Abstract
The evidence from clinical trials concerning the efficacy of dietary polyphenols on cardiometabolic health is divergent. Therefore, this review aimed to determine the pooled effect of dietary polyphenols on cardiometabolic risk markers and compare the difference in efficacy between whole polyphenol-rich foods and purified food polyphenol extracts. We conducted a random-effect model meta-analysis of randomized controlled trials (RCTs) on the effect of polyphenols on blood pressure, lipid profile, flow-mediated dilation (FMD), fasting blood glucose (FBG), waist circumference, and markers of inflammation. Effect size was expressed as weighted mean difference and 95% CI. RCTs published in English between 2000 and 2021 involving adult participants with cardiometabolic risks were searched in electronic databases. Forty-six RCTs involving 2494 participants with a mean age of 53.3 ±10 y were included in this review. Whole polyphenol-rich food but not purified food polyphenol extracts significantly reduced systolic blood pressure (SBP, −3.69 mmHg; 95% CI: −4.24, −3.15 mmHg; P = 0.00001) and diastolic blood pressure (DBP, −1.44 mmHg; 95% CI: −2.56, −0.31 mmHg; P = 0.0002). Concerning waist circumference, purified food polyphenol extracts led to a larger effect (−3.04 cm; 95% CI: −7.06, −0.98 cm; P = 0.14). Significant effects on total cholesterol (−9.03 mg/dL; 95% CI: −16.46, −1.06 mg/dL; P = 0.02) and TGs (−13.43 mg/dL; 95% CI: −23.63, −3.23; P = 0.01) were observed when purified food polyphenol extracts were considered separately. None of the intervention materials significantly affected LDL-cholesterol, HDL-cholesterol, FBG, IL-6, and CRP. When both whole food and extracts were pooled together, there was a significant reduction in SBP, DBP, FMD, TGs, and total cholesterol. These findings suggest that polyphenols both as whole food and purified extracts can be efficacious in reducing cardiometabolic risks. However, these results must be interpreted with caution because of high heterogeneity and risk of bias among RCTs. This study was registered on PROSPERO as CRD42021241807.
Keywords: polyphenols, cardiometabolic risks, dyslipidemia, blood pressure, inflammation, vascular function, central adiposity, blood glucose
Statements of Significance.
To the best of our knowledge, this is the first meta-analysis to make a comparison between whole polyphenol-rich foods and purified polyphenol extracts. Additionally, it summarizes current evidence on the effect of dietary polyphenols on several cardiometabolic risk markers.
Introduction
The current global surge in cardiometabolic risks [1] necessitates investing in both preventive and curative interventions [[2], [3], [4]]. Over the last 2 decades (2000–2020), there has been an exponential rise in the importance of polyphenols as potential modulators for CVDs [2]. Consequently, this has increased the inclusion of polyphenols in the human diet as either whole polyphenol-rich food or purified extracts often used as supplements [5].
Polyphenols are a diverse category of plant secondary metabolites with a wide range of complex structures [5]. The main classes of polyphenols are phenolic acids, flavonoids, stilbenes, phenolic alcohols, and lignans. Flavonoids are by far the most abundant group of polyphenols in the diet and are comprised of flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols [6]. Flavonoids are widespread constituents of fruits; vegetables; cereals; olives; dry legumes; chocolate; beverages, such as tea, coffee, and red wine; and some spices [4]. Polyphenols could attenuate a number of cardiometabolic risk markers, such as blood pressure, blood glucose, abdominal obesity, and lipid, oxidative, inflammation, and vascular health markers [5,[7], [8], [9], [10], [11]]. These health benefits are essentially because of the antioxidant and anti-inflammatory properties of dietary polyphenols [2].
Although the case for the biological functions of polyphenols in humans is accumulating, there remains insufficient evidence on the health effects related to their consumption and cardiometabolic health. The available evidence from clinical trials linked to the efficacy of polyphenols on cardiometabolic health is divergent. First, the diversity of polyphenolic compounds in food. The intensity of the biological activities of polyphenols primarily depends on their chemical structure and bioavailability, which account for the proportion absorbed, digested, and metabolized after entering the circulatory system [12,13]. In whole food, most polyphenols are present as glycosides with complex oligomeric structures and require several biotransformations to reach the target tissue, which could reduce its bioavailability or completely alter its bioactivity. Ultimately, the focus is increasingly shifting to the use of purified extracts whose bioavailability is said to be superior [5]. Second, the high interindividual variation in terms of absorption, metabolism, and excretion patterns because of both genetic and nongenetic factors (gender, gut microbiota profile, age, and [epi]genotype), may explain the heterogeneity in the efficacy of dietary polyphenols [2,5]. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) on the effect of dietary polyphenols on selected markers of suboptimal cardiometabolic health and compared the efficacy of whole polyphenol-rich foods and purified food polyphenol extracts.
Methods
Search strategy
Electronic databases of PubMed, EMBASE, Scopus, and Web of Science were searched. Additional studies were identified from the bibliographies of published reviews and included articles (snowballing). The initial search string (Supplementary Table S1) was developed in PubMed using the PICO criteria in Table 1. The search string was adapted for use in the different databases. Searches were conducted from January 2000 to December 2021. All identified studies were imported to endnote. Before conducting data extraction, the review protocol was registered on PROSPERO as CRD42021241807.
TABLE 1.
PICOS criteria used for systematic literature review
| Participants | People (≥18 y) with cardiometabolic risks (prehypertension, hypertension, metabolic syndrome, overweight, obesity, dyslipidemia, insulin resistance, type 2 visceral adiposity, prediabetes, and type 2 diabetes mellitus) |
| Intervention | Dietary polyphenols (whole food and purified polyphenol extracts) |
| Comparisons | Placebo |
| Outcomes | Lipid profile (reduction of Total cholesterol, TGs, LDL-c and an increase in HDL-c), glucose homeostasis (FBG), endothelial function (measured by Flow-Mediated Dilation-FMD), blood pressure (SBP and DBP) and inflammation markers (CRP and IL-6). reduction in waist circumference |
| Study design | Only RCTs (both parallel and cross-over designs) were eligible |
FBG, fasting blood glucose; PICOS, participants, intervention, comparisons, outcomes, and study design.
Eligibility criteria
The review was limited to RCTs (both parallel and cross-over designs) conducted among populations aged ≥18 y living with cardiometabolic risks (Supplementary Table S2). Only human studies published in English from January 2000 and December 2021 have been included.
Data extraction
Study selection
Two independent reviewers (TK and CM) screened the identified studies according to the inclusion criteria to select the relevant ones. Prior to this, duplicates were removed. First, titles and abstracts were assessed. In case of insufficient information in the title and abstract, full text was read. The full text of the selected studies was then retrieved and read to determine whether they met the inclusion criteria. In the event of a disagreement between the reviewers, “the discussion model until consensus is reached” was applied. Where this failed, a third reviewer was consulted. Excluded full-text studies were listed with the reason for their exclusion.
Data coding
Using a structured excel form, we extracted the following information from the studies on variables such as study title, author(s), journal, year of publication, study design, study setting (country and city), sample size (number of participants), and participants’ characteristics (age, sex and health status, and study eligibility criteria), and doses. Classes of polyphenols (defined according to Tomas-Barberan et al. [5]), baseline and endline mean and SD of primary and secondary outcome measures. Trialists were contacted for data if the published result details were insufficient. Where SDs were missing but either CIs, SEM, or P values were provided, SDs were then calculated following the Cochrane Handbook guidelines [14]. Other variables included study duration, metabolism of polyphenols, and mechanism of action of polyphenols. Any discrepancies encountered during data extraction were resolved through discussion with a third reviewer (PY).
Risk of bias (quality) assessment
Studies were assessed independently for methodological validity by 2 reviewers (TK and CM); prior to inclusion in the review using the Cochrane tool for assessing the risk of bias in randomized trials (RoB tool) [15]. The following domains were assessed: random sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. We judged the RoB in each study as “low,” “high,” or “uncertain.” We judged a domain as “Unclear risks of bias” if there was no information or uncertainty over the potential of threat to validity.
Data analysis
We performed a meta-analysis of mean differences [15] for total cholesterol, LDL-cholesterol, HDL-cholesterol, TGs, FBG, waist circumference, CRP, IL-6, systolic blood pressure (SBP), and diastolic blood pressure (DBP) between the intervention and control groups using random-effect models at 95% CI. The heterogeneity in the studies was assessed by the I2 statistic (a measure of the proportion of between-study variability) and judged as low, moderate, or high if it reached 25%, 50%, and 75%, respectively [16]. Potential sources of variability and whether or not it could affect the mean differences in risk markers were investigated in subgroup analyses. Subgroups were predefined based on the following 4 criteria: 1) study size–studies were stratified into small studies (<30 participants) and larger studies (≥30 participants); 2) study duration–distinction was made between studies with follow-up duration of maximum 4 wk and studies with follow-up time beyond 4 wk; 3) study design–studies were subgrouped into parallel and cross-over designs; and 4) intervention material given –whole food-rich in polyphenols, or purified food polyphenol extracts. Publication bias was qualitatively assessed by visually inspecting funnel plots and quantitatively by performing Egger’s regression test. Trim-and-Fill correction was applied to adjust the analysis for the effects of publication bias. Sensitivity analysis was on the other hand done by removing studies with a high RoB. The analyses were performed in Review Manager 5.4.1 Software. Effect size was expressed as weighted mean difference and 95% CI and statistical significance was considered for P value of <0.05.
Results
Study search and screening results
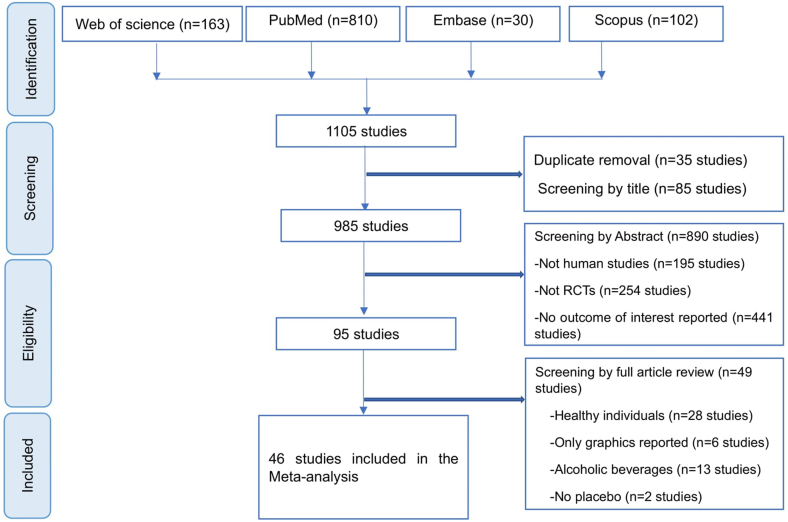
The search yielded 1104 studies. After removal of duplicate studies (n = 35), a total of 975 studies were judged irrelevant by their title (n = 856) and abstract (n = 119) screening. A total of 94 studies were screened for full-text review. Out of the 94 studies, 49 studies were eliminated for various reasons as reported in PRISMA flow diagram (Figure 1). A total of 46 studies met the inclusion criteria and were included in quality assessment and meta-analysis. Studies were mainly from Europe, South America, United States, and Asia. No study was found in Africa.
FIGURE 1.
PRISMA flowchart showing the screening process of the studies included in the meta-analysis.
Characteristics of the studies included
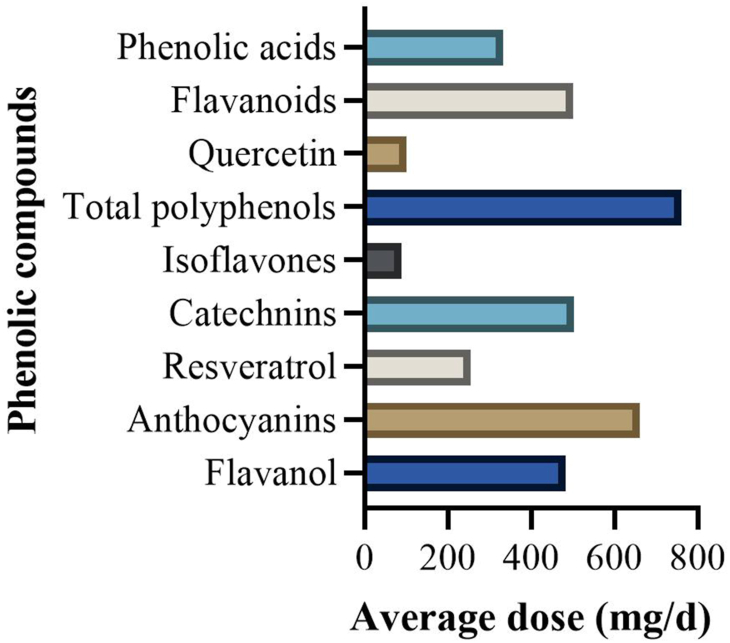
Overall, the included studies comprised 2494 participants spread into the intervention arm (n = 1254) and placebo (n = 1240) with a mean age of 53.3 ±10 y. The intervention material was either a whole polyphenols-rich food (n = 28 studies) or purified food polyphenol extracts (n = 18 studies). The size for the studies ranged from 16 to 270 participants and the study duration was between 3 d and 12 mo. Berries were the most frequently studied food items, followed by green/black tea, cocoa/chocolates, and grape/grape seeds (Supplementary Table S3). On average, the portion sizes of fruits as an intervention material were 5 servings per day (Supplementary Table S1). Flavonoids were the most studied group of polyphenols (n = 36 studies), whereas there were 6 studies on nonflavonoids and 4 studies on total polyphenols. Among the flavonoids, flavanols constituted the highest number of studies, whereas the stilbenes were the most studied class in the nonflavonoid group (Supplementary Figure S1). The average doses of the different phenolic compounds as administered in the included studies are illustrated in Figure 2. The doses ranged from 20 to 1000 mg/d, 30 to 1177 mg/d, and 6.2 to 1000 mg/d for nonflavonoids, total polyphenols, and flavonoids, respectively. An elaborate description of the characteristics of each included study is provided in Supplementary Table S1.
FIGURE 2.
Average daily dosage of the different phenolic compounds in the included studies. RCT, randomized controlled trial.
Although neither weight nor BMI was an intended outcome for our review, we provide details of these 2 parameters (Supplementary Table S2). In 15 of 46 studies that measured body weight, only 4 studies reported significant weight loss in the intervention groups. In 3 of these 4 studies, participants were required to follow an isocaloric diet alongside the test food or supplement.
Mostly food intake measurements were performed as a control tool for adherence to the intervention and quite often results of dietary intake were never reported. For the 20 studies that reported dietary intake results, none of them indicated significant changes in total energy and macronutrient intake (carbohydrates, proteins, fats, fiber, SFA, and PUFA) across study arms. Details of dietary intake and restrictions/modifications are shown in Supplementary Table S2. Participants were often encouraged to maintain habitual diet. Only 15 studies forbade participants from consuming other foods that might have similar concentrations of polyphenols similar to the test food/supplement.
Risk of bias assessment
Overall, studies demonstrated a high risk of selection, performance, and detection bias (Supplementary Figures S2 and S3). Thirteen studies did not provide details of random sequence generation, 15 studies either did not report allocation concealment or the details were unclear, whereas in 7 studies there was no blinding of participants and personnel or outcome assessors. Consequently, 8 studies [8,[17], [18], [19], [20], [21], [22], [23]] were rated “high,” whereas 5 studies [[24], [25], [26], [27], [28]] were judged to have “some concerns” for overall RoB.
Publication bias assessment
Visual inspection of funnel plots of mean difference and SE (Supplementary Figures S4–S14) suggested potential publication bias for the effect of polyphenols on SBP, DBP, LDL-cholesterol, flow-mediated dilation (FMD), IL-6, and waist circumference. Additionally, results of Egger’s regression-based test presented in Supplementary Table S4 confirmed publication bias for SBP, DBP, LDL-cholesterol, and IL-6. Using trim-and-fill correction, 3, 9, 2, 4, 1, 3, 4, and 3 potentially missing studies were imputed for the analysis of SBP, LDL-cholesterol, HDL-cholesterol, TG, FBG, waist circumference, IL-6, and CRP, respectively. The imputed effect sizes are presented in Supplementary Table S5.
Meta-analysis results
The overall results of the meta-analyses for the different outcomes are presented in Table 2 [29–51]. Subgroup analyses are reported in TABLE 3, TABLE 4, TABLE 5, TABLE 6 for intervention materials, study duration, study size, and study design, respectively. Forest plots for the intervention material subgroup analysis of TGs, total cholesterol, and waist circumference are presented in FIGURE 3, FIGURE 4, FIGURE 5, respectively, and in Supplementary Figures S15–S22 for SBP, DBP, LDL-cholesterol, HDL-cholesterol, FBG, FMD, IL-6, and CRP.
TABLE 2.
Random-effects model meta-analysis of mean difference of blood pressure, lipid profile, fasting blood glucose, waist circumferences, endothelial function, and markers of inflammation
| Outcomes | WMD (95% CI) | P value | Studies, n | Participants, n | I2(%) | References |
|---|---|---|---|---|---|---|
| Blood pressure (mmHg) | ||||||
| SBP | −3.53 (−3.90, −3.16) | 0.00001 | 27 | 1313 | 3 | [8,[19], [20], [21], [22],[26], [27], [28],29,33,35,36,39,43,46,71] |
| DBP | −1.41 (−2.38, −0.44) | 0.004 | 26 | 1618 | 60 | [8,[19], [20], [21], [22],27,28,29,33,35,36,39,43,46,71] |
| Lipid profile (mg/dL) | ||||||
| LDL-c | −5.4 (−17.94, 7.14) | 0.40 | 26 | 1523 | 98 | [19,20,[22], [23], [24],26,27,29,31,32,38,40,41,[44], [45], [46], [47], [48], [49], [50], [51], [52], [53],57,71,72] |
| HDL-c | 0.79 (−0.43, 2.02) | 0.20 | 28 | 1621 | 68 | [19,20,[22], [23], [24],[26], [27], [28],29,31,32,57,38,[40], [41], [42],[44], [45], [46], [47], [48], [49], [50], [51], [52], [53],71,72] |
| TGs | −10.12 (−19.40, −0.84) | 0.03 | 29 | 1657 | 80 | [19,20,[22], [23], [24],[26], [27], [28],29,31,32,57,38,[40], [41], [42],[44], [45], [46], [47], [48], [49], [50], [51], [52], [53],71,72] |
| Total cholesterol | −5.11 (−9.19, −1.02) | 0.01 | 27 | 1570 | 76 | [19,23,24,26,27,29,31,32,38,40,41,[44], [45], [46], [47], [48], [49], [50], [51], [52], [53],57,71,72] |
| Glucose (FBG) (mg/dL) | −1.67 (−3.91, 0.58) | 0.15 | 25 | 1643 | 84 | [8,19,20,22,26,[28], [29], [30], [31], [32],37,38,41,42,[45], [46], [47],50,[52], [53], [54], [55],57,71,72] |
| Waist circumference (cm) | −1.42 (−3.40, 0.56) | 0.16 | 13 | 1055 | 77 | [22,[28], [29], [30], [31],37,45,46,51,[53], [54], [55],72] |
| FMD (%) | 2.22 (0.66, 3.77) | 0.005 | 9 | 338 | 92 | [7,28,32,34,44,51,[70], [71], [72]] |
| Inflammation markers | ||||||
| IL-6 (pg/mL) | −0.14 (−1.16, 0.87) | 0.78 | 10 | 502 | 97 | [17,18,25,27,31,38,47,49,51,56] |
| CRP (mg/L) | −0.16 (−0.35, 0.03) | 0.09 | 16 | 829 | 0 | [8,[17], [18], [19],24,32,38,41,46,47,49,52,56,57,72,80] |
I2 statistic (statistical heterogeneity).
DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; SBP, systolic blood pressure; WMD, weighted mean difference.
Significance level at P < 0.05.
TABLE 3.
Random-effects model meta-analysis of mean difference of blood pressure, lipid profile, fasting blood glucose, waist circumferences, endothelial function, and markers of inflammation according to trial intervention material
| Subgroups | WMD (95% CI) | P value | Studies, n | Participants, n | I2 (%) |
|---|---|---|---|---|---|
| SBP (mmHg) | |||||
| Whole polyphenol-rich food | −3.69 (−4.24, −3.15) | 0.00001 | 16 | 846 | 24 |
| Purified food polyphenol extracts | −1.45 (−3.19, −0.28) | 0.10 | 11 | 467 | 0 |
| DBP (mmHg) | |||||
| Whole polyphenol-rich food | −1.44 (−2.56, −0.31) | 0.0002 | 14 | 773 | 66 |
| Purified food polyphenol extracts | −1.13 (−3.32, 1.07) | 0.31 | 12 | 845 | 53 |
| LDL-c (mg/dL) | |||||
| Whole polyphenol-rich food | −5.03 (−25.87, 15.80) | 0.64 | 15 | 823 | 99 |
| Purified food polyphenol extracts | −4.98 (−10.31, 0.34) | 0.07 | 11 | 700 | 54 |
| HDL-c (mg/dL) | |||||
| Whole polyphenol-rich food | 0.44 (−1.30, 2.18) | 0.62 | 16 | 871 | 75 |
| Purified food polyphenol extract | 1.15 (−0.66, 2.96) | 0.21 | 12 | 750 | 51 |
| Glucose (FBG) (mg/dL) | |||||
| Whole polyphenol-rich food | −2.06 (−6.05, 1.94) | 0.31 | 14 | 784 | 83 |
| Purified food polyphenol extracts | −1.06 (−3.37, 1.24) | 0.37 | 11 | 839 | 74 |
| FMD (%) | |||||
| Whole polyphenol-rich food | 1.44 (0.35, 2.52) | 0.009 | 6 | 207 | 84 |
| Purified food polyphenol extract | 4.92 (−6.30, 16.14) | 0.39 | 3 | 131 | 97 |
| IL-6 (pg/mL) | |||||
| Whole polyphenol-rich food | −0.21 (−0.85, 0.43) | 0.52 | 4 | 165 | 0 |
| Purified food polyphenol extracts | −0.27 (−1.61, 1.07) | 0.69 | 6 | 337 | 98 |
| CRP (mg/L) | |||||
| Whole polyphenol-rich food | −0.21 (−0.48, 0.06) | 0.12 | 8 | 298 | 10 |
| Purified food polyphenol extracts | −0.19 (−0.57, 0.20) | 0.34 | 8 | 531 | 0 |
I2 statistic (statistical heterogeneity).
DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; SBP, systolic blood pressure; WMD, weighted mean difference.
Significance level at P < 0.05.
TABLE 4.
Random-effects model meta-analysis of mean difference of blood pressure, lipid profile, fasting blood glucose, waist circumferences, endothelial function and markers of inflammation according to the duration of trials
| Subgroups | WMD (95% CI) | P value | Studies, n | Participants, n | I2 (%) |
|---|---|---|---|---|---|
| SBP (mmHg) | |||||
| Longer study duration | −3.68 (−4.30, −3.06) | 0.00001 | 18 | 1004 | 0 |
| Shorter study duration | −3.50 (−5.78, −1.22) | 0.003 | 9 | 309 | 33 |
| DBP (mmHg) | |||||
| Longer study duration | −1.30 (−2.50, −0.10) | 0.03 | 17 | 1310 | 44 |
| Shorter study duration | −2.40 (−2.94, −1.87) | 0.00001 | 9 | 308 | 0 |
| LDL-c (mg/dL) | |||||
| Longer study duration | −9.64 (−29.59, 10.32) | 0.34 | 17 | 1163 | 98 |
| Shorter study duration | 3.15 (−2.69, 8.99) | 0.4 | 9 | 360 | 74 |
| HDL-c (mg/dL) | |||||
| Longer study duration | 1.29 (−0.36, 2.94) | 0.13 | 18 | 1213 | 62 |
| Shorter study duration | 0.00 (−1.76, 1.76) | 0.2 | 10 | 408 | 44 |
| TGs (mg/dL) | |||||
| Longer study duration | −13.56 (−24.73, −2.39) | 0.02 | 19 | 1249 | 77 |
| Shorter study duration | −3.94 (−20.44, 12.56) | 0.64 | 10 | 408 | 77 |
| Total cholesterol (mg/dL) | |||||
| Longer study duration | −7.59 (−12.06, −3.11) | 0.0009 | 18 | 1210 | 47 |
| Shorter study duration | 1.54 (−3.43, 6.51) | 0.54 | 9 | 360 | 56 |
| Glucose (FBG) (mg/dL) | |||||
| Longer study duration | −2.62 (−5.53, 0.29) | 0.08 | 16 | 1272 | 79 |
| Shorter study duration | 0.78 (−0.92, 2.49) | 0.37 | 9 | 351 | 35 |
| Waist circumference (cm) | |||||
| Longer study duration | −2.05 (−4.18, 0.08) | 0.06 | 10 | 903 | 80 |
| Shorter study duration | 2.16 (−2.07, 6.40) | 0.32 | 3 | 146 | 24 |
| FMD (%) | |||||
| Longer study duration | 4.72 (−1.82, 11.26) | 0.16 | 3 | 140 | 97 |
| Shorter study duration | 1.73 (0.71, 2.74) | 0.0009 | 6 | 198 | 72 |
| IL-6 (pg/mL) | |||||
| Longer study duration | 0.32 (−1.22, 1.86) | 0.69 | 4 | 253 | 70 |
| Shorter study duration | −0.42 (−1.73, 0.88) | 0.53 | 6 | 249 | 96 |
| CRP (mg/L) | |||||
| Longer study duration | −0.11 (−0.33, 0.11) | 0.32 | 9 | 553 | 0 |
| Shorter study duration | −0.33 (−0.71, 0.05) | 0.09 | 7 | 276 | 0 |
Longer study duration (>4 wk) and shorter study duration (3 d to 4 wk).
I2 statistic (statistical heterogeneity).
DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; SBP, systolic blood pressure; WMD, weighted mean difference.
Significance level at P<0.05.
TABLE 5.
Random-effects model meta-analysis of mean difference of blood pressure, lipid profile, fasting blood glucose, waist circumferences, endothelial function, and markers of inflammation according to the study size
| Subgroups | WMD (95% CI) | P value | Studies, n | Participants, n | I2 (%) |
|---|---|---|---|---|---|
| SBP (mmHg) | |||||
| Large studies | −3.42 (−4.75, −2.09) | 0.00001 | 14 | 959 | 14 |
| Small studies | −3.49 (−3.67, −3.31) | 0.00001 | 13 | 354 | 0 |
| DBP (mmHg) | |||||
| Large studies | −0.43 (−0.85, −0.01) | 0.05 | 14 | 1329 | 0 |
| Small studies | −2.34 (−3.84, −0.84) | 0.002 | 12 | 289 | 39 |
| Lipid profile (mg/dL) | |||||
| LDL-c | |||||
| Large studies | −12.09 (−30.49, 6.32) | 0.2 | 17 | 1301 | 99 |
| Small studies | 6.13 (−0.03, 12.29) | 0.05 | 9 | 222 | 55 |
| HDL-c | |||||
| Large studies | 1.16 (−0.33, 2.64) | 0.13 | 18 | 135 | 74 |
| Small studies | 0.15 (−2.14, 1.84) | 0.88 | 10 | 270 | 27 |
| TGs | |||||
| Large studies | −14.19 (−26.78, −1.60) | 0.03 | 19 | 1387 | 82 |
| Small studies | −4.6 (−13.86, 4.65) | 0.33 | 10 | 270 | 31 |
| Total cholesterol | |||||
| Large studies | −7.55 (−12.47, −2.64) | 0.003 | 18 | 1348 | 81 |
| Small studies | 1.29 (−4.63, 7.22) | 0.67 | 9 | 222 | 45 |
| Glucose (FBG) (mg/dL) | |||||
| Large studies | −2.75 (−5.85, 0.34) | 0.08 | 17 | 1430 | 77 |
| Small studies | 0.09 (−2.40, 2.58) | 0.95 | 8 | 193 | 77 |
| Waist circumference (cm) | |||||
| Large studies | −2.08 (−4.17, 0.02) | 0.05 | 11 | 978 | 78 |
| Small studies | 2.46 (−2.78, 7.70) | 0.36 | 2 | 77 | 60 |
| FMD (%) | |||||
| Large studies | 3.87 (0.92, 6.82) | 0.01 | 5 | 271 | 94 |
| Small studies | 0.57 (−1.51, 2.66) | 0.59 | 4 | 67 | 90 |
| IL6 (pg/mL) | |||||
| Large studies | −0.11 (−1.35, 1.14) | 0.86 | 8 | 455 | 98 |
| Small studies | −0.24(−0.65, 0.17) | 0.25 | 2 | 47 | 0 |
Large studies (≥30 participants) and small studies (<30 participants).
I2 statistic (statistical heterogeneity).
DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; SBP, systolic blood pressure; WMD, weighted mean difference.
Significance level at P < 0.05.
TABLE 6.
Random-effects model meta-analysis of mean difference of blood pressure, lipid profile, fasting blood glucose, waist circumferences, endothelial function, and markers of inflammation according to study design
| Outcomes and subgroups | WMD (95% CI) | P value | Studies, n | Participants, n | I2 (%) |
|---|---|---|---|---|---|
| SBP (mmHg) | |||||
| Parallel Design | −3.28 (−4.52, −2.03) | 0.00001 | 17 | 949 | 18 |
| Cross-over Design | −3.49 (−3.67, −3.31) | 0.00001 | 10 | 364 | 0 |
| DBP (mmHg) | |||||
| Parallel design | −1.14 (−2.38, 0.09) | 0.07 | 16 | 1333 | 43 |
| Cross-over design | −2.41 (−2.94, −1.88) | 0.00001 | 10 | 285 | 0 |
| LDL-c (mg/dL) | |||||
| Parallel design | −10.31 (−31.13, 10.50) | 0.00001 | 16 | 1179 | 98 |
| Cross-over design | 2.93 (−2.29, 8.16) | 0.27 | 10 | 344 | 70 |
| HDL-c (mg/dL) | |||||
| Parallel design | 0.58 (−1.05, 2.22) | 0.4 | 16 | 876 | 39 |
| Cross-over design | 1.18 (−0.84, 3.19) | 0.25 | 11 | 585 | 82 |
| TGs (mg/dL) | |||||
| Parallel design | −18.25 (−28.73, −7.78) | 0.0006 | 18 | 1265 | 58 |
| Cross-over design | −0.25 (−10.29, 9.79) | 0.05 | 11 | 392 | 58 |
| Total cholesterol (mg/dL) | |||||
| Parallel design | −9.05 (−14.03, −4.08) | 0.0004 | 17 | 1226 | 49 |
| Cross-over design | 1.93 (−2.06, 5.92) | 0.34 | 10 | 344 | 45 |
| Glucose (FBG) mg/dL | |||||
| Parallel design | −3.31 (−6.52, −0.10) | 0.04 | 15 | 1288 | 78 |
| Cross-over design | 1.28 (0.19, 2.37) | 0.02 | 10 | 335 | 12 |
| Waist circumference (cm) | |||||
| Parallel design | −1.57 (−3.74, 0.60) | 0.16 | 11 | 967 | 81 |
| Cross-over design | −0.17 (−4.41, 4.06) | 0.94 | 2 | 88 | 0 |
| FMD (%) | |||||
| Parallel design | 3.28 (0.13, 6.43) | 0.04 | 4 | 181 | 96 |
| Cross-over design | 2.16 (1.26, 3.06) | 0.00001 | 5 | 157 | 39 |
| IL-6 (pg/mL) | |||||
| Parallel design | 0.32 (−1.22, 1.86) | 0.69 | 4 | 253 | 70 |
| Cross-over design | −0.42 (−1.73, 0.88) | 0.53 | 6 | 249 | 96 |
| CRP (mg/L) | |||||
| Parallel design | −0.16 (−0.48, 0.16) | 0.33 | 7 | 528 | 6 |
| Cross-over design | −0.26 (−0.55, 0.04) | 0.17 | 9 | 263 | 0 |
I2 statistic (statistical heterogeneity).
DBP, diastolic blood pressure; FBG, fasting blood glucose; FMD, flow-mediated dilation; SBP, systolic blood pressure; WMD, weighted mean difference.
Significance level at P < 0.05.
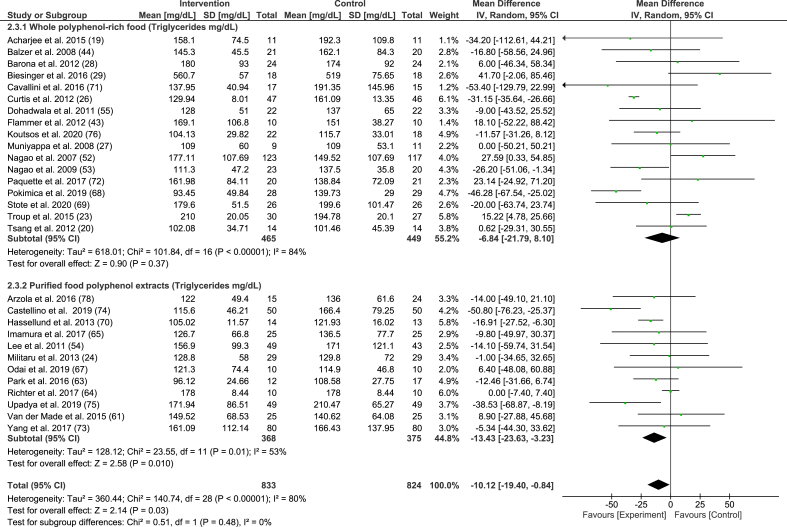
FIGURE 3.
Random-effects model meta-analysis of mean differences of trials measuring TGs (mg/dL) in participants given whole polyphenol-rich foods and purified food polyphenol extracts.
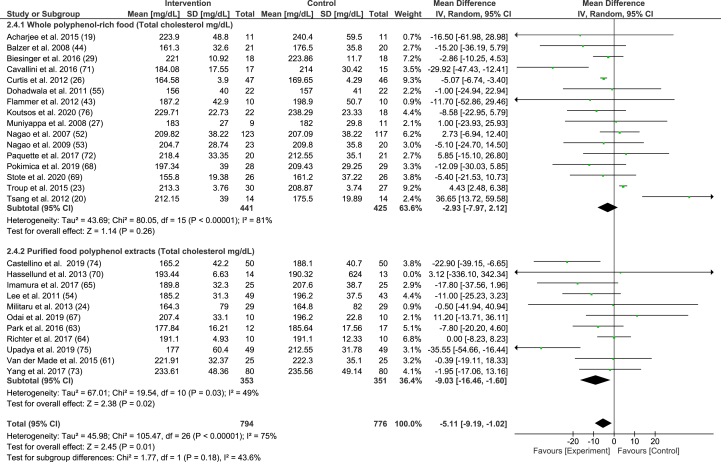
FIGURE 4.
Random-effects model meta-analysis of mean differences of trials measuring total cholesterol (mg/dL) in participants given whole polyphenol-rich foods and purified food polyphenol extracts.
FIGURE 5.
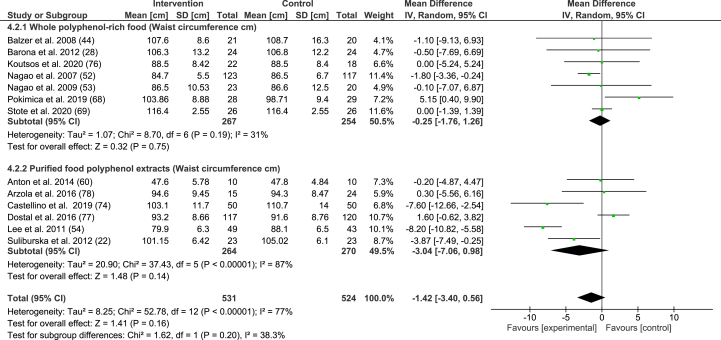
Random-effects model meta-analysis of mean differences of trials measuring waist circumference (cm) in participants given whole polyphenol-rich foods and purified food polyphenol extracts.
Effect of polyphenols on blood pressure
Polyphenols significantly reduced SBP (−3.53 mmHg; 95% CI: −3.90, −3.16 mmHg; P = 0.00001). In a sensitivity analysis, the removal of studies with high RoB [8,[19], [20], [21], [22]], yielded an effect size of −3.51 mmHg; 95% CI: −3.68, −3.33 mmHg; P = 0.00001. Both shorter and longer follow-up durations had similar effect sizes. Whole polyphenol-rich foods but not purified food polyphenol extracts conferred a significant effect on SBP (−3.69 mmHg; 95% CI: −4.24, −3.15 mmHg; P = 0.00001).
Regarding DBP an overall significant reduction (−1.41 mmHg; 95% CI: −2.38, −0.44 mmHg; P = 0.004) was observed. In sensitivity analysis, removal of studies [8,[19], [20], [21],57] with high RoB, marginally reduced the effect size (−1.21 mmHg; 95% CI: −2.24, −0.18 mmHg; P = 0.02). When studies were stratified according to study size, small studies demonstrated a higher effect size (−2.34 mmHg; 95% CI: −3.84, −0.84 mmHg; P = 0.002) than larger studies (−0.43 mmHg; 95% CI: −0.85, −0.01 mmHg; P= 0.05). Only whole polyphenol-rich foods had a significant reducing effect on DBP. Studies with shorter durations had a larger effect size (−2.40 mmHg; 95% CI: −2.94, −1.87 mmHg; P = 0.00001) than longer duration studies (−1.32 mmHg; 95% CI: −2.56, −0.09 mmHg; P = 0.03).
Effect of polyphenols on blood lipid metabolism
Regarding LDL-cholesterol, a nonsignificant reduction (−5.4 mg/dL; 95% CI: −17.94, 7.14 mg/dL, P = 0.40) was observed. In parallel design subanalysis, the effect size increased to significant levels. None of the intervention materials had a significant effect on LDL-cholesterol. When study size was considered separately, larger studies showed a bigger effect (−12.09 mg/dL, 95% CI: −30.49, 6.32 mg/dL, P = 0.20) on LDL-cholesterol, whereas there was an increase in LDL-cholesterol in the subset of small studies (6.13 mg/dL, 95% CI: −0.03, 2.29 mg/dL, P = 0.05). When study duration was considered, only longer studies had reducing effect on LDL-cholesterol (−9.64 mg/dL 95% CI: −29.59, 10.32 mg/dL, P = 0.34).
Overall, HDL-c increased by 0.79 mg/dL, 95% CI: −0.43, 2.02 mg/dL, P = 0.20; which increased to 1.16 mg/dL, 95% CI: −0.33, 2.64 mg/dL, P = 0.13 upon pooling large studies. Neither intervention materials nor study duration subanalyses had a significant effect on HDL-cholesterol.
There was a significant reduction of TGs (−10.12 mg/dL, 95% CI: −19.40, −0.84 mg/dL, P = 0.03), which became even larger when subgroups of larger studies and longer follow-up duration were considered (−14.19 mg/dL, 95% CI: −26.78, −1.60 mg/dL, P = 0.03), (−13.56 mg/dL 95% CI: −24.73, −2.39 mg/dL, P = 0.02), respectively. When purified food polyphenol extracts were considered separately, TGs reduced by −13.43 mg/dL 95% CI: −23.63, −3.23 mg/dL, P = 0.01 (Figure 3).
Similarly, there was a significant reduction in total cholesterol (−5.11 mg/dL, 95% CI: −9.19, −1.02 mg/dL, P = 0.01). When only purified food polyphenol extracts were pooled, the effect significantly increased to −9.03 mg/dL, 95% CI: −16.46, −1.60, P = 0.02 (Figure 4). In addition, large studies and longer follow-up duration had a large reducing effect on total cholesterol (−7.55 mg/dL, 95% CI: −12.47, −2.64 mg/dL, P = 0.003, and −7.59 mg/dL 95% CI: −12.06, −3.11 mg/dL, P = 0.0009), respectively.
Effect of polyphenols on glucose homeostasis
Overall, polyphenols reduced fasting blood glucose (FBG) by −1.67 mg/dL, 95% CI: −3.91, 0.58 mg/dL, P = 0.15. Considering parallel trials separately, the effect grew significantly (−3.31 mg/dL, 95% CI: −6.52, −0.10 mg/dL, P = 0.04). When the effect was estimated independently in large and longer studies, it increased to −2.08 mg/dL, 95% CI: −4.17, 0.02 mg/dL, P = 0.05 and −2.37 mg/dL, 95% CI: −5.20, 0.45 mg/dL, P = 0.1, respectively. None of the intervention materials had a significant effect on FBG.
Effect of polyphenols on waist circumference
There was a nonsignificant reduction in waist circumference (−1.42 cm; 95% CI: −3.40, 0.56 cm; P = 0.16) and this effect size further reduced to −1.18 cm; 95% CI: −3.28, 0.92 cm; P = 0.27 when Buscemi et al. [17] was removed (due to high RoB). The pooled effect in only large studies was −2.08 cm; 95% CI: −4.17, 0.02 cm; P = 0.05. However, when intervention materials were considered separately, purified food polyphenol extracts had a larger effect (−3.04 cm; 95% CI: −7.06, 0.98 cm, P = 0.14) than whole polyphenol-rich foods (−0.25 cm; 95% CI: −1.76, 1.26 cm, P = 0.75) (Figure 5).
Effect of polyphenols on vascular function
Polyphenols increased FMD (2.22 %; 95% CI: 0.66, 3.77%; P = 0.005) which rose when only large (3.87%; 95% CI: 0.26, 8.31 %; P = 0.01), longer studies (4.72% 95% CI: −1.82, 11.26; P = 0.16) and purified food polyphenol extracts (4.92%; 95% CI: −6.30, 16.14 %; P = 0.39) subgroups were considered. In sensitivity analysis, removal of Buscemi et al.’s study [17] due to high RoB reduced the overall effect size to 2.14%; 95% CI: 0.43, 3.86%; P = 0.01.
Effect of polyphenols on inflammation markers
An overall nonsignificant IL-6 reduction (−0.14 pg/mL; 95% CI: −1.16, 0.87 pg/mL; P = 0.78) was observed. In a sensitivity analysis, when 3 studies were removed [17,18,25] due to high RoB, effect size plummeted to −0.03 pg/mL; 95% CI: −0.44, 0.39 pg/mL; P = 0.91. Similarly, a nonsignificant reduction (−0.16 mg/L; 95% CI: −0.34, 0.05 mg/L; P = 0.09) was observed in CRP. When studies (8,17–19] were removed owing to the high RoB, the effect size was almost unchanged (−0.13 mg/L; 95% CI: −0.34, 0.08 mg/L; P = 0.22. Study duration, study design, intervention material, and study size subanalyses did not have a significant effect on IL-6 and CRP.
Discussion
This meta-analysis aimed to consolidate the available evidence on the impact of dietary polyphenols on blood pressure, lipid profile, blood glucose, waist circumference, vascular function, and markers of inflammation. Overall, compared with placebo, polyphenols demonstrated beneficial effects on the selected outcomes. Most importantly, significant reduction was observed in blood pressure, TGs, and total cholesterol. Similarly, significant improvement was noted in vascular function marked by the increase in the FMD. No substantial effect on waist circumference and glucose homeostasis was observed. Except for SBP, a high heterogeneity was observed in all outcomes.
Polyphenols are the most ubiquitous phytochemicals in a regular human diet with a total daily intake of approximately 1 g [5], and are becoming popular in both primary and secondary prevention of cardiometabolic risks, especially high blood pressure and dyslipidemia. Although the evidence that polyphenols can improve HDL-cholesterol was unconvincing, our review demonstrated that polyphenols are quite efficacious on lowering LDL-cholesterol, total cholesterol, and TGs. Katan et al. [58] showed that a daily intake of 2–2.4 g of phytochemicals, such as plant sterols, resulted into an average reduction of LDL-cholesterol by 8.9%; 95% CI: 7.4, −10.5, an effect closer to the clinical significance of 10% LDL-cholesterol–lowering effect following a daily intake of 2 g of phytosterols [59]. There is compelling evidence that lowering blood cholesterol regulates the risk of CVDs [59,60]. For example, every 1% reduction in cholesterol levels attracts a 1%–2% decrease in cardiovascular events and mortality [61]. Owing to their antioxidant properties, dietary polyphenols can mitigate oxidative damage of blood lipids [62]. Oxidation of LDL-cholesterol to oxLDL is a critical step in atherosclerosis and the associated endothelial dysfunction and inflammations. Anthocyanins are considered by USDA as the most potent flavonoids against LDL oxidation [63,64]. Concomitantly, flavonoids attenuate acute postprandial inflammations associated with Western diets [65]. In fact, the European Food Safety Authority confirms that a daily intake of 200 mg of cocoa flavanols ameliorate endothelial function and inflammations [66].
Regarding blood pressure, the observed treatment effect on SBP (−3.53 mmHg) following polyphenol consumption was similar to the documented SBP reduction on adherence to moderate alcohol and dietary sodium consumption [67]. An overall 5 mmHg reduction in SBP is shown to reduce the risk of stroke by 13% [68] and considerably reduce CVD mortality [69]. There was a small but rather significant reduction in DBP −1.41 mmHg, 95%CI: −2.38, −0.44. A 2 mmHg reduction in DBP coincides with an 11.5% decrease in the risk of stroke [68]. It can be argued that such small reductions could complement the pharmacologic treatment outcomes of hypertension and contribute to the overall reduction of the risk of CVDs both at the clinical and population levels. Our results on DBP are corroborated by a previous Cochrane review [9] that investigated the hypotensive properties of cocoa. Two studies [27,70] from this review were also included in our meta-analysis. A potential mechanism of action by which polyphenols confer hypotensive effects is by increasing the bioavailability of NO and upregulating endothelial NO-synthase activity. NO is a potent vasodilator that reduces blood pressure [9,69,71,72]. Additionally, flavonoids especially flavanols may block ACE activity, reducing blood pressure [9] In fact, flavanols, anthocyanins, phenolic acids, tannins, and resveratrol have been identified as green ACE inhibitors [73]. These putative benefits have been shown to vary with the duration of the intervention. There is evidence that long-term interventions with flavanol supplementation can avert 27% of CVD deaths [74].
Based on study follow-up duration subanalyses, longer studies yielded a bigger reduction in total cholesterol, LDL-cholesterol, TGs but not HDL-cholesterol. Evidence from human trials has shown that the treatment effect of polyphenols is larger following an intake longer than 1 mo [75,76]. Likewise, intake of purified (poly)phenolic extracts in the form of supplements coincided with a larger treatment effect in dyslipidemia; TGs, total cholesterol, and a marginal increase in HDL-cholesterol.
Concerning waist circumference, there was a larger reduction following the intake of purified food polyphenol extracts compared with polyphenol-rich foods. Polyphenols especially refined isoflavone have been shown to increase fat loss [77]. The superior bioactivity of purified (poly)phenolic supplements hinges on their ability to overcome bioavailability constraints associated with whole foods [5].
Since participants were often encouraged to maintain habitual diet, and where dietary restrictions were imposed, they applied both to both the study arms, and because an average adherence of 94% to the interventions was registered, it is possible that the observed effects are attributable to polyphenol intake.
Study strength and limitations
This meta-analysis included only RCTs. We closely followed the rigorous methodology of the Cochrane Collaboration for systematic reviews for interventions [15]. The search strategy was comprehensive and involved both computerized and manual searches. The uniqueness of this review is the comparison of the efficacy between whole polyphenol-rich foods and purified food polyphenol extracts often used as supplements. However, the review also has some limitations. A number of trials did not follow the CONSORT 2010 guidelines of reporting an RCT [78,79] and this resulted in a number of methodological flaws. Subgroup analysis on the basis of dosage was not possible because both polyphenol-rich food and purified food polyphenol extracts were too diverse for any meaningful stratification. In some studies, measurement techniques for certain parameters were either not reported or varied across studies. Notably, blood pressure measurements varied with respect to the position (sitting, standing, and supine) and method (clinical and ambulatory). Adherence to intervention materials was mostly self-reported or by counting emptied packages and seldom did studies measure plasma or urine metabolites. Whole food products studied often lacked proper taxonomic and composition profiles. Moreover, the chemical composition of such foods postulates variations across subspecies, preservation and preparation methods, and time of harvest. Such variations can have potential alterations in the test effects [5]. The search strategy did not include terms of individual food items used as intervention materials; hence some food intervention trials may have been left out. Finally, this review only focused on surrogate markers but not clinical outcomes, such as CVD mortality.
Conclusions
Evidence in this meta-analysis suggests that dietary polyphenol interventions can suppress several cardiometabolic risks in patient populations. The extent of treatment effect varies considerably from one risk marker to the other. A larger effect size was observed with LDL-cholesterol, TGs, total cholesterol, and SBP. Purified (poly)phenolic extracts are generally more efficacious than whole polyphenol-rich foods. Larger interventions with longer follow-up durations are more effective than shorter and small ones. These findings point to the potential application of polyphenol-rich foods in the management of cardiometabolic risks. However, because of high heterogeneity and RoB among RCTs in the study, caution must be taken while interpreting the results. In addition, there is a need for more well-designed large RCTs with longer duration to increase confidence in conclusions regarding the efficacy of polyphenols on cardiometabolic risk markers. More mechanistic studies are required to substantiate the effect of polyphenols on especially glucose and cholesterol regulation and delineate it from dietary fibers.
Author disclosures
The authors have no competing interests to declare. The funder had no role in designing of the review, data extraction, analysis, interpretation, and writing of the manuscript.
Acknowledgments
TK, CM, and PY conceived and designed the study. TK, CM, BM, and PY contributed to the screening of studies, RoB assessment, and data extraction analysis. TK, CM, PY, BV, OP, and BM interpreted the data, wrote, edited, and reviewed the manuscript. All authors read and approved the final version.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.01.002.
Funding
This study was funded by the Belgian Directorate General for Development Cooperation and Humanitarian Aid (DGD), for funding through the VLIR-UOS framework.
Data Availability
Data described in the manuscript will be made available upon request pending approval from the corresponding author.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Micha R, Mannar V, Afshin A, Allemandi L, Baker P, Battersby J, et al., 2020 global nutrition report: action on equity to end malnutrition.
- 2.Marino M., Del Bo’ C., Martini D., Porrini M., Riso P. A review of registered clinical trials on dietary (poly) phenols: past efforts and possible future directions. Foods. 2020;9(11):1606. doi: 10.3390/foods9111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valenzuela P.L., Carrera-Bastos P., Gálvez B.G., Ruiz-Hurtado G., Ordovas J.M., Ruilope L.M., et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021;18(4):251–275. doi: 10.1038/s41569-020-00437-9. [DOI] [PubMed] [Google Scholar]
- 4.Goszcz K., Duthie G.G., Stewart D., Leslie S.J., Megson I.L. Bioactive polyphenols and cardiovascular disease: chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017;174(11):1209–1225. doi: 10.1111/bph.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas-Barberan F.A., González-Sarrías A., García-Villalba R. John Wiley & Sons; Hoboken, USA: 2020. Dietary polyphenols: metabolism and health effects. [Google Scholar]
- 6.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll K.S., Appathurai A., Jois M., Radcliffe J.E. Effects of herbs and spices on blood pressure: a systematic literature review of randomised controlled trials. J. Hypertens. 2019;37(4):671–679. doi: 10.1097/HJH.0000000000001952. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Luna R., Munoz-Hernandez R., Miranda M.L., Costa A.F., Jimenez-Jimenez L., Vallejo-Vaz A.J., et al. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012;25(12):1299–1304. doi: 10.1038/ajh.2012.128. [DOI] [PubMed] [Google Scholar]
- 9.Ried K., Sullivan T.R., Fakler P., Frank O.R., Stocks N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD008893.pub2. 8. [DOI] [PubMed] [Google Scholar]
- 10.Serban M.C., Sahebkar A., Zanchetti A., Mikhailidis D.P., Howard G., Antal D., et al. Effects of quercetin on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016;5(7) doi: 10.1161/JAHA.115.002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Ling W., Du Z., Chen Y., Li D., Deng S., et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017;8(5):684–693. doi: 10.3945/an.116.014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N., Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst. 2019;24 doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas M., Saeed F., Anjum F.M., Afzaal M., Tufail T., Bashir M.S., et al. Natural polyphenols: an overview. Int. J. Food Prop. 2017;20(8):1689–1699. doi: 10.1080/10942912.2016.1220393. [DOI] [Google Scholar]
- 14.Higgins J.P.T., Li T., Deeks J.J. Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions. 2019:143–176. [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.Publisher (John Wiley & Sons, Hoboken, USA) (Accessed 13 February 2023).
- 16.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buscemi S., Rosafio G., Arcoleo G., Mattina A., Canino B., Montana M., et al. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012;95(5):1089–1095. doi: 10.3945/ajcn.111.031088. [DOI] [PubMed] [Google Scholar]
- 18.Nogueira L.D.P., Nogueira Neto J.F., Klein M.R.S.T., Sanjuliani A.F. Short-term effects of green tea on blood pressure, endothelial function, and metabolic profile in obese prehypertensive women: a crossover randomized clinical trial. J. Am. Coll. Nutr. 2017;36(2):108–115. doi: 10.1080/07315724.2016.1194236. [DOI] [PubMed] [Google Scholar]
- 19.Acharjee S., Zhou J.R., Elajami T.K., Welty F.K. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism. 2015;64(2):236–243. doi: 10.1016/j.metabol.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsang C., Smail S N.F. lmoosawi, I. Davidson, E.A.S. Al-Dujaili, Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012;1:e9. doi: 10.1017/jns.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudarma V., Sukmaniah S., Siregar P. Effect of dark chocolate on nitric oxide serum levels and blood pressure in prehypertension subjects. Acta Med. Indones. 2011;43(4):224–228. [PubMed] [Google Scholar]
- 22.Suliburska J., Bogdanski P., Szulinska M., Stepien M., Pupek-Musialik D., Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace. Elem. Res. 2012;149(3):315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troup R., Hayes J.H., Raatz S.K., Thyagarajan B., Khaliq W., Jacobs D.R., Jr., et al. Effect of black tea intake on blood cholesterol concentrations in individuals with mild hypercholesterolemia: a diet-controlled randomized trial. J. Acad. Nutr. Diet. 2015;115(2):264–271. doi: 10.1016/j.jand.2014.07.021. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Militaru C., Donoiu I., Craciun A., Scorei I.D., Bulearca A.M., Scorei R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29(1):178–183. doi: 10.1016/j.nut.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Marín-Echeverri C., Blesso C.N., Fernández M.L., Galvis-Pérez Y., Ciro-Gómez G., Núñez-Rangel V., et al. Vol. 7. Antioxidants; Basel: 2018. Effect of agraz (vaccinium meridionale swartz) on high-density lipoprotein function and inflammation in women with metabolic syndrome; p. 14. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis P.J., Dhatariya K., Sampson M., Kroon P.A., Potter J., Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diab. Care. 2012;35(2):226–232. doi: 10.2337/dc11-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muniyappa R., Hall G., Kolodziej T.L., Karne R.J., Crandon S.K., Quon M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008;88(6):1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barona J., Aristizabal J.C., Blesso C.N., Volek J.S., Fernandez M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012;142(9):1626–1632. doi: 10.3945/jn.112.162743. [DOI] [PubMed] [Google Scholar]
- 29.Nagao T., Hase T., Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15(6):1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 30.Nagao T., Meguro S., Hase T., Otsuka K., Komikado M., Tokimitsu I., et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. (Silver Spring) 2009;17(2):310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.H., Park E., Lee H.J., Kim M.O., Cha Y.J., Kim J.M., et al. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr. Res. Pract. 2011;5(1):28–33. doi: 10.4162/nrp.2011.5.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohadwala M.M., Holbrook M., Hamburg N.M., Shenouda S.M., Chung W.B., Titas M., et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011;93(5):934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis P.J., Potter J., Kroon P.A., Wilson P., Dhatariya K., Sampson M., et al. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013;97(5):936–942. doi: 10.3945/ajcn.112.043745. [DOI] [PubMed] [Google Scholar]
- 34.Asher G.N., Viera A.J., Weaver M.A., Dominik R., Caughey M., Hinderliter A.L. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement. Altern. Med. 2012;12:26. doi: 10.1186/1472-6882-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong W.W., Taylor A.A., Smith E.O., Barnes S., Hachey D.L. Effect of soy isoflavone supplementation on nitric oxide metabolism and blood pressure in menopausal women. Am. J. Clin. Nutr. 2012;95(6):1487–1494. doi: 10.3945/ajcn.111.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ras R.T., Zock P.L., Zebregs Y.E.M.P., Johnston N.R., Webb D.J., Draijer R. Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre- and stage I. hypertension. Br. J. Nutr. 2013;110(12):2234–2241. doi: 10.1017/S000711451300161X. [DOI] [PubMed] [Google Scholar]
- 37.Anton S.D., Embry C., Marsiske M., Lu X., Doss H., Leeuwenburgh C., et al. Safety and metabolic outcomes of resveratrol supplementation in older adults: results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014;57:181–187. doi: 10.1016/j.exger.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Made S.M., Plat J., Mensink R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: a randomized, placebo-controlled crossover trial. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grassi D., Draijer R., Desideri G., Mulder T., Ferri C. Black tea lowers blood pressure and wave reflections in fasted and postprandial conditions in hypertensive patients: a randomised study. Nutrients. 2015;7(2):1037–1051. doi: 10.3390/nu7021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park E., Edirisinghe I., Choy Y.Y., Waterhouse A., Burton-Freeman B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: a randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br. J. Nutr. 2016;115(2):226–238. doi: 10.1017/S0007114515004328. [DOI] [PubMed] [Google Scholar]
- 41.Richter C.K., Skulas-Ray A.C., Fleming J.A., Link C.J., Mukherjea R., E.S Krul, et al. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: a dose-response randomised controlled trial. Br. J. Nutr. 2017;117(10):1403–1413. doi: 10.1017/S000711451700143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imamura H., Yamaguchi T., Nagayama D., Saiki A., Shirai K., Tatsuno I. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with Type 2 diabetes mellitus. Int. Heart. J. 2017;58(4):577–583. doi: 10.1536/ihj.16-373. [DOI] [PubMed] [Google Scholar]
- 43.Boix-Castejon M., Herranz-Lopez M., Gago A.P., Olivares-Vicente M., Caturla N., Roche E., et al. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: a randomized controlled trial. Food. Funct. 2018;9(6):3173–3184. doi: 10.1039/c8fo00367j. [DOI] [PubMed] [Google Scholar]
- 44.Odai T., Terauchi M., Kato K., Hirose A., Miyasaka N. Effects of grape seed proanthocyanidin extract on vascular endothelial function in participants with prehypertension: a randomized, double-blind, placebo-controlled study. Nutrients. 2019;11(12) doi: 10.3390/nu11122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pokimica B., García-Conesa M.T., Zec M., Debeljak-Martačić J., Ranković S., Vidović N., et al. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients. 2019;11(4) doi: 10.3390/nu11040850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stote K.S., Wilson M.M., Hallenbeck Thomas K., Rourke J.M., Sweeney M.I., et al. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: an 8-week, double-blind, randomized, placebo-controlled trial. Curr. Dev. Nutr. 2020;4(4) doi: 10.1093/cdn/nzaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassellund S.S., Flaa A., Kjeldsen S.E., Seljeflot I., Karlsen A., Erlund I., et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013;27(2):100–106. doi: 10.1038/jhh.2012.4. [DOI] [PubMed] [Google Scholar]
- 48.Cavallini D.C., Manzoni M.S., Bedani R., Roselino M.N., Celiberto L.S., Vendramini R.C., et al. Probiotic soy product supplemented with isoflavones improves the lipid profile of moderately hypercholesterolemic men: a randomized controlled trial. Nutrients. 2016;8(1) doi: 10.3390/nu8010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paquette M., Larque A.S.M., Weisnagel S.J., Desjardins Y., Marois J., Pilon G., et al. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017;117(4):519–531. doi: 10.1017/S0007114517000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L., Ling W., Yang Y., Chen Y., Tian Z., Du Z., et al. Role of purified anthocyanins in improving cardiometabolic risk factors in Chinese men and women with prediabetes or early untreated diabetes–a randomized controlled trial. Nutrients. 2017;9(10) doi: 10.3390/nu9101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellino G., Nikolic D., Magán-Fernández A., Malfa G.A., Chianetta R., Patti A.M., et al. Altilix® supplement containing chlorogenic acid and luteolin improved hepatic and cardiometabolic parameters in subjects with metabolic syndrome: a 6 month randomized, double-blind, placebo-controlled study. Nutrients. 2019;11(11) doi: 10.3390/nu11112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upadya H., Prabhu S., Prasad A., Subramanian D., Gupta S., Goel A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia 11 medical and health sciences 1103 clinical sciences. BMC Complement. Altern. Med. 2019;19(1) doi: 10.1186/s12906-019-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koutsos A., Riccadonna S., Ulaszewska M.M., Franceschi P., Trošt K., Galvin A., et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: a randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020;111(2):307–318. doi: 10.1093/ajcn/nqz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dostal A.M., Samavat H., Espejo L., Arikawa A.Y., Stendell-Hollis N.R., Kurzer M.S. Green tea extract and catechol-o-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J. Nutr. 2016;146(1):38–45. doi: 10.3945/jn.115.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arzola-Paniagua M.A., García-Salgado López E.R., Calvo-Vargas C.G., Guevara-Cruz M. Vol. 24. Obesity; Silver Spring: 2016. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: a randomized controlled trial; pp. 1454–1463. 7. [DOI] [PubMed] [Google Scholar]
- 56.Chew B., Mathison B., Kimble L., McKay D., Kaspar K., Khoo C., et al. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial. Eur. J. Nutr. 2019;58(3):1223–1235. doi: 10.1007/s00394-018-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biesinger S., Michaels H.A., Quadros A.S., Qian Y., Rabovsky A.B., Badger R.S., et al. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur. J. Clin. Nutr. 2016;70(1):10–16. doi: 10.1038/ejcn.2015.88. [DOI] [PubMed] [Google Scholar]
- 58.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R., et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003;78(8):965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 59.Bresson J.-L., Flynn A., Heinonen M., Hulshof K., Korhonen H., Lagiou P., et al. Plant sterols and blood cholesterol–scientific substantiation of a health claim related to plant sterols and lower/reduced blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies. EFSA J. 2008;781:1–12. doi: 10.2903/j.efsa.2008.781. [DOI] [Google Scholar]
- 60.National Cholesterol Education Program (NCEP) expert panel on detection evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 61.Ross S.D., Allen I.E., Connelly J.E., Korenblat B.M., Smith M.E., Bishop D., et al. Clinical outcomes in statin treatment trials: a meta-analysis. Arch. Intern. Med. 1999;159(15):1793–1802. doi: 10.1001/archinte.159.15.1793. [DOI] [PubMed] [Google Scholar]
- 62.Medina-Vera I., Gómez-de-Regil L., Gutiérrez-Solis A.L., Lugo R., Guevara-Cruz M., Pedraza-Chaverri J., et al. Dietary strategies by foods with antioxidant effect on nutritional management of dyslipidemias: a systematic review. Antioxidants. Basel. 2021;10(2):225. doi: 10.3390/antiox10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shebeko S.K., Zupanets I.A., Popov O.S., Tarasenko O.O. Polyphenols: Mechanisms of Action in Human Health and Disease. Academic Press; London, UK: 2018. pp. 373–394. [Google Scholar]
- 64.Bhagwat D.B., Haytowitz J.M., Holden USDA database for the flavonoid content of selected foods, Release 3.1. U.S. Department of Agriculture, Agricultural Research Service. Nutrient Data Laboratory. 2014 http://www.ars.usda.gov/nutrientdata/flav [Internet) Available from. [Google Scholar]
- 65.Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit. Rev. Food. Sci. Nutr. 2016;56(3):419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- 66.EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012;10(7):2809. doi: 10.2903/j.efsa.2012.2809. [DOI] [Google Scholar]
- 67.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr., et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 68.Reboldi G., Gentile G., Angeli F., Ambrosio G., Mancia G., P Verdecchia Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J. Hypertens. 2011;29(7):1253–1269. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- 69.Bahadoran Z., Mirmiran P., Kabir A., Azizi F., Ghasemi A. The nitrate-independent blood pressure–lowering effect of beetroot juice: a systematic review and meta-analysis. Adv. Nutr. 2017;8(6):830–838. doi: 10.3945/an.117.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heiss C., Jahn S., Taylor M., Real W.M., Angeli F.S., Wong M.L., et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J. Am. Coll. Cardiol. 2010;56(3):218–224. doi: 10.1016/j.jacc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 71.Flammer A.J., Sudano I., Wolfrum M., Thomas R., Enseleit F., Périat D., et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012;33(17):2172–2180. doi: 10.1093/eurheartj/ehr448. [DOI] [PubMed] [Google Scholar]
- 72.Balzer J., Rassaf T., Heiss C., Kleinbongard P., Lauer T., Merx M., et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008;51(22):2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 73.Hussain F., Jahan N. K.-u. Rahman, B. Sultana, S. Jamil, Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/4643736. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sesso H.D., Manson J.E., Aragaki A.K., Rist P.M., Johnson L.G., Friedenberg G., et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COSMOS randomized clinical trial. Am. J. Clin. Nutr. 2022;115(6):1490–1500. doi: 10.1093/ajcn/nqac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kord-Varkaneh H., Ghaedi E., Nazary-Vanani A., Mohammadi H., Shab-Bidar S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019;59(15):2349–2362. doi: 10.1080/10408398.2018.1451820. [DOI] [PubMed] [Google Scholar]
- 76.Onakpoya E., Spencer C., Heneghan M., Thompson The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2014;24(8):823–836. doi: 10.1016/j.numecd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Llaha F., Zamora-Ros R. The effects of polyphenol supplementation in addition to calorie restricted diets and/or physical activity on body composition parameters: a systematic review of randomized trials. Front. Nutr. 2020;7(84) doi: 10.3389/fnut.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending approval from the corresponding author.