Abstract
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative disorders, affecting the basal nuclei, causing impairment of motor and cognitive functions. Loss of dopaminergic (DAergic) neurons or their degeneration and the aggregation of Lewy bodies is the hallmark of this disease. The medications used to treat PD relieve the symptoms and maintain quality of life, but currently, there is no cure. There is a need for the development of therapies that can cease or perhaps reverse neurodegeneration effectively. With the rapid advancements in cell replacement therapy techniques, medical professionals are trying to find a cure by which restoration of dopamine neurotransmitters can occur. Researchers have started focusing on cell-based therapies using mesenchymal stem cells (MSCs) due to their abundance in the body, the ability of proliferation, and immunomodulation. Here we review the MSC-based treatment in Parkinson's disease and the various mechanisms it repairs DAergic neurons in parkinsonian patients.
Keywords: Parkinson’s disease, dopaminergic neurons, stem cell, therapy, mesenchymal stem cells
1. INTRODUCTION
Parkinson's disease (PD) is the second most common progressive neurodegenerative disease and the most prevalent form of primary Parkinsonism. Approximately 1% of people over the age of 50 and around 2.5% of people over 70 are affected by PD. Men have a 2.0 percent lifetime risk of developing PD, while women have a 1.3 percent lifetime risk [1]. Familial PD is genetically inherited in either an autosomal dominant or recessive fashion, while sporadic (idiopathic) PD is thought to arise due to interaction between genetics and environment. Alpha-synuclein (aSyn), phosphatase and tensing homolog-induced Kinase 1 (PINK1), Glucocerebrosidase (GBA), Parkin RBR E3 ubiquitin-protein ligase (PARK2), Vacuolar protein sorting-associated protein 35 (VPS35), Leucine-rich repeat Kinase 2 (LRRK2), and Parkinson protein 7 (PARK7) have been identified as causal genes for familial PD. These genes, along with some specific metabolites and biomarkers linked to PD, have been utilized to determine possible early detection strategies for the disease [2].
PD's motor and non-motor symptoms result from the loss or damage to striatal DAergic and non-DAergic neurons [3]. The appearance of bradykinesia, rigidity, loss of postural reflexes, and rest tremor is the most frequently encountered motor symptoms. However, clinical manifestations such as neuro-ophthalmological disorders, bulbar dysfunction, and respiratory abnormalities can also be recognized as the disease progresses. The fact that most motor symptoms respond to DAergic medication is one of the primary indicators that can help differentiate PD from other parkinsonian illnesses, hence disease identification [4]. Hyposmia, constipation, rapid eye movement (REM) behaviour disorder, depression, and olfactory impairment are other nonmotor symptoms [5]. Moreover, disturbances in odour, sleep, mood, and gastrointestinal function may signal the onset of Parkinson's disease or associated synucleinopathies. They may occur five or more years before these neurodegenerative diseases manifest [6]. Parkinson's disease has been linked to several risk factors and genetic mutations. Oxidative stress, the generation of free radicals, and various environmental pollutants are risk factors for the disease. Some studies demonstrate genetic links to PD, with several gene mutations being identified. Intriguingly, there is an inverse link between cigarette smoking, caffeine use, and the chance of acquiring PD [7].
The drugs which attenuate the pathology of α-synuclein or show favourable activity on other processes involved in PD can be used to treat PD successfully. Drug repurposing (using existing medications for a new indication) has sparked a lot of interest, as it could result in an accelerated path to the clinic, provided that safety, as well as pharmacokinetic studies, is already established [8]. Currently, pharmacological therapies and surgery are unable to fix the problem effectively. Treatment by restoring DAergic neurotransmitters in individuals with PD using DAergic neurons and cell transplantation is being investigated, which has been made possible by the discovery of cell replacement therapy [9]. The concept behind clinical trials of cell therapy in PD patients is that restoring striatal DAergic transmission with transplanted DAergic neurons would result in clinical improvement, even if the condition is chronic and damages other regions of the brain and neuronal systems [10].
2. PD AS A CANDIDATE FOR STEM CELL THERAPY
The progressive death of neurons in the nervous system is referred to as neurodegeneration. Recent research suggests that neurons lose their regular structures and functions even before their death, implying that simply withholding these neurons from dying is not likely to be a successful treatment technique. As a result, stem cell therapy appears to be the most successful treatment option [11]. In 1979, prenatal rat DAergic neurons were transplanted to ameliorate motor impairments in a PD model of rats with good graft survival and axonal outgrowth. This study proved that cell therapy could restore deficiencies caused by minor brain tissue damage [12]—a procedure for converting Bone marrow-derived MSC (BM-MSCs) into Neurotrophic factors-secreting cells (NTFSCs) that release Brain-derived neurotrophic factors (BNDF) and Glial cell line-derived neurotrophic factors (GDNF) has been established. In a PD cellular model, the NTF-SC conditioned medium provides significant protection, and grafting of the NTF-SCs into a rat model after impairment led to the relocation of the transplanted cells to the affected site and restoration of the impaired DAergic nerve terminal system in the striatum [13].
3. TYPES OF STEM CELLS
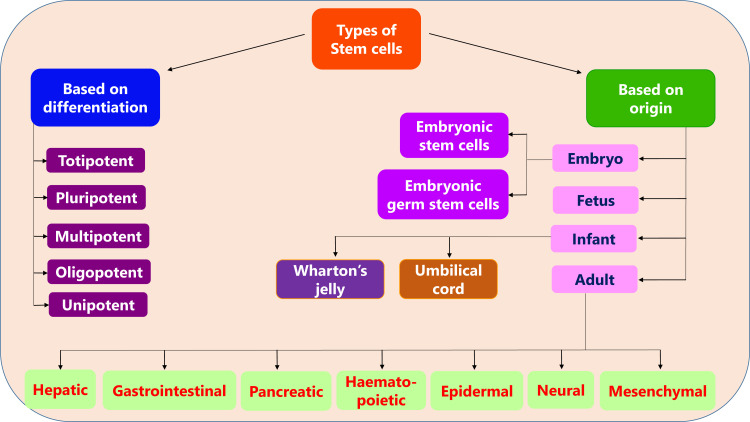
The classification of stem cells based on their differentiation and origin is shown in Fig. (1).
Fig. (1).
Classification of stem cells.
4. DIFFERENT TYPES OF MSCS
MSCs derived from different sources were found to have certain similarities, such as identical surface markers and some differences. Hence it is crucial to collate these similarities and differences to determine which one is best suited for cell therapy. Furthermore, for stemness-related genes, BM-MSCs and Adipose tissue-derived MSC (AD-MSCs) had a remarkably similar transcriptional profile. Surface antigen expression, differentiation ability, and immunosuppressive activity were identical in Umbilical cord-derived stem cells (UC-MSCs), BM-MSCs, and AD-MSCs, but UC-MSCs exhibited a higher proliferation rate than the others and the lowest expression of senescence markers. AD-MSCs, on the other hand, had the shortest culture time and the lowest proliferation rate. Other studies have found that ADMSCs had a more substantial proliferation potential than BM-MSCs and a higher expression of neural markers in differentiated AD-MSCs. The secretion levels of certain factors like Vascular endothelial growth factors (VEGF), Transforming growth factors-β 1 (TGFβ1) were considerably higher in BM-MSCs than those of AD-MSCs.
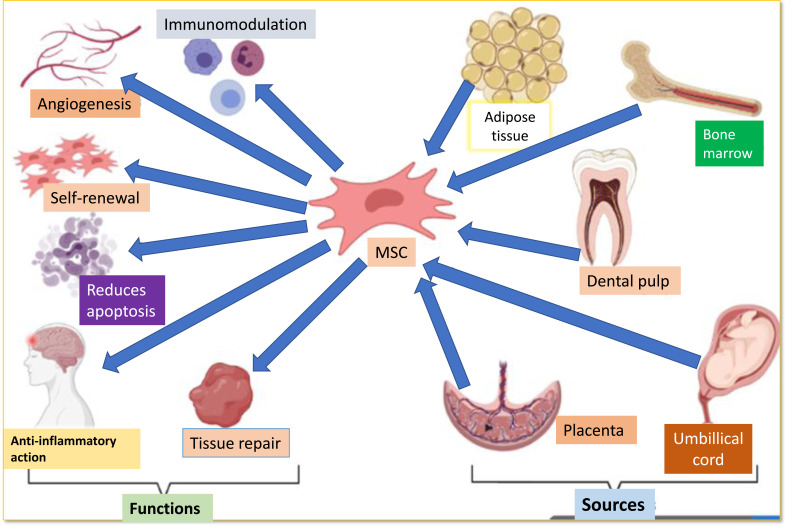
Furthermore, UCMSCs had a higher rate of proliferation than Dental pulp-derived MSC (DP-MSCs), while DP-MSCs had a lower incidence of apoptosis and senescence, and their cell shape was maintained following subculture. Furthermore, compared with BM-MSCs, DP-MSCs and stem cells derived from human exfoliated deciduous teeth (SHEDs) demonstrated a better differentiating capability for neurogenesis [14]. Human endometrium-derived stem cells (hEDSCs) are a new source of cell therapy in PD treatment. They seem to be widely available and collected by a simple, safe, and painless method like Pap smears. After being injected into the striatum, the cells can implant into the striatum of (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) MPTP-exposed monkeys, relocate to the substantia nigra, and autonomously develop into DAergic neuron-like cells [15]. The sources and functions of MSCs are depicted in Fig. (2).
Fig. (2).
Sources and functions of MSC.
4.1. Bone-Marrow Derived MSCs
Due to their relative efficiency and safety, the lack of immunological complications with autografts, and the absence of religious and legal obstacles, adult bone marrow-derived cells, especially MSC (BM-MSCs), have gained enormous interest in PD research. Due to the tissue-repair capabilities and “homing” ability of these cells to the damaged site, studies have highlighted the transplantation of BM-MSC as a viable method for treating neurological diseases [16]. BM-MSCs are the most well-studied of the various types of MSCs. In vitro, BMSCs can be cultured as plastic adherent cells from bone marrow samples. The main advantage of BM-MSCs is that they are widely obtainable through aspiration of the patient's bone marrow, so they can be used for both auto- and allotransplantation without raising ethical concerns. The ability of BM-MSCs to release cytotrophic mediators, including Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and NGF-3; neurturin and Glial-derived neurotrophic factor (GDNF) is well recognized. Neurogenesis, neuroprotection, neuronal survival, and differentiation are all dependent on these neurotrophic substances [17].
BMMSC were shown to have positive benefits on PD patients and exhibited differentiation into DAergic neurons, with a detectable amount of Dopamine in the differentiated cells' culture media. Furthermore, three months after transplanting, PD rat models showed considerable per cent improvement. This demonstrates that BM-MSC can differentiate into DAergic precursors and offer many of their features, resulting in behavioural improvements in the hemiparkinsonian rat after transplantation in an animal model. Trzaska et al. [18] confirmed these findings, demonstrating that adult MSC has DAergic characteristics [19]. Lately, a clinical trial in advanced PD patients involving unilateral transplantation of autologous BM-MSC into the sublateral ventricular zone demonstrated significant clinical improvement with no side effects like tumour formation at 12 months [20].
4.2. Dental Tissue-Derived MSC
Many biological features of dental pulp stem cells (DPSC) are similar to those of MSC, such as bone marrow MSC (BM-MSC), adipose tissue-derived stem cells (ADSC), and umbilical cord MSCs (UCMSC); however, there are some differences in their proliferative capacity, differentiation, immunomodulatory effect, secretome characteristics, and secretory capacity [21]. DPSC are stem cells derived from ectoderm that emerge from migrating neural crest cells and have a high degree of plasticity [22]. DPSC release several substances that are important for neuronal survival, proliferation, and differentiation. Dental pulp conditioned medium (DPCM) included various neurotrophic factors, including neurotrophin-3 (NT-3) and nerve growth factor (NGF), which led to neuroprotective action against neurodegenerative disorders. The use of DPSC may be beneficial to people with PD. Exosomes generated by DPSC protect midbrain neurons against 6-hydroxydopamine (6OHDA) induced apoptosis, while neurotrophic factors including NGF and GDNF protect midbrain neurons from 6-OHDA in vitro [23].
DPSCs were transplanted intrathecally into the MPTP-induced old-age mouse model of PD, which favored behavioral recovery, repaired DAergic functions, and lessened MPTP-induced damage by minimizing the release of pro-inflammatory factors such as IL-1α, IL-1β, IL6, IL8, and tumor necrosis factor TNF-α and increased expression of anti-inflammatory factors like TNF-β, IL2, and IL4. In an in vitro model of PD, DPSC displayed neuro-immuno-modulatory action by minimizing MPTP-induced impairments associated with Reactive oxygen species (ROS), DNA damage, and nitric oxide release [24].In Parkinsonian mice, the therapeutic efficacy of SHED was investigated. According to the researchers, SHED possessed DAergic differentiation capacities, and SHED transplantation into the striatum of Parkinsonian rats reduced their behavioral abnormalities. When compared to SHED, DPSC have better neuronal plasticity toward DAergic neurons [25].
4.3. Adipose Tissue-Derived MSC
Adipose-derived stem cells were identified as MSCs in adipose tissue for the first time in 2001, and adipose tissue has been studied as a cell source for tissue engineering and in regenerative medicine since then [26]. They have a surface antigen marker profile and differentiation capacity similar to BM-MSC, in addition to being more heterogeneous [27]. ADMSC have a comprehensive immune regulatory function, making them ideal candidates for cellular treatment [28]. They can be cultured ex vivo in a short amount of time, but their 'stemness,' which is defined by their ability to proliferate and differentiate, declines over time [29]. They discovered that ADMSC have properties similar to those of BM-MSCs. Similar to BMSC, adipose tissue contains stem cells [30]. Even though ADMSC and BMMSC are pretty comparable, it has been noted that ADMSC is not entirely identical to BMMSC [31].
4.4. Umbilical Cord-Derived MSCs
The UC of a human being is a potential source of MSC. UC-MSC have demonstrated the ability to differentiate into three germ layers, aggregate in damaged tissue or inflammatory sites, stimulate tissue repair, and influence immune response, in addition to their obvious benefits, such as a painless collecting technique and rapid self-renewal [32]. Fast cell multiplication and high proliferative fitness at UC-MSC harvesting allow for the preparation of larger batches that can be stored for future administration [33]. UC-MSCs are less immunogenic and have a more decisive immunosuppressive action [34].
The umbilical cord and adipose tissues offer significant advantages over BM-MSC. The umbilical cord is derived from postnatal tissue discarded after delivery, making cell collection for donors less invasive. Adipose tissue, which may be retrieved readily through liposuction, has a substantial number of MSC. Because of their potential to differentiate into other cell types and proliferate, UC-MSC and ADMSC are viable sources for cell-based therapies [35].
4.5. Placenta Derived MSC
The placenta is a stable and abundant source of fetal MSCs. These cells have the potential to differentiate into several cell types and possess immunological features [36]. Placenta-derived MSC (P-MSC) is a homogenous group of cells with strong homing and cellular lineage priming capabilities, as well as a high proliferation rate [37]. Amniotic fluid-derived MSC (AF-MSC) is a type of P-MSCs. Second-trimester amniotic fluid (AF) has been identified as a novel and rich source of foetal MSC that can be used in clinical applications [38]. In terms of developmental phases, AF-MSC is beneficial, but the invasiveness of the collecting procedures—amniocentesis and fetal infections—are problematic [39]. Because of their differentiation potential, in vitro culture aspects, absence of tumorigenic potential, and ethical problems, AF-MSC has a lot of potential for clinical applications [40].
The applications of MSC obtained from different sources are listed in Table 1.
Table 1.
Different sources of MSC and their applications.
| Source | Differential Potential | Clinical Applications | References |
|---|---|---|---|
| Bone marrow | [1] Adipose cells [2] Embryonic tissue [3] Astrocytic glial cells [4] Cardiac muscle cells [5] Osteoblasts [6] Cartilage cells [7] Hepatocytes [8] Stromal cells [9] Myocytes [10] Neural cells [11] Mesangial cells |
➢ Therapeutic potential against PD pathophysiology via multi-mechanistic actions. ➢ Chronic ischemic stroke ➢ Spinal cord injury ➢ Diabetes mellitus type 1 ➢ Cirrhosis in rats ➢ Treatment of the radiation-induced gastrointestinal syndrome ➢ Hematological malignancy ➢ Organ repair ➢ Regeneration of tissue-engineered urinary bladder and prevention of fibrosis ➢ Treatment of intrauterine adhesions in a rat |
[41-49] |
| Adipose tissue | ➢ Adipose cells ➢ Bone cells ➢ Cartilage cells ➢ Muscle cells |
➢ Repopulate cardiac muscles after a heart attack ➢ Myocardium restoration ➢ Bone regeneration ➢ Nerve system rebuilding ➢ Skin reconstruction ➢ Cartilage repair ➢ Liver reconstruction ➢ Enhance radiotherapy effects on hepatocellular carcinoma. ➢ Osteoarthritis ➢ Promotes wound healing ➢ Stroke |
[50-56] |
| Dental pulp | ➢ Odontoblasts ➢ Osteoblasts ➢ Adipocytes ➢ Chondrocytes ➢ Neurogenic cells ➢ Myogenic cells |
➢ Irreversible pulpitis ➢ Treat alveolar bone defects in human ➢ Regenerate periodontal tissues and bone damage ➢ Formation of blood vessels in the myogenic lineage ➢ Genetic disorders ➢ Endodontic regeneration |
[57-62] |
| Amniotic fluid and placenta | AF-MSCs: ➢ Neural stem cells ➢ Adipocytes ➢ Osteoblasts ➢ Chondrocytes ➢ Hepatocytes P-MSCs: ➢ Pancreatic cells |
AF-MSCs: ➢ Spinal cord injuries and demyelinating diseases ➢ Neuronal regenerative therapy ➢ Cancer treatment ➢ Improve ovarian function by resisting DNA damage ➢ Hyperoxic lung injury ➢ Ischemic heart injury ➢ Tubular necrosis of kidney and acute bladder injury P-MSCs: ➢ Cardiac diseases, ulcers, bone repair ➢ Autoimmune disorders ➢ Bronchopulmonary dysplasia |
[63-69] |
| Birth derived tissue | UC-MSCs: ➢ Adipocytes ➢ Chondrocytes WJ-MSCs: ➢ DAergic neurons ➢ Chondrocytes |
UC-MSCs: ➢ Musculoskeletal diseases ➢ Spinal cord injury ➢ APP and presenilin (PS1) double-transgenic mice ➢ Improve anticancer effects ➢ Cartilage regenration WJ-MSCs: ➢ Kidney injury, lung injury, orthopedic injury, and cancer therapy ➢ Wound healing and hair growth |
[44, 56, 70-74] |
Abbreviations: AF-MSCs = Amniotic fluid-derived MSCs, WJ-MSCs = Wharton’s jelly derived MS.
5. ROLE OF MSC IN THE TREATMENT OF PD
MSC are multipotent progenitor cells that have already been extensively used to treat bone diseases, autoimmune and inflammatory disorders, spinal cord injuries, severe pneumonia, myocardial infarction, allotransplant rejection, extensive burns, degenerative conditions, and severe chronic wounds [75]. MSCs can be differentiated into dopamine neurons, which would assist in the functional recovery of the lost neurons in people with PD. MSC participate in tissue repair and regeneration by activating various mechanisms once they have been mobilized to the damaged site. In several studies, pleiotropic effects of MSC have been revealed, offering them considerable therapeutic potential [76].
MSC can self-renewal and differentiate into various mesenchymal cells like adipocytes, tenocytes, osteoblasts, skeletal myocytes, chondrocytes, and visceral mesoderm cells. Furthermore, several investigations have revealed that MSCs can develop into neuron-like cells of ectodermal and endodermal origin, hepatocytes, and cardiomyocytes, in addition to the mesodermal lineage [77].
• Because of their broad availability in the body, proliferative capacity, and immunomodulatory characteristics, MSC has gained enormous attention from researchers interested in PD. MSC have various pathways that are responsible for their therapeutic potential. Protective neurotrophic factors, growth factors such as vascular endothelial growth factors (VEGF), insulin-like growth factor 1 (IGF-1), hepatocyte growth factors (HGF), brain-derived neurotrophic factors (BNDF), transforming growth factors-β (TGF-β), beta-nerve growth factors (β-NGF), glial cell-derived neurotrophic factors and fibroblast growth factor 2 (FGF-2), anti-apoptotic factors, and cytokines are secreted by MSCs at the injured site. MSCs could aid repair in this way by producing anti-inflammatory cytokines like interleukin (IL)-10 and TGF-β39, as well as inhibiting pro-inflammatory cytokines like tumour necrosis factor-α, interferon-gamma (IFN- γ), and IL-1 β, found in PD patient’s brain.
• Through their paracrine signalling and multipotency, MSC also regulates hematopoiesis and facilitates tissue regeneration.
• MSC can differentiate into a neural lineage, which includes DAergic neuron precursors.
• Because of the crucial role of damaged mitochondria in the neurodegeneration of DAergic neurons, recent studies demonstrating that MSC can transport mitochondria to the injured site indicate another potential mechanism that could prove to be advantageous in PD therapy.
• There is no risk of neoplastic transformation with MSC therapy, and it is safe [78].
• Autophagic dysfunction due to lack of autophagolysosome generation may be linked to DAergic cell death in PD models treated with neurotoxin. However, MSC therapy enhanced autophagolysosome production and suppressed α-syn expression, possibly increasing DAergic neuron survival against environmental neurotoxins [79].
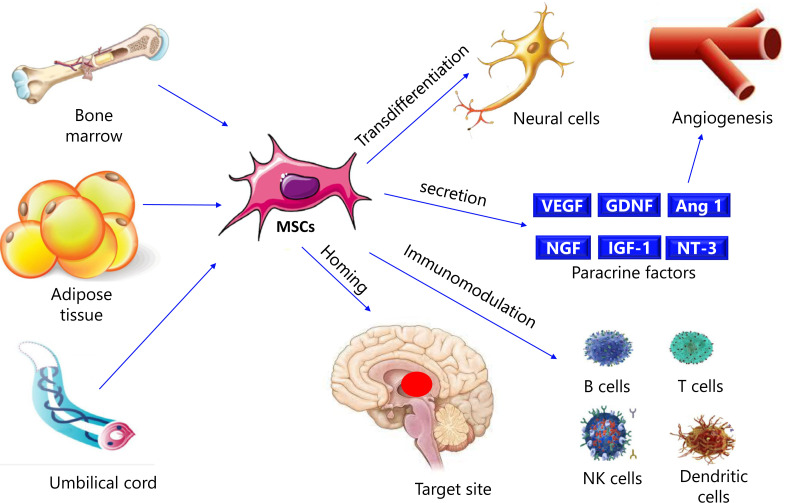
According to studies, the immune system can identify and destroy abnormally accumulated α-synuclein in the brain of PD patients as an antigen. Multiple inflammatory cytokines are increased in the patients. MSC's role in cell migration and immunological modulation would become highly significant in treating various disorders. Under certain culture conditions, MSC can also develop into neuron-like cells. Motor function was greatly improved, and trabecular meshwork MSC could differentiate into DAergic neurons, according to the findings [80]. Fig. (3) shows the mechanisms of MSC in PD.
Fig. (3).
Mechanisms of MSC in PD. VEGF: Vascular endothelial growth factor; GDNF: Glial cell line-derived neurotrophic factor; Ang 1: Angiopoietin 1; NGF: Nerve growth factor; IGF-1: Insulin-like growth factor 1; NT-3: Neurotrophin-3; NK cells: Natural killer cells.
6. DOSAGE AND TIMING OF MSC TREATMENT
MSC treatment dose and timing are issues that need to be resolved and confirmed for translational applications. Dosing for a particular disease entity may be determined by the potency and efficacy of the MSC preparation, as well as the disease's condition and phenotype. A single dose of MSC was employed in the in vivo asthma experiment, and it had a significant influence on the murine model of both acute and chronic illness. This holds for the other in vivo models as well. In clinical trials, multiple infusions were required to assist or cause clinical improvement using MSCs in Crohn's disease, osteogenesis imperfecta, and acute steroid-refractory graft versus host disease (GvHD) [81].
7. ROUTES OF ADMINISTRATION
The following procedures are included in mesenchymal stem cell transplantation: (a) stereotaxic injection, (b) intravenous injection, (c) subsequent intranasal injection [82].
8. INDUCTION AND TRANSPLANTATION OF DAERGIC NEURONS
MSC can be extracted from various embryonic and adult organs, including bone marrow and adipose tissue. BM-MSC has the potential to develop into a variety of cell types. The quantity of colony-forming unit of fibroblast (CFU-F) is measured to estimate the extent of proliferation of the mesenchymal stem cell [83].
8.1. Induction
It was reported that BM-MSC might be used to precisely generate dopamine neurons. This technique forms post-mitotic functioning neuronal cells with high efficiency while avoiding glial cell contamination. The developed neuronal cells are then converted into dopamine neurons. The insertion of the Notch1 intracellular domain (NICD) causes the induction. After cytokine stimulation (basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CNTF), and forskolin (FSK), MSC becomes like neural progenitor cells (NPCs), displaying NPC markers. GDNF causes neurons to develop into dopamine neurons tyrosine hydroxylase (TH), which are beneficial in the PD model [84].
8.2. Transplantation
Induced dopamine neurons were transplanted into the striatum of a PD model rat from either rodent or human BM-MSC. The synthesis of dopamine in the transplanted brains was demonstrated in brain slice culture experiments. MSC promoted neurogenesis (progenitor cells), decreased inflammation, gliosis, and death signals, and elevated neuronal plasticity (neurorescue) with more remarkable cell survival (and an elevated striatal dopamine level). There was no evidence of tumour formation in the brain, indicating that DAergic neurons produced from BM-MSC cannot form tumors [85].
9. POSSIBLE MECHANISMS INVOLVED IN MSC-EVOKED REPAIR OF DAERGIC NEURONS
Multivariate mechanisms are involved in a successful MSC therapy. Exogenous and endogenous factors work together in a variety of ways to assist recovery. The external factors are fundamental to stem cells (SCs) and through which the injected cells alter the underlying microenvironment and even integrate into the host neuronal networks. The endogenous factors are associated with the host's response to the introduction of SCs and the impact this has on SC behavior and survival [86].
MSC has two main effects: a trophic effect mediated by the numerous types of trophic factors and cytokines secreted by MSCs, and differentiation to form a wide range of cells to restore lost cells. They can inhibit the growth and activation of immune system cells. These interactions can occur either directly (i.e., cell-cell interaction) or indirectly (via soluble factors) [87]. MSC can regulate the immune system by being engulfed by antigen-presenting cells. According to a recent study, the consequent display of MSC antigens by antigen-presenting cells (APCs) leads to a series of anti-inflammatory actions, thus enhancing the therapeutic process outcome [88].
9.1. Trans-differentiation and Fusion
The underlying mechanisms remain unclear, even though functional recovery after MSC transplantation has been found in various animal models of neurodegenerative disorder. MSC obtained from multiple tissues are incredibly adaptable, and under different culture conditions, they can adopt the morphological and phenotypic traits of neuronal cells. Co-culture with glial, neuronal, and neuronal stem cells, as well as astrocyte or neuronal conditioned media, can improve MSC's ability to differentiate into neurons. Overexpression of genes including Noggin, brain-derived neurotrophic factor (BDNF), and Notch intracellular domain (NICD), which are essential for neural development and function, can also accelerate MSC neural transdifferentiation. Similarly, diverse techniques based on chemical induction, gene transfection, co-culturing, and conditioned media can be used to convert MSC into DAergic neurons [89].
9.2. Secretion of Paracrine Factors
MSC have been found to secrete a variety of growth factors and cytokines, including BDNF, nerve growth factor (NGF), glial-cell-line-derived neurotrophic factor (GDNF), fibroblast growth factor 2 and 8 (FGF2 and FGF8), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), ciliary neurotrophic factor (CNTF), and stromal cell-derived factor-1(SDF-1). GDNF, BDNF, basic fibroblast growth factor (bFGF), and CNTF, among other growth factors produced by MSC, have neurotrophic and neuroprotective effects on DAergic neurons. Thus, growth factors released by transplanted MSC and activated host cells are likely to be involved in MSC's therapeutic benefits in many animal PD models [81].
9.3. Interaction with Host Cells
Transplanted MSC has been shown to influence endogenous neural stem cells (NSC), glial cells, and blood vessels via the release of paracrine factors, which aids in neuronal tissue repair and functional recovery, in addition to their effects on host DAergic neurons. Endogenous neuronal stem cells were induced to proliferate, migrate, and differentiate when human MSC was transplanted into the mouse hippocampus. Recent studies have also shown that transplantation of the intrahippocampal and intracerebroventricular MSC promotes neurogenesis. MSC' ability to enhance endothelial cell proliferation and angiogenesis is a crucial characteristic of their regenerative activities. Angiogenic factors such as VEGF, FGF, HGF, Stromal cell-derived factor-1 (SDF1), Angiopoietin-1 (Ang-1), Angiopoietin-2 (Ang-2), and matrix metalloproteinase-1 may mediate the MSC action on blood vessels [90].
9.4. Immunomodulation and Anti-inflammatory Effects of MSC
The role of inflammation in the etiology of PD suggests that MSC's immunomodulatory and anti-inflammatory actions may contribute to their therapeutic effects following transplantation in PD animal models. Several lines of evidence imply that MSC plays a dual role in the immune system: suppressing or activating immunological responses. Allogeneic MSC transplantation into a rat model of PD has been shown to elicit a cellular immune response. MSC, on the other hand, can inhibit immune cells such as B lymphocytes, T lymphocytes, dendritic cells, and natural killer (NK) cells [89]. Experimental investigations of ischemic heart disease and autoimmune disorders show that MSCs protect tissue by acting as anti-inflammatory mediators. MSC, on the other hand, can reduce inflammatory responses through a variety of mechanisms. MSC anti-inflammatory effects include a) expression of an Interleukine-1 (IL-1) receptor antagonist, b) release of anti-TNFα, c) secretion of PGE2, which changes macrophages to a phenotype that releases Interleukine-10 (IL-10), and d) expression of stanniocalcin-1, which minimizes ROS [91].
9.5. Homing
The exact mechanisms by which MSCs move and reside within tissues are still unknown. The cells tether and roll against the endothelium in the first step, causing the cells to decelerate in the blood flow. The cells are activated by G-protein-coupled receptors in the second step, which is succeeded by an integrin-mediated, activation-dependent arrest in the third step. Finally, in the fourth step, the cells transmigrate through the endothelium and the underlying basement membrane [92]. Several factors influence the therapeutic efficacy of MSC homing. Culture conditions, passage count, donor age, delivery method, and host receptivity are crucial factors [87].
10. PRECLINICAL STUDIES
Li and colleagues were among the first to investigate MSC's therapeutic benefits in PD models in 2001. PD was induced in adult male mice by giving MPTP hydrochloride for a week (intraperitoneally). Undifferentiated BM-MSC were stereotaxically injected intrastriatal into the mice after this week. The motor behavior of animals was assessed with the help of a rotarod test, a month following transplantation. Their brains were removed for immunohistochemistry examination. MSCs infused intrastriatally survive and improve functional recovery, according to the results [93]. Riecke et al. performed a meta-analysis in 2015 that evaluated the impact of MSC treatment on behavioral impairment in PD models. MSC may be explored for clinical studies in the treatment of early-stage PD, according to the results [94].
One more study investigated the impact of human umbilical cord-derived MSC (hUC-MSC) on the MPTP-induced PD model. This hUC-MSC was previously stimulated by curcumin, a pigment that possesses anti-apoptotic and anti-inflammatory properties. In comparison to the control group, the supernatant of PC12 cells after hUC-MSC injection contained relatively low levels of NO and proinflammatory cytokines, but more differentiated DAergic neurons. These cells were discovered to represent a possible approach for promoting DAergic neuronal cell multiplication and differentiation [95]. In a PD model, AD-MSC had persistent anti-inflammatory and neurogenic effects, as well as improving cognitive function [96].
11. CLINICAL TRIALS
Dopamine replacement medication (L-DOPA or levodopa) is the primary treatment for PD in humans; however, it has side effects and the drawback that it fails to cease the advancement of DAergic neuronal loss in the substantia nigra. In search of an approach for DAergic neuron regeneration, the positive outcomes achieved from treating experimental PD models with MSC in humans highlighted the potential of adopting this strategy [97]. Clinical trials using MSCs as a therapeutic agent are being carried out due to their low immunogenicity, lack of teratoma risk and ethical issue, and low likelihood of tumorigenicity after transplantation into the human body [98]. However, none are successful. Clinical trials focusing on MSC-mediated therapy for PD, currently set time from diagnosis and age as the primary inclusion criteria. The primary outcome measures for evaluating MSC therapy effectiveness differ in various studies as can be noted from Table 2, while progress has lately been made in proposing a worldwide agreement of PD51 outcome measures. Furthermore, scales such as the Hoehn and Yahr scale, the Unified PD Rating Scale (UPDRS), and the non-motor symptoms questionnaire (NMS-Quest), etc., are used not only to recruit individuals but also to track their clinical progress [79].
Table 2.
Clinical trials of MSC in PD [97].
| Title | Study Type | Study Design | No. of Participants | Route of Administration | Outcome Measures | Clinicaltrials.gov ID |
|---|---|---|---|---|---|---|
| To Study the Safety and Efficacy of Bone Marrow-Derived MSC Transplant in PD | Interventional | Single group assignment | 5 | Cerebral | Improvement in the clinical condition of the patient assessed using UPDRS (UNIFIED PARKINSON'S DISEASE RATING SCALE) and Time Tests. | NCT00976430 |
| Phase I/II Trial of Autologous Bone Marrow-Derived MSC to Patients With PD. | Interventional | Single group assignment | 20 | Intravenous | Number of participants who experienced adverse effects | NCT01446614 |
| Autologous Mesenchymal Stem Cell Therapy in Progressive Supranuclear Palsy: a Randomized, Double-blind, Controlled Clinical Trial | Interventional | Randomized | 25 | Arterial | Incidence of adverse effects | NCT01824121 |
| Pilot Phase I Study of Allogeneic Bone Marrow-Derived MSC Therapy for Idiopathic PD | Interventional | Non-randomized | 20 | Intravenous | safety of allogeneic MSC therapy in patients with iPD as indicated by the presence of adverse events that are confirmed to be related to the therapy | NCT02611167 |
| Safety and Efficacy Investigation of Patients With PD by Transplantation of Umbilical Cord Derived MSC | Interventional | Single group assignment | 20 | Intravenous | Changes of the Unified Parkinson's Disease Rating Scale (UPDRS) | NCT03550183 |
| A Safety and Efficacy Study of the Effects of MSC (MSCs) Differentiated Into Neural Stem Cells (NSCs) on the Motor and Non-motor Symptoms in People With PD (PD). | Interventional | Randomized | 10 | Intrathecal and intravenous | Incidence of Treatment-Emergent Adverse Events (TAEAs) as a result of the injection | NCT03684122 |
| Allogeneic Bone Marrow-derived MSC as a Disease-modifying Therapy for Idiopathic PD: Phase IIa Double-blind Randomized Placebo-Controlled Trial | Interventional | Randomized | 45 | Not defined | Safest number of effective doses of MSC as measured by the Part III of the Movement Disorder Society Unified Parkinson's disease Rating Scale (MDS-UPDRS) scale | NCT04506073 |
12. LIMITATIONS
Even though MSC transplantation has been a successful therapy for PD treatment, this is primarily owing to their trophic effects that are not long-lasting. Furthermore, intravenous infusion of MSCs at a higher concentration has been documented to produce pulmonary thrombosis [99]. As a result, from the standpoint of cell-based treatment, stereotaxic transplantation of MSC-derived DAergic neurons directly into the striatum of the patients is preferred. Since plasmid incorporation is required for DAergic neuron production utilizing NICD transfection, additional long-term research is required to assure tumour growth and effectiveness of modified MSCs.
MSCs are a diverse group of stem cells since they are frequently obtained from mesenchymal tissues as adherent cells [100]. MSC population might be infected by these adhering cells, especially early in the growth process. Following subcultures, the cells appear to cluster on general MSCs, with other cells being overlooked; however, MSCs are still not a single homogenous cell type. As a result, the concept of MSCs is still unclear, and there is a need to discover a distinct molecular marker that is uniquely expressed by MSCs. Because of all these factors, the differentiation of MSCs into DAergic neurons is still a mystery. MSCs do display trilineage differentiation, although the differentiation ratio is often low, and hence a subgroup of MSCs is believed to be linked to differentiation. The prospect for MSCs to be used to treat PD would be considerably enhanced if the cells accountable for differentiating into DAergic neurons are identified.
Despite the abovementioned limitations, MSC therapy remains a therapeutic reality, because MSCs can be effectively cultivated in the laboratory to obtain the required therapeutic scale and are freely available cells with minimal moral issues. Since they are already routinely employed in clinical conditions such as myocardial infarction and osteoarthritis, MSCs have a proven record in these settings. Moreover, they may be procured readily from patients, marrow banks, as well as through autologous transplantation, which may reduce the risk of rejection.
CONCLUSION AND FUTURE PROSPECTS
PD is a debilitating, degenerative condition for which there is presently no cure. The substantia nigra pars compacta, which characterized pathologically by persistent degeneration of DAergic neurons. PD can be treated in a variety of ways, including medication therapy and surgery. However, there is currently no effective treatment for PD. Through multi-mechanistic techniques, the current study highlighted MSCs' ability to ameliorate PD pathogenesis (immunomodulatory, anti-inflammatory, and anti-apoptotic effects as well as neurotrophic and neurogenic potentials). These encouraging findings pave the path for clinical trials of MSCs in treating neurodegenerative disorders, especially PD. Although these cells have been widely regarded as safe, future research should focus on constant monitoring and long-term follow-up to avoid the chance of tumor growth after MSC therapy. Previous clinical trial data should influence future paths, emphasizing the critical necessity for a systematic approach to finding optimal combinations of circumstances to develop reliable and effective treatment designs for PD.
ACKNOWLEDGEMENTS
The Authors are grateful to the dean of Pharmacy college and rector of University of Hail for their continues encouragement.
LIST OF ABBREVIATIONS
- 6-OHDA
6-Hydroxydopamine
- AD-MSC
Adipose Tissue-derived mesenchymal Stem Cells
- AF-MSC
Amniotic Fluid-derived Mesenchymal Stem Cells
- Ang-1
Angiopoietin-1
- Ang-2
Angiopoietin-2
- APCs
Antigen-presenting Cells
- aSyn
Alpha-synuclein
- bFGF
Basic Fibroblast Growth Factor
- BM-MSC
Bone Marrow-derived Mesenchymal Stem Cells
- BNDF
Brain-derived Neurotrophic Factors
- CFU-F
Colony-forming Unit of Fibroblast
- CNTF
Ciliary Neurotrophic Factor
- DAergic
Dopaminergic
- DP-MSC
Dental Pulp Derived Mesenchymal Stem Cells
- DPCM
Dental Pulp Conditioned Medium
- FGF-2
Fibroblast Growth Factor 2
- FSK
Forskolin
- GBA
Glucocerebrosidase
- GDNF
Glial Cell Line-derived Neurotrophic Factors
- GvHD
Graft Versus Human Disease
- hEDSC
Human Endometrium-derived Stem Cells
- HGF
Hepatocyte Growth Factors
- IGF-1
Insulin-like Growth Factor 1
- IL
Interleukine
- LRRK2
Leucine-rich Repeat Kinase 2
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSCs
Mesenchymal Stem Cells
- NGF
Nerve Growth Factor
- NICD
Notch1 Intracellular Domain
- NK cells
Natural Killer Cells
- NPCs
Neural Progenitor Cells
- NT-3
Neurotrophin-3
- NTFSC
Neurotrophic Factors-secreting Cells
- P-MSC
Placenta Derived Mesenchymal Stem Cells
- PARK2
Parkin RBR E3 Ubiquitin-protein Ligase
- PARK7
Parkinson Protein 7
- PD
Parkinson’s Disease
- PDGF
Platelet-derived Growth Factor
- PINK1
Phosphatase and Tensing Homolog-induced Kinase 1
- REM
Rapid Eye Movement
- ROS
Reactive Oxygen Species
- SDF-1
Stromal Cell-derived Factor-1
- SHED
Stem Cells Derived From Human Exfoliated Deciduous Teeth
- TGF-β
Transforming Growth Factors-β
- TNF
Tumour Necrosis Factor
- UC-MSC
Umbilical Cord-derived Stem Cells
- VEGF
Vascular Endothelial Growth Factors
- VPS35
Vacuolar Protein Sorting-associated Protein 35
- WJ-MSC
Wharton’s Jelly Derived Mesenchymal Stem Cells
- β-NGF
Beta-nerve Growth Factors
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Marvanova M. Introduction to Parkinson disease (PD) and its complications. Ment. Health Clin. 2016;6(5):229–235. doi: 10.9740/mhc.2016.09.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball N., Teo W.P., Chandra S., Chapman J. Parkinson’s disease and the environment. Front. Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fayyaz M., Jaffery S.S., Anwer F., Zil-E-Ali A., Anjum I. The effect of physical activity in Parkinson’s disease: A mini-review. Cureus. 2018;10(7):e2995. doi: 10.7759/cureus.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Váradi C. Clinical features of Parkinson’s disease: The evolution of critical symptoms. Biology (Basel) 2020;9(5):103. doi: 10.3390/biology9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massano J., Bhatia K.P. Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012;2(6):a008870. doi: 10.1101/cshperspect.a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman J.G., Postuma R. Premotor and nonmotor features of Parkinson’s disease. Curr. Opin. Neurol. 2014;27(4):434–441. doi: 10.1097/WCO.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMaagd G., Philip A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P&T. 2015;40(8):504–532. [PMC free article] [PubMed] [Google Scholar]
- 8.Stoker T.B., Barker R.A. Recent developments in the treatment of Parkinson’s disease. F1000Res. 2020;9:F1000 Faculty Rev-862. doi: 10.12688/f1000research.25634.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salem N.A. Mesenchymal stem cell based therapy for Parkinson’s disease. Int. J. Stem Cell Res. Ther. 2019;6:62. doi: 10.23937/2469-570X/1410062. [DOI] [Google Scholar]
- 10.Lindvall O., Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1(4):382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y., Winanto N., Ng S.Y. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl. Neurodegener. 2020;9(1):2. doi: 10.1186/s40035-019-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuhara T., Kameda M., Sasaki T., Tajiri N., Date I. Cell Therapy for Parkinson’s Disease. Cell Transplant. 2017;26(9):1551–1559. doi: 10.1177/0963689717735411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawitan J.A. Prospect of cell therapy for Parkinson’s disease. Anat. Cell Biol. 2011;44(4):256–264. doi: 10.5115/acb.2011.44.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gugliandolo A., Bramanti P., Mazzon E. Mesenchymal stem cell therapy in Parkinson’s disease animal models. Curr. Res. Transl. Med. 2017;65(2):51–60. doi: 10.1016/j.retram.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Wang X., Li J., Huang R., Yu X., Dong C., Liu P., Zhang F., Hu J., Qi Y., Zhang J., Li Q., Yan B. The preclinical research progress of stem cells therapy in Parkinson’s disease. BioMed Res. Int. 2016;2016:5683097. doi: 10.1155/2016/5683097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capitelli C.S., Lopes C.S., Alves A.C., Barbiero J., Oliveira L.F., da Silva V.J., Vital M.A. Opposite effects of bone marrow-derived cells transplantation in MPTP-rat model of Parkinson’s disease: A comparison study of mononuclear and mesenchymal stem cells. Int. J. Med. Sci. 2014;11(10):1049–1064. doi: 10.7150/ijms.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataramana N.K., Pal R., Rao S.A., Naik A.L., Jan M., Nair R., Sanjeev C.C., Kamble R.B., Murthy D.P., Chaitanya K. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: A pilot clinical study. Stem Cells Int. 2012;2012:931902. doi: 10.1155/2012/931902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trzaska K.A., Kuzhikandathil E.V., Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25(11):2797–2808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- 19.Venkataramana N.K., Kumar S.K., Balaraju S., Radhakrishnan R.C., Bansal A., Dixit A., Rao D.K., Das M., Jan M., Gupta P.K., Totey S.M. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl. Res. 2010;155(2):62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Politis M., Lindvall O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012;10(1):1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan X., Sun Z., Chu C., Boltze J., Li S. Dental pulp stem cells: An attractive alternative for cell therapy in ischemic stroke. Front. Neurol. 2019;10:824. doi: 10.3389/fneur.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z., Lei T., Liu Y., Yang Y., Bi W., Du H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson’s disease. Stem Cell Res. Ther. 2021;12(1):5. doi: 10.1186/s13287-020-01957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda T., Inden M., Ito T., Kurita H., Hozumi I. Characteristics and therapeutic potential of dental pulp stem cells on neurodegenerative diseases. Front. Neurosci. 2020;14:407. doi: 10.3389/fnins.2020.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priyan G.L.S., Ramalingam S., Udhayakumar Y. Human dental pulp stem cells and its applications in regenerative medicine – A literature review. J Global Oral Health. 2019;2(1):59–67. doi: 10.25259/JGOH_54_2019. [DOI] [Google Scholar]
- 25.Yamada Y., Nakamura-Yamada S., Kusano K., Baba S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: A concise review. Int. J. Mol. Sci. 2019;20(5):1132. doi: 10.3390/ijms20051132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji W., Rubin J.P., Marra K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells. 2014;6(3):312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Quinn G., Okochi H., Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y., Hoogduijn M.J., Baan C.C., Korevaar S.S., de Kuiper R., Yan L., Wang L., van Besouw N.M. Adipose tissue-derived mesenchymal stem cells have a heterogenic cytokine secretion profile. Stem Cells Int. 2017;2017:4960831. doi: 10.1155/2017/4960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobita M., Tajima S., Mizuno H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: stem cell transplantation methods that enhance stemness. Stem Cell Res. Ther. 2015;6(1):215. doi: 10.1186/s13287-015-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin L., Zhu Y., Yang J., Ni Y., Zhou Z., Chen Y., Wen L. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol. Med. Rep. 2015;11(3):1722–1732. doi: 10.3892/mmr.2014.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakao N., Nakayama T., Yahata T., Muguruma Y., Saito S., Miyata Y., Yamamoto K., Naoe T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am. J. Pathol. 2010;177(2):547–554. doi: 10.2353/ajpath.2010.091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamura-Inoue T., He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells. 2014;6(2):195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesarova L., Jaresova K., Simara P., Koutna I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int. J. Mol. Sci. 2020;21(15):5366. doi: 10.3390/ijms21155366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao Y.H., Wu H.P., Chan C.K., Tsai C., Peng C.T., Wu K.H. Umbilical cord-derived mesenchymal stem cells for hematopoietic stem cell transplantation. J. Biomed. Biotechnol. 2012;2012:759503. doi: 10.1155/2012/759503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitada M., Dezawa M. Parkinson’s disease and mesenchymal stem cells: potential for cell-based therapy. Parkinsons Dis. 2012;2012:873706. doi: 10.1155/2012/873706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira M.S., Barreto-Filho J.B. Placental-derived stem cells: Culture, differentiation and challenges. World J. Stem Cells. 2015;7(4):769–775. doi: 10.4252/wjsc.v7.i4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddesh S.E., Gowda D.M., Jain R., Gulati A., Patil G.S., Anudeep T.C., Jeyaraman N., Muthu S., Jeyaraman M. Placenta-derived mesenchymal stem cells (P-MSCs) for COVID-19 pneumonia-a regenerative dogma. Stem Cell Investig. 2021;8:3. doi: 10.21037/sci-2020-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wouters G., Grossi S., Mesoraca A., Bizzoco D., Mobili L., Cignini P., Giorlandino C. Isolation of amniotic fluid-derived mesenchymal stem cells. J. Prenat. Med. 2007;1(3):39–40. [PMC free article] [PubMed] [Google Scholar]
- 39.Spitzhorn L.S., Rahman M.S., Schwindt L., Ho H.T., Wruck W., Bohndorf M., Wehrmeyer S., Ncube A., Beyer I., Hagenbeck C., Balan P., Fehm T., Adjaye J. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017;2017:5932706. doi: 10.1155/2017/5932706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava M., Ahlawat N., Srivastava A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. J. Obstet. Gynaecol. India. 2018;68(1):15–19. doi: 10.1007/s13224-017-1034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed H.H., Salem A.M., Atta H.M., Eskandar E.F., Farrag A.R., Ghazy M.A., Salem N.A., Aglan H.A. Updates in the pathophysiological mechanisms of Parkinson’s disease: Emerging role of bone marrow mesenchymal stem cells. World J. Stem Cells. 2016;8(3):106–117. doi: 10.4252/wjsc.v8.i3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrzejewska A., Dabrowska S., Lukomska B., Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. (Weinh.) 2021;8(7):2002944. doi: 10.1002/advs.202002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajpoot S., Tewar G. Review on stem cells: Basics classification and applications. Int. J. Pharm. Sci. Rev. Res. 2018;49(2):48–52. [Google Scholar]
- 44.Barky AR., Ali E.M.M., Mohamed T.M. Stem cells, classifications and their clinical applications. Am. J. Pharmacol. Ther. 2017;1(1):001-007. [Google Scholar]
- 45.Yang C., Dai W., Chen H., Wu B. Application of human bone marrow-derived mesenchymal stem cells in the treatment of radiation-induced Gastrointestinal syndrome. Sci. China Life Sci. 2014;57(12):1177–1182. doi: 10.1007/s11427-014-4721-3. [DOI] [PubMed] [Google Scholar]
- 46.Kemp K.C., Hows J., Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk. Lymphoma. 2005;46(11):1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 47.Ming Li., Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drzewiecki B.A., Thomas J.C., Tanaka S.T. Bone marrow-derived mesenchymal stem cells: Current and future applications in the urinary bladder. Stem Cells Int. 2010;2010:765167. doi: 10.4061/2010/765167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Ju B., Pan C., Gu Y., Zhang Y., Sun L., Zhang B., Zhang Y. Application of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions in Rats. Cell. Physiol. Biochem. 2016;39(4):1553–1560. doi: 10.1159/000447857. [DOI] [PubMed] [Google Scholar]
- 50.Miana V.V., González E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018;12:822. doi: 10.3332/ecancer.2018.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Liu Y., Chen Y., Yuan L., Liu H., Wang J., Liu Q., Zhang Y. Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020;2020:8810813. doi: 10.1155/2020/8810813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu L., Tang Q., Yin X., Yan D., Tang M., Xin J., Pan Q., Ma C., Yan S. The therapeutic potential of adipose tissue-derived mesenchymal stem cells to enhance radiotherapy effects on hepatocellular carcinoma. Front. Cell Dev. Biol. 2019;7:267. doi: 10.3389/fcell.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minteer D., Marra K.G., Rubin J.P. Adipose-derived mesenchymal stem cells: biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013;129:59–71. doi: 10.1007/10_2012_146. [DOI] [PubMed] [Google Scholar]
- 54.Ma H., Lam P.K., Siu W.S., Tong C.S.W., Lo K.K.Y., Koon C.M., Wu X.X., Li X., Cheng W., Shum W.T., Leung P.C. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model - A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 2021;14:753–764. doi: 10.2147/CCID.S298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krawczenko A., Klimczak A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022;23(5):2425. doi: 10.3390/ijms23052425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frese L., Dijkman P.E., Hoerstrup S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016;43(4):268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso-Goulart V., Ferreira L.B., Duarte C.A., de Lima I.L., Ferreira E.R. Mesenchymal stem cells from human adipose tissue and bone repair: a literature review. Biotechnology Research and Innovation. 2018;2(1):74–80. doi: 10.1016/j.biori.2017.10.005. [DOI] [Google Scholar]
- 58.Aly R.M. Current state of stem cell-based therapies: an overview. Stem Cell Investig. 2020;7:8. doi: 10.21037/sci-2020-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potdar P.D., Jethmalani Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells. 2015;7(5):839–851. doi: 10.4252/wjsc.v7.i5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ledesma-Martínez E., Mendoza-Núñez V.M., Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int. 2016;2016:4709572. doi: 10.1155/2016/4709572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B., Ouchi T., Cao Y., Zhao Z., Men Y. Dental-derived mesenchymal stem cells: state of the art. Front. Cell Dev. Biol. 2021;9:654559. doi: 10.3389/fcell.2021.654559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masuda K., Han X., Kato H., Sato H., Zhang Y., Sun X., Hirofuji Y., Yamaza H., Yamada A., Fukumoto S. Dental pulp-derived mesenchymal stem cells for modeling genetic disorders. Int. J. Mol. Sci. 2021;22(5):2269. doi: 10.3390/ijms22052269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi X., Mao J., Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020;9(4):445–464. doi: 10.1002/sctm.19-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deedwania P., Deka D., Mohanty S., Dadhwal V., Sharma A. Isolation and characterization of mesenchymal stem cells derived from amniotic fluid: A prospective study. Int. J. MolImmuno. Oncol. 2020;5(2):67–72. doi: 10.25259/IJMIO_22_2019. [DOI] [Google Scholar]
- 65.Kim E.Y., Lee K.B., Kim M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47(3):135–140. doi: 10.5483/BMBRep.2014.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jafari A., Rezaei-Tavirani M., Farhadihosseinabadi B., Zali H., Niknejad H. Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin. Stem Cell Res. Ther. 2021;12(1):126. doi: 10.1186/s13287-021-02196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang B., Ding C., Zou Q., Lu J., Wang W., Li H. Human amniotic fluid mesenchymal stem cells improve ovarian function during physiological aging by resisting DNA damage. Front. Pharmacol. 2020;11:272. doi: 10.3389/fphar.2020.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J., Wang D., Liang T., Guo Q., Zhang G. Amniotic fluid-derived mesenchymal stem cells: characteristics and therapeutic applications. Arch. Gynecol. Obstet. 2014;290(2):223–231. doi: 10.1007/s00404-014-3231-7. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Y., Yang Y., Zhang Y., Hao G., Liu T., Wang L., Yang T., Wang Q., Zhang G., Wei J., Li Y. Placental mesenchymal stem cells of fetal and maternal origins demonstrate different therapeutic potentials. Stem Cell Res. Ther. 2014;5(2):48. doi: 10.1186/scrt436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chia W.K., Cheah F.C., Abdul Aziz N.H., Kampan N.C., Shuib S., Khong T.Y., Tan G.C., Wong Y.P. A review of placenta and umbilical cord-derived stem cells and the immunomodulatory basis of their therapeutic potential in bronchopulmonary dysplasia. Front Pediatr. 2021;9:615508. doi: 10.3389/fped.2021.615508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farini A., Sitzia C., Erratico S., Meregalli M., Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014;2014:306573. doi: 10.1155/2014/306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbaspanah B., Reyhani S., Mousavi S.H. Applications of Umbilical Cord Derived Mesenchymal Stem Cells in Autoimmune and Immunological Disorders: From Literature to Clinical Practice. Curr. Stem Cell Res. Ther. 2021;16(4):454–464. doi: 10.2174/1574888X16999201124153000. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.Y., Jeon H.B., Yang Y.S., Oh W., Chang J.W. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J. Stem Cells. 2010;2(2):34–38. doi: 10.4252/wjsc.v2.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marino L., Castaldi M.A., Rosamilio R., Ragni E., Vitolo R., Fulgione C., Castaldi S.G., Serio B., Bianco R., Guida M., Selleri C. Mesenchymal Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int. J. Stem Cells. 2019;12(2):218–226. doi: 10.15283/ijsc18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabapathy V., Sundaram B. v M, S.; Mankuzhy, P.; Kumar, S. Human Wharton’s Jelly Mesenchymal Stem Cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9(4):e93726. doi: 10.1371/journal.pone.0093726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rocha J.L.M., de Oliveira W.C.F., Noronha N.C., Dos Santos N.C.D., Covas D.T., Picanço-Castro V., Swiech K., Malmegrim K.C.R. Mesenchymal Stromal Cells in Viral Infections: Implications for COVID-19. Stem Cell Rev. Rep. 2021;17(1):71–93. doi: 10.1007/s12015-020-10032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hmadcha A., Martin-Montalvo A., Gauthier B.R., Soria B., Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim N., Cho S.G. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2013;28(4):387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fričová, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020;5(1):20. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin J.Y., Lee P.H. Mesenchymal stem cells modulate misfolded α-synuclein in parkinsonian disorders: A multitarget disease-modifying strategy. Stem Cell Res. (Amst.) 2020;47:101908. doi: 10.1016/j.scr.2020.101908. [DOI] [PubMed] [Google Scholar]
- 81.Yao P., Zhou L., Zhu L., Zhou B., Yu Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur. Neurol. 2020;83(3):235–241. doi: 10.1159/000509268. [DOI] [PubMed] [Google Scholar]
- 82.Dimarino A.M., Caplan A.I., Bonfield T.L. Mesenchymal stem cells in tissue repair. Front. Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y., Shen J., Ke K., Gu X. Clinical potential and current progress of mesenchymal stem cells for Parkinson’s disease: a systematic review. Neurol. Sci. 2020;41(5):1051–1061. doi: 10.1007/s10072-020-04240-9. [DOI] [PubMed] [Google Scholar]
- 84.Dezawa M., Kanno H., Hoshino M., Cho H., Matsumoto N., Itokazu Y., Tajima N., Yamada H., Sawada H., Ishikawa H., Mimura T., Kitada M., Suzuki Y., Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Invest. 2004;113(12):1701–1710. doi: 10.1172/JCI200420935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park H.J., Lee P.H., Bang O.Y., Lee G., Ahn Y.H. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J. Neurochem. 2008;107(1):141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 86.Hima Bindu A., Srilatha B. Potency of Various Types of Stem Cells and their Transplantation. J. Stem Cell Res. Ther. 2011;1(3):115. doi: 10.4172/2157-7633.1000115. [DOI] [Google Scholar]
- 87.Berrío Sánchez J., Cucarian Hurtado J., Barcos Nunes R., de Oliveira A.A. Mesenchymal stem cell transplantation and aerobic exercise for Parkinson’s disease: therapeutic assets beyond the motor domain. Rev. Neurosci. 2019;30(2):165–178. doi: 10.1515/revneuro-2018-0011. [DOI] [PubMed] [Google Scholar]
- 88.Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28(7):801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J., Moll G., Cox C.S., Jr Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi Y., Su J., Roberts A.I., Shou P., Rabson A.B., Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33(3):136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glavaski-Joksimovic A., Bohn M.C. Mesenchymal stem cells and neuroregeneration in Parkinson’s disease. Exp. Neurol. 2013;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Lee P.H., Park H.J. Bone marrow-derived mesenchymal stem cell therapy as a candidate disease-modifying strategy in Parkinson’s disease and multiple system atrophy. J. Clin. Neurol. 2009;5(1):1–10. doi: 10.3988/jcn.2009.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y., Chen J., Wang L., Zhang L., Lu M., Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci. Lett. 2001;316(2):67–70. doi: 10.1016/S0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 94.Riecke J., Johns K.M., Cai C., Vahidy F.S., Parsha K., Furr-Stimming E., Schiess M., Savitz S.I. A Meta-Analysis of Mesenchymal Stem Cells in Animal Models of Parkinson’s Disease. Stem Cells Dev. 2015;24(18):2082–2090. doi: 10.1089/scd.2015.0127. [DOI] [PubMed] [Google Scholar]
- 95.Jinfeng L., Yunliang W., Xinshan L., Yutong W., Shanshan W., Peng X., Xiaopeng Y., Zhixiu X., Qingshan L., Honglei Y., Xia C., Hongwei W., Bingzhen C. Therapeutic Effects of CUR-Activated Human Umbilical Cord Mesenchymal Stem Cells on 1-Methyl-4-phenylpyridine-Induced Parkinson’s Disease Cell Model. BioMed Res. Int. 2016;2016:9140541. doi: 10.1155/2016/9140541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwerk A., Altschüler J., Roch M., Gossen M., Winter C., Berg J., Kurtz A., Akyüz L., Steiner B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen. Med. 2015;10(4):431–446. doi: 10.2217/rme.15.17. [DOI] [PubMed] [Google Scholar]
- 97.De Becker A., Riet I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells. 2016;8(3):73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mendes Filho D., Ribeiro P.D.C., Oliveira L.F., de Paula D.R.M., Capuano V., de Assunção T.S.F., da Silva V.J.D. Therapy With Mesenchymal Stem Cells in Parkinson Disease: History and Perspectives. Neurologist. 2018;23(4):141–147. doi: 10.1097/NRL.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 99.Ramot Y., Steiner M., Morad V., Leibovitch S., Amouyal N., Cesta M.F., Nyska A. Pulmonary thrombosis in the mouse following intravenous administration of quantum dot-labeled mesenchymal cells. Nanotoxicology. 2010;4(1):98–105. doi: 10.3109/17435390903470093. [DOI] [PubMed] [Google Scholar]
- 100.Kuroda Y., Kitada M., Wakao S., Nishikawa K., Tanimura Y., Makinoshima H., Goda M., Akashi H., Inutsuka A., Niwa A., Shigemoto T., Nabeshima Y., Nakahata T., Nabeshima Y., Fujiyoshi Y., Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl. Acad. Sci. USA. 2010;107(19):8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]