Abstract
The subcellular location of activity and protein of ADP-glucose pyrophosphorylase (AGPase) in developing tomato (Lycopersicon esculentum) fruit was determined following a report that the enzyme might be present inside and outside the plastids in this organ. Plastids prepared from crude homogenates of columella and pericarp, the starch-accumulating tissues of developing fruit, contained 8% to 18% of the total activity of enzymes known to be confined to plastids, and 0.2% to 0.5% of the total activity of enzymes known to be confined to the cytosol. The proportion of the total activity of AGPase in the plastids was the same as that of the enzymes known to be confined to the plastid. When samples of plastid and total homogenate fractions were subjected to immunoblotting with an antiserum raised to AGPase, most or all of the protein detected was plastidial. Taken as a whole, these data provide strong evidence that AGPase is confined to the plastids in developing tomato fruit.
The aim of this study was to determine the subcellular distribution of ADP-Glc pyrophosphorylase (AGPase) in the starch-accumulating tissues of developing tomato (Lycopersicon esculentum) fruit. The activity of this enzyme is likely to be an important factor in determining the starch content of the tomato fruit (Schaffer et al., 2000). The high starch content of fruit of a line of tomato developed from a cross between L. hirsutum and a low starch, commercial line of tomato was shown to be associated with a considerably higher AGPase activity than the parental tomato, and the possession of an L. hirsutum-derived allele encoding the large subunit of AGPase.
AGPase is confined to the plastid in many organs (Okita, 1992; ap Rees, 1995), but there are plastidial and extra-plastidial isoforms in the developing endosperm of maize, barley, and other cereals (Denyer et al., 1996; Thorbjørnsen et al., 1996; Beckles et al., 2001). Chen et al. (1998) have proposed, on the basis of immunogold labeling experiments, that plastidial and cytosolic isoforms of AGPase also occur in the columella and placenta of young tomato fruit. The existence of cytosolic as well as plastidial AGPase would have important consequences for the regulation of starch synthesis in the fruit. However, we have discovered that the ratio of ADP-Glc to UDP-Glc in the developing fruit is very low—a feature that is consistent with a primarily or exclusively plastidial location for AGPase activity (Beckles et al., 2001). To resolve these apparently contradictory findings we prepared plastids from the starch-accumulating tissues of developing fruit and used these to gain direct, quantitative information about the subcellular location of enzyme activity and protein.
RESULTS
Starch Accumulation and AGPase Activity through Fruit Development
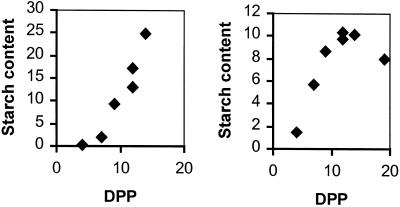
To select an appropriate stage of fruit development for preparation of plastids we initially studied changes in starch content and AGPase activity through development. The starch content of the fruit increased on a fresh weight basis between 8 and about 15 d post-pollination (DPP) and declined thereafter, reaching undetectably low levels at maturity (not shown). Measurements on separate samples of pericarp and of columella plus placenta (subsequently referred to as columella) revealed net rates of starch accumulation between 4 and 12 DPP of 4.4 and 7.4 nmol Glc equivalents min−1 g−1 fresh weight for the pericarp and the columella, respectively (Fig. 1). Activity of AGPase was highest on a fresh weight basis early in development. For fruits of between 8 and 11 DPP, activity of AGPase in the pericarp and the columella was 100 and 220 nmol min−1 g−1 fresh weight, respectively (means from four fruit). Activity then fell and was below the level of detection at about 30 DPP in pericarp and columella (not shown).
Figure 1.
Starch content of developing tomato fruit. Starch contents of columellar plus placental tissue (left) and pericarp tissue (right) were measured during the phase of net starch accumulation. Each data point represents a measurement on a sample made up of tissue from at least four fruit. Values are milligrams per gram of fresh weight. Rates of starch synthesis presented in the text were calculated from the difference in starch content between tissue from fruit at 4 and 12 DPP.
Subcellular Distribution of AGPase Activity
Plastids were prepared from columella and from pericarp during the linear phase of starch accumulation. A homogenate made by chopping the tissues in the presence of 0.5 m sorbitol was centrifuged to yield supernatant and plastid-enriched pellet fractions. The following is evidence that the preparations were suitable for determining enzyme localization. First, for AGPase and for the plastidial and cytosolic marker enzymes (alkaline pyrophosphatase and soluble starch synthase for the plastid, and pyrophosphate, Fru 6-P 1-phosphotransferase and alcohol dehydrogenase for the cytosol), the sum of the activities in the supernatant and pellet fractions was between 91% and 113% of the activity in the original homogenate (Table I). Thus, there was no serious loss of enzyme activity during preparation of the fractions. Second, the yield and purity of plastids indicated by the proportion of activity of plastidial and cytosolic marker enzymes in the pellet was adequate to allow robust interpretations of data from these preparations. An approximate 10% and 17% of the total activity of the plastidial marker enzymes was recovered in the pellet fractions from columella and pericarp tissues, respectively (Table I). For both tissues, less than 0.5% of the total activity of cytosolic marker enzymes was recovered in the pellet. This value is much lower than, and significantly different (P < 0.001) from that of the plastidial marker enzymes, indicating that the level of contamination of plastids by cytosol was low.
Table I.
Activity and latency of AGPase and marker enzymes in fractions from plastid preparations
| Enzyme | Columella and Placenta (Activity as a Percentage of That in the Homogenate)
|
Latency | Pericarp (Activity as a Percentage of That in the Homogenate)
|
Latency | ||
|---|---|---|---|---|---|---|
| Pellet | Pellet + supernatant | Pellet | Pellet + supernatant | |||
| % | % | |||||
| AGPase | 9.5 ± 0.7 | 102 ± 3 (9) | 77 ± 3 | 19.8 ± 2.5 | 113 ± 32 (5) | 61 ± 20 |
| Plastidial marker enzymes | ||||||
| Alkaline pyrophosphatase | 9.8 ± 0.6 | 100 ± 6 (7) | 79 ± 6 | 17.5 ± 2.4 | 93 ± 12 (5) | 64 ± 9 |
| Soluble starch synthase | 8.2 ± 1.2 | 97 ± 5 (5) | NDa | 15.6 ± 1.4 | 110 ± 3 (5) | ND |
| Cytosolic marker enzymes | ||||||
| Alcohol dehydrogenase | 0.23 ± 0.12 | 91 ± 3 (9) | 9 ± 3 | 0.46 ± 0.15 | 110 ± 3 (5) | 7 ± 0.01 |
| PPi: fructose 6-P 1-phosphotransferase | 0.16 ± 0.13 | 96 ± 5 (7) | ND | 0.22 ± 0.11 | 102 ± 6 (5) | ND |
In each experiment, about 15 g of pericarp or 5 g of columella from fruit of 8 to 11 DPP was chopped to produce a homogenate. Pellet and supernatant fractions were derived from homogenates by centrifugation. The activities of AGPase and marker enzymes in the pellet fraction were expressed as percentages of the activities in the original homogenates. For each enzyme, the recovery during the fractionation is estimated as the percentage of the activity in the original homogenate that was recovered in the pellet and supernatant fractions. Values in these columns are means ± se of estimates from the no. of separate plastid preparations shown in parentheses. Values of latency were derived by assaying duplicate samples of unfractionated homogenate in the presence of 0.6 m sorbitol (AGPase and alcohol dehydrogenase) or 0.6 m Suc (alkaline pyrophosphatase). The organelles in one sample were kept intact, whereas in the other sample they were lysed by the addition of detergent. The activity in the lysed sample minus that in the intact sample is expressed as a percentage of that in the lysed sample to give the latency value. In each experiment, each enzyme was assayed three times in intact and in lysed samples. Values are means ± se of estimates from three separate experiments.
ND, Not determined.
To determine the subcellular distribution of AGPase activity we compared the proportion of the total AGPase activity recovered in the pellet with that of the plastidial and cytosolic marker enzymes. An approximate 10% and 19% of the AGPase activity in the columella and pericarp tissues, respectively, was recovered in the pellet (Table I). There is no significant difference between these values and those for the plastid marker enzymes (10% and 17%, respectively; Table I). The values are strikingly different from those for cytosolic marker enzymes. This indicates that most or all of the AGPase activity is associated with plastids.
The association of AGPase activity with the pellet fraction does not necessarily mean that the activity was contained within the plastid. AGPase in the pellet could, for example, be attached to the surface of some component of the pellet. To discover whether AGPase activity in the pellet fraction was actually inside a membrane-bound organelle, the latency of AGPase was compared with the latency of a plastidial marker enzyme, alkaline pyrophosphatase, and a cytosolic marker enzyme, alcohol dehydrogenase. In these experiments, the activity of an enzyme is compared in assays containing intact organelles or organelles that have been deliberately lysed. An enzyme within an organelle will not be accessible to substrates in the assay, and thus its activity will be lower in the assay with intact organelles than in the assay with lysed organelles. The difference in activity between the intact and lysed samples is expressed as a percentage of the activity in the lysed assay and is described as the latency of the enzyme. As expected, the plastidial marker enzyme was substantially latent (about 78% for columella and 63% for pericarp), and the cytosolic marker enzyme was not (8% latent; Table I). The latency values for AGPase were essentially the same as those for the plastidial marker enzyme (Table I). This indicates strongly that most or all of the AGPase activity in the pellet is contained within plastids, rather than simply attached to the surface of a component of the pellet.
Further evidence about the location of AGPase activity in the pellet fractions was provided by experiments in which samples of homogenate were treated with Triton X-100 prior to centrifugation. This detergent lyses the plastids, releasing plastidial enzymes so that they no longer appear in the pellet after centrifugation. As expected, treatment with Triton reduced the yield of plastidial marker enzymes in the pellet from the usual value of about 17% (Table I) to only 1% to 5%. The yield of AGPase in the pellet was reduced by Triton treatment in exactly the same way (not shown). This result is again consistent with the idea that most or all of the AGPase activity in the pellet fraction is contained inside plastids.
Subcellular Distribution of AGPase Protein
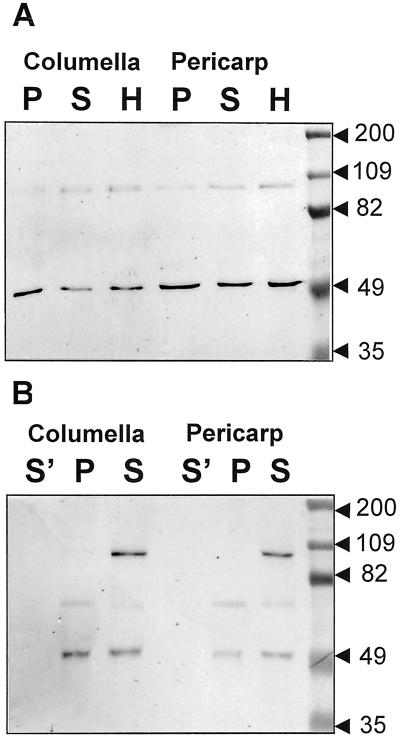
The above results indicate that most or all of the AGPase activity is plastidial in the starch-storing tissues of tomato fruit. The difference between these results and those of Chen et al. (1998), who suggested from immunogold labeling experiments that there is a cytosolic form of the enzyme in tomato fruit, might be explained if the cytosol contains AGPase protein, which is inactive in our assays. Therefore, we investigated the distribution of AGPase protein in the homogenate, pellet, and supernatant fractions from columella and pericarp by immunoblotting with an antiserum raised against the major small subunit of AGPase from maize endosperm, and with an antiserum raised against AGPase from spinach leaf. This same spinach leaf antiserum has been shown to recognize AGPase protein purified from tomato leaf and fruit (Chen and Janes, 1997, 1998), and both of the antisera have been shown to recognize the small subunit of AGPase from a variety of other monocotyledonous and dicotyledonous species (Giroux and Hannah, 1984; Morell et al., 1987; Hylton and Smith, 1992; Thorbjørnsen et al., 1996). The gels from which blots were prepared were loaded in two ways. First, lanes were loaded with samples of pellet, homogenate, and supernatant fractions, each of which contained the same activity of the plastidial marker enzyme, alkaline pyrophosphatase (Fig. 2A, lanes P, S, and H; Fig. 2B, lanes P and S). If the AGPase protein is plastidial, the intensity of the AGPase band on the blot should be the same for these fractions. However, if the AGPase protein is wholly or substantially extraplastidial, the intensity of the band should be much greater in the homogenate and supernatant fractions than in the pellet fraction. Second, lanes were loaded with supernatant and pellet fractions that contained the same activity of the cytosolic marker enzyme, alcohol dehydrogenase (Fig. 2B, lanes S' and P). If the AGPase protein is cytosolic, the intensity of the AGPase band on the blot should be the same for the two fractions. However, if the AGPase protein is wholly or substantially plastidial, the intensity of the band should be much greater in the pellet fraction than in the supernatant fraction.
Figure 2.
Immunoblots of fractions from plastid preparations. Samples of homogenate (H), supernatant (S and S'), and pellet (P) fractions from plastid preparations were loaded onto a 7.5% (w/v) SDS-polyacrylamide gel, subjected to electrophoresis, and electroblotted onto nitrocellulose. For each tissue, sample sizes for lanes H, S, and P were adjusted so that on a given gel each lane contained the same activity of the plastid marker enzyme alkaline pyrophosphatase. Sample sizes for lanes S' were adjusted so that these lanes contained the same activity of the cytosolic marker enzyme alcohol dehydrogenase as the adjacent lanes P. AGPase is the band of approximately 50 kD. Checks on the nature of other bands on the blot are described in “Materials and Methods.” A, Immunoblot developed with antiserum raised against the BT2 protein of maize (small subunit of cytosolic AGPase), at a dilution of 1/500. Each lane contained an AGPase activity of approximately 12.5 nmol min−1. On the right are prestained molecular mass markers, the masses of which are indicated in kilodaltons. B, Immunoblot developed with antiserum raised against AGPase from spinach leaf, at a dilution of 1/6,667. For pericarp, lanes P and S contained an AGPase activity of 39 nmol min−1, and lane S' contained an AGPase activity of 0.23 nmol min−1. For columella, lanes P and S contained an AGPase activity of 17 nmol min−1, and lane S' contained an AGPase activity of 0.41 nmol min−1. On the right are prestained molecular mass markers, the masses of which are indicated in kilodaltons.
In samples from pericarp and columella, both antisera strongly recognized only one band of an appropriate molecular mass to be a subunit of AGPase (about 50 kD, Chen and Janes, 1997, 1998). Where lanes were loaded with equal activities of plastidial marker enzyme, the band was of approximately equal intensity in homogenate, pellet, and supernatant fractions (compare lanes P, S, and H in Fig. 2A, and lanes P and S in Fig. 2B). However, where lanes contained equal activities of cytosolic marker enzyme, the band was visible in the pellet and not the supernantant fraction (compare lanes P and S' in Fig. 2B). These data indicate that AGPase protein is primarily or exclusively plastidial in the pericarp and the columella.
DISCUSSION
Our study provides strong evidence that AGPase activity and protein is mainly or exclusively plastidial in the pericarp and the columella of the developing tomato fruit. This conclusion is consistent with our observation that the ratio of ADP-Glc to UDP-Glc in developing fruit is very low (Beckles et al., 2001). We suggest that ADP-Glc in tomato fruit is synthesized via a plastidial AGPase from Glc phosphate imported from the cytosol. Consistent with this idea, envelopes of plastids from tomato fruit are reported to have a hexose-phosphate-phosphate exchange transporter (Schünemann and Borchert, 1994). Extraplastidial isoforms of AGPase have thus far been identified only in the endosperms of cereals (Denyer et al., 1996; Thorbjørnsen et al., 1996; Shannon et al., 1998; Beckles et al., 2001). The pathway we suggest for tomato appears to occur in all other organs for which reliable information is available, including the embryos of oilseed rape and pea and the tubers of potato (Hill and Smith, 1991; Kang and Rawsthorne, 1994, 1995; Naeem et al., 1997).
Our results are at variance with those of Chen and colleagues, who reported that the stroma and the cytosol were labeled in sections of developing pericarp challenged with an antiserum to tomato AGPase. Chen et al. (1998) suggested that the cytosolic protein they detected might be an untransported precursor of the plastidial AGPase. It is likely that the antisera we used recognized primarily or exclusively the small subunit(s) of the tomato enzyme. The amino acid sequences of small subunits are highly conserved between species, whereas those of large subunits are divergent (Smith-White and Preiss, 1994). In studies with purified AGPase from tomato fruit, Chen and Janes (1998) found that the spinach antiserum recognized only the small subunit. It is possible, therefore, that the cytosolic protein detected by Chen and colleagues was an inactive form of the large subunit, which we did not detect. Regardless of the nature of the cytosolic antigen detected by Chen et al. (1998), our results provide strong evidence that little or no active AGPase is present outside the plastid in developing fruit.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum L. cv Moneymaker) plants were grown in a greenhouse with a minimum temperature of approximately 15°C and supplementary lighting to provide a 16-h photoperiod. For all experiments, fruit was harvested onto ice and used immediately.
Measurement of Starch Content
Samples were of pericarp or columella plus placenta taken from at least four fruits. Measurements were made by enzymatic conversion of starch to Glc (Smith, 1988).
AGPase Assay
Assay components and pH were optimized to give maximum rates, at 25°C. Rates were linear with respect to time over the period of the assay and were proportional to the amount of extract within the range of amounts used. The assay contained 100 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (pH 7.9), 5 mm MgCl2, 0.4 mm NAD, 2.5 mm ADP-Glc, 1.5 mm Na pyrophosphate, 1 mm 3-phosphoglycerate, 10 units Glc 6-P dehydrogenase (from Leuconostoc mesenteroides), and 4 units phosphoglucomutase, in a final volume of 1 mL.
Extracts for assay of activity through development of the fruit were prepared by homogenization of representative, longitudinal segments of fruit in a mortar followed by an all-glass homogenizer in 3 to 5 volumes of ice-cold 100 mm MOPS [3-(N-morpholino)-propanesulfonic acid] (pH 7.2), 2 mm EDTA, 6 mm dithiothreitol (DTT), 100 mL L−1 ethanediol, and 100 g L−1 polyvinylpolypyrrolidone. The homogenate was centrifuged for 10 min at 10,000g.
Mixing experiments were used to check whether activity of AGPase was lost during extract preparation. Replicate samples of starch-storing tissues of tomato and of developing pea cotyledon (an organ with a high activity of AGPase; Smith et al., 1989) were prepared. One sample of each tissue was extracted separately and the remaining two were extracted together. In two separate experiments with fruit of 8 to 11 DPP, activity of AGPase in the mixed extracts was 99% and 82% of that predicted from the separate extracts. For fruit of 30 DPP these values were 98% and 86%. These results provide good evidence that no major loss of enzyme activity occurred during preparation of tomato fruit extracts.
Assays of Plastidial and Cytosolic Marker Enzymes
Soluble Starch Synthase
The assay was according to Jenner et al. (1994) using the resin method.
Alkaline Pyrophosphatase
The assay was according to Gross and ap Rees (1986). The assay contained 50 mm Bicine [N,N′-bis(2-hydroxyethylglycine)] (pH 8.9), 20 mm MgCl2, and 1.25 mm Na pyrophosphate, in a final volume of 0.2 mL.
Alcohol Dehydrogenase
The assay was according to Denyer and Smith (1988).
Pyrophosphate, Fru 6-P 1-Phosphotransferase
The assay was according to Foster and Smith (1993).
Preparation of Amyloplasts
Procedures were carried out at 0°C to 4°C. The pericarp and columellar plus placental tissue were removed from fruits between 8 and 11 DPP and were placed separately in amyloplast isolation medium containing 0.5 m sorbitol, 50 mm HEPES (pH 7.5), 1 mm EDTA, 1 mm KCl, 2 mm MgCl2, 10 mL L−1 ethanediol, 1 mm DTT, and 20 g L−1 fat-free bovine serum albumin. An approximate 15 g of pericarp or 5 g of columellar plus placental tissue were finely chopped with razor blades and filtered through two layers of Miracloth (Chicopee Mills, Milltown, NJ) to produce a homogenate fraction. This was centrifuged at 48g for 10 min for pericarp and for 8 min for columella plus placenta, in a swing-out rotor, to give a pellet and a supernatant fraction. The pellet was resuspended in 2 to 3 mL of amyloplast isolation medium. Prior to assay, plastids in the homogenate, supernatant, and pellet fractions were ruptured by addition of Triton X-100 (0.1 mL L−1) or by repeated extrusion through a fine needle, followed by centrifugation at 10,000g for 5 min. Latency measurements were done as described by Entwistle and ap Rees (1988).
SDS-PAGE and Immunoblotting
Samples were diluted with double-strength gel sample buffer (as in Laemmli, 1970, except that it contained 70 mm DTT in place of mercaptoethanol), heated to 100°C for 2 min, and centrifuged at 10,000g for 5 min before loading onto 7.5% (w/v) SDS-polyacrylamide gels. Immunoblots were done according to Bhattacharyya et al. (1990).
The spinach leaf and the maize antisera used in this study recognized, in addition to AGPase, proteins of higher molecular mass than authentic AGPase from tomato fruit (Chen and Janes, 1997). Further evidence that these proteins are not AGPase was provided by examination of the correlation between the intensity of the 50-, 66-, and 100-kD bands and the activity of AGPase at five stages of development (data not shown; described in Beckles, 1999). This revealed a very good correlation for the 50-kD band (R2 = 0.82 or higher), but a poor correlation for the 66- and 100-kD bands (R2 = 0.48 or less).
ACKNOWLEDGMENTS
We thank Dr. Kim Hammond-Kosack (Sainsbury Laboratory, Norwich) for the gift of tomato cv Moneymaker plants, and Chris Hylton, Kay Denyer, Sam Zeeman (John Innes Centre), and Prof. Enid MacRobbie (University of Cambridge) for their advice and support.
Footnotes
This work was supported by a Competitive Strategic Grant from the Biotechnology and Biological Sciences Research Council, and by the Commonwealth Scholarship Commission, the Association of Commonwealth Universities, and the Cambridge Philosophical Society (to D.M.B.).
LITERATURE CITED
- ap Rees T. Where do plants make ADPglucose? In: Pontis HG, Salerno GL, Echeverria EJ, editors. Sucrose: Metabolism, Biochemistry, Physiology and Molecular Biology. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 143–155. [Google Scholar]
- Beckles DM. The subcellular location of ADPglucose pyrophosphorylase in starch storing cells. PhD thesis. UK: University of Cambridge; 1999. [Google Scholar]
- Beckles DM, Smith AM, ap Rees T. A cytosolic ADPglucose pyrophosphorylase is a feature of Graminaceous endosperms but not of other starch-storing organs. Plant Physiol. 2001;125:818–827. doi: 10.1104/pp.125.2.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya MK, Smith AM, Ellis THN, Hedley C, Martin C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch branching enzyme. Cell. 1990;60:115–122. doi: 10.1016/0092-8674(90)90721-p. [DOI] [PubMed] [Google Scholar]
- Chen B-Y, Janes HW. Multiple forms of ADP-glucose pyrophosphorylase from tomato fruit. Plant Physiol. 1997;113:235–241. doi: 10.1104/pp.113.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B-Y, Janes HW. Multiple forms of ADP-glucose pyrophosphorylase from tomato leaf. Physiol Plant. 1998;103:491–496. doi: 10.1104/pp.113.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B-Y, Wang Y, Janes HW. ADP-glucose pyrophosphorylase is localized to both the cytoplasm and plastids in developing pericarp of tomato fruit. Plant Physiol. 1998;116:101–106. doi: 10.1104/pp.116.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjørnsen T, Keeling P, Smith AM. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extraplastidial. Plant Physiol. 1996;112:779–785. doi: 10.1104/pp.112.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Smith AM. The capacity of plastids from developing pea cotyledons to synthesize acetyl CoA. Planta. 1988;173:172–182. doi: 10.1007/BF00403008. [DOI] [PubMed] [Google Scholar]
- Entwistle G, ap Rees T. Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm. Biochem J. 1988;255:391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J, Smith AM. Metabolism of glucose 6-phosphate by plastids from developing pea embryos. Planta. 1993;190:17–24. [Google Scholar]
- Giroux MJ, Hannah LC. ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet. 1984;243:400–408. doi: 10.1007/BF00280470. [DOI] [PubMed] [Google Scholar]
- Gross P, ap Rees T. Alkaline inorganic pyrophosphatase and starch synthesis in amyloplasts. Planta. 1986;167:140–145. doi: 10.1007/BF00446381. [DOI] [PubMed] [Google Scholar]
- Hill LM, Smith AM. Evidence that glucose 6-phosphate is imported as the substrate for starch synthesis by the plastids of developing pea embryos. Planta. 1991;185:91–96. doi: 10.1007/BF00194519. [DOI] [PubMed] [Google Scholar]
- Hill LM, Smith AM. Coupled movements of glucose 6-phosphate and triose phosphate through the envelopes of plastids from developing embryos of pea (Pisum sativum L.) J Plant Physiol. 1995;146:411–417. [Google Scholar]
- Hylton CM, Smith AM. The rb mutation of peas causes structural and regulatory changes in ADP-glucose pyrophosphorylase from developing embryos. Plant Physiol. 1992;99:1626–1634. doi: 10.1104/pp.99.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner CF, Denyer K, Hawker JS. Caution on the use of the generally accepted methanol precipitation technique for the assay of soluble starch synthase in crude extracts of plant tissues. Aust J Plant Physiol. 1994;21:17–22. [Google Scholar]
- Kang F, Rawsthorne S. Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.) Plant J. 1994;6:795–805. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morell MK, Bloom M, Knowles V, Preiss J. Subunit structure of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol. 1987;85:182–187. doi: 10.1104/pp.85.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M, Tetlow IJ, Emes MJ. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997;11:1095–1103. [Google Scholar]
- Okita T. Is there an alternative pathway for starch synthesis? Plant Physiol. 1992;100:560–564. doi: 10.1104/pp.100.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Levin I, Oguz I, Petreikov M, Cincarevsky F, Yeselson Y, Shen S, Gilboa N, Bar M. ADPglucose pyrophosphorylase activity and starch accumulation in immature tomato fruit: the effect of a Lycopersicon hirsutum-derived introgression encoding for the large subunit. Plant Sci. 2000;152:135–144. [Google Scholar]
- Schünemann D, Borchert S. Specific transport of inorganic phosphate and C3- and C6-sugar-phosphates across the envelope membranes of tomato (Lycopersicon esculentum) leaf chloroplasts, tomato fruit chloroplasts and fruit chromoplasts. Bot Acta. 1994;107:461–467. [Google Scholar]
- Shannon JC, Pien F-M, Cao H, Liu K-C. Brittle-1, an adenylate translocator, facilitates transfer of extraplastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol. 1998;117:1235–1252. doi: 10.1104/pp.117.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM. Major differences in isoforms of starch-branching enzyme in embryos of round- and wrinkled-seeded peas (Pisum sativum L.) Planta. 1988;175:270–279. doi: 10.1007/BF00392437. [DOI] [PubMed] [Google Scholar]
- Smith AM, Bettey M, Bedford ID. Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiol. 1989;89:1279–1284. doi: 10.1104/pp.89.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-White B, Preiss J. Comparisons of proteins of ADP-glucose pyrophosphorylase from diverse sources. J Mol Evol. 1994;34:449–464. doi: 10.1007/BF00162999. [DOI] [PubMed] [Google Scholar]
- Thorbjørnsen T, Villand P, Denyer K, Olsen O-A, Smith AM. Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm. Plant J. 1996;10:243–250. [Google Scholar]