Abstract
The use of electronic (e)-cigarettes was initially considered a beneficial solution to conventional cigarette smoking cessation. However, paradoxically, e-cigarette use is rapidly growing among nonsmokers, including youth and young adults. In 2019, this rapid growth resulted in an epidemic of hospitalizations and deaths of e-cigarette users (vapers) due to acute lung injury; this novel disease was termed e-cigarette or vaping use-associated lung injury (EVALI). Pathophysiologic mechanisms of EVALI likely involve cytotoxicity and neutrophilic inflammation caused by inhaled chemicals, but further details remain unknown. The undiscovered mechanisms of EVALI are a barrier to identifying biomarkers and developing therapeutics. Furthermore, adverse effects of e-cigarette use have been linked to chronic lung diseases and systemic effects on multiple organs. In this comprehensive review, we discuss the diverse spectrum of vaping exposures, epidemiological and clinical reports, and experimental findings to provide a better understanding of EVALI and the adverse health effects of chronic e-cigarette exposure.
Keywords: e-cigarette or vaping use-associated lung injury, EVALI, electronic nicotine delivery systems, ENDS, e-cigarette aerosols, acute lung injury, inflammatory lung disease, systemic inflammation
1. INTRODUCTION
Electronic (e)-cigarettes represent the newest tobacco product increasingly used by the public (1–4). However, despite their popularity, the effect of e-cigarette use (vaping) remains poorly understood, and chronic e-cigarette use will likely lead to serious health effects. A variety of e-cigarettes have been marketed to the public over the past decade in the context of a lack of regulatory action. E-cigarette or vaping use-associated lung injury, or EVALI, is a recently described entity at the forefront of current investigations and represents an epidemiologic outbreak that focused attention of clinicians and researchers on vaping products. Though the cause of EVALI has been linked to vitamin E acetate (VEA), which is added to some vaping products, aerosols generated by e-cigarettes (termed electronic nicotine delivery systems or ENDS) have the potential to cause numerous pulmonary toxicities, both acute and chronic. The pathophysiology of EVALI and the host responses that correlate with chronic inflammation are described in this review, as well as the general pulmonary toxicity and pathophysiologic profile of vaping tetrahydrocannabinol (THC) products and ENDS.
2. THE SPECTRUM OF VAPING EXPOSURES
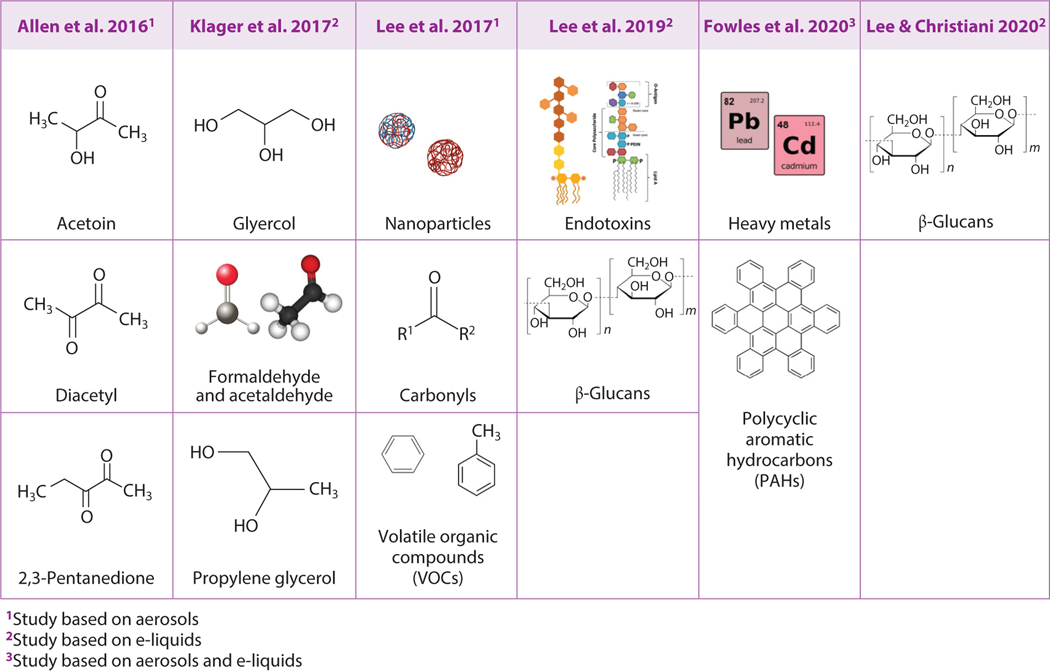
Modern e-cigarettes were invented in 2003 and entered the international market in 2007 (1–4). They have rapidly evolved from the first-generation “cig-a-like,” designed to look like a conventional tobacco cigarette, to the second-generation vape pens, third-generation box Mods, and the currently popular fourth-generation pod-based devices (5, 6). With the rapid evolution of the hardware of these ENDS devices, four core components have remained the same: a liquid reservoir (called a tank, cartridge, or pod) to hold the e-liquid, a power source (most commonly a lithium rechargeable battery), a heating element (atomizer), and a mouthpiece (7). But the voltage, wattage, temperature, metals, plastics, and other factors differ across devices. Some of these factors have been found to play a major role in the formation of toxins, such as the production of high levels of formaldehyde with the application of high wattage or high temperature (8, 9) and production of carbonyls with combinations of wick length and coil design (10). Toxic metals and other substances have also been detected in e-liquids and e-cigarette aerosols, which may be due to the materials used to make the devices (11–13, 14–19) (Figure 1).
Figure 1.
Toxic substances detected in e-cigarette aerosols and e-liquids. Toxic substances detected in e-cigarettes include toxicants (chemicals, nanoparticles, and heavy metals) and toxins (endotoxin and β-glucans). Figure adapted from images created with BioRender.com.
While e-devices have gone through four generations of evolution, the composition changes of e-liquids are innumerable. With hundreds of chemicals added to e-liquids to produce flavors appealing to every man, woman, and child from every country and culture on Earth, there is almost an infinite combination of chemicals being used to create the e-liquids available on the market. Some chemical additives have been approved for ingestion via the gastrointestinal tract, whereas others have never been approved for human consumption. Approval for gastrointestinal consumption does not confer safety for inhalation of the chemicals, as the gastrointestinal tract has evolved to protect the body from absorbing toxins and being harmed by them entering the body in this manner. The lungs, however, have evolved to allow passage of molecules entering the airways directly into the bloodstream, such that adding chemicals to e-liquids for aerosolization and inhalation into the airways is hijacking this evolutionary process to rapidly deliver chemicals contained in e-cigarette aerosols into the bloodstream (20).
Another aspect of vaping exposure is puff topography, which affects coil temperature and thus the composition of e-cigarette emissions. While the topography of smoking conventional tobacco cigarettes is similar between users—rapid (1–1.5 s) puffs, spaced by intervals of 20–30 s until the cigarette is finished, followed by either another cigarette or a break in between cigarettes—vaping of e-cigarettes involves a longer inhalation (2.3–4.3 s),with many different intervals between puffs (21–23) owing to the ability to take a puff at any time throughout the day without needing to light a new one or commit to smoking a full cigarette. Although some e-cigarette users (vapers) are social vapers, only using e-cigarettes around friends or at parties, others vape continuously, with the first use before getting out of bed and the last use before bedtime. In addition, some people are exposed to secondhand e-cigarette aerosols, such that they are primarily inhaling aerosols that have entered someone else’s lungs first. With the lack of sidestream vapor, because e-cigarettes only generate aerosols while the user is actively applying negative pressure to the mouthpiece, secondhand exposure to e-cigarette aerosols is likely to be less intense than that seen with cigarette smoke (which includes both sidestream and exhaled residual smoke).However, studies to date have confirmed that individuals standing close to e-cigarette users or within a confined space (a car or room without good ventilation) undergo significant exposure to e-cigarette aerosols (24–26).
Interestingly, many e-cigarette users are not committed to a single device or a single flavor. Thus, they expose themselves to chemicals produced by multiple e-devices, plus the multitude of chemicals within the flavored e-liquids they choose to use. This complexity of e-cigarette use makes it more challenging to track sources of lung injury and inflammation caused by any single device or chemical. Finally, many e-cigarette users are also conventional tobacco smokers, marijuana smokers, or vapers of THC. Each of these inhalants has its own range of host effects, known and unknown, and the consequences of combining the various inhalants are yet unknown.
3. EPIDEMIOLOGY OF ACUTE LUNG INJURY FROM ELECTRONICVAPING
In 2019, there were several outbreaks of acute respiratory failure of mysterious cause in persons who vape THC, nicotine, or both. Layden et al. (27) reported in the New England Journal of Medicine a cluster of cases from Illinois and Wisconsin in which patients presented with acute, severe respiratory distress after using e-cigarette products. Two letters published at the same time added further evidence of this new vaping-induced respiratory disease: a six-case cluster from Utah (28) and a report of imaging changes seen in a range of cases (29). The syndrome has been since termed EVALI by the US Centers for Disease Control and Prevention (CDC). As of January 9, 2020, the CDC reported a total of 2,602 hospitalized EVALI cases across all 50 states, the District of Columbia (DC), and two territories (Puerto Rico and US Virgin Islands). Fifty-seven deaths by then had been confirmed in 27 states and Washington, DC.
Inhalation of toxic environmental agents causes injury, both acute and subacute, to the airways and lung parenchyma (30–38). The pathologic outcomes of lung injuries depend on the dose of the inhaled toxic compound(s) and their physicochemical properties, including solubility and chemical composition (39–43). Much of our current knowledge about toxic inhalation syndromes derives from both occupational and community settings. In occupational settings, toxic compounds include acids, bases, metals, solvents, ozone, phosgene, or chlorine dioxide at high levels. In community settings, exposures occur during derailments of chemical-bearing train cars, factory explosions, and overexposure to household cleaning agents (30–38). Depending on levels and types of inhaled chemicals, patients may develop a wide range of symptoms, including minor respiratory tract discomfort, acute airway injury and damage, parenchymal pneumonitis, alveolar edema, hypoxemic respiratory failure, and death (Figure 2). This constellation of damage and host responses is clinically called acute respiratory distress syndrome (ARDS) (44–46).
Figure 2.
Mechanistic overview of the adverse effects of electronic cigarettes on the lung. As a primary organ, the lung is damaged and impaired by electronic cigarette use. Figure adapted from images created with BioRender.com. Abbreviations: γH2AX, gamma H2AX; ROS, reactive oxygen species.
Prior to 2019, numerous vaping-associated lung diseases had been reported, with a heterogeneous collection of pneumonitis patterns, including acute eosinophilic pneumonia, organizing pneumonia, lipoid pneumonia, diffuse alveolar damage and ARDS, diffuse alveolar hemorrhage, hypersensitivity pneumonitis, peribronchiolar granulomatous pneumonitis, and the rare giant-cell interstitial pneumonitis (47–51). Though pathologic manifestations of respiratory injury caused by e-cigarette aerosol inhalation may be diverse, the recent EVALI epidemic was different in that pathologic patterns were consistent with a single common etiology. Most people diagnosed with EVALI reported having used products with THC or cannabidiol (CBD) that are formulated with other terpene oils (83%), while the remaining 17% reported using only nicotine-containing vaping products, which are not routinely mixed with terpenes, including VEA. In a report published in the New England Journal of Medicine, Blount and colleagues (52) found VEA in the bronchoalveolar lavage (BAL) fluid of 48 of 51 EVALI patients in a convenience sampling. Coconut oil and limonene were also found in a few patients. THC or its metabolites were found in the BAL of 94% of this group. In bulk samples seized by law enforcement, VEA was found in 20 of 20 seized samples in 2019 but in 0 of 10 seized samples in 2018. Hence, the most prevalent culprit appeared to be the additive VEA. It remains unknown whether VEA or its pyrolysis products is the causal agent of EVALI (52, 53). It is believed that the non-THC/CBD vapers who were diagnosed with EVALI were actually suffering from disparate vaping-associated lung diseases caused by different toxins within the aerosols or different host responses to the inhalants.
Common histopathologic features in EVALI include lipid-laden alveolar macrophages that frequently coincide with vacuolization and vacuolated pneumocytes (27, 52). These findings are typically observed in patients with chemical-induced pneumonitis. Although VEA itself may be the key common exposure culprit for EVALI, underlying mechanisms of toxicity may be more complicated. Specifically, severe inflammatory responses and pulmonary edema may be caused by pyrolysis products of vitamin E oil rather than the parent compound itself. However, examining the toxicity of the pyrolysis products is difficult because some are gases, such as ketene, which are not easily measured in biological samples. Controlled studies using animal models have provided early insight into whether exposure to VEA alone can directly cause acute lung injury (54). In mice exposed to VEA, the level of albumin in BAL fluid (a surrogate marker of lung epithelial damage) and the total number of leukocytes in the lungs were increased to a greater extent than those in mice exposed to air or propylene glycol (PG) and vegetable glycerin (VG). Moreover, cells isolated from the BAL fluid of mice exposed to VEA contained numerous lipid-laden alveolar macrophages, a finding consistent with clinical observations in patients with EVALI (27, 52).
An autopsy series of 23 suspected cases showed that 21 met the EVALI definition and had histological evidence of acute to subacute lung injury, including diffuse alveolar damage or organizing pneumonia (55). Transbronchial and surgical lung biopsies from eight men aged 19–61 years with respiratory symptoms following e-cigarette use showed acute lung injury, including organizing pneumonia and/or diffuse alveolar damage (56). This is the predominant pattern in the acute process we call EVALI. Additional common features seen in the lung biopsies were fibroblast plugs, hyaline membranes, fibrinous exudates, type 2 pneumocyte hyperplasia, and interstitial organization. Some cases featured a sparse interstitial chronic inflammatory infiltrate. Although macrophages were present within the airspaces in all cases, this feature was not prominent, and findings typical of exogenous lipoid pneumonia were not present (56). While acute features of vaping-induced lung injury are becoming clearer, pathological aspects of chronic lung disease due to vaping are still unclarified.
The epidemiologic profile of EVALI patients who were hospitalized was published in a large series (n = 2,558) in 2020 by Werner and colleagues (57). Most EVALI patients were male [32 of 60 (53%) in fatal and 1,666 of 2,498 (67%) in nonfatal cases, respectively]. The proportion of patients was higher among those who were non-Hispanic white [39 of 49 (80%) in fatal and 1,104 of 1,818 (61%) in nonfatal cases, respectively] than among those in other race or ethnic groups.In fatal cases, the proportion was higher among those 35 years of age or older [44 of 60 (73%)] than among those younger than 35 years. Among the patients who had an available medical history, a higher proportion of those with fatal cases than those with nonfatal cases had a history of asthma [13 of 57 (23%) versus 102 of 1,297 (8%)], cardiac disease [26 of 55 (47%) versus 115 of 1,169 (10%)], or a mental health condition [32 of 49 (65%) versus 575 of 1,398 (41%)]. A total of 26 of 50 patients (52%) with fatal cases were obese. The study highlighted that premorbid chronic conditions, including cardiac and respiratory diseases, as well as mental health conditions, were common among hospitalized patients with EVALI.
Although the numbers of EVALI cases dropped dramatically during 2020, cases are still occurring. Unfortunately, the onset of the coronavirus disease 2019 (COVID-19) pandemic and its progress in the United States have presented challenges to both clinicians and epidemiologists in diagnosing, treating, and accounting for disease incidence (58, 59). Moreover, a recent study in youth demonstrated an increased risk of COVID-19 among vapers,most of whom use only ENDS and not THC/VEA solutions. However, it remains unknown whether smoking of cigarettes and vaping e-cigarettes in youth increase risk of COVID-19. To address this question, in May 2020, Gaiha et al. (60) conducted a national online survey among adolescents and young adults aged 13–24 years (n = 4,351). Multivariable logistic regression was performed to determine the relationships between COVID-19-related symptoms, testing, and diagnosis with multiple variables, including use of e-cigarettes only,dual use (e-cigarettes and cigarettes),sociodemographic factors, obesity, and complying with shelter-in-place. COVID-19 diagnosis was five times higher among ever-users of e-cigarettes only, seven times higher among ever-dual-users, and seven times higher among past 30-day dual-users. Frequency of positive COVID-19 testing was nine times higher among past 30-day dual-users and 2.6 times higher among past 30-day e-cigarette only users. Symptoms of COVID-19 were 4.7 times higher among past 30-day dual-users. This study revealed that while COVID-19 is less common in youth, use of e-cigarettes only or the dual use of e-cigarettes and cigarettes increases the risk of COVID-19 in this demographic.
4. EPIDEMIOLOGY OF CHRONIC RESPIRATORY ILLNESS IN VAPERS
The focus of the outbreak in 2019 turned to THC vaping products, but it is important not to lose sight of the larger health issues around vaping. Global usage of ENDS has increased in the last decade, especially among youth and young adults (61). In 2019, the prevalence of ENDS among middle and high school studentsin the United States was 10.5 and 27.5%,respectively (62).ENDS are noncombustible tobacco products that heat and aerosolize a liquid containing humectants and solvents (5–7, 63). The liquid contained in the tanks, cartridges, and pods used in the e-devices is commonly referred to as e-liquid, and commercial labels list the primary ingredients as PG and VG (also known as glycerol), plus flavorings and nicotine (15, 16). The e-devices heat the liquid via activation of the battery and conduction of the energy through a heating coil within the liquid. Application of negative pressure via the mouthpiece is used to pull the e-liquid through a mesh to create a fine aerosol. Recently, analyses of commonly used vaping fluids have shown that e-cigarette fluids contain at least seven groups of potentially toxic compounds: nicotine, carbonyls, volatile organic compounds (VOCs, such as benzene and toluene), particles, trace metal elements according to flavor (14, 17), and bacterial endotoxins and β-glucans (18, 19) (Figure 1). Additive compounds without nicotine can cause lung damage by eliciting cellular toxicity. For example, two flavoring chemicals alone, diacetyl and 2,3-pentanediol, have been shown to perturb transcriptomic changes related to ciliogenesis and cytoskeletal structure in well-differentiated primary normal human bronchial epithelial (NHBE) cells (64). The literature contains many reports of acute lung disease caused by the vaping of nicotine-containing ENDS, including acute eosinophilic pneumonia, respiratory bronchiolitis-associated interstitial lung disease, and hypersensitivity pneumonitis (47, 55, 56). The heterogeneity in the response to inhaled insults is not unexpected, given the numerous chemicals contained in e-cigarette aerosols and variability in how hosts respond to different insults due to underlying genetic and environmental factors. However, some commonalities in acute lung injury pathology related to THC products containing VEA emerged during the outbreak (55).
Population-based data on individuals who vape e-liquids chronically are sparse. There are a number of reports on known toxic exposures to humans generated by actual products on the market and growing evidence of adverse human health effects (65). As with cigarette smoking, the inhalation of chemicals contained within ENDS aerosols can elicit inflammatory responses in the lungs. Vaping has thus far been associated with asthma (39, 66–69), bronchiolitis (55, 56), and alteration of airway defenses (64). In the study including the Population Assessment of Tobacco and Health (PATH) study Wave 4 data on 33,606 US adult participants who indicated ever using e-cigarettes, the risk of wheezing and other respiratory symptoms was greater in ENDS users as compared to nonusers and lower compared to smokers (70). Comparisons of adults who ever vaped without marijuana versus those who ever vaped with marijuana (at least sometimes or rarely) showed that self-reported respiratory symptoms over the past 12 months were significantly increased when vaping with marijuana, including wheezing/whistling in the chest, wheezing in the chest during or after exercise, and a dry cough at night (71). This study revealed that lifetime use of e-cigarettes with marijuana associates with self-reported respiratory symptoms over the past 12 months among adults.
Studies of e-cigarette exposure in humans are limited but have demonstrated pulmonary and cardiac toxicities. In one study of healthy never smokers who were exposed to ENDS aerosols for a short time, 10 subjects were assessed at baseline with questionnaires, chest X-rays, lung function tests, plasma levels of endothelial microparticles, and bronchoscopy to obtain small airway epithelial cells and alveolar macrophages. One week later, subjects inhaled 10 puffs of Blu brand e-cigarettes two times. Following repeated exposure, both clinical and biological parameters were examined. Although no significant changes in clinical parameters were observed, biological changes were observed. Compared to baseline, inhalation of e-cigarette aerosol with nicotine caused altered transcriptomes of small airway epithelial cells and alveolar macrophages among all subjects and elevated plasma microparticle levels, providing in vivo human data demonstrating that acute inhalation of e-cigarette aerosols dysregulates normal human lung homeostasis in a limited cohort of healthy naïve individuals (72).
Human exposure studies have also generally shown increased sympathetic nerve activity, platelet hemostasis processes, reactive oxygen species (ROS) generation, and endothelial dysfunction. Studies with conflicts of interest vis-à-vis industry sponsorship were less likely to report such effects, whereas almost all nonconflicted studies did (20). Interestingly, adverse cardiac effects were also noted in a study of healthy, nonsmoking, and nonvaping adults who were exposed to secondhand vaping emissions (25). In this randomized, repeated measures cross-over study, total heart rate variability [measured by the standard deviation of beat-to-beat (NN) intervals (SDNN)], heart rate variability over short cycles [the average of SDNN (ASDNN)], and heart rate correct QT intervals (QTc) were assessed. Nicotine from these e-cigarette exposures were associated with a 7.8% decrease in SDNN, a 7.7% decrease in ASDNN, and a 3.8-ms decrease in QTc. Greater nicotine over a longer exposure (15–30 min) was associated with greater QTc reductions. These results were the first evidence of short-term, secondhand e-cigarette vapor-induced cardiac autonomic effects in healthy nonsmokers.
In another study (26), exhaled breath was collected from 17 e-cigarette vapers and analyzed for nicotine, PG, VG, formaldehyde, acetaldehyde, acrolein, tobacco-specific nitrosamines, and heavy metals. Among the analytes in exhaled breath, levels of nicotine, PG, tobacco-specific nitrosamines, and copper were increased. Based upon the initial assessment of toxicants in exhaled breath, bystander exposure was estimated for two different exposure scenarios. Each of the two scenarios simulated daily exposures during either a daily commute in a small unventilated car with two e-cigarette users or a daily office hour in an office-sized space with one e-cigarette user. Results showed that bystanders may experience irritation of the respiratory tract and systemic effects, including palpitations and increased systolic blood pressure. The irritation of the respiratory tract was associated with exposure to PG and VG, and systemic effects were associated with exposure to nicotine (26).
Early life exposure to e-cigarettes/ENDS is a public health concern. There are no human studies, asyet, of maternal ENDS use and birth/development outcomes, but the principal toxicants delivered by these devices raise considerable public health concern (73). Exposure of female mice to e-cigarettes in early pregnancy significantly impaired embryo implantation, as evidenced by the nearly complete absence of implantation sites in e-cigarette-exposed animals at day 5, despite exhibiting high levels of progesterone, an indicator of pregnancy (74). Effects of nicotine, a key substance of concern but by no means the only one in ENDS, on the fetus are well known (75–78). Hence, first- and second-hand e-cigarette aerosols may well pose significant hazards to the fetus. How these adverse fetal responses impair pulmonary function and disease risk in later life is a subject of ongoing research.
5. MODELS AND MECHANISMS OF LUNG INJURYAND INFLAMMATION
Clinical observations and epidemiological studies confirming the adverse biological effects of e-cigarettes on human health, and increasing cases of EVALI-related deaths, emphasize the urgent need to understand the pathophysiologic mechanisms of EVALI and acute and chronic effects of e-cigarette use. However, due to the rapid growth of e-cigarette use worldwide, these mechanisms and the long-term consequences of e-cigarette use remain unknown. Unknown pathophysiologic mechanisms result in a lack of biomarkers, which are needed as diagnostic tools. To identify potential biomarkers and targeted treatments and to prevent vaping-induced diseases including EVALI, both in vitro and in vivo approaches are required to understand the causes and pathophysiologic mechanisms of EVALI.
E-cigarettes elicit adverse health effects through direct contact of aerosols (also called e-cigarette vapor) with tissues or cells in the oral cavity and lung or through systemic effects on multiple organs including the heart, brain, eyes, and kidneys (65, 79, 80, 81–86) (Figure 3). Because the most substantial toxicity of e-cigarettes is expected in the lung, and systemic effects may be propagated by injured lung, many studies have focused on the lung using both in vitro and in vivo models (Figure 2). In vitro models (82, 87) are less likely to replicate real-life exposure to e-cigarettes but provide mechanistic insight into molecular and cellular pathways impacted by specific chemicals contained in e-cigarette aerosols. Despite the known limitations of in vitro models, multiple studies have found similarities of transcriptomic profiles in airway epithelial cells from e-cigarette users and cultured NHBE cells exposed to e-cigarette aerosols (72, 88, 89). In the earliest in vitro studies, cells were exposed to e-liquids, which do not recapitulate the chemical composition of aerosols generated by ENDS. Recently, researchers transitioned to systems in which mammalian cells were directly exposed to e-cigarette aerosols, increasing the relevance of these studies to real-life exposures.
Figure 3.
Adverse effects of electronic cigarettes on human health. As the site of the contact with inhaled toxic chemicals, the lungs are directly damaged by electronic cigarette use, but multiple organs are damaged by systemic adverse effects. Figure adapted from images created with BioRender.com.
In vivo exposures have been done primarily in rodents, with early models also using e-liquids instead of aerosols (90, 91). Researchers have now universally transitioned to nose-only or whole-body exposures of animals to freshly generated e-cigarette aerosols. Use of commercially available e-cigarettes has increased the translatability of these studies to the general population but has made it difficult for researchers to keep up as new generations of devices emerge every 2–4 years. Studies focused on flavors popular in the vaping community also increase the relevance of results to e-cigarette users. Alternatively, some researchers focus on core components of e-liquids (PG, VG, and nicotine) that are present in all e-liquid solutions and thus have high relevance to the entire vaping community. As specific chemicals have been identified as harm inducing, such as VEA as the causal agent of EVALI, researchers introduce these into vaping exposure systems to rapidly assess for lung injury and inflammation. Because inhalants can alter the immune and inflammatory state of the lungs and body (e.g., tobacco smoke), researchers also assess for alterations in the responses of e-cigarette-exposed animals to common clinical challenges using models of acute lung injury, bacterial and viral pneumonia, and airway reactivity.
5.1. Inflammatory Cytokines and Mediators
Overall, exposure to e-cigarettes induces secretion of proinflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), from epithelial cells and immune cells in the upper airway and lung (80, 92–96). A particular pattern of neutrophil signals has been detected across studies. In the sputum of e-cigarette users, the neutrophilic granule proteins neutrophil elastase, proteinase 3, azurocidin 1, and myeloperoxidase are significantly increased (97),suggesting activation of neutrophils by e-cigarette exposure. Exposure of neutrophils to e-cigarette vapor extract markedly increases expression of CD11b and CD66b, which play a critical role in the activation of neutrophils (95). Furthermore, exposure to e-cigarette vapor extract leads to increased IL-8 secretion and protease activity, including that of neutrophil elastase and matrix metalloproteinase 9 (95). Increased proteases can damage lung basement membrane and extracellular matrix, leading to emphysema (98, 99). In one of the first studies of VEA using alveolar type II epithelial cells cultured in an air-liquid interface, VEA was incorporated into the cells with subsequent release of monocyte and neutrophil chemokines, demonstrating the direct role of VEA in inducing inflammatory responses (96).
Across in vivo exposures, the main cells found to be recruited to the lungs in response to e-cigarette aerosol inhalation include macrophages (94, 100, 101), neutrophils (102), eosinophils, and T cells (101, 103). As evidence of the importance of both e-device type and the composition of the heating element, Kleinman et al.’s (102) histologic analysis found acute lung injury in rats exposed to e-cigarette aerosols generated by a heating element made of nickel-chromium alloy (at 70 W) but not one with a stainless-steel atomizer. To assess how e-cigarette exposure alters airway inflammation, two distinct mouse models of allergic asthma had been used: house dust mite and ovalbumin (104–106). In a mouse model of allergic asthma using house dust mite, which induces type 2-low airway inflammation, exposure to e-cigarette aerosols containing 12 mg/mL nicotine suppressed allergic inflammatory responses (105).In a mouse model of allergic asthma using ovalbumin, which induces type 2-high airway inflammation, exposure to e-cigarette aerosols containing 18 mg/mL nicotine increased allergic inflammatory responses (106).Because these two groups used two different asthma models (type 2-low inflammation versus type-2 high inflammation), it is possible that e-cigarette aerosol effects vary based on asthma phenotype. Alternatively, differences could also be due to different e-devices and wattage applied (Joyetech eVic-VT e-cigarette with a maximum of 45 W versus the Kanger Mini ProTank 2 with 5.76 W). As mentioned above, much work has focused on the e-cigarette effects on the inflammatory state of the lungs. Investigators have identified changes in numerous cytokines within the airways and lung parenchyma of e-cigarette-exposed rodents, but consistent patterns have not been identified, likely owing to the heterogeneity of exposure models, e-devices, and e-liquids used (65).
5.2. Cellular Damage
Vaping results in the generation of increased reactive aldehyde species (13, 107) causing the cellular accumulation of 4-hydroxynonenal, which induces apoptosis, mitochondria dysfunction, and protein inactivation (108–110).E-cigarette exposure also directly induces cellular damage by driving increased ROS generation during oxidative burst (92,111,112) and DNA damage (80,88,113). In multiple epithelial cell lines, e-cigarette exposure increases comet tail length and the accumulation of gamma H2AX (γH2AX) foci, suggesting single-strand and double-strand DNA breaks caused by e-cigarettes (113). E-cigarette-exposed cells also show increased rates of apoptosis and necrosis, regardless of nicotine content (113). Higher levels of apoptosis in lung cells of mice exposed to e-cigarette aerosols daily have also been observed, which suggests an increased risk of developing emphysema (94), as well as apoptosis within cardiac tissue (114). Finally, Canistro et al. (115) demonstrated the comutagenic and cancer-initiating effects of e-cigarette aerosol exposures in a rat model. They found that e-cigarette aerosol inhalation caused a boost in phase I carcinogen-bioactivating enzymes, including activators of polycyclic aromatic hydrocarbons, and increased oxygen free radical production and DNA oxidation to 8-hydroxy-2′-deoxyguanosine.
Inflammation and DNA damage are also associated with compromised oral health (80).In both human periodontal ligament fibroblasts and human gingival epithelium progenitors, exposure to e-cigarettes increases secretion of inflammatory mediators [IL-8 and prostaglandin E2 (PGE2)] and DNA damage as marked by γH2AX (80). E-cigarette exposure increases generation of ROS that induces inflammatory responses in both human epithelial cells and mouse models (116). In H292 cells maintained in air-liquid interface culture, exposure to flavored e-cigarette aerosols induces cellular toxicity and ROS generation (111). In primary NHBE cells differentiated in air-liquid interface culture, exposure to e-cigarette aerosols induces a marker of ROS, 8-isoprostane, in a dose-dependent manner (88). Gene ontology analysis suggests that cellular regeneration and differentiation are impaired, while DNA damage and ROS generation are increased in NHBE cells. In small airway epithelial cells, exposure to e-cigarette emissions generates eight times more ROSthanincontrolcells(117).Murineexposuresarealsoassociatedwithgeneexpressionchanges consistent with increased oxidative burst and apoptosis in cardiac tissue in particular, raising concern for the development of cardiomyopathy (114), and e-cigarette exposures lead to increased lipid peroxidation, which is evidence of oxidative stress (118).
E-cigarette aerosols also impact mitochondria, cilia, and fibrosis. E-cigarette exposure induces mitochondria dysfunction, resulting in reduced ATP production, which is linked to compromised ciliary functions (119). Mitochondrial dysfunction may be due to increased ROS within mitochondria and may lead to insufficient energy production in cells. Chronic exposure to e-cigarette aerosols can be associated with the development of organ fibrosis, with increases in both profibrotic and oxidative stress markers (93).
5.3. Transcriptional and Proteomic Modifications
E-cigarette aerosols induce transcriptional alterations in both oral and airway epithelial cells (64, 80, 88, 89, 94, 120–122). As identified by RNA sequencing analysis and confirmed by quantitative polymerase chain reaction (qPCR), two tumor suppressor genes (NOTCH1 and HERC2) are downregulated in e-cigarette users (120). Transcriptional analysis of oral epithelial cells from e-cigarette users suggests that cancer risk is increased. E-cigarette exposure reduces the ciliated-cell marker gene (FOXJ1) while increasing expression of the genes involved in xenobiotic metabolism (CYP1A1 and CYP1B1) and oxidative stress (DNAH10) (88).
Proteomic analysis of airway epithelial cells from biopsies revealed unique protein expression profiles in vapers compared to analysis in nonsmokers (89). In airway epithelial cells from vapers, expressions of MUC5AC, MUC4, and CYP1B1 proteins were higher than in those from nonsmokers. These findings from human tissues were further validated in NHBE cells after exposure to aerosolized PG/VG. Exposure to PG/VG for four days induced MUC5AC protein in well-differentiated NHBE cells. These data suggest that e-cigarette exposure may cause airway obstruction by increased secretion of gel-forming mucin in vapers (89).Interestingly, e-cigarette exposure reduces ribosomal proteins and subsequent protein biogenesis in NHBE cells (121), indicating the potential detrimental effect of e-cigarette smoke on ribosomes and the associated protein synthesis in the airway epithelium. In NHBE cells, the phospholipid and fatty acid triacylglycerol metabolism pathways are found among the cellular pathways with the most significantly enriched gene expression following e-cigarette exposure (122). These data suggest that alterations in cellular glycerophospholipid biosynthesis are an important consequence of e-cigarette exposure.
5.4. Impaired Host Defense: Barrier Dysfunctions, Mucociliary Clearance,Bacterial Clearance, and Viral Defense
Epithelial integrity is the first line of lung defense. However, exposure to e-cigarette aerosols not only causes sloughing of epithelial cells, but it also disrupts epithelial barrier integrity (88, 93, 96, 111, 112). In mice, VEA inhalation leads to lung damage similar to that seen in humans (54). Specifically, VEA inhalation causes lung edema, neutrophilia, epithelial cell death, and lymphocyte-predominant perivascular inflammation (96) and also reduces the production of surfactant protein A (96, 112).
Mucociliary clearance is critical for protection of the respiratory tract against inhaled toxic substances (123). Impaired mucociliary clearance results in chronic inflammation by establishing a favorable environment for pathogenic bacterial colonization and growth (123, 124). In both in vitro and in vivo models, e-cigarette exposures compromise mucociliary clearance by reducing ciliary beating frequency (94, 119, 125, 126). In NHBE cells, e-cigarette exposure reduces not only the number of ciliated cells (64, 94) but also ciliary beating frequency (94, 119, 125, 126). Exposure to aerosolized, nicotine-containing e-cigarette fluids reduces ion conductance and mucociliary function in human bronchial epithelial cells and induces airway hyperreactivity and air space enlargement in exposed mice (note that e-liquids were aerosolized with a medication nebulizer, not an e-device) (94). A transient reduction of ciliary beating frequency was also observed after exposure to cinnamaldehyde-containing e-liquid, vaped aerosol, or cinnamaldehyde alone (119). Reduced ciliary beating frequency occurred secondary to reduced ATP production, which resulted from dysregulated mitochondria (119). In well-differentiated NHBE cells, exposure to e-cigarette vapor reduced airway surface liquid hydration and increased mucus viscosity in a nicotine-dependent manner (126). Impaired mucociliary clearance appears to be mediated by TRPA1, an ion channel, and not nicotinic acetylcholine receptors.
Multiple cells in the lungs contribute to host defense through direct antimicrobial activities. E-cigarette vapor exposure has been found to inhibit antibacterial function of epithelial cells, macrophages, and neutrophils (63, 93, 127). Inhibition of phagocytosis in macrophages (92, 128) is one mechanism by which vaping impacts bacterial clearance. The other impaired host defense mechanism is inhibition of neutrophil extracellular trap formation (NETosis) (127). Exposure of NHBE cells to e-liquids reduces the expression of SPLUNC1,a host defense molecule,providing further evidence of the impaired bacterial host defenses in e-cigarette users (129). In particular, NHBE cells were isolated from young healthy donors aged 8–10 years. Thus, these in vitro studies support epidemiological studies demonstrating the increased rates of chronic bronchitis symptoms in adolescent e-cigarette users compared to nonsmokers (130). Finally, e-cigarette aerosol exposure has been found to promote biofilm formation and virulence of common bacterial colonizers and pathogens (63, 131), which indicates that vapers, like conventional tobacco smokers before them, may develop higher rates of invasive bacterial infections.
In vitro e-cigarette aerosol exposure causes apoptosis, secondary necrosis, and necrosis in lung epithelial cells and apoptosis and inflammatory caspase–mediated cell death in macrophages (132). Exposure to e-cigarette aerosols containing nicotine inhibits phagocytic and efferocytic abilities of primary macrophages, leading to decreased bacterial clearance when challenged with a bacterial pathogen (118). Exposure of neutrophils, which are the first cells recruited to the site of infection, to e-cigarette aerosols with and without nicotine leads to decreased phagocytosis and decreased bactericidal activity (63, 127). Suppression of antimicrobial functions of both macrophages and neutrophils by e-cigarette aerosols in vitro and ex vivo supports the concept that e-cigarette use damages host defenses and will lead to increased susceptibility to pulmonary infections.
Alveolar macrophages cultured with either e-liquid or e-cigarette vapor condensate result in a dose-dependent reduction in cell viability (92). E-cigarette vapor condensate is significantly more toxic to alveolar macrophages than nonvaped e-liquid. Excessive production of ROS, inflammatory cytokines, and chemokines induced by e-cigarette aerosols may induce an inflammatory state in alveolar macrophages within the lung that is partly dependent on nicotine. Inhibition of phagocytosis also suggests that users may suffer from impaired bacterial clearance. The two primary chemicals found in e-liquids are PG and VG. These additives act as vehicles and carriers for nicotine, which is highly insoluble in water, as well as for flavorings. Although some researchers view these chemicals as unimportant, both have been found to have toxic effects at both the cellular and host levels. PG and VG reduce glucose uptake in NHBE cells in an air-liquid interface culture (133). This is relevant in that glucose transports move extracellular glucose into airway cells to maintain luminal surface with a low concentration of glucose in which bacterial growth is prohibited. Thus, reduced glucose uptake by PG/VG suggests compromised innate immunity against bacterial infections in e-cigarette users (134). In NHBE cells in vitro, PG/VG also reduce membrane fluidity and impaired protein diffusion, suggesting that PG/VG could alter cellular endocytosis and exocytosis (89).
In terms of viral immunity, airway epithelial cells from vapers were found to have decreased expression of Toll-like receptor 3, suggesting that viral immunity is impaired by e-cigarette use (89).Infectionofe-cigarette-exposedmicewithinfluenzaleadstoincreasedlunginflammationand injury, consistent with an inability to control the viral infection and the potential to develop an immunomodulated state leading to excessive lung inflammation in response to viral infection (118, 135). Due to the COVID-19 pandemic, some groups have assessed specifically for the effects of vaping on molecules that play a role in SARS-CoV-2 infections, finding that female (but not male) mice exposed to e-cigarette aerosols containing nicotine had increased angiotensin-converting enzyme 2 (ACE2) levels in the lung (101).
6. CONCLUSION
Since the appearance of ENDS on the market, the prevalence of e-cigarette use has become a growing public health concern. Despite the expected toxicity of inhaled nicotine and various chemical additives (Figure1), the impact on human health has been controversial. To date, a growing body of evidence indicates that e-cigarettes cause lung inflammation and injury (Figure 2) as well as systemic adverse effects in multiple organs (Figure 3). However, the pathophysiological mechanisms by which the lung and various organs are damaged remain unknown. Thus far, most evidence was collected in observational studies, some of which showed contradictory outcomes. Incongruous outcomes may be attributable to multiple factors, including frequency of vaping, e-device type, e-liquid composition, age, sex, and underlying health conditions. Henceforth, we should prioritize our efforts toward controlled studies to elucidate the pathophysiologic mechanisms behind the adverse health effects caused by e-cigarettes. Advanced knowledge will allow us to develop biomarkers and treatments of vaping-related diseases.
ACKNOWLEDGMENTS
The authors thank Ira Advani and Josephine Pham for generating the figures.
Glossary
- E-cigarette
electronic cigarette
- EVALI
electronic cigarette or vaping use-associated lung injury
- VEA
vitamin E acetate
- ENDS
electronic nicotine delivery systems
- THC
tetrahydrocannabinol
- CBD
cannabidiol
- BAL
bronchoalveolar lavage
- PG
propylene glycol (propane-1,2-diol)
- VG
vegetable glycerine (propane-1,2,3-triol)
- NHBE cells
normal human bronchial epithelial cells
- ROS
reactive oxygen species
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Dockrell M, Morrison R, Bauld L, McNeill A. 2013. E-cigarettes: prevalence and attitudes in GreatBritain. Nicotine Tob. Res 15:1737–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grana R, Benowitz N, Glantz SA. 2014. E-cigarettes: a scientific review. Circulation 129:1972–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, et al. 2014. Four hundred and sixty brands ofe-cigarettes and counting: implications for product regulation. Tob. Control 23(Suppl. 3):iii3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. 2014. Electronic cigarettes: review of use,content, safety, effects on smokers and potential for harm and benefit. Addiction 109:1801–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahandeh N, Chowdhary H, Middlekauff HR. 2021. Vaping and cardiac disease. Heart 107:1530–35 [DOI] [PubMed] [Google Scholar]
- 6.Choi H, Lin Y, Race E, Macmurdo MG. 2021. Electronic cigarettes and alternative methods of vaping. Ann. Am. Thorac. Soc 18:191–99 [DOI] [PubMed] [Google Scholar]
- 7.DeVito EE, Krishnan-Sarin S. 2018. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr. Neuropharmacol 16:438–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, et al. 2014. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16:1319–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Chen W, Liao J, Matsuo T, Ito K, et al. 2017. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLOS ONE 12:e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vreeke S, Zhu X, Strongin RM. 2020. A simple predictive model for estimating relative e-cigarette toxic carbonyl levels. PLOS ONE 15:e0238172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D, Aravindakshan A, Hilpert M, Olmedo P, Rule AM, et al. 2020. Metal/metalloid levels in electronic cigarette liquids, aerosols, and human biosamples: a systematic review. Environ. Health Perspect 128:36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, et al. 2018. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ. Health Perspect 126:027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, et al. 2015. Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. Environ. Pollut 198:100–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowles J, Barreau T, Wu N. 2020. Cancer and non-cancer risk concerns from metals in electronic cigarette liquids and aerosols. Int. J. Environ. Res. Public Health 17:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, et al. 2016. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ. Health Perspect 124:733–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klager S, Vallarino J, MacNaughton P, Christiani DC, Lu Q, Allen JG 2017. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ. Sci. Technol 51:10806–13 [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. 2017. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ. Health 16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MS,Allen JG,Christiani DC.2019.Endotoxin and (1 → 3)-β-d-glucan contamination in electronic cigarette products sold in the United States. Environ. Health Perspect 127:47008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MS, Christiani DC. 2020. Microbial toxins in nicotine vaping liquids. Am. J. Respir. Crit. Care Med 201:741–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy CD,van Schalkwyk MCI,McKee M,Pisinger C.2019.The cardiovascular effects of electronic cigarettes: a systematic review of experimental studies. Prev. Med 127:105770. [DOI] [PubMed] [Google Scholar]
- 21.Bergeria CL, Heil SH, Bunn JY, Sigmon SC, Higgins ST. 2018. Comparing smoking topography and subjective measures of usual brand cigarettes between pregnant and non-pregnant smokers.Nicotine Tob. Res 20:1243–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox S, Goniewicz ML, Kosmider L, McRobbie H, Kimber C, Dawkins L. 2021. The time course of compensatory puffing with an electronic cigarette: secondary analysis of real-world puffing data with highandlownicotineconcentrationunderfixedandadjustablepowersettings.NicotineTob.Res 23:1153–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasser AM, Johnson AL, Niaura RS, Abrams DB, Pearson JL. 2021. Youth vaping and tobacco use in context in the United States: results from the 2018 National Youth Tobacco Survey. Nicotine Tob. Res 23:447–53 [DOI] [PubMed] [Google Scholar]
- 24.Ballbe M, Martinez-Sanchez JM, Sureda X, Fu M, Perez-Ortuno R, et al. 2014. Cigarettes versus e-cigarettes: passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res 135:76–80 [DOI] [PubMed] [Google Scholar]
- 25.Lee MS, Rees VW, Koutrakis P, Wolfson JM, Son YS, et al. 2019. Cardiac autonomic effects of second-hand exposure to nicotine from electronic cigarettes: an exploratory study. Environ. Epidemiol 3:e033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser WF, Klerx WN, Cremers H, Ramlal R, Schwillens PL, Talhout R. 2019. The health risks of electronic cigarette use to bystanders. Int. J. Environ. Res. Public Health 16:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, et al. 2020. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—Final Report. N. Engl. J. Med 382:903–16 [DOI] [PubMed] [Google Scholar]
- 28.Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, et al. 2019. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med 381:1488–89 [DOI] [PubMed] [Google Scholar]
- 29.Henry TS, Kanne JP, Kligerman SJ. 2019. Imaging of vaping-associated lung disease. N. Engl. J. Med 381:1486–87 [DOI] [PubMed] [Google Scholar]
- 30.Rhee J, Dominici F, Zanobetti A, Schwartz J, Wang Y, et al. 2019. Impact of long-term exposures to ambient PM2.5 and ozone on ARDS risk for older adults in the United States. Chest 156:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue K. 2011. Promoting effects of nanoparticles/materials on sensitive lung inflammatory diseases. Environ. Health Prev. Med 16:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach A,Hernandez ML.2012.The effect of environmental oxidative stress on airway inflammation. Curr. Opin. Allergy Clin. Immunol 12:133–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hailemariam Y, Amiri HM, Nugent K. 2012. Acute respiratory symptoms following massive carbon black exposure. Occup. Med 62:578–80 [DOI] [PubMed] [Google Scholar]
- 34.Johannson KA, Balmes JR, Collard HR. 2015. Air pollution exposure: A novel environmental risk factor for interstitial lung disease? Chest 147:1161–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schelegle ES, Walby WF, Alfaro MF, Wong VJ, Putney L, et al. 2003. Repeated episodes of ozone inhalation attenuates airway injury/repair and release of substance P, but not adaptation. Toxicol. Appl. Pharmacol 186:127–42 [DOI] [PubMed] [Google Scholar]
- 36.D’Amato G, Cecchi L. 2008. Effects of climate change on environmental factors in respiratory allergic diseases. Clin. Exp. Allergy 38:1264–74 [DOI] [PubMed] [Google Scholar]
- 37.Alexis NE, Carlsten C. 2014. Interplay of air pollution and asthma immunopathogenesis: a focused review of diesel exhaust and ozone. Int. Immunopharmacol 23:347–55 [DOI] [PubMed] [Google Scholar]
- 38.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, et al. 2019. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, part 2: air pollution and organ systems. Chest 155:417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kales SN, Christiani DC. 2004. Acute chemical emergencies. N. Engl. J. Med 350:800–8 [DOI] [PubMed] [Google Scholar]
- 40.Puisney C, Baeza-Squiban A, Boland S. 2018. Mechanisms of uptake and translocation of nanomaterials in the lung. Adv. Exp. Med. Biol 1048:21–36 [DOI] [PubMed] [Google Scholar]
- 41.Manke A, Wang L, Rojanasakul Y. 2013. Pulmonary toxicity and fibrogenic response of carbon nanotubes. Toxicol. Mech. Methods 23:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, et al. 2017. Toxicology of silica nanoparticles: an update. Arch. Toxicol 91:2967–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braakhuis HM, Park MV, Gosens I, De Jong WH, Cassee FR. 2014. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, et al. 2019. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laffey JG, Kavanagh BP. 2017. Fifty years of research in ARDS. Insight into acute respiratory distress syndrome: from models to patients. Am. J. Respir. Crit. Care Med 196:18–28 [DOI] [PubMed] [Google Scholar]
- 46.Thompson BT, Chambers RC, Liu KD. 2017. Acute respiratory distress syndrome. N. Engl. J. Med 377:562–72 [DOI] [PubMed] [Google Scholar]
- 47.Mull ES, Erdem G, Nicol K, Adler B, Shell R. 2020. Eosinophilic pneumonia and lymphadenopathy associated with vaping and tetrahydrocannabinol use. Pediatrics 145:e20193007 [DOI] [PubMed] [Google Scholar]
- 48.Collins BN, Lepore SJ, Winickoff JP, Nair US, Moughan B, et al. 2018. An office-initiated multilevel intervention for tobacco smoke exposure: a randomized trial. Pediatrics 141:S75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galiatsatos P, Gomez E, Lin CT, Illei PB, Shah P, Neptune E. 2020. Secondhand smoke from electronic cigarette resulting in hypersensitivity pneumonitis. BMJ Case Rep. 13:e233381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christiani DC. 2020. Vaping-induced acute lung injury. N. Engl. J. Med 382:960–62 [DOI] [PubMed] [Google Scholar]
- 51.De Giacomi F, Vassallo R, Yi ES, Ryu JH. 2018. Acute eosinophilic pneumonia. Causes, diagnosis, and management. Am. J. Respir. Crit. Care Med 197:728–36 [DOI] [PubMed] [Google Scholar]
- 52.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, et al. 2020. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med 382:697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman R, Meiman J, Stanton M, Gummin DD. 2020. Culprit or correlate? An application of the Bradford Hill criteria to Vitamin E acetate. Arch. Toxicol 94:2249–54 [DOI] [PubMed] [Google Scholar]
- 54.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. 2020. An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N. Engl. J. Med 382:1175–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reagan-Steiner S, Gary J, Matkovic E, Ritter JM, Shieh WJ, et al. 2020. Pathological findings in suspected cases of e-cigarette, or vaping, product use-associated lung injury (EVALI): a case series. Lancet Respir. Med 8:1219–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, et al. 2020. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping). Am. J. Clin. Pathol 153:30–39 [DOI] [PubMed] [Google Scholar]
- 57.Werner AK, Koumans EH, Chatham-Stephens K, Salvatore PP, Armatas C, et al.2020.Hospitalizationsand deaths associated with EVALI. N. Engl. J. Med 382:1589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazachkov M, Pirzada M. 2020. Diagnosis of EVALI in the COVID-19 era. Lancet Respir. Med 8:1169–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callahan SJ, Harris D, Collingridge DS, Guidry DW, Dean NC, et al. 2020. Diagnosing EVALI in the time of COVID-19. Chest 158:2034–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaiha SM, Cheng J, Halpern-Felsher B. 2020. Association between youth smoking, electronic cigarette use, and COVID-19. J. Adolesc. Health 67:519–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Besaratinia A, Tommasi S. 2019. Vaping: a growing global health concern. E Clinical Medicine 17:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, et al. 2019. E-cigarette use among youth in the United States, 2019. JAMA 322:2095–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, et al. 2016. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med 94:667–79 [DOI] [PubMed] [Google Scholar]
- 64.Park HR, O’Sullivan M, Vallarino J, Shumyatcher M, Himes BE, et al. 2019. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci. Rep 9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bozier J, Chivers EK, Chapman DG, Larcombe AN, Bastian NA, et al. 2020. The evolving landscape of e-cigarettes: a systematic review of recent evidence. Chest 157:1362–90 [DOI] [PubMed] [Google Scholar]
- 66.Schweitzer RJ, Wills TA, Tam E, Pagano I, Choi K. 2017. E-cigarette use and asthma in a multi-ethnic sample of adolescents. Prev. Med 105:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, et al. 2019. The association between e-cigarette use and asthma among never combustible cigarette smokers: behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm. Med 19:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho JH, Paik SY. 2016. Association between electronic cigarette use and asthma among high school students in South Korea. PLOS ONE 11:e0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi K, Bernat D. 2016. E-cigarette use among Florida youth with and without asthma. Am. J. Prev. Med 51:446–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D, Xie Z. 2020. Cross-sectional association of lifetime electronic cigarette use with wheezing and related respiratory symptoms in U.S. adults. Nicotine Tob. Res 22:S85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie Z, Li D. 2020.Cross-sectional association between lifetime use of electronic cigarettes with or without marijuana and self-reported past 12-month respiratory symptoms as well as lifetime respiratory diseases in U.S. adults. Nicotine Tob. Res 22:S70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. 2018. Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir. Res 19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larcombe AN. 2019. Early-life exposure to electronic cigarettes: cause for concern. Lancet Respir. Med 7:985–92 [DOI] [PubMed] [Google Scholar]
- 74.Wetendorf M, Randall LT, Lemma MT, Hurr SH, Pawlak JB, et al. 2019. E-cigarette exposure delays implantation and causes reduced weight gain in female offspring exposed in utero.J.Endocr.Soc 3:1907–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGrath-Morrow SA,Gorzkowski J,Groner JA,Rule AM,Wilson K,et al.2020.The effects of nicotine on development. Pediatrics 145:e20191346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer KF, Verkaik-Schakel RN, Timens W, Kobzik L, Plosch T, Hylkema MN. 2017. The fetal programming effect of prenatal smoking on Igf1r and Igf1 methylation is organ- and sex-specific. Epigenetics 12:1076–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holbrook BD. 2016. The effects of nicotine on human fetal development. Birth Defects Res. C Embryo Today 108:181–92 [DOI] [PubMed] [Google Scholar]
- 78.Wong MK, Barra NG, Alfaidy N, Hardy DB, Holloway AC. 2015. Adverse effects of perinatal nicotine exposure on reproductive outcomes. Reproduction 150:R185–93 [DOI] [PubMed] [Google Scholar]
- 79.Figueredo CA, Abdelhay N, Figueredo CM, Catunda R, Gibson MP. 2021. The impact of vaping on periodontitis: a systematic review. Clin. Exp. Dent. Res 7:376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundar IK, Javed F, Romanos GE, Rahman I. 2016. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 7:77196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. 2017. Impact of electronic cigarettes on the cardiovascular system. J. Am. Heart Assoc 6:e006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai M, Byun MK, Shin J, Crotty Alexander LE. 2020. Effects of e-cigarettes and vaping devices on cardiac and pulmonary physiology. J. Physiol 598:5039–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heldt NA, Reichenbach N, McGary HM, Persidsky Y. 2021. Effects of electronic nicotine delivery systems and cigarettes on systemic circulation and blood-brain barrier: implications for cognitive decline. Am. J. Pathol 191:243–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S, Chen J, Cheng T, Gindulyte A, He J, et al. 2019. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 47:D1102–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Breland AB, Spindle T, Weaver M, Eissenberg T. 2014. Science and electronic cigarettes: current data, future needs. J. Addict. Med 8:223–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li G, Chan YL, Nguyen LT, Mak C, Zaky A, et al. 2019. Impact of maternal e-cigarette vapor exposure on renal health in the offspring. Ann. N. Y. Acad. Sci 1452:65–77 [DOI] [PubMed] [Google Scholar]
- 87.Gotts JE, Jordt SE, McConnell R, Tarran R. 2019. What are the respiratory effects of e-cigarettes? BMJ 366:l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moses E,Wang T,Corbett S,Jackson GR,Drizik E,et al.2017.Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol. Sci 155:248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, et al. 2018. Chronic e-cigarette exposurealters the human bronchial epithelial proteome. Am. J. Respir. Crit. Care Med 198:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lim HB,Kim SH.2014.Inhallation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicol. Res 30:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marczylo T 2020.How bad are e-cigarettes? What can we learn from animal exposure models? J. Physiol 598:5073–89 [DOI] [PubMed] [Google Scholar]
- 92.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, et al.2018.Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73:1161–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, et al. 2018. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 314:R834–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, et al. 2016. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71:1119–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higham A, Rattray NJ, Dewhurst JA, Trivedi DK, Fowler SJ, et al. 2016. Electronic cigarette exposuretriggers neutrophil inflammatory responses. Respir. Res 17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsumoto S, Fang X, Traber MG, Jones KD, Langelier C, et al. 2020. Dose-dependent pulmonary toxicity of aerosolized vitamin E acetate. Am. J. Respir. Cell Mol. Biol 63:748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, et al. 2018. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am. J. Respir. Crit. Care Med 197:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Janoff A, White R, Carp H, Harel S, Dearing R, Lee D. 1979. Lung injury induced by leukocytic proteases. Am. J. Pathol 97:111–36 [PMC free article] [PubMed] [Google Scholar]
- 99.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277:2002–4 [DOI] [PubMed] [Google Scholar]
- 100.Glynos C, Bibli SI, Katsaounou P, Pavlidou A, Magkou C, et al. 2018. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 315:L662–72 [DOI] [PubMed] [Google Scholar]
- 101.Wang Q, Sundar IK, Li D, Lucas JH, Muthumalage T, et al. 2020. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARSCoV-2 Covid-19 ACE2 receptor regulation. Respir. Res 21:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kleinman MT, Arechavala RJ, Herman D, Shi J, Hasen I, et al. 2020. E-cigarette or vaping product use associated lung injury produced in an animal model from electronic cigarette vapor exposure without tetrahydrocannabinol or vitamin E oil. J. Am. Heart Assoc 9:e017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szafran BN, Pinkston R, Perveen Z, Ross MK, Morgan T, et al. 2020. Electronic-cigarette vehicles and flavoring affect lung function and immune responses in a murine model. Int. J. Mol. Sci 21:6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fallon PG, Schwartz C. 2020. The high and lows of type 2 asthma and mouse models. J. Allergy Clin. Immunol 145:496–98 [DOI] [PubMed] [Google Scholar]
- 105.Chapman DG, Casey DT, Ather JL, Aliyeva M, Daphtary N, et al. 2019. The effect of flavored e-cigarettes on murine allergic airways disease. Sci. Rep 9:13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taha HR, Al-Sawalha NA, Alzoubi KH, Khabour OF. 2020. Effect of e-cigarette aerosol exposure on airway inflammation in a murine model of asthma. Inhal. Toxicol 32:503–11 [DOI] [PubMed] [Google Scholar]
- 107.Azimi P, Keshavarz Z, Lahaie Luna M, Cedeno Laurent JG, Vallarino J, et al. 2021. An unrecognized hazard in e-cigarette vapor: preliminary quantification of methylglyoxal formation from propylene glycol in e-cigarettes. Int. J. Environ. Res. Public Health 18:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Breitzig M, Bhimineni C, Lockey R, Kolliputi N. 2016. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol 311:C537–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bahmed K, Messier EM, Zhou W, Tuder RM, Freed CR, et al. 2016. DJ-1 modulates nuclear erythroid2-related factor-2-mediated protection in human primary alveolar type II cells in smokers. Am. J. Respir. Cell Mol. Biol 55:439–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao M, Zhong H, Xia L, Tao Y, Yin H. 2017. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria.Free Radic.Biol.Med 111:316–27 [DOI] [PubMed] [Google Scholar]
- 111.Noel A, Hossain E, Perveen Z, Zaman H, Penn AL 2020. Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air-liquid interface. Respir. Res 21:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muthumalage T, Lucas JH, Wang Q, Lamb T, McGraw MD, Rahman I. 2020. Pulmonary toxicity and inflammatory response of e-cigarette vape cartridges containing medium-chain triglycerides oil and vitamin E acetate: implications in the pathogenesis of EVALI. Toxics 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, et al. 2016. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral. Oncol 52:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Espinoza-Derout J, Hasan KM, Shao XM, Jordan MC, Sims C, et al. 2019. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am. J. Physiol. Heart Circ. Physiol 317:H445–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Canistro D, Vivarelli F, Cirillo S, Marquillas CB, Buschini A, et al. 2017. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep 7:2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, et al. 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLOS ONE 10:e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, et al. 2018. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater 344:549–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, et al. 2015. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLOS ONE 10:e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, Jaspers I. 2019. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell. Mol. Physiol 316:L470–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tommasi S,Caliri AW,Caceres A,Moreno DE,Li M,et al.2019.Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int. J. Mol. Sci 20:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park HR, Vallarino J, O’Sullivan M, Wirth C, Panganiban RA, et al.2021.Electronic cigarette smoke reduces ribosomal protein gene expression to impair protein synthesis in primary human airway epithelial cells. Sci. Rep 11:17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen Y, Wolkowicz MJ, Kotova T, Fan L, Timko MP. 2016. Transcriptome sequencing reveals e-cigarette vapor and mainstream-smoke from tobacco cigarettes activate different gene expression profiles in human bronchial epithelial cells. Sci. Rep 6:23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jing JC, Chen JJ, Chou L, Wong BJF, Chen Z. 2017. Visualization and detection of ciliary beating pattern and frequency in the upper airway using phase resolved Doppler optical coherence tomography. Sci. Rep 7:8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siegel SJ, Weiser JN. 2015. Mechanisms of bacterial colonization of the respiratory tract. Annu. Rev. Microbiol 69:425–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carson JL, Zhou L, Brighton L, Mills KH, Zhou H, et al.2017.Temporal structure/function variation in cultured differentiated human nasal epithelium associated with acute single exposure to tobacco smoke or E-cigarette vapor. Inhal. Toxicol 29:137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chung S, Baumlin N, Dennis JS, Moore R, Salathe SF, et al. 2019. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am. J. Respir. Crit. Care Med 200:1134–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, et al. 2020. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am. J. Physiol. Cell Physiol 318:C205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, et al. 2017. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell. Mol. Physiol 313:L278–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Q, Jiang D, Minor M, Chu HW. 2014. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLOS ONE 9:e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, et al. 2017. Electronic cigarette use and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med 195:1043–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bagale K, Paudel S, Cagle H, Sigel E, Kulkarni R. 2020. Electronic cigarette (e-cigarette) vapor exposure alters the Streptococcus pneumoniae transcriptome in a nicotine-dependent manner without affecting pneumococcal virulence. Appl. Environ. Microbiol 86:e02125–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serpa GL, Renton ND, Lee N, Crane MJ, Jamieson AM. 2020. Electronic nicotine delivery system aerosol-induced cell death and dysfunction in macrophages and lung epithelial cells. Am. J. Respir. Cell Mol. Biol 63:306–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woodall M, Jacob J, Kalsi KK, Schroeder V, Davis E, et al. 2020. E-cigarette constituents propyleneglycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol 319:L957–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pezzulo AA, Gutierrez J, Duschner KS, McConnell KS, Taft PJ, et al. 2011. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLOS ONE 6:e16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, et al. 2019.Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig 129:4290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]