Abstract
Folate is hypothesized to accelerate cell proliferation in colorectal cancer (CRC) by supporting DNA synthesis, while alcohol is also linked to gastrointestinal epithelial proliferation, despite biological antagonism of folate. We report associations between folate and alcohol consumption with the proliferation marker Ki67 in CRC tumors from the Southern Community Cohort Study. Tumor samples were obtained from formalin-fixed paraffin-embedded tissue blocks. The percentage of cells expressing Ki67 was measured immunohistochemically. Exposures were assessed via questionnaire pre-diagnosis. Associations were assessed via linear regression. In 248 cases (40–78 years), neither dietary folate, folic acid supplements, nor total folate intake were associated with Ki67. Folic acid supplement use was associated with Ki67 in distal/rectal tumors (β [95% confidence interval]: 7.5 [1.2–13.8], p=.02) but not proximal tumors (−1.4 [−7.1–4.3], p=.62). A positive trend for total folate was observed for distal/rectal tumors (1.6 [0.0–3.3] per 200 μcg, p-trend=.05). Heavy drinking (women: ≥1 drink/day, men: ≥2 drinks/day) was associated with higher Ki67 (6.4 [1.0–11.9], versus non-drinkers, p=.02), especially for distal/rectal tumors (10.4 [1.6–19.1], p=.02). Negative interaction between alcohol, total folate was observed for distal/rectal tumors (p-interaction=.06). Modest associations between folate, alcohol consumption and distal/rectal tumor Ki67 expression suggest accelerated proliferation, consistent with folate’s role in DNA synthesis.
Keywords: folate, folic acid, alcohol, colorectal cancer, Ki67
Introduction
A majority of epidemiological studies report an inverse association between dietary folate (vitamin B9) and risk for incident colorectal cancer (CRC) (1–3), the third most common cancer in the United States for men and women (4). These findings are supported by evidence of a protective biological mechanism, as folate’s role in one-carbon metabolism is required for normal DNA methylation and synthesis of purines and thymidine required for DNA synthesis in mitotic cells (5). Inadequate intracellular folate may cause uracil misincorporation in DNA (6–8), leading to strand breaks and chromosomal instability (7,9). The evidence for a causal, protective association between folate and CRC is bolstered by genetic studies showing that a common variant in a folate metabolism gene is associated with lower risk for CRC (10).
However, there is concern that high folate consumption, including dietary fortification and supplementation with folic acid, which has higher bioavailability than folate (11) but is enzymatically converted to folate upon absorption (5), may accelerate growth of colorectal neoplasms via synthesis of nucleotides required for proliferation (12,13). This hypothesis is supported by data from studies in CRC mouse models (13–15), clinical trials showing increased risk for adenoma recurrence (16) or colorectal cancer (17) with folic acid supplements, and the success of anti-folate chemotherapies, including methotrexate and 5-fluorouracil. These chemotherapies work by inhibiting folate metabolic enzymes required to synthesize thymidine and purines for DNA (18,19). However, there has been limited investigation into identifying the role of folate consumption in CRC tumor proliferation in humans.
Similar to high folate consumption, results from cross-sectional studies and animal models suggest that heavy alcohol consumption is associated with greater proliferation of the rectal mucosa (20–22). Intriguingly, these studies indicate that alcohol may accelerate proliferation of the mucosa despite well-known biological antagonism of folate (23,24). Alcohol triggers folate catabolism in the large intestine (25) and reduces the expression of folate-transport proteins in multiple tissues (24). Currently, data are lacking from studies of alcohol intake and human CRC, and it is not known whether regular alcohol consumption is associated with accelerated cell proliferation in CRC tumors, or whether drinking alcohol modifies the proliferative effect of folate.
Better understanding the influence of folate and alcohol consumption on CRC tumor proliferation is important given mandatory folic acid fortification in the United States and across the world (26,27), high rates of folic acid supplementation (28), and alcohol use (29). One way to address this gap is to investigate associations between folate, alcohol consumption and the expression of Ki67 in CRC tumor samples, as Ki67 is an objective marker of tumor proliferation and consistently associated with mortality in epidemiological studies (see recent meta-analysis by Luo et al. (30)). In the present study, we report associations between folate/folic acid consumption from diet and supplements and Ki67 expression in CRC tumor samples, as well as associations and the combined impact of folate and alcohol intake alcohol. These results may shed light on the role of modifiable lifestyle factors underlying the molecular attributes of CRC.
Materials and methods
Study participants
Participants were recruited from the Southern Community Cohort Study (SCCS), a prospective study from the southeastern United States designed to investigate racial and socioeconomic disparities in health (31,32). The SCCS study design, aims, and recruitment have been described previously (31). In brief, more than 85,000 English-speaking participants (age 40–79) were enrolled between 2002 and 2009. Upon enrollment, participants provided a serum sample and completed interviews and questionnaires concerning medical history, diet, lifestyle, physical activity, and demographic background. Incident CRC cases through December 31, 2017 were identified through linkage to state cancer registries (International Classification of Diseases-Oncology-3 (ICD-O-3) codes C180 – C189, C199, C209, and C260). There were 114 proximal tumors (ascending or transverse colon: ICD-O-3 codes C180-C185), 122 distal or rectal tumors (descending or sigmoid colon, rectum: C186, C187, C199, and C209), and 12 tumors with indeterminate site (C188, C189, or missing site). Tumor stage was determined via the TNM staging system (American Joint Committee on Cancer, Seventh Edition) (33). Tumor samples were obtained from formalin-fixed paraffin-embedded tissue blocks from the primary tumor. Samples were collected at the time of surgical resection from each case’s treating medical facility.
The Institutional Review Boards of Vanderbilt University Medical Center and Meharry Medical College provided approval for this study. All participants provided written informed consent, and all study procedures conformed to the tenets of the Declaration of Helsinki.
Participant eligibility and colorectal cancer assessment
Participants eligible for this analysis had an incident CRC tumor diagnosed subsequent to SCCS enrollment and an available tumor sample (n = 287). Participants were excluded who did not complete the food frequency questionnaire (FFQ) or had ≥ 10 missing FFQ items (n = 28), or reported unrealistic total energy intake (< 800 kcal/day or > 8,000 kcal/day) (n = 11), leaving 248 cases for the analysis.
Dietary assessment and alcohol consumption
Typical diet from the past year was measured at enrollment via computer-assisted FFQ. The FFQ developed for the SCCS has been described previously (34) and consisted of 89 line-items across 12 major headings. For each line-item, participants were asked to select from nine frequency categories, ranging from “never” or “rarely” up to “2+ servings/day”. While the FFQ did not query portion sizes, race and sex-specific portion size estimates were used to estimate nutrient intakes. The approach to estimate the nutrient composition for each FFQ item has been previously described and accounted for mandatory fortification of selected micronutrients (including folic acid) that began in 1998 (35). Dietary folate and folic acid are reported as dietary folate equivalents (DFEs), a unit of measurement that accounts for greater bioavailability of folic acid relative to folate (DFEs = folate (mcg) + (folic acid (mcg) * 1.7)) (11,36). In an independent sample of 255 SCCS participants, estimated dietary folate was validated against serum levels of total folate (measured via validated microbiological assay (37)), showing a modest correlation (partial correlation coefficient = 0.26) (38).
The FFQ also included questions about supplement use in the past year including multivitamins and other supplements containing folic acid, with response options ranging from “never” to “2 or more times per day”. Folic acid supplement use was defined as consumption of a multivitamin or other folic acid supplement at least once weekly, with a majority of these participants (84.2%) taking these supplements once daily. Total consumption of folate and folic acid (including diet and supplements) was calculated as DFEs, assuming 400 mcg folic acid (667 DFEs) per serving from supplements (36).
Alcohol use over the past year was also assessed via baseline questionnaire. Participants who reported consuming alcohol in the previous year were queried about their intake in five categories, including 1) light beer, 2) regular beer, ale, malt liquor, or stout, 3) white wine, 4) red wine, and 5) liquor or mixed drinks. For each category, participants reported the consumption frequency (nine categories from “never” to “2+ per day”) and the number of drinks per drinking occasion, with total daily drinks calculated through multiplication. Total alcohol consumption (as drinks per day) was calculated by summing across categories. Participants were categorized as “non-drinkers” (0 drinks/day), “light drinkers” (alcohol consumption > 0 and < 1 drink/day for women, and < 2 drinks/day for men), or “heavy drinkers” (≥ 1 drink/day for women, and ≥ 2 drinks/day for men).
Tumor immunohistochemistry
A tyramide signal amplification-based fluorescent multiplex immunohistochemistry (mIHC/IF) technique (39) was applied for the detection of Ki67 in CRC tissues, and the stained slides were imaged using an automated fluorescence multispectral imaging system consisting of an Olympus BX-61 motorized microscope with six filter sets, X-Cite XYLIS Fluorescence Illuminator and imaging software (Q-Capture, ImageJ, CellProfiler). Ki67 staining, imaging, and quantification in CRC tumor samples have been described previously (40). The Ki67 tumor expression outcome was reported as the percentage of tumor cells expressing Ki67.
Statistical analysis
Multivariable linear regression was used to measure associations with tumor Ki67 expression for dietary folate, folic acid supplement use (≥1x vs. < 1x per week), total folate intake, and alcohol consumption (non-drinker, light drinker, and heavy drinker), and interactions between total folate intake and alcohol. Estimates were obtained in the full sample of CRC cases and separately by tumor location (proximal colon vs. distal colon and rectum). For dietary folate and total folate, regression estimates (β-coefficient with 95% confidence interval) were obtained by quartile of exposure (with quartile 1 as reference) and per 1000 DFE increase to determine the linear trend, with p-trend was obtained utilizing the continuous exposure variables. For alcohol consumption, estimates were obtained relative to the ‘non-drinker’ category, without test for linear trend. Model 1 was adjusted for age at enrollment (in 5-year categories), race (African American or non-African American), sex, and total energy intake (kcal/day). Models were adjusted for energy intake (as a continuous variable) to account for measurement error in the assessment of folate intake and to mitigate potential confounding by energy intake, physical activity, and body size (41). To identify additional confounding variables, additional covariates were added to model 1 and retained for model 2 if there was evidence for significant confounding (i.e. change in β-coefficient ≥ 10%) across multiple associations of interest, including associations for dietary folate, folic acid supplement use total folate, and alcohol consumption. The following covariates were tested: tumor stage (0-I, II, III, IV, missing); alcohol consumption (folate models only); total folate intake (alcohol models only); smoking status (never, current, former); physical activity (metabolic equivalents per week), education (< 12 years, completed high school or GED, some college, or college graduate); income (< $15,000, $15,000–24,999, $25,000–49,999, or ≥ $50,000); diabetes (yes, no), body mass index (BMI, < 25.0, 25.0–29.9, 30.0–34.9, and ≥35.0 kg/m2); ever screened for CRC (yes, no); and family history of CRC (yes, no). Alcohol/folate and tumor stage met the criterion for confounding, and subsequently were included in model 2. As a sensitivity analysis, the potential for residual confounding was investigated by adding previously listed covariates to model 2. To test for interaction between total folate intake and alcohol consumption (categorized as ‘non-drinker’ vs. ‘light/heavy drinker’), an interaction term was added to model 2. Likewise, to assess interactions with tumor site (‘proximal’ vs. ‘distal/rectal’) for each exposure, a separate model was run including an interaction term for tumor site with the exposure of interest.
For all models, residual plots were inspected to confirm that the assumptions required for linear regression analysis were met. To test for non-linearity in the relationship between continuous folate exposures and tumor Ki67, the linear models described above were compared to models with cubic-spline terms for dietary folate, total folate using Akaike’s information criteria (AIC) to evaluate model fit. Because the AIC statistic was consistently higher for the cubic-spline models (indicating no improvement in model fit), results from linear models are presented. As a relatively small proportion of covariate data was missing (less than 1–2% of observations), missing values for covariates were imputed using the sex and race-specific mode (for categorical categorical) or median (continuous covariates). All analyses were completed in the year 2022 using SAS version 9.4 (SAS Inc., Cary, NC). P < .05 was used as the threshold for statistical significance.
Results
Sample characteristics
In total, 248 CRC cases (40–78 years at enrollment) were analyzed (mean±standard deviation: 57±9 years). Cases were 56.0% female and 67.7% African American. The median time [inter-quartile range (IQR)] between enrollment and CRC diagnosis was 69.5 [41.0–99.0] months, which was similar for proximal tumors (73.0 [46.0–103.0] months) and tumors of the distal colon and rectum (67.0 [39.0–94.0] months). Tumor staging and other demographic, lifestyle, and health history characteristics of this sample are shown in Table 1. The median consumption of dietary folate was 586 DFE per day [IQR: 371–857] and 795 DFE [IQR: 433–1257] for total folate. Seventy-six cases (30.6%) reported folic acid supplement use at least once weekly. There were 134 participants categorized as ‘non-drinkers’ (54.0%), 79 ‘light drinkers’ (31.9%), and 35 ‘heavy drinkers’ (14.1%). Higher total folate intake was positively associated with physical activity, fruit and vegetable consumption, fiber, total energy intake, and the healthy eating index score (Table 1). Covariate levels by quartile of tumor Ki67 expression are reported in Supplementary Table S1.
Table 1:
Covariate levels a by quartile of total folate consumption, including folate/folic acid from diet and supplements b
| Total folate intake (DFE/day) b | |||||
|---|---|---|---|---|---|
| All cases N = 248 |
Q1 (125.7–429.9) N = 62 |
Q2 (436.6–793.3) N = 62 |
Q3 (797.1–1256.2) N = 62 |
Q4 (1258.3–3146.0) N = 62 |
|
| Enrollment age (years) | 57±9 | 56±9 | 57±9 | 57±10 | 58±9 |
| Tumor stage | |||||
| 0-I | 49 (20%) | 6 (10%) | 12 (19%) | 16 (26%) | 15 (24%) |
| II | 42 (17%) | 13 (21%) | 10 (16%) | 14 (23%) | 5 (8%) |
| III | 60 (24%) | 17 (27%) | 15 (24%) | 10 (16%) | 18 (29%) |
| IV | 61 (25%) | 15 (24%) | 13 (21%) | 13 (21%) | 20 (32%) |
| Missing | 36 (15%) | 11 (18%) | 12 (19%) | 9 (15%) | 4 (6%) |
| Sex | |||||
| Female | 139 (56%) | 37 (60%) | 34 (55%) | 37 (60%) | 31 (50%) |
| Male | 109 (44%) | 25 (40%) | 28 (45%) | 25 (40%) | 31 (50%) |
| Race | |||||
| White | 77 (31%) | 15 (24%) | 20 (32%) | 19 (31%) | 23 (37%) |
| African American | 168 (68%) | 46 (74%) | 42 (68%) | 41 (66%) | 39 (63%) |
| Hispanic/Latino | 1 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Asian or Pacific Islander | 1 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| 2+ race | 1 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Education | |||||
| < 12 years | 72 (29%) | 20 (32%) | 19 (31%) | 18 (29%) | 15 (24%) |
| Completed HS or GED | 76 (31%) | 16 (26%) | 22 (36%) | 23 (37%) | 15 (24%) |
| Some college | 69 (28%) | 21 (34%) | 14 (23%) | 17 (27%) | 17 (27%) |
| College graduate | 30 (12%) | 5 (8%) | 6 (10%) | 4 (6%) | 15 (24%) |
| Household income ($/year) | |||||
| < $15,000 | 133 (55%) | 32 (52%) | 32 (52%) | 38 (63%) | 31 (53%) |
| $15,000–24,999 | 49 (20%) | 13 (21%) | 12 (20%) | 13 (22%) | 11 (19%) |
| $25,000–49,999 | 38 (16%) | 10 (16%) | 11 (18%) | 7 (12%) | 10 (17%) |
| ≥ $50,000 | 21 (9%) | 6 (10%) | 6 (10%) | 2 (3%) | 7 (12%) |
| CRC screening (% yes) | 66 (27%) | 11 (18%) | 22 (35%) | 17 (27%) | 16 (26%) |
| CRC family history (% yes) | 23 (9%) | 7 (11%) | 5 (8%) | 5 (8%) | 6 (10%) |
| Aspirin in the last year (% yes) | 78 (31%) | 18 (29%) | 23 (37%) | 19 (31%) | 18 (29%) |
| Body mass index (kg/m2) | 30±7 | 30±6 | 31±8 | 30±6 | 31±7 |
| Diabetes (% yes) | 78 (31%) | 16 (26%) | 27 (44%) | 13 (21%) | 22 (35%) |
| Smoking status | |||||
| Current | 88 (35%) | 24 (39%) | 19 (31%) | 24 (39%) | 21 (34%) |
| Past | 71 (29%) | 20 (32%) | 13 (21%) | 22 (35%) | 16 (26%) |
| Never | 89 (36%) | 18 (29%) | 30 (48%) | 16 (26%) | 25 (40%) |
| Alcohol b,c | |||||
| Non-drinker | 134 (54%) | 37 (60%) | 36 (58%) | 30 (48%) | 31 (50%) |
| Light/moderate | 79 (32%) | 17 (27%) | 18 (29%) | 21 (34%) | 23 (37%) |
| Heavy | 35 (14%) | 8 (13%) | 8 (13%) | 11 (18%) | 8 (13%) |
| Physical activity (MET/wk) | 22±18 | 17±13 | 23±20 | 21±17 | 26±19 |
| Sedentary activity (Hours/day) | 9±5 | 9±5 | 9±5 | 9±5 | 9±5 |
| Energy intake (kcal/day) | 2476±1403 | 1419±394 | 2140±675 | 2748±1246 | 3597±1790 |
| Meat (servings/day) | 2±1 | 2±1 | 2±1 | 2±1 | 2±1 |
| Fruits/vegetables (servings/day) | 3±2 | 3±1 | 3±1 | 3±2 | 4±2 |
| Fiber (g/day) | 23±14 | 12±4 | 19±6 | 23±11 | 36±18 |
| Healthy Eating Index score d | 60±13 | 58±13 | 60±13 | 58±14 | 65±11 |
Abbreviations: CRC – colorectal cancer, MET – metabolic equivalent, DFE – dietary folate equivalents
Data presented as mean±SD for continuous variables, or as N (%) for categorical variables
Consumption of alcohol and folate from diet and supplements was assessed for the year prior to enrollment.
Non-drinker: 0 drinks per day; Light-drinker: alcohol consumption > 0 and < 1 drink/day for women, < 2 drink/day for men; Heavy drinker: ≥ 1 drink/day for women, ≥ 2 drink/day for men
Calculation of the Healthy Eating Index score, reflecting adherence to the Dietary Guidelines for Americans, has been described previously in the Southern Community Cohort Study (see reference 34).
Folate consumption and Ki67
In the full sample (n = 248), there were modest, positive associations with tumor Ki67 expression for dietary folate (β [95% CI]: = 1.2 [−0.6 – 2.9] per 200 DFE, p-trend=.18), folic acid supplement use (β [95% CI]: 1.9 [−2.0 – 5.8] compared to non-use, p=.34), and total folate intake (β [95% CI]: 0.4 [−0.5 – 1.3] per 200 DFE, p-trend=.34) after adjustment for covariates (Table 2). Results were similar in the subgroup of participants with proximal tumors (n = 114, p-trend≥.37 for all associations) (Table 3). Associations were similar after adjustment for additional covariates (Supplementary Table S3-S4).
Table 2:
Colorectal tumor marker Ki67 expression (% positive) by consumption of dietary folate, use of folic acid supplements, total folate (including folate from diet and supplements) and alcohol consumption a. Data presented as β (95% CI) relative to the reference level (N = 248)
| Tumor Ki67 (% positive) | |||
|---|---|---|---|
| N | Model 1 b | Model 2 c | |
| Dietary folate (DFE) a | |||
| Q1 (125.3–369.5) | 62 | 19.8 (mean) - Ref d | 21.2 (mean) - Ref d |
| Q2 (372.8–586.1) | 62 | 1.5 (−3.5–6.5) | 1.8 (−3.2–6.7) |
| Q3 (586.3–854.8) | 62 | 2.2 (−3.4–7.9) | 2.1 (−3.5–7.8) |
| Q4 (859.9–3146.0) | 62 | 0.9 (−6.8–8.6) | 0.5 (−7.3–8.3) |
| β (95% CI) per 200 DFE | 248 | 0.7 (−1.0–2.4) | 1.2 (−0.6–2.9) |
| P-trend | .42 | .18 | |
| Folic acid supplements a | |||
| < 1x per week | 172 | 20.5 (mean) - Ref d | 21.8 (mean) - Ref d |
| ≥ 1x per week | 76 | 1.6 (−2.2–5.4) | 1.9 (−2.0–5.8) |
| P-value | .40 | .34 | |
| Total folate (DFE) a,e | |||
| Q1 (125.7–429.9) | 62 | 20.7 (mean) - Ref d | 22.2 (mean) - Ref d |
| Q2 (436.6–793.3) | 62 | 0.7 (−4.4–5.7) | 0.4 (−4.6–5.5) |
| Q3 (797.1–1256.2) | 62 | 0.1 (−5.3–5.6) | −0.4 (−5.9–5.1) |
| Q4 (1258.3–3146.0) | 62 | 0.2 (−6.0–6.4) | 0.8 (−5.5–7.2) |
| β (95% CI) per 200 DFE | 248 | 0.3 (−0.6–1.1) | 0.4 (−0.5–1.3) |
| P-trend | .56 | .34 | |
| Alcohol a,f | |||
| Non-drinker | 134 | 19.7 (mean) - Ref d | 19.8 (mean) - Ref d |
| Light drinker | 79 | 1.4 (−2.5–5.4) | 1.5 (−2.5–5.4) |
| Heavy drinker | 35 | 5.7 (0.4–11.0) | 6.4 (1.0–11.9) |
| P-value (heavy vs. non) | .04 | .02 | |
Abbreviation: CI – confidence interval; DFE – dietary folate equivalent
Consumption of alcohol and folate from diet and supplements was assessed for the year prior to enrollment.
Model 1: Adjusted for age at enrollment, race, sex, and total energy intake (kcal/day)
Model 2: Further adjusted for total folate (alcohol model only), alcohol (folate models only), and tumor stage
Values for the reference group are the mean value for quartile 1 adjusted for the covariates included in the statistical model.
Incudes dietary folate/folic acid and folic acid from supplements
Non-drinker: 0 drinks per day; Light-drinker: alcohol consumption > 0 and < 1 drink/day for women, < 2 drink/day for men; Heavy drinker: ≥ 1 drink/day for women, ≥ 2 drink/day for men
Table 3:
Colorectal tumor marker Ki67 expression (% positive) by consumption of dietary folate, use of folic acid supplements, total folate (including folate from diet and supplements) and alcohol consumption a stratified by CRC tumor site. Data presented as β (95% CI) relative to the reference level
| Proximal tumors b
(n = 114) |
Distal tumors b
(n = 122) |
|||||
|---|---|---|---|---|---|---|
| N |
Model 1
c β (95% CI) |
Model 2
d β (95% CI) |
N |
Model 1
c β (95% CI) |
Model 2
d β (95% CI) |
|
| Dietary folate (DFE) a | ||||||
| Q1 (125.3–369.5) | 31 | 20.8 (mean) - Ref e | 22.3 (mean) - Ref e | 29 | 20.4 (mean) - Ref e | 22.4 (mean) - Ref e |
| Q2 (372.8–586.1) | 27 | −0.2 (−7.2–6.8) | 1.2 (−6.0–8.3) | 32 | 2.8 (−5.2–10.7) | 3.4 (−4.5–11.3) |
| Q3 (586.3–854.8) | 27 | −0.5 (−8.5–7.4) | −0.3 (−8.5–7.8) | 32 | 3.3 (−5.8–12.4) | 3.6 (−5.8–12.9) |
| Q4 (859.9–3146.0) | 29 | −5.0 (−16.7–6.7) | −5.3 (−17.3–6.7) | 29 | 4.0 (−8.5–16.4) | 3.6 (−9.0–16.3) |
| β (95% CI) per 200 DFE | 114 | 0.8 (−1.4–3.0) | 1.1 (−1.3–3.4) | 122 | 0.5 (−2.3–3.3) | 1.3 (−1.5–4.2) |
| P-trend f | .47 | .37 | .73 | .36 | ||
| Folic acid supplements a | ||||||
| < 1x per week | 79 | 20.0 (mean) - Ref e | 21.7 (mean) - Ref e | 84 | 21.3 (mean) - Ref e | 23.1 (mean) - Ref e |
| ≥ 1x per week | 35 | −1.6 (−7.0–3.7) | −1.4 (−7.1–4.3) | 38 | 5.3 (−0.7–11.3) | 7.5 (1.2–13.8) |
| P-value f | .55 | .62 | .08 | .02 | ||
| Total folate (DFE) a,g | ||||||
| Q1 (125.7–429.9) | 28 | 22.5 (mean) - Ref e | 24.1 (mean) - Ref e | 30 | 20.6 (mean) - Ref e | 21.7 (mean) - Ref e |
| Q2 (436.6–793.3) | 30 | −1.1 (−8.0–5.9) | 0.2 (−6.9–7.3) | 30 | 0.6 (−7.4–8.7) | 1.5 (−6.6–9.6) |
| Q3 (797.1–1256.2) | 24 | −6.3 (−14.3–1.8) | −7.4 (−15.5–0.7) | 34 | 3.7 (−4.8–12.2) | 5.4 (−3.3–14.1) |
| Q4 (1258.3–3146.0) | 32 | −5.2 (−13.7–3.2) | −4.5 (−13.3–4.2) | 28 | 5.1 (−5.2–15.5) | 8.5 (−2.6–19.6) |
| β (95% CI) per 200 DFE | 114 | −0.1 (−1.2–1.0) | 0.0 (−1.2–1.2) | 122 | 0.9 (−0.6–2.4) | 1.6 (0.0–3.3) |
| P-trend f | .82 | .98 | .23 | .05 | ||
| Alcohol a,h | ||||||
| Non-drinker | 63 | 18.7 (mean) - Ref e | 19.4 (mean) - Ref e | 64 | 21.0 (mean) - Ref e | 22.1 (mean) - Ref e |
| Light drinker | 34 | 0.3 (−5.5–6.1) | 0.0 (−6.0–6.0) | 42 | 2.0 (−4.0–8.0) | 0.4 (−5.8–6.6) |
| Heavy drinker | 17 | 5.0 (−2.7–12.8) | 5.4 (−2.6–13.4) | 16 | 10.0 (1.5–18.5) | 10.4 (1.6–19.1) |
| P-value (heavy vs. non) f | .20 | .18 | .02 | .02 | ||
Abbreviation: CI – confidence interval; CRC – colorectal cancer; DFE – dietary folate equivalent
Consumption of alcohol and folate from diet and supplements was assessed for the year prior to enrollment.
Proximal colon tumors include ICD-O-3 codes C180-C185. Distal colon or rectal tumors include ICD-O-3 codes C186, C187, C199, and C209.
Model 1: Adjusted for age at enrollment, race, sex, and total energy intake (kcal/day)
Model 2: Further adjusted for total folate (alcohol model only), alcohol (folate models only), and tumor stage
Values for the reference group are the mean value for quartile 1 adjusted for the covariates included in the statistical model.
For the interaction with CRC tumor site, all p-interaction > .05
Incudes dietary folate/folic acid and folic acid from supplements
Non-drinker: 0 drinks per day; Light-drinker: alcohol consumption > 0 and < 1 drink/day for women, < 2 drink/day for men; Heavy drinker: ≥ 1 drink/day for women, ≥ 2 drink/day for men
Among cases with distal/rectal tumors (n = 122), folic acid supplement use was associated with higher tumor Ki67 expression in model 2 (β [95% CI]: 7.5 [1.2 – 13.8] compared to non-use, p=.02) (Table 3). For total folate intake, there was a positive but non-statistically significant association with Ki67 expression in distal/rectal tumors (β [95% CI]: 1.6 [0.0 – 3.3] per 200 DFE, p-trend=.05), while a weaker association was observed for dietary folate (β [95% CI]: 1.3 [−1.5 – 4.2] per 200 DFE, p-trend=.36). Results were similar after adjustment for additional covariates (Supplementary Table S5). No significant interactions between folate exposures and tumor site were observed (p-interaction > .05 for all).
Alcohol consumption and Ki67, and interaction with folate
Heavy alcohol consumption was positively associated with tumor Ki67 expression in the full sample (β [95% CI]: 6.4 [1.0 – 11.9], compared to non-drinkers, p=.02) (Table 2) and in the subgroup of distal/rectal tumors (β [95% CI|: 10.4 [1.6 – 19.1], p=.02) (Table 3). A weaker association was observed in proximal tumors (β [95% CI|: 5.4 [−2.6 – 13.4], p=.18). The association between alcohol consumption and Ki67 expression was modestly attenuated after further adjustment for variables related to socioeconomic status (Supplementary Table S3-S5).
In the full sample, there was no interaction between alcohol consumption and total folate (p-interaction=.63, Supplementary Table S2). There was modest evidence for an interaction in distal/rectal tumors, with a positive association for total folate observed in ‘non-drinkers’ (β [95% CI|: 14.2 [0.4 – 28.0] for quartile 4 vs. 1), but not in ‘light/heavy’ drinkers (p-interaction=.06). No interaction was observed in proximal tumors (p-interaction=.45).
Discussion
In this cross-sectional analysis of CRC tumor samples from the SCCS, we observed modest evidence that use of folic acid supplements is associated with greater expression of Ki67 in tumors of the distal colon and rectum. These results give weight to the hypothesis that folate supports the proliferation of CRC tumors, not previously reported in epidemiological studies. Further, the observed positive association between heavy alcohol consumption and tumor Ki67 expression provides novel evidence that regular alcohol consumption may promote proliferative tumor phenotypes.
Consistent with our findings, results from animal models suggest that dietary folate may accelerate the growth and development of adenomas when administered after initiation (13–15), potentially through the provision of methyl units required for rapid DNA synthesis (12). Likewise, increased risk for CRC or adenomas with folic acid supplements has been reported in some human clinical trials (16,17) though not all RCTS (42–44). Cole et al. (2007) reported that participants with a recent history of adenoma given high-dose folic acid supplements (1 mg/day) were more likely to develop an advanced adenoma after 6–8 years of follow-up (risk ratio (RR) [95% confidence interval (CI)]: 1.67 [1.00–2.88]) compared to placebo, and had higher risk for developing three or more adenomas (RR [95% CI]: 2.32 [1.23–4.35]) (16). In the B-PROOF study, older adults (age 65+) given folic acid and vitamin B12 were more likely to develop incident CRC after long-term follow-up than participants given placebo (Hazard Ratio [95% CI]:1.77 [1.08–2.90]) (17). Conversely, epidemiological studies show that high folate intake is associated with 20–30% reduced risk for CRC compared to low intake (1–3,45), and this protective association is supported by a plausible biological mechanism whereby adequate cellular folate levels reduce uracil misincorportion into DNA in lieu of thymidine (6–8), thereby preventing DNA strand breaks (7,9). Consequently, a ‘dual effect’ of folate has been proposed, whereby folate may reduce risk for incident CRC by preventing chromosomal instability, but accelerate the growth of established neoplasms by participating in one-carbon metabolic pathways essential for nucleotide synthesis (12). The potential dual effect of folate is concerning given mandatory folic acid fortification in much of the world (46), high rates of folic acid supplement use (approximately 35% of U.S. adults (28)), and the high prevalence of adenomas in older adults, which may approach 30–40% of adults by age 70 (47).
However, to the authors’ knowledge there are currently no studies that directly investigate associations between folate exposures and molecular characteristics that reflect tumor growth and prognosis, which would be valuable to better understand the proposed dual effect of folate in CRC. In our analysis, we show that pre-diagnostic use of folic acid supplement was modestly associated with higher CRC Ki67 expression for distal/rectal tumors, while a positive but non-statistically significant association with Ki67 expression was observed for total folate intake (including dietary folate). These results suggest that consuming high doses of folic acid may accelerate the growth of existing neoplasms, as Ki67 is an objective marker of tumor proliferation (48). Higher CRC tumor Ki67 expression is associated with increased mortality and risk for liver metastasis in epidemiological studies (30,49), suggesting that excessive folate exposure may promote adverse outcomes in CRC patients. Our findings were specific to the distal/rectal tumor site, and it is worth noting that the mean (±SE) Ki67 expression was higher in distal/rectal tumors compared to proximal tumors (22.9±1.3% vs. 19.7±1.2%, respectively), and consequently these tumors may have been more dependent on folate to maintain high rates of cell division. There was approximately a six-month difference in the median incidence time for cases with proximal tumors vs. distal/rectal tumors, and therefore it is possible that differences in the timing of dietary assessment may have influenced this result. Notably, we observed stronger positive associations for folic acid supplement use compared to dietary folate (although a positive trend was still observed for this exposure with Ki67 expression in distal/rectal tumors). This is unlikely to represent a distinct effect of folic acid relative to folate, as the dietary folate exposure includes substantial folic acid from fortification. However, this discrepancy may be related to the large dose of folic acid contained in dietary supplements, which is often 400 mcg folic acid per serving and more than 100% of the recommended dietary allowance. Importantly, the similar trends observed for dietary folate and folic acid supplements supports the hypothesis that high consumption of folate may drive proliferation of CRC tumors in the distal colon and rectum.
Greater alcohol consumption was independently associated with higher Ki67 expression, with a stronger association for distal/rectal tumors compared to tumors of the proximal colon. This finding is consistent with mechanistic studies that have demonstrated that alcohol exposure increases nuclear β-catenin, a key transcription factor in CRC that increases the expression of genes that regulate cell proliferation (50), as well as data showing greater proliferation of the healthy rectal mucosa from heavy drinkers (≥ 100 g/day alcohol) compared to non-drinkers or moderate drinkers (20). Also consistent with our results, experimental studies in rodents show increased mucosal proliferation in the rectum after chronic alcohol treatment, but not in the colon (21,22). Likewise, large-scale epidemiological studies suggest that heavy alcohol use is more strongly associated with risk for distal/rectal tumors compared to proximal tumors (51–53). The influence of alcohol on mucosal proliferation is likely mediated by the extraordinary increase in intestinal acetaldehyde after alcohol consumption (54,55). Alcohol’s influence on acetaldehyde concentration appears to be more pronounced in the rectum compared to the colon (55,56), reflecting the higher concentration of alcohol metabolizing enzymes in the rectum (56,57). Acetaldehyde is classified by the International Agency for Research on Cancer (IARC) as ‘possibly carcinogenic to humans’ (58), and acetaldehyde concentrations in the distal colon and rectum have been shown to be directly associated with mucosal proliferation after alcohol administration in rodents (22). Although not fully understood, there is also growing evidence that heavy drinking (i.e. more than 2–3 drinks/day) is associated with increased mortality in individuals with CRC. In a meta-analysis of nine cohort studies, Cai et al. (2014) reported that heavy alcohol consumption (≥ 50 g/day) was associated with significantly increased risk for CRC-specific mortality compared to non-drinking (RR [95% CI] = 1.21 [1.01–1.46]) (59). While a more recent meta-analysis reported that pre-diagnostic heavy drinking was not associated with greater risk for overall or CRC-specific mortality (60), there was clear evidence for a J-shaped association between alcohol consumption and overall mortality, with increased risk for mortality at the higher end of the exposure distribution. Our data suggest that alcohol may increase mortality via increased tumor proliferation, which is associated with worse survival and higher risk for metastasis (30). Notably, heavy alcohol consumption was associated with greater CRC proliferation despite considerable biological evidence that alcohol antagonizes folate activity by increasing folate catabolism (25), decreasing folate absorption from the small intestine (61), decreasing renal reabsorption (62), and decreasing transport of folate into target tissues (24). Consistent with these biological findings, we found evidence for effect modification such that total folate intake was positively associated with tumor Ki67 expression in distal/rectal tumors among non-drinkers only (Supplementary Table S2). Consequently, future studies concerning the relations between folate intake and CRC tumor proliferation may benefit from stratifying by alcohol consumption.
This study is attended by several limitations. Because our analysis is strictly observational, we are unable to make conclusions about causal relationships between folate, alcohol and tumor proliferation, despite the existence of plausible biological mechanisms and supportive evidence from experimental models. While it is possible that correlated dietary exposures (e.g. nutrients found in a multivitamin) may have partially confounded our results, we are unaware of any research to suggest that other micronutrients can independently drive tumor cell proliferation. In a previously published validation study from the SCCS, estimated dietary folate consumption was weakly associated with serum folate levels (Pearson’s R = 0.26) (38). This may reflect the challenges of accurately measuring nutrient intake via FFQ or significant inter-individual variability in the absorption and metabolism of folate, both of which would be expected to cause exposure misclassification and bias towards the null hypothesis. Our analyses included adjustment for total energy intake, which is recommended for nutritional analyses to mitigate multiple sources of confounding and to correct for over or under-reporting in the exposure of interest (41). Total folate intake was strongly collinear with total energy intake in our sample (Pearson’s R = 0.62), which may have limited our ability to identify independent associations for folate in the multivariable model (63). However, in analysis of distal/rectal tumors, relatively strong associations for total folate intake and folate supplement use remained after adjusting for energy intake. Because alcohol is a source of energy, simultaneously including total energy intake in models for alcohol consumption may be an over-adjustment, or may indicate the effect of substituting other sources of energy for alcohol. However, estimates for the main effect of alcohol on tumor Ki67 expression, or interactions with folate were not substantially affected by adjusting for energy intake in our analyses. Our analysis is also limited by small sample size, which may have limited statistical power, especially for subgroup analyses. Despite evidence linking the MTHFR C677T variant to lower risk for colorectal cancer through effects on intracellular folate metabolism (10), we did not incorporate this polymorphism into our analysis due to its known rarity in African Americans (64) and because approximately half of SCCS participants did not provide a serum sample for genetic analysis (31). Lastly, we adjusted for race in multivariate models using broad categories (African American, yes/no), which may have led to residual confounding by race. However, only three participants self-reported non-African American, non-White racial ancestry, and consequently this is unlikely to lead to significant bias.
In conclusion, pre-diagnostic use of folic acid supplements by CRC cases was associated with higher tumor Ki67 expression in distal/rectal tumors, reflecting a more proliferative tumor phenotype that is consistent with folate’s role in one-carbon metabolism and DNA synthesis. A positive association between heavy alcohol consumption and tumor Ki67 expression is consistent with earlier findings and adds to a growing body of evidence that excessive alcohol use may promote adverse outcomes for CRC patients.
Supplementary Material
Figure 1.
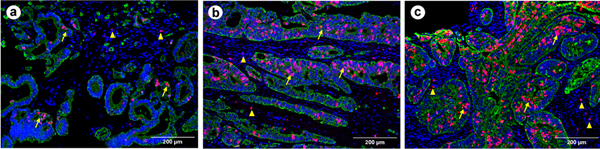
Representative images of Ki67 expression in colorectal cancer (CRC) tissue stained by multiplex fluorescent immunohistochemistry. The cancer cells, cell nuclei and Ki67 are labeled in green, blue and red, respectively. The Ki67 positive cells exhibit in both intratumoral (↑) and stromal (Δ) areas. A measurement pipeline is established using CellProfiler (v3.1.5) or automated quantification of Ki67 in cancer cells, excluding stromal tissues, as described in our previous study40. The images a, b and c are scored as 7.79%, 19.87% and 32.54%, respectively, representing weak, moderate and strong expression levels of Ki67 in CRC cells.
Acknowledgements
Data on SCCS cancer cases used in this publication were provided by the Alabama Statewide Cancer Registry; Kentucky Cancer Registry, Lexington, KY; Tennessee Department of Health, Office of Cancer Surveillance; Florida Cancer Data System; North Carolina Central Cancer Registry, North Carolina Division of Public Health; Georgia Comprehensive Cancer Registry; Louisiana Tumor Registry; Mississippi Cancer Registry; South Carolina Central Cancer Registry; Virginia Department of Health, Virginia Cancer Registry; Arkansas Department of Health, Cancer Registry, 4815 W. Markham, Little Rock, AR 72205. The Arkansas Central Cancer Registry is fully funded by a grant from the National Program of Cancer Registries, Centers for Disease Control and Prevention (CDC). Data on SCCS cancer cases from Mississippi were collected by the Mississippi Cancer Registry which participates in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the Mississippi Cancer Registry. Cancer data for SCCS cancer cases from West Virginia have been provided by the West Virginia Cancer Registry. The opinions expressed are those of the authors and do not necessarily represent those of the CDC or the West Virginia Cancer Registry.
Financial support:
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R00 CA207848 and R01 CA255318 to SWA); the University of Wisconsin-Madison, Office of Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation and the University of Wisconsin Carbone Cancer (grant number P30 CA014520 to HH Bailey). The Southern Community Cohort Study (SCCS) is supported by the National Cancer Institute at the National Institutes of Health (grant numbers R01 CA92447, U01 CA202979 to W Zheng), including special allocations from the American Recovery and Reinvestment Act (grant number 3R01 CA092447-08S1 to WJ Blot). The funding source had no role in the study design, in the collection, analysis, and interpretation of data, or in the decision to submit the article for publication.
Footnotes
Declaration of interests: The authors report that there are no competing interests to declare.
Data availability:
Data are available to qualified researchers who submit a proposal via the SCCS online submission system (https://www.southerncommunitystudy.org/for-researchers.html).
References
- 1.Kennedy DA, Stern SJ, Moretti M, Matok I, et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. 2011;35(1):2–10. doi: 10.1016/j.canep.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010;21(11):1919–1930. doi: 10.1007/s10552-010-9620-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazzen S, Dolatkhah R, Tabrizi JS, Shaarbafi J, et al. Folic acid intake and folate status and colorectal cancer risk: A systematic review and meta-analysis. Clin Nutr. 2018;37(6 Pt A):1926–1934. doi: 10.1016/j.clnu.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 5.Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res. 2007;51(3):267–292. doi: 10.1002/mnfr.200600191 [DOI] [PubMed] [Google Scholar]
- 6.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998;12(14):1491–1497. [PubMed] [Google Scholar]
- 7.Duthie SJ, Grant G, Narayanan S. Increased uracil misincorporation in lymphocytes from folate-deficient rats. Br J Cancer. 2000;83(11):1532–1537. doi: 10.1054/bjoc.2000.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blount BC, Mack MM, Wehr CM, MacGregor JT, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–3295. doi: 10.1073/pnas.94.7.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YI, Shirwadkar S, Choi SW, Puchyr M, et al. Effects of dietary folate on DNA strand breaks within mutation-prone exons of the p53 gene in rat colon. Gastroenterology. 2000;119(1):151–161. doi: 10.1053/gast.2000.8518 [DOI] [PubMed] [Google Scholar]
- 10.Taioli E, Garza MA, Ahn YO, Bishop DT, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: a HuGE-GSEC review. Am J Epidemiol. 2009;170(10):1207–1221. doi: 10.1093/aje/kwp275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang TL, Hung J, Caudill MA, Urrutia TF, et al. A long-term controlled folate feeding study in young women supports the validity of the 1.7 multiplier in the dietary folate equivalency equation. J Nutr. 2005;135(5):1139–1145. doi: 10.1093/jn/135.5.1139 [DOI] [PubMed] [Google Scholar]
- 12.Mason JB, Tang SY. Folate status and colorectal cancer risk: A 2016 update. Mol Aspects Med. 2017;53:73–79. doi: 10.1016/j.mam.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 13.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44(1):10–25. doi: 10.1002/em.20025 [DOI] [PubMed] [Google Scholar]
- 14.Song J, Medline A, Mason JB, Gallinger S, et al. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60(19):5434–5440. [PubMed] [Google Scholar]
- 15.Song J, Sohn KJ, Medline A, Ash C, et al. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000;60(12):3191–3199. [PubMed] [Google Scholar]
- 16.Cole BF, Baron JA, Sandler RS, Haile RW, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351 [DOI] [PubMed] [Google Scholar]
- 17.Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, Swart KMA, et al. Folic Acid and Vitamin B12 Supplementation and the Risk of Cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28(2):275–282. doi: 10.1158/1055-9965.EPI-17-1198 [DOI] [PubMed] [Google Scholar]
- 18.Kim YI. 5,10-Methylenetetrahydrofolate reductase polymorphisms and pharmacogenetics: a new role of single nucleotide polymorphisms in the folate metabolic pathway in human health and disease. Nutr Rev. 2005;63(11):398–407. doi: 10.1111/j.1753-4887.2005.tb00377.x [DOI] [PubMed] [Google Scholar]
- 19.Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15(4):183–210. doi: 10.1016/j.drup.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Simanowski UA, Homann N, Knühl M, Arce L, et al. Increased rectal cell proliferation following alcohol abuse. Gut. 2001;49(3):418–422. doi: 10.1136/gut.49.3.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simanowski UA, Seitz HK, Baier B, Kommerell B, et al. Chronic ethanol consumption selectively stimulates rectal cell proliferation in the rat. Gut. 1986;27(3):278–282. doi: 10.1136/gut.27.3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simanowski UA, Suter P, Russell RM, Heller M, et al. Enhancement of ethanol induced rectal mucosal hyper regeneration with age in F344 rats. Gut. 1994;35(8):1102–1106. doi: 10.1136/gut.35.8.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma J, Krupenko SA. Folate pathways mediating the effects of ethanol in tumorigenesis. Chem Biol Interact. 2020;324:109091. doi: 10.1016/j.cbi.2020.109091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development. FEBS J. 2009;276(8):2175–2191. doi: 10.1111/j.1742-4658.2009.06959.x [DOI] [PubMed] [Google Scholar]
- 25.Shaw S, Jayatilleke E, Herbert V, Colman N. Cleavage of folates during ethanol metabolism. Role of acetaldehyde/xanthine oxidase-generated superoxide. Biochem J. 1989;257(1):277–280. doi: 10.1042/bj2570277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choumenkovitch SF, Selhub J, Wilson PWF, Rader JI, et al. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002;132(9):2792–2798. doi: 10.1093/jn/132.9.2792 [DOI] [PubMed] [Google Scholar]
- 27.Quinlivan EP, Gregory JF. Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77(1):221–225. doi: 10.1093/ajcn/77.1.221 [DOI] [PubMed] [Google Scholar]
- 28.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91(1):231–237. doi: 10.3945/ajcn.2009.28427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner JB, Zirnheld KH, Hu H, Floyd A, et al. Analysis of alcohol use, consumption of micronutrient and macronutrients, and liver health in the 2017–2018 National Health and Nutrition Examination Survey. Alcohol Clin Exp Res. 2022;46(11):2025–2040. doi: 10.1111/acer.14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, et al. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta analysis. BMC Cancer. 2019;19(1):123. doi: 10.1186/s12885-019-5324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972–979. [PMC free article] [PubMed] [Google Scholar]
- 32.Yu D, Sonderman J, Buchowski MS, McLaughlin JK, et al. Healthy Eating and Risks of Total and Cause-Specific Death among Low-Income Populations of African-Americans and Other Adults in the Southeastern United States: A Prospective Cohort Study. PLoS Med. 2015;12(5):e1001830; discussion e1001830. doi: 10.1371/journal.pmed.1001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 34.Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, et al. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol (Noisy-le-grand). 2003;49(8):1295–1304. [PubMed] [Google Scholar]
- 35.Signorello LB, Munro HM, Buchowski MS, Schlundt DG, et al. Estimating nutrient intake from a food frequency questionnaire: incorporating the elements of race and geographic region. Am J Epidemiol. 2009;170(1):104–111. doi: 10.1093/aje/kwp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US); 1998. Accessed May 7, 2022. http://www.ncbi.nlm.nih.gov/books/NBK114310/ [PubMed] [Google Scholar]
- 37.Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34(11):2357–2359. [PubMed] [Google Scholar]
- 38.Signorello LB, Buchowski MS, Cai Q, Munro HM, et al. Biochemical validation of food frequency questionnaire-estimated carotenoid, alpha-tocopherol, and folate intakes among African Americans and non-Hispanic Whites in the Southern Community Cohort Study. Am J Epidemiol. 2010;171(4):488–497. doi: 10.1093/aje/kwp402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 40.Lawler T, Su T, Cai Q, Steinwandel MD, et al. Associations between serum vitamin D biomarkers and tumor expression of Ki67, p53, and COX-2 in colorectal cancer cases from the Southern Community Cohort Study. J Steroid Biochem Mol Biol. 2023;225:106201. doi: 10.1016/j.jsbmb.2022.106201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willett WC. Implications of total energy intake for epidemiologic analyses. In: Epidemiology Nutritional. Vol 13. Oxford University Press; 2013:260–286. [Google Scholar]
- 42.Hankey GJ, Eikelboom JW, Yi Q, Lees KR, et al. Treatment with B vitamins and incidence of cancer in patients with previous stroke or transient ischemic attack: results of a randomized placebo-controlled trial. Stroke. 2012;43(6):1572–1577. doi: 10.1161/STROKEAHA.111.641613 [DOI] [PubMed] [Google Scholar]
- 43.Vollset SE, Clarke R, Lewington S, Ebbing M, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381(9871):1029–1036. doi: 10.1016/S0140-6736(12)62001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin T, Du M, Du H, Shu Y, et al. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci Rep. 2015;5:12044. doi: 10.1038/srep12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovannucci E.Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132(8 Suppl):2350S–2355S. doi: 10.1093/jn/132.8.2350S [DOI] [PubMed] [Google Scholar]
- 46.Murphy ME, Westmark CJ. Folic Acid Fortification and Neural Tube Defect Risk: Analysis of the Food Fortification Initiative Dataset. Nutrients. 2020;12(1):E247. doi: 10.3390/nu12010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corley DA, Jensen CD, Marks AR, Zhao WK, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11(2):172–180. doi: 10.1016/j.cgh.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45. doi: 10.1016/j.cca.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 49.Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118(5):544–552. doi: 10.1016/j.acthis.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 50.Johnson CH, Golla JP, Dioletis E, Singh S, et al. Molecular Mechanisms of Alcohol-Induced Colorectal Carcinogenesis. Cancers (Basel). 2021;13(17):4404. doi: 10.3390/cancers13174404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SY, Wilkens LR, Setiawan VW, Monroe KR, et al. Alcohol Intake and Colorectal Cancer Risk in the Multiethnic Cohort Study. Am J Epidemiol. 2019;188(1):67–76. doi: 10.1093/aje/kwy208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrari P, Jenab M, Norat T, Moskal A, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer. 2007;121(9):2065–2072. doi: 10.1002/ijc.22966 [DOI] [PubMed] [Google Scholar]
- 53.McNabb S, Harrison TA, Albanes D, Berndt SI, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020;146(3):861–873. doi: 10.1002/ijc.32377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000;86(2):169–173. doi: [DOI] [PubMed] [Google Scholar]
- 55.Seitz HK, Simanowski UA, Garzon FT, Rideout JM, et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology. 1990;98(2):406–413. doi: 10.1016/0016-5085(90)90832-l [DOI] [PubMed] [Google Scholar]
- 56.Pronko P, Bardina L, Satanovskaya V, Kuzmich A, et al. Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol Alcohol. 2002;37(3):229–235. doi: 10.1093/alcalc/37.3.229 [DOI] [PubMed] [Google Scholar]
- 57.Seitz HK, Egerer G, Oneta C, Krämer S, et al. Alcohol dehydrogenase in the human colon and rectum. Digestion. 1996;57(2):105–108. doi: 10.1159/000201322 [DOI] [PubMed] [Google Scholar]
- 58.Agents Classified by the IARC Monographs, Volumes 1–131 – IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Accessed May 10, 2022. https://monographs.iarc.who.int/agents-classified-by-the-iarc/
- 59.Cai S, Li Y, Ding Y, Chen K, et al. Alcohol drinking and the risk of colorectal cancer death: a meta-analysis. Eur J Cancer Prev. 2014;23(6):532–539. doi: 10.1097/CEJ.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 60.Kim Y, Je Y, Giovannucci EL. Association between Alcohol Consumption and Survival in Colorectal Cancer: A Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1891–1901. doi: 10.1158/1055-9965.EPI-19-0156 [DOI] [PubMed] [Google Scholar]
- 61.Wani NA, Kaur J. Reduced levels of folate transporters (PCFT and RFC) in membrane lipid rafts result in colonic folate malabsorption in chronic alcoholism. J Cell Physiol. 2011;226(3):579–587. doi: 10.1002/jcp.22525 [DOI] [PubMed] [Google Scholar]
- 62.Wani NA, Thakur S, Najar RA, Nada R, et al. Mechanistic insights of intestinal absorption and renal conservation of folate in chronic alcoholism. Alcohol. 2013;47(2):121–130. doi: 10.1016/j.alcohol.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 63.Willett WC. Issues in analysis and presentation of dietary data. In: Epidemiology Nutritional. Vol 13. Oxford University Press; 2013:305–333. [Google Scholar]
- 64.Le Marchand L, Wilkens LR, Kolonel LN, Henderson BE. The MTHFR C677T polymorphism and colorectal cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1198–1203. doi: 10.1158/1055-9965.EPI-04-0840 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available to qualified researchers who submit a proposal via the SCCS online submission system (https://www.southerncommunitystudy.org/for-researchers.html).