Abstract
Menthol and tobacco flavors are available for almost all tobacco products, including electronic cigarettes (e-cigs). These flavors are a mixture of chemicals with overlapping constituents. There are no comparative toxicity studies of these flavors produced by different manufacturers. We hypothesized that acute exposure to menthol and tobacco-flavored e-cig aerosols induces inflammatory, genotoxicity, and metabolic responses in mouse lungs. We compared two brands, A and B, of e-cig flavors (PG/VG, menthol, and tobacco) with and without nicotine for their inflammatory response, genotoxic markers, and altered genes and proteins in the context of metabolism by exposing mouse strains, C57BL/6J (Th1-mediated) and BALB/cJ (Th2-mediated). Brand A nicotine-free menthol exposure caused increased neutrophils and differential T-lymphocyte influx in bronchoalveolar lavage fluid and induced significant immunosuppression, while brand A tobacco with nicotine elicited an allergic inflammatory response with increased Eotaxin, IL-6, and RANTES levels. Brand B elicited a similar inflammatory response in menthol flavor exposure. Upon e-cig exposure, genotoxicity markers significantly increased in lung tissue. These inflammatory and genotoxicity responses were associated with altered NLRP3 inflammasome and TRPA1 induction by menthol flavor. Nicotine decreased surfactant protein D and increased PAI-1 by menthol and tobacco flavors, respectively. Integration of inflammatory and metabolic pathway gene expression analysis showed immunometabolic regulation in T cells via PI3K/Akt/p70S6k-mTOR axis associated with suppressed immunity/allergic immune response. Overall, this study showed the comparative toxicity of flavored e-cig aerosols, unraveling potential signaling pathways of nicotine and flavor-mediated pulmonary toxicological responses, and emphasized the need for standardized toxicity testing for appropriate premarket authorization of e-cigarette products.
Keywords: e-cigarettes, ENDS, flavors, menthol, tobacco, nicotine, immunometabolism, hypersensitivity
Electronic nicotine delivery systems (ENDS) or electronic cigarettes (e-cigs) have become popular as a cessation method, with declined use of combustible cigarettes. However, the use of ENDS by nonsmokers is on the rise, especially among youth (Galderisi et al., 2020; Levy et al., 2019). Contradictory to the intended use of ENDS as a cessation tool, flavored ENDS has become a gateway to nicotine use and nicotine/vaping addiction, as more than 20% of high schoolers and middle-schoolers were using e-cigs in 2021 (Hamberger and Halpern-Felsher, 2020; Miech et al., 2021). Despite all efforts by regulatory agencies, a plethora of nicotine-containing ENDS (vape bars, e-liquids, pods) is available in retail stores and online. Similarly, menthol-, mint-, cooling-, and tobacco-flavored ENDS are available in many forms, such as e-liquids, disposable vapes, and pod-based products. However, with increased regulation policies by the Food and Drug Administration, such as the flavor ban and premarket authorization, menthol- and tobacco-flavored ENDS sales and consumption has been rising, while other flavored ENDS sales have been declining (Ali et al., 2022; Diaz et al., 2021).
E-liquids contain humectants, flavoring chemicals, and additives, such as flavor enhancers forming secondary pyrolytic products upon heating. These degradation products depend on many factors, such as the temperature of the coil and the power (wattage) of the device (Bitzer et al., 2018; Li et al., 2021b). We have shown that certain flavoring chemicals cause more toxicity and inflammation than others (Kaur et al., 2018; Lamb et al., 2020, 2022; Muthumalage et al., 2017, 2019). We and others have shown that secondary degradation products, such as aldehydes in menthol, tobacco, cinnamon, and fruit-flavored e-liquids, cause mitochondrial dysfunction, cellular toxicity, inflammation, and impaired phagocytosis (Hickman et al., 2019; Hua et al., 2019; Jabba et al., 2020; Khlystov and Samburova, 2016; Omaiye et al., 2019). Subacute to subchronic exposure to flavored ENDS has shown moderate levels of lung inflammation and immune response in mice (Crotty Alexander et al., 2018; Szafran et al., 2020). In our previous studies, we have shown that flavoring chemicals generate reactive oxygen species, causing epithelial barrier dysfunction, oxidative stress, cytotoxicity, inflammation, mitochondrial dysfunction, DNA damage, and cellular senescence in vitro and in vivo in mouse lungs (Lamb et al., 2020, 2022; Lei et al., 2017; Lerner et al., 2015; Lucas et al., 2020; Muthumalage et al., 2019; Wang et al., 2020). E-cigarette, or Vaping-Induced lung Injury epidemic was primarily associated with e-cigarettes using THC-containing cartridges, but we have found the presence of nicotine and flavors in those cartridges (Muthumalage et al., 2020). While researchers have identified individual cellular processes, the exact mechanism of vaping-induced lung injury is unclear (Alexander et al., 2020).
Currently, no standardized manufacturing guidelines are practiced during e-cigarette flavor production for chemical ingredients, such as PG/VG, nicotine, and flavoring agents. Different manufacturers/vendors produce the same e-cig flavor using various proportions of humectants, flavoring agents, and nicotine and label them as a particular flavor, eg, tobacco and menthol. While we have observed acute lung injury and priming of pathogenic processes, such as extracellular matrix remodeling due to PG/VG and nicotine exposure, flavor interaction-induced lung damage has not yet been studied. Furthermore, comparative toxicity assessment of the same flavor by two brands has not been investigated. E-cigs of the same flavor by different vendors may have differential toxicity. Hence, we assessed two locally purchased e-liquid brands of PG/VG, menthol, and tobacco flavors with and without nicotine, using two strains of mice (C57BL/6J and BALB/cJ, both male and female). These variables were selected to minimize bias and generalization by using just one brand of e-liquid. In this study, we performed a comparative toxicological analysis, focusing on inflammation, genotoxicity, and cellular metabolism to shed light on acute phase lung injury and resolution.
As the chemosensory cation channel receptors have been observed to play a role in cough reflex sensitivity in lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF), we assessed transient receptor potential ankyrin 1 (TRPA1) expression in lung homogenates. Flavoring chemicals in e-cig aerosols have not been well studied as an agonist of TRPA1, though expressed in bronchial epithelial cells and lung fibroblasts and have been associated with allergic asthma (Caceres et al., 2009). The pivotal roles of these intracellular immune receptors/sensors, and inflammasomes, in sensing systemic metabolic perturbations have recently become evident. We have shown augmented plasminogen activator inhibitor 1 (PAI-1) with PG/VG + nicotine exposure in mouse lungs. Lung inflammation is signified by acute lung injury biomarkers such as surfactant proteins, SP-A, SP-D, SP-B, and SP-C. These surfactant proteins lower the surface tension, preventing the alveolar from collapsing and play a critical role in the innate immune response. Thus, we determined the potential correlations of mechanisms of inflammatory and injurious responses by acute menthol and tobacco flavor exposure. Acute lung injury markers, NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, surfactant protein D (SP-D), and plasminogen activator inhibitor 1 (PAI-1) were determined in mouse lungs postexposure to e-cigs (Koivisto et al., 2022; Moilanen et al., 2012). As metabolic homeostasis has been shown to play a role in the incidence of asthma, we integrated innate and adaptive immune responses with cellular metabolism to understand the metabolic reprogramming of immune cells, which is essential for both inflammatory and antiinflammatory responses(Brumpton et al., 2013; O'Neill, 2017; O'Neill et al., 2016; Palsson-McDermott and O'Neill, 2020).
In this study, we hypothesized that both C57BL/6J and BALB/cJ mouse strains would elicit Th1 and Th2 inflammatory responses upon e-cigarette aerosol exposures, respectively. We also hypothesized that PG/VG, menthol, and tobacco flavors would cause differential proinflammatory and correlated acute phase responses regardless of brand differences, and that nicotine would exacerbate the inflammatory response.
Methods
Scientific rigor and reproducibility
We applied a robust, unbiased experimental design, and data analysis approach throughout the study. We validated the methods and ensured reproducibility with repeated experiments. All methods are presented in detail with transparency. Results were reported and interpreted without bias. For all assays, laboratory-grade biological and chemical resources were purchased from commercial sources. Our methodologies, data, and results adhered to strict NIH reproducibility standards and scientific rigor. Exposures had male and female mice of the same age, N = 8–10 per strain. For e-liquid exposures, instead of generalizing the effects with just one brand, we included two brands. All analytes were assayed with N = 2–3 technical replicates per group. Assays were performed with unique IDs and self-blinded to avoid bias.
Ethics statement: institutional biosafety and animal protocol approval
Experiments in this study were performed according to the standards and guidelines approved by The University of Rochester Institutional Biosafety Committee (study approval number: Rahman/102054/09-167/07-186; identification code: 07-186; date of approval: 5 January 2019 and 3 February 2020).
All mouse housing, handling, exposure, and procedure protocols used in this study were approved by the University Committee on Animal Research (UCAR) Committee of the University of Rochester, Rochester, NY (UCAR protocol 102204/UCAR-2007-070E, date of approval: January 5, 2019, and February 3, 2020).
Animals
For in vivo exposures, 8- to 10-week-old male and female C57BL/6J and BALB/cJ mice (body weight ∼25 g) from Jackson labs were purchased and housed at the University of Rochester vivarium under normal light and dark cycles and ad libitum feeding according to UCAR guidelines.
Procurement of ENDS e-liquids
Two nationwide commercially available e-liquid brands were de-identified as A and B for propylene glycol (PG), vegetable glycerin (VG), menthol 0 mg nicotine, menthol with 6 mg nicotine, tobacco flavor 0 mg nicotine, and tobacco flavor with 6 mg nicotine procured from local vendors. E-liquids were stored in a dark room in a cooler until use. PG/VG (1:1) was prepared fresh before each exposure by mixing overnight on a rocker. Hence, the products were obtained from different vendors with matching humectants, nicotine, and flavors.
Characterization of liquid and vapor phase constituents
E-liquids (menthol and tobacco) were chemically characterized by gas chromatography and mass spectrometry (GC-MS). Aerosols from menthol and tobacco flavors were sampled in 1-l vacuum bottles, and each cartridge was sampled for 10 min with 10 puffs each. These samples were sent to ALS Environmental, CA, for analysis of constituents remaining in the vapor phase after storage and shipping were analyzed by EPA method TO-15, which focuses on a standard suite of terpenes and volatile organic compounds (VOCs). In addition, a mass spectral library search was used for tentatively identified compounds.
Whole-body ENDS aerosol exposure
The whole-body mouse exposure was performed using the InExpose vaping system attached to a Joytech (eVIC VTCmini) (SCIREQ). The 3rd generation e-cig device was automatically triggered and controlled by the SCIREQ Flexiware software (Version 8.0). The e-cig exposure regimen was based on Behar topography, simulating realistic exposure of 2 puffs/min (70 ml puff volume, 2 s puff duration, 30 s inter-puff interval, bias flow of 2 l/min) for a total of 2 h exposure time for three consecutive days. The e-liquid heating temperate was set to 220°C (80 W and 11–15 mA) with a 0.15-ohm coil. Mice were placed inside the exposure chamber, and real-time humidity, oxygen, carbon dioxide, and ambient temperature (∼20°C) were automatically recorded and monitored (Table 1). Using qTRAK (TSI) real-time probes for carbon monoxide (CO) and VOCs were recorded (Table 1). Mouse numbers were designated into air (control), PG/VG (1:1), menthol 0 mg nicotine, menthol 6 mg nicotine, tobacco 0 mg nicotine, and tobacco 6 mg nicotine. Exposure to e-cig aerosols was conducted for three consecutive days, 2 h/day. Air (control) group mice went through the same procedure as other mice with clean tubing with bias air flow without any aerosol exposures, and they were housed in a clean-air room post-sham exposure. Each mouse group exposed to respective flavored aerosols had separate designated tubing and pump heads to avoid residual contamination from other flavors, and the mice were housed in a clean-air room postexposure. Two hours after the third day of exposure to aerosols, blood was collected by submandibular method for serum cotinine analysis. After the last day, approximately 24 h later, the mice were sacrificed and blood (by vena cava collection method), bronchoalveolar lavage fluid (BALF), and lung tissues were collected. Cotinine levels in serum were estimated by ELISA (CalBiotech, Cat No. CO096D) to ensure the absence or presence of nicotine exposure in menthol and tobacco flavors.
Table 1.
Mouse exposure chamber conditions during aerosol exposures
| Temperature | RH% | CO (ppm) | CO2 | VOC (ppm) | |
|---|---|---|---|---|---|
| PG/VG | 21.38 ± 0.31 | 67.13 ± 19.47 | 33.00 ± 6.99 | 145.75 ± 159.22 | 13.33 ± 1.89 |
| Menthol | 21.85 ± 0.34 | 84.23 ± 8.56 | 2.99 ± 6.44 | 342.45 ± 327.98 | 46.55 ± 23.84 |
| Menthol + Nic | 21.74 ± 0.18 | 87.24 ± 21.57 | 0.52 ± 1.79 | 334.67 ± 310.92 | 29.67 ± 10.58 |
| Tobacco | 21.03 ± 0.43 | 74.55 ± 4.79 | 14.86 ± 15.14 | 14.92 ± 33.31 | 11.00 ± 8.50 |
| Tobacco + Nic | 21.78 ± 0.47 | 57.76 ± 7.07 | 21.86 ± 26.90 | 30.00 ± 38.92 | 15.46 ± 6.07 |
| Overall | 21.56 ± 0.35 | 74.18 ± 12.16 | 14.65 ± 13.46 | 173.56 ± 158.93 | 23.20 ± 14.94 |
Bronchoalveolar lavage collection
Upon anesthesia, 0.6 ml of 0.9% NaCl saline solution was instilled 3 times (1.8 ml cumulative volume) into the trachea and the recovered BALF was centrifuged at 1000 rpm for 7 min. The acellular fraction of the BALF was stored at −80°C for cytokine analysis by Luminex assay. The pelleted cells were then used for flow cytometry analysis to obtain differential cell counts.
Immunoblot analyses
Approximately 30 mg of lungs were homogenized in RIPA lysis buffer using a magnetic bullet blender. Protein in lung homogenate samples was determined by BCA assay (Thermo Scientific, Cat No. 23227), and approximately 20 µg of total protein per well was loaded onto 8%–15% SDS polyacrylamide gels for protein separation by electrophoresis (SDS-PAGE). Separated proteins were electroblotted onto nitrocellulose transfer membranes (Bio-Rad, 1620112). Membranes were blocked with 5% nonfat dry milk (1 h ambient temp) and probed with 1:1000 TRPA1 (Invitrogen, PA588615), 1:1000 SP-D (Abcam, ab220422), 1:1000 PAI-1 (Abcam, ab182973), and NLRP3 (Abcam, ab210491) primary antibodies overnight at 4°C. Goat Anti-Rabbit IgG (H + L) secondary antibody was used at a dilution of 1:10 000. 1:5000 GAPDH (Abcam, ab9482) and 1:5000 β-actin (Abcam, ab20272) were used as loading controls for the normalization of target proteins during densitometry analysis.
Inflammatory mediators by Luminex assay
Fifty microliters of BALF or homogenized lung tissue were used with BioRad 23-plex-Group I kit to quantify secreted inflammatory mediators (BioRad Catalog No. M60009RDPD) according to the manufacturer’s instructions. Briefly, capture antibody-coupled magnetic beads were added to the plate, followed by the addition of samples and the standards. After incubating, the detection antibody and streptavidin-PE were added. The appropriate number of washing steps and incubation steps were followed as instructed. After resuspending the sample in 125 μl of assay buffer, the plate was read on a FLEXMAP 3D system (Luminex, Austin, Texas). The concentrations of each analyte were compared to the unexposed air group and the analytes that showed significant differences were reported.
Genotoxicity assessment
To assess genotoxicity caused by ENDS exposures, frozen lung tissues were homogenized in RIPA buffer and the protein levels were determined by Pierce BCA assay (Thermofisher, 23227). Subsequently, ATR, Chk1, Chk2, H2A.X, MDM2, p21, and P53 protein levels were measured by the magnetic bead panel (Cell Signaling Multiplex Assay, 48-621MAG, EMD Millipore) on FLEXMAP 3D. The data were normalized by protein levels for each analyte and reported as net median florescence intensity.
Flow cytometry analysis
Collected cells from the BALF recovery were counted by acridine orange/propidium iodide (AO/PI) assay to obtain total cell counts. The cells were then blocked with anti-CD16/32 (Fc block) for 10 min. Followed by a PBS wash step, cells were stained with CD45, F4/80, Ly6B.2, CD4, and CD8 cell surface markers in the staining buffer to identify approximate counts of cell populations. After 30 min of incubation in the dark at 4°C, the cells were washed twice in PBS and resuspended in 100 μl buffer. Appropriate FMO controls and compensation beads were used for compensation. Sample acquisition was performed using a Guava easyCyte 8 flow cytometer (Luminex ). Data analysis was performed using GuavaSoft 3.3.
Gene expression profiling by NanoString sprint profiler
RNA samples isolated using Direct-zol Zymo kit according to manufacturer’s protocol from mouse lung lobes were quantified through NanoDrop spectrophotometer (ND-1000, NanoDrop Technologies), and 30 ng RNA samples were prepared for NanoString analysis. Premade NanoString codesets for metabolic and inflammation genes were purchased and hybridized with the samples according to the manufacturer’s guidelines. Gene expressions were assessed after quality check and normalization using nSolver 4.0 software. Significantly upregulated genes (p < .05) curated and Venn diagram was prepared using https://bioinformatics.psb.ugent.be/webtools/Venn/
Proteomics analysis
Approximately 20 mg of snap-frozen mouse lungs were tested for suitability (no blood contamination) by hemoglobin SDS-PAGE and the provided Max Quant Log2 fold change values were provided by the University of Rochester proteomics core facility.
In brief, protein extraction, protein concentration estimation, sample trypsinization, and S-Trap centrifugation were performed to collect the digested peptides. Subsequently, data collection performed for peptides from each fraction were injected onto a homemade 30 cm C18 column with 1.8 um beads (Sepax), with an Easy nLC-1200 HPLC (Thermo Fisher), connected to a Fusion Lumos Tribrid mass spectrometer (Thermo Fisher). Solvent A was 0.1% formic acid in water, while solvent B was 0.1% formic acid in 80% acetonitrile. Ions were introduced to the mass spectrometer using a Nanospray Flex source operating at 2 kV. The gradient began at 3% B and held for 2 min, increased to 10% B over 7 min, increased to 38% B over 64 min, then ramped up to 90% B in 5 min and was held for 3 min, before returning to starting conditions in 2 min and re-equilibrating for 7 min, for a total run time of 90 min. Raw data were processed with DIA-NN version 1.8.1 (https://github.com/vdemichev/DIA-NN). For all experiments, data analysis was carried out using library-free analysis mode in DIA-NN. To annotate the library, the mouse UniProt “one protein sequence per gene” database (UP000000589, downloaded 4/7/2021) was used with “deep learning-based spectra and RT prediction” enabled. Protein quantification carried out using the MaxLFQ algorithm as implemented in the DIA-NN R package (https://github.com/vdemichev/diann-rpackage) and the number of peptides quantified in each protein group was counted as implemented in the DiannReportGenerator Package (https://github.com/kswovick/DIANN-Report-Generator).
Subsequently, data for each exposure group (air, PG/VG, menthol, menthol 6 mg nicotine, tobacco, tobacco 6 mg nicotine) were curated to identify ±1.5-fold change. Data presented in a Venn diagram using https://bioinformatics.psb.ugent.be/webtools/Venn/
Mitochondrial bioenergetics of MLE15 cells treated with extracellular vesicles isolated from tobacco flavor exposed mouse lung
Murine type II epithelial cells (MLE-15) were cultured in DMEM/F12K medium and L2: F12K medium supplemented with 10% FBS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2 until confluency. Cells were seeded at 20 000 seeding density on 6-well seahorse plates (Cat No. 103025-100). We isolated exosomes, by digesting ∼40 mg of lung tissue from mice exposed to tobacco flavor and air (control) groups in 1x Liberase, and sequentially centrifuging at 300 × g, 2000 × g, 10 000 × g, and finally at 100 000 × g via ultracentrifugation method. MLE cells in seahorse plates were then treated with isolated exosomes at 5 µg/ml. Twenty-four hours posttreatment, MitoStress assay (Cat No. 13015-100, Agilent) was performed per manufacturer’s instructions to measure the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) of MLE cells upon treatment. The data were normalized by live cell count in each well and plotted by Wave software.
Statistical analysis
The statistical differences between treatment groups were analyzed through t-tests, one-way ANOVA, and two-way ANOVA in GraphPad Prism software (version 9). Results were presented as the mean ± SEM. A p-value of <.05 was considered significant.
Results
Chemical composition of menthol and tobacco flavors based on two e-liquid brands A and B
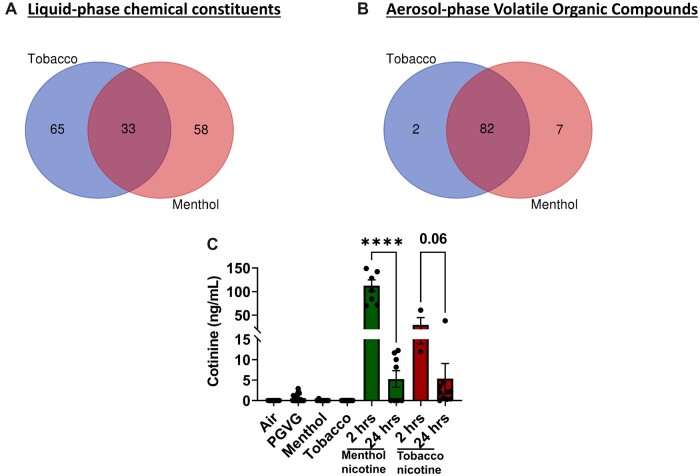
Chemical characterization of liquid and vapor phases showed high levels of alcohol-ethanol, PG, propene, 1,3-butadiene, acetone, acetaldehyde, propene, acrolein, methacrolein, sulfur dioxide, n-propanal, maltol, ethyl ether, menthanone, levomenthol, isoprene, ethyl acetate, alpha pinene, and beta pinene. Menthol derivatives were found in both tobacco and menthol flavors (Tables 2 and 3). Tobacco and menthol flavors had 33 common chemical constituents in the liquid phase and 82 common VOCs in the aerosol phase (Figures 1A and 1B). Further, we detected less than 1 mg/ml of nicotine in menthol and tobacco bottles labeled as zero nicotine. Our data suggest that similar flavors from different brands/vendors have an inconsistent formulation of chemicals attributable to the differences in the toxicological assessment. We confirmed nicotine exposure by measuring serum cotinine levels 2 h postexposure. In menthol 6 mg nicotine-exposed mice, the serum cotinine level was approximately 113 ng/ml and in the tobacco 6 mg nicotine group, the cotinine level was approximately 29 ng/ml. In contrast, 24 h postexposure significantly reduced serum cotinine levels (Figure 1C).
Table 2.
Tobacco and menthol E-liquid flavor chemical constituents
| Tobacco Flavor |
Menthol Flavor |
||
|---|---|---|---|
| CAS | Chemical Name | CAS | Chemical Name |
| 54-11-5 | Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (S)- | 54-11-5 | Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (S)- |
| 4940-11-08 00:00:00 | Ethyl maltol | 4940-11-08 00:00:00 | Ethyl maltol |
| 71-55-6 | Ethane, 1,1,1-trichloro- | 71-55-6 | Ethane, 1,1,1-trichloro- |
| 623-37-0 | Hexan-3-ol | 623-37-0 | Hexan-3-ol |
| 1000152-79-7 | Cyclopentane, 1,2,3,4,5-pentamethyl- | 1000152-79-7 | Cyclopentane, 1,2,3,4,5-pentamethyl- |
| 26456-76-8 | 2-Hexene, 3,5,5-trimethyl- | 26456-76-8 | 2-Hexene, 3,5,5-trimethyl- |
| 75-85-4 | Amylene hydrate | 75-85-4 | Amylene hydrate |
| 40467-04-7 | 2-Hexene, 2,5,5-trimethyl- | 40467-04-7 | 2-Hexene, 2,5,5-trimethyl- |
| 693-65-2 | Amyl ether | 693-65-2 | Amyl ether |
| 110-98-5 | 2-Propanol, 1,1′-oxybis- | 110-98-5 | 2-Propanol, 1,1′-oxybis- |
| 1678-82-6 | Menthane <trans-para-> | 1678-82-6 | Menthane <trans-para-> |
| 116-09-6 | Hydroxyacetone | 116-09-6 | Hydroxyacetone |
| 2568-25-4 | 1,3-Dioxolane, 4-methyl-2-phenyl- | 2568-25-4 | 1,3-Dioxolane, 4-methyl-2-phenyl- |
| 60-12-8 | Phenylethyl alcohol | 60-12-8 | Phenylethyl alcohol |
| 104-67-6 | 2(3H)-Furanone, 5-heptyldihydro- | 104-67-6 | 2(3H)-Furanone, 5-heptyldihydro- |
| 78-70-6 | Linalool | 78-70-6 | Linalool |
| 98-55-5 | Terpineol <alpha-> | 98-55-5 | Terpineol <alpha-> |
| 104-76-7 | 1-Hexanol, 2-ethyl- | 104-76-7 | 1-Hexanol, 2-ethyl- |
| 121-33-5 | Vanillin | 1490-04-6 | Menthol |
| 118-71-8 | Maltol | 121-32-4 | Vanillal |
| 106-62-7 | 1-Propanol, 2-(2-hydroxypropoxy)- | 14371-10-9 | Cinnamaldehyde <(E)-> |
| 56-81-5 | Glycerin | 2890-62-2 | Ethanone, 1-(1-methylcyclohexyl)- |
| 6214-01-03 00:00:00 | 1,2-Propanediol, 2-acetate | 563-80-4 | 2-Butanone, 3-methyl- |
| 68527-74-2 | Vanillin propylene glycol acetal | 53951-43-2 | 1,3-Dioxolane-2-methanol,2,4-dimethyl- |
| 706-14-9 | 2(3H)-Furanone, 5-hexyldihydro- | 107-87-9 | Propyl methyl ketone |
| 100-51-6 | Benzyl alcohol | 7149-26-0 | Linalyl anthranilate |
| 106-61-6 | 1,2,3-Propanetriol, 1-acetate | 80-71-7 | 2-Cyclopenten-1-one,2-hydroxy-3-methyl- |
| 627-69-0 | 1,2-Propanediol, 1-acetate | 765-70-8 | Cyclopentane-1,2-dione <3-methyl-> |
| 1000378-33-1 | 1-[(1-Propoxypropan-2-yl)oxy]propan-2-yl acetate | 928-96-1 | Hex-(3Z)-enol |
| 1754-62-7 | 2-Propenoic acid, 3-phenyl-, methyl ester, (E)- | 67634-12-2 | Lyral |
| 102-62-5 | Glycerol 1,2-diacetate | 119-61-9 | Benzophenone |
| 1120-36-1 | Tetradec-1-ene | 84-66-2 | Diethyl phthalate |
| 102-76-1 | Triacetin | 928-97-2 | 3-Hexen-1-ol, (E)- |
| 77-93-0 | Triethyl citrate | 54120-69-3 | 1,4-Dioxane-2,6-dimethanol |
| 23726-91-2 | Damascone <(E)-beta-> | 1319-88-6 | Benzaldehyde glyceryl acetal |
| 621-59-0 | Benzaldehyde, 3-hydroxy-4-methoxy- | 105-68-0 | Propanoate <isopentyl-> |
| 1490-04-6 | Cyclohexanol, 5-methyl-2-(1-methylethyl)- | 556-52-5 | Glycidol |
| 121-32-4 | Ethyl vanillin | 106-27-4 | Butanoic acid, 3-methylbutyl ester |
| 14371-10-9 | Cinnamaldehyde <(E)-> | 3623-51-6 | Neomenthol |
| 2890-62-2 | Ethanone, 1-(1-methylcyclohexyl)- | 491-07-6 | Isomenthone |
| 563-80-4 | 2-Butanone, 3-methyl- | 7786-67-6 | Isopulegol |
| 53951-43-2 | 1,3-Dioxolane-2-methanol, 2,4-dimethyl- | 15932-80-6 | Pulegone |
| 107-87-9 | Propyl methyl ketone | 470-82-6 | Eucalyptol |
| 7149-26-0 | Linalyl anthranilate | 51174-12-0 | 2,4,4-Trimethyl-1-hexene |
| 80-71-7 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 584-03-2 | 1,2-Butanediol |
| 765-70-8 | Cyclopentane-1,2-dione <3-methyl-> | 1455-20-5 | Butylthiophene <2-> |
| 928-96-1 | Hex-(3Z)-enol | 99-49-0 | Carvone |
| 67634-12-2 | Lyral | 16409-45-3 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate |
| 119-61-9 | Benzophenone | 29141-10-4 | (1R,2R,5S)-5-Methyl-2-(prop-1-en-2-yl)cyclohexanol |
| 84-66-2 | Diethyl phthalate | 75-07-0 | Acetic aldehyde |
| 928-97-2 | 3-Hexen-1-ol, (E)- | 6485-40-1 | (−)-Carvone |
| 65-85-0 | Benzoic acid | 15356-70-4 | d(+) Menthol |
| 120-57-0 | Piperonal | 1197-07-5 | Carveol <trans-> |
| 97-54-1 | Isoeugenol | 14073-97-3 | l-Menthone |
| 97-53-0 | Eugenol | 589-98-0 | 3-Octanol |
| 2051-49-2 | Hexanoic acid, anhydride | 4819-67-4 | Delphone |
| 6290-17-1 | Ethyl acetoacetate propylene glycol ketal | 2216-51-5 | Levomenthol |
| 93-58-3 | Benzoic acid, methyl ester | 89-81-6 | 2-Cyclohexen-1-one,3-methyl-6-(1-methylethyl)- |
| 554-12-1 | Methyl propionate | 89-82-7 | Pulegone |
| 68527-76-4 | Ethylvanillin propylene glycol acetal, cis- | 20405-60-1 | Dihydrocarvyl acetate |
| 123-11-5 | Benzaldehyde, 4-methoxy- | 112-30-1 | Decyl alcohol |
| 554-14-3 | Thiophene <alpha-methyl-> | 2230-87-7 | Neomenthyl acetate |
| 120-51-4 | Benzyl benzoate | 3391-87-5 | (+)-Menthone |
| 166273-38-7 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | 104-46-1 | Anethole |
| 104-61-0 | 2(3H)-Furanone, dihydro-5-pentyl- | 122-00-9 | Acetophenone <4′-methyl-> |
| 584-02-1 | Pentanol <3-> | 103-54-8 | Acetic acid, cinnamyl ester |
| 61683-99-6 | 1,3-Benzodioxole, 5-(4-methyl-1,3-dioxolan-2-yl)- | 21040-45-9 | Cinnamyl acetate <(E)-> |
| 134-20-3 | Methyl anthranilate | 52154-82-2 | Mentha-2,8-dien-1-ol <trans-, para-> |
| 74421-06-0 | 2-Heptene, 5-ethyl-2,4-dimethyl- | 127-91-3 | Pinene <beta-> |
| 111-27-3 | 1-Hexanol | 13877-93-5 | Caryophyllene <(E)-> |
| 104-50-7 | Octalactone <gamma-> | 1000155-47-0 | 3-Cyclohexen-1-one, 2-isopropyl-5-methyl- |
| 616-44-4 | Thiophene, 3-methyl- | 4180-23-8 | Anethole <(E)-> |
| 77-83-8 | Ethyl 3-methyl-3-phenylglycidate (Z) | 7212-40-0 | 2-Cyclohexen-1-ol,1-methyl-4-(1-methylethenyl)-, trans- |
| 104-21-2 | Benzenemethanol, 4-methoxy-, acetate | 57-71-6 | 2,3-Butanedione, monooxime |
| 105-13-5 | Benzenemethanol, 4-methoxy- | 1000364-16-7 | 3-Methylbenzyl alcohol, TBDMS derivative |
| 76-09-5 | 2,3-Butanediol, 2,3-dimethyl- | 1671-77-8 | 1-Pentanone, 1-(4-methylphenyl)- |
| 106-24-1 | Geraniol | 35852-46-1 | Pentanoate <cis-3-hexenyl-> |
| 105-21-5 | 2(3H)-Furanone, dihydro-5-propyl- | 40625-96-5 | 5-Methyl-2,4-diisopropylphenol |
| 19464-92-7 | Ethyl methylphenylglycidate | 1000400-22-0 | 4H-thiopyran-4-one, 2,3-dihydro-2-phenyl- |
| 1126-51-8 | 5-Oxotetrahydrofuran-2-carboxylic acid, ethyl ester | 1139-30-6 | Caryophyllene oxide |
| 10482-56-1 | l-.alpha.-Terpineol | 619-01-2 | Cyclohexanol,2-methyl-5-(1-methylethenyl)- |
| 17003-99-5 | 2-Nonene, 3-methyl-, (E)- | 80-56-8 | Pinene <alpha-> |
| 532-12-7 | Pyridine, 3-(3,4-dihydro-2H-pyrrol-5-yl)- | 118-65-0 | Isocaryophyllene |
| 7145-23-5 | 3-Hexene, 2,3-dimethyl- | 18172-67-3 | Bicyclo[3.1.1]heptane,6,6-dimethyl-2-methylene-, (1S)- |
| 10473-13-9 | 3-Buten-2-ol, 2,3-dimethyl- | 42436-07-7 | cis-3-Hexenyl phenyl acetate |
| 118-93-4 | Acetophenone <2′-hydroxy-> | 50639-00-4 | 2-Hexen-1-ol, 2-ethyl- |
| 2785-89-9 | Phenol, 4-ethyl-2-methoxy- | 1000145-04-8 | 1-Methyl-4-isopropyl-cyclohexyl 2-hydroperfluorobutanoate |
| 486-56-6 | Cotinine | 1000139-76-4 | Pentanoic acid, 4-methyl-, 1-buten-1-yl ester |
| 614-97-1 | 5-Methylbenzimidazole | 13466-78-9 | 3-Carene |
| 92-48-8 | 2H-1-Benzopyran-2-one, 6-methyl- | 149-57-5 | Hexanoic acid <2-ethyl-> |
| 108-87-2 | Hexahydrotoluene | 4403-13-8 | Ethylene glycol, TMS derivative |
| 864685-64-3 | 2,3,4,4-Tetramethyl-5-methylidenecyclopent-2-en-1-one | ||
| 1000307-63-7 | p-Anisic acid, 4-cyanophenyl ester | ||
| 1000343-91-2 | Isophthalic acid, ethyl tridec-2-ynyl ester | ||
| 65079-19-8 | 6-Quinolinamine, 2-methyl- | ||
| 13588-28-8 | 1-Propanol, 2-(2-methoxypropoxy)- | ||
| 54644-41-6 | Propanoic acid, 2,2-dimethyl-, 2-(1,1-dimethylethyl)phenyl ester | ||
| 7150-55-2 | 1-Butanone, 4-chloro-1-(4-hydroxyphenyl)- | ||
Table 3.
VOCs in tobacco- and menthol-flavored e-cigarette aerosols
| Tobacco Flavor |
Menthol Flavor |
||||
|---|---|---|---|---|---|
| CAS | Component | Conc (μg/m3) | CAS | Component | Conc. (μg/m3) |
| 64-17-5 | Ethanol | 590 000 | 64-17-5 | Ethanol | 2 200 000 |
| 14073-97-3 | l-Menthone | 4400 | 67-64-1 | Acetone | 330 000 |
| 107-02-8 | Acrolein | 3500 | 107-02-8 | Acrolein | 150 000 |
| 75-07-0 | Acetaldehyde | 3100 | 75-07-0 | Acetaldehyde | 150 000 |
| 67-64-1 | Acetone | 2600 | 123-38-6 | n-Propanal | 77 000 |
| 57-55-6 | Propylene glycol | 1800 | 115-07-1 | Propene | 24 000 |
| 108-05-4 | Vinyl acetate | 1500 | 80-56-8 | alpha-Pinene | 7800 |
| 123-38-6 | n-Propanal | 1300 | 78-79-5 | Isoprene | 7000 |
| 107-18-6 | 2-Propen-1-ol | 710 | 107-18-6 | 2-Propen-1-ol | 7000 |
| 2216-51-5 | Levomenthol | 620 | 57-55-6 | Propylene glycol | 5600 |
| 67-63-0 | 2-Propanol (isopropyl alcohol) | 600 | 108-05-4 | Vinyl acetate | 5100 |
| 460-00-4 | Bromofluorobenzene | 514 | 74-99-7 | Propyne | 5000 |
| 2037-26-5 | Toluene-d8 | 507 | 127-91-3 | beta-Pinene | 4200 |
| 17060-07-0 | 1,2-Dichloroethane-d4 | 469 | 141-78-6 | Ethyl acetate | 3900 |
| 80-56-8 | alpha-Pinene | 430 | 115-11-7 | 2-Methylpropene | 3900 |
| 75-15-0 | Carbon disulfide | 310 | 7446-09-5 | Sulfur dioxide | 2700 |
| 78-93-3 | 2-Butanone (MEK) | 310 | 78-93-3 | 2-Butanone (MEK) | 2500 |
| 141-78-6 | Ethyl acetate | 310 | 106-99-0 | 1,3-Butadiene | 2300 |
| 110-82-7 | Cyclohexane | 310 | 78-84-2 | 2-Methylpropanal | 2200 |
| 80-62-6 | Methyl methacrylate | 310 | 67-63-0 | 2-Propanol (isopropyl alcohol) | 2000 |
| 179601-23-1 | m,p-Xylenes | 310 | 60-29-7 | Ethyl ether | 1800 |
| 100-44-7 | Benzyl chloride | 310 | 75-15-0 | Carbon disulfide | 1000 |
| 75-34-3 | 1,1-Dichloroethane | 160 | 110-82-7 | Cyclohexane | 1000 |
| 109-99-9 | Tetrahydrofuran (THF) | 160 | 80-62-6 | Methyl methacrylate | 1000 |
| 123-86-4 | n-Butyl acetate | 160 | 179601-23-1 | m,p-Xylenes | 1000 |
| 115-07-1 | Propene | 150 | 100-44-7 | Benzyl chloride | 1000 |
| 75-71-8 | Dichlorodifluoromethane (CFC 12) | 150 | 75-34-3 | 1,1-Dichloroethane | 520 |
| 74-87-3 | Chloromethane | 150 | 109-99-9 | Tetrahydrofuran (THF) | 520 |
| 76-14-2 | 1,2-Dichloro-1,1,2,2-tetrafluoroethane (CFC 114) | 150 | 123-86-4 | n-Butyl acetate | 520 |
| 75-01-4 | Vinyl chloride | 150 | 460-00-4 | Bromofluorobenzene | 517 |
| 106-99-0 | 1,3-Butadiene | 150 | 75-01-4 | Vinyl chloride | 510 |
| 74-83-9 | Bromomethane | 150 | 74-83-9 | Bromomethane | 510 |
| 75-00-3 | Chloroethane | 150 | 75-00-3 | Chloroethane | 510 |
| 75-05-8 | Acetonitrile | 150 | 75-35-4 | 1,1-Dichloroethene | 510 |
| 75-69-4 | Trichlorofluoromethane | 150 | 107-05-1 | 3-Chloro-1-propene (Allyl Chloride) | 510 |
| 107-13-1 | Acrylonitrile | 150 | 76-13-1 | Trichlorotrifluoroethane | 510 |
| 75-35-4 | 1,1-Dichloroethene | 150 | 156-60-5 | trans-1,2-Dichloroethene | 510 |
| 75-09-2 | Methylene chloride | 150 | 1634-04-4 | Methyl tert-Butyl Ether | 510 |
| 107-05-1 | 3-Chloro-1-propene (allyl chloride) | 150 | 110-54-3 | n-Hexane | 510 |
| 76-13-1 | Trichlorotrifluoroethane | 150 | 67-66-3 | Chloroform | 510 |
| 156-60-5 | trans-1,2-Dichloroethene | 150 | 107-06-2 | 1,2-Dichloroethane | 510 |
| 1634-04-4 | Methyl tert-butyl ether | 150 | 71-55-6 | 1,1,1-Trichloroethane | 510 |
| 156-59-2 | cis-1,2-Dichloroethene | 150 | 78-87-5 | 1,2-Dichloropropane | 510 |
| 110-54-3 | n-Hexane | 150 | 75-27-4 | Bromodichloromethane | 510 |
| 67-66-3 | Chloroform | 150 | 79-01-6 | Trichloroethene | 510 |
| 107-06-2 | 1,2-Dichloroethane | 150 | 123-91-1 | 1,4-Dioxane | 510 |
| 71-55-6 | 1,1,1-Trichloroethane | 150 | 142-82-5 | n-Heptane | 510 |
| 71-43-2 | Benzene | 150 | 79-00-5 | 1,1,2-Trichloroethane | 510 |
| 56-23-5 | Carbon tetrachloride | 150 | 108-88-3 | Toluene | 510 |
| 78-87-5 | 1,2-Dichloropropane | 150 | 591-78-6 | 2-Hexanone | 510 |
| 75-27-4 | Bromodichloromethane | 150 | 124-48-1 | Dibromochloromethane | 510 |
| 79-01-6 | Trichloroethene | 150 | 106-93-4 | 1,2-Dibromoethane | 510 |
| 123-91-1 | 1,4-Dioxane | 150 | 111-65-9 | n-Octane | 510 |
| 142-82-5 | n-Heptane | 150 | 108-90-7 | Chlorobenzene | 510 |
| 10061-01-5 | cis-1,3-Dichloropropene | 150 | 100-41-4 | Ethylbenzene | 510 |
| 108-10-1 | 4-Methyl-2-pentanone | 150 | 75-25-2 | Bromoform | 510 |
| 10061-02-6 | trans-1,3-Dichloropropene | 150 | 95-47-6 | o-Xylene | 510 |
| 79-00-5 | 1,1,2-Trichloroethane | 150 | 111-84-2 | n-Nonane | 510 |
| 108-88-3 | Toluene | 150 | 79-34-5 | 1,1,2,2-Tetrachloroethane | 510 |
| 591-78-6 | 2-Hexanone | 150 | 98-82-8 | Cumene | 510 |
| 124-48-1 | Dibromochloromethane | 150 | 103-65-1 | n-Propylbenzene | 510 |
| 106-93-4 | 1,2-Dibromoethane | 150 | 622-96-8 | 4-Ethyltoluene | 510 |
| 111-65-9 | n-Octane | 150 | 95-63-6 | 1,2,4-Trimethylbenzene | 510 |
| 127-18-4 | Tetrachloroethene | 150 | 541-73-1 | 1,3-Dichlorobenzene | 510 |
| 108-90-7 | Chlorobenzene | 150 | 106-46-7 | 1,4-Dichlorobenzene | 510 |
| 100-41-4 | Ethylbenzene | 150 | 95-50-1 | 1,2-Dichlorobenzene | 510 |
| 75-25-2 | Bromoform | 150 | 5989-27-5 | d-Limonene | 510 |
| 100-42-5 | Styrene | 150 | 120-82-1 | 1,2,4-Trichlorobenzene | 510 |
| 95-47-6 | o-Xylene | 150 | 75-71-8 | Dichlorodifluoromethane (CFC 12) | 500 |
| 111-84-2 | n-Nonane | 150 | 74-87-3 | Chloromethane | 500 |
| 79-34-5 | 1,1,2,2-Tetrachloroethane | 150 | 76-14-2 | 1,2-Dichloro-1,1,2,2-tetrafluoroethane (CFC 114) | 500 |
| 98-82-8 | Cumene | 150 | 75-05-8 | Acetonitrile | 500 |
| 103-65-1 | n-Propylbenzene | 150 | 75-69-4 | Trichlorofluoromethane | 500 |
| 622-96-8 | 4-Ethyltoluene | 150 | 107-13-1 | Acrylonitrile | 500 |
| 108-67-8 | 1,3,5-Trimethylbenzene | 150 | 75-09-2 | Methylene chloride | 500 |
| 95-63-6 | 1,2,4-Trimethylbenzene | 150 | 156-59-2 | cis-1,2-Dichloroethene | 500 |
| 541-73-1 | 1,3-Dichlorobenzene | 150 | 71-43-2 | Benzene | 500 |
| 106-46-7 | 1,4-Dichlorobenzene | 150 | 56-23-5 | Carbon tetrachloride | 500 |
| 95-50-1 | 1,2-Dichlorobenzene | 150 | 108-10-1 | 4-Methyl-2-pentanone | 500 |
| 5989-27-5 | d-Limonene | 150 | 10061-02-6 | trans-1,3-Dichloropropene | 500 |
| 96-12-8 | 1,2-Dibromo-3-chloropropane | 150 | 100-42-5 | Styrene | 500 |
| 120-82-1 | 1,2,4-Trichlorobenzene | 150 | 108-67-8 | 1,3,5-Trimethylbenzene | 500 |
| 91-20-3 | Naphthalene | 150 | 96-12-8 | 1,2-Dibromo-3-chloropropane | 500 |
| 87-68-3 | Hexachlorobutadiene | 150 | 87-68-3 | Hexachlorobutadiene | 500 |
| 2037-26-5 | Toluene-d8 | 494 | |||
| 10061-01-5 | cis-1,3-Dichloropropene | 490 | |||
| 127-18-4 | Tetrachloroethene | 490 | |||
| 91-20-3 | Naphthalene | 490 | |||
| 17060-07-0 | 1,2-Dichloroethane-d4 | 475 | |||
Figure 1.
A, Venn diagram depicting the number of detected chemicals tobacco and menthol flavors. E-liquids, tobacco and menthol, from two brands (A and B) were analyzed by GC-MS for their chemical composition. Tobacco and menthol flavors have distinctly 65 and 58 flavor constituents with 33 being common constituents. B, Venn diagram depicting the number of detected volatile organic compounds in tobacco and menthol flavored aerosols. Aerosols, tobacco and menthol, from two brands (A and B), were analyzed by GC-MS for their chemical composition. Tobacco and menthol flavors had two and seven constituents exclusively, with 82 being common overlapping constituents. C, Serum cotinine levels of mice exposed to menthol and tobacco aerosols with and without nicotine. Cotinine levels in serum quantified in C57BL/6J and BALB/cJ male and female mice exposed to air, PG/VG, menthol 0 and 6 mg nicotine, and tobacco 0 and 6 mg nicotine of brand A. Serum from menthol and tobacco groups with nicotine were collected at 2 and 24 h post 3-day exposure (N = 3–8 per group, ****p < .0001, t-test).
Acute exposure to brand A, PG/VG, menthol, and tobacco with and without nicotine, induced immune cell infiltration in BALF, irrespective of the mouse strain
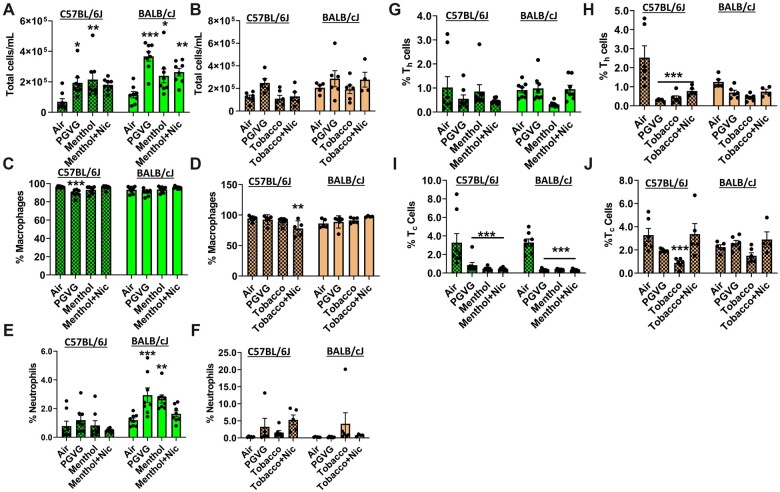
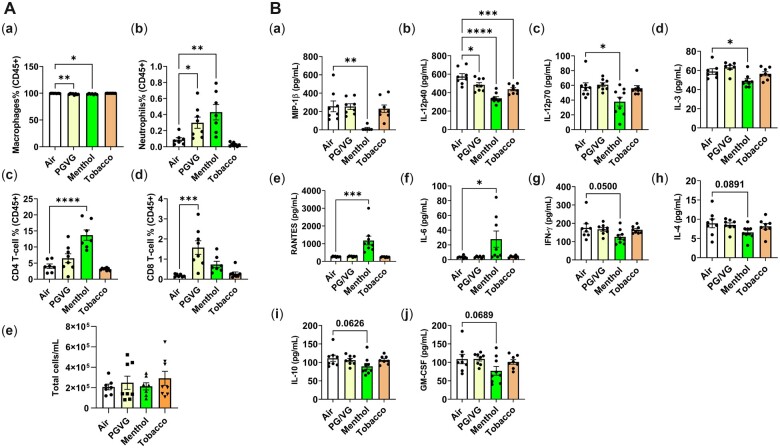
To assess the elicited inflammatory response by exposure to ENDS, total cell counts and differential cell count were determined in BALF. Exposure to PG/VG, menthol 0 mg nicotine, and menthol 6 mg nicotine, all of which are from brand A e-liquid, caused significant immune cell influx in the lung as the total cell number has increased in both C57BL/6J and BALB/cJ strains compared to the air group (Figure 2A). BALF cells were constituted mostly of alveolar macrophages and a slight reduction was seen in C57BL/6J mice exposed to tobacco with nicotine (Figures 2C and 2D). Greater differential influxes in neutrophils were observed in flavor-exposed groups, particularly in BALB/cJ mice. Both PG/VG and menthol 0 mg nicotine exposure caused a significant influx up to 2.9% (by 60% increase) in neutrophils compared to the air group, particularly in BALB/cJ mice (Figures 2E and 2F). Overall, CD4 lymphocyte percentages were decreased in PG/VG, tobacco ± nicotine exposed groups compared to the air group (by 88% significant decrease in C57BL/6J mice) (Figures 2G and 2H). In both C57BL/6J and BALB/cJ mice, PG/VG, menthol ± nicotine groups significantly reduced Tc cells to 0.4% (reduced by 74% compared to the air group; Figures 2I and 2J). Overall, menthol flavor induced inflammatory influx in BALB/cJ mice, while tobacco flavor exposure affected C57BL/6J mice more. These data suggest that acute exposure to brand A, PG/VG tobacco menthol flavors ± nicotine, elicited an inflammatory response.
Figure 2.
Differential cell counts in bronchoalveolar lavage fluid by exposure to brand A, PG/VG, menthol, and tobacco flavors with and without nicotine in C57BL/6J and BALB/cJ mice. C57BL/6J and BALB/cJ male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and the BALF was collected. A and B, Total cell counts were performed by staining with AO/PI dye. C and D, Macrophages (F4/80+), E and F, neutrophils (Ly6B.2), G and H, Th lymphocytes (CD4+), and I and J, Tc lymphocytes (CD8+) were assessed as percentages of CD45+ parent cell population by flow cytometry. Data are shown as mean ± SEM. *p < .05, **p < .01, and ***p < .001 versus respective air group per strain (2-way ANOVA) (N = 4–8 per group).
Exposure to brand A, PG/VG, menthol, and menthol with nicotine caused immunosuppression
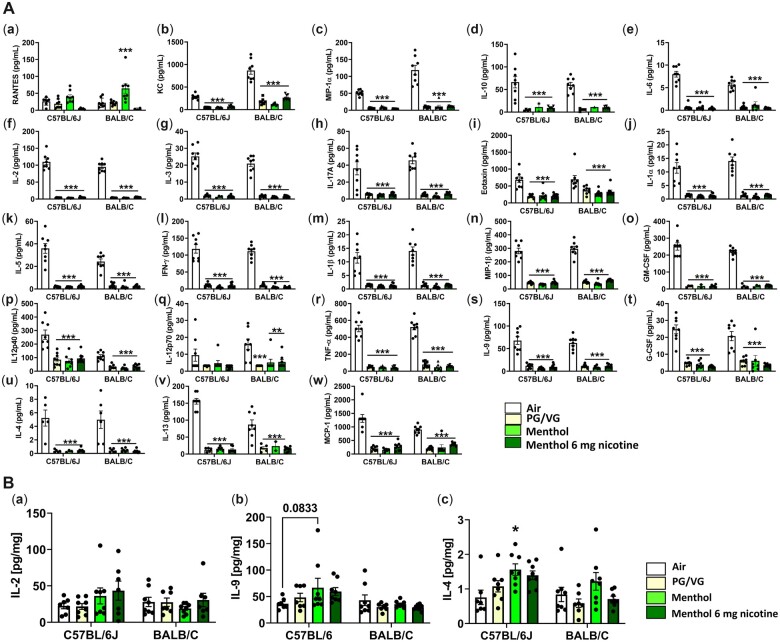
Exposure to brand A, PG/VG, menthol 0 mg nicotine, and menthol 6 mg nicotine, caused highly significant suppression of major inflammatory cytokines, KC (Figure 3A, b), MIP-1α (Figure 3A, c), IL-1α (Figure 3A, j), IFNγ (Figure 3A, i), IL-1β (Figure 3A, m), IL-2 (Figure 3A, j), IL-3 (Figure 3A, g), IL-4 (Figure 3A, u), IL-5 (Figure 3A, k), IL-6 (Figure 3A, e), IL-9 (Figure 3A, s), IL-10 (Figure 3A, d), IL12 (p40) (Figure 3A, p), IL-12 (p70) (Figure 3A, q), IL-13 (Figure 3A, v), IL-17A (Figure 3A, h), Eotaxin (Figure 3A, i), G-CSF(Figure 3A, o), GM-CSF (Figure 3A, t), TNFα (Figure 3A, r), MIP-1β (Figure 3A, n), and MCP-1 (Figure 3A, w), in both C57BL/6J and BALB/cJ mouse BALF. However, RANTES levels were increased by menthol 0 mg nicotine exposure in both mouse strains but significantly in BALB/cJ (Figure 3A, a). However, this increase was reversed in the presence of nicotine (Figure 3A, a). In the lung homogenate, IL2 (NS), IL4 (p < .05), and IL9 (p = .08) were increased in C57BL/6J mice by 64%, 81%, and 50% increases respectively by exposure to menthol 0 mg ENDS flavor compared to the air group (Figures 3B, a–c).
Figure 3.
A, Exposure to brand A, PG/VG, menthol, and menthol with nicotine flavors in C57BL/6J and BALB/cJ mice elicited a regulatory inflammatory cytokine response in bronchoalveolar lavage. C57BL/6J and BALB/cJ, male and female, mice were exposed to PG/VG, menthol, and menthol 6 mg nicotine, 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and bronchoalveolar lavage was collected by instilling 0.6 ml 3× and pooled. Cytokine levels were measured by Luminex. a, RANTES; b, KC; c, MIP-1α; d, IL-10; i, IL-6; f, IL2; g, IL-3; h, IL-17A; i, Eotaxin; j, IL-1α; k, IL5; l, IFNγ; m, IL-1β; n, MIP-1β; o, GM-CSF; p, IL12p40; q, IL12p70; r, TNFα; s, IL9; t, G-CSF; u, IL-4; v, IL-13; w, MCP-1. Data are shown as mean ± SEM. *p < .05, **p < .01, ***p < .001 versus respective air group per strain (2-way ANOVA) (N = 7–8 per group). B, Exposure to brand A, PG/VG, menthol, and tobacco flavors with and without nicotine in C57BL/6J and BALB/cJ mice elicited a cytokine response in mouse lung homogenate. C57BL/6J and BALB/cJ, male and female, mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and lung tissues were collected. Homogenized lungs in RIPA buffer were used to determine inflammatory mediators by Luminex and normalized by total protein (BCA assay). a, IL-2; b, IL-9; c, IL-4. Datat are shown as mean ± SEM. *p < .05 versus respective air group per strain (2-way ANOVA) (N = 7–8 per group).
Exposure to brand A, PG/VG, tobacco, and tobacco with nicotine caused cytokine suppression
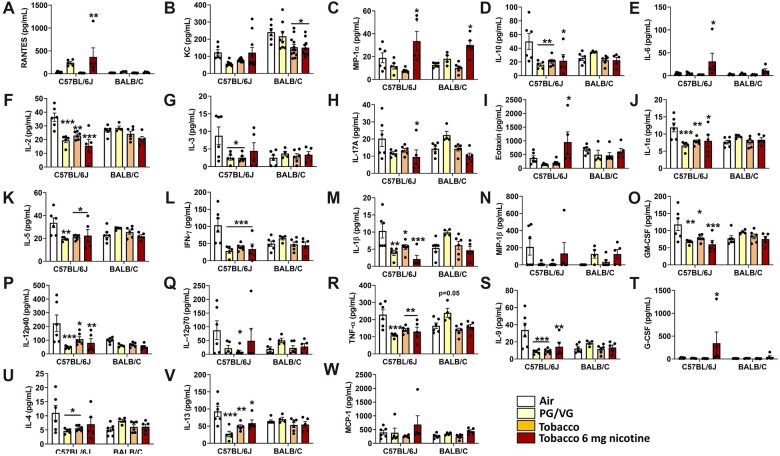
PG/VG caused a nearly significant increase in TNFα (p = .05) (Figure 4R) and incremental nonsignificant increases in IL-1α (Figure 4J), IL-1β (Figure 4M), MIP-1β (Figure 4N), IFNγ (Figure 4L), GM-CSF (Figure 4O), IL17A (Figure 4H), IL5 (Figure 4K), and IL4 (Figure 4U) in BALB/cJ mice compared to C57BL/6J mice. Exposure to tobacco 0 mg, in general, caused immunosuppression significantly in C57BL/6J mice, including IL-1α (Figure 4J), IFNγ (Figure 4L), IL-1β (Figure 4M), GM-CSF (Figure 4O), IL4 (Figure 4U), IL-2 (Figure 4F), IL-3 (Figure 4G), IL-13 (Figure 4V), IL10 (Figure 4D), IL12p70 (Figure 4Q), IL12p40 (Figure 4P), IL9 (Figure 4S), and IL5 (Figure 4K) compared to the air group. In contrast, compared to the air group, tobacco 6 mg nicotine exposure significantly elevated levels of MIP-1α (Figure 4C) in both strains. Significant increases in RANTES (Figure 4A), IL-6 (Figure 4E), eotaxin (Figure 4I), and G-CSF (Figure 4T) were observed in C57BL/6J mice. This significantly suppressed cytokine response with the presence of nicotine demarcates the augmented response by flavor, which may be due to interaction with nicotine of the flavor alone.
Figure 4.
Exposure to brand A, PG/VG, tobacco, and tobacco with nicotine flavors in C57BL/6J and BALB/cJ mice elicited a regulatory inflammatory cytokine response in bronchoalveolar lavage. C57BL/6J and BALB/cJ, male and female mice, were exposed to PG/VG, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and bronchoalveolar lavage was collected by instilling 0.6 ml 3× and pooled. Cytokine levels were measured by Luminex. A, RANTES; B, KC; C, MIP-1α; D, IL-10; E, IL-6; F, IL2; G, IL-3; H, IL-17A; I, Eotaxin; J, IL-1α; K, IL5; L, IFNγ; M, IL-1β; N, MIP-1β; O, GM-CSF; P, IL12p40; Q, IL12p70; R, TNFα; S, IL9; T, G-CSF; U, IL-4; V, IL-13; W, MCP-1. Data are presented as mean ± SEM. *p < .05, **p < .01, and ***p < .001 versus respective air group per strain (2-way ANOVA) (N = 7–8 per group).
These data suggest that while there are some differences in the immune response between the strains, overall, a similar inflammatory response was elicited by brand A, PG/VG, tobacco, and tobacco with nicotine in both strains. The interaction of nicotine with tobacco demonstrated an altered response. These responses are driven toward allergic inflammation and acute lung injury in the presence of nicotine and tobacco flavor.
Acute exposure to brand B, PG/VG, menthol, and tobacco flavors induced immune cell infiltration in BALF
To elucidate the flavor-specific immune response, an identical 3-day exposure to PG/VG, menthol, and tobacco without any nicotine from brand B was performed on C57BL/6J mice and the BALF differential cell counts were performed. Compared to the unexposed counterparts, the PG/VG-exposed mice had increased neutrophils (Ly6.b+) (p < .05), CD4 T-cells (NS), and CD8-T-cells (p < .001) compared to the air group (Figures 5A, b–d). Menthol flavor exposed groups had significantly increased neutrophils and both CD4 and CD8 T-lymphocytes (Figures 5A, c and d). In contrast, tobacco flavor exposure did not cause any changes in macrophage, neutrophil, or T-lymphocyte counts in BALF compared to the air group (Figures 5A, a–e). These data demonstrate that PG/VG and menthol from brand B caused a greater immune cell influx and an immune response compared to the tobacco flavor and air groups.
Figure 5.
A, Differential cell counts in bronchoalveolar lavage by exposure to brand B, PG/VG, menthol, and tobacco flavors in C57BL/6J. C57BL/6J male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and the BALF was collected. a, Macrophages (F4/80+); b, neutrophils (Ly6B.2); c, Th lymphocytes (CD4+); d, Tc lymphocytes (CD8+), were assessed as percentages of CD45+ parent cell population by flow cytometry based on the (e) total cell counts determined by staining with AO/PI dye. Data are shown as mean ± SEM. *p < .05, **p < .01, ***p < .001 versus respective air group per strain (2-way ANOVA) (N = 8 per group). B, Exposure to brand B, PG/VG, menthol, and tobacco flavors in C57BL/6J mice elicited a differential inflammatory cytokine response in bronchoalveolar lavage. C57BL/6J male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and bronchoalveolar lavage was collected by instilling 0.6 ml 3× and pooled. Cytokine levels were measured by Luminex. a, MIP-1β; b, IL-12p40; c, IL-12p70; d, IL-3; e, RANTES; f, IL-6; g, IFNγ; h, IL-4; i, IL-10; j, GM-CSF. Data are shown as mean ± SEM. *p < .05, **p < .01, ***p < .001, and ****p < .0001 versus respective air group per strain (2-way ANOVA) (N = 8 per group).
Exposure to brand B nicotine-free menthol flavor induced immune suppression
To further recapitulate the menthol and tobacco flavor-specific immune response, BALF from C57BL/6J mice exposed to brand B aerosols of PG/VG, menthol, and tobacco, were evaluated for inflammatory mediators. While most cytokines were not changed (data not shown), menthol flavor caused significant attenuation of MIP-1β, IL-12p40, IL-12p70, and IL3 (Figures 5B, a–d). Albeit not significant, IFNγ (p = .05), IL4, IL10, and GM-CSF levels were attenuated by the menthol exposure compared to the air control group (Figures 5B, g–j). Similar to the response elicited by brand A, RANTES levels were significantly elevated (p < .001) by brand B menthol flavor-exposed mice (Figure 5B, a). Furthermore, IL-6 levels were significantly elevated (p < .001) by menthol exposure (Figure 5B, f). These increased IL-6 and RANTES levels, along with decreased proinflammatory cytokines, suggest the inflammatory response may be allergic and immunosuppressive.
Exposure to menthol flavor-induced chemosensory cation channel TRPA1 and nicotine-augmented biomarkers of acute lung injury
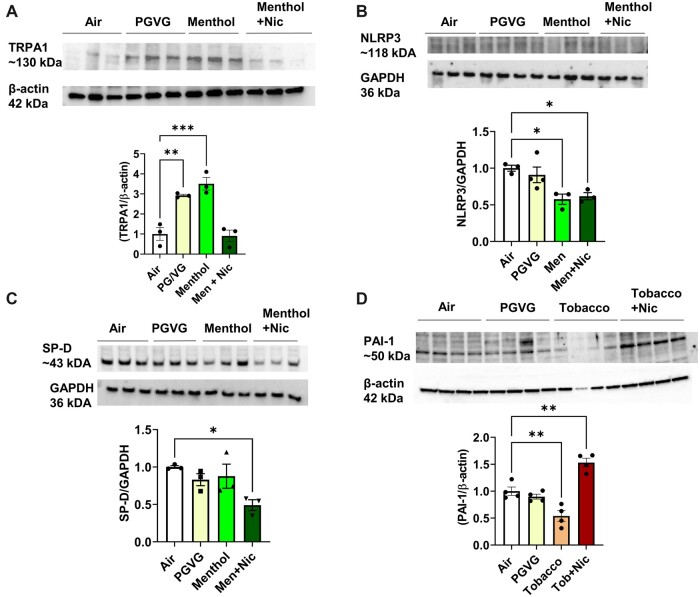
TRPA1 abundance was significantly increased by PG/VG and menthol compared to the air group. In contrast, menthol 6 mg nicotine group TRPA1 level was reduced/inhibited compared to the menthol group reversing its level similar to the air group (Figure 6A) with full blots (Supplementary Figure 1). NLRP3 inflammasome was altered by menthol exposure (Figure 6B) with full blot (Supplementary Figure 2). Surfactant protein SP-D was significantly reduced by menthol 6 mg nicotine exposure group (Figure 6C) with full blot (Supplementary Figure 2). While PAI-1 level was significantly reduced by tobacco 0 mg nicotine, this was reversed in the presence of nicotine, augmenting the PAI-1 protein abundance significantly (Figure 6D) with full blot (Supplementary Figure 1). Data suggest exposure to PG/VG and flavors with nicotine may cause acute lung injury (Figures 6B–D).
Figure 6.
ENDS flavors induced acute lung injury-associated biomarkers. C57BL/6J male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure, and lung tissues were homogenized for immunoblotting by SDS-PAGE. A, TRPA-1; B, NLRP3; C, SP-D; D, PAI-1 protein abundance were measured and normalized to GAPDH and β-actin loading controls. Data shown for respective bands and the densitometry values were plotted as mean ± SEM. *p < .05, **p < .01, and ***p < .001 versus respective air group per strain (2-way ANOVA) (N = 3–4 per group).
Exposure to PG/VG, menthol, and tobacco flavors caused genotoxicity
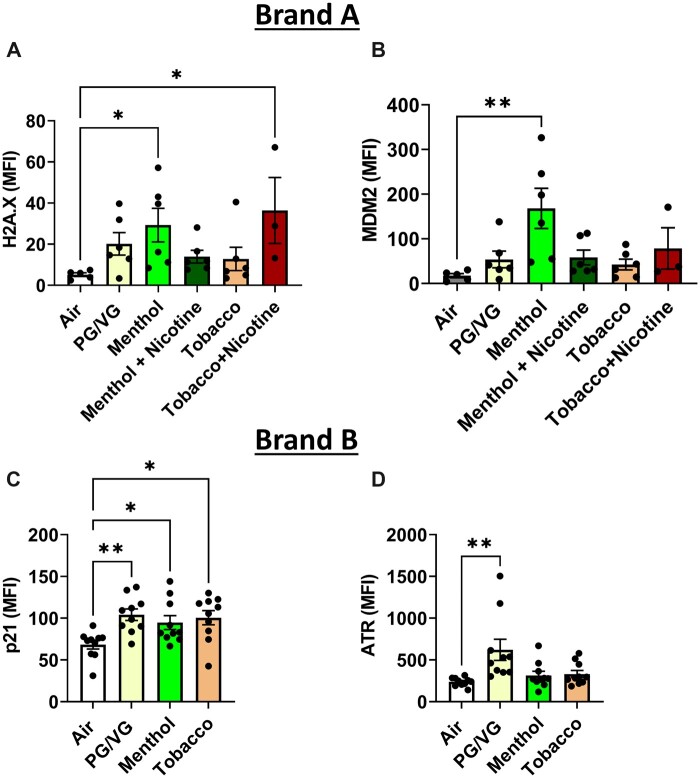
Genotoxicity markers by acute exposure to brand A aerosols (flavors sold as 0 and 6 mg nicotine) and brand B aerosols (flavors are nicotine-free) were determined in mouse lung tissue. Brand B PG/VG exposure caused a significant increase in p21 and ATR (Figures 7C and D). Mice exposed to menthol (0 mg nicotine) increased H2A.X, MDM2, and p21 significantly (Figures 7A–C). Mice exposed to tobacco 0 mg nicotine caused a significant increase in p21 by brand B aerosol exposure (Figure 7C). Tobacco (0 mg nicotine brand B) increased p21 levels while brand A tobacco flavor with 6 mg nicotine increased H2A.X levels (Figures 7A and C). Overall, aerosols (PG/VG, menthol, tobacco with and without nicotine), demonstrated significantly altered genotoxic parameters while there were differences between brand, flavor, and the presence of nicotine (Figure 7). These data suggest that acute exposure to e-cig aerosols may cause genotoxicity.
Figure 7.
Genotoxicity by exposure to PG/VG, menthol, and tobacco flavors in C57BL/6J mouse lung tissue. C57BL/6J male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and mouse lung tissues were homogenized and measured genotoxicity markers, A, H2AX; B, MDM2; C, p21; and D, ATR by measuring net median florescence intensity (MFI) normalized to total protein. Data are shown as mean ± SEM. *p < .05, **p < .01, and ***p < .001 versus respective air group per strain (1-way ANOVA) (N = 3–10 per group).
Exposure to aerosols-induced intracellular p38-p70s6k kinase-associated signaling
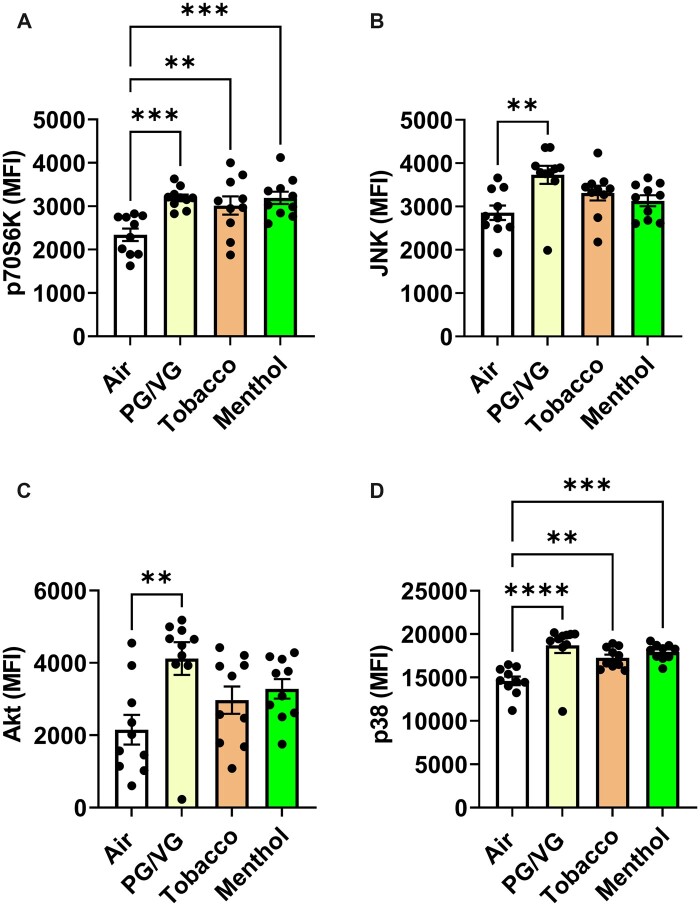
To determine potential pathways of intracellular signaling upon exposure to nicotine-free brand B ENDS aerosols, prepared cell lysates were assessed for major signaling receptor tyrosine kinases, which are key for cellular metabolism. PG/VG significantly elevated p70S6K, JNK, Akt, and p38 (Figures 8A–D). Menthol and tobacco flavors significantly increased P70S6K and p38 protein levels (Figures 8A and D). Overall, p70S6K and p38 were increased by all aerosols (Figures 8A and D).
Figure 8.
Exposure to PG/VG, menthol, and tobacco flavors in C57BL/6J mouse lung tissue induced PI3K-Akt-mTOR pathway signaling. C57BL/6J male and female mice were exposed to PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and mouse lung tissues were homogenized and measured cell signaling pathway markers, A, P70S6K; B, JNK; C, Akt; and D, p38 by measuring net median florescence intensity (MFI) normalized to total protein. Data shown as mean ± SEM. *p < .05, **p < .01, and ***p < .001 versus respective air group per strain (1-way ANOVA) (N = 6–10 per group).
Acute exposure to PG/VG, menthol, and tobacco caused gene alterations related to immunometabolism
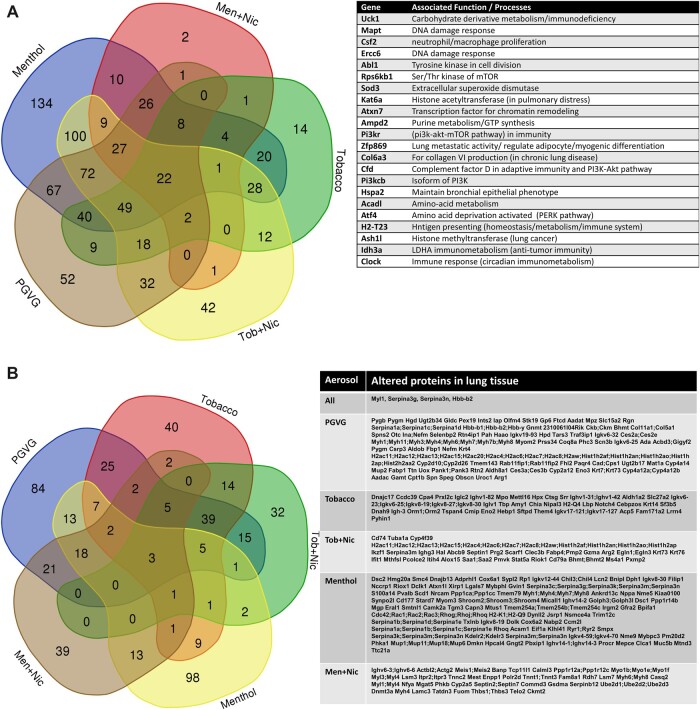
Lung tissues of C57BL/6J from acute exposure to PG/VG, menthol, and tobacco flavors from both brands of e-liquids were assessed for gene expression changes related to inflammation and metabolism. After individually analyzing each flavor exposed group, 22 genes that changed significantly (p < .05) by acute exposure to ENDS aerosols compared to the air group were identified. These genes include Uck1, Mapt, Csf2, Ercc6, Abl1, Rps6kb1, Sod3, Kat6a, Atxn7, Ampd2, Pi3kr, Zfp869, Col6a3, Cfd, Pi3kcb, Hspa2, Acadl, Atf4, H2-T23, Ash1, Ldh3a, and Clock. Individually PG/VG, tobacco, tobacco + Nic, menthol, and menthol + Nic groups dysregulated 52,134, 2,14, and 42 genes, respectively, and altered 22 commonly overlapped genes by all exposures, as depicted in a Venn diagram (Figure 9A). These genes are involved in metabolism, DNA damage response, oxidative stress, and tumor suppression/cell division homeostasis.
Figure 9.
A, Inflammatory and Metabolic gene expression alterations by acute exposure to PG/VG, menthol, menthol with nicotine, tobacco, tobacco with nicotine aerosols. C57BL/6J male and female mice were exposed to three days (2 h/day) air, PG/VG, menthol, menthol + nicotine, tobacco, and tobacco + nicotine aerosols. RNA isolated from lungs were hybridized with Nanostring codesets and the inflammatory and metabolic gene alterations were determined. Genes that ±1.5-fold change altered significantly compared to the Air group were compared. p < .05, 1-way ANOVA, N = 6 per group. B, Protein alterations by acute exposure to PG/VG, menthol, menthol with nicotine, tobacco, tobacco with nicotine aerosols. C57BL/6J male and female mice were exposed to three days (2h/day) air, PG/VG, menthol, menthol + nicotine, tobacco, and tobacco flavor + nicotine aerosols. Protein changes were determined by proteomics analysis and presented in a Venn diagram. Proteins significantly altered by 1.5-fold are listed (p < .05 vs air group), N = 3 per group.
Acute exposure to PG/VG, menthol, and tobacco altered key acute phase and metabolic proteins
Mouse lungs analyzed for protein alterations upon each exposure to PG/VG, menthol 0 mg nicotine, menthol 6 mg nicotine, tobacco 0 mg, and tobacco 6 mg were curated in a Venn diagram to identify commonly altered proteins at least 1.5-fold by exposure to all aerosols as well as individual flavor for brand A. Serpin3a, Myl1, and Hbb-b2 proteins were altered by flavors (Figure 9B).
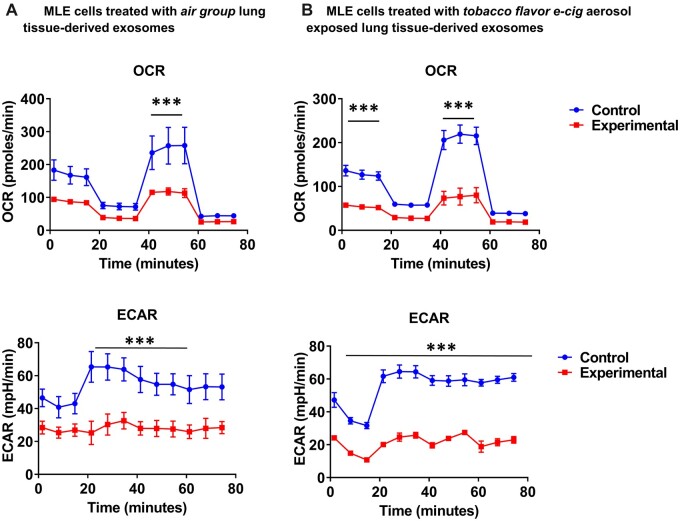
Treatment of MLE cells from extracellular vesicles isolated from mice exposed to tobacco flavor caused alterations in mitochondrial bioenergetics
As the acute exposure to flavors caused significant alterations in genes related to cellular metabolism, mitochondrial respiration of MLE cells treated with exosomes isolated from air and tobacco groups was assessed. We assessed the size of isolated exosomes, which were about 100–125 nm. Twenty-four hours after exosome treatment, MLE cells treated with exosomes derived from tobacco flavor exposed mice (Figure 10B) showed significantly lower OCR and ECAR compared to exomes derived from the air/control mice (Figure 10A). These data suggest that exosome cargo from tobacco-exposed mice may contain metabolic abrogating miRNA or proteins.
Figure 10.
Mitochondrial respiration is affected by extracellular vesicles derived from the lungs of mice exposed to brand B tobacco flavor. C57BL/6J male and female mice were exposed air and tobacco-flavored e-cig aerosols 2 h/day for three consecutive days in SCIREQ whole-body exposure chamber. Mice were euthanized ∼24 h postexposure and exosomes were isolated from the lung tissue. MLE15 cells were grown in Seahorse plates and treated with isolated exosomes. Twenty-four hours later, Sea horse mitostress assay was run and oxygen consumption rate (OCR) extracellular acidification rate (ECAR) parameters were acquired, and normalized by total cells. A, MLE cells treated with air group extracellular vesicles (EVs) and B, MLE cells treated with tobacco flavor exposed EVs. t-test, ***p < .001 versus control, N = 3.
Immunometabolism-associated signaling by ingenuity pathway analysis
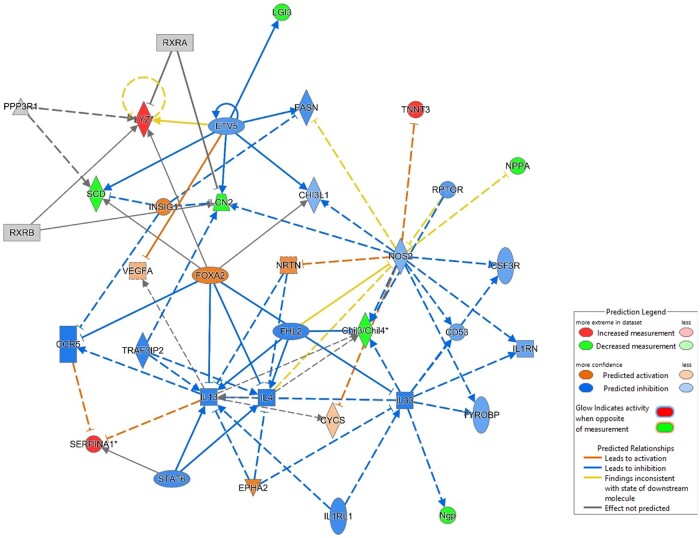
Core analysis of proteomics data by ingenuity pathway analysis (IPA) trial version (Krämer et al., 2014) from menthol 0 mg nicotine-exposed mice showed inhibition and deactivation pathways of proinflammatory transcription factors (NOS2, RAPTOR signaling) and increased negative regulators such as serpins (Figure 11).
Figure 11.
Potential pathways of immunometabolic and allergic inflammatory response via PI3K-Akt-mTOR. C57BL/6J male and female representative canonical pathway of menthol e-cig flavor exposure-induced lung response by ingenuity pathway analysis of proteomics data demonstrating inhibition of inflammatory pathways and upregulating immunoregulatory pathways.
Discussion
According to a 2018–2019 tobacco user survey, over 23.1% of e-cigarette users were never smokers (Mayer et al., 2020). In this study, using an in vivo mouse exposure model, we conducted an acute exposure to ENDS aerosols to determine the inflammatory response, genotoxicity, and metabolic changes in the lung, simulating the inhalation toxicological effects of a nonsmoker response to first-time acute e-cig exposure (naïve exposure). The acute phase immune response and its resolution upon exposure to carrier fluid/humectant (PG/VG), menthol, and tobacco flavors with and without nicotine from different brands has been demonstrated in this study. We hypothesized that a proinflammatory response would be elicited upon exposure to aerosols, and the two brands would be consistent or differential in their responses to PG/VG, menthol, and tobacco with and without nicotine flavors aerosol exposures.
In our tested tobacco and menthol flavored product chemical analysis, we determined that flavored e-liquids contain a mixture of chemicals, including significant overlap in liquid and aerosol phase constituents. Therefore, it must be noted that the toxicity and biological responses result from these chemical constituents and is not attributable to a certain flavor per se. The additive or synergistic toxicity and effects of these chemicals in the flavors are important to be determined.
We observed cell infiltration in BALF by all flavors of both brands A and B. Among the BALF leukocytes, an increase in neutrophils (significantly with menthol) and differential CD4 and CD8 counts were seen by brand A and B flavors. Small changes in neutrophilic cellular patterns may still mean great biological significance as greater than 3% can suggest collagen vascular diseases, IPF, aspiration pneumonia, infections, bronchitis, acute respiratory disease syndrome, and diffused-alveolar damage (Meyer et al., 2012). There was a decrease in T lymphocytes with the exposure to brand A and an increase in CD4-T cell counts by brand B. Interestingly, all inflammatory mediators were suppressed by brand A PG/VG, menthol, and menthol 6 mg nicotine, except RANTES. While the same brand tobacco flavor exposure resulted in similar immunosuppression, tobacco with nicotine elevated G-CSF, RANTES, IL-6, MIP1α, and eotaxin levels. This suggests that though most inflammatory mediators were suppressed, there was an inflammatory response and the potential of nicotine interaction with flavors to augment the immune response. According to Alam et al., asthmatic patients are often seen with increased MCP-1, RANTES, and MIP-1α levels in BALF (Alam et al., 1996). Upregulation of RANTES mRNA and an increase in RANTES protein levels in asthmatics has been demonstrated, suggesting an association between RANTES expression and eosinophilia in asthma and allergic disease (Koya et al., 2006). The response we observed with increased RANTES and intricacies of other inflammatory mediators associated with flavored aerosols-induced dampened proinflammation is consistent with recent studies that have demonstrated the T-lymphocytic correlation in the resolution of allergic asthma in mice exposed to house dust mites (Li et al., 2021a). Consistent with the inflammatory response, proteomics analysis showed a 3.2-fold increase of Muc5b levels in menthol flavor-exposed mice, indicating potential mucociliary dysfunction (Hancock et al., 2018).
With brand A, PG/VG showed some elevated proinflammatory cytokines, such as increased TNFα in BALB/cJ group, though the increases were not significant in other mediators. This augmented response was unique to BALB/cJ mice. These small but differential changes in the PG/VG exposure may be attributed to PG/VG batch differences and chamber/coil heating conditions. Overall, exposure to brand A, PG/VG, menthol, and tobacco, demonstrated a controlled inflammatory response leaning toward immunosuppression or immune tolerance. Consistent with the results and the inflammatory changes observed in this study, regulatory processes, such as immunometabolism are critical to maintaining the homeostasis of an immune response (Chou et al., 2022; Gotts et al., 2019; Lloyd and Murdoch, 2010; Martin et al., 2016). For the comparative analysis of the same flavor manufactured by 2 different brands, brand B flavor exposures were conducted. In PG/VG, menthol, and tobacco-exposed mice, there was a significantly increased neutrophil and CD4-T cell count compared to the air group. While PG/VG and tobacco exposures did not elicit any significant inflammatory cytokine response, menthol exposure caused a significant attenuation in MIP-1β, IL-12p40, and IL12-p70, and a highly significant increase in IL-6 and RANTES levels. These changes once again suggest that there may be an allergic inflammatory response upon exposure to brand B, PG/VG, and menthol flavors, as these immune phenomena have been exemplified by IL-6 mediated asthma/allergic diseases (Neveu et al., 2010; Rincon and Irvin, 2012). Overall, acute e-cig aerosol exposure elicited an immune response demonstrating a tolerogenic response with significant immune dampening.
Exposure to menthol flavor significantly increased TRPA1, which has been shown to play a role in response to exogenous irritants such as acrolein, cinnamaldehyde, and capsaicin. Consistent with our study, TRPA1 has been shown to play a complex role in basal airway function regulation, inflammatory mechanisms, and acute lung injury (Birrell et al., 2009; Caceres et al., 2009; Hajna et al., 2020; Koivisto et al., 2022; Moilanen et al., 2012). With aerosol exposures, a reduction of surfactant protein D (SP-D) was seen, and SP-D has been shown to directly modulate innate immune cell function, pulmonary inflammation, and migration of peripheral monocyte/macrophages into the lung through GM-CSF–dependent pathways during indirect lung injury (King and Kingma, 2011). Further, this may explain slight but significant changes in BALF macrophages in flavor aerosol-exposed mice. Further, tobacco-flavored nicotine-containing aerosol exposure caused a PAI-1 abundance increase in the lung tissues. Nicotine has been shown to increase PAI-1 and to have a direct causal effect in acute lung injury edema fluid-associated mortality (Prabhakaran et al., 2003; Zidovetzki et al., 1999).
Menthol and menthol with nicotine altered NLRP3 inflammasome protein abundance in mice. This is consistent with the immunosuppressive effects and immunomodulatory pathways we identified. NLRP3 inflammasome is the most prominent inflammasome promoting innate and adaptive immune responses and has been seen to destabilize by ubiquitination with cigarette smoke (Han et al., 2017). Growing evidence indicates that NLRP3 may create a metabolic loop in which glycolysis is induced upstream and downstream of NLRP3 inflammasome activation, corroborating the immunometabolic response upon exposure to aerosols in this study (Chou et al., 2022; Finucane et al., 2019).
Further, in the assessment of genotoxicity by brand A menthol and tobacco with nicotine exposures, DNA damage marker H2A.X protein levels were significantly increased. As H2AX formation is intertwined with kinases such as ATM and ATR, it is plausible for the response to be PI3K mediated (Kuo and Yang, 2008; Marechal and Zou, 2013; Ward and Chen, 2001). MDM2, the primary negative regulator of p53, was significantly elevated by all aerosols, PG/VG, menthol and tobacco. This suggests MDM2-p53 may play a critical role in long-term exposure to these flavored ENDS in tumorigenesis, as p53 suppression has been associated with non-small-cell lung cancer (Deben et al., 2016, 2015). We observed significantly elevated levels of p70s6k, a serine/threonine kinase downstream target of mTOR. The mammalian target of rapamycin (mTOR) is pivotal for cellular nutrient processing, anabolic and catabolic homeostasis, and autophagy (Sabatini, 2017). Thus, the increase in p70s6k suggests mTOR involvement in the immunometabolic response we observed. Sinclair et al. demonstrated the function of mTOR in allergic inflammation by mediating the metabolic adaptation of such tissue-resident antigen-presenting cells and the immunological function of allergic inflammation (Sinclair et al., 2017; Wang and Wu, 2018). Our data suggest that aerosol exposure has resulted in an immunological defense and dormancy state due to mTORs mediated immunometabolic processes is consistent with existing research (Linke et al., 2017). The observed increase in p38 protein levels suggests aerosol exposure-induced inflammation, cell cycle, cell differentiation, and tumorigenesis by p38-MAP kinase pathway(Zarubin and Han, 2005).
We used Nanostring technology gene expression analyses to elucidate inflammatory and metabolic changes in aerosol-exposed mice. By assessing genes that are significantly altered (upregulated or downregulated) in DNA damage, cellular metabolism, and inflammation, causally networking responses were observed. These data suggested that the immunosuppressive effects maybe correlated to immunometabolism. Our gene expression analysis corroborated PI3K-mTOR mediated signaling responsible for mitochondrial function, metabolism, and immune response regulation, as we observed significant changes in Rps6kb1 serine/threonine kinase of mTOR and PI3K isoforms.
Further, gene expression analysis also showed Arginase-1 alterations, specifically by menthol flavor exposure. Recent studies have shown arginase 1,2 involvement in effector T-cell mediator immunity (O’Neill et al., 2016). Arginine plays a critical distinction between inflammatory and tolerant cell phenotypes via nitic oxide synthase pathway, consistent with the immunoregulatory effects we observed post aerosol exposure. Extracellular vesicles (EVs) carrying Arg have also been found to cause T-cell immunity in antitumor immune responses (Czystowska-Kuzmicz et al., 2019; Singer and Chandel, 2019; Sosnowska et al., 2019). This is consistent with our gene expression changes where antitumorigenic and metastatic activity was targeted, eg, ldh3a, Zfp869, and Ash1l. This corroborates our data from the treatment of MLE epithelial cells with isolated EVs from tobacco flavor-exposed mice, which showed significantly altered OCR and ECAR compared to the air group, suggesting mitochondrial dysfunction and metabolic dysregulation.
Curated proteomics data from each exposure further solidified changes in protein associated with DNA damage, metabolic changes, and immune cell signaling. All aerosol exposures induced significant upregulation of serpinA3. SerpinA3/Antichymotrypsin has been shown to play a role in the inhibition of proteolytic enzymes as well as modulate oncogenic processes by negatively regulating PI3K-Akt-mTOR pathway (Herrero-Sánchez et al., 2016). Further, serpin3a is associated with extracellular matrix remodeling as well as mutations that have been associated with COPD. Aerosol exposures also significantly downregulated Myl protein levels (up to log2 8-fold change). Allergic inflammation-induced pulmonary vascular remodeling by OVA challenge has been shown to downregulate these muscle proteins significantly (Fan et al., 2015).
Overall, the comparative toxicological analysis of brands A and B containing PG/VG, menthol, menthol 6 mg nicotine, tobacco, and tobacco 6 mg nicotine showed differential immune cell influx in BALF, allergic inflammation, and metabolic reprograming in acute exposure with more immunosuppressive effects by menthol. Flavor interactions with nicotine altered these responses. Potential pathways of allergic inflammation include PI3K-Akt-mTOR mediated immunometabolism. More immunosuppressive effects were observed with menthol flavor from both brands A and B. Though tobacco flavor caused more significantly altered cytokines in C57BL/6J compared to BALB/cJ mice, this strain still showed differential responses in inflammatory mediators and cell counts, suggesting immunoregulatory effects and perhaps is not as susceptible to tobacco flavor constituents. Further, RANTES was identified as a unique player for both tobacco and menthol flavor exposure-induced inflammatory response. Though we did not segregate our data into sex-dependent differences, for specific parameters, we observed more susceptibility in assessed parameters in 1 sex over the other. However, as the differences were statistically not significant due to sample size, sex-specific differences were not a primary focus of this study. The data suggest that T cells were possibly undergoing metabolic reprograming, thus resulting in the suppressed immune response. Our data suggest that chronic exposure to these aerosols may cause lung pathogenesis, including disease susceptibility, reduced bacterial clearance, and potential tumorigenesis.
In conclusion, two brands of the same flavor can cause a differential inflammatory response, genotoxicity, and immunometabolic changes due to flavoring chemical constituents present in the flavored e-liquids and their secondary products in the aerosol. This is likely due to manufacturers utilizing different flavoring chemical compositions containing different constituents, which impart flavor profiles and the purity of ingredients, eg, humectants, nicotine, and solvents. For example, we found high levels of ethanol in the e-liquid aerosols, similar to other studies showing levels of up to 206 mg/ml ethanol in e-liquids (Poklis et al., 2017). Despite our initial hypothesis, by and large, both strains showed similar immunoregulatory responses, but C57BL/6J showed more significant changes in this 3-day acute exposure model. Chronic exposures are needed to assess strain-dependent differences. The potential mechanism of immune-inflammatory responses is associated with the activation of TRPA1 and PAI-1. As demonstrated by significant adverse responses, Brand A is currently not premarket authorization approved. Our study emphasizes the need to characterize the chemicals in flavored e-liquids and ENDS and perform appropriate toxicological testing in vitro and in vivo to provide insights into regulating e-cig liquids.
Limitations of the current study and future directions
As e-cig aerosols are complex mixtures that depend on many factors, such as temperature, humidity, and airflow, it was challenging to conduct acute toxicity estimates for the mixture. Further, we intend to conduct repeat-dose studies (28–90 days) using menthol (and cooling) and tobacco-flavored aerosols and measure lung mechanics parameters with whole-body plethysmography, pulse oximetry, body weight, micronuclei assessment as nonlethal toxicity parameters and indices. Further, in our next studies, we plan to identify additive and synergistic effects of common chemical constituents present in the liquid and the aerosol. Determining the most toxic chemicals and ascertaining their toxicological parameters and indices would be useful in predictive toxicity assessments.
While we kept an on-average record of the particle size distribution (250 mg/m3, PM 1.0), conducting real-time particle size and distribution during exposures was challenging. Hence, in future studies, we intend to integrate a scanning mobility particle sizer for real-time particle size distribution measurements within the exposure chamber.
Another limitation of the study would be inconsistency or incorrect labeling of ingredients on the bottles by manufacturers, such as the nicotine concentration, as we determined marginal levels of nicotine in zero mg flavor liquids, which further emphasizes the need for the proper regulation of e-liquid constituents and enforcements of policies.
Supplementary Material
Acknowledgments
We thank URMC proteomics core for analyzing the lung samples. We thank Dr. Gagandeep Kaur for isolating exosomes from lung tissue from exposed mice. Qiagen IPA trial version was used to generate the canonical pathway diagram.
Contributor Information
Thivanka Muthumalage, Department of Environmental Medicine, School of Medicine and Dentistry, University of Rochester Medical Center, Rochester, New York 14642, USA.
Irfan Rahman, Department of Environmental Medicine, School of Medicine and Dentistry, University of Rochester Medical Center, Rochester, New York 14642, USA.
Disclaimer: The authors have nothing to claim or disclaim about any products used here to test their toxicological and biological effects. The authors have no personal interests or gains from the outcome of this study. No commercial interests with e-liquid manufacturers.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Declaration of conflicting interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest, and no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Author contributions
T.M. and I.R. conceived and designed the experiments. T.M. performed the experiments, analyzed data, and wrote the article. I.R. edited the article.
Funding
US Food and Drug Administration and NIH National Cancer Institute (Tobacco Centers of Regulatory Science, Western New York Center for Research on Flavored Tobacco Products; U54CA238110) and National Institute of Environmental Health Sciences (K99ES033835). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Association.
Data availability
We declare that we have provided all the data, but the primary data will be available upon request.
References
- Alam R., York J., Boyars M., Stafford S., Grant J., A., Lee J., Forsythe P., Sim T., Ida N. (1996). Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 153, 1398–1404. [DOI] [PubMed] [Google Scholar]
- Alexander L. E. C., Bellinghausen A. L., Eakin M. N. (2020). What are the mechanisms underlying vaping-induced lung injury? J. Clin. Invest. 130, 2754–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F. R. M., Seaman E. L., Diaz M. C., Ajose J., King B. A. (2022). Trends in unit sales of cooling flavoured e-cigarettes, USA, 2017-2021. Tob. Control. 1052–1056. doi: 10.1136/tc-2022-057395. [DOI] [PubMed] [Google Scholar]
- Birrell M. A., Belvisi M. G., Grace M., Sadofsky L., Faruqi S., Hele D. J., Maher S., A., Freund-Michel V., Morice A. H. (2009). TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am. J. Respir. Crit. Care Med. 180, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer Z., T., Goel R., Reilly S. M., Foulds J., Muscat J., Elias R. J., Richie J. P. Jr (2018). Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol. 31, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumpton B. M., Camargo C. A. Jr, Romundstad P. R., Langhammer A., Chen Y., Mai X. M. (2013). Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur. Respir. J. 42, 1495–1502. [DOI] [PubMed] [Google Scholar]
- Caceres A. I., Brackmann M., Elia M. D., Bessac B. F., del Camino D., D’Amours M., Witek J. S., Fanger C. M., Chong J. A., Hayward N. J., et al (2009). A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U S A 106, 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W. C., Rampanelli E., Li X., Ting J. P. (2022). Impact of intracellular innate immune receptors on immunometabolism. Cell. Mol. Immunol. 19, 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty Alexander L. E., Drummond C. A., Hepokoski M., Mathew D., Moshensky A., Willeford A., Das S., Singh P., Yong Z., Lee J. H., et al (2018). Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czystowska-Kuzmicz M., Sosnowska A., Nowis D., Ramji K., Szajnik M., Chlebowska-Tuz J., Wolinska E., Gaj P., Grazul M., Pilch Z., et al (2019). Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 10, 3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deben C., Deschoolmeester V., Lardon F., Rolfo C., Pauwels P. (2016). TP53 and MDM2 genetic alterations in non-small cell lung cancer: evaluating their prognostic and predictive value. Crit. Rev. Oncol. Hematol. 99, 63–73. [DOI] [PubMed] [Google Scholar]
- Deben C., Wouters A., Op de Beeck K., van Den Bossche J., Jacobs J., Zwaenepoel K., Peeters M., Van Meerbeeck J., Lardon F., Rolfo C., et al (2015). The MDM2-inhibitor Nutlin-3 synergizes with cisplatin to induce p53 dependent tumor cell apoptosis in non-small cell lung cancer. Oncotarget 6, 22666–22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. C., Donovan E. M., Schillo B. A., Vallone D. (2021). Menthol e-cigarette sales rise following 2020 FDA guidance. Tob. Control. 30, 700–703. [DOI] [PubMed] [Google Scholar]
- Fan C., Meuchel L. W., Su Q., Angelini D. J., Zhang A., Cheadle C., Kolosova I., Makarevich O. D., Yamaji-Kegan K., Rothenberg M. E., et al (2015). Resistin-like molecule alpha in allergen-induced pulmonary vascular remodeling. Am. J. Respir. Cell Mol. Biol. 53, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane O. M., Sugrue J., Rubio-Araiz A., Guillot-Sestier M. V., Lynch M. A. (2019). The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1beta-dependent manner in macrophages. Sci. Rep. 9, 4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi A., Ferraro V. A., Caserotti M., Quareni L., Perilongo G., Baraldi E. (2020). Protecting youth from the vaping epidemic. Pediatr. Allergy Immunol. 31Suppl 26, 66–68. [DOI] [PubMed] [Google Scholar]
- Gotts J. E., Jordt S. E., McConnell R., Tarran R. (2019). What are the respiratory effects of e-cigarettes? Bmj 366, l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajna Z., Cseko K., Kemeny A., Kereskai L., Kiss T., Perkecz A., Szitter I., Kocsis B., Pinter E., Helyes Z. (2020). Complex regulatory role of the TRPA1 receptor in acute and chronic airway inflammation mouse models. Int J Mol Sci 21, 4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger E. S., Halpern-Felsher B. (2020). Vaping in adolescents: epidemiology and respiratory harm. Curr. Opin. Pediatr. 32, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Jerome J. A., Gregory A. D., Mallampalli R. K. (2017). Cigarette smoke destabilizes NLRP3 protein by promoting its ubiquitination. Respir. Res. 18, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock L. A., Hennessy C. E., Solomon G. M., Dobrinskikh E., Estrella A., Hara N., Hill D. B., Kissner W. J., Markovetz M. R., Grove Villalon D. E., et al (2018). Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat. Commun. 9, 5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Sánchez M. C., Rodríguez-Serrano C., Almeida J., San-Segundo L., Inogés S., Santos-Briz Á., García-Briñón J., SanMiguel J. F., Del Cañizo C., Blanco B. (2016). Effect of mTORC1/mTORC2 inhibition on T cell function: potential role in graft-versus-host disease control. Br. J. Haematol. 173, 754–768. [DOI] [PubMed] [Google Scholar]
- Hickman E., Herrera C. A., Jaspers I. (2019). Common E-cigarette flavoring chemicals impair neutrophil phagocytosis and oxidative burst. Chem. Res. Toxicol. 32, 982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M., Omaiye E. E., Luo W., McWhirter K. J., Pankow J. F., Talbot P. (2019). Identification of cytotoxic flavor chemicals in top-selling electronic cigarette refill fluids. Sci. Rep. 9, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabba S. V., Diaz A. N., Erythropel H. C., Zimmerman J. B., Jordt S. E. (2020). Chemical adducts of reactive flavor aldehydes formed in E-cigarette liquids are cytotoxic and inhibit mitochondrial function in respiratory epithelial cells. Nicotine Tob. Res. 22, S25–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Muthumalage T., Rahman I. (2018). Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 288, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A., Samburova V. (2016). Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol. 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- King B. A., Kingma P. S. (2011). Surfactant protein D deficiency increases lung injury during endotoxemia. Am. J. Respir. Cell Mol. Biol. 44, 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto A. P., Belvisi M. G., Gaudet R., Szallasi A. (2022). Advances in TRP channel drug discovery: from target validation to clinical studies. Nat. Rev. Drug Discov. 21, 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya T., Takeda K., Kodama T., Miyahara N., Matsubara S., Balhorn A., Joetham A., Dakhama A., Gelfand E. W. (2006). RANTES (CCL5) regulates airway responsiveness after repeated allergen challenge. Am. J. Respir. Cell Mol. Biol. 35, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., , GreenJ., , PollardJ., Jr, and , Tugendreich S. (2014). Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L. J., Yang L. X. (2008). Gamma-H2AX – a novel biomarker for DNA double-strand breaks. In Vivo 22, 305–309. [PubMed] [Google Scholar]
- Lamb T., Muthumalage T., Meehan-Atrash J., Rahman I. (2022). Nose-only exposure to cherry- and tobacco-flavored e-cigarettes induced lung inflammation in mice in a sex-dependent manner. Toxics 10, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T., Muthumalage T., Rahman I. (2020). Pod-based menthol and tobacco flavored e-cigarettes cause mitochondrial dysfunction in lung epithelial cells. Toxicol. Lett. 333, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W., Lerner C., Sundar I. K., Rahman I. (2017). Myofibroblast differentiation and its functional properties are inhibited by nicotine and e-cigarette via mitochondrial OXPHOS complex III. Sci. Rep. 7, 43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C. A., Sundar I. K., Watson R. M., Elder A., Jones R., Done D., Kurtzman R., Ossip D. J., Robinson R., McIntosh S., et al (2015). Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. Environ. Pollut. 198, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. T., Yuan Z., Li Y., Mays D., Sanchez-Romero L. M. (2019). An examination of the variation in estimates of e-cigarette prevalence among U.S. adults. Int J Environ Res Public Health 16, 3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Mirzakhani H., Kiefer A., Koelle J., Vuorinen T., Rauh M., Yang Z., Krammer S., Xepapadaki P., Lewandowska-Polak A., et al (2021a). Regulated on Activation, Normal T cell Expressed and Secreted (RANTES) drives the resolution of allergic asthma. iScience 24, 103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Burns A. E., Tran L. N., Abellar K. A., Poindexter M., Li X., Madl A. K., Pinkerton K. E., Nguyen T. B. (2021b). Impact of e-liquid composition, coil temperature, and puff topography on the aerosol chemistry of electronic cigarettes. Chem. Res. Toxicol. 34, 1640–1654. [DOI] [PubMed] [Google Scholar]
- Linke M., Fritsch S. D., Sukhbaatar N., Hengstschlager M., Weichhart T. (2017). mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. 591, 3089–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. M., Murdoch J. R. (2010). Tolerizing allergic responses in the lung. Mucosal Immunol. 3, 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. H., Muthumalage T., Wang Q., Friedman M. R., Friedman A. E., Rahman I. (2020). E-liquid containing a mixture of coconut, vanilla, and cookie flavors causes cellular senescence and dysregulated repair in pulmonary fibroblasts: implications on premature aging. Front. Physiol. 11, 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal A., Zou L. (2013). DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5, a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. M., Clapp P. W., Rebuli M. E., Pawlak E. A., Glista-Baker E., Benowitz N. L., Fry R. C., Jaspers I. (2016). E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M., Reyes-Guzman C., Grana R., Choi K., Freedman N. D. (2020). Demographic characteristics, cigarette smoking, and e-cigarette use among US adults. JAMA Netw. Open. 3, e2020694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. C., Raghu G., Baughman R. P., Brown K. K., Costabel U., du Bois R. M., Drent M., Haslam P. L., Kim D. S., Nagai S., et al; American Thoracic Society Committee on BAL in Interstitial Lung Disease. (2012). An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 185, 1004–1014. [DOI] [PubMed] [Google Scholar]
- Miech R., Leventhal A., Johnston L., O’Malley P. M., Patrick M. E., Barrington-Trimis J. (2021). Trends in use and perceptions of nicotine vaping among US youth from 2017 to 2020. JAMA Pediatr. 175, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen L. J., Laavola M., Kukkonen M., Korhonen R., Leppanen T., Hogestatt E. D., Zygmunt P. M., Nieminen R. M., Moilanen E. (2012). TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci. Rep. 2, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Friedman M. R., McGraw M. D., Ginsberg G., Friedman A. E., Rahman I. (2020). Chemical constituents involved in e-cigarette, or vaping product use-associated lung injury (EVALI). Toxics 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Lamb T., Friedman M. R., Rahman I. (2019). E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 9, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T., Prinz M., Ansah K. O., Gerloff J., Sundar I. K., Rahman I. (2017). Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 8, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu W. A., Allard J. L., Raymond D. M., Bourassa L. M., Burns S. M., Bunn J. Y., Irvin C. G., Kaminsky D. A., Rincon M. (2010). Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir. Res. 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L. (2017). Immunometabolism and the land of milk and honey. Nat. Rev. Immunol. 17, 217. [DOI] [PubMed] [Google Scholar]
- O’Neill L. A., Kishton R. J., Rathmell J. (2016). A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye E. E., McWhirter K. J., Luo W., Tierney P. A., Pankow J. F., Talbot P. (2019). High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 9, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-McDermott E. M., O’Neill L. A. J. (2020). Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 30, 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis J. L., Wolf C. E. 2nd, Peace M. R. (2017). Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID). Drug Test. Anal. 9, 1637–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran P., Ware L. B., White K. E., Cross M. T., Matthay M. A., Olman M. A. (2003). Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L20–8. [DOI] [PubMed] [Google Scholar]
- Rincon M., Irvin C. G. (2012). Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 8, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. M. (2017). Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. U S A 114, 11818–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair C., Bommakanti G., Gardinassi L., Loebbermann J., Johnson M. J., Hakimpour P., Hagan T., Benitez L., Todor A., Machiah D., et al (2017). mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science 357, 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. D., Chandel N. S. (2019). Immunometabolism of pro-repair cells. J. Clin. Invest. 129, 2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowska A., Czystowska-Kuzmicz M., Golab J. (2019). Extracellular vesicles released by ovarian carcinoma contain arginase 1 that mitigates antitumor immune response. Oncoimmunology 8, e1655370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafran B. N., Pinkston R., Perveen Z., Ross M. K., Morgan T., Paulsen D. B., Penn A. L., Kaplan B. L. F., Noel A. (2020). Electronic-cigarette vehicles and flavoring affect lung function and immune responses in a murine model. Int J Mol Sci 21, 6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Sundar I. K., Li D., Lucas J. H., Muthumalage T., McDonough S. R., Rahman I. (2020). E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR alpha7 receptor: role of nAChR alpha7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res. 21, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wu H. (2018). T cells in adipose tissue: critical players in immunometabolism. Front. Immunol. 9, 2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward I. M., Chen J. (2001). Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47759–47762. [DOI] [PubMed] [Google Scholar]
- Zarubin T., Han J. (2005). Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Chen P., Fisher M., Hofman F. M., Faraci F. M. (1999). Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-associated pathway. Stroke 30, 651–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We declare that we have provided all the data, but the primary data will be available upon request.