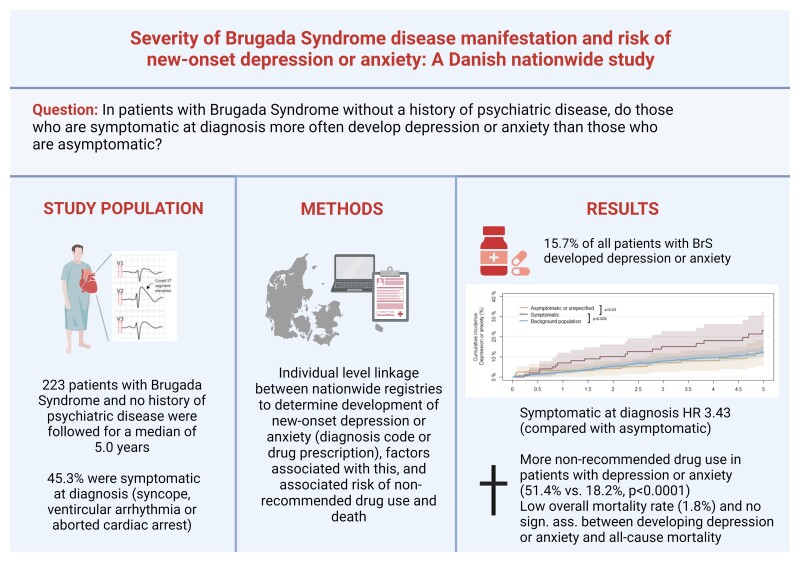
Abstract
Aims
Reduced psychological health is associated with adverse patient outcomes and higher mortality. We aimed to examine if a Brugada syndrome (BrS) diagnosis and symptomatic disease presentation were associated with an increased risk of new-onset depression or anxiety and all-cause mortality.
Methods and results
All Danish patients diagnosed with BrS (2006–2018) with no history of psychiatric disease and available for ≥6 months follow-up were identified using nationwide registries and followed for up to 5 years after diagnosis. The development of clinical depression or anxiety was evaluated using the prescription of medication and diagnosis codes. Factors associated with developing new-onset depression or anxiety were determined using a multivariate Cox proportional hazards regression model. Disease manifestation was categorized as symptomatic (aborted cardiac arrest, ventricular tachycardia, or syncope) or asymptomatic/unspecified at diagnosis. A total of 223 patients with BrS and no history of psychiatric disease were identified (72.6% male, median age at diagnosis 46 years, 45.3% symptomatic). Of these, 15.7% (35/223) developed new-onset depression or anxiety after BrS diagnosis (median follow-up 5.0 years). A greater proportion of symptomatic patients developed new-onset depression or anxiety compared with asymptomatic patients [21/101 (20.8%) and 14/122 (11.5%), respectively, P = 0.08]. Symptomatic disease presentation (HR 3.43, 1.46–8.05) and older age (lower vs. upper tertile: HR 4.41, 1.42–13.63) were significantly associated with new-onset depression or anxiety. All-cause mortality in this group of patients treated according to guidelines was low (n = 4, 1.8%); however, 3/4 developed depression or anxiety before death.
Conclusion
Approximately, one-sixth of patients with BrS developed new-onset depression or anxiety following a diagnosis of BrS. Symptomatic BrS disease manifestation was significantly associated with new-onset depression or anxiety.
Keywords: BrS, Arrhythmia, Sudden cardiac death, Psychiatric disease
Graphical Abstract
Graphical abstract.
What’s new?
Approximately one in six patients with Brugada syndrome developed new-onset depression or anxiety during up to 5 years of follow-up after their Brugada syndrome diagnosis.
Being symptomatic and older at diagnosis are factors significantly associated with the development of depression or anxiety.
Patients with Brugada syndrome who developed depression or anxiety are significantly more often treated with drugs that should be avoided in patients with Brugada syndrome.
Introduction
Brugada syndrome (BrS) is a rare inherited cardiac disease with an increased risk of developing malignant ventricular arrhythmias, and BrS represents an important cause of sudden cardiac death in young people.1,2 Several aspects of being diagnosed with BrS may negatively impact the psychological health and well-being of patients with BrS. This includes both the initial diagnostic process perhaps resulting from the sudden death of a close relative, living with the disease in itself with the inherent risk of malignant arrhythmias, and the risk of adverse events that are associated with the treatment options [e.g. inappropriate therapy by implantable cardioverter defibrillators (ICDs)].3,4 Identification of patients with BrS who may be at risk of psychological difficulties and who require additional psychological support is critical for improving patient outcomes.5,6 Furthermore, patients with BrS are advised to avoid treatment with drugs that may increase their risk of ventricular arrhythmias including many antidepressants,2,7,8 and therefore early identification of patients at risk of developing depression is important so that non-pharmacological interventions (e.g. cognitive behaviour therapy9) can be initiated.
To date, most studies of BrS have focused on the risk of adverse cardiovascular outcomes (e.g. arrhythmias and mortality) and treatment options (e.g. pharmacotherapy and ICDs). Less attention has been directed at psychological outcomes although reduced psychological well-being (e.g. depression or anxiety) is associated with adverse patient outcomes and higher mortality.10,11 However, as most patients with BrS and other inherited cardiac diseases live for a long time with their disease, there has been an increasing awareness of the psychological health of these patients,12 especially for patients at the highest risk for poor outcomes (i.e. patients with inherited cardiac diseases and an ICD implanted). In a patient survey conducted by the European Heart Rhythm Association, more than one-third of patients reported worries in relation to their implanted devices, e.g. fear of device activation and fear of failure of the device.13 A recent review article by O’Donovan et al.14 on patient perceptions in inherited cardiac conditions found that one-third of patients report levels of psychological distress indicative of the need for clinical intervention (e.g. pharmacological). To assess the relationship between the patient’s underlying disease and the development of depression or anxiety as compared to the symptoms of their disease, there is a need to examine this issue in a broad cohort of patients with BrS, including patients with BrS that are asymptomatic at the time of diagnosis.
In this study, we used the Danish nationwide registries to investigate whether patients with BrS had an increased risk of developing clinically significant new-onset depression or anxiety (i.e. diagnosis of depression or anxiety or started pharmacological treatment) after the time of BrS diagnosis and particularly if being symptomatic at the time of diagnosis (i.e. having experienced aborted cardiac arrest, ventricular tachycardia, or syncope) represents a useful clinical marker. Moreover, we evaluated whether appropriate ICD therapy was associated with the development of depression or anxiety after BrS diagnosis and whether the development of depression or anxiety after BrS diagnosis was associated with a higher risk of use of drugs that are not recommended for patients with BrS and all-cause mortality.
Methods
Registries
All Danish citizens receive a unique and permanent identification number through the civil registration system upon birth or immigration. This allows for nationwide cross-linkage amongst the Danish registries on an individual level. The Danish healthcare system is a government tax-funded single-payer system that guarantees unrestricted access to medical services.
Since 1978, all admissions to and discharges from Danish hospitals have been registered in the Danish National Patient Registry. For each admission and discharge, one primary diagnosis and relevant secondary diagnoses are registered according to the International Classification of Diseases [from 1994 and onwards: the 10th revision (ICD-10)].15 Since 1995, all drug prescriptions from Danish pharmacies have been registered in the Danish National Prescription Register using the Anatomical Therapeutic Chemical (ATC) system. Due to the partial reimbursement of drug expenses by the government-financed healthcare system, it is a requirement by law for Danish pharmacies to register all dispensed prescriptions, making this register both accurate and valid.16
For a subset of the patients followed at the specialized inherited cardiac disease clinic at Copenhagen University Hospital, Rigshospitalet, we also had access to additional clinical information through manual chart review including information on symptoms at the time of disease manifestation and results of genetic testing.
Efforts were made not to report data that may aid in the identification of individuals as Danish law prohibits reporting of low group numbers from the nationwide registries (n ≤ 3), and, thus, such low group numbers were replaced with ‘≤3’ throughout the paper. The exact numbers are known to the investigators.
Study population
We identified all Danish patients with a first-time ICD-10 code of BrS (DI472M) between 2006 and 2018 through the Danish National Patient Registry available for at least 6 months of follow-up. In this study, we included patients with BrS with no history of depression, anxiety, or other psychiatric diseases prior to BrS diagnosis, and who did not claim prescriptions of antidepressants, anxiolytics, or antipsychotics (see Supplementary material online, Appendix for specific ICD-10 and ATC codes).
The DI472M diagnosis code was introduced to the nationwide registries in 2006 and specifically allocated to diagnose patients with BrS. We have validated the diagnosis code for BrS in the Danish National Patient Registry and found a high positive predictive value of 95.8%.17
Matched background population
To compare the rate of depression or anxiety after BrS diagnosis with the background population, each patient with BrS was matched on age and sex in a 1:4 ratio with population controls from the entire Danish population using a greedy matching algorithm. Background controls were assigned the same index date as the BrS diagnosis date for the case they were matched upon and were without known psychiatric disease before the assigned index date.
Disease manifestation
We determined BrS disease manifestation through hospital discharge diagnosis from the Danish National Patient Registry and from medical records where available, as done previously.18–20 Patients were grouped according to disease manifestation at the time of BrS diagnosis as being ‘symptomatic at BrS diagnosis’ [i.e. had experienced aborted cardiac arrest (ICD-10 codes: DI490B, DI490, DI469, or DI460), ventricular tachycardia (ICD-10 codes: DI472A, DI472B, DI472D, DI470, or DI472), or syncope (ICD-code: DR559) in relation to BrS diagnosis], or as ‘asymptomatic or unspecified disease manifestation at diagnosis’. Chart review had to be available for patients to be classified as asymptomatic, as they were defined as not having any clinical symptoms according to the manual chart review and not having any outpatient clinic or in-hospital diagnoses for cardiac events (listed above) prior to BrS diagnosis. Patients with unspecified disease manifestation were patients who did not have any of the pre-specified diagnoses for cardiac events prior to diagnosis (i.e. no hospital or outpatient clinic contacts), and for whom chart review may not have been available to confirm that the patient was truly asymptomatic or revealed unspecific pre-diagnosis symptoms such as dizziness or palpitations.
Implantable cardioverter defibrillator data
All Danish patients with an ICD implanted are registered in the Danish Pacemaker and ICD Register. This register holds nationwide information on implant and explant and information on a follow-up including ICD therapy [i.e. appropriate and inappropriate shocks and anti-tachycardia pacing (ATP)]. The time of the first appropriate therapy (either ATP, shock therapy, or both) was identified.21
Comorbidity, pharmacotherapy, social factors
Information on patient comorbidity up to 10 years prior to the time of BrS diagnosis was obtained through the Danish National Patient Registry (see Supplementary material online, Appendix for specific ICD-10 codes). Included in this was information on a diagnosis code of depression, anxiety, and any psychiatric disease. Concomitant pharmacotherapy, including antidepressants and anxiolytics, in the 90 days leading up to diagnosis was identified through the Danish National Prescription Register of the Danish Medicines Agency (see Supplementary material online, Appendix for specific ATC codes). Diabetes was defined as either presence of a diagnosis code for diabetes in the registries prior to diagnosis or a dispensed prescription of an antidiabetic drug within the 180 days leading up to diagnosis.22 Hypertension was defined as having dispensed prescriptions of two or more antihypertensive drugs within the 180 days leading up to diagnosis, as done previously.22 Drugs not recommended for use in patients with BrS were identified through the brugadadrugs.org website,7 and the use of them during follow-up was identified through the Danish National Prescription Register.
Social factors (i.e. living alone and educational level) at the time of diagnosis was obtained from Statistics Denmark. Educational levels were grouped into three according to the International Standard Classification of Education (ISCED): basic school (ISCED 0–2), high school or vocational education (ISCED 3), and higher education (ISCED 5–8).18
Statistical analyses
Continuous variables were compared using the Kruskal–Wallis test and categorical variables using the χ2 test or Fisher’s exact test where appropriate. To estimate the time to depression or anxiety, cumulative incidence curves were generated using the Aalen–Johansen estimator. Differences in outcomes in the cumulative incidence curves were evaluated using Gray’s test.
Factors associated with the development of new-onset depression or anxiety were assessed using multivariable Cox proportional hazards regression models. Time after BrS diagnosis was used as the underlying time scale. The end of the study was defined as the occurrence of the primary study outcome, passing the end of the observational period (31 December 2018), loss to follow-up (e.g. emigration), or death, whichever came first. Patients were followed for up to 5 years after BrS diagnosis. Clinically relevant variables included in the model were age at diagnosis (tertiles), sex, year of diagnosis, disease manifestation (i.e. symptomatic or asymptomatic/unspecified), ICD, diabetes, ischemic heart disease, atrial fibrillation, and social factors (marital status and level of education). A sensitivity analysis was conducted in which any shock therapy was included as a variable in the multivariable Cox regression model along with the abovementioned variables. The association between ICD shock therapy and developing depression or anxiety was evaluated using a logistic regression model adjusted for the same factors as listed above, whereas the association between developing depression or anxiety and all-cause mortality was evaluated using a Cox proportional hazards regression model also adjusted for the factors listed above.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R Core Team (2022) version 4.0.3. For all analyses, a two-sided P-value <0.05 was considered statistically significant.
Ethics
The present study was approved by the Danish Data Protection Agency (P-2019-348). Registry-based analyses, using de-identifiable data, are exempt from ethics approval in Denmark. The collection of additional clinical data from medical records for a subset of the patients was approved by the regional ethics committee (journal no.: H-17032105) and with consent from the patients. Approval of the use of data from the Danish Pacemaker and ICD Register was also obtained (DPICD-2022-06-25).
Results
Patient characteristics
For this study, 263 patients with BrS diagnosed between 2006 and 2018 with at least 6 months of follow-up were identified. Of these, 223/263 (84.8%) were not known with any psychiatric disease, including depression and anxiety, before the time of BrS diagnosis, and comprised the study population. Patients were predominantly male (n = 162/223, 72.6%) and had a median age at diagnosis of 46 years [interquartile range (IQR) 32.2–57.1 years] (Table 1). Overall, 101 of 223 patients with BrS (45.3%) were symptomatic at the time of BrS diagnosis (i.e. had experienced aborted cardiac arrest, ventricular tachycardia, or syncope), whereas 54.7% were either confirmed asymptomatic (n = 17) or had an unspecified disease manifestation (n = 105). Patients who were symptomatic at BrS diagnosis were more likely to have an ICD implanted (60.4%) compared with patients with BrS who were asymptomatic or had unspecified disease manifestation (14.8%) (P < 0.0001). We did not find other differences in comorbidity and pharmacotherapy between symptomatic and asymptomatic patients with BrS (Table 1). Supplementary material online, Table S1 shows baseline characteristics of patients with BrS and a history of depression, anxiety, or other psychiatric diseases.
Table 1.
Baseline characteristics of all patients with Brugada syndrome stratified by disease manifestation
| Asymptomatic or unspecified disease manifestation (n = 122) | Symptomatic disease manifestation (n = 101) | All (n = 223) | P-value | |
|---|---|---|---|---|
| Sex (male) | 84 (68.9) | 78 (77.2) | 162 (72.6) | 0.2 |
| Age at diagnosis | 45.8 (30.3, 57.4) | 46.1 (32.6, 55.9) | 46 (32.2, 57.1) | 0.6 |
| ICD implanted | 18 (14.8) | 61 (60.4) | 79 (35.4) | < 0.0001 |
| Disease manifestation | ||||
| Asymptomatic or unspecified | 122 (100) | — | 122 (54.7) | |
| Symptomatic (total) | — | 101 (100) | 101 (45.3) | |
| Syncope | — | 56 (55.4) | 56 (25.1) | |
| Ventricular tachycardia | — | 17 (16.8) | 17 (7.6) | |
| Aborted cardiac arrest | — | 28 (27.7) | 28 (12.6) | |
| Social factors | ||||
| Living together/cohabiting | 82 (68.3) | 69 (69.7) | 151 (68.9) | 0.9 |
| Education level 1—ISCED 0–2, basic school | 23 (20.4) | 21 (21.9) | 44 (21.1) | |
| Education level 2—ISCED 3, high school or vocational education | 55 (48.7) | 47 (49.0) | 102 (48.8) | |
| Education level 3—ISCED 5–8, higher education | 35 (31.0) | 28 (29.2) | 63 (30.1) | 0.9 |
| Comorbidities | ||||
| Cancer | 4 (3.3.) | 5 (5.0) | 9 (4.0) | 0.8 |
| Hypertension | 9 (7.4) | 14 (11.6) | 20 (9.0) | 0.5 |
| Ischemic heart disease | 7 (5.7) | 5 (5.0) | 12 (5.4) | 1 |
| Atrial fibrillation | 10 (8.2) | 6 (5.9) | 16 (7.2) | 0.7 |
| Concomitant pharmacotherapy (90 days prior to diagnosis) | ||||
| Beta blockers | 10 (8.2) | 13 (12.9) | 23 (10.3) | 0.4 |
| Calcium antagonist | 4 (3.3) | 7 (6.9) | 11 (4.9) | 0.3 |
| ACE inhibitor | 6 (4.9) | 6 (5.9) | 12 (5.4) | 1 |
ICD, implantable cardioverter defibrillator; ISCED, International Standard Classification of Education.
There were only a few differences between patients with BrS and age- and sex-matched background populations without previous psychiatric disease. More patients with BrS than people in the background population had ischemic heart disease (5.4% vs. 2.0%, P = 0.01), atrial fibrillation (7.2% vs. 1.0%, P < 0.0001), and epilepsy (2.2% vs. 0.2%, P = 0.003) at baseline (Table 2).
Table 2.
Baseline characteristics of all patients with Brugada syndrome and matched background population
| Patients with Brugada syndrome (n = 223) | Matched background population (n = 892) | P-value | |
|---|---|---|---|
| Sex (male) | 162 (72.6) | 648 (72.6) | 1 |
| Age at diagnosis | 46 (32.2, 57.1) | 45.6 (32.4, 57.1) | 0.9 |
| ICD implanted | 79 (35.4) | ≤3 | <0.0001 |
| Social factors | |||
| Living together/cohabiting | 151 (68.9) | 525 (70.9) | 0.6 |
| Education level 1—ISCED 0–2, basic school | 44 (21.1) | 199 (27.6) | |
| Education level 2—ISCED 3, high school or vocational education | 102 (48.8) | 339 (47.0) | |
| Education level 3—ISCED 5–8, higher education | 63 (30.1) | 184 (25.5) | 0.1 |
| Comorbidities | |||
| Cancer | 9 (4.0) | 24 (2.7) | 0.4 |
| Diabetes | 5 (2.2) | 31 (3.5) | 0.5 |
| Epilepsy | 5 (2.2) | ≤3 | 0.003 |
| Hypertension | 20 (9.0) | 62 (7.0) | 0.4 |
| Ischemic heart disease | 12 (5.4) | 18 (2.0) | 0.01 |
| Atrial fibrillation | 16 (7.2) | 9 (1.0) | <0.0001 |
| Concomitant pharmacotherapy (90 days prior to diagnosis) | |||
| Beta blockers | 23 (10.3) | 28 (3.1) | <0.0001 |
| Calcium antagonist | 11 (4.9) | 25 (2.8) | 0.2 |
| ACE inhibitor | 12 (5.4) | 27 (3.0) | 0.1 |
ICD, implantable cardioverter defibrillator; ISCED, International Standard Classification of Education.
Risk of new-onset depression or anxiety after a Brugada syndrome diagnosis
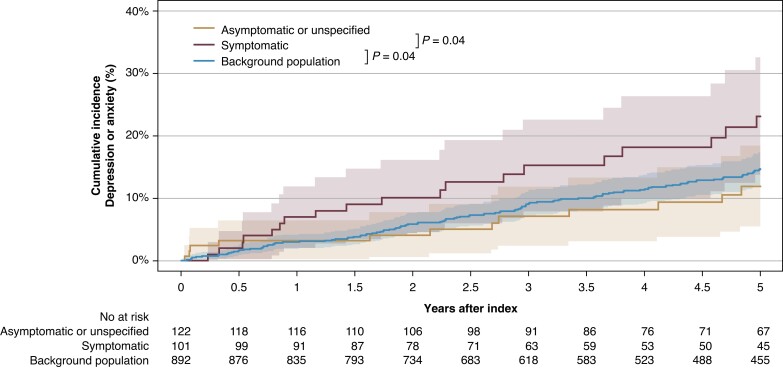
Patients were followed for a median of 5.0 years (IQR 3.1–5.0 years). Among the 223 patients with BrS without a history of depression, anxiety, or other psychiatric diseases prior to BrS diagnosis, 35 patients (15.7%) developed new-onset depression or anxiety during follow-up. Of these, 16 patients (7.2%) initiated treatment with an antidepressant, 27 patients (12.1%) initiated treatment with an anxiolytic, 3 patients (1.3%) had a hospital admission for or received a diagnosis of depression or anxiety at an outpatient clinic. There were no significant differences in groups of antidepressants and anxiolytics prescribed when comparing patients with BrS to the background population (see Supplementary material online, Table S2). Patients who developed new-onset depression or anxiety had a median time to depression or anxiety of 2.1 years (IQR 0.8–3.6 years). Figure 1 depicts the cumulative incidence curves for risk of new-onset depression or anxiety during follow-up in patients with BrS who were symptomatic at diagnosis, patients with BrS who were asymptomatic or had unspecified disease manifestation, and the background population. We identified a significant difference between the cumulative incidence curves of symptomatic patients with BrS compared with those of asymptomatic patients with BrS (P = 0.04) and between the cumulative incidence curves of symptomatic patients with BrS compared with those of the background population (P = 0.04). A crude number of patients who developed new-onset depression or anxiety during up to 5 years of follow-up after BrS diagnosis were 14/122 patients (11.5%) who were asymptomatic or had unspecified disease manifestation at the time of diagnosis compared with 21/101 patients (20.8%) who were symptomatic at the time of diagnosis. Among patients who were followed up for at least 3 years (n = 171), the 3-year risk of developing new-onset depression or anxiety was 12.9% for all patients with BrS. For patients with BrS symptomatic at diagnosis, the 3-year risk was 16.4%, and for patients asymptomatic at diagnosis, it was 10.2%, while it was 11.8% for the background population. The 5-year risk in patients who were followed up for 5 years (n = 134) was 15.7% for all patients with BrS, 19.3% for symptomatic patients with BrS, 13.0% for asymptomatic patients, and 14.3% for the background population.
Figure 1.
Cumulative incidence curves of new-onset depression or anxiety after a diagnosis of Brugada syndrome according to disease manifestation compared with those of the background population.
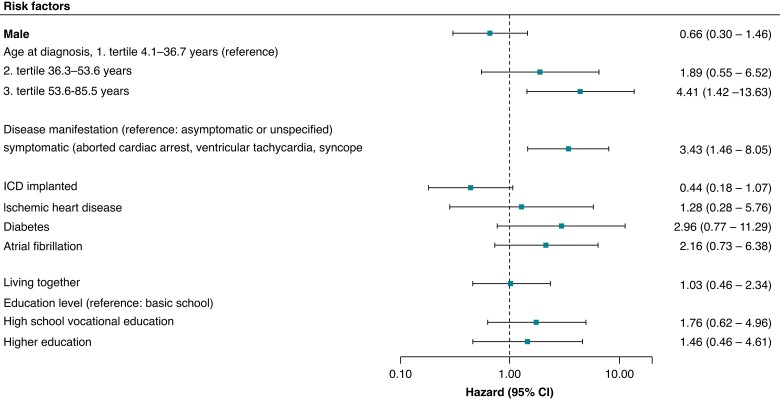
In a multivariable Cox proportional hazards regression model, we found that symptomatic patients with BrS were significantly more likely to develop new-onset depression or anxiety after BrS diagnosis compared with patients who were asymptomatic or had unspecified disease manifestation [hazard ratio (HR) 3.43, 95% confidence interval (CI) 1.46–8.05] (Figure 2). Older age at the time of diagnosis (lower tertile compared with upper tertile: HR 4.30, 95% CI 1.39–13.33) was also significantly associated with a relatively higher risk of developing new-onset depression or anxiety, whereas having an ICD implanted was not (HR 0.44, 95% CI 0.18–1.07). In a sensitivity analysis additionally adjusting for any ICD shock therapy, the results were similar (see Supplementary material online, Figure S2).
Figure 2.
Factors associated with the development of new-onset depression or anxiety after a diagnosis of Brugada syndrome according to multivariable Cox proportional hazards regression analysis.
Genotype status was known for patients with clinical information available (n = 51); of the 51 patients with clinical information available, 13 patients (25.5%) had a pathogenic or likely pathogenic mutation in SCN5A. Among the 51 patients with BrS with clinical information available, ≤3 patients developed new-onset depression or anxiety during follow-up. None of the patients that developed depression or anxiety during follow-up had an SCN5A mutation.
Implantable cardioverter defibrillator therapy during follow-up
Overall, 79/223 patients with BrS had an ICD implanted, of which 13 (16.5%) experienced at least one episode of appropriate therapy (i.e. shock or ATP) during a median follow-up of 5 years. Nine patients received one or more shock therapies. Four patients received inappropriate therapy. Few patients (≤3/9) received appropriate shock therapy, and no patients who received inappropriate shock therapy developed depression or anxiety during follow-up compared with 10/70 patients (14.3%) with an ICD but without shock therapy. No significant association between the appropriate shock therapy or any shock therapy and development of depression or anxiety during follow-up was identified [odds ratio (OR) 0.7, 95% CI 0.09–5.5 and OR 0.6, 95% CI 0.08–4.6, respectively].
Treatment with drugs not recommended for patients with Brugada syndrome during follow-up
There was a significant difference in the proportion of patients who were treated with drugs not recommended for patients with BrS during follow-up between patients with BrS who developed new-onset depression or anxiety (n = 18/35, 51.4%) and patients with BrS who did not develop new-onset depression or anxiety (n = 34/188, 18.1%) (P < 0.0001).
Four patients with BrS who developed new-onset depression or anxiety during follow-up were treated with one or more antidepressants not recommended for use in patients with BrS (i.e. lamotrigine, amitriptyline, and nortriptyline, ≤ 3 patients were treated with each of the drugs). The majority of patients (n = 16/18, 89%) were treated with other types of non-recommended drugs including metoclopramide, tramadol, or fexofenadine. Among patients with an ICD, 12% (3/25) of patients who were treated with non-recommended drugs during follow-up received appropriate therapy compared to 18.5% (10/54) of patients who were not treated with non-recommended drugs (P = 0.7).
All-cause mortality during follow-up
During a median follow-up of 5 years, 4 of 223 (1.8%) patients with BrS died. Patients with BrS had a median age at the time of death of 74.4 years (IQR 69.3–79.6 years) [median time to death 2.8 years (IQR 2.7–3.1 years)]. Three of four patients with BrS that died during follow-up had developed new-onset depression or anxiety after the time of BrS diagnosis [median time to depression or anxiety 2.2 years (IQR 1.2–2.5 years)], and none of the patients who died were assigned a cardiovascular death. There was no significant association between developing depression or anxiety after BrS diagnosis and all-cause mortality (HR 29.4, 95% CI 0.7–1167, P = 0.07), but events were few. In the background population, 17 of 946 (1.9%) died, and the median age at death was 68.3 years (IQR 63.2–72.7). This was not significantly different from the age at death for cases (P = 0.24).
Discussion
In this nationwide study, we examined the risk of developing new-onset depression or anxiety in patients with BrS and whether there was an impact of BrS disease manifestation on the risk of clinical new-onset depression or anxiety. Our study had four principal findings. First, a significant proportion of patients with BrS developed new-onset depression or anxiety after BrS diagnosis (15.7%). Second, patients who were symptomatic at the time of BrS diagnosis had a higher risk of developing depression or anxiety compared with that in both patients with BrS who were asymptomatic or had unspecified disease manifestation and people from the background population. Notably, the patients who were asymptomatic or had unspecified disease manifestation had a similar risk of developing depression or anxiety as the background population. Third, compared with patients who did not develop new-onset depression or anxiety, patients who did develop new-onset depression or anxiety were significantly more often treated with drugs not recommended for use in patients with BrS. Fourth, we found that the risk of all-cause mortality was low as only four patients died during the 5-year follow-up. The low risk, which is equal to the risk of the background population, is probably due to timely ICD implantation, as nine patients received appropriate shock therapy during follow-up.
Previous reports of psychologic health have shown high rates of depression or anxiety in groups of patients with different inherited cardiac diseases23–25 as well as separate inherited cardiac diseases.19,26,27 However, to improve patient outcomes, it is important to identify subgroups of patients at risk to be able to help these patients timely. Some of the medication (e.g. several tricyclic antidepressants and some selective serotonin reuptake inhibitors7,28) that is used to treat patients with depression is not recommended in patients with BrS due to their possible proarrhythmic properties. While physicians in some cases make a weighted choice to initiate non-recommended pharmacologic treatment despite the patients having a BrS diagnosis (e.g. if the patient has an ICD), early recognition of depression in these patients with BrS for non-pharmacologic treatment to be initiated should be strived at.9 In our study, patients symptomatic at BrS diagnosis were more likely to develop depression or anxiety than patients who were asymptomatic or had unspecified disease manifestation at diagnosis. In fact, patients who were asymptomatic at diagnosis or had unspecified disease manifestation had a risk of developing depression or anxiety similar to the background population indicating that it may primarily be the course of the disease rather than the diagnosis of the disease itself that is of importance. As non-pharmacological treatment is not recorded in the nationwide registries and commonly is handled by a primary care physician, it is possible that the total number of patients with depression or anxiety may be higher than reported which may have affected study findings. This highlights the need for increased awareness of depressive symptoms in especially the symptomatic subgroup of patients. In the present study, 45.3% of patients with BrS were reported to be symptomatic, which is higher compared with the finding in previous studies reporting approximately one in three to be symptomatic.29 The difference could owe to differences in how symptomatic is defined (i.e. some of the previous studies did not include patients with aborted cardiac arrest) or differences in the implementation of systematic cascade screening. Our results are in line with previous studies in patients with long QT syndrome (LQTS) on the development of depression or anxiety19,30 and workforce attachment,18 probably reflecting a common burden of stressful life events in patients with BrS and LQTS, which are similar in terms of an immediate risk of ventricular arrhythmias and consequently sudden cardiac death. Moreover, patients with BrS who developed new-onset depression or anxiety received significantly more drugs that are not recommended for use in patients with BrS, as their use may increase the risk of ventricular arrhythmias in patients with BrS. The difference was not driven by non-recommended antidepressant therapy; however, it highlights an increased need for awareness in this vulnerable group of patients.
Varying results have been reported on the effects on psychological health of having an ICD implanted. ICD therapy (i.e. appropriate and inappropriate shocks) has been associated with increased risk of depression and anxiety both in patients with inherited arrhythmias and the general cardiology population, while other patients with ICDs are satisfied with their devices and report quality of life similar to age-matched controls.24,25,31–34 Probst et al.26 found that patients with BrS reported significantly lower general and mental health scores compared with those of the French general population; however, they did not find any significant differences in the psychological impact of ICDs in implanted and non-implanted patients with BrS. A recent prospective study by van den Heuvel et al.23 in patients with genetic heart diseases and ICDs found that while some patients adjust well to living with an ICD, other subgroups including females and patients with comorbidity had clinically elevated levels of anxiety and depression, and in patients with ICDs, Frydensberg et al.35 found females and patients with secondary prophylactic indication for having an ICD (e.g. due to previous ACA) to report higher anxiety and depression scores. In our study, while also adjusting for disease manifestation, we did not find ICD implantation to be associated with an increased risk of developing depression or anxiety after a diagnosis of BrS. Furthermore, we did not find an increased risk of developing depression or anxiety in patients who experienced appropriate or any shock therapy; however, the numbers were low.
We found that patients with BrS in Denmark have a low overall risk of all-cause mortality, and a tendency towards an association between developing depression or anxiety after BrS diagnosis and all-cause mortality (HR 29.4, P = 0.07); however, events were few. In general, patients with depression and anxiety have a higher mortality rate,10,11 and even though the association between developing new-onset depression or anxiety and mortality in our study was not significant, there is a need for detection of patients at risk of developing depression or anxiety.
Most patients with BrS are followed closely in outpatient clinics. When treating these patients, mental health should be of just as much importance as cardiac health. The findings of this study can help guide clinicians towards patients at an increased risk of developing depression or anxiety after a BrS diagnosis (i.e. patients symptomatic at diagnosis and of older age). For future studies, it would be interesting to investigate whether a systematic approach to the detection of the development of depression and anxiety in these patients could make a difference, e.g. earlier detection of disease, to be able to initiate non-pharmacological interventions.
Limitations
This was a retrospective register-based study with inherent limitations. Thus, we cannot exclude the possibility of residual confounding or confounding by indication. Although this was a nationwide study, the sample size was limited as BrS is a rare disease. As a result of the relatively low sample size, a limited follow-up time, and the relatively low absolute risk of events in patients with BrS,36 the number of cardiac events (e.g. shock therapy) during follow-up was low. In this study, we focused on clinically significant depression or anxiety defined by a relevant diagnosis code in the Danish National Patient Registry or the initiation of relevant pharmacotherapy identified through the Danish National Prescription Register. Thus, information on pre-depressive or pre-anxiety symptoms from the general practitioner or psychologist of patients that did not lead to a diagnosis or initiation of relevant pharmacotherapy were not available, and this may have influenced our findings. Furthermore, we did not have access to information on treatment other than medication, e.g. different forms of psychotherapy. Disease manifestation was defined through diagnosis codes and chart review; however, as chart review was not available for all patients, some of them had to be classified as unspecified disease manifestation, and this may have influenced our results. Due to the lack of clinical information on all patients (e.g. circumstances of type I ECG, family history, and results of genetic testing), it was not possible to calculate a Shanghai score. The diagnosis of BrS was validated against the previous guidelines from 201537 and not the most recent,2 which may have had an impact on the PPV. We included all patients with a diagnosis code of BrS, and thus patients with BrS overlap syndromes were also included.
Conclusions
Approximately one in six patients diagnosed with BrS developed new-onset depression or anxiety during a median follow-up of 5 years after they had been diagnosed with BrS. Patients symptomatic at diagnosis had a more than three-fold greater likelihood of developing new-onset depression or anxiety compared with that in patients that were asymptomatic at the time of diagnosis. By contrast, patients who were asymptomatic or had an unspecified disease manifestation at diagnosis had a risk of developing depression or anxiety similar to the background population 1 year after diagnosis of BrS, suggesting that most focus should be on identifying patients at risk of new-onset depression or anxiety in the group of patients that are symptomatic at the time of BrS diagnosis. Moreover, significantly more patients who developed new-onset depression or anxiety were treated with drugs that are not recommended for use in patients with BrS compared with patients who did not develop depression or anxiety. The overall mortality rate among patients with BrS was low after the time of BrS diagnosis in a group of patients who are treated according to guidelines.
Supplementary Material
Contributor Information
Camilla H B Jespersen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Johanna Krøll, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Priya Bhardwaj, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Bo Gregers Winkel, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Peter Karl Jacobsen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Christian Jøns, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Jens Haarbo, Department of Cardiology, Copenhagen University Hospital - Herlev and Gentofte, Gentofte Hospitalsvej 1, 2900 Hellerup, Denmark.
Jens Kristensen, Department of Cardiology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, 8200 Aarhus N, Denmark.
Jens Brock Johansen, Department fo Cardiology, Odense University Hospital, J B Winsløws Vej 4, 5000 Odense C, Denmark.
Berit T Philbert, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Sam Riahi, Department of Cardiology, Aalborg University Hospital, Hobrovej 18-22, 9000 Aalborg, Denmark; Department of Clinical Medicine, Aalborg University Hospital, Hobrovej 18-22, 9000 Aalborg, Denmark.
Christian Torp-Pedersen, Department of Cardiology, Nordsjaellands Hospital, Dyrehavevej 29, 3400 Hillerød, Denmark; Department of Public Health, University of Copenhagen, Øster Farimagsgade 5, 1353 Copenhagen K, Denmark.
Lars Køber, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Jacob Tfelt-Hansen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Forensic Medicine, Faculty of Medical Sciences, University of Copenhagen, Frederik V's Vej 11, 2100 Copenhagen Ø, Denmark.
Peter E Weeke, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The project was supported by the Novo Nordisk Foundation (Tandem Programme; #31634) and the John and Birthe Meyer Foundation.
Data Availability
Due to restrictions related to Danish law and protecting patient privacy, the combined set of data used in this study can only be made available through a trusted third party, Statistics Denmark. Data will be shared on request to the corresponding author with the permission of Statistics Denmark. More information regarding data access is available at https://www.dst.dk/en/TilSalg/Forskningsservice.
References
- 1. Lahrouchi N, Raju H, Lodder EM, Papatheodorou E, Ware JS, Papadakis Met al. . Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol 2017;69:2134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022:43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 3. Hendriks KSWH, Hendriks MMWB, Birnie E, Grosfeld FJM, Wilde AAM, van den Bout Jet al. . Familial disease with a risk of sudden death: a longitudinal study of the psychological consequences of predictive testing for long QT syndrome. Heart Rhythm 2008;5:719–24. [DOI] [PubMed] [Google Scholar]
- 4. Holst AG, Jensen HK, Eschen O, Henriksen FL, Kanters J, Bundgaard Het al. . Low disease prevalence and inappropriate implantable cardioverter defibrillator shock rate in Brugada syndrome: a nationwide study. Europace 2012;14:1025–9. [DOI] [PubMed] [Google Scholar]
- 5. Dunbar SB, Langberg JJ, Reilly CM, Viswanathan B, McCarty F, Culler SDet al. . Effect of a psychoeducational intervention on depression, anxiety, and health resource use in implantable cardioverter defibrillator patients. Pacing Clin Electrophysiol 2009;32:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med 2005;165:2508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DMet al. . Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website ( www.brugadadrugs.org ). Heart Rhythm 2009;6:1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yap YG, Behr ER, Camm AJ. Drug-induced Brugada syndrome. Europace 2009;11:989–94. [DOI] [PubMed] [Google Scholar]
- 9. Lett HS, Davidson J, Blumenthal JA. Nonpharmacologic treatments for depression in patients with coronary heart disease. Psychosom Med 2005;67:S58–62. [DOI] [PubMed] [Google Scholar]
- 10. Meier SM, Mattheisen M, Mors O, Mortensen PB, Laursen TM, Penninx BW. Increased mortality among people with anxiety disorders: total population study. Br J Psychiatry J Ment Sci 2016;209:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laursen TM, Musliner KL, Benros ME, Vestergaard M, Munk-Olsen T. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord 2016;193:203–7. [DOI] [PubMed] [Google Scholar]
- 12. Aktaa S, Tzeis S, Gale CP, Ackerman MJ, Arbelo E, Behr ERet al. . European Society of Cardiology quality indicators for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2023;25:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haugaa KH, Potpara TS, Boveda S, Deharo J-C, Chen J, Dobreanu Det al. . Patients’ knowledge and attitudes regarding living with implantable electronic devices: results of a multicentre, multinational patient survey conducted by the European Heart Rhythm Association. Europace 2018;20:386–91. [DOI] [PubMed] [Google Scholar]
- 14. O’Donovan C, Ingles J, Broadbent E, Skinner JR, Kasparian NA. How patient perceptions shape responses and outcomes in inherited cardiac conditions. Heart Lung Circ 2020;29:641–52. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 17. Jespersen CHB, Krøll J, Bhardwaj P, Hansen CJ, Svane J, Winkel BGet al. . Use of non-recommended drugs in patients with Brugada syndrome: a Danish nationwide cohort study. J Am Heart Assoc 2023;12:e028424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jespersen CHB, Butt JH, Krøll J, Winkel BG, Kanters JK, Gislason Get al. . Workforce attachment after a congenital long QT syndrome diagnosis: a Danish nationwide study. Open Heart 2022;9:e002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krøll J, Jensen HK, Jespersen C, Kanters JK, Hansen MS, Christiansen Met al. . Severity of congenital long QT syndrome disease manifestation and risk of depression, anxiety, and mortality: a nationwide study. Europace 2022;24:620–629. [DOI] [PubMed] [Google Scholar]
- 20. Weeke PE, Kellemann JS, Jespersen CB, Theilade J, Kanters JK, Hansen MSet al. . Long-term proarrhythmic pharmacotherapy among patients with congenital long QT syndrome and risk of arrhythmia and mortality. Eur Heart J 2019;40:3110–7. [DOI] [PubMed] [Google Scholar]
- 21. Weeke P, Johansen JB, Jørgensen OD, Nielsen JC, Møller M, Videbæk Ret al. . Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter-defibrillators for primary prevention: data from the Danish ICD Register. Europace 2013;15:1150–7. [DOI] [PubMed] [Google Scholar]
- 22. Krøll J, Butt JH, Jensen HK, Fosbøl EL, Jespersen C, Winkel BGet al. . β-blocker adherence among patients with congenital long QT syndrome: a nationwide study. Eur Heart J Qual Care Clin Outcomes 2022;9:76–84. [DOI] [PubMed] [Google Scholar]
- 23. van den Heuvel LM, Sarina T, Sweeting J, Yeates L, Bates K, Spinks Cet al. . A prospective longitudinal study of health-related quality of life and psychological wellbeing after an implantable cardioverter-defibrillator in patients with genetic heart diseases. Heart Rhythm O2 2022;3:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh SM, Murray B, Tichnell C, McClellan R, James CA, Barth AS. Anxiety and depression in inherited channelopathy patients with implantable cardioverter-defibrillators. Heart Rhythm O2 2021;2:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ingles J, Sarina T, Kasparian N, Semsarian C. Psychological wellbeing and posttraumatic stress associated with implantable cardioverter defibrillator therapy in young adults with genetic heart disease. Int J Cardiol 2013;168:3779–84. [DOI] [PubMed] [Google Scholar]
- 26. Probst V, Plassard-Kerdoncuf D, Mansourati J, Mabo P, Sacher F, Fruchet Cet al. . The psychological impact of implantable cardioverter defibrillator implantation on Brugada syndrome patients. Europace 2011;13:1034–9. [DOI] [PubMed] [Google Scholar]
- 27. Hintsa T, Keltikangas-Järvinen L, Puttonen S, Ravaja N, Toivonen L, Kontula Ket al. . Depressive symptoms in the congenital long QT syndrome. Ann Med 2009;41:516–21. [DOI] [PubMed] [Google Scholar]
- 28. Postema PG, Neville J, de Jong JSSG, Romero K, Wilde AAM, Woosley RL. Safe drug use in long QT syndrome and Brugada syndrome: comparison of website statistics. Europace 2013;15:1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mascia G, Bona RD, Ameri P, Canepa M, Porto I, Parati Get al. . Brugada syndrome and syncope: a practical approach for diagnosis and treatment. Europace 2021;23:996–1002. [DOI] [PubMed] [Google Scholar]
- 30. Hintsa T, Jokela M, Elovainio M, Määttänen I, Swan H, Hintsanen Met al. . Stressful life events and depressive symptoms among symptomatic long QT syndrome patients. J Health Psychol 2016;21:505–12. [DOI] [PubMed] [Google Scholar]
- 31. Jacq F, Foulldrin G, Savouré A, Anselme F, Baguelin-Pinaud A, Cribier Aet al. . A comparison of anxiety, depression and quality of life between device shock and nonshock groups in implantable cardioverter defibrillator recipients. Gen Hosp Psychiatry 2009;31:266–73. [DOI] [PubMed] [Google Scholar]
- 32. Magyar-Russell G, Thombs BD, Cai JX, Baveja T, Kuhl EA, Singh PPet al. . The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: a systematic review. J Psychosom Res 2011;71:223–31. [DOI] [PubMed] [Google Scholar]
- 33. Bostwick JM, Sola CL. An updated review of implantable cardioverter/defibrillators, induced anxiety, and quality of life. Heart Fail Clin 2011;7:101–8. [DOI] [PubMed] [Google Scholar]
- 34. Rhodes AC, Murray B, Tichnell C, James CA, Calkins H, Sears SF. Quality of life metrics in arrhythmogenic right ventricular cardiomyopathy patients: the impact of age, shock and sex. Int J Cardiol 2017;248:216–20. [DOI] [PubMed] [Google Scholar]
- 35. Frydensberg VS, Johansen JB, Möller S, Riahi S, Wehberg S, Haarbo Jet al. . Anxiety and depression symptoms in Danish patients with an implantable cardioverter-defibrillator: prevalence and association with indication and sex up to 2 years of follow-up (data from the national DEFIB-WOMEN study). Europace 2020;22:1830–40. [DOI] [PubMed] [Google Scholar]
- 36. Delise P, Probst V, Allocca G, Sitta N, Sciarra L, Brugada Jet al. . Clinical outcome of patients with the Brugada type 1 electrocardiogram without prophylactic implantable cardioverter defibrillator in primary prevention: a cumulative analysis of seven large prospective studies. Europace 2018;20:f77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to restrictions related to Danish law and protecting patient privacy, the combined set of data used in this study can only be made available through a trusted third party, Statistics Denmark. Data will be shared on request to the corresponding author with the permission of Statistics Denmark. More information regarding data access is available at https://www.dst.dk/en/TilSalg/Forskningsservice.