Abstract
BACKGROUND:
Preservation of brain health has emerged as a leading public health priority for the aging world population. Advances in neurovascular biology have revealed an intricate relationship among brain cells, meninges, and the hematic and lymphatic vasculature (the neurovasculome) that is highly relevant to the maintenance of cognitive function. In this scientific statement, a multidisciplinary team of experts examines these advances, assesses their relevance to brain health and disease, identifies knowledge gaps, and provides future directions.
METHODS:
Authors with relevant expertise were selected in accordance with the American Heart Association conflict-of-interest management policy. They were assigned topics pertaining to their areas of expertise, reviewed the literature, and summarized the available data.
RESULTS:
The neurovasculome, composed of extracranial, intracranial, and meningeal vessels, as well as lymphatics and associated cells, subserves critical homeostatic functions vital for brain health. These include delivering O2 and nutrients through blood flow and regulating immune trafficking, as well as clearing pathogenic proteins through perivascular spaces and dural lymphatics. Single-cell omics technologies have unveiled an unprecedented molecular heterogeneity in the cellular components of the neurovasculome and have identified novel reciprocal interactions with brain cells. The evidence suggests a previously unappreciated diversity of the pathogenic mechanisms by which disruption of the neurovasculome contributes to cognitive dysfunction in neurovascular and neurodegenerative diseases, providing new opportunities for the prevention, recognition, and treatment of these conditions.
CONCLUSIONS:
These advances shed new light on the symbiotic relationship between the brain and its vessels and promise to provide new diagnostic and therapeutic approaches for brain disorders associated with cognitive dysfunction.
Keywords: AHA Scientific Statements; aging; Alzheimer disease; cerebrovascular circulation; cognition; dementia, vascular; leukoaraiosis
Dementia is one of the world’s largest public health problems. In the United States, >500 000 individuals are diagnosed with dementia each year.1 More than 1.5% of the US population is currently living with dementia.1 The costs for providing care for individuals with dementia are enormous, exceeding those for cardiovascular disease or cancer.2
The predominant research focus in Alzheimer disease (AD), the most common cause of clinically diagnosed dementia in the elderly, has been on the pathobiology of amyloid plaques and neurofibrillary tangles, with relatively little attention paid to the neurovascular components of the disease. It is notable that disturbances in blood flow delivery3 and blood-brain barrier (BBB) permeability4 appear to occur before the onset of symptoms and before evidence of neurodegeneration. Furthermore, recent autopsy studies have revealed that brain infarcts are found in almost half of patients diagnosed with AD during life, and many brains also have evidence of arterial lipid deposits (atherosclerosis) or arteriolar wall thickening (arteriolosclerosis).5 At the same time, improved cardiovascular health has been shown to correlate with improved brain health and reduced incidence of dementia later in life.6 These converging observations highlight the critical relationship between the neurovasculature and brain health.7 However, the mechanisms and pathogenic relevance of this apparent linkage remain elusive. Therefore, the question remains: Is cerebrovascular dysfunction an epiphenomenon of the neurodegenerative process or a pathogenic contributor?
The brain has high energy requirements that are fulfilled by oxygen and glucose delivered to a large number of functionally distinct brain regions by an intricate network of blood vessels stretching >400 miles that are densely packed into the brain substance.8 Considering that the brain’s requirements for oxygen and glucose vary in space and time depending on the level of brain activity, delivering the appropriate amount of blood to each region poses a staggering logistical challenge. Accordingly, the structural, functional, and molecular diversity of the cerebral vasculature is astounding, and the interaction between brain cells and the local vasculature is remarkably complex compared with that of other organs.8,9
Historically, the brain and its vasculature have been considered separate domains, with neuroscientists concentrating on brain cells and vascular biologists on blood vessels. As a result, a rigid distinction was placed between cerebrovascular diseases (eg, stroke) and neurodegenerative diseases (eg, AD), considered mutually exclusive conditions. Over the past decade, a wealth of basic science observations have shed light on the close structural and functional interaction between brain cells and vascular cells, culminating in the establishment of the neurovascular unit concept.10 At the same time, epidemiological and clinical-pathological studies have revealed an unanticipated overlap between vascular and neurodegenerative pathologies, especially those underlying dementia, including AD.11 In this scientific statement, we explore the emerging data on the cellular, molecular, and functional bases for these interactions in health and disease, and using this evidence, we will seek to identify unresolved issues and outstanding questions. Last, we highlight potential ramifications for preventing and treating the rapidly expanding burden of dementia in the United States and the world.
METHODS
This statement was commissioned by the American Heart Association’s Stroke Council and is in accordance with the American Heart Association’s conflict-of-interest and ethics policies.
Authors with expertise in vascular biology, cellular and molecular biology, neurology, neurophysiology, neuroimmunology, cognitive science, and neuropathology pertaining to the structure, function, and neurovascular pathobiology were selected to contribute to this statement. All current members of the American Heart Association/American Stroke Association were eligible for selection. Topics for the statement were identified by the chair and vice chair and revised with input from the writing group. Subgroups of experts for each of the topics were established and charged with writing the section of the statement pertaining to their expertise. Each subgroup performed a search of the relevant English literature considered for inclusion in this statement using an up-to-date search strategy of reference databases and appropriate search terms. Conflicts were resolved by group consensus. The search also included a review of bibliographies and manual searches of key articles. Drafts of each section were written and sent to the chair and vice chair of the writing group for editing and elimination of redundancy. The edited sections were returned to the group members for clarification and revisions and sent back to the chair and vice chair. The sections were then assembled in a single document that was sent back to the members for discussion and comments. On the basis of these discussions and consensus, sections were then edited accordingly by the primary author and returned to the chair and vice chair for further editing. The document was circulated among all members of the writing group, and once consensus was reached, the final document was submitted for independent peer review. After peer review, the final document was approved for publication by the relevant American Heart Association councils and the Science Advisory and Coordinating Committee.
NEUROVASCULOME AND NEUROVASCULAR UNIT
Throughout this statement, the term neurovasculome refers to the entire extracranial (aortic arch to base of the skull) and intracranial vasculature and associated cells pertaining to skull, brain, and meninges (arteries, capillaries, veins, and lymphatics). The inclusion of extracranial arteries is justified by their contribution to cerebrovascular regulation9 and of extracranial veins for their role in cerebrospinal fluid (CSF) homeostasis and clearance.12 Neurovascular unit refers to the specific neurovascular associations occurring at each segment of the neurovasculome. Therefore, the neurovasculome is constituted by multiple neurovascular units that vary by cell type composition, depending on the specific vascular segment.9
THE NEUROVASCULOME: EMERGING MOLECULAR AND CELLULAR DIVERSITY
Single-cell and cell type–restricted molecular approaches have been increasingly applied to study the neurovasculome. Although low sensitivity for low-abundance transcripts (eg, ion channels and G-coupled receptors) is a limitation, these techniques have proven crucial for revealing the identity and heterogeneity of vascular cells in the brain. A list of the major relevant databases is provided in Supplemental Table 1.
Single-Cell Methods
Single-cell RNA sequencing (RNA-seq) methods allow the transcriptome of thousands of cells to be interrogated simultaneously, making it possible to define the brain vessels at unprecedented molecular resolution.13-15 Single-cell RNA-seq combined with bioinformatics is a robust approach to define transcriptional cell signatures, uncovering previously uncharacterized cellular diversity that, together with in situ techniques, allows the developmental, functional, and topographic classification of vascular and vascular-associated cells. In addition, it is now possible to study normal and abnormal brain vessels in postmortem human brains using single-nucleus RNA-seq,16-18 and spatially resolved transcriptomics allows merging molecular data with tissue imaging approaches.19 These techniques have been essential in classifying subpopulations of vascular and vascular-associated cells along the neurovascular tree13-15 and are starting to reveal regional differences in vascular transcriptomics, for example, between the neocortex and hippocampus.9
Heterogeneity and Spatial Organization of Vascular and Vascular-Associated Cells
In the mouse brain, endothelial cells (ECs) exhibit significant transcriptional heterogeneity yet form a seamless continuum of shifting transcriptional states along the arteriole-capillary-venous network.13 An initial single-cell transcriptomic study in mouse brain identified 3 EC clusters corresponding to arterial, capillary, and venous zones, and subsequent work expanded this to 7 endothelial clusters, further dividing arterial ECs into 3 subgroups and adding transitional zones between arterioles-capillaries and capillaries-venules.20 The arterial ECs exhibit enriched expression of genes for cellular plasticity (Mgp, Fbln5, Eln, Igfbp4, Clu, Sema3g) and signal transduction (Bmx, Efnb2, Vegfc, Hey1), whereas capillary ECs express genes for transport, metabolism, and O2 response (Mfsd2a, Tfrc, Slc16a1, Fmo2). Venous ECs exhibit heightened expression of genes for inflammatory signaling (Cfh, Il1r1, Vwf, Vcam1). Emerging evidence demonstrates regional heterogeneity also in capillary ECs. For example, a Plvap-positive capillary EC cluster has been identified in the circumventricular organs (CVOs) where capillaries are more permeable than in other brain regions.21
In contrast to the continuum of transcriptional states with ECs, murine mural cell types segregate into 2 distinct subclasses.13 One corresponds to smooth muscle cells (SMCs) on the arterial side, which are further subdivided into arterial/large arteriole and small arteriole groups. Reflecting their dynamic roles in blood flow regulation, arterial SMCs/arteriolar SMCs express genes encoding contractility and cytoskeletal reorganization (Acta2, Tagln, Cnn1, Pdlim3). The arteriolar SMC class may be synonymous with ensheathing pericytes22,23 or precapillary SMCs24 found in the transition zone between arteriole and capillary branches.25 A second class corresponds to a broad continuum of transcriptional states between pericytes and venular SMCs, which express little to no Acta2 but are more enriched in the expression of genes for signaling (Pdgfrb, Vtn), metabolic sensing and transport (Abcc9, Kcnj8, Atp13a5, Art3, Ggt1, Grm3),26 and cell growth and survival (Ptn).27 The identification of mural class-specific markers has enabled the creation of mice for improved genetic targeting of pericyte/venous SMC subclasses.28
Several additional vascular-associated cell types reside within the perivascular space, delimited by the vascular basement membrane in which the mural cells are embedded and the glial basement membrane next to the astrocytic endfeet. On larger arterioles, the perivascular space is more evident, but as the diameter of vessels decreases, the glial and pial basement membrane layers fuse together, and the perivascular space disappears. Cell types within the perivascular space include perivascular fibroblasts and perivascular macrophages (PVMs). Seven clusters of fibroblast-like cells have been identified from whole-brain single-cell transcriptomic studies,20 with 2 classes representing perivascular fibroblasts identified by studies of vascular-enriched preparations.13 Like PVMs, perivascular fibroblasts are found on arterioles and large venules but not capillaries, except in the leaky capillaries of the CVO in the brain.13,21,29 Like mural cells, perivascular fibroblasts express Pdgfrβ but are unique in their expression of genes for proteins of the extracellular matrix and basement membrane (Col1a1, Col1a2, Col6a1, Col6a2, Lama1, Efemp1) and modulators of its stability (Itih5, Spp1), suggesting a role in the regulation of vascular basement membrane. Brain macrophages reside at the border regions of the central nervous system (CNS), including the meninges, choroid plexus, CVO, and perivascular space. Similar to microglia, these border-associated macrophages, including PVMs, are derived from early erythromyeloid precursors emerging in the yolk sac30,31 but can be distinguished from microglia throughout embryogenesis and adulthood by their location, transcriptome, and phenotype.32 PVMs, which may originate from neonatal meningeal macrophages,33 are characterized by their expression of scavenger-receptors (Mrc1, Cd163, Cd36, Lyve1) not found in microglia and are distinguishable from choroid plexus macrophages by low expression of major histocompatibility complex class II genes (H2-Aa, CD74). However, bona fide parenchymal PVMs cannot be distinguished from pial macrophages by their transcriptome.34 Topographically, PVMs are located in perivascular spaces surrounding parenchymal venules and arterioles, as well as pial vessels (pial macrophages), but are not associated with brain capillaries. In contrast, some microglial cells are located near vessels but within brain parenchyma (juxtavascular microglia) and are associated primarily with capillaries. They lie abluminal to the vessel wall and come in direct contact with the capillary basement membrane without intervening astrocytic endfeet.35,36 These cells are prominent during embryonic development and early postnatal life when they exhibit motility along the vessel wall. In the mature mouse brain, juxtavascular microglia become predominantly stationary.35-37
Astrocytes have complex physiological roles in the CNS, including modulation of synapse formation, mediating immune cell interactions,38 metabolic support, and neurovascular functions.39 Transcriptomic studies have revealed a functional diversity among astrocytes across brain regions,40,41 cortical layers,42 or even the specific neural circuits in which they reside.43 Whereas astrocytes surrounding blood vessels exhibit different phenotypes and transcriptomes depending on regional vascular anatomy,21 diversity may also exist in distinct microvascular zones within brain regions, for example, in the CVO.21 In addition, because astrocytes exhibit highly complex morphologies and extensive levels of ramification, whole-cell transcriptomics might not capture the complexity of astrocytic cell organization, as shown by the existence of a specialized transcriptome and proteome in astrocytic endfeet.44,45
Oligodendrocyte precursor cells, identified by expression of Pdgfrα among other genes in transcriptomic studies, are critical regulators of vascular development46 and are occasionally found in close proximity to the vascular wall in the adult brain. Both oligodendrocyte precursor cells and mature oligodendrocytes (identified by Opalin) have been identified by vascular-enriched transcriptomic studies.17
Human Neurovasculome
Recently, the development of improved vascular-enrichment procedures made it possible to map the cerebral blood vessels in human postmortem brain and freshly isolated surgical specimen tissues with single-nucleus RNA-seq.16-18 These studies revealed that the human cerebrovasculature recapitulates many features of murine arteriole-capillary-venous organization of neurovascular cell types, including heterogeneity of EC types, and punctuation of mural cell types into 2 categories. However, these studies also revealed greater diversity in cell types in humans such as 2 pericyte types dedicated to transport function and matrix regulation and showed that different brain regions can have distinct cerebrovascular features. It is remarkable that good correspondence was observed between freshly isolated and postmortem tissue,16,18 but additional studies are needed to assess the influence of confounders typical of autopsy material such as postmortem intervals and other factors. The data also heed caution for the use and interpretation of murine studies because transcriptomic signatures of human vascular cell types differ in many ways from those in the murine brain.16,18 Validation of some of these data is a critical next step in the field. The growth of this technology may reveal a wealth of information on neurovascular changes associated with diseases such as AD and paths forward for improvement of preclinical models in translational research.
Targeting the Neurovasculome in Mice
The discovery of cell type– and subpopulation-specific marker genes has led to the development of mouse lines that selectively and conditionally express recombinases in various vascular cells (Figure 1), which can be used for gene deletion (Cre/lox system), activation of reporter genes for cellular tagging and fate mapping, functional transcriptomics (translatome),47 and cell type–restricted proteomics.48 The growth of single-cell approaches described previously will enable the development of mouse lines for improved targeting of cellular subclasses through identification of more selective gene markers or design of combinatorial approaches with multiple genes. For example, approaches that rely on Cre enzyme complementation have been developed to increase cell-type specificity and have been used to discern between microglia and PVMs.49 A list of Cre driver mice for targeting cerebral blood vessels and associated cells is provided in Supplemental Table 2. Gene targeting of brain ECs with viral gene transfer–based approaches is also feasible and is becoming increasingly popular.50-53
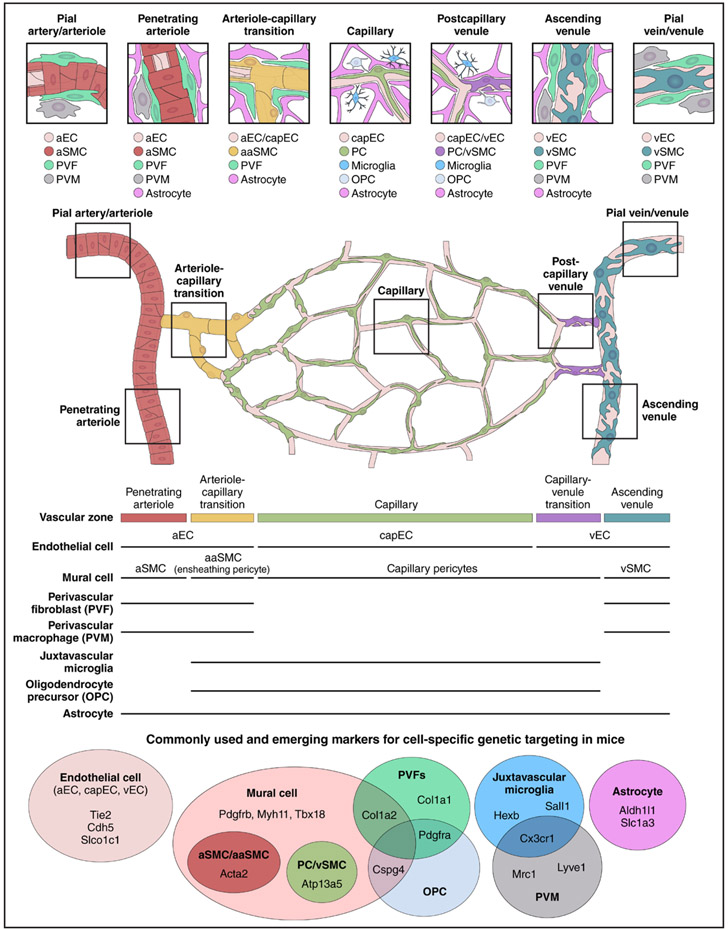
Figure 1. Vascular architecture and zonation from pial arteriole and penetrating branches diving into the brain parenchyma to ascending venule and pial venule emerging from the brain.
Schematic representation of the cerebral microvasculature and associated cells in neocortex. In the well-studied cerebral cortex, a 2-dimensional plexus of arterioles on the pial surface dives into the cortical parenchyma to form penetrating arterioles. Small offshoots emerge from penetrating arterioles to supply the capillary bed. The first few branches of these offshoots are distinct from both the penetrating arteriole and true capillaries and are called the arteriole-capillary transition. In mice, capillaries make up >90% of the total vascular length and form a dense 3-dimensional network throughout the parenchyma.23 Capillaries then coalesce into a larger capillary-venous transition vessel, which drains into ascending venules and eventually back into venules of the pial surface. Cell types identified by single-cell transcriptomics (Supplemental Table 1) remain to be precisely mapped onto the cerebrovascular architecture and complemented by proteomic studies. However, knowledge derived from in situ RNA hybridization, immunohistochemistry, and transgenic mouse lines begins to build the picture of where the cell types reside within the arteriole-capillary-venous circuit and their positions within the vascular wall. Available murine Cre drivers still target principal vascular and vascular-associated cell types, but mouse lines that target cell type subclusters are emerging (Supplemental Table 2). aaSMC indicates arteriolar smooth muscle cell; aEC, arterial endothelial cell; aSMC, arterial smooth muscle cell; capEC, capillary endothelial cell; PC, pericyte; vEC, venous endothelial cell; and vSMC, venous smooth muscle cell.
Brain on Chip
There is also considerable interest in developing “brain on chip” microfluidic models of the BBB and neurovascular unit that commonly include human induced pluripotent stem cells,54 with an emerging appreciation for the potential need for transcriptomic validation of vascular cell subtypes9 because a commonly used and validated EC differentiation protocol may yield cells more closely related to epithelial cells.55 Nevertheless, these methods have started to provide new insights into the cellular biology of the cerebral vasculature.56 For example, human induced pluripotent stem cell–derived ECs have helped establish a link between the AD risk gene PICALM (phosphatidylinositol-binding clathrin assembly) and enhanced amyloid-β (Aβ) clearance through BBB transcytosis.57 Bioengineered human microvessels unveiled a role of brain apolipoprotein E and circulating high-density lipoprotein in facilitating Aβ42 clearance from the brain.58 Furthermore, human induced pluripotent stem cell–derived 3-dimensional microvascular cultures (EC, mural cells, and astrocytes) revealed an involvement of calcineurin–nuclear factor of activated T cells signaling in pericytes in the mechanisms by which the ApoE4 allele may promote cerebral amyloid angiopathy.59 Methodological developments in this area have been rapid, and more complex models involving different neuronal types, astrocytes, and microglia, in addition to vascular cells (cerebral organoids, assembloids), are fast approaching.60
Beyond RNA-Seq
Epigenomic DNA signatures can be studied by determining chromatin accessibility (eg, ATAC sequencing61) or DNA methylation state62 at single-cell resolution, and long-read DNA sequencing of single-cell RNA libraries can be used to determine differential splicing at the single-cell level.63 Combining these techniques together with oligonucleotide-labeled antibodies directed at cell surface proteins (cellular indexing of transcriptomes and epitopes by sequencing64) yields epigenomic, transcriptomic, and targeted proteomic data from individual cells. Spatial information of the brain transcriptome can be acquired by multiplexed or sequential in situ hybridization or in situ sequencing that allows targeted detection of several hundred genes at single-cell resolution.65 Genome-wide spatial transcriptomics (slide-Seq, 10X Visium) can be studied at the level of tissue microdomains (<5 cells).66 Although single-cell epigenomic and transcriptomic methods are now widely available and are applied to study the cerebral blood vessels in health and disease across species,13,17,67 untargeted mass spectrometry techniques are under development to study the proteome,68 lipidome,69 and metabolome70 at single-cell resolution. To date, these methods have low through-put and sensitivity and have not been applied to study the neurovasculome.
THE NEUROVASCULOME: ROLES IN BRAIN HOMEOSTASIS AND COGNITIVE HEALTH
Cerebral blood vessels have an intimate and complex relationship with brain cells that reflects their critical role in maintaining the homeostasis of the brain microenvironment. The brain’s high metabolic demands, high susceptibility to energy deficit, and low regenerative capacity require a highly regulated supply of energy substrates, removal of waste, and careful regulation of the composition of the interstitial milieu. The neurovasculome achieves this goal through diverse mechanisms that engage both brain and vascular cells with segmental specificity along the cerebrovascular tree.9 Its preeminent goals are to provide the brain with the energy substrates needed to support the functions of neurons and glia, to control the composition of the brain’s microenvironment, and to enable immune surveillance. These critical functions are achieved by regulating cerebral blood flow (CBF), controlling the bidirectional molecular transfer across the cerebral endothelium through the BBB, removing the large amounts of byproducts generated by brain activity,10,71 and directing the traffic of immune cell into and out of the brain.72
Cerebral Blood Flow
CBF is regulated by a wide variety of factors. Here, we briefly discuss cerebrovascular autoregulation and neurovascular coupling. More exhaustive reviews of these topics have been published recently.71,73
Cerebrovascular autoregulation is a relatively slow adaptive process that aims to stabilize CBF in the face of changing arterial pressure, the main determinant of cerebral perfusion pressure.73 Within a physiologically relevant range, which varies from subject to subject and is estimated to be ±20 mm Hg mean arterial pressure,73 increases in perfusion pressure cause resistance arteries to constrict, whereas decreases in pressure cause vasodilation, aimed at counteracting the changes in CBF resulting from changes in perfusion pressure. However, the process has a time constant on the order of seconds, and rapid changes in blood pressure escape autoregulation.73 Cerebral autoregulation is mediated largely by the property of arterial SMCs to contract in response to increases in pressure (myogenic response). The mechanisms of the myogenic response are thought to involve pressure-sensitive ion channels and receptors on arterial SMCs, although neurogenic mechanisms mediated by the sympathetic cerebrovascular innervation, metabolic factors,74 and possibly astrocytes75 may modulate the response.
Neurovascular coupling is a dynamic process that provides CBF to different brain regions in response to changes in local neural activity. These mechanisms ensure a healthy supply of energy substrates and waste and heat clearance and deliver blood-borne modulatory signals such as insulin-like growth factor 1 to active regions.9,10,76,77 It is interesting, however, that in severe acute brain injuries, neural activity induced by waves of spreading depolarizations can lead to reductions in CBF (inverse neurovascular coupling), which can induce neuronal damage.78 Neurovascular coupling is mediated by coordinated signaling between different segments of the neurovasculome. Active neurons can trigger changes in CBF directly by releasing vasoactive molecules such as ions (eg, K+, H+), prostaglandins (prostaglandin E2), epoxyeicosatrienoic acids, and nitric oxide onto SMCs to dilate arterioles.71 Both excitatory and inhibitory neurons signal to cerebral vessels, but current evidence supports a more dominant role for inhibitory neurons, particularly nitric oxide synthase–positive interneurons.71,79,80 Neurovascular coupling may also occur indirectly through neuronal signaling to astrocytes, which are thought to regulate the contractility of ensheathing pericytes on the arteriole-capillary transitional segment.81 These signals can be bidirectional, with signals such as prostaglandin E2, epoxyeicosatrienoic acids, and moderate K+ efflux dilating and 20-hydroxyeicosatetraenoic acids and large K+ effluxes constricting vessels.81,82 ECs also sense neuronal or astrocytic signals to facilitate coupling of neural activity to CBF.83 Both ECs and mural cells are important conduits that propagate vasoactive signals along the vascular tree.84-86 Activation of inward-rectifier K+ and transient receptor potential ankyrin 1 channels has been implicated in endothelial hyperpolarization, which propagates retrogradely to relax contractile mural cells upstream.87,88 Coordinated dilatation of upstream and downstream vascular segments is essential for increasing flow efficiently in interconnected vascular networks.9
Other factors that have a profound and more global effect on CBF include arterial Pco2 and Po2. Hypercapnia increases and hypocapnia decreases CBF, and hypoxia increases CBF.73 In addition, numerous other factors can influence CBF globally, either directly or indirectly, for example, posture, circulating glucose, hormones and peptides, hematocrit, blood viscosity, and cold stress.73,89 These regulatory mechanisms, intrinsic and extrinsic to the brain, work in concert to ensure that the brain is adequately perfused.
The BBB
The BBB is formed by the EC layer that lines the walls of brain microvessels and serves as a physiological barrier between the blood and neural tissue.90 These specialized ECs are different from ECs in other circulatory districts because they (1) have specialized tight junctions that prohibit paracellular passage of water-soluble molecules; (2) have a low rate of vesicular transport (transcytosis) to prevent free transcellular trafficking; (3) express specific influx and efflux transporters, which allow molecule-specific exchange between the blood and the CNS; and (4) have low expression of leukocyte adhesion molecules and play a role in regulating the influx of immune cells. The restricted permeability of brain vasculature has been attributed historically to the specialized tight junctions,91 but recent work demonstrated that vesicular transport (transcytosis) is actively inhibited in cerebral ECs.92-95 Although CNS ECs constitute the BBB, BBB properties are not intrinsic to ECs but require active induction and maintenance from the CNS environment.96 For example, endothelial influx and efflux transporters contribute to maintenance of brain Aβ homeostasis.4,12 During development, Wnt from neural tissues is a key BBB induction cue and continues to play a role in BBB maintenance.97 Pericyte and astrocyte deficiency also results in BBB leakage,98-101 and pericyte-secreted vitronectin may regulate BBB through integrin receptors on the neighboring ECs.102 The specific astrocytic cues that regulate the BBB are poorly understood, but in vitro data suggest a role for tissue-type plasminogen activator.103
Clearance Mechanisms and Proteostasis
Besides meeting the high metabolic demand of the brain by delivering oxygen and nutrients to the tissue through blood flow, the neurovasculome also plays a major role in conveying proteins and metabolites from the brain parenchyma and to the lymphatic system. Various clearance mechanisms include transport across the BBB, clearance through dural lymphatic vessels, and drainage of soluble waste products along the perivascular spaces surrounding penetrating blood vessels.12 The relative contribution of each of these pathways in health and disease remains to be defined, but the role of perivascular clearance has received increasing attention as a potential key mediator in the pathophysiology of AD and related dementias. Although there are outstanding controversies concerning the exact anatomic pathways of perivascular clearance and how they function, the leading paradigms in the field suggest that soluble waste products travel along spaces surrounding individual penetrating vessels (Figure 2). According to the glymphatic model, CSF flows inward through arteriolar perivascular spaces, where it mixes with interstitial fluid and drains along perivenular spaces in the same direction as blood flow.104 Glymphatic clearance is most active during sleep, and impaired clearance of toxins, possibly caused by disordered sleep structure, has been implicated in neurodegenerative diseases.105 In contrast, the intramural periarterial drainage model suggests that solutes within the interstitial fluid drain along the basement membranes of the same arterioles in the opposite direction of blood flow.106 Whether perivascular pathways for fluid also exist along the smallest blood vessels in the brain, including the arteriole-capillary transition and at the level of the capillaries, remains unclear. However, these small vessels represent the majority of total vascular length and may have a role in the drainage of interstitial fluid. The microarchitecture of the perivascular spaces along different vascular zones, as well as the cell types that contribute to the formation of the spaces, remains poorly defined. More recently, an alternative model was proposed that does not rely on perivascular drainage routes. It suggests that there is mixing of CSF and interstitial fluid at the pial surface, which allows soluble waste products to be cleared without the need for unidirectional flow.107 Additional experiments designed to test assumptions underlying these proposed models are needed next steps to harmonizing conflicting experimental observations that may partially be attributable to heterogeneous experimental setups across laboratories.
Figure 2. Schematic representation of neurovascular clearance pathways from the brain.
Simplified depiction of the major clearance pathways operating through the neurovasculature. These include intramural periarterial drainage, mixing between cerebrospinal fluid (CSF) and interstitial fluid (ISF) and glymphatic flow. See text for details and references.
Another area of ongoing debate concerns the driving forces that move solutes along various proposed perivascular clearance routes. Several studies have suggested that arterial pulsations, generated by the heartbeat and breathing, can generate unidirectional fluid flow in perivascular spaces.108,109 In direct imaging, fluorescently labeled tracers injected into the cisterna magna were observed to travel into the brain along periarteriolar spaces in a pulsatile manner in phase with the cardiac cycle.109 Whether these rather subtle arteriolar wall displacements are strong enough to drive fluid out of the brain along perivenular or intramural pathways has been disputed.110
An alternative candidate to drive fluid efflux is vasomotion, which is observed during resting wakefulness as spontaneous large fluctuations in arterial diameter that occur at low frequency (0.1 Hz).111-113 Low-frequency hemodynamics have been found to increase during sleep, which may facilitate waste drainage during the night. Of note, preclinical studies investigating perivascular fluid flow often rely on invasive techniques (including cranial window surgeries and injection of tracers) and the use of anesthesia, which interfere with the brain’s natural homeostatic environment and cerebral hemodynamics.114 Thus, there is a great need for the development of noninvasive imaging techniques to track fluid flow dynamics, preferably in the awake resting or sleep state.
Immune Cell Trafficking
Immune cell trafficking into the brain is restricted by tight junctions in ECs, choroid plexus epithelium, arachnoid cells, and CVO tanycytes, each acting as a barrier.115-117 However, tight junctions are largely absent in blood vessels of the dura mater, choroid plexus, and CVOs, resulting in free bidirectional exchange of macromolecules and immune cells between the blood and the surrounding tissue. In particular, choroid plexus and dura mater are well perfused118 and have emerged as key sites of neuroimmune regulation.115-117
Several immune cells are found in the extravascular space of the dura mater, including dendritic cells, monocytes, neutrophils, natural killer cells, T cells, and B cells.34 Cell tracking studies have shown that intestinal immune cells contribute to the pool of dural immune cells.119 Besides extravasating from dural vessels, immune cells might also migrate directly from the skull bone marrow along penetrating diploic veins120 and bridging vessels121 that connect subarachnoid vessels to dural lacunar veins and the skull bone marrow.121-125 Under pathological conditions, they can penetrate the arachnoid layer and enter the brain parenchyma.121,122
Compared with other brain myeloid cells, stromal choroid plexus macrophages, also yolk sac derived,126 are short-lived (weeks in the adult mouse) and are at least partially replaced by blood monocyte–derived macrophages.30,127 Other cells found in the choroid plexus stroma are lymphocytes, natural killer cells, monocytes, and dendritic cells.34 Peripheral immune cells are found in CVOs under homeostatic conditions.128
The normal CSF contains mainly memory T cells.129 Under homeostatic conditions, CSF T cells are transitory129 and egress from the CSF through dural lymphatic vessels to reach deep cervical lymph nodes and through transcribrosal lymphatics to reach the superficial cervical lymph nodes.130 Extravasation of effector T cells from pial vessels and choroid plexus and transarachnoid migration from the dural extracellular space have been observed in diverse pathologies.116 Resident brain immune cells such as microglia and macrophages also interact with infiltrating intlammatory cells in the setting of brain diseases and can lead to pathology by producing cytokines, chemokines, and other proinflammatory and toxic molecules.32
These observations collectively highlight the diverse nature and mechanistic heterogeneity through which the neurovasculome maintains the homeostasis of the working brain.
HOW DOES THE NEUROVASCULOME INFLUENCE COGNITIVE FUNCTION
Alterations of structure and function of the neurovasculome have a profound effect on brain health, and a growing body of evidence suggests a link between neurovascular dysfunction and cognitive impairment in humans.
Flow-Dependent Mechanisms Causing Ischemia and Brain Injury
Hypoperfusion is a key feature of vascular cognitive impairment and dementia (VCID) and leads to ischemic injury to the white matter (WM) and gray matter. WM lesions (WMLs) are commonly seen in aging and, when the burden becomes high, are a substrate for VCID. Data from epidemiological and postmortem pathological studies show that vasculopathy and reduced WM perfusion are significant features of WMLs,131 which tend to accumulate in the deep watershed territories of the WM and are a major contributor to increased risk for dementia.131 There are also data to suggest flow-independent mechanisms that stem from the injured cerebral endothelium and result in inflammation and BBB leakage within and adjacent to WMLs.132 Epidemiological data show a strong association between WMLs and vascular risk factors such as hypertension and diabetes but also vascular diseases such as aortic stiffness and atrial fibrillation without stroke.133 Postmortem studies demonstrate extensive pathological changes across the WM microvascular bed.134 These include wall thickening by hyalinosis and fibroid necrosis of the arterioles, SMC loss, rarefaction of the capillaries, and thickening of the venule walls by collagen deposition (venous collagenosis), which are associated with pallor of adjacent perivascular myelin and astrogliosis.135,136 Perivascular accumulation of axonal structural proteins, neurofilament heavy in particular, has also been reported.137
Recent imaging and postmortem pathological studies highlight changes in astrocytes and pericytes that are shared across various neurological conditions associated with WMLs and cognitive dysfunction. Fragmentation of the distal processes and swollen cell bodies of astrocytes (clasmatodendrosis)138 may be observed in neuropathological specimens, but their contribution to the development of cognitive dysfunction in disorders associated with WMLs such as stroke and AD remains unclear. Earlier changes such as swollen cell bodies, cytoplasmic vacuolation, and amoeboid shape139 could indicate that astrocytes are dysfunctional and no longer able to maintain the homeostatic environment of the surrounding WM. In addition, experimental studies have suggested a role of reactive astrocytes in neuroinflammation and Aβ production in AD models.140 Similarly, capillary pericytes are decreased in the deep WM, but not the overlying cortex, in age-related dementias, including poststroke dementia.141 The emerging picture suggests disrupted gliovascular and neurovascular interactions with compensatory microvascular remodeling as a substrate for a disconnection syndrome that manifests as cognitive impairment and dementia in individuals with WMLs. The interplay among WM injury, network connectivity, and cognition is supported by data from multimodal brain imaging studies that include structural connectivity, resting-state functional magnetic resonance imaging (MRI), and WML segmentation.142 Studies that use transcranial Doppler ultrasound or arterial spin labeling–MRI to measure CBF and neurovascular coupling in individuals with WML provide additional support for neurovascular interactions and network connectivity as a substrate for preserving healthy brain aging.143
Hypoperfusion in the gray matter may cause ischemic injury in the form of microinfarcts, which easily escape detection on conventional MRI because of their small size (on average, ≈200 μm on postmortem examination).144 They are twice as frequent in individuals with dementia as in those who die without dementia.145 Clinical and animal studies suggest that small-vessel disease (including venular disease), hypoperfusion, and embolism can cause them.146,147 Although the volume of each individual microinfarct is miniscule, the fact that they can number in the thousands in an individual brain148 and have effects on connectivity and neurovascular function beyond their borders149 suggests that they can have direct adverse effects on brain networks subserving cognition.
Flow-Dependent Mechanisms Not Related to Ischemia
Recent experimental evidence has raised the possibility that neurovascular dysfunction has the potential to also alter cognitive function by disrupting the homeostasis of neuronal proteins or failing to deliver neurotrophic factors needed to maintain a healthy cognition. For example, a salt-rich diet induces cognitive impairment in mice in the absence of elevated blood pressure.150 Cognitive dysfunction depends on the gut-brain axis through which gut Th17 lymphocytes elevate circulating levels of interleukin-17, leading to a deficit in cerebral endothelial nitric oxide, endothelial dysfunction, and reduced resting CBF.150 However, these hemodynamic alterations do not play a role in cognitive dysfunction, which is instead mediated by a deficit in endothelial nitric oxide, which, in turn, leads to hyperphosphorylation and aggregation of tau, a protein involved in AD.150 Other studies have suggested that the increase in CBF induced by brain activity (functional hyperemia) does more than deliver oxygen and glucose to the brain. For example, hippocampal neurogenesis, which is essential for cognitive health, relies on functional hyperemia to deliver angiocrine factors to the neurogenic niche to support neuroblast survival.77,151 Failure of delivery of endothelial or circulating neurotrophic factors through blood flow has also been implicated in cognitive deficits in a model of autism (6p11.2 deletion syndrome).152 The relevance of these mechanisms to cognitive impairment in humans awaits further study.
Failure of Clearance Mechanisms
Impaired perivascular clearance has been implicated in the pathophysiology of various neurological disorders. Reduced tracer movement was observed in experimental models of cerebral amyloid angiopathy,112,153 microinfarcts,154 apolipoprotein E isoforms trafficking,155 and hypertension.109 What these models have in common is impaired vessel function. In patients with cerebral small-vessel disease, loss of vessel function may contribute to the accumulation of proteins in the walls of blood vessels such as Aβ in cerebral amyloid angiopathy.156 This could lead into a feed-forward cycle of SMC loss, impaired clearance, and continued protein buildup, eventually resulting in vessel wall breakdown and ischemia or hemorrhage. A deeper understanding of perivascular waste clearance systems may elucidate their involvement in cognitive dysfunction and provide novel targets for intervention.
Neuroimmune Mechanisms
Innate and adaptive immunity can have profound effects on cognitive function. Neutrophils, innate immune cells, have been shown to contribute to capillary stalling in a mouse model of AD, and blocking neutrophil-endothelium interactions improved cognitive function.157-159 In humans, hyperactivated neutrophils correlate with AD progression, whereas a higher neutrophil-to-lymphocyte ratio correlates with any dementia.160-162 PVMs are increased in aged and hypertensive rats160 and contribute to neurovascular and cognitive dysfunction in models of hypertension or amyloid accumulation through oxidative stress.52,163 Microglia are involved in the deleterious effects of amyloid and tau pathology and neuroinflammation in mouse models.164 Increased reactive oxygen species and proinflammatory cytokines, coupled with impaired migratory capacity and reduced Aβ phagocytosis, were found in blood monocytes of individuals with mild cognitive impairment and AD.165 As for adaptive immunity, loss of naive T cells during aging decreases T-cell diversity and favors the expansion of memory T cells, including memory effector T cells. The number of adaptive immune cells in brain increases with age. For example, age-related increases in vascular-associated T cells were observed within the WM of monkeys.166 Clonally expanded interferon-γ–producing CD8+ T cells have been described in the subventricular zone of aged mice.167 Similarly, a higher proportion of activated CD8+ T cells are found in the CSF of patients with AD compared with healthy age-matched control subjects, which correlated with AD-related cognitive decline.168 An increased lymphocyte-to-monocyte ratio was positively correlated with dementia incidence.169 In conclusion, although few animal studies have suggested a clear cause-effect relationship between increased immune cell trafficking to the brain and cognitive decline, correlative studies support an association of activated innate and adaptive immune cells with cognitive dysfunction. Furthermore, the recent discovery that CNS antigens accumulate in dural sinuses and are presented to circulating lymphocytes, a process disrupted during neuroinflammatory states and aging, suggests the possibility that alteration of the cross-talk between CNS and systemic immunity may have a role in brain disease.170
THE NEUROVASCULOME IN STROKE, AD, AND RELATED DEMENTIAS
Abundant epidemiological evidence implicates neurovascular dysfunction as a risk factor for age-related cognitive decline, vascular cognitive impairment, AD, and related dementias. Of the 12 modifiable risk factors identified by the Lancet Commission with the strongest evidence for potential prevention of dementia, 9 are also risk factors for cardiovascular disease.171 Hypertension, diabetes, smoking, obesity, and hypercholesterolemia alter neurovascular regulation172,173 and increase the risk for dementia.11 It is likely that neurovascular dysfunction mediates at least some of the associations of these risk factors with dementia. For many of these risk factors, the elevation in risk can be detected in midlife,174 suggesting that early neurovascular dysfunction begins several decades before clinical symptoms.
Stroke
Symptomatic stroke is a major cause of VCID.175 Cerebrovascular disease can, in addition to stroke, cause covert vascular damage to the brain, detectable on neuroimaging as lacunar infarcts, WMLs, cortical microinfarcts, and cerebral microbleeds. In patients with cerebral small-vessel disease, damage to the cerebral WM may begin with increased BBB permeability, followed by suppression of cerebrovascular responses to CO2 and then ischemia,176 although other studies suggest that ischemia in watershed areas between cortical and subcortical arterial territories is the initial pathogenic event.172,173
Cognitive Decline
Stroke and covert cerebrovascular damage are not the only ways by which neurovascular dysfunction causes cognitive decline. Evidence from human studies suggests that neurovascular dysfunction occurs early in the AD pathophysiological process, when it may possibly serve as an initiator or accelerator of AD pathology. For example, a study in the general population found that higher total vascular risk factor burden was associated with greater burden of Aβ 20 years later.177 A drop in cerebral perfusion is one of the earliest detectable alterations in the brains of individuals with AD.3 Many, but not all, studies suggest that cerebrovascular reactivity to CO2 is reduced in AD.178 Decreased clearance of Aβ is hypothesized to be the initial step in vascular Aβ formation; human studies suggest that sporadic AD is the result of failure to adequately clear Aβ peptide from the extracellular space into the CSF or the blood.179 This suggests a potential role for neurovasculome-related clearance mechanisms in the initiation of AD, although the exact causes of this failure to clear Aβ and tau remain elusive. Studies in animal models strongly implicate failure of perivascular, glymphatic, and dural lymphatic pathways in the clearance impairment.105,112,180,181 A growing ability to use biomarkers of neurovascular and clearance functions in living human subjects, discussed in more detail in the subsequent section, may further clarify which mechanisms are at play in the pathogenesis of AD and related dementias.
NEUROVASCULAR BIOMARKERS OF COGNITIVE DYSFUNCTION
Emerging technologies are increasingly offering new windows into human neurovasculome function, either directly in the brain—often through neuroimaging—or by identifying peripheral surrogates of these processes and linking them with cognitive decline. Some of these biomarkers are summarized in the Table, focusing on those associated with specific neurovasculome cell types and neurovasculome function. These biomarkers are in various stages of validation, but all of them have some evidence of proof of concept (ie, that they measure a change related to a process) and proof of principle (ie, that they have been associated with cognitive decline or with cognitive disorders such as AD).
Table.
Selected In Vivo Human Neuroimaging and Fluid Biomarkers of Neurovasculome Dysfunction and Cognitive Decline
| Pathophysiological process | Biomarker | |
|---|---|---|
| Imaging | Biofluid | |
| Astrocyte dysfunction | … | Glial fibrillary acidic protein182 |
| Pericyte stress | … | Platelet-derived growth factor β183 |
| Functional connectivity | Resting-state fMRI184 Task-related fMRI185 |
… |
| Low CBF | ASL MRI186 15Oxygen PET187 |
… |
| Altered cerebrovascular reactivity to CO2 | Evoked TCD flow velocity178 Evoked MRI ASL or fMRI BOLD responses188 |
… |
| Vascular stiffness and pulsatility | Ankle-brachial index189 Pulse-wave velocity measurements190 Phase-contrast MRI191 |
… |
| BBB permeability | MR DCE and DSC permeability192 | CSF:serum albumin ratio193 Matrix metalloproteinases194 |
| CSF bulk flow and clearance | Ultralong echo time diffusion MRI195 Phase-contrast MRI196 BOLD MRI197 |
… |
| Inflammation | TSPO PET (microglial activation)197 | Biochemical markers, including C-reactive protein and interleukins198 |
| Lipid dysmetabolism | … | Blood cholesterol199,200 Blood lipidomics199 |
| Endothelial dysfunction | Brachial flow-mediated dilation201 | Circulating microparticles202 Various blood markers (including fibrinogen, PAI, ICAM-1, ADMA, and others)203 |
Emerging biomarkers of vascular-mediated processes, measured in the brain or in the periphery, that have been associated with cognitive decline, dementia, clinically diagnosed Alzheimer disease, or mixed dementia.
ADMA indicates asymmetric dimethylarginine; ASL, arterial spin label; BBB, blood-brain barrier; BOLD, blood oxygen level dependent; CBF, cerebral blood flow; CSF, cerebrospinal fluid; DCE, dynamic contrast enhanced; DSC, dynamic susceptibility contrast; fMRI, functional magnetic resonance imaging; ICAM-1, intercellular adhesion molecule 1; MR, magnetic resonance; MRI, magnetic resonance imaging; PAI, plasminogen activator inhibitor type 1; PET, positron emission tomography; TCD, transcranial Doppler; and TSPO, translocator protein.
Many studies have examined cerebral and systemic vascular hemodynamic measures, typically derived from ultrasonography or MRI, in relation to cognition. These studies demonstrate that impairments in several vascular hemodynamic measures are associated with cognitive function, cerebral small-vessel disease, and CSF tau, Aβ, and inflammatory biomarkers.178,188,204,205 Pilot studies using various MRI techniques to measure bulk movement of CSF, which may play a role in Aβ clearance, are beginning to emerge.195 Fluid biomarkers of lipid metabolism—including ceramides, cholesterol esters, low-density lipoprotein C, apolipoprotein B, and lipoprotein(a)—have been linked to cerebral WM changes and cognition.199,200 Putative blood markers of microvascular dysfunction and inflammation have been associated with cerebral small-vessel disease and cognitive decline.198,203 Soluble platelet-derived growth factor receptor β, released by pericytes when under stress, has been detected in CSF in patients with AD.183 Circulating microparticles released from ECs are an emerging vascular biomarker class and seem to be elevated in patients with AD with vascular risk factors compared with patients with AD without vascular risk factors, although they are not associated with cognitive decline.202 Despite the growing panoply of useful biomarkers, they still fail to explain all of the associations between vascular risk and cognitive decline,206 indicating that either the biomarkers are insufficient markers of the underlying processes or additional processes remain to be discovered.
CONCLUSIONS AND FUTURE DIRECTIONS
This overview provides a glimpse of the great strides that have been made in defining the critical role of the neurovasculome in brain health and its pathogenic involvement in brain diseases. Proteomic and transcriptomic studies have revealed a remarkable molecular diversity of vascular and vascular-associated cells. Functional roles of cerebral blood vessels beyond the delivery of O2 and critical nutrients have been identified such as clearance of toxic byproducts of brain activity, regulation of neuroimmune trafficking, and provision of factors essential for maintaining neuronal homeostasis. Failure of these mechanisms has been convincingly linked to brain diseases in animal models, especially those involving impaired cognition. These advances have also unveiled an unexpected level of complexity that raises additional questions, requiring new approaches and strategies to find answers. Some of these are listed here.
Single-Cell Studies
Cell type– and subtype-specific marker genes have generally been defined by studying healthy young mice, and more work is needed to confirm their validity across brain regions, age, sex, species, and disease states.17,67,207 Although cell type–specific Cre-driver mouse lines have become indispensable in neurovascular studies, to date, they lack the specificity that would allow targeting of subclusters of vascular cell types. Intersectional approaches using mice harboring >1 recombinase are still needed to obtain cellular specificity for some vascular cell types.27,49 Furthermore, new neurovascular omics studies are likely to provide new markers for specifically targeting vascular cell subpopulations. In particular, investigations of the proteome, epigenome, lipidome, and metabolome, in different brain regions, as well as gray matter versus WM, would be valuable. These approaches are essential to elucidate the functional role of vascular cell subclusters identified by single-cell–single-nuclei RNA-seq studies and will be facilitated by methods for spatially resolved omics.208,209
Neurovascular Coupling
A major question in the field is how vasoactive signals from neurons, astrocytes, ECs, pericytes, and perivascular cells are integrated to shape the highly orchestrated segmental hemodynamic adjustments underlying the coupling response. For example, astrocytes may not be necessary for the CBF response to occur, but they may trigger a larger increase in CBF when engaged.210 Perhaps they are engaged only during more sustained neural activity211 or to help restore resting diameter.212 Similarly, the mechanisms of the retrograde propagation of vasoactive signals from microvessels within the activated brain region to pial vessels upstream, needed for the full expression of functional hyperemia, remain to be elucidated. The relative contribution of each cell type to the propagation and expression of vasodilatation and to focusing the vasodilatation strictly to vessels within activated regions remains to be assessed. Regional differences in CBF regulation, already suggested by some studies, need to be explored at the cellular and molecular levels.213-215 New imaging technologies (3-photon microscopy, optical coherence tomography, functional ultrasound imaging) will provide the tools to explore neurovascular function in regions located deep in the brain that are not accessible noninvasively by conventional imaging methods.216-218 Answering these questions opens the way to elucidating which aspect of neurovascular coupling is altered in disease states and to defining its specific contributions to brain dysfunction at the regional level.
Clearance
The involvement of the cerebrovascular and lymphatic vessels in brain protein clearance implicates the neurovasculome in proteostasis. Because accumulation of protein aggregates is a critical pathogenic mechanism in a large number of brain diseases, there is a great need to gain a better understanding of the underlying pathways. What is the relative contribution of the different pathways thus implicated (Figure 2)? Are they preferentially active at specific neurovascular segments? What is the anatomy of the perivascular compartments across these segments? What are the dominant driving forces of clearance? How are they influenced by biological rhythms (sleep-awake cycle, estrogen cycle)? How do these pathways interact, and how do they ultimately drain into the lymphatic system? How do these pathways contribute to the overall fluid dynamics of the brain, from CSF and interstitial fluid production, bidirectional transfer at the BBB, and lymphatic drainage? The development of noninvasive imaging techniques of fluid flow dynamics in animal models, preferably in the awake state or sleep, is sorely needed. Such approaches will also facilitate translation of experimental insights to the human brain now that examination of fluid flow dynamics with noninvasive brain imaging is becoming a reality.219-221
Models of VCID and AD and Related Dementias
There is a pressing need to develop clinically relevant animal models to study the role of the neurovasculome in cognitive health. No single model to date recapitulates the comorbidities underlying cognitive impairment on vascular or neurodegenerative bases. Models are needed to reproduce the multiplicity of vascular lesions underlying VCID (microinfarcts, microhemorrhages, hypertensive lipohyalinosis, arteriolosclerosis, atherosclerosis, cerebral amyloid angiopathy, vascular inflammation). These need to be combined with models of neurodegenerative pathologies (Aβ, tau, TDP-43, α-synuclein) to reproduce mixed causes of cognitive impairment, as well as with aging, metabolic disorders (eg, obesity and diabetes), and lifestyle (eg, diet and physical activity) and genetic (ApoE4, TREM2) risk factors213,222 common to both neurodegenerative and vascular pathologies. Models to investigate WM pathology, a major contributor to cognitive impairment on vascular and neurodegenerative bases, are also needed, the development of which will be facilitated by emerging technologies to image the deep cortical WM in vivo213,217 and for gene targeting WM and associated cells.223,224
Prevention and Therapy
Identifying and treating vascular risk factors, which should preserve neurovasculome function, are widely acknowledged to be key components for public health strategies to optimize brain health.7 However, definitive evidence for dementia prevention from large randomized trials is not yet available. The FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) showed that an intervention package that included vascular risk reduction, healthy diet, and physical activity enhanced performance on neuropsychologic testing in older individuals but was not designed to have the sample size to detect reduction in dementia.225 The results of other multidomain prevention trials have been mixed,7 potentially indicating that the manner in which the interventions are applied and to what populations may be important. Hypertension is the strongest risk factor for stroke and is also a strong risk factor for dementia.226 In the MIND substudy (Memory and Cognition in Decreased Hypertension) of the SPRINT trial (Systolic Blood Pressure Intervention Trial), intensive systolic blood pressure lowering to a target of <120 mm Hg reduced the combined incidence of mild cognitive impairment and dementia.227 Lowering blood pressure also reduced the progression of WML228 and improved CBF,229 but whether these were the reasons for the reduced incidence of mild cognitive impairment and dementia has not been determined. Nevertheless, currently, the best clinical evidence for preventing dementia is treating elevated blood pressure. A more nuanced understanding of how risk factors for cognitive impairment affect the neurovasculome in humans would provide new leads that may unveil new preventive strategies and, possibly, therapeutic targets.
Need for Integrated Approaches
Neurovascular biology in relation to cognitive impairment and neurodegenerative diseases has been explored only in discrete studies and specialized contexts. Consequently, a comprehensive understanding of the neurovascular underpinnings of neurodegeneration has remained elusive. We are now at the point where technical advances to explore neurovascular function in vivo at the cellular and molecular levels, coupled with cutting edge imaging modalities to probe both cerebrovascular and brain function in humans, provide the bases for potentially transformative leaps in elucidating the role of vascular health in brain health at the mechanistic level. An ideal research approach would be sufficiently comprehensive to support exploration of the integrated brain system together, rather than maintaining the shortcomings of research to date, whereby specialists focus on their own individual elements: neurons, astrocytes, vascular cells, inflammation, and cell lineage tracing. The resulting discoveries could inspire human interventions in collaboration with vascular neurologists, neurointensivists, and neurosurgeons. The great opportunity is to look to neurovascular interactions from the developmental-cellular-molecular level to the network-system-organ level in health and disease, bringing together technologies, approaches, and expertise from different fields to gain an understanding unattainable within a single discipline.
Supplementary Material
Footnotes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STR.0000000000000431.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on October 18, 2022, and the American Heart Association Executive Committee on January 24, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or Meredith.Edelman@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Iadecola C, Smith EE, Anrather J, Gu C, Mishra A, Misra S, Perez-Pinzon MA, Shih AY, Sorond FA, van Veluw SJ, Wellington CL; on behalf of the American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; and Council on Lifestyle and Cardiometabolic Health. The neurovasculome: key roles in brain health and cognitive impairment: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2023;54:e•••e•••. doi: 10.1161/STR.0000000000000431
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Disclosures
| Writing group member |
Employment | Research grant | Other research support |
Speakers’ bureau/ honoraria |
Expert witness |
Ownership interest |
Consultant/ advisory board |
Other |
|---|---|---|---|---|---|---|---|---|
| Costantino Iadecola | Weill Cornell Medicine Feil Family Brain and Mind Research Institute | NIH† | None | None | None | None | Broadview Ventures† | None |
| Eric E. Smith | University of Calgary, Clinical Neurosciences Foothills Medical Centre (Canada) | None | None | None | None | None | None | None |
| Josef Anrather | Weill Cornell Medical College Feil Family Brain and Mind Research Institute | None | None | None | None | None | None | None |
| Chenghua Gu | Harvard Medical School, Howard Hughes Medical Institute | NIH†; Howard Hughes Medical Institute†; Fidelity Biosciences Research Institute†; Paul G. Allen Frontiers Group†; AHA† | None | None | None | None | None | None |
| Anusha Mishra | Oregon Health & Science University Neurology, Jungers Center | NIH†; John and Tami Marick Foundation* | None | None | None | None | None | None |
| Sanjay Misra | Mayo Clinic | NIH/NHLBI (R01 HL098967)† | None | None | None | Pavaj Vascular†; Inova Vascular* | Medtronic* | None |
| Miguel A. Perez-Pinzon | University of Miami Miller School of Medicine | NIH† | None | None | None | None | None | None |
| Andy Y. Shih | Seattle Children’s Research Institute Center for Developmental Biology and Regenerative Medicine | None | None | None | None | None | None | None |
| Farzaneh A. Sorond | Northwestern University, Feinberg School of Medicine | NIH (PI)† | None | None | None | None | None | None |
| Susanne J. van Veluw | Massachusetts General Hospital | NIH† | None | None | None | None | Biogen* | Therini Bio (sponsored research agreement)† |
| Cheryl L. Wellington | University of British Columbia (Canada) | Heart and Stroke Foundation of Canada (funded for lipoprotein studies in Alzheimer disease)†; BrightFocus Foundation (funded for lipoprotein studies in Alzheimer disease)† | None | None | None | None | None | None |
| Reviewer | Employment | Research grant | Other research support |
Speakers’ bureau/ honoraria |
Expert witness |
Ownership interest |
Consultant/ advisory board |
Other |
|---|---|---|---|---|---|---|---|---|
| Frank M. Faraci | University of Iowa Carver College of Medicine | None | None | None | None | None | None | None |
| Philip B. Gorelick | Northwestern University | None | None | None | None | None | None | None |
| Atticus H. Hainsworth | St. George’s University of London (United Kingdom) | None | None | None | None | None | None | None |
| Abbie C. Johnson | University of Vermont, Larner College of Medicine | NIH/NINDS (PI on 1R01 NS127284-01 titled “The Role of the Hippocampal Vasculature in Vascular Cognitive Impairment and Dementia” that investigates how aging and chronic hypertension effect hippocampal vascular function, hippocampal perfusion, neurovascular coupling, and cognition)*; AHA (PI on AHA Career Development Award 20CDA35310239 titled “Hippocampal Vascular Function in Chronic Hypertension and Post-Stroke Dementia” that investigates vascular reactivity and perfusion, neurovascular coupling, and blood-brain barrier function in the hippocampus after ischemic stroke as underlying mechanisms of poststroke dementia)* | None | None | None | None | None | None |
| Anne Joutel | INSERM (France) | None | None | None | None | None | None | None |
| Mohammed B. Khan | Augusta University | None | None | None | None | None | None | None |
REFERENCES
- 1.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC; Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016;7:11934. doi: 10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, Bennett DA. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85:114–124. doi: 10.1002/ana.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. ; on behalf of the American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. ; on behalf of the American Heart Association/American Stroke Association. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipolla MJ. The cerebral circulation. In: Integrated Systems Physiology: From Molecule to Function. 1st ed. Morgan & Claypool Life Sciences Publishers; 2009:1–59. [PubMed] [Google Scholar]
- 9.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–1209. doi: 10.1038/s41593-021-00904-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Munoz Sanchez JL, Anstey KJ, Brayne C, Dartigues JF, Engedal K, Kivipelto M, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 12.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, et al. Clearance systems in the brain: implications for Alzheimer’s disease. Nat Rev Neurol. 2016;12:248. doi: 10.1038/nrneurol.2016.36 [DOI] [PubMed] [Google Scholar]
- 13.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- 14.He L, Vanlandewijck M, Mäe MA, Andrae J, Ando K, Gaudio FD, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data. 2018;5:180160. doi: 10.1038/sdata.2018.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 16.Garcia FJ, Sun N, Lee H, Godlewski B, Mathys H, Galani K, Zhou B, Jiang X, Ng AP, Mantero J, et al. Single-cell dissection of the human brain vasculature. Nature. 2022;603:893–899. doi: 10.1038/s41586-022-04521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang AC, Vest RT, Kern F, Lee DP, Agam M, Maat CA, Losada PM, Chen MB, Schaum N, Khoury N, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022;603:885–892. doi: 10.1038/s41586-021-04369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler EA, Kim CN, Ross JM, Garcia JH, Gil E, Oh I, Chen LQ, Wu D, Catapano JS, Raygor K, et al. A single-cell atlas of the normal and malformed human brain vasculature. Science. 2022;375:eabi7377. doi: 10.1126/science.abi7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Schroeder A, Coleman K, Chen C, Auerbach BJ, Li M. Statistical and machine learning methods for spatially resolved transcriptomics with histology. Comput Struct Biotechnol J. 2021;19:3829–3841. doi: 10.1016/j.csbj.2021.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030.e16. doi: 10.1016/j.cell.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfau SJ, Langen UH, Fisher TM, Prakash I, Nagpurwala F, Lozoya RA, Lee W-CA, Wu Z, Gu C. Vascular and perivascular cell profiling reveals the molecular and cellular bases of blood-brain barrier heterogeneity. bioRxiv. Preprint posted online April 27, 2021. doi: 10.1101/2021.04.26.44146 [DOI] [Google Scholar]
- 22.Grant RI, Hartmann DA, Underly RG, Berthiaume A-A, Bhat NR, Shih AY Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab. 2017;39:411–425. doi: 10.1177/0271678X17732229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann DA, Berthiaume AA, Grant RI, Harrill SA, Koski T, Tieu T, McDowell KP, Faino AV, Kelly AL, Shih AY Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci. 2021;24:633–645. doi: 10.1038/s41593-020-00793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol. 2022;84:331–354. doi: 10.1146/annurev-physiol-061121-040127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariharan A, Weir N, Robertson C, He L, Betsholtz C, Longden TA. The ion channel and GPCR toolkit of brain capillary pericytes. Front Cell Neurosci. 2020;14:601324. doi: 10.3389/fncel.2020.601324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, Huuskonen MT, Sagare AP, Lazic D, Sweeney MD, Kong P, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089–1098. doi: 10.1038/s41593-019-0434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Ge T, Xia S, Wu H, Colt M, Xie X, Zhang B, Zeng J, Chen J, Zhu D. Atp13a5 marker reveals pericytes of the central nervous system in mice. bioRxiv. Preprint posted online July 10, 2021. doi: 10.1101/2021.07.09.451694 [DOI] [Google Scholar]
- 29.Bonney SK, Sullivan LT, Cherry TJ, Daneman R, Shih AY. Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. J Cereb Blood Flow Metab. 2022;42:966–978. doi: 10.1177/0271678X211068528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. doi: 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utz SG, See P, Mildenberger W, Thion MS, Silvin A, Lutz M, Ingelfinger F, Rayan NA, Lelios I, Buttgereit A, et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181:557–573. e18. doi: 10.1016/j.cell.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 32.Prinz M, Masuda T, Wheeler MA, Quintana FJ. Microglia and central nervous system-associated macrophagesL from origin to disease modulation. Annu Rev Immunol. 2021;39:251–277. doi: 10.1146/annurev-immunol-093019-110159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda T, Amann L, Monaco G, Sankowski R, Staszewski O, Krueger M, Del Gaudio F, He L, Paterson N, Nent E, et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature. 2022;604:740–748. doi: 10.1038/s41586-022-04596-2 [DOI] [PubMed] [Google Scholar]
- 34.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4 [DOI] [PubMed] [Google Scholar]
- 35.Mondo E, Becker SC, Kautzman AG, Schifferer M, Baer CE, Chen J, Huang EJ, Simons M, Schafer DP A developmental analysis of juxtavascular microglia dynamics and interactions with the vasculature. J Neurosci. 2020;40:6503–6521. doi: 10.1523/JNEUROSCI.3006-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisht K, Okojie KA, Sharma K, Lentferink DH, Sun Y-Y, Chen H-R, Uweru JO, Amancherla S, Calcuttawala Z, Campos-Salazar AB, et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat Commun. 2021;12:5289. doi: 10.1038/s41467-021-25590-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- 38.Han RT, Kim RD, Molofsky AV, Liddelow SA. Astrocyte-immune cell interactions in physiology and pathology. Immunity. 2021;54:211–224. doi: 10.1016/j.immuni.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 39.Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci. 2021;24:1475–487. doi: 10.1038/s41593-021-00905-6 [DOI] [PubMed] [Google Scholar]
- 40.Batiuk MY, Martirosyan A, Wahis J, de Vin F, Marneffe C, Kusserow C, Koeppen J, Viana JF, Oliveira JF, Voet T, et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun. 2020;11:1220. doi: 10.1038/s41467-019-14198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borggrewe M, Grit C, Vainchtein ID, Brouwer N, Wesseling EM, Laman JD, Eggen BJL, Kooistra SM, Boddeke E. Regionally diverse astrocyte subtypes and their heterogeneous response to EAE. Glia. 2021;69:1140–1154. doi: 10.1002/glia.23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, Ben Haim L, Young AMH, Batiuk MY, Prakash K, et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci. 2020;23:500–509. doi: 10.1038/s41593-020-0602-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron. 2017;95:531–549.e9. doi: 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stokum JA, Shim B, Huang W, Kane M, Smith JA, Gerzanich V, Simard JM. A large portion of the astrocyte proteome is dedicated to perivascular endfeet, including critical components of the electron transport chain. J Cereb Blood Flow Metab 2021;41:2546–2560. doi: 10.1177/0271678X211004182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulay A-C, Saubaméa B, Adam N, Chasseigneaux S, Mazaré N, Gilbert A, Bahin M, Bastianelli L, Blugeon C, Perrin S, et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov 2017;3:17005. doi: 10.1038/celldisc.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavali M, Ulloa-Navas MJ, Perez-Borreda P, Garcia-Verdugo JM, McQuillen PS, Huang EJ, Rowitch DH. Wnt-dependent oligodendroglial-endothelial interactions regulate white matter vascularization and attenuate injury. Neuron. 2020;108:1130–1145.e5. doi: 10.1016/j.neuron.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cleuren ACA, van der Ent MA, Jiang H, Hunker KL, Yee A, Siemieniak DR, Molema G, Aird WC, Ganesh SK, Ginsburg D. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc Natl Acad Sci U S A. 2019;116:23618–23624. doi: 10.1073/pnas.1912409116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krogager TP, Ernst RJ, Elliott TS, Calo L, Beránek V, Ciabatti E, Spillantini MG, Tripodi M, Hastings MH, Chin JW. Labeling and identifying cell-specific proteomes in the mouse brain. Nat Biotechnol. 2018;36:156–159. doi: 10.1038/nbt.4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J-S, Kolesnikov M, Peled-Hajaj S, Scheyltjens I, Xia Y, Trzebanski S, Haimon Z, Shemer A, Lubart A, Van Hove H, et al. A binary Cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity. 2021;54:176–190.e7. doi: 10.1016/j.immuni.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 50.Nikolakopoulou AM, Wang Y, Ma Q, Sagare AP, Montagne A, Huuskonen MT, Rege SV, Kisler K, Dai Z, Korbelin J, et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J Exp Med. 2021;218:e20202207. doi: 10.1084/jem.20202207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korbelin J, Dogbevia G, Michelfelder S, Ridder DA, Hunger A, Wenzel J, Seismann H, Lampe M, Bannach J, Pasparakis M, et al. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol Med. 2016;8:609–625. doi: 10.15252/emmm.201506078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, Poon C, Schaeffer S, Segarra SG, Korbelin J, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76:795–807. doi: 10.1161/HYPERTENSIONAHA.120.15581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krolak T, Chan KY, Kaplan L, Huang Q, Wu J, Zheng Q, Kozareva V, Beddow T, Tobey IG, Pacouret S, et al. A high-efficiency AAV for endothelial cell transduction throughout the central nervous system. Nat Cardiovasc Res. 2022;1:389–400. doi: 10.1038/s44161-022-00046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24:995–1005.e6. doi: 10.1016/j.stem.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Lu TM, Houghton S, Magdeldin T, Duran JGB, Minotti AP, Snead A, Sproul A, Nguyen DT, Xiang J, Fine HA, et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc Natl Acad Sci U S A. 2021;118:e2016950118. doi: 10.1073/pnas.2016950118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanchard JW, Victor MB, Tsai L-H. Dissecting the complexities of Alzheimer disease with in vitro models of the human brain. Nat Rev Neurol. 2022;18:25–39. doi: 10.1038/s41582-021-00578-6 [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, et al. Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A, Stukas S, Zhao W, Kulic I, Wellington CL. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. eLife. 2017;6:e29595. doi: 10.7554/eLife.29595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanchard JW, Bula M, Davila-Velderrain J, Akay LA, Zhu L, Frank A, Victor MB, Bonner JM, Mathys H, Lin YT, et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med. 2020;26:952–963. doi: 10.1038/s41591-020-0886-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H, Zhou J, Tian W, Luo C, Bartlett A, Aldridge A, Lucero J, Osteen JK, Nery JR, Chen H, et al. DNA methylation atlas of the mouse brain at single-cell resolution. Nature. 2021;598:120–128. doi: 10.1038/s41586-020-03182-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joglekar A, Prjibelski A, Mahfouz A, Collier P, Lin S, Schlusche AK, Marrocco J, Williams SR, Haase B, Hayes A, et al. A spatially resolved brain region- and cell type-specific isoform atlas of the postnatal mouse brain. Nat Commun. 2021;12:463–416. doi: 10.1038/s41467-020-20343-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W-T, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell. 2020;182:976–991.e19. doi: 10.1016/j.cell.2020.06.038 [DOI] [PubMed] [Google Scholar]
- 66.Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363:1463–1467. doi: 10.1126/science.aaw1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao L, Li Z, Vong JSL, Chen X, Lai H-M, Yan LYC, Huang J, Sy SKH, Tian X, Huang Y, et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun. 2020;11:4413–4415. doi: 10.1038/s41467-020-18249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vistain LF, Tay S. Single-cell proteomics. Trends Biochem Sci. 2021;46:661–672. doi: 10.1016/j.tibs.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Cheng S, Lin Q, Cao W, Yang J, Zhang M, Shen A, Zhang W, Xia Y, Ma X, et al. Single-cell lipidomics with high structural specificity by mass spectrometry. Nat Commun. 2021;12:2869. doi: 10.1038/s41467-021-23161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali A, Abouleila Y, Shimizu Y, Hiyama E, Emara S, Mashaghi A, Hankemeier T. Single-cell metabolomics by mass spectrometry: advances, challenges, and future applications. TRAC Trends Anal Chem. 2019;120:115436. doi: 10.1016/j.trac.2019.02.033 [DOI] [Google Scholar]
- 71.Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci. 2020;21:416–432. doi: 10.1038/s41583-020-0322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mapunda JA, Tibar H, Regragui W, Engelhardt B. How does the immune system enter the brain? Front Immunol. 2022;13:805657 doi: 10.3389/fimmu.2022.805657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep. 2014;6:14. doi: 10.12703/P6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2016;323:96–109. doi: 10.1016/j.neuroscience.2015.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hosford PS, Gourine AV. What is the key mediator of the neurovascular coupling response? Neurosci Biobehav Rev. 2019;96:174–181. doi: 10.1016/j.neubiorev.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nunez A, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 78.Dreier JP The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- 79.Howarth C, Mishra A, Hall CN. More than just summed neuronal activity: how multiple cell types shape the BOLD response. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190630. doi: 10.1098/rstb.2019.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Echagarruga CT, Gheres KW, Norwood JN, Drew PJ. nNOS-expressing interneurons control basal and behaviorally evoked arterial dilation in somatosensory cortex of mice. eLife. 2020;9:e60533. doi: 10.7554/eLife.60533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mishra A. Binaural blood flow control by astrocytes: listening to synapses and the vasculature. J Physiol. 2017;595:1885–1902. doi: 10.1113/JP270979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, Prat A, Drapeau P, Di Polo A. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585:91–95. doi: 10.1038/s41586-020-2589-x [DOI] [PubMed] [Google Scholar]
- 85.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chow BW, Nunez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL, Kumar P, Sabatini BL, Gu C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579:106–110. doi: 10.1038/s41586-020-2026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, Pritchard HA, Isakson BE, Tran CHT, Earley S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. eLife. 2021;10:e63040. doi: 10.7554/eLife.63040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnes JN, Charkoudian N. Integrative cardiovascular control in women: regulation of blood pressure, body temperature, and cerebrovascular responsiveness. FASEB J. 2021;35:e21143. doi: 10.1096/fj.202001387R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood CAP, Zhang J, Aydin D, Xu Y, Andreone BJ, Langen UH, Dror RO, Gu C, Feng L. Structure and mechanism of blood-brain-barrier lipid transporter MFSD2A. Nature. 2021;596:444–448. doi: 10.1038/s41586-021-03782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94:581–594.e5. doi: 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chow BW, Gu C. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron. 2017;93:1325–1333.e3. doi: 10.1016/j.neuron.2017.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1 [DOI] [PubMed] [Google Scholar]
- 97.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- 98.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 100.Heithoff BP, George KK, Phares AN, Zuidhoek IA, Munoz-Ballester C, Robel S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia. 2021;69:436–472. doi: 10.1002/glia.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ayloo S, Lazo CG, Sun S, Zhang W, Cui B, Gu C. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron. 2022;110:1641–1655.e6. doi: 10.1016/j.neuron.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tran ND, Schreiber SS, Fisher M. Astrocyte regulation of endothelial tissue plasminogen activator in a blood-brain barrier model. J Cereb Blood Flow Metab. 1998;18:1316–1324. doi: 10.1097/00004647-199812000-00006 [DOI] [PubMed] [Google Scholar]
- 104.Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiol Rev. 2022;102:1025–1151. doi: 10.1152/physrev.00031.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136:139–152. doi: 10.1007/s00401-018-1862-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bakker E, Naessens DMP, VanBavel E. Paravascular spaces: entry to or exit from the brain? Exp Physiol. 2019;104:1013–1017. doi: 10.1113/EP087424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878. doi: 10.1038/s41467-018-07318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diem AK, MacGregor Sharp M, Gatherer M, Bressloff NW, Carare RO, Richardson G. Arterial pulsations cannot drive intramural periarterial drainage: significance for Abeta drainage. Front Neurosci. 2017;11:475. doi: 10.3389/fnins.2017.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci. 2019;11:1. doi: 10.3389/fnagi.2019.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, Greenberg SM, Bacskai BJ. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron. 2020;105:549–561.e5. doi: 10.1016/j.neuron.2019.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kedarasetti RT, Turner KL, Echagarruga C, Gluckman BJ, Drew PJ, Costanzo F. Functional hyperemia drives fluid exchange in the paravascular space. Fluids Barriers CNS. 2020;17:52. doi: 10.1186/s12987-020-00214-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hablitz LM, Vinitsky HS, Sun Q, Staeger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5:eaav5447. doi: 10.1126/sciadv.aav5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410 [DOI] [PubMed] [Google Scholar]
- 116.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol. 2019;4:eaav0492. doi: 10.1126/sciimmunol.aav0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666 [DOI] [PubMed] [Google Scholar]
- 118.Faraci FM, Kadel KA, Heistad DD. Vascular responses of dura mater. Am J Physiol. 1989;257:H157–H161. doi: 10.1152/ajpheart.1989.257.1.H157 [DOI] [PubMed] [Google Scholar]
- 119.Brea D, Poon C, Benakis C, Lubitz G, Murphy M, Iadecola C, Anrather J. Stroke affects intestinal immune cell trafficking to the central nervous system. Brain Behav Immun. 2021;96:295–302. doi: 10.1016/j.bbi.2021.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toriumi H, Shimizu T, Shibata M, Unekawa M, Tomita Y, Tomita M, Suzuki N. Developmental and circulatory profile of the diploic veins. Microvasc Res. 2011;81:97–102. doi: 10.1016/j.mvr.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 121.Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560:55–60. doi: 10.1038/s41586-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schläger C, Körner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T, Cabañas C, Nelson PJ, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939 [DOI] [PubMed] [Google Scholar]
- 123.Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–1217. doi: 10.1038/s41593-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brioschi S, Wang W-L, Peng V, Wang M, Shchukina I, Greenberg ZJ, Bando JK, Jaeger N, Czepielewski RS, Swain A, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373:eabf9277. doi: 10.1126/science.abf9277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, Baker W, Papadopoulos Z, Drieu A, Blackburn S, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373:eabf7844. doi: 10.1126/science.abf7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056–3074. e21. doi: 10.1016/j.cell.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cui J, Xu H, Lehtinen MK. Macrophages on the margin: choroid plexus immune responses. Trends Neurosci. 2021;44:864–875. doi: 10.1016/j.tins.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH. Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2−/− mice. Brain Behav Immun. 2016;57:161–172. doi: 10.1016/j.bbi.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213 [DOI] [PubMed] [Google Scholar]
- 130.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sorond FA, Gorelick PB. Brain white matter: a substrate for resilience and a substance for subcortical small vessel disease. Brain Sci. 2019;9:193. doi: 10.3390/brainsci9080193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134:226–239. doi: 10.1016/j.neuropharm.2017.12.030 [DOI] [PubMed] [Google Scholar]
- 133.Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, Berman SE, Biessels GJ, Black SE, Bos I, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement (NY). 2019;5:107–117. doi: 10.1016/j.trci.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hase Y, Ding R, Harrison G, Hawthorne E, King A, Gettings S, Platten C, Stevenson W, Craggs LJL, Kalaria RN. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol Commun. 2019;7:16. doi: 10.1186/s40478-019-0666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Humphreys CA, Smith C, Wardlaw JM. Correlations in post-mortem imaging-histopathology studies of sporadic human cerebral small vessel disease: a systematic review. Neuropathol Appl Neurobiol. 2021;47:910–930. doi: 10.1111/nan.12737 [DOI] [PubMed] [Google Scholar]
- 136.Blevins BL, Vinters HV, Love S, Wilcock DM, Grinberg LT, Schneider JA, Kalaria RN, Katsumata Y, Gold BT, Wang DJJ, et al. Brain arteriolosclerosis. Acta Neuropathol. 2021;141:1–24. doi: 10.1007/s00401-020-02235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anad A, Barker MK, Katanga JA, Arfanakis K, Bridges LR, Esiri MM, Isaacs JD, Prpar Mihevc S, Pereira AC, Schneider JA, et al. Vasculocentric axonal NfH in small vessel disease. J Neuropathol Exp Neurol. 2022;81:182–192. doi: 10.1093/jnen/nlab134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hase Y, Chen A, Bates LL, Craggs LJL, Yamamoto Y, Gemmell E, Oakley AE, Korolchuk VI, Kalaria RN. Severe white matter astrocytopathy in CADASIL. Brain Pathol. 2018;28:832–843. doi: 10.1111/bpa.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen A, Akinyemi RO, Hase Y, Firbank MJ, Ndung’u MN, Foster V, Craggs LJ, Washida K, Okamoto Y, Thomas AJ, et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and poststroke dementia. Brain. 2016;139:242–258. doi: 10.1093/brain/awv328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frost GR, Li YM. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017;7:170228. doi: 10.1098/rsob.170228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ding R, Hase Y, Ameen-Ali KE, Ndung’u M, Stevenson W, Barsby J, Gourlay R, Akinyemi T, Akinyemi R, Uemura MT, et al. Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer’s disease. Brain Pathol. 2020;30:1087–1101. doi: 10.1111/bpa.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jenkins LM, Kogan A, Malinab M, Ingo C, Sedaghat S, Bryan NR, Yaffe K, Parrish TB, Nemeth AJ, Lloyd-Jones DM, et al. Blood pressure, executive function, and network connectivity in middle-aged adults at risk of dementia in late life. Proc Natl Acad Sci U S A. 2021;118:e2024265118. doi: 10.1073/pnas.2024265118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, et al. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, Greenberg SM, Biessels GJ. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017;16:730–740. doi: 10.1016/s1474-4422(17)30196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–436. doi: 10.1038/jcbfm.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Z, van Veluw SJ, Wong A, Liu W, Shi L, Yang J, Xiong Y, Lau A, Biessels GJ, Mok VC. Risk factors and cognitive relevance of cortical cerebral microinfarcts in patients with ischemic stroke or transient ischemic attack. Stroke. 2016;47:2450–2455. doi: 10.1161/STROKEAHA.115.012278 [DOI] [PubMed] [Google Scholar]
- 147.Hartmann DA, Hyacinth HI, Liao FF, Shih AY. Does pathology of small venules contribute to cerebral microinfarcts and dementia? J Neurochem. 2018;144:517–526. doi: 10.1111/jnc.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 2013;80:1365–1369. doi: 10.1212/WNL.0b013e31828c2f52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Summers PM, Hartmann DA, Hui ES, Nie X, Deardorff RL, McKinnon ET, Helpern JA, Jensen JH, Shih AY. Functional deficits induced by cortical microinfarcts. J Cereb Blood Flow Metab. 2017;37:3599–3614. doi: 10.1177/0271678X16685573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Faraco G, Hochrainer K, Segarra SG, Schaeffer S, Santisteban MM, Menon A, Jiang H, Holtzman DM, Anrather J, Iadecola C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature. 2019;574:686–690. doi: 10.1038/s41586-019-1688-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen J, Wang D, Wang X, Gupta S, Ayloo B, Wu S, Prasad P, Xiong Q, Xia J, Ge S. Neurovascular coupling in the dentate gyrus regulates adult hippocampal neurogenesis. Neuron. 2019;103:878–890.e3. doi: 10.1016/j.neuron.2019.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ouellette J, Toussay X, Comin CH, Costa LDF, Ho M, Lacalle-Aurioles M, Freitas-Andrade M, Liu QY, Leclerc S, Pan Y, et al. Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nat Neurosci. 2020;23:1090–1101. doi: 10.1038/s41593-020-0663-1 [DOI] [PubMed] [Google Scholar]
- 153.Kim SH, Ahn JH, Yang H, Lee P, Koh GY, Jeong Y. Cerebral amyloid angiopathy aggravates perivascular clearance impairment in an Alzheimer’s disease mouse model. Acta Neuropathol Commun. 2020;8:181. doi: 10.1186/s40478-020-01042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, Nedergaard M. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37:2870–2877. doi: 10.1523/JNEUROSCI.2112-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11:74. doi: 10.1186/s13024-016-0138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease: one peptide, two pathways. Nat Rev Neurol. 2020;16:30–42. doi: 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci. 2019;22:413–420. doi: 10.1038/s41593-018-0329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bracko O, Njiru BN, Swallow M, Ali M, Haft-Javaherian M, Schaffer CB. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. J Cereb Blood Flow Metab. 2020;40:1441–1452. doi: 10.1177/0271678X19873658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- 160.Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, Feuerstein G, Hallenbeck JM, Siren AL. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab. 1994;14:348–352. doi: 10.1038/jcbfm.1994.43 [DOI] [PubMed] [Google Scholar]
- 161.Ramos-Cejudo J, Johnson AD, Beiser A, Seshadri S, Salinas J, Berger JS, Fillmore NR, Do N, Zheng C, Kovbasyuk Z, et al. The neutrophil to lymphocyte ratio Is associated with the risk of subsequent dementia in the Framingham Heart Study. Front Aging Neurosci. 2021;13:773984. doi: 10.3389/fnagi.2021.773984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Willik KD, Fani L, Rizopoulos D, Licher S, Fest J, Schagen SB, Ikram MK, Ikram MA. Balance between innate versus adaptive immune system and the risk of dementia: a population-based cohort study. J Neuroinflammation. 2019;16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 165.Munawara U, Catanzaro M, Xu W, Tan C, Hirokawa K, Bosco N, Dumoulin D, Khalil A, Larbi A, Lévesque S. Hyperactivation of monocytes and macrophages in MCI patients contributes to the progression of Alzheimer’s disease. Immun Ageing. 2021;18:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Batterman KV, Cabrera PE, Moore TL, Rosene DL. T Cells actively infiltrate the white matter of the aging monkey brain in relation to increased microglial reactivity and cognitive decline. Front Immunol. 2021;12:607691. doi: 10.3389/fimmu.2021.607691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, George BM, Boutet SC, Hebestreit K, Pluvinage JV, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571:205–210. doi: 10.1038/s41586-019-1362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, Groger J, Minnerup J, Wiendl H, Meuth SG, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging. 2015;36:81–89. doi: 10.1016/j.neurobiolaging.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 169.Lombardi G, Paganelli R, Abate M, Ireland A, Molino-Lova R, Sorbi S, Macchi C, Pellegrino R, Di Iorio A, Cecchi F. Leukocyte-derived ratios are associated with late-life any type dementia: a cross-sectional analysis of the Mugello study. GeroScience. 2021;43:2785–2793. doi: 10.1007/s11357-021-00474-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184:1000–1016.e27. doi: 10.1016/j.cell.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Phoenix A, Chandran R, Ergul A. Cerebral microvascular senescence and inflammation in diabetes. Front Physiol. 2022;13:864758. doi: 10.3389/fphys.2022.864758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Peters R, Peters J, Booth A, Anstey KJ. Trajectory of blood pressure, body mass index, cholesterol and incident dementia: systematic review. Br J Psychiatry. 2020;16:16–28. doi: 10.1192/bjp.2019.156 [DOI] [PubMed] [Google Scholar]
- 175.Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018;14:1416–1426. doi: 10.1016/j.jalz.2018.06.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/s1474-4422(19)30079-1 [DOI] [PubMed] [Google Scholar]
- 177.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Keage HA, Churches OF, Kohler M, Pomeroy D, Luppino R, Bartolo ML, Elliott S. Cerebrovascular function in aging and dementia: a systematic review of transcranial Doppler studies. Dement Geriatr Cogn Dis Extra. 2012;2:258–270. doi: 10.1159/000339234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen X, Liu X, Koundal S, Elkin R, Zhu X, Monte B, Xu F, Dai F, Pedram M, Lee H. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat Aging. 2022;2:214–223. doi: 10.1038/s43587-022-00181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen C-H, Cheng Y-W, Chen Y-F, Tang S-C, Jeng J-S. Plasma neurofilament light chain and glial fibrillary acidic protein predict stroke in CADASIL. J Neuroinflammation. 2020;17:124. doi: 10.1186/s12974-020-01813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miners JS, Kehoe PG, Love S, Zetterberg H, Blennow K. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res Ther. 2019;11:81. doi: 10.1186/s13195-019-0534-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schulz M, Malherbe C, Cheng B, Thomalla G, Schlemm E. Functional connectivity changes in cerebral small vessel disease: a systematic review of the resting-state MRI literature. BMC Med. 2021;19:103. doi: 10.1186/s12916-021-01962-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, Rosand J, Viswanathan A, Salat DH, Polimeni JR, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJ, van Buchem MA, Wardlaw JM. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2016;36:1653–1667. doi: 10.1177/0271678x16662891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.De Reuck J, Decoo D, Marchau M, Santens P, Lemahieu I, Strijckmans K. Positron emission tomography in vascular dementia. J Neurol Sci. 1998;154:55–61. doi: 10.1016/s0022-510x(97)00213-x [DOI] [PubMed] [Google Scholar]
- 188.Catchlove SJ, Pipingas A, Hughes ME, Macpherson H. Magnetic resonance imaging for assessment of cerebrovascular reactivity and its relationship to cognition: a systematic review. BMC Neurosci. 2018;19:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guerchet M, Aboyans V, Nubukpo P, Lacroix P, Clement JP, Preux PM. Ankle-brachial index as a marker of cognitive impairment and dementia in general population: a systematic review. Atherosclerosis. 2011;216:251–257. doi: 10.1016/j.atherosclerosis.2011.03.024 [DOI] [PubMed] [Google Scholar]
- 190.Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH, Heiss G. Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J Alzheimers Dis. 2017;57:195–204. doi: 10.3233/JAD-161041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shi Y, Thrippleton MJ, Marshall I, Wardlaw JM. Intracranial pulsatility in patients with cerebral small vessel disease: a systematic review. Clin Sci. 2018;132:157–171. doi: 10.1042/CS20171280 [DOI] [PubMed] [Google Scholar]
- 192.Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, Fazekas F, Ropele S, Frayne R, van Oostenbrugge RJ, et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement. 2019;15:840–858. doi: 10.1016/j.jalz.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, Nahavandi P, Thomas DL, Lythgoe MF, Wells JA. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. eLife. 2018;7:e34028. doi: 10.7554/eLife.34028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Attier-Zmudka J, Sérot J-M, Valluy J, Saffarini M, Macaret A-S, Diouf M, Dao S, Douadi Y, Malinowski KP, Balédent O. Decreased cerebrospinal fluid flow is associated with cognitive deficit in elderly patients. Front Aging Neurosci. 2019;11:87. doi: 10.3389/fnagi.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628–631. doi: 10.1126/science.aax5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Low A, Mak E, Rowe JB, Markus HS, O’Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. doi: 10.1016/j.arr.2019.100916 [DOI] [PubMed] [Google Scholar]
- 199.Liu Y, Chan DKY, Thalamuthu A, Wen W, Jiang J, Paradise M, Lee T, Crawford J, Wai Kin Wong M, Hua Xu Y, et al. Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia. Comput Struct Biotechnol J. 2020;18:1613–1624. doi: 10.1016/j.csbj.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pokharel Y, Mouhanna F, Nambi V, Virani SS, Hoogeveen R, Alonso A, Heiss G, Coresh J, Mosley T, Gottesman RF, et al. ApoB, small-dense LDL-C, Lp(a), LpPLA2 activity, and cognitive change. Neurology. 2019;92:e2580–e2593. doi: 10.1212/WNL.0000000000007574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Naiberg MR, Newton DF, Goldstein BI. Flow-mediated dilation and neurocognition: Systematic review and future directions. Psychosom Med. 2016;78:192–207. doi: 10.1097/PSY.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 202.Hosseinzadeh S, Noroozian M, Mortaz E, Mousavizadeh K. Plasma microparticles in Alzheimer’s disease: the role of vascular dysfunction. Metab Brain Dis. 2018;33:293–299. doi: 10.1007/s11011-017-0149-3 [DOI] [PubMed] [Google Scholar]
- 203.Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab. 2016;36:72–94. doi: 10.1038/jcbfm.2015.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alvarez-Bueno C, Cunha PG, Martinez-Vizcaino V, Pozuelo-Carrascosa DP, Visier-Alfonso ME, Jimenez-Lopez E, Cavero-Redondo I. Arterial stiffness and cognition among adults: a systematic review and meta-analysis of observational and longitudinal studies. J Am Heart Assoc. 2020;9:e014621. doi: 10.1161/JAHA.119.014621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bangen KJ, Smirnov DS, Delano-Wood L, Wierenga CE, Bondi MW, Salmon DP, Galasko D. Arterial stiffening acts synergistically with APOE genotype and AD biomarker status to influence memory in older adults without dementia. Alzheimers Res Ther. 2021;13:121. doi: 10.1186/s13195-021-00851-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Eira M, Bensenor IM, Dorea EL, Cunha RS, Mill JG, Lotufo PA. Potent antiretroviral therapy for human immunodeficiency virus infection increases aortic stiffness. Arq Bras Cardiol. 2012;99:1100–1107. doi: 10.1590/s0066-782x2012005000110 [DOI] [PubMed] [Google Scholar]
- 207.Schaffenrath J, Huang SF, Wyss T, Delorenzi M, Keller A. Characterization of the blood-brain barrier in genetically diverse laboratory mouse strains. Fluids Barriers CNS. 2021;18:34. doi: 10.1186/s12987-021-00269-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ganesh S, Hu T, Woods E, Allam M, Cai S, Henderson W, Coskun AF. Spatially resolved 3D metabolomic profiling in tissues. Sci Adv. 2021;7:eabd0957. doi: 10.1126/sciadv.abd0957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Larsson L, Frisen J, Lundeberg J. Spatially resolved transcriptomics adds a new dimension to genomics. Nat Methods. 2021;18:15–18. doi: 10.1038/s41592-020-01038-7 [DOI] [PubMed] [Google Scholar]
- 210.Gu X, Chen W, Volkow ND, Koretsky AP, Du C, Pan Y. Synchronized astrocytic Ca(2+) responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell Rep. 2018;23:3878–3890. doi: 10.1016/j.celrep.2018.05.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Institoris A, Rosenegger DG, Gordon GR. Arteriole dilation to synaptic activation that is sub-threshold to astrocyte endfoot Ca2+ transients. J Cereb Blood Flow Metab. 2015;35:1411–1415. doi: 10.1038/jcbfm.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tran CHT, Peringod G, Gordon GR. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron. 2018;100:1133–1148.e3. doi: 10.1016/j.neuron.2018.09.045 [DOI] [PubMed] [Google Scholar]
- 213.Koizumi K, Hattori Y, Ahn SJ, Buendia I, Ciacciarelli A, Uekawa K, Wang G, Hiller A, Zhao L, Voss HU, et al. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun. 2018;9:3816. doi: 10.1038/s41467-018-06301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760 [DOI] [PubMed] [Google Scholar]
- 215.Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, Crombag HS, Hall CN. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat Commun. 2021;12:3190. doi: 10.1038/s41467-021-23508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Park KS, Shin JG, Qureshi MM, Chung E, Eom TJ. Deep brain optical coherence tomography angiography in mice: in vivo, noninvasive imaging of hippocampal formation. Sci Rep. 2018;8:11614. doi: 10.1038/s41598-018-29975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hontani Y, Xia F, Xu C. Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain. Sci Adv. 2021;7:eabf3531. doi: 10.1126/sciadv.abf3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deffieux T, Demene C, Tanter M. Functional ultrasound imaging: a new imaging modality for neuroscience. Neuroscience. 2021;474:110–121. doi: 10.1016/j.neuroscience.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 219.Albayram MS, Smith G, Tufan F, Tuna IS, Bostanciklioglu M, Zile M, Albayram O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. 2022;13:203. doi: 10.1038/s41467-021-27887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mehta NH, Sherbansky J, Kamer AR, Carare RO, Butler T, Rusinek H, Chiang GC, Li Y, Strauss S, Saint-Louis LA, et al. The brain-nose interface: a potential cerebrospinal fluid clearance site in humans. Front Physiol. 2021;12:769948. doi: 10.3389/fphys.2021.769948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Benveniste H, Lee H, Ozturk B, Chen X, Koundal S, Vaska P, Tannenbaum A, Volkow ND. Glymphatic cerebrospinal fluid and solute transport quantified by MRI and PET imaging. Neuroscience. 2021;474:63–79. doi: 10.1016/j.neuroscience.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tsai HH, Chen YF, Yen RF, Lo YL, Yang KC, Jeng JS, Tsai LK, Chang CF. Plasma soluble TREM2 is associated with white matter lesions independent of amyloid and tau. Brain. 2021;144:3371–3380. doi: 10.1093/brain/awab332 [DOI] [PubMed] [Google Scholar]
- 223.Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, Schifferer M, Gouna G, Usifo F, Kannaiyan N, et al. White matter aging drives microglial diversity. Neuron. 2021;109:1100–1117.e10. doi: 10.1016/j.neuron.2021.01.027 [DOI] [PubMed] [Google Scholar]
- 224.Guo Q, Scheller A, Huang W. Progenies of NG2 glia: what do we learn from transgenic mouse models? Neural Regener Res. 2021;16:43–48. doi: 10.4103/1673-5374.286950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 226.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, et al. ; on behalf of the American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534. doi: 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dolui S, Detre JA, Gaussoin SA, Herrick JS, Wang DJJ, Tamura MK, Cho ME, Haley WE, Launer LJ, Punzi HA, et al. Association of intensive vs standard blood pressure control with cerebral blood flow: Secondary analysis of the SPRINT MIND randomized clinical trial. JAMA Neurol. 2022;79:380–389. doi: 10.1001/jamaneurol.2022.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.