Abstract
The increasing global emergence of zoonoses warrants improved awareness of activities that predispose vulnerable communities to greater risk of disease. Zoonotic disease outbreaks regularly occur within Myanmar and at its borders partly due to insufficient knowledge of behavioral risks, hindering participatory surveillance and reporting. This study employed a behavioral surveillance strategy among high-risk populations to understand the behavioral risks for zoonotic disease transmission in an effort to identify risk factors for pathogen spillover. To explore behavioral mechanisms of spillover in Myanmar, we aimed to: (1) evaluate the details around animal contact and types of interaction, (2) assess the association between self-reported unusual symptoms (i.e., any illness or sickness that is not known or recognized in the community or diagnosed by medical providers) and animal contact activities and (3) identify the potential risk factors including behavioral practices of self-reported illness. Participants were enrolled at two community sites: Hpa-An and Hmawbi in Southern Myanmar. A behavioral questionnaire was administered to understand participants’ animal exposures, behaviors and self-reported illnesses. From these responses, associations between (1) animal contact activities and self-reported unusual illnesses, and (2) potential risk factors and self-reported unusual illness were tested. Contact with poultry seemed to be very frequent (91.1%) and many participants reported raising, handling and having poultry in their houses as well as slaughtering or being scratched/bitten by them, followed by contact with rodents (57.8%) and swine (17.9%). Compared to participants who did not have any unusual symptoms, participants who had unusual symptoms in the past year were more likely to have sold dead animals (OR = 13.6, 95% CI 6.8–27.2), slaughtered (OR = 2.4, 95% CI 1.7–3.3), raised (OR = 3.4, 95% CI 2.3–5.0) or handled animals (OR = 2.1, 95% CI 1.2–3.6), and had eaten sick (OR = 4.4, 95% CI 3.0–6.4) and/or dead animals (OR = 6.0, 95% CI 4.1–8.8) in the same year. Odds of having reported unusual symptoms was higher among those involved in animal production business (OR = 3.4, 95% CI 1.9–6.2) and animal-involved livelihoods (OR = 3.3, 95% CI 1.5–7.2) compared to other livelihoods. The results suggest that there is a high level of interaction between humans, livestock and wild animals in communities we investigated in Myanmar. The study highlights the specific high-risk behaviors as they relate to animal contact and demographic risk factors for zoonotic spillover. Our findings contribute to human behavioral data needed to develop targeted interventions to prevent zoonotic disease transmission at human–animal interfaces.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10393-023-01636-9.
Keywords: Zoonoses, Infectious disease transmission, Risk factors, Behavioral risk, Community, Myanmar
Introduction
Among the recognized human pathogens, approximately 60% are known to be zoonotic, i.e., able to transmit between species from animals and humans, which is the most likely sources of emerging and reemerging infectious diseases (Cleaveland et al., 2001; Taylor et al., 2001; Woolhouse and Gowtage-Sequeria, 2005; Jones et al, 2008). Southeast Asia (SEA) is widely considered an emerging infectious diseases (EID) hot spot, having experienced several major endemic and epidemic outbreaks within the last 15 years, including avian influenza, severe acute respiratory syndrome, leptospirosis and rabies (Bhatia and Narain, 2010; Coker et al., 2011).
Zoonotic outbreaks are often precipitated by landscape-level drivers common to developing regions, including increased trade and mobility, commercialization of agriculture and animal production, forest development and ecotourism (Bhatia and Narain, 2010; Coker et al., 2011; Hassell et al., 2017; Liebler et al., 2009). These factors are present in Myanmar, a developing SEA nation (Myanmar Ministry of Agriculture, Livestock and Irrigation, 2016). Despite relatively high forest coverage, estimated 44.5–63.0% of its land area (The Republic of the Union of Myanmar, 2013; The World Bank, 2015), recent socioeconomic factors have accelerated the development of Myanmar’s forests at unprecedented rates. Forest decline increased from 0.3 to 0.55% yearly from the early 2000s to 2014 (a loss of two million hectares) due to agricultural conversion, logging and other consumptive or extractive industries (Bhagwat et al., 2017; Leimgruber et al., 2005). Land-use change of this magnitude can create human–wildlife interfaces and increase opportunities for pathogen spillover through mass migration and displacement of people, and industrialization (Hassell et al., 2017; Liebler et al., 2009; Murray and Daszak, 2013).
At a community level, “high-risk behaviors”—sociocultural or occupational practices involving human–animal interactions—can predispose habitants to zoonotic disease risks. Myanmar is home to many at-risk communities, with those living on the margins of poverty disproportionately residing in rural areas (Food and Agriculture Organization of the United Nations, 2018; The World Bank Group, 2017a, b). In 2017, about 64.8–70.0% of the population resided in rural areas, and 46.0% lived in or near poverty (Food and Agriculture Organization of the United Nations, 2018; The World Bank Group, 2017a, b; Thant and Winn, 2016). Historically, much of the zoonotic disease burden falls to these populations, due to various socioeconomic, exposure and infrastructure factors, including limited human health services, underreporting of illnesses and insufficient surveillance (Cascio et al., 2011; Leimgruber et al., 2005; Osbjer et al., 2015).
Following the 2006 avian influenza (H5N1) outbreak, the One Health concept—emphasizing the interrelatedness of human, animal and environmental health—was introduced to Myanmar to address the burden of endemic zoonoses (World Health Organization, 2018). Myanmar’s first One Health plan was developed in 2013 to establish communication systems to notify public health authorities during disease outbreaks, prioritizing surveillance in early stages of detection and prevention (Myanmar Ministry of Health and Sports, 2013; Draft One Health Plan, 2013). However, implementing this plan proved challenging due to several factors, including limited knowledge of zoonotic disease transmission (Myanmar Ministry of Health and Sports, 2013).
To explore behavioral mechanisms of pathogen spillover in Myanmar, we aimed to: (1) evaluate the details around animal contact and types of interaction, (2) assess the association between self-reported unusual symptoms and animal contact activities and (3) identify the potential risk factors including behavioral practices of self-reported illness.
Materials and Methods
Site Characterization and Study Populations
Site selection was based on concurrent presence of wildlife and livestock, agricultural land use, low access to health care and infrastructure, and observation of behaviors incorporating animals (e.g., bat consumption, ecotourism). Wildlife species of concern included bats, rodents and non-human primates, which are more likely to be the source of major zoonoses of public health concern such as severe acute respiratory syndrome, Nipah virus and Ebola hemorrhagic fever (Devaux et al., 2019; Han et al., 2015; Meerburg et al., 2009). These taxa have higher pathogen spillover potential due to various ecological traits and increased opportunities for contact with humans (Olival et al., 2017). Concurrent wildlife viral surveillance targeted coronaviruses, filoviruses, paramyxoviruses and influenza viruses, which may be carried by our targeted species of interest.
Two study sites and their respective populations were targeted: (1) residents near bat caves in Hpa-an of Kayin State and (2) villagers of Hmawbi, near Hlawga National Park (HNP) in Yangon State. Locals at both sites depend heavily on commercial agriculture and livestock production and engage in religious practices involving wildlife contact. Ecotourism is prominent at both locations.
Site 1. Hpa-an
Situated in a rural agricultural region characterized by crop production and free-roaming livestock, Hpa-an features extensive, sacred caves that attract humans for guano harvesting, religious pilgrimages and ecotourism. These caves house colonies of approximately 300,000–500,000 bats (Chaerephon sp., Eonycteris sp., Craseonycteris sp. and Hipposideros sp.) throughout the year, with highest capacities in the wet season (Valitutto et al., 2020). Additionally, one cave complex maintains a resident colony of 200 macaques that regularly interact with visitors through hand-feeding. Each cave hosts approximately 100–500 local and international tourists daily with seasonal fluctuations. Residents of these communities participate in traditional and religious behaviors including wildlife consumption and removal of footwear to visit sacred caves. This study focused on several villages within a 12 km radius in this region, including residents who were occupationally exposed to bats through guano collection or visited the caves for religious purposes.
Site 2. Hmawbi
Occupying 1,540 acres (Ministry of Hotels and Tourism, 2018), HNP harbors many wildlife species, including over 2000 free-roaming macaques (Macaca mulatta and M. nemestrina), numerous bat species (Cynopterus sp., Pteropus sp., Taphozous sp. and Hipposideros sp.), deer, boar, gaur, birds and rodents according to Hlawga National Park official website (https://www.hlawgapark.com/). Domesticated species like elephant, cattle, chickens, ducks and dogs are also present. The park receives approximately 200 to 1,000 visitors daily. With the exception of one community due to its further location for recruitment, this study focused on residents of villages in Hmawbi clustered within 10 km of HNP, including HNP staff and those working in animal production, hunting and crop production.
Recruitment and Informed Consent
A cross-sectional study was performed at the two sites from March 2017 to September 2018. Introductory visits were made by the study staff to each of the selected sites prior to the commencement of the study. Community visits began with discussions with local authorities and community leaders, who subsequently assisted with recruitment of community members by introducing the study, followed by community meetings to discuss study details with community members, including the voluntary nature of participation in the study, inclusion and exclusion criteria, as well as future dates, times and locations relevant to study participation.
Participants who met the criteria for enrollment at community sites, together with their parents or legal guardians if applicable, were invited to speak with the study staff regarding the details of the study, to review the study information and informed consent form, and to ask any relevant questions. All study documents were in Myanmar language and study team members were fluent in local languages, ensuring the participants fully understood the study and procedures. One-on-one structured questionnaires were administered by Myanmar midwives and community health workers trained and supervised by Township Health Officers. Only consented participants were enrolled in the study. Participation in the study was completely voluntary, and all participants were informed that they could withdraw from the study at any time without consequences. Children younger than 17 years old were eligible to participate in the study if they provided assent and were accompanied by a parent or legal guardian who provided informed consent and remained present during the entire consent process.
Data Analysis
We first conducted descriptive analysis by locations to describe demographic factors (gender, age, education, primary livelihood, length of time living at current location, number of people living with), living environment and practices (drinking water treated, food storage), travel history, animal contact and self-reported unusual symptoms in the past year. For univariate descriptive analyses, we used Chi-square and Fisher’s exact tests. The unadjusted and adjusted associations were assessed by locations between types of animal contact activities as independent variables and whether or not participants had self-reported unusual symptoms in the past year as the outcome variable. Further, the unadjusted and adjusted associations between potential risk factors and whether or not participants had self-reported unusual symptoms were also assessed by locations. A p-value < 0.05 was considered statistically significant. Categorical variables between groups were compared by Chi-square tests or Fisher’s exact tests and t-tests. All analyses were done using STATA/IC version 16.0 (StataCorp., College, TX, USA).
Ethics Statement
The study protocols were reviewed and approved by the Ethics Review Committee on Medical Research Involving Human Subjects, Department of Medical Research, Ministry of Health and Sports (ERC Number: 002617 and Approval Number: Ethics/DMR/2017/062); and the Institutional Review Board Administration of the University of California, Davis (No. 804522-20). Only consented participants were enrolled in the study. Participation in the study was completely voluntary, and all participants were informed that they could withdraw from the study at any time without consequences.”
Results
Characteristics of Participants and Household
A total of 708 participants were enrolled in the study from March 2017 to September 2018 at two community sites—306 participants from Hpa-An site and 402 participants from Hmawbi site. There was an equal number of male and female participants. The majority of participants were aged between 40 and 59 (44.8%) in Hpa-An site while the majority was between 20 and 39 years old in Hmawbi site. Most participants in Hmawbi site had completed secondary school and above (39.4%) compared to those in Hpa-an site (29.4%). Most participants at Hpa-an site were involved in non-animal-related primary livelihood (33.7%) while most participants at Hmawbi site were involved in animal production business. More than half of the participants have been living at their current location for more than ten years in both Hpa-an site (79.1%) and Hmawbi site (58.5%) and almost all participants lived with one or more people in the same dwelling. Most participants reported treating drinking water and indicated that they had a dedicated location for human waste and used containers with covers for food storage in households in both sites. About half of the participants (46.1%) from Hpa-an site and 82.8% from Hmawbi site self-reported unusual symptoms in the past year. Almost all participants had contact with animals in the past year (Table 1).
Table 1.
Demographic characteristics of participants.
| Total (n = 708) | Hpa-an site (n = 306) | Hmawbi site (n = 402) | |
|---|---|---|---|
| Gender# | |||
|
Female Male |
354 (50%) | 167 (54.6%) | 187 (46.5%) |
| 354 (50%) | 139 (45.4%) | 215 (53.5%) | |
| Age group (in years)^ | |||
|
< 10 10–19 20–39 40–59 60–79 |
34 (4.8%) 45 (6.4%) 255 (36.0%) 317 (44.8%) 57 (8.1%) |
13 (4.3%) 15 (4.9%) 79 (25.8%) 166 (54.3%) 33 (10.8%) |
21 (5.2%) 30 (7.5%) 176 (43.8%) 151 (37.6%) 24 (6%) |
| Education | |||
|
None Primary school Secondary school College/university/professional |
75 (10.6%) 350 (49.4%) 257 (36.3%) 26 (3.7%) |
57 (18.6%) 159 (52%) 82 (26.8%) 8 (2.6%) |
18 (4.5%) 191 (47.5%) 175 (43.5%) 18 (4.5%) |
| Primary livelihood | |||
|
(i) Non-animal business, construction worker, migrant laborer (ii) Crop production (iii) Child/student (iv) Homemaker/unemployed (v) Nurse, doctor, traditional healer, community clinic workers (vi) Animal production business (Rancher/farmer animal production business, meat processing/slaughter house/abattoir) |
58 (8.2%) 188 (26.6%) 37 (5.2%) 86 (12.1%) 3 (<1%) 252 (35.6%) |
37 (12.1%) 80 (26.1%) 12 (3.9%) 53 (17.3%) 1 (<1%) 87 (28.4%) |
21 (5.2%) 108 (26.6%) 25 (6.2%) 33 (8.2%) 2 (0.5%) 165 (41%) |
| (vii) Animal-related livelihood (wild/exotic animal trade/market business, bat guano harvester, hunter/trapper/fisher, livestock/domestic animal/product trade, zoo/sanctuary animal health care, animal health provider/veterinarian) | 63 (8.9%) | 16 (2%) | 47 (11.7%) |
| (viii) forager/gatherer/non-timber forest product collector/protected area worker | 21 (3%) | 20 (6.5%) | 1 (0.3%) |
| Primary livelihood | |||
|
Crop production Animal production Other animal-involved occupation Other non-animal-related occupation |
188 (26.5%) 252 (35.6%) 84 (11.9%) 184 (26%) |
80 (26.1%) 87 (28.4%) 36 (11.8%) 103 (33.7%) |
108 (26.9%) 165 (41%) 48 (12%) 81 (20.1%) |
| Length of time living at the location | |||
|
<1 year 1–5 years 5–10 years >10 years |
52 (7.3%) 113 (16.0%) 66 (9.3%) 477 (67.4%) |
17 (5.6%) 37 (12.1%) 10 (3.3%) 242 (79.1%) |
35 (8.7%) 76 (18.9%) 56 (13.9%) 235 (58.5%) |
| No. of people living in the same dwelling | |||
|
None 1–4 5–9 10 and above |
1 (< 1%) 352 (49.7%) 328 (46.3%) 27 (3.8%) |
0 (0%) 121 (39.5%) 169 (55.2%) 16 (5.3) |
1 (0.2%) 231 (57.5%) 159 (39.6%) 11 (2.7%) |
| Water is treated | |||
|
Yes No |
661 (93.4%) 47 (6.6%) |
287 (93.8%) 19 (6.2%) |
374 (93.0%) 28 (7%) |
| Dedicated location for human waste | |||
|
Yes No |
677 (95.6%) 31 (4.4%) |
283 (92.5%) 23 (7.5%) |
394 (98%) 8 (2%) |
| Containers for food storage in household | |||
|
Yes, with covers Yes, without covers No |
643 (91.0%) 26 (3.7%) 39 (5.5%) |
246 (80.4%) 23 (7.5%) 37 (12.1%) |
397 (98.8%) 3 (0.7%) 2 (0.5%) |
| Self-reported unusual symptoms in the past year* | |||
|
Yes No |
474 (67%) 234 (33%) |
141 (46.1%) 165 (53.9%) |
333 (82.8%) 69 (17.2%) |
| Contact with any type of animals in the past year | |||
|
Yes No |
698 (98.6%) 10 (1.4%) |
297 (97.1%) 9 (2.9%) |
401 (99.8%) 1 (0.2%) |
#Observed.
^Self-reported.
*Unusual symptoms mean any illness or sickness that is not known or recognized in the community or diagnosed by medical providers.
Specific Forms of Animal Contact in the Past Year
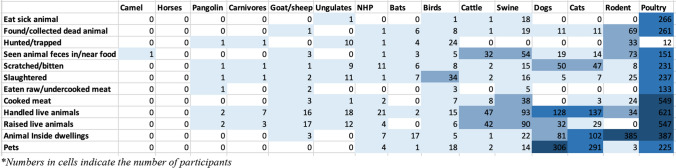
To understand details around animal contacts, participants were asked about the types of animals they came in contact with within the past year and the nature of the interaction. Interaction with poultry seemed to be very common among the participants and many reported handling, raising and having poultry in their houses as well as being scratched/bitten by or slaughtering them. Contact with rodents and swine was also frequently observed among the participants (57.7% & 18%) and many reported seeing feces of rodents (n = 73), swine (n = 54) and cattle (n = 32) in or near their food. Seventeen participants said they had bats in their house, 6 participants reported being scratched or bitten by bats and 7 participants reported slaughtering bats for unknown purposes (Fig. 1).
Figure 1.
Animal contact activities in the past year.
Association Between Self-Reported Unusual Symptoms and Animal Contact Activities by Sites
At Hmawbi site, participants who reported unusual symptoms in the past year were more likely to have sold dead animals (OR = 6.39, 95% CI 3.07–13.3), slaughtered animals (OR = 3.89, 95% CI 2.11–7.17), raised animals (OR = 5.05, 95% CI 2.7–9.44), eaten sick (OR = 9.48, 95% CI 4.79–19.76) or dead animals (OR = 6.56, 95% CI 3.72–11.59) or scratched/bitten by animals (OR = 1.88, 95% CI 1.11–3.18). At Hpa-an site, participants who reported unusual symptoms in the past year were more likely to have shared water with animals (OR = 2.12, 95% CI: 1.05–4.3) or raised animals (OR = 1.89, 95% CI 1.11–3.22). The associations remained significant when adjusted for primary livelihood, age and education with the exception of the association between scratched/bitten by animals and unusual symptoms at Hmawbi site (Table 2).
Table 2.
Self-reported unusual symptoms among participants given high-risk animal contact activities.
| Type of contact activity | Odds ratio by sites (95% CI) | Adjusted odds ratios by sites (95% CI) | ||
|---|---|---|---|---|
| Self-reported unusual symptoms (Hmawbi site) N = (402) | Self-reported unusual symptoms (Hpa-an site) N = (306) | Self-reported unusual symptoms (Hmawbi site)a | Self-reported unusual symptoms (Hpa-an site)a | |
| Sold dead animals | 6.39 (3.07–13.3) | – | 4.3 (1.96–9.43) | – |
| Slaughtered animals | 3.89 (2.11–7.17) | 1.56 (0.98–2.5) | 2.02 (1.0–4.04) | 1.65 (1.0–2.69) |
| Shared water with animals for washing | 0.69 (0.28–1.67) | 2.12 (1.05–4.3) | 0.64 (0.25–1.63) | 2.1 (1.04–4.27) |
| Raised live animals | 5.05 (2.7–9.44) | 1.89 (1.11–3.22) | 3.13 ( 1.52–6.45) | 1.98 (1.14–3.45) |
| Hunted/trapped | 0.97 (0.41–2.3) | 1.32 (0.52–3.35) | 0.52 (0.2–1.34) | 1.39 (0.53–3.61) |
| Handled live animals | 1.21 (0.25–5.84) | 1.07 (0.57–2.0) | 0.67 (0.13–3.58) | 1.08 (0.56–2.08) |
| Eaten sick animals | 9.48 (4.79–18.76) | 0.71 (0.38–1.32) | 6.65 (3.23–13.67) | 0.72 (0.38–1.35) |
| Eaten raw or undercooked meat or organ or blood | 1.38 (0.46–4.08) | 0.83 (0.51–1.33) | 1.23 (0.39–3.87) | 0.84 (0.52–1.35) |
| Eaten dead animals | 6.56 (3.72–11.59) | 0.91 (0.45–1.88) | 4.43 (2.27–8.62) | 0.93 (0.45–1.92) |
| Animal inside dwelling | 0.80 (0.17–3.65) | 1.17 (0.69–2.0) | 0.56 (0.11–2.79) | 1.22 (0.71–2.09) |
| Scratched/bitten | 1.88 (1.11–3.18) | 0.93 (0.54–1.61) | 1.20 (0.68–2.14) | 0.91 (0.52–1.59) |
Significant results given in bold
a: Adjusted for primary livelihood, age, education.
–: Dropped due to low n.
Risk Factor Analysis of Self-Reported Symptoms
Of all participants in the study, 67% (474/708) reported having unusual symptoms in the past year (Table 1). Females from Hpa-an sites were more likely to have reported unusual symptoms compared to males. Participants from Hmawbi site aged 20–39 (OR = 2.75, 95% CI 1.33–5.69) were more likely to have reported unusual symptoms compared to those less than 10 years old. Those involved in crop production (OR = 4.38, 95% CI 2.2–8.72), animal production (OR = 3.42, 95% CI 1.88–6.21) and other animal-involved livelihoods (OR = 3.27, 95% CI 1.49–7.17) were more likely to have reported unusual symptoms compared to those in non-animal-involved livelihoods but only among those recruited from Hmawbi site. In both sites, those who had unusual self-reported illness were more likely to have family members who also had unusual symptoms in the same year (OR = 8.97, 95% CI 6.14–13.12). When adjusted for whether or not other people they lived with had symptoms in the past year, the associations remained significant between participants’ self-reported unusual symptoms and gender and primary livelihood. There was no significant associations between age, education level, whether participants had traveled in the same year or whether drinking water was treated with participants’ self-reported illness in both sites (Table 3).
Table 3.
Association of potential risk factors with self-reported illness.
| Unadjusted odds ratio by site (95% CI) | Adjusted odds ratio by site (95% CI)# | |||
|---|---|---|---|---|
| Odds ratio by site (95% CI) | ||||
| Hpa-An (0) (n = 306) | Hmawbi (1) (n = 402) | Hpa-An (0) (n = 306) | Hmawbi (1) (n = 402) | |
| Gender (female vs male) | ||||
|
Male Female |
1 2.01(1.27–3.19) |
1 0.94 (0.56–1.58) |
1 1.96 (1.23–3.12) |
1 1.07 (0.58–1.98) |
| Age (in years) | ||||
|
< 10* 10–19 20–39 40–59 60–79 |
1 0.25 (0.04–1.58) 1.64 (0.49–5.46) 1.64 (0.51–5.22) 0.7 |
1 1.5 (0.6–3.7) 2.8 (1.3–5.7) 2.1 (1.0–4.2) 1.0 (0.4–2.4) |
1 0.29 (0.04–1.93) 1.86 (0.55–6.33) 1.97 (0.6–6.46) 0.86 (0.22–3.37) |
1 1.36 (0.3–6.15) 1.35 (0.45– 4.06) 1.86 (0.61–5.7) 1.02 (0.21–4.87) |
| Education | ||||
|
None* Primary school Secondary school College/university/professional |
1 0.81 (0.44–1.49) 1.29 (0.65–2.53) 0.67 (0.15–3.06) |
1 2.23 (0.78–6.34) 2.87 (0.99–8.31) 4 (0.68–23.41) |
1 0.82 (0.44–1.52) 1.27 (0.64–2.52) 0.61 (0.13–2.85) |
1 1.47 (0.41–5.22) 1.83 (0.51–6.61) 2.3 (0.29–18.01) |
| Primary livelihood | ||||
|
Crop production Animal production Other animal-involved occupation Other non-animal-related occupation |
0.73 (0.41–1.33) 1.18 (0.67–2.09) 0.79 (0.37–1.69) 1 |
4.38 (2.2–8.72) 9.7 (4.66–20.21) 5.33 (2.04–13.93) 1 |
0.85 (0.47–1.57) 1.25 (0.7–2.24) 0.8 (0.37–1.75) 1 |
2.21 (0.96–5.08) 5.26 (2.27–12.22) 5.65 (1.92–16.62) 1 |
| Traveled | ||||
|
Yes No |
1.04 (0.65–1.64) 1 |
1.46 (0.81–2.61) 1 |
1.07 (0.67–1.72) 1 |
1.05 (0.53–2.1) 1 |
| Water treated | ||||
|
Yes No |
0.76 (0.3–1.92) 1 |
0.79 (0.27–2.36) 1 |
0.82 (0.32–2.1) 1 |
1.32(0.36–4.9) 1 |
| Symptoms in other people whom participants lived with | ||||
|
Yes No |
2.08 (1.23–3.5) 1 |
21.61 (10.92–42.76) 1 |
– – |
– – |
#Adjusted for whether or not people participants lived with had symptoms in the past year
Discussion
We used a behavioral surveillance approach among high-risk communities to study the association between behavioral practices and the risk of zoonotic viral spillover in Myanmar. Our study provides important insights into the level of animal contact in the community that is understudied and highlights the potential pathways of human–animal interaction. We found the association between self-reported unusual symptoms among participants and participants’ animal contact activities as well as evidence of potential risk factors of self-reported unusual symptoms. The largest part of surveillance data in the region has been focusing on virological results while epidemiological and behavioral investigations have been lacking and poorly understood (Trevennec, 2011). Our findings contribute to much needed human behavioral data for targeted interventions and strategies to prevent zoonotic disease transmission at human–animal interfaces.
A high prevalence of animal contact with poultry, rodents, swine and cattle was observed among study participants. These animals carry a wide range of zoonotic viral pathogens such as swine influenza, hepatitis E virus, avian influenza and hantaviruses. Hepatitis E virus (HEV) has been previously reported in parts of Southeast Asia, especially among those living rural areas with direct exposure to swine (Hinjoy, 2012, Meng 2002, Christou, 2013, Drobeniuc, 2001) or among those who consumed raw and uncooked pork (Wibawa, 2004, Blacksell, 2007). Similarly, avian influenza viruses have been frequently detected in pigs in Asian countries including China, Korea and Myanmar (Cao, 2013, Nidon, 2010, Zhou, 2014, Mon, 2012), and in domestic poultry in Southeast Asia (WHO, timeline major events, OIE, 2016, Avian Influenza), and these viruses including H9N2 and H5N1 had been reported to infect humans (WHO, 2020). Since backyard pig farming and background poultry production play an important role in the livelihood of rural and peri-urban populations in Southeast Asia, understanding the local population’s behavioral practices and measures to protect themselves from zoonotic spillover will help develop the risk mitigation interventions. Our study population reported regular contact with rodents which may carry hantaviruses that can be transmitted to humans and have been previously identified in humans in Southeast Asia (Groen, 2002, Rollins, 1986, Lee 1999). Infections caused by hemorrhagic fever with renal syndrome-related hantaviruses can be life-threatening; thus, it is of public health interest to tailor sanitation, proper hygiene and rodent intervention strategies to reduce the frequent occurrence of rodents in households and occupational settings to reduce the risk of acquiring the disease.
Animal Contact Activities in the Past Year
Considering the high prevalence of animal contact among the study population, we next sought to evaluate the overall patterns around human–animal interactions and the pathways of those interactions. The most common type of contact was with poultry through raising, handling or having them in or near participants’ houses. Myanmar has first reported HPAI in 2006, and multiple waves of H5Nx HPAI outbreaks have been reported in duck farms throughout several states in Myanmar, including Yangon where we conducted our study (Mon et al, 2012). In addition, HPAIV-H5N1 were found in a live bird market in Yangon, Myanmar, from 2017 to 2018 (Thurain et al, 2020). Even though human infections rarely occur, it has been reported that approximately 60% of the cases have died (Claas et al, 1998). This presents a significant risk of HPAIV-H5N1 transmission in poultry and humans via live markets and poultry farms and of public health threats. It is of importance to note that contact with bats inside the dwelling was also observed among our participants in both of our sites because bats are reservoirs of several zoonotic pathogens of global concern including SARS (Menachery et al, 2015), Nipah virus (Luby et al, 2009) and Ebola (Leroy et al, 2005). It is also alarming that participants reported handling and being scratched/bitten by NHPs because it has been well reported that several outbreaks of Ebola hemorrhagic fever were the consequence of handling and butchering NHPs (Leroy et al., 2004). Therefore, interventions to raise awareness of zoonotic risks and to connect individuals to local resources for disease reporting will be critical for early reporting of diseases and responses.
Self-reported Unusual Symptoms Among Participants Given High-Risk Animal Contact Activities
Even though serology studies would be able to pinpoint prior exposure to zoonotic viral pathogens, self-reported illness data have been widely used in disease surveillance and risk factor studies when serology data are not available. Our finding of increased odds of self-reported unusual illness among those who had different animal contact activities is consistent with that of other studies (Li, 2019, Krueger, 2015, Rinsky, 2019). Animal contact activities that were associated with self-reported unusual symptoms differed across sites. Increased odds of self-reported illness among those who slaughtered and sold dead animals were observed only among participants in Hmawbi site. The fact that Hmawbi is located in a close proximity to different slaughterhouses in the industrialized peri-urban zone might have played a role in these associations. Increased odds of unusual symptoms were associated with raising live animals in both locations. It might be due to the fact that very few farmers in Myanmar have received any formal training on pig or poultry farming and their knowledge about animal diseases is very limited (Belton et al., 2020).
To our surprise, those who reported eating raw meat had lower odds of having self-reported unusual symptoms. However, we were limited in our data to know whether the majority of those who did not report self-reported unusual illness but had eaten raw/undercooked meat, organ or blood were already clinically diagnosed for having food-borne illness.
Association of Potential Risk Factors with Self-reported Illness
Regardless, our findings observed the clear increased association between self-reported unusual illness and primary livelihood in peri-urban Hmawbi site, particularly crop production, animal production and other animal-involved occupations, which is consistent with other studies in the region (Patel 2019, Li, 2019). This highlights the importance of targeted surveillance and especially serology studies in these occupational groups in more peri-urban locations where medium- and large-scale farms exist. Even though the majority of the participants had dedicated location for human waste, we did not follow up in our questionnaire if that included open defecation in a preferred location.
Participants aged 20–39 were more likely to have reported unusual symptoms compared to younger participants and this could be due to the fact that these aged groups are more likely to be involved in livelihood activities that have exposure to animals, especially in peri-urban locations. There also seemed to be transmission risk to other household members since there was a significantly increased odds of symptoms in household members of participants who reported symptoms themselves. Behavioral interventions such as social distancing and isolating will be central to reducing the risk of household transmission if such interventions are possible and could be beneficial to avoid clusters of potential community transmission.
Conclusion
Although this study could not establish a direct link between prior exposure to zoonotic pathogens and participants’ high-risk behaviors and practices in their daily lives or in livelihood activities due to the lack of serology data, the results suggest that there is a very high interaction among humans and domestic and wild animals among these communities. Our study provides important insight into community-level animal contact in a period (2017–18) prior to major behavioral changes during COVID-19. We highlight the most common pathways through which individuals come into contact with different animals, in a local context. Our findings on increased odds of having unusual symptoms among those who had different animal contact activities via their likelihood activities or daily practices suggest a need for more targeted surveillance among these populations for early detection and behavioral interventions for the prevention of emerging and reemerging zoonotic diseases. Future studies should include serology testing of a wide range of viral pathogens in addition to the screening of potential zoonotic viruses. More studies in the region to understand the behavioral risk factors associated with zoonotic transmission are needed, especially in Myanmar that has a weak surveillance infrastructure as well as more efforts for laboratory capacity building.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Livestock Breeding and Veterinary Department (LBVD) within the Ministry of Agriculture, Livestock, and Irrigation (MOALI); Ministry of Natural Resources and Environmental Conservation (MONREC); and the Department of Medical Research (DMR) within the Ministry of Health and Sports (MOHS), Myanmar, with whom we collaborated closely on all research activities. Additional support for this project was provided by the Smithsonian Institution. Support for the preparation of this manuscript was provided by the Morris Animal Foundation and Dennis and Connie Keller through a training partnership, as well as Judy and John W. McCarter, Jr., and James and Jamie Coss. This content has not been reviewed or endorsed by the Morris Animal Foundation, and the views expressed herein do not necessarily reflect the views of the Foundation, its officers, directors, affiliates or agents.
Author Contributions
SY carried out formal analysis and interpretation, contributed to methodology and writing—reviewing edits and revising, and wrote the original draft; MTV and OA were responsible for conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision and writing—reviewing and editing; L-ACH did formal analysis and writing—reviewing and editing; JHY participated in data curation and writing—reviewing and editing; TWM, HL, MMH and HMT were involved in data curation, investigation, supervision and writing—reviewing and editing; EH and LF took part in conceptualization, methodology, resources and writing—reviewing and editing; SM was involved in conceptualization, funding acquisition, supervision and writing—reviewing and editing.
Funding
This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT project (cooperative agreement number AID-OAA-A-14-00102), by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI151797 and R01AI163118. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Declarations
Ethical Approval
Ethical approvals were granted by the Department of Medical Research under the Ministry of Health and Sports of Myanmar (Ethics Code Ethic/DMR/ 2017/062) and the Institutional Review Board of the University of California at Davis (IRB ID 804522-32). Surveys were voluntary, with full informed written consent obtained from each participant. Data were de-identified to protect respondent confidentiality.
References
- Belton B, Cho A, Payongayong E, Mahrt K, Abaidoo E. Commercial poultry and pig farming in Yangon’s peri-urban zone. Food Security Policy Project (FSPP)
- Bhagwat T, Hess A, Horning N, Khaing T, Thein ZM, Aung KM, et al. Losing a jewel–rapid declines in Myanmar’s intact forests from 2002–2014. PLoS One. 2017;12:e0176364. doi: 10.1371/journal.pone.0176364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Narain JP. Review paper: the challenge of emerging zoonoses in Asia Pacific. Asia Pacific Journal of Public Health. 2010;22(4):388–394. doi: 10.1177/1010539510370908. [DOI] [PubMed] [Google Scholar]
- Blacksell SD, Myint KSA, Khounsy S, Phruaravanh M, Mammen MP, Day NPJ, Newton PN. Prevalence of hepatitis E virus antibodies in pigs: implications for human infections in village-based subsistence pig farming in the Lao PDR. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:305–307. doi: 10.1016/j.trstmh.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N, Zhu W, Chen Y, Tan L, Zhou P, Cao Z, Ke C, Li Y, Wu J, Qi W, Jiao P, Zhang G. Avian influenza A (H5N1) virus antibodies in pigs and residents of swine farms, southern China. Journal of Clinical Virology. 2013;58(4):647–651. doi: 10.1016/j.jcv.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Cascio A, Bosilkovski M, Rodriguez-Morales AJ, Pappas G. The socio-ecology of zoonotic infections. Clinical Microbiology and Infection. 2011;17:336–342. doi: 10.1111/j.1469-0691.2010.03451. [DOI] [PubMed] [Google Scholar]
- Claas ECJ, Osterhaus ADME, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou L, Kosmidou M. Hepatitis E virus in the Western world–a pork-related zoonosis. Clinical Microbiology and Infection. 2013;19:600–604. doi: 10.1111/1469-0691.12214. [DOI] [PubMed] [Google Scholar]
- Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet. 2011;377:599–609. doi: 10.1016/S0140-6736(10)62004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux CA, Mediannikov O, Medkour H, Raoult D. Infectious disease risk across the growing human-non human primate interface: a review of the evidence. Frontiers Public Health. 2019;7:305. doi: 10.3389/fpubh.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draft One Health Plan . Central epidemiology unit department of public. Myanmar: Health Ministry of Health and Sports; 2013. [Google Scholar]
- Drobeniuc J, Favorov MO, Shapiro CN, Bell BP, Mast EE, Dadu A, Culver D, Iarovoi P, Robertson BH, Margolis HS. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis. 2001;184:1594–1597. doi: 10.1086/324566. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOStat: Myanmar. FAO; 2018. (http://www.fao.org/faostat/en/#country/28, accessed 25 January 2019).
- Forest area (% of land area). The World Bank; 2015. (https://data.worldbank.org/indicator/AG.LND.FRST.ZS, accessed 13 January 2019).
- Formulation and Operationalization of National Action Plan for Poverty Alleviation and Rural Development through Agriculture (NAPA), Working Paper 2: Livestock Production, Extension and Applied Research. Government of the Republic of the Union of Myanmar: Ministry of Agriculture, Livestock and Irrigation; 2016. (http://www.fao.org/3/a-bl829e.pdf, accessed 12 January 2019).
- Groen J, Suharti C, Koraka P, van Gorp ECM, Sutaryo J, Lundkvist A, Osterhaus ADME. Serological evidence of human hantavirus infections in Indonesia. Infection. 2002;30:326–327. doi: 10.1007/s15010-002-2194-y. [DOI] [PubMed] [Google Scholar]
- Han H-J, Wen H-L, Zhou C-M, Chen F-F, Luo L-M, Liu J-W, et al. Bats as reservoirs of severe emerging infectious diseases. Virus Research. 2015;205:1–6. doi: 10.1016/j.virusres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife-livestock-human interface. Trends in Ecology & Evolution. 2017;32(1):55–67. doi: 10.1016/j.tree.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinjoy S, Nelson KE, Gibbons RV, Jarman RG, Mongkolsirichaikul D, Smithsuwan P, Fernandez S, Labrique AB, Patchanee P. A cross-sectional study of hepatitis e virus infection in healthy people directly exposed and unexposed to pigs in a rural community in northern Thailand. Zoonoses Public Health. 2012;60:555–562. doi: 10.1111/zph.12030. [DOI] [PubMed] [Google Scholar]
- Japan International Cooperation Agency (JICA). Final Report of the Data Collection Survey on Agriculture Sector in the Republic of the Union of Myanmar. The Republic of the Union of Myanmar; 2013
- Joint external evaluation of IHR Core Capacities of the Republic of the Union of Myanmar. Geneva: World Health Organization; 2018. License: CC BY-NC-SA 3.0 IGO. https://extranet.who.int/sph/sites/default/files/documentlibrary/document/JEE%20Report%20Myanmar%202017.pdf
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger WS, Hilborn ED, Dufour AP, Sams EA, Wade TJ. Self-reported acute health effects and exposure to companion animals. Zoonoses and Public Health. 2015;63(4):311–319. doi: 10.1111/zph.12233. [DOI] [PubMed] [Google Scholar]
- Lee HW. Epidemiology and epizootiology. In: Lee HW, Calisher C, Schmaljohn CS, editors. Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Seoul: WHO Collaborating Centre for Virus Reference and Research; 1999. pp. 40–48. [Google Scholar]
- Leimgruber P, Kelly DS, Steininger MK, Brunner J, Müller T, Songer M. Forest cover change patterns in Myanmar (Burma) 1990–2000. Environ Conservation. 2005;32(4):356–364. doi: 10.1017/S0376892905002493. [DOI] [Google Scholar]
- Leroy EM, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment J-M et al (2004) Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303(5656):387–390. 10.1126/science.1092528 [DOI] [PubMed]
- Li H, et al. Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosafety and Health. 2019;1(2):84–90. doi: 10.1016/j.bsheal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler JH, Otte J, Roland-Holst D, Pfeiffer DU, SoaresMagalhaes R, Rushton J, et al. Industrial food animal production and global health risks: exploring the ecosystems and economics of avian influenza. EcoHealth. 2009;6(1):58–70. doi: 10.1007/s10393-009-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah virus. Clinical Infectious Diseases. 2009;49(11):1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Critical Reviews in Microbiology. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- Menachery VD, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature Medicine. 2015;21:1508–1513. doi: 10.1038/nm.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis e virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Hotels and Tourism. Yangon. MOHT; 2018. (http://tourism.gov.mm/attractions/destinations/yangon/, accessed 25 January 2019).
- Ministry of Planning and Finance. An Analysis of Poverty in Myanmar Part 01: Trends Between 2004/05 and 2015. World Bank Group; 2017a.
- Ministry of Planning and Finance. An Analysis of Poverty in Myanmar Part 02: Poverty Profile. World Bank Group; 2017b
- Mon PP, Lapkuntod J, Maw MT, Nuansrichay B, Parchariyanon S, Tiensin T, Htun T, Padungtod P, Kalpravidh W, Sunn K, Maclean M, Amonsin A. Highly pathogenic avian influenza (H5N1) in Myanmar, 2006–2010. Archives of Virology. 2012;157:2113–2123. doi: 10.1007/s00705-012-1411-y. [DOI] [PubMed] [Google Scholar]
- Murray KA, Daszak P. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Current Opinion in Virology. 2013;3(1):79–83. doi: 10.1016/j.coviro.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE World Organization for Animal Health. Avian Influenza. See https://www.oie.int/en/disease/avian-influenza/#ui-id-1. Assessed on Feb 27, 2022.
- Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbjer K, Boqvist S, Sokerya S, Kannarath C, San S, Davun H, et al. Household practices related to disease transmission between animals and humans in rural Cambodia. BMC Public Health. 2015;15:476. doi: 10.1186/s12889-015-1811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Saxena D. Self-reported selected zoonotic diseases among animal handlers in Urban Ahmedabad, India. Veterinary World. 2019;12(1):176–182. doi: 10.14202/vetworld.2019.176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinsky JL, et al. Animal production, insecticide use and self-reported symptoms and diagnoses of COPD, including chronic bronchitis, in the agricultural health study. Environment International. 2019;127:764–772. doi: 10.1016/j.envint.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin PE, Nawrocka E, Rodhain F, Sureau P, McCormick JB. Serological data on hemorrhagic fever with renal syndrome in Southeast Asia. Bulletin De La Societe De Pathologie Exotique Et De Ses Filiales. 1986;79:473–475. [PubMed] [Google Scholar]
- Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London. Series B: Biological Science. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thant YZM, Win HH (2016) Myanmar agricultural and rural statistics system and development plans: country paper. United Nations Economic and Social Commission for Asia and the Pacific Statistical Institute for Asia and the Pacific (SIAP). Accessed 13 January 2019. http://www.unsiap.or.jp/e-learning/el_material/Agri/1606_Advocacy_KOR/cr_Myanmar.pdf
- Thurain K, Mon PP, Nasamran C, Charoenkul K, Boonyapisitsopa S, Tun TN, San YY, Aye AM, Amonsin A. Surveillance of influenza A virus subtype H5N1 in a live bird market in Yangon, Myanmar: 2017–2018. Transboundary and Emerging Diseases. 2020 doi: 10.1111/tbed.13618. [DOI] [PubMed] [Google Scholar]
- Trevennec K, Cowling B, Peyre M, Baudon E, Martineau G, Roger F. Swine influenza surveillance in East and Southeast Asia: a systematic review. Animal Health Research Reviews. 2011;12(2):213–223. doi: 10.1017/S1466252311000181. [DOI] [PubMed] [Google Scholar]
- Valitutto MT, Aung O, Tun KYN, Vodzak ME, Zimmerman D, et al. Detection of novel coronaviruses in bats in Myanmar. Plos One. 2020;15(4):e0230802. doi: 10.1371/journal.pone.0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibawa IDN, Muljono DH, Mulyanto Suryadarma IG, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73:38–44. doi: 10.1002/jmv.20059. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerging Infectious Diseases. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. H5N1 avian influenza: timeline of major events. See http://www.who.int/csr/disease/avian_influenza/ai_timeline/en/index.html.
- World Organisation for Animal Health (OIE) (2016) Final report of the chief veterinary officer of Myanmar. H5N1 HPAI outbreak in Monywa poultry production zone. Last Updated August 2017.
- Zhou P, Hong M, Merrill MM, He H, Sun L, Zhang G. Serological report of influenza A (H7N9) infections among pigs in Southern China. BMC Veterinary Research. 2014;2014(10):203. doi: 10.1186/s12917-014-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.