This systematic review and meta-analysis investigates the prevalence of urinary tract abnormalities detected on kidney ultrasonography after first febrile urinary tract infection in children.
Key Points
Question
What is the prevalence of urinary tract abnormalities detected on kidney ultrasonography after the first febrile urinary tract infection (UTI) in children?
Findings
In this systematic review and meta-analysis of 29 studies and 9170 children, the prevalence of abnormalities detected on kidney ultrasonography was 22.1%. Of the 8 studies with 2569 children that reported clinically important abnormalities, the prevalence was 3.1%.
Meaning
Study results suggest that 1 in 4 to 5 children with a first febrile UTI will have a urinary tract abnormality detected on kidney ultrasonography and 1 in 32 will have an abnormality that changes clinical management.
Abstract
Importance
Controversy exists on the clinical utility of kidney ultrasonography after first febrile urinary tract infection (UTI), and clinical practice guideline recommendations vary.
Objective
To determine the prevalence of urinary tract abnormalities detected on kidney ultrasonography after the first febrile UTI in children.
Data Sources
The MEDLINE, EMBASE, CINAHL, PsycINFO, and Cochrane Central Register of Controlled Trials databases were searched for articles published from January 1, 2000, to September 20, 2022.
Study Selection
Studies of children with first febrile UTI reporting kidney ultrasonography findings.
Data Extraction and Synthesis
Two reviewers independently screened titles, abstracts, and full texts for eligibility. Study characteristics and outcomes were extracted from each article. Data on the prevalence of kidney ultrasonography abnormalities were pooled using a random-effects model.
Main Outcomes and Measures
The primary outcome was prevalence of urinary tract abnormalities and clinically important abnormalities (those that changed clinical management) detected on kidney ultrasonography. Secondary outcomes included the urinary tract abnormalities detected, surgical intervention, health care utilization, and parent-reported outcomes.
Results
Twenty-nine studies were included, with a total of 9170 children. Of the 27 studies that reported participant sex, the median percentage of males was 60% (range, 11%-80%). The prevalence of abnormalities detected on renal ultrasonography was 22.1% (95% CI, 16.8-27.9; I2 = 98%; 29 studies, all ages) and 21.9% (95% CI, 14.7-30.1; I2 = 98%; 15 studies, age <24 months). The prevalence of clinically important abnormalities was 3.1% (95% CI, 0.3-8.1; I2 = 96%; 8 studies, all ages) and 4.5% (95% CI, 0.5-12.0; I2 = 97%; 5 studies, age <24 months). Study recruitment bias was associated with a higher prevalence of abnormalities. The most common findings detected were hydronephrosis, pelviectasis, and dilated ureter. Urinary tract obstruction was identified in 0.4% (95% CI, 0.1-0.8; I2 = 59%; 12 studies), and surgical intervention occurred in 1.4% (95% CI, 0.5-2.7; I2 = 85%; 13 studies). One study reported health care utilization. No study reported parent-reported outcomes.
Conclusions and Relevance
Results suggest that 1 in 4 to 5 children with first febrile UTI will have a urinary tract abnormality detected on kidney ultrasonography and 1 in 32 will have an abnormality that changes clinical management. Given the considerable study heterogeneity and lack of comprehensive outcome measurement, well-designed prospective longitudinal studies are needed to fully evaluate the clinical utility of kidney ultrasonography after first febrile UTI.
Introduction
Urinary tract infection (UTI) is a common bacterial infection in children that occurs in 1.7% of boys and 8.4% of girls before 7 years of age.1 It is the eighth most common reason for hospitalization in children in the US and is cumulatively costly.2 In North America, performing ultrasonography of the kidneys and bladder, a noninvasive, nonionizing, and readily available imaging modality, is common practice in all children younger than 2 years after the first febrile UTI.3,4 The rationale is identifying urinary tract abnormalities and severe vesicoureteral reflux predisposing children to recurrent UTIs and voiding dysfunction. After detection of abnormalities on kidney ultrasonography, further investigation and intervention may prevent future UTIs, kidney damage, and chronic kidney disease.3
American Academy of Pediatrics and Canadian Paediatrics Society guidelines recommend performing kidney ultrasonography in all children 2 to 24 months of age and younger than 24 months of age, respectively, after the first febrile UTI.3,4 However, guidelines in other regions differ.5 Some recommend performing kidney ultrasonography after first febrile UTI even beyond 24 months (eg, up to 36 months or at any age),6,7,8 and others are more selective.9,10 The UK’s National Institute of Clinical Excellence (NICE) recommends kidney ultrasonography in children younger than 6 months and only in children older than 6 months to 16 years if the UTI is recurrent or atypical.10
The variation in guideline recommendations reflects uncertainty around the clinical utility of kidney ultrasonography after first febrile UTI, ie, the measurable net-positive effect on a child’s clinical outcome.11 Kidney ultrasonography has poor sensitivity (40%) and only fair specificity (75%) in detecting even high-grade vesicoureteral reflux.12,13,14,15 In the current era of routine and later trimester prenatal ultrasonography, the detection rate of new, serious urinary tract abnormalities in children presenting with their first UTI is estimated to be low.4 There is also growing recognition and concern with overdiagnosis, detecting an abnormality from which a patient does not receive a net benefit.16 Detecting insignificant abnormalities on kidney ultrasonography may result in a cascade of unnecessary care and costs.
To better understand the clinical utility of kidney ultrasonography, we conducted a systematic review and meta-analysis of the prevalence of urinary tract abnormalities and clinically important abnormalities detected on kidney ultrasonography after the first febrile UTI in children. We also reviewed studies for secondary outcomes, including the specific types of abnormalities detected, need for surgical intervention, health care utilization after kidney ultrasonography, and parent-reported outcomes.
Methods
This systematic review and meta-analysis was conducted from May 3, 2021, to March 10, 2023. The protocol was registered at PROSPERO (CRD42021265011). This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.17
Information Sources and Search Strategy
A medical librarian (Q.M.) searched MEDLINE (OVID), EMBASE (OVID), CINAHL (EBSCO), PsycINFO (OVID), and Cochrane Central Register of Controlled Trials (OVID) databases for articles published from January 1, 2000, to September 20, 2022, with the terms “urinary tract infection,” “ultrasonography,” and “infant.” We limited our search to articles published after 2000 given the advancements in ultrasonography technology (eg, higher resolution), which might affect the detection of urinary tract abnormalities.18 The search strategies for all electronic databases can be found in the online-only supplement (eMethods in Supplement 1). We also searched on clinical trial registries of (ClinicalTrials.gov, World Health Organization International Clinical Trials Portal), checked reference lists of included studies, and contacted experts in the field to identify any unpublished studies.
Study Selection
Studies were included if they reported ultrasonography findings in children younger than 24 months of age with first febrile UTI. We also included studies of older children (as long as they included children younger than 24 months) as some practice guidelines recommend kidney ultrasonography in children older than 24 months with a febrile UTI.5,6,7,8 All study designs, including observational studies and randomized clinical trials, were eligible for inclusion. Studies were excluded if the population (1) included children who did not have fever or if studies did not state that fever was an inclusion criterion; (2) included children who had recurrent UTI (ie, not the first episode UTI) or if studies did not state that first episode UTI was an inclusion criterion; (3) were case series of fewer than 50 patients; or (4) included non-English language. Titles and abstracts were first screened independently for eligibility by 2 reviewers (S.Y. and M.R.A.). The same 2 authors then reviewed the full texts of potentially relevant articles. Any discrepancies were resolved through discussion and adjudication with a third reviewer (S.M.) if necessary.
Data Extraction
For full-text articles meeting the inclusion criteria, data extraction occurred independently by 3 authors (S.Y., M.R.A., S.M.), and discrepancies were resolved through discussion until agreement was reached. Data were extracted on study characteristics and methods, participant characteristics, ultrasonography intervention details, and primary and secondary outcomes. As few studies reported participant race and ethnicity, we did not include these data. We did not have any a priori hypothesis around race and ethnicity and detection of abnormalities on kidney ultrasonography.
Outcomes
The primary outcomes were the prevalence of any urinary tract abnormality and clinically important abnormality on kidney ultrasonography. Although a priori we considered a classification scheme to define clinically important abnormalities, only a few studies reported this outcome. For the remainder, the adjudication of clinically important outcomes was not possible due to insufficient detail from the data reported in studies.19 Therefore, we defined clinically important abnormality as defined by each study. Broadly, studies defined this outcome as an ultrasonography finding that changed clinical management (eTable 1 in Supplement 1). One study by Wallace et al19 had 2 levels of abnormalities (moderate and severe) that could lead to a change in management. We dichotomized their outcome to severe (clinically important) and nonsevere, as was done in their study. Secondary outcomes included the specific types of abnormalities detected (eg, urinary tract obstruction), need for surgical intervention, health care utilization associated with kidney ultrasonography (eg, specialist visits, further imaging investigations), and parent-reported outcomes (eg, anxiety with abnormal test result).
Quality Assessment
The quality of included studies was assessed independently by 3 authors (S.Y., M.R.A., and S.M.) for all included studies, with any disagreement resolved by discussion. The Joanna Briggs Institute Prevalence Critical Appraisal Tool (JBI) was used to assess observational studies with prevalence as the primary outcome. This tool consists of 10 items rated yes, no, unclear, or not applicable.20 We adapted the tool to include 11 items, expanding “evaluating whether objective, standard criteria were used for the measurement of the condition” to 2 separate items: 1 for UTI diagnosis and 1 for urinary tract abnormality detection on ultrasonography (eTable 2 in Supplement 1).
Statistical Analyses
To obtain the pooled prevalence of any urinary tract abnormality, clinically important abnormality detected on kidney ultrasonography, and secondary outcomes, we used a random-effects model using the restricted maximum-likelihood procedures.21 Proportions were transformed using the double arcsine transformation before pooling study estimates.22 This transformation method avoids an unduly large weight for studies with very low or high proportions.23 For the outcome of any urinary tract abnormality, we conducted a sensitivity analysis by removing outlier studies and reestimating the pooled prevalence.24 For the outcome of clinically important abnormality, we conducted a sensitivity analysis by using an alternate prevalence of clinically important abnormality in the Wallace et al19 study, defined by both moderate and severe abnormalities.
To investigate heterogeneity, we conducted prespecified subgroup analyses to obtain the pooled prevalence of any urinary tract abnormality for the following groups: age (inclusion of children <24 months vs <24 months and ≥24 months) as children with urinary tract abnormalities might present earlier in childhood with UTI; recruitment end date (2000 to 2010 vs ≥2011) given the advances in ultrasonography technology; study design (retrospective vs prospective); study setting (hospitalized vs ambulatory with or without hospitalized) as the higher severity of illness hospitalized population may have a higher prevalence of abnormalities; and whether the UTI definition included pyuria (yes vs no) as the absence of pyuria may be associated with false-positive detection of infection (eg, contaminated specimens) and, thus, lower detection of abnormalities. We also conducted subgroup analysis of 2 study quality items: “were study participants recruited in an appropriate and clear way?” (yes vs no or unclear) and “were standard criteria described for abnormalities on renal ultrasound?” (yes vs no). We also conducted a subgroup analysis to obtain the pooled prevalence of clinically important abnormality by age (inclusion of children <24 months vs <24 months and ≥24 months). In the subgroup analyses, a mixed-effects model was used. In this model, the random effects were used to model variability in study effects within each subgroup, and fixed effects were used to model the difference in effects across subgroups.25 Post hoc, a random-effects meta-regression26 of proportions using the double arcsine transformation, was undertaken to further explore sources of heterogeneity in the prevalence of any abnormality. Based on the number of studies, 3 variables were investigated: sex, the percentage of female children in each study (continuous); study recruitment year end date (continuous); and the study quality item related to appropriate and clear patient recruitment (dichotomous).
All reported CIs represent 95% CIs, and a P value <.05 was considered significant. Heterogeneity was evaluated using the I2 statistic. Heterogeneity was classified as mild (0%-30%), moderate (31%-50%), substantial (51%-80%), and considerable (81%-100%). All statistical analyses were conducted with R software, version 4.0.3 (R Project for Statistical Computing) using the meta and metafor packages.27,28
Results
Search Results
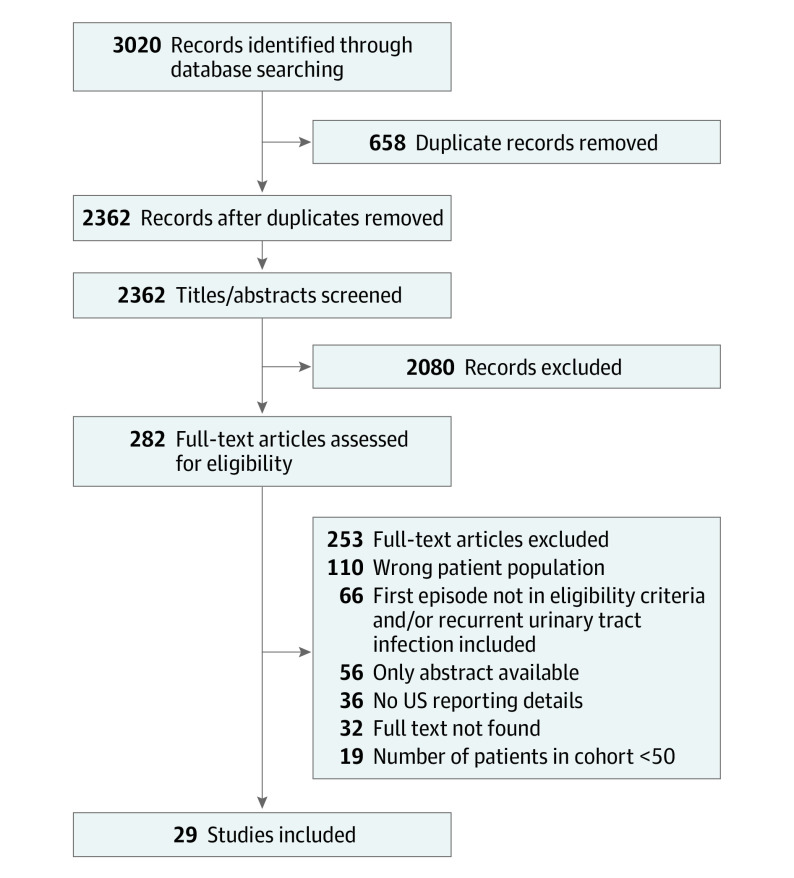
The search resulted in a total of 3020 studies. After removing 658 duplicates, 2362 studies were included for screening. A total of 2080 studies were excluded during title and abstract screening, leaving 282 studies for full-text screening. After 253 full-text articles were excluded, 29 studies remained for the final analysis with a total of 9170 children (Figure 1).14,19,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55
Figure 1. Flow Diagram of Study Selection.
Quality Assessment
Of the 29 included studies, 16 studies14,19,29,30,34,35,36,37,42,43,44,46,48,50,52,53 were representative of the target population of children with first febrile UTI who were younger than 24 months of age; those studies that were not included older children as well (eTable 3 in Supplement 1). Twenty studies14,19,29,30,34,35,36,37,39,40,41,43,46,47,49,50,51,52,53,54 recruited participants appropriately and clearly; those that did not commonly included children based on the performance of an additional imaging test (eg, voiding cystourethrogram). All but 3 studies43,50,54 reported the study participants and setting in detail. Two studies46,50 did not report criteria for the diagnosis of UTI (eTable 4 in Supplement 1). Twenty-three studies14,19,29,30,31,32,33,34,35,36,37,39,40,41,42,43,44,48,50,51,52,53,54 reported criteria for abnormalities seen on kidney ultrasonography, and 10 studies19,29,32,37,38,41,42,46,48,51 reported criteria and/or a grading system for hydronephrosis, of which 6 studies19,29,37,40,42,48 used the Society of Fetal Urology system (eTable 5 in Supplement 1).56 No study reported a statistical analysis plan for prevalence with 95% CIs. Only 2 studies19,51 reported the rationale for the study sample size relevant to the outcome of prevalence of abnormalities. Only 1 study37 had insufficient coverage of the identified sample for the outcome data of ultrasonography abnormalities (ie, missing data). Seven studies19,32,33,34,44,47,54 reported subgroup differences by age and/or sex.
Study Characteristics
Of the 29 included studies, 9 studies14,30,41,48,49,50,51,52,53 were published between 2000 and 2010, whereas 20 studies19,29,31,32,33,34,35,36,37,38,39,40,42,43,44,45,46,47,54,55 were published between 2011 and 2022 (Table). Nineteen studies29,30,31,32,33,36,37,38,40,41,43,44,46,47,48,49,53,54,55 were retrospective, and 10 studies14,19,34,35,39,42,45,50,51,52 were prospective cohorts. No randomized clinical trials or cohort studies compared kidney ultrasonography with first febrile UTI vs targeted strategies (eg, deferring until second febrile UTI). Twenty-four studies19,29,30,31,32,35,36,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,55 were conducted in children hospitalized for UTI, 1 in children exclusively in an ambulatory setting,14 and 3 in both settings.33,34,37 Fifteen studies14,18,29,30,34,35,36,37,42,43,44,46,48,52,53 only included children younger than 24 months. Of the 27 studies14,19,29,30,31,32,33,34,35,36,38,39,40,41,42,43,44,45,46,47,48,50,51,52,53,54,55 that reported sex, the median percentage of females was 40% (range, 20%-89%), and the median percentage of males was 60% (range, 11%-80%). Thirteen studies14,19,29,31,34,35,39,40,42,44,52,53,55 included pyuria in their UTI diagnostic criteria, and 23 studies14,19,29,30,31,32,33,34,35,36,37,39,40,41,42,43,44,48,50,51,52,53,54 defined abnormal ultrasonography results. The study sample size ranged from 66 to 820 children (median, 300 children). Of the 21 studies14,19,29,30,31,32,33,34,35,36,37,39,40,41,42,43,45,47,51,53,54 that reported the causative bacteria, Escherichia coli was the main causative bacteria (median, 86% [range, 67%-96%]). Fifteen14,19,30,34,35,36,38,39,41,43,45,47,48,51,55 of 2514,19,29,30,32,34,35,36,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55 studies that included data on kidney ultrasonography timing reported performing ultrasonography during the hospital admission or within a week after diagnosis of UTI. No studies reported delaying the ultrasonography for concern of false-positive detection of abnormalities during the acute infection.
Table. Study Characteristics.
| Author and publication year | Country | Study design | Recruitment end date | Study setting (hospitalized, nonhospitalized, both) | Sample size, No. | Study age range eligibility, mo | Mean or median age of study population, moa | Female sex, No. (%) | Male sex, No. (%) | Pyuria included in UTI definition | Abnormal ultrasonography definition | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Described in study | Hydronephrosis grading described in study | |||||||||||
| Arlen et al,37 2014 | US | Retrospective | 2012 | Both | 234 | 2-24 | 9 | NR | NR | N | Y | Yb |
| Breinbjerg et al,32 2021 | Denmark | Retrospective | 2013 | Hospitalized | 421 | 0-168 | 52.8 | 299 (71) | 122 (29) | N | Y | Y |
| Cheng et al,38 2011 | Taiwan | Retrospective | 2007 | Hospitalized | 545 | 0-60 | 8.1 | 209 (38) | 336 (62) | N | N | Y |
| Han et al,55 2016 | South Korea | Retrospective | 2014 | Hospitalized | 298 | 0-36 | 4.4 | 84 (28) | 214 (72) | Y | N | N |
| Harper et al,43 2016 | France | Retrospective | 2012 | Hospitalized | 318 | 2-24 | 6.9 | 174 (55) | 144 (45) | N | Y | N |
| Hoberman et al,14 2003 | US | Prospective | 1997 | Nonhospitalized | 309 | 1-24 | 8.4 | 276 (89) | 33 (11) | Y | Y | N |
| Hsu et al,34 2016 | Taiwan | Prospective | NR | Both | 388 | 0-24 | 6.1 | 133 (34) | 255 (66) | Y | Y | N |
| Huang et al,49 2008 | Taiwan | Retrospective | 2006 | Hospitalized | 390 | 0-60 | NR | NR | NR | N | N | N |
| Hung et al,35 2016 | Taiwan | Prospective | NR | Hospitalized | 310 | 0-24 | 5a | 115 (37) | 195 (63) | Y | Y | N |
| Ipek et al,45 2012 | Turkey | Prospective | 2009 | Hospitalized | 66 | 1-168 | 38.0 | 47 (71) | 19 (29) | N | N | N |
| Ismaili et al,39 2011 | Belgium | Prospective | 2008 | Hospitalized | 209 | 0.2-204 | 10a | 132 (63) | 77 (37) | Y | Y | N |
| Jahnukainen et al,41 2006 | Finland | Retrospective | 2000 | Hospitalized | 155 | 0-192 | 6.0 | 91 (59) | 64 (41) | N | Y | Y |
| Kawai et al,42 2019 | Japan | Prospective | 2018 | Hospitalized | 74 | 0-12 | 4.5a | 22 (30) | 52 (70) | Y | Y | Yb |
| Kobayashi et al,29 2019 | Japan | Retrospective | 2011 | Hospitalized | 231 | 0-24 | 4.2a | 78 (34) | 153 (66) | Y | Y | Yb |
| Lee et al,48 2009 | Taiwan | Retrospective | 2004 | Hospitalized | 699 | 2-24 | 5.8 | 216 (31) | 483 (69) | N | Y | Yb |
| Lertdumrongluk and Lertdumrongluk,31 2021 | Thailand | Retrospective | 2019 | Hospitalized | 260 | 0-72 | 4.0a | 88 (34) | 172 (66) | Y | Y | N |
| Lytzen et al,47 2011 | Denmark | Retrospective | 2006 | Hospitalized | 96 | 0.6-179 | 8.4a | 61 (64) | 35 (36) | N | N | N |
| Miron et al,50 2007 | Israel | Prospective | NR | Hospitalized | 209 | 0-60 | 10a | 161 (77) | 48 (23) | N | Y | N |
| Montini et al,52 2009 | Italy | Prospective | 2005 | Hospitalized | 300 | 0-24 | 7.0a | 188 (63) | 112 (37) | Y | Y | N |
| Pauchard et al,36 2017 | Switzerland | Retrospective | 2013 | Hospitalized | 122 | 0-3 | 1.4 | 25 (20) | 97 (80) | N | Y | N |
| Pennesi et al,40 2012 | Italy | Retrospective | NR | Hospitalized | 406 | 1-36 | 9.5 | 276 (68) | 130 (32) | Y | Y | Nb |
| Pennesi et al,33 2021 | Italy | Retrospective | 2018 | Both | 263 | 2-36 | 8.5a | 167 (64) | 96 (36) | NR | Y | Y |
| Sasaki et al,46 2012 | US | Retrospective | 2009 | Hospitalized | 471 | 0-12 | NR | 161 (34) | 310 (66) | NR | N | N |
| Soccorso et al,30 2010 | UK | Retrospective | 2006 | Hospitalized | 427 | 0-12 | 5.2a | 169 (40) | 258 (60) | N | Y | N |
| Wallace et al,19 2020 | US | Prospective | 2018 | Hospitalized | 211 | 0-24 | 1.0a | 84 (40) | 127 (60) | Y | Y | Yb |
| Wongbencharat et al,44 2016 | Thailand | Retrospective | 2010 | Hospitalized | 387 | 0-12 | NR | 150 (39) | 237 (61) | Y | Y | N |
| Wong et al,53 2010 | Hong Kong | Retrospective | 2006 | Hospitalized | 820 | 0-24 | 3.8a | 244 (30) | 576 (70) | Y | Y | N |
| Yilmaz et al,54 2016 | Turkey | Retrospective | 2013 | Not reported | 300 | 0->60 | 11a | 233 (78) | 67 (22) | N | Y | N |
| Zamir et al,51 2004 | Israel | Prospective | 2000 | Hospitalized | 255 | 0-60 | 16 | 192 (75) | 63 (25) | N | Y | Y |
Abbreviations: N, no; NR, not reported; UTI, urinary tract infection; Y, yes.
Median.
Used the Society of Fetal Urology grading system of hydronephrosis.
Any Kidney Ultrasonography Abnormality
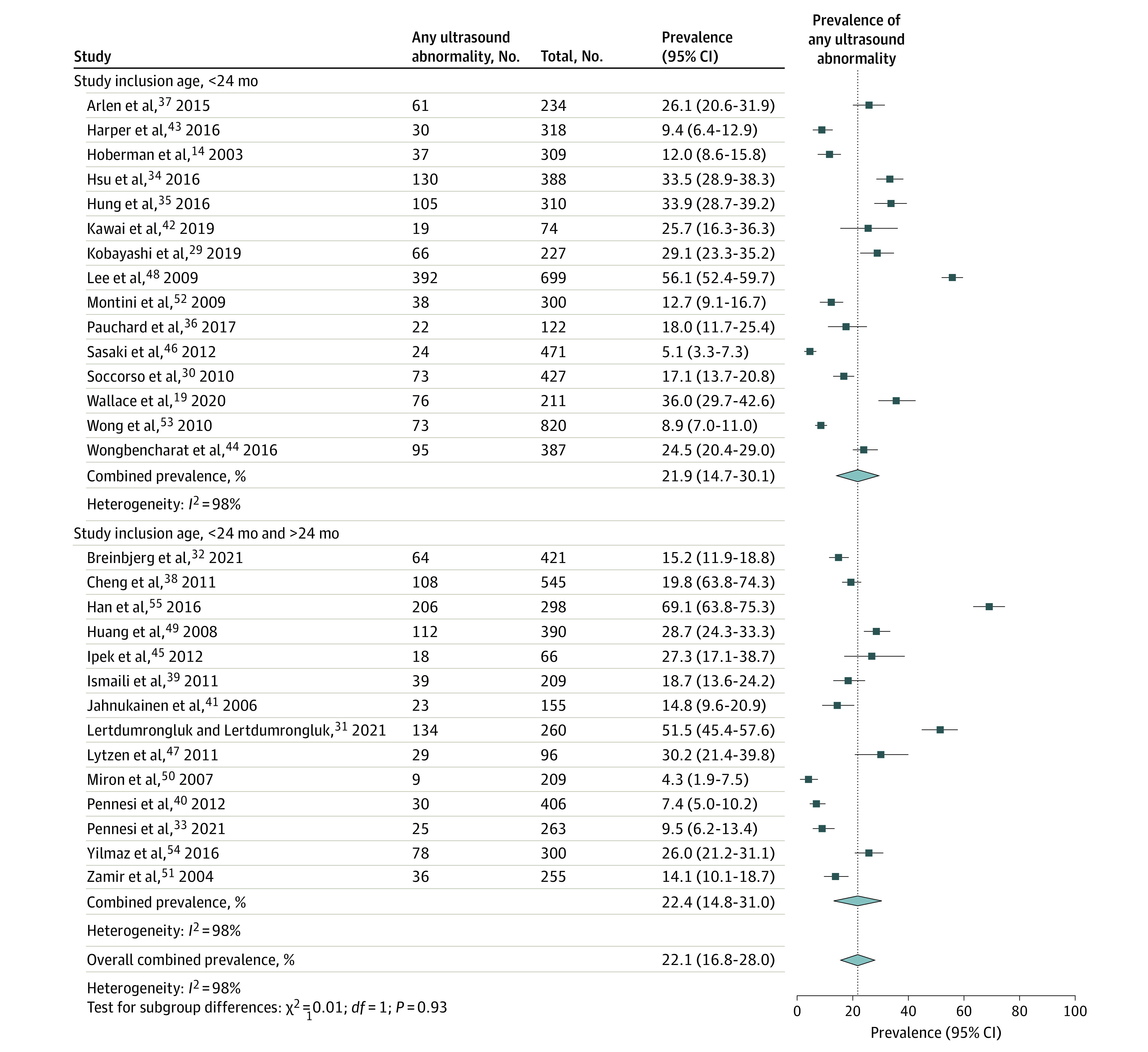
Of the 29 included studies with 9170 children, the pooled prevalence of urinary tract abnormality was 22.1% (95% CI, 16.8-27.9; I2 = 98%). The prevalence of any abnormality was not statistically different in studies limited to children younger than 24 months (21.9%; 95% CI, 14.7-30.1; I2 = 98%) compared with studies that also included children older than 24 months (22.4%; 95% CI, 14.8-31.0; I2 = 98%; P = .93) (Figure 2). In a sensitivity analysis that removed 2 outlier studies,48,55 the pooled prevalence of urinary tract abnormality was similar (eResults in Supplement 1).
Figure 2. Pooled Prevalence of Urinary Tract Abnormalities Detected on Kidney Ultrasonography After First Febrile Urinary Tract Infection in Children.
Subgroup Analyses
The prevalence of abnormalities detected on kidney ultrasonography was not statistically different in prospective studies (20.6%; 95% CI, 12.1-30.7; I2 = 95%) compared with retrospective studies (22.9%; 95% CI, 16.3-30.3; I2 = 98%; P = .71) (eFigure 1 in Supplement 1); in studies that ended recruitment before 2010 (17.8%; 95% CI, 11.2-25.6; I2 = 98%) compared with studies whose recruitment ended after 2010 (27.2%; 95% CI, 18.7-36.7; I2 = 98%; P = .11); in studies that only included children who were hospitalized (22.5%; 95% CI, 16.5-29.1; I2 = 98%) compared with studies that included children in the ambulatory setting (19.3%; 95% CI, 7.1-35.6; I2 = 96%; P = .70); and in studies that included pyuria as a criterion for UTI diagnosis (26.4%; 95% CI, 18.3-35.4; I2 = 98%) compared with studies that did not include pyuria (18.8%; 95% CI, 12.4-26.2; I2 = 98%; P = .18).
In subgroup analyses exploring study quality as a source of heterogeneity, studies with clear and appropriate recruitment had a significantly lower prevalence of abnormalities on kidney ultrasonography (18.1%; 95% CI, 12.8-24.2; I2 = 96%) compared with studies that did not (32.1%; 95% CI, 22.2-42.7; I2 = 99%; P = .02). The prevalence of abnormalities for studies that reported criteria for abnormal ultrasonography results (20.6%; 95% CI, 15.0-27.0; I2 = 98%) was not statistically different compared with those that did not report criteria (28.3%; 95% CI, 16.1-42.2; I2 = 99%; P = .29).
Meta-regression
In a multivariable meta-regression, there was no statistically significant association between prevalence of abnormality and study female sex percentage (inverse association), year of recruitment end (direct association), and the quality item related to appropriate and clear recruitment (inverse association).
Clinically Important Kidney Ultrasonography Abnormalities
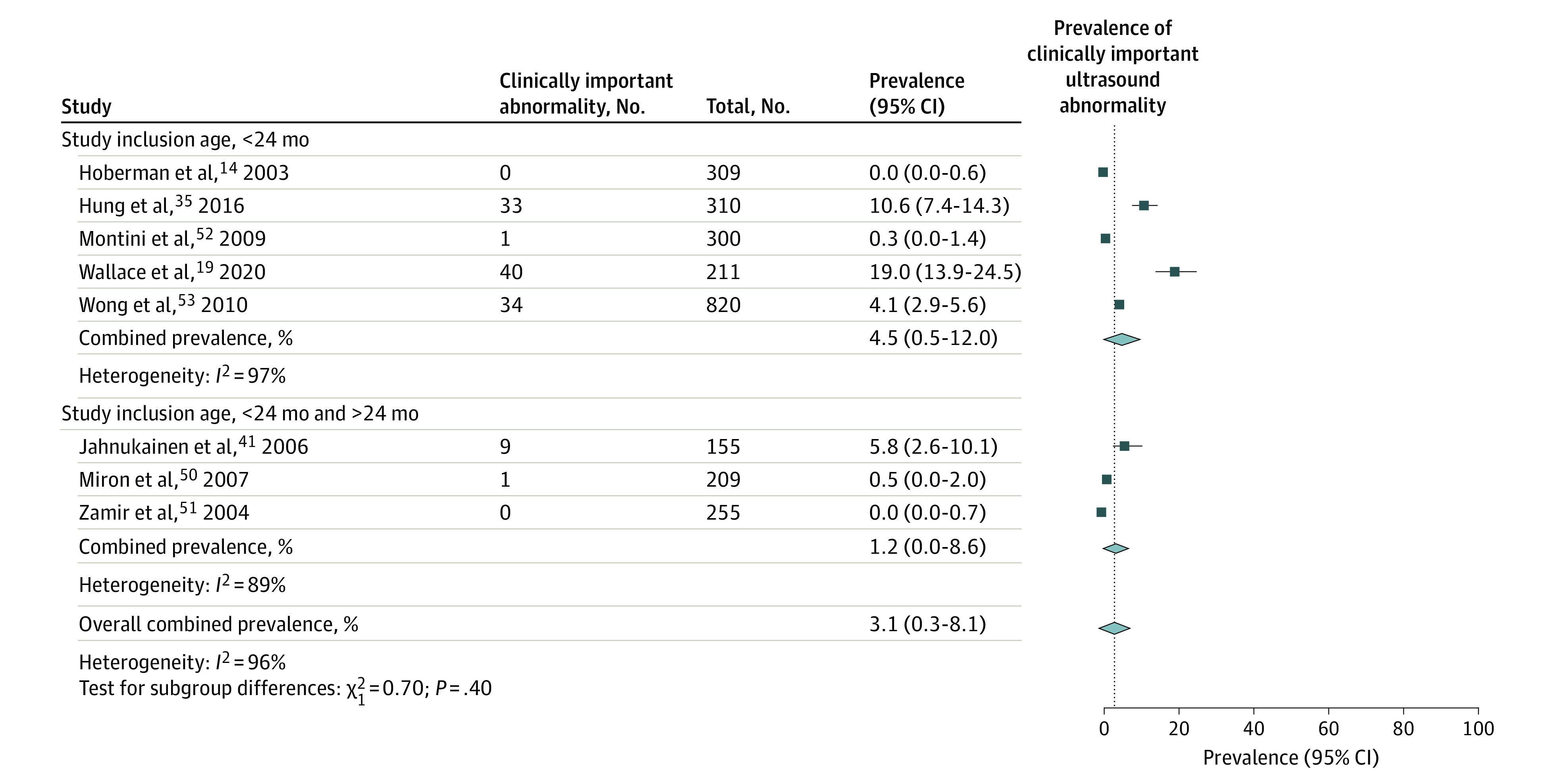
For the outcome of clinically important abnormalities detected on kidney ultrasonography, which included 8 studies14,19,35,41,50,51,52,53 with 2569 children, the pooled prevalence of clinically important abnormalities was 3.1% (95% CI, 0.3-8.1; I2 = 96%). The prevalence of clinically important abnormalities was not statistically different in studies limited to children younger than 24 months (4.5%; 95% CI, 0.5-12.0; I2 = 97%) compared with studies that also included children older than 24 months (1.2%; 95% CI, 0-8.6; I2 = 97%; P = .40) (Figure 3). In a sensitivity analysis that used an alternate prevalence of clinically important abnormality for the Wallace et al19 study defined by both moderate and severe abnormalities, the pooled prevalence was 4.0% (95% CI, 0.2-11.6; I2 = 98%).
Figure 3. Pooled Prevalence of Clinically Important Urinary Tract Abnormalities Detected on Kidney Ultrasonography After First Febrile Urinary Tract Infection in Children.
Secondary Outcomes
eTable 6 and eFigure 2 in Supplement 1 report the pooled prevalence of specific abnormalities detected on kidney ultrasonography across studies. The most common abnormalities reported were hydronephrosis (9.2%; 95% CI, 5.1-14.3; I2 = 98%; 21 studies, 6848 children), pelviectasis/dilated pelvis (6.0%; 95% CI, 2.3-11.2; I2 = 96%; 10 studies, 3920 children), and dilated ureter (2.9%; 95% CI, 1.3-5.2; I2 = 89%; 10 studies, 3401 children). The pooled prevalence of urinary tract obstruction was 0.4% (95% CI, 0.1-0.8; I2 = 59%; 12 studies, 3761 children) and for surgical intervention was 1.4% (95% CI, 0.5-2.7; I2 = 85%; 13 studies, 4153 children) (eFigure 3 in Supplement 1). One study reported health care utilization related to kidney ultrasonography,19 and no studies reported parent-reported outcomes.
Discussion
In this systematic review and meta-analysis, the prevalence of urinary tract abnormalities on kidney ultrasonography across 29 studies and 9170 children with first febrile UTI was 22.1% in studies of all ages and similar in studies of children younger than 24 months. Of the 8 studies of 2569 children that further classified the abnormalities based on whether the ultrasonography findings changed clinical management, the prevalence of clinically important abnormalities was 3.1%. The results from this meta-analysis suggest that 1 in 4 to 5 children will have an abnormality detected on kidney ultrasonography with first febrile UTI, and 1 in 32 will have an abnormality that changes clinical management. Given the considerable study heterogeneity and lack of comprehensive outcome measurement (ie, the impact on health outcomes, related health care utilization, and parent-reported value), well-designed prospective studies are needed to fully evaluate the clinical utility of kidney ultrasonography imaging after first febrile UTI.
In 2007, the UK’s NICE guidelines recommended against screening ultrasonography after first febrile UTI in infants older than 6 months if the child responds well to treatment within 48 hours and has a “typical” infection (eg, Escherichia coli with no bacteremia).10 However, no studies have comprehensively evaluated outcomes before and after the NICE guideline’s ultrasonography recommendations were implemented. Using simulation modeling, a cost-effectiveness study by Gaither et al57 compared routine kidney bladder ultrasonography after first vs second febrile UTI. They concluded that performing kidney ultrasonography in all children aged 2 to 24 months after a first febrile UTI does not meet cost-effectiveness thresholds, supporting the strategy of deferring screening until a second UTI.57
There was considerable heterogeneity across studies in our meta-analysis despite restricting the inclusion of studies of children with UTI to those with first episode UTI and with fever. Ultrasonography reporting of abnormalities across studies was not uniform, as no universally accepted standard exists. This likely was an important source of heterogeneity as the use of a standard reporting criteria by radiologists has been associated with improved accuracy of identifying and classifying abnormalities.58 Studies may have underreported minor abnormalities, thus, the pooled prevalence of abnormalities from this meta-analysis of 22.1% may be an underestimation. Subgroup analyses investigating heterogeneity found that an important study quality item related to the appropriate recruitment of study participants. Studies that required additional imaging (eg, voiding cystourethrogram) for study inclusion had a higher prevalence of abnormalities. This may have biased the study population to those with abnormal ultrasonography results who were referred for further imaging. The other subgroup analyses and meta-regression did not identify reasons to explain heterogeneity based on study age, inclusion criteria, recruitment era, study setting (hospitalized vs ambulatory), pyuria as a UTI diagnosis criterion, and sex of the study population.
Understanding what proportion of children will have a clinically important abnormality on kidney ultrasonography after first episode UTI is important to understanding its clinical utility. Studies broadly defined clinically important abnormalities based on whether it changed patient treatment; however, there was variability in how this outcome was defined and reported. Some studies specifically included a change in medical interventions (testing, consultation, parent counseling) in addition to surgical intervention, whereas other studies did not specify. It is important to note that a change in patient treatment (eg, further testing, consultation) does not always translate to improved health outcomes.11,59 Thus, to fully understand the clinical utility of kidney ultrasonography and build on this systematic review’s findings, well-designed, longitudinal, prospective studies are needed to evaluate health outcomes, health services utilization associated with minor abnormalities and overdiagnosis, and parent-reported value of testing. In addition, future studies should use clearly defined criteria to estimate clinically important abnormalities.19,60
Limitations
There are several limitations to this study. All studies were observational cohort studies, and most were retrospective. Although the highest level of evidence would come from a clinical trial that compares universal screening kidney ultrasonography with alternative strategies, we did not identify such studies. There was also considerable heterogeneity across studies. Studies provided limited information on the timing of kidney ultrasonography, therefore, we could not account for this potential source of heterogeneity. It has been postulated that there may be an increased prevalence of abnormalities (eg, urinary tract dilatation) when the ultrasonography is performed during the acute infection when the inflammatory process is active, compared with several weeks after.3,61 We could also not account for differences in the severity of illness at presentation (eg, sepsis, bacteremia) to understand heterogeneity due to a lack of reporting in the primary studies. Some analyses on the prevalence of specific abnormalities detected on ultrasonography are limited by the smaller number of studies that reported these outcomes. Furthermore, for the outcome of surgical intervention, the follow-up period varied across studies. All but 4 studies were limited to hospitalized children, therefore, our study results may not be generalizable to nonhospitalized children diagnosed with first febrile UTI.
Conclusions
In this systematic review and meta-analysis of kidney ultrasonography findings after first febrile UTI in children, the prevalence of urinary tract abnormality was 22%, and the prevalence of clinically important abnormalities that changed clinical management was 3%. Given the considerable study heterogeneity and lack of comprehensive outcome measurement, well-designed prospective longitudinal studies are needed to fully evaluate the clinical utility of kidney ultrasonography after first febrile UTI.
eMethods. Search Strategy
eTable 1. Definition of Clinically Important Abnormalities on Ultrasound Reported in Studies
eTable 2. Study Quality Assessment Tool and Response Explanation
eTable 3. Quality Assessment of Studies
eTable 4. Additional Study Characteristics
eTable 5. Kidney Ultrasound Reporting in Studies
eResults. Sensitivity Analyses for Outcome of Urinary Tract Abnormalities
eTable 6. Prevalence of Specific Abnormalities Detected on Kidney Ultrasound After First Episode Febrile Urinary Tract Infection
eFigure 1. Pooled Prevalence of Urinary Tract Abnormalities Detected on Kidney Ultrasound After First Febrile Urinary Tract Infection in Children, Subgroup Analyses
eFigure 2. Pooled Prevalence of Specific Abnormalities Detected on Kidney Ultrasound After First Febrile Urinary Tract Infection in Children
eFigure 3. Pooled Prevalence of Surgical Intervention of the Urinary Tract
Nonauthor Collaborators. Canadian Paediatric Inpatient Research Network (PIRN)
Data Sharing Statement
References
- 1.Tullus K, Shaikh N. Urinary tract infections in children. Lancet. 2020;395(10237):1659-1668. doi: 10.1016/S0140-6736(20)30676-0 [DOI] [PubMed] [Google Scholar]
- 2.Kaiser SV, Rodean J, Coon ER, Mahant S, Gill PJ, Leyenaar JK. Common diagnoses and costs in pediatric hospitalization in the US. JAMA Pediatr. 2022;176(3):316-318. doi: 10.1001/jamapediatrics.2021.5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts KB; Subcommittee on Urinary Tract Infection; Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330 [DOI] [PubMed] [Google Scholar]
- 4.Robinson JL, Finlay JC, Lang ME, Bortolussi R; Canadian Paediatric Society, Infectious Diseases and Immunization Committee, Community Paediatrics Committee . Urinary tract infections in infants and children: diagnosis and management. Paediatr Child Health. 2014;19(6):315-325. doi: 10.1093/pch/19.6.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu B, Liu Y, Wang H, Duan F, Mi L, Liang Y. Clinical guidelines of UTIs in children: quality appraisal with AGREE II and recommendations analysis. BMJ Open. 2022;12(4):e057736. doi: 10.1136/bmjopen-2021-057736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein R, Dogan HS, Hoebeke P, et al. ; European Association of Urology; European Society for Pediatric Urology . Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67(3):546-558. doi: 10.1016/j.eururo.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Ammenti A, Alberici I, Brugnara M, et al. ; Italian Society of Pediatric Nephrology . Updated Italian recommendations for the diagnosis, treatment and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020;109(2):236-247. doi: 10.1111/apa.14988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayakumar M, Kanitkar M, Nammalwar BR, Bagga A; Indian Society of Pediatric Nephrology . Revised statement on management of urinary tract infections. Indian Pediatr. 2011;48(9):709-717. [PubMed] [Google Scholar]
- 9.McTaggart S, Danchin M, Ditchfield M, et al. ; Kidney Health Australia - Caring for Australasians with Renal Impairment . KHA-CARI guideline: diagnosis and treatment of urinary tract infection in children. Nephrology (Carlton). 2015;20(2):55-60. doi: 10.1111/nep.12349 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence (NICE) . Urinary Tract Infection in Under 16s: Diagnosis and Management. National Institute for Health and Clinical Excellence; 2018. [PubMed] [Google Scholar]
- 11.Kennedy AG. Evaluating the effectiveness of diagnostic tests. JAMA. 2022;327(14):1335-1336. doi: 10.1001/jama.2022.4463 [DOI] [PubMed] [Google Scholar]
- 12.Mahant S, Friedman J, MacArthur C. Renal ultrasound findings and vesicoureteral reflux in children hospitalised with urinary tract infection. Arch Dis Child. 2002;86(6):419-420. doi: 10.1136/adc.86.6.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westwood ME, Whiting PF, Cooper J, Watt IS, Kleijnen J. Further investigation of confirmed urinary tract infection (UTI) in children under five years: a systematic review. BMC Pediatr. 2005;5(1):2. doi: 10.1186/1471-2431-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348(3):195-202. doi: 10.1056/NEJMoa021698 [DOI] [PubMed] [Google Scholar]
- 15.Nelson CP, Johnson EK, Logvinenko T, Chow JS. Ultrasound as a screening test for genitourinary anomalies in children with UTI. Pediatrics. 2014;133(3):e394-e403. doi: 10.1542/peds.2013-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. doi: 10.1542/peds.2014-1778 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 18.Viteri B, Calle-Toro JS, Furth S, Darge K, Hartung EA, Otero H. State-of-the-art renal imaging in children. Pediatrics. 2020;145(2):e20190829. doi: 10.1542/peds.2019-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace SS, Ban K, Singh A, et al. Clinical predictors for abnormal renal bladder ultrasound in hospitalized young children with a first febrile urinary tract infection. Hosp Pediatr. 2020;10(5):392-400. doi: 10.1542/hpeds.2019-0240 [DOI] [PubMed] [Google Scholar]
- 20.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147-153. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. 2005;30(3):261-293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- 22.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 23.Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesizers of evidence. BMC Med Res Methodol. 2021;21(1):189. doi: 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 25.Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prev Sci. 2013;14(2):134-143. doi: 10.1007/s11121-013-0377-7 [DOI] [PubMed] [Google Scholar]
- 26.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559-1573. doi: 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 27.R Project for Statistical Computing. R: A Language and Environment for Statistical Computing. Accessed April 8, 2022. https://www.R-project.org/
- 28.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 29.Kobayashi Y, Mishina H, Michihata N, Miyasaka M, Takayama JI. Indication for voiding cystourethrography during first urinary tract infection. Pediatr Int. 2019;61(6):595-600. doi: 10.1111/ped.13835 [DOI] [PubMed] [Google Scholar]
- 30.Soccorso G, Wagstaff J, Blakey K, et al. Investigating febrile UTI in infants: is a cystogram necessary? J Pediatr Urol. 2010;6(2):148-152. doi: 10.1016/j.jpurol.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Lertdumrongluk K, Lertdumrongluk P. Predictive score for vesicoureteral reflux in children with a first febrile urinary tract infection. Int J Urol. 2021;28(5):573-577. doi: 10.1111/iju.14515 [DOI] [PubMed] [Google Scholar]
- 32.Breinbjerg A, Jørgensen CS, Frøkiær J, Tullus K, Kamperis K, Rittig S. Risk factors for kidney scarring and vesicoureteral reflux in 421 children after their first acute pyelonephritis, and appraisal of international guidelines. Pediatr Nephrol. 2021;36(9):2777-2787. doi: 10.1007/s00467-021-05042-7 [DOI] [PubMed] [Google Scholar]
- 33.Pennesi M, Amoroso S, Pennesi G, et al. Is ultrasonography mandatory in all children at their first febrile urinary tract infection? Pediatr Nephrol. 2021;36(7):1809-1816. doi: 10.1007/s00467-020-04909-5 [DOI] [PubMed] [Google Scholar]
- 34.Hsu CC, Tsai JD, Ku MS, et al. Antimicrobial resistance and diagnostic imaging in infants younger than 2 months old hospitalized with a first febrile urinary tract infection: a population-based comparative study. Pediatr Infect Dis J. 2016;35(8):840-845. doi: 10.1097/INF.0000000000001184 [DOI] [PubMed] [Google Scholar]
- 35.Hung TW, Tsai JD, Liao PF, Sheu JN. Role of renal ultrasonography in predicting vesicoureteral reflux and renal scarring in children hospitalized with a first febrile urinary tract infection. Pediatr Neonatol. 2016;57(2):113-119. doi: 10.1016/j.pedneo.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 36.Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. doi: 10.1136/archdischild-2016-311587 [DOI] [PubMed] [Google Scholar]
- 37.Arlen AM, Merriman LS, Kirsch JM, et al. Early effect of American Academy of Pediatrics urinary tract infection guidelines on radiographic imaging and diagnosis of vesicoureteral reflux in the emergency room setting. J Urol. 2015;193(5)(suppl):1760-1765. doi: 10.1016/j.juro.2014.06.100 [DOI] [PubMed] [Google Scholar]
- 38.Cheng CH, Hang JF, Tsau YK, Lin TY. Nephromegaly is a significant risk factor for renal scarring in children with first febrile urinary tract infections. J Urol. 2011;186(6):2353-2357. doi: 10.1016/j.juro.2011.07.112 [DOI] [PubMed] [Google Scholar]
- 39.Ismaili K, Wissing KM, Lolin K, et al. Characteristics of first urinary tract infection with fever in children: a prospective clinical and imaging study. Pediatr Infect Dis J. 2011;30(5):371-374. doi: 10.1097/INF.0b013e318204dcf3 [DOI] [PubMed] [Google Scholar]
- 40.Pennesi M, L’erario I, Travan L, Ventura A. Managing children under 36 months of age with febrile urinary tract infection: a new approach. Pediatr Nephrol. 2012;27(4):611-615. doi: 10.1007/s00467-011-2087-3 [DOI] [PubMed] [Google Scholar]
- 41.Jahnukainen T, Honkinen O, Ruuskanen O, Mertsola J. Ultrasonography after the first febrile urinary tract infection in children. Eur J Pediatr. 2006;165(8):556-559. doi: 10.1007/s00431-006-0113-4 [DOI] [PubMed] [Google Scholar]
- 42.Kawai S, Nakai H, Kanai T, et al. Prevention of recurrent febrile urinary tract infection in infants: ultrasonography-oriented approach is more practical than a top-down approach. Pediatr Int. 2019;61(10):1007-1014. doi: 10.1111/ped.13970 [DOI] [PubMed] [Google Scholar]
- 43.Harper L, Delforge X, Maurin S, et al. A novel approach to evaluating the benefit of post–urinary tract infection renal ultrasonography, using decision curve analysis. Pediatr Nephrol. 2016;31(10):1631-1636. doi: 10.1007/s00467-016-3410-9 [DOI] [PubMed] [Google Scholar]
- 44.Wongbencharat K, Tongpenyai Y, Na-Rungsri K. Renal ultrasound and DMSA screening for high-grade vesicoureteral reflux. Pediatr Int. 2016;58(3):214-218. doi: 10.1111/ped.12803 [DOI] [PubMed] [Google Scholar]
- 45.Ipek IO, Sezer RG, Senkal E, Bozaykut A. Relationship between procalcitonin levels and presence of vesicoureteral reflux during first febrile urinary tract infection in children. Urology. 2012;79(4):883-887. doi: 10.1016/j.urology.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 46.Sasaki J, Parajuli N, Sharma P, et al. Utility of post–urinary tract infection imaging in patients with normal prenatal renal ultrasound. Clin Pediatr (Phila). 2012;51(3):244-246. doi: 10.1177/0009922811420712 [DOI] [PubMed] [Google Scholar]
- 47.Lytzen R, Thorup J, Cortes D. Experience with the NICE guidelines for imaging studies in children with first pyelonephritis. Eur J Pediatr Surg. 2011;21(5):283-286. doi: 10.1055/s-0031-1277212 [DOI] [PubMed] [Google Scholar]
- 48.Lee MD, Lin CC, Huang FY, Tsai TC, Huang CT, Tsai JD. Screening young children with a first febrile urinary tract infection for high-grade vesicoureteral reflux with renal ultrasound scanning and technetium-99m-labeled dimercaptosuccinic acid scanning. J Pediatr. 2009;154(6):797-802. doi: 10.1016/j.jpeds.2008.12.045 [DOI] [PubMed] [Google Scholar]
- 49.Huang HP, Lai YC, Tsai IJ, Chen SY, Tsau YK. Renal ultrasonography should be done routinely in children with first urinary tract infections. Urology. 2008;71(3):439-443. doi: 10.1016/j.urology.2007.10.049 [DOI] [PubMed] [Google Scholar]
- 50.Miron D, Daas A, Sakran W, Lumelsky D, Koren A, Horovitz Y. Is omitting post urinary tract infection renal ultrasound safe after normal antenatal ultrasound—an observational study. Arch Dis Child. 2007;92(6):502-504. doi: 10.1136/adc.2006.108662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89(5):466-468. doi: 10.1136/adc.2002.019182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montini G, Zucchetta P, Tomasi L, et al. Value of imaging studies after a first febrile urinary tract infection in young children: data from Italian Renal Infection Study 1. Pediatrics. 2009;123(2):e239-e246. doi: 10.1542/peds.2008-1003 [DOI] [PubMed] [Google Scholar]
- 53.Wong SN, Tse NKC, Lee KP, et al. Evaluating different imaging strategies in children after first febrile urinary tract infection. Pediatr Nephrol. 2010;25(10):2083-2091. doi: 10.1007/s00467-010-1569-z [DOI] [PubMed] [Google Scholar]
- 54.Yılmaz S, Özçakar ZB, Kurt Şükür ED, et al. Vesicoureteral reflux and renal scarring risk in children after the first febrile urinary tract infection. Nephron. 2016;132(3):175-180. doi: 10.1159/000443536 [DOI] [PubMed] [Google Scholar]
- 55.Han SY, Lee IR, Park SJ, Kim JH, Shin JI. Usefulness of neutrophil-lymphocyte ratio in young children with febrile urinary tract infection. Korean J Pediatr. 2016;59(3):139-144. doi: 10.3345/kjp.2016.59.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23(6):478-480. doi: 10.1007/BF02012459 [DOI] [PubMed] [Google Scholar]
- 57.Gaither TW, Selekman R, Kazi DS, Copp HL. Cost-effectiveness of screening ultrasound after a first, febrile urinary tract infection in children aged 2-24 months. J Pediatr. 2020;216:73-81.e1. doi: 10.1016/j.jpeds.2019.06.049 [DOI] [PubMed] [Google Scholar]
- 58.Swartz S, Thakrar P, Kolinski J, et al. Imaging practices and implications in young infants with urinary tract infection. Hosp Pediatr. 2022;12(11):922-932. doi: 10.1542/hpeds.2021-006507 [DOI] [PubMed] [Google Scholar]
- 59.Staub LP, Lord SJ, Simes RJ, et al. Using patient management as a surrogate for patient health outcomes in diagnostic test evaluation. BMC Med Res Methodol. 2012;12:12. doi: 10.1186/1471-2288-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen HT, Benson CB, Bromley B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol. 2014;10(6):982-998. doi: 10.1016/j.jpurol.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 61.Mattoo TK, Shaikh N, Nelson CP. Contemporary management of urinary tract infection in children. Pediatrics. 2021;147(2):e2020012138. doi: 10.1542/peds.2020-012138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy
eTable 1. Definition of Clinically Important Abnormalities on Ultrasound Reported in Studies
eTable 2. Study Quality Assessment Tool and Response Explanation
eTable 3. Quality Assessment of Studies
eTable 4. Additional Study Characteristics
eTable 5. Kidney Ultrasound Reporting in Studies
eResults. Sensitivity Analyses for Outcome of Urinary Tract Abnormalities
eTable 6. Prevalence of Specific Abnormalities Detected on Kidney Ultrasound After First Episode Febrile Urinary Tract Infection
eFigure 1. Pooled Prevalence of Urinary Tract Abnormalities Detected on Kidney Ultrasound After First Febrile Urinary Tract Infection in Children, Subgroup Analyses
eFigure 2. Pooled Prevalence of Specific Abnormalities Detected on Kidney Ultrasound After First Febrile Urinary Tract Infection in Children
eFigure 3. Pooled Prevalence of Surgical Intervention of the Urinary Tract
Nonauthor Collaborators. Canadian Paediatric Inpatient Research Network (PIRN)
Data Sharing Statement