobis et al. (2018), they found that apnea was most reliably induced when the most medial electrodes were stimulated, corresponding to activation of the central nucleus of the amygdala. Prior research stimulating the central nucleus of the amygdala in rabbits (Applegate et al., 1983) and cats (Harper et al., 1984) found that brief pulses of stimulation (< 1 second in duration) often led to transient increases in respiration, whereas the human neurosurgical studies all delivered trains of electrical stimulation over a prolonged period (between 5 to 60 seconds). This suggests that the amygdala has the potential to both excite and inhibit respiration, depending on the duration of stimulation.
While most neurosurgical studies have found that apnea was only elicited following amygdala stimulation, there have been sporadic reports of apnea following stimulation to other regions in the medial temporal lobe (Lacuey et al., 2019) and adjacent limbic structures (Kaada & Jasper, 1952). A retrospective analysis of epilepsy patients who had seizures while being monitored with intracranial electrodes found a high incidence of ictal central apnea as the seizure spread into the amygdala (Nobis et al., 2019). These findings have important implications for understanding the etiology of sudden unexpected death in epilepsy (SUDEP), the most common cause of death in patients with refractory epilepsy (Buchanan, 2019; Massey, Sowers, Dlouhy, & Richerson, 2014). In line with this notion, a mouse model of SUDEP found that electrolytic lesions to the amygdala significantly reduced the incidence of seizure-induced respiratory arrest (Marincovich, Bravo, Dlouhy, & Richerson, 2019), adding further evidence that amygdala-driven seizures are inducing apnea and playing a causal role in the sudden death of patients with epilepsy, often times without their awareness.
It is quite remarkable that we are only learning about this strong relationship between the amygdala and apnea in recent years, as researchers have been stimulating the amygdala for many decades. However, a close inspection of the literature reveals several documented instances of amygdala-driven apnea. The first report by W.G. Spencer in 1894 found that respiration in non-human animals “can be slowed and arrested by excitation of a certain spot… to the outer side of the olfactory tract just in front of the junction of the tract with the uncinate. And this arrest can be constantly obtained and the experiment repeated again and again under certain conditions” (Spencer, 1894, p.629). In 1899, Hughlings Jackson commented on this prescient observation, “I presume that the arrest is by great cortical inhibition of the respiratory medulla… watching patients in slight paroxysms of epilepsy with regard to degrees of asphyxia… there is sometimes turning blue, as the friends may say. Here comes, I submit, the importance of that part of Mr. W.G. Spencer’s research which I have quoted; his is very near to the uncinate gyrus. So that if an epileptic discharge does begin in some part of that gyrus it may spread to and may soon reach the arrest centre” (Jackson, 1899). The first report of intracranial stimulation in humans causing apnea came in 1952, where mechanical stimulation “in and about the anterior portion of the hippocampal gyrus resulted in complete respiratory arrest… in one instance for as long as 56 seconds” (Kaada & Jasper, 1952). There was also another case where respiratory arrest for as long as 40 seconds could be reliably evoked following stimulation “near the center of the left amygdala” (Nelson & Ray, 1968). In rats, the intensity of hypercapnic response to CO2 was inversely correlated with c-fos expression in the medial amygdala consistent with the amygdala having an inhibitory influence on chemoreception (Tenorio-Lopes et al., 2017). In anesthetized squirrel monkeys, stimulation of the amygdala caused prolonged apneas, leading to significant increases in PCO2 and decreases in pH (Reis & McHugh, 1968). Remarkably, the respiratory inhibition “persisted long enough to cause myocardial hypoxia and even death from lethal arrythmias”, yet signs of the monkey struggling were notably absent. Similar changes in PCO2 and pH were reproduced via occlusion of the trachea, however without concurrent stimulation of the amygdala, the tracheal occlusion elicited a strong increase in respiratory effort and behavioral signs of struggle in the monkey. Reis & McHugh (1968) concluded that the amygdala stimulation had selectively blocked respiratory and behavioral chemoreceptor responses.
Amygdala projections to the brainstem are inhibitory
The amygdala is one of the most highly connected structures in the brain (Pessoa, 2008). It is comprised of 13 different subnuclei, each with their own unique pattern of connectivity (Freese & Amaral, 2009). Neuroanatomical connections linking the amygdala with the brainstem are inhibitory in nature and prominently feature brainstem nuclei involved in both respiration and chemoreception. Much of our understanding of amygdala neuroanatomy comes from studies conducted in non-human animals such as rats and monkeys (Freese & Amaral, 2009). Since there are differences between species in the physiological properties of amygdala neurons (Dumont et al., 2002), it will be important to conduct more anatomical studies in human brains (including postmortem approaches) to verify the connectivity patterns (Mori et al., 2017).
The central nucleus provides the amygdala’s primary output to the brainstem and is quite distinct from other amygdala nuclei due to its ‘striatal-like’ medium spiny neurons that form a continuum with the bed nucleus of the stria terminalis (BNST) in what has come to be known as the extended amygdala (Davis, Walker, Miles, & Grillon, 2010; Shackman & Fox, 2016). Notably, the central nucleus and BNST share many connections to brainstem nuclei and also appear to share an overlapping timing of impulses to these nuclei (Nagy & Paré, 2008). It is important to recognize that the cells in the central nucleus of the amygdala are primarily GABAergic inhibitory neurons (Babaey, Chatain, & Krueger-Berg, 2018; Ciocchi et al., 2010). These GABAergic neurons are innervated by GABAergic interneurons from the adjacent intercalated cell islands, as well as GABAergic projections from the insula (Cassell, Freedman, & Shi, 1999; Sun, Yi, & Cassell, 1994), and this dense GABAergic circuitry appears to underlie the anxiolytic effect of benzodiazepines (Griessner et al., 2018; Paulus et al., 2005).
As a consequence of this arrangement, the projections from the central nucleus of the amygdala to the brainstem are also GABAergic, and thus, inhibitory in nature (Jongen‐Rêlo & Amaral, 1998; Liu et al., 2021). The most prominent projections are to brainstem nuclei critically involved in regulating autonomic functioning (Price & Amaral, 1981). These include direct projections to nuclei that regulate the rhythm of respiration including the parabrachial nucleus and preBötzinger complex, as well as projections to a site in the midline of the medulla that has been shown to elicit apnea (Dutschmann & Dick, 2012; Jia, Zhang, & Wan, 2005; Verner, Pilowsky, & Goodchild, 2008; Yang et al., 2020). The central nucleus also innervates nuclei that mediate sympathetic arousal, including asphyxia-induced and CO2-induced arousal; these include projections to adrenergic and noradrenergic neurons in the rostral ventrolateral medulla, locus coeruleus, and the dorsal motor nucleus of the vagus (Cassel & Gray, 1989; Guyenet & Abbott, 2013; Liu et al., 2021; Wallace, Magnuson, & Gray, 1992). The central nucleus has additional projections to the dorsal periaqueductal gray (PAG), dorsal raphe, as well as the lateral and dorsomedial hypothalamus, regions which are integrally involved in orchestrating the full repertoire of defensive behaviors that characterize states of fear, anxiety, and panic including freezing, avoidance, and escape (Del-Ben & Graeff, 2009; Keifer et al., 2015; Spiacci, Coimbra, & Zangrossi, 2012; Schimitel et al., 2012; Weera et al., 2021; Weissbourd et al., 2014). In terms of chemoreception, the central nucleus has projections to both the retrotrapezoid nucleus (Rosin, Chang, & Guyenet, 2006) and the medullary raphe (Mason, 2001; Richerson, 2004), the primary sites of the brain’s central chemoreceptors (Nattie & Li, 2012). It also projects to the NTS (Saha, Batten, & Henderson, 2000), a region which is the primary recipient of signals from peripheral chemoreceptors (Zoccal et al., 2014) and vagal sensory afferents from the lungs (Chang et al., 2015). This unique pattern of connectivity combined with strong GABAergic projections make the central nucleus of the amygdala ideally situated to: (1) inhibit respiration, (2) inhibit chemoreceptive awareness for elevations in CO2, and (3) inhibit the associated fear and arousal spurred by CO2-induced suffocation alarms.
Apnea-induced Anxiety
In the current section we discuss how amygdala activity fits within a model of Apnea-induced Anxiety (AiA). The AiA model predicts that similar to the neurosurgical stimulation studies, states of apnea are being unconsciously triggered by amygdala activation, resulting in transient spikes in CO2 that can provoke anxiety and lead to avoidance behavior. We hypothesize that varying degrees of AiA is happening on a recurring basis in day-to-day life in all individuals who have a functioning amygdala capable of inhibiting downstream structures within the brainstem. The basis of the model is that once amygdala activation surpasses a certain threshold, breathing will be inhibited and a transient episode of apnea will be triggered. One prediction stemming from this model is that individuals who have a hyperactive amygdala (Swartz, Knodt, Radtke & Hariri, 2015), especially patients with clinical anxiety (Bruehl, Delsignore, Komossa, & Weidt, 2014; Etkin & Wager, 2007; Hayes, Hayes, & Mikedis, 2012; Ipser, Singh, & Stein, 2013), will be most at risk of having recurrent episodes of amygdala-driven apnea. Like the neurosurgical stimulation studies, the model predicts that most individuals will be entirely unaware of the fact that their breathing has temporarily stopped. Their first sign of disturbance would only become apparent after amygdala activation had subsided and its inhibition over the brainstem was lifted, at which point they would resume breathing but would suddenly feel anxious due to the unforeseen build-up of CO2 generated during the episode of apnea. From this perspective, the well-documented hypersensitivity that anxious patients have in response to CO2 may not entirely be due to suffocation false alarms, as originally theorized (Klein, 1993), but rather real alarms firing to transient spikes in CO2 caused by recurring episodes of amygdala-driven apnea (Figure 3).
Figure 3. A model of Apnea-induced Anxiety.
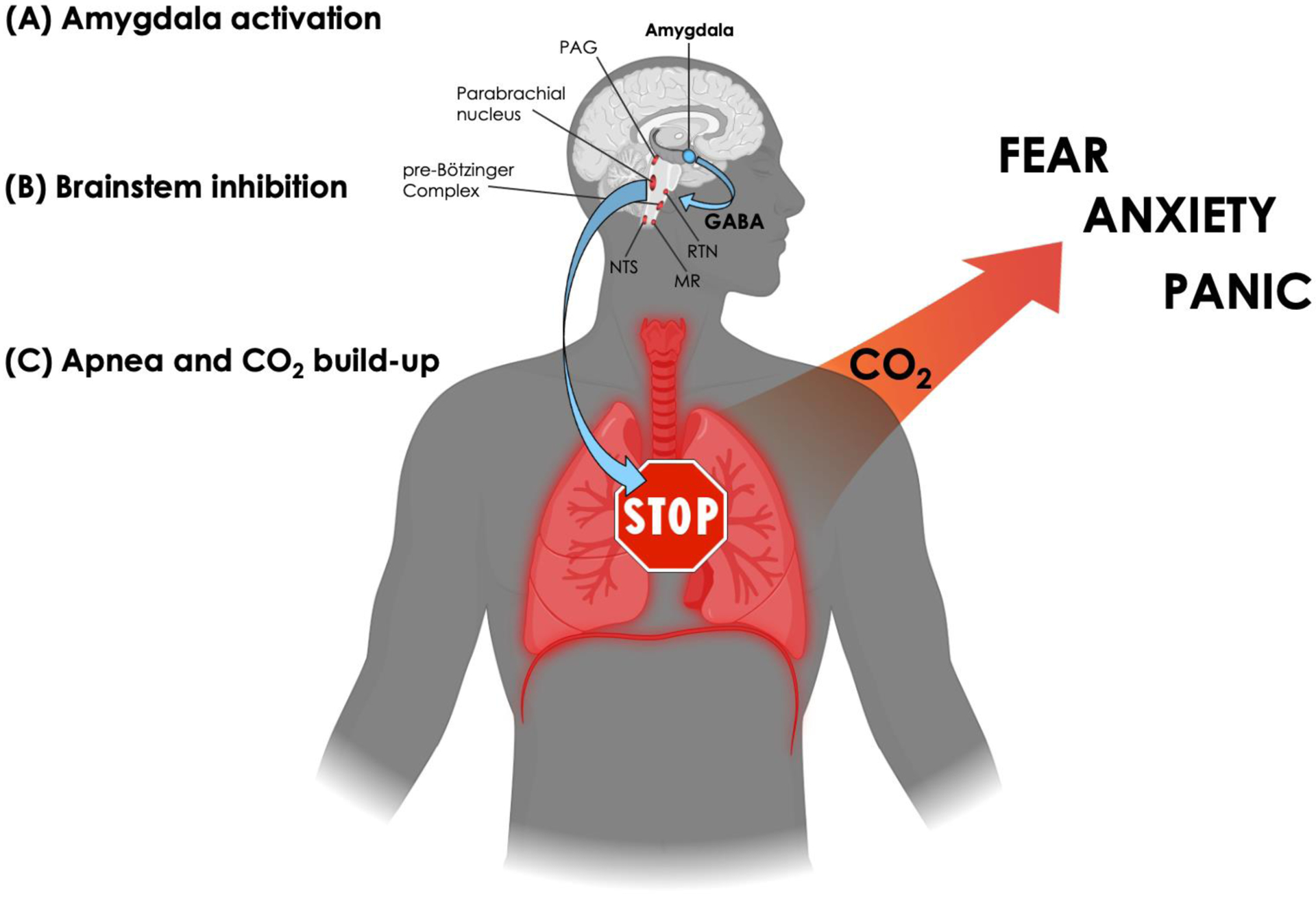
(A) Amygdala activation. The model applies to situations in which amygdala activation surpasses the necessary threshold to elicit apnea. Since a myriad of emotionally laden stimuli can activate the amygdala (Costafreda et al., 2008), the model allows for the possibility that any individual, healthy or anxious, could find themselves in a situation that triggers an episode of apnea.
(B) Brainstem inhibition. The central nucleus of the amygdala will flood downstream targets in the brainstem with GABA, leading to inhibition over respiratory-related nuclei that regulate the rate of breathing (including the periaqueductal gray (PAG) in the midbrain, the parabrachial nucleus in the pons, and the pre-Bötzinger complex in the medulla) as well as inhibition over chemoreceptive-related nuclei that sense changes in CO2 (both centrally in the retrotrapezoid nucleus (RTN) and medullary raphe (MR), and peripherally in the nucleus tractus solitarii (NTS) via projections from the carotid and aortic bodies).
(C) Apnea and CO2 build-up. As inhibition over brainstem respiratory centers accumulates, breathing eventually stops, leading to a transient state of apnea and rising levels of CO2. However, due to amygdalar inhibition over chemoreceptive centers in the brainstem, the individual remains unaware of the CO2 build-up and unaware that their breathing has stopped. Once amygdala activation subsides, and inhibition over the brainstem is lifted, chemoreceptive awareness and respiratory drive returns. The unforeseen build-up of CO2 generated during the episode of apnea rapidly activates suffocation alarms, triggering a state of anxiety characterized by varying degrees of fear and panic, hyperventilation, and avoidance/escape behavior. (Created with BioRender.com)
The amygdala’s ability to elicit apnea could be an evolutionarily determined manifestation of the broader freezing response that the amygdala is well-known to coordinate (Janak & Tye, 2015). For instance, when central amygdala circuits receive signals of imminent threat, respiration might be reflexively paused to help the prey avoid being detected by the predator. Given the amygdala’s strong inhibitory input over key respiratory and chemoreceptive nuclei, this arrest of respiration can be rapidly enacted and sustained, even as CO2 levels escalate. The amygdala-lesioned patients may have lost this ability, as their lesion has effectively severed the amygdala’s inhibitory connections to the brainstem. As a consequence, they experience a disinhibited state of fear to elevations in CO2 characterized by excessive escape behavior and hyperventilation. When queried afterwards, the lesion patients all reported that the feelings induced by CO2 could be best described as a profound fear of suffocation (Feinstein et al., 2013).
These observations support aspects of Donald Klein’s suffocation false alarm theory of spontaneous panic, which hypothesizes that “a physiologic misinterpretation by a suffocation monitor misfires an evolved suffocation alarm system. This produces sudden respiratory distress followed swiftly by a brief hyperventilation, panic, and the urge to flee. Carbon dioxide hypersensitivity is seen as due to the deranged suffocation alarm monitor” (Klein, 1993, p. 306). The data from the lesion patients as well as the intracranial stimulation studies all point to the amygdala as being integrally involved in monitoring and regulating suffocation alarms emerging from chemoreceptive centers in the brainstem. However, the proposed physiologic misinterpretation that leads to “false” suffocation alarms may not be a misfire after all, but rather, an entirely appropriate response to an actual alarm. In other words, the amygdala could be triggering episodes of apnea outside of one’s awareness, and the subsequent build-up of CO2 would activate chemoreceptors and trigger the suffocation alarm system. This reinterpretation of Klein’s theory suggests that CO2 hypersensitivity may actually be a byproduct of real suffocation alarms being repeatedly incited by episodes of amygdala-driven apnea.
CO2 hypersensitivity across the spectrum of anxiety
According to the AiA model, CO2 hypersensitivity likely develops over time as a consequence of recurring episodes of amygdala-driven apnea leading to hypercapnia. Due to the amygdala’s inhibition over the chemoreceptive system during the actual episode of apnea, hypercapnic states would seem to suddenly emerge out of the blue, after amygdala activation has subsided and its inhibition over the brainstem has lifted. This in turn triggers a much larger suffocation alarm than if the chemoreceptors’ warnings had been heeded during the initial moments of apnea. Over time, these unforeseen homeostatic disruptions to the acid-base balance may cause the chemoreceptive system to respond disproportionately to small increases in CO2, such that even short periods of apnea can induce feelings of anxiety and suffocation fear in anxious individuals (such as during a voluntary breath hold: Asmundson & Stein, 1994; Lapidus et al., 2020). Moreover, since a key component of anxiety is apprehensive worry about uncertain events in the future, the repeated occurrence of unexpected hypercapnic states would undoubtedly heighten one’s level of apprehension, leading to more anxiety and greater levels of hypersensitivity toward CO2.
CO2 hypersensitivity is most commonly tested by having patients inhale an air mixture containing elevated levels of CO2. This simple procedure will often trigger anxiety across the spectrum including in patients with panic disorder (Colasanti et al., 2012; Rassovsky & Kushner, 2003), posttraumatic stress disorder (Kellner et al., 2018; Muhtz et al., 2011; Telch et al., 2012), and social anxiety disorder (Blechert et al., 2010; Caldirola et al., 1997; Gorman et al., 1990). Importantly, in the studies above, the demographically-matched healthy participants experienced significantly less anxiety than the patients when exposed to the same exact dose of CO2, confirming that the anxious patients’ CO2-response was indeed hypersensitive. In addition, patients will often report that the feelings induced by CO2 are remarkably similar to the anxiety and panic that they experience in everyday life (Rapee et al., 1992; Schruers et al., 2004). This same observation was actually highlighted in one of the first studies to ever expose anxious patients to CO2 (Cohen & White, 1951), with the patients reporting that the feeling induced by CO2 was “identical” to the surges in anxiety that they felt in everyday life.
The fact that CO2 induces anxiety across the spectrum of anxiety disorders is sometimes overshadowed by early research efforts that aimed to use CO2-reactivity as a specific marker for panic disorder. In these early studies, the primary outcome was usually focused on whether or not the manipulation provoked a panic attack, often times using high doses of 35% CO2 (Colasanti et al., 2012; Perna et al., 1995; Rassovsky & Kushner, 2003; Verburg et al., 1998). This panicogenic focus may have caused the field to overlook CO2’s ability to induce less intense forms of acute anxiety. Indeed, one study found that the clearest way to discriminate panic disordered patients from healthy controls during a 35% CO2 challenge was using an anxiety scale rather than the number or intensity of induced symptoms of panic (Battaglia & Perna, 1995); a modest 20-point change in ratings of subjective anxiety on a 100-point visual analogue scale proved to be the ideal threshold for differentiating the two groups.
CO2 is not just a panicogen, but it is also anxiogenic, especially at lower doses. Studies that have utilized lower doses of CO2 (ranging from 5.5% to 20%) have all found that CO2 induces heightened levels of anxiety in anxious patients and little to no anxiety in healthy participants (Blechert et al., 2010; Rapee et al., 1992; Seddon et al., 2011). These studies lend support to a continuum model of CO2 sensitivity. On one end of the continuum are healthy individuals who report minimal anxiety in response to CO2, especially healthy participants with no family history of panic disorder (Colasanti et al., 2012). In the middle of the continuum are patients with generalized anxiety disorder and social phobia, who typically report mild-to-moderate levels of anxiety when exposed to CO2 (Blechert et al., 2010; Rapee et al., 1992; Seddon et al., 2011). At the extreme end of the continuum are patients with panic disorder and agoraphobia who will often report the highest levels of anxiety to CO2 (Blechert et al., 2010; Colasanti et al., 2012; Rapee et al., 1992; Rassovsky & Kushner, 2003). Regardless of diagnosis, we postulate that individuals who have a hypersensitivity to CO2 will attempt to minimize chemoreceptive disturbances by deploying a range of strategies to prevent hypercapnia. These compensatory strategies (described in the subsequent sections) may help abate hypercapnia in the short-term, but over time will lead to maladaptive outcomes that may contribute to the chronicity of anxiety disorders.
CO2-induced avoidance behavior
Heightened experiences of fear and anxiety and the pervasive avoidance of stimuli capable of inducing such experiences are perhaps the two most common themes that unite all anxiety disorders. As the previous section described, CO2 inhalation elicits a heightened experience of fear and anxiety across the spectrum of anxiety disorders. As it turns out, CO2 is also a potent stimulus for eliciting avoidance behavior. The innate avoidance triggered by CO2 appears to be evolutionarily hardwired into the nervous system and conserved across species. Drosophila fruit flies have specialized olfactory sensory neurons that are able to detect minute changes in the outside levels of CO2 in their local environment, and rapidly trigger a change in flight pattern in order to avoid that area of air space (Suh et al., 2004; Suh et al., 2007). Similar patterns of innate avoidance to environments containing high levels of CO2 can be found in species ranging from C. elegans (Bretscher et al., 2011) to rats (Améndola & Weary, 2020; Kirkden et al., 2008). In humans, although speculative, the AiA model highlights the possibility that certain patterns of avoidance behavior are actually a byproduct of the specific situations and circumstances that activate one’s amygdala and trigger episodes of apnea and spikes in CO2. And similar to the fruit fly, heightened levels of CO2 may motivate the anxious patient to escape from their surrounding environment. For example, in the case of social phobia, the AiA model would predict that social contexts would most readily stimulate states of amygdala-driven apnea, and the subsequent increases in CO2 would provoke socially anxious patients to escape from these environments and avoid them in the future. This largely unconscious process of attributing internal suffocation alarms to external environmental cues is really a form of fear generalization that could lead to avoidance of a multitude of different stimuli and contexts (McMurray et al., 2020), culminating in the widespread pattern of avoidance characteristic of agoraphobia.
Hyperventilation to minimize AiA
Individuals struggling to adapt to the repeated and unforeseen buildups of CO2 stemming from AiA may compensate by simply lowering their baseline level of PCO2 so that future spikes are less likely to elicit a full-blown suffocation alarm. Consistent with this prediction, it has long been known that anxious patients have a tendency toward hyperventilation at rest (Kaplan, 1997; Saltzman, Heyman, & Sieker, 1963), and patients with panic disorder (Meuret & Ritz, 2010), as well as other types of anxiety disorders (Henje Blom et al., 2014; Van den Hout et al., 1992), have been found to have low resting end-tidal CO2. This has led to much discussion and debate as to whether hyperventilation is a cause or consequence of panic (Klein, 1993; Ley, 1985; Meuret & Ritz, 2010). According to the AiA model, hyperventilation would be a consequence of recurring episodes of amygdala-driven apnea and a form of chemoreceptive avoidance aimed at reducing the occurrence of chemoreceptors firing following an episode of apnea. Unfortunately, this compensatory strategy is maladaptive and may actually lead to more episodes of apnea, and even greater levels of anxiety and panic. For example, patients with low resting end-tidal CO2 have an even greater panic response to elevations in CO2 than patients with normal levels of CO2 (Papp et al., 1997) and hyperventilation often leads to more apneas rather than less (Meah & Gardner, 1994). Hyperventilation can also acutely induce symptoms of panic, including heart palpitations, shortness of breath, dizziness, trembling, tingling or numbness in the extremities, and depersonalization/derealization (Meuret & Ritz, 2010). And prolonged hyperventilation can lead to an enduring state of hypocapnia and a host of health issues (Meuret, Kroll, & Ritz, 2017), including exacerbation of anxiety (Brashear, 1983). In addition, low baseline levels of CO2 across the spectrum of anxiety disorders is a predictor of poor treatment response to psychotherapy (Davies & Craske, 2014; Tolin et al., 2017), highlighting the need for a more targeted treatment approach. Remarkably, when such an approach was undertaken by combining capnometry-assisted biofeedback with hypoventilation breathing training, not only were panic disordered patients able to normalize their levels of end-tidal CO2, but by doing so they were able to significantly reduce their symptoms of panic disorder (Meuret, Wilhelm, Ritz, & Roth, 2008).
Amygdala volumetric reductions in anxious psychopathology
A cursory review of the literature examining amygdala volumes in clinically anxious patients shows that the notion of a larger amygdala being associated with greater levels of fear and anxiety is not just overly simplistic, but in many cases opposite to reality. This was most evident in panic disorder, where four studies showed that panic patients have significantly smaller amygdala volumes than demographically-matched healthy individuals (Asami et al., 2018; Hayano et al., 2009; Massana et al., 2003; Yoon et al, 2016). Smaller amygdala volumes were also found in a group of participants with spider phobia (Fisler et al., 2013). The findings were mixed with regard to generalized anxiety disorder and social anxiety disorder, with some studies showing significant decreases in amygdala volume (Irle et al., 2010; Milham et al., 2005) and others showing significant increases or no significant differences (De Bellis et al., 2000; Machado-de-Sousa et al., 2014; Suor et al., 2020). In general, most of these studies were underpowered, with average sample sizes ranging between 20 to 30 participants per group. A meta-analysis of studies in patients with major depression (which is often comorbid with anxiety), found significantly reduced amygdala volumes in depressed patients who were unmedicated (Hamilton et al., 2008). More recently, an analysis of nearly 1000 individuals with and without alcohol use disorder (which is also highly comorbid with anxiety), found that alcohol-dependent males had significantly smaller amygdala volumes, especially in the basolateral nucleus (Grace et al., 2021). There is also a high comorbidity between asthma and panic disorder (Meuret & Ritz, 2010; Meuret, Kroll, & Ritz, 2017), and it was recently found that asthmatics with smaller amygdala volumes tended to catastrophize more about future asthma attacks and reported less ability to control their asthma (Ritz et al., 2019). Smaller amygdala volumes were also found in autistic children, but only in those who had clinically significant anxiety (Herrington et al., 2017). Finally, with regard to trauma exposure, there is further evidence of decreased amygdala volume, both in children exposed to trauma (Hanson & Nacewicz, 2021; Nogovitsyn et al., 2020; Weissman et al., 2020), as well as in adults with posttraumatic stress disorder (Morey et al., 2012; O’Doherty et al., 2015). Thus, across the spectrum of anxiety disorders, as well as in other disorders that are often comorbid with anxiety, a relatively consistent pattern of reduced amygdala volume has been found. The etiology, cellular mechanism, and developmental trajectory underlying these volumetric reductions remains to be determined. It will also be important for future studies to examine the volume of specific amygdala subnuclei, especially the central nucleus given its direct projections to the brainstem. Based on the AiA model and the data from the amygdala-lesioned patients, it seems plausible that a smaller amygdala volume would confer less downstream inhibition over brainstem chemoreceptive centers, making it harder to inhibit suffocation alarms. Viewed in this light, the smaller amygdala volumes found in clinically anxious patients may be a neural marker for CO2 hypersensitivity.
Evidence of apnea in real-world anxiogenic contexts
The AiA model predicts that chronically anxious individuals will experience frequent episodes of apnea induced centrally via the amygdala, especially when exposed to anxiogenic contexts. Of note, this centrally-induced form of apnea during waking states is quite different than the apnea that occurs during sleep, which is most commonly caused by an obstruction of the airways and also appears to be associated with anxiety (Garbarino et al., 2020). While evidence for amygdala hyperactivity has been found across the spectrum of anxiety disorders (Bruehl et al., 2014; Etkin & Wager, 2007; Hayes, Hayes, & Mikedis, 2012; Ipser, Singh, & Stein, 2013), very little research has examined whether recurring episodes of apnea are also evident in anxious populations. There are, however, some notable exceptions. An ambulatory monitoring study assessing individuals with flying phobia while onboard an actual airplane found evidence of increased pauses in breathing, sometimes lasting up to 20 seconds (Wilhelm & Roth, 1998). A sleep study conducted in a group of patients with panic disorder found an increased rate of “microapneas” entailing brief 5–10 second pauses in breathing (Stein et al., 1995). Another study in patients with panic disorder found evidence of frequent “breathing pauses” lasting anywhere between 20–60 seconds (Bystritsky et al., 2000). Notably, the pauses were longest in those patients who experienced panic attacks during a subsequent CO2 challenge. These data add to other research showing marked breathing irregularities in panic disorder (Papp et al., 1997), including hyperventilation (Meuret & Ritz, 2010) and frequent sighs (Abelson et al., 2001), which might be part of a compensatory response to help clear the build-up of CO2 following an episode of apnea. Another ambulatory monitoring study was able to capture a series of panic attacks in a group of patients with panic disorder (Meuret et al., 2011). Shortly before the onset of the panic attack, they found significant decreases in tidal volume followed by abrupt increases in PCO2. In contrast, the panic attack itself was characterized by heart rate and tidal volume increases and a corresponding reduction in PCO2. Although the occurrence of apnea was not specifically analyzed, it remains possible that the noted drop in tidal volume and increase in PCO2 that preceded a panic attack was actually triggered by episodes of apnea.
There have also been real-world studies using ambulatory monitoring in healthy individuals that found a significant increase in patterns of “sustained inhibitory breathing” while participants were in anxiogenic settings such as at work or during social gatherings as compared to when they were alone (Anderson et al., 1992). Interestingly, these patterns of inhibitory breathing were also associated with higher blood pressure (Anderson et al., 1993), which is in line with other studies that have found frequent episodes of apnea of 10–20 seconds in duration in individuals with hypertension (Anderson et al., 2008; Novak et al., 1994; Wu et al., 2016), suggesting that even short periods of apnea are sufficient to elicit hypercapnia-induced increases in blood pressure. There have also been anecdotal reports of apnea arising while reading emails or engaging in social media, with one empirical study finding evidence that individuals “held their breath” at the onset of receiving a text message (Lin & Peper, 2009). Taken together, these initial findings suggest that apneas can spontaneously occur in everyday life, especially in anxiogenic contexts. And although it was not explicitly explored in these prior studies, it remains possible that most individuals were entirely unaware of the fact that their breathing had stopped.
It is important to emphasize that the proposed model of AiA is still speculative and there are many unknowns that need to be tested. While the above studies provide initial evidence that episodes of apnea can occur in everyday life, they also highlight the importance of utilizing ambulatory monitoring to assess respiration in real-world anxiogenic contexts where AiA is most likely to occur. Future studies will need to conduct more granular analyses on the respiratory data to discern the presence, frequency, and duration of the apnea episodes. In addition, the above studies all lacked a clear definition of apnea making it difficult to determine whether a true episode of apnea was actually induced. In the sleep apnea literature, apnea is defined as a period of breathing cessation that is ≥ 10 seconds based on a drop in the peak signal excursion by ≥ 90% of pre-event baseline (Berry et al., 2012), and a similar definition could potentially be applied to future ambulatory monitoring studies. Finally, experience sampling will need to be collected in conjunction with ambulatory monitoring in order to ascertain whether the episodes of apnea are temporally-linked to fluctuations in state anxiety as the AiA model predicts.
Treatment implications of AiA
In the event that AiA is a real phenomenon being surreptitiously driven by amygdala hyperactivity, then new interventions could be readily developed to interrupt or prevent the apnea before it cascades into CO2-induced anxiety. Wireless wearable sensors could be used to identify apneas, relaying respiratory-associated signals to monitoring algorithms that can quickly alert the patient whenever an episode of apnea or hypercapnia is detected. For example, a wireless and portable pulse oximeter could send real-time feedback using vibrations or sounds anytime levels of O2 saturation drop, alerting the patient to resume breathing. Likewise, a portable capnometry system could detect sudden spikes in end-tidal CO2 and alert the patient to exhale before anxiety escalates or to breathe slower if abnormally low levels of CO2 were detected (Meuret & Ritz, 2021). Devices that incorporate accelerometry or respiratory inductive plethysmography into the fabric of one’s shirt may be able to rapidly detect an apnea at its onset. One noteworthy observation from the recent neurosurgical stimulation studies found that patients could stop amygdala-driven apnea simply by purposefully breathing through their mouth (Nobis et al., 2018), suggesting that there are regulatory circuits that can override the amygdala’s inhibition over nasal breathing. Tapping into these alternate respiratory circuits could potentially help overcome AiA, but real-time feedback will be imperative since most people will be unaware that their breathing has stopped.
Conclusion
The physiological processes driving the chronic and unrelenting nature of anxiety remain elusive. Here we outlined the parameters of Apnea-induced Anxiety (AiA), a neurobiological model that attempts to provide a plausible explanation for the chronicity of anxiety disorders. The model is predicated on the newly discovered ability of the amygdala to induce apnea and inhibit suffocation fear. This dual brain-behavior relationship is not well-known in the fields of psychiatry and psychology, but it can be formally tested in future studies in both humans and non-human animals, and it may have substantial implications with regard to both the etiology and treatment of anxiety.
Acknowledgements
The authors would like to thank Dr. Paul Davenport for his expert consultation on respiratory physiology. This work was partially supported by the William K. Warren Foundation and grants from NIGMS P20GM121312 (JSF and SSK), NIMH K23MH112949 (SSK), and NIMH R01MH127225 (SSK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing financial interests.
References
- Abelson JL, Weg JG, Nesse RM, & Curtis GC (2001). Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry, 49(7), 588–595. 10.1016/S0006-3223(00)01078-7 [DOI] [PubMed] [Google Scholar]
- Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, … & Deisseroth K (2015). Basomedial amygdala mediates top-down control of anxiety and fear. Nature, 527(7577), 179–185. 10.1038/nature15698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Améndola L, & Weary DM (2020). Understanding rat emotional responses to CO2. Translational Psychiatry, 10(1), 1–12. 10.1038/s41398-020-00936-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Austin J, & Haythornthwaite JA (1993). Blood pressure during sustained inhibitory breathing in the natural environment. Psychophysiology, 30(2), 131–137. 10.1111/j.1469-8986.1993.tb01726.x [DOI] [PubMed] [Google Scholar]
- Anderson DE, Coyle K, & Haythornthwaite JA (1992). Ambulatory monitoring of respiration: inhibitory breathing in the natural environment. Psychophysiology, 29(5), 551–557. 10.1111/j.1469-8986.1992.tb02029.x [DOI] [PubMed] [Google Scholar]
- Anderson DE, McNeely JD, Chesney MA, & Windham BG (2008). Breathing variability at rest is positively associated with 24-h blood pressure level. American Journal of Hypertension, 21(12), 1324–1329. 10.1038/ajh.2008.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, & McNall CL (1983). Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiology & Behavior, 31(3), 353–360. 10.1016/0031-9384(83)90201-9 [DOI] [PubMed] [Google Scholar]
- Asami T, Nakamura R, Takaishi M, Yoshida H, Yoshimi A, Whitford TJ, & Hirayasu Y (2018). Smaller volumes in the lateral and basal nuclei of the amygdala in patients with panic disorder. PLoS ONE, 13(11), e0207163. 10.1371/journal.pone.0207163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, & Stein MB (1994). Triggering the false suffocation alarm in panic disorder patients by using a voluntary breath-holding procedure. The American Journal of Psychiatry, 151(2), 264–266. 10.1176/ajp.151.2.264 [DOI] [PubMed] [Google Scholar]
- Babaev O, Chatain CP, & Krueger-Burg D (2018). Inhibition in the amygdala anxiety circuitry. Experimental & Molecular Medicine, 50(4), 1–16. 10.1038/s12276-018-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, & Michaelis S (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335. 10.31887/dcns.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, & Perna G (1995). The 35% CO2 challenge in panic disorder: optimization by receiver operating characteristic (ROC) analysis. Journal of Psychiatric Research, 29(2), 111–119. 10.1016/0022-3956(94)00045-S [DOI] [PubMed] [Google Scholar]
- Battisti‐Charbonney A, Fisher J, & Duffin J (2011). The cerebrovascular response to carbon dioxide in humans. The Journal of Physiology, 589(12), 3039–3048. 10.1113/jphysiol.2011.206052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Vos T, Scott KM, Ferrari AJ, & Whiteford HA (2014). The global burden of anxiety disorders in 2010. Psychological Medicine, 44(11), 2363–2374. 10.1017/S0033291713003243 [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, & Damasio AR (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269(5227), 1115–1118. 10.1126/science.7652558 [DOI] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, … & Hurlemann R (2012). Fear processing and social networking in the absence of a functional amygdala. Biological Psychiatry, 72(1), 70–77. 10.1016/j.biopsych.2011.11.024 [DOI] [PubMed] [Google Scholar]
- Berntson GG, & Khalsa SS (2021). Neural circuits of interoception. Trends in Neurosciences, 44(1), 17–28. 10.1016/j.tins.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, … & Tangredi MM (2012). Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Journal of Clinical Sleep Medicine, 8(5), 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, & Blanchard RJ (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology, 81(2), 281–290. 10.1037/h0033521 [DOI] [PubMed] [Google Scholar]
- Blechert J, Wilhelm FH, Meuret AE, Wilhelm EM, & Roth WT (2010). Respiratory, autonomic, and experiential responses to repeated inhalations of 20% CO2 enriched air in panic disorder, social phobia, and healthy controls. Biological Psychology, 84(1), 104–111. 10.1016/j.biopsycho.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear RE (1983). Hyperventilation syndrome. Lung, 161(1), 257–273. 10.1007/BF02713872 [DOI] [PubMed] [Google Scholar]
- Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, & de Bono M (2011). Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron, 69(6), 1099–1113. 10.1016/j.neuron.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews, 47(1), 260–280. 10.1016/j.neubiorev.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Buchanan GF (2019). Impaired CO2-induced arousal in SIDS and SUDEP. Trends in Neurosciences, 42(4), 242–250. 10.1016/j.tins.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A, Craske M, Maidenberg E, Vapnik T, & Shapiro D (2000). Autonomic reactivity of panic patients during a CO2 inhalation procedure. Depression and Anxiety, 11(1), 15–26. [DOI] [PubMed] [Google Scholar]
- Caldirola D, Perna G, Arancio C, Bertani A, & Bellodi L (1997). The 35% CO2 challenge test in patients with social phobia. Psychiatry Research, 71(1), 41–48. 10.1016/S0165-1781(97)00038-3 [DOI] [PubMed] [Google Scholar]
- Cassell MD, & Gray TS (1989). The amygdala directly innervates adrenergic (C1) neurons in the ventrolateral medulla in the rat. Neuroscience Letters, 97(1–2), 163–168. 10.1016/0304-3940(89)90157-2 [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, & Shi C (1999). The intrinsic organization of the central extended amygdala. Annals of the New York Academy of Sciences, 877(1), 217–241. 10.1111/j.1749-6632.1999.tb09270.x [DOI] [PubMed] [Google Scholar]
- Chang C, & Glover GH (2009). Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage, 47(4), 1381–1393. 10.1016/j.neuroimage.2009.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, & Liberles SD (2015). Vagal sensory neuron subtypes that differentially control breathing. Cell, 161(3), 622–633. 10.1016/j.cell.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, … & Lüthi A (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Cohen ME, & White PD (1951). Life situations, emotions, and neurocirculatory asthenia (anxiety neurosis, neurasthenia, effort syndrome). Psychosomatic Medicine, 13(6), 335–357. 10.1097/00006842-195111000-00001 [DOI] [PubMed] [Google Scholar]
- Colasanti A, Esquivel G, J Schruers K, & J Griez E (2012). On the psychotropic effects of carbon dioxide. Current Pharmaceutical Design, 18(35), 5627–5637. 10.2174/138161212803530745 [DOI] [PubMed] [Google Scholar]
- Collins KA, Westra HA, Dozois DJ, & Burns DD (2004). Gaps in accessing treatment for anxiety and depression: challenges for the delivery of care. Clinical Psychology Review, 24(5), 583–616. 10.1016/j.cpr.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Conde SV, Sacramento JF, & Martins FO (2020). Immunity and the carotid body. Bioelectronic Medicine, 6(24), 1–20. 10.1186/s42234-020-00061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, & Lydiard RB (1998). Brain circuits in panic disorder. Biological Psychiatry, 44(12), 1264–1276. 10.1016/s0006-3223(98)00300-x [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, & Fu CH (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. 10.1016/j.brainresrev.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Davenport PW, & Vovk A (2009). Cortical and subcortical central neural pathways in respiratory sensations. Respiratory Physiology & Neurobiology, 167(1), 72–86. 10.1016/j.resp.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Davies CD, & Craske MG (2014). Low baseline PCO2 predicts poorer outcome from behavioral treatment: Evidence from a mixed anxiety disorders sample. Psychiatry Research, 219(2), 311–315. 10.1016/j.psychres.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15(1), 353–375. 10.1146/annurev.ne.15.030192.002033 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … & Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. 10.1016/S0006-3223(00)00835-0 [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, & Feldman JL (2018). Breathing matters. Nature Reviews Neuroscience, 19(6), 351–367. 10.1038/s41583-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Ben CM, & Graeff FG (2009). Panic disorder: is the PAG involved? Neural Plasticity, 2009(2), 1–9. 10.1155/2009/108135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, … & Richerson GB (2015). Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. Journal of Neuroscience, 35(28), 10281–10289. 10.1523/JNEUROSCI.0888-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J (2005). Role of acid-base balance in the chemoreflex control of breathing. Journal of Applied Physiology, 99(6), 2255–2265. 10.1152/japplphysiol.00640.2005 [DOI] [PubMed] [Google Scholar]
- Dumont ÉC, Martina M, Samson RD, Drolet G, & Paré D (2002). Physiological properties of central amygdala neurons: species differences. European Journal of Neuroscience, 15(3), 545–552. 10.1046/j.0953-816x.2001.01879.x [DOI] [PubMed] [Google Scholar]
- Dutschmann M, & Dick TE (2012). Pontine mechanisms of respiratory control. Comprehensive Physiology, 2(4), 2443–2469. 10.1002/cphy.c100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, & Tranel D (2011). The human amygdala and the induction and experience of fear. Current Biology, 21(1), 34–38. 10.1016/j.cub.2010.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, & Tranel D (2016). A tale of survival from the world of Patient SM. In Amaral DG & Adolphs R (Eds.), Living without an amygdala (pp. 1–38). The Guilford Press. [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, … & Wemmie JA (2013). Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience, 16(3), 270–272. 10.1038/nn.3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisler MS, Federspiel A, Horn H, Dierks T, Schmitt W, Wiest R, … & Soravia LM (2013). Spider phobia is associated with decreased left amygdala volume: a cross-sectional study. BMC Psychiatry, 13(1), 1–7. 10.1186/1471-244X-13-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, & Amaral DG (2009). Neuroanatomy of the primate amygdala. In Whalen PJ & Phelps EA (Eds.), The human amygdala (pp. 3–42). The Guilford Press. [Google Scholar]
- Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Bonanni E, & Magnavita N (2020). Association of anxiety and depression in obstructive sleep apnea patients: a systematic review and meta-analysis. Behavioral Sleep Medicine, 18(1), 35–57. 10.1080/15402002.2018.1545649 [DOI] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, … & Barhanin J (2010). Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proceedings of the National Academy of Sciences, 107(5), 2325–2330. 10.1073/pnas.0910059107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, & Coplan JD (2000). Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry, 157(4), 493–505. 10.1176/appi.ajp.157.4.493 [DOI] [PubMed] [Google Scholar]
- Gorman JM, Papp LA, Martinez J, Goetz RR, Hollander E, Liebowitz MR, & Jordan F (1990). High-dose carbon dioxide challenge test in anxiety disorder patients. Biological Psychiatry, 28(9), 743–757. 10.1016/0006-3223(90)90510-9 [DOI] [PubMed] [Google Scholar]
- Grace S, Rossetti MG, Allen N, Batalla A, Bellani M, Brambilla P, … & Lorenzetti V (2021). Sex differences in the neuroanatomy of alcohol dependence: hippocampus and amygdala subregions in a sample of 966 people from the ENIGMA Addiction Working Group. Translational Psychiatry, 11(156), 1–15. 10.1038/s41398-021-01204-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessner J, Pasieka M, Böhm V, Grössl F, Kaczanowska J, Pliota P, … & Haubensak W (2018). Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Molecular Psychiatry, 26(2), 534–544. 10.1038/s41380-018-0310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griez E, Van den Hout MA, & Verstappen F (1987). Body fluids after CO2 inhalation: Insight into panic mechanisms? European Archives of Psychiatry and Neurological Sciences, 236(6), 369–371. 10.1007/BF00377427 [DOI] [PubMed] [Google Scholar]
- Guyenet PG, & Abbott SB (2013). Chemoreception and asphyxia-induced arousal. Respiratory Physiology and Neurobiology, 188(3), 333–343. 10.1016/j.resp.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, & Bayliss DA (2015). Neural control of breathing and CO2 homeostasis. Neuron, 87(5), 946–961. 10.1016/j.neuron.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, & Gotlib IH (2008). Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13(11), 993–1000. 10.1038/mp.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, & Nacewicz BM (2021). Amygdala allostasis and early life adversity: considering excitotoxicity and inescapability in the sequelae of stress. Frontiers in Human Neuroscience, 15(624705), 1–23. 10.3389/fnhum.2021.624705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Frysinger RC, Trelease RB, & Marks JD (1984). State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Research, 306(1–2), 1–8. 10.1016/0006-8993(84)90350-0 [DOI] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, … & Hirayasu Y. (2009). Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry and Clinical Neurosciences, 63(3), 266–276. 10.1111/j.1440-1819.2009.01960.x [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1), 1–13. 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson Y (1940). Carbon dioxide. Cyclopedia of Medicine (Vol. 3). Philadelphia, PA: F. A. Davis. [Google Scholar]
- Henje Blom E, Serlachius E, Chesney MA, & Olsson EM (2014). Adolescent girls with emotional disorders have a lower end‐tidal CO2 and increased respiratory rate compared with healthy controls. Psychophysiology, 51(5), 412–418. 10.1111/psyp.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Maddox BB, Kerns CM, Rump K, Worley JA, Bush JC, … & Miller JS (2017). Amygdala volume differences in autism spectrum disorder are related to anxiety. Journal of Autism and Developmental Disorders, 47(12), 3682–3691. 10.1007/s10803-017-3206-1 [DOI] [PubMed] [Google Scholar]
- Ipser JC, Singh L, & Stein DJ (2013). Meta‐analysis of functional brain imaging in specific phobia. Psychiatry and Clinical Neurosciences, 67(5), 311–322. 10.1111/pcn.12055 [DOI] [PubMed] [Google Scholar]
- Irle E, Ruhleder M, Lange C, Seidler-Brandler U, Salzer S, Dechent P, … & Leichsenring F (2010). Reduced amygdalar and hippocampal size in adults with generalized social phobia. Journal of Psychiatry & Neuroscience, 35(2), 126–131. 10.1503/jpn.090041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J, Chapleau MW, & Somers VK (2021). Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiological Reviews, 101(3), 1177–1235. 10.1152/physrev.00039.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH (1899). On asphyxia in slight epileptic paroxysms. On the symptomatology of slight epileptic fits supposed to discharge-lesions of uncinate gyrus. Lancet, 1(1), 79–80. [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HG, Zhang GY, & Wan Q (2005). A GABAergic projection from the central nucleus of the amygdala to the parabrachial nucleus: an ultrastructural study of anterograde tracing in combination with post-embedding immunocytochemistry in the rat. Neuroscience Letters, 382(1–2), 153–157. 10.1016/j.neulet.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Jongen‐Rêlo AL, & Amaral DG (1998). Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. European Journal of Neuroscience, 10(9), 2924–2933. 10.1111/j.1460-9568.1998.00299.x [DOI] [PubMed] [Google Scholar]
- Kaada BR, & Jasper H (1952). Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Archives of Neurology & Psychiatry, 68(5), 609–619. 10.1001/archneurpsyc.1952.02320230035004 [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience, 24(24), 5506–5515. 10.1523/JNEUROSCI.0292-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan NM (1997). Anxiety-induced hyperventilation: a common cause of symptoms in patients with hypertension. Archives of Internal Medicine, 157(9), 945–948. 10.1001/archinte.1997.00440300023002 [DOI] [PubMed] [Google Scholar]
- Keifer OP Jr, Hurt RC, Ressler KJ, & Marvar PJ (2015). The physiology of fear: reconceptualizing the role of the central amygdala in fear learning. Physiology, 30(5), 389–401. 10.1152/physiol.00058.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner M, Muhtz C, Nowack S, Leichsenring I, Wiedemann K, & Yassouridis A (2018). Effects of 35% carbon dioxide (CO2) inhalation in patients with post-traumatic stress disorder (PTSD): A double-blind, randomized, placebo-controlled, cross-over trial. Journal of Psychiatric Research, 96(1), 260–264. 10.1016/j.jpsychires.2017.10.019Get [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, … & Merikangas KR (2012). Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 69(4), 372–380. 10.1001/archgenpsychiatry.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, … & Zucker N (2018). Interoception and mental health: a roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 501–513. 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, & Hurlemann R (2016). Panic anxiety in humans with bilateral amygdala lesions: pharmacological induction via cardiorespiratory interoceptive pathways. Journal of Neuroscience, 36(12), 3559–3566. 10.1523/JNEUROSCI.4109-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Horovitz O, Pellman BA, Tan LM, Li Q, Richter-Levin G, & Kim JJ (2013). Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proceedings of the National Academy of Sciences, 110(36), 14795–14800. 10.1073/pnas.1310845110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkden RD, Niel L, Lee G, Makowska IJ, Pfaffinger MJ, & Weary DM (2008). The validity of using an approach-avoidance test to measure the strength of aversion to carbon dioxide in rats. Applied Animal Behaviour Science, 114(1–2), 216–234. 10.1016/j.applanim.2008.03.001 [DOI] [Google Scholar]
- Klein DF (1993). False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Archives of General Psychiatry, 50(4), 306–317. 10.1001/archpsyc.1993.01820160076009 [DOI] [PubMed] [Google Scholar]
- Lacuey N, Hampson JP, Harper RM, Miller JP, & Lhatoo S (2019). Limbic and paralimbic structures driving ictal central apnea. Neurology, 92(7), e655–e669. 10.1212/WNL.0000000000006920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Londono L, & Lhatoo SD (2017). Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology, 88(7), 701–705. 10.1212/WNL.0000000000003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus RC, Puhl M, Kuplicki R, Stewart JL, Paulus MP, Rhudy JL, … & Tulsa 1000 Investigators. (2020). Heightened affective response to perturbation of respiratory but not pain signals in eating, mood, and anxiety disorders. PLoS ONE, 15(7), e0235346. 10.1371/journal.pone.0235346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: a two-system framework. American Journal of Psychiatry. 173(11), 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Ley R (1985). Blood, breath, and fears: A hyperventilation theory of panic attacks and agoraphobia. Clinical Psychology Review, 5(4), 271–285. 10.1016/0272-7358(85)90008-X [DOI] [Google Scholar]
- Lin IM, & Peper E (2009). Psychophysiological patterns during cell phone text messaging: A preliminary study. Applied Psychophysiology and Biofeedback, 34(1), 53–57. 10.1007/s10484-009-9078-1 [DOI] [PubMed] [Google Scholar]
- Liu J, Hu T, Zhang MQ, Xu CY, Yuan MY, & Li RX (2021). Differential efferent projections of GABAergic neurons in the basolateral and central nucleus of amygdala in mice. Neuroscience Letters, 745(2), 135621. 10.1016/j.neulet.2020.135621 [DOI] [PubMed] [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, & Craske MG (2015). Response rates for CBT for anxiety disorders: Need for standardized criteria. Clinical Psychology Review, 42, 72–82. 10.1016/j.cpr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Machado-de-Sousa JP, de Lima Osório F, Jackowski AP, Bressan RA, Chagas MH, Torro-Alves N, … & Hallak JE (2014). Increased amygdalar and hippocampal volumes in young adults with social anxiety. PLoS ONE, 9(2), e88523. 10.1371/journal.pone.0088523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincovich A, Bravo E, Dlouhy B, & Richerson GB (2019). Amygdala lesions reduce seizure-induced respiratory arrest in DBA/1 mice. Epilepsy & Behavior, 121(Pt. B), 106440. 10.1016/j.yebeh.2019.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P (2001). Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annual Review of Neuroscience, 24(1), 737–777. 10.1146/annurev.neuro.24.1.737 [DOI] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gastó C, Junqué C, Massana J, … & Salamero M (2003). Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage, 19(1), 80–90. 10.1016/s1053-8119(03)00036-3 [DOI] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, & Richerson GB (2014). Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nature Reviews Neurology, 10(5), 271. 10.1038/nrneurol.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray KM, Gray A, Horn P, & Sah R (2020). High behavioral sensitivity to carbon dioxide associates with enhanced fear memory and altered forebrain neuronal activation. Neuroscience, 429(1), 92–105. 10.1016/j.neuroscience.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meah MS, & Gardner WN (1994). Post‐hyperventilation apnoea in conscious humans. The Journal of Physiology, 477(3), 527–538. 10.1113/jphysiol.1994.sp020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, & Mishkin M (1999). Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience, 11(12), 4403–4418. 10.1046/j.1460-9568.1999.00854.x [DOI] [PubMed] [Google Scholar]
- Meuret AE, & Ritz T (2010). Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. International Journal of Psychophysiology, 78(1), 68–79. 10.1016/j.ijpsycho.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, & Ritz T (2021). Capnometry‑Assisted Respiratory Training. In Principles and Practice of Stress Management (Fourth Edition), 303–326. [Google Scholar]
- Meuret AE, Kroll J, & Ritz T (2017). Panic disorder comorbidity with medical conditions and treatment implications. Annual Review of Clinical Psychology, 13(1), 209–240. 10.1146/annurev-clinpsy-021815-093044 [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Wilhelm FH, Zhou E, Conrad A, Ritz T, & Roth WT (2011). Do unexpected panic attacks occur spontaneously? Biological Psychiatry, 70(10), 985–991. 10.1016/j.biopsych.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, & Roth WT (2008). Feedback of end-tidal PCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research, 42(7), 560–568. 10.1016/j.jpsychires.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DP, Leibenluft E, Ernst M, Charney D, & Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological Psychiatry, 57(9), 961–966. 10.1016/j.biopsych.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Mobbs D, Adolphs R, Fanselow MS, Barrett LF, LeDoux JE, Ressler K, & Tye KM (2019). Viewpoints: Approaches to defining and investigating fear. Nature Neuroscience, 22(8), 1205–1216. 10.1038/s41593-019-0456-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, … & McCarthy G (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry, 69(11), 1169–1178. 10.1001/archgenpsychiatry.2012.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kageyama Y, Hou Z, Aggarwal M, Patel J, Brown T, … & Troncoso JC (2017). Elucidation of white matter tracts of the human amygdala by detailed comparison between high-resolution postmortem magnetic resonance imaging and histology. Frontiers in Neuroanatomy, 11(1), 16. 10.3389/fnana.2017.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhtz C, Yassouridis A, Daneshi J, Braun M, & Kellner M (2011). Acute panicogenic, anxiogenic and dissociative effects of carbon dioxide inhalation in patients with post-traumatic stress disorder (PTSD). Journal of Psychiatric Research, 45(7), 989–993. 10.1016/j.jpsychires.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Nagy FZ, & Paré D (2008). Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. Journal of Neurophysiology, 100(6), 3429–3436. 10.1152/jn.90936.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, & Li A (2012). Central chemoreceptors: locations and functions. Comprehensive Physiology, 2(1), 221–254. 10.1002/cphy.c100083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, & Ray CD (1968). Respiratory arrest from seizure discharges in limbic system: report of cases. Archives of Neurology, 19(2), 199–207. 10.1001/archneur.1968.00480020085008 [DOI] [PubMed] [Google Scholar]
- Nobis WP, Otárula KAG, Templer JW, Gerard EE, VanHaerents S, Lane G, … & Schuele S (2019). The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. Journal of Neurosurgery, 132(5), 1313–1323. 10.3171/2019.1.JNS183157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, & Zelano C (2018). Amygdala‐stimulation‐induced apnea is attention and nasal‐breathing dependent. Annals of Neurology, 83(3), 460–471. 10.1002/ana.25178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogovitsyn N, Addington J, Souza R, Placsko TJ, Stowkowy J, Wang J, … & MacQueen G (2020). Childhood trauma and amygdala nuclei volumes in youth at risk for mental illness. Psychological Medicine, 1–8. 10.1017/S0033291720003177 [DOI] [PubMed] [Google Scholar]
- Novak V, Novak P, De Champlain J, & Nadeau R (1994). Altered cardiorespiratory transfer in hypertension. Hypertension, 23(1), 104–113. 10.1161/01.hyp.23.1.104 [DOI] [PubMed] [Google Scholar]
- O’Doherty DC, Chitty KM, Saddiqui S, Bennett MR, & Lagopoulos J (2015). A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 232(1), 1–33. 10.1016/j.pscychresns.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, … & Kalin NH (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature, 466(7308), 864–868. 10.1038/nature09282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, … & Gorman JM (1997). Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. American Journal of Psychiatry, 154(11), 1557–1565. 10.1176/ajp.154.11.1557 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, & Khalsa SS (2019). An active inference approach to interoceptive psychopathology. Annual Review of Clinical Psychology, 15(1), 97–122. 10.1146/annurev-clinpsy-050718-095617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, & Stein MB (2005). Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry, 62(3), 282–288. 10.1001/archpsyc.62.3.282 [DOI] [PubMed] [Google Scholar]
- Perna G, Barbini B, Cocchi S, Bertani A, & Gasperini M (1995). 35% CO2 challenge in panic and mood disorders. Journal of Affective Disorders, 33(3), 189–194. 10.1016/0165-0327(94)00088-q [DOI] [PubMed] [Google Scholar]
- Pessoa L (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158. 10.1038/nrn2317 [DOI] [PubMed] [Google Scholar]
- Price JL, & Amaral DG (1981). An autoradiographic study of the projections of the central nucleus of the monkey amygdala. Journal of Neuroscience, 1(11), 1242–1259. 10.1523/JNEUROSCI.01-11-01242.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Brown TA, Antony MM, & Barlow DH (1992). Response to hyperventilation and inhalation of 5.5% carbon dioxide-enriched air across the DSM-III—R anxiety disorders. Journal of Abnormal Psychology, 101(3), 538–552. 10.1037//0021-843x.101.3.538 [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, & Kushner MG (2003). Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. Journal of Anxiety Disorders, 17(1), 1–32. 10.1016/s0887-6185(02)00181-0 [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Abrams K, & Kushner MG (2006). Suffocation and respiratory responses to carbon dioxide and breath holding challenges in individuals with panic disorder. Journal of Psychosomatic Research, 60(3), 291–298. 10.1016/j.jpsychores.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Reis DJ, & McHugh PR (1968). Hypoxia as a cause of bradycardia during amygdala stimulation in monkey. American Journal of Physiology, 214(3), 601–610. 10.1152/ajplegacy.1968.214.3.601 [DOI] [PubMed] [Google Scholar]
- Rhone AE, Kovach CK, Harmata GI, Sullivan AW, Tranel D, Ciliberto MA, … & Dlouhy BJ (2020). A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy. JCI Insight, 5(6), e134852. 10.1172/jci.insight.134852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews Neuroscience, 5(6), 449–461. 10.1038/nrn1409 [DOI] [PubMed] [Google Scholar]
- Ritz T, Kroll JL, Aslan S, Janssens T, Khan DA, Pinkham AE, & Brown ES (2019). Subcortical gray matter volumes in asthma: associations with asthma duration, control, and anxiety. Brain Imaging and Behavior, 14(6), 2341–2350. 10.1007/s11682-019-00188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, & Guyenet PG (2006). Afferent and efferent connections of the rat retrotrapezoid nucleus. Journal of Comparative Neurology, 499(1), 64–89. 10.1002/cne.21105 [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TFC, & Henderson Z (2000). A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience, 99(4), 613–626. 10.1016/s0306-4522(00)00240-2 [DOI] [PubMed] [Google Scholar]
- Saltzman HA, Heyman A, & Sieker HO (1963). Correlation of clinical and physiologic manifestations of sustained hyperventilation. New England Journal of Medicine, 268(26), 1431–1436. 10.1056/NEJM196306272682602 [DOI] [PubMed] [Google Scholar]
- Scheele D, Mihov Y, Kendrick KM, Feinstein JS, Reich H, Maier W, & Hurlemann R (2012). Amygdala lesion profoundly alters altruistic punishment. Biological Psychiatry, 72(3), e5–e7. 10.1016/j.biopsych.2012.01.028 [DOI] [PubMed] [Google Scholar]
- Schimitel FG, de Almeida GM, Pitol DN, Armini RS, Tufik S, & Schenberg LC (2012). Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat. Neuroscience, 200, 59–73. 10.1016/j.neuroscience.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Schruers KRJ, Van De Mortel H, Overbeek T, & Griez E (2004). Symptom profiles of natural and laboratory panic attacks. Acta Neuropsychiatrica, 16(2), 101–106. 10.1111/j.0924-2708.2004.0084.x [DOI] [PubMed] [Google Scholar]
- Seddon K, Morris K, Bailey J, Potokar J, Rich A, Wilson S, … & Nutt DJ (2011). Effects of 7.5% CO2 challenge in generalized anxiety disorder. Journal of Psychopharmacology, 25(1), 43–51. 10.1177/0269881110364270 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016). Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36(31), 8050–8063. 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J, Azar T, & Lawson D (2006). Comparison of carbon dioxide, argon, and nitrogen for inducing unconsciousness or euthanasia of rats. Journal of the American Association for Laboratory Animal Science, 45(2), 21–25. [PubMed] [Google Scholar]
- Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, … & Mizuno K (2012). Clinical significance of acid–base balance in an emergency setting in patients with acute heart failure. Journal of Cardiology, 60(4), 288–294. 10.1016/j.jjcc.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Spencer WG (1894). The effect produced upon respiration by faradic excitation of the cerebrum in the monkey, dog, cat, and rabbit. Philosophical Transactions of the Royal Society of London B., 185, 609–657. 10.1098/rstb.1894.0014 [DOI] [Google Scholar]
- Spiacci A Jr, Coimbra NC, & Zangrossi H Jr (2012). Differential involvement of dorsal raphe subnuclei in the regulation of anxiety-and panic-related defensive behaviors. Neuroscience, 227, 350–360. 10.1016/j.neuroscience.2012.09.061 [DOI] [PubMed] [Google Scholar]
- Springer KS, Levy HC, & Tolin DF (2018). Remission in CBT for adult anxiety disorders: a meta-analysis. Clinical Psychology Review, 61, 1–8. 10.1016/j.cpr.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Stein MB, Millar TW, Larsen DK, & Kryger MH (1995). Irregular breathing during sleep in patients with panic disorder. American Journal of Psychiatry, 152(8), 1168–1173. 10.1176/ajp.152.8.1168 [DOI] [PubMed] [Google Scholar]
- Suh GS, de Leon SBT, Tanimoto H, Fiala A, Benzer S, & Anderson DJ (2007). Light activation of an innate olfactory avoidance response in Drosophila. Current Biology, 17(10), 905–908. 10.1016/j.cub.2007.04.046 [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, … & Anderson DJ (2004). A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature, 431(7010), 854–859. 10.1038/nature02980 [DOI] [PubMed] [Google Scholar]
- Sun N, Yi H, & Cassell MD (1994). Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. Journal of Comparative Neurology, 340(1), 43–64. 10.1002/cne.903400105 [DOI] [PubMed] [Google Scholar]
- Suor JH, Jimmy J, Monk CS, Phan KL, & Burkhouse KL (2020). Parsing differences in amygdala volume among individuals with and without social and generalized anxiety disorders across the lifespan. Journal of Psychiatric Research, 128, 83–89. 10.1016/j.jpsychires.2020.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, & Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. 10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersfield AE, & McNicol MW (1969). Salbutamol and isoproterenol: A double-blind trial to compare bronchodilator and cardiovascular activity. New England Journal of Medicine, 281(24), 1323–1326. 10.1056/NEJM196912112812402 [DOI] [PubMed] [Google Scholar]
- Taugher RJ, Dlouhy BJ, Kreple CJ, Ghobbeh A, Conlon MM, Wang Y, & Wemmie JA (2020). The amygdala differentially regulates defensive behaviors evoked by CO2. Behavioural Brain Research, 377(4), 112236. 10.1016/j.bbr.2019.112236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, … & Wemmie JA (2014). The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. Journal of Neuroscience, 34(31), 10247–10255. 10.1523/JNEUROSCI.1680-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Lüthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16(6), 317–331. 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- Telch MJ, Rosenfield D, Lee HJ, & Pai A (2012). Emotional reactivity to a single inhalation of 35% carbon dioxide and its association with later symptoms of posttraumatic stress disorder and anxiety in soldiers deployed to Iraq. Archives of General Psychiatry, 69(11), 1161–1168. 10.1001/archgenpsychiatry.2012.8 [DOI] [PubMed] [Google Scholar]
- Tenorio‐Lopes L, Henry MS, Marques D, Tremblay MÈ, Drolet G, Bretzner F, & Kinkead R (2017). Neonatal maternal separation opposes the facilitatory effect of castration on the respiratory response to hypercapnia of the adult male rat: Evidence for the involvement of the medial amygdala. Journal of Neuroendocrinology, 29(12), e12550. 10.1111/jne.12550 [DOI] [PubMed] [Google Scholar]
- Tolin DF, Billingsley AL, Hallion LS, & Diefenbach GJ (2017). Low pre-treatment end-tidal CO2 predicts dropout from cognitive-behavioral therapy for anxiety and related disorders. Behaviour Research and Therapy, 90, 32–40. 10.1016/j.brat.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Van den Hout MA, Hoekstra R, Arntz A, Christiaanse M, Ranschaert W, & Schouten E (1992). Hyperventilation is not diagnostically specific to panic patients. Psychosomatic Medicine, 54(2), 182–191. 10.1097/00006842-199203000-00005 [DOI] [PubMed] [Google Scholar]
- Verburg K, Pols H, de Leeuw M, & Griez E (1998). Reliability of the 35% carbon dioxide panic provocation challenge. Psychiatry Research, 78(3), 207–214. 10.1016/s0165-1781(98)00009-2 [DOI] [PubMed] [Google Scholar]
- Verner TA, Pilowsky PM, & Goodchild AK (2008). Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Research, 1208(3), 128–136. 10.1016/j.brainres.2008.02.028 [DOI] [PubMed] [Google Scholar]
- Vickers K, Jafarpour S, Mofidi A, Rafat B, & Woznica A (2012). The 35% carbon dioxide test in stress and panic research: Overview of effects and integration of findings. Clinical Psychology Review, 32(3), 153–164. 10.1016/j.cpr.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, & Gray TS (1992). Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Research Bulletin, 28(3), 447–454. 10.1016/0361-9230(92)90046-z [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, & Wisniewski SR (2007). The STAR* D Project results: a comprehensive review of findings. Current Psychiatry Reports, 9(6), 449–459. 10.1007/s11920-007-0061-3 [DOI] [PubMed] [Google Scholar]
- Weera MM, Shackett RS, Kramer HM, Middleton JW, & Gilpin NW (2021). Central amygdala projections to lateral hypothalamus mediate avoidance behavior in rats. Journal of Neuroscience, 41(1), 61–72. 10.1523/JNEUROSCI.0236-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, & Luo L (2014). Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron, 83(3), 645–662. 10.1016/j.neuron.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Lambert HK, Rodman AM, Peverill M, Sheridan MA, & McLaughlin KA (2020). Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depression and Anxiety, 37(9), 916–925. 10.1002/da.23062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, & Welsh MJ (2006). Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends in Neurosciences, 29(10), 578–586. 10.1016/j.tins.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Wiest G, Lehner-Baumgartner E, & Baumgartner C (2006). Panic attacks in an individual with bilateral selective lesions of the amygdala. Archives of Neurology, 63(12), 1798–1801. 10.1001/archneur.63.12.1798 [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, & Roth WT (1998). Taking the laboratory to the skies: Ambulatory assessment of self‐report, autonomic, and respiratory responses in flying phobia. Psychophysiology, 35(5), 596–606. 10.1017/s0048577298970196 [DOI] [PubMed] [Google Scholar]
- Wu H, Zhan X, Zhao M, & Wei Y (2016). Mean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnea. Medicine, 95(48), e5493. 10.1097/MD.0000000000005493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Kim EJ, Callaway EM, & Feldman JL (2020). Monosynaptic projections to excitatory and inhibitory preBötzinger complex neurons. Frontiers in Neuroanatomy, 14(58), 1–11. 10.3389/fnana.2020.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Kim JE, Kim GH, Kang HJ, Kim BR, Jeon S, … & Lyoo IK (2016). Subregional shape alterations in the amygdala in patients with panic disorder. PLoS ONE, 11(6), e0157856. 10.1371/journal.pone.0157856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, … & Wemmie JA (2009). The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell, 139(5), 1012–1021. 10.1016/j.cell.2009.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Furuya WI, Bassi M, Colombari DS, & Colombari E (2014). The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology, 5(238), 1–12. 10.3389/fphys.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]


