Abstract
Background
Patients with hypertrophic cardiomyopathy (HCM) and atrial fibrillation (AF) are at increased stroke risk in comparison to those with non-valvular AF not affected by HCM.
Objectives
To investigate the role of left atrial appendage closure (LAAC) in patients with HCM and AF.
Methods and results
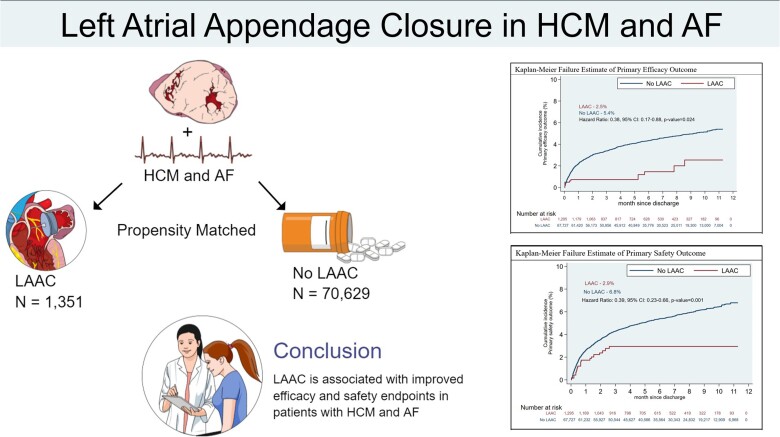
We identified patients with HCM and AF using the National Readmission Dataset. Patients were stratified based on LAAC status. The primary efficacy outcome was a composite of ischaemic and haemorrhagic stroke, TIA, and all-cause mortality. The primary safety outcome was a composite of major bleeding and pericardial complications. Patients were matched using inverse probability of treatment weighting. Cox-proportional hazard regression was applied to calculate the hazard ratio (HR) with a 95% confidence interval (CI) on matched cohorts. We identified 71 980 patients with HCM and AF. 1351 (1.9%) patients underwent LAAC. Two hundred and eighty-seven (21.2%) underwent transcatheter LAAC. LAAC was associated with a lower risk of the primary efficacy outcome (2.5% vs. 5.4%, HR: 0.38; 95% CI: 0.17–0.88; P = 0.024), the primary safety outcome (2.9% vs. 6.8%, HR: 0.39; 95% CI: 0.23–0.66, P = 0.001), and reduced major bleeding. The LAAC group trended towards a lower risk of ischaemic stroke and all-cause mortality.
Conclusion
Surgical and transcatheter LAAC was associated with a lower risk of haemorrhagic stroke and major bleeding.
Keywords: Atrial fibrillation, Hypertrophic cardiomyopathy, Left atrial appendage closure, Stroke, Bleeding
Graphical Abstract
Graphical Abstract.
What’s new?
Hypertrophic cardiomyopathy patients with AF who undergo LAAC have a significantly lower composite endpoint of ischaemic or haemorrhagic stroke, TIA, and mortality.
Hypertrophic cardiomyopathy and AF patients who undergo LAAC also have a lower composite endpoint of major bleeding and pericardial complications.
Left atrial appendage closure patients were likely to be younger, have coagulopathy, obesity, and were treated in large hospitals or teaching hospitals.
Amongst patients who did not undergo concurrent myectomy, LAAC was still associated with a reduction in the primary efficacy outcome, suggesting that LAAC is efficacious independent of septal myectomy.
We observed a lower rate of heart failure admissions in the LAAC group, which is counter to previously reported data.
Introduction
Atrial fibrillation (AF) represents the most common arrhythmia in patients with hypertrophic cardiomyopathy (HCM), presenting in up to 28% of patients.1 AF has been identified as an independent predictor of mortality in HCM patients due to stroke, thromboembolism, and heart failure.1–3 Ischaemic strokes are up to eight times more common when HCM patients are in AF than when they are in sinus rhythm.1 Traditional stroke risk prediction models, such as the CHA2DS2-VASc score, have failed to demonstrate high predictive accuracy for ischaemic strokes in HCM patients with a significant number of strokes occurring in patients with a CHA2DS2-VASc score of 0.2,4 The factors that have been associated with a high risk of systemic thromboembolism in HCM patients with AF include left atrial size, increasing age, heart failure symptoms, and vascular disease.4 Additionally, the stroke risk in HCM patients with AF is independent of the duration of AF episodes (paroxysmal or permanent) or the number of paroxysmal episodes.5
The 2020 EHRA/EAPCI Expert Consensus Statement on Catheter-Based Left Atrial Appendage Occlusion supports the use of percutaneous LAAC in patients with an increased stroke risk and a contraindication to long-term anticoagulation.6 The 2019 AHA/ACC Focused Update of the 2014 Guidelines for the Management of Patients with AF assigns a class IIb indication for LAAC in this population.7 LAAC has demonstrated clinical benefits in large, prospective, randomized controlled trials, with respect to stroke prevention, cardiac mortality, and all-cause mortality in patients with non-valvular AF, however, HCM patients are not-represented in these studies.7 Additionally, careful patient selection for LAAC is important as those with heart failure have been demonstrated to suffer from adverse effects when undergoing LAAC.8 Furthermore, for patients with AF undergoing cardiac surgery, LAAC at the time of surgery has demonstrated a reduction in stroke or systemic thromboembolism risk.9
The 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy recommends anticoagulation for systemic thromboembolism prevention in HCM patients with AF.5 However, no recommendations were made for LAAC due to the under-representation of HCM patients in trials evaluating the safety and efficacy of transcatheter LAAC. Similarly, there is a paucity of data regarding the efficacy of surgical LAAC in HCM patients.
Given the high prevalence of AF in HCM patients, the associated high morbidity and mortality, and the under-representation of HCM patients in LAAC studies, we sought to use this retrospective cohort to evaluate the efficacy and safety of LAAC in HCM patients with AF.
Methods
Data source
Data were extracted from 2016 to 2019 Nationwide Readmission Database (NRD). NRD is an all-payer database, a subset of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality. NRD from 2016–19 contains data from approximately 17 million discharges per year, across 28 geographically dispersed states. This dataset accounts for 60% of the total U.S. resident population and 58.2% of all U.S. hospitalizations.10 The NRD utilizes a de-identified unique number for every patient, tracking patients and determining readmissions across hospitals within a calendar year. NRD has been studied and validated in multiple previous studies.11,12 Due to the use of de-identified patients, institutional review board approval was not required. This study followed the reporting guidelines specified by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.13
Patient selection
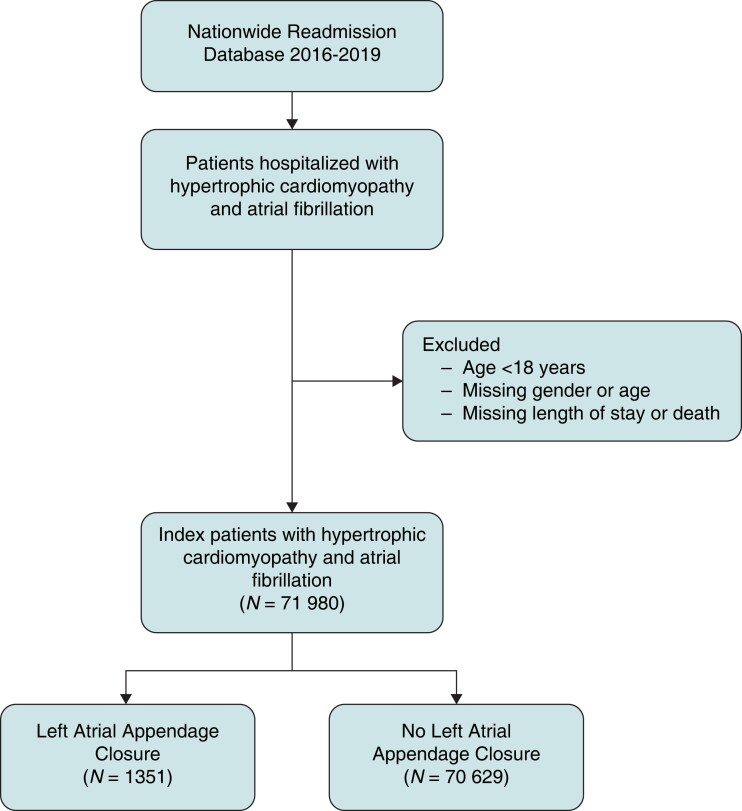
We identified patients with HCM using previously described ICD-10 CM codes (I42.1, I42.2) in the primary or secondary diagnosis fields.14 AF was identified using appropriate ICD-10 CM codes (I48) in either the primary or secondary diagnosis fields. Patients with missing information on age, gender, mortality, length of stay, or age <18 were removed (Figure 1).
Figure 1.
Patient selection and study design. Depiction of the patient selection algorithm and study design that was implemented.
Baseline variables
We used the variables provided in the NRD by HCUP to identify baseline characteristics including age, sex, hospital characteristics (location, bed size, and teaching status), and patient-specific aspects including primary payer.15,16 We utilized the ICD-10-CM codes provided by the Elixhauser comorbidity index calculator given by HCUP to identify obesity, hypertension, diabetes, heart failure, chronic lung disease, chronic liver disease, alcohol disorder, peripheral vascular disease, cancer, coagulopathy, and anaemia (see Supplementary material online, Table S1).17,18 Other comorbidities such as chronic kidney disease stage 3 or more (CKD), prior coronary artery bypass grafting (CABG), prior percutaneous coronary intervention (PCI), hyperlipidaemia, previous stroke/transient ischaemic attack, and tobacco use were identified using appropriate ICD-10-CM codes (see Supplementary material online, Table S1).
Intervention & outcomes
The intervention of interest was LAAC (surgical or percutaneous). LAAC was identified using appropriate ICD-10 PCS codes (02L7) in either the primary or secondary procedure codes. The primary efficacy outcome was a composite of the incidences of any (ischaemic or haemorrhagic) stroke, transient ischaemic attack (TIA), and all-cause mortality. The primary safety outcome was a composite of major bleeding (composite of gastrointestinal bleeding, intracerebral haemorrhage (ICH), hospitalization for bleeding, and blood transfusion), pericardiocentesis, and cardiac tamponade. Individual primary outcomes and heart failure readmissions were considered secondary outcomes. Details regarding outcomes are provided in Supplementary material online, Table S2.
Statistical analysis
We presented categorical variables as numbers and percentages and continuous variables as means with standard deviation or medians with interquartile range (IQR). Categorical variables were compared between the two groups using Chi-square, while continuous variables were compared using the t-test or Wilcoxon rank-sum test. A non-parsimonious multivariable logistic regression model was developed to estimate the propensity score using all variables mentioned in Table 1 to match patients with or without LAAC. Then, a double-robust method was used to generate treatment weights, and the inverse probability of the treatment weighting method was applied to match patients. Time-to-event was determined from discharge date to readmission date for all outcomes. If patients were not readmitted, they were censored, and censored time was calculated from discharge month to the end of the year (see Supplementary material online, Table S3). On matched cohorts, Cox-proportional hazard regression was applied to calculate the hazard ratio (HR) with a 95% confidence interval (CI) for post-discharge outcomes. The global test of proportionality assumption was not violated (global test P > 0.05). Kaplan–Meier graphs were constructed and Kaplan–Meier estimates were calculated for outcomes. The balance of baseline variables before and after matching are presented in Supplementary material online, Figure S1. A two-tailed P-value of 0.05 was designated as statistically significant. Interaction testing was performed between LAAC and surgical myectomy on heart failure readmissions. We adhered to the methodological standards of HCUP. All statistical analyses were conducted using appropriate weighting, stratifying, and clustering samples to obtain national estimates using the svy package of STATA, version 16.1 (StataCorp, College Station, TX).
Table 1.
Baseline characteristics
| Patient populations | No-LAAC | LAAC | All patients | P-valuea |
|---|---|---|---|---|
| 70 629 | 1351 | 71 980 | ||
| Age, mean ± SD | 70 ± 13.1 | 67.5 ± 10.1 | 69.9 ± 13.1 | <0.001 |
| Sex | <0.001 | |||
| Male | 46.6% | 54.6% | 46.7% | |
| Female | 53.4% | 45.4% | 53.3% | |
| Comorbiditiesb | ||||
| Hypertension | 82.1% | 82.8% | 82.1% | 0.678 |
| Diabetes | 28.4% | 27.2% | 28.4% | 0.449 |
| Hyperlipidaemia | 56.1% | 64.9% | 56.3% | <0.001 |
| Heart failure | 57.5% | 51.0% | 57.4% | 0.001 |
| Prior PCI | 7.5% | 7.3% | 7.4% | 0.843 |
| Prior CABG | 4.9% | 3.2% | 4.8% | 0.030 |
| Stroke/TIA | 12.3% | 12.6% | 12.3% | 0.800 |
| Chronic lung disease | 30.4% | 21.6% | 30.2% | <0.001 |
| Chronic kidney disease | 30.5% | 22.1% | 30.3% | <0.001 |
| Chronic liver disease | 5.9% | 5.7% | 5.9% | 0.843 |
| Peripheral vascular disease | 25.4% | 23.8% | 25.4% | 0.368 |
| Tobacco use disorder | 27.9% | 35.7% | 28.1% | <0.001 |
| Coagulopathy | 11.2% | 28.3% | 11.5% | <0.001 |
| Obesity | 21.4% | 27.7% | 21.6% | <0.001 |
| Cancer | 6.2% | 1.3% | 5.2% | <0.001 |
| Anaemia | 6.4% | 5.9% | 6.4% | 0.560 |
| CHA2DS2-VASc | 4.1 ± 1.7 | 3.9 ± 1.7 | <0.001 | |
| Elixhauser comorbidity index, median (IQR) | 5 (4–7) | 6 (4–7) | 0.272 | |
| Concurrent procedures | ||||
| Myectomy | 2.2% | 19.5% | 2.57% | |
| CABG | 1.5% | 18.5% | 1.84% | |
| Transcatheter aortic valve replacement | 0.5% | <0.8% | <0.51% | |
| Surgical aortic valve replacement | 1.6% | 19.4% | 1.9% | |
| Mitral valve surgery | 2.1% | 40.1% | 2.8% | |
| Pulmonary valve surgery | <0.02% | <0.8% | <0.1% | |
| Tricuspid valve surgery | 0.2% | 4.7% | 0.3% | |
| Atrioventricular septal closure | 0.2% | 3.0% | 0.3% | |
| Primary payer | <0.001 | |||
| Private/self | 22.9% | 31.8% | 23.1% | |
| Medicare/Medicaid | 77.1% | 68.3% | 76.9% | |
| Hospital size | <0.001 | |||
| Small/medium | 40.9% | 22.1% | 40.6% | |
| Large | 59.1% | 77.9% | 59.4% | |
| Teaching hospitalc | <0.001 | |||
| Non-teaching | 24.2% | 14.2% | 24.0% | |
| Teaching | 75.8% | 85.9% | 76.0% | |
| Hospital location | 0.169 | |||
| Non-urban | 40.3% | 36.8% | 40.2% | |
| Urban | 59.7% | 63.2% | 59.8% | |
| Income per zip code | 0.068 | |||
| 0–25th | 23.3% | 20.7% | 23.2% | |
| 26–50th | 25.3% | 27.0% | 25.4% | |
| 51 = 75th | 26.0% | 22.8% | 25.9% | |
| 76–100th | 24.1% | 27.6% | 24.2% |
Values are mean ± SD or %.
The P-value comparing LAAC to no-LAAC.
International classification of diseases-10th revision codes was used to identify respective comorbidities as per Supplementary material online, Table S2. The bed size cut-off points divided into small, medium, and large have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category (https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp).
A hospital is considered to be a teaching hospital if it has an American Medical Association–approved residency programme (https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.).
CABG, coronary artery bypass graft; IQR, interquartile range; LAAC, left atrial appendage closure; PCI, percutaneous coronary intervention; SD, standard deviation; TIA, transient ischaemic attack.
Results
A total of 71 980 patients were included in the final analysis, of which 1351 (1.9) underwent LAAC (Table 1). 287 (21.2%) underwent transcatheter LAAC. The mean age of the cohort was 69.9 ± 13.1 years and 53.3% were female. The most common comorbidities were hypertension (82.1%), heart failure (57.4%), hyperlipidaemia (56.3%), CKD (30.3%), and chronic lung disease (30.2%). The median follow-up was 165 days.
Those who underwent LAAC were significantly younger than those who did not (67.5 ± 10.1 years vs. 70.0 ± 13.1 years, P < 0.001) and were more likely to be male than female (54.6% vs. 45.4%, P < 0.001). Patients who underwent LAAC were more likely to have a history of coagulopathy (28.3% vs. 11.2%, P < 0.001), obesity (27.7% vs. 21.4% P < 0.001), tobacco use disorder (35.7% vs. 27.9%, P < 0.001), and hyperlipidaemia (64.9% vs. 56.1%, P < 0.0001). Additionally, patients who underwent LAAC were more likely to be admitted to large hospitals (77.9% vs. 59.1%, P < 0.001) or teaching hospitals (85.9% vs. 75.8%, P < 0.001). They were less likely to have a history of heart failure (51.0% vs. 57.5%, P = 0.001), prior CABG (3.2% vs. 4.9%, P-0.030), chronic lung disease (21.6% vs. 30.4%, P < 0.001), CKD (22.1% vs. 30.5%, P < 0.001), or cancer (1.3% vs. 6.2%, P < 0.001) as compared to patients who did not undergo LAAC (Table 1).
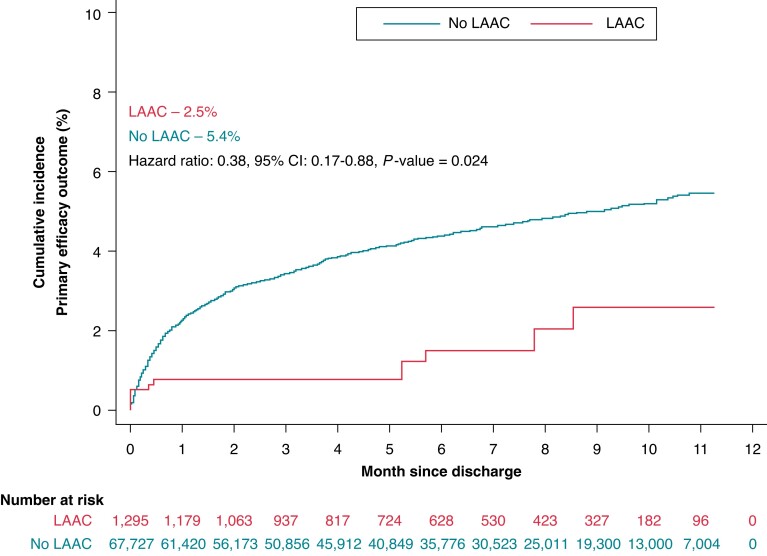
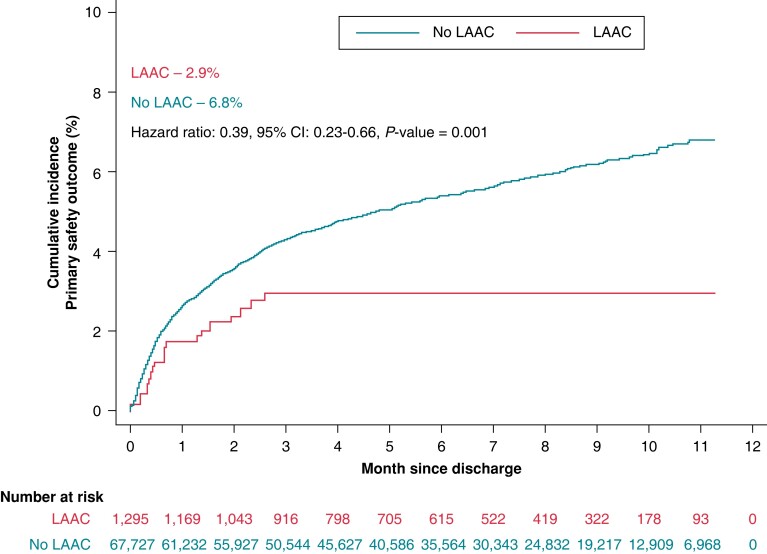
The primary efficacy outcome (a composite of ischaemic or haemorrhagic stroke, TIA, and mortality) was significantly lower in patients who underwent LAAC (2.5% vs. 5.4%, HR: 0.38; 95% CI: 0.17–0.88; P = 0.024) compared to those who did not (Table 2, Central Illustration). The primary safety outcome was significantly lower in patients who underwent LAAC (2.9% vs. 6.8%, HR 0.39; 95% CI: 0.23–0.66, P = 0.001) (Table 2, Figure 2).
Table 2.
Primary and secondary outcomes by LAAC status
| No-LAAC | LAAC | IPTW method | P-value | |
|---|---|---|---|---|
| n = 70 629 | n = 1351 | HR (95% CI) | ||
| Primary efficacy outcome | 5.4% | 2.5% | 0.38 (0.17–0.88) | 0.024 |
| Primary safety outcome | 6.8% | 2.9% | 0.39 (0.23–0.66) | 0.001 |
| Ischaemic stroke | 1.9% | 0.8% | 0.20 (0.04–1.12) | 0.067 |
| Intracerebral haemorrhage | 0.2% | 0.0% | NA | <0.001 |
| All-cause mortality | 3.5% | 2.6% | 0.48 (0.19–1.23) | 0.124 |
| Major bleeding | 6.0% | 1.6% | 0.34 (0.18–0.64) | 0.001 |
| Gastrointestinal bleeding | 1.3% | 0.2% | 0.22 (0.06–0.79) | 0.020 |
| Blood transfusions | 4.90% | 2.10% | 0.37 (0.19–0.73) | 0.004 |
| Admission for bleeding | 2.30% | 0.50% | 0.20 (0.07–0.58) | 0.003 |
| Pericardiocentesis | 0.30% | 0.20% | 0.44 (0.06–3.19) | 0.42 |
| Cardiac tamponade | 0.30% | 0.50% | 1.33 (0.41–4.35) | 0.635 |
| Heart failure hospitalizations | 13.2% | 6.4% | 0.52 (0.33–0.82) | 0.005 |
The primary efficacy outcome was a composite of ischaemic or haemorrhagic stroke, TIA, and/or mortality.
The primary safety outcome was a composite of major bleeding, pericardiocentesis, and pericardial tamponade.
Major bleeding was a composite of intracranial haemorrhage, gastrointestinal bleeding, blood transfusion, and/or admission for bleeding.
Cumulative percentages using Kaplan–Meier curve time-to-event analysis.
Cox-proportional hazards regression model was used to generate hazard ratios.
CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LAAC, left atrial appendage closure.
Central illustration.
Kaplan-Meier Failure Estimate of Primary Efficacy Outcome. Time-to-event analysis of primary efficacy outcome of stroke (ischemic or hemorrhagic), TIA, and mortality comparing LAAC (red) with no-LAAC (blue). Event rates represent Kaplan Meier estimates. CI – confidence interval; HR – hazard ratio; LAAC – left atrial appendage closure.
Figure 2.
Kaplan–Meier failure estimate of primary safety outcome. Time-to-event analysis of primary safety outcome of major bleeding (gastrointestinal bleeding, intracerebral haemorrhage, hospitalization for bleeding, and blood transfusion), pericardiocentesis, and cardiac tamponade comparing LAAC (red) with no-LAAC (blue). CI, confidence interval; HR, hazard ratio; LAAC, left atrial appendage closure.
Individually, ischaemic stroke (0.8% vs. 1.9%, HR 0.20; 95% CI: 0.04–1.12, P = 0.067) and all-cause mortality (2.6% vs. 3.5%, HR: 0.48; 95% CI: 0.19–1.23; P = 0.124) were numerically lower with LAAC but did not reach statistical significance. LAAC was associated with lower rates of ICH (0.0% vs. 0.2%; P < 0.001), major bleeding (1.6% vs. 6.0%, HR 0.34, 95% CI 0.18–0.64, P = 0.001), GI bleeding (0.2% vs. 1.3%, HR 0.22; 95% CI: 0.06–0.79, P = 0.020), blood transfusions requiring admission (2.10% vs. 4.90%, HR 0.37, 95% CI 0.19–0.73, P = 0.004), and admissions for bleeding (0.50% vs. 2.30%, HR 0.20, 95% CI 0.07–0.58, P = 0.003). The incidence of pericardiocentesis and cardiac tamponade were similar in both groups. An exploratory secondary outcome of heart failure readmissions (6.4% vs. 13.2%, HR 0.52, 95% CI 0.33–0.82, P = 0.005), was lower with LAAC (Table 2). A significant interaction between LAAC and myectomy upon heart failure readmissions was not present (P-value for interaction = 0.76). LAAC without myectomy (HR 0.575, 95% CI 0.348–0.949, P = 0.030) and myectomy without LAAC (HR 0.495, 95% CI 0.328–0.745, P = 0.001) were both independently associated with reduced heart failure readmissions. The combination of LAAC and myectomy was also significantly associated with heart failure readmissions (HR 0.242, 95% CI 0.109–0.537, P < 0.001).
In the subgroup analysis, patients who underwent LAAC and had a history of septal myectomy (0.8% vs. 1.2%, HR: 0.27; 95% CI: 0.11–0.66; P = 0.004), non-concurrent myectomy (3.1% vs. 5.5%, HR: 0.37; 95% CI: 0.15–0.92; P = 0.033), and persistent AF (1.0% vs. 5.9%, HR: 0.07; 95% CI: 0.01–0.48; P = 0.007) had a significantly lower risk for the primary efficacy outcome (Table 3). Patients who underwent LAAC and had a history of obstructive HCM or paroxysmal AF had a lower risk for the primary efficacy outcome but did not reach statistical significance (Table 3).
Table 3.
Subgroup analysis of primary efficacy outcome
| Subgroups | n | No-LAAC | LAAC | IPTW method | |
|---|---|---|---|---|---|
| HR (95% CI) | P-value | ||||
| Obstructive HCM | 15 551 | 5% | 1.7% | 0.36 (0.10–1.24 | 0.104 |
| Septal myectomy | 1848 | 1.2% | 0.8% | 0.27 (0.11–0.66) | 0.004 |
| Non-concurrent myectomy | 33 593 | 5.5% | 3.1% | 0.37 (0.15–0.92) | 0.033 |
| Paroxysmal AF | 33 363 | 4.9% | 2.9% | 0.50 (0.20–1.24) | 0.133 |
| Persistent AF | 38 582 | 5.9% | 1.0% | 0.07 (0.01–0.48) | 0.007 |
The primary efficacy outcome was a composite of ischaemic or haemorrhagic stroke, TIA, and/or mortality.
Cumulative percentages using Kaplan–Meier curve time-to-event analysis.
Cox-proportional hazards regression model was used to generate hazard ratios.
CI, confidence interval; HCM, hypertrophic cardiomyopathy; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LAAC, left atrial appendage closure.
Discussion
Our study demonstrates that HCM patients with AF who undergo LAAC have a significantly lower composite endpoint of ischaemic or haemorrhagic stroke, TIA, and mortality which was primarily driven by a reduction in haemorrhagic stroke. There was also a significantly lower rate of the primary safety outcome in those who underwent LAAC. Interestingly, we observed a lower rate of heart failure admissions in the LAAC group, which is counter to previously reported data that LAAC may increase the rate of heart failure admissions.19 Those who underwent LAAC were likely to be younger and as young patients are more likely to undergo myectomy, this cohort includes a large proportion of surgical LAAC. In contrast, percutaneous LAAC is often more commonly performed in the elderly.20 We also found that these patients were more likely to be obese, a known risk factor for AF.7 Males underwent LAAC more frequently, a trend similar to past cohorts that exclusively underwent percutaneous LAAC.20 Patients who underwent LAAC were also more likely to have been treated in large or teaching hospitals, suggesting a referral bias for these complex HCM patients to tertiary institutions for multidisciplinary care.
We also found that patients with HCM, AF, and coagulopathy were more likely to undergo LAAC, a trend consistent with EHRA/EAPCI and AHA/ACC guidelines for the use of LAAC in patients unable to tolerate anticoagulation.6,7 The need for both blood transfusions and admissions for bleeding was significantly lower in the LAAC group, suggesting that LAAC may be an appropriate intervention for those at high risk of bleeding due to coagulopathy. Given this, the more frequent use of LAAC in those with coagulopathy seems appropriate.
The mechanisms of AF in HCM are likely multifactorial.21 The elevated left ventricular filling pressures found in HCM resulting in elevated LA afterload with subsequent LA dilation and shortening of the effective atrial refractory period are thought to be major promotors of left atrial remodelling, however, atrial myopathy secondary to HCM is also recognized. The high burden of fibrosis may promote an electrical substrate that contributes to increased arrhythmias and AF.21 The higher rates of recurrent AF following successful pulmonary vein isolation, particularly in those with persistent AF, and the important predictive value of LA size in HCM support this theory of atrial myopathy.21,22 In our subgroup analysis, we saw that even when septal myectomy is performed, LAAC further reduced the incidence of our primary efficacy outcome, reinforcing the above theory that elevated LA afterload alone is not solely responsible for AF in this population. Furthermore, in patients who did not undergo concurrent myectomy, LAAC was still associated with a reduction in the primary efficacy outcome, suggesting that LAAC is efficacious independent of septal myectomy and the potential benefits of a reduction in outflow tract gradient. Despite the concern that embolic stroke in HCM patients with AF may not be solely LAA in origin, our results suggest that LAAC is efficacious and safe.
Preliminary data from a small single-arm retrospective pilot study involving 36 HCM patients with AF undergoing percutaneous LAAC for primary or secondary stroke prevention demonstrated the safety and efficacy of this approach.23 At a mean follow-up time of 28.4 months, no thromboembolic events occurred in either the primary or secondary stroke prevention groups and 97.2% of patients achieved freedom from anticoagulation.
Our large retrospective data support the efficacy and safety of LAAC for stroke prevention in HCM patients with AF. The benefits are driven primarily by a reduction in ICH, GI bleeding, blood transfusions, and admissions for bleeding. Importantly, there was no significant increase in the rate of pericardiocentesis or cardiac tamponade in the LAAC group. Although the patients in our cohort were treated with predominantly surgical LAAC, reflecting the availability of this therapy long before transcatheter LAAC, we believe that the benefits of LAAC seen in this cohort may be extrapolated to transcatheter LAAC. This is further highlighted by the efficacy associated with LAAC without concurrent myectomy, suggesting that myectomy alone is not responsible for the improvement in the primary efficacy outcome with LAAC.
Limitations
First, this is an observational, retrospective analysis using an administrative database, thus it is subject to errors related to coding discrepancies, and the fidelity of data is dependent on the rigour of coding practices of institutions submitting data to this database. Furthermore, we do not have left atrial size data, which has been demonstrated to predict systemic thromboembolic events in HCM-AF patients.24 We also do not have information regarding the success rate of LAAC as assessed by the presence of flow into the appendage following closure. Anticoagulation and procedural and peri-procedural stroke details independent of long-term stroke prevention benefits are not available in this predominantly surgical cohort. Furthermore, most patients underwent LAAC during surgical myectomy, however, our subgroup analysis showed an association with improvement in the primary efficacy outcome in the absence of concurrent myectomy. Although the groups compared in our analysis underwent propensity matching, there may be variables that are not accounted for that are different between the groups, resulting in confounding. This can only be completely overcome with a randomized controlled study. Finally, our mean follow-up time of 165 days is relatively short, resulting in a relatively low event rate.
Conclusions
Left atrial appendage closure is associated with a significantly lower rate of a primary efficacy outcome of ischaemic or haemorrhagic stroke, TIA, and mortality that was driven by a reduction in ICH. There was also a significant reduction in the primary safety outcome. Overall, our findings indicate that surgical and transcatheter LAAC is appropriate in HCM patients with AF and an elevated bleeding risk or contraindication to anticoagulation. Furthermore, in patients referred for surgical myectomy who have a history of AF, concomitant surgical LAAC should be considered. Future LAAC registries may provide further information on the impact of left atrial size on patient selection and long-term outcomes of surgical and transcatheter LAAC in this patient population.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
Tasveer Khawaja, Department of Internal Medicine, Case Western Reserve University, University Hospitals, Cleveland, OH, USA.
Monil Majmundar, Department of Cardiovascular Medicine, University of Kansas Medical Center, Kansas City, KS, USA.
Zachary Zuzek, Division of Cardiovascular Health and Disease, University of Cincinnati Medical Center, Cincinnati, OH, USA.
Shilpkumar Arora, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Guilherme F Attizzani, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Steven J Filby, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Yasir Abu-Omar, Department of Cardiothoracic Surgery, Case Western Reserve University, University Hospitals, Cleveland, OH, USA.
Mehdi H Shishehbor, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Judith A Mackall, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Anene Ukaigwe, Department of Cardiology, Case Western Reserve University, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Lakeside 5038, Cleveland, OH 44106, USA.
Funding
None.
Data Availability
The data used for this analysis is from the National Readmission Database, thus it is available to the public at cost.
References
- 1. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517–24. [DOI] [PubMed] [Google Scholar]
- 2. Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fauchier L, Bisson A, Bodin A, Herbert J, Spiesser P, Pierre B, et al. . Ischemic stroke in patients with hypertrophic cardiomyopathy according to presence or absence of atrial fibrillation. Stroke 2022;53:497–504. [DOI] [PubMed] [Google Scholar]
- 4. Guttmann OP, Pavlou M, O'Mahony C, Monserrat L, Anastasakis A, Rapezzi Cet al. . Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM risk-CVA). Eur J Heart Fail 2015;17:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott Pet al. . 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:e558–631. [DOI] [PubMed] [Google Scholar]
- 6. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYHet al. . EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. Europace 2020;22:184. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jret al. . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 8. Munir MB, Khan MZ, Darden D, Abideen Asad ZU, Osman M, Singh GDet al. . Association of heart failure with procedural complications and in-hospital outcomes from left atrial appendage occlusion device implantation in patients with atrial fibrillation: insights from the national inpatient sample of 62 980 procedures. Europace 2022;24:1451–9. [DOI] [PubMed] [Google Scholar]
- 9. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma Met al. . Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
- 10. NRD Overview . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality, 2019. [PubMed] [Google Scholar]
- 11. Bambhroliya AB, Donnelly JP, Thomas EJ, Tyson JE, Miller CC, McCullough LDet al. . Estimates and temporal trend for US nationwide 30-day hospital readmission among patients with ischemic and hemorrhagic stroke. JAMA Netw Open 2018;1:e181190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolte D, Kennedy KF, Shishehbor MH, Abbott JD, Khera S, Soukas Pet al. . Thirty-Day readmissions after endovascular or surgical therapy for critical limb ischemia: analysis of the 2013 to 2014 nationwide readmissions databases. Circulation 2017;136:167–76. [DOI] [PubMed] [Google Scholar]
- 13. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13:S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Souza A, Pezzin L, Laud P, Singh A. Racial disparities in patients diagnosed with light chain (AL) amyloidosis. Blood Cancer J 2021;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue Pet al. . Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008;117:1897–907. [DOI] [PubMed] [Google Scholar]
- 16. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jret al. . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- 17. Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the charlson comorbidity Index and elixhauser score work. Med Care 2015;53:e65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. (HCUP) HCaUP . Elixhauser Comorbidity Software for ICD-10-CM (beta version). Rockville, MD; 2019. [Google Scholar]
- 19. Kim DY, Kim MJ, Seo J, Cho I, Shim CY, Hong GRet al. . Predictors of subsequent heart failure after left atrial appendage closure. Circ J 2022;86:1129–36. [DOI] [PubMed] [Google Scholar]
- 20. Khalil F, Arora S, Killu AM, Tripathi B, DeSimone CV, Egbe Aet al. . Utilization and procedural adverse outcomes associated with watchman device implantation. Europace 2021;23:247–53. [DOI] [PubMed] [Google Scholar]
- 21. Rowin EJ, Sridharan A. Thinking outside the heart to treat atrial fibrillation in hypertrophic cardiomyopathy. J Am Heart Assoc 2020;9:e016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Creta A, Elliott P, Earley MJ, Dhinoja M, Finlay M, Sporton Set al. . Catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a European observational multicentre study. Europace 2021;23:1409–17. [DOI] [PubMed] [Google Scholar]
- 23. Mo BF, Zhang R, Yuan JL, Sun J, Zhang PP, Li Wet al. . Left atrial appendage closure for primary and secondary stroke prevention in patients with hypertrophic cardiomyopathy and atrial fibrillation: A pilot study. Front Cardiovasc Med 2021;8:719755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tani T, Yagi T, Kitai T, Kim K, Nakamura H, Konda Tet al. . Left atrial volume predicts adverse cardiac and cerebrovascular events in patients with hypertrophic cardiomyopathy. Cardiovasc Ultrasound 2011;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this analysis is from the National Readmission Database, thus it is available to the public at cost.