Abstract
Gibberellins (GAs) initiate a series of events that culminate in programmed cell death, whereas abscisic acid (ABA) prevents this process. Reactive oxygen species (ROS) are key elements in aleurone programmed cell death. Incubation of barley (Hordeum vulgare) aleurone layers in H2O2 causes rapid death of all cells in GA- but not ABA-treated layers. Sensitivity to H2O2 in GA-treated aleurone cells results from a decreased ability to metabolize ROS. The amounts and activities of ROS scavenging enzymes, including catalase (CAT), ascorbate peroxidase, and superoxide dismutase are strongly down-regulated in aleurone layers treated with GA. CAT activity, protein, and Cat2 mRNA decline rapidly following exposure of aleurone layers to GA. In ABA-treated layers, on the other hand, the amount and activity of CAT and Cat2 mRNA increases. Incubation in ABA maintains high amounts of ascorbate peroxidase and superoxide dismutase, whereas GA brings about a rapid reduction in the amounts of these enzymes. These data imply that GA-treated cells loose their ability to scavenge ROS and that this loss ultimately results in oxidative damage and cell death. ABA-treated cells, on the other hand, maintain their ability to scavenge ROS and remain viable.
The cereal endosperm differentiates into the starchy endosperm and the aleurone layer. As the grain matures all cells of the starchy endosperm die. The only endosperm cells alive in mature grain are aleurone cells (Olsen et al., 1999). During germination and seedling establishment, aleurone cells synthesize and secrete hydrolytic enzymes into the starchy endosperm to mobilize stored reserves that nourish the growing embryo. Cells of the aleurone layer die after they have fulfilled their secretory function. This process was first described in the nineteenth century by Haberlandt (1884). Aleurone cell death is a form of programmed cell death (PCD) and is the culmination of a developmental program that is initiated by gibberellins (GAs) produced by the embryo of the germinating grain (Appleford and Lenton, 1997). This program can be arrested by abscisic acid (ABA; Wang et al., 1996), and in dormant grain ABA is thought to prevent germination and endosperm mobilization (Schuurink et al., 1993; Bewley, 1997).
The synthesis and secretion of hydrolases brings about dramatic changes in aleurone cells that contribute to PCD of the aleurone layer. The amino acids required for the de novo synthesis of secreted enzymes come from storage proteins found in aleurone protein storage vacuoles (PSVs). Following GA treatment, aleurone PSVs become lytic compartments containing proteases and other hydrolases that are used to mobilize the contents of the PSV (Bethke et al., 1996, 1998). In mature grain these vacuoles are approximately 5 μm in diameter. As the contents of the PSVs are hydrolyzed, however, the PSVs fuse to form one large central vacuole (Bethke et al., 1998; Swanson et al., 1998). Barley (Hordeum vulgare) aleurone cells die in response to GA only after this large central vacuole has been formed (Bethke et al., 1999).
The metabolism of triglycerides also plays a key role in aleurone PCD. Although the cereal aleurone layer is adjacent to abundant carbohydrate reserves in the starchy endosperm, these reserves are not immediately available to aleurone cells. To become available they must first be hydrolyzed by enzymes secreted from the aleurone layer. The aleurone layer relies on its store of triglycerides to provide energy and substrates for the synthesis and secretion of hydrolases. The lipid reserves of the aleurone cell are extensive, and oleosomes occupy as much as 25% of the volume of the aleurone cell (Jones, 1969). Fatty acids in wheat aleurone are metabolized via β-oxidation and gluconeogensis following GA treatment (Doig et al., 1975). H2O2 is a major by-product of β-oxidation and we have shown that H2O2 is a major contributor to the reactive oxygen species (ROS) that lead to aleurone PCD (Bethke and Jones, 2000). Tenets of this hypothesis are explored in this paper.
ROS are scavenged by many enzymes including superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT; Bowler et al., 1994; Asada, 1997; Scandalios et al., 1997). Several reports have suggested a link between ROS and PCD. ROS are thought to be key players in cell death that accompanies senescence in plants (Pastori and del Rio, 1997; del Rio et al., 1998; Jimenez et al., 1998). During leaf senescence, CAT activity decreases, whereas the activity of enzymes that generate ROS such as urate oxidase and xanthine oxidase are stimulated (del Rio et al., 1998). Cell death that occurs during the pathogen-induced hypersensitive response (HR) is accompanied by an increase in the production of ROS, mainly due to the activation of a plasma-membrane associated NAD(P) H oxidase (Jabs, 1999). The activities of APX and CAT were suppressed during the HR, perhaps leading to localized increases in the amount of ROS (Dorey et al., 1998; Mittler et al., 1998). Additional support for a role of ROS in pathogen-induced cell death comes from experiments with tobacco plants expressing antisense CAT or antisense APX (Mittler et al., 1999). These plants were hyper-responsive to pathogen attack. These data support the idea that suppression of ROS-scavenging enzymes during the HR plays an important role in pathogen-induced PCD (Mittler et al., 1999). However, other data indicate that ROS may not be required for PCD during the HR (Xie and Chen, 2000).
Cell death in the cereal aleurone layer shares many features with organ senescence in plants (Fath et al., 2000). Thus, the aleurone cell mobilizes almost all of its carbon, nitrogen, and potassium reserves before death. GA-induced nucleases degrade the DNA of living cells as part of the cell death program (Fath et al., 1999). Aleurone cells become highly vacuolate, and the volume of cytoplasm and associated organelles declines prior to death (Bethke et al., 1999). In this communication we report on hormone-induced changes in the amounts of the principle ROS metabolizing enzymes of the barley aleurone layer. We show that ABA maintains high CAT, APX, and SOD activities and prevents PCD, whereas GA brings about a rapid reduction in these enzyme activities and promotes cell death. We propose that GA-treated aleurone cells become increasingly sensitive to ROS and that ROS are among the agents that bring about PCD in the aleurone cell.
RESULTS
Death of Cells in the Barley Aleurone Layer Is Tightly Regulated by ABA and GA
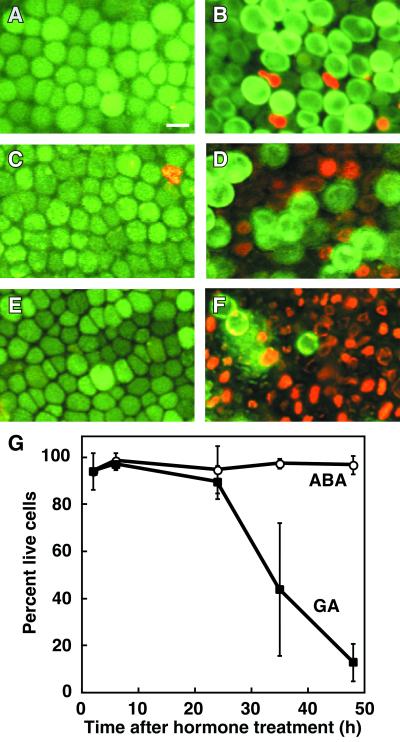
Although PCD of barley aleurone protoplasts treated with GA and ABA has been described (Bethke et al., 1999), the time course of GA-induced PCD in barley aleurone layers has not been quantified. To do this we monitored cell death and viability of aleurone cells in intact aleurone layers by simultaneously staining living and dead cells with fluorescent probes (Fig. 1) and by measuring metabolic activity of aleurone cells as a function of oxygen consumption (Fig. 2). Non-fluorescent fluorescein diacetate (FDA) is taken up by living aleurone cells and hydrolyzed to yield fluorescein, which fluoresces green (Fig. 1A). N-(3-triethylammoniumpropyl)-4-{6-[4-(diethylamino) phenyl]-hexatrienyl} pyridinium dibromide (FM 4-64) partitions slowly into live aleurone cells, but accumulates rapidly in dead cells and gives orange-red fluorescence (Fig. 1F). As determined by staining with these two fluorescent probes, almost no aleurone cells died when aleurone layers were incubated in 5 μm ABA for up to 48 h (Fig. 1, A, C, and E). Treatment of aleurone layers with 5 μm GA, on the other hand, brought about a decrease in the number of living cells (green fluorescence) and an increase in the number of dead cells (orange/red fluorescence; Fig. 1, B, D, and F). The extent of cell death at several time points was quantified by counting the number of living and dead cells (Fig. 1G). Virtually all cells in GA-treated aleurone layers die between 24 and 48 h of incubation (Fig. 1, B, D, F, and G).
Figure 1.
Time course of PCD in barley aleurone layers determined by staining with FDA and FM 4-64. A through F, Representative images from aleurone layers incubated in ABA (A, C, and E) or GA (B, D, and F) for 24 (A and B), 35 (C and D), or 48 (E and F) h and visualized by epifluorescence microscopy. Live cells appear green and dead cells appear orange or red. G, Quantification of viability and death for ABA- and GA-treated barley aleurone layers. The data at each time point are from four aleurone layers, with two determinations per layer and approximately 150 cells per determination. Plotted is the mean percentage of live cells in each determination ± sd from the mean.
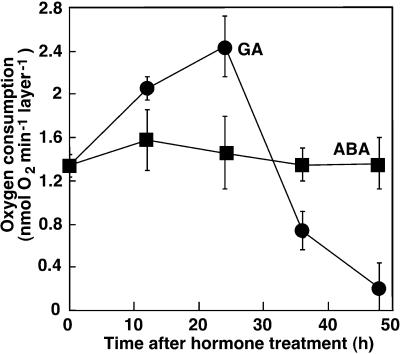
Figure 2.
The rate of oxygen consumption by aleurone layers is used as a measure for metabolic activity. Aleurone layers were incubated in GA (●) or ABA (▪) for the indicated times. The rate of oxygen consumption for individual layers in 2 mL of water was measured polarographically for 20 min. Bars represent the means ± sd of three independent measurements from different experiments.
We also measured polarographically the rate of oxygen consumption by intact aleurone layers to determine metabolic activity (Fig. 2). The rate of oxygen consumption by aleurone layers incubated in ABA remained constant for 48 h and did not differ significantly from that of freshly isolated tissue (Fig. 2). GA treatment stimulated oxygen uptake by about 90% relative to untreated controls or ABA-treated tissue during the first 24 h of incubation, but thereafter oxygen uptake declined. By 48 h of incubation in GA, oxygen consumption was reduced by about 85% compared with freshly isolated layers and by about 90% compared with layers treated with GA for 24 h. The kinetics of oxygen consumption between 24 and 48 h of incubation in GA parallel changes in cell viability as measured using FDA and FM 4-64 (compare Figs. 1G and 2).
GA Sensitizes Cells of the Intact Aleurone Layer to H2O2
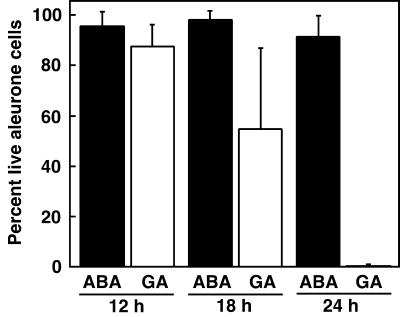
Barley aleurone protoplasts pretreated with GA die when exposed to H2O2, but protoplasts incubated in ABA are much less sensitive to H2O2 (Bethke and Jones, 2001). We tested the hypothesis that cells of the intact aleurone layer also become more sensitive to H2O2 following GA treatment. Aleurone layers were incubated in GA or ABA for 12, 18, or 24 h, exposed to 1% (v/v; 325 mm) H2O2 for 1 h, stained with FDA and FM 4-64, and living and dead cells were counted (Fig. 3). Almost all cells in layers treated with ABA for up to 24 h remained alive following H2O2 exposure. GA-treated cells, however, became progressively more sensitive to H2O2. For layers treated with GA for 12 h, approximately 15% of cells were dead following H2O2 exposure. For aleurone layers incubated in GA for 18 h, the percentage of dead cells increased to about 50%. Virtually all cells in aleurone layers incubated in GA for 24 h died in response to H2O2, even though 90% of GA-treated cells incubated in the absence of H2O2 were still alive at this time (compare Figs. 1G and 3).
Figure 3.
GA treatment of barley aleurone layers reduces their ability to tolerate H2O2. H2O2 (1% [v/v]; 325 mm) was added to aleurone layers incubated in ABA or GA for 12, 18, or 24 h. Layers were stained with FDA and FM 4-64 1 h after H2O2 addition and cells were scored live or dead. The data at each time point are from six aleurone layers, with two determinations per layer and approximately 150 cells per determination. Bars are the mean percentage of live cells in each determination ± sd from the mean.
Down-Regulation of CAT by GA
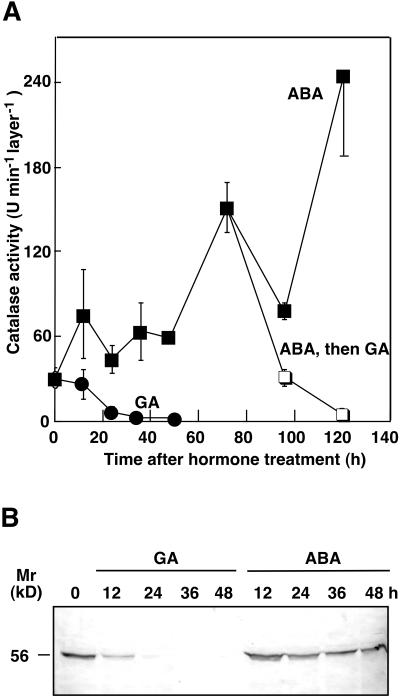
Because GA-, but not ABA-treated, aleurone cells die when exposed to H2O2 we tested the hypothesis that cells of GA-treated tissue contain lower amounts of enzymes that can metabolize H2O2. CAT catalyzes the disproportionation of H2O2 to oxygen and water and is one of the principal H2O2-metabolizing enzymes in plant cells (Scandalios et al., 1997). Using a polarographic assay we found that CAT activity declined in aleurone layers incubated in GA, but not ABA (Fig. 4A). For layers incubated in GA for 24 h, CAT activity was almost not detectable (Fig. 4A). We confirmed this observation using antibodies raised against maize CAT, which react with all three maize CAT isoforms (Fig. 4B). CAT protein declined as early as 12 h after GA treatment and by 24 h of incubation in GA CAT was not detectable.
Figure 4.
CAT activity and protein decline in GA-treated but not ABA-treated aleurone layers over time. A, CAT activity in extracts of barley aleurone layers incubated in GA (●) or ABA (▪) for the indicated times or from aleurone layers preincubated in 5 μm ABA with the addition of 25 μm GA at 72 h (□) and then further incubated for the indicated time. CAT activity was measured polarographically. One unit was defined as the decomposition of 1 μmol H2O2 per minute. Data are means ± sd of three independent samples. B, Immunoblot of homogenates of GA- or ABA-treated aleurone layers. The blot was probed with a PAb against maize CAT. Size of an Mr marker is indicated on the left.
ABA treatment brings about an increase in CAT activity in barley aleurone layers (Fig. 4A). During the first 48 h of incubation in ABA, CAT activity and CAT protein remained approximately constant (Fig. 4, A and B). Prolonged incubation in ABA brought about an increase in the activity of this enzyme. In three separate experiments CAT activity increased about 5-fold by 72 h and about 8-fold above the initial activity by 120 h of incubation in ABA (Fig. 4A). When aleurone layers incubated in 5 μm ABA for 72 h were transferred to medium containing 5 μm ABA and 25 μm GA, CAT activity dropped by more than 80% in 24 h (Fig. 4A). At the same time, oxygen consumption of layers transferred to GA-containing medium increased relative to ABA-treated layers, indicating that these layers were metabolically active and remained alive (data not shown). CAT activity was not detectable 48 h after transfer to GA-containing medium (Fig. 4A), and oxygen consumption ceased (data not shown).
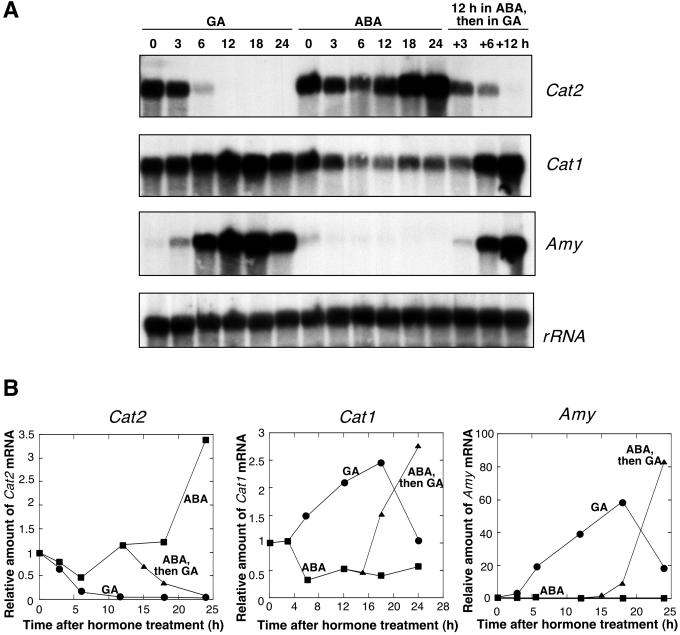
To investigate the roles of ABA and GA in regulating CAT synthesis we monitored changes in Cat mRNA. Barley has two CAT genes, Cat1 and Cat2, and cDNAs encoding these genes were generously provided by Ronald Skadsen (Skadsen et al., 1995). RNA was isolated from aleurone layers incubated in GA or ABA for up to 24 h and probed with Cat1- and Cat2-specific barley cDNAs, as well as cDNA probes for barley α-amylase (Amy) and maize 28s rRNA. Changes in the amounts of Cat1, Cat2, and Amy mRNAs were qualitatively and quantitatively the same in three independent experiments, and the results of one of these experiments is shown in Figure 5. Whereas the amount of Cat1 mRNA increased slightly in response to GA treatment, the amount of Cat2 mRNA declined rapidly following exposure of aleurone layers to GA (Fig. 5). The decline in Cat2 mRNA is dramatic, with a 35% decline in 3 h and an 85% reduction by 6 h of exposure to GA (Fig. 5). By 12 h after GA addition, the amount of Cat2 mRNA had fallen by more than 95%, whereas the amount of Cat1 mRNA had increased approximately 110%. When the same blot was probed with a barley Amy cDNA, there was about a 60-fold increase in the amount of Amy mRNA by 18 h incubation in GA.
Figure 5.
Effect of GA and ABA on the amount of Cat1 and Cat2 mRNA in aleurone. RNA was isolated from layers incubated in GA or ABA, or from layers preincubated in 5 μm ABA with the addition of 25 μm GA at 12 h. A, RNA gel blot was probed with barley Cat1 cDNA, Cat2 cDNA, Amy cDNA probes, and maize 28s rRNA probe. B, The abundance of each RNA was determined by laser densitometry (●, GA; ▪, ABA; ▴, 12 h ABA, then addition of 5-fold excess GA).
Cat1 and Cat2 mRNA accumulation in ABA-treated tissue had the opposite pattern from that found in GA-treated tissue. Cat1 mRNA declined following incubation in ABA, but Cat2 mRNA increased (Fig. 5). After 24 h in ABA the amount of Cat1 mRNA was 45% below that of freshly isolated aleurone layers, whereas the amount of Cat2 was almost 250% higher. GA reversed the effects of ABA on the accumulation of Cat1, Cat2, and Amy mRNAs (Fig. 5). When aleurone layers were incubated in ABA for 12 h and then transferred to a 5-fold excess of GA (25 μm), GA brought about a large increase in Cat1 mRNA and a corresponding decrease in Cat2 mRNA. As a control the blot was probed with a barley Amy cDNA and, as predicted, GA reversed the effects of ABA on Amy mRNA accumulation.
Down-Regulation of APX and SOD by GA
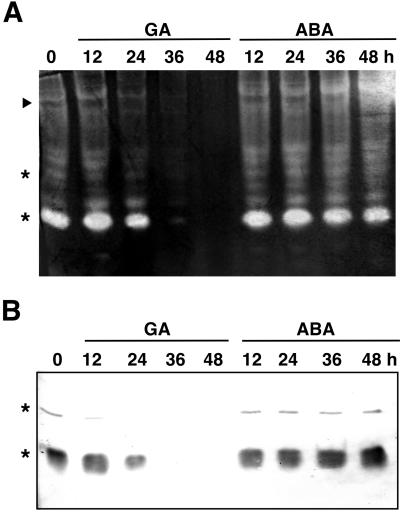
Although CATs are important for scavenging H2O2, APX and SOD also play vital roles in the metabolism of ROS in plants (Bowler et al., 1994; Asada, 1997). APX uses two molecules of ascorbate to reduce H2O2 to water with the generation of two molecules of monodehydroascorbate. To determine whether APX activity is hormonally regulated in barley aleurone, native APX activity gel assays of homogenates from GA- or ABA-treated aleurone layers were performed (Fig. 6A). Several isoforms of APX activity were detected in freshly isolated aleurone layers. GA treatment brought about a decline in the activity of these isoforms, whereas APX activities did not change after ABA application. When a duplicate native gel was subjected to electroblotting and probed with a polyclonal antibody raised against peroxisomal APX (pAPX) from cucumber cotyledons, immunological staining coincided with the most active isoform of APX and a minor APX activity detected by the gel assay (Fig. 6, asterisks).
Figure 6.
APX activity and protein decline in GA-treated but not ABA-treated aleurone layers over time. A, APX activity gel of homogenates of GA- or ABA-treated aleurone layers. Arrowhead indicates the position of an activity not inhibited by the APX specific inhibitor p-chloromercuribenzoic acid, and asterisks indicate the position of APX activities recognized by the anti-pAPX-PAb. B, Immunoblot of homogenates of GA- or ABA-treated aleurone layers probed with a PAb against pAPX.
A decrease in the most active APX isoform could be detected in aleurone layers 24 h after GA treatment (Fig. 6A), and this was reflected in a reduction of the corresponding pAPX protein (Fig. 6B). A minor APX activity identified as a pAPX and the matching pAPX protein were also lower in layers exposed to GA for 12 h (Fig. 6). Very little APX activity or pAPX protein could be found in layers incubated in GA for 36 h (Fig. 6), even though more than 40% of cells are still alive (Fig. 1G). APX activities did not change over time in ABA-treated layers (Fig. 6A), although an increase in pAPX was detected in ABA-treated layers by protein blotting (Fig. 6B). The slowest migrating activity on the APX activity gel was unlikely to be APX since this activity was not inhibited by the APX-specific inhibitor p-chloromercuribenzoic acid (Fig. 6A, arrowhead and data not shown). Possible candidates for this activity were ascorbate oxidase and guaiacol preoxidase, which use ascorbate as electron donor, but their activities are unaffected by p-chloromercuribenzoic acid (Asada, 1997).
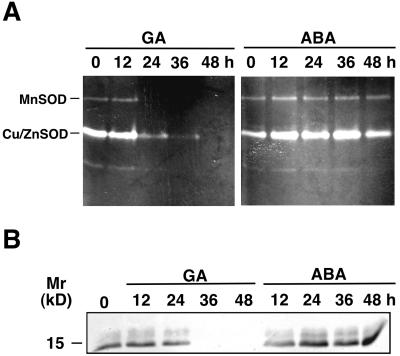
SODs catalyze the dismutation of superoxide radicals to oxygen and H2O2. There were dramatic changes in the activities of SOD isoforms and in the amounts of SOD protein and mRNA in GA-treated aleurone layers (Figs. 7 and 8). At least three SOD activities were detected after electrophoresis under native conditions (Fig. 7A). Cell fractionation experiments showed that the strongest band of activity is a cytosolic SOD and the two minor bands are organellar SODs (data not shown). SOD isoforms can be classified based on their sensitivity to cyanide and H2O2 (Scandalios, 1993). When SOD activity gels were incubated in 4 mm KCN or 5 mm H2O2, the cytosolic band of SOD activity was abolished, identifying this activity as a Cu/ZnSOD. The slowest migrating band was resistant to both inhibitors, a characteristic of MnSOD (data not shown). The most rapidly migrating band of activity could not be unequivocally classified by our inhibitor studies. All three SOD activities declined in GA-treated aleurone layers. The MnSOD activity was absent and the Cu/ZnSOD activity was dramatically reduced in homogenates of layers exposed to GA for 24 h. In contrast, SOD activities increased slightly following incubation in ABA for up to 48 h (Fig. 7A).
Figure 7.
SOD activity and protein decline in GA-treated but not ABA-treated aleurone layers over time. A, SOD activity gel of homogenates of GA- or ABA-treated aleurone layers. B, Immunoblot of homogenates of GA- or ABA-treated aleurone layers probed with an anti-maize cytosolic Cu/ZnSOD PAb. Size of an Mr marker is indicated on the left. The positions of MnSOD and Cu/ZnSOD on the activity gel are indicated.
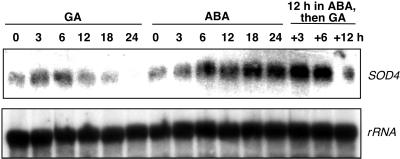
Figure 8.
Effect of GA and ABA on the amount of Sod4-cognate mRNA in aleurone layers. RNA was isolated from layers incubated in GA or ABA for the indicated times or from layers preincubated in 5 μm ABA with the addition of 25 μm GA at 12 h. RNA blots were hybridized with maize Sod4 cDNA and maize 28s rRNA probes.
Protein (Fig. 7B) and RNA (Fig. 8) blots support our observations on the effects of GA and ABA on SOD activity. Protein blots using antibodies raised against maize cytosolic Cu/ZnSOD4, which recognize all maize cytosolic SOD isoforms, show that a cognate of this protein decreases following GA treatment and is not present in aleurone layers incubated in GA for 36 h or longer. The amount of this enzyme remains unchanged or increased slightly in ABA-treated layers (Fig. 7B). Changes in SOD activity and protein are mirrored by changes in mRNA that hybridized to a maize Sod4 cDNA, though there were small and important differences. When RNA blots were probed with a maize Sod4 cDNA, RNA amounts were low in freshly isolated aleurone layers, increased slightly during the first 6 h of incubation in GA, and then declined to undetectable levels at 24 h (Fig. 8). In layers incubated in ABA there was a sustained increase in cognate Sod4 mRNA up to 24 h, when the amount of RNA was about twice that found in fresh layers. When layers pretreated with ABA for 12 h were transferred to medium containing 5 μm ABA and 25 μm GA, the amount of RNA declined (Fig. 8).
DISCUSSION
The results described in this paper support the hypothesis that death of GA-treated barley aleurone cells results from an inability to scavenge ROS. We show that PCD of cells in intact, GA-treated aleurone layers begins approximately 24 h after hormone treatment, with approximately 50% of the cells dead in 36 h and virtually all of the cells dead in 48 h. Cells in ABA-treated aleurone layers, however, remain alive for 48 h. The extent of cell death was quantified using fluorescent probes that simultaneously identify live and dead cells (Fig. 1) and these data were complementary to those when rates of oxygen consumption were used as a measure of cellular respiration (Fig. 2). The viability of ABA-treated aleurone cells was correlated with their ability to tolerate high concentrations of H2O2 (Fig. 3) and with relatively high rates of CAT, APX, and SOD activity (Figs. 4, 6, and 7). GA-induced death of aleurone cells, on the other hand, is correlated with rapid death on exposure to H2O2 (Fig. 3), and with reduced rates of activity for CAT, APX, and SOD (Figs. 4, 6, and 7). These data point to a strong causal relationship between ROS-metabolizing enzymes and PCD in intact aleurone layers. Furthermore, these data confirm earlier observations about the role of ROS in regulating death of aleurone protoplasts. GA-treated protoplasts are more susceptible to H2O2 than ABA-treated protoplasts and incubation of GA-treated protoplasts with the antioxidant butylated hydroxy toluene reduces the rate of hormonally induced cell death (Bethke and Jones, 2001).
A decline in the enzymes of ROS metabolism in GA-treated aleurone layers precedes death of cells within the layer. A decrease in the amount of CAT protein was seen as early as 12 h after incubation in GA (Fig. 4B), and by 24 h CAT was below the level of detection (Fig. 4B). pAPX proteins were also noticeably reduced 12 to 24 h after GA-treatment and were not detectable in layers incubated in GA for 36 h (Fig. 6B). Likewise, an SOD4-cognate was below the limit of detection 36 h after GA treatment (Fig. 7B). The changes in CAT, APX, and SOD protein mimic changes in CAT, APX, and SOD activity (Figs. 4, 6, and 7). In marked contrast, the amounts and activities of CAT, APX, and SOD do not decline during incubation in ABA for up to 48 h (Figs. 4, 6, and 7). Rather, total CAT activity increased when layers were incubated in ABA, and after 120 h in ABA CAT activity was 7- to 8-fold higher than in freshly isolated layers (Fig. 4A). The ABA-induced rise in total CAT activity could be reversed by incubation in GA. Transfer of layers preincubated in ABA for 3 d to GA brought about a rapid decline in CAT activity, as well as cell death (Fig. 4A).
Although cells in aleurone layers exposed to GA for up to 24 h are viable under normal conditions of incubation (Fig. 1), we demonstrated that they become progressively more susceptible to H2O2 during the time when the activities of ROS-metabolizing enzymes decline. Aleurone layers treated with GA or ABA for 12 h are insensitive to added H2O2 (Fig. 3). At later times of incubation, however, cell death results from addition of H2O2 to GA-treated layers, but death does not occur in ABA-treated layers (Fig. 3). The viability or death of aleurone layers exposed to H2O2 correlates strongly with the amounts of CAT, APX, and SOD (Figs. 4, 6, and 7) and foreshadows the eventual PCD of cells in GA-treated layers and prolonged life of cells in ABA-treated layers (Fig. 1). These data suggest that one aspect of the response of barley aleurone cells to GA is their increased vulnerability to damage and death resulting from ROS.
Of the ROS-metabolizing enzymes that we examined, CAT activity and protein declined the fastest following GA treatment of barley aleurone layers. To learn more about this response we determined the amount of Cat1 and Cat2 mRNA in barley aleurone layers treated with GA or ABA. Skadsen et al. (1995) showed that Cat1 and Cat2 are differentially expressed in barley. Cat1 was found to be the predominant Cat gene in the developing seed and in mature starchy endosperm and aleurone layers (Skadsen et al., 1995). On the other hand, Cat2 mRNA was virtually absent from the starchy endosperm and aleurone layer of germinating grain and from isolated aleurone layers treated with GA for 5 d (Skadsen et al., 1995). Here we show that Cat2 mRNA is present in freshly isolated barley aleurone layers and in layers incubated in ABA for up to 24 h, but that it declines rapidly following GA treatment. Cat2 mRNA was virtually absent in aleurone layers incubated in GA for 12 h or longer (Fig. 5).
Additional data suggest that the decline in Cat2 mRNA was a specific response to GA. First, we showed that addition of GA to aleurone layers that had been preincubated in ABA for 12 h brought about a rapid decline in the amount of Cat2 mRNA. This decline was comparable with the decline in Cat2 mRNA seen after GA treatment of freshly isolated aleurone layers (Fig. 5). Second, we showed that when the same RNA blots were probed with Amy cDNA probe, the GA-induced decline in Cat2 mRNA was matched, as would be expected, with a dramatic accumulation of Amy mRNA (Fig. 5). This experiment demonstrates that the decline in the amount of Cat2 mRNA did not result from a lack of GA-responsiveness in the aleurone layers used in our experiments. Hormonal regulation of Cat gene expression has also been demonstrated in maize. The promoter of maize Cat1 has a functional ABA response element (Guan et al., 2000). A putative GA-responsive element was found in the promoter of maize Cat2, but its functionality has not been established (Guan et al., 1996). In addition, a putative antioxidant response element motif is present in the promoter of all three maize Cat genes and it has been demonstrated that the expression of maize Cat genes can be regulated by H2O2 (Guan et al., 2000). Although our data do not provide evidence for a direct effect of H2O2 on Cat gene expression in barley aleurone, this possibility cannot be excluded.
The down-regulation of barley Cat2 mRNA by GA is unusual since only a few genes are repressed by GA in the barley aleurone layer. It is of interest that two of these genes are also related to stress responses: alcohol dehydrogenase and peroxiredoxin (Nolan and Ho, 1988; Stacy et al., 1996). Peroxiredoxins may protect barley embryos and aleurone cells against desiccation-induced free radical damage during late seed development and early imbibition (Stacy et al., 1996).
Changes in the amounts of Cat2 mRNA are consistent with changes in CAT activity and protein in our experiments. The observation that Cat1 mRNA persists in GA-treated layers is inconsistent with our data on CAT protein and CAT activity. Although GA stimulates Cat1 mRNA accumulation, our data show that CAT activity declines after 12 h in GA, and CAT activity and protein are absent from layers treated with GA for 24 h (Figs. 4 and 5). We interpret these data as showing that Cat1 mRNA is not translated or that CAT1 protein is turned over rapidly. This conclusion has precedent in other species, as the amount of Cat mRNA does not reflect the amount of CAT protein or activity in senescing peas (Distefano et al., 1999), maize shoots subject to low temperature (Auh and Scandalios, 1997), and tobacco leaves undergoing the HR (Chen et al., 1993).
APX is an important H2O2-scavenging enzyme that is found in the cytosol, chloroplasts, mitochondria, and peroxisomes of higher plants (Asada, 1997). We identified several APX isoforms in GA- and ABA-treated barley aleurone layers (Fig. 6). The activity of individual APX isoforms and the amount of pAPX decreased in GA-treated aleurone layers (Fig. 6). Because APX has a much higher affinity for H2O2 than CAT, the decrease in APX activity is likely to contribute to an accumulation of H2O2 and the eventual death of the aleurone cell. ABA increases the amount of pAPX protein and APX activity (Fig. 6). The idea that changes in APX activity may be important in preventing or promoting death is supported by experiments using tobacco plants undergoing the HR. The amount of cytosolic APX protein declined due to post-transcriptional suppression during pathogen-induced PCD in tobacco (Mittler et al., 1998), and pathogen-induced PCD was enhanced in transgenic tobacco plants expressing antisense Apx (Mittler et al., 1999). These data lead Mittler et al. (1999) to conclude that a reduced capability to detoxify ROS may lead to PCD. Studies with senescing daylily flowers, where CAT and APX activities were found to decrease, support this conclusion (Panavas and Rubinstein, 1998).
SOD is also widely recognized as an important ROS-scavenging enzyme in plants, and our data show a strong positive correlation between the amount of Sod4 mRNA, enzyme activity, and the effects of GA and ABA on cell viability in aleurone layers (Figs. 1, 7, and 8). The three types of SODs known to exist in plants differ in their functional metals and subcellular localization (Bowler et al., 1994). Cu/ZnSODs are generally found in the cytosol, but have also been detected in peroxisomes and chloroplasts. FeSOD is located in the chloroplast, and MnSOD has been found in the mitochondrial matrix and peroxisomes (Bowler et al., 1994). The abundance of SOD isoforms is highly variable and is regulated by environmental and developmental stimuli (Bowler et al., 1992). We show that barley aleurone cells contain at least three different SOD isoforms, and we have identified the major activity as cytosolic Cu/ZnSOD and one of the minor activities as an organellar MnSOD (Fig. 7A). All three SOD activities are down-regulated by GA at a time when aleurone cells are still alive. SOD activities increased in ABA-treated layers (Fig. 7A).
We probed RNA blots with a maize cDNA clone encoding a cytosolic Cu/Zn Sod4 (Fig. 8). We showed that the GA-induced decrease in SOD activity was paralleled by a decrease in the amount of Sod4-cognate mRNA (Fig. 8). ABA increased the amount of Sod4-cognate mRNA in aleurone layers (Fig. 8), but addition of GA to aleurone layers preincubated in ABA for 12 h led to a decline in the amount of Sod4-cognate mRNA. These data support the hypothesis that Sod4 gene expression is hormonally regulated. ABA has been shown previously to regulate Sod4 gene expression in maize, as well as cytosolic Cu/Zn SodCc2 gene expression in rice (Sakamoto et al., 1994; Zhu and Scandalios, 1994; Guan and Scandalios, 1998). To our knowledge, however, this is the first report of GA-regulated SOD gene expression.
In this paper we show a direct correlation between the amounts of ROS-scavenging enzymes and hormonal regulation of cell death in aleurone cells. As a consequence of normal metabolism, especially lipid metabolism, O2.− and H2O2 are produced by barley aleurone layers. The GA-induced disappearance of CAT activity in barley aleurone layers occurs at a time when all cells of the layer are alive and metabolically active and malate synthase, a glyoxysomal enzyme of the glyoxylate cycle, is still detectable (Fig. 2 and data not shown). Cell death does not occur until 12 to 24 h later. We speculate that other H2O2-scavenging systems can partially compensate the loss of CAT activity over the next 12 to 24 h. As APX and SOD activities diminish, however, there is an inevitable increase in the amount of ROS in GA-treated aleurone cells. ROS may then damage membrane lipids and DNA, or might effect the function of cellular proteins. Peroxidation of membrane lipids may result in the loss of membrane integrity and function. This is in agreement with earlier observations showing that GA-induced PCD in barley aleurone protoplasts was rapid and resulted in rupture of the plasma membrane (Bethke et al., 1999).
MATERIALS AND METHODS
Plant Material
Aleurone layers were prepared from de-embryonated barley (Hordeum vulgare cv Himalaya, 1991 harvest, Washington State University, Pullman) grains as described by Schuurink et al. (1996). Isolated aleurone layers were treated with 20 mm CaCl2 and 5 μm GA or 5 μm ABA and incubated for the indicated time.
Determination of Cell Viability
Viability of cells in intact aleurone layers was determined by staining living aleurone layers with FDA (2 μg mL−1 in 20 mm CaCl2; Molecular Probes, Eugene, OR) for 15 min followed by FM 4-64 (20 μm in 20 mm CaCl2; Molecular Probes) for 3 min. Layers were observed with a microscope (Axiophot, Zeiss, Thornwood, NJ) and images where captured using film (Ektachrome 160T, Eastman Kodak, Rochester, NY). A template containing five separate regions was superimposed on these images and live and dead cells in each region were counted.
Oxygen Consumption by Aleurone Tissue
At the indicated times a single aleurone layer was placed in an oxygen electrode unit containing 2 mL of water (Rank Brothers, Bottisham, UK) and oxygen uptake was measured at room temperature for 20 min. Oxygen consumption remained linear over the investigated 20 min.
CAT Activity Assay
CAT activity was determined by measuring H2O2-dependent oxygen evolution at room temperature with an oxygen electrode unit (Rank Brothers). Ten aleurone layers were ground to a fine powder in liquid nitrogen extracted in 300 μL buffer (60 mm potassium phosphate, pH 7.8, 0.1 mm EDTA, 20 μm E64, 20 μm pepstatin, and 20 μm leupeptin) and the homogenate was centrifuged in an Eppendorf tabletop centrifuge (model 5415C) for 15 min at 4,600 rpm at 4°C. Assays were performed in a 5-mL aqueous solution containing 17.6 mm H2O2 to which 10 μL of the supernatant was added. Commercially available bovine liver CAT (Sigma, St. Louis) was used to calibrate the electrode. One unit was defined as the decomposition of 1 μmol H2O2 per minute.
Immunoblotting
Samples (30 μL per lane) prepared as described above (CAT activity assay) were separated by SDS-PAGE (for SOD and CAT western blots) or PAGE (for APX western blots). After electrophoresis on 12.5% (w/v) SDS-PAGE or 10% (w/v) PAGE, proteins were electrotransferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Protein blots were blocked with 3% (w/v) skim milk powder in phosphate-buffered saline, and the appropriate first antibody was added at a 1:200 dilution and incubated overnight. Secondary antibody (goat-anti-rabbit IgG) coupled to horseradish peroxidase (Sigma) was visualized chromogenically.
RNA Isolation and RNA Gel Blotting
Aleurone layers (15) were ground to a fine powder in liquid nitrogen and total barley aleurone RNA was isolated according to the manufacturer's instruction using the RNeasy plant mini kit (Qiagen, Valencia, CA). RNA gel blots were made by separating RNA (5 μg) on a formaldehyde agarose gel (1.2%, w/v) followed by blotting onto a nylon membrane (msi, Micron Separations Inc., Westboro, MA) by capillary action (Sambrook et al., 1989). Membranes were hybridized at 65°C in 7% (w/v) SDS, 0.5 m sodium phosphate, pH 7.2, and 5 mm EDTA. cDNA probes were labeled with [α32-P]dCTP (ICN Biomedicals, Costa Mesa, CA) by random priming using the Prime-it RmT random primer labeling kit (Stratagene, La Jolla, CA). After hybridization the membrane was washed for 20 min at 65°C in 2× SSC (1× SSC is 0.15 m NaCl, 15 mm sodium citrate) and 0.1% (w/v) SDS, then washed for 20 min at 65°C in 0.5× SSC and 0.1% (w/v) SDS, followed by a wash for 20 min at 65°C in 0.1× SSC and 0.1% (w/v) SDS. The blots were exposed to film (X-Omat AR-5, Eastman Kodak) with an intensifying screen at −80°C for 30 min to 48 h. The amount of 32P-labeled cDNA probe hybridizing to specific mRNAs was determined semiquantitatively by exposing the membrane to a Phosphor Imager screen, which was then analyzed by laser densitometry (Molecular Dynamics, Sunnyvale, CA). The blot was stripped and reprobed with maize s28 rRNA cDNA probe to standardize loading. For each replicate experiment, the same membrane was used for all cDNAs.
APX Activity Gels
APX activity was assayed using the method described by Mittler and Zilinskas (1993). In brief, aleurone layers (10) were ground to a fine powder in liquid nitrogen extracted in 300 μL of buffer (60 mm sodium phosphate, pH 7.8, 0.1 mm EDTA, 5 mm ascorbate, and 10% [w/v] glycerol) at 4°C, the homogenate was centrifuged for 15 min at 4,600 rpm, and samples of the supernatant (30 μL) were separated by 10% (w/v) PAGE under non-denaturing and non-reducing conditions. The gels were pre-run for 30 min in a carrier buffer containing 2 mm ascorbate. After electrophoresis at 100 V and 4°C using carrier buffer containing 2 mm ascorbate, the gel was immersed in 50 mm sodium phosphate, pH 7.0, and 2 mm ascorbate for 30 min, changing the solution every 10 min. The gel was soaked in 50 mm sodium phosphate, pH 7.0, 4 mm ascorbate, and 2 mm H2O2 for an additional 20 min before a brief washing with 50 mm sodium phosphate, pH 7.0. The gel was incubated in 50 mm sodium phosphate, pH 7.8, 28 mm tetramethylethylenediamine (Sigma) and 2.45 mm nitroblue tetrazolium (Sigma) until the gel turned uniformly blue except at positions exhibiting APX activity. When maximum contrast was achieved, the reaction was stopped by rinsing the gel with water. To determine the specificity of the APX activity, the gel was incubated with 50 mm sodium phosphate, pH 7.0, and 2 mm ascorbate in the presence of 0.5 mm p-chloromercuribenzoic acid (Nutritional Biochemicals, Cleveland) for 30 min, changing the solution every 10 min (Chen and Asada, 1989; Mittler and Zilinskas, 1993). The gel was processed as described above with the exception that 0.5 mm p-chloromercuribenzoic acid was added to every incubation step except the developing step.
SOD Activity Gels
SOD activity was assayed using the method described by Beauchamp and Fridovich (1971). In brief, aleurone layers were prepared as described above for CAT activity assay and samples of the supernatant (30 μL per lane) were separated by PAGE under non-denaturing and non-reducing conditions. After electrophoresis on a 12.5% (w/v) native PAGE at 100 V and room temperature the gel was immersed in 2.45 mm nitroblue tetrazolium for 20 min, followed by a 15-min soak in a solution containing 28 mm tetramethylethylenediamine, 28 μm riboflavin (Sigma), and 36 mm potassium phosphate, pH 7.8. SOD activity was detected by illuminating the gel with bright light. This caused the gel to turn uniformly blue except at positions exhibiting SOD activity. When maximum contrast was achieved, the reaction was stopped by rinsing the gel with water. CuZn, Fe, or MnSOD activities were distinguished from each other by their sensitivity to 4 mm KCN or 5 mm H2O2 (Scandalios, 1993). In brief, samples were incubated in freshly prepared 4 mm KCN or 5 mm H2O2 for 30 min at 4°C prior to loading on the gel. After electrophoresis, the gels were incubated as described above with the exception that 4 mm KCN or 5 mm H2O2 was added to each incubation buffer.
ACKNOWLEDGMENTS
The authors thank Steven Huang for technical assistance and Eleanor Crump for editorial assistance. We also thank Dr. Ronald Skadsen (U.S. Department of Agriculture, Madison, WI) for providing the barley Cat1 and Cat2 cDNAs, Dr. John Scandalios (North Carolina State University, Raleigh) for the generous gift of the maize Sod4 cDNA as well as the anti-maize SOD4 antibody and anti-maize CAT antibody, and Dr. Richard Trelease (Arizona State University, Tempe) for supplying anti-cucumber pAPX antibody.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9818047) and by Novartis Agricultural Discovery Institute (to R.L.J.).
LITERATURE CITED
- Appleford NEJ, Lenton JR. Hormonal regulation of α-amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiol Plant. 1997;100:534–542. [Google Scholar]
- Asada K. The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2 scavenging in plants. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 715–735. [Google Scholar]
- Auh C-K, Scandalios JG. Spatial and temporal responses of the maize catalases to low temperature. Physiol Plant. 1997;101:149–156. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Hillmer S, Jones RL. Isolation of intact protein storage vacuoles from barley aleurone. Plant Physiol. 1996;110:521–529. doi: 10.1104/pp.110.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001;25:19–29. doi: 10.1046/j.1365-313x.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Lonsdale JE, Fath A, Jones RL. Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell. 1999;11:1033–1045. doi: 10.1105/tpc.11.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Swanson SJ, Hillmer S, Jones RL. From storage compartment to lytic organelle: the metamorphosis of the aleurone protein storage vacuole. Ann Bot. 1998;82:399–412. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Van Camp W, Van Montagu M, Inze D. Superoxide dismutase in plants. Crit Rev Plant Sci. 1994;13:199–218. [Google Scholar]
- Bowler C, Van Montagu M, Inze D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:83–116. [Google Scholar]
- Chen G-X, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the difference in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jimenez A, Lopez-Huertas E, Hernandez JA. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998;116:1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano S, Palma JM, McCarthy I, del Rio LA. Proteolytic cleavage of plant proteins by peroxisomal endoproteases from senescent pea leaves. Planta. 1999;209:308–313. doi: 10.1007/s004250050637. [DOI] [PubMed] [Google Scholar]
- Doig RI, Colborne AJ, Morris G, Laidman DL. The induction of glyoxysomal enzyme activities in the aleurone cells of germinating wheat. J Exp Bot. 1975;26:387–398. [Google Scholar]
- Dorey S, Baillieul F, Saindrenan P, Fritig B, Kauffmann S. Tobacco class I and II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol Plant-Microbe Interact. 1998;11:1102–1109. [Google Scholar]
- Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R. Programmed cell death in cereal aleurone. Plant Mol Biol. 2000;44:255–266. doi: 10.1023/a:1026584207243. [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke PC, Jones RL. Barley aleurone cell death is not apoptotic: characterization of nuclease activities and DNA degradation. Plant J. 1999;20:305–315. [PubMed] [Google Scholar]
- Guan L, Polidoros AN, Scandalios JG. Isolation, characterization and expression of the maize Cat2 catalase gene. Plant Mol Biol. 1996;30:913–924. doi: 10.1007/BF00020803. [DOI] [PubMed] [Google Scholar]
- Guan L, Scandalios JG. Two structurally similar maize cytosolic superoxide dismutase genes, Sod4 and Sod4A, respond differentially to abscisic acid and high osmoticum. Plant Physiol. 1998;117:217–224. doi: 10.1104/pp.117.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan LM, Zhao J, Scandalios JG. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000;22:87–95. doi: 10.1046/j.1365-313x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Haberlandt G. Physiologische Pflanzenanatomie. Ed 1. Leipzig, Germany: W. Engelman; 1884. [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharm. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Hernandez JA, Pastori G, Del Rio LA, Sevilla F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL. The effect of ultracentrifugation on fine structure and α-amylase production in barley aleurone cells. Plant Physiol. 1969;44:1428–1438. doi: 10.1104/pp.44.10.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Feng X, Cohen M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell. 1998;10:461–473. doi: 10.1105/tpc.10.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inze D, Ellis B. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hypersensitive to pathogen infection. Proc Natl Acad Sci USA. 1999;96:14165–14170. doi: 10.1073/pnas.96.24.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zilinskas BA. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem. 1993;212:540–546. doi: 10.1006/abio.1993.1366. [DOI] [PubMed] [Google Scholar]
- Nolan RC, Ho T-HD. Hormonal regulation of gene expression in barley aleurone layers: induction and suppression of specific genes. Planta. 1988;174:551–560. doi: 10.1007/BF00634486. [DOI] [PubMed] [Google Scholar]
- Olsen O-A, Lemmon BE, Brown RC. Developmental biology of the cereal endosperm. Trends Plant Sci. 1999;4:253–257. doi: 10.1016/s1360-1385(99)01431-4. [DOI] [PubMed] [Google Scholar]
- Panavas T, Rubinstein B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998;133:125–138. [Google Scholar]
- Pastori GM, del Rio LA. Natural senescence of pea leaves: an activated oxygen-mediated function for peroxisomes. Plant Physiol. 1997;113:411–418. doi: 10.1104/pp.113.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Okumura T, Kaminaka H, Sumi K, Tanaka K. Structure and differential response to abscisic acid of two promoters for the cytosolic copper/zinc-superoxide dismutase genes, SodCc1 and SodCc2, in rice protoplasts. FEBS Lett. 1995;358:62–66. doi: 10.1016/0014-5793(94)01396-i. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG, Guan L, Polidoros AN. Catalases in plants: gene structure, properties, regulation, and expression. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 343–406. [Google Scholar]
- Schuurink RC, Chan PV, Jones RL. Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiol. 1996;111:371–380. doi: 10.1104/pp.111.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink RC, van Duijn G, Heidekamp F. Is abscisic acid involved in barley aleurone dormancy? In: Walker-Simmons MK, Ried JL, editors. Pre-Harvest Sprouting in Cereals 1992. American Association of Cereal Chemists, St. Paul. 1993. [Google Scholar]
- Skadsen RW, Schulze-Lefert P, Herbst JM. Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Mol Biol. 1995;29:1005–1014. doi: 10.1007/BF00014973. [DOI] [PubMed] [Google Scholar]
- Stacy RAP, Munthe E, Steinum T, Sharma B, Aalaen RB. A peroxiredoxin antioxidant is encoded by a dormancy-related gene, Per1, expressed during late development in the aleurone and embryo of barley grains. Plant Mol Biol. 1996;31:1205–1216. doi: 10.1007/BF00040837. [DOI] [PubMed] [Google Scholar]
- Swanson S, Bethke PC, Jones RL. Barley aleurone cells contain two types of vacuoles: characterization of lytic compartments using fluorescent probes. Plant Cell. 1998;13:685–698. doi: 10.1105/tpc.10.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Oppedijk B, Lu X, Van Duijn B, Schilperoort RA. Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol Biol. 1996;32:1125–1134. doi: 10.1007/BF00041396. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chen Z. Harpin-induced hypersensitive cell death is associated with altered mitochondrial functions in tobacco cells. Mol Plant-Microbe Interact. 2000;13:183–190. doi: 10.1094/MPMI.2000.13.2.183. [DOI] [PubMed] [Google Scholar]
- Zhu D, Scandalios JG. Differential accumulation of manganese-superoxide dismutase transcripts in maize in response to abscisic acid and high osmoticum. Plant Physiol. 1994;106:173–178. doi: 10.1104/pp.106.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]