Abstract
Phenotypic features such as ataxia and loss of motor function, which are characteristics of Parkinson’s disease (PD), are expected to be very closely related to cerebellum function. However, few studies have reported the function of the cerebellum. Since the cerebellum, like the cerebrum, is known to undergo functional and morphological changes due to neuroinflammatory processes, elucidating key functional factors that regulate neuroinflammation in the cerebellum can be a beneficial therapeutic approach. Therefore, we employed PD patients and MPTP-induced PD mouse model to find cytokines involved in cerebellar neuroinflammation in PD and to examine changes in cell function by regulating related genes. Along with the establishment of a PD mouse model, abnormal shapes such as arrangement and number of Purkinje cells in the cerebellum were confirmed based on histological finding, consistent with those of cerebellums of PD patients. As a result of proteome profiling for neuroinflammation using PD mouse cerebellar tissues, fetuin-A, a type of cytokine, was found to be significantly reduced in Purkinje cells. To further elucidate the function of fetuin-A, neurons isolated from cerebellums of embryos (E18) were treated with fetuin-A siRNA. We uncovered that not only the population of neuronal cells, but also their morphological appearances were significantly different. In this study, we found a functional gene called fetuin-A in the PD model’s cerebellum, which was closely related to the role of cerebellar Purkinje cells of mouse and human PD. In conclusion, morphological abnormalities of Purkinje cells in PD mice and patients have a close relationship with a decrease of fetuin-A, suggesting that diagnosis and treatment of cerebellar functions of PD patients might be possible through regulation of fetuin-A.
Keywords: Cerebellum, Fetuin-A, MPTP, Parkinson’s disease, Purkinje cell
INTRODUCTION
Parkinson’s disease (PD) is already well known as a progressive chronic degenerative neurological symptom characterized by decreased motor skills, resting tremors, rigidity, and gait disorder phenotype (1). PD also causes non-motor symptomatic problems regarding mood disorders such as depression and anxiety (2-4). Pathological changes caused by PD are attributed to continuously decreased dopaminergic neurons within the substantia nigra (SNr), resulting in a remarkable decrease of dopamine concentration in the striatum (5). Therefore, most studies have focused on the basal ganglia, although reciprocal anatomical connections between basal ganglia and cerebellum have been fully identified thus far (1, 6, 7). Notably, effects of morphological and pathological changes in the cerebellum on clinical symptom changes in PD patients have recently attracted attention (1). Many studies agree that the severity of these neurodegenerative diseases is promoted by reduced frontal cortex volume, blood-brain barrier (BBB) damage, progressing synaptic dysfunction, reduced striatal dopamine receptors, calcium impairment, mitochondrial alteration, and reactive oxygen species (ROS) increment (8-10). The persistent inflammatory state listed above as the concept of “neuroinflammation” has been noted as a significant concern for degenerative brain diseases caused by aging (11-13). Therefore, we hypothesize that specific inflammatory cytokines known to modulate neuroinflammation might exist in these pathological mechanisms, considering that symptoms of PD patients might be closely related to the function of the cerebellum. In the present study, we found morphological abnormalities of Purkinje cells in PD patients. We predicted that these fatal abnormalities might have appeared due to sustained long-lasting neuroinflammation and that they might have a very close relationship with motor dysfunction in PD.
Meanwhile, fetuin-A mainly appears in embryonic cells and adult hepatocytes (14, 15). It can bind to many receptors (15, 16). It exhibits multifaceted physiological and pathological functions (15, 17). In addition, recent studies have demonstrated that fetuin-A concentration is reduced in the pathogenesis of assorted brain pathologies, including cerebral ischemic injury and neurodegenerative diseases (15). These results show that fetuin-A contributes to neuroprotection and anti-inflammatory reaction (15, 18). In other words, the function of fetuin-A might be involved in the pathological mechanism of PD.
Researchers have devised an insult called 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) and used it to induce PD in animal models. Repeated chronic injection of MPTP is known to cause neuroinflammation in the animal brain for a long time (19). An MPTP insult can accelerate the decrease of Purkinje cell population and the loss of calcium-binding positive Purkinje cells (20). Loss of induced hyper-activation of Purkinje cells has been reported (21). Purkinje cells’ ability to maintain normal morphological shape and functional performance can be seen as an indicator of synaptic plasticity (22), a major consideration for the cerebellum’s motor and non-motor functional mechanisms (23). Thus, we observed cerebellar tissues of PD patients and constructed an MPTP-induced PD mouse model.
In this study, we focused on fetuin-A expressed in Purkinje cells of the cerebellum. The function of fetuin-A in the cerebellum is not yet well known. To induce silencing of fetuin-A in embryonic cerebellar primary neurons, RNAi method was used. We provide strong evidence that fetuin-A is a novel functional biomarker in the cerebellum of PD through examination of MPTP-induced PD mice, PD human patients, and an in vitro model.
RESULTS
Fetuin-A levels are decreased in cerebellar Purkinje cells of patients with PD
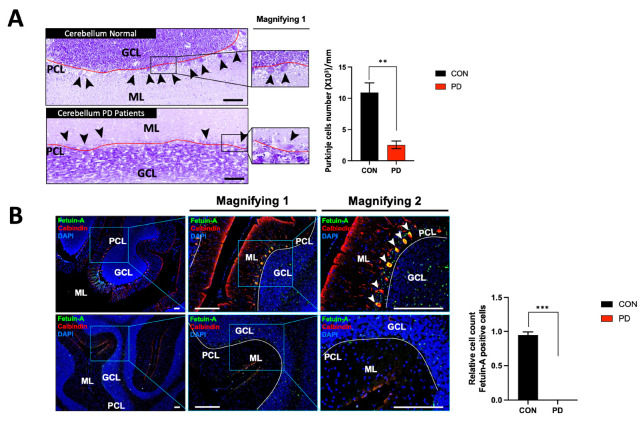
As a result of observing PD patients’ cerebella after Nissl staining, we encountered clear differences in the shape and distribution of Purkinje cells on the Purkinje cell layer (PCL) line compared to those appeared in the cerebella of normal subjects (Normal) (Fig. 1A). When the expression of fetuin-A expressed in Purkinje cells of PD patients was compared to that in normal controls observed through immunofluorescence, expression levels of calbindin and fetuin-A were well observed in Purkinje cells of the cerebella of normal subjects. Conversely, they were almost absent in PD patients (Fig. 1B).
Fig. 1.
Histological features of the cerebella in human PD patients and reduction of fetuin-A expression in Purkinje cells. (A) Nissl staining of the cerebella in PD patients. (B) Expression of fetuin-A in cerebellar Purkinje cells. The solid-red line is an imaginary extension of PCL. Black arrow heads indicate Purkinje cells. White arrow heads indicate fetuin-A and/or calbindin positive Purkinje cells. “Magnifying 1” is the enlarged image. “Magnifying 2” is the maximum magnification of the image in the box. GCL = granule cell layer; ML = molecular layer; PCL = Purkinje cell layer; ***P < 0.005; **P < 0.01. Scale bar = 100 μm. Error bar represents mean ± standard deviation.
Fetuin-A levels are suppressed in the cerebellum of a PD mouse model
PD mice established by chronic MPTP exposure are summarized in Supplementary Fig. 1.
When the cerebella of these selected PD mice and normal cerebella (CON) were compared by Nissl staining, histological findings were remarkable like those observed in the cerebella of human patients with PD (Fig. 2A). As a result of observing expression characteristics of 111 inflammatory cytokines expressed in the lysate of the cerebella of PD mice by proteome array, several proteins showed expression differences, with fetuin-A showing the most remarkable differences in brain tissues and sera (Fig. 2B and Supplementary Fig. 1D). Osteopontin (OPN) and myeloperoxidase (MPO) showed common changes in the cerebellum (Fig. 2B and Supplementary Fig. 1D). Since the difference in fetuin-A protein expression between CON and PD groups was confirmed in proteome profiling, the difference in fetuin-A expression in the cerebellar tissue between CON and PD groups was analyzed again by Western blot (Fig. 2C).
Fig. 2.
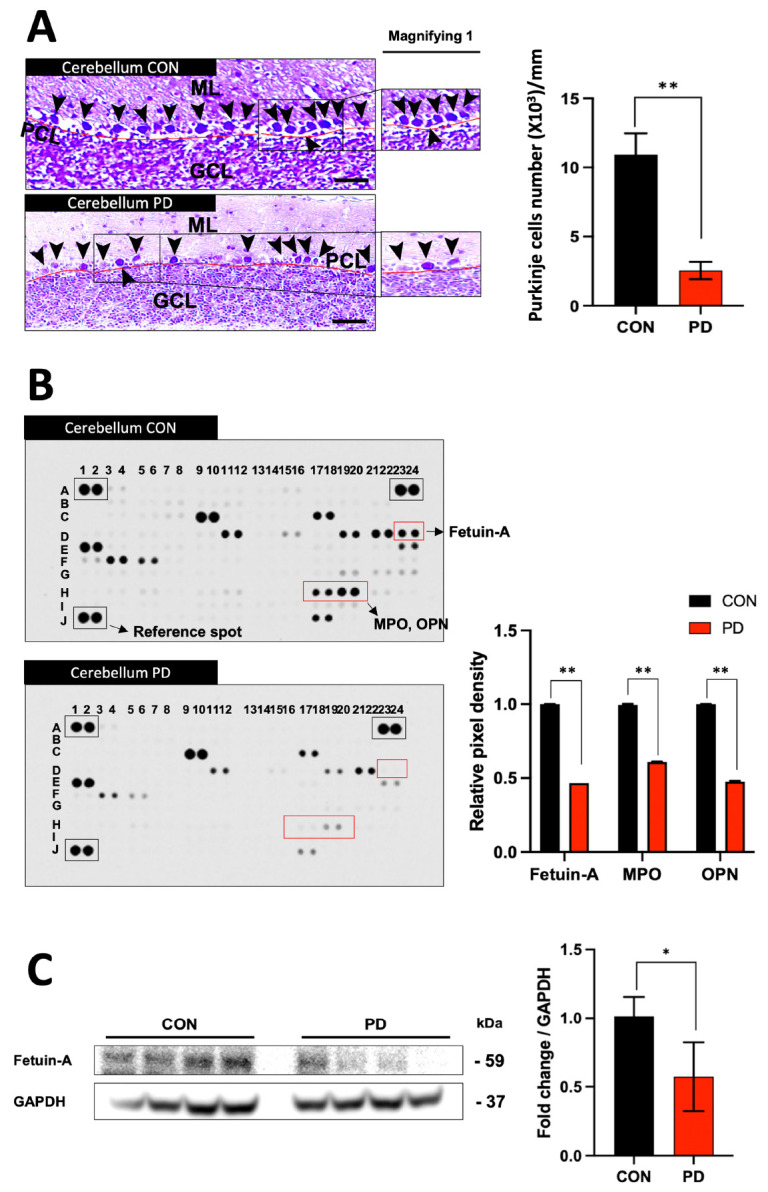
Histological features and proteome profiling of the cerebellum in MPTP-induced PD mice. (A) Histological traits of Nissl staining of Purkinje cells in normal and PD mouse cerebellum. (B) Proteome profiling to observe changes in inflammatory cytokines in the cerebella of normal and PD mice. (C) Protein expression of fetuin-A was significantly decreased in the cerebella of PD mice. Deep blue boxes are reference genes. “Magnifying” indicates enlarged image. **P < 0.01; *P < 0.05 vs. CON. Scale bar = 100 μm. Error bar represents mean ± standard deviation.
Impaired Purkinje cells show reduced fetuin-A levels in a PD mouse model
Immunohistochemistry and immunofluorescence staining of fetuin-A were performed to determine its expression location in the cerebellum. In the CON, fetuin-A was mainly expressed in Purkinje cells. However, in the PD model, the expression of fetuin-A was considerably reduced or disappeared (Fig. 3A, B). This result was consistent in the cerebella of PD mice and human PD. Double-merged images of calbindin and fetuin-A established that fetuin-A’s intensity in the PD mouse model was significantly reduced in Purkinje cells (Fig. 3C).
Fig. 3.
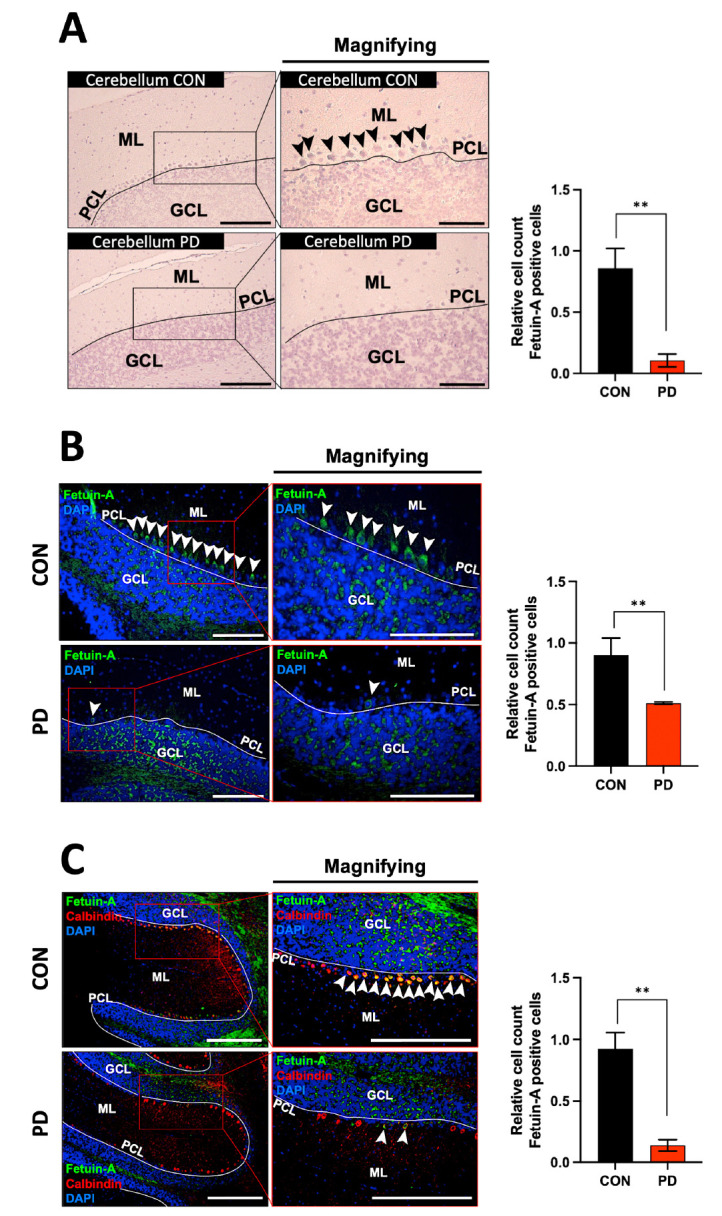
Immunohistochemistry and immunofluorescence for observing fetuin-A expressing cells in the cerebella of PD mice. (A, B) Fetuin-A in the cerebellum is well expressed in Purkinje cells in CON, but not in PD mice. (C) Double merged images of calbindin and fetuin-A confirmed that fetuin-A’s intensity in the PD model was significantly reduced in Purkinje cells. Black arrow heads indicate fetuin-A positive Purkinje cells. White arrow heads indicate fetuin-A and/or calbindin positive Purkinje cells. “Magnifying” indicates enlarged image of the left square of each image. White line indicates Purkinje cell layer. Scale bar = 100 μm. **P < 0.01 vs. CON. Error bar represents mean ± standard deviation.
Knockdown of fetuin-A decreases cellular survival of Purkinje cells
Results shown above suggest that the decrease of fetuin-A expression in the cerebellum of PD model might have led to the decline in the shape and number of Purkinje cells. Therefore, if the survival of neuronal cells derived from the cerebellum could be determined throughout fetuin-A gene silencing, the function of fetuin-A in PD could be newly identified. Results showed that the absolute number of neurons derived from the cerebellum of an 18-day-old embryo was reduced due to fetuin-A siRNA treatment (Fig. 4).
Fig. 4.
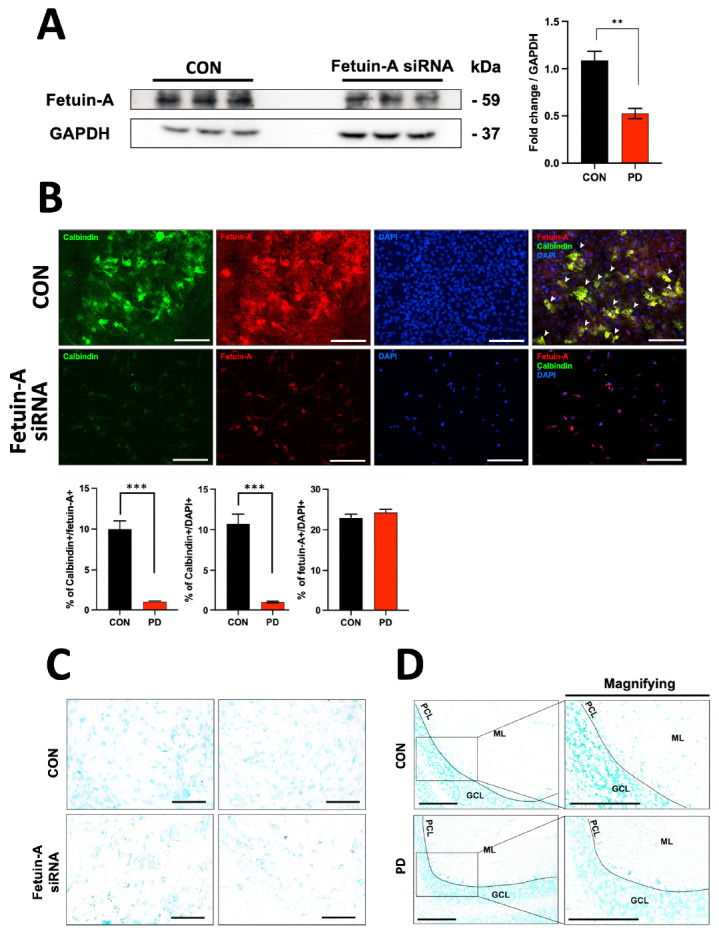
Observation of morphological changes in embryonic neuronal cells after treatment with fetuin-A siRNA. (A) Significant silencing of fetuin-A expression was observed in cells treated with siRNA. (B) In 18-day embryonic (E18) cerebellar neuronal cells, strong signals of calbindin and fetuin-A were observed in the CON group. However, the shape of cells treated with fetuin-A siRNA transfection looked different from CON and the number of cells expressing calbindin or fetuin-A was significantly decreased. (C, D) TUNEL assay results for measuring apoptosis in Purkinje cells. ***P < 0.005; **P < 0.01 vs. CON. Error bar represents mean ± standard deviation.
TUNEL assay
As a result of the TUNEL assay, there were no apoptosis-positive changes in fetuin-A silenced neurons in Purkinje cells of PD animals (Fig. 4C, D).
DISCUSSION
Recently, interest in the pathological mechanism and additional phenotypes of PD is significantly increasing. However, studies on the cerebellum remain insufficient. Screening inflammatory cytokines that participate in significant pathological mechanisms in the cerebellum for ataxia in PD patients is expected to provide diagnostic and therapeutic value as mentioned earlier. We confirmed histological abnormalities and a significant reduction of fetuin-A expression in the cerebella of PD patients (Fig. 1). Effects of fetuin-A expression on Purkinje cells were determined using mouse and cell models. MPTP administration substantially reduced animals’ motor performance with a significant decrease in dopaminergic neurons in the SNr based on histological findings (Supplementary Fig. 1). Nissl staining of MPTP-induced PD mice also showed remarkably similar histological changes in the cerebella of PD patients (Fig. 2A). By proteome array, we found significant expression changes of some proteins in the cerebellum and serum of MPTP-induced PD, including fetuin-A, MPO, and OPN (Fig. 2B and Supplementary Fig. 1D). Although it has been continuously reported that MPO and OPN can be significant biomarkers in degenerative brain diseases (24, 25), the discovery of fetuin-A in the cerebellum is novel. Notably, fetuin-A’s expression change was the most prominent among these proteins that showed expression changes (Fig. 2B and Supplementary Fig. 1D). When the expression of fetuin-A in the protein homogenate of the cerebellum was examined, the same result as the proteome array was obtained (Fig. 2C). As mentioned earlier, since fetuin-A has a neuroprotective effect (26, 27), it seems likely that the expression of fetuin-A is proportionally reduced according to the severity of PD. As a result of analyzing the expression of fetuin-A in the cerebellum using IHC and IF methods, it was found that the expression of fetuin-A in PD mice was significantly reduced (Fig. 3). Based on our fetuin-A siRNA results using the RNAi technique, suppression of fetuin-A expression was predicted to affect Purkinje cells’ death (Fig. 4). However, a decrease in fetuin-A expression did not lead to cell apoptosis (Fig. 4D). Thus, further investigation is needed.
In summary, we compared characteristic changes of the cerebella of MPTP-induced PD mice with those of human PD patients. The role of fetuin-A gene expression in Purkinje cells was demonstrated. Along with the fact that the function of the fetuin-A gene expressed in Purkinje cells was important in both mouse models and humans, we found out regulating fetuin-A expression is very important for maintaining the function of Purkinje cells. Our study is the first to filter out significant biomarkers by performing histological structure and proteomic analyses of MPTP-induced PD mouse and the cerebella of human PD patients. Further studies are needed to elucidate specifics and additional molecules that drive fetuin-A.
MATERIALS AND METHODS
Animals and MPTP-induced Parkinson’s disease mouse model
C57BL/6J mice (n = 28, male 25-30 g, 8-week-old) were purchased from Orient Bio (Seongnam, Korea). These animals were housed in a room maintained humidity at 60% and temperature of 22 ± 2°C under a 12 h light:12 h dark cycle. Animals had free access to food and water. These mice (MPTP; n = 14) were intraperitoneally injected with MPTP (S47312, Selleck Chemical, Houston, USA) at 30 mg/kg/day dissolved in 0.9% saline for 30 consecutive days. Remaining animals (CON; n= 14) were administered with only vehicle (0.9% saline) under the same conditions. Motor performance was inspected by rotarod (B.S Technolab, Daejeon, Korea) for a chronic MPTP-induced PD mouse model at 40 rpm compared to CON to indicate movement ability. Result was calculated as average latency on the rotating rod with speed accelerating gradually from 10 rpm/min to 40 rpm/min. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Soonchunhyang University (Approval number: SCH22-0005).
Histology
Mice were anesthetized with 20% Urethane (Daejung chemical & metals, Korea) at a volume of 10 ml/kg. They were then perfused transcardially with 0.9% saline, followed by treatment with 4% paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M phosphate buffer (PB, pH7.4). Brain tissues were removed and post-fixed overnight in 4% PFA at 4°C. They were then sectioned at 4-μm in thickness after paraffin-embedding. Sections contained the Purkinje cell layer of the cerebellum area corresponding to the bregma between −5.80 and −6.24 mm of the mouse brain atlas (28). Additionally, coordinates of SNr to identify dopaminergic neurons were identified at bregma −2.70 mm to −2.92 mm. For Nissl staining, brain tissues were stained with 1% cresyl violet (Daejung chemicals & metals, Korea). Cerebellar tissues of normal human subjects (#NBP2-77754) and PD patients (#NBP2-77999, Novus Biologicals, Centennial, CO, USA) were obtained and compared with MPTP-induced PD mouse phenotypes. Human subject study was conducted in accordance with the Helsinki Declaration. The protocol was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (SCHCA 2020-03-030-001).
Proteome arrays
To explore protein profiles of released cytokines and chemokines in mice cerebella and sera, semi-quantitatively analyses were conducted using a Proteome profiler mouse XL cytokine Array Kit (R&D systems, USA). Samples were incubated with spotted nitrocellulose membranes according to the manufacturer’s instructions. Membrane HRP luminescence signal intensities were detected using a chemi-luminance bioimaging instrument (CheBI2, CBI004, NeoScience, Korea) with an exposure time of 10 min. Images were recorded and intensities obtained for dots on the membrane were individually determined in duplicate with an Image Studio software (version 5.2). Fold change was measured compared to the average value of the control.
Western blot
Proteins were lysed in RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA) with phosphatase inhibitor (GenDEPOT, USA). Protein concentration was determined using BSA standard. After quantification, equal amounts of protein samples were loaded onto each lane with 10% Tris-glycine and transferred to PVDF membranes (Bio-Rad, CA, USA). After transfer, the membrane was blocked with 1X TBST (10X TBS with Tween 20, Biosesang, Korea) containing 5% BSA. Membranes were incubated with monoclonal mouse anti-fetuin A (1:100 dilution, Santa Cruz Biotechnology, Dallas, TX, USA) and polyclonal rabbit anti-GAPDH (1:10,000 dilution, Cell Signaling, MA, USA) at 4°C overnight. These membranes were then incubated with HRP-conjugated horse anti-mouse IgG or goat anti-rabbit IgG (1:2,000, Vector Laboratories, Burlingame, CA, USA) for 2 h at room temperature. Immunoreactive bands were visualized with a SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA, USA).
Immunohistochemistry
Tissues were deparaffinized, rehydrated, sequentially treated with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 30 min, and then blocked with CAS-Block Histochemical Reagent (Thermo Fisher Scientific, Waltham, MA, USA). They were then incubated with the following primary antibodies with diluent (PBS, 0.3% Triton X-100) at 4°C overnight: polyclonal rabbit anti-tyrosine hydroxylase (1:500, abcam, Cambridge, UK) and monoclonal mouse anti-fetuin-A (1:100, Santa Cruz Biotechnology, Dallas, TX, USA). Tissues were incubated with biotinylated goat anti-rabbit IgG or goat anti-mouse IgG (1:300, Vector Laboratories, Burlingame, CA, USA) for 2 h at room temperature. Sections were visualized using 3,3’-diaminobenzidine tetrachloride (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer. Coverslip was mounted using Eukitt Quick hardening mounting medium. Stained brain sections were quantified with ImageJ software v1.52a (Bethesda, MD, USA).
Immunofluorescence
Sections were permeabilized in 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), blocked in CAS-BlockTM Histochemical Reagent (Thermo Fisher Scientific, Waltham, MA, USA), and then stained with the following antibodies: polyclonal rabbit anti-calbindin (1:500, CB38, Swant, Switzerland) and monoclonal mouse anti-fetuin-A (diluted 1:100, Santa Cruz Biotechnology, Dallas, TX, USA). Sections were then incubated with donkey anti-mouse IgG (H + L) Alexa Fluor 488 or donkey anti-rabbit IgG (H + L) Alexa Fluor 594 (1:300, Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. FluoroshieldTM with DAPI (Sigma-Aldrich, St. Louis, MO, USA) was used for nuclear staining. Stained brain sections were analyzed with an immunofluorescence microscope (Thermo Fisher Scientific, InvitrogenTM EVOSTM M7000 Imaging System, USA). These stained brain sections were quantified with the ImageJ software v1.52a (Bethesda, MD, USA).
Cells were plated onto sterilized glass coverslips placed in 6- or 24-well culture plates. Cells were fixed with 4% PFA, permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, USA), blocked with CAS-BlockTM Histochemical Reagent (ThermoFisher Scientific, Waltham, MA, USA), and stained as described above.
Primary cell culture
Embryos (E18) were removed from three pregnant C57BL/6J mice and anesthetized with isoflurane (Ifran, Hana Pharmaceuticals, Hwasung, Korea). These isolated embryos were sacrificed and embryonic cerebella were dissected and stored in 10 ml of cold Ca2+ and Mg2+ free Hank’s balanced salt solution (HBSS) (Gibco, Grand Island, NY, USA) containing 10 μg/ml gentamicin (Gibco, Grand Island, NY, USA). Tubes were centrifuged at 500 rpm for 5-min. The supernatant was aspirated and digested with 2.5 ml HBSS containing 0.1% trypsin (Gibco, Grand Island, NY, USA) at 4°C for 15 min. The cerebellum was gently ground into small aggregates with magnesium sulfate (12 mM, Sigma-Aldrich, St. Louis, MO, USA) and DNase I (5 U/ml, Zymoresearch, USA). Five-milliliter of HBSS was added to the cell suspension and tubes were centrifuged at 500 rpm at 4°C for 5 min. After removing the supernatant, cells were seeded into 24-well culture plates at a concentration of 1 × 105 cells/cm2 with Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, putrescine (100 μM, Sigma-Aldrich, St. Louis, MO, USA), sodium selenite (30 nM, Sigma-Aldrich, St. Louis, MO, USA), and gentamicin (10 μg/ml, Gibco, Grand Island, NY, USA) at 37°C. Cells were kept in the incubator. After half of the old medium was removed, a new fresh culture medium containing transferrin (200 μg/ml, Sigma-Aldrich, St. Louis, MO, USA), progesterone (40 nM, Sigma-Aldrich, St. Louis, MO, USA), triiodothyronine (0.5 ng/ml, Sigma-Aldrich, St. Louis, MO, USA), and cytosine arabinoside (4 μM, Sigma-Aldrich, St. Louis, MO, USA) was added.
Fetuin-A gene silencing
Fetuin-A siRNA was complexed with Lipofectamine RNAiMAX Reagent (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. Transfection medium was removed and replaced with DMEM containing 10% fetal bovine serum at 8 h after transfection. Fetuin-A siRNA (Fetuin-A Ahsg) was purchased from Santa Cruz Biotechnology (sc-39443, CA, USA).
TUNEL assay
TUNEL assay was performed to verify apoptosis that might appear in Purkinje cells of cerebellar tissues of MPTP-induced PD mice. A TUNEL kit (ab206386, Abcam, Cambridge, USA) commercially available was used for this analysis. All processes were performed according to the manufacturer’s directions. An optical microscope was used to take photomicrographs.
Statistics
Data are presented as mean ± standard deviation (SD). Statistics were analyzed using a two-tailed Student’s t-test for comparison of two groups. For comparison of multiple groups, analysis of variance (ANOVA) with post-hoc comparisons using Duncan’s multiple range test of the SPSS (IBM Corp., ver.22) statistical software package was used. P < 0.05 were considered statistically significant.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by a National Research Foundation (NRF) grant (NRF-2018R1D1A3B07047960) and Soonchunhyang University Research Fund.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dissanayaka NN, Sellbach A, Matheson S, et al. Anxiety disorders in Parkinson's disease: prevalence and risk factors. Mov Disord. 2010;25:838–845. doi: 10.1002/mds.22833. [DOI] [PubMed] [Google Scholar]
- 3.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;23:183–189. quiz 313. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- 4.Riedel O, Klotsche J, Spottke A, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson's disease. J Neurol. 2010;257:1073–1082. doi: 10.1007/s00415-010-5465-z. [DOI] [PubMed] [Google Scholar]
- 5.Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl. 2006:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Su W, Li S, et al. Cerebellar atrophy in different subtypes of Parkinson's disease. J Neurol Sci. 2018;392:105–112. doi: 10.1016/j.jns.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Mirdamadi JL. Cerebellar role in Parkinson's disease. J Neurophysiol. 2016;116:917–919. doi: 10.1152/jn.01132.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton J, van Amelsvoort T, Murphy D. HRT and its effect on normal ageing of the brain and dementia. Br J Clin Pharmacol. 2001;52:647–653. doi: 10.1046/j.0306-5251.2001.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 11.Di Benedetto S, Müller L, Wenger E, Düzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 13.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 14.Siegel-Axel DI, Ullrich S, Stefan N, et al. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014;57:1057–1066. doi: 10.1007/s00125-014-3177-0. [DOI] [PubMed] [Google Scholar]
- 15.Chekol Abebe E, Tilahun Muche Z, Mengie Ayele T, et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front Cell Dev Biol. 2022;10:945287. doi: 10.3389/fcell.2022.945287.a6a0ccad43c84cb9af73facab29080d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trepanowski J, Mey J, Varady K. Fetuin-A: a novel link between obesity and related complications. Int J Obes (Lond) 2015;39:734–741. doi: 10.1038/ijo.2014.203. [DOI] [PubMed] [Google Scholar]
- 17.Wojtysiak-Duma B, Malecha Jędraszek A, Burska A, Duma D, Donica H. Serum fetuin-A levels in patients with type 2 diabetes mellitus. Ann UMCS Sect. 2010;23:93–99. [Google Scholar]
- 18.Wang H, Li W, Zhu S, et al. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J Cereb Blood Flow Metab. 2010;30:493–504. doi: 10.1038/jcbfm.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lofrumento DD, Saponaro C, Cianciulli A, et al. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation. 2011;18:79–88. doi: 10.1159/000320027. [DOI] [PubMed] [Google Scholar]
- 20.Vignola C, Necchi D, Scherini E, Bernocchi G. MPTP-induced changes in the monkey cerebellum-immunohistochemistry of calcium-binding and cytoskeletal proteins. Neurodegeneration. 1994;3:25–31. [Google Scholar]
- 21.Heman P, Barcia C, Gómez A, et al. Nigral degeneration correlates with persistent activation of cerebellar Purkinje cells in MPTP-treated monkeys. Histol Histopathol. 2012;27:89–94. doi: 10.14670/HH-27.89. [DOI] [PubMed] [Google Scholar]
- 22.Hirano T. Purkinje neurons: development, morphology, and function. Cerebellum. 2018;17:699–700. doi: 10.1007/s12311-018-0985-7. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Takahara D, Hirata Y, et al. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur J Neurosci. 2010;31:1402–1413. doi: 10.1111/j.1460-9568.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- 24.Carecchio M, Comi C. The role of osteopontin in neurodegenerative diseases. J Alzheimers Dis. 2011;25:179–185. doi: 10.3233/JAD-2011-102151. [DOI] [PubMed] [Google Scholar]
- 25.Gellhaar S, Sunnemark D, Eriksson H, Olson L, Galter D. Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson's and Alzheimer's disease. Cell Tissue Res. 2017;369:445–454. doi: 10.1007/s00441-017-2626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chekol Abebe E, Tilahun Muche Z, Behaile TMA, et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front Cell Dev Biol. 2022;10:945287. doi: 10.3389/fcell.2022.945287.a6a0ccad43c84cb9af73facab29080d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinen MC, Babler A, Weis J, et al. Fetuin-A protein distribution in mature inflamed and ischemic brain tissue. PLoS One. 2018;13:e0206597. doi: 10.1371/journal.pone.0206597.b351d3ab60394cf2880eedb8389fa803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Franklin KB. Paxinos and Franklin's the mouse brain in stereotaxic coordinates. 5th edn. Academic press; United States: 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.