Abstract
Aims
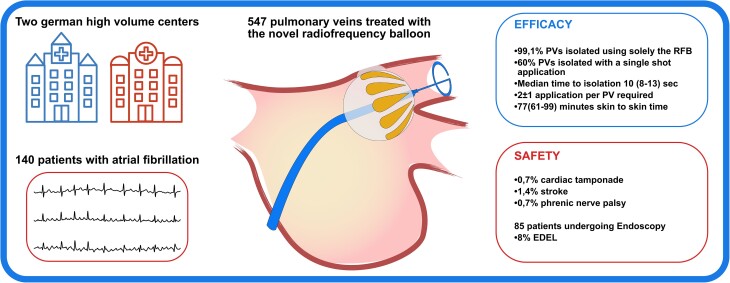
A novel irrigated radiofrequency (RF) balloon (RFB) for pulmonary vein (PV) isolation (PVI) was released in selected centres. We pooled the procedural data on efficacy and safety of RFB-PVI from two high volume German centres.
Methods and results
Consecutive patients with RFB procedures were enrolled. A 3D electroanatomical left atrial map guided the RFB navigation. Every RF delivery lasted 60 s, and duration was automatically reduced to 20 s for electrodes facing the posterior wall. Procedural data and post-procedural endoscopy data (<48 h) were analysed. Data from 140 patients were collected (57% male, 67 ± 11 years, 57% paroxysmal atrial fibrillation). There were 547 PVs identified, and 99.1% could be isolated using solely the RFB. Single-shot PVI was recorded in 330/547 (60%) PVs. Median time to isolation during the first application was 10 s (IQR 8–13). A total of 2.1 ± 1.8 applications per PV were delivered, with the left superior PV requiring more application compared to other PVs. Median procedure and fluoroscopy time were 77 min (61–99) and 13 min (10–17), respectively. Major safety events were recorded only in the first 25 cases at each centre and included 1/140(0.7%) cardiac tamponade, 1/140(0.7%) phrenic nerve palsy, and 2/140 strokes (1.4%). An oesophageal temperature rise was recorded in 81/547 (15%) PVs, and endoscopy detected oesophageal lesions in 7/85 (8%) patients undergoing endoscopy.
Conclusion
The RFB showed a high efficacy allowing for fast PVI procedures, and 60% of PVs could be isolated at the first application. Most safety events were recorded during the learning phase. An oesophageal temperature monitoring is suggested: oesophageal lesions were detected in 8% of patients.
Keywords: Atrial Fibrillation, Ablation, Radiofrequency, Balloon
Graphical Abstract
Graphical Abstract.
What’s new?
This is the largest report on atrial fibrillation patients treated with the novel radiofrequency balloon from two high volume centres.
99.1% of pulmonary veins were isolated using solely the radiofrequency balloon.
60% of pulmonary veins could be isolated with a single-shot application, after a median of 10 s.
Safety events mainly occurred during the learning phase.
Endoscopy detected oesophageal lesions in 8% of patients undergoing endoscopy.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults,1 and its incidence and prevalence are increasing with the aging of the population. Catheter ablation demonstrated to be superior to antiarrhythmic drugs for rhythm control,2 and pulmonary vein isolation (PVI) represents the cornerstone of catheter ablation of AF.3 The PVI is traditionally performed using radiofrequency current (RFC) delivered in a point-by-point fashion in combination with a 3D mapping system, but the procedure is technically demanding, has a long learning curve and great disparities among the results of different centres, and operators were reported.4 Balloon catheter technologies were therefore developed to ease and uniform the results of catheter ablation of AF, demonstrating non-inferiority in terms of efficacy compared to RFC.5,6 Recently, several single-shot catheters were developed:7 among these new technologies, the radiofrequency balloon (RFB) tried to put together the well-known energy form of RFC and the ease of use of balloon catheters, in combination with the navigation in a 3D mapping system. After initial promising results,8,9 the RFB was released for clinical use in selected centres, and two high volume German centres decided to share their results in a prospective registry (AURORA collaboration): the aim of this study is to report initial shared data on efficacy and safety of the new RFB.
Methods
All patients treated with the new RFB in two high volume German centres (Cardioangiologisches Centrum Bethanien—Frankfurt am Main/Germany and University Heart and Vascular Centre Hamburg Eppendorf—Hamburg/Germany) were enrolled. Patients with symptomatic AF between 18 and 85 years underwent RFB ablation, exclusion criteria consisted in left-atrial (LA) diameter >55 mm, AF duration >12 months, evidence of a thrombus in the LA appendage in preprocedural transoesophageal echocardiography (TEE), and contraindication to anticoagulation. No other preprocedural imaging (computer tomography/cardiac magnetic resonance) was performed. All patients gave written informed consent to the procedure, and the study was approved by the institutional review board.
Ablation procedure
Oral anticoagulation was interrupted the morning of the procedure. Preprocedural TEE was performed at centre discretion to rule out intracardiac thrombi (one centre skipping it in in case of SR and CHADSVASC <3). Procedures were performed under deep sedation using boluses of midazolam, fentanyl, and a continuous infusion of propofol. In the beginning of the sedation, a temperature probe for luminal oesophageal temperature (LET) measurement was introduced. After two venous accesses, a deflectable diagnostic catheter (10P Inquiry, Abbott, USA or 8P Webster, Biosense Webster, USA) was positioned in the CS. Transeptal puncture was performed under fluoroscopy via a 8.5F transseptal sheath (SL-1, Abbot, USA) in conjunction with a BRK1 transseptal needle. After transseptal puncture, 100-IU/kg unfractionated Heparin was administrated, with further additional boli to achieve an activated clotting time >300 s. Selective pulmonary vein (PV) angiograms were then performed using a multipurpose catheter and served as a reference for mapping and ablation.
A 3D high resolution mapping of the left atrium was obtained using a multipolar circumferential catheter (LASSO NAV, Biosense Webster, USA). The SL1 sheath was then exchanged for the RFB deflectable delivery sheath (13,5F–14F, GuideStar, BiosenseWebster, USA) over a wire placed in the left superior pulmonary vein (LSPV), and the RFB was introduced in the left atrium.
The radiofrequency balloon: technology description
The RFB is a 28-mm balloon (HELIOSTAR, BiosenseWebster, USA). The deflectable delivery sheath (GuideStar, BiosenseWebster, USA) was initially delivered with a 13,5F outer diameter, and during the study, its diameter was increased to 14F.
The RFB incorporates 10 flexible electrodes (14.5-mm length) on its distal hemisphere, with a tear shape to ensure uniform interelectrode distance (4.4 mm proximal to 1.1-mm distal). Each electrode has several holes in the midline to ensure open irrigation during inflation and ablation. The balloon is flushed via an irrigation pump that can vary the flow from 5 mm/min (inflation phase) to 35 mL/min during the ablation phase. The RFB is compliant, and despite a fixed flow, its diameter can therefore show variations: the distance between the electrodes is continuously measured by the system that provides the so called ‘Inflation Index’ (1 when the RFB reaches the 28-mm diameter, and decreases proportionally in case of smaller diameters). The RFB has a proximal magnetic navigation sensor, and the distal electrodes are displayed based on impendence mapping in the 3D mapping system.
On the RFB, there are radiopaque markers to identify the different electrodes fluoroscopically even without the need of the 3D map. The catheter has a central lumen to host a guidewire or an inner lumen 10-pole spiral catheter (SC—LassoStar, BiosenseWebster, USA), available in 15 and 20-mm diameter. The first-generation SC was not equipped with a magnetic sensor, therefore not allowing the acquisition of the 3D shell. The central lumen distal injection of contrast medium can be used to prove balloon position and grade of occlusion.
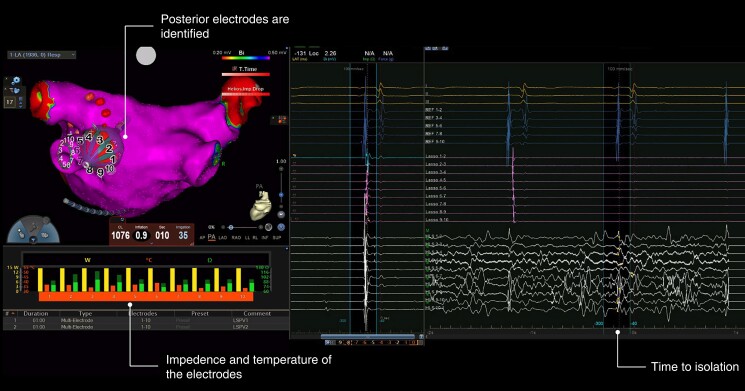
An example of RFB ablation is displayed in Figure 1. Ablation is performed via the N-GEN RF generator with unipolar energy delivered via each activated electrode. The energy is set at 15 W and delivered in a temperature-controlled mode. The system mandates identification of 2–4 electrodes facing the posterior LA wall. At these electrodes, ablation is automatically stopped after 20 s, otherwise the energy delivery lasted 60 s per application. During ablation at septal PVs, the multipolar diagnostic catheter must be placed in the superior vena cava for phrenic nerve (PN) stimulation. In addition, stimulation from the anterior electrodes on the RFB surface was carried out before energy delivery to rule out local PN capture.
Figure 1.
An example of real time isolation after 10 s of a left inferior pulmonary vein using the RFB. The RFB is displayed in the 3D map of the left atrium in the posteroanterior view. The intracardiac electrograms (EGMs) of the coronary sinus are displayed above, the EGMs of the Lasso Star are displayed in the middle, the EGMs from the RFB surface (note the ablation artifacts) are displayed below. RFB, radiofrequency balloon.
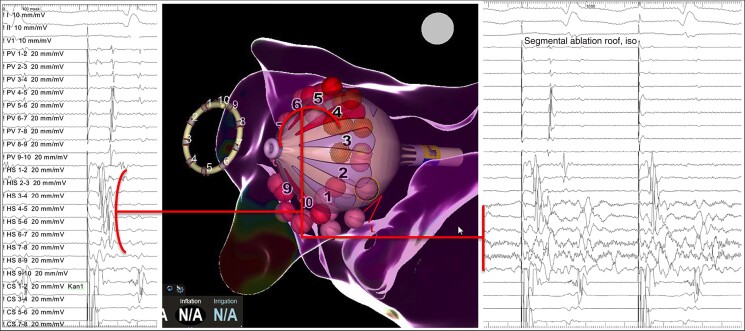
Single-shot isolation was defined as isolation after the first circumferential attempt. In case of failed isolation or early reconnection, further circumferential applications could be delivered after repositioning of the balloon. Alternatively, the connection gap was identified (based on the electrograms derived from the RFB electrodes and from the inner lumen SC), and a segmental ablation was performed (Figure 2). A cross talk manoeuvre (with isolation of the ipsilateral PV) was attempted in case of suspected activation deriving from the carena. In case of futile ablation attempts with the RFB, a switch to point by point touch up ablation could be performed at operator discretion.
Figure 2.
Example of a segmental isolation of a left superior pulmonary vein. Notice the earliest activation of the PV in the Heliostar (HS) electrodes 4 to 7 (left panel), preceding the signals collected distally by the Lasso-star (PV). In the right panel, real time PVI could be recorded during localized application of RFC at these electrodes. PV, pulmonary vein; PVI, pulmonary vein isolation; RFC, radiofrequency current.
After ablation, a 3D voltage map was acquired to confirm ostial PV isolation at operator’s discretion.
Post-ablation care
An echocardiogram was performed to rule out pericardial effusion. A figure of eight suture served to reach haemostasis, and no protamine was routinely used. Oral anticoagulation was resumed ≤6 h after the procedure. An unselected subset of patients underwent oesophagoscopy the day after ablation.
Statistical analysis
Data mean ± standard deviation was used to describe continuous variables with normal distribution; otherwise median and interquartile ranges were used. For nominal parameters, the absolute and relative frequency were counted. Data were summarized in an Excel sheet and analysed with SPSS Version 20 and Graphpad-Prizm Version 7.
Results
A total of 140 patients (73 from centre 1 and 67 from centre 2) were included; 80/140 (57%) patients were male, mean age 67 ± 11 years, and 80/140 (57%) with paroxysmal AF (PAF). Detailed baseline characteristics are given in Table 1. Four electrophysiologists per centre were involved as first operator.
Table 1.
Baseline characteristics
| Baseline characteristics | |
|---|---|
| Male sex, n (%) | 80/140 (57%) |
| Age (years) | 67 ± 11 |
| Body mass index | 28 ± 6 |
| Persistent AF, n (%) | 60/140 (43%) |
| Arterial hypertension, n (%) | 86/140 (61%) |
| Diabetes mellitus, n (%) | 21/140 (15%) |
| Stroke, n (%) | 7/140 (5%) |
| NYHA > 1, n (%) | 36/140 (26%) |
| Coronary artery disease, n (%) | 30/140 (21%) |
| LVEF (%) | 58 ± 9 |
AF, atrial fibrillation; LVEF, left ventricular ejection fraction.
Procedural efficacy
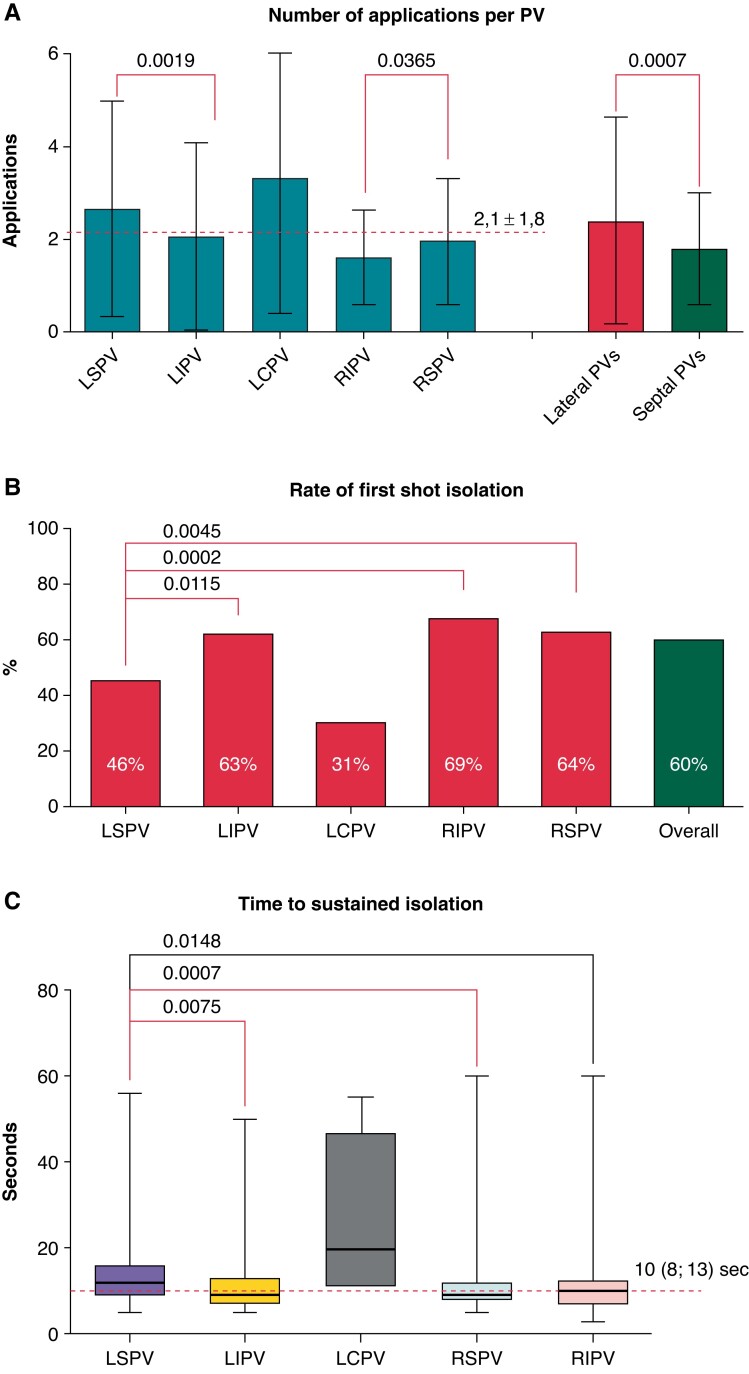
In 140 patients, 547 PVs were identified [13 left common pulmonary vein (LCPVs)] and 542/547 (99.1%) PVs could be isolated solely using the RFB. Two left superior pulmonary veins (LSPVs), two left inferior pulmonary veins (LIPVs) and one right inferior pulmonary veins (RIPV) could not be isolated with the RFB (after 8,8,17,11, and 1 applications): three PVs were left non-isolated; in one LSPV and in one RIPV, an RF touch-up ablation was performed. A mean of 8.1 ± 3.8 applications per patient and a mean of 2.1 ± 1.8 applications per PV were delivered. Excluding the 13 identified LCPV that required 3.3 ± 2.9 applications, the LSPV (2.6 ± 2.3 applications) was the PV requiring more applications compared to LIPV (2.1 ± 2.0), right superior pulmonary vein (RSPV) (2.0 ± 1.4) and RIPV (1.6 ± 1.0). Less applications were needed in the inferior PVs compared to the ipsilateral superior PVs; lateral PVs required significantly more applications than septal PVs (Figure 3A). Single-shot isolation, defined as durable isolation of the target PV at the first attempt, was overall recorded in 330/547 PVs (60%). Once again, LCPV was excluded, and the LSPV showed the lowest rate of single-shot isolation (46%) in comparison to LIPV (63%), RSPV (64%), and RIPV (69%), (Figure 3B). In case of PVI with the first attempt, real time to isolation (TTI) was recorded in 234/330 (71%) PVs after a median of 10 sec (Q1 = 8; Q3 = 13), with the LSPV exposing longer TTI compared to LIPV and RSPV (Figure 3C).
Figure 3.
Graphical representation of the procedural efficacy in the different PVs, with the LSPV often requiring more applications to achieve PVI. PVs, pulmonary veins; LSPV left superior pulmonary vein; PVI, pulmonary vein isolation.
Procedural safety
Procedural complications were recorded in 5/140 patients (3.6%)—Table 2. In 2/140 (1.4%) patients, a periprocedural stroke was recorded. One patient developed dysarthria and a left-sided hemiparesis, and an MRI revealed a mesencephalic infarction. The other patient presented a paresis of the left arm related to an embolic infarction in the right precentral gyrus. Both patients underwent preprocedural TEE with no evidence of LA/LAA thrombi. Both were treated conservatively, and the symptoms resolved at the 3 months follow up. In both cases, no charring of the catheter was observed, and the operators suspected difficulties in handling of the Guidestar delivery sheath as the probable origin of the cardioembolic complications. One patient (0.7%) experienced a cardiac tamponade that was treated with pericardiocentesis; one patient developed a phrenic nerve palsy (PNP) during ablation of an RSPV. To notice, this was the third patient treated with the RFB in one of the two centres, and no pacing from the balloon surface to detect PN proximity was performed. In general, all complications happened in the first 25 patients treated, since patient 25 in both centres recorded no further major complication. After case 25, the only complication consisted was a groin haematoma: a false aneurysm was diagnosed and treated with a thrombin injection.
Table 2.
Safety events
| Safety events | |
|---|---|
| Cardiac tamponade, n (%) | 1/140 (0.7) |
| Vascular complications, n (%) | 1/140 (0.7%) |
| Stroke, n (%) | 2/140 (1.4%) |
| Acute PV stenosis/narrowing, n (%) | 0/140 (0%) |
| Phrenic nerve paralysis, n (%) | 1/140 (0.7%) |
| Death, n (%) | 0/140 (0%) |
PV, pulmonary vein.
Oesophageal temperature monitoring
A temperature rise >39°C, which was defined as a cutoff to prematurely discontinue RFC delivery at the posterior electrodes, was recorded in 81/547 (15%) PVs and in 62/140 (44%) patients, most commonly at the LIPV [28% vs. LSPV (17%)], RIPV (11%), and RSPV (1%)).
Procedural data
The overall median skin to skin procedure time was 77 min (61–99), and the overall median RFB dwell time was 30 min (21–44). In one centre, the procedure time was significantly shorter (63(53–76) vs. 94(80–115), P = 0.0001), but the difference in the balloon dwell time was less pronounced [27(19–33) vs. 34(25–49), P = 0.0014]. The median fluoroscopy time was 13 min (10–17).
Post-procedural oesophago-gastroscopy
An oesophageal endoscopy was performed in unselected 85 patients after a median of 1 day after the procedure. Seven thermic endoscopy-detected esophageal lesions (EDEL) (7/85–8%) were recorded, all consisting in ulcers of 0.2–1 cm in diameter. The biggest ulcer was described in a patient with a marked temperature rise (47°C) during ablation at the LIPV, when the LET acoustic alarm did not work properly. A control endoscopy after 4 weeks revealed an almost complete healing of this lesion. Six out of seven patients (86%) with EDEL had a temperature rise in the oesophagus, vs. 31/78 (40%) patients without EDEL. Patients with EDEL were in trend treated with more applications at the left-sided PVs (7 ± 3 vs. 4 ± 3, P = 0.07), with significantly more applications at the LSPV (5 ± 3 vs. 2 ± 2, P = 0.04) compared to patients without EDEL (Table 3). Procedural time in patients with EDEL was significantly longer.
Table 3.
Procedural data of patients with EDEL
| EDEL | No EDEL | P | |
|---|---|---|---|
| Number of patients, n (%) | 7/85 (8%) | 78/85 (92%) | |
| Total number of applications per patient | 11 ± 5 | 8 ± 4 | 0.146 |
| Number of application left PVs | 7 ± 3 | 4 ± 3 | 0.068 |
| Number of application right PVs | 4 ± 2 | 4 ± 2 | 0.938 |
| LSPV total applications | 5 ± 3 | 2 ± 2 | 0.043 |
| LIPV total applications | 2 ± 1 | 2 ± 2 | 0.609 |
| LCPV total applications | 11 | 2 ± 1 | NA |
| RSPV total applications | 2 ± 1 | 2 ± 1 | 0.502 |
| RIPV total applications | 2 ± 1 | 2 ± 1 | 0.524 |
| Oesophageal temp rise >39°C | 6/7 (86%) | 31/78 (40%) | 0.040 |
| T rise left-PVs | 5/7 (71%) | 21/78 (27%) | 0.026 |
| T rise right-PVs | 2/7 (29%) | 10/78 (13%) | 0.256 |
| Procedure time (min) | 105 ± 31 | 75 ± 27 | 0.007 |
| LA balloon time (min) | 46 ± 22 | 32 ± 18 | 0.058 |
EDEL, endoscopy-detected esophageal lesions; LA, left-atrial; LCPV, left common pulmonary; LIPV, left interior pulmonary vein; vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right interior pulmonary veins; RSPV; right superior pulmonary vein.
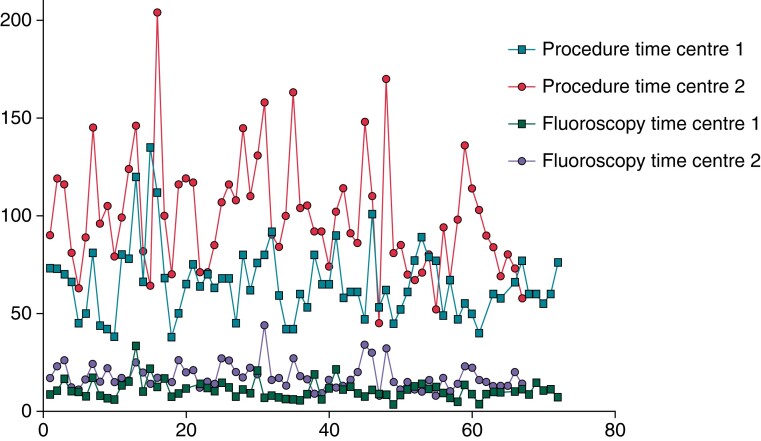
Learning curve
No clear learning curve effect, expressed by significant changes in procedure time, or fluoroscopy times could be noticed for both centres (Figure 4). An analysis of the performance of the two centres using the RFB was performed based on tertile subdivision of each centre experience. No significant difference was found in procedure time, fluoroscopy time, and number of RFB applications per patient (Supplemental Material 1). The rate of single-shot isolation increased numerically among the tertiles of experience in the two centres (centre 1: 57%, 67%, and 72%; centre 2: 53%, 52%, and 58%) without reaching a statistical significance.
Figure 4.
Learning curve regarding procedural and fluoroscopy time in the two centres.
Discussion
To the best of our knowledge, this is the largest report on the acute efficacy and safety of consecutive patients treated with the recently released RFB. Main findings are (i) a high procedure efficacy with 99.1% PVs isolated using solely the RF-Balloon; (ii) single-shot isolation recorded in the 60% of PVs after a median of 10 s; (iii) a safety profile in line with other balloon technologies, with a call for caution about exchanging mapping and ablation catheters in the large RF balloon delivery sheath; (iv) a call for the reintroduction of oesophageal temperature measurement during RFB-PVI; and (v) procedure time in line to what is reported in other multicentric experiences using different technologies.
Procedural efficacy
We recorded a very high rate of PV isolation using solely the newly introduced RFB. Compared to other balloon technologies, the RFB displayed an acute PV isolation rate similar to the cryoballoon10 and laserballoon,11 despite including the learning curve of multiple operators. With the cryoballoon technology, the contact of the balloon with the PV antrum is assessed via an occlusion PV angiogram; furthermore, the temperature reached during freezing is considered a marker of complete occlusion.12 The laserballoon technology relies on direct visualization of the PV antrum.13 With the RFB, the electrodes on its surface give operators impedance values that quantify the grade of contact of the ablation-electrodes with the target tissue. Based on previous studies,8,9 the operators are now instructed to achieve a minimal impedance value of >90 Ω to deliver optimal RF current to the tissue. As a consequence, 60% of all PVs in this report could be isolated with a single circumferential RFB application of 60 s.
Such a short application duration could lead to a reduction of the ablation time compared to the cryoballoon (180–240 s per PV) or the laserballoon (160 s per PV with the current ×3 generation14).
Rapid real time PV isolation (after a median of 10 s) was recorded in most of treated PVs. This may translate into very short procedure times, comparable to other single-shot PVI technologies such as pulsed field ablation.15
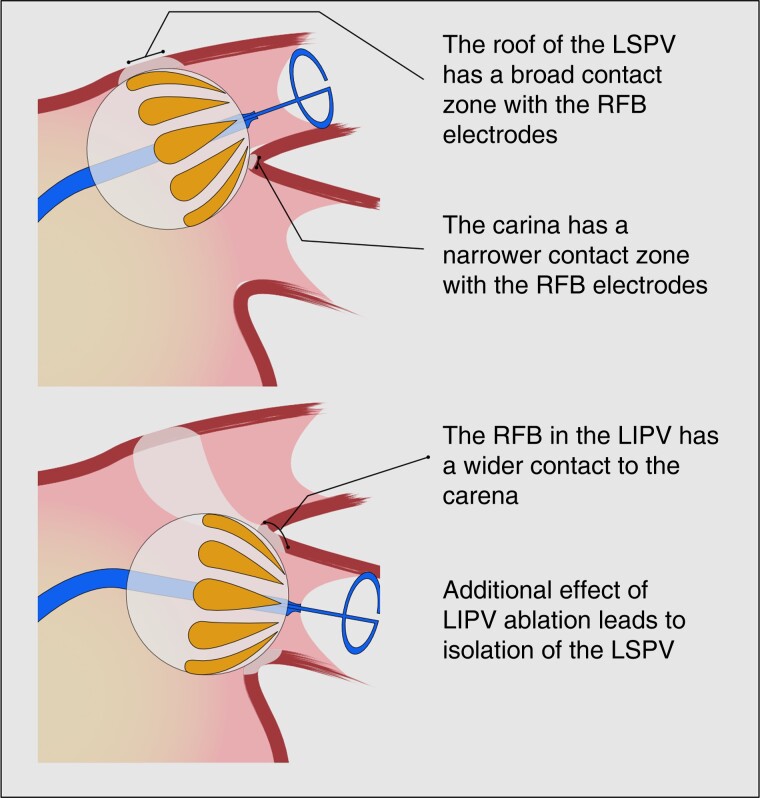
To notice, the current 60% rate of single-shot PVI is inferior to the 74% reported in the SHINE study. This difference is difficult to be explained, but may involve different operators, different generation of the RFB, or difference in patients’ characteristics/anatomy. Among the PVs acutely isolated in this study, excluding left common PVs, the LSPV demonstrated to be the PV recording the greatest number of applications to be isolated and the lowest rate of single-shot PV isolation. The reasons are probably to find in the anatomy of the LSPV. The ridge to the LAA anterior and the carina to the LIPV inferior are thick and edgy structures, with a reduced amount of tissue in contact with the RFB electrodes, probably leading to a greater amount of energy dissipated in the blood flow. Stability of the catheter may also play a role: different from cryoablation, RF energy delivery does not cause adhesion of the balloon to the tissue, increasing the probability of catheter instability at this location. Therefore, the carina may require an RFB application from the LIPV to be blocked (crosstalk manoeuvre—Figure 5), but the ridge to the LA appendage may simply require multiple or longer applications to achieve a transmural lesion.
Figure 5.
A graphical representation of the cross talk manoeuvre, used to overcome the narrow contact zone between the balloon and the carena.
In this study, 13 left common PVs were identified: interestingly, all LCPVs could be isolated with the RFB. Even if more applications were required, the RFB demonstrated to adapt to different anatomical scenarios. The possibility to vary the inflation flow and the RFB compliancy may be useful in the context of PVs with smaller diameter and/or elliptical ostia; the combination with the 3D mapping system may allow to deliver sequential segmental ablations in case of PVs bigger than 28 mm.
Procedural safety
We recorded a single cardiac tamponade that required percardiocentesis: the involved operator could not indicate a specific cause for this mechanical complication, since no steam pop was audible and the transseptal access was uneventful. The two recorded strokes should be a signal of caution. The probable association to the handling of the Guidestar transseptal sheath led to a redesign of the haemostatic valve to avoid leakage and air embolisms. Additionally, an ACT >350 s was targeted after these complications occurred. To notice, the generation of the Lasso-Star used in this report was without a magnetic sensor, so the 3D map was obtained via an exchange for a standard multipolar Lasso-Nav catheter in the 14F Guidestar. The newly released Lasso-Star-Nav spiral catheter will avoid the need of exchanging catheters within the transseptal sheath, potentially reducing the risk for air embolisms.
Phrenic nerve palsy is a common complication of balloon technologies for PVI. In the current report, we recorded 1/140 PNP (0.7%). A large multicentric registry using the CB revealed a rate of PN injury of 4.2%, with a palsy at discharge still present in 1.9%.16 Compared to the experience with the CB, thermal lesions using RF energy may have a smaller time window for reversible PN injury, as demonstrated for the laserballoon.17 In this context, prevention of PN injury remains crucial: the stimulation from the RFB electrodes oriented anteriorly should be mandatory as well as continuous stimulation from the superior vena cava during ablation of the septal PVs.
Applying RF energy at the posterior wall of the left atrium is always concerning due to the proximity to the oesophagus. Despite switching off RF energy delivery at the posterior wall after 20 s, a temperature rise above 39°C was recorded in 14% of targeted PVs. Seven EDEL were recorded among the 85 patients undergoing post-procedural endoscopy, a lower rate compared to the 12.5% previously described in the RADIANCE study. Interestingly, EDEL were detected in patients with more applications delivered at the LSPV. Using RF energy and a point-by-point ablation, randomized data showed no benefit for the routine use of an oesophageal temperature probe;18 conversely, the use of a LET cutoff during CB PVI demonstrated to reduce the rate of EDEL.19 In the current report, the biggest EDEL was found in a patient with an unnoticed increase of LET: we therefore think that an oesophageal temperature monitoring should be suggested. Further studies focusing on energy titration at the posterior wall and safety/efficacy are needed.
This is a call for the reintroduction of LET measurement, abandoned by many centres using RF ablation, in patients undergoing RFB PVI. Nearby, following the observation that lesions delivered adjacent to each other may result in ‘heat stacking’,20 a waiting time between two consecutive applications should be considered to reduce the increase of LET.
Procedural data
Single-shot devices were introduced to ease and facilitate PVI. The Fire and Ice trial demonstrated how the CB could reduce the procedure time compared to RF ablation even in high volume centres, at the cost of an increased fluoroscopy exposure.5 The procedure duration using the RFB in this report is in line with modern single-shot ablation technologies with median procedure times of 63 and 90 min in the two centres involved. Interestingly, the difference in the balloon LA time was less marked, confirming how balloon technologies can homogenize the experience of different centres.4 The residual time consists in the preparation time and in the 3D mapping time. The recent introduction of an inner lumen spiral catheter equipped with a magnetic sensor may impact the duration of these phases, potentially further reducing the skin-to-skin time.
Limitations
The current study reports on a collective of consecutive patients undergoing RFB PVI in two high volume centres, the results are preliminary, and include multiple learning curves. The use of segmental applications was not systematically collected so no analysis on this topic could be performed. No waiting time after PVI was applied and no adenosin challenge was performed, so the true rate of acute PVI may be overestimated. The patients undergoing oesophagoscopy were unselected but bias cannot be excluded. During the study, several technological updates were delivered by the manufacturer in response to the operator’s suggestions; if the efficacy and safety profile will be impacted by these, changes should be subject to further investigations.
Conclusions
In this multicentric study, we recorded a high procedural efficacy of the RFB for PVI, with 99% of PVs successfully isolated with the RFB and single-shot isolation in 60% of PVs. The LSPV seems to require more applications compared to the other PVs. After a short learning curve (25 cases), the safety profile seems to be favourable, but caution in the handling of the large diameter sheath is warranted. Oesophageal lesions were detected in 8% of patients: the use of a temperature probe is advised.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
Stefano Bordignon, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Ilaria My, Universitäres Herz- und Gefäßzentrum - Klinik für Kardiologie - Universitätsklinikum Hamburg-Eppendorf, Germany.
Shota Tohoku, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Andreas Rillig, Medizinische Klinik 3 - Universitätsklinikum der Goethe Universität, Frankfurt, Germany.
David Schaack, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Shaojie Chen, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Bruno Reißmann, Medizinische Klinik 3 - Universitätsklinikum der Goethe Universität, Frankfurt, Germany.
Lukas Urbanek, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Jun Hirokami, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Tolga Efe, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Ramin Ebrahimi, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Mahi Butt, Universitäres Herz- und Gefäßzentrum - Klinik für Kardiologie - Universitätsklinikum Hamburg-Eppendorf, Germany.
Feifan Ouyang, Universitäres Herz- und Gefäßzentrum - Klinik für Kardiologie - Universitätsklinikum Hamburg-Eppendorf, Germany.
Julian K R Chun, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany.
Andreas Metzner, Universitäres Herz- und Gefäßzentrum - Klinik für Kardiologie - Universitätsklinikum Hamburg-Eppendorf, Germany.
Boris Schmidt, Medizinische Klinik III, CCB am Agaplesion Markus Krankenhaus, Frankfurt am Main, Germany; Medizinische Klinik 3 - Universitätsklinikum der Goethe Universität, Frankfurt, Germany.
Funding
This register is partially founded by an unrestricted research grant from Biosense Webster. T.E. received a fellowship grant from the European Heart Rhythm Association.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JEet al. . Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metzner A, Kuck K-H, Chun JKR. What we have learned: is pulmonary vein isolation still the cornerstone of atrial fibrillation ablation? EP Europace 2022;24:ii8–13. [DOI] [PubMed] [Google Scholar]
- 4. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi Fet al. . Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
- 5. Kuck K-HH, Fürnkranz A, Chun KRJKRJ, Metzner A, Ouyang F, Schlüter Met al. . Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. . Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 7. Boersma L. New energy sources and technologies for atrial fibrillation catheter ablation. Europace 2022;24:ii44–51. [DOI] [PubMed] [Google Scholar]
- 8. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini Fet al. . Safety, effectiveness, and quality of life following pulmonary vein isolation with a multi-electrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhillon GS, Honarbakhsh S, di Monaco A, Coling AE, Lenka K, Pizzamiglio Fet al. . Use of a multi-electrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol 2020;31:1259–69. [DOI] [PubMed] [Google Scholar]
- 10. Bordignon S, Chen S, Bologna F, Thohoku S, Urbanek L, Willems Fet al. . Optimizing cryoballoon pulmonary vein isolation: lessons from >1000 procedures— the Frankfurt approach. Europace 2021;23:868–77. [DOI] [PubMed] [Google Scholar]
- 11. Dukkipati SR, Cuoco F, Kutinsky I, Aryana A, Bahnson TD, Lakkireddy Det al. . Pulmonary vein isolation using the visually guided laser balloon: a prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol 2015;66:1350–60. [DOI] [PubMed] [Google Scholar]
- 12. Andrade JG. Cryoablation for atrial fibrillation. Heart Rhythm O2 2020;1:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt B, Gunawardene M, Urban V, Kulikoglu M, Schulte-Hahn B, Nowak Bet al. . Visually guided sequential pulmonary vein isolation: insights into techniques and predictors of acute success. J Cardiovasc Electrophysiol 2012;23:576–82. [DOI] [PubMed] [Google Scholar]
- 14. Tohoku S, Bordignon S, Chen S, Zanchi S, Bianchini L, Trolese Let al. . Single-sweep pulmonary vein isolation using the new third-generation laser balloon—evolution in ablation style using endoscopic ablation system. J Cardiovasc Electrophysiol 2021;32:2923–32. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt B, Bordignon S, Tohoku S, Chen S, Bologna F, Urbanek Let al. . 5S Study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol 2022;15:E010817. [DOI] [PubMed] [Google Scholar]
- 16. Heeger C-H, Sohns C, Pott A, Metzner A, Inaba O, Straube Fet al. . Phrenic nerve injury during cryoballoon-based pulmonary vein isolation: results of the worldwide YETI registry. Circ Arrhythm Electrophysiol 2022;15:e010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tohoku S, Chen S, Last J, Bordignon S, Bologna F, Trolese Let al. . Phrenic nerve injury in atrial fibrillation ablation using balloon catheters: incidence, characteristics, and clinical recovery course. J Cardiovasc Electrophysio 2020;31:1932–41. [DOI] [PubMed] [Google Scholar]
- 18. Schoene K, Arya A, Grashoff F, Knopp H, Weber A, Lerche Met al. . Oesophageal probe evaluation in radiofrequency ablation of atrial fibrillation (OPERA): results from a prospective randomized trial. Europace 2020;22:1487–94. [DOI] [PubMed] [Google Scholar]
- 19. Fürnkranz A, Bordignon S, Böhmig M, Konstantinou A, Dugo D, Perrotta Let al. . Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm Elsevier 2015;12:268–74. [DOI] [PubMed] [Google Scholar]
- 20. Steiger N, Qian PC, Foley G, Bomma T, Kreidieh O, Whitaker Jet al. . Measured temperatures using uninterrupted and interrupted sequences of radiofrequency applications in a phantom gel model: implications for esophageal injury. J Interv Card Electrophysiol 2022; Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.